Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - AveXis, Inc. | a16-10739_1ex99d2.htm |
| 8-K - 8-K - AveXis, Inc. | a16-10739_18k.htm |
Exhibit 99.1
AVXS-101 Phase 1 Gene Therapy Clinical Trial In Spinal Muscular Atrophy Type 1 Jerry R Mendell, MD Center for Gene Therapy Research Institute Nationwide Children’s Hospital
Disclosures: Jerry R Mendell, MD I have never had any investment in any product that I have tested in Clinical Trial I serve as a consultant to AveXis, Inc. who is sponsoring this SMA gene therapy trial I also serve as a consultant to Sarepta Therapeutics in the eteplirsen trial for DMD

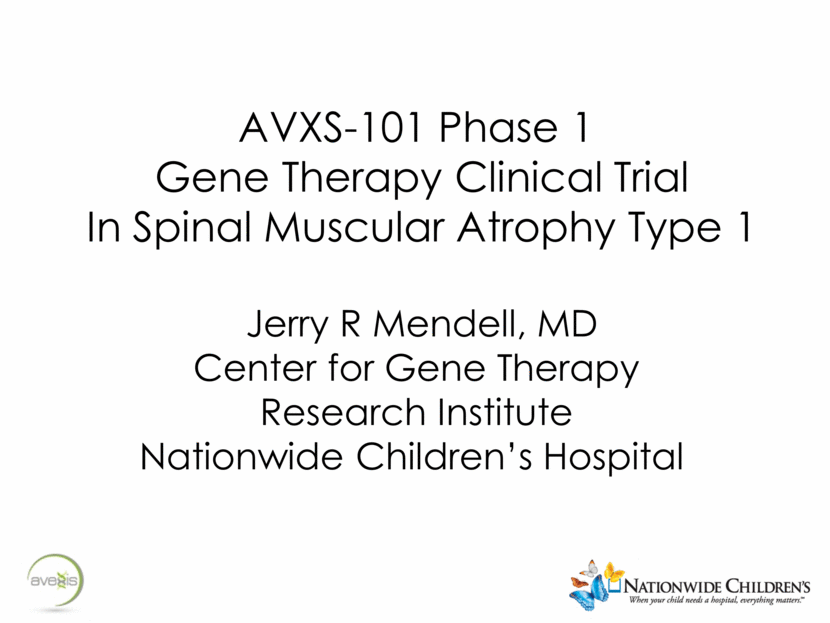
Spinal Muscular Atrophy (SMA) Type 1 Werdnig-Hoffman Onset < 6 mos, never sit!; death <2years Hypotonia, weakness; legs > arms; typical frog leg position Bulbar muscle weakness: weak suck and swallow Require substantial nutritional support Nutritional failure is a major complication - 50% on feeding support by 8 mos age1 Bell-shaped chest; abdominal breathing Inexorable progression to respiratory failure is expected Median age to permanent ventilation or death is 10.5 months, IQR 8.1-13.6 months1 By 13.6 months only 25% of SMA type 1 patients are alive and free of permanent ventilation1 Tongue fasciculation characteristic Absent Reflexes 1. Finkel, R. et al. Observational study of spinal muscular atrophy type I and implications for clinical trials. Neurology 83,810–817 (2014).
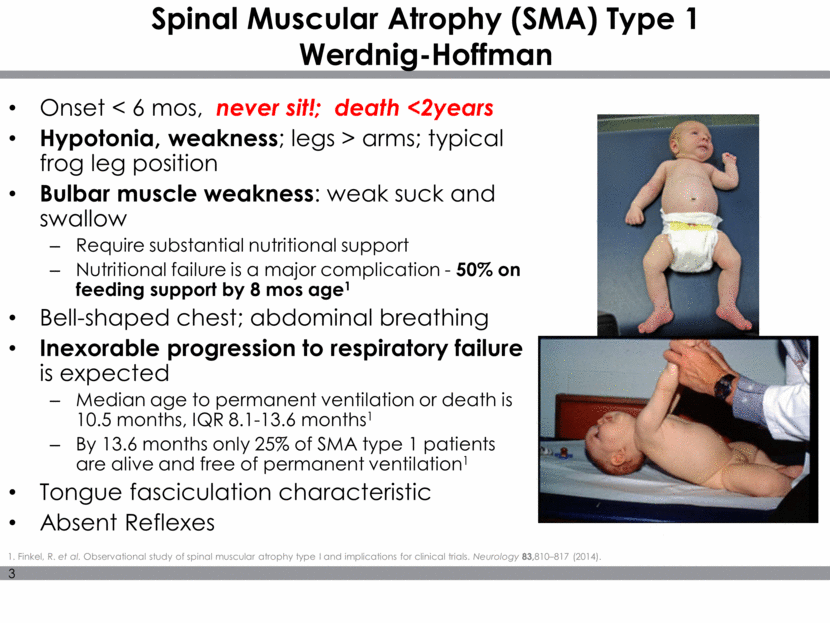
Major Milestones in SMA History Leading Genetic Cause of Infant Mortality ~1 in 10,000 live births 1995: Homozygous Deletion SMN1 Chr 5q13 Two Forms SMN in humans: SMN1(T), SMN2(C) NORMAL INDIVIDUAL SMN genes SMN protein SMN1 SMN2 SMN2 SMA INDIVIDUAL Multiple Copies of SMN2 modify Phenotype Functional SMN Protein Not-functional SMN Protein C to T creates ESS
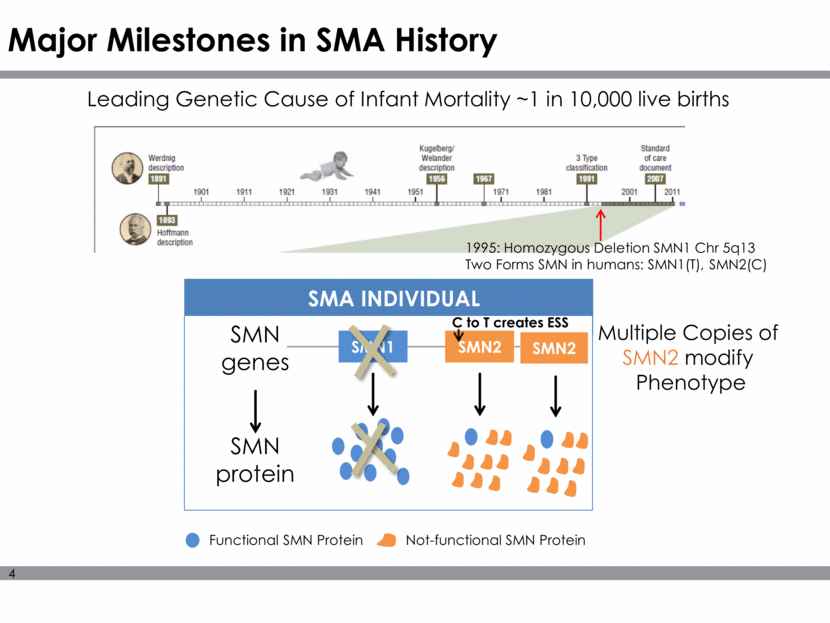
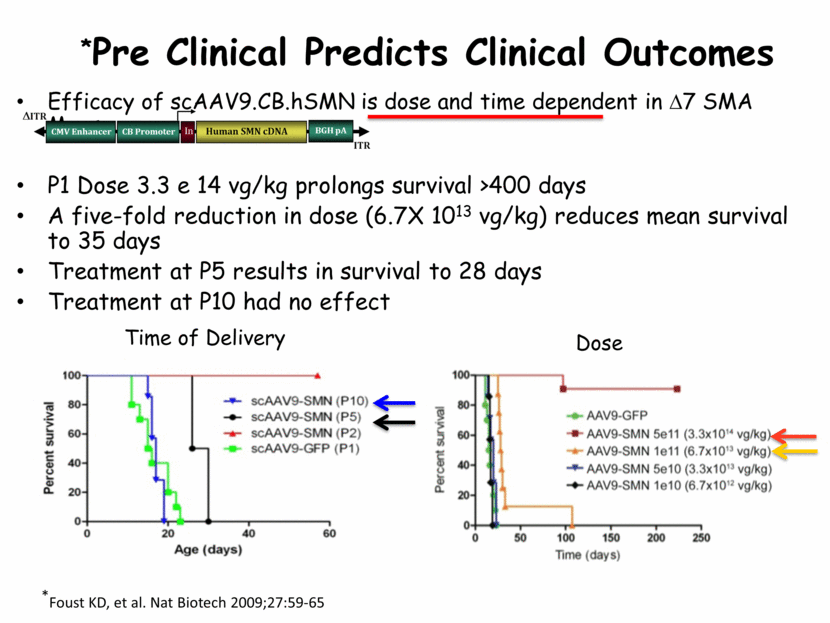
