Attached files
| file | filename |
|---|---|
| EX-99.2 - EXHIBIT 99.2 - Cytosorbents Corp | v428735_ex99-2.htm |
| 8-K - CURRENT REPORT - Cytosorbents Corp | v428735_8k.htm |
Exhibit 99.1

Cyto Sorbents Corporation NASDAQ: CTSO Working to Save Lives Through Blood Purification Biotech Showcase Presentation January 2016

Safe Harbor Statement Statements in this presentation regarding CytoSorbents Corporation and its operating subsidiaries CytoSorbents Medical Inc . and CytoSorbents Europe GmbH that are not historical facts are forward - looking statements and are subject to risks and uncertainties that could cause actual future events or results to differ materially from such statements . Any such forward - looking statements are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 . It is routine for our internal projections and expectations to change . Although these expectations may change, we are under no obligation to inform you if they do . Actual events or results may differ materially from those contained in the projections or forward - looking statements . The following factors, among others, could cause our actual results to differ materially from those described in a forward - looking statement : our history of losses ; potential fluctuations in our quarterly and annual results ; competition, inability to achieve regulatory approval for our device, technology systems beyond our control and technology - related defects that could affect the companies’ products or reputation ; risks related to adverse business conditions ; our dependence on key employees ; competition for qualified personnel ; the possible unavailability of financing as and if needed ; and risks related to protecting our intellectual property rights or potential infringement of the intellectual property rights of third parties . This list is intended to identify only certain of the principal factors that could cause actual results to differ from those discussed in the forward - looking statements . Readers are referred to a discussion of important risk factors detailed in the Company’s Form 10 - K filed with the Securities and Exchange Commission on March 31 , 2015 and other reports and documents filed from time to time by us, which are available online at www . sec . gov .

3 Cyto Sorbents is A Leader in Critical Care Immunotherapy Leading the Prevention or Treatment of Life - Threatening Inflammation in the ICU and Cardiac Surgery using CytoSorb ® Blood Purification

4 Severe Inflammation is Deadly in the ICU Millions of people are admitted to the intensive care unit in hospitals worldwide each year with deadly inflammatory conditions • In these conditions, massive inflammation causes cell death and organ failure. Patients are kept alive with “life support” – machines like mechanical ventilation, dialysis, and vasopressors, with the hope that the body starts to heal itself • Because of the lack of effective therapies, approximately 1 in every 3 patients dies • The costs can be staggering: Lack of “active” therapies lead to patients lingering days to weeks in the ICU at $2,000 - 3,000 per day in the ICU* on average • Not surprising that we spend nearly 1% of our GDP on critical care** Sepsis ARDS Burn Injury Trauma Pancreatitis Influenza Cancer Immunotherapy * Cooper, L, et al, Crit Care Med 2004, 32(11):2247 - 2253. ** Halpern, NA, et al., Crit Care Med 2010, 38(1):65 - 71.

5 Severe Inflammation is Dangerous in Open Heart Surgery Cytokine Storm SIRS Multiple Organ Failure Free Hemoglobin Vascular / Kidney Injury ~1 million Open Heart Surgeries in US and EU annually • Coronary artery bypass graft surgery • Valve repair or replacement • Heart or lung transplantation • Congenital defect repair • Aortic reconstruction In complex cardiac surgeries, patients are on the heart - lung machine and operating table for a long time which can cause destruction of blood cells and can trigger a cytokine storm and severe inflammation Organ dysfunction and failure, particularly lung and kidney failure, frequently result Before now, there were no effective ways to prevent this from happening

6 Cytokines Fuel the Fire of Inflammation • Cytokines are small proteins that normally help stimulate and regulate the immune system and control inflammation • Cytokines are a dual edged sword • They are required for proper immune system function • However, in mild to moderate excess, cytokines can cause or exacerbate disease (e.g. autoimmune diseases) • But cytokines in vast excess, called “cytokine storm” can lead to a massive uncontrolled systemic inflammatory response syndrome (SIRS).

