Attached files
| file | filename |
|---|---|
| 8-K - FOUNDATION MEDICINE, INC. 8-K - Foundation Medicine, Inc. | a51256334.htm |
| EX-99.1 - EXHIBIT 99.1 - Foundation Medicine, Inc. | a51256334ex99_1.htm |
Exhibit 99.2

2016 J.P. Morgan Annual Healthcare Conference

These slides and the accompanying oral presentation contain forward-looking statements and information. These materials, including any accompanying oral presentation, contain forward-looking statements about our business. You should not place undue reliance on forward-looking statements as these statements are based upon our current expectations, forecasts and assumptions and are subject to significant risks and uncertainties. These statements may be identified by words such as “may,” “will,” “should,” “could,” “expect,” “intend,” “plan,” “anticipate,” “believe,” “estimate,” “predict,” “potential,” “forecast,” “continue” or the negative of these terms or other words or terms of similar meaning. Risks and uncertainties that could cause our actual results to differ materially from those set forth in any forward-looking statements include, but are not limited to, the matters listed under “Risk Factors” in our Quarterly Report on Form 10-Q for the three months ended March 31, 2015, and our subsequent filings with the Securities and Exchange Commission. These reports are available at www.sec.gov or by contacting our investor relations department at ir@foundationmedicine.com. Statements, including forward-looking statements, speak only to the date they are provided (unless an earlier date is indicated), and we do not undertake any obligation to publicly update any statements, including forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law. Forward Looking Statements

Who We Are FOUNDATION MEDICINE is a molecular information company that’s leading a transformation in cancer care.

