Attached files
| file | filename |
|---|---|
| EX-32 - EX-32 - ACURA PHARMACEUTICALS, INC | v212664_ex32.htm |
| EX-23.1 - EX-23.1 - ACURA PHARMACEUTICALS, INC | v212664_ex23-1.htm |
| EX-31.2 - EX-31.2 - ACURA PHARMACEUTICALS, INC | v212664_ex31-2.htm |
| EX-31.1 - EX-31.1 - ACURA PHARMACEUTICALS, INC | v212664_ex31-1.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
|
(Mark One)
|
|
|
x
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE
ACT OF 1934 FOR THE FISCAL YEAR ENDED DECEMBER 31, 2010
|
|
Or
|
|
|
¨
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from _____ to _____
|
Commission file number 1-10113
ACURA PHARMACEUTICALS, INC.
(Exact name of registrant as specified in its charter)
|
New York
|
11-0853640
|
|
(State or other jurisdiction of Incorporation or organization)
|
(I.R.S. Employer Identification No.)
|
|
616 N. North Court, Suite 120, Palatine, Illinois
|
60067
|
|
(Address of principal administrative office)
|
(Zip code)
|
Registrant's telephone number, including area code: 847 705 7709
Securities registered pursuant to section 12(b) of the Act:
Common Stock, par value $0.01 per share
Securities registered pursuant to section 12(g) of the Act:
(Title of Class)
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
Yes ¨ No x
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ¨ No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company.
¨ Large Accelerated Filer ¨ Accelerated Filer x Non-Accelerated Filer ¨ Smaller Reporting Company.
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
Based on the last sale price on the NASDAQ Capital Market of the Common Stock on June 30, 2010 ($2.51) (the last business day of the registrant's most recently completed second fiscal quarter), the aggregate market value of the voting stock held by non-affiliates of the registrant was approximately $26.0 million.
As of February 28, 2011, the registrant had 44,640,268 shares of Common Stock, par value $0.01, outstanding.
DOCUMENTS INCORPORATED BY REFERENCE: Portions of the Proxy Statement for the registrant’s Annual Meeting of Shareholders to be held on or about April 28, 2011 are incorporated by reference into Part III of this Annual Report on Form 10-K.
Acura Pharmaceuticals, Inc.
Form 10-K
For the Fiscal Year Ended December 31, 2010
Tablet of Contents
|
PAGE
|
|||
|
PART I
|
|||
|
Item 1.
|
Business
|
3
|
|
|
Item 1A.
|
Risk Factors
|
21
|
|
|
Item 1B.
|
Unresolved Staff Comments
|
33
|
|
|
Item 2.
|
Properties
|
33
|
|
|
Item 3.
|
Legal Proceedings
|
33
|
|
|
Item 4.
|
Reserved
|
34
|
|
|
PART II
|
|||
|
Item 5.
|
Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
|
34
|
|
|
Item 6.
|
Selected Financial Data
|
35
|
|
|
Item 7.
|
Management's Discussion and Analysis of Financial Condition and Results of Operations
|
36
|
|
|
Item 7A.
|
Quantitative and Qualitative Disclosures About Market Risk
|
42
|
|
|
Item 8.
|
Financial Statements and Supplementary Data
|
42
|
|
|
Item 9.
|
Changes in and Disagreement with Accountants on Accounting and Financial Disclosure
|
43
|
|
|
Item 9A.
|
Controls and Procedures
|
43
|
|
|
Item 9B.
|
Other Information
|
43
|
|
|
PART III
|
|||
|
Item 10.
|
Directors, Executive Officers and Corporate Governance
|
44
|
|
|
Item 11.
|
Executive Compensation
|
44
|
|
|
Item 12.
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
|
44
|
|
|
Item 13.
|
Certain Relationships and Related Transactions, and Director Independence
|
44
|
|
|
Item 14.
|
Principal Accountant Fees and Services
|
44
|
|
|
PART IV
|
|||
|
Item 15.
|
Exhibits and Financial Statement Schedules
|
44
|
|
|
Signatures
|
45
|
||
|
Index to Financial Statements
|
F-1
|
||
2
Forward-Looking Statements
Certain statements in this Report constitute "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements involve known and unknown risks, uncertainties and other factors which may cause our actual results, performance or achievements to be materially different from any future results, performance, or achievements expressed or implied by such forward-looking statements. The most significant of such factors include, but are not limited to, our ability and the ability of King Pharmaceuticals Research and Development, Inc. (“King”), a wholly-owned subsidiary of King Pharmaceuticals, Inc., (to whom we have licensed our
Aversion® Technology for certain opioid analgesic products in the United States, Canada and Mexico) and the ability other pharmaceutical companies, if any, to whom we may license our Aversion® Technology or Impede™ Technology, to obtain necessary regulatory approvals and commercialize products utilizing such technologies, the ability to avoid infringement of patents, trademarks and other proprietary rights of third parties, and the ability to fulfill the U.S. Food and Drug Administration’s (“FDA”) requirements for approving our product candidates for commercial manufacturing and distribution in the United States, including, without limitation, the adequacy of the results of the laboratory and clinical studies completed to date and the results of laboratory and clinical studies we may complete in the future to support FDA approval of our product
candidates, the adequacy of the development program for our product candidates, including whether additional clinical studies will be required to support FDA approval of our product candidates, changes in regulatory requirements, adverse safety findings relating to our product candidates, the risk that the FDA may not agree with our analysis of our clinical studies and may evaluate the results of these studies by different methods or conclude that the results of the studies are not statistically significant, clinically meaningful or that there were human errors in the conduct of the studies or the risk that further studies of our product candidates are not positive or otherwise do not support FDA approval, whether or when we are able to obtain FDA approval of labeling for our product candidates for the proposed indications or for abuse deterrent features, whether our product candidates
will ultimately deter abuse in commercial settings, and the uncertainties inherent in scientific research, drug development, laboratory and clinical trials and the regulatory approval process. Other important factors that may also affect future results include, but are not limited to: our ability to attract and retain skilled personnel; our ability to secure and protect our patents, trademarks and other proprietary rights; litigation or regulatory action that could require us to pay significant damages or change the way we conduct our business; our ability to compete successfully against current and future competitors; our dependence on third-party suppliers of raw materials; our ability to secure U.S. Drug Enforcement Administration ("DEA") quotas and source the active ingredients for our products in development; difficulties or delays in conducting clinical trials for our
product candidates or in the commercial manufacture and supply of our products; and other risks and uncertainties detailed in this Report. When used in this Report, the words "estimate," "project," "anticipate," "expect," "intend," "believe," and similar expressions identify forward-looking statements.
PART I
ITEM 1. BUSINESS
Overview
We are a specialty pharmaceutical company engaged in research, development and manufacture of product candidates intended to provide abuse deterrent features and benefits utilizing our proprietary Aversion® and Impede™ Technologies. Our Aversion® Technology opioid analgesic product candidates are intended to effectively relieve pain while simultaneously discouraging common methods of opioid product misuse and abuse, including the:
|
|
·
|
intravenous injection of dissolved tablets or capsules;
|
|
|
·
|
nasal snorting of crushed tablets or capsules; and
|
|
|
·
|
intentional swallowing of excess quantities of tablets or capsules (when product candidates are formulated with niacin).
|
We and our licensee, King are jointly developing opioid analgesic product candidates both with and without niacin utilizing our patented Aversion® Technology. In addition to Acurox® (oxycodone HCl) Tablets, we and King are developing Vycavert® (hydrocodone bitartrate/acetaminophen) Tablets, Acuracet® (oxycodone HCl/ acetaminophen) Tablets, Acurox® with Niacin (oxycodone HCl/niacin) Tablets and additional undisclosed opioid product candidates utilizing our Aversion® Technology. We and King have submitted a
New Drug Application (NDA) for Acurox® with Niacin Tablets. Four opioid product candidates (both with and without niacin) are licensed to King under our License, Development and Commercialization Agreement dated October 30, 2007. We are also developing an undisclosed benzodiazepine tablet product candidate utilizing Aversion® Technology intended for the treatment of anxiety disorders, and a pseudoephedrine HCl tablet product utilizing Impede™ Technology intended for treatment of nasal congestion.
3
All of our opioid product candidates utilize Aversion® Technology (both with and without niacin) and are covered by issued U.S. patents, which in combination with our anticipated product labeling and drug product listing strategies are anticipated to provide our opioid products with barriers to market entry for generic competition through the expiration of our patents in 2025.
In addition to our Aversion® Technology, as part of our continuing research efforts we are investigating and developing novel mechanisms to incorporate abuse deterrent features into abused and misused pharmaceutical products. In this regard we have developed Impede™ PSE, a pseudoephedrine hydrochloride (“PSE”) tablet product candidate utilizing our Impede™ Technology. Impede™ Technology utilizes a proprietary mixture of functional inactive ingredients intended to limit or impede extraction of PSE from tablets for use as a starting material in producing the illicit drug methamphetamine. An 18 subject
clinical study demonstrated our Impede™ PSE Tablets are bioequivalent to Sudafed® brand PSE tablets and a leading generic PSE store brand tablet. We are currently negotiating with a contract manufacturer for the scale up and commercial manufacture of our Impede™ PSE Tablets. It is our current expectation to market our Impede™ PSE Tablets directly to national and regional drug store chains.
Acurox® Tablets is an orally administered immediate release tablet containing oxycodone hydrochloride (HCl) as its sole active analgesic ingredient and is intended for the relief of moderate to severe pain. On December 17, 2010, King submitted a New Drug Application (“NDA”) for Acurox® Tablets to the FDA, including a request for priority review classification. On February 10, 2011 the FDA notified King of the FDA’s acceptance for filing of the Acurox® Tablets NDA and the grant of a priority review classification. The priority review classification establishes a non-binding date of June 17, 2011 for the FDA to complete its review of the Acurox® Tablets NDA under the Prescription Drug User Fee Act (PDUFA). In addition to filing acceptance and assignment of a Priority review classification, the FDA’s filing communication letter to King also includes preliminary comments about potential review issues relating to an intranasal abuse liability study included in the NDA and requests additional information relating to this study and other issues. The preliminary notice of potential review issues is not indicative of deficiencies that may be identified during the FDA’s review of the
NDA. No assurance can be given that any issues raised as part of the FDA’s review of the Acurox® NDA (including the potential review issues in the FDA’s filing communication letter) will be addressed to the FDA’s satisfaction or that the Acurox® NDA will be approved by the FDA. Acurox® Tablets utilizes our patented Aversion® Technology which is designed to limit or impede abuse by intravenous injection of dissolved tablets and nasal snorting of crushed tablets. A separate NDA for Acurox® with Niacin
(oxycodone HCl/niacin) Tablets, which is designed to deter intravenous, nasal, and abuse by excess oral consumption, is subject to an FDA Complete Response Letter. See below under the caption “Acurox® Tablets Development Program - Acurox® with Niacin Tablets” for the status of our response to the FDA’s Complete Response Letter.
The misuse and abuse of pharmaceutical products in general, and opioid analgesics in particular, is a significant societal problem described as epidemic in nature. It is estimated that 75 million people in the U.S. suffer from pain, and, according to U.S. government surveys, 35.0 million people, or more than 10% of the U.S. population, have used prescription opioid analgesics non-medically at some point in their lifetime. We expect our Aversion® Technology opioid product candidates to compete primarily in the market for immediate release opioid products (“IR Opioid Products”) which are commonly prescribed for relief of pain for
durations generally less than 30 days. In 2010, IMS Health reported 260 million prescriptions dispensed for opioid analgesic tablets and capsules, of which approximately 244 million were for IR Opioid Products and 16 million were for extended release opioid tablet and capsule products (“ER Opioid Products”) which are commonly prescribed for relief of chronic pain for durations ranging from several weeks to several months or longer. We have contracted, through an independent market research firm, numerous market research studies including two which surveyed 401 and 435 opioid analgesic prescribing U.S. based physicians, respectively. These studies revealed that physicians are keenly aware of opioid analgesic abuse and are personally concerned with the potential impact of drug abuse on their respective medical practices. Our study of 401
physicians indicated that of the prescriptions likely to be written for our product candidates that utilize the analgesic oxycodone, 59% will be switched from immediate release products containing either hydrocodone or oxycodone, with the remaining 41% being switched from other currently marketed opioid analgesic products such as codeine, propoxyphene, morphine, and tramadol. Ninety-four percent (94%) of 401 physicians surveyed indicated they would either prescribe one of the Aversion® Technology products (with or without niacin) profiled in the market research questionnaire for one of their last five patients receiving an opioid prescription or they are aware of a patient in their practice for whom
Aversion® Technology opioid analgesic products (with or without niacin) would be an appropriate choice. These studies suggest that regardless of whether Acurox® Tablets contain or do not contain niacin, Acurox® has the potential to garner a substantial share of immediate release opioid analgesic prescriptions, although there can be no assurance in this regard.
4
We have established and intend to pursue future strategic alliances and licensing agreements with pharmaceutical companies to augment and enhance our ability to develop and commercialize our product candidates. We also intend to pursue the in-license or acquisition of product candidates and technologies to expand our portfolio of abuse deterrent technologies and product candidates. In October 2007, we entered into a License, Development and Commercialization Agreement with King to develop and commercialize certain opioid analgesic products utilizing our proprietary Aversion® Technology, including Acurox® Tablets. The King Agreement initially provided King with an exclusive license in the United States, Canada and Mexico (the “King Territory”) to Acurox® Tablets (with and without niacin) and Acuracet® (oxycodone HCl/acetaminophen) Tablets (with and without niacin), and an option to license future opioid analgesic product candidates utilizing our Aversion® Technology (with and without niacin) in the King Territory. In May and December 2008, King exercised its option and licensed an undisclosed opioid analgesic tablet product and Vycavert® with Niacin (hydrocodone bitartrate/niacin/acetaminophen) Tablets,
respectively. Under the terms of the King Agreement, King made an upfront cash payment to us of $30 million. As of February 28, 2011, we had received an additional $28.4 million from King in the form of milestone payments, option fees and reimbursement for research and development expenses. In addition, we are eligible for future regulatory and sales milestone payments, reimbursement for certain research and development expenses and royalties on combined annual net sales of all products commercialized under the King Agreement. On January 31, 2011, Pfizer Inc. (“Pfizer”) announced completion of a tender offer acquisition of King Pharmaceuticals, Inc., resulting in King becoming a majority-owned subsidiary of Pfizer. Pfizer publicly announced it intends to complete a short-form merger with King on or about February 28, 2011, pursuant
to which King will become a wholly-owned subsidiary of Pfizer.
We conduct research, development, laboratory, manufacturing, and warehousing activities at our operations facility in Culver, Indiana and lease an administrative office in Palatine, Illinois. In addition to internal capabilities and activities, we engage numerous clinical research organizations (“CROs”) with expertise in regulatory affairs, clinical trial design and monitoring, clinical data management, biostatistics, medical writing, laboratory testing and related services. Such CROs perform, under our direction, development and regulatory services relating to our technologies and product candidates. We also intend to contract with a third-party pharmaceutical product
manufacturer and packager to supply commercial requirements for our Impede™ PSE Tablets.
Our Strategy
Our goal is to become a leading specialty pharmaceutical company focused on addressing the growing societal problem of pharmaceutical drug abuse by developing a broad portfolio of products with abuse deterrent features and benefits. Specifically, we intend to:
|
|
·
|
Capitalize on our Experience and Expertise in the Research and Development of Pharmaceutical Products with Abuse Deterrent Features and Benefits. Our strategy is to facilitate rapid product development and minimize risk by utilizing active pharmaceutical ingredients with proven safety and efficacy profiles with known potential for abuse, and develop new products utilizing our proprietary technologies using the FDA’s 505(b)(2) NDA and other regulatory pathways and processes.
|
|
|
·
|
Emerge as a Leader in Developing and Commercializing Products with Abuse Deterrent Features and Benefits Able to Uniquely Address the Growing Problem of Abuse of Prescription Drugs. We believe that Acurox® Tablets and our other opioid product candidates in development have demonstrated that Aversion® Technology allows products to provide the analgesic benefit they were intended to deliver, while simultaneously having features that are intended to deter misuse and abuse. We believe these benefits will be attractive to physicians, third party payers, and public advocacy groups sensitive to the problem of prescription drug
abuse.
|
|
·
|
Optimize Shareholder Value and Temper Risk by Licensing our Product Candidates to Strategically Focused Pharmaceutical Companies in the U.S. and Other Geographic Territories. On October 30, 2007, we and King entered into the King Agreement to develop and commercialize in the United States, Canada and Mexico opioid analgesic products utilizing Aversion® Technology. We believe opportunities exist to enter into similar agreements with other partners for these same opioid products outside the King Territory, and in the United States and worldwide for developing additional Aversion® Technology and Impede™ Technology product
candidates for other abusable drugs such as tranquilizers, stimulants, sedatives and decongestants. By licensing our product candidates to strategically focused companies with expertise and infrastructure in commercialization of pharmaceuticals, we are able to leverage our expertise, intellectual property rights and Aversion® and Impede™ Technologies without the need to invest in and build costly sales and manufacturing infrastructure. We anticipate that our future revenue, if any, will be derived from milestone and royalty payments related to the commercialization of products utilizing our Aversion® and Impede™ Technologies and from commercialization of our Impede™ PSE Tablets.
|
|
|
·
|
In-license or Acquire Alternative Technologies and Product Candidates to Expand our Portfolio of Abuse Deterrent Technologies and Products. We intend to pursue the in-license or acquisition of product candidates and technologies that will allow us to expand our portfolio of products with abuse deterrent features and benefits. Such in-licensing or acquisition transactions, if successfully completed, of which no assurance can be given, may include product candidates or technologies for pain relief, and other drugs susceptible to misuse and abuse.
|
5
|
|
·
|
Apply our Aversion® and Impede™ Technologies to Non-Opioid Drugs Susceptible to Abuse. We intend to first develop a portfolio of opioid analgesic products, and thereafter we intend to expand to other pharmaceutical product categories containing potentially abusable active ingredients such as tranquillizers (brand products such as Valium®, Xanax®, Klonopin® and Ativan®), stimulants (brand products such as Dexedrine®, Adderall®, Ritalin® and Concerta®), sedatives (brand products such as Nembutal®, Butisol®, and Seconal®) and decongestants (brand products such as Sudafed®, Zyrtec-D®, Allegra-D®, and
Claritin-D®). These products, like opioid analgesics, may also be prone to misuse and abuse.
|
|
|
·
|
Maintain our Efficient Internal Cost Structure. We maintain a streamlined and highly efficient cost structure focused on: (i) selection, formulation development, laboratory evaluation, manufacture, quality assurance, and stability testing of certain finished dosage form product candidates; (ii) development and prosecution of our patent applications; (iii) negotiation and execution of license and development agreements with strategically focused pharmaceutical companies and; (iv) utilizing third-party contract manufacturers/packagers to supply our commercial requirements for our Impede™ PSE Tablet product. By outsourcing the high cost elements of our product development and commercialization process, we believe that we
substantially minimize required fixed overhead and capital investment and thereby reduce our business risk. We currently do not intend to use a physician focused sales force to commercialize products on our own.
|
Aversion® Technology Opioid Product Candidates in Development
Aversion® Technology opioid analgesic product candidates which have demonstrated Proof of Concept1 are set forth in the table below.
|
Our Opioid Product Candidates
|
Stage of Development
|
|
|
Acurox® (oxycodone HCl) Tablets
|
NDA submitted by King to FDA on 12/17/10; FDA accepted the NDA for filing on February 10, 2011 and granted a Priority review classification. Prescription Drug User Fee Act target date for completion of FDA’s review is June 17, 2011.
|
|
|
Acurox® with Niacin (oxycodone HCl/niacin) Tablets
|
NDA submitted to FDA on 12/30/08; Complete Response Letter (CRL) received 6/30/09; FDA Advisory Committee meeting held April 22, 2010; Resubmission of the NDA responding to the CRL expected after completion of the analysis of Study AP-ADF-114 and analyses of food and non-steroidal anti-inflammatory drug (“NSAID”) effects.
|
|
|
Acuracet® with Niacin (oxycodone HCl/niacin/acetaminophen) Tablets
|
Investigational New Drug Application ("IND") filed with FDA and active beginning 6-1-08. IND subsequently transferred to King
|
|
Vycavert® with Niacin (hydrocodone bitartrate/niacin/acetaminophen) Tablets
|
Proof of Concept complete. King exercised its option to license and is responsible for development
|
|
|
4th (undisclosed opioid analgesic/niacin) Tablets
|
Proof of Concept complete. King exercised its option to license and is responsible for development
|
|
|
5th (undisclosed opioid analgesic/niacin) Tablets
|
Proof of Concept complete
|
|
|
6th (undisclosed opioid analgesic/niacin) Tablets
|
Proof of Concept complete
|
|
|
7th (undisclosed opioid analgesic/niacin) Tablets
|
Proof of Concept complete
|
|
|
8th (undisclosed opioid analgesic/niacin) Tablets
|
Proof of Concept complete
|
1 Proof of concept is attained upon demonstration of certain product stability and bioavailability parameters defined in the King Agreement. Refer to description of the King Agreement in this Report. With three exceptions, King has either licensed or has an option to license all opioid product candidates listed above in the U.S., Canada and Mexico.
6
Aversion® Technology Overview
Aversion® Technology is a proprietary platform technology providing abuse deterrent features and benefits to orally administered pharmaceutical drug products containing potentially abusable active ingredients. Aversion® Technology may be utilized both with and without niacin. Our focus has been to utilize our Aversion® Technology with opioid analgesics administered in tablet form. In addition, we believe Aversion® Technology is a versatile technology which may be applicable to non-opioid active ingredients susceptible to abuse and administered in tablet or capsule form, including tranquilizers, sedatives and stimulants (See “Aversion®
Technology Non-Opioid Product Candidates in Development” below).
Aversion® Technology opioid analgesic product candidates include a unique composition of active and inactive pharmaceutical ingredients. The opioid active ingredients are intended to provide effective relief from pain while the unique mixture of inactive ingredients provides non-therapeutic functionality. When dissolved in water or other solvents, the functional inactive ingredients quickly form a viscous gel, which increases the difficulty of extracting the opioid active ingredient in a form and volume suitable for injection. In addition, the combination of functional inactive ingredients is intended to induce nasal passage discomfort and disliking effects if the tablets are
crushed and snorted. Aversion® Technology opioid product candidates may also include niacin, an active ingredient in vitamins, cholesterol reducers and nutritional supplements, in amounts determined by us to be well tolerated when our product candidates are administered at recommended doses but which are intended to induce temporary disliking effects as increasing numbers of tablets are swallowed in excess of the recommended analgesic dose. When Aversion® Technology is utilized, it is intended that the resulting product provides the same therapeutic benefits as the non Aversion® Technology product, while simultaneously discouraging the most common methods of pharmaceutical product misuse and abuse.
Intended to Deter I.V. Injection of Opioids Extracted from Dissolved Tablets
Prospective drug abusers may attempt to dissolve currently marketed opioid-containing tablets or capsules in water, alcohol, or other common solvents, filter the dissolved solution, and then inject the resulting fluid intravenously to obtain euphoric effects. In product candidates utilizing Aversion® Technology, extracting the active ingredient using generally available solvents, including water or alcohol, into a volume and form suitable for intravenous (“I.V.”) injection, converts the tablet into a viscous gel mixture and traps the active ingredient in the gel. Additionally, it is not possible, without difficulty, to draw this viscous gel through a needle into a syringe
for I.V. injection. We believe that this gel forming feature will limit or impede the ability of prospective I.V. drug abusers from extracting and injecting opioid active ingredients from product candidates developed utilizing Aversion® Technology.
Intended to Deter Nasal Snorting
Prospective drug abusers may crush or grind currently marketed pharmaceutical opioid-containing tablets or capsules and snort the resulting powder. The abused active ingredient in the powder is absorbed through the lining of the nasal passages providing the abuser with a rapid onset of euphoric effects. Aversion® Technology products are intended to discourage nasal snorting by burning and irritating the nasal passages of a prospective drug abuser who crushes and snorts such products. We believe products which utilize Aversion® Technology will discourage prospective nasal drug abusers from snorting crushed tablets.
7
Intended to Deter Swallowing Excess Quantities of Tablets
Niacin, an active ingredient in vitamins, cholesterol reducers and nutritional supplements, may also be included in opioid analgesic product candidates utilizing Aversion® Technology. We believe that should a person swallow excess quantities of tablets utilizing Aversion® Technology with niacin they will experience disliking symptoms, including intense flushing, itching, sweating and/or chills, headache and a general feeling of discomfort as a result of the increasing dose of niacin. It is expected that these niacin-induced disliking symptoms will begin approximately 10 to 15 minutes after the excess dose is swallowed and will dissipate approximately 75 to 90 minutes
later. In addition, we believe it is generally recognized by physicians, nurses, and other health care providers that niacin has a well established safety profile in long term administration at doses far exceeding the amounts in each product candidate utilizing Aversion® Technology with niacin. We believe the undesirable niacin effects at escalating doses will not prevent, but are expected to deter, swallowing excess quantities of product candidates utilizing Aversion® Technology with niacin, although there can be no assurance in this regard. It has been known for years that niacin side effect may be mitigated if niacin is taken with food or with certain doses of non-steroidal anti-inflammatory drugs. The addition of niacin to products utilizing Aversion® Technology will expose legitimate pain patients to low, safe levels of niacin without
expected impact on the desired opioid analgesic effects but may cause a mild flushing in some of these pain patients.
U.S. Market Opportunity for Opioid Analgesic Products Utilizing Aversion® Technology
The misuse and abuse of prescription drug products in general, and opioid analgesics in particular, is a significant societal problem that has been described as epidemic in nature by Joseph A. Califano, Jr., Chairman and President, National Center for Addiction and Substance Abuse at Columbia University, July 2005. Results from the 2009 National Survey on Drug Use and Health, estimated that 35.0 million people, or more than 10% of the population, have used prescription opioid analgesics non-medically at some point in their lifetime. In addition, it is estimated that more
than 75 million people in the U.S. suffer from pain, which is more than the number of people with diabetes, heart disease and cancer combined. For many pain sufferers, opioid analgesics provide their only pain relief. As a result, opioid analgesics are among the largest prescription drug classes in the U.S. with over 260 million tablet and capsule prescriptions dispensed in 2010 of which approximately 244 million were for IR Opioid Products and 16 million were for ER Opioid Products. However, physicians and other health care providers at times are reluctant to prescribe opioid analgesics for fear of misuse, abuse, and diversion of legitimate prescriptions for illicit use.
