Attached files
| file | filename |
|---|---|
| 8-K - 8-K - PALISADE BIO, INC. | v433235_8k.htm |
Exhibit 99.01

March 2016 Corporate Presentation

NEURALSTEM, INC. Safe Harbor Statement Safe Harbor statements under the Private Securities Litigation Reform Act of 1995 : This presentation contains forward - looking statements as defined in Section 27 A of the Securities Act of 1933 as amended, and section 21 E of the Securities Exchange Act of 1934 , as amended . Such forward - looking statements are based upon Neuralstem , Inc . ’s management’s current expectations, estimates, beliefs, assumptions, and projections about Neuralstem’s business and industry . Words such as “anticipates,” “expects,” “intends,” “plans,” “predicts,” “believes,” “seeks,” “estimates,” “may,” “will,” “should,” “would,” “potential,” “continue,” and variations of these words (or negatives of these words) or similar expressions, are intended to identify forward - looking statements . In addition, any statements that refer to expectations, projections, or other characterizations of future events or circumstances, including any underlying assumptions, are forward - looking statements . These forward - looking statements are not guarantees of future performance and are subject to certain risks, uncertainties, and assumptions that are difficult to predict . Therefore, our actual results could differ materially and adversely from those expressed in any forward - looking statements as a result of various risk factors . These risks and uncertainties include the risks associated with the effect of changing economic conditions, trends in the products markets, variations in Neuralstem’s cash flow, market acceptance risks, technical development risks and other risk factors detailed in Neuralstem’s Securities and Exchange Commission filings . For links to SEC documents please visit the company’s Web site : neuralstem . com . For links to SEC documents please visit the company’s Web site : neuralstem . com .
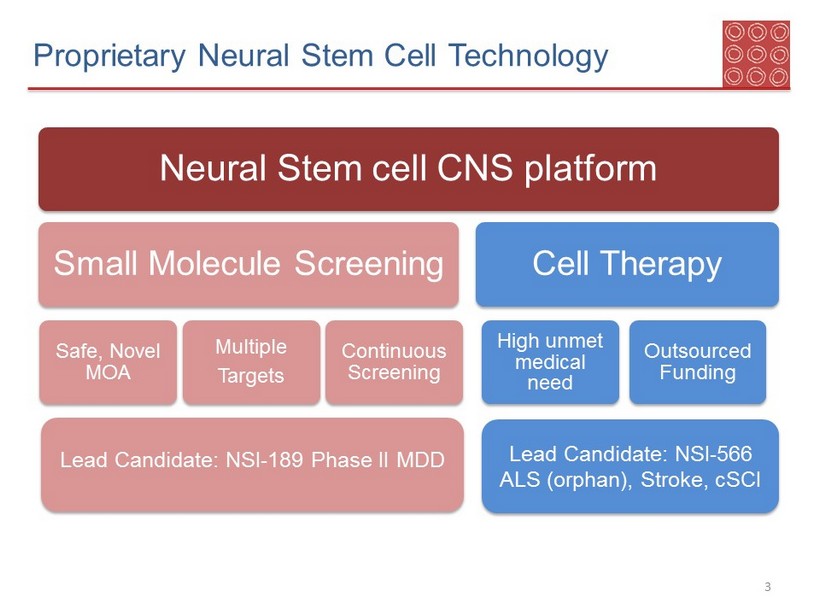
Proprietary Neural Stem Cell Technology 3 Neural Stem cell CNS platform Small Molecule Screening Safe, Novel MOA Multiple Targets Continuous Screening Cell Therapy High unmet medical need Outsourced Funding Lead Candidate: NSI - 189 Phase II MDD Lead Candidate: NSI - 566 ALS (orphan), Stroke, cSCI

Pipeline 4

Clinical Corporate Goals 5 • MDD Ph II Trial, Data 2H 2017 • Exploration of additional safety studies NSI - 189 • Partnerships for continuing clinical development • Expedite regulatory pathways NSI - 566 • Expansion of clinical & regulatory personnel • Business development initiatives Corporate

