Attached files
| file | filename |
|---|---|
| 8-K - 8-K - CAPRICOR THERAPEUTICS, INC. | v442729_8k.htm |
Exhibit 99.1

NASDAQ: CAPR A Translational Medicine Company www.capricor.com Investor Presentation June 2016

2 This presentation contains forward - looking statements and information that are based on the beliefs of the management of Capricor Therapeutics, Inc . (Capricor) as well as assumptions made by and information currently available to Capricor . All statements other than statements of historical fact included in this presentation are forward - looking statements, including but not limited to statements identified by the words “anticipates,” “believes,” “estimates,” and “expects” and similar expressions . Such forward - looking statements also include any expectation of or dates for commencement of clinical trials, IND filings, similar plans or projections and other matters that do not relate strictly to historical facts . These statements reflect Capricor’s current views with respect to future events, based on what we believe are reasonable assumptions ; however, the statements are subject to a number of risks, uncertainties and assumptions . There are a number of important factors that could cause actual results or events to differ materially from those indicated by such forward - looking statements . More information about these and other risks that may impact Capricor's business are set forth in Capricor's Annual Report on Form 10 - K for the year ended December 31 , 2015 , as filed with the Securities and Exchange Commission on March 30 , 2016 , and in its Registration Statement on Form S - 3 , as filed with the Securities and Exchange Commission on September 28 , 2015 and in our Quarterly Report on Form 10 - Q for the period ending March 31 , 2016 as filed with the Securities and Exchange Commission on May 13 , 2016 . Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those in the forward - looking statements . Further, Capricor’s management does not intend to update these forward - looking statements and information after the date of this presentation . Forward - Looking Statements

3 Capricor – Portfolio at a Glance Diseases of Inflammation and Fibrosis Outpatient Management of Heart Failure Cardiosphere - Derived Cells CDC Exosomes Natriuretic Peptides Adult Cardiac Conditions Duchenne Muscular Dystrophy P ROGRAM Internal & External Janssen Option TBD Phase I / II Phase II Preclinical Phase II Potential Outlicense
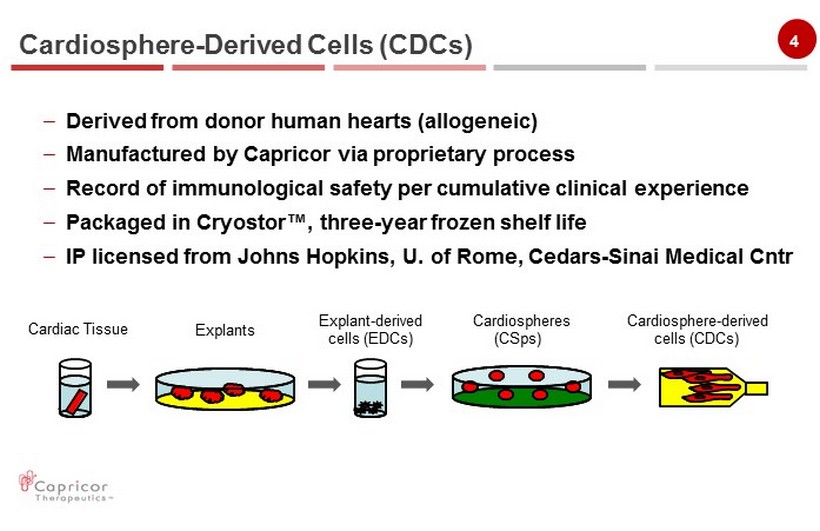
4 – Derived from donor human hearts (allogeneic) – Manufactured by Capricor via proprietary process – Record of immunological safety per cumulative c linical experience – Packaged in Cryostor ™, three - year frozen shelf life – IP licensed from Johns Hopkins, U. of Rome, Cedars - Sinai Medical Cntr Cardiosphere - Derived Cells ( CDCs) Cardiosphere - derived cells (CDCs) Cardiospheres (CSps) Explant - derived cells (EDCs) Explants Cardiac Tissue

