Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Ignyta, Inc. | d202568d8k.htm |
| EX-99.1 - EX-99.1 - Ignyta, Inc. | d202568dex991.htm |
Exhibit 99.2
A phase 1 dose escalation study of RXDX-105, an oral RET and BRAF inhibitor, in patients with advanced solid
tumors
Manish R. Patel1, Marwan Fakih2, A. Craig Lockhart3, Anthony J. Olszanski4, Siqing Fu5, Lyudmila Bazhenova6, Alexander Drilon7, Rupal Patel8, Jennifer W.
Oliver8, Pratik S. Multani8, and Ding Wang9
1Sarah Cannon Research Institute/Florida Cancer Specialists, Sarasota, FL; 2City of Hope Comprehensive Cancer Center,
Duarte, CA; 3Washington University Medical Center, St. Louis, MO; 4Fox Chase Cancer Center, Philadelphia, PA; 5MD Anderson Cancer Center, Houston, TX; 6University of California San Diego Moores Cancer Center, San Diego, CA; 7Memorial Sloan Kettering
Cancer Center, New York, NY; 8Ignyta, Inc., San Diego, CA; 9Henry Ford Hospital, Detroit, Michigan
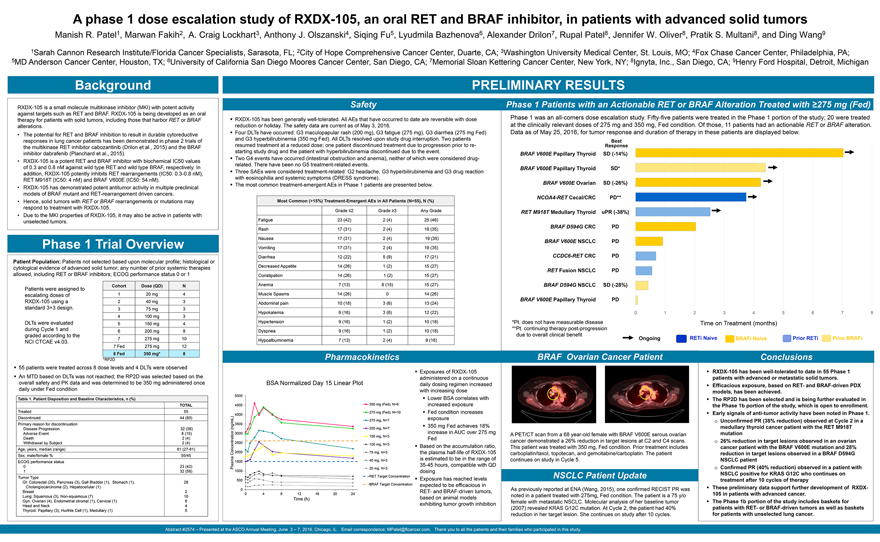
Background
RXDX-105 is a small molecule multikinase inhibitor (MKI) with potent activity against targets such as RET and BRAF. RXDX-105 is being developed as an oral therapy for patients with
solid tumors, including those that harbor RET or BRAF alterations.
The potential for RET and BRAF inhibition to result in durable cytoreductive responses in lung
cancer patients has been demonstrated in phase 2 trials of the multikinase RET inhibitor cabozantinib (Drilon et al., 2015) and the BRAF inhibitor dabrafenib (Planchard et al., 2015).
RXDX-105 is a potent RET and BRAF inhibitor with biochemical IC50 values of 0.3 and 0.8 nM against wild type RET and wild type BRAF, respectively. In addition, RXDX-105 potently
inhibits RET rearrangements (IC50: 0.3-0.8 nM), RET M918T (IC50: 4 nM) and BRAF V600E (IC50: 54 nM).
RXDX-105 has demonstrated potent antitumor activity in
multiple preclinical models of BRAF mutant and RET-rearrangement driven cancers.
Hence, solid tumors with RET or BRAF rearrangements or mutations may respond to
treatment with RXDX-105.
Due to the MKI properties of RXDX-105, it may also be active in patients with unselected tumors.
