Attached files
| file | filename |
|---|---|
| EX-24.1 - EXHIBIT 24.1 - Symmetry Surgical Inc. | ssrg-ex241_2015q4.htm |
| EX-10.19 - EXHIBIT 10.19 - Symmetry Surgical Inc. | ssrg-ex1019_2015q4.htm |
| EX-32.1 - EXHIBIT 32.1 - Symmetry Surgical Inc. | ssrg-ex321_2015q4.htm |
| EX-23.1 - EXHIBIT 23.1 - Symmetry Surgical Inc. | ssrg-ex231_2015q4.htm |
| EX-31.2 - EXHIBIT 31.2 - Symmetry Surgical Inc. | ssrg-ex312_2015q4.htm |
| EX-31.1 - EXHIBIT 31.1 - Symmetry Surgical Inc. | ssrg-ex311_2015q4.htm |
| EX-21.1 - EXHIBIT 21.1 - Symmetry Surgical Inc. | ssrg-ex211_2015q4.htm |
| EX-10.18 - EXHIBIT 10.18 - Symmetry Surgical Inc. | ssrg-ex1018_2015q4.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended January 2, 2016
Commission File Number: 001-36770
SYMMETRY SURGICAL INC.
(Exact name of registrant as specified in its charter)
Delaware | 47-1523659 |
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) |
3034 Owen Drive, Antioch, Tennessee | 37013 |
(Address of principal executive offices) | (Zip Code) |
(800) 251-3000 | |
(Registrant’s telephone number, including area code) | |
Securities registered pursuant to Section 12(b) of the Act:
Title of Each Class: | Name of Each Exchange on Which Registered: | |
Common Stock, Par Value $0.0001 Per Share | The NASDAQ Global Market | |
Securities registered pursuant to section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities
Act. Yes o No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the
Act. Yes o No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. ý Yes ¨ No
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (S232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). ý Yes ¨ No
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer ¨ | Accelerated filer ý | ||
Non-accelerated filer ¨ (Do not check if a smaller reporting company) | Smaller reporting company ¨ | ||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). ¨ Yes ý No
The aggregate market value of the voting stock of Symmetry Surgical, Inc. held by non-affiliates of the Registrant as of July 4, 2015, based on the closing price of $8.68, as reported by The NASDAQ Global Market, was approximately $89.4 million.
The number of shares outstanding of the registrant’s common stock as of February 29, 2016 was 10,731,139 shares.
DOCUMENTS INCORPORATED BY REFERENCE
Certain information is incorporated into Part III of this report by reference to the Registrant's 2016 Proxy Statement to be filed with the Securities and Exchange Commission not later than 120 days after the end of the fiscal year covered by this Form 10-K.
SYMMETRY SURGICAL INC.
TABLE OF CONTENTS
PART I | |||
Item 1. | Business | ||
Item 1A. | Risk Factors | ||
Item 1B. | Unresolved Staff Comments | ||
Item 2. | Properties | ||
Item 3. | Legal Proceedings | ||
Item 4. | Mine Safety Disclosure | ||
PART II | |||
Item 5. | Market for the Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities | ||
Item 6. | Selected Financial Data | ||
Item 7. | Management's Discussion and Analysis of Financial Condition and Results of Operations | ||
Item 7A. | Quantitative and Qualitative Disclosures About Market Risk | ||
Item 8. | Financial Statements and Supplementary Data | ||
Item 9. | Changes in and Disagreements With Accountants on Accounting and Financial Disclosure | ||
Item 9A. | Controls and Procedures | ||
Item 9B. | Other Information | ||
Part III | |||
Item 10. | Directors, Executive Officers and Corporate Governance | ||
Item 11. | Executive Compensation | ||
Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | ||
Item 13. | Certain Relationships and Related Transactions and Director Independence | ||
Item 14. | Principal Accounting Fees and Services | ||
PART IV | |||
Item 15. | Exhibits, Financial Statement Schedules | ||
Signatures | |||
Cautionary Note Regarding Forward-Looking Statements
Throughout this Annual Report on Form 10-K, or in our other reports or registration statements filed from time to time with the Securities and Exchange Commission under the Securities Exchange Act of 1934, as amended, or under the Securities Act of 1933, as amended, as well as in documents we incorporate by reference or in press releases or oral statements made by our officers or representatives, we may make statements that express our opinions, expectations, or projections regarding future events or future results, in contrast with statements that reflect historical facts. These predictive statements, which we generally precede or accompany by typical conditional words such as “anticipate,” “intend,” “believe,” “estimate,” “plan,” “seek,” “project,” “potential,” or “expect,” or by the words “may,” “will,” “could,” or “should,” and similar expressions or terminology are intended to operate as “forward-looking statements” of the kind permitted by the Private Securities Litigation Reform Act of 1995. That legislation protects such predictive statements by creating a “safe harbor” from liability in the event that a particular prediction does not turn out as anticipated.
Forward-looking statements convey our current expectations or forecast future events. While we intend to express our best judgment when we make statements about what we believe will occur in the future, and although we base these statements on assumptions that we believe to be reasonable when made, these forward-looking statements are not a guarantee of performance, and you should not place undue reliance on such statements. Forward-looking statements are subject to many uncertainties and other variable circumstances, many of which are outside of our control, that could cause our actual results and experience to differ materially from those we thought would occur.
We also refer you to, and believe that you should carefully read, the "Risk Factors" portion of this report to better understand the risks and uncertainties that are inherent in our business and in owning our securities.
Any forward-looking statements which we make in this report or in any of the documents that are incorporated by reference herein speak only as of the date of such statement, and we undertake no ongoing obligation to update such statements. Comparisons of results between current and any prior periods are not intended to express any future trends or indications of future performance, unless expressed as such, and should only be viewed as historical data.
PART I
Dollars are in thousands unless otherwise noted. We are an "emerging growth company" as that term is defined in the Jumpstart Our Business Startups Act of 2012 and, as such, we have elected to comply with certain reduced public company reporting requirements.
Item 1. Business
Separation from Symmetry Medical Inc.
In August 2014, Symmetry Medical Inc. (“SMI”) announced that it had entered into an Agreement and Plan of Merger, dated as of August 4, 2014 (the “Merger Agreement”), by and among SMI, TecoStar Holdings, Inc., Tecomet, Inc., and TecoSym, Inc. (“Merger Sub”), pursuant to which Merger Sub was merged with and into SMI, with SMI continuing as the surviving corporation (the “Merger”). As a result of the Merger, all of the outstanding shares of common stock of Symmetry Surgical Inc. ("Symmetry Surgical," the "Company", "we," "us," or "our") were spun-off to SMI’s stockholders and the holder of each outstanding share of common stock of SMI received one-quarter of a share of common stock of Symmetry Surgical. We refer to this transaction as the “Spin-Off.”
In connection with the Spin-Off, SMI entered into a Separation Agreement, dated as of August 4, 2014 (the “Separation Agreement”) with the Company. Pursuant to the Separation Agreement, SMI transferred all of the assets and liabilities of its Symmetry Surgical Business to the Company prior to the consummation of the Merger and the Spin-Off. The Separation Agreement provides for various agreements and arrangements governing Symmetry Surgical's relationship with SMI after the Spin-Off, including a Supply Agreement, dated as of August 4, 2014 (the "Supply Agreement") which governs pricing and supply requirements for products each company sources from the other. Following the completion of the Merger and the Spin-Off on December 5, 2014, Symmetry Surgical began to operate as an independent publicly-traded company.
Overview
Symmetry Surgical is a Delaware corporation headquartered in Nashville, Tennessee. We are a global marketer and distributor of medical devices with some limited manufacturing focused on the general surgery market. We offer over 20,000 products and sell primarily to hospitals and surgical centers in the United States and countries worldwide through direct representatives, dealers and distributors. Specifically, our current product portfolio includes a broad range of reusable stainless steel and titanium, hand-held general and specialty surgical instruments, single use and disposable instruments, electro-surgery instruments, retractor systems, ligation clips and appliers, and containers and sterilization devices sold directly to hospitals and other sites of care.
We expect the global healthcare industry to continue to grow as populations age, access to care in developing nations increases, and treatment advances in medical care are achieved. We also believe that cost containment in healthcare is becoming an increasingly critical issue in both government-funded and privately insured populations. At the same time, we believe that there is an expectation of continued improvements in the standard of care and prevention, although there will now be an economic component of the discussion. Our company competes in the General Surgery market of medical devices. The General Surgery market includes the following market segments: Open Surgery Instruments, Minimally Invasive Surgery Instruments, Energy Based and Powered Instruments, Adhesion Prevention Products, Disposable Surgical Supplies, and Medical Robotics and Computer Assisted Surgery. Our primary historical focus has been on reusable general surgery instruments and sterilization containers, a market which includes the Open Surgery, Minimally Invasive Surgery, and Energy segments. Looking ahead, we are planning to expand our target market to include general surgery devices used across a range of specialties in which we already have a presence, including: Arthroscopy, Bariatric, Colo-Rectal, Neurosurgery, OB/Gyn, Orthopedics, Pediatrics, Spine, Thoracic, Urology, General Surgery, Ophthalmology, Otolaryngology/ENT, Plastics/Reconstructive, Peripheral Vascular, Cardiovascular, Neurovascular, and Endovascular. We intend to focus on devices which are reusable, single-use/disposable, or re-posable, or devices with a reusable handle and disposable active component.
We compete with divisions of large, multi-national branded medical device companies including segments of Aesculap, a division of B. Braun Medical, Inc., V. Mueller, a division of CareFusion, Jarit/Miltex, the surgical instrument brands of Integra Life Sciences, Teleflex Incorporated, and Karl Storz, as well as hundreds of smaller, independent suppliers of specific instruments located throughout the world. The incremental markets which we intend to pursue are dominated by these and other large multi-national corporations as well as smaller regional global competitors and start-up/entrepreneurial companies. We expect that growth in our historical market will be driven by the following factors:
• | Macro-economics and demographics driving overall hospital procedural growth; |
• | Capital investment in new hospital and/or new operating room construction, especially in developing countries; |
• | Customer cost pressures increasing the use of reusable or “re-posable” surgical instruments versus single use/disposable instruments; and |
• | Innovations that result in a reduction of labor required during surgery, decreased operating room times, or other reductions in costs of service. |
We expect market growth in the incremental markets which we intend to pursue to be driven by a number of factors, including but not limited to:
• | Macro-economics and demographics driving overall hospital procedural growth; |
• | Innovations that result in a reduction in labor required during surgery, decreased operating room times, or other reductions in costs of service or increased efficiency in providing services; and |
• | The migration of care to lower acuity sites and the demand for single use instruments at those sites. |
We are dedicated to developing high-quality surgical instruments that respond to the needs of clinicians as they arise, making a real difference in the lives of patients. Our broad portfolio brings together the rich history and distinctive product lines of three SMI acquisitions: the surgical instruments and sterilization container business of Specialty Surgical Instrumentation, Inc. ("SSI"); the electro-surgery instrument business of Olsen Medical, LLC ("Olsen Medical"); and the stainless steel and titanium surgical instrument portfolio of Codman & Shurtleff, Inc., a subsidiary of Johnson & Johnson; as well as our acquisitions of key assets of Vesocclude Medical, LLC. ("Vesocclude Medical" or "Vesocclude") and the assets from Insightra Medical, Inc. ("Insightra") that will become the Symmetry AccessTM Low Profile Retractor.
Our business was established in 1976 as Specialty Surgical Instrumentation, Inc. SMI acquired Specialty Surgical Instrumentation, Inc. in 2007 and added two additional acquisitions, Olsen Medical and the Codman & Shurtleff, Inc. instruments portfolio, to create the Symmetry Surgical division of SMI in 2011. Symmetry Surgical Inc. was incorporated in July 2014 as a wholly-owned subsidiary of SMI. We followed these acquisitions with our 2015 acquisitions of key assets of Vesocclude Medical and selected assets from Insightra.
In 2011, we acquired certain assets of Olsen Medical, a division of PSC Industries, Inc. Olsen Medical manufactures a full line of single-use and reusable bipolar and monopolar forceps, cords, electrosurgical pens/pencils, electrodes, and accessories.
Also in 2011, we acquired the surgical instruments product portfolio from Codman & Shurtleff, Inc., a subsidiary of Johnson & Johnson. The assets acquired in this transaction included certain U.S. and German-based personnel, as well as inventory, intellectual property, trademarks, regulatory approvals, and an instrument procurement center located in Tuttlingen, Germany. The addition of this product portfolio allowed us to offer a broader array of medical instruments and related products, expand our global distribution, increase our intellectual property, trademarks, and regulatory approvals, and obtain an instrument procurement center and personnel located in Tuttlingen, Germany.
From 2012 to 2014 we executed on the integration of our 2011 acquisitions. This process included the establishment of a new global distribution center at our Nashville, Tennessee headquarters, the implementation of a new ERP system for order to cash and supply chain processing, label changes for all acquired products, the establishment of a global distributor network, the integration of an instrument quality and procurement facility in Tuttlingen, Germany, the integration of the Louisville, Kentucky instrument finishing and packaging facility (primarily devoted to Olsen Medical products), and the initiation of cross training our direct selling force in the United States. In 2013, we further expanded our ability to provide global supply chain management and international commercial operations with the opening of our Symmetry Surgical Switzerland subsidiary based in Schaffhausen, Switzerland. Additionally, we began the process of establishing country-specific regulatory approvals for those of our legacy products which were not historically sold outside the U.S. We also engaged in the education and training of our international distributor network and made our first Symmetry Surgical comprehensive catalog available to the U.S. and international markets.
On August 28, 2015, we acquired key assets of Vesocclude Medical located in Raleigh, North Carolina for $4,055 in cash and up to an additional $2,200 in contingent consideration (based on achievements of milestones discussed in Note 4 of the financial statements); each of which are gross of the Company's 4.8% existing minority interest in Vesocclude. Vesocclude's portfolio of ligation clips and appliers are used in surgical procedures to close tubal anatomic structures such as blood vessels or ducts. The portfolio consists of several sizes of titanium clips used to close the structures, which come loaded in a patented "push and click" cartridge system that facilitates easy loading into either open surgical or endoscopic appliers. Vesocclude products are primarily sold in the U.S. direct to hospitals but in some territories are sold through stocking distributors and kit packers. Internationally, Vesocclude products are sold through stocking distributors.
On December 1, 2015, we acquired the patent protected, ultra-low profile, soft-tissue retraction technology and related product portfolio and other assets from privately held Insightra Medical for $400 in cash as well as a royalty on future sales. The portfolio, formerly known as ReeTrakt, will be re-launched under the Symmetry Surgical brand as the Symmetry Access™ Low Profile Retractor beginning in the first half of 2016. The Symmetry Access™ Low Profile Retractor is a single-use product with an ultra-low profile that supports minimal/small incision surgical procedures by providing better surgical access and field of view. The product is designed to minimize tissue damage by providing gentle and adjustable tissue retraction without a bridge, arms or handles that reduce access to the surgical site. The portfolio offers a variety of tissue retraction tips and adheres securely to the surgical drape or skin, eliminating the need for hand-held retraction during a case. We plan to launch this product through our existing sales channel in the U.S and internationally.
Products from all of the above described acquisitions are now sold by Symmetry Surgical’s sales force in the U.S. and internationally through country-specific distributors.
Symmetry Surgical’s products are common to a wide range of surgical procedures, enabling clinicians to expose, grasp, cut, clamp, and repair during virtually all types of surgery, as well as clean and sterilize following surgery. They are subject to our quality standards and are only made available to the commercial marketplace after passing inspection tests and obtaining appropriate regulatory approvals. Our collective one-hundred-year-plus heritage is well represented by our brands, which we believe are respected by clinicians and hospital customers and many of which are backed by intellectual property rights.
Our U.S.-based marketing team collaborates with internal and external engineers and product developers to create a product pipeline that addresses unmet needs for the surgical specialties which we serve in the product categories in which we compete. Our new product development team also collaborates with surgeon innovators to develop products that fill needs and are well-suited to surgeons’ preferences. Once product designs are finalized, they are sourced by Symmetry Surgical from a broad range of instrument manufacturers in the U.S., Germany, and other regions of the world, or manufactured internally if appropriate.
Commercial demand is generated by both direct sales representatives and geographically defined authorized dealers in the U.S. as well as local market distributors outside the U.S. Symmetry Surgical does not maintain a direct sales force outside the U.S., although we employ regionally-based business development Teammates to collaborate with country-based distributors to generate demand and reinforce Symmetry Surgical’s standards for marketing, sales, and compliance. Sales outside the U.S. are accomplished through authorized distributors who purchase products from us and then sell the products to the final customer and are accountable for inventory and accounts receivable in local markets. In the U.S., our direct representatives are compensated in a variety of manners, but primarily via commission. U.S.-based dealers are compensated via commission for sales processed by Symmetry Surgical. Customer orders are received by customer service and physically processed at our Nashville, Tennessee headquarters and thereafter distributed by third party carriers and freight forwarders worldwide.
Mission/Vision
Our mission is to be the future of surgical instruments. Our goal, through organic growth and acquisition, is to grow into a company with stronger gross margins that delivers consistent cash flow and is recognized for being customer centric and developing high-quality surgical instruments that respond to the needs of clinicians - making a real difference in the lives of patients and the economics of care. We believe we can deliver on this goal by achieving our aspirations and capitalizing on what we believe to be our competitive advantages, as outlined in our Strategy and Approach.
We are dedicated to improving patient clinical and economic outcomes and delivering exceptional quality and service to our customers. We are collaborative with our customers, responding directly to clinician and provider needs with new innovations that help them deliver exceptional care in a total value environment. We strive to be more responsive than our competition, which often consists of minor divisions in much larger corporate entities.
Strategy and Approach
We aspire to:
• | Be recognized as a leading global medical device company that is customer-centric and brand-driven, with a unique clinical and health economic focus on surgical instruments needed routinely in operating rooms, surgery centers, and sites of care that are not reimbursement specific. |
• | Create an attractive environment for our engaged employees that is appealing and challenging, while providing opportunities for empowerment and growth that makes us an employer of choice with a culture that is data, process, compliance, and results focused, action oriented, accountable, proud and innovative. |
• | Provide flawless quality, global regulatory registration and compliance with integrated quality systems and excellent supplier quality management. |
• | Earn long-term customer loyalty through engaging customer experiences. |
• | Achieve sustained profitable growth by innovating and launching new products, gaining market share through our sales and professional education efforts, in-licensing new products and Alliance Partners Products, and seamlessly integrating acquisitions. |
• | Be a recognized acquirer and integrator with the talent and infrastructure to drive the benefits of acquisitions quickly. |
• | Manage an advanced supply chain built on lean thinking and continuous improvement that maximizes customer service and achieves competitive costs and cash flow generation while managing a comprehensive instrument and device portfolio. |
• | Nourish enduring brand health through a differentiated portfolio of products and services that deliver economic and clinical value accomplished through external and internal innovation developed and brought to market on a consistent, predictable rhythm. |
We intend to focus our energies in the following areas, which we believe will create competitive advantages for our business:
• | A Direct Sales Channel in the U.S. We believe our talented sales force (both employee and dealer) and Professional Education Teammates will build long term relationships with a wide range of customers - clinicians, operating room directors, sterile processing, and materials management - with an offering based on total value, customer centric responsiveness, and training to drive market share growth. |
• | Strategic Relationships with Group Purchasing Organizations ("GPOs") and Integrated Delivery Networks though Access to Key Decision Makers. We believe our breadth and expansive scope of products positions us as an important partner with our customers in reusable general surgical instruments and other general surgery products. We believe this position will give us access to key decision makers with whom we intend to continue to build strategic relationships and serve their multiple members and sites of care. |
• | Sales Synergies by Cross-Selling Products and Offerings. Symmetry Surgical offers over 20,000 products to our global customers. We believe we can leverage the sales synergies created by this expansive product offering - and complementary additions - to generate increased revenue. |
• | Broad Portfolio of Products and Trusted Brands. We believe our extensive product portfolio and distribution capability allow us to meet our customers’ needs across numerous locations on a timely basis from our distribution center located in Nashville, TN. We believe our breadth and scope of products allows our customers to reduce their number of suppliers and streamline their procurement. We believe our trusted brands, which include Bookwalter®, Greenberg®, Olsen®, Magna-Free®, The Ultra System®, Access Surgical International®, Classic® /Classic Plus®, Opti-Length®, RapidClean®, VesoccludeTM, and Symmetry AccessTM are attractive as physician preference items. Our products capitalize on our portfolio of patents and trademarks. |
• | Quality and Regulatory Compliance. Our quality systems are based upon and in compliance with International Organization for Standardization (ISO) requirements and, where applicable, United States Food and Drug Administration (FDA) regulations. We believe our quality and regulatory compliance systems meet or exceed our customers’ expectations. |
• | Global Reach. Commercial demand is generated by scores of geographically defined authorized distributors worldwide. |
• | Rationalized and Reliable Supply Chain. Under the direction of Symmetry Surgical Switzerland, our Tuttlingen, Germany facility provides sourcing and quality services for products procured in Germany, as well as other regions of the world through strategic suppliers. We will strive to build a lean supply chain that achieves high service levels, with optimal working capital investment and competitive margins. |
• | Innovation. We believe our product portfolio will continue to expand through the addition of products identified to solve our customers’ unmet needs - both clinical and economic. We intend to collaborate with surgeons to innovate, design, develop, prototype, source, register, and market proprietary products based on intellectual property. |
• | Increased Presence In General Surgery By Diversifying Our Revenue Base and Expanding Our Sales Channels to Market. We believe we have a large footprint in the acute care surgical instruments market and a presence in a wide array of surgical interventions - both domestically and abroad. We believe we will continue to grow our go to market channel by serving adjacent clinicians beyond the operating room setting, operating room directors, hospital material managers, and hospital central sterilization in the acute care market as well as expanding to other sites of care. |
• | Continue to Expand Our Offerings Through External Collaboration. We believe that our current portfolio, U.S. direct sales force, professional education capabilities, and global customer access offer new and innovative medical companies a meaningful channel to market their products, enabling us to realize revenue through licensing, distribution agreements, or acquisition. We will look to expand our offerings across the general surgery market and plan to be an acquirer/licensor of strategically appropriate companies and products. |
• | Being Responsive. We believe we can be more responsive to our customers’ needs than our competitors, which in many cases are relatively minor divisions in much larger corporate entities. |
Products Overview - Surgical Instruments and Related Products
The medical devices we offer are predominantly classified as general surgery instruments and are commonly found in operating rooms and utilized by our customers in a wide range of surgical interventions. Our portfolio of surgical instruments, which includes retractor instruments / systems of instruments, electro-surgery instruments, ligation clips and appliers, general instruments and specialty instruments are complemented by our offering of containers and sterilization devices which are used to enable the sterilization and storage of surgical instruments. We also offer ancillary products, including fiber optic light sources for surgery and non-toxic enzymatic detergents for cleaning instruments. We offer all these products to our customers as a comprehensive surgical instruments portfolio. While we manage our business in one segment we report on revenue by two categories:
• | Symmetry Surgical Branded Products, which include products with Symmetry Surgical-owned brands sold in the United States and worldwide. Today, this includes surgical instruments such as retractor instruments/systems, electro-surgery instruments, ligation clips and appliers, containers and sterilization devices and general and specialty instruments. |
• | Alliance Partners Products, which include complementary products offered by other manufacturers which Symmetry Surgical distributes, primarily in markets within the United States. Today, this includes complementary general surgery instruments used in surgical lighting, laparoscopic surgery, and accessories. We report on revenue in this category separately because we offer these products for sale in our capacity as a distributor, and we do not own the underlying intellectual property, nor are we the manufacturer of record and the gross margin profile is different than our Symmetry Surgical branded products. |
We believe our collective heritage is well represented by our brands (which include Symmetry®, Bookwalter®, RapidClean®, Classic Plus® and Classic®, Microsect®, Olsen®, Secto®, Opti-Length®, Magna-Free®, Access Surgical International®, Greenberg®, Riley Medical®, Quad-Lock®, FlashPak®, The Ultra System®, VesoccludeTM, and Symmetry AccessTM). We believe that these brands are respected by clinicians and hospital customers and they are all backed by intellectual property. These general surgery instruments typically fall into five categories (described below), however, we offer them as a comprehensive portfolio.
• | Retractor Instruments/Systems, which include the original, market-leading Bookwalter® retractor system and Greenberg® neurosurgical retractor systems. Additionally, our broader retractor portfolio provides access and visualization for spinal, orthopedic, plastic and general surgery procedures. The portfolio includes both reusable as well as single-use / disposable items. |
• | Electro-surgery Instruments, which includes the Olsen Medical® line of bipolar and mono polar single-use and reusable instruments, including the Midas Touch® non-stick cermet coating as well as attachment configurations for most generators. |
• | Containers & Sterilization Devices, which include the market leader in immediate-use sterilization, the Flash Pak®, which was designed specifically to allow delivery of sterile instruments to the point of care within minutes of sterilization. We also offer a range of products including the Quad-Lock® container system, developed to address sterilization integrity during surgical instrument transportation into the operating room, and The Ultra Container System, designed to meet needs for durability in a lightweight container system. |
• | General and Specialty Instruments are offered in one of the most comprehensive portfolios of general instrument devices in the industry - carrying innovative, hard-to-find products across a range of surgical interventions. |
• | Ligation Clips and Appliers are used in surgical procedures to close tubal anatomic structures such as blood vessels or ducts. The portfolio consists of several sizes of titanium clips used to close the structures, which come loaded in a patented "push and click" cartridge system that facilitates easy loading into either open surgical or endoscopic appliers. |
In total, we offer over 20,000 products in our catalog.
Competition
Our business competes with divisions of large, multi-national branded medical device companies including segments of Aesculap division of B. Braun Medical, Inc., V. Mueller, a division of CareFusion Becton Dickinson, Jarit/Miltex, the surgical instrument brands of Integra Life Sciences, Teleflex Incorporated, and Karl Storz, as well as hundreds of smaller, independent suppliers of specific instruments located throughout the world. We compete with our larger competitors on the basis of product quality, breadth of product offering, reputation for sourcing from quality manufacturers, clinically trained sales force, training/education, product performance, value/cost relationship, product availability, innovation, and responsiveness to tender opportunities and other customer needs. We compete with the smaller independent competitors on the basis of breadth of product offering, clinically trained sales force, training/education, product quality, product performance, value/cost relationship, product availability, innovation, and responsiveness to tender opportunities and other customer needs. We also compete with substitute devices such as re-processed single use instruments or substitute therapies.
Research & Development
In response to a product portfolio roadmap created by our strategic marketing team in our innovation process, our Research and Development Teammates design, develop, prototype, commercialize (in collaboration with our Supply Chain and Quality and Regulatory teams), contribute to the development of training and education materials (in collaboration with our Professional Education Team), and prepare proprietary products for market. Our engineers and product developers strive to create products and capabilities that address unmet clinical, physician and patient satisfaction, and economic needs in the surgical specialties and sites of care which we serve. We focus on products which are regulated as Class I and II devices in the United States and require a less significant investment in research and development. We aim to invest approximately 1% of revenue in research & development efforts. We can collaborate with surgeon innovators from conception through launch to ensure that our products will meet the needs of healthcare providers in the clinical setting. We compensate health care professionals for their contributions of intellectual property or consulting services in the product development process consistently with our healthcare compliance guidelines and all applicable laws and regulations. Additionally, we collaborate with external design and development firms and inventors, as well as strategic suppliers in the innovation process. We perform research and development at our Nashville, TN and Raleigh, NC locations.
Intellectual Property
We rely on a combination of trademarks and patents, together with non-disclosure and confidentiality agreements, to establish and protect the proprietary rights in our technologies, products and brands. Our current intellectual property portfolio is comprised of 113 issued and 53 pending U.S. and non-U.S. patents (including in-licensed patents) and 60 issued and pending U.S. and non-U.S. trademarks. Our patents and patent applications cover the following areas of our technology:
• | Branded Containers and Sterilization Devices; |
• | Electro-surgery; |
• | Branded Retractor Systems; |
• | Ligation Clips and Appliers; and |
• | Specialty Instruments. |
Our current patents expire between January 30, 2017 and November 26, 2033. We actively monitor our intellectual property by periodically reviewing new developments to identify extensions to our patent portfolio and employ external patent attorneys to assist us in managing our intellectual property portfolio.
Some of the intellectual property used in our business has been cross-licensed on a perpetual royalty-free basis from our former parent company, SMI, since the closing of the Merger. Intellectual property that we own and that is licensed to SMI cannot be used by SMI in a manner which is competitive with Symmetry Surgical and we have similar restrictions on the use of SMI's intellectual property that we license. Both companies’ use of the intellectual property is governed by the shared IP cross license agreement described in the Merger Agreement.
The following are representative of our registered trademarks, and we believe that all of these provide significant value via customer recognition and perception: Symmetry®, Bookwalter®, RapidClean®, Classic Plus® and Classic®, Microsect®, Olsen®, Secto®, Opti-Length®, Magna-Free®, Access Surgical International®, Greenberg®, Riley Medical®, Quad-Lock®, FlashPak® Symmetry Sharp KerrisonTM , The Ultra System®, VesoccludeTM, and Symmetry AccessTM.
While we believe our patents are valuable, we believe our knowledge, experience, proprietary and trade secret information, manufacturing processes, product design and development staff and sales staff have been equally or more important in maintaining our competitive position. We seek to protect our non-protected know-how, trade secrets, processes and other proprietary confidential information principally through confidentiality, non-compete and invention assignment agreements.
Government Regulation and Regulatory Compliance
We are a manufacturer and marketer of medical devices, and therefore are subject to governmental regulation (including the Food and Drug Administration and the Center for Medicare Services in the United States, and other United States and international agencies). We are also subject to federal, state and local environmental laws and regulations governing the emission, discharge, use, storage and disposal of hazardous materials and the remediation of contamination associated with the release of these materials at our facilities and at off-site disposal locations. We are also subject to various other environmental, transportation and labor laws as well as various other directives and regulations both in the U.S. and abroad.
We maintain a regulatory program to assure compliance with all applicable U.S. and international regulatory standards and directives with regard to our business. Our regulatory program focuses primarily on minimizing any risks associated with noncompliance with requirements or standards that could impact our products’ fit, form and function (including design, manufacture, testing, labeling, promotion and sales of the devices, maintenance of records, and traceability). We also place great emphasis on maintaining and following effective auditing practices and procedures to assure compliance with all internal and external standard operating procedures and 510(k) process requirements. The vast majority of our products are Class I or 510(k) devices. Finally, we conduct ongoing due diligence to monitor and assure compliance with all country of origin requirements, import and export, certifications with regard to international regulatory agencies, and customer complaints/product defects.
