Attached files
| file | filename |
|---|---|
| 8-K - 8-K - PALISADE BIO, INC. | v428765_8k.htm |
| EX-99.01 - EXHIBIT 99.01 - PALISADE BIO, INC. | v428765_ex99-01.htm |
Exhibit 99.02

January 12, 2016 Biotech Showcase Corporate Presentation

NEURALSTEM, INC. Safe Harbor Statement Safe Harbor statements under the Private Securities Litigation Reform Act of 1995 : This presentation contains forward - looking statements as defined in Section 27 A of the Securities Act of 1933 as amended, and section 21 E of the Securities Exchange Act of 1934 , as amended . Such forward - looking statements are based upon Neuralstem , Inc . ’s management’s current expectations, estimates, beliefs, assumptions, and projections about Neuralstem’s business and industry . Words such as “anticipates,” “expects,” “intends,” “plans,” “predicts,” “believes,” “seeks,” “estimates,” “may,” “will,” “should,” “would,” “potential,” “continue,” and variations of these words (or negatives of these words) or similar expressions, are intended to identify forward - looking statements . In addition, any statements that refer to expectations, projections, or other characterizations of future events or circumstances, including any underlying assumptions, are forward - looking statements . These forward - looking statements are not guarantees of future performance and are subject to certain risks, uncertainties, and assumptions that are difficult to predict . Therefore, our actual results could differ materially and adversely from those expressed in any forward - looking statements as a result of various risk factors . These risks and uncertainties include the risks associated with the effect of changing economic conditions, trends in the products markets, variations in Neuralstem’s cash flow, market acceptance risks, technical development risks and other risk factors detailed in Neuralstem’s Securities and Exchange Commission filings . For links to SEC documents please visit the company’s Web site : neuralstem . com . For links to SEC documents please visit the company’s Web site : neuralstem . com .

Overview 2 • Founding science utilizing regionally specific stem cells • Novel small molecule drug screening platform targeting neurogenesis; identified multiple compounds • Phase II lead compound (NSI - 189) targeting major depressive disorder (MDD) • Novel neurogenic, synaptogenic MOA • Clinical stage transplantation cell therapy platform targeting: ALS, cSCI , & stroke Neuralstem’s proprietary technology uses regionally specific neural stem cells for the development of CNS therapies.
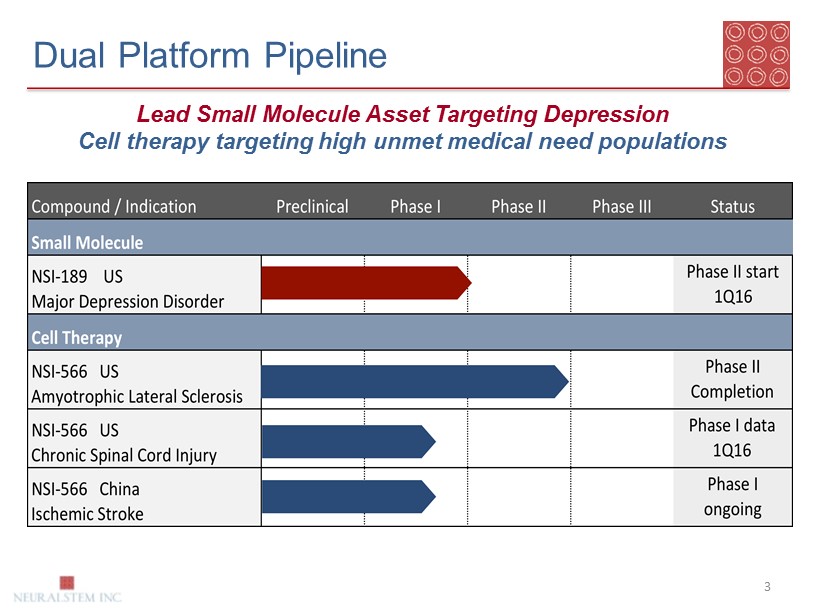
Dual Platform Pipeline 3 Lead Small Molecule Asset Targeting Depression Cell therapy targeting high unmet medical need populations

NSI - 189 Small Molecule Overview 4 NSI - 189 Small Molecule • Novel MOA increasing synaptic connections • Patent composition of matter (US Pat.No . 7,560,553) till 2029 NSI - 189 Phase II Major Depressive Disorder (MDD) • Commencement early 2016 • Data 2H 2017 Compelling early clinical data • Randomized, placebo - controlled, double blinded Phase Ib data • Excellent Safety Profile • Clinical efficacy on multiple scales; large effect size Large MDD Market • Peak Sales Forecast in G7 at 2029: US$ 1bn+ NSI - 189: First - in - class Neurogenic Small Molecule Drug

