Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - KERYX BIOPHARMACEUTICALS INC | d147280dex991.htm |
| 8-K - FORM 8-K - KERYX BIOPHARMACEUTICALS INC | d147280d8k.htm |

Fourth Quarter and Year End 2015 Financial Results February 25, 2016 Keryx Biopharmaceuticals, Inc. Exhibit 99.2

Safe Harbor Statement Various remarks that we make about our future expectations, plans and prospects constitute forward-looking statements for purposes of the safe harbor provisions under the Private Securities Litigation Reform Act of 1995. Important factors may cause our actual results to differ materially, including: whether Auryxia™ (ferric citrate), will be successfully marketed in the U.S.; whether we can successfully obtain reimbursement coverage for Auryxia; whether we can adjust our operating expense to projected levels while maintaining our current clinical and commercial activities; whether we will be able to identify and negotiate acceptable terms with a commercialization partner in the E.U.; whether a partner can successfully launch Fexeric® in the E.U.; whether Riona® will be successfully marketed by our Japanese partner, Japan Tobacco, Inc. and Torii Pharmaceutical Co., Ltd; the risk that we may not be successful in the development of Auryxia for the treatment of iron deficiency anemia in non-dialysis chronic kidney disease patients; and other risk factors identified from time to time in our reports filed with the Securities and Exchange Commission. These and other important factors that may affect our results are discussed under the