Attached files
| file | filename |
|---|---|
| 8-K - 8-K - MADRIGAL PHARMACEUTICALS, INC. | a16-10338_18k.htm |
Exhibit 99.1
The New Madrigal Pharmaceuticals NASDAQ: SNTA | May 2016 1

Forward-Looking Statements Any statements made in this presentation relating to future financial or business performance, conditions, plans, prospects, trends, or strategies and other financial and business matters, including without limitation, whether and when our recently announced merger with Madrigal will close; the ability of the combined company to raise needed capital; the success of our merger with Madrigal, if consummated; the estimated size of the market for product candidates, the timing and success of the combined company’s development and commercialization of its anticipated product candidates; and the availability of alternative therap ies for the combined company’s target market, are forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. In addition, when or if used in this presentation, the words “may,” “could,” “should,” “anticipate,” “believe,” “estimate,” “expect,” “intend,” “plan,” “predict” and similar expressions and their variants, as they relate to Madrigal, Synta or the management of either company, before or after the aforementioned merger, may identify forward-looking statements. Madrigal and Synta caution that these forward-looking statements are subject to numerous assumptions, risks, and uncertainties, which change over time. Important factors that may cause actual results to differ materially from the results discussed in the for ward-looking statements or historical experience include risks and uncertainties, including the timing and completion of the merge r, including the parties’ ability to satisfy the closing conditions of the Merger Agreement with Madrigal, including the closing condition that Synta have a minimum net cash amount of $28.5 million, Synta’s continued listing on NASDAQ, the failure by Madrigal or Synta to secure and maintain relationships with collaborators, risks relating to clinical trials; risks relating to the commercialization, if any, of Madrigal or Synta proposed product candidates (such as marketing, regulatory, product liability, supply, competition, and other risks); dependence on the efforts of third parties; dependence on intellectual property; and r isks that Madrigal or Synta may lack the financial resources and access to capital to fund proposed operations. Further information on the factors and risks that could affect Synta’s business, financial conditions and results of operations are contained in Synta’s filings with the U.S. Securities and Exchange Commission, which are available at www.sec.gov. The forward-looking statements represent the estimate of Madrigal and Synta as of the date hereof only, and Madrigal and Synta specifically disclaim any duty or obligation to update forward-looking statements. 2

