Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Jaguar Health, Inc. | a16-10635_18k.htm |
Exhibit 99.1
Corporate Presentation May 2016

This presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements regarding the anticipated timing of the commercial launch of Canalevia, and the timing of expanding the indication for Canalevia to acute diarrhea and the timing of data from planned proof of concept, field and other studies are forward-looking statements. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “expect,” “plan,” “aim,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplate,” “believe,” “estimate,” “predict,” “potential” or “continue” or the negative of these terms or other similar expressions. The forward-looking statements in this presentation are only predictions. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our business, financial condition and results of operations. These forward-looking statements speak only as of the date of this presentation and are subject to a number of risks, uncertainties and assumptions, some of which cannot be predicted or quantified and some of which are beyond our control. The events and circumstances reflected in our forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. Moreover, we operate in a dynamic industry and economy. New risk factors and uncertainties may emerge from time to time, and it is not possible for management to predict all risk factors and uncertainties that we may face. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. 1 Forward-Looking Statements

GI Product Development Strategy Approved for use in humans Fulyzaq1 (crofelemer) Intellectual property applies globally to all products across species 2 1Fulyzaq was approved by the FDA in 2012 for the symptomatic relief of noninfectious diarrhea in adults with HIV/AIDS on antiretroviral therapy. The product is a registered trademark of and is marketed by Salix Pharmaceuticals, Inc. Neonorm™ (non-prescription) Canalevia™ (Rx) Species-specific formulations of crofelemer (Rx)
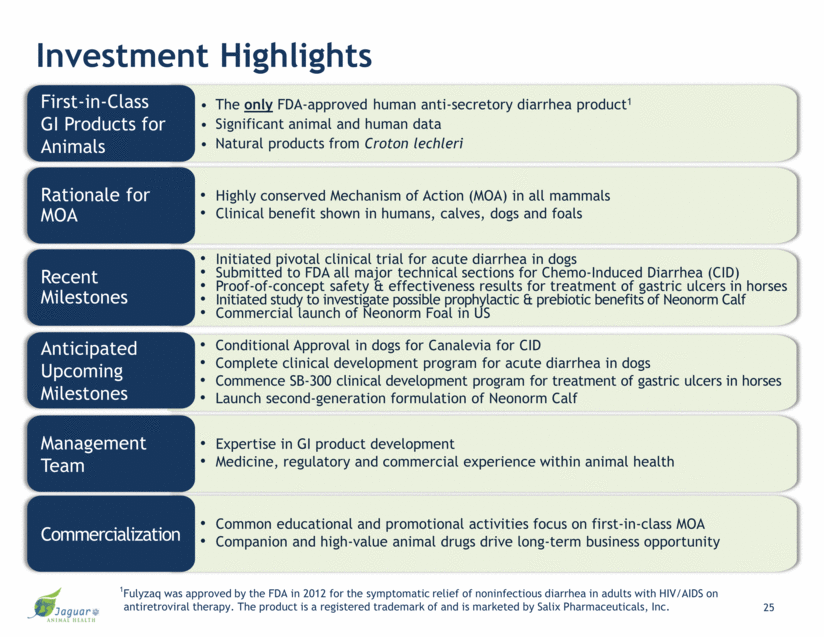
Investment Highlights The only FDA-approved human anti-secretory diarrhea product1 Significant animal and human data Natural products from Croton lechleri First-in-Class GI Products for Animals Conditional Approval in dogs for Canalevia for CID Complete clinical development program for acute diarrhea in dogs Continue SB-300 clinical development program for treatment of gastric ulcers in horses Launch second-generation formulation of Neonorm Calf Anticipated Upcoming Milestones Highly conserved Mechanism of Action (MOA) in all mammals Clinical benefit shown in humans, calves, dogs and foals Rationale for MOA Initiated pivotal clinical trial for acute diarrhea in dogs Submitted to FDA all major technical sections for Chemo-Induced Diarrhea (CID) Proof-of-concept safety & effectiveness results for treatment of gastric ulcers in horses Initiated study to investigate possible prophylactic & prebiotic benefits of Neonorm Calf Commercial launch of Neonorm Foal in US Recent Milestones Expertise in GI product development Medicine, regulatory and commercial experience within animal health Management Team Common educational and promotional activities focus on first-in-class MOA Companion and high-value animal drugs drive long-term business opportunity Commercialization 3 1Fulyzaq was approved by the FDA in 2012 for the symptomatic relief of noninfectious diarrhea in adults with HIV/AIDS on antiretroviral therapy. The product is a registered trademark of and is marketed by Salix Pharmaceuticals, Inc.
