Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - ACURA PHARMACEUTICALS, INC | v436920_8k.htm |
Exhibit 99.1

CONFIDENTIAL Nasdaq: ACUR April 13, 2016 Topline Interim Cohort 1 Results Study AP - LTX - 400 (Study 400) © 2016 Acura Pharmaceuticals, Inc. All Rights Reserved

Nasdaq: ACUR General Caution Regarding Forward Looking Statements Certain statements in this presentation constitute “forward - looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 . Such forwarding - looking statements involve known and unknown risks, uncertainties and other factors which may cause our actual results, performance or achievements to be materially different from any future results, performance, or achievements expressed or implied by such forward - looking statements . These statements reflect our current views with respect to future events and are based on assumptions and subject to risks and uncertainties . Given these uncertainties, you should not place undue reliance on these forward - looking statements . We discuss many of these risks in greater detail in our filings with the Securities and Exchange Commission . Unless required by law, we undertake no obligation to update or revise any forward - looking statements to reflect new information or future events or developments . Forward - looking statements may include, but are not limited to: • The expected results of clinical studies relating to our LTX - 04 formulation, the date by which such study results will be available and whether LTX - 04 will ultimately receive FDA approval; • whether LTX - 04 will retard release of the active ingredients as dose levels increase; • w hether we will be able to reformulate LTX - 04 to provide increased blood level at a 1 or 2 tablet dose; • whether our Limitx™ technology can e expanded to extended - release products; • the ability to fund, or obtain funding, for our continuing operations; • the ability to enter into future partnerships or maintain our current partnerships; • the results and timing of future development efforts, whether the FDA will accept those results and completeness of our studies, whether FDA will approve the products for marketing, and whether our technologies will actually reduce abuse if marketed ; and • exposure to infringement of patents, trademarks and other proprietary rights of third parties . 2

Nasdaq: ACUR Study 400 Objectives 3 Objectives: • Demonstrate LIMITX technology can retard the release of the active ingredient as 3 or more doses are administered in humans • Demonstrate the LTX - 04 formulation achieves blood levels of drug with 1 and/or 2 tablets equivalent to DILAUDID Observations: • 3 tablets of LTX - 04P demonstrated an approximate 22% proportional reduction in maximum plasma concentration ( Cmax ) than non - LIMITX tablets • LTX - 04S had proportional Cmax to DILAUDID • LTX - 04S & P did not achieve equivalent peak levels of drug ( Cmax ) at 1 and 2 tablets compared to DILAUDID • Cmax was ~50% lower than DILAUDID at both doses • Tmax was approximately the same as DILAUDID at both doses • AUC 0 - inf was approximately the same as DILAUDID at both doses
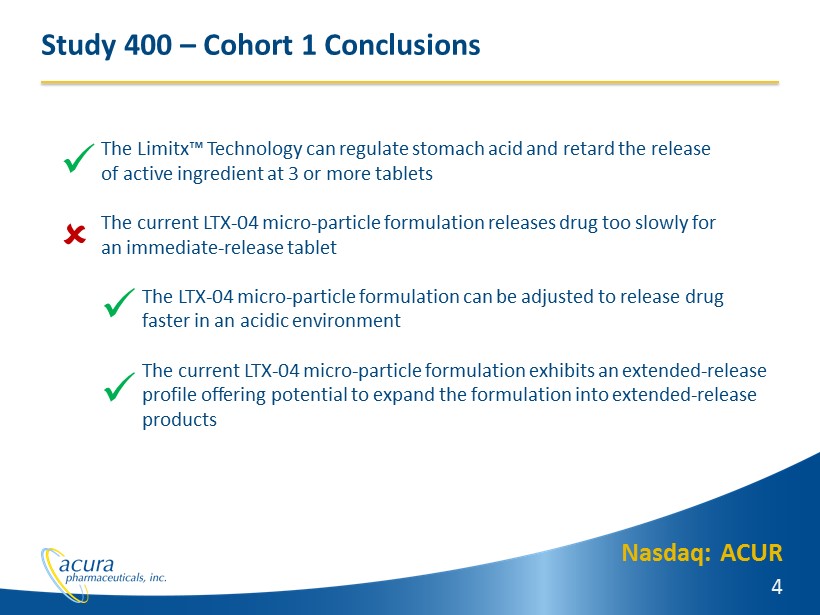
Nasdaq: ACUR 4 Study 400 – Cohort 1 Conclusions The Limitx™ Technology can regulate stomach acid and retard the release of active ingredient at 3 or more tablets The current LTX - 04 micro - particle formulation releases drug too slowly for an immediate - release tablet The LTX - 04 micro - particle formulation can be adjusted to release drug faster in an acidic environment The current LTX - 04 micro - particle formulation exhibits an extended - release p rofile offering potential to expand the formulation into extended - release products x x x