*Pre Clinical Predicts Clinical Outcomes Efficacy of scAAV9.CB.hSMN is dose and time dependent in D7 SMA Mouse P1 Dose 3.3 e 14 vg/kg prolongs survival >400 days A five-fold reduction in dose (6.7X 1013 vg/kg) reduces mean survival to 35 days Treatment at P5 results in survival to 28 days Treatment at P10 had no effect Dose Time of Delivery *Foust KD, et al. Nat Biotech 2009;27:59-65
Phase 1 Trial Design 6 *Patients may be put on noninvasive ventilator support (BiPAP) for <16 hours/day at discretion of their physician or study staff TRIAL OVERVIEW Route of Administration IV infusion via peripheral limb vein Prednisolone 1 mg/kg 1day Pre-GT Trial Design Open-label, dose-escalation Principal Investigator Jerry R Mendell, MD TRIAL OVERVIEW Study Site Primary Safety and Tolerability Secondary Time from birth until death or time to 16-hour ventilation continuously for >2 weeks in absence of an acute reversible illness or peri-operatively OBJECTIVES Inclusion Age <9 months/6 months at day of vector infusion with SMA Type 1 as defined by: Bi-allelic SMN1 gene mutations (deletion or point mutations) 2 copies of SMN2 Onset of disease at birth to 6 months of age Symptomatic SMA (Hypotonia delay in motor skills, poor head control, round shoulder posture and hypermobility of joints) Exclusion Active viral infection (includes HIV or serology positive for hep B or C) Use of invasive ventilatory support (tracheotomy with positive pressure)* or pulse oximetry <95% saturation Patients with binding anti-AAV9 antibody titers >1:50 as determined by ELISA immunoassay Abnormal laboratory values considered to be clinically significant SMN2 single base substitution in exon 7, c.859G>C (modifies disease) Key Enrollment Criteria Exploratory CHOP INTEND (infant test of neuromuscular disease) Bayley Motor Scales of Infant/Toddler development – Gross Motor Interactive Video Evaluation-mini (ACTIVE-mini) Comp Motor Action Pot (CMAP) Motor Unit Number (MUNE) Electrical Impedance Myography (EIM) AVXS-101 Phase 1 Trial Design Clinicaltrials.gov Identifier = NCT02122952 ¹ Inclusion criteria was 9 months of age and younger for the first nine patients. 6 months of age and younger for the last six patients.
