Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - CAPRICOR THERAPEUTICS, INC. | v426685_8k.htm |
Exhibit 99.1

NASDAQ: CAPR December 2015 Transformative Therapies from Bench to Bedside

2 Forward - Looking Statements This presentation contains forward - looking statements and information that are based on the beliefs of the management of Capricor Therapeutics, Inc. (Capricor) as well as assumptions made by and information currently available to Capricor. All statements other than statements of historical fact included in this presentation are forward - looking statements, including but not limited to statements identified by the words “anticipates,” “believes,” “estimates,” and “expects” and similar expressions. Such forward - looking statements also include any expectation of or dates for commencement of clinical trials, IND filings, similar plans or projections and other matters that do not relate strictly to historical facts. These statements reflect Capricor’s current views with respect to future events, based on what we believe are reasonable assumptions; however, the statements are subject to a number of risks, uncertainties and assumptions. There are a number of important factors that could cause actual results or events to differ materially from those indicated by such forward - looking statements. More information about these and other risks that may impact Capricor's business are set forth in Capricor's Annual Report on Form 10 - K for the year ended December 31, 2014, as filed with the Securities and Exchange Commission on March 16, 2015, in its Registration Statement on Form S - 3, as filed with the Securities and Exchange Commission on September 28, 2015, and in its Quarterly Report on Form 10 - Q for the quarter ended September 30, 2015, as filed with the Securities and Exchange Commission on November 13, 2015. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those in the forward - looking statements. Further, Capricor’s management does not intend to update these forward - looking statements and information after the date of this presentation.

3 Capricor Financial Highlights – Capricor, Inc. founded in 2005 (Baltimore, MD; JHU spinoff) – Completed reverse merger with Nile Therapeutics in November 2013 – Uplisted to NASDAQ in March 2015 – Total Cash as of Sept. 30 , 2015: $17.2M (current cash out: ῀ Q3 2016) – Non - dilutive capital funding to date: $39.5M – Shares Outstanding: 16.3M – Headquarters: Los Angeles, CA

4 Capricor Opportunity – Pioneer in Cardiac - Derived Cell (CDC) Technology and CDC - Derived Exosomes – Solid IP position in CDC and E xosome Technology – Diversified platform and therapeutic pipeline – Orphan indication: DMD - associated cardiomyopathy program – Meaningful clinical milestones over the next 12 months – License Option and Development Collaboration with Johnson and Johnson

5 Cardiosphere - Derived Cells CAP - 1002 Micro - RNA Platform Natriuretic Peptide therapy Capricor’s Platform & Therapeutic Pipeline
6 Cardiosphere - Derived Cells (CDCs): Adult Heart disease
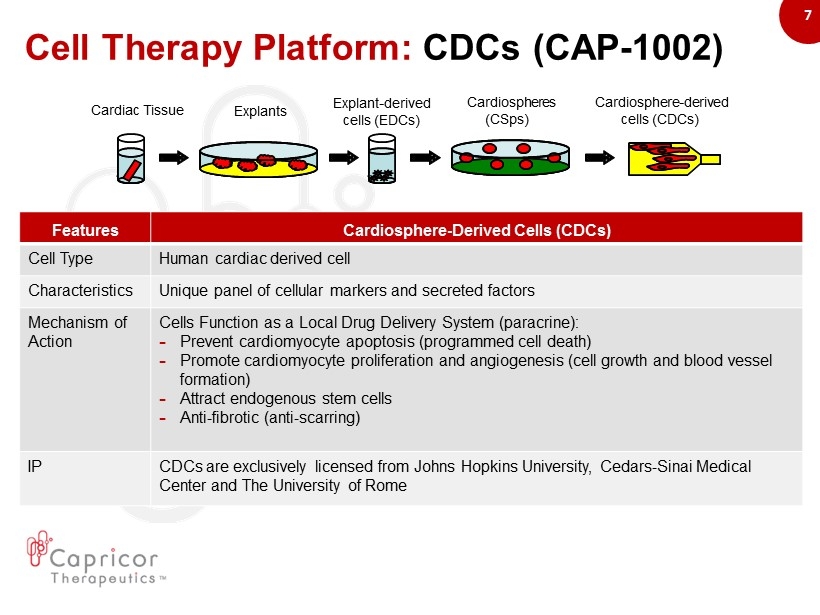
7 Cell Therapy Platform: CDCs (CAP - 1002) Cardiosphere - derived cells (CDCs) Cardiospheres (CSps) Explant - derived cells (EDCs) Explants Cardiac Tissue Features Cardiosphere - Derived Cells (CDCs) Cell Type Human cardiac derived cell Characteristics Unique panel of cellular markers and secreted factors Mechanism of Action Cells Function as a Local Drug Delivery System (paracrine) : – Prevent cardiomyocyte apoptosis (programmed cell death) – Promote cardiomyocyte proliferation and angiogenesis (cell growth and blood vessel formation) – Attract endogenous stem cells – Anti - fibrotic (anti - scarring) IP CDCs are exclusively licensed from Johns Hopkins University, Cedars - Sinai Medical Center and The University of Rome
8 CADUCEUS: Proof of Concept First - in - Man Data – Intracoronary delivery of autologous CDCs - 25M cells – Patients with reduced ejection fraction following MI – 25 patients (17 CDCs, 8 Controls) – Sponsored by Cedars - Sinai Medical Center – Results : CDCs reduced the amount of scar in the heart caused by a heart attack; therefore, smaller scars may lead to better outcomes Lancet , 2012, 21(6): 1121 - 1135.
