Attached files
| file | filename |
|---|---|
| 8-K - 8-K - PALISADE BIO, INC. | v429794_8k.htm |
| EX-99.01 - EXHIBIT 99.01 - PALISADE BIO, INC. | v429794_ex99-01.htm |
Exhibit 99.02

Phacilitate Cell & Gene Therapy World
January 25-27, 2016 Washington D.C.

NEURALSTEM, INC.
Safe Harbor Statement
Safe Harbor statements under the Private Securities Litigation Reform Act of 1995: This presentation contains forward-looking statements as defined in Section 27A of the Securities Act of 1933 as amended, and section 21E of the Securities Exchange Act of 1934, as amended. Such forward-looking statements are based upon Neuralstem, Inc.’s management’s current expectations, estimates, beliefs, assumptions, and projections about Neuralstem’s business and industry. Words such as “anticipates,” “expects,” “intends,” “plans,” “predicts,” “believes,” “seeks,” “estimates,” “may,” “will,” “should,” “would,” “potential,” “continue,” and variations of these words (or negatives of these words) or similar expressions, are intended to identify forward-looking statements. In addition, any statements that refer to expectations, projections, or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking statements. These forward-looking statements are not guarantees of future performance and are subject to certain risks, uncertainties, and assumptions that are difficult to predict. Therefore, our actual results could differ materially and adversely from those expressed in any forward-looking statements as a result of various risk factors. These risks and uncertainties include the risks associated with the effect of changing economic conditions, trends in the products markets, variations in Neuralstem’s cash flow, market acceptance risks, technical development risks and other risk factors detailed in Neuralstem’s Securities and Exchange Commission filings. For links to SEC documents please visit the company’s Web site: neuralstem.com.
For links to SEC documents please visit the company’s Web site: neuralstem.com.

Overview
Neuralstem’s proprietary technology uses regionally specific
neural stem cells for the development of CNS therapies.
| · | Neuralstem (Nasdaq: CUR) founded in 1996 |
| · | Human neural stem cell technology, discovered by its founding Scientist and Chairman, Karl Johe, Ph.D., while at NINDS at NIH |
| · | Issued patents worldwide |
| · | Two different product pipelines and fields: |
| · | Regenerative therapies: Injection of lab-cultured neural stem cells into CNS for treatment of neurodegenerative diseases -NSI-566 for the treatment of ALS, cSCI, & stroke |
· Pharmaceuticals: Small molecule compounds for psychiatric and neurodegenerative diseases -NSI-189 for MDD 2

Dual Platform Pipeline
Lead Small Molecule Asset Targeting Depression Cell therapy targeting high unmet medical need populations