7 Massive Inflammation Causes Organ Failure Organ failure occurs when vital organs stop working, causing nearly half of all deaths in the ICU . Little can be done to prevent or treat it today

8 Cyto Sorb ® Removes the Fuel to the Fire • CytoSorb ® targets the $20+ billion opportunity in critical care and cardiac surgery • Approved in the European Union as the only specifically approved extracorporeal cytokine filter for use in any situation where cytokines are elevated • Clinically proven to remove key cytokines in the blood of critically - ill patients • Removes many other inflammatory mediators such as free hemoglobin, bacterial toxins, and complement • Safe and well - tolerated: In 9,000+ human treatments, mainly in critically - ill patients, including 1,000+ cardiac surgeries *CytoSorb is not yet approved in the U.S.
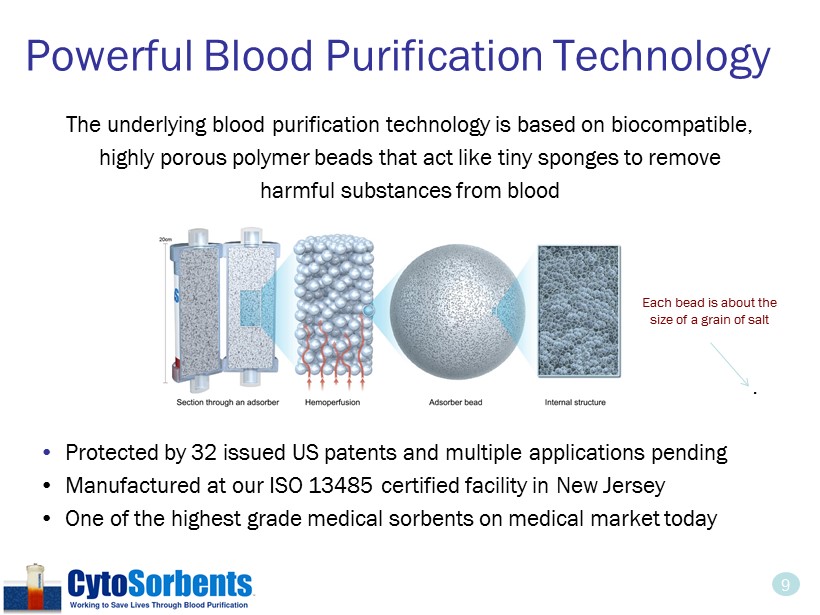
9 The underlying blood purification technology is based on biocompatible, highly porous polymer beads that act like tiny sponges to remove harmful substances from blood • Protected by 32 issued US patents and multiple applications pending • Manufactured at our ISO 13485 certified facility in New Jersey • One of the highest grade medical sorbents on medical market today Powerful Blood Purification Technology . Each bead is about the size of a grain of salt