We expect our Aversion® Technology opioid product candidates to compete primarily in the IR Opioid Product segment of the US opioid analgesic market, a segment which has grown at a 4% compounded annual rate over the last five years. On average, an IR Opioid Product prescription contains approximately 57 tablets or capsules. According to the 2009 National Survey on Drug Use and Health, prescription drug abusers have supplanted abusers of all illicit drugs except marijuana. Of these abused prescription products, IR Opioid Products, which typically provide rapid onset of analgesia and require dosing every 4 to 6 hours, comprise the
vast majority of this abuse compared with ER Opioid Products, which release their opioids gradually, generally over a 12 to 24 hour period. Due to fewer identified competitors and the significantly larger market for dispensed prescriptions for IR Opioid Products compared to ER Opioid Products, we have initially focused on developing IR Opioid Products utilizing Aversion® Technology.
According to IMS Health, in 2010, sales in the IR Opioid Product segment, comprised of 97% generic products, were $2.0 billion. Assuming the FDA approves differentiated label claims of the abuse deterrent features and benefits of our product candidates, of which no assurance can be given, we anticipate that our Aversion® Technology IR Opioid Products licensed to King will be premium priced compared to generic products resulting in the growth of sales in the IR Opioid Product market segment. All definitive pricing decisions relating to such Aversion® opioid products will be made by King.
Despite considerable publicity regarding the abuse of OxyContin® Tablets and other ER Opioid Products, U.S. government statistics suggest that far more people have used IR Opioid Products non-medically than ER Opioid Products. These statistics estimate that nearly 4 times as many people have misused the IR Opioid Products Vicodin®, Lortab® and Lorcet® (hydrocodone
bitartrate/acetaminophen brands and generics) as have ever abused OxyContin®. We estimate 60-95% of the 35.0 million life time US opioid abusers have non-medically used the active ingredients in our IR Opioid product candidates. As indicated in the following chart, the top five abused opioid products are available only as IR Opioid Products.
8
Lifetime Non-Medical Use of Selected Pain Relievers, Age 12 or Older: 2009
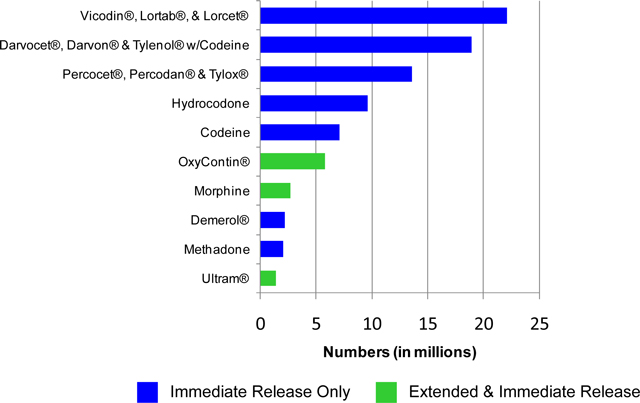
Source: SAMHSA, Office of Applied Studies, 2009 National Survey on Drug Use and Health.
We have completed, through an independent market research firm, three physician market research studies with 282, 401 and 435 opioid prescribing U.S. based physicians, respectively. A sampling of key findings from these approximately 1,100 physicians includes:
Physicians are keenly aware of opioid analgesic abuse
|
|
·
|
The 282 physicians surveyed estimated on average that about one out of six prescriptions for oxycodone and hydrocodone containing products are abused.
|
|
|
·
|
94% of the 435 physicians surveyed experienced at least one suspicious incident regarding opioid abuse in the past month, while nearly 64% experienced four or more discretely different incidents regarding opioid abuse in the past month.
|
Physicians are personally concerned with opioid abusers impact to their respective practices
|
|
·
|
Following the survey of 282 physicians, the researchers concluded, “abuse [of opioid analgesics] is a particular problem for physicians because many are not fully sure who is abusing these opioids, and they view such abuse as a legal threat to their practice.” “More than half [of the physicians surveyed] believe their physician colleagues are more concerned about avoiding state review [of their opioid prescribing habits] than meeting [professional association] pain guidelines [for their patients]”.
|
|
|
·
|
After the survey of 435 physicians the researchers concluded “the primary motive for prescribing the Aversion® Technology product[s] is the concern physicians have about opioid abuse and the threat it represents to their practice.”
|
9
Physicians are favorably inclined toward prescribing opioids with abuse deterrent features and benefits
|
|
·
|
94% of the 401 physicians surveyed indicated that they would either prescribe one of the Aversion® Technology products profiled in the market research questionnaire for one of their last five patients receiving an opioid prescription or they are aware of a patient in their practice for whom Aversion® Technology products would be an appropriate choice.
|
|
|
·
|
57% of the 435 physicians indicated that their opioid analgesic prescribing would increase if they were more certain they were not aiding abusers.
|
|
|
·
|
Following the survey of 401 physicians, the researchers concluded “these [Aversion® Technology oxycodone products] would disproportionately replace current immediate release oxycodone [and oxycodone/acetaminophen] prescriptions, but would also draw substantial volume from hydrocodone/acetaminophen products.”
|
Overall, we believe the availability of opioid analgesics with abuse deterrent features, including products using our Aversion® Technology (with or without niacin), will impact the selection of products used for relief of pain. Our market research survey of the 401 physicians indicated that of the prescriptions likely to be written for our product candidates that utilize oxycodone, 59% will be switched from immediate release products containing either oxycodone or hydrocodone, with the remaining 41% being switched from other currently marketed opioid analgesic products such as codeine, propoxyphene, morphine and tramadol. Our market research surveys of 401 physicians and 435 physicians,
respectively, suggest that the particular combination of ingredients [i.e., with or without niacin] does not appear to have a substantial effect on the estimated brand market share potential of our Acurox® product candidates.
A majority of pharmaceutical products in the U.S. are paid for by third party payers such as insurers, pharmacy benefit managers, self-insured companies and the federal and state governments through Medicare, Medicaid and other entitlement programs. We believe our product candidates must demonstrate a clinical benefit to the patient and/or an economic benefit to third party payers and/or a benefit to health care providers to receive favored reimbursement status by the third party payers, of which no assurance can be given.
Independent estimates have been made assessing the potentially significant cost impact of prescription opioid abuse to insurers. An analysis of health and pharmacy insurance claims between 1998 and 2002 for almost 2 million Americans conducted by Analysis Group, Inc. and others indicated that enrollees with a diagnosis of opioid abuse had average claims of approximately $14,000 per year higher than an age-gender matched non-opioid abuse sample. A 2007 report by the Coalition Against Insurance Fraud inflated this excess cost per patient to more than $16,000 for 2007, and by applying the U.S. government’s estimated 4.4 million annual opioid abusers, concluded that opioid abuse could
costs health insurers up to $72.5 billion a year.
Acurox® Tablets Development Program
Acurox® Tablets)
On December 17, 2010, King (our licensee under the King Agreement) submitted a NDA for Acurox® (oxycodone HCI) Tablets to the FDA, including a request for priority review classification On February 10, 2011, the FDA notified King of the FDA’s acceptance for filing of the Acurox® Tablets NDA and the grant of a priority review classification. The Prescription Drug User Fee Act non-binding target date for completion of FDA’s review is June 17, 2011.
The NDA for Acurox® Tablets includes results from numerous clinical and laboratory studies assessing the efficacy and safety of Acurox® Tablets and to demonstrate the abuse deterrent features and benefits, including the data and results from the studies set forth in the table below.
10
|
Studies in the Acurox® Tablets (without niacin) 505(b)(2) NDA Submission
|
Summary of Results
|
|||
|
AP-ADD-100
Phase I
|
Bioequivalence to currently marketed oxycodone HCl Reference Listed Drug
|
Acurox® Tablets are bioequivalent to the Reference Listed Drug and to Acurox® with Niacin Tablets
|
||
|
K###-##-####
Phase I
|
Dose Proportionality and the Effects of Food on the Bioavailability
|
Acurox® Tablets are dose proportional between 5 mg and 15 mg. Food resulted in a decrease in oxycodone peak exposure (Cmax), an increase in total exposure (AUC), and a delay to peak oxycodone (Tmax) compared with fasted conditions.
|
||
|
K###-##-####
Phase I
|
Evaluate effects of nasal snorting in subjects with a history of snorting and nasal drug abuse
|
Refer to summary in this Report
|
||
|
PR-381
|
Laboratory test quantifying I.V. abuse deterrent properties (syringe test)
|
Refer to summary in this Report
|
||
|
PR-382
|
Laboratory tests quantifying I.V. abuse deterrent properties (extraction test)
|
Refer to summary in this Report
|
||
In addition to filing acceptance and assignment of a priority review classification, the FDA’s filing communication letter to King also includes preliminary comments about potential review issues relating to the intranasal abuse liability study included in the Acurox® Tablets NDA and requests additional information relating to this study and other issues. The preliminary notice of potential review issues is not indicative of deficiencies that may be identified during the FDA’s review of the NDA. No assurance can be given that any issues raised as part of the FDA’s review of the Acurox® Tablets NDA (including the potential review issues in the FDA’s filing communication letter) will be addressed to the FDA’s satisfaction or that the Acurox® Tablets NDA will be approved by the FDA.
Study K###-##-#### or Study 1002: Study 1002 is entitled “Randomized, Double-Blind, Active-Controlled Study to Evaluate the Relative Abuse Potential and Safety of Intranasally Administered Crushed Acurox® Tablets in Non-Dependent Recreational Opioid Users”. A total of 40 adult subjects with a history of recreational drug abuse successfully completed screening, a naloxone challenge (demonstrating no addiction to opioids), and a discrimination test (demonstrating the ability to discern drug liking between snorting oxycodone HCl 15mg and placebo) and were
randomized into the treatment phase of the study. 39 subjects were analyzed.
In the treatment phase, subjects intranasally administered crushed tablets of: (a) 3 x 5mg Roxicodone® tablets, and (b) 2 x 7.5mg Acurox® Tablets. Subjects received all doses in a randomized, cross-over manner with 48 hours between doses. Subjects rated each dose for 6 hours post-administration for drug liking/disliking on a 100mm bipolar visual analogue scale (100mm bi-polar VAS where 100=”like a lot”, 50=”neutral, and 0 = “dislike a lot”) as well as 6-point measures of several nasal discomfort measures. At 8 hours, subjects rated their Overall Drug Liking and Take Drug Again measures (both 100mm bipolar VAS). The maximum drug
like/dislike effect (Emax) was designated in the study protocol as the primary endpoint.
For the Drug Liking Emax, Roxicodone® Tablets had statistically significant difference (p<0.0001) in mean like/dislike score (93.4) compared to Acurox® Tablets (70.6). The mean Overall Drug Liking VAS and Take Drug Again VAS scores at 8 hours were close to or slightly below the neutral point of 50 with Acurox® Tablets, compared to Roxicodone® Tablet values of 87.2 and 91.2, respectively (p<0.0001 for both). The study VAS score results were supported by the assessments for nasal discomfort. The inferential analyses showed significant treatment effects (p ≤ 0.008) for all nasal assessment scales except for facial pain/ pressure. Except for nasal discomfort, crushed and snorted Acurox® Tablets were generally well tolerated. The majority of adverse events were mild and consistent with the intended nasal discomforting effects of crushed Acurox® Tablets. There were no serious adverse events reported.
Study PR-382 - Extraction Test – Study PR-382 is entitles “Evaluation of the Potential for Extraction of Oxycodone HCl from Dissolved Acurox® Tablets for the Preparation of an Intravenous Solution Suitable for Injection in Humans”. An independent pharmaceutical laboratory utilizing a professionally trained pharmaceutical chemist attempted to extract the oxycodone HCl from: (a) 8 x 5mg generic oxycodone HCl tablets and (b) 8 x 5mg Acurox® Tablets. The chemist was allowed to use any processes, procedures and equipment to prepare a solution of 10mL’s suitable for injection that could be drawn through a
hypodermic needle into a syringe. The chemist was limited to 8 hours of laboratory time per drug [i.e. 8 hours for (a) and 8 hours for (b)]. Endpoints in the study were the percent of oxycodone HCl extracted from each drug, a chemist rating of the ease/difficult of the extraction procedure (1=easy to execute; 10=complex to execute) and the relative availability of the equipment or materials used (1=readily available; 10=difficult to obtain).
11
The results from this study suggest that generic oxycodone HCl tablets are easily (ease/difficulty rating =1) dissolved in water in less than 10 minutes for potential abuse via IV injection. The drug concentration is sufficiently high (~28mg of 40mg extracted or a 72% yield) for such purposes. No Acurox® Tablet solutions meeting the pre-specified criteria for a successful oxycodone extraction were obtained using either water, organic solvents, or organic solvent mixtures from 8 different extraction procedures.
Study PR-381 - Syringe Test: – Study PR-381 is entitled “Demonstration of the Ability of Acurox® (oxycodone HCl, USP) Tablets to Resist Direct Conversion into an Injectable Solution”. An independent pharmaceutical laboratory attempted to prepare a solution suitable for intravenous injection from: (a) 2 x 5mg generic oxycodone HCl tablets and (b) 2 x 5mg Acurox® Tablets. The tablets were crushed and dissolved in increasing amounts of seven different common solvents safe for humans to inject until at least 3mL’s of the resulting mixture could be drawn through a hypodermic needle into a
syringe. In all solvents except one, the generic tablets could be drawn into the syringe following the addition of just 4mL’s of solvent with the seventh solvent requiring 20mL’s. Acurox® Tablets required 14mL’s or more with six solvents and 6mL’s for the seventh solvent to prepare a solution suitable for injecting. With these volumes an abuser would theoretically need to perform between 4 and 10 injections of the drug solutions obtained from crushed Acurox® Tablets using a 3mL syringe.
Acurox® with Niacin Tablets
We submitted a NDA for Acurox with Niacin Tablets on December 30, 2008 and received a Complete Response Letter (CRL) from the FDA on June 30, 2009. An FDA Advisory Committee meeting was held on April 22, 2010 to discuss Acurox® with Niacin Tablets and the result of the studies evaluating the addition of niacin for the purpose of reducing misuse of oxycodone by excess oral consumption. The FDA Advisory Committee voted at such meeting that they did not have sufficient evidence to support the approval of the NDA for Acurox® with
Niacin Tablets for the treatment of moderate to severe pain, considering the deterrent effects of niacin and the potential abuse deterrent features specific to Acurox® with Niacin Tablets. The FDA questioned: (a) the perceived increased incidence of flushing when Acurox® with Niacin Tablets are taken by pain patients at recommended doses, (b) a lack of evidence supporting the effectiveness of niacin to reduce peak drug liking (Emax) when taken at abused (high) doses in the fasted state, and (c) the potential to mitigate the effectiveness of niacin with food or NSAIDS.
To provide additional support for the abuse deterrent benefits of niacin in Acurox® with Niacin Tablets, we and King conducted an additional oral abuse liability study (AP-ADF-114 (Study 114)). Study 114 was not included in the original NDA filing for Acurox® with Niacin Tablets. We and King are continuing to evaluate the results of Study 114 and intend to submit a response to the FDA’s June 2009 Complete Response Letter for Acurox® with Niacin Tablets.
Study AP-ADF-114 or Study 114: Study 114 is entitled “A Randomized, Double-Blind, Placebo- and Active-Controlled Study to Assess the Relative Abuse Potential of Acurox® [with Niacin] Tablets (formerly known as Acurox® Tablets) in Non-Dependent Recreational Opioid Users”. A total of 46 healthy adult subject with a history of recreational opioid abuse successfully completed the screening, a naloxone challenge (demonstrating no addiction to opioids), a drug
discrimination test (demonstrating the ability to discern drug liking between ingesting oxycodone HCl 40mg and placebo), and the treatment phase. In the treatment phase fasted subjects orally administered, in randomized and cross-over manner: (a) 8 x 5mg Roxicodone® tablets, (b) 8 x 10mg Roxicodone® tablets, (c) 8 x 5/30mg Acurox® with Niacin Tablets, (d) 8 x 10/30 Acurox® with Niacin Tablets, and (e) placebo. The primary efficacy assessment was the 100-mm bipolar visual analog scale Drug Liking/Disliking Assessment taken over 12 hours post-dosing. Secondary assessments included a Take Drug Again Assessment (TDAA) and a Global Assessment of Overall Drug Liking (both 100mm bipolar VAS).
We and King continue to analyze the results from Study 114. However, topline results indicate statistically significant differences between Acurox® with Niacin Tablets (40/240 mg and 80/480 mg) and the respective equivalent Roxicodone® doses (40/0 mg and 80/0 mg) for the peak liking (Emax) as measured by Like/Dislike scores for 8 hours post-administration (p=0.003 and p<0.0001 , respectively). There were statistically significant and clinically
meaningful decreases between Acurox® with Niacin Tablets and the respective equivalent Roxicodone® doses in the Take Drug Again Assessment (at 1, 2, and 8 hours; p<=0.001) and the Global Assessment of Overall Drug Liking (assessed at 12 hours; p<=.0032). No serious adverse events were reported with the majority of adverse events are consistent with the expected impediment effects of niacin (skin burning sensation, skin warm, and flushing).
12
Expectations for Acurox® Tablets Product Labeling
The FDA has publicly stated that an explicit indication or claims of abuse deterrence will not be permitted in product labeling unless such indication or claims are supported by double blind controlled clinical studies demonstrating an actual reduction in product abuse by patients or drug abusers. We believe the cost, time and practicality of designing and implementing clinical studies adequate to support explicit labeling claims of abuse deterrence are prohibitive. The FDA has stated that scientifically derived data and information describing the physical characteristics of a product candidate and/or the results of laboratory and clinical
studies simulating product abuse may be acceptable to include in the product label. We intend to include in the labels of our Aversion® Technology product candidates (whether with or without niacin) both a physical description of the abuse deterrent characteristics and information from our laboratory and clinical studies designed to simulate the relative difficulty of abusing our product candidates. The extent to which such information will be included in the FDA approved product label will be the subject of our discussions with an agreement by the FDA as part of the NDA review process for each of our product candidates. Further, because FDA closely regulates promotional materials, even if FDA initially approves labeling that includes a description of the abuse deterrent characteristics of the product, the FDA’s Division Drug Marketing,
Advertising, and Communication (i.e. DDMAC) will continue to review the acceptability of promotional labeling claims and product advertising campaigns for our marketed products, if any.
King Agreement
On October 30, 2007, we and King Pharmaceuticals Research and Development, Inc. (“King”), a wholly-owned subsidiary of King Pharmaceuticals, Inc., entered into a License, Development and Commercialization Agreement (the “King Agreement”) to develop and commercialize in the United States, Canada and Mexico (the "King Territory") certain opioid analgesic products utilizing our proprietary Aversion® Technology. The King Agreement initially provided King with an exclusive license in the King Territory for Acurox® (oxycodone HCl) Tablets with and
without niacin and Acuracet® (oxycodone HCl/acetaminophen) Tablets with and without niacin utilizing Aversion® Technology. In addition, the King Agreement provides King with an option to license in the King Territory certain future opioid analgesic products developed utilizing Aversion® Technology (with and without niacin). As of December 31, 2010, King exercised its option to license two additional product candidates including an undisclosed opioid analgesic tablet product and Vycavert® (hydrocodone bitartrate/acetaminophen) Tablets with and without niacin, all of which utilize our Aversion® Technology . We are responsible for using commercially reasonable efforts to develop
Acurox® with Niacin Tablets through regulatory approval by the FDA. The King Agreement provides that we or King may develop additional opioid analgesic product candidates utilizing our Aversion® Technology (with and without niacin) and, if King exercises its option to license such additional product candidates, they will be subject to the milestone and royalty payments and other terms of the King Agreement.
Pursuant to the King Agreement, we and King formed a joint steering committee to oversee development and commercialization strategies for Aversion® opioid analgesic products licensed to King. We are responsible for all Acurox® with Niacin Tablet development activities, the expenses for which we are reimbursed by King, through FDA approval of a NDA. If the FDA approves the NDA for Acurox® with Niacin Tablets, of which no assurance can be given, King will be responsible for
commercializing such product in the U.S. With respect to all other products licensed by King pursuant to the Agreement in all King Territories, including Acurox® Tablets, King will be responsible, at its own expense, for development, regulatory, and commercialization activities. All products developed pursuant to the King Agreement will be manufactured by King or a third party contract manufacturer under the direction of King. Subject to the King Agreement, King will have final decision making authority with respect to all development and commercialization activities for all licensed products. We have reviewed our participation in the King-Acura joint steering committee in light of the requirements of Emerging Issues Task Force, Issue No. 00-21, “Revenue Arrangements with
Multiple Deliverables” (“EITF 00-21”) and concluded that this activity has no standalone value therefore it does not meet the criteria to be considered a separate unit of accounting.
13
At December 31, 2010, we had received aggregate payments of $58.3 million from King, consisting of a $30.0 million non-refundable upfront cash payment, $17.3 million in reimbursed research and development expenses relating to Acurox® with Niacin Tablets and Acurox® Tablets, $6.0 million in fees relating to King’s exercise of its option to license an undisclosed opioid analgesic tablet product and Vycavert® with Niacin Tablets, and a $5.0 million milestone fee relating to our successful achievement of the primary endpoints for our pivotal Phase III clinical study for
Acurox® with Niacin Tablets. The King Agreement also provides for King’s payment to us of a $3.0 million fee upon King’s exercise of its option for each future opioid analgesic utilizing Aversion® Technology (“Future Product”). In the event that King does not exercise its option for a Future Product, King may be required to reimburse us for certain of our expenses relating to such Future Product. Further, we may receive up to $23 million in additional non-refundable milestone payments for each active opioid analgesic ingredient licensed to King which achieves certain regulatory milestones in specific countries in the King Territory. An opioid analgesic product candidate formulated with and without niacin is considered a single product candidate for purposes of the option fees and milestone payments payable
under the King Agreement. We can also receive a one-time $50 million sales milestone payment upon the first attainment of $750 million in net sales of all of our licensed products across all King Territories. In addition, for sales occurring following the one year anniversary of the first commercial sale of the first licensed product sold, King will pay us a royalty at one of 6 rates ranging from 5% to 25% based on the level of combined annual net sales for all products licensed by us to King across all King Territories, with the highest applicable royalty rate applied to such combined annual sales. King’s royalty payment obligations expire on a product by product and country-by-country basis upon the later of (i) the expiration of the last valid patent claim covering such product in such country, or (ii) fifteen (15) years from the first commercial sale of such product
in such country. No minimum annual fees are payable by either party under the King Agreement. Reference is made to Item 7 of Note A of the Notes to Consolidated Financial Statements included as a part of this Report, entitled “Revenue Recognition, Deferred Program Fee Revenue, and Collaboration Revenue” for a description of the revenue recognition method employed by the Company under the King Agreement.
The King Agreement expires upon the expiration of King’s royalty payment and other payment obligations under the King Agreement. King may terminate the King Agreement (i) in its entirety at any time by written notice to us, and (ii) with respect to any product at any time upon the provision of not less than 12 months’ prior written notice. We may terminate the King Agreement with respect to a product in the United States in the event such product is not commercially launched by King within 120 days after receipt of regulatory approval of such product or in its entirety if King commences any interference or opposition proceeding
challenging the validity or enforceability of any of our patent rights licensed to King under the King Agreement. Either party has the right to terminate the King Agreement on a product by product and country-by-country basis if the other party is in material breach of its obligations under the King Agreement relating to such product and such country, and to terminate the Agreement in its entirety in the event the other party makes an assignment for the benefit of creditors, files a petition in bankruptcy or otherwise seeks relief under applicable bankruptcy laws, in each case subject to applicable cure periods.
In the event of termination, no payments are due except those royalties and milestones that have accrued prior to termination under the King Agreement and all licenses under the King Agreement are terminated. For all Acura terminations and termination by King where we are not in breach, the King Agreement provides for the transition of development and marketing of the licensed products from King to us, including the conveyance by King to us of the trademarks and all regulatory filings and approvals solely used in connection with the commercialization of such licensed products and, in certain cases, for King’s supply of such licensed products for a
transitional period at King’s cost plus a mark-up.
The foregoing description of the King Agreement contains forward-looking statements about Acurox® Tablets, and other product candidates being developed pursuant to the King Agreement. As with any pharmaceutical products under development or proposed to be developed, substantial risks and uncertainties exist in development, regulatory review and commercialization process. There can be no assurance that the King Agreement will not be terminated by its terms prior to receipt of regulatory approval for any product developed pursuant to the King
Agreement. Further, there can be no assurance that any product developed, in whole or in part, pursuant to the King Agreement will receive regulatory approval or prove to be commercially successful. Accordingly, investors in the Company should recognize that there is no assurance that the Company will receive the milestone payments or royalty revenues described in the King Agreement or even if such milestones are achieved, that the related products will be successfully commercialized and that any royalty revenues payable to us by King will materialize. For further discussion of other risks and uncertainties associated with the Company, see Item 1A in this Report under the heading “Risks Factors”.
On October 12, 2010, Pfizer, Inc. announced a tender offer to acquire all of the outstanding shares of King Pharmaceuticals, Inc. Pfizer’s tender offer acquisition of King Pharmaceuticals was completed on January 31, 2011, resulting in King becoming a majority-owned subsidiary of Pfizer. Pfizer has advised that it intends to complete a short-form merger with King on or about February 28, 2011, pursuant to which King will become a wholly-owned subsidiary of Pfizer. King will remain the responsible party under the King Agreement following such merger transaction.
14
Patents and Patent Applications
In April 2007, the United States Patent and Trademark Office (“USPTO”), issued to us a patent titled “Methods and Compositions for Deterring Abuse of Opioid Containing Dosage Forms” (the “920 Patent”). The 54 allowed claims in the 920 Patent encompass certain pharmaceutical compositions intended to deter the most common methods of prescription opioid analgesic product misuse and abuse. These patented pharmaceutical compositions include the mixture of functional inactive ingredients and specific opioid analgesics such as oxycodone HCl and hydrocodone bitartrate among others.
In January 2009, the USPTO issued to us a patent (the “402 Patent”) with 18 allowed claims. The 402 Patent encompasses certain combinations of kappa and mu opioid receptor agonists and other ingredients intended to deter opioid analgesic product misuse and abuse.
In March 2009, the USPTO issued to us a patent (the “726 Patent”) with 20 allowed claims. The 726 Patent encompasses a wider range of abuse deterrent compositions than our 920 Patent.
Neither of the 920 Patent, 402 Patent or 726 Patent requires niacin to be a constituent of a product for the product to be within the scope of the patent claims.
In addition to our issued U.S. patents, we have filed multiple U.S. patent applications and international patent applications relating to compositions containing abusable active pharmaceutical ingredients. Except for those rights conferred in the King Agreement, we have retained all intellectual property rights to our Aversion® Technology, Impede™ Technology, and related product candidates.
