Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Synthetic Biologics, Inc. | v434170_8k.htm |
Exhibit 99.1

Investor Slide Deck March 2016

Forward - Looking Statements This presentation includes forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 , as amended, on Synthetic Biologics’ current expectations and projections about future events . In some cases forward - looking statements can be identified by terminology such as "may," "should," "potential," "continue," "expects," "anticipates," "intends," "plans," "believes,“ "estimates,” “indicates,” and similar expressions . These statements are based upon management’s current beliefs, expectations and assumptions and are subject to a number of risks and uncertainties, many of which are difficult to predict and include statements regarding our timeline for our SYN - 004 and SYN - 010 clinical trials and reporting of data, the size of the market, benefits to be derived from use of SYN - 004 and SYN - 010 , our anticipated patent portfolio, and our execution of our growth strategy . The forward - looking statements are subject to risks and uncertainties that could cause actual results to differ materially from those set forth or implied by any forward - looking statements . Important factors that could cause actual results to differ materially from those reflected in Synthetic Biologics’ forward - looking statements include, among others, our product candidates demonstrating safety and effectiveness, as well as results that are consistent with prior results, our ability to initiate clinical trials and if initiated, our ability to complete them on time and achieve the desired results and benefits, our clinical trials continuing enrollment as expected, our ability to obtain regulatory approval for our commercialization of product candidates or to comply with ongoing regulatory requirements, regulatory limitations relating to our ability to promote or commercialize our product candidates for the specific indications, acceptance of our product candidates in the marketplace and the successful development, marketing or sale of our products, our ability to maintain our license agreements, the continued maintenance and growth of our patent estate, us to becoming or remaining profitable, our ability to establish and maintain collaborations, our ability to obtain or maintain the capital or grants necessary to fund our research and development activities, a loss of any of our key scientists or management personnel, and other factors described in Synthetic Biologics’ annual report on Form 10 - K for the year ended December 31 , 2015 , subsequent quarterly reports on Form 10 - Qs and any other filings we make with the SEC . The information in this presentation is provided only as of the date presented, and Synthetic Biologics undertakes no obligation to update any forward - looking statements contained in this presentation on account of new information, future events, or otherwise, except as required by law . 2

Investment Considerations NYSE MKT: SYN • Lead candidates in Phase 2 development focused on protecting the gut microbiome while targeting pathogen - specific diseases • SYN - 010 = innovative approach to target an underlying cause of irritable bowel syndrome with constipation (IBS - C), not just the symptoms • Intended to reduce impact of methane producing organisms in the gut • SYN - 004 = category creator for the prevention of C. difficile infection (CDI) and antibiotic - associated diarrhea (AAD) • Intended to degrade certain intravenous (IV) beta - lactam antibiotics in the gut • Targeting multi - billion dollar market opportunities addressing significant unmet medical needs • Multiple near - term and long - term clinical milestones • Experienced management team with extensive clinical and commercial track record 3

Management Team • Jeffrey Riley, CEO Pfizer, Nichols Institute (Quest), SmithKline Beecham, QIC • Steven Shallcross , CFO Vanda Pharmaceuticals, Inc., Empire Petroleum Partners, LLC, Innocoll AG (formerly privately held Innocoll Holdings, Inc.) • Joseph Sliman, MD, MPH, SVP Clinical/Regulatory Vanda Pharmaceuticals, Inc., MedImmune , Inc., DynPort Vaccine • Raymond Stapleton, PhD, SVP, Manufacturing Merck & Co., Inc. • Michael Kaleko, MD, PhD, SVP R&D Genetic Therapy, Inc. (Novartis), Advanced Vision Therapies (currently known as Wellstat Ophthalmics ) • Klaus Gottlieb, MD, FACG, VP Clinical/Regulatory Quintiles, U.S. Food & Drug Administration • Maureen Early, MBA, VP Commercial Rhone Poulenc Rorer/Aventis, Upside Endeavors 4

