Attached files
| file | filename |
|---|---|
| EX-23.2 - EX-23.2 - Foundation Medicine, Inc. | d118961dex232.htm |
| EX-31.2 - EX-31.2 - Foundation Medicine, Inc. | d118961dex312.htm |
| EX-21.1 - EX-21.1 - Foundation Medicine, Inc. | d118961dex211.htm |
| EX-23.1 - EX-23.1 - Foundation Medicine, Inc. | d118961dex231.htm |
| EX-32.1 - EX-32.1 - Foundation Medicine, Inc. | d118961dex321.htm |
| EX-31.1 - EX-31.1 - Foundation Medicine, Inc. | d118961dex311.htm |
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
(Mark One)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2015
or
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to .
Commission File Number 001-36086
FOUNDATION MEDICINE, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 27-1316416 | |
| (State or other jurisdiction of incorporation or organization) |
(IRS Employer Identification No.) |
150 Second Street
Cambridge MA, 02141
(Address of principal executive offices, including zip code)
Registrant’s Telephone Number, Including Area Code:
(617) 418-2200
Securities registered pursuant to Section 12(b) of the Act:
| Common Stock, $0.0001 Par Value | The NASDAQ Global Select Market | |
| (Title of each class) | (Name of each exchange on which registered) |
Securities registered pursuant to Section 12(g) of the Act: NONE
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). x Yes ¨ No
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in 12b-2 of the Exchange Act.
| Large accelerated filer | ¨ | Accelerated filer | x | |||
| Non-accelerated filer | ¨ | Smaller reporting company | ¨ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
As of June 30, 2015, the last business day of the registrant’s most recently completed second fiscal quarter, the aggregate market value of common stock held by non-affiliates of the registrant computed by reference to the last reported sale price of the registrant’s common stock on the Nasdaq Global Select Market as of such date was approximately $436.7 million. As of February 26, 2016 there were 34,540,998 shares of the registrant’s common stock, $0.0001 par value per share, outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
The registrant intends to file a definitive proxy statement pursuant to Regulation 14A within 120 days of the end of the fiscal year ended December 31, 2015. Portions of such definitive proxy statement are incorporated by reference into Part III of this Annual Report on Form 10-K.
Table of Contents
ANNUAL REPORT ON FORM 10-K
For the Year Ended December 31, 2015
Table of Contents
This Annual Report on Form 10-K, or this Annual Report, contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, and is subject to the “safe harbor” created by those sections. Any statements about our expectations, beliefs, plans, objectives, assumptions or future events or performance are not historical facts and may be forward-looking. Some of the forward-looking statements can be identified by the use of forward-looking terms such as “believes,” “expects,” “may,” “will,” “should,” “seek,” “intends,” “plans,” “estimates,” “projects,” “anticipates,” or other comparable terms. These forward-looking statements involve risk and uncertainties. We cannot guarantee future results, levels of activity, performance or achievements, and you should not place undue reliance on our forward-looking statements. Our actual results may differ significantly from the results discussed in the forward-looking statements. Factors that might cause such a difference include, but are not limited to, those set forth in “Item 1A. Risk Factors” and elsewhere in this Annual Report. Except as may be required by law, we have no plans to update our forward-looking statements to reflect events or circumstances after the date of this Annual Report. We caution readers not to place undue reliance upon any such forward-looking statements, which speak only as of the date made.
Unless the content requires otherwise, references to “Foundation,” “the Company,” “we,” “our,” and “us,” in this Annual Report refer to Foundation Medicine, Inc. and its subsidiaries.
| ITEM 1. | BUSINESS |
Overview
We are a molecular information company focused on fundamentally changing the way in which patients with cancer are evaluated and treated. We believe an information-based approach to making clinical treatment decisions based on comprehensive genomic profiling will become a standard of care for patients with cancer. We derive revenue from selling products that are enabled by our molecular information platform to physicians and biopharmaceutical companies. Our platform includes proprietary methods and algorithms for analyzing specimens across all types of cancer, and for incorporating that information into clinical care in a concise and user-friendly fashion. Our products provide genomic information about each patient’s individual cancer, enabling physicians to optimize treatments in clinical practice and biopharmaceutical companies to develop targeted oncology therapies more effectively. We believe we have a significant first mover advantage in providing comprehensive genomic profiling and molecular information products on a commercial scale.
Our flagship clinical molecular information products, FoundationOne for solid tumors, and FoundationOne Heme for blood-based cancers, or hematologic malignancies, including leukemia, lymphoma, myeloma, and advanced sarcomas, are, to our knowledge, the only widely available comprehensive genomic profiles designed for use in the routine care of patients with cancer. To accelerate our commercial growth and enhance our competitive advantage, we are continuing to develop and commercialize new molecular information products for physicians and biopharmaceutical companies, strengthen our commercial organization, introduce new marketing, education and provider engagement efforts, grow our molecular information knowledgebase, called FoundationCORE, pursue reimbursement from regional and national third-party payors and government payors, publish scientific and medical advances, and foster relationships across the oncology community.
The cancer treatment paradigm is evolving rapidly, and we believe there is now widespread recognition that cancer is a disease of the genome, rather than a disease defined solely by its specific anatomical location in the body. Today, physicians increasingly use precision medicines to target cancers based on the specific genomic alterations driving their growth. We believe physicians need molecular information about their patients’ unique cancers to determine the optimal course of treatment.
We believe the oncology community needs comprehensive molecular information products that can assess the known biologically relevant genomic alterations from a patient’s tumor and distill complex molecular
1
Table of Contents
information into a concise and actionable format, and we designed FoundationOne and FoundationOne Heme to be such products. We believe a comprehensive approach to providing molecular information for use in clinical settings addresses an area of significant unmet medical need for patients suffering from advanced, or active metastatic, cancers. We estimate that there are approximately 1.1 million patients per year in the United States with newly-diagnosed or recurrent active metastatic cancers who fall into challenging treatment categories, including patients who have rare or aggressive diseases, patients whose disease has progressed after standard treatments, and patients who have tested negative under, or been ineligible for, traditional molecular diagnostic tests. We are initially focusing on these patients because we believe this patient population will benefit most from comprehensive molecular information products.
To maintain our market leadership position and offer physicians and biopharmaceutical companies a full suite of innovative molecular information solutions, we developed our third comprehensive genomic profiling product, FoundationACT (Assay for Circulating Tumor DNA), which we believe is a best-in-class, blood-based (liquid biopsy) assay to evaluate circulating tumor DNA, or ctDNA, which is DNA shed from tumors that circulates in blood plasma outside of cells. We launched FoundationACT for research use to our biopharmaceutical partners in December 2015. We plan to initiate the commercial launch of FoundationACT to ordering physicians in March 2016. We believe FoundationACT will become an important molecular information solution for oncologists because it will provide a new option for comprehensive genomic profiling when a tissue biopsy is not feasible or when tissue is not available. By analyzing cell-free DNA isolated from a patient’s blood, we can identify clinically relevant genomic alterations in the circulating tumor DNA and match these alterations to targeted therapies and clinical trials.
As the number of available targeted therapies expands and as physicians gain further experience using comprehensive molecular information in their routine treatment decisions, we believe that the potentially addressable market for comprehensive molecular information products will expand over the next five years to include most patients who have metastatic disease, patients with earlier stage disease, and patients from whom a tissue biopsy is not available. We estimate that this potential U.S. market expansion could include an additional 900,000 total patients for FoundationOne, FoundationOne Heme, and FoundationACT, bringing the total number of patients who could benefit from our approach in the U.S. to approximately 2 million on an annual basis.
We believe we have a significant first mover advantage in building the only commercially available molecular information platform that comprehensively assesses cancer simultaneously for all four classes of genomic alterations (i.e., base pair substitutions, copy number alterations, short insertions and deletions, or indels, and gene rearrangements) across all cancer-related genes with the sensitivity and specificity required for routine medical practice. We published our analytic validation for FoundationOne in Nature Biotechnology in October 2013, and we anticipate publishing a similar validation for FoundationOne Heme in 2016. We presented analytic validation data for FoundationACT at the 2016 Advances in Genome Biology and Technology Conference demonstrating that our assay achieves very high unique coverage from limited ctDNA input which enables accurate detection of base substitutions, indels, and genomic rearrangements at very low tumor content, as well as copy number amplifications with performance specifications equivalent to those for FoundationOne. FoundationOne and FoundationOne Heme deliver, and FoundationACT, when commercialized, will deliver this complex molecular information in a concise report that matches detected molecular alterations with potentially relevant treatment options, including clinical trials.
We believe the genomic alterations identified for each patient should be accompanied by the most current and relevant scientific and medical literature related to those alterations, and that this information should be presented in a clear and concise manner. Our molecular information knowledgebase, FoundationCORE, stores this genomic alteration data and currently includes more than 68,000 genomic profiles. Test reports are now available to physicians using FoundationICE, the newest version of our online Interactive Cancer Explorer, which we made available to select providers in December 2014 and launched in May 2015 at the annual meeting of the American Society of Clinical Oncology, or ASCO. We believe FoundationICE will help physicians to more efficiently use our clinical products and will enhance the utility of comprehensive genomic profiling.
2
Table of Contents
We believe our unique and proprietary decision support applications are a competitive differentiator of our comprehensive molecular information solutions and may accelerate the broad adoption of our products and create an important network effect among users. These applications are designed to integrate with physician workflow and enhance the actionability of our molecular information, enabling physicians to make informed therapeutic decisions. For example, an important component of FoundationICE is PatientMatch, a technology application that enables physician-to-physician connections to facilitate sharing of treatment decisions and clinical outcomes data for patients with similar genomic profiles, in a manner compliant with the Health Insurance Portability and Accountability Act, or HIPAA. In September 2015, we announced the expansion of our suite of molecular information-based products with GeneKit. GeneKit is a pathology-focused genomics solution that facilitates rapid interpretation and report generation for next generation sequencing, or NGS, assays of up to 50 genes. GeneKit provides a reflex option to our assays, FoundationOne, FoundationOne Heme, and FoundationACT, should the more narrowly focused third-party molecular diagnostics tests fail to identify a genomic alteration in a patient’s biopsy.
Our molecular information solutions address significant unmet needs in the market and enable providers and their patients to identify relevant treatment options, including potential access to clinical trials. We believe the current system for patients to access clinical trials in the United States is highly inefficient. Approximately 3% of patients with cancer in the United States are enrolled in clinical trials. To overcome this challenge of patient access to clinical trials and precision therapies, we launched a pilot program in October 2015 called SmartTrials. This program provides physicians with information on certain enrolling studies of investigational, targeted therapies, and immunotherapies based on the genomic alterations identified in the patient’s tumor sample. We believe this program may be a catalyst for precision medicine by enabling greater patient access to personalized therapies and simultaneously, enabling our biopharmaceutical partners to accelerate drug development.
Our clinical products have been rapidly adopted in the marketplace, and several thousand physicians from large academic centers to community-based practices have ordered FoundationOne or FoundationOne Heme. We believe this rapid adoption, which has been accomplished in the early stages of our commercial growth, demonstrates the demand for and utility of our comprehensive solutions that help oncologists effectively implement the promise of precision medicine. We believe our current and future molecular information products address a market opportunity of $12-15 billion over the next five years.
We believe our suite of molecular information products have a sustainable competitive advantage because they:
| • | Provide comprehensive and reliable identification of clinically relevant information — FoundationOne currently assesses 315 biologically relevant cancer genes for all classes of genomic alterations with high sensitivity and specificity. We believe FoundationOne identifies genomic alterations that other commercially available diagnostic tests cannot. FoundationOne Heme employs RNA sequencing of 265 genes in addition to DNA sequencing of 405 genes to detect all classes of genomic alterations across genes known to be altered other than through inherited genetic characteristics, which are also known as somatic alterations, in hematologic malignancies, pediatric cancers, and sarcomas, which we believe also will lead to the identification of clinically relevant information. FoundationACT, which we launched to our biopharmaceutical partners in December 2015 for research use, identifies in a cancer patient’s blood all known clinically relevant alterations in 62 genes altered in human solid tumors that are validated targets for therapy or are unambiguous drivers of cancer. The assay has been optimized to overcome the tremendous challenges of detecting low quantities of ctDNA in blood and has been analytically validated for high accuracy across all classes of genomic alterations; |
| • | Promote physician interaction to create a powerful network effect — We are continually augmenting FoundationCORE and expanding the functionality of FoundationICE to allow for sharing of genomic and treatment data in a HIPAA-compliant fashion. We believe these efforts will create a network effect of more users and ultimately more actionable information; |
3
Table of Contents
| • | Incorporate the latest scientific and medical advances — We have extensive relationships across the scientific and medical oncology communities, including with key thought leaders and leading biopharmaceutical companies. These relationships help us incorporate new cancer genes, the latest scientific findings, newly available targeted therapeutics, and relevant clinical trials into FoundationOne, FoundationOne Heme, and FoundationACT test results; |
| • | Readily integrate into routine clinical practice — Our proprietary sample preparation processes and computational biology algorithms allow us to utilize small amounts of tumor tissue from a wide variety of sample types, including tissue with low tumor purity and from liquid (blood) biopsies, so as to allow for routine specimen collection. We detect and report the clinically relevant genomic alterations, generally within 11 to 14 days for FoundationOne and within 15 to 18 days for FoundationOne Heme, in each case from the time the specimen is received. We expect to report results in less than 14 days for FoundationACT once it is launched commercially. We are dedicated to providing high-quality support to our customers, from order initiation and sample acquisition through report delivery and follow-up with our medical affairs team; and |
| • | Provide clinically relevant information and decision support applications that physicians can use— In a concise report, our products communicate the relevant genomic alterations in a patient’s cancer and based on peer-reviewed literature and clinical and governmental databases, match these alterations with targeted therapies and relevant clinical trials. We continue to develop innovative new decision support applications, like PatientMatch, GeneKit, and SmartTrials, that support informed treatment decision-making and enable improved access to therapies. |
We believe we are a unique and highly differentiated molecular information company. The combination of our world-class lab, FoundationCORE, our bioinformatics capabilities, and our technology applications enable us to be a best-in-class and reliable partner to biopharmaceutical partners. Our molecular information platform is currently used by more than 25 biopharmaceutical partners to enhance their development of targeted oncology therapies, and we believe we are well positioned to capitalize on a market opportunity with biopharmaceutical partners in excess of $600 million over the next five years. Our biopharmaceutical partners leverage our molecular information platform in three primary ways:
| • | Molecular profiling: We use our core proprietary testing platform, computational biology, and information technology capabilities to analyze patient samples from both retrospective and prospective clinical trials. We provide our biopharmaceutical partners comprehensive genomic profiling to enable novel clinical trial designs, enhance patient selection, expand patient populations, and identify novel gene targets. We believe the market opportunity for molecular profiling services to biopharmaceutical partners is in excess of $200 million. A selection of our biopharmaceutical partners for molecular profiling includes Agios Pharmaceuticals, Inc., or Agios, Clovis Oncology, Inc., or Clovis, Novartis Pharmaceuticals Corporation, or Novartis, and Roche Holdings, Inc. and its affiliates, or Roche. |
| • | FoundationCORE Insights: We have more than 68,000 real-world, clinical profiles within FoundationCORE. We assist our biopharmaceutical partners with novel target identification, clinico-genomics data and unique analytic offerings. We believe the market opportunity for FoundationCORE Insights is in excess of $300 million. An example of one of our biopharmaceutical partners for FoundationCORE Insights includes Loxo Oncology, or Loxo. |
| • | Companion Diagnostics: We provide our partners with companion diagnostic development and support to help drive their precision medicine strategies. We utilize the FoundationOne laboratory platform under quality system regulation, or QSR, to enable access to individual targeted therapies with the aim of developing companion diagnostic assays having a premarket approval, or PMA, from the United States Food and Drug Administration, or FDA. We are currently working with Clovis and Mirati Therapeutics, Inc., or Mirati, among others, to develop their companion diagnostic approach. We believe the total addressable market for companion diagnostics is in excess of $100 million over the next five years. |
4
Table of Contents
We believe our companion diagnostic development work is an integral component of our regulated products strategy and the development of a “universal” companion diagnostic that is built on the FoundationOne platform. We believe that by enabling physicians in the future to use one, FDA-approved companion diagnostic platform for multiple drugs requiring a companion diagnostic, we can eliminate much of the guesswork from testing and provide assurance to physicians and their patients that they have the information necessary to make an informed treatment decision.
In addition to generating revenue, these relationships often enable us to identify new cancer genes under investigation that can be incorporated into our platform at an early stage, as well as to participate in the development of the newest oncology therapeutics and practice. We are actively working to expand these relationships.
Our Molecular Information Business

We are dedicated to ongoing innovation in our molecular information platform and new product pipeline. Our product development investments have already yielded enhancements to FoundationOne and the platform generally, enabling us to analyze more genes, using less tissue, while reducing turn-around time. We also utilize RNA-based sequencing technology to analyze additional gene fusions commonly found in hematologic malignancies and sarcomas, and we launched FoundationOne Heme, which incorporates this technology, in December 2013. In addition, our efforts to reliably and accurately detect ctDNA led to the launch of FoundationACT for our biopharmaceutical partners for research use in 2015. We plan to commence our commercial launch of FoundationACT to ordering physicians in March 2016. We are exploring and developing new scientifically-advanced and clinically relevant products that include, for example, assays to analyze circulating tumor cells and products that expand our offerings into additional areas such as immune response and disease monitoring. We believe that our development of a suite of product offerings will be instrumental in providing patients and physicians with the comprehensive molecular information needed to evaluate a patient’s cancer and enable more informed treatment decisions.
The increasing availability and understanding of molecular information within the practice of oncology is driving a revolution in the treatment of cancer. We seek to leverage the vast array of genomic data generated by
5
Table of Contents
our molecular information platform together with clinical data to position ourselves at the nucleus of this new treatment paradigm. Our biopharmaceutical partners have already begun using our data to further refine clinical trial design and drug development. In an example of the power of our molecular information platform, after a biopharmaceutical partner’s Phase II trial that used a narrowly focused molecular diagnostic test to screen trial subjects failed to meet its primary endpoint, we performed our comprehensive genomic profile on the tissue collected from trial participants. Our analysis helped our biopharmaceutical partner predict a response to the drug and create new hypotheses to test in Phase III trials, which led to the subsequent U.S. and European approval of the drug and may have increased the target population who could benefit from this therapeutic approach.
Over time, we intend to expand our ability to capture, aggregate, analyze, and facilitate the broader exchange of genomic data across the global oncology community. We are investing in our technology architecture to allow oncologists to share clinical data. Through FoundationICE, PatientMatch, GeneKit, and SmartTrials, as well as data partnerships with leading technology and software companies such as Flatiron Health, Clinical Outcomes and Tracking Analysis, or COTA, and IMS Health, we are building a data platform that efficiently captures and allows for the analysis of data that we believe will create a network effect leading to more users and, in turn, more relevant genomic and clinical data. If we, in conjunction with oncologists, pathologists, biopharmaceutical companies, and academic researchers, can successfully capture and utilize this data, we believe we will play an even more integral role in transforming care for the millions of patients suffering from cancer.
Our Strategic Collaboration with Roche
On January 12, 2015, we announced a broad, strategic collaboration with Roche in the field of molecular information for oncology. The collaboration is a broad multi-part agreement that includes an R&D collaboration, a U.S. educational support collaboration, an ex-U.S. commercial collaboration, a binding term sheet for an in vitro diagnostic product development collaboration and an equity investment with certain governance provisions. We believe this strategic collaboration with Roche will accelerate many aspects of our business strategy and further advance our leadership position in molecular information solutions globally.
Under the terms of the R&D collaboration agreement, Roche may pay potentially more than $150 million over five years to access our molecular information platform and to fund R&D product development programs. Roche will utilize our molecular information platform to standardize its clinical trial testing, enable comparability of clinical trial results for R&D purposes, and better understand the potential for combination therapies. The initial focus of the R&D product development programs will be to develop genomic profiling tests for continuous blood-based monitoring and cancer immunotherapies, and to develop next generation companion diagnostics. As our capabilities increase through our collaboration with Roche, we expect to have more offerings available to existing and new clients.
The commercial collaborations are designed to significantly broaden our reach across international clinical and molecular information markets. Specifically, under the ex-U.S. commercial collaboration, Roche obtained ex-U.S. commercialization rights to our existing products and to future co-developed products. Under the U.S. educational support collaboration, while we remain solely responsible for commercialization of our products within the United States, Roche will engage its medical education team to provide non-branded information to pathologists across the United States specific to comprehensive genomic profiling in cancer.
We closed our strategic transaction with Roche on April 7, 2015. As a result of (1) a primary investment by Roche of $250 million in cash to purchase 5 million newly issued shares of our common stock at a purchase price of $50.00 per share and (2) a tender offer in which Roche acquired 15,604,288 outstanding shares of our common stock at a price of $50.00 per share, Roche became our majority shareholder, and as of December 31, 2015, Roche owned approximately 61% of our outstanding common stock. We maintain our operational independence, with Roche having minority representation on our Board of Directors, or the Board.
6
Table of Contents
Our Strategy
Our objective is to transform the care of patients with cancer by leading the development and commercialization of proprietary molecular information products that inform the diagnosis and treatment of cancer, and that enhance the development of cancer therapies. To achieve this objective, our strategy is to:
| • | Drive awareness and adoption of our suite of molecular information products including FoundationOne, FoundationOne Heme, FoundationACT, and future products we may develop — We have built an experienced, oncology-focused commercial organization, and we continue to collaborate with thought leaders to validate our platform and influence utilization of our products, promote physician interaction, engage with patient advocacy and other key oncology stakeholders, and continue to pursue payment and reimbursement for our products. |
| • | Empower the broader cancer community with molecular information — We are investing in technologies to allow oncologists and pathologists to collaborate and share treatment and other clinical information. We launched FoundationICE, and we continue to develop new and innovative decision support offerings, including PatientMatch, GeneKit and SmartTrials to further this goal. Over time, we intend to expand our capacity to capture, aggregate, analyze and facilitate the broader exchange of genomic and clinical data across the global oncology community—a strategy that we believe eventually will create a network effect encouraging physician participation and the development of substantial amounts of data that, in turn, will positively impact the treatment of cancer. |
| • | Demonstrate the value of our products to patients, physicians, and payors — To illustrate the value of our products, we are educating physicians and payors about the patients most likely to benefit from our products, conducting clinical trials and health economic studies, and communicating our data through peer-reviewed journals and conference presentations. |
| • | Enable biopharmaceutical companies to more effectively develop new cancer therapies — We are continuing to expand our commercial relationships with biopharmaceutical partners to enable us to discover and interrogate new cancer genes, to assist in the development of novel targeted therapeutics and companion diagnostic tests, to improve clinical trial efficiency and outcomes, and to continue our involvement at the cutting edge of cancer treatment. |
| • | Invest in product enhancements and new product innovations — We are developing new molecular information products and conducting research and development into potential products to evaluate and monitor disease progression and better understand markers of response to targeted therapies and immunotherapies. We are also investing resources to advance our companion diagnostics development and our FDA-regulated products strategy. |
Our Industry
Despite enormous investment in research and the introduction of new treatments, cancer remains a critical area of unmet medical need. According to the 2015 American Cancer Society report, “Cancer Facts & Figures 2015,” in 2014 in the United States, nearly 14.5 million people were suffering from cancer, and more than 1.6 million people were expected to be diagnosed with the disease during 2015. Hematologic malignancies, most commonly leukemias, lymphomas, and myelomas, are cancers that affect the body’s blood, lymphatic system, or bone marrow. Taken together, hematologic malignancies account for approximately 10% of new cancer diagnoses in the United States.
The global cancer burden is growing. The World Health Organization predicts in its Global Action Against Cancer publication that in 2020 there will be 16 million new cancer cases and 10 million cancer deaths globally. A recent report by the American Cancer Society, “The Global Economic Cost of Cancer,” estimates that the total annual economic impact of premature death and disability from cancer worldwide is approximately $900 billion.
7
Table of Contents
According to ASCO, there were more than 12,000 practicing oncologists treating patients with cancer in the United States in 2014. Whereas a small portion of oncologists practice in major academic-based cancer centers, the National Cancer Institute estimates that approximately 85% of the oncologists in the United States practice in community-based settings where the vast majority of patients with cancer are treated.
The diagnosis of cancer is complex and multidimensional. Practicing oncologists order multiple tests, including currently available molecular diagnostic tests, to better understand the genomic alterations that are driving their patients’ cancer growth.
Cancer Treatment is Evolving to a Molecular-Based Paradigm
Cancer is not a single disease. The term ‘cancer’ describes a class of diseases characterized by uncontrolled cell growth. Cells can experience uncontrolled growth if there are alterations to DNA, such as damage or mutations, and, therefore, disruption to the genes and proteins regulating cell division.
Surgery is often the first line of therapy for cancer where possible, and, according to the American Cancer Society, most patients with cancer will have some type of surgery. Surgery often presents the greatest chance for a cure, especially if the cancer has been detected early in its development and has not spread to other parts of the body. Many patients, however, require therapeutic intervention beyond surgery alone.
Physicians have used radiation as a cancer therapy since the early 20th century, and modern radiation techniques deliver therapy with significant precision. Nevertheless, even today, radiation’s use and efficacy is limited because the high doses necessary to kill cancer cells often cause damage to healthy cells in the treatment area and fail to kill all cancer cells, particularly if the cancer has spread to other parts of the body.
Physicians began using chemotherapy in the 1940s as a drug therapy approach that acts by killing cells that divide rapidly, one of the main properties of most cancer cells. These cytotoxic therapies are often prescribed by a trial and error approach — both because certain chemotherapies have limited efficacy in some patients and the treatment effect might thus be inconsistent, and because the therapies’ indiscriminate destruction of healthy cells involved in critical biological functions can cause severe toxic side effects in some patients.
More recently, oncologists are integrating a precision medicine approach by utilizing therapeutics that target cancers based on the specific genomic alterations driving their growth. We believe the oncology community is generally beginning to change clinical practice so that oncologists treat each individual’s cancer according to its unique genomic alterations that impact the underlying biological pathways within the patient’s cancer, rather than treating a patient’s cancer based solely on its initial anatomical location in the body, such as the breast, colon or lung. In addition, as a result of advancements in cancer biology and genomic technology that enable the identification of new cancer genes, biopharmaceutical companies are directing more research and development resources towards targeted therapies. There are currently more than 50 approved targeted oncology therapies on the market and approximately 3,100 active clinical trials. More than 830 compounds are in development for the treatment of cancer and, of these compounds, 73% are personalized medicines. In 2014, according to IMS Health, global sales of targeted oncology therapies totaled approximately $50 billion, compared to less than $4.4 billion in 2003.
The rapid increase in molecular information related to cancer, as well as the increasing array of targeted oncology therapeutics, is making it more difficult for physicians to make treatment decisions. The National Comprehensive Cancer Network estimates that 50% to 75% of cancer therapies in the United States are used off label, meaning that physicians prescribe therapies for clinical indications in manners different from those approved by FDA. Off-label usage of traditional cytotoxic therapies is often driven by physicians struggling to treat a patient’s disease after it fails to respond to initial treatment regimens. Targeted therapies are used off label by oncologists who have expertise in genomics or access to diagnostic tools that allow them to make informed decisions about off-label use of targeted therapies.
8
Table of Contents
In order to maximize the utility of diverse cancer-related molecular information to better guide the use of targeted therapies, we believe a new approach is needed. Specifically, the oncology community needs comprehensive genomic information products that can assess the known and biologically relevant genomic alterations, and distill complex molecular information into a concise and actionable format.
Current Challenges of Evaluating Cancer on a Molecular Level
Today, physicians are faced with numerous challenges when making decisions on how to best utilize currently available molecular diagnostics for cancer, including:
| • | the inherent limitations of certain molecular diagnostic tissue tests that analyze only a single or a limited number of genomic markers from tumor tissue and that only identify a subset of the four classes of genomic alterations found in cancers; |
| • | in the case of solid tumors, insufficient and/or poor quality tumor biopsy tissue relative to the amount and quality needed to perform all desired or required tests; and |
| • | difficulty of integrating existing molecular diagnostic tests into clinical practice, including the decisions about which tests to order and how to effectively match the genomic information provided by tests with current targeted therapies or clinical trials. |
Single-Marker or Limited Gene Tissue-based Hotspot Panel Tests Can Be Useful Tools But Often Miss Relevant Information
Most currently available molecular diagnostic tests are single-marker or limited gene “hotspot” panel tests that capture only one or a limited number of the most common, well-known gene alterations that these tests are designed to target. There are four classes of genomic alterations that are clinically relevant to the treatment of cancer: base pair substitutions; copy number alterations; short indels; and gene rearrangements and fusions. Hotspot panel tests generally are only able to identify base pair substitutions and specific gene rearrangements, do not routinely detect copy number alterations, and often lack the sensitivity to identify short indels. In addition, hotspot panel tests are typically incapable of detecting gene fusions, a type of alteration that is a common driver of hematologic malignancies, sarcomas and pediatric cancers, and certain solid tumors.
The following table summarizes the uses and inherent limitations of the current testing methods utilized in commercially available single-marker and hotspot panel tests for cancer, including the most commonly ordered according to results of a 2008 survey of oncologists and hematologists published in the Journal of Clinical Pathology article, “Molecular testing for somatic cancer mutations: a survey of current and future testing in UK laboratories.” Although oncologists may order these tests to look for one or a limited number of specific gene alterations, we believe the inherent limitations of tests using these methods are understood by pathologists and genomicists who perform the tests and the oncologists who order them.
| Name |
Uses |
Limitations | ||
| Polymerase chain reaction, or PCR-based tests, a technology used for amplifying DNA sequences | Enable the detection of short fragment DNA or RNA sequences. | Single-gene tests for specific and limited number of mutations.
Only identify known and select base substitutions and short indels, such as BRAF V600E. | ||
| Immunohistochemical, or IHC, stains, a process used to diagnose abnormal cells | Utilize antibody proteins to identify certain antigens that are unique to various types of cancer. | Only identify the expressed presence of a known and select protein or specific protein marker, such as HER2, related to a particular genomic alteration. | ||
9
Table of Contents
| Name |
Uses |
Limitations | ||
| FISH-based DNA probes, a mechanism for detecting DNA sequences through the use of fluorescent technology | Reveal specific genomic abnormalities, including insertion/deletions and rearrangements. | Only detect select gene rearrangements, such as EML4-ALK.
Difficult to test for multiple markers. | ||
| Flow Cytometry | Detection of tumor cell DNA aneuploidy, the analysis of tumor cell proliferation and the immunophenotyping of leukemias. | Only looks at a limited number of cells, and does not detect broader range of genomic alterations. | ||
| Cytogenetics | Determination of which chromosomal translocations and fusion genes are present in malignant cells. | Cannot detect other types of genomic abnormalities. | ||
Limited Tissue Availability and Poor Tissue Quality Restrict Testing Options
Many clinical tumor samples are provided from standard biopsies, needle biopsies or fine needle aspirates that yield very small tissue amounts. Small amounts of tissue samples limit the number of diagnostic tests a physician can order, and ordering one or a limited number of tests that look for one or a limited number of genomic alterations necessarily increases the likelihood that a physician may fail to identify other genomic alterations and ultimately therapeutic options.
Clinical tumor specimens also often have low tumor purity, meaning that the relevant genomic alterations occur in low frequencies within the sample and are difficult to detect. Moreover, the vast majority of clinical samples are stored as formalin-fixed and paraffin-embedded, or FFPE, specimens. FFPE preservation can damage DNA and RNA. Low tumor purity or damage to DNA or RNA may limit the availability of hotspot panel tests to identify certain genomic alterations.
A Growing Number of Molecular Diagnostics are Often Difficult to Integrate into Clinical Practice
Physicians today face an increasingly difficult decision about which single-marker or hotspot panel tests to order. There are a growing number of tests, each specific to a different cancer type and each having limited ability to detect multiple genomic alterations. Often, in the case of solid tumors, only a small amount of tumor biopsy is available, forcing the physician to order only a subset of desired diagnostic tests, often one test at a time in a serial manner. Furthermore, tests are usually selected based on the traditional treatment paradigm of the cancer’s location in the body or by simple trial and error. Integration of molecular diagnostics into clinical practice is particularly challenging with hematologic cancers in which lack of tissue distinctions within the cancers often lead to misdiagnosis, lack of prognostic information and, thus, mistreatment.
Running multiple, disjointed tests also poses logistical challenges associated with routing samples to several different laboratories and high costs associated with conducting multiple tests. Moreover, limited tissue availability may prevent relevant tests from being ordered, tests conducted may miss genomic alterations, and the results may not be delivered soon enough to be used during the typical treatment cycle for a patient. Even if a physician has enough cancer specimen to order a sufficient number of hotspot panel tests and single gene molecular tests to identify relevant genomic alterations and receives the results of all of these tests in a timely fashion, the physician would commonly receive a series of uncoordinated individual reports from different laboratories that are difficult for non-specialized pathologists or other physicians to interpret and synthesize. Compounding these challenges, especially in the community oncology setting, is how to effectively match the genomic information provided by tests with current targeted therapies or clinical trials for a particular patient. As a result of one or a combination of these current limitations, physicians may fail to identify or to prescribe a potentially appropriate targeted oncology therapy or to direct a patient to a potentially appropriate clinical trial.
10
Table of Contents
The Opportunity for a Single, Comprehensive Molecular Information Solution
In order to harness the potential of understanding the genomic drivers of a patient’s cancer and new therapies targeted at specific genomic alterations, we believe the oncology community needs a new approach: a single molecular information platform that can assess a solid tumor or hematologic malignancy for the presence of biologically relevant genomic alterations from either a tissue specimen or a liquid biopsy. This solution would also provide assistance to physicians in matching the genomic alterations identified in their patients’ cancers with relevant available therapeutic alternatives and clinical trials.
Our molecular information platform, which includes proprietary technology, methods and computational algorithms, is the product of years of research and development and significant capital investment. Through this platform we deliver comprehensive genomic profiling to support physicians in the improvement of clinical patient care and to support biopharmaceutical companies in the development of novel cancer therapeutics. The first molecular information products enabled by our platform are FoundationOne, which is optimized for use with solid tumors, FoundationOne Heme, which is optimized for hematologic cancers, including leukemia, lymphoma and myeloma, as well as many sarcomas, and FoundationACT, which is optimized for use with liquid (blood) samples when solid tumor tissue specimens are insufficient. FoundationOne, our initial clinical product launched in June 2012, is a comprehensive genomic profile that identifies the individual molecular alterations present in a patient’s cancer tumor and matches them with relevant targeted therapies and clinical trials. FoundationOne Heme, our second commercially available product, which we developed in collaboration with Memorial Sloan-Kettering Cancer Center, or MSKCC, and launched in December 2013, is a comprehensive genomic profile that identifies the individual molecular alterations present in a patient’s blood-based cancer and matches them with relevant targeted therapies and clinical trials. FoundationACT, our third molecular information product, enables comprehensive genomic profiling from a blood sample when a tissue biopsy cannot be obtained. FoundationACT was launched to our biopharmaceutical partners for research use in December 2015, and we plan to launch to ordering physicians in March 2016.
Our Suite of Molecular Information Products Integrates Complex Insights into Routine Clinical Care
FoundationOne, FoundationOne Heme, and FoundationACT are, to our knowledge, the first commercially available comprehensive genomic profiles used in the analysis of routine cancer specimens in a clinical setting. We believe these products are the only molecular information products that can comprehensively assess cancer tissue simultaneously for all four classes of genomic alterations with sufficient sensitivity and specificity for routine medical practice. Moreover, these products deliver this complex molecular information in a contextualized report that matches detected molecular alterations with potentially relevant treatment options and clinical trials. We perform our clinical tests in our laboratory located in Cambridge, Massachusetts, which is certified under the Clinical Laboratory Improvement Amendments of 1988, or CLIA, accredited by the College of American Pathologists, or CAP, and licensed by New York, Massachusetts and other states. Optimization and automation enable workflows to deliver medical reports to ordering physicians generally within 11 to 14 days for
11
Table of Contents
FoundationOne and within 15 to 18 days for FoundationOne Heme, in each case from the time the specimen is received. We expect to deliver medical reports to ordering physicians in less than 14 days for FoundationACT once it is launched commercially.

12
Table of Contents
A Comprehensive Clinical Assessment of Relevant Alterations in Cancer Genes from Tissue Biopsies
FoundationOne interrogates the genes known to be somatically altered in human solid tumors that are validated targets for therapy or are unambiguous drivers of cancer. We have selected this set of genes based upon the advice of an international group of key opinion leaders, or KOLs, in oncology and cancer biology, input offered by our biopharmaceutical partners and an extensive review of the relevant literature. The current version of FoundationOne interrogates the entire coding sequence of 315 cancer-related genes for base substitutions, short indels, and copy number alterations, as well as select intronic regions of 28 genes commonly involved in rearrangements. FoundationOne Heme interrogates genes somatically altered in hematologic malignancies and sarcomas that are validated targets for therapy or unambiguous drivers of cancer based on current scientific knowledge. The current version of FoundationOne Heme interrogates 265 cancer-related genes for gene fusions through RNA sequencing and 405 cancer-related genes through DNA sequencing for base substitutions, short indels, and copy number alterations, as well as certain intronic regions of 31 commonly rearranged genes. FoundationOne Heme has been specifically validated to detect gene fusions, a type of alteration that is a common driver in hematologic malignancies, sarcomas, and pediatric cancers, with high accuracy. Both tests include those genes implicated in cancers for which a targeted therapy is FDA-approved and for which targeted therapies are in current or near-term clinical development. We update our tests periodically to reflect new scientific and medical knowledge about cancer biology, including newly relevant cancer genes along with those genes for which there are newly available targeted therapeutics and clinical trials.
The ability of our molecular diagnostic products to identify genomics alterations is greater than other commercially available molecular tests, in part, because we believe our products:
| • | examine the entire coding region of each gene analyzed, enabling much broader interrogation of potential alterations for each gene; |
| • | are the only molecular diagnostic products that can comprehensively assess cancer tissue simultaneously for all classes of genomic alterations; |
| • | assess samples with high sensitivity and specificity across all four classes of genomic alterations for a wide array of cancer-related genes; and |
| • | interrogate more cancer-related genes than many other molecular diagnostic tests. |
A Validated and Highly Precise Process of Testing for Tissue and Liquid Biopsies
Our proprietary methods and workflow make our suite of clinical molecular products suitable for clinical use at a commercial scale. Standard biopsies and needle biopsies obtained in a clinical setting often yield very small tissue amounts that have a low concentration of tumor cells and are preserved in a FFPE format. We have developed proprietary techniques for optimizing pre-sequencing sample preparation and have built post-sequencing computational algorithms that enable our products to be sufficiently sensitive to perform comprehensive genomic profiling on routine clinical tumor samples. We have optimized our processes to maximize throughput, efficiency, and quality.
FoundationOne has undergone extensive analytic validation that demonstrates test performance using both reference specimens and hundreds of actual FFPE clinical cancer specimens having results derived from prior standard diagnostic tests. We performed validation studies in which FoundationOne testing was conducted on previously characterized cell lines known to contain various base substitutions and cancer specimens known to contain various indels and copy number alterations to evaluate whether FoundationOne was capable of detecting these pre-defined genomic alterations. FoundationOne was found to be highly sensitive in identifying these genomic alterations even where the percentage of cells in test samples containing the alterations (versus normal cells not containing the alterations) was very low. Specifically, FoundationOne was able to detect 99% of base substitutions contained in test samples in which less than 10% of the cells contained the base substitutions, 97% of indels in samples in which 10% to 20% of the cells contained the indels, and 99% of copy number alterations of at least 8-fold in which 30% of the cells contained the alterations. In aggregate, FoundationOne detected
13
Table of Contents
greater than 99% of the genomic alterations contained in the samples tested in the validation study. We believe these results demonstrate the importance of our proprietary methods, algorithms, and advanced bioinformatics, and are helping to set the industry standards for validation of comprehensive genomic profiling. The analytic validation results of our studies on FoundationOne were published in Nature Biotechnology in October 2013.
FoundationOne Heme has also undergone extensive analytic validation for both the DNA and RNA sequencing components of the test. Test performance of the DNA component matched the high accuracy achieved by FoundationOne for all classes of genomic alterations. Test performance of the RNA component demonstrated a sensitivity to detect greater than 99% of known gene fusions at 20% tumor content and 97% of known gene fusions at 10% tumor content. The results of our analytic validation studies on FoundationOne Heme were presented at the annual meeting of the American Society of Hematology, or ASH, in December 2013, and we are in the process of submitting this validation work for publication.
FoundationACT, an assay which enables genomic profiling of ctDNA, has undergone extensive analytic validation demonstrating that the assay exceeds requirements for clinical use. Circulating cell-free DNA is highly fragmented, present at very low concentrations in the blood, and contains only a small fraction of ctDNA. We believe many cancer patients may not shed enough detectable tumor DNA into their bloodstream and may thus test negative using ctDNA assays. Therefore, the isolation and identification of ctDNA is extremely challenging. Assay sensitivity and specificity is directly dependent on cell-free DNA extraction and circulating tumor evaluation, high efficiency sample preparation, and capture efficiency combined with custom low frequency variant calling algorithms. We have optimized FoundationACT for sensitivity and specificity of base substitutions, indels, genomic rearrangements, and copy number amplifications. The workflow for FoundationACT is highly reproducible, achieves the required turnaround time, and is compatible with whole blood, plasma and cell-free DNA inputs.
At the annual meeting of the Advances In Genome Biology and Technology, in February 2016, we reported on aspects of our analytic validation study in a presentation entitled, “Assessment of the Relative Clinical Utility of ctDNA and Tissue Biopsies for the Detection of Actionable Genomic Alterations in Routine Clinical Oncology Specimens.” Our analytic validation study demonstrated that FoundationACT results were 100% concordant with FoundationOne and digital droplet PCR results across 87 base substitutions (43 at <5% mutant allele frequency), 3 indels and 5 genomic alterations. The precision and sensitivity observed with FoundationACT passed target performance specifications. We believe, based on the analysis of the literature, that we have the most accurate, highly sensitive and specific, analytically validated assay to measure ctDNA from blood. We are in the process of submitting this analytic validation work for publication.
To support our commercial efforts for FoundationACT and to give providers confidence in using a liquid biopsy assay when tissue is not available, we launched a large, multi-center study to assess the potential utility of this test across various cancers and stages of the disease as well as to refine indications for use. The study will provide additional analytic validation by establishing concordance of the test in detecting genomic alterations from ctDNA as compared to alterations detected in tissue biopsies assessed by FoundationOne. We expect to publish results from this study.
Reports Physicians Can Readily Understand and Use to Guide Patient Care
We designed our test reports, in collaboration with leading oncologists, to deliver clinically relevant information in a manner that seamlessly integrates into their practices. We present the results from our tests in a medically relevant and, we believe, practice-friendly manner that empowers physicians to make informed treatment decisions. During a period of active treatment, patients typically visit their physician every three to four weeks. We report the clinically relevant genomic alterations to a physician for use, generally within 11 to 14 days for FoundationOne and within 15 to 18 days for FoundationOne Heme, in each case from the time the specimen is received. We expect to report results in less than 14 days for FoundationACT.
The first page of the report clearly illustrates the test’s key findings. Specifically, it lists the analyzed cancer’s relevant genomic alterations and matches them with either FDA-approved therapies or open clinical
14
Table of Contents
trials for therapies targeting these alterations. The report also identifies noteworthy absences of genomic alterations typically associated with anatomical tumors of the same type. In addition, the report includes summaries of and references to supporting data from peer-reviewed publications and clinical trial information. All of the information on the report can also be accessed by ordering physicians through the newest version of our online Interactive Cancer Explorer, FoundationICE.
An Example of Page One Findings from a FoundationOne Report

| PATIENT RESULTS |
TUMOR TYPE: BLADDER
UROTHELIAL | |
| 4 genomic alterations |
Genomic Alterations Identified† | |
| 4 therapies associated with potential clinical benefit 0 therapies associated with lack of response 8 clinical trials |
NF2 Y153fs*1 ATM V2119fs*8 ATR splice site 7349+2T>C TP53 R280K | |
|
†For a complete list of the genes assayed, please refer to the Appendix *See Appendix for details |
| THERAPEUTIC IMPLICATIONS
|
|
| ||||
| Genomic Alterations Detected |
FDA Approved Therapies (in patient’s tumor type) |
FDA Approved Therapies (in another tumor type) |
Potential Clinical Trials | |||
| NF2 Y153fs*1 |
None | Everolimus Lapatinib Temsirolimus Trametinib |
Yes, see clinical trials section | |||
| ATM V2119fs*8 |
None | None | Yes, see clinical trials section | |||
| ATR splice site 7349+2T>C |
None | None | Yes, see clinical trials section | |||
| TP53 R280K |
None | None | Yes, see clinical trials section | |||
Note: Genomic alterations detected may be associated with activity of certain FDA-approved drugs; however, the agents listed in this report may have varied clinical evidence in the patient’s tumor type. Neither the therapeutic agents nor the trials identified are ranked in order of potential or predicted efficacy for this patient, nor are they ranked in order of level of evidence for this patient’s tumor type.
We deliver our test reports along with easy access to current information about the reported genomic alterations, associated therapies, and clinical trials. We intend to continue updating our Interactive Cancer Explorer with new important features and applications, such as our recently implemented PatientMatch application, as we gather feedback from our ordering clients over time.
Strong Evidence of Clinically Relevant Findings with FoundationOne
We designed our suite of molecular information products to address challenges associated with the everyday clinical management of patients diagnosed with cancer. We have experienced rapid adoption of our clinical products, and several thousand physicians from large academic centers and community-based practices have ordered FoundationOne or FoundationOne Heme.
15
Table of Contents
We believe that the following case studies illustrate the power of FoundationOne to impact treatment regimens for patients in a clinical setting.
Case Study 1: FoundationOne Heme Identifies a Clinically Relevant Genomic Fusion in Sarcmoa—Patient Receives Targeted Therapy in Clinical Trial
A 41-year-old woman presented with a firm mass in her left groin, which was diagnosed on biopsy as a sarcoma. This tumor was 10 cm in greatest dimension. Multiple nodules ranging in size from 4-13 mm were present in both lungs, and were consistent with widely disseminated disease.
After the initial diagnosis, she began an aggressive treatment plan, including enrolling in a clinical trial with a non-genomically matched targeted therapy, chemotherapy, pre-operative radiation, and limb-sparing surgery. The primary tumor was excised and demonstrated 90% necrosis after five weeks. However, imaging of her chest showed worsening metastatic disease, and the largest nodule was 18 mm. The disease continued to progress and subsequent imaging showed multiple lung nodules greater than 3 cm, with the largest nearly 7 cm, a large increase in size. A FoundationOne Heme test was administered and detected an NTRK1 fusion. Based on this information, the patient enrolled in a Phase I trial of an investigational NTRK inhibitor. Prior to enrolling in this trial, the patient had significant shortness of breath on exertion and required supplemental oxygen. Moreover, imaging showed continued tumor progression with multiple nodules in both lungs. After one cycle of the investigational NTRK inhibitor, the patient exhibited a partial response. After the fourth cycle of this therapy, the patient no longer required supplemental oxygen and imaging demonstrated almost complete tumor disappearance.
Case Study 2: FoundationOne Identifies Clinically Relevant Mutation in Endometrial Cancer—Patient is Enrolled in Clinical Trial of a Check Point Inhibitor
A 54 year old woman presented with irregular vaginal bleeding and was diagnosed with endometrial cancer. She underwent a hysterectomy and was diagnosed with an early stage but high grade endometrioid-type endometrial cancer. The patient declined to undergo radiation therapy and thereafter rapidly developed new abdominal masses, with biopsy showing recurrent cancer. She was then treated with a combination of chemotherapy and radiation therapy, and did well for two years. At that time, the patient developed bulky lymph nodes near her neck, which when biopsied showed a second recurrence of the endometrial tumor. Imaging showed the disease encased the large veins from the lower half of her body, resulting in swelling of both legs, which significantly impeded her quality of life.
The patient then enrolled in a clinical trial of a check point inhibitor. Following administration with the check point inhibitor, she rapidly felt better, as the swelling in her legs was dramatically reduced. Imaging showed a partial response at 8 weeks, and this response has been maintained for 10 months.
To understand this dramatic response, the primary tumor was sent for FoundationOne testing. There were 32 genomic alterations (GA) identified which is a high number relative to an average of 3-5 across cancers. Also, a mutation in DNA polymerase epsilon (POLE) was identified, which impairs how this enzyme corrects errors in the tumor genome. This GA could thus explain the hypermutated phenotype. This is about -5-10 times higher than the ‘average’ tumor. In addition, there were 113 “variants of unknown significance”, also a very high number relative to other cancer cases. Therefore, this patient’s tumor exhibits an extraordinarily high mutation burden consistent with the hypermutator phenotype described for POLE mutant endometrial cancers, which likely correlates to durable clinical benefit she received from a checkpoint inhibitor.
Our Platform for Biopharmaceutical Research and Development
For many of the same reasons our products provide information that is well suited for the clinical setting, our molecular information platform enhances the ability of our biopharmaceutical partners to develop targeted
16
Table of Contents
oncology therapies. We deploy our molecular information platform to analyze tissue samples provided by biopharmaceutical partners from their clinical trials, and we provide our partners access to our FoundationCORE knowledgebase to provide insight into molecular information derived from our analysis. We use our core proprietary platform testing, computational biology, and information technology capabilities to provide our biopharmaceutical partners with comprehensive genomic profiling and information relevant to precision medicine strategies for both retrospective and prospective clinical studies and other drug development activities. Our platform capabilities enable our biopharmaceutical partners to:
| • | accelerate clinical development timelines and increase the likelihood of patient response by prospectively analyzing tumor specimens to identify patients with certain genomic alterations for enrollment in clinical trials for targeted cancer therapeutics; |
| • | understand complex combinations of genomic alterations to facilitate development of companion diagnostic tests that may be necessary to bring a targeted cancer therapeutic to market; |
| • | guide usage and inform future development opportunities for experimental and marketed therapies by retrospectively analyzing clinical trial patients to stratify them as responders or non-responders based on the presence or absence of certain genomic alterations; |
| • | create opportunities for drug combination studies or new target discovery by identifying mechanisms of primary and acquired resistance; and |
| • | inform improvements to clinical trial design by contributing to the understanding of why some clinical studies did not meet their primary endpoints. |
We currently have ongoing relationships with more than 25 biopharmaceutical partners, many of which are leaders in developing targeted cancer therapies, and those relationships have expanded over time. Our publicly announced biopharmaceutical customers include Agios, ARIAD Pharmaceuticals, Inc., Array BioPharma Inc., AstraZeneca UK Limited, Celgene, Clovis, Eisai Co., Ltd., Loxo, Johnson & Johnson, Mirati, Novartis, and Roche.
In addition to customary clinical settings in which physicians prescribe an FDA-approved therapy, approximately 3% of patients with cancer in the United States are currently enrolled in clinical trials of new experimental therapies sponsored by biopharmaceutical companies. By broadening our relationships with our biopharmaceutical partners, we expect to deploy our molecular information platform for an increasing portion of patients with cancer enrolled in clinical trials both inside and outside the United States. In 2014, we announced a collaboration with WuXi PharmaTech Inc., or WuXi, who is performing the laboratory component of our tests for our biopharmaceutical partners in its laboratories in Shanghai. Our partnership with WuXi allows us to offer our comprehensive genomic profiles to biopharmaceutical companies conducting clinical trials in China. We expect our biopharmaceutical partner relationships will continue to expand and will provide more opportunities to sell our molecular information products for companion diagnostic development, research and development projects, and new target discovery and validation. In addition, we believe our strategic collaboration with Roche represents a significant opportunity to further standardize clinical trial testing across many cancer therapeutics development programs, develop additional companion diagnostic products, and grant access to our molecular information knowledgebase, FoundationCORE.
In addition to generating near and long term revenue, we believe our relationships with our biopharmaceutical partners provide us with important strategic opportunities, including enabling us to identify new genes under investigation that can be incorporated early into our molecular information platform and our products, and more broadly allowing us to actively participate in the newest oncology therapeutics and practice. For example, we are providing comprehensive genomic profiling for the Novartis Signature program, which rapidly matches patients to treatments that target their tumor’s molecular alterations and brings the trial to the patient rather than the patient traveling to a trial site. We are also participating in the Lung Cancer Master Protocol (LUNG-MAP) trial, a genomically-driven clinical trial sponsored by a unique collaboration of various
17
Table of Contents
public and private entities. We believe our activities with leading drug development companies that are focused on cancer therapeutics further our relationships with the broader oncology community, including thought leaders who are important to the adoption of our commercial products.
Market Opportunities for FoundationOne, FoundationOne Heme, and FoundationACT
We believe our suite of comprehensive molecular information products will continue to serve as a valuable tool for a greater number of physicians. Aggregating and delivering complex molecular, medical and scientific information in a single report that can be understood and acted upon has become increasingly important, especially as a majority of our test volume is driven by physicians in community-based practices rather than academic medical centers. Physicians in the community setting often see patients across a wide spectrum of cancer types, which can be particularly challenging as new data emerges that is specific to each cancer type. In 2013, approximately 30% of our test volume was driven by physicians in community practices. In each of 2014 and 2015, approximately 60% of our test volume was driven by community physicians. Approximately 80% of patients with cancer in the United States today are treated in community practice settings.
As we deploy our commercialization strategy, we continue to work with our growing network of oncology thought leaders to identify the subsets of patients with metastatic cancer for whom FoundationOne, FoundationOne Heme, and FoundationACT are most likely to positively inform treatment decisions. We are focused on driving awareness of the potential utility of our tests in these subsets of patients, defined as:
| • | patients who test negative under traditional single marker or hotspot panel tests for their tumor type, such as negative for alterations in the genes EGFR, ALK, and KRAS in non-small cell lung cancer, or NSCLC; |
| • | patients from whom a tissue biopsy cannot be obtained or who have insufficient available tissue to perform multiple hotspot panel tests, such as patients with NSCLC with very little tumor tissue left in archive; |
| • | patients for whom standard treatments have been tried and failed, such as patients whose breast cancer continues to progress despite multiple chemotherapy regimens; |
| • | patients with rare or uncommon tumors, such as certain sarcomas or non-colon/small-bowel gastrointestinal tumors, for whom no standard treatment approach exists; and |
| • | patients who have aggressive disease and failed conventional chemotherapy but who maintain adequate functional status, such as some individuals with urothelial cancers or choangiocarcincoma. |
While these groups are not mutually exclusive, we estimate that annually in the United States there are approximately one million patients who suffer from the above-described or similar cancers and who would most benefit from FoundationOne testing, and another approximately one hundred thousand patients who would most benefit from FoundationOne Heme. These estimates are based upon a combination of feedback from our network of oncology thought leaders, data published by the National Cancer Institute in the Cancer Statistics Review, and focused market research that we commissioned.
As the number of available targeted therapies expands and as physicians gain further experience using comprehensive genomic profiling in their routine treatment decisions, we believe that the potential addressable market for comprehensive genomic profiling will expand over the next five years to include most patients who have metastatic disease, not only those patients limited to the challenging treatment categories noted above. These patients may include, for example, those who are earlier in the treatment cycle, those who suffer from a broader set of disease conditions, those patients diagnosed with rare and uncommon cancers regardless of stage, and those for whom tissue is not available and who would benefit from a liquid biopsy assay. We estimate that this potential market expansion in the United States could include an additional 900,000 total patients for FoundationOne, FoundationOne Heme, and FoundationACT annually based upon the same combination of
18
Table of Contents
sources of information we used to estimate the size of the patient population we are initially targeting. Although we expect existing and future diagnostic testing providers to also target these patient populations, we believe our tests are currently the only commercially available comprehensive genomic profiles that comprehensively assess cancer tissue simultaneously for all four classes of genomic alterations across all cancer-related genes with the sensitivity and specificity required for routine medical practice. In addition, we believe that our ex-U.S. commercialization agreement with Roche provides us with expert access to global markets and an estimated additional two million patients worldwide.
