Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Intra-Cellular Therapies, Inc. | d147828d8k.htm |
| EX-99.1 - EX-99.1 - Intra-Cellular Therapies, Inc. | d147828dex991.htm |
| EX-99.4 - EX-99.4 - Intra-Cellular Therapies, Inc. | d147828dex994.htm |
| EX-99.3 - EX-99.3 - Intra-Cellular Therapies, Inc. | d147828dex993.htm |
Exhibit 99.2
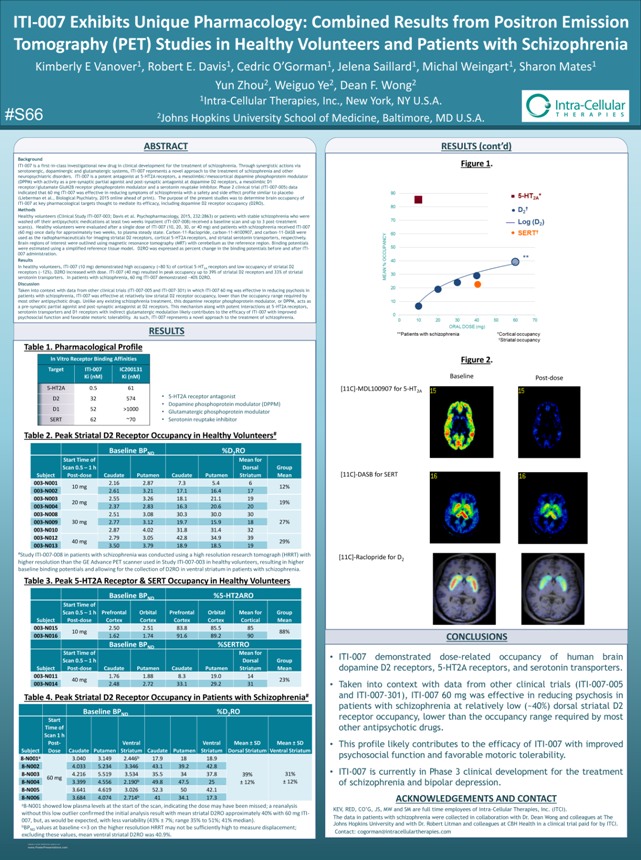
ITI-007 Exhibits Unique Pharmacology: Combined Results from Positron Emission Tomography (PET) Studies in Healthy Volunteers and Patients with Schizophrenia Kimberly E Vanover1, Robert E. Davis1, Cedric O’Gorman1, Jelena Saillard1, Michal Weingart1, Sharon Mates1 Yun Zhou2, Weiguo Ye2, Dean F. Wong2 1Intra-Cellular Therapies, Inc., New York, NY U.S.A. #S66 2Johns Hopkins University School of Medicine, Baltimore, MD U.S.A. Intra-Cellular THERAPIES Background ITI-007 is a first-in-class investigational new drug in clinical development for the treatment of schizophrenia. Through synergistic actions via serotonergic, dopaminergic and glutamatergic systems, ITI-007 represents a novel approach to the treatment of schizophrenia and other neuropsychiatric disorders. ITI-007 is a potent antagonist at 5-HT2A receptors, a mesolimbic/mesocortical dopamine phosphoprotein modulator (DPPM) with activity as a pre-synaptic partial agonist and post-synaptic antagonist at dopamine D2 receptors, a mesolimbic D1 receptor/glutamate GluN2B receptor phosphoprotein modulator and a serotonin reuptake inhibitor. Phase 2 clinical trial (ITI-007-005) data indicated that 60 mg ITI-007 was effective in reducing symptoms of schizophrenia with a safety and side effect profile similar to placebo (Lieberman et al., Biological Psychiatry, 2015 online ahead of print). The purpose of the present studies was to determine brain occupancy of ITI-007 at key pharmacological targets thought to mediate its efficacy, including dopamine D2 receptor occupancy (D2RO). Methods Healthy volunteers (Clinical Study ITI-007-003; Davis et al. Psychopharmacology, 2015, 232:2863) or patients with stable schizophrenia who were washed off their antipsychotic medications at least two weeks inpatient (ITI-007-008) received a baseline scan and up to 3 post-treatment scan(s). Healthy volunteers were evaluated after a single dose of ITI-007 (10, 20, 30, or 40 mg) and patients with schizophrenia received ITI-007 (60 mg) once daily for approximately two weeks, to plasma steady state. Carbon-11-Raclopride, carbon-11-M100907, and carbon-11-DASB were used as the radiopharmaceuticals for imaging striatal D2 receptors, cortical 5-HT2A receptors, and striatal serotonin transporters, respectively. Brain regions of interest were outlined using magnetic resonance tomography (MRT) with cerebellum as the reference region. Binding potentials were estimated using a simplified reference tissue model. D2RO was expressed as percent change in the binding potentials before and after ITI-007 administration. Results In healthy volunteers, ITI-007 (10 mg) demonstrated high occupancy (>80 %) of cortical 5-HT2A receptors and low occupancy of striatal D2 receptors (~12%). D2RO increased with dose. ITI-007 (40 mg) resulted in peak occupancy up to 39% of striatal D2 receptors and 33% of striatal serotonin transporters. In patients with schizophrenia, 60 mg ITI-007 demonstrated ~40% D2RO. Discussion Taken into context with data from other clinical trials (ITI-007-005 and ITI-007-301) in which ITI-007 60 mg was effective in reducing psychosis in patients with schizophrenia, ITI-007 was effective at relatively low striatal D2 receptor occupancy, lower than the occupancy range required by most other antipsychotic drugs. Unlike any existing schizophrenia treatment, this dopamine receptor phosphoprotein modulator, or DPPM, acts as a pre-synaptic partial agonist and post-synaptic antagonist at D2 receptors. This mechanism along with potent interactions at 5-HT2A receptors, serotonin transporters and D1 receptors with indirect glutamatergic modulation likely contributes to the efficacy of ITI-007 with improved psychosocial function and favorable motoric tolerability. As such, ITI-007 represents a novel approach to the treatment of schizophrenia. RESULTS