Additional Information about the Merger and Where to Find It This presentation does not constitute an offer to sell or the solicitation of an offer to buy any securities or a solicitatio n of any vote or approval. A definitive proxy statement and a proxy card will be filed with the SEC and will be mailed to Synta’s stockholders seeking any required stockholder approvals in connection with the proposed transactions. The proxy statement will contain important information about Synta, Madrigal, the transaction and related matters. BEFORE MAKING ANY VOTING OR INVESTMENT DECISION, INVESTORS AND STOCKHOLDERS ARE URGED TO READ THE PROXY STATEMENT (INCLUDING ANY AMENDMENTS OR SUPPLEMENTS THERETO) AND ANY OTHER RELEVANT DOCUMENTS THAT SYNTA MAY FILE WITH THE SEC WHEN THEY BECOME AVAILABLE BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION ABOUT THE PROPOSED TRANSACTIONS. Stockholders may obtain, free of charge, copies of the definitive proxy statement and any other documents filed by Synta with the SEC in connection with the proposed transactions at the SEC's website (http://www.sec.gov), at Synta’s website under the heading “Investors / SEC Filings”, or by directing a written request to: Synta Pharmaceuticals Corp., 45 Hartwell Avenue, Lexington, MA 02421, Attention: Wendy Rieder, Esq. Synta and its directors and executive officers and Madrigal and its directors and executive officers may be deemed to be participants in the solicitation of proxies from the stockholders of Synta in connection with the proposed transaction. Information regarding the special interests of these directors and executive officers in the merger will be included in the proxy statement referred to above. Additional information regarding the directors and executive officers of Synta is also included in Synta’s Annual Report on Form 10-K, as amended, which was filed with the SEC on March 16, 2016, and amended on April 29, 2016. This document is available free of charge at the SEC web site (www.sec.gov), at Synta’s website under the heading “Investors / SEC Filings”, or by directing a written request to Synta as described above. 3

Transaction Terms • Synta to acquire all outstanding shares of Madrigal in exchange for approximately 253.9 million newly issued shares of Synta common stock Synta shareholders expected to own 36.0% of the combined company and Madrigal shareholders expected to own 64.0% Upon closing of the transaction, the combined company will be known as Madrigal Pharmaceuticals Investor syndicate committed to invest up to $9 million in Madrigal prior to the closing of the Merger All Stock Transaction • Name and Ownership • • Cash and Funding – Combined anticipated Synta cash balance and private placement provides sufficient cash to fund several clinical readouts Approval • Approved by the boards of directors of both companies and Madrigal shareholders Expected to close by the end of 3Q 2016, subject to the approval of the stockholders of Synta as well as other customary closing conditions, including satisfaction of Synta having at least $28.5 million in net cash at closing • Close 4