Nasdaq: ACUR Study 400 Cohort 1 Design 5 30 subjects enrolled 10 taking 2 tablets 1 0 taking 1 tablets 1 0 taking 3 tablets Single doses of LTX - 04P, LTX - 04S and DILAUDID taken 1 week a apart with blood samples taken and pH monitoring Single doses of LTX - 04P, LTX - 04S and DILAUDID taken 1 week a apart with blood samples taken and pH monitoring Single doses of LTX - 04P, LTX - 04S and DILAUDID taken 1 week a apart with blood samples taken and pH monitoring 10 Completers 8 Completers 8 Completers

Nasdaq: ACUR 6 Study 400 LTX - 04P and LTX - 04S • Identical micro - particle formulation in identical amounts in each dose of LTX - 04P and LTX - 04S • LTX - 04S has 45% less the buffering capacity than LTX - 04P • The LTX - 04 test formulations were designed to neutralize different amounts of stomach acid to test the parameters of the oral abuse deterrence of LIMITX LTX-04S LTX-04P 45% Less Buffering (acid neutralizing) Capacity
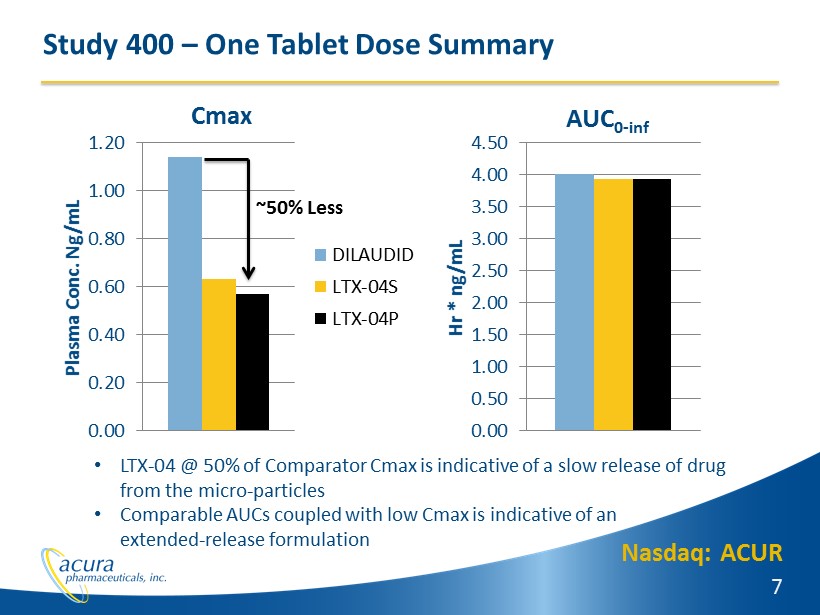
Nasdaq: ACUR 7 Study 400 – One Tablet Dose Summary 0.00 0.20 0.40 0.60 0.80 1.00 1.20 Plasma Conc. Ng/mL DILAUDID LTX-04S LTX-04P ~50% Less 0.00 0.50 1.00 1.50 2.00 2.50 3.00 3.50 4.00 4.50 Hr * ng/mL Cmax AUC 0 - inf • LTX - 04 @ 50% of Comparator Cmax is indicative of a slow release of drug from the micro - particles • Comparable AUCs coupled with low Cmax is indicative of an extended - release formulation
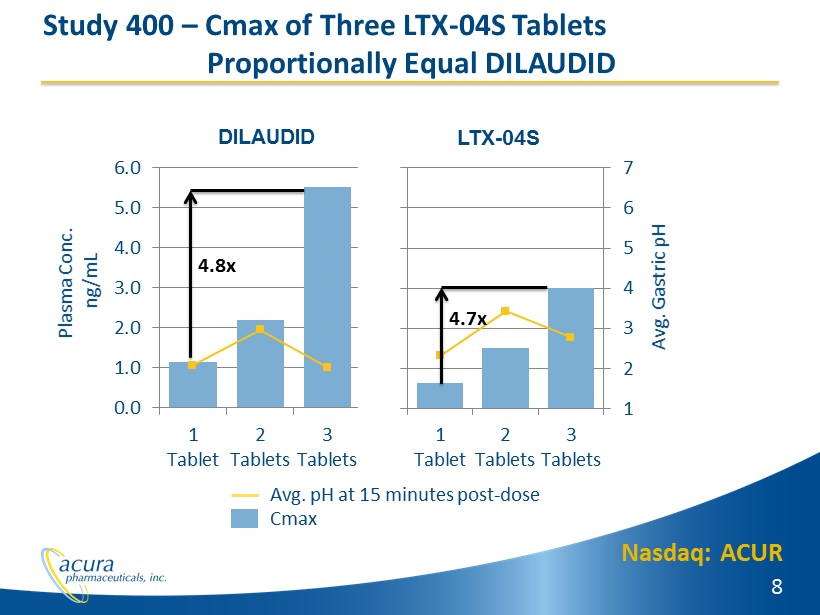
Nasdaq: ACUR 8 Study 400 – Cmax of Three LTX - 04S Tablets Proportionally Equal DILAUDID 1 2 3 4 5 6 7 0.0 1.0 2.0 3.0 4.0 5.0 6.0 1 Tablet 2 Tablets 3 Tablets Avg. Gastric pH Plasma Conc. ng/mL DILAUDID 1 2 3 4 5 6 7 0.0 1.0 2.0 3.0 4.0 5.0 6.0 1 Tablet 2 Tablets 3 Tablets LTX - 04S Avg. pH at 15 minutes post - dose Cmax 4.8x 4.7x