Table 1. Pharmacological Profile In Vitro Receptor Binding Affinities Target ITI-007 IC200131 Ki (nM) Ki (nM) 5-HT2A 0.5 61 D2 32 574 D1 52 >1000 SERT 62 70 • 5-HT2A receptor antagonist • Dopamine phosphoprotein modulator (DPPM) • Glutamatergic phosphoprotein modulator • Serotonin reuptake inhibitor Table 2. Peak Striatal D2 Receptor Occupancy in Healthy Volunteers# Baseline BPND %D2RO Start Time of Mean for Scan 0.5 – 1 h Dorsal Group Subject Post-dose Caudate Putamen Caudate Putamen Striatum Mean 003-N001 2.16 2.87 7.3 5.4 6 10 mg 12% 003-N002 2.61 3.21 17.1 16.4 17 003-N003 2.55 3.26 18.1 21.1 19 20 mg 19% 003-N004 2.37 2.83 16.3 20.6 20 003-N008 2.51 3.08 30.3 30.0 30 003-N009 30 mg 2.77 3.12 19.7 15.9 18 27% 003-N010 2.87 4.02 31.8 31.4 32 003-N012 2.79 3.05 42.8 34.9 39 40 mg 29% 003-N013 3.50 3.79 18.9 18.5 19 #Study ITI-007-008 in patients with schizophrenia was conducted using a high resolution research tomograph (HRRT) with higher resolution than the GE Advance PET scanner used in Study ITI-007-003 in healthy volunteers, resulting in higher baseline binding potentials and allowing for the collection of D2RO in ventral striatum in patients with schizophrenia. Table 3. Peak 5-HT2A Receptor & SERT Occupancy in Healthy Volunteers (*p<0.05; **p<0.01; ***p<0.001 compared with vehicle (0) ANOVA with Newman-Ks post-hoc + SEM) Baseline BPND %5-HT2ARO Start Time of Scan 0.5 – 1 h Prefrontal Orbital Prefrontal Orbital Mean for Group Subject Post-dose Cortex Cortex Cortex Cortex Cortical Mean 003-N015 2.50 2.51 83.8 85.5 85 10 mg 88% 003-N016 1.62 1.74 91.6 89.2 90 Baseline BPND %SERTRO Start Time of Mean for Scan 0.5 – 1 h Dorsal Group Subject Post-dose Caudate Putamen Caudate Putamen Striatum Mean 003-N011 1.76 1.88 8.3 19.0 14 40 mg 23% 003-N014 2.48 2.72 33.1 29.2 31 Table 4. Peak Striatal D2 Receptor Occupancy in Patients with Schizophrenia(#) Baseline BPND %D2RO Start Time of Scan 1 h Post- Ventral Ventral Mean ± SD Mean ± SD Subject Dose Caudate Putamen Striatum Caudate Putamen Striatum Dorsal Striatum Ventral Striatum 8-N001(a) 3.040 3.149 2.446(b) 17.9 18 18.9 8-N002 4.033 5.234 3.346 43.1 39.2 42.8 8-N003 4.216 5.519 3.534 35.5 34 37.8 39% 31% 60 mg 8-N004 3.399 4.556 2.190(b) 49.8 47.5 25 ± 12% ± 12% 8-N005 3.641 4.619 3.026 52.3 50 42.1 8-N006 3.684 4.074 2.714(b) 41 34.1 17.3 a8-N001 showed low plasma levels at the start of the scan, indicating the dose may have been missed; a reanalysis without this low outlier confirmed the initial analysis result with mean striatal D2RO approximately 40% with 60 mg ITI- 007, but, as would be expected, with less variability (43% ± 7%; range 35% to 51%; 41% median). bBP values at baseline <=3 on the higher resolution HRRT may not be sufficiently high to measure displacement; ND excluding these values, mean ventral striatal D2RO was 40.9%. RESEARCH POSTER PRESENTATION DESIGN © 2012 www.PosterPresentations.com RESULTS (cont’d) Figure 1. Figure 2. Baseline Post-dose (*p<0. [11C]-MDL100907 05; **p< .01; ***p<0. for 5-HT0012A co (0) ANOV post-hoc + SEM) [11C]-DASB for SERT [11C]-Raclopride for D2 CONCLUSIONS • ITI-007 demonstrated dose-related occupancy of human brain dopamine D2 receptors, 5-HT2A receptors, and serotonin transporters. • Taken into context with data from other clinical trials (ITI-007-005 and ITI-007-301), ITI-007 60 mg was effective in reducing psychosis in patients with schizophrenia at relatively low (~40%) dorsal striatal D2 receptor occupancy, lower than the occupancy range required by most other antipsychotic drugs. • This profile likely contributes to the efficacy of ITI-007 with improved psychosocial function and favorable motoric tolerability. • ITI-007 is currently in Phase 3 clinical development for the treatment of schizophrenia and bipolar depression. ACKNOWLEDGEMENTS AND CONTACT KEV, RED, CO’G, JS, MW and SM are full time employees of Intra-Cellular Therapies, Inc. (ITCI). The data in patients with schizophrenia were collected in collaboration with Dr. Dean Wong and colleagues at The Johns Hopkins University and with Dr. Robert Litman and colleagues at CBH Health in a clinical trial paid for by ITCI. Contact: cogorman@intracellulartherapies.com