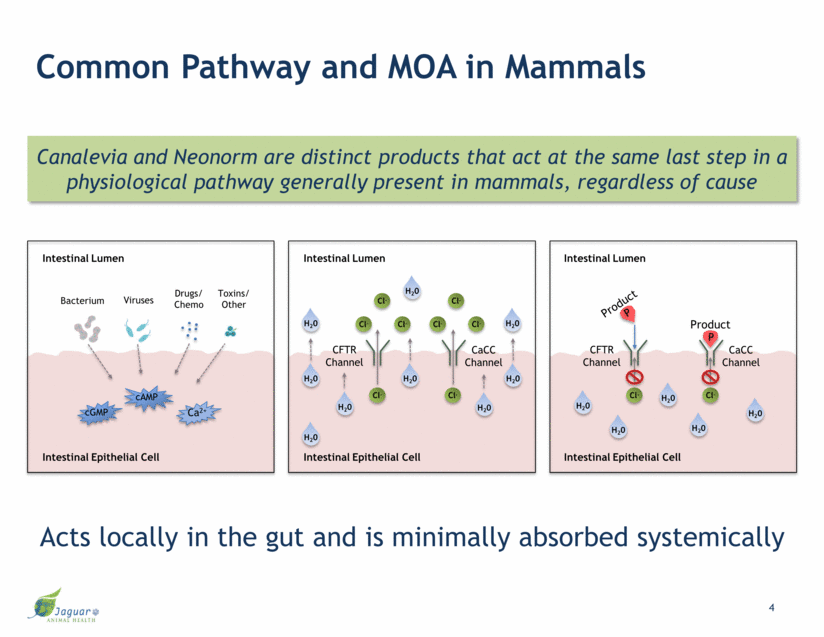
Common Pathway and MOA in Mammals Canalevia and Neonorm are distinct products that act at the same last step in a physiological pathway generally present in mammals, regardless of cause Acts locally in the gut and is minimally absorbed systemically P P Intestinal Lumen Intestinal Epithelial Cell Cl- CFTR Channel CaCC Channel Cl- H20 H20 H20 H20 H20 Cl- Cl- Cl- Cl- Cl- Cl- Intestinal Lumen Intestinal Epithelial Cell Cl- CFTR Channel CaCC Channel Cl- H20 H20 H20 H20 H20 H20 H20 H20 H20 Bacterium Viruses Drugs/ Chemo Toxins/ Other cAMP cGMP Ca2+ Intestinal Epithelial Cell Intestinal Lumen Product Product 4
Intellectual Property Portfolio Exclusive worldwide veterinary license to all Napo IP for all veterinary treatment uses and indications for all species of animals Zero to low royalties Exclusive global veterinary license to 2,300+ medicinal plants Eight provisional patent applications, three pending patent applications under the Patent Cooperation Treaty, and one U.S. non-provisional patent application Notices of Allowance for two NP-500 patent applications Jaguar’s drug product candidate to treat diseases related to insulin-resistance in dogs, horses & cats Prebiotic benefits of polyphenols/Croton lechleri-derived products Rifaximin combination 5
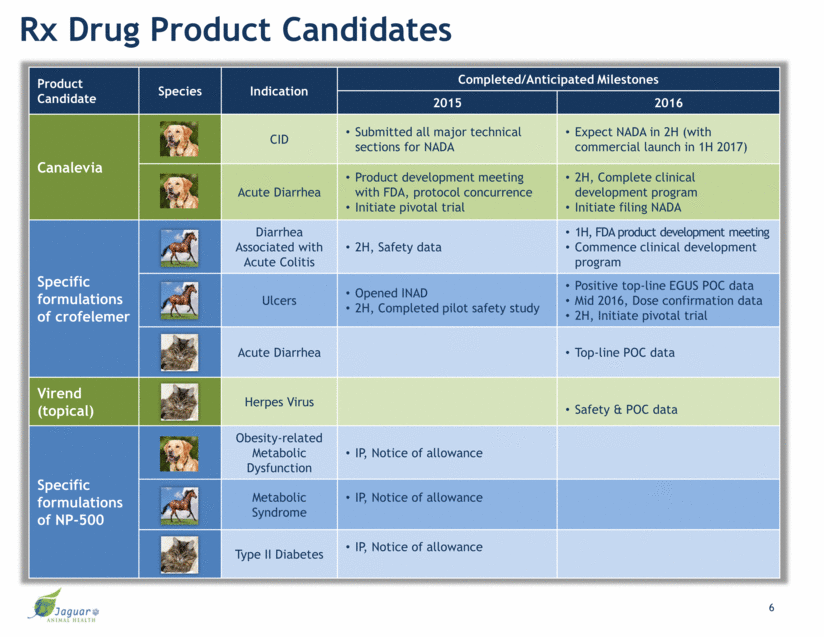
Rx Drug Product Candidates Product Candidate Species Indication Completed/Anticipated Milestones 2015 2016 Canalevia CID Submitted all major technical sections for NADA Expect NADA in 2H (with commercial launch in 1H 2017) Acute Diarrhea Product development meeting with FDA, protocol concurrence Initiate pivotal trial 2H, Complete clinical development program Initiate filing NADA Specific formulations of crofelemer Diarrhea Associated with Acute Colitis 2H, Safety data 1H, FDA product development meeting Commence clinical development program Ulcers Opened INAD 2H, Completed pilot safety study Positive top-line EGUS POC data Mid 2016, Dose confirmation data 2H, Initiate pivotal trial Acute Diarrhea Top-line POC data Virend (topical) Herpes Virus Safety & POC data Specific formulations of NP-500 Obesity-related Metabolic Dysfunction IP, Notice of allowance Metabolic Syndrome IP, Notice of allowance Type II Diabetes IP, Notice of allowance 6
Canalevia: Diarrhea in Dogs Diarrhea is one of the most common reasons for veterinary and emergency visits for dogs in the US Six million cases annually of acute and chronic watery diarrhea No FDA-approved anti-secretory products for dogs Current treatments Rehydration Diet change Absorbents/binding agents (Pepto-Bismol) Anti-motility agents (Imodium) Antibiotics No current treatments directly address dehydration and watery flow 7