NSI - 189 Scientific Advisory Board 6 Dr. Maurizio Fava Harvard, MGH, Executive Vice Chair, Dept. of Psychiatry Principal Investigator: NSI - 189 Phase 2 MDD clinical trial Dr. Michael Thase Univ. of Pennsylvania, Chief. Division of Mood and Anxiety Disorders Treatment and Research Program Dr. Mark Frye Mayo Clinic, Chair, Psychiatry and Psychology Dr. John Greden Univ. of Michigan , Founder and Executive Director, Healthy System Depression Center Dr. Richard Keefe Duke Institute for Brain Sciences, Director Schizophrenia Research Group Dr. Thomas Laughren Harvard, MGH, Director, Regulatory Affairs, Former Director of Psychiatric Division, CDER, FDA World Class Psychiatric, Clinical and Regulatory Experts

NSI - 189 Target Product Profile 7 Indication Monotherapy and Adjunctive treatment of Major Depressive Disorder (MDD), with improvement of cognition Efficacy • Primary: MADRS • Key secondary: Onset of effect, s ustained effect, cognitive symptom improvement Superiority vs. active comparator at day 28 and sustained for 90 days & post - dosing durability Tolerability • Based on clinical wellness • Safe and well tolerated • No major adverse events: body weight gain or sexual dysfunction Safety: • Warnings & Precautions Standard suicidality warning Administration Oral, 4 - 12 weeks episodic course of treatment Health Outc ome Measures (Payer requirement) • Economic modelling for reduced cost to payer • Patient reported outcomes - reduction in symptoms of depression, durability, and cognitive improvement
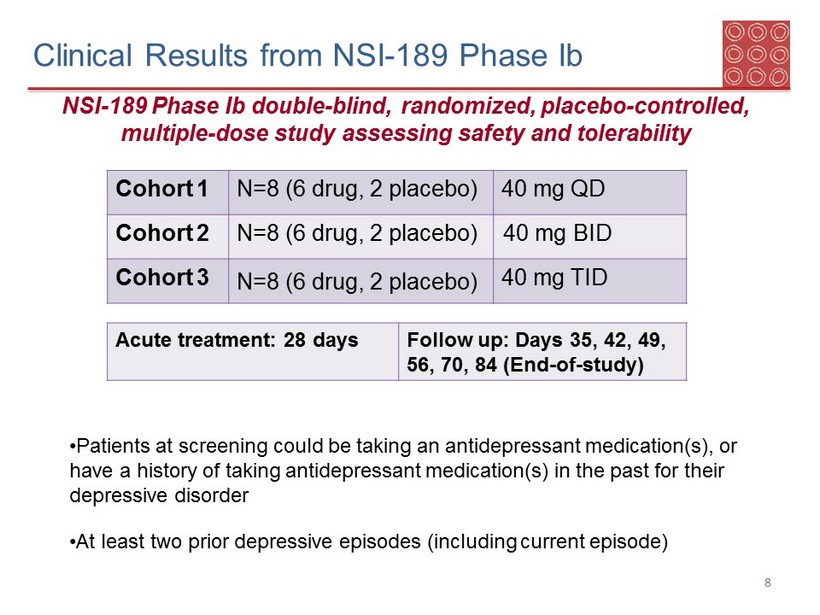
Cohort 1 N=8 (6 drug, 2 placebo) 40 mg QD Cohort 2 N=8 (6 drug, 2 placebo) 40 mg BID Cohort 3 N=8 (6 drug, 2 placebo) 40 mg TID Acute treatment: 28 days Follow up: Days 35, 42, 49, 56, 70, 84 (End - of - study) Clinical Results from NSI - 189 Phase Ib NSI - 189 Phase Ib double - blind, randomized, placebo - controlled, multiple - dose study assessing safety and tolerability • Patients at screening could be taking an antidepressant medication(s), or have a history of taking antidepressant medication(s) in the past for their depressive disorder • At least two prior depressive episodes (including current episode) 8