Commercialization Strategy
We continue to create awareness and drive adoption of our comprehensive molecular information products through our commercialization strategy to:
| • | build an experienced, fully-integrated, oncology-focused commercial organization in the United States; |
| • | expand our footprint globally through our ex-U.S. commercial agreement with Roche; |
| • | collaborate with oncology thought leaders, leading academic institutions, and community-based oncology networks/practices on FoundationOne, FoundationOne Heme and FoundationACT clinical cases, clinical research, publications, and product development; |
| • | drive broader adoption and increased ordering frequency per physician by offering differentiated programs, ongoing product innovation, new technology tools, applications, and services such as FoundationICE, PatientMatch, GeneKit, and SmartTrials, that provide easier access to clinically relevant information and an active medical affairs team to build and support new and ongoing client relationships; |
| • | publish important medical and scientific data in top-tier peer-reviewed journals and present at major industry conferences; |
| • | demonstrate improved clinical outcomes and health economic data to support broad reimbursement from third-party and government payors for comprehensive genomic profiling; |
| • | work with patient advocacy groups and medical societies to create greater awareness of our products and the importance of incorporating molecular diagnostics into cancer treatment; and |
| • | facilitate broader access to care through our patient assistance and access programs, including FoundationACCESS, and our technology tools and applications. |
Through these efforts, we are creating greater awareness of the unique capabilities of our suite of molecular information products throughout the oncology community—from patients suffering from cancer, to the physicians treating them, to the third-party payors for these treatments and to biopharmaceutical companies developing new treatments—all with the goal of facilitating better-informed treatment decisions for the greatest number of patients with cancer. We believe that by driving physician and patient demand for FoundationOne, FoundationOne Heme and FoundationACT, and by being part of improving patient outcomes, we will continue to increase utilization of our molecular information products and obtain favorable reimbursement decisions by third-party payors.
Building an Experienced, Oncology-Focused Commercial Organization
United States
Our commercial organization in the United States works with oncologists and pathologists at hospitals and cancer centers in both the community and academic settings. We launched FoundationOne in June 2012 with a sales force of only two people. As of December 31, 2015, we have built a fully integrated commercial
19
Table of Contents
organization including sales, sales management and support, client services, marketing, and reimbursement. We will continue to grow this highly specialized, oncology-focused commercial team and support it with medical specialists who bring extensive knowledge in the design and use of molecular information products.
Our current sales efforts focus on building relationships with thought leaders at leading academic research institutions, pathologists and physicians in community practice settings, and leading physician networks to demonstrate the clinical usefulness of our molecular information solutions. We continue to optimize our partnership with ION Solutions, a diversified physician services organization whose membership represents more than half of the private practice oncologists in the United States. In 2014, we initiated our marketing services agreement that supports joint marketing efforts across the ION network. In 2015, we were selected as ION’s preferred partner for comprehensive genomic profiling services, and over the past twelve months we have experienced significant volume growth within the various ION practices. Other oncology networks, such as Cancer Treatment Centers of America, have also chosen to use FoundationOne and FoundationOne Heme.
As we continue to grow and optimize our commercial organization, and streamline ordering, data collection, and clinical trial enrollment, we will increasingly have the capacity to support community hospitals and community-based cancer centers that we believe require a reliable and collaborative partner for molecular information solutions.
In addition, we are focusing on developing relationships with pathologists so that we can better serve the pathology community by providing value-added molecular information products and technology and integration services.
Further, we believe that our U.S. Education Collaboration Agreement (as described in detail in our other public filings and below in the section entitled “Our Strategic Collaboration with Roche,”) with Genentech, Inc., a wholly owned subsidiary of Roche, or Genentech, will drive awareness of comprehensive genomic profiling and thereby supplement the education provided today by our field sales team. Genentech will develop unbranded education materials related to NGS and comprehensive genomic profiling, and provide materials and related education to clinicians and hospitals. We believe this effort will have a meaningful impact on awareness and adoption of comprehensive genomic profiling, especially among pathologists and in community-based practices. Following the execution of our strategic collaboration with Roche in April 2015, the Genentech team completed education and training on comprehensive genomic profiling, developed educational materials for use with pathologists, and they are currently executing a pathology-focused medical education strategy. We believe the addition of the Genentech medical education efforts will help to drive adoption and utilization of our suite of molecular information products.
International
Prior to the closing of our strategic collaboration with Roche in April 2015, our international sales strategy had been focused on partnering with leading distributors and building our direct selling efforts. We believe that our ex-U.S. Commercial Agreement with Roche accelerates our access to these and other global markets.
Under the terms of our ex-U.S. Commercial Agreement with Roche, Roche has the right to commercialize FoundationOne and FoundationOne Heme in all countries outside of the United States. Once we decide to commercialize FoundationACT outside of the United States, Roche also has the right to commercialize FoundationACT in all countries outside of the United States. Roche has significant experience, expertise, and local market knowledge across many regions around the world, and we believe they are uniquely positioned to understand local market dynamics, payor systems, and regulatory systems.
Our ex-U.S. commercialization agreement with Roche includes a one-year planning period during which we have worked together with Roche in 2015 to identify an initial set of countries in which Roche would begin
20
Table of Contents
commercializing our products. Roche has initially divided its ex-U.S. commercial launch into two phases. In the first phase, Roche commenced commercial activities for our tests in Israel in November 2015 and expects to launch our products in 2016 in Germany, Switzerland, Austria, Brazil and Canada.
Collaborating with Thought Leaders to Shape the New Cancer Treatment Paradigm
We believe physicians look to peers and key thought leaders in the medical community when evaluating a new technology. Oncology thought leaders have historically been early adopters of new technologies because they have greater access to new therapies, clinical trials, and diagnostic tools as compared to many community oncologists. Since our inception, our founders, medical affairs group, senior management, and commercial team have leveraged existing and built new relationships with these early adopters.
KOLs and leading cancer researchers have embraced our comprehensive molecular information approach, including oncologists at premier cancer institutions such as MSKCC, Vanderbilt-Ingram Cancer Center, and the Taussig Cancer Institute at the Cleveland Clinic. In addition to using FoundationOne and FoundationOne Heme for clinical cases, these individuals and institutions collaborate with us on clinical studies, peer-reviewed publications, and medical and scientific conference presentations. We believe our relationships with KOLs help validate our platform, drive adoption of our clinical products in community oncology settings and international markets, establish our leadership position in the field of molecular information about cancer, and thereby further our ultimate goal—to facilitate better-informed treatment decisions for the greatest number of patients with cancer.
We believe that a significant barrier to the achievement of precision medicine in oncology is that patient information is trapped in silos across the healthcare system. To facilitate cross-organizational sharing of clinico-genomic data in oncology as a means to drive better insights and improved clinical outcomes, we launched a precision medicine partner program in September 2015. The Precision Medicine Exchange Consortium™, or PMEC, facilitates data exchange, establishes an enhanced clinical trials network, advances research, and supports education and applications of precision medicine in oncology and molecular pathology. PMEC brings together oncology thought leaders from leading U.S. academic medical centers, regional hospital systems, and community oncology networks, who share a desire to improve clinical outcomes in oncology treatment through molecular profiling. The consortium intends to improve the practice of precision medicine through a collaborative exchange of molecular information and clinical outcomes data, and through a broader integration of comprehensive genomic profiling in cancer treatment.
Founding members of PMEC include The Cleveland Clinic’s Taussig Cancer Institute, Hackensack University Medical Center, The Robert H. Lurie Comprehensive Cancer Center of Northwestern University, Sidney Kimmel Cancer Center at Thomas Jefferson University, UC Davis Health Comprehensive Cancer Center, the University of North Carolina Lineberger Comprehensive Cancer Center, and Vanderbilt-Ingram Cancer Center, The Wake Forest Baptist Comprehensive Cancer Center and the Medical College of Wisconsin / Froedert Cancer Center. We believe this consortium can significantly advance precision medicine in cancer and we believe our role in its formation is a differentiator for our business and will potentially enable us to drive increased adoption and utilization of our products.
We believe the creation of PMEC and the associated, planned establishment of research opportunities, clinical trials, tracking outcomes, and sharing of clinico-genomic information, will help drive increased utilization of our suite of molecular information products, showcase us as a reliable and collaborative partner to community-based providers and academic medical centers, and further strengthen our position as thought leader in cancer.
Our relationships with KOLs in oncology have been instrumental in driving the adoption of FoundationOne and FoundationOne Heme. We believe awareness of FoundationOne within the community oncology setting was largely created through our publications and presentations with KOLs and the resulting peer-to-peer interaction
21
Table of Contents
they generated. We will continue to nurture these relationships with thought leaders as we drive adoption of FoundationOne, FoundationOne Heme and FoundationACT, and as we develop new, innovative products that enable providers to deliver the best cancer care.
Promoting Physician Interaction and Creating a Network Effect
We believe that if we can continue to integrate the results of our products into the everyday clinical practice of oncologists, we will become an even more important partner in their efforts to treat patients with cancer. Our goal is for physicians to use the latest version of our Interactive Cancer Explorer, FoundationICE, in the context of their busy clinical practices, to shape each patient’s treatment plan. Through FoundationICE we deliver the key genomic information identified by our tests in an organized fashion along with access to current information about the reported genomic alterations, associated therapies, and clinical trials. FoundationICE presents complex genomic information in what was designed as a practice-friendly interface that links directly into publicly available databases, such as PubMed and clinicaltrials.gov. The portal also provides direct links or references to journal articles and clinical trials information relevant to a patient’s identified genomic alterations. Over time, we intend for FoundationICE to link to additional public and private data sources as we continue to rationalize, correlate, and incorporate disparate sources of information into our products. By making this information more readily accessible to physicians, we aim to make it easier for them to bring new, relevant information to each patient’s treatment plan.
An important new component of FoundationICE is PatientMatch, a technology application that leverages the experience of more than 68,000 genomic profiles already in FoundationCORE to increase the utility of our test results. Using PatientMatch, physicians within the FoundationICE network are able to connect with other physicians treating patients with similar disease profiles (tumor type and genomic alteration(s)) and share treatment and outcome information in a HIPAA-compliant manner. FoundationICE also has a number of important efficiency-improving features to help physicians care for their patients, including the ability to track samples through our pipeline before we report the results and in-application notifications. Another new feature in FoundationICE is advanced search, which allows physicians to search across all of their patients by gene, alteration, and tumor type. Practice administrators and researchers can use this same capability to query patients across their entire institution. We believe FoundationICE and PatientMatch will be important applications to support and accelerate the broad adoption of our products, build client relationships, and create an important network effect among users. We believe the better we can integrate our solutions into a physician’s routine clinical practice, including through EMR integration, the more likely a physician is to order our products.
Supporting Adoption through Publications and Clinical Trials
We believe the successful completion of multiple clinical trials, our publication of scientific and medical results in peer-reviewed journals, and presentations at leading conferences are critical to the broad adoption of products enabled by our proprietary platform. Our publications and presentations to date have helped communicate our capabilities and the clinical results that early adopters of our platform have achieved. We will continue to use these channels to drive commercial adoption of our products and obtain favorable reimbursement decisions.
Since the beginning of 2012, we have had:
| • | over 100 peer-reviewed articles published or accepted for publication, including by Nature Medicine, Nature Biotechnology, Clinical Cancer Research, Cancer Discovery, Cancer Research, Clinical Breast Cancer, Hematology & Oncology, American Journal of Surgical Pathology, Journal of Clinical Oncology, Journal of Thoracic Oncology, Blood, and Genome Medicine; |
| • | over 175 poster presentations based on clinical and research data that have been accepted and presented at major scientific conferences on themes that include the identification of multiple novel actionable drug targets, known drug targets in novel tumor types, novel resistance mechanisms to targeted therapies, new insights into models of metastasis, and novel hypotheses on the molecular basis of response or resistance to certain targeted therapies; and |
22
Table of Contents
| • | more than 75 speaking presentations at scientific meetings such as ASCO, American Association of Cancer Research, San Antonio Breast Cancer Symposium, US and Canadian Association of Pathology, or USCAP, Advances in Genome Biology and Technology, or AGBT, and ASH, among others. |
We have a number of clinical trials sponsored by us and clinical trials sponsored by individual physicians, or investigator-initiated clinical trials, underway or completed, such as:
| • | The MD Anderson Randomized Phase III Trial. This ongoing study aims to compare the progression free survival of individuals who receive a targeted matched therapy based on FoundationOne testing versus those who receive an empiric therapy without knowledge of the FoundationOne results. |
| • | Our Study with Horizon Healthcare Services and Clinical Outcomes Tracking and Analysis (COTA). This is a prospective clinical study measuring changes in survival benefit and total cost savings achieved among patients with previously untreated metastatic NSCLC who undergo comprehensive genomic profiling with FoundationOne versus another molecular test. The study data is designed to enable Horizon Healthcare Services, Inc., or Horizon Healthcare Services, to provide its members with coverage for FoundationOne as a critical component of clinical care pathways in the evaluation of patients with metastatic lung cancer. |
| • | Prospective CUP trial. This is a prospective, ongoing, single arm observational study of FoundationOne-directed targeted therapy in newly diagnosed and previously treated patients with cancers of unknown primary origin, or CUP. The primary feasibility objective of this study is to determine the utility of comprehensive molecular profiling using FoundationOne to identify molecular abnormalities in CUP patients and direct therapy with matched targeted agents. The primary efficacy objective is to demonstrate an improvement in overall survival in a predefined subset of CUP patients receiving matched targeted therapy based on FoundationOne testing as compared to a historical control. |
| • | Circulating Tumor DNA clinical study. This ongoing multicenter study is a clinical validation study designed to identify the clinical settings for which FoundationACT can be indicated and explore the concordance of genomic alterations between primary/metastatic surgical resections/biopsies and ctDNA in multiple solid tumor types. |
| • | Our Study with MSKCC on FoundationOne Heme. In this study, MSKCC and our researchers used FoundationOne Heme to analyze routine cancer specimens from 746 patients with a range of hematologic malignancies, including leukemia, lymphoma and myeloma. The data from the study, which was presented at ASH’s annual meeting in December 2014, demonstrated the feasibility of comprehensive genomic profiling in patients with hematologic malignancies. FoundationOne Heme was shown to enable the identification of genomic alterations that are molecular drivers of the observed patients’ cancers which may help to expand and inform treatment options for individual patients, advance the development of new therapies against these targets, and ultimately improve patient outcomes. In addition, robust analytic validity was demonstrated. Publication of the associated manuscript is anticipated later this year. |
| • | The MD Anderson Prospective Study. This completed study aimed to compare the clinical outcomes of patients who are treated with targeted therapy after testing with FoundationOne compared to historical outcomes for patients treated with chemotherapy. The study was designed to enroll a group of 300 patients with advanced solid tumors who were to be screened at enrollment with FoundationOne. These patients were then treated with a targeted therapy selected on the basis of the FoundationOne report. We presented an abstract describing the initial findings from this study at ASCO’s annual meeting in June 2015 which demonstrated improved outcomes across a variety of important clinical endpoints. Publication of the associated manuscript is anticipated later this year. |
23
Table of Contents
| • | The FoundationOne Registry. The objective of this study was to better understand the impact of FoundationOne on a clinical population including, importantly, how physicians act on the results and how the results impact care and outcomes. Enrollment of the initial 500 patient study was completed in July 2014, and the study is currently in the final stages of data analysis. |
| • | The US Oncology Decision Impact Study. This study assessed the impact of FoundationOne on physician decision-making in a real world setting. FoundationOne was performed on solid tumors from 300 patients during their second or later line of therapy. When the patient’s cancer progressed, the impact of FoundationOne in switching a physician’s recommended next course of treatment was evaluated. The study demonstrated that FoundationOne testing resulted in an altered therapeutic choice in 28 percent of advanced solid tumor patients. |
Engaging With Patient Advocacy Groups and Other Important Stakeholders to Drive Awareness
We have established relationships with many patient advocacy groups to drive awareness of our products and to educate the advocacy community and other key stakeholders, including major medical societies and networks, about the shifting oncology paradigm towards precision medicine.
Patient advocates are important stakeholders in the cancer community because they have influence within the patient community and with health care providers, KOLs, and policy makers. We established our advocacy relations program with the following goals:
| • | develop awareness around genomic testing; |
| • | position us as a patient-centered company within the patient community by creating goodwill and becoming a trustworthy corporate partner; |
| • | effectively shape the dialogue around cancer genomics with key constituents; and |
| • | work with advocates to help increase genomics conversation and drive the use of molecular information testing. |
We have successfully established key relationships to help educate advocates about us and our capabilities in oncology. Some of the organizations we engage with include Friends of Cancer Research, Patient Advocate Foundation, Clearity Foundation, American Cancer Society Cancer Action Network, Lung Cancer Foundation of America, Bonnie J. Addario Lung Cancer Foundation, Uniting Against Lung Cancer, Pancreatic Cancer Action Network, and many others. We continue to engage representatives from several patient advocacy organizations, establishing our commitment to understanding patients’ needs and positioning us as a neutral facilitator of oncology stakeholders, with important insight and relationships across industry, advocacy, and regulatory bodies. Through these activities, we have developed the basis for a meaningful advocacy relations program, with opportunities to more strategically engage advocates moving forward. During 2014 and 2015, we worked with 16 lung cancer advocacy groups in a campaign called “Don’t Guess. Test.” to raise awareness about the importance of comprehensive genomic profiling for patients with lung cancer. We expect to continue similar efforts in 2016 and beyond.
Our relationships with other influential organizations that shape the delivery of care are also critical as we work to develop and educate the market. We currently work with and aim to work with many organizations, including the National Comprehensive Cancer Network, ASCO, USCAP, CAP and others regarding the role of NGS and broader molecular profiling in the evaluation of patients and their tumors. We are working with these organizations both on potential educational initiatives as well as the evolution of guidelines, which today are very much tumor-type specific, to recognize the growing importance of the molecular characterization of the collection of diseases known as cancer.
Driving Adoption by Enabling Broader Access to Care
Adoption of comprehensive genomic profiling by academic and community-based oncologists supports the clinical utility required to improve health outcomes and quality of life for select groups of patients that can
24
Table of Contents
benefit from targeted therapy treatment. However, it can sometimes be difficult for patients and their physicians to gain access to these potentially life-saving treatments. In 2015, we strengthened our offerings to provide a suite of patient-centric services that we call FoundationAccess that support increased utilization of comprehensive genomic profiling and enable access to targeted therapies.
Together with EmergingMed, we launched an innovative patient-centric program called FoundationAccess Trial Navigator through which we are offering personalized clinical trial navigation services to help health care providers and their patients who have received our tests to identify and access appropriate clinical trials based on their specific genomic profiles and clinical characteristics. We believe these clinical trial enrollment support services will increase the ability of physicians to act on comprehensive genomic profiling results and more frequently consider clinical trial enrollment while making treatment decisions. Additionally, through FoundationAccess Careline, which we offer through our partnership with the Patient Advocate Foundation, we provide personalized case management services to patients who are uninsured, underinsured or insured, but who are facing obstacles obtaining prescribed targeted therapies that are approved in an indication other than their tumor type. We help these patients navigate appropriate channels to secure treatments by leveraging peer-reviewed literature supporting their use, as well as the reimbursement system to help cover treatment costs and the costs associated with our tests. We believe this will increase the ability of physicians to act on our test results. Further, through FoundationICE and PatientMatch we are helping to connect physicians with their peers who have seen patients with similar genomic profiles in order to learn about their peers’ treatment decisions and related outcomes. Lastly, through our collaboration with Roche, we are uniquely positioned to deliver access programs when the results of our comprehensive genomic profiles reveal molecular alterations that can be treated by one of Roche’s oncologic agents. In these situations, we offer drug access support, financial assistance programs, and clinical trial connectivity and enrollment support in close collaboration with Roche. We believe this patient-centric service is unique to our business and gives patients access to targeted anti-cancer agents from one of the leading drug developers in cancer. Each of these areas of focus provides tangible steps our clinical partners can take to enhance the actionability of the results of our comprehensive genomic profiling assays.
Payment and Reimbursement for Our Molecular Information Products
The principal groups that currently pay us, or that we expect to pay us in the future, for our molecular information products include:
| • | our biopharmaceutical customers, with whom we have individual agreements; |
| • | certain hospitals, cancer centers, and other institutions that pay us directly at negotiated rates for their physicians’ test orders; |
| • | international patients and distributors who pay us directly at agreed-upon prices; |
| • | commercial third-party payors who currently pay us based on Current Procedural Terminology, or CPT®, codes; |
| • | government payors, including Medicare, to whom we have been submitting claims, and state Medicaid plans; and |
| • | patients who make self-payments, or co-payment, co-insurance or deductible payments and other amounts that we have been unable to collect from their third-party payors. |
We believe that our products present unique solutions for commercial third-party payors and government payors who are faced with an increasingly complex and dynamic cancer diagnostic and treatment environment. These complexities include a growing number of single-marker and hotspot panel tests, the increasing number and cost burden of targeted oncology therapies, and an underlying shift in physicians’ treatment of cancer that is based on molecular pathways rather than tumor location. In addition, this shifting treatment paradigm comes at a time when commercial third-party payors and government payors are increasingly making significant efforts to contain healthcare costs. We believe the use of our products aligns with payors’ goals to improve the safety, efficacy, and affordability of cancer diagnosis and treatment.
25
Table of Contents
Adequate reimbursement is an important factor in achieving broad clinical adoption of our molecular information products. At the same time, we believe broad clinical adoption will help drive favorable reimbursement decisions. To achieve broad reimbursement coverage with commercial third-party and government payors, including Medicare and Medicaid, we are focused on demonstrating the economic and clinical value of our products to payors by:
| • | Setting a High Bar for Validation and Performance. Our tests provide clinically relevant results that are highly reproducible and sensitive, and we believe that a majority of our relevant findings would not be detected by many other commercial tests on the market today. Patients may benefit from our detection of otherwise unknown genomic alterations that can lead to their physicians choosing alternate therapies. We have presented data on the reproducibility, sensitivity, specificity, and comprehensive scope of our products at numerous conferences and in peer-reviewed journals. In October 2013, the analytic validation results of our studies on FoundationOne were published in Nature Biotechnology. In 2014, we received approval from the New York State Department of Health for FoundationOne and FoundationOne Heme, which represents a high standard for quality in large-scale, multi-gene sequencing-based testing in cancer. Also in 2014, Palmetto GBA, or Palmetto, an influential Medicare Administrative Contractor, or MAC, published validation guidelines for NGS tests. In May 2015, Palmetto published a final local coverage determination, or LCD, that outlines the rigorous validation requirements for coverage and reimbursement of comprehensive genomic profiles in certain forms of cancer, and we believe our products meet those validation criteria. |
| • | Increasing Utilization by Driving Repeat Physician Demand. Several thousand physicians from large academic centers and community-based practices have ordered FoundationOne or FoundationOne Heme since their respective formal commercial launches in June 2012 and December 2013. The expansion of our commercial organization is driving volume growth by strengthening customer relationships with academic and community-based oncologists. We believe that this adoption, including repeat usage, demonstrates growing physician demand for a comprehensive solution to help in the treatment of their patients. In addition, we believe that increasing adoption and consistent utilization of our products and their impact on improving outcomes for patients with cancer will lead to favorable reimbursement decisions. |
| • | Engaging Key Members of the Oncology Community. We will continue to work with oncology thought leaders, professional societies, patient advocacy groups, and cancer networks. We believe these relationships help validate our platform, drive adoption of our products in the broad community oncology setting, and establish our leadership position in the field of molecular information about cancer. In addition, we believe adoption of our products by key members of the oncology community will help to influence the inclusion of FoundationOne, FoundationOne Heme, and FoundationACT in practice guidelines as well as coverage decisions by commercial third-party payors and government payors. |
| • | Publishing in Peer-Reviewed Publications. We seek to publish in scientific and medical journals such as Nature Medicine, Nature Biotechnology, Journal of Thoracic Oncology, Cancer Discovery, Clinical Cancer Research, Blood, Hematology & Oncology, and others. Our publications have covered novel scientific findings, clinical utility of test results, individual patient outcomes, and common traits of genomic alterations in primary and metastatic tumors, among many others. We believe that our approach, which we have designed to be rigorous and data-driven, is important in establishing requisite evidence of the clinical and analytical validity and clinical utility of our products with payors. |
| • | Demonstrating Clinical Utility. To demonstrate the impact of our products on physician treatment decisions and patient outcomes, we have conducted and will continue to conduct a number of clinical studies with organizations such as US Oncology, MD Anderson Cancer Center, MSKCC, and other leading academic and community-based medical centers. We are also enrolling patients into a registry through which we will track changes in physician treatment patterns as well as longitudinal patient outcome data for patients with cancer of an unknown primary origin. |
26
Table of Contents
| • | Improving Health Economics. We have built economic models to measure the financial benefits of using our products in guiding patient treatment by selecting targeted therapies for each patient and for minimizing the use of drugs that will not likely have a positive impact. Additionally, we launched several health economics studies in 2015. We plan to use the data we gather through the use of these models and studies in our discussions with commercial third-party payors and government payors. |
We are initially focused on obtaining reimbursement coverage from third-party payors for patients in six specific clinical indications, including (1) patients with newly-diagnosed stage IV NSCLC, (2) patients with newly-diagnosed cancer of unknown origin or unknown primary anatomic site, (3) patients with newly-diagnosed stage IV rare or uncommon solid tumors for whom very limited or no systemic treatment options exist in clinical care guidelines or pathways, (4) patients with newly-diagnosed stage IV solid tumors who have received limited to no benefit from standard chemotherapy and whose tumors typically include a high prevalence of clinically relevant genomic alterations, (5) patients with solid tumors whose available biopsy sample proves insufficient for traditional molecular diagnostic testing, and (6) patients with stage IV solid tumors who have exhausted the established guideline driven systemic therapy or therapies but who maintain adequate functional status as deemed by their treating physician. After obtaining reimbursement coverage for one or more of these six initial clinical indications, we may seek broader coverage for additional indications.
In October 2014, we announced a formal agreement with Priority Health, a regional commercial third-party payor in Michigan, for broad coverage of FoundationOne and FoundationOne Heme. We believe this was an important initial coverage agreement given that it includes both of these products and covers five of the six initial clinical indications noted above for FoundationOne. We also publicly announced that HealthChoice Oklahoma and one additional regional payer in southern California had each enacted broad, pan-cancer coverage policies for FoundationOne and FoundationOne Heme for their members. Several large, self-insuring organizations also enacted coverage as part of broad, best-in-class employee benefits packages. In December 2015, we announced an agreement with UnitedHealthcare, one of the largest insurers in the United States. The agreement covers FoundationOne for patients with initially diagnosed metastatic stage IV NSCLC. We believe this agreement is another important step towards broader reimbursement for our comprehensive genomic profiling assays as we continue to demonstrate clinical and economic evidence supporting coverage and payment for our products in additional cancer types.
In May 2015, Palmetto published a final LCD, which includes reimbursement for comprehensive genomic profiles for highly validated testing in an initial subset of patients diagnosed with NSCLC. We believe this is an important first step towards broader coverage, and we believe Palmetto is considered a key thought leader among several other MACs.
We believe that demonstrating value in clinical outcomes and healthcare economics will be important drivers of broad reimbursement coverage for our molecular information products. In January 2016, we announced a three-way collaboration among us, Horizon Healthcare Services, New Jersey’s oldest and largest health insurer, and COTA to initiate a prospective clinical study measuring changes in survival benefit and total cost savings achieved among patients with previously untreated metastatic NSCLC who undergo comprehensive genomic profiling with FoundationOne versus other molecular testing or no testing. The study enables Horizon Healthcare Services to provide its members with coverage for FoundationOne as a critical component of clinical care pathways in the evaluation of patients with metastatic lung cancer. We believe this is a model that could potentially support coverage and payment agreements with other third-party payors.
The reimbursement environment is evolving as regulators and payors try to establish new rules and frameworks for the reimbursement of molecular diagnostic tests. Our continued ability to participate in the development and implementation of those rules and frameworks is important in order to differentiate between our products and other third-party tests in the marketplace.
27
Table of Contents
The current list price for the FoundationOne test is $5,800, and the current list price for the FoundationOne Heme test is $7,200. The list price for FoundationACT will be released when we commercially launch this test, which we expect to do in March 2016. Payment for the tests is not certain and may come from various sources. Actual payment will often be less than the list prices. Sources of current or potential payment include: (1) commercial third-party payors, such as health insurance or managed care plans; (2) government health benefit programs such as Medicare and Medicaid; (3) other healthcare providers, such as hospitals, accountable care organizations, cancer centers, and other institutions; (4) international distributors; and (5) individual patients.
Currently, the significant majority of commercial third-party payors reimburse our claims based upon stacked CPT codes, the predominant methodology, or based on other methods such as percentages of charges or other formulas that are not made known to us. In addition, a small portion of payors outsource our claims to preferred provider organizations or third-party administrators, who process our claims and pay us directly at negotiated rates. Coverage and payment is determined by the third-party payor on a case-by-case basis.
As of December 31, 2015, we were not a participating provider in any state Medicaid program, and, therefore did not have coverage decisions under which our tests were covered by these Medicaid programs. We are a participating provider in the Medicare program, but, as described below, currently no specific Medicare national or finalized local coverage determinations in our region for FoundationOne or FoundationOne Heme with established payment rates have been issued.
We do have agreements in place with various healthcare providers and with international distributors pursuant to which we process FoundationOne and FoundationOne Heme tests on specimens submitted, and the providers or distributors pay us for the test results based on negotiated rates. Those rates vary but are less than our list prices. We may also negotiate rates with individual patients, if the patient is responsible for payment.
We submit claims for our products to commercial third-party payors using CPT codes for molecular testing services. We receive payments on some, but not all, of our claims submitted. In 2014, new CPT codes were approved by the American Medical Association, or AMA, that are intended to classify with greater specificity those molecular tests that rely on NGS and evaluate multiple genes at once. These new CPT Codes became effective on January 1, 2015. We do not believe that these new CPT codes describe our tests since they do not appropriately differentiate our comprehensive genomic profiles from hotspot panel tests. Therefore, unless payors specifically request use of these new CPT codes, we intend to continue submitting claims for our tests utilizing methodologies that we believe are more effective under the circumstances, including stacked CPT codes, McKesson Z-Codes (as described below), and other potential approaches.
Since we are not currently a participating provider with many commercial third-party payors, payment for our test remains largely uncertain. We request that physicians discuss with their patients the potential responsibility of the patient for the cost of our products should the patient’s insurance policy not cover those costs. We undertake the primary responsibility for obtaining third-party reimbursement on behalf of patients, including appeals for any initial denials, prior to billing a patient. With this practice established, we believe that most patients receiving our tests know that they may be responsible for some portion of the cost of the test should their third-party payor deny or limit coverage. We also offer a comprehensive patient assistance program, FoundationAccess, to support patients whose incomes are below certain thresholds and to allow for extended payment terms, as necessary, given the patient’s economic situation.
We are enrolled in the Medicare program in order to bill Medicare for the tests we perform for the benefit of Medicare patients. There is currently no national coverage determination in effect or under consideration to our knowledge that provides for or contemplates a consideration for coverage of our tests by Medicare. In the absence of national coverage determinations for our tests, MACs have some discretion in determining coverage, and, therefore, payment for our tests is based on using an LCD process. When FoundationOne was launched in 2012, our then-current MAC requested that we not submit claims for services provided to Medicare patients to allow our MAC time to assess the appropriate coding, coverage and payment for FoundationOne. To
28
Table of Contents
accommodate this request, we deferred the submission of claims until November 2013, when we initiated the process of submitting claims to National Government Services, for FoundationOne for Medicare patients with dates of service on or after November 1, 2013. We began submitting claims to National Government Services for FoundationOne Heme in December 2013 immediately upon launch of this product.
The claims submitted to National Government Services use a miscellaneous CPT code, which is used for submitting claims for services or procedures that do not have specific CPT codes to provide the means of reporting and tracking services and procedures until a more specific code is established. We have not yet received any payments from Medicare for the claims that have been submitted with that miscellaneous code. The claims submitted to National Government Services may be denied or paid, in whole or in part. If a claim is denied or paid in part, we may decide to appeal the denied claim or any denied portion of the claim. National Government Services may also issue a non-coverage determination for any or all of our products that would apply to future claims, or National Government Services may defer processing or paying a claim pending the issuance of a coverage determination for any or all of our products. Once a coverage determination is made, we will have the opportunity to submit additional materials to National Government Services in support of an adjustment to such determination, if needed.
In February 2016, National Government Services announced a final LCD, effective in April 1, 2016, which includes reimbursement for hotspot panel tests of 5 to 50 genes for patients with metastatic NSCLC. We do not believe this LCD reflects coverage for our validated comprehensive genomic profiling products. We will continue to engage National Government Services to pursue coverage and reimbursement for comprehensive genomic profiling, and given the multiple potential outcomes that could result from the submission of our claims to National Government Services, we may determine to provide appropriate advance notices to patients covered by Medicare to enable us to bill a Medicare patient for all or part of a claim that is denied coverage by National Government Services. Based on the response of National Government Services to our submitted claims, we will also assess our ability to submit claims to Medicare, or bill Medicare patients, for previously reported tests. If we submit claims for these tests, and if Medicare denies coverage for these previously reported tests, our ability to bill Medicare patients for such tests will be limited.
To assist payors in making reimbursement and coverage decisions, the McKesson Diagnostics Exchange™, or McKesson DEX, has been created. Tests that are registered with McKesson DEX are assigned, based on the uniqueness of each test, a five-digit alphanumeric McKesson Z-Code™ identifier, or Z-Code. These identifiers are intended to enable the consistent identification of tests across systems by laboratories, providers, and payors. Because we believe the current CPT codes associated with our tests do not adequately address the comprehensive and precise characteristics of FoundationOne and FoundationOne Heme, we have established unique Z-Codes for FoundationOne and FoundationOne Heme, and expect to establish a unique Z-code for FoundationACT.
Palmetto, which currently plays a leading role in in many states about coverage determinations for complex molecular tests, has established the Molecular Diagnostic Services program, or MolDX, which is currently used to help determine coverage and payment for Medicare in 17 states. Z-Codes have been adopted by Palmetto as part of MolDX.
The AMA is responsible for establishing CPT codes. In 2013 the AMA began working with McKesson DEX to map Z-Codes to CPT code sets in a project known as CPT CodeBridge™. We expect the map between CPT code sets and Z-Codes to facilitate coverage and payment decisions by payors, but the CPT CodeBridge project is still in process, and it is unclear how this project will impact payors or the reimbursement of our tests.
Investing in Ongoing and New Product Innovations
We were founded as a scientifically and medically driven company and are dedicated to ongoing innovation in our molecular information platform and commercial product pipeline. We have invested, and will continue to
29
Table of Contents
invest, significant time and resources into the improvement of our platform and the development and introduction of new products. We are focused on new clinical products and on new technologies and features that will increase the actionability, connectivity and utilization of our clinical products.
We believe we have a first mover advantage in offering comprehensive genomic profiling products that interrogate with precision the genes known to be altered in human cancer. Since our formal commercial launch of FoundationOne in June 2012, we have continued to invest in its improvement, including periodic updates to increase the number of relevant cancer-related genes, decrease tissue size requirements, increase sensitivity, and improve turn-around time. We have incorporated RNA-based sequencing technology into FoundationOne Heme to identify additional gene fusions commonly found in hematologic malignancies and sarcomas. We have also innovated and launched a third assay, FoundationACT, which enables highly accurate molecular profiling from a blood sample when a tissue biopsy is not available or accessible.
We endeavor to stay at the cutting edge of molecular information and genomic testing and to maintain our advantages by continuously exploring and developing new clinically relevant approaches to improving cancer care. Our ongoing research and technology development efforts to advance our product pipeline and expand the impact of molecular information for improving cancer care include:
| • | developing new products to evaluate and monitor cancer progression over time, including products that enable validated testing of circulating tumor cells; |
| • | building and validating our QSR laboratory to pursue our companion diagnostics and FDA-regulated products strategy; |
| • | enhancing our understanding of markers of response to targeted therapies and immunotherapies, and researching immuno-diagnostic assays; |
| • | enhancing our Interactive Cancer Explorer and developing decision support applications such as PatientMatch, GeneKit, and SmartTrials; and |
| • | creating advanced search capabilities within FoundationCORE and across the public domain to enhance the utility and value of our aggregated datasets. |
FoundationICE and PatientMatch
Our online Interactive Cancer Explorer, the latest release of which is FoundationICE, allows physicians to access the key genomic information identified by our products along with current information about the reported genomic alterations, associated therapies, and clinical trials. Interactive Cancer Explorer presents complex genomic information in what was designed as a practice-friendly interface that links directly into publicly available databases, such as PubMed and clinicaltrials.gov, to provide additional information relevant to a patient’s identified genomic alterations.
FoundationICE, launched in December 2014, includes a new feature called PatientMatch, which is an application that leverages more than 68,000 genomic profiles already stored in the FoundationCORE knowledgebase. Using PatientMatch, physicians within our network are able to connect with other physicians treating patients with similar genomic profiles to share treatment and outcome information in a HIPAA-compliant manner. We believe FoundationICE and PatientMatch are important applications to support and accelerate the broad adoption of our products, and to create an important network effect among users. We anticipate building additional features in FoundationICE to continue to improve efficiency of patient care and the actionability of our tests, including genomic dashboards, and integration with FoundationACCESS Careline and FoundationACCESS Trial Navigator, our clinical trial matching service collaboration with EmergingMed.
FoundationCORE
The increasing availability and understanding of molecular information about cancer is driving a revolution in treating the entire class of diseases. FoundationCORE is our knowledgebase that includes genomic data,
30
Table of Contents
medical and scientific literature, clinical trial information, and a small but growing amount of clinical outcome information. We believe that FoundationCORE, which is driven by our molecular information platform, positions us at the nucleus of this new treatment paradigm in cancer care.
Some of our biopharmaceutical partners currently utilize FoundationCORE to further refine their clinical trial design and drug discovery and development activities. For example, they use this data to better understand the prevalence of certain genomic alterations, or combinations thereof, across various cancer types or patient profiles. FoundationCORE powers FoundationICE, PatientMatch, and GeneKit, enabling oncologists and pathologists in clinical practice to utilize this aggregated data set to collaborate and share treatment choices, response rates, and other clinical information in a HIPAA-compliant manner. Over time, we plan to expand our capacity to capture, aggregate, analyze, and facilitate the broader exchange of genomic data across the global oncology community. Examples of such initiatives include our collaborations with Flatiron Health and COTA, each of which is anticipated to yield broader clinical datasets that will reside in FoundationCORE. We believe FoundationICE will eventually create a network effect as more data is shared among physicians, thereby driving further utilization and ultimately leading to a larger and more impactful dataset.
We believe that our molecular information platform will continue to add to the collective knowledgebase of cancer biology and clinical practice and potentially contribute to advancements in the treatment of cancer by:
| • | creating additional utility for our physician customers by delivering new potentially actionable information through FoundationICE; |
| • | informing data-driven patient care decisions; |
| • | providing molecular epidemiology for novel and known targets for target validation; |
| • | identifying known drug targets in novel tumor types; |
| • | identifying novel resistance mechanisms to targeted therapies; |
| • | discovering new insights into the biologic basis of metastasis; |
| • | illuminating new cancer targets, including determining the role of genomic variants of previously unknown significance; |
| • | enabling combination therapy; and |
| • | enhancing clinical trial design. |
We expect the importance of our molecular information strategy will increase with the number of our patient cases and as we augment FoundationCORE with additional clinical data. If we, in conjunction with our partners, oncologists, pathologists, biopharmaceutical companies, and academic researchers, can successfully capture and utilize this data, we believe we can continue to play an even more integral role in transforming care for the millions of patients suffering from cancer.
Operations
Composition of FoundationOne, FoundationOne Heme, and FoundationACT Analyses
We currently perform all of our tests in our clinical laboratory located in Cambridge, Massachusetts. When a physician orders our products, he or she does not need to alter the standard surgical technique or tissue handling processes. The physician’s staff typically completes an order form (by hand, electronically, or via electronic medical records technology), packages the specimen in a kit we provide, and then ships the kit via overnight carrier. Once we receive the specimen at our laboratory and enter all pertinent information about the specimen into our clinical laboratory information management system, we prepare the specimen for testing. Each FoundationOne, FoundationOne Heme and FoundationACT analysis consists of three parts: specimen preparation, sequencing, and data analysis.
31
Table of Contents
Specimen Preparation
For FoundationOne, where samples are submitted as FFPE blocks or slides, our first step is pathology review, in which we assess the quality of the tissue sample to determine if it is suitable for testing using FoundationOne. We are able to process samples for testing using a very small amount of DNA. In general, the sample must be at least 40 microns in thickness and consist of at least 20% tumor cells. Almost all samples meeting our tissue requirements will allow extraction of enough high-quality DNA (50 nanograms) for FoundationOne for solid tumor analysis.
For FoundationOne Heme samples submitted as FFPE blocks or slides, the process is exactly as outlined for FoundationOne above except that we extract both DNA and RNA from these specimens, 50 nanograms and 300 nanograms, respectively, and the requisite tissue thickness is 80 microns. For FoundationOne Heme samples that are received as fresh peripheral blood or bone marrow aspirate, we prepare a smear and perform a morphologic review in order to confirm adequate cellularity and tumor/lesional tissue content (e.g., 20%) before the specimen is sequenced.
Following FoundationOne test ordering, pathology review, and DNA extraction, the extracted DNA is broken down into small fragments which we then manipulate using standard and molecular biology techniques, some of which represent our trade secrets and know-how, to create a complex mixture of DNA molecules. We then separate DNA fragments from the relevant cancer genes through our proprietary hybrid capture process. After hybrid capture, we are ready to interrogate the DNA content to determine where the critical genomic alterations exist.
The DNA component of the FoundationOne Heme test is identical to FoundationOne described above, but the RNA component has an additional step where the RNA must first be converted into cDNA. Once in this state, the RNA component of the FoundationOne Heme test progresses side by side with the DNA component.
Sequencing
The content of each DNA or cDNA molecule is determined using a process called sequencing in which sequences of DNA molecules, or nucleotides, are identified in every position of every molecule. NGS involves the massively parallel sequencing of DNA or RNA isolated from human cells that, in the context of cancer, can be applied to genes throughout the entire cancer genome. Our products are able to detect genomic alterations that may be present in as low as 1% of all cells being tested. We have made substantial modifications to our process in order to maximize throughput, efficiency, and quality based upon the NGS technology we currently use that is supplied by Illumina, Inc., or Illumina.
Data Analysis
At the end of the sequencing process, we have identified the entire coding regions of the relevant genes, and that data is entered into a sophisticated series of our proprietary computational algorithms designed to detect and identify all genomic alterations present in the cancer sample.
Sequence analysis involves a careful alignment of every DNA sequence with a known reference sequence. We have validated our algorithms that perform this alignment by running tens of thousands of samples through the process. Once all DNA sequences are aligned against the reference and quality filters have been applied, specific algorithms search for differences between the sequenced DNA and the reference. These differences represent potential genomic alterations.
Not all of the genomic alterations that are detected are responsible for driving the cancer; therefore, we further distill the alterations to a point where we have a list of only those alterations that are cancer drivers and where there is a therapy, FDA-approved drug, or available clinical trial for which the patient is eligible based on the genomic characteristics of his or her sample. A qualified computational biologist further scrutinizes identified alterations to ensure accuracy.
32
Table of Contents
The last part of our process involves synthesizing the data into clinically relevant information. This is a multi-faceted procedure performed by a team of trained scientists that culminates in the production and review of an integrated report, which contains information about the alterations detected and what therapeutic options may be available based on the genomic findings in a given patient. This report is reviewed and signed by our medical staff and is returned to the ordering physician who can use the data in conjunction with a clinical assessment to inform his or her treatment decisions.
A report is typically delivered to the physician generally within 11 to 14 days for FoundationOne and within 15 to 18 days for FoundationOne Heme, in each case from the time the specimen is received. We expect to report results in less than 14 days for FoundationACT.
Quality Assurance
We are committed to providing reliable and accurate molecular information to our customers. Accurate specimen identification, timely communication of results, and prompt correction of errors is critical. We monitor our quality through a variety of methods, including performance improvement indicators, proficiency testing, internal and external audits, and satisfaction surveys. Any quality concerns and incidents are subject to risk assessment, root cause analysis, and a corrective action plan that is reviewed monthly with department management to ensure that we are providing the best products possible to our customers. Protection of patient results from misuse and improper access is important and thus patient confidential information is limited to necessary personnel.
We have established a comprehensive quality assurance program for our laboratory designed to produce accurate and timely test results and to ensure the consistent high quality of our tests. Our quality assurance program includes policies and procedures covering personnel qualifications and training requirements, process and test validation, quality control of reagents and test processes, proficiency testing, routine monitoring, and internal audit. Quality control metrics are assessed at various points in the testing process and final disposition of patient results requires adherence to quality control metrics that meet and exceed recommendations by professional organizations and regulatory authorities. Additionally, the long-term trends in quality control metrics are reviewed monthly by management. Our quarterly internal quality assurance audits cover pre-analytic, analytic and post-analytic functions, assess improvement indicators, and set new metrics for the following quarter. We also have an extensive, internally administered program of specimen proficiency testing to ensure that test performance is reproducible and functioning optimally.
Policies and procedures have been developed to satisfy all applicable requirements necessary for federal and state licensures and accreditation for clinical diagnostic laboratories. We follow the policies and procedures for patient and employee safety, hazardous waste disposal, and fire codes stated in the general laboratory procedure manuals. We believe that all pertinent regulations of CLIA, Occupational Safety and Health Administration, Environmental Protection Agency, and FDA are satisfied by following the established guidelines and procedures of our quality assurance program.
Reproducibility
Our ability to reproduce high quality results is critical to ensuring that we deliver better-informed treatment options to the greatest number of patients with cancer. We have worked to ensure the results of FoundationOne and FoundationOne Heme are commensurate by conducting an extensive analytical validation that robustly demonstrates test performance using both reference specimens and hundreds of routine FFPE clinical cancer specimens with results derived from prior standard diagnostic tests. For example, in validation studies on actual clinical cancer specimens, including samples where as few as 20% of the nuclei in the specimen were derived from tumor cells, high accuracy for FoundationOne was observed across all classes of genomic alterations, including sensitivity greater than 99% for detection of base substitutions, greater than 98% for detection of indels, and greater than 95% for detection of copy number alterations. Our specificity was greater than 99% across all classes of alterations.
33
Table of Contents
Supply Agreement
In July 2013, we entered into a five-year supply, service and support agreement with Illumina, or the supply agreement, for Illumina to provide products and services that support and can be used for the gene sequencing component of our molecular testing activities. During the term of the supply agreement, Illumina will supply us with sequencers, reagents and other consumables for use with the Illumina sequencers, and service contracts for the maintenance and repair of the sequencers.
During the term of the supply agreement, we are required to make a rolling forecast of our expected needs for reagents and other consumables, and we may place purchase orders for reagents and other consumables that conform to such forecast. Illumina may not unreasonably reject conforming purchase orders and will, in its reasonable discretion, accept additional purchase orders for quantities of reagents and other consumables beyond our forecast requirements. During each six-month period we have a binding obligation to purchase an amount of reagents and other consumables equal to the greater of a percentage of our six-month forecast and a fixed minimum amount. Subject to discounts that vary depending on the volume of hardware and reagents and other consumables ordered, the price for sequencers and for service contracts is based on Illumina list prices, and the price for reagents and other consumables is based on contract prices that are fixed for a set period of time and may increase thereafter subject to limitations. The supply agreement does not require us to order minimum amounts of hardware, or to use exclusively the Illumina platform for conducting our sequencing.
We may use equipment, reagents and other materials supplied by third parties in the operation of our business. The agreement contains customary use limitations, representations and warranties, indemnification, limitations of liability, and other provisions.
Intellectual Property
Our business relies upon proprietary technologies, methods and processes, product designs and branding that we have invented, developed, or licensed. Our policy is to seek patent protection and trademark registration for commercially valuable assets we develop, as appropriate, and maintain as trade secrets other aspects of our proprietary platform, processes, and know-how.
Patents
Our patent portfolio includes a single issued U.S. patent, pending U.S. provisional and utility applications, and strategically focused corresponding international applications filed via the Patent Cooperation Treaty, or PCT, and foreign national and regional counterpart applications. We believe our portfolio of patents and patent applications serve to protect our business in the United States and in foreign jurisdictions in which we elect to pursue and are successful in obtaining patent rights. These applications fall into three broad categories:
| • | applications relating to our genomic analysis methodologies and procedures, including claims directed to process innovations and advances in solution hybridization, bait selection and capture, variant calling, and somatic versus germline alteration differentiation; |
| • | applications relating to genomic discoveries, including claims relating to novel genomic alterations correlated to various cancers and associated methods of treatment of patients harboring such genomic alterations; and |
| • | applications relating to genomic information and correlated treatment and outcome information management, matching, and delivery, including claims directed to web-mediated systems for capturing, managing, tracking and reporting genomic information and associated clinical outcome data, as well as other applications relating to annotation and curation of genomic results for medical reporting. |
A number of our patent applications that pertain to genomic alterations and associated methods of treatment provide us with potential royalty-bearing licensing opportunities. These opportunities arise primarily with
34
Table of Contents
companies developing or selling therapeutic products for cancer treatment. These companies may determine that the products or tests they are developing or selling require a license to the methods claimed in our patents and patent applications.
Trade Secrets and Trademarks
In addition to patents, we rely on trade secrets and know-how to develop and maintain our competitive position. For example, significant elements of FoundationOne, FoundationOne Heme, and FoundationACT, including aspects of sample preparation, computational-biological algorithms, and related processes and software, are based on unpatented trade secrets and know-how that are not publicly disclosed. We protect trade secrets and know-how by establishing confidentiality agreements and invention assignment agreements with our employees, consultants, scientific advisors, contractors, and commercial partners. These agreements generally provide that all confidential information developed or made known during the course of an individual or entity’s relationship with us must be kept confidential during and after the relationship and that all inventions or developments resulting from work performed for us or relating to our business and conceived or completed during the period of employment or assignment, as applicable, shall be our exclusive property. In addition, we take other appropriate precautions, such as physical and technological security measures, to guard against misappropriation of our proprietary technology by third parties. We have also adopted policies and conduct training that provides guidance on our expectations, and our advice for best practices, in protecting our trade secrets.
We also seek trademark protection in the United States and in foreign jurisdictions where available and when appropriate. Foundation Medicine®, FoundationOne®, Interactive Cancer Explorer®, Once. And for All®, and The Molecular Information Company® are all registered trademarks of Foundation in the United States, and several of these marks are at various stages of the registration process in other countries. FoundationACT™, FoundationICE™, FoundationCORE™, PatientMatch™, GeneKit™, Precision Medicine Exchange Consortium™, and SmartTrials™ are also trademarks of Foundation. Other trademarks or service marks that may appear in this Annual Report are the property of their respective holders. For convenience, we do not use the ® and ™ symbols in each instance in which one of our trademarks appears throughout this Annual Report, but this should not be construed as any indication that we will not assert, to the fullest extent under applicable law, our rights thereto.
Competition
We believe our products are currently the only commercially available comprehensive molecular information products that provide a fully informative genomic profile in a concise and actionable format designed for use in the clinical setting. Our principal competition comes from existing large and small commercial diagnostic companies, as well as certain medical institutions that offer single-marker or hotspot panel tests that can capture only the most common and known gene alterations and a limited set of gene rearrangements. Although these tests assess only a limited number of genes and are unable to detect copy number alterations and often miss short indels, in many circumstances, these are the diagnostic methods that physicians use and have used for many years. It may be difficult to change the methods or behavior of the referring physicians to incorporate our products into their practices. In addition, academic research centers and NGS platform developers are offering or developing NGS-based testing intended to be comprehensive for known cancer genes that may seek to compete with our products on the number of genes they interrogate. We have also observed increased competition from companies that offer similar molecular information tests. However, we are not aware of any of these third-party tests having sufficient sensitivity and specificity, operational scale, or reporting elements to fit the realities of current clinical practice, including volume and quality limits of tumor samples, demands on turnaround time, and ease of use.
35
Table of Contents
Single-Marker and Hotspot Panel Tests
We may face competition from companies that offer products or have conducted research to profile genes and gene expression in various cancers. Personalized genetic diagnostics is a new area of science, and we cannot predict what tests others will develop that may compete with or provide results comparable or superior to the results we are able to achieve. Our competitors include laboratory companies such as Bio-Reference Laboratories, Inc., Laboratory Corporation of America Holdings, Quest Diagnostics Incorporated, Molecular Health, Caris Life Sciences, Guardant Health, Paradigm, NeoGenomics Laboratories, as well as companies that manufacture or may manufacture diagnostic testing kits such as Abbott Laboratories, Qiagen N.V. and Sequenom, Inc. These kits, which companies often include with capital equipment and reagents to local pathology laboratories, can be used directly by the physician, which can facilitate adoption. In addition, companies such as Genomic Health, Inc. and Myriad Genetics, Inc. have well-established commercial organizations that sell molecular diagnostic tests to physicians and may develop tests which compete with our products on price.
Academic Research Centers and NGS Platforms
Many hospitals and academic centers may look to internalize the type of comprehensive molecular testing we perform. Our competition may include entities such as the MD Anderson Cancer Center, MSKCC, University of Michigan, Baylor Medical Genetics Laboratories, Washington University in St. Louis, University of Washington, and other academic hospitals and research centers. Although these academic centers could have greater access to, and ability to drive adoption with, certain key thought leaders than we do, we expect that the competition from these academic centers will, for the most part, be restricted to their local markets.
In addition to developing kits, certain life sciences and diagnostic companies also provide NGS platforms. Illumina, Thermo Fisher Scientific, Inc., and other companies develop NGS platforms that are being sold directly to research centers, pharmaceutical companies, and clinical laboratories. While many of the applications for these platforms are focused on the research and development markets and others are focused on testing for non-cancer conditions, each of these companies has launched and may continue to commercialize products used in the clinical oncology market. We believe diagnostic platform providers will seek to place sequencing machines in laboratories to develop sequencing-based laboratory based testing services. In addition, Illumina has received FDA-approval for a diagnostic kit for clinical use outside of oncology, which is sold to clients who have purchased its platforms. We believe Illumina and other diagnostic platform providers may develop additional FDA-approved diagnostic kits for clinical use by clients who have purchased their platforms, potentially including for these clients to identify genomic alterations in samples from solid tumors or blood-based cancers. Many private companies are developing information technology-based tools to support the integration of NGS testing into the clinical setting. These companies could have substantially greater financial, technical, and other resources than we do and may be more successful than we may be in achieving widespread market acceptance. Any tests they develop may be more effective, or more effectively marketed and sold, than FoundationOne, FoundationOne Heme, or FoundationACT.
Liquid Biopsy Assays
There is tremendous excitement within the oncology community about the promise of liquid biopsy assays for their potential to improve cancer diagnosis and optimize patient care. We believe that a ctDNA assay is an important tool for providers when a tissue biopsy cannot be obtained or when tissue has been depleted. There are a variety of techniques for analyzing ctDNA for a single gene or multiple genes. We believe we have developed a best-in-class, reliable, and comprehensive ctDNA assay, FoundationACT, that provides new options for patients when tissue is not accessible. Developed with input from our biopharmaceutical partners, we believe FoundationACT includes the most clinically relevant gene list that is technologically feasible without being cost-prohibitive. We believe our ctDNA assay has unparalleled accuracy with 99% positive predictive value. FoundationACT has been analytically validated for all four types of alterations.
36
Table of Contents
Several diagnostic companies have developed and launched, or plan to launch, ctDNA assays. Some of these companies include Biocept, Exosome Diagnostics, Guardant Health, Genomic Health, Inivata, Personal Genome Diagnostics, and Qiagen, among others.