5 CDCs Exert Their Effects Via Paracrine Mechanisms – Do not act by ‘ stemness ’ – do not engraft – Act as local drug delivery vehicles – Are epigenetic modulators of gene expression and cell function – Release a wide variety of regulatory bio - molecules to effect their actions – RNAs – Proteins – Secrete these bio - molecules within exosomes
6 – A large body of reproducible data shows that CDCs: Induce cardiac myogenesis Prevent cardiomyocyte apoptosis (programmed cell death) Promote new blood vessel formation Exert anti - scarring activity Attract endogenous progenitor cells – Clinical trials of CDCs have shown durable cardiac improvements: Post - myocardial infarction (MI) cardiac dysfunction – Scar mass – Viable heart mass Advanced heart failure – NYHA class – Ventricular dimensions CDCs: Heart Cells for Heart Disease – Quality of life – Functional status – Regional contractility – Systolic wall thickening

7 Clinical Proof - of - Concept was Provided by CADUCEUS – Patients with reduced ejection fraction (EF ) following myocardial infarction (MI ) – N=25 (17 active, 8 control ) – One - time intracoronary delivery of autologous CDCs (25 million cells) – Sponsored by Cedars - Sinai Medical Center, with Johns Hopkins University Lancet , 2012, 21(6): 1121 - 1135.

8 Capricor – Detail by Program Cardiosphere - Derived Cells CDC Exosomes Natriuretic Peptides Duchenne Muscular Dystrophy P ROGRAM TBD Phase I / II

9 DMD Cardiomyopathy is #1 Cause of Death in DMD – DMD results from mutation of calcium - regulating dystrophin gene – 1 per 3,500 male births – Induces profound, progressive dysfunction of all muscle types – Cardiac involvement is universal and #1 cause of death – DMD cardiomyopathy results in dilated, non - compliant failing hearts due to abnormal transmembrane calcium flux – Consequence is inflammation, necrosis, cardiomyocyte death and progressive cardiac fibrosis

10 Menon et al, Pediatr Cardiol 2014 ; 35 :1279 - 85. Heart Failure is the End Result of Progressive Scarring Tandon et al, J Am Heart Assoc 2015 ; 4 :e001338. – In DMD, myocardial fibrosis ( scar): – Independently predicts adverse cardiac remodeling, ventricular arrhythmia, death – Increases linearly with age and strongly correlates with LV ejection fraction
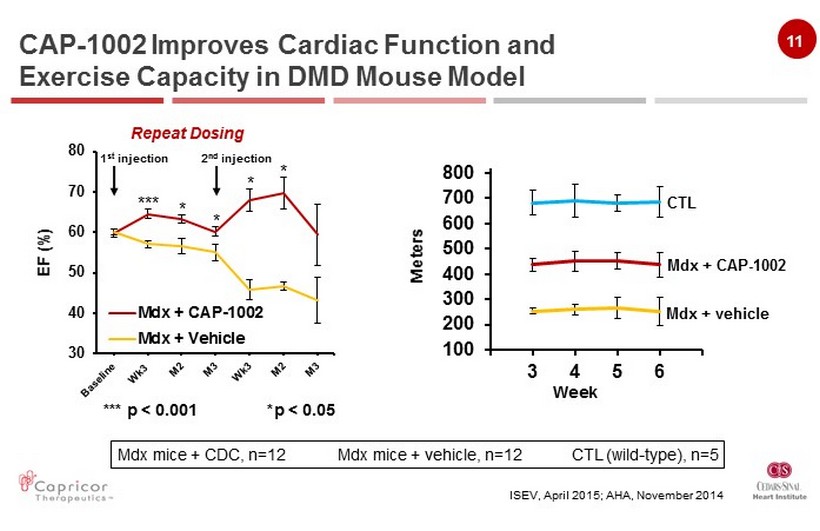
11 30 40 50 60 70 80 Mdx + CAP-1002 Mdx + Vehicle *** p < 0.001 * p < 0.05 100 200 300 400 500 600 700 800 3 4 5 6 CTL Mdx + CAP - 1002 Mdx + vehicle Week *** 1 st injection EF (%) Repeat Dosing Meters 2 nd injection Mdx mice + CDC, n=12 Mdx mice + vehicle, n=12 CTL (wild - type), n=5 CAP - 1002 Improves Cardiac Function and Exercise Capacity in DMD Mouse Model ISEV, April 2015; AHA , November 2014 * * * *