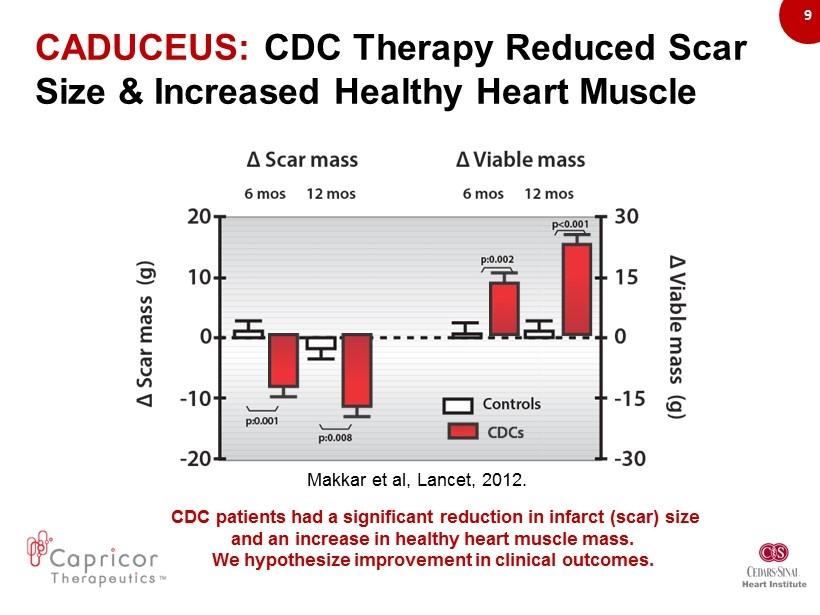
9 Makkar et al, Lancet, 2012. CDC p atients had a s ignificant r eduction in i nfarct (scar) s ize and an increase in healthy heart muscle mass. We hypothesize improvement in clinical outcomes . CADUCEUS : CDC Therapy Reduced Scar Size & Increased Healthy Heart Muscle

10 Effect of Infarct (Scar) Size on Events Wu, E. et al. Heart 94, 730 - 736 (2008 ).
11 Conclusions from CADUCEUS – CDCs reduced the amount of scar in the heart caused by a heart attack – Smaller scars may lead to better outcomes – CDCs increase healthy heart muscle mass – Autologous manufacturing is not a viable business model in this indication – Multiple clinical programs underway using allogeneic cells
12 Janssen (J&J) Deal Highlights Collaboration Agreement and Exclusive License Option with Janssen Biotech for Cell Therapy Program for Cardiovascular Applications – Received upfront payment of $12.5M – Collaboration on elements of cell manufacturing – Exclusive license agreement option for CAP - 1002 up to 60 days after receipt of six month ALLSTAR Phase II data ▪ If exercised, Capricor to receive an upfront license fee and additional milestone payments which may total up to $325M ▪ If exercised, Janssen to pay all future clinical trial and development costs of CAP - 1002 ▪ Double - digit royalty to be paid to Capricor on commercial sales of licensed products
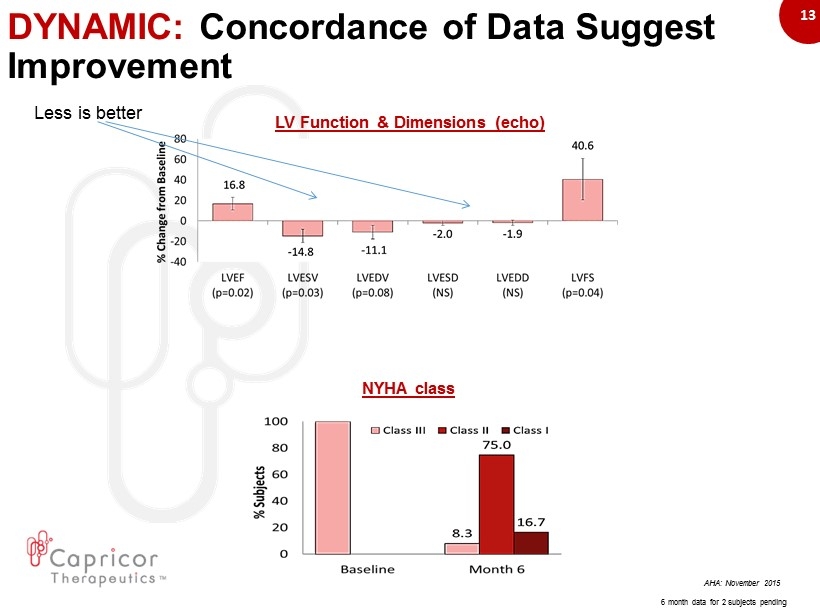