Our Competitive Strengths
Our molecular information platform enables us to offer comprehensive molecular information products that interrogate with precision the genes known to be altered in human cancer. Our products are uniquely differentiated from other oncology diagnostic products because, to our knowledge, they are the first and only products to comprehensively address all solid tumors and hematologic malignancies, and to deliver a clear, concise report detailing clinically relevant treatment alternatives. We believe our products have a sustainable competitive advantage on the basis of:
| • | our ability, with FoundationOne, to assess 315 cancer-related genes for all classes of genomic alterations with high sensitivity and specificity, as described in our October 2013 Nature Biotechnology publication, unlike currently available single gene tests and hotspot panel tests, which focus only on a limited numbers of genes and a subset of genomic alteration types; |
| • | our ability, with FoundationOne Heme, to detect all classes of genomic alterations, including base pair substitutions, indels, copy number alterations and select gene rearrangements in 405 cancer-related genes, in addition to utilizing RNA sequencing across 265 genes to capture a broad range of gene fusions, a type of alteration that is a common driver of hematologic cancers and sarcomas; |
| • | our ability, with FoundationACT, our ctDNA assay, to reliably and accurately find all the clinically relevant alterations in 62 genes from a blood sample when a tissue biopsy is not an option. We believe the introduction of FoundationACT enables us to capitalize on our first mover advantage and the strength of our established Foundation Medicine brand to be the molecular information solution of choice for providers with a full suite of highly validated and reliable products to aid in cancer diagnosis and treatment; |
| • | our proprietary optimizations allow us to utilize a wide variety of sample types, including small biopsies and fine needle aspirates, and samples with low tumor purity; |
| • | our ability to leverage our expertise and relationships with oncology thought leaders to keep pace with scientific and medical advances to, among other things, incorporate newly relevant cancer genes along with newly available targeted therapeutics and clinical trials; |
| • | our ability to deliver, in a concise report, actionable information regarding the relevant genomic alterations in a patient’s cancer and to match these alterations with targeted therapies based on peer-reviewed literature in a medically relevant time frame; |
| • | our ability to deliver complex information through the convenience and utility of our FoundationICE; |
| • | our ability, through a complete suite of molecular information decision support applications including PatientMatch, to connect physicians with peers who have seen patients with similar genomic profiles and learn about their treatment decisions and outcomes to facilitate their own decision making; |
| • | our efforts to capture, aggregate, analyze, and facilitate the broader exchange of genomic data across the global oncology community, including our efforts with PMEC, to create a network effect as we gather more data which will lead to more users and ultimately more comprehensive datasets; |
| • | our ability to leverage the vast array of genomic data generated by our molecular information platform, included in FoundationCORE, together with clinical data, for example, through our relationship with Flatiron Health, to position ourselves at the nucleus of this new treatment paradigm; |
| • | our strategic collaboration with Roche, which we believe accelerates all aspects our business, including advancing our leadership position in precision medicine, supporting our innovative product |
37
Table of Contents
| development efforts, contributing to education and awareness among providers in the United States, significantly expanding our commercial opportunities globally, and strengthening our balance sheet and operating leverage; |
| • | our ability to actively participate in the development of the newest oncology therapeutics and practice through our relationships with our more than 25 biopharmaceutical partners; and |
| • | our ability to provide value-added services to help our physician customers and patients act on the results of their comprehensive genomic profiling test results, including through FoundationAccess, FoundationAccess Careline and FoundationAccess Clinical Trials, to help patients access prescribed therapies and our collaboration with EmergingMed to provide a clinical trial matching service for patients. |
Strategic Collaboration with Roche
On April 7, 2015, we closed, or the Closing, our broad, strategic collaboration with Roche and certain of its affiliates in the field of molecular information for oncology, including an R&D collaboration, a U.S. educational support collaboration, an ex-U.S. commercial collaboration, a binding term sheet for an in vitro diagnostic product development collaboration, and an equity investment with certain governance provisions.
Transaction Agreement
Under the terms of the Transaction Agreement, dated January 11, 2015, by and between us and Roche Holdings, Inc., or the Transaction Agreement, Roche made an equity investment, or the Investment, including (1) a primary investment of $250 million in cash to purchase 5 million newly issued shares of our common stock at a purchase price of $50.00 per share and (2) a tender offer in which Roche acquired 15,604,288 outstanding shares of our common stock at a price of $50.00 per share. As of December 31, 2015, Roche owned approximately 61% of our outstanding common stock.
Collaboration
In connection with the Investment and simultaneously with the execution of the Transaction Agreement, we entered into a strategic collaboration with certain affiliates of Roche to develop and commercialize comprehensive molecular information and genomic analysis products for the treatment of cancer patients, or the Collaboration, pursuant to (a) a Collaboration Agreement, or the R&D Collaboration Agreement, by and among us, F. Hoffmann-La Roche Ltd, or Roche Basel, and Hoffmann-La Roche Inc., or Roche U.S., (b) a US Education Collaboration Agreement, or the U.S. Education Collaboration Agreement, by and between us and Genentech, (c) an Ex-US Commercialization Agreement, or the Ex-U.S. Commercialization Agreement, by and between us and Roche Basel, and (d) a Binding Term Sheet for an In Vitro Diagnostics (IVD) Agreement, or the IVD Term Sheet, by and between us and Roche Basel, each of which became effective April 7, 2015. We refer to the R&D Collaboration Agreement, the U.S. Education Collaboration Agreement, the Ex-U.S. Commercialization Agreement, and the IVD Term Sheet collectively as the Collaboration Agreements.
Pursuant to the R&D Collaboration Agreement, we are collaborating with Roche on multiple programs related to the use and development of products and services for use in molecular information, immunotherapy, ctDNA and companion diagnostics. These programs are being conducted pursuant to agreed-upon work plans and are subject to the oversight of a joint research and development committee. Under the molecular information platform program, Roche will pay us $85 million over five years for the molecular genomic profiling of a minimum number of cancer samples and to access our molecular information database. We could also receive up to approximately $74 million for the scope of activities related to the immunotherapy testing platform program, the ctDNA platform program, and the companion diagnostics platform program. The initial term of the R&D Collaboration Agreement is five years. However, certain provisions of the R&D Collaboration Agreement may
38
Table of Contents
remain in effect after such initial five-year term so long as Roche and its affiliates own at least a majority of the outstanding shares of our common stock. We or Roche may terminate the R&D Collaboration Agreement in the event of a breach of the agreement by the other party.
Pursuant to the U.S. Education Collaboration Agreement, we and Genentech, a wholly owned subsidiary of Roche, are conducting an education support program for healthcare professionals and laboratories in the United States regarding the use of next-generation sequencing and comprehensive genomic profiling technology. Genentech will develop education materials based upon information related to comprehensive genomic profiling provided by us, and we and Genentech share the costs related to the education support program activities. In addition, in the event we seek to promote any companion diagnostic product for use with a Genentech therapeutic in the United States, Genentech will have a right of first negotiation, subject to certain conditions, with respect to the co-promotion of such companion diagnostic product. The U.S. Education Collaboration Agreement will remain in effect for five years, and either party may terminate the agreement without cause upon six months’ written notice during the first year of the term and upon three months’ written notice thereafter, and either party may terminate in the event of a breach by the other party.
Pursuant to the Ex-U.S. Commercialization Agreement, Roche will have the right to commercialize our existing clinical diagnostic testing products, including FoundationOne and FoundationOne Heme, any clinical diagnostic products developed under the R&D Collaboration Agreement, and any other products upon mutual agreement. Beginning on April 7, 2016, Roche will have the exclusive right to commercialize such products worldwide, excluding the United States and any countries Roche elects to exclude prior to April 7, 2016. Roche also holds a right of first negotiation with respect to the commercialization of our future clinical diagnostic products, excluding in vitro diagnostic tests, companion diagnostic products developed by us for third parties, and any standalone data or molecular information products. Roche may pay us an agreed amount for the right to extend the launch timeline for FoundationOne and FoundationOne Heme by specified periods in specified countries. Roche will also pay agreed upon royalties and commercial milestones. Further, if Roche fails to meet certain minimum revenue requirements for FoundationOne or FoundationOne Heme tests for three consecutive years in a specified country, we have the right to terminate Roche’s exclusive commercialization rights in the applicable country. The Ex-U.S. Commercialization Agreement will remain in effect for five years and may be extended by Roche for additional two-year periods. Roche shall have the right to terminate the agreement without cause upon six months’ written notice after the initial five year term, and either party may terminate the agreement in the event of breach by the other party.
Pursuant to the IVD Term Sheet, we have agreed with Roche to use commercially reasonable efforts to negotiate and enter into a definitive agreement by April 7, 2016 (unless such period is extended by mutual agreement of the parties) regarding a non-exclusive collaboration to develop and commercialize in vitro diagnostic versions of certain of our existing products, including FoundationOne and FoundationOne Heme, and our future products, including those developed under the R&D Collaboration Agreement, or the IVD Kits. The IVD Term Sheet provides that we may not develop IVD Kits that use a sequencing platform owned or controlled by Roche for internal purposes or with third parties without the prior written consent of Roche. The IVD Term Sheet contemplates that Roche will bear all development costs related to the development of the Roche sequencing platform. The IVD Term Sheet will control this relationship between the parties until the parties enter into a definitive agreement or mutually agree to terminate their relationship.
Investor Rights Agreement
In connection with the Investment and simultaneously with the execution of the Transaction Agreement, we entered into an Investor Rights Agreement, or the Investor Rights Agreement, which became effective April 7, 2015, with Roche and Third Rock Ventures, L.P., affiliates of KPCB Holdings, Inc. and Google Ventures 2011, L.P., or the Existing VC Investors.
39
Table of Contents
Under the terms of the Investor Rights Agreement, our Board was increased to nine directors, consisting of (a) three directors designated by Roche, (b) four independent directors, (c) Michael Pellini, our Chief Executive Officer, and (d) one independent director, whose seat has not yet been filled.
Following the Closing: (a) so long as Roche beneficially owns at least 10% of the outstanding shares of our common stock, it will be entitled to the lesser of (i) the number of seats representing 33.34% of our Board and (ii) proportionate representation on our Board and (b) so long as Roche is entitled to appoint at least one director to our Board, it will be entitled to proportionate representation on each committee of our Board, subject to compliance with the applicable rules of the SEC and the NASDAQ Stock Market.
Until such time as Roche and its affiliates beneficially owns less than a majority of the outstanding shares of our common stock (subject to a cure period), we may not take certain actions without Roche’s prior written consent, including any of the following: (a) appoint a new Chief Executive Officer; (b) incur any indebtedness (as defined in the Investor Rights Agreement) that would result in the outstanding aggregate principal amount of the indebtedness of us and our subsidiaries exceeding the lesser of (A) $200 million and (B) 20% of our aggregate market capitalization at the time of such incurrence; (c) issue or sell any equity securities (including any securities convertible or exercisable into such equity securities), other than (X) common stock issued upon the exercise or settlement of equity awards granted as of the date of the Investor Rights Agreement in accordance with their terms, (Y) equity awards granted after the date of the Investor Rights Agreement pursuant to our 2013 Stock Option and Incentive Plan or any permitted new equity incentive plan or equity incentive plan amendment, and (Z) in connection with permitted acquisitions, certain shares of our common stock issued as stock consideration as long as such issuance does not result in Roche beneficially owning less than 50.5% of the outstanding shares of common stock on a fully diluted basis; (d) establish or amend any of our equity incentive plans, except for certain permitted equity incentive plans and permitted equity incentive plan amendments; (e) acquire any entity, business or assets if the aggregate consideration payable by us exceeds the lesser of (X) $200 million and (Y) 20% of our aggregate market capitalization at the time of such transaction, unless Roche is separately contemplating acquiring the same entity, business or assets; (f) dispose of any entity, business or assets if the aggregate consideration payable to us exceeds $50 million; (g) change the scope and nature of our business; (h) amend our organizational documents; (i) take any action that would impair in any material respect our ability to perform our obligations under the Investor Rights Agreement or Roche’s rights thereunder; or (j) voluntarily dissolve or liquidate or make any voluntary bankruptcy filings.
Voting Obligations. As long as Roche is entitled to appoint at least one director to our Board, Roche will be required to (a) cause all of its shares of our common stock to be present for quorum purposes at any meeting of our stockholders, (b) vote all of its shares of our common stock to approve any matter requiring approval by Roche described in the preceding paragraph that Roche has approved within the previous six months, and (c) vote all of its shares of our common stock in connection with the election of directors or the adoption of certain equity incentive plans either (i) in accordance with the recommendation of our Board or (ii) in the same proportion as the votes cast by all of our stockholders other than Roche and its affiliates.
Standstill Provisions. Under the terms of the Investor Rights Agreement, from April 7, 2015 until April 7, 2018, or the Restricted Period, Roche will be restricted from acquiring additional shares of our common stock, except in order to offset dilution and maintain its aggregate percentage ownership in us. During the Restricted Period and for as long as Roche has the right to designate a director, Roche shall not make any proxy solicitations in connection with the election or removal of our directors, or knowingly encourage or facilitate a third party to engage in any such solicitation, subject to certain limited exceptions.
Following the Restricted Period, Roche will be permitted to make an offer to purchase all remaining shares of our common stock held by our stockholders, or a Roche Buyout Offer. Prior to April 7, 2020, any Roche Buyout Offer shall be made on a confidential basis, subject to the review, evaluation and approval of a special committee of independent directors who are unaffiliated with Roche and are not our officers or employees, or the Disinterested Directors, and subject to a non-waivable condition that a majority of the shares of our common
40
Table of Contents
stock held by stockholders not affiliated with Roche approve the Roche Buyout Offer. From and after April 7, 2020, any Roche Buyout Offer may be made directly to our stockholders without the review, evaluation or approval of our Board or the Disinterested Directors, as long as it is subject to the non-waivable condition that a majority of the shares of our common stock held by stockholders not affiliated with Roche approve the Roche Buyout Offer. If, from and after April 7, 2020, Roche makes a Roche Buyout Offer, then at any subsequent annual meeting of our stockholders (or special meeting called for the purpose of electing directors), Roche will be entitled to nominate any individuals who qualify as independent directors under the terms of the Investor Rights Agreement.
The standstill restrictions on Roche will automatically terminate if Roche ceases to own at least 20% of our outstanding common stock or we enter into a definitive agreement with respect to, or our Board recommends to our stockholders, a transaction pursuant to which any person or group would acquire, directly or indirectly, voting securities representing more than 20% of the aggregate voting power of all of our then-outstanding voting securities.
Anti-dilution Protections. We have agreed to establish and maintain a stock repurchase program and to repurchase shares of our common stock in order to maintain Roche’s aggregate percentage ownership at no less than 50.5% of the outstanding shares of our common stock on a fully diluted basis, less any shares transferred by Roche until the earlier of (a) any transfer by Roche of shares of our common stock, following which Roche beneficially owns less than 40% of the outstanding shares of our common stock on a fully diluted basis and (b) Roche beneficially owning less than 30% of the outstanding shares of our common stock.
Until the date on which Roche beneficially owns less than 30% of the outstanding shares of our common stock, Roche also will hold a continuing option to purchase shares of common stock directly from us or in the open market, at prevailing market prices, in order to maintain its aggregate percentage ownership at no less than 50.5% of the outstanding shares of our common stock on a fully diluted basis, less any shares transferred by Roche, or the Share Percentage Cap. If we fail to or are unable to satisfy our repurchase obligations under the stock repurchase program described above, and Roche purchases shares of common stock from us or in the open market, the Share Percentage Cap will be increased by the percentage of the outstanding shares of common stock, on a fully diluted basis, represented by the shares of common stock that Roche was required to purchase at its cost in order to maintain its aggregate percentage ownership.
In the event that we issue any securities and, as a result thereof, Roche beneficially owns less than 50.1% of the outstanding shares of our common stock on a fully diluted basis, the restrictions on Roche under the Investor Rights Agreement (including with respect to the agreement to vote Roche’s shares of common stock, the standstill restrictions and the transfer restrictions), but not the rights of Roche under the Investor Rights Agreement, will immediately terminate, and Roche will thereafter have the ability to exercise in full its rights as a stockholder.
Restrictions on Transfer of Shares. Roche may not transfer any shares of our common stock during the Restricted Period. Thereafter, subject to certain exceptions, Roche may not transfer any shares of our common stock to any person or group, if such person or group would beneficially own in excess of 10% of the outstanding shares of our common stock following such transfer, without the prior consent of a special committee of Disinterested Directors. Following April 7, 2020, Roche will be permitted to transfer all (but not less than all) of its shares of our common stock to a third party that has made an offer to us or our stockholders (including pursuant to a tender offer) to purchase all of the outstanding shares of our common stock if the price, form of consideration and other terms and conditions of the transfer offered to Roche are the same (or no more favorable) than the price, form of consideration and other terms and conditions offered to all of our other stockholders, other than (a) fair market consideration payable in exchange for entering into restrictive covenants and (b) commercial agreements (including with respect to transition services) on arms’-length terms, in each case that the purchaser requires as a condition to the transaction.
41
Table of Contents
Registration Rights. Following April 7, 2018, Roche will be entitled to customary demand and piggyback registration rights, subject to customary underwriter cutbacks.
Matters Reserved for Approval of the Disinterested Directors. For as long as there is at least one director designated by Roche on our Board, the following actions will require approval of a majority of the Disinterested Directors (or a special committee of Disinterested Directors): (a) any transaction between Roche or any of its affiliates, on the one hand, and us, on the other hand; (b) any enforcement or waiver of our rights under any agreement between us, on the one hand, and Roche or any of its affiliates, on the other hand; and (c) any purchase of shares of our common stock by Roche or any of its affiliates, except as otherwise expressly set forth in the Investor Rights Agreement.
Freedom to Pursue Opportunities. As a general matter, neither we nor Roche will be required to offer a corporate opportunity to the other, and except as agreed in connection with the Collaboration or as part of the Investor Rights Agreement, there will be no restrictions on our or Roche’s ability to engage in similar activities or lines of business.
Termination. The Investor Rights Agreement will automatically terminate on the later of the date Roche beneficially owns less than 10% of the outstanding shares of our common stock or the date that Roche owns no Registrable Securities (as defined in the Investor Rights Agreement).
The foregoing summary descriptions of the Transaction Agreement, the Investment, the Collaboration and the Investor Rights Agreement do not purport to be complete and are qualified in their entirety by reference to the applicable agreements, copies of which are filed with this Annual Report. Additional descriptions on the transactions contemplated by the Transaction Agreement have been included in our Current Report on Form 8-K, filed with the Securities and Exchange Commission, or the SEC, on January 12, 2015, our Current Report on Form 8-K/A filed with the SEC on February 2, 2015, our Current Report on Form 8-K filed with the SEC on April 7, 2015, our Current Report on Form 8-K/A filed with the SEC on August 21, 2015, our Solicitation/Recommendation Statement on Schedule 14D-9 relating to the Investment filed with the SEC on February 2, 2015 (including any amendments thereto on Schedule 14D-9/A), and our definitive Proxy Statement on Schedule 14A relating to the Investment filed with the SEC on February 19, 2015 (including any amendments thereto).
Governmental Regulations
Clinical Laboratory Improvement Amendments of 1988 and State Regulation
As a clinical laboratory, we are required to hold certain federal and state licenses, certifications, and permits to conduct our business. As to federal certifications, in 1988, Congress passed the Clinical Laboratory Improvement Amendments, or CLIA, establishing quality standards for all laboratory testing to ensure the accuracy, reliability, and timeliness of patient test results regardless of where the test was performed. Our laboratory is CLIA certified and accredited by the College of American Pathologists, or CAP, a CLIA approved accrediting organization. In addition, we are required to meet certain laboratory licensing requirements for states with regulations beyond CLIA. For more information on state licensing requirements, see below in the section entitled “Governmental Regulations—State Laboratory Testing.”
Under CLIA, a laboratory is any facility which performs laboratory testing on specimens derived from humans for the purpose of providing information for the diagnosis, prevention or treatment of disease, or the impairment of, or assessment of health. CLIA also requires that we hold a certificate applicable to the type of work we perform and comply with certain standards. CLIA further regulates virtually all clinical laboratories by requiring they be certified by the federal government and comply with various operational, personnel, facilities administration, quality, and proficiency requirements intended to ensure that their clinical laboratory testing services are accurate, reliable, and timely. Laboratories must register and list their tests with The Centers for Medicare & Medicaid Services, or CMS, the agency that oversees CLIA. CLIA compliance and certification is
42
Table of Contents
also a prerequisite to be eligible to bill for services provided to governmental payor program beneficiaries and for many private payors. CLIA is user-fee funded. Therefore, all costs of administering the program must be covered by the regulated facilities, including certification and survey costs.
We are subject to survey and inspection every two years to assess compliance with program standards, and may be subject to additional unannounced inspections. Laboratories performing high complexity testing are required to meet more stringent requirements than laboratories performing less complex tests. In addition, a laboratory like ours that is certified as “high complexity” under CLIA may develop, manufacture, validate, and use proprietary tests referred to as laboratory developed tests, or LDTs. To date, the FDA has taken the position that LDTs currently do not require FDA approval; however, CLIA requires full validation, including accuracy, precision, specificity, sensitivity, and establishment of a reference range for any LDT used in clinical testing.
In addition to CLIA requirements, we elect to participate in the accreditation program of CAP. CMS has deemed CAP standards to be equally or more stringent than CLIA regulations and has approved CAP as a recognized accrediting organization. Inspection by CAP is performed in lieu of CMS for accredited laboratories. Because we are accredited by the CAP Laboratory Accreditation Program, we are deemed to also comply with CLIA.
CLIA provides that a state may adopt laboratory regulations that are more stringent than those under federal law, and a number of states have implemented their own more stringent laboratory regulatory requirements. State laws may require that laboratory personnel meet certain qualifications, specify certain quality control procedures, facility requirements, or prescribe record maintenance requirements.
State Laboratory Testing
Several states require the licensure of out-of-state laboratories that accept specimens from those states. For example, New York requires a laboratory to hold a permit which is issued after an on-site inspection and approval of testing methodology, and has various requirements over and above CLIA and CAP, including those for personnel qualifications, proficiency testing, physical facility, equipment, and quality control standards. Our laboratory holds the required licenses for these states, which include Massachusetts, Maryland, Rhode Island, Pennsylvania, Florida, California, and New York.
From time to time, other states may require out of state laboratories to obtain licensure in order to accept specimens from the state. If we identify any other state with such requirements, or if we are contacted by any other state advising us of such requirements, we intend to follow instructions from the state regulators as to how we should comply with such requirements.
FDA
The FDA regulates the sale and distribution in interstate commerce of medical devices under the Federal Food, Drug, and Cosmetic Act, or the FDCA, including in vitro diagnostic devices, reagents, and instruments used to perform diagnostic testing. Devices must undergo premarket review by FDA prior to commercialization unless the device is of a type exempted from such review by statute, regulation, or pursuant to FDA’s exercise of enforcement discretion. FDA, to date, has generally not attempted to apply all of the requirements of the FDCA on LDTs, which are tests that are designed, manufactured, validated, and used within a single laboratory, and therefore we do not believe that our LDTs currently require pre-market clearance or approval. It is likely that FDA will more actively regulate LDTs, which could lead to premarket and post-market obligations. Indeed, in July 2010, FDA held a two-day public meeting on the oversight of LDTs in which the agency stated it decided to exercise authority over LDTs, but had not decided how it would exercise that authority. In October 2014, FDA issued draft guidance documents stating that FDA intends to change its policy and describing an approach to regulating LDTs using a risk-based, phased-in approach. If finalized, the guidance documents would impose premarket review and other medical device requirements under the FDCA on LDTs classified as high and moderate risk. Enforcement of premarket review and QSR requirements would be phased-in based on the risk of
43
Table of Contents
the LDT over a period of several years, but premarket review would begin immediately for newly developed LDTs, the highest-risk group of LDTs. Medical Device Reporting requirements and compliance with either a new notification procedure in which the laboratory must provide FDA with certain basic information about each LDT offered by their laboratory or FDA’s device registration and listing requirements would be required within six months of finalization of the guidance documents (with limited exceptions). In addition, clinical investigations of LDTs would need to be conducted in compliance with FDA’s regulations, including the requirement to obtain an investigational device exemption, or IDE, when required. There is no time frame in which FDA must finalize the draft guidance documents, but FDA stated that it intends to issue a final guidance in fiscal year 2016. In the meantime, we maintain our CLIA certification, which permits the use of LDTs for diagnostic purposes.
FDA regulations pertaining to medical devices govern, among other things, the research, design, development, pre-clinical and clinical testing, manufacture, safety, effectiveness, clearance or approval, record-keeping, packaging, labeling, storage, adverse event reporting, advertising, promotion, marketing, sales, distribution, and import and export of medical devices. Pursuant to the FDCA, medical devices are subject to varying degrees of regulatory control and are classified in one of three classes depending on the controls FDA determines necessary to reasonably ensure their safety and effectiveness.
Class I devices are those for which reasonable assurance of safety and effectiveness can be provided by adherence to FDA’s general controls for medical devices, which include applicable portions of FDA’s QSR, facility registration and product listing, reporting of adverse medical events, and appropriate, truthful, and non-misleading labeling, advertising and promotional materials. Many Class I devices are exempt from premarket regulation; however, some Class I devices require premarket clearance by FDA through the 510(k) premarket notification process described below.
Class II devices are subject to FDA’s general controls, and any other special controls, such as performance standards, postmarket surveillance, and FDA guidelines, deemed necessary by FDA to provide reasonable assurance of the devices’ safety and effectiveness. Premarket review and clearance by FDA for Class II devices are accomplished through the 510(k) premarket notification procedure, although some Class II devices are exempt from the 510(k) requirements. Premarket notifications are subject to user fees, unless a specific exemption applies. To obtain 510(k) clearance, a manufacturer must submit a premarket notification demonstrating that the proposed device is “substantially equivalent” to a predicate device, which is a previously cleared 510(k) device or a preamendment device that was in commercial distribution before May 28, 1976, for which FDA has not yet called for the submission of a premarket approval, or PMA, application. In determining substantial equivalence, FDA assesses whether the proposed device has the same intended use and technical characteristic as the predicate device, or whether the proposed device has different technological characteristics, but the information submitted in the premarket notification demonstrates the device is as safe and effective as a legally marketed device and does not raise different questions of safety and effectiveness than the predicate device. FDA may request additional information, including clinical data. Under the FDCA, a manufacturer must submit a premarket notification at least 90 days before introducing a device into interstate commerce, but FDA’s review of the premarket notification can take significantly longer. If FDA determines that the device is substantially equivalent to the predicate device(s), the subject device may be marketed. However, if FDA determines that a device is not substantially equivalent to the predicate device(s), then the device would be regulated as a Class III device, discussed below. If a manufacturer obtains a 510(k) clearance for its device and then makes a modification that could significantly affect the device’s safety or effectiveness, a new premarket notification must be submitted to FDA.
Class III devices are those deemed by FDA to pose the greatest risk, such as those for which reasonable assurance of the device’s safety and effectiveness cannot be assured solely by the general controls and special controls described above and that are life-sustaining or life-supporting. Some preamendment Class III devices for which FDA has not yet required a PMA require FDA’s clearance of a premarket notification in order to be marketed. However, most Class III devices are required to undergo the PMA process in which the manufacturer
44
Table of Contents
must demonstrate reasonable assurance of the safety and effectiveness of the device to FDA’s satisfaction. A PMA application must provide valid scientific evidence, typically extensive preclinical and clinical trial data, and information about the device and its components regarding, among other things, device design, manufacturing, and labeling. PMA applications (and supplemental PMA applications) are subject to significantly higher user fees than are 510(k) premarket notifications. Some PMA applications are exempt from a user fee, for example a small business’s first PMA.
After a PMA application is submitted and found to be sufficiently complete, FDA begins an in-depth review of the submitted information. During this review period, FDA may request additional information or clarification of information already provided. FDA also may convene an advisory panel of outside experts to review and evaluate the application and provide recommendations to FDA as to the approvability of the device. In addition, FDA generally will conduct a pre-approval inspection of the manufacturing facility to ensure compliance with the QSR. FDA can delay, limit, or deny approval of a PMA application for many reasons.
If the FDA’s evaluations of both the PMA application and the manufacturing facilities are favorable, FDA will either issue an approval letter authorizing commercial marketing or an approvable letter that usually contains a number of conditions that must be met in order to secure final approval. If the FDA’s evaluations are not favorable, FDA will deny approval of the PMA or issue a not approvable letter. The agency may determine that additional clinical trials are necessary, in which case the PMA approval may be delayed while the trials are conducted and the data acquired and submitted in an amendment to the PMA. Even with additional trials, FDA may not approve the PMA application. The PMA process, including the gathering of clinical and nonclinical data, and the submission to FDA for review, can take several years, and the process can be expensive and uncertain.
Even if FDA approves a PMA, the agency can impose post approval conditions that it believes necessary to ensure the safety and effectiveness of the device, including, among other things, restrictions on labeling, promotion, sale, and distribution. After approval of a PMA, a new PMA or PMA supplement may be required for a modification to the device, its labeling, or its manufacturing process.
A clinical trial may be required in support of a 510(k) submission and generally is required for a PMA application. These trials generally require an Investigational Device Exemption, or IDE, approved by FDA for a specified number of patients, unless the product is exempt from IDE requirements or deemed a non-significant risk device eligible for more abbreviated IDE requirements. The IDE application must be supported by appropriate data, such as animal and laboratory testing results, showing that it is safe to test the device in humans and that the testing protocol is scientifically sound. Clinical trials may begin 30 days after the submission of the IDE application unless FDA disapproves the IDE or places the trial on clinical hold. Additionally, clinical trials may not begin until their protocol and informed consent receive approval from the appropriate institutional review boards at the clinical trial sites. All clinical trials must be conducted in accordance with the FDA’s IDE regulations.
Even if regulatory approval or clearance of a device is granted, FDA may impose limitations on the uses and indications for which the device may be labeled and promoted, and the device remains subject to significant regulatory requirements. Medical devices may be marketed only for the uses and indications for which they are cleared or approved. Device manufacturers must register their facilities and list their devices with FDA. A device manufacturer’s manufacturing processes and those of some of its suppliers are required to comply with the applicable portions of the QSR, which covers quality management, design, production and process controls, quality assurance, labeling, packaging, shipping, and complaint handling. Device manufacturers must submit to the FDA medical device reports for deaths, serious injuries, and certain malfunctions and report certain field corrections and product recalls or removals. Some manufacturers also may be subject to post-market surveillance regulations. Facility records and manufacturing processes are subject to periodic unscheduled inspections by FDA.
Failure to comply with applicable regulatory requirements can result in enforcement action by FDA, which may include any of the following sanctions: public warning letters, fines, injunctions, civil or criminal penalties,
45
Table of Contents
recall or seizure of products, operating restrictions, partial suspension or total shutdown of production, delays in or denial of 510(k) clearance or PMA applications for new products, challenges to existing 510(k) clearances or PMA applications, and a recommendation by FDA to disallow a device manufacturer from entering into government contracts. FDA also has the authority to request repair, replacement, or refund of the cost of any device manufactured or distributed. In the event that a supplier fails to maintain compliance with a device manufacturer’s quality requirements, the manufacturer may have to qualify a new supplier and could experience manufacturing delays as a result.
We believe that our LDTs would likely be regulated as either a Class II or Class III device. Accordingly, some level of premarket review—either a 510(k) or a PMA—would likely be required for our test if FDA no longer applies its enforcement discretion to LDTs. While the data requirements are typically greater for Class III devices, the data required for Class II devices has increased, and it is possible that some amount of clinical data (retrospective or prospective or both) would be required for either type of submission. Currently, FDA is undertaking a review of the adequacy of the 510(k) process. It is difficult to predict what changes may result, but it should be assumed that any changes will increase, not decrease, the regulatory requirements. We believe that products we may develop in the future for use as companion diagnostic tests may be regulated as Class II devices requiring 510(k) clearance or Class III devices requiring PMA approval. We cannot assure you that our current products and other future products will not require 510(k) clearance or PMA approval in the future, or, in such an event, that such approval or clearance would be forthcoming.
HIPAA and HITECH
Under the administrative simplification provisions of HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or the HITECH Act, the United States Department of Health and Human Services issued regulations that establish uniform standards governing the conduct of certain electronic health care transactions and protecting the privacy and security of protected health information used or disclosed by health care providers and other covered entities. Three principal regulations with which we are required to comply have been issued in final form under HIPAA: privacy regulations, security regulations, and standards for electronic transactions, which establish standards for common health care transactions. The privacy and security regulations were extensively amended in 2013 to incorporate requirements from the HITECH Act.
The privacy regulations cover the use and disclosure of protected health information by health care providers and other covered entities. They also set forth certain rights that an individual has with respect to his or her protected health information maintained by a health care provider, including the right to access or amend certain records containing protected health information, or to request restrictions on the use or disclosure of protected health information. The security regulations establish requirements for safeguarding the confidentiality, integrity, and availability of protected health information that is electronically transmitted or electronically stored. The HITECH Act, among other things, established certain protected health information security breach notification requirements. A covered entity must notify affected individual(s) and the United States Department of Health and Human Services when there is a breach of unsecured protected health information. The HIPAA privacy and security regulations establish a uniform federal “floor” that health care providers must meet and do not supersede state laws that are more stringent or provide individuals with greater rights with respect to the privacy or security of, and access to, their records containing protected health information. Massachusetts, for example, has a state law that protects the privacy and security of personal information of Massachusetts residents that is more prescriptive than HIPAA.
These laws contain significant fines and other penalties for wrongful use or disclosure of protected health information. Additionally, to the extent that we submit electronic health care claims and payment transactions that do not comply with the electronic data transmission standards established under HIPAA and the HITECH Act, payments to us may be delayed or denied.
46
Table of Contents
Federal, State and Foreign Fraud and Abuse Laws
In the United States, there are various fraud and abuse laws with which we must comply and we are potentially subject to regulation by various federal, state and local authorities, including CMS, other divisions of the U.S. Department of Health and Human Services (e.g., the Office of Inspector General), the U.S. Department of Justice, and individual U.S. Attorney offices within the Department of Justice, and state and local governments. We also may be subject to foreign fraud and abuse laws.
In the United States, the federal Anti-Kickback Statute prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving remuneration to induce or in return for patient referrals for, or purchasing, leasing, ordering or arranging for the purchase, lease or order of, any health care item or service reimbursable under a governmental payor program. Courts have stated that a financial arrangement may violate the Anti-Kickback Statute if any one purpose of the arrangement is to encourage patient referrals or other federal health care program business, regardless of whether there are other legitimate purposes for the arrangement. The definition of “remuneration” has been broadly interpreted to include anything of value, including gifts, discounts, credit arrangements, payments of cash, consulting fees, waivers of co-payments, ownership interests, and providing anything at less than its fair market value. Recognizing that the Anti-Kickback Statute is broad and may technically prohibit many innocuous or beneficial arrangements within the health care industry, the U.S. Department of Health and Human Services issued a series of regulatory “safe harbors.” These safe harbor regulations set forth certain provisions, which, if met, will assure health care providers and other parties that they will not be prosecuted under the federal Anti- Kickback Statute. Although full compliance with these provisions ensures against prosecution under the federal Anti-Kickback Statute, the failure of a transaction or arrangement to fit within a specific safe harbor does not necessarily mean that the transaction or arrangement is illegal or that prosecution under the federal Anti-Kickback Statute will be pursued. Penalties for federal anti-kickback violations are severe, and include imprisonment, criminal fines, civil money penalties, and exclusion from participation in federal health care programs. Many states also have anti-kickback statutes, some of which may apply to items or services reimbursed by any third-party payor, including commercial insurers.
Legislation defining two new federal crimes related to health care were recently enacted: health care fraud and false statements relating to health care matters. The health care fraud statute prohibits knowingly and willfully executing a scheme to defraud any health care benefit program, including private payors. A violation of this statute is a felony and may result in fines, imprisonment, or exclusion from governmental payor programs such as the Medicare and Medicaid programs. The false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact, or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for health care benefits, items, or services. A violation of this statute is a felony and may result in fines, imprisonment, or exclusion from governmental payor programs.
Finally, another development affecting the health care industry is the increased enforcement of the federal False Claims Act and, in particular, actions brought pursuant to the False Claims Act’s “whistleblower” or “qui tam” provisions. The False Claims Act imposes liability on any person or entity that, among other things, knowingly presents, or causes to be presented, a false or fraudulent claim for payment by a federal governmental payor program. The qui tam provisions of the False Claims Act allow a private individual to bring actions on behalf of the federal government alleging that the defendant has defrauded the federal government by submitting a false claim to the federal government and permit such individuals to share in any amounts paid by the entity to the government in fines or settlement. When an entity is determined to have violated the False Claims Act, it may be required to pay up to three times the actual damages sustained by the government, plus civil penalties ranging from $5,500 to $11,000 for each false claim.
In addition, various states have enacted false claim laws analogous to the federal False Claims Act, although many of these state laws apply where a claim is submitted to any third-party payor and not merely a governmental payor program.
47
Table of Contents
In Europe various countries have adopted anti-bribery laws providing for severe consequences, in the form of criminal penalties and/or significant fines, for individuals and/or companies committing a bribery offence. Violations of these anti-bribery laws, or allegations of such violations, could have a negative impact on our business, results of operations and reputation. For instance, in the United Kingdom, under the Bribery Act 2010, which went into effect in July 2011, a bribery occurs when a person offers, gives or promises to give a financial or other advantage to induce or reward another individual to improperly perform certain functions or activities, including any function of a public nature. Bribery of foreign public officials also falls within the scope of the Bribery Act 2010. Under the new regime, an individual found in violation of the Bribery Act 2010, faces imprisonment of up to 10 years. In addition, the individual can be subject to an unlimited fine, as can commercial organizations for failure to prevent bribery.
Physician Referral Prohibitions
Under a federal law directed at “self-referral,” commonly known as the “Stark Law,” there are prohibitions, with certain exceptions, on referrals for certain designated health services, including laboratory services, that are covered by the Medicare and Medicaid programs by physicians who personally, or through a family member, have an investment or ownership interest in, or a compensation arrangement with, an entity performing the tests. The prohibition also extends to payment for any testing referred in violation of the Stark Law. A person who engages in a scheme to circumvent the Stark Law’s referral prohibition may be fined up to $100,000 for each such arrangement or scheme. In addition, any person who presents or causes to be presented a claim to the Medicare or Medicaid programs in violation of the Stark Law is subject to civil monetary penalties of up to $15,000 per bill submission, an assessment of up to three times the amount claimed and possible exclusion from participation in federal governmental payor programs. Bills submitted in violation of the Stark Law may not be paid by Medicare or Medicaid, and any person collecting any amounts with respect to any such prohibited bill is obligated to refund such amounts. Many states have comparable laws that are not limited to Medicare and Medicaid referrals.
Corporate Practice of Medicine
Numerous states have enacted laws prohibiting business corporations, such as us, from practicing medicine and employing or engaging physicians to practice medicine, generally referred to as the prohibition against the corporate practice of medicine. These laws are designed to prevent interference in the medical decision-making process by anyone who is not a licensed physician. For example, California’s Medical Board has indicated that determining what diagnostic tests are appropriate for a particular condition and taking responsibility for the ultimate overall care of the patient, including providing treatment options available to the patient, would constitute the unlicensed practice of medicine if performed by an unlicensed person. Violation of these corporate practice of medicine laws may result in civil or criminal fines, as well as sanctions imposed against us and/or the professional through licensure proceedings. Typically such laws are only applicable to entities that have a physical presence in the state.
Other Regulatory Requirements
Our laboratory is subject to federal, state and local regulations relating to the handling and disposal of regulated medical waste, hazardous waste and biohazardous waste, including chemical, biological agents and compounds, blood and bone marrow samples, and other human tissue. Typically, we use outside vendors who are contractually obligated to comply with applicable laws and regulations to dispose of such waste. These vendors are licensed or otherwise qualified to handle and dispose of such waste.
The U.S. Occupational Safety and Health Administration has established extensive requirements relating to workplace safety for health care employers, including requirements to develop and implement programs to protect workers from exposure to blood-borne pathogens by preventing or minimizing any exposure through needle stick or similar penetrating injuries.
48
Table of Contents
Segment, Revenue and Geographical Information
We operate in one reportable business segment and derive revenue from multiple countries, with 92.7%, 85.8%, and 83.5% of our total revenue coming from the United States in fiscal years 2015, 2014 and 2013, respectively. In 2015, we generated total revenue of $93.2 million from the sale of our products, compared to $61.1 million in 2014 and $29.0 million in 2013.
In January 2015, we announced a broad strategic collaboration with Roche Holdings, Inc. and certain of its affiliates. This relationship has continued to expand and Roche accounted for more than 10% of our revenues in the year ended December 31, 2015.
Employees
As of January 31, 2016, we had 417 full-time employees, with 356 in technology, research and development, business development and laboratory and commercial operations, and 61 in general and administrative functions. We had 340 full-time employees in our Cambridge, Massachusetts facilities, and 77 of our full-time employees work remotely. None of our employees is represented by a labor union with respect to his or her employment with us.
Research and Development Expenses
Research and development expenses increased to $43.9 million for the year ended December 31, 2015 from $30.6 million for the year ended December 31, 2014. The increase was due to a $5.4 million increase in employee and contractor-related expenses, a $3.9 million increase in laboratory supply costs, including reagents utilized in research and development activities, a $1.6 million increase in laboratory management expenses, a $1.6 million increase in rent and other facilities costs, and a $0.8 million increase in clinical trial costs.
Research and development expenses increased to $30.6 million for the year ended December 31, 2014 from $24.9 million for the year ended December 31, 2013. The increase was primarily due to a $4.5 million increase in employee and contractor-related expenses, a $1.4 million increase in technology-related support and maintenance, a $0.2 million increase in facilities, partially offset by a $0.3 million decrease in clinical trials expense, and a $0.1 million decrease in research-related lab supplies and materials.
Where You Can Find More Information
Our website address is www.foundationmedicine.com. Our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, including exhibits, proxy and information statements and amendments to those reports filed or furnished pursuant to Sections 13(a), 14, and 15(d) of the Securities Exchange Act of 1934, as amended, or the Exchange Act, are available through the “Investors” portion of our website free of charge as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. Information on our website is not part of this Annual Report on Form 10-K or any of our other securities filings unless specifically incorporated herein by reference. The public may read and copy these materials at the SEC’s Public Reference Room at 100 F Street, NE, Washington, DC 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. In addition, our filings with the SEC may be accessed through the SEC’s Interactive Data Electronic Applications system at http://www.sec.gov. All statements made in any of our securities filings, including all forward-looking statements or information, are made as of the date of the document in which the statement is included, and we do not assume or undertake any obligation to update any of those statements or documents unless we are required to do so by law.
49
Table of Contents
| ITEM 1A. | RISK FACTORS |
The following risks and uncertainties, together with all other information in this Annual Report, including our consolidated financial statements and related notes, should be considered carefully. Any of the risk factors we describe below could adversely affect our business, financial condition or results of operations, and could cause the market price of our common stock to fluctuate or decline.
Risks Relating to Our Business and Strategy
We may not be able to generate sufficient revenue from FoundationOne, FoundationOne Heme, FoundationACT, or our relationships with our biopharmaceutical partners to achieve and maintain profitability.
We believe our commercial success is dependent upon our ability to successfully market and sell our first molecular information products, FoundationOne for solid tumors, FoundationOne Heme for blood-based cancers, or hematologic malignancies, and FoundationACT, our blood-based (liquid biopsy) assay to measure ctDNA, which we plan to launch commercially to ordering physicians in March 2016, to physicians in clinical practice, to continue to expand our current relationships and develop new relationships with biopharmaceutical partners, and to develop and commercialize new molecular information products. The demand for our existing products may decrease or may not continue to increase at historical rates for a number of reasons, including among others increased competition from companies that offer similar molecular diagnostic tests. In addition, FoundationOne and FoundationOne Heme have coverage decisions from only a few commercial third-party payors and do not have coverage contracts with or coverage decisions from most commercial third-party payors or any government payors, including Medicare. FoundationACT does not yet have coverage contracts with or coverage decisions from any commercial third-party payors or any government payors. Certain commercial third-party payors have declined to reimburse FoundationOne and FoundationOne Heme because they have designated our products as experimental and investigational. This designation is customarily assigned to a product or service by a third-party payor pending the development of clinical information deemed sufficient to support a positive coverage decision. During this assessment period our products do not have the benefit of a positive coverage decision or a coverage contract from these third-party payors, resulting, in the aggregate, in a material loss of revenue to us. We have experienced revenue growth from the sale of each of FoundationOne and FoundationOne Heme to physicians since their formal commercial launches in June 2012 and December 2013, respectively. We may not be able to continue revenue growth or maintain existing revenue levels for a number of reasons, including because increased competition and lack of positive coverage decisions or coverage contracts from government or third-party payors may make it less likely that physicians will order our products.
Our biopharmaceutical partners may decide to decrease or discontinue their use of our molecular information platform due to changes in research and product development plans, failures in their clinical trials, financial constraints, or utilization of internal molecular testing resources or molecular tests performed by other parties, which are circumstances outside of our control. In addition, biopharmaceutical companies may decline to do business with us or decrease or discontinue their use of our molecular information platform due to our broad strategic collaboration with certain affiliates of Roche and the fact that Roche is our largest stockholder and beneficially owns a majority of our outstanding stock. In addition to reducing our revenue, if our biopharmaceutical partners decide to decrease or discontinue their use of our molecular information platform, this may reduce our exposure to early stage research that facilitates the incorporation of newly developed information about cancer into our molecular information platform and products.
We are currently not profitable. Even if we succeed in increasing adoption of our existing products by physicians, obtaining additional coverage decisions from commercial third-party payors and government payors, maintaining and creating relationships with our existing and new biopharmaceutical partners, and developing and commercializing additional molecular information products, we may not be able to generate sufficient revenue to achieve profitability.
50
Table of Contents
FoundationOne, FoundationOne Heme, and FoundationACT may never achieve significant commercial market acceptance.
FoundationOne, FoundationOne Heme, and FoundationACT may never gain significant acceptance in the marketplace and, therefore, may never generate substantial revenue or profits for us. Our ability to achieve commercial market acceptance for our existing products will depend on several factors, including:
| • | our ability to convince the medical community of the clinical utility of our products and their potential advantages over existing and new molecular tests; |
| • | the willingness of physicians and patients to utilize our products; and |
| • | the agreement by commercial third-party payors and government payors to reimburse our products, the scope and amount of which will affect patients’ willingness or ability to pay for our products and likely heavily influence physicians’ decisions to recommend our products. |
In addition, physicians may rely on guidelines issued by industry groups, such as the National Comprehensive Cancer Network, medical societies, such as the College of American Pathologists, or CAP, or other key oncology-related organizations before utilizing any diagnostic test. Although we have a number of clinical trials sponsored by us and clinical trials sponsored by individual physicians, or investigator-initiated clinical trials, underway to demonstrate the clinical utility of each of our existing products, none of our products are, and may never be, listed in any such guidelines.
We believe that the successful completion of clinical trials, publication of scientific and medical results in peer-reviewed journals, and presentations at leading conferences are critical to the broad adoption of our products. Publication in leading medical journals is subject to a peer-review process, and peer reviewers may not consider the results of studies involving our products sufficiently novel or worthy of publication.
The failure to be listed in physician guidelines or the failure of our trials to produce favorable results or to be published in peer-reviewed journals could limit the adoption of our products. Failure to achieve widespread market acceptance of our products would materially harm our business, financial condition, and results of operations.
We rely on a limited number of suppliers or, in some cases, sole suppliers, for some of our laboratory instruments and materials and may not be able to find replacements or immediately transition to alternative suppliers.
We rely on several sole suppliers, including Illumina, for certain laboratory substances used in the chemical reactions incorporated into our processes, or reagents, sequencers, equipment, and other materials which we use in our laboratory operations. The terms upon which we are able to purchase these supplies and materials from any supplier could be adversely affected by our broad strategic collaboration with Roche and the fact that Roche is our largest stockholder and beneficially owns a majority of our outstanding stock. An interruption in our laboratory operations could occur if we encounter delays or difficulties in securing these reagents, sequencers, or other laboratory materials, and if we cannot then obtain an acceptable substitute. Any such interruption could significantly affect our business, financial condition, results of operations, and reputation.
We rely on Illumina as the sole supplier of sequencers and various associated reagents, and as the sole provider of maintenance and repair services for these sequencers. Any disruption in Illumina’s operations could impact our supply chain and laboratory operations of our molecular information platform and our ability to conduct our business and generate revenue.
We believe that there are only a few other equipment manufacturers that are currently capable of supplying and servicing the equipment necessary for our laboratory operations, including sequencers and various associated reagents. The use of equipment or materials furnished by these replacement suppliers would require us to significantly alter our laboratory operations. Transitioning to a new supplier would be time-consuming and
51
Table of Contents
expensive, may result in interruptions in our laboratory operations, would likely affect the performance specifications of our laboratory operations, and would require that we revalidate our existing products. There can be no assurance that we would be able to secure alternative equipment, reagents, and other materials, and bring such equipment, reagents, and materials on line and revalidate them without experiencing interruptions in our workflow. In the case of an alternative supplier for Illumina, there can be no assurance that replacement sequencers and various associated reagents would be available or would meet our quality control and performance requirements for our laboratory operations. If we should encounter delays or difficulties in securing, reconfiguring, or revalidating the equipment and reagents we require for our products, our business, financial condition, results of operations and reputation could be adversely affected.
If our sole laboratory facility becomes damaged or inoperable, if we are required to vacate our laboratory facility, or if we are delayed in obtaining or unable to obtain additional laboratory space, our ability to conduct our genomic analyses, pursue our research and development efforts or our companion diagnostics partnerships, and fulfill our contractual obligations may be jeopardized.
We currently derive substantially all of our revenue from tests conducted at a single laboratory facility located in Cambridge, Massachusetts. Our facility and equipment could be harmed or rendered inoperable by natural or man-made disasters, including war, fire, earthquake, power loss, communications failure, or terrorism, which may render it difficult or impossible for us to operate our molecular information platform for some period of time. The inability to perform our molecular tests or to reduce the backlog of analyses that could develop if our facility is inoperable, for even a short period of time, may result in the loss of customers or harm to our reputation, and we may be unable to regain those customers or repair our reputation in the future. Furthermore, our facility and the equipment we use to perform our research and development work could be unavailable or costly and time-consuming to repair or replace. It would be difficult, time-consuming, and expensive to rebuild our facility or license or transfer our proprietary technology to a third party, particularly in light of the licensure and accreditation requirements for a commercial laboratory like ours. Even in the unlikely event we are able to find a third party with such qualifications to enable us to conduct our molecular tests, we may be unable to negotiate commercially reasonable terms with such third parties.
We may construct, acquire or enter into relationships with third parties to procure additional laboratory space inside and outside the United States to support our existing and new tests. Our Ex-U.S. Commercialization Agreement with Roche contemplates that we will provide additional laboratory space in Europe and Asia to perform genomic sequencing for FoundationOne and FoundationOne Heme in those geographies. In addition, our R&D Collaboration Agreement with Roche contemplates that we will collaborate with Roche on multiple programs related to the development of products and services for use in molecular information, immunotherapy, ctDNA, and companion diagnostics. If we are unable to obtain or are delayed in obtaining new laboratory space to support these commercialization and development efforts, we could fail to meet certain contractual obligations and agreed upon timelines with certain of our biopharmaceutical partners, including Roche, or provide existing products and develop and launch new products in certain territories, which could result in harm to our business and reputation, and adversely affect our business, financial condition and results of operations.
We carry insurance for damage to our property and laboratory and the disruption of our business, but this insurance may not cover all of the risks associated with damage to our property or laboratory or disruption to our business, may not provide coverage in amounts sufficient to cover our potential losses, and may not continue to be available to us on acceptable terms, if at all.
If we are unable to support demand for our existing and our future products, including ensuring that we have adequate capacity to meet increased demand, or we are unable to successfully manage the evolution of our molecular information platform, our business could suffer.
As our volume grows, including any potential increases in volume due to our collaboration with Roche, we will need to continue to increase our workflow capacity for sample intake, customer service, billing and general process improvements, expand our internal quality assurance program, and extend our molecular information
52
Table of Contents
platform to support comprehensive genomic analyses at a larger scale within expected turnaround times. We will need additional certified laboratory scientists and technicians and other scientific and technical personnel to process higher volumes of our molecular information products. Portions of our process are not automated and will require additional personnel to scale. We will also need to purchase additional equipment, some of which can take several months or more to procure, set up, and validate, and increase our software and computing capacity to meet increased demand. There is no assurance that any of these increases in scale, expansion of personnel, equipment, software and computing capacities, or process enhancements will be successfully implemented, or that we will have adequate space in our laboratory facility to accommodate such required expansion.
As we commercialize additional products, we will need to incorporate new equipment, implement new technology systems and laboratory processes, and hire new personnel with different qualifications. For example, we may procure additional laboratory space to allow us to further develop new products. Failure to manage this growth or transition could result in turnaround time delays, higher product costs, declining product quality, deteriorating customer service, and slower responses to competitive challenges. A failure in any one of these areas could make it difficult for us to meet market expectations for our products, and could damage our reputation and the prospects for our business.
New product development involves a lengthy and complex process, and we may be unable to successfully commercialize FoundationACT or any other products we may develop on a timely basis, or at all, and the development and commercialization of additional products may negatively affect the commercialization of existing products.
FoundationACT, our blood-based (liquid biopsy) assay to measure ctDNA, which we plan to launch commercially to ordering physicians in March 2016, will take time to successfully commercialize, and there can be no assurance that these products will be successful in the evaluation of cancers for a variety of technical and market reasons. Our future molecular information products, which are in various stages of early development, will take time to develop and commercialize, if we are able to commercialize them at all. There can be no assurance that our new products will be capable of reliably identifying relevant genomic alterations in various forms of cancer. Before we can commercialize any new products, we will need to expend significant funds in order to:
| • | conduct substantial research and development, including validation studies and potentially clinical trials; |
| • | build additional laboratory space for new products; |
| • | further develop and scale our laboratory processes to accommodate different products; and |
| • | further develop and scale our infrastructure to be able to analyze increasingly large amounts of data. |
Our product development process involves a high degree of risk, and product development efforts may fail for many reasons, including:
| • | failure of the product to perform as expected at the research or development stage; |
| • | lack of validation data; or |
| • | failure to demonstrate the clinical utility of the product. |
As we develop products, we will have to make significant investments in product development, marketing, and selling resources. In addition, the commercialization of newer products, such as FoundationACT, may also negatively affect the sales of existing products, such as FoundationOne or FoundationOne Heme, where the diagnostic applications overlap, to the extent physicians decide to order the newer product in lieu of our existing products.
53
Table of Contents
If we cannot compete successfully with our competitors, including new entrants in the market, we may be unable to increase or sustain our revenue or achieve and sustain profitability.
Personalized genomic diagnostics is a new area of science, and we face intense competition from companies that offer products or have conducted research to profile genes and gene expression in various cancers. Our principal competition comes from diagnostic companies that offer molecular diagnostic tests that capture only a single-marker or hotspot panel tests that capture a limited number of the most well-known gene alterations, as well as academic research centers, diagnostic companies and NGS, platform developers that are offering or developing NGS-based testing. In addition, there are an increasing number of sophisticated commercial competitors who are selling hot spot panel or NGS-based tests that they are marketing as comparable to, and/or more cost effective than, our existing products.
Our competitors include laboratory companies such as Bio-Reference Laboratories, Inc., Laboratory Corporation of America Holdings, Quest Diagnostics Incorporated, Molecular Health, Caris Life Sciences, Guardant Health, Paradigm, NeoGenomics Laboratories, as well as companies such as Abbott Laboratories, Qiagen N.V., and Sequenom, Inc. that manufacture or may manufacture diagnostic testing kits. In addition, companies such as Genomic Health, Inc. and Myriad Genetics, Inc. have well-established commercial organizations that sell molecular diagnostic tests for cancer to physicians and may develop tests which compete with our products. Recently, these competitors have been increasingly marketing their tests as comparable to, and/or more cost effective than, our products, and if such marketing strategies are successful, they could result in physicians determining not to order our products which, in turn, would have an adverse effect on our results of operations.
Many hospitals and academic medical centers may also seek to perform the type of molecular testing we perform at their own facilities. As such, our competition may include entities such as the MD Anderson Cancer Center, Memorial Sloan-Kettering Cancer Center, University of Michigan, Baylor Medical Genetics Laboratories, Washington University in St. Louis, University of Washington, and other academic hospitals and research centers.