Manufacturing and Materials
We perform limited manufacturing and packaging in the U.S. at our Louisville, Kentucky and Nashville, Tennessee facilities and operate quality and procurement centers in the U.S., Switzerland, and Germany, all under the leadership of our Swiss subsidiary. These centers engage with suppliers to manufacture to our specifications. Our manufacturers use raw materials, including stainless steels, plastics, titanium, and various other components in the manufacture of our products that have a long history of use in clinical settings. We currently acquire certain services, materials, products or other assets
from third parties and outsource certain aspects of our business. While we believe we have a reliable supplier base with alternative sources for our products, we do not normally maintain multiple sources for products and transferring a product from one supplier to another can only be done after an appropriate period to ensure quality and regulatory compliance. Thus, our performance is often a reflection of the performance of our supplier base and our efforts to manage them.
Customers
Products are sold primarily to the tertiary hospital operating room environment, although growth is coming increasingly from a migration of site of care to ambulatory surgery centers and physician offices for select procedures. We believe that our well-established customer relationships - based on total value, responsiveness, and training - with GPOs, Integrated Delivery Networks, hospital materials management, operating room directors, and clinicians has positioned us to drive growth. In the U.S., we sell through a combination of direct representatives and authorized dealers (who do not take title to the products) in specific geographies. Internationally, we sell through country specific distributors who take title and represent us in their respective local markets.
During fiscal 2015, we sold products to nearly 5,000 customers. We have relationships with several GPOs with sales on their contracts representing, in the aggregate, 38%, 32% and 20% of revenue in fiscal 2015, 2014 and 2013, respectively.
We sell our products to customers both domestically and in approximately 100 countries worldwide, with the vast majority of our sales in less than 30 countries. Approximately 87.3%, 87.5% and 88.1% of our revenue in fiscal 2015, 2014 and 2013, respectively, was from sales to customer in the United States. Approximately 12.7%, 12.5% and 11.9% of our revenue in fiscal 2015, 2014 and 2013, respectively, was from sales to customers internationally, based on the location of the customer to which we shipped our products. For additional geographic information see Note 20 to the Financial Statements.
Sales and Marketing
Our sales and marketing efforts emphasize the quality, clinical performance, and comprehensive breadth of our product line. Sales and marketing personnel are predominantly located in the U.S., although we have regionally-based business development leaders to assist in driving growth through our global network of distributors. U.S. sales are through a combination of direct representatives and dealers who generate demand which is fulfilled directly by Symmetry Surgical. Our hospital customers include clinicians, Operating Room Directors, hospital Materials Management, hospital Central Sterilization, multi-hospital strategic sourcing entities, and GPOs. Additionally, we are generating demand in ambulatory surgery centers, physician’s offices, and other evolving sites of care through our e-commerce efforts. Our commercial focus capitalizes on: tender opportunities for new or updated operating rooms where customers seek to outfit a full range of capabilities, new surgeons or new services being added to an existing operating room that require a specific clinical focus of instruments, introduction of specialized clinical innovation and new products, and replacement of existing products which have reached the end of their life cycle. Our customer interactions often involve training and education in the use of our products. Our sales personnel are technically trained and are based in the territories they serve. This enables us to be responsive to the needs of our customers and actively involved in the planning and developing of future opportunities.
Quality Assurance
We maintain a comprehensive quality assurance and quality control program, which includes the control and documentation of all product specifications, material specifications, operating procedures, equipment maintenance and quality control methods. Our quality systems are based upon FDA requirements and the ISO standards for medical device manufacturers. We believe that all of our facilities are currently in substantial compliance with regulations applicable to them. For example, for our U.S. and German facilities these regulations include the current Good Manufacturing Practice regulations and other quality system regulations administered by the FDA. Our facilities in Louisville, Kentucky, Nashville, Tennessee, and Tuttlingen, Germany are currently ISO 13485 certified and registered and subject to inspection by the FDA. Our facility in Louisville, Kentucky was inspected by the FDA in 2015 and received four Form 483 observations. These have subsequently been addressed. All facilities were inspected by notified bodies in 2015. Our U.S. facilities have also obtained Certificates of Foreign Government (CFG), which are required for importing medical devices to many foreign markets, and Canadian Medical Device Regulations (CMDR) certificates, certifying them to the Canadian Medical Devices Conformity Assessment System, which is a prerequisite for incrementally acquiring device licenses from Health Canada.
All aspects of the supply chain are integrated into our overall quality system. Our suppliers are evaluated and audited to assure compliance with all international trade compliance quality standards. Relative to our manufacturing processes, we maintain and adhere to specific standard operating procedures within our quality systems to ensure compliance with our requirements for our products. The suppliers we utilize in the distribution process are evaluated to assure compliance with all international trade compliance quality standards.
Employees
As of February 29, 2016 the Company had 196 employees, of which 192 are full-time employees and four are part-time or seasonal, with dozens of independent dealers and distributors to supplement this team. None of our U.S. employees are represented by a labor union or covered by a collective bargaining agreement. Our Swiss employees are not unionized and we are not aware of any employee consideration of or efforts to become unionized. Our German employees are represented by a works council pursuant to applicable local country laws and regulations. We consider our relations with our employees to be good.
Environmental Issues
Our facilities and operations are subject to extensive federal, state, local and foreign environmental and occupational health and safety laws and regulations. These laws and regulations govern, among other things, air emissions; wastewater discharges; the generation, storage, handling, use and transportation of hazardous materials; the handling and disposal of hazardous wastes; the cleanup of contamination; and the health and safety of our employees. Under such laws and regulations, we are required to obtain permits from governmental authorities for some of our operations. If we violate or fail to comply with these laws, regulations or permits, we could be fined or otherwise sanctioned by regulators. We could also be held responsible for costs and damages arising from any contamination at our past or present facilities or at third-party waste disposal sites. We cannot completely eliminate the risk of contamination or injury resulting from hazardous materials, and we may incur material liability as a result of any contamination or injury.
Capital Investment
Information concerning our capital expenditures is presented under the caption “Capital Expenditures” in Item 7, Management’s Discussion and Analysis of Financial Condition and Results of Operations in this Form 10-K.
Executive Officers of the Registrant
Set forth below are the name, age, position and a brief account of the business` experience of each of the Company’s executive officers as of March 1, 2016.
Name | Age | Position | ||
Executive Officers: | ||||
Thomas J. Sullivan | 52 | President, Chief Executive Officer and Director | ||
Scott D. Kunkel | 42 | Senior Vice President, Chief Financial Officer | ||
David C. Milne | 48 | Senior Vice President, General Counsel, Chief Administrative Officer, Corporate Secretary and Chief Compliance Officer | ||
Ronda L. Harris | 45 | Vice President, Chief Accounting Officer | ||
Jose E. Fernandez | 47 | Senior Vice President, Chief Technology Officer | ||
Thomas J. Sullivan is Symmetry Surgical's President and Chief Executive Officer and a Director. Mr. Sullivan served in these capacities for SMI from 2011 to 2014. From June of 2007 through January 2011, Mr. Sullivan was the President of the Supply Chain & Business Process Division of Johnson & Johnson Health Care Systems Inc. (“J&J”). He held numerous executive and functional leadership roles at J&J from 1990 when he joined as an Intern through January 2011. From 2005 to 2007, Mr. Sullivan was the President of DePuy Orthopaedics, Inc. From 2002 to 2005 he served as President of J&J Medical Products Canada. From 1999 to 2001, Mr. Sullivan served as General Manager for J&J Gateway LLC and Worldwide Vice President of e-Business. Prior to J&J, Mr. Sullivan served in management roles at Bell Atlantic / Verizon. Mr. Sullivan graduated as a Palmer Scholar from The Wharton School in 1991 where he earned an MBA in Strategic Management and Information Technology. He also holds a Bachelor of Science magna cum laude in Applied Mathematics and Computer Science from the University of Pittsburgh. He is a member of the National Association of Corporate Directors (“NACD”) and in 2013 earned
his NACD Governance Fellow certification. Mr. Sullivan is also a Director of Span-America Medical Systems Inc. (NASDAQ: SPAN).
Scott D. Kunkel is Symmetry Surgical's Senior Vice President, Chief Financial Officer, a position he assumed on December 5, 2014. Mr. Kunkel joined SMI in March 2009 as a Director of Finance, a position he served until 2011. From 2012 through December 5, 2014, Mr. Kunkel served as Vice President Finance at SMI's Symmetry Surgical division. Before joining SMI, Mr. Kunkel was the Vice President Finance & Administration and Member at EnergyLogic, LLC from February 2004 to March 2009. Mr. Kunkel came to EnergyLogic from General Electric, where he was a Finance Manager at GE Consumer & Industrial from April 2000 to February 2004. Mr. Kunkel started his career in January 1996 as a Financial Analyst for Ford Motor Company. Mr. Kunkel graduated magna cum laude from the University of Notre Dame with a Bachelor of Business Administration degree and received his MBA from the Indiana University Kelley School of Business.
David C. Milne is Symmetry Surgical's Senior Vice President, General Counsel, Chief Administrative Officer, Corporate Secretary and Chief Compliance Officer. Mr. Milne joined SMI in 2009 as Senior Vice President of Human Resources, General Counsel and Corporate Secretary. From 2000 through 2009, Mr. Milne was employed by The Steak ‘n Shake Company (NYSE: SNS), where he most recently served as Vice President, General Counsel and Corporate Secretary. After graduating cum laude from the Indiana University School of Law, Mr. Milne practiced with Bose, McKinney & Evans and Scopelitis, Garvin, Light, Hanson & Feary where he concentrated on representing employers in labor and employment law matters. Mr. Milne also received his undergraduate degree from Wabash College and his M.A. in English Literature from Indiana University, Bloomington.
Ronda L. Harris is Symmetry Surgical's Vice President, Chief Accounting Officer. Ms. Harris joined SMI in 2008 as Chief Accounting Officer with extensive experience in financial management, planning and implementation of effective financial reporting and financial control processes. Prior to joining SMI, Ms. Harris served as Assistant Controller of General Electric’s Consumer and Industrial Business. Ms. Harris began her career at PricewaterhouseCoopers. She received a Bachelor of Science degree in Accounting from Indiana University and became a Certified Public Accountant in 1997.
Jose E. Fernandez is Symmetry Surgical's Chief Technology Officer. Mr. Fernandez joined SMI in 2010 as Senior Vice President of New Product Development with extensive experience in the development and commercialization of orthopaedic, spine, and interventional vascular products. Prior to joining SMI, Mr. Fernandez was employed by Disc Motion Technologies, Inc., where he most recently served as Vice President, Research and Development. Prior to his tenure at Disc Motion, he served in senior Research and Development capacities for Cordis, a division of Johnson & Johnson, GMP Companies, Inc. and Exactech, Inc. Mr. Fernandez attended the University of Florida and is a U.S. Navy veteran.
For information regarding our directors, see Part III of this Form 10-K filing.
Family Relationships
There are no family relationships between any of the executive officers or directors of the Company.
Available Information
Our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934,as amended, are available free of charge through our website as soon as reasonably practicable after we electronically file such material with, or furnish it to, the Securities and Exchange Commission (SEC). Our internet address is www.symmetrysurgical.com (access the filings by using the “Investor Relations” link on the home page, and “SEC Filings” within the “Investor Relations” box located in the text). You may read and copy any materials we file with the SEC at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC maintains an internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC, and our filings are available there as well. The address of that site is http://www.sec.gov.
Information relating to our corporate governance, including our Corporate Governance Guidelines, Code of Business Conduct and Ethics and information concerning our executive officers, directors and Board committees (including committee charters),
and transactions in Symmetry Surgical Inc. securities by directors and officers, is available on or through our website at www.symmetrysurgical.com under “Investor Relations” then “Corporate Governance.”
We are not including the information on our website as a part of, or incorporating it by reference into, our Form 10-K.
Item 1A. Risk Factors
Our business faces many risks. We believe the risks described below are the material risks that we face. However, the risks described below may not be the only risks that we face. Additional unknown risks, or risks that we currently consider immaterial, may also impair our business operations or financial condition. If any of the events or circumstances described below actually occurs, our business, financial condition or results of operations could suffer, and the price of our common stock could decline. Some of these risks include:
• | The potential impact of an increase in prices we pay for our products or decrease in the prices that we may charge for them; |
• | Symmetry Surgical’s ability to obtain and maintain financing on reasonable terms; |
• | Symmetry Surgical’s ability to adapt to a shift in technologies or methods used in surgery; |
• | Fluctuations in demand for Symmetry Surgical’s products; |
• | Symmetry Surgical’s ability to maintain contracts with its large customers and dealers; and |
• | Symmetry Surgical’s ability to operate as a micro-cap public company. |
Risks Related to the Spin-Off
Our Supply Agreement with SMI will expire after five years and prices may increase after two years.
We have historically procured goods and services from SMI through internal agreements that now are formalized in our Supply Agreement with SMI dated August 4, 2014. As part of our separation from SMI on December 5, 2014, we entered into a five-year supply agreement for products which they supply to us and we supply to them. We have limited revenue resulting from sales to SMI. The current prices paid by us for SMI’s products were frozen for two years from the date of the Supply Agreement, after that time, prices will adjust to a pre-set SMI’s “cost plus 25%,” and will be in place for three additional years. Were the day of the Spin-Off's mix and volume to be subject to this price increase, and if we were not able to find alternative, lower cost providers, we would have incurred approximately $1,000 of incremental expenses for products during 2015. Management believes that mix and volume has and will continue to change over the coming year, and that the current projected impact could instead be between $500 and $700 if were unable to find alternative, lower cost providers. There is no guarantee that we will be able to re-source this volume at a lower cost, nor that the mix and volume impact will not be greater or that prices will not rise further after the expiration of the Supply Agreement.
Risks Related to Our Business
Loss of a large GPO contract, a proprietary hospital system contract, a large U.S. dealer or a significant, country-specific international distributor (or their poor performance) could adversely affect revenue and could adversely affect our business.
We maintain positive relationships with several GPOs and large proprietary hospital systems. As these organizations continue to pursue cost reduction opportunities, they may demand contractual concessions which we are not willing to accept or they may consolidate their purchases into one or more suppliers, excluding us in the process. Additionally, inside the U.S. we are represented in some significant geographic areas by independent dealers and outside the U.S. we sell through country-specific distributors, either of whom may also demand contractual concessions which may be undesirable for us in that market, may perform poorly or may decide to terminate their relationships with us. While we believe we could pursue other dealers and distributors in local and global markets and engage GPO or hospital system hospitals directly, the loss of their contracts would impede our ability to generate demand and revenue, or could impair our margins, and could adversely affect sales and profitability.
A significant shift in technologies or methods used in surgery could make our products obsolete or less attractive or enable surgical procedures to move to a site of care in which we do not have a significant presence.
The development of new technologies could reduce or shift demand for our products. For example, new surgical procedures such as natural orifice surgery or growth in robotic surgery and the associated increase in demand for proprietary surgical instruments could reduce demand for our surgical instruments. New sterilization methods could also limit the demand for our sterilization cases. Adoption of advanced or differentiated energy forms could similarly reduce demand for our electro-surgery instruments. Provider concerns with infection associated with reusable instruments or the shift to using only single use instruments could reduce demand for our reusable instruments. Any of these or other shifts in technologies or methods used in surgery could adversely affect demand for our products. Additionally, the re-use/reprocessing of single use surgical instruments could reduce demand for traditional reusable instruments and place pricing pressure on some instrument products, leading to reduced revenue.
The development of new technologies or greater patient and insurer acceptance of non-hospital or office-based surgical interventions could result in a shift in site of care, reducing demand for our products or shifting demand to customers or distributors with whom we have little experience, resulting in reduced revenue, demand or pricing pressure.
The medical device industry and surgical instrument segment in which we operate is highly competitive, and we may be unable to compete effectively with other companies.
The medical device industry is intensely competitive. We compete with subsidiaries of larger, more established medical device companies as well as smaller, private companies and international manufacturers that purport to provide low-cost solutions for our primary customers. Many of our competitors have access to greater financial, technical, research and development, marketing, manufacturing, sales, distribution, administrative, consulting and other resources than we do. Our competitors may be more effective in marketing or pricing or contracting, and may have a longer history of developing, sourcing, and gaining regulatory approval of products. Our competitors may be also able to gain market share by offering lower-cost products or by offering larger bundles of products across additional clinical areas, or by entering into sole-source agreements with customers, to our exclusion.
Our success will depend on our ability to achieve market acceptance for our products, innovate new products that meet market needs, implement sourcing and production plans, execute commercial plans, gain regulatory approval for products under development, obtain Intellectual Property protection and source or produce products consistently in sufficient quantities to meet demand. We must compete against current technologies on the market as well as respond to new innovations brought by existing or unknown competitors. We may need to invest in clinical or health economic research to support our technologies and may not be as well-resourced or effective in our research as our competition. Competitive pressures could adversely affect our profitability.
We may not realize all of the revenue expected from new products we develop or those we distribute.
We incur expenses in developing and testing new products and related medical devices. These expenses are projected to continue to increase. Our realization of additional revenue from new product development efforts is inherently subject to a number of important risks and uncertainties, including, directly or indirectly, end-user acceptance of the product, reimbursement approval of the product or the procedure in which it is used by third-party payers such as Medicaid, Medicare and private insurers and, in some cases, FDA or comparable foreign regulatory approval of the product. In addition, our customers typically have no contractual requirement to purchase the products that we develop from us. We also incur costs for new product development and production based upon certain estimates of volume for our existing and anticipated products. If the actual demand for our products is less than planned, our revenue and net income may decline.
We also enter into contractual relationships with Alliance Partners under which we distribute products for third parties. The initial work to develop customer acceptance of these products, as well as the sales cycle for new products can divert our attention from current products, or result in reduced sales if these efforts are not successful. In addition, we may purchase inventories of Alliance Partners products which may not sell, or which may sell at lower than anticipated prices, in each case potentially resulting in reduced revenues and profits.
If product liability lawsuits are brought against us, our suppliers or our customers our business may be harmed.
The manufacture and sale of our healthcare and other products exposes us and our suppliers to potential product liability claims and product recalls, including those which may arise from misuse or malfunction of, or design or manufacturing flaws in, our products, or use of our products with components or systems not manufactured by us. Product liability claims or product recalls, regardless of their ultimate outcome, could require us to spend significant time and money in litigation or otherwise require us to pay significant damages or costs of settlement, which could adversely affect our earnings and financial condition. Such lawsuits or claims against our suppliers could also impair our supply of products or increase the prices we must pay for
them. The product liability insurance that we carry is limited in scope and amount and may not be adequate to protect us against the full extent of costs or damages related to product liability claims. Further, significant litigation or adverse awards could render us unable to maintain this insurance at reasonable costs and on reasonable terms, if at all.
We rely on our independent sales dealers, sales representatives, and international distributors to market and sell our products and their efforts, success or decisions to transition to other product lines or employers could adversely impact our business.
Our success in both the U.S. and international markets depends largely upon marketing arrangements with independent sales dealers (U.S.), sales representatives (U.S.), and international distributors (outside U.S.) under which we receive their sales and service expertise and relationships with the customers in the marketplace. Any of these partners may terminate their relationships with us or devote insufficient sales efforts to our products. We do not control our independent dealers or international distributors, and they may not be successful in implementing our marketing plans. Our failure to maintain our existing relationships with our independent dealers, sales representatives, or international distributors could have an adverse effect on our operations. We have experienced turnover with some of our independent sales dealers and international distributors in the past, which adversely affected short-term financial results while we transitioned to new independent sales dealers and international distributors. While we believe these transitions have been managed effectively, similar occurrences could happen in the future, with different results, which could have a greater adverse effect on our operations than we have previously experienced.
Our operating results are subject to significant potential fluctuation and historical results should not be relied on as an indication of our future results.
Our operating results have fluctuated in the past and may vary significantly from quarter-to-quarter or year-to-year in the future due to a combination of factors, many of which are beyond our control. These factors include, but are not limited to:
• | the number, timing and significance of new products and product introductions and enhancements achieved by us or our competitors; |
• | potential acquisitions by us or any acquisition of our business; |
• | changes in pricing policies by us and/or our competitors; |
• | changes in medical treatment or regulatory practices; |
• | delays caused by the regulatory approval process for our new products; |
• | our ability to meet customer demand for certain products or types of products; |
• | decisions regarding the utilization of our manufacturing assets; |
• | significantly changing quality and regulatory requirements mandated by the FDA and/or our customers; |
• | disruption in our supply network or demand greater than supply; and |
• | availability and cost of raw materials. |
Our quarterly revenue and operating results may vary significantly in the future and period-to-period comparisons of our results of operations may not necessarily be meaningful and should not be relied upon as indications of our future performance. We cannot assure you that our revenue will increase or be sustained in future periods or that we will be profitable in any future period. Any shortfalls in revenue or earnings from levels expected by securities or industry analysts could have an immediate and significant adverse effect on the trading price of our common stock in any given period.
If we do not retain and/or attract key individuals, skilled professionals and sales representatives we may not be able to operate successfully, and we may not be able to meet our strategic objectives.
Our success depends in part upon the retention of key managerial, sales and technical personnel, financial professionals, and skilled supply chain professionals and operators. We and our key suppliers, dealers and distributors compete for such personnel with other companies and organizations, many of which are larger and have greater name recognition and financial and other resources than we or our key suppliers, dealers. and distributors do. Many of these competitors are located in the same geographic areas in which our current operations are located or can attract personnel to work virtually globally or relocate. There can be no assurance that we will be successful in retaining our current personnel, or in hiring or retaining qualified personnel in the future. The loss by us, our suppliers, dealers or distributors of key personnel or the inability to hire or retain qualified personnel in the future could have a material adverse effect on our ability to operate successfully. We also do not maintain key man life insurance on any of our executive officers, senior management or other key personnel, so we could incur financial costs associated with their death or disability related to paying obligations to their estates and replacing them, as well as operating without their abilities.
Our efforts to differentiate our products in the marketplace with breadth, innovation, intellectual property, quality, service, education/training, or branding may fail, resulting in reduced demand for our products.
Our comprehensive portfolio, complemented by our commercial efforts, generates demand in the marketplace. Should we fail to differentiate ourselves with portfolio breadth, product innovation, patented technologies, and quality, demand for our products could erode. We must continue to have a broad offering of proprietary products as well as Alliance Partners products offered on behalf of other manufacturers that are complementary in nature. In 2015, Alliance Partners products represented 9.0% of our sales. In May 2014, an Alliance Partner ended its contract with us with respect to its New Wave products. Relationships with our Alliance Partners are dynamic in nature and while we have contractual agreements in place, there is no guarantee that this revenue cannot be lost with or without compensation due to acquisition of the manufacturer by another party, termination of the relationship by either party, or other factors. The loss of any or all of such products, or inability to replace such products, could negatively impact our financial results.
We depend on suppliers, and in some cases a single third-party supplier, and our key suppliers in turn can depend on a single supplier, for key products and raw materials. The loss of these sources or our inability to source a product from a new supplier in a timely fashion should the need arise due to demand or supplier performance could harm our business. Additionally, commodity price fluctuations in key metals and plastics could impact profitability.
We sell products which are sourced from specific manufacturers. Additionally the products we sell are made of plastic, titanium, stainless steel and various other raw materials. While we generally believe that the raw materials used in our products are readily available from multiple sources, from time to time we rely on a limited number of suppliers and in some cases on a single source vendor. Additionally, our suppliers will sometimes, in turn, rely on a limited number of raw material suppliers. For example, our supply chain requires the supply of a patented Radel® R plastic, which is designed to withstand the intense heat produced during frequent sterilizations, for use in our instrument handles and plastic cases. This plastic is sourced from a single supplier. Further, some of our raw materials are produced in areas of the world that are subject to political and other disruptions that could impair the supply of raw materials to our suppliers or the supply of their products to us. Any supply interruption in a limited or sole-sourced component or raw material could materially harm our ability to produce or source our products until a new source of supply, if any, could be found. Further, our efforts to cover such materials could be costly and impair our ability to meet our contractual obligations for certain products on a profitable basis, if at all. Additionally, while the finished products we procure can often be sourced from multiple vendors, sourcing of products from a new supplier can often take significant time to allow for appropriate development, knowledge transfer, quality certification and regulatory approvals, thus making it difficult to respond rapidly to disruptions. We may be unable to find a sufficient alternative supply channel in a reasonable time period or on commercially reasonable terms, if at all. This challenge could interrupt our business, cause us to become involved in litigation with suppliers or customers, impair our profitability and/or reduce the quality, price or availability of our products. In addition, changes in manufacturing or supply processes may require regulatory approval, which could delay the production and sale of the products we manufacture and source.
If our suppliers experience issues with their ability to supply the products we require, raise the price of those products (including as a result of global commodity price increases), or otherwise impair our ability to obtain the products, it would impair our ability to sell our products to our customers and impact our sales and profit. We source a large number of products under the Supply Agreement with SMI, and plan to continue to do so for several years. If SMI experiences issues with its ability to supply the products we require, raises the price of those products upon termination of the two-year price freeze in the Supply Agreement, or otherwise impairs our ability to obtain the products, it would delay or prevent products from reaching our customers or result in our prices increasing, all negatively impacting sales and profit.
Our future capital needs are uncertain and we may need to raise additional funds in the future.
Our future capital needs are uncertain and we may need to raise additional funds in the future through debt or equity offerings. Our future capital requirements will depend on many factors, including, but not limited to:
• | revenue generated by sales of our products; |
• | expenses incurred in acquiring, manufacturing and selling our products; |
• | costs of developing new products or technologies; |
• | costs associated with capital expenditures; |
• | costs associated with our expansion; |
• | costs associated with regulatory compliance, including maintaining compliance with the quality system regulations imposed by the FDA; |
• | the number, cost and timing of acquisitions and other strategic transactions; |
• | working capital requirements related to growing new acquisitions or existing business; |
• | expansion of our international or domestic facilities; and |
• | costs associated with litigation, judicial or administrative awards or other legal issues that arise. |
As a result of these factors, we may need to raise additional funds, and these funds may not be available on favorable terms, if at all. Furthermore, if we issue equity or convertible debt securities to raise additional funds, our existing stockholders may experience dilution, and the new equity or convertible debt securities may have rights, preferences and privileges senior to those of our existing stockholders. If we cannot raise funds on acceptable terms, we may not be able to develop or enhance our products, execute our business strategy, take advantage of future opportunities, or respond to competitive pressures or unanticipated customer requirements, or we could be unable to continue our business operations.
If we are unable to protect our intellectual property and property rights, or are subject to intellectual property claims by third parties, our business could be harmed.
We rely on a combination of patents, trade secrets, copyrights, know-how, trademarks, license agreements and contractual provisions to establish and protect our proprietary rights to our technologies and products. Additionally, we share a significant amount of intellectual property with SMI through our cross license agreement. We cannot guarantee that the steps we have taken or will take to protect our intellectual property rights will be adequate or that they will deter infringement, misappropriation or violation of our intellectual property. Litigation may be necessary to enforce our intellectual property rights and to determine the validity and scope of our proprietary rights. Any litigation could result in substantial expenses and may not adequately protect our intellectual property rights or may result in our loss of those rights. In addition, the laws of some of the countries in which our products are or may be sold may not protect our products and intellectual property to the same extent as U.S. laws, if at all, and we may be unable to protect our rights in trade secrets and unpatented proprietary technology in these countries. If our trade secrets become known, we may lose the competitive advantages associated with them.
We seek to protect our trade secrets, know-how and other unpatented proprietary technology, in part, with confidentiality agreements with our employees, independent dealers and distributors and customers. We cannot be certain, however, that:
• | these agreements will not be breached; |
• | these agreements will be enforced by a court or other judicial body; |
• | we will have adequate remedies for any breach; or |
• | trade secrets, know-how and other unpatented proprietary technology will not otherwise become known to or independently developed by our competitors through an undiscovered breach of an agreement or otherwise. |
In addition, third parties may claim that we are infringing, misappropriating or violating their intellectual property rights. We could be found to infringe those intellectual property rights, which could affect our ability to manufacture any affected product. In addition, any litigation to defend or prosecute our intellectual property rights could require substantial financial resources, divert the time and effort of our management and cause customers to delay or limit their purchases of the affected product until resolution of the litigation.
Any litigation or claims against us, whether or not successful, could result in substantial costs and could harm our reputation. In addition, intellectual property litigation or claims could force us to do one or more of the following:
• | cease selling or using any of our products that incorporate the challenged intellectual property, which could adversely affect our revenue; |
• | obtain a license from the holder of the intellectual property right alleged to have been infringed, which license may not be available on reasonable terms, if at all; and |
• | re-design or, in the case of trademark claims, rename our products to avoid infringing the intellectual property rights of third parties, which may not be possible and could be costly and time-consuming if it is possible to do so. |
We are subject to risks associated with our foreign operations.
We have significant international operations and we continue to expand and grow these operations. We have operations in Switzerland and Germany and sales into approximately 100 countries worldwide through local market distributors. Certain risks are inherent in international operations that could have an adverse impact on our business, results of operations or profitability, including, but not limited to:
• | difficulties in enforcing agreements and collecting receivables through certain foreign legal systems; |
• | foreign customers who may have longer payment cycles than do our customers in the U.S.; |
• | tax rates in certain foreign countries may exceed those in the U.S. and foreign earnings may be subject to withholding requirements or the imposition of tariffs, exchange controls or other restrictions including transfer pricing restrictions when products produced in one country are sold to an affiliated entity in another country; |
• | general economic and political conditions in countries where we operate or where end users of our products reside; |
• | difficulties associated with managing a large organization spread throughout various countries; |
• | changes in governmental approaches to foreign industry; |
• | changes in tax, training or other incentives upon which we relied (or rely) in deciding to do business in a particular country; |
• | wars, insurrections or other strife; |
• | difficulties in enforcing intellectual property rights; |
• | compliance with the Foreign Corrupt Practices Act and similar anti-bribery laws in non-U.S. jurisdictions; |
• | compliance obligations under a variety of foreign laws and regulations; and |
• | compliance with complex international laws and regulations. |
If a natural or man-made disaster (including cyber-attacks) strikes one or more of our offices, procurement/distribution facilities, key suppliers’ facilities, our information technology infrastructure or software, or our global carrier network we may be unable to manufacture/procure certain products or receive, process, ship and deliver customer orders for a substantial amount of time and our revenue could decline.
The efficient operation of our business is dependent on the support of our information technology systems. Our global business operations reside on a central enterprise resource planning system and internal server network. Failure of this system, the reliability of the data maintained in it, our telephony infrastructure, or our customer connectivity could result in significant disruption to our business. In addition, despite our security measures and our best attempts, our systems may be damaged by cyber-attacks, viruses, disasters, hackers, hardware failure, power failure or other disruptions. Any significant disruption could adversely affect our ability to operate efficiently, which could negatively impact our sales and profits.