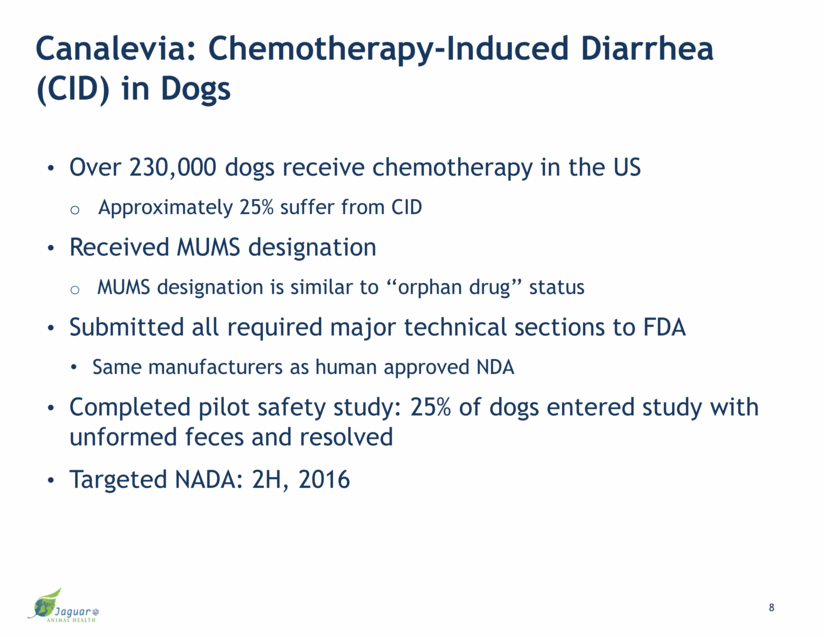
Canalevia: Chemotherapy-Induced Diarrhea (CID) in Dogs Over 230,000 dogs receive chemotherapy in the US Approximately 25% suffer from CID Received MUMS designation MUMS designation is similar to ‘‘orphan drug’’ status Submitted all required major technical sections to FDA Same manufacturers as human approved NDA Completed pilot safety study: 25% of dogs entered study with unformed feces and resolved Targeted NADA: 2H, 2016 8
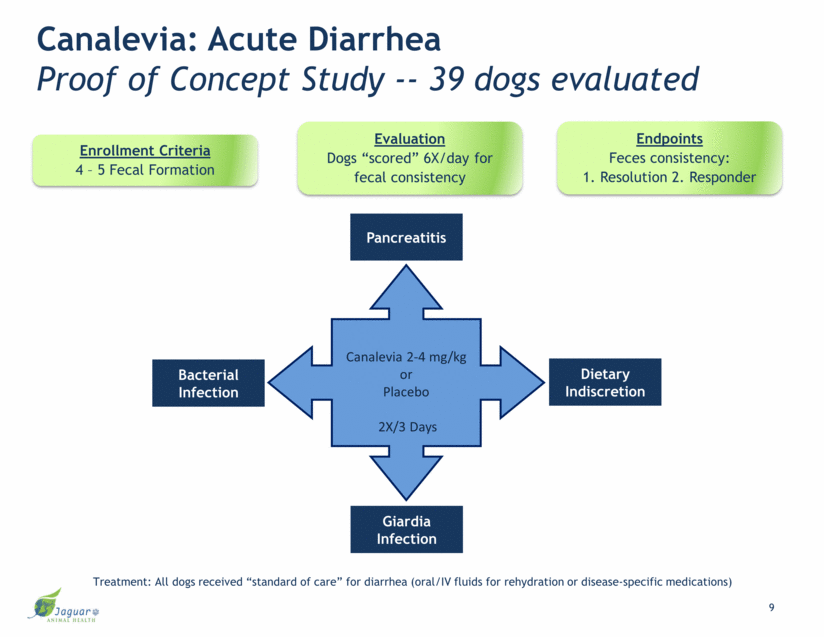
Canalevia 2-4 mg/kg or Placebo 2X/3 Days Canalevia: Acute Diarrhea Proof of Concept Study -- 39 dogs evaluated Bacterial Infection Pancreatitis Dietary Indiscretion Giardia Infection 9 Treatment: All dogs received “standard of care” for diarrhea (oral/IV fluids for rehydration or disease-specific medications) Evaluation Dogs “scored” 6X/day for fecal consistency Endpoints Feces consistency: 1. Resolution 2. Responder Enrollment Criteria 4 – 5 Fecal Formation
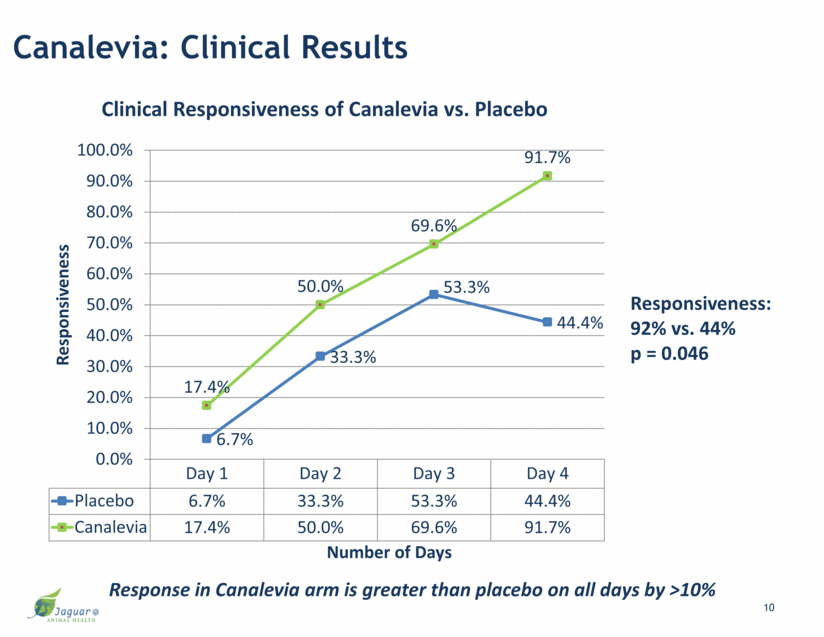
Canalevia: Clinical Results Response in Canalevia arm is greater than placebo on all days by >10% Responsiveness: 92% vs. 44% p = 0.046 10 Day 1 Day 2 Day 3 Day 4 Placebo 6.7% 33.3% 53.3% 44.4% Canalevia 17.4% 50.0% 69.6% 91.7% 6.7% 33.3% 53.3% 44.4% 17.4% 50.0% 69.6% 91.7% 0.0% 10.0% 20.0% 30.0% 40.0% 50.0% 60.0% 70.0% 80.0% 90.0% 100.0% Responsiveness Number of Days Clinical Responsiveness of Canalevia vs. Placebo
Number of Dogs Achieving Resolution* of Diarrhea 11 *Resolution of diarrhea is defined as achieving a fecal score of 1 or 2 and no relapse during the study The difference in proportions for crofelemer and control is 32% The Chi-square p-value = 0.0470
Canalevia: Pathway to Commercialization 12 Expand label indication for Canalevia to acute diarrhea, regardless of cause Development Meeting with FDA, Protocol Concurrence, 2015 Pivotal efficacy initiation in 2015 Commercial launch for CID expected in 2017 Commercial launch expected in 2017
Equine Ulcer Opportunity (SB-300) Positive top-line EGUS POC data 97% of high performance horses have either gastric or colonic ulcers* 63% of high performance horses have colonic ulcers* 87% of high performance horses have gastric ulcers* 54% of high performance horses have both colonic and gastric ulcers* No marketed FDA-approved treatments for colonic ulcers in horses Chronic treatment cost ~$50/day International synergies for market awareness and demand ~4 million high performance horses in US 13 *Pellegrini, Franklin L., Results of a large-scale necroscopic study of equine colonic ulcers. J Equine Vet Sci 2005; v. 25, no. 3; 113–117.
