Attached files
| file | filename |
|---|---|
| EX-10.2 - EX-10.2 - Presbia PLC | d887068dex102.htm |
| EX-23.1 - EX-23.1 - Presbia PLC | d887068dex231.htm |
| EX-32.1 - EX-32.1 - Presbia PLC | d887068dex321.htm |
| EX-21.1 - EX-21.1 - Presbia PLC | d887068dex211.htm |
| EX-31.1 - EX-31.1 - Presbia PLC | d887068dex311.htm |
| EX-31.2 - EX-31.2 - Presbia PLC | d887068dex312.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
(Mark One)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2014
or
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to .
Commission File Number 001-36824
PRESBIA PLC
(Exact name of registrant as specified in its charter)
| Ireland | 98-1162329 | |
| (State or other jurisdiction of incorporation or organization) |
(IRS Employer Identification No.) |
120/121 Baggot Street Lower
Dublin 2 Ireland
(Address of principal executive offices, including zip code)
Registrant’s Telephone Number, Including Area Code:
+353 (1) 659 9446
Securities registered pursuant to Section 12(b) of the Act:
| Ordinary Shares, $0.001 Par Value | The NASDAQ Global Market | |
| (Title of each class) | (Name of each exchange on which registered) |
Securities registered pursuant to Section 12(g) of the Act: NONE
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ¨ No x
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). ¨ Yes ¨ No
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in 12b-2 of the Exchange Act.
| Large accelerated filer | ¨ | Accelerated filer | ¨ | |||
| Non-accelerated filer | x | Smaller reporting company | ¨ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
The aggregate market value of the Registrant’s ordinary shares (the only common equity of the registrant) held by non-affiliates for the last business day of the Registrant’s most recently completed second fiscal quarter: Not applicable because the Registrant’s common equity was not publicly traded as of such date.
As of March 24, 2015, there were 13,351,874 ordinary shares outstanding.
PRESBIA PLC
2014 ANNUAL REPORT ON FORM 10-K
Cautionary Note Regarding Forward-Looking Statements
This Annual Report on Form 10-K contains forward-looking statements, within the meaning of the Private Securities Litigation Reform Act of 1995, that involve substantial risks and uncertainties. The forward-looking statements are contained principally in the sections of this Annual Report on Form 10-K titled “Business,” “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” but are also contained elsewhere in this Annual Report on Form 10-K. In some cases, you can identify forward-looking statements by the words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “objective,” “ongoing,” “plan,” “predict,” “project,” “potential,” “should,” “will,” or “would,” and or the negative of these terms, or other comparable terminology intended to identify statements about the future. These statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements. Although we believe that we have a reasonable basis for each forward-looking statement contained in this Annual Report on Form 10-K, we caution you that these statements are based on a combination of facts and factors currently known by us and our expectations of the future, about which we cannot be certain.
The forward-looking statements in this Annual Report on Form 10-K include, among other things, statements about:
| • | the initiation, timing, progress and results of our clinical trials, our regulatory submissions and our research and development programs; |
| • | our ability to advance our products into, and successfully complete, clinical trials; |
| • | our ability to obtain pre-market approvals; |
| • | the commercialization of our products; |
| • | the implementation of our business model, strategic plans for our business, products and technology; |
| • | the scope of protection we are able to establish and maintain for intellectual property rights covering our products and technology; |
| • | estimates of our expenses, future revenues, capital requirements and our needs for additional financing; |
| • | the timing or likelihood of regulatory filings and approvals; |
| • | our financial performance; and |
| • | developments relating to our competitors and our industry. |
You should refer to “Part I, Item 1A. Risk Factors” of this Annual Report on Form 10-K for a discussion of important factors that may cause our actual results to differ materially from those expressed or implied by our forward-looking statements. As a result of these factors, we cannot assure you that the forward-looking statements in this Annual Report on Form 10-K will prove to be accurate. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame or at all. We undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
You should read this Annual Report on Form 10-K and the documents that we reference in this Annual Report on Form 10-K and have filed as exhibits to this Annual Report on Form 10-K completely and with the understanding that our actual future results may be materially different from what we expect. We qualify all of our forward-looking statements by these cautionary statements.
1
Industry and Market Data
We obtained the industry and market data in this Annual Report on Form 10-K from our own research as well as from industry and general publications and surveys and studies conducted by third parties. Industry and general publications, studies and surveys generally state that the information contained therein has been obtained from sources believed to be reliable. These third parties may, in the future, alter the manner in which they conduct surveys and studies regarding the markets in which we operate our business. As a result, you should carefully consider the inherent risks and uncertainties associated with the industry and market data contained in this Annual Report on Form 10-K, including those discussed in “Part I, Item 1A. Risk Factors.”
Trademarks
This Annual Report on Form 10-K includes trademarks, service marks and trade names owned by us or other companies. All trademarks, service marks and trade names included in this Annual Report on Form 10-K are the property of their respective owners. Our principal trademark or trade name that we use is PresbiaTM.
2
Part I
In January 2015, we completed a series of corporate reorganization transactions described in “Item 1. Business—Corporate History and Information,” which we refer to herein as the Reorganization Transactions. Unless we state otherwise, the terms “we,” “us,” “our,” “Presbia” and the “company” refer to Presbia PLC and its consolidated subsidiaries after giving effect to the Reorganization Transactions. Prior to the completion of the Reorganization Transactions, the foregoing terms refer to the entities that became the consolidated subsidiaries of Presbia PLC upon consummation of the Reorganization Transactions.
Overview
We are an ophthalmic device company which has developed and is currently marketing a proprietary optical lens implant for treating presbyopia, the age-related loss of the ability to focus on near objects. Our lens, which we refer to as our microlens, is a miniature lens designed to be surgically implanted in a patient’s eye to improve that patient’s ability to see objects at close distances. Our current strategy is to continue to commercialize our microlens in certain strategic countries where we currently have marketing approval and to continue to seek to obtain marketing approval in other key markets, including the United States. Our goal is to become a leading provider of corneal inlay presbyopia-correcting treatment worldwide.
According to Market Scope, an ophthalmic market research organization, presbyopia is a common vision disorder that affects approximately 1.8 billion people worldwide. Presbyopia is associated with the inability of the eye’s natural lens to change shape, or accommodate, in order to see clearly objects in the near and middle distance ranges. According to Market Scope, the worldwide presbyopic population is expected to grow to approximately 2.1 billion by 2020. According to Market Scope, spending on devices, equipment and procedure fees for presbyopia-correcting surgery is expected to increase from approximately $408 million in 2014 to approximately $750 million in 2019 at the manufacturer level. We do not currently have marketing approval in many jurisdictions included in the foregoing global data, which jurisdictions collectively represent a majority of the worldwide presbyopic population. We have marketing approval in a number of strategic countries that we are targeting for commercialization and we are actively seeking marketing approval in certain other strategic countries that we are targeting for commercialization, including the United States.
We believe that our solution offers each of the following benefits:
| • | our solution is effective as a standalone solution for plano presbyopes, or those individuals who suffer from presbyopia but do not have any other visual disorder, and may also be used in conjunction with laser procedures or lens replacement procedures for those individuals who in addition to being presbyopic suffer from other visual disorders. |
| • | our solution is minimally invasive; our microlens can be implanted and removed in simple, surgical procedures. |
| • | we believe that our solution offers significant near vision improvement with little or no loss of binocular distance visual acuity (the ability to see distant objects with both eyes without prescription enhancement) and minimal risk of adverse side effects. |
| • | our solution offers a wide range of corrective power, from +1.5 diopters to +3.5 diopters, in 0.25 diopter increments (a diopter is a unit of measurement of the optical power of a lens). |
| • | once implanted, our microlens is invisible to the naked eye. |
In addition, our microlens and the procedure to implant our microlens are not currently reimbursed through private or governmental third-party payors in any country, nor do we anticipate that they will be reimbursable in the foreseeable future. Although the commercialization of our microlens depends on a prospective patient’s ability to cover the costs of our microlens and the implantation procedure and we believe that a substantial
3
portion of presbyopes worldwide do not have the financial means to cover the costs of our microlens, we believe that a direct patient pay model enables medical providers to avoid pricing pressure from private or governmental third-party payors.
In 2012, we completed a 12-month, multicenter, post-marketing evaluation of our microlens in Italy and Greece in presbyopic patients between the ages of 45 and 60. We designed, and oversaw the implementation of, the protocol for this evaluation, which was conducted at our request by a surgeon at the Vardinoyannion Eye Institute at the University of Crete in Crete, Greece and by a surgeon at Prato Hospital in Prato, Italy. The average uncorrected near visual acuity (the ability to see close objects without prescription enhancement), or UCVA-near, in the operated eye of the 70 patients who completed the study was 20/110 (Snellen). Following implantation with our microlens, such patients had an average UCVA-near of 20/27 12 months post-surgery. Although there was a small loss in uncorrected distance vision in the operated eye, there was no significant change in binocular distance visual acuity after 12 months post-surgery. Our microlens has also been the subject of certain third party studies. We did not commission these studies or design, review or oversee the implementation of their protocols, and we have limited information with respect to these studies.
In addition to being an effective standalone treatment for presbyopia, we believe that our solution can also be used in conjunction with other surgical approaches that are used to treat vision disorders other than presbyopia. For example, we believe that our microlens procedure can be combined with laser in-situ keratomileusis, or LASIK, procedures, which are used to treat certain near distance and far distance visual disorders, as well as lens replacement procedures used to treat cataracts (whereby the natural lens is replaced with an intraocular lens, or IOL implant). We also believe that our microlens can be used to treat presbyopia in certain post-LASIK and post-cataract surgery patients. We believe that, having undergone eye surgery in the past, certain patients are more likely than the general population to consider eye surgery to treat presbyopia. Moreover, as we believe that our solution can be performed at the same time that certain other forms of vision-correction treatments are being administered, we believe that our solution provides an integrated treatment option for patients and an additional source of revenue for the patient’s ophthalmic surgeon. In this way, we believe that our solution complements existing surgical treatments for vision problems.
Our microlens procedure is performed using a 150 kilohertz or greater frequency femtosecond laser, which is a laser that is currently used in certain LASIK surgeries, cataract surgeries and cornea replacement surgeries. In commercializing our solution, we intend to target those markets with a well-established presence of refractive laser centers equipped with femtosecond lasers. We believe that the existing infrastructure in most such laser centers is sufficient to make our solution an attractive opportunity for such laser centers and our commercialization strategy includes working closely with such laser centers to train and qualify ophthalmic surgeons on the use of our solution.
Through our European Union CE Mark, we are generally authorized to market our microlens throughout the European Economic Area (27 of the 28 European Union member states plus Iceland, Liechtenstein and Norway), or EEA, and Switzerland. We currently market our microlens in certain strategic EEA countries as well as certain strategic countries outside of the EEA in which we possess marketing approval. Through March 15, 2015, ophthalmic surgeons have implanted over 600 of our microlenses outside of the United States. For geographic information regarding our revenues and long-lived assets, please see Note 10 to our audited financial statements included elsewhere in this Annual Report on Form 10-K.
We are presently seeking marketing approval in other strategic countries, including the United States. In order to commercialize our microlens and our proprietary insertion tool, which we refer to as our microlens inserter, in the United States, we must first obtain a pre-market approval, or PMA, from the U.S. Food and Drug Administration, or the FDA. In December 2013, we received approval of our investigational device exemption, or IDE, to commence a staged pivotal clinical trial in order to obtain clinical data necessary to obtain FDA approval to market our microlens and microlens inserter in the United States. We began enrollment for this study in May 2014 and began treating patients in June 2014. Initially, 75 subjects underwent insertion of our microlens
4
at six investigational sites in the first stage of this study. Based on six-month data on 52 of these subjects, in January 2015, we submitted an interim safety report to the FDA along with a supplement to our IDE with the request for approval to begin second stage enrollment. In February 2015, we received approval from the FDA to commence second stage enrollment in this trial. We are permitted to enroll up to an additional 337 subjects at up to nine additional investigational sites. Through March 15, 2015, 25 subjects underwent insertion of our microlens in the second stage of this study. We do not expect to receive approval from the FDA and commence commercial activity in the United States before the fourth quarter of 2017.
The Eye and Vision Problems
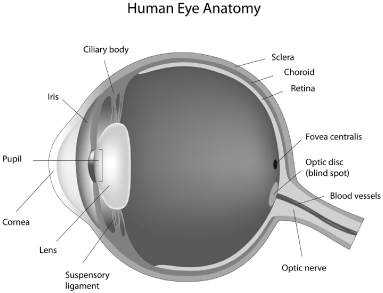
The human eye is a specialized sensory organ capable of receiving visual images and transmitting them to the visual center in the brain. Among the main parts of the eye are the cornea, the iris, the lens and the retina. The cornea is the clear window in the front of the eye through which light first passes. The interior surface of the cornea is lined with a single layer of flat, tile-like endothelial cells, whose function is to maintain the transparency of the cornea. The iris is a pigmented muscular curtain located behind the cornea that opens and closes to regulate the amount of light entering the eye through the pupil, an opening at the center of the iris. The lens, known in medical terminology as the “crystalline lens,” is a clear structure located behind the cornea that changes shape, or accommodates, to focus light on the back of the eye. The retina is a layer of nerve tissue in the back of the eye that senses the light image and transmits it to the brain via the optic nerve. The figure below illustrates certain elements of the basic anatomy of the human eye.
The eye may be affected by common visual disorders, disease or trauma. A normal, well-functioning eye receives images of objects at varying distances and focuses the images on the retina. Refractive errors (including myopia, hyperopia, presbyopia and astigmatism, each described below) occur when the eye cannot properly focus an image on the retina. In addition to presbyopia, common vision problems include:
| • | myopia, or nearsightedness, which occurs when the eye’s lens focuses images in front of the retina; |
| • | hyperopia, or farsightedness, which occurs when the eye’s lens focuses images behind the plane of the retina; |
| • | astigmatism, an optical defect in which vision is blurred due to an oval-shaped cornea or, in some cases, an oval-shaped natural lens, producing a distorted image on the retina. Astigmatism may accompany myopia or hyperopia; and |
5
| • | cataracts, a clouding of the lens, which worsens with time and gradually occludes incoming light images. |
Cataracts are age-related, while myopia, hyperopia and astigmatism are not age-related. The most common surgical treatment for myopia, hyperopia and astigmatism is LASIK surgery, in which the surface of the cornea is carefully mapped and then a computerized optical laser uses this mapping to reshape the surface of the cornea by ablation to permit proper focusing. Cataracts are most often treated by surgically removing the affected lens and replacing it with a monofocal (that is, a single focus) IOL.
Presbyopia is an age-related refractive disorder that generally begins to develop when a person reaches the age of 35. The disorder may go unnoticed for several years after its initial onset and can worsen with age. The first symptoms of presbyopia are typically experienced when a person begins to have difficulty reading fine print. Presbyopia is associated with a loss of lens “elasticity,” the ability of the lens to change shape in order to focus incoming light on the retina from objects in near and middle distance ranges. Elasticity is slowly lost as people age, resulting in a slow decrease in the ability of the eye to focus on nearby objects. Presbyopia is a natural part of aging and affects substantially all people at some point in their adult lives.
Presbyopia Market
According to Market Scope, presbyopia currently affects approximately 1.8 billion people worldwide, or approximately 25% of the global population. According to Market Scope, the worldwide presbyopic population is expected to grow to approximately 2.1 billion people by the end of 2020. The global market opportunity for surgical treatment of presbyopia is large and growing due to the aging of the population. Globally, the median age is projected to increase from 29 years in 2011 to 38 years by 2050. Consistent with the expected growth in the worldwide presbyopic population, according to Market Scope, the annual number of presbyopia-correcting surgeries performed globally is expected to increase from approximately 625,000 procedures in 2014 to approximately 1.2 million procedures by 2019. According to Market Scope, corneal inlays are projected to be the fastest growing segment of this market and are expected to grow from approximately 18,000 procedures in 2014 to approximately 204,000 procedures in 2019. In addition, according to Market Scope, spending on devices, equipment and procedure fees for presbyopia-correcting surgery is expected to increase from approximately $408 million in 2014 to approximately $750 million in 2019 at the manufacturer level. We do not have marketing approval in many jurisdictions included in the foregoing global data, which jurisdictions collectively represent a majority of the worldwide presbyopic population. We have marketing approval in a number of strategic countries that we are targeting for commercialization and we are actively seeking marketing approval in certain other strategic countries that we are targeting for commercialization, including the United States.
Approaches for Treating Presbyopia
Although reading glasses and contact lenses have historically been, and remain, the most common solution for presbyopia, there are significant drawbacks associated with these non-surgical approaches. Eyeglasses can easily be lost, misplaced, broken or scratched and require frequent cleaning. Also, many people wish to avoid the inconvenience of keeping reading glasses close at hand. Contact lenses require daily insertion, removal and maintenance, which can be problematic for an increasingly mobile population and for people living and working in dusty environments or in unsanitary conditions.
There are presently four surgical correction categories for treating presbyopia:
Monovision. Monovision treatments correct one eye, typically the dominant eye, for distance vision and correct the other, non-dominant eye for near vision. While monovision may be accomplished through the use of glasses with two different lenses with varying thickness, that approach can cause bothersome symptoms when a person looks through the edges of the glasses. A more typical approach to monovision is the use of two different contact lenses. A more permanent monovision approach is to undergo laser or IOL-
6
based refractive surgeries adapted for presbyopia correction. A significant drawback of monovision surgical treatments is the complexity of achieving additional correction, if vision further deteriorates. Additional drawbacks include occasional patient adaptation issues, whereby patients have difficulty adjusting to the monovision arrangement and suffer from blurring of vision, difficulty driving at night and loss of stereopsis, or the ability to focus upon an object with both eyes and create a single stereoscopic image.
Multifocal. Multifocal approaches are designed to provide both distance and near focus at the same time in each eye. Generally, both depth perception, or the ability to judge the distance of an object, and contrast sensitivity, or the ability to detect detail having subtle color gradations, are generally improved when two eyes can focus on an object. In addition, these approaches are intended to be improvements over constantly taking glasses off and putting them back on (possibly by wearing glasses around the neck) or by using bifocal or varifocal glasses or contact lenses, in which the eye is trained to look through the top part for distance vision and the bottom part for near vision. Multifocal effects can be achieved by lens replacement, including multifocal IOLs (IOLs with different zones of varying power), or through the creation of a multifocal cornea using laser refractive surgery (to create two or more refractive zones on the central cornea) or intrastromal ablation (laser used to make small changes in the thickness of the cornea). As with monovision, a significant drawback of these multifocal approaches is the complexity of achieving additional correction, if vision further deteriorates. In addition, some patients may experience halos, or rings around lights, at night, and it may also take time for multifocal patients to adapt to the different focal areas.
Restoring Accommodation. Accommodating approaches generally attempt to replace the natural lens with an accommodating IOL, which is an artificial lens that is designed to mimic the movement of the natural crystalline lens of the eye. All IOL-based surgeries are susceptible to opacification, or clouding, of the lens capsule, which is the part of the natural lens covering that remains after surgery, decreasing vision and requiring a laser procedure to cut a hole in the clouded back lining of the lens capsule to allow light to pass through the membrane to the retina. Accommodating IOLs are also subject to certain other complications pertaining to the shrinkage, closure or clouding of the capsule that can reduce the mode of action of the accommodating IOLs, rendering them less effective. Other less common accommodating techniques include lens softening and scleral relaxation techniques, which are designed to improve near vision by restoring the function of the eye’s own accommodative system. Lens-softening techniques use pharmaceuticals or lasers to soften or change the structure of the natural crystalline lens, allowing it to flex better to increase accommodation. Scleral relaxation techniques use implants in the sclera of the eye to increase the eye’s ability to focus at near distances. To date, these procedures have had little documented success.
Corneal Inlays. Corneal inlays include miniature surgically implanted lenses (such as our microlens), optical devices inserted into the cornea to reshape the front surface of the eye, and small implants to reduce the size of the opening into the eye to reset the angle of the light rays entering the eye and reduce both the number of rays and the light scatter, each of which is designed to improve near vision.
Our Solution
We have designed our microlens to address certain limitations of other surgical approaches to treat presbyopia. The critical aspects of our solution include:
| • | Effective Treatment Option for Plano Presbyopes. The largest sub-group of the presbyopic population is plano presbyopes, or those individuals without significant refractive error who suffer from presbyopia. Plano presbyopes account for approximately 38% of the total presbyopic population. We believe that ophthalmologists are generally reluctant to recommend a LASIK or IOL procedure as a solution for a plano presbyope given the inherent risks and visual compromises of such procedures. Because our procedure does not involve the removal of the natural lens, the reshaping of the cornea or the removal of corneal tissue, we believe that ophthalmologists will be more likely to recommend our microlens as a solution for plano presbyopes than a LASIK or IOL procedure. |
| • | Complementary Solution. In addition to being a treatment option for plano presbyopes, we believe that our solution can be used in conjunction with other surgical procedures that treat vision disorders other |
7
| than presbyopia, including LASIK procedures for near and/or distance vision correction and the implantation of traditional monofocal IOLs used to treat cataracts. In addition, we believe that our microlens can be used to treat presbyopia in certain post-LASIK and post-cataract surgery patients. |
| • | Minimally Invasive. Our microlens is implanted in a pocket in the cornea created with a femtosecond laser. The pocket seals itself within a few days, holding the lens in place. The procedure does not require the reshaping of the cornea and no corneal tissue is removed. Moreover, the nature of our solution permits normal nutrient flow to the cornea, enabling corneal metabolism. As a result, there is less potential for dry-eye symptoms and less damage to the collagen fibers that support corneal shape and structure. |
| • | Removable. We have designed our microlens and procedure to be easily removable. We believe that designing a lens that is removable gives patients the ease of mind of knowing that if they are uncomfortable with the results, or if technological advances produce different solutions in the future, they have not taken a step that prevents them from being able to undergo future procedures. The design of our microlens will also permit removal in the event that a patient’s presbyopia significantly progresses with age and the patient wishes to have a lens with additional diopter power implanted. We believe that by designing our microlens and procedure in a manner that allows our microlens to easily be replaced, ophthalmic surgeons will be able to choose the lens most appropriate for a given patient as the patient ages and the patient’s presbyopia progresses. In the United States, our IDE does not permit replacement of a microlens in the event that a patient’s microlens is removed after implantation. Also, in the United States, our IDE requires any removal of the microlens to be reported as an adverse event. |
| • | Correction Options. The range of optical power corrections available in our microlens allows the ophthalmic surgeon to choose the correction most appropriate for the patient’s specific near vision requirements, as opposed to a unilateral “one size fits all” approach. |
| • | Invisible. The clear nature of our microlens renders it invisible to the naked eye which we believe will make it appealing to patients. |
| • | Does Not Hinder Certain Other Procedures. Our microlens does not hinder examination of the retina and other structures in the eye necessary to diagnose other ocular health disorders. |
| • | Minimal Side Effects. In the limited number of procedures performed to date outside of the United States and the limited number of procedures performed as part of our U.S. staged pivotal clinical trial, healing and adaptation to the microlens generally begin immediately after the surgery, with the immediate common side effects of such a procedure generally being mild eye dryness and irritation, transient elevated intraocular pressure due to standard post-surgery medication regimen, corneal haze (the activation of inflammatory cells in connection with surgery), transient light sensitivity (an abnormal occurrence of photosensitivity associated with the femtosecond laser) and certain visual symptoms, such as halos or glare. |
We believe that surgical treatment for presbyopia represents a large new market opportunity for ophthalmic surgeons. The market for traditional surgical ophthalmic treatments, such as LASIK for myopia, hyperopia and astigmatism, and traditional monofocal IOLs for cataracts, is highly mature. Our procedure utilizes the femtosecond laser currently used for certain LASIK surgeries, cataract surgeries and cornea replacement surgeries. We believe that many refractive laser centers equipped with such lasers are not operating at full capacity, and we hope to utilize such untapped capacity. Our procedure would allow these laser centers and ophthalmic surgeons to introduce a new treatment modality using their existing laser equipment, adding incremental revenue without the need for significant new capital commitments.
We believe that patient demand for our microlens may be driven in part by the large and growing number of individuals who have become comfortable with and undergone LASIK procedures, which we believe have lost their “experimental” reputation. We expect that these individuals are more likely to be early adopters of our microlens solution to the extent they require additional vision correction in connection with the onset of
8
presbyopia. We believe demand is likely to be fueled further by the ever-evolving, near-vision needs resulting from the increasing reliance on smart phones, tablets and other technological advances requiring good near vision.
Our Technology
Our microlens is a disc shaped lens that has a refractive zone in the periphery designed to improve near vision problems associated with presbyopia and a central zone that is designed to maintain distance vision. The two figures below illustrate the design of our microlens.
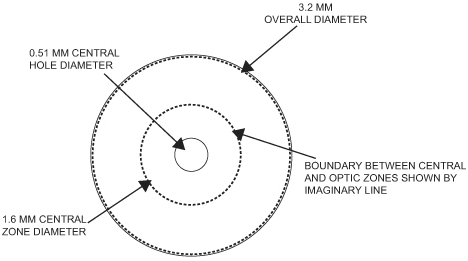
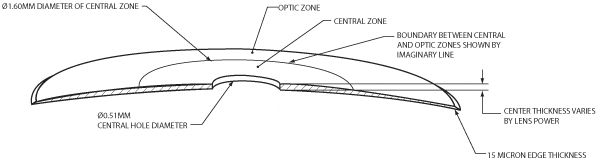
Our microlens is 3.2 millimeters (mm) in diameter, has an edge thickness of 0.015 mm and has a center thickness that ranges from approximately 0.03 mm to 0.05 mm (depending on the lens power). Once implanted, it is invisible to the naked eye. Our microlens is made of a hydrophilic acrylic material, similar to the kind that has been used to make IOLs for over 20 years. Our microlens is designed to be removable. In addition, our microlens is designed to reduce the risk of permanent corneal tissue loss and is designed to be biocompatible with the cornea, allowing for corneal metabolism, which is essential to the health and normal functioning of the cornea.
Ocular dominance is the tendency to prefer visual input from one eye to the other. Our microlens is implanted in a patient’s non-dominant eye to minimize impact to binocular uncorrected distance vision. Through implantation in the patient’s non-dominant eye, our solution seeks to exploit the brain’s ability to perceptually suppress central vision in one eye when the two eyes are receiving disparate stimuli and focus on the clearer images while ignoring the blurrier images. Prior to implantation, we require patients to wear a contact lens for near vision correction in their non-dominant eye for a minimum of three to five days before insertion of our microlens in order to assess whether or not the patient is able to adapt to the change in the visual system. Not all prospective
9
patients are able to adapt to the change in the visual system. Based on feedback that we have received from surgeons to date, we believe that approximately 40% of prospective patients who underwent monofocal contact lens correction in their non-dominant eye were unable to adapt to the change in the visual system and approximately 25% of prospective patients who underwent multifocal contact lens correction in their non-dominant eye were unable to adapt to the change in the visual system.
To improve near vision, as shown below, the refractive peripheral portion of our microlens is designed to help focus light from near objects (darker shaded light) onto the retina.
Near Vision with Microlens
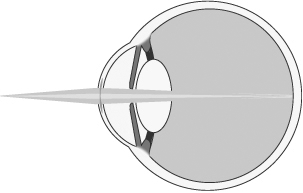
To maintain maximum distance vision, the central portion of our microlens is designed to permit light from distant objects to pass through the cornea and lens and focus on the retina (lighter shaded light shown below). The refractive peripheral portion of the lens causes some distant light rays to focus in front of the retina, instead of on it (darker shaded light shown below). However, when the brain receives dual visual stimulus from the corrected non-dominant eye, as well as the uncorrected dominant eye, it is able to correctly combine the information into an image for the patient.
Distance Vision with Microlens
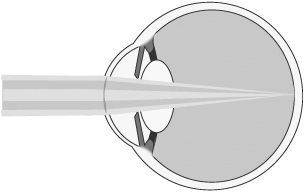
Insertion of our Microlens
Our microlens is surgically implanted, using our proprietary microlens inserter, in an outpatient setting. The procedure, requiring only topical anesthesia, typically takes a trained ophthalmic surgeon approximately 10 minutes. The procedure and equipment needed to create a corneal pocket to insert the microlens are similar to those currently used in LASIK procedures. We believe that the existing infrastructure in most refractive laser centers equipped with femtosecond lasers is sufficient for our procedure.
10
The figures below illustrate our microlens inserter and the insertion of our microlens.
| Illustration of our Proprietary Insertion Tool
|
Illustration of Insertion of Our Microlens
|
The ophthalmic surgeon starts the procedure by making a mark on the cornea at the center of the visual axes in order to determine the most appropriate location of the corneal pocket as well as the microlens placement and alignment once in place. Then, using a 150 kilohertz or greater frequency femtosecond laser, the ophthalmic surgeon creates a pocket, approximately four to 5.5 mm in diameter, in the cornea of the patient’s non-dominant eye. Using our microlens inserter, the ophthalmic surgeon then inserts our microlens into the corneal pocket. Finally, the ophthalmic surgeon centers and checks the position of the implanted microlens before completing the surgery. The corneal pocket automatically seals itself within a few days, holding the microlens in place at the center of the eye’s visual axis.
We have designed our microlens and procedure to be removable in a minimally invasive manner in the event that a patient wishes to have a stronger prescription microlens implanted. This may occur if a patient’s presbyopia significantly progresses over time or in the event that a patient wishes to have the microlens removed for any other reason, including if the patient is uncomfortable with the results, if neural adaptation is not achieved, or if technological advances produce alternative solutions in the future. In the United States, our IDE does not permit replacement of a microlens in the event that a patient’s microlens is removed after implantation. Also, in the United States, our IDE requires any removal of the microlens to be reported as an adverse event.
The procedure to remove our microlens may take place in an outpatient setting, using only topical anesthesia. The removal procedure consists of opening the corneal pocket entry point and, using a fluid to lubricate the pocket of the lens, sliding the lens from the corneal pocket. This procedure typically takes a trained ophthalmic surgeon approximately 10 minutes. A new microlens can be immediately inserted into a patient’s existing corneal pocket.
Through March 15, 2015, ophthalmic surgeons have implanted over 600 of our microlenses outside of the United States.
Clinical Studies
We have completed a multicenter clinical study outside the United States. In addition, several third parties have conducted limited studies of our microlens. These studies are summarized below.
Evaluation Conducted Outside of the United States
In early 2012, we completed a 12-month multicenter, post-market evaluation in Italy and Greece of our microlens in presbyopic patients between the ages of 45 and 60 to evaluate the safety and effectiveness of our microlens. We designed, and oversaw the implementation of, the protocol for this evaluation, which was conducted at our
11
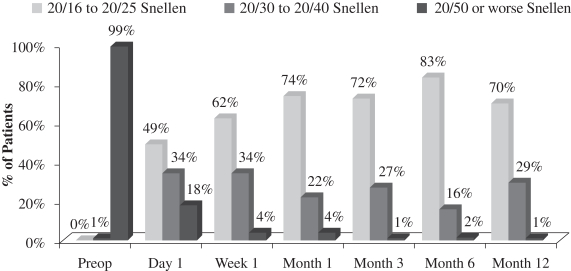
request by a surgeon at the Vardinoyannion Eye Institute at the University of Crete in Crete, Greece and by a surgeon at Prato Hospital in Prato, Italy. The 12-month data for the 70 patients who completed the study demonstrated successful patient outcomes and a low rate of post-operative adverse events. The average UCVA-near in the operated eye pre-surgery for those 70 patients was 20/110 and 99% of those patients started the study with UCVA-near in the operated eye of 20/50 or worse. Key effectiveness findings from this evaluation included the following:
| • | the average UCVA-near in the operated eye for such patients post-surgery was 20/27, 99% of such patients completed the study with 20/40 or better UCVA-near in the operated eye and 70% of such patients completed the study with 20/25 or better UCVA-near in the operated eye (see Figure 1 below); |
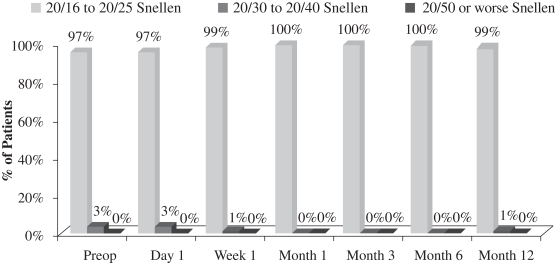
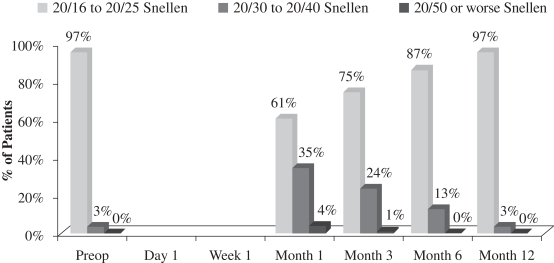
| • | although there was a slight loss in uncorrected distance visual acuity (the ability to see distant objects without prescription enhancement), or UCVA-distance, in the operated eye (see Figure 4 below), there was no significant change in binocular UCVA-distance (UCVA-distance when using both eyes) from before treatment to after treatment in this study (see Figure 2 below); and |
| • | there was no significant change in best corrected distance visual acuity (distance vision using prescription enhancement), or BCVA-distance, in the operated eye after 12 months (see Figure 3 below). |
A Snellen chart is an eye chart used by eye care professionals and others to measure visual acuity. It usually consists of letters printed in lines of decreasing size which a person is asked to read at a fixed distance. 20/20 is a term used to define normal visual acuity, which relates to the Snellen chart. The first number denotes a certain distance, and the second number denotes the distance at which a person with normal visual acuity could read clearly those letters that the subject of the assessment can read clearly at the distance denoted in the first number. The standard distance for testing distance visual acuity is 20 feet. Thus, with respect to distance vision, if an individual has 20/100 vision, it means that a person with normal distance vision can read at 100 feet what the patient can only read at 20 feet (poor distance vision). 20/10 vision, on the other hand, would mean the individual has better than normal distance vision, being able to read at 20 feet what a person with normal distance vision could only read at 10 feet. With respect to near vision, the 20/20 nomenclature is used with the distances in the first number and the second number scaled to the distance used in the study. Thus, an individual with 20/20 near vision means the patient can read clearly those letters at the distance tested (usually 40 centimeters (cm) in the United States and 33 cm outside of the United States) that a person with normal near visual acuity could read clearly at that distance. In our post-market evaluation, we tested visual acuity using an Early Treatment Diabetic Retinopathy Chart, or ETDRS, Snellen chart; the distance used to test distance visual acuity was 20 feet and the distance used to test near visual acuity was 33 cm.
12
An important measurement is the number of patients who reach better visual acuity levels, or visual correction, after treatment. Before surgery, the 70 patients who completed the study had an average UCVA-near in the eye to be operated on of 20/110 and 99% of such patients started the study with UCVA-near measurements of 20/50 or worse in that eye. After treatment with our microlens, such patients had an average UCVA-near in the operated eye of 20/27 and 99% of such patients achieved UCVA-near measurements of 20/40 or better in the operated eye and 70% of such patients achieved UCVA-near measurements of 20/25 or better in the operated eye. The following chart summarizes the positive UCVA-near results in this post-market evaluation:
Figure 1
Uncorrected Near Visual Acuity Operated Eye (33 cm chart)
(N=70 at Month 12)
13
Another important measurement is the number of patients who maintain binocular UCVA-distance levels post-treatment. There was no significant change in binocular UCVA-distance from before treatment to after treatment in this study. This stability in binocular vision is important because it indicates that patients in the study did not experience a significant compromise in binocular UCVA-distance as a result of the insertion of our microlens, meaning that their normal binocular far vision was not compromised. The following chart summarizes the binocular UCVA-distance findings in this post-market evaluation:
Figure 2
Uncorrected Binocular Distance Visual Acuity
(N=70 at Month 12)
14
In addition, patients in this study experienced no significant change in BCVA-distance in the operated eye at 12 months post-implantation, which indicates that there was no compromise in the operated eye’s optical system at 12 months. The following chart summarizes BCVA-distance findings with respect to the patient’s operated eye in this post-market evaluation:
Figure 3
Best Corrected Distance Vision Acuity Operated Eye
(N=70 at Month 12)
15
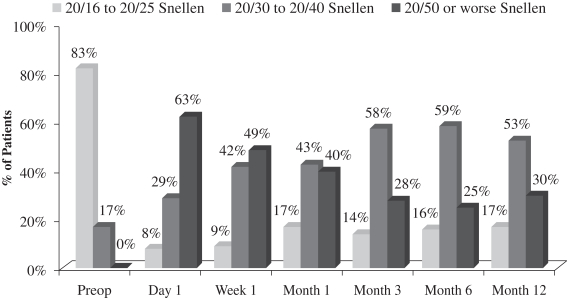
There was a slight loss of UCVA-distance in the operated eye in the study population. Before surgery, 83% of patients achieved UCVA-distance in the operated eye of 20/16 to 20/25 and 17% achieved UCVA-distance in the operated eye of 20/30 to 20/40. After treatment with our microlens, 17% of patients achieved UCVA-distance in the operated eye of 20/16 to 20/25, 53% achieved UCVA-distance in the operated eye of 20/30 to 20/40, and 30% achieved UCVA-distance in the operated eye of 20/50 or worse. Although, as mentioned above, there was no significant change in binocular UCVA-distance from before treatment to after treatment in this study, far distance vision in the operated eye is also important, particularly as it relates to overall patient satisfaction. In the study population, 78% of patients who responded reported that they perceived their UCVA-distance in the operated eye as “excellent” to “good.” This generally correlates to the data at month 12, where 70% of patients achieved 20/40 vision or better in the operated eye. The remaining 30% of patients achieved UCVA-distance in the operated eye of 20/50 or worse, and consistent with such results, 20% of patients who responded reported that they perceived their UCVA-distance in the operated eye as “fair.” One patient who responded considered his outcome with respect to UCVA-distance in the operated eye as “poor.” The following chart summarizes UCVA-distance findings with respect to the patient’s operated eye in this post-market evaluation:
Figure 4
Uncorrected Distance Visual Acuity Operated Eye
(N=70 at Month 12)
There are several possible explanations for the loss of distance visual acuity in the operated eye, including, but not limited to, the following:
| • | the time required for neural adaptation, or the time it takes the brain to adapt to the change in the visual system; |
| • | improper patient selection, or the selection of patients who are intolerant of monovision, impatient or not willing to wait for the neural adaptation time period; and |
| • | improper lens power selection, meaning the patient is difficult to refract. |
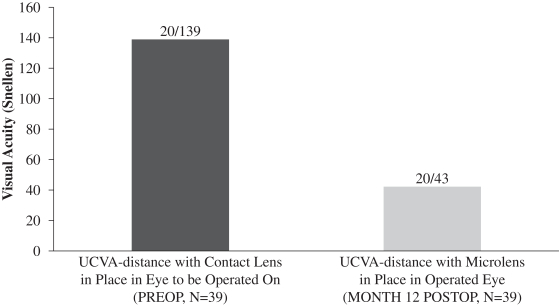
A subset of 39 patients enrolled in this evaluation underwent a monovision simulation, whereby pre-surgery UCVA-distance in the eye to be operated on with near vision contact lens correction in place was compared to UCVA-distance in the operated eye following implantation of our microlens. The purpose of this simulation was
16
to evaluate whether UCVA-distance in the operated eye following implantation of our microlens is better than pre-surgery UCVA-distance in such eye with the use of a near vision correction contact lens. These patients were found to have an average UCVA-distance in the operated eye of 20/43 12 months post-surgery, while the same patients pre-surgery had an average UCVA-distance in the eye with near vision contact lens correction of 20/139. We believe that this result is due to the nature of the design of our microlens which is intended to maintain distance vision in the operated eye to the greatest extent possible. The central portion of our microlens is designed to allow for light from distant objects to enter the eye and focus on the retina, and the retina ultimately transmits that image to the brain. Figure 5 below illustrates the findings in this evaluation with respect to post-surgery UCVA-distance in the operated eye compared to pre-surgery UCVA-distance in the eye to be operated upon with near vision contact lens correction in place:
Figure 5
Overall, patient satisfaction with the procedure was generally high. 97% of patients who responded reported “excellent” or “good” perception of UCVA-near, and 97% of patients who responded reported “excellent” or “good” perception of binocular UCVA-distance. 75% of patients who responded reported no use of glasses for near tasks while the remaining 25% reported use of glasses less than 50% of the time. 78% of patients who responded indicated that they used glasses for near tasks more than 50% of the time prior to implantation.
Key safety findings from the evaluation over a 12-month period included the following:
| • | low rate of post-operative adverse events; |
| • | one patient complained one week after implantation of significant halos and glare when driving at night and requested removal of the microlens (the lens was removed one month post-surgery); |
| • | one case of transient light sensitivity syndrome was reported: this represents an abnormal occurrence of photosensitivity associated with the femtosecond laser, which resolved after application of a topical steroid regimen; |
| • | one case of epithelial ingrowth was reported: this represents an abnormal growth of corneal epithelium in an area where it does not belong, associated with the femtosecond laser, which resolved after the ingrowth was surgically cleared; and |
17
| • | four cases of transient stromal haze were reported: these cases involved the activation of inflammatory cells in connection with surgery, which resolved after application of a topical steroid regimen. |
There was no significant change in:
| • | intraocular pressure, or the fluid pressure in the eye; |
| • | endothelial cell density, or the tissue layer undersurface of the cornea and which regulates corneal water content; |
| • | pachymetry, or the measure of corneal thickness; or |
| • | binocular contrast sensitivity. |
We continue to evaluate our microlens through clinical studies and marketing and post-marketing evaluations in connection with regulatory requirements and our commercialization efforts. In addition, through March 15, 2015, ophthalmic surgeons have implanted over 600 of our microlenses outside of the United States.
Third Party Studies
Our microlens has been the subject of certain third party studies that have been conducted to assess the efficacy and safety of our microlens. We did not commission these studies or design, review or oversee the implementation of their protocols (although we paid the annual fees of the institutional review board, or IRB, reviewing one such study in Japan), and we have limited information with respect to these studies. These studies have reported certain adverse effects relating to the safety and efficacy of our microlens and microlens inserter. In connection with the findings in certain of such studies and observations of other surgeons regarding our procedure, we have undertaken certain investigative actions as part of our ongoing risk mitigation efforts. See “Risk Factors—Risks Related to Our Business—If concerns regarding side effects from presbyopia-correcting surgery generally, or our products specifically, develop, including as a result of third-party studies and publications, our business, results of operations and financial condition will be materially and adversely affected.”
U.S. Staged Pivotal Clinical Trial
In May 2014, we began a staged pivotal clinical trial to seek marketing approval for our microlens and microlens inserter in the United States. See “—Clinical Development and Commercialization Targets” below for a description of this study. For a description of adverse events to date in this study, see “Risk Factors—Risks Related to Our Business—If concerns regarding side effects from presbyopia-correcting surgery generally, or our products specifically, develop, including as a result of third-party studies and publications, our business, results of operations and financial condition will be materially and adversely affected.”
Upgraded Microlens Inserter
We are developing upgraded versions of our microlens inserter, including a disposable microlens inserter, that will be designed to be used only one time, and ultimately, a pre-loaded disposable microlens inserter, that will be designed to be used one time and to be equipped with a microlens already loaded to make the insertion process easier and quicker for surgeons. Our new designs will utilize a common universal molded activation hand piece that we developed that is intended for use with any lens delivery assemblies that we design. We have already molded the activation hand piece, and we are now focusing our efforts on design evaluation and optimization of the lens delivery assembly. We intend to select a lens delivery assembly by the first half of 2015. We intend to apply for marketing authorizations for the disposable and preloaded disposable systems in the United States and the European Union by the third quarter of 2015 and the third quarter of 2016, respectively. We expect marketing authorization of these products in the United States will require submission of a PMA supplement.
18
Clinical Development and Commercialization Targets
In December 2013, we received approval from the FDA to commence a staged pivotal clinical trial of our microlens in the United States. This clinical trial is a prospective, non-randomized, unmasked, multicenter clinical investigation. Beginning in May 2014, we enrolled a total of 75 subjects at six investigational sites in the United States. Beginning in June 2014, each of these subjects underwent insertion of our microlens in the subject’s non-dominant eye. Based on six-month data on 52 of these subjects, in January 2015, we submitted to the FDA an interim safety report as a supplement to our IDE. In February 2015, we received approval from the FDA to commence second stage enrollment in this trial. We are permitted to enroll up to an additional 337 subjects at up to nine additional investigational sites. Through March 15, 2015, 25 subjects underwent insertion of our microlens in the second stage of this study. We currently anticipate that in order to file our PMA we will need 24-month post-surgery data on each of at least 300 patients. All subjects will be followed for three years following implantation. Subjects from outside the United States will not be enrolled in this study. The primary endpoint will be UCVA-near at 24 months post- implantation, together with safety objectives such as a low rate of ocular adverse events, endothelial cell loss over time in the operated eye, and an assessment of BCVA-distance and contrast sensitivity in the operated eye (the visual ability, with distance vision correction in place, to see objects that may not be outlined clearly or that do not stand out from their background). Although our microlens is designed to be removable, our IDE requires any removal to be reported as an adverse event. We are targeting submission of our final PMA, containing 24-month data on 300 subjects, to the FDA in the second quarter of 2017. We are pursuing a modular PMA submission strategy whereby we intend to submit to the FDA information regarding preclinical testing, engineering, and manufacturing in the fourth quarter of 2015 or the first quarter of 2016, prior to the submission of our final PMA. We are targeting PMA approval of our microlens in the fourth quarter of 2017. We are also targeting submission to the FDA of a final report with 36-month data on these 300 subjects in the second quarter of 2018. These milestones could be delayed by further interactions with the FDA or by a variety of other factors, including the final design of the study that is approved by the FDA, and are subject to risks and uncertainties. There can be no assurance that the FDA will grant our PMA approval or, if granted, that it will be granted in accordance with our anticipated time schedule. In addition, the FDA may require us to conduct post-approval studies as a condition of approval.
We plan to continue to work closely with refractive laser centers and hospitals in order to secure commercialization commitments in those countries in which we currently have marketing approval that we are targeting for commercialization. In addition, we plan to continue to seek marketing approval in additional countries that we determine present appropriate opportunities to commercialize our microlens and microlens inserter.
Strategy
Our goal is to become the leading provider of corneal lens implants for patients with presbyopia. We are currently commercializing our microlens internationally in select countries. In December 2013, we gained approval to begin a staged pivotal clinical trial in order to seek marketing approval for our microlens in the United States. We completed the first stage of this study in July 2014, and submitted to the FDA an interim safety report on 52 subjects in January 2015. In February 2015, we received approval from the FDA to commence second stage enrollment in this trial.
We believe that most presbyopic individuals are not aware of procedures intended to decrease their dependence on reading glasses, the traditional solution for presbyopia, and therefore do not seek treatment from an ophthalmic professional.
We believe that refractive surgery center operators, impacted by sharp declines in LASIK procedure volumes, are searching for new technologies that can be used in elective (patient-pay) procedures to grow revenues. We
19
believe that these centers have existing infrastructure (femtosecond lasers) and databases of patients who may be eligible for these presbyopia procedures. We believe that a significant opportunity rests in the population of presbyopic individuals who are unaware of these new treatment options. Our entry strategy is to identify refractive laser surgery centers and partner with them through our comprehensive commercialization strategy to attempt to create patient awareness of and demand for our microlens.
Once a commercialization agreement is signed with a clinic, we intend to become an active partner and assign a commercialization team to the clinic. The process begins with comprehensive training of all surgeons and staff, and identification of potential patients, which is intended to drive demand to the clinic for our microlens procedure.
The surgeon goes through a comprehensive training and certification program, which includes didactic sessions, wet-lab training, and surgery performed with members of our surgical training team present during the certification process. Thereafter, patients are seen at regular intervals, and individual patient results are collected. These data are then analyzed by our clinical services team to ensure proper patient selection was achieved, that patient expectations were managed appropriately, and that patients achieve optimal visual outcomes. Once the surgeon is certified, our goal is for the practice to continue to grow its business, using the tools and methods provided by our commercialization team. In addition, we will train one surgeon to become a peer trainer to train additional surgeons in the practice, thereby increasing capacity at the practice. We have utilized this model in certain jurisdictions, including Ireland and Brazil, which resulted in adoption of our technology into the practice. Implementation of the process is designed to yield the best possible patient selection, which in turn is designed to lead to the highest possible patient satisfaction.
We currently have an arrangement with laser centers in Ireland, Australia and Brazil (and Canada as permitted under Canada’s Special Access Program) whereby the laser center has committed to help promote our microlens in the applicable jurisdiction and to target a set number of procedures per annum in each such jurisdiction. In addition, we have ongoing interactions with other laser centers, including laser centers in the EEA, Asia and South America, whereby we are seeking commercialization commitments from certain laser centers to adopt our unique process and the integration of our technology in their respective centers.