Clinical Results from NSI - 189 MDD Phase Ib 9 p=0.02 d=0.90 Study Day -20 0 20 40 60 80 100 Symptoms of Depression Questionnaire 2.0 2.2 2.4 2.6 2.8 3.0 3.2 3.4 3.6 3.8 Placebo NS-189 NS-189 1x per day NS-189 2x per day NS-189 3x per day p=0.03 d=1.10 Day 84 Day 28 Symptoms of Depression Questionnaire (SDQ) p=0.09 d=0.95 Study Day 0 20 40 60 80 100 Montgomery and Asberg Depression Rating Scale 5 10 15 20 25 30 Placebo NS-189 NS-189 1x per day NS-189 2x per day NS-189 3x per day p=0.19 d=0.84 Montgomery - Asberg Depression Rating Scale (MADRS) Day 28 Day 84 • Statistically significant improvement, p=0.02, by SDQ • Large effect size of Cohen’s d = 0.95 by MADRS • Responder (≥50% reduction in MADRS): 10/18 or 56%; Remission ( ≤10 score in MADRS): 9/18 or 50% All: A Phase 1B, Randomized, Double - Blind, Placebo - Controlled, Multiple - Dose Escalation Study Evaluating the Effects of NSI - 189 Phosp hate, a Neurogenic Compound, in Patients with Major Depressive Disorder (MDD) , presented June 2014, by Maurizio Fava, M.D., Karl Johe, Ph.D., Lev G. Gertsik , MD, Larry Ereshefsky , PharmD , Bettina Hoeppner , Ph.D., Martina Flynn, David Mischoulon , M.D., Ph.D., Gustavo Kinrys , M.D., and Marlene Freeman, M.D.

p=0.01 d=0.94 Study Day -20 0 20 40 60 80 100 Cognitive and Physical Functioning Questionnaire 2.0 2.5 3.0 3.5 4.0 4.5 5.0 Placebo NS-189 NS-189 1x per day NS-189 2x per day NS-189 3x per day p<0.01 d=1.20 Day 28 Day 84 Clinical Results from NSI - 189 MDD Phase Ib 10 All: A Phase 1B, Randomized, Double - Blind, Placebo - Controlled, Multiple - Dose Escalation Study Evaluating the Effects of NSI - 189 Phosp hate, a Neurogenic Compound, in Patients with Major Depressive Disorder (MDD) , presented June 2014, by Maurizio Fava, M.D., Karl Johe, Ph.D., Lev G. Gertsik , MD, Larry Ereshefsky , PharmD , Bettina Hoeppner , Ph.D., Martina Flynn, David Mischoulon , M.D., Ph.D., Gustavo Kinrys , M.D., and Marlene Freeman, M.D. • Significant (p=0.01) and Large effect size (d=0.94) in cognitive function improvement • Persistent improvement over the drug - free 8 weeks in CPFQ Cognitive and Physical Functioning Questionnaire (CPFQ)

Topographs of High Frequency alpha (10 - 12 Hz): Day 28 from Baseline Left posterior temporal (T5) (t=2.45, p=0.02) Left parietal regions (P3) (t=3.31, p=0.004) qEEG Biomarker Results from NSI - 189 MDD Phase Ib Quantitative EEG ( qEEG ) biomarker: • Increases coherence activity between prefrontal cortex and hippocampus • Two coordinating brain centers utilized for depression and cognition Blood biomarker panel : • Blood panel analysis (78%) correlates to MADRS r esponse r ate: (78%) p artial r esponder (<14) + responders (≥ 50% ) A Phase 1B, Randomized, Double - Blind, Placebo - Controlled, Multiple - Dose Escalation Study Evaluating the Effects of NSI - 189 Phosp hate, a Neurogenic Compound, in Patients with Major Depressive Disorder (MDD) , presented June 2014, by Maurizio Fava, M.D., Karl Johe, Ph.D., Lev G. Gertsik , MD, Larry Ereshefsky , PharmD , Bettina Hoeppner , Ph.D., Martina Flynn, David Mischoulon , M.D., Ph.D., Gustavo Kinrys , M.D., and Marlene Freeman, M.D. 11