Our facilities or those of our key suppliers may be affected by natural or man-made disasters. In the event that one or more of our facilities, or those operated by a key supplier, were affected by a disaster, we would be forced to attempt to shift sourcing to another source or rely on third-party manufacturers, who may or may not have the capability to effectively supply the affected products. We provide global distribution from our Nashville, Tennessee facility. Should a disaster strike this facility, we would be forced to attempt to shift distribution to another facility in the U.S. or Europe, which could adversely affect our ability to ship and invoice product for a substantial time period and potentially increase costs associated with distribution. Destruction of this facility could also result in our credit agreement being terminable by the lender. Disruptions to the global transportation network could also affect our ability to procure ship and invoice products. Although we have insurance for damage to our property and the interruption of our business, this insurance may not be sufficient in scope or amount to cover all of our potential losses and may not continue to be available to us on acceptable terms, if at all.
We may be adversely impacted by work stoppages, other labor matters, or new labor laws.
Currently, our U.S. and Swiss facilities are not unionized and we are not aware of any employee consideration of or efforts to become unionized. Our German facility is represented by a works council pursuant to applicable local country laws and regulations. While we have not experienced any adverse effects from work stoppages or slow-downs, work stoppages or slow-downs experienced by us, our suppliers, or their suppliers could result in the interruption of production at facilities where our products are made or used. We cannot assure you that we will not encounter strikes, further unionization efforts, new labor laws, or other types of conflicts with labor unions or our employees, any or all of which could have an adverse effect on our financial results.
As we continue to expand our business globally, our success will depend, in part, on our ability to anticipate and effectively manage these and other risks. We cannot assure you that these and other factors will not have a material adverse effect on our international operations or our business as a whole.
We are subject to complex and costly regulation that can increase costs or impair our ability to bring products to market.
Our products are subject to regulation by the U.S. Food and Drug Administration, or FDA, and other national, federal and state governmental authorities in the U.S. and abroad. It can be costly and time-consuming to obtain regulatory clearance and/or approval to market medical products. Clearance and/or approval might not be granted for a new or modified device or other product on a timely basis, if at all. Regulations are subject to change as a result of legislative, administrative or judicial action or interpretation, which may further increase our costs or reduce sales. Unless an exception applies, the FDA requires that the manufacturer of new medical products or a new indication for use of, or other significant change in, an existing medical device
obtain either 510(k) pre-market notification clearance or pre-market approval before those products can be marketed or sold in the U.S. Modifications or enhancements to a product that could significantly affect its safety or effectiveness, or that would constitute a major change in the intended use of the product, technology, materials, labeling, packaging, or manufacturing process may also require a new 510(k) clearance.
In addition, we are subject to regulations covering manufacturing practices, product labeling and advertising, and adverse-event reporting that apply after we have obtained clearance or approval to sell a product. Our failure to maintain clearances or approvals for existing products, to obtain clearance or approval for new or modified products, or to adhere to regulations for manufacturing, labeling, advertising or adverse event reporting could adversely affect our results of operations and financial condition. Further, if we determine a product manufactured or marketed by us does not meet our specifications, published standards or regulatory requirements, we may seek to correct the product or withdraw the product from the market, which could have an adverse effect on our business. Many of our facilities and procedures, and those of our suppliers, are subject to ongoing oversight, including periodic inspection by governmental authorities. Compliance with production, safety, quality control and quality assurance regulations can be costly and time-consuming.
The sales and marketing of medical products is coming under increased scrutiny by the FDA and other regulatory agencies and enforcement bodies, including but not limited to the federal Anti-Kickback Statute, state anti-kickback laws and the federal Physician Payment Sunshine Act. If our sales and marketing activities fail to comply with FDA regulations or guidelines, or other applicable laws, we may be subject to warnings or enforcement actions from the FDA or other enforcement bodies.
Competitor and distributor consolidation could adversely affect demand and pricing, which could adversely affect our business.
Competitor consolidation may increase downward pricing pressure as a consequence of the resulting larger competitor’s greater product and service offerings or its ability to purchase or supply on a more cost-efficient scale. Distributor consolidation, domestically or in specific countries, may increase margin pressure or reduce our revenue, either of which could impact our operating results.
Our commercial efforts may not be successful.
We rely upon our ability to provide products to customers on competitive quality, clinical education/training, service, differentiated innovation, price, and quantity terms. If our sales efforts are unable to bring our value proposition to our customers, customers may consider competitive products. Some of our customers utilize a single or small group of suppliers and may choose to rationalize their supplier base if our commercial team does not successfully execute our value proposition. Further, we may be unable to secure distribution rights for products required by our customers, causing them to consolidate their purchasing with competitors who are able to provide such broader array of products. If any of these events should occur, it would impair our direct sales business and cause a decline in revenue and profit.
Efforts to acquire additional companies or product lines may divert our managerial resources away from our business operations, and if we complete additional acquisitions, we may incur or assume additional liabilities or experience integration problems resulting in a failure to realize the anticipated benefits.
In addition to internally generated growth, our current strategy involves growth through acquisitions. In the future, we may seek to acquire additional businesses or product lines for various reasons, including in order to obtain new product manufacturing capabilities, add new customers, increase penetration with existing customers or expand into new geographic markets. Our ability to successfully grow through additional acquisitions depends upon our ability to identify, negotiate, complete and integrate suitable acquisitions and to obtain any necessary financing.
We may be unable to continue to implement our acquisition strategy or the strategy we implement ultimately may be unsuccessful. We intend to pursue the acquisition of businesses and product lines complementary to our own; both products which are consistent with those we sell today as well as medical device adjacencies in the hospital environment. Acquisitions could range in size from a single product to a large product family to an entirely new clinical line which, if consummated, could be significant to us.
If we pursue and/or complete acquisitions, we may experience the following, any of which could materially adversely affect our operating results:
• | material transaction expenses; |
• | debt or increased interest and amortization expense; |
• | increased depreciation expense; |
• | increased operating expense; |
• | increased capital investment; |
• | possible in-process research and development charges for acquisitions that do not meet the definition of a ‘‘business;’’ |
• | difficulties integrating any acquired companies, personnel and products into our existing business; |
• | delays in realizing the benefits of the acquired company or products; |
• | diversion of our management’s time and attention from other business concerns; |
• | limited or no direct prior experience in new markets or countries we may enter; |
• | higher costs of integration than we anticipated; |
• | difficulties in retaining key employees of the acquired business who are necessary to manage these businesses; |
• | difficulties in maintaining uniform standards, controls, procedures and policies throughout our acquired companies; |
• | loss of customers or suppliers; |
• | litigation and/or other disputes brought with the acquired business could result in negative consequences; and |
• | adverse customer reaction to such acquisition. |
Some acquisition target businesses or products may not have adequate financial, disclosure, regulatory, quality or other compliance controls at the time we acquire them. As we grow by acquisition, we must manage any new businesses to integrate them into our systems for financial, disclosure, compliance, regulatory and quality control, realize synergies, and control costs. Acquisitions also involve other risks, including diversion of management resources otherwise available for execution and development of our business and risks associated with entering clinical or geographic markets in which our commercial and product development teams have limited experience or where experienced distribution partners are not available.
Our ability to develop our resources to adapt to new products or business areas and to identify and enter into or maintain satisfactory distribution networks for new acquisitions will in part determine our future success. We may fail to identify suitable acquisition candidates in the future, obtain acceptable financing or consummate an acquisition. If we cannot integrate acquired operations, manage the cost of providing our products or price our products appropriately, our profitability and return on investment could suffer. In addition, as a result of our acquisitions of other medical device products or businesses, we may be subject to the risk of unanticipated uncertainties, regulatory and other compliance matters or legal liabilities relating to those acquired businesses for which the sellers of the acquired businesses may not indemnify us, for which we may not be able to obtain insurance (or adequate insurance), or for which the indemnification provided may not be sufficient to cover the ultimate liabilities.
Our earnings would be negatively impacted if we write off goodwill or intangible assets created as a result of our various acquisitions.
We have accumulated goodwill amounting to $9,341 as of January 2, 2016, or 6% of our total assets as of such date. Goodwill is not amortized but rather tested for impairment by us annually or more frequently if an event occurs or circumstances develop that would likely result in impairment. Examples of such events or circumstances include, but are not limited to, a significant adverse change in legal or business climate, an adverse regulatory action, or unanticipated competition or financial restatements. Additional acquisitions could result in increased risk of further impairments.
During fiscal 2015, we did impair certain definite-lived intangible assets aggregating $1,014. We conducted our annual impairment test and determined that no impairment of goodwill existed. During fiscal 2014, impairment charges were an aggregate of $55,817 for goodwill and other assets. These charges are recorded in the consolidated and combined statements of operations within asset impairment. See Note 7 to the consolidated and combined financial statements for additional information.
If we are unable to continue to improve our current products, develop new products or achieve customer quality expectations, we may experience a decrease in demand for our products, our products could become obsolete, or we may incur higher costs in attempts to respond to customer expectations.
We sell our products to customers in markets that are characterized by technological change, product innovation and evolving industry standards and expectations. We are continually engaged in product development and improvement programs, both in collaboration with our customers and independently. In addition, our competitors may produce products that are more appealing to our customers and thereby impair our ability to compete effectively with them. Our competitors’ product development capabilities could also become more effective than ours, and their new products may get to market before our products, may be more effective or less expensive than our products or render our products obsolete. Compliance with the lengthy regulatory process may impair our ability to develop innovative products, as well as our ability to do so on a commercially effective timeline. If our customers change or increase quality expectations or requirements, and we are unable to achieve them, whereas
our competitors are, we may lose volume and revenue. Additionally, we may significantly increase our costs in attempts to achieve product quality expectations. If one or more of these events were to occur, our business, financial condition and results of operation could be adversely affected.
Regulations related to conflict minerals may force us to incur additional expenses, may result in damage to our business reputation and may adversely impact our ability to conduct our business.
Certain minerals that are mined in the Democratic Republic of the Congo and adjoining countries are restricted from use in manufacturing our products, and the Company is required to investigate its supply chain to confirm that there are no such minerals used in its products. There are costs associated with complying with these disclosure requirements, including for diligence in regards to the sources of any conflict minerals used in our products, in addition to the potential cost of remediation and other changes to products, processes, or sources of supply as a consequence of such verification activities. These requirements may increase our cost for products or render some products less marketable.
Currency exchange rate fluctuations could have an adverse effect on our revenue and financial results.
We incur a significant portion of our expenses in currencies other than U.S. dollars. We have operations in Switzerland and Germany, as well as sales to many countries worldwide. While our sales to international customers are in U.S. dollars, their purchases may be affected by the cost of acquisition. Currency exchange rates are subject to fluctuation due to, among other things, changes in local, regional or global economic conditions, the imposition of currency exchange restrictions and unexpected changes in regulatory or taxation environments. Exchange rate fluctuations could have an adverse effect on our margins and financial results.
If we fail to obtain, or experience significant delays in obtaining, FDA clearances or approvals to commercially distribute our future products our ability to sell our products could suffer.
Some of our products are subject to rigorous regulatory pre-approval by the FDA and other federal, state and foreign governmental authorities. We are responsible for obtaining the applicable regulatory approval for the commercial distribution of our products. The process of obtaining this approval, particularly from the FDA, can be costly and time consuming, and there can be no assurance that we will obtain the required approvals on a timely basis, if at all. The FDA, for example, assigns medical devices to one of three classes which determine, among other things, the type and degree of FDA approval required to commercially distribute the device in the U.S. We produce Class I, II and III devices. Class I devices are deemed to present little risk to patients and are generally exempt from FDA approval requirements. Class II devices can generally be commercially distributed only after the device has received 510(k) clearance. The FDA will clear marketing of a medical device through the 510(k) process if certain design, testing and validation requirements are met and it is demonstrated that the device is ‘‘substantially equivalent’’ to a device that was legally marketed prior to May 28, 1976, or to another commercially available device subsequently cleared through the 510(k) Pre-Market Notification process. This process generally takes three to six months, but recently has taken substantially longer, up to nine months or more, due to increased review time and scrutiny
of requirements to assure safe and effective products for consumers. Before a Class III device can be commercially distributed in the U.S., a pre-market approval, or PMA, must be obtained from the FDA. The PMA process can be expensive and uncertain, requires detailed and comprehensive scientific and other data and generally takes between one and three years, but may take significantly longer. The commercial distribution of any products we develop that require regulatory clearance may be delayed. In addition, because we cannot assure you that any new products or any product enhancements we develop for commercial distribution in the U.S. will be exempt from the FDA market clearance requirements or subject to the shorter 510(k) clearance process, the regulatory approval process for our products or product enhancements may take significantly longer than anticipated by us or our customers. In addition, our products are or will be required to have a Universal Device Indicator on the package and device by 2018. If we are unable to achieve these labeling requirements our product distribution could be restricted, impairing our operations and financial condition. Similarly, we may incur significant costs in complying with this requirement, which could negatively impact our pricing to customers or our revenue.
Any claims in excess of our insurance coverage limits may result in substantial costs and a reduction in our available capital resources.
We maintain a wide array of insurance coverage, including property insurance policies covering physical damage to our equipment, facilities, buildings and inventory; employer’s liability insurance generally covering our employees’ workplace death or injury; product liability insurance covering product liability claims arising from the use, consumption or operation of our products; general liability insurance covering certain incidents to third parties that occur on or in our premises; business interruption insurance, and directors and officers liability insurance, among others. Our insurance coverage, however, may not be sufficient to cover all claims or coverage may be denied or unavailable in certain circumstances. As we expand our sales
efforts into multiple international countries and product categories such expansion could increase the risk of claims that may not be covered sufficiently, if at all, by our policies of insurance.
We may experience difficulties, delays, performance impacts or unexpected costs from facility infrastructure changes or outsourcing.
We regularly evaluate the location and function of our facilities. In the future, we may be required to consolidate, move or outsource our operations in order to improve our cost structure, achieve increased operating efficiencies, and improve our competitive standing or results of operations and/or to address unfavorable economic conditions. We may also lose favorable tax incentives or not be able to renew a lease on acceptable terms, resulting in the need to consolidate or relocate. As part of these actions, we may further reduce staff, make changes to certain capital projects, close certain operations or abandon leases for certain facilities that will not be used in our operations. In conjunction with any actions, we will continue to make significant investments and build the framework for our future growth and business continuity. We may not realize, in full or in part, all of the anticipated benefits and savings from these efforts due to unforeseen difficulties, delays or unexpected costs. If we are unable to achieve or maintain all of the resulting savings or benefits to our business or other unforeseen events occur, our business and results of operations may be adversely affected.
Implementation and achievement of our growth objectives may be impeded by political, social, and economic uncertainties or unrest in countries in which we conduct operations or market or distribute our products. In addition, compliance with multiple, and potentially conflicting, international laws and regulations, import and export limitations, anti-corruption laws, and exchange controls may be difficult, burdensome or expensive.
We have significant international efforts and we continue to expand and grow these operations. We have operations in Switzerland and Germany and sales into many countries worldwide through local market distributors. Should disruptions, including those related to political or economic circumstances in those countries occur, or should there be unrest, social disruption, terrorist attacks, political upheaval, revolutions, wars or conflicts, they may adversely affect our business, performance, prospects, value, financial condition, and results of operations.
We are also subject to compliance with various laws and regulations, including but not limited to, the Foreign Corrupt Practices Act in the United States and similar anti-bribery laws in other countries, which generally prohibit companies and their intermediaries from making improper payments to officials for the purpose of obtaining or retaining business. While our employees and agents are required to comply with these laws, we cannot assure you that our internal policies and procedures will always protect us from violations of these laws, despite our commitment to legal compliance and corporate ethics and our training and monitoring efforts. The occurrence or allegation of violations of these laws or regulations may result in legal action against our Company or our employees and the consequences thereof, as well as the cost of addressing such a claim, could adversely affect our business, performance, prospects, value, financial condition, and results of operations.
We may be adversely affected by the negative impact of social media and customer/market perception.
We utilize social media to provide an information channel to customers, prospective customers, employees, and investors. Despite our best efforts, we may be adversely affected by negative commentary generated (accurate or not) by other users of social media. This may adversely affect our reputation, the reputation of our brands, and customer perception, any or all of which could negatively impact our sales and profits.
We may be adversely affected by the impact of environmental and safety regulations.
We are subject to federal, state, local and foreign laws and regulations governing the protection of the environment and occupational health and safety, including laws regulating air emissions, wastewater discharges, and the management and disposal of hazardous materials and wastes, and the health and safety of our employees. We are also required to obtain permits from governmental authorities for certain operations. If we violate or fail to comply with these laws, regulations or permits, we could incur fines, penalties or other sanctions, which could have a material adverse effect on us. Environmental laws tend to become more stringent over time, and we could incur material expenses in the future relating to compliance with future environmental laws. In addition, we could be held responsible for costs and damages arising from any contamination or injury resulting from hazardous materials at our past or present facilities or at third-party waste disposal sites. Such costs could be material.
Future levels of indebtedness may limit our ability to operate our business, finance acquisitions and pursue new business strategies.
Our possible future indebtedness could:
• | make us more vulnerable to unfavorable economic conditions; |
• | make it more difficult to obtain additional financing (or maintain current levels of financing) in the future for working capital, capital expenditures or other general corporate purposes; |
• | make us susceptible to fluctuations in market interest rates that affect the cost of our borrowings to the extent that our variable rate debt is not covered by interest rate derivative agreements; and |
• | make it more difficult to pursue strategic acquisitions, alliances and collaborations. |
Our ability to service any future indebtedness will depend on our future performance, which will be affected by prevailing economic conditions and financial, business, regulatory and other factors, including but not limited to all of the factors and risks discussed herein. Some of these factors are beyond our control. Our ability to service any future indebtedness also relies on certain assumptions including, among others, that we will continue to be successful in implementing our business strategy and that there will be no material adverse developments in our business, liquidity or capital requirements. If we cannot generate sufficient cash flow from operations to meet our other obligations and commitments, we may be required to refinance our debt or to dispose of assets to obtain funds for such purpose. If necessary, we cannot be certain that refinancing or asset dispositions could be effected on a timely basis or on satisfactory terms, if at all, or would be permitted by the terms of our debt instruments. To the extent we incur additional indebtedness or other obligations in the future, the risks associated with our indebtedness described above, including our possible inability to service our debt, would increase.
Failure to satisfy the obligations and maintain compliance with our lending agreements could have a material adverse effect on our business.
Our lending arrangement includes various restrictive covenants where compliance is essential for credit availability. We may be unable to comply with the financial ratios or covenants and, if we fail to do so, we may be unable to obtain waivers from our lender. Failure to comply with any payment or compliance requirements of our debt would entitle the lender to, among other things, accelerate the maturity or terminate the availability of credit commitments. Such an action could render our business unsustainable.
Our credit agreement and future lending agreements may contain restrictions that limit our ability to pay dividends, incur additional debt, make acquisitions and make other investments.
Our Credit Agreement includes customary covenants for facilities of its type, including limitations on indebtedness, liens, investments and acquisitions, loans and advances, mergers and consolidations, sales of assets, and dividends, stock repurchases and other restricted payments. If we are successful in obtaining additional financing in the future, any such lending agreement into which we enter may similarly contain covenants that restrict our ability to make distributions to stockholders or other payments unless we satisfy certain financial tests and comply with various financial ratios. These lending agreements may also contain covenants that limit our ability to incur additional indebtedness, invest in our foreign operations, acquire other businesses and make capital expenditures and impose various other restrictions. These covenants could affect our ability to operate our business and may limit our ability to take advantage of potential business opportunities as they arise.
Our future capital needs are uncertain and we may need to raise additional funds in the future.
Our future capital needs are uncertain and we may need to raise additional funds in the future through debt or equity offerings. Our future capital requirements will depend on many factors, including, but not limited to:
• | cost of acquisitions; |
• | revenue generated by sales of our products; |
• | expenses incurred in manufacturing and selling our products; |
• | costs of developing new products or technologies; |
• | costs associated with capital expenditures; |
• | costs associated with our expansion; |
• | costs associated with regulatory compliance, including maintaining compliance with the quality |
• | system regulations imposed by the FDA; |
• | the number and timing of acquisitions and other strategic transactions; |
• | working capital requirements related to growing new acquisitions or existing business; |
• | expansion of our international or domestic facilities; and |
• | costs associated with litigation, judicial or administrative awards or other legal issues that arise. |
As a result of these factors, we may need to raise additional funds, and these funds may not be available on favorable terms, or at all. Furthermore, if we issue equity or convertible debt securities to raise additional funds, our existing stockholders may experience dilution, and the new equity or convertible debt securities may have rights, preferences and privileges senior to those of our existing stockholders. If we cannot raise funds on acceptable terms, we may not be able to develop or enhance our products, execute our business strategy, take advantage of future opportunities, or respond to competitive pressures or unanticipated customer requirements.
Risks Related to Our Industry
Our business is subject to healthcare industry cost containment measures and other industry trends affecting pricing that could result in reduced sales of, or prices for, our products.
Acceptance of our products by hospitals, outpatient centers, physicians and other customers depends on, among other things, reimbursement approval of third-party payers such as Medicaid, Medicare and private insurers (or other governmental and non-governmental sources outside the United States). The continuing efforts of government, insurance companies and other payers of healthcare costs to contain or reduce those costs could lead to lower reimbursement rates or non-reimbursement for medical procedures that use our products or for our products themselves. As that occurs, customers might insist that we lower prices on products related to the affected medical device or they might significantly reduce or eliminate their purchases from us of such products. We also have relationships with GPOs which negotiate pricing for member hospitals, and which require price discounts for certain products. Pricing pressure from all of the foregoing sources may have an impact on our financial results.
Changes in the healthcare industry may eliminate or reduce the size of the market for our products, which could have a negative impact on our financial performance.
In the United States the movement toward managed care and healthcare cost containment, as well as other global government and private sector initiatives in markets in which we do business, are placing increased emphasis on the delivery of more cost effective care that could adversely affect the sale and/or the prices of our products. Examples of potentially problematic movements include:
• | a trend toward site of care outside the tertiary hospital where our resources are mainly focused; |
• | alignment of physicians with healthcare institutions, reducing physician preference and increasing commoditization of our products; |
• | reduced funding constraining capital and operating budgets at our customers’ businesses; and |
• | gainsharing proposals, physician profiling, and collaboration with service providers which could alter current standards of care. |
Reduced hospital or operating room construction and customer consolidation could adversely affect demand and pricing, which could adversely affect our business.
Many healthcare providers are consolidating to create new companies that possess greater regional or national market power. As the healthcare industry continues to consolidate, our customers may delay purchases or reduce their future needs as they integrate operations and consolidate facilities/operating rooms. Customer consolidation may also impact demand for our products, as the consolidated company implements centralized procurement to reduce inventory. Larger customers may increase pricing pressure. Additionally, reduced capital budgets in the U.S. or decreased government funding abroad could result in fewer new hospital constructions or the addition of operating rooms to existing hospitals which would reduce large tender opportunities and reduce sales opportunities.
Significant changes to U.S. federal, state and foreign tax laws and regulations that apply to our operations and activities could have a material adverse effect on our financial results.
Our operations are subject to the tax laws, regulations and administrative practices of the U.S., U.S. state jurisdictions and other countries in which we do business. Significant changes in these rules could have a material adverse effect on the results of operations. For example, our effective tax rate reflects the impact of our Schaffhausen, Switzerland global supply chain operations and undistributed foreign earnings for which no U.S. taxes have been provided because such earnings are intended
to be invested indefinitely outside the U.S. Substantial changes in U.S. tax law regarding tax on certain foreign profits could result in an increase in our effective tax rate, which could have a material adverse effect on our financial results.
The full impact of the Patient Protection and Affordable Health Care Act on our business remains uncertain.
The Patient Protection and Affordable Health Care Act, as amended by the Health Care and Education Reconciliation Act (collectively the “PPACA”) continues to significantly impact the medical device industry, and its terms and date(s) of effective regulations remain uncertain. For example, PPACA imposed a 2.3% excise tax on all domestic sales of medical devices after December 31, 2012. In 2015 and 2014, we incurred $847 and $767, respectively in excise tax, which was included as a general and administrative expense. The tax has been suspended for 2016 and 2017 although there is no guarantee that the suspension will continue (or that it will not return at a higher figure). We determine revenue subject to the excise tax on the basis of which entity is the registered owner of the device, the first sale in the U.S. and other factors. With the exception of revenue growth and product mix subject to the excise tax, we do not expect a material change in our excise tax responsibility going forward, although some excise tax that was paid by SMI prior to the Spin-Off may be shifted to us under the terms of our Supply Agreement and is already reflected in our pricing agreements to the extent it is re-implemented. We cannot predict with certainty whether regulators will implement any changes to the excise tax or the ultimate effect of any changes to the excise tax or the federal health care reform in general will have on us when fully implemented. Specifically, unless and until the law and associated regulations are finalized, fully implemented and not subject to further legislative efforts to modify or overturn them, we are unable to accurately estimate the future impact of the PPACA on our business, as its continued implementation could lead to an impact on our customers’ business, including surgical volumes, hospital construction or capital spending. The legislation could have a material adverse effect on our customers’ businesses and our business, cash flows, financial condition and results of operations.
Many significant parts of the law will be phased in during the next decade and require further clarification in the form of regulations. As a result, many impacts will not be known until those regulations are enacted (if at all).
Effective August 1, 2013, certain manufacturers of medical devices covered by Medicare, Medicaid, and the Children’s Health Insurance Program who make payments or other transfers of value to physicians and teaching hospitals, including us, were required to begin tracking and reporting such payments and transfers under the regulations known as the National Physician Payment Transparency Program. Efforts to comply with these requirements on an ongoing basis may result in an increase in operational expenses and a diversion of management’s time from other business activities. Failure to comply fully could cause us to incur costs and expenses associated with remedial compliance or fines (or both).
Changing laws and increasingly complex corporate governance and public disclosure requirements could have an adverse effect on our business and operating results.
Changing laws, regulations and standards, including those relating to corporate governance and public disclosure such as the Dodd-Frank Wall Street Reform and Consumer Protection Act and recently enacted and proposed SEC regulations, have created additional compliance requirements for companies such as ours. These include, but are not limited to, the reporting of the use of conflict minerals. Our efforts to comply with these requirements have resulted in, and are likely to continue to result in, an increase in operational expenses and a diversion of management’s time from other business activities.
Risks Relating to Our Common Stock
Our common stock may be volatile and could decline substantially.
There has been volatility in the market price and trading volume of securities of companies operating in the medical device industry, which has often been unrelated to the operating performance of particular companies. These broad market fluctuations may adversely affect the trading price of our common stock. Price declines in our common stock could result from general market and economic conditions and a variety of other factors, including:
• | actual or anticipated fluctuations in our operating results; |
• | our announcements or our competitors’ announcements regarding new products, significant contracts, acquisitions or strategic investments; |
• | loss of any of our key management or technical personnel; |
• | conditions affecting the medical device industry generally; |
• | product liability lawsuits against us or our customers; |
• | clinical trial results with respect to our or our suppliers' or customers’ medical devices; |
• | changes in our growth rates or our competitors’ growth rates; |
• | developments regarding our patents or proprietary rights, or those of our competitors; |
• | FDA and international actions with respect to the government regulation of medical devices and third-party reimbursement practices; |
• | public concern as to the safety of our products; |
• | changes in health care policy in the U.S. and internationally; |
• | conditions in the financial markets in general or changes in general economic conditions; |
• | our inability to raise additional capital; |
• | changes in stock market analyst recommendations regarding our common stock, other comparable companies or the medical device industry generally, or lack of analyst coverage of our common stock; |
• | sales of our common stock by our executive officers, directors and five percent stockholders or sales of substantial amounts of common stock; |
• | changes in accounting principles; and |
• | the announcement of financial restatements. |
In the past, following periods of volatility in the market price of a particular company’s securities or financial restatements, litigation has often been brought against that company. If litigation of this type is brought against us, it could be extremely expensive, not covered by insurance (in full or in part) and divert management’s attention and the Company’s resources.
Our Certificate of Incorporation, our Bylaws and Delaware law contain provisions that could discourage another company from acquiring us and may prevent attempts by our stockholders to replace or remove our current board of directors or management.
Provisions of the Delaware General Corporation Law, our Certificate of Incorporation and our Bylaws may discourage, delay or prevent a merger or acquisition that stockholders may consider favorable, including transactions through which stockholders might otherwise receive a premium for their shares. In addition, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace or remove our board of directors. These provisions include:
• | providing for a classified board of directors with staggered terms; |
• | requiring supermajority stockholder voting to effect certain amendments to our certificate of incorporation and by-laws; |
• | eliminating the ability of stockholders to call special meetings of stockholders; |
• | prohibiting stockholder action by written consent; |
• | establishing advance notice requirements for nominations for election to the board of directors or for proposing matters that can be acted on by stockholders at stockholder meetings; |
• | limiting the ability of stockholders to amend, alter or repeal the by-laws; and |
• | authorizing the board of directors to issue, without stockholder approval, shares of preferred stock and common stock with such terms as the board of directors may determine. |
We are also protected by Section 203 of the Delaware General Corporation Law, which prevents us from engaging in a business combination with a person who becomes a 15.0% or greater stockholder for a period of three years from the date such person acquired such status unless certain board or stockholder approvals were obtained.
We are an emerging growth company, and we cannot be certain if the reduced disclosure requirements applicable to emerging growth companies will make our common stock less attractive to investors.
For so long as we remain an emerging growth company as defined in the JOBS Act, we may take advantage of certain exemptions from various requirements that are applicable to public companies that are not emerging growth companies, including not being required to comply with the independent auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. We may take advantage of these exemptions for so long as we are an emerging growth company, which could be as long as five years following the completion of the Spin-Off. Investors may find our common stock less attractive because we rely on these exemptions. If some investors find our common stock less
attractive as a result, there may be a less active trading market for our common stock, and our stock price may be more volatile and may decline.
In addition, Section 107 of the JOBS Act also provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. We chose to "opt in" to the extended transition period, and as a result, we may delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. Our financial statements, therefore, may not be comparable to those companies that comply with public company effective dates.
Other General Risks.
Our future viability and profitability is also dependent on a number of other factors that affect the performance of all industries and not just the medical device industry, including (but not limited to) the following:
• | financial failure or default by a party to any contract to which we are, or may become, a party; |
• | insolvency or other managerial failure by any of our customers or suppliers; |
• | industrial disputes; |
• | litigation; |
• | natural disasters; and |
• | acts of terrorism or an outbreak of international hostilities. |
Item 1B. Unresolved Staff Comments
None.