U.S. Staged Pivotal Clinical Trial
Gaining approval to market our products in the United States is a critical element in our strategy. In order to obtain such approval, we must obtain a PMA from the FDA. We cannot assure you when or whether we will obtain such an approval, or what expenditures we will incur whether or not we obtain such approval, given the many significant risks associated with seeking such an approval from the FDA.
International Commercialization
Through our European Union CE Mark, we are generally authorized to market our microlens throughout the EEA and Switzerland (certain EEA countries also require additional in-country registration). We currently market our microlens in certain strategic EEA countries, as well as certain strategic non-EEA countries in which we currently possess marketing approval. We will continue to seek marketing approval in other strategic countries that we believe are appropriate to further our commercialization strategy.
Sales & Marketing
Sales and marketing for our microlens is led by our chief commercial officer and dedicated business development directors in Australia and Brazil, and our clinical services director in Ireland, who currently focus on refractive laser centers that we have identified in selected target markets. They are supported in the process by a surgical trainer and our commercialization team.
20
We believe the existing infrastructure in most refractive laser centers equipped with a 150 kilohertz or greater femtosecond laser is sufficient to support our commercialization efforts, minimizing the need to establish a significant sales representative structure at this juncture in our development. We intend to utilize a distributor / agent structure only in those countries that will require us to do so.
We also intend to sponsor ophthalmic surgeons to speak and present data at numerous conferences throughout Europe, Latin America, Asia and the United States. We believe this marketing strategy will help promote our microlens and increase demand from both clinicians and patients worldwide.
Our microlens and the procedure to implant our microlens are not currently reimbursed through private or governmental third-party payors in any country, nor do we anticipate that our microlens and the procedure to implant our microlens will be reimbursable through private or governmental third-party payors in the foreseeable future. Although the commercialization of our microlens depends on a prospective patient’s ability to cover the costs of our microlens and the implantation procedure and we believe that a substantial portion of presbyopes worldwide do not have the financial means to cover the costs of our microlens, we believe that a direct patient-pay model enables medical providers to avoid pricing pressure from private or governmental third-party payors. We do not have control over the prices that medical providers charge patients for our microlens and the implantation procedure.
Research and Development
We believe that it is essential for us to remain focused on advancing our technology and continuing to improve our microlens, as well as our microlens inserter and other auxiliary Presbia products. We maintain an active internal research and development process, which also includes clinical activities and regulatory affairs. In order to achieve our business objectives, we will continue our investment in research and development. Our research and development team, consisting of eight persons as of December 31, 2014, communicates with ophthalmic surgeons who are currently utilizing the Presbia system, enabling us to make design changes as we receive feedback. Over the last three years, we have made a number of modifications to our microlens inserter to enable ophthalmic surgeons to more quickly and efficiently remove our microlens from its sealed container and prepare it for insertion into the laser-cut corneal pocket.
Over the next 12 months, our goal is to continue our focus on research and development, particularly with respect to developing a disposable microlens inserter and a pre-loaded disposable microlens inserter for our microlens and evaluating the development of a single microlens that includes both presbyopic and hyperopic correction. Additionally, as we regard our microlens to be an optical platform, we will continue to explore new approaches to correct vision problems, including evaluating the development of a corneal inlay for the treatment of macular degeneration, a chronic eye disease that causes vision loss in the center of a patient’s field of vision.
We expended $4.2 million, $2.1 million, and $1.0 million for research and development during the years ended December 31, 2014, December 31, 2013, and December 31, 2012, respectively.
Intellectual Property
Our commercial success depends, in part, on our ability to obtain and maintain proprietary protection for our products, technologies and other know-how, to operate without infringing the proprietary rights of others and to prevent others from infringing our proprietary rights. We strive to protect our investment in the research, development, manufacturing and marketing of our products through the use of patents, trademarks, copyrights and trade secrets, as well as customary confidentiality and other contractual protections. We own, or have rights to, several patents, several patent applications, trademarks, copyrights, trade secrets and other intellectual property directly related and important to our business. However, the extent to which our intellectual property will provide us with protection and enable us to commercialize our proprietary technology without interference from others is subject to numerous risks. See “Risk Factors—Risk Relating to Our Intellectual Property.”
21
Patents
We currently own two issued US patents, two U.S. patent applications which have been allowed and will be issued shortly and five other pending U.S. patent applications; all of which we consider material to our business. The first of our existing patents relates to our microlens inserter; this patent expires in September 2030. One of the allowed patent applications expands upon the subject of the microlens inserter and we are awaiting the issuance of this patent. The second patent relates to the method and apparatus to package and transport our microlens and making it readily accessible; this patent expires in June 2032. The other allowed patent application expands upon the subject of the method and apparatus to package our microlens and we are awaiting the issuance of this patent. Our five pending applications relate to the method and apparatus to inject our microlens, to the method for laser cutting a corneal pocket to insert our microlens, to the apparatus and method to use a preloaded inserter to insert our microlens, to the combination of the packaging apparatus and the inserter to remove the lens, and to our proprietary data collection software.
Additionally, we have a total of thirty-three foreign patent applications, twenty-eight of which are still pending in Australia, Brazil, Canada, China, Europe, Israel, India, Japan, Korea and Russia. The foreign applications correspond to the content of the two issued U.S. patents. We currently own a patent in Canada which corresponds to the U.S. patent which covers the method and apparatus to insert our microlens, and we have granted applications in Israel and China which correspond to the same U.S. patent. We also own a patent in Japan which corresponds to the U.S. patent which covers the microlens inserter, and we have a granted application in Australia which corresponds to the same U.S. patent.
Our patents and patent applications may allow us to exclude others from practicing our proprietary inventions and may provide us with an opportunity to obtain royalties or cross-licenses of intellectual property from other manufacturers. Because we have limited knowledge of the research and development efforts and strategic plans of our competitors, we can only estimate the value of our patents and patent applications. Competitors may be able to design products and/or processes that avoid infringing our patent portfolio as it may exist from time to time.
Trademarks
Worldwide, we have several registered trademarks and pending trademark applications that we consider to be important to our business. The scope and duration of trademark protection varies widely throughout the world. In some countries, trademark protection continues only as long as the mark is used. Other countries require registration of trademarks and the payment of registration fees. Trademark registrations are generally for fixed but renewable terms.
Confidentiality Agreements
We protect our proprietary technology, in part, through confidentiality and nondisclosure agreements with employees, consultants and other parties. Our confidentiality agreements with employees and consultants generally contain standard provisions requiring those individuals to assign to Presbia, without additional consideration, inventions conceived or reduced to practice by them while employed or retained by Presbia, subject to customary exceptions.
Competition
The medical device industry in general, and the ophthalmic medical device market in particular, are highly competitive, subject to rapid technological change and significantly affected by new product introductions and market activities of other participants. Our currently marketed products are, and any future products we commercialize will be, subject to intense competition.
We expect to compete against companies that are developing corneal inlay surgical solutions for presbyopia, including AcuFocus, Inc., ReVision Optics, Inc., Neoptics AG and LensGen, Inc. AcuFocus, Inc.’s corneal inlay
22
approach attempts to use small aperture optics to reduce distortion by eliminating peripheral light rays and limiting the width of diverging light rays. ReVision Optics, Inc.’s corneal inlay approach attempts to produce a smooth variation in focal power across the pupil by microscopically altering the surface shape of the cornea. Neoptics AG’s corneal inlay approach consists of a bifocal lens with a peripheral zone designed to improve near vision and a central zone for distance vision. LensGen, Inc. is a newer company with little publicly available information regarding its intraocular lens which is being designed to harness fluidics and displacement to manipulate curvature to better capture light. Both AcuFocus, Inc. and ReVision Optics, Inc. have been conducting clinical trials in the United States for years and have more experience than Presbia in conducting such trials. AcuFocus, Inc. has completed a pivotal clinical trial in the United States and is thus further along in the FDA approval process than Presbia. Also, both AcuFocus, Inc. and ReVision Optics, Inc., like Presbia, have marketing approval in certain jurisdictions outside the United States, including the EEA, and can be expected to compete with Presbia in such jurisdictions. We believe that AcuFocus, Inc. may have more commercial activities to date than Presbia in certain jurisdictions, including Japan. We do not believe that Neoptics AG has approval in the U.S. to conduct clinical trials or marketing approval in any jurisdiction. In addition to being an effective treatment option for presbyopia, we believe that our microlens is less invasive than ReVision’s Optics, Inc.’s corneal inlay, offers a range of optical power corrections not offered by ReVision’s Optics, Inc.’s corneal inlay or AcuFocus, Inc.’s corneal inlay, is less conspicuous than AcuFocus, Inc.’s corneal inlay and is more easily removable than ReVision Optics, Inc.’s corneal inlay.
In June 2014, the FDA released data presented by AcuFocus to the FDA in its PMA submission with respect to its KAMRA device. The data related to a study conducted by AcuFocus with respect to 508 subjects (of which the FDA determined that there were 494 evaluable subjects). We reviewed that data against the data that we compiled from the post-market surveillance study that we conducted in Italy and Greece in 2012 with respect to 70 patients who underwent implantation of our microlens. For further information regarding this post-market evaluation, see “—Our Solution—Evaluation Conducted Outside of the United States.”
We note the following with respect to the AcuFocus study and our post-market surveillance study:
| • | Approximately 80.8% of AcuFocus’ 494 evaluable subjects achieved UCVA-near of 20/40 or better in the operated eye 12 months postoperative, which was the primary efficacy endpoint of the AcuFocus study. As discussed in “—Our Solution—Clinical Studies—Evaluation Conducted Outside of the United States”, approximately 99% of the 70 patients in Presbia’s post-market surveillance study achieved UCVA-near of 20/40 or better in the operated eye 12 months postoperative. |
| • | Both the KAMRA, in AcuFocus’ study, and Presbia’s microlens, in Presbia’s post-market surveillance study, generally had minimal effect on a subject’s binocular distance vision. |
| • | Included among the adverse events in each study at 12 months postoperative, 3.0% of subjects experienced device explantations in AcuFocus’ study, and 1.4% of subjects experienced device explantations in Presbia’s post-market surveillance study. |
We note that AcuFocus’ study includes a substantially larger sample size (number of patients that received the implant) than Presbia’s post-marketing evaluation and that comparability of the results of AcuFocus’ study and Presbia’s post-marketing evaluation could also be adversely affected by differences in patient demographics, such as gender and ethnicity, as well as differences in study protocols, site location and other conditions. We also note that we are currently conducting our U.S. staged pivotal clinical trial, and that trial might provide different results than those observed in our post-market evaluation described above and elsewhere in this Annual Report on Form 10-K.
We expect to compete against companies that offer alternative surgical treatment methodologies, including monovision, multifocal and accommodating approaches, and companies that promote reading glasses and/or contact lenses as approaches for responding to presbyopia. At any time, our known competitors and other potential market entrants may develop new devices or treatment alternatives that may compete directly with our
23
products. In addition, they may gain a market advantage by developing and patenting competitive products or processes earlier than we can or by obtaining regulatory approvals/clearances or market registrations more rapidly than we can.
Certain of our current and potential competitors may have significantly greater financial, technical, marketing and other resources than we do and may be able to devote greater resources to the development, regulatory approval, promotion, sale and support of their products. Our competitors may also have more extensive customer bases and broader customer relationships than we do, including relationships with our potential customers. In addition, many of these companies have longer operating histories and greater brand recognition than we do. Because of the size of the presbyopia market and the high growth profile of that market, we anticipate that companies will dedicate significant resources to developing competing products. We believe that the principal competitive factors in our market include:
| • | improved outcomes for patients and other product quality issues; |
| • | product innovation; |
| • | acceptance by ophthalmic surgeons; |
| • | ease of use and reliability; |
| • | regulatory status and speed to market; |
| • | product price and procedure price; and |
| • | reputation for technical leadership. |
We cannot assure you that we will be able to compete effectively against our competitors in regard to any one or all of these factors.
Manufacturing
Our microlens is manufactured using hydrophilic acrylic material that has been utilized in the lens manufacturing market for the last 20 years. This material is well known and has an established safety profile. High precision lathing machines are used to generate sub-micro level accuracy of convex/concave radii. Like other traditional IOL manufacturing processes, the manufacturing of the microlens is divided into a dry and a wet process.
At present, our microlens is manufactured by a third-party original equipment manufacturer, or OEM, in Israel. This supplier has committed to a guaranteed minimum production level that we believe is adequate to meet our current needs. The agreement with this supplier is set to expire in January 2017. We expect that our Israeli OEM, or another supplier located overseas, will be utilized to satisfy all international demand for our microlens for the foreseeable future. We have also constructed a manufacturing facility in Irvine, California. We plan to use our Irvine, California manufacturing plant to supply the microlens for our U.S. clinical study and believe that that facility is scalable to meet future U.S. demand once it has received all applicable regulatory registrations, approvals and certifications. Our U.S. facility has received regulatory approval from the State of California to manufacture our microlens for our U.S. staged pivotal trial. In addition, we have received the necessary regulatory approval to use such facility to provide backup manufacturing capacity for sales in the EEA, should such capacity be needed in the future. Our microlens inserter is manufactured by a third-party OEM in the United States, according to our specifications. Although we do not have a guaranteed supply commitment from this supplier, we believe that this supplier will be able to meet our foreseeable needs.
We believe that our current manufacturing arrangements are sufficient to support our foreseeable manufacturing needs. The manufacturing by the Israeli OEM includes in-house sterilization, packaging, and inventory. Inventory of our microlens is ultimately stored at our facilities in the Netherlands and Ireland.
24
Sources and Availability of Raw Materials
We use a wide range of raw materials in the production of our products. We purchase most of the raw materials and components from external suppliers. The hydrophilic acrylic material used to manufacture our microlens is supplied to us by a single supplier located in the United Kingdom. We would be required to obtain approval from the FDA in the event that we wished to use different material or similar material from a different supplier with respect to any products to be offered and sold in the United States. Although we do not have a guaranteed supply commitment from our sole supplier of such hydrophilic acrylic material, we believe that such supplier will be able to sufficiently meet our currently anticipated supply needs. Although we do not currently have any long-term agreements in place for the supply of any other raw materials that we use, such materials are currently readily available from a number of suppliers, both in the United States and abroad.
Government Regulation
Our medical device products are subject to extensive regulation by the FDA and various other federal, state and non-U.S. governmental authorities, such as the competent authorities of the countries of the EEA. Government regulation of medical devices is meant to assure their safety and effectiveness, and includes regulation of, among other things:
| • | design, development and manufacturing; |
| • | testing, labeling, content and language of instructions for use and storage; |
| • | clinical trials; |
| • | product safety; |
| • | marketing, sales and distribution; |
| • | regulatory clearances and approvals, including premarket clearance and approval; |
| • | conformity assessment procedures; |
| • | product traceability and record keeping procedures; |
| • | advertising and promotion; |
| • | product complaints, complaint reporting, recalls and field safety corrective actions; |
| • | post-market surveillance, including reporting of deaths or serious injuries and malfunctions that, if they were to recur, could lead to death or serious injury; |
| • | post-market studies; and |
| • | product import and export. |
To market and sell our products in any country, we must first seek and obtain regulatory approvals, certifications or registrations and comply with the laws and regulations of that country. These laws and regulations, including the requirements for approvals, certifications or registrations and the time required for regulatory review, vary from country to country. Obtaining and maintaining regulatory approvals, certifications and/or registrations are expensive, and we cannot be certain that we will receive regulatory approvals, certifications and/or registrations in any country for which we have yet to receive such approvals, certifications and/ or registrations or that we will be able to maintain any regulatory approvals, certifications or registrations that we currently possess in any country. If we fail to obtain or maintain regulatory approvals, certifications or registrations in any country in which we currently market or plan to market our products or if we fail to comply with all applicable regulatory laws, rules and regulations, our ability to sell our products could be jeopardized and we could be subject to enforcement actions. See “Part I, Item 1a. Risk Factors—Risks Related to Regulatory Requirements” for a discussion of the risks and uncertainties that apply to Presbia in connection with government regulation of its products.
Regulatory Requirements in the United States
Under the U.S. Food, Drug and Cosmetic Act, or the FD&C Act, manufacturers of medical devices must comply with extensive regulation relating to the issues described above, including regulations governing the design, testing, manufacturing, packaging, quality, servicing and marketing of medical products. Our immediate focus is upon the steps that we must take before our products can be marketed and sold in the United States.
25
FDA’s Premarket Clearance and Approval Requirements
Unless an exemption applies, each medical device that is distributed commercially in the United States requires either prior 510(k) clearance or prior approval of a PMA application from the FDA. The FDA classifies medical devices into one of three classes. Devices deemed to pose low to moderate risk are placed in either Class I or II, which, absent an exemption, requires the manufacturer to submit to the FDA a premarket notification requesting permission for commercial distribution. This process is known as 510(k) clearance. Some low risk devices are exempt from this requirement. Devices deemed by the FDA to pose the greatest risk, such as life-sustaining, life-supporting or implantable devices, or devices deemed not substantially equivalent to a previously cleared 510(k) device, are placed in Class III, requiring approval of a PMA application. Our current products are Class III devices requiring prior approval of a PMA application from the FDA. Both premarket clearance and PMA applications are subject to the payment of user fees, paid at the time of submission for FDA review. The FDA can also impose restrictions on the sale, distribution or use of devices at the time of their clearance or approval, or subsequent to marketing.
Premarket Approval
A PMA application must be submitted if, as is the case with the microlens and our microlens inserter, the device cannot be cleared through the 510(k) process. The PMA application process is generally more costly and time consuming than the 510(k) process and requires proof of the safety and effectiveness of the device to the FDA’s satisfaction. Accordingly, a PMA application must be supported by extensive data including, but not limited to, technical information regarding device design and development, pre-clinical and clinical trials, data and labeling to support the FDA’s determination that the device is safe and effective for its intended use. After a PMA application is complete, the FDA will accept the application and begin an in-depth review of the submitted information. By statute, the FDA has 180 days to review the “accepted application,” although, generally, review of the application takes between one and three years, and may take significantly longer. During this review period, the FDA may request additional information and/or clarification of information already provided. Also, during the review period, an advisory panel of experts from outside the FDA may be convened to review and evaluate the application and provide recommendations to the FDA as to the approvability of the device. In addition, the FDA will conduct a preapproval inspection of the manufacturing facility to ensure compliance with its Quality System Regulations, or QSRs, which impose elaborate design development, testing, control, documentation and other quality assurance procedures in the design and manufacturing process. The FDA may approve a PMA application with post-approval conditions intended to ensure the safety and effectiveness of the device, including, among other things, restrictions on labeling, promotion, sale and distribution and collection of long-term follow-up data from patients in the clinical study that supported approval. Failure to comply with the conditions of approval can result in materially adverse enforcement actions, including the loss or withdrawal of the approval. New PMA applications or PMA application supplements are required for significant modifications to the manufacturing process, as well as for modifications that affect the safety or effectiveness of the device, including, for example, certain types of modifications to the device’s indication for use, manufacturing process, labeling and design. PMA supplements often require submission of the same type of information as a PMA application, except that the supplement is limited to information needed to support any changes from the device covered by the original PMA application, and may not require as extensive clinical data or the convening of an advisory panel.
Our microlens, as an implanted device, and our microlens inserter, as an instrument directly associated with the implantation process, cannot be marketed and sold in the United States without PMA approval. We anticipate that other products that we may develop in the future, as well as modifications to our existing products, will also be associated with the implantation process and thus in all likelihood will be subject to PMA approval rather than 510(k) clearance.
26
IDE Applications
A clinical trial is almost always required to support a PMA application. In the United States, absent certain limited exceptions, human clinical trials intended to support product clearance or approval require an IDE application. Some types of studies deemed to present “non-significant risk” are deemed to have an approved IDE once certain requirements are addressed and IRB approval is obtained. If the device presents a “significant risk” to human health, as defined by the FDA, the sponsor must submit an IDE application to the FDA and obtain IDE approval prior to commencing the human clinical trials. The IDE application must be supported by appropriate data, such as animal and laboratory testing results, showing that it is safe to evaluate the device in humans and that the testing protocol is scientifically sound. The IDE application must be approved in advance by the FDA for a specified number of subjects, unless the product is deemed a non-significant risk device and eligible for more abbreviated IDE requirements.
In December 2013, we received approval of our IDE from the FDA to begin a staged pivotal clinical trial of our microlens in the United States.
Clinical Trials
Clinical trials for a Class III device may begin once the IDE application is approved by the FDA and the responsible IRBs at the clinical trial sites. There can be no assurance that submission of an IDE will result in the ability to commence clinical trials. Additionally, after a trial begins, the FDA may place it on hold or terminate it if, among other reasons, it concludes that the clinical subjects are exposed to unacceptable health risks that outweigh the benefits of participation in the study. During a study, sponsors are required to comply with the FDA’s IDE requirements for investigator selection, trial monitoring, reporting and record-keeping and with prohibitions on promoting investigational devices or making safety or efficacy claims for them. Sponsors are also responsible for the appropriate labeling and distribution of investigational devices.
We began enrollment for our U.S. staged pivotal clinical trial in May 2014 and began treating patients in June 2014. Initially, 75 subjects underwent insertion of our microlens at six investigational sites in the first stage of this study. Based on six-month data on 52 of these subjects, in January 2015, we submitted an interim safety report to the FDA along with a supplement to our IDE requesting approval to begin second stage enrollment. In February 2015, we received approval from the FDA to commence second stage enrollment in this trial. We are permitted to enroll up to an additional 337 subjects at up to nine additional investigational sites. Through March 15, 2015, 25 subjects underwent insertion of our microlens in the second stage of this study. The clinical trial that we have commenced for our microlens and our microlens inserter is expected to extend at least through 2017. Even if we do receive PMA approval, we do not anticipate receiving that approval for our microlens and our microlens inserter before the fourth quarter of 2017.
Our clinical trials must be conducted in accordance with FDA regulations and federal and state regulations concerning human subject protection, including informed consent and healthcare privacy. The investigators must also obtain patient informed consents, rigorously follow the investigational plan and study protocol, control the disposition of investigational devices and comply with all reporting and record-keeping requirements.
In addition, the FDA’s grant of permission to proceed with clinical testing does not constitute a binding commitment that the FDA will consider our study design adequate to support PMA approval. In addition, there can be no assurance that the data that we generate during a clinical study will meet chosen safety and effectiveness endpoints or otherwise produce results that will lead the FDA to grant marketing approval.
Pervasive and Continuing FDA Regulation
After a device is placed on the market, regardless of its classification or premarket pathway, numerous regulatory requirements apply. These include, but are not limited to:
| • | establishment registration and device listings with the FDA, which helps facilitate FDA inspections and other regulatory action; |
27
| • | QSRs, which requires manufacturers, including third-party manufacturers, to follow stringent design, testing, process control, documentation and other quality assurance procedures during all aspects of the development and manufacturing process; |
| • | labeling control and advertising regulations, which prohibit the promotion of products for uncleared or unapproved, or off-label, uses or indications, and impose other restrictions on labeling; |
| • | approval or clearance of product modifications that could significantly affect safety or efficacy or that would constitute a major change in intended use; |
| • | medical device reporting regulations, which require that manufacturers report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if it were to recur; |
| • | corrections and removal reporting regulations, which require that manufacturers report to the FDA field corrections and product recalls or removals if undertaken to reduce a risk to health posed by the device or to remedy a violation of the FDCA that may present a risk to health. In addition, the FDA may order a mandatory recall if there is a reasonable probability that the device would cause serious adverse health consequences or death; and |
| • | post-approval restrictions or conditions, including requirements to conduct post-market surveillance studies to establish continued safety data. |
The FDA has broad post-market and regulatory enforcement powers. We will be required to register with the FDA as a medical device manufacturer. As such, our manufacturing facilities will be subject to FDA inspections for compliance with QSRs. These regulations will require that we manufacture our products and maintain our documents in a prescribed manner with respect to design, manufacturing, testing and quality control activities. As a medical device manufacturer, we will also be required to comply with FDA requirements regarding the reporting of adverse events associated with the use of our medical devices, as well as product malfunctions that would likely cause or contribute to death or serious injury if the malfunction were to recur. FDA regulations also govern product labeling and prohibit a manufacturer from marketing a medical device for unapproved applications. The FDA may conduct unannounced inspections to determine compliance with the QSR and other regulations, and these inspections may include the manufacturing facilities of subcontractors. Failure by us or our suppliers to comply with applicable regulatory requirements can result in enforcement actions by the FDA or other regulatory authorities, which may result in sanctions and related consequences including, but not limited to:
| • | untitled letters or warning letters; |
| • | fines, injunctions, consent decrees and civil penalties; |
| • | recall, detention or seizure of our products; |
| • | operating restrictions, partial suspension or total shutdown of production; |
| • | refusal of or delay in granting our requests for premarket approval or clearances of new products or modified products; |
| • | once we have received premarket approvals or clearances, withdrawing those approvals or clearances that are already granted; |
| • | refusal to grant export approval for our products; |
| • | criminal prosecution; and |
| • | unanticipated expenditures to address or defend such actions. |
Regulatory Requirements Outside of the United States
Sales of medical devices outside the United States are subject to non-U.S. regulatory requirements that vary widely from country to country. These laws and regulations range from simple product registration requirements
28
in some countries to complex clearance and production controls in others. As a result, the processes and time periods required to obtain foreign marketing approval may be longer or shorter than those necessary to obtain FDA market approval. These differences may affect the efficiency and timeliness of international market introduction of our products.
In order to be placed on the market within the EEA, medical devices must meet the essential requirements set out in the relevant medical device legislation. The principal legislation regulating general medical devices in the EEA is Directive 93/42/EEC, referred to herein as the EU Medical Devices Directive. In the case of low risk (Class I) medical devices, such as our microlens inserter, the manufacturer may self-certify conformity with the EU Medical Devices Directive by issuing a declaration of conformity. In the case of medium to high risk (Class IIa, IIb and III) medical devices, including our microlens which is a Class IIb medical device, the certificate of conformity issues from a notified body. Where a medical device meets the essential requirements set out in the EU Medical Devices Directive and complies with the appropriate conformity assessment procedure, based on the classification of the medical device, a declaration or certificate of conformity will issue and a CE Mark may then be affixed to the product. Once a CE Mark has been affixed to the medical device, it may then be placed on the market in any country within the EEA and Switzerland (subject to certain localized registration and language requirements).
In February 2010, we received a certificate of conformity from our notified body for our microlens allowing the CE mark to be affixed to our microlens. In May 2013, we issued a declaration of conformity for our microlens inserter allowing the CE Mark to be affixed to our microlens inserter. We have also obtained an ISO 13485 quality system certification, which confirms that our medical device manufacturing quality management system is compliant with globally recognized standards set forth by the International Organization for Standardization. We are required to keep up-to-date and remain compliant with the most recently issued standards. In order to maintain our certificate of conformity and CE Mark, we must continue to comply with the EU Medical Devices Directive and pass annual facilities audit inspections by an inspection agency of the EEA to ISO 13485 standards. In addition, a notified body or other competent authority in an EEA country may perform post-marketing audits on our products and premises from time to time. Failure to comply with such requests in a timely manner, and any adverse findings in any such audit, could result in the withdrawal of our certificate of conformity and our CE Mark, and the recall or withdrawal of our products from the EEA market. Each certificate of conformity may be valid for a maximum of five years but would typically be valid for three years. Our existing certificate of conformity for our microlens is valid until November 2019. At the end of each period of validity, we are required to apply to the notified body for a renewal of our certificate of conformity. There may be delays in the renewal of our certificate of conformity and the notified body may require modifications to our products or to the related technical files before it agrees to issue a new certificate of conformity.
On September 26, 2012, the European Commission adopted a package of legislative proposals designed to replace the existing regulatory framework for medical devices in the EEA. The European Commission’s proposals may undergo significant amendments as they are reviewed by the European Council and European Parliament as part of the EEA legislative process. If and when adopted, the proposed new legislation may prevent or delay the EEA approval or clearance of any future products we may develop or impact our ability to modify currently EEA approved or cleared products on a timely basis.
In addition, we have obtained marketing authorization for our microlens and microlens inserter in certain countries outside of the EEA, including certain countries in which our microlens is currently commercially available. We are subject to the regulatory laws and regulations of each such country in order to maintain our marketing authorization. In addition, we will be subject to the regulatory laws and regulations of any additional country in which we obtain marketing approval to maintain such approval. These regulatory laws are complex and vary from country to country. Failure to comply with applicable laws and regulations could jeopardize our ability to sell our products and result in a variety of enforcement actions, all of which would negatively impact our business, results of operations and financial condition.
29
Corruption Laws
The U.S. Foreign Corrupt Practices Act and similar foreign anti-corruption laws generally prohibit companies and their intermediaries from making improper payments or providing anything of value to improperly influence foreign government officials for the purpose of obtaining or retaining business, or obtaining an unfair advantage. In recent years, there has been a substantial increase in the global enforcement of anti-corruption laws. Our ongoing non-U.S. operations and our expansion into additional countries outside the United States, including in developing countries, could increase the risk of such violations. Violations of these laws may result in severe criminal or civil sanctions, could disrupt our business, and could adversely affect our reputation, business and results of operations or financial condition.
Environmental Matters
Our activities currently require the controlled use of potentially harmful biological materials and hazardous materials and chemicals. We are subject to U.S. federal, state and local and non-U.S. environmental and pollution control laws and regulations governing the use, storage, handling and disposal of these materials and specified waste products. We believe that our operations comply in all material respects with applicable environmental laws and regulations in each country where we conduct business or have operations. We do not expect compliance with these laws to affect materially our capital expenditures, earnings or competitive position. We have no plans to invest in material capital expenditures for environmental control facilities for the remainder of our current fiscal year or for the next fiscal year. We are not aware of any pending actions, litigation or significant financial obligations arising from current or past environmental practices that are likely to have a material adverse impact on our financial position. However, environmental problems relating to our properties could develop in the future, and such problems could require significant expenditures. In addition, we cannot predict changes in environmental legislation or regulations that may be adopted or enacted in the future and that may adversely affect us.
Employees
As of December 31, 2014, we had 33 employees, one of whom holds a Bachelor of Optometry degree, and five of whom hold other advanced degrees. Of our total workforce, eight employees are engaged in research and development, and 23 employees are engaged in business development, manufacturing, finance, legal, human resources, facilities, information technology administration and general management. We have no collective bargaining agreements with our employees and we have not experienced any work stoppages. We believe that our relations with our employees are good.
Facilities
Our operations are currently conducted at three leased facilities. We lease or sublease an aggregate of approximately 14,800 square feet of office, laboratory and manufacturing space in Irvine, California, we lease approximately 300 square feet of office and storage space in Amsterdam, the Netherlands, and we lease approximately 305 square feet of office and warehouse space in Dublin, Ireland. Our corporate headquarters are currently located at our Dublin location.
We believe that our existing facilities are adequate for our current needs. When our leases expire, we may seek to renew our leases or look for additional or alternate space for our operations. We believe that suitable additional or alternative space will be available in the future on commercially reasonable terms.
Corporate History and Information
In February 2015, Presbia PLC consummated its initial public offering of ordinary shares. Prior to our initial public offering, we effected a series of reorganization transactions described below.
Our controlling shareholder, Presbia Holdings, was organized in the Cayman Islands in 2007 as an exempted company with limited liability. In 2009, Presbia Holdings acquired Visitome, Inc., a California corporation and the developer of our corneal inlay technology.
30
In October 2013, we completed a restructuring which involved the establishment of our interim holding company, Presbia Ireland, Limited, that directly or indirectly owns 100% of our business, assets and subsidiaries. Presbia Ireland, Limited is organized under the laws of Ireland as a private limited company. At the time of the restructuring, Presbia Ireland, Limited was wholly-owned by Presbia Holdings and certain intercompany debt was owed to Presbia Holdings by certain of its other subsidiaries. As part of the restructuring, approximately $12.2 million of such outstanding intercompany debt owed to Presbia Holdings was converted to equity of such subsidiaries. We refer to this transaction as the 2013 Restructuring.
In November 2014, Presbia Holdings converted all the remaining indebtedness owed to Presbia Holdings by certain subsidiaries of Presbia Ireland, Limited at that time to equity. In this transaction, approximately $23.5 million of outstanding intercompany debt owed to Presbia Holdings was converted to equity of such subsidiaries. We refer to this transaction as the 2014 Debt Conversion.
In January 2015, Presbia Holdings converted all the remaining indebtedness owed by a subsidiary of Presbia Ireland, Limited at that time to equity. In this transaction, approximately $1.6 million of outstanding intercompany debt owed to Presbia Holdings was converted to equity of such subsidiary. We refer to this transaction as the 2015 Debt Conversion. In addition, immediately following the 2015 Debt Conversion, Presbia Holdings contributed all the share capital in issue in Presbia Ireland, Limited to Presbia PLC, an Irish incorporated public limited company formed in February 2014 for the purpose of consummating our initial public offering, in exchange for 9,166,667 ordinary shares of Presbia PLC. We refer to this transaction as the 2015 Capital Contribution. Presbia PLC previously issued 40,000 ordinary shares to Presbia Holdings upon its formation, in order to satisfy statutory requirements for the incorporation of all Irish public limited companies, which were re-designated as deferred shares under our memorandum and articles of association prior to the consummation of our initial public offering. We refer to the 2014 Debt Conversion, the 2015 Debt Conversion and the 2015 Capital Contribution, collectively, as the 2014-2015 Restructuring.
31
We refer to the 2013 Restructuring, the formation and initial capitalization of Presbia PLC, and the 2014-2015 Restructuring, collectively, as the Reorganization Transactions.
Our corporate structure is set forth below.
Our principal executive offices are located at 120/121 Baggot Street Lower, Dublin 2 Ireland, and our telephone number is +353 (1) 659 9446.
Our website address is http://www.presbia.com. The information in, or that can be accessed through, our website is not part of this Annual Report on Form 10-K. Our annual reports on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K and amendments to those reports are available, free of charge, on or through our website as soon as reasonably practicable after we electronically file such material with, or furnish it to, the Securities Exchange Commission, or the SEC. The public may read and copy any materials we file with the SEC at the SEC’s Public Reference Room at 100 F Street, NE, Washington, D.C. 20549. Information on the operation of the Public Reference Room can be obtained by calling 1-800-SEC-0330. The SEC maintains an Internet site that contains reports, proxy and information statements and other information regarding our filings at www.sec.gov.
32
We are providing the following cautionary discussion of risk factors, uncertainties and assumptions that we believe are relevant to our business. These are factors that, individually or in the aggregate, we think could cause our actual results to differ materially from expected and historical results and our forward-looking statements. We note these factors for investors as permitted by Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act, and Section 27A of the Securities Act of 1933, as amended, or the Securities Act. You should understand that it is not possible to predict or identify all such factors. Consequently, you should not consider this section to be a complete discussion of all potential risks or uncertainties that may substantially impact our business. Moreover, we operate in a competitive and rapidly changing environment. New factors emerge from time to time and it is not possible to predict the impact of all of these factors on our business, financial condition or results of operations.
Risks Related to Our Business
We anticipate that we will continue to incur significant losses for the foreseeable future and, if we are unable to achieve and sustain profitability, the market value of our ordinary shares will likely decline.
We are a development stage ophthalmic device company with a limited operating history. We do not possess the regulatory approvals necessary to market our products in the United States, and we continue to incur significant research and development, sales and marketing and general and administrative expenses related to our operations. We are not profitable and have incurred losses in each year since our formation. Our net losses for the years ended December 31, 2014, 2013 and 2012 were $15.7 million, $9.5 million and 5.0 million, respectively. As of December 31, 2014, we had an accumulated deficit of $37.4 million.
We expect to continue to incur significant losses for the foreseeable future. We expect that these losses and our cash needs will increase in the near term as we continue to conduct our staged pivotal clinical trial in the United States, seek marketing approval in other countries, and commercialize our products in those non-U.S. markets where we are permitted to sell our microlens and microlens inserter. We may never achieve profitability, and unless and until we do, we will need to continue to raise capital. We expect to finance future cash needs through public or private equity offerings, debt financings or corporate collaborations and licensing arrangements. Additional funds may not be available when we need them on terms that are acceptable to us, or at all. If adequate funds are not available, we may be required to delay, reduce the scope of, or curtail, our operations. To the extent that we raise additional funds by issuing equity securities, our shareholders will experience dilution, and debt financing, if available, may involve restrictive covenants. We may not be able to enter into collaborations that we seek to establish. To the extent that we raise additional funds through collaborations and licensing arrangements, it may be necessary to relinquish some rights to our technologies or our product candidates or grant licenses on terms that may not be favorable to us. We may seek to access the public or private capital markets whenever conditions are favorable, even if we do not have an immediate need for additional capital at that time.
We expect to incur substantial expenses in our pursuit of regulatory approval in the United States and can provide no assurances that we will obtain the necessary approvals from the FDA to market our products in the United States.
The United States is a key market for commercialization of our microlens. Before we can market our products in the United States, we must conduct and successfully complete extensive clinical trials and then receive premarketing approval, or PMA, from the FDA. The earliest that we can reasonably expect to receive a PMA for our microlens and microlens inserter is in the fourth quarter of 2017, and it is possible that none of our existing products or any products we may seek to develop in the future will ever obtain a PMA. Furthermore, even if we were to obtain a PMA, neither approval by the FDA nor our existing CE Mark ensures approval by regulatory authorities in other countries or jurisdictions that we are targeting for commercialization of our microlens and microlens inserter, and approval by one regulatory authority does not ensure approval by regulatory authorities in other countries or by the FDA.
33
The time required to obtain approval by the FDA and comparable non-U.S. regulatory authorities is unpredictable and depends upon numerous factors, including the substantial discretion of such regulatory authorities. In addition, approval policies, regulations or the type and amount of preclinical and clinical data necessary to gain approval may change during the course of a product’s development and may vary among jurisdictions. We will be required to undertake and complete certain studies to generate data required to support submissions to the FDA and certain other regulatory authorities, which studies may require additional capital and time. If we do not receive or maintain regulatory approvals for our products in the United States and other jurisdictions that we target for commercialization of our products, we will not be able to successfully commercialize our products, which would substantially impair our ability to generate revenues and materially harm our business, results of operations and financial condition.
Our microlens and microlens inserter are currently our sole products and we are highly dependent on the successful marketing and sales of these products. There is no assurance that we will be able to develop any additional products.
Our microlens and microlens inserter are currently our sole products. We may fail to successfully commercialize our products. Successfully commercializing medical devices such as our microlens is a complex and uncertain process, dependent on the efforts of management, distributors, outside consultants and general economic conditions, among other factors. Any factors that adversely impact the commercialization of our microlens including, but not limited to, the delay or denial of regulatory approvals that we seek, competition or acceptance in the marketplace, will have a negative impact on our business, results of operations and financial condition. We cannot assure you that we will be successful in developing or commercializing any potential enhancements to our microlens or any other products. Our inability to successfully commercialize our current products and/or successfully develop and commercialize additional products or any enhancements to our products which we may develop would have a material adverse effect on our business, results of operations and financial condition.
Our planned clinical trials may be delayed, suspended or terminated, which could delay or prohibit us from obtaining regulatory approvals or make obtaining such regulatory approvals more costly.
In February 2015, we received approval from the FDA to commence second stage enrollment in our U.S. staged pivotal clinical trial. We estimate it will take approximately an additional six months to select, qualify and arrange for the implantation of our microlens for all the additional patients for the clinical trial we plan to undertake in connection with our PMA submission for our microlens. Delays in the commencement or completion of clinical testing could significantly affect our product development costs. We do not know whether planned clinical trials will begin on time or be completed on schedule, if at all. The commencement and completion of clinical trials can be delayed for a number of reasons, including delays related to:
| • | obtaining regulatory authorization to commence a clinical trial or complying with conditions imposed by a regulatory authority regarding the scope or design of a clinical trial; |
| • | reaching agreement on acceptable terms with prospective clinical research organizations, or CROs, and trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites; |
| • | manufacturing, including manufacturing sufficient quantities of a product or other materials for use in clinical trials; |
| • | recruiting and enrolling patients to participate in clinical trials for a variety of reasons, including the size of the patient population, the complexity of clinical trial protocols, changed standards of care during the conduct of the trial, and competition from other clinical trial programs for similar indications; |
| • | unexpected adverse effects experienced by patients in a clinical trial; and |
| • | retaining patients who have initiated a clinical trial, but may withdraw due to treatment protocol, adverse effects from the therapy, lack of efficacy from the treatment, personal issues or who are lost during follow-up. |
34
Clinical trials may also be delayed, suspended or terminated as a result of ambiguous or negative interim results, or results that are inconsistent with earlier results. In addition, a clinical trial may be suspended or terminated by us, the FDA or other regulatory authorities due to a number of factors, including:
| • | failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols; |
| • | inspection of the clinical trial operations, trial sites or manufacturing sites by the FDA or other regulatory authorities, resulting in the imposition of a clinical hold; |
| • | unforeseen safety issues or any determination that a clinical trial presents unacceptable health risks; and |
| • | lack of adequate funding to continue the clinical trial, including the incurrence of unforeseen costs due to enrollment delays, requirements to conduct additional clinical trials or increased expenses associated with the services of our CROs and other third parties. |
Our product development costs will increase if we experience delays in testing or if we need to perform more or larger clinical trials than planned. Additionally, changes in regulatory requirements and policies may occur in any jurisdiction and we may need to amend clinical trial protocols to reflect these changes. Amendments may require us to resubmit our clinical trial protocols to independent ethical committees, known as institutional review boards, or IRBs, for reexamination, which may impact the costs, timing or successful completion of a clinical trial. In addition, IRBs or other regulatory authorities may order the temporary discontinuation or termination of our clinical trials at any time if they believe that the clinical trials are not being conducted in accordance with applicable regulatory requirements, including if they present an unacceptable safety risk to patients. If we experience delays in completion of, or if we, the FDA or other regulatory authorities, an IRB or other reviewing entities, or any of our clinical trial sites suspend or terminate any of, our clinical trials, the commercial prospects for our products may be harmed and our ability to generate revenues will be delayed. In addition, many of the factors that cause, or lead to, termination or suspension of, or a delay in the commencement or completion of, clinical trials may also ultimately lead to the denial of regulatory approval of a product. Also, if one or more clinical trials are delayed, our competitors may be able to bring products to market before we do or further entrench their products in the market, and the commercial viability of our product candidates could be significantly reduced.
If concerns regarding side effects from presbyopia correction surgery generally, or our products specifically, develop, including as a result of third-party studies and publications, our business, results of operations and financial condition will be materially and adversely affected.
Concerns about potential side effects and long-term results may negatively impact market acceptance of presbyopia correction surgery generally or our products specifically, result in potential liability for us and prevent us from growing our business. Any undesirable side effects that may be discovered in our clinical trials or evaluations or in any third party studies or evaluations could delay or prevent regulatory approval, including FDA approval, could prevent us from maintaining our existing regulatory approvals, including our CE mark, or limit marketability of our products.
In early 2012, we completed a 12-month multicenter, post-market evaluation in Italy and Greece of our microlens in presbyopic patients between the ages of 45 and 60. The 12-month data for 70 patients indicated certain post-operative adverse events, including: one removal of the microlens, as a result of a patient’s complaints of significant halos and glare when driving at night; one case of transient light sensitivity syndrome (an abnormal occurrence of photosensitivity associated with the femtosecond laser); one case of epithelial ingrowth (an abnormal growth of corneal epithelium in an area where it does not belong, associated with the femtosecond laser); and four cases of transient stromal haze (the activation of inflammatory cells in connection with surgery). In addition, certain patients experienced a slight loss in uncorrected visual acuity-distance, or UCVA-distance, which is distance vision in the operated eye without prescription enhancement. For further information regarding this post-market evaluation, see “Part I, Item 1. Business—Our Solution—Evaluation Conducted Outside of the United States.”
35
In addition, our microlens has been the subject of certain third party studies that have been conducted to assess the efficacy and safety of our microlens. We did not commission these studies or design, review or oversee the implementation of their protocols, and we have limited information with respect to these studies. These studies have reported certain adverse effects relating to the safety and efficacy of our microlens and microlens inserter. With respect to the below referenced third-party study conducted in Japan, we paid the annual fees of the IRB which reviews the study’s protocol.
One ongoing third-party study being conducted in Italy from 2011 to present by a group of ophthalmic surgeons evaluated the efficacy and safety of our microlens in 81 patients. Of the 81 patients evaluated, six patients underwent removal of our microlens within 12 months of implantation due to patient-reported reduction in distance vision and the presence of halos and glare. Findings that our microlens compromises distance vision could result in the suspension of our U.S. clinical trial, delay, make it more difficult and expensive for us to receive and/or prevent us from receiving, or prevent us from maintaining, regulatory approvals, including FDA approval or our CE mark, or limit marketability of our microlens and microlens inserter.
Another third-party study conducted in Japan from 2012 to April 2014 by one ophthalmic surgeon evaluated the efficacy and safety of our microlens in 38 patients. Such study reports a number of adverse events, including: three cases of inlay defect (a mark or defect seen on the edge of the microlens that is made by the microlens inserter due to improper microlens loading prior to insertion); two cases of epithelial ingrowth (an abnormal growth of corneal epithelium in an area where it does not belong associated with the femtosecond laser); two cases of microlens removal (related to halos and glare); one case of meibomianitis (inflammation of the meibomian glands, a group of oil-secreting glands in the eyelids); one case of moderate foreign debris (the presence of material in the pocket after using a laser and inserting the microlens); one case of severe keratic precipitates (an accumulation of white blood cells on the corneal endothelium which arises as a result of inflammatory reactions); one case of superficial punctate keratisis (a non-inflammatory condition of the cornea with discrete opacities of the cornea, without ulceration); and one case of vertical gas bubbles (escape of gas bubbles from the dissection plane into the trabecular meshwork then to the anterior chamber during laser-assisted flap or tunnel creation). Such study also documented 17 observations of “foreign debris” of unknown composition and origin under high magnification slit lamp examination (three of such 17 patients also experienced one of the other adverse events noted above, including the moderate case of foreign debris noted above which was reported as an adverse event). The ophthalmic surgeon performing the testing initially reported such foreign debris to be metallic.
As a result of the foreign debris adverse event noted in this study, the other foreign debris observations noted in this study and in the study in Russia discussed below, as well as anecdotal comments made by certain other surgeons regarding observations of foreign debris, we opened a corrective action and preventive action investigation to assess possible sources of the foreign debris. We developed a matrix of all possible sources of the foreign debris, including our microlens inserter, and conducted analysis and performed a literature review to determine the source of the foreign debris. As part of this analysis, we collected nine sterilized (but not cleaned) microlens inserters used in the Japan study (as well as an additional microlens inserter that had not been opened or used in the Japan study) and arranged for a third party to analyze such microlens inserters for the presence of foreign debris. Of the 10 microlens inserters that were tested, one microlens inserter was found to have two particles present, and two microlens inserters were found to have one particle present. These particles were determined to be series 300 stainless steel. The quantity and shape of these particles did not appear to be consistent with the characteristics of the foreign particles noted in the study conducted by the ophthalmic surgeon in Japan.
Based on the outcome of this initial analysis, additional testing was conducted to evaluate all possible sources that could lead to the observation of foreign debris as reported in the study conducted by the ophthalmic surgeon in Japan. Actuation tests were performed on 12 of our microlens inserters by a third party. The microlens inserters were not cleaned or sterilized between actuation ranges. Five of the 12 microlens inserters included in this testing came directly from the manufacturer and had never undergone cleaning or sterilization. Of the 12
36
microlens inserters tested, five microlens inserters did not produce particles in any of the actuation ranges. One microlens inserter that had not been cleaned or sterilized produced particles in the one to 10 actuation range, four of the 12 microlens inserters produced particles in the 11 to 25 actuation range, two of the 12 microlens inserters produced particles in the 26 to 50 range, and six of the 12 microlens inserters produced particles in the 51 to 100 range. Certain of the particles were determined to be series 300 stainless steel and others were determined to be titanium.
Following the actuation tests, additional testing was conducted to evaluate the cause of the foreign debris noted in third-party studies. As part of our ongoing corrective action and preventative action plan, we arranged for an analysis of the microlens of a patient (who was one of the two patients noted above in the Japanese study who had their microlens removed due to halos and glare) who was noted to have foreign debris present following implantation. This patient’s microlens was removed following implantation for reasons unrelated to the presence of the foreign debris (the patient’s microlens was removed due to repeated decentering of the microlens post-implantation). The removed microlens was found to have five foreign particles present. Initial testing concluded that the foreign particles were likely composed of stainless steel, which suggested that one or more metal instruments present in the surgical environment was likely the source of the foreign particles. Initial testing also concluded that galling, or chafing, may be occurring between the plunger and head assembly components of our microlens inserter, which could be the cause of the production of metal debris. As a result of these initial findings, additional testing was conducted by a third-party metallurgist at our request to determine whether the composition of the foreign particles in the removed microlens is consistent with the elements and concentrations thereof found in our microlens inserter. Such additional testing confirmed that the foreign particles in the explanted lens were of stainless steel composition, but also determined that the composition of the foreign particles was not consistent with the composition of our microlens inserter.