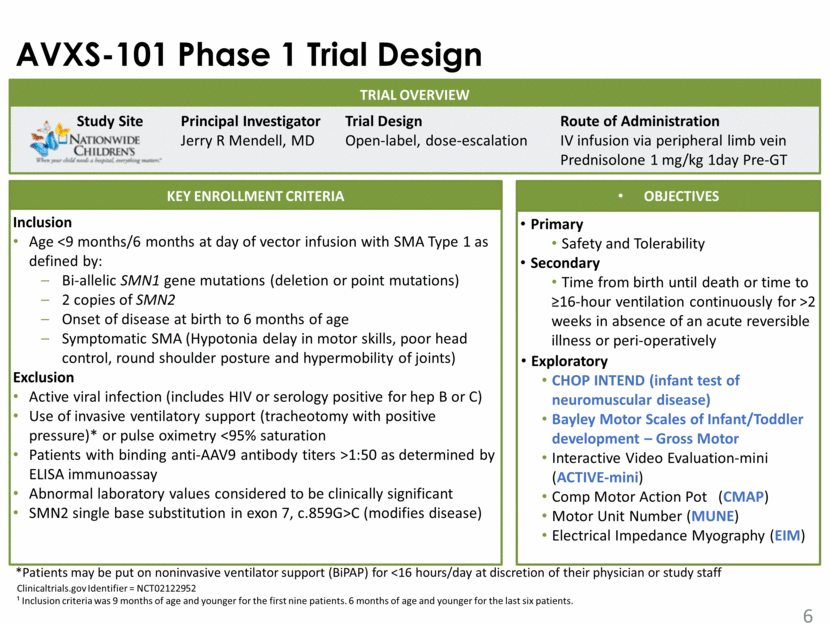
Sample Size and Dosing Cohorts Phase1 SMA Gene Therapy Trial 15 SMA patients have been enrolled - Ages 0.9 to 7.9 months 9 patients in original IND with amendment for an additional 6 IV infusion (AAV9) in peripheral vein - Cohort 1 6.7 X 1013 vg/kg n=3 - Cohort 2: 2.0 X 1014 vg/kg n=12 Prednisolone for immune suppression one-day prior to gene transfer 1 mg/kg (modified after Pt 1)
Intravenous Delivery x1 Hour
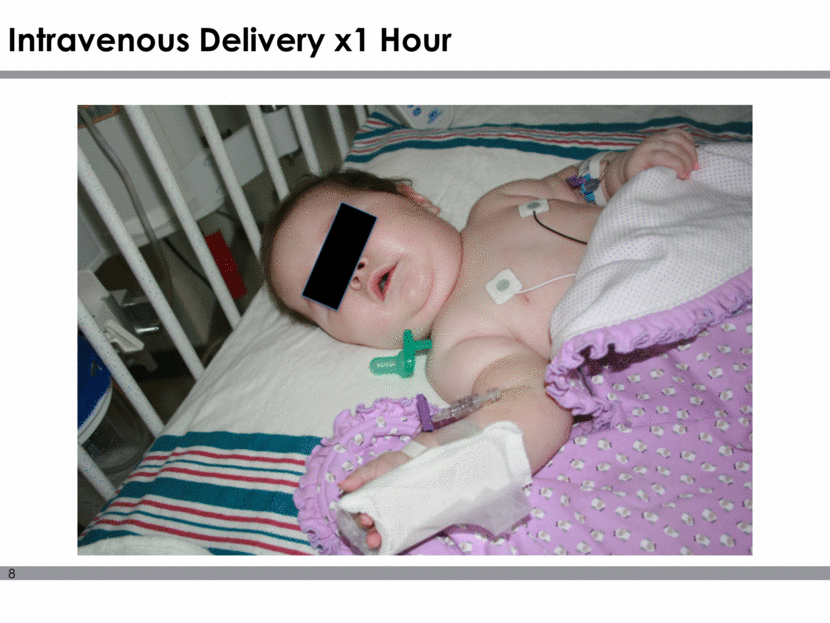
Natural History of SMA Type 1 9 *Survival = no death, or no need for 16-hr/day ventilation continuously for 2 weeks, in the absence of an acute reversible illness Finkel RS, et al. Neurology 2014;83:810–7 Pediatric NM Clinical Research Network for SMA 90% of SMA Type 1 patients will not survive to the age of 2 SMA Type 1 survival rates % Event-free survival* Age (mos) 50% survival* 10.5 mos 25% survival* 13.6 mos 8% survival* 20 mos Holds head steady alone; brings hands to mouth Rolls over in both directions Cruises; may stand alone Walks alone; may run and walk up stairs; eats with a spoon Climb furniture alone; kicks and throws a ball Onset of SMA Type 1 by 6 months Symptoms may present Milestone for a healthy infant Sits alone; crawls 0 25 50 75 100 0 2 4 6 8 10 12 14 16 18 20 22 24
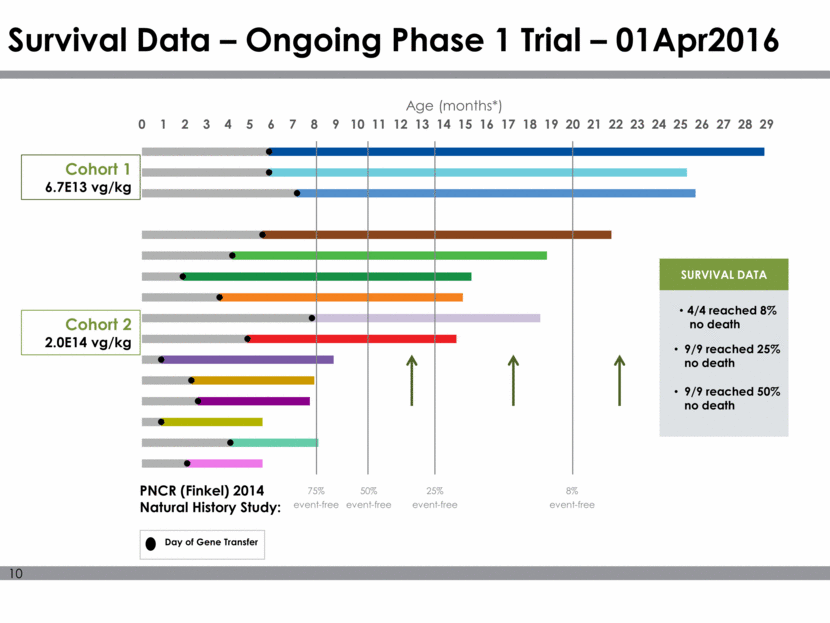
Survival Data – Ongoing Phase 1 Trial – 01Apr2016 Cohort 1 6.7E13 vg/kg Cohort 2 2.0E14 vg/kg PNCR (Finkel) 2014 Natural History Study: Day of Gene Transfer SURVIVAL DATA * A month is defined as 30 days 75% event-free 50% event-free 25% event-free 8% event-free 4/4 reached 8% no death 9/9 reached 25% no death 9/9 reached 50% no death 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 Age (months*)
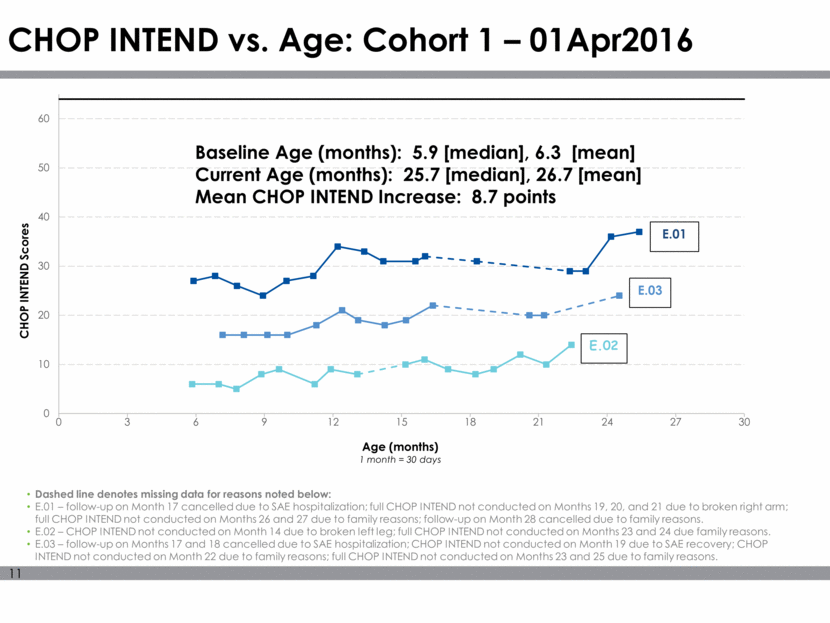