Item 2. Properties
We lease approximately 43,000 square feet in Antioch, Tennessee, a suburb of Nashville, Tennessee, for our corporate headquarters and warehouse space. The term of the lease lasts until May 2018, with an option to renew for a successive three-year term. We also lease approximately 25,000 square feet of warehouse and light manufacturing space in Louisville, Kentucky pursuant to a lease that is due to expire in September 2018. In addition, we lease approximately 5,400 square feet of warehouse space in Tuttlingen, Germany, pursuant to a lease that is due to expire in January 2019. We lease approximately 900 square feet of space for our office in Schaffhausen, Switzerland, which may be terminated at any time upon six months’ prior notice. We also lease approximately 3,400 square feet of product development office space in Raleigh, North Carolina that is due to expire in December 2017. Finally, we lease approximately 1,100 square feet of office space in Fort Wayne, Indiana on a month-to-month basis.
Location | Principal Use | Approximate Square Footage | Own/ Lease | ||||
Nashville, Tennessee | Medical products distribution; Symmetry Surgical Corporate Headquarters | 43,000 | Lease | ||||
Louisville, Kentucky | Instrument finishing and packaging operation | 25,000 | Lease | ||||
Tuttlingen, Germany | Instrument procurement and quality center | 5,400 | Lease | ||||
Schaffhausen, Switzerland | Symmetry Surgical global supply chain management | 900 | Lease | ||||
Raleigh, North Carolina | Product development offices | 3,400 | Lease | ||||
Fort Wayne, Indiana | Administrative offices | 1,100 | Lease | ||||
Total square footage | 78,800 | ||||||
Item 3. Legal Proceedings
Information pertaining to legal proceedings is provided in Note 18 entitled "Commitments and Contingencies" of the Notes to consolidated and combined financial statements included under Item 8, "Financial Statements and Supplementary Data," and is incorporated by reference herein.
Item 4. Mine Safety Disclosures
Not Applicable
PART II
Item 5. Market for the Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
The information required by Item 5 with respect to securities authorized for issuance under Equity Compensation Plans is set forth in Part III, Item 12 of this Form 10-K.
Principal Market
Our common stock trades on The NASDAQ Global Market ("NASDAQ") under the trading symbol SSRG. Our common stock has been listed on NASDAQ since December 5, 2014, the first business day following Spin-off. The following table sets forth, for the periods indicated, the highest and lowest sale price for our common stock by quarter for 2015 and 2014, as reported by NASDAQ:
2015 | 2014 | ||||||||||||||
High | Low | High | Low | ||||||||||||
Fourth Quarter (Beginning December 5, 2014) | $ | 9.74 | $ | 8.38 | $ | 7.96 | $ | 7.30 | |||||||
Third Quarter | $ | 9.20 | $ | 7.87 | $ | — | $ | — | |||||||
Second Quarter | $ | 9.21 | $ | 6.83 | $ | — | $ | — | |||||||
First Quarter | $ | 8.17 | $ | 7.15 | $ | — | $ | — | |||||||
The closing sale price for our common stock on February 29, 2016 was $9.54.
Stockholders
As of February 29, 2016, there were 147 registered holders of record of our common stock. The transfer agent and registrar for our common stock is Computershare Trust Company, N.A., 211 Quality Circle, Suite 210, College Station, Texas 77842-3170, telephone (800) 962-4284.
We currently do not have a share repurchase plan or program.
Dividends
We have never declared or paid any dividends on our capital stock and do not currently anticipate declaring or paying dividends in the foreseeable future. We currently intend to retain all of our future earnings, if any, to finance the operation and expansion of the Company. Any future determination relating to our dividend policy will be made at the discretion of our Board of Directors, and will depend on a number of factors, including future earnings, capital requirements, financial conditions, future prospects, contractual restrictions and covenants and other factors.
Performance Graph (Unaudited)
The following graph compares the cumulative total return on the Company's common stock with the Russell MicroCap Index and the Dow Jones U.S. Medical Equipment Index during the same period. This graph covers the period from December 5, 2014 (the first day our common stock began trading following the Spin-Off on December 5, 2014) through December 31, 2015. This graph assumes $100 was invested in the stock or the index on January 5, 2014 and also assumes the reinvestment of all dividends. No dividends have been declared or paid on the Company's common stock. Returns over the indicated period should not be considered indicative of future stock price performance.
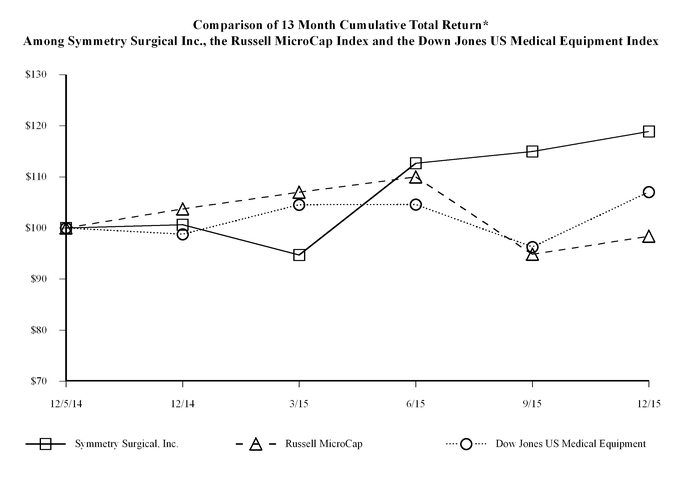
Item 6. Selected Financial Data
The following table sets forth Symmetry Surgical's selected financial information derived from its (i) audited consolidated financial statements as of and for the year ended January 2, 2016; (ii) audited consolidated and combined financial statements as of and for the year ended January 3, 2015; and (iii) audited combined financial statements as of and for the year ended December 28, 2013. The historical financial statements for periods prior to the Spin-Off on December 5, 2014 were prepared on a stand-alone basis and were derived from SMI's consolidated financial statements and accounting records as if the Symmetry Surgical business had been stand-alone for all periods presented. Accordingly, Symmetry Surgical's financial statements for periods prior to December 5, 2014 are presented on a combined basis and reflect Symmetry Surgical's financial position, results of operations and cash flows as its business was operated as part of SMI prior to the separation, in conformity with generally accepted accounting principles (GAAP) in the United States. The historical financial statements for periods prior to December 5, 2014 also reflected an allocation of expenses related to certain SMI corporate functions, including senior management, legal, human resources, finance, information technology and quality assurance. These expenses were allocated to Symmetry Surgical based on direct usage or benefit where identifiable, with the remainder allocated on a pro-rata basis of revenue. Symmetry Surgical considers the expense allocation methodology and results to be reasonable. However, the allocations may not be indicative of the actual expenses that would have been incurred had Symmetry Surgical operated as an independent, stand-alone, publicly-traded company for the periods presented. Accordingly, the historical financial information presented for periods prior to December 5, 2014 may not be indicative of the results of operations or financial position that
would have been achieved if Symmetry Surgical had been an independent, stand-alone, publicly-traded company during the periods shown or of Symmetry Surgical's performance for periods subsequent to the Spin-Off on December 5, 2014. Refer to "Basis of Historical Presentation" included under Item 7, "Management's Discussion and Analysis of Financial Condition and Results of Operations" for additional information.
Fiscal Year | ||||||||||||||||
2015 | 2014 | 2013 | 2012 | |||||||||||||
(in thousands, except per share data) | ||||||||||||||||
Consolidated and Combined Statements of Operations Data: | ||||||||||||||||
Revenue - third parties | $ | 84,527 | $ | 81,104 | $ | 88,876 | $ | 106,663 | ||||||||
Revenue - Symmetry OEM Solutions | — | 678 | 71 | 342 | ||||||||||||
Total revenue | 84,527 | 81,782 | 88,947 | 107,005 | ||||||||||||
Cost of revenue | 45,077 | 46,098 | 48,394 | 55,175 | ||||||||||||
Gross profit | 39,450 | 35,684 | 40,553 | 51,830 | ||||||||||||
General and administrative expenses | 19,546 | 18,643 | 20,515 | 18,186 | ||||||||||||
Sales and marketing expenses | 16,920 | 17,402 | 18,279 | 19,114 | ||||||||||||
Asset impairment | 1,014 | 55,817 | 20,105 | — | ||||||||||||
Operating income (loss) | 1,970 | (56,178 | ) | (18,346 | ) | 14,530 | ||||||||||
Interest expense | 222 | 24 | — | — | ||||||||||||
Derivative valuation loss | — | — | 242 | (242 | ) | |||||||||||
Other expense | 269 | 229 | 38 | 4 | ||||||||||||
Income (loss) before income taxes | 1,479 | (56,431 | ) | (18,626 | ) | 14,768 | ||||||||||
Income tax expense (benefit) | 279 | (20,656 | ) | (6,441 | ) | 5,647 | ||||||||||
Net income (loss) | $ | 1,200 | $ | (35,775 | ) | $ | (12,185 | ) | $ | 9,121 | ||||||
Net income (loss) per share: | ||||||||||||||||
Basic | $ | 0.12 | $ | (3.73 | ) | $ | (1.27 | ) | $ | 0.95 | ||||||
Diluted | $ | 0.12 | $ | (3.73 | ) | $ | (1.27 | ) | $ | 0.95 | ||||||
Weighted average common shares and equivalent shares outstanding: | ||||||||||||||||
Basic (a) | 9,645 | 9,587 | 9,587 | 9,587 | ||||||||||||
Diluted (a) | 9,688 | 9,587 | 9,587 | 9,587 | ||||||||||||
Consolidated Balance Sheet Data: | ||||||||||||||||
Cash | $ | 8,072 | $ | 2,994 | $ | 648 | $ | 162 | ||||||||
Working capital | 32,881 | 26,986 | 20,186 | 27,856 | ||||||||||||
Total assets | 153,981 | 152,049 | 185,451 | 217,648 | ||||||||||||
Total debt | — | 3,876 | — | — | ||||||||||||
Total equity | 141,013 | 138,306 | 171,828 | 200,947 | ||||||||||||
Other Financial Data: | ||||||||||||||||
Depreciation and amortization | $ | 6,482 | $ | 6,331 | $ | 6,102 | $ | 6,557 | ||||||||
(a) On December 5, 2014, SMI distributed 9,587 shares of Symmetry Surgical common stock. For periods prior to the separation, the weighted-average basic and diluted shares outstanding were based on the number of shares of Symmetry Surgical common stock outstanding on the distribution date. Refer to Note 19 to the consolidated and combined financial statements for information regarding the calculation of basic and diluted earnings per share for the year ended January 3, 2015.
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
(Dollars in thousands, unless otherwise noted)
You should read the following discussion in conjunction with the audited consolidated and combined financial statements and corresponding notes appearing in Item 8, "Financial Statements and Supplementary Data." This Management’s Discussion and Analysis of Financial Condition and Results of Operations contains forward-looking statements. The matters discussed in these forward-looking statements are subject to risks, uncertainties, and other factors that could cause actual results to differ materially from those stated, projected or implied in the forward-looking statements. Please see “Risk Factors” and “Special Note Regarding Forward-Looking Statements” for a discussion of the uncertainties, risks, and assumptions associated with these statements.
Separation from Symmetry Medical Inc.
In August 2014, Symmetry Medical Inc. (“SMI”) announced that it had entered into an Agreement and Plan of Merger, dated as of August 4, 2014 (the “Merger Agreement”), by and among SMI, TecoStar Holdings, Inc., Tecomet, Inc., and TecoSym, Inc. (“Merger Sub”), pursuant to which Merger Sub was merged with and into SMI, with SMI continuing as the surviving corporation (the “Merger”). As a result of the Merger, all of the outstanding shares of common stock of Symmetry Surgical Inc. ("Symmetry Surgical," the “Company,” "we," "us," or "our") were spun-off to SMI’s stockholders and the holder of each outstanding share of common stock of SMI received one-quarter of a share of common stock of the Company. This is referred to as the “Spin-Off.”
In connection with the Spin-Off, SMI entered into a Separation Agreement, dated as of August 4, 2014 (the “Separation Agreement”) with the Company. Pursuant to the Separation Agreement, SMI transferred all of the assets and liabilities of its Symmetry Surgical Business to the Company prior to the consummation of the Merger and the Spin-Off. The Separation Agreement provides for various agreements and arrangements governing the Company’s relationship with SMI after the Spin-Off. Following the completion of the Merger and the Spin-Off on December 5, 2014, Symmetry Surgical began to operate as an independent publicly-traded company.
Company Overview
Symmetry Surgical is a global marketer and distributor of medical devices with some limited manufacturing focused on the general surgery market. We offer over 20,000 products and sell primarily to hospitals and surgical centers in the U.S. and countries worldwide. Our current product portfolio includes a broad range of reusable stainless steel and titanium, hand-held general and specialty surgical instruments, single use and disposable instruments (vein strippers, SECTO® dissectors, tonsil sponges and surgical marker pens), electro-surgery instruments, retractor systems, ligation clips and appliers, and containers and sterilization devices sold directly to hospitals and other sites of care. Symmetry Surgical is dedicated to developing and delivering high-quality; innovative surgical instruments that meet clinicians' needs and improve patients' lives. Our team collaborates with healthcare providers around the world to provide medical devices that exceed our customers' expectations and provide solutions for today's needs and tomorrow's growth. Our products are typically used in the surgical specialties of neurosurgery, spine, general surgery - open and laparoscopy, microsurgery, OB/Gyn, ophthalmology, otolaryngology / ENT, plastic / reconstructive, peripheral vascular, arthroscopy, orthopedic, pediatrics, cardiovascular, thoracic, and urology in the hospital setting as well as surgery centers and in select physician offices. Our products are viewed in two categories by management: Symmetry Surgical Branded Products and Alliance Partners Products. Symmetry Surgical Branded Products include all products sold under brand names managed by the Company whereas Alliance Partners Products are brands the Company distributes for others in defined markets.
• | Symmetry Surgical Branded Products include products with Symmetry Surgical-owned brands sold in the United States and worldwide. Today, these products include general surgery instruments such as retractor instruments/systems of instruments, electro-surgery instruments, ligation clips and appliers, containers and sterilization devices, as well as general instruments and specialty instruments. The Company’s rich and diverse history creates one of the industry's most comprehensive surgical instrument portfolios, which includes our well-known brands such as BOOKWALTER®, GREENBERG®, RILEY™, OLSEN™, ULTRA™, QUAD-LOCK®, FLASHPAK®, RAPIDCLEAN®, OPTI-LENGTH®, CLASSIC® and CLASSIC PLUS®, VESOCCLUDE™, and SYMMETRY ACCESS™. |
• | Alliance Partners Products include complementary products offered by other manufacturers which Symmetry Surgical distributes, primarily in markets within the United States. Today, these products include complementary general surgery instruments used in surgical lighting, laparoscopic surgery, and accessories. Revenue in this category is reported separately because these products are offered for sale in the Company’s capacity as a distributor, and the Company does not own the underlying intellectual property, nor is the Company the manufacturer of record. |
On August 28, 2015, we acquired key assets of Vesocclude Medical located in Raleigh, North Carolina for $4,055 in cash and up to an additional $2,200 in contingent consideration; each of which are gross of the Company's 4.8% existing minority interest in Vesocclude. The contingent consideration is payable based on achieving certain milestones related to new product introduction and specified new product commercial sales levels. Vesocclude's portfolio of ligation clips and appliers are used in surgical procedures to close tubal anatomic structures such as blood vessels or ducts. Vesocclude products are primarily sold in the U.S. direct to hospitals but in some territories are sold through stocking distributors. Internationally, Vesocclude products are sold through stocking distributors. Prior to the acquisition, we had represented the Vesocclude portfolio in select global territories as part of our Alliance Partners products.
On December 1, 2015, we acquired the patent protected, ultra-low profile, soft-tissue retraction technology and related product portfolio and other assets from privately held Insightra® Medical for $400 in cash as well as a royalty on future sales. The portfolio, formerly known as ReeTrakt, will be re-launched under the Symmetry Surgical brand as the Symmetry Access™ Low Profile Retractor beginning in the first half of 2016. The Symmetry Access™ Low Profile Retractor is a single-use product with an ultra-low profile that supports minimal/small incision surgical procedures by providing better surgical access and field of view. The product is designed to minimize tissue damage by providing gentle and adjustable tissue retraction without a bridge, arms or handles that reduce access to the surgical site. The portfolio offers a variety of tissue retraction tips and adheres securely to the surgical drape or skin, eliminating the need for hand-held retraction during a case. The Company plans to launch this product as a Symmetry Surgical branded product offering through its existing sales channel in the U.S and internationally.
Symmetry Surgical has operations in the U.S., Germany and Switzerland, and includes the consolidated operations of Specialty Surgical Instrumentation, Inc. (d/b/a Symmetry Surgical Inc.), Olsen Medical, LLC, Symmetry Surgical Vesocclude, LLC, Symmetry Surgical Netherlands, CV, Symmetry Surgical Netherlands, BV, Symmetry Surgical, GmbH and Symmetry Surgical Switzerland GmbH.
We expect the global healthcare industry to continue to grow as the population ages, access to care in developing nations increases, and treatment advances in medical care are achieved. We also believe that cost containment in healthcare is becoming an increasingly critical issue in both government-funded and privately insured populations. At the same time, we believe that there is an expectation of continued improvements in the standard of care and prevention, although there will now be an economic component of the discussion. Our company competes in the general surgery market of medical devices. The General Surgery market includes the following market segments: Open Surgery Instruments, Minimally Invasive Surgery Instruments, Energy Based and Powered Instruments, Adhesion Prevention Products, Disposable Surgical Supplies, and Medical Robotics and Computer Assisted Surgery. Our primary historical focus has been on reusable general surgery instruments and sterilization containers, a market which includes the Open Surgery, Minimally Invasive Surgery and Energy segments.
The Company’s sales and marketing efforts emphasize the quality, clinical performance, and comprehensive breadth of its product line. Sales and marketing personnel are predominantly located in the U.S., although there are regionally-based business development leaders to assist in driving growth through a global network of distributors. U.S. sales are through a combination of direct representatives and dealers who generate demand which is fulfilled directly by Symmetry Surgical. Hospital customers include clinicians, operating room directors, hospital materials management, hospital central sterilization, multi-hospital strategic sourcing entities, and GPOs. Additionally, the Company is generating demand in ambulatory surgery centers, physicians’ offices, and other evolving sites of care through our e-commerce efforts. The Company aims to capitalize on: tender opportunities for new or updated operating rooms, where customers seek to outfit a full range of capabilities, new surgeons or new services being added to an existing operating room that require a specific clinical focus of instruments, introduction of specialized clinical innovation and new products, and replacement of existing products which have reached the end of their life cycle. Customer interactions often involve training and education on the use of our products. Sales personnel are technically trained and are based in the territories they serve. This enables the Company to be responsive to the needs of customers and become actively involved in the planning and development of future opportunities.
In the U.S., Symmetry Surgical continues to focus on salesforce execution, customer responsiveness, and launching new products. Outside of the U.S., Symmetry Surgical continues to work to increase business with our distributor network. We have successfully transferred regulatory approvals for products labeled in legacy graphics in the vast majority of countries we sell into and continue the process of registering the new Symmetry Surgical labeling of these products in all relevant countries. We also continue a similar process for the former SSI, Olsen, Vesocclude, and the former Insightra product lines, which previously had only been in limited international distribution, so that we may offer these products on a wider basis to customers throughout the world. Beginning in 2014 and continuing in 2015, Symmetry Surgical experienced growth through its global e-commerce site to serve customers. Additionally, we continued to execute on our strategic initiatives to
position the business for growth in the U.S. and international markets, while also focusing on prudent expense management.
Our revenue in fiscal 2015 increased $2,745, or 3.4%, compared to fiscal 2014. This increase was primarily driven by improved sales execution efforts resulting in mid-single digits volume growth, incremental revenue from the Vesocclude Medical acquisition, and improved service levels, partially offset by very low single digit price erosion stemming from targeted product promotions and the loss of the New Wave Surgical product line, which was acquired by a third party in the first quarter of fiscal 2014 and was no longer distributed by Symmetry Surgical after April 30, 2014.
During fiscal 2015, we sold products to nearly 5,000 customers. Approximately 87.3% and 87.5% of our revenue was from sales to customers in the United States in fiscal 2015 and 2014, respectively, as compared to 12.7% and 12.5% attributable to international sales.
Our revenue from Symmetry Surgical branded products represented 91.0% and 88.5% of our total revenue in fiscal 2015 and 2014, respectively as compared to 9.0% and 11.5% attributable to Alliance Partners products. Prior to the acquisition of the assets of Vesocclude Medical in August 2015, the revenue from the sales of Vesocclude product were depicted in the Alliance Partners product category. Upon acquisition, this revenue is now Symmetry Surgical branded product. For the purposes of comparability between periods, the historical product categories have been reclassified to include Vesocclude revenue in Symmetry Surgical branded product category.
Basis for Historical Presentation
For the periods prior to the Spin-Off on December 5, 2014, our financial information has been prepared on a stand-alone basis derived from SMI’s consolidated financial statements and accounting records. Therefore, as of and for the year ended January 3, 2015, our financial statements are audited consolidated and combined. For the year ended December 28, 2013, our financial statements are audited combined. Beginning in fiscal 2015, our financial statements are consolidated. The combined financial statements reflect Symmetry Surgical’s results of operations and cash flows as our business was operated as part of SMI prior to the Spin-Off, in conformity with U.S. generally accepted accounting principles ("GAAP").
The combined financial statements included the allocation of certain assets and liabilities that historically had been held at the SMI corporate level but which were specifically identifiable or allocable to Symmetry Surgical. Cash and cash equivalents held by SMI at the effective time of the Merger remained with SMI and were not allocated to Symmetry Surgical. Long-term debt and short-term borrowings were not allocated to Symmetry Surgical as none of the debt recorded by SMI was directly attributable to or guaranteed by Symmetry Surgical. All intracompany transactions and accounts have been eliminated. All intercompany transactions between Symmetry Surgical and SMI's OEM Solutions business have been reflected as related party transactions. All intercompany transactions between Symmetry Surgical and SMI corporate are considered to be effectively settled in the combined financial statements of cash flows as financing activity and in the combined balance sheet as net parent investment.
The historical financial statements do not necessarily include all of the expenses that would have been incurred and may not necessarily reflect Symmetry Surgical’s results of operations, financial position and cash flows had Symmetry Surgical been a stand-alone company during the periods presented. Symmetry Surgical’s historical financial statements include an allocation of expenses related to certain general and administrative, selling and marketing expenses from SMI to Symmetry Surgical. These expenses have been allocated to Symmetry Surgical first on the basis of direct usage when identifiable, with the remainder allocated on basis of their respective revenues. Symmetry Surgical considers the expense allocation methodology and results to be reasonable for all periods presented. However, the allocation may not be indicative of the actual expenses that would have been incurred had Symmetry Surgical operated as an independent, publicly-traded company for the periods presented.
Results of Operations
The following table summarizes our consolidated and combined results of operations for each of the past three fiscal years. Our historical results are not necessarily indicative of the operating results that may be expected in the future.
Fiscal Year | ||||||||||||||||||||
2015 | 2014 | 2013 | ||||||||||||||||||
(in thousands) | ||||||||||||||||||||
Dollars | % of Revenue | Dollars | % of Revenue | Dollars | % of Revenue | |||||||||||||||
Statement of Operations Data: | ||||||||||||||||||||
Revenue - third parties | $ | 84,527 | 100.0 | % | $ | 81,104 | 99.2 | % | $ | 88,876 | 99.9 | % | ||||||||
Revenue - Symmetry OEM Solutions | — | — | % | 678 | 0.8 | % | 71 | 0.1 | % | |||||||||||
Total revenue | 84,527 | 100.0 | % | 81,782 | 100.0 | % | 88,947 | 100.0 | % | |||||||||||
Cost of revenue | 45,077 | 53.3 | % | 46,098 | 56.4 | % | 48,394 | 54.4 | % | |||||||||||
Gross profit | 39,450 | 46.7 | % | 35,684 | 43.6 | % | 40,553 | 45.6 | % | |||||||||||
Sales and marketing expenses | 16,920 | 20.0 | % | 17,402 | 21.3 | % | 18,279 | 20.6 | % | |||||||||||
General and administrative expenses | 19,546 | 23.1 | % | 18,643 | 22.8 | % | 20,515 | 23.1 | % | |||||||||||
Asset impairment | 1,014 | 1.2 | % | 55,817 | 68.3 | % | 20,105 | 22.6 | % | |||||||||||
Operating income (loss) | 1,970 | 2.3 | % | (56,178 | ) | (68.7 | )% | (18,346 | ) | (20.6 | )% | |||||||||
Other (income) expense: | ||||||||||||||||||||
Interest expense | 222 | 0.3 | % | 24 | — | % | — | — | % | |||||||||||
Derivatives valuation loss | — | — | % | — | — | % | 242 | 0.3 | % | |||||||||||
Other | 269 | 0.3 | % | 229 | 0.3 | % | 38 | — | % | |||||||||||
Income (loss) before income taxes | 1,479 | 1.7 | % | (56,431 | ) | (69.0 | )% | (18,626 | ) | (20.9 | )% | |||||||||
Income tax expense (benefit) | 279 | 0.3 | % | (20,656 | ) | (25.3 | )% | (6,441 | ) | (7.2 | )% | |||||||||
Net income (loss) | $ | 1,200 | 1.4 | % | $ | (35,775 | ) | (43.7 | )% | $ | (12,185 | ) | (13.7 | )% | ||||||
Fiscal 2015 Compared to Fiscal 2014
Revenue. Revenue for fiscal 2015 increased $2,745, or 3.4%, to $84,527 from $81,782 for the comparable 2014 period. Revenue for each of our product categories in these periods was as follows:
Fiscal Year | |||||||||||||||
Sales by product | 2015 | 2014 | Dollar Change | Percent Change | |||||||||||
Symmetry Surgical branded | $ | 76,935 | $ | 72,362 | $ | 4,573 | 6.3 | % | |||||||
Alliance Partners | 7,592 | 9,420 | (1,828 | ) | (19.4 | )% | |||||||||
Total revenues | $ | 84,527 | $ | 81,782 | $ | 2,745 | 3.4 | % | |||||||
Fiscal Year Ended | |||||||||||||||
Sales by geography | 2015 | 2014 | Dollar Change | Percent Change | |||||||||||
United States | $ | 73,821 | $ | 71,578 | $ | 2,243 | 3.1 | % | |||||||
International | 10,706 | 10,204 | 502 | 4.9 | % | ||||||||||
Total Revenue | $ | 84,527 | $ | 81,782 | $ | 2,745 | 3.4 | % | |||||||
Total revenue increased $2,745 in fiscal 2015 as compared to the comparable fiscal 2014 period, primarily driven by improved sales execution efforts resulting in mid-single digits volume growth, incremental revenue from the Vesocclude Medical acquisition, and improved service levels, partially offset by very low single digit price erosion stemming from targeted product promotions and the loss of the New Wave Surgical product line, which was acquired by a third party in the first quarter of fiscal 2014 and was no longer distributed by Symmetry Surgical after April 30, 2014. Revenue
attributable to Symmetry Surgical branded products increased $4,573, primarily driven by improved sales execution efforts and the incremental revenue from the Vesocclude Medical acquisition. Alliance products revenue was down $1,828 primarily driven by a $2,684 reduction in revenue from the New Wave Surgical product line, partially offset by growth in our other Alliance products. Both of these growth rates have been historically adjusted to reflect the transition of the Vesocclude product line from the Alliance Partners products category to the Symmetry Surgical branded category due to Symmetry’s acquisition of the assets of Vesocclude Medical.
U.S. sales increased $2,243 as a result of improved sales execution and the incremental revenue from the Vesocclude Medical acquisition, partially offset by the loss of the New Wave Surgical product line. International sales improved, increasing $502, or 4.9%, from the prior fiscal year primarily as a result of the incremental revenue from the Vesocclude Medical acquisition.
Gross Profit. Gross profit for fiscal 2015 increased $3,766, or 10.6%, to 39,450 from $35,684 for fiscal 2014, primarily due to the $2,745 increase in revenue and an improvement in gross margin as a percentage of revenue. Gross margin as a percentage of revenue increased 3.1%, to 46.7% for fiscal 2015 from 43.6% for fiscal 2014. Gross margin as a percentage of revenue was favorably impacted by improved product mix, lower product costs due to favorable exchange rates, tight cost controls and volume leverage on higher sales, partially offset by pricing pressures related to product specific promotions.
Sales and Marketing Expenses. For fiscal 2015, sales and marketing expenses decreased $482 or 2.8% to $16,920 from $17,402 in fiscal 2014, due to a reduction in employee compensation and benefit levels, reduced professional fees and efficiencies in exhibition, advertising and product sample expenses.
General and Administrative Expenses. For fiscal 2015, general and administrative expenses increased $903 or 4.8%, to $19,546 from $18,643 in fiscal 2014. Significant items that impacted general and administrative expenses included:
Fiscal 2015 | ||||||
Dollars | As a % of Revenue | |||||
2014 period reported General &Administrative expenses | $ | 18,643 | 22.8 | % | ||
Employee compensation and benefit costs, excluding stock compensation | (1,307 | ) | ||||
Amortization of intangible assets | 187 | |||||
Medical device excise tax expense | 80 | |||||
Research and development | 453 | |||||
Stock compensation | 814 | |||||
Other | 676 | |||||
2015 period reported General & Administrative expenses | $ | 19,546 | 23.1 | % | ||
During fiscal 2015, employee compensation and benefit costs paid in cash decreased $1,307 due to the absence of a cash bonus plan for fiscal 2015 which resulted in a decrease in bonus expense of $446 and a reduction in severance costs of $380 as well as cost reduction actions taken in the second half of 2014 to improve the cost structure of the business given lower revenue. Research and development costs in fiscal 2014 were allocated from SMI. In fiscal 2015, these costs represented direct costs incurred by Symmetry Surgical which have increased as we have invested in a dedicated team along with additional research and development activities as part of the Vesocclude Medical acquisition. Stock compensation expense in fiscal 2014 was an allocation from SMI. In fiscal 2015, these costs represented direct costs incurred by Symmetry Surgical which have increased due to shares granted to our board of directors. Other expenses increased $676 in fiscal 2015 driven by higher professional fees related to the Company's separation from SMI and costs incurred as a stand-alone public company.
Asset Impairment. During fiscal 2015, we recorded a pre-tax non-cash charge in the amount of $1,014 as compared to $55,817 during fiscal 2014. The 2015 impairment was primarily driven by intangible assets that are no longer a key focus of the product portfolio given recent acquisitions and other areas of revenue growth.
Other (Income) Expense. Interest expense for fiscal 2015 increased $198, or 825%, to $222 from $24 in fiscal 2014 related to the new financial arrangement put into place December 5, 2014 in connection with the Spin-off.
Other income for the periods ended January 2, 2016 and January 3, 2015 represents foreign currency exchange rate fluctuations on transactions denominated in foreign currencies as well as a non-cash gain of $67 representing the difference between the fair value and the equity method carrying value of our minority interest in Vesocclude Medical LLC.