| Clinical Development History/Plan of HSSC | ||||||
| (Human Spinal Cord-Derived Neural Stem Cells) | ||||||
| Cell Line | Indication | Location | Trial | ROA | Doses | Study Status |
| Phase | ||||||
| NSI-566, CCB | ALS | USA | I | Intraspinal | 0.5x106/5 inj- | Completed in Feb |
| 061005 | (n=15) | 1.5x106/15 inj | 2013 | |||
| NSI-566, CCB | ALS | USA | II (n=15) | Intraspinal | 2x106/10 inj- | Completed in Dec |
| 061005 | 16x106/40 inj | 2015 | ||||
| NSI-566, CCB | ALS | USA | IIb | Intraspinal | 12x106/40 inj | Update Spring 2016 |
| 141026 | (n=TBD) | |||||
| NSI-566, CCB | SCI-chronic (12- | USA | I | Intraspinal | 1.2x106/6 inj | Group A completed in |
| 061005 | 24 months post | (n=8) | Jan 2016 | |||
| injury) | ||||||
| NSI-566, CCB | Stroke-chronic | China | I | Intracerebral | 10x106/15 inj- | Group C dosed |
| SZ | (4-24 months | (n=9) | 72x106/45 inj | 1Q 2016 | ||
| post injury) | ||||||
| NSI-566, CCB | Stroke-chronic | China | II/III | Intracerebral | 72x106/45 inj | Planned start in 2H |
| SZ | (6-24 months | (n=TBD) | 2016 | |||
| post injury) | ||||||
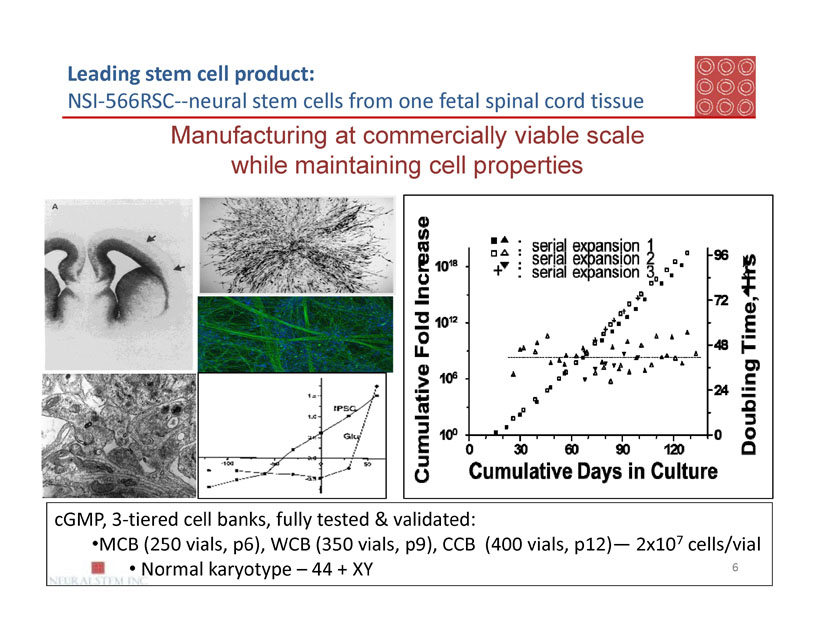
Leading stem cell product:
NSI-566RSC--neural stem cells from one fetal spinal cord tissue
Manufacturing at commercially viable scale
while maintaining cell properties
cGMP, 3-tiered cell banks, fully tested & validated:
· MCB (250 vials, p6), WCB (350 vials, p9), CCB (400 vials, p12)— 2x107cells/vial
· Normal karyotype – 44 + XY

NSI-566: SOD-1 Rat Model of ALS
· Koliatsos et al.,
Johns Hopkins
| · | Marsala et al., UCSD |
| 1. | Xu L, et al. Neurosci Lett. 2011; 494(3): 222-6. |
| 2. | Xu L, et al. J Comp Neurol. 2009; 514(4):297-309. |
| 3. | Yan J, et al. PLoS Med. 2007 4(2): e39. |
| 4. | Xu L, et al. Transplantation. 2006; 82(7):865-75. |
| 5. | Yan J, et al. Stem Cells. 2006; 24(8):1976-85. |
| 6. | Hefferan MP, et al. PLoS One. 2012;7(8):e42614. |
| 7. | Hefferan MP, et al. Cell Transplant. 2011;20(8):1153-61. |

NSI-566: Intraspinal Injections in Mini-pigs:
· Boulis et al, Emory
· Marsala et al., UCSD
| 1. | Gutierrez J, et al. Neurosurgery. 2015 Oct;77(4):604-12; discussion 612. |
| 2. | Federici T, et al. J Vis Exp. 2012 Dec 7;(70):e4371. |
| 3. | Riley JP, et al. Neurosurgery. 2011 Dec;69(2 Suppl Operative):ons147-54; discussion ons155. |
| 4. | Raore B, et al. Spine (Phila Pa 1976). 2011;36(3):E164-71. |
| 5. | Dolezalova D, et al. J Comp Neurol. 2014; 522(12):2784-801. |
| 6. | Usvald D, et al. Cell Transplant. 2010;19(9):1103-22. |