heading “Risk Factors” in public filings including our 2014 Annual Report on Form 10-K, Form 10-Qs, as well as other filings we periodically make with the SEC. In addition, any forward-looking statements made during this presentation speak only as of the date of this presentation. While we may update these forward-looking statements to reflect events or circumstances that occur after this date, we specifically disclaim any obligation to do so, even if our estimates and expectations change.
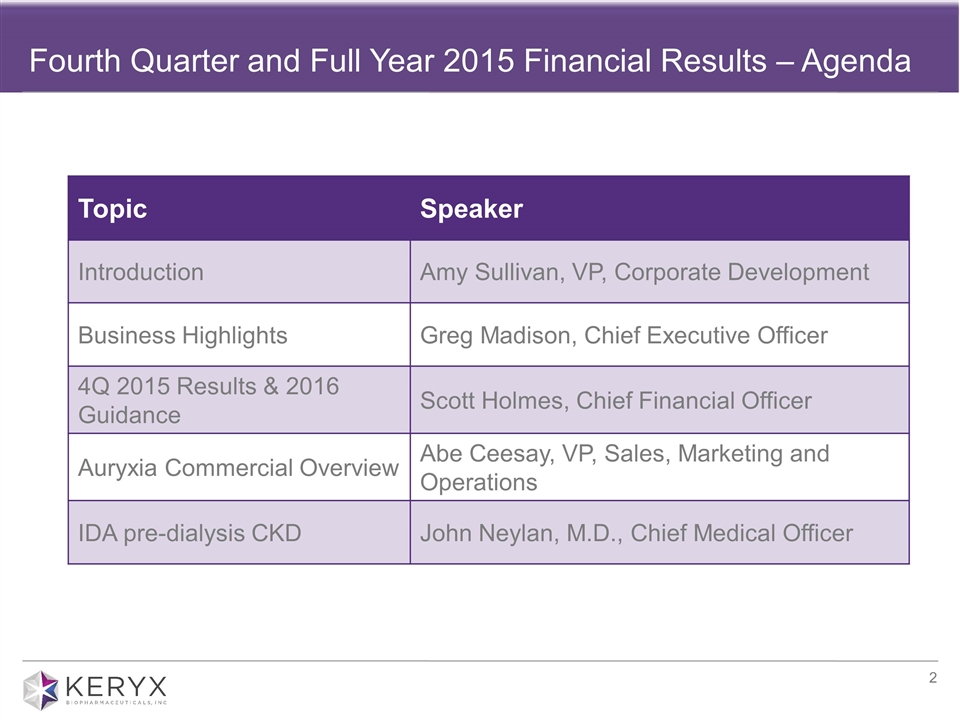
Fourth Quarter and Full Year 2015 Financial Results – Agenda Topic Speaker Introduction Amy Sullivan, VP, Corporate Development Business Highlights Greg Madison, Chief Executive Officer 4Q 2015 Results & 2016 Guidance Scott Holmes, Chief Financial Officer Auryxia Commercial Overview Abe Ceesay, VP, Sales, Marketing and Operations IDA pre-dialysis CKD John Neylan, M.D., Chief Medical Officer
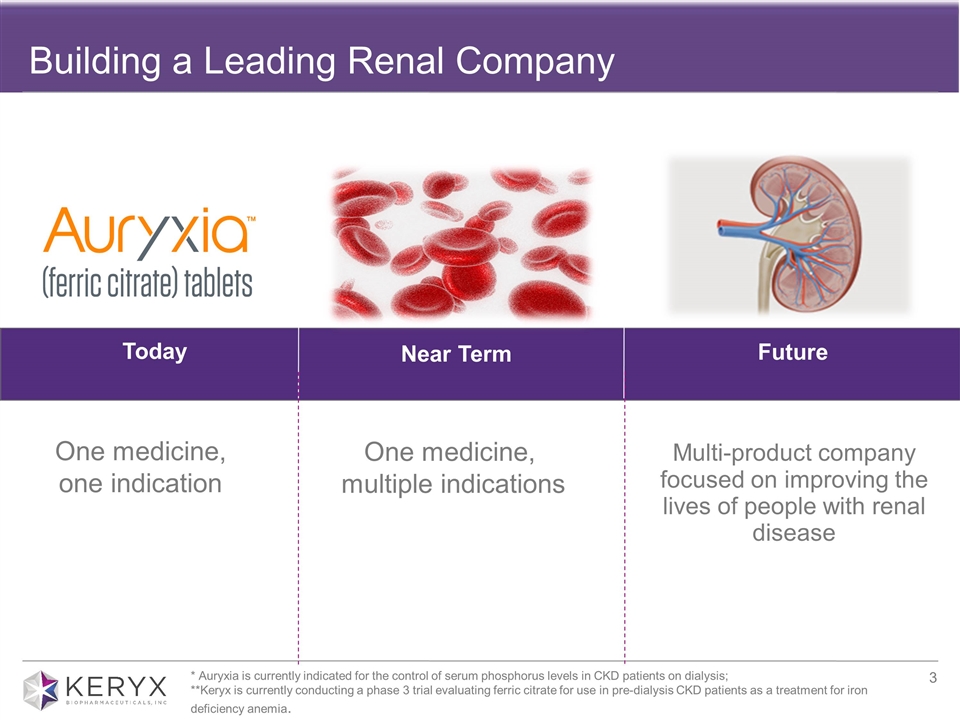
Building a Leading Renal Company Today Near Term Future One medicine, multiple indications Multi-product company focused on improving the lives of people with renal disease One medicine, one indication * Auryxia is currently indicated for the control of serum phosphorus levels in CKD patients on dialysis; **Keryx is currently conducting a phase 3 trial evaluating ferric citrate for use in pre-dialysis CKD patients as a treatment for iron deficiency anemia.
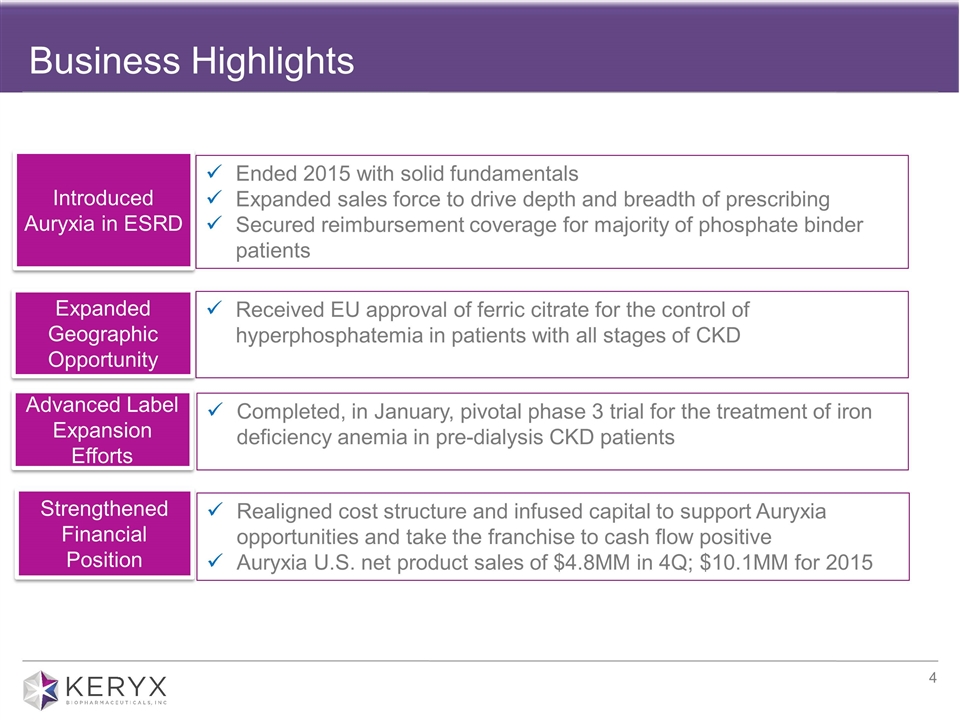