CHOP INTEND vs. Age: Cohort 1 – 01Apr2016 Dashed line denotes missing data for reasons noted below: E.01 – follow-up on Month 17 cancelled due to SAE hospitalization; full CHOP INTEND not conducted on Months 19, 20, and 21 due to broken right arm; full CHOP INTEND not conducted on Months 26 and 27 due to family reasons; follow-up on Month 28 cancelled due to family reasons. E.02 – CHOP INTEND not conducted on Month 14 due to broken left leg; full CHOP INTEND not conducted on Months 23 and 24 due family reasons. E.03 – follow-up on Months 17 and 18 cancelled due to SAE hospitalization; CHOP INTEND not conducted on Month 19 due to SAE recovery; CHOP INTEND not conducted on Month 22 due to family reasons; full CHOP INTEND not conducted on Months 23 and 25 due to family reasons. Baseline Age (months): 5.9 [median], 6.3 [mean] Current Age (months): 25.7 [median], 26.7 [mean] Mean CHOP INTEND Increase: 8.7 points 0 10 20 30 40 50 60 0 3 6 9 12 15 18 21 24 27 30 CHOP INTEND Scores Age (months) 1 month = 30 days E.02 E.03 E.01
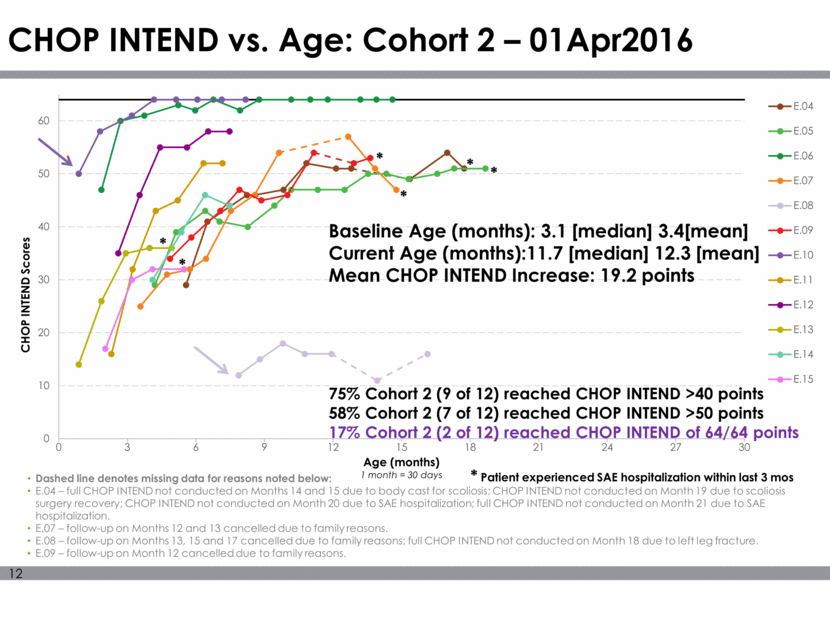
CHOP INTEND vs. Age: Cohort 2 – 01Apr2016 Dashed line denotes missing data for reasons noted below: E.04 – full CHOP INTEND not conducted on Months 14 and 15 due to body cast for scoliosis; CHOP INTEND not conducted on Month 19 due to scoliosis surgery recovery; CHOP INTEND not conducted on Month 20 due to SAE hospitalization; full CHOP INTEND not conducted on Month 21 due to SAE hospitalization. E.07 – follow-up on Months 12 and 13 cancelled due to family reasons. E.08 – follow-up on Months 13, 15 and 17 cancelled due to family reasons; full CHOP INTEND not conducted on Month 18 due to left leg fracture. E.09 – follow-up on Month 12 cancelled due to family reasons. Baseline Age (months): 3.1 [median] 3.4[mean] Current Age (months):11.7 [median] 12.3 [mean] Mean CHOP INTEND Increase: 19.2 points 75% Cohort 2 (9 of 12) reached CHOP INTEND >40 points 58% Cohort 2 (7 of 12) reached CHOP INTEND >50 points 17% Cohort 2 (2 of 12) reached CHOP INTEND of 64/64 points * * * * * Patient experienced SAE hospitalization within last 3 mos * * 0 10 20 30 40 50 60 0 3 6 9 12 15 18 21 24 27 30 CHOP INTEND Scores Age (months) 1 month = 30 days E.04 E.05 E.06 E.07 E.08 E.09 E.10 E.11 E.12 E.13 E.14 E.15