Study Objective: Evaluate the safety and effectiveness of SB-300 for treatment of equine gastrointestinal ulcers SB-300 Proof-of-Concept Study for Equine Ulcers 14 Placebo 2.5BID 10QID 30 racehorses were randomized to one of three groups (10 horses per group). Horses in the TRT5 group received 5 grams of SB-300 divided into 2 doses per day; and those in the TRT40 group received 40 grams of SB-300 divided into 4 doses per day. GLANDULAR: DAY 14 % of Horses with Improvement (1 Grade Decrease) GLANDULAR: DAY 35 % of Horses with No Resolution (p-value of 0.03) Placebo 2.5BID 10QID Published studies1,2 with omeprazole demonstrate that between 14% and 34% of horses diagnosed with EGUS are observed with resolution or improvement of glandular ulcers when used at the manufacturer’s recommended treatment duration of 28 days *Sykes BW, Sykes KM, Hallowell GD. A comparison of three doses of omeprazole in the treatment of equine gastric ulcer syndrome: A blinded, randomised, dose-response clinical trial. Equine Vet J. 2015;47(3):285-290. 2Sykes BW, Sykes KM, Hallowell GD. A comparison of two doses of omeprazole in the treatment of equine gastric ulcer syndrome: a blinded, randomised, clinical trial. Equine Vet J. 2014;46(4):416-421. Conclusions: Glandular Ulcers Resolution and improvement vs. placebo at Day 14, with a p-value of 0.0286

SB-300 Proof-of-Concept Study for Equine Ulcers 15 Drug testing in horses that received SB-300 did not detect any substances commonly disallowed in horse racing—enabling continued therapy SB-300 acts locally in the gut with minimal systemic absorption Feed does not appear to interfere with local availability of SB-300 SB-300 did not alter gastric pH Maintaining normal gastric pH is essential for: Digestion Gut immunity First line defense against pathogens Absorption of vitamins and minerals Additional Advantages:

Neonorm: Non-Prescription Products Products Species Use Anticipated Milestones 2015 2016 Neonorm Calf Improve gut health and normalize stool formation in pre-weaned dairy calves with scours Field studies: Include evaluation of herd-wide prophylactic & prebiotic benefits. Initial results support prebiotic effect and beneficial results of Neonorm on weight gain in preweaned calves Launch second-generation formulation (administered in liquid) South American commercial launch Species-specific formulations of Neonorm Normalize fecal formation (horse foals) 2H, POC positive results 2H, Soft-launched at American Association of Equine Practitioners Trade Show 1H, Commercial launch Normalize fecal formation (adult horses & other farm/production animals) Initiate POC studies in various species based on market research 16