NSI-566: ALS Phase I
A Phase I, Open-label, First-in-human, Feasibility
and Safety Study of Human
Spinal Cord derived Neural Stem Cell
Transplantation for the Treatment of ALS
Eva L. Feldman1, Jonathan D. Glass2, Nicholas M. Boulis2, Thais Federici2, MeraidaPolak2, C. Kelly2and Karl Johe3
1University of Michigan, Ann Arbor, MI 2Emory University, 3Neuralstem, Inc

NSI-566: ROA intraspinal injections
| 1. | Feldman EL, et al. Ann Neurol. 2014 Mar;75(3):363-73. |
| 2. | Riley J, et al. Neurosurgery. 2014 Jan;74(1):77-87. |

NSI-566: Long-term graft survival with transient immunosuppression
| # of | # of | # of Days IM Meds | ||||
| days on | days on | |||||
| Patient | FK506 | MMF | Discontinued | |||
| number | ||||||
| Gender | before death | Survival Days | % Donor DNA | |||
| 1 | M | 177 | 165 | 216 | 394 | 0.06 – 5.40 |
| 2 | M | 107 | 503 | 67 | 572 | 0.18 – 0.93 |
| 3 | M | 259 | 259 | 0 | 259 | 0.03 – 2.39 |
| 4 | M | 189 | 192 | 133 | 325 | 0.07 – 4.20 |
| 5 | M | 94 | 283 | 638 | 921 | 0.14 – 0.67 |
| 6 | F | 139 | 134 | 57 | 196 | 0.06 - 0.96 |
| FK506 = tacrolimus, MMF=mycophenolate mofetil, IM=immunomodulatory | ||||||
Tadesse T, et al. Ann Clin Transl Neurol. 2014 Nov;1(11):900-8.

NSI-566 Phase I
Clear treatment-emergent improvement of function
Feldman EL, et al. Ann Neurol. 2014 Mar;75(3):363-73.

NSI-566: ALS Phase II
A Phase II, Open-label, Dose Escalation and Safety Study of Human Spinal Cord derived Neural Stem Cell Transplantation for the Treatment of Amyotrophic Lateral Sclerosis
Study Sites: University of Michigan, Emory University, Mass General Hospital
N=15 of variable disease profiles
Up to 16 million cells injected, intraspinal, C3-C5/L2-L5

NSI-566: ALS Phase II Results
50% of study patients with positive change of ALSFRS slope

NSI-566: ALS Phase II Results
40-80% of study patients better than historical dataset
ALSFRS-R FVC (% predicted) Grip strength

NSI-566 SCI: Motor improvement, scar reduction & cavity-filling effect grafted at Day 3 post-SCI
van Gorp S, et al., Stem Cell Res Ther. 2013 May 28;4(3):57.

NSI-566 SCI: Motor improvement & functional integration grafted at day 7 post-SCI in rat with complete spinal transection
Lu P, et al., Cell. 2012 Sep 14;150(6):1264-73.

NSI-566 cSCI: Phase I Safety Trial
| 1 – 2 years after injury |
| AIS A only |
| Group A: 4 patients with T2-T12 (completed) |
| Removal of instruments to enable MRI survey |
| 1.2 x 106 cells in 6 bilateral injections at and below injury |
| 6 month follow-up: No SAE |
| 4.5 year post-study follow-up |
Study Site: University of California San Diego
Next Phase I study in Group B: 4 more patients