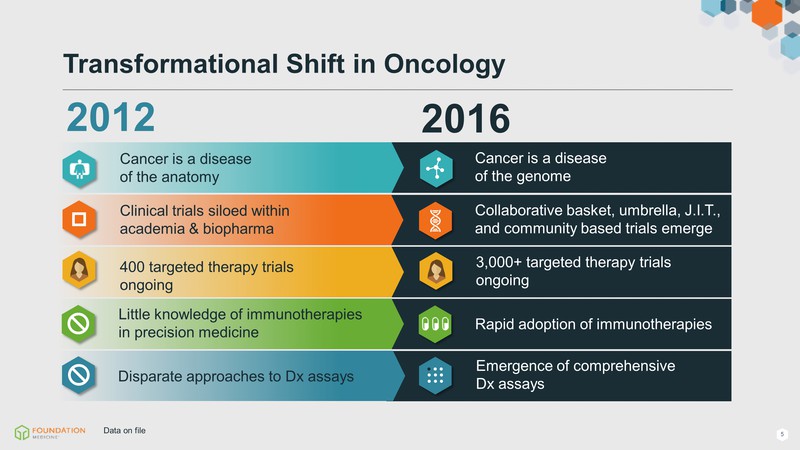
2012 2016 Little knowledge of immunotherapies in precision medicine Rapid adoption of immunotherapies Cancer is a disease of the anatomy Cancer is a disease of the genome Collaborative basket, umbrella, J.I.T., and community based trials emerge Clinical trials siloed within academia & biopharma 400 targeted therapy trials ongoing 3,000+ targeted therapy trials ongoing Transformational Shift in Oncology Emergence of comprehensive Dx assays Disparate approaches to Dx assays Data on file
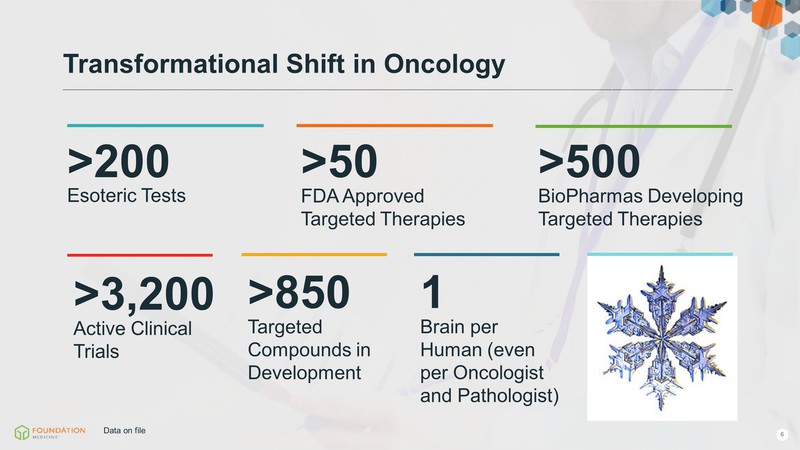
>200 Esoteric Tests >50 FDA Approved Targeted Therapies >500 BioPharmas Developing Targeted Therapies >3,200 Active Clinical Trials >850 Targeted Compounds in Development Unique Patients 1 Brain per Human (even per Oncologist and Pathologist) Transformational Shift in Oncology Data on file
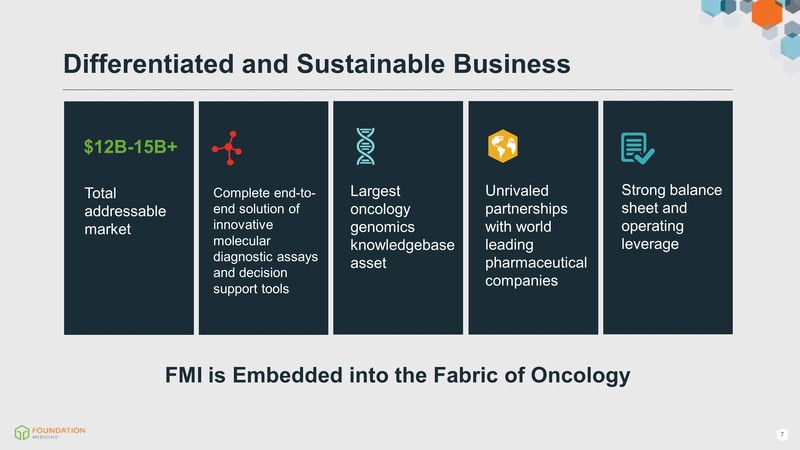
Differentiated and Sustainable Business FMI is Embedded into the Fabric of Oncology $12B-15B+ Total addressable market Complete end-to-end solution of innovative molecular diagnostic assays and decision support tools Largest oncology genomics knowledgebase asset Strong balance sheet and operating leverage Unrivaled partnerships with world leading pharmaceutical companies
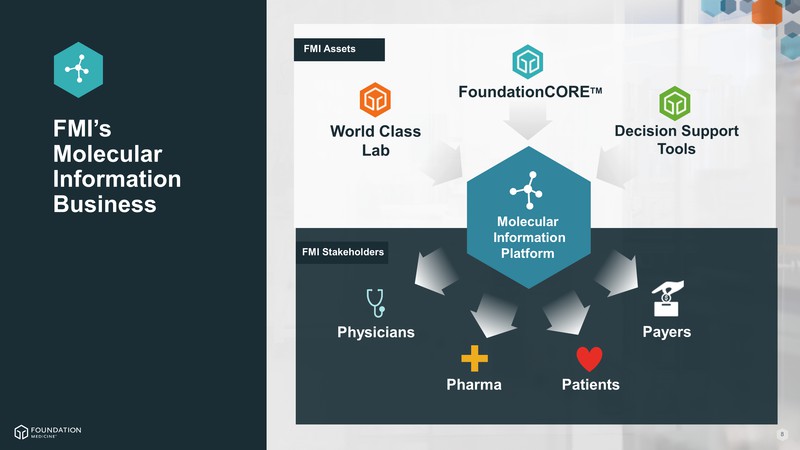
FMI Assets FMI Stakeholders FMI’s Molecular Information Business FoundationCORETM Physicians Pharma Patients Molecular Information Platform World Class Lab Decision Support Tools Payers