10 Cyto Sorb ® is Plug and Play with Existing Machines Place a temporary dialysis catheter in a major vein Connect the device to a standard dialysis machines found in hospitals worldwide Pump blood out of the body and through the cartridge The polymer beads directly contact blood and remove unwanted or toxic substances “Purified” blood is pumped back into the patient Can treat 70+ total blood volumes per 24 hr treatment Each treatment uses a new cartridge Easy to Use, No Special Equipment or Training Required
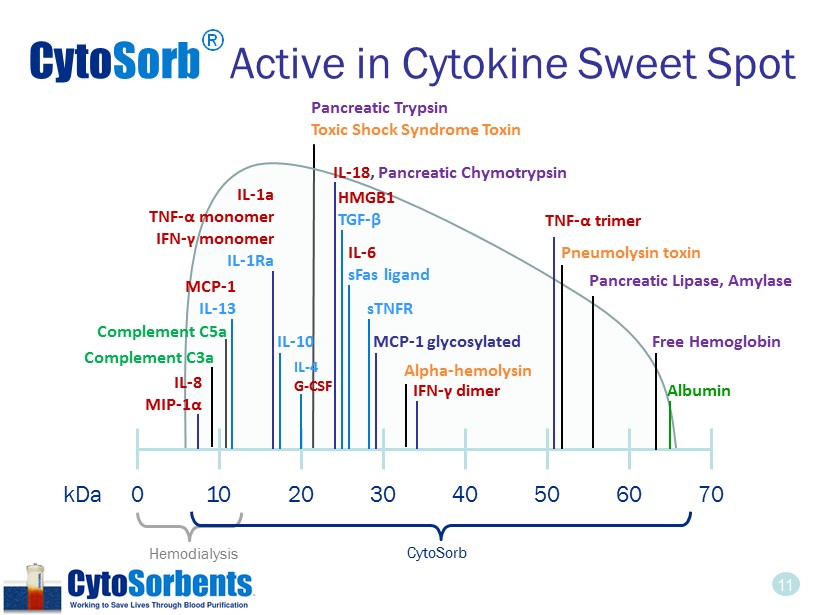
11 Cyto Sorb ® Active in Cytokine Sweet Spot Hemodialysis 0 kDa 10 20 30 40 50 60 70 IL - 1a TNF - α monomer IFN - γ monomer IL - 1Ra IL - 10 TNF - α trimer IFN - γ dimer HMGB1 TGF - β IL - 6 sFas ligand MCP - 1 IL - 13 MCP - 1 glycosylated Albumin IL - 8 MIP - 1α IL - 18 , Pancreatic Chymotrypsin sTNFR IL - 4 G - CSF CytoSorb Free Hemoglobin Pancreatic Trypsin Toxic Shock Syndrome Toxin Pancreatic Lipase, Amylase Complement C5a Complement C3a Pneumolysin toxin Alpha - hemolysin
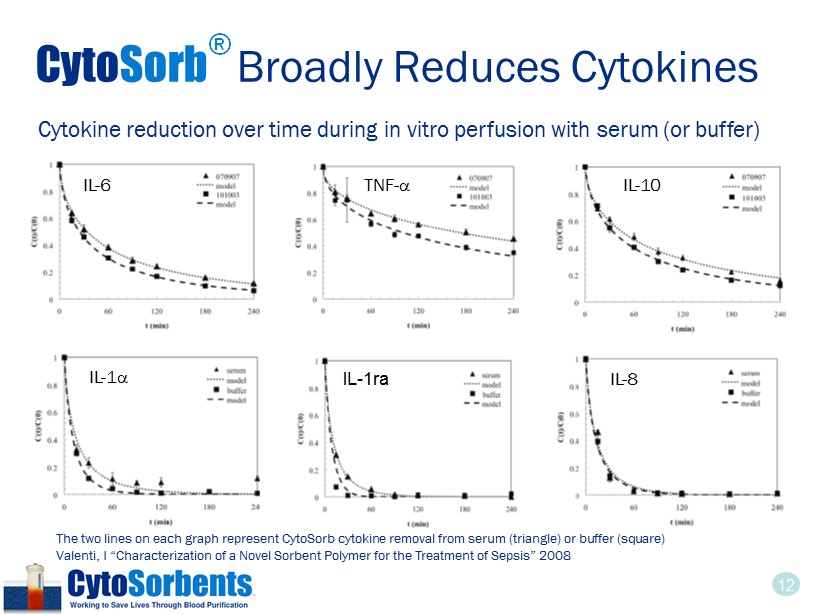
12 Cyto Sorb ® Broadly Reduces Cytokines IL - 6 TNF - a IL - 10 IL - 1 a IL - 1ra IL - 8 Cytokine reduction over time during in vitro perfusion with serum (or buffer) The two lines on each graph represent CytoSorb cytokine removal from serum (triangle) or buffer (square) Valenti, I “Characterization of a Novel Sorbent Polymer for the Treatment of Sepsis” 2008

13 Goal: To Prevent or Treat Organ Failure Sepsis ARDS Burn Injury Trauma Pancreatitis Influenza Surgical The Potential to Revolutionize Critical Care Medicine Improve Patient Outcome and Survival Decrease Costs Of ICU and Patient Care

14 Cyto Sorb Is A New Immunomodulation Strategy to Control Severe Inflammation in the ICU NSAIDS Aspirin Anti - cytokine antibodies Anti - integrin antibodies Anti - oxidants Anti - Inflammatory (too weak) Immunosuppressive (too strong) Immunomodulatory (“balanced”) Corticosteroids Chemotherapy Organ transplant Anti - rejection drugs Radiation Immune system ablation Anti - leukocyte Antibodies