Favorable NSI - 189 DMPK Characteristics 12 Human PK supports QD dosing in clinic • t 1/2 is 17 - 20 hours • Total clearance is low (less than hepatic blood flow) • No gender difference in exposure profiles • No difference in AUC and t 1/2 between fasted and fed states Attractive metabolic profile • Few metabolites, multiple pathways, no unique human metabolites (hepatocytes) Attractive pharmaceutical properties • Good solubility and high permeability, single crystalline polymorph, optimized salt form

NSI - 189 Phase II MDD Trial 13 Study Objectives • Primary: Montgomery - Asberg Depression Rating Scale (MADRS) • Secondary: SDQ , HAMD17, CGI - S, CPFQ, SFI (sexual dysfunction), Cogscreen Battery (including Digit Symbol Coding Task), Cogstate Brief Battery Innovative Study Design • Independent , remote, confirmation of MADRS diagnosis by MGH • Placebo - reducing prescreen process • Fewer, quality MDD trial sites (n=12 ) • Three arm: 40mg BID, 40mg QD, & placebo (n=220 randomized) • Potential registration study if successful in either active arm • Power: >80%, 2 - sid ed p ≤ 0.05; d=0.5 Principal Investigator: Maurizio Fava, M.D. Slater Family Professor of Psychiatry at Harvard Medical School, Massachusetts General Hospital Double - Blind, Placebo - Controlled, 2 - Dose Study

Preclinical Data 14 • Orally active, neurogenic, neuroregenerative , compound for the treatment of depression, cognitive impairment, and neurodegeneration • A new chemical entity with novel mechanism of action, molecular target yet unknown, but not mediated by SSRI or SNRI or by BDNF release or via any known GPCRs, kinases, or channels • Stimulates hippocampal neurogenesis and increases hippocampal volume in young, healthy, normal mouse • Shows antidepressant effects in mouse models of depression

P - o - P Activity at Multiple Doses in Novelty Suppressed Feeding Model (Mouse) 15 • NSI - 189 (10 - 100mg/kg) and imipramine significantly decreased the latency to eat compared to vehicle (water) after 28 days of oral dosing but not 1 day (now shown) • No significant treatment effects on either body weight or neurological observations Doses : 10 - 100mg/kg
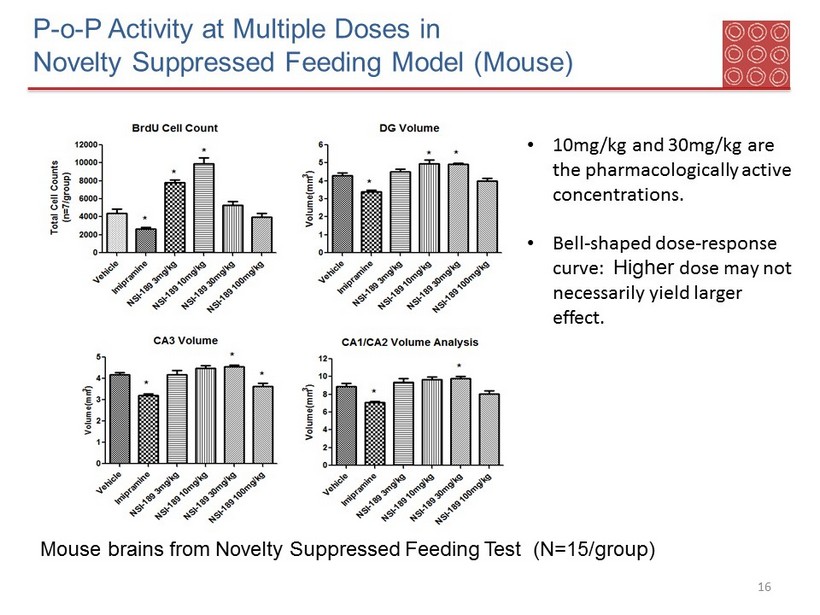
P - o - P Activity at Multiple Doses in Novelty Suppressed Feeding Model (Mouse) 16 Mouse brains from Novelty Suppressed Feeding Test (N=15/group) • 10mg/kg and 30mg/kg are the pharmacologically active concentrations. • Bell - shaped dose - response curve: Higher dose may not necessarily yield larger effect.