17 A new and unique anti-diarrheal for foals Commercial launch in Q1 2016 Premiered product at Dec. 2015 American Association of Equine Practitioners Trade Show There are currently no other anti-secretory products commercially available for the foal market
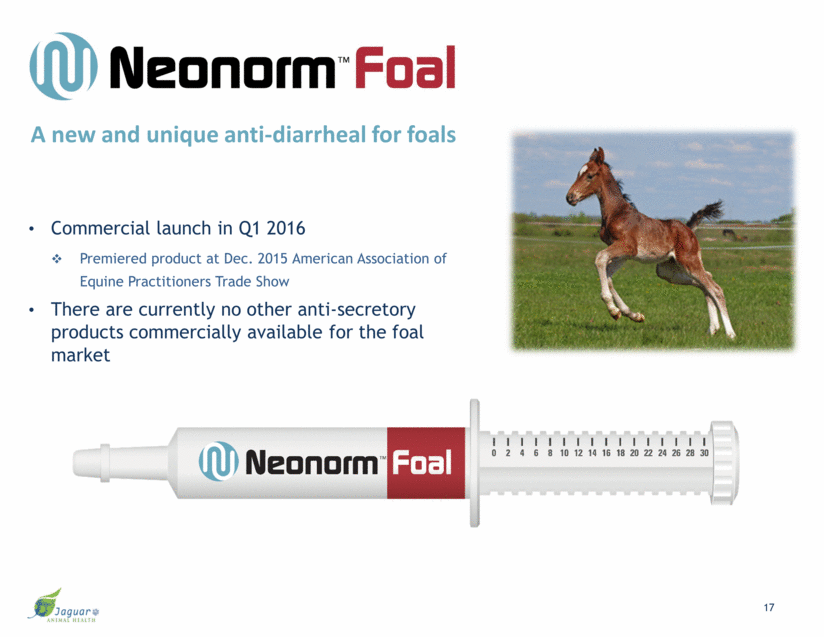
Study Objective: Determine the safety, tolerability, and efficacy of Neonorm Foal Neonorm Foal Proof-of-Concept Study Conclusion: This exploratory study demonstrated the effectiveness of Neonorm Foal vs. placebo in the treatment of diarrhea amongst foals from birth to 16 weeks. 1Responder: A foal who achieved a formed stool by the end of the treatment period. 2Resolution: A foal who achieved a formed stool (1 or 2) at any point at any post-baseline assessment. 18
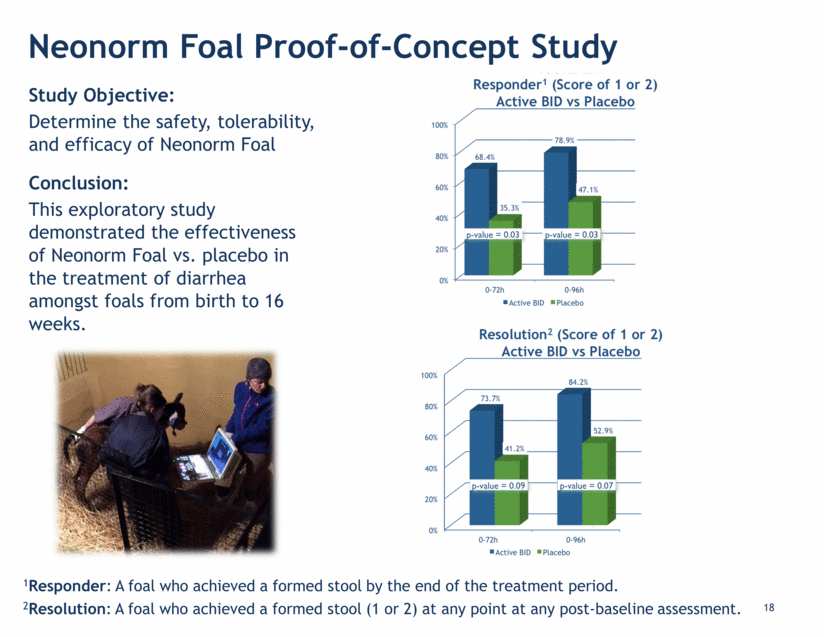
19 Study Objective: Evaluate severity and incidence of diarrhea, mortality and weight gain. Conclusion: The total economic benefit from health endpoints, such as weight gain, from Neonorm could be ~$110 per calf. Neonorm Calf E. coli Challenge Study* Average Daily Calf Fecal Scores Neonorm significantly decreased severity of watery diarrhea (p=0.0133) 3 # of Calves Mortality Average Daily Weight Gain After 25 Days Neonorm Calf 20 5% (1 calf) 15.5 pounds (281 g/day) Placebo 19 21% (4 calves) 12.1 pounds (219 g/day) Average Duration of Watery Diarrhea (Score >2) Average Duration of Severe Watery Diarrhea (Score 3 or 4) Neonorm Calf 3.03 days 1.10 days Placebo 5.16 days 2.42 days Health and Economic Impacts *Study published in American Dairy Science Association’s Journal of Dairy Science, fall 2015
Neonorm Calf: Field Studies1 Two recently completed field studies: Study conducted in association with Cornell University College of Veterinary Medicine and field study in Wisconsin further supports benefits related to supporting reductions in water loss associated with diarrhea and supporting weight gain in preweaned calves Recently completed Cornell study supports benefit on optimization of intestinal microbiome in calves Cornell trial to investigate this potential prebiotic and prophylactic benefit Second-generation formulation of Neonorm—for entire herd management—that can be administered in water and/or milk replacer 20 1These are results from the two field studies conducted to support Jaguar’s commercial launch of Neonorm.