At this juncture, we have not reached any definitive conclusions as to the source of the foreign debris noted in the third-party studies. The final report of our corrective action and preventative action investigation is expected to be submitted to the FDA in November 2015. As a result of additional testing that we have completed as part of our investigation, we believe that our microlens inserter has the potential to produce metallic debris, although the debris noted during such testing was generally environmental in nature and was not considered clinically significant. In addition, there have been 19 observations of foreign debris in the 75 subjects implanted in the first stage of our U.S. staged pivotal clinical trial. None of these 19 observations was reported as an adverse event, none of these patients was reported to have any other adverse event and the debris was not considered clinically significant. We have reported these 19 observations in our interim safety report submitted to the FDA.
A common source of non-metallic debris is the general surgical environment. Several articles written over the last 10 years report that interface debris is a relatively common finding in patients who have undergone LASIK surgery or phacoemulsification (cataract surgery in which the eye’s internal lens is emulsified with an ultrasonic hand piece and aspired from the eye).
Other than the one case of moderate foreign debris reported as an adverse event in the third party study conducted in Japan, at this time, we are not aware of any additional adverse events reported with respect to the foreign debris observations noted in the third party studies or our U.S. staged pivotal clinical trial. We have developed additional cleaning and sterilization procedures and packaging procedures which are designed to provide microlens inserters in a clean initial condition prior to use. As part of our ongoing risk mitigation efforts, we are continuing to develop a disposable microlens inserter for our microlens and a pre-loaded disposable microlens inserter. In addition, with respect to our staged pivotal clinical trial that we are conducting in the United States, each microlens inserter is continuing to only being used one time in order to mitigate the potential risk associated with the possible creation of metal foreign debris during insertion of our microlens. If our microlens inserter or any other equipment supplied by us is determined to pose a health risk through the deposit of metal debris in a patient’s eye, such determination could result in the suspension of our U.S. clinical trial, delay, make it more difficult and expensive for us to receive and/or prevent us from receiving, or prevent us from maintaining, regulatory approvals, including FDA approval or our CE mark, limit marketability of our products and subject us to lawsuits or claims.
37
Three additional third-party studies conducted at three locations in Brazil beginning in 2012 evaluated the efficacy and safety of our microlens. The first ongoing study reported that four of 22 patients lost three or more lines of UCVA-distance at one year postoperative compared to an average UCVA-distance of 20/20 before the study. No additional adverse events were reported in that study. The second study reported that two of 10 patients lost two lines of UCVA-distance vision at three months postoperative compared to an average UCVA-distance of 20/20 before the study. No additional adverse events were reported in the study. The third ongoing study did not provide visual acuity data and no adverse events were reported.
One additional third-party study of the efficacy and safety of our microlens was conducted in 2012 in Russia. This study reported that two of 10 patients experienced a decrease in UCVA-distance and BCVA-distance at three months postoperative due to night glare compared to before the study (preoperative visual acuity was not provided). One of these patients underwent removal of our microlens, and no additional adverse events were reported for the patient after lens removal. This study also noted minimal debris inclusion in two cases. No additional postoperative adverse events were reported in this study.
We are aware of one additional case of a patient undergoing microlens removal in a commercial setting in the Czech Republic and three additional cases of a patient undergoing microlens removal in commercial settings in Brazil. In addition, in March 2014, we became aware of a request for removal of a microlens from a patient who was implanted in Brazil. We have been informed that after treatment with a topical steroid, the patient’s inflammation issues have resolved and the patient does not wish to have the microlens removed.
In connection with the 75-patient implants as part of the first stage of our staged pivotal clinical trial in the United States, we had four adverse events in the implanted eyes, each of which has resolved: one case of minor corneal abrasion occurring prior to surgery, which resolved one day later without treatment; one case of elevated intraocular pressure due to a postoperative medication tapering regimen that included a steroid, which resolved six days later at the second interim postoperative visit (the subject was determined to be a steroid responder, and was prescribed an intraocular pressure-lowering medication until the intraocular pressure was back to baseline); one case of dry eye symptoms, which resolved two months later following treatment with artificial tears; and one case of corneal haze, which resolved three months later after being treated with a topical steroid. No unanticipated adverse device effects in the implanted eyes have been reported in this study to date. We have had two serious adverse events reported for subjects unrelated to our microlens. One subject underwent an unspecified outpatient procedure unrelated to the microlens or implantation procedure, experienced a complication during the procedure and was hospitalized overnight. Another patient fell off an all-terrain vehicle and was hospitalized for six days, which was unrelated to the microlens or implantation procedure. In addition, there have been 19 observations of foreign debris in the 75 subjects implanted in the first stage of our U.S. staged pivotal clinical trial. None of these 19 observations was reported as an adverse event and the debris was not considered clinically significant.
If our microlens or microlens inserter are ultimately determined to produce undesirable side effects, including posing a health risk through the deposit of foreign particles in a patient’s eye, such determination could result in the suspension of our U.S. staged pivotal clinical trial, delay, make it more difficult and expensive for us to receive and/or prevent us from receiving, or prevent us from maintaining, regulatory approvals, including FDA approval or our CE mark, limit marketability of our products and subject us to lawsuits or claims.
Adverse findings in post-marketing vigilance or regulatory audits could subject us to suspension or withdrawal of our certificates of conformity, mandatory product recalls and significant legal liability, which would materially and adversely affect our business, results of operations and financial condition.
In February 2010, we received a certificate of conformity from our notified body (a third-party organization designated by competent authorities of the European Economic Area (27 of the 28 European Union member states plus Iceland, Liechtenstein and Norway), or EEA, to conduct regulatory oversight on medical devices) for our microlens allowing the CE Mark to be affixed to our microlens, permitting our microlens to be placed on the market within any state in the EEA and Switzerland (subject to certain localized registration and language
38
requirements). Manufacturers of medical devices in the EEA are required to implement post-marketing vigilance procedures with respect to their CE Marked medical devices. Such post-marketing vigilance procedures include surveillance of patient and user complaints and alleged adverse events associated with the use of CE Marked medical devices. Serious incidents associated with the use of a medical device must be reported to the competent authority in whose territory the incident occurred. Incidents that must be reported include any malfunction or deterioration in the characteristics and/or performance of a device, as well as any inadequacy in the labeling or the instructions for use which, directly or indirectly, might lead to or might have led to the death of a patient, user or other person, or to a serious deterioration in their state of health. Incidents must be reported as soon as possible, and in some cases immediately, after the manufacturer becomes aware of the incident. In addition to reporting the incident, the manufacturer must investigate the incident and take any corrective action required. The required corrective action depends on the seriousness of the incident, and varies from the issuance of advisory notices to the implementation of product recalls. Minor incidents not requiring notification to the competent authorities must be documented, reviewed, investigated and analyzed on a regular basis by the manufacturer to determine whether trending conclusions can be made concerning the safety or performance of the medical device and whether actions must be taken in relation to the continued marketing of medical devices currently on the market. We expect to incur ongoing costs to comply with these post-market vigilance obligations in EEA markets for so long as we continue to market and sell products in those markets. Moreover, any patient or user complaints and/or adverse events discovered during such post-market vigilance could subject us to suspension or withdrawal of our certificates of conformity, mandatory product recalls and significant legal liability, which would materially and adversely affect our business, results of operation and financial condition. In addition, a notified body or other competent authority in an EEA country may perform post-marketing audits on our products and premises from time to time. Failure to comply with such requests in a timely manner, and any adverse findings in any such audit, could subject us to suspension or withdrawal of our certificates of conformity, mandatory product recalls and significant legal liability, which would materially and adversely affect our business, results of operations and financial condition.
We were previously subject to certain legal proceedings relating to the ownership of certain assets, including intellectual property. As demonstrated by such proceedings, future claims regarding intellectual property may be costly and time consuming to defend and future claims may delay or prevent the development and commercialization of our products or place our patent portfolio and other proprietary rights at risk.
In June 2008, Biovision AG, a Swiss company, was liquidated in bankruptcy in Switzerland. Vladimir Feingold, our Chief Technology Officer and one of our directors, was a minority owner of Biovision AG and served as its President and Chief Executive Officer. During the bankruptcy auction in Switzerland, Thomke Invest AG, or Thomke, purchased certain assets of Biovision AG with the stated intention of transferring those assets to Biovision Technology AG, which is now Neoptics AG, a competitor developing an alternative corneal inlay surgical lens to treat presbyopia. Disputes arose as to the scope of the purchased assets, the propriety of the Swiss auction, and what persons or entities had superior rights with respect to certain property, data, know-how, processes, and technology relating to a specialized surgically implanted lens to treat presbyopia, which we refer to collectively as the Disputed Assets.
Two competing lawsuits were filed in July 2008. The first lawsuit, which we refer to as the Thomke Action, was filed in the Superior Court of the State of California, County of Orange, by Thomke against Mr. Feingold, Biovision AG, our Visitome, Inc. subsidiary, Zohar Loshitzer, our Chief Business Development Officer and one of our directors, Orchard Capital Corporation, which is owned by Richard Ressler (one of our directors and the controlling shareholder of Presbia Holdings, our controlling shareholder), and Swiss Investment Corporation. In its complaint, Thomke alleged, among other things, wrongful possession of personal property and conversion of the Disputed Assets. The second lawsuit, which we refer to as the Visitome Action, was filed on the same day in the same court by our Visitome, Inc. subsidiary seeking a declaration of rights with respect to the Disputed Assets. In November 2012, Swiss Investment Corporation and Mr. Feingold filed a lawsuit in Switzerland to invalidate certain orders issued by the Swiss Insolvency Office with respect to the Disputed Assets.
39
The Thomke Action and the Visitome Action were eventually consolidated. On December 12, 2012, the parties to the various lawsuits and certain of their affiliates entered into a settlement agreement to settle the three lawsuits. The parties to the settlement agreement included certain of our affiliates, including Mr. Feingold, Mr. Loshitzer, Visitome, Inc., PresbiBio, LLC, Presbia Holdings and Orchard Capital Corporation. The resulting settlement agreement included, among other things, (i) dismissals (with prejudice) of the three lawsuits, (ii) mutual releases of all matters arising prior to the date of the settlement agreement, including, without limitation, claims regarding the Disputed Assets, (iii) mutual waivers of all known or unknown matters subject to the mutual releases, (iv) mutual covenants not to sue in connection with matters released under the settlement agreement, and (v) acknowledgements and agreements to the terms of the settlement agreement by certain of our other affiliates not party thereto.
We cannot guarantee that we will not be subject to future claims regarding intellectual property. If successful, any such claims could place our patent portfolio and other proprietary rights at risk, which would have a material and adverse effect on our business, results of operations and financial condition. Even if such claims are not successful, they could be costly and time consuming to defend and they could delay or prevent the development and commercialization of our products.
We have a limited operating history and may face difficulties encountered by early stage companies in new and rapidly evolving markets.
Our controlling shareholder, Presbia Holdings, was formed in 2007 and acquired our Visitome, Inc. subsidiary in 2009. Accordingly, we have a limited operating history upon which to base an evaluation of our business and prospects. In assessing our prospects, you should consider the risks and difficulties frequently encountered by early stage companies in new and rapidly evolving markets, particularly companies engaged in the development and sales of medical devices. These risks include our ability to:
| • | manage expectations during the lengthy process of obtaining PMA approval from the FDA; |
| • | establish and increase awareness of our brand and strengthen customer loyalty; |
| • | grow our business in targeted markets outside of the United States while awaiting FDA approval; |
| • | implement and successfully execute our commercialization strategy; |
| • | respond effectively to competitive pressures and developments; |
| • | continue to develop and enhance our products in development; |
| • | obtain regulatory approval to commercialize our products and, when and if approved, enhance those products; |
| • | maintain compliance with all applicable regulatory statutes and regulations; |
| • | expand our global presence; |
| • | perform clinical research and trials on our existing products and future product candidates; |
| • | attract, retain and motivate qualified personnel; and |
| • | raise additional capital, if additional capital is needed, on favorable or acceptable terms. |
As a result of these or other risks, our business strategy might not be successful.
We are engaged in an intensely competitive business with competitors that may enjoy significant competitive advantages over us and if we are unable to compete successfully against our existing or potential competitors, our sales and operating results may be negatively affected and we may not grow.
The market for surgical presbyopia correction is intensely competitive, both in and outside of the United States, and competition may increase. In addition to our company, there are at least four companies currently developing
40
competing corneal inlay surgical solutions—AcuFocus, Inc., Revision Optics, Inc., Neoptics AG and LensGen, Inc. Other non-corneal inlay procedures also offer solutions to presbyopia, including: monovision approaches (whereby one eye, typically the dominant eye, is corrected for distance vision and the other eye is corrected for near vision using glasses, contact lenses or surgical procedures); multifocal approaches (whereby both a distance focus and a near focus are provided at the same time in each eye using glasses, contact lenses, surgically implanted artificial lenses or laser surgery); and accommodating approaches (whereby surgically implanted artificial lenses are designed to mimic the movement of the natural crystalline lens of the eye or techniques are used to attempt to restore the function of the eye’s own accommodative system). Certain companies enjoy competitive advantages over us, including: significantly greater name recognition; established relations with healthcare professionals and customers; established distribution networks; additional lines of products; greater experience in conducting research and development, manufacturing, clinical trials, obtaining regulatory approval for products, and marketing approved products; greater financial and human resources for product development, sales and marketing, and patent litigation; and earlier commencement of U.S. pivotal clinical trials. To compete in this market requires an ongoing, extensive search for technological innovation and the ability to respond to rapid technological change. It also requires, among other things, the ability to effectively discover, develop, test and obtain regulatory approvals for products, as well as the ability to effectively commercialize, market and promote approved products, including communicating the effectiveness, safety and value of products to actual and prospective patients and medical professionals. A better-financed or lower-cost provider of corneal inlay surgical solutions or a competing vision treatment could take market share away from us or force us to lower product prices, causing our revenues and results of operations to decline materially.
If we do not convince ophthalmic surgeons that our products are attractive alternatives to our competitors’ products as well as a complementary solution to other existing vision correction procedures, we will not be commercially successful.
Ophthalmic surgeons play a significant role in determining the course of treatment and, ultimately, the type of products that will be used to treat a patient for presybyopia. As a result, it will be important for us to effectively market our products to them. Acceptance of our products depends on educating ophthalmic surgeons as to the distinctive characteristics, perceived clinical benefits, safety and cost effectiveness of our products as compared to our competitors’ products as well as the utility of our microlens to be used as a complementary procedure to existing surgical treatments for visual problems. It also depends on training ophthalmic surgeons in the proper application of our products. If we are not successful in convincing ophthalmic surgeons of the merits of our products or educating them on the use of our products, they may not use our products and we will be unable to fully commercialize our products or reach profitability. Ophthalmic surgeons may be hesitant to change their medical treatment practices for the following reasons, among others:
| • | lack of experience with our products; |
| • | existing relationships with competitors and distributors that sell their products; |
| • | lack or perceived lack of evidence supporting additional patient benefits; |
| • | perceived liability risks generally associated with the use of new products and procedures; and |
| • | the time commitment that may be required for training. |
In addition, we believe recommendations and support of our products by influential ophthalmic surgeons are important for market acceptance and adoption. If we do not receive support from such ophthalmic surgeons or long term data does not show the benefits of using our products, ophthalmic surgeons may not use our products. In such circumstances, we may not be able to grow our revenues or achieve profitability.
If we are unable to train ophthalmic surgeons and their clinical staff on the safe and appropriate use of our products, we may be unable to achieve revenue growth or profitability.
An important part of our sales process includes the ability to train ophthalmic surgeons and their clinical staff on the safe and appropriate use of our products. We have very limited experience in training and retaining qualified
41
independent ophthalmic surgeons to perform presbyopia correction surgery using our products. If we are unable to attract ophthalmic surgeons to our training programs, we may be unable to achieve growth or profitability.
There is a learning process involved in ophthalmic surgeons and their clinical staff becoming proficient in the use of our products. It is critical to the success of our commercialization efforts to train a sufficient number of ophthalmic surgeons and to provide them with adequate instruction in the use of our microlens and microlens inserter. This training process may take longer than expected and may therefore affect our ability to increase sales. Following completion of training, we expect to rely on the trained ophthalmic surgeons to advocate the benefits of our products in the broader marketplace. Convincing ophthalmic surgeons to dedicate the time and energy necessary for adequate training is challenging, and we cannot assure you we will be successful in these efforts. If ophthalmic surgeons and their clinical staff are not properly trained, they may misuse or ineffectively use our products. Such uses may result in unsatisfactory patient outcomes, patient injury, negative publicity or lawsuits against us, any of which would have a material adverse effect on our business, results of operations and financial condition.
Our reliance on a single third-party supplier for sales of our microlens outside of the United States and our reliance on a limited number of third-party suppliers for our microlens inserter could harm our ability to meet demand for our products in a timely and cost effective manner.
We rely on a single supplier located in Israel (Hanita Lenses) to manufacture and supply our microlens that we sell outside of the United States. This supplier has committed to a guaranteed minimum production level that we believe is adequate to meet our current needs. The agreement with this supplier is set to expire in January 2017. We have manufacturing capacity in Irvine, California, but items manufactured in that facility to date have been used solely for pre-IDE testing in the United States. Our U.S. facility has received regulatory approval from the State of California to manufacture our microlens for our U.S. staged pivotal trial. Also, our U.S. facility recently received regulatory approval to manufacture our microlens for sale in the EEA. We expect to continue to utilize our existing Israeli supplier for products sold outside of the United States, including in the EEA, unless and until we determine that it is more efficient for our company to manufacture our microlens for sale outside the United States and we obtain any additional regulatory approvals that may be required. Given the location of our Israeli supplier, the supply of our microlens could be disrupted if events were to occur in the Middle East that resulted in social, political, economic or military instability. Given our reliance on this supplier and our limited experience manufacturing our microlens at our California facility, we cannot assure you that we will be able to obtain sufficient quantities of our microlens in the future, which could have a material adverse effect on our business, results of operations and financial condition.
Our microlens inserter is manufactured by a third-party original equipment manufacturer in the United States (Total Titanium, Inc.). We do not have a guaranteed supply commitment from this supplier. Although we believe that this supplier will be able to meet our foreseeable needs, we cannot assure you that we will be able to obtain sufficient quantities of our microlens inserter in the future, which could have a material adverse effect on our business, results of operations and financial condition.
For us to be successful, our suppliers must be able to provide us with products in desired quantities, in compliance with regulatory requirements, in accordance with agreed-upon detailed specifications, at acceptable costs and on a timely basis. Reliance on third party suppliers entails risks to which we would not be subject if we manufactured all of our products ourselves, including reliance on the third parties for regulatory compliance and quality assurance, the possibility that products will not be delivered on a timely basis, the possibility of increases in pricing for our products, the possibility of breach of the applicable manufacturing agreement by third parties and the possibility of termination or non-renewal of the agreement by third parties. If any of these risks materialize, it could significantly increase our costs and impact our ability to meet demand for our products and could have a material adverse effect on our business, results of operations and financial condition. If we are unable to satisfy commercial demand for our products in a timely manner, our ability to generate revenue would be impaired, market acceptance of our products could be adversely affected and customers may instead purchase or use our competitors’ products. Securing a replacement supplier could be difficult, time-consuming and expensive.
42
There are a limited number of suppliers and third-party manufacturers that operate under the FDA’s current Good Manufacturing Practices, or cGMP, maintain certifications of the International Standards Organization, or ISO, that are recognized as harmonized standards in the EEA, and have the necessary expertise and capacity to manufacture our products. As a result, if it were necessary to terminate our relationship with our existing suppliers, it may be difficult for us to locate another supplier that could promptly fulfill our anticipated future needs. If we are unable to arrange for third-party manufacturing of our products, or are unable to do so on commercially reasonable terms, our sales may be materially and adversely affected.
We rely on a single third-party supplier to supply the raw material used to manufacture our microlens.
The hydrophilic acrylic material used to manufacture our microlens is supplied to us by a single supplier located in the United Kingdom. We do not have a guaranteed supply commitment from this supplier. Although we believe that such supplier will be able to sufficiently meet our currently anticipated supply needs, we cannot assure you that we will be able to obtain sufficient quantities of the hydrophilic acrylic material in the future, which could have a material adverse effect on our business, results of operations and financial condition. In addition, we would be required to obtain approval from the FDA in the event that we wished to use different material or similar material from a different supplier with respect to any products to be offered and sold in the United States.
The global nature of our business may result in fluctuations and declines in our sales and profits.
Our products are currently available in several countries outside of the United States. Because we have received a CE Mark for our microlens, we have the ability presently to market that product within the EEA and in Switzerland. For the foreseeable future, pending receipt of the necessary FDA approvals to market our products in the United States, we expect that sales outside of the United States will represent 100% of our revenues. We may be exposed to transaction risk because some of our sales and expenses will be incurred in a different currency than the local currency. To date, we have not attempted to offset our exposure to this risk by investing in derivatives or engaging in other hedging transactions.
Economic, social and political conditions, laws, practices and local customs vary widely among the countries in which we sell our products. Our operations outside of the United States face a number of risks and potential costs, enjoy less stringent protection of intellectual property and face economic, political and social uncertainty in some countries, especially in emerging markets. We have limited experience developing and manufacturing our products to comply with the commercial and legal requirements of markets outside of the United States. Our success in markets outside of the United States will depend, in part, on our ability to manufacture products that meet applicable regulatory and commercial requirements, our ability to enforce contractual commitments and our ability to develop and implement policies and strategies that are effective in anticipating and managing these and other risks in the countries where we do business. Such risks may have a material adverse effect on our operations in any particular country and on our business as a whole. Inflation in emerging markets also may make our products more expensive there and increase the credit risks to which we will be exposed.
If we do not successfully implement our commercialization strategy, our business, results of operations and financial condition will be adversely affected.
We have developed our commercialization strategy based on assumptions about the presbyopia market that might prove to be wrong. We believe that various demographics and industry-specific trends, including adults noticing the onset of presbyopia as they reach their forties, the demands upon our eyes resulting from the increased use of electronic devices and increasing acceptance of eye surgeries as alternatives to reading glasses and contact lenses, will help drive growth in our market and our business, but these demographics and trends are uncertain. Actual demand for our products could differ materially from projected demand if our assumptions regarding these factors prove to be incorrect or do not materialize, or if alternative treatments to those offered by our products gain widespread acceptance.
43
We may not be able to successfully implement our commercialization strategy. To implement our commercialization strategy of initially dealing directly with laser centers, we must, among other things, educate the decision-makers within these organizations regarding the advantages of our products and processes, train professionals working in those centers on how to use our products, enter into commercially reasonable agreements with those centers and engage in careful follow-up to capture relevant experience and demonstrate our goal to partner with our laser center customers. Our strategy of focusing exclusively on the presbyopia market may limit our ability to grow. Moreover, even if we successfully implement our commercialization strategy, our operating results may not improve or may decline. We may decide to alter or discontinue aspects of our commercialization strategy and may adopt different strategies due to business or competitive factors not currently foreseen, such as new medical technologies that would make our products obsolete. Any failure to implement our business strategy may materially and adversely affect our business, results of operations and financial condition.
If the market does not accept and endorse presbyopia correction surgery, we will not be able to successfully execute our business plan.
We believe that our profitability and our ability to expand depend to a large extent on the acceptance of vision correction surgeries in general, as well as presbyopia correction surgery specifically, as a safe and effective treatment option. Even if we obtain FDA and other required regulatory approvals, if presbyopia correction surgery does not gain broad market acceptance, our opportunity to achieve profitability and sustained growth will be severely limited. We cannot assure you that presbyopia correction surgery will be accepted widely, if at all, by ophthalmic surgeons, ophthalmologists, optometrists or the general population as an alternative to existing or future methods of treating presbyopia or other refractive vision disorders. Market acceptance depends on a number of factors, including:
| • | the efficacy and safety of our products as demonstrated in clinical trials, as well as by actual usage in jurisdictions where our products are authorized for marketing and sale; |
| • | the clinical indications for which our products are approved if and when approvals are granted; |
| • | acceptance by ophthalmic surgeons, ophthalmologists, optometrists and ophthalmic centers; |
| • | third-party publications reporting findings with respect to the efficacy and safety of our products; |
| • | the potential and demonstrable advantages of our products and of competitive products and processes; |
| • | relative convenience and ease of administration; |
| • | the tolerance of our products by patients, including prevalence and severity of side effects; and |
| • | the effectiveness of our sales and marketing efforts. |
Any factor that adversely impacts market acceptance of presbyopia correction surgery will have a negative impact on our business, results of operations and financial condition.
We do not anticipate that our microlens and the procedure to implant our microlens will be reimbursable through private or governmental third-party payors, which could limit market acceptance.
Our microlens and the procedure to implant our microlens are not currently reimbursable through private or governmental third-party payors in any country. In addition, we do not anticipate that our microlens and the procedure to implant our microlens will be reimbursable through private or governmental third-party payors in the foreseeable future. The commercialization of our microlens depends on prospective patients’ ability to cover the costs of our microlens and the implantation procedure. We believe that a substantial portion of presbyopes worldwide do not have the financial means to cover the costs of our microlens. A general regional or worldwide economic downturn could negatively impact demand for our microlens. In the event that medically eligible patients deem the costs of our procedure to be prohibitively high or consider alternative treatment options to be more affordable, our business, results of operations and financial condition would be negatively impacted.
44
Our ability and the ability of our subsidiaries to use net operating loss carryforwards and certain other tax attributes may be limited.
Our ability and the ability of our subsidiaries to utilize United States federal net operating loss carryforwards and federal tax credits may be limited under Sections 382 and 383 of the Internal Revenue Code of 1986, as amended, or the Code. The limitations apply if an “ownership change,” as defined by Section 382, occurs. Generally, an ownership change occurs if the percentage of the value of the stock that is owned by one or more direct or indirect “five percent shareholders” increases by more than 50 percentage points over their lowest ownership percentage at any time during the applicable testing period (typically three years). The ownership change of a parent entity may result in the ownership change of a subsidiary. If we or any of our subsidiaries have experienced an “ownership change” at any time since formation, that corporation may already be subject to limitations on the ability to utilize existing net operating losses and other tax attributes to offset taxable income. In addition, future changes in our stock ownership, which may be outside of our control, may trigger an “ownership change” and, consequently, Section 382 and 383 limitations. As a result, if we or our subsidiaries earn net taxable income, the ability to use pre-change net operating loss carryforwards and other tax attributes to offset United States federal taxable income may be subject to limitations, which could potentially result in increased future tax liability to us or our subsidiaries.
We may not be able to achieve a competitive worldwide effective corporate tax rate.
We cannot give any assurance as to what our effective tax rate will be, because of, among other things, uncertainty regarding the geographic mix of any income we generate and the tax policies of the jurisdictions where we operate. Our actual effective tax rate may vary from our expectation and that variance may be material. Additionally, the tax laws of Ireland and other jurisdictions could change in the future, and such changes could cause a material change in our effective tax rate, which may negatively impact our business, results of operations and financial condition.
Presbia PLC and its Presbia Ireland, Limited subsidiary are incorporated in and resident for tax purposes in Ireland. Accordingly, they are subject to Irish corporation tax on their worldwide income and gains. The current rates of Irish corporation tax are 12.5% for certain trading income, 25% for all other income, and 33% for capital gains. It is anticipated that we will be subject to the lower rate of Irish corporation tax applicable to our trading income (currently 12.5%) on the basis that we will be carrying on a trade in Ireland for Irish tax purposes. However, we cannot guarantee that our activities in Ireland will be sufficient to qualify for trading status in respect of all or any portion of our income. There is no comprehensive definition of what constitutes “trading” for Irish tax purposes, and whether or not a company is carrying on a trade in Ireland for Irish tax purposes is determined on the facts of each individual case. Consequently, we cannot assure you that the Irish Revenue (Tax) authorities would accept our trading status for Irish tax purposes in respect of all or any portion of our income. If it is determined that we are not in fact carrying on a trade in Ireland for Irish tax purposes, our income in Ireland could be subject to a 25% tax rate, including future royalty income from the U.S. market.
Our status as a foreign corporation for U.S. federal income tax purposes could be affected by changes in applicable laws.
We believe that, under current law, we are treated as a foreign corporation for U.S. federal income tax purposes. However, changes to the inversion rules in Section 7874 of the Code or the U.S. Treasury Regulations promulgated thereunder or other U.S. Internal Revenue Service, or IRS, or U.S. Treasury Department guidance could adversely affect our status as a foreign corporation for U.S. federal income tax purposes, and any such changes could have prospective or retroactive application to us and/or our respective shareholders and affiliates. Most recently, the U.S. Treasury Department issued Notice 2014-52, which applies stricter “anti-inversion” rules to inversion transactions occurring on or after September 22, 2014. Although the Notice in its current form would not affect our status as a foreign corporation, the U.S. Congress may enact legislation in the future to change the inversion rules, possibly retroactively. In addition, recent legislative proposals have aimed to expand the scope of U.S. corporate tax residence, and such legislation, if passed, could have a material and adverse effect on us.
45
We depend on key employees, the loss of which could substantially damage our business and our ability to compete.
We depend on the continued service of our chief technology officer, Vladimir Feingold, and other key employees. The loss of a key employee could hurt our business substantially. Mr. Feingold is an employee at will and is not subject to a non-compete obligation. We could be particularly damaged if he or any other key employee or employees went to work for our competitors. Our future success depends on our ability to identify, attract, train, motivate and retain other highly skilled personnel. Failure to do so may adversely affect our results. Other than with respect to Mr. Feingold, we do not maintain insurance policies to cover the cost of replacing the services of any of our key employees who may unexpectedly die or become disabled.
We may seek to grow our business through acquisitions of or investments in new or complementary businesses, products or technologies, and the failure to manage any acquisitions or investments, or the failure to integrate them with our existing business, could have a material adverse effect on us.
From time to time, we expect to consider opportunities to acquire or make investments in other technologies, products and businesses that may enhance our capabilities, complement our current products or expand the breadth of our markets or customer base. Potential and completed acquisitions and strategic investments involve numerous risks, including:
| • | problems assimilating the purchased technologies, products or business operations; |
| • | maintaining uniform standards, procedures, controls and policies; |
| • | unanticipated costs associated with acquisitions; |
| • | diversion of management’s attention from our core business; |
| • | adverse effects on existing business relationships with suppliers; |
| • | risks associated with entering new markets in which we have limited or no experience; |
| • | potential loss of key employees of acquired businesses; and |
| • | increased legal and accounting compliance costs. |
We have no current commitments or intentions with respect to any acquisition or investment. We do not know if we will be able to identify suitable acquisitions, complete any such acquisitions on favorable terms or at all, successfully integrate any acquired business, product or technology into our business or retain any key personnel, suppliers or distributors. Our ability to grow through acquisitions successfully depends upon our ability to identify, negotiate, complete and integrate suitable target businesses and to obtain any necessary financing. These efforts could be expensive and time-consuming, and may disrupt our ongoing business and prevent management from focusing on our operations. If we are unable to integrate any acquired businesses, products or technologies effectively, our business, results of operations and financial condition would be materially and adversely affected.
We may need to increase the size of our organization, and we may experience difficulties in managing growth.
As of December 31, 2014, we had 33 full-time employees. Whether or not we grow by acquisition or internal growth, we expect that it will be necessary to expand our managerial, operational, financial and other resources in order to manage our operations and clinical trials, continue our development activities and fully commercialize our products. Our systems currently in place may not be adequate to support this future growth. Our need to effectively execute our business strategy requires that we:
| • | manage our clinical trials effectively; |
| • | provide substantial support to ophthalmic centers at the time that we enter into contractual relationships with them and provide ongoing support even after the centers are fully trained; |
46
| • | manage our internal development efforts effectively; |
| • | continue to improve our operational, financial and management controls, reporting systems and procedures; and |
| • | identify, recruit, maintain, motivate and integrate additional employees. |
If we are unable to expand our managerial, operational, financial and other resources to the extent required to manage our development and commercialization activities, our business, results of operations and financial condition would be materially and adversely affected.
We may be subject to costly product liability claims related to our clinical trials and products and, if we are unable to obtain adequate insurance or are required to pay for liabilities resulting from a claim excluded from, or beyond the limits of, our insurance coverage, a material liability claim could adversely affect our financial condition.
We face the risk that the use of our products may result in adverse side effects to patients in our clinical trials. We face even greater risks in connection with the commercialization of our products, including our current sales outside of the United States. Although we maintain product liability insurance and request that laser centers and hospitals offering our products, and the physicians at such facilities, maintain product liability insurance, any such insurance coverage may be insufficient to reimburse us for any expenses or losses we may suffer, and we may be required to increase our product liability insurance coverage for trials that we initiate in the future. We do not know whether we will be able to continue to obtain product liability coverage and obtain expanded coverage if we require it, on acceptable terms, or at all. We may not have sufficient resources to pay for any liabilities resulting from a claim excluded from, or beyond the limits of, our insurance coverage. To the extent that we provide indemnities in favor of third parties under our agreements with them, there is also a risk that these third parties could incur liability and bring a claim under such indemnities. An individual may bring a product liability claim against us alleging that one of our products caused an injury or is found to be unsuitable for consumer use. Any product liability claim brought against us, with or without merit, could result in:
| • | withdrawal of clinical trial volunteers, investigators, patients or trial sites; |
| • | difficulties in commercializing our products; |
| • | decreased demand for our products; |
| • | regulatory investigations that could require costly recalls or product modifications; |
| • | loss of revenues; |
| • | substantial costs of litigation; |
| • | liabilities that substantially exceed our product liability insurance, which we would then be required to pay ourselves; |
| • | an increase in our product liability insurance rates or the inability to maintain insurance coverage in the future on acceptable terms, if at all; |
| • | the diversion of management’s attention from our business; and |
| • | damage to our reputation and the reputation of our products. |
Product liability claims may subject us to the foregoing and other risks, which could have a material adverse effect on our business, results of operations and financial condition.
47
If we use biological and hazardous materials in a manner that causes injury or violates applicable laws or regulations, we could be liable for damages.
Our activities currently require the controlled use of potentially harmful biological materials and hazardous materials and chemicals. We cannot eliminate the risk of accidental contamination or injury to employees or third parties from the use, storage, handling or disposal of these materials. In the event of contamination or injury, we could be held liable for any resulting damages, and any liability could exceed our resources or any applicable insurance coverage we may have. Additionally, we are subject to, on an ongoing basis, a variety of federal, state and non-U.S. environmental and pollution control laws and regulations governing the use, storage, handling and disposal of these materials and specified waste products. The cost of compliance with these laws and regulations may become increasingly significant and could have a material adverse effect on our business, results of operations and financial condition. In the event of an accident or if we otherwise fail to comply with applicable regulations, we could lose our permits or approvals or be held liable for damages or penalized with fines.
Our employees may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements.
We are exposed to the risk of employee fraud or other misconduct. Misconduct by employees could include intentional failures to comply with FDA or other governmental regulations, to provide accurate information to the FDA or other governmental authorities, to comply with manufacturing standards we have established or to report financial information or data accurately. Employee misconduct could involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. It is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of significant fines or other sanctions.
Our sales volumes and our operating results may fluctuate from quarter to quarter, which may make our performance more difficult to understand and may make our future performance more difficult to predict.
We may experience meaningful variability in our sales and operating expenses among quarters, as well as within each quarter, as a result of a number of factors, including, among other things:
| • | the timing of or failure to obtain regulatory approvals or clearances for products; |
| • | the number of products sold in the quarter; |
| • | the demand for, and pricing of, our products and the products of our competitors; |
| • | costs, benefits and timing of new product introductions; |
| • | increased competition; |
| • | the availability and cost of components and materials; |
| • | the number of selling days in the quarter; and |
| • | impairment and other special charges. |
Such quarterly fluctuations may make it difficult to understand our performance and predict our future performance.
48
If we experience material weaknesses in the future or otherwise fail to maintain an effective system of internal controls in the future, we may not be able to accurately report our financial condition or results of operations which may adversely affect investor confidence in us and, as a result, the value of our ordinary shares.
As a result of becoming a public company, we will be required, under Section 404 of the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, to establish adequate internal control over financial reporting and disclosure controls and procedures and to furnish a report by management on, among other things, the effectiveness of our internal control over financial reporting beginning with our Annual Report on Form 10-K for the year ending December 31, 2016. This assessment must include disclosure of any material weaknesses identified by our management in our internal control over financial reporting. A material weakness is a deficiency or combination of deficiencies in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of a company’s annual and interim financial statements will not be detected or prevented on a timely basis.
We are analyzing the computer systems processes and related documentation necessary to perform the evaluations needed to comply with Section 404. We may not be able to complete our evaluation, testing and any required remediation in a timely fashion. During the evaluation and testing process, if we identify one or more material weaknesses in our internal control over financial reporting, we will be unable to assert that our internal controls are effective. The effectiveness of our controls and procedures may be limited by a variety of factors, including:
| • | faulty human judgment and simple errors, omissions or mistakes; |
| • | fraudulent action of an individual or collusion of two or more people; |
| • | inappropriate management override of procedures; and |
| • | the possibility that any enhancements to controls and procedures may still not be adequate to assure timely and accurate financial control. |
If we are unable to conclude that our internal control over financial reporting is effective, we could lose investor confidence in the accuracy and completeness of our financial reports, which would likely cause the price of our ordinary shares to decline.
When we cease to be an “emerging growth company” under the federal securities laws, our auditors will be required to express an opinion on the effectiveness of our internal controls. If we are unable to confirm that our internal control over financial reporting is effective, or if our auditors are unable to express an opinion on the effectiveness of our internal controls, we could lose investor confidence in the accuracy and completeness of our financial reports, which could cause the price of our ordinary shares to decline.
We expect to incur significant costs as a result of being a public company, which may adversely affect our operating results and financial condition.
We expect to incur costs associated with corporate governance requirements, including requirements under the Sarbanes-Oxley Act, as well as rules implemented by the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010, or the Dodd-Frank Act, the SEC, and the NASDAQ listing rules. These rules and regulations are expected to increase our accounting, legal and financial compliance costs and make some activities more time-consuming and costly. In addition, we will incur additional costs associated with our public company reporting requirements and we expect those costs to increase in the future. We also expect these rules and regulations to make it more expensive for us to maintain directors’ and officers’ liability insurance and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. As a result, it may be more difficult for us to attract and retain qualified persons to serve on our board of directors, or our Board, committees of our Board, or as executive officers. We cannot predict or estimate the amount of additional costs we may incur or the timing of such costs.
49
If we experience significant disruptions in our information technology systems, our business may be adversely affected.
We depend on our information technology systems for the efficient functioning of our business, including accounting, data storage, compliance, purchasing and inventory management. Although we attempt to mitigate interruptions, we may experience difficulties in implementing certain upgrades, which would impact our business operations, or experience difficulties in operating our business during the upgrade, either of which could disrupt our operations, including our ability to timely ship and track product orders, project inventory requirements, manage our supply chain and otherwise adequately service our customers. In the event we experience significant disruptions as a result of the implementation of our information technology systems, we may not be able to repair our systems in an efficient and timely manner. Accordingly, such events may disrupt or reduce the efficiency of our entire operation and have a material adverse effect on our results of operations and cash flows.
Fluctuations in insurance cost and availability could adversely affect our profitability or our risk management profile.
We hold a number of insurance policies, including product liability insurance, directors’ and officers’ liability insurance, general liability insurance, property insurance and workers’ compensation insurance. If the costs of maintaining adequate insurance coverage increase significantly in the future, our operating results could be materially and adversely affected. Likewise, if any of our current providers should no longer be able to provide coverage to us, we may not be able to find another provider that provides comparable coverage for comparable costs, which could impact our coverage and materially and adversely affect our operating results.
Risks Related to our Regulatory Requirements
Our products are subject to extensive governmental regulation both in the United States and in other countries, and our failure to comply with applicable requirements could cause our business to suffer.
Our products are subject to extensive regulation by the FDA and various other federal, state and non-U.S. governmental authorities, such as the competent authorities of the countries of the EEA and other countries in which we currently have marketing approval and/or conduct operations. Government regulation of medical devices is meant to assure their safety and effectiveness, and includes regulation of, among other things:
| • | design, development and manufacturing; |
| • | testing, labeling, content and language of instructions for use and storage; |
| • | clinical trials; |
| • | product safety; |
| • | marketing, sales and distribution; |
| • | regulatory approvals and clearances, including premarket approval and clearance; |
| • | conformity assessment procedures; |
| • | product traceability and record keeping procedures; |
| • | advertising and promotion; |
| • | product complaints, complaint reporting, recalls and field safety corrective actions; |
| • | post-market surveillance, including reporting of deaths or serious injuries and malfunctions that, if they were to recur, could lead to death or serious injury; |
| • | post-market studies; and |
| • | product import and export. |
50
Failure to comply with applicable laws and regulations could jeopardize our ability to sell our products and result in enforcement actions such as:
| • | delays in the introduction of products into the market; |
| • | warning letters; |
| • | injunctions; |
| • | fines and other civil penalties; |
| • | termination of distribution; |
| • | recalls or seizures of products; |
| • | total or partial suspension of production; |
| • | refusal of the FDA or other regulators to grant necessary approvals or clearances; |
| • | withdrawals or suspensions of then current approvals or clearances, resulting in prohibitions on sales of our products; |
| • | withdrawal of the CE Certificates of Conformity granted by the notified body or delay in obtaining these certificates; and/or |
| • | in the most serious cases, criminal penalties. |
Any of these sanctions could result in higher than anticipated costs or lower than anticipated sales and have a material adverse effect on our reputation, business, results of operations and financial condition.
We are subject to complex regulations which have tended to become more stringent over time. Regulatory changes could result in restrictions on our ability to carry on or expand our operations, higher than anticipated costs or lower than anticipated sales.
Our current products are Class III devices under the U.S. Food, Drug and Cosmetic Act, or FDCA, and thus subject to more stringent regulatory controls than other medical devices. Before we can market or sell our microlens and our microlens inserter in the United States, we must obtain approval of a PMA application from the FDA. Our Investigational Device Exemption, or IDE, enables us to use our microlens and our microlens inserter in clinical studies in order to begin to collect safety and effectiveness data for the PMA application. In the PMA approval process, the FDA must determine that a proposed device is safe and effective for its intended use based, in part, on extensive data, including, but not limited to, technical, pre-clinical, clinical trial, manufacturing and labeling data. The PMA process is typically required for devices that are deemed to pose the greatest risk, such as implantable devices, as well as life-sustaining and life-supporting devices. The process of obtaining a PMA generally takes from one to four years, or even longer, from the time the application is submitted to the FDA until an approval is obtained. We do not expect to receive our PMA any earlier than in the fourth quarter of 2017.
Future products that we may develop, as well as material modifications to our existing products, will require a new PMA. If the FDA requires us to go through a lengthier, more rigorous examination for future products or modifications to existing products than we had expected, our product introductions or modifications could be delayed or canceled, which could cause our sales to decline. Outcomes under the PMA process are difficult to predict, as are the time and expense associated with that process. Further, even if any of our future products do not require a PMA, we cannot assure you that we will be able to obtain clearances under Section 510(k) of the FDCA, or 510(k) clearances, which is a less onerous approval process than the PMA process, with respect to those products.
The FDA can delay, limit or deny approval or clearance of a device for many reasons, including:
| • | our inability to demonstrate to the FDA’s satisfaction that our products are safe and effective for their intended uses; |
51
| • | the data from our pre-clinical studies and clinical trials may be insufficient to support clearance or approval, where required; and |
| • | the manufacturing process or facilities we use may not meet applicable requirements. |
Significant delays in receiving approval or clearance, or the failure to receive approval or clearance for our products, would adversely affect our ability to generate revenues and negatively impact our business, results of operations and financial condition.
In addition, the FDA may change its approval and clearance policies, adopt additional regulations or revise existing regulations, or take other actions that may prevent or delay approval or clearance of our products under development or impact our ability to modify any products that may be approved or cleared. For example, in 2011, the FDA announced a Plan of Action to modernize and improve the FDA’s premarket review of medical devices, and has implemented, and continues to implement, reforms intended to streamline the premarket review process. In addition, as part of the U.S. Food and Drug Administration Safety and Innovation Act of 2012, or FDASIA, the U.S. Congress enacted several reforms entitled “Medical Device Regulatory Improvements” and additional miscellaneous provisions which will further affect medical device regulation both pre- and post-approval. Any change in the laws or regulations that govern the approval and clearance processes relating to our current and future products could make it more difficult and costly to obtain approval or clearance for new products, or to produce, market and distribute existing products.
Any delay in, or failure to receive or maintain, approval or clearance for our products under development could prevent us from generating revenue in the United States from these products or achieving profitability. Additionally, the FDA and other regulatory authorities have broad enforcement powers. Regulatory enforcement or inquiries, or other increased scrutiny on us, could dissuade some ophthalmic surgeons from using our products and adversely affect our reputation and the perceived safety and efficacy of our products.
In addition, even if we obtain the proper regulatory approval or clearance to market a product, the FDA has the power to require us to conduct post-market surveillance systems, which are designed to identify adverse events, device malfunctions or complaints from patients implanted with the device during a specified period after the commencement of commercial use in the United States. The FDA may also require us to conduct post-marketing studies to further monitor the safety and/or efficacy of our products. Failure to conduct required surveillance systems or studies in a timely manner could result in the revocation of the PMA approval or 510(k) clearance for the product that is subject to such a requirement and could also result in the recall or withdrawal of the product, which would prevent us from generating sales from that product in the United States.
In order to be placed on the market within the EEA, medical devices must meet the essential requirements set out in the relevant medical device legislation. The principal legislation regulating general medical devices in the EEA is Directive 93/42/EEC, referred to herein as the EU Medical Devices Directive. In the case of low risk (Class I) medical devices, such as our microlens inserter, the manufacturer may self-certify conformity with the EU Medical Devices Directive by issuing a declaration of conformity. In the case of medium to high risk (Class IIa, IIb and III) medical devices, including our microlens which is a Class IIb medical device, the certificate of conformity issues from a notified body. Where a medical device meets the essential requirements set out in the EU Medical Devices Directive and complies with the appropriate conformity assessment procedure, based on the classification of the medical device, a declaration or certificate of conformity will issue and a CE Mark may then be affixed to the product. Once a CE Mark has been affixed to the medical device, it may then be placed on the market in any country within the EEA and Switzerland (subject to certain localized registration and language requirements).
In February 2010, we received a certificate of conformity from our notified body for our microlens allowing the CE Mark to be affixed to our microlens. In May 2013, we issued a declaration of conformity for our microlens inserter allowing the CE Mark to be affixed to our microlens inserter. We have also obtained an ISO 13485
52
quality system certification, which confirms that our medical device manufacturing quality management system is compliant with globally recognized standards set forth by the International Organization for Standardization. We are required to keep up-to-date and remain compliant with the most recently issued standards. In order to maintain our certificate of conformity and CE Mark, we must continue to comply with the EU Medical Devices Directive and pass annual facilities audit inspections by an inspection agency of the EEA to ISO 13485 standards. In addition, a notified body or other competent authority in an EEA country may perform post-marketing audits on our products and premises from time to time. Failure to comply with such requests in a timely manner, and any adverse findings in any such audit, could result in the withdrawal of our certificate of conformity and our CE Mark, and the recall or withdrawal of our products from the EEA market. Each certificate of conformity may be valid for a maximum of five years but would typically be valid for three years. Our existing certificate of conformity for our microlens is valid until November 2019. At the end of each period of validity, we are required to apply to the notified body for a renewal of our certificate of conformity. There may be delays in the renewal of our certificate of conformity and the notified body may require modifications to our products or to the related technical files before it agrees to issue a new certificate of conformity.
On September 26, 2012, the European Commission adopted a package of legislative proposals designed to replace the existing regulatory framework for medical devices in the EEA. The European Commission’s proposals may undergo significant amendments as they are reviewed by the European Council and European Parliament as part of the EEA legislative process. If and when adopted, the proposed new legislation may prevent or delay the EEA approval or clearance of any future products we may develop or impact our ability to modify currently EEA approved or cleared products on a timely basis.
The United States, in which we are seeking marketing approval, those countries which recognize our CE mark, and those other countries in which we have marketing approval, collectively, only represent a portion of the worldwide presbyopic population. To market and sell our products in other countries, including those countries that may represent a substantial portion of the worldwide presbyopic population, we must seek and obtain regulatory approvals, certifications and/or registrations and comply with the laws and regulations of those countries. These laws and regulations, including the requirements for approvals, certifications and/or registrations and the time required for regulatory review, vary from country to country. Obtaining and maintaining regulatory approvals, certifications and/or registrations are expensive, and we cannot be certain that we will receive regulatory approvals, certifications and/or registrations in any country for which we have yet to receive such approvals, certifications and/or registrations or that we will be able to maintain any regulatory approvals, certifications and/or registrations that we currently possess. If we fail to obtain or maintain regulatory approvals, certifications and/or registrations in any country in which we plan to market our products, our ability to generate revenue will be harmed.