Building the Case for MOA 17 No appreciable binding activity against 52 neurotransmitter related receptors/ion channels/ enzymes – Novoscreen : Adenosine , GABA, Glutamate, Histamine, Muscarinic, Nicotinic, norepinephrine, opioid, or and serotonin receptors, Ca++, Cl - , K+ channels, PKA, PKC, CRF, MAO - A/B, or CREB and ERK pathways (related to BDNF release) No binding or functional activities against 900 Other kinases (DISCOVERX KinomeScan ) NSI - 189 Binding Activities ≥ 50% at 10µM Target IC50 (µM) Dopamine Transporter (h) 14.2 Norepinephrine Transporter (h) 1.1 5 - HT Transporter (h) >30 5 - HT3 Receptor 2.1 5 - HT7 Receptor (h) 11.1 Opioid mu Receptor (h) 15.7 Opioid delta 1 Receptor 12.7
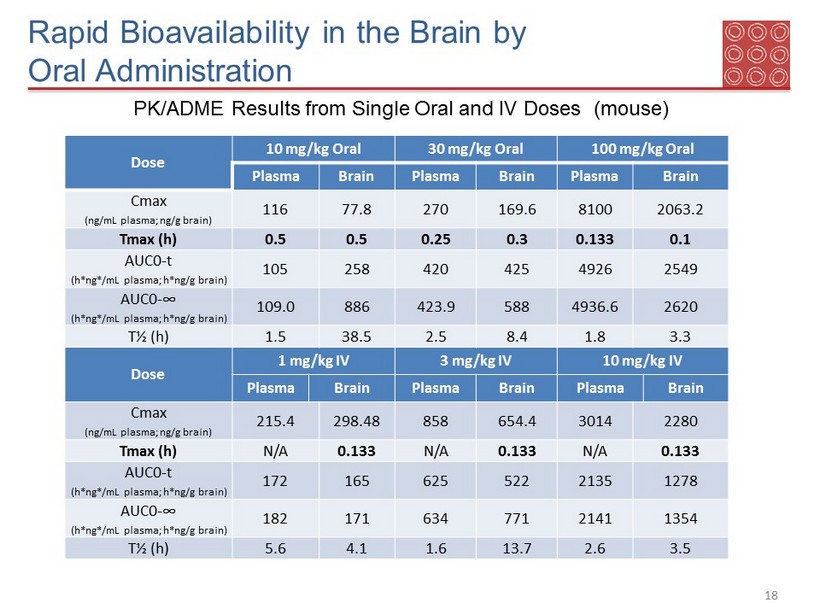
Rapid Bioavailability in the Brain by Oral Administration 18 PK/ADME Results from Single Oral and IV Doses (mouse) Dose 10 mg/kg Oral 30 mg/kg Oral 100 mg/kg Oral Plasma Brain Plasma Brain Plasma Brain Cmax ( ng /mL plasma; ng /g brain) 116 77.8 270 169.6 8100 2063.2 Tmax (h) 0.5 0.5 0.25 0.3 0.133 0.1 AUC0 - t (h* ng */mL plasma; h* ng /g brain) 105 258 420 425 4926 2549 AUC0 - ∞ (h* ng */mL plasma; h* ng /g brain) 109.0 886 423.9 588 4936.6 2620 T½ (h) 1.5 38.5 2.5 8.4 1.8 3.3 Dose 1 mg/kg IV 3 mg/kg IV 10 mg/kg IV Plasma Brain Plasma Brain Plasma Brain Cmax ( ng /mL plasma; ng /g brain) 215.4 298.48 858 654.4 3014 2280 Tmax (h) N/A 0.133 N/A 0.133 N/A 0.133 AUC0 - t (h* ng */mL plasma; h* ng /g brain) 172 165 625 522 2135 1278 AUC0 - ∞ (h* ng */mL plasma; h* ng /g brain) 182 171 634 771 2141 1354 T½ (h) 5.6 4.1 1.6 13.7 2.6 3.5