In addition, Illumina, Thermo Fisher, and other companies market NGS platforms that are being sold directly to research centers, biopharmaceutical companies, and clinical laboratories. While these platforms have been largely utilized in research and development settings or testing for non-cancer conditions, each of these companies has launched and will likely continue to commercialize products for focused application in the clinical oncology market. We believe diagnostic platform providers will seek to place sequencing machines in laboratories and to develop NGS-based laboratory-developed tests, or LDTs, for use in clinical oncology, including by seeking to decrease the cost, size, and complexity of their platforms. In addition, Illumina has received approval by the FDA for a diagnostic kit for clinical use outside of oncology which is sold to clients who have purchased its platforms. We believe Illumina and other diagnostic platform providers may develop additional FDA-approved diagnostic kits for clinical use by clients who have purchased their platforms, potentially including for these clients to identify genetic alterations in samples of solid tumors or blood-based cancers. Also, many private companies are developing information technology-based tools to support the integration of NGS testing into the clinical setting. The successful development and marketing of these products by diagnostic platform providers could enable some of our potential customers to perform clinical-grade, comprehensive genomic analyses, which could have a material adverse effect on our business and financial condition. These companies may also use their patent portfolios, developed in connection with developing their tests, to allege that our products infringe their patents, and we could face litigation with respect to such allegations and the validity of such patents.
Because our proprietary molecular information platform consists largely of trade-secret protected technology and know-how and has only limited patent protection, new and existing companies could seek to develop molecular tests that compete with ours. These competitors could have technological, financial, and
54
Table of Contents
market access advantages that are not currently available to us and they could develop and commercialize competing products faster than we are able to do so. Additional competition, including price competition, could have a material adverse impact on our net revenues and profitability.
The molecular diagnostic industry is subject to rapidly changing technology, which could make our molecular information platform, FoundationOne, FoundationOne Heme, FoundationACT, and other products we develop obsolete.
Our industry is characterized by rapid technological changes, frequent new product introductions and enhancements, and evolving industry standards, all of which could make our molecular information platform and existing and future molecular information products obsolete. Our future success will depend on our ability to keep pace with the evolving needs of our customers on a timely and cost-effective basis and to pursue new market opportunities that develop as a result of technological and scientific advances. In recent years, there have been numerous advances in technologies relating to the diagnosis and treatment of cancer. There have also been advances in methods used to analyze very large amounts of genomic information, including methods used by our competitors, such as diagnostic testing of ctDNA. We must continuously enhance our molecular information platform and develop new products to keep pace with evolving standards of care. If we do not update our molecular information platform to reflect new scientific knowledge about cancer biology, information about new cancer therapies, or relevant clinical trials, our molecular information platform could become obsolete and sales of our existing and any new products could decline, which would have a material adverse effect on our business, financial condition, and results of operations.
If our products do not perform as expected, we may not be able to achieve widespread market adoption among physicians, which would cause our operating results, reputation, and business to suffer.
Our success depends on the market’s confidence that we can provide reliable, high-quality molecular information products. There is no guarantee that the accuracy and reproducibility we have demonstrated to date will continue, particularly for clinical samples, as our test volume increases. We believe that our customers are likely to be particularly sensitive to product defects and errors, including if our products fail to detect genomic alterations with high accuracy from clinical specimens or if we fail to list, or inaccurately include, certain treatment options and available clinical trials in our product reports. As a result, the failure of our products to perform as expected would significantly impair our operating results and our reputation. We may be subject to legal claims arising from any defects or errors.
We refer to the efficiency of our sequencing process as its yield. The sequencing process yields that we achieve depend on the design and operation of our sequencing process, which uses a number of complex and sophisticated biochemical, informatics, optical, and mechanical processes, many of which are highly sensitive to external factors. An operational or technological failure in one of these complex processes or fluctuations in external variables may result in sequencing processing yields that are lower than we anticipate or that vary between sequencing runs. In addition, we are regularly evaluating and refining our sequencing process. These refinements may initially result in unanticipated issues that further reduce our sequencing process yields or increase the variability of our sequencing process yields. Low sequencing process yields, or higher than anticipated variability of our sequencing processing yields, increase total sequencing costs and reduce the number of samples we can sequence in a given time period, which can cause variability in our operating results and damage our reputation.
In addition, our product reports match identified genomic alterations with FDA-approved targeted therapies or relevant clinical trials of targeted therapies. If a patient or physician who orders one of our products is unable to obtain, or be reimbursed for the use of, targeted therapies because they are not indicated in the FDA-approved product label for treatment of a patient’s cancer, the patient is unable to enroll in an identified clinical trial due to the enrollment criteria of the trial, or some other reason, the patient or ordering physician may conclude the test
55
Table of Contents
report does not contain actionable information. If physicians do not believe our products consistently generate actionable information about their patients’ cancers, they may be less likely to order our products, our reputation could be harmed, and our business and results of operations could suffer.
Furthermore, we have made significant investments in various technologies to support adoption of our molecular information products. For example, our test reports are now available to physicians using FoundationICE, the newest version of our online Interactive Cancer Explorer. We believe FoundationICE will help physicians to more efficiently use our clinical products and will enhance the utility of comprehensive genomic profiling. We also continue to develop innovative new decision support applications, like PatientMatch, GeneKit and SmartTrials, that support informed treatment decision-making and enable improved access to therapies. If these technologies do not work as expected or fail to gain broad utilization among clinical physicians, they will not drive adoption of our molecular information products, which would have an adverse impact on our revenues and business operations.
If we lose the support of key thought leaders, it may be difficult to establish products enabled by our molecular information platform as a standard of care for patients with cancer, which may limit our revenue growth and ability to achieve profitability.
We have established relationships with leading oncology thought leaders at premier cancer institutions and oncology networks such as the Memorial Sloan-Kettering Cancer Center and the Taussig Cancer Institute at the Cleveland Clinic. If these key thought leaders determine that our molecular information platform, our existing products or other products that we develop are not clinically effective, that alternative technologies are more effective, or if they elect to use internally developed products, we would encounter significant difficulty validating our testing platform, driving adoption, or establishing our molecular information platform and tests as a standard of care, which would limit our revenue growth and our ability to achieve profitability.
If we cannot maintain our current relationships, or enter into new relationships, with biopharmaceutical companies, our product development could be delayed.
We deploy our molecular information platform to analyze tissue samples provided by biopharmaceutical partners from their clinical trials. We have entered into agreements with biopharmaceutical companies in the cancer field including, for example, Agios, ARIAD Pharmaceuticals, Inc., Array BioPharma Inc., AstraZeneca UK Limited, Celgene Corporation, Clovis Oncology, Eisai Co., Ltd., Johnson & Johnson, and Novartis, among others, as well as our broad collaboration with Roche which closed in April 2015. For the years ended December 31, 2014 and 2013, our alliance with Novartis accounted for more than 10% of our total revenue. For the year ended December 31, 2015, our broad collaboration with Roche accounted for more than 10% of our total revenue. We expect that our broad collaboration with Roche will account for a material portion of our revenue in future years. The revenue attributable to Novartis or Roche may fluctuate in the future, which could have an adverse effect on our financial condition and results of operations. In addition, the termination of either of these relationships could result in a temporary or permanent loss of revenue to us.
Our success in the future depends in part on our ability to maintain these relationships and to enter into new relationships. This can be difficult due to several factors, including internal and external constraints placed on these organizations that can limit the number and type of relationships with companies like us that can be considered and consummated; the agreements governing our relationships are generally terminable at will by our biopharmaceutical customers; our biopharmaceutical customers may be dissatisfied with our products; and continued usage of our products among particular biopharmaceutical customers may depend on whether the partner obtains positive data in its clinical trials or other administrative factors that are outside our control. Additionally, some of our biopharmaceutical partners have contracted with us to provide testing for large numbers of samples, which could strain our testing capacity and restrict our ability to perform additional tests for other customers. If we fail to maintain these relationships, or enter into new ones, our business could suffer.
56
Table of Contents
In addition, certain biopharmaceutical companies, including those with which we currently have agreements, may choose not to do business with us or may seek out other partners for molecular information, due to our broad strategic collaboration with Roche and the fact that Roche is our largest stockholder and beneficially owns a majority of our outstanding stock, particularly if they are actual or potential competitors with Roche. If we are unable to continue to grow our business with biopharmaceutical companies, our business and results of operations would be adversely affected.
From time to time, we expect to engage in discussions with biopharmaceutical companies regarding commercial opportunities. There is no assurance that any of these discussions will result in a commercial agreement, or if an agreement is reached, that the resulting engagement will be successful or that any clinical studies conducted as part of the engagement will produce successful outcomes. Speculation in the industry about our existing or potential engagements with biopharmaceutical companies can be a catalyst for adverse speculation about us, our products, and our technology, which can result in harm to our reputation and our business.
We may be unable to manage our future growth effectively, which could make it difficult to execute our business strategy.
We anticipate growth in our business operations, including the addition of new laboratory and office space inside and outside the United States. This future growth could create strain on our organizational, administrative, and operational infrastructure, including laboratory operations, quality control, customer service, and sales force management. We may not be able to maintain the quality or expected turnaround times of our products or satisfy customer demand as it grows. Our ability to manage our growth properly will require us to continue to improve our operational, financial, and managerial controls, as well as our reporting systems and procedures. We plan to implement new enterprise software systems in a number of areas affecting a broad range of business processes and functional areas. The time and resources required to implement these new systems is uncertain, and failure to complete this in a timely and efficient manner could adversely affect our operations.
We have limited experience in marketing and selling our products, and if we are unable to expand our direct sales and marketing force to adequately address our customers’ needs, our business may be adversely affected.
We have limited experience in marketing and selling FoundationOne Heme, which launched in December 2013, and FoundationACT, which was launched for research use to our biopharmaceutical partners in December 2015 and which we plan to launch commercially to ordering physicians in March 2016. We may not be able to market, sell, or distribute our existing products or other products we may develop effectively enough to support our planned growth.
Our future sales in the United States will depend in large part on our ability to develop, and substantially expand, our sales force and to increase the scope of our marketing efforts. Our target market of physicians is a large and diverse market. As a result, we believe it is necessary to develop a sales force that includes sales representatives with specific technical backgrounds. We will also need to attract and develop marketing personnel with industry expertise. Competition for such employees is intense. We may not be able to attract and retain personnel or be able to build an efficient and effective sales and marketing force, which could negatively impact sales and market acceptance of our products and limit our revenue growth and potential profitability.
Our expected future growth will impose significant added responsibilities on members of management, including the need to identify, recruit, maintain, and integrate additional employees. Our future financial performance and our ability to commercialize our products and to compete effectively will depend in part on our ability to manage this potential future growth effectively, without compromising quality.
Pursuant to our Ex-U.S. Commercialization Agreement with Roche, commencing in April 2016, Roche will have the exclusive right to commercialize FoundationOne, FoundationOne Heme, any clinical diagnostic products developed under our R&D Collaboration Agreement with Roche, including FoundationACT, and any
57
Table of Contents
other products upon mutual agreement, in each case outside of the United States to the extent Roche has not elected to exclude any countries from its territory. Subject to satisfaction of certain performance milestones, the Ex-U.S. Commercialization Agreement will remain in effect for five years and may be extended by Roche for additional two-year periods. Roche shall have the right to terminate the agreement without cause upon six months’ written notice after the initial five year term, and either party may terminate the agreement in the event of breach by the other party. During the term of the Ex-U.S. Commercialization Agreement, we will be relying on Roche’s efforts to sell and market FoundationOne, FoundationOne Heme, FoundationACT, and potentially other future molecular information products outside of the United States, and if Roche’s sales and marketing efforts are not successful, we may not achieve significant market acceptance of our products outside the United States, which would materially and adversely impact our business operations.
For any jurisdictions outside of the United States that Roche elects to exclude from its territory, if we believe a significant market opportunity for our products exists, we intend to enlist distribution partners and local laboratories to assist with sales, distribution, and customer support. We may not be successful in finding, attracting, and retaining distribution partners or laboratories, or we may not be able to enter into such arrangements on favorable terms. Sales practices utilized by our distribution partners that are locally acceptable may not comply with sales practices standards required under United States laws that apply to us, which could create additional compliance risk. If these additional sales and marketing efforts are not successful, we may not achieve significant market acceptance for our products in these markets, which could harm our business.
The loss of any member of our senior management team or our inability to attract and retain highly skilled scientists, clinicians, and salespeople, or the diversion of management’s attention due to the implementation of our collaboration with Roche, could adversely affect our business.
Our success depends on the skills, experience and performance of key members of our senior management team, including Michael J. Pellini, M.D., our Chief Executive Officer. The individual and collective efforts of these employees will be important as we continue to develop our molecular information platform and additional products, and as we expand our commercial activities. The loss or incapacity of existing members of our senior management team could adversely affect our operations if we experience difficulties in hiring qualified successors. All members of our senior management team have employment agreements; however, the existence of an employment agreement does not guarantee the retention of the employee for any period of time. We do not maintain “key person” insurance on any of our employees.
Our research and development programs and laboratory operations depend on our ability to attract and retain highly skilled scientists and technicians. We may not be able to attract or retain qualified scientists and technicians in the future due to the intense competition for qualified personnel among life science businesses, particularly in Cambridge, Massachusetts. We also face competition from universities and public and private research institutions in recruiting and retaining highly qualified scientific personnel. We may have difficulties locating, recruiting, or retaining qualified sales people. In addition, our obligation to repurchase shares of our common stock pursuant to Roche’s anti-dilution protections set forth in the Investor Rights Agreement may result in changes to our equity compensation programs, which could impact our ability to attract and retain key personnel. Recruitment and retention difficulties can limit our ability to support our research and development and sales programs. All of our employees are at will, which means that either we or the employee may terminate their employment at any time.
The implementation of our broad strategic collaboration with Roche may also divert management’s focus and resources from other strategic opportunities and operational matters. In addition, this implementation could cause management and employee disruption, resulting in the possible loss of key management, sales and marketing, technical or other personnel. If we experience any of these implementation-related issues, our business could be harmed.
58
Table of Contents
If we were sued for product liability or professional liability, we could face substantial liabilities that exceed our resources.
The marketing, sale, and use of our products could lead to the filing of product liability claims were someone to allege that our products identified inaccurate or incomplete information regarding the genomic alterations of the tumor or malignancy analyzed, reported inaccurate or incomplete information concerning the available therapies for a certain type of cancer, or otherwise failed to perform as designed. We may also be subject to liability for errors in, a misunderstanding of, or inappropriate reliance upon the information we provide in the ordinary course of our business activities. A product liability or professional liability claim could result in substantial damages and be costly and time-consuming for us to defend.
We maintain product and professional liability insurance, but this insurance may not fully protect us from the financial impact of defending against product liability or professional liability claims. Any product liability or professional liability claim brought against us, with or without merit, could increase our insurance rates or prevent us from securing insurance coverage in the future. Additionally, any product liability lawsuit could damage our reputation or cause current clinical or biopharmaceutical partners to terminate existing agreements and potential clinical or biopharmaceutical partners to seek other partners, any of which could impact our results of operations.
We depend on our information technology systems, and any failure of these systems could harm our business.
We depend on information technology and telecommunications systems for significant elements of our operations, including our laboratory information management system, our computational biology system, our knowledge management system, our customer reporting, and our FoundationICE and GeneKit portals. We have installed and are expanding a number of enterprise software systems that affect a broad range of business processes and functional areas, including, for example, systems handling human resources, financial controls and reporting, contract management, regulatory compliance, and other infrastructure operations. In addition to the aforementioned business systems, we continue to extend the capabilities of both our preventative and detective security controls by augmenting the monitoring and alerting functions, the network design, and the automatic countermeasure operations of our technical systems. These information technology and telecommunications systems support a variety of functions, including laboratory operations, test validation, sample tracking, quality control, customer service support, billing and reimbursement, research and development activities, scientific and medical curation, and general administrative activities. In addition, our third-party billing and collections provider depends upon technology and telecommunications systems provided by outside vendors. Information technology and telecommunications systems are vulnerable to damage from a variety of sources, including telecommunications or network failures, malicious human acts, and natural disasters. Moreover, despite network security and back-up measures, some of our servers are potentially vulnerable to physical or electronic break-ins, computer viruses, and similar disruptive problems. Failures or significant downtime of our information technology or telecommunications systems, or those used by our third-party service providers, could prevent us from conducting our comprehensive genomic analyses, preparing and providing reports and data to pathologists and oncologists, billing payors, processing reimbursement appeals, handling patient or physician inquiries, conducting research and development activities, and managing the administrative aspects of our business. Any disruption or loss of information technology or telecommunications systems on which critical aspects of our operations depend could have an adverse effect on our business.
Security breaches, loss of data, and other disruptions could compromise sensitive information related to our business or prevent us from accessing critical information and expose us to liability, which could adversely affect our business and our reputation.
In the ordinary course of our business, we and our third-party billing and collections provider collect and store sensitive data, including legally protected health information, personally identifiable information, intellectual property, and proprietary business information owned or controlled by us or our customers, payors,
59
Table of Contents
and biopharmaceutical partners. We manage and maintain our applications and data utilizing a combination of on-site systems, managed data center systems, and cloud-based data center systems. We also communicate, and facilitate the exchange of, sensitive patient data to and between customers and their contracted or affiliated health care providers through our FoundationICE and GeneKit portals. These applications and related data encompass a wide variety of business-critical information including research and development information, commercial information, and business and financial information. We face four primary risks relative to protecting this critical information, including: unauthorized access risk; inappropriate disclosure risk; inappropriate modification risk; and the risk of our being unable to adequately monitor our controls over the first three risks.
The secure processing, storage, maintenance, and transmission of this critical information is vital to our operations and business strategy, and we devote significant resources to protecting such information. Although we take measures to protect sensitive information from unauthorized access or disclosure, our information technology and infrastructure, and that of our third-party billing and collections provider, may be vulnerable to attacks by hackers or viruses or breached due to personnel error, malfeasance, or other disruptions. Any such breach or interruption could compromise the security or integrity of our networks, and the information stored there could be accessed by unauthorized parties, publicly disclosed, corrupted, lost, or stolen. Any such access, disclosure, corruption or other loss or theft of information could result in governmental investigations, class action legal claims or proceedings, liability under laws that protect the privacy of personal information, such as HIPAA, and regulatory penalties. Although we have implemented security measures and a formal, dedicated enterprise security program to prevent unauthorized access to patient data, FoundationICE and GeneKit are currently accessible through online portals and may, in the future, be accessible through dedicated mobile applications, and there is no guarantee we can absolutely protect our online portals or our mobile applications from breach. Unauthorized access to, or loss or dissemination of, the data embedded in or transferred via these applications could also disrupt our operations, including our ability to conduct our analyses, provide test results, bill payors or patients, process claims and appeals, provide customer assistance services, conduct research and development activities, collect, process, and prepare company financial information, provide information about our products and other patient and physician education and outreach efforts through our website, manage the administrative aspects of our business, and damage our reputation, any of which could adversely affect our business.
The U.S. Office of Civil Rights may impose penalties on a covered entity for a failure to comply with a requirement of HIPAA. Penalties will vary significantly depending on factors such as the date of the violation, whether the covered entity knew or should have known of the failure to comply, or whether the covered entity’s failure to comply was due to willful neglect. These penalties include civil monetary penalties of $100 to $50,000 per violation, up to an annual cap of $1,500,000. A person who knowingly obtains or discloses individually identifiable health information in violation of HIPAA may face a criminal penalty of up to $50,000 and up to one year imprisonment. The criminal penalties increase to $100,000 and up to five years imprisonment if the wrongful conduct involves false pretenses, and to $250,000 and up to 10 years imprisonment if the wrongful conduct involves the intent to sell, transfer, or use identifiable health information for commercial advantage, personal gain, or malicious harm. The U.S. Department of Justice is responsible for criminal prosecutions under HIPAA. Furthermore, in the event of a breach as defined by HIPAA, the covered entity has specific reporting requirements under HIPAA regulations. In the event of a significant breach, the reporting requirements could include notification to the general public.
In addition, the interpretation and application of consumer, health-related, and data protection laws in the United States, Europe, and elsewhere are often uncertain, contradictory, and in flux. It is possible that these laws may be interpreted and applied in a manner that is inconsistent with our practices. If so, this could result in government-imposed fines or orders requiring that we change our practices, which could adversely affect our business. In addition, these privacy regulations may differ from country to country, and may vary based on whether testing is performed in the United States or in the local country. Our operations or business practices may not comply with these regulations in each country, and complying with these various laws could cause us to incur substantial costs or require us to change our business practices and compliance procedures in a manner adverse to our business.
60
Table of Contents
Compliance with changing European privacy laws could require us to incur significant costs or experience significant business disruption and failure to so comply could result in an adverse impact on our business.
In Europe, Directive 95/46/EC of the European Parliament and of the Council of 24 October 1995 on the protection of individuals with regard to the processing of personal data and on the free movement of such data, or the Directive, has required European Union, or EU, member states to implement data protection laws to meet the strict privacy requirements of the Directive. Among other requirements, the Directive regulates transfers of personally identifiable data that is subject to the Directive, or Personal Data, to third countries, such as the United States, that have not been found to provide adequate protection to such Personal Data. We have in the past relied upon adherence to the U.S. Department of Commerce’s Safe Harbor Privacy Principles and compliance with the U.S.-EU and U.S.-Swiss Safe Harbor Frameworks as agreed to and set forth by the U.S. Department of Commerce, and the European Union and Switzerland, which established a means for legitimating the transfer of Personal Data by data controllers in the European Economic Area, or the EEA, to the United States. As a result of the October 6, 2015 European Union Court of Justice, or ECJ, opinion in Case C-362/14 (Schrems v. Data Protection Commissioner) regarding the adequacy of the U.S.-EU Safe Harbor Framework, the U.S. – EU Safe Harbor Framework is no longer deemed to be a valid method of compliance with requirements set forth in the Directive (and member states’ implementations thereof) regarding the transfer of Personal Data outside of the EEA.
Recently, it was announced that negotiators from Europe and the United States reached political agreement on a successor to the Safe Harbor framework that will be referred to as the EU-US Privacy Shield. However, it is likely to be months before all of the details regarding the Privacy Shield program are finalized and a procedure is introduced to allow interested companies to participate in the program. While the details regarding the Privacy Shield program continue to be finalized, we will continue to face uncertainty as to whether our efforts to comply with our obligations under European privacy laws will be sufficient. If we are investigated by a European data protection authority, we may face fines and other penalties. Any such investigation or charges by European data protection authorities could have a negative effect on our existing business and on our ability to attract and retain new customers.
In light of the ECJ opinion in the Schrems case, we anticipate undertaking efforts to conform transfers of Personal Data from the EEA based on current regulatory obligations, the guidance of data protection authorities, and evolving best practices. Despite these efforts, we may be unsuccessful in establishing conforming means of transferring such data from the EEA, including due to ongoing legislative activity, which may vary the current data protection landscape.
We may also experience hesitancy, reluctance, or refusal by European or multi-national customers to continue to use our services due to the potential risk exposure to such customers as a result of the ECJ ruling in the Schrems case and the current data protection obligations imposed on them by certain data protection authorities. Such customers may also view any alternative approaches to compliance as being too costly, too burdensome, too legally uncertain or otherwise objectionable and therefore decide not to do business with us.
We and our customers are at risk of enforcement actions taken by certain EU data protection authorities until such point in time that we may be able to ensure that all transfers of Personal Data to us from the EEA are conducted in compliance with all applicable regulatory obligations, the guidance of data protection authorities, and evolving best practices. We may find it necessary to establish systems to maintain Personal Data originating from the EU in the EEA, which may involve substantial expense and may cause us to need to divert resources from other aspects of our business, all of which may adversely affect our business.
The Directive may be replaced in time with the pending European General Data Protection Regulation, which may impose additional obligations and risk upon our business and which may increase substantially the penalties to which we could be subject in the event of any non-compliance. We may incur substantial expense in complying with the new obligations to be imposed by the European General Data Protection Regulation and we may be required to make significant changes in our business operations, all of which may adversely affect our revenues and our business overall.
61
Table of Contents
We may acquire other businesses, form joint ventures, or make investments in other companies or technologies that could negatively affect our operating results, dilute our stockholders’ ownership, increase our debt, or cause us to incur significant expense.
Our business strategy may, from time to time, include pursuing acquisitions of businesses and assets. We also may pursue strategic alliances and joint ventures that leverage our proprietary molecular information platform and industry experience to expand our offerings or distribution. We have no experience with acquiring other companies. Negotiating these transactions and the formation of strategic alliances or joint ventures can be time-consuming, difficult and expensive, and our ability to close these transactions may be subject to third-party approvals, including, in some cases, the approval of Roche pursuant to the terms of the Investor Rights Agreement, as well as governmental authorities, which are beyond our control. In addition, some third parties may choose not to enter into partnership arrangements with us because of our relationship with Roche. Consequently, we may not be able to complete such transactions on favorable terms or at all, and we can make no assurance that these transactions, once undertaken and announced, will close.
An acquisition or investment may result in unforeseen operating difficulties and expenditures. Specifically, we may not be able to integrate the businesses, products, personnel, or operations of the acquired companies, particularly if key personnel of the acquired business choose not to work for us, we could assume unknown or contingent liabilities, and we may have difficulty retaining the customers of any acquired business. Acquisitions also could result in the incurrence of debt, contingent liabilities, or future write-offs of intangible assets or goodwill, any of which could have a material adverse effect on our financial condition, results of operations, and cash flows. Integration of an acquired company also may disrupt ongoing operations and require management resources that we would otherwise focus on developing our existing business. As a result, we cannot be assured that the anticipated benefits of any acquisition, technology license, strategic alliance, or joint venture would be realized or that we would not be exposed to unknown liabilities. These challenges related to acquisitions or investments could adversely affect our business, results of operations, and financial condition.
To finance any acquisitions or joint ventures, we may choose to issue shares of our common stock as consideration, which could dilute the ownership of our stockholders, if the issuance does not trigger our repurchase obligations under the Investor Rights Agreement, and be subject to the prior consent of Roche, which might not be given. Additional funds may not be available on terms that are favorable to us, or at all. If the price of our common stock is low or volatile, or if Roche does not provide consent for transactions requiring their approval, we may not be able to acquire other companies or fund a joint venture project using our stock as consideration.
International expansion of our business exposes us to business, regulatory, political, operational, financial, and economic risks associated with doing business outside of the United States.
We currently have limited international operations, but our business strategy incorporates plans for significant international expansion through our collaboration with Roche. Pursuant to our Ex-U.S. Commercialization Agreement with Roche, commencing in April 2016, Roche will have the exclusive right to commercialize FoundationOne, FoundationOne Heme, any clinical diagnostic products developed under our R&D Collaboration Agreement with Roche, including FoundationACT, and any other products upon mutual agreement, in each case outside of the United States to the extent Roche has not elected to exclude any countries from its territory. Subject to satisfaction of certain performance milestones, the Ex-U.S. Commercialization Agreement will remain in effect for five years and may be extended by Roche for additional two-year periods. Roche shall have the right to terminate the agreement without cause upon six months’ written notice after the initial five year term, and either party may terminate the agreement in the event of breach by the other party. Until Roche assumes responsibility for commercialization of our products outside of the United States, we intend to continue to rely on distributor relationships to conduct physician and patient association outreach activities and to expand payor relationships outside of the United States.
62
Table of Contents
Doing business internationally involves a number of risks, including:
| • | multiple, conflicting, and changing laws and regulations such as data protection laws, privacy regulations, tax laws, export and import restrictions, employment laws, regulatory requirements, and other governmental approvals, permits, and licenses; |
| • | failure by us, Roche or our distributors to obtain regulatory approvals for the manufacture, sale and use of our products in various countries; |
| • | any decisions by us or our distributors to terminate our distribution relationships in various countries; |
| • | additional, potentially relevant third-party patent rights; |
| • | complexities and difficulties in obtaining protection for and enforcing our intellectual property; |
| • | difficulties in staffing and managing foreign operations; |
| • | complexities associated with managing multiple payor reimbursement regimes, government payors, or patient self-pay systems; |
| • | logistics and regulations associated with preparing, shipping, importing and exporting tissue samples, including infrastructure conditions, transportation delays, and customs; |
| • | limits in our ability to penetrate international markets if we are not able to conduct our molecular tests locally; |
| • | financial risks, such as longer payment cycles, difficulty collecting accounts receivable, the impact of local and regional financial crises on demand and payment for our products, and exposure to foreign currency exchange rate fluctuations; |
| • | natural disasters, political and economic instability, including wars, terrorism, and political unrest, outbreak of disease, boycotts, curtailment of trade, and other business restrictions; and |
| • | regulatory and compliance risks that relate to maintaining accurate information and control over sales and distribution activities that may fall within the purview of the U.S. Foreign Corrupt Practices Act, or FCPA, including its books and records provisions, or its anti-bribery provisions. |
Any of these factors could significantly harm our future international expansion and operations and, consequently, our revenue and results of operations.
We could be adversely affected by violations of the FCPA and other worldwide anti-bribery laws.
International customers have ordered, or may, in the future, order our products, and we are, therefore, subject to the FCPA, which prohibits companies and their intermediaries from making payments in violation of law to non-U.S. government officials for the purpose of obtaining or retaining business or securing any other improper advantage. Our current reliance on third-party distributors to sell our products internationally demands a high degree of vigilance in maintaining our policy against participation in corrupt activity, because these distributors could be deemed to be our agents, and we could be held responsible for their actions. Other U.S. companies in the medical device and pharmaceutical field have faced criminal penalties under the FCPA for allowing their agents to deviate from appropriate practices in doing business with these individuals. We are also subject to similar anti-bribery laws in the jurisdictions in which we operate, including the United Kingdom’s Bribery Act of 2010, which prohibits commercial bribery and makes it a crime for companies to fail to prevent bribery. These laws are complex and far-reaching in nature, and, as a result, we cannot assure you that we would not be required in the future to alter one or more of our practices to be in compliance with these laws, any changes in these laws, or the interpretation thereof. Any violations of these laws, or allegations of such violations, could disrupt our operations, involve significant management distraction, involve significant costs and expenses, including legal fees, and could result in a material adverse effect on our business, prospects, financial condition, or results of operations. We could also suffer severe penalties, including criminal and civil penalties, disgorgement, and other remedial measures.
63
Table of Contents
Our employees, principal investigators, consultants, and commercial partners may engage in misconduct or other improper activities, including non-compliance with regulatory standards and requirements, and insider trading.
We are exposed to the risk of fraud or other misconduct by our employees, principal investigators, consultants, and commercial partners. Misconduct by these parties could include intentional failures to comply with the regulations of the FDA and non-U.S. regulators, comply with healthcare fraud and abuse laws and regulations in the United States and abroad, report financial information or data accurately, or disclose unauthorized activities to us. In particular, sales, marketing, and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing, and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs, and other business arrangements. Such misconduct could also involve the improper use of information obtained in the course of clinical studies, which could result in regulatory sanctions and cause serious harm to our reputation. We currently have a code of conduct applicable to all of our employees, but it is not always possible to identify and deter employee misconduct, and our code of conduct and the other precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses, or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could result in the imposition of significant fines or other sanctions, which could have a significant impact on our business. Whether or not we are successful in defending against such actions or investigations, we could incur substantial costs, including legal fees, and divert the attention of management in defending ourselves against any of these actions or investigations.
If we use hazardous materials in a manner that causes injury, we could be liable for damages.
Our activities currently require the use of hazardous chemicals. We cannot eliminate the risk of accidental contamination or injury to employees or third parties from the use, storage, handling, or disposal of these materials. In the event of contamination or injury, we could be held liable for any resulting damages, and any liability could exceed our resources or any applicable insurance coverage we may have. Additionally, we are subject on an ongoing basis to federal, state, and local laws and regulations governing the use, storage, handling, and disposal of these materials and specified waste products. The cost of compliance with these laws and regulations may become significant, and could negatively affect our operating results.
Our income tax provision and other tax liabilities may be insufficient if taxing authorities are successful in asserting tax positions that are contrary to our position.
Significant judgment is required by us to determine our provision for income taxes and our liabilities for taxes. From time to time, we are reviewed or audited by various federal, state, local and foreign authorities regarding income tax matters. Although we believe our judgment in determining the appropriate tax treatment is supportable and in accordance with relevant guidance, it is possible that the final tax authority may take a tax position that is different than that which is reflected in our income tax provision or different than the taxes we previously paid. Such differences could have a material adverse effect on our income tax provision or benefit or otherwise require the payment of additional taxes and, consequently, have a material adverse effect on our results of operations, financial position, and/or cash flows for such period.
64
Table of Contents
Reimbursement and Regulatory Risks Relating to Our Business
If commercial third-party payors or government payors fail to provide coverage or adequate reimbursement, or if there is a decrease in the amount of reimbursement for our existing products or future products we develop, if any, our revenue and prospects for profitability would be harmed.
In both domestic and many international markets, sales of our existing and any future products we develop will depend, in large part, upon the availability of reimbursement from third-party payors. These third-party payors include government healthcare programs such as Medicare, managed care providers, accountable care organizations, private health insurers, and other organizations. In particular, we believe that obtaining a positive national coverage decision and favorable reimbursement rate from CMS, for each of our existing products will be a necessary element in achieving material commercial success. Physicians and patients may not order our products unless commercial third-party payors and government payors authorize such ordering and pay for all, or a substantial portion, of the list price, and certain commercial third-party payors may not agree to reimburse our existing products if CMS or our local MAC, does not issue a positive coverage decision.
There is currently no national coverage decision that determines whether and how our products are covered by Medicare. In the absence of a national coverage determination, local MACs that administer the Medicare program in various regions have some discretion in determining coverage and, therefore, payment for tests. At the time FoundationOne was launched in 2012, our local MAC, National Government Services, initially requested that we not submit claims for services provided to Medicare patients while the MAC assessed the appropriate coding, coverage, and payment for FoundationOne as a whole. To accommodate this request, we deferred the submission of claims until November 2013, when we commenced the process of submitting claims to National Government Services for FoundationOne and FoundationOne Heme tests for Medicare patients with dates of service on or after November 1, 2013.
We are submitting claims to National Government Services using a miscellaneous CPT code. When submitting claims for services or procedures that do not have specific CPT codes, providers may submit those claims using a CPT code, referred to as the miscellaneous CPT code, to provide the means of reporting and tracking services and procedures until a more specific CPT code is established. We are not submitting claims to National Government Services using stacked CPT codes in the manner currently used in submitting claims to commercial third-party payors. The use of a miscellaneous CPT code for claims submitted to CMS may decrease the likelihood of reimbursement given that a miscellaneous CPT code is a single CPT code that does not represent an identified service or procedure. We have not received any correctly processed payments for FoundationOne, FoundationOne Heme, or FoundationACT provided to patients covered by Medicare to date. If CMS does not issue a positive national coverage determination, or National Government Services does not issue a local coverage determination, with respect to one or more of these products or if National Government Services denies reimbursement of one or more of these products, withdraws its coverage policies after reimbursement is obtained, reviews and adjusts the rate of reimbursement, or stops paying for one or more of these products altogether, our revenue and results of operations would be adversely affected both because we will not receive revenue for tests performed but also because physicians may be less likely to order a test for a patient if the test is not subject to a coverage determination such that the patient could ultimately be responsible for all or substantially all of the cost of the test.
We have not received any correctly processed payments from Medicare for the claims submitted. The response to date of National Government Services to the submission of our claims has been to deny payment, or make erroneously processed payments, which we have refunded, and we have decided to appeal these claims. The response to those appeals is uncertain. National Government Services may deny paying a claim pending a coverage or payment determination. Even if we do receive payments from National Government Services on these appeals, the reimbursement rate may be lower than we expect, and if such rate is then adopted by commercial third-party payors, it would have an adverse effect on our revenues and results of operations. In addition, National Government Services may issue a non-coverage determination for one or more of our existing products that would apply to future claims. Although we would have the opportunity to submit additional materials in support of a positive coverage determination for our products to National Government Services and
65
Table of Contents
to CMS through the Office of Medicare Hearings and Appeals (OMHA) on appeal, there is no guarantee that National Government Services or CMS will provide us with a positive coverage decision or reverse a non-coverage decision that it already issued.
Commercial third-party payors and government payors are increasingly attempting to contain healthcare costs by demanding price discounts and limiting both coverage on which diagnostic products they will pay for and the amounts that they will pay for new molecular diagnostic products. Because of the cost-containment trends, commercial third-party payors and government payors that currently provide reimbursement for, or in the future cover, one or more of our existing products may reduce, suspend, revoke, or discontinue payments or coverage at any time, including those payors that designate one or more of our existing products as experimental and investigational. The percentage of submitted claims that are ultimately paid, the length of time to receive payment on claims, and the average reimbursement of those paid claims, is likely to vary from period to period.
As a result, there is significant uncertainty surrounding whether the use of products that incorporate new technology, such as FoundationOne, FoundationOne Heme, and FoundationACT, will be eligible for coverage by commercial third-party payors and government payors or, if eligible for coverage, what the reimbursement rates will be for these products. The fact that a diagnostic product has been approved for reimbursement in the past, for any particular indication or in any particular jurisdiction, does not guarantee that such diagnostic product will remain approved for reimbursement or that similar or additional diagnostic products will be approved in the future. We have had claims for reimbursement denied by certain commercial third-party payors, in some cases because they have designated FoundationOne and FoundationOne Heme as experimental and investigational. In addition, in March 2015, Palmetto, a MAC, published a final local coverage determination effective July 6, 2015 outlining guidelines for coverage of well-validated comprehensive genomic profiles for certain patients diagnosed with NSCLC. The Palmetto website lists FoundationOne as a covered test under these guidelines. Our local MAC, National Government Services, has elected not to follow these guidelines from Palmetto and instead issued their own LCD for patients with NSCLC, which will be effective April 1, 2016. This LCD provides coverage for hotspot panel tests of between 5 and 50 genes, and therefore does not provide coverage for our comprehensive genomic profiling products. Reimbursement of NGS-based cancer tests by commercial third-party payors and government payors may depend on a number of factors, including a payor’s determination that our existing and future products are:
| • | not experimental or investigational; |
| • | medically reasonable and necessary; |
| • | appropriate for the specific patient; |
| • | cost effective; |
| • | supported by peer-reviewed publications; |
| • | included in clinical practice guidelines; and |
| • | supported by clinical utility studies demonstrating improved outcomes. |
As a result, our efforts to receive reimbursement on behalf of patients will take a substantial amount of time, and various commercial third-party payors and government payors may never cover or provide adequate authorization for orders or payment for our existing and future products. Our strategy to achieve broad reimbursement coverage is focused on demonstrating the clinical utility and economic benefits of our products including engagement with key members of the oncology community and increasing physician demand, but there is no assurance that we will succeed in any of these areas or that, even if we do succeed, we will receive favorable reimbursement decisions. If adequate third-party authorization for ordering and reimbursement is unavailable, we may not be able to maintain volume and price levels sufficient to realize an appropriate return on investment in product development. Furthermore, if a commercial third-party payor or government payor denies coverage and payment, it may be difficult for us to collect from the patient, and we may not be successful.
66
Table of Contents
In addition, we are currently considered a “non-contracted provider” by all but a few commercial third-party payors because we have not entered into specific contracts to provide reimbursement for one or more of our existing products for their covered patients, and as a result we take on primary responsibility for obtaining reimbursement on behalf of patients. If we were to become a contracted provider with additional commercial third-party payors in the future, the amount of overall reimbursement we receive may decrease if we were to be required to limit test ordering and/or be reimbursed less money per test performed at a contracted rate than at a non-contracted rate, which could have a negative impact on our revenue. We may also be unable to collect payments from patients beyond that which is paid by their coverage, and will experience lost revenue as a result. In addition, coverage in a specific tumor type such as NSCLC may result in our inability to accept orders and non-payment for other non-covered tumor types, resulting in lost volume and revenue. Finally, our contracts with current and any additional third-party payors will be subject to renewal, and the renewal process could result in lower reimbursement rates or elimination of reimbursement to us if the parties fail to agree to the terms of renewal and the contract is terminated.
The United States and foreign governments continue to propose and pass legislation designed to reduce the cost of healthcare. For example, in some foreign markets, the government controls the pricing of many healthcare products. We expect that there will continue to be federal and state proposals to implement governmental controls or impose healthcare requirements. In addition, the Medicare program and increasing emphasis on managed or accountable care in the United States will continue to put pressure on product utilization and pricing. Utilization and cost control initiatives could decrease the volume of orders and payment that we would receive for any products in the future, which would limit our revenue and profitability.
Changes in the way that the FDA regulates laboratory tests developed, manufactured, validated, and performed by laboratories like ours could result in additional expense in offering our current and any future products or even possibly delay or suspend development, manufacture, or commercialization of such products.
The FDA does not currently regulate most laboratory developed tests, or LDTs, such as FoundationOne, FoundationOne Heme, and FoundationACT. The FDA historically has taken the position that, although such LDTs are medical devices, it exercises enforcement discretion by not requiring compliance with its regulations. However, on July 31, 2014, the FDA announced that it intends to change this policy. The FDA previously announced in June 2010 that it intended to no longer exercise enforcement discretion for LDTs and subsequently stated that it would publish guidance documents describing an approach to regulating LDTs. In October 2014, the FDA published two draft guidance documents that, if finalized, would implement a regulatory approach for most LDTs. In the draft guidance documents, the FDA stated that it had serious concerns regarding the lack of independent review of the evidence of clinical validity of LDTs and asserted that the requirements under CLIA, do not address the clinical validity of any LDT. If published and finalized in the same form, the guidance documents would impose a risk-based, phased-in approach for LDTs similar to the existing in vitro diagnostic devices framework.
Under the risk-based approach described in the draft guidance documents, the FDA would rely upon its existing medical device classification system to evaluate the risk of LDTs. Subject to certain limited exemptions, the FDA would require that laboratories providing LDTs, within six months after the guidance documents are finalized, comply with (i) either a new notification procedure in which the laboratory must provide the FDA with certain basic information about each LDT offered by their laboratory or the FDA’s device registration and listing requirements, and (ii) the medical device reporting requirements for LDTs offered by that laboratory. The FDA’s premarket review requirements would begin twelve months after finalization of the guidance documents for the highest risk tests, including LDTs with the same intended use as a companion diagnostic or LDTs with the same intended use as an FDA-approved Class III medical device. Premarket review would begin immediately for newly developed LDTs in this highest-risk group. Premarket review for other LDTs classified as high-risk by the FDA would be phased in over the next four years and the FDA expects to announce the priority list for premarket review for the remaining Class III LDTs within 24 months from finalization of this guidance. The FDA identified certain tests as higher risk, including LDTs that act like companion diagnostics, LDTs that screen for serious diseases or conditions for use in asymptomatic patients with no other available confirmatory diagnostic product
67
Table of Contents
or procedure, and LDTs for certain infectious diseases with high-risk intended uses. Such higher risk LDTs would likely receive higher priority during the phased-in enforcement period. Premarket review of moderate-risk (Class II) LDTs would be phased-in over a period of four years following completion of the premarket review period for LDTs classified as high-risk.
If classified as Class III medical devices, our products would likely be required to be approved by the FDA under a premarket approval application or PMA, which must be supported by valid scientific evidence to demonstrate a reasonable assurance of safety and effectiveness of the subject product, typically including the results of human clinical trials that demonstrate the clinical utility of our products. If classified as Class II medical devices, we would need to submit premarket notifications or 510(k)s that demonstrate that our products are substantially equivalent in technological characteristics and intended use to legally marketed predicate devices. If we are unable to identify an appropriate predicate that is substantially equivalent to our device, we would be required to submit a PMA or a de novo reclassification request.
Until premarket review is required for a test, the LDT could remain on the market while the FDA reviews the applications or premarket notifications for such test. In addition, once a premarket application is submitted to FDA or FDA issues a 510(k) clearance order, the laboratory must also comply with FDA’s quality system regulation.
If the FDA requires us to seek clearance or approval to offer FoundationOne, FoundationOne Heme, FoundationACT, or any of our future products for clinical use, we may not be able to obtain such approvals on a timely basis, or at all. If premarket review is required, our business could be negatively impacted if we are required to stop selling molecular information products pending their clearance or approval, or the launch of any new products that we develop could be delayed by new requirements. The cost of conducting clinical trials and otherwise developing data and information to support premarket applications may be significant. In addition, future regulation by the FDA could subject our business to further regulatory risks and costs. Failure to comply with applicable regulatory requirements of the FDA could result in enforcement action, including untitled or warning letters, fines, injunctions, or civil or criminal penalties. In addition, we could be subject to a recall or seizure of current or future products, operating restrictions, a partial suspension or a total shutdown of production. Any such enforcement action would have a material adverse effect on our business, financial condition and operations.
The FDA’s draft guidance documents for LDTs were published on October 3, 2014, and the FDA accepted comments from the public through February 2, 2015. The FDA will consider such comments before deciding whether to issue final guidance documents implementing the same or a modified version of the regulatory approach described in the draft guidance documents. While there is no time frame in which the FDA must issue final guidance documents, the FDA included a final guidance on its framework for regulating LDTs as part of a list of priority guidance documents it intends to issue in fiscal year 2016. Legislative proposals have been introduced in Congress or publicly circulated, each of which would implement differing approaches to the regulation of LDTs. We cannot predict whether any of these legislative proposals will be enacted into law or the impact such new legal requirements would have on our business.
In addition, in November 2013, the FDA finalized guidance regarding the sale and use of products labeled for research or investigational use only. Among other things, the guidance states that the FDA continues to be concerned about distribution of research- or investigational-use only products intended for clinical diagnostic use. The guidance states that the FDA will assess whether a manufacturer of such research- or investigational-use only products intends its products be used for clinical diagnostic purposes by examining the totality of circumstances, including advertising, instructions for clinical interpretation, presentations that describe clinical use, and specialized technical support such as assistance performing clinical validation, surrounding the distribution of the product in question. The FDA has advised that if evidence demonstrates that a product is inappropriately labeled for research- or investigational-use only, the device could be deemed misbranded and adulterated within the meaning of the Federal Food, Drug and Cosmetic Act. Some of the reagents and other
68
Table of Contents
components we use in FoundationOne, FoundationOne Heme, and FoundationACT are currently labeled as research-use only products. If the FDA were to undertake enforcement actions, some of our suppliers may cease selling research-use only products to us, and any failure to obtain an acceptable substitute could significantly and adversely affect our business, financial condition and results of operations.
Healthcare policy changes, including recently enacted legislation reforming the U.S. health care system, may have a material adverse effect on our financial condition, results of operations, and cash flows.
In March 2010, legislation collectively referred to as the Affordable Care Act, or ACA, was enacted in the United States. The ACA made a number of substantial changes in the way healthcare is financed by both governmental and private insurers. Among other things, the ACA:
| • | requires each medical device manufacturer to pay a sales tax equal to 2.3% of the price for which such manufacturer sells its medical devices. In 2015, Congress imposed a 2-year moratorium on this medical device tax, so that medical device sales during the period between January 1, 2016 and December 31, 2017 are exempt from the tax. Absent further legislative action, the tax will be automatically reinstated for medical device sales starting on January 1, 2018. If the tax is reinstated and if our products become regulated as medical devices, we could be required to begin paying this tax on the sales of our products for which we submit a marketing application, such as a 510(k) or PMA, to the FDA; and |
| • | mandates a reduction in payments for clinical laboratory services paid under the Medicare Clinical Laboratory Fee Schedule, or CLFS, of 1.75% for the years 2011 through 2015. In addition, a productivity adjustment is made to the fee schedule payment amount. |
On April 1, 2013, cuts to the federal budget were implemented, requiring a 2% cut in Medicare payments for all services, including clinical laboratory testing. In December 2013, Congress extended this 2% cut for an additional two years until 2023. Many CPT procedure codes that we use to bill our products were revised by the AMA, effective January 1, 2013. These new CPT codes were developed and implemented for individual genes, or the components of a multi-gene panel. In the Final Rule for 2013, CMS announced that it decided to keep the new molecular codes on the CLFS rather than move them to the Physician Fee Schedule. CMS then announced that for 2013, it would price the new codes using a “gap filling” process. Under this approach, CMS referred the CPT codes to the MACs to allow them to determine an appropriate price. CMS then calculated the median of the pricing provided by the MACs to establish and publish a National Limit Amount, or NLA, by CPT code for 2014.
In 2014, the AMA approved and implemented new CPT codes for genomic sequencing-based panel tests, effective January 1, 2015. In 2015, CMS used a “gap filling” process to price some of these new codes, which included referring the new codes to the MACs to allow them to determine and submit to CMS an appropriate price if they deemed a code to be a covered service. CMS then established and published for 2016 an NLA for these codes as calculated by determining the median price as provided by the MACs for the applicable code. If CMS reduces reimbursement for the new CPT codes for individual genes or fails to price new multi-gene panel codes which cover our products, or if commercial payors who often base pricing on Medicare fee schedules reduce non-contracted payment rates below the new NLA amount for CPT codes corresponding to individual genes, mandate use of the new sequencing-based panel CPT codes, or decide to stop payment on specific CPT codes altogether, our revenue could be adversely affected.
Additionally, in April 2014 the Protecting Access to Medicare Act of 2014, or the Act, was enacted into law which reforms the Medicare payment system for clinical laboratory tests paid through the CLFS. The Act rescinds CMS statutory authority to make adjustments to future payments for tests based on “technological changes,” which CMS had intended to apply to certain test codes on the CLFS beginning in calendar year 2015. Beginning in January 2017, Medicare payment for clinical diagnostic laboratory tests will be established by a market-based payment system. Under this new methodology, CMS will establish Medicare payment for each test based on the weighted median of the payment rates for private payors for the test. The Act creates a new class of
69
Table of Contents
Advanced Diagnostic Laboratory Tests defined as sole source multi-analyte tests with a unique algorithm yielding a single result or a test that is cleared or approved by FDA or other such criteria developed by the Secretary of Health and Human Services. For Medicare payment prior to 2017, the Act creates a transitional period for both new and existing clinical diagnostic laboratory tests. Our average commercial payor reimbursement starting in 2017 could be adversely affected depending upon if and how payors adopt this new CMS pricing methodology and resulting rates. However, CMS has not published the Final Rules describing how the Act will be implemented, and as a result, the full impact of the Act on new and existing tests is uncertain.
We cannot predict whether future health care initiatives will be implemented at the federal or state level, or how any future legislation or regulation may affect us. The taxes imposed by the new federal legislation and the expansion of the government’s role in the U.S. health care industry, as well as changes to the reimbursement amounts paid by payors for our existing and future products may reduce our profits, and have a material adverse effect on our business, financial condition, results of operations, and cash flows. Moreover, Congress has proposed, on several occasions, to impose a significant reduction in payment rates and/or 20% coinsurance on patients for clinical laboratory tests reimbursed under the CLFS. These adjustments would require us to bill patients for these amounts, which could have a material adverse effect on our business, financial condition, results of operations, and cash flows.
If we fail to comply with the complex federal, state, local and foreign laws and regulations that apply to our business, we could suffer severe consequences that could materially and adversely affect our operating results and financial condition.
We are subject to CLIA, a federal law that regulates clinical laboratories that perform testing on specimens derived from humans for the purpose of providing information for the diagnosis, prevention, or treatment of disease. CLIA regulations mandate specific standards in the areas of personnel qualifications, administration, participation in proficiency testing, patient test management, quality control, quality assurance, and inspections. We have a current certificate of accreditation under CLIA to conduct our genomic analyses through our accreditation by CAP. To renew this certificate, we are subject to survey and inspection every two years. Moreover, CLIA inspectors may make unannounced inspections of our clinical reference laboratory at any time.
We are also required to maintain a license to conduct testing in Massachusetts. Massachusetts laws establish standards for day-to-day operation of our clinical laboratory, including the training and skills required of personnel and quality control over and above that required by CLIA. We are also licensed to conduct testing by the states of California, Pennsylvania, Maryland, Florida, Rhode Island, and in New York, where we have received a permit from the New York State Department of Health to conduct FoundationOne and FoundationOne Heme testing and deliver the related test report for specimens originating from New York. If we do not maintain these licenses or if our approval is revoked, our business would suffer. Moreover, other states may adopt similar requirements in the future. We may be subject to regulation in foreign jurisdictions as we expand international distribution of our products and seek to expand clinical laboratory operations outside the United States. International regulation may require prior review or approval of our products or services, may impose limits on the export of tissue necessary for us to perform our tests, and, as we establish laboratory operations outside the United States, may require us to obtain licenses and other operating permits. This additional regulation may affect our ability to provide our products and services and to conduct laboratory operations outside of the United States. If we are unable to comply with existing laws and regulations or changes to the laws and regulations, our business could be materially adversely affected.
Any sanction imposed under CLIA, its implementing regulations, or state or foreign laws or regulations governing licensure, or our failure to renew a CLIA certificate, a state or foreign license, or accreditation, could have a material adverse effect on our business. Most CLIA deficiencies are not classified as “condition-level” deficiencies, and there are no adverse effects upon the laboratory operations as long as the deficiencies are corrected. Remediation of these deficiencies are routine matters, with corrections occurring within several hours or weeks. More serious CLIA deficiencies could rise to the level of “condition-level” deficiencies, and CMS has
70
Table of Contents
the authority to impose a wide range of sanctions, including revocation of the CLIA certification along with a bar on the ownership or operation of a CLIA certified laboratory by any owners or operators of the deficient laboratory. There is an administrative hearing procedure that can be pursued by the laboratory in the event of imposition of such sanctions, during which the sanctions are stayed, but the process can take a number of years to complete. If we were to lose our CLIA certification or CAP accreditation, we would not be able to operate our clinical laboratory and conduct our molecular tests, which would result in material harm to our business and results of operations.
We furnish to biopharmaceutical partners and academic researchers genomic information that has been de-identified in accordance with HIPAA and relevant international health information privacy regulations. The laws of certain states may require specific consent either to retain or utilize certain genetic information for research or other purposes even if such information has been de-identified. A finding that we have failed to comply with any such laws and any remedial activities required to ensure compliance with such laws could cause us to incur substantial costs, to change our business practices, or to limit the retention or use of genetic information in a manner that, individually or collectively, could be adverse to our business.
Our operations are subject to other extensive federal, state, local, and foreign laws and regulations, all of which are subject to change. These laws and regulations currently include, among others:
| • | HIPAA, which established comprehensive federal standards with respect to the privacy and security of protected health information and requirements for the use of certain standardized electronic transactions, particularly with respect to our online portals, FoundationICE and GeneKit; |
| • | amendments to HIPAA under the HITECH Act, which strengthen and expand HIPAA privacy and security compliance requirements, increase penalties for violators, extend enforcement authority to state attorneys general, and impose requirements for breach notification; |
| • | the federal Anti-Kickback Statute, which prohibits knowingly and willfully offering, paying, soliciting, or receiving remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual, or the furnishing, arranging for, or recommending of an item or service that is reimbursable, in whole or in part, by a federal health care program; |
| • | the federal Stark physician self-referral law, which prohibits a physician from making a referral for certain designated health services covered by the Medicare program, including laboratory and pathology services, if the physician or an immediate family member has a financial relationship with the entity providing the designated health services, unless the financial relationship falls within an applicable exception to the prohibition; |
| • | the federal False Claims Act, which imposes liability on any person or entity that, among other things, knowingly presents, or causes to be presented, a false or fraudulent claim for payment to the federal government; |
| • | the federal Civil Monetary Penalties Law, which prohibits, among other things, the offering or transfer of remuneration to a Medicare or state health care program beneficiary if the person knows or should know it is likely to influence the beneficiary’s selection of a particular provider, practitioner, or supplier of services reimbursable by Medicare or a state health care program, unless an exception applies; |
| • | other federal and state fraud and abuse laws, such as anti-kickback laws, prohibitions on self-referral, fee-splitting restrictions, prohibitions on the provision of products at no or discounted cost to induce physician or patient adoption, and false claims acts, which may extend to services reimbursable by any third-party payor, including private insurers; |
| • | the prohibition on reassignment of Medicare claims, which, subject to certain exceptions, precludes the reassignment of Medicare claims to any other party; |
71
Table of Contents
| • | the rules regarding billing for diagnostic tests reimbursable by the Medicare program, which in certain circumstances prohibit laboratories from charging the Medicare program directly for services provided to hospital inpatients, and also prohibit a physician or other supplier from marking up the price of the technical component or professional component of a diagnostic test ordered by the physician or other supplier and supervised or performed by a physician who does not “share a practice” with the billing physician or supplier; |
| • | state laws that prohibit other specified practices, such as billing physicians for testing that they order; waiving coinsurance, copayments, deductibles, and other amounts owed by patients; billing a state Medicaid program at a price that is higher than what is charged to one or more other payors; and |
| • | similar foreign laws and regulations that apply to us in the countries in which we operate. |
Our failure to comply could lead to civil or criminal penalties, exclusion from participation in government health care programs, or prohibitions or restrictions on our ability to conduct commercial activities. We believe that we are in material compliance with all statutory and regulatory requirements, but there is a risk that one or more government agencies could take a contrary position. These laws and regulations are complex and are subject to interpretation by the courts and by government agencies. If one or more such agencies alleges that we may be in violation of any of these requirements, regardless of the outcome, it could damage our reputation and adversely affect important business relationships with third parties, including managed care organizations and other commercial third-party payors.