We are currently in the process of seeking marketing authorization for our microlens in China and India.
Before a medical device can be marketed in China, the device must be registered with the China Food and Drug Administration, or CFDA. Our microlens is classified as a Class III device by the CFDA. We will be required to submit evidence of home country product approval and manufacturing compliance as a prerequisite to registration in China. A class III device must be submitted to the CFDA for testing, and the CFDA may, in certain circumstances, require that additional clinical trials be conducted in China. A final application with supporting data must be submitted to the CFDA, and, following the CFDA’s review of that application, the CFDA may issue an Import Medical Device Registration Certificate, or an IMDRC. Upon receipt of an IMDRC, a CFDA number can be placed on the device label, packaging material and user manual, and the device can be commercially distributed in China. The IMDRC is valid for five years. Even after receipt of an IMDRC, the applicable medical device is subject to ongoing regulation by the CFDA with respect to design, development, manufacturing, labeling, storage, record keeping, adverse event reporting, sale, promotion, distribution and shipping. We are in the process of preparing the supporting documentation that the CFDA will require as well as preparing samples for testing by the CFDA.
Before a medical device can be marketed in India, the device must be registered with the Drug Controller General of India, or the DCGI, or confirmation must be received from the DCGI that registration is not
53
required. We are in the process of seeking confirmation from the DCGI that registration of our microlens is not required. If registration is required, we will be required to submit the product registration approvals in any of the Global Harmonization Task Force countries (the United States, Europe, Canada, Australia and Japan) as a prerequisite to registration in India. In addition, if registration is required, we will be required to comply with applicable DCGI regulations pertaining to the design, development, manufacturing, labeling, storage, record keeping, adverse event reporting, sale, promotion, distribution and shipping of our microlens. If registration is not required, we would still be required to comply with the DCGI’s product labeling requirements.
Failure to comply with applicable laws and regulations could jeopardize our ability to sell our products and result in a variety of enforcement actions, all of which would negatively impact our business, results of operations and financial condition.
Modifications to our products may require new premarket approvals or may require us to cease marketing or recall the modified products until approvals are obtained.
Any modification to a PMA-approved device that could significantly affect its safety or effectiveness, including significant design and manufacturing changes, or that would constitute a major change in its intended use, design or manufacture, may require approval of a new PMA. The FDA requires every manufacturer to make this determination in the first instance, but the FDA may review any manufacturer’s decision. The FDA may not agree with our decisions regarding whether new approvals are necessary. If the FDA disagrees with any determination that we may make in the future and requires us to seek new PMA approvals for modifications to any previously approved or cleared products for which we have concluded that new approvals are unnecessary, we may be required to cease marketing or distribution of our products or to recall the modified product until we obtain approval, and we may be subject to significant regulatory fines or penalties. We have commenced the development of a disposable microlens inserter and a pre-loaded disposable microlens inserter which may require an additional PMA.
In the EEA, we are required to inform the notified body that carried out the conformity assessment of the medical devices we market or sell in the EEA of any planned changes to our quality system or changes to our devices which could affect compliance with the essential requirements set forth in the EU Medical Devices Directive or the devices’ intended purpose. The notified body will then assess the changes and verify whether they affect the products’ conformity with the essential requirements set forth in the EU Medical Devices Directive or the conditions for the use of the device. If the assessment is favorable, the notified body will issue a new CE Certificate of Conformity or an addendum to the existing CE Certificate of Conformity attesting compliance with the essential requirements set forth in the EU Medical Devices Directive. If it is not, we may not be able to continue to market and sell the product in the EEA.
We may fail to obtain or maintain regulatory approvals to market our products in countries outside of the United States.
We market our products in certain countries outside of the United States and intend to expand our non-U.S. marketing. Each jurisdiction that we target for commercialization of our products requires regulatory approvals and compliance with numerous and sometimes varying regulatory requirements. In addition to the countries in which we currently have marketing approval, we are seeking regulatory approval or clearance to market our products in China, Taiwan, Peru, Saudi Arabia, India and Turkey. The approval procedures vary among countries and may involve requirements for additional testing, and the time required to obtain approval may differ from country to country and from that required to obtain clearance or approval in the United States and the necessary CE Certificates of Conformity in the EEA countries.
Approval or clearance in the United States and/or a CE Certificate of Conformity in the EEA countries does not ensure approval or certification by regulatory authorities in other countries or jurisdictions, and approval or certification by one regulatory authority does not ensure approval or certification by regulatory authorities in
54
other countries or by the FDA. Any non-U.S. regulatory approval or certification process may include similar risks associated with obtaining FDA clearance or approval. In addition, some countries only approve or certify a product for a certain period of time, in which case we will be required to re-approve or re-certify our products in a timely manner prior to the expiration of our prior approval or certification. We may not obtain regulatory approvals that we seek on a timely basis, if at all. We may not be able to file for regulatory approvals or certifications and may not receive or maintain necessary approvals to commercialize our products in any market. If we fail to receive or maintain necessary approvals or certifications to commercialize our products in any non-U.S. jurisdiction on a timely basis, or at all, or if we fail to have our products re-approved or re-certified, our business, results of operations and financial condition could be materially and adversely affected.
If we or our suppliers fail to comply with ongoing EEA and FDA or other regulatory authority requirements, or if we experience unanticipated problems with our products, these products could be subject to restrictions or withdrawal from the market.
Any product for which we obtain approval or clearance, and the manufacturing processes, reporting requirements, post-approval clinical data and promotional activities for such product, will be subject to continued regulatory review, oversight and periodic inspections by the FDA and other U.S. and non-U.S. regulatory authorities. In particular, we and our third-party suppliers will be required to comply with the FDA’s Quality System Regulation, or QSR. In EEA countries, compliance with harmonized standards is also recommended as this is often interpreted as a presumption of conformity with the relevant essential requirements set forth in Annex I to the EU Medical Devices Directive. These FDA regulations and EU standards cover the methods and documentation of the design, testing, production, control, quality assurance, labeling, packaging, sterilization, storage and shipping of our products. Compliance with applicable regulatory requirements is subject to continual review and is monitored rigorously through periodic inspections by the FDA. Compliance with harmonized standards in the EEA is also subject to regular review through the conduct of inspections by notified bodies or other certification bodies. If we, or our suppliers, fail to adhere to QSR requirements in the United States or other harmonized standards in the EEA, this could delay production of our products and lead to fines, difficulties in obtaining regulatory clearances and CE Certificates of Conformity, recalls, enforcement actions, including injunctive relief or consent decrees, or other consequences, which could, in turn, have a material adverse effect on our business, financial condition or results of operations.
In addition, the FDA audits compliance with the QSR through periodic announced and unannounced inspections of manufacturing and other facilities. The failure by our company or any of our suppliers to comply with applicable statutes and regulations administered by the FDA, or the failure to timely and adequately respond to any adverse inspectional observations or product safety issues, could result in any of the following enforcement actions:
| • | untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties; |
| • | unanticipated expenditures to address or defend such actions; |
| • | customer notifications or repair, replacement, refunds, recalls, detention or seizure of our products; |
| • | operating restrictions or partial suspension or total shutdown of production; |
| • | refusal of or delaying our requests for PMA approval of new products or modified products; |
| • | withdrawing PMA approvals that have already been granted; |
| • | refusal to grant export approval for our products; and |
| • | criminal prosecution. |
Any of these sanctions could have a material adverse effect on our reputation, business, results of operations and financial condition. Furthermore, our key component suppliers may not currently be or may not continue to be in compliance with all applicable regulatory requirements, which could result in our failure to produce our products on a timely basis and in the required quantities, if at all.
55
Outside the EEA and the United States, our products and operations are required to comply with standards set by the applicable regulatory authorities in each jurisdiction that we target for commercialization of our products, and those standards, types of evaluation and scope of review differ among such regulatory authorities. We intend to comply with the standards enforced by such regulatory authorities as needed to commercialize our products. If we fail to comply with any of these standards adequately, a regulatory authority may take adverse actions similar to those within the power of a notified body or competent authority or the FDA. Any such action may harm our reputation and business, and could have a material adverse effect on our business, results of operations and financial condition.
If our products, or the malfunction of our products, cause or contribute to a serious injury or a death, we will be subject to medical device reporting regulations, which can result in voluntary corrective actions or agency enforcement actions.
Under the FDA medical device reporting regulations, medical device manufacturers are required to report to the FDA information that a device has or may have caused or contributed to a serious injury or death or has malfunctioned in a way that would likely cause or contribute to serious injury or death if the malfunction of the device or a similar device were to recur. All manufacturers placing medical devices in the market in the EEA are legally bound to report any serious or potentially serious incidents involving devices they produce or sell to the competent authority in whose jurisdiction the incident occurred. Were this to happen to us, the relevant competent authority would file an initial report, and there would then be a further inspection or assessment if there were particular issues. This would be carried out either by the competent authority or it could require that the notified body carry out the inspection or assessment.
Any such adverse event involving our products could result in future voluntary corrective actions, such as recalls or customer notifications, or agency action, such as inspection or enforcement action. Any corrective action, whether voluntary or involuntary, will require the dedication of our time and capital, distract management from operating our business and may harm our business, results of operations and financial condition.
In the EEA, we must comply with the EU Medical Device Vigilance System. Under this system, incidents must be reported to the relevant authorities of the EEA countries, and manufacturers are required to take Field Safety Corrective Actions, or FSCAs, to reduce a risk of death or serious deterioration in the state of health associated with the use of a medical device that is already placed on the market. An incident is defined as any malfunction or deterioration in the characteristics and/or performance of a device, as well as any inadequacy in the labeling or the instructions for use which, directly or indirectly, might lead to or might have led to the death of a patient or user or of other persons or to a serious deterioration in their state of health. An FSCA may include the recall, modification, exchange, destruction or retrofitting of the device. FSCAs must be communicated by the manufacturer or its European Authorized Representative to its customers and/or to the end users of the device through Field Safety Notices.
Our products may in the future be subject to product recalls. A recall of our products, either voluntarily or at the direction of governmental authorities, or the discovery of serious safety issues with our products, could have a significant adverse impact on us.
Governmental authorities, including the FDA, have the authority to require the recall of commercialized products in the event of material deficiencies or defects in design or manufacture. In the case of the FDA, the authority to require a recall must be based on an FDA finding that there is reasonable probability that the device would cause serious injury or death. In addition, non-U.S. governmental authorities have the authority to require the recall of our products in the event of material deficiencies or defects in design or manufacture. Manufacturers may, under their own initiative, recall a product if any material deficiency in a device is found. A government-mandated or voluntary recall by us could occur as a result of an unacceptable risk to health, product failures, malfunctions, manufacturing errors, design or labeling defects or other deficiencies and issues. Recalls of any of our products would divert managerial and financial resources and would have an adverse effect on our reputation, results of
56
operations and financial condition, which could impair our ability to produce our products in a cost-effective and timely manner in order to meet our customers’ demands. We may also be subject to liability claims, be required to bear other costs, or take other actions that may have a negative impact on our future sales and our ability to reach profitability.
We rely on third parties to conduct our clinical trials and assist us with pre-clinical development. If these third parties do not perform as contractually required or expected, we may not be able to obtain regulatory clearance or approval for, or commercialize, our products.
We rely on third parties, such as contract research organizations, medical institutions, clinical investigators and contract laboratories, to conduct our clinical trials and to assist in the preparation of our PMA submissions. If these third parties do not successfully carry out their contractual duties or regulatory obligations or meet expected deadlines, if these third parties need to be replaced, or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements or for other reasons, our pre-clinical development activities or clinical trials may be extended, delayed, suspended or terminated, and we may not be able to obtain regulatory clearance or approval for, or successfully commercialize, our products on a timely basis, if at all, and our business, operating results and prospects may be materially and adversely affected. Furthermore, our third-party clinical trial investigators may be delayed in conducting our clinical trials for reasons outside of their control.
The results of our clinical trials may not support our product claims or may result in the discovery of adverse side effects.
Our ongoing research and development, pre-clinical testing, clinical trial and post-market evaluation activities will be subject to extensive regulation and review by numerous governmental authorities, both in and outside of the United States. We are currently conducting a pivotal clinical trial under our IDE for our microlens and microlens inserter, to gather information about these products’ safety, efficacy or optimal use. In the future we may conduct clinical trials to support approval of new products. All such clinical studies must be conducted in compliance with applicable regulations or the applicable regulatory authorities may take enforcement action. The data collected from these clinical studies may ultimately be used to support market clearance for these products. Even if our clinical trials are completed as planned, we cannot be certain that their results will support our product claims or that the applicable regulatory authorities and notified bodies will agree with our conclusions regarding them. Success in pre-clinical studies and early clinical trials does not ensure that later clinical trials will be successful, and we cannot be sure that later trials will replicate the results of prior trials and pre-clinical studies. The clinical trial process may fail to demonstrate that our products are safe and effective for the proposed indicated uses, which could cause us to abandon a product and may delay development of others. Any delay or termination of our clinical trials will delay the filing of our product submissions and, ultimately, our ability to commercialize our products and generate revenues. It is also possible that patients enrolled in clinical trials will experience adverse side effects that are not currently part of the product’s profile.
We may be subject to enforcement action if we engage in improper marketing or promotion of our products.
The marketing and promotion of our products is subject to EEA Member States laws implementing the EU Medical Devices Directive, Directive 2006/114/EC concerning misleading and comparative advertising, and Directive 2005/29/EC on unfair commercial practices, as well as other EEA Member State legislation governing the advertising and promotion of medical devices. In addition, we are subject to EU and national Codes of Conduct. These laws and Codes of Conduct may limit or restrict the advertising and promotion of our products to the general public and may impose limitations on our promotional activities with healthcare professionals.
Further, once our products are approved, our promotional materials and training methods must comply with FDA and other applicable laws and regulations, including the prohibition of the promotion of unapproved, or off-label, use. If the FDA determines that our promotional materials or training constitutes promotion of an off-label use, it
57
could request that we modify our training or promotional materials or subject us to regulatory or enforcement actions, including the issuance of an untitled letter, a warning letter, injunction, seizure, civil fine or criminal penalties. It is also possible that other federal, state or non-U.S. enforcement authorities might take action if they consider our promotional or training materials to constitute promotion of an off-label use. In that event, our reputation could be damaged and adoption of the products could be impaired. In addition, the off-label use of our products may increase the risk of product liability claims, which are expensive to defend and could divert our management’s attention, result in substantial damage awards against us, and harm our reputation.
Regulatory healthcare reforms may make it more difficult and costly for us to obtain regulatory approval or clearance of our products and to produce, market and distribute our products after approval or clearance is obtained.
FDA regulations and guidance are often revised or reinterpreted by the FDA in ways that may significantly affect our business and our products. Any new regulations or revisions or reinterpretations of existing regulations may impose additional costs or lengthen review times of our products. Delays in receipt of, or failure to receive, regulatory approvals or clearances for our products would have a material adverse effect on our business, results of operations and financial condition.
Risks Related to Our Intellectual Property
We may become subject to third parties’ claims alleging infringement of their patents and proprietary rights or seeking to invalidate our patents or proprietary rights, or we may need to become involved in lawsuits to protect or enforce our patent portfolio, which could be costly, time consuming, delay or prevent the development and commercialization of our products, or put our patent portfolio and other proprietary rights at risk.
Litigation relating to infringement or misappropriation of patent and other intellectual property rights in the medical device industry is common. For example, we were previously a party to legal proceedings relating to the ownership of certain assets, including intellectual property. See “—Risks Related to Our Business—We were previously subject to certain legal proceedings relating to the ownership of certain assets, including intellectual property. As demonstrated by such proceedings, future claims regarding intellectual property may be costly and time consuming to defend and future claims may delay or prevent the development and commercialization of our products or place our patent portfolio and other proprietary rights at risk.” We may be subject to third-party claims in the future that would cause us to incur substantial expenses and which, if successful, could cause us to pay substantial damages. These damages potentially include increased damages and attorneys’ fees if we are found to have infringed such rights willfully. Further, if a patent infringement suit is brought against us, our research, development, manufacturing or sales activities relating to the product that is the subject of the suit may be delayed or terminated. As a result of patent infringement claims, or in order to avoid potential infringement claims, we may choose to seek, or be required to seek, a license from the claimant, which would be likely to include a requirement to pay license fees or royalties or both. These licenses may not be available on acceptable terms, or at all. Even if a license can be obtained on acceptable terms, the rights may be nonexclusive, which would give our competitors access to the same intellectual property rights. If we are unable to enter into a license on acceptable terms, we could be prevented from commercializing one or more of our products, or forced to modify such products, or to cease some aspect of our business operations, which could harm our business significantly.
U.S. and non-U.S. issued patents and pending patent applications controlled by third parties may relate to areas in which we are developing products. In such an instance, because all issued patents are entitled to a presumption of validity in many countries, including the United States and many European countries, issued patents held by others that claim our products or technology may limit our freedom to operate unless and until those patents expire or are declared invalid or unenforceable in a court of applicable jurisdiction, if we do not obtain a license or other right to practice the claimed inventions. Pending patent applications controlled by third parties may result in additional issued patents claiming our products and technology. In addition, the publication of patent
58
applications occurs with a certain delay after the date of filing, so we may not be aware of all relevant patent applications of third parties at a given point in time. Further, publication of discoveries in the scientific or patent literature often lags behind actual discoveries, so we may not be able to determine whether inventions claimed in patent applications of third parties have been made before or after the date on which inventions claimed in our patent applications and patents have been made. If third parties prepare and file patent applications in the United States that also claim technology or therapeutics claimed by our patent applications or patents, we may have to participate in interference proceedings in the U.S. Patent and Trademark Office, or USPTO, to determine the priority of invention. An unfavorable outcome could require us to attempt to license rights from the prevailing party, or to cease using the related technology or developing or commercializing the related product candidate. We may also become involved in opposition proceedings in the European Patent Office regarding our intellectual property rights with respect to our products and technology.
Competitors may infringe our patent rights, or misappropriate or violate our other intellectual property rights. To counter infringement or unauthorized use, we may find it necessary to file infringement or other claims to protect our intellectual property rights. In addition, in any infringement proceeding brought by us against a third party to enforce our rights, a court may decide that a patent of ours is invalid or unenforceable, or may refuse to stop the other party from using the technology at issue on the basis that our patent does not cover the technology in question. An adverse result in any such litigation proceeding could put our patent protections at risk of being invalidated or interpreted narrowly, which could open us up to additional competition and have a material adverse effect on our business.
The cost to us of any patent litigation or other proceedings, such as interference proceedings, which are meant to determine who first invented any of the claims covered by the patent, even if resolved in our favor, could be substantial. Such litigation or proceedings could substantially increase our operating losses and reduce our resources available for development activities. We may not have sufficient financial or other resources to adequately conduct such litigation or proceedings. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than us because of their substantially greater financial resources. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments, and, if securities analysts or investors perceive these results to be negative, there could be a substantial adverse effect on the price of our ordinary shares. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace. Patent litigation and other proceedings may also require significant time and attention of management and technical staff, which may materially and adversely impact our financial position and results of operations. Furthermore, because of the substantial amount of discovery required in connection with most intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation.
Our proprietary rights may not adequately protect our technologies and product candidates. If we are unable to protect our product candidates and our intellectual property rights, our position in the market may be materially and adversely affected.
Our commercial success may depend on our ability to obtain patents and maintain adequate protection for our technologies, intellectual property and product candidates in the United States and other countries. Our patent portfolio consists of certain U.S. patents, patents issued in other jurisdictions and patent applications in the United States and other jurisdictions relating to our technologies. There is no guarantee that any of our patent applications will result in issued patents, or that any patents, if issued, will include claims that are sufficiently broad to cover our existing products or products in development, or to provide meaningful protection from our competitors. We will be able to protect our proprietary rights from unauthorized use by third parties only to the extent that our proprietary technologies and future products are covered by valid and enforceable patents or are effectively maintained as trade secrets within our organization. If third parties disclose or misappropriate our proprietary rights, it may materially and adversely impact our position in the market.
59
We have applied for patents covering both our technologies and the products we are developing. We may fail to apply for patents on important technologies or products in development in a timely fashion, or at all. Our existing patents and any future patents we obtain may not be sufficiently broad to prevent others from using our technologies or from developing competing products and technologies. Moreover, the patent positions of many medical device companies are highly uncertain and involve complex legal and factual questions for which important legal principles remain unresolved. As a result, the validity and enforceability of our patent portfolio cannot be predicted with certainty. In addition, we cannot guarantee you that:
| • | we were the first to make the inventions covered by our issued patents and our pending patent applications; |
| • | we were the first to file patent applications for these inventions; |
| • | others will not independently develop similar or alternative technologies or duplicate any of our technologies by inventing around our claims; |
| • | a third party will not challenge our proprietary rights, and, if challenged, that a court will hold that our existing or future patents are valid and enforceable; |
| • | any patents issued to us will cover our products as ultimately developed, or provide us with any competitive advantages; |
| • | we will develop additional proprietary technologies that are patentable; or |
| • | the patents of others will not have a material adverse effect on our business. |
In addition, there are numerous recent changes to the patent laws and proposed changes to the rules of the USPTO which may have a significant impact on our ability to protect our technology and enforce our intellectual property rights. For example, on September 16, 2011, United States President Obama signed the America Invents Act which codifies several significant changes to the U.S. patent laws, including, among other things, changing from a “first to invent’ to a “first inventor to file” system, limiting where a patentee may file a patent suit, requiring the apportionment of patent damages, eventually eliminating interference proceedings while maintaining derivation actions, and creating a post-grant opposition process to challenge patents after they have issued. The effects of these changes are currently uncertain as the USPTO must still implement various regulations, and the courts have yet to address many of these provisions in the context of a dispute.
Restrictions on our patent rights relating to our products may limit our ability to prevent third parties from competing against us.
Our success will depend, in part, on our ability to obtain and maintain patent protection for our products, preserve our trade secrets, prevent third parties from infringing upon our proprietary rights and operate without infringing upon the proprietary rights of others. We cannot be certain that the claims in our patent applications to inventions covering our current or future products will be considered patentable by the USPTO and courts in the United States or by the patent offices and courts in countries outside of the United States.
We have filed a method-of-use patent application and may file additional method-of-use patent applications in the future. This type of patent protects the use of the product only for the specified method and does not prevent a competitor from making and marketing a product that is identical to our product for an indication that is outside the scope of the patented method. Moreover, even if these competitors do not actively promote their product for our targeted indication, ophthalmic surgeons and ophthalmologists may use these products “off-label.” Although off-label use may infringe or contribute to the infringement of method-of-use patents, the practice is difficult to prevent or prosecute.
Patent applications in the United States and most other countries are confidential for a period of time until they are published, and publication of discoveries in scientific or patent literature typically lags actual discoveries by several months or more. As a result, we cannot be certain that we and the inventors of the issued patents and
60
applications that we may in-license were the first to conceive of the inventions covered by such patents and pending patent applications or that we and those inventors were the first to file patent applications covering such inventions. Also, patent protection may lapse before we manage to obtain commercial value from patents that we may obtain, which might result in increased competition and materially and adversely affect our position in the market.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting and defending patents on our products and technologies throughout the world would be prohibitively expensive. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories where we have patent protection, but where enforcement is not as strong as that in the United States. These products may compete with our future products in jurisdictions where we do not have any issued patents and our patent claims or other intellectual property rights may not be effective or sufficient to prevent them from so competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in jurisdictions outside of the United States. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection, which could make it difficult for us to stop the infringement of any patent issued to us or the marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in jurisdictions outside of the United States could result in substantial cost and divert our efforts and attention from other aspects of our business.
Obtaining and maintaining our patents depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
The USPTO and various non-U.S. governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other provisions during the patent process. There are situations in which noncompliance can result in abandonment or lapse of a patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, competitors may be able to enter the market earlier than would otherwise have been the case.
If our trademarks and trade names are not adequately protected, then we may not be able to build name recognition in our markets of interest and our business may be adversely affected.
Our registered or unregistered trademarks or trade names may be challenged, infringed, circumvented or declared generic or determined to be infringing on other marks. We may not be able to protect our rights to these trademarks and trade names, which we need to build name recognition by potential partners or customers in our markets of interest. Over the long term, if we are unable to establish name recognition based on our trademarks and trade names, then we may not be able to compete effectively and our business may be materially and adversely affected.
We may be subject to claims that we or our employees or consultants have wrongfully used or disclosed alleged trade secrets of our employees’ or consultants’ former employers or their clients. These claims may be costly to defend and, if we do not successfully do so, we may be required to pay monetary damages and may lose valuable intellectual property rights or personnel.
Although no claims against us are currently pending, we may be subject to claims that our employees or our company have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of the former employers of our employees. Litigation may be necessary to defend against these claims. If we fail in
61
defending such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. A loss of key research personnel or their work product could hamper our ability to commercialize, or prevent us from commercializing, our products, which could severely harm our business. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a significant distraction to management.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to seeking patents for some of our technology and products, we will also rely on trade secrets, including unpatented know-how, technology and other proprietary information, to maintain our competitive position. We seek to protect our trade secrets, in part, by entering into non-disclosure and confidentiality agreements with parties who have access to them, such as our employees, contract manufacturers, consultants and other third parties. We also enter into confidentiality and invention or patent assignment agreements with our employees and consultants that obligate them to assign their inventions to us. Despite these efforts, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, some courts inside and outside the United States are less willing or unwilling to protect trade secrets. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent them from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to or independently developed by a competitor, our competitive position would be harmed.
Risks Related to Ownership of Our Ordinary Shares
We believe that we may be a passive foreign investment company, or PFIC, for U.S. federal income tax purposes, which could subject U.S. Holders to adverse U.S. federal income tax consequences.
Although neither we nor any of our subsidiaries was a PFIC in 2014 or any prior taxable year, we believe that we may be a PFIC for U.S. federal income tax purposes in the current taxable year and for foreseeable future taxable years. A PFIC generally is a foreign corporation if either at least (i) 75% of its gross income is “passive income” (the “PFIC Income Test”) or (ii) 50% of the gross value of its assets is attributable to assets that produce, or are held for the production of, passive income (the “PFIC Asset Test”). The proceeds from our initial public offering are a passive asset under these rules, and if substantial enough, may cause us to meet the PFIC Asset Test for 2015 (and in later years if we are not deploying the cash at a rate that would allow us to avoid meeting the PFIC Asset Test in such later years). Similarly, any earnings received from investments made with the proceeds from our initial public offering will be passive income under these rules, and if substantial enough, may cause us to meet the PFIC Income Test for 2015 and in later years. If we are a PFIC in any taxable year in which you hold shares and you are a U.S. Holder, we always will be a PFIC with respect to your stock ownership (subject to the QEF election discussed immediately below) unless you make an election to “purge” PFIC status as of the beginning of the first taxable year that we are not a PFIC (a year in which we do not meet the PFIC Income Test or the PFIC Asset Test) or the first taxable year that you make a QEF election, if such election is made after the first year in which you held our shares and in which we are a PFIC, all as discussed further below. If we are a PFIC and you are a U.S. Holder and do not make a Qualified Electing Fund election, or QEF election, with respect to us or a mark-to-market election with respect to our ordinary shares, you will be subject to adverse tax consequences, including deferred tax and interest charges with respect to certain distributions on our ordinary shares, any gain realized on a disposition of our ordinary shares and certain other events. The effect of these adverse tax consequences could be materially adverse to you. If you are a U.S. Holder and make a valid, timely QEF election for us, you will not be subject to those adverse tax consequences, but could recognize taxable income in a taxable year with respect to our ordinary shares in excess of any distributions that we make to you in that year, thus giving rise to so-called “phantom income” and to a potential out-of-pocket tax liability. If we are a PFIC with respect to any tax year, we will provide information to all electing shareholders needed to comply with
62
the QEF election in time for each electing shareholder to make and maintain a timely QEF election, taking into account available extensions. If you are a U.S. Holder and make a valid, timely mark-to-market election with respect to our ordinary shares, you will recognize as ordinary income or loss in each year that we are a PFIC an amount equal to the difference between your basis in our ordinary shares and the fair market value of the ordinary shares, thus also possibly giving rise to phantom income and a potential out-of-pocket tax liability. Ordinary loss generally is recognized only to the extent of net mark-to-market gains previously included in income. We believe that one or more of our subsidiaries may be PFICs in the current taxable year and for foreseeable future taxable years based on their current and projected assets and income; also, we may form or acquire a subsidiary that is a PFIC in the future. In such event, U.S. Holders will also need to make the QEF election with respect to each such subsidiary in order to avoid the adverse tax consequences described above. We intend to provide on a timely basis all information necessary for U.S. Holders to make the QEF election with respect to any of our subsidiaries that may be classified as a PFIC in any tax year. U.S. Holders should also be aware that the mark-to-market election generally will not be available with respect to any of our subsidiaries that is a PFIC, rendering such election less beneficial to U.S. Holders than the QEF election. We will determine on an annual basis whether we will be a PFIC with respect to any taxable year. As noted above, if we are a PFIC in any taxable year in which you own shares and you are a U.S. holder, we will remain a PFIC with respect to your stock ownership unless you make a “purging election.” The resulting liability from such an election could be substantial.
If the IRS determines that we are not a PFIC, and you previously paid taxes pursuant to a QEF election or a mark-to-market election, you may pay more taxes than you legally owe.
If the IRS makes a determination that we are not a PFIC and you previously paid taxes pursuant to a QEF election or mark-to-market election, then you may have paid more taxes than you legally owed due to such election. If you do not, or are not able to, file a refund claim before the expiration of the applicable statute of limitations, you will not be able to claim a refund for those taxes.
An active, liquid and orderly trading market for our ordinary shares may not develop and you may not be able to resell your shares at or above the price that you paid for them.
Prior to our initial public offering, there was no public market for our ordinary shares. Although our ordinary shares are listed on the NASDAQ Global Market, an active, liquid, and orderly trading market for our shares may never develop or be sustained. If an active market for our ordinary shares does not continue to develop or is not sustained, it may be difficult for investors in our ordinary shares to sell shares without depressing the market price for the shares or to sell the shares at all.
Our share price may be volatile, and you may not be able to resell your shares at or above the price that you paid for them.
Since our initial public offering, the trading price of our ordinary shares has been volatile, and it is likely that the trading price of our ordinary shares will continue to be volatile. As a result of this volatility, investors may not be able to sell their ordinary shares at or above the price paid for the shares. The market price for our ordinary shares may be influenced by many factors, including:
| • | announcements regarding the initiation, timing, progress or results of our clinical trials, post-market evaluation studies, research and development programs and commercialization efforts; |
| • | fluctuations in our quarterly financial results or the quarterly financial results of companies perceived to be similar to us; |
| • | actual or anticipated fluctuations in our key operating metrics, financial condition and operating results; |
| • | third-party publications reporting findings with respect to the efficacy and safety of our products; |
| • | difficulties in establishing relationships with refractive laser centers; |
63
| • | actual or anticipated changes in our growth rate; |
| • | announcements of technological innovations or new offerings by us or our competitors; |
| • | our announcement of actual results for a fiscal period that are worse than projected or expected or our announcement of revenue or earnings guidance that is lower than expected; |
| • | changes in estimates of our financial results or recommendations by securities analysts; |
| • | failure of any of our products to achieve or maintain market acceptance; |
| • | changes in market valuations of similar companies; |
| • | success of competitive products or services; |
| • | changes in our capital structure, such as future issuances of securities or the incurrence of debt; |
| • | announcements by us or our competitors of significant products or services, contracts, acquisitions or strategic alliances; |
| • | regulatory developments in the United States or other countries; |
| • | actual or threatened litigation involving us or our industry; |
| • | additions or departures of key personnel; |
| • | share price and volume fluctuations attributable to inconsistent trading volume levels of our shares; |
| • | further issuances of ordinary shares by us; |
| • | sales of ordinary shares by our shareholders; |
| • | repurchases or redemptions of ordinary shares; and |
| • | changes in general economic, industry and market conditions. |
In addition, the stock market in general, and the market for medical device companies in particular, has experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of those companies. Securities class action litigation has often been instituted against companies following periods of volatility in the overall market and in the market price of a company’s securities. Any such litigation, if instituted against us, could result in very substantial costs, divert our management’s attention and resources, and harm our business, operating results and financial condition.
A significant portion of our total issued shares may be sold into the public market in the near future, which could cause the market price of our ordinary shares to drop significantly, even if our business is doing well.
As of March 24, 2015, we had 13,351,874 ordinary shares issued and outstanding. Of these shares, 9,166,667 shares were outstanding before our initial public offering. These 9,166,667 shares are held by Presbia Holdings, our controlling shareholder. In addition, our controlling shareholder purchased 500,000 ordinary shares in our initial public offering. As of March 24, 2015, our controlling shareholder owned 9,666,667 of our outstanding ordinary shares.
If our controlling shareholder, sells, or indicates an intention to sell, or if our controlling shareholder distributes our shares to its equity holders and those equity holders sell or indicate an intention to sell, substantial amounts of our ordinary shares in the public market, the trading price of our ordinary shares could decline. The perception in the market that these sales may occur could also cause the trading price of our ordinary shares to decline. Of the 9,666,667 shares held by our controlling shareholder, 9,166,667 shares are subject to lockup restrictions set forth in a lockup agreement entered into by Presbia Holdings and the underwriter for our initial public offering, which restrictions will expire on July 28, 2015. After the lock-up period expires, up to an additional 9,166,667 ordinary shares will be eligible for sale in the public market, subject to volume limitations under Rule 144 under the Securities Act.
64
Our controlling shareholder, and its permitted transferees, is entitled to rights with respect to the registration of the ordinary shares that it holds under the Securities Act, subject to the lock-up agreement described above with respect to the 9,166,667 shares that it holds that were acquired prior to our initial public offering. See “Part II, Item 13. Certain Relationships and Related Party Transactions—Registration Rights Agreement.” Registration of these shares under the Securities Act would result in the shares becoming freely tradable without restriction under the Securities Act, except for shares purchased by affiliates.
On January 29, 2015, we filed a registration statement on Form S-8 registering the issuance of 1.8 million ordinary shares subject to options or other equity awards issued or reserved for issuance under our Presbia Incentive Plan. Shares registered under this registration statement on Form S-8 will be available for sale in the public market subject to applicable vesting arrangements and the exercise of options, the lock-up restrictions set forth in the applicable lock-up agreements between our officers and directors and the underwriter of our initial public offering and, in the case of our affiliates, the restrictions of Rule 144. If these additional ordinary shares are sold, or if it is perceived that they will be sold, in the public market, the trading price of our ordinary shares could decline.
In addition, after the expiration of the applicable lock-up agreements between our controlling shareholder, our officers and our directors and the underwriter for our initial public offering, which are set to expire on July 28, 2015, our controlling shareholder, our directors and our executive officers may establish programmed selling plans under Rule 10b5-1 of the Exchange Act with respect to shares that they hold or thereafter acquire, for the purpose of effecting sales of our ordinary shares. Any sales of ordinary shares by these shareholders, or the perception that those sales may occur, including the entry into such programmed selling plans, could have a material adverse effect on the trading price of our ordinary shares.
If securities or industry analysts do not publish or cease publishing research or reports about us, our business, or our market, or if they change their recommendations regarding our shares adversely, our share price and trading volume could decline.
The trading market for our ordinary shares will be influenced by the research and reports that industry or securities analysts may publish about us, our business, our market or our competitors. Securities and industry analysts do not currently, and may never, publish research on us. If no securities or industry analysts commence coverage of our company, our share price and trading volume would likely be negatively impacted. If any of the analysts who may cover us change their recommendation regarding our shares adversely, or provide more favorable relative recommendations about our competitors, our share price would likely decline. If any of the analysts who may cover us were to cease coverage or fail to regularly publish reports on us, we could lose visibility in the financial markets, which in turn could cause our share price or trading volume to decline.
Our controlling shareholder has substantial control over us and beneficially owns a majority of our issued ordinary shares, which could delay or prevent a change in corporate control.
Presbia Holdings, which is controlled, directly and/or indirectly, by Richard Ressler, one of our directors, holds a majority of our issued ordinary shares. As of March 24, 2015, Presbia Holdings held 9,666,667 of our issued and outstanding ordinary shares. As a result, our controlling shareholder has the ability to control the outcome of matters submitted to our shareholders for approval, including the election of directors and any sale, merger, consolidation or sale of all or substantially all of our assets. In addition, our controlling shareholder has the ability to control or influence our management and our affairs. Furthermore, the concentration of voting power in our controlling shareholder may have an adverse effect on our share price.
We are an “emerging growth company” and we cannot be certain if the reduced disclosure requirements applicable to emerging growth companies will make our ordinary shares less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act, and we are taking advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not
65
“emerging growth companies,” including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes Oxley Act for an extended period of time, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. We may take advantage of these exemptions until we are no longer an “emerging growth company.” We will remain an “emerging growth company” until December 31, 2020, although if the market value of our ordinary shares that are held by non-affiliates exceeds $700 million as of any June 30 before that time and in certain other circumstances, we would cease to be an “emerging growth company” as of the following December 31. We cannot predict if investors will find our ordinary shares less attractive because we may rely on these exemptions. If some investors find our ordinary shares less attractive as a result, there may be a less active trading market for our ordinary shares.
We are a “controlled company” under the NASDAQ listing rules, and as such we are entitled to exemption from certain NASDAQ corporate governance standards, and you may not have the same protections afforded to shareholders of companies that are subject to all NASDAQ corporate governance requirements.
Presbia Holdings, our controlling shareholder, controls a majority of the voting power of our issued ordinary shares. As a result, we are a “controlled company” within the meaning of the corporate governance rules of NASDAQ. Under these rules, a controlled company may elect not to comply with certain corporate governance requirements, including: the requirement that we have a compensation committee that is composed entirely of independent directors; the requirement that we have a nominating/corporate governance committee that is composed entirely of independent directors; and the requirement that a majority of the members of our Board be independent directors. In addition, we are relying on the phase-in rules of the SEC and NASDAQ with respect to the independence of our audit committee. These rules permit us to have an audit committee that has one member that is independent by the NASDAQ listing date, a majority of members that are independent within 90 days of the NASDAQ listing date, and all members that are independent within one year of the NASDAQ listing date. We are utilizing and intend to continue to utilize some or all of those exemptions. Accordingly, you will not be similarly situated to shareholders of companies that are subject to all of the corporate governance requirements of NASDAQ. Our status as a controlled company could make our ordinary shares less attractive to some investors or otherwise harm our stock price.
We do not currently intend to pay dividends on our ordinary shares and, consequently, your ability to achieve a return on your investment will depend on appreciation in the price of our ordinary shares.
We have never declared or paid any cash dividends on our ordinary shares and do not intend to do so for the foreseeable future. We currently intend to retain all available funds and any future earnings to support the operation of, and to finance the growth and development of, our business. Any future determination to declare cash dividends will be made at the discretion of our Board, subject to compliance with applicable laws (including the Irish Companies Acts, which require Irish companies to have “profits available for distribution” before they can pay dividends) and covenants under credit facilities, which may restrict or limit our ability to pay dividends and will depend on our financial condition, operating results, capital requirements, general business conditions and other factors that our Board may deem relevant. As a result, any return to shareholders will be limited to the appreciation, if any, of their ordinary shares.
Provisions contained in our articles of association, as well as provisions of Irish law, could impair a takeover attempt.
Our articles of association and certain provisions of the Irish Companies Acts contain provisions that could have the effect of delaying or preventing changes in control or changes in our management without the consent of our Board.
There are a number of approaches for acquiring an Irish public limited company, including a court-approved scheme of arrangement under the Irish Companies Acts, through a tender offer by a third party under the Irish
66
Takeover Panel Act 1977 (as amended) and the Irish Takeover Rules 2007 (as amended) made thereunder, which we refer to herein as the Irish Takeover Rules, and by way of a merger with a company incorporated in the EEA under the European Communities (Cross-Border Mergers) Regulations 2008 (as amended). Each method requires shareholder approval or acceptance and different thresholds apply.
The Irish Takeover Rules will govern a takeover or attempted takeover of our company by means of a court-approved scheme of arrangement or a tender offer. These Rules contain detailed provisions for takeovers including as to disclosure, dealing and timetable. The Irish Takeover Rules could discourage an investor from acquiring 30% or more of the outstanding ordinary shares of our company unless such investor were prepared to make a bid to acquire all outstanding ordinary shares.
Our Board may be limited by the Irish Takeover Rules in its ability to defend an unsolicited takeover attempt.
Under the Irish Takeover Rules, we will not be permitted to take certain actions that might “frustrate” an offer for our ordinary shares once our Board has received an offer, or has reason to believe an offer is or may be imminent, without the approval of more than 50% of shareholders entitled to vote at a general meeting of our shareholders and/or the consent of the Irish Takeover Panel. This could limit the ability of our Board to take defensive actions even if it believes that such defensive actions would be in the best interests of our company and shareholders.
Irish law differs from the laws in effect in the U.S. and may afford less protection to holders of our securities.
It may not be possible to enforce court judgments obtained in the U.S. against us in Ireland based on the civil liability provisions of the U.S. federal or state securities laws. In addition, there is some uncertainty as to whether the courts of Ireland would recognize or enforce judgments of U.S. courts obtained against us or our directors or officers based on the civil liabilities provisions of the U.S. federal or state securities laws or hear actions against us or those persons based on those laws. We have been advised that the United States currently does not have a treaty with Ireland providing for the reciprocal recognition and enforcement of judgments in civil and commercial matters. Therefore, a final judgment for the payment of money rendered by any U.S. federal or state court based on civil liability, whether or not based solely on U.S. federal or state securities laws, would not automatically be enforceable in Ireland.
As an Irish company, we are governed by the Irish Companies Acts, which differ in some material respects from laws generally applicable to U.S. corporations and shareholders, including, among others, differences relating to interested director and officer transactions and shareholder lawsuits. Likewise, the duties of directors and officers of an Irish company generally are owed to the company only. Shareholders of Irish companies generally do not have a personal right of action against directors or other officers of the company and may exercise such rights of action on behalf of the company only in limited circumstances. Accordingly, holders of our ordinary shares may have more difficulty protecting their interests than would holders of shares of a corporation incorporated in a jurisdiction of the United States.
The rights of our shareholders may differ from the rights typically offered to shareholders of a U.S. corporation and these differences may make our ordinary shares less attractive to investors.
We are incorporated under Irish law and, therefore, certain of the rights of holders of our shares are governed by Irish law, including the provisions of the Irish Companies Acts, and by our articles of association. These rights differ in certain respects from the rights of shareholders in typical U.S. corporations and these differences may make our ordinary shares less attractive to investors. The principal differences include the following:
| • | under Irish law, dividends may only be declared if we have, on an individual entity basis, profits available for distribution, within the meaning of the Irish Companies Acts; |
| • | under Irish law, each shareholder generally has preemptive rights to subscribe on a proportionate basis to any issuance of shares. Under U.S. law, shareholders generally do not have preemptive rights unless |
67
| specifically granted in the certificate of incorporation or otherwise. Pre-emption rights may be disapplied under Irish law for renewable five-year periods by Irish companies by way of a provision in their articles of association or special resolution of their shareholders, which is an option we have availed ourselves of prior to the consummation of our initial public offering; |
| • | under Irish law, certain matters require the approval of holders of 75% of the votes cast at a general meeting of our shareholders, including amendments to our articles of association. This may make it more difficult for us to complete certain types of corporate transactions deemed advisable by our Board. Under U.S. law, generally only majority shareholder approval is required to amend the certificate of incorporation or to approve other significant transactions; |
| • | under Irish law, a bidder seeking to acquire us would need, on a tender offer, to receive shareholder acceptance in respect of 80% of our outstanding shares. If this 80% threshold is not achieved in the offer, under Irish law, the bidder cannot complete a “second step merger” to obtain 100% control of us. Accordingly, tender of 80% of our outstanding shares will likely be a condition in a tender offer to acquire us, not 50% as is more common in tender offers for corporations organized under U.S. law; and |
| • | under Irish law, shareholders may be required to disclose information regarding their equity interests upon our request, and the failure to provide the required information could result in the loss or restriction of rights attaching to the shares, including prohibitions on the transfer of the shares, as well as restrictions on voting, dividends and other payments. Comparable provisions generally do not exist under U.S. law. |
A future transfer of your ordinary shares, other than one effected by means of the transfer of book entry interests in DTC, may be subject to Irish stamp duty.
Transfers of ordinary shares effected by means of the transfer of book entry interests in the Depository Trust Company, or DTC, should not be subject to Irish stamp duty. It is anticipated that the majority of ordinary shares will be traded through DTC through brokers who hold such ordinary shares on behalf of customers through DTC. This exemption should be available because our ordinary shares will be traded on a recognized stock exchange in the United States. However, if you hold your ordinary shares as of record rather than beneficially through DTC or through a broker that holds your ordinary shares through DTC, any transfer of your ordinary shares could be subject to Irish stamp duty (currently at the rate of 1% of the higher of the price paid or the market value of the ordinary shares acquired). Payment of Irish stamp duty is generally a legal obligation of the transferee. The potential for stamp duty to arise could adversely affect the price of our ordinary shares.
Item 1B. Unresolved Staff Comments.
None.
Our operations are currently conducted at three leased facilities. We lease or sublease an aggregate of approximately 14,800 square feet of office, laboratory and manufacturing space in Irvine, California. The lease covering approximately two-thirds of this space expires in May 2017 and the sublease covering approximately one-third of this spaces expires in July 2016. In addition, we lease approximately 300 square feet of office and storage space in Amsterdam, the Netherlands, and we lease approximately 305 square feet of office and storage space in Dublin, Ireland. Our corporate headquarters are currently located at our Dublin location.
We believe that our current facilities are suitable and adequate to meet our current needs.
We are not aware of any pending or threatened legal proceeding against us that could have a material adverse effect on our business, operating results or financial condition. However, the medical device industry is
68
characterized by frequent claims and litigation, including claims regarding patent and other intellectual property rights as well as improper hiring practices. As a result, we may be involved in various legal proceedings from time to time.
Item 4. Mine Safety Disclosures.
Not applicable.
69
Part II
Item 5. Market for Registrant’s Common Equity, Related Shareholder Matters and Issuer Purchases of Equity Securities.
Market Information for Ordinary Shares
Our ordinary shares are traded on The NASDAQ Global Market under the ticker symbol “LENS” and began trading on January 29, 2015. Prior to our initial public offering, there was no public market for our ordinary shares. As a result, we have not set forth quarterly information with respect to the high and low prices for our ordinary shares for the two most recent fiscal years.
The last reported sale price of our ordinary shares as reported on The NASDAQ Global Market on March 24, 2015 was $8.25 per share.
Shareholders
As of March 24, 2014, there were two holders of record of our ordinary shares. This number does not reflect the beneficial holders of our ordinary shares who hold shares in street name through brokerage accounts or other nominees.
Dividend Policy
We have never declared or paid any cash dividends on our ordinary shares. We currently intend to retain all available funds and any future earnings to support our operations and finance the growth and development of our business. We do not intend to pay cash dividends on our ordinary shares for the foreseeable future. Any future determination relating to our dividend policy will be made at the discretion of our Board and will depend on, among other factors, our results of operations, financial condition, capital requirements, contractual restrictions, business prospects and other factors our Board may deem relevant.
Securities Authorized for Issuance Under Equity Compensation Plans
At December 31, 2014, we did not have any equity compensation plans. In January 2015, our Board adopted our Presbia Incentive Plan, which was then approved by our shareholders in January 2015. A total of 1,800,000 of our ordinary shares are authorized for issuance under our Presbia Incentive Plan. In connection with our initial public offering, in January 2015, we issued options to certain officers and directors covering 920,000 of our ordinary shares, each with an exercise price of $10.00 per share. Since our initial public offering, we have issued 18,540 restricted shares to certain directors, and we have issued options to certain officers and employees covering 102,500 of our ordinary shares, each with an exercise price of $8.63 per share.
Share Performance Graph
Our ordinary shares began being trading on the NASDAQ Global Market on January 29, 2015. We will provide a share performance graph beginning with our 2015 Annual Report on Form 10-K.
Recent Sales of Unregistered Securities
Since its formation on February 6, 2014, Presbia PLC has issued the following securities which were not registered under the Securities Act:
(1) Upon its formation on February 6, 2014, Presbia PLC issued 40,000 ordinary shares of €1.00 each to Presbia Holdings (six of such shares are held by six nominee shareholders on behalf of Presbia Holdings), in order to satisfy statutory requirements for the incorporation of all Irish public limited companies. Prior to our initial public offering, these shares were re-designated as deferred shares under our memorandum and articles of association.