13 DYNAMIC: Concordance of Data Suggest Improvement AHA: November 2015 NYHA class LV Function & Dimensions (echo) 6 month data for 2 subjects pending Less is better
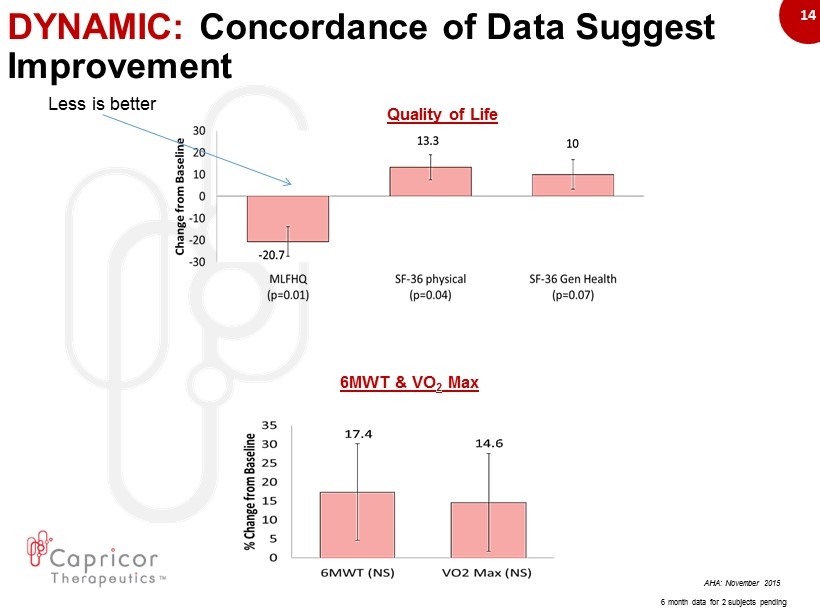
14 DYNAMIC: Concordance of Data Suggest Improvement AHA: November 2015 6MWT & VO 2 Max Quality of Life 6 month data for 2 subjects pending Less is better
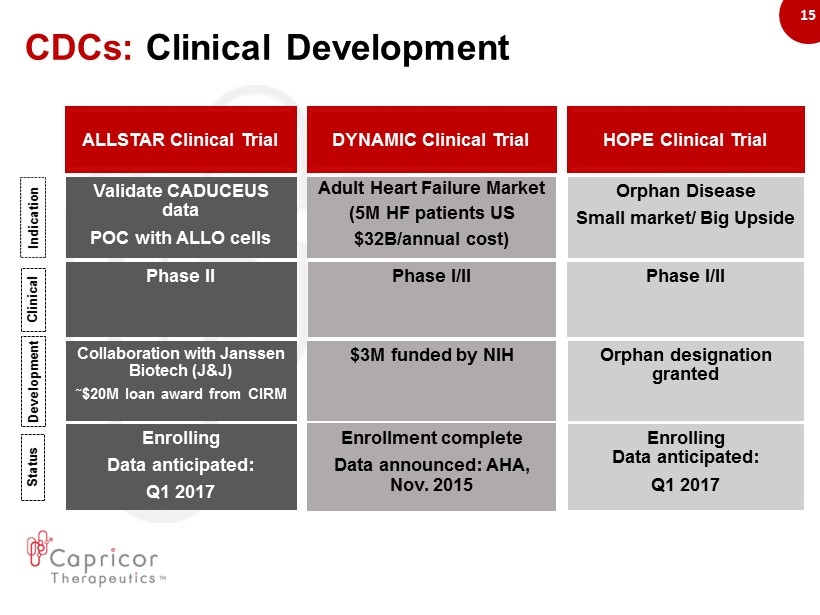
15 ALLSTAR Clinical Trial Collaboration with Janssen Biotech (J&J) ῀ $20M loan award from CIRM Phase II Phase I/II Enrolling Data anticipated: Q1 2017 Phase I/II Validate CADUCEUS data POC with ALLO cells Adult Heart Failure Market (5M HF patients US $32B/annual cost) Orphan Disease Small market/ Big Upside Orphan designation granted Enrollment complete Data announced: AHA, Nov. 2015 Enrolling Data anticipated: Q1 2017 DYNAMIC Clinical Trial HOPE Clinical Trial $3M funded by NIH Indication Clinical Development Status CDCs: Clinical Development
16 Duchenne Muscular Dystrophy Prevalence : 1 in 3,500 male births worldwide ~20,000 male children affected in the US (~275,000 worldwide) DMD is fatal with most deaths due to cardiomyopathy Reference: McNeil et. al, Muscle & Nerve, 2010
17 Orphan Drug Designation Granted to CAPR – CAP - 1002 can be used in CONJUNCTION with ANY other dystrophin - correcting therapies targeting skeletal muscle ▪ These therapies do not appear effective in cardiac muscle ▪ Very few clinical trials to treat DMD cardiomyopathy – Presents potential billion dollar market opportunity