Premier Lab Deep Expertise in Computational Biology Proprietary Algorithms Unmatched Sensitivity and Specificity World Class Lab Deep Expertise and Unmatched Scale for a Distinct Competitive Advantage
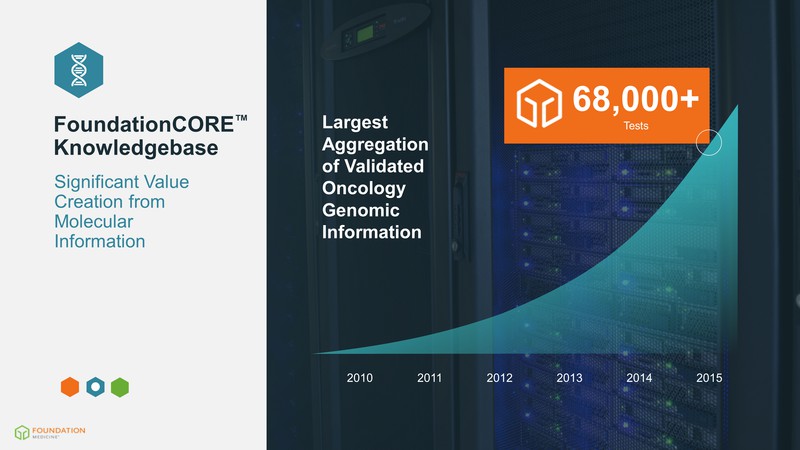
2010 2011 2012 2013 2014 2015 Largest Aggregation of Validated Oncology Genomic Information 68,000+ Tests FoundationCORE(TM) Knowledgebase Significant Value Creation from Molecular Information
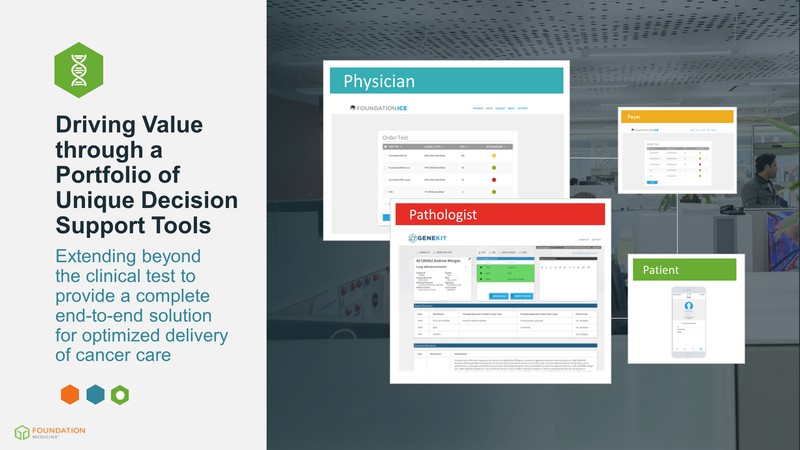
Driving Value through a Portfolio of Unique Decision Support Tools Extending beyond the clinical test to provide a complete end-to-end solution for optimized delivery of cancer care Physician Patient Payer Pathologist
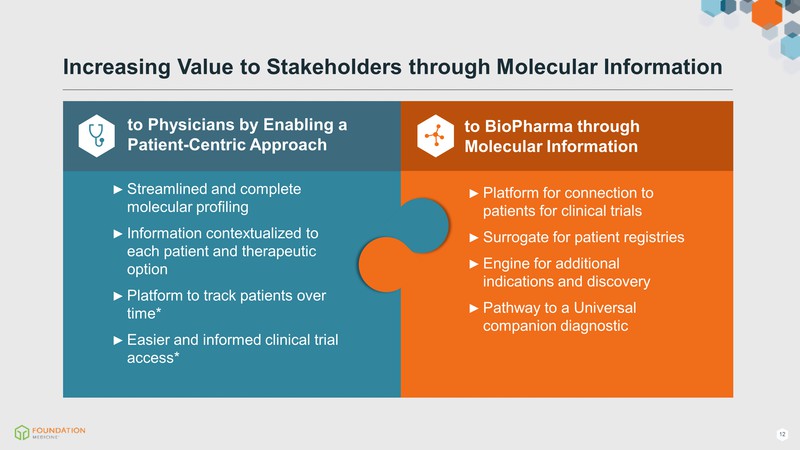
to BioPharma through Molecular Information Platform for connection to patients for clinical trials Surrogate for patient registries Engine for additional indications and discovery Pathway to a Universal companion diagnostic to Physicians by Enabling a Patient-Centric Approach Streamlined and complete molecular profiling Information contextualized to each patient and therapeutic option Platform to track patients over time* Easier and informed clinical trial access* Increasing Value to Stakeholders through Molecular Information

Initial focus for FoundationOne® and FoundationOne® Heme (US unmet need population) ~1.1 million patients $3.5B Patient Need is Large and Unmet Global Addressable Market in 2020: >$15B