Nasdaq: ACUR 9 Study 400 – Cmax ~22% Lower at 3 Tablets for LTX - 04P than expected 1 2 3 4 5 6 7 0.0 1.0 2.0 3.0 4.0 5.0 6.0 1 Tablet 2 Tablets 3 Tablets Avg. Gastric pH Plasma Conc. ng/mL DILAUDID 1 2 3 4 5 6 7 0.0 1.0 2.0 3.0 4.0 5.0 6.0 1 Tablet 2 Tablets 3 Tablets LTX - 04P Avg. pH at 15 minutes post - dose Cmax 4.8x 3 .8x

Nasdaq: ACUR 10 LTX - 04 - Micro - particle Dissolution versus an earlier prototype (MP - 3)
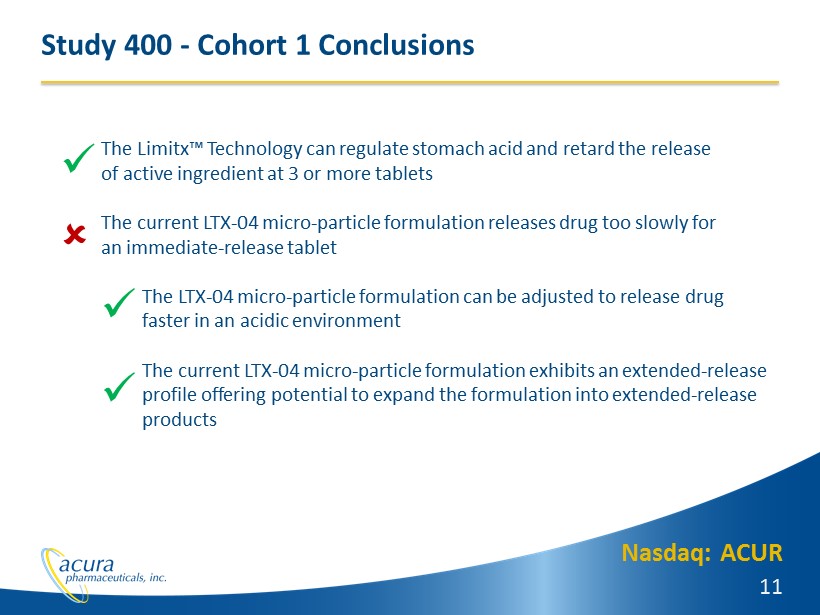
Nasdaq: ACUR 11 Study 400 - Cohort 1 Conclusions The Limitx™ Technology can regulate stomach acid and retard the release of active ingredient at 3 or more tablets The current LTX - 04 micro - particle formulation releases drug too slowly for an immediate - release tablet The LTX - 04 micro - particle formulation can be adjusted to release drug faster in an acidic environment The current LTX - 04 micro - particle formulation exhibits an extended - release p rofile offering potential to expand the formulation into extended - release products x x x

Nasdaq: ACUR 12 LTX - 04 Development Next Steps • Complete Study AP - LTX - 400 Cohort #2 to confirm results for oral abuse deterrence • Results expected end of June 2016 • Fine tune LTX - 04 micro - particle formulation • Use additional insights from Cohort #1 to assess tablet formulation • Discuss Study AP - LTX - 400 results with FDA under FAST TRACK designation • Commence 1 and/or 2 tablet p harmacokinetic study for bioequivalence in the 2 nd half 2016

CONFIDENTIAL Nasdaq: ACUR Acura Pharmaceuticals, Inc. 616 N. North Court, Suite 120 Palatine, IL 60067 (847) 705 - 7709 www.AcuraPharm.com www.Nexafed.com www.Oxaydo.com