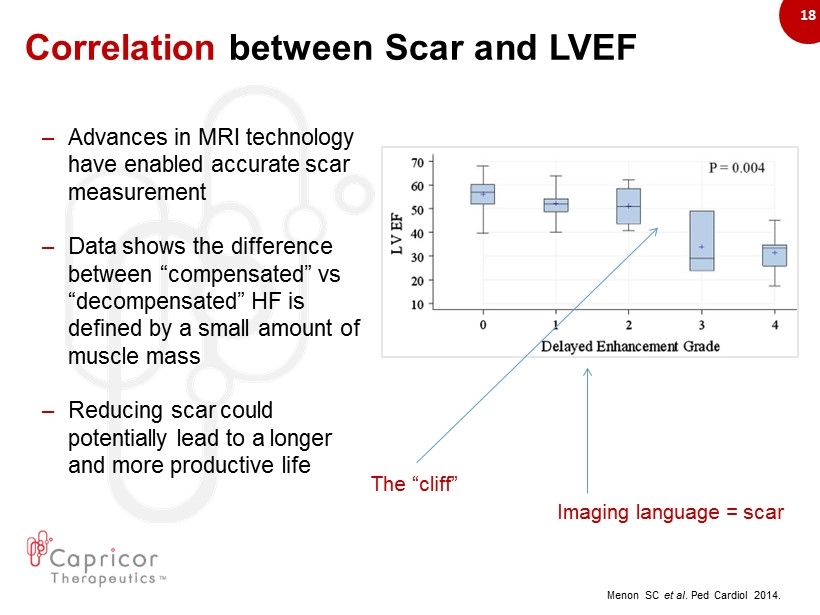
18 Correlation between Scar and LVEF – Advances in MRI technology have enabled accurate scar measurement – Data shows the difference between “compensated” vs “decompensated” HF is defined by a small amount of muscle mass – Reducing scar could potentially lead to a longer and more productive life Menon SC et al . Ped Cardiol 2014. Imaging language = scar The “cliff”

19 – M imics the target indication, DMD – A ged to the point at which cardiac dysfunction becomes evident: ≥10 months old Reference: Cedars - Sinai Heart Institute mdx Mouse Model of Duchenne Muscular Dystrophy
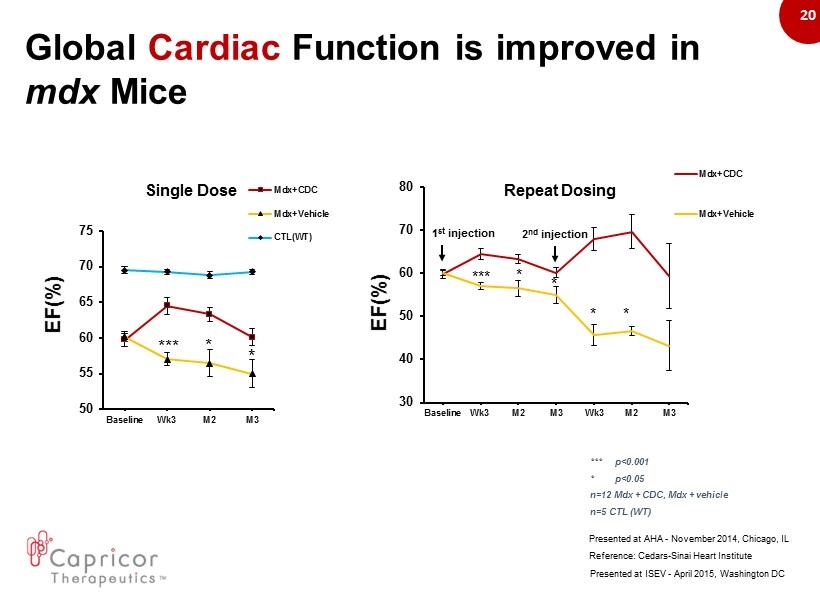
20 * *** * 50 55 60 65 70 75 Baseline Wk3 M2 M3 Mdx+CDC Mdx+Vehicle CTL(WT) Reference: Cedars - Sinai Heart Institute Presented at AHA - November 2014, Chicago, IL * * 30 40 50 60 70 80 Baseline Wk3 M2 M3 Wk3 M2 M3 Mdx+CDC Mdx+Vehicle *** * * 1 st injection 2 nd injection EF(%) Presented at ISEV - April 2015, Washington DC Repeat Dosing Single Dose Global Cardiac Function is improved in mdx Mice EF(%) *** p<0.001 * p<0.05 n=12 Mdx + CDC, Mdx + vehicle n=5 CTL (WT)
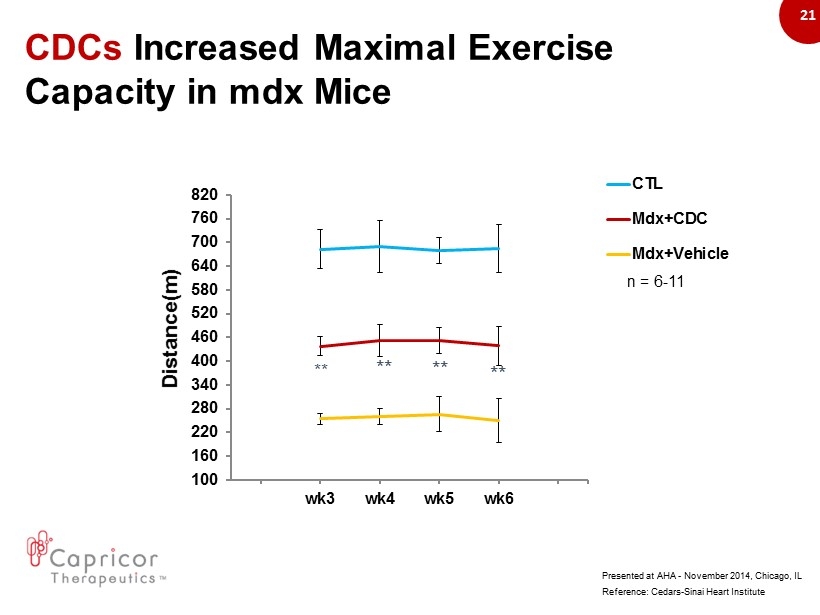