70
(2) In anticipation of our initial public offering, on January 15, 2015, Presbia PLC issued 9,166,667 ordinary shares to Presbia Holdings in exchange for the contribution by Presbia Holdings of all the share capital in issue in Presbia Ireland, Limited to Presbia PLC. See “Part I, Item I, Business—Corporate History and Information.”
No underwriters were used in the foregoing transactions. The sales of securities described in paragraphs (1) and (2) above were deemed to be exempt from registration pursuant to Section 4(a)(2) of the Securities Act as transactions by an issuer not involving a public offering and/or Rule 506 promulgated under the Securities Act. All of the participants in these transactions represented to us in connection with their acquisition that they were acquiring the shares for investment and not distribution, and that they could bear the risks of the investment and could hold the securities for an indefinite period of time. Such recipients received written disclosures that the securities had not been registered under the Securities Act and that any resale must be made pursuant to a registration or an available exemption from such registration. All of the foregoing securities are deemed restricted securities for the purposes of the Securities Act.
Purchases of Equity Securities by the Issuer and Affiliated Purchasers
None.
Use of Proceeds
On January 28, 2015, our registration statement on Form S-1 (File No 333-194713), as amended, was declared effective by the SEC for our initial public offering. Upon the closing of our initial public offering on February 3, 2015, we sold 4,166,667 ordinary shares, $0.001 par value per share, at a public offering price of $10.00 per share, for an aggregate public offering price of $41.7 million. Jefferies LLC acted as the sole book-running manager for the offering.
As a result of the initial public offering, we received net proceeds of approximately $36.6 million, after deducting approximately $2.9 million of underwriting discounts and commissions and estimated offering expenses of approximately $2.2 million payable by us. None of such payments were direct or indirect payments to any of (i) our directors or officers or their associates, (ii) persons owning 10 percent or more of our common stock, or (iii) our affiliates.
There has been no material change in the planned use of proceeds from our initial public offering from that described in the final prospectus related to the offering, which we filed with the SEC on January 29, 2015.
71
Item 6. Selected Financial Data.
The following consolidated statement of operations for the years ended December 31, 2014, 2013 and 2012 and the consolidated balance sheets data for the years ended December 31, 2014 and 2013 have been derived from our audited consolidated financial statements included elsewhere in this Annual Report on Form 10-K. The consolidated balance sheet data for the year ended December 31, 2012 is derived from the audited financial statements included in our prospectus filed with the SEC on January 29, 2015. Our historical results for any prior periods are not necessarily indicative of results to be expected for any future period. The information set forth in the following table should be read in conjunction with “Part II, Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and related notes thereto included elsewhere in this Annual Report on Form 10-K.
| YEAR ENDED DECEMBER 31, | ||||||||||||
| (in thousands, except per share amounts) | 2014 | 2013 | 2012 | |||||||||
| CONSOLIDATED STATEMENT OF OPERATIONS DATA: |
||||||||||||
| Revenues |
$ | 161 | $ | 98 | $ | 95 | ||||||
| Cost of goods sold |
61 | 111 | 71 | |||||||||
|
|
|
|
|
|
|
|||||||
| Gross profit (loss) |
100 | (13 | ) | 24 | ||||||||
| Operating expenses: |
||||||||||||
| Research and development |
4,243 | 2,136 | 1,024 | |||||||||
| Sales and marketing |
1,652 | 1,044 | 632 | |||||||||
| General and administrative |
7,577 | 4,088 | 1,957 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
13,472 | 7,268 | 3,613 | |||||||||
|
|
|
|
|
|
|
|||||||
| Operating loss |
(13,372 | ) | (7,281 | ) | (3,589 | ) | ||||||
| Interest expense |
2,288 | 2,161 | 1,458 | |||||||||
| Other income |
6 | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Loss before income tax provision |
(15,654 | ) | (9,442 | ) | (5,047 | ) | ||||||
| Income tax provision |
10 | 20 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
$ | (15,664 | ) | $ | (9,462 | ) | $ | (5,047 | ) | |||
|
|
|
|
|
|
|
|||||||
| Net loss per share—basic and diluted |
$ | (1.71 | ) | $ | (1.03 | ) | $ | (0.55 | ) | |||
|
|
|
|
|
|
|
|||||||
| DECEMBER 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| CONSOLIDATED BALANCE SHEET DATA: |
||||||||||||
| Cash |
$ | 138 | $ | 584 | $ | 176 | ||||||
| Total Assets |
2,342 | 3,765 | 1,371 | |||||||||
| Payable due to Presbia Holdings |
417 | 9,425 | 13,043 | |||||||||
| Accumulated deficit |
(37,386 | ) | (21,722 | ) | (12,260 | ) | ||||||
| Total shareholders’ deficit |
(706 | ) | (8,882 | ) | (12,122 | ) | ||||||
72
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
You should read the following discussion and analysis of our financial condition and results of operations together with our financial statements and the related notes appearing elsewhere in this Annual Report on Form 10-K. Some of the information contained in this discussion and analysis or set forth elsewhere in this Annual Report on Form 10-K, including information with respect to our plans and strategy for our business and related financing, includes forward-looking statements that involve risks and uncertainties. You should read “Cautionary Note Regarding Forward-Looking Statements” and “Part I, Item 1A. Risk Factors” of this Annual Report on Form 10-K for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
Overview
We are an ophthalmic device company which has developed and is currently marketing a proprietary optical lens implant for treating presbyopia, the age-related loss of the ability to focus on near objects. Our microlens is a miniature lens designed to be surgically implanted in a patient’s eye to improve that patient’s ability to see objects at close distances. Our current strategy is to continue to commercialize our microlens in certain strategic countries where we currently have marketing approval and to continue to seek to obtain marketing approval in other key markets, including the United States. Our goal is to become a leading provider of corneal inlay presbyopia-correcting treatment worldwide.
Although reading glasses and contact lenses have historically been, and remain, the most common solution for presbyopia, there are significant drawbacks associated with these approaches, as well as with alternative surgical approaches. We believe that our microlens provides an alternative solution to those presbyopic individuals who desire greater freedom from glasses and wish to avoid the daily maintenance and other complications of contact lenses. We believe that our microlens can be both an effective standalone solution for presbyopia and an effective complementary solution that can be used in conjunction with certain other surgical approaches that are used to treat vision disorders other than presbyopia.
Through our European Union CE Mark, we are generally authorized to market our microlens throughout the European Economic Area (27 of the 28 European Union member states plus Iceland, Liechtenstein and Norway), or EEA, and Switzerland. We currently market our microlens in certain strategic EEA countries as well as certain strategic countries outside of the EEA in which we possess marketing approval. Through March 15, 2015, ophthalmic surgeons have implanted over 600 of our microlenses outside of the United States.
We are presently seeking marketing approval in other strategic countries, including the United States. In December 2013, we received approval to commence a staged pivotal clinical trial as part of the U.S. Food and Drug Administration, or FDA, approval process. Beginning in May 2014, we enrolled a total of 75 subjects at six investigational sites in the United States and each subject underwent insertion of our microlens in the non-dominant eye. Based on six-month data on 52 subjects, in January 2015, we submitted an interim safety report as a supplement to our investigational device exemption, or IDE, to the FDA. In February 2015, we received approval from the FDA to commence second stage enrollment in this trial. We are permitted to enroll up to an additional 337 subjects at up to nine additional investigational sites. Through March 15, 2015, 25 subjects underwent insertion of our microlens in the second stage of this study. This study is necessary in order to obtain clinical data to provide the primary support for a safety and effectiveness evaluation to support a pre-market approval, or PMA, for marketing clearance in the United States. Data on a minimum of 300 subjects with 24-month data will be submitted as part of the PMA, and all subjects will be followed for three years following implantation. We are targeting submission of our final PMA, containing 24-month data on 300 subjects, to the FDA in the second quarter of 2017. We are pursuing a modular PMA submission strategy whereby we intend to submit to the FDA information regarding preclinical testing, engineering, and manufacturing in the fourth quarter of 2015 or the first quarter of 2016, prior to the submission of our final PMA. We are targeting PMA approval of our microlens in the fourth quarter of 2017. We are also targeting submission to the FDA of a final report with
73
36-month data on these 300 subjects in the second quarter of 2018. These milestones could be delayed by further interactions with the FDA or by a variety of other factors. In addition, no assurance can be given that the FDA will grant us PMA approval or, if granted, that it will be granted in accordance with our anticipated time schedule. Also, the FDA may require us to conduct post-approval studies as a condition of approval.
We are a development stage ophthalmic device company with a limited operating history. We are not profitable and have incurred losses in each year since our formation. We have reported recurring net losses and negative cash flow from operating activities since inception and, as of December 31, 2014, we had an accumulated deficit of $37.4 million. We expect to continue to incur significant losses for the foreseeable future.
On February 3, 2015, we closed the initial public offering of our ordinary shares. We sold a total of 4,166,667 ordinary shares in the offering at a public offering price of $10.00 per share. The aggregate public offering price was $41.7 million, and we received net proceeds of approximately $36.6 million from the offering, after deducting $5.1 million of underwriting discounts and commissions and estimated offering expenses payable by us.
Emerging Growth Company Status
The Jumpstart Our Business Startups Act of 2012, or the JOBS Act, permits an “emerging growth company” such as us to take advantage of an extended transition period to comply with new or revised accounting standards applicable to public companies. We have chosen to “opt out” of this provision and, as a result, we will comply with new or revised accounting standards as required when they are adopted. This decision to opt out of the extended transition period under the JOBS Act is irrevocable.
Factors Affecting our Industry
The medical device industry in general, and the ophthalmic medical device market in particular, are highly competitive. In order for us to succeed in this market as a development stage company, we must:
| • | incur substantial expenditures to obtain regulatory approvals necessary to commence marketing our products in particular jurisdictions; |
| • | develop a commercialization strategy that is responsive both to the needs of laser centers and ophthalmic surgeons and to our own requirements and limitations; |
| • | invest in our future by continuing to advance our technology and improve our microlens, our microlens inserter and other auxiliary products; |
| • | focus on, and respond to, the feedback we receive in post-operative situations and comply with various post-marketing reporting obligations; and |
| • | invest in our infrastructure, to assure that if we are successful in gaining necessary regulatory approvals, commercializing our products and advancing our technology, we will be able to grow our business accordingly. |
We expect to compete against companies that develop corneal inlay surgical solutions for presbyopia, companies that offer alternative surgical methodologies for the treatment of presbyopia, including monovision, multifocal and accommodating approaches, and companies that promote reading glasses and/or contact lenses as approaches for treating presbyopia. At any time, our known competitors and other potential market entrants, many of which have greater resources and experience in the ophthalmic medical device market than we have, may develop new devices or treatment alternatives that may compete directly with our products. In addition, they may gain a market advantage by developing and patenting competitive products or processes earlier than we can or by obtaining regulatory approvals/clearances or market registrations more rapidly than we can.
74
The competitive nature of the market, the high degree of government regulation, the importance of technological innovation and the significance that most people place on near vision combine to elevate the risks facing any development stage company seeking to enter our market.
Factors Affecting our Revenues
We believe that the principal factors affecting our revenues will include:
| • | our ability to obtain pre-market approval for our microlens and microlens inserter in the United States and, if we are able to obtain that approval, the time that it will take us to obtain that approval and the associated expenses; |
| • | our ability to obtain necessary regulatory approvals in other jurisdictions that we target to commercialize our products, and, if we are able to obtain those approvals, the time that it will take us to obtain those approvals and the associated expenses; |
| • | the growth in the worldwide presbyopic population and the increasing needs of significant elements of that population to view small print on a variety of electronic devices; |
| • | our ability to maintain the regulatory approvals that we currently possess and may acquire in the future and the associated expense; |
| • | our ability to obtain commercialization commitments from laser centers in the jurisdictions in which we are authorized to market our products; |
| • | our ability to gain acceptance by ophthalmic surgeons, to train those ophthalmic surgeons and to cause those ophthalmic surgeons to train other ophthalmic surgeons; and |
| • | the effects that our competitors will have on us, in terms of our ability to meet price competition, to respond to product announcements and developments by others and to respond to other developments in the market. |
Factors Affecting Our Expenses
Our expenses are principally driven by the following factors:
| • | Cost of goods sold. At present, our cost of goods sold relates principally to amounts that we pay to our microlens supplier in Israel and our OEM microlens inserter supplier in the United States. Although we have developed manufacturing capacity in our California facility, all output from that facility will be used for evaluation purposes and for clinical trials unless there is an alternative use for that facility or we receive the necessary governmental approvals to sell products that we manufacture in the United States. Until there is an alternative use for that facility or we receive those approvals, the direct manufacturing costs that we incur in producing products in the United States will be included within research and development expenses. When and if we receive those approvals and manufacture products in the United States for sale in the United States, our cost of goods sold will also include raw material costs, labor expenses and other expenses that we incur to manufacture our products. We do not expect to ramp up production at our California facility beyond what would be required for the clinical trials unless and until we receive approval of our PMA application from the FDA. Our costs of goods sold are directly impacted by the terms of our supply agreements, which may obligate us to pay additional costs if we do not reach our suppliers’ production expectations, and by shipping and handling expenses. When and if we ourselves manufacture products for sale, our costs of goods sold will also be directly impacted by: |
| • | the number of employees that will be engaged in manufacturing and the wages and benefits, including stock-based compensation, that we will pay to those employees; |
| • | to the extent we invest in fixed assets associated with manufacturing activities, the depreciation expenses associated with those fixed assets; |
75
| • | the costs we incur to purchase raw materials; |
| • | inventory write-downs for excess or obsolete inventory; |
| • | the costs of non-production materials; and |
| • | lease expenses associated with our production facilities. |
| • | Research and development. Our research and development expenses consist of the expenses we incur to develop our products, to pursue patent and trademark protection, to respond to technological challenges, to conduct clinical trials and post-market evaluation studies and to pursue governmental approvals. We expect to continue to expense all research and development costs as they are incurred with the exception of capital expenditures that would have alternative uses. Our research and development expenses consist of employee salaries and related benefits, including stock-based compensation, third-party contract costs relating to research, manufacturing, preclinical studies, clinical trial activities and post-market evaluation studies, and allocated facility costs. We expect that our research and development expenses will increase substantially as we continue our staged pivotal clinical trial necessary for us to obtain PMA approval in the United States. Such expenses will also increase as we advance products and projects into further development and continue our early stage research. The process of conducting preclinical studies and clinical trials necessary to obtain regulatory approval is costly and time consuming. We may not succeed in achieving certain marketing approvals that we seek for our products. The probability of success of each product may be affected by numerous factors, including preclinical data, clinical data, post-market and third-party evaluation studies, competition, manufacturing capability and commercial viability. |
As of December 31, 2014, we had eight employees directly engaged in research and development for at least a portion of their responsibilities. We also engage outside advisors and counsel to assist in development projects and in prosecuting patent and trademark applications. Our research and development expenses will be directly impacted by:
| • | the number of employees that will be engaged in research and development and the wages and benefits, including stock-based compensation, that we will pay to those employees; |
| • | the extent to which we will rely on outside sources to provide research and development assistance and the fees charged to us for those services; |
| • | the extent to which we pursue clinical trials, our ability to sign-up patients for those trials and retain patients in those trials, the outcomes arising from those trials and the regulatory responses to those trials; |
| • | the extent to which we pursue post-market evaluation studies, our ability to sign-up patients for those studies and retain patients in those studies, the outcomes arising from those studies and the regulatory responses to those studies; |
| • | the results of third-party evaluation studies; |
| • | the size and geographical scope of the patent and trademark portfolio and the maintenance fees required to maintain that portfolio; and |
| • | to the extent that we invest in fixed assets associated with our clinical trials, the depreciation expenses associated with those fixed assets. |
| • | Sales and marketing. Our sales and marketing expenses consist of costs associated with our sales efforts. Our commercialization strategy involves engaging laser centers to ultimately sponsor our products after gaining confidence in our products and processes. We will train the staff of these centers in practice integration, support patient recruitment with direct response advertising campaigns, surgical performance, patient management and post-operative reporting, enabling the centers to perform a substantial portion of the commercialization process on their own. If we are successful in implementing |
76
| this strategy, our principal expenses will be in furnishing training teams to laser centers and then arranging for a smaller Presbia team to remain available to the center once the center is able to perform the necessary skills on its own. We have incurred, and will continue to incur, expenses in connection with conferences, seminars and trade shows that we attend and/or sponsor. We may also enter into marketing campaigns with participating laser centers, which will add to our sales and marketing expenses. Our sales and marketing expenses will be directly impacted by: |
| • | the volume of our revenues; |
| • | the number of countries in which we obtain authorization to market our products and the associated regulations; |
| • | the number of laser centers that will be willing to partner with us; |
| • | the extent to which we are successful in training ophthalmic surgeons to train other ophthalmic surgeons; |
| • | the extent to which we will be required to develop a distributorship network in countries that mandate that approach; |
| • | the number of employees that will be engaged in sales and marketing and the wages and benefits, including stock-based compensation, that we will pay to those employees; |
| • | the extent to which we identify advertising opportunities that we believe are likely to produce revenue growth; and |
| • | the extent to which we continue to participate in conferences, seminars and similar opportunities. |
| • | General and administrative expenses. Our general and administrative expenses consist of finance, human resources, purchasing and information technology services, other administrative services, foreign exchange costs and expenses associated with planning for and implementing the Reorganization Transactions. To date, our general and administrative expenses have been our largest single cost element, reflecting our approach of concentrating our own efforts on research and development and contracting with third-parties, principally affiliated entities and outside professionals, to provide administrative services to us. Over time, we expect to build our own infrastructure and perform more of these services in-house, in which case our general and administrative expenses will relate more to our own payroll than to the amount that we pay to third-parties. Furthermore, we expect to incur additional expenses as a result of operating as a public company, including costs to comply with the rules and regulations applicable to companies listed on The NASDAQ Global Market and costs related to compliance and reporting obligations pursuant to the rules and regulations of the SEC. In addition, as a public company, we expect to incur increased expenses related to additional insurance, investor relations and other increases related to needs for additional human resources and professional services. Our general and administrative expenses will be directly impacted by: |
| • | the extent to which we purchase services from third-parties; |
| • | the costs we incur to build an infrastructure capable of performing services in-house; |
| • | the number of employees that will be engaged in general and administrative functions and the wages and benefits, including stock-based compensation, that we will pay to those employees; |
| • | the geographical breadth of our company; and |
| • | the extent to which costs associated with being a public company increase over time. |
| • | Interest expense. From inception through November 30, 2014, our interest expense reflects the interest charges that we have incurred through borrowings from Presbia Holdings and, prior to 2009, Orchard Investments, LLC (which is wholly-owned, directly or indirectly, by our director, Richard Ressler, his immediate family and trusts established for his immediate family). From inception through November 30, 2014, Presbia Holdings and Orchard Investments, LLC funded the cash that we required |
77
| to operate our business, at an interest expense of 15% per annum, compounding daily. From November 2014 to our initial public offering consummated in February 2015, we funded our operations through borrowings from Presbia Holdings, at an interest rate equal to the then applicable monthly federal rate of interest for short-term loans, adjusted monthly, compounding daily. As of January 2015, all such debt has been converted to our equity. The amount of interest expense that we incur in the future will be directly impacted by: |
| • | our need for external debt financing; |
| • | the terms we will be able to attract when and if we require external debt financing; and |
| • | our ability, which cannot be assured, to attract equity financing as an alternative to debt financing. |
Critical Accounting Polices and Estimates
Our financial statements are prepared in accordance with generally accepted accounting principles in the United States, or GAAP. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues, costs and expenses and related disclosures. We have based and will base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances. In many instances, we could have reasonably used different accounting estimates, and in other instances changes in the accounting estimates are reasonably likely to occur from period to period. Accordingly, actual results could differ significantly from the estimates made by our management. To the extent that there are material differences between these estimates and actual results, our future financial statement presentation, financial condition, results of operations and cash flows will be affected. We believe that the accounting policies discussed below are critical to understanding our historical and future performance, as these policies relate to the more significant areas involving management’s judgments and estimates.
Revenue Recognition
We recognize revenue when there is persuasive evidence that an arrangement exists with our customers, selling prices are fixed or determinable, title or risk of loss has passed, and collection is reasonably assured. Revenue is recognized upon shipment and payments are either received in advance, or net 30 days for lenses or net 14 days for accessories. Distributor arrangements include certain perfunctory acceptance provisions and a one-year warranty, from the date of shipment, that products are free from defects in material workmanship. Under such provisions customers may reject shipments via written notifications ranging from 14-45 days or exchange defective product under warranty for the same non-defective product. We have not had any significant rejected shipments or warranty claims. We do not grant price concessions to our distributors.
In 2012, we changed our commercialization strategy from exclusively using distributors to also targeting refractive laser centers equipped with femtosecond lasers, except in countries that require the use of distributors or sales representatives. We recognize revenue from laser centers based upon an analysis of the terms of each customer arrangement and upon determination that persuasive evidence of an arrangement exists, selling prices are fixed or determinable, title or risk of loss has passed, and collection is reasonably assured. Revenues from laser centers during the years ended December 31, 2014, 2013 and 2012 were not material.
Stock-Based Compensation
Prior to our initial public offering, our stock-based compensation was derived from option grants and stock awards made by Presbia Holdings, our controlling shareholder, and those expenses have been allocated to our company and reflected in our research and development, general and administrative and sales and marketing expenses.
In connection with our initial public offering, Presbia PLC made certain equity grants to certain of our directors and officers and, in the future, our option grants and stock awards will be granted by Presbia PLC. We record compensation costs related to stock options and restricted shares granted to employees based on the estimated
78
fair value of the awards on the date of grant, net of estimated forfeitures. The grant date fair value of the stock-based awards will generally be recognized on a straight-line basis over the requisite service period, which is generally the vesting period of the respective awards. Stock-based compensation related to stock options granted to non-employees is recognized as the stock options are earned. The fair value of non-employee stock options are calculated at each reporting date, until the award vests or there is a substantial disincentive for the non-employee not to perform the required services. We estimate the fair value of stock options, and the resulting stock-based compensation expense, using the Black-Scholes option-pricing model.
The Black-Scholes option-pricing model requires the use of highly subjective and complex assumptions that determine the fair value of stock-based awards, including the expected term and the price volatility of the underlying stock. These assumptions include the estimated fair value of our ordinary shares and the following additional inputs:
| • | Expected term—the expected term represents the period that the stock-based awards are expected to be outstanding. For options granted to employees, we use the simplified method to determine the expected term. The simplified method calculates the expected term as the average of the time-to-vesting and the contractual life of the options. For options granted to non-employees, we use the contractual term of 10 years as the expected term. |
| • | Expected volatility—the expected volatility will be derived from historical volatilities of unrelated publicly listed peer companies over a period approximately equal to the expected term of the award. We will look to other companies because we have limited information on the volatility of our ordinary shares due to our lack of trading history. When making the selections of our industry peer companies to be used in the volatility calculation, we will consider the size, stage in the life cycle, and financial leverage of peer companies in comparison to our company. |
| • | Risk-free interest rate—the risk-free interest rate is based on the U.S. Treasury yield in effect at the time of grant for zero coupon U.S. Treasury notes with maturities approximately equal to the expected term of the awards. |
| • | Expected dividend—the expected dividend is assumed to be zero as we have no current plans to pay any dividends on our ordinary shares. |
Prior to completion of our initial public offering in February 2015, we were required to estimate the fair value of the ordinary shares underlying our stock-based awards when performing the fair value calculations with the Black-Scholes option-pricing model. The fair value of the ordinary shares underlying our stock-based awards will be determined on each grant date by our Board or our Board’s compensation committee, with input from management. Before an active public trading market for our ordinary shares was realized through our initial public offering in February 2015, our Board or our Board’s compensation committee considered numerous objective and subjective factors, such as third party valuation studies, to determine the best estimate of the fair value of our ordinary shares.
The fair value of each stock option is based upon estimates of the fair value of the shares underlying each option as described further below. The assumptions noted in the following table provide the basis for determining the fair value of the non-employee options as of December 31, 2014 and 2013.
| YEAR ENDED DECEMBER 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| Stock price per share |
$ | 0.40 | $ | 0.54 | $ | 0.07 | ||||||
| Term |
8.8 years | 9.8 years | 7.1-7.8 years | |||||||||
| Volatility |
77 | % | 69 | % | 70 | % | ||||||
| Dividends |
— | — | — | |||||||||
| Discount rate |
2.1 | % | 2.6 | % | 1.2 | % | ||||||
79
We are also required to estimate forfeitures at the time of grant and revise those estimates in subsequent periods if actual forfeitures differ from our estimates. We use historical data to estimate pre-vesting option forfeitures and record stock-based compensation expense only for those awards that are expected to vest. To the extent that actual forfeitures differ from our estimates, the difference is recorded as a cumulative adjustment in the period the estimates are revised. Stock-based compensation expense recognized in the financial statements is based on awards that are ultimately expected to vest. Through December 31, 2014, actual forfeitures have not been material.
Stock-based compensation expense associated with stock options granted to employees and non-employees was $0.3 million, $0.5 million and $47,000 for the years ended December 31, 2014, 2013 and 2012, respectively. At December 31, 2014, we had $0.2 million of total unrecognized stock-based compensation expense related to service-based option awards which we expect to recognize over a weighted-average remaining vesting period of approximately 2.8 years. In addition, as of December 31, 2014, we had unrecognized compensation expense related to the issuance of restricted stock awards of $0.1 million, which we expect to recognize over the remaining vesting period of 3.8 years. Our stock-based compensation expense is expected to increase as a result of recognizing our existing unrecognized stock-based compensation for awards that will vest and as we issue additional stock-based awards to attract and retain our employees.
For the years ended December 31, 2014, 2013 and 2012, we allocated stock-based compensation expense as follows (in thousands of dollars):
| YEAR ENDED DECEMBER 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| Research and development |
$ | 213 | $ | 112 | $ | 33 | ||||||
| General and administrative |
81 | 373 | 14 | |||||||||
| Sales and marketing |
19 | 4 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
$ | 313 | $ | 489 | $ | 47 | ||||||
|
|
|
|
|
|
|
|||||||
Fair Value of Presbia Holdings Ordinary Shares
Prior to completion of our initial public offering in February 2015, we were required to estimate the fair value of our ordinary shares underlying our stock-based awards when performing the fair value calculations using the Black-Scholes option pricing model. In the absence of an active public trading market for our ordinary shares, on each grant date, we will estimate the fair value of our ordinary shares in order to determine an exercise price for our option grants. Presbia Holdings considered various objective and subjective factors, along with input from management and contemporaneous and retrospective valuations, to determine the fair value of its ordinary shares, including:
| • | external market conditions affecting the ophthalmic and medical device industries; |
| • | our results of operations and financial position; |
| • | the status of our commercial and sales development efforts; |
| • | the status of our research and development efforts; |
| • | the status and timing of our FDA clinical trials and regulatory affairs; |
| • | the lack of an active public market for our capital stock; and |
| • | the likelihood of achieving a liquidity event, such as an initial public offering, or sale of our company in light of prevailing market conditions. |
Valuation at December 31, 2014. The valuation method at December 31, 2014 utilized probability—weighted income and market approaches, in which it assigned 10% weight to the outcome of the income approach of $1.06
80
per share and 90% weight to the outcome of the market approach of $0.33 per share to reach a concluded value of $0.40 per share of the ordinary shares of Presbia Holdings. The income approach considers future cash flows on a discounted basis (DCF) as one indicator of current value while the market approach considers recent transactions in recent initial public offerings of similar equities, such as for those enterprises in early stages of development and in the biopharmaceutical or medical device industries. Greater weight was given to the market approach, compared to the December 31, 2013 valuation below, as the Company was nearing completion of its initial public offering, which was consummated in February 2015. The market approach measures the value of an asset through the analysis of recent sales or offerings of companies operating in the same or similar industry.
The income approach consisted of a base case scenario, which was assigned a 70% probability of success, in which the U.S. FDA clinical trials are concluded in 2017 and revenues commenced in the United States in March 2018. The income approach also assigned additional probabilities of 25% and 5% to a delayed case where entry into the U.S. market is delayed by one year to March 2019 and a pessimistic case where FDA approval is not achieved and there is no entry into the U.S. market, respectively. In each scenario, the DCF was discounted by applying a 27.5% discount rate to free cash flows generated in each of a ten-year projection period and a terminal value that represents future cash flows past 2023, the last year in the ten year projection. The weighted-average enterprise value was further discounted by 22% to account for the lack of marketability of Presbia Holdings’ ordinary shares to arrive at a concluded value of $0.40 per share. The discount rate of 27.5% applied in the DCF model is based on our stage of development. The lack of marketability discount of 22% was determined by applying a protective put option analysis reflecting the expected timing of a liquidity event of Presbia Holdings’ ordinary shares.
The market approach considers recent initial public offerings in ophthalmology and general biotech markets. The initial public offering analysis develops values of a company through the analysis of pre-money values of comparable public companies as of their respective initial public offering dates. This method provides a range of equity values as of an initial public offering date. The range of enterprise values, when attributed to the ordinary shares of Presbia Holdings, indicated an average of $0.42 per share. The same lack of marketability discount of 22% was applied to indicate a value per ordinary share of $0.33.
Valuation at December 31, 2013. The valuation method at December 31, 2013 utilized probability—weighted income and market approaches, in which it assigned an 80% weight to the outcome of the income approach of $0.54 per share and 20% weight to the outcome of the market approach of $0.55 per share to reach a concluded value of $0.54 per share of the ordinary shares of Presbia Holdings. The income approach considers future cash flows on a discounted basis (DCF) as one indicator of current value while the market approach considers recent transactions in recent initial public offerings of similar equities, such as for those enterprises in early stages of development, in the biopharmaceutical or medical device industries.
The income approach consisted of a base case scenario, which was assigned a 65% probability of success, in which the U.S. FDA clinical trials are concluded in 2018 and revenues commenced in the U.S. in March 2019. The income approach also assigned additional probabilities of 25%, 5% and 5% to a delayed case where entry into the U.S. market is delayed by one year to March 2020, a pessimistic case where FDA approval is not achieved and there is no entry into the U.S. market and an optimistic case where FDA approval is received sooner than expected with entry into the U.S. market by December 31, 2018, respectively. In each scenario, the DCF was discounted by applying a 30% discount rate to free cash flows generated in each of a ten-year projection period and a terminal value that represents future cash flows past 2023, the last year in the ten year projection. The weighted-average enterprise value was further discounted by 29% to account for the lack of marketability of Presbia Holding’s ordinary shares to arrive at a concluded value of $0.54 per share. The discount rate of 30% applied in the DCF model is based on the Company’s stage of development. The lack of marketability discount of 29% was determined by applying a protective put option analysis reflecting the expected timing of a liquidity event of the Presbia Holding’s ordinary shares. The market approach considers recent initial public offerings in ophthalmology and general biotech markets. The initial public offering analysis develops values of a company through the analysis of pre-money values of comparable public companies as of
81
their respective initial public offering dates. This method provides a range of equity values as of an initial public offering date. The range of enterprise values, when attributed to the ordinary shares of Presbia Holdings, indicated an average of $0.78 per share. The same lack of marketability discount of 29% was applied to indicate a value per ordinary share of $0.55.
Valuation at September 1, 2013 and Prior. The valuation method used at September 1, 2013, each of December 31, 2012, 2011 and 2010, and February 1, 2010, the date Presbia Holdings first granted share options, was a historical cost method. For these valuations, consideration was given to three generally recognized valuation approaches—the income, market and cost valuation approaches. The cost approach was ultimately used to value the ordinary shares. Since our operations were in such an early stage, as evidenced by the recognition of insignificant revenues, and because we and Presbia Holdings lacked arm’s length third party financings, use of the cost approach was determined to be the best technique. Under this approach, the estimated fair value of the ordinary shares was derived by applying a rate of return consistent with a development stage entity to the historical expenses associated with our development stage activities. The estimated fair value of the enterprise was then allocated to the ordinary shares after consideration of all classes of capital stock and debt, if any, and then reduced by a discount for lack of marketability. The significant assumptions under this approach reflected in these valuations include a rate of return of 50%, which was determined based upon the early development stage, and a 27% discount for lack of marketability based upon quantitative analysis using a protective put analysis reflecting the expected timing of a liquidity event on the valuation dates. For each of the valuations, a study of peer group public companies in the medical device industry was performed to estimate the volatility of Presbia Holdings’ ordinary shares, which was estimated to be in the range of 70% for the periods covered by the valuations. These valuations resulted in estimated fair values of Presbia Holdings’ ordinary shares ranging from $0.01 per share in 2010 to $0.08 per share in September 2013.
A combination of income and market approaches was utilized in the valuation of Presbia Holdings’ ordinary shares as of December 31, 2013 rather than the cost method previously used primarily due to significant developments in its business that occurred during the fourth quarter of 2013, including FDA approval to commence immediately a pivotal trial in the United States using a staged enrollment and the hiring of key executives in marketing, engineering, operations and finance.
Compensation Cost of 2013 Awards. During 2010, Presbia Holdings granted options to employees and non-employees to purchase 4,490,000 of its ordinary shares at exercise prices ranging from $0.01 to $0.049 per share. In October 2013, the 4,180,000 options granted in 2010 that remained outstanding were canceled and replaced with an equivalent number of share options with an exercise price of $0.08 per share. Also, in October 2013, Presbia Holdings granted options to employees and non-employees to purchase 900,000 of its ordinary shares at an exercise price of $0.30 per share and an aggregate 1,500,000 shares of its restricted ordinary shares to Zohar Loshitzer and two other board members. For purposes of recognizing stock-based compensation, the grant-date fair value of all of our October 2013 option awards, the determination that there was no incremental fair value for the modification of original 2010 awards replaced by the 4,108,000 awards in 2013, both of which were measured using the Black-Scholes option-pricing model, and the estimated fair value of the restricted stock awards granted in October 2013, used an estimated fair value of $0.30 per share. As of December 31, 2013, when we re-measured the fair value of our non-employee awards using the Black-Scholes option-pricing model, we used an estimated fair value of $0.54 per share based on the valuation at December 31, 2013.
Research and Development Expenses
We recognize research and development expenses as they are incurred. With respect to capital expenditures for property and equipment used in conducting research and development activities, these costs are generally capitalized on the balance sheet as part of property and equipment and depreciated over their useful lives to research and development expense provided these assets have future alternative uses.
During the first quarter of 2014, we began incurring costs in connection with the FDA staged-enrollment pivotal clinical trial, which is expected to continue into 2017 or later. During this trial, we will continue to incur costs for
82
patient recruiting, acquisition of clinical test equipment to be used in the trial, outside experts to read and interpret the results of the studies, third party costs to monitor the investigational sites and perform data collection activities and surgeon and patient fees in connection with surgical procedures and follow-up visits. Our policy with respect to the recognition of these expenses is to record such expenses as research and development expense in the period in which the services are provided. We will evaluate the purchases of clinical test equipment, on a case by case basis, to determine if there exists an alternative use for the equipment following the clinical trial. In the event we determine that there is no alternative use for the test equipment, then that cost will be expensed as part of research and development expense in the period in which we take title to the equipment from the supplier.
Impairment of Long-Lived Assets
We review the recoverability of long-lived and finite-lived intangible assets when circumstances indicate that the carrying amount of assets might not be recoverable. This evaluation compares the carrying value of the long-lived asset to the undiscounted cash flow projections associated with an asset or group of assets. In the event undiscounted cash flow projections indicate impairment, we would record an impairment loss on the statements of operations in the period in which the impairment occurred and adjust the carrying value of the asset or group of assets to its fair value.
Income Taxes
Deferred income tax assets and liabilities are recorded for the expected future tax consequences of temporary differences between the financial statement carrying amounts and the income tax basis of assets and liabilities. A valuation allowance is recorded against all of our net deferred tax asset balance due to uncertainties related to the realizability of our deferred tax assets as a result of our history of operating losses.
Segment Information
In accordance with generally accepted accounting principles, we identify operating segments as components or elements of an enterprise about which separate discrete financial information is available for evaluation by the chief operating decision-maker in making decisions regarding resource allocation and assessing performance. To date, the Company has viewed its operations and manages its business as one segment.
Results of Operations
Comparison of the Years Ended December 31, 2014 and 2013
| YEAR ENDED DECEMBER 31, | ||||||||||||
| 2014 | 2013 | Change | ||||||||||
| Revenues |
$ | 161 | $ | 98 | $ | 63 | ||||||
| Cost of goods sold |
61 | 111 | (50 | ) | ||||||||
| Operating expenses: |
||||||||||||
| Research and development |
4,243 | 2,136 | 2,107 | |||||||||
| Sales and marketing |
1,652 | 1,044 | 608 | |||||||||
| General and administrative |
7,577 | 4,088 | 3,489 | |||||||||
|
|
|
|
|
|
|
|||||||
| Operating loss |
(13,372 | ) | (7,281 | ) | (6,091 | ) | ||||||
| Interest expense |
2,288 | 2,161 | 127 | |||||||||
| Other expense |
(6 | ) | — | (6 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Loss before income tax provision |
(15,654 | ) | (9,442 | ) | (6,212 | ) | ||||||
| Income tax provision |
10 | 20 | (10 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
$ | (15,664 | ) | $ | (9,462 | ) | $ | (6,202 | ) | |||
|
|
|
|
|
|
|
|||||||
83
Revenue
Revenue for the year ended December 31, 2014 was $161,000 as compared to $98,000 in 2013. Revenues were insignificant in both periods due to the transitioning of the sales strategy from a solely distributor-based strategy to refractive laser centers that began in 2012. Until we receive FDA approval to sell and market our microlens within the United States, we are focusing our sales and marketing resources to sell our microlens to refractive laser centers outside the United States. Sales in 2014 and 2013 were to existing customers representing refractive laser centers outside of the United States.
Cost of Goods Sold
Cost of goods sold was $61,000 in the year ended December 31, 2014 as compared to $111,000 in the year ended December 31, 2013. During the year ended December 31, 2014, cost of goods sold consisted of $12,000 for cost of units shipped to customers, $61,000 for provision of inventory obsolescence, partially offset by $12,000 for favorable inventory adjustments. For the year ended December 31, 2013, cost of goods sold included the cost of units shipped to customers and inventory write-downs of $56,000.
Research and Development
Research and development expense increased by $2.1 million, or 99%, for the year ended December 31, 2014 as compared to 2013, due to increased expenditures in principally three areas: (i) a $417,000 increase in expenses related to our pilot manufacturing facility; (ii) a $1.2 million cost increase related to our U.S. staged pivotal clinical trial that commenced in 2014, which consists of costs incurred to establish and monitor the initial six investigational sites, costs related to the recruitment of the 75 subjects enrolled in the first stage of the trial and purchases of equipment used to monitor patient outcomes, and (iii) product development costs, which were higher by $469,000 due to additional personnel costs of $305,000, increased professional fees of $94,000 related to intellectual property filings and other overhead costs of $70,000. In February 2015, we received approval from the FDA to commence the second phase of the staged pivotal trial.
Sales and Marketing
Sales and marketing expense increased by $0.6 million, or 58%, for the year ended December 31, 2014 as compared to the year ended December 31, 2013. We are not authorized to sell our microlens in the United States until we receive FDA approval to do so; therefore, our sales and marketing activities are limited to developing the markets outside the United States. During the year ended December 31, 2014, we incurred higher costs than for the same period in 2013 for sales and marketing personnel of $262,000, an increase of 49%, and higher travel expenses related to supporting clients outside the United States of $343,000, an increase of 496%, and a slight increase in other marketing costs of $5,000. We expect sales and marketing costs to increase throughout 2015 and into 2016 as we continue to develop the outside United States (OUS) market.
General and Administrative
General and administrative expenses increased by $3.5 million, or 85%, in the year ended December 31, 2014 as compared to the year ended December 31, 2013 due primarily to the recognition of $3.4 million of deferred offering costs related to our initial public offering as general and administrative expenses. These costs were previously classified on the balance sheet in other assets commencing in the third quarter of 2013 and continuing into the third quarter of 2014. This expense consists primarily of legal and audit professional fees and printing costs in connection with our initial public offering that was delayed during the second and third quarters of 2014 for a period that exceeded 90 days. As a result of this delay, the accumulated costs recognized prior to September 2014 were reclassified as expense. The increase in general and administrative costs also included higher personnel related costs of $0.2 million, or 12%, relating to finance, information technology, operations and administration costs, offset by a $0.1 million decrease in other operating expenses, including telephone, facility, Internet, travel and supply costs.
84
Interest and Other Expense
Interest and other expense for the year ended December 31, 2014 increased $121,000, or 6%, as compared to the year ended December 31, 2013. The change is due primarily to the October 2013 contribution of $12.2 million of indebtedness from Presbia Holdings to certain of its subsidiaries as part of the 2013 Restructuring, which resulted in less principal due to Presbia Holdings and less accrued interest in the year ended December 31, 2013. In addition, the change resulted from accrued interest on total additional borrowings of $13.0 million from Presbia Holdings, comprised of $11.7 million and $1.3 million in borrowings, respectively, during the year ended December 31, 2014 and the period from the 2013 Restructuring to December 31, 2013. Through November 2014, interest accrued on principal due to Presbia Holdings at an annual rate of 15% compounded daily. In November 2014, the Company entered into an Interest Rate Agreement wherein any borrowings shall accrue interest at a rate equal to the then applicable monthly federal rate of interest for short-term loans, adjusted monthly, compounding daily. For additional information, see discussion above under the caption “ Factors Affecting Our Expenses; Interest Expense. “
Net Loss
Our net loss of $15.7 million for the year ended December 31, 2014 was $6.2 million greater, or 66% greater, than the loss in the corresponding period of 2013 of $9.5 million, due primarily to increased operating expenses of $2.7 million, the write off of the deferred offering costs of $3.4 million and $0.1 million increase in interest expense. We expect that losses will continue through 2015 and 2016, and possibly further, due to anticipated costs related to our U.S. staged pivotal clinical trial and ongoing costs required to develop the market outside of the United States for our microlens.
Comparison of the Years Ended December 31, 2013 and 2012
| YEAR ENDED DECEMBER 31, | ||||||||||||
| 2013 | 2012 | Change | ||||||||||
| Revenues |
$ | 98 | $ | 95 | $ | 3 | ||||||
| Cost of goods sold |
111 | 71 | 40 | |||||||||
| Operating expenses: |
||||||||||||
| Research and development |
2,136 | 1,024 | 1,112 | |||||||||
| Sales and marketing |
1,044 | 632 | 412 | |||||||||
| General and administrative |
4,088 | 1,957 | 2,131 | |||||||||
|
|
|
|
|
|
|
|||||||
| Operating loss |
(7,281 | ) | (3,589 | ) | (3,692 | ) | ||||||
| Interest expense |
2,161 | 1,458 | 703 | |||||||||
|
|
|
|
|
|
|
|||||||
| Loss before income tax provision |
(9,442 | ) | (5,047 | ) | (4,395 | ) | ||||||
| Income tax provision |
20 | — | 20 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
$ | (9,462 | ) | $ | (5,047 | ) | $ | (4,415 | ) | |||
|
|
|
|
|
|
|
|||||||
Revenue
Revenue in 2013 was $98,000, representing an increase of $3,000, or 3.2%, when compared to 2012. During 2013, we transitioned our sales and marketing plan from an exclusive distributor-based strategy to a selling strategy that focuses on sales to refractive laser centers in those countries outside the United States where our microlens is approved. Approximately $75,000 of our sales in 2013 were to laser centers and $23,000 of our sales in 2013 represented sales to distributors. In certain countries outside the United States, a local distributor is the only way to market our product into those countries. In 2012, all of our sales were distributor-based revenues. Also, in 2012, we terminated our arrangement with a distributor and repurchased all of our products held by the distributor on the termination date for $30,000, an amount equal to the sales price we recognized in 2011 upon the original sale to the distributor. As a result of the repurchase, revenues for the year ended December 31, 2012 were reduced by $30,000.
85
Cost of Goods Sold
Our cost of goods sold, which increased by $40,000, or 56%, was insignificant in each of the past two fiscal years. Although our cost of goods sold will increase as revenues increase, we do not expect cost of goods sold to be a principal expense driver for us until such time as our revenues become substantial. Cost of goods sold includes the purchased cost of the units that were shipped to our customers and inventory write-downs reflecting excess quantities on hand. In 2012 and 2013, we recorded write-downs of $22,000 and $56,000, respectively, based on the age of the lens inventory and refractive mix of the inventory.
Research and Development
Research and development expenses increased significantly, by $1,112,000, or 109%, from the year ended December 31, 2012 to the year ended December 31, 2013, reflecting higher costs related to stock-based compensation costs of approximately $0.1 million, the startup costs and related operating expenses of our U.S. manufacturing facility that support the 2014 launch of our FDA clinical trials of approximately $0.4 million and $0.5 million for evaluation studies and new product development.
Sales and Marketing
Our sales and marketing expenses increased by $412,000, or 65%, from the year ended December 31, 2012 to the year ended December 31, 2013. The increase in sales and marketing expenses reflected additional expenses incurred in developing a refractive laser center commercial strategy.
General and Administrative
General and administrative expenses increased by $2,131,000, or 109%, between the year ended December 31, 2012 and the year ended December 31, 2013, as a result of organizational costs of $0.5 million incurred to complete the 2013 Restructuring which resulted in the formation of Presbia Ireland, Limited, stock-based compensation expense increase of approximately $0.4 million, audit fees related to fiscal year 2012 and prior years of approximately $0.3 million and higher costs of $0.9 million related to increased costs of additional staff and related costs associated with the development of our company.
Interest Expense
Interest expense increased by approximately $703,000, or 48%, from the year ended December 31, 2012 to the year ended December 31, 2013 due to additional funding from our controlling shareholder of $5.4 million offset partially by the 2013 Restructuring that converted approximately $12.2 million of debt due to Presbia Holdings to equity.
Net Loss
Our net loss increased from $5.0 million during the year ended December 31, 2012 to $9.5 million during the year ended December 31, 2013, reflecting the increase in research and development expenses associated with the increase in clinical trial expenses during 2013 and the increase in general and administrative expenses discussed above.
Liquidity and Capital Resources
On February 3, 2015, we closed the initial public offering of our ordinary shares. We sold a total of 4,166,667 ordinary shares in the offering at a public offering price of $10.00 per share. The aggregate proceeds from our initial public offering was $41.7 million, and we received net proceeds of approximately $36.6 million from the offering, after deducting $5.1 million of underwriting discounts and commissions and estimated offering expenses payable by us.
86
As of December 31, 2014, we have generated an accumulated deficit of $37.4 million. We funded operations through November 2014 by means of borrowings from Presbia Holdings and at an interest cost of 15% per annum, compounding daily. From November 2014 to the consummation of our initial public offering, we funded our operations through borrowings from Presbia Holdings, at an interest rate equal to the then applicable monthly federal rate of interest for short-term loans, adjusted monthly, compounding daily.
On November 30, 2014, as part of the 2014 Debt Conversion, Presbia Holdings converted all the remaining indebtedness owed to Presbia Holdings by certain subsidiaries of Presbia Ireland, Limited at that time to equity. In the 2014 Debt Conversion, approximately $23.5 million of outstanding intercompany debt owed to Presbia Holdings was converted to equity of such subsidiaries. On January 14, 2015, as part of the 2015 Debt Conversion, Presbia Holdings converted all the remaining indebtedness owed to Presbia Holdings by a subsidiary of Presbia Ireland, Limited at that time to equity. In the 2015 Debt Conversion, approximately $1.6 million of outstanding intercompany debt owed to Presbia Holdings was converted to equity of such subsidiary.
Our primary uses of cash are to fund operating expenses, primarily general and administrative expenditures and research and development expenditures. Cash used to fund operating expenses is impacted by the timing of when we pay these expenses, as reflected in the change in our outstanding accounts payable and accrued expenses.
Our future capital requirements are difficult to forecast and will depend on many factors, including:
| • | the initiation, progress, timing and completion of clinical trials for our products; |
| • | the number and characteristics of products that we pursue; |
| • | the progress, costs and results of our clinical trials; |
| • | the outcome, timing and cost of regulatory approvals; and |
| • | delays that may be caused by changing regulatory requirements. |
The following table summarizes our cash flows for the periods indicated (in thousands):
| YEAR ENDED DECEMBER 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| Net cash used in operating activities |
$ | (9,514 | ) | $ | (4,821 | ) | $ | (3,044 | ) | |||
| Net cash used in investing activities |
(179 | ) | (147 | ) | (694 | ) | ||||||
| Net cash provided by financing activities |
9,247 | 5,376 | 3,760 | |||||||||
Prior to February 2015, we relied on funding from Presbia Holdings to fund our all of our operations including offering costs to complete the our initial public offering on February 3, 2015. At December 31, 2014, we had an accumulated deficit of approximately $37.4 million and we expect to incur additional operating losses during fiscal years 2015 and 2016, and possibly further. As we continue to incur losses, our transition to profitability will depend on the successful development, approval and commercialization of our microlens. We may never achieve profitability, and unless and until we do, we will need to continue to raise additional capital. With existing cash at December 31, 2014 and the proceeds from the our initial public offering, we expect to be able to fund our operations and have sufficient cash reserves for the next 12 months.