Reference is made to Item 1A, “Risk Factors” for a discussion, among other things, of pending patent applications owned by third parties including claims that may encompass our Acurox® Tablets and other product candidates. If such third party patent applications result in valid and enforceable issued patents containing claims in their current form, we or our licensees could be required to obtain a license to such patents, should one be available, or alternatively, to alter our product candidates to avoid infringing such third-party patents.
Competition in the Opioid Product Market
We compete to varying degrees with numerous companies in the pharmaceutical research, development, manufacturing and commercialization fields. Many of our competitors have substantially greater financial and other resources and are able to expend more funds and effort than us in research and development of their competitive technologies and products. Although a larger company with greater resources than us will not necessarily have a higher likelihood of receiving regulatory approval for a particular product or technology as compared to a smaller competitor, the company with a larger research and development expenditure will be in a position to support more development projects simultaneously, thereby potentially
improving the likelihood of obtaining regulatory approval of a commercially viable product or technology than its smaller rivals.
We believe potential competitors may be developing opioid abuse deterrent technologies and products. Such potential competitors include, but may not be limited to, Pain Therapeutics of South San Francisco, CA, (in collaboration with King Pharmaceuticals Inc.), Purdue Pharma of Stamford, CT, Atlantic Pharmaceuticals, of Atlanta, GA, Egalet a/s, of Verlose, Denmark, KemPharm of North Liberty, Iowa and Collegium Pharmaceuticals, Inc., of Cumberland, RI. These companies appear to be focusing their development efforts on ER Opioid Products, except for Atlantic Pharmaceuticals, while the majority of our Aversion® Technology opioid analgesic product candidates under development, are IR Opioid
Products.
Aversion® Technology Non-Opioid Product Candidates in Development
We are developing a benzodiazepine tranquilizer product candidate utilizing our Aversion® Technology intended for the treatment of anxiety disorders. Benzodiazepine products are classified as Schedule IV controlled substances by the DEA. According to Drugs of Abuse, published by the DEA, tranquilizers are abused in manners similar to opioid analgesics. The 2009 National Survey on Drug Use and Health estimates that 21.7 million people have abused prescription tranquilizers (including benzodiazepines) at some point in
their lifetime and 5.5 million have abused tranquilizers in the past year.
15
We have had a face-to-face meeting with the FDA’s Division of Psychiatry Products (DPP) regarding our proposed strategy for developing a benzodiazepine containing product candidate with abuse deterrent features and benefits. At that meeting, DPP recommended our benzodiazepine containing product candidate and proposed investigational new drug application strategy be reviewed by the Division of Anesthesia and Analgesia Products (DAAP). DPP’s view was that although our proposed product contains a benzodiazepine indicated for a psychiatric condition, that it would be more appropriate for DAAP to review our proposed development strategy
due to their broader experience with products intended to reduce abuse. We intend to schedule a meeting with the DAAP to discuss our benzodiazepine containing product candidate.
Our benzodiazepine product candidate is intended to be encompassed by numerous pending U.S. patent applications. There can be no assurances that such pending patent applications will result in issued patent claims encompassing our benzodiazepine product candidate.
Impede™ Technology Product Candidates in Development
We have developed Impede™ PSE, a pseudoephedrine hydrochloride (“PSE”) tablet product candidate utilizing our Impede™ Technology. Impede™ Technology utilizes a proprietary mixture of functional inactive ingredients intended to limit or impede extraction of PSE from the tablets for use as a starting material in producing the illicit drug methamphetamine. The unique mixture of inactive ingredients in the Impede™ PSE product candidate are generally recognized as safe.
Most PSE containing products are classified by the FDA for sale Over-The-Counter (without a doctor’s prescription) and most product formulations do not require the approval of a New Drug Application by the FDA to initiate commercial distribution and marketing. In 2006 regulations relating to over-the counter sale of PSE products were amended with the enactment of the Federal Combat Methamphetamine Epidemic Act (CMEA). The CMEA was enacted in response to an alarming increase in and widespread conversion of PSE containing products into methamphetamine. Among other things, the CMEA requires retail stores to maintain their inventory of PSE containing products in a secured
location and restricts the amount of PSE products a store can sell to an individual customer. Implementation of the CMEA initially reduced the number of illegal methamphetamine laboratories as the then most commonly used process for conversion of PSE to methamphetamine required substantial quantities of PSE. However, a newer, more efficient process for converting PSE to methamphetamine requires less PSE. Possibly as a result of the more efficient process, the DEA reported 2009 clandestine methamphetamine laboratory seizures increased 62% over the low reported in 2007. Impede™ Technology is designed to deter a wide range of processes of methamphetamine production including both the older and newer processes. In response to the ongoing methamphetamine problem, several local jurisdictions (state, counties and/or local
municipalities) have enacted or propose to enact legislation to require a physician’s prescription to obtain a PSE containing product.
We sponsored an independent laboratory test of our Impede™ PSE tablets compared to Sudafed®* brand PSE tablets in an attempt to extract PSE from 100 x 30 mg tablets for conversion to methamphetamine using what we believe to be the three most commonly used extraction processes. The results of these tests demonstrated that while PSE was readily extracted from Sudafed® tablets, Impede™ PSE effectively impeded the extraction of the PSE for conversion into methamphetamine. The results of these tests are summarized in the table below:
|
% Pseudoephedrine HCl extracted
from 100 x 30mg tablets
|
||||||||||||
|
Product Tested
|
Method 1
|
Method 2
|
Method 3
|
|||||||||
|
Impede™ PSE Tablets
|
0 | % | 0 | % |
~0
|
% | ||||||
|
Sudafed® Tablets
|
96 | % | 89 | % | 79 | % | ||||||
*Sudafed® is a registered trademark of Johnson and Johnson Corporation
We have a completed an 18 subject crossover pharmacokinetic study to evaluate the plasma concentrations of: (a) 2 x 30mg Impede™ PSE Tablets, (b) 2 x 30mg Sudafed® Tablets, and (c) 2 x 30mg generic PSE tablets (also known as a “store brand”). The study demonstrated that Impede™ PSE is bioequivalent to Sudafed® and to pseudoephedrine HCl tablets manufactured by the Perrigo Company.
Tablet products containing 60 mg or less of PSE are considered by the FDA to be safe and effective for use by the general public without a prescription. We believe our 30 mg PSE tablet product developed utilizing Impede™ Technology meets or will meet the FDA’s requirements for “Over-the-Counter Human Drugs Which are Generally Recognized as Safe and Effective and Not Misbranded” as set forth in the Code of Federal Regulations at 21 CFR 330.1 which will allow us to commercialize our Impede™ PSE Tablets without submitting a NDA to the FDA.
16
We are currently negotiating with our preferred contract manufacturer for the scale up, manufacture and packaging of commercial quantities of our Impede™ PSE Tablets. It is our expectation to market, sale and distribute our Impede™ PSE Tablets directly to national and regional drug store chains.
Government Regulation
All pharmaceutical firms, including us, are subject to extensive regulation by the federal government, principally by the FDA under the Federal Food, Drug and Cosmetic Act (the “FD&C Act”), and, to a lesser extent, by state and local governments. Before our prescription products may be marketed in the U.S., they must be approved by the FDA for commercial distribution. Additionally, we are subject to extensive regulation by the DEA under the Controlled Substances Act for research, development and manufacturing of controlled substances. We are also subject to regulation under federal, state and local laws, including requirements regarding occupational safety,
laboratory practices, environmental protection and hazardous substance control, and may be subject to other present and future local, state, federal and foreign regulations, including possible future regulations of the pharmaceutical industry. We cannot predict the extent to which we may be affected by legislative and other regulatory developments concerning our products and the healthcare industry in general.
However, because of recent developments in the legislative and regulatory framework within which drug products are reviewed and approved by FDA, approval of drug products by FDA may be subject to continuing obligations intended to assure safe use of the products. Specifically, effective March 25, 2008, under Title IX of Subtitle A of the Food and Drug Administration Amendments Act of 2007 (“FDAAA”), FDA may require a Risk Evaluation and Mitigation Strategy (“REMS”) to manage known or potential serious risks associated with drugs or biological products. If FDA finds that a REMS is necessary to ensure that the benefits of our products outweigh the risks associated with the
products, FDA will require a REMS and, consequently, that we take additional measures to ensure safe use of the product. Components of a REMS may include, but are not limited to a Medication Guide, a marketing and sales communication plan, elements to assure safe product use, a REMS implementation system, and a timetable for assessment of the effectiveness of the REMS.
The FD&C Act, the Controlled Substances Act and other federal statutes and regulations govern the testing, manufacture, quality control, export and import, labeling, storage, record keeping, approval, pricing, advertising, promotion, sale and distribution of pharmaceutical products. Noncompliance with applicable requirements both before and after approval, can subject us, our third party manufacturers and other collaborative partners to administrative and judicial sanctions, such as, among other things, warning letters, fines and other monetary payments, recall or seizure of products, criminal proceedings, suspension or withdrawal of regulatory approvals, interruption or cessation of clinical trials, total or
partial suspension of production or distribution, injunctions, limitations on or the limitation of claims we can make for our products, and refusal of the government to enter into supply contracts for distribution directly by governmental agencies, or delay in approving or refusal to approve new drug applications. The FDA also has the authority to revoke or withhold approvals of new drug applications.
The Federal Controlled Substances Act imposes various registration, record-keeping and reporting requirements, procurement and manufacturing quotas, labeling and packaging requirements, security controls and a restriction on prescription refills on certain pharmaceutical products. Establishments may not handle controlled drug substances until they have been inspected and registered by the DEA and must be equipped to meet DEA security requirements. A principal factor in determining the particular requirements, if any, applicable to a product is its actual or potential abuse profile. A pharmaceutical product may be “scheduled” as a C-I, C-II, C-III, C-IV or C-V controlled substance, with C-I
substances considered to present the highest risk of substance abuse and C-V substances the lowest. Because of the potential for abuse, opioid analgesic active pharmaceutical ingredients and finished drug products, including all of our Aversion® Technology opioid product candidates, are regulated, or scheduled, under the Controlled Substances Act. Because they contain oxycodone HCl, we believe that Acurox® Tablets and Acurox® with Niacin Tablets will be DEA C-II products.
17
FDA approval is required before any "new drug," can be marketed. A "new drug" is one not generally recognized as safe and effective for its intended use. Our products are new drugs. Such approval must be based on adequate and well controlled laboratory and clinical investigations. In addition to providing required safety and effectiveness data for FDA approval, a drug manufacturer's practices and procedures must comply with current Good Manufacturing Practices (“cGMPs”), which apply to the manufacturing, receiving, holding and shipping. Accordingly, manufacturers must continue to expend time, money and effort in all applicable areas relating to quality assurance and regulatory compliance, including
production and quality control to comply with cGMPs. Failure to so comply risks delays in approval of drug products and possible FDA enforcement actions, such as an injunction against shipment of products, the seizure of non-complying products, criminal prosecution and/or any of the other possible consequences described above. We are subject to periodic inspection by the FDA and DEA.
The FDA Drug Approval Process
The process of drug development is complex and lengthy. The activities undertaken before a new pharmaceutical product may be marketed in the U.S. generally include, but are not limited to, preclinical studies; submission to the FDA of an IND, which must become active before human clinical trials commence; adequate and well-controlled human clinical trials to establish the safety and efficacy of the product; submission to the FDA of an NDA; acceptance for filing of the NDA by FDA; satisfactory completion of an FDA pre-approval inspection of the clinical trial sites and manufacturing facility or facilities at which the both the active ingredients and finished drug product are produced to assess compliance with
cGMPs; and FDA review and approval of the NDA prior to any commercial sale and distribution of the product in the U.S.
Preclinical studies include laboratory evaluation of product chemistry and formulation, and in some cases, animal studies and other studies to preliminarily assess the potential safety and efficacy of the product candidate. The results of preclinical studies together with manufacturing information, and analytical data are then submitted to the FDA as a part of an IND. An IND must become effective prior to the commencement of human clinical trials. The IND becomes effective 30 days following its receipt by the FDA unless the FDA objects to, or otherwise raises concerns or questions and imposes a clinical hold. In the event that FDA objects to the IND and imposes a clinical hold, the IND
sponsor must address any outstanding FDA concerns or questions to the satisfaction of the FDA before clinical trials can proceed. There can be no assurance that submission of an IND will result in FDA authorization to commence clinical trials. Once an IND is in effect, the protocol for each clinical trial to be conducted under the IND must be submitted to the FDA, which may or may not allow the trial to proceed.
Human clinical trials are typically conducted in three phases that often overlap:
Phase I: This phase is typically the first involving human participants, and involves the smallest number of human participants (typically, 20-50). The investigational drug is initially introduced into healthy human subjects or patients and tested for safety, dosage tolerance, absorption, metabolism, distribution and excretion. In addition, it is sometimes possible to gain a preliminary indication of efficacy.
Phase II: Once the preliminary safety and tolerability of the drug in humans is confirmed during phase I, phase II involves studies in a somewhat larger group of study subjects. Unlike phase I studies, which typically involve healthy subjects, participants in phase II studies maybe affected by the disease or condition for which the product candidate is being developed. Phase II studies are intended to identify possible adverse effects and safety risks, to evaluate the efficacy of the product for specific targeted diseases, and to determine appropriate dosage and tolerance.
Phase III: Phase III trials typically involve a large numbers of patients affected by the disease or condition for which the product candidate is being developed. Phase III clinical trials are undertaken to evaluate clinical efficacy and safety under conditions resembling those for which the product will be used in actual clinical practice after FDA approval of the NDA. Phase III trials are typically the most costly and time-consuming of the clinical phases.
Phase IV: Phase IV trials may be required by FDA after the approval of the NDA for the product, as a condition of the approval, or may be undertaken voluntarily by the sponsor of the trial. The purpose of phase IV trials is to continue to evaluate the safety and efficacy of the drug on a long-term basis and in a much larger and more diverse patient population than was included in the prior phases of clinical investigation.
18
After clinical trials have been completed, the sponsor must submit an NDA to the FDA including the results of the preclinical and clinical testing, together with, among other things, detailed information on the chemistry, manufacturing, quality controls, and proposed product labeling. There are two types of NDAs; a 505(b)(1) and a 505(b)(2). A 505(b)(1) NDA is also known as a "full NDA" and is described by section 505(b)(1) of the FD&C Act as an application containing full reports of investigations of safety and effectiveness, in addition to other information. The data in a full NDA is either owned by the applicant or are data for which the applicant has obtained a right of reference. A 505(b)(2) application
is one described under section 505(b)(2) of the FD&C Act as an application for which information, or one or more of the investigations relied upon by the applicant for approval "were not conducted by or for the applicant and for which the applicant has not obtained a right of reference or use from the person by or for whom the investigations were conducted". This provision permits the FDA to rely for approval of an NDA on data not developed by the applicant, such as published literature or the FDA's finding of safety and effectiveness of a previously approved drug. 505(b)(2) applications are submitted under section 505(b)(1) of the FD&C Act and are therefore subject to the same statutory provisions that govern 505(b)(1) applications that require among other things, "full reports" of safety and effectiveness. Our regulatory submission for Acurox® with Niacin Tablets and
King’s submission for Acurox ® Tablets were both accepted for filing by FDA as 505(b)(2) NDA’s.
Each NDA requires a user fee, pursuant to the requirements of the Prescription Drug User Fee Act (“PDUFA”), as amended. According to FDA’s fee schedule, effective on October 1, 2010, for the 2011 fiscal year, the user fee for an application fee requiring clinical data (such as an NDA) is $1,542,000. The FDA adjusts PDUFA user fees on an annual basis. PDUFA also imposes annual product and facility fees. The annual product fee for prescription drugs and biologics for the 2011 fiscal year is $86,520 and the annual facility fee for facilities used to manufacture prescription drugs and biologics for the 2011 fiscal year is $497,200. A written request can be submitted
for a waiver of the application fee for the first human drug application that is filed by a small business, but no waivers for product or establishment fees are available. Where we are subject to these fees, they are significant expenditures that may be incurred in the future and must be paid at the time of submission of each application to FDA. The King Agreement provides that King will reimburse us for the NDA application fee for Acurox® with Niacin Tablets and pay user fees for Acurox® with Niacin Tablets and all other products licensed by the Company to King pursuant to the King Agreement.
After an NDA is submitted by an applicant, and if it is accepted for filing by the FDA, the FDA will then review the NDA and, if and when it determines that the data submitted are adequate to show that the product is safe and effective for its intended use, the FDA will approve the product for commercial distribution in the U.S. There can be no assurance that any of our product candidates will receive FDA approval or that even if approved, they will be approved with labeling that includes descriptions of its abuse deterrent features. Moreover, even if our product candidates are approved with labeling that includes descriptions of the abuse deterrent features of our products, advertising and promotion
for the products will be limited to the specific claims and descriptions in the FDA approved product labeling.
The FDA requires drug manufacturers to establish and maintain quality control procedures for manufacturing, processing and holding drugs and investigational products, and products must be manufactured in accordance with defined specifications. Before approving an NDA, the FDA usually will inspect the facility(ies) at which the active pharmaceutical ingredients and finished drug product is manufactured, and will not approve the product unless it finds that cGMP compliance at those facility(ies) are satisfactory. If the FDA determines the NDA is not acceptable, the FDA may outline the deficiencies in the NDA and often will request additional information, thus delaying the approval of a
product. Notwithstanding the submission of any requested additional testing or information, the FDA ultimately may decide that the application does not satisfy the criteria for approval. After a product is approved, changes to the approved product, such as adding new indications, manufacturing changes, or changes in or additions to the approved labeling for the product, may require submission of a new NDA or, in some instances, an NDA amendment, for further FDA review. Post-approval marketing of products in larger or different patient populations than those that were studied during development can lead to new findings about the safety or efficacy of the products. This information can lead to a product sponsor’s requesting approval for and/or the FDA requiring changes in the labeling of the product or even the withdrawal of the product
from the market.
The Best Pharmaceuticals for Children Act, or BPCA, became law in 2002 and was subsequently reauthorized and amended by FDAAA. The reauthorization of BPCA provides an additional six months of patent protection to NDA applicants that conduct acceptable pediatric studies of new and currently-marketed drug products for which pediatric information would be beneficial, as identified by FDA in a Pediatric Written Request. The Pediatric Research Equity Act (“PREA”), became law in 2003, and was also subsequently reauthorized and amended by FDAAA. The reauthorization of PREA requires that most applications for drugs and biologics include a pediatric assessment (unless waived or deferred)
to ensure the drugs' and biologics' safety and effectiveness in children. Such pediatric assessment must contain data, gathered using appropriate formulations for each age group for which the assessment is required, that are adequate to assess the safety and effectiveness of the drug or the biological product for the claimed indications in all relevant pediatric subpopulations, and to support dosing and administration for each pediatric subpopulation for which the drug or the biological product is safe and effective. The pediatric assessments can only be deferred provided there is a timeline for the completion of such studies. FDA may waive (partially or fully) the pediatric assessment requirement for several reasons, including if the applicant can demonstrate that reasonable attempts to produce a pediatric formulation necessary for that age group have
failed. We and King requested deferral of the pediatric assessment in the NDA filings for Acurox® with Niacin Tablets and Acurox® Tablets.
19
The terms of approval of any NDA for our product candidates, including the indication and product labeling (and, consequently advertising and promotion) may be more restrictive than what is sought in the NDA or what is desired by us. Additionally, FDA may condition approval of abuse deterrence statements and claims for Acurox® with Niacin and Acurox® Tablets on phase IV clinical studies for continued assessment of such statements or claims. The testing and FDA approval process for our product candidates requires substantial time, effort, and financial resources, and we cannot be sure that any approval will be granted on a timely basis, if at all.
Further, as a condition of approval of any NDA, FDA may require a REMS to ensure the safe use and monitoring of any of our products. If required, a REMS may include, but may not be limited to, use of an FDA-approved Medication Guide and/or Patient Package Insert, a communication plan for patients or healthcare providers concerning the drug, a description of elements to assure safe use of the product, and a timetable for FDA’s assessment of the effectiveness of the REMS.
In addition, we, our suppliers and our licensees are required to comply with extensive FDA requirements both before and after approval. For example, we or our licensees are required to report certain adverse reactions and production problems, if any, to the FDA, and to comply with certain requirements concerning advertising and promotion for our products, which, as discussed above, may significantly affect the extent to which we can include statements or claims referencing our abuse deterrent technology in product labeling and advertising. Also, quality control and manufacturing procedures must continue to conform to cGMP after approval to avoid the product being rendered misbranded and/or
adulterated under the FD&C Act as a result of manufacturing problems. In addition, discovery of any material safety issues may result in changes to product labeling or restrictions on a product manufacturer, potentially including removal of the product from the market.
Whether or not FDA NDA approval in the U.S. has been obtained, approvals from comparable governmental regulatory authorities in foreign countries must be obtained prior to the commencement of commercial activities in those countries. The approval procedure varies in complexity from country to country, and the time required may be longer or shorter than that required for FDA approval.
Facilities for Research, Development, and Manufacturing
We conduct research, development, laboratory, clinical supplies manufacturing and related activities for product candidates utilizing our technologies at our Culver, Indiana facility. The 25,000 square foot facility is registered with the DEA to perform research, development and manufacture of certain DEA Scheduled active pharmaceutical ingredients and finished dosage form products. We obtain quotas for supply of DEA-scheduled active pharmaceutical ingredients from the DEA and develop finished dosage forms in our Culver facility. We manufacture clinical trial supplies of drug products in our Culver facility in volumes sufficient to meet FDA standards for
NDAs. King is responsible for commercial manufacture of the product candidates licensed under the King Agreement. We expect that future product candidates developed and licensed by us will be commercially manufactured by our licensees or other qualified third party contract manufacturers.
We expect to rely on contract manufacturers to manufacture, package and supply our commercial quantities of our Impede™ PSE Tablet product once commercialized. Initially, we intend to source our commercial requirements of Impede™ PSE Tablets from a single manufacturer and do not currently have a second source for this product. Although we believe that there are alternate sources of supply that can satisfy our commercial requirements, replacing a contract manufacturer may result in delays and additional costs.
Segment Reporting
We operate in one business segment; the research, development and manufacture of innovative abuse deterrent, orally administered pharmaceutical product candidates.
20
Environmental Compliance
We are subject to regulation under federal, state and local environmental laws and believe we are in material compliance with such laws. We incur the usual waste disposal cost associated with a pharmaceutical research, development and manufacturing operation.
Raw Materials
To purchase certain active ingredients required for our development and manufacture of product candidates utilizing our Aversion® Technology, we are required to file for and obtain supply quotas from the DEA. No assurance can be given that we will be successful in obtaining adequate DEA quotas in a timely manner. Even assuming adequate and timely DEA quotas, there can be no assurances that the approved manufacturers of raw materials for our product candidates will supply us with our requirements for the active or inactive ingredients required for the development and manufacture of our product candidates.
Subsidiaries
Our Culver, Indiana research, development, and manufacturing operations are conducted by Acura Pharmaceutical Technologies, Inc., an Indiana corporation and our wholly-owned subsidiary.
ITEM 1A. RISK FACTORS
Our future operating results may vary substantially from anticipated results due to a number of factors, many of which are beyond our control. The following discussion highlights some of these factors and the possible impact of these factors on future results of operations. If any of the following factors actually occur, our business, financial condition or results of operations could be materially harmed. In that case, the value of our common stock could decline substantially.
Risks Relating to Our Business and Industry
We have a history of operating losses and may not achieve profitability sufficient to generate a positive return on shareholders’ investment.
We had a net loss of $12.7 million for the year ended December 31, 2010, a net loss of $15.8 million for the year ended December 31, 2009 and net income of $14.5 million for the year ended December 31, 2008. Our future profitability will depend on several factors, including:
|
|
·
|
our receipt of milestone payments and royalties relating to products developed and commercialized under our license agreement with King (as more fully described under the caption “Item 1. Business — King Agreement”); and
|
|
|
·
|
the receipt of FDA approval and the successful commercialization by King and other future licensees (if any) of products utilizing our Aversion® and Impede™ Technologies without infringing the patents and other intellectual property rights of third parties.
|
We cannot assure you that we will ever have a product approved for commercialization by the FDA or that we or our licensees will bring any product to market.
We recognized revenues of $3.3 and $3.8 million in the years ended December 31, 2010 and 2009, respectively, from payments received under the King Agreement. However, we have not yet generated any revenues from Aversion® Technology product sales. Even if we succeed in commercializing one or more of our Aversion® Technology product candidates, we expect to continue using cash reserves for the foreseeable future. Our expenses may increase in the foreseeable future as a result of continued research and development of additional product candidates, maintaining and expanding the scope of our intellectual property, commercializing our Impede™ PSE product, and hiring of additional
research and development staff.
21
We will need to generate revenues from direct product sales or indirectly from royalties on sales to achieve and maintain profitability. If we cannot successfully develop, obtain regulatory approval for and commercialize our product candidates licensed to King under the King Agreement or other product candidates under similar license agreements anticipated to be negotiated and executed with other pharmaceutical companies, of which no assurance can be given, we will not be able to generate such royalty revenues or achieve future profitability. Our failure to achieve or maintain profitability would have a material adverse impact on the market price of our common stock.
We must rely on current cash reserves, technology licensing fees and third party financing to fund operations.
Pending the receipt of milestone payments and royalties, if any, under the King Agreement or under similar license agreements anticipated to be negotiated and executed with other pharmaceutical companies, of which no assurance can be given, we must rely on our current cash reserves, revenues from sales of our Impede™ PSE product, if any, and third-party financing to fund operations and product development activities. No assurance can be given that current cash reserves or revenues from Impede™ PSE product sales will be sufficient to fund the continued operations and development of our product candidates until such time as we generate additional revenue from the King Agreement or similar license
agreements anticipated to be negotiated and executed with the other pharmaceutical companies. Moreover, no assurance can be given that we will be successful in raising additional financing or, if funding is obtained, that such funding will be sufficient to fund operations until product candidates utilizing our Aversion® and Impede™ Technologies may be commercialized.
Our product candidates are unproven and may not be approved by the FDA.
We are committing a majority of our resources to the development of Acurox® Tablets and other product candidates utilizing our Aversion® and Impede™ Technologies. There can be no assurance that the FDA will ultimately approve Acurox® Tablets or any other product candidate utilizing Aversion® Technology for commercial distribution. Further there can be no assurance that other product candidates developed using Aversion® Technology or Impede™ Technology will achieve the targeted end points
in the required clinical studies or perform as intended in other pre-clinical and clinical studies or lead to a NDA submission or filing acceptance. Our failure to successfully develop and achieve final FDA approval of a product candidate utilizing Aversion® Technology will have a material adverse effect on our financial condition.