Business Highlights Ended 2015 with solid fundamentals Expanded sales force to drive depth and breadth of prescribing Secured reimbursement coverage for majority of phosphate binder patients Received EU approval of ferric citrate for the control of hyperphosphatemia in patients with all stages of CKD Completed, in January, pivotal phase 3 trial for the treatment of iron deficiency anemia in pre-dialysis CKD patients Realigned cost structure and infused capital to support Auryxia opportunities and take the franchise to cash flow positive Auryxia U.S. net product sales of $4.8MM in 4Q; $10.1MM for 2015 Introduced Auryxia in ESRD Expanded Geographic Opportunity Advanced Label Expansion Efforts Strengthened Financial Position
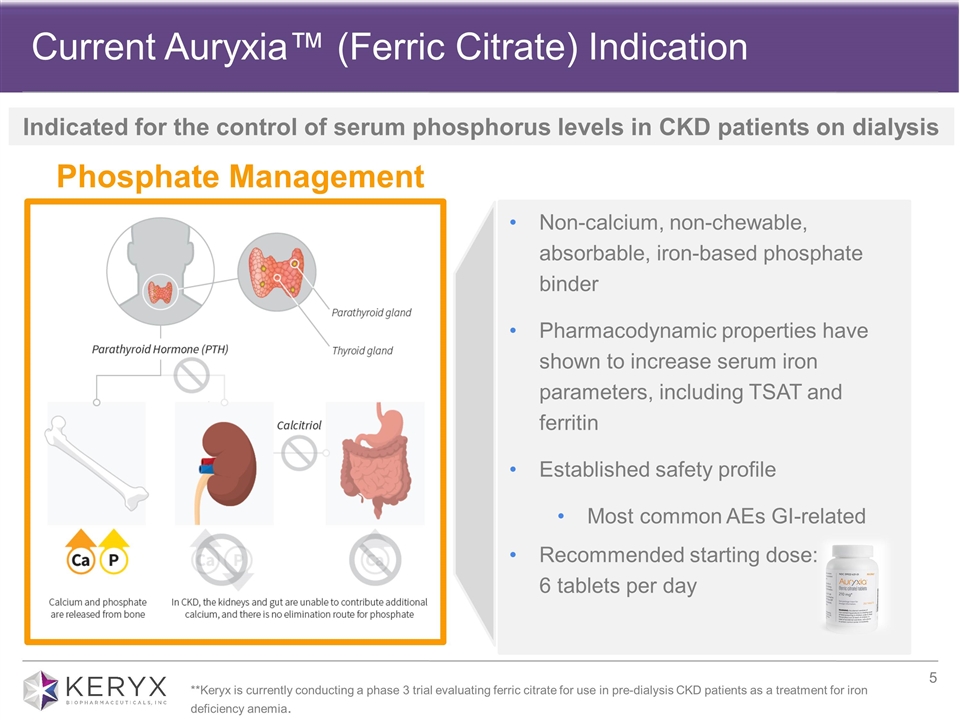
Current Auryxia™ (Ferric Citrate) Indication Phosphate Management Non-calcium, non-chewable, absorbable, iron-based phosphate binder Pharmacodynamic properties have shown to increase serum iron parameters, including TSAT and ferritin Established safety profile Most common AEs GI-related Recommended starting dose: 6 tablets per day **Keryx is currently conducting a phase 3 trial evaluating ferric citrate for use in pre-dialysis CKD patients as a treatment for iron deficiency anemia. Indicated for the control of serum phosphorus levels in CKD patients on dialysis
A Potential New Treatment for Iron Deficiency Anemia Anemia Management In January, completed pivotal phase 3 trial evaluating ferric citrate for treatment of IDA in pre-dialysis stages 3-5 CKD Plan to provide top-line safety and efficacy results in early 2Q 2016 If successful, plan to: submit regulatory application