Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Cardiff Oncology, Inc. | a15-23097_18k.htm |
Exhibit 99.1
November 2015 Better Cancer Monitoring with Cell-Free DNA

Forward-Looking Statements Statements in this presentation about the Company's expectations, applications of its technology, markets, launch of tests and other statements that are not historical facts are “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934 and are based on management's current beliefs, assumptions, estimates and projections. Actual results may differ materially from those projected in the forward-looking statements for various reasons, including risks associated with product and test development, test transfer to contracting labs, government regulation, market acceptance, limited commercial experience, dependence on key personnel, obtaining financing and other factors discussed in the Company's periodic reports filed with the Securities and Exchange Commission. 2 Copyright ©2015 Trovagene, Inc. Confidential

3 Our Goal Improve Treatment Outcomes with Noninvasive Cancer Monitoring Trovagene’s technology noninvasively detects and quantitates circulating tumor DNA in urine and plasma for improved disease management

Copyright ©2015 Trovagene, Inc. Confidential 4 Clinical collaborations with leading cancer centers and pharmaceutical partners CLIA certified, CAP accredited, high complexity lab to offer diagnostic services Trovagene’s noninvasive liquid biopsy assays are used to detect and monitor oncogene mutation status, response to therapy, disease progression, minimal residual disease and recurrence Proprietary methods of extracting, purifying, detecting and quantifying oncogene mutations in cell-free DNA NASDAQ: TROV Founded in 1999 NASDAQ listing in 2012 Technology feasibility established 2013 Clinical feasibility established 2014 Marketing launch initiated 2015 Cash: $74M as of Sept 30, 2015 Company Overview