12 – Ongoing HOPE - Duchenne study designed to support registration – CAP - 1002 is distinct from the exon skipping therapies Apparent lack of mutation dependence supports use in any genotype – FDA Orphan Drug Designation for the treatment of DMD CAP - 1002 in Clinical Development for DMD - Associated Cardiomyopathy

13 – Boys with cardiomyopathy secondary to DMD – N=24, parallel - group study (3 - 4 sites) 1:1 randomization to one - time CAP - 1002 (75 million cells) or ‘usual care’ Randomize Usual Care M12 W2 W6 M3 M6 30 d Randomized Phase I / II HOPE - Duchenne Trial Ongoing Screen * FDA Draft Guidance, June 2015. CAP - 1002 Infusion – Expect to report six - month top - line data in Q1 2017 Structural (cardiac MRI)* and functional endpoints Quality of Life endpoints Passed interim DSMB safety review x Success in HOPE may enable Capricor to discuss a BLA with FDA by late 2017.

14 Capricor – Detail by Program Cardiosphere - Derived Cells CDC Exosomes Natriuretic Peptides Adult Cardiac Conditions P ROGRAM Janssen Option Phase II

15 – > 5 million U.S. patients estimated to have heart failure Current treatments largely fail to address the progressive underlying disease – In a preclinical model of non - ischemic dilated cardiomyopathy, CDCs have been shown to: reverse abnormalities in cell signaling prevent adverse remodelling improve survival – DYNAMIC I study evaluated CAP - 1002 in patients with advanced heart failure Open - label clinical trial conducted at Cedars - Sinai Medical Center Subjects treated with a one - time, triple coronary infusion (37.5 – 75 million cells) Six - and 12 - month follow - up CAP - 1002’s Opportunity in Advanced Heart Failure CAP - 1002 demonstrated an efficacy signal with concordant improvements in functional status , quality - of - life, and left ventricular function and size
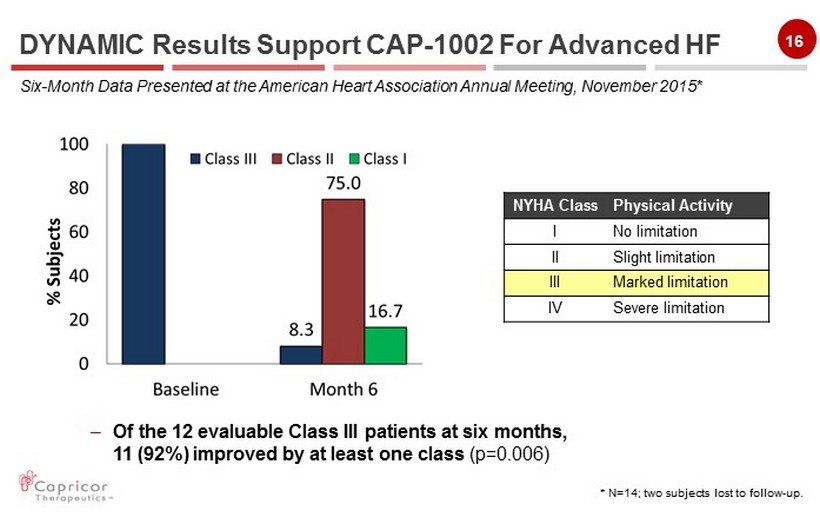
16 NYHA Class Physical Activity I No limitation II Slight limitation III Marked limitation IV Severe limitation * N=14 ; two subjects lost to follow - up. DYNAMIC Results Support CAP - 1002 For Advanced HF Six - Month Data Presented at the American Heart Association Annual Meeting, November 2015* – Of the 12 evaluable Class III patients at six months, 11 (92%) improved by at least one class (p=0.006)
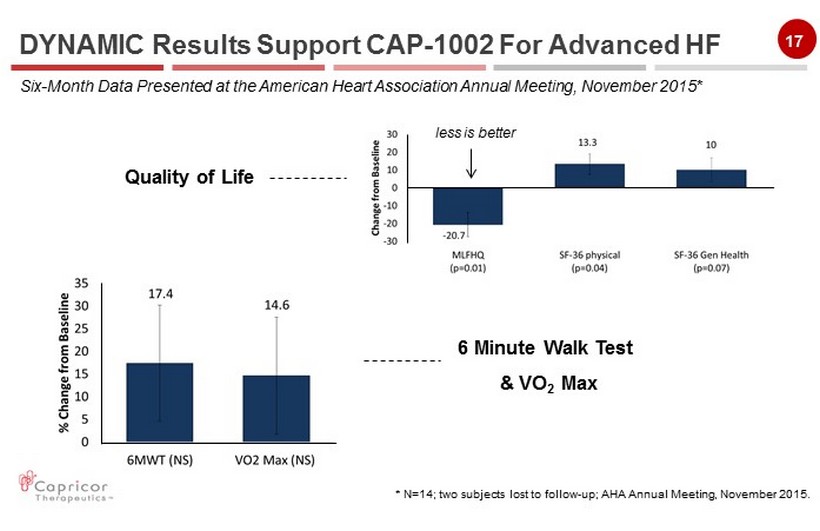
17 6 Minute Walk Test & VO 2 Max Quality of Life less is better * N=14 ; two subjects lost to follow - up; AHA Annual Meeting, November 2015. DYNAMIC Results Support CAP - 1002 For Advanced HF Six - Month Data Presented at the American Heart Association Annual Meeting, November 2015*