CHOP INTEND vs. Age – 01Apr2016 COHORT 1 (n=3) Baseline Age (months): 5.9 [median], 6.3 [mean] Current Age (months): 25.7 [median], 26.7 [mean] Mean CHOP INTEND Increase: 8.7 points COHORT 2 (n=12) Baseline Age (months): 3.1 [median],3.4 [mean] Current Age (months):11.7 [median],12.3 [mean] Mean CHOP INTEND Increase: 19.2 points Cohort 1 Cohort 2 0 10 20 30 40 50 60 0 3 6 9 12 15 18 21 24 27 30 CHOP INTEND Scores Age (months) 1 month = 30 days E.01 E.02 E.03 E.04 E.05 E.06 E.07 E.08 E.09 E.10 E.11 E.12 E.13 E.14 E.15 19.2 point avg increase in CHOP INTEND 8.7 point avg increase in CHOP INTEND

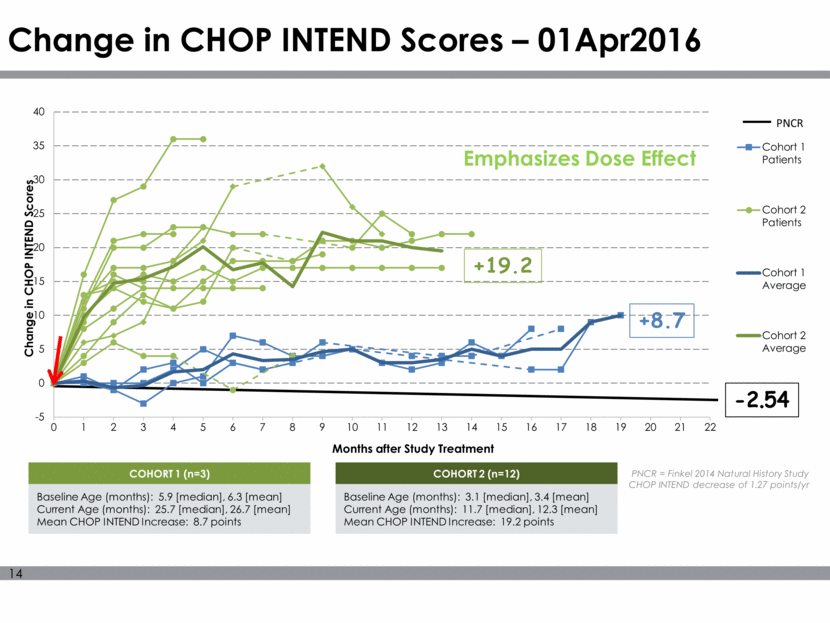
Change in CHOP INTEND Scores – 01Apr2016 COHORT 1 (n=3) Baseline Age (months): 5.9 [median], 6.3 [mean] Current Age (months): 25.7 [median], 26.7 [mean] Mean CHOP INTEND Increase: 8.7 points COHORT 2 (n=12) Baseline Age (months): 3.1 [median], 3.4 [mean] Current Age (months): 11.7 [median], 12.3 [mean] Mean CHOP INTEND Increase: 19.2 points PNCR = Finkel 2014 Natural History Study CHOP INTEND decrease of 1.27 points/yr Emphasizes Dose Effect +19.2 +8.7 -2.54 PNCR
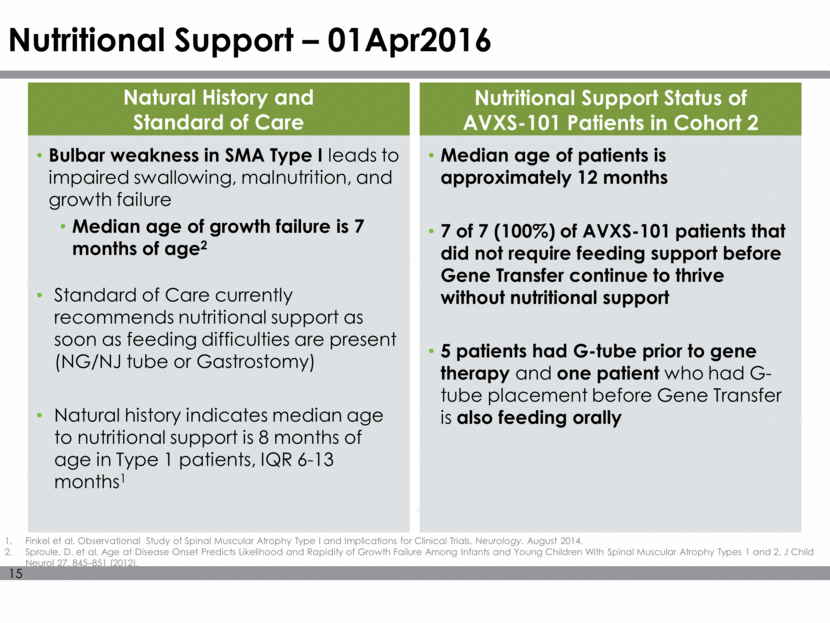
Nutritional Support – 01Apr2016 Bulbar weakness in SMA Type I leads to impaired swallowing, malnutrition, and growth failure Median age of growth failure is 7 months of age2 Standard of Care currently recommends nutritional support as soon as feeding difficulties are present (NG/NJ tube or Gastrostomy) Natural history indicates median age to nutritional support is 8 months of age in Type 1 patients, IQR 6-13 months1 Natural History and Standard of Care Nutritional Support Status of AVXS-101 Patients in Cohort 2 Median age of patients is approximately 