The New Combined Company: Investment Highlights • Phase 2-ready liver-directed thyroid hormone receptor-ß agonist (THR-ß); efficacy and safety profile validated by preclinical and clinical data MGL-3196: First-in-Class THR-ß Agonist • Initial indications are NASH and familial hypercholesterolemia; possibility to expand indications Large & Underserved Markets in NASH & Genetic Lipid Disorders • Multiple potential clinical trial initiations in 2016 and data readouts throughout 2017 for NASH, HeFH & HoFH Multiple Possible Value-Creating Catalysts over Next 18 Months Expected Funding to Key Inflection Points • Combined cash resources sufficient to reach key clinical inflection points in NASH, HeFH & HoFH • Experienced management team with proven track record in drug discovery, development and commercialization; expertise in liver diseases Seasoned Management Team 5
Synta-Madrigal Leadership • Combined company will be led by an experienced management team with multiple successful NDA/EMAs and marketed products – Paul Friedman, M.D. - Chairman and CEO • Former CEO of Incyte Pharmaceuticals; former President of DuPont Pharmaceuticals Research Rebecca Taub, M.D. - Chief Medical Officer, Executive Vice President, R&D • Led teams that discovered Eliquis and MGL-3196, Madrigal’s lead compound • Recognized expert in liver regeneration and diseases of the liver Marc Schneebaum - Chief Financial Officer • SVP, CFO of Synta since 2014 – – • Board comprised of seven directors: five from Madrigal, one from Synta, and one mutually agreed upon designee Corporate headquarters will be located in the Philadelphia area • 6
MGL-3196: Building a Platform in Lipid and Liver Disorders 7

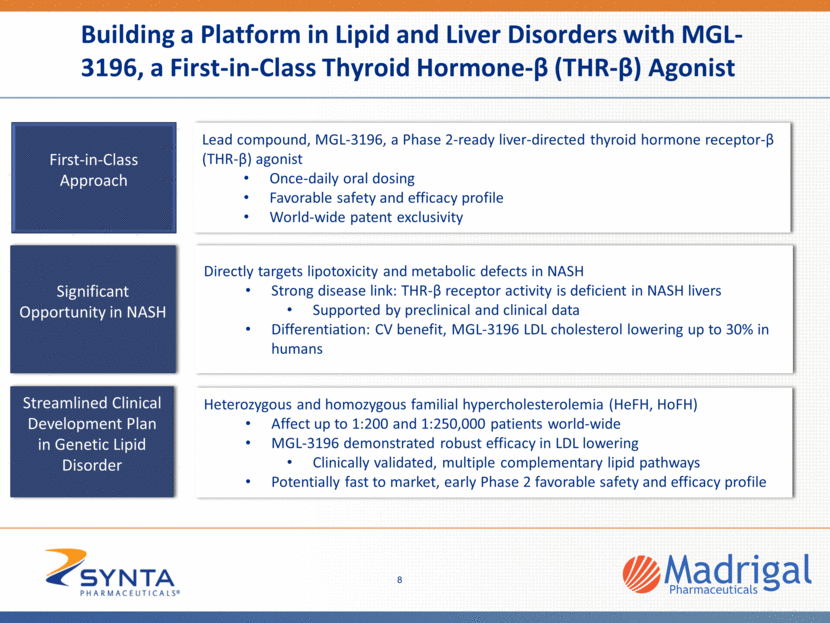
Building a Platform in Lipid and Liver Disorders with MGL-3196, a First-in-Class Thyroid Hormone-β (THR-β) Agonist Lead compound, MGL-3196, a Phase 2-ready liver-directed thyroid hormone receptor-β (THR-β) agonist Once-daily oral dosing Favorable safety and efficacy profile World-wide patent exclusivity First-in-Class Approach Streamlined Clinical Development Plan in Genetic Lipid Disorder Directly targets lipotoxicity and metabolic defects in NASH Strong disease link: THR-β receptor activity is deficient in NASH livers Supported by preclinical and clinical data Differentiation: CV benefit, MGL-3196 LDL cholesterol lowering up to 30% in humans Significant Opportunity in NASH Heterozygous and homozygous familial hypercholesterolemia (HeFH, HoFH) Affect up to 1:200 and 1:250,000 patients world-wide MGL-3196 demonstrated robust efficacy in LDL lowering Clinically validated, multiple complementary lipid pathways Potentially fast to market, early Phase 2 favorable safety and efficacy profile 8
Madrigal Pipeline • Thyroid analogue platform (MGL-3196, backup, new molecules) • • • • Biliary disorders (progressive familial intrahepatic cholestasis-PFIC) Subclinical hypothyroidism Genetic dyslipidemias Orphan thyroid diseases, RTH, MCT8 deficiency: new molecule, brain penetrant Nature Rev. Endocrinol. 10, 164, 2014 9 Compound/Target Disease State Pre-Clinical Phase 1 Phase 2 Phase 3 MGL-3196 Thyroid Hormone Receptor- (THR-) Agonist NASH FH MGL-3745 Thyroid Hormone – Receptor- (THR-) Agonist (Backup) (Same as 3196)
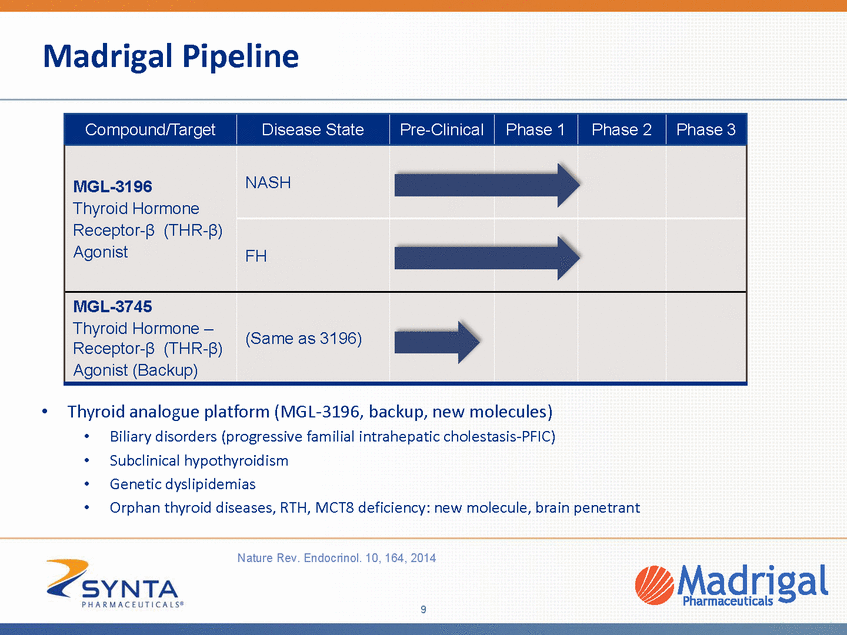