Matt Posard Chief Commercial Officer Karsten Schmidt, PhD Senior VP, Technology Operations Illumina Inc., Biosite Dx, Gen-Probe BA Quantitative Economics and Decision Sciences, UCSD Sequenom, Inc., Sanofi-Aventis Deutschland GmbH (formerly: Rhône-Poulenc Rorer), Fisons plc PhD The University of Bonn Experienced Leadership Team Copyright ©2015 Trovagene, Inc. Confidential 5 Antonius Schuh, PhD Chief Executive Officer Stephen Zaniboni Chief Financial Officer Mark Erlander, PhD Chief Scientific Officer Sorrento Therapeutics, AviaraDx, Arcturus, Sequenom PhD Pharmaceutical Chemistry, University of Bonn Awarepoint, XIFIN, Sorrento Therapeutics, AviaraDx, Arcturus, Sequenom CPA, BS Accounting Boston University, MBA Boston College BioTheranostics, AviaraDx, Arcturus, J&J, Scripps Research Institute BS UCDavis, MS Biochemstry Iowa State University, PhD Neuroscience UCLA

Strong Scientific Advisory Board and Board of Directors Copyright ©2015 Trovagene, Inc. Confidential 6 Alberto Bardelli, PhD Department of Oncology, Torino Medical School, Institute for Cancer Research Paul Billings, MD, PhD Chief Medical Officer, Omica Scientific Advisors Charles Cantor, PhD Founder and Retired Chief Scientific Officer Sequenom Carlo M. Croce, MD Chairman, Department of Molecular Virology, Immunology and Medical Genetics at Ohio State University, and Director of the Institute of Genetics at the Ohio State University Comprehensive Cancer Center Riccardo Dalla-Favera, MD Director, Institute for Cancer Genetics and Herbert Irving Comprehensive Cancer Center, Columbia University Brunangelo Falini, MD Director, Institute Hematology, University of Perugia Peter Hirth, PhD Co-founder and Retired Chief Executive Officer of Plexxikon Thomas Adams, PhD Chairman Paul R. Billings, MD, PhD Director, Member Executive Compensation and Nominating Committees John P. Brancaccio, CPA Director, Chair Audit Committee Carl Feldbaum Director, Chair Nominating Committee Gary Jacob, PhD Director, Member Executive Compensation Committee Rodney S. Markin, MD, PhD Director, Member Executive Compensation, Nominating and Audit Committees Antonius Schuh, PhD Director, Chief Executive Officer Stanley Tennant, MD Director, Chair Executive Compensation Committee and Member Audit Committee Board of Directors
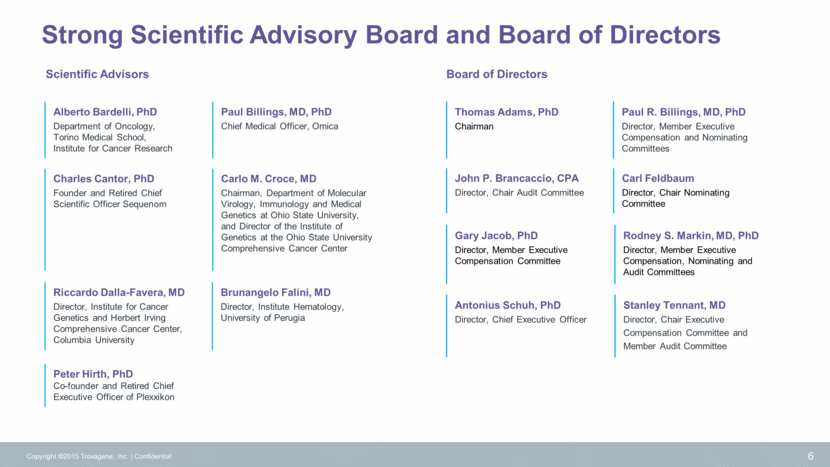