• Based on human neural stem cell differentiation in vitro • Captures large window of neurodevelopment: neurogenesis to synaptogenesis • Multiple Potential Sites of Action During Stages of Neurogenesis 19 Proprietary Drug Screening Platform Hippocampal Neural Stem Cells 1. Proliferation Immature Neurons Glial Progenitor 2. Differentiation Mature Neurons Committed Neuronal Progenitor Glia Increased Cognition/Function NSI - 106, 127, 144, 149, 150, 158 NSI - 106 NSI - 106, 127, 130 144, 149, 158 183, 185, 190 NSI - 127, 130, 137, 143, 144, 182, 189 3. Maturation/Function 7 days 8 - 21 days

Neuralstem's Library Selection: Potentially CNS - Active Compounds 20 Libraries selected to target neurogenesis – Kinases & phosphatases – Nuclear receptors – Peptide mimetics – GPCRs Five structural libraries chosen for diversity (scaffolds) Selected ~2000 compounds per library – Predict in advance for CNS - Availability – Cover max chemical space within each library In - Licensed Compound Libraries

21 10,269 Small Molecule Compounds through High Content Screen 16 Neurogenic Compounds in vitro 7 Orally Active Neurogenic Leads ( 3 Structural Classes) 16 Tested for Acute Toxicity in Mice 15 Tested for Neurogenesis in Healthy, Adult Mice 1 Development Candidate Selected NSI - 189 Screening Path to NSI - 189 4 Leads Tested in 3 Mouse Depression Models

Neuralstem’s Operational Team 22 CMC Jim Zeller, Ph.D., API Process Chemistry 22 years in Warner - Lambert/Parke Davis/Pfizer, in Holland, MI, NDAs for gabapentin, atorvastatin, quinapril.; 12 years consulting – NDAs for carfilzomib , vismodegib , tedizolid phosphate, lifitegrast , obitecholic acid Sam McClintock, Ph.D., Drug Product Development/Analytical Chemistry 23 years in Merck, 9 years in biotech. Merck Key Contributor to approval & launch of Singulair , Vioxx , and Arcoxia Richard Pariza, Ph.D., Analog Chemistry 30 years in Abbott/Cedarburg Pharm - Tox Grace Furman, Ph.D. , Toxicology 25 years in Pfizer/ Biotechs , NDA for DepoCyt ( cytarabine liposomal), Sutent ( sunitinib malate), Macugen ( pegaptanib ), Sivextro ( tedizolid phosphate) Ronald Christopher, Ph.D., Metabolism/ Clin . Pharmacology 25 years in Merck/Takeda/ Biotechs , NDA for Ultram (tramadol), Nesina ( alogliptin ), and Belviq ( lorcaserin ) William Kramer, Ph.D., PK/PD/Clinical Pharmacology 11 years in Boehringer Mannheim/Schering - Plough, 18 years consulting. Dennis Fisher, M.D., Population PK > 25 years in academia (UCSF) and industry consulting Clinical Trials Andrew Moniz, V.P., 25 years in global CROs, special emphasis in CNS indications: 4 NDAs and 3 ANDAs in CNS Strategic Marketing Terry Frangiosa 20 years in Boehringer Mannheim /Janssen/J&J, Launched /prelaunch Risperdal (Bipolar), Invega Sustenna (Schizophrenia), Esketamine (Depression)