MGL-3196, a First-in-Class Liver-Directed THR- β Agonist We believe MGL-3196 is the first bona fide THR-β selective molecule Discovery of MGL-3196 and backups at Roche utilized a novel functional assay that went beyond what previous companies had done (simple receptor binding assay) Earlier compounds purported to be THR-β selective show no functional selectivity and, like thyroid hormone, activate the THR-α receptor equally well as the β receptor β-selectivity and liver targeting are key to beneficial metabolic actions and avoiding safety issues MGL-3196: excellent safety; unlike prior thyroid receptor agonist, no cartilage findings in chronic toxicology or ALT increases in human studies J Med Chem. 2014;57(10):3912-3923 Thyroid Gland Liver T4è T3 T4 T3 Nuc Thyroid Hormone Receptor α or β TSH Thyroid Hormone Pathway T4 T4, prohormone T3, active hormone TSH, thyroid stimulating hormone less α potent more ß selective α-potency (nM) β/α relative to T3 KB GC-1 10 -5 0 5 10 15 20 25 30 35 -500 500 1500 2500 3500 4500 Thyroid Hormone (T3) MB07811 (GC1) MGL-3196 Eprotirome
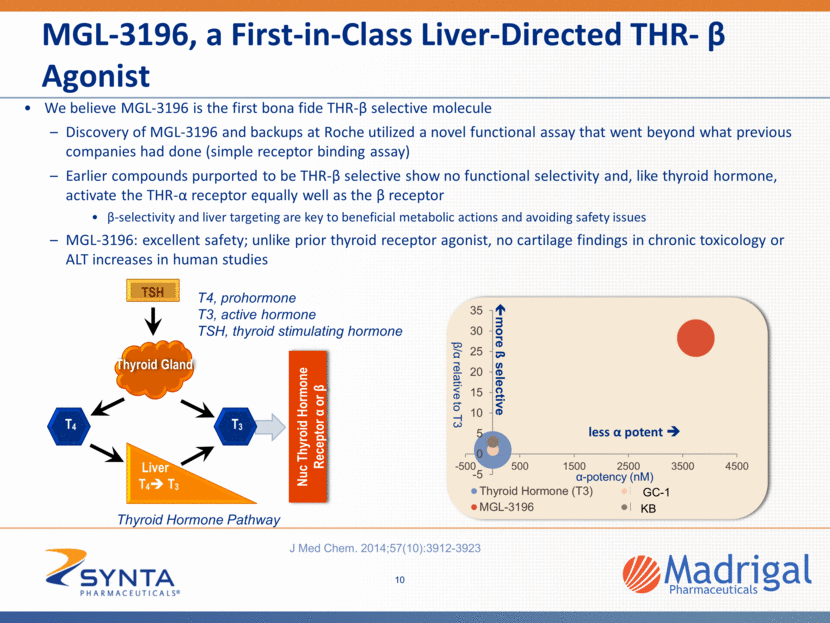
MGL-3196, a First-in-Class Liver-Directed THR- β Agonist MGL-3196’s demonstrated and potential properties: Once daily oral dosing* High liver uptake; low tissue penetration outside liver** Demonstrated and potential THR-β actions and benefits in the liver Insulin resistance** LDL-C, TGs, ApoB* Lp(a) ***, * NASH: Reduction of fatty liver, inflammation and fibrosis** Favorable safety profile in human, preclinical and animal toxicology studies No suppression of the central thyroid axis* No THR-α effects (heart rate, bone, CNS effects) *, ** No bone or cartilage findings in long-term animal toxicology studies** Not mutagenic** No clinical liver enzyme elevations* *Atherosclerosis 230 (2013) 373-380 **MGL-3196- preclinical studies ***MGL-3196-Phase 1 11
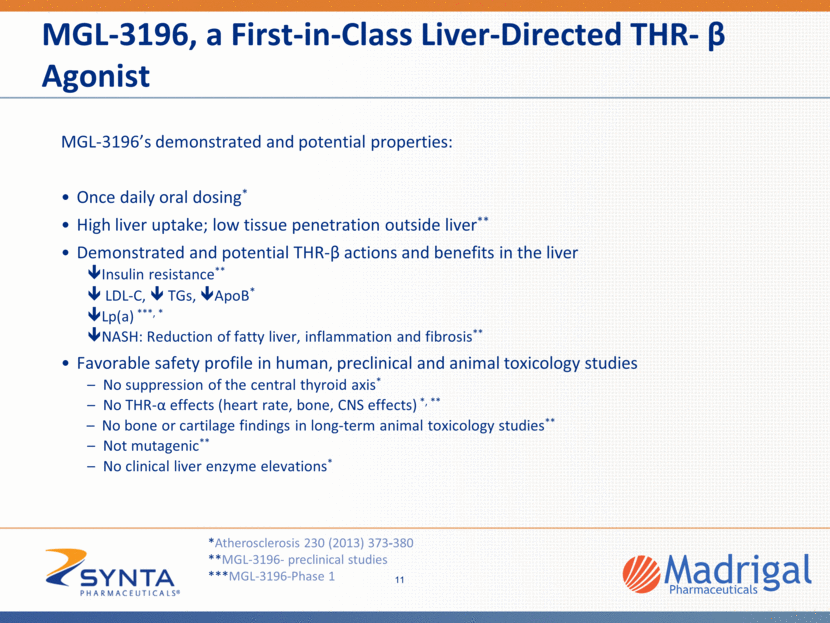
Differentiated NASH Opportunity • WW NASH market estimated at $35bn-$40bn • While epidemiology data varies, ~2-3% of the US population is thought to have NASH, and expected to grow given the high rate of growth of obesity and diabetes No FDA approved treatment options • • MGL-3196 is a novel differentiated cardio-protective therapy • Strong disease link: THR- receptor activity is deficient in NASH livers • Supported by preclinical and clinical data • • Differentiation: CV benefit, MGL-3196 LDL cholesterol lowering up to 30% in humans Critical differentiation in NASH, a disease more likely to lead to death by cardiovascular disease than liver disease • Treating NASH, rather than fibrosis, is key to addressing the disease • Recent FDA guidance indicates that resolution of NASH, without reducing fibrosis, is an approvable endpoint • Recognition that liver fibrosis will decrease with time after NASH resolves (similar to reduction of fibrosis as the liver regenerates after cure of HCV) 12 Defined, Regulatory Path Unique & Differentiated Approach Large & Growing Unserved Market