Significant Market Opportunity Physicians lack tools to effectively monitor and react to changes in cancer over time Copyright ©2015 Trovagene, Inc. Confidential 7 Source: 2010 BCC Research Trovagene PCM delivers critical information to detect and measure actionable cancer mutations over time Insufficient Imaging Tools in Oncology Still Yield Huge Market Opportunity for Trovagene to Capture Liquid Biopsy Market Share $15B 2015 est. Cancer already in advanced state No molecular data delivered Source: 2015 Liquid Biopsy Report Recurrence Monitoring Treatment Monitoring Therapy Selection Liquid Biopsy Market – $13.6B
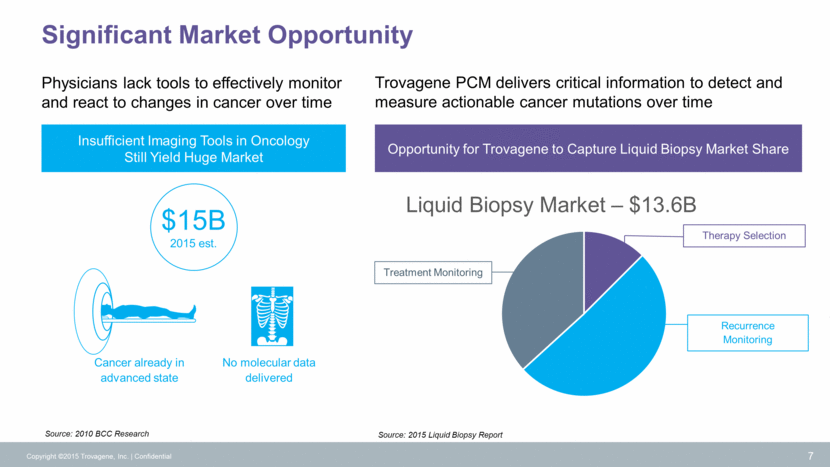
Detection Minimal Residual Disease (MRD) Quantitate Drug Response Monitoring New Standard of Care Enabled by Trovagene Technology Copyright ©2015 Trovagene, Inc. Confidential 8 Biopsy Surgery Treatment Treatment Biopsy PROCEDURE IMAGING Every 6-8 weeks Every 6-8 weeks Trovagene platform allows physicians to make clinically actionable decisions in near “real-time” Monitoring for minimal residual disease and tumor recurrence Earlier detection of metastatic disease Detection of clinically actionable mutations Alternative to tissue biopsy Assists in identifying most effective treatment Eliminates risk of adverse events associated with tissue biopsy procedure Assess tumor cell kill by therapy and monitor tumor mutation burden Immediate assessment of therapy effect Switch from ineffective therapy weeks in advance of imaging Monitoring for disease progression and emergence of resistance Anticipates and enables next therapy to target tumor resistance PCM CLINICAL UTILITY
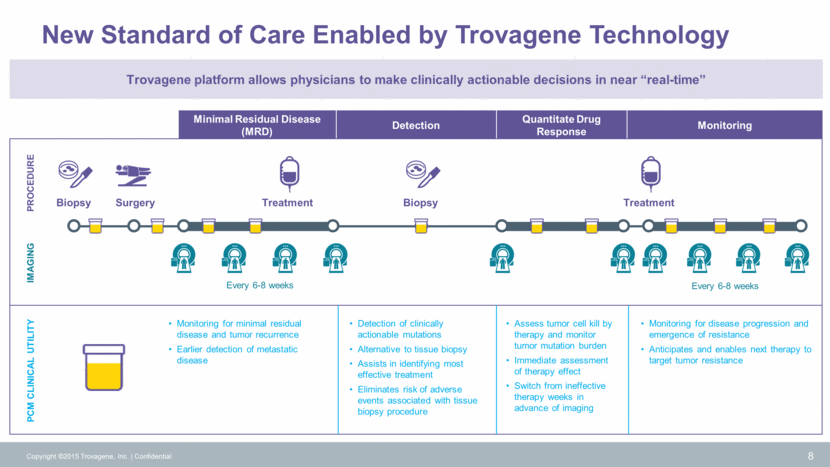
Tumor cells Blood Urine Advantages of Circulating Tumor DNA (ctDNA) 9 Copyright ©2015 Trovagene, Inc. Confidential Apoptosis or necrosis occurs, ctDNA is released ctDNA enters blood stream and kidneys High-depth systemic understanding of tumor dynamics Delivers clinically actionable data in real time Provides diagnostic and prognostic information before, during treatment and at disease progression Captures intra- and inter-tumor heterogeneity
Trovagene Precision Cancer Monitoring (PCM ) Platform 10 SM Copyright ©2015 Trovagene, Inc. Confidential 100-1000 fold enrichment ~300,000 genome equivalents of cfDNA/200 mL of urine Collection, Extraction and Isolation of ctDNA Mutant Allele Enrichment Detection and Quantification Enables monitoring over time Ultra-Sensitive Detection and Quantitative Monitoring Integrates with NGS and digital PCR platforms LDT and CLIA compliant Compatible for tech transfer agreements
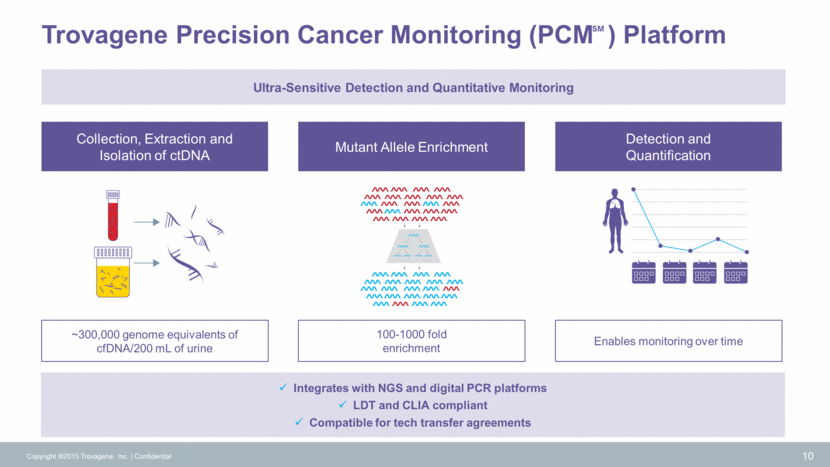
Intellectual Property Copyright ©2015 Trovagene, Inc. Confidential 11 51-110 bp TrNA Family Concentrating Urine Family Patent Term 2035 US provisional app filed 2034 US provisional app filed 2029 US & EU 2018 US & EU Small Footprint Family Monitoring Disease Family 20-50 bp TrNA Family Anion Exchange Purification Family Viral and Pathogen TrNA Families Patent Families TrNA Patent Family 2026 US, EU, JP, China, Australia, Canada, India 2034 US provisional app filed 2034 US provisional app filed 2027 US, EU, Canada
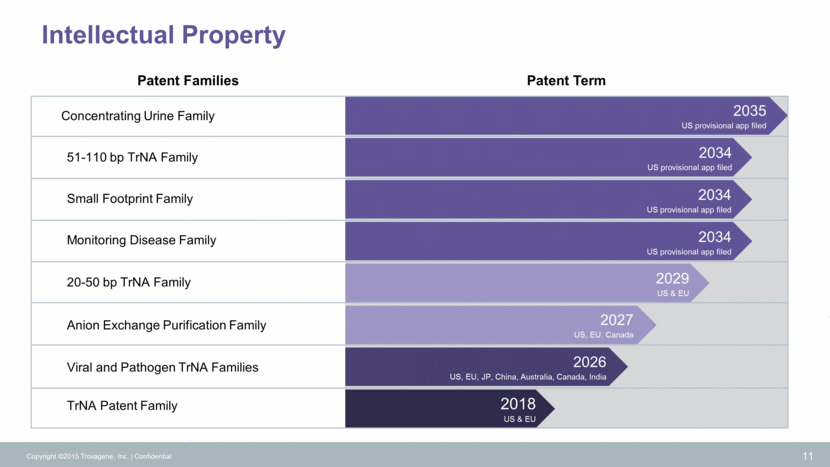
Copyright ©2015 Trovagene, Inc. Confidential 12 Technology Portfolio Evolution Feasibility Analysis In Development Commercially Available KRAS (Exon 2) BRAF (V600E) EGFR (Exon 19 Del) EGFR (Exon 20 T790M) EGFR (Exon 21 L858R) KRAS (5 assays) NRAS (5 assays) EGFR (6 assays) BRAF (1 assays) PIK3CA (2 assays) Find-It Follow-It ALK RET ROS Single Assays for Monitoring (Ultrasensitive, Quantitative) Multi-gene Panel Gene Rearrangements
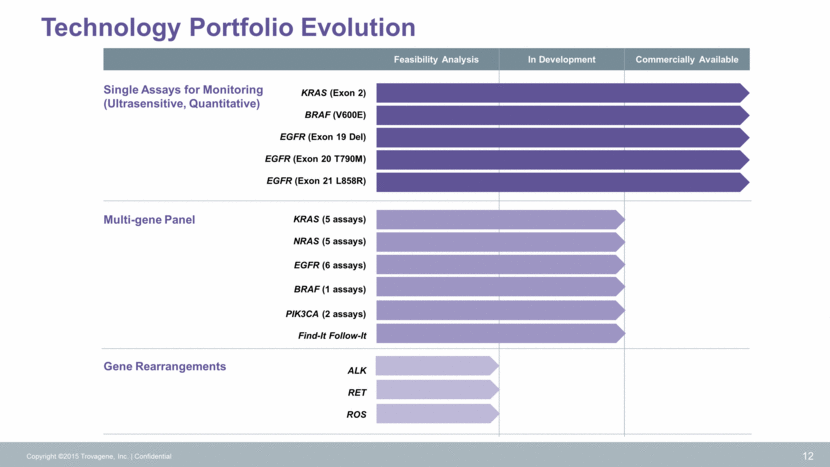
Distribution and Service Options Copyright ©2015 Trovagene, Inc. Confidential 13 CLIA Lab Pharma Services Tech Transfer Cloud Exchange Centralized Democratized
Trovagene Research Institute (TRI) World Class Team & Technology to Enhance Our Clinical Expertise and Commercial Reach Copyright ©2015 Trovagene, Inc. Confidential 14 Goal: Accelerate adoption of Trovagene's Precision Cancer Monitoring platform in translational research and clinical applications World leader in cancer genomic research and translational science Scientific Chair European Subsidiary of Trovagene Collaboration with University of Torino Alberto Bardelli, PhD Accomplished translational research team Center of Excellence in Europe Established network with pharmaceutical and life science companies Technology and intellectual property transferred to TRI Leverage relationships with key opinion leaders and pharmaceutical companies Conduct clinical and translational studies in Europe
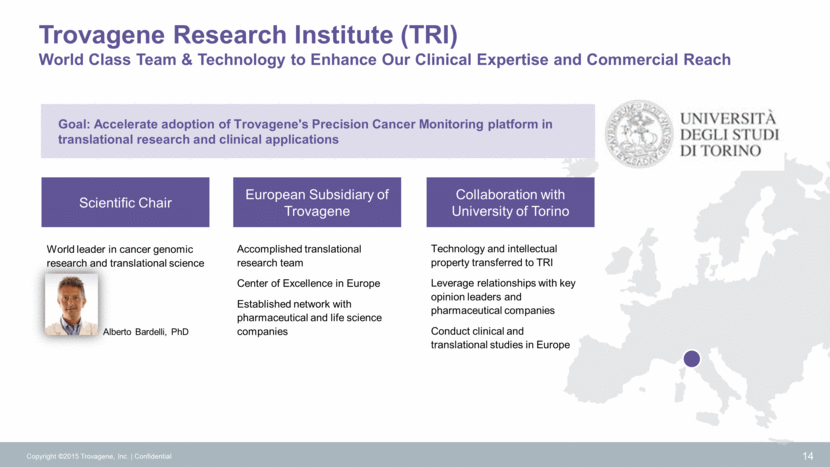
Strategy to Drive Clinical Adoption and Reimbursement Copyright ©2015 Trovagene, Inc. Confidential 15 Demonstrate concordance of oncogene mutation status between ctDNA and tumor tissue Clinical Utility: Determine mutational status of actionable biomarkers in ctDNA when a tissue biopsy is not available Expand mutational coverage of the PCM platform Clinical Utility: Quantitatively assess mutational status in ctDNA longitudinally as an indicator of responsiveness to therapy, disease status and emergence of resistance mutations Demonstrate, in multi-institutional trials, that results from ctDNA-based monitoring of actionable mutations increases patient progression-free survival (PFS) and overall survival (OS) Demonstrate health economic benefits of noninvasive oncogene mutation monitoring Clinical Utility: Quantitatively assess patient mutational status in ctDNA longitudinally for mutational status and early detection of resistance to therapy as a decision tool for therapy selection QUALITATIVE QUANTITATIVE STANDARD OF CARE Stage 1 Tumor Status Stage 2 Tumor Dynamics Stage 3 Improve Patient Outcomes
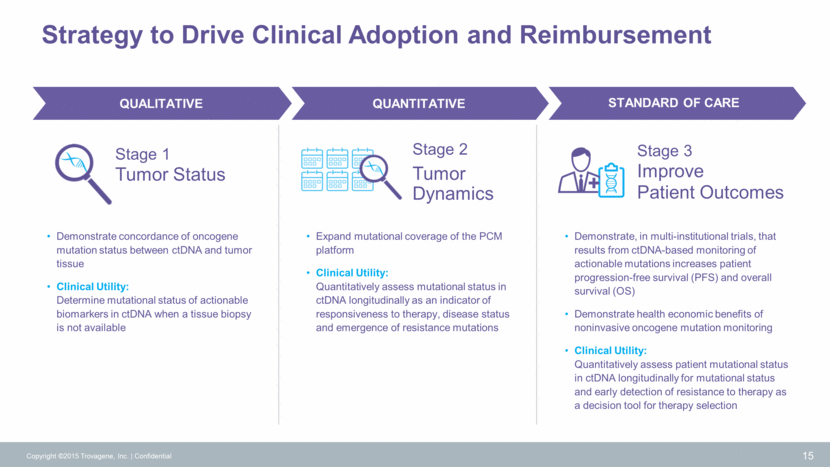
ECD/LCH Tissue Biopsy First Line Treatment Imaging (every 2-3 months) Copyright ©2015 Trovagene, Inc. Confidential 16 Monitoring Therapeutic Response BRAF V600E Detection BRAF V600E TROVAGENE ECD/LCH ALL STAGES Molecular diagnosis of BRAF mutation – patients are placed on targeted therapy Re-staging of cancer CLINICAL YTILITY MSKCC, MDACC, NIH (30 patients) STUDIES Histiocytic Disorders: ECD/LCH Standard of Care
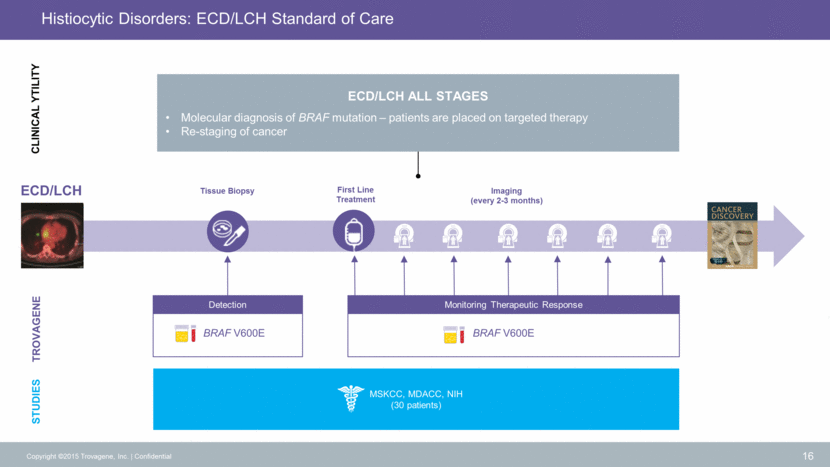
Urinary ctDNA Outperforms Tissue Biopsies1 1Hyman et al., Cancer Discov. 2015 Jan;5(1):64-71 17 Copyright ©2015 Trovagene, Inc. Confidential Detection Urinary ctDNA BRAF V600E genotype Tissue BRAF V600E genotype Indeterminate Genotype BRAFV600E Mutant BRAF Wildtype n=9 n=6 n=15 BRAFV600E Mutant BRAF Wildtype n=14 n=16 70% BRAF mutational status obtained 100% BRAF mutational status obtained Urine Tissue Biopsies 100% concordance between tissue, urine and plasma in treatment naive patients.
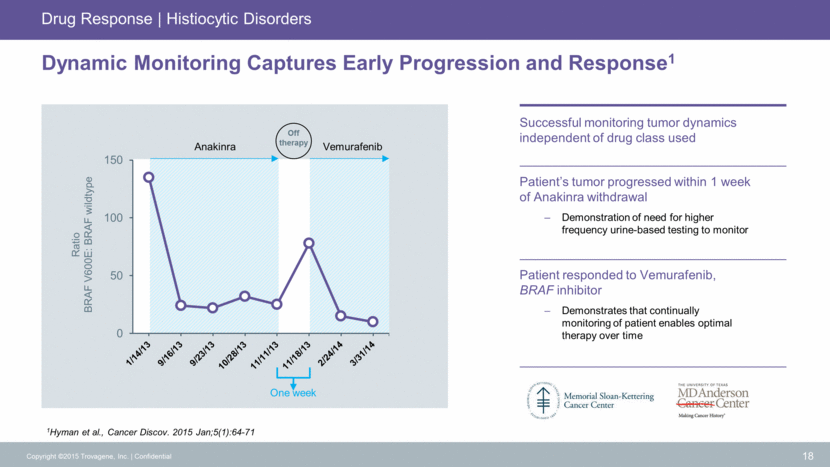
Dynamic Monitoring Captures Early Progression and Response1 Successful monitoring tumor dynamics independent of drug class used One week Patient’s tumor progressed within 1 week of Anakinra withdrawal Demonstration of need for higher frequency urine-based testing to monitor Patient responded to Vemurafenib, BRAF inhibitor Demonstrates that continually monitoring of patient enables optimal therapy over time 1Hyman et al., Cancer Discov. 2015 Jan;5(1):64-71 18 Copyright ©2015 Trovagene, Inc. Confidential Drug Response Histiocytic Disorders Anakinra Vemurafenib Off therapy Ratio BRAF V600E: BRAF wildtype 0 50 100 150
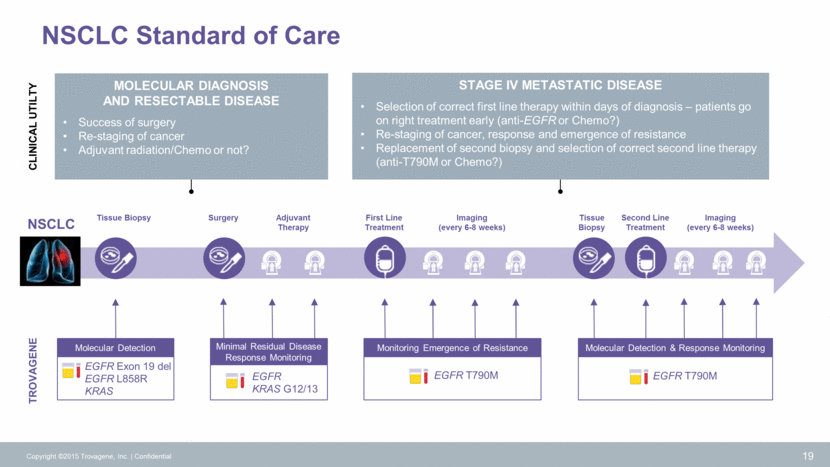
Molecular Detection & Response Monitoring EGFR T790M Monitoring Emergence of Resistance EGFR T790M NSCLC Standard of Care TROVAGENE NSCLC Tissue Biopsy First Line Treatment Tissue Biopsy Second Line Treatment Imaging (every 6-8 weeks) Surgery Imaging (every 6-8 weeks) Adjuvant Therapy 19 Molecular Detection EGFR Exon 19 del EGFR L858R KRAS EGFR KRAS G12/13 Minimal Residual Disease Response Monitoring CLINICAL UTILTY Molecular DIAGNOSIS and RESECTABLE DISEASE Success of surgery Re-staging of cancer Adjuvant radiation/Chemo or not? Stage IV Metastatic DISEASE Selection of correct first line therapy within days of diagnosis – patients go on right treatment early (anti-EGFR or Chemo?) Re-staging of cancer, response and emergence of resistance Replacement of second biopsy and selection of correct second line therapy (anti-T790M or Chemo?) Copyright ©2015 Trovagene, Inc. Confidential
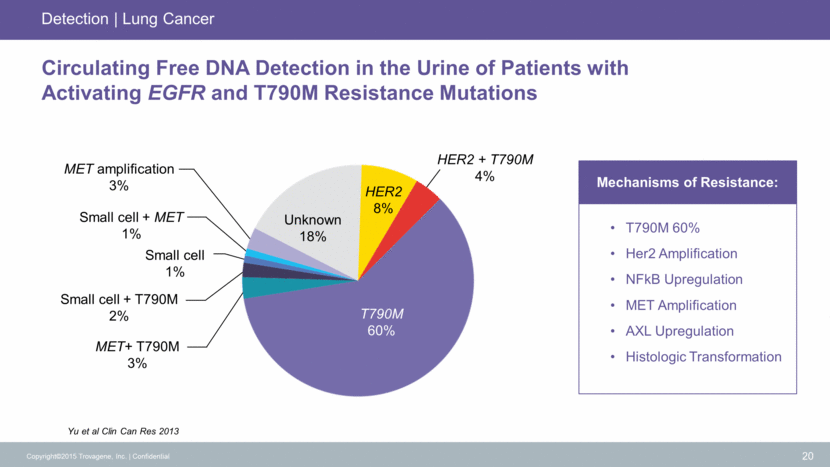
Yu et al Clin Can Res 2013 Copyright©2015 Trovagene, Inc. Confidential 20 Circulating Free DNA Detection in the Urine of Patients with Activating EGFR and T790M Resistance Mutations T790M 60% Her2 Amplification NFkB Upregulation MET Amplification AXL Upregulation Histologic Transformation Mechanisms of Resistance: Detection Lung Cancer Small cell 1% HER2 + T790M 4% MET amplification 3% Small cell + MET 1% Small cell + T790M 2% MET+ T790M 3% HER2 8% T790M 60% Unknown 18%
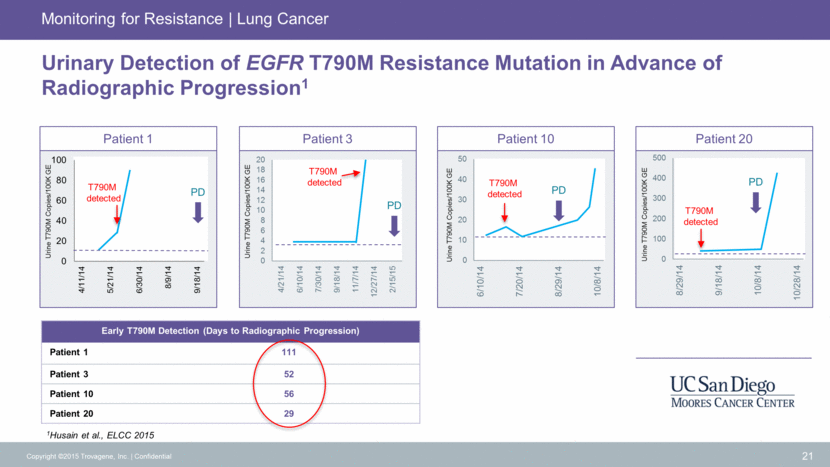
Urinary Detection of EGFR T790M Resistance Mutation in Advance of Radiographic Progression1 21 Copyright ©2015 Trovagene, Inc. Confidential Monitoring for Resistance Lung Cancer Patient 20 Patient 10 Patient 3 PD PD PD T790M detected T790M detected PD T790M detected T790M detected Urine T790M Copies/100K GE Patient 1 Urine T790M Copies/100K GE Urine T790M Copies/100K GE Urine T790M Copies/100K GE 1Husain et al., ELCC 2015 Early T790M Detection (Days to Radiographic Progression) Patient 1 111 Patient 3 52 Patient 10 56 Patient 20 29 0 2 4 6 8 10 12 14 16 18 20 4/21/14 6/10/14 7/30/14 9/18/14 11/7/14 12/27/14 2/15/15 0 20 40 60 80 100 4/11/14 5/21/14 6/30/14 8/9/14 9/18/14 0 10 20 30 40 50 6/10/14 7/20/14 8/29/14 10/8/14 0 100 200 300 400 500 8/29/14 9/18/14 10/8/14 10/28/14
Monitoring Urinary ctDNA EGFR Mutation Within First 2 Weeks of Targeted Therapy Predicts Subsequent Radiographic Response in Patients 22 Copyright ©2015 Trovagene, Inc. Confidential Quantitate Drug Response Lung Cancer T790M Ex19 del Size on CT scan Sum of Longest Diameters (mm) Week 1 on drug 6 wks CT scan 12 wks CT scan 1Husain et al., ELCC 2015 Patient 1 Best Overall Response – PR Copies/100K GE (95% CI) Time on Drug T790M Exon 19 del Baseline 24 (19-38) 167 (125-267) Day 1 (4 h on drug) < LOD 8 (6-13) Day 1 221 (168-361) 87 (65-139) Day 2 34 (28-55) 117 (88-187) Day 3 48 (39-78) 36 (27-58) Day 4 < LOD < LOD Day 5 15 (13-25) < LOD Day 6 < LOD 19 (14-30) Day 7 < LOD < LOD Week 2 < LOD < LOD Week 3 < LOD < LOD Week 4 < LOD < LOD Week 6 < LOD < LOD Week 12 < LOD < LOD LOD (T790M) = 2 copies (12 copies/100K GE) LOD (Ex 19 del) = 1 copy (6 copies/100K GE) SLD = Sum of the Longest Diameter of Lesions 0 10 20 30 40 50 60 70 80 0 50 100 150 200 250 10/20/2014 10/27/2014 11/3/2014 11/10/2014 11/17/2014 11/24/2014 12/1/2014 12/8/2014 12/15/2014 12/22/2014 12/29/2014 1/5/2015 1/12/2015 1/19/2015 Urine EGFR Copies/100K GE
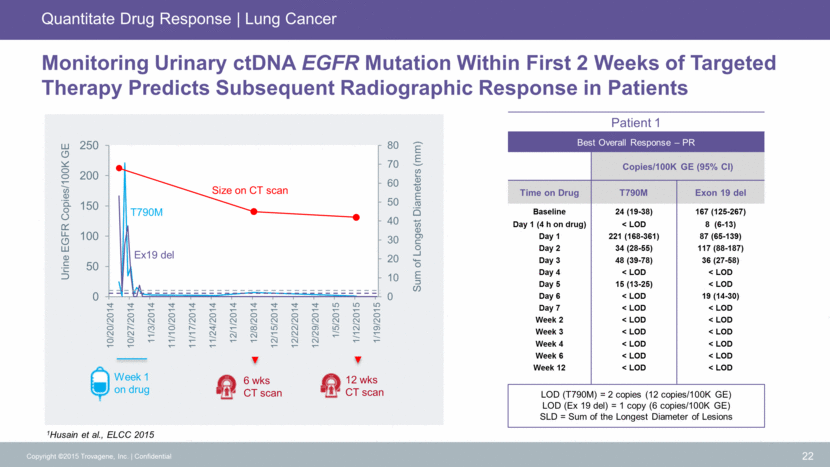
Case Study Copyright ©2015 Trovagene, Inc. Confidential 23 September 2013 December 2013 March 2014 May 2014 August 2014 November 2014 January 2015 February 2015 Needle tissue biopsy performed, confirming NSCLC Patient started on erlotinib Scan confirmed decrease in tumor and metastases Scan confirmed stable disease Scan confirmed new lesion Patient declined re-biopsy and remained on erlotinib Tissue biopsy performed Sample insufficient for comprehensive genomic evaluation Imaging confirmed progression Afatinib started Re-biopsy performed Results not reportable - insufficient amount of extracted DNA Patient started on chemotherapy and experienced intolerable side effects Urine obtained for ctDNA T790M mutation identified Patient started on AZ9291 – showed good symptomatic and imaging response The value of higher sensitivity of urine-based liquid biopsy compared to tissue biopsy Patient: 71 year old female Disease: Non-Small Cell Lung Cancer Trovagene PCM Applied: Detection of L858R and T790M Mutations
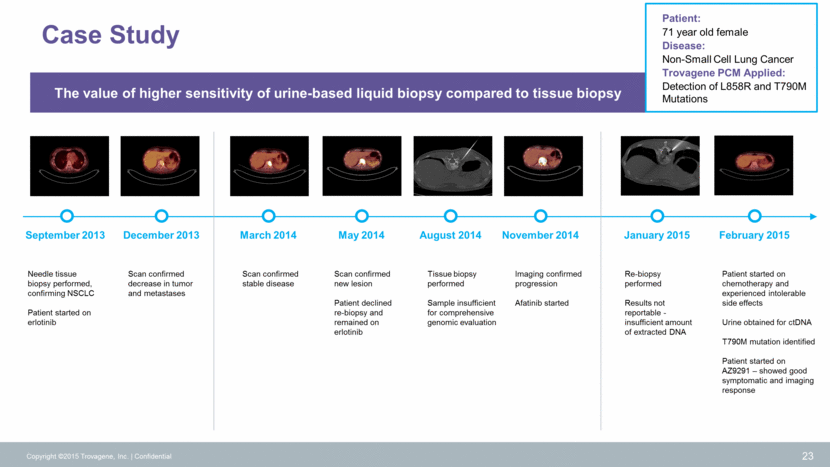
TROVAGENE Adjuvant Therapy Imaging (every 6 weeks) Systemic Therapy Imaging (every 6 wks-6 months) Surgery Molecular Detection 24 KRAS, NRAS, BRAF Minimal Residual Disease Response Monitoring Molecular Detection KRAS, NRAS, BRAF KRAS, NRAS, BRAF Response Monitoring CRC CLINICAL UTILTY Stage I-III Resectable Stage IV Resectable Liver Metastases Success of surgery Re-staging of cancer Chemo or not? Advanced and metastatic unresectable Replacement of biopsy performed with sole purpose of molecular diagnosis of RAS: selection of correct therapy within days of diagnosis – patients go on right treatment early (anti-EGFR/ VEGFR/ BRAF or Chemotherapy?) Re-staging of cancer, response and emergence of resistance Colorectal Cancer Standard of Care Copyright ©2015 Trovagene, Inc. Confidential
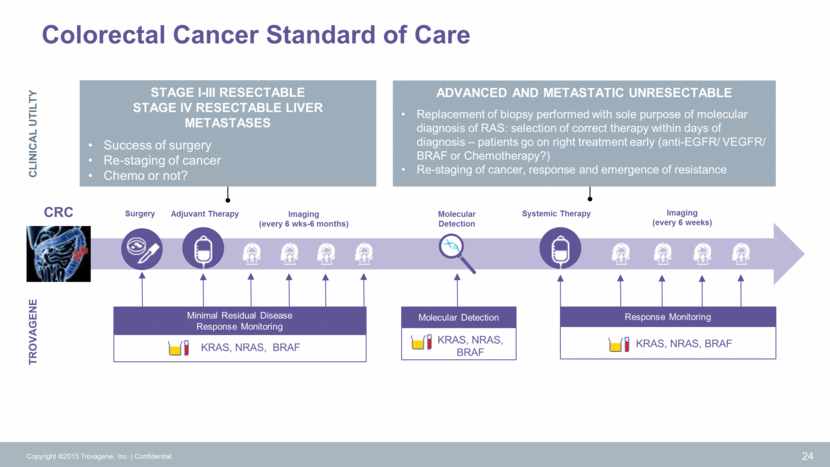
Monitoring ctDNA KRAS for Minimum Residual Disease Post Surgery1 1Melnikova et al, AACR 2015 25 Copyright ©2015 Trovagene, Inc. Confidential Minimal Residual Disease (MRD) Colorectal Cancer Curative surgery correlates with undetectable levels of urinary ctDNA KRAS post-surgery in 4 of 5 patients Palliative surgery correlates with high levels of urinary ctDNA KRAS post-surgery in 10 of 12 patients Colorectal Cancer with Resectable Liver Metastases Surgery Days Alive > 1967 Patient 9 Days Alive = 292 Surgery 1.2 1.0 0.8 0.6 0.4 0.2 0.0 Fold Change KRAS Copies (Plasma) 250 200 150 100 50 0 Fold Change KRAS Copies (Plasma) Urine Plasma Urine Plasma Patient 12 0.00 0.50 1.00 1.50 2.00 Fold Change KRAS Copies (Urine) Collection Date 0.00 2.00 4.00 6.00 8.00 10.00 12.00 14.00 16.00 18.00 Fold Change KRAS Copies (Urine) Collection Date
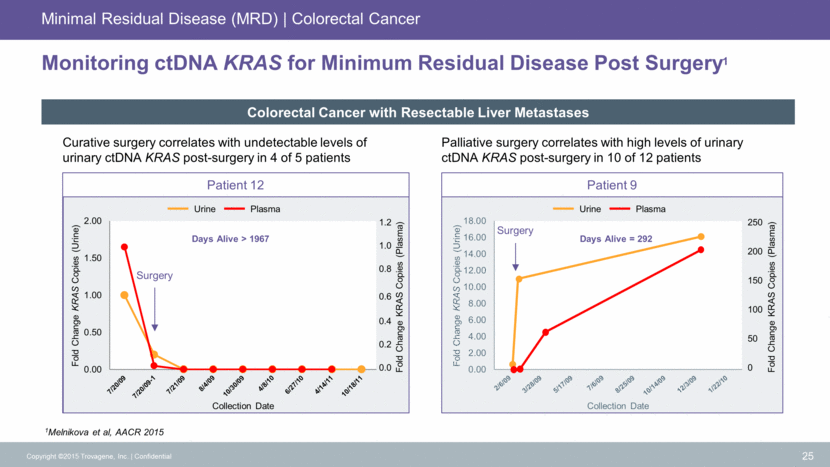
Pancreatic Cancer Standard of Care 26 TROVAGENE Diagnosis Biopsy Adjuvant Therapy Imaging (every 6-12 weeks) Imaging (every 6-12 weeks) Resectable Disease Surgery Non