Product Pipeline 5 C - Cedars - Sinai Medical Center collaboration I - Intrexon Corporation collaboration T - The University of Texas at Austin collaboration Completed Planned – 2016 Therapeutic Area Proprietary Technology Product Candidate Discovery Preclinical Phase 1 Phase 2 Phase 3 IBS - C Oral modified - release lovastatin lactone SYN - 010 C Prevention of CDI and AAD [Degrade IV Beta - lactam Antibiotics] Oral enzyme SYN - 004 Prevention of CDI and AAD [Degrade Oral Beta - lactam Antibiotics] Oral enzyme SYN - 007 Prevention of CDI and AAD [Degrade IV Carbapenem Antibiotics] Oral enzyme SYN - 006 Prevention/Treatment of Pertussis (whooping cough) Monoclonal antibodies SYN - 005 I,T PKU Biotherapeutics SYN - 200 I Preserve gut microbiome and barrier/Treat inflammation Oral intestinal alkaline phosphatase SYN - 020

Milestones: Achieved & Upcoming Therapeutic Area/Product Candidate Timeline IBS - C – SYN - 010: Phase 2 (1 st study; acute, placebo - controlled) 2Q 2015 – Initiated Phase 2 4Q 2015 – Reported Phase 2 topline data Phase 2 (2 nd study; extension, SYN - 010 42mg) 4Q 2015 – Initiated Phase 2 1Q 2016 – Reported Phase 2 topline data Request end of Phase 2 Meeting with FDA Summer 2016 Pivotal Phase 3 trial(s) 2H 2016 – Initiate Phase 3 trial(s) CDI/AAD Prevention – SYN - 004: Phase 1a/1b 1Q 2015 – Positive topline Phase 1b results 1Q 2015 – Positive Phase 1a/1b PK data Phase 2a open - label (1 st ileostomy study; ceftriaxone) 1Q 2015 – Initiated Phase 2a 4Q 2015 – Reported supportive Phase 2a topline data Phase 2a open - label (2 nd ileostomy study; ceftriaxone + PPI) 2Q 2015 – Initiated Phase 2a 1H 2016 – Report Phase 2a topline data Phase 2b proof - of - concept ( double - blind, placebo - controlled) 3Q 2015 – Initiated Phase 2b trial 1H 2016 – Interim analysis of blinded data by independent monitor committee Pivotal Phase 3 trial(s) 2H 2016 – Initiate Phase 3 trial(s) 6

Source: Genome Medicine 2011, 3 :14 http://genomemedicine.com/content/3/3/14 Diseases Directly Influenced by the Gut Microbiome 7

Human Microbiome The body has 10 times as many microbe cells as human cells Source: http://commonfund.nih.gov/hmp/overview.aspx Human Microbiome > 1,000,000 Genes Human Genome 23,000 Genes 99% of Genes in the body are Microbial, NOT Human Leveraging the microbiome could significantly change medicine 8

Source: US National Library of Medicine. Image source: Ottman N, et al. (2012) The function of our microbiota : who is out there and what do they do? Front. Cell. Inf. Microbio . 2:104. Human Microbiome Over Time Response to environmental conditions and life stages 9

SYN - 010 Treatment of IBS - C

Pathogen - Specific Microbiome Therapeutic Treating the underlying cause not symptoms SYN APPROACH: Anti - Archaea specific therapeutic 11

SYN - 010: Proprietary Modified - Release Lovastatin Lactone Designed to reduce methane production by M. smithii in the intestine Bacteroides thetaiotaomicron is one of many bacterium that ferments carbohydrates in the gut which releases H 2 and CO 2 Carbohydrates Methanobrevibacter smithii archea consumes hydrogen gas from Bacteroides and produces methane, which is lost from gut as “gas” Gas H 2 Source: http://commons.wikimedia.org/wiki/File:Intestine_and_stomach_ - _transparent_ - _cut.png 12

