Attached files
| file | filename |
|---|---|
| 8-K - 8-K - PALISADE BIO, INC. | v436843_8k.htm |
Exhibit 99.01

April 2016 Corporate Presentation

NEURALSTEM, INC. Safe Harbor Statement Safe Harbor statements under the Private Securities Litigation Reform Act of 1995 : This presentation contains forward - looking statements as defined in Section 27 A of the Securities Act of 1933 as amended, and section 21 E of the Securities Exchange Act of 1934 , as amended . Such forward - looking statements are based upon Neuralstem , Inc . ’s management’s current expectations, estimates, beliefs, assumptions, and projections about Neuralstem’s business and industry . Words such as “anticipates,” “expects,” “intends,” “plans,” “predicts,” “believes,” “seeks,” “estimates,” “may,” “will,” “should,” “would,” “potential,” “continue,” and variations of these words (or negatives of these words) or similar expressions, are intended to identify forward - looking statements . In addition, any statements that refer to expectations, projections, or other characterizations of future events or circumstances, including any underlying assumptions, are forward - looking statements . These forward - looking statements are not guarantees of future performance and are subject to certain risks, uncertainties, and assumptions that are difficult to predict . Therefore, our actual results could differ materially and adversely from those expressed in any forward - looking statements as a result of various risk factors . These risks and uncertainties include the risks associated with the effect of changing economic conditions, trends in the products markets, variations in Neuralstem’s cash flow, market acceptance risks, technical development risks and other risk factors detailed in Neuralstem’s Securities and Exchange Commission filings . For links to SEC documents please visit the company’s Web site : neuralstem . com .

Key Highlights • Refocused Business Strategy: – New, experienced management team – Focus on lead program: NSI - 189 – Partner NSI - 566 programs • NSI - 189: Novel Neurogenic Small Molecule targeting MDD – Compelling early clinical data – I ntellectual property portfolio, protection expiring from 2016 - 2034 – Near term milestones: Phase II MDD Data expected 2H17 – Renowned scientific advisory team • NSI - 566 – Partner for continuing development 2

Management 3 Richard Daly, CEO • President, AstraZeneca Diabetes, US; President, BMS Diabetes, US; Co - Founder & Partner, SagePath Partners; EVP , Takeda Pharmaceuticals (North and South America); VP, Commercial Strategy, TAP Pharmaceuticals • Boards: Catalyst Pharmaceuticals; Synergy Pharmaceuticals • Education: MBA, Kellogg Graduate School of Management; BS, Microbiology, University of Notre Dame Dr. Karl Johe , CSO • Co - founder , Chairman of the Board of Neuralstem ; NIH/NINDS Staff Scientist • Education: Post - doctoral fellow, UCSF; Ph.D, Biochemistry, Albert Einstein College of Medicine; MA/BA , Biochemistry , University of Kansas Jonathan Lloyd Jones, CFO • Sr . Director, Corporate Development, Genzyme Corporation; V.P Finance, TransMolecular ; CFO and V.P Corporate Development, TetraLogic ; CFO, Columbia Laboratories, Inc. • Professional Qualification: Chartered Accountant, Institute of Chartered Accountants in England & Wales. • Education: MBA , University of Pennsylvania Wharton School of Business ; BSc. Business Studies, University of Bradford

NSI - 189 Scientific Advisory Board Dr. Maurizio Fava Harvard, MGH, Executive Vice Chair, Dept. of Psychiatry Principal Investigator: NSI - 189 Phase 2 MDD clinical trial Dr. Michael Thase Univ. of Pennsylvania, Chief. Division of Mood and Anxiety Disorders Treatment and Research Program Dr. Mark Frye Mayo Clinic, Chair, Psychiatry and Psychology Dr. John Greden Univ. of Michigan , Founder and Executive Director, Healthy System Depression Center Dr. Richard Keefe Duke Institute for Brain Sciences, Director Schizophrenia Research Group Dr. Thomas Laughren Harvard, MGH, Director, Regulatory Affairs, Former Director of Psychiatric Division, CDER, FDA World Class Psychiatric, Clinical and Regulatory Experts 4