Even if the FDA Approves Acurox® Tablets for commercial distribution, if Acurox® Tablets are not approved with labeling describing its abuse deterrent features, we will be unable to refer to the abuse deterrent characteristics of Acurox® to promote the product.
Our strategy for Acurox® Tablets depends upon our ability to distinguish Acurox® Tablets from other immediate release oxycodone HCl containing products based primarily on abuse deterrent features. As with all of our product candidates utilizing Aversion® Technology, even if Acurox® Tablets are approved by the FDA, our failure to achieve approval of product labeling that sufficiently differentiates Acurox® Tablets from other immediate release oxycodone HCl containing tablets may adversely affect our business and results of operations. The FDA has publicly stated that explicit indications or claims of abuse deterrence will not be permitted unless such indications or claims are supported by double blind controlled clinical studies demonstrating an actual reduction in product abuse by patients or drug abusers. Because the cost, time and practicality of designing and implementing clinical studies adequate to support explicit claims of abuse deterrence are prohibitive, we are not pursuing and have not conducted the clinical trials necessary to include an explicit product label claim of abuse deterrence. Instead, we intend to rely on certain clinical and laboratory studies to support the inclusion of information about the abuse deterrent
features of Acurox® Tablets to support promotion by our licensee(s) of the product. We intend to include in the product labels of our product candidates both a physical description of the abuse deterrent features and information from laboratory and clinical studies designed to simulate the relative difficulty of abusing our product candidates. However, the extent to which such information will be included in the FDA approved product label will be the subject of our discussions with, and agreement by, the FDA as part of the NDA review process for each of our product candidates. The outcome of those discussions with the FDA will determine whether we will be able to market our product candidates with labeling that sufficiently differentiates them from other products that have
comparable therapeutic profiles. If the FDA does not approve the Acurox® Tablet labeling with such information, our licensee will not be able to promote Acurox® Tablets based on its abuse deterrent features, may not be able to differentiate Acurox® Tablets from other oxycodone HCl containing immediate release products, and may not be able to charge a premium above the price of such other products which could materially adversely affect our business and results of operations.
Because FDA closely regulates promotional materials and other promotional activities, even if FDA initially approves product labeling that includes a description of the abuse deterrent characteristics of our product, FDA may object to our or our licensee’s marketing claims and product advertising campaigns.
22
Relying on third party contract research organizations ("CROs") may result in delays in our pre-clinical, clinical or laboratory testing. If pre-clinical, clinical or laboratory testing for our product candidates are unsuccessful or delayed, we will be unable to meet our anticipated development and commercialization timelines.
To obtain FDA approval to commercially sell and distribute in the U.S. any of our prescription product candidates, we or our licensees must submit to the FDA an NDA demonstrating, among other things, that the product candidate is safe and effective for its intended use. This demonstration requires significant testing. As we do not possess the resources or employ all the personnel necessary to conduct such testing, we rely on CROs for the majority of this testing with our product candidates. As a result, we have less control over our development program than if we performed the testing entirely on our own. Third parties may not perform their responsibilities on our anticipated schedule. Delays in our development
programs could significantly increase our product development costs and delay product commercialization.
The commencement of clinical trials with our product candidates may be delayed for several reasons, including but not limited to delays in demonstrating sufficient pre-clinical safety required to obtain regulatory approval to commence a clinical trial, reaching agreements on acceptable terms with prospective licensees, manufacturing and quality assurance release of a sufficient supply of a product candidate for use in our clinical trials and/or obtaining institutional review board approval to conduct a clinical trial at a prospective clinical site. Once a clinical trial has begun, it may be delayed, suspended or terminated by us or regulatory authorities due to several factors, including ongoing discussions with
regulatory authorities regarding the scope or design of our clinical trials, failure to conduct clinical trials in accordance with regulatory requirements, lower than anticipated recruitment or retention rate of patients in clinical trials, inspection of the clinical trial operations or trial sites by regulatory authorities, the imposition of a clinical hold by FDA, lack of adequate funding to continue clinical trials, and/or negative or unanticipated results of clinical trials.
Clinical trials required by the FDA for commercial approval may not demonstrate safety or efficacy of our product candidates. Success in pre-clinical testing and early clinical trials does not assure that later clinical trials will be successful. Results of later clinical trials may not replicate the results of prior clinical trials and pre-clinical testing. Even if the results of our pivotal phase III clinical trials are positive, we and our licensees may have to commit substantial time and additional resources to conduct further pre-clinical and clinical studies before we or our licensees can submit NDAs or obtain regulatory approval for our product candidates.
Clinical trials are expensive and at times difficult to design and implement, in part because they are subject to rigorous regulatory requirements. Further, if participating subjects or patients in clinical studies suffer drug-related adverse reactions during the course of such trials, or if we, our licensees or the FDA believes that participating patients are being exposed to unacceptable health risks, we or our licensees may suspend the clinical trials. Failure can occur at any stage of the trials, and we or our licensees could encounter problems causing the abandonment of clinical trials or the need to conduct additional clinical studies, relating to a product candidate.
Even if our clinical trials and laboratory testing are completed as planned, their results may not support commercially viable product label claims. The clinical trial process may fail to demonstrate that our product candidates are safe and effective for their intended use. Such failure may cause us or our licensees to abandon a product candidate and may delay the development of other product candidates.
We have no commercial manufacturing capacity and rely on third-party contract manufacturers to produce our commercial quantities.
We do not have the facilities, equipment or personnel to manufacture commercial quantities of our product candidates and therefore must rely on our licensees or other qualified third party contract manufactures with appropriate facilities and equipment to contract manufacture commercial quantities of products utilizing our Aversion® and Impede™ Technologies. These licensees and third party contract manufacturers are also subject to cGMP regulations. Any performance failure on the part of our licensees or contract manufacturers could delay commercialization of any approved products, depriving us of potential product
revenue.
Our drug candidates, including our Impede™ PSE Tablets, require precise, high quality manufacturing. Failure by our contract manufacturers to achieve and maintain high manufacturing standards could result in patient injury or death, product recalls or withdrawals, delays or failures in testing or delivery, cost overruns, or other problems that could materially adversely affect our business. Contract manufacturers may encounter difficulties involving production yields, quality control, and quality assurance. These manufacturers are subject to ongoing periodic unannounced inspection by the FDA and corresponding state and foreign
agencies to ensure strict compliance with cGMP and other applicable government regulations; however, we do not have control over third-party manufacturers’ compliance with these regulations and standards.
23
If for some reason our contract manufacturers cannot perform as agreed, we may be required to replace them. Although we believe there are a number of potential replacements, we may incur added costs and delays in identifying and qualifying any such replacements. In addition, a new manufacturer would have to be educated in, or develop substantially equivalent processes for, production of our drug candidates.
We or our licensees may not obtain required FDA approval; the FDA approval process is time-consuming and expensive.
The development, testing, manufacturing, marketing and sale of pharmaceutical products are subject to extensive federal, state and local regulation in the United States and other countries. Satisfaction of all regulatory requirements typically takes years, is dependent upon the type, complexity and novelty of the product candidate, and requires the expenditure of substantial resources for research, development and testing. Substantially all of our operations are subject to compliance with FDA regulations. Failure to adhere to applicable FDA regulations by us or our licensees would have a material adverse effect on our operations and financial condition. In addition, in the event we are successful in developing
product candidates for distribution and sale in other countries, we would become subject to regulation in such countries. Such foreign regulations and product approval requirements are expected to be time consuming and expensive.
We or our licensees may encounter delays or rejections during any stage of the regulatory review and approval process based upon the failure of clinical or laboratory data to demonstrate compliance with, or upon the failure of the product candidates to meet, the FDA’s requirements for safety, efficacy and quality; and those requirements may become more stringent due to changes in regulatory agency policy or the adoption of new regulations. After submission of an NDA the FDA may refuse to file the application, deny approval of the application, require additional testing or data and/or require post-marketing testing and surveillance to monitor the safety or efficacy of a product. The FDA commonly takes more
than a year to grant final approval for an NDA. The Prescription Drug User Fee Act (“PDUFA”) sets time standards for FDA’s review of NDA’s. The FDA's timelines described in the PDUFA guidance are flexible and subject to change based on workload and other potential review issues and may delay the FDA’s review of an NDA. Further, the terms of approval of any NDA, including the product labeling, may be more restrictive than we or our licensees desire and could affect the marketability of our products.
Even if we comply with all the FDA regulatory requirements, we or our licensees may never obtain regulatory approval for any of our product candidates. If we or our licensees fail to obtain regulatory approval for any of our product candidates, we will have fewer commercialized products and correspondingly lower revenues. Even if regulatory approval of our products is received, such approval may involve limitations on the indicated uses or promotional claims we or our licensees may make for our products, or otherwise not permit labeling that sufficiently differentiates our product candidates from competitive products with comparable therapeutic profiles but without abuse deterrent features. Such events
would have a material adverse effect on our operations and financial condition. We may market certain of our product without the prior application to and approval by the FDA. The FDA may subsequently require us to withdraw such products and submit NDA’s for approval prior to re-marketing.
The FDA also has the authority to revoke or suspend approvals of previously approved products for cause, to debar companies and individuals from participating in the drug-approval process, to request recalls of allegedly violative products, to seize allegedly violative products, to obtain injunctions to close manufacturing plants allegedly not operating in conformity with current Good Manufacturing Practices (“cGMP”) and to stop shipments of allegedly violative products. In the event the FDA takes any such action relating to our products (if any are approved by FDA), such actions would have a material adverse effect on our operations and financial condition.
24
We must maintain FDA approval to manufacture clinical supplies of our product candidates at our facility; failure to maintain compliance with FDA requirements may prevent or delay the manufacture of our product candidates and costs of manufacture may be higher than expected.
We have installed the equipment necessary to manufacture clinical trial supplies of our Aversion® and Impede™ Technology product candidates in tablet formulations at our Culver, Indiana facility. To be used in clinical trials, all of our product candidates must be manufactured in conformity with cGMP regulations. All such product candidates must be manufactured, packaged, and labeled and stored in accordance with cGMPs. Modifications, enhancements or changes in manufacturing sites of marketed products are, in many circumstances, subject to FDA approval, which may be subject to a lengthy application process or which we may be unable to obtain. Our Culver, Indiana facility, and those of any third-party
manufacturers that we or our licensees may use, are periodically subject to inspection by the FDA and other governmental agencies, and operations at these facilities could be interrupted or halted if the FDA deems such inspections are unsatisfactory. Failure to comply with FDA or other governmental regulations can result in fines, unanticipated compliance expenditures, recall or seizure of products, total or partial suspension of production or distribution, suspension of FDA review of our product candidates, termination of ongoing research, disqualification of data for submission to regulatory authorities, enforcement actions, injunctions and criminal prosecution.
We develop our products, and manufacture clinical supplies, at a single location. Any disruption at this facility could adversely affect our business and results of operations.
We rely on our Culver, Indiana facility for developing our product candidates and the manufacture of clinical supplies of our product candidates. If the Culver, Indiana facility were damaged or destroyed, or otherwise subject to disruption, it would require substantial lead-time to repair or replace. If our Culver facility were affected by a disaster, we would be forced to rely entirely on CROs and third party contract manufacturers while repairs were being made. Although we believe we possess adequate insurance for damage to our property and for the disruption of our business from casualties, such insurance may not be sufficient to cover all of our potential losses and may not
continue to be available to us on acceptable terms, or at all. Moreover, any disruptions or delays at our Culver, Indiana facility could impair our ability to develop our product candidates utilizing the Aversion® or Impede™ Technologies, which could adversely affect our business and results of operations.
Our operations are subject to environmental, health and safety, and other laws and regulations, with which compliance is costly and which exposes us to penalties for non-compliance.
Our business, properties and product candidates are subject to federal, state and local laws and regulations relating to the protection of the environment, natural resources and worker health and safety and the use, management, storage and disposal of hazardous substances, waste and other regulated materials. Because we own and operate real property, various environmental laws also may impose liability on us for the costs of cleaning up and responding to hazardous substances that may have been released on our property, including releases unknown to us. These environmental laws and regulations also could require us to pay for environmental remediation and response costs at third-party
locations where we dispose of or recycle hazardous substances. The costs of complying with these various environmental requirements, as they now exist or may be altered in the future, could adversely affect our financial condition and results of operations.
If our licensees do not satisfy their obligations, we will be unable to develop our licensed product candidates.
On October 30, 2007, we entered into an Agreement with King (as more fully described under the caption “Item 1. Business – King Agreement”). At December 31, 2010 we had received aggregate payments of $58.3 million from King, consisting of a $30.0 million non-refundable upfront cash payment, $17.3 million in reimbursed research and development expenses relating to our licensed product candidates, $6.0 million in option exercise fees relating to King’s exercise of its option to license an undisclosed opioid analgesic tablet product and Vycavert® with Niacin Tablets, and a $5.0 million
milestone fee relating to our successful achievement of the primary end points for our pivotal Phase III clinical study for Acurox® with Niacin Tablets . Our future revenue, if any, will be derived from milestone payments and royalties under the King Agreement and under similar license agreements anticipated to be potentially negotiated and executed with other pharmaceutical companies. No assurance can be given that we will receive the milestone and royalty payments provided for in the King Agreement, or that we will be successful in entering into similar agreements with other pharmaceutical companies to develop and commercialize products utilizing our Aversion® or Impede™ Technologies.
25
As part of such license agreements, we will not have day-to-day control over the activities of our licensees with respect to any product candidate. If a licensee fails to fulfill its obligations under an agreement with us, we may be unable to assume the development of the product candidate covered by that agreement or to enter into alternative arrangements with another third-party. In addition, we may encounter delays in the commercialization of the product candidate that is the subject of a license agreement. Accordingly, our ability to receive any revenue from the product candidates covered by such agreements will be dependent on the efforts of our licensee. We could be involved in disputes with a
licensee, which could lead to delays in or termination of, our development and commercialization programs and result in time consuming and expensive litigation or arbitration. In addition, any such dispute could diminish our licensee’s commitment to us and reduce the resources they devote to developing and commercializing our products. If any licensee terminates or breaches its agreement, or otherwise fails to complete its obligations in a timely manner, our chances of successfully developing or commercializing our product candidates would be materially adversely effected. Additionally, due to the nature of the market for our product candidates, it may be necessary for us to license all or a significant portion of our product candidates to a single company thereby eliminating our opportunity to commercialize other product candidates with other licensees.
If we fail to maintain our license agreement with King, we may have to reduce or delay our product candidate development.
Our plan for developing, manufacturing and commercializing Acurox® Tablets and other opioid analgesic product candidates utilizing our Aversion® Technology currently requires us to successfully maintain our license agreement with King. In addition to other customary termination provisions, the King Agreement provides that King may terminate the King Agreement at any time upon written notice to us. If King elects to terminate the King Agreement, or if we are otherwise unable to maintain our existing relationship with King, we may have to limit the size or scope of, or delay or abandon the development of, Acurox® Tablets (with and without niacin) and other opioid analgesic product
candidates or undertake and fund development of these product candidates ourselves. If we were required to fund development and commercialization efforts with respect to Acurox® Tablets and other opioid analgesic product candidates on our own, we may need to obtain additional financing, which may not be available on acceptable terms, or at all.
If King is not successful in commercializing Acurox® Tablets (with or without niacin) and other licensed product candidates incorporating the Aversion® Technology our revenues and our business will suffer.
Pursuant to the King Agreement, King is responsible for manufacturing, marketing, pricing, promoting, selling, and distributing certain of our product candidates in the US, Canada and Mexico. If such agreement is terminated in accordance with its terms, including due to a party’s failure to perform its obligations or responsibilities under the agreement, then we would need to commercialize the products ourselves for which we currently have no infrastructure or alternatively enter into a new agreement with another pharmaceutical company, of which no assurance can be given. In this event our revenues and/or royalties for these products could be adversely impacted.
King’s manufacturing facility is currently the sole commercial source of supply for Acurox® Tablets and our other product candidates licensed to King. If King’s manufacturing facility fails to obtain sufficient DEA quotas for the opioid active ingredients contained in such product candidates, fails to source adequate quantities of active and inactive ingredients, fails to comply with regulatory requirements, or otherwise experiences disruptions in commercial supply of our product candidates, product revenue and our royalties could be adversely impacted.
King has a diversified product line for which Acurox® Tablets and our other product candidates licensed to King will vie for King’s promotional, marketing, and selling resources. If King fails to commit sufficient promotional, marketing and selling resources to our products, product revenue and our royalties could be adversely impacted. Additionally, in view of Pfizer, Inc.’s recent acquisition of King Pharmaceuticals in February, 2011, there can be no assurance that Pfizer will commit the resources required for the successful development and commercialization of our product
candidates.
The market for our opioid product candidates is highly competitive with many marketed non abuse deterrent brand and generic products and other abuse deterrent product candidates in development. If King prices our product candidates inappropriately, fails to position our products properly, targets inappropriate physician specialties, or otherwise does not provide sufficient promotional support, product revenue and our royalties could be adversely impacted.
The market may not be receptive to products incorporating our Aversion® Technology.
The commercial success of our products will depend on acceptance by health care providers and others that such products are clinically useful, cost-effective and safe. There can be no assurance given that our products utilizing the Aversion® or Impede™ Technologies would be accepted by health care providers and others. Factors that may materially affect market acceptance of our product candidates include but are not limited to:
26
|
|
·
|
the relative advantages and disadvantages of our products compared to competitive products;
|
|
|
·
|
the relative timing to commercial launch of our products compared to competitive products;
|
|
|
·
|
the relative safety and efficacy of our products compared to competitive products;
|
|
|
·
|
the product labeling approved by the FDA for our products;
|
|
·
|
the willingness of third party payers to reimburse for our prescription products; and
|
|
·
|
the willingness of consumers to pay for our products.
|
Our product candidates, if successfully developed and commercially launched, will compete with both currently marketed and new products launched in the future by other companies. Health care providers may not accept or utilize any of our products. Physicians and other prescribers may not be inclined to prescribe our products unless our products demonstrate commercially viable advantages over other products currently marketed for the same indications. Consumers may not be willing to purchase our products. If our products do not achieve market acceptance, we may not be able to generate significant revenues or become profitable.
If we, our licensees or others identify serious adverse events or deaths relating to any of our products once on the market, we may be required to withdraw our products from the market, which would hinder or preclude our ability to generate revenues.
We or our licensees are required to report to relevant regulatory authorities all serious adverse events or deaths involving our product candidates or approved products. If we, our licensees, or others identify such events, regulatory authorities may withdraw their approvals of such products; we or our licensees may be required to reformulate our products; we or our licensees may have to recall the affected products from the market and may not be able to reintroduce them onto the market; our reputation in the marketplace may suffer; and we may become the target of lawsuits, including class actions suits. Any of these events could harm or prevent sales of the affected products and could
materially adversely affect our business and financial condition.
In the event that we or our licensees are successful in bringing any products to market, our revenues may be adversely affected if we fail to obtain insurance coverage or adequate reimbursement for our products from third-party payers.
The ability of our licensees to successfully commercialize our products may depend in part on the availability of reimbursement for our prescription products from government health administration authorities, private health insurers, and other third-party payers and administrators, including Medicaid and Medicare. We cannot predict the availability of reimbursement for newly-approved products utilizing our Aversion® Technology. Third-party payers and administrators, including state Medicaid programs and Medicare, are challenging the prices charged for pharmaceutical products. Government and other third-party payers increasingly are limiting both coverage and the level of reimbursement for new
drugs. Third-party insurance coverage may not be available to patients for any of our products candidates. The continuing efforts of government and third-party payers to contain or reduce the costs of health care may limit our commercial opportunity. If government and other third-party payers do not provide adequate coverage and reimbursement for any product utilizing our Aversion® Technology, health care providers may not prescribe them or patients may ask their health care providers to prescribe competing products with more favorable reimbursement. In some foreign markets, pricing and profitability of pharmaceutical products are subject to government control. In the United States, we expect there may be federal and state proposals for similar controls. In addition, we expect that increasing emphasis on managed care in the United States will continue to put pressure on
the pricing of pharmaceutical products. Cost control initiatives could decrease the price that we or our licensees charge for any of our products in the future. Further, cost control initiatives could impair our ability or the ability of our licensees to commercialize our products and our ability to earn revenues from commercialization.
Consolidation in the healthcare industry could lead to demands for price concessions or to the exclusion of some suppliers from certain of our markets, which could have an adverse effect on our business, financial condition or results of operations.
Because healthcare costs have risen significantly, numerous initiatives and reforms by legislatures, regulators and third-party payers to curb these cost increases have resulted in a trend in the healthcare industry to consolidate product suppliers and purchasers. As the healthcare industry consolidates, competition among suppliers to provide products to purchasers has become more intense. This in turn has resulted and will likely continue to result in greater pricing pressures and the exclusion of certain suppliers from important market segments as group purchasing organizations, and large single accounts continue to use their market power to influence product pricing and purchasing
decisions. We expect that market demand, government regulation, third-party reimbursement policies and societal pressures will continue to influence the worldwide healthcare industry, resulting in further business consolidations, which may exert further downward pressure on the prices of our anticipated products. This downward pricing pressure may adversely impact our business, financial condition or results of operations.
27
Our success depends on our ability to protect our intellectual property.
Our success depends on our ability to obtain and maintain patent protection for products developed utilizing our technologies, in the United States and in other countries, and to enforce these patents. The patent positions of pharmaceutical firms, including us, are generally uncertain and involve complex legal and factual questions. Notwithstanding our receipt of U.S. Patent No. 7,201,920, U.S. Patent No. 7,476,402 and U.S. Patent No. 7,510,726 from the United States Patent and Trademark Office (“USPTO”) encompassing our opioid product candidates utilizing our Aversion® Technology, there is no assurance that any of our patent claims in our
other pending non-provisional and provisional patent applications relating to our technologies will issue or if issued, that any such patent claims will be valid and enforceable against third-party infringement or that our products will not infringe any third-party patent or intellectual property. Moreover, any patent claims relating to our technologies may not be sufficiently broad to protect our products. In addition, issued patent claims may be challenged, potentially invalidated or potentially circumvented. Our patent claims may not afford us protection against competitors with similar technology or permit the commercialization of our products without infringing third-party patents or other intellectual property rights.
Our success also depends on our not infringing patents issued to others. We may become aware of patents belonging to competitors and others that could require us to obtain licenses to such patents or alter our technologies. Obtaining such licenses or altering our technology could be time consuming and costly. We may not be able to obtain a license to any technology owned by or licensed to a third party that we or our licensees require to manufacture or market one or more of our products. Even if we can obtain a license, the financial and other terms may be disadvantageous.
Our success also depends on maintaining the confidentiality of our trade secrets and know-how. We seek to protect such information by entering into confidentiality agreements with employees, potential licensees, raw material suppliers, contract research organizations, contract manufacturers, potential investors, consultants and other parties. These agreements may be breached by such parties. We may not be able to obtain an adequate, or perhaps, any remedy to such a breach. In addition, our trade secrets may otherwise become known or be independently developed by our competitors. Our inability to protect our intellectual property or to commercialize our products without infringing third-party patents or other
intellectual property rights would have a material adverse affect on our operations and financial condition.
We may become involved in patent litigation or other intellectual property proceedings relating to our Aversion® or Impede™ Technologies or product candidates which could result in liability for damages or delay or stop our development and commercialization efforts.
The pharmaceutical industry has been characterized by significant litigation and other proceedings regarding patents, patent applications and other intellectual property rights. The situations in which we may become parties to such litigation or proceedings may include:
|
|
·
|
litigation or other proceedings we may initiate against third parties to enforce our patent rights or other intellectual property rights;
|
|
|
·
|
litigation or other proceedings we may initiate against third parties seeking to invalidate the patents held by such third parties or to obtain a judgment that our products do not infringe such third parties’ patents;
|
|
|
·
|
litigation or other proceedings third parties may initiate against us to seek to invalidate our patents or to obtain a judgment that third party products do not infringe our patents;
|
28
|
|
·
|
if our competitors file patent applications that claim technology also claimed by us, we may be forced to participate in interference or opposition proceedings to determine the priority of invention and whether we are entitled to patent rights on such invention; and
|
|
|
·
|
if third parties initiate litigation claiming that our products infringe their patent or other intellectual property rights, we will need to defend against such proceedings.
|
The costs of resolving any patent litigation or other intellectual property proceeding, even if resolved in our favor, could be substantial. Many of our potential competitors will be able to sustain the cost of such litigation and proceedings more effectively than we can because of their substantially greater resources. Uncertainties resulting from the initiation and continuation of patent litigation or other intellectual property proceedings could have a material adverse effect on our ability to compete in the marketplace. Patent litigation and other intellectual property proceedings may also consume significant management time.
Our technologies or products may be found to infringe claims of patents owned by others. If we determine or if we are found to be infringing a patent held by another party, we, our suppliers or our licensees might have to seek a license to make, use, and sell the patented technologies and products. In that case, we, our suppliers or our licensees might not be able to obtain such license on acceptable terms, or at all. The failure to obtain a license to any third party technology that may be required would materially harm our business, financial condition and results of operations. If a legal action is brought against us, we could incur substantial defense costs, and any such action might not be resolved in our
favor. If such a dispute is resolved against us, we may have to pay the other party large sums of money and use of our technology and the testing, manufacturing, marketing or sale of one or more of our products could be restricted or prohibited. Even prior to resolution of such a dispute, use of our technology and the testing, manufacturing, marketing or sale of one or more of our products could be restricted or prohibited.
We are aware of certain United States and international pending patent applications owned by third parties with claims potentially encompassing our product candidates. While we do not expect the claims contained in such pending patent applications will issue in their present form, there can be no assurance that such patent applications will not issue as patents with claims encompassing one or more of our product candidates. If such patent applications result in valid and enforceable issued patents, containing claims in their current form or otherwise encompassing our products we or our licensees may be required to obtain a license to such patents, should one be available, or alternatively, alter our
products so as to avoid infringing such third-party patents. If we or our licensees are unable to obtain a license on commercially reasonable terms, or at all, we or our licensees could be restricted or prevented from commercializing our products. Additionally, any alterations to our products or our technologies could be time consuming and costly and may not result in technologies or products that are non-infringing or commercially viable.
We cannot assure you that our technologies, products and/or actions in developing our products will not infringe third-party patents. Our failure to avoid infringing third-party patents and intellectual property rights in the development and commercialization of our products would have a material adverse affect on our operations and financial condition.