in 3Q 2016 present data at medical conference in 4Q 2016 * Auryxia is currently indicated for the control of serum phosphorus levels in CKD patients on dialysis; **Keryx is currently conducting a phase 3 trial evaluating ferric citrate for use in pre-dialysis CKD patients as a treatment for iron deficiency anemia.
A Potential New Treatment Category for CKD Phosphate Management Anemia Management * Auryxia is currently indicated for the control of serum phosphorus levels in CKD patients on dialysis; **Keryx is currently conducting a phase 3 trial evaluating ferric citrate for use in pre-dialysis CKD patients as a treatment for iron deficiency anemia.
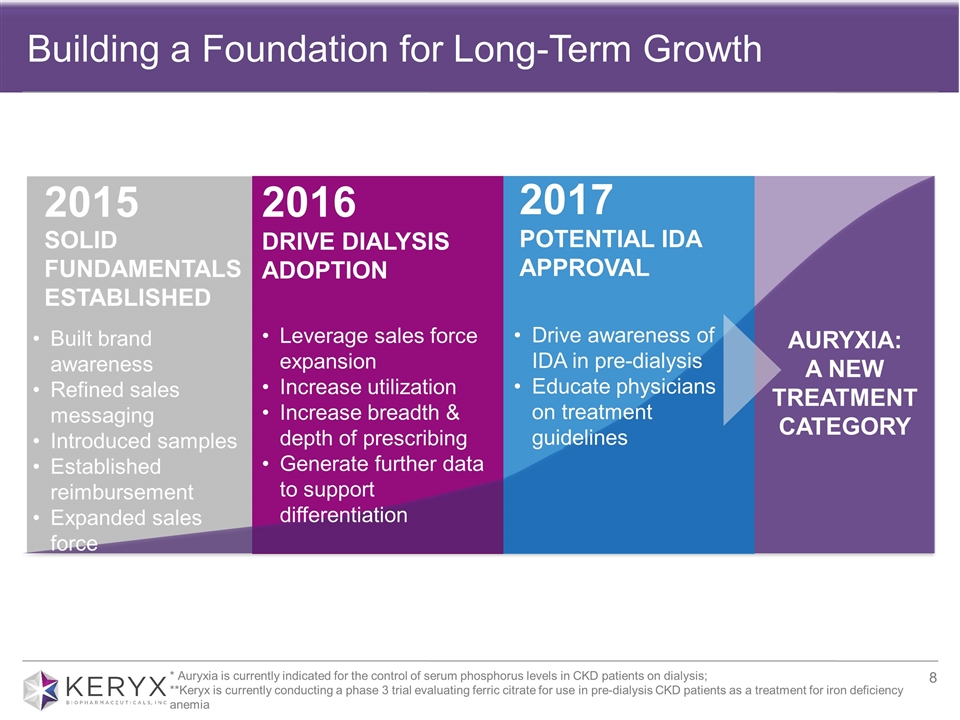
Building a Foundation for Long-Term Growth SOLID FUNDAMENTALS ESTABLISHED 2015 2017 POTENTIAL IDA APPROVAL 2016 DRIVE DIALYSIS ADOPTION AURYXIA: A NEW TREATMENT CATEGORY Leverage sales force expansion Increase utilization Increase breadth & depth of prescribing Generate further data to support differentiation Drive awareness of IDA in pre-dialysis Educate physicians on treatment guidelines 2017 POTENTIAL IDA APPROVAL * Auryxia is currently indicated for the control of serum phosphorus levels in CKD patients on dialysis; **Keryx is currently conducting a phase 3 trial evaluating ferric citrate for use in pre-dialysis CKD patients as a treatment for iron deficiency anemia Built brand awareness Refined sales messaging Introduced samples Established reimbursement Expanded sales force

Financial Highlights Scott Holmes Chief Financial Officer