Income Tax Expense. Our effective tax rate was 18.9% for fiscal 2015 as compared to a benefit of 36.6% for fiscal 2014. Provision for income taxes increased by $20,935 to $279 for fiscal 2015 from $(20,656) in fiscal 2014, primarily due to a $57,910 increase in pre-tax income. The effective tax rate in fiscal 2015 differs from the U.S. Federal tax rate of 34% primarily due to the impact of adjusting our deferred tax assets for a change in state tax rate as a result of changes in our state apportionment of income.
Fiscal 2014 Compared to Fiscal 2013
Revenue. Revenue for fiscal 2014 decreased $7,165, or 8.1%, to $81,782 from $88,947 for the comparable fiscal 2013 period. Revenue for each of our product categories in these periods was as follows:
Fiscal Year | ||||||||||||||
Sales by product | 2014 | 2013 | Dollar Change | Percent Change | ||||||||||
Symmetry Surgical branded | $ | 72,362 | $ | 74,671 | $ | (2,309 | ) | (3.1 | )% | |||||
Alliance Partners | 9,420 | 14,276 | (4,856 | ) | (34.0 | )% | ||||||||
Total revenues | $ | 81,782 | $ | 88,947 | $ | (7,165 | ) | (8.1 | )% | |||||
Fiscal Year | ||||||||||||||
Sales by geography | 2014 | 2013 | Dollar Change | Percent Change | ||||||||||
United States | $ | 71,578 | $ | 78,358 | $ | (6,780 | ) | (8.7 | )% | |||||
International | 10,204 | 10,589 | (385 | ) | (3.6 | )% | ||||||||
Total Revenue | $ | 81,782 | $ | 88,947 | $ | (7,165 | ) | (8.1 | )% | |||||
Total revenue decreased $7,165 in fiscal 2014 as compared to fiscal 2013. Revenue attributable to Symmetry Surgical branded products was down $2,309, primarily impacted by unfavorable pricing, as the Company sold more products under GPO contract agreements ("contracted sales") than in fiscal 2013. The sluggish hospital spending environment in the U.S. was partially offset by increased sales of $607 to SMI's OEM Solutions Business, which was discontinued as part of our separation from SMI in December 2014. Alliance products revenue was down $4,856 in fiscal 2014, primarily driven by a $4,204 reduction in revenue from the New Wave Surgical product line, which was acquired by a third party in the first quarter of fiscal 2014 and was no longer distributed by the Company after April 2014. For historical comparability, both product categories have been adjusted to reflect the transition of the Vesocclude product line from the Alliance Partners products category to the Symmetry Surgical branded category due to Symmetry’s acquisition of the assets of Vesocclude Medical in fiscal 2015.
U.S. sales were down $6,780 as a result of the reduction in New Wave Surgical revenue and unfavorable pricing from contracted sales. International sales remained relatively flat, decreasing $385, or 3.6%, in fiscal 2014 compared to the prior fiscal year.
Gross Profit. Gross profit for fiscal 2014 decreased $4,869, or (12.0)%, to $35,684 from $40,553 for fiscal 2013, primarily due to the $7,165 reduction in revenue and a decline in gross margin as a percentage of revenue. Gross margin as a percentage of revenue decreased 2.0%, to 43.6% for fiscal 2014 from 45.6% for fiscal 2013. Gross margin as a percentage of revenue was impacted by unfavorable pricing due to heavier mix of contracted sales and higher product costs in fiscal 2014, as the Company purchased many items from outside our normal supply chain as a result of supplier disruptions in order to meet customer delivery expectations. Partially offsetting these reductions to gross margin as a percentage of revenue was a favorable impact of product mix driven by less New Wave Surgical product revenues, which generated below average gross margins. Finally, the sales to SMI's OEM Solutions Business negatively impacted gross margins as those products were sold at minimal gross margin.
Sales and Marketing Expenses. For fiscal 2014, sales and marketing expenses decreased $877, or 4.8%, to $17,402 from $18,279 in fiscal 2013, due to a reduction in employee compensation and benefits as a result of lower sales in fiscal 2014, a reduction in advertising expenses. The Company launched its global distribution of an updated and comprehensive catalog in the third quarter of fiscal 2013 and improved collection efforts which allowed for the release of certain reserves for the allowance for doubtful accounts.
General and Administrative Expenses. For fiscal 2014, general and administrative expenses decreased $1,872 or 9.1%, to $18,643 from $20,515 in fiscal 2013. Significant items that impacted general and administrative expenses included:
Fiscal Year 2014 | ||||||
Dollars | As a % of Revenue | |||||
2013 period reported General &Administrative expenses | $ | 20,515 | 23.1 | % | ||
Symmetry Surgical infrastructure additional costs | (337 | ) | ||||
Employee compensation and benefit costs, excluding stock compensation | (924 | ) | ||||
Amortization of intangible assets | 348 | |||||
Medical device excise tax expense | 18 | |||||
Research and development | (142 | ) | ||||
Stock compensation | 92 | |||||
Other | (927 | ) | ||||
2014 period reported General & Administrative expenses | $ | 18,643 | 22.8 | % | ||
During fiscal 2014, employee compensation and benefit costs paid in cash decreased $924 due to a decrease of $478 in employee severance as well as cost reduction actions taken in both fiscal 2013 and fiscal 2014 to improve the cost structure of the business given lower revenues. Other expenses decreased $927 in fiscal 2014, driven by lower acquisition related expenses and loss on the sale of assets in fiscal 2013 of $411.
Asset Impairment. During fiscal 2014, we recorded a pre-tax non-cash charge in the amount of $55,817 as compared to $20,105 during fiscal 2013. The fiscal 2014 impairment was primarily driven by the market valuation of the Company combined with lower estimates of future cash flows due to a sluggish hospital spending environment in the U.S., previously disclosed integration challenges related to the 2011 acquisition of the surgical instruments portfolio of Codman and Shurtleff, Inc. from which the business has not recovered as quickly as previously expected, and reduced cost savings estimates for the post Spin-Off operations of the Company which have also been previously disclosed.
Other (Income) Expense. Interest expense of $24 was incurred for fiscal 2014 related to the new financial arrangement put into place December 5, 2014 in connection with the Spin-off.
Derivatives valuation consists of foreign currency forward contracts which were used to mitigate the effect of changes in the foreign exchange rates on net income. As of December 28, 2013, we had settled all of our outstanding forward swap contracts and we recorded a loss of $242 in fiscal 2013, which resulted from fluctuation in the Euro versus the US Dollar.
Other income for the periods ended January 3, 2015 and December 28, 2013 represents foreign currency exchange rate fluctuations on transactions denominated in foreign currencies.
Income Tax Expense. Our effective tax rate was a benefit of 36.6% for fiscal 2014 as compared to a benefit of 34.6% for fiscal 2013. Provision for income taxes decreased by $14,215, or 220.7%, to $(20,656) for fiscal 2014 from $(6,441) in fiscal 2013, primarily due to a $37,805 decrease in pre-tax income. The effective tax rate in fiscal 2014 differs from the U.S. Federal tax rate of 35% primarily due to the impact of state taxes.
Liquidity and Capital Resources
Liquidity
Our principal source of liquidity in fiscal 2015 was cash generated from operations. Principal uses of cash in fiscal 2015 were for payments on our Revolving Loan and for acquisitions. We expect that our principal uses of cash in the future will be to pay for capital expenditures, to finance working capital, and to fund possible future acquisitions.
We believe our cash flows from operations, as well as our bank financing arrangement, will permit us to stay committed to our strategic plan of growing the current business as well as growing through acquisitions.
The following table summarizes our primary sources and uses of cash in the periods presented:
Fiscal Year | |||||||||
2015 | 2014 | 2013 | |||||||
Net Cash Flow provided by (used in): | |||||||||
Operating activities | $ | 13,551 | $ | (570 | ) | $ | 19,067 | ||
Investing activities | (4,427 | ) | (540 | ) | (928 | ) | |||
Financing activities | (3,876 | ) | 3,293 | (17,656 | ) | ||||
Effect of exchange rate changes on cash | (170 | ) | 163 | 3 | |||||
Net increase in cash | $ | 5,078 | $ | 2,346 | $ | 486 | |||
Operating Activities. Operating activities generated cash of $13,551 in fiscal 2015 compared to cash usage of $570 for fiscal 2014, an increase of $14,121. The increase in cash from operations is primarily a result of an increase in net cash provided by working capital of $11,191 as well as an increase in net income adjusted for non-cash items. Net cash provided by working capital for fiscal 2015 was $3,145 compared to net cash used in working capital of $8,046 for fiscal 2014 primarily driven by a decrease in inventory levels and an increase in accounts payable.
In fiscal 2014, operating activities used cash of $570 compared to cash generation of $19,067 for fiscal 2013, a decrease of $19,637. The decrease in cash from operations was primarily a result of a reduction in net cash provided by working capital and an increase in net loss of $23,590 adjusted for non-cash items. Significant working capital was used to increase inventory levels to better service customer demands and to reduce accounts payable outstanding. Net income adjusted for non-cash items decreased $2,766 from fiscal 2013 due primarily to reduced sales and related gross profit.
Investing Activities. Net cash used in investing activities was $4,427 in fiscal 2015 compared to a usage of $540 for fiscal 2014. Investing activities during fiscal 2015 consisted of $3,860 related to the acquisition of Vesocclude, $400 related to the acquisition of Insightra as well as capital expenditures of $171.
Financing Activities. Financing activities used $3,876 of cash in fiscal 2015 compared to a cash generation of $3,293 in fiscal 2014. Financing activities during fiscal 2015 related to net payments on the Revolving Loan. Financing activities during 2013 resulted solely from cash transfers to SMI. Subsequent to the Spin-Off, the Company no longer participates in cash management and funding arrangements with SMI. The Company completed the period ending January 2, 2016 with no borrowings on its Revolving Loan.
Capital Expenditures. Capital expenditures of $171 were $381 lower in fiscal 2015 compared to fiscal 2014, due to one-time investments in e-commerce and information systems infrastructure that did not repeat in fiscal 2015.
Capital expenditures totaled $552 in fiscal 2014 and primarily consisted of information systems and infrastructure expansion to support the Spin-Off from SMI, compared to $1,477 in fiscal 2013. The fiscal 2013 capital expenditures primarily comprised of costs incurred in connection with implementing information systems to allow us to eliminate Johnson & Johnson transition services in fiscal 2012 which continued into the first half of fiscal 2013.
Debt and Credit Facilities
On December 5, 2014, the Company, Specialty Surgical Instrumentation, Inc. and Olsen Medical, LLC (collectively, the “Borrowers”) entered into a Credit Agreement (the “Credit Agreement”) with Capital One (formerly known as General Electric Capital Corporation ("GECC")), which provides for a Revolving Loan of up to $20,000, subject to a borrowing base limitation. Any amounts outstanding under the Revolving Loan are due and payable on December 5, 2019. Amounts outstanding under the Credit Agreement bear interest at LIBOR plus 3.25%, or the Base Rate (which will generally be based on the prime rate as published by the Wall Street Journal from time to time) plus 2.25%. Borrowings under this revolving loan are classified as current as of January 3, 2015 due to the existence of cash dominion by Capital One. In February 2015, we amended the Credit Agreement to allow for a collateral cash account which is excluded from the cash dominion. This collateral cash account is included in other assets in the consolidated balance sheets. In August 2015, we amended the Credit Agreement to allow for the acquisition of Vesocclude Medical. As of January 2, 2016, there were no outstanding borrowings under our Credit Agreement.
The Credit Agreement is guaranteed by Symmetry Surgical International, Inc., Symmetry Surgical Vesocclude, LLC, S.S.I.I. LP, LLC and Symmetry Medical SSI Real Estate LLC (collectively the “Guarantors”). The Revolving Loan is secured by a
first-priority perfected security interest in substantially all of the tangible and intangible assets of the Borrowers and the Guarantors, including a pledge of 100% of the equity interests of each domestic subsidiary.
The Credit Agreement includes customary covenants for facilities of this type, including limitations on indebtedness, liens, investments and acquisitions, loans and advances, mergers and consolidations, sales of assets, and dividends, stock repurchases and other restricted payments. In addition, the Credit Agreement requires that Borrowers maintain a minimum fixed charge coverage ratio of 1.25 to 1.00 tested at the close of each fiscal quarter beginning March 31, 2015, based on the preceding four fiscal quarters, and maintain at all times minimum liquidity (the sum of cash, cash equivalent and available borrowing capacity under the borrowing base) of $1,500, tested monthly. As of January 2, 2016, the borrowing base limitation was $13,735 and fully available. The Company was in compliance with all covenants as of January 2, 2016.
We believe that cash flow from operating activities and borrowings on our Bank Revolver will be sufficient to fund currently anticipated working capital, planned capital spending and debt service requirements for the foreseeable future, including at least the next twelve months.
Contractual Obligations and Commercial Commitments
The following table reflects our contractual obligations as of January 2, 2016:
Payments Due by Period | |||||||||||||||
Total | Less than 1 year | 1 - 3 years | 4 - 5 years | More than 5 years | |||||||||||
Debt obligations (1) | $ | — | $ | — | $ | — | $ | — | $ | — | |||||
Operating lease obligations | 1,195 | 527 | 663 | 5 | — | ||||||||||
Employee benefit pension obligations (2) | 804 | — | — | — | 804 | ||||||||||
Purchase obligations (3) | 1,494 | 221 | 1,273 | — | — | ||||||||||
Contingent liabilities (4) | $ | 2,183 | $ | 1,047 | $ | 1,136 | $ | — | $ | — | |||||
Total | $ | 5,676 | $ | 1,795 | $ | 3,072 | $ | 5 | $ | 804 | |||||
(1) | Represents principal maturities only and, therefore, excludes the effects of interest which is settled monthly on outstanding borrowings. There are no scheduled principal payments for our Revolving Loan prior to maturity at January 2, 2016 as there was no amount outstanding under the Revolving Loan. Borrowings under this revolving loan were classified as current as of January 3, 2015 due to the existence of cash dominion by Capital One. Borrowings under the Revolving Loan bear interest at LIBOR plus 3.25%, or the Base Rate (which will generally be based on the prime rate as published by the Wall Street Journal from time to time) plus 2.25%. |
(2) | Represents benefit liability related to the one sponsored defined benefit pension plan for the benefit of the Company's employees at its German subsidiary. There are no significant benefits under the plan which are expected to be paid within the next 10 years. |
(3) | For the purposes of this table, contractual obligations for purchases of goods or services are defined as agreements that are enforceable and legally binding and that specify all significant terms, including: fixed or minimum quantities, fixed, minimum or variable price provisions; and the approximate timing of the transaction. Our purchase orders are normally based on our current manufacturing needs and are fulfilled by our vendors within a short time. We enter into blanket orders with vendors that have preferred pricing terms; however, these orders are normally cancelable by us without penalty. Amounts represent a purchase agreement to minimum quantities of finished instruments through August 2019. |
(4) | Represents the estimated fair value of contingent consideration associated with the assets acquired from Vesocclude Medical, LLC and Insightra Medical. The Vesocclude Medical contingent consideration involves two contingent purchase payments. Contingent purchase liability No. 1 is based on achieving certain milestones related to new product introduction. Contingent purchase liability No. 2 is based on reaching specified new product commercial sales levels. The potential undiscounted amount of each future payment that the Company could be required to make under the agreement is between $0 and $2,200 in the aggregate. The Insightra Medical contingent purchase payment is based as a percent of future sales. |
Off-Balance Sheet Arrangements
Off-balance sheet arrangements include our operating leases.
Environmental
We incurred almost zero capital expenditures specifically for environmental compliance and health and safety during 2015 and 2014. In connection with past acquisitions, we completed Phase I environmental assessments and did not find any significant issues that we believe needed to be remediated. Based on information currently available, we do not believe that we have any material environmental liabilities. We cannot be certain, however, that environmental issues will not be discovered or arise in the future related to these acquisitions or existing facilities.
Critical Accounting Policies and Estimates
The preparation of our consolidated and combined financial statements in accordance with accounting principles generally accepted in the United States requires management to make estimates, judgments and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses during the periods presented. On an ongoing basis, we evaluate these estimates. We base our estimates on historical experiences and assumptions believed to be reasonable under the circumstances. Those estimates form the basis for our judgments that affect the amounts reported in the consolidated and combined statements. Actual results could differ from our estimates under different assumptions or conditions. Our significant accounting policies, which may be affected by our estimates and assumptions, are more fully described in Note 3 to our consolidated and combined financial statements that appear elsewhere in this Form 10-K.
On April 5, 2012, the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, was enacted. Section 107 of the JOBS Act provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act of 1933, as amended, or the Securities Act, for complying with new or revised accounting standards. In other words, an “emerging growth company” may delay the adoption of certain accounting standards until those standards would otherwise apply to private companies and we have chosen to take advantage of this extended transition period. As a result, our financial statements may not be comparable to those companies that comply with public company effective dates.
Revenue Recognition. We recognize revenue, on orders received from customers, when there is persuasive evidence of an arrangement with the customer that is supportive of revenue recognition, the customer has made a fixed commitment to purchase the product for a fixed or determinable sales price, collection is reasonably assured under our normal billing and credit terms, and ownership and all risks of loss have been transferred to the buyer which is normally upon shipment or delivery to the customer. In certain circumstances, customer terms require receipt of product prior to the transfer of the risk of ownership. In such circumstances, revenue is not recognized upon shipment, but rather upon confirmation of delivery. For product sales to distributors, Symmetry Surgical recognizes revenue upon shipment to the distributor under standard contract terms stating that title to the goods passes to the distributors at point of shipment to the distributor’s location. All shipments to distributors are at contract prices and payment is not contingent upon resale of the product. We offer certain customers volume rebates, which are reflected as a reduction in revenue when it is probable the rebate will be earned based on current and forecasted sales and the amount can be estimated. Product returns are estimated based upon historical return rates and are reflected as a reduction of revenue in the same period revenue is recognized. These estimates have historically been materially consistent with actual results. We do not believe our estimates are reasonably likely to materially change in the future.
Inventories. Inventories are stated at the lower of cost, determined primarily on the first-in, first-out (FIFO) method, or market (net realizable value). Costs include material, labor and manufacturing overhead costs, as applicable. We review our inventory balances quarterly for excess products or obsolete inventory levels and write down, if necessary, the inventory to net realizable value.
Goodwill. We test goodwill for impairment annually on the first day of the fourth fiscal quarter and more frequently if circumstances warrant. We determine fair values using an income approach. When available and appropriate, we use comparative market multiples to corroborate discounted cash flow results. For purposes of the income approach, fair value is determined based on the present value of estimated future cash flows, discounted at an appropriate risk-adjusted rate. We use our internal forecasts to estimate future cash flows and include an estimate of long-term future growth rates based on our most recent views of the long-term outlook for each reporting unit. Actual results may differ from those assumed in our forecasts. We derive our discount rates using a capital asset pricing model and analyzing published rates for industries relevant to our reporting units to estimate the cost of equity financing. We use discount rates that are commensurate with the risks and uncertainty inherent in the respective businesses and in our internally developed forecasts. The discount rate used in most recent valuation was 12.0%. Valuations using the market approach reflect prices and other relevant observable information generated by market transactions involving comparable businesses. Compared to the market approach, the income approach more closely aligns valuation to our business profile, including geographic markets served
and product offerings. Required rates of return, along with uncertainty inherent in the forecasts of future cash flows, are reflected in the selection of the discount rate.
For fiscal 2015, we conducted our annual impairment tests, noting no impairment. To derive the fair value of the reporting unit, as required in step one of the impairment test, we used the income approach, specifically the discounted cash flow method, to determine the fair value of the reporting unit. The results of the step one impairment test resulted in the requirement for us to perform step two of the annual impairment test. In the second step, the Company assigned the reporting unit's fair value to all of its assets and liabilities, including any unrecognized intangible assets, in a hypothetical analysis that calculates the implied fair value of goodwill in the same manner as if the reporting unit were being acquired in a business combination. If the implied fair value of the reporting unit's goodwill is less than the carrying value, the difference is recorded as an impairment charge. This allocation process was performed only for purposes of measuring the goodwill impairment and not to adjust the carrying values of the recognized assets and liabilities. The results of the step two impairment test indicated that no impairment existed. Revenue growth rates assumed were 0.0% to 5.7% over the next five years with a terminal growth rate of 3.0%. These growth rates are driven by new product launches as well as continued strong sales execution and low single-digit market growth. A significant decline in our revenue and earnings or a significant decline in the price of common stock could result in an impairment charge in the future. Such events that could result in a decline in our revenue and earnings in the future would be a loss of a significant customer or product line, pricing pressure on products as we attempt to gain market share, and further disruptions in our manufacturing supplier base, among other events.
During 2014, we conducted our interim and annual impairment tests, and recorded an impairment charge of $55,817. The impairment was primarily driven by the market valuation of the Company combined with lower estimates of future cash flows due to the sluggish hospital spending environment in the U.S., previously disclosed integration challenges related to the 2011 acquisition of the surgical instruments portfolio of Codman and Shurtleff, Inc. from which the Company had not recovered as quickly as previously expected and reduced cost savings estimates for the post Spin-off operations of the Company which had also been previously disclosed.
Definite-Lived Long-Lived Assets. We assess the impairment of definite-lived long-lived assets when circumstances indicate the carrying value of an asset may not be recoverable based on the undiscounted future cash flows of an asset. If the carrying amount of the asset is determined not to be recoverable, a write-down to fair value is recorded. Fair values are determined based on quoted market values, undiscounted cash flows, or external appraisals, as applicable. We review long-lived assets for impairment at the individual asset or the asset group level for which the lowest level of independent cash flows can be identified. Intangible assets subject to amortization consist of trademarks and technology and patents which are amortized using the straight-line method, as well as customer related intangible assets which are amortized on an accelerated method. Straight-line methods are used for income tax purposes. All of our intangible assets were acquired in connection with our various acquisitions. We are required to reassess the expected useful lives of existing definite-lived assets annually. Impairment charges were recorded in 2015 related to two patents and one trademark of $252 and $762, respectively. We reviewed all other definite-lived long-lived assets and did not record any impairment related to the remaining assets for fiscal 2015, 2014 or 2013.
Income Taxes. The consolidated and combined financial statements of Symmetry Surgical have been prepared using the asset and liability method in accounting for income taxes, which requires the recognition of deferred income taxes for the expected future tax consequences of net operating losses, credits, and temporary differences between the financial statement carrying amounts and the tax basis of assets and liabilities. Income taxes prior to the Spin-Off are presented and calculated on a separate tax return basis (inclusive of certain loss benefits), although Symmetry Surgical’s operations have historically been included in SMI’s U.S. federal and state tax returns or the tax returns of non-U.S. jurisdictions. Accordingly, the income taxes presented may not be reflective of the results that would have occurred on a standalone basis prior to Spin-Off. Differences between the anticipated and actual outcomes of these future tax consequences could have a material impact on our consolidated and combined results of operations or financial position. Additionally, we use tax planning strategies as part of our global tax compliance program. Judgments and interpretation of statutes are inherent in this process. Symmetry Surgical provides related valuation reserves, where applicable, in accounting for uncertain tax positions. Interest and penalties associated with reserves for uncertain tax positions are classified in income tax expense in the consolidated and combined statements of operations.
Impact of Recently Issued and Adopted Accounting Standards
Revenue from Contracts with Customers: In May 2014, the FASB issued ASU No. 2014-09, Revenue from Contracts with Customers (Topic 606), which supersedes the revenue recognition requirements in Accounting Standards Codification (“ASC”) 605, Revenue Recognition. This ASU is based on the principle that revenue is recognized to depict
the transfer of goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods and services. The ASU also requires additional disclosure about the nature, amount, timing and uncertainty of revenue and cash flows arising from customer contracts, including significant judgments and changes in judgments and assets recognized from costs incurred to obtain or fulfill a contract. ASU 2014-09 was originally effective for fiscal periods (including interim periods) beginning after December 15, 2016. In August 2015, the FASB issued ASU 2015-14, Revenue from Contracts with Customers (Topic 606): Deferral of the Effective Date, which defers the effective date by one year, with early adoption permitted as of the original effective date. ASU 2014-09 allows for either a full retrospective or a modified retrospective transition method. We are currently assessing the potential impact of the adoption of ASU 2014-09 on its financial statements and related disclosures.
Reporting Discontinued Operations and Disclosures of Disposals of Components of an Entity: In April 2014, the FASB issued ASU 2014-08, Presentation of Financial Statements (Topic 205) and Property, Plant and Equipment (Topic 360), Reporting Discontinued Operations and Disclosures of Disposals of Components of an Entity. This update modifies the requirements for reporting discontinued operations. Under the amendments in ASU 2014-08, the definition of discontinued operation has been modified to only include those disposals of an entity that represent a strategic shift that has (or will have) a major effect on an entity’s operations and financial results. This update also expands the disclosure requirements for disposals that meet the definition of a discontinued operation and requires entities to disclose information about disposals of individually significant components that do not meet the definition of discontinued operations. This update is effective for annual and interim periods beginning after December 15, 2014. Our adoption of this ASU in 2015 did not have an impact on our financial position, results of operations or cash flows.
Accounting for Share-Based Payments When the Terms of an Award Provide That a Performance Target Could Be Achieved after the Requisite Service Period. In June, 2014, the FASB issued ASU 2014-12, Accounting for Share-Based Payments When the Terms of an Award Provide That a Performance Target Could Be Achieved after the Requisite Service Period. This new guidance states that a performance target that affects vesting of a share-based payment and that could be achieved after the requisite service period is a performance condition under ASC 718. This update is effective for annual and interim periods beginning after December 15, 2015. We do not believe this ASU will have an impact on our financial statements.
Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern. In August, 2014, the FASB issued ASU 2014-15, Disclosure of Uncertainties about an Entity's Ability to Continue as a Going Concern. This new guidance requires management to evaluate whether there is substantial doubt about the entity’s ability to continue as a going concern and, if so, disclose that fact. Management will also be required to evaluate and disclose whether its plans alleviate that doubt. The standard is effective for annual periods ending after December 15, 2016 with early adoption permitted. We do not believe this ASU will have an impact on our financial statements.
Business Combinations: Simplifying the Accounting for Measurement-Period Adjustments. In September 2015, the FASB issued ASU No. 2015-16, Business Combinations (Topic 805): Simplifying the Accounting for Measurement-Period Adjustments. This new guidance requires that an acquirer recognize adjustments to provisional amounts that are identified during the measurement period in the reporting period in which the adjustment amounts are determined. The amendments of this standard are effective prospectively for the fiscal years, including interim periods, beginning after December 15, 2015, and early adoption is permitted. We will adopt this ASU on January 3, 2016.
Income Taxes (Topic 740) Related to the Balance Sheet Classification of Deferred Taxes. In November 2014 the FASB issued ASU 2015-17, Income Taxes (Topic 740) Related to the Balance Sheet Classification of Deferred Taxes which will require entities to present deferred tax assets (DTAs) and deferred tax liabilities (DTLs) as noncurrent in a classified balance sheet. The ASU simplifies the current guidance (ASC 740-10-45-4), which requires entities to separately present DTAs and DTLs as current and noncurrent in a classified balance sheet. The ASU is effective for annual reporting periods beginning on or after December 15, 2016, and interim periods within those annual periods. The Board decided to allow all entities to early adopt the ASU for financial statements that had not been issued. Therefore, we adopted this ASU at January 2, 2016, and have reclassified all prior periods to be consistent with the requirements outlined in the ASU. The impact of the prior period reclassification was a $2,816 reduction of current assets, a $15 reduction of current liabilities and a net working capital decrease of $2,801 at January 3, 2015.
Technical Corrections and Improvements. In June 2015, the FASB issued ASU No. 2015-10, Technical Corrections and Improvements to clarify that, for nonrecurring measurements estimated at a date during the reporting period other than the end
of the reporting period, an entity should clearly indicate that the fair value information presented is not as of the period's end as well as the date or period that the measurement was taken. We adopted ASU 2015-10, effective June 12, 2015, as the change was effective upon issuance. The adoption did not have an impact on our consolidated financial statements.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
Interest Rate Risk
We are exposed to market risk from fluctuations in interest rates. For variable rate debt, interest rate changes generally do not affect the fair market value of such debt, but do impact future earnings and cash flows, assuming other factors are held constant. As of January 2, 2016, we had no outstanding variable rate debt; therefore, we have no exposure to interest rate changes on indebtedness.
Foreign Currency Risk
Foreign currency risk is the risk that we will incur economic losses due to adverse changes in foreign currency exchange rates. As a global company, our primarily exposure to foreign currency results from our Euro denominated inventory purchases. A hypothetical 10 percent change in the Euro to U.S. dollar exchange rate would have impacted our earnings for the twelve months ended January 2, 2016 by approximately $1,100.
At January 2, 2016, the potential impact on earnings from a hypothetical instantaneous 10.0% increase or decrease in quoted foreign currency spot rates applied to foreign currency sensitive instruments would be approximately $150. This foreign currency sensitivity model is limited by the assumption that all of the foreign currencies to which we are exposed would simultaneously increase or decrease by 10.0% because such synchronized changes are unlikely to occur.
Item 8. Financial Statements and Supplemental Data
Page | |
Management’s Report on Internal Control Over Financial Reporting | |
Report of Independent Registered Public Accounting Firm | |
Consolidated and Combined Financial Statements: | |
Consolidated Balance Sheets | |
Consolidated and Combined Statements of Operations | |
Consolidated and Combined Statements of Comprehensive Income | |
Consolidated and Combined Statements of Shareholders’ Equity | |
Consolidated and Combined Statements of Cash Flow | |
Notes to Consolidated and Combined Financial Statements | |
All schedules have been omitted because they are not required or applicable or the information is included in the consolidated and combined financial statements or note thereto.
Management’s Report on Internal Control Over Financial Reporting.
Our management is responsible for establishing and maintaining effective internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act. This system is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of consolidated financial statements for external purposes in accordance with U.S. GAAP. Because of its inherent limitations, internal control over financial reporting, may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Our management performed an assessment of the effectiveness of our internal control over financial reporting as of January 2, 2016, utilizing the criteria discussed in the “Internal Control - Integrated Framework (2013)” issued by the Committee of Sponsoring Organizations of the Treadway Commission. The objective of this assessment was to determine whether our internal control over financial reporting was effective as of January 2, 2016. Based on management's assessment, we have concluded that our internal control over financial reporting was effective as of January 2, 2016.
By | /s/ Thomas J. Sullivan | |
Thomas J. Sullivan, | ||
Chief Executive Officer | ||
By | /s/ Scott D. Kunkel | |
Scott D. Kunkel, | ||
Chief Financial Officer | ||
March 1, 2016 | ||
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders of Symmetry Surgical Inc.:
We have audited the accompanying consolidated balance sheets of Symmetry Surgical Inc. as of January 2, 2016 and January 3, 2015, and the related consolidated and combined statements of operations, comprehensive income (loss), equity and cash flows for each of the three years in the period ended January 2, 2016. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company’s internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Symmetry Surgical Inc. at January 2, 2016 and January 3, 2015, and the consolidated and combined results of its operations and its cash flows for each of the three years in the period ended January 2, 2016, in conformity with US generally accepted accounting principles.