21 100 160 220 280 340 400 460 520 580 640 700 760 820 wk3 wk4 wk5 wk6 CTL Mdx+CDC Mdx+Vehicle CDCs Increased M aximal E xercise Capacity in mdx Mice Distance(m) ** ** ** ** n = 6 - 11 Reference: Cedars - Sinai Heart Institute Presented at AHA - November 2014, Chicago, IL
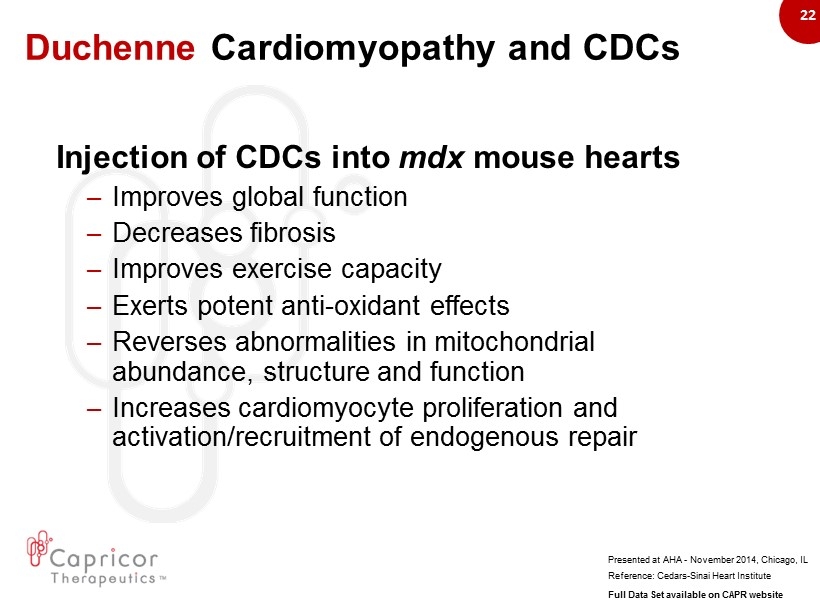
22 Duchenne Cardiomyopathy and CDCs Injection of CDCs into mdx mouse hearts – Improves global function – D ecreases fibrosis – Improves exercise capacity – Exerts potent anti - oxidant effects – R everses abnormalities in mitochondrial abundance, structure and function – I ncreases cardiomyocyte proliferation and activation/recruitment of endogenous repair Reference: Cedars - Sinai Heart Institute Presented at AHA - November 2014, Chicago, IL Full Data Set available on CAPR website
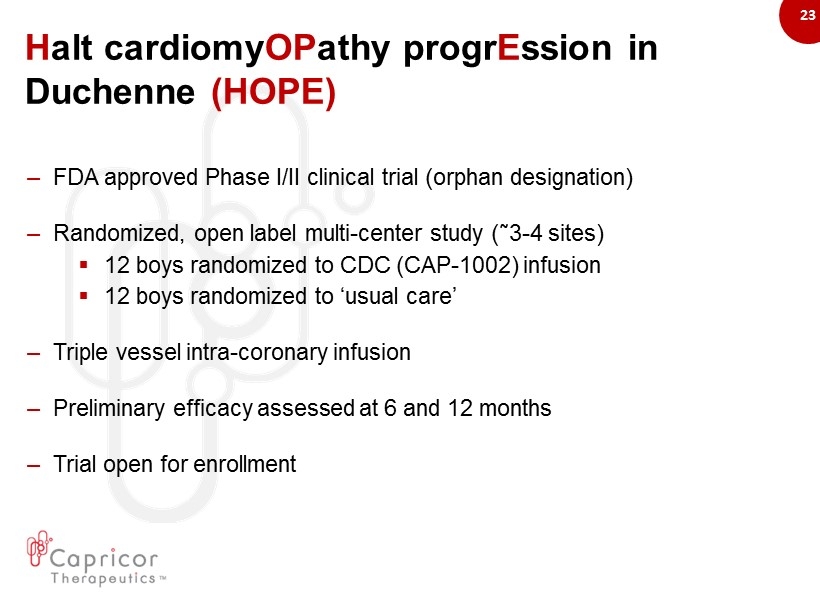
23 H alt cardiomy OP athy progr E ssion in Duchenne (HOPE) – FDA approved Phase I/II clinical trial (orphan designation) – R andomized , open label multi - center study ( ῀ 3 - 4 sites) ▪ 12 boys randomized to CDC (CAP - 1002) infusion ▪ 12 boys randomized to ‘usual care’ – Triple vessel intra - coronary infusion – Preliminary efficacy assessed at 6 and 12 months – Trial open for enrollment
24 Exosomes: Next Generation Regenerative Medicine Therapeutic Platform
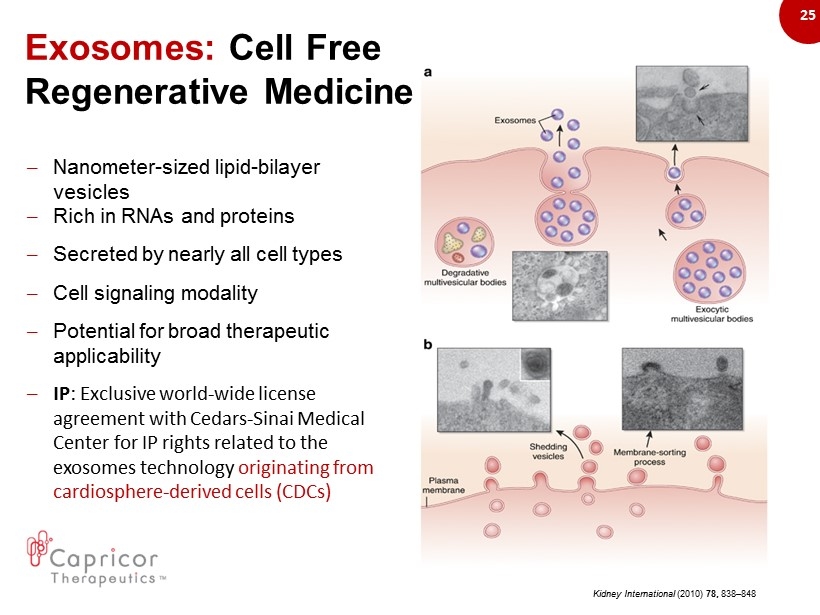