SYN - 010 Phase 2 trial design for IBS - C – multiple sites in U.S. ~60 patients SYN - 010 High Dose 20 patients SYN - 010 Low Dose 20 patients Placebo 20 patients SYN - 010 High Dose 54 patients* Study #1 – 4 Week Acute Treatment Study #2 – 8 Week Extension Treatment Topline Analysis Study #1 Topline Analysis Study #2 Primary Endpoint: • Reduction of breath methane Secondary Endpoints: • Reduction in abdominal pain and bloating • Increase in complete spontaneous bowel movement (CSBM) 12 Weeks *Study #1 completers rolled into Study #2 13

SYN - 010 in IBS - C Patients 12 Weeks of Treatment Increased % of Monthly Responders with time & in placebo transferred to SYN - 010 14 0% 10% 20% 30% 40% 50% Placebo (Wk 1-4) SYN-010 42 mg (5-12) SYN-010 21 mg SYN-010 42 mg SYN-010 42 mg SYN-010 42 mg All Subjects Combined Monthly Responders (%) Response improved when changed from Placebo to SYN - 010 Weeks 1 - 4|5 - 8|9 - 12 Weeks 1 - 4|5 - 8|9 - 12 Weeks 1 - 4|5 - 8|9 - 12 Weeks --- |5 - 8|9 - 12 Monthly Responders* had a Weekly Response in at least 50% of weeks A Monthly Responder is defined as a subject who has a weekly response in at least 50% of the weeks of treatment during the mo nth . A Weekly Responder is defined as a subject who experiences: 1. A decrease in weekly average score for worst abdominal pain in the past 24 hours of at least 30% compared with Study 1 Baseli ne, AND 2. A stool frequency increase of 1 or more complete spontaneous bowel movements per week compared with Study 1 Baseline

SYN - 010 Improves Symptoms in IBS - C Patients Response frequency increased over time and in placebo transferred to SYN - 010* 15 Study Period % Weeks Subjects Had a Response Placebo / 42 mg 21 mg / 42 mg 42 mg / 42 mg Increase of ≥1 CSBM per Week vs Baseline Weeks 1 - 4 Weeks 5 - 8 Weeks 9 - 12 0% a 25% b 37.5% c 37.5% d 75% e 75% e 25% a 25% a 12.5 % b ≥30% Decrease in Abdominal Pain Score vs Baseline Weeks 1 - 4 Weeks 5 - 8 Weeks 9 - 12 0% b 50% f 75% g 0% d 50% e 75% e 0% a 100% a 100% f ≥30% Decrease in Bloating Score vs Baseline Weeks 1 - 4 Weeks 5 - 8 Weeks 9 - 12 0% b 50% f 75% g 25% d 75% e 100% e 25% a 100% a 100% f *Phase 2 clinical studies were not powered for statistical significance for this endpoint Data are medians (range 0 - 100%); No. of subjects a 17 b 16 c 14 d 20 e 19 f 15 g 13 Response criteria based on FDA approval endpoints for IBS - C No serious adverse events, and no related cases of diarrhea, were reported

SYN - 010 • Continued evaluation of SYN - 010 clinical pharmacokinetics • Determine dose(s) for advanced clinical testing • Submitted two Type C Meeting requests with FDA • Chemistry, Manufacturing, & Controls meeting (1H 2016) • Clinical meeting to confirm 505(b)(2) clinical pathway (1H 2016) • Request end of Phase 2 meeting with FDA (Summer 2016) • Designing Phase 3 clinical trial (ongoing) • Targeting Phase 3 initiation (2H 2016) Ongoing clinical trial development 16