NSI-566 cSCI: Phase I Safety Trial
Subject #4, self-reported https://www.instagram.com/p/9161Cag9vK/
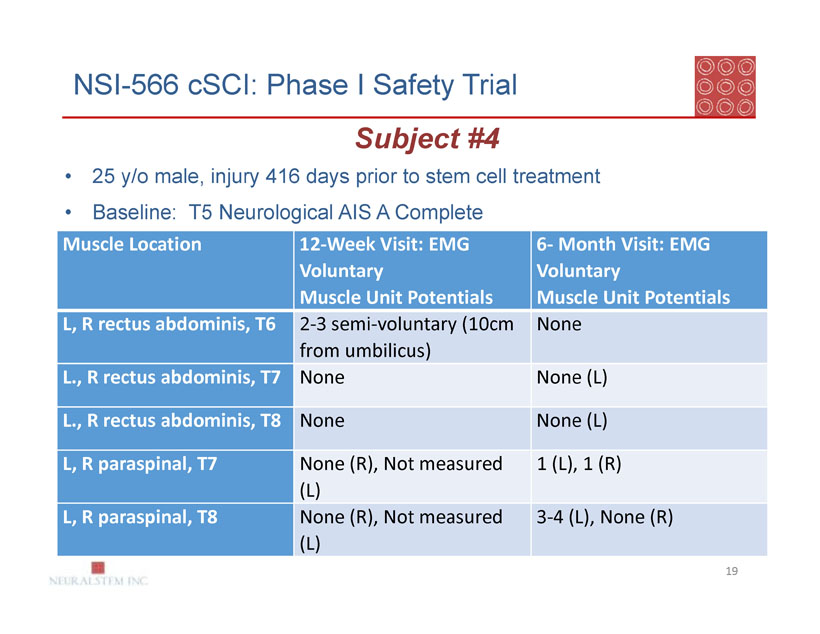
NSI-566 cSCI: Phase I Safety Trial
Subject #4
| 25 y/o male, injury 416 days prior to stem cell treatment |
| Baseline: T5 Neurological AIS A Complete |
| Muscle Location | 12-Week Visit: EMG | 6- Month Visit: EMG |
| Voluntary | Voluntary | |
| Muscle Unit Potentials | Muscle Unit Potentials | |
| L, R rectus abdominis, T6 | 2-3 semi-voluntary (10cm | None |
| from umbilicus) | ||
| L., R rectus abdominis, T7 | None | None (L) |
| L., R rectus abdominis, T8 | None | None (L) |
| L, R paraspinal, T7 | None (R), Not measured | 1 (L), 1 (R) |
| (L) | ||
| L, R paraspinal, T8 | None (R), Not measured | 3-4 (L), None (R) |
| (L) |
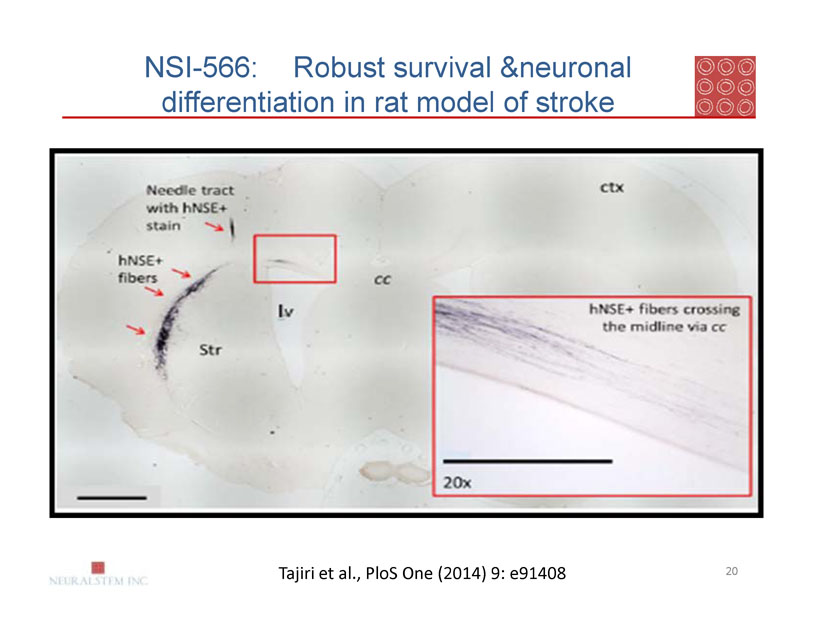
NSI-566: Robust survival &neuronal differentiation in rat model of stroke
Tajiri et al., PloS One (2014) 9: e91408