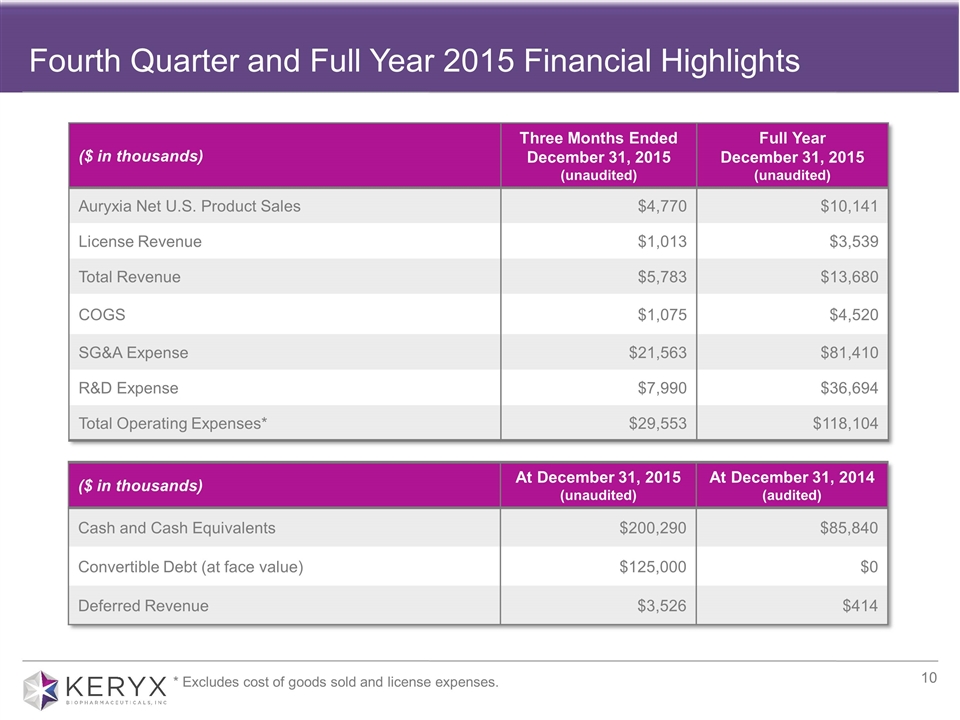
Fourth Quarter and Full Year 2015 Financial Highlights ($ in thousands) Three Months Ended December 31, 2015 (unaudited) Full Year December 31, 2015 (unaudited) Auryxia Net U.S. Product Sales $4,770 $10,141 License Revenue $1,013 $3,539 Total Revenue $5,783 $13,680 COGS $1,075 $4,520 SG&A Expense $21,563 $81,410 R&D Expense $7,990 $36,694 Total Operating Expenses* $29,553 $118,104 ($ in thousands) At December 31, 2015 (unaudited) At December 31, 2014 (audited) Cash and Cash Equivalents $200,290 $85,840 Convertible Debt (at face value) $125,000 $0 Deferred Revenue $3,526 $414 * Excludes cost of goods sold and license expenses.
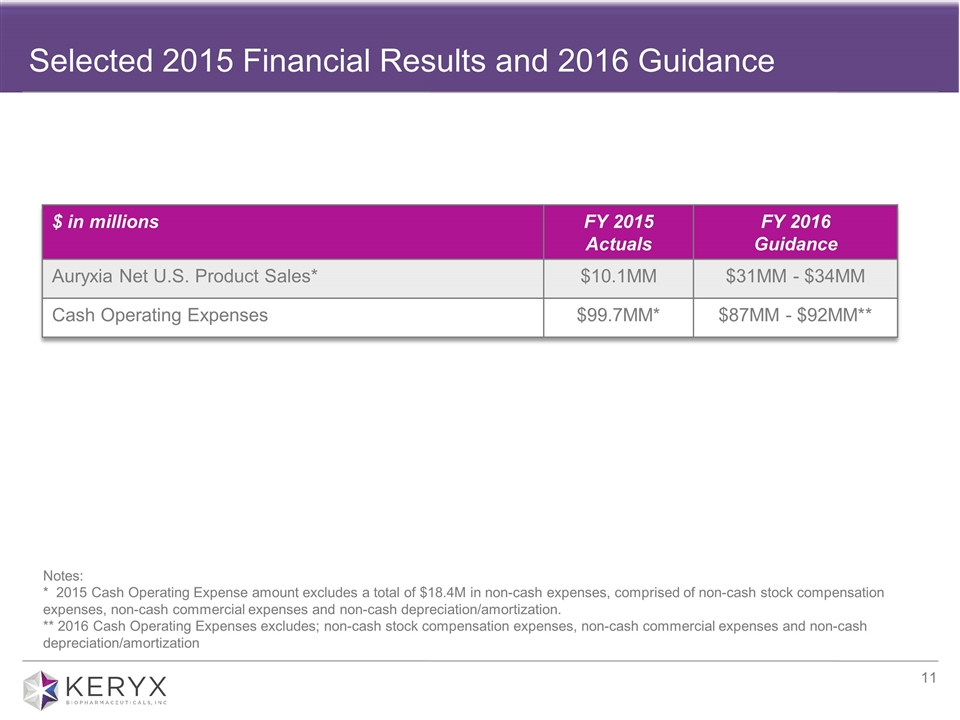
Selected 2015 Financial Results and 2016 Guidance $ in millions FY 2015 Actuals FY 2016 Guidance Auryxia Net U.S. Product Sales* $10.1MM $31MM - $34MM Cash Operating Expenses $99.7MM* $87MM - $92MM** Notes: * 2015 Cash Operating Expense amount excludes a total of $18.4M in non-cash expenses, comprised of non-cash stock compensation expenses, non-cash commercial expenses and non-cash depreciation/amortization. ** 2016 Cash Operating Expenses excludes; non-cash stock compensation expenses, non-cash commercial expenses and non-cash depreciation/amortization

Commercial Progress Abe Ceesay VP, Sales, Marketing and Operations
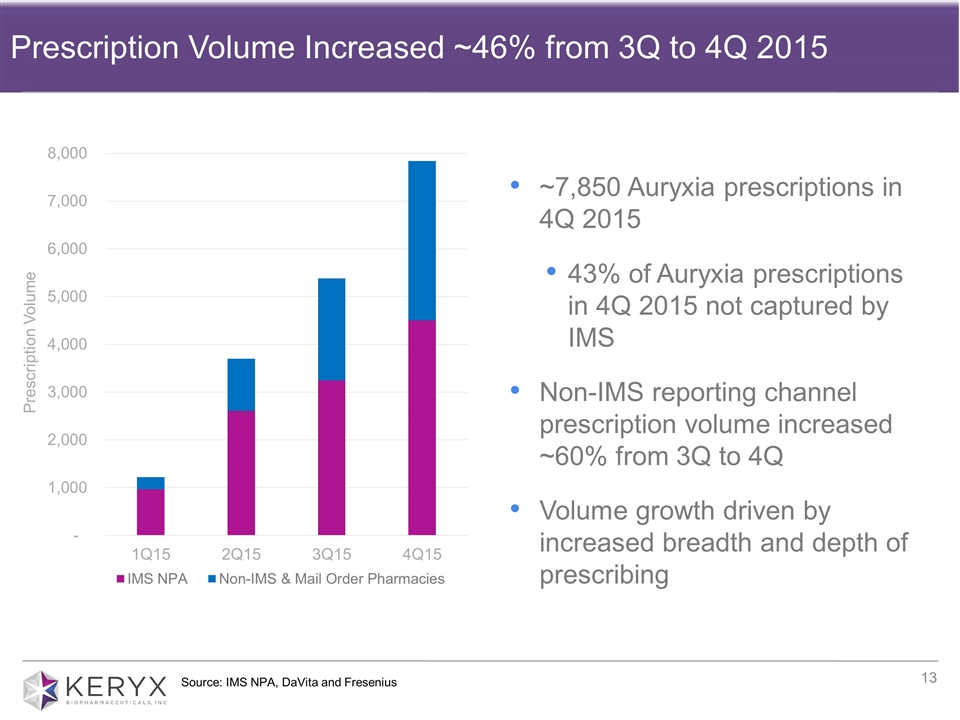
Source: IMS NPA, DaVita and Fresenius Prescription Volume Increased ~46% from 3Q to 4Q 2015 ~7,850 Auryxia prescriptions in 4Q 2015 43% of Auryxia prescriptions in 4Q 2015 not captured by IMS Non-IMS reporting channel prescription volume increased ~60% from 3Q to 4Q Volume growth driven by increased breadth and depth of prescribing