IBS Market Overview Source: IMS Audited Sales Data, Midas Global Sales (Analytics Link) 17 2015 IBS Global Therapeutic Sales Forecast ~$669.3M Global IBS Sales in 2023 are expected to be greater than $1.5B Market growth attributed to: • Increased uptake of Linzess ® and label expansion of Xifaxan ® • Launch of 4 late - stage pipeline products including 2 late - stage for IBS - C: ̶ Plecanatide - Synergy ̶ Tenapanor - Ardelyx $146.0 $141.9 $220.9 $61.4 $30.3 $68.8 rifaximin lubiprostone linaclotide alosetron ramosetron others

IBS - C Market Overview 1 SYN - 010 targets an underlying cause vs competition Product Candidate SYN - 010 * Linzess Amitiza OTC Laxatives Plecanatide Tenapanor Company Synthetic Allergan Takeda Various Synergy Ardelyx Phase/Status Phase 2 Marketed Marketed Marketed Phase 3 Phase 3 Treat underlying cause of IBS - C Treat symptoms Relieves constipation Relieves pain Causes more regular bowel movements Does not cause severe diarrhea h h h h h h h h h h h h *Based on preclinical data and Company expectations 1 Not a comprehensive list of pipeline products; representative of compounds that are the farthest along in clinical developmen t Source: www.clinicaltrials.gov ; GlobalData ; Corporate pipeline websites 18 h h

SYN - 010 Intellectual Property Strategy

Foundational SYN - 010 Patents Exclusively licensed from Cedars - Sinai 20 Patented Methods of Treatment (Broadest Indication) Expires 2023 • U.S. Patent No. 9,192,618 Claim 1 : A method of treating IBS - C, comprising administering an effective amount of a selective inhibitor of methanogenesis to the distal gut of a human patient in need thereof, wherein : the IBS - C is characterized by elevated intestinal methane levels and the selective inhibitor of methanogenesis is a HMG - CoA reductase inhibitor . • Patent application is being mined further in U.S. • Comparable patents granted in Europe, Canada, and Australia

SYN - 010 Specific Treatment Patent Family Exclusively licensed from Cedars - Sinai 21 • Allowed in U.S. and soon to issue (US 14/211,197) Claims cover t reating c onstipation with statins in patients h aving s tool m ethanogen l evels w hich are indicative of d isease • Patent application is being m ined f urther in U.S. • Pending in Europe, Japan, China, Canada, Australia, Brazil and Mexico • U.S. Patent expires in 2034 and comparable t erm is expected in various international j urisdictions Patented and Pending Methods of Treating Constipation with SYN - 010 in Screened Patients Expires 2034

SYN - 010 Formulation Compositions Patent Family Co - owned with, and exclusively licensed from, Cedars - Sinai 22 • Patent applications r ecently f iled • Pending in U.S. and PCT ( i.e. can b e f iled b roadly internationally) • Future patents expected to cover SYN - 010 formulations (Composition of Matter), among o thers , and have t erm to at least 2035 Pending Patent Applications on SYN - 010 Formulations, Methods of Use in Specific Patient Populations, and Clinical Dosing Expires 2035

SYN - 010 Minimally Absorbed in Canine Model Mean concentration vs time (beta - hydroxyacid ; n=5) 23 0.0 5.0 10.0 15.0 20.0 25.0 0 4 8 12 16 20 24 28 32 36 40 Mean lovastatin ß - hydroxyacid (ng/mL) Time (h) 6 x pH 5.5 minitablets (42 mg) 6 x pH 7.0 minitablets (42 mg) 1 x pH 5.5 + 5 x pH 7.0 minitablets (42 mg) Mevacor® (40 mg) Altoprev® (40 mg) One huge outlier Significantly reduced ß - hydroxyacid levels with pH 7.0 minitablets alone or in combination

SYN - 010 Plasma Concentration • After 28 days of treatment, 65% of patients had no detectable plasma trough levels of lovastatin lactone or lovastatin beta - hydroxyacid • In the few patients with detectable trough levels at Day 28, concentrations of both lactone and beta - hydroxyacid were significantly lower than observed for commercial lovastatin formulations • Consistent with limited absorption, there were no statistically significant changes in LDL - C levels relative to placebo after 28 days First Phase 2 clinical trial data 24