18 Left Ventricular Dynamics & Dimensions less is better * N=14; two subjects lost to follow - up; measurements assessed by echocardiography; AHA Annual Meeting, November 2015. DYNAMIC Results Support CAP - 1002 For Advanced HF Six - Month Data Presented at the American Heart Association Annual Meeting, November 2015* – Efficacy signal continued to be observed at 12 months LVEF + 17.5% (median) from baseline (p=0.02)
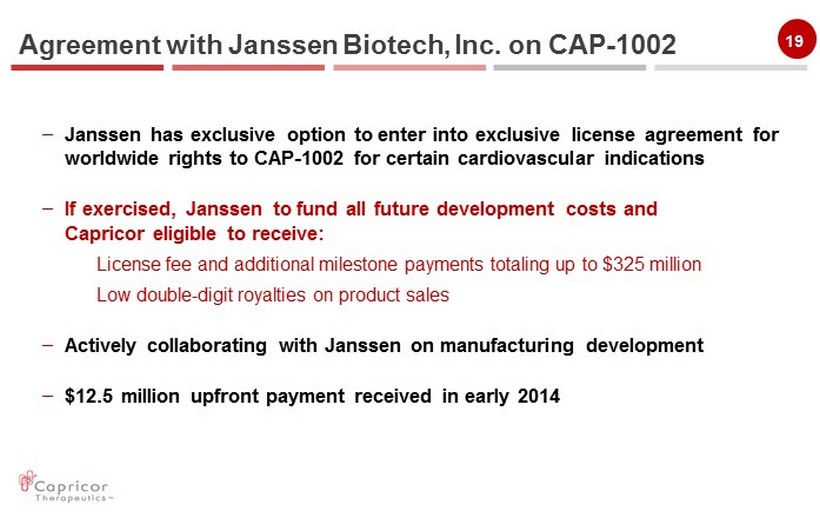
19 – Janssen has exclusive option to enter into exclusive license agreement for worldwide rights to CAP - 1002 for certain cardiovascular indications – If exercised, Janssen to fund all future development costs and Capricor eligible to receive: License fee and additional milestone payments totaling up to $325 million Low double - digit royalties on product sales – Actively collaborating with Janssen on manufacturing development – $12.5 million upfront payment received in early 2014 Agreement with Janssen Biotech, Inc. on CAP - 1002