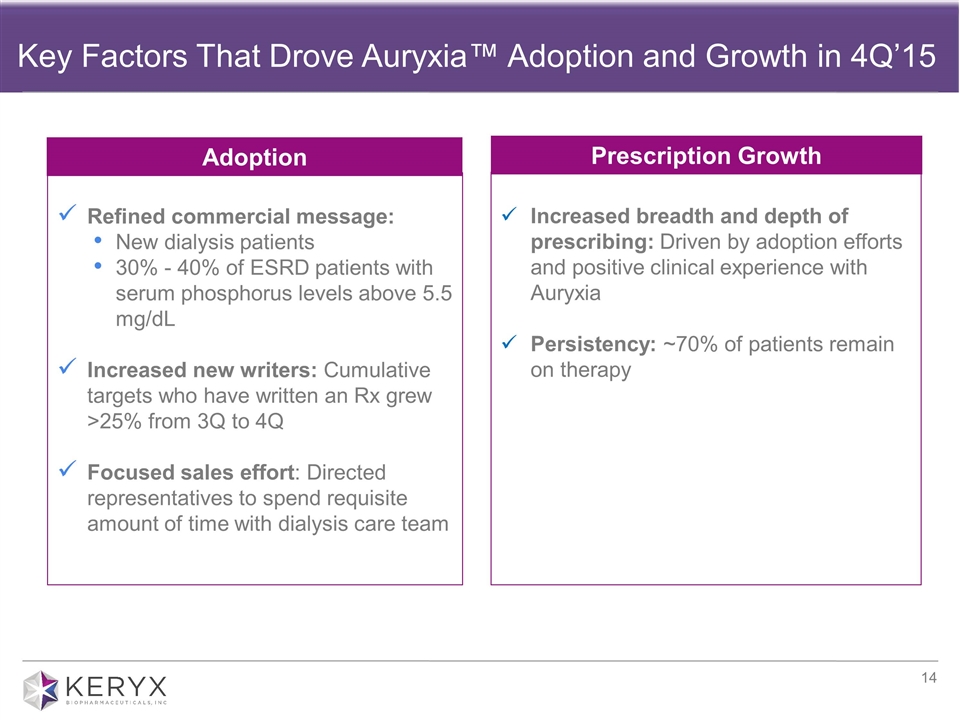
Key Factors That Drove Auryxia™ Adoption and Growth in 4Q’15 Refined commercial message: New dialysis patients 30% - 40% of ESRD patients with serum phosphorus levels above 5.5 mg/dL Increased new writers: Cumulative targets who have written an Rx grew >25% from 3Q to 4Q Focused sales effort: Directed representatives to spend requisite amount of time with dialysis care team Increased breadth and depth of prescribing: Driven by adoption efforts and positive clinical experience with Auryxia Persistency: ~70% of patients remain on therapy Prescription Growth Adoption
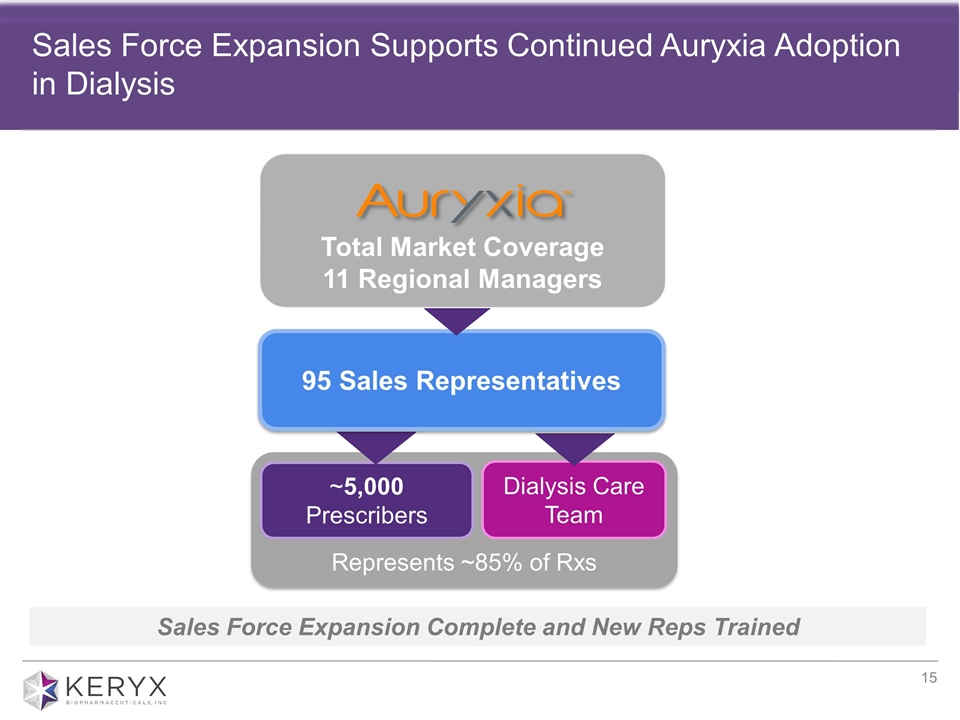
Represents ~85% of Rxs Sales Force Expansion Supports Continued Auryxia Adoption in Dialysis ~5,000 Prescribers Sales Force Expansion Complete and New Reps Trained Dialysis Care Team 95 Sales Representatives Total Market Coverage 11 Regional Managers
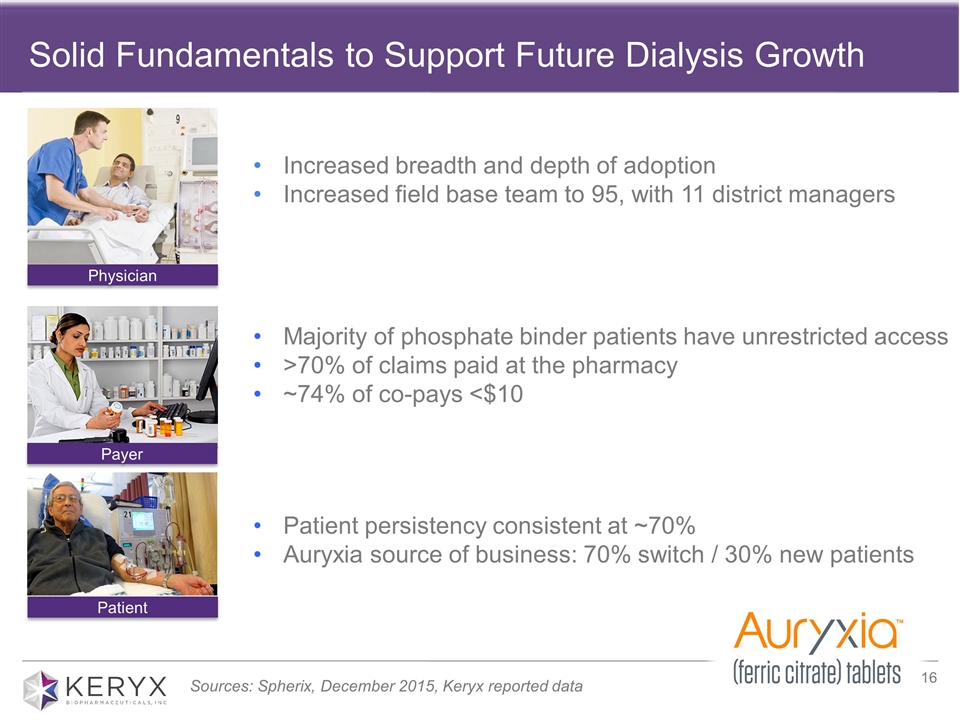
Solid Fundamentals to Support Future Dialysis Growth Physician Payer Patient Increased breadth and depth of adoption Increased field base team to 95, with 11 district managers Majority of phosphate binder patients have unrestricted access >70% of claims paid at the pharmacy ~74% of co-pays <$10 Patient persistency consistent at ~70% Auryxia source of business: 70% switch / 30% new patients Sources: Spherix, December 2015, Keryx reported data