Phase 1 Trial Overview
Patient Population: Patients not selected based upon molecular profile;
histological or cytological evidence of advanced solid tumor; any number of prior systemic therapies allowed, including RET or BRAF inhibitors; ECOG performance status 0 or 1
Patients were assigned to escalating doses of RXDX-105 using a standard 3+3 design.
DLTs were
evaluated during Cycle 1 and graded according to the NCI CTCAE v4.03.
Cohort
1 2 3 4 5 6 7
7 Fed
8 Fed
Dose (QD)
20 mg 40 mg 75 mg 100 mg 150 mg 200 mg 275 mg 275 mg
350 mg*
N
4 3 3 3 4 8 10 12
8
*RP2D
55 patients were treated across 8 dose levels and 4 DLTs were observed
An MTD
based on DLTs was not reached; the RP2D was selected based on the overall safety and PK data and was determined to be 350 mg administered once daily under Fed condition
Table 1. Patient Disposition and Baseline Characteristics, n (%)
TOTAL
Treated 55
Discontinued 44 (80)
Primary reason for discontinuation
Disease Progression 32 (58)
Adverse Event 8 (15)
Death 2 (4)
Withdrawal by Subject 2 (4)
Age, years, median (range) 61 (27-81)
Sex, male/female % 55/45
ECOG performance status
0 23 (42)
1 32 (58)
Tumor Type
GI: Colorectal (20), Pancreas (3), Gall Bladder (1), Stomach (1), 28
Cholangiocarcinoma (2), Hepatocellular (1)
Breast 2
Lung: Squamous (3), Non-squamous (7) 10
Gyn: Ovarian (4), Endometrial stromal (1), Cervical
(1) 6
Head and Neck 4
Thyroid: Papillary (3), Hurthle Cell (1), Medullary (1)
5
PRELIMINARY RESULTS
Safety
RXDX-105 has been generally well-tolerated. All AEs that have occurred to date are reversible with dose reduction or holiday. The safety data are current as of May 3, 2016.
Four DLTs have occurred: G3 maculopapular rash (200 mg), G3 fatigue (275 mg), G3 diarrhea (275 mg Fed) and G3 hyperbilirubinemia (350 mg Fed). All DLTs resolved
upon study drug interruption. Two patients resumed treatment at a reduced dose; one patient discontinued treatment due to progression prior to restarting study drug and the patient with hyperbilirubinemia discontinued due to the event.
Two G4 events have occurred (intestinal obstruction and anemia), neither of which were considered drug-related. There have been no G5 treatment-related events.
Three SAEs were considered treatment-related: G2 headache, G3 hyperbilirubinemia and G3 drug reaction with eosinophilia and systemic symptoms (DRESS syndrome).
The most common treatment-emergent AEs in Phase 1 patients are presented below.
Most Common
(>15%) Treatment-Emergent AEs in All Patients (N=55), N (%)
Grade £2 Grade
³3 Any Grade
Fatigue 23 (42) 2 (4) 25 (46)
Rash 17 (31) 2 (4) 19 (35)
Nausea 17 (31) 2 (4) 19 (35)
Vomiting 17 (31) 2 (4) 19 (35)
Diarrhea 12 (22) 5 (9) 17 (21)
Decreased Appetite 14 (26) 1 (2) 15 (27)
Constipation 14 (26) 1 (2) 15 (27)
Anemia 7 (13) 8 (15) 15 (27)
Muscle Spasms 14 (26) 0 14 (26)
Abdominal pain 10 (18) 3 (6) 13 (24)
Hypokalemia 9 (16) 3 (6) 12 (22)
Hypertension 9 (16) 1 (2) 10 (18)
Dyspnea 9 (16) 1 (2) 10 (18)
Hypoalbuminemia 7 (13) 2 (4) 9 (16)
Pharmacokinetics
BSA Normalized Day 15 Linear Plot
Exposures of RXDX-105 administered on a continuous daily
dosing regimen increased with increasing dose Lower BSA correlates with increased exposure Fed condition increases exposure 350 mg Fed achieves 18% increase in AUC over 275 mg Fed
Based on the accumulation ratio, the plasma half-life of RXDX-105 is estimated to be in the range of 35-45 hours, compatible with QD dosing Exposure has reached levels expected to