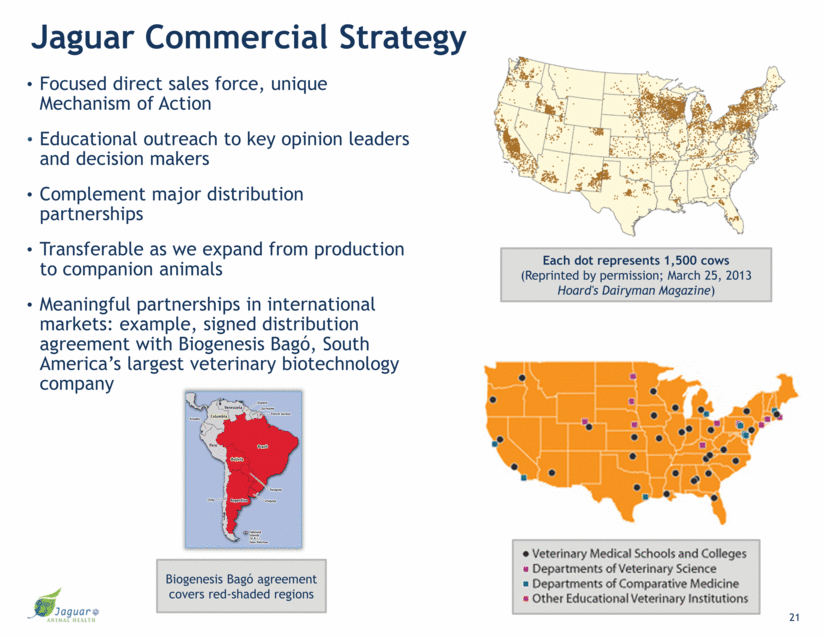
Jaguar Commercial Strategy Focused direct sales force, unique Mechanism of Action Educational outreach to key opinion leaders and decision makers Complement major distribution partnerships Transferable as we expand from production to companion animals Meaningful partnerships in international markets: example, signed distribution agreement with Biogenesis Bagó, South America’s largest veterinary biotechnology company 21 Biogenesis Bagó agreement covers red-shaded regions Each dot represents 1,500 cows (Reprinted by permission; March 25, 2013 Hoard's Dairyman Magazine)
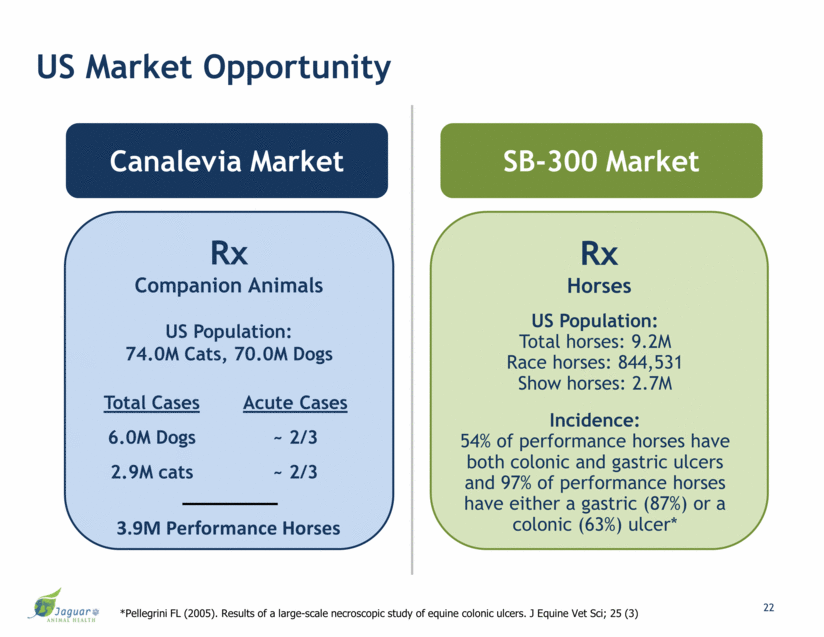
US Market Opportunity Canalevia Market SB-300 Market Rx Companion Animals US Population: 74.0M Cats, 70.0M Dogs Rx Horses Total Cases Acute Cases 6.0M Dogs ~ 2/3 2.9M cats ~ 2/3 3.9M Performance Horses 22 US Population: Total horses: 9.2M Race horses: 844,531 Show horses: 2.7M Incidence: 54% of performance horses have both colonic and gastric ulcers and 97% of performance horses have either a gastric (87%) or a colonic (63%) ulcer* *Pellegrini FL (2005). Results of a large-scale necroscopic study of equine colonic ulcers. J Equine Vet Sci; 25 (3)
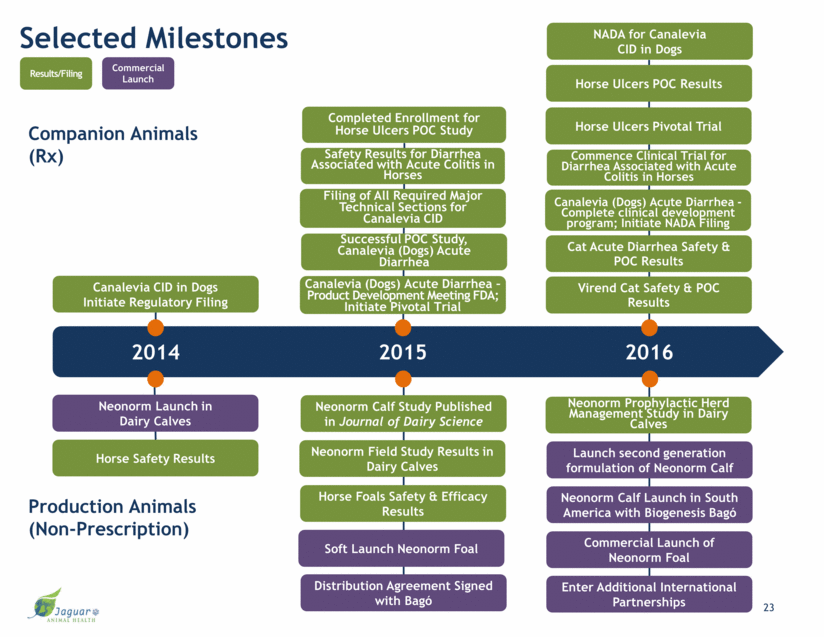
Selected Milestones 2014 2015 2016 Companion Animals (Rx) Production Animals (Non-Prescription) Neonorm Field Study Results in Dairy Calves Neonorm Launch in Dairy Calves Canalevia CID in Dogs Initiate Regulatory Filing Horse Foals Safety & Efficacy Results Commercial Launch of Neonorm Foal Virend Cat Safety & POC Results Filing of All Required Major Technical Sections for Canalevia CID Horse Safety Results Results/Filing Commercial Launch Horse Ulcers POC Results Soft Launch Neonorm Foal Cat Acute Diarrhea Safety & POC Results Canalevia (Dogs) Acute Diarrhea – Product Development Meeting FDA; Initiate Pivotal Trial Commence Clinical Trial for Diarrhea Associated with Acute Colitis in Horses Canalevia (Dogs) Acute Diarrhea - Complete clinical development program; Initiate NADA Filing Horse Ulcers Pivotal Trial Neonorm Calf Launch in South America with Biogenesis Bagó Neonorm Prophylactic Herd Management Study in Dairy Calves Distribution Agreement Signed with Bagó Completed Enrollment for Horse Ulcers POC Study Safety Results for Diarrhea Associated with Acute Colitis in Horses Neonorm Calf Study Published in Journal of Dairy Science Successful POC Study, Canalevia (Dogs) Acute Diarrhea Launch second generation formulation of Neonorm Calf Enter Additional International Partnerships NADA for Canalevia CID in Dogs 23