We may be exposed to product liability claims and may not be able to obtain adequate product liability insurance.
Our business exposes us to potential product liability risks, which are inherent in the testing, manufacturing, marketing and sale of pharmaceutical products. Product liability claims might be made by patients, or health care providers or others that sell or consume our products. These claims may be made even with respect to those products that possess regulatory approval for commercial sale. We are currently covered by clinical trial product liability insurance on a claims-made basis. This coverage may not be adequate to cover any product liability claims. Product liability coverage is expensive. In the future, we may not be able to maintain or obtain such product liability insurance at a reasonable
cost or in sufficient amounts to protect us against losses due to product liability claims. Any claims that are not covered by product liability insurance could have a material adverse effect on our business, financial condition and results of operations. Reference is made to the discussion under the caption “Item 3 – Legal Proceedings – Reglan®/Metoclopramide Litigation” for a discussion of pending product liability litigation filed against the Company in each of Pennsylvania, New Jersey and California.
The pharmaceutical industry is characterized by frequent litigation. Those companies with significant financial resources will be better able to bring and defend any such litigation. No assurance can be given that we would not become involved in such litigation. Such litigation may have material adverse consequences to our financial condition and results of operations.
29
We face significant competition which may result in others developing or commercializing products before or more successfully than we do.
The pharmaceutical industry is highly competitive and is affected by new technologies, governmental regulations, health care legislation, availability of financing, litigation and other factors. If our product candidates receive FDA approval, they will compete with a number of existing and future drug products and therapies developed, manufactured and marketed by others. Existing or future competing products may provide greater therapeutic convenience, clinical or other benefits for a specific indication than our products, or may offer comparable performance at lower costs. If our products are unable to capture and maintain market share, we or our licensees may not achieve significant product revenues and our
financial condition and results of operations will be materially adversely affected.
We or our licensees will compete for market share against fully integrated pharmaceutical companies or other companies that collaborate with larger pharmaceutical companies, academic institutions, government agencies and other public and private research organizations. Many of these competitors have products already approved, marketed or in development. In addition, many of these competitors, either alone or together with their collaborative partners, operate larger research and development programs, have substantially greater financial resources, experience in developing products, obtaining FDA and other regulatory approvals, formulating and manufacturing drugs, and commercializing products than we
do.
We are concentrating a substantial majority of our efforts and resources on developing product candidates utilizing our Aversion® and Impede™ Technologies. The commercial success of products utilizing such technologies will depend, in large part, on the intensity of competition, FDA approved product labeling for our products compared to competitive products, and the relative timing and sequence for commercial launch of new products by other companies developing, marketing, selling and distributing products that compete with the products utilizing our Aversion® and Impede™ Technologies. Alternative technologies and non-opioid products are being developed to improve or replace the
use of opioid analgesics. In the event that such alternatives to opioid analgesics are widely adopted, then the market for products utilizing our Aversion® and Impede™ Technologies may be substantially decreased thus reducing our ability to generate future profits.
Key personnel are critical to our business and our success depends on our ability to retain them.
We are dependent on our management and scientific team, including Andrew D. Reddick, our President and Chief Executive Officer, Robert Jones, our Senior Vice President and Chief Operating Officer, and Albert W. Brzeczko, Ph.D., our Vice President of Technical Affairs. We may not be able to attract and retain personnel on acceptable terms given the competition for such personnel among biotechnology, pharmaceutical and healthcare companies, universities and non-profit research institutions. While we have employment agreements with certain employees, all of our employees are at-will employees who may terminate their employment at any time. We do not have
key personnel insurance on any of our officers or employees. The loss of any of our key personnel, or the inability to attract and retain such personnel, may significantly delay or prevent the achievement of our product and technology development and business objectives and could materially adversely affect our business, financial condition and results of operations.
The U.S. Drug Enforcement Administration (“DEA”) limits the availability of the active ingredients used in our product candidates and, as a result, our quota may not be sufficient to complete clinical trials or may result in development delays.
The DEA regulates certain finished drug products and active pharmaceutical ingredients, including certain opioid active pharmaceutical ingredients and pseudoephedrine HCl in our current product candidates. Consequently, their manufacture, research, shipment, storage, sale and use are subject to a high degree of regulation. Furthermore, the amount of active ingredients we can obtain for our clinical trials is limited by the DEA and our quota may not be sufficient to complete clinical trials. There is a risk that DEA regulations may interfere with the supply of the products used in our clinical trials.
30
Prior ownership changes limit our ability to use our tax net operating loss carryforwards.
Significant equity restructuring often results in an Internal Revenue Section 382 ownership change that limits the future use of Net Operating Loss (“NOL”) carryforwards and other tax attributes. We have determined that an ownership change (as defined by Section 382 of the Internal Revenue Code) did occur as a result of restructuring that occurred in 2004. Neither the amount of our NOL carryforwards nor the amount of limitation of such carryforwards claimed by us have been audited or otherwise validated by the Internal Revenue Service, which could challenge the amount we have calculated. The recognition and measurement of our tax benefit includes estimates and judgment by
our management, which includes subjectivity. Changes in estimates may create volatility in our tax rate in future periods based on new information about particular tax positions that may cause management to change its estimates.
Risks Relating to Our Common Stock
Our quarterly results of operations will fluctuate, and these fluctuations could cause our stock price to decline.
Our quarterly and annual operating results are likely to fluctuate in the future. These fluctuations could cause our stock price to decline. The nature of our business involves variable factors, such as the timing of the research, development and regulatory submissions of our product candidates that could cause our operating results to fluctuate. The forecasting of the timing of sales of our product candidates is difficult due to the uncertainty inherent in seeking FDA and other necessary approvals for our product candidates. As a result, in some future quarters or years our clinical, financial or operating results may not meet the expectations of securities analysts and investors which
could result in a decline in the price of our stock.
Volatility in stock prices of other companies may contribute to volatility in our stock price.
The market price of our common stock, like the market price for securities of pharmaceutical and biotechnology companies, has historically been highly volatile. The stock market from time to time experiences significant price and volume fluctuations unrelated to the operating performance of particular companies. Factors, such as fluctuations in our operating results, future sales of our common stock, announcements of technological innovations or new therapeutic products by us or our competitors, announcements regarding collaborative agreements, laboratory or clinical trial results, government regulation, FDA determinations on the approval of a product candidate NDA submission, developments in patent or other
proprietary rights, public concern as to the safety of drugs developed by us or others, changes in reimbursement policies, comments made by securities analysts and general market conditions may have a substantial effect on the market price of our common stock. In the past, following periods of volatility in the market price of a company’s securities, securities class action litigation and shareholder derivative litigation has often been instituted. A securities class action suit or shareholder derivative suit against us could result in substantial costs, potential liabilities and the diversion of management’s attention and resources and result in a material adverse affect on our financial condition and results of operations. Reference is made to the discussion under the caption “Item 3 –Legal Proceedings – Securities Class Action and Derivative
Litigation” for a discussion of a pending securities class action litigation filed against us in the United States District Court for the Northern District of Illinois, Eastern Division, and three shareholder derivative suits filed in the Circuit Court of Cook County, Illinois, Chancery Division.
Our stock price has been volatile and there may not be an active, liquid trading market for our common stock.
Our stock price has experienced significant price and volume fluctuations and may continue to experience volatility in the future. Factors that have a material impact on the price of our common stock, in addition to the other issues described herein, include results of or delays in our pre-clinical and clinical studies, any delays in, or failure to receive FDA approval of our product candidates, the success of our license agreement with King, announcements of technological innovations or new commercial products by us or others, developments in patents and other proprietary rights by us or others, future sales of our common stock by existing stockholders, regulatory developments or changes in regulatory
guidance, the departure of our officers, directors or key employees, and period-to-period fluctuations in our financial results. Also, you may not be able to sell your shares at the best market price if trading in our stock in not active or if the volume is low. There is no assurance that an active trading market for our common stock will be maintained on the NASDAQ Capital Market.
31
The National Association of Securities Dealers, Inc., or NASD, and the Securities and Exchange Commission, or SEC, have adopted rules relating to the listing of publicly traded stock. If we were unable to continue to comply with such rules, we could be delisted from trading on the NASDAQ Capital Market and thereafter trading in our common stock, if any, would be conducted through the Over-the-Counter Bulletin Board of the NASD. As a consequence of such delisting, an investor would likely find it more difficult to dispose of, or to obtain quotations as to the price of, our common stock. Delisting of our common stock from the NASDAQ Capital Market could also result in lower prices per share of our common stock than
would otherwise prevail.
We do not have a history of paying dividends on our common stock.
Historically we have not declared and paid any cash dividends on our common stock. We intend to retain all of our earnings for the foreseeable future to finance the operation and expansion of our business. As a result, you may only receive a return on your investment in our common stock if the market price of our common stock increases.
GCE Holdings LLC can control all matters requiring approval by shareholders.
GCE Holdings LLC beneficially owns approximately 74.7% of our outstanding common stock as of December 31, 2010 (calculated in accordance with Rule 13d-3 promulgated under the Securities Exchange Act of 1934, as amended). As a result, GCE Holdings LLC, in view of its ownership percentage of our common stock, will be able to control all matters requiring approval by our shareholders, including the approval or rejection of mergers, sales or licenses of all or substantially all of our assets, or other business combination transactions. The interests of GCE Holdings LLC may not always coincide with the interests of our other shareholders and as such we may take action in advance of its interests to the
detriment of our other shareholders. Accordingly, you may not be able to influence any action we take or consider taking, even if it requires a shareholder vote.
We are currently a “Controlled Company” within the meaning of the NASDAQ Capital Market Listing Requirements and, as a result, are exempt from certain corporate governance requirements.
Because GCE Holdings LLC controls more than 50% of the voting power of our common stock, we are currently considered to be a “controlled company” for purposes of a NASDAQ Capital Market listing requirements. As such, we are permitted, and have elected, to opt out of the NASDAQ Capital Market listing requirements that would otherwise require our board of directors to have a majority of independent directors, our board nominations to be selected, or recommended for the board’s selection either by a nominating committee comprised entirely of independent directors or by a majority of independent directors, and our compensation committee to be comprised entirely of independent
directors. Accordingly, you may not have the same protections afforded to stockholders of companies that are subject to all of the NASDAQ Capital Market corporate governance requirements.
Any future sale of a substantial number of shares included in our current registration statement could depress the trading price of our stock, lower our value and make it more difficult for us to raise capital.
In accordance with the terms of the Securities Purchase Agreement dated August 20, 2007 between us and the investors named therein, we filed a registration statement with the SEC to register the shares included in our Units issued pursuant to the Securities Purchase Agreement, including shares underlying warrants included in the Units. In addition, pursuant to the exercise of previously granted piggyback registration rights, each of GCE Holdings, LLC, Galen Partners III, L.P., Galen Partners International III, L.P., Galen Employee Fund III, L.P., Care Capital Investments II, LP, Care Capital Offshore Investments II, LP and Essex Woodlands Health Ventures V, L.P. have exercised their piggyback
registration rights to include an aggregate of 26,584,016 shares in such registration statement. As a result, 34,243,273 shares (representing approximately 74.7% of our shares outstanding on a fully-diluted basis – including all derivative securities, whether or not currently exercisable) are included in the registration statement for resale by selling stockholders. Such registration statement was declared effective by the SEC on November 20, 2007. If some or all of such shares included in such registration statement are sold by our affiliates and others it may have the effect of depressing the trading price of our common stock. In addition, such sales could lower our value and make it more difficult for us to raise capital if needed in the future.
32
ITEM 1B. UNRESOLVED STAFF COMMENTS
The Company has received no written comments regarding periodic or current reports from the staff of the SEC that were issued 180 days or more preceding the end of its 2010 fiscal year that remain unresolved.
ITEM 2. PROPERTIES
We lease from an unaffiliated Lessor, approximately 1,600 square feet of administrative office space at 616 N. North Court, Suite 120, Palatine, Illinois 60067. The lease agreement has a term expiring March 31, 2012. The lease agreement provides for rent, property taxes, common area maintenance, and janitorial services on an annualized basis of approximately $29,000 per year. We utilize this lease space for our administrative, marketing and business development functions.
We conduct research, development, laboratory, development scale and NDA submission batch scale manufacturing and other activities relating to developing product candidates using Aversion® and Impede™ Technologies at our facility located at 16235 State Road 17, Culver, Indiana. At this location, our wholly-owned subsidiary Acura Pharmaceutical Technologies, Inc., owns a 25,000 square foot facility with 7,000 square feet of warehouse, 8,000 square feet of manufacturing space, 4,000 square feet of research and development labs and 6,000 square feet of administrative and storage space. The facility is located on 28 acres of land.
ITEM 3. LEGAL PROCEEDINGS
Securities Class Action and Derivative Litigation
A lawsuit captioned Bang v. Acura Pharmaceuticals, et al, was filed on September 10, 2010 in the United States District Court for the Northern District of Illinois, Eastern Division (Case 1:10-cv-05757) against us and certain of our current and former officers seeking unspecified damages on behalf of a putative class of persons who purchased our common stock between February 21, 2006 and April 22, 2010. The complaint alleges that certain of our officers made false or misleading statements, or failed to disclose material facts in order to make statements not misleading, relating to our Acurox® with Niacin Tablets product candidate, resulting in
violations of Section 10(b) of the Securities Exchange Act of 1934 (the “Exchange Act”), Rule 10b-5 under the Exchange Act and Section 20(a) of the Exchange Act. The complaint further alleges that such false or misleading statements or omissions had the effect of artificially inflating the price of our common stock. On January 11, 2011, the Court appointed a group of three stockholders as Lead Plaintiffs. The Lead Plaintiffs have not yet filed their amended complaint. We believe that the allegations in the complaint are without merit and intend to vigorously defend the litigation.
On October 25, 2010, Kiley Hill, a purported stockholder of the Company filed a shareholder derivative action in the Circuit Court of Cook County, Illinois, Chancery Division captioned Hill v. Acura Pharmaceuticals et al. (Case No. 2010-CH-46380), against our directors and certain of our executive officers, generally relating to the same events that are the subject of the class action litigation described above. The complaint purports
to be brought on our behalf and names us as a nominal defendant. The complaint seeks unspecified damages from the individual defendants for breaches of fiduciary duty, abuse of control, gross mismanagement, contribution and indemnification, waste of corporate assets and unjust enrichment for actions occurring from at least February 21, 2006 through April 22, 2010. Substantively similar complaints captioned Hagan v. Acura Pharmaceuticals et al. (Case No. 2010-CH-46621) and Newell v. Reddick et al (Case No. 2010-CH-46873) were filed on October 27, 2010 and October 28, 2010, respectively, in the Circuit Court of Cook County,
Illinois, Chancery Division, by other purported stockholders of the Company. We have agreed to a temporary stay of these actions.
Reglan®/Metoclopramide Litigation
Halsey Drug Company, as predecessor to the Company, has been named along with numerous other companies as a defendant in cases filed in three separate state coordinated litigations pending in Pennsylvania, New Jersey and California, respectively captioned In re: Reglan® /Metoclopramide Mass Tort Litigation, Philadelphia County Court of Common Pleas, January Term, 2010, No. 01997; In re: Reglan® Litigation, Superior Court of New Jersey, Law Division, Atlantic County, Case No. 289, Master Docket No. ATL-L-3865-10; and Reglan®/Metoclopramide Cases, Superior Court of California, San Francisco County, Judicial Council Coordination Proceeding No. 4631, Superior Court No.: CJC-10-004631.
In this product liability litigation against numerous pharmaceutical product manufacturers and distributors, including the Company, plaintiffs claim injuries from their use of the Reglan® brand of metoclopramide and generic metoclopramide. In the Pennsylvania state court mass tort proceeding, over 50 lawsuits have been filed against the Company and Halsey Drug Company alleging that Plaintiffs developed neurological disorders as a result of their use of the Reglan® brand and/or generic metoclopramide. Plaintiffs have not yet served any individual lawsuits upon the Company in the New Jersey and California actions. In the lawsuits filed to date, Plaintiffs have not confirmed they ingested any of the generic metoclopramide manufactured by the Company. The Company discontinued manufacture and distribution of generic metoclopramide more than 15 years
ago. The Company believes these claims are without merit and will vigorously defend these actions.
33
ITEM 4. (RESERVED)
PART II
ITEM 5. MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market and Market Prices of Common Stock
Set forth below for the periods indicated are the high and low sales prices for trading in our common stock on the NASDAQ Capital Market as reported by the NASDAQ Capital Market.
|
Period
|
Sale Prices
|
|||||||
|
|
High
|
Low
|
||||||
| 2009 Fiscal Year | ||||||||
|
First Quarter
|
7.69 | 4.07 | ||||||
|
Second Quarter
|
9.00 | 4.85 | ||||||
|
Third Quarter
|
6.51 | 4.85 | ||||||
|
Fourth Quarter
|
5.79 | 4.00 | ||||||
|
2010 Fiscal Year
|
||||||||
|
First Quarter
|
6.06 | 4.33 | ||||||
|
Second Quarter
|
9.13 | 2.50 | ||||||
|
Third Quarter
|
2.95 | 2.25 | ||||||
|
Fourth Quarter
|
3.98 | 2.20 | ||||||
|
2011 Fiscal Year
|
||||||||
|
First Quarter (through January 31, 2011)
|
3.62 | 2.91 | ||||||
Holders
There were approximately 600 holders of record of our common stock on February 28, 2011. This number, however, does not reflect the ultimate number of beneficial holders of our common stock.
Dividend Policy
The payment of cash dividends is subject to the discretion of our Board of Directors and is dependent upon many factors, including our earnings, our capital needs and our general financial condition. Historically we have not paid any cash dividends.
Securities Authorized for Issuance under Equity Compensation Plans
Reference is made to the Company’s Proxy Statement for its 2011 Annual Meeting of Shareholders under the caption “Compensation of Executive Officers and Directors - Restricted Stock Unit Award Plan; and Securities Authorized for Issuance under Equity Compensation Plans”.
34
ITEM 6. SELECTED FINANCIAL DATA
The selected consolidated financial data presented below for the years ended December 31, 2010, 2009, 2008, 2007 and 2006 are derived from our audited Consolidated Financial Statements. The Consolidated Financial Statements as of December 31, 2010 and 2009 and for each of the years in the three-year period ended December 31, 2010, and the reports thereon, are included elsewhere in this Report. The selected financial information presented for our 2007 and 2006 operations and for our 2008, 2007 and 2006 balance sheets are derived from our audited Consolidated Financial Statements not presented in this Report.
The information set forth below is qualified by reference to, and should be read in conjunction with, the Consolidated Financial Statements and related notes thereto included elsewhere in this Report and "Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations". All share data gives effect to the 1 for 10 reverse stock split implemented December 5, 2007.
|
OPERATING DATA (in thousands) except per share data
|
2010
|
2009
|
2008
|
2007
|
2006
|
|||||||||||||||
|
Revenues
|
$ | 3,311 | $ | 3,835 | $ | 44,437 | $ | 6,404 | $ | — | ||||||||||
|
Operating expenses:
|
||||||||||||||||||||
|
Research and development(1)
|
7,177 | 5,673 | 14,322 | 7,169 | 5,172 | |||||||||||||||
|
Marketing, general and administrative(2)
|
8,858 | 11,662 | 9,133 | 4,141 | 5,654 | |||||||||||||||
|
Interest expense
|
— | — | — | (1,207 | ) | (1,140 | ) | |||||||||||||
|
Interest income
|
42 | 147 | 780 | 268 | 18 | |||||||||||||||
|
Amortization of debt discount & deferred private debt offering costs
|
— | — | — | (2,700 | ) | (183 | ) | |||||||||||||
|
(Loss) gain on fair value change of conversion features
|
— | — | — | (3,483 | ) | 4,235 | ||||||||||||||
|
(Loss) gain on fair value change of common stock warrants
|
— | — | — | (1,905 | ) | 2,164 | ||||||||||||||
|
Other (expense) income
|
(14 | ) | (3 | ) | (3 | ) | 19 | (235 | ) | |||||||||||
|
(Loss) income before income tax
|
(12,696 | ) | (13,356 | ) | 21,759 | (13,914 | ) | (5,967 | ) | |||||||||||
|
Income tax expense (benefit)
|
11 | 2,479 | 7,285 | (9,600 | ) | — | ||||||||||||||
|
Net (loss) income applicable to common stockholders
|
$ | (12,707 | ) | $ | (15,835 | ) | $ | 14,474 | $ | (4,314 | ) | $ | (5,967 | ) | ||||||
|
(Loss) earnings per share: Basic
|
$ | (0.27 | ) | $ | (0.35 | ) | $ | 0.32 | $ | (0.11 | ) | $ | (0.75 | ) | ||||||
|
(Loss) earnings per share: Diluted
|
$ | (0.27 | ) | $ | (0.35 | ) | $ | 0.29 | $ | (0.11 | ) | $ | (0.75 | ) | ||||||
|
Weighted average shares used in computing net earnings (loss) per share: Basic
|
47,029 | 45,932 | 45,675 | 39,157 | 34,496 | |||||||||||||||
|
Weighted average shares used in computing net earnings (loss) per share: Diluted
|
47,029 | 45,932 | 49,416 | 39,157 | 34,496 | |||||||||||||||
(1) Includes stock-based compensation expense of approximately $1,700, $1,900, $600, $400 and $2,100 for years 2010, 2009, 2008, 2007 and 2006, respectively.
(2) Includes stock-based compensation expense of approximately $5,100, $7,300, $3,300, $500 and $3,500 for years 2010, 2009, 2008, 2007 and 2006, respectively.
|
BALANCE SHEET DATA(3)
(in thousands)
|
2010
|
2009
|
2008
|
2007
|
2006
|
|||||||||||||||
|
Working capital (deficiency)
|
$ | 23,289 | $ | 28,750 | $ | 35,991 | $ | 22,306 | $ | (28,641 | ) | |||||||||
|
Total assets
|
25,493 | 31,917 | 42,961 | 45,628 | 1,619 | |||||||||||||||
|
Total debt, net (4)
|
— | — | — | — | 28,787 | |||||||||||||||
|
Total liabilities
|
1,152 | 2,007 | 5,897 | 26,908 | 39,899 | |||||||||||||||
|
Accumulated deficit
|
(335,928 | ) | (323,221 | ) | (307,386 | ) | (321,860 | ) | (317,543 | ) | ||||||||||
|
Stockholders' equity (deficit)
|
24,341 | $ | 29,910 | $ | 37,064 | $ | 18,720 | $ | (38,280 | ) | ||||||||||
(3) Reflects $30 million received from King in December, 2007.
(4) Includes estimated fair value of conversion features of convertible debt outstanding as of December 31, 2006.
35
This discussion and analysis should be read in conjunction with our financial statements and accompanying notes included elsewhere in this Report. Operating results are not necessarily indicative of results that may occur in the future periods. Certain statements in this Report under this Item 7, Item 1, "Business", Item 1A, “Risk Factors” and elsewhere in this Report constitute "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements involve known and unknown risks, uncertainties and other factors which may cause our actual results, performance or achievements or industry results, to be materially different from any
future results, performance, or achievements expressed or implied by such forward-looking statements. See page 3 of this Report for a description of the most significant of such factors.
Company Overview
We are a specialty pharmaceutical company engaged in research, development and manufacture of product candidates intended to provide abuse deterrent features and benefits utilizing our proprietary Aversion® and Impede™ Technologies. Our Aversion® Technology opioid analgesic product candidates are intended to effectively relieve pain while simultaneously discouraging common methods of opioid product misuse and abuse, including:
|
|
·
|
intravenous injection of dissolved tablets or capsules;
|
|
|
·
|
nasal snorting of crushed tablets or capsules; and
|
|
|
·
|
intentional swallowing of excess quantities of tablets or capsules (when product candidates are formulated with niacin).
|
In addition to our opioid product candidates utilizing Aversion® Technology, we are investigating and developing novel mechanisms to incorporate abuse deterrent features into additional abused and misused pharmaceutical products. In this regard we have developed Impede™ PSE, a pseudoephedrine hydrochloride (“PSE”) tablet product utilizing our Impede™ Technology. Impede™ Technology utilizes a proprietary mixture of functional inactive ingredients intended to limit or impede extraction of PSE from the tablet for use as a starting material in producing the illicit drug methamphetamine. We are also developing an undisclosed benzodiazepine product
candidate utilizing Aversion® Technology intended for the treatment of anxiety disorders.
Company’s Present Financial Condition
At December 31, 2010, we had cash and cash equivalents of $24.0 million compared to $30.2 million of cash and cash equivalents at December 31, 2009. We had working capital of $23.3 million at December 31, 2010 compared to working capital of $28.8 million at December 31, 2009. We had an accumulated deficit of approximately $335.9 million and $323.2 million at December 31, 2010 and December 31, 2009, respectively. We had a loss from operations of approximately $12.7 million and a net loss of $12.7 million for the year ended December 31, 2010, compared to a loss from operations of $13.5 million and a net loss of $15.8 million for the year ended December 21, 2009. As of February 28, 2011 we had
cash and cash equivalents of approximately $22.2 million.
During the year ended December 31, 2010, we recognized revenues of $3.3 million derived from the $1.1 million amortized portion of the $30.0 million upfront cash payment received from King in December 2007 and $2.2 million for reimbursement of research and development expenses for Acurox® Tablets and Acurox® with Niacin Tablets licensed to King under the King Agreement. During the year ended December 31, 2009, we recognized revenues of $3.8 million derived from the $3.0 million amortized portion of the $30.0 million upfront cash payment received from King in December 2007
and $0.8 million for reimbursement of reimbursement of research and development expenses for Acurox® Tablets. We have yet to generate any product sales or royalty revenues from product sales. To fund our continued operations, we expect to rely on our current cash resources, additional payments that may be made under the King Agreement and under future license agreements with other pharmaceutical company partners, of which there can be no assurance of obtaining, and revenues, if any, from our commercialization of our Impede™ PSE Tablets. Our cash requirements for operating activities may increase in the future as we continue to conduct pre-clinical studies and clinical trials for our product candidates, maintain, defend, if necessary and expand the scope of our
intellectual property, hire additional personnel, contract with third party contract manufacturers for the commercial supply of our Impede™ PSE Tablets, commercialize our Impede™ PSE Tablets, or invest in other areas.