NSI-566: Ameliorates Stroke – induced motor deficits in rat model of stroke
Tajiri et al., PloS One (2014) 9: e91408

NSI-566:Stroke
NSI-566 engraftment at 4 weeks post transplantation in ischemia-induced mini-pig brain
K299-HuNu K299-H&E Human NF-specific staining

NSI-566: Phase I for treatment of paralysis from chronic stroke
| Single Study Site: BaYi Hospital, Beijing PLA |
| Open label feasibility & safety study |
| Cohort | Treatment | Cell | Deposits | No of | Status |
| ID | Number/Deposit | per Track | Cannula | ||
| Tracks | |||||
| A (n=3) | 1.2x107 | 40,000 | 5 | 3 | Dosed |
| cells/μL×20μL | |||||
| B (n=3) | 2.4x107 | 80,000 | 5 | 3 | Dosed |
| cells/μL×20μL | |||||
| C (n=3) | 7.2x107 | 80,000 | 15 | 3 | Ongoing |
| cells/μL×20μL |
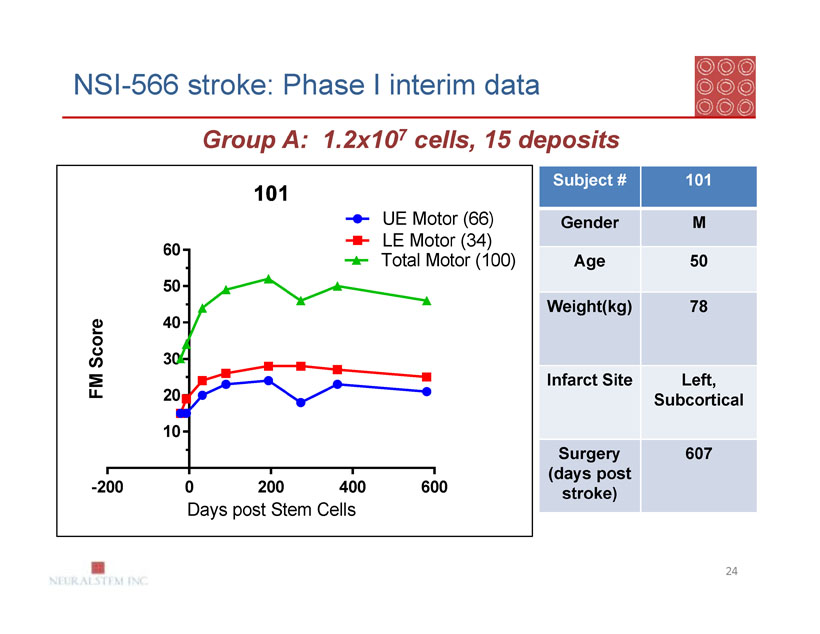
NSI-566 stroke: Phase I interim data
Group A: 1.2x107 cells, 15 deposits
| Subject # | 101 |
| Gender | M |
| Age | 50 |
| Weight(kg) | 78 |
| Infarct Site | Left, |
| Subcortical | |
| Surgery | 607 |
| (days post | |
| stroke) |
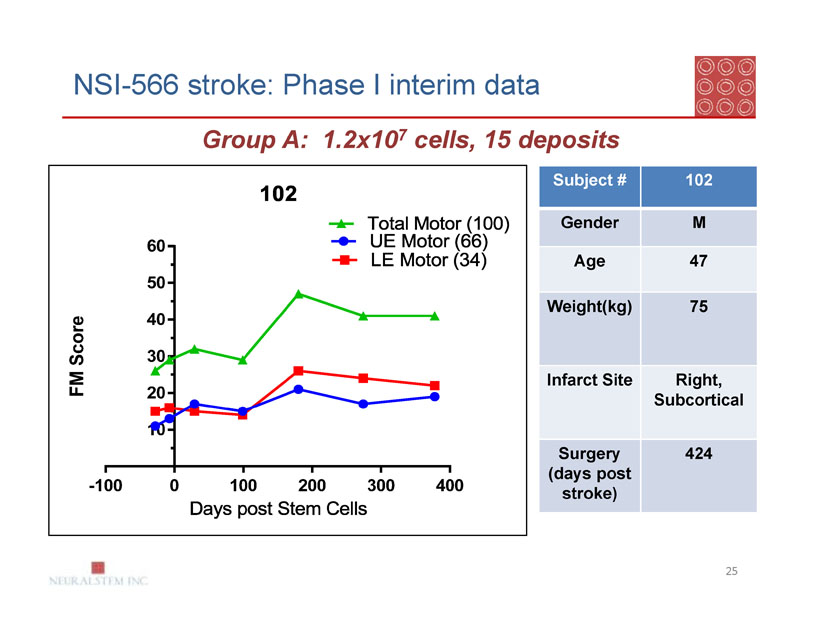
NSI-566 stroke: Phase I interim data
Group A: 1.2x107 cells, 15 deposits
| Subject # | 102 |
| Gender | M |
| Age | 47 |
| Weight(kg) | 75 |
| Infarct Site | Right, |
| Subcortical | |
| Surgery | 424 |
| (days post | |
| stroke) |