Contractual Obligations and Other Commitments
The following table summarizes our contractual obligations as of December 31, 2014 (in thousands).
| CONTRACTUAL OBLIGATIONS | LESS THAN 1 YEAR |
1 TO 3 YEARS |
3 TO 5 YEARS |
MORE THAN 5 YEARS |
TOTAL | |||||||||||||||
| Facility leases |
$ | 195 | $ | 165 | $ | 50 | $ | — | $ | 410 | ||||||||||
| Payable due to Presbia Holdings(1) |
— | (1) | — | (1) | — | (1) | — | (1) | 417 | (1) | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total contractual obligations |
$ | 195 | $ | 165 | $ | 50 | $ | — | $ | 817 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (1) | This debt had no fixed maturity. This debt and all additional debt owed to Presbia Holding incurred since December 31, 2014 was converted to equity upon the consummation of the 2014-2015 Restructuring. |
87
Off-Balance Sheet Arrangements
We have not entered into any off-balance sheet arrangements and do not have any holdings in variable interest entities.
Recent Accounting Pronouncements
In May 2014, the Financial Accounting Standards Board (“FASB”) and the International Accounting Standard Board (“IASB”) jointly issued a new revenue recognition standard that will supersede nearly all existing revenue recognition guidance under GAAP. The revenue recognition standard will allow for the recognition of revenue when a company transfers promised goods or services to customers in an amount that reflects the consideration to which the company expects to be entitled in exchange for those goods or services. The standard is effective for public entities for annual and interim periods beginning after December 15, 2016. Early adoption is not permitted under GAAP. We are currently evaluating the impact that the adoption of this guidance will have on our consolidated financial statements.
On June 10, 2014, the FASB issued Accounting Standards Update No. 2014-10 (ASU 2014-10), which eliminates the definition of a development stage entity, eliminates the development stage presentation and disclosure requirements under ASC 915, and amends provisions of existing variable interest entity guidance under ASC 810. As a result of the changes, the financial statements of entities which meet the former definition of a development stage entity will no longer include the following:
| • | Inception-to-date income, cash flow and equity information |
| • | Label indicating that the financial statements are those of a development stage entity |
| • | Disclosures of the nature of the entity’s development stage activities as well as the first year in which the entity is no longer considered a development stage entity |
Additionally, ASU 2014-10 clarified that the lack of commencement of principal operations represents a risk and uncertainty under ASC 275 and, accordingly, the financial statements should reflect appropriate disclosures.
Finally, variable interest entity rules no longer contain an exception for development stage entities and, as a result, development stage entities will have to be evaluated for consolidation in the same manner as non-development stage entities.
Public entities are no longer required to apply the presentation and disclosure provisions of ASC 915 during annual periods beginning after December 15, 2014, and the revisions to the consolidation standards are effective for annual periods beginning after December 15, 2015. We adopted this standard during the fourth quarter of 2014. The adoption of this guidance impacted the financial statement presentation, but did not have a material impact on our financial position or results of operations and cash flows.
The FASB issued ASU 2014-15 on August 27, 2014, providing guidance on determining when and how to disclose going-concern uncertainties in the financial statements. The new standard requires management to perform interim and annual assessments of an entity’s ability to continue as a going concern within one year of the date the financial statements are issued. An entity must provide certain disclosures if “conditions or events raise substantial doubt about the entity’s ability to continue as a going concern.” The ASU applies to all entities and is effective for annual periods ending after December 15, 2016, and interim periods thereafter, with early adoption permitted. We are currently evaluating the impact that the adoption of this guidance will have on our consolidated financial statements.
88
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
We are exposed to market risks in the ordinary course of our business. These risks primarily include interest rate and foreign exchange sensitivities as follows:
Interest Rate Risk
We had cash of $138,000 and $584,000 as of December 31, 2014 and 2013, respectively, which consists of checking account deposits. Historically, we have maintained minimal cash balances. Our levels of interest income on the unused balances will continue to be subject to fluctuations in market interest rates.
We do not enter into investments for trading or speculative purposes and have not used any derivative financial instruments to manage our interest rate risk exposure. We have not been exposed nor do we anticipate being exposed to material risks due to changes in interest rates. A hypothetical 10% change in interest rates during any of the periods presented would not have had a material impact on our financial statements.
Through November 30, 2014, we incurred interest expense at a fixed rate of 15% per annum, compounding daily, on amounts advanced by our controlling shareholder. From December 1, 2014 to our initial public offering, we funded our operations through borrowings from Presbia Holdings, at an interest rate equal to the then applicable monthly federal rate of interest for short-term loans, adjusted monthly, compounding daily.
Transaction Risk
We may be exposed to transaction risk because some of our expenses will be incurred in a different currency than our reporting currency, the U.S. Dollar. To date, we have not attempted to offset our exposure to this risk by investing in derivatives or engaging in other hedging transactions.
89
Item 8. Financial Statements and Supplementary Data.
INDEX TO FINANCIAL STATEMENTS
| PAGE | ||||
| PRESBIA PLC |
||||
| Consolidated Financial Statements: |
||||
| 91 | ||||
| Consolidated Balance Sheets as of December 31, 2014 and 2013 |
92 | |||
| 93 | ||||
| Consolidated Statement of Shareholders’ Deficit for the Years ended December 31, 2014, 2013 and 2012 |
94 | |||
| Consolidated Statements of Cash Flows for the Years Ended December 31, 2014, 2013 and 2012 |
95 | |||
| 96 | ||||
90
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of Presbia PLC
Dublin, Ireland
We have audited the accompanying consolidated balance sheets of Presbia PLC, an Irish public limited company, (the “Company”) as of December 31, 2014 and 2013, and the related consolidated statements of operations and comprehensive loss, shareholders’ deficit, and cash flows for each of the three years ended December 31, 2014. Our audits also included the financial statement schedule listed in the index at Item 15(a)(2) of the Company’s Annual Report on Form 10-K. These financial statements and the financial statement schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements and the financial statement schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, such consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2014 and 2013, and the results of its operations and its cash flows for each of the three years ended December 31, 2014 in conformity with accounting principles generally accepted in the United States of America. Also, in our opinion, such financial statement schedule, when considered in relation to the basic consolidated financial statements taken as a whole, presents fairly, in all material respects, the information set forth therein.
As discussed in Note 1 to the consolidated financial statements, the financial statements include allocations of expenses from Presbia Holdings, the Company’s ultimate controlling shareholder. These allocations may not be reflective of the actual level of costs or debt which would have been incurred had the Company operated as a separate entity apart from Presbia Holdings. Also as disclosed in Note 9, the Company has arrangements with related parties, including Presbia Holdings.
/s/ Deloitte & Touche LLP
Los Angeles, California
March 31, 2015
91
CONSOLIDATED FINANCIAL STATEMENTS
PRESBIA PLC
(in thousands)
| DECEMBER 31, | ||||||||
| 2014 | 2013 | |||||||
| Assets |
||||||||
| Current assets |
||||||||
| Cash |
$ | 138 | $ | 584 | ||||
| Accounts receivable |
25 | 20 | ||||||
| Inventory |
378 | 199 | ||||||
| Prepaid expenses and other current assets |
122 | 146 | ||||||
|
|
|
|
|
|||||
| Total current assets |
663 | 949 | ||||||
| Property and equipment, net |
747 | 820 | ||||||
| Intangible asset |
46 | 3 | ||||||
| Other assets |
886 | 1,993 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 2,342 | $ | 3,765 | ||||
|
|
|
|
|
|||||
| Liabilities and shareholders’ deficit |
||||||||
| Current liabilities |
||||||||
| Accounts payable |
$ | 1,882 | $ | 2,652 | ||||
| Due to related parties |
12 | 132 | ||||||
| Other current liabilities |
700 | 405 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
2,594 | 3,189 | ||||||
| Payable due to the Parent |
417 | 9,425 | ||||||
| Deferred rent |
37 | 33 | ||||||
|
|
|
|
|
|||||
| Total liabilities |
3,048 | 12,647 | ||||||
| Commitments and contingencies (note 11) |
||||||||
| Shareholder’s deficit |
||||||||
| Euro ordinary shares |
||||||||
| One Euro (US$1.35) par value, 40,000 shares authorized, 40,000 and 0 issued and outstanding at December 31, 2014 and 2013, respectively |
54 | — | ||||||
| Capital |
36,626 | 12,840 | ||||||
| Accumulated deficit |
(37,386 | ) | (21,722 | ) | ||||
|
|
|
|
|
|||||
| Total shareholder’s deficit |
(706 | ) | (8,882 | ) | ||||
|
|
|
|
|
|||||
| Total liabilities and shareholders’ deficit |
$ | 2,342 | $ | 3,765 | ||||
|
|
|
|
|
|||||
The accompanying notes are an integral part of these Consolidated Financial Statements
92
PRESBIA PLC
Consolidated Statements of Operations and Comprehensive Loss
(in thousands, except share and per share data)
| YEAR ENDED DECEMBER 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| Revenues |
$ | 161 | $ | 98 | $ | 95 | ||||||
| Cost of goods sold |
61 | 111 | 71 | |||||||||
|
|
|
|
|
|
|
|||||||
| Gross profit (loss) |
100 | (13 | ) | 24 | ||||||||
| Operating expenses: |
||||||||||||
| Research and development |
4,243 | 2,136 | 1,024 | |||||||||
| Sales and marketing |
1,652 | 1,044 | 632 | |||||||||
| General and administrative |
7,577 | 4,088 | 1,957 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
13,472 | 7,268 | 3,613 | |||||||||
|
|
|
|
|
|
|
|||||||
| Operating loss |
(13,372 | ) | (7,281 | ) | (3,589 | ) | ||||||
| Interest expense |
2,288 | 2,161 | 1,458 | |||||||||
| Other income |
6 | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Loss before income tax provision |
(15,654 | ) | (9,442 | ) | (5,047 | ) | ||||||
| Income tax provision |
10 | 20 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
(15,664 | ) | (9,462 | ) | (5,047 | ) | ||||||
| Other comprehensive loss |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total comprehensive loss |
$ | (15,664 | ) | $ | (9,462 | ) | $ | (5,047 | ) | |||
|
|
|
|
|
|
|
|||||||
| Net loss per share-basic and diluted |
$ | (1.71 | ) | $ | (1.03 | ) | $ | (0.55 | ) | |||
|
|
|
|
|
|
|
|||||||
| Shares used to compute basic and diluted net loss per share |
9,166,667 | 9,166,667 | 9,166,667 | |||||||||
|
|
|
|
|
|
|
|||||||
The accompanying notes are an integral part of these Consolidated Financial Statements
93
PRESBIA PLC
Consolidated Statement of Shareholders’ Deficit
(in thousands, except share data)
| Euro Ordinary Shares |
Accumulated Deficit |
|||||||||||||||||||
| Shares | Amount | Capital | Total | |||||||||||||||||
| Balance, December 31, 2011 |
— | $ | — | $ | 91 | $ | (7,213 | ) | $ | (7,122 | ) | |||||||||
| Stock-based compensation allocated from the Parent |
— | — | 47 | — | 47 | |||||||||||||||
| Net loss |
— | — | — | (5,047 | ) | (5,047 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balance, December 31, 2012 |
— | — | 138 | (12,260 | ) | (12,122 | ) | |||||||||||||
| Stock-based compensation allocated from Parent |
— | — | 489 | — | 489 | |||||||||||||||
| Capitalization of amounts due to Parent Pursuant to 2013 Restructuring of Presbia Ireland, Limited. |
— | — | 12,213 | — | 12,213 | |||||||||||||||
| Net loss |
— | — | — | (9,462 | ) | (9,462 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balance, December 31, 2013 |
— | — | 12,840 | (21,722 | ) | (8,882 | ) | |||||||||||||
| Stock-based compensation allocated from the Parent |
313 | — | 313 | |||||||||||||||||
| Capitalization of Presbia PLC |
40,000 | 54 | — | — | 54 | |||||||||||||||
| Capitalization of amounts due to Parent Pursuant to 2014 Debt Conversion of Presbia Holdings |
— | — | 23,473 | — | 23,473 | |||||||||||||||
| Net loss |
— | — | — | (15,664 | ) | (15,664 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balance, December 31, 2014 |
40,000 | $ | 54 | $ | 36,626 | $ | (37,386 | ) | $ | (706 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
The accompanying notes are an integral part of these Consolidated Financial Statements
94
Consolidated Statements of Cash Flows
(in thousands)
| YEAR ENDED DECEMBER 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| Cash flow from operating activities: |
||||||||||||
| Net loss |
$ | (15,664 | ) | $ | (9,462 | ) | $ | (5,047 | ) | |||
| Adjustments to reconcile net loss to cash used in operating activities: |
||||||||||||
| Depreciation |
135 | 91 | 29 | |||||||||
| Amortization |
3 | 5 | 5 | |||||||||
| Inventory provisions |
61 | 56 | 22 | |||||||||
| Write-off of deferred offering costs |
3,369 | — | — | |||||||||
| Stock-based compensation allocated from the Parent |
313 | 489 | 47 | |||||||||
| Non-cash interest expense on funding from the Parent |
2,288 | 2,161 | 1,458 | |||||||||
| Non-cash operating expenses allocated from the Parent |
183 | 1,016 | 500 | |||||||||
| Changes in operating assets and liabilities: |
||||||||||||
| Accounts receivable |
(5 | ) | (10 | ) | 37 | |||||||
| Inventory |
(239 | ) | (56 | ) | (85 | ) | ||||||
| Prepaid expenses and other current assets |
23 | 34 | (136 | ) | ||||||||
| Other assets |
(9 | ) | 50 | (80 | ) | |||||||
| Accounts payable and other current liabilities |
136 | 755 | 121 | |||||||||
| Income taxes payable |
8 | — | — | |||||||||
| Deferred rent |
4 | (2 | ) | 28 | ||||||||
| Due to related parties |
(120 | ) | 52 | 57 | ||||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in operating activities |
(9,514 | ) | (4,821 | ) | (3,044 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Cash flow from investing activities: |
||||||||||||
| Website development costs |
(46 | ) | — | — | ||||||||
| Purchase of property and equipment |
(133 | ) | (147 | ) | (694 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in investing activities |
(179 | ) | (147 | ) | (694 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Cash flow from financing activities: |
||||||||||||
| Deferred offering costs |
(2,151 | ) | (42 | ) | — | |||||||
| Capitalization of Presbia PLC |
54 | — | — | |||||||||
| Funding from the Parent |
11,344 | 5,418 | 3,760 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by financing activities |
9,247 | 5,376 | 3,760 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net increase (decrease) in cash |
(446 | ) | 408 | 22 | ||||||||
| Cash balance at beginning of period |
584 | 176 | 154 | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash balance at end of period |
$ | 138 | $ | 584 | $ | 176 | ||||||
|
|
|
|
|
|
|
|||||||
| Supplemental disclosure of cash flow information: |
||||||||||||
| Cash paid for income taxes |
6 | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Supplemental disclosures of non-cash investing and financing activities: |
||||||||||||
| Purchase of property and equipment included in accounts payable |
— | 46 | 24 | |||||||||
|
|
|
|
|
|
|
|||||||
| Allocated operating expenses funded by the Parent |
183 | 1,016 | 500 | |||||||||
|
|
|
|
|
|
|
|||||||
| Deferred offering costs included in accounts payable and other current liabilities |
1,365 | 1,922 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Capitalization of amounts due to Parent persuant to the 2013 Restructuring |
— | 12,213 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Capitalization of amounts due to Parent persuant to the 2014 Debt Conversion |
23,473 | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
The accompanying notes are an integral part of these Consolidated Financial Statements
95
PRESBIA PLC
Notes to the Consolidated Financial Statements
(1) Basis of Presentation and Description of the Business
Presbia PLC (the “Company” or “Presbia PLC”), an Irish public limited company, was formed on February 6, 2014 through the issuance of 40,000 shares for 40,000 Euros (approximately $54,000) for the purpose of consummating an initial public offering (“IPO”) of its ordinary shares. Presbia PLC’s ultimate controlling shareholder, Presbia Holdings (the “Parent”), was organized in the Cayman Islands in 2007 as an exempted company with limited liability. On October 21, 2013, the Parent completed a restructuring (the “2013 Restructuring”) which involved the formation on September 13, 2013 of an interim holding company, Presbia Ireland, Limited, and the contribution by the Parent of 100% of its direct and indirect ownership interests in its business, assets and subsidiaries to Presbia Ireland, Limited.
On November 30, 2014, Presbia Holdings converted all the remaining indebtedness owed to Presbia Holdings by certain subsidiaries of Presbia Ireland, Limited at that time to equity (“2014 Debt Conversion”). In the 2014 Debt Conversion, approximately $23.5 million of outstanding intercompany debt owed to Presbia Holdings was converted to equity of Presbia Ireland, Limited. On January 14, 2015, Presbia Holdings converted all the remaining indebtedness owed to Presbia Holdings by a subsidiary of Presbia Ireland, Limited at that time to equity (the “2015 Debt Conversion”) in the amount of approximately $1.6 million.
On January 14, 2015, the Parent contributed all the share capital in issue in Presbia Ireland, Limited to Presbia PLC (the “2015 Capital Contribution”) in exchange for 9,166,667 ordinary shares of Presbia PLC. The aggregate 9,166,667 ordinary shares of Presbia PLC have been reflected as issued and outstanding as of the earliest date of the periods presented for purposes of computation of basic and diluted net loss per share.
On February 3, 2015, Presbia PLC completed its initial public offering (“IPO”) of 4,166,667 of its ordinary shares at a price to the public of $10.00 per ordinary share and commenced trading on The NASDAQ Global Market under the symbol LENS (See Note 14). The Parent acquired 500,000 ordinary shares from the public offering. The net proceeds from the IPO consisted of aggregate gross proceeds of approximately $41.7 million less underwriting discounts and commissions of approximately $2.9 million and other issuance costs of approximately $2.2 million resulting in net proceeds of approximately $36.6 million.
The accompanying consolidated financial statements have been derived from the historical cost basis of the assets and liabilities, financial condition and cash flows of Presbia PLC, Presbia Ireland, Limited, organized in Ireland, and Presbia USA, Inc., and OPL, LLC. Presbia USA, Inc. and OPL, LLC are both entities organized in the United States, and include Presbia USA, Inc.’s subsidiaries, Visitome, Inc. and PresbiBio, LLC, both organized in the United States, and OPL, LLC’s direct and indirect subsidiaries, PIP Holdings, C.V and Presbia Cooperatief U.A., both organized in the Netherlands, and PresbiOptical LLC, organized in the United States (collectively, including Presbia PLC, the “Company”). The accompanying consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America. The Company’s fiscal year ends on December 31. The entities presented in the consolidated financial statements have been under common control during the periods presented. All intercompany accounts have been eliminated in consolidation.
The Company, which began to be formed on June 29, 2007, is developing and marketing a surgical solution to the age-related vision condition known as presbyopia. The Company’s primary objectives have been twofold: (1) achieve commercial success in those parts of the world where the Company’s surgical procedure has been approved by regulatory agencies and (2) successfully apply to the United States Food and Drug Administration (the “FDA”) for the authorization to market and manufacture the surgical procedure in the United States. This procedure is currently not authorized by the FDA and may not be manufactured, sold, distributed or surgically performed on any individual in the United States. The Company’s principal revenue generating activity is the sale of the Company’s microlens to approved ophthalmologist physicians who, in turn, perform the surgical
96
procedure on their patient base. Presbia is authorized to sell, market and perform this procedure in 38 countries through its wholly-owned Dutch subsidiary, Presbia Cooperatief U.A. Activities to-date have consisted primarily of research and development.
Stock-based compensation expenses recognized by the Parent that were incurred to benefit the Company have been allocated to the Company (see Note 8). In December 2012, an entity affiliated with the Parent’s chairman paid cash of $100,000 to settle litigation involving various parties to the litigation, including the Parent and Visitome Inc. The litigation settlement in the amount of $100,000 is reflected as general and administrative expense in the Company’s statement of operations for the year ended December 31, 2012 (see Note 9). The financial information in these statements may not include all of the expenses that would have been incurred had the Company been a separate, stand-alone publicly traded entity.
Liquidity
The Company has incurred significant operating losses since inception and has relied on funding from its Parent to fund our operations prior to its initial public offering on February 3, 2015. At December 31, 2014, the Company has an accumulated deficit of $37.4 million. As the Company continues to incur losses, its transition to profitability will depend on the successful development, approval and commercialization of its product and on the achievement of sufficient revenues to support its cost structure. The Company may never achieve profitability, and unless and until it does, it will need to continue to raise additional capital. Management expects that existing cash as of December 31, 2014 plus the proceeds of its offering in February 2015 will be sufficient to fund the Company’s operations for the next 12 months.
(2) Summary of Significant Accounting Policies
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires that management makes estimates and assumptions that are included in the consolidated financial statements and accompanying notes. The actual results may differ from those estimates.
Segment Information
In accordance with Accounting Standard Codification (“ASC”) 280-10-50, Segment Reporting, operating segments are identified as components of an enterprise about which separate discrete financial information is available for evaluation by the chief operating decision-maker in making decisions regarding resource allocation and assessing performance. Since 2012, the Company has viewed and managed its operations and business as one segment through its wholly-owned Dutch subsidiary, Presbia Cooperatief U.A., and operating primarily in the United States of America and in Brazil, Canada, Ireland, Israel, Italy, Japan, and Turkey.
Foreign Currency
The functional currency of subsidiaries outside the United States of America is the U.S. Dollar. Transactions in foreign currencies during the year are re-measured at rates of exchange on the dates of the transactions. Gains and losses related to re-measurement of items arising through operating activities are accounted for in the statement of operations and comprehensive loss and included in general and administrative expense. Aggregate foreign exchange losses were $19,000, $4,000 and $4,000 for the years ended December 31, 2014, 2013 and 2012, respectively.
Risks and Uncertainties
The Company’s product requires the approval of the FDA and regulatory agencies in the countries where the Company operates or expects to establish operations in the future. There is no assurance that the Company’s
97
products will receive or maintain the necessary approvals to begin or continue operations. If the approvals are denied, delayed or withdrawn, there may be a material adverse impact on the Company’s results of operations and related cash flows.
Cash is generally deposited in demand deposit accounts that, from time to time, may exceed insurable limits. The Company has not experienced any losses from its deposits of cash.
Fair Value of Financial Instruments
The carrying values of certain of the Company’s financial instruments, such as prepaid expenses, accounts payable and accrued expenses, approximate fair value due to their short maturities. Amounts payable to related parties, including the payable due to the Parent, which has no fixed maturity or expiration date, do not have readily determinable fair values.
Cash
The Company considers highly liquid investments with original maturities less than 90 days to be cash equivalents. As of December 31, 2014 and 2013, the Company had no such short-term investment instruments and maintained its cash in bank demand deposit accounts.
Inventory
The Company accounts for inventory at the lower of market or cost. Inventory is stated at weighted average cost, which is determined by applying the current average cost to the ending inventory. Inventory consists of lenses and lens inserters and other accessories used by physicians in the surgical process associated with the lenses. The Company maintains serialized records of all lenses, including the five-year expiration date of each lens, after which the lens cannot be sold. The Company considers the expiration dates of lenses, in addition to comparing the carrying amount of inventory to expected demand, and will write-down inventory for amounts determined to be excess or obsolete.
Property and Equipment
Property and equipment are stated at cost less accumulated depreciation and amortization. Depreciation of property and equipment is computed using the straight-line method over their estimated useful lives of three to ten years. Leasehold improvements are amortized over the lesser of the useful life of the asset or the remaining term of the lease. Repairs and maintenance of property and equipment are expensed as incurred. Upon retirement or sale, the original cost and accumulated depreciation are removed and any gain or loss is recognized in the consolidated statements of operations and comprehensive loss.
Impairment of Long-Lived Assets
The Company reviews the recoverability of long-lived and finite-lived intangible assets when circumstances indicate that the carrying amount of assets might not be recoverable. This evaluation is based on various analyses, including undiscounted cash flow projections. In the event undiscounted cash flow projections indicate impairment, the Company would record an impairment based on the fair value of the assets at the date of the impairment.
Intangible Assets
In 2009, the Parent acquired Visitome, Inc. by issuing capital stock valued at $50,000. The Parent assigned a fair value of $50,000 to the net assets acquired which included a finite-lived intangible asset of $24,000, property and equipment of $11,000 and goodwill of $15,000. The intangible asset was fully amortized through August 2014 on a straight-line basis over its estimated useful life of five years. The Company amortized $3,200, $4,800 and $4,800 in 2014, 2013 and 2012, respectively.
98
Costs incurred to develop software for the Company’s website are capitalized and amortized over the estimated useful life of the software and are included in intangible assets. Costs related to design or maintenance of website development is expensed as incurred. For the years ended December 31, 2014 and 2013, the Company capitalized $46,000 and $0 of costs, respectively, associated with website development. No amortization expense was recorded in 2014, 2013 and 2012.
Comprehensive Loss
Comprehensive income or loss is defined as a change in equity of a company attributable to all transactions excluding those transactions resulting from investment with owners and distributions to owners. There were no differences between net loss and comprehensive loss in the years ended December 31, 2014, 2013 and 2012.
Revenue Recognition
The Company recognizes revenue when there is persuasive evidence that an arrangement exists with the customer, selling prices are fixed or determinable, title or risk of loss has passed, and collection is reasonably assured. Revenue is recognized upon shipment and payments are either received in advance, or net 30 days. In the years ended December 31, 2014, 2013 and 2012 there were four customers, respectively, that represented 100% of total sales recognized for each year. As of December 31, 2014, the Company was not authorized to manufacture or sell any of its products or services within the United States and, as a result, all of the Company’ revenues are derived from foreign customers.
Years ended December 31, 2014 and 2013
The Company recognized revenues of $153,000 and $75,000 from laser centers during the years ended December 31, 2014 and 2013, respectively. The Company recognizes revenue from these laser centers based upon an analysis of the terms of each customer arrangement and upon determination that persuasive evidence of an arrangement exists, selling prices are fixed or determinable, title or risk of loss has passed, and collection is reasonably assured.
Year ended December 31, 2012
During the year ended December 31, 2012, all revenues recognized were from distributor arrangements. The distributor arrangements include certain perfunctory acceptance provisions and a one-year warranty, from the date of shipment, that products are free from defects in material workmanship. Under such provisions customers may reject shipments via written notifications ranging from 14-45 days or exchange defective product under warranty for the same non-defective product. The Company has not had any significant rejected shipments or warranty claims.
In 2012, the Company terminated its arrangement with a foreign distributor and repurchased all of the Company’s products held by the distributor on the termination date for $30,000, an amount equal to the sales price recognized by the Company in 2011 upon the original sale to the distributor. As a result of the repurchase, revenues for the year ended December 31, 2012 were reduced by $30,000.
In 2012 the Company changed its commercialization strategy from exclusively using distributors to also targeting refractive laser centers equipped with femtosecond lasers, except in countries that require the use of distributors or sales representatives.
Clinical Trials
During 2014, the Company received approval from the FDA to commence a staged-enrollment pivotal FDA clinical trial of its Microlens involving the implantation of presbyopic patients. The first staged-enrollment commenced during the first quarter of 2014, and during these trials, the Company incurred costs for patient
99
recruiting, acquisition of clinical test equipment to be used in the trials, outside experts to read and interpret the results of the studies, third party costs to monitor the investigational sites and perform data collection activities and surgeon and patient fees in connection with the surgical procedures and follow-up visits. In February 2015, the Company received approval from the FDA to commence second stage enrollment in this trial. The Company’s policy with respect to the recognition of these costs is to record such costs as research and development expense in the consolidated statements of operations and comprehensive loss in the period in which the services are provided. The Company will evaluate the purchases of clinical test equipment, on a case by case basis, to determine if there exists an alternative use for the equipment following the clinical trials. In the event that the Company determines that there is no alternative use for the test equipment, that cost will be expensed as part of research and development expense.
Stock-Based Compensation
The compensation cost of stock-based awards granted to employees is measured at grant date, based on the estimated fair value of the award. The Company estimates the fair value of stock options using a Black-Scholes option pricing model. Compensation expense for options granted to non-employees is determined as the fair value of consideration received or the fair value of the equity instruments issued, whichever is more reliably measured. Stock-based compensation costs are expensed on a straight-line basis (net of estimated forfeitures) over the service period. The fair value of awards granted to non-employees is remeasured each period until the related service is complete or there exists a significant disincentive not to perform the required services. Stock-based compensation costs are reflected in the accompanying statements of operations and comprehensive loss based upon the underlying employees’ roles within the Company.
Deferred Offering Costs
Generally, costs incurred such as legal, accounting and other professional fees including printing costs in the course of preparing for an IPO are deferred on the balance sheet as deferred offering costs, which are eventually reclassified when the IPO is concluded as a reduction of the proceeds of the IPO as part of additional paid in capital. In the event the IPO is delayed or postponed for a period longer than 90 days or the Company determines that it no longer expects to conclude the IPO for any reason, the deferred offering costs are expensed as general and administrative expense on the consolidated statements of operations and comprehensive loss in the period in which the delay occurs or the period in which management determines that the public offering is postponed, delayed indefinitely or cancelled. In the second and third quarters of 2014, approximately $3.4 million of such IPO costs was expensed as general and administrative expense due to a postponement of the Company’s IPO that exceeded 90 days. During September 2014, the Company resumed preparations for the IPO and incurred approximately $0.8 million between the resumption date and December 31, 2014, and recognized this amount as part of other assets on the balance sheet as of December 31, 2014.
Income Taxes
For the year ended December 31, 2012, the Company was generally not subject to income taxes, as income or loss was either passed-through and included in the income tax returns of the Company’s shareholders or otherwise not subject to tax under local statute or rulings. As described in Note 1, the Parent completed a restructuring on October 21, 2013. As a result, some of the entities are no longer pass-through entities or were restructured as taxable entities. Provisions for federal, foreign, state, and local income taxes are calculated on pre-tax income based on current tax law and include the cumulative effect of any changes in tax rates from those used previously in determining deferred tax assets and liabilities. Deferred income tax assets and liabilities are recorded for the expected future tax consequences of temporary differences between the financial statement carrying amounts and the income tax basis of assets and liabilities. A valuation allowance is recorded to reduce net deferred income tax assets to amounts that are more likely than not to be realized.
100
Advertising Costs
The Company incurs direct response advertising expense outside the United States in order to create awareness of the Company’s Microlens solution to presbyopia. The Company’s policy with respect to direct response advertising is to defer costs related to media and setup costs and amortize those costs to the statement of operations in the period in which the related revenue is recognized. Due to the early stages of market development and the uncertainty around the impact of advertising campaigns to-date, the Company expensed approximately $244,000, $3,000 and $36,000 as part of sales and marketing expense in the consolidated statements of operations for the years ended December 31, 2014, 2013 and 2012, respectively.
Recently Issued Accounting Standards
In May 2014, the Financial Accounting Standards Board (“FASB”) and the International Accounting Standard Board (“IASB”) jointly issued a new revenue recognition standard that will supersede nearly all existing revenue recognition guidance under GAAP. The revenue recognition standard will allow for the recognition of revenue when a company transfers promised goods or services to customers in an amount that reflects the consideration to which the company expects to be entitled in exchange for those goods or services. The standard is effective for public entities for annual and interim periods beginning after December 15, 2016. Early adoption is not permitted under GAAP. The Company is currently evaluating the impact that the adoption of this guidance will have on its consolidated financial statements.
On June 10, 2014, the FASB issued Accounting Standards Update No. 2014-10 (ASU 2014-10), which eliminates the definition of a development stage entity, eliminates the development stage presentation and disclosure requirements under Accounting Standards Codification No 915 (ASC 915), and amends provisions of existing variable interest entity guidance under ASC 810. As a result of the changes, the financial statements of entities which meet the former definition of a development stage entity will no longer include the following:
| • | Inception-to-date income, cash flow and equity information |
| • | Label indicating that the financial statements are those of a development stage entity |
| • | Disclosures of the nature of the entity’s development stage activities as well as the first year in which the entity is no longer considered a development stage entity |
Additionally, ASU 2014-10 clarified that the lack of commencement of principal operations represents a risk and uncertainty under ASC 275 and, accordingly, the financial statements should reflect appropriate disclosures.
Finally, variable interest entity rules no longer contain an exception for development stage entities and, as a result, development stage entities will have to be evaluated for consolidation in the same manner as non-development stage entities.
Public entities are no longer required to apply the presentation and disclosure provisions of ASC 915 during annual periods beginning after December 15, 2014, and the revisions to the consolidation standards are effective for annual periods beginning after December 15, 2015. The Company adopted this standard during the fourth quarter of 2014. The adoption of this guidance impacted the financial statement presentation, but did not have a material impact on the Company’s financial position or results of operations and cash flows.
The FASB issued ASU 2014-15 on August 27, 2014, providing guidance on determining when and how to disclose going-concern uncertainties in the financial statements. The new standard requires management to perform interim and annual assessments of an entity’s ability to continue as a going concern within one year of the date the financial statements are issued. An entity must provide certain disclosures if “conditions or events raise substantial doubt about [the] entity’s ability to continue as a going concern.” The ASU applies to all entities and is effective for annual periods ending after December 15, 2016, and interim periods thereafter, with early adoption permitted. The Company is currently evaluating the impact that the adoption of this guidance will have on its consolidated financial statements.
101
(3) Inventory
The Company maintains finished goods inventory for the purpose of supporting sales activities outside the United States. Inventory consists of ready-to-use lenses of various refractive powers and accessories required by ophthalmologists to carry out the surgical procedures. The ready-to-use lenses are manufactured, inspected and then delivered to a central warehouse outside the United States for the purpose of supporting such commercial activity. The Company performs no lens or accessory manufacturing within the United States for purpose of sale anywhere in the world. The Company maintains a serial number tracking system that measures shelf life such that no lens that has aged beyond four years can be delivered to a customer. During the years ended December 31, 2014, 2013 and 2012, inventory write-downs reflecting excess quantities on hand were recognized in the amount of $61,000, $56,000 and $22,000, respectively, based on the age of the lens inventory, inserters, accessories and the refractive mix of the inventory. Finished goods inventory consists of the following as of dates set forth below (in thousands):
| DECEMBER 31, | ||||||||
| 2014 | 2013 | |||||||
| Lenses |
$ | 371 | $ | 178 | ||||
| Accessories |
7 | 21 | ||||||
|
|
|
|
|
|||||
| Total |
$ | 378 | $ | 199 | ||||
|
|
|
|
|
|||||
(4) Property and Equipment
Property and equipment consists of the following as of the dates set forth below (in thousands):
| DECEMBER 31, | ||||||||
| 2014 | 2013 | |||||||
| Office equipment and computers |
$ | 51 | $ | 45 | ||||
| Leasehold improvements |
132 | 129 | ||||||
| Production equipment and facilities |
682 | 664 | ||||||
| Software |
7 | 0 | ||||||
| Furniture and vehicles |
155 | 127 | ||||||
|
|
|
|
|
|||||
| 1,027 | 965 | |||||||
| Less: accumulated depreciation |
(280 | ) | (145 | ) | ||||
|
|
|
|
|
|||||
| Property & Equipment, net |
$ | 747 | $ | 820 | ||||
|
|
|
|
|
|||||
Depreciation and amortization expense related to property and equipment for the years ended December 31, 2014, 2013 and 2012 was $135,000, $91,000 and $29,000 respectively.
(5) Other Current Liabilities
Other current liabilities consist of the following as of the dates set forth below (in thousands):
| DECEMBER 31, | ||||||||
| 2014 | 2013 | |||||||
| Costs related to the initial public offering |
$ | 327 | $ | 214 | ||||
| Accrued professional fees |
20 | 82 | ||||||
| Accrued payroll & vacation |
114 | 77 | ||||||
| Other accrued liabilities |
230 | 32 | ||||||
|
|
|
|
|
|||||
| Total |
$ | 691 | $ | 405 | ||||
|
|
|
|
|
|||||
102
(6) Other Assets
Other assets consist of the following as of the dates set forth below (in thousands):
| DECEMBER 31, | ||||||||
| 2014 | 2013 | |||||||
| Prepaid rent and other |
$ | 47 | $ | 28 | ||||
| Deferred costs related to initial public offering |
839 | 1,965 | ||||||
|
|
|
|
|
|||||
| Total |
$ | 886 | $ | 1,993 | ||||
|
|
|
|
|
|||||
(7) Shareholders’ Deficit
Euro Ordinary Shares
Capital at December 31, 2014 consisted of ordinary shares (“Euro Ordinary Shares”) of $54,000, one Euro par value, 40,000 authorized and issued and paid-in on February 6, 2014 related to the formation of Presbia PLC, compared to zero shares at December 31, 2013. The rights and preferences of the Euro Ordinary Shares are as follows: (i) subject to the right of the Company to set record dates for the purposes of determining the identity of shareholders entitled to notice of and/or to vote at a general meeting, the right to attend and speak at any general meeting of the Company and to exercise one vote per Euro Ordinary Share held at any general meeting of the Company; (ii) the right to participate pro rata in all dividends declared by the Company; and (iii) the right, in the event of the Company’s winding up, to participate pro rata in the total assets of the Company. The rights attaching to the Euro Ordinary Shares may be subject to the terms of issue of any series or class of preferred shares allotted by the Company’s board of directors from time to time in accordance with article. On January 14, 2015 the Company amended its memorandum and articles of association, resulting in the re-designation of the 40,000 Euro Ordinary Shares as deferred ordinary shares. See Note 14 for a description of the amended rights and preferences of the deferred ordinary shares.
Capital
Capital of $36.7 million as of December 31, 2014, compared to $12.8 million at December 31, 2013, consists of the capital contribution of approximately $12.2 million pursuant to the 2013 Restructuring, the capital contribution of approximately $23.5 million pursuant to the 2014 Debt Conversion and the allocation of stock-based compensation from the Parent of approximately $1.0 million.
(8) Stock Options
Stock-Based Compensation Plans
As of December 31, 2014, the Company did not have any active or inactive stock-based compensation plans; however, during the year ended December 31, 2010 and in October 2013, the Parent granted options to purchase its ordinary shares and granted awards of restricted ordinary shares, to both employees and non-employees of the Company and stock-based compensation related to such awards has been recognized as expense in the accompanying financial statements as described further below.
On January 28, 2015, as part of the Company’s IPO, the Company established the Presbia Incentive Plan for the purpose of granting equity-based awards to its directors and employees up to an authorized level of 1.8 million shares (See Note 14).
2010 Original Awards
During 2010, the Parent granted stock options for 4,490,000 ordinary shares (the “2010 Original Awards”) to employees, consultants, medical advisory board members and board members in exchange for future services, granted at exercise prices ranging from $0.01 to $0.049 per ordinary share of the Parent. As described below, in October 2013, the Parent granted replacement stock options covering 4,180,000 of the 2010 Original Awards that
103
remained outstanding on the date of the replacement grant. The 2010 Original Awards vested 20% annually over a five-year period and had a contractual term of ten years. The Parent’s ordinary shares have never traded publicly; a valuation study determined that the fair value of the ordinary shares during February and October 2010 was $0.014 and $0.024 per share, respectively. For a discussion of the valuation methods and assumptions utilized in the 2010 Valuation, see “Valuation of the Parent’s Ordinary Shares—Valuation at September 1, 2013 and Prior” below.
2013 Replacement Awards
In October 2013, the Parent granted stock options (the “2013 Replacement Awards”) covering 4,180,000 ordinary shares at an exercise price of $0.08 per share, based on a valuation of the ordinary shares of the Parent using the methodology described for the 2010 Original Awards above that indicated a value of $0.08 per share at September 1, 2013, to replace those shares of the 2010 Original Awards that remained outstanding on the date of the replacement grant. For a discussion of the valuation methods and assumptions utilized in the 2010 Valuation, see “Valuation of the Parent’s Ordinary Shares—Valuation at September 1, 2013 and Prior” below. The 2010 Original Awards that were replaced had a weighted-average exercise price of $0.03 per share. The 2013 Replacement Awards have a contractual term of 10 years, compared to a remaining contractual term of approximately 6.5 years for the 2010 Original Awards, and remaining vesting terms commensurate with the remaining vesting terms of the 2010 Original Awards on the date of the replacement grant. The 2010 Original Awards that were replaced with the 2013 Replacement Awards were cancelled and the transaction is a modification of the 2010 Original Awards for accounting purposes. The Company determined that the modification did not result in any incremental stock-based compensation expense by measuring the fair value of the 2010 Original Awards immediately before and after the modification using the Black-Scholes option-pricing model. The absence of any incremental stock-based compensation expense is primarily due to the 2010 Original Awards having a weighted-average exercise price of $0.03 per share compared to a higher exercise price of $0.08 per share for the 2013 Replacement Awards.
2013 Stock Option Grants and Restricted Stock Awards
During October 2013, the Parent granted new stock options to employees and non-employees covering 900,000 ordinary shares of the Parent with an exercise price of $0.30 per share, a contractual term of ten years, and annual vesting of 20% over a five-year period. The grant-date fair value of the awards was estimated to be $182,000 using the Black-Scholes option-pricing model. For a discussion of the valuation methods and assumptions utilized in the valuation of the ordinary shares for the 2013 stock option grants, see “Valuation of the Parent’s Ordinary Shares—Valuation at December 31, 2013” below. In addition to the fair value of an ordinary share, other significant inputs include estimated volatility of 72%, term of 6.5 years for employees and 10 years for nonemployees, risk-free rate of 2% and expected dividends of zero.
In October 2013, the Parent awarded Zohar Loshitzer a 1,000,000 restricted share stock award that will vest on the expiration of all lockup restrictions related to the IPO (July 28, 2015), unless the recipient’s employment is terminated for cause. The fair value of the 1,000,000 shares of $300,000, or $0.30 per share, was determined as described under “Valuation of the Parent’s Ordinary Shares—Valuation at December 31, 2013” below and expensed in the year ended December 31, 2013 since the terms of the award permit continued vesting if Mr. Loshitzer’s employment is terminated without cause. In October 2013, the Parent granted 250,000 restricted share stock awards to each of two members of its board of directors with 20% annual vesting over a five-year period. The fair value of the 500,000 shares of $150,000, or $0.30 per share, is expected to be expensed ratably over five years. At December 31, 2014, the number of non-vested restricted shares totaled 400,000, the weighted-average remaining vesting period was 3.8 years and all are expected to vest.
Non-Employee Stock-Based Compensation
Stock-based compensation expense related to stock options granted to non-employees is recognized as the stock options are earned. It was concluded that the fair value of the stock options is more reliably measurable than the fair value of the services received. The fair value of non-employee stock options are calculated at each reporting
104
date, using the Black-Scholes option-pricing model, until the award vests or there is a substantial disincentive for the non-employee not to perform the required services. For a discussion of the valuation methods and assumptions utilized for the remeasurement of non-employee awards during the years ended December 31, 2014 and 2013, see “Valuation of Parent’s Ordinary Shares” below.
The following table sets forth the required inputs to determine the fair values for the non-employee stock options:
| YEAR ENDED DECEMBER 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| Stock price per share |
$ | 0.40 | $ | 0.54 | $ | 0.07 | ||||||
| Term |
8.8 years | 9.8 years | 7.1-7.8 years | |||||||||
| Volatility |
77 | % | 69 | % | 70 | % | ||||||
| Dividends |
— | — | — | |||||||||
| Discount rate |
2.1 | % | 2.6 | % | 1.2 | % | ||||||
Employee Stock Awards
During 2010 and in October 2013, the Parent granted stock options for shares of its common stock and restricted shares to employees, consultants, medical advisory members and board members in exchange for future services. Since the stock-based compensation expenses that were recognized by the Parent were incurred to benefit the Company, these expenses have been allocated to the Company. The options expire 10 years from the date of the grant and have vesting periods of five years with 20% vesting in each of the five years. These options were awarded by the Parent to employees and non-employees and the stock-based compensation expense was allocated to the Company as follows in the consolidated statements of operations and comprehensive loss for all periods presented:
Compensation cost for employee stock-based awards is based on the estimated grant date fair value and is recognized over the service period of the applicable award on a straight-line basis. The Black-Scholes option pricing model was used to determine the fair value of employee stock options. To apply the Black-Scholes option pricing model, the grant-date fair value of the underlying common stock price or value of the shares is adjusted based on the assumptions regarding the volatility of the common stock prices or share values, the expected term of the option and the risk-free interest rate corresponding to a time period equivalent to the expected term of the option. Volatility was based on the stock volatility of comparable public companies within the same industry. Included in the 2010 Original Awards, the Parent granted stock options for 1,810,000 shares of its common stock to employees. Due to the limited history available to measure the expected term of stock option grants, the expected term was estimated to be 6.5 years using the simplified expected term method. A risk-free rate of 3.0% was used that corresponds to the expected term of 6.5 years.
The employee, non-employee and restricted stock stock-based compensation expense was allocated to the Company in the accompanying statements of operations and comprehensive loss for all the periods presented as follows (in thousands):
| YEAR ENDED DECEMBER 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| Research and development |
$ | 213 | $ | 112 | $ | 33 | ||||||
| General and administrative |
81 | 373 | 14 | |||||||||
| Sales and marketing |
19 | 4 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
$ | 313 | $ | 489 | $ | 47 | ||||||
|
|
|
|
|
|
|
|||||||
105
The following table sets forth stock option activity for the year ended December 31, 2014:
| NUMBER OF PARENT’S SHARES |
WEIGHTED AVERAGE EXERCISE PRICE PER SHARE |
AGGREGATE INTRINSIC VALUE |
||||||||||
| Balance, January 1, 2014 |
5,080,000 | $ | 0.12 | |||||||||
| Granted |
— | $ | — | |||||||||
| Exercised |
(75,000 | ) | $ | 0.09 | $ | 23 | ||||||
| Forfeited/cancelled |
(170,000 | ) | $ | 0.30 | ||||||||
|
|
|
|||||||||||
| Balance, December 31, 2014 |
4,835,000 | $ | 0.11 | $ | 1,388 | |||||||
|
|
|
|||||||||||
| Vested and expected to vest, December 31, 2014 |
4,794,000 | $ | 0.11 | $ | 1,383 | |||||||
| Exercisable, December 31, 2014 |
3,419,000 | $ | 0.09 | $ | 1,062 | |||||||
Intrinsic value is measured using the estimated fair value at the date of grant, or as of December 31 (for outstanding options), less the applicable exercise price. The weighted-average remaining contractual terms for options outstanding, vested and expected to vest, and exercisable as of December 31, 2014 was 8.8 years.
Unrecognized Stock-based Compensation
As of December 31, 2014, there was $100,000 and $46,000 of unrecognized compensation expense related to employee and non-employee options, respectively, which is expected to be recognized by the Company over the weighted average vesting period of 2.7 years. Unrecognized compensation expense related to restricted stock was $114,000 as of December 31, 2014 and is expected to be recognized over the weighted average vesting period of 3.8 years.
Valuation of the Parent’s Ordinary Shares
Valuation at December 31, 2014. The valuation method at December 31, 2014 utilized probability—weighted income and market approaches, in which it assigned an 10% weight to the outcome of the income approach of $1.06 per share and 90% weight to the outcome of the market approach of $0.33 per share to reach a concluded value of $0.40 per share of the ordinary shares of the Parent. The income approach considers future cash flows on a discounted basis (DCF) as one indicator of current value while the market approach considers recent transactions in recent initial public offerings of similar equities including the Company’s anticipated IPO such as for those enterprises in early stages of development, in the biopharmaceutical or medical device industries.
The income approach consisted of a base case scenario, which was assigned a 70% probability of success, in which the U.S. FDA clinical trials are concluded in 2017 and revenues commenced in the U.S. in March 2018. The income approach also assigned additional probabilities of 25% and 5% to a delayed case where entry into the U.S. market is delayed by one year to March 2019 and a pessimistic case where FDA approval is not achieved and there is no entry into the U.S. market, respectively. In each scenario, the DCF was discounted by applying a 27.5% discount rate to free cash flows generated in each of a ten-year projection period and a terminal value that represents future cash flows past 2023, the last year in the ten year projection. The weighted-average enterprise value was further discounted by 22% to account for the lack of marketability of the Parent’s ordinary shares to arrive at a concluded value of $0.40 per share. The discount rate of 27.5% applied in the DCF model is based on the Company’s stage of development. The lack of marketability discount of 22% was determined by applying a protective put option analysis reflecting the expected timing of a liquidity event of the Parent’s ordinary shares.