/s/ Ernst & Young LLP
Indianapolis, Indiana
March 1, 2016
SYMMETRY SURGICAL INC.
CONSOLIDATED BALANCE SHEETS
(In Thousands)
January 2, 2016 | January 3, 2015 | ||||||
ASSETS: | |||||||
Current Assets: | |||||||
Cash | $ | 8,072 | $ | 2,994 | |||
Accounts receivable, net | 10,922 | 10,070 | |||||
Inventories | 23,076 | 24,141 | |||||
Other current assets | 1,624 | 2,417 | |||||
Total current assets | 43,694 | 39,622 | |||||
Property and equipment, net | 2,258 | 2,768 | |||||
Deferred income taxes | 24,291 | 24,411 | |||||
Goodwill | 9,341 | 7,126 | |||||
Intangible assets, net of accumulated amortization | 73,719 | 77,903 | |||||
Other assets | 678 | 219 | |||||
Total Assets | $ | 153,981 | $ | 152,049 | |||
LIABILITIES AND EQUITY: | |||||||
Current Liabilities: | |||||||
Accounts payable | $ | 6,240 | $ | 5,283 | |||
Accrued wages and benefits | 1,785 | 1,902 | |||||
Other accrued expenses | 1,540 | 1,417 | |||||
Contingent purchase liability, current | 1,047 | — | |||||
Accrued income taxes | 201 | 158 | |||||
Revolving line of credit | — | 3,876 | |||||
Total current liabilities | 10,813 | 12,636 | |||||
Contingent purchase liability, non-current | 1,136 | — | |||||
Other liabilities | 1,019 | 1,107 | |||||
Total Liabilities | 12,968 | 13,743 | |||||
Commitments and contingencies | |||||||
Equity: | |||||||
Stockholders' Equity: | |||||||
Common Stock, $.0001 par value; 50,000 shares authorized; 10,535 shares issued January 2, 2016; 10,313 shares issued January 3, 2015 | 1 | 1 | |||||
Additional paid-in capital | 140,460 | 138,928 | |||||
Retained earnings (accumulated deficit) | 1,084 | (116 | ) | ||||
Accumulated other comprehensive loss | (532 | ) | (507 | ) | |||
Total Stockholders' Equity | 141,013 | 138,306 | |||||
Total Liabilities and Equity | $ | 153,981 | $ | 152,049 | |||
See accompanying notes to consolidated and combined financial statements.
SYMMETRY SURGICAL INC.
CONSOLIDATED AND COMBINED STATEMENTS OF OPERATIONS
(In Thousands, Except Per Share Data)
Fiscal Year Ended | ||||||||||||
2015 | 2014 | 2013 | ||||||||||
Revenue - third parties | $ | 84,527 | $ | 81,104 | $ | 88,876 | ||||||
Revenue - Symmetry OEM Solutions | — | 678 | 71 | |||||||||
Total revenue | 84,527 | 81,782 | 88,947 | |||||||||
Cost of revenue | 45,077 | 46,098 | 48,394 | |||||||||
Gross profit | 39,450 | 35,684 | 40,553 | |||||||||
Sales and marketing expenses | 16,920 | 17,402 | 18,279 | |||||||||
General and administrative expenses | 19,546 | 18,643 | 20,515 | |||||||||
Asset impairment | 1,014 | 55,817 | 20,105 | |||||||||
Operating income (loss) | 1,970 | (56,178 | ) | (18,346 | ) | |||||||
Other (income) expense: | ||||||||||||
Interest expense | 222 | 24 | — | |||||||||
Derivatives valuation loss | — | — | 242 | |||||||||
Other | 269 | 229 | 38 | |||||||||
Income (loss) before income taxes | 1,479 | (56,431 | ) | (18,626 | ) | |||||||
Income tax expense (benefit) | 279 | (20,656 | ) | (6,441 | ) | |||||||
Net income (loss) | $ | 1,200 | $ | (35,775 | ) | $ | (12,185 | ) | ||||
Net income (loss) per share: | ||||||||||||
Basic | $ | 0.12 | $ | (3.73 | ) | $ | (1.27 | ) | ||||
Diluted | $ | 0.12 | $ | (3.73 | ) | $ | (1.27 | ) | ||||
Weighted average common shares and equivalent shares outstanding: | ||||||||||||
Basic (a) | 9,645 | 9,587 | 9,587 | |||||||||
Diluted (a) | 9,688 | 9,587 | 9,587 | |||||||||
(a) On December 5, 2014, SMI distributed 9,587 shares of Symmetry Surgical common stock. For periods prior to the separation, the weighted-average basic and diluted shares outstanding were based on the number of shares of Symmetry Surgical common stock outstanding on the distribution date. Refer to Note 19 for information regarding the calculation of basic and diluted net income per share for the year ended January 3, 2015.
See accompanying notes to consolidated and combined financial statements.
SYMMETRY SURGICAL INC.
CONSOLIDATED AND COMBINED STATEMENTS OF COMPREHENSIVE INCOME (LOSS)
(In Thousands)
Fiscal Year Ended | |||||||||||||
2015 | 2014 | 2013 | |||||||||||
Net income (loss) | $ | 1,200 | $ | (35,775 | ) | $ | (12,185 | ) | |||||
Other comprehensive income (loss) | |||||||||||||
Foreign currency translation adjustments | (109 | ) | (305 | ) | 33 | ||||||||
Pension plan actuarial gain (loss), net of taxes | 84 | (41 | ) | 64 | |||||||||
Comprehensive income (loss) | $ | 1,175 | $ | (36,121 | ) | $ | (12,088 | ) | |||||
See accompanying notes to consolidated and combined financial statements.
SYMMETRY SURGICAL INC.
CONSOLIDATED AND COMBINED STATEMENTS OF EQUITY
(In Thousands)
Common Shares Outstanding | Common Stock | Additional Paid-in Capital | Retained Earnings | Net Parent Investment | Accumulated Other Comprehensive Income (Loss) | Total | ||||||||||||||
Balance at December 29, 2012 | — | $ | — | $ | — | $ | — | $ | 201,205 | $ | (258 | ) | $ | 200,947 | ||||||
Comprehensive income: | ||||||||||||||||||||
Net loss | — | — | — | — | (12,185 | ) | — | (12,185 | ) | |||||||||||
Other comprehensive income (loss) | — | — | — | — | — | 97 | 97 | |||||||||||||
Net transfers to Parent | — | — | — | — | (17,031 | ) | (17,031 | ) | ||||||||||||
Balance at December 28, 2013 | — | — | — | — | 171,989 | (161 | ) | 171,828 | ||||||||||||
Comprehensive income: | ||||||||||||||||||||
Net transfers from Parent prior to separation | — | — | — | — | 2,599 | — | 2,599 | |||||||||||||
Net loss prior to separation | — | — | — | — | (35,659 | ) | — | (35,659 | ) | |||||||||||
Reclassification of parent company investment in connection with separation | — | — | 138,929 | — | (138,929 | ) | — | — | ||||||||||||
Issuance of common stock at separation | 9,587 | 1 | (1 | ) | — | — | — | — | ||||||||||||
Net loss | — | — | — | (116 | ) | — | — | (116 | ) | |||||||||||
Other comprehensive income (loss) | — | — | — | — | — | (346 | ) | (346 | ) | |||||||||||
Stock-based compensation | 726 | — | — | — | — | — | — | |||||||||||||
Balance at January 3, 2015 | 10,313 | $ | 1 | $ | 138,928 | $ | (116 | ) | $ | — | $ | (507 | ) | $ | 138,306 | |||||
Comprehensive income: | ||||||||||||||||||||
Net income | — | — | — | 1,200 | — | — | 1,200 | |||||||||||||
Other comprehensive income (loss) | — | — | — | — | — | (25 | ) | (25 | ) | |||||||||||
Stock-based compensation | 222 | — | 1,532 | — | — | 1,532 | ||||||||||||||
Balance at January 2, 2016 | 10,535 | $ | 1 | $ | 140,460 | $ | 1,084 | $ | — | $ | (532 | ) | $ | 141,013 | ||||||
See accompanying notes to consolidated and combined financial statements.
SYMMETRY SURGICAL INC.
CONSOLIDATED AND COMBINED STATEMENTS OF CASH FLOW
(In Thousands)
Fiscal Year Ended | |||||||||||
2015 | 2014 | 2013 | |||||||||
Operating activities | |||||||||||
Net income (loss) | $ | 1,200 | $ | (35,775 | ) | $ | (12,185 | ) | |||
Adjustments to reconcile net income to net cash provided by operating activities: | |||||||||||
Depreciation | 920 | 956 | 1,075 | ||||||||
Amortization of intangible assets | 5,562 | 5,375 | 5,027 | ||||||||
Amortization of debt issuance costs | 100 | 7 | — | ||||||||
Net loss on sale of assets | 35 | 114 | 423 | ||||||||
Asset impairment | 1,014 | 55,817 | 20,105 | ||||||||
Deferred income tax provision | 97 | (19,822 | ) | (5,187 | ) | ||||||
Stock-based compensation | 1,532 | 717 | 625 | ||||||||
Derivative valuation loss | — | — | 242 | ||||||||
Unrealized foreign currency transaction (gain) loss | (54 | ) | 87 | 117 | |||||||
Change in operating assets and liabilities: | |||||||||||
Accounts receivable | (1,187 | ) | 3,131 | 5,952 | |||||||
Due to Symmetry OEM Solutions | — | (1,946 | ) | 665 | |||||||
Other assets | 701 | (333 | ) | (36 | ) | ||||||
Inventories | 2,125 | (7,127 | ) | 2,321 | |||||||
Current income taxes | 57 | 143 | 8 | ||||||||
Accounts payable | 1,066 | (1,674 | ) | (316 | ) | ||||||
Accrued expenses and other | 383 | (240 | ) | 231 | |||||||
Net cash provided by (used in) operating activities | 13,551 | (570 | ) | 19,067 | |||||||
Investing activities | |||||||||||
Purchases of property and equipment | (171 | ) | (552 | ) | (1,477 | ) | |||||
Proceeds from the sale of property and equipment | 4 | 12 | 549 | ||||||||
Acquisitions | (4,260 | ) | — | — | |||||||
Net cash used in investing activities | (4,427 | ) | (540 | ) | (928 | ) | |||||
Financing activities | |||||||||||
Proceeds from Revolving Loan | 141 | 7,559 | — | ||||||||
Payments on Revolving Loan | (4,017 | ) | (3,683 | ) | — | ||||||
Debt issuance costs | — | (441 | ) | — | |||||||
Net transfers to Symmetry Medical Inc. | — | (142 | ) | (17,656 | ) | ||||||
Net cash (used in) provided by financing activities | (3,876 | ) | 3,293 | (17,656 | ) | ||||||
Effect of exchange rate changes on cash | (170 | ) | 163 | 3 | |||||||
Net increase in cash | 5,078 | 2,346 | 486 | ||||||||
Cash at beginning of period | 2,994 | 648 | 162 | ||||||||
Cash at end of period | $ | 8,072 | $ | 2,994 | $ | 648 | |||||
Supplemental disclosures: | |||||||||||
Cash paid for interest | $ | 119 | $ | 9 | $ | — | |||||
Cash paid for income taxes | $ | 139 | $ | — | $ | — | |||||
See accompanying notes to consolidated and combined financial statements.
SYMMETRY SURGICAL INC.
NOTES TO CONSOLIDATED AND COMBINED FINANCIAL STATEMENTS
(In Thousands, Except Per Share Data)
1. Description of Operations
Overview
Symmetry Surgical Inc. (the “Company”) is a global marketer and distributor of medical devices with some limited manufacturing focused on the surgery market. The Company offers over 20,000 products and sells primarily to hospitals and surgical centers in the U.S. and countries worldwide. The Company's current product portfolio includes a broad range of reusable stainless steel and titanium, hand-held general and specialty surgical instruments, single use and disposable instruments, electro-surgery instruments, retractor systems, containers and sterilization devices, and ligation clips sold directly to hospitals and other sites of care. These products are typically used in the surgical specialties of neurosurgery, spine, general surgery- open and laparoscopy, microsurgery, OB/Gyn, ophthalmology, otolaryngology / ENT, plastic / reconstructive, peripheral vascular, arthroscopy, orthopedic, pediatrics, cardiovascular, thoracic, and urology in a hospital setting as well as surgery centers and in select physician offices. These products are viewed in two categories by management; Symmetry Surgical Branded Products and Alliance Partners Products. Symmetry Surgical Branded Products include all products sold under brand names managed by the Company whereas Alliance Partners Products are brands the Company distributes for others in defined markets.
On August 28, 2015, the Company acquired the assets of Vesocclude Medical, LLC ("Vesocclude Medical" or "Vesocclude") for $4,055 in cash, gross of the Company's 4.8% existing minority interest in Vesocclude, and up to an additional $2,200 in contingent consideration based on the achievement of milestones discussed in Note 4. Vesocclude's portfolio of titanium ligation clips and appliers are used in surgical procedures to close tubal anatomic structures such as blood vessels or ducts. Vesocclude products are primarily sold in the U.S. direct to hospitals but in some territories are sold through stocking distributors and kit packers. Internationally, Vesocclude products are sold through stocking distributors.
On December 1, 2015, the Company acquired the patent protected, ultra-low profile, soft-tissue retraction technology and related product portfolio and other assets from privately held Insightra® Medical for $400 in cash as well as a royalty on future sales with a fair value of $107 as of the date of acquisition. The portfolio, formerly known as ReeTrakt, will be re-launched under the Symmetry Surgical brand as the Symmetry Access™ Low Profile Retractor beginning in the first half of 2016. The Symmetry Access™ Low Profile Retractor is a single-use product with an ultra-low profile that supports minimal/small incision surgical procedures by providing better surgical access and field of view. Symmetry Surgical plans to launch this product through our existing sales channel in the U.S and internationally.
Separation from Symmetry Medical Inc.
In August 2014, Symmetry Medical Inc. (“SMI”) announced that it had entered into an Agreement and Plan of Merger, dated as of August 4, 2014 (the “Merger Agreement”), by and among SMI, TecoStar Holdings, Inc., Tecomet, Inc., and TecoSym, Inc. (“Merger Sub”), pursuant to which Merger Sub was merged with and into SMI, with SMI continuing as the surviving corporation (the “Merger”). As a result of the Merger, all of the outstanding shares of common stock of Symmetry Surgical Inc. were spun-off to SMI’s stockholders and the holder of each outstanding share of common stock of SMI received one-quarter of a share of common stock of Symmetry Surgical. This is referred to as the “Spin-Off.”
In connection with the Spin-Off, SMI entered into a Separation Agreement, dated as of August 4, 2014 (the “Separation Agreement”) with the Company. Pursuant to the Separation Agreement, SMI transferred all of the assets and liabilities of its Symmetry Surgical Business to the Company prior to the consummation of the Merger and the Spin-Off. The Separation Agreement provides for various agreements and arrangements governing the Company's relationship with SMI after the Spin-Off.
The Company includes the consolidated operations of Specialty Surgical Instrumentation, Inc. (d/b/a Symmetry Surgical Inc.), Olsen Medical, LLC, Symmetry Surgical Vesocclude, LLC, Symmetry Surgical Netherlands, CV, Symmetry Surgical Netherlands, BV, Symmetry Surgical, GmbH and Symmetry Surgical Switzerland, GmbH.
2. Basis for Historical Presentation
The consolidated and combined financial statements prior to Separation on December 5, 2014 include the allocation of certain assets and liabilities that were historically held at the SMI corporate level but which are specifically identifiable or allocable to Symmetry Surgical. Cash and cash equivalents held by SMI was not allocated to Symmetry Surgical as they were not expected to be transferred to Symmetry Surgical. Long-term debt and short-term borrowings were not allocated to Symmetry Surgical as none of the debt recorded by SMI was directly attributable to or guaranteed by Symmetry
Surgical. All historical intracompany transactions and accounts have been eliminated. All historical intercompany transactions between Symmetry Surgical and the OEM Solutions Business have been reflected as related party transactions. All intercompany transactions between Symmetry Surgical and SMI corporate are considered to be effectively settled in the consolidated and combined financial statements of cash flows as financing activity.
The historical financial statements of Symmetry Surgical prior to Separation do not necessarily include all of the expenses that would have been incurred had Symmetry Surgical been a separate, stand-alone entity and may not necessarily reflect Symmetry Surgical’s results of operations, financial position and cash flows had Symmetry Surgical been a stand-alone company during the periods presented. These historical financial statements include an allocation of expenses related to certain general and administrative, selling and marketing expenses from SMI to Symmetry Surgical. These expenses have been allocated to Symmetry Surgical first on the basis of direct usage when identifiable, with the remainder allocated on the basis of their respective revenues. Symmetry Surgical considers the expense allocation methodology and results to be reasonable for all pre-separation periods presented. However, the allocation may not be indicative of the actual expenses that would have been incurred had Symmetry Surgical operated as an independent, publicly-traded company for these periods.
3. Summary of Significant Accounting Policies
Principles of Consolidation. The consolidated and combined financial statements include the accounts of the Company and its wholly-owned subsidiaries. All significant intercompany accounts and transactions have been eliminated in consolidation.
Year End. The Company’s fiscal year is the 52 or 53 week period ending on the Saturday closest to December 31. Fiscal year 2015 was a 52 week year (ending January 2, 2016), fiscal year 2014 was a 53 week year (ending January 3, 2015), and fiscal year 2013 was a 52 week year (ending December 28, 2013). References in these consolidated and combined financial statements to 2015, 2014 and 2013 refer to these financial periods, respectively.
Use of Estimates. Preparation of these consolidated and combined financial statements requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results could differ from those estimates, but management does not believe such differences will materially affect the Company's financial position or results of operations.
Business Combinations. The Company records its business combinations under the acquisition method of accounting. Under the acquisition method of accounting, the Company allocates the purchase price of each acquisition to the tangible and identifiable intangible assets acquired and liabilities assumed based on their respective fair values at the date of acquisition. The fair value of identifiable intangible assets is based upon detailed valuations that use various assumptions made by management. Any excess of the purchase price over the fair value of the net tangible and intangible assets acquired is allocated to goodwill.
Revenue Recognition. The Company recognizes revenue on orders received from its customers when there is persuasive evidence of an arrangement with the customer that is supportive of revenue recognition, the customer has made a fixed commitment to purchase the product for a fixed or determinable price, collection is reasonably assured under the Company’s normal billing and credit terms and ownership and all risks of loss have been transferred to the buyer which is normally upon shipment or delivery to the customer. In certain circumstances, customer terms require receipt of product prior to the transfer of the risk of ownership. In such circumstances, revenue is not recognized upon shipment, but rather upon confirmation of delivery. For product sales to distributors, the Company recognizes revenue upon shipment to the distributor under standard contract terms stating that title to the goods passes to the distributors at point of shipment to the distributor’s location. All shipments to distributors are at contract prices and payment is not contingent upon resale of the product. The Company offers certain customers volume rebates, which are reflected as a reduction in revenue when it is probable the rebate will be earned based on current and forecasted sales and the amount can be estimated. Product returns are estimated based upon historical return rates and are reflected as a reduction of revenue in the same period revenue is recognized.
Cash. Cash and cash equivalents include all highly liquid investments with a maturity of three months or less at the time of purchase.
Allowance for Doubtful Accounts. The Company performs periodic credit evaluations of customers' financial condition and generally does not require collateral. Receivables are generally due within 30 to 90 days. The Company maintains an allowance for doubtful accounts for estimated losses in the collection of accounts receivable. The Company makes estimates
regarding the future ability of its customers to make required payments based on historical credit experience and expected future trends. Provisions to the allowance for doubtful accounts are charged to current selling and marketing expenses. Actual losses are charged against this allowance when incurred. The activity in the allowance for doubtful accounts was as follows:
Fiscal Year Ended | |||||||||||
January 2, 2016 | January 3, 2015 | December 28, 2013 | |||||||||
Balance as of beginning of period | $ | 306 | $ | 225 | $ | 483 | |||||
Provision (recoveries) | 37 | 481 | (69 | ) | |||||||
Write-offs | (139 | ) | (400 | ) | (189 | ) | |||||
Balance as of end of period | $ | 204 | $ | 306 | $ | 225 | |||||
Concentration of Credit Risk. On an on-going basis, the Company has no financial instruments that represent a concentration of credit risk.
Inventories. Inventories are stated at the lower of cost, determined primarily on the first-in, first-out (FIFO) method, or market (net realizable value). Costs include material, labor and overhead costs, as applicable. Inventory balances are reviewed quarterly for excess products or obsolete inventory levels and written down, if necessary, to net realizable value.
Property and Equipment. Property and equipment are stated on the basis of cost. Depreciation is calculated on the straight-line method over the estimated useful lives of the respective assets. Accelerated methods are used for income tax purposes. Repair and maintenance costs are charged to expense as incurred. Upon retirement or sale of an asset, its cost and related accumulated depreciation are removed from the consolidated balance sheet and any gain or loss is recorded in operating income or expense.
Definite-Lived Long-Lived Assets. The Company assesses the impairment of definite-lived long-lived assets when circumstances indicate the carrying value of an asset may not be recoverable based on the undiscounted future cash flows of an asset. If the carrying amount of the asset is determined not to be recoverable, a write-down to fair value is recorded. Fair values are determined based on quoted market values, undiscounted cash flows, or external appraisals, as applicable. The Company reviews long-lived assets for impairment at the individual asset or the asset group level for which the lowest level of independent cash flows can be identified. Intangible assets subject to amortization consist of trademarks and technology and patents which are amortized using the straight-line method, as well as customer related intangible assets which are amortized on an accelerated method. Straight-line methods are used for income tax purposes. All of the Company’s intangible assets were acquired in connection with its various acquisitions. The Company is required to reassess the expected useful lives of existing definite-lived assets annually. Impairment charges were recorded in fiscal 2015 related to two patents and one trademark of $252 and $762, respectively. These assets were transitioned from Symmetry Medical during the Spin-Off and are no longer a key focus of the product portfolio given recent acquisitions and other areas of revenue growth. The Company reviewed all other definite-lived long-lived assets and did not record any impairment charges related to the remaining assets for fiscal 2015, 2014 or 2013.
The assessment of the recoverability of long-lived assets reflects management’s assumptions and estimates. Factors that management must estimate when performing impairment tests include sales volume, prices, inflation, discount rates, exchange rates, tax rates and capital spending. Significant management judgment is involved in estimating these factors, and they include inherent uncertainties. Measurement of the recoverability of these assets is dependent upon the accuracy of the assumptions used in making these estimates, as well as how the estimates compare to the eventual future operating performance of the specific reporting unit to which the assets are attributed. Changes in these estimates could change the conclusion regarding the impairment of other intangible assets or goodwill (as described below) and potentially result in a non-cash impairment in the future period.
During fiscal 2013, all indefinite life intangible assets were evaluated and determined to be definite-lived assets and therefore assigned an appropriate useful life. In connection with the annual impairment test during fiscal 2013, the Company recognized an impairment charge related to certain trademarks and in-process research and development of $1,245 and $610, respectively.
Goodwill. Goodwill recorded by the Company represents the purchase price in excess of the net assets of businesses acquired. Goodwill is not amortized but is tested for impairment annually on the first day of the fourth fiscal quarter and more frequently if circumstances warrant using a two-step process. The first step is a screen for potential impairment, while the second step measures the amount of impairment. Potential impairment is determined by comparing estimated fair value to the net book value of the reporting unit. Fair value is calculated using an income approach based on the present value of estimated future cash flows. The Company completed its impairment test and no impairment of goodwill existed in fiscal 2015. The Company recorded goodwill impairment charges in fiscal 2014 fiscal and 2013 totaling $55,817 and $18,250, respectively.
Income Taxes. Income taxes prior to the Spin-Off are presented on a separate return basis. Prior to the Spin-Off, the Company was included in the consolidated tax return of SMI. The consolidated and combined financial statements of the Company have been prepared using the assets and liability method in accounting for income taxes, which requires the recognition of deferred income taxes for the expected future tax consequences of net operating losses, credits, and temporary differences between the financial statement carrying amounts and the tax basis of assets and liabilities. Differences between the anticipated and actual outcomes of these future tax consequences could have a material impact on the consolidated results of operations or financial position of the Company. As a stand-alone entity, the Company files tax returns on its own behalf and its deferred taxes and effective tax rate may differ from those used in the historical periods. The Company did not maintain an income taxes payable to/from account with SMI. With the exception of certain entities outside the U.S. that transferred to the Company at separation, the Company is deemed to settle current tax balances with the SMI tax paying entities in the respective jurisdictions. These settlements are reflected as changes in net parent investment or net transfers to Symmetry Medical Inc. Additionally, tax planning strategies are utilized as part of the global tax compliance program of the Company. Judgments and interpretation of statutes are inherent in this process. The Company provides related reserves, where applicable, in accounting for uncertain tax positions. Interest and penalties associated with reserves for uncertain tax positions are classified in income tax expense in the statements of operations.
During fiscal 2015, the Company identified an immaterial error in which deferred tax assets and additional paid-in capital were understated by $352 at the separation date from SMI in December 2014. The Company corrected the error in the accompanying January 3, 2015 consolidated balance sheet and consolidated and combined statements of equity.
Pensions. The Company engages outside actuaries to assist in the determination of the obligation and cost under the plan which are direct obligations of the Company. The valuation of the funded status and the net period benefit cost for this plan is calculated using actuarial assumptions. The significant assumptions, which are reviewed annually, include the discount rate, compensation increases, turnover rates and health care cost trend rates. The significant assumptions used in determining these calculations are disclosed in Note 8 to the consolidated and combined financial statements. Actuarial losses and gains are amortized over the remaining service attribution periods of the employees under the corridor method, in accordance with the rules for accounting for post-employment benefits.
Foreign Currency Translation. The financial statements of the Company's foreign subsidiaries are accounted for in local currency and have been translated into U.S. dollars in accordance with accounting guidance on foreign currency translation. Assets and liabilities have been translated using the exchange rate in effect at the balance sheet date. Revenues and expenses have been translated using a weighted-average exchange rate for the period. Currency translation adjustments have been recorded as a separate component of equity.
Foreign currency transaction gains and losses resulting from a subsidiary's foreign currency denominated assets and liabilities included in other income were losses of $309, $195 and $49 in fiscal 2015, 2014 and 2013, respectively.
Shipping and Handling Costs. The Company reflects freight costs associated with shipping its products to customers as a component of cost of revenue.
Advertising Costs. Advertising costs are expensed as incurred and were $552, $444 and $728 in fiscal 2015, 2014 and 2013, respectively.
Research and Development Costs. The Company recognizes costs associated with research and development (R&D) within general and administrative expenses in the consolidated and combined statements of operations. R&D costs were $1,174, $721 and $864 in fiscal 2015, 2014 and 2013, respectively.
Derivative Financial Instruments. The Company recognizes all derivative instruments in its consolidated and combined financial statements at fair value. Changes in the fair value of derivatives are recorded each period in the derivative valuation loss line item of the consolidated and combined statements of operations unless the derivative qualifies for hedge accounting in which case the realized changes in fair value are reflected in the same financial statement line item of the item being hedged or the effective portion of changes in fair value of hedges is recorded each period in accumulated other comprehensive income (loss), net of tax, until the related hedge transaction occurs. Any ineffective portion of changes in fair value of the hedges is recorded in the derivative valuation loss line item of the statement of operations.
Stock-based compensation. The Company measures stock-based compensation cost at the grant date based on the estimated fair value of the award. Compensation cost for service-based awards is recognized ratably over the applicable service period. Compensation cost for performance-based awards is reassessed each period and recognized based upon the probability that the performance targets will be achieved. For restricted stock subject to service conditions or with performance targets, the fair market value of the award is determined based upon the closing value of the Company’s stock price on the grant date and the amount of stock-based compensation expense recognized during a period is based on the portion of the awards that are ultimately expected to vest. The Company estimates forfeitures at the time of grant by analyzing historical data and revises those estimates in subsequent periods if actual forfeitures differ from those estimates. For restricted stock with service conditions or with performance targets, the total expense recognized for each grant is only for those awards that ultimately vest.
Product Warranty. The Company offers a limited warranty for its products with a coverage period that ranges between 1 year to lifetime for materials and workmanship. Warranty coverage varies depending on the product family. The Company’s policy is to accrue the estimated cost of warranty coverage at the time of the sale. The following table presents the change in the product warranty accrual included in other accrued expenses.
Fiscal Year Ended | |||||||||||
January 2, 2016 | January 3, 2015 | December 28, 2013 | |||||||||
Balance as of beginning of period | $ | 321 | $ | 555 | $ | 235 | |||||
Provision for warranties issued in current year | 157 | 201 | 105 | ||||||||
Provision for (recover of) pre-existing warranty, net | — | (157 | ) | 325 | |||||||
Payments | (129 | ) | (278 | ) | (110 | ) | |||||
Balance as of end of period | $ | 349 | $ | 321 | $ | 555 | |||||
Recently Issued and Adopted Accounting Pronouncements
On April 5, 2012, the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, was enacted. Section 107 of the JOBS Act provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act of 1933, as amended, or the Securities Act, for complying with new or revised accounting standards. In other words, an “emerging growth company” may delay the adoption of certain accounting standards until those standards would otherwise apply to private companies and the Company has chosen to take advantage of this extended transition period.
Revenue from Contracts with Customers: In May 2014, the FASB issued ASU No. 2014-09, Revenue from Contracts with Customers (Topic 606), which supersedes the revenue recognition requirements in Accounting Standards Codification (“ASC”) 605, Revenue Recognition. This ASU is based on the principle that revenue is recognized to depict the transfer of goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods and services. The ASU also requires additional disclosure about the nature, amount, timing and uncertainty of revenue and cash flows arising from customer contracts, including significant judgments and changes in judgments and assets recognized from costs incurred to obtain or fulfill a contract. ASU 2014-09 was originally effective for fiscal periods (including interim periods) beginning after December 15, 2016. In August 2015, the FASB issued ASU 2015-14, Revenue from Contracts with Customers (Topic 606): Deferral of the Effective Date, which defers the effective date by one year, with early adoption permitted as of the original effective date. ASU 2014-09 allows for either a full retrospective or a modified retrospective transition method. The Company is currently assessing the potential impact of the adoption of ASU 2014-09 on its financial statements and related disclosures.