Pipeline 5 Status* (1) Phase II MDD clinical trial results to be provided 2H17 (2) Second Indication to be determined in 2Q16 (3) Ongoing preclinical studies in NSI - 189 and other undisclosed compounds. Multiple NSI - 189 POP publications to be submitted in 2016 (4) NSI - 566: Active efforts to pursue partnerships
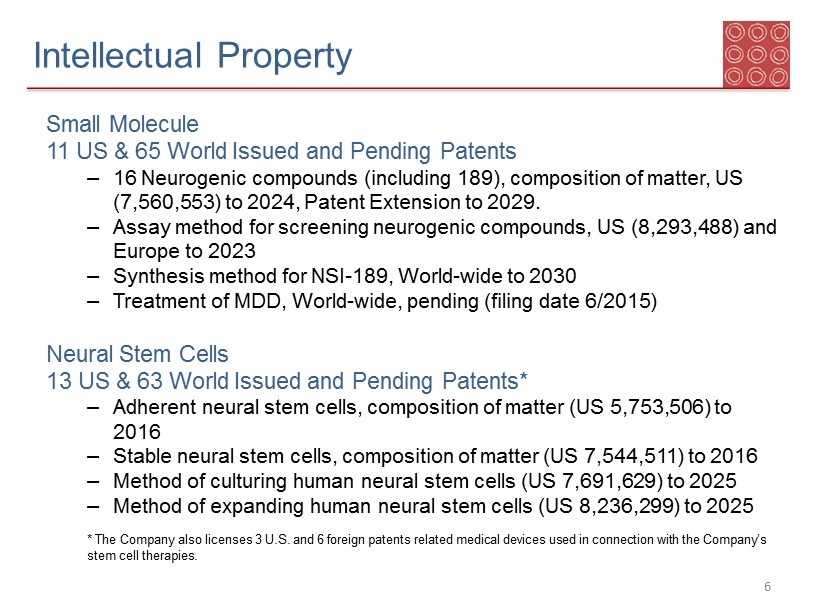
Intellectual Property S mall M olecule 11 US & 65 World Issued and Pending Patents – 16 Neurogenic compounds (including 189), composition of matter, US ( 7,560,553) to 2024, Patent Extension to 2029. – Assay method for screening neurogenic compounds, US ( 8,293,488) and Europe to 2023 – Synthesis method for NSI - 189, World - wide to 2030 – Treatment of MDD, World - wide, pending (filing date 6/2015) N eural Stem Cells 13 US & 6 3 World Issued and Pending Patents* – Adherent neural stem cells, composition of matter (US 5,753,506) to 2016 – Stable neural stem cells, composition of matter (US 7,544,511) to 2016 – Method of culturing human neural stem cells (US 7,691,629) to 2025 – Method of expanding human neural stem cells (US 8,236,299) to 2025 * The Company also licenses 3 U.S. and 6 foreign patents related medical devices used in connection with the Company’s stem cell therapies. 6

MDD Market Opportunity Patients First Line TRD I TRD II 4th line + % patients in given line of therapy 33% 17% 10% 40% % patients that fail given line of therapy 67% 75% 80% N/A Information regarding patient failure derived from: Rush AJ, Fava M, et al; Am J Psychiatry 163:11, November 2006 * Anxiety and Depression Association of America (ADAA), website: http:// www.adaa.org /about - adaa /press - room/facts - statistics retrieved March 2016 7 TRD II - Adjunct Rx - Rx Switch - Other 4 th / 5 th - New combos TRD I - Rx Switch - Adjunct Rx - SSRI / SNRI First Line - Monotherapy - SSRI/NSRI 67% p t. failure 75% p t. failure 80% p t. failure US Market: Estimated 14.8 mn patients*

NSI - 189 Overview • Unique New Chemical Entity (NCE) • Novel neurogenic MOA • Highly stable and well characterized • MDD Market Opportunity • U nsatisfied patient population* • High patient turnover rate in MDD* • Strong IP position through 2024 (2029 with patent term extension) • Efficacy: • Compelling Phase Ib MDD randomized, double - blind data • L arge effect size • Cognitive benefit profile • Potential disease modifying, durability profile • Excellent safety profile • Multiple Asset pipeline expansion opportunities • Preclinical Proof of Principal (POP) studies 8 • Gaynes BN, et al; A direct comparison of presenting characteristics of depressed outpatients from primary vs. specialty care settings: prelimin ary findings from the STAR*D clinical trial. Gen Hosp Psychiatry. 2005 Mar - Apr;27(2):87 - 96 and Rush AJ, Fava M, et al; STAR*D Investigators Group. Sequenced treatment alternatives to relieve depression (STAR*D): rationale and design. Control Clin Trials. 2004 Feb;25(1):119 - 42 .