25 Exosomes: Cell Free Regenerative Medicine – Nanometer - sized lipid - bilayer vesicles – Rich in RNAs and proteins – Secreted by nearly all cell types – Cell signaling modality – Potential for broad therapeutic applicability – IP : Exclusive world - wide license agreement with Cedars - Sinai Medical Center for IP rights related to the exosomes technology originating from cardiosphere - derived cells (CDCs) Kidney International (2010) 78, 838 – 848

26 Exosomes: Paracrine Signals Suggest C ells MOA – Autologous and allogeneic cells show a similar potency – Delivered cells are cleared shortly after administration (< 1 month) – The therapeutic benefit is maintained after delivered cells are gone – Administered cells promote endogenous regenerative pathways – Paracrine signals play an essential role in the effects mediated by these cells
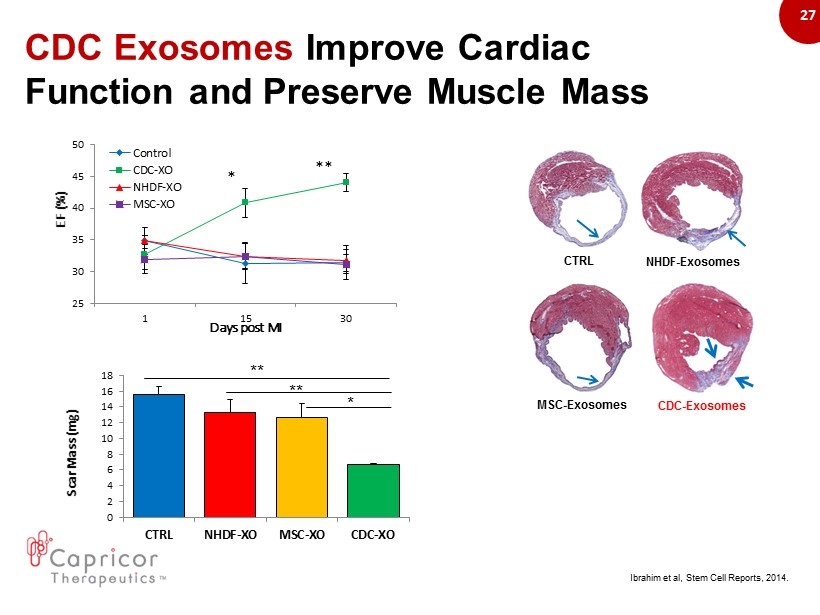
27 CDC E xosomes Improve Cardiac Function and Preserve M uscle Mass 0 2 4 6 8 10 12 14 16 18 CTRL NHDF-XO MSC-XO CDC-XO Scar Mass (mg) ** ** * CTRL NHDF - Exosomes MSC - Exosomes CDC - Exosomes 25 30 35 40 45 50 1 15 30 EF (%) Days post MI Control CDC-XO NHDF-XO MSC-XO ** * Ibrahim et al, Stem Cell Reports, 2014.

28 – Targeting announcing First - in - Man clinical indication: Q1 2016 – Indications under consideration: ▪ Eyes ▪ Skin ▪ Cancer – Meet with FDA: Q1 2016 – Targeting IND submission: 2016 Exosomes: Targeted Milestones
29 NATRIURETIC PEPTIDE TECHNOLOGY
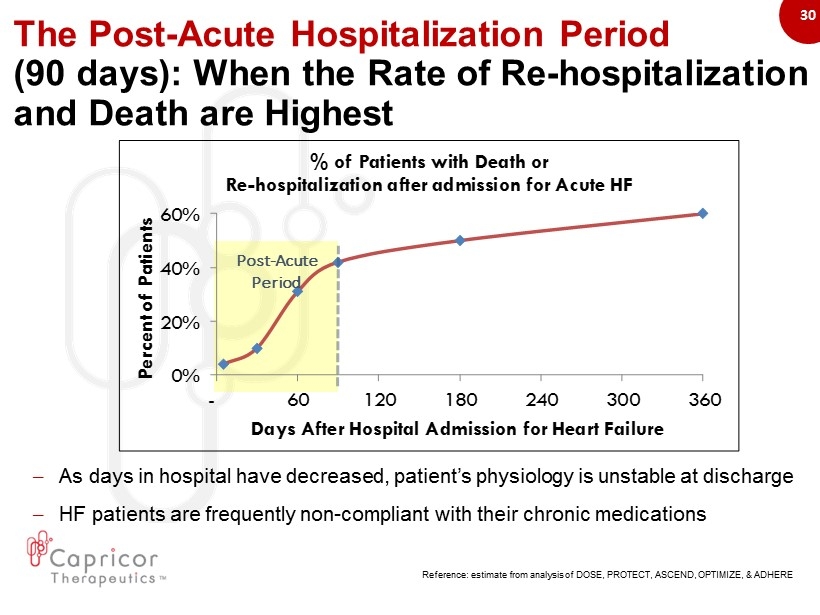