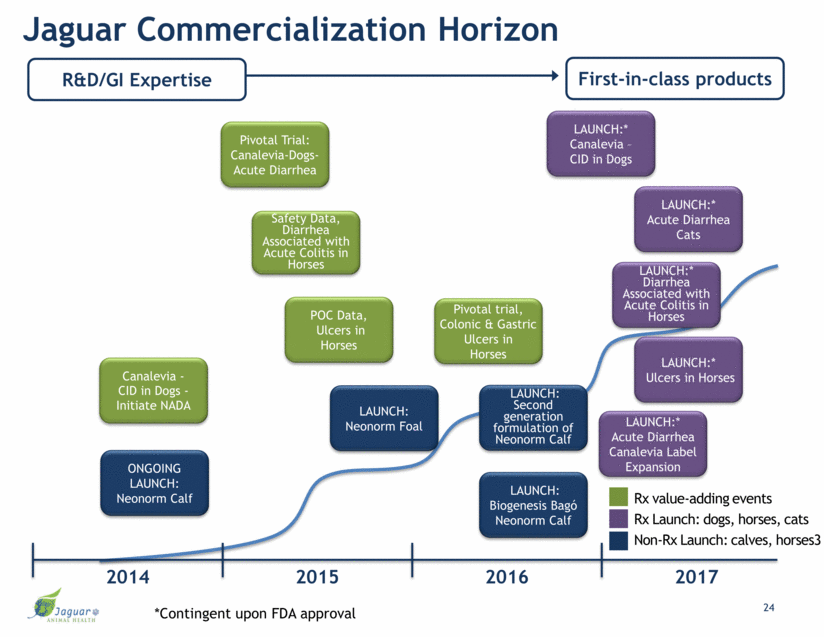
Jaguar Commercialization Horizon R&D/GI Expertise First-in-class products ONGOING LAUNCH: Neonorm Calf Pivotal Trial: Canalevia-Dogs- Acute Diarrhea Canalevia - CID in Dogs - Initiate NADA LAUNCH: Neonorm Foal LAUNCH:* Canalevia – CID in Dogs LAUNCH:* Diarrhea Associated with Acute Colitis in Horses LAUNCH:* Acute Diarrhea Canalevia Label Expansion LAUNCH:* Acute Diarrhea Cats Rx value-adding events Rx Launch: dogs, horses, cats Non-Rx Launch: calves, horses3 2015 2017 2016 2014 *Contingent upon FDA approval POC Data, Ulcers in Horses LAUNCH:* Ulcers in Horses LAUNCH: Biogenesis Bagó Neonorm Calf Safety Data, Diarrhea Associated with Acute Colitis in Horses Pivotal trial, Colonic & Gastric Ulcers in Horses 24 LAUNCH: Second generation formulation of Neonorm Calf
Investment Highlights The only FDA-approved human anti-secretory diarrhea product1 Significant animal and human data Natural products from Croton lechleri First-in-Class GI Products for Animals Conditional Approval in dogs for Canalevia for CID Complete clinical development program for acute diarrhea in dogs Commence SB-300 clinical development program for treatment of gastric ulcers in horses Launch second-generation formulation of Neonorm Calf Anticipated Upcoming Milestones Highly conserved Mechanism of Action (MOA) in all mammals Clinical benefit shown in humans, calves, dogs and foals Rationale for MOA Initiated pivotal clinical trial for acute diarrhea in dogs Submitted to FDA all major technical sections for Chemo-Induced Diarrhea (CID) Proof-of-concept safety & effectiveness results for treatment of gastric ulcers in horses Initiated study to investigate possible prophylactic & prebiotic benefits of Neonorm Calf Commercial launch of Neonorm Foal in US Recent Milestones Expertise in GI product development Medicine, regulatory and commercial experience within animal health Management Team Common educational and promotional activities focus on first-in-class MOA Companion and high-value animal drugs drive long-term business opportunity Commercialization 25 1Fulyzaq was approved by the FDA in 2012 for the symptomatic relief of noninfectious diarrhea in adults with HIV/AIDS on antiretroviral therapy. The product is a registered trademark of and is marketed by Salix Pharmaceuticals, Inc.