Building the Case for MOA Extensive screening showed novel MOA vs. currently marketed therapies Screening: • 52 neurotransmitter related receptors/ion channels/ enzymes Novoscreen : Adenosine, GABA, Glutamate, Histamine, Muscarinic, Nicotinic, norepinephrine, opioid, or and serotonin receptors, Ca ++, Cl - , K+ channels, PKA, PKC, CRF, MAO - A/B, or CREB and ERK pathways (related to BDNF release ) • 900 o ther kinases NSI - 189 Binding Activities ≥ 50% at 10µM Target IC50 (µM) Dopamine Transporter (h) 14.2 Norepinephrine Transporter (h) 1.1 5 - HT Transporter (h) >30 5 - HT3 Receptor 2.1 5 - HT7 Receptor (h) 11.1 Opioid mu Receptor (h) 15.7 Opioid delta 1 Receptor 12.7 9

Cohort 1 N=8 (6 drug, 2 placebo) 40 mg QD Cohort 2 N=8 (6 drug, 2 placebo) 40 mg BID Cohort 3 N=8 (6 drug, 2 placebo) 40 mg TID Acute treatment: 28 days Follow up: Days 35, 42, 49, 56, 70, 84 (End - of - study) Clinical Results from NSI - 189 MDD Phase Ib NSI - 189 Phase Ib double - blind, randomized, placebo - controlled, dose - escalating study assessing safety and tolerability • Early indication of efficacy in MDD and Cognition • L arge effect size 10

Clinical Results from NSI - 189 MDD Phase Ib p=0.02 d=0.90 Study Day -20 0 20 40 60 80 100 Symptoms of Depression Questionnaire 2.0 2.2 2.4 2.6 2.8 3.0 3.2 3.4 3.6 3.8 Placebo NS-189 NS-189 1x per day NS-189 2x per day NS-189 3x per day p=0.03 d=1.10 Day 84 Day 28 Symptoms of Depression Questionnaire (SDQ) p=0.09 d=0.95 Study Day 0 20 40 60 80 100 Montgomery and Asberg Depression Rating Scale 5 10 15 20 25 30 Placebo NS-189 NS-189 1x per day NS-189 2x per day NS-189 3x per day p=0.19 d=0.84 Montgomery - Asberg Depression Rating Scale (MADRS) Day 28 • Large effect size (d = 0.95) MADRS • Responder (≥50% reduction in MADRS): 10/18 or 56%; • Remission ( ≤10 score in MADRS): 9/18 or 50% • Encouraging durable effect All: A Phase 1B, Randomized, Double - Blind, Placebo - Controlled, Multiple - Dose Escalation Study Evaluating the Effects of NSI - 189 Phosp hate, a Neurogenic Compound, in Patients with Major Depressive Disorder (MDD) , presented June 2014, by Maurizio Fava, M.D., Karl Johe, Ph.D., Lev G. Gertsik , MD, Larry Ereshefsky , PharmD , Bettina Hoeppner , Ph.D., Martina Flynn, David Mischoulon , M.D., Ph.D., Gustavo Kinrys , M.D., and Marlene Freeman, M.D. Day 84 11

p=0.01 d=0.94 Study Day -20 0 20 40 60 80 100 Cognitive and Physical Functioning Questionnaire 2.0 2.5 3.0 3.5 4.0 4.5 5.0 Placebo NS-189 NS-189 1x per day NS-189 2x per day NS-189 3x per day p<0.01 d=1.20 Day 28 Day 84 Clinical Results from NSI - 189 MDD Phase Ib All: A Phase 1B, Randomized, Double - Blind, Placebo - Controlled, Multiple - Dose Escalation Study Evaluating the Effects of NSI - 189 Phosp hate, a Neurogenic Compound, in Patients with Major Depressive Disorder (MDD) , presented June 2014, by Maurizio Fava, M.D., Karl Johe, Ph.D., Lev G. Gertsik , MD, Larry Ereshefsky , PharmD , Bettina Hoeppner , Ph.D., Martina Flynn, David Mischoulon , M.D., Ph.D., Gustavo Kinrys , M.D., and Marlene Freeman, M.D. • Large effect size (d=0.94) in cognitive function improvement • Persistent improvement over the drug - free 8 weeks in CPFQ Cognitive and Physical Functioning Questionnaire (CPFQ) 12