15 Case Report: Toxic Shock Syndrome • Recently, a 3 year old, 38 pound girl was stung on her leg by an insect and became infected with Staph aureus • Despite antibiotics, her condition rapidly worsened and she developed a systemic inflammatory response syndrome with toxic shock syndrome and multiple organ failure requiring life support • Extensive capillary leak syndrome, broad drop in her blood cell levels with hemorrhage, and progression towards scalded skin syndrome • She was stabilized within 72 hours by a combination of CytoSorb and dialysis • She made a complete recovery in 3 weeks and CytoSorb was credited with saving her life and preventing the need for amputation Site of insect sting

16 • US Dept of Health and Human Services awarded $0.5M grant (2010) for therapies that can save lives and reduce costs under the QTDP Program • NIH grant awarded $7M five year (2006 - 2010) to University of Pittsburgh and Dr. John Kellum to research CytoSorb bead for treatment of sepsis • NIH/NHLBI awarded $1.7M Phase I & II SBIR to advance the HemoDefend purification technology intended to improve the quality and safety of blood transfusions (2013 - present) ~$17 Million in US Government Support • DARPA awarded $3.8M five year (2012 - present) contract as part of “Dialysis - Like Therapeutics” program to treat sepsis by removing cytokines and pathogen - derived toxins • U.S. Army awarded $1.15M SBIR contracts for trauma and burn injury research (2011 - present) • U.S. Air Force is funding a 30 - patient human pilot study in trauma (2013 - present). FDA approved trial that has begun enrollment valued at ~$3M

17 Drive Usage and Sales Of CytoSorb

18 • Huge market CytoSorb ® is sold to hospitals and critical care physicians, targeting a “need to have” $ 20 + billion worldwide critical care opportunity addressing organ failure • Little to no competition • Critical care physicians understand the problem • It is a plug and play high margin disposable “razorblade” Hospital’s existing hemodialysis infrastructure is the “razor”, no new hardware • Technicians already know how to use the device • CytoSorb ® is reimbursed in Germany/Austria at > $ 500 /cartridge . Depending on the application and devices used, revenue potential per patient ~ $ 1 - 5 K • Affordable yet profitable with blended gross margins of 63 % , target > 80 % • Intensive care units are highly centralized easy for a small sales force to access Cyto Sorb ® An Excellent Business Model

19 Direct Sales: Germany, Austria, Switzerland We are selling CytoSorb to most of the major university and public hospitals in Germany, Austria and Switzerland. The market in Germany alone is $1.0 - 1.5 billion

20 Distributed in 32 countries including the Germany, UK, France, Italy, Netherlands, Turkey, Russia, India, Israel, Poland, Romania, Scandanavia, Denmark, Middle East, Vietnam, Australia and New Zealand covering 2.1 billion lives. Expanding to other EU countries and countries outside the EU that accept the CE Mark WMC Intensiv Med E.U. Approval Opens Global Distribution Australia and New Zealand

21 Quarterly “Euro - Adjusted” Product Sales $13,679 $87,960 $176,098 $127,969 $203,561 $314,159 $569,243 $663,233 $1,031,761 $871,150 $816,109 $920,041 $1,221,694 $1,750,000 $0 $200,000 $400,000 $600,000 $800,000 $1,000,000 $1,200,000 $1,400,000 $1,600,000 $1,800,000 $2,000,000 Q3 2012 Q4 2012 Q1 2013 Q2 2013 Q3 2013 Q4 2013 Q1 2014 Q2 2014 Q3 2014 Q4 2014 Q1 2015 Q2 2015 Q3 2015 Q4 2015 CytoSorb® "Euro Adjusted" Product Sales Preliminary Actual Range $1.4 - 1.6M * Preliminary *

22 Product Sales Growth, “Euro Adjusted” $151,574 $310,779 $405,706 $595,588 $821,787 $1,214,932 $1,750,196 $2,578,396 $3,135,387 $3,382,253 $3,639,061 $3,828,994 $4,700,000 $- $500,000 $1,000,000 $1,500,000 $2,000,000 $2,500,000 $3,000,000 $3,500,000 $4,000,000 $4,500,000 $5,000,000 Q4 2012 Q1 2013 Q2 2013 Q3 2013 Q4 2013 Q1 2014 Q2 2014 Q3 2014 Q4 2014 Q1 2015 Q2 2015 Q3 2015 Q4 2015 Trailing Twelve Month Product Sales Impact of Euro Reported Sales Preliminary Actual Range $3.9 - 4.1M * * Preliminary