12 months 7 of 7 (100%) of AVXS-101 patients that did not require feeding support before Gene Transfer continue to thrive without nutritional support 5 patients had G-tube prior to gene therapy and one patient who had G-tube placement before Gene Transfer is also feeding orally Finkel et al. Observational Study of Spinal Muscular Atrophy Type I and Implications for Clinical Trials. Neurology. August 2014. Sproule, D. et al. Age at Disease Onset Predicts Likelihood and Rapidity of Growth Failure Among Infants and Young Children With Spinal Muscular Atrophy Types 1 and 2. J Child Neurol 27, 845–851 (2012).
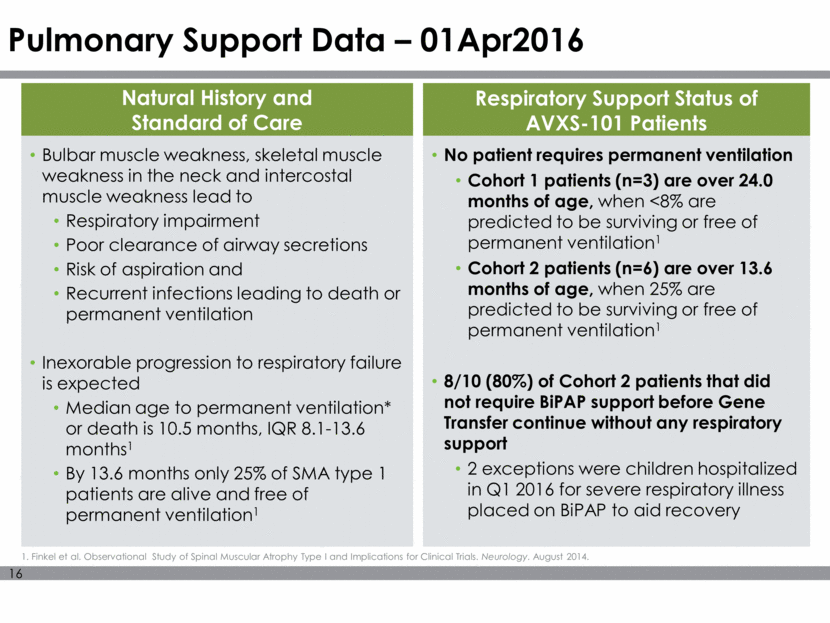
Pulmonary Support Data – 01Apr2016 Respiratory Support Status of AVXS-101 Patients No patient requires permanent ventilation Cohort 1 patients (n=3) are over 24.0 months of age, when <8% are predicted to be surviving or free of permanent ventilation1 Cohort 2 patients (n=6) are over 13.6 months of age, when 25% are predicted to be surviving or free of permanent ventilation1 8/10 (80%) of Cohort 2 patients that did not require BiPAP support before Gene Transfer continue without any respiratory support 2 exceptions were children hospitalized in Q1 2016 for severe respiratory illness placed on BiPAP to aid recovery Bulbar muscle weakness, skeletal muscle weakness in the neck and intercostal muscle weakness lead to Respiratory impairment Poor clearance of airway secretions Risk of aspiration and Recurrent infections leading to death or permanent ventilation Inexorable progression to respiratory failure is expected Median age to permanent ventilation* or death is 10.5 months, IQR 8.1-13.6 months1 By 13.6 months only 25% of SMA type 1 patients are alive and free of permanent ventilation1 Natural History and Standard of Care 1. Finkel et al. Observational Study of Spinal Muscular Atrophy Type I and Implications for Clinical Trials. Neurology. August 2014.