Why MGL-3196 for NASH? We believe that MGL-3196 will treat the underlying disease in NASH patients • MGL-3196 has pleiotropic effects characteristic of an “ideal” NASH drug –Potentially fixes components of the metabolic syndrome, treats insulin sensitivity, dyslipidemia, and underlying fatty liver disease • Hypothyroidism at the level of the thyroid gland is common in NASH (20-30%) and liver-specific hypothyroidism is present in human NASH (Endocrinology. 2014;155(11):4591-4601) –Liver specific hypothyroidism is caused by degradation of thyroid hormone (increased deiodinase (DIO) 3 produced by stellate cells) in the NASH liver –Treatment with MGL-3196 in human NASH may be a hormone replacement therapy •Important that the replacement be specific for THR- •Thyroid hormone treatment is generally not effective for NASH because thyroid hormone: 1-is degraded in the NASH liver, and 2-causes systemic THR- effects • NASH patients with advanced fibrosis have increased CV risk and primarily die of CV (not liver) disease (Ekstedt, Hepatology, 2014; Angulo et al, Gastroenterology, 2015 ) –MGL-3196 lowers LDL-cholesterol and may provide CV benefit to NASH patients 13
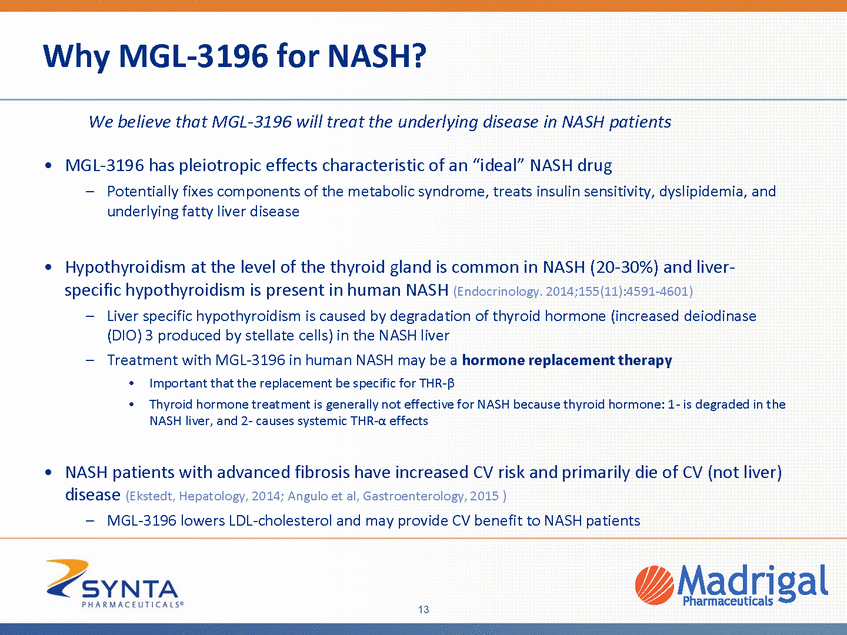
THR- Benefits Across the Spectrum of Early to Advanced NASH Evidence from human and animal data from Madrigal and multiple published studies support pleiotropic actions of THR-ß NASH inflammation and fibrosis Normal Liver Fatty Liver Cirrhosis METABOLIC BENEFITS liver fat & lipotoxicity insulin sensitivity LDL cholesterol atherosclerosis ANTI-INFLAMMATORY ANTI-FIBROTIC apoptosis, liver enzymes regeneration fibrosis & inflammation Lipid Res. 2014;55(11):2408-2415 ; REDUCED NASH COMPLICATIONS CV disease HCC Effects of MGL-3196: Hepatology. 2009;49(2):407-417; FASEB J. Endocrinology. 2014;155(11):4591-4601; PLoS One. 2013;8(12):e78534 2008;22(8):2981-2989; Taub et al,, So et al, unpub; Trends Endocrinol Metab. 2014;25(10):538-545PLoS One. 2010;5(1):e8710 Toxicol Pathol. 2003;31(1):113-120; J 14
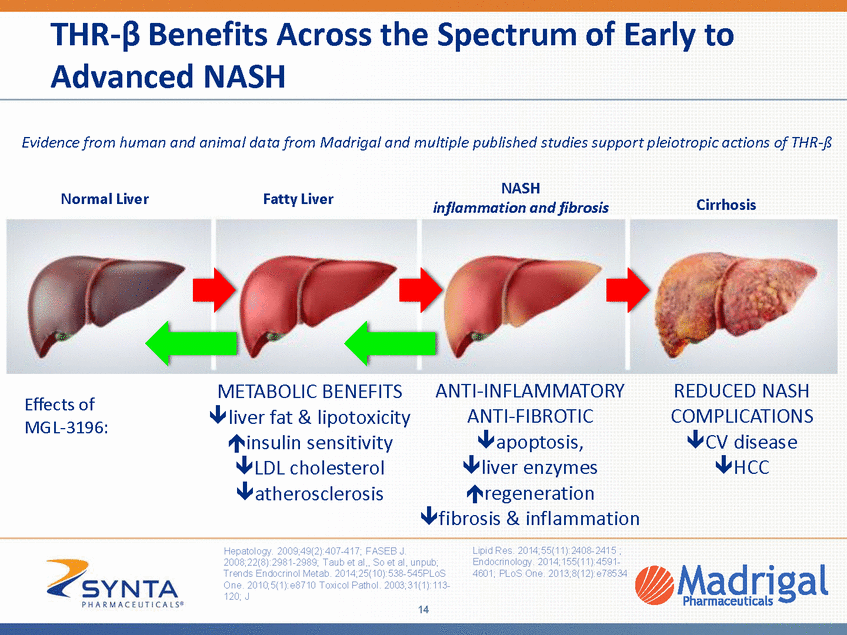
MGL-3196: Preclinical Data Support NASH and Lipid Indications • • Improved safety profile relative to T3 (thyroid hormone) and other thyroid agonists Reduced markers of NASH in diabetic and obese animals including: – – – – Reduction in fatty liver Improved liver enzyme levels Decreased inflammation and fibrosis markers Improvement in NASH scores • • • Cholesterol, triglyceride lowering in a variety of animal species Complementary lipid lowering mechanism to statins Insulin sensitizing action comparable to rosiglitazone 15
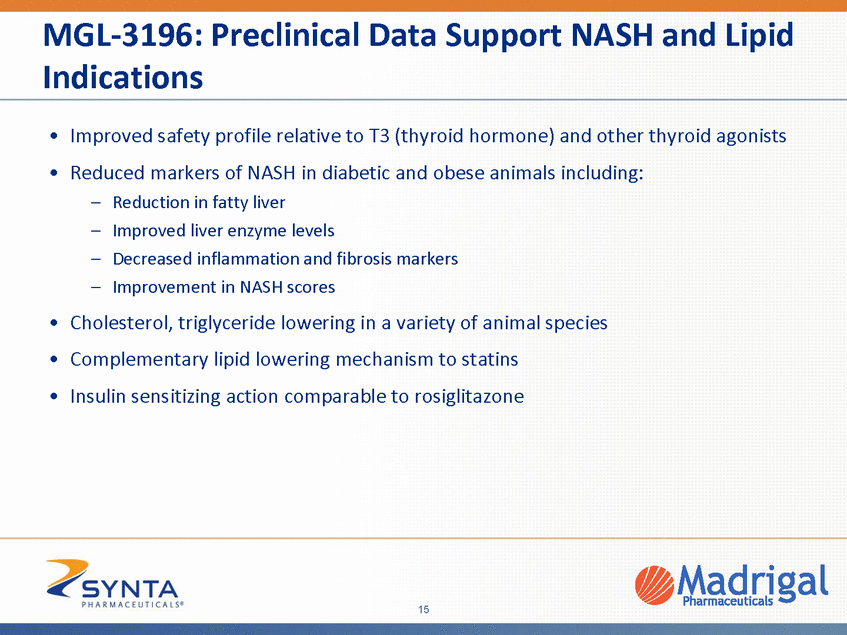
MGL-3196: Improved Safety Profile Relative to T3 T3 Bone Mineral Density Cholesterol 300.0 0.06 250.0 0.05 ** *** *** Thyroid hormone (T3, thyroxine) treatment may cause osteoporosis Significantly reduced bone mineral density with T3 200.0 *** 0.04 150.0 0.03 *** *** 100.0 0.02 50.0 0.01 0.0 0.00 Control T3 : 30 ug/kg T3 : 10 ug/kg T3 : 100 ug/kg p<.05* p<.01** P<.001*** MGL-3196 Cholesterol Bone Mineral Density 300.0 0.06 250.0 0.05 200.0 *** *** 0.04 150.0 0.03 *** 100.0 *** 0.02 50.0 0.01 0.0 0.00 Control MGL-3196 1 mg/kg MGL-3196 10 mg/kg MGL-3196 .3 mg/kg MGL-3196 3 mg/kg BMJ 2011;342:d2238 16 Cholesterol (mg/dl±s.d.) Cholesterol (mg/dl±s.d.) Bone Density (g/cm*cm) Bone Density (g/cm*cm) 24d study in 40 week old diet-induced obese (DIO) mice on High Fat Diet (HFD) for 38 weeks