SYN - 010 Phase 2 Trial Completion/Phase 3 Initiation Projected ~2016 Patented Methods of Treatment (Broadest Indication) Expires 2023 Patented and Pending Methods of Treating Constipation with SYN - 010 in Screened Patients Expires 2034 Pending Patent Applications on SYN - 010 Formulations, Methods of Use in Specific Patient Populations, and Clinical Dosing Expires 2035 SYN - 010’s Strategic Patent Position 25 Patents : ~55 U.S. and Foreign Granted and ~15 U.S. and Foreign Pending Additionally, expected 3 Year New Use Marketing Exclusivity , measured from FDA Approval

SYN - 004 Prevention of C. difficile Infection and AAD

Collateral Damage Caused by Antibiotic Use • Antibiotics • Prevent/treat primary infections • Carried to liver, transported to bile and excreted via large intestine • May unintentionally upset natural balance of gut microbiome by killing off good bacteria • A microbial imbalance in the gut microbiome provides an opportunity for overgrowth of harmful pathogenic organisms (e.g., C. difficile ) which may cause severe diarrhea, damage to the colon and in some cases death Imbalance of the gut microbiome 24 million patients are administered IV antibiotics annually in the U.S. 1 1 - This information is an estimate derived from the use of information under license from the following IMS Health Incorporated inf ormation service: CDM Hospital database for full year 2012. IMS expressly reserves all rights, including rights of copying, distribution and repu blication. 27
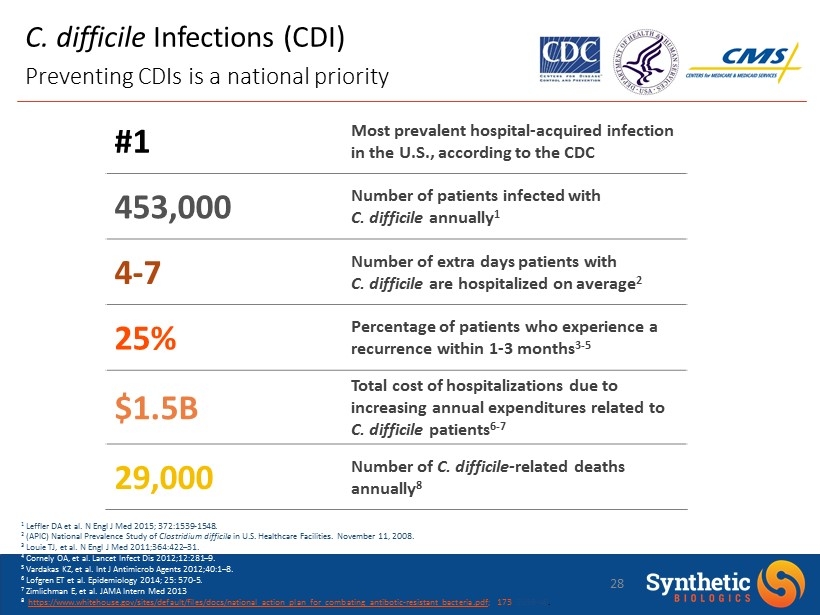
C. difficile Infections (CDI) Preventing CDIs is a national priority 1 Leffler DA et al. N Engl J Med 2015; 372:1539 - 1548. 2 (APIC) National Prevalence Study of Clostridium difficile in U.S. Healthcare Facilities. November 11, 2008. 3 Louie TJ, et al. N Engl J Med 2011;364:422 – 31. 4 Cornely OA, et al. Lancet Infect Dis 2012;12:281 – 9. 5 Vardakas KZ, et al. Int J Antimicrob Agents 2012;40:1 – 8. 6 Lofgren ET et al. Epidemiology 2014; 25: 570 - 5. 7 Zimlichman E, et al. JAMA Intern Med 2013 8 https://www.whitehouse.gov/sites/default/files/docs/national_action_plan_for_combating_antibotic - resistant_bacteria.pdf ; 173 : 2039 - 46 . 28 #1 Most prevalent hospital - acquired infection in the U.S., according to the CDC 453,000 Number of patients infected with C. difficile annually 1 4 - 7 Number of extra days patients with C. difficile are hospitalized on average 2 25% Percentage of patients who experience a recurrence within 1 - 3 months 3 - 5 $1.5B Total cost of hospitalizations due to increasing annual expenditures related to C. difficile patients 6 - 7 29,000 Number of C. difficile - related deaths annually 8