Reporting Discontinued Operations and Disclosures of Disposals of Components of an Entity: In April 2014, the FASB issued ASU 2014-08, Presentation of Financial Statements (Topic 205) and Property, Plant and Equipment (Topic 360), Reporting Discontinued Operations and Disclosures of Disposals of Components of an Entity. This update modifies the requirements for reporting discontinued operations. Under the amendments in ASU 2014-08, the definition of discontinued operation has been modified to only include those disposals of an entity that represent a strategic shift that has (or will have) a major effect on an entity’s operations and financial results. This update also expands the disclosure requirements for disposals that meet the definition of a discontinued operation and requires entities to disclose information about disposals of individually significant components that do not meet the definition of discontinued operations. This update is effective for annual and interim periods beginning after December 15, 2014. The adoption of this ASU in fiscal 2015 by the Company did not have an impact on its financial position, results of operations or cash flows.
Accounting for Share-Based Payments When the Terms of an Award Provide That a Performance Target Could Be Achieved after the Requisite Service Period. In June, 2014, the FASB issued ASU 2014-12, Accounting for Share-Based Payments When the Terms of an Award Provide That a Performance Target Could Be Achieved after the Requisite Service Period. This new guidance states that a performance target that affects vesting of a share-based payment and that could be achieved after the requisite service period is a performance condition under ASC 718. This update is effective for annual and interim periods beginning after December 15, 2015. The Company does not believe this ASU will have an impact on the Company's financial statements.
Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern. In August, 2014, the FASB issued ASU 2014-15, Disclosure of Uncertainties about an Entity's Ability to Continue as a Going Concern. This new guidance requires management to evaluate whether there is substantial doubt about the entity’s ability to continue as a going concern and, if so, disclose that fact. Management will also be required to evaluate and disclose whether its plans alleviate that doubt. The standard is effective for annual periods ending after December 15, 2016 with early adoption permitted. The Company does not believe this ASU will have an impact on the Company’s financial statements.
Business Combinations: Simplifying the Accounting for Measurement-Period Adjustments. In September 2015, the FASB issued ASU No. 2015-16, Business Combinations (Topic 805): Simplifying the Accounting for Measurement-Period Adjustments. This new guidance requires that an acquirer recognize adjustments to provisional amounts that are identified during the measurement period in the reporting period in which the adjustment amounts are determined. The amendments of this standard are effective prospectively for the fiscal years, including interim periods, beginning after December 15, 2015, and early adoption is permitted. The Company adopted this ASU on January 3, 2016.
Income Taxes (Topic 740) Related to the Balance Sheet Classification of Deferred Taxes. In November 2014 the FASB issued ASU 2015-17, Income Taxes (Topic 740) Related to the Balance Sheet Classification of Deferred Taxes which will require entities to present deferred tax assets (DTAs) and deferred tax liabilities (DTLs) as noncurrent in a classified balance sheet. The ASU simplifies the current guidance (ASC 740-10-45-4), which requires entities to separately present DTAs and DTLs as current and noncurrent in a classified balance sheet. The ASU is effective for annual reporting periods beginning on or after December 15, 2016, and interim periods within those annual periods. The Board decided to allow all entities to early adopt the ASU for financial statements that had not been issued. Therefore, the Company adopted this ASU at January 2, 2016, and has reclassified all prior periods to be consistent with the requirements outlined in the ASU. The impact of the prior period reclassification was a $2,816 reduction of current assets, a $15 reduction of current liabilities and a net working capital decrease of $2,801 at January 3, 2015.
Technical Corrections and Improvements. In June 2015, the FASB issued ASU No. 2015-10, Technical Corrections and Improvements to clarify that, for nonrecurring measurements estimated at a date during the reporting period other than the end of the reporting period, an entity should clearly indicate that the fair value information presented is not as of the period's end as well as the date or period that the measurement was taken. The Company adopted ASU 2015-10, effective June 12, 2015, as the change was effective upon issuance. The adoption did not have an impact on the Company's consolidated financial statements.
4. Acquisitions
On August 28, 2015, the Company acquired the assets of Vesocclude for $4,055 in cash and up to an additional $2,200 in contingent consideration; each of which are gross of the Company's 4.8% existing minority interest in Vesocclude. The contingent consideration involves two contingent purchase payments. Contingent purchase liability No. 1 is based on achieving certain milestones related to new product introduction. Contingent purchase liability No. 2 is based on reaching specified new product commercial sales levels. The settlement of these contingent purchase liabilities can be made in cash or
the Company's common stock or any combination thereof at the Company's option. Vesocclude's portfolio of titanium ligation clips and appliers are used in surgical procedures to close tubal anatomic structures such as blood vessels or ducts. Vesocclude products are primarily sold in the U.S. direct to hospitals but in some territories are sold through stocking distributors and kit packers. Internationally, Vesocclude products are sold through stocking distributors. The Company believes the Vesocclude acquisition further expands its Symmetry Surgical branded product offerings and augments the geographic coverage of its sales force as well as allows Symmetry Surgical to extend its brand through the legacy Vesocclude sales channels both in the US and internationally.
Prior to the acquisition date, the Company accounted for its 4.8% minority interest as an equity-method investment. Pursuant to current accounting standards, the Company recorded 100% of the assets and liabilities acquired at their fair values, and recognized a $67 non-cash gain representing the difference between the fair value (based on the purchase price less a control premium) and the equity method carrying value of the investment at the acquisition date. The gain is included in the line item "Other income" in the consolidated statements of operations.
The following table summarizes the fair value of the total consideration which has been preliminarily allocated to the fair value of the assets acquired and liabilities assumed based on the Company's internal operational assessments and other analyses which are Level 3 measurements. The preliminary purchase price allocation has been completed based on all information currently available.
Net cash consideration | $ | 3,860 | ||
Contingent purchase liability No. 1, current | 1,034 | |||
Contingent purchase liability No. 2, non-current | 1,013 | |||
Pre-existing ownership interest | 186 | |||
Gain on pre-existing ownership interest | 67 | |||
Fair value of total consideration | $ | 6,160 | ||
Inventory | $ | 962 | ||
Property and equipment | 252 | |||
Acquired customers | 1,010 | |||
Trademarks | 280 | |||
Acquired technology and patents | 800 | |||
Other assets, non-current | 637 | |||
Non-compete | 25 | |||
Net identifiable assets acquired | $ | 3,966 | ||
Goodwill | $ | 2,194 | ||
Cash paid for acquisition | $ | 4,055 | ||
Cash received from seller related to previous ownership interest | 195 | |||
Net cash transferred to seller upon closing | $ | 3,860 | ||
In accordance with accounting guidance, the Company estimated the fair value of the contingent consideration as of the acquisition date and included such contingent purchase liabilities as a component of total purchase price as noted above. The potential undiscounted amount of each future payment that the Company could be required to make under the agreement is between $0 and $2,200 in the aggregate. The fair value of the contingent consideration arrangement of $2,047 was estimated by applying a market approach. That measure is based on significant inputs that are not observable in the market, also referred to as Level 3 inputs. Key assumptions include a discount rate of 18.61% and an estimated level of probability. Changes to the fair value of the contingent consideration, subsequent to the initial purchase date, have been reflected in the statement of operations, which totaled $26 in fiscal 2015.
The purchase price of Vesocclude exceeded the fair value of net identifiable tangible and intangible assets. Goodwill recorded is deductible for U.S. Federal income taxes. The purchase was accounted for as a business combination and the results of Vesocclude have been included in the Company's consolidated statements of operations since the date of acquisition. The pro forma impact of the acquisition on the Company's prior period consolidated statements of operations was not material.
The acquisition includes a 20% minority interest in a medical device company and an option to purchase the remaining ownership interest. The Company has accounted for its 20% ownership interest as an equity method investment.
On December 1, 2015, the Company acquired the patent protected, ultra-low profile, soft-tissue retraction technology and related product portfolio and other assets from privately held Insightra® Medical for $400 in cash as well as a royalty on future sales. This royalty has been deemed a contingent purchase liability with a fair value of $107 as of the date of acquisition. The portfolio, formerly known as ReeTrakt, will be re-launched under the Symmetry Surgical brand as the Symmetry Access™ Low Profile Retractor beginning in the first half of 2016. The Symmetry Access™ Low Profile Retractor is a single-use product with an ultra-low profile that supports minimal/small incision surgical procedures by providing better surgical access and field of view. The product is designed to minimize tissue damage by providing gentle and adjustable tissue retraction without a bridge, arms or handles that reduce access to the surgical site. The portfolio offers a variety of tissue retraction tips and adheres securely to the surgical drape or skin, eliminating the need for hand-held retraction during a case. The Company believes this acquisition further expands its Symmetry Surgical branded product offerings. The Company plans to launch this product as a Symmetry Surgical branded product offering through its existing sales channel in the U.S and internationally.
The following table summarizes the fair value of the total consideration which has been preliminarily allocated to the fair value of the assets acquired and liabilities assumed based on the Company's internal operational assessments and other analyses which are Level 3 measurements. The preliminary purchase price allocation has been completed based on all information currently available.
Cash consideration | $ | 400 | ||
Contingent purchase liability No. 3, non-current | 107 | |||
Fair value of total consideration | $ | 507 | ||
Inventory | $ | 102 | ||
Property and equipment | 30 | |||
Acquired technology and patents | 341 | |||
Non-compete | 5 | |||
Net identifiable assets acquired | $ | 478 | ||
Goodwill | $ | 29 | ||
The purchase price of Insightra Medical exceeded the fair value of net identifiable tangible and intangible assets. Goodwill recorded is deductible for U.S. Federal income taxes. The purchase was accounted for as a business combination and the results of this acquisition did not have any significant impact to the 2015 consolidated statements of operations since its date of acquisition. The pro forma impact of the acquisition on the Company's prior period consolidated statements of operations was not material.
5. Inventories
Inventories consist of the following:
January 2, 2016 | January 3, 2015 | ||||||
Raw materials | $ | 1,032 | $ | 880 | |||
Work-in-process | 141 | 530 | |||||
Finished goods | 21,903 | 22,731 | |||||
$ | 23,076 | $ | 24,141 | ||||
6. Property and Equipment
Property and equipment, including depreciable lives, consists of the following:
January 2, 2016 | January 3, 2015 | ||||||
Buildings and improvements (20 to 40 years) | $ | 560 | $ | 575 | |||
Machinery and equipment (5 to 15 years) | 1,816 | 1,499 | |||||
Office equipment (3 to 5 years) | 3,686 | 4,039 | |||||
Construction-in-progress | 10 | 43 | |||||
6,072 | 6,156 | ||||||
Less accumulated depreciation | (3,814 | ) | (3,388 | ) | |||
$ | 2,258 | $ | 2,768 | ||||
7. Goodwill and Other Intangible Assets
The Company has one operating segment that represents the lowest level for which discrete financial information is available and the operating results are regularly reviewed by management.
Prior to performing the annual goodwill impairment test step 1 analysis, the Company tested long-lived assets to be held and used for impairment on an undiscounted cash flow basis using a risk-adjusted discount rate. See Note 3 for the results of this testing.
To derive the fair value of the reporting unit, as required in step one of the goodwill impairment test, the Company uses the income approach, specifically the discounted cash flow method, to determine the fair value of the reporting unit. This approach calculates fair value by estimating the after-tax cash flows attributable to the reporting unit and then discounting these after-tax cash flows to a present value using a risk-adjusted discount rate. Inputs used to fair value the Company's reporting unit are considered Level 3 inputs of the fair value hierarchy and include the following:
• | The Company's financial projections for its reporting unit are based on management's assessment of macroeconomic variables, industry trends and market opportunities, as well as the Company's strategic objectives and future growth plans. Revenue growth rates assumed in fiscal 2015 for the Company's reporting unit were approximately 0.0% - 5.7% for fiscal 2016 and beyond. Revenue growth rates assumed in fiscal 2014 and fiscal 2013 for the Company's reporting unit were approximately 3-5% and 3-6%, respectively, for fiscal 2015 and beyond. |
• | The discount rate used to measure the present value of the projected future cash flows is set using a weighted-average cost of capital method that considers market and industry data, as well as the Company's specific risk factors that are likely to be considered by a market participant. The weighted-average cost of capital is the Company's estimate of the overall after-tax rate of return required by equity and debt holders of a business enterprise. The discount rate used was approximately 12.0% in fiscal 2015, 15.0% in fiscal 2014 and 12.5% in fiscal 2013. |
For Level 3 measurements, significant increases or decreases in long-term growth rates or discount rates in isolation or in combination could result in a significantly lower or higher fair value measurement.
In the second step, the Company assigned the reporting unit's fair value to all of its assets and liabilities, including any unrecognized intangible assets, in a hypothetical analysis that calculates the implied fair value of goodwill in the same manner as if the reporting unit were being acquired in a business combination. If the implied fair value of the reporting unit's goodwill is less than the carrying value, the difference is recorded as an impairment charge. This allocation process was performed only for the purposes of measuring the goodwill impairment and not to adjust the carrying values of the recognized assets and liabilities.
During fiscal 2015, we determined that no impairment of goodwill existed. During fiscal 2015, we did impair certain definite-lived intangible assets aggregating $1,014 transitioned from Symmetry Medical during the Spin-Off that are no longer a key focus of the product portfolio given recent acquisitions and other areas of revenue growth. Accordingly, the fair value of these assets was determined to be negligible and was written off in full. During fiscal 2013, we impaired certain definite-lived intangible assets totaling $1,855. There were no definite-lived intangible impairments in fiscal 2014.
During fiscal 2014 and fiscal 2013, the Company determined that the expected operating results for the Symmetry Surgical reporting unit were projected to be substantially lower than previous forecasts. Given this information, the Company conducted its interim and annual impairment tests, as applicable, and determined that impairment existed. The Company recorded pre-tax
non-cash charges of $55,817 in fiscal 2014 for goodwill and $20,105 in total for fiscal 2013, $18,250 for goodwill and $1,855 of other assets (as described above) and these charges have been recorded in the Company's consolidated and combined statements of operations within the asset impairment line item. The fiscal 2014 impairment was primarily driven by the market valuation of the Company combined with lower revenue due to a sluggish hospital spending environment in the U.S. and previously disclosed integration challenges related to the 2011 acquisition of the surgical instruments portfolio of Codman and Shurtleff, Inc., from which the Company has not recovered as quickly as previously expected and reduced cost savings estimates for the post Spin-off operations of the Company which have also been previously disclosed.
As of January 2, 2016, the balances of intangible assets, other than goodwill, were as follows:
Weighted-Average Amortization Period | Gross Intangible Assets | Accumulated Amortization | Net Intangible Assets | ||||||||||
Acquired customers | 19 years | $ | 92,355 | $ | (22,961 | ) | $ | 69,394 | |||||
Trademarks | 13 years | 4,014 | (866 | ) | 3,148 | ||||||||
Acquired technology, patents and other | 13 years | 1,521 | (344 | ) | 1,177 | ||||||||
Intangible assets subject to amortization | 19 years | $ | 97,890 | $ | (24,171 | ) | $ | 73,719 | |||||
As of January 3, 2015, the balances of intangible assets, other than goodwill, were as follows:
Weighted-Average Amortization Period | Gross Intangible Assets | Accumulated Amortization | Net Intangible Assets | ||||||||||
Acquired customers | 19 years | $ | 91,438 | $ | (17,986 | ) | $ | 73,452 | |||||
Trademarks | 10 years | 4,815 | (730 | ) | 4,085 | ||||||||
Acquired technology and patents | 13 years | 1,220 | (854 | ) | 366 | ||||||||
Intangible assets subject to amortization | 19 years | $ | 97,473 | $ | (19,570 | ) | $ | 77,903 | |||||
Intangible asset amortization expense for fiscal 2015, 2014 and 2013 was $5,562, $5,375 and $5,027, respectively, and is included in general and administrative expenses in the consolidated and combined statements of operations. At January 2, 2016, the annual amortization expense for intangible assets recorded as of January 2, 2016 is anticipated to be as follows for each of the next 5 fiscal years:
2016 | $ | 5,400 | |
2017 | 5,427 | ||
2018 | 5,378 | ||
2019 | 5,321 | ||
2020 | 5,262 | ||
The reconciliation of the beginning and ending carrying amounts of goodwill are as follows:
Balance as of December 28, 2013 | $ | 62,995 | ||
Impairment of goodwill | (55,817 | ) | ||
Effects of foreign currency | (52 | ) | ||
Balance as of January 3, 2015 | $ | 7,126 | ||
Acquisitions | 2,223 | |||
Effects of foreign currency | (8 | ) | ||
Balance as of January 2, 2016 | $ | 9,341 | ||
Accumulated goodwill impairment losses as of January 2, 2016 and January 3, 2015 were $74,067.
8. Employee Benefit Plan
The Company sponsors one defined benefit pension plan for the benefit of its employees at its German subsidiary, which is a legacy plan of the Codman surgical instruments business acquired during the year ended December 31, 2011. The plan is unfunded and provides defined benefits based on the final average salary of the employees as defined in the plan. The components of net periodic pension cost for fiscal 2015 are as follows:
Fiscal Year Ended | |||||||||||||
2015 | 2014 | 2013 | |||||||||||
Components of Net Periodic Pension Cost: | |||||||||||||
Service cost | $ | 40 | $ | 46 | $ | 50 | |||||||
Interest cost | 20 | 26 | 22 | ||||||||||
Amortization of net actuarial loss | 19 | 9 | 16 | ||||||||||
Net periodic pension cost | $ | 79 | $ | 81 | $ | 88 | |||||||
Weighted average assumptions used to determine net periodic pension cost: | |||||||||||||
Discount rate | 2.40 | % | 3.60 | % | 3.00 | % | |||||||
Salary increases | 2.50 | % | 2.50 | % | 2.50 | % | |||||||
The change in the projected benefit obligation and the funded status of the plan and a reconciliation of such funded status to the amounts reported in the consolidated balance sheets as of January 2, 2016 and January 3, 2015 is as follows:
Fiscal Year Ended | |||||||||||||
2015 | 2014 | 2013 | |||||||||||
Change in projected benefit obligation | |||||||||||||
Benefit obligation, beginning of year | $ | 900 | $ | 813 | $ | 793 | |||||||
Service cost | 40 | 46 | 50 | ||||||||||
Interest cost | 20 | 26 | 22 | ||||||||||
Actuarial (gain) loss | (85 | ) | 106 | (117 | ) | ||||||||
Foreign currency exchange rate changes | (71 | ) | (91 | ) | 65 | ||||||||
Benefit obligation, end of year | $ | 804 | $ | 900 | $ | 813 | |||||||
Change in fair value of assets: | |||||||||||||
Fair value of plan assets, beginning of year | $ | — | $ | — | $ | — | |||||||
Fair value of plan assets, end of year | — | — | — | ||||||||||
Funded status at end of year - under funded | $ | (804 | ) | $ | (900 | ) | $ | (813 | ) | ||||
The following summarizes the Company's balance sheet related pension and other benefit plan accounts at January 2, 2016 as compared to accounts at January 3, 2015:
Fiscal Year Ended | |||||||||
2015 | 2014 | ||||||||
Non-current benefit liability | $ | 804 | $ | 900 | |||||
Accumulated other comprehensive loss, net of tax | $ | 154 | $ | 238 | |||||
No contributions were made during fiscal 2015 to the plan, nor are any contributions expected to be made to the plan in fiscal 2016, since the plan is unfunded. The accumulated benefit obligation for the plan was $660 and $732 at January 2, 2016 and January 3, 2015, respectively.
The amounts recognized in accumulated other comprehensive income as of January 2, 2016 and January 3, 2015 was as follows:
Fiscal Year Ended | |||||||||||||
2015 | 2014 | 2013 | |||||||||||
Net actuarial (gain) loss | $ | (85 | ) | $ | 106 | $ | (117 | ) | |||||
Less: Tax expense (benefit) | 1 | (65 | ) | 53 | |||||||||
Accumulated other comprehensive (income) loss impact | $ | (84 | ) | $ | 41 | $ | (64 | ) | |||||
Weighted average assumptions used to determine benefit obligation: | |||||||||||||
Discount rate | 2.49 | % | 2.40 | % | 3.60 | % | |||||||
Salary increases | 2.80 | % | 2.50 | % | 2.50 | % | |||||||
The net actuarial loss for the pension plan required to be amortized from accumulated other comprehensive income into net periodic pension cost in fiscal 2015 is expected to be $13.
There are no significant benefits under the plan which are expected to be paid from fiscal 2016 through fiscal 2020.
9. Fair Value of Financial Instruments
Accounting guidance establishes a three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value. These tiers include: Level 1, defined as observable inputs such as quoted prices in active markets; Level 2, defined as inputs other than quoted prices in active markets that are either directly or indirectly observable; and Level 3, defined as unobservable inputs for which little or no market data exists, therefore requiring an entity to develop its own assumptions.
As of January 2, 2016, the Company held contingent purchase liabilities required to be measured at fair value on a recurring basis. The Company's contingent purchase liabilities associated with the acquisitions of Vesocclude Medical and Insightra Medical are not observable. The fair value of these liabilities have unobservable inputs in which little or no market data exists, therefore requiring the Company to categorize these liabilities as Level 3 and to re-measure the liabilities using its own assumptions in accordance with ASC 820. The contingent liabilities will continue to be accounted for and measured at fair value until the contingencies are settled or expire.
The fair value and carrying value of the Company's liabilities measured at fair value on a recurring basis is as follows (None at January 3, 2015):
January 2, 2016 | |||||||||||||||
Fair Value Measurements | |||||||||||||||
Level 1 | Level 2 | Level 3 | Total | ||||||||||||
Liabilities | |||||||||||||||
Contingent purchase liability No. 1 | $ | — | $ | — | $ | 1,047 | $ | 1,047 | |||||||
Contingent purchase liability No. 2 | 1,029 | 1,029 | |||||||||||||
Contingent purchase liability No. 3 | — | — | 107 | 107 | |||||||||||
Total liabilities | $ | — | $ | — | $ | 2,183 | $ | 2,183 | |||||||
Certain nonfinancial assets and liabilities are measured at fair value on a nonrecurring basis and are subject to fair value adjustments in certain circumstances, such as when there is evidence of impairment. Assets and liabilities acquired in business combinations (see Note 4) are recorded at their fair value as of the date of acquisition.
The Company has performed annual and interim impairment tests for goodwill. The fair value of the reporting unit is determined using the income approach. The income approach focuses on the income-producing capability of an asset, measuring the current value of the asset by calculating the present value of its future economic benefits such as cash earnings, cost savings, tax structure and product offerings. Value indications are developed by discounting expected cash flows to their present value at a rate of return that incorporates the risk-free rate for the use of funds, the expected rate of inflation and risks associated with the reporting unit. These assets are classified within Level 3, in the event that the
Company was required to measure and record such assets at fair value within its consolidated and combined financial statements, as discussed in Note 7, Goodwill and Other Intangible Assets.
The Company periodically evaluates the carrying value of long-lived assets to be held and used, including definite-lived intangible assets and property and equipment, when events or circumstances warrant such a review. Fair value is determined primarily using anticipated cash flows assumed by a market participant, discounted at a rate commensurate with the risk involved and these assets would generally be classified within Level 3, in the event that the Company were required to measure and record such assets at fair value within its consolidated and combined financial statements.
The interest rates on the Company's borrowings under the revolving credit facility are adjusted regularly to reflect current market rates and thus carrying value approximates fair value for these borrowings as there is no active market for our Revolving Loan which is classified as Level 3.
10. Derivatives
The Company utilizes derivative instruments to minimize the volatility of cash flows and statement of operations impacts associated with the impact of fluctuations in foreign currency. The Company recognizes all derivative instruments as either assets or liabilities at fair value on the consolidated balance sheets. Derivative asset and liability amounts with the same counterparty are netted against each other for financial reporting purposes. The Company utilizes third party valuations to assist in the determination of the fair value of these derivatives. The Company considered its derivative instrument valuations to be Level 2 fair value measurements.
In June and July 2012, the Company entered into forward swap contracts to mitigate the impact of fluctuations in foreign currency on the statement of operations. As of January 2, 2016 and January 3, 2015, the Company had settled all of its outstanding forward swap contracts. These swap contracts were not designated as cash flow hedges and therefore the change in the fair value was immediately recorded in derivatives valuation (gain) loss in the consolidated and combined statements of operations for the period ended December 28, 2013.
11. Debt Arrangements
On December 5, 2014, the Company, Specialty Surgical Instrumentation, Inc. and Olsen Medical, LLC (collectively, the “Borrowers”) entered into a Credit Agreement (the “Credit Agreement”) with Capital One (formerly known as General Electric Capital Corporation "GECC"), which provides for a revolving loan of up to $20,000, subject to a borrowing base limitation (the "Revolving Loan"). The Credit Agreement, which is senior and secured, had $0 outstanding as of January 2, 2016 and an aggregate of $3,876 outstanding as of January 3, 2015. Any amounts outstanding under the Revolving Loan are due and payable on December 5, 2019. Amounts outstanding under the Credit Agreement bear interest at LIBOR plus 3.25%, or the Base Rate (which will generally be based on the prime rate as published by the Wall Street Journal from time to time) plus 2.25%. Borrowings under the Revolving Loan are classified as current as of January 3, 2015 due to the existence of cash dominion by Capital One. In February 2015, the Company amended the Credit Agreement to allow for a collateral cash account which is excluded from the cash dominion. This collateral cash account is included in current other assets in the consolidated balance sheets. In August 2015, the Company amended the Credit Agreement to allow for the acquisition of Vesocclude Medical.
The Company has deferred debt issuance costs related to the Revolving Loan of $346 and $434 as of January 2, 2016 and January 3, 2015, respectively, reflected in current other assets in the consolidated balance sheets.
The Credit Agreement is guaranteed by Symmetry Surgical International, Inc., Symmetry Surgical Vesocclude LLC, S.S.I.I. LP, LLC and Symmetry Medical SSI Real Estate LLC (collectively the “Guarantors”). The Revolving Loan is secured by a first-priority perfected security interest in substantially all of the tangible and intangible assets of the Borrowers and the Guarantors, including a pledge of 100% of the equity interests of each domestic subsidiary.
The Credit Agreement includes customary covenants for facilities of this type, including limitations on indebtedness, liens, investments and acquisitions, loans and advances, mergers and consolidations, sales of assets, and dividends, stock repurchases and other restricted payments. In addition, the Credit Agreement requires that Borrowers maintain a minimum fixed charge coverage ratio of 1.25 to 1.00 tested at the close of each fiscal quarter beginning March 31, 2015, based on the preceding four fiscal quarters, and maintain at all times minimum liquidity (the sum of cash, cash equivalent and available borrowing capacity under the borrowing base) of $1,500, tested monthly. As of January 2, 2016, the borrowing base limitation was $13,735 and fully available.
The Company was in compliance with all covenants as of January 2, 2016.
See Note 4 for contingent purchase liabilities related to the Vesocclude and Insightra acquisitions.
12. Accumulated Other Comprehensive Income
Accumulated other comprehensive income is comprised of gains (losses) resulting from currency translations of foreign entities and pension plan actuarial gains and losses, net of tax. Other comprehensive income consists of the following:
Foreign Currency Translation | Pension Plan Actuarial Loss, net of tax | Other Comprehensive Income (loss) | |||||||||
Balance at December 28, 2013 | $ | 36 | $ | (197 | ) | $ | (161 | ) | |||
Other comprehensive income (loss) | (305 | ) | (41 | ) | (346 | ) | |||||
Balance at January 3, 2015 | (269 | ) | (238 | ) | (507 | ) | |||||
Other comprehensive income (loss) | (109 | ) | 84 | (25 | ) | ||||||
Balance at January 2, 2016 | $ | (378 | ) | $ | (154 | ) | $ | (532 | ) | ||
13. Net Transfers to Symmetry Medical Inc.
Net transfers to Symmetry Medical Inc. in the consolidated and combined statements of cash flow represent changes in amounts owed to or due from SMI. Prior to the Spin-Off, these were presented within net parent investment on the combined balance sheet and represented SMI's initial investment in the Company, subsequent allocations of expenses, and advances and receipts of cash resulting from the operations of the U.S. business with SMI. The balance reflected the U.S. based Company’s participation in SMI’s U.S. centralized cash management program under which all of the U.S. Company’s cash receipts were remitted to SMI and all cash disbursements were funded by SMI. Other transactions that affected net parent investment included general and administrative expenses incurred by SMI and allocated to the Company. Subsequent to the Spin-Off, the Company no longer participates in cash management and funding arrangements with SMI.
14. Related Party Transactions
Related party transactions of the Company include the purchase and sale of surgical instruments and cases between SMI's OEM Solutions Business and the Company, centralized cash and debt management in the U.S. and the allocation of certain general and administrative, selling and marketing and research and development expenses from SMI to the Company, prior to separation.
Sales of surgical instruments and cases from the Company to SMI's OEM Solutions business were recorded at intercompany transfer prices and totaled $678 and $71 in fiscal 2014 and 2013, respectively.
Purchases of surgical instruments and cases from SMI's OEM Solutions business by the Company were recorded at intercompany transfer prices and totaled $6,714 and $5,381 in fiscal 2014 and 2013, respectively.
The U.S. based portion of the Company participated in SMI's U.S. centralized cash management program under which all of the U.S. Company’s cash receipts were remitted to SMI and all cash disbursements were funded by SMI. The cash receipts were not kept at specific accounts and instead commingled with cash from other SMI entities. As cash was disbursed and received by SMI prior to separation, it has been accounted for through net parent investment.
During fiscal 2014 and 2013, SMI provided various general and administrative and selling and marketing services to the Company including legal assistance, marketing services, human resources, financial reporting and analysis, information technology and insurance management, as well as research and development services. It was SMI's policy to charge these expenses first on the basis of direct usage when identifiable, with the remainder allocated among SMI’s subsidiaries on the basis of their respective revenue. These allocations totaled $5,996 and $6,832 for fiscal 2014 and 2013, respectively. These charges may not be indicative of the actual expense that would have been incurred had the Company operated as an independent, publicly-traded company for the periods presented.