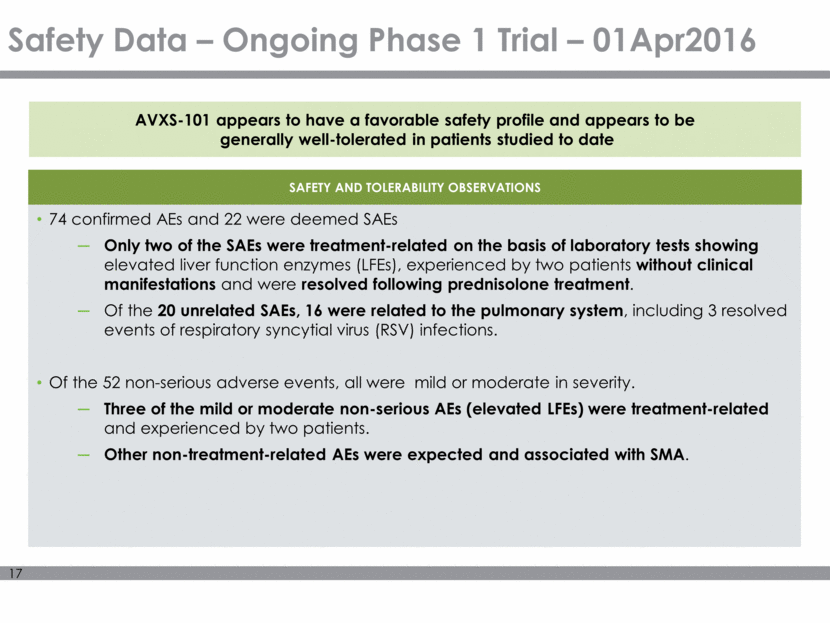
Safety Data – Ongoing Phase 1 Trial – 01Apr2016 SAFETY AND TOLERABILITY OBSERVATIONS 74 confirmed AEs and 22 were deemed SAEs Only two of the SAEs were treatment-related on the basis of laboratory tests showing elevated liver function enzymes (LFEs), experienced by two patients without clinical manifestations and were resolved following prednisolone treatment. Of the 20 unrelated SAEs, 16 were related to the pulmonary system, including 3 resolved events of respiratory syncytial virus (RSV) infections. Of the 52 non-serious adverse events, all were mild or moderate in severity. Three of the mild or moderate non-serious AEs (elevated LFEs) were treatment-related and experienced by two patients. Other non-treatment-related AEs were expected and associated with SMA. AVXS-101 appears to have a favorable safety profile and appears to be generally well-tolerated in patients studied to date
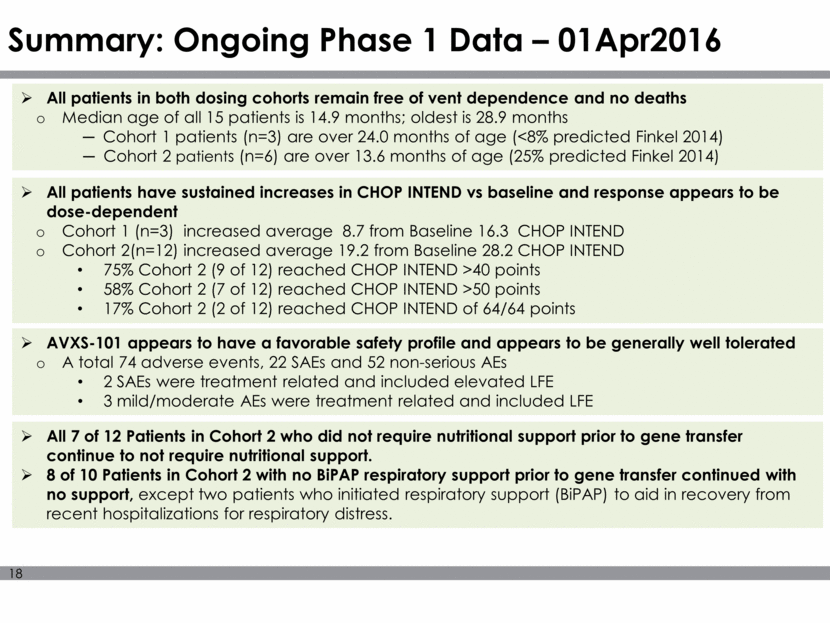
Summary: Ongoing Phase 1 Data – 01Apr2016 AVXS-101 appears to have a favorable safety profile and appears to be generally well tolerated A total 74 adverse events, 22 SAEs and 52 non-serious AEs 2 SAEs were treatment related and included elevated LFE 3 mild/moderate AEs were treatment related and included LFE All patients in both dosing cohorts remain free of vent dependence and no deaths Median age of all 15 patients is 14.9 months; oldest is 28.9 months Cohort 1 patients (n=3) are over 24.0 months of age (<8% predicted Finkel 2014) Cohort 2 patients (n=6) are over 13.6 months of age (25% predicted Finkel 2014) All patients have sustained increases in CHOP INTEND vs baseline and response appears to be dose-dependent Cohort 1 (n=3) increased average 8.7 from Baseline 16.3 CHOP INTEND Cohort 2(n=12) increased average 19.2 from Baseline 28.2 CHOP INTEND 75% Cohort 2 (9 of 12) reached CHOP INTEND >40 points 58% Cohort 2 (7 of 12) reached CHOP INTEND >50 points 17% Cohort 2 (2 of 12) reached CHOP INTEND of 64/64 points All 7 of 12 Patients in Cohort 2 who did not require nutritional support prior to gene transfer continue to not require nutritional support. 8 of 10 Patients in Cohort 2 with no BiPAP respiratory support prior to gene transfer continued with no support, except two patients who initiated respiratory support (BiPAP) to aid in recovery from recent hospitalizations for respiratory distress.