The FDA and other regulatory agencies strictly regulate the promotional claims that may be made about prescription products. If we are found to have improperly promoted off-label uses, we may become subject to significant fines and other liability.
Our molecular information products include a report to physicians that describes the tested cancer’s genomic alterations and, based on peer-reviewed literature and a government-sponsored list of clinical trials (clinicaltrials.gov) matches them with FDA-approved therapies or open clinical trials for therapies targeting cancers driven by those alterations. In some cases, the therapies identified in our report are not approved for the patient’s cancer or disease state. The FDA and other regulatory agencies strictly regulate the promotional claims that may be made about prescription drug and device products. In particular, a product may not be promoted for uses or indications beyond those contained in such product’s approved labeling. The U.S. government has levied large civil and criminal fines against companies for alleged improper promotion and has enjoined several companies from engaging in off-label promotion. The FDA has also requested that companies enter into consent decrees or permanent injunctions under which specified promotional conduct is changed or curtailed. If the FDA determines that we have engaged in off-label promotion in our test reports by providing information regarding approved therapies, we may be subject to civil or criminal fines.
In addition, incentives exist under applicable laws that encourage competitors, employees, and physicians to report violations of rules governing promotional activities for pharmaceutical products. These incentives could lead to so-called whistleblower lawsuits as part of which such persons seek to collect a portion of monies allegedly overbilled to government agencies due to, for example, promotion of pharmaceutical products beyond labeled claims. These incentives could also lead to suits that we have mischaracterized a competitor’s product in the marketplace and, as a result, we could be sued for alleged damages to our competitors. Such lawsuits, whether with or without merit, are typically time-consuming and costly to defend. Such suits may also result in related stockholder lawsuits, which are also costly to defend.
We may be subject to fines, penalties, licensure requirements, or legal liability, if it is determined that through our test reports we are practicing medicine without a license.
Our test reports delivered to physicians provide information regarding FDA-approved therapies and clinical trials that oncologists may use in making treatment decisions for their patients. We make members of our organization available to discuss the information provided in the reports. State laws prohibit the practice of
72
Table of Contents
medicine without a license. Our customer service representatives and medical affairs team provide support to our customers, including assistance in interpreting the test report results. A governmental authority or individual actor could allege that the identification of available therapies and clinical trials in our reports and the related customer service we provide constitute the practice of medicine. A state may seek to have us discontinue the inclusion of certain aspects of our test reports or the related services we provide, or subject us to fines, penalties, or licensure requirements. Any determination that we are practicing medicine without a license may result in significant liability to us.
If the validity of an informed consent from a patient enrolled in a clinical trial with one of our biopharmaceutical partners was challenged, we could be forced to stop using some of our resources, which would hinder our molecular information product development efforts.
We have implemented measures to ensure that all clinical data and genetic and other biological samples that we receive from our biopharmaceutical partners have been collected from subjects who have provided appropriate informed consent for purposes which extend to our product development activities. We seek to ensure that these data and samples are provided to us on a subject de-identified manner. We also have measures in place to ensure that the subjects from whom the data and samples are collected do not retain or have conferred on them any proprietary or commercial rights to the data or any discoveries derived from them. Our biopharmaceutical partners conduct clinical trials in a number of different countries, and, to a large extent, we rely upon them to comply with the subject’s informed consent and with applicable local laws and international regulations. The collection of data and samples in many different countries results in complex legal questions regarding the adequacy of informed consent and the status of genetic material under a large number of different legal systems. The subject’s informed consent obtained in any particular country could be challenged in the future, and those informed consents could prove invalid, unlawful, or otherwise inadequate for our purposes. Any findings against us, or our biopharmaceutical partners, could deny us access to or force us to stop using some of our clinical samples, which would hinder our molecular information product development efforts. We could become involved in legal challenges, which could consume our management and financial resources.
Ethical, legal, and social concerns related to the use of genomic information could reduce demand for our molecular information products.
Genomic testing, like that conducted using our molecular information platform and products, has raised ethical, legal, and social issues regarding privacy and the appropriate uses of the resulting information. Governmental authorities could, for social or other purposes, limit or regulate the use of genomic information or genomic testing or prohibit testing for genetic predisposition to certain conditions, particularly for those that have no known cure. Similarly, these concerns may lead patients to refuse to use genomic tests even if permissible.
Ethical and social concerns may also influence U.S. and foreign patent offices and courts with regard to patent protection for technology relevant to our business. These and other ethical, legal, and social concerns may limit market acceptance of our products or reduce the potential markets for products enabled by our molecular information platform, either of which could have an adverse effect on our business, financial condition, or results of operations.
Intellectual Property Risks Related to Our Business
Litigation or other proceedings or third-party claims of intellectual property infringement could require us to spend significant time and money and could prevent us from selling our products or impact our stock price.
Third parties have asserted and may in the future assert that we are employing their proprietary technology without authorization. As we continue to commercialize each of FoundationOne, FoundationOne Heme, and FoundationACT in their current or updated forms, launch new products, and enter new markets, we expect that
73
Table of Contents
competitors will claim that our products infringe their intellectual property rights as part of business strategies designed to impede our successful commercialization and entry into new markets. We occasionally receive letters from third parties inviting us to take licenses under, or alleging that we infringe, their patents. Third parties may have obtained, and may in the future obtain, patents under which such third parties may claim that the use of our technologies constitutes patent infringement.
We could incur substantial costs and divert the attention of our management and technical personnel in defending ourselves against any of these claims. Any adverse ruling or perception of an adverse ruling in defending ourselves against these claims could have a material adverse impact on our cash position and stock price. Furthermore, parties making claims against us may be able to obtain injunctive or other relief, which could block our ability to develop, commercialize, and sell products, and could result in the award of substantial damages against us. In the event of a successful claim of infringement or misappropriation against us, we may be required to pay damages and obtain one or more licenses from third parties, or be prohibited from selling certain products, all of which could have a material adverse impact on our cash position and business and financial condition.
In addition, we may be unable to obtain these licenses at a reasonable cost, if at all. We could, therefore, incur substantial costs related to royalty payments for licenses obtained from third parties, which could negatively affect our gross margins. Moreover, we could encounter delays in product introductions while we attempt to develop alternative methods or products. Defense of any lawsuit or failure to obtain any of these licenses on favorable terms could prevent us from commercializing products, and the prohibition of sale of any of our products would materially affect our ability to grow and maintain profitability and have a material adverse impact on our business.
Developments in patent law could have a negative impact on our business.
From time to time, the United States Supreme Court, or the Supreme Court, other federal courts, the United States Congress, or the United States Patent and Trademark Office, or the USPTO, may change the standards of patentability and any such changes could have a negative impact on our business.
Two cases involving diagnostic method claims and “gene patents” have recently been decided by the Supreme Court. On March 20, 2012, the Supreme Court issued a decision in Mayo Collaborative v. Prometheus Laboratories, or Prometheus, a case involving patent claims directed to optimizing the amount of drug administered to a specific patient. According to that decision, Prometheus’ claims failed to incorporate sufficient inventive content above and beyond mere underlying natural correlations to allow the claimed processes to qualify as patent-eligible processes that apply natural laws. On June 13, 2013, the Supreme Court subsequently decided Association for Molecular Pathology v. Myriad Genetics, or Myriad, a case brought by multiple plaintiffs challenging the validity of patent claims held by Myriad Genetics, Inc. relating to the breast cancer susceptibility genes BRCA1 and BRCA2, holding that isolated genomic DNA that exists in nature, such as the DNA constituting the BRCA1 and BRCA2 genes, is not patentable subject matter, but that cDNA, which is an artificial construct created from RNA transcripts of genes, may be patent eligible.
On December 16, 2014, the USPTO issued interim guidance, entitled “2014 Interim Guidance on Patent Subject Matter Eligibility,” which is for use by USPTO personnel in examining patent claims reciting judicially recognized exceptions to patentable subject matter, including laws of nature, natural phenomena, or abstract ideas, for patent eligibility in view of the Supreme Court decisions in Prometheus, Myriad, and Alice Corporation Pty. Ltd. V. CLS Bank International, or Alice Corp. The guidance indicates that claims reciting a judicial exception to patent-eligible subject matter must amount to significantly more than the judicial exception itself in order to be patent-eligible subject matter. We cannot assure you that our efforts to seek patent protection for our technology and products will not be negatively impacted by this interim guidance issued by the USPTO, the decisions described above, rulings in other cases, or changes in guidance or procedures issued by the USPTO.
74
Table of Contents
We cannot fully predict what impact the Supreme Court’s decisions in Prometheus, Myriad, and Alice Corp. may have on the ability of biopharmaceutical companies or other entities to obtain or enforce patents relating to DNA, genes, or genomic-related discoveries in the future. Despite the USPTO interim guidance described above, the contours of when claims reciting laws of nature, natural phenomena, or abstract ideas may meet the patent eligibility requirements are not clear and may take years to develop via interpretation at the USPTO and in the courts. There are many previously issued patents claiming nucleic acids and diagnostic methods based on natural correlations that issued before the recent Supreme Court decisions discussed, and although many of these patents may be invalid under the standards set forth in the Supreme Court’s recent decisions, until successfully challenged, these patents are presumed valid and enforceable, and certain third parties could allege that we infringe, or request that we obtain a license to, these patents. Whether based on patents issued prior to or after these Supreme Court decisions, we could have to defend ourselves against claims of patent infringement, or choose to license rights, if available, under patents claiming such methods. In particular, although the Supreme Court has held in Myriad that isolated genomic DNA is not patent-eligible subject matter, certain third parties could allege that activities that we may undertake infringe other classes of gene-related patent claims, and we could have to defend ourselves against these claims by asserting non-infringement and/or invalidity positions, or pay to obtain a license to these claims. In any of the foregoing or in other situations involving third-party intellectual property rights, if we are unsuccessful in defending against claims of patent infringement, we could be forced to pay damages or be subjected to an injunction that would prevent us from utilizing the patented subject matter in question if we are unable to obtain a license on reasonable terms. Such outcomes could materially affect our ability to offer our products and have a material adverse impact on our business. Even if we are able to obtain a license or successfully defend against claims of patent infringement, the cost and distraction associated with the defense or settlement of these claims could have a material adverse impact on our business.
In addition, the Leahy-Smith America Invents Act, or the America Invents Act, which was signed into law in 2011, includes a number of significant changes to U.S. patent law. These changes include a transition from a “first-to-invent” system to a “first-to-file” system, changes to the way issued patents are challenged, and changes to the way patent applications are disputed during the examination process. These changes may favor larger and more established companies that have greater resources to devote to patent application filing and prosecution. The USPTO has developed new and untested regulations and procedures to govern the full implementation of the America Invents Act, and many of the substantive changes to patent law associated with the America Invents Act, and, in particular, the first-to-file provisions, became effective on March 16, 2013. Substantive changes to patent law associated with the America Invents Act may affect our ability to obtain patents, and, if obtained, to enforce or defend them. Accordingly, it is not clear what, if any, impact the America Invents Act will ultimately have on the cost of prosecuting our patent applications, our ability to obtain patents based on our discoveries and our ability to enforce or defend any patents that may issue from our patent applications, all of which could have a material adverse effect on our business.
We may be unable to protect or enforce our intellectual property effectively, which could harm our competitive position.
Obtaining and maintaining a strong patent position is important to our business. Many of our patent applications are in the early stages of prosecution. Patent law relating to the scope of claims in the technology fields in which we operate is complex and uncertain, so we cannot be assured that we will be able to obtain or maintain patent rights, or that the patent rights we may obtain will be valuable, provide an effective barrier to competitors or otherwise provide competitive advantages. Others have filed, and in the future are likely to file, patent applications that are similar or identical to ours or those of our licensors. To determine the priority of inventions, or demonstrate that we did not derive our invention from another, we may have to participate in interference or derivation proceedings in the USPTO or in court that could result in substantial costs in legal fees and could substantially affect the scope of our patent protection. We cannot be assured our patent applications will prevail over those filed by others. Also, our intellectual property rights may be subject to other challenges by third parties. Patents we obtain could be challenged in litigation or in administrative proceedings such as ex parte reexam, inter partes review, or post-grant review in the United States or opposition proceedings in Europe or other jurisdictions.
75
Table of Contents
Obtaining and maintaining a patent portfolio entails significant expense and resources. Part of the expense includes periodic maintenance fees, renewal fees, annuity fees, various other governmental fees on patents and/or applications due in several stages over the lifetime of patents and/or applications, as well as the cost associated with complying with numerous procedural provisions during the patent application process. We may or may not choose to pursue or maintain protection for particular inventions. In addition, there are situations in which failure to make certain payments or noncompliance with certain requirements in the patent process can result in abandonment or lapse of a patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. If we choose to forego patent protection or allow a patent application or patent to lapse purposefully or inadvertently, our competitive position could suffer.
Legal actions to enforce our patent rights can be expensive and may involve the diversion of significant management time. In addition, these legal actions could be unsuccessful and could also result in the invalidation of our patents or a finding that they are unenforceable. We may or may not choose to pursue litigation or interferences against those that have infringed our patents, due to the associated expense and time commitment of monitoring these activities. If we fail to protect or to enforce our intellectual property rights successfully, our competitive position could suffer, which could harm our results of operations.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to patent protection, we also rely upon copyright and trade secret protection, as well as non-disclosure agreements and invention assignment agreements with our employees, consultants and third parties, to protect our confidential and proprietary information. For example, significant elements of FoundationOne, FoundationOne Heme, and FoundationACT, including aspects of sample preparation, computational-biological algorithms, and related processes and software, are based on unpatented trade secrets and know-how that are not publicly disclosed. In addition to contractual measures, we try to protect the confidential nature of our proprietary information using physical and technological security measures. Such measures may not, for example, in the case of misappropriation of a trade secret by an employee or third party with authorized access, provide adequate protection for our proprietary information. Our security measures may not prevent an employee or consultant from misappropriating our trade secrets and providing them to a competitor, and recourse we take against such misconduct may not provide an adequate remedy to protect our interests fully. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret can be difficult, expensive, and time-consuming, and the outcome is unpredictable. In addition, trade secrets may be independently developed by others in a manner that could prevent legal recourse by us. If any of our confidential or proprietary information, such as our trade secrets, were to be disclosed or misappropriated, or if any such information were independently developed by a competitor, our competitive position could be harmed.
We may not be able to enforce our intellectual property rights throughout the world.
The laws of some foreign countries do not protect intellectual property rights to the same extent as the laws of the United States. Many companies have encountered significant problems in protecting and defending intellectual property rights in certain foreign jurisdictions. The legal systems of some countries, particularly developing countries, do not favor the enforcement of patents and other intellectual property protection, especially those relating to biotechnology. This could make it difficult for us to stop the infringement of our patents, if obtained, or the misappropriation of our other intellectual property rights. For example, many foreign countries have compulsory licensing laws under which a patent owner must grant licenses to third parties. In addition, many countries limit the enforceability of patents against third parties, including government agencies or government contractors. In these countries, patents may provide limited or no benefit. Patent protection must ultimately be sought on a country-by-country basis, which is an expensive and time-consuming process with uncertain outcomes. Accordingly, we may choose not to seek patent protection in certain countries, and we will not have the benefit of patent protection in such countries.
76
Table of Contents
Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business. Accordingly, our efforts to protect our intellectual property rights in such countries may be inadequate. In addition, changes in the law and legal decisions by courts in the United States and foreign countries may affect our ability to obtain adequate protection for our technology and the enforcement of intellectual property.
Third parties may assert ownership or commercial rights to inventions we develop.
Third parties may in the future make claims challenging the inventorship or ownership of our intellectual property. For example, we rely on certain third parties to provide us with tissue samples and biological materials that we use to conduct our genomic analyses. We have written agreements with collaborators that provide for the ownership of intellectual property arising from our collaborations. These agreements provide that we must negotiate certain commercial rights with collaborators with respect to joint inventions or inventions made by our collaborators that arise from the results of the collaboration. In some instances, there may not be adequate written provisions to address clearly the resolution of intellectual property rights that may arise from a collaboration. If we cannot successfully negotiate sufficient ownership and commercial rights to the inventions that result from our use of a third-party collaborator’s materials where required, or if disputes otherwise arise with respect to the intellectual property developed with the use of a collaborator’s samples, we may be limited in our ability to capitalize on the market potential of these inventions. In addition, we may face claims that our agreements with employees, contractors, or consultants obligating them to assign intellectual property to us are ineffective, or in conflict with prior or competing contractual obligations of assignment, which could result in ownership disputes regarding intellectual property we have developed or will develop and interfere with our ability to capture the commercial value of such inventions. Litigation may be necessary to resolve an ownership dispute, and if we are not successful, we may be precluded from using certain intellectual property, or may lose our exclusive rights in that intellectual property. Either outcome could have an adverse impact on our business.
Third parties may assert that our employees or consultants have wrongfully used or disclosed confidential information or misappropriated trade secrets.
We employ individuals who were previously employed at universities or other diagnostic or biopharmaceutical companies, including our competitors or potential competitors. Although we try to ensure that our employees and consultants do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or our employees, consultants or independent contractors have inadvertently or otherwise used or disclosed intellectual property, including trade secrets or other proprietary information, of a former employer or other third parties. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
Risks Related to Our Relationship with Roche
We may not fully realize the anticipated benefits of our broad strategic collaboration with Roche or realize them in the expected time frame.
Implementation of the collaboration agreements and related corporate governance agreements with Roche may result in material unanticipated problems, expenses, liabilities, competitive responses, loss of customer relationships, and diversion of management’s attention, including, among others:
| • | difficulties in achieving anticipated business opportunities and growth prospects; |
| • | difficulties in managing the expanded operations of a more complex company; |
| • | challenges related to adhering to our obligations to repurchase shares pursuant to Roche’s anti-dilution protections contained in the Investor Rights Agreement; |
77
Table of Contents
| • | challenges resulting from increased complexities in accounting and tax matters related to our obligations under the Investor Rights Agreement and Tax Sharing Agreement by and between us and Roche Holdings, Inc., dated January 11, 2015, or the Tax Sharing Agreement, including our compliance with certain financial, accounting and tax reporting obligations, practices and procedures, and the transition to a new independent public company accounting firm; |
| • | challenges in keeping existing customers and obtaining new customers, including any biopharmaceutical customers that are actual or potential competitors with Roche; |
| • | challenges in our relationships with collaboration partners, suppliers, distributors, and patients; and |
| • | challenges in attracting and retaining key personnel that may arise from working in a more complex company or due to changes in our equity incentive program that we adopted to maintain Roche’s percentage ownership interest pursuant to our obligations under the Investor Rights Agreement. |
Many of these factors will be outside of our control, and any one of them could result in increased costs, decreases in the amount of expected revenues and diversion of management’s time and energy, which could materially impact our business, financial condition and results of operations. In addition, even if the full benefits of our relationship with Roche are realized, including the sales and growth opportunities that are expected, these benefits may not be achieved within the anticipated time frame and additional unanticipated costs may be incurred in connection with the relationship. All of these factors could negatively impact the price of our common stock. As a result, we cannot assure you that our relationship with Roche will result in the realization of the anticipated benefits.
As long as Roche owns greater than a majority of our outstanding shares of common stock, other holders of our common stock may have limited ability to affect the outcome of certain matters requiring stockholder approval and Roche’s interest may conflict with ours and other stockholders’.
As of December 31, 2015, Roche owned approximately 61% of our outstanding common stock. As a result, until such time as Roche holds shares representing less, and potentially a material portion less, than a majority of the votes entitled to be cast by the holders of our outstanding common stock at a stockholder meeting, Roche generally will have the ability to control the outcome of any matter submitted for the vote of our stockholders, except in certain circumstances set forth in our certificate of incorporation, bylaws and the Investor Rights Agreement.
The interests of Roche may not coincide with the interests of our other stockholders. Roche’s ability, subject to the limitations in our certificate of incorporation, bylaws and the Investor Rights Agreement, to control all matters submitted to our stockholders for approval limits the ability of other stockholders to influence corporate matters and, as a result, we may take actions that our minority stockholders do not view as beneficial. As a result, the market price of our common stock could be adversely affected.
In addition, the existence of a majority stockholder could discourage third parties from acquiring since the third party would be required to negotiate any such transaction with Roche, and the interests of Roche with respect to such transaction may be different from the interests of our other stockholders. In particular, it is possible that our minority stockholders may not receive a control premium for their shares upon any eventual sale of our business. In addition, the performance of Roche or speculation about the possibility of future actions Roche may take in connection with us may adversely affect our share price.
We have entered into contractual provisions that may significantly limit our ability to undertake certain business opportunities, conduct certain operations, raise capital or require us to make material expenditures.
Pursuant to the Investor Rights Agreement, until such time as Roche and its affiliates beneficially owns less than a majority of the outstanding shares of our common stock (subject to a cure period), we may not take certain actions without Roche’s prior written consent, including any of the following: (a) appoint a new Chief Executive
78
Table of Contents
Officer; (b) incur any indebtedness (as defined in the Investor Rights Agreement) that would result in the outstanding aggregate principal amount of the indebtedness of us and our subsidiaries exceeding the lesser of (A) $200 million and (B) 20% of our aggregate market capitalization at the time of such incurrence; (c) issue or sell any equity securities (including any securities convertible or exercisable into such equity securities), other than (X) common stock issued upon the exercise or settlement of equity awards granted as of the date of the Investor Rights Agreement in accordance with their terms, (Y) equity awards granted after the date of the Investor Rights Agreement pursuant to our 2013 Stock Option and Incentive Plan or any permitted new equity incentive plan or equity incentive plan amendment, and (Z) in connection with permitted acquisitions, certain shares of our common stock issued as stock consideration as long as such issuance does not result in Roche beneficially owning less than 50.5% of the outstanding shares of our common stock on a fully diluted basis; (d) establish or amend any of our equity incentive plans, except for certain permitted equity incentive plans and permitted equity incentive plan amendments; (e) acquire any entity, business or assets if the aggregate consideration payable by us exceeds the lesser of (X) $200 million and (Y) 20% of our aggregate market capitalization at the time of such transaction, unless Roche is separately contemplating acquiring the same entity, business or assets; (f) dispose of any entity, business or assets if the aggregate consideration payable to us exceeds $50 million; (g) change the scope and nature of our business; (h) amend our organizational documents; (i) take any action that would impair in any material respect our ability to perform our obligations under the Investor Rights Agreement or Roche’s rights thereunder; or (j) voluntarily dissolve or liquidate or make any voluntary bankruptcy filings. Our Board or management team could believe that taking any one of these actions would be in our best interests and the best interests of our stockholders. As such, if we are unable to complete any of these actions because Roche does not provide its consent, it could adversely impact our business and results of operations.
The Investor Rights Agreement also requires us to establish and maintain a stock repurchase program and to repurchase shares of our common stock in order to maintain Roche’s aggregate percentage ownership at no less than 50.5% of the outstanding shares of our common stock on a fully diluted basis, less any shares transferred by Roche. Our obligation to maintain such stock repurchase program may involve material expenditures of cash by us. If we fail to or are unable to satisfy our repurchase obligations under the stock repurchase program and we issue any securities and, as a result thereof, Roche beneficially owns less than 50.1% of the outstanding shares of our common stock on a fully diluted basis, the restrictions on Roche under the Investor Rights Agreement (including with respect to the agreement to vote Roche’s shares of common stock, the standstill restrictions and the transfer restrictions), but not the rights of Roche under the Investor Rights Agreement, will immediately terminate, and Roche will thereafter have the ability to exercise in full its rights as a stockholder.
In addition, the Transaction Agreement by and between us and Roche Holdings, Inc., dated January 11, 2015, or the Transaction Agreement, provides for us to indemnify Roche for breaches of the Transaction Agreement by us subject to negotiated limitations. If we are required to indemnify Roche for any such breaches, it could have a material adverse impact on our results of operations.
Roche’s majority ownership of us and contractual restrictions on their ability to purchase and sell our common stock could have a material negative impact on the liquidity of our common stock.
As of December 31, 2015, Roche owned approximately 61% of our outstanding common stock. In addition, under the terms of the Investor Rights Agreement, until April 2018, Roche is restricted from acquiring additional shares, except in order to offset dilution and maintain its aggregate percentage ownership in us at no less than 50.5% of the outstanding shares of our common stock on a fully diluted basis. The Investor Rights Agreement also requires us to establish a stock repurchase program designed to maintain Roche’s percentage ownership interest in our common stock. This ownership and these provisions will likely result in a less liquid trading market for our shares. This lack of liquidity may make it more difficult for investors to transact in our shares and the price of our stock may suffer as a result.
79
Table of Contents
The independent members of our Board, who are not our employees and are not Roche designees, do not represent a majority of our Board.
We have determined that the directors designated by Roche, who represent a minority on our Board, and our Chief Executive Officer are not independent directors under NASDAQ Rule 5605(a)(2). NASDAQ Rule 5605(b) requires that a majority of the board of directors of NASDAQ listed companies be comprised of independent directors. In connection with the closing of our transactions with Roche, the size of our Board was increased to nine seats. The Board currently consists of three directors designated by Roche, or the Roche Designees, and five of our directors who served prior to the closing, including our Chief Executive Officer. We intend that the vacancy on the Board will be filled in accordance with the Investor Rights Agreement with an independent director to be agreed upon by us and Roche.
Four members of our Board are independent under NASDAQ Rule 5605(a)(2), which does not constitute a majority of our Board at this time. However, due to Roche’s majority ownership of our stock, we intend to rely upon the “Controlled Company” exemption (as set forth in NASDAQ Rule 5615(c)(2)) from the majority Board independence requirement of NASDAQ Rule 5605(b) until the new independent director is appointed to the Board. If we are not able to identify and appoint a new independent director to our Board in a timely fashion, the perception of us as an independent entity could be diminished and our business could be harmed.
Pursuant to our agreements with Roche, certain development and commercialization decisions for our products are determined by Roche and we do not retain full control, which presents a number of risks including, among others:
| • | Roche may conduct multiple product development efforts within areas that are the subject of our collaboration with Roche, and Roche may develop, either alone or with others, products in related fields that are competitive with the current and future products that are the subject of our collaboration with Roche. Competing products, either developed by Roche, or to which Roche has rights, may result in withdrawal of or reduction in Roche’s support for our programs or products, or otherwise impair the development, marketability and commercialization of our products; |
| • | Collaborations often require the parties to cooperate, and failure to do so effectively could adversely affect or result in the termination or delay of development, regulatory approval or sales of our products, or result in litigation or arbitration; |
| • | Roche’s interests may not always be aligned with our interests, and Roche may not market our products in the same manner or to the same extent that we would or on the time schedule we currently contemplate, which could adversely affect our business; |
| • | If our interests come into conflict with those of Roche, Roche may choose to act in its self-interest, which may limit our ability to implement our strategies or result in delays and other obstacles in the development or commercialization of our current and future products; |
| • | Roche will have control and broad discretion over certain aspects of the commercialization of our products in its territory, and we will have little, if any, influence on how this commercialization will be conducted. This lack of control could cause delays or other difficulties, which may prevent us from receiving any royalty, milestone or other payments, or limit such payments, with respect to such commercialization; |
| • | Roche may be unable to successfully commercialize our products in its territory, which would negatively impact our revenues and our strategy to develop these products; |
| • | Any failure by Roche to comply with applicable laws and regulatory requirements in the marketing, sale and maintenance of regulatory approvals of our products could adversely affect our revenues or give rise to legal proceedings in which we would be involved; |
80
Table of Contents
| • | Roche has certain rights to terminate our agreements with them. If Roche terminates one or more of our agreements, the actual milestone, royalty and other payments that we receive under these agreements may be lower than expected, and our ability to generate revenue under such agreements could be delayed or could cease entirely; and |
| • | There is no guarantee that the various milestone events set forth in our agreements with Roche will be achieved. To the extent we or Roche do not succeed in developing and commercializing our products, or if we or Roche fail to achieve such milestones, our revenues under these agreements will be limited. |
These risks introduce considerable uncertainty regarding the success of our current and future collaborative efforts with Roche. If these efforts fail, our product development or commercialization of new and existing products in various territories could be delayed, and revenues from our current and future products could be adversely affected.
Risks Relating to Our Financial Condition and Capital Requirements
We are an early, commercial-stage company and have a limited operating history, which may make it difficult to evaluate our current business and predict our future performance.
We are an early, commercial-stage company and have a limited operating history. We were incorporated in Delaware and began operations in November 2009. Our limited operating history, particularly in light of our business model based upon sales of novel products enabled by our molecular information platform and the rapidly evolving genomic analysis industry, may make it difficult to evaluate our current business and predict our future performance. Any assessment of our profitability or prediction about our future success or viability is subject to significant uncertainty. We have encountered and will continue to encounter risks and difficulties frequently experienced by early commercial-stage companies in rapidly evolving industries. If we do not address these risks successfully, our business will suffer.
We have a history of net losses. We expect to incur net losses in the future and we may never achieve sustained profitability.
We have historically incurred substantial net losses, including a net loss of $89.6 million in 2015. From our inception in 2009 through December 31, 2015, we had an accumulated deficit of $231.6 million. We expect our losses to continue as a result of ongoing research and development expenses and increased selling and marketing costs. These losses have had, and will continue to have, an adverse effect on our working capital, total assets, and stockholders’ equity. Because of the numerous risks and uncertainties associated with our research, development, and commercialization efforts, we are unable to predict when we will become profitable, and we may never become profitable. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our inability to achieve and then maintain profitability would negatively affect our business, financial condition, results of operations, and cash flows.
We may need to raise additional capital to fund our existing operations, develop our molecular information platform, commercialize new products, and expand our operations.
If our available cash balances, net proceeds from our initial public offering, or IPO, and the investment from Roche, and anticipated cash flow from operations are insufficient to satisfy our liquidity requirements including because of lower demand for our products as a result of lower than currently expected rates of reimbursement from commercial third-party payors and government payors or other risks described in this Annual Report, we may seek to sell common or preferred equity or convertible debt securities, enter into a credit facility or another form of third-party funding, or seek other debt financing.
81
Table of Contents
We may consider raising additional capital in the future to expand our business, to pursue strategic investments, to take advantage of financing opportunities, or for other reasons, including to:
| • | increase our sales and marketing efforts to drive market adoption of our products and address competitive developments; |
| • | fund development and marketing efforts of any future products; |
| • | further expand our laboratory operations domestically and outside of the United States; |
| • | expand our technologies into other types of cancers; |
| • | acquire, license or invest in technologies, including information technologies; |
| • | acquire or invest in complementary businesses or assets; and |
| • | finance capital expenditures and general and administrative expenses. |
Our present and future funding requirements will depend on many factors, including:
| • | our ability to achieve revenue growth; |
| • | our rate of progress in establishing reimbursement arrangements with domestic and international commercial third-party payors and government payors; |
| • | the cost of expanding our laboratory operations and offerings, including our sales and marketing efforts; |
| • | our rate of progress in, and cost of the sales and marketing activities associated with, establishing adoption of and reimbursement for our products; |
| • | our rate of progress in, and cost of research and development activities associated with, products in research and early development; |
| • | the effect of competing technological and market developments; |
| • | costs related to international expansion; and |
| • | the potential cost of and delays in product development as a result of any regulatory oversight applicable to our products. |
The various ways we could raise additional capital carry potential risks and are, in certain cases as set forth in the Investor Rights Agreement, subject to the prior consent of Roche. If we raise funds by issuing equity securities, dilution to our stockholders could result. Any equity securities issued also could provide for rights, preferences, or privileges senior to those of holders of our common stock. If we raise funds by issuing debt securities, those debt securities would have rights, preferences, and privileges senior to those of holders of our common stock. The terms of debt securities issued or borrowings pursuant to a credit agreement could impose significant restrictions on our operations. If we raise funds through collaborations and licensing arrangements, we might be required to relinquish significant rights to our platform technologies or products, or grant licenses on terms that are not favorable to us.
The credit markets and the financial services industry have experienced a period of unprecedented turmoil and upheaval characterized by the bankruptcy, failure, collapse, or sale of various financial institutions and an unprecedented level of intervention from the United States federal government. Accordingly, additional equity or debt financing might not be available on reasonable terms, if at all. If we cannot secure additional funding when needed, we may have to delay, reduce the scope of, or eliminate one or more research and development programs or sales and marketing initiatives. In addition, we may have to work with a partner on one or more of our development programs, which could lower the economic value of those programs to us.
82
Table of Contents
We incur significant costs as a result of operating as a public company and our management devotes substantial time to public company compliance programs.
As a public company, we incur significant legal, accounting, and other expenses due to our compliance with regulations and disclosure obligations applicable to us, including compliance with the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, as well as rules implemented by the SEC, and the NASDAQ Stock Market, or NASDAQ. The SEC and other regulators have continued to adopt new rules and regulations and make additional changes to existing regulations that require our compliance. In July 2010, the Dodd-Frank Wall Street Reform and Consumer Protection Act, or the Dodd-Frank Act, was enacted. There are significant corporate governance and executive compensation related provisions in the Dodd-Frank Act that have required the SEC to adopt additional rules and regulations in these areas. Stockholder activism, the current political environment, and the current high level of government intervention and regulatory reform may lead to substantial new regulations and disclosure obligations, which may lead to additional compliance costs and impact, in ways we cannot currently anticipate, the manner in which we operate our business. Our management and other personnel devote a substantial amount of time to these compliance programs and monitoring of public company reporting obligations, and as a result of the new corporate governance and executive compensation related rules, regulations, and guidelines prompted by the Dodd-Frank Act, and further regulations and disclosure obligations expected in the future, we will likely need to devote additional time and costs to comply with such compliance programs and rules. These rules and regulations will cause us to incur significant legal and financial compliance costs and will make some activities more time-consuming and costly.
To comply with the requirements of being a public company, we may need to undertake various actions, including implementing new internal controls and procedures and hiring new accounting or internal audit staff. The Sarbanes-Oxley Act requires that we maintain effective disclosure controls and procedures and internal control over financial reporting. We are continuing to develop and refine our disclosure controls and other procedures that are designed to ensure that information required to be disclosed by us in the reports that we file with the SEC is recorded, processed, summarized, and reported within the time periods specified in SEC rules and forms, and that information required to be disclosed in reports under the Exchange Act is accumulated and communicated to our principal executive and financial officers. Our current controls and any new controls that we develop may become inadequate, and weaknesses in our internal control over financial reporting may be discovered in the future. Any failure to develop or maintain effective controls could adversely affect the results of periodic management evaluations and annual independent registered public accounting firm attestation reports regarding the effectiveness of our internal control over financial reporting, which we may be required to include in our periodic reports we file with the SEC under Section 404 of the Sarbanes-Oxley Act, and could harm our operating results, cause us to fail to meet our reporting obligations, or result in a restatement of our prior period financial statements. In the event that we are not able to demonstrate compliance with the Sarbanes-Oxley Act, that our internal control over financial reporting is perceived as inadequate, or that we are unable to produce timely or accurate financial statements, investors may lose confidence in our operating results, and the price of our common stock could decline.
We are required to comply with certain of the SEC rules that implement Section 404 of the Sarbanes-Oxley Act, which requires management to certify financial and other information in our quarterly and annual reports and provide an annual management report on the effectiveness of our internal control over financial reporting. This assessment needs to include the disclosure of any material weaknesses in our internal control over financial reporting identified by our management or our independent registered public accounting firm. During the evaluation and testing process, if we identify one or more material weaknesses in our internal control over financial reporting or if we are unable to complete our evaluation, testing, and any required remediation in a timely fashion, we will be unable to assert that our internal control over financial reporting is effective.
Our independent registered public accounting firm will not be required to formally attest to the effectiveness of our internal control over financial reporting until the first annual report required to be filed with the SEC following the date we are no longer an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, depending on whether we choose to rely on certain exemptions set forth
83
Table of Contents
in the JOBS Act. We cannot assure you that there will not be material weaknesses or significant deficiencies in our internal controls in the future. If we are unable to assert that our internal control over financial reporting is effective, or if our independent registered public accounting firm is unable to express an opinion on the effectiveness of our internal control over financial reporting, we could lose investor confidence in the accuracy and completeness of our financial reports, which could have a material adverse effect on the price of our common stock.
In addition, adherence to our obligations to repurchase shares of our common stock pursuant to Roche’s anti-dilution protections in the Investor Rights Agreement may be costly and require substantial resources and efforts. Furthermore, adherence to our other obligations under the Investor Rights Agreement and the Tax Sharing Agreement, including our compliance with certain financial, accounting and tax reporting obligations, may be costly and time-consuming, require substantial resources and efforts, and result in changes to our existing business practices.
Our ability to use our net operating loss carryforwards to offset future taxable income may be subject to certain limitations.
In general, under Section 382 of the Internal Revenue Code of 1986, as amended, or the Code, a corporation that undergoes an “ownership change” is subject to annual limitations on its ability to use its pre-change net operating loss carryforwards or other tax attributes, or NOLs, to offset future taxable income or reduce taxes. In 2015, we completed a Section 382 analysis and believe we experienced one or more ownership changes in our corporate history, within the meaning of Section 382 of the Code, including in connection with our transaction with Roche that closed in April 2015; accordingly, our pre-change NOLs are subject to certain annual limitations under Section 382. Future changes in our stock ownership could result in additional ownership changes under Section 382 of the Code further limiting our ability to utilize our NOLs. Furthermore, our ability to use NOLs of companies that we may acquire in the future may be subject to limitations. For these reasons, we may not be able to use a material portion of the NOLs even if we attain profitability.
Risks Related to Our Common Stock
We expect that our stock price may fluctuate significantly.
There has been a public market for our common stock for only a short period of time. Although our common stock is listed on the NASDAQ Global Select Market, an active public market for our common stock may not be sustained, among other reasons, due to the ownership of a majority of our outstanding stock by Roche and contractual restrictions set forth in the Investor Rights Agreement on Roche’s and our ability to transact our shares.
In addition, the market price of shares of our common stock could be subject to wide fluctuations in response to many risk factors listed in this section, and others beyond our control, including:
| • | actual or anticipated fluctuations in our financial condition and operating results; |
| • | actual or anticipated changes in our growth rate relative to our competitors; |
| • | competition from existing products or new products that may emerge; |
| • | announcements by us, our biopharmaceutical partners, or our competitors of significant acquisitions, strategic partnerships, joint ventures, collaborations, or capital commitments; |
| • | failure to meet or exceed financial estimates and projections of the investment community or that we provide to the public and the revision of any financial estimates and projections that we provide to the public; |
| • | issuance of new or updated research or reports by securities analysts; |
| • | fluctuations in the valuation of companies perceived by investors to be comparable to us; |
84
Table of Contents
| • | share price and volume fluctuations attributable to inconsistent trading volume levels of our shares; |
| • | additions or departures of key management or scientific personnel; |
| • | disputes or other developments related to proprietary rights, including patents, litigation matters, and our ability to obtain patent protection for our technologies; |
| • | changes to reimbursement levels by commercial third-party payors and government payors, including Medicare, and any announcements relating to reimbursement levels; |
| • | announcement or expectation of additional debt or equity financing efforts; |
| • | sales of our common stock by us, our insiders, or our other stockholders; |
| • | the perception of us in the marketplace as an independent entity; |
| • | the performance of Roche or speculation about the possibility of future actions Roche may take in connection with us; and |
| • | general economic and market conditions. |
These and other market and industry factors may cause the market price and demand for our common stock to fluctuate substantially, regardless of our actual operating performance, which may limit or prevent investors from readily selling their shares of our common stock and may otherwise negatively affect the liquidity of our common stock. In addition, the stock market in general, and NASDAQ and biopharmaceutical companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. In the past, when the market price of a stock has been volatile, holders of that stock have instituted securities class action litigation against the company that issued the stock. If any of our stockholders brought a lawsuit against us, we could incur substantial costs defending the lawsuit. Such a lawsuit could also divert the time and attention of our management.
We are an “emerging growth company” and will be able to avail ourselves of reduced disclosure requirements applicable to emerging growth companies, which could make our common stock less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act, and we intend to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies,” including not being required to comply with the auditor attestation requirements of Section 404(b) of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. In addition, Section 107 of the JOBS Act also provides that an emerging growth company can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act of 1933, or the Securities Act, for complying with new or revised accounting standards. In other words, an emerging growth company can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. However, we are electing not to take advantage of such extended transition period, and as a result we will comply with new or revised accounting standards on the relevant dates on which adoption of such standards is required for non-emerging growth companies. Section 107 of the JOBS Act provides that our decision to not take advantage of the extended transition period for complying with new or revised accounting standards is irrevocable.
We cannot predict if investors will find our common stock less attractive because we may rely on any of the exemptions available under the JOBS Act. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile. We may take advantage of these reporting exemptions until we are no longer an emerging growth company. We will remain an emerging growth company until the earliest of (i) the last day of the fiscal year in which we have total
85
Table of Contents
annual gross revenue of $1.0 billion or more; (ii) December 31, 2018; (iii) the date on which we have issued more than $1.0 billion in nonconvertible debt during the previous three years; and (iv) the date on which we are deemed to be a large accelerated filer under the rules of the SEC.
We have never paid dividends on our capital stock, and we do not anticipate paying any dividends in the foreseeable future. Consequently, any gains from an investment in our common stock will likely depend on whether the price of our common stock increases.
We have not paid dividends on any of our classes of capital stock to date, and we currently intend to retain all of our future earnings, if any, to fund the development and growth of our business. As a result, capital appreciation, if any, of our common stock will be your sole source of gain for the foreseeable future. Consequently, in the foreseeable future, you will likely only experience a gain from your investment in our common stock if the price of our common stock increases.
If equity research analysts do not publish research or reports about our business or if they issue unfavorable commentary or downgrade our common stock, the price of our common stock could decline.
The trading market for our common stock relies in part on the research and reports that equity research analysts publish about us and our business. We do not control these analysts. The price of our common stock could decline if one or more equity research analysts downgrade our common stock or if they issue other unfavorable commentary or cease publishing reports about us or our business.
Our relationship with Roche and anti-takeover provisions contained in our certificate of incorporation and bylaws, as well as provisions of Delaware law, could impair a takeover attempt.
As of December 31, 2015, Roche beneficially owned approximately 61% of our outstanding common stock. As a result, until such time as Roche and its affiliates hold shares representing less, and potentially a material portion less, than a majority of the votes entitled to be cast by the holders of our outstanding common stock at a stockholder meeting, Roche generally will have the ability to control the outcome of matters submitted for the vote of our stockholders related to a proposed takeover attempt. The existence of a majority stockholder could discourage third parties from acquiring our business since the third party would be required to negotiate any such transaction with Roche, and the interests of Roche with respect to such transaction may be different from the interests of our other stockholders. In particular, it is possible that our minority stockholders may not receive a control premium for their shares upon any eventual sale of our business.
Our certificate of incorporation, bylaws, and Delaware law contain provisions which could have the effect of rendering more difficult, delaying or preventing an acquisition deemed undesirable by our Board. Our corporate governance documents include provisions:
| • | authorizing “blank check” preferred stock, which could be issued by our Board without stockholder approval and may contain voting, liquidation, dividend, and other rights superior to our common stock; |
| • | limiting the liability of, and providing indemnification to, our directors and officers; |
| • | limiting the ability of our stockholders to call and bring business before special meetings; |
| • | requiring advance notice of stockholder proposals for business to be conducted at meetings of our stockholders and for nominations of candidates for election to our Board; |
| • | controlling the procedures for the conduct and scheduling of Board and stockholder meetings; and |
| • | providing our Board with the express power to postpone previously scheduled annual meetings and to cancel previously scheduled special meetings. |
These provisions, alone or together, could delay or prevent hostile takeovers and changes in control or changes in our management.
86
Table of Contents
As a Delaware corporation, we are also subject to provisions of Delaware law, including Section 203 of the Delaware General Corporation Law, which prevents some stockholders holding more than 15% of our outstanding common stock from engaging in certain business combinations without approval of the holders of substantially all of our outstanding common stock.
Any provision of our certificate of incorporation, bylaws or Delaware law that has the effect of delaying or deterring a change in control could limit the opportunity for our stockholders to receive a premium for their shares of our common stock, and could also affect the price that some investors are willing to pay for our common stock.
Our certificate of incorporation designates the Court of Chancery of the State of Delaware as the sole and exclusive forum for certain types of actions and proceedings that may be initiated by our stockholders, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers, or other employees.
Our certificate of incorporation provides that, unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware shall be the sole and exclusive forum for (i) any derivative action or proceeding brought on our behalf, (ii) any action asserting a claim of breach of a fiduciary duty owed by any of our directors, officers, or other employees to us or our stockholders, (iii) any action asserting a claim arising pursuant to any provision of the Delaware General Corporation Law, our certificate of incorporation or our bylaws, or (iv) any action asserting a claim against us governed by the internal affairs doctrine. Any person or entity purchasing or otherwise acquiring any interest in shares of our capital stock shall be deemed to have notice of and consented to the provisions of our certificate of incorporation described above. This choice of forum provision may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers or other employees, which may discourage such lawsuits against us and our directors, officers, and other employees. Alternatively, if a court were to find these provisions of our certificate of incorporation inapplicable to, or unenforceable in respect of, one or more of the specified types of actions or proceedings, we may incur additional costs associated with resolving such matters in other jurisdictions, which could adversely affect our business, financial condition or results of operations.
ITEM 1B. UNRESOLVED STAFF COMMENTS
Not applicable.
We have leased approximately 62,000 square feet of laboratory and office space at 150 Second Street, Cambridge, Massachusetts through February 2021, 38,411 square feet of office space at Ten Canal Park in Cambridge, Massachusetts through approximately September 2020, and 1,975 square feet of office space in Palo Alto, California through May 2016. When each of our leases expire, we may renew the applicable existing lease or look for additional or alternate space for our operations. We believe that any additional space we may require will be available on commercially reasonable terms.
From time to time, we are party to litigation arising in the ordinary course of its business. As of December 31, 2015, we were not party to any material litigation.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
87
Table of Contents
| ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Market Information
Our common stock commenced trading under the symbol “FMI” on the NASDAQ Global Select Market on September 25, 2013. Prior to that time, there was no public market for our common stock.
The following table sets forth on a per share basis, for the periods indicated, the low and high close prices of our common stock as reported by the NASDAQ Global Select Market.
| High | Low | |||||||
| Year ended December 31, 2015 |
||||||||
| First Quarter |
$ | 49.76 | $ | 21.88 | ||||
| Second Quarter |
$ | 50.93 | $ | 31.64 | ||||
| Third Quarter |
$ | 34.13 | $ | 17.94 | ||||
| Fourth Quarter |
$ | 23.93 | $ | 15.97 | ||||
| High | Low | |||||||
| Year ended December 31, 2014 |
||||||||
| First Quarter |
$ | 43.62 | $ | 22.87 | ||||
| Second Quarter |
$ | 31.52 | $ | 20.30 | ||||
| Third Quarter |
$ | 29.30 | $ | 18.96 | ||||
| Fourth Quarter |
$ | 27.62 | $ | 18.67 | ||||
Holders of Record
On February 26, 2016, the last reported sales price of our common stock on the Nasdaq Global Select Market was $15.01 and as of February 26, 2016, there were approximately 26 holders of record of our common stock. However, because many of our outstanding shares are held in accounts with brokers and other institutions, we believe we have more beneficial owners.
Dividend Policy
We have never declared or paid dividends on our common stock and do not expect to pay dividends on our common stock for the foreseeable future. Instead, we anticipate that all of our earnings in the foreseeable future will be used for the operation and growth of our business. Any future determination to declare dividends will be subject to the discretion of our Board and will depend on various factors, including applicable laws, our results of operations, financial condition, future prospects, and any other factors deemed relevant by our Board. In addition, any future indebtedness that we may incur could preclude us from paying dividends.
Stock Performance Graph
This graph is not “soliciting material,” is not deemed “filed” with the SEC and is not to be incorporated by reference into any of our filings under the Securities Act or the Exchange Act, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing.
The following graph shows the total stockholder return of an investment of $100 in cash on September 25, 2013 (the first day of trading of our common stock), through December 31, 2015 for (i) our common stock, (ii) the NASDAQ Composite Index (U.S.), and (iii) the NASDAQ Biotechnology Index. Pursuant to applicable SEC rules, all values assume reinvestment of the full amount of all dividends, however no dividends have been declared on our common stock to date. The stockholder return shown on the graph below is not necessarily indicative of future performance, and we do not make or endorse any predictions as to future stockholder returns.
88
Table of Contents
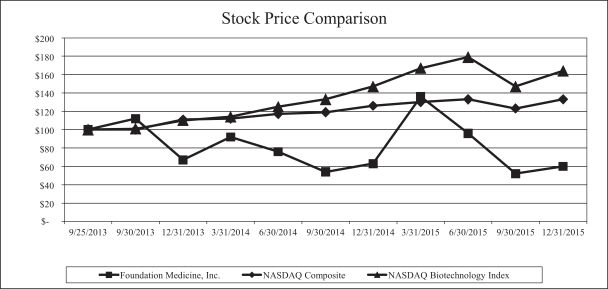
Equity Compensation Plan Information
For information regarding securities authorized for issuance under equity compensation plans, see Part III “Item 12—Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.”
Sales of Unregistered Securities
None.
Use of Proceeds
On September 30, 2013, we closed the sale of 6,772,221 shares of common stock to the public (inclusive of 883,333 shares of common stock sold by us pursuant to the full exercise of an overallotment option granted to the underwriters) at a price of $18.00 per share, before underwriting discounts. The offer and sale of the shares in our IPO was registered under the Securities Act pursuant to registration statements on Form S-1 (File No. 333-190226), which was filed with the SEC on July 29, 2013 and amended subsequently and declared effective by the SEC on September 24, 2013, and Form S-1MEF (File No. 333-191333), which was filed with the SEC on September 24, 2013 and automatically effective upon filing. Following the sale of the shares in connection with the closing of our IPO, the offering terminated. The offering did not terminate before all the securities registered in the registration statements were sold. Goldman, Sachs & Co. and J.P. Morgan Securities LLC acted as joint book-running managers of the offering, and Leerink Swann LLC and Sanford C. Bernstein & Co., LLC acted as co-managers of the offering.
We raised approximately $110.4 million in net proceeds after deducting underwriting discounts and commissions of approximately $8.5 million and other offering expenses of approximately $3.0 million. None of these expenses consisted of direct or indirect payments made by us to directors, officers or persons owning 10% or more of our common stock or to their associates, or to our affiliates. There were no material changes in our use of the proceeds from our IPO as described in our final prospectus filed with the SEC on September 25, 2013. As of December 31, 2015, we had used all of the net proceeds from our IPO.
Issuer Purchases of Equity Securities by the Issuer and Affiliated Purchasers
None.
89
Table of Contents
ITEM 6. SELECTED FINANCIAL DATA
The selected financial data set forth below as of December 31, 2015 and 2014, and for each of the years ended December 31, 2015, 2014, and 2013 has been derived from the audited consolidated financial statements of the Company, which are included elsewhere in this Annual Report on Form 10-K. We derived the consolidated financial data for the years ended December 31, 2012 and 2011, and as of December 31, 2013, 2012, and 2011 from our audited consolidated financial statements that are not included elsewhere in this Annual Report on Form 10-K.
The information set forth below should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the audited consolidated financial statements, and the notes thereto, and other financial information included herein. Our historical results are not necessarily indicative of our future results.
| Year Ended December 31, | ||||||||||||||||||||
| 2015 | 2014 | 2013 | 2012 | 2011 | ||||||||||||||||
| (in thousands, except share and per share data) | ||||||||||||||||||||
| Statement of Operations Data: |
||||||||||||||||||||
| Revenue |
$ | 79,759 | $ | 60,221 | $ | 28,136 | $ | 10,358 | $ | 2,057 | ||||||||||
| Related-party revenue from Roche |
13,444 | 858 | 854 | 287 | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total revenue |
93,203 | 61,079 | 28,990 | 10,645 | 2,057 | |||||||||||||||
| Costs and expenses |
||||||||||||||||||||
| Cost of revenue |
36,695 | 27,434 | 11,659 | 5,681 | 258 | |||||||||||||||
| Cost of Roche related-party revenue |
2,736 | — | — | — | — | |||||||||||||||
| Selling and marketing |
49,030 | 28,997 | 12,326 | 3,454 | 1,555 | |||||||||||||||
| General and administrative |
50,614 | 27,302 | 21,865 | 8,644 | 6,992 | |||||||||||||||
| Research and development |
43,883 | 30,629 | 24,901 | 14,777 | 9,023 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total costs and expenses |
182,958 | 114,362 | 70,751 | 32,556 | 17,828 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Loss from operations |
(89,755 | ) | (53,283 | ) | (41,761 | ) | (21,911 | ) | (15,771 | ) | ||||||||||
| Interest income (expense), net |
124 | (42 | ) | (235 | ) | (421 | ) | (421 | ) | |||||||||||
| Other income (expense), net |
— | 1,103 | (948 | ) | (61 | ) | (845 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net loss |
(89,631 | ) | (52,222 | ) | (42,944 | ) | (22,393 | ) | (17,037 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Accretion of convertible preferred stock |
— | — | (139 | ) | (286 | ) | (296 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net loss applicable to common stockholders |
$ | (89,631 | ) | $ | (52,222 | ) | $ | (43,083 | ) | $ | (22,679 | ) | $ | (17,333 | ) | |||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net loss per common share applicable to common stockholders, basic and diluted |
$ | (2.73 | ) | $ | (1.87 | ) | $ | (4.64 | ) | $ | (10.47 | ) | $ | (14.06 | ) | |||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Weighted average common shares outstanding, basic and diluted |
32,836,219 | 27,954,327 | 9,294,730 | 2,166,832 | 1,232,658 | |||||||||||||||
| December 31, | ||||||||||||||||||||
| 2015 | 2014 | 2013 | 2012 | 2011 | ||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Balance Sheet Data: |
||||||||||||||||||||
| Cash, cash equivalents, and marketable securities |
$ | 232,309 | $ | 72,080 | $ | 124,293 | $ | 54,838 | $ | 10,852 | ||||||||||
| Working capital |
199,748 | 73,202 | 117,551 | 49,856 | 7,521 | |||||||||||||||
| Total assets |
297,989 | 111,938 | 157,268 | 66,039 | 18,065 | |||||||||||||||
| Notes payable, excluding current portion |
— | — | — | 1,441 | 3,041 | |||||||||||||||
| Redeemable convertible preferred stock warrant liability |
— | — | — | 225 | 94 | |||||||||||||||
| Redeemable convertible preferred stock |
— | — | — | 98,658 | 32,455 | |||||||||||||||
| Accumulated deficit |
(231,616 | ) | (141,985 | ) | (89,763 | ) | (46,819 | ) | (24,426 | ) | ||||||||||
| Total stockholders’ equity (deficit) |
$ | 257,689 | $ | 86,169 | $ | 131,711 | $ | (43,397 | ) | $ | (22,303 | ) | ||||||||
90
Table of Contents
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
The following discussion and analysis of our financial condition and results of operations should be read together with our “Selected Financial Data” and our consolidated financial statements, related notes, and other financial information included elsewhere in this Annual Report on Form 10-K. This discussion contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those described in, or implied by, the forward-looking statements. Factors that could cause or contribute to those differences include, but are not limited to, those identified below and those discussed above in the section entitled “Risk Factors.”