MGL-3196: Health Data Supports Improvement in Liver lower panels: 24d study in 40 wk old DIO mice on HFD 35 wks Liver Size Liver Triglycerides Insulin Tolerance Test 5 70 4 * **80 ** 3 60 * 80 2 20 1 Time,0(min) 0 0 *** p<0.001 ** p<0.01*p<0.05 Control MGL-3196 3 mg/kg Control MGL-3196 .3mg/kg MGL-3196 1 ALT 300 250 200 150 100 50 0 *** *** *** *** Control MGL-3196 1mg/kg MGL-3196 10mg/kg MGL-3196 .3mg/kg MGL-3196 3mg/kg NASH score 17 IU/L Liver % Body Weight % Time 0 Glucose Triglycerides mg/g % Time 0 Glucose 120 100 40 0 0 60 50 40 30 20 6010 (0.5 U/kg insulin) 120 100 * 60 * 40 20 * ** ** 12 0 MGL-3196 .3mg/kg MGL-3196 1mg/kg Rosiglitazone: 10 mg/kg Liver Fat (Histology 0 60 120 Time, (min) * p<0.05 MGL-3196 )MGL-3196 3 mg/kg •RoRseigdlituazconeed: 1h0 empg/akgtic triglycerides (>50%), normalized liver size • Insulin sensitivity improved at all doses • Reduced liver enzymes (ALT, AST) • Improved liver histology, reduced Control MGL-3196 * Upper panels: 24d study in 17 wk old DIO mice (po, qd) on high fat diet (HFD) 13 wks;
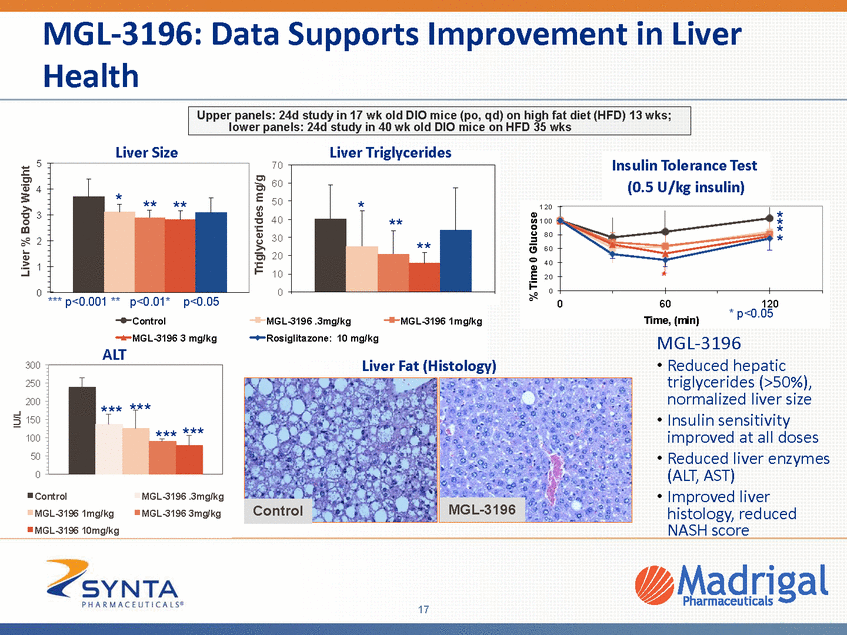
MGL-3196: Reduction of Key NASH, Fibrosis Pathway Genes at Human Comparable Drug Levels MGL-3196 (mg/kg) HFD mouse model •Up-regulation of NASH and fibrosis related transcripts in HFD as compared with lean mice controls Effects of MGL-3196 in 25 week HFD mouse model Inflammation MCP-1/CCL2 MIP-2/CXCL2 MIP-2ß/CXLCL3 A20/TNFaip3 CRP Annexin 2 SAA1 Fibrosis Collagen 1 Galectin-3 TIMP1 Collagen 4a2 SMA Collagen 4a1 CTGF Keratin 18 Collagen 3 Galectin-1 HFD Lean 0.1 0.3 1 3 Rosi • Downregulation of key NASH, inflammation and fibrosis pathway genes •Reduced to lean mouse level •Highly statistically significant Reduction of ALT More improvement than rosiglitazone • • • Rosiglitazone has been shown to be modestly efficacious in human NASH (HEPATOLOGY 2011;54:1631-1639) • Drug exposures at .3-1 mg/kg in HFD mice are similar to human dose where maximum lipid lowering is observed •Maximum efficacy ~ .3-1 mg/kg in 25 week study 12 3 4 5 6 7 Bad Good TIMP1 tissue inhibitor metalloproteinase CTGF connective tissue growth factor SMA smooth muscle actin SAA serum amyloid A CRP C-reactive protein “HFD”, lane 1 mean HFD gene expression normalized to mean Lean; Lanes (2-7) mean gene expression normalized to mean of DIO; “Rosi” (rosiglitazone, 3 mg/kg, 24 wks) Red, higher expression; blue decreased expression 18 25 week study in DIO, lean control mice and HFD mice treated with 0.1 to 3 mg/kg MGL-3196 or Rosiglitazone (3mg/kg)
MGL-3196: Completed: Long-term Dosing in Humans is Enabled • Single Ascending Dose (SAD) study • Multiple Ascending Dose (MAD) study – Six dose cohorts, 36 total HV dosed daily with MGL-3196 (5, 20, 50, 80, 100, or 200 mg) and 12 with placebo for 14 days •Healthy volunteers with slightly elevated LDL cholesterol (> 110 mg/dL) Well-tolerated, appeared safe at all doses tested No effect on vital signs, heart rate, central thyroid axis, or liver enzymes – – • Phase 1 study dosing MGL-3196 with statins • Series of GLP toxicology and CMC studies support all indications – – – Manufacturing and product formulation Chronic toxicology package Phase 2-enabling Atherosclerosis 230 (2013) 373-380 19
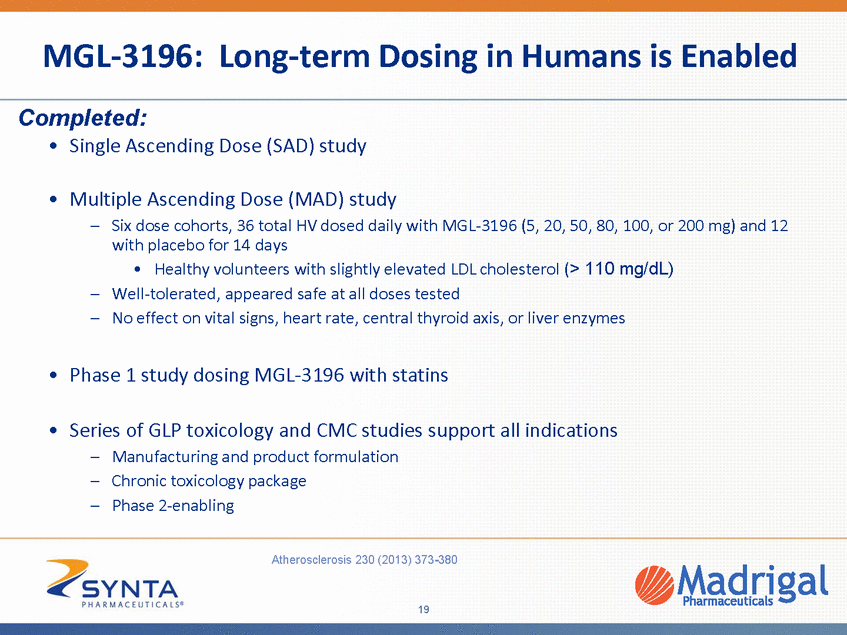