36
In 2008, we recognized revenue of $44.4 million derived from the $21.9 million amortized portion of the $30.0 million non-refundable cash payment received from King in December 2007, $6.0 million in option exercise fees paid to us by King for the licenses to the third and fourth opioid analgesic product candidates licensed to King under the King Agreement, $5.0 million in an Acurox® with Niacin Tablets development milestone payment received from King, and $11.5 million paid to us by King for reimbursement of research and development expenses for Acurox® with Niacin Tablets . Our losses have resulted principally from costs incurred in connection with research and development activities,
salaries and other personnel-related costs and general corporate expenses. Research and development activities include costs of pre-clinical studies, clinical trials, and clinical trial product supplies associated with our product candidates. Salaries and other personnel-related costs include non-cash, stock-based compensation associated with stock options and restricted stock units granted to employees and non-employee directors.
Results of Operations for the Years Ended December 31, 2010 and 2009
|
December 31
|
||||||||||||||||
|
2010
|
2009
|
Change
|
||||||||||||||
|
|
$000’s
|
$000’s
|
Percent
|
|||||||||||||
| Revenues | ||||||||||||||||
|
Program fee revenue
|
1,088 | 3,077 | (1,989 | ) | (65 | ) | ||||||||||
|
Collaboration fee revenue
|
2,223 | 758 | 1,465 | 193 | ||||||||||||
|
Total revenue
|
3,311 | 3,835 | (524 | ) | (14 | ) | ||||||||||
|
Operating expenses
|
||||||||||||||||
|
Research and development
|
7,177 | 5,673 | 1,504 | 27 | ||||||||||||
|
Marketing, general and administrative
|
8,858 | 11,662 | (2,804 | ) | (24 | ) | ||||||||||
|
Total operating expenses
|
16,035 | 17,335 | (1,300 | ) | (8 | ) | ||||||||||
|
Loss from operations
|
(12,724 | ) | (13,500 | ) | (776 | ) | (6 | ) | ||||||||
|
|
||||||||||||||||
|
Other income (expense)
|
||||||||||||||||
|
Interest income
|
42 | 147 | (105 | ) | (71 | ) | ||||||||||
|
Other expense
|
(14 | ) | (3 | ) | 11 | 367 | ||||||||||
|
Total other income
|
28 | 144 | (116 | ) | (81 | ) | ||||||||||
|
Loss before income tax
|
(12,696 | ) | (13,356 | ) | (660 | ) | (5 | ) | ||||||||
|
Income tax expense
|
11 | 2,479 | (2,468 | ) | (100 | ) | ||||||||||
|
Net loss
|
(12,707 | ) | (15,835 | ) | (3,128 | ) | (20 | ) | ||||||||
Revenue
In December 2007, King paid us a $30.0 million upfront fee in connection with the closing of the King Agreement. Program fee revenue recognized during 2010 from amortizing this upfront fee was $1.1 million compared to $3.0 million in 2009. We have assigned an equal portion of the program fee revenue to each of three product candidates identified under the King Agreement. We have completed our development activities on 2 of the 3 product candidates and fully amortized the portion of the upfront fee for those two product candidates in 2008. We currently estimate the development period for the third product candidate to extend through June 2011. We had no milestone revenue in 2010 or
2009.
Collaboration revenue recognized in 2010 was $2.2 million for reimbursement, pursuant to the King Agreement, of our Acurox® Tablets and Acurox® with Niacin Tablets development and regulatory expenses incurred during 2010. We invoice King in arrears on a calendar quarter basis for our reimbursable development and regulatory expenses under the King Agreement. We expect the amount and timing of collaboration revenue to fluctuate in relation to the amount and timing of the underlying research and development expenses. The Company had collaboration revenue of $0.8 million for 2009.
37
Operating Expenses
Research and development expense during 2010 and 2009 were primarily for product candidates utilizing our Aversion® and Impede™ Technologies, including costs of preclinical studies, clinical trials, clinical supplies and related formulation and design costs, salaries and other personnel related expenses, and facility costs. Included in the 2010 and 2009 results are non-cash stock-based compensation charges of $1.7 million and $1.9 million, respectively, associated with the grant of stock options and restricted stock units. Excluding the stock-based compensation expense, in 2010 there was a $1.7 million increase in development expenses compared to 2009 primarily attributable to
development activities for our benzodiazepine product candidate, an extended release opioid product candidate, and our Impede™ PSE tablet product.
Marketing expenses during 2010 and 2009 consisted of market research studies on our Aversion® and Impede™ Technologies. Our general and administrative expenses primarily consisted of legal, audit and other professional fees, corporate insurance, and payroll. Included in the 2010 and 2009 results are non-cash stock-based compensation charges of $5.1 million and $7.3 million, respectively, associated with the grant of stock options and restricted stock units. Excluding the stock-based compensation expense, in 2010 there was a decrease of $0.6 million in marketing, general and administrative expenses compared to 2009.
Other Income (Expense)
During 2010 and 2009, we had no debt and cash proceeds received pursuant to the King Agreement were invested in U.S. Treasury Bills and money market funds in accordance with the investment policy approved by our Board of Directors, resulting in minimal interest income in 2010 and $0.1 million in 2009 due to the prevailing low variable, market rates of interest.
Net Loss
The Company records its tax provision using a 40% effective tax rate. The net loss of $12.7 million for 2010 includes no federal or state income tax benefit provisions due to uncertainty of their future utilization. A state tax provision was recorded for the Company’s subsidiary operations apportioned to one state jurisdiction. The Company’s net loss of $15.8 million for 2009 included income tax expense of $2.5 million recorded when we adjusted our deferred income tax asset valuation reserve. We determined it was more likely than not that the Company’s net operating loss carryforwards may not be utilized.
Results of Operations for the Years Ended December 31, 2009 and 2008
|
December 31
|
||||||||||||||||
|
2009
|
2008
|
Change
|
||||||||||||||
|
$000’s
|
$000’s
|
Percent
|
||||||||||||||
|
Revenues
|
||||||||||||||||
|
Program fee revenue
|
3,077 | 27,941 | (24,864 | ) | (89 | )% | ||||||||||
|
Collaboration fee revenue
|
758 | 11,496 | (10,738 | ) | (93 | )% | ||||||||||
|
Milestone revenue
|
— | 5,000 | (5,000 | ) | (100 | )% | ||||||||||
|
Total revenue
|
3,835 | 44,437 | (40,602 | ) | (91 | )% | ||||||||||
|
Operating expenses
|
||||||||||||||||
|
Research and development
|
5,673 | 14,322 | (8,649 | ) | (60 | )% | ||||||||||
|
Marketing, general and administrative
|
11,662 | 9,133 | 2,529 | 28 | % | |||||||||||
|
Total operating expenses
|
17,335 | 23,455 | (6,120 | ) | (26 | )% | ||||||||||
|
(Loss) income from operations
|
(13,500 | ) | 20,982 | (34,482 | ) | (164 | )% | |||||||||
|
|
||||||||||||||||
|
Other income (expense)
|
||||||||||||||||
|
Interest income
|
147 | 780 | (633 | ) | (81 | )% | ||||||||||
|
Other expense
|
(3 | ) | (3 | ) | — | — | ||||||||||
|
Total other income
|
144 | 777 | (633 | ) | (81 | )% | ||||||||||
|
(Loss) income before income tax
|
(13,356 | ) | 21,759 | (35,115 | ) | (161 | )% | |||||||||
|
Income tax expense
|
2,479 | 7,285 | (4,806 | ) | (66 | )% | ||||||||||
|
Net (loss) income
|
(15,835 | ) | 14,474 | (30,309 | ) | (209 | )% | |||||||||
38
Revenue
In December 2007, King paid us a $30.0 million upfront fee in connection with the closing of the King Agreement. Program fee revenue recognized during 2009 from amortizing this upfront fee was $3.0 million compared to $21.9 million in 2008. We have assigned an equal portion of the program fee revenue to each of three product candidates identified under the King Agreement. We have completed our development activities on 2 of the 3 product candidates and have fully amortized the portion of the upfront fee for those two product candidates in 2008. At that time, we estimated the development period for the third product candidate would end in December, 2010. Also, included in program fee revenue
in 2008 are two $3.0 million option exercise fees paid by King to us in May 2008 and December 2008, upon the exercise of its option to license a third and fourth opioid analgesic product candidate under the King Agreement. In June 2008, King paid us a $5.0 million milestone payment for successfully achieving the primary end points in our pivotal Phase III study, AP-ADF-105 for Acurox® with Niacin Tablets. We had no milestone revenue in 2009.
Collaboration revenue recognized in 2009 was $0.8 million for reimbursement, pursuant to the King Agreement, of our Acurox® with Niacin Tablets development and regulatory expenses incurred during 2009. We invoice King in arrears on a calendar quarter basis for our reimbursable development and regulatory expenses under the King Agreement. We expect the amount and timing of collaboration revenue to fluctuate in relation to the amount and timing of the underlying research and development expenses. The Company had collaboration revenue of $11.5 million for 2008.
Operating Expenses
Research and development expense during 2009 and 2008 were primarily for product candidates utilizing our Aversion® Technology, including costs of preclinical, clinical trials, clinical supplies and related formulation and design costs, salaries and other personnel related expenses, and facility costs. Included in the 2009 and 2008 results are non-cash stock-based compensation charges of $1.9 million and $0.6 million, respectively, associated with the grant of stock options and restricted stock units. Excluding the stock-based compensation expense, there is a $10.0 million decrease in development expenses primarily attributable to a reduction of our clinical study costs. During 2008, we conducted
and completed our pivotal Phase III clinical trial for Acurox® with Niacin Tablets.
Marketing expenses during 2009 and 2008 consisted of Aversion® Technology customized market data research studies. Our general and administrative expenses primarily consisted of legal, audit and other professional fees, corporate insurance, and payroll. Included in the 2009 and 2008 results are non-cash stock-based compensation charges of $7.3 million and $3.3 million, respectively, associated with the grant of stock options and restricted stock units. Excluding the stock-based compensation expense, there was a decrease of $1.5 million in marketing, general and administrative expenses including reductions of $0.9 million in payroll, $0.3 million legal and accounting professional services, and $0.3
million state franchise taxes.
Other Income (Expense)
During 2009 and 2008, we had no debt and cash proceeds received pursuant to the King Agreement were primarily invested in money market funds, U.S. Treasury Bills, bank commercial paper, and overnight sweep investments, resulting in interest income of $0.1 million and $0.8 million, respectively.
Net Income (Loss)
Deferred income taxes have been recognized in prior years for temporary differences between financial statement and income tax bases of assets and liabilities and loss carry-forwards for which income tax benefits are expected to be realized in future years. During 2009 we recorded a valuation allowance to reduce our net deferred income tax assets to the amount that is more likely than not to be realized. Our net loss of $15.8 million for 2009 includes a provision for income tax expense of $2.5 million for such adjustment to our valuation allowance as we determined that the utilization of certain deferred tax assets recorded in prior years
could not be reasonably predicted based upon financial forecast for succeeding years.
Our net income of $14.5 million for 2008 includes a provision for income tax expense of $8.5 million which has been partially offset by $1.2 million of income tax benefits recorded from the anticipated utilization of some of our deferred tax assets arising from net operating loss carryforwards.
39
Liquidity and Capital Resources
At December 31, 2010, we had cash and cash equivalents of $24.0 million compared to $30.2 million in cash and cash equivalents at December 31, 2009. We had working capital of $23.3 million at December 31, 2010 compared to $28.8 million at December 31, 2009. The decreases in our 2010 cash position and working capital of $6.2 million and $5.5 million, respectively, and the cash flows used in operating activities of $6.5 million for the year ended December 31, 2010, are primarily due to development work conducted on product candidates not licensed to King. Our investing activities in 2010 were less than $0.1 million in capital expenditures and in 2009 our investing activities included capital
expenditures of $0.2 million and net maturities of short-term investments of $5.0 million.
At February 28, 2011, we had cash and cash equivalents of approximately $22.2 million. We estimate that such cash reserves will be sufficient to fund the development of Aversion® Technology and Impede™ Technology product candidates, and related operating expenses at least through the next 12 months.
Pending our receipt of milestone payments and royalties from King related to product candidates developed under the King Agreement, other milestone and royalty payments under similar license agreements anticipated to be negotiated and executed with other pharmaceutical company partners, and revenues from the commercialization of our Impede™ PSE tablets, of which no assurance can be given, we must rely on our current cash reserves, including interest income from the investment of our cash reserves, to fund the development of our Aversion® Technology, Impede™ Technology and related administrative and operating expenses. Our future sources of revenue, if any, will be derived from milestone payments
and royalties under the King Agreement and under similar license agreements with other pharmaceutical company partners, and from the commercialization of our Impede™ PSE tablets, for which there can be no assurance.
The amount and timing of our future cash requirements will depend on regulatory and market acceptance of our product candidates and the resources we devote to the development and commercialization of our product candidates.
The following table presents our expected cash payments on contractual obligations outstanding as of December 31, 2010:
|
Payments due by period (in thousands)
|
||||||||||||||||||||
|
Total
|
Less than
1 year
|
1-3
years
|
3-5
years
|
More than
5 years
|
||||||||||||||||
|
Operating leases
|
$ | 36 | $ | 29 | $ | 7 | $ | — | $ | — | ||||||||||
|
Clinical studies(1)
|
68 | 68 | — | — | — | |||||||||||||||
|
Employment agreements
|
889 | 889 | — | — | — | |||||||||||||||
|
Total
|
$ | 993 | $ | 986 | $ | 7 | $ | — | $ | — | ||||||||||
Off-Balance Sheet Arrangements
We do not engage in transactions or arrangements with unconsolidated or other special purpose entities.
40
Critical Accounting Policies
The preparation of our financial statements in accordance with United States generally accepted accounting principles requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses in our financial statements and accompanying notes. We evaluate our estimates on an ongoing basis, including those estimates related to contract agreements, research collaborations and investments. We base our estimates on historical experience and various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from
other sources. Actual results may differ from these estimates under different assumptions or conditions. The following items in our financial statements require significant estimates and judgments:
Revenue Recognition, Deferred Program Fee Revenue and Collaboration Revenue
We recognize revenue when there is persuasive evidence that an arrangement exists, delivery has occurred, the price is fixed and determinable, and collection is reasonably assured. In connection with King Agreement, we recognize program fee revenue, collaboration revenue and milestone revenue.
Program fee revenue is derived from amortized upfront payments, such as the $30.0 million upfront payment from King received in December 2007, and license fees, such as the $3.0 million option exercise fee paid by King to us in each of May 2008 and December 2008 upon the exercise of its option to license a third and fourth opioid analgesic product candidate under the King Agreement. We have assigned an equal portion of King’s $30.0 million upfront payment to each of three product candidates identified in the King Agreement and recognize the upfront payment as program fee revenue ratably over our estimate of the development period for each identified product candidate. We recognized $1.1 million, $3.1
million and $21.9 million of this program fee revenue in 2010, 2009 and 2008, respectively.
Collaboration revenue is derived from reimbursement of development expenses, which are invoiced quarterly in arrears, and are recognized when costs are incurred pursuant to the King Agreement. The ongoing research and development services being provided to King under the collaboration are priced at fair value based upon the reimbursement of expenses incurred pursuant to the collaboration with King. We recognized $2.2 million, $0.8 million and $11.5 million of collaboration revenue in 2010, 2009 and 2008, respectively of which $0.1 million and $0.4 million were current receivables at December 31, 2010 and 2009, respectively
Milestone revenue is contingent upon the achievement of certain pre-defined events in the development of Acurox® Tablets and other product candidates licensed to King under the King Agreement. Milestone payments from King are recognized as revenue upon achievement of the “at risk” milestone events, which represent the culmination of the earnings process related to that milestone. Milestone payments are triggered either by the results of our research and development efforts or by events external to us, such as regulatory approval to market a product. As such, the milestones are substantially at risk at the inception of the King Agreement, and the amounts of the payments assigned thereto are
commensurate with the milestone achieved. In addition, upon the achievement of a milestone event, we have no future performance obligations related to that milestone payment. Each milestone payment is non-refundable and non-creditable when made. In June 2008, King paid us a $5.0 million milestone payment for successfully achieving the primary endpoints in our pivotal Phase III study, AP-ADF-105 for Acurox® with Niacin Tablets.
Research and Development
Research and Development (“R&D”) expenses include internal R&D activities, external Contract Research Organization (“CRO”) services and their clinical research sites, and other activities. Internal R&D activity expenses include facility overhead, equipment and facility maintenance and repairs, laboratory supplies, pre-clinical laboratory experiments, depreciation, salaries, benefits, and share-based compensation expenses. CRO activity expenses include preclinical laboratory experiments and clinical trial studies. Other activity expenses include regulatory consulting, and regulatory legal counsel. Internal R&D activities and other activity expenses are charged to
operations as incurred. We make payments to the CRO's based on agreed upon terms and may include payments in advance of the study starting date. We review and accrue CRO expenses and clinical trial study expenses based on services performed and rely upon estimates of those costs applicable to the stage of completion of a study as provided by the CRO. Accrued CRO costs are subject to revisions as such studies progress to completion. Revisions are charged to expense in the period in which the facts that give rise to the revision become known. We have entered into several cancelable CRO arrangements and our obligations under these arrangements were approximately $68,000 and $1.4 million at December 31, 2010 and 2009, respectively, for services to be incurred as subjects are enrolled and progress through the studies.
41
Income Taxes
We account for income taxes under the liability method. Under this method, deferred income tax assets and liabilities are determined based on differences between financial reporting and income tax basis of assets and liabilities and are measured using the enacted income tax rates and laws that will be in effect when the differences are expected to reverse. Additionally, net operating loss and tax credit carryforwards are reported as deferred income tax assets. The realization of deferred income tax assets is dependent upon future earnings. A valuation allowance against deferred income tax assets is required if, based on the weight of available evidence, it is more likely than not that some or all of
the deferred income tax assets may not be realized. We recorded adjustments by way of increase of $2.5 million to the deferred income tax asset valuation allowance during 2009. This adjustment recognized a $2.5 million tax expense from income taxes in our income for 2009. At December 31, 2010, 100% of the deferred income tax assets are offset by a valuation allowance due to uncertainties with respect to future utilization of net operating loss carryforwards. If in the future it is determined that amounts of our deferred income tax assets would likely be realized, the valuation allowance would be reduced in the period in which such determination is made and a benefit from income taxes in such period would be recognized.
Stock Compensation
Compensation cost related to stock-based payment transactions is measured based on fair value of the equity or liability instrument issued. For purposes of estimating the fair value of each stock option unit on the date of grant, we utilized the Black-Scholes option-pricing model. The Black-Scholes option valuation model was developed for use in estimating the fair value of traded options, which have no vesting restrictions and are fully transferable. In addition, option valuation models require the input of highly subjective assumptions including the expected volatility factor of the market price of our common stock (as determined by reviewing its historical public market closing prices). Our accounting for
stock-based compensation for restricted stock units (“RSUs”) is based on the fair-value method. The fair value of the RSUs is the market price of our common stock on the date of grant, less its exercise cost.
Capital Expenditures
Our capital expenditures during 2010, 2009 and 2008 were $41,000, $220,000 and $143,000, respectively. Capital expenditures in each such year were primarily attributable to the purchase of scientific equipment and improvements to the Culver, Indiana facility.
Impact of Inflation
We believe that inflation did not have a material impact on our operations for the periods reported.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Some of the securities that we invest in may be subject to market risk. Our primary objective in our cash management activities is to preserve principal while at the same time maximizing income we receive from our investments. A change in the prevailing interest rates may cause the principal amount of our investments to fluctuate. We have no holdings of derivative financial and commodity instruments. As of December 31, 2010, our investments consisted primarily of investments in U.S. Treasury Bills and money market accounts with variable, market rates of interest.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The consolidated financial statements of Acura Pharmaceuticals, Inc. and Subsidiary and the Report of the Independent Registered Public Accounting Firm thereon, to be filed pursuant to Item 8 are included in Item 15.
42
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures. We have conducted an evaluation, under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer of the effectiveness of the design and operation of our disclosure controls and procedures pursuant to Exchange Act Rule 13a-15(e). Based upon that evaluation, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures are effective in timely alerting them to material information relating to the Company (including our subsidiary) required to be included in our periodic Securities and Exchange
Commission filings. No significant changes were made in our internal controls or in other factors that could significantly affect these controls subsequent to the date of their evaluation.
Management’s Report on Internal Control Over Financial Reporting. Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rule 13a-15(f) and 15d-15(f) promulgated under the Exchange Act as a process designated by, or under the supervision of, our principal executive and principal financial officers and effected by our board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in
accordance with generally accepted accounting principles and includes those policies and procedures that:
|
|
·
|
Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and disposition of our assets;
|
|
|
·
|
Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and
|
|
|
·
|
Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements.
|
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2010. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control – Integrated Framework. Based on our assessment, management believes that, as of December 31, 2010, our internal control over financial reporting is effective based on those criteria.
Changes in Internal Control Over Financial Reporting. There was no change in our internal control over financial reporting that occurred during the Fourth Quarter 2010 that materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. OTHER INFORMATION
Not Applicable.
43
PART III
|
Item 10.
|
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
|
Reference is made to 2011 Proxy Statement to be filed on or about March 15, 2011 with respect to Directors, Executive Officers and Corporate Governance, which is incorporated herein by reference and made a part hereof in response to the information required by Item 10.
|
Item 11.
|
EXECUTIVE COMPENSATION.
|
Reference is made to our 2011 Proxy Statement to be filed on or about March 15, 2011with respect to Executive Compensation, which is incorporated herein by reference and made a part hereof in response to the information required by Item 11.
|
Item 12.
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS.
|
Reference is made to our 2011 Proxy Statement to be filed on or about March 15, 2011with respect to the to the security ownership of certain beneficial owners and management and related stockholder matters, which is incorporated herein by reference and made a part hereof in response to the information required by Item 12.
|
Item 13.
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
|
Reference is made to our 2011 Proxy Statement to be filed on or about March 15, 2011with respect to certain relationships and related transactions and direct independence, which is incorporated herein by reference and made a part hereof in response to the information required by Item 13.
|
Item 14.
|
AUDITOR FEES.
|
Reference is made to our 2011 Proxy Statement to be filed on or about March 15, 2011with respect to auditor fees, which is incorporated herein by reference and made a part in response to the information required by Item 14.
PART IV
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(a) The following documents are filed as part of this report:
1. All Financial Statements: See Index to Financial Statements
2. Financial Statement Schedules: None
3. Exhibits: See Index to Exhibits
44
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
Date: March 1, 2011
|
ACURA PHARMACEUTICALS, INC.
|
|
|
By:
|
ANDREW D. REDDICK
|
|
|
Andrew D. Reddick
President and Chief Executive Officer
(Principal Executive Officer)
|
||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
|
Signature
|
Title(s)
|
Date
|
||
|
/s/Andrew D. Reddick
|
President, Chief Executive Officer and Director
|
March 1, 2011
|
||
|
Andrew D. Reddick
|
(Principal Executive Officer)
|
|||
|
/s/Peter A. Clemens
|
Senior Vice President and Chief Financial Officer
|
March 1, 2011
|
||
|
Peter A. Clemens
|
(Principal Financial and Accounting Officer)
|
|||
|
/s/William G. Skelly
|
Director
|
March 1, 2011
|
||
|
William G. Skelly
|
||||
|
/s/Bruce F Wesson
|
Director
|
March 1, 2011
|
||
|
Bruce F. Wesson
|
||||
|
/s/William A. Sumner
|
Director
|
March 1, 2011
|
||
|
William A. Sumner
|
||||
|
/s/Richard J. Markham
|
Director
|
March 1, 2011
|
||
|
Richard J. Markham
|
||||
|
/s/Immanuel Thangaraj
|
Director
|
March 1, 2011
|
||
|
Immanuel Thangaraj
|
||||
|
/s/George K. Ross
|
Director
|
March 1, 2011
|
||
|
George K. Ross
|
45
INDEX TO FINANCIAL STATEMENTS
|
|
Page
|
|
Report of Independent Registered Public Accounting Firm
|
F-2
|
|
Consolidated Balance Sheets
|
F-3
|
|
Consolidated Statements of Operations
|
F-4
|
|
Consolidated Statements of Stockholders' Equity
|
F-5
|
|
Consolidated Statements of Cash Flows
|
F-6 to F-7
|
|
Notes to Consolidated Financial Statements
|
F-8 to F-17
|
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Shareholders
Acura Pharmaceuticals, Inc.
Palatine, Illinois
We have audited the accompanying consolidated balance sheets of Acura Pharmaceuticals, Inc. as of December 31, 2010 and 2009 and the related consolidated statements of operations, stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2010. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designating audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over
financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and the significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Acura Pharmaceuticals, Inc. at December 31, 2010 and 2009, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2010, in conformity with accounting principles generally accepted in the United States of America.
/s/ BDO USA, LLP
Chicago, Illinois
March 1, 2011
F-2
ACURA PHARMACEUTICALS, INC. AND SUBSIDIARY
CONSOLIDATED BALANCE SHEETS
DECEMBER 31, 2010 and 2009
(in thousands except par values)
|
2010
|
2009
|
|||||||
|
Assets
|
||||||||
|
Current assets
|
||||||||
|
Cash and cash equivalents
|
$ | 24,045 | $ | 30,174 | ||||
|
Collaboration revenue receivable
|
126 | 357 | ||||||
|
Prepaid insurance
|
213 | 193 | ||||||
|
Prepaid expenses and other current assets
|
57 | 33 | ||||||
|
Total current assets
|
24,441 | 30,757 | ||||||
|
Property, plant and equipment, net
|
1,052 | 1,160 | ||||||
|
Total assets
|
$ | 25,493 | $ | 31,917 | ||||
|
Liabilities and Stockholders’ Equity
|
||||||||
|
Current liabilities
|
||||||||
|
Accrued expenses
|
$ | 686 | $ | 452 | ||||
|
Deferred program fee revenue
|
466 | 1,555 | ||||||
|
Total current liabilities
|
1,152 | 2,007 | ||||||
|
Commitments and contingencies (Note H)
|
||||||||
|
Stockholders’ equity
|
||||||||
|
Common stock - $.01 par value; 100,000 shares authorized; 43,894 and 43,728 shares issued and outstanding in 2010 and 2009, respectively
|
439 | 437 | ||||||
|
Additional paid-in capital
|
359,830 | 352,694 | ||||||
|
Accumulated deficit
|
(335,928 | ) | (323,221 | ) | ||||
|
Total stockholders’ equity
|
24,341 | 29,910 | ||||||
|
Total liabilities and stockholders’ equity
|
$ | 25,493 | $ | 31,917 | ||||
See accompanying notes to the consolidated financial statements.