Paradigm Shift Fewer CDIs expected with co - administration of SYN - 004 Treatments • β - lactam • Fluoroquinolone • Clindamycin • Other • Metronidazole • Vancomycin • Fidaxomicin SYN - 004 Paradigm SYN - 004 + beta - lactam antibiotics* SYN - 004 designed to protect the natural balance of the gut microbiome during antibiotic use PREVENTION * I ntended to include penicillins plus cephalosporins Current Paradigm Antibiotics Treatments • β - lactam • Fluoroquinolone • Clindamycin • Other • Metronidazole • Vancomycin • Fidaxomicin C. difficile Infections (CDI) 29
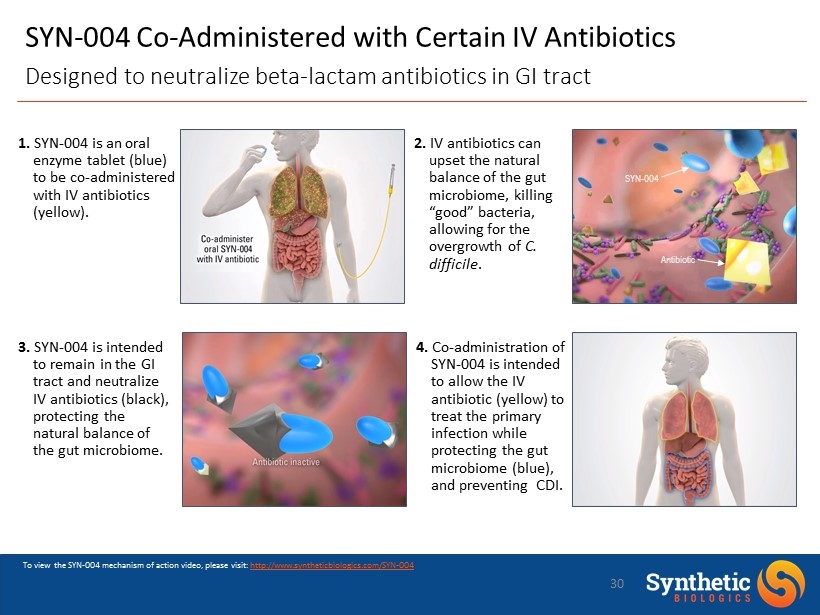
SYN - 004 Co - Administered with Certain IV Antibiotics Designed to neutralize beta - lactam antibiotics in GI tract SYN - 004 Antibiotic To view the SYN - 004 mechanism of action video, please visit: http://www.syntheticbiologics.com/SYN - 004 1. SYN - 004 is an oral enzyme tablet (blue) to be co - administered with IV antibiotics (yellow). 3. SYN - 004 is intended to remain in the GI tract and neutralize IV antibiotics (black), protecting the natural balance of the gut microbiome . 2. IV antibiotics can upset the natural balance of the gut microbiome , killing “good” bacteria, allowing for the overgrowth of C. difficile . 4. Co - administration of SYN - 004 is intended to allow the IV antibiotic (yellow) to treat the primary infection while protecting the gut microbiome (blue), and preventing CDI. 30