23 How This Can Get Big Quickly • In Germany alone, there are ~154,000 cases of severe sepsis or septic shock each year. Here, we have: x Established reimbursement x Most of the major university and public hospitals as customers x Significant key opinion leader support throughout the country x Strong direct sales team and support infrastructure 2,100 acute care hospitals in Germany. The top 400 have >400 beds 300 - 600 patients a year with sepsis per hospital CytoSorb ASP is > $1,000 per cartridge. Average sepsis patient uses $3,000 - 5,000 worth of cartridges If adopted as standard of care, each hospital ≈ $1 - 3 million for sepsis alone

24 Geographic Expansion – A Growth Engine Q4 2015 to Q1 2016 Start 131M people *Mayr, FB, Yende S, Angus DC, “Epidemiology of severe sepsis” Virulence 2014 5(1):4 - 11 Estimated 2016 Start
25 Growth Driven by Direct, Distributor, Partner Sales * This graph is provided only to demonstrate the concept of revenue layering. It does NOT represent revenue forecasts or guidance

26 2 nd CytoSorb Germany User’s Meeting

27 1 st International CytoSorb User’s Meeting

28 2 nd International CytoSorb User’s Meeting

29 International Society of Intensive Care & Emergency Medicine – Brussels 2015

30 Drive the Data that will Make CytoSorb Standard of Care

31 50+ Investigator Initiated Studies Additional Sepsis Trials Case Reports Germany Dosing Study Director of Clinical Operations Medical Director Trauma Advisory Board Sepsis Advisory Board Cardiac Surgery Advisory Board Chief Medical Officer Statistician Cardiac Surgery Sepsis Trauma Director of European Scientific Affairs Robust Clinical Program and Team International CytoSorb Registry

32 • 40 - patient, eight - center feasibility study evaluating the safety of CytoSorb ® intra - operatively in a bypass circuit in a heart - lung machine during complex cardiac surgery in elective, non - emergent surgery where the patient is expected to under cardio - pulmonary bypass > 3 hours • Aortic reconstruction, CABG redos, multiple valve replacements, etc • The goal is the safe reduction of plasma - free hemoglobin and other inflammatory mediators that can cause post - operative complications REFRESH I Trial To Start in US RE duction in FRE e H emoglobin

33 REFRESH I Update RE duction in FRE e H emoglobin Trial • Working with major cardiac surgery centers in this trial • Baylor College of Medicine • Baystate Medical Center • Columbia University • Cooper University Hospital • University of Kentucky • University of Maryland • University of Pennsylvania • University of Pittsburgh Medical Center • 6 out of 8 sites are currently eligible to enroll patients • The trial is 10% enrolled and expected to be completed in 1H 2016 at which point the data will be discussed with the FDA and a direction for a potential pivotal trial (REFRESH 2) will be decided (de novo 510(k) vs PMA)

34 Severe Sepsis or Septic Shock US Trial • Severe sepsis and septic shock are the overzealous immune response to a serious infection that can cause organ injury, organ failure and death • It is the largest, but also the most complex, critical care market, afflicting 27 million people worldwide, killing at least a third. There are currently no products approved to treat sepsis • We plan to do US clinical trials in sepsis to advance towards US approval • Studies are expected to begin in 2016

35 Many Published Peer - Reviewed Articles

36 CytoSorb Website – A Wealth of Info Visit us at www.cytosorb.com

37 International CytoSorb Registry • The home of 50+ planned investigator initiated studies with many already enrolling

38 Leverage Pipeline to Establish Strategic Partnerships

39 Fresenius Medical Care • In December 2014, entered into a multi - year, 6 country partnership with Fresenius, the largest dialysis company in the world for exclusive distribution of CytoSorb® in critical care in France, Sweden, Norway, Finland, Denmark and Poland • CytoSorb® is a key part of Fresenius’ growth strategy in critical care • Fresenius has committed to annual minimum purchases to maintain exclusivity in these countries • Leveraging Fresenius’ #1 or #2 position in critical care in these territories and elsewhere in the world with an industry - leading sales force and distribution • Potential for much broader synergy and expansion in the future