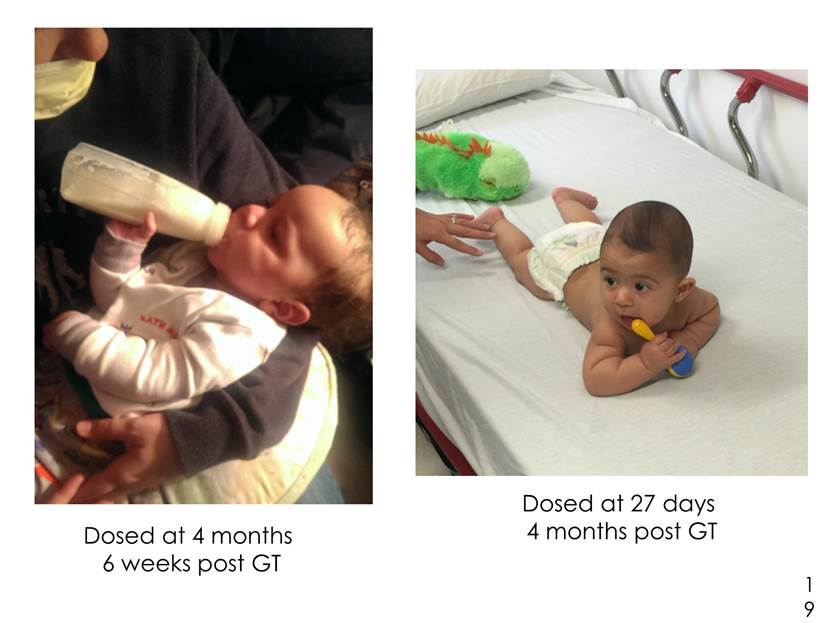
Dosed at 4 months 6 weeks post GT Dosed at 27 days 4 months post GT 19
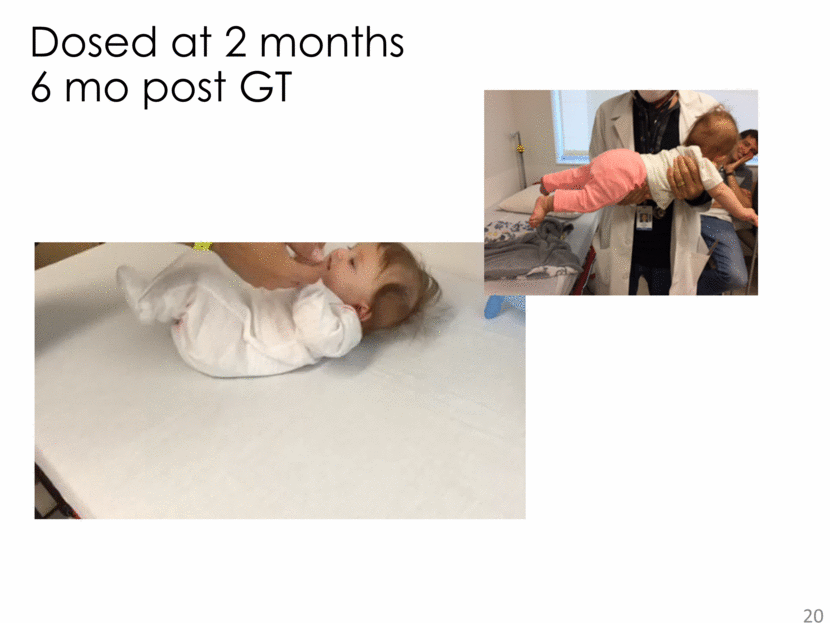
20 Dosed at 2 months 6 mo post GT
Making Great Progress !! Thanks to the Team at NCH Brian Kaspar Tom Prior Samiah Al-Zaidy Carlos Miranda Richard Shell Chris Petek Dave Arnold Katie Church Louise Rodino-Klapac Sarah Corcoran Linda Lowes Kathrin Meyer Lindsay Alfano John Kissel Kate Berry Kevin Foust Arthur Burghes Lindsey Braun Team at AveXis Sukumar Nagendran Minna Du Nancy Sacco Sarah Leonard Doug Sproule Jessica Cardenas Courtney Wells 21