Overview
We are a molecular information company focused on fundamentally changing the way in which patients with cancer are evaluated and treated. We believe an information-based approach to making clinical treatment decisions based on comprehensive genomic profiling will become a standard of care for patients with cancer. We derive revenue from selling products that are enabled by our molecular information platform to physicians and biopharmaceutical companies. Our platform includes proprietary methods and algorithms for analyzing specimens across all types of cancer, and for incorporating that information into clinical care in a concise and user-friendly fashion. Our products provide genomic information about each patient’s individual cancer, enabling physicians to optimize treatments in clinical practice and biopharmaceutical companies to develop targeted oncology therapies more effectively. We believe we have a significant first mover advantage in providing comprehensive genomic profiling and molecular information products on a commercial scale.
Our flagship clinical molecular information products, FoundationOne for solid tumors and FoundationOne Heme for blood-based cancers, or hematologic malignancies, including leukemia, lymphoma, myeloma, and advanced sarcomas, are, to our knowledge, the only widely available comprehensive genomic profiles designed for use in the routine care of patients with cancer. To accelerate our commercial growth and enhance our competitive advantage, we are continuing to develop and commercialize new molecular information products for physicians and biopharmaceutical companies, strengthen our commercial organization, introduce new marketing, education and provider engagement efforts, grow our molecular information knowledgebase, called FoundationCORE, aggressively pursue reimbursement from regional and national third-party payors and government payors, publish scientific and medical advances, and foster relationships across the oncology community. We believe our molecular information products address a market opportunity of $12-15 billion.
The cancer treatment paradigm is evolving rapidly, and we believe there is now widespread recognition that cancer is a disease of the genome, rather than a disease defined solely by its specific anatomical location in the body. Today, physicians increasingly use precision medicines to target cancers based on the specific genomic alterations driving their growth. We believe physicians need molecular information about their patients’ unique cancers to determine the optimal course of treatment.
Since our inception in 2009, we have devoted substantially all of our resources to the development of our molecular information platform, the commercialization of FoundationOne, and the development of new products such as FoundationOne Heme and FoundationACT. We have incurred significant losses since our inception, and as of December 31, 2015 our accumulated deficit was approximately $231.6 million. We expect to continue to incur operating losses over the near term as we expand our commercial operations, conduct clinical trials, and invest in our molecular information platform and additional products, including the anticipated commercial launch of FoundationACT in March 2016.
91
Table of Contents
Recent Developments
In January 2016, we announced a three-way collaboration with Horizon Healthcare Services, New Jersey’s oldest and largest health insurer, and COTA to advance precision medicine, improve clinical outcomes, and deliver enhanced value to the healthcare system in the treatment of patients with metastatic NSCLC. Together, the organizations initiated a prospective clinical study measuring changes in survival benefit and total cost savings achieved among patients with previously untreated metastatic NSCLC who undergo comprehensive genomic profiling with FoundationOne versus another assay. The study is designed to enable Horizon Healthcare Services to provide its members with coverage for FoundationOne as a critical component clinical care pathway in the evaluation of patients with metastatic lung cancer.
In December 2015, we launched our third comprehensive genomic profiling product, FoundationACT (Assay for Circulating Tumor DNA), for our biopharmaceutical partners for research use. We believe that FoundationACT is a best-in-class, blood-based (liquid biopsy) assay to measure ctDNA. We plan to initiate the commercial launch of FoundationACT to ordering physicians in March 2016. We believe FoundationACT will become an important molecular information solution for oncologists because it will provide a new option for comprehensive genomic profiling when tissue biopsy is not feasible. By analyzing cell-free DNA isolated from a patient’s blood, we can identify clinically relevant genomic alterations in the cell-free DNA that is ctDNA and match these alterations to targeted therapies and clinical trials.
Financial Operations Overview
Revenue
We derive our revenue from selling products that are enabled by our molecular information platform. The information provided in our test results is branded as FoundationOne, FoundationOne Heme or FoundationACT for our clinical customers and is not branded for our biopharmaceutical customers. The principal focus of our commercial operations is to continue to drive adoption of products enabled by our molecular information platform. In particular, we seek to increase sales volume of FoundationOne and FoundationOne Heme and, once launched, FoundationACT, in the clinical setting and increase the volume of tests and other services enabled by our molecular information platform that we perform for our biopharmaceutical customers.
For many physician orders within the United States, the payment we ultimately receive depends upon the rate of reimbursement from commercial third-party payors and government payors. We are not currently a participating provider with most commercial third-party payors and, therefore, do not have specific coverage decisions from those third-party payors for our products with established payment rates. Currently, most of the commercial third-party payors that reimburse our claims do so based upon the stacked CPT codes, the predominant methodology, or based on other methods such as percentages of charges or other formulas that are not made known to us. In addition, a small portion of commercial third-party payors outsource our claims to preferred provider organizations or third-party administrators, who process our claims and pay us directly at negotiated rates. Coverage and payment is determined by each third-party payor on a case-by-case basis. As of December 31, 2015, we were not a participating provider in any state Medicaid program, and, therefore did not have coverage decisions under which our tests were covered by these Medicaid programs. We are a participating provider in the Medicare program, but we do not have a coverage decision within our jurisdiction. At the end of 2013, we began the process of submitting claims for our tests to Medicare. We may also negotiate rates with patients, if the patient is responsible for payment. Our efforts in obtaining reimbursement based on individual claims, including pursuing appeals or reconsiderations of claim denials, take a substantial amount of time, and bills may not be paid for many months or at all. Furthermore, if a third-party payor denies coverage after final appeal, payment may not be received at all.
We currently recognize revenue on a cash basis from commercial third-party payors and from patients who make co-payments, pay deductibles, or pay other amounts that we have been unable to collect from their third-party payors because the payment is not fixed or determinable and collectability is not reasonably assured, as a result of the fact that we do not have coverage decisions in place with most third-party payors and have a limited
92
Table of Contents
history of collecting claims. We expect to use judgment in assessing whether the fee is fixed or determinable and whether collectability is reasonably assured as we continue to gain payment experience with third-party payors and patients. Costs associated with performing tests are recorded as tests are processed. These costs are recorded regardless of when or whether revenue is recognized with respect to those tests. Because we currently recognize revenue on a cash basis from commercial third-party payors, the costs of those tests are recognized in advance of any associated revenues. Our revenue from these payors is generally lower and our net loss is higher than if we were recognizing revenue from these payors on an accrual basis in the period during which the work was performed and costs were incurred.
There are currently no national or local coverage determinations in our region that determine whether and how our tests are covered by Medicare. In the absence of national coverage decisions, local MACs that administer the Medicare program in various regions have some discretion in determining coverage and, therefore, payment for tests. For example, while one leading MAC covering another region issued a decision to cover well-validated comprehensive genomic profiles for a subset of NSCLC patients effective July 2015, our local MAC has not yet elected to follow the same standards for determining coverage at this time. Our local MAC, who would process our claims on behalf of Medicare, initially requested that we not submit claims for FoundationOne tests provided to Medicare patients while the contractor assessed the appropriate coverage and payment for FoundationOne as a whole. Based on the volume of our Medicare claims, we began the process of submitting claims to Medicare in November 2013, but we have not generated any revenue from Medicare for our tests to date. As a result, our net loss is higher than if we were recognizing revenue from the sale of our tests for patients covered by Medicare. FoundationOne and FoundationOne Heme tests for patients covered by Medicare represented approximately 31%, 31%, and 30% of total tests reported to physicians in the United States during the years ended December 31, 2015, 2014, and 2013, respectively.
At the end of 2013, we commenced the process of submitting claims to Medicare for FoundationOne tests provided to Medicare patients, and subsequently during the first quarter of 2014 we commenced the process of submitting claims to Medicare for FoundationOne Heme tests provided to Medicare patients. As of December 31, 2015, our MAC has not correctly processed and reimbursed us for any of the claims that we have submitted, and we are in the process of appealing these unpaid claims. In the future, our MAC may issue a negative coverage determination for one or more of our tests that would apply to future claims or may defer processing a claim pending a coverage or payment determination. If a claim is paid by our MAC, either upon acceptance of the claim or following a successful appeal of a denied claim, we will generate revenue from Medicare for our testing. In February 2016, National Government Services announced a final LCD, effective April 1, 2016, which includes reimbursement for hotspot tests of 5 to 50 genes for patients with metastatic NSCLC. We do not believe this LCD reflects coverage for our validated comprehensive genomic profiling products, and we will continue to seek a positive coverage determination from our MAC, which, if obtained, will establish a standard for the reimbursement of our Medicare claims.
We expect that our current lack of significant coverage decisions and the general uncertainty around reimbursement for our tests will continue to negatively impact our revenue and earnings, both because we will not recognize revenue for tests performed, particularly if our test volumes increase period-to-period, and because the absence of Medicare or other significant coverage decisions may lead physicians to not order a meaningful number of tests. Following our achievement of a coverage decision from a commercial third-party payor or a government payor or once we have a sufficient history of claims collections with any such payor that we conclude the fees for our tests for individuals insured by such payor are sufficiently fixed or determinable and collectability is reasonably assured, we will begin to recognize revenue from such payor on an accrual basis. As of December 31, 2015, we had cash, cash equivalents, and marketable securities of approximately $232.3 million. If we are not able to obtain coverage decisions from additional commercial third-party payors and government payors over the longer term, and our available cash and marketable security balances and cash flows from operations are insufficient to satisfy our liquidity requirements, we may require additional capital beyond our currently anticipated amounts. Additional capital may not be available on reasonable terms, or at all, and may be subject to the prior consent of Roche pursuant to our Investor Rights Agreement.
93
Table of Contents
We recognize revenue from the sale of our tests to certain hospitals, cancer centers, other institutions, and patients at the time results are reported to physicians if all revenue recognition criteria have been met.
We also receive a small portion of revenue from patients who make co-payments and pay deductibles. In addition, while we take on the primary responsibility for obtaining third-party reimbursement on behalf of patients, including appeals for any initial denials, we ultimately do bill patients for amounts that we have been unable to collect from their third-party payors. We initiated the process to seek reimbursement from Medicare at the end of 2013, and we may also decide to provide appropriate notices to patients covered by Medicare to enable us to bill a patient for all or part of a claim that is denied coverage by Medicare. We offer a comprehensive patient assistance program to support patients whose incomes are below certain thresholds and to allow for extended payment terms, as necessary, given the patient’s economic situation.
Revenue from our biopharmaceutical customers is based on a negotiated price per test or on the basis of agreements to provide certain testing volumes or other deliverables over defined periods. We recognize revenue upon delivery of the test results, or over the period that testing volume or other deliverables are provided, as appropriate, assuming all other revenue recognition criteria have been met.
Certain of our arrangements include multiple deliverables. We analyze multiple-element arrangements based on the guidance in Financial Accounting Standards Board, or FASB, Accounting Standards Codification Topic 605-25, Revenue Recognition-Multiple-Element Arrangements, or ASC 605-25. Pursuant to the guidance in ASC 605-25, we evaluate multiple-element arrangements to determine (1) the deliverables included in the arrangement and (2) whether the individual deliverables represent separate units of accounting or whether they must be accounted for as a combined unit of accounting. This evaluation involves subjective determinations and requires management to make judgments about the individual deliverables and whether such deliverables are separable from the other aspects of the contractual relationship. Deliverables are considered separate units of accounting provided that: (i) the delivered items have value to the customer on a standalone basis and (ii) if the arrangement includes a general right of return relative to the delivered items and delivery or performance of the undelivered items is considered probable and substantially in our control. In assessing whether an item has standalone value, we consider factors such as the research, development and commercialization capabilities of a third party and the availability of the associated expertise in the general marketplace. In addition, we consider whether the other party in the arrangement can use the other deliverables for their intended purpose without the receipt of the remaining elements, whether the value of the deliverable is dependent on the undelivered items, and whether there are other vendors that can provide the undelivered elements.
Arrangement consideration that is fixed or determinable is allocated among the separate units of accounting using the relative selling price method. Then, the applicable revenue recognition criteria in ASC 605-25 is applied to each of the separate units of accounting in determining the appropriate period and pattern of recognition. We determine the selling price of a unit of accounting following the hierarchy of evidence prescribed by ASC 605-25. Accordingly, we determine the estimated selling price for units of accounting within each arrangement using vendor-specific objective evidence, or VSOE, of selling price, if available, third-party evidence, or TPE, of selling price if VSOE is not available, or best estimate of selling price, or BESP, if neither VSOE nor TPE is available. We typically use BESP to estimate the selling price, since we generally do not have VSOE or TPE of selling price for our units of accounting under multiple-element arrangements. Determining the BESP for a unit of accounting requires significant judgment. In developing the BESP for a unit of accounting, we consider applicable market conditions and estimated costs. We validate the BESP for units of accounting by evaluating whether changes in the key assumptions used to determine the BESP will have a significant effect on the allocation of arrangement consideration between multiple units of accounting. We recognize arrangement consideration allocated to each unit of accounting when all of the revenue recognition criteria in ASC 605-25 are satisfied for that particular unit of accounting.
At the inception of an arrangement that includes milestone payments, we evaluate whether each milestone is substantive and at risk to both parties on the basis of the contingent nature of the milestone. This evaluation includes an assessment of whether: (i) the consideration is commensurate with either our performance to achieve
94
Table of Contents
the milestone or the enhancement of the value of the delivered items as a result of a specific outcome resulting from our performance to achieve the milestone, (ii) the consideration relates solely to past performance, and (iii) the consideration is reasonable relative to all of the deliverables and payment terms within the arrangement. We evaluate factors such as the scientific, clinical, regulatory, commercial, and other risks that must be overcome to achieve the respective milestone and the level of effort and investment required to achieve the respective milestone in making this assessment. There is considerable judgment involved in determining whether a milestone satisfies all of the criteria required to conclude that a milestone is substantive. Generally, once a substantive milestone has been achieved, we will recognize revenue related to that milestone using a proportional performance model over the period which the unit of accounting is delivered or based on the level of effort expended to date over the total expected effort, whichever is considered the most appropriate measure of performance. Revenue from commercial milestone payments are accounted for as royalties and recorded as revenue upon achievement of the milestone, assuming all other revenue recognition criteria are met.
We also recognize royalty revenue in the period of sale of the related product(s), based on the underlying contract terms, provided that the reported sales are reliably measurable and we have no remaining performance obligations, assuming all other revenue recognition criteria are met.
For some multiple-element arrangements, we are reimbursed for either all or a portion of the research and development costs incurred. We perform research and development services as part of our revenue activities and, therefore, believe such activities are a part of our primary business. Therefore, we record these reimbursements as revenue in the statement of operations using a proportional performance model over the period which the unit of accounting is delivered or based on the level of effort expended to date over the total expected effort, whichever is considered the most appropriate measure of performance.
We expect our revenue to increase over time as we expand our commercial efforts within the United States, and outside the United States pursuant to our Ex-U.S. Commercialization Agreement with Roche. Positive reimbursement decisions from additional commercial third-party payors and government payors, such as Medicare and Medicaid, would eliminate much of the uncertainty around payment and could allow us to recognize revenue earlier and potentially increase our overall revenue growth and test volume growth from ordering physicians within the United States. We also expect to grow our biopharmaceutical customer base. Over time, we expect that our revenue from ordering physicians within and outside of the United States will exceed revenue from our biopharmaceutical customers, given the higher percentage of patients with cancer who are treated outside of clinical trial settings.
Cost of Revenue and Operating Expenses
We allocate certain overhead expenses, such as rent, utilities, and depreciation to cost of revenue and operating expense categories based on headcount and facility usage. As a result, an overhead expense allocation is reflected in cost of revenue and each operating expense category.
Cost of Revenue
Cost of revenue consists of personnel expenses, including salaries, bonuses, employee benefits and stock-based compensation expenses, cost of laboratory supplies, depreciation of laboratory equipment and amortization of leasehold improvements, shipping costs, and certain allocated overhead expenses. We expect these costs will increase in absolute dollars as we increase our sales volume, but will decrease as a percentage of revenue over time as our sales increase and we gain operating efficiencies.
Costs associated with performing tests are recorded as tests are processed. These costs are recorded regardless of whether revenue is recognized with respect to those tests. Because we currently recognize revenue on a cash basis from commercial third-party payors and patients who make co-payments, pay deductibles or pay other amounts that we have been unable to collect from their insurers, the costs of those tests are often recognized in advance of any associated revenues.
95
Table of Contents
Selling and Marketing Expenses
Our selling and marketing expenses include costs associated with our sales organization, including our direct sales force and sales management, client services, marketing, reimbursement, and business development personnel who are focused on our biopharmaceutical customers. These expenses consist principally of salaries, commissions, bonuses, employee benefits, travel, and stock-based compensation, as well as marketing and educational activities, and allocated overhead expenses. We expense all selling and marketing costs as incurred.
During the years ended December 31, 2015, 2014, and 2013, our selling and marketing expenses represented approximately 53%, 47%, and 43%, respectively, of our total revenue. We expect our selling and marketing expenses to continue to increase in absolute dollars as we expand our sales force, grow our client service infrastructure, and increase our marketing and medical affairs activities to drive further awareness and adoption of FoundationOne, FoundationOne Heme, FoundationACT, and any future products we may develop.
General and Administrative Expenses
Our general and administrative expenses include costs for our executive, accounting and finance, legal, and human resources functions. These expenses consist principally of salaries, bonuses, employee benefits, travel, and stock-based compensation, as well as professional services fees such as consulting, audit, tax, legal and billing fees, general corporate costs and allocated overhead expenses. We expense all general and administrative expenses as incurred.
We expect that our general and administrative expenses will continue to increase, primarily due to the costs associated with increased infrastructure and headcount, as well as the costs associated with fulfilling our obligations under our collaboration with Roche. These costs include additional legal and accounting expenses, and an increase in billing costs related to our anticipated increase in revenues.
Research and Development Expenses
Our research and development expenses consist primarily of costs incurred for the development of new and enhanced products and services including FoundationACT, immunotherapy testing, companion diagnostic development, significant product improvements, clinical trials to evaluate the clinical utility of our tests, the development of our FoundationCORE knowledgebase, and various technology applications such as FoundationICE, Patient Match, GeneKit, and SmartTrials. Costs to develop our technology capabilities are recorded as research and development unless they meet the criteria to be capitalized as internal-use software costs. Our research and development activities include the following costs:
| • | personnel-related expenses such as salaries, bonuses, employee benefits, and stock-based compensation; |
| • | fees for contractual and consulting services; |
| • | costs to manage and synthesize our medical data and to expand FoundationCORE; |
| • | clinical trials; |
| • | laboratory supplies; and |
| • | allocated overhead expenses. |
We expect that our overall research and development expenses will continue to increase in absolute dollars as we continue to innovate our molecular information platform, develop additional products, expand our genomic and medical data management resources, and conduct our ongoing and new clinical trials.
96
Table of Contents
Interest Income (Expense), Net
Interest income (expense), net includes interest income and interest expense. Interest income is earned on our cash, cash equivalents, and marketable securities. Interest expense consists primarily of interest incurred on our loan balance, which was paid in full during the year ended December 31, 2014.
Other Income (Expense), Net
Other income (expense), net for the year ended December 31, 2014 resulted from a tax incentive award from the Massachusetts Life Sciences Center. Other income (expense), net for the year ended December 31, 2013 resulted from changes in the fair value of our preferred stock warrant liability, which was exercised in October 2013, partially offset by a tax incentive award from the Massachusetts Life Sciences Center.
Results of Operations
Comparison of Years Ended December 31, 2015 and 2014
| Year Ended December 31, | Change | |||||||||||||||
| 2015 | 2014 | $ | % | |||||||||||||
| (in thousands, except percentages) | ||||||||||||||||
| Statement of Operations Data: |
||||||||||||||||
| Revenue |
$ | 79,759 | $ | 60,221 | $ | 19,538 | 32 | % | ||||||||
| Related-party revenue from Roche |
13,444 | 858 | 12,586 | 1467 | % | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total revenue |
93,203 | 61,079 | 32,124 | 53 | % | |||||||||||
| Costs and expenses |
||||||||||||||||
| Cost of revenue |
36,695 | 27,434 | 9,261 | 34 | % | |||||||||||
| Cost of Roche related-party revenue |
2,736 | — | 2,736 | 100 | % | |||||||||||
| Selling and marketing |
49,030 | 28,997 | 20,033 | 69 | % | |||||||||||
| General and administrative |
50,614 | 27,302 | 23,312 | 85 | % | |||||||||||
| Research and development |
43,883 | 30,629 | 13,254 | 43 | % | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total costs and expenses |
182,958 | 114,362 | 68,596 | 60 | % | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss from operations |
(89,755 | ) | (53,283 | ) | (36,472 | ) | (68 | %) | ||||||||
| Interest income (expense), net |
124 | (42 | ) | 166 | 395 | % | ||||||||||
| Other income (expense), net |
— | 1,103 | (1,103 | ) | 100 | % | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
$ | (89,631 | ) | $ | (52,222 | ) | $ | (37,409 | ) | (72 | %) | |||||
|
|
|
|
|
|
|
|
|
|||||||||
Reclassifications
Certain reclassifications have been made to conform the prior period’s data to the current format. These reclassifications had no net effect on our consolidated financial statements.
Revenue
Total revenue increased to $93.2 million for the year ended December 31, 2015 from $61.1 million during the year ended December 31, 2014. Revenue from tests reported for our ordering physicians increased to $49.2 million for the year ended December 31, 2015 from $36.6 million for the year ended December 31, 2014. The increase was driven by our growing test volumes and expanding commercialization efforts. The increase in revenue from our biopharmaceutical customers to $44.0 million from $24.4 million for the years ended December 31, 2015 and 2014, respectively, resulted from increased business development activity among our new and existing biopharmaceutical customers and a broadening of the services we offer to existing clients.
97
Table of Contents
During the year ended December 31, 2015, we reported 32,998 clinical tests to ordering physicians, including 3,922 FoundationOne Heme tests, as compared to 24,271 tests reported during the year ended December 31, 2014, including 3,716 FoundationOne Heme tests. We also reported 8,827 and 5,586 tests to our biopharmaceutical customers during the years ended December 31, 2015 and 2014, respectively.
The average revenue per test for clinical use that met our revenue recognition criteria during the year ended December 31, 2015 was approximately $3,300. This average revenue per test does not include 8,779 FoundationOne and FoundationOne Heme tests reported during the period for patients covered by Medicare and for which claims were not yet submitted or paid, 246 tests that were reported and not billed, and 11,758 tests that were reported and billed to commercial third-party payors during the period but were not paid during the period. This average revenue includes 2,820 tests reported in prior years for which revenue was recognized during the year ended December 31, 2015.
The average revenue per test for clinical use that met our revenue recognition criteria during the year ended December 31, 2014 was approximately $3,600. This average revenue per test does not include 6,363 FoundationOne and FoundationOne Heme tests reported during the period for patients covered by Medicare and for which claims were not yet submitted or paid, 348 tests that were reported and not billed, and 8,830 tests that were reported and billed to commercial third-party payors during the period but were not paid during the period. This average revenue includes 1,559 tests reported in prior years for which revenue was recognized during the year ended December 31, 2014.
Our average revenue per test excludes tests for which we have not yet recognized revenue. Because we recognize revenue on a cash basis from commercial third-party payors and from patients who make co-payments, pay deductibles, or pay other amounts that we have been unable to collect from their third-party payors because the payment is not fixed or determinable and collectability is not reasonably assured, and our efforts to obtain payment for individual claims can take a substantial amount of time, there is typically a significant lag between the time the test is reported and the time we actually recognize the revenue from such test. As a result, if we were to include tests for which we have not recognized revenue in our average revenue per test calculation for a particular period, it would imply that we will not receive any revenue for such tests. Despite our lack of coverage decisions, we have been reasonably successful in securing reimbursement from many commercial third-party payors for tests reported in prior periods. With respect to tests reported for patients covered by Medicare, we commenced the process of submitting claims to Medicare for these tests in November 2013 and have not yet been reimbursed based on properly processed submissions for these claims. We also expect to record revenue from patients who make co-payments, pay deductibles, or pay other amounts that we have been unable to collect from third-party payors. While receipt of payment from third-party payors and patients in respect of these claims is not currently fixed or determinable and collectability is not reasonably assured, we do expect to record revenue in the future for some of the tests reported in this period. However, it is difficult to predict future revenue from the previously reported tests because we are in an early stage of commercialization and we have limited payment history. As a result, we cannot be certain that the revenue per test we recognize in the future will remain consistent with the average revenue per test reported above.
The cumulative amount of FoundationOne and FoundationOne Heme tests that have been billed to commercial third-party payors and reported for patients covered by Medicare but for which we had not recognized revenue was 19,409 and 17,623, respectively, as of December 31, 2015. If commercial third-party payors or government payors agree to pay us for these tests in the future, we will recognize revenue for such tests in the period in which our revenue recognition criteria are met. Any revenue that we receive in respect of these previously reported tests will favorably impact our liquidity and results of operations in future periods.
For our biopharmaceutical customer revenue that was based on a negotiated price per test, the average revenue per test was approximately $3,700 and $3,300 for the years ended December 31, 2015 and 2014, respectively. This increase in average revenue per test was primarily due to entering into agreements with new biopharmaceutical customers during the year. We expect our average revenue per test for biopharmaceutical customers to remain fairly consistent with prior periods over time. Approximately $29.5 million and $11.6
98
Table of Contents
million of our biopharmaceutical revenue for the years ended December 31, 2015 and 2014, respectively, represented payments under contracts with multiple-element arrangements that were not negotiated on a price per test basis.
Cost of Revenue
Total cost of revenue, including the cost of Roche related-party revenue, increased to $39.4 million for the year ended December 31, 2015 from $27.4 million for the year ended December 31, 2014. This increase was driven by increasing test volumes from our ordering physicians and biopharmaceutical customers. The average cost per test does not differ materially by customer. Additional volume led to higher reagent and consumable costs, additional laboratory personnel-related costs, facilities costs, and higher depreciation expense related to new equipment purchases. During the years ended December 31, 2015 and 2014, our total cost of revenue represented approximately 42.3% and 44.9%, respectively, of our total revenue. We expect to make additional investments in personnel, infrastructure, and systems to scale our laboratory operations to meet future anticipated demand for existing and new products.
Selling and Marketing Expenses
Selling and marketing expenses increased to $49.0 million for the year ended December 31, 2015 from $29.0 million for the year ended December 31, 2014. The increase was primarily related to a 43% increase in headcount during the year and consisted of an increase of $10.0 million in personnel-related costs, including $2.3 million in stock-based compensation charges, for new employees in our sales, marketing, client service, and reimbursement departments to support our commercialization efforts, a $4.6 million increase in marketing and consulting costs, a $3.6 million increase in rent and other facilities costs, and a $1.8 million increase in travel-related costs.
General and Administrative Expenses
General and administrative expenses increased to $50.6 million for the year ended December 31, 2015 from $27.3 million for the year ended December 31, 2014. The increase was primarily related to a 51% increase in headcount during the year and included $14.4 million in advisor fees related to closing the Roche transaction, a $5.1 million increase in personnel costs to support and expand our legal, finance, and human resources infrastructure, including $1.6 million in stock-based compensation charges, a $2.2 million combined increase in legal, consulting, audit, and billing fees, and a $1.6 million increase in rent and other facilities costs.
Research and Development Expenses
Research and development expenses increased to $43.9 million for the year ended December 31, 2015 from $30.6 million for the year ended December 31, 2014. The increase was primarily related to a 43% increase in headcount during the year and consisted of a $5.4 million increase in employee and contractor-related expenses, a $3.9 million increase in laboratory supply costs, including reagents utilized in research and development activities, a $1.6 million increase in laboratory management expenses, a $1.6 million increase in rent and other facilities costs, and a $0.8 million increase in clinical trial costs.
Interest Income (Expense), Net
Interest income (expense), net was $0.1 million for the year ended December 31, 2015, consisting of interest earned on cash, cash equivalents, and marketable securities. Interest income (expense), net was ($42,000) for the year ended December 31, 2014, consisting primarily of interest expense on our loan balance, which was paid in full during the year ended December 31, 2014.
Other Income (Expense), Net
Other income (expense), net was $0 and $1.1 million for the years ended December 31, 2015 and 2014, respectively, resulting from a tax incentive award granted by the Massachusetts Life Sciences Center.
99
Table of Contents
Comparison of Years Ended December 31, 2014 and 2013
| Year Ended December 31, | Change | |||||||||||||||
| 2014 | 2013 | $ | % | |||||||||||||
| (in thousands, except percentages) | ||||||||||||||||
| Statement of Operations Data: |
||||||||||||||||
| Revenue |
$ | 60,221 | $ | 28,136 | $ | 32,085 | 114 | % | ||||||||
| Related-party revenue from Roche |
858 | 854 | 4 | 0 | % | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total revenue |
61,079 | 28,990 | 32,089 | 111 | % | |||||||||||
| Costs and expenses |
||||||||||||||||
| Cost of revenue |
27,434 | 11,659 | 15,775 | 135 | % | |||||||||||
| Selling and marketing |
28,997 | 12,326 | 16,671 | 135 | % | |||||||||||
| General and administrative |
27,302 | 21,865 | 5,437 | 25 | % | |||||||||||
| Research and development |
30,629 | 24,901 | 5,728 | 23 | % | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total costs and expenses |
114,362 | 70,751 | 43,611 | 62 | % | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss from operations |
(53,283 | ) | (41,761 | ) | (11,522 | ) | (28 | %) | ||||||||
| Interest income (expense), net |
(42 | ) | (235 | ) | 193 | (82 | %) | |||||||||
| Other income (expense), net |
1,103 | (948 | ) | 2,051 | 216 | % | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
$ | (52,222 | ) | $ | (42,944 | ) | $ | (9,278 | ) | (22 | %) | |||||
|
|
|
|
|
|
|
|
|
|||||||||
Revenue
Total revenue increased to $61.1 million for the year ended December 31, 2014 from $29.0 million during the year ended December 31, 2013. Revenue from tests reported for our ordering physicians increased to $36.6 million for the year ended December 31, 2014 from $14.8 million for the year ended December 31, 2013. The increase was driven by our growing test volumes and expanding commercialization efforts. The increase in revenue from our biopharmaceutical customers to $24.4 million from $14.2 million for the years ended December 31, 2014 and 2013, respectively, resulted from increased activity among our new and existing biopharmaceutical customers.
During the year ended December 31, 2014, we reported 24,271 clinical tests to ordering physicians, including 3,716 FoundationOne Heme tests, as compared to 9,095 FoundationOne tests and no FoundationOne Heme tests reported during the year ended December 31, 2013. We also reported 5,586 and 3,776 tests to our biopharmaceutical customers during the years ended December 31, 2014 and 2013, respectively.
The average revenue per test for clinical use that met our revenue recognition criteria during the year ended December 31, 2014 was approximately $3,600. This average revenue per test does not include 6,363 FoundationOne and FoundationOne Heme tests reported during the period for patients covered by Medicare and for which claims were not yet submitted or paid, 348 tests that were reported and not billed, and 8,830 tests that were reported and billed to commercial third-party payors during the period but were not paid during the period. This average revenue includes 1,559 tests reported in prior years for which revenue was recognized during the year ended December 31, 2014.
The average revenue per test for clinical use that met our revenue recognition criteria during the year ended December 31, 2013 was approximately $3,500. This average revenue per test does not include 2,080 FoundationOne tests reported during the period for patients covered by Medicare and for which claims were not yet submitted or paid, 198 tests that were reported and not billed, and 2,963 tests that were reported and billed to commercial third-party payors during the period but were not paid during the period. This average revenue includes 435 tests reported in prior years for which revenue was recognized during the year ended December 31, 2013.
100
Table of Contents
Our average revenue per test excludes tests for which we have not yet recognized revenue. Because we recognize revenue on a cash basis from commercial third-party payors and from patients who make co-payments, and our efforts to obtain payment for individual claims can take a substantial amount of time, there is typically a significant lag between the time the test is reported and the time we actually recognize the revenue from such test. As a result, if we were to include tests for which we have not recognized revenue in our average revenue per test calculation for a particular period, it would imply that we will not receive any revenue for such tests. Despite our lack of coverage decisions, we have been reasonably successful in securing reimbursement from many commercial third-party payors for tests reported in prior periods. With respect to tests reported for patients covered by Medicare, we commenced the process of submitting claims to Medicare for these tests in November 2013 and have not yet been reimbursed based on properly processed submissions for these claims. We also expect to record revenue from patients who make co-payments, pay deductibles, or pay other amounts that we have been unable to collect from third-party payors. While receipt of payment from third-party payors and patients in respect of these claims is not currently fixed or determinable and collectability is not reasonably assured, we do expect to record revenue in the future for some of the tests reported in this period. However, it is difficult to predict future revenue from the previously reported FoundationOne and FoundationOne Heme tests because we are in an early stage of commercialization and do not yet have broad coverage decisions. As a result, we cannot be certain that the revenue per test we recognize in the future will remain consistent with the average revenue per test reported above.
The cumulative amount of FoundationOne and FoundationOne Heme tests that have been billed to commercial third-party payors and reported for patients covered by Medicare but for which we have not recognized revenue was 10,471 and 8,844, respectively, as of December 31, 2014. If commercial third-party payors or government payors agree to pay us for these tests in the future, we will recognize revenue for such tests in the period in which our revenue recognition criteria are met. Any revenue that we receive in respect of these previously reported tests will favorably impact our liquidity and results of operations in future periods.
For our biopharmaceutical customer revenue that was based on a negotiated price per test, the average revenue per test was approximately $3,300 and $3,700 for the years ended December 31, 2014 and 2013, respectively. This decrease in average revenue per test was due in part to several large projects that included certain volume-based discounts. We expect our average revenue per test for biopharmaceutical customers to remain fairly consistent going forward. Approximately $11.6 million and $8.9 million of our biopharmaceutical revenue for the years ended December 31, 2014 and 2013, respectively, represented payments under contracts with multiple-element arrangements that were not negotiated on a price per test basis.
Cost of Revenue
Cost of revenue increased to $27.4 million for the year ended December 31, 2014 from $11.7 million for the year ended December 31, 2013. This increase was driven by increasing test volumes from our ordering physicians and biopharmaceutical customers. The average cost per test does not differ materially by customer. Additional volume led to higher reagent and consumable costs, additional laboratory personnel-related costs, and higher depreciation expense related to new equipment purchases. During the years ended December 31, 2014 and 2013, our cost of revenue represented approximately 44.9% and 40.2%, respectively, of our total revenue. We expect to make additional investments in personnel, infrastructure, and systems to scale our laboratory operations to meet future anticipated demand.
Selling and Marketing Expenses
Selling and marketing expenses increased to $29.0 million for the year ended December 31, 2014 from $12.3 million for the year ended December 31, 2013. The increase was primarily due to an increase of $13.5 million in personnel expenses related to an increase in headcount in our sales, marketing, client service, and reimbursement departments, a $2.2 million increase in marketing expenditures on market development and product marketing activities to support our growing commercial and clinical business, a $0.7 million increase in consulting, and a $0.3 million increase in various other expenses.
101
Table of Contents
General and Administrative Expenses
General and administrative expenses increased to $27.3 million for the year ended December 31, 2014 from $21.9 million for the year ended December 31, 2013. The increase was primarily due to a $3.7 million increase in legal, consulting, audit, and billing fees, a $3.6 million increase in personnel and related costs to support and expand our legal, finance, and human resources infrastructure, and a $1.8 million increase in rent and other facilities costs, partially offset by a $3.7 million decrease in stock-based compensation primarily as a result of restricted stock that fully vested during 2013.
Research and Development Expenses
Research and development expenses increased to $30.6 million for the year ended December 31, 2014 from $24.9 million for the year ended December 31, 2013. The increase was primarily due to a $4.5 million increase in employee and contractor-related expenses, a $1.4 million increase in technology-related support and maintenance, and a $0.2 million increase in facilities, partially offset by a $0.4 million decrease in clinical trials expense and research-related lab supplies and materials.
Interest Income (Expense), Net
Interest expense, net was $42,000 and $235,000 for the years ended December 31, 2014 and 2013, respectively. The decrease was due to the reduction in our outstanding loan balance, as the amount was paid in full in December 2014.
Other Income (Expense), Net
Other income (expense), net was $1.1 million for the year ended December 31, 2014 resulting from a tax incentive award granted by the Massachusetts Life Sciences Center. Other income (expense), net was $(0.9) million for the year ended December 31, 2013 resulting from $1.4 million of other expense associated with the change in the fair value of our warrant liability immediately before it converted into a warrant to purchase common stock and was reclassified into additional paid-in capital, partially offset by $0.4 million of other income resulting from a tax incentive award granted by the Massachusetts Life Sciences Center.
Liquidity and Capital Resources
We have incurred losses and negative cash flows from operations since our inception in November 2009, and as of December 31, 2015, we had an accumulated deficit of $231.6 million.
We have funded our operations principally from the sale of common stock, preferred stock and revenue from clinical testing and our biopharmaceutical partners. Since we have received only a few coverage decisions for our existing tests from commercial third-party payors and have a limited history of collecting claims, we currently recognize revenue on a cash basis from most commercial third-party payors. We will continue to make requests for payment and/or appeal payment decisions made by commercial third-party payors. In addition, none of our existing tests are currently covered by Medicare, and we have not received correctly processed payments on the claims we have submitted to Medicare. If commercial third-party payors or government payors agree to pay us for any of these tests in the future, we would recognize revenue for any such tests in the period in which our revenue recognition criteria are met.
On September 30, 2013, we completed our IPO, which resulted in the sale of 6,772,221 shares of our common stock at a public offering price of $18.00 per share, before underwriting discounts, including 883,333 shares of common stock issued upon the exercise in full by the underwriters of their option to purchase additional shares at the public offering price to cover over-allotments. We received net proceeds from the IPO of approximately $110.4 million after deducting underwriting discounts, commissions, and expenses payable by us. As of December 31, 2015, we had used all of the net proceeds from our IPO.
102
Table of Contents
On April 7, 2015, we closed our strategic collaboration with Roche. The transaction included a primary investment by Roche of $250.0 million in cash to purchase 5 million newly issued shares of our common stock at a purchase price of $50.00 per share.
As of December 31, 2015, we had cash, cash equivalents, and marketable securities of approximately $232.3 million. Cash in excess of immediate requirements is invested in accordance with our investment policy, primarily with a view to liquidity and capital preservation. These excess funds are held in U.S. government agency securities, U.S. treasuries, and money market mutual funds consisting of U.S. government-backed securities and treasuries.
We have occasionally received letters from third parties inviting us to take licenses under, or alleging that we infringe, their patents. While any potential infringement claims could pose an uncertainty for our business, no notice of alleged infringement that we have received to date has led to a lawsuit or a license, and, as a result, no such claim has had an impact on our results of operations.
Cash Flows
The following table sets forth the primary sources and uses of cash for each of the periods set forth below:
| Year Ended December 31, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| (in thousands) | ||||||||||||
| Net cash (used in) provided by: |
||||||||||||
| Operating activities |
$ | (63,726 | ) | $ | (42,736 | ) | $ | (30,766 | ) | |||
| Investing activities |
(140,717 | ) | (8,827 | ) | (8,494 | ) | ||||||
| Financing activities |
250,126 | (650 | ) | 108,715 | ||||||||
|
|
|
|
|
|
|
|||||||
| Net increase (decrease) in cash and cash equivalents |
$ | 45,683 | $ | (52,213 | ) | $ | 69,455 | |||||
|
|
|
|
|
|
|
|||||||
Operating Activities
Net cash used in operating activities in all periods resulted primarily from our net losses adjusted for non-cash charges and changes in components of working capital. The net cash used in operating activities was $63.7 million for the year ended December 31, 2015 compared to $42.7 million for the year ended December 31, 2014. The increase in cash used in operating activities was driven primarily by an increase in net loss of $37.4 million, which included $14.4 million in advisor fees related to the Roche transaction, partially offset by an $8.5 million increase in cash provided by changes in working capital driven by Roche-related activity, an increase in stock-based compensation expense of $5.2 million, and an increase in depreciation and amortization expense of $2.4 million.
The net cash used in operating activities was $42.7 million for the year ended December 31, 2014 compared to $30.8 million for the year ended December 31, 2013. The increase in cash used in operating activities was driven primarily by an increase in net loss of $9.3 million and a $2.7 million increase in cash used to support working capital requirements during the year ended December 31, 2014 as compared to the year ended December 31, 2013.
Investing Activities
Net cash used in investing activities for the year ended December 31, 2015 was $140.7 million and consisted of purchases of marketable securities of $114.8 million, purchases of property and equipment of $25.4 million, and an increase in restricted cash of $0.5 million. Net cash used in investing activities for the year ended December 31, 2014 was $8.8 million and consisted of purchases of property and equipment of $9.7 million,
103
Table of Contents
partially offset by a decrease in restricted cash of $0.9 million due to the release of a portion of the restricted cash balance related to our laboratory and office facilities. Net cash used in investing activities for the year ended December 31, 2013 was $8.5 million and consisted of an increase in restricted cash of $1.6 million related to our laboratory and office facilities, and purchases of property and equipment of $6.9 million.
Financing Activities
Net cash provided by financing activities for the year ended December 31, 2015 was $250.1 million and consisted of $245.4 million of proceeds received from the sale and issuance of common stock to Roche, net of issuance costs, as well as $4.7 million from the exercise of stock options during the period. Net cash used in financing activities for the year ended December 31, 2014 was $0.7 million and consisted primarily of $1.3 million of loan principal payments, partially offset by $0.6 million of proceeds from the exercise of stock options. Net cash provided by financing activities for the year ended December 31, 2013 was $108.7 million and reflects the net proceeds from our IPO of $110.4 million, offset in part by $1.7 million in loan principal repayments.
Operating Capital Requirements
We expect to incur additional operating losses in the near future and our operating expenses will increase as we continue to expand our sales force, increase our marketing efforts to drive market adoption of FoundationOne, FoundationOne Heme, and FoundationACT, invest in clinical trials, innovate our molecular information platform, and develop new product offerings. Our liquidity requirements have and will continue to consist of selling and marketing expenses, research and development expenses, capital expenditures, expenses incurred fulfilling our obligations under the Ex-U.S. Commercialization Agreement with Roche, working capital, and general corporate expenses. If demand for our products continues to increase, we anticipate that our capital expenditure requirements will also increase in order to build additional capacity. We expect that our planned expenditures will be funded from our ongoing operations and from our existing cash, cash equivalents, and marketable securities.
In April 2015, the Roche transaction was consummated and we received $250.0 million in gross proceeds from the sale of 5,000,000 shares of our common stock to Roche at a price of $50.00 per share. Based on our current business plan, we believe our cash, cash equivalents, and marketable securities as of December 31, 2015 and anticipated cash flows from operations will be sufficient to meet our anticipated cash requirements over the next 12 months. We may consider raising additional capital to pursue strategic investments or for other reasons, subject to certain consent rights of Roche contained in the Investor Rights Agreement. In the future, we expect our operating and capital expenditures to increase as we increase our headcount, expand our selling and marketing activities and continue to invest in new product offerings.
If our available cash, cash equivalent, and marketable securities balances and anticipated cash flow from operations are insufficient to satisfy our liquidity requirements, including because of lower demand for our products, lower than currently expected rates of reimbursement from commercial third-party payors and government payors, increased competition from other providers of molecular diagnostic tests, or other risks described in this Annual Report on Form 10-K, we may seek to sell common or preferred equity or convertible debt securities, enter into a credit facility or another form of third-party funding, or seek other debt financing. The sale of equity and convertible debt securities may result in dilution to our stockholders and those securities may have rights senior to those of our common stock. If we raise additional funds through the issuance of equity, convertible debt securities or other debt financing, these securities or other debt could contain covenants that would restrict our operations, and certain of these transactions will be subject to the prior consent of Roche as set forth in the Investor Rights Agreement. Any other third-party funding arrangement could require us to relinquish valuable rights. We may require additional capital beyond our currently anticipated amounts. Additional capital may not be available on reasonable terms, or at all.
104
Table of Contents
These estimates are forward-looking statements and involve risks and uncertainties and actual results could vary materially and negatively as a result of a number of factors, including the factors discussed in Part I, Item 1A. Risk Factors of this Annual Report on Form 10-K. We have based our estimates on assumptions that may prove to be wrong and we could utilize our available capital resources sooner than we currently expect. If we cannot expand our operations or otherwise capitalize on our business opportunities because we lack sufficient capital, our business, financial condition, and results of operations could be materially adversely affected.
Contractual Obligations and Commitments
The following table summarizes our principal contractual obligations at December 31, 2015.
| Total | 2016 | 2017-2018 | 2019-2020 | Thereafter | ||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Operating lease obligations(1)(2)(3)(4)(5) |
$ | 32,894 | $ | 6,362 | $ | 13,081 | $ | 12,707 | $ | 744 | ||||||||||
| Purchase obligations(6) |
6,197 | 4,697 | 1,500 | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total |
$ | 39,091 | $ | 11,059 | $ | 14,581 | $ | 12,707 | $ | 744 | ||||||||||
| (1) | In 2013, we leased 61,591 square feet for office and laboratory space in Cambridge, Massachusetts under an operating lease that expires in February 2021. On June 30, 2014 we signed an amendment to the lease adding 8,164 square feet of additional office and laboratory space under an operating lease which also expires in February 2021. |
| (2) | In April 2014, we leased 1,975 square feet for office space in Palo Alto, California under an operating lease that expires in May 2016. |
| (3) | In September 2014, we extended the lease of a corporate apartment in Cambridge, Massachusetts under an operating lease that expired in September 2015. |
| (4) | In September 2015, we executed a new lease for a corporate apartment in Cambridge, Massachusetts under an operating lease that expires in September 2016. |
| (5) | In March 2015, we leased 38,411 square feet for office space in Cambridge, Massachusetts under an operating lease that expires in August 2020. |
| (6) | In July 2013, we entered into a five-year supply, service and support agreement, or the supply agreement, with Illumina for Illumina to provide products and services that support and can be used for the gene sequencing component of our molecular testing activities. During the remaining term of this supply agreement, the actual amount of the purchase obligation shall be determined during each period set forth in the table but will not be lower than the amounts set forth in the table. The actual amount of the purchase obligation shall be based upon a percentage of the forecasts we submit for Illumina product and services. |
Net Operating Loss Carryforwards
We had deferred tax assets of approximately $99.0 million and deferred tax liabilities of $6.9 million as of December 31, 2015. The deferred tax assets have been fully offset by a valuation allowance due to uncertainties surrounding our ability to realize these tax benefits. The deferred tax assets are primarily composed of federal and state tax net operating loss, or NOL, carryforwards and research and development tax credit carryforwards. As of December 31, 2015, we had federal NOL carryforwards of approximately $175.3 million and state NOL carryforwards of $159.1 million available to reduce future taxable income, if any. These federal NOL carryforwards expire at various times through 2035 and the state NOL carryforwards begin to expire in 2024. In general, if we experience a greater than 50 percent aggregate change in ownership of certain significant stockholders over a three-year period, or a Section 382 ownership change, utilization of our pre-change NOL carryforwards are subject to an annual limitation under Section 382 of the Internal Revenue Code of 1986, as amended, and similar state laws. Such limitations may result in expiration of a portion of the NOL carryforwards before utilization and may be substantial. Following the Roche transaction, as discussed in
105
Table of Contents
detail throughout this Annual Report on Form 10-K, we conducted a Section 382 study covering the period from corporate inception through April 7, 2015, which was the closing date of the Roche transaction. This study concluded that limitations on our NOL carryforwards are not restrictive, with the exception of approximately $1.5 million of pre-March 31, 2010 NOLs. The restrictions on the NOLs are expected to free up for use by December 31, 2016.
Off-Balance Sheet Arrangements
We did not have during the periods presented, and we do not currently have, any off-balance sheet arrangements, as defined in the rules and regulations of the SEC.
Application of Critical Accounting Policies
We have prepared our consolidated financial statements in accordance with accounting principles generally accepted in the United States. The preparation of these consolidated financial statements requires us to make estimates, assumptions, and judgments that affect the reported amounts of assets, liabilities, expenses, and related disclosures at the date of the consolidated financial statements, as well as revenue and expenses recorded during the reporting periods. We evaluate our estimates and judgments on an ongoing basis. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results could therefore differ materially from these estimates under different assumptions or conditions.
While our significant accounting policies are described in more detail in Note 2 to our consolidated financial statements included later in this Annual Report on Form 10-K, we believe the following accounting policies to be critical to the judgments and estimates used in the preparation of our consolidated financial statements.
Revenue Recognition
We currently derive revenue from selling products that are enabled by our molecular information platform. We recognize revenue in accordance with ASC 605. Accordingly, revenue is recognized when all of the following criteria are met: persuasive evidence of an arrangement exists; delivery has occurred; the fee is fixed or determinable; and collectability is reasonably assured. We receive payments from: commercial third-party payors; certain hospitals and cancer centers with which we have direct bill relationships; individual patients; and our biopharmaceutical customers.
We currently recognize revenue on a cash basis for sales of our products for which we receive payments from commercial third-party payors and patients who make co-payments, pay deductibles or other amounts that we have been unable to collect from their third-party payors since the fee is not fixed or determinable and collectability is not reasonably assured. Our products are delivered electronically and, as such, there are no shipping and handling fees incurred by us or billed to our customers. We believe our products are exempt from state sales taxation due to the nature of our products. As a result, we do not charge our customers state sales tax. Because we have limited payment experience with third-party payors and patients, we have not concluded that the fee is fixed or determinable or that collectability is reasonably assured, and therefore, we recognize revenue on a cash basis. We expect to use judgment in our assessment of whether the fee is fixed or determinable and whether collectability is reasonably assured in determining when to recognize revenue in the future as we continue to gain payment experience with third-party payors and patients.
We recognize revenue from the sale of our products to certain hospitals, cancer centers, other institutions, and patients at the time results of our tests are reported to physicians, assuming all revenue recognition criteria have been met.
106
Table of Contents
Our arrangements with biopharmaceutical customers are based on a negotiated price per test or on the basis of agreements to provide certain testing volumes or other deliverables over defined periods. We recognize revenue upon delivery of the test results, or over the period that testing volume or other deliverables are provided, as appropriate, assuming all other revenue recognition criteria have been met. Amounts received prior to satisfying the revenue recognition criteria are recorded as deferred revenue in our consolidated balance sheets. Amounts expected to be recognized as revenue within the 12 months following the balance sheet date are classified as deferred revenue, current portion. Amounts not expected to be recognized as revenue within the 12 months following the balance sheet date are classified as deferred revenue, net of current portion.
Certain of our arrangements include multiple deliverables. We analyze multiple-element arrangements based on the guidance in ASC 605-25. Pursuant to the guidance in ASC 605-25, we evaluate multiple-element arrangements to determine (1) the deliverables included in the arrangement and (2) whether the individual deliverables represent separate units of accounting or whether they must be accounted for as a combined unit of accounting. This evaluation involves subjective determinations and requires us to make judgments about the individual deliverables and whether such deliverables are separable from the other aspects of the contractual relationship. Deliverables are considered separate units of accounting provided that: (i) the delivered items have value to the customer on a standalone basis and (ii) if the arrangement includes a general right of return relative to the delivered items and delivery or performance of the undelivered items is considered probable and substantially in our control. In assessing whether an item has standalone value, we consider factors such as the research, development and commercialization capabilities of a third party and the availability of the associated expertise in the general marketplace. In addition, we consider whether the other party in the arrangement can use the other deliverables for their intended purpose without the receipt of the remaining elements, whether the value of the deliverable is dependent on the undelivered items and whether there are other vendors that can provide the undelivered elements.
Arrangement consideration that is fixed or determinable is allocated among the separate units of accounting using the relative selling price method. Then, the applicable revenue recognition criteria in ASC 605-25 is applied to each of the separate units of accounting in determining the appropriate period and pattern of recognition. We determine the selling price of a unit of accounting following the hierarchy of evidence prescribed by ASC 605-25. Accordingly, we determine the estimated selling price for units of accounting within each arrangement using VSOE of selling price, if available, TPE of selling price if VSOE is not available, or BESP, if neither VSOE nor TPE is available. We typically use BESP to estimate the selling price, since we generally do not have VSOE or TPE of selling price for our units of accounting under multiple-element arrangements. Determining the BESP for a unit of accounting requires significant judgment. In developing the BESP for a unit of accounting, we consider applicable market conditions and estimated costs. We validate the BESP for units of accounting by evaluating whether changes in the key assumptions used to determine the BESP will have a significant effect on the allocation of arrangement consideration between multiple units of accounting. We recognize arrangement consideration allocated to each unit of accounting when all of the revenue recognition criteria in ASC 605-25 are satisfied for that particular unit of accounting.
At the inception of an arrangement that includes milestone payments, we evaluate whether each milestone is substantive and at risk to both parties on the basis of the contingent nature of the milestone. This evaluation includes an assessment of whether: (i) the consideration is commensurate with either our performance to achieve the milestone or the enhancement of the value of the delivered items as a result of a specific outcome resulting from our performance to achieve the milestone, (ii) the consideration relates solely to past performance and (iii) the consideration is reasonable relative to all of the deliverables and payment terms within the arrangement. We evaluate factors such as the scientific, clinical, regulatory, commercial and other risks that must be overcome to achieve the respective milestone and the level of effort and investment required to achieve the respective milestone in making this assessment. There is considerable judgment involved in determining whether a milestone satisfies all of the criteria required to conclude that a milestone is substantive. Generally, once a substantive milestone has been achieved, we will recognize revenue related to that milestone using a proportional performance model over the period which the unit of accounting is delivered or based on the level of effort
107
Table of Contents
expended to date over the total expected effort, whichever is considered the most appropriate measure of performance. Revenue from commercial milestone payments are accounted for as royalties and recorded as revenue upon achievement of the milestone, assuming all other revenue recognition criteria are met.
We also recognize royalty revenue in the period of sale of the related product(s), based on the underlying contract terms, provided that the reported sales are reliably measurable and we have no remaining performance obligations, assuming all other revenue recognition criteria are met.
For some multiple-element arrangements, we are reimbursed for either all or a portion of the research and development costs incurred. We perform research and development services as part of our revenue activities and, therefore, believe such activities are a part of our primary business. Therefore, we record these reimbursements as revenue in the statement of operations using a proportional performance model over the period which the unit of accounting is delivered or based on the level of effort expended to date over the total expected effort, whichever is considered the most appropriate measure of performance.
Stock-based Compensation
Prior to becoming a public company, we utilized significant estimates and assumptions in determining the fair value of our common stock. We granted stock options at exercise prices not less than the fair value of our common stock as determined by our Board, with input from management. The Board determined the estimated fair value of our common stock based on a number of objective and subjective factors, including external market conditions affecting the biotechnology industry sector and the prices at which we sold shares of redeemable convertible preferred stock, the superior rights and preferences of securities senior to our common stock at the time, our stage of commercialization, our historical operating results, and the likelihood of achieving a liquidity event, such as an initial public offering or sale.
We utilized various valuation methodologies in accordance with the framework of the 2004 American Institute of Certified Public Accountants Technical Practice Aid, or Practice Aid, Valuation of Privately-Held Company Equity Securities Issued as Compensation, to estimate the fair value of our common stock. Our Board estimated our enterprise value as of the various valuation dates using a market approach and an income approach, which are acceptable valuation methods in accordance with the Practice Aid. Under the market approach, enterprise value can be estimated by evaluating recent arm’s length transactions involving the sale of our preferred stock to investors and by comparisons to similar publicly traded companies. Under the income approach, enterprise value can be estimated using the discounted cash flow method. Additionally, each valuation reflects a marketability discount, resulting from the illiquidity of our common stock. We performed these contemporaneous valuations as of August 31, 2011, November 5, 2012, February 15, 2013, and May 10, 2013.
Since becoming a public company, we have accounted for stock-based compensation arrangements with our employees, consultants, and non-employee directors using a fair value method in accordance with Accounting Standards Codification Topic 718, Compensation—Stock Compensation, or ASC 718, which requires us to recognize compensation expense for costs related to all share-based payments. To date, our stock-based awards have included grants of stock options, restricted stock units, or RSUs, and restricted stock.
The fair value method for stock option awards requires us to estimate the fair value of stock-based awards to employees and non-employees on the date of grant using the Black-Scholes option-pricing model. The fair value is then recognized, net of estimated forfeitures, as stock-based compensation expense over the requisite service period, which is typically the vesting period, of the award. Stock-based awards granted to non-employees are subject to periodic revaluation over their vesting term.