40 Fresenius Launch Expected in Q1 2016 • In 2015, we worked with Fresenius to complete all of the documentation, country registrations, certifications, development of marketing materials, and training needed for the launch • Fresenius is currently marketing the technology to critical care key opinion leaders in multiple countries • They have purchased product inventory from us in anticipation of a formal roll - out of CytoSorb in the next several months • CytoSorb will also be certified on the newly launched multiFiltratePRO

41 Strategic Partnership: The Most Comprehensive Treatment for Sepsis Treat the Massive Inflammatory Response Treat the Primary Infection • Biocon is the largest biotechnology company in India • Partnered with Biocon since late 2013 • Significantly growing usage in India with now expansion into Sri Lanka • Expanded the partnership to include co - development and funding of small trials

42 • In November 2014, we established an initial partnership in France with one of the top 4 cardiac surgery companies in the world. The partner successfully completed its test evaluation of CytoSorb with one of the leading cardiac surgeons in France. • We are currently having discussions with multiple players in this space • CytoSorb ® has been used safely in more than 1,000 intra - operative cardiac surgery cases to date in Europe Cardiac Surgery Partner Update
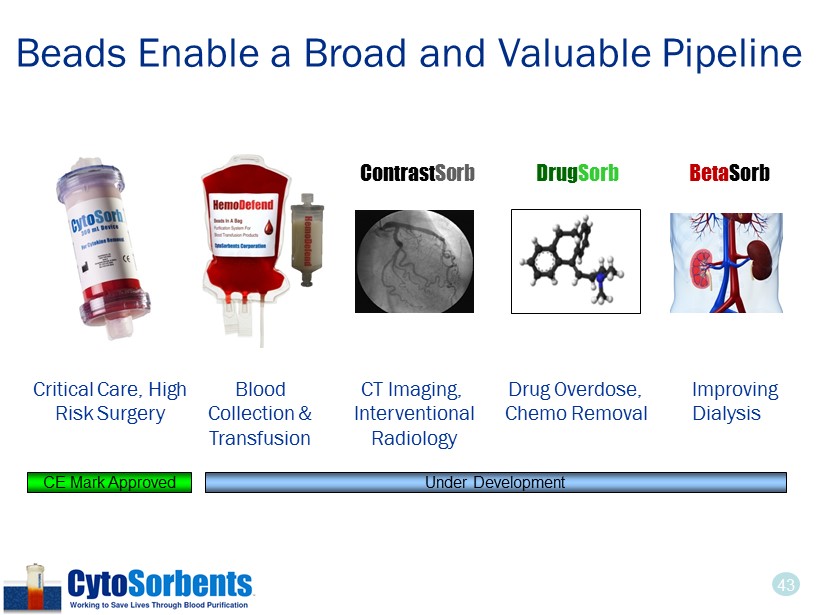
43 Critical Care, High Risk Surgery Blood Collection & Transfusion CT Imaging, Interventional Radiology Contrast Sorb Drug Overdose, Chemo Removal Drug Sorb Beta Sorb Improving Dialysis Under Development CE Mark Approved Beads Enable a Broad and Valuable Pipeline

44 Potential for More Strategic Partnerships* Cardiac Surgery Renal Dialysis Blood Transfusion Biotech and Immunotherapy Critical Care or Catheters *Companies listed here are used simply as examples of companies in these respective verticals. We make no other representations to our relationship with any of these companies .

45 Increase Investor Awareness

46 NASDAQ Capital Market Listed • Clean capital structure with good liquidity for investors

47 Added to the Russell Microcap Index June 2015

48 Increase Institutional Ownership Source: NASDAQ

49 Increase Analyst Coverage

50 Increase Media Coverage

51 Cyto Sorb ® may help revolutionize critical care medicine, saving lives, and reducing costs • Massive untapped $20 billion unmet, medical need in critical care • CytoSorb ® sales are generating significant growth with attractive 60+% gross margins • Continued geographic expansion throughout the world • Two pathways to US FDA approval for CytoSorb ® : Cardiac Surgery and critical illnesses such as sepsis • Expansion of existing strategic partnerships and potential new ones CytoSorb is helping to save lives throughout the world Cyto Sorbents Has Tremendous Potential

NASDAQ: CTSO Dr. Phillip Chan, MD, PhD – CEO pchan@cytosorbents.com