Biomarker Results from NSI - 189 MDD Phase Ib Blood biomarker panel : • Blood panel analysis correlates to MADRS r esponse rate • MDD panel was developed based on SSRIs activity profile • Rapid and persistent efficacy 13 Response Rate Partial Responder (<14) +Responders ( ≥ 50%) Responder ( ≥ 50%) Remission ( ≤ 10) By MADRS 13/ 18 ( 72% ) 10/18 (56%) 9/18 (50%) By Blood Panel 13/ 18 ( 72% ) • A1AT • ApoC3 • BDNF • Cortisol • EGF • MPO • Prolactin • Resistin • TNFR2 • TSH Biomarker Profiling of NSI - 189 Phosphate, a Neurogenic Compound, in Patients with Major Depressive Disorder (MDD) during a Phase Ib Randomized Double - Blind, Placebo - Controlled Trial JA . Bilello1, X. Feng1, LM.Thurmond1 , L. Gertsik2, BA. English3, L. Ereshefsky3, M. Fava4, B. Hoeppner4, M. Flynn4, D. Mischou lon 4, G. Kinrys4, M. Freeman4, and K. Johe5 10 Biomarkers:
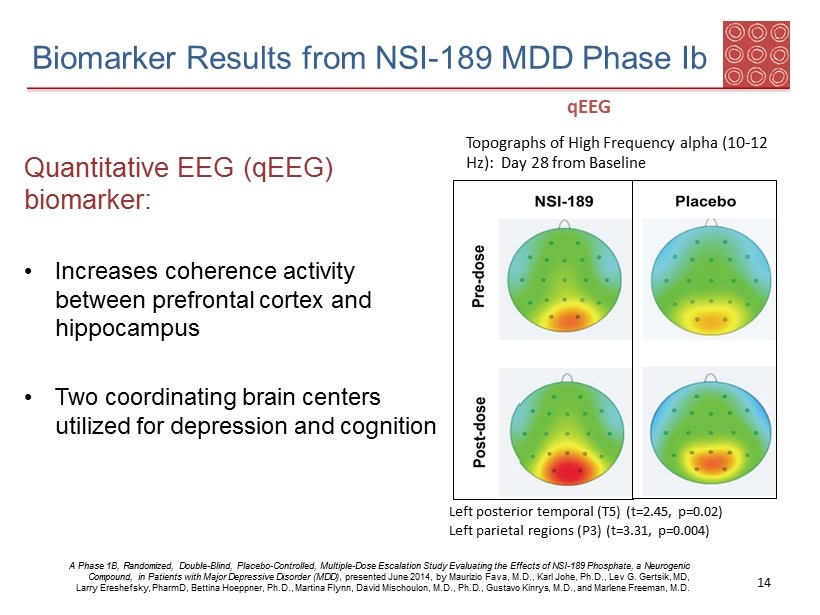
Topographs of High Frequency alpha (10 - 12 Hz): Day 28 from Baseline Left posterior temporal (T5) (t=2.45, p=0.02) Left parietal regions (P3) (t=3.31, p=0.004) qEEG Biomarker Results from NSI - 189 MDD Phase Ib Quantitative EEG ( qEEG ) biomarker : • Increases coherence activity between prefrontal cortex and hippocampus • Two coordinating brain centers utilized for depression and cognition A Phase 1B, Randomized, Double - Blind, Placebo - Controlled, Multiple - Dose Escalation Study Evaluating the Effects of NSI - 189 Phosp hate, a Neurogenic Compound, in Patients with Major Depressive Disorder (MDD) , presented June 2014, by Maurizio Fava, M.D., Karl Johe, Ph.D., Lev G. Gertsik , MD, Larry Ereshefsky , PharmD , Bettina Hoeppner , Ph.D., Martina Flynn, David Mischoulon , M.D., Ph.D., Gustavo Kinrys , M.D., and Marlene Freeman, M.D. 14