MGL-3196: Robust LDL and Triglyceride Lowering in 14 Day Multiple Dose Phase 1 Study Change in Lipids After 14 Days 20 10 0 Chol LDL-C n-HD -C TG -10 ** *** -20 ** ** ** *** *** -30 *** -40 -50 -60 5mg 20mg 50mg 80mg 100mg 200mg * Change from Baseline (CFB) by mean % CFB calculated for each individual subject 24h after 14th dose; baseline value obtained just prior to first dose; ApoB, apolipoprotein B; Chol, total cholesterol; LDL-C, LDL cholesterol directly measured; Non-HDL-C, non-HDL cholesterol; TG, triglycerides (median %CFB) Once daily oral treatment led to highly significant and dose-dependent up to ~30% reduction of apolipoprotein B (ApoB), total, LDL, non-HDL cholesterol; Strong trends in triglyceride reduction up to 60%; Near maximal effect at 80mg dose Atherosclerosis 230 (2013) 373-380 20 %Change From Baseline MGL-3196, Placebo Subtracted Ap oB No L * * * ** *** ** *** *** “ *** p<0.001 ** p<0.01 * p0.05 “p0.1 “
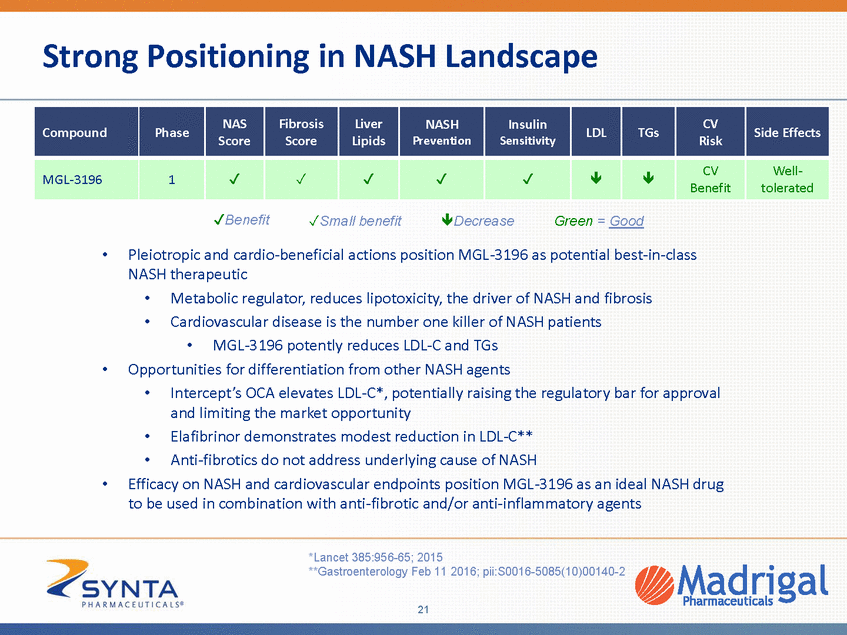
Strong Positioning in NASH Landscape tolerated Benefit Small benefit Decrease Green = Good • Pleiotropic and cardio-beneficial actions position MGL-3196 as potential best-in-class NASH therapeutic • • Metabolic regulator, reduces lipotoxicity, the driver of NASH and fibrosis Cardiovascular disease is the number one killer of NASH patients • MGL-3196 potently reduces LDL-C and TGs • Opportunities for differentiation from other NASH agents • Intercept’s OCA elevates LDL-C*, potentially raising the regulatory bar for approval and limiting the market opportunity Elafibrinor demonstrates modest reduction in LDL-C** Anti-fibrotics do not address underlying cause of NASH • • • Efficacy on NASH and cardiovascular endpoints position MGL-3196 as an ideal NASH drug to be used in combination with anti-fibrotic and/or anti-inflammatory agents *Lancet 385:956-65; 2015 **Gastroenterology Feb 11 2016; pii:S0016-5085(10)00140-2 21 Compound Phase NAS Score Fibrosis Score Liver Lipids NASH Prevention Insulin Sensitivity LDL TGs CV Risk Side Effects MGL-3196 1 CV Benefit Well-
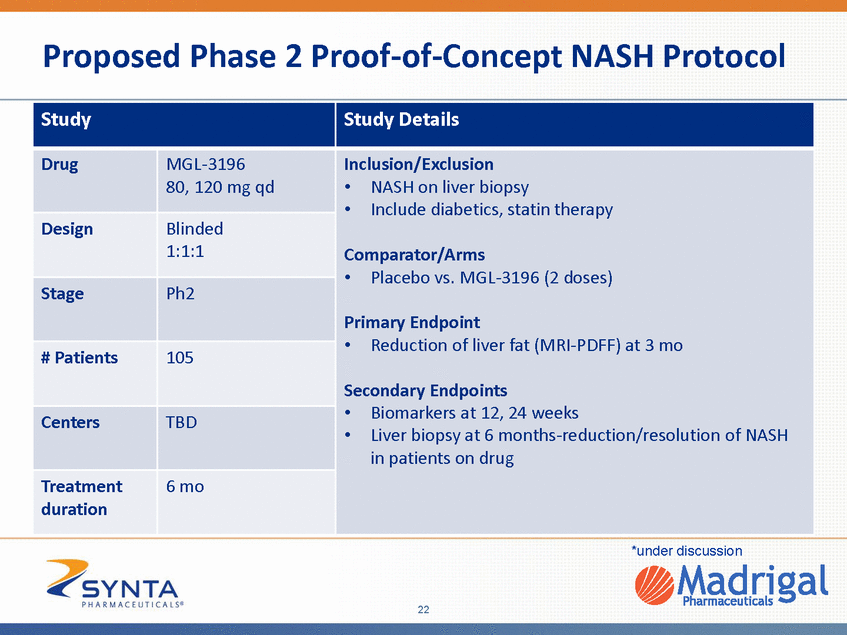
Proposed Phase 2 Proof-of-Concept NASH Protocol *under discussion 22 Study Study Details Drug MGL-3196 80, 120 mg qd Inclusion/Exclusion •NASH on liver biopsy •Include diabetics, statin therapy Comparator/Arms •Placebo vs. MGL-3196 (2 doses) Primary Endpoint •Reduction of liver fat (MRI-PDFF) at 3 mo Secondary Endpoints •Biomarkers at 12, 24 weeks •Liver biopsy at 6 months-reduction/resolution of NASH in patients on drug Design Blinded 1:1:1 Stage Ph2 # Patients 105 Centers TBD Treatment duration 6 mo
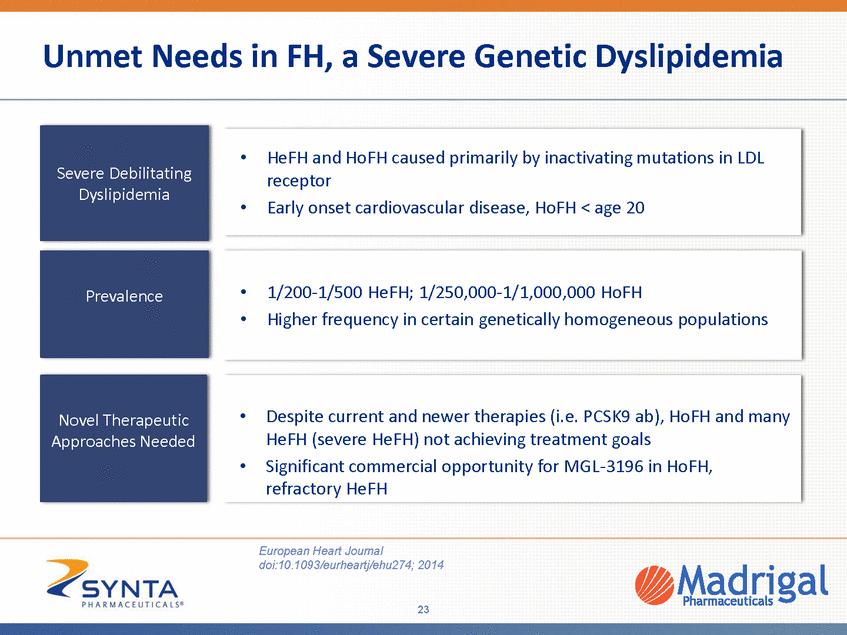
Unmet Needs in FH, a Severe Genetic Dyslipidemia • HeFH and HoFH caused primarily by inactivating mutations in LDL receptor Early onset cardiovascular disease, HoFH < age 20 • • • 1/200-1/500 HeFH; 1/250,000-1/1,000,000 HoFH Higher frequency in certain genetically homogeneous populations • Despite current and newer therapies (i.e. PCSK9 ab), HoFH and many HeFH (severe HeFH) not achieving treatment goals Significant commercial opportunity for MGL-3196 in HoFH, refractory HeFH • European Heart Journal doi:10.1093/eurheartj/ehu274; 2014 23 Novel Therapeutic Approaches Needed Prevalence Severe Debilitating Dyslipidemia
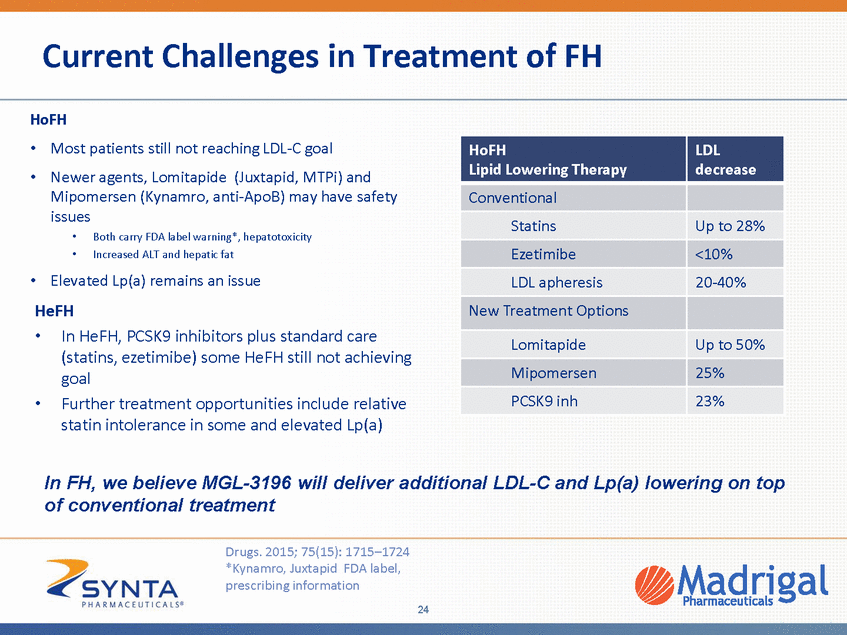