The market approach considers recent initial public offerings in ophthalmology and general biotech markets. The initial public offering analysis consisted of inputs provided by the Company’s lead underwriter which considered a range of pre-IPO equity valuation ranging from $90 million to $140 million. This method provides a range of
106
equity values as of an initial public offering date. The range of enterprise values, when attributed to the ordinary shares of the Parent, indicated an average of $0.42 per share. The same lack of marketability discount of 22% was applied to indicate a value per ordinary share of $0.33.
Valuation at December 31, 2013. The valuation method at December 31, 2013 utilized probability—weighted income and market approaches, in which it assigned an 80% weight to the outcome of the income approach of $0.54 per share and 20% weight to the outcome of the market approach of $0.55 per share to reach a concluded value of $0.54 per share of the ordinary shares of the Parent. The income approach considers future cash flows on a discounted basis (DCF) as one indicator of current value while the market approach considers recent transactions in recent initial public offerings of similar equities, such as for those enterprises in early stages of development, in the biopharmaceutical or medical device industries.
The income approach consisted of a base case scenario, which was assigned a 65% probability of success, in which the U.S. FDA clinical trials are concluded in 2018 and revenues commenced in the U.S. in March 2019. The income approach also assigned additional probabilities of 25%, 5% and 5% to a delayed case where entry into the U.S. market is delayed by one year to March 2020, a pessimistic case where FDA approval is not achieved and there is no entry into the U.S. market and an optimistic case where FDA approval is received sooner than expected with entry into the U.S. market by December 31, 2018, respectively. In each scenario, the DCF was discounted by applying a 30% discount rate to free cash flows generated in each of a ten-year projection period and a terminal value that represents future cash flows past 2023, the last year in the ten year projection. The weighted-average enterprise value was further discounted by 29% to account for the lack of marketability of the Parent’s ordinary shares to arrive at a concluded value of $0.54 per share. The discount rate of 30% applied in the DCF model is based on the Company’s stage of development. The lack of marketability discount of 29% was determined by applying a protective put option analysis reflecting the expected timing of a liquidity event of the Parent’s ordinary shares. The market approach considers recent initial public offerings in ophthalmology and general biotech markets. The initial public offering analysis develops values of a company through the analysis of pre-money values of comparable public companies as of their respective initial public offering dates. This method provides a range of equity values as of an initial public offering date. The range of enterprise values, when attributed to the ordinary shares of the Parent, indicated an average of $0.78 per share. The same lack of marketability discount of 29% was applied to indicate a value per ordinary share of $0.55.
Valuation at September 1, 2013 and Prior. The valuation method used at September 1, 2013, each of December 31, 2012, 2011 and 2010, and February 1, 2010, the date the Parent first granted share options, was a historical cost method. For these valuations, consideration was given to three generally recognized valuation approaches—the income, market and cost valuation approaches. The cost approach was ultimately used to value the ordinary shares. Since the Company’s operations were in such an early stage, as evidenced by the recognition of insignificant revenues, and because the Parent and the Company lacked arm’s length third party financings, use of the cost approach was determined to be the best technique. Under this approach, the estimated fair value of the ordinary shares was derived by applying a rate of return consistent with a development stage entity to the historical expenses associated with the development stage activities, which comprise those of the Company. The estimated fair value of the enterprise was then allocated to the ordinary shares after consideration of all classes of capital stock and debt, if any, and then reduced by a discount for lack of marketability. The significant assumptions under this approach reflected in these valuations include a rate of return of 50%, which was determined based upon the early development stage and a 27% discount for lack of marketability based upon quantitative analysis using a protective put analysis reflecting the expected timing of a liquidity event on the valuation dates. For each of the valuations, a study of peer group public companies in the medical device industry was performed to estimate the volatility of the Parent’s ordinary shares, which was estimated to be in the range of 70% for the periods covered by the valuations. These valuations resulted in estimated fair values of the Parent’s ordinary shares ranging from $0.01 per share in 2010 to $0.08 per share in September 2013.
A combination of income and market approaches was utilized in the valuation of the Parent’s ordinary shares as of December 31, 2013 rather than the cost method previously used primarily due to significant developments in
107
its business that occurred during the fourth quarter of 2013, including FDA approval to commence immediately a pivotal trial in the United States using a staged enrollment and the hiring of key executives in marketing, engineering, operations and finance.
Compensation Cost of 2013 Awards. During 2010, the Parent granted options to employees and non-employees to purchase 4,490,000 of its ordinary shares at exercise prices ranging from $0.01 to $0.049 per share. In October 2013, the 4,180,000 options granted in 2010 that remained outstanding were canceled and replaced with an equivalent number of share options with an exercise price of $0.08 per share. Also in October 2013, the Parent granted options to employees and non-employees to purchase 900,000 of its ordinary shares at an exercise price of $0.30 per share and an aggregate 1,500,000 shares of its restricted ordinary shares to Zohar Loshitzer and two other board members. For purposes of recognizing share-based compensation, the grant-date fair value of all of October 2013 option awards, the determination that there was no incremental fair value for the modification of 2010 Original Awards replaced by the 2013 Replacement Awards, both of which were measured using the Black-Scholes option-pricing model, and the estimated fair value of the restricted stock awards granted in October 2013, used an estimated fair value of $0.30 per share. As of December 31, 2013, when the Parent re-measured the fair value of its non-employee awards using the Black-Scholes option-pricing model, it used an estimated fair value of $0.54 per share based on the December 31, 2013 valuation.
(9) Related Party Transactions
The following table sets forth the amounts due to related parties reflected in the accompanying consolidated balance sheets (in thousands):
| DECEMBER 31, | ||||||||
| 2014 | 2013 | |||||||
| Payable to related parties—current: |
||||||||
| Management services provided by related parties |
$ | 12 | $ | 132 | ||||
|
|
|
|
|
|||||
| Total |
$ | 12 | $ | 132 | ||||
|
|
|
|
|
|||||
| Payable due to the Parent—noncurrent: |
||||||||
| Principal amounts due to the Parent |
$ | 417 | $ | 7,180 | ||||
| Accrued interest due to the Parent |
0 | 2,245 | ||||||
|
|
|
|
|
|||||
| Total |
$ | 417 | $ | 9,425 | ||||
|
|
|
|
|
|||||
Richard S. Ressler
Mr. Ressler has served as the Chairman of the Board of the Parent since the Parent’s formation in 2007 and along with immediate family members has a controlling interest in a partnership that controls the Parent. As of December 31, 2014 and 2013, net principal amounts due to the Parent amounted to $0.4 million and $7.2 million, respectively. In addition, the Company has recorded accrued interest due to the Parent on the accumulated funding outstanding at a rate (through November 25, 2014) of 15% per annum, compounding daily, totaling zero and approximately $2.2 million as of December 31, 2014 and 2013, respectively. On October 21, 2013, pursuant to the 2013 Restructuring, approximately $12.2 million of debt due to the Parent was converted from debt to capital. The funding debt has no fixed maturity or expiration date and is classified as a noncurrent liability in the accompanying consolidated balance sheets.
On November 30, 2014, Presbia Holdings converted all the remaining indebtedness owed to Presbia Holdings by certain subsidiaries of Presbia Ireland, Limited at that time to equity (“2014 Debt Conversion”). In the 2014 Debt Conversion, approximately $23.5 million of outstanding intercompany debt owed to Presbia Holdings was converted to equity of such subsidiaries.
108
Also on November 30, 2014, the Company’s subsidiaries entered into an Interest Rate Agreement (the “Interest Rate Agreement”) with its Parent. Pursuant to the terms of the agreement, the parties agreed that any future borrowings from the Parent shall accrue at a rate, to be adjusted each calendar month, equal to the then applicable monthly federal rate of interest for short-term loans, as defined in Section 1274 of the Internal Revenue Code of 1986, and such interest should accrue daily on the basis of a 365 day year until such amount is repaid in full or such amount has been contributed to capital by the Parent to the Company’s subsidiaries. The agreement provides that any future borrowings shall have no fixed maturity.
Since 2011, Orchard Capital Corporation (“Orchard”), which is wholly-owned by Mr. Ressler, has provided financial analysis and bookkeeping, accounting, legal and compliance services to the Company pursuant to a Services Agreement. During the years ended December 31, 2014, 2013 and 2012, the Company recognized general and administrative expense of $18,000, $242,000 and $212,000, respectively, for services invoiced by Orchard. As of December 31, 2014 and 2013, amounts due to Orchard for management and accounting services amounted to $0 and $23,000, respectively.
Mr. Ressler serves in various senior management capacities at CIM Group, L.P. (together with its affiliates, “CIM Group”). An affiliate of CIM Group leased office space to the Company in Los Angeles, California from September 2009 through November 2011. The Company continued to lease such space through September 2012, but beginning in November 2011, the Company leased such space from a new landlord which was not a related party. As of December 31, 2012, amounts due to CIM Group for use of office space amounted to $38,000.
Commencing during the second quarter of 2013, the Company has received human resources management services, payroll services, IT support and risk management services from CIM Group. The Company has incurred charges of $152,000 and $142,000 payable to CIM Group for such services for the years ended December 31, 2014 and 2013, respectively. As of December 31, 2014 and 2013, amounts due to CIM Group for human resources, payroll, information technology and legal services amounted to $12,000 and $109,000, respectively.
On December 19, 2012, an entity affiliated with Mr. Ressler, a defendant in a legal matter entitled Thomke Invest AG v. Vladimir Feingold, Visitome, Inc., Zohar Loshitzer and Orchard Capital (“the Visitome Parties” as defendant), paid cash of $100,000 to the plaintiff as part of a Settlement Agreement and General Release and is included in payable due to the Parent in the Company’s consolidated balance sheet as of December 31, 2014 and 2013.
Zohar Loshitzer
In January 2011, the Company entered into a consulting agreement with MTP Consulting, Inc., or MTP Consulting, pursuant to which MTP Consulting provided certain management consulting services to the Company. Mr. Loshitzer, the Company’s Chief Business Development Officer and one of its directors, and a director and shareholder of the Parent, was the founder, owner and President of MTP Consulting. The agreement provided for the payment of bi-monthly consulting fees of $10,400 to MTP Consulting. This agreement was terminated in August 2013 and no additional amounts are due or payable to MTP Consulting pursuant to this agreement. The Company made aggregate payments of $0, $300,000 and $250,000 either directly to Mr. Loshitzer as a consultant or to MTP Consulting for the years ended December 31, 2014, 2013 and 2012, respectively. Effective November 1, 2013, Mr. Loshitzer became an employee of the Company.
(10) Operating Segments and Geographic Information
The Company operates in one operating segment, the restoration of clear vision caused by presbyopia. The Company provides the refractive lens for patient surgeries and accessories for procedures performed exclusively outside the United States. Revenue originating in the United States is limited to intercompany transactions that do not result in any revenue generating activities to any individual or physician in the United States, and these amounts are eliminated upon consolidation. The operating losses in the United States result primarily from
109
research and development and general and administrative costs while the operating losses in the foreign operations result primarily from sales and marketing costs and an allocation of general and administrative costs to foreign operations.
Revenues from external customers to individual countries are allocated based on the location of the customer. For the periods presented, there was no more than one customer in each individual country.
The following table sets forth the Company’s revenues generated from external customers located in foreign countries and long-lived assets by area (in thousands):
| YEAR ENDED DECEMBER 31, | ||||||||||||
| REVENUES |
2014 | 2013 | 2012 | |||||||||
| Ireland |
$ | 149 | $ | 66 | $ | — | ||||||
| Israel |
— | — | 22 | |||||||||
| Italy |
4 | 6 | 21 | |||||||||
| Spain |
— | — | (30 | ) | ||||||||
| Japan |
4 | 9 | — | |||||||||
| Brazil |
— | 17 | 43 | |||||||||
| Turkey |
— | — | 39 | |||||||||
| Canada |
4 | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
$ | 161 | $ | 98 | $ | 95 | ||||||
|
|
|
|
|
|
|
|||||||
| DECEMBER 31, | ||||||||
| LONG-LIVED ASSETS |
2014 | 2013 | ||||||
| U.S. |
716 | $ | 817 | |||||
| Foreign |
31 | 6 | ||||||
|
|
|
|
|
|||||
| Total |
$ | 747 | $ | 823 | ||||
|
|
|
|
|
|||||
(11) Commitments and Contingencies
Facility Leases
In addition to the related party lease arrangements described in Note 9, in May 2012, the Company entered into a five year non-cancelable lease for office and manufacturing space in Irvine, California that expires in May 2017, a 26-month sublease of office space in the same California location that commenced in June 2014 and will expire in July 2016 and a one-year lease (which is now month to month) in Dublin, Ireland that commenced on December 1, 2013. Rent expense for the years ended December 31, 2014, 2013 and 2012 was $234,000, $166,000 and $194,000, respectively. The following table shows the annual base rental cost over the term of the leases (in thousands).
| YEARS ENDED DECEMBER 31, |
OBLIGATIONS UNDER FACILITY LEASES |
|||
| 2015 |
$ | 195 | ||
| 2016 |
165 | |||
| 2017 |
50 | |||
| 2018 |
— | |||
| 2019 |
— | |||
| 2020 and Thereafter |
— | |||
|
|
|
|||
| Total |
$ | 410 | ||
|
|
|
|||
110
Contingencies
From time to time, the Company may be subject to legal proceedings and claims arising in the ordinary course of business. Management does not believe that the outcome of any of these matters will have a material effect on the Company’s consolidated financial operations.
(12) Income Taxes
Income (loss) before income tax consisted of the following (in thousands):
| YEAR ENDED DECEMBER 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| United States |
$ | (1,339 | ) | $ | (4,810 | ) | $ | (2,146 | ) | |||
| Foreign |
(14,315 | ) | (4,632 | ) | (2,901 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total |
$ | (15,654 | ) | $ | (9,442 | ) | $ | (5,047 | ) | |||
|
|
|
|
|
|
|
|||||||
The provision for income taxes is as follows (in thousands):
| YEAR ENDED DECEMBER 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| Current provision: |
||||||||||||
| Federal |
$ | — | $ | — | $ | — | ||||||
| State |
2 | 5 | — | |||||||||
| Foreign |
8 | 15 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total current |
10 | 20 | — | |||||||||
| Deferred provision |
||||||||||||
| Federal |
|
— |
|
— | — | |||||||
| State |
— | — | — | |||||||||
| Foreign |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total deferred |
|
— |
|
— | — | |||||||
| Valuation allowance |
|
— |
|
— | — | |||||||
|
|
|
|
|
|
|
|||||||
| Total deferred |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total income tax provision |
$ | 10 | $ | 20 | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
A reconciliation of the federal statutory rate to the effective rate is as follows:
| YEAR ENDED DECEMBER 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| Expected income tax benefit at federal statutory rate |
35.0 | % | 34.0 | % | 34.0 | % | ||||||
| State tax provision, net of federal benefit |
— | % | — | % | — | % | ||||||
| Foreign rate differential |
(24.0 | %) | (16.1 | %) | (15.4 | %) | ||||||
| Pass-through entities |
— | % | (13.5 | %) | (17.8 | %) | ||||||
| Change in valuation allowance |
(6.2 | %) | (2.3 | %) | (0.0 | %) | ||||||
| Nondeductible expenses |
(5.1 | %) | — | % | — | % | ||||||
| Other |
0.2 | % | (2.3 | %) | (0.8 | %) | ||||||
|
|
|
|
|
|
|
|||||||
| Income tax provision |
(0.1 | %) | (0.2 | %) | — | % | ||||||
|
|
|
|
|
|
|
|||||||
111
The components of the Company’s deferred tax assets are summarized as follows (in thousands):
| YEAR ENDED DECEMBER 31, | ||||||||
| 2014 | 2013 | |||||||
| Deferred tax assets: |
||||||||
| Net operating loss carryforwards |
$ | 1,201 | $ | 141 | ||||
| Accrued expenses |
44 | 80 | ||||||
| Stock based compensation |
186 | 79 | ||||||
|
|
|
|
|
|||||
| Deferred tax assets |
1,431 | 300 | ||||||
| Valuation allowance |
(1,366 | ) | (262 | ) | ||||
|
|
|
|
|
|||||
| Net deferred tax assets |
65 | 38 | ||||||
| Deferred tax liabilities: |
||||||||
| Depreciation |
(65 | ) | (38 | ) | ||||
|
|
|
|
|
|||||
| Total deferred tax liabilities |
(65 | ) | 38 | |||||
|
|
|
|
|
|||||
| Net deferred tax asset |
$ | — | $ | — | ||||
|
|
|
|
|
|||||
The valuation allowance has been established to offset the Company’s net deferred tax assets, as realization of such assets is not considered to be more likely than not due to the Company’s history of losses and uncertainties regarding the Company’s ability to generate future taxable income sufficient to realize the benefit of these deferred tax assets.
At December 31, 2014, the Company has U.S. Federal net operating loss (“NOL”) carryforwards of approximately $729,000 subject to potential limitations pursuant to Internal Revenue Code section 382 as discussed below. The federal NOL carryforwards will begin to expire in 2033, unless previously utilized. The Company has NOL carryforwards in Ireland of $7.6 million that can be carried forward indefinitely.
Pursuant to Sections 382 of the Internal Revenue Code (the “Code”), annual use of the Company’s NOL carryforwards may be limited in the event a cumulative change in ownership of 50% of certain shareholders occurs within a three-year period. An ownership change may limit the amount of NOL carryforwards that can be utilized annually to offset future taxable income and tax. In general, an “ownership change” as defined by Section 382 of the Code results from a transaction or series of transactions over a three-year period resulting in an ownership change of more than 50 percentage points of the outstanding stock of a company by certain shareholders.
For the year ended December 31, 2012, the Company was generally not subject to income taxes, as income or loss was either passed-through and included in the income tax returns of the Company’s shareholders or otherwise not subject to tax under local statute or rulings. As described in Note 1, the Parent completed a restructuring on October 21, 2013. As a result, some of the entities are no longer pass-through entities or were restructured as taxable entities. The impact of the change in tax status in 2013 has been reflected in the Company’s deferred tax summary above and is offset by a full valuation allowance.
Presbia Cooperatief U.A. is a Dutch Company that has received an advance ruling with the Tax and Customs Administration that is in effect from January 1, 2012 to December 31, 2015.
As of December 31, 2014 and 2013, the Company did not have any unrecognized tax benefits and does not anticipate a significant increase in the unrecognized tax benefits over the next 12 months. The Company’s policy is to recognize interest expense and penalties related to income tax matters as a component of the income tax provision. As of December 31, 2014 and 2013, the Company did not have any tax related accrued interest and penalties on its balance sheet or on its statement of operations and comprehensive loss.
112
Due to net operating loss carryovers, the U.S. federal and state returns are open to examination by the Internal Revenue Service and state jurisdictions for years 2008 through 2014. The foreign income tax returns are open to examination for the years 2011 through 2014.
The Company recognizes tax benefits associated with the exercise of stock options directly to stockholders’ equity only when realized. A windfall tax benefit occurs when the actual tax benefit realized upon an employee’s disposition of a share based award exceeds the deferred tax asset, if any, associated with the award. At December 31, 2014, deferred tax assets did not include $39,000 of excess tax benefits from stock based compensation.
(13) Quarterly Financial Data (unaudited)
The following tables present the unaudited financial data for each of the interim periods in 2014 and 2013:
| QUARTER ENDED (unaudited) | ||||||||||||||||
| 2014 | 03/31/14 | 06/30/14 | 09/30/14 | 12/31/14 | ||||||||||||
| Revenues |
$ | 26 | $ | 61 | $ | 38 | $ | 36 | ||||||||
| Cost of goods sold |
6 | 33 | 6 | 16 | ||||||||||||
| Operating expenses: |
||||||||||||||||
| Research and development |
732 | 1,675 | (a) | 1,016 | 820 | |||||||||||
| Sales and marketing |
249 | 394 | 463 | 546 | ||||||||||||
| General and administrative |
1,376 | 4,272 | (b) | 881 | 1,048 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Operating loss |
(2,337 | ) | (6,313 | ) | (2,328 | ) | (2,394 | ) | ||||||||
| Interest expense |
433 | 584 | 719 | 552 | ||||||||||||
| Other income |
— | 6 | — | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss before income tax provision |
(2,770 | ) | (6,891 | ) | (3,047 | ) | (2,946 | ) | ||||||||
| Income tax provision |
2 | 3 | 5 | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
$ | (2,772 | ) | $ | (6,894 | ) | $ | (3,052 | ) | $ | (2,946 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss per share-basic and diluted |
$ | (0.30 | ) | $ | (0.75 | ) | $ | (0.33 | ) | $ | (0.32 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Weighted average shares basic and diluted |
9,166,667 | 9,166,667 | 9,166,667 | 9,166,667 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| QUARTER ENDED (unaudited) | ||||||||||||||||
| 2013 | 03/31/13 | 06/30/13 | 09/30/13 | 12/31/13 | ||||||||||||
| Revenues |
$ | — | $ | 43 | $ | 11 | $ | 44 | ||||||||
| Cost of goods sold |
2 | 35 | 29 | 45 | ||||||||||||
| Operating expenses: |
||||||||||||||||
| Research and development |
482 | 472 | 656 | 526 | ||||||||||||
| Sales and marketing |
275 | 267 | 229 | 273 | ||||||||||||
| General and administrative |
523 | 489 | 1,351 | (c) | 1,725 | (c) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Operating loss |
(1,282 | ) | (1,220 | ) | (2,254 | ) | (2,525 | ) | ||||||||
| Interest expense |
516 | 594 | 667 | 384 | ||||||||||||
| Other expense |
— | — | — | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss before income tax provision |
(1,798 | ) | (1,814 | ) | (2,921 | ) | (2,909 | ) | ||||||||
| Income tax provision |
— | — | — | 20 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
$ | (1,798 | ) | $ | (1,814 | ) | $ | (2,921 | ) | $ | (2,929 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss per share-basic and diluted |
$ | (0.20 | ) | $ | (0.20 | ) | $ | (0.32 | ) | $ | (0.32 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Weighted average shares basic and diluted |
9,166,667 | 9,166,667 | 9,166,667 | 9,166,667 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
113
| (a) | Includes $1.2 million related to commencement of U.S clinical trials |
| (b) | Includes $3.4 million write-off of deferred offering costs |
| (c) | Includes legal and professional fees in connection with the Company’s 2012 and 2011 financial statement audits and the October 2013 restructuring. |
(14) Subsequent Events
2015 Capital Contribution
On January 14, 2015, the Company amended its memorandum and articles of association, resulting in the redesignation of the 40,000 Euro ordinary shares as deferred ordinary shares. As of January 14, 2015, the share capital of the Company was $400,000 and €40,000 divided into 350,000,000 ordinary shares of $0.001 each (the “Ordinary Shares”), 9,166,667 of which are outstanding and were issued in connection with the 2015 Capital Contribution disclosed in Note 1, 50,000,000 preferred shares of $0.001 (the “Preferred Shares”) each, all of which are authorized but unissued, and 40,000 deferred ordinary shares of €1.00 each (the “Deferred Ordinary Shares”), all of which are issued and outstanding.
The rights and restrictions attaching to the Ordinary Shares are as follows: the right to attend and speak at any general meeting of the Company and to exercise one vote per Ordinary Share held at any general meeting of the Company; the right to participate pro rata in all dividends declared by the Company; and the right, in the event of the Company’s winding up, to participate pro rata in the total assets of the Company.
The rights and restrictions attaching to the Deferred Ordinary Shares are as follows:
Income: The holder of a Deferred Ordinary Share shall not be entitled to receive any dividend or distribution declared, made or paid or any return of capital (save as provided below) and shall not entitle its holder to any further or other right of participation in the assets of the Company;
Capital: On a winding up of, or other return of capital (other than on a redemption of any class of shares in the capital of the Company) by the Company, the holders of Deferred Ordinary Shares shall be entitled to participate in such return of capital or winding up of the Company, such entitlement to be limited to the repayment of the amount paid up or credited as paid up on such Deferred Ordinary Shares and shall be paid only after the holders of Ordinary Shares shall have received payment in respect of such amount as is paid up or credited as paid up on those Ordinary Shares held by them at that time, plus the payment in cash of $5,000,000 on each such Ordinary Share.
Acquisition of Deferred Ordinary Shares: The Company as agent for the holders of Deferred Ordinary Shares shall have the irrevocable authority to authorise and instruct the Company’s secretary (or any other person appointed for the purpose by the Company’s board of directors) to acquire, or to accept the surrender of, the Deferred Ordinary Shares for no consideration and to execute on behalf of such holders such documents as are necessary in connection with such acquisition or surrender, and pending such acquisition or surrender to retain the certificates, to the extent issued, for such Deferred Ordinary Shares.
Voting: The holders of Deferred Ordinary Shares shall not be entitled to receive notice of, nor attend, speak or vote at, any general meeting.
The rights attaching to the Preferred Shares will be determined by the Company’s board of directors. The directors are authorized to issue all or any of the authorized but unissued Preferred Shares from time to time in one or more classes or series, and to fix for each such class or series such voting power, full or limited, or no voting power, and such designations, preferences and relative, participating, optional or other special rights and such qualifications, limitations or restrictions thereof, as shall be stated and expressed in the resolution or resolutions adopted by the Board providing for the issuance of such class or series, including, without limitation, the authority to provide that any such class or series may be: redeemable at the option of the Company, or the holders, or both, with the manner of the redemption to be set by the Company’s board of directors, and redeemable at such time or times, including upon a fixed date, and at such price or prices,
114
as shall be determined by the Board; entitled to receive dividends (which may be cumulative or non-cumulative) at such rates, on such conditions and at such times, and payable in preference to, or in such relation to, the dividends payable on any other class or classes of shares or any other series all as shall be determined by the Board; entitled to such rights upon the dissolution of, or upon any distribution of the assets of, the Company, as shall be determined by the Board; or convertible into, or exchangeable for, shares of any other class or classes of shares, or of any other series of the same or any other class or classes of shares, of the Company, at such price or prices or at such rates of exchange and with such adjustments as the Company’s board of directors determines.
On January 14, 2015, the Company entered into a Debt Contribution Agreement pursuant to which all outstanding amounts owed to the Parent under the Interest Rate Agreement at that time were contributed to capital of the Company’s subsidiaries.
Presbia Incentive Plan
On January 14, 2015, the Company approved a compensation incentive plan (the “Presbia Incentive Plan”). The Presbia Incentive Plan permits the Company to grant awards of stock options, restricted shares, stock appreciation rights, restricted share units, performance shares, performance share units, dividend equivalent rights in respect of awards and other share-based and cash-based awards, including annual and long-term cash incentive awards.
Awards under the Presbia Incentive Plan may be granted to employees, directors, consultants and other persons who perform services for the Company or a subsidiary of the Company. A total of 1,800,000 ordinary shares are authorized for issuance under the Presbia Incentive Plan.
On January 28, 2015, the Company’s board of directors approved grants to the following officers and directors, effective upon the execution of the underwriting agreement relating to the Company’s initial public offering: to Ralph Thurman, options to purchase 250,000 ordinary shares; to Zohar Loshitzer, options to purchase 100,000 ordinary shares; to Vladimir Feingold, options to purchase 100,000 ordinary shares; to Todd Cooper, options to purchase 450,000 ordinary shares; to Richard Ressler, options to purchase 10,000 ordinary shares; and to Mark Blumenkranz, options to purchase 10,000 ordinary shares. All such options were granted pursuant to the Presbia Incentive Plan at an exercise price of $10.00 per share and equal to the price at which the Company’s ordinary shares were offered to the public in its initial public offering. The options granted to Messrs. Loshitzer, Feingold and Cooper will vest in five equal annual installments commencing one year after the date on which such price is determined pursuant to the Company’s execution of the underwriting agreement relating to its initial public offering. The options granted to Messrs. Thurman, Ressler and Blumenkranz will vest with respect to one third of the underlying ordinary shares on the grant date and one third of the underlying ordinary shares on each of the next two anniversaries of that date.
On March 16, 2015, the Company’s board of directors approved grants of 9,270 restricted ordinary shares of the Company to Robert J. Cresci and to Mark Blumenkranz, respectively, and stock option awards of 10,000 options to Richard Fogarty and Jake Vander Zanden, respectively, at an exercise price of $8.63 per share. The restricted shares and stock options will vest in five equal, annual installments commencing one year after the date of grant. Further on March 16, 2015, the board of directors approved an aggregate 82,500 stock options to be granted to other employees vesting in five equal, annual installments commencing one year following the date of grant.
Initial Public Offering
On February 3, 2015, the Presbia PLC completed its IPO in which 4,166,667 ordinary shares were offered to the public at $10.00 per share. The Parent acquired 500,000 of the ordinary shares sold in the IPO.
Presbia Investments
On March 26, 2015, Presbia Investments, a Cayman Islands entity, was formed as a wholly-owned subsidiary of Presbia PLC for the purpose of investing the proceeds from the initial public offering.
115
Item 9. Changes in and Disagreements With Accountants on Accounting and Financial Disclosure.
None.
Item 9A. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures.
Our management, with the participation of our Chief Executive Officer and Chief Accounting Officer, has evaluated the effectiveness of our disclosure controls and procedures (as such term is defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended) as of the end of the period covered by this Annual Report on Form 10-K. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost benefit relationship of possible controls and procedures. Based on such evaluation, our Chief Executive Officer and Chief Accounting Officer have concluded that, as of the end of the period covered by this report, our disclosure controls and procedures were effective.
Management’s Report on Internal Control Over Financial Reporting
This annual report does not include a report of management’s assessment regarding internal control over financial reporting or an attestation report of the Company’s registered public accounting firm due to a transition period established by rules of the SEC for newly public companies.
Changes in Internal Control Over Financial Reporting
There was no change in our internal control over financial reporting identified in connection with the evaluation required by Rule 13a-15(d) and 15d-15(d) of the Exchange Act that occurred during the quarter ended December 31, 2014 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
None.
116
Part III
Item 10. Directors, Executive Officers and Corporate Governance.
The following table sets forth information regarding our executive officers and directors as of March 27, 2015.
| NAME |
AGE | POSITION(S) | ||||
| Ralph “Randy” Thurman(1)(2)(3)(4) |
65 | Executive Chairman of the Board | ||||
| Todd Cooper(1) |
50 | Chief Executive Officer, President and Director | ||||
| Zohar Loshitzer |
57 | Chief Business Development Officer and Director | ||||
| Vladimir Feingold |
65 | Chief Technology Officer, Executive Vice President and Director | ||||
| John Jacob (“Jake”) Vander Zanden |
53 | Chief Commercial Officer | ||||
| Richard Fogarty |
64 | Chief Accounting Officer, Vice President, Finance and Secretary | ||||
| Mark S. Blumenkranz(2)(3)(4) |
63 | Director | ||||
| Richard Ressler(3)(4) |
56 | Director | ||||
| Robert J. Cresci(2)(3)(4) |
71 | Director | ||||
| (1) | Member of our executive committee. |
| (2) | Member of our audit committee. |
| (3) | Member of our nominating and corporate governance committee. |
| (4) | Member of our compensation committee. |
Ralph “Randy” Thurman has served as a director of Presbia Holdings since October 2013 and has served as its Executive Chairman since January 2014. From October 2014 through January 2015, Mr. Thurman served as the Chief Executive Officer of Presbia PLC. He commenced serving as the Executive Chairman of Presbia PLC in January 2015. Mr. Thurman has served as a consulting advisor in private equity since 2008. Mr. Thurman served as an Operating Executive and Partner at AEA Investments LP (“AEA Investments”), a private equity firm, from October 2012 through March 2014, and during that period, was the Executive Chairman of Cogent HMG, a portfolio company of AEA Investments engaged in healthcare technology. Before joining AEA Investments, Mr. Thurman was a Senior Advisor at New Mountain Capital, LLC, a private and public equity investment firm, since May 2008. From July 2008 to October 2011, Mr. Thurman served as a director of CardioNet, Inc., a publicly-traded global medical technology company focused on diagnosing and monitoring cardiac arrhythmias, where he also served as Executive Chairman from July 2008 to January 2009, as President and Chief Executive Officer from February 2009 to June 2010, and as Chairman from June 2009 until his resignation from the board of directors in October 2011. From 2001 to 2007, he was a Founder, Chairman and Chief Executive Officer of VIASYS Healthcare Inc., a privately-held healthcare technology company. Mr. Thurman served as a director of Enzon, Inc., a biotechnology company, from 1993 to 2001 and served as Chairman from 1996 to 2001. From 1997 to 2001, Mr. Thurman served as Chairman and Chief Executive Officer of Strategic Reserves LLC, a privately-held company providing funding and strategic direction to healthcare technology companies. Prior to that, he served as Chairman and Chief Executive Officer of Corning Life Sciences, Inc., a manufacturer of laboratory products for life sciences research (1993 to 1997), and held various positions at Rhone-Poulenc Rorer Pharmaceuticals, Inc., a global pharmaceutical company (1984 to 1993). Mr. Thurman currently serves on the board of directors of each of the following publicly-traded companies: Arno Therapeutics, Inc. and Allscripts Healthcare Solutions, Inc. From November 2013 to February 2014, Mr. Thurman served as a director of Orthofix International N.V. Mr. Thurman received a B.S. in economics from Virginia Polytechnic Institute and an M.A. in management from Webster University.
Mr. Thurman has been chosen to serve on our Board due to his experience and expertise as an investor in medical device and healthcare companies.
Todd Cooper has served as the President and Chief Executive Officer of Presbia PLC and as a director of Presbia PLC since January 2015. From July 2011 to November 2014, Mr. Cooper served as the Chief Executive Officer of NVISION, which operates a network of ophthalmological surgical centers. From October 2008 to June 2010, Mr. Cooper served as the Vice President of Marketing and the General Manager of Henry Schein Medical, a
117
division of Henry Schein, Inc., a global provider of health care products and services to office-based practitioners. Prior to that, Mr. Cooper served as: the Senior Vice President, Global Sales and Marketing of Discus, a global manufacturer of dental products (2003 to 2008); a Senior Vice President of IMG Holdings, a boutique management consulting firm (1997-2003); the Director of Marketing at The Franklin Mint, a consumer products company (1995 to 1997); and the Senior Marketing Manager at Canadian Tire Corporation, Limited, a retailer which sells a wide range of automotive, sports, leisure, and home products (1993 to 1995). Mr. Cooper received a B.A. from the University of Alberta in 1994.
Mr. Cooper has been chosen to serve on our Board due to his extensive management experience in ophthalmological and other medical device companies and his prior chief executive officer experience.
Zohar Loshitzer has served as the President and Chief Executive Officer and a director of Presbia Holdings since May 2007, served as the Chief Executive Officer of Presbia PLC from February 2014 to October 2014, served as the President of Presbia PLC from February 2014 to January 2015 and has served as a director of Presbia PLC since February 2014. Mr. Loshitzer assumed the position of Chief Business Development Officer of Presbia PLC upon Mr. Cooper’s election as the Chief Executive Officer and President of Presbia PLC in January 2015. Since January 2005, Mr. Loshitzer has served as a principal at Orchard Capital, where he supports the portfolio companies of Orchard Capital by designing operational efficiencies and cost reductions, and he has served since August 2000 as the President and Chief Executive Officer of Universal Telecom Services, Inc., a provider of telecommunications services and solutions to emerging markets. He has served as Executive Vice President of Corporate Strategy of j2 Global since June 2001 and from July 1997 through June 2001 he served as the Chief Information Officer at j2 Global. Mr. Loshitzer was the founder and President of MTP Consulting, Inc., a business consulting firm, from January 2011 to August 2013, and he was the founder and President of Imali, Inc., another business consulting firm, from January 2007 to December 2010. Since 1995, he has been a Managing Director at Orchard Telecom, a provider of telecommunications products. He previously served as a consultant to MAI Systems Corporation, a provider of information technology solutions, and as a General Manager and Managing Director at Life Alert Emergency Response, Inc., a provider of security services for the elderly, which Mr. Loshitzer co-founded. He has been a director of the publicly-traded Advanced Cell Technology Inc. since December 2011 and publicly-traded Environmental Solutions Worldwide, Inc. since January 2011. He graduated from Tel Aviv University with a degree in Electronics Engineering.
Mr. Loshitzer has been chosen to serve on our Board as a result of his finance and business management knowledge and experience and his investment experience in start-ups and early stage financings.
Vladimir Feingold has served as the Chief Technology Officer and as a director of Presbia Holdings since September 2009, the Chief Technology Officer of Presbia PLC since February 2014 and an Executive Vice President of Presbia PLC since June 2014, and he commenced serving as a director of Presbia PLC in January 2015. He has more than 30 years’ experience as an executive in the medical technology field and in research and development. Beginning in September 2001, he was the Chief Executive Officer of Visitome, Inc. and Biovision AG until Biovision AG was liquidated in bankruptcy in Switzerland in 2008 and the operations of Visitome were merged into Presbia in 2009. Previously, Mr. Feingold served as President and Chairman of Staar Surgical AG, Switzerland (1994-1999) and held positions of increasing responsibility with its parent, Staar Surgical Co., a U.S. public company (1991-1999). From 1995 to 2000, Mr. Feingold was also a director of Canon-Staar, Japan, and from 1984 to 2001, Mr. Feingold was managing director and chairman of the board of Bionica Pty Ltd., Australia, a company which produced and distributed an ambulatory medication delivery system, and marketed ophthalmic products. From 1975 to 1984, Mr. Feingold held various research, engineering and manufacturing executive positions at Telectronics Pty. Ltd., Australia. At that time, Telectronics manufactured miniature advance cardiac pacemakers. Mr. Feingold received his B.E. (Mechanical) and B.Sc. (Computer Science and Mathematics) from the University of Sydney, Australia.
Mr. Feingold has been chosen to serve on our Board as a result of his scientific background, his familiarity with presbyopia and his understanding of our industry.
118
John Jacob (“Jake”) Vander Zanden has served as the Chief Commercial Officer of Presbia Holdings since February 2013 and has served as the Chief Commercial Officer of Presbia PLC since February 2014. As Chief Commercial Officer at Presbia, Mr. Vander Zanden is responsible for expanding the commercial growth of our microlens. From September 2009 to July 2012, Mr. Vander Zanden served as President of the Americas and as Senior Vice President of Global Marketing of Hoya Surgical Optics, Inc., an ophthalmic medical device company. Previously, Mr. Vander Zanden served as the Corporate Vice President, Senior Vice President and General Manager at Lumenis Ltd., a medical laser company, from March 2007 to June 2008, and as Vice President and General Manager, Global Pain Management, at Medtronic, Inc., a medical technology company, from September 2004 to May 2006. In 2006, he co-founded Vander Zanden Group, a consulting firm focused on start-up as well as pre- and post-merger and acquisition activities, strategy, and growth initiatives. He held numerous roles in pharmaceuticals, biologics, medical devices and over-the-counter consumer products at Allergan, Inc., a multi-specialty health care company, from March 1989 to September 2004. Mr. Vander Zanden received his B.A. in Marketing and Management from the College of St. Thomas in St. Paul, Minnesota. He serves on the Board of Advisors of the Sand Hill Incubator.
Richard T. Fogarty has served as the Chief Accounting Officer of Presbia Holdings since August 2013 and has served as the Secretary of Presbia PLC since formation, the Chief Accounting Officer of Presbia PLC since February 2014 and the Vice President, Finance of Presbia PLC since June 2014. From February 2010 to August 2013, Mr. Fogarty was Vice President, Finance and Administration and Chief Financial Officer for Plainfield Precision, Inc., a provider of contract manufacturing services to the automotive and medical device industries. Mr. Fogarty held corporate controller and corporate finance positions in publicly-held companies Collectors Universe, Inc., which provides authentication and grading services for high-value collectibles, from March 2006 to February 2010, and Impco Technologies, Inc. (now Fuel Systems Solutions, Inc.), which designs, manufactures and supplies alternative fuel products and systems, from November 2002 to March 2006. Mr. Fogarty holds an M.B.A. from Fairleigh Dickinson University and a B.S. degree from Union College. Mr. Fogarty is a Certified Management Accountant.
Dr. Mark S. Blumenkranz has served as a director of Presbia Holdings since October 2013 and commenced serving as a director of Presbia PLC in January 2015. He is the H.J. Smead Professor and Chairman of the Department of Ophthalmology at Stanford University, where he has served since January 1998. He is the founding director of the Byers Eye Institute, a nationally-recognized eye care center dedicated to combating blindness and preserving sight. Dr. Blumenkranz is a founder and director of several privately-held early stage companies in the ophthalmic field. He was a founder and director of Peak Surgical, Inc., an innovator in pulsed plasma mediated electrosurgery that was acquired by Medtronic, Inc. in 2011. In 2004, he co-founded Optimedica Corporation, which was acquired by Abbott Medical Optics, Inc. in 2013. Dr. Blumenkranz co-founded, and has served as a director since July 2006 of, Avalanche Biotechnologies, Inc., which company has been publicly traded since August 2014. He received his Baccalaureate, Master of Medical Science, and M.D. degrees at Brown University, followed by a Residency in Ophthalmology at Stanford University and a fellowship in vitreoretinal diseases at the Bascom Palmer Eye Institute of the University of Miami, where he served on the faculty for five years. Dr. Blumenkranz is a past-President of the American University Professors of Ophthalmology, Retina Society and Macula Society, and a Fellow of the Corporation of Brown University, where he serves as chairman of the Medical School Committee.
Dr. Blumenkranz has been chosen to serve on our Board due to his expertise in ophthalmic matters and his experience in early stage biomedical company development.
Richard S. Ressler has served as an officer and a director of Presbia Holdings since May 2007 and commenced serving as a director of Presbia PLC in January 2015. Mr. Ressler serves on the board of Presbia Holdings pursuant to a services agreement between our company and Orchard Capital Corporation (“Orchard Capital”). Mr. Ressler is the founder, owner and President of Orchard Capital, a firm that provides consulting and advisory services to companies (including Presbia) in which Orchard Capital or its affiliates invest. He has been President of Orchard Capital since 1994. Mr. Ressler has been Chairman of the Board of Directors of j2 Global, Inc. (“j2
119
Global”), a publicly-traded provider of services through the internet, since 1997, and served as j2 Global’s Chief Executive Officer from 1997 to 2000, serving in each of these capacities pursuant to a consulting agreement between j2 Global and Orchard Capital. Since March 2014, Mr. Ressler has been Chairman of the Board of Directors of CIM Commercial Trust Corporation, a publicly-traded real estate investment trust. Through an agreement with Orchard Capital, Mr. Ressler serves in various senior capacities with, among other companies, CIM Group, L.P. (together with its affiliates, “CIM Group”), a real estate investment and management company co-founded by Mr. Ressler, and Orchard First Source Asset Management (together with its affiliates, “OFSAM”), an investment adviser focusing on middle market debt investments co-founded by Mr. Ressler. Both OFSAM and its wholly owned subsidiary, OFS Capital Management, LLC, are registered with the U.S. Securities and Exchange Commission as registered investment advisers. Mr. Ressler also serves as a board member for various private companies in which Orchard Capital or its affiliates invest. Mr. Ressler holds a B.A. from Brown University and J.D. and M.B.A. degrees from Columbia University.
Mr. Ressler has been chosen to serve on our Board as a result of his extensive experience with, and knowledge of, business management and finance.
Robert J. Cresci has served as a director of Presbia PLC since March 2015. Robert J. Cresci has been a managing director of Pecks Management Partners Ltd., an investment management firm, since 1990. Mr. Cresci currently serves on the boards of j2 Global, Inc., Luminex Corporation, OFS Capital Corporation, CIM Commercial Trust Corporation, and several private companies. Mr. Cresci previously served on the board of Continucare Corporation until 2011 and the board of Sepracor, Inc. until 2009. By virtue of his time with Pecks Management Partners and the other business entities mentioned, Mr. Cresci brings to our board of directors his broad expertise and experience in accounting issues, and public company matters. Mr. Cresci holds an undergraduate degree in Engineering from the United States Military Academy at West Point and holds a M.B.A. in Finance from the Columbia University Graduate School of Business.
Each of our executive officers and directors also serves as an executive officer and/or director of our Presbia USA, Inc. subsidiary. Certain of our executive officers and directors are also officers and/or directors of other subsidiaries of our company.
There are no family relationships among any of our directors or executive officers.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act requires persons who own more than ten percent of a registered class of our equity securities and our directors and executive officers to file with the SEC initial reports of ownership and reports in changes in ownership of any Presbia PLC equity securities. Because we were not publicly traded in 2014, no reports were required to be filed in 2014.
Code of Business Conduct and Ethics
We have adopted a code of business conduct and ethics that applies to all of our employees, officers and Board members, including those officers responsible for financial reporting. Our code of business conduct and ethics is available on our website (http://www.presbia.com). We expect that any amendments to the code, or any waivers of its requirements, will be disclosed on our website.
Board Composition and Committees
Our controlling shareholder, Presbia Holdings, a Cayman Islands entity, controls a majority of our issued and outstanding ordinary shares. As a result, we are a “controlled company” within the meaning of NASDAQ listing rules, and thus, we are exempt from the following NASDAQ requirements:
| • | the requirement that a majority of our Board consist of independent directors; |
120
| • | the requirement that director nominees be selected, or recommended for the Board’s selection, either by independent directors constituting a majority of the Board’s independent directors in a vote in which only independent directors participate, or by a nominating committee comprised solely of independent directors; and |
| • | the requirement that we have a compensation committee that is composed entirely of independent directors with a written charter addressing the committee’s purposes and responsibilities. |
We intend to rely on these exemptions. As a result, we do not have a majority of independent directors and we do not have a compensation committee or nominating and corporate governance committee consisting entirely of independent directors. Accordingly, our investors do not have the same protections afforded to shareholders of companies that are subject to all of NASDAQ’s corporate governance requirements.
Executive Committee. The executive committee of our Board shall consist of two directors, which directors shall be Mr. Cooper and Mr. Thurman, provided that Mr. Cooper and Mr. Thurman shall have the right to designate another member of our Board to serve on the executive committee for any meeting for which either of Mr. Cooper or Mr. Thurman is unavailable. The chairman of the executive committee shall be the individual appointed by the committee members as the chairman at the commencement of each meeting of the committee. The executive committee is authorized to exercise all functions of our Board in the intervals between meetings of our Board to the extent permitted by law.
Audit Committee. The audit committee of our Board consists of Dr. Blumenkranz and Messrs. Cresci and Thurman, and Mr. Cresci serves as the chairman of this committee. The audit committee assists our Board in its oversight responsibilities relating to the integrity of our financial statements, the qualifications, independence, compensation and performance of our independent auditors, our systems of internal accounting and financial controls, the performance of our internal audit function, the compliance of our company with legal and regulatory requirements and compliance with our company’s Code of Business Conduct and Ethics. We currently have two independent directors serving on our audit committee. We are required and intend to have a completely independent audit committee within one year of our listing on The NASDAQ Global Market.
Subject to transition rules satisfied by the time schedule described above, our audit committee members must satisfy both NASDAQ and SEC independence criteria. Under the NASDAQ listing rules, a director will only qualify as an “independent director” if (i) the director is not disqualified under certain objective tests established by the NASDAQ listing rules and (ii) in the opinion of the issuer’s board of directors, that person does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. To be considered independent for purposes of the SEC’s rules, a member of an audit committee of a listed company may not, other than in his or her capacity as a member of the audit committee, the board of directors, or any other board committee: (1) accept, directly or indirectly, any consulting, advisory, or other compensatory fee from the listed company or any of its subsidiaries or (2) be an affiliated person of the listed company or any of its subsidiaries.
Our Board has determined that Dr. Mark S. Blumenkranz and Mr. Robert J. Cresci meet both the SEC and the NASDAQ definitions of an independent director with respect to their service on our audit committee. In making this determination, our Board considered the relationships that Dr. Blumenkranz and Mr. Cresci have with our company and all other facts and circumstances our Board deemed relevant in determining their independence. Our Board has determined that Mr. Cresci qualifies as an “audit committee financial expert” under SEC rules and regulations.
Compensation Committee. The compensation committee of our Board consists of Dr. Blumenkranz and Messrs. Ressler, Cresci and Thurman, and Mr. Thurman serves as the chairman of this committee. The primary purpose of the compensation committee of our Board is to (i) facilitate our Board’s discharge of its responsibilities relating to the evaluation and compensation of our executives, (ii) oversee the administration of our
121
compensation plans, including the Presbia Incentive Plan, (iii) review and determine Board member compensation and (iv) prepare any report on executive compensation required by the rules and regulations of the SEC and the listing rules of NASDAQ.
Nominating and Corporate Governance Committee. The nominating and corporate governance committee of our Board consists of Dr. Blumenkranz and Messrs. Thurman, Cresci and Ressler, and Mr. Thurman serves as the chairman of this committee. The primary purpose of our nominating and corporate governance committee is to (i) review the qualifications of, and recommend to our Board, proposed nominees for election to our Board, consistent with criteria approved by our Board, (ii) select, or recommend that our Board select, the director nominees for the next annual meeting of shareholders, (iii) develop, evaluate and recommend to our Board corporate governance practices applicable to our company and (iv) lead our Board in its annual review of the Board and management.