The Black-Scholes option-pricing model requires the input of subjective assumptions, including expected stock price volatility and the expected life of stock options. We do not have sufficient history to estimate the volatility of our common stock price or the expected term of our stock options. We calculate expected volatility based on historical volatility data of a representative group of companies that are publicly traded. We selected
108
Table of Contents
representative companies with comparable characteristics to us, including risk profiles, position within the industry, and with historical stock price information sufficient to meet the expected term of the stock-based awards. We compute the historical volatility of this selected group using the daily closing prices for the selected companies’ shares during the equivalent period of the calculated expected term of our stock-based awards. We will continue to use the representative group volatility information until the historical volatility of our common stock is relevant to measure expected volatility for future stock option grants.
We determine the expected term of stock options according to the “simplified” method. Under this method, the expected term is calculated as the average of the time-to-vesting and the contractual life of the stock option. The assumed dividend yield is based on our expectation that we will not pay dividends in the foreseeable future, which is consistent with our history of not paying dividends. We determine the risk-free interest rate by using the equivalent to the expected term based on the U.S. Treasury yield curve in effect as of the date of grant. We estimate forfeitures at the time of grant and revise our estimates, as appropriate, but actual future forfeiture rates may differ. If actual results differ significantly from these estimates, stock-based compensation expense and our statements of operations and comprehensive loss could be materially impacted.
We have also granted performance-based stock options with terms that allow the recipients to vest in a specific number of shares based upon the achievement of performance-based milestones as specified in the grants. Stock-based compensation expense associated with these performance-based stock options is recognized if the performance condition is considered probable of achievement using management’s best estimates of the time to vesting for the achievement of the performance-based milestones. If the actual achievement of the performance-based milestones varies from our estimates, stock-based compensation expense could be materially different than what is recorded in the period. The cumulative effect on current and prior periods of a change in the estimated time to vesting for performance-based stock options will be recognized as compensation cost in the period of the revision, and recorded as a change in estimate.
The fair values of our restricted stock and RSUs are based on the market value of our stock on the date of grant. Compensation expense for restricted stock and RSU’s is recognized on a straight-line basis over the applicable service period.
While the assumptions used to calculate and account for share-based compensation awards represents management’s best estimates, these estimates involve inherent uncertainties and the application of management’s judgment. As a result, if revisions are made to our underlying assumptions and estimates, our share-based compensation expense could vary significantly from period to period.
We have included stock-based compensation as part of our cost of revenue and our operating expenses in our statements of operations and comprehensive loss as follows:
| Year Ended December 31, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Cost of revenue |
$ | 980 | $ | 466 | $ | 67 | ||||||
| Selling and marketing |
3,474 | 1,130 | 142 | |||||||||
| General and administrative |
4,272 | 2,635 | 6,346 | |||||||||
| Research and development |
2,450 | 1,758 | 761 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
$ | 11,176 | $ | 5,989 | $ | 7,316 | ||||||
|
|
|
|
|
|
|
|||||||
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We are exposed to market risk related to changes in interest rates. As of December 31, 2015, we had cash, cash equivalents, and marketable securities of $232.3 million primarily invested in U.S. government agency securities, U.S. treasuries, and money market mutual funds invested in U.S. government-backed securities and treasuries. Our primary exposure to market risk is interest rate sensitivity, which is affected by changes in the
109
Table of Contents
general level of U.S. interest rates, particularly because our investments are primarily in short-term securities. Our marketable securities are subject to interest rate risk and will fall in value if market interest rates increase. If market interest rates were to increase immediately and uniformly by 100 basis points, or one percentage point, from levels at December 31, 2015, the net fair value of our interest-sensitive marketable securities would have resulted in a hypothetical decline of $0.7 million.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
Our consolidated financial statements, together with the reports of our independent registered public accounting firms, appear at pages F-1 through F-33 of this Annual Report on Form 10-K for the year ended December 31, 2015.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
On April 6, 2015, Ernst & Young LLP, or Ernst & Young, resigned as our independent registered public accounting firm, and Ernst & Young’s resignation was accepted and approved by the audit committee of the Board. Ernst & Young performs certain non-audit services for Roche and/or its affiliates, and, as such, determined that it may have ceased to qualify as being “independent” from us within the meaning of the federal securities laws and the rules and regulations thereunder following April 7, 2015, when we closed our transaction with Roche. Ernst & Young’s reports on our financial statements for the years ended December 31, 2014 and December 31, 2013 did not contain an adverse opinion or disclaimer of opinion, nor were they qualified or modified as to uncertainty, audit scope, or accounting principles. In addition, there were no disagreements between us and Ernst & Young on accounting principles, financial statements disclosures or audit scope, which, if not resolved to the satisfaction of Ernst & Young, would have caused them to make reference to the disagreement in their reports.
On April 6, 2015, the audit committee of the Board approved KPMG LLP, or KPMG, as our new independent registered public accounting firm to audit our consolidated financial statements for the fiscal year ended December 31, 2015. During the fiscal years ended December 31, 2013 and December 31, 2014, and through the date of KPMG’s appointment, we did not consult with KPMG with respect to the application of accounting principles to a specified transaction, either completed or proposed, or the type of audit opinion that might be rendered on our consolidated financial statements, or any other matters or reportable events as set forth in Items 304(a)(2)(i) and (ii) of Regulation S-K.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
Disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) are designed only to provide reasonable assurance that they will meet their objectives. Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of the effectiveness, as of December 31, 2015, of the design and operation of our disclosure controls and procedures, as such term is defined in Exchange Act Rules 13a-15(e) and 15d-15(e). Based on this evaluation, our principal executive officer and principal financial officer have concluded that, as of such date, our disclosure controls and procedures are effective to ensure that information required to be disclosed by us in our Exchange Act reports is recorded, processed, summarized, and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate to allow timely decisions regarding required disclosure.
110
Table of Contents
Management’s Annual Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as such term is defined in Exchange Act Rule 13a–15(f) or 15d-15(d). Our internal control system was designed to provide reasonable assurance to our management and our Board regarding the preparation and fair presentation of published financial statements. All internal control systems, no matter how well designed have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation. Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2015. In making this assessment, our management used the criteria set forth in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission in 2013 (COSO criteria). Based on this assessment, management concluded that our internal control over financial reporting was effective as of December 31, 2015. This Annual Report on Form 10-K does not include an attestation report of the Company’s independent registered public accounting firm due to a transition period established by rules of the Securities and Exchange Commission for newly public companies.
Changes in Internal Control Over Financial Reporting
There were no changes in our internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) or 15d-15(d)) during the quarter ended December 31, 2015 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
None.
111
Table of Contents
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The information required under this item is incorporated herein by reference to the Company’s definitive proxy statement to be filed in connection with its annual stockholder meeting pursuant to Regulation 14A, which proxy statement will be filed with the SEC not later than 120 days after the close of the Company’s fiscal year ended December 31, 2015.
ITEM 11. EXECUTIVE COMPENSATION
The information required under this item is incorporated herein by reference to the Company’s definitive proxy statement to be filed in connection with its annual stockholder meeting pursuant to Regulation 14A, which proxy statement will be filed with the SEC not later than 120 days after the close of the Company’s fiscal year ended December 31, 2015.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information required under this item is incorporated herein by reference to the Company’s definitive proxy statement to be filed in connection with its annual stockholder meeting pursuant to Regulation 14A, which proxy statement will be filed with the SEC not later than 120 days after the close of the Company’s fiscal year ended December 31, 2015.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information required under this item is incorporated herein by reference to the Company’s definitive proxy statement to be filed in connection with its annual stockholder meeting pursuant to Regulation 14A, which proxy statement will be filed with the SEC not later than 120 days after the close of the Company’s fiscal year ended December 31, 2015.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
The information required under this item is incorporated herein by reference to the Company’s definitive proxy statement to be filed in connection with its annual stockholder meeting pursuant to Regulation 14A, which proxy statement will be filed with the SEC not later than 120 days after the close of the Company’s fiscal year ended December 31, 2015.
112
Table of Contents
| ITEM 15. | EXHIBITS, FINANCIAL STATEMENT SCHEDULES |
| (a) | 1. Consolidated Financial Statements. |
For a list of the consolidated financial statements included herein, see Index on page F-1 of this report.
2. Financial Statement Schedules.
All required information is included in the financial statements or notes thereto.
3. List of Exhibits.
See the Exhibit Index in Item 15(b) below.
| (b) | Exhibit Index. |
| Exhibit |
Exhibit Index | |
| 2.1 | Transaction Agreement, by and between the Company and Roche Holdings, Inc., dated January 11, 2015 (incorporated by reference to Exhibit 2.1 of the Company’s Form 8-K filed on January 12, 2015) | |
| 3.1 | Seventh Amended and Restated Certificate of Incorporation of the Company (incorporated by reference to Exhibit 3.1 of the Company’s Form 8-K filed on April 7, 2015) | |
| 3.2 | Amended and Restated Bylaws of the Company (incorporated by reference to Exhibit 3.2 of the Company’s Form 8-K filed on October 2, 2013) | |
| 4.1 | Form of Common Stock certificate of the Company (incorporated by reference to Exhibit 4.1 to the Company’s Registration Statement on Form S-1/A (File No. 333-190226) filed on September 12, 2013) | |
| 4.2 | Second Amended and Restated Investors’ Rights Agreement, by and between the Company and the Investors named therein, dated as of June 20, 2013 (incorporated by reference to Exhibit 4.3 to the Company’s Registration Statement on Form S-1 (File No. 333-190226) filed on July 29, 2013) | |
| 4.3 | Investor Rights Agreement, by and between the Company and Roche Holdings, Inc., dated January 11, 2015 (incorporated by reference to Exhibit 4.1 of the Company’s Form 8-K filed on January 12, 2015) | |
| 4.4 | Amendment to Second Amended and Restated Investors’ Rights Agreement, by and between the Company and the Investors named therein, dated January 11, 2015 (incorporated by reference to Exhibit 4.2 of the Company’s Form 8-K filed on January 12, 2015) | |
| 10.1† | Amended and Restated 2010 Stock Incentive Plan and forms of agreements thereunder (incorporated by reference to Exhibit 10.1 to the Company’s Registration Statement on Form S-1 (File No. 333-190226) filed on July 29, 2013) | |
| 10.2† | 2013 Stock Option and Incentive Plan and forms of agreements thereunder (incorporated by reference to Exhibit 10.2 to the Company’s Registration Statement on Form S-1/A (File No. 333-190226) filed on September 12, 2013) | |
| 10.3† | Executive Employee Offer Letter issued by the Company to Michael J. Pellini, dated as of September 9, 2013 (incorporated by reference to Exhibit 10.3 to the Company’s Registration Statement on Form S-1/A (File No. 333-190226) filed on September 12, 2013) | |
| 10.4† | Executive Employee Offer Letter issued by the Company to Robert W. Hesslein, dated as of March 7, 2013 (incorporated by reference to Exhibit 10.5 to the Company’s Registration Statement on Form S-1 (File No. 333-190226) filed on July 29, 2013) | |
113
Table of Contents
| Exhibit |
Exhibit Index | |
| 10.5† | Executive Employee Offer Letter issued by the Company to Jason Ryan, dated as of March 7, 2013 (incorporated by reference to Exhibit 10.6 to the Company’s Registration Statement on Form S-1 (File No. 333-190226) filed on July 29, 2013) | |
| 10.6† | Executive Employee Offer Letter issued by the Company to Steven J. Kafka, dated as of May 21, 2013, as amended (incorporated by reference to Exhibit 10.7 to the Company’s Registration Statement on Form S-1/A (File No. 333-190226) filed on September 12, 2013) | |
| 10.7 | Form of Indemnification Agreement, to be entered into between the Company and its directors and officers (incorporated by reference to Exhibit 10.8 to the Company’s Registration Statement on Form S-1/A (File No. 333-190226) filed on August 16, 2013) | |
| 10.8 | Lease, by and between the Company and 150 Second Street, LLC, dated as of February 4, 2013 (incorporated by reference to Exhibit 10.10 to the Company’s Registration Statement on Form S-1 (File No. 333-190226) filed on July 29, 2013) | |
| 10.9 | Lease, by and between the Company and 150 Second Street, LLC, dated as of March 27, 2013 (incorporated by reference to Exhibit 10.11 to the Company’s Registration Statement on Form S-1 (File No. 333-190226) filed on July 29, 2013) | |
| 10.10# | Supply and Support Agreement, by and between the Company and Illumina, Inc., effective as of July 25, 2013 (incorporated by reference to Exhibit 10.13 to the Company’s Registration Statement on Form S-1/A (File No. 333-190226) filed on August 2, 2013) | |
| 10.11† | Executive Employee Offer Letter issued by the Company to Vincent A. Miller, dated as of August 1, 2011, as amended (incorporated by reference to Exhibit 10.15 to the Company’s Registration Statement on Form S-1/A (File No. 333-190226) filed on September 12, 2013) | |
| 10.12† | 2013 Employee Stock Purchase Plan (incorporated by reference to Exhibit 10.16 to the Company’s Registration Statement on Form S-1/A (File No. 333-190226) filed on September 12, 2013) | |
| 10.13 | First Amendment to Lease, by and between the Company and 150 Second Street, LLC, dated November 27, 2013 (incorporated by reference to Exhibit 10.1 of the Company’s Form 8-K filed on December 4, 2013) | |
| 10.14 | Second Amendment to Lease, by and between the Company and ARE-MA REGION NO. 50, LLC, dated June 30, 2014 (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on July 2, 2014). | |
| 10.15 | Tax Sharing Agreement, by and between the Company and Roche Holdings, Inc., dated January 11, 2015 (incorporated by reference to Exhibit 10.1 of the Company’s Form 8-K filed on January 12, 2015) | |
| 10.16# | Collaboration Agreement, by and among the Company, F. Hoffman-La Roche Ltd and Hoffman-La Roche Inc., dated January 11, 2015 (incorporated by reference to Exhibit 10.2 of the Company’s Form 8-K/A filed on August 21, 2015) | |
| 10.17# | Ex-US Commercialization Agreement, by and between the Company and F. Hoffmann-La Roche Ltd, dated January 11, 2015 (incorporated by reference to Exhibit 10.3 of the Company’s Form 8-K/A filed on August 21, 2015) | |
| 10.18# | US Education Collaboration Agreement, by and between the Company and Genentech, Inc., dated January 11, 2015 (incorporated by reference to Exhibit 10.4 of the Company’s Form 8-K/A filed on August 21, 2015) | |
| 10.19# | Binding Term Sheet for an In Vitro Diagnostics Collaboration, by and between the Company and F. Hoffman-La Roche Ltd, dated January 11, 2015 (incorporated by reference to Exhibit 10.5 of the Company’s Form 8-K/A filed on August 21, 2015) | |
114
Table of Contents
| Exhibit |
Exhibit Index | |
| 10.20† | Executive Employee Offer letter issued by the Company to David J. Daly, dated as of November 20, 2014 (incorporated by reference to Exhibit (e)(26) to the Company’s Solicitation/Recommendation Statement on Schedule 14D-9 filed on February 2, 2015) | |
| 10.21 | Deed of Lease, by and between the Company and BCSP Cambridge Ten Property LLC, dated March 11, 2015 (incorporated by reference to Exhibit 10.1 of the Company’s Form 8-K filed on March 12, 2015) | |
| 21.1* | Subsidiaries of the Company | |
| 23.1* | Consent of KPMG LLP | |
| 23.2* | Consent of Ernst & Young LLP | |
| 31.1* | Certification of Principal Executive Officer pursuant to Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | |
| 31.2* | Certification of Principal Financial Officer pursuant to Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | |
| 32.1** | Certification of Principal Executive Officer and Principal Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | |
| 101 | Interactive Data Files regarding (a) our Consolidated Balance Sheets as of December 31, 2015 and 2014, (b) our Consolidated Statements of Operations and Comprehensive Loss for the Years Ended December 31, 2015, 2014 and 2013, (c) our Consolidated Statements of Redeemable Convertible Preferred Stock and Stockholders’ Equity (Deficit) for the Years Ended December 31, 2015, 2014 and 2013, (d) our Consolidated Statements of Cash Flows for the Years Ended December 31, 2015, 2014 and 2013, and (e) the Notes to such Consolidated Financial Statements | |
| * | Filed herewith. |
| ** | Furnished herewith. |
| † | Indicates a management contract or any compensatory plan, contract or arrangement. |
| # | Confidential treatment has been requested or granted for certain information contained in this exhibit. Such information has been omitted and filed separately with the Securities and Exchange Commission. |
115
Table of Contents
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| FOUNDATION MEDICINE, INC. | ||||||
| Date: March 1, 2016 |
By: | /s/ Michael J. Pellini, M.D. | ||||
| Michael J. Pellini, M.D. | ||||||
| Chief Executive Officer (Principal Executive Officer) | ||||||
| Date: March 1, 2016 |
By: | /s/ Jason Ryan | ||||
| Jason Ryan | ||||||
| Chief Financial Officer (Principal Financial and Accounting Officer) | ||||||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Name |
Title |
Date | ||
| /s/ Michael J. Pellini, M.D. Michael J. Pellini, M.D. |
Chief Executive Officer and Director (Principal Executive Officer) |
March 1, 2016 | ||
| /s/ Jason Ryan Jason Ryan |
Chief Financial Officer (Principal Financial and Accounting Officer) |
March 1, 2016 | ||
| /s/ Alexis Borisy Alexis Borisy |
Director |
March 1, 2016 | ||
| /s/ Sandra Horning, M.D. Sandra Horning, M.D. |
Director |
March 1, 2016 | ||
| /s/ Evan Jones Evan Jones |
Director |
March 1, 2016 | ||
| /s/ Daniel O’Day Daniel O’Day |
Director |
March 1, 2016 | ||
| /s/ David Schenkein, M.D. David Schenkein, M.D. |
Director |
March 1, 2016 | ||
| /s/ Michael D. Varney, Ph.D. Michael D. Varney, Ph.D. |
Director |
March 1, 2016 | ||
| /s/ Krishna Yeshwant, M.D. Krishna Yeshwant, M.D. |
Director |
March 1, 2016 | ||
116
Table of Contents
Index to Consolidated Financial Statements
F-1
Table of Contents
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of
Foundation Medicine, Inc.:
We have audited the accompanying consolidated balance sheet of Foundation Medicine, Inc. and subsidiary (the Company) as of December 31, 2015, and the related consolidated statement of operations and comprehensive loss, redeemable convertible preferred stock and stockholders’ equity (deficit), and cash flows for the year then ended. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Foundation Medicine, Inc. and subsidiary as of December 31, 2015, and the results of their operations and their cash flows for the year then ended, in conformity with U.S. generally accepted accounting principles.
/s/ KPMG LLP
Boston, Massachusetts
March 1, 2016
F-2
Table of Contents
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of
Foundation Medicine, Inc.
We have audited the accompanying consolidated balance sheets of Foundation Medicine, Inc. (the Company) as of December 31, 2014, and the related consolidated statements of operations and comprehensive loss, redeemable convertible preferred stock and stockholders’ equity (deficit) and cash flows for each of the two years in the period ended December 31, 2014. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company’s internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Foundation Medicine, Inc. at December 31, 2014, and the consolidated results of its operations and its cash flows for each of the two years in the period ended December 31, 2014, in conformity with U.S. generally accepted accounting principles.
/s/ Ernst & Young LLP
Boston, Massachusetts
March 13, 2015
F-3
Table of Contents
Consolidated Balance Sheets
(In thousands, except share and per share data)
| December 31, | ||||||||
| 2015 | 2014 | |||||||
| Assets |
||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 117,763 | $ | 72,080 | ||||
| Marketable securities |
89,607 | — | ||||||
| Accounts receivable, net of allowance of $171 and $0 at December 31, 2015 and 2014, respectively |
7,362 | 9,894 | ||||||
| Receivable due from Roche |
403 | — | ||||||
| Inventory |
7,992 | 4,809 | ||||||
| Prepaid expenses and other current assets |
6,517 | 2,865 | ||||||
|
|
|
|
|
|||||
| Total current assets |
229,644 | 89,648 | ||||||
| Marketable securities |
24,939 | — | ||||||
| Property and equipment, net |
41,333 | 21,015 | ||||||
| Restricted cash |
1,395 | 864 | ||||||
| Other assets |
678 | 411 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 297,989 | $ | 111,938 | ||||
|
|
|
|
|
|||||
| Liabilities and stockholders’ equity |
||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 10,469 | $ | 7,263 | ||||
| Accrued expenses and other current liabilities |
12,822 | 7,414 | ||||||
| Deferred revenue |
717 | 340 | ||||||
| Roche related-party deferred revenue |
3,742 | — | ||||||
| Current portion of deferred rent |
2,146 | 1,429 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
29,896 | 16,446 | ||||||
| Deferred rent, net of current portion and other non-current liabilities |
10,404 | 9,323 | ||||||
| Commitments and contingencies (Note 12 ) |
||||||||
| Stockholders’ equity: |
||||||||
| Preferred stock, $0.0001 par value, 5,000,000 authorized; no shares issued and outstanding at December 31, 2015 and 2014 |
— | — | ||||||
| Common stock, $0.0001 par value, 150,000,000 shares authorized; 34,513,845 and 28,223,958 shares issued and outstanding at December 31, 2015 and 2014, respectively |
3 | 3 | ||||||
| Additional paid-in capital |
489,480 | 228,151 | ||||||
| Accumulated other comprehensive loss |
(178 | ) | — | |||||
| Accumulated deficit |
(231,616 | ) | (141,985 | ) | ||||
|
|
|
|
|
|||||
| Total stockholders’ equity |
257,689 | 86,169 | ||||||
|
|
|
|
|
|||||
| Total liabilities and stockholders’ equity |
$ | 297,989 | $ | 111,938 | ||||
|
|
|
|
|
|||||
The accompanying notes are an integral part of these consolidated financial statements
F-4
Table of Contents
Consolidated Statements of Operations and Comprehensive Loss
(In thousands, except share and per share data)
| Year Ended December 31, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Revenue |
$ | 79,759 | $ | 60,221 | $ | 28,136 | ||||||
| Related-party revenue from Roche |
13,444 | 858 | 854 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total revenue |
93,203 | 61,079 | 28,990 | |||||||||
| Costs and expenses: |
||||||||||||
| Cost of revenue |
36,695 | 27,434 | 11,659 | |||||||||
| Cost of Roche related-party revenue |
2,736 | — | — | |||||||||
| Selling and marketing |
49,030 | 28,997 | 12,326 | |||||||||
| General and administrative |
50,614 | 27,302 | 21,865 | |||||||||
| Research and development |
43,883 | 30,629 | 24,901 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total costs and expenses |
182,958 | 114,362 | 70,751 | |||||||||
|
|
|
|
|
|
|
|||||||
| Loss from operations |
(89,755 | ) | (53,283 | ) | (41,761 | ) | ||||||
| Other income (expense): |
||||||||||||
| Interest income (expense), net |
124 | (42 | ) | (235 | ) | |||||||
| Other income (expense), net |
— | 1,103 | (948 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Total other income (expense), net |
124 | 1,061 | (1,183 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
(89,631 | ) | (52,222 | ) | (42,944 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Other comprehensive loss: |
||||||||||||
| Unrealized loss on available-for-sale securities |
(178 | ) | — | — | ||||||||
|
|
|
|
|
|
|
|||||||
| Total other comprehensive loss |
(178 | ) | — | — | ||||||||
|
|
|
|
|
|
|
|||||||
| Comprehensive loss |
$ | (89,809 | ) | $ | (52,222 | ) | $ | (42,944 | ) | |||
|
|
|
|
|
|
|
|||||||
| Reconciliation of net loss to net loss applicable to common stockholders: |
||||||||||||
| Net loss |
(89,631 | ) | (52,222 | ) | (42,944 | ) | ||||||
| Accretion of redeemable convertible preferred stock |
— | — | (139 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Net loss applicable to common stockholders |
$ | (89,631 | ) | $ | (52,222 | ) | $ | (43,083 | ) | |||
|
|
|
|
|
|
|
|||||||
| Net loss per common share applicable to common stockholders, basic and diluted |
$ | (2.73 | ) | $ | (1.87 | ) | $ | (4.64 | ) | |||
|
|
|
|
|
|
|
|||||||
| Weighted average common shares outstanding, basic and diluted |
32,836,219 | 27,954,327 | 9,294,730 | |||||||||
|
|
|
|
|
|
|
|||||||
The accompanying notes are an integral part of these consolidated financial statements
F-5
Table of Contents
Consolidated Statements of Redeemable Convertible Preferred Stock and Stockholders’ Equity (Deficit)
(In thousands, except share and per share data)
| Series A Redeemable Convertible Preferred Stock |
Series B Redeemable Convertible Preferred Stock |
Common Stock | Additional Paid-In Capital |
Accumulated Other Comprehensive Loss |
Accumulated Deficit |
Total Stockholders’ (Deficit) Equity |
||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | |||||||||||||||||||||||||||||||||||
| Balance at December 31, 2012 |
43,750,000 | 42,962 | 24,762,134 | 55,696 | 2,727,443 | — | 3,422 | — | (46,819 | ) | (43,397 | ) | ||||||||||||||||||||||||||||
| Issuance costs related to Series B preferred stock offering |
— | — | — | (10 | ) | — | — | — | — | — | — | |||||||||||||||||||||||||||||
| Accretion of redeemable convertible preferred stock to redemption value |
— | 125 | — | 14 | — | — | (139 | ) | — | — | (139 | ) | ||||||||||||||||||||||||||||
| Conversion of redeemable convertible preferred stock into common stock |
(43,750,000 | ) | (43,087 | ) | (24,762,134 | ) | (55,700 | ) | 17,128,024 | 2 | 98,785 | — | — | 98,787 | ||||||||||||||||||||||||||
| Reclassification of warrant to purchase redeemable convertible preferred stock into warrant to purchase common stock |
— | — | — | — | — | — | 1,605 | — | — | 1,605 | ||||||||||||||||||||||||||||||
| Issuance of common stock from initial public offering, net of underwriters’ discounts and issuance costs |
— | — | — | — | 6,772,221 | 1 | 110,380 | — | — | 110,381 | ||||||||||||||||||||||||||||||
| Issuance of common stock in exchange for services |
— | — | — | — | 633 | — | 4 | — | — | 4 | ||||||||||||||||||||||||||||||
| Exercise of warrant for common stock |
— | — | — | — | 44,566 | — | — | — | — | — | ||||||||||||||||||||||||||||||
| Exercise of stock options |
— | — | — | — | 48,863 | — | 49 | — | — | 49 | ||||||||||||||||||||||||||||||
| Vesting of restricted stock |
— | — | — | — | 909,031 | — | 49 | — | — | 49 | ||||||||||||||||||||||||||||||
| Stock-based compensation expense |
— | — | — | — | — | — | 7,316 | — | — | 7,316 | ||||||||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | — | — | (42,944 | ) | (42,944 | ) | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Balance at December 31, 2013 |
— | — | — | — | 27,630,781 | 3 | 221,471 | — | (89,763 | ) | 131,711 | |||||||||||||||||||||||||||||
| Exercise of stock options |
— | — | — | — | 212,822 | — | 637 | — | — | 637 | ||||||||||||||||||||||||||||||
| Vesting of restricted stock |
— | — | — | — | 380,355 | — | 54 | — | — | 54 | ||||||||||||||||||||||||||||||
| Stock-based compensation expense |
— | — | — | — | — | — | 5,989 | — | — | 5,989 | ||||||||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | — | — | (52,222 | ) | (52,222 | ) | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Balance at December 31, 2014 |
— | — | — | — | 28,223,958 | 3 | 228,151 | — | (141,985 | ) | 86,169 | |||||||||||||||||||||||||||||
| Exercise of stock options |
— | — | — | — | 1,070,457 | — | 4,739 | — | — | 4,739 | ||||||||||||||||||||||||||||||
| Vesting of restricted stock |
— | — | — | — | 219,430 | — | 27 | — | — | 27 | ||||||||||||||||||||||||||||||
| Issuance of common stock from Roche transaction, net of issuance costs |
— | — | — | — | 5,000,000 | — | 245,387 | — | — | 245,387 | ||||||||||||||||||||||||||||||
| Stock-based compensation expense |
— | — | — | — | — | — | 11,176 | — | — | 11,176 | ||||||||||||||||||||||||||||||
| Other comprehensive loss |
— | — | — | — | — | — | — | (178 | ) | — | (178 | ) | ||||||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | — | — | (89,631 | ) | (89,631 | ) | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Balance at December 31, 2015 |
— | $ | — | — | $ | — | 34,513,845 | $ | 3 | $ | 489,480 | $ | (178 | ) | $ | (231,616 | ) | $ | 257,689 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements
F-6
Table of Contents
Consolidated Statements of Cash Flows
(In thousands)
| Year Ended December 31, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Operating activities |
||||||||||||
| Net loss |
$ | (89,631 | ) | $ | (52,222 | ) | $ | (42,944 | ) | |||
| Adjustments to reconcile net loss to cash used in operating activities: |
||||||||||||
| Depreciation and amortization expense |
10,600 | 8,195 | 5,006 | |||||||||
| Stock-based compensation |
11,176 | 5,989 | 7,316 | |||||||||
| Provision for doubtful accounts on accounts receivable |
171 | — | — | |||||||||
| Provision for inventory excess and obsolescence |
105 | — | — | |||||||||
| Purchased premiums and interest on marketable securities |
33 | — | — | |||||||||
| Impairment of fixed assets |
— | — | 439 | |||||||||
| Change in fair value of warrant liability |
— | — | 1,380 | |||||||||
| Common stock issued in exchange for professional services |
— | — | 4 | |||||||||
| Non-cash interest expense |
— | 3 | 58 | |||||||||
| Changes in operating assets and liabilities: |
||||||||||||
| Accounts receivable |
2,361 | (3,632 | ) | (4,067 | ) | |||||||
| Receivable from Roche |
(403 | ) | — | — | ||||||||
| Inventory |
(3,288 | ) | (3,046 | ) | (960 | ) | ||||||
| Prepaid expenses and other current assets |
(3,652 | ) | (1,873 | ) | (442 | ) | ||||||
| Other assets |
(267 | ) | (282 | ) | (102 | ) | ||||||
| Accounts payable |
(1,559 | ) | 3,119 | 1,672 | ||||||||
| Accrued expenses and other current liabilities |
4,704 | 1,724 | 1,515 | |||||||||
| Deferred rent and other non-current liabilities |
1,805 | (133 | ) | 1,219 | ||||||||
| Deferred revenue |
377 | (578 | ) | (860 | ) | |||||||
| Roche related-party deferred revenue |
3,742 | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in operating activities |
$ | (63,726 | ) | $ | (42,736 | ) | $ | (30,766 | ) | |||
| Investing activities |
||||||||||||
| Purchases of property and equipment |
(25,430 | ) | (9,688 | ) | (6,930 | ) | ||||||
| Purchases of marketable securities |
(114,756 | ) | — | — | ||||||||
| (Increase) decrease in restricted cash |
(531 | ) | 861 | (1,564 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in investing activities |
(140,717 | ) | (8,827 | ) | (8,494 | ) | ||||||
| Financing activities |
||||||||||||
| Proceeds from issuance of common stock to Roche, net of issuance costs |
245,387 | — | — | |||||||||
| Proceeds from issuance of restricted stock and stock option exercises |
4,739 | 637 | 49 | |||||||||
| Payments of notes payable |
— | (1,287 | ) | (1,705 | ) | |||||||
| Proceeds from issuance of common stock, net of issuance costs |
— | — | 110,381 | |||||||||
| Proceeds from issuance of Series B Preferred Stock, net of issuance costs |
— | — | (10 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by (used in) financing activities |
250,126 | (650 | ) | 108,715 | ||||||||
| Net increase (decrease) in cash and cash equivalents |
45,683 | (52,213 | ) | 69,455 | ||||||||
| Cash and cash equivalents at beginning of period |
72,080 | 124,293 | 54,838 | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash and cash equivalents at end of period |
$ | 117,763 | $ | 72,080 | $ | 124,293 | ||||||
|
|
|
|
|
|
|
|||||||
| Supplemental disclosure of cash flow information |
||||||||||||
| Cash paid for interest |
$ | — | $ | 263 | $ | 171 | ||||||
|
|
|
|
|
|
|
|||||||
| Supplemental disclosure of non-cash investing and financing activities |
||||||||||||
| Conversion of convertible preferred stock into common stock |
$ | — | $ | — | $ | 98,787 | ||||||
|
|
|
|
|
|
|
|||||||
| Acquisition of property and equipment included in accounts payable and accrued expenses |
$ | 6,822 | $ | 1,334 | $ | 3,916 | ||||||
|
|
|
|
|
|
|
|||||||
| Reclassification of warrant liability to additional paid-in capital |
$ | — | $ | — | $ | 1,605 | ||||||
|
|
|
|
|
|
|
|||||||
| Accretion of convertible preferred stock to redemption value |
$ | — | $ | — | $ | 139 | ||||||
|
|
|
|
|
|
|
|||||||
The accompanying notes are an integral part of these consolidated financial statements
F-7
Table of Contents
Notes to Consolidated Financial Statements
| 1. | Nature of Business |
Foundation Medicine, Inc. and its wholly-owned subsidiary, Foundation Medicine Securities Corporation (collectively, the “Company”), is a molecular information company focused on fundamentally changing the way in which patients with cancer are evaluated and treated. The Company believes an information-based approach to making clinical treatment decisions based on comprehensive genomic profiling will become a standard of care for patients with cancer. The Company derives revenue from selling products that are enabled by its molecular information platform to physicians and biopharmaceutical companies.
The Company’s flagship clinical molecular information products, FoundationOne for solid tumors and FoundationOne Heme for blood-based cancers, or hematologic malignancies, including leukemia, lymphoma, myeloma, and advanced sarcomas, are, to its knowledge, the only widely available comprehensive genomic profiles designed for use in the routine care of patients with cancer. To accelerate its commercial growth and enhance its competitive advantage, the Company is continuing to develop and commercialize new molecular information products for physicians and biopharmaceutical companies, strengthen its commercial organization, introduce new marketing, education and provider engagement efforts, grow its molecular information knowledgebase, called FoundationCORE, aggressively pursue reimbursement from regional and national third-party payors, publish scientific and medical advances, and foster relationships throughout the oncology community. Examples of new products include GeneKit, a cloud-based genomics solutions portal for pathologists and FoundationACT, a blood-based (liquid biopsy) assay to measure circulating tumor DNA (“ctDNA”). The Company launched FoundationACT for research use to its biopharmaceutical partners in December 2015 and anticipates a commercial launch of this test to ordering physicians in March 2016.
The Company believes that its existing cash, cash equivalents, and marketable securities at December 31, 2015 will be sufficient to allow the Company to fund its current operating plan through at least January 1, 2017. As the Company continues to incur losses, its transition to profitability is dependent upon a level of revenues adequate to support the Company’s cost structure. If the Company’s transition to profitability is not consistent with its current operating plan, the Company may have to seek additional capital.
| 2. | Summary of Significant Accounting Policies and Basis of Presentation |
| A. | Basis of Presentation and Consolidation |
The Company’s consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States (“GAAP”). Any reference in these notes to applicable guidance is meant to refer to the authoritative United States generally accepted accounting principles as found in the Accounting Standards Codification (“ASC”) and Accounting Standards Update (“ASU”) of the Financial Accounting Standards Board (“FASB”). The Company’s consolidated financial statements include the accounts of Foundation Medicine, Inc. and its wholly-owned subsidiary, Foundation Medicine Securities Corporation. All intercompany balances and transactions have been eliminated.
| B. | Use of Estimates |
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenue and expenses during the reporting period. The Company bases its estimates on historical experience and other market-specific or other relevant assumptions that it believes to be reasonable under the circumstances. This process may result in actual results differing materially from those estimated amounts used in the preparation of the financial statements if these results differ from historical experience, or other assumptions do not turn out to
F-8
Table of Contents
be substantially accurate, even if such assumptions are reasonable when made. Significant estimates relied upon in preparing these consolidated financial statements include revenue recognition, the determination of the fair value of stock awards issued prior to the initial public offering in September 2013, stock-based compensation expense, and the valuation allowance on the Company’s deferred tax asset.
| C. | Off-Balance Sheet Risk and Concentrations of Credit Risk |
The Company has no off-balance sheet risk, such as foreign exchange contracts, option contracts, or other foreign hedging arrangements. Financial instruments, which potentially subject the Company to significant concentrations of credit risk, consist primarily of cash, cash equivalents, marketable securities designated as available-for-sale, and accounts receivable. The primary objectives for the Company’s investment portfolio are the preservation of capital and the maintenance of liquidity. The Company’s investment policy includes guidelines on the quality of the institutions and financial instruments and defines allowable investments that the Company believes minimizes the exposure to concentration of credit risk. The Company’s marketable securities are designated as available-for-sale and primarily consist of U.S. government agency securities and U.S. treasuries.
The Company routinely assesses the creditworthiness of its customers. The Company has not experienced any material losses related to receivables from individual customers, or groups of customers. The Company does not require collateral. Due to these factors, no additional credit risk beyond amounts provided for collection losses is believed by management to be probable in the Company’s accounts receivable.
| D. | Segment Information |
Operating segments are defined as components of an enterprise about which separate discrete information is available for evaluation by the chief operating decision maker, or decision-making group, in deciding how to allocate resources and in assessing performance. The Company views its operations and manages its business in one operating segment, which is the business of delivering molecular information about cancer to its customers.
The Company’s revenue is generated primarily in the United States. The majority of the Company’s revenue from customers located outside the United States, which was generated from four customers, was $3,170,000, $4,450,000, and $3,234,000 for the years ended December 31, 2015, 2014, and 2013, respectively. All of the Company’s long-lived assets are located in the United States.
| E. | Cash and Cash Equivalents |
The Company considers all highly liquid investments with original maturity from the date of purchase of three months or less to be cash equivalents. Cash and cash equivalents include bank demand deposits and money market funds that invest primarily in U.S. government-backed securities and treasuries. Cash equivalents are carried at cost, which approximates their fair value.
| F. | Marketable Securities |
The Company classifies its marketable securities as available-for-sale. Marketable securities with a remaining maturity date greater than one year are classified as non-current. Available-for-sale securities are maintained by an investment manager and consist of U.S. treasury securities, U.S. government agency securities, and money market funds invested in U.S. government-agency securities or U.S. treasuries. Available-for-sale securities are carried at fair value with the unrealized gains and losses included in accumulated other comprehensive loss as a component of stockholders’ equity until realized. Any premium or discount arising at purchase is amortized and/or accreted to interest income and/or expense. Realized gains and losses are determined using the specific identification method and are included in other income (expense).
F-9
Table of Contents
If any adjustment to fair value reflects a decline in value of the investment, the Company considers all available evidence to evaluate the extent to which the decline is “other-than-temporary” and, if so, mark the investment to market through a charge to the Company’s statement of operations and comprehensive loss.
| G. | Accounts Receivable and Allowance for Doubtful Accounts |
The Company’s accounts receivable consist primarily of amounts due from biopharmaceutical customers, and from certain hospitals, cancer centers and other institutions with whom it has negotiated price per test (direct bill) relationships for tests performed using its molecular information platform. There are no accounts receivable associated with amounts that are billed to commercial third-party payors or directly to patients, because this revenue is recognized on a cash basis (see Note 2 Section L). The Company determines its allowance by considering a number of factors, including the length of time accounts receivable are past due, previous loss history, a specific customer’s ability to pay its obligations to the Company, and the condition of the general economy and industry as a whole. As of December 31, 2015 and 2014, the Company recorded an allowance for doubtful accounts of $171,000 and $0, respectively.
Two customer account balances consisting of $2,423,000 and $825,000 were greater than 10% of the total accounts receivable balance representing 31% and 11%, respectively, of total accounts receivable at December 31, 2015. Four customer account balances consisting of $1,673,000, $1,168,000, $1,128,000 and $1,073,000 were greater than 10% of the total accounts receivable balance representing 17%, 12%, 11% and 11%, respectively, of total accounts receivable at December 31, 2014.
| H. | Inventory |
Inventories are stated at the lower of cost or market on a first-in, first-out basis. In order to assess the ultimate realization of inventories, the Company is required to make judgments as to future demand requirements compared to current or committed inventory levels. The Company evaluates its inventories for excess quantities and obsolescence. Inventories that are considered excess or obsolete are expensed. At December 31, 2015 and 2014, inventory consisted of the following (in thousands):
| December 31, | ||||||||
| 2015 | 2014 | |||||||
| Raw materials |
$ | 6,604 | $ | 3,851 | ||||
| Work-in-process |
1,388 | 958 | ||||||
|
|
|
|
|
|||||
| $ | 7,992 | $ | 4,809 | |||||
|
|
|
|
|
|||||
| I. | Property and Equipment |
Property and equipment are stated at cost, less accumulated depreciation and amortization. Property and equipment are depreciated and amortized using the straight-line method over the estimated useful lives of the assets. Maintenance and repairs are expensed as incurred. The following estimated useful lives are used to depreciate the Company’s assets:
| Estimated Useful Life | ||
| Computer equipment and software |
3 years | |
| Lab equipment |
3 years | |
| Furniture and fixtures |
5 years | |
| Leasehold improvements |
Shorter of the useful life or remaining lease term |
The Company capitalizes certain costs incurred for software developed or obtained for internal use, including external direct material and service costs. Capitalized internal-use software costs, which are included in property and equipment, are generally depreciated over three years.
F-10
Table of Contents
The Company reviews long-lived assets when events or changes in circumstances indicate the carrying value of the assets may not be recoverable. Recoverability is measured by comparison of the assets’ book value to future net undiscounted cash flows that the assets are expected to generate. If such assets are considered to be impaired, the impairment to be recognized is measured by the amount by which the book value of the assets exceeds their fair value, which is measured based on the projected discounted future net cash flows arising from the assets. No impairment losses were recorded during the years ended December 31, 2015 and 2014. In connection with the One Kendall Square lease termination, the Company recorded an impairment charge of $439,000 associated with fixed assets left at the facility during the year ended December 31, 2013. (See Note 12)
| J. | Restricted Cash |
Restricted cash consists of deposits securing collateral letters of credit issued in connection with the Company’s operating leases. The Company had restricted cash of $1,395,000 and $864,000 as of December 31, 2015 and 2014, respectively.
| K. | Fair Value of Financial Instruments |
The Company is required to disclose information on all assets and liabilities reported at fair value that enables an assessment of the inputs used in determining the reported fair values. FASB ASC Topic 820, Fair Value Measurements and Disclosures (“ASC 820”), establishes a hierarchy of inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the observable inputs be used when available. Observable inputs are inputs that market participants would use in pricing the asset or liability based on market data obtained from sources independent of a company. Unobservable inputs are inputs that reflect a company’s assumptions about the inputs that market participants would use in pricing the asset or liability, and are developed based on the best information available in the circumstances. The fair value hierarchy applies only to the valuation inputs used in determining the reported fair value of the investments and is not a measure of the investment credit quality. The hierarchy defines three levels of valuation inputs:
| Level 1 inputs | Quoted prices in active markets for identical assets or liabilities | |||||
| Level 2 inputs | Inputs other than quoted prices included within Level 1 that are observable for the asset or liability, either directly or indirectly | |||||
| Level 3 inputs | Unobservable inputs that reflect a company’s own assumptions about the assumptions market participants would use in pricing the asset or liability | |||||
The fair value hierarchy prioritizes valuation inputs based on the observable nature of those inputs. Assets and liabilities measured at fair value are classified in their entirety based on the lowest level of input that is significant to the fair value measurement. The Company’s assessment of the significance of a particular input to the fair value measurement in its entirety requires management to make judgments and consider factors specific to the asset or liability. (See Note 8)
| L. | Revenue Recognition |
The Company derives revenue from selling products that are enabled by its molecular information platform. The Company currently receives payments from: commercial third-party payors; certain hospitals and cancer centers with which it has direct-bill relationships; individual patients; distributors; and its biopharmaceutical customers.
The Company recognizes revenue in accordance with FASB ASC Topic 605, Revenue Recognition (“ASC 605”). Accordingly, the Company recognizes revenue when all of the following criteria are met: (i) persuasive evidence of an arrangement exists; (ii) delivery has occurred; (iii) the fee is fixed or determinable; and (iv) collectability is reasonably assured. Criterion (i) is satisfied when the Company has an arrangement or
F-11
Table of Contents
contract in place. Criterion (ii) is satisfied when the Company delivers a report to the ordering physician or the biopharmaceutical customer. Determination of criteria (iii) and (iv) are based on management’s judgments regarding whether the fee is fixed or determinable, and whether the collectability of the fee is reasonably assured.
The Company recognizes revenue on a cash basis when it cannot conclude that criteria (iii) and (iv) have been met. The Company currently recognizes revenue on a cash basis from sales of its products for which the Company receives payments from commercial third-party payors and from patients who make co-payments, pay deductibles or from other amounts that the Company has been unable to collect from third-party payors. The Company uses judgment in its assessment of whether the fee is fixed or determinable and whether collectability is reasonably assured in determining when to recognize revenue in the future as it continues to gain payment experience with third-party payors and patients. Accordingly, the Company expects to recognize revenue on a cash basis for these customers until it has sufficient history to reliably estimate payment patterns. The Company’s products are delivered electronically, and as such there are no shipping and handling fees incurred by the Company or billed to customers. The Company’s products are exempt from state sales taxation due to the nature of the products. As a result, the Company does not charge customers state sales tax.
The Company recognizes revenue from the sale of its products to certain hospitals, cancer centers, other institutions and patients at the time results of the test are reported to physicians, if criteria (i) through (iv) above are met.
Revenue from sales of the Company’s products to biopharmaceutical customers are based on a negotiated price per test or on the basis of an agreement to provide certain testing volume, data access, or other services over a defined period. The Company recognizes revenue upon delivery of the test results, or over the period the testing volume or other deliverables are provided, as appropriate.
The Company analyzes multiple-element arrangements based on the guidance in FASB ASC Topic 605-25, Revenue Recognition-Multiple-Element Arrangements (“ASC 605-25”). Pursuant to the guidance in ASC 605-25, the Company evaluates multiple-element arrangements to determine (1) the deliverables included in the arrangement and (2) whether the individual deliverables represent separate units of accounting or whether they must be accounted for as a combined unit of accounting. This evaluation involves subjective determinations and requires management to make judgments about the individual deliverables and whether such deliverables are separable from the other aspects of the contractual relationship. Deliverables are considered separate units of accounting if: (i) the delivered items have value to the customer on a standalone basis and (ii) the arrangement includes a general right of return relative to the delivered items and delivery or performance of the undelivered items is considered probable and substantially in the control of the Company. In assessing whether an item has standalone value, the Company considers factors such as the research, development and commercialization capabilities of a third party and the availability of the associated expertise in the general marketplace. In addition, the Company considers whether the other party in the arrangement can use the other deliverables for their intended purpose without the receipt of the remaining elements, whether the value of the deliverable is dependent on the undelivered items, and whether there are other vendors that can provide the undelivered elements.
Arrangement consideration that is fixed or determinable is allocated among the separate units of accounting using the relative selling price method. Then, the applicable revenue recognition criteria in ASC 605-25 is applied to each of the separate units of accounting in determining the appropriate period and pattern of recognition. The Company determines the selling price of a unit of accounting following the hierarchy of evidence prescribed by ASC 605-25. Accordingly, the Company determines the estimated selling price for units of accounting within each arrangement using vendor-specific objective evidence (“VSOE”) of selling price, if available, third-party evidence (“TPE”) of selling price if VSOE is not available, or best estimate of selling price (“BESP”) if neither VSOE nor TPE is available. The Company typically uses BESP to estimate the selling price, since it generally does not have VSOE or TPE of selling price for its units of accounting under multiple-element arrangements. Determining the BESP for a unit of accounting requires significant judgment. In developing the BESP for a unit of accounting, the Company considers applicable market conditions and estimated costs. The
F-12
Table of Contents
Company validates the BESP for units of accounting by evaluating whether changes in the key assumptions used to determine the BESP will have a significant effect on the allocation of arrangement consideration between multiple units of accounting. The Company recognizes arrangement consideration allocated to each unit of accounting when all of the revenue recognition criteria in ASC 605-25 are satisfied for that particular unit of accounting.
At the inception of an arrangement that includes milestone payments to the Company, the Company evaluates whether each milestone is substantive and at risk to both parties on the basis of the contingent nature of the milestone. This evaluation includes an assessment of whether: (i) the consideration is commensurate with either the Company’s performance to achieve the milestone or the enhancement of the value of the delivered items as a result of a specific outcome resulting from the Company’s performance to achieve the milestone, (ii) the consideration relates solely to past performance, and (iii) the consideration is reasonable relative to all of the deliverables and payment terms within the arrangement. The Company evaluates factors such as the scientific, clinical, regulatory, commercial, and other risks that must be overcome to achieve the respective milestone and the level of effort and investment required to achieve the respective milestone in making this assessment. There is considerable judgment involved in determining whether a milestone satisfies all of the criteria required to conclude that a milestone is substantive. Generally, once a substantive milestone has been achieved, the Company will recognize revenue related to that milestone using a proportional performance model over the period which the unit of accounting is delivered or based on the level of effort expended to date over the total expected effort, whichever is considered the most appropriate measure of performance. Revenue from commercial milestone payments are accounted for as royalties and recorded as revenue upon achievement of the milestone, assuming all other revenue recognition criteria are met.
The Company also recognizes royalty revenue in the period of sale of the related product(s), based on the underlying contract terms, provided that the reported sales are reliably measurable and the Company has no remaining performance obligations, assuming all other revenue recognition criteria are met.
For some multiple-element arrangements, the Company will be reimbursed for either all or a portion of the research and development costs incurred. The Company performs research and development services as part of its revenue activities and, therefore, believes such activities are a part of its primary business. Therefore, the Company records these reimbursements as revenue in the statement of operations using a proportional performance model over the period which the unit of accounting is delivered or based on the level of effort expended to date over the total expected effort, whichever is considered the most appropriate measure of performance.
Two customer arrangements, consisting of $13,444,000 and $10,618,000 of revenue, were greater than 10% of total revenue, representing 14% and 11%, respectively, of the Company’s total revenue for the year ended December 31, 2015. One customer arrangement, consisting of $7,996,000 of revenue was greater than 10% of total revenue, representing 13% of the Company’s total revenue for the year ended December 31, 2014. Two customer arrangements, consisting of $6,813,000 and $3,652,000 of revenue, were greater than 10% of total revenue, representing 23% and 13%, respectively, of the Company’s total revenue for the year ended December 31, 2013.
| M. | Research and Development Expenses |
Research and development costs are expensed as incurred and include salaries and benefits, facilities costs, reagent costs, overhead costs, clinical trial costs, contract services and other related costs.
| N. | Stock-Based Compensation |
The Company accounts for stock-based compensation awards in accordance with FASB ASC Topic 718, Compensation—Stock Compensation (“ASC 718”). ASC 718 requires all stock-based compensation to
F-13
Table of Contents
employees, including grants of employee stock options and restricted stock and modifications to existing stock options, to be recognized in the statement of operations and comprehensive loss based on their fair values.
Compensation expense related to awards to employees is recognized on a ratable straight-line basis, based on the grant date fair value, over the requisite service period of the award, which is generally the vesting term. Awards to non-employees are adjusted through stock-based compensation expense as the awards vest to reflect the current fair value of such awards, and are expensed using the straight-line method.
The Company expenses restricted stock awards based on the fair value of the award on a ratable straight-line basis over the requisite service period of the award. The unvested portion of awards of restricted stock to non-employees is subject to remeasurement over the vesting term.
The Company estimates the fair value of its stock-based awards to employees and directors using the Black-Scholes option pricing model, which requires subjective assumptions, including (a) the expected stock price volatility, (b) the expected term of the award, (c) the risk-free interest rate, and (d) expected dividends. Due to the lack of Company-specific historical and implied volatility data, the Company has based its estimate of expected volatility on the historical volatility of a representative group of companies that are publicly traded. The Company selected a representative group of companies with comparable characteristics to it, including risk profiles, position within the industry, and with historical share price information sufficient to meet the expected term of the stock-based awards. The Company computes the historical volatility of this selected group using the daily closing prices for the selected companies’ shares during the equivalent period of the calculated expected term of the stock-based awards. The Company will continue to apply this process until a sufficient amount of historical information regarding the volatility of its own stock price becomes available. The Company estimates the expected life of its employee stock options using the “simplified” method, whereby, the expected life equals the arithmetic average of the vesting term and the original contractual term of the option due to its lack of sufficient historical data. The risk-free interest rates for periods within the expected term of the option are based on the U.S. Treasury securities with a maturity date commensurate with the expected term of the associated award. The Company has never paid, and does not expect to pay, dividends in the foreseeable future; therefore, the expected dividend yield is assumed to be zero.
Forfeitures are required to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. The term “forfeitures” is distinct from “cancellations” and represents only the unvested portion of the surrendered option. Ultimately, the actual expense recognized over the vesting period will only be for those options that vest. (See Note 10)
| O. | Income Taxes |
Income taxes are recorded in accordance with FASB ASC Topic 740, Income Taxes (“ASC 740”), which provides for deferred taxes using an asset and liability approach. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases using enacted tax rates in effect for the year in which the differences are expected to affect taxable income. Tax benefits are recognized when it is more likely than not that a tax position will be sustained during an audit. Deferred tax assets are reduced by a valuation allowance if current evidence indicates that it is considered more likely than not that these benefits will not be realized.
| P. | Net Loss Per Share |
Basic net loss per share is calculated by dividing net loss applicable to common stockholders by the weighted-average shares outstanding during the period, without consideration for common stock equivalents. Diluted net loss per share is calculated by adjusting the weighted-average shares outstanding for the dilutive effect of common stock equivalents outstanding for the period, determined using the treasury-stock method. For purposes of the diluted net loss per share calculation, preferred stock, stock options, restricted stock awards, and
F-14
Table of Contents
warrants are considered to be common stock equivalents but are excluded from the calculation of diluted net loss per share because their effect would be anti-dilutive. Therefore, basic and diluted net loss per share applicable to common stockholders were the same for all periods presented.
| Q. | Comprehensive Loss |
Comprehensive loss is defined as the change in equity of a business enterprise during a period from transactions, other events, and circumstances from non-owner sources. Comprehensive loss consists of net loss and other comprehensive loss, which includes unrealized losses on available-for-sale securities. Comprehensive loss is included in the accompanying statements of operations and comprehensive loss.
| R. | Application of New or Revised Accounting Standards |
On April 5, 2012, the Jump-Start Our Business Startups Act (the “JOBS Act”) was signed into law. The JOBS Act contains provisions that, among other things, reduce certain reporting requirements for an “emerging growth company.” As an emerging growth company, the Company has elected to not take advantage of the extended transition period afforded by the JOBS Act for the implementation of new or revised accounting standards, and as a result, will comply with new or revised accounting standards on the relevant dates on which adoption of such standards is required for non-emerging growth companies.
| S. | Recent Accounting Pronouncements |
From time to time, new accounting pronouncements are issued by the FASB or other standard setting bodies and adopted by the Company as of the specified effective date. Unless otherwise discussed, the Company believes that the impact of recently issued standards that are not yet effective will not have a material impact on its financial position or results of operations upon adoption.
In May 2014, the FASB and the International Accounting Standards Board jointly issued ASU No. 2014-09, Revenue from Contracts with Customers (“ASU 2014-09”), which supersedes the revenue recognition requirements in Accounting Standards Codification 605 (“ASC 605”) and most industry-specific guidance. The new standard requires that an entity recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. The update also requires additional disclosure about the nature, amount, timing, and uncertainty of revenue and cash flows arising from customer contracts, including significant judgments and changes in judgments and assets recognized from costs incurred to obtain or fulfill a contract. In July 2015, the FASB decided to delay the effective date of ASU 2014-09 by one year until January 1, 2018. The FASB also permitted entities to choose to adopt the standard as of the original effective date (January 1, 2017). The Company intends to adopt ASU 2014-09 on January 1, 2018, and is currently evaluating the method of adoption and the potential impact that ASU 2014-09 may have on its financial position and results of operations.