NSI-566 stroke: Phase I interim data
Group A: Subject 102

NSI-566 stroke: Phase I interim data
Group A: Subject 102
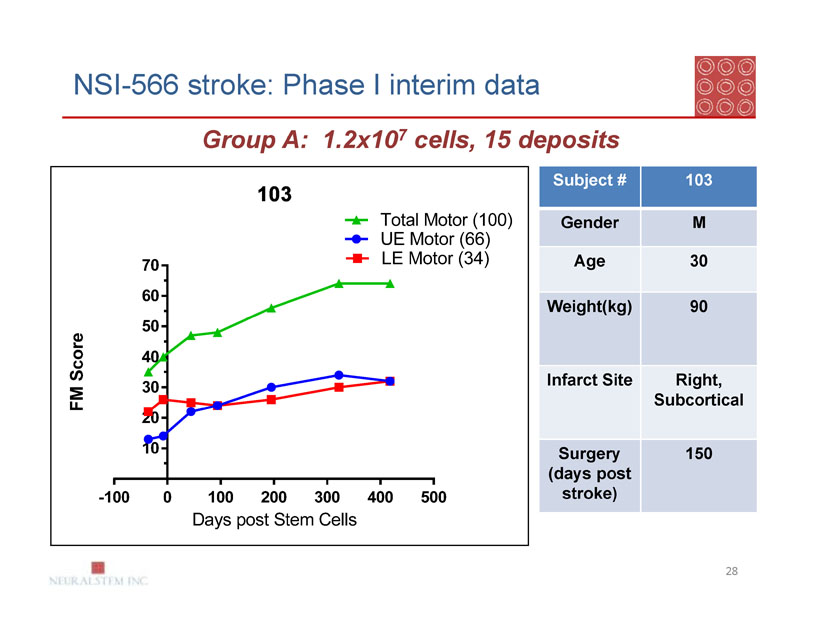
NSI-566 stroke: Phase I interim data
Group A: 1.2x107 cells, 15 deposits
| Subject # | 103 |
| Gender | M |
| Age | 30 |
| Weight(kg) | 90 |
| Infarct Site | Right, |
| Subcortical | |
| Surgery | 150 |
| (days post | |
| stroke) |

Group A: Subject 103

Conclusion
| Human spinal cord-derived neural stem cells (NSI-566) has demonstrated consistent biological activities across multiple disease conditions: ALS, stroke, cSCI |
| Next steps: Pivotal trials in multiple indications to demonstrate efficacy as the primary objective. |
| The trials will be randomized, sham-surgery controlled, double-blinded studies of adequate size to qualify as registration studies per discussions with the FDA. |