Our Board has adopted written charters under which the audit committee, compensation committee and nominating and corporate governance committee operate. A copy of each of these charters, which satisfy the applicable standards and rules of the SEC and NASDAQ, is available on our website (http://www.presbia.com).
Compensation Committee Interlocks and Insider Participation
None of our executive officers serves as a member of the compensation committee or board of directors of any other entity that has an executive officer serving or expected to serve as a member of our Board or compensation committee.
Board Diversity
Our nominating and corporate governance committee is responsible for reviewing with the Board, on an annual basis, the appropriate characteristics, skills and experience required for the Board as a whole and its individual members. In evaluating the suitability of individual candidates (both new candidates and current members), the nominating and corporate governance committee, in recommending candidates for election, and the Board, in approving (and, in the case of vacancies, appointing) such candidates, will take into account many factors, including the following:
| • | diversity of personal and professional background, perspective and experience; |
| • | personal and professional integrity, ethics and values; |
| • | experience in corporate management, operations or finance, such as serving as an officer or former officer of a publicly-traded company, and a general understanding of marketing, finance and other elements relevant to the success of a publicly-traded company in today’s business environment; |
| • | experience relevant to our industry and with relevant social policy concerns; |
| • | experience as a board member or executive officer of another publicly-traded company; |
| • | relevant academic expertise or other proficiency in an area of our operations; |
| • | practical and mature business judgment, including ability to make independent analytical inquiries; |
| • | promotion of a diversity of business or career experience relevant to the success of our company; and |
| • | any other relevant qualifications, attributes or skills. |
Our Board intends to evaluate each individual in the context of the Board as a whole, with the objective of assembling a group that can best maximize the success of the business and represent shareholder interests through the exercise of sound judgment using its diversity of experience in these various areas.
122
Item 11. Executive Compensation.
This section discusses the material components of the executive compensation program for our named executive officers who are identified in the Summary Compensation Table below. This discussion may contain forward-looking statements that are based on our current plans, considerations, expectations and determinations regarding future compensation programs. Actual compensation programs that we adopt following the completion of this offering may differ materially from the currently planned programs summarized in this discussion.
Summary Compensation Table
As an emerging growth company, we have opted to comply with the executive compensation disclosure rules applicable to “smaller reporting companies,” as such term is defined in the rules promulgated under the Securities Act, which require compensation disclosure for each person who served as our principal executive officer during 2014 and our two most highly compensated executive officers other than our principal executive officers whose total compensation for 2014 exceeded $100,000. During 2014, excluding Mr. Loshitzer and Mr. Thurman, each of whom served as our principal executive officer during a portion of 2014, the two most highly compensated executive officers whose total compensation exceeded $100,000 were Vladimir Feingold and Jake Vander Zanden. Messrs. Thurman, Loshitzer, Feingold and Vander Zanden are referred to in this Annual Report on Form 10-K as our named executive officers.
The following table provides information regarding the compensation awarded to, or earned by, our named executive officers during 2014 and 2013.
SUMMARY COMPENSATION TABLE
| NAME AND PRINCIPLE |
YEAR | SALARY ($) |
BONUS ($) |
STOCK AWARDS ($) |
OPTION AWARDS ($) |
NON-EQUITY INCENTIVE PLAN COMPENSA- TION ($) |
NON-QUALI- FIED DEFERRED COMPENSA- TION EARNINGS ($) |
ALL OTHER COMPENSA- TION ($) |
TOTAL ($) |
|||||||||||||||||||||||||||
| Ralph Thurman |
2014 | (1) | $ | 120,000 | — | — | — | — | — | — | $ | 120,000 | ||||||||||||||||||||||||
| Chief Executive Officer |
2013 | — | — | $ | 75,000 | (3) | — | — | — | — | 75,000 | |||||||||||||||||||||||||
| (October 2014 - January 2015) |
||||||||||||||||||||||||||||||||||||
| Zohar Loshitzer |
2014 | (2) | 300,000 | — | — | — | — | — | — | 300,000 | ||||||||||||||||||||||||||
| Chief Executive Officer |
2013 | (2) | 300,000 | (4) | — | 300,000 | (5) | — | (6) | — | — | — | 600,000 | |||||||||||||||||||||||
| (2013 & through October 2014) and President (2013 & 2014) |
||||||||||||||||||||||||||||||||||||
| Vladimir Feingold |
2014 | 300,000 | — | — | — | — | — | — | 300,000 | |||||||||||||||||||||||||||
| Chief Technology |
2013 | 300,000 | — | — | — | (6) | — | — | — | 300,000 | ||||||||||||||||||||||||||
| Officer |
||||||||||||||||||||||||||||||||||||
| Jake Vander Zanden |
2014 | 200,000 | 20,000 | — | — | — | — | — | 220,000 | |||||||||||||||||||||||||||
| Chief Commercial |
2013 | 183,000 | (7) | — | — | 99,280 | (8) | — | — | — | 282,280 | |||||||||||||||||||||||||
| Officer |
||||||||||||||||||||||||||||||||||||
| (1) | Mr. Thurman was appointed Chief Executive Officer in October 2014 and, as a result, his compensation for 2014 is based on service for less than an entire year. |
| (2) | Mr. Loshitzer served as Chief Executive Officer and President from February 2014 to October 2014. In October 2014, Mr. Loshitzer was succeeded as Chief Executive Officer by Mr. Thurman, but continued as President. Prior to his appointment as Chief Executive Officer and President, Mr. Loshitzer served as a consultant to the company until becoming an employee on November 1, 2013. |
| (3) | Mr. Thurman was awarded 250,000 restricted ordinary shares of Presbia Holdings, our principal shareholder, in 2013. The amount reported represents the aggregate grant date fair value calculated in accordance with FASB ASC 718. Information concerning the assumptions used to calculate this amount is set forth in Note 8 of the audited consolidated financial statements presented elsewhere in this Annual Report on Form 10-K. |
123
| (4) | A portion of such amount represents consulting fees paid to Mr. Loshitzer’s wholly-owned consulting company or directly to Mr. Loshitzer. Effective November 1, 2013, Mr. Loshitzer became an employee of our company. |
| (5) | Mr. Loshitzer was awarded 1,000,000 restricted ordinary shares of Presbia Holdings. The amount reported represents the aggregate grant date fair value calculated in accordance with FASB ASC 718. Information concerning the assumptions used to calculate this amount is set forth in Note 8 of the audited consolidated financial statements presented elsewhere in this Annual Report on Form 10-K. |
| (6) | In October 2013, replacement options covering 710,000 ordinary shares of Presbia Holdings were granted to each of Messrs. Loshitzer and Feingold, which had a grant date incremental fair value of zero. Additional information concerning the replacement options and the assumptions used to calculate these amounts is set forth in Note 8 of the audited consolidated financial statements presented elsewhere in this Annual Report on Form 10-K. |
| (7) | Mr. Vander Zanden commenced employment with our company during 2013 and, as a result, his compensation for 2013 is based on service for less than an entire year. |
| (8) | Mr. Vander Zanden was awarded options to purchase 500,000 ordinary shares of Presbia Holdings in October 2013. The amount reported represents the aggregate grant date fair value calculated in accordance with FASB ASC 718. Information concerning the assumptions used to calculate this amount is set forth in Note 8 of the audited consolidated financial statements presented elsewhere in this Annual Report on Form 10-K. |
Mr. Loshitzer served as our Chief Executive Officer throughout 2013 and until October 2014, when he was succeeded by Mr. Thurman, who currently serves as our Executive Chairman. When Mr. Thurman accepted the position of Chief Executive Officer, it was with the understanding that we would conduct an active search to identify a suitable successor with executive experience in the medical device industry. As a result of that search, in January 2015, our Board named Todd Cooper as our new Chief Executive Officer and President. Effective upon Mr. Cooper’s appointment, Mr. Thurman was named our Executive Chairman and Mr. Loshitzer was named our Chief Business Development Officer.
Cash Bonus Program
Our Board has established for 2015, and expects to continue in subsequent years, a cash bonus program for our employees. Pursuant to this program, employees will be eligible to receive an annual cash target bonus based on a specified percentage of the employee’s salary, which bonus will be earned upon the achievement of certain specified individual and corporate milestones.
Equity Awards
Presbia PLC
No equity awards were granted by Presbia PLC to our named executive officers during the year ended December 31, 2014. At December 31, 2014, none of our named executive officers held any options to purchase our ordinary shares or held any other share awards in respect of our ordinary shares. In connection with our initial public offering, in January 2015, we granted options to our named executive officers and Todd Cooper as follows:
| • | to Ralph Thurman, options to purchase 250,000 ordinary shares; |
| • | to Zohar Loshitzer, options to purchase 100,000 ordinary shares; |
| • | to Vladimir Feingold, options to purchase 100,000 ordinary shares; and |
| • | to Todd Cooper, options to purchase 450,000 ordinary shares. |
All such options were granted pursuant to the Presbia Incentive Plan at an exercise price of $10.00 per share and expire ten years from the grant date. With the exception of Mr. Thurman’s options, such options vest in five equal annual installments commencing one year after the grant date. Mr. Thurman’s options vested with respect to one third of the underlying ordinary shares on the grant date and will vest with respect to one third of the underlying ordinary shares on each of the next two anniversaries of that date. None of such options are exercisable while the recipient is an employee, officer or director of our company, unless our Board, in its sole discretion, waives such restriction after determining that such exercise shall not trigger a Rule 9 mandatory takeover under the Irish Takeover Panel Act 1977 (as amended) and the related Irish takeover rules by the recipient or any other officer, employee, director or shareholder of our company.
124
We expect from time to time to make equity grants under the Presbia Incentive Plan to our employees to align the interests of our employees with our company.
Presbia Holdings
No equity awards were granted by Presbia Holdings to our named executive officers during 2014.
The following table sets forth information regarding holdings by our named executive officers, as of December 31, 2014, of unexercised stock options granted by Presbia Holdings and outstanding restricted stock awards granted by Presbia Holdings.
OUTSTANDING EQUITY AWARDS AT FISCAL YEAR-END
| OPTIONS AWARDS(1) | STOCK AWARDS | |||||||||||||||||||||||
| NAME |
NUMBER OF ORDINARY SHARES UNDERLYING UNEXERCISED OPTIONS (EXERCISABLE) |
NUMBER OF ORDINARY SHARES UNDERLYING UNEXERCISED OPTIONS (UNEXERCIS- ABLE) |
OPTION EXERCISE PRICE |
OPTION EXPIRATION DATE |
NUMBER OF RESTRICTED SHARES THAT HAVE NOT VESTED |
DECEMBER 31, 2014 MARKET VALUE OF RESTRICTED SHARES THAT HAVE NOT VESTED |
||||||||||||||||||
| Ralph Thurman |
— | — | — | — | 200,000 | (2) | $ | 80,000 | (3) | |||||||||||||||
| Zohar Loshitzer |
568,000 | (4) | 142,000 | $ | 0.08 | 10/21/23 | 1,000,000 | (5) | $ | 400,000 | (3) | |||||||||||||
| Vladimir Feingold |
568,000 | (4) | 142,000 | $ | 0.08 | 10/21/23 | — | — | ||||||||||||||||
| Jake Vander Zanden |
100,000 | 400,000 | (6) | $ | 0.30 | 10/21/23 | — | — | ||||||||||||||||
| (1) | There were no option exercises by any of our named executive officers during the year ended December 31, 2014. |
| (2) | Such shares will vest in four equal annual installments commencing on October 21, 2015, 2016, 2017 and 2018. In addition, such shares will vest upon a “change of control” of Presbia Holdings unless the Board of Directors of Presbia Holdings determines that Mr. Thurman has been offered substantially similar replacement restricted stock and a comparable position at any acquiring company. |
| (3) | There was no established market value for Presbia Holdings’ restricted shares as of December 31, 2014; the amount set forth in the table above represents our estimate of fair market value of $0.40 per ordinary share. |
| (4) | Such options (covering an aggregate of 710,000 shares) were immediately vested and exercisable with respect to 60% of the ordinary shares subject thereto on the date of grant, vested and became exercisable with respect to an additional 20% of the ordinary shares subject thereto on February 1, 2014 and vested and became exercisable with respect to an additional 20% of the ordinary shares subject thereto on February 1, 2015. |
| (5) | Such shares will vest on July 28, 2015 (the end of the lockup period for our initial public offering), unless Mr. Loshitzer’s employment is terminated by us for cause. In addition, such shares will vest upon a “change in control” of Presbia Holdings unless the board of directors of Presbia Holdings determines that the recipient has been offered substantially similar replacement restricted stock and a comparable position at any acquiring company. |
| (6) | Such options vest with respect to 25% of the ordinary shares subject thereto on October 21, 2015, 2016, 2017 and 2018. In addition, such options will vest and become exercisable upon a “change in control” of Presbia Holdings unless the board of directors of Presbia Holdings determines that the recipient has been offered substantially similar replacement options and a comparable position at any acquiring company. |
125
Retirement Benefits
We do not maintain, and during 2014 and 2013 did not maintain, any tax-qualified or non-qualified plans that provide for the payment of retirement benefits or benefits paid primarily following retirement to any of our named executive officers.
Agreements with Named Executive Officers
Each of our named executive officers is an employee at will. Other than as described below, we are not party to employment agreements with any of our named executive officers.
We entered into a letter agreement, which includes a severance arrangement, with Mr. Cooper, our President and Chief Executive Officer, who will be a named executive officer for the year ending December 31, 2015. The agreement provides that Mr. Cooper’s annual base salary shall be $400,000, with a target annual bonus of 50% of base pay based on agreed to objectives of our Board. Also, pursuant to this letter agreement, in connection with our initial public offering, we issued to Mr. Cooper an option to purchase 450,000 of our ordinary shares at an exercise price of $10.00 per share. In addition, if Mr. Cooper is terminated without cause or if he resigns because his base salary is unilaterally reduced or his title is diminished, he will be entitled to six months base pay, six months of reimbursement of certain medical benefits and vesting of stock options that would otherwise vest during that six-month period.
In 2013, we made aggregate payments of $200,000 to MTP Consulting, Inc., a consulting company wholly-owned by Zohar Loshitzer pursuant to a consulting agreement with MTP Consulting, Inc. that terminated in August 2013. We have reported the fees paid to MTP Consulting, Inc. in respect of the year ended December 31, 2013 as part of the salary paid to Mr. Loshitzer in the “Summary Compensation Table.”
Presbia Incentive Plan
In January 2014, prior to the consummation of our initial public offering, we adopted a stock plan, which we refer to as the Presbia Incentive Plan. Unless sooner terminated by the Board, the Presbia Incentive Plan will expire 10 years after its adoption.
The Presbia Incentive Plan permits us to grant awards of stock options, restricted shares, stock appreciation rights, restricted share units, performance shares, performance share units, dividend equivalent rights in respect of awards and other share-based and cash-based awards, including annual and long-term cash incentive awards. Awards under the Presbia Incentive Plan may be granted to employees, directors, consultants and other persons who perform services for our company or a subsidiary of our company.
A total of 1,800,000 of our ordinary shares are authorized for issuance under the Presbia Incentive Plan. For purposes of calculating the number of shares available under the Presbia Incentive Plan, shares covered by forfeited, terminated, or cancelled awards are available for future awards under the Presbia Incentive Plan, as are shares that are surrendered or withheld from any award to satisfy tax withholding obligations or the exercise price of an award or that are tendered by an award recipient to pay the exercise price of any awards. Such shares may be authorized but unissued shares or authorized and issued shares held in our treasury or acquired by our company for purposes of the Presbia Incentive Plan.
The Presbia Incentive Plan is administered by the Board’s compensation committee. The compensation committee has the authority to:
| • | determine which individuals shall be granted awards and the provisions of award agreements; |
| • | interpret the Presbia Incentive Plan and award agreements; |
| • | prescribe, amend and rescind rules and regulations, if any, relating to the Presbia Incentive Plan; |
126
| • | make all determinations necessary or advisable for the administration of the Presbia Incentive Plan; and |
| • | correct any defect, supply any omission and reconcile any inconsistency in the Presbia Incentive Plan or any award agreement. |
Payments to our company upon the grant, exercise or payment of an award may be made in such form as our compensation committee determines, including cash, ordinary shares, net share exercise, other securities, other awards or other property.
Options granted pursuant to the Presbia Incentive Plan will have an exercise price that is not less than 100% of the fair market value of the shares subject to the option on the date of grant and a term of not more than 10 years from the date of grant. In general, unless an award agreement specifies otherwise, options will become exercisable with respect to 20% of the shares subject thereto on each of the first five anniversaries of the date of grant. However, each option will become fully exercisable upon a “change in control” of our company (as defined in the Presbia Incentive Plan), unless the Board determines that the optionee has been offered substantially identical replacement options and a comparable position at the acquiring company. In general, upon an optionee’s termination of employment, any then exercisable options held by the optionee may be exercised for a period of three months following such termination (one year in the case of death), but in no event beyond the stated expiration date of such options; provided that all options shall immediately terminate upon termination of an optionee’s employment for cause.
Restricted shares granted pursuant to the Presbia Incentive Plan may not be sold, assigned or otherwise transferred during the restricted period determined by our compensation committee at grant. Except as otherwise determined by our compensation committee, upon a recipient’s termination of employment prior to the expiration of the applicable restricted period, all shares for which the restricted period has not lapsed shall be forfeited and reacquired by us at no cost (or for nil consideration). Our compensation committee may accelerate the vesting of all or any restricted shares at any time on such terms as it shall determine by cancelling the outstanding restrictions to which such shares are subject prior to the expiration of the restricted period of such shares. In addition, all restricted shares will become fully vested, and the restrictions to which shares are subject shall lapse, upon a “change in control” of our company (as defined in the Presbia Incentive Plan) unless the Board determines that the recipient has been offered substantially identical replacement restricted shares and a comparable position at the acquiring company. During the restricted period, the recipient shall possess all incidents of ownership of the restricted shares, including the right to receive dividends on and vote such shares; provided that, unless otherwise set forth in an award agreement, any cash or share dividends with respect to restricted shares shall be withheld by us for the recipient’s account and shall be subject to the same restrictions as the corresponding restricted shares to which such dividends relate.
Share appreciation rights granted pursuant to the Presbia Incentive Plan will confer the right to receive, for each ordinary share with respect to which the share appreciation right is exercised, an amount equal to (i) the excess of the fair market value of an ordinary share on the date of exercise over (ii) the base price of the share appreciation right. The base price of share appreciation rights will not be less than 100% of the fair market value of the ordinary shares subject to the share appreciation right on the date of grant. Share appreciation rights will become exercisable at such time or times as our compensation committee shall determine. Payment upon exercise of a share appreciation right may be made in cash or in our ordinary shares or both, as determined by our compensation committee.
Restricted share units granted pursuant to the Presbia Incentive Plan will be subject to such terms as the compensation committee may determine. At the time of grant, our compensation committee will specify the date or dates on which restricted share units will vest and the conditions to vesting and will specify the date on which ordinary shares will be transferred to a recipient in respect of vested restricted share units (which date may be later than the vesting date or dates of such award). Except as otherwise determined by our compensation committee, upon a recipient’s termination of employment, restricted share units that have not vested shall be forfeited and cancelled (or reacquired by us for nil consideration). Our compensation committee may at any time accelerate the vesting dates of all or any restricted share units or waive or amend any conditions of such awards.
127
Our compensation committee may grant performance shares in the form of actual ordinary shares or performance share units having a value equal to an identical number of ordinary shares, in such amounts and subject to such terms as the compensation committee may determine. The performance conditions and the length of the performance period applicable to performance shares and performance share unit awards shall be determined by our compensation committee. In addition, our compensation committee shall determine whether performance share units will be paid in cash, ordinary shares or a combination of both.
Our compensation committee may award other types of share-based or cash-based awards under the Presbia Incentive Plan in such amounts and subject to such terms and conditions as our compensation committee may determine. Such awards may entail the transfer of actual ordinary shares or payment in cash or otherwise of amounts based on the value of our ordinary shares or the payment of cash pursuant to annual and long-term incentive awards approved by our compensation committee that may or may not be based on the value of our ordinary shares.
The Board may amend the Presbia Incentive Plan at any time, but no amendment may materially alter or adversely impair rights and obligations under previously granted awards without consent. Amendments to the Presbia Incentive Plan require shareholder approval to the extent required by applicable laws, regulations or rules.
This description is not complete. For more information, we refer you to the full text of the Presbia Incentive Plan, which we filed as an exhibit to this Annual Report on Form 10-K.
Securities Laws and U.S. Federal Income Taxes.
The Presbia Incentive Plan is designed to comply with various U.S. federal securities and tax laws as follows:
| • | Securities Laws. The Presbia Incentive Plan is intended to conform to all provisions of the Securities Act and the Exchange Act and any and all regulations and rules promulgated by the SEC thereunder, including without limitation, Rule 16b-3. The Presbia Incentive Plan will be administered, and options will be granted and may be exercised, only in such a manner as to conform to such laws, rules and regulations. |
| • | Section 162(m) of the Code. In general, under Section 162(m) of the Code, income tax deductions of publicly held corporations may be limited to the extent total compensation for certain executive officers exceeds $1,000,000 in any taxable year of the corporation. However, under Section 162(m), the deduction limit does not apply to certain “performance-based compensation” established by an independent compensation committee that is adequately disclosed to and approved by shareholders. Under a Section 162(m) transition rule for compensation plans of corporations that are privately held and that become publicly held in an initial public offering, the Presbia Incentive Plan will not be subject to Section 162(m) until a specified transition date, which is the earliest of: |
| • | the date on which the Presbia Incentive Plan is materially modified; |
| • | the date on which all of the ordinary shares reserved for issuance and other compensation allocated under the Presbia Incentive Plan are issued; |
| • | the date on which the Presbia Incentive Plan expires; or |
| • | the date of the first meeting of our shareholders at which members of our Board are to be elected that occurs after the close of the third calendar year following the calendar year in which our initial public offering occurred. |
Prior to the transition date, the deduction limitation under Section 162(m) of the Code will not apply to compensation received pursuant to rights or awards granted under the Presbia Incentive Plan.
128
After the transition date, rights or awards granted under the Presbia Incentive Plan, other than compensation received pursuant to options and stock appreciation rights or the vesting of restricted shares granted prior to the transition date, will not qualify as “performance-based compensation” for purposes of Section 162(m), unless such rights or awards are granted or vest upon pre-established objective performance goals, the material terms of which are disclosed to and approved by our shareholders.
Director Compensation
No compensation was awarded to, or earned by, the non-employee directors of Presbia PLC during 2014 for their service on our Board. In addition, no compensation was awarded to, or earned by, the non-employee directors of Presbia PLC, who also serve on the board of directors of Presbia Holdings, during 2014 for their service on the Presbia Holdings’ board of directors.
For information regarding the compensation that we paid to our named executive officers who serve on the Board of Presbia PLC and the board of directors of Presbia Holdings, Messrs. Thurman, Loshitzer and Feingold, see “—Summary Compensation Table.”
At present, directors of Presbia PLC are compensated as follows: (i) any director who is an employee of Presbia PLC or any of its subsidiaries or who is otherwise not independent with respect to his or her service on the Board under NASDAQ rules will not receive any compensation for serving as a director; (ii) each other director who is independent with respect to his or her service on the Board under NASDAQ rules will receive an annual board cash fee of $60,000; and (iii) we will reimburse each director for out-of-pocket expenses incurred in connection with attending Board and committee meetings. In addition, we intend to grant new directors a one-time restricted share award valued at $80,000 in connection with their appointment to our Board, which shares will vest ratably over a five year vesting period, and we intend to grant our independent non-employee directors an annual restricted share award valued at $40,000 for their continued service on our Board, which shares will vest ratably over a five year vesting period.
Director Equity Awards
Presbia PLC
No equity awards were granted by Presbia PLC to its directors during the year ended December 31, 2014. At December 31, 2014, none of our directors held any options to purchase our ordinary shares or held any other share awards in respect of our ordinary shares. In connection with our initial public offering, in January 2015, we granted options to our non-employee directors as follows:
| • | to Richard Ressler, options to purchase 10,000 ordinary shares; and |
| • | to Mark Blumenkranz, options to purchase 10,000 ordinary shares. |
All such options were granted pursuant to the Presbia Incentive Plan at an exercise price of $10.00 per share and expire ten years from the grant date. Such options vested with respect to one third of the underlying ordinary shares on the grant date and will vest with respect to one third of the underlying ordinary shares on each of the next two anniversaries of that date. None of such options shall be exercisable while the recipient is an employee, officer or director of our company, unless our Board, in its sole discretion, waives such restriction after determining that such exercise shall not trigger a Rule 9 mandatory takeover under the Irish Takeover Panel Act 1977 (as amended) and the related Irish takeover rules by the recipient or any other officer, employee, director or shareholder of our company.
On March 16, 2015, we granted 9,270 restricted ordinary shares to each of Robert J. Cresci and Mark Blumenkranz. The restricted shares will vest in five equal, annual installments commencing one year after the date of grant.
129
Presbia Holdings
No equity awards were granted by Presbia Holdings to our non-employee directors during 2014.
The following table sets forth information regarding holdings by Richard Ressler and Mark Blumenkranz, as of December 31, 2014, of unexercised stock options granted by Presbia Holdings and outstanding restricted stock awards granted by Presbia Holdings.
OUTSTANDING EQUITY AWARDS AT FISCAL YEAR-END
| OPTIONS AWARDS(1) | STOCK AWARDS | |||||||||||||||||||||||
| NAME |
NUMBER OF ORDINARY SHARES UNDERLYING UNEXERCISED OPTIONS (EXERCISABLE) |
NUMBER OF ORDINARY SHARES UNDERLYING UNEXERCISED OPTIONS (UNEXERCIS- ABLE) |
OPTION EXERCISE PRICE |
OPTION EXPIRATION DATE |
NUMBER OF RESTRICTED SHARES THAT HAVE NOT VESTED |
DECEMBER 31, 2014 MARKET VALUE OF RESTRICTED SHARES THAT HAVE NOT VESTED |
||||||||||||||||||
| Richard Ressler |
60,000 | (1) | — | $ | 0.08 | 10/21/23 | — | $ | — | |||||||||||||||
| Mark Blemenkranz |
— | — | — | — | 200,000 | (2) | $ | 180,000 | (3) | |||||||||||||||
| (1) | Such options (covering an aggregate of 60,000 shares) were immediately vested and exercisable with respect to 60% of the ordinary shares subject thereto on the date of grant, vested and became exercisable with respect to an additional 20% of the ordinary shares subject thereto on February 1, 2014 and vested and became exercisable with respect to an additional 20% of the ordinary shares subject thereto on February 1, 2015. |
| (2) | Such shares will vest in four equal annual installments on October 21, 2015, 2016, 2017 and 2018. In addition, such shares will vest upon a “change in control” of Presbia Holdings unless the board of directors of Presbia Holdings determines that Mr. Blumenkranz has been offered substantially similar replacement restricted stock and a comparable position at any acquiring company. |
| (3) | There was no established market value for Presbia Holdings’ restricted shares as of December 31, 2014; the amount set forth in the table above represents our estimate of fair market value of $0.40 per ordinary share. |
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
The following table sets forth certain information as of March 27, 2015 with respect to the beneficial ownership of our ordinary shares by:
| • | each of our named executive officers and directors; |
| • | all of our executive officers and directors as a group; and |
| • | each person or group of affiliated persons who is known by us to beneficially own more than 5% of our outstanding shares. |
The amounts and percentages of shares beneficially owned are reported on the basis of regulations of the SEC governing the determination of beneficial ownership of securities. Under the SEC’s rules, a person is deemed to be a beneficial owner of a security if that person has or shares voting power, which includes the power to vote or to direct the voting of such security, or investment power, which includes the power to dispose of or to direct the disposition of such security. Unless otherwise indicated below, each beneficial owner named in the table below has sole voting and sole investment power with respect to all shares beneficially owned, subject to community property laws where applicable.
130
Unless otherwise indicated, the address of each person listed below is c/o Presbia PLC, 120/121 Baggot Street Lower, Dublin 2 Ireland.
| ORDINARY SHARES BENEFICIALLY OWNED |
||||||||
| NAME OF BENEFICIAL OWNER |
NUMBER | PERCENT | ||||||
| Todd Cooper |
— | (1) | — | |||||
| Zohar Loshitzer |
— | (2) | — | |||||
| Vladimir Feingold |
— | (3) | — | |||||
| Richard Ressler |
9,670,000 | (4) | 72.5 | % | ||||
| Mark Blumenkranz |
12,603 | (5) | * | |||||
| Ralph Thurman |
83,333 | (6) | * | |||||
| John Jacob Vander Zanden |
— | (7) | — | |||||
| Robert Cresci |
9,270 | (8) | * | |||||
| Executive officers and directors as a group (8 persons) |
9,775,206 | (9) | 72.8 | % | ||||
| * | Less than one percent. |
| (1) | Does not include 450,000 ordinary shares covered by options, none of which will be exercisable within 60 days of March 27, 2015. |
| (2) | Does not include 100,000 ordinary shares covered by options, none of which are exercisable within 60 days of March 27, 2015, or any shares owned by Presbia Holdings. On an as-converted, fully-diluted basis, Presbia Holdings had 261,344,633 ordinary shares outstanding as of March 27, 2015, which we refer to as the Presbia Holdings Aggregate Shares. With respect to the Presbia Holdings Aggregate Shares, as of March 27, 2015, Mr. Loshitzer (i) owned 11.5% of the equity interests in Orchard Presbia, LLC, which in turn owned approximately 23.0% of the Presbia Holdings Aggregate Shares, (ii) owned restricted shares issued by Presbia Holdings that represent approximately 0.4% of the Presbia Holdings Aggregate Shares and (iii) owned options to purchase Presbia Holdings’ ordinary shares covering approximately 0.3% of the Presbia Holdings Aggregate Shares. Mr. Loshitzer disclaims beneficial ownership of any of our ordinary shares held by Presbia Holdings. |
| (3) | Does not include 100,000 ordinary shares covered by options, none of which are exercisable within 60 days of March 27, 2015, or any shares owned by Presbia Holdings. With respect to the Presbia Holdings Aggregate Shares, as of March 27, 2015, Mr. Feingold (i) and his family members owned 100% of Feingold Investments, LLC, which in turn owned approximately 14.8% of the Presbia Holdings Aggregate Shares, and (ii) owned options to purchase Presbia Holdings’ ordinary shares covering approximately 0.3% of the Presbia Holdings Aggregate Shares. Mr. Feingold disclaims beneficial ownership of any of our ordinary shares held by Presbia Holdings. |
| (4) | Includes 3,333 ordinary shares covered by options which are exercisable within 60 days of March 27, 2015. As of March 27, 2015, Presbia Holdings holds 9,666,667 of our ordinary shares. Richard Ressler is a director of Presbia Holdings and beneficially owns all of the ordinary shares of Presbia PLC held by Presbia Holdings by virtue of the following: (i) Richard Ressler and trusts for his family members own 100% of the equity interests in Orchard Investments, LLC, which in turn owns approximately 83.3% of the equity interests in Orchard Presbia, LLC, (ii) Orchard Investments, LLC owns approximately 19.1% of the Presbia Holdings Aggregate Shares, (iii) Orchard Presbia, LLC owns approximately 23.0% of the Presbia Holdings Aggregate Shares, (iv) Orchard Alternative Investments, LP owns approximately 8.5% of the Presbia Holdings Aggregate Shares, (v) Orchard Capital Investments, LLC is the managing member of Orchard Alternative Investments GP, LLC, which is the general partner of Orchard Alternative Investments, LP, (vi) Mr. Ressler is the President of Orchard Capital Corporation, which is the Manager of each of Orchard Investments, LLC, Orchard Presbia, LLC and Orchard Capital Investments, LLC, and (vii) Mr. Ressler directly owns approximately 30.5% of the Presbia Holdings Aggregate Shares, which includes options to purchase Presbia Holdings’ ordinary shares covering less than 0.1% of the Presbia Holdings Aggregate Shares. |
131
| (5) | Consists of 3,333 and 9,270 ordinary shares, respectively, covered by options which are exercisable within 60 days of March 27, 2015 and restricted shares awarded on March 16,2015. Does not include any shares owned by Presbia Holdings. As of March 27, 2015, Dr. Blumenkranz owned restricted shares issued by Presbia Holdings that represented approximately 0.1% of the Presbia Holdings Aggregate Shares. Dr. Blumenkranz disclaims beneficial ownership of any ordinary shares held by Presbia Holdings. |
| (6) | Consists of 83,333 ordinary shares covered by which are exercisable within 60 days March 27, 2015. Does not include any shares owned by Presbia Holdings. As of March 27, 2015, Mr. Thurman owned restricted shares issued by Presbia Holdings that represented approximately 0.1% of the Presbia Holdings Aggregate Shares. Mr. Thurman disclaims beneficial ownership of any ordinary shares held by Presbia Holdings. |
| (7) | With respect to the Presbia Holdings Aggregate Shares, as of March 27, 2015, Mr. Vander Zanden owned options to purchase Presbia Holdings’ ordinary shares that cover approximately 0.2% of the Presbia Holdings Aggregate Shares. Does not include any shares held by Presbia Holdings. Mr. Vander Zanden disclaims beneficial ownership of any of our ordinary shares held by Presbia Holdings. |
| (8) | Consists of 9,270 ordinary shares covered by restricted shares awarded on March 16, 2015. |
| (9) | Includes the 9,666,667 ordinary shares held by Presbia Holdings as of March 27, 2015, all of which are deemed to be beneficially owned by Richard Ressler. Includes 89,999 ordinary shares covered by options which are exercisable within 60 days of March 27, 2015. |
Item 13. Certain Relationships and Related Transactions and Director Independence.
The following is a description of transactions since January 1, 2014 to which we have been a party, in which the amount involved exceeds $120,000, and in which any of our directors, executive officers or holders of more than 5% of our share capital, or an affiliate or immediate family member thereof, had or will have a direct or indirect material interest.
Transactions with Presbia Holdings
From 2009 through our initial public offering, Presbia Holdings funded our operations with cash and payments of operating expenses on our behalf. The aggregate principal amount due Presbia Holdings in connection with such funding as of December 31, 2014 was $0.4 million. Such funding accrued interest at a rate of 15% per annum, compounding daily, through November 30, 2014. From December 1, 2014 to the 2015 Capital Contribution in January 2015, such funding accrued interest at a rate equal to the then applicable monthly federal rate of interest for short-term loans, adjusted monthly, compounding daily. All of such funding debt was converted to equity prior to the consummation of our initial public offering, as described below.
As part of the Reorganization Transactions, in October 2013, we effected the 2013 Restructuring described under “Part I, Item I, Business—Corporate History and Information.” To effect the 2013 Restructuring, we entered into certain agreements with Presbia Holdings. Pursuant to those agreements and other intercompany agreements, Presbia Ireland, Limited, our interim holding company, acquired, directly or indirectly, 100% of our business, assets and subsidiaries from Presbia Holdings. At the time of the 2013 Restructuring, Presbia Ireland, Limited was wholly-owned by Presbia Holdings and certain intercompany debt was owed to Presbia Holdings by certain of its other subsidiaries. As part of the 2013 Restructuring, Presbia Holdings converted approximately $12 million of outstanding intercompany debt owed to Presbia Holdings into equity of certain of such subsidiaries.
In November 2014, we effected the 2014 Debt Conversion described under “Part I, Item I, Business—Corporate History and Information.” In the 2014 Debt Conversion, approximately $23.5 million, representing the balance of outstanding intercompany debt owed to Presbia Holdings by certain subsidiaries of Presbia Ireland, Limited at that time, was converted to equity of such subsidiaries.
In January 2015, we effected the 2015 Debt Conversion described under “Part I, Item I, Business—Corporate History and Information.” In the 2015 Debt Conversion, approximately $1.6 million, representing the balance of outstanding intercompany debt owed to Presbia Holdings by a subsidiary of Presbia Ireland, Limited at that time, was converted to equity of such subsidiary.
132
In addition, in January 2015, Presbia Holdings contributed all of the shares in issue of Presbia Ireland, Limited to Presbia PLC, an Irish incorporated public limited company, in exchange for 9,166,667 ordinary shares of Presbia PLC.
Also, Presbia Holdings purchased an aggregate of 500,000 ordinary shares in our initial public offering in February 2015.
Presbia Holdings is our controlling shareholder. For information regarding the relationship of Richard Ressler (one of our directors and the beneficial owner of the ordinary shares of our company held by Presbia Holdings) with Presbia Holdings, see “Part III, Item 10. Directors, Executive Officers and Corporate Governance.”
Transactions with Orchard Capital and its Affiliates
Orchard Capital has provided financial analysis and bookkeeping, accounting, legal and compliance services to Presbia since January 2011 pursuant to a Services Agreement. Such agreement will remain in effect until terminated by either party thereto upon 30 days’ notice. Orchard Capital invoices us quarterly for such services at cost. During the year ended December 31, 2014, we recognized general and administrative expenses of $18,000 for services invoiced by Orchard Capital. As of December 31, 2014, no amounts were due to Orchard Capital for management and accounting services.
Also, commencing during the second quarter of 2013, we have received human resources management services, payroll services, IT support and risk management services from CIM Group. We have incurred charges of $152,000 payable to CIM Group for such services for the year ended December 31, 2014. As of December 31, 2014, amounts due to CIM Group for human resources, payroll, information technology and legal services amounted to $12,000.
For information regarding the relationship of Richard Ressler (one of our directors and the beneficial owner of the ordinary shares of our company held by Presbia Holdings) with Orchard Capital and CIM Group, see “Part III, Item 10. Directors, Executive Officers and Corporate Governance.”
Registration Rights Agreement
Presbia Holdings, our controlling shareholder, and certain of its transferees, have rights to cause our company to register their ordinary shares, including any ordinary shares that Presbia Holdings transfers to its equity owners, under the Securities Act. These rights are provided under the terms of a registration rights agreement between us and Presbia Holdings and includes demand registration rights and piggyback registration rights. These registration rights are assignable, subject to certain conditions, including that the assignee be bound by the terms and conditions of the registration rights agreement. To the extent permitted by applicable law, we will pay, or if not permitted by applicable law, we will cause one of our non-Irish subsidiaries to pay, all registration expenses in connection with registrations under this agreement.
Demand registration rights
Under the terms of the registration rights agreement, at any time beyond six months after the consummation of our initial public offering, we are required, upon the written request of the holders of the shares that are entitled to rights under the registration rights agreement, to use our best efforts to register all or a portion of these shares for public resale. We are not required to effect a registration pursuant to this provision of the registration rights agreement (i) if the shares requested to be registered do not represent (a) at least 10% of the shares that are entitled to registration rights under the agreement or (b) an anticipated aggregate public offering price of at least $10 million; or (ii) during the period starting with the date 30 days prior to our good faith estimate of the date of filing of, and ending on a date 180 days following the effective date of, any company-initiated registration under the Securities Act. If such a registration is to be an underwritten offering, then the holders’ registration rights are conditioned upon the holders’ participation in that underwriting. We may defer the filing of a registration
133
statement once during any 12-month period for a period of not more than ninety days, if we provide written notice stating that in the good faith judgment of our Board, a disadvantageous condition exists, including the existence of certain material transactions or financings, the unavailability of any required financial statements, or the possession by our company of any material information which would not be in the best interests of our company to disclose.
Piggyback registration rights
Presbia Holdings, as well as its equity holders and other permitted transferees, are entitled to piggyback registration rights. If we register any of our securities for our own account, the holders of these shares are entitled to include their shares in the registration. If such registration is to be an underwritten offering, then the holders’ registration rights are conditioned on such holders’ participation in that underwriting.
Director and Executive Officer Compensation
See “Item 11. Executive Compensation” for information regarding compensation of our directors and executive officers.
Indemnification Agreements and Directors’ and Officers’ Liability Insurance
We have entered into indemnification agreements with each of our directors and executive officers. Also, our Presbia USA, Inc. subsidiary has entered into an indemnification agreement with each of our executive officers and directors (each of our executive officers and directors is also an officer and/or director of our Presbia USA, Inc. subsidiary). These agreements, among other things, require us to indemnify an indemnitee to the fullest extent permitted by applicable law, including indemnification of expenses such as attorneys’ fees, judgments, fines and settlement amounts incurred by the indemnitee in any action or proceeding, including any action or proceeding by us or in our right, arising out of the person’s services as a director or executive officer. We also maintain directors’ and officers’ liability insurance for our directors and officers.
Policies and Procedures for Related Party Transactions
Our Board has adopted a written related person transaction policy to set forth the policies and procedures for the review and approval or ratification of related person transactions. This policy covers, with certain exceptions, any transaction, arrangement or relationship, or any series of similar transactions, arrangements or relationships in which we were or are to be a participant, where the amount involved exceeds $120,000 and a related person had, has or will have a direct or indirect material interest, including, without limitation, purchases of goods or services by or from the related person or entities in which the related person has a material interest, indebtedness, guarantees of indebtedness and employment by us of a related person.
As provided by our audit committee charter, our audit committee is responsible for reviewing and approving in advance the related party transactions covered by our company’s related transaction policies and procedures.
Item 14. Principal Accountant Fees and Services.
The following table presents fees for professional services provided by Deloitte & Touche LLP for the years ended December 31, 2014 and 2013 (amounts in thousands):
| YEAR ENDED DECEMBER 31, | ||||||||
| 2014 | 2013 | |||||||
| Audit fees |
$ | 403 | $ | 440 | ||||
| Audit-related fees |
622 | 561 | ||||||
| Tax fees |
268 | 145 | ||||||
| All other fees |
— | — | ||||||
|
|
|
|
|
|||||
| Total |
$ | 1,293 | $ | 1,146 | ||||
|
|
|
|
|
|||||
134
Audit fees. Audit fees consist of the aggregate fees billed for professional services rendered for (i) the audit of our annual financial statements and (ii) accounting consultations.
Audit-Related Fees. Audit related fees consist of fees billed for professional services rendered for the filing of our Registration Statement on Form S-1 related to our initial public offering.
Tax Fees. Tax fees consisted of professional services rendered for tax compliance, tax advice and tax planning. The services for the fees disclosed under this category include tax return preparation and technical tax advice.
All Other Fees. No other fees were incurred in 2014 or 2013.
Consistent with SEC policies regarding auditor independence and the audit committee’s charter, the audit committee has responsibility for engaging, setting compensation for and reviewing the performance of the independent registered public accounting firm.
Prior to our initial public offering in February 2015, we had not constituted an audit committee of the board of directors. However, the board of directors as a whole approved all of the professional services provided by Deloitte & Touche LLP for the years ended December 31, 2014 and 2013.
Part IV
Item 15. Exhibits and Financial Statement Schedules.
(a)(1) Financial Statements. The financial statements filed as part of this report are listed on the Index to the Consolidated Financial Statements in “Part II, Item 8 Financial Statements and Supplementary Data.”
(a)(2) Financial Statement Schedules. The following financial statement schedule is filed as part of this Form 10-K:
Schedule II — Consolidated Valuation and Qualifying Accounts
All other schedules have been omitted because they are not applicable or not required, or the information is included in the Consolidated Financial Statements or Notes thereto.
(a)(3) Exhibits required by Item 601 of Regulation S-K. The information required by this Section (a)(3) of Item 15 is set forth on the exhibit index that follows the signatures page of this Annual Report on Form 10-K.
Schedule II – Consolidated Valuation and Qualifying Accounts
| Balance at Beginning of Period |
Additional Allowance |
Adjustments | Balance at End of Period |
|||||||||||||
| Deferred Income Tax Asset Valuation Allowance |
||||||||||||||||
| Year ended December 31, 2014 |
$ | 262 | $ | 1,045 | $ | 68 | $ | 1,375 | ||||||||
| Year ended December 31, 2013 |
— | 262 | — | 262 | ||||||||||||
| Year ended December 31, 2012 |
— | — | — | — | ||||||||||||
135
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| Date: March 31, 2015 | PRESBIA PLC | |||
| By: | /s/ Todd Cooper | |||
| Todd Cooper | ||||
| President and Chief Executive Officer | ||||
Power of Attorney
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Todd Cooper and Richard Fogarty, jointly and severally, his attorneys-in-fact, each with the power of substitution, for him in any and all capacities, to sign any amendments to this Annual Report on Form 10-K, and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, hereby ratifying and confirming all that each of said attorneys-in-fact, or his substitute or substitutes, may do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated:
| Name |
Title |
Date | ||
| /s/ Todd Cooper Todd Cooper |
President, Chief Executive Officer and Director (Principal Executive Officer) |
March 31, 2015 | ||
| /s/ Richard Fogarty Richard Fogarty |
Chief Accounting Officer, Vice President, Finance and Secretary (Principal Financial and Accounting Officer) |
March 31, 2015 | ||
| /s/ Mark Blumenkranz Mark Blumenkranz |
Director | March 31, 2015 | ||
| /s/ Vladimir Feingold Vladimir Feingold |
Director | March 31, 2015 | ||
| /s/ Zohar Loshitzer Zohar Loshitzer |
Director | March 31, 2015 | ||
| /s/ Richard Ressler Richard Ressler |
Director | March 31, 2015 | ||
| /s/ Ralph Thurman Ralph Thurman |
Director | March 31, 2015 | ||
| /s/ Robert Cresci Robert Cresci |
Director | March 31, 2015 | ||
136
EXHIBIT INDEX
| Exhibit No. |
Description of Exhibit | |
| 3.1(1) | Memorandum and Articles of Association of Presbia PLC | |
| 10.1(1) | Share Exchange Deed between the Registrant and Presbia Holdings, dated January 14, 2015 | |
| 10.2+ | Registration Rights Agreement between the Registrant and Presbia Holdings | |
| 10.3#(1) | Presbia Incentive Plan | |
| 10.4#(2) | Form of Stock Option Agreement (to be issued under the Presbia Incentive Plan) | |
| 10.5#(2) | Form of Restricted Stock Agreement (to be issued under the Presbia Incentive Plan) | |
| 10.6#(2) | Presbia Holdings Stock Plan | |
| 10.7#(2) | Amendment No. 1 to Presbia Holdings Stock Plan | |
| 10.8#(2) | Restricted Stock Grant Notice and Restricted Stock Award Agreement between Presbia Holdings and Zohar Loshitzer | |
| 10.9(2) | Lease, dated April 23, 2012, between PresbiBio LLC and Image Holdings, Inc. | |
| 10.10(3) | Sublease, dated May 6, 2014, by and between Trustwave Holdings, Inc. and PresbiBio, LLC | |
| 10.11(3) | First Amendment to Sublease, dated July 16, 2014, by and between Trustwave Holdings, Inc. and PresbiBio, LLC | |
| 10.12(3) | Form of Indemnification Agreement between Presbia USA, Inc. and its officers and directors | |
| 10.13(3) | Form of Indemnification Agreement between Presbia PLC and its directors | |
| 10.14(3) | Form of Indemnification Agreement between Presbia PLC and its executive officers | |
| 10.15(3) | Services Agreement, dated as of January 1, 2011, between PresbiBio, LLC and Orchard Capital Corporation | |
| 10.16#(4) | Offer Letter with Todd Cooper | |
| 21.1+ | Subsidiaries of the Registrant | |
| 23.1+ | Consent of Deloitte & Touche, LLP, Independent Registered Public Accounting Firm. | |
| 24.1+ | Power of Attorney (included on the signature page) | |
| 31.1+ | Certification of Chief Executive Officer of Presbia PLC pursuant to Rule 13a-14(a)/15d-14(a) of the Securities Exchange Act of 1934 | |
| 31.2+ | Certification of Chief Accounting Officer of Presbia PLC pursuant to Rule 13a-14(a)/15d-14(a) of the Securities Exchange Act of 1934 | |
| 32.1+ | Certifications of Chief Executive Officer and Chief Accounting Officer of Presbia PLC pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. (furnished herewith) | |
| # | Indicates management contract or compensatory plan. |
| + | Indicates filed herewith. |
| (1) | Previously filed as an exhibit to the registrant’s Registration Statement on Form S-1 (File No. 333-194713) filed with the Securities and Exchange Commission on January 23, 2015 and incorporated herein by reference. |
| (2) | Previously filed as an exhibit to the registrant’s Registration Statement on Form S-1 (File No. 333-194713) filed with the Securities and Exchange Commission on October 9, 2014 and incorporated herein by reference. |
137
| (3) | Previously filed as an exhibit to the registrant’s Registration Statement on Form S-1 (File No. 333-194713) filed with the Securities and Exchange Commission on January 12, 2015 and incorporated herein by reference. |
| (4) | Previously filed as an exhibit to the registrant’s Registration Statement on Form S-1 (File No. 333-194713) filed with the Securities and Exchange Commission on January 15, 2015 and incorporated herein by reference. |
138