be efficacious in RET- and BRAF-driven tumors, based on animal models exhibiting tumor growth inhibition
5000
4500
4000
(ng/mL) 3500
3000
2500
Concentration 2000 Plasma 1500
1000
500
0
0 4 8 12 16 20 24
Time (h)
350 mg (Fed), N=6 275 mg (Fed), N=10 275 mg, N=7 200 mg, N=7 150 mg, N=3 100 mg, N=3
75 mg, N=3 40 mg, N=3 20 mg, N=3 RET Target Concentration BRAF Target Concentration
Phase 1 Patients with an Actionable RET or BRAF Alteration Treated with ³275 mg (Fed)
Phase 1 was an all-comers dose escalation study. Fifty-five patients were treated in the Phase 1 portion of
the study; 20 were treated at the clinically relevant doses of 275 mg and 350 mg, Fed condition. Of those, 11 patients had an actionable RET or BRAF alteration. Data as of May 25, 2016, for tumor response and duration of therapy in these patients
are displayed below.
Best Response
BRAF V600E Papillary Thyroid SD (-14%)
BRAF V600E Papillary Thyroid SD* BRAF V600E Ovarian SD (-26%) NCOA4-RET Cecal/CRC PD** RET M918T Medullary Thyroid uPR (-38%)
BRAF D594G CRC PD
BRAF V600E NSCLC PD
CCDC6-RET CRC PD
RET Fusion NSCLC PD
BRAF D594G NSCLC SD (-28%)
BRAF V600E Papillary Thyroid PD
0 1 2 3 4 5 6 7 8
*Pt. does not have measurable disease **Pt. continuing therapy post-progression due to overall clinical benefit
Time on Treatment (months)
Ongoing RETi Naive BRAFi Naive Prior RETi Prior BRAFi
BRAF Ovarian Cancer Patient
A PET/CT scan from a 68 year-old female with BRAF V600E
serous ovarian cancer demonstrated a 26% reduction in target lesions at C2 and C4 scans. This patient was treated with 350 mg, Fed condition. Prior treatment includes carboplatin/taxol, topotecan, and gemcitabine/carboplatin. The patient continues
on study in Cycle 5.
NSCLC Patient Update
As previously reported at ENA
(Wang, 2015), one confirmed RECIST PR was noted in a patient treated with 275mg, Fed condition. The patient is a 75 y/o female with metastatic NSCLC. Molecular analysis of her baseline tumor (2007) revealed KRAS G12C mutation. At Cycle 2, the
patient had 40% reduction in her target lesion. She continues on study after 10 cycles.
Conclusions
RXDX-105 has been well-tolerated to date in 55 Phase 1 patients with advanced or metastatic solid tumors. Efficacious exposure, based on RET- and BRAF-driven PDX models, has been
achieved.
The RP2D has been selected and is being further evaluated in the Phase 1b portion of the study, which is open to enrollment. Early signals of anti-tumor
activity have been noted in Phase 1. Unconfirmed PR (38% reduction) observed at Cycle 2 in a medullary thyroid cancer patient with the RET M918T mutation 26% reduction in target lesions observed in an ovarian cancer patient with the BRAF V600E
mutation and 28% reduction in target lesions observed in a BRAF D594G
NSCLC patient Confirmed PR (40% reduction) observed in a patient with NSCLC positive for KRAS
G12C who continues on treatment after 10 cycles of therapy These preliminary data support further development of RXDX-105 in patients with advanced cancer.
The
Phase 1b portion of the study includes baskets for patients with RET- or BRAF-driven tumors as well as baskets for patients with unselected lung cancer.
Abstract
#2574 – Presented at the ASCO Annual Meeting, June 3 – 7, 2016, Chicago, IL.
Email correspondence: MPatel@flcancer.com. Thank you to all the
patients and their families who participated in this study.