SYN - 004 • First generation candidate, P1A, was evaluated in four Phase 1 and one Phase 2 clinical trials conducted in Europe • In total, 112 patients and 143 healthy normal subjects participated in the studies • Completed Phase 1a (40 participants) and 1b (24 participants) trials • PK data supports SYN - 004 should have no effect on the antibiotic in the bloodstream • No clinically significant safety events were observed; well tolerated by participants • Completed first Phase 2a trial (10 evaluable participants) • Demonstrated the ability of SYN - 004 to degrade ceftriaxone in the chyme of healthy participants with functioning ileostomies without affecting ceftriaxone in the bloodstream • Second Phase 2a trial (ongoing) • Characterize SYN - 004 activity on ceftriaxone in the small intestine in the presence of esomeprazole, an approved, OTC proton pump inhibitor Completed and ongoing clinical trials 31

SYN - 004 Phase 2b proof - of - concept trial design (ongoing) 32 ~75 Global Clinical Sites 370 patients SYN - 004 + Ceftriaxone Placebo + Ceftriaxone Primary Endpoint: • Prevention of CDI and C. difficile associated diarrhea (CDAD) Secondary Endpoints: • Prevention of AAD* Interim Analysis of Blinded Data by Independent Monitor Committee: • First 120 patients, and 10 cases of CDI • Evaluate baseline rate of CDI in placebo group 1:1 98 Patients enrolled as of 3/10/2016 *In addition to our primary and secondary endpoints, the Phase 2b study includes an exploratory endpoint to evaluate SYN - 004’s ability to potentially limit the disruption of gut microbiome diversity, also known as dysbiosis .

SYN - 004 • FDA Type C Meeting requested (trial design and endpoints) • Phase 3 trial vision • Prevention of CDI and AAD among hospitalized patients receiving IV ceftriaxone and other beta - lactam antibiotics • Global study; multiple indications for IV beta - lactam therapy • Demonstrate no effect on blood levels of antibiotic or primary diagnosis cure rates Ongoing clinical trial development 33
SYN - 004 • ~35 U.S. and foreign patents; ~30 U.S. and foreign patents pending • Composition of matter claims and pharmaceutical compositions of beta - lactamases, including SYN - 004, was issued in November 2014 (U.S. Patent 8,894,994) • Carries a term to at least 2031 • An extensive portfolio of granted use patents and pending patent applications for SYN - 004 - related technology • Additional patent filings covering composition of matter claims could extend patent protection of SYN - 004 to at least 2035 Broad patent estate 34

SYN - 004: Market Potential Intended to target certain IV beta - lactam antibiotics * Estimate based on the following assumptions: Cost of SYN - 004/day of $100 x 117M days of “SYN - 004 target” β - lactam antibiotics in 2014 1 - Arlington Medical Resources (AMR), a Decision Resources Group Company, 2014 Audits of Acute Care Hospital Antibiotic Utilizat ion . 35 117M 16.7M 453K SYN - 004 Potential U.S. Market ~$12 Billion* 117M: Average days of SYN - 004 administered with target IV beta - lactam antibiotics 1 16.7M: Hospitalized patients 1 453K: New patients infected with C. difficile annually in the U.S.

C. difficile Market Overview 1 SYN - 004 is a prophylactic approach vs treatment Currently the only methods for preventing primary C. difficile infection are through antibiotic stewardship and infection control 1 Not a comprehensive list of pipeline products; representative of compounds that are the farthest along in clinical developmen t Sources: www.clinicaltrials.gov ; GlobalData ; Corporate pipeline websites Product Candidate SYN - 004 * Dificid MK - 3451A ACAM - CDIFF™ SER - 109 PF - 06425090 Company Synthetic Merck Merck Sanofi Seres Pfizer Compound Enzyme to protect microbiome Macrocyclic antibiotic Monoclonal antibody Vaccine Microbiome therapeutic Vaccine Phase/Status Phase 2 Marketed Phase 3 Phase 3 Phase 2 Phase 2 Prophylactic/ Primary prevention Treatment N/A Prevention of recurrence N/A N/A N/A Route of administration Oral Oral Infusion Injections (3X over 30 days) Oral Injections (3X over 30 days) h h h h h *Based on preclinical & Phase 1 data and Company expectations 36