Current Challenges in Treatment of FH HoFH • • Most patients still not reaching LDL-C goal Newer agents, Lomitapide (Juxtapid, MTPi) and Mipomersen (Kynamro, anti-ApoB) may have safety issues • • Both carry FDA label warning*, hepatotoxicity Increased ALT and hepatic fat • Elevated Lp(a) remains an issue HeFH • In HeFH, PCSK9 inhibitors plus standard care (statins, ezetimibe) some HeFH still not achieving goal Further treatment opportunities include relative statin intolerance in some and elevated Lp(a) • In FH, we believe MGL-3196 will deliver additional LDL-C and Lp(a) lowering on top of conventional treatment Drugs. 2015; 75(15): 1715–1724 *Kynamro, Juxtapid FDA label, prescribing information 24 HoFH Lipid Lowering Therapy LDL decrease Conventional Statins Up to 28% Ezetimibe <10% LDL apheresis 20-40% New Treatment Options Lomitapide Up to 50% Mipomersen 25% PCSK9 inh 23%
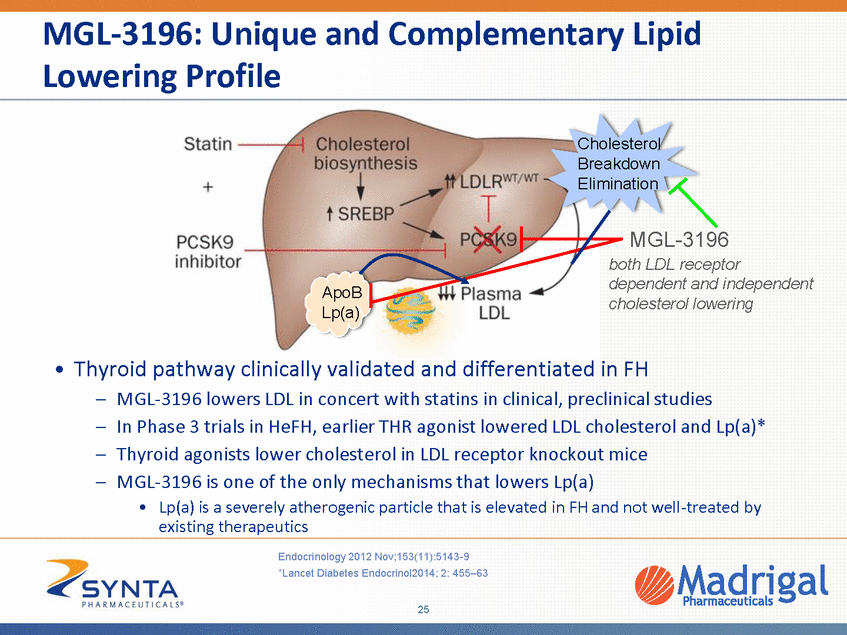
MGL-3196: Unique and Complementary Lipid Lowering Profile Cholesterol Breakdown Elimination MGL-3196 both LDL receptor dependent and independent cholesterol lowering ApoB Lp(a) • Thyroid pathway clinically validated and differentiated in FH – – – – MGL-3196 lowers LDL in concert with statins in clinical, preclinical studies In Phase 3 trials in HeFH, earlier THR agonist lowered LDL cholesterol and Lp(a)* Thyroid agonists lower cholesterol in LDL receptor knockout mice MGL-3196 is one of the only mechanisms that lowers Lp(a) •Lp(a) is a severely atherogenic particle that is elevated in FH and not well-treated by existing therapeutics Endocrinology 2012 Nov;153(11):5143-9 *Lancet Diabetes Endocrinol2014; 2: 455–63 25
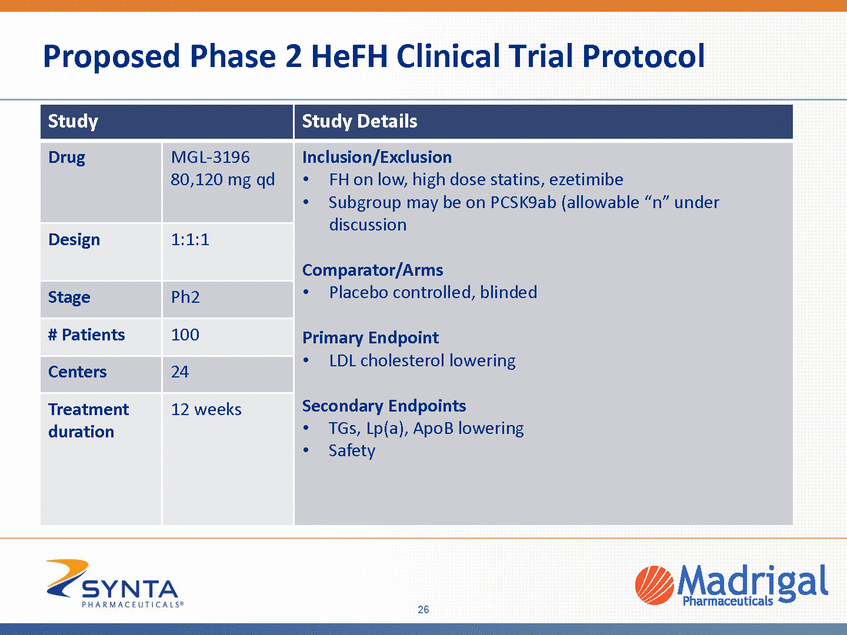
Proposed Phase 2 HeFH Clinical Trial Protocol duration 26 Study Study Details Drug MGL-3196 80,120 mg qd Inclusion/Exclusion •FH on low, high dose statins, ezetimibe •Subgroup may be on PCSK9ab (allowable “n” under discussion Comparator/Arms •Placebo controlled, blinded Primary Endpoint •LDL cholesterol lowering Secondary Endpoints •TGs, Lp(a), ApoB lowering •Safety Design 1:1:1 Stage Ph2 # Patients 100 Centers 24 Treatment 12 weeks
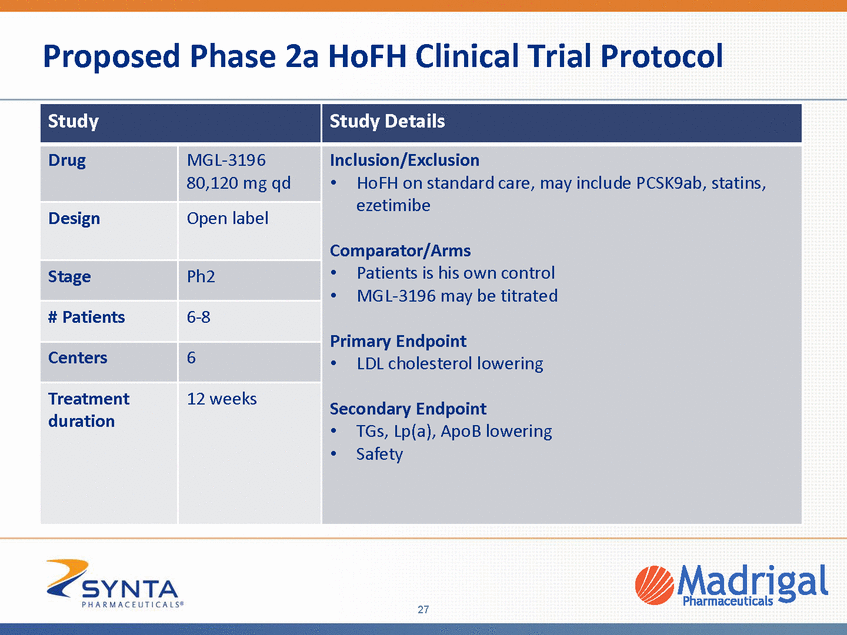
Proposed Phase 2a HoFH Clinical Trial Protocol 27 Study Study Details Drug MGL-3196 80,120 mg qd Inclusion/Exclusion •HoFH on standard care, may include PCSK9ab, statins, ezetimibe Comparator/Arms •Patients is his own control •MGL-3196 may be titrated Primary Endpoint •LDL cholesterol lowering Secondary Endpoint •TGs, Lp(a), ApoB lowering •Safety Design Open label Stage Ph2 # Patients 6-8 Centers 6 Treatment duration 12 weeks
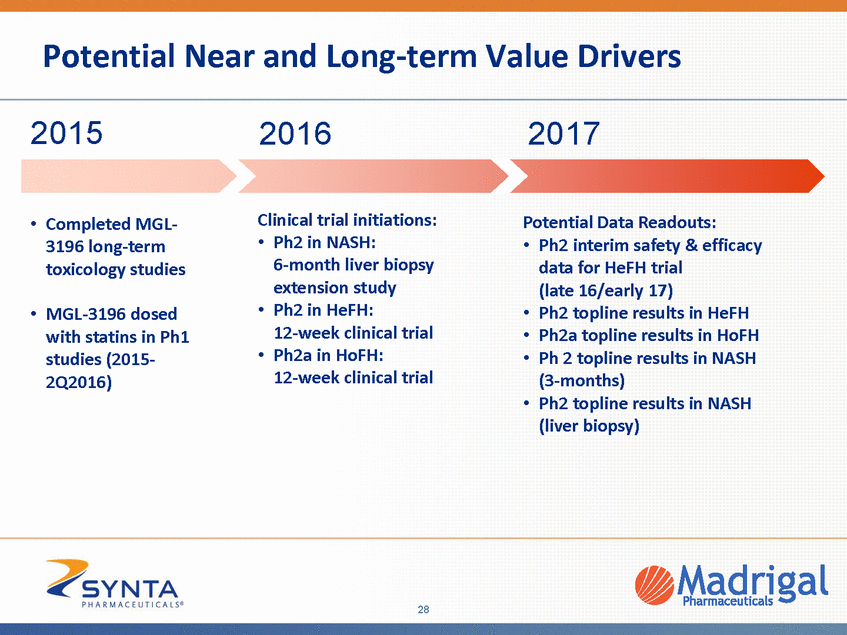
Potential Near and Long-term Value Drivers 2015 2016 2017 Clinical trial initiations: • Potential Data Readouts: Completed MGL-3196 long-term toxicology studies • Ph2 in NASH: 6-month liver biopsy extension study Ph2 in HeFH: 12-week clinical trial Ph2a in HoFH: 12-week clinical trial • Ph2 interim safety & efficacy data for HeFH trial (late 16/early 17) Ph2 topline results in HeFH Ph2a topline results in HoFH Ph 2 topline results in NASH (3-months) Ph2 topline results in NASH (liver biopsy) • • • • • MGL-3196 dosed with statins in Ph1 studies (2015-2Q2016) • • 28
Thank you NASDAQ: SNTA | May 2016 29