NSI - 189 Differentiation 5 N ovel neurogenic MOA • I ncreasing synaptic connections in the hippocampus • Neurogenesis in the hippocampus is linked to depression and other psychiatric and neurologic disorders No major s ide affects; better than current depression therapies • No metabolic or sexual dysfunction side effects Additional benefit in cognition f unction in MDD • Large effect size Persisted Improvements for MDD and cognition • Improvements persisted over the non - dosing 8 - week follow up period NSI - 189 is highly differentiated from current commercial depression therapies

Cohort 1 N=8 (6 drug, 2 placebo) 40 mg QD Cohort 2 N=8 (6 drug, 2 placebo) 40 mg BID Cohort 3 N=8 (6 drug, 2 placebo) 40 mg TID Acute treatment: 28 days Follow up: Days 35, 42, 49, 70, 84 (End - of - study) NSI - 189 Phase Ib NSI - 189 Phase Ib double - blind, randomized, placebo - controlled, multiple - dose study assessing safety and tolerability • Patients at screening could be taking an antidepressant medication(s), or have a history of taking antidepressant medication(s) in the past for their depressive disorder • At least two prior depressive episodes (including current episode ) 6

NSI - 189 MDD Phase Ib 7 p=0.02 d=0.90 Study Day -20 0 20 40 60 80 100 Symptoms of Depression Questionnaire 2.0 2.2 2.4 2.6 2.8 3.0 3.2 3.4 3.6 3.8 Placebo NS-189 NS-189 1x per day NS-189 2x per day NS-189 3x per day p=0.03 d=1.10 Day 84 Day 28 Symptoms of Depression Questionnaire (SDQ) p=0.09 d=0.95 Study Day 0 20 40 60 80 100 Montgomery and Asberg Depression Rating Scale 5 10 15 20 25 30 Placebo NS-189 NS-189 1x per day NS-189 2x per day NS-189 3x per day p=0.19 d=0.84 Montgomery - Asberg Depression Rating Scale (MADRS) Day 28 Day 84 Clinically Meaningful Results • SDQ combined treatment group showed statistically significant improvement (p=0.02) • Clinically meaningful reduction in depressive & cognitive symptoms (40mg, 80mg) • Persistent efficacy over the non - dosing 8 - week follow - up period All: A Phase 1B, Randomized, Double - Blind, Placebo - Controlled, Multiple - Dose Escalation Study Evaluating the Effects of NSI - 189 Phosp hate, a Neurogenic Compound, in Patients with Major Depressive Disorder (MDD) , presented June 2014, by Maurizio Fava, M.D., Karl Johe, Ph.D., Lev G. Gertsik , MD, Larry Ereshefsky , PharmD , Bettina Hoeppner , Ph.D., Martina Flynn, David Mischoulon , M.D., Ph.D., Gustavo Kinrys , M.D., and Marlene Freeman, M.D .