NSI - 189 MDD Phase Ib : Adverse Events 15
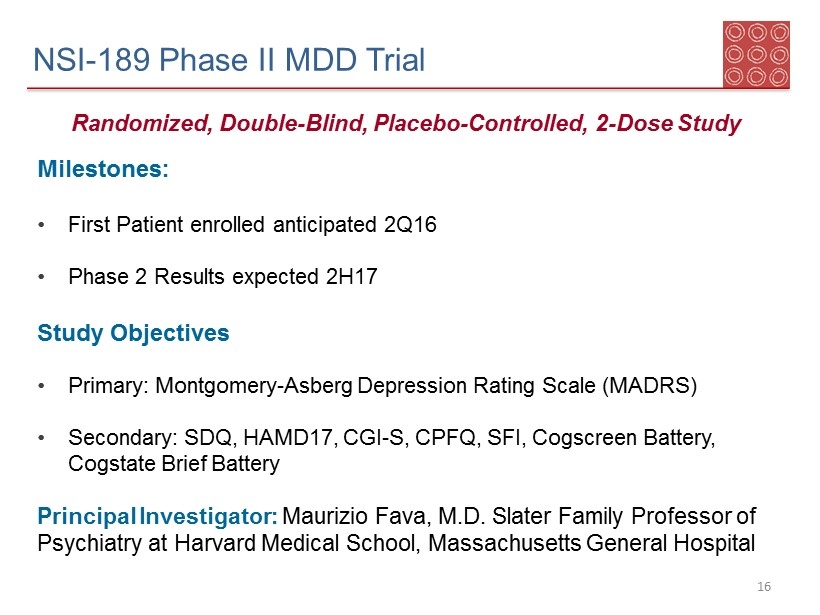
NSI - 189 Phase II MDD Trial Milestones: • First Patient enrolled anticipated 2Q16 • Phase 2 Results expected 2H17 Study Objectives • Primary: Montgomery - Asberg Depression Rating Scale (MADRS ) • Secondary: SDQ , HAMD17, CGI - S, CPFQ, SFI, Cogscreen Battery, Cogstate Brief Battery Principal Investigator: Maurizio Fava, M.D. Slater Family Professor of Psychiatry at Harvard Medical School, Massachusetts General Hospital Randomized, Double - Blind, Placebo - Controlled, 2 - Dose Study 16

NSI - 189 Phase II MDD Trial Highlights • Experienced MDD trial sites (n=12) • Dual patient screening requirement – Secondary confirmatory screen: i ndependent, remote MADRS diagnosis by MGH • P lacebo - reducing prescreen process, re - randomization Study Design • Three arm: 40mg BID, 40mg QD, & placebo (n=220 randomized ) • Power : >80%, 2 - sid ed p ≤ 0.05; d= 0.5 – Potential registration study • 12 week study; 6 month follow - up study Placebo - reducing, Study Design 17

Preclinical Data • Orally active, neurogenic, neuroregenerative , compound for the treatment of depression, cognitive impairment, and neurodegeneration • A new chemical entity with novel mechanism of action, molecular target yet unknown, but not mediated by SSRI or SNRI or by BDNF release or via any known GPCRs, kinases, or channels • Stimulates hippocampal neurogenesis and increases hippocampal volume in young, healthy, normal mice • Shows antidepressant effects in mouse models of depression 18 BDNF: brain - derived neurotrophic factor. GPCR: G - protein coupled receptor. SSRI: selective serotonin re - uptake inhibitor. SNRI: selective norepinephrine re - uptake inhibitor.

• Based on human neural stem cell differentiation in vitro • Through the use of our proprietary drug screening platform • Captures large window of neurodevelopment: neurogenesis to synaptogenesis • Multiple potential s ites of action d uring s tages of neurogenesis • Anticipated to identify additional indications Pipeline Expansion Hippocampal Neural Stem Cells 1. Proliferation Immature Neurons Glial Progenitor 2. Differentiation Mature Neurons Committed Neuronal Progenitor Glia Increased Cognition/Function 3. Maturation/Function 7 days 8 - 21 days 19

Key Highlights • Experienced Management Team • Renowned Advisory Board • Lead Candidate: NSI - 189 novel neurogenic small molecule • Protected IP: 2024 (2029 with patent term extension) – New Chemical Entity • Near term milestones: – Phase II MDD trial; results expected 2H17 – Preclinical POP publications; expected 2016 – Pipeline expansion o pportunities • Compelling randomize, double blind, Phase Ib MDD data, large effect size – Supporting biomarker data – Potential disease modifying • Phase II MDD innovative trial design • Cell therapy business development opportunities 20