30 The Post - Acute Hospitalization Period ( 90 days): When the Rate of Re - hospitalization and Death are Highest 0% 20% 40% 60% - 60 120 180 240 300 360 Percent of Patients Days After Hospital Admission for Heart Failure % of Patients with Death or Re - hospitalization after admission for Acute HF Reference: estimate from analysis of DOSE, PROTECT, ASCEND, OPTIMIZE, & ADHERE Post - Acute Period – As days in hospital have decreased, patient’s physiology is unstable at discharge – HF patients are frequently non - compliant with their chronic medications
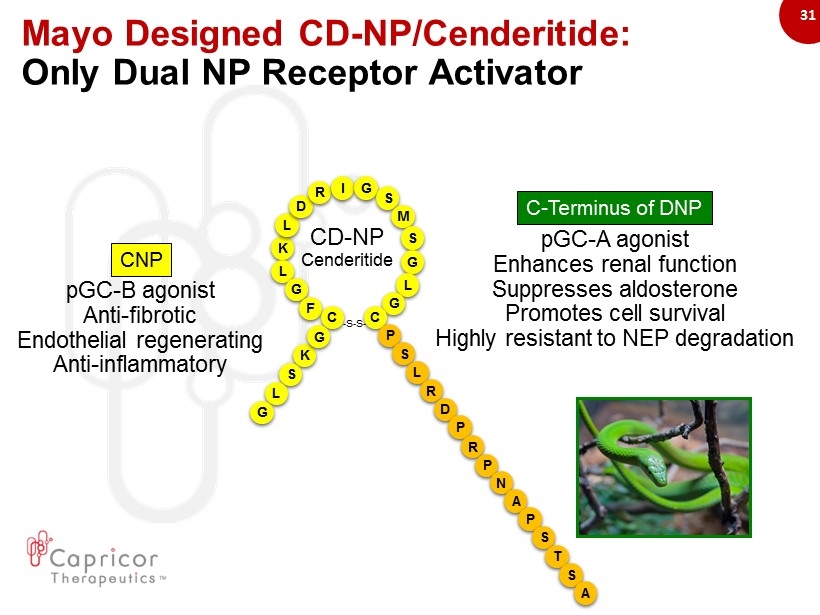
31 Mayo Designed CD - NP/Cenderitide: Only Dual NP Receptor Activator - S - S - K L L D R I G S M S G L G G F C C K G S L G pGC - B agonist Anti - fibrotic Endothelial regenerating Anti - inflammatory P S L R D CD - NP Cenderitide P R P N A P S T S A pGC - A agonist Enhances renal function Suppresses aldosterone Promotes cell survival Highly r esistant to NEP degradation CNP C - Terminus of DNP
32 - S - S - K L L D R I G S M S G L G G F C C K G S L G N H 2 P S L R D P R P N A P S T S A Cenderitide The Opportunity + 1 st arm of Phase II complete
33 Cenderitide for Outpatient and Ambulatory Heart Failure Target Indication Prevention of re - hospitalization in patients with a recent acute heart failure admission as well as other potential indications – Phase IIa PK/PD Trial ▪ 1 st arm - 14 patients treated, enrollment complete ▪ Patients with stable chronic heart failure ▪ Trial assessed the safety and tolerability, pharmacokinetics