Label Expansion Efforts John Neylan, M.D. Chief Medical Officer
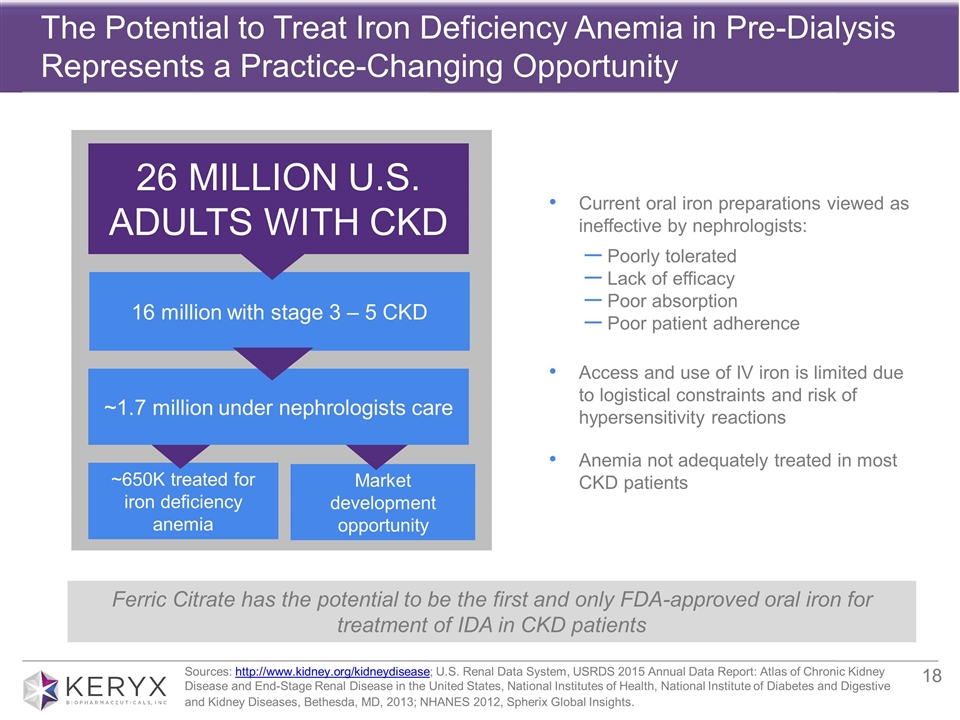
The Potential to Treat Iron Deficiency Anemia in Pre-Dialysis Represents a Practice-Changing Opportunity Sources: http://www.kidney.org/kidneydisease; U.S. Renal Data System, USRDS 2015 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2013; NHANES 2012, Spherix Global Insights. 26 MILLION U.S. ADULTS WITH CKD 16 million with stage 3 – 5 CKD ~650K treated for iron deficiency anemia Current oral iron preparations viewed as ineffective by nephrologists: Poorly tolerated Lack of efficacy Poor absorption Poor patient adherence Access and use of IV iron is limited due to logistical constraints and risk of hypersensitivity reactions Anemia not adequately treated in most CKD patients Market development opportunity ~1.7 million under nephrologists care Ferric Citrate has the potential to be the first and only FDA-approved oral iron for treatment of IDA in CKD patients
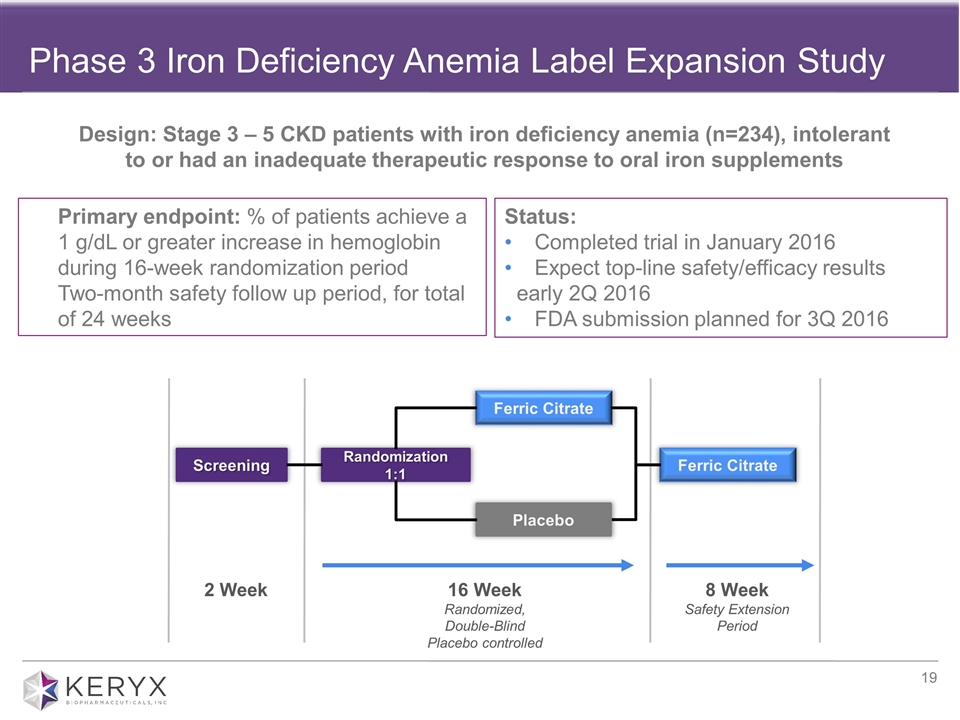
Phase 3 Iron Deficiency Anemia Label Expansion Study Primary endpoint: % of patients achieve a 1 g/dL or greater increase in hemoglobin during 16-week randomization period Two-month safety follow up period, for total of 24 weeks Screening Randomization 1:1 2 Week 16 Week Randomized, Double-Blind Placebo controlled 8 Week Safety Extension Period Ferric Citrate Placebo Ferric Citrate Status: Completed trial in January 2016 Expect top-line safety/efficacy results early 2Q 2016 FDA submission planned for 3Q 2016 Design: Stage 3 – 5 CKD patients with iron deficiency anemia (n=234), intolerant to or had an inadequate therapeutic response to oral iron supplements