15. Income Taxes
Income before income taxes consisted of:
Fiscal Year Ended | |||||||||||
2015 | 2014 | 2013 | |||||||||
Domestic | $ | 795 | $ | (57,545 | ) | $ | (19,016 | ) | |||
Foreign | 684 | 1,114 | 390 | ||||||||
$ | 1,479 | $ | (56,431 | ) | $ | (18,626 | ) | ||||
Significant components of the Company’s net deferred tax assets are as follows:
January 2, 2016 | January 3, 2015 | |||||||
Deferred tax asset | ||||||||
Compensation | $ | 708 | $ | 162 | ||||
Inventory | 2,320 | 2,317 | ||||||
Loss carryforwards | 3,555 | 2,555 | ||||||
Intangibles | 17,442 | 19,139 | ||||||
Other | 514 | 624 | ||||||
Total deferred tax asset | 24,539 | 24,797 | ||||||
Deferred tax liability | ||||||||
Property and equipment | (254 | ) | (401 | ) | ||||
Total deferred tax liabilities | (254 | ) | (401 | ) | ||||
Deferred tax assets, net | $ | 24,285 | $ | 24,396 | ||||
Significant components of the income tax provision are as follows:
Fiscal Year | |||||||||||
2015 | 2014 | 2013 | |||||||||
Current: | |||||||||||
Federal | $ | — | $ | — | $ | (1,435 | ) | ||||
State | 31 | 40 | 159 | ||||||||
Foreign | 176 | 148 | 50 | ||||||||
207 | 188 | (1,226 | ) | ||||||||
Deferred | 72 | (20,844 | ) | (5,215 | ) | ||||||
$ | 279 | $ | (20,656 | ) | $ | (6,441 | ) | ||||
The provision for income taxes differs from that computed at the federal statutory rate of 34% in 2015 and 35% in 2014 and 2013 as follows:
Fiscal Year | |||||||||||
2015 | 2014 | 2013 | |||||||||
Tax at Federal statutory rate | $ | 503 | $ | (19,747 | ) | $ | (6,519 | ) | |||
State income taxes | 81 | (1,618 | ) | (552 | ) | ||||||
Change in state deferred tax rate | (231 | ) | — | — | |||||||
Foreign income taxes | (95 | ) | (250 | ) | 103 | ||||||
Goodwill impairment | — | 871 | 310 | ||||||||
Other | 21 | 88 | 217 | ||||||||
$ | 279 | $ | (20,656 | ) | $ | (6,441 | ) | ||||
At January 2, 2016, the Company had a Federal net operating loss carryforward of approximately $8,432 and various multistate income tax net operating loss carryforwards of approximately $18,160 which have been recorded as a deferred tax asset of approximately $3,555. The net operating loss carryforwards begin to expire in 2032. No provision has been made for United States federal and state or foreign taxes that may result from future remittances of undistributed earnings of foreign subsidiaries because it is expected that such earnings will be reinvested in these foreign operations indefinitely. At January 2, 2016, we had an aggregate of $1,702 of unremitted earnings of foreign subsidiaries that have been or are intended to be permanently reinvested for continued use in foreign operations.
As of January 2, 2016, the Company is subject to unexpired statutes of limitations for U.S. federal income taxes for the years 2012-2014. The Company is also subject to unexpired statutes of limitations for various states, most significantly Tennessee, generally for the years 2012-2014.
The Company assesses, on a quarterly basis, the realizability of its deferred tax assets by evaluating all available evidence, both positive and negative, including: (1) the cumulative results of operations in recent years, (2) the nature of recent losses, (3) estimates of future taxable income and (4) the length of operating loss carryforward periods. After considering both the positive and negative evidence management determined that it was more-likely-than-not that it would realize the value of its deferred tax assets. As a result, the Company did not record a valuation allowance against its net deferred tax assets as of January 2, 2016. Realization of the deferred tax asset is dependent upon the Company achieving approximately $63,000 of income before income taxes prior to expiration.
16. Profit Sharing Plan
During fiscal 2015, the Company maintained a profit sharing plan, which qualifies for favorable tax treatment under Section 401(k) of the Internal Revenue Code. During fiscal 2014 and 2013, SMI maintained a profit sharing plan, which qualified for favorable tax treatment under Section 401(k) of the Internal Revenue Code. Contributions by both the Company and SMI were based upon both discretionary and matching nondiscretionary amounts. The matching amounts represent a 50% match of employees’ contributions, up to a maximum of $4 per participant per year. Expense recorded by the Company for the plans was $222, $260 and $265 for 2015, 2014 and 2013, respectively.
17. Stock-Based Compensation Plans
The Equity Incentive Plan. The Equity Incentive Plan ("the Plan") is designed to enable us to attract, retain and motivate our directors, officers, employees and others who perform services for the Company and its subsidiaries, and to further align their interests with those of the Company’s stockholders, by providing for or increasing their ownership interests in our Company. The Plan provides for the issuance of stock options, stock appreciation rights (“SARs”), restricted stock, deferred stock, dividend equivalents, other stock-based awards and performance awards. Performance awards are based on the achievement of one or more business or personal criteria or goals, as determined by the compensation committee and approved by the Board of Directors (and independent directors for awards made to executive officers and the Board of Directors). An aggregate of 1,250 shares of common stock are now reserved for issuance under the Plan, subject to certain adjustment reflecting changes in the Company's capitalization. The Company provides newly issued shares to satisfy stock option exercises and issuance of SARs, restricted stock, deferred stock, dividend equivalents, other stock-based awards and performance awards.
Restricted stock is a grant of shares of common stock that may not be sold or disposed of, and that may be forfeited in the event of certain terminations of employment, prior to the end of a restricted period set by the compensation committee. A participant granted restricted stock generally has all of the rights of a shareholder, unless the compensation committee determines otherwise.
During fiscal 2015, the Company awarded 234 shares of performance-based restricted stock to employees that vest three years from the date the performance targets are met. An additional 5 shares of performance-based restricted stock were granted to various employees that vest in one year if the performance targets are met. Additionally, an aggregate of 1 shares of non-performance based restricted stock were granted to several employees during fiscal 2015 that vest in one year. Awards that are subject to performance conditions are expensed based on the probability that these conditions will be achieved. The aggregate fair value of shares granted in fiscal 2015 was $2,183.
During fiscal 2014, the Company awarded 500 shares of performance-based restricted stock to employees that vest three years from the date the performance targets are met. An additional 84 shares of performance-based restricted stock were granted to various employees that vest in one year if the performance targets are met. Additionally, an aggregate of 27 shares of non-
performance-based restricted stock were granted to several employees during fiscal 2014 that have vesting schedules that vary by grant and range from one through three years. The Company also granted 115 shares of non-performance-based restricted stock to directors, the terms of which allow for pro-rata vesting upon a director's retirement during the year in which the shares were issued. As all the directors are eligible for retirement, the Company recognized the compensation expense associated with these awards during 2015. Awards that are subject to performance conditions are expensed based on the probability that these conditions will be achieved. The aggregate fair value of 2014 granted shares was $5,674.
In fiscal 2015, the Company recorded compensation expense related to restricted stock grants of $1,532. In fiscal 2014, compensation expense related to restricted stock grants was immaterial due to the awards only being outstanding for one day. The Company's policy is to recognize expense for awards subject to graded or cliff vesting using the straight-line attribution method. As of January 2, 2016, the Company had unearned compensation cost related to unvested restricted stock awards of $6,207 which will be expensed through 2018.
A summary of all restricted stock activity for the period indicated below is as follows:
Number of Shares | Weighted Average Grant Date Fair Value | |
Unvested at January 3, 2015 | 726 | $7.81 |
Vested | — | $— |
Granted | 240 | $9.09 |
Canceled | (18) | $7.81 |
Unvested at January 2, 2016 | 948 | $8.13 |
No shares have vested since the Company's Spin-Off from SMI through January 2, 2016.
18. Commitments and Contingencies
Operating Leases. The Company has various operating leases, primarily for leased facilities. Total rental expense for these operating leases amounted to $500, $551 and $571 in 2015, 2014 and 2013, respectively. At January 2, 2016, future minimum payments for operating leases with initial terms of one year or more are as follows: $527 in 2016; $494 in 2017; $154 in 2018; $15 in 2019; $5 in 2020 and nil thereafter.
Unconditional Purchase Obligations. The Company has a contract to purchase finished instruments through August 2019. Based on contractual pricing at January 2, 2016, the minimum purchase obligation totals $1,494. Purchases under the contract totaled approximately $672 for fiscal 2015. These purchases are not in excess of our forecasted requirements.
Legal & Environmental Matters. The Company is involved, from time to time, in various contractual, product liability, intellectual property and other claims and disputes incidental to its business. Currently, there is no environmental or other litigation pending or, to the knowledge of the Company, threatened, that the Company expects to have a material adverse effect on its financial condition, results of operations or liquidity. While litigation is subject to uncertainties and the outcome of litigated matters is not predictable with assurance, the Company currently believes that the disposition of all pending or, to the knowledge of the Company, threatened claims and disputes, individually or in the aggregate, should not have a material adverse effect on the Company’s consolidated financial position, results of operations or liquidity.
Under the terms of the separation agreement between the Company and SMI, the Company agreed to indemnify SMI and its related entities from and against any and all liabilities relating to, arising out of or resulting from: any failure by the Company to pay, perform or otherwise promptly discharge any of the liabilities the Company agreed to assume with the Spin-Off; any of the Company's liabilities defined in the Merger Agreement; and any breach of any of the Merger Agreement, Separation Agreement or IP Cross-License Agreement. Liabilities that result from these obligations could result in significant, and in some cases uninsured, financial obligations. The Company does not believe this will have a significant impact on its financial position, results of operations or cash flows.
In September 2013, Xodus Medical Inc., Alessio Pigazzi and Glenn Keilar (collectively “Xodus”) filed suit against Prime Medical, LLC (“Prime”), and Specialty Surgical Instrumentation, Inc. d/b/a Symmetry Surgical Inc. in the United States District Court for the Western District of Pennsylvania under cause number 2:13-cv-01372-AJS. In the lawsuit Xodus
alleged that Prime, a manufacturer of products Symmetry Surgical distributes, had infringed on two of its patents, U.S. Patent No. 8,511,314 and US Patent No. 8,464,720 (the “Xodus Patents”) and that Symmetry Surgical was liable to it for damages resulting from selling products that infringed on the Xodus Patents. Symmetry Surgical’s Distribution Agreement with Prime provides Symmetry Surgical with full indemnification for these claims, and Prime has paid for all costs of litigation thus far. On February 24, 2014 the U.S. Patent and Trademarks Office ("USPTO") found substantial questions regarding the patentability of the Xodus Patents and on July 10, 2014 the USPTO rejected both of the Xodus Patents. Xodus has appealed to the full Board of the USPTO, a process that should be concluded in fiscal year 2016. Should the USPTO reverse its prior two findings, and should the US District Court find that Prime’s products infringe on the Xodus Patents, then Symmetry Surgical may be found liable to Xodus for some percentage of its sales of the Prime products, to the extent that Prime is unable to satisfy its indemnity obligations.
On September 29, 2014, a purported class action complaint challenging the company’s former parent’s merger and the Company’s spin-out as a stand-alone public company was filed by Resolution Partners, an alleged stockholder of SMI, and all others similarly situated, in the Kosciusko Circuit Court in the state of Indiana. The complaint named as defendants Symmetry Medical Inc. (“SMI”), the members of the board of directors of SMI, Genstar Capital LLC, Tecomet’s sponsor (‘‘Genstar’’), Tecomet, Holdings and TecoSym Inc. The complaint generally alleges, among other things, that the members of the SMI board of directors breached their fiduciary duties to Resolution Partners and SMI stockholders during merger negotiations and by entering into the Merger Agreement and approving the Merger, and that Genstar and Tecomet allegedly aided and abetted such alleged breaches of fiduciary duties. The complaint further alleges that the joint proxy statement/prospectus filed by Symmetry Surgical with the SEC on September 5, 2014, which contained the preliminary proxy statement of SMI, was misleading or omitted certain allegedly material information. The complaint sought, among other relief, injunctive relief enjoining consummation of the Merger, compensatory and/or rescissory damages in an unspecified amount and costs and fees. The parties settled the suit prior to the consummation of the transaction, for no additional consideration and a few additional disclosures filed in a Form 8-k, although left the issue of a claim for fees and costs for resolution at a later time, either through agreement or via a court hearing. The Company has agreed with SMI to share equally in any fee award, up to 50% of the remaining insurance deductible. The Company does not believe this will have a significant impact on its financial position, results of operations or cash flows.
19. Net Income(Loss) Per Share
The following table sets forth the computation of earnings per share.
Fiscal Year | |||||||||||
2015 | 2014 | 2013 | |||||||||
Net income (loss) for basic earnings per share: | |||||||||||
Income available to common shares -- Basic | $ | 1,200 | $ | (35,775 | ) | $ | (12,185 | ) | |||
Basic weighted average common shares outstanding | 9,645 | 9,587 | 9,587 | ||||||||
Net income (loss) attributable to common shareholders | $ | 0.12 | $ | (3.73 | ) | $ | (1.27 | ) | |||
Net income (loss) for diluted earnings per share: | |||||||||||
Income available to common shares -- Diluted | $ | 1,200 | $ | (35,775 | ) | $ | (12,185 | ) | |||
Basic weighted average common shares outstanding | 9,645 | 9,587 | 9,587 | ||||||||
Effect of dilution | 43 | — | — | ||||||||
Diluted weighted average common shares outstanding | 9,688 | 9,587 | 9,587 | ||||||||
Net income (loss) attributable to common shareholders | $ | 0.12 | $ | (3.73 | ) | $ | (1.27 | ) | |||
As of January 2, 2016, the diluted weighted average share calculation does not include performance based restricted stock awarded in fiscal 2014 and 2015 totaling 735 shares due to the performance criteria not being completed.
For periods prior to the separation, the numerator for both basic and diluted earnings per share was net income (loss) attributable to the Company. The denominator for basic and diluted earnings per share was calculated using the number of shares of Symmetry Surgical common shares outstanding on the distribution date.
20. Product and Geographic Information
The Company has one operating segment which is the lowest level for which discrete financial information is available and the operating results are regularly reviewed by management. The Company sells a broad range of reusable stainless steel and titanium surgical hand-held instruments and retractor systems, sterile disposable surgical products (vein strippers, SECTO dissectors, tonsil sponges and surgical marker pens), and sterilization containers. These products are typically used in the surgical specialties of spine, general/OB-GYN, microsurgery/neurosurgery, orthopedics, laparoscopy, cardiovascular, thoracic and general surgery in the hospital setting as well as surgery centers and in select physician offices. These products are viewed in two categories by management; Symmetry Surgical Branded Products and Alliance Partners Products. Symmetry Surgical Branded Products include all products sold under brand names managed by the Company whereas Alliance Partners Products are brands the Company distributes for others in defined markets.
Prior to the acquisition of the assets of Vesocclude Medical in August 2015, the revenue from the sales of Vesocclude product was depicted in the Alliance Partners product category. Upon acquisition, this revenue is now Symmetry Surgical branded product. For the purposes of comparability between periods, the historical product categories have been reclassified to include Vesocclude revenue in Symmetry Surgical branded product category.
Revenues are attributed to geographic locations based on the location to which the Company ships its products.
Revenues to External Customers:
Fiscal Year | |||||||||||
2015 | 2014 | 2013 | |||||||||
United States | $ | 73,821 | $ | 71,578 | $ | 78,358 | |||||
International | 10,706 | 10,204 | 10,589 | ||||||||
Total revenues | $ | 84,527 | $ | 81,782 | $ | 88,947 | |||||
Revenues by Product:
Fiscal Year | |||||||||||
2015 | 2014 | 2013 | |||||||||
Symmetry Surgical branded | $ | 76,935 | $ | 72,362 | $ | 74,671 | |||||
Alliance Partners | 7,592 | 9,420 | 14,276 | ||||||||
Total revenues | $ | 84,527 | $ | 81,782 | $ | 88,947 | |||||
Substantially all of the Company's long-lived tangible assets are located in the United States.
21. Quarterly Results of Operations (Unaudited)
The Company’s fiscal year end is the 52 or 53 week period ending the Saturday closest to December 31. Fiscal year 2015 is a 52 week year ending January 2, 2016. Fiscal year 2014 was a 53 week year ending January 3, 2015. The Company’s interim quarters for fiscal 2015 and 2014 were 13 weeks long, except for the fourth quarter of fiscal 2014 which was 14 weeks. The following quarterly results of operations refer to these financial periods (in thousands, except per share data):
Fiscal 2015 | ||||||||||||||||||||
First Quarter | Second Quarter | Third Quarter | Fourth Quarter (1) | Fiscal Year | ||||||||||||||||
(in thousands except per share data) | ||||||||||||||||||||
Total revenue | $ | 20,774 | $ | 20,662 | $ | 20,951 | $ | 22,140 | $ | 84,527 | ||||||||||
Gross profit | 9,885 | 9,809 | 9,956 | 9,800 | 39,450 | |||||||||||||||
Net income (loss) | 756 | 341 | 255 | (152 | ) | 1,200 | ||||||||||||||
Earnings per share: | ||||||||||||||||||||
Basic | $ | 0.08 | $ | 0.04 | $ | 0.03 | $ | (0.02 | ) | $ | 0.12 | |||||||||
Diluted | $ | 0.08 | $ | 0.04 | $ | 0.03 | $ | (0.02 | ) | $ | 0.12 | |||||||||
Fiscal 2014 | ||||||||||||||||||||
First Quarter | Second Quarter (2) | Third Quarter | Fourth Quarter (2) | Fiscal Year | ||||||||||||||||
(in thousands except per share data) | ||||||||||||||||||||
Total revenue | $ | 20,562 | $ | 20,473 | $ | 20,453 | $ | 20,294 | $ | 81,782 | ||||||||||
Gross profit | 8,476 | 9,842 | 9,017 | 8,349 | 35,684 | |||||||||||||||
Net income (loss) | (368 | ) | (6,146 | ) | 275 | (29,536 | ) | (35,775 | ) | |||||||||||
Earnings per share: | ||||||||||||||||||||
Basic | $ | (0.04 | ) | $ | (0.64 | ) | $ | 0.03 | $ | (3.08 | ) | $ | (3.73 | ) | ||||||
Diluted | $ | (0.04 | ) | $ | (0.64 | ) | $ | 0.03 | $ | (3.08 | ) | $ | (3.73 | ) | ||||||
(1) The fourth quarter of 2015 net loss includes a pre-tax impairment charge of $1,014.
(2) The second and fourth quarters of 2014 net income (loss) include pre-tax asset impairment charges of $10,500 and $45,317, respectively.
The sum of the quarters may not equal the year to date amounts due to rounding.
For periods prior to the separation, the weighted-average basic and diluted shares outstanding were based on the number of shares of Symmetry Surgical common stock outstanding on the distribution date.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
ITEM 9A. CONTROLS AND PROCEDURES
(a) Evaluation of Disclosure Controls and Procedures
Evaluation of Disclosure Controls and Procedures. In accordance with Rule 13a-15(b) of the Securities Exchange Act of 1934 (the “Exchange Act”), as of the end of the period covered by this Annual Report on Form 10-K, the Company’s management evaluated, with the participation of the Company's Chief Executive Officer and Senior Vice President and Chief Financial Officer, the effectiveness of the design and operation of the Company’s disclosure controls and procedures (as defined in Rule 13a-15(e) under the Exchange Act). Based upon their evaluation of these disclosure controls and procedures, the Chief Executive Officer and Senior Vice President and Chief Financial Officer concluded that the disclosure controls and procedures were effective as of the end of the period covered by this Annual Report on Form 10-K to ensure that information required to be disclosed by the Company in the reports it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time period specified in the Securities and Exchange Commission rules and forms, and to ensure that information required to be disclosed by the Company in the reports it files or submits under the Exchange Act is accumulated and communicated to the Company’s management, including its Chief Executive Officer and Senior Vice President and Chief Financial Officer, as appropriate, to allow timely decisions regarding disclosure.
(b) Changes in Internal Control over Financial Reporting
Changes in Internal Controls. There were no changes in the Company's “internal control over financial reporting" that occurred during the quarter ended January 2, 2016 that have materially affected, or are reasonably likely to materially affect, the Company's internal control over financial reporting.
Management's Report on Internal Controls Over Financial Reporting. The report of management required under this Item 9A can be found on page 45 of this Form 10-K under the heading "Management's Report on Internal Control over Financial Reporting."
This annual report does not include an attestation report of the company’s registered public accounting firm regarding internal control over financial reporting due to the Company’s status as an emerging growth company.
Item 9B. Other Information
None.
PART III
As noted above in Part I, we are an "emerging growth company" as that term is defined in the Jumpstart Our Business Startups Act of 2012 and, as such, we have elected to comply with certain reduced public company reporting requirements in this Form 10-K, and particularly as they pertain to certain disclosures in this Part III.
Item 10. Directors, Executive Officers and Corporate Governance
The information required to be furnished pursuant to Item 10 with respect to directors and corporate governance is incorporated herein by reference from the sections entitled "Governance of the Corporation" and "Information Regarding Our Directors" in our Proxy Statement for the 2016 Annual Meeting of Shareholders, which we will file with the Securities and Exchange Commission no later than 120 days after the end of our fiscal year.
Item 11. Executive Compensation
The information required to be furnished pursuant to Item 11 is incorporated herein by reference from the sections entitled "Executive Compensation" and "Compensation Discussion and Analysis" in our Proxy Statement for the 2016 Annual Meeting of Shareholders which we will file with the Securities and Exchange Commission no later than 120 days after the end of our fiscal year.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The information required to be furnished pursuant to Item 12 is incorporated herein by reference from the sections entitled "Stock Ownership of Directors and Executive Officers" and "Stock Ownership of Certain Beneficial Owners" in our Proxy Statement for the 2016 Annual Meeting of Shareholders which we will file with the Securities and Exchange Commission no later than 120 days after the end of our fiscal year.
An aggregate of 1,250 shares of common stock are now reserved for issuance under the 2014 Equity Incentive Plan. The following table sets forth, as of January 2, 2016, certain information related to our outstanding equity:
Plan Category | Number of securities to be issued upon exercise of outstanding options, warrants and rights | Weighted-average exercise price of outstanding options, warrants and rights | Number of Securities remaining available for future issuance under equity incentive plans (excluding securities reflected in column (a) |
Equity compensation plans approved by security holders | --- | --- | 302 |
Equity compensation plans not approved by security holders | --- | --- | --- |
Total | --- | --- | 302 |
Item 13. Certain Relationships and Related Transactions and Director Independence
The information required to be furnished pursuant to Item 13 is incorporated herein by reference from the sections entitled "Governance of the Corporation" and "Related Party Transactions" in our Proxy Statement for the 2016 Annual Meeting of Shareholders which we will file with the Securities and Exchange Commission no later than 120 days after the end of our fiscal year.
Item 14. Principal Accounting Fees and Services
The information required to be furnished pursuant to Item 14 is incorporated herein by reference from the section entitled "Audit and Non-Audit Fees" in our Proxy Statement for the 2016 Annual Meeting of Shareholders which we will file with the Securities and Exchange Commission no later than 120 days after the end of our fiscal year.
PART IV
Item 15. Exhibits, Financial Statement Schedules
(a) | See Part II, Item 8 for an index of the Company’s consolidated and combined financial statements. | |
(b) | Exhibits: | |
2.1 | Separation Agreement between Symmetry Medical Inc. and Symmetry Surgical Inc. (f/k/a Racecar Spinco, Inc.) (incorporated by reference to Exhibit 2.1 of Symmetry Surgical Inc. Registration Statement on Form S-4 (File No. 333-198596), as amended, which became effective on November 4, 2014 (the "Registration Statement"). | |
Articles of Incorporation and Bylaws | ||
3.1 | Certificate of Incorporation of Symmetry Surgical Inc. (f/k/a Racecar Spinco, Inc.) (incorporated by reference to Exhibit 3.1 of the Registration Statement). | |
3.2 | Certificate of Amendment to the Certificate of Incorporation of Symmetry Surgical Inc. (f/k/a Racecar Spinco, Inc.) (incorporated by reference to Exhibit 3.2 of Registration Statement). | |
3.3 | Form of Amended and Restated Certificate of Incorporation of Symmetry Surgical Inc. (incorporated by reference to Exhibit 3.3 of the Registration Statement). | |
3.4 | Bylaws of Symmetry Surgical Inc. as amended through December 17, 2014 (incorporated by reference to Exhibit 3.4 to our Form 10-K/A filed August 10, 2015). | |
3.5 | Form of Amended and Restated Bylaws of Symmetry Surgical Inc. (incorporated by reference to Exhibit 3.5 of the Registration Statement). | |
Instruments Defining the Rights of Security Holders, Including Indentures | ||
4.1 | Form of Common Stock Certificate, $0.0001 par value per share (incorporated by reference to Exhibit 4.1 of the Registration Statement). | |
Material Contracts | ||
10.1 | Form of Quality Agreement between Symmetry Medical Inc. and Symmetry Surgical Inc. (f/k/a Racecar Spinco, Inc.) (incorporated by reference to Exhibit 10.1 of the Registration Statement). | |
10.2 | Form of Shared IP Cross License Agreement between Symmetry Medical Inc. and Symmetry Surgical Inc. (f/k/a Racecar Spinco, Inc.) (incorporated by reference to Exhibit 10.2 of the Registration Statement). | |
10.3 | Form of Supply Agreement between Symmetry Medical Inc. and Symmetry Surgical Inc. (f/k/a Racecar Spinco, Inc.) (incorporated by reference to Exhibit 10.3 of the Registration Statement). | |
10.4 | Form of Transition Services Agreement Symmetry Medical Inc. and Symmetry Surgical Inc. (f/k/a Racecar Spinco, Inc.) (incorporated by reference to Exhibit 10.4 of the Registration Statement). | |
10.5† | Form of Symmetry Surgical Inc. 2014 Equity Incentive Plan (incorporated by reference to Exhibit 10.5 of the Registration Statement). | |
10.6 | Credit Agreement dated as of December 5, 2014, among Symmetry Surgical Inc., Specialty Surgical Instrumentation, Inc. and Olsen Medical, LLC as Borrowers, General Electric Capital Corporation as a Lender and Swingline Lender and as Agent for all Lenders, the other financial institutions party thereto as Lenders and GE Capital Markets, Inc. as Sole Lead Arranger and Bookrunner. (incorporated by reference to Exhibit 10.1 to our Form 10-Q filed on December 19, 2014). | |
10.7† | Form of Restricted Stock Agreement issued under 2014 Equity Incentive Plan. (incorporated by reference to Exhibit 10.7 to our Form 10-K filed March 12, 2015). | |
10.8† | Form of Indemnity Agreement. (incorporated by reference to Exhibit 10.7 to our Form 10-K filed March 12, 2015). | |
10.9† | Employment and Executive Benefit Agreement by and between Symmetry Surgical Inc. and Thomas J. Sullivan, dated November 28, 2014. (incorporated by reference to Exhibit 10.7 to our Form 10-K filed March 12, 2015). | |
10.10† | Employment and Executive Benefit Agreement by and between Symmetry Surgical Inc. and Scott D. Kunkel, dated November 28, 2014. (incorporated by reference to Exhibit 10.7 to our Form 10-K filed March 12, 2015). | |
10.11† | Employment and Executive Benefit Agreement by and between Symmetry Surgical Inc. and David C. Milne, dated November 28, 2014. (incorporated by reference to Exhibit 10.7 to our Form 10-K filed March 12, 2015). | |
10.12† | Employment and Executive Benefit Agreement by and between Symmetry Surgical Inc. and Ronda L. Harris, dated November 28, 2014. (incorporated by reference to Exhibit 10.7 to our Form 10-K filed March 12, 2015). | |
10.13† | Employment and Executive Benefit Agreement by and between Symmetry Surgical Inc. and Jose Fernandez, dated November 28, 2014. (incorporated by reference to Exhibit 10.7 to our Form 10-K filed March 12, 2015). | |
10.14† | Amendment to the Employment and Executive Benefit Agreement by and between Symmetry Surgical Inc. and Thomas J. Sullivan, dated May 9, 2015. (incorporated by reference to Exhibit 10.1 to our Form 10-Q filed April 4, 2015). | |
10.15† | Form Amendment to Restricted Stock and Cash Incentive Agreement issued under 2014 Equity Incentive Plan. (incorporated by reference to Exhibit 10.16 to our Form 10-Q filed November 9, 2015). | |
10.16† | Form Amendment to Restricted Stock and Cash Incentive Agreement issued under 2014 Equity Incentive Plan. (incorporated by reference to Exhibit 10.17 to our Form 8-K filed December 2, 2015). | |
10.17† | Form of Restricted Stock Agreement issued under 2014 Equity Incentive Plan. (incorporated by reference to Exhibit 10.8 to our Form 8-K filed January 28, 2016). | |
10.18 | First Amendment to Credit Agreement entered into by Symmetry Surgical Inc. as Borrowers, General Electric Capital Corporation as a Lender dated February 26, 2015.* | |
10.19 | Second Amendment to Credit Agreement entered into by Symmetry Surgical Inc. as Borrowers, General Electric Capital Corporation as a Lender dated August 28, 2015.* | |
Other | ||
21.1 | List of Subsidiaries.* | |
23.1 | Consent of Independent Registered Public Accounting Firm, Ernst & Young LLP.* | |
24.1 | Power of Attorney.* | |
Executive Officer Certifications | ||
31.1 | Certification of Chief Executive Officer required by Item 307 of Regulation S-K as promulgated by the Securities and Exchange Commission and pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.* | |
31.2 | Certification of Chief Financial Officer required by Item 307 of Regulation S-K as promulgated by the Securities and Exchange Commission and pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.* | |
32.1 | Certification of Chief Executive Officer and Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.* | |
XBRL | ||
101.INS | XBRL Instance Document.~ | |
101.SCH | XBRL Taxonomy Extension Schema Document.~ | |
101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document.~ | |
101.DEF | XBRL Taxonomy Extension Definition Linkbase Document.~ | |
101.LAB | XBRL Taxonomy Extension Label Linkbase Document.~ | |
101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document.~ | |
† | Indicates management contract or compensatory plans or arrangements required to be filed as an exhibit. | |
* | Filed currently herewith. | |
~ | In accordance with Rule 406T under Regulation S-T, the XBRL-related information in Exhibit 101 shall be deemed “furnished” and not “filed.” | |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
SYMMETRY SURGICAL INC. | ||
By | /s/ Thomas J. Sullivan | |
Thomas J. Sullivan, | ||
President and Chief Executive Officer | ||
(Principal Executive Officer) | ||
By | /s/ Scott D. Kunkel | |
Scott D. Kunkel, | ||
Senior Vice President and Chief Financial Officer | ||
(Principal Financial Officer) | ||
March 1, 2016 | ||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the date indicated.
Name | Title | Date | |||
/s/ Thomas J. Sullivan | |||||
Thomas J. Sullivan | Chief Executive Officer, President and Director (Principal Executive Officer) | March 1, 2016 | |||
/s/ Scott D. Kunkel | |||||
Scott D. Kunkel | Senior Vice President, Chief Financial Officer (Principal Financial Officer) | March 1, 2016 | |||
/s/ Ronda L. Harris | |||||
Ronda L. Harris | Chief Accounting Officer | March 1, 2016 | |||
* | Craig B. Reynolds | Director | March 1, 2016 | ||
* | Francis T. Nusspickel | Director | March 1, 2016 | ||
* | James S. Burns | Director | March 1, 2016 | ||
* | John S. Krelle | Director | March 1, 2016 | ||
* | Robert G. Deuster | Director | March 1, 2016 | ||
* | By: /s/ Scott D. Kunkel Scott D. Kunkel Attorney-in-fact Pursuant to Power of Attorney (Exhibit 24.1 hereto) | ||||