Initial focus for FoundationOne® and FoundationOne® Heme (US unmet need population) ~1.1 million patients $3.5B Patient Need is Large and Unmet Global Addressable Market in 2020: >$15B Expanded use in U.S. and International ~4 million patients $9B
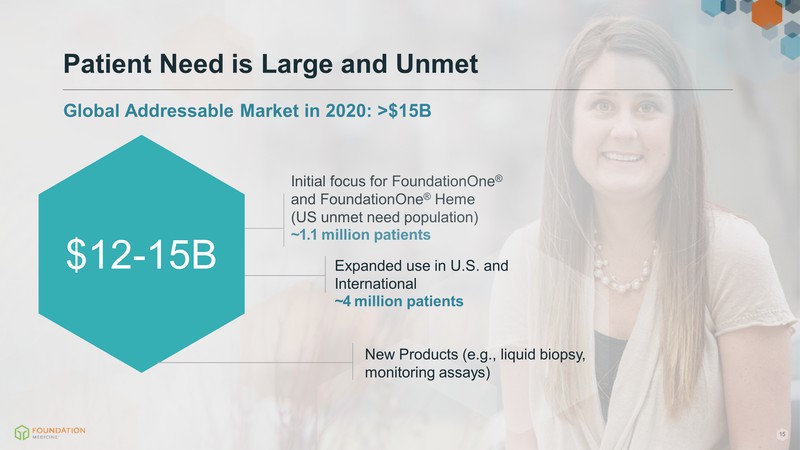
Initial focus for FoundationOne® and FoundationOne® Heme (US unmet need population) ~1.1 million patients $3.5B Patient Need is Large and Unmet Global Addressable Market in 2020: >$15B Expanded use in U.S. and International ~4 million patients $9B $12-15B New Products (e.g., liquid biopsy, monitoring assays)
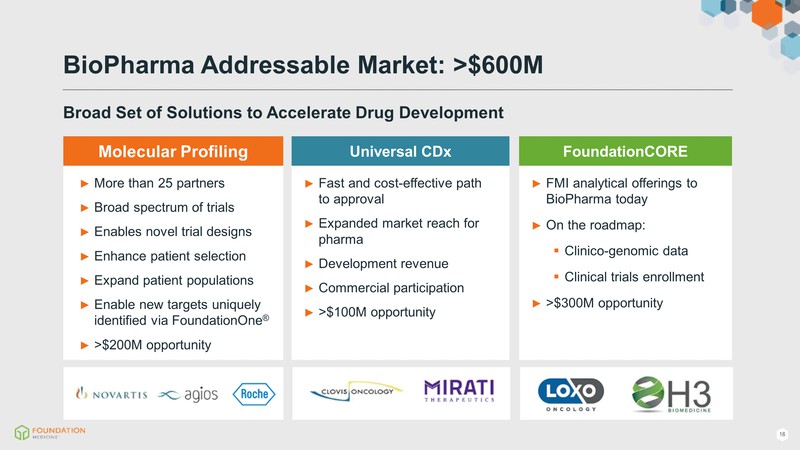
BioPharma Addressable Market: >$600M Broad Set of Solutions to Accelerate Drug Development More than 25 partners Broad spectrum of trials Enables novel trial designs Enhance patient selection Expand patient populations Enable new targets uniquely identified via FoundationOne >$200M opportunity Molecular Profiling Fast and cost-effective path to approval Expanded market reach for pharma Development revenue Commercial participation >$100M opportunity Universal CDx FMI analytical offerings to BioPharma today On the roadmap: Clinico-genomic data Clinical trials enrollment >$300M opportunity(Gp:) FoundationCORE
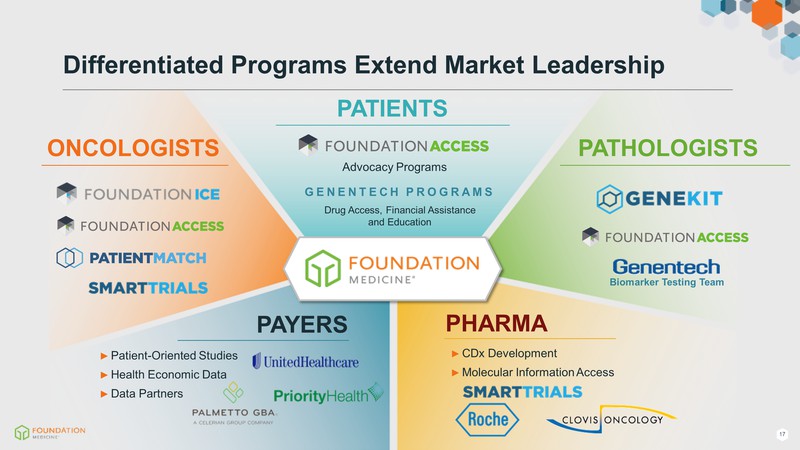
