Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Corium International, Inc. | a16-3487_18k.htm |
| EX-99.1 - EX-99.1 - Corium International, Inc. | a16-3487_1ex99d1.htm |
Exhibit 99.2
Corplex™ Donepezil TDS Phase 1 Interim Topline Results Corium International, Inc.

Forward-looking Statements This presentation and the accompanying oral presentation contain forward-looking statements within the meaning of the U.S. Private Securities Litigation Reform Act of 1995, including statements regarding our business strategy, clinical trial timing and plans, the achievement of clinical and commercial milestones, and the advancement of our technologies and our proprietary, co-developed and partnered products and product candidates. Forward-looking statements are based on management’s current expectations and projections and are subject to risks and uncertainties, which may cause Corium’s actual results to differ materially from the statements contained herein. Further information on potential risk factors that could affect Corium’s business and its results are detailed in Corium's Annual Report on Form 10-K for the year ended September 30, 2015, filed with the Securities and Exchange Commission on December 16, 2015, and other reports as filed from time to time with the Securities and Exchange Commission. Undue reliance should not be placed on forward-looking statements, especially guidance on future financial or operating performance, which speaks only as of the date they are made. Corium undertakes no obligation to update publicly any forward-looking statements to reflect new information, events or circumstances after the date they were made or to reflect the occurrence of unanticipated events.

Phase 1 Study Overview Open label, two-period crossover study Two sequential treatments: Donepezil TDS, single-dose, once-weekly patch targeted to deliver 5 mg /day Oral Aricept 5 mg as comparator, one 5 mg tablet administered once daily for 7 days 9 healthy volunteers (50-80 years of age) received both treatments Primary endpoints: Assess transdermal feasibility Compare Donepezil TDS and oral Aricept PK Secondary endpoints: Safety Skin tolerability