20 ALLSTAR Trial Nearing Enrollment Completion – Randomized, double - blind, placebo - controlled; 30 - 35 U.S. centers – 120 subjects with recent (30 – 90 d) or chronic (91 – 365 d) STEMI or NSTEMI – Left ventricular scar size ≥15% of LV mass – Left ventricular ejection fraction ≤45% – 25 million CDCs or saline infused one time into infarct - associated coronary artery – Followed for 12 months post - dosing – Efficacy evaluated by centrally - read cardiac MRI and other measures – Six - month data to be available in Q1 2017 – Janssen has until 60 days following delivery of six - month data to exercise option
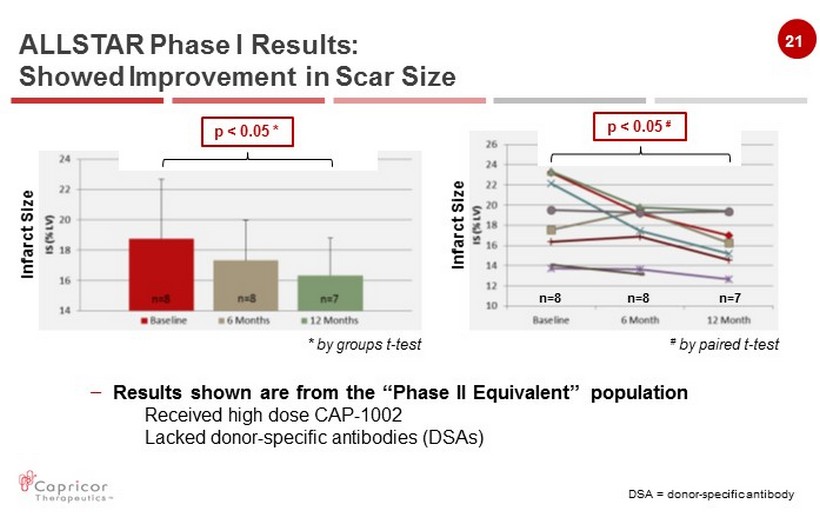
21 ALLSTAR Phase I Results: Showed Improvement in Scar Size * by groups t - test # by paired t - test – Results shown are from the “Phase II Equivalent” population Received high dose CAP - 1002 Lacked donor - specific antibodies (DSAs) DSA = donor - specific antibody p < 0.05 # p < 0.05 * n=8 n=8 n=7 Infarct Size Infarct Size
22 Capricor – Detail by Program Diseases of Inflammation and Fibrosis Cardiosphere - Derived Cells CDC Exosomes Natriuretic Peptides P ROGRAM Internal & External Preclinical

23 Capricor’s CDC Exosomes – CAP - 2003 – Exosomes represent a potential next - generation, cell - free therapeutic platform – Exosomes mediate the regenerative and cardioprotective effects of CDCs Were discovered in the course of elucidating CDCs’ mechanism of action – Like exosomes from other cells, CDC Exosomes: are rich in RNAs and proteins readily cross cell membranes f unction as a cell signaling modality – Have demonstrated striking activity in several inflammatory models (cardiac and non - cardiac) – Capricor has an exclusive WW license to CDC Exosomes technology from Cedars - Sinai Medical Center
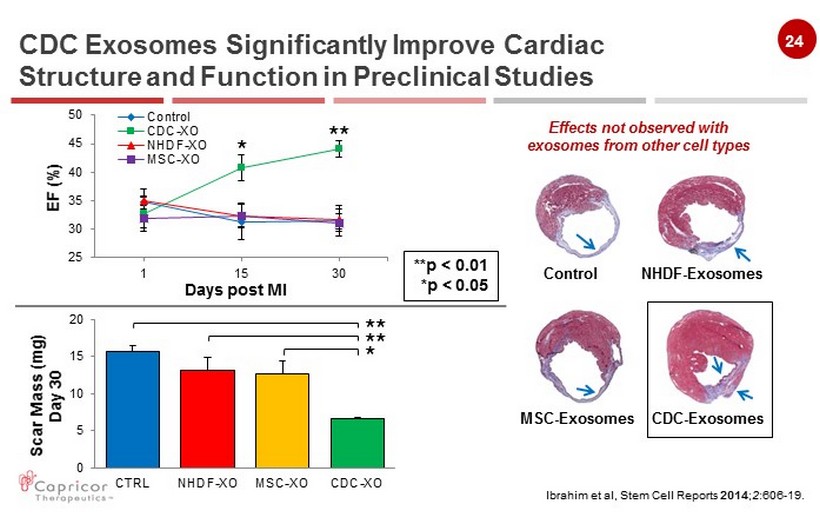
24 Ibrahim et al, Stem Cell Reports 2014 ; 2 :606 - 19 . Effects not observed with exosomes from other cell types CDC Exosomes Significantly Improve Cardiac Structure and Function in Preclinical Studies 0 5 10 15 20 CTRL NHDF-XO MSC-XO CDC-XO Scar Mass (mg ) Day 30 * ** ** 25 30 35 40 45 50 1 15 30 EF (%) Days post MI Control CDC-XO NHDF-XO MSC-XO * ** Control NHDF - Exosomes MSC - Exosomes CDC - Exosomes **p < 0.01 *p < 0.05