Differentiated Programs Extend Market Leadership PATHOLOGISTS Biomarker Testing Team GENENTECH PROGRAMS Drug Access, Financial Assistance and Education PATIENTS Advocacy Programs PAYERS Patient-Oriented Studies Health Economic Data Data Partners ONCOLOGISTS PHARMA CDx Development Molecular Information Access
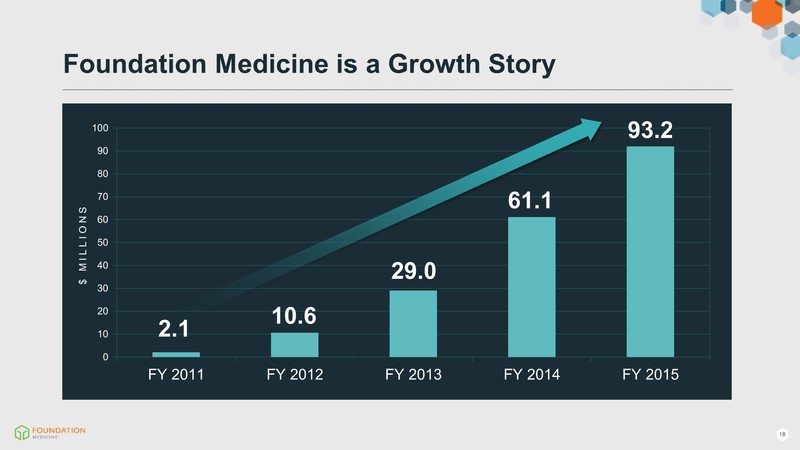
Foundation Medicine is a Growth Story $ MILLIONS 10.6 29.0 61.1 93.2 2.1
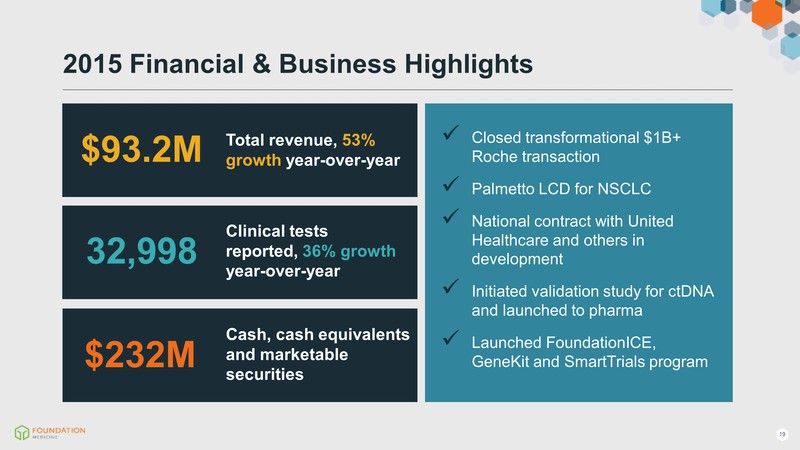
2015 Financial & Business Highlights $93.2M $232M Total revenue, 53% growth year-over-year 32,998 Clinical tests reported, 36% growth year-over-year Cash, cash equivalents and marketable securities Closed transformational $1B+ Roche transaction Palmetto LCD for NSCLC National contract with United Healthcare and others in development Initiated validation study for ctDNA and launched to pharma Launched FoundationICE, GeneKit and SmartTrials program
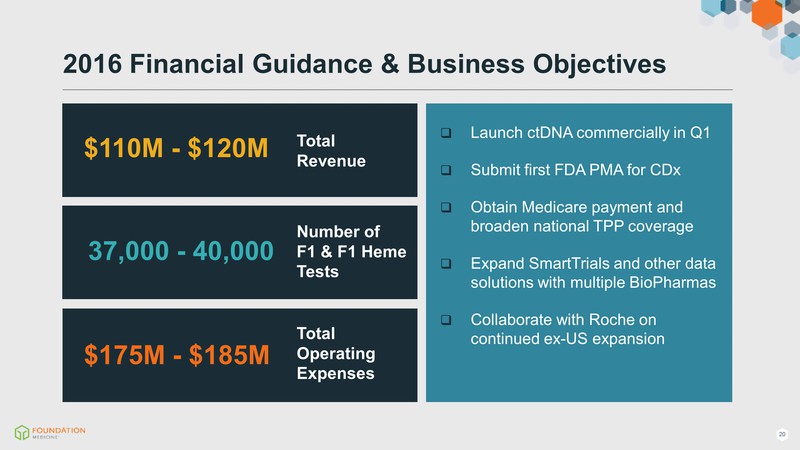
2016 Financial Guidance & Business Objectives $110M - $120M $175M - $185M 37,000 - 40,000 Total Revenue Total Operating Expenses Number of F1 & F1 Heme Tests Launch ctDNA commercially in Q1 Submit first FDA PMA for CDx Obtain Medicare payment and broaden national TPP coverage Expand SmartTrials and other data solutions with multiple BioPharmas Collaborate with Roche on continued ex-US expansion

Kristen’s Story is Foundation Medicine’s Story.

2016 JP Morgan Annual Healthcare Conference