F-3
ACURA PHARMACEUTICALS, INC. AND SUBSIDIARY
CONSOLIDATED STATEMENTS OF OPERATIONS
YEARS ENDED DECEMBER 31, 2010, 2009 and 2008
(in thousands except per share data)
|
2010
|
2009
|
2008
|
||||||||||
|
Revenues
|
||||||||||||
|
Program fee revenue
|
$ | 1,088 | $ | 3,077 | $ | 27,941 | ||||||
|
Collaboration revenue
|
2,223 | 758 | 11,496 | |||||||||
|
Milestone revenue
|
- | - | 5,000 | |||||||||
|
Total revenue
|
3,311 | 3,835 | 44,437 | |||||||||
|
Operating expenses
|
||||||||||||
|
Research and development
|
7,177 | 5,673 | 14,322 | |||||||||
|
Marketing, general and administrative
|
8,858 | 11,662 | 9,133 | |||||||||
|
Total operating expenses
|
16,035 | 17,335 | 23,455 | |||||||||
|
(Loss) income from operations
|
(12,724 | ) | (13,500 | ) | 20,982 | |||||||
|
Other income (expense)
|
||||||||||||
|
Interest income
|
42 | 147 | 780 | |||||||||
|
Other expense
|
(14 | ) | (3 | ) | (3 | ) | ||||||
|
Total other income
|
28 | 144 | 777 | |||||||||
|
(Loss) income before income tax
|
(12,696 | ) | (13,356 | ) | 21,759 | |||||||
|
Income tax expense
|
11 | 2,479 | 7,285 | |||||||||
|
Net (loss) income applicable to common stockholders
|
$ | (12,707 | ) | $ | (15,835 | ) | $ | 14,474 | ||||
|
Earnings (loss) per share
|
||||||||||||
|
Basic
|
$ | (0.27 | ) | $ | (0.35 | ) | $ | 0.32 | ||||
|
Diluted
|
$ | (0.27 | ) | $ | (0.35 | ) | $ | 0.29 | ||||
|
Weighted average shares
|
||||||||||||
|
Basic
|
47,029 | 45,932 | 45,675 | |||||||||
|
Diluted
|
47,029 | 45,932 | 49,416 | |||||||||
See accompanying notes to the consolidated financial statements.
F-4
ACURA PHARMACEUTICALS, INC. AND SUBSIDIARY
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY
YEARS ENDED DECEMBER 31, 2010, 2009 and 2008
(in thousands except par values)
|
Common Stock
$0.01 Par Value
|
Additional
Paid-in
|
Accumulated
|
||||||||||||||||||
|
Shares
|
$ Amount
|
Capital
|
Deficit
|
Total
|
||||||||||||||||
|
Balance at Dec. 31, 2007
|
42,706 | $ | 427 | $ | 340,153 | $ | (321,860 | ) | $ | 18,720 | ||||||||||
|
Net income for the year ended Dec. 31, 2008
|
- | - | 14,474 | 14,474 | ||||||||||||||||
|
Other stock-based compensation
|
- | - | 3,850 | - | 3,850 | |||||||||||||||
|
Issuance of common shares for exercise of warrant
|
17 | - | 20 | - | 20 | |||||||||||||||
|
Balance at Dec. 31, 2008
|
42,723 | $ | 427 | $ | 344,023 | $ | (307,386 | ) | $ | 37,064 | ||||||||||
|
Net loss for the year ended Dec. 31, 2009
|
- | - | - | (15,835 | ) | (15,835 | ) | |||||||||||||
|
Other stock-based compensation
|
- | - | 9,204 | - | 9,204 | |||||||||||||||
|
Issuance of common shares for cashless exercise of warrants
|
730 | 7 | (7 | ) | - | - | ||||||||||||||
|
Issuance of common shares for exercise of warrant
|
50 | 1 | 159 | - | 160 | |||||||||||||||
|
Issuance of common shares for cashless exercise of options and payroll taxes
|
225 | 2 | (685 | ) | - | (683 | ) | |||||||||||||
|
Balance at Dec. 31, 2009
|
43,728 | $ | 437 | $ | 352,694 | $ | (323,221 | ) | $ | 29,910 | ||||||||||
|
Net loss for the year ended Dec. 31, 2010
|
- | - | - | (12,707 | ) | (12,707 | ) | |||||||||||||
|
Other stock-based compensation
|
- | - | 6,746 | - | 6,746 | |||||||||||||||
|
Issuance of common shares for cashless exercise of warrants
|
43 | 1 | (1 | ) | - | - | ||||||||||||||
|
Issuance of common shares for exercise of warrant
|
123 | 1 | 391 | - | 392 | |||||||||||||||
|
Balance at Dec. 31, 2010
|
43,894 | $ | 439 | $ | 359,830 | $ | (335,928 | ) | $ | 24,341 | ||||||||||
See accompanying notes to the consolidated financial statements.
F-5
ACURA PHARMACEUTICALS, INC. AND SUBSIDIARY
CONSOLIDATED STATEMENTS OF CASH FLOWS
YEARS ENDED DECEMBER 31, 2010, 2009, and 2008
(in thousands, except supplemental data)
|
|
2010
|
2009
|
2008
|
|||||||||
|
Cash flows from operating activities:
|
||||||||||||
|
Net (loss) income
|
$ | (12,707 | ) | $ | (15,835 | ) | $ | 14,474 | ||||
|
Adjustments to reconcile net (loss) income to net cash (used in) provided by operating activities:
|
||||||||||||
|
Depreciation and amortization
|
135 | 130 | 143 | |||||||||
|
Non-cash stock compensation expense
|
6,746 | 9,204 | 3,850 | |||||||||
|
(Gain) loss on asset disposals
|
14 | (3 | ) | 1 | ||||||||
|
Deferred income taxes
|
- | 2,479 | 7,109 | |||||||||
|
Change in fixed asset impairment reserve
|
- | - | (29 | ) | ||||||||
|
Changes in assets and liabilities
|
||||||||||||
|
Collaboration revenue receivable
|
231 | 3,172 | (553 | ) | ||||||||
|
Prepaid expenses and other current assets
|
(37 | ) | 222 | 207 | ||||||||
|
Accounts payable
|
- | (382 | ) | 382 | ||||||||
|
Accrued expenses
|
226 | (1,113 | ) | 549 | ||||||||
|
Deferred program fee revenue
|
(1,088 | ) | (3,077 | ) | (21,942 | ) | ||||||
|
Net cash (used in) provided by operating activities
|
(6,480 | ) | (5,203 | ) | 4,191 | |||||||
|
Cash flows from investing activities:
|
||||||||||||
|
Purchases of short-term investments
|
- | - | (26,039 | ) | ||||||||
|
Maturities of short-term investments
|
- | 5,039 | 21,000 | |||||||||
|
Capital expenditures
|
(41 | ) | (220 | ) | (143 | ) | ||||||
|
Proceeds from asset disposals
|
- | - | 1 | |||||||||
|
Net cash (used in) provided by investing activities
|
(41 | ) | 4,819 | (5,181 | ) | |||||||
|
Cash flows from financing activities:
|
||||||||||||
|
Proceeds from exercise of warrant
|
392 | 160 | 20 | |||||||||
|
Net cash provided by financing activities
|
392 | 160 | 20 | |||||||||
|
Net decrease in cash and cash equivalents
|
(6,129 | ) | (224 | ) | (970 | ) | ||||||
|
Cash and cash equivalents at beginning of period
|
30,174 | 30,398 | 31,368 | |||||||||
|
Cash and cash equivalents at end of period
|
$ | 24,045 | $ | 30,174 | $ | 30,398 | ||||||
|
Cash paid during the period:
|
||||||||||||
|
Interest
|
$ | - | $ | - | $ | 2 | ||||||
|
Income taxes
|
$ | 20 | $ | 108 | $ | 82 | ||||||
See accompanying notes to the consolidated financial statements.
F-6
ACURA PHARMACEUTICALS, INC. AND SUBSIDIARY
CONSOLIDATED STATEMENTS OF CASH FLOWS (CONTINUED)
YEAR ENDED DECEMBER 31, 2010, 2009, and 2008
Supplemental disclosures of noncash investing and financing activities presented on a reverse stock split basis:
Year ended December 31, 2010
|
1.
|
Warrants to purchase 65,000 shares of common stock were exercised in cashless exercise transactions resulting in the issuance of 43,000 shares of common stock.
|
Year ended December 31, 2009
|
1.
|
Warrants to purchase 1,479,000 shares of common stock were exercised in cashless exercise transactions resulting in the issuance of 730,000 shares of common stock.
|
|
2.
|
Options to purchase 525,000 shares of common stock were exercised in cashless exercise transactions and after withholding shares for statutory minimum payroll taxes calculated at $685,000, the transactions resulted in the issuance of 225,000 shares of common stock.
|
Year ended December 31, 2008
|
1.
|
Impaired fixed assets with a $52,000 net book value were disposed and a $29,000 reduction in the impairment allowance was favorably recognized.
|
|
2.
|
A $1,177,000 valuation allowance against deferred income tax assets was removed which resulted in an equal amount recorded as a benefit against current income tax expense.
|
See accompanying notes to the consolidated financial statements.
F-7
ACURA PHARMACEUTICALS, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2010, 2009 and 2008
NOTE A - DESCRIPTION OF BUSINESS AND SUMMARY OF ACCOUNTING POLICIES
Acura Pharmaceuticals, Inc., a New York corporation, and its subsidiary (the “Company”, “We”, or “Our”) is a specialty pharmaceutical company engaged in research, development and manufacture of product candidates intended to provide abuse deterrent features and benefits utilizing our proprietary Aversion® and Impede™ Technologies.
Amounts presented are rounded to the nearest thousands except per share data.
Summary of Significant Accounting Policies
A summary of the significant accounting policies consistently applied in the preparation of the accompanying consolidated financial statements follows.
|
|
1. Principles of Consolidation
|
The consolidated financial statements include the accounts of the Company and its wholly-owned subsidiary, Acura Pharmaceutical Technologies, Inc. All significant intercompany accounts and transactions are eliminated in consolidation.
2. Cash and Cash Equivalents
We consider all highly liquid securities with an original maturity of three months or less to be cash equivalents. Our cash and cash equivalent balances consist of U.S. Treasury Bills or money market accounts and checking funds with variable, market rates of interest. From time to time, amounts may exceed the Federal Reserve insurance limits however we believe our credit risk exposure is not material. We believe the financial risks associated with these instruments are minimal and we have not experienced any losses from our investments in these securities. Our cash and cash equivalents are governed by our investment policy as approved by our Board of Directors. The carrying amount of cash and cash equivalents
approximates its fair value due to its short-term nature.
3. Concentration of Credit Risk
We invest our excess cash in accordance with the investment policy approved by our Board of Directors that seeks both liquidity and safety of principal. The policy provides for investments in instruments issued by the United States government and by commercial institutions with strong investment grade credit ratings and places restrictions on maturity terms and concentrations by type and issuer.
4. Use of Estimates in Consolidated Financial Statements
The preparation of consolidated financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and use assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent liabilities at the date of the consolidated financial statements, as well as the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. Management periodically evaluates estimates used in the preparation of the consolidated financial statements for continued reasonableness. Appropriate adjustments, if any, to the estimates used are made prospectively based on
such periodic evaluations.
5. Inventories
We had no inventories at each of December 31, 2010 and 2009. Purchases of active pharmaceutical ingredients and raw materials required for our development and clinical trial manufacture of product candidates utilizing our Aversion® or Impede™ Technologies are expensed as incurred.
|
|
6. Property, Plant and Equipment
|
Property, plant and equipment are recorded at cost. Depreciation is recorded on a straight-line basis over the estimated useful lives of the related assets. Leasehold improvements are amortized on a straight-line basis over the shorter of their useful lives or the terms of their respective leases. Betterments are capitalized and maintenance and repairs are charged to operations as incurred. The estimated lives of the major classification of depreciable assets are:
F-8
|
Building and improvements
|
10 - 40 years
|
|
Land improvements
|
20 - 40 years
|
|
Machinery and equipment
|
7 - 10 years
|
|
Scientific equipment
|
5 - 10 years
|
|
Computer hardware and software
|
3 - 10 years
|
|
Office equipment
|
5 - 10 years
|
|
|
7.
|
Revenue Recognition, Deferred Program Fee Revenue and Collaboration Revenue
|
We recognize revenue when there is persuasive evidence that an arrangement exists, delivery has occurred, the price is fixed and determinable, and collection is reasonably assured. In connection with our License, Development, and Commercialization Agreement dated October 30, 2007 (the “King Agreement”) with King Pharmaceuticals Research and Development, Inc. (“King”), we recognize program fee revenue, collaboration revenue and milestone revenue.
Program fee revenue is derived from amortized upfront payments, such as the $30.0 million upfront payment from King received in December 2007, and license fees, such as the $3.0 million option exercise fee paid by King to us in each of May 2008 and December 2008 upon the exercise of its option to license a third and fourth opioid analgesic product candidate under the King Agreement. We have assigned an equal portion of King’s $30.0 million upfront payment to each of three product candidates identified in the King Agreement and recognize the upfront payment as program fee revenue ratably over our estimate of the development period for each identified product candidate. We expect to recognize the
remainder of the program fee revenue for the third product candidate ratably over its remaining development period which we currently estimate to end in June 2011. We recognized $1.1 million, $3.1 million, and $21.9 million of this program fee revenue in 2010, 2009 and 2008, respectively.
Collaboration revenue is derived from reimbursement of development expenses, which are invoiced quarterly in arrears, and are recognized when costs are incurred pursuant to the King Agreement. The ongoing research and development services being provided to King under the collaboration are priced at fair value based upon the reimbursement of expenses incurred pursuant to the collaboration with King. We recognized $2.2 million, $0.8 million, and $11.5 million of collaboration revenue in 2010, 2009, and 2008, respectively, of which $0.1 and $0.4 million were current receivables at December 31, 2010 and 2009, respectively.
Milestone revenue is contingent upon the achievement of certain pre-defined events in the development of Acurox® Tablets and other product candidates licensed to King under the King Agreement. Milestone payments from King are recognized as revenue upon achievement of the “at risk” milestone events, which represent the culmination of the earnings process related to that milestone. Milestone payments are triggered either by the results of our research and development efforts or by events external to us, such as regulatory approval to market a product. As such, the milestones are substantially at risk at the inception of
the King Agreement, and the amounts of the payments assigned thereto are commensurate with the milestone achieved. In addition, upon the achievement of a milestone event, we have no future performance obligations related to that milestone payment. Each milestone payment is non-refundable and non-creditable when made. In June 2008, we recognized milestone revenue when King paid us a $5.0 million payment for successfully achieving the primary endpoints in our pivotal Phase III study, AP-ADF-105 for Acurox® with Niacin Tablets.
|
8.
|
Research and Development
|
Research and Development (“R&D”) expenses include internal R&D activities, external contract research organization (“CRO”) activities, and other activities. Internal R&D activity expenses include facility overhead, equipment and facility maintenance and repairs, depreciation, laboratory supplies, pre-clinical laboratory experiments, depreciation, salaries, benefits, and incentive compensation expenses. CRO activity expenses include preclinical laboratory experiments and clinical trial studies. Other activity expenses include clinical trial studies and regulatory consulting, and regulatory counsel. Internal R&D activities and other activity expenses are charged to
operations as incurred. We make payments to the CRO's based on agreed upon terms and may include payments in advance of the study starting date. We review and accrue CRO and clinical trial study expenses based upon estimates of those costs applicable to the stage of completion of a study as provided by the CRO. At December 31, 2010 we have accrued $0.3 million of CRO and clinical trial study expenses.
F-9
9. Income Taxes
We account for income taxes under the liability method. Under this method, deferred income tax assets and liabilities are determined based on differences between the financial reporting and the income tax basis of assets and liabilities and are measured using the enacted income tax rates and laws that will be in effect when the differences are expected to reverse. Additionally, net operating loss and tax credit carryforwards are reported as deferred income tax assets. The realization of deferred income tax assets is dependent upon future earnings. A valuation allowance is required against deferred income tax assets if, based on the weight of available evidence, it is more likely than not that some or
all of the deferred income tax assets may not be realized. During 2009 the we determined it was more likely than not that we would not be able to realize our recorded deferred income tax assets and we recorded an adjustment of $2.5 million to the deferred income tax asset valuation allowance and recognized an expense from income taxes in such period. During 2008 we determined it was more likely than not that we would be able to realize some of its deferred income tax assets in the near future and recorded an adjustment of $1.2 million to the deferred income tax asset valuation allowance. This adjustment recognized a benefit from income taxes in our income for such period. At both December 31, 2010 and 2009, 100% of all remaining net deferred income tax assets were offset by a valuation allowance due to uncertainties with respect to future utilization of net
operating loss carryforwards. If in the future it is determined that additional amounts of our deferred income tax assets would likely be realized, the valuation allowance would be reduced in the period in which such determination is made and an additional benefit from income taxes in such period would be recognized.
10. Earnings (Loss) Per Share
The computation of basic earnings (loss) per share of common stock is based upon the weighted average number of common shares outstanding during the period, including shares related to vested restricted stock units (See Note I). The computation of diluted earnings (loss) per share is based on the same number of shares used in the basic share calculation adjusted for the effect of other potentially dilutive securities. No such adjustments were made for 2010 or 2009 as their effects would have been antidilutive.
|
Year ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Basic earnings per share
|
||||||||||||
|
Numerator:
|
||||||||||||
|
Net (loss) income applicable to common stockholders
|
$ | (12,707 | ) | $ | (15,835 | ) | $ | 14,474 | ||||
|
Denominator:
|
||||||||||||
|
Common shares (weighted)
|
43,842 | 42,912 | 42,719 | |||||||||
|
Vested restricted stock units (weighted)
|
3,187 | 3,020 | 2,956 | |||||||||
|
Weighted average number of shares outstanding
|
47,029 | 45,932 | 45,675 | |||||||||
|
Basic earnings (loss) per common share
|
$ | (0.27 | ) | $ | (0.35 | ) | $ | 0.32 | ||||
|
Diluted earnings (loss) per share
|
||||||||||||
|
Denominator:
|
||||||||||||
|
Common shares (weighted)
|
43,842 | 42,912 | 42,719 | |||||||||
|
Vested restricted stock units (weighted)
|
3,187 | 3,020 | 2,952 | |||||||||
|
Stock options
|
- | - | 1,443 | |||||||||
|
Common stock warrants
|
- | - | 2,302 | |||||||||
|
Weighted average number of shares outstanding
|
47,029 | 45,932 | 49,416 | |||||||||
|
Diluted earnings (loss) per common share
|
$ | (0.27 | ) | $ | (0.35 | ) | $ | 0.29 | ||||
|
Excluded potentially dilutive securities:
|
||||||||||||
|
Common stock issuable (1):
|
||||||||||||
|
Employee and director stock options
|
4,243 | 3,671 | 1,149 | |||||||||
|
Common stock warrants
|
2,193 | 2,380 | - | |||||||||
|
Non-vested restricted stock units
|
49 | 204 | 30 | |||||||||
|
Total excluded dilutive shares
|
6,485 | 6,255 | 1,179 | |||||||||
(1) Number of shares issuable represents those securities which were either i) nonvested at year end or ii) were vested but antidilutive. The number of shares is based on maximum number of shares issuable on exercise or conversion of the related securities as of year end. Such amounts have not been adjusted for the treasury stock method or weighted average outstanding calculations as required if the securities were dilutive.
F-10
|
|
11.
|
Stock-Based Compensation
|
We have four stock-based compensation plans covering stock options and restricted stock units for our employees and directors, which are described more fully in Note G.
We measure our compensation cost related to share-based payment transactions based on fair value of the equity or liability instrument issued. For purposes of estimating the fair value of each stock option unit on the date of grant, we utilize the Black-Scholes option-pricing model. The Black-Scholes option valuation model was developed for use in estimating the fair value of traded options, which have no vesting restrictions and are fully transferable. In addition, option valuation models require the input of highly subjective assumptions including the expected volatility factor of the market price of our common stock (as determined by reviewing our historical public market closing prices).
Our accounting for stock-based compensation for restricted stock units (“RSUs”) is based on the fair-value method. The fair value of the RSUs is the market price of our common stock on the date of grant, less its exercise cost.
|
12.
|
Accounting Developments
|
In October 2009, the FASB issued an amendment to its previously released guidance on revenue arrangements with multiple deliverables. This guidance becomes effective for the Company at the beginning of its 2011 fiscal year. The pronouncement addresses how to determine whether an arrangement involving multiple deliverables contains more than one unit of accounting and how the arrangement consideration should be allocated among the separate units of accounting. The pronouncement may be applied retrospectively or prospectively for new or materially modified arrangements and early adoption is permitted. The Company is currently assessing the impact of adopting this guidance.
NOTE B – LICENSE, DEVELOPMENT, AND COMMERCIALIZATION AGREEMENT
On October 30, 2007, we and King Pharmaceuticals Research and Development, Inc. (“King”), a wholly-owned subsidiary of King Pharmaceuticals, Inc., entered into a License, Development and Commercialization Agreement (the “King Agreement”) to develop and commercialize in the United States, Canada and Mexico (the "King Territory") certain opioid analgesic products utilizing our proprietary Aversion® Technology. In addition, the King Agreement provides King with an option to license in the King Territory certain future opioid analgesic products developed utilizing Aversion® Technology. As of
December 31, 2010, King had exercised its option to license two additional product candidates including an undisclosed opioid analgesic tablet product and Vycavert® (hydrocodone bitartrate/niacin/acetaminophen) Tablets, each of which utilize our Aversion® Technology. We are responsible for using commercially reasonable efforts to develop Acurox® with Niacin Tablets through regulatory approval by the FDA. The King Agreement provides that we or King may develop additional opioid analgesic product candidates utilizing our Aversion® Technology and, if King exercises its option to license such additional product candidates, they will be subject to the milestone and royalty payments and other terms of the King Agreement.
At December 31, 2010, we had received aggregate payments of $58.3 million from King, consisting of a $30.0 million non-refundable upfront cash payment, $17.3 million in reimbursed research and development expenses relating to Acurox® Tablets and Acurox® with Niacin Tablets, $6.0 million in fees relating to King’s exercise of its option to license an undisclosed opioid analgesic tablet product and Vycavert® Tablets, and a $5.0 million milestone fee relating to our successful achievement of the primary endpoints for our pivotal Phase III clinical study for Acurox® with Niacin Tablets. The King Agreement also provides for King’s payment to us of a $3.0 million fee upon King’s exercise of its option for each future opioid product candidate that utilizes our Aversion® Technology (Future Product). In the event that King does not exercise its option for a Future Product, King may be required to reimburse us for certain of our expenses relating to such Future Product. Further, we may receive up to $23 million in additional non-refundable milestone payments for each product candidate licensed to King which achieve certain regulatory milestones in specific countries in the King Territory. An opioid analgesic product candidate formulated with and without niacin is considered a single product candidate for purposes of the option fees and milestone payments payable
under the King Agreement. We can also receive a one-time $50 million sales milestone payment upon the first attainment of $750 million in net sales of all of our licensed products across all King Territories. In addition, for sales occurring following the one year anniversary of the first commercial sale of the first licensed product sold, King will pay us a royalty at one of six rates ranging from 5% to 25% based on the level of combined annual net sales for all products licensed by us to King across all King Territories, with the highest applicable royalty rate applied to such combined annual sales. King’s royalty payment obligations expire on a product by product and country-by-country basis upon the later of (i) the expiration of the last valid patent claim covering such product in such country, or (ii) fifteen (15) years from the first
commercial sale of such product in such country.
F-11
The King Agreement expires upon the expiration of King’s royalty payment and other payment obligations under the King Agreement. King may terminate the King Agreement (i) in its entirety at any time by written notice to us, and (ii) with respect to any product at any time upon the provision of not less than 12 months’ prior written notice. We may terminate the King Agreement with respect to a product in the United States in the event such product is not commercially launched by King within 120 days after receipt of regulatory approval of such product or in its entirety if King commences any interference or opposition proceeding challenging the validity or enforceability of any of
our patent rights licensed to King under the King Agreement
NOTE C – PROPERTY, PLANT AND EQUIPMENT
Property, plant and equipment are summarized as follows:
|
December 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Building and improvements
|
$ | 1,243 | $ | 1,465 | ||||
|
Land and improvements
|
161 | 161 | ||||||
|
Machinery and equipment
|
43 | 26 | ||||||
|
Scientific equipment
|
583 | 569 | ||||||
|
Computer hardware and software
|
251 | 251 | ||||||
|
Office equipment
|
27 | 27 | ||||||
|
Other personal property
|
68 | 60 | ||||||
| 2,375 | 2,559 | |||||||
|
Less accumulated depreciation and amortization
|
(1,323 | ) | (1,399 | ) | ||||
|
Total property, plant and equipment, net
|
$ | 1,052 | $ | 1,160 | ||||
Depreciation and amortization expense for the years ended December 31, 2010, 2009, and 2008 was $0.14 million, $0.13 million, and $0.14 million, respectively.
NOTE D – ACCRUED EXPENSES
Accrued expenses are summarized as follows:
|
December 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Payroll, payroll taxes and benefits
|
$ | 95 | $ | 89 | ||||
|
Professional services
|
193 | 160 | ||||||
|
Franchise taxes
|
12 | 21 | ||||||
|
Property taxes
|
19 | 19 | ||||||
|
Clinical and regulatory services
|
307 | 75 | ||||||
|
Other fees and services
|
60 | 88 | ||||||
| $ | 686 | $ | 452 | |||||
NOTE E – COMMON STOCK WARRANTS
The Company has outstanding common stock purchase warrants at December 31, 2010 exercisable for 2.2.million shares of common stock, all of which contain a cashless exercise feature. These warrants have an exercise price of $3.40 per share and expiration date of August 2014.
F-12
NOTE F – INCOME TAXES
Provision for Income Taxes
The reconciliation between our provision for income taxes and the amounts computed by multiplying income (loss) before taxes by the U.S. statutory tax rate of 34% is as follows:
|
December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Tax (benefit) at U.S. statutory tax rate
|
$ | (4,320 | ) | $ | (4,541 | ) | $ | 7,398 | ||||
|
State tax (benefit), net of federal effect
|
(56 | ) | (416 | ) | 1,518 | |||||||
|
Research and development tax credits
|
(45 | ) | (100 | ) | (129 | ) | ||||||
|
Wage reported stock-based compensation
|
- | (587 | ) | - | ||||||||
|
Change in valuation allowance
|
4,748 | 8,453 | (1,177 | ) | ||||||||
|
Other
|
(315 | ) | (330 | ) | (325 | ) | ||||||
|
Provision for income taxes
|
$ | 11 | $ | 2,479 | $ | 7,285 | ||||||
Tax expense for 2010 is current state taxes and for 2009 it is deferred taxes. The tax expense for 2008 is comprised of $0.2 million of current state taxes and $7.1 million of deferred taxes.