profiles, and pharmacodynamic response to increasing dose levels of Cenderitide ▪ No significant safety issues and Insulet pump proved effective ▪ Early results suggest tolerability and physiologic effect – Initiating a second study to further assess higher doses – Announce further plans following results of second study

34 Senior Management – Chief Executive Officer Linda Marbán, Ph.D. (Founder, JHU, Cleveland Clinic) – Chief Medical Officer Deborah Ascheim, M.D. (Mount - Sinai, Columbia University) – EVP & General Counsel Karen Krasney, J.D. – Acting VP Clinical Operations: Jeff Rudy, (Amgen, Celladon) – VP of New Therapies Houman Hemmati, M.D., Ph.D. (Allergan) – VP of R&D for Regenerative Therapies Luis Rodriguez - Borlado, Ph.D . (Coretherapix) – VP of Research and Development Rachel Smith, Ph.D. (Johns Hopkins) – VP of Finance AJ Bergmann, M.B.A. Board of Directors – Executive Chairman Frank Litvack, M.D. (ConorMed) – Linda Marbán, Ph.D. – Dave Musket (ProMed Partners) – Earl M. (Duke) Collier, Jr . (Genzyme) – George W. Dunbar, Jr . (Aastrom) – Joshua Kazam (Kite, Two - River) – Gregory Schafer (Aduro, Onyx) – Louis Manzo (Investor) – Louis J. Grasmick (Investor ) Scientific Advisory Board – Chairman Eduardo Marbán, M.D., Ph.D. (Founder , JHU , Cedars - Sinai) Senior Management & Board of Directors

NASDAQ: CAPR December 2015 Transformative Therapies from Bench to Bedside www.capricor.com