In August 2014, the FASB issued ASU 2014-15, Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern (“ASU 2014-15”). ASU 2014-15 requires management to evaluate, at each annual or interim reporting period, whether there are conditions or events that exist that raise substantial doubt about the Company’s ability to continue as a going concern within one year after the date the financial statements are issued and provide related disclosures. ASU 2014-15 is effective for annual periods ending after December 15, 2016 and earlier application is permitted. The adoption of ASU 2014-15 is not expected to have a material effect on the Company’s consolidated financial statements or disclosures.
In November 2015, the FASB issued ASU No. 2015-17, Balance Sheet Classification of Deferred Taxes (“ASU 2015-17”), which provides guidance for balance sheet classification of deferred taxes. This standard requires that deferred tax assets and liabilities be classified as non-current on the balance sheet, and eliminates the prior guidance which required an entity to separate deferred tax liabilities and assets into a current amount
F-15
Table of Contents
and a noncurrent amount on the balance sheet. ASU 2015-17 is effective for fiscal years, and interim periods within those years, beginning after December 15, 2016. Earlier application is permitted as of the beginning of an interim or annual period. The amendments in ASU 2015-17 may be applied either prospectively to all deferred tax liabilities and assets or retrospectively to all periods presented. The Company elected to early adopt ASU 2015-17 for the year ended December 31, 2015 on a prospective basis. The adoption of ASU 2015-17 did not have a material effect on the Company’s consolidated financial statements or disclosures.
In February 2016, the FASB issued ASU No. 2016-02, Leases (“ASU 2016-02”), to increase transparency and comparability among organizations by recognizing lease assets and lease liabilities, including for operating leases, on the balance sheet and disclosing key information about leasing arrangements. ASU 2016-02 is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2018. The Company is currently evaluating the impact that adopting ASU 2016-02 will have on its consolidated financial statements.
| T. | Subsequent Events |
The Company considers events or transactions that occur after the balance sheet date but prior to the issuance of the consolidated financial statements to provide additional evidence relative to certain estimates or to identify matters that require additional disclosure.
| U. | Reclassifications |
Certain reclassifications have been made to conform the prior period’s data to the current format. These reclassifications had no net effect on the Company’s consolidated financial statements.
| 3. | Significant Agreements |
Roche Holdings, Inc. and its affiliates
Summary of the Transaction
On January 11, 2015, the Company signed a broad strategic collaboration with Roche Holdings, Inc. and certain of its affiliates (collectively, “Roche”) to further advance the Company’s leadership position in genomic analysis and molecular information solutions in oncology. The transaction, which is a broad multi-part arrangement that includes a research & development (“R&D”) collaboration, a commercial collaboration, a U.S. medical education collaboration, and an equity investment with certain governance provisions, closed on April 7, 2015.
Under the terms of the transaction, Roche (a) made a primary investment of $250,000,000 in cash through the purchase of 5,000,000 newly issued shares of the Company’s common stock at a purchase price of $50.00 per share and (b) completed a tender offer to acquire 15,604,288 outstanding shares of the Company’s common stock at a price of $50.00 per share (collectively (a) and (b), the “Investment”). Immediately following the closing of the transaction, Roche owned approximately 61.3% of the outstanding shares. As of December 31, 2015, Roche’s ownership was approximately 60.9% of the outstanding shares. Upon the closing of the transaction, the size of the Board of Directors of the Company (“Board”) was increased to nine, including three designees of Roche. Four existing independent directors and the Company’s Chief Executive Officer, Michael Pellini, M.D., have continued as directors, and the Company expects that one new independent director will be added.
The Company assessed the agreements related to each of the R&D collaboration, commercial collaboration, and the U.S. medical education collaboration and determined they should be treated as a single contract for accounting purposes.
F-16
Table of Contents
Summary of the R&D Collaboration Agreement
Under the terms of the Collaboration Agreement by and among the Company, F. Hoffmann-La Roche Ltd, and Hoffmann-La Roche Inc., dated January 11, 2015 (the “R&D Collaboration Agreement”), Roche could pay the Company more than $150,000,000 over a period of five years to access its molecular information platform, to reserve capacity for sample profiling, and to fund R&D programs. Amounts under the R&D Collaboration Agreement will be received as services are performed and obligations are fulfilled under each platform program. Roche will utilize the Company’s molecular information platform to standardize sample profiling conducted as part of its clinical trials, to enable comparability of clinical trial results for R&D purposes, and to better understand the potential for combination therapies. In addition, Roche and the Company will jointly develop solutions related to cancer immunotherapy testing, blood-based genomic analysis using ctDNA assays, and next generation companion diagnostics, each of which represents a distinct platform within the R&D Collaboration Agreement. The R&D Collaboration Agreement is governed by a Joint Management Committee (“JMC”) formed by an equal number of representatives from the Company and Roche. There are also other sub-committees for each platform that will be established to oversee the day to day responsibilities of the respective platform. The JMC will, among other activities, review and approve R&D plans and establish and set expectations for the other platform sub-committees. The JMC and other sub-committees, although considered deliverables under the arrangement, are immaterial in relation to the entire arrangement and therefore were not considered when allocating consideration.
Molecular Information Platform Program
Under the molecular information platform program within the R&D Collaboration Agreement, the following deliverables were identified: (i) cross-licenses for access to relevant intellectual property (“IP”), (ii) reserved capacity for sample profiling, (iii) access to the Company’s molecular information database, (iv) full-time equivalent persons (“FTEs”) per year for performance of database queries and delivery of results, and (v) sample profiling above the reserved capacity limit.
The Company determined which deliverables within the arrangement have standalone value from the other undelivered elements, and identified the following separate units of accounting: (i) reserved capacity for sample profiling, (ii) access to the Company’s molecular information database and FTEs per year for the performance of database queries and the delivery of results, and (iii) sample profiling above the reserved capacity limit. The cross-licenses grant each party access to relevant IP to perform under the contract or to exploit the deliverables. The licenses are delivered at the inception of the arrangement and relate to development and sample profiling work performed under the platform. The Company does not sell the licenses separately as they are closely connected to the development and sample profiling activities and have little value to Roche without these other deliverables. Therefore, the licenses are combined with the other units of accounting identified under the molecular information platform program and do not have standalone value.
The Company identified allocable consideration of approximately $85,000,000 related to the molecular information platform program, which was allocated to the individual units of accounting based on the BESP. Revenue related to reserved capacity for sample profiling will be recognized on a straight-line basis as the capacity is available for each individual contract year within the arrangement. The database access and FTE payments will be recognized ratably over the five year contract life. The FTEs will perform database queries and will deliver results of the requested database queries. The value to Roche is not only the access to the database, but also the service being performed by the FTEs. Therefore, the Company concluded the FTEs should be combined with the database access as one unit of accounting. For any sample profiling provided above the reserved capacity, the Company will recognize revenue as the service is provided based on the BESP.
Immunotherapy Testing Platform Development Program
Under the immunotherapy testing platform development program within the R&D Collaboration Agreement, the following deliverables were identified: (i) cross-licenses for access to relevant IP, (ii) obligations
F-17
Table of Contents
to perform R&D services for immuno-biomarker discovery and signature identification, and (iii) obligations to provide sample profiling using immunotherapy clinical study assays.
The Company determined which deliverables within the arrangement have standalone value from the other undelivered elements, and identified the following separate units of accounting: (i) obligations to perform R&D services for immuno-biomarker discovery and signature identification and (ii) obligations to provide sample profiling using immunotherapy clinical study assays. The cross-licenses grant each party access to relevant IP of the other party to perform such party’s obligations under the contract and to exploit the deliverables. The licenses are delivered at the inception of the arrangement and relate to R&D work performed under the platform. The Company does not sell the licenses separately as they are closely connected to the R&D activities and have little value to Roche without these other deliverables. Therefore, the licenses are combined with the other units of accounting identified under the immunotherapy testing platform development program and do not have standalone value.
Under this platform, Roche will reimburse the Company for certain R&D costs incurred related to the immuno-biomarker discovery and signature identification activities, as well as costs incurred in the development of immunotherapy assays for clinical studies. In addition, Roche will be required to make certain milestone payments upon the achievement of specified clinical events under the immunotherapy testing platform development program. Clinical milestone payments up to $6,600,000 in the aggregate are triggered upon the initiation of Roche clinical trials using immunotherapy assays developed under the R&D Collaboration Agreement and are considered substantive. The R&D reimbursements and clinical milestone payments will be recognized using a proportional performance model when earned by the Company.
Circulating Tumor DNA (ctDNA) Platform Development Program
Under the ctDNA platform development program within the R&D Collaboration Agreement, the following deliverables were identified: (i) cross-licenses for access to relevant IP, (ii) obligations to perform R&D services for the development of a ctDNA clinical trial assay, including its analytical validation, and (iii) sample profiling resulting from the development of a ctDNA clinical assay.
The Company determined which deliverables within the arrangement have standalone value from the other undelivered elements, and identified the following separate units of accounting: (i) obligations to perform R&D services for the development of a ctDNA clinical trial assay and (ii) delivery of clinical sample profiling resulting from the development of a ctDNA clinical assay. The cross-licenses grant each party access to relevant IP of the other party to perform such party’s obligations under the contract and to exploit the deliverables. The licenses are delivered at the inception of the arrangement and relate to R&D work performed under the platform. The Company does not sell the licenses separately as they are closely connected to the R&D activities and have little value to Roche without these other deliverables. Therefore, the licenses are combined with the other units of accounting identified under the ctDNA platform development program and do not have standalone value.
The Company is responsible for all R&D costs under the ctDNA platform development program. Roche will be required to make certain milestone payments upon the achievement of specified events. Milestone payments up to $12,000,000 in the aggregate are triggered upon successful analytical validation of a ctDNA assay and delivery of a ctDNA clinical trial assay for use in Roche clinical trials. All milestones are considered substantive and will be recognized using a proportional performance model when earned by the Company.
Companion Diagnostics (CDx) Development Program
Under the CDx development program within the R&D Collaboration Agreement, the following deliverables were identified: (i) cross-licenses for access to relevant IP and (ii) obligations to perform R&D services for the development of CDx assays for use in connection with certain Roche products.
F-18
Table of Contents
The Company determined which deliverables within the arrangement have standalone value from the other undelivered elements, and concluded all deliverables under the CDx development program represent a single unit of accounting. The cross-licenses grant each party access to relevant IP of the other party to perform such party’s obligations under the contract and to exploit the deliverables. The licenses are delivered at the inception of the arrangement and relate to R&D work performed under the platform. The Company does not sell the licenses separately as they are closely connected to the R&D activities and have little value to Roche without these other deliverables. Therefore, the licenses are combined with the obligation to perform R&D services for the development of a CDx assay as a single unit of accounting.
Under this platform, Roche will reimburse the Company for certain costs incurred related to R&D under the CDx development program with respect to investigational markers. In addition, Roche will be required to make certain milestone payments upon the achievement of specified regulatory and commercial events under the CDx development program. Regulatory milestone payments of $600,000 are triggered upon obtaining FDA approval of a premarket approval application for each CDx product developed under the arrangement and are considered substantive. The R&D reimbursements and regulatory milestone payments will be recognized using a proportional performance model when earned by the Company. Commercial milestone payments are triggered upon the performance of a specified number of CDx assays for certain commercial clinical diagnostic uses. Any commercial milestone payments received by the Company will be treated similar to royalties and recognized in their entirety when earned.
Termination of the R&D Collaboration Agreement
The R&D Collaboration Agreement may be terminated by either the Company or Roche on a program-by-program basis, upon written notice, in the event of the other party’s uncured material breach. Roche may also terminate the entire R&D Collaboration Agreement or an individual program under the R&D Collaboration Agreement for any reason upon written notice to the Company, subject to certain exceptions. If the R&D Collaboration Agreement is terminated, license and IP rights are returned to each party and the Company must return to Roche or dispose of any unused samples delivered for profiling purposes. If Roche terminates the R&D Collaboration Agreement as a result of a breach by the Company, Roche retains the license rights granted to certain IP of the Company, and the Company shall refund to Roche any reserved capacity fees and database access fees previously received by the Company that were unused based on the passage of time up to termination for the given contract year. If the R&D Collaboration Agreement is terminated by Roche without cause, or by the Company due to a breach by Roche, the Company has a right to receive the contractual payments it would have expected to receive for each program had the agreement not been terminated.
Summary of the Ex-U.S. Commercialization Agreement
In addition to the R&D Collaboration Agreement, the Company entered into a commercial collaboration agreement with Roche designed to facilitate the delivery of the Company’s products and services outside the United States (“Ex-U.S.”) in partnership with Roche (the “Ex-U.S. Commercialization Agreement”). Specifically, Roche will obtain Ex-U.S. commercialization rights to the Company’s existing products and services and to future co-developed products and services, and the Company will remain solely responsible for commercialization of its products and services within the United States. Roche will have specified time periods to determine if it desires to exercise its commercialization rights in selected geographic areas Ex-U.S., which selected geographic areas together constitute the “Roche Territory.” For those geographic areas that Roche does not select, the commercialization rights for such geographic areas will revert to the Company. The Ex-U.S. Commercialization Agreement is governed by the JMC. There is also a Joint Operational Committee (“JOC”) that has been established to oversee the activities under the Ex-U.S. Commercialization Agreement. The JMC will have the responsibilities as outlined under the R&D Collaboration Agreement. The JMC and JOC, although considered deliverables under the arrangement, are immaterial in relation to the entire arrangement and therefore were not considered when allocating consideration.
F-19
Table of Contents
Under the Ex-U.S. Commercialization Agreement, the following deliverables were identified: (i) the right, granted by means of a license, for Roche to market and sell the Company’s products in the Roche Territory and (ii) obligations to perform sample profiling and other services relating to Company products and services sold by Roche in the Roche Territory. The Company concluded that the license is delivered at the inception of the arrangement. The Company does not sell the license separately as it is closely connected to the sample profiling and other services and has little value to Roche without these services being performed. Therefore, the deliverables identified will be combined as a single unit of accounting under the Ex-U.S. Commercialization Agreement and revenue will be recognized as the service is performed for each product sold by Roche.
Roche will be required to make a one-time milestone payment of $10,000,000 when the aggregate gross margin on sales of certain of the Company’s products reaches $100,000,000 in the Roche Territory in any calendar year. Roche may also pay delay fees to the extent Roche fails to launch Company products in specific countries within a specified timeframe. This milestone payment and these fees will be treated similarly to royalties and recognized in their entirety when earned.
The Company will be entitled to receive, on a quarterly basis, tiered royalty payments ranging from the mid-single digits to high-teens based on a percentage of the aggregate gross margin generated on sales of specified products in the Roche Territory during any calendar year. Royalty payments will be recognized in the period when earned.
The Ex-U.S. Commercialization Agreement may be terminated by either the Company or Roche in its entirety or on a country-by-country or product-by-product basis, upon written notice, in the event of the other party’s uncured material breach. Roche may also terminate the Ex-U.S. Commercialization Agreement without cause on a product-by-product and/or country-by-country basis, upon written notice to the Company, after the initial five year term. If the Ex-U.S. Commercialization Agreement is terminated, the license and IP rights granted by the Company to Roche terminate. In addition, if Roche terminates the Ex-U.S. Commercialization Agreement as a result of a breach by the Company, Roche may seek damages via arbitration or be eligible to receive either a one-time payment reflecting the value of the terminated products or a royalty on sales of the terminated products based on the royalty Roche would have paid the Company for the terminated products had the Ex-U.S. Commercialization Agreement not been terminated.
Summary of the U.S. Education Agreement
Within the United States, the Company has entered into the U.S. Education Collaboration Agreement (the “U.S. Education Agreement”) with Genentech, Inc. (“Genentech”), an affiliate of Roche. Genentech has agreed to engage its pathology education team to provide information and medical education to health care providers regarding comprehensive genomic profiling in cancer. The Company will pay Genentech on a quarterly basis for costs incurred by Genentech in conducting the education activities based on a number of factors. The total amount of payments to be made over the course of the arrangement is immaterial and all payments will be expensed as incurred.
Biopharmaceutical Customer
In November 2011, the Company entered into a Master Services Agreement (“MSA”) with a biopharmaceutical customer (the “Customer”) establishing the legal and administrative framework for collaboration. In May 2012, the Company and the Customer amended the MSA to include certain guaranteed quarterly minimum payments by the Customer in return for the Company providing sufficient laboratory capacity to perform up to a maximum number of tests. The amendment defined an initial two year term beginning on the amendment date, during which the Customer agreed to pay the Company $14,200,000. The Company and the Customer executed a second amendment to the MSA, which was effective in October 2013, and extended the term through at least September 2016 during which time the Customer agreed to pay the Company $17,100,000. The Company concluded that the second amendment represented a material modification to the arrangement
F-20
Table of Contents
pursuant to ASC 605. At the date of the modification, there was no deferred revenue balance on the consolidated balance sheet. The Company identified three deliverables under the amended agreement: (i) project management and Joint Steering Committee (“JSC”) services, (ii) the provision of molecular information developed by the Company upon the Customer’s request, and (iii) the provision of sufficient laboratory capacity to test a minimum number of samples provided by the Customer. The Company determined that none of the deliverables under the amended agreement had standalone value since these services were not sold separately by any vendor, including the Company, and a customer could not resell these deliverables on a standalone basis. Thus, the Company determined the arrangement included a single unit of accounting, with revenue being recognized ratably over the three-year term of the amended MSA based on the passage of time, as there is no evidence to suggest revenue is earned or obligations are fulfilled in a different pattern. Under the amended MSA, the Company recognized revenue of $6,968,000, $7,996,000, and $6,812,000 for the years ended December 31, 2015, 2014, and 2013, respectively.
In July 2015, the Company was notified by the Customer of its desire to discontinue the obligation for the Company to provide molecular information upon the Customer’s request. These services were discontinued effective October 1, 2015. All remaining deliverables under the amended MSA, as described above, remained unchanged.
Biopharmaceutical Partner
In July 2012, the Company entered into a Master Services Agreement (“Services Agreement”) with a biopharmaceutical partner (“Partner”) to perform sample profiling at the Partner’s request. The Services Agreement established the legal and administrative framework for the partnership between the entities. The Services Agreement also included a right for the Partner to initiate an exclusive negotiation with the Company for the development of a Companion Diagnostic (“CDx”). In March 2014, the Company and Partner expanded the scope of work by executing a Companion Diagnostic Agreement (“Amended Agreement”), thereby amending the Services Agreement to include the joint development and regulatory approval for a CDx. The Amended Agreement defined the term of the arrangement as the earlier of five years or receipt of certain regulatory approvals of a CDx. The Company concluded that the amendment to the original Services Agreement represented a material modification to the arrangement pursuant to ASC 605 as the Amended Agreement increased total consideration by a significant amount. Additionally, the deliverables under the Amended Agreement changed significantly. At the date of the modification, there was no deferred revenue balance on the consolidated balance sheet related to the original Services Agreement with this Partner.
The Company identified seven deliverables under the Amended Agreement: (i) cross-licenses for access to relevant IP, (ii) obligations to continue to perform sample profiling pursuant to the original Services Agreement, (iii) obligations to perform specific R&D activities for the development of a CDx assay for use in connection with the Partner’s product, (iv) obligations to assist in obtaining regulatory approval of the Partner’s product at its request, (v) participation on a JSC to manage the overall development of the CDx assay, (vi) obligations to perform analytical validation of the CDx assay, and (vii) obligations to make the CDx assay commercially available, following any required regulatory approval. The obligation to make the CDx assay commercially available is dependent on successful development and regulatory approval. As such, the Company determined that this was a contingent deliverable and therefore arrangement consideration was not allocated to this deliverable.
The Company then determined the following deliverables were separate units of accounting: (i) obligations to continue to perform sample profiling pursuant to the original Services Agreement, (ii) obligations to perform specific R&D activities for the development of a CDx assay for use in connection with the Partner’s product and to provide assistance in obtaining regulatory approval of the Partner’s product at its request, (iii) obligations to perform analytical validation of the CDx assay, and (iv) obligations to make the CDx assay commercially available, following any regulatory approval obtained. The cross-licenses grant each party access to relevant IP of the other party to perform such party’s obligations under the contract and to exploit the deliverables. The
F-21
Table of Contents
licenses are delivered at the inception of the arrangement and primarily relate to the R&D development activities performed under the Amended Agreement. The Company does not sell the licenses separately as they are closely connected to the R&D development activities and have little value to the Partner without the performance of such activities. The JSC obligations do not have standalone value and are also closely connected to the R&D development activities under the Amended Agreement. The JSC obligations, although considered deliverables under the arrangement, are immaterial in relation to the entire arrangement. Therefore, the licenses and JSC obligations were combined with the R&D development activities, or unit (ii) identified above.
Under the Amended Agreement, the Partner pays a fixed fee for each sample to be profiled; will reimburse the Company for a portion of costs incurred in performing analytical validation of the CDx assay; and will be required to make certain substantive milestone and other payments upon the achievement of specified regulatory and clinical events tied to the development and commercialization of the CDx. The fixed or determinable consideration under the Amended Agreement was allocated to the units of accounting based on the BESP. Consideration allocated to sample profiling is recognized as samples are delivered, which is when the recognition criteria in ASC 605-25 has been satisfied. Consideration allocated to the R&D development activities and the analytical validation work is recognized using the proportional performance method. Both the Company and the Partner expect to negotiate the strategy and specifics of any commercial partnership upon obtaining regulatory approval of a CDx assay, at which point the fair value of any consideration obtained will be assessed.
Under the Amended Agreement, the Company recognized revenue of $10,618,000 for the year ended December 31, 2015, primarily related to sample profiling and milestone payments received upon the achievement of specified regulatory and clinical events tied to the R&D development activities of the CDx. Under the Amended Agreement and Services Agreement, the Company recognized combined revenue of $2,689,000 for the year ended December 31, 2014, primarily related to sample profiling. Under the Services Agreement, the Company recognized revenue of $1,017,000 for the year ended December 31, 2013.
| 4. | Marketable Securities |
The following table summarizes the available-for-sale securities held at December 31, 2015 (in thousands):
| Amortized Cost |
Unrealized Gains |
Unrealized Losses |
Fair Value | |||||||||||||
| Description: |
||||||||||||||||
| U.S. government agency securities and treasuries |
$ | 114,724 | $ | — | $ | (178 | ) | $ | 114,546 | |||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 114,724 | $ | — | $ | (178 | ) | $ | 114,546 | |||||||
|
|
|
|
|
|
|
|
|
|||||||||
The estimated market value of marketable securities by maturity date is as follows (in thousands):
| December 31, 2015 | ||||
| Due in one year or less |
$ | 89,607 | ||
| Due after one year through two years |
24,939 | |||
|
|
|
|||
| Total |
$ | 114,546 | ||
|
|
|
|||
The Company did not hold any available-for-sale securities as of December 31, 2014. The amortized cost of available-for-sale securities is adjusted for amortization of premiums and accretion of discounts to maturity. At December 31, 2015, the balance in the Company’s accumulated other comprehensive loss was composed solely of activity related to the Company’s available-for-sale marketable securities. There were no realized gains or losses recognized during the year ended December 31, 2015, and as a result, the Company did not reclassify any amount out of accumulated other comprehensive loss for the same period.
F-22
Table of Contents
The aggregate fair value of securities held by the Company in an unrealized loss position for less than twelve months as of December 31, 2015 was $114,546,000, which consisted of 14 U.S. government agency securities and 10 U.S. treasury securities. To determine whether an other-than-temporary impairment exists, the Company performs an analysis to assess whether it intends to sell, or whether it would more likely than not be required to sell, the security before the expected recovery of the amortized cost basis. Where the Company intends to sell a security, or may be required to do so, the security’s decline in fair value is deemed to be other-than-temporary and the full amount of the unrealized loss is recognized on the statement of comprehensive loss as an other-than-temporary impairment charge. When this is not the case, the Company performs additional analysis on all securities with unrealized losses to evaluate losses associated with the creditworthiness of the security. Credit losses are identified where the Company does not expect to receive cash flows, based on using a best estimate, sufficient to recover the amortized cost basis of a security and amount of the loss recognized in other income (expense). The Company has the intent and ability to hold all securities until recovery.
The Company does not intend to sell and it is unlikely that the Company will be required to sell the above investments before recovery of their amortized cost bases, which may be maturity. The Company determined there was no material change in the credit risk of the above investments, and as a result, the Company determined it did not hold any investments with an other-than-temporary impairment as of December 31, 2015.
| 5. | Property and Equipment |
Property and equipment and related accumulated depreciation and amortization are as follows (in thousands):
| December 31, | ||||||||
| 2015 | 2014 | |||||||
| Lab equipment |
$ | 27,883 | $ | 14,843 | ||||
| Computer equipment |
10,542 | 6,673 | ||||||
| Software |
3,703 | 2,111 | ||||||
| Furniture and office equipment |
3,376 | 1,974 | ||||||
| Leasehold improvements |
18,677 | 12,834 | ||||||
| Construction in process |
5,172 | — | ||||||
|
|
|
|
|
|||||
| 69,353 | 38,435 | |||||||
| Less accumulated depreciation and amortization |
(28,020 | ) | (17,420 | ) | ||||
|
|
|
|
|
|||||
| $ | 41,333 | $ | 21,015 | |||||
|
|
|
|
|
|||||
Depreciation and amortization expense for the years ended December 31, 2015, 2014, and 2013 was $10,600,000, $8,195,000, and $5,006,000, respectively. The Company classifies capitalized internal-use software in lab equipment, computer equipment and software based on its intended use. Depreciation expense related to all capitalized internal-use software for the years ended December 31, 2015, 2014, and 2013 was $238,000, $459,000 and $459,000, respectively. The remaining unamortized capitalized internal-use software costs at December 31, 2015 and 2014 were $0 and $238,000, respectively.
| 6. | Accrued Expenses and Other Current Liabilities |
Accrued expenses consisted of the following (in thousands):
| December 31, | ||||||||
| 2015 | 2014 | |||||||
| Payroll and employee-related costs |
$ | 8,375 | $ | 5,011 | ||||
| Professional services |
2,038 | 1,123 | ||||||
| Property and equipment purchases |
1,194 | 471 | ||||||
| Other |
1,215 | 809 | ||||||
|
|
|
|
|
|||||
| $ | 12,822 | $ | 7,414 | |||||
|
|
|
|
|
|||||
F-23
Table of Contents
| 7. | Net Loss Per Common Share |
The following potential common stock equivalents were not included in the calculation of diluted net loss per common share because the inclusion thereof would be antidilutive.
| Year Ended December 31, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Outstanding stock options |
1,684,783 | 2,792,021 | 2,314,284 | |||||||||
| Unvested restricted stock |
969,758 | 335,933 | 517,237 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
2,654,541 | 3,127,954 | 2,831,521 | |||||||||
|
|
|
|
|
|
|
|||||||
| 8. | Fair Value Measurements |
As referenced in Note 2, accounting principles provide guidance for using fair value to measure assets and liabilities based on a hierarchy of inputs and requires management to make judgments and consider factors specific to the asset or liability.
As of December 31, 2015 and 2014, the Company’s financial instruments consisted of cash, cash equivalents, marketable securities, restricted cash, accounts receivable, accounts payable, and accrued liabilities. The carrying amount of cash and cash equivalents, accounts receivable, accounts payable, and accrued liabilities approximate their fair values because of the short-term nature of the instruments.
The following tables present information about the Company’s assets and liabilities that are measured at fair value on a recurring basis as of December 31, 2015 and 2014, and indicate the fair value hierarchy of the valuation techniques utilized to determine such fair value (in thousands):
| Fair Value Measurement at December 31, 2015 | ||||||||||||||||
| Quoted Prices in Active Markets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
Total | |||||||||||||
| Assets: |
||||||||||||||||
| Cash equivalents |
$ | 94,741 | $ | 8,400 | $ | — | $ | 103,141 | ||||||||
| Marketable securities: |
||||||||||||||||
| U.S. government agency securities and treasuries |
54,954 | 59,592 | — | 114,546 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 149,695 | $ | 67,992 | $ | — | $ | 217,687 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Fair Value Measurement at December 31, 2014 | ||||||||||||||||
| Quoted Prices in Active Markets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
Total | |||||||||||||
| Assets: |
||||||||||||||||
| Cash equivalents |
$ | 68,016 | $ | — | $ | — | $ | 68,016 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 68,016 | $ | — | $ | — | $ | 68,016 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
The Company measures eligible assets and liabilities at fair value, with changes in value recognized in the statement of operations and comprehensive loss. Fair value treatment may be elected either upon initial recognition of an eligible asset or liability or, for an existing asset or liability, if an event triggers a new basis of
F-24
Table of Contents
accounting. Items measured at fair value on a recurring basis during the year ended December 31, 2015 include marketable securities. The Company did not elect to remeasure any other existing financial assets or liabilities, and did not elect the fair value option for any other financial assets and liabilities transacted during the years ended December 31, 2015 and 2014.
The fair values of the Company’s marketable securities are determined through market and observable sources and have been classified as Level 1 and Level 2. These assets have been initially valued at the transaction price and subsequently valued utilizing third-party pricing services. The pricing services use many inputs to determine value, including reportable trades, benchmark yields, credit spreads, broker/dealer quotes, and other industry and economic events. The Company validates the prices provided by third-party pricing services by reviewing their pricing methods and obtaining market values from other pricing sources. After completing these validation procedures, the Company did not adjust or override any fair value measurements provided by third-party pricing services as of December 31, 2015.
| 9. | Notes Payable |
In November 2010, the Company entered into a Loan Agreement with Lighthouse Capital Partners VI, L.P., whereby the Company had access to borrow up to $5,000,000. The Company borrowed all $5,000,000 available under the Loan Agreement in increments greater than $100,000 through January 31, 2012 (the “Commitment Termination Date”). For each advance, the Company paid interest only payments at a fixed rate of 8.25% for six months, followed by 36 months of interest and principal payments and a final payment of 4.5% of the advance. The debt was secured by certain property and equipment. The final payments were amortized over the term of the advances and recorded in interest expense. At December 31, 2014, the loan was paid in full. In connection with the Loan Agreement, the Company recorded interest expense in the accompanying consolidated statements of operations and comprehensive loss totaling $61,000 and $228,000 during the years ended December 31, 2014 and 2013, respectively.
In connection with the Loan Agreement, the Company issued a warrant to purchase up to 200,000 shares of the Series A Preferred Stock at a purchase price of $1.00 per share. As of December 31, 2012, the warrant was exercisable for 200,000 shares of Series A Preferred Stock. In conjunction with the closing of the Company’s initial public offering (“IPO”), the warrant was automatically converted into a warrant exercisable for 50,000 shares of common stock at a purchase price of $4.00 per share, which resulted in the reclassification of the related convertible preferred stock warrant liability to additional paid-in capital as the warrant met the criteria for equity classification upon conversion to a warrant for the purchase of common stock. In October 2013, Lighthouse Capital Partners completed a cashless exercise of the warrant and received 44,566 shares of the Company’s common stock.
| 10. | Stockholders’ Equity |
Common Stock
On September 30, 2013, the Company closed its IPO whereby the Company sold 6,772,221 shares of common stock (inclusive of 883,333 shares of common stock sold by the Company pursuant to the full exercise of an overallotment option granted to the underwriters in connection with the offering) at a price of $18.00 per share, for net proceeds of $110,381,000. The shares began trading on the Nasdaq Global Select Market on September 25, 2013. Upon the closing of the IPO, all outstanding shares of convertible preferred stock converted into 17,128,024 shares of common stock.
On April 7, 2015, the Company closed a strategic collaboration with Roche. The transaction included (1) a primary investment by Roche of $250,000,000 in cash to purchase 5,000,000 newly issued shares of the Company’s common stock at a purchase price of $50.00 per share and (2) a tender offer in which Roche acquired 15,604,288 outstanding shares of the Company’s common stock at a price of $50.00 per share.
F-25
Table of Contents
Common stockholders are entitled to one vote per share. Holders of common stock are entitled to receive dividends, when and if declared by the Board. The voting, dividend, and liquidation rights of the holders of the common stock are subject to, and qualified by, the rights of the holders of the preferred stock, of which no shares were outstanding at December 31, 2015.
The Company has reserved for future issuance the following number of shares of common stock:
| December 31, | ||||||||
| 2015 | 2014 | |||||||
| Unvested restricted stock |
969,758 | 335,933 | ||||||
| Common stock options outstanding |
1,684,783 | 2,792,021 | ||||||
| Shares available for issuance under the 2013 Stock Plan |
1,694,077 | 1,375,555 | ||||||
| Shares available for issuance under the 2013 Employee Stock Purchase Plan |
788,503 | 788,503 | ||||||
|
|
|
|
|
|||||
| 5,137,121 | 5,292,012 | |||||||
|
|
|
|
|
|||||
In November 2009, the Company issued 2,125,000 shares of common stock to the founders of the Company for consideration equal to the par value per share, the then estimated fair value of the common stock. The founders entered into restricted stock agreements whereby the shares of common stock issued were subject to vesting and became fully vested in November 2013. An additional 112,500 shares of common stock subject to repurchase were issued to employees and consultants at fair value during the year ended December 31, 2010. Shares subject to repurchase by the Company are recorded as a liability at their original purchase price. Shares subject to repurchase that were issued to non-employees are revalued at each vesting date and at the end of the reporting period, with changes in fair value recorded as stock-based compensation expense on a straight-line basis. As the Company’s right to repurchase the shares lapses, the liability is reclassified to additional paid-in capital. There was no restricted stock activity outside of the 2010 Stock Plan and the 2013 Stock Plan (each, as more fully described under the section below entitled 2010 and 2013 Stock Incentive Plans) during each of the years ended December 31, 2015 and 2014.
Total stock-based compensation expense recognized for restricted stock issued outside of the 2010 Stock Plan was $5,764,000 for the year ended December 31, 2013. There was no stock-based compensation expense outside of the 2010 Stock Plan or the 2013 Stock Plan for the years ended December 31, 2015 and 2014. The total fair value of the restricted stock issued outside of the 2010 Stock Plan and the 2013 Stock Plan that vested during the years ended December 31, 2015, 2014 and 2013 was $0, $78,000 and $5,943,000, respectively.
2010 and 2013 Stock Incentive Plans
In 2010, the Company adopted the Foundation Medicine, Inc. 2010 Stock Incentive Plan (the “2010 Stock Plan”) under which it granted restricted stock, incentive stock options (“ISOs”) and non-statutory stock options to eligible employees, officers, directors and consultants to purchase up to 1,162,500 shares of common stock. In the year ended December 31, 2013, the Company amended the 2010 Stock Plan to increase the number of shares of common stock available for issuance to 4,232,500.
In 2013, in conjunction with its IPO, the Company adopted the Foundation Medicine, Inc. 2013 Stock Option and Incentive Plan (the “2013 Stock Plan”) under which it may grant restricted and unrestricted stock, restricted stock units, ISOs, non-statutory stock options, stock appreciation rights, cash-based awards, performance share awards and dividend equivalent rights to eligible employees, officers, directors and consultants to purchase up to 1,355,171 shares of common stock. In connection with the establishment of the 2013 Stock Plan, the Company terminated the 2010 Stock Plan and the 512,568 shares which remained available for grant under the 2010 Stock Plan were included in the number of shares authorized under the 2013 Stock Plan. Shares forfeited or repurchased from the 2010 Stock Plan are returned to the 2013 Stock Plan for future issuance. On January 1, 2015 and 2014, the number of shares reserved and available for issuance under the 2013 Stock
F-26
Table of Contents
Plan increased by 1,134,996 and 1,125,921 shares of common stock, respectively, pursuant to a provision in the 2013 Stock Plan that provides that the number of shares reserved and available for issuance will automatically increase each January 1, beginning on January 1, 2014, by 4% of the number of shares of common stock issued and outstanding on the immediately preceding December 31 or such lesser number as determined by the compensation committee of the Board.
The terms of stock award agreements, including vesting requirements, are determined by the Board, or permissible designee thereof, subject to the provisions of the 2010 Stock Plan and the 2013 Stock Plan. Options granted by the Company typically vest over a four-year period. The options are exercisable from the date of grant for a period of 10 years. The exercise price for stock options granted is equal to the closing price of the Company’s common stock on the applicable date of grant.
Restricted Stock
The 2010 Stock Plan and the 2013 Stock Plan allow for granting of restricted stock awards. For restricted stock awards granted to employees, the intrinsic value on the date of grant is recognized as stock-based compensation expense ratably over the period in which the restrictions lapse. For restricted stock awards granted to non-employees the intrinsic value is remeasured at each vesting date and at the end of the reporting period. The following table shows a roll forward of restricted stock activity pursuant to the 2010 Stock Plan and the 2013 Stock Plan:
| Number of Shares |
||||
| Unvested at December 31, 2014 |
219,617 | |||
| Granted |
910,652 | |||
| Vested |
(113,008 | ) | ||
| Cancelled |
(57,397 | ) | ||
|
|
|
|||
| Unvested at December 31, 2015(1) |
959,864 | |||
|
|
|
|||
| (1) | Excludes 9,894 shares of unvested restricted stock remaining from the early exercise of stock options. |
Total stock-based compensation expense recognized for restricted stock awards was $5,615,000, $740,000, and $153,000 for the years ended December 31, 2015, 2014, and 2013, respectively. The total grant date fair value of the restricted stock awards that vested during the years ended December 31, 2015, 2014, and 2013 approximated the stock-based compensation expense recorded during the respective periods.
Stock Options
A summary of stock option activity under the 2010 Stock Plan and the 2013 Stock Plan is as follows (in thousands, except share and per share amounts):
| Number of Shares |
Weighted- Average Exercise Price |
Weighted- Average Remaining Contractual Term (In Years) |
Aggregate Intrinsic Value(2) |
|||||||||||||
| Outstanding as of December 31, 2014 |
2,792,021 | $ | 10.82 | 8.3 | $ | 35,390 | ||||||||||
| Granted |
163,000 | 45.34 | ||||||||||||||
| Exercised |
(1,070,457 | ) | 4.43 | |||||||||||||
| Cancelled |
(199,781 | ) | 18.50 | |||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Outstanding as of December 31, 2015 |
1,684,783 | $ | 17.31 | 7.7 | $ | 13,965 | ||||||||||
| Exercisable as of December 31, 2015 |
836,024 | $ | 12.94 | 7.4 | $ | 8,487 | ||||||||||
| Vested and expected to vest at December 31, 2015(1) |
1,593,339 | $ | 16.96 | 7.7 | $ | 13,520 | ||||||||||
F-27
Table of Contents
| (1) | This represents the number of vested options plus the number of unvested options expected to vest at the respective dates, based on unvested options adjusted for estimated forfeitures. |
| (2) | The aggregate intrinsic value was calculated based on the positive difference between the estimated fair value of the Company’s common stock on December 31, 2015, or the date of exercise, as appropriate, and the exercise price of the underlying options. |
Certain stock options contain provisions allowing for the early exercise of such options into shares subject to repurchase by the Company. For the years ended December 31, 2014 and 2013, 116,316 and 8,750 options, respectively, were exercised prior to vesting. At December 31, 2015, 9,894 shares, which were early exercised, remain subject to repurchase.
The weighted-average fair value of options granted for the years ended December 31, 2015, 2014, and 2013 was $26.67, $15.88, and $4.89, respectively. The Company recorded total stock-based compensation expense for stock options granted to employees, directors and non-employees from the 2010 Stock Plan and the 2013 Stock Plan of $5,561,000, $5,249,000, and $1,399,000 during the years ended December 31, 2015, 2014, and 2013, respectively. The aggregate intrinsic value of stock options exercised during the years ended December 31, 2015, 2014, and 2013 was $42,325,000, $4,799,000, and $791,000, respectively. As of December 31, 2015, unrecognized compensation cost of $27,946,000 related to non-vested employee stock-based compensation arrangements is expected to be recognized over a weighted-average period of 2.1 years.
The Company recorded stock-based compensation expense in the consolidated statements of operations and comprehensive loss as follows (in thousands):
| Year Ended December 31, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Cost of revenue |
$ | 980 | $ | 466 | $ | 67 | ||||||
| Selling and marketing |
3,474 | 1,130 | 142 | |||||||||
| General and administrative |
4,272 | 2,635 | 6,346 | |||||||||
| Research and development |
2,450 | 1,758 | 761 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
$ | 11,176 | $ | 5,989 | $ | 7,316 | ||||||
|
|
|
|
|
|
|
|||||||
The weighted-average assumptions used to estimate the fair value of stock options using the Black-Scholes option pricing model were as follows:
| Year Ended December 31, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Expected volatility |
63.1 | % | 64.2 | % | 66.9 | % | ||||||
| Risk-free interest rate |
1.52 | % | 1.99 | % | 1.49 | % | ||||||
| Expected option term (in years) |
6.25 | 6.25 | 6.25 | |||||||||
| Expected dividend yield |
0.0 | % | 0.0 | % | 0.0 | % | ||||||
2013 Employee Stock Purchase Plan
In September 2013, the Company adopted the Foundation Medicine, Inc. Employee Stock Purchase Plan (the “2013 Employee Stock Purchase Plan”), under which the Company is authorized to issue and sell shares of its common stock to participating employees. A total of 788,503 shares of common stock are available for issuance under the 2013 Employee Stock Purchase Plan.
All employees who have been employed by the Company or its designated subsidiaries for at least six weeks and whose customary employment is for more than 20 hours a week are eligible to participate in the 2013
F-28
Table of Contents
Employee Stock Purchase Plan. Any employee who owns, or would own upon such purchase under the 2013 Employee Stock Purchase Plan, 5% or more of the voting power or value of the Company’s stock is not eligible to purchase shares under the 2013 Employee Stock Purchase Plan.
The Company may make one or more offerings to its employees to purchase stock under the 2013 Employee Stock Purchase Plan. The period of any offering shall not shall exceed six months in duration or overlap with another offering period. Each employee who is a participant in the 2013 Employee Stock Purchase Plan may purchase shares by authorizing payroll deductions of up to 10% of his or her eligible compensation during any offering. Unless the participating employee has previously withdrawn from the offering, his or her accumulated payroll deductions will be used to purchase common stock on the last business day of the offering period at a price equal to 85% of the fair market value of the common stock on the first business day or the last business day of the offering period, whichever is lower. During any offering period, an employee may not acquire shares constituting 5% or more of the voting power of the Company’s stock, or such other maximum number established by the Company. During any calendar year, an employee may not purchase more than $25,000 worth of common stock, valued at the start of the offering period. An employee’s participation under the 2013 Employee Stock Purchase Plan terminates upon voluntary withdrawal or upon termination of employment. Accumulated payroll deductions at the time the employee’s participation ends shall be refunded to the employee.
Although authorized, the 2013 Employee Stock Purchase Plan has not been implemented by the Company. As of December 31, 2015, no employees were participating in, and no shares have been purchased under, the 2013 Employee Stock Purchase Plan.
| 11. | Income Taxes |
The Company accounts for income taxes under ASC 740. Deferred income tax assets and liabilities are determined based upon differences between financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse.
For the years ended December 31, 2015, 2014, and 2013, the Company has not recorded a provision for federal or state income taxes as it has incurred cumulative net operating losses since inception.
As of December 31, 2015, the Company had federal and state net operating loss carryforwards of approximately $175,300,000 and $159,100,000, respectively, which were available to reduce future taxable income. The federal and state net operating loss carryforwards exclude approximately $36,600,000 and $32,400,000 of deductions related to the exercise of stock options, respectively. This amount represents an excess tax benefit and has not been included in the gross deferred tax asset reflected for net operating losses and is credited directly to additional paid-in capital when realized. The net operating loss carryforwards expire at various times beginning in 2029 for federal purposes and 2024 for state purposes.
The Company also had federal and state tax credits of approximately $6,200,000 and $5,100,000, respectively, which may be used to offset future tax liabilities. These tax credit carryforwards will expire at various times beginning in 2029 for federal purposes and 2015 for state purposes.
The net operating loss and tax credit carryforwards are subject to review and possible adjustment by the Internal Revenue Service and state tax authorities. Net operating loss and tax credit carryforwards may become subject to an annual limitation in the event of certain cumulative changes in the ownership interest of significant stockholders over a three-year period in excess of 50%, as defined under Sections 382 and 383 of the Internal Revenue Code, respectively, as well as similar state provisions. This could limit the amount of tax attributes that can be utilized annually to offset future taxable income or tax liabilities. The amount of the annual limitation is determined based on the value of the Company immediately prior to the ownership change. Subsequent ownership changes may further affect the limitation in future years. Following the Roche transaction, as discussed in detail in Note 3, the Company conducted a Section 382 study covering the period from corporate inception through April 7, 2015, which was the closing date of the Roche transaction. This study concluded that
F-29
Table of Contents
limitations on the Company’s NOL carryforwards are not restrictive, with the exception of approximately $1,500,000 of pre-March 31, 2010 NOLs. The restrictions on the NOLs are expected to free up for use by December 31, 2016.
The Company has not recorded any reserves for uncertain tax positions as of December 31, 2015 or 2014. If the Company did record a reserve, it would be a component of income tax expense. The Company has not yet conducted a study of research and development credit carryforwards. This study may result in an adjustment to the Company’s research and development credit carryforwards; however, until a study is completed and any adjustment is known, no amounts are being presented as an uncertain tax position. As of December 31, 2015, the Company had no accrued interest or penalties related to uncertain tax positions. Since the Company is in a loss carryforward position, the Company is generally subject to examination by the U.S. federal, state and local income tax authorities for all tax years in which a loss carryforward is available. The Company is not currently under examination by the Internal Revenue Service or any other jurisdictions for any tax years.
The principal components of the Company’s deferred tax assets are as follows:
| As of December 31, | ||||||||
| 2015 | 2014 | |||||||
| Deferred tax assets: |
||||||||
| Net operating loss carryforwards |
$ | 67,958 | $ | 37,245 | ||||
| Deferred revenue |
1,732 | 130 | ||||||
| Accrued bonus |
1,822 | 1,162 | ||||||
| Deferred rent |
4,789 | 4,110 | ||||||
| Nonaccrual receivables |
9,353 | 13,372 | ||||||
| Other |
3,801 | 1,702 | ||||||
| Research and development credits |
9,534 | 5,544 | ||||||
|
|
|
|
|
|||||
| Gross deferred tax assets |
98,989 | 63,265 | ||||||
| Deferred tax liability |
(6,862 | ) | (8,429 | ) | ||||
| Valuation allowance |
(92,127 | ) | (54,836 | ) | ||||
|
|
|
|
|
|||||
| Net deferred tax assets |
$ | — | $ | — | ||||
|
|
|
|
|
|||||
ASC 740 requires a valuation allowance to reduce the deferred tax assets reported if, based on the weight of available evidence, it is more likely than not that some portion or all of the deferred tax assets will not be realized. After consideration of all the evidence, both positive and negative, the Company has recorded a valuation allowance against its deferred tax assets at December 31, 2015 and 2014, respectively, because the Company’s management has determined that is it more likely than not that these assets will not be fully realized. The increase in the valuation allowance of $37,300,000 in 2015 primarily relates to the net loss incurred by the Company during that period.
A reconciliation of the income tax expense at the federal statutory tax rate to the Company’s effective income tax rate follows:
| Year Ended December 31, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Statutory tax rate |
34.0 | % | 34.0 | % | 34.0 | % | ||||||
| State taxes, net of federal benefit |
4.8 | % | 3.7 | % | 4.0 | % | ||||||
| Permanent differences |
(1.0 | %) | (1.9 | %) | (6.7 | %) | ||||||
| Research and development credits |
4.5 | % | 4.9 | % | 5.0 | % | ||||||
| Other |
(0.6 | %) | — | 0.1 | % | |||||||
| Change in valuation allowance |
(41.7 | %) | (40.7 | %) | (36.4 | %) | ||||||
|
|
|
|
|
|
|
|||||||
| Effective tax rate |
0.0 | % | 0.0 | % | 0.0 | % | ||||||
|
|
|
|
|
|
|
|||||||
F-30
Table of Contents
| 12. | Commitments and Contingencies |
One Kendall Square
In May 2010, the Company commenced a facility lease which was due to expire in October 2015. In November 2013, the Company terminated this lease effective October 31, 2013 (the “Surrender Date”). The Company paid rent, operating expenses and other charges due under the facility lease through the Surrender Date. In exchange for terminating the facility lease, the Company paid the landlord an additional $338,000 equal to two additional months of rent, operating expenses, and all other charges due under the facility lease, plus reimbursement to the landlord for a portion of the brokerage commissions incurred by the landlord in leasing the facility to a new tenant. In connection with the lease termination, the Company recorded an impairment charge of $439,000 associated with fixed assets left at the facility. The Company also reversed $290,000 of deferred rent associated with the lease. The Company recorded $730,000 of rent expense in the year ended December 31, 2013 associated with this lease.
150 Second Street
In 2013, the Company signed two separate facility leases. The first lease commenced in March 2013 and had a one year expected term which was terminated in October 2013. The second lease (the “Headquarters Lease”) for office and laboratory space at 150 Second Street in Cambridge, Massachusetts commenced in September 2013 and initially had an eight year expected term. The Headquarters Lease is subject to fixed rate escalation increases and the landlord waived the Company’s rent obligation for the first 10.5 months of the lease, having an initial value of $3,300,000. The landlord also agreed to fund up to $9,239,000 in tenant improvements. The Company recorded the tenant improvements as leasehold improvements and deferred rent on the consolidated balance sheet. Deferred rent is amortized as a reduction in rent expense over the term of the Headquarters Lease. The Company recognizes rent expense on a straight-line basis over the expected lease term. In connection with the Company’s termination of its prior lease at One Kendall Square, the rent abatement was reduced to approximately $1,841,000 and the expected term of the Headquarters Lease was reduced to 7.5 years. The Company began to record rent expense in April 2013 upon gaining access to and control of the space. Upon execution of the Headquarters Lease, the Company paid a security deposit of $1,725,000 which was reduced to approximately $864,000 in 2014. The security deposit is included in restricted cash in the accompanying balance sheet as of December 31, 2015 and 2014.
On June 30, 2014, the Company executed a Second Amendment to the Headquarters Lease, resulting in 8,164 square feet of additional space commencing in November 2014. The Company began recording rent expense upon gaining access to and control of the space in July 2014. The landlord also agreed to fund up to $1,020,500 in normal tenant improvements.
The Company recorded rent expense of $2,534,000, $2,411,000, and $1,640,000 for the years ended December 31, 2015, 2014 and 2013, respectively, associated with the Headquarters Lease.
Ten Canal Park Lease
The Company signed a facility lease (the “Lease”) on March 11, 2015 for office space at Ten Canal Park in Cambridge, Massachusetts (the “Premises”). The Lease commenced on March 12, 2015, which was the date the landlord received the Letter of Credit (as defined in the Lease), and expires on August 31, 2020. The Company will pay rent of $172,850 per month for the first year with scheduled escalating rent payments thereafter, and shall receive up to $1,995,550 from the landlord for tenant improvements to the Premises. In connection with the Lease, the Company provided a security deposit in the amount of $1,037,000, which was reduced to approximately $530,550 in June 2015. The security deposit is included in restricted cash in the accompanying balance sheet as of December 31, 2015.
The Company recorded rent expense of $1,279,000 for the year ended December 31, 2015, associated with the Lease.
F-31
Table of Contents
As of December 31, 2015, the minimum future rent payments under the Company’s lease agreements were as follows:
| 2016 |
$ | 6,362 | ||
| 2017 |
6,456 | |||
| 2018 |
6,625 | |||
| 2019 |
6,797 | |||
| 2020 |
5,910 | |||
| Thereafter |
744 | |||
|
|
|
|||
| Total minimum lease payments |
$ | 32,894 | ||
|
|
|
Supply Agreement
In July 2013, the Company entered into a five-year supply, service and support agreement (the “Supply Agreement”) with Illumina, Inc. (“Illumina”) for Illumina to provide products and services that support and can be used for the gene sequencing component of its molecular testing activities. During the term of the Supply Agreement, Illumina will supply the Company with sequencers, reagents and other consumables for use with the Illumina sequencers, and service contracts for the maintenance and repair of the sequencers.
During the term of the Supply Agreement, the Company is required to make a rolling forecast of its expected needs for reagents and other consumables, and it may place purchase orders for reagents and other consumables that conform to such forecast. Illumina may not unreasonably reject conforming purchase orders and will, in its reasonable discretion, accept additional purchase orders for quantities of reagents and other consumables beyond the Company’s forecast requirements. During each six-month period the Company has a binding obligation to purchase an amount of reagents and other consumables equal to the greater of a percentage of its six-month forecast and a fixed minimum amount. Subject to discounts that vary depending on the volume of hardware and reagents and other consumables ordered, the price for sequencers and for service contracts is based on Illumina list prices, and the price for reagents and other consumables is based on contract prices that are fixed for a set period of time and may increase thereafter subject to limitations. The Supply Agreement does not require the Company to order minimum amounts of hardware, or to use exclusively the Illumina platform for conducting its sequencing. As of December 31, 2015, the Company’s minimum remaining purchase commitment for the remainder of the term of the Supply Agreement was not lower than approximately $6,197,000.
Legal Matters
From time to time, the Company is party to litigation arising in the ordinary course of its business. As of December 31, 2015, the Company was not party to any material litigation.
| 13. | Related Party Transactions |
Roche Holdings, Inc. and its affiliates
Revenue from Roche was $13,444,000 during the year ended December 31, 2015, which primarily consisted of payments made for the reserved capacity arrangement, access to the Company’s molecular information platform, and the reimbursement of R&D costs under the R&D Collaboration Agreement. Roche-related revenue represented 14.4% of the Company’s total revenue in the year ended December 31, 2015. Costs of related party revenue from Roche were $2,736,000 during the year ended December 31, 2015, which consisted of costs incurred under the molecular information platform program within the R&D Collaboration Agreement. Deferred revenue from Roche was $3,742,000 for the year ended December 31, 2015.
Revenue from Roche was $858,000 and $854,000 during the years ended December 31, 2014 and 2013, respectively, under a previous contractual relationship between Roche and the Company. Roche-related revenue represented 1.4% and 2.9% of the Company’s total revenue in the years ended December 31, 2014 and 2013, respectively.
F-32
Table of Contents
There were no other material Roche-related transactions as of December 31, 2015 and 2014 and for each of the years ended December 31, 2015, 2014, and 2013.
Other related party transactions
The Company recognized revenue of $1,716,000 and $641,000 in the years ended December 31, 2015 and 2014, respectively, and no revenue for the year ended December 31, 2013 from an arrangement with an entity affiliated with a member of the Company’s Board executed in the year ended December 31, 2013. Of these amounts, $825,000 and $419,000 was included in accounts receivable as of December 31, 2015 and 2014, respectively.
| 14. | Selected Quarterly Financial Data (Unaudited) |
The following table contains quarterly financial information for fiscal years 2015 and 2014. The Company believes that the following information reflects all normal recurring adjustments necessary for a fair statement of the information for the periods presented.
| First Quarter |
Second Quarter |
Third Quarter |
Fourth Quarter |
|||||||||||||
| 2015 |
||||||||||||||||
| Revenue |
$ | 19,295 | $ | 22,458 | $ | 25,399 | $ | 26,051 | ||||||||
| Costs and expenses |
$ | 36,267 | $ | 55,517 | $ | 46,010 | $ | 45,164 | ||||||||
| Net loss |
$ | (16,965 | ) | $ | (33,050 | ) | $ | (20,596 | ) | $ | (19,020 | ) | ||||
| Basic and diluted net loss per share |
$ | (0.59 | ) | $ | (0.98 | ) | $ | (0.60 | ) | $ | (0.55 | ) | ||||
| 2014 |
||||||||||||||||
| Revenue |
$ | 11,455 | $ | 14,496 | $ | 16,448 | $ | 18,680 | ||||||||
| Costs and expenses |
$ | 23,596 | $ | 28,259 | $ | 29,426 | $ | 33,081 | ||||||||
| Net loss |
$ | (12,166 | ) | $ | (13,779 | ) | $ | (12,980 | ) | $ | (13,297 | ) | ||||
| Basic and diluted net loss per share |
$ | (0.44 | ) | $ | (0.49 | ) | $ | (0.46 | ) | $ | (0.47 | ) | ||||
F-33