Deferred Tax Assets and Valuation Allowance
Deferred tax assets reflect the tax effects of net operating losses (“NOLs”), tax credit carryovers, and temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. The most significant item of our deferred tax assets is derived from our Federal NOLs. We have approximately $25.1 million federal income tax benefits at December 31, 2010 derived from $73.8 million Federal NOLs at the U.S. statutory tax rate of 34%, available to offset future taxable income, some of which have limitations for use as prescribed under IRC Section 382. Our NOLs will expire in varying amounts between 2011 and 2030 if not
used, and those expirations will cause fluctuations in our valuation allowance. The components of our deferred tax assets are as follows:
|
December 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Deferred tax assets:
|
||||||||
|
Estimated future value of NOLs
|
||||||||
|
- Federal
|
$ | 25,101 | $ | 23,114 | ||||
|
- State
|
3,260 | 3,862 | ||||||
|
Research and development tax credits
|
234 | 194 | ||||||
|
Deferred program fee revenue
|
183 | 611 | ||||||
|
Stock-based compensation
|
13,080 | 10,505 | ||||||
|
Other, net
|
30 | 41 | ||||||
|
Total deferred taxes
|
41,888 | 38,327 | ||||||
|
Valuation allowance
|
(41,888 | ) | (38,327 | ) | ||||
|
Net deferred tax assets
|
$ | - | $ | - | ||||
Realization of deferred tax assets is dependent upon future earnings, if any, the timing and amount of which may be uncertain. Valuation allowances are placed on deferred tax assets when uncertainty exist on their near term utilization. Periodic reviews are made by us on the valuation allowances and fluctuations can occur. Those fluctuations may be reflected as income tax expenses or benefits in the period they occur. In 2008 we concluded that it was likely that we would be able to utilize $1.2 million of these deferred tax assets based upon the economics of the King Agreement and we reduced the valuation allowance by that amount. In 2008 $11.9 million of deferred tax assets were used to offset our 2008
federal and state tax liabilities. In 2009, we increased the valuation allowance by $2.5 million for the then available deferred tax assets and placed a valuation allowance against the 2009 operating results. We continue to place a valuation allowance against the current year’s operating results and at December 31, 2010, 100% of the deferred income tax assets are offset by a valuation allowance due to uncertainties with respect to future utilization of net operating loss carryforwards. If in the future it is determined that amounts of our deferred income tax assets would likely be realized, the valuation allowance would be reduced in the period in which such determination is made and a benefit from income taxes in such period would be recognized.
F-13
Uncertainty in Income Taxes
We adopted FASB’s statement regarding accounting for uncertainty in income taxes which defined the threshold for recognizing the benefits of tax-return positions in the financial statements as "more-likely-than-not" to be sustained by the taxing authorities. Our adoption of the standard did not result in establishing a contingent tax liability reserve or a corresponding charge to retained earnings. At each of December 31, 2010, 2009 and 2008, we had no liability for income tax associated with uncertain tax positions. Our practice will be to recognize interest and penalties related to uncertain tax positions in interest expense and other non operating expense, respectively. We file federal and state income
tax returns. In the normal course of business the Company is subject to examination by these taxing authorities. With few exceptions, we believe we are no longer subject to U.S. federal income tax examinations for years before 2009 and for state income tax examinations for years before 2007.
NOTE G – EMPLOYEE BENEFIT PLANS
401(k) and Profit-Sharing Plan
We have a 401(k) and Profit-Sharing Plan (the “Plan”) for our employees. Employees may elect to make a basic contribution of up to 15% of their annual earnings. The Plan provides that the Company can make discretionary matching contributions equal to 25% of the first 6% of employee contributions for an aggregate employee contribution of 1.5%, along with a discretionary profit-sharing contribution. We did not contribute matching or profit sharing contributions for the Plan in years 2010, 2009, and 2008.
Stock Option Plans
We maintain various stock option plans. A summary of our stock option plans as of December 31, 2010, 2009, and 2008, and for the years then ended consisted of the following:
|
Years Ended December 31,
|
||||||||||||||||||||||||
|
2010
|
2009
|
2008
|
||||||||||||||||||||||
|
Number
of
Options
(000’s)
|
Weighted
Average
Exercise
Price
|
Number
of
Options
(000’s)
|
Weighted
Average
Exercise
Price
|
Number
of
Options
(000’s)
|
Weighted
Average
Exercise
Price
|
|||||||||||||||||||
|
Outstanding, beginning
|
3,671 | $ | 5.90 | 2,968 | $ | 4.93 | 1,858 | $ | 2.60 | |||||||||||||||
|
Granted
|
649 | 3.36 | 1,312 | 6.38 | 1,160 | 9.58 | ||||||||||||||||||
|
Exercised
|
- | - | (525 | ) | 1.30 | - | - | |||||||||||||||||
|
Forfeited or expired
|
(77 | ) | 10.10 | (84 | ) | 7.95 | (50 | ) | 23.69 | |||||||||||||||
|
Outstanding, ending
|
4,243 | $ | 5.40 | 3,671 | $ | 5.90 | 2,968 | $ | 4.93 | |||||||||||||||
|
Options exercisable
|
3,528 | $ | 5.71 | 2,712 | $ | 5.49 | 2,215 | $ | 3.26 | |||||||||||||||
The following table summarizes information about nonvested stock options outstanding at December 31, 2010:
|
Number of
Options
Not
Exercisable
(000)’s
|
Weighted
Average
Fair
Value
|
|||||||
|
Outstanding at December 31, 2009
|
959 | $ | 6.77 | |||||
|
Granted
|
649 | 3.17 | ||||||
|
Vested
|
(893 | ) | 4.37 | |||||
|
Forfeited or expired
|
- | - | ||||||
|
Outstanding at December 31, 2010
|
715 | $ | 3.64 | |||||
We estimate the option’s fair value on the date of grant using the Black - Scholes option-pricing model. Black-Scholes utilizes assumptions related to volatility, the risk-free interest rate, the dividend yield (which is assumed to be zero, as we have not paid any cash dividends) and employee exercise behavior. Expected volatilities utilized in the Black-Scholes model are based on the historical volatility of our common stock price. The risk-free interest rate is derived from the U.S. Treasury yield curve in effect at the time of grant. The expected life of the grants is derived from historical factors. The assumptions used in the Black Scholes model to determine fair value for the 2010 and 2009
stock option grants were:
F-14
|
2010
|
2009
|
|||||||
|
Expected dividend yield
|
0.0 | % | 0.0 | % | ||||
|
Risk-free interest rates
|
3.5% to 3.9
|
% |
2.4% to 3.1
|
% | ||||
|
Average expected volatility
|
119 | % | 124 | % | ||||
|
Expected term (years)
|
10 | 10 | ||||||
|
Weighted average grant date fair value
|
$ | 3.18 | $ | 6.06 | ||||
As of December 31, 2010, 2009, and 2008 the aggregate intrinsic value of the option awards vested was $2.4 million, $4.8 million, and $10.5 million, respectively. In addition, the aggregate intrinsic value of option awards exercised during the year ended December 31, 2009 was $1.7 million. The total remaining unrecognized compensation cost related to the unvested option awards at December 31, 2010 was $2.6 million and is expected to be recognized over the next twenty-four month remaining requisite service period. The Company recognized stock-based compensation cost from the option awards of $5.8 million, $8.2 million, and $3.7 million during the years ended December 31, 2010, 2009, and 2008, respectively.
Stock-based compensation from option awards in the amount of $1.4 million and $1.7 million and $0.6 million is included in research and development expense in the years ended December 31, 2010, 2009 and 2008, respectively. Stock-based compensation from option awards in the amount of $4.4 million, $6.5 million, and $3.1 million is included in general and administrative expenses in the years ended December 31, 2010, 2009, and 2008, respectively.
Restricted Stock Unit Award Plan
We have a Restricted Stock Unit Award Plan (“2005 RSU Plan”) for our employees and non-employee directors. Vesting of an RSU entitles the holder to receive a share of common stock of the Company on a distribution date. A summary of the RSU Plan as of December 31, 2010, 2009, and 2008, and for the years then ended consisted of the following:
|
Years Ended December 31,
|
||||||||||||||||||||||||
|
2010
|
2009
|
2008
|
||||||||||||||||||||||
|
Number
of RSUs
|
Number
of Vested
RSUs
|
Number
of RSUs
|
Number
of Vested
RSUs
|
Number
of
RSUs
|
Number
of Vested
RSUs
|
|||||||||||||||||||
|
Outstanding, beginning
|
3,316 | 3,112 | 3,000 | 2,970 | 2,950 | 2,950 | ||||||||||||||||||
|
Granted
|
- | - | 330 | - | 50 | - | ||||||||||||||||||
|
Exercised
|
- | - | - | - | - | - | ||||||||||||||||||
|
Vested
|
- | 155 | - | 142 | - | 20 | ||||||||||||||||||
|
Forfeited or expired
|
- | - | (17 | ) | - | - | - | |||||||||||||||||
|
Outstanding, ending
|
3,316 | 3,267 | 3,316 | 3,112 | 3,000 | 2,970 | ||||||||||||||||||
The stock-based compensation cost to be incurred on the RSUs is the RSU’s fair value, which is the market price of the Company’s common stock on the date of grant, less its exercise cost. The fair value of the RSU grants made in 2009 and 2008 was $2.1 million and $0.4 million, respectively. The weighted average fair value of RSUs outstanding at end of 2010, 2009 and 2008 was $3.75, $3.75 and $3.49 per award, respectively. The total remaining unrecognized compensation cost related to the unvested RSU awards amounted to $0.3 million at December 31, 2010. The Company recognized compensation cost from the RSU awards of $1.0 million, $1.0 million, and $0.2 million, during the years ended December 31, 2010,
2009, and 2008, respectively. Stock-based compensation from RSU awards in the amount of $0.3 million and $0.2 million is included in research and development expense in the years ended December 31, 2010 and 2009, respectively and no stock-based compensation in 2008. Stock-based compensation from RSU awards in the amount of $0.7 million, $0.8 million, and $0.2 million is included in general and administrative expense in the years ended December 31, 2010, 2009, and 2008, respectively. No related tax benefits were recorded in calendar year 2010, 2009, or 2008.
The 2005 RSU Plan provides that upon a change in control of the Company or upon termination of an employee’s employment with the Company without cause, vesting will accelerate and the RSUs will fully vest. Absent a change of control, one-fourth of vested shares of common stock underlying an RSU award will be distributed (after payment of $0.01 par value per share) on January 1 of each of 2011, 2012, 2013 and 2014. If a change in control occurs, the vested shares underlying the RSU award will be distributed at or about the time of the change in control. On January 1, 2011, 0.54 million vested shares were distributed to our employees and 0.29 million shares were withheld by the Company upon our
employees’ election to exchange RSUs in satisfaction of $1.0 million withholding tax obligations.
F-15
NOTE H – COMMITMENTS AND CONTINGENCIES
Securities and Class Action Litigation
A lawsuit captioned Bang v. Acura Pharmaceuticals, et al, was filed on September 10, 2010 in the United States District Court for the Northern District of Illinois, Eastern Division (Case 1:10-cv-05757) against us and certain of our current and former officers seeking unspecified damages on behalf of a putative class of persons who purchased our common stock between February 21, 2006 and April 22, 2010. The complaint alleges that certain Company officers made false or misleading statements, or failed to disclose material facts in order to make statements not misleading, relating to our Acurox® with Niacin Tablet product candidate, resulting in violations of Section 10(b) of the Securities Exchange Act of 1934 (the “Exchange Act”), Rule 10b-5 under the Exchange Act and Section 20(a) of the Exchange Act. The complaint further alleges that such false or misleading statements or omissions had the effect of artificially inflating the price of our common stock. We believe that the allegations in the complaint are without merit and intend to vigorously defend the litigation
On October 25, 2010, Kiley Hill, a purported stockholder of the Company filed a shareholder derivative action in the Circuit Court of Cook County, Illinois, Chancery Division captioned Hill v. Acura Pharmaceuticals et al. (Case No. 2010-CH-46380), against our directors and certain of our executive officers, generally relating to the same events that are the subject of the class action litigation described above. The complaint purports to be brought on our behalf and names us as a nominal defendant. The complaint seeks unspecified damages from the individual defendants for breaches of fiduciary duty, abuse of control, gross mismanagement, contribution and
indemnification, waste of corporate assets and unjust enrichment for actions occurring from at least February 21, 2006 through April 22, 2010. Substantively similar complaints captioned Hagan v. Acura Pharmaceuticals et al. (Case No. 2010-CH-46621) and Newell v. Reddick et al (Case No. 2010-CH-46873) were filed in the Circuit Court of Cook County, Illinois, Chancery Division, by other purported stockholders of the Company on October 27, 2010 and October 28, 2010, respectively. We have agreed to a temporary stay of these derivative actions
Reglan®/Metoclopramide Litigation
Halsey Drug Company, as predecessor to the Company, has been named along with numerous other companies as a defendant in cases filed in three separate state coordinated litigations pending in Pennsylvania, New Jersey and California, respectively captioned In re: Reglan® /Metoclopramide Mass Tort Litigation, Philadelphia County Court of Common Pleas, January Term, 2010, No. 01997; In re: Reglan® Litigation, Superior Court of New Jersey, Law Division, Atlantic County, Case No. 289, Master Docket No. ATL-L-3865-10; and Reglan®/Metoclopramide Cases, Superior Court of California, San Francisco County, Judicial Council Coordination Proceeding No. 4631, Superior Court No.: CJC-10-004631. In this product liability litigation against numerous pharmaceutical product manufacturers and distributors, including the Company, plaintiffs claim injuries from their use of the Reglan® brand of metoclopramide and generic metoclopramide. In the Pennsylvania state court mass tort proceeding, over 50 lawsuits have been filed against the Company and Halsey Drug Company alleging that Plaintiffs developed neurological disorders as a result of their use of the Reglan® brand and/or generic metoclopramide. Plaintiffs have not yet served any individual lawsuits upon the Company in the New Jersey and California actions. In the
lawsuits filed to date, Plaintiffs have not confirmed they ingested any of the generic metoclopramide manufactured by the Company. The Company discontinued manufacture and distribution of generic metoclopramide more than 15 years ago. The Company believes these claims are without merit and will vigorously defend these actions.
Employment Agreements
Each of Andrew D. Reddick, Robert B. Jones and Peter A. Clemens are parties to employment agreements containing similar terms expiring December 31, 2011, which provide annual salaries of $377,000, $300,000 and $211,500, respectively, plus the payment of annual bonuses, in the discretion of the Company’s Compensation Committee or Board of Directors, based on the achievement of targets, conditions, or parameters as set by the Compensation Committee or the Board of Directors.
Statutory Minimum Withholding Tax Obligations
Under our stock option plans and our 2005 RSU plan, our employees may elect to have shares withheld upon exercise of options and upon the exchange of RSUs in satisfaction of the statutory minimum withholding tax obligations of such employees relating to such option exercises or RSU exchanges. On January 1, 2011, certain of our employees elected to have 0.29 million of their common shares withheld by the Company upon the exchange of RSUs in satisfaction of their $1.0 million withholding tax obligations.
F-16
Financial Advisor Agreement
In connection with our August 2007 Unit Offering, we are obligated to pay a fee to our then financial advisor upon each exercise of the warrants issued in the Unit Offering, in proportion to the number of warrants exercised. The amount of the fee assuming 100% exercise of the remaining 1.2 million warrants is $0.25 million. We have not reflected this obligation as a liability in our consolidated financial statements as the payment is contingent upon the timing and exercise of the warrants by each of the warrant holders. Such fee, if any, will be paid to the financial advisor and be offset against the equity proceeds as the warrants are exercised.
NOTE I – SELECTED QUARTERLY FINANCIAL DATA (UNAUDITED)
|
Three Month Period Ended
|
||||||||||||||||
|
Calendar Year 2010
|
Mar. 31
|
Jun. 30
|
Sept. 30
|
Dec. 31
|
||||||||||||
|
Total revenue (i)
|
$ | 2,040 | $ | 620 | $ | 292 | $ | 359 | ||||||||
|
Loss from operations
|
(4,035 | ) | (3,186 | ) | (2,566 | ) | (2,937 | ) | ||||||||
|
Net loss (ii)
|
(4,035 | ) | (3,192 | ) | (2,553 | ) | (2,927 | ) | ||||||||
|
Loss per common share - basic and diluted
|
$ | (0.09 | ) | $ | (0.07 | ) | $ | (0.05 | ) | $ | (0.06 | ) | ||||
|
Calendar Year 2009
|
Mar. 31
|
Jun. 30
|
Sept. 30
|
Dec. 31
|
||||||||||||
|
Total revenue (i)
|
$ | 1,380 | $ | 897 | $ | 808 | $ | 750 | ||||||||
|
Loss from operations
|
(2,197 | ) | (3,256 | ) | (3,970 | ) | (4,077 | ) | ||||||||
|
Net loss (ii)
|
(1,277 | ) | (6,520 | ) | (3,954 | ) | (4,084 | ) | ||||||||
|
Loss per common share - basic and diluted
|
$ | (0.03 | ) | $ | (0.14 | ) | $ | (0.09 | ) | $ | (0.09 | ) | ||||
(i) See Note A(7) for revenue recognition.
(ii) We recorded a valuation adjustment on our deferred income tax assets and recorded income tax expense of $2.5 million in quarter ended June 30, 2009.
F-17
ACURA PHARMACEUTICALS, INC.
EXHIBIT INDEX
The following exhibits are included as a part of this Annual Report on Form 10-K or incorporated herein by reference.
|
Exhibit
Number
|
Exhibit Description
|
|
|
3.1
|
Restated Certificate of Incorporation of the Registrant (incorporated by reference to Exhibit 3.1 to the Registrant’s Form 8-K filed on June 25, 2009).
|
|
|
3.2
|
Certificate of Amendment Reverse Splitting Common Stock and restating but not changing text of part of Article III of Restated Certificate of Incorporation (incorporated by Reference to Exhibit 3.1 to the Form 8-K filed December 4, 2007).
|
|
|
3.3
|
Restated Bylaws of the Registrant (incorporated by reference to Exhibit 3.1 to the Form 8-K filed on March 3, 2009).
|
|
|
10.1
|
License, Development and Commercialization Agreement by and between the Registrant and King Pharmaceuticals Research and Development, Inc. (incorporated by reference to Exhibit 10.1 of the Form 8-K filed on November 2, 2007) (confidential treatment has been requested for portions of this Exhibit).
|
|
|
10.2
|
Securities Purchase Agreement dated as of August 20, 2007 (“PIPE SPA”) among the Registrant, Vivo Ventures Fund VI, L.P., Vivo Ventures VI Affiliates Fund, L.P. (collectively “Vivo”), GCE Holdings LLC, and certain other signatories thereto (incorporated by reference to Exhibit 10.1 to the Form 8-K filed on August 21, 2007).
|
|
|
10.3
|
Form of Warrant dated as of August 20, 2007 issued pursuant to the PIPE SPA (incorporated by reference to Exhibit 4.1 to the Form 8-K filed on August 21, 2007).
|
|
|
10.4
|
Form of Warrants dated May 5, 2003 issued to Galen Partners III, L.P., Galen Partners International, III, L.P., Galen Employee Fund III, L.P., Essex Woodlands Health Ventures Fund V, L.P. and Care Capital Investments II, LP and others) (incorporated by reference to Exhibit 10.6 to the October 2007 S-3).
|
|
|
10.5
|
Amended and Restated Voting Agreement dated as of February 6, 2004 among the Registrant, Care Capital Investments II, LP, Essex Woodlands Health Ventures Fund V, L.P., Galen Partners III, L.P., and others (incorporated by reference to Exhibit 10.5 of the Form 8-K filed on February 10, 2004 (the “February 2004 Form 8-K”)).
|
|
|
10.6
|
Joinder and Amendment to Amended and Restated Voting Agreement dated November 9, 2005 between the Registrant, GCE Holdings, Essex Woodlands Health Ventures Fund V, L.P., Care Capital Investments II, LP, Galen Partners III, L.P. and others (incorporated by reference to Exhibit 10.1 to the Form 8-K filed November 10, 2005).
|
|
|
10.7
|
Second Amendment to Amended and Restated Voting Agreement dated as of January 24, 2008 between the Registrant and GCE Holdings, LLC (incorporated by reference to Exhibit 10.1 to the Form 8-K filed January 28, 2008).
|
|
|
10.8
|
Registrant’s 1995 Stock Option and Restricted Stock Purchase Plan (incorporated by reference to Exhibit 4.1 to the Registrant's Registration Statement on Form S-8, File No. 33-98396).
|
|
|
10.9
|
Registrant’s 1998 Stock Option Plan, as amended (incorporated by reference to Appendix C to the Registrant’s Proxy Statement filed on May 12, 2009).
|
E-1
|
Exhibit
Number
|
Exhibit Description
|
|
|
10.10
|
Registrant’s 2005 Restricted Stock Unit Award Plan, as amended (incorporated by reference to Appendix B to the Registrant’s Proxy Statement filed on April 2, 2008).
|
|
|
10.11
|
Registrant’s 2008 Stock Option Plan, as amended on June 25, 2009 (incorporated by reference to Appendix B to our Proxy Statement filed on May 12, 2009).
|
|
|
10.12
|
Executive Employment Agreement dated as of August 26, 2003 between the Registrant and Andrew D. Reddick (“Reddick”) (incorporated by reference to Exhibit 10.2 to the Form 10-Q for the quarter ended June 30, 2004 (the “June 2004 10-Q”)).
|
|
|
10.13
|
Amendment to Executive Employment Agreement between the Registrant and Reddick, dated May 27, 2004 (incorporated by reference to Exhibit 10.4 to the June 2004 10-Q).
|
|
|
10.14
|
Second Amendment to Executive Employment Agreement between the Registrant and Reddick, dated May 24, 2005 incorporated by reference to Exhibit 10.116 to the Form 10-K for the year ending December 31, 2005 filed on February 21, 2006 (the “2005 Form 10-K”)).
|
|
|
10.15
|
Third Amendment to Executive Employment Agreement between the Registrant and Reddick, dated December 22, 2005 (incorporated by reference to Exhibit 10.1 to the Form 8-K filed December 23, 2005 (the “December 2005 Form 8-K”)).
|
|
|
10.16
|
Fourth Amendment to Executive Employment Agreement between the Registrant and Reddick dated December 16, 2007 (incorporated by reference to Exhibit 10.20 to the Form 10-K for the year ending December 31, 2007, filed on March 5, 2008).
|
|
|
10.17
|
Fifth Amendment to Executive Employment Agreement between the Registrant and Reddick executed July 9, 2008 (incorporated by reference to Exhibit 10.1 to our Form 8-K filed on July 10, 2008)
|
|
|
10.18
|
Executive Employment Agreement dated as of April 5, 2004 between the Registrant and Ron J. Spivey (incorporated by reference to Exhibit 10.3 to the June 2004 10-Q).
|
|
|
10.19
|
Amendment to Executive Employment Agreement dated December 22, 2005 between Registrant and Ron J. Spivey (incorporated by reference to Exhibit 10.2 to the December 2005 Form 8-K).
|
|
|
10.20
|
Second Amendment to Executive Employment Agreement dated December 19, 2007 between the Registrant and Ron J. Spivey (incorporated by reference to Exhibit 10.23 to the Form 10-K for the year ending December 31, 2007, filed on March 5, 2008).
|
|
|
10.21
|
Third Amendment to Employment Amendment to Executive Employment Agreement executed July 9, 2008 (incorporated by reference to Exhibit 10.2 to our Form 8-K filed on July 10, 2008).
|
|
|
10.22
|
Amended and Restate Employment Agreement effective as of January 1, 2009 between the registrant and Ron J. Spivey (incorporated by reference to Exhibit 10.3 to our Form 8-K filed on July 10, 2008)
|
|
|
10.23
|
Employment Agreement dated as of March 10, 1998 between the Registrant and Peter Clemens (“Clemens”) (incorporated by reference to Exhibit 10.44 to the Form 10-K for the period ending December 31, 2007, filed on April 15, 1998).
|
|
|
10.24
|
First Amendment to Employment Agreement made as of June 28, 2000 between the Registrant and Clemens (incorporated by reference to Exhibit 10.44A to the Registrant’s 2005 Form 10-K).
|
E-2
|
Exhibit
Number
|
Exhibit Description
|
|
|
10.25
|
Second Amendment to Executive Employment Agreement between Registrant and Clemens, dated as of January 5, 2005 (incorporated by reference to Exhibit 99.1 to the Registrant's Form 8-K filed January 31, 2005).
|
|
|
10.26
|
Third Amendment to Executive Employment Agreement dated December 22, 2005 between Registrant and Clemens (incorporated by reference to Exhibit 10.3 to the December 2005 Form 8-K).
|
|
|
10.27
|
Fourth Amendment to Executive Employment Agreement dated December 16, 2007 between Registrant and Clemens (incorporated by reference to Exhibit 10.28 to the Form 10-K for the year ending December 31, 2007, filed on March 5, 2008).
|
|
|
10.28
|
Fifth Amendment to Executive Employment Agreement executed July 9, 2008 between Registrant and Clemens (incorporated by reference to Exhibit 10.4 to our Form 8-K filed on July 10, 2008).
|
|
|
10.29
|
Employment Agreement dated as of March 18, 2008 between the Registrant and Robert B. Jones (incorporated by reference to Exhibit 10.1 to our Form 8-K filed on March 24, 2008).
|
|
|
10.30
|
Consulting Agreement dated as of December 10, 2009 between Registrant and Garth Boehm (incorporated by reference to our Annual Report on Form 10-K for the year ended December 31, 2009 filed on March 2, 2010).
|
|
|
14.1
|
Code of Ethics (incorporated by reference to Exhibit 14.1 of the Form 8-K filed on December 10, 2007).
|
|
|
21
|
Subsidiaries of the Registrant (incorporated by reference to the Form 10-K for the fiscal year ended December 31, 2006 filed on March 15, 2007).
|
|
|
*23.1
|
Consent of BDO USA LLP, Independent Registered Public Accounting Firm.
|
|
|
*31.1
|
Certification of Periodic Report by Chief Executive Officer pursuant to Rule 13a-14 and 15d-14 of the Securities Exchange Act of 1934.
|
|
|
*31.2
|
Certification of Periodic Report by Chief Financial Officer pursuant to Rule 13a-14 and 15d-14 of the Securities Exchange Act of 1934.
|
|
|
*32
|
Certification of Chief Executive Officer and Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
*Filed or furnished herewith.
E-3