p=0.01 d=0.94 Study Day -20 0 20 40 60 80 100 Cognitive and Physical Functioning Questionnaire 2.0 2.5 3.0 3.5 4.0 4.5 5.0 Placebo NS-189 NS-189 1x per day NS-189 2x per day NS-189 3x per day p<0.01 d=1.20 Day 28 Day 84 NSI - 189 MDD Phase Ib 8 All: A Phase 1B, Randomized, Double - Blind, Placebo - Controlled, Multiple - Dose Escalation Study Evaluating the Effects of NSI - 189 Phosp hate, a Neurogenic Compound, in Patients with Major Depressive Disorder (MDD) , presented June 2014, by Maurizio Fava, M.D., Karl Johe, Ph.D., Lev G. Gertsik , MD, Larry Ereshefsky , PharmD , Bettina Hoeppner , Ph.D., Martina Flynn, David Mischoulon , M.D., Ph.D., Gustavo Kinrys , M.D., and Marlene Freeman, M.D . • Significant effect size in cognitive function improvement; improvement persisted over 8 - week follow up period • NSI - 189 CPFQ was significantly better than the placebo group (p=0.01) at Day 28; Large effect size of 0.94 Cognitive and Physical Functioning Questionnaire (CPFQ)
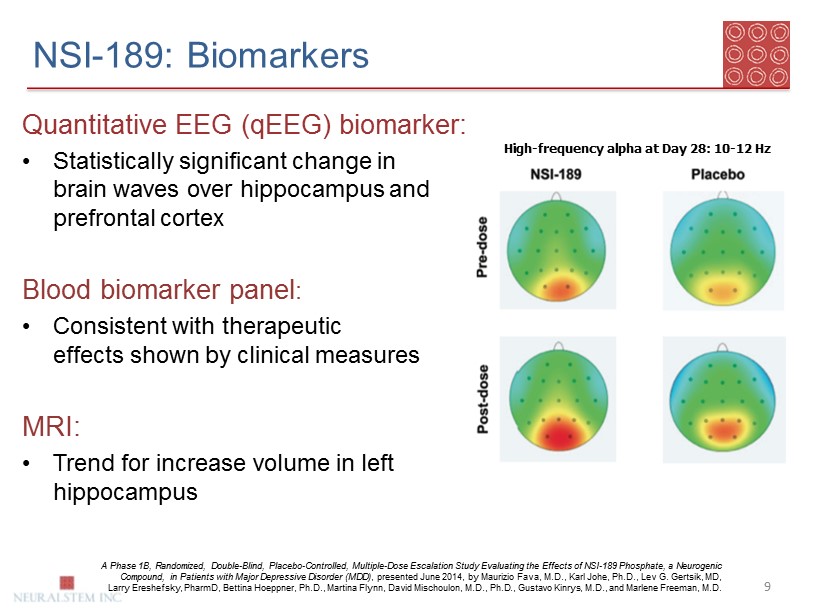
NSI - 189: Biomarkers 9 Quantitative EEG ( qEEG ) biomarker: • Statistically significant change in brain waves over hippocampus and prefrontal cortex Blood biomarker panel : • Consistent with therapeutic effects shown by clinical measures MRI: • Trend for increase volume in left hippocampus High - frequency alpha at Day 28: 10 - 12 Hz A Phase 1B, Randomized, Double - Blind, Placebo - Controlled, Multiple - Dose Escalation Study Evaluating the Effects of NSI - 189 Phosp hate, a Neurogenic Compound, in Patients with Major Depressive Disorder (MDD) , presented June 2014, by Maurizio Fava, M.D., Karl Johe, Ph.D., Lev G. Gertsik , MD, Larry Ereshefsky , PharmD , Bettina Hoeppner , Ph.D., Martina Flynn, David Mischoulon , M.D., Ph.D., Gustavo Kinrys , M.D., and Marlene Freeman, M.D.
NSI - 189 Phase II Trial Design 10 Study Objectives • Primary: Montgomery - Asberg Depression Rating Scale ( MADRS ) • Secondary: response and remission rates, durability effect Principal Investigator: Maurizio Fava, M.D. Slater Family Professor of Psychiatry at Harvard Medical School, Massachusetts General Hospital Sequential Comparison Parallel Trial Design* • 220 subjects, 12 sites • Three arm: 80mg (40mg BID), 40mg QD, placebo • 14 - 28 day screening including SAFER interview* • 12 week out - patient treatment period A Phase II, Double - Blind, Placebo - Controlled Study of NSI - 189 Phosphate (NS2014 - 1 ) *Fava, M. Massachusetts General Hospital Dept. of Psychiatry, Clinical Trials Network and Institute MedAvante , Inc .

Biological Activity: • Multiple mechanisms of action: neuroprotection , bridging , regeneration • Definitive evidence of long - term graft survival without immunosuppression in ALS patients NSI - 566 spinal cord stem cell - derived programs • Phase Ib Chronic Spinal Cord Injury ( cSCI ) – Funded by UCSD • Phase I/II Ischemic Stroke: Phase I/ II – BaYi Brain Hospital, Beijing PLA • Phase II Amyotrophic Lateral Sclerosis ( ALS ) – Secure grant funding for registration trial – Recent data presented in Sept 2015 Cell Therapy Overview 11 Neuralstem's technology enables the isolation, expansion and controlled differentiation of neural stem cells into mature, physiologically relevant human neurons and glial cells.
Neuralstem Catalysts 12 Program Milestones • Phase II MDD FDA Approval 1Q16 • Sept 2015 FDA protocol submission • Phase I cSCI d ata 1Q16 • Ongoing Phase I/II stroke trial 2016 • Phase II MDD d ata 2H17 Other Initiatives • Exploring additional NSI - 189 possible indications • Secure grant money for the next possible ALS clinical study