25 Control CAP - 2003 (low - dose) CAP - 2003 (high - dose) – Keratoconjunctivitis induced in rabbits (wound followed by LPS application) – After development of severe inflammation, eyes were treated with a single administration of CAP - 2003 or control, then followed for three days – Day three data show that CAP - 2003 can rapidly improve : – corneal wound injury – ocular surface inflammation – conjunctivitis – corneal edema LPS = lipopolysaccharide CDC Exosomes Provide Rapid and Dose - Dependent Improvement in Ocular Inflammation Model

26 – CDC exosomes have regenerative and cell - modulating capabilities – Broad treatment potential in inflammatory and fibrotic conditions Supported by growing body of preclinical data – Capricor plans to announce the first indication for CAP - 2003 in mid - 2016 CAP - 2003 Moving into the Clinic

27 Capricor – Detail by Program Outpatient Management of Heart Failure Cardiosphere - Derived Cells CDC Exosomes Natriuretic Peptides P ROGRAM Phase II Potential Outlicense

28 Cenderitide (CD - NP) – For Potential Outlicense Dual Natriuretic Peptide Receptor Activator for Cardio - Renal Disease States – Provides a first - in - class product licensing opportunity Positioned to address a large heart failure market segment lacking in therapeutic options – Proof - of - concept for chronic patch pump delivery Two Phase II PK/PD studies have been completed Well - tolerated at pharmacologically - active levels Flexible dosing for individual dose titration – Designed for outpatient management of heart failure OmniPod ® ( Insulet )

29 Cash, cash equivalents, and marketable securities reported at March 31, 2016 $14.3 million Net cash used in operations in 2015 $10.8 million Shares outstanding (May 12, 2016) 18.0 million Cash Runway Through Early 2017 Data Readouts

30 * Janssen has the right to exercise its option to CAP - 1002 at any time until 60 days after the delivery by Capricor of the six - month follow - up results from the ALLSTAR clinical trial. Upcoming Milestones 2016 2017 Anticipated Event Mid Announce first indication for CAP - 2003 Q3 Complete enrollment in HOPE - Duchenne trial of CAP - 1002 Complete enrollment in ALLSTAR trial of CAP - 1002 H2 Report Phase II data (higher dose range) on Cenderitide Q1 Report six - month data from HOPE - Duchenne trial Six - month data from ALLSTAR trial to be available to Capricor* H1 Submit IND for CAP - 2003

31 AJ Bergmann, MBA VP of Finance Leland Gershell, MD PhD Chief Financial Officer Deborah Ascheim, MD Chief Medical Officer Karen Krasney, JD EVP & General Counsel Rachel Smith, PhD VP of Research & Development Houman Hemmati, MD PhD VP of New Therapy Development Luis Rodriguez - Borlado, PhD VP of Regenerative Therapies Linda Marbán, PhD Chief Executive Officer Senior Management Frank Litvack, MD – Executive Chairman

NASDAQ: CAPR A Translational Medicine Company www.capricor.com 8840 Wilshire Boulevard – 2 nd floor Beverly Hills, CA 90211 (310) 358 - 3200