Financial Highlights

Financial Snapshot • Cash balance (as of 12/31/15): ~$20.8 million • Current Price: $1.87 (as of 3/7/16) • 52 Week Range: $0.93 - $4.32 • Average Volume (3 months): 1,190,070 • Shares Outstanding: ~91 million (as of 3/7/16) • Options Outstanding: ~8.9 million* • Warrants Outstanding: ~7.9 million** • Market Capitalization: ~$170 million • Headquarters in Rockville, Maryland * As of 12/31/2015 weighted average exercise price is $2.14 ** As of 12/31/2015 weighted average exercise price is $1.79 38

Investor Slide Deck March 2016 SYN – Mar 2016 Slide Deck (3.14.2016) – FINAL - FINAL

Appendix SYN - 010: Phase 2 Clinical Trial Information

SYN - 010 • Treatment period completed in December 2015 (63 patients) • Primary endpoint was the change from baseline in the area under the curve (AUC) of breath methane production at Day 7, based on a 180 - minute lactulose breath test (LBT) • A necessary normalization of the severely left - skewed breath test data, not prespecified in the statistical analysis plan, was accomplished by square root transformation; paired t - tests were performed allowing each subject to serve as their own control • SYN - 010 effects on breath methane were evaluated in a within group analysis by comparing the methane AUC on a LBT at Day 7 vs baseline Day 1 or Day 28 vs baseline Day 1 • After 7 days reductions in breath methane levels were seen in the 42 mg dose group (p=0.02) but not the 21 mg dose group (p=0.64) • In contrast, after 28 days both the 21 mg (p=0.03) and 42 mg (p=0.01) dose groups showed reductions in breath methane levels • Secondary efficacy assessments included change in methane AUC at Day 28, stool frequency and consistency, abdominal pain, and safety data • Preliminary analysis of secondary efficacy assessment data indicates that an improvement in the stool frequency response for the 21 mg dose group (p=0.02) was apparent, while the 42 mg dose group (p=0.54) was numerically better demonstrating a positive trend • Additionally, an improvement in weekly abdominal pain intensity for the 42 mg dose group (p=0.08) was seen, while the 21 mg dose group (p=0.26) was numerically better demonstrating a positive trend • There were no serious adverse events observed First Phase 2 clinical trial (4 week acute study) 41

SYN - 010 • Treatment period completed in January 2016 (54 patients) • Met primary endpoint of evaluating the ability of SYN - 010 to sustain reduction in breath methane levels • Topline data from all patients who completed clinical trial showed a statistically significant decrease in methane production (p=0.002) • Measured from the beginning of the first Phase 2 study (Study 1 baseline - Day 1) to the end of the second Phase 2 study (12 weeks of treatment - Day 84) • Secondary endpoints included evaluating abdominal pain, bloating and CSBM • A statistically significant reduction in the mean IBS Symptom Severity Score (IBS - SSS; p<0.0001), which includes abdominal pain, bloating, stool frequency and quality of life scores, for all patients from Study 1 baseline to the end of the second Phase 2 study • An increase in the percentage of patients identified as Monthly Responders, an FDA - defined composite measure incorporating improvements in CSBMs and abdominal pain 1 Second Phase 2 clinical trial (8 week extension study) 42 1 A Monthly Responder is defined as a patient who has a Weekly Response in at least 50% of the weeks of treatment during the mo nt h. A Weekly Responder is defined as a patient who experiences a decrease in weekly average score for worst abdominal pain in the past 24 hou rs of at least 30% compared with Study 1 Baseline and a stool frequency increase of 1 or more CSBM per week compared with Study 1 Baseline.