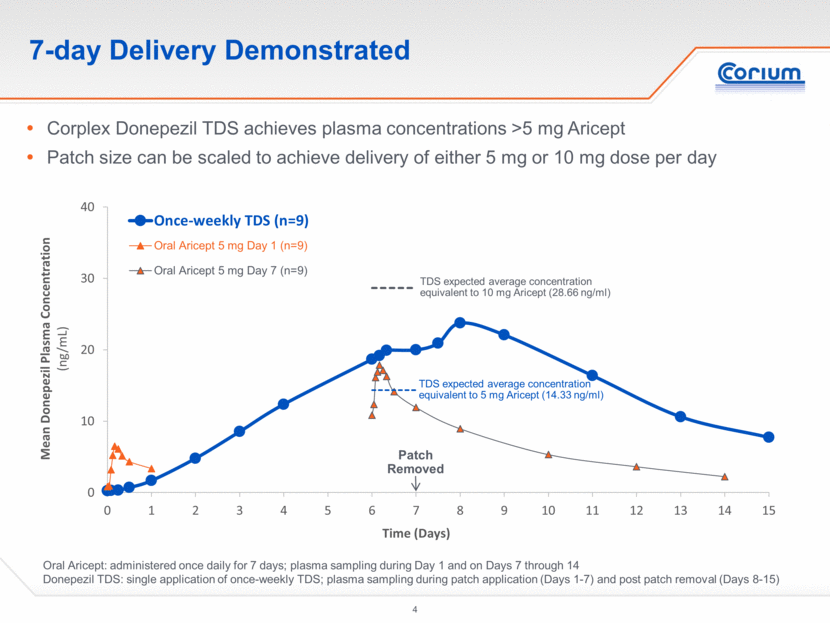
7-day Delivery Demonstrated Oral Aricept: administered once daily for 7 days; plasma sampling during Day 1 and on Days 7 through 14 Donepezil TDS: single application of once-weekly TDS; plasma sampling during patch application (Days 1-7) and post patch removal (Days 8-15) Corplex Donepezil TDS achieves plasma concentrations >5 mg Aricept Patch size can be scaled to achieve delivery of either 5 mg or 10 mg dose per day Patch Removed TDS expected average concentration equivalent to 10 mg Aricept (28.66 ng/ml) TDS expected average concentration equivalent to 5 mg Aricept (14.33 ng/ml)
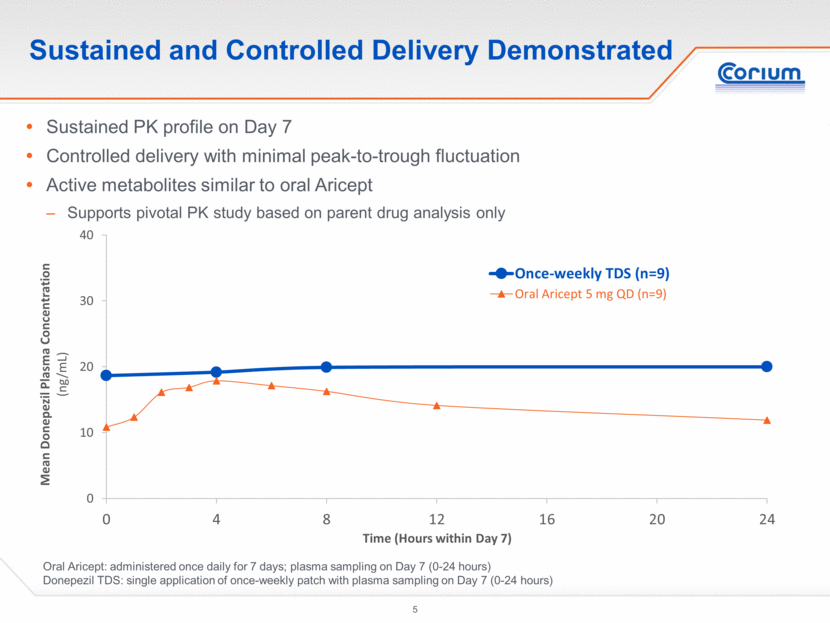
Sustained and Controlled Delivery Demonstrated Oral Aricept: administered once daily for 7 days; plasma sampling on Day 7 (0-24 hours) Donepezil TDS: single application of once-weekly patch with plasma sampling on Day 7 (0-24 hours) Sustained PK profile on Day 7 Controlled delivery with minimal peak-to-trough fluctuation Active metabolites similar to oral Aricept Supports pivotal PK study based on parent drug analysis only 0 10 20 30 40 0 4 8 12 16 20 24 Mean Donepezil Plasma Concentration ( ng/mL) Time (Hours within Day 7) Once-weekly TDS (n=9) Oral Aricept 5 mg QD (n=9)
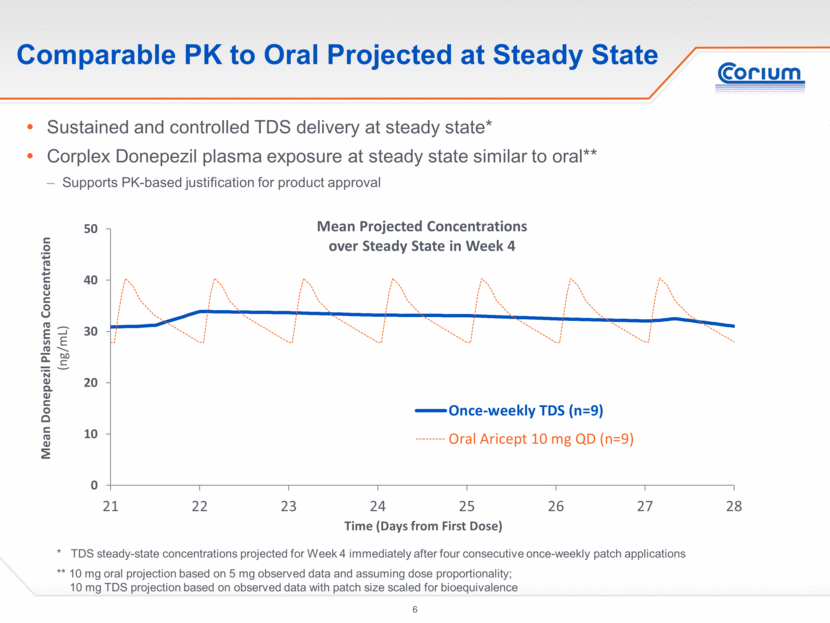
Sustained and controlled TDS delivery at steady state* Corplex Donepezil plasma exposure at steady state similar to oral** Supports PK-based justification for product approval Comparable PK to Oral Projected at Steady State * TDS steady-state concentrations projected for Week 4 immediately after four consecutive once-weekly patch applications ** 10 mg oral projection based on 5 mg observed data and assuming dose proportionality; 10 mg TDS projection based on observed with patch size scaled for bioequivalence 0 10 20 30 40 50 21 22 23 24 25 26 27 28 Mean Donepezil Plasma Concentration (ng/mL ) Time ( Days from First Dose) Mean Projected Concentrations over Steady State in Week 4 Once-weekly TDS (n=9) Oral Aricept 10 mg QD (n=9)