26 Jaguar and Napo Pharmaceuticals are engaged in discussions to review a potential merger and/or other ways to cooperate with their respective business endeavors. Potential Merger

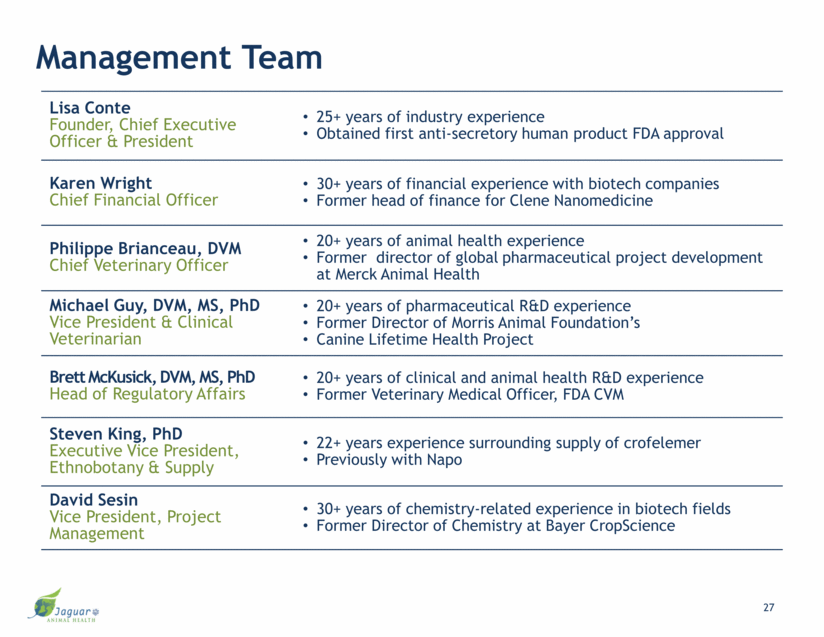
Management Team Lisa Conte Founder, Chief Executive Officer & President 25+ years of industry experience Obtained first anti-secretory human product FDA approval Karen Wright Chief Financial Officer 30+ years of financial experience with biotech companies Former head of finance for Clene Nanomedicine Philippe Brianceau, DVM Chief Veterinary Officer 20+ years of animal health experience Former director of global pharmaceutical project development at Merck Animal Health Michael Guy, DVM, MS, PhD Vice President & Clinical Veterinarian 20+ years of pharmaceutical R&D experience Former Director of Morris Animal Foundation’s Canine Lifetime Health Project Brett McKusick, DVM, MS, PhD Head of Regulatory Affairs 20+ years of clinical and animal health R&D experience Former Veterinary Medical Officer, FDA CVM Steven King, PhD Executive Vice President, Ethnobotany & Supply 22+ years experience surrounding supply of crofelemer Previously with Napo David Sesin Vice President, Project Management 30+ years of chemistry-related experience in biotech fields Former Director of Chemistry at Bayer CropScience 27
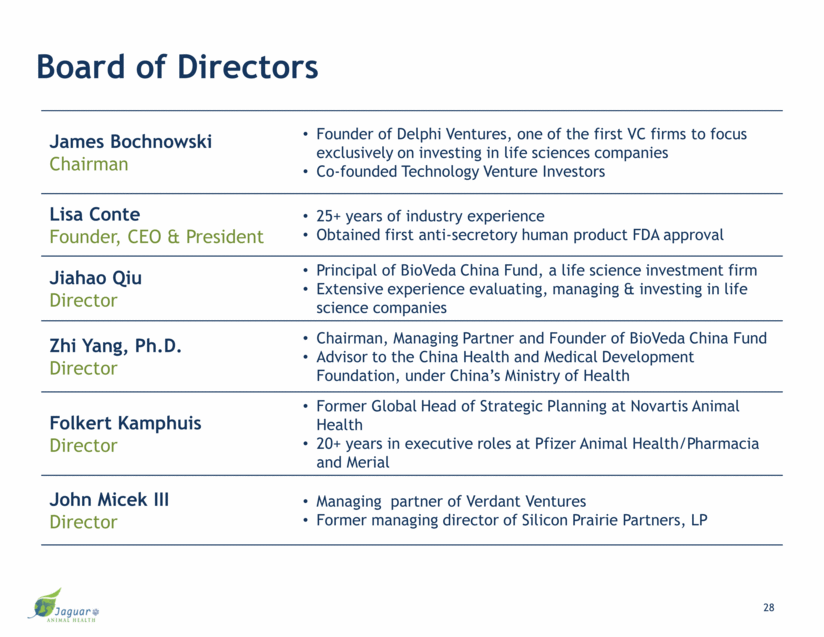
Board of Directors James Bochnowski Chairman Founder of Delphi Ventures, one of the first VC firms to focus exclusively on investing in life sciences companies Co-founded Technology Venture Investors Lisa Conte Founder, CEO & President 25+ years of industry experience Obtained first anti-secretory human product FDA approval Jiahao Qiu Director Principal of BioVeda China Fund, a life science investment firm Extensive experience evaluating, managing & investing in life science companies Zhi Yang, Ph.D. Director Chairman, Managing Partner and Founder of BioVeda China Fund Advisor to the China Health and Medical Development Foundation, under China’s Ministry of Health Folkert Kamphuis Director Former Global Head of Strategic Planning at Novartis Animal Health 20+ years in executive roles at Pfizer Animal Health/Pharmacia and Merial John Micek III Director Managing partner of Verdant Ventures Former managing director of Silicon Prairie Partners, LP 28
[LOGO]