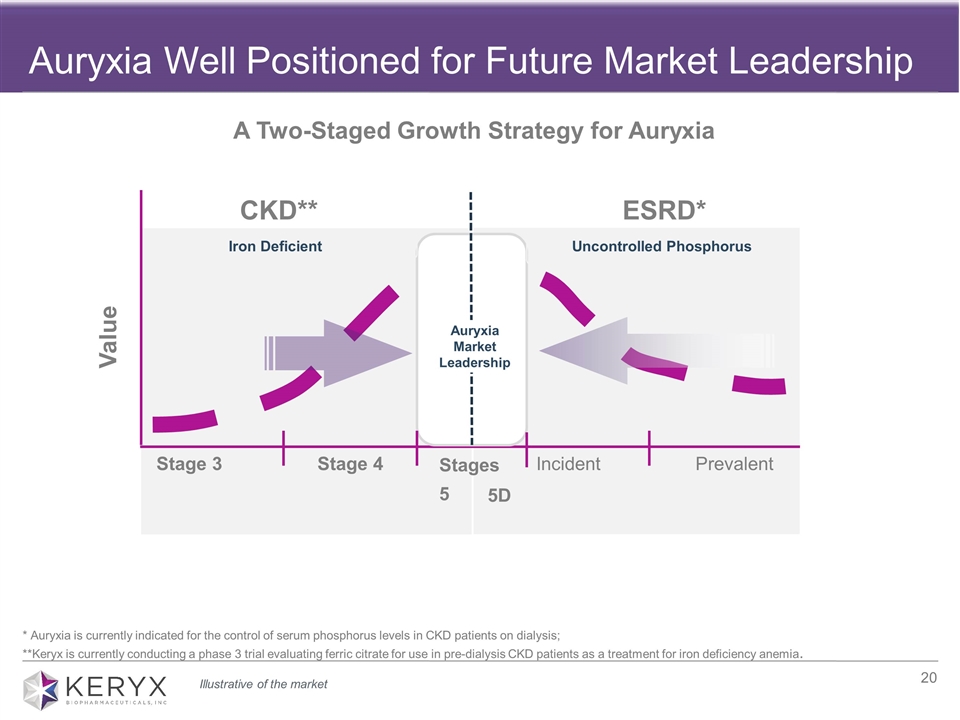
Auryxia Well Positioned for Future Market Leadership CKD** ESRD* Stage 3 Stage 4 Iron Deficient Value A Two-Staged Growth Strategy for Auryxia Uncontrolled Phosphorus * Auryxia is currently indicated for the control of serum phosphorus levels in CKD patients on dialysis; **Keryx is currently conducting a phase 3 trial evaluating ferric citrate for use in pre-dialysis CKD patients as a treatment for iron deficiency anemia. Auryxia Market Leadership Illustrative of the market Stages 5 5D Incident Prevalent
Priorities for the Future Drive Auryxia’s path to success in dialysis: Increase awareness Drive breadth and depth of prescribing Address the unmet need in pre-dialysis CKD patients with iron deficiency anemia** Expand global reach of ferric citrate * Auryxia is currently indicated for the control of serum phosphorus levels in CKD patients on dialysis; **If approved for the treatment of iron deficiency anemia in pre-dialysis CKD patients. Keryx is currently conducting a phase 3 trial evaluating ferric citrate for use in pre-dialysis CKD patients as a treatment for iron deficiency anemia. Establish Auryxia as a new treatment category Understand if we can better manage kidney patient care during the transition to dialysis with Auryxia treatment in the pre-dialysis setting 2017 and Beyond 2016

Fourth Quarter and Year End 2015 Financial Results February 25, 2016 Keryx Biopharmaceuticals, Inc.