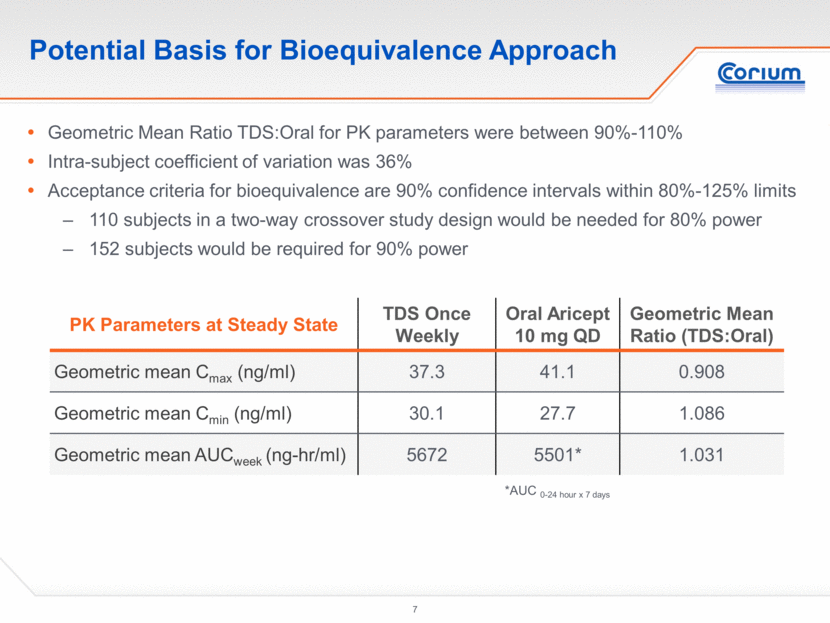
Potential Basis for Bioequivalence Approach Geometric Mean Ratio TDS:Oral for PK parameters were between 90%-110% Intra-subject coefficient of variation was 36% Acceptance criteria for bioequivalence are 90% confidence intervals within 80%-125% limits 110 subjects in a two-way crossover study design would be needed for 80% power 152 subjects would be required for 90% power PK Parameters at Steady State TDS Once Weekly Oral Aricept 10 mg QD Geometric Mean Ratio (TDS:Oral) Geometric mean Cmax (ng/ml) 37.3 41.1 0.908 Geometric mean Cmin (ng/ml) 30.1 27.7 1.086 Geometric mean AUCweek (ng-hr/ml) 5672 5501* 1.031 *AUC 0-24 hour x 7 days
Acceptable Safety and Skin Tolerability Local skin tolerability No/mild skin irritation (erythema) Erythema transient and self-resolving Mild and self-resolving itching (pruritus) in some subjects Systemic safety Adverse events characteristic of Aricept and expected for the dosages used No adverse events unique to transdermal delivery

Key Phase 1 Conclusions Acceptable safety and skin tolerability Sustained and controlled PK in target age group (50-80) with once-weekly patch Achievement of plasma concentrations in excess of 5 mg oral Aricept Mean plasma concentrations at steady state projected to approximate 10 mg oral Similar active metabolite profile to oral Steady-state projections show similar donepezil exposure to oral Data supports pre-IND discussions with FDA Estimated timing for pre-IND meeting Q2 2016 Results support advancing into Phase 1b to finalize formulation/patch size for 10 mg/day dose Expected start of Phase 1b: March 2016 All in vitro work for Phase 1b has been completed; clinical supply manufacturing has commenced
Nasdaq: CORI Corium International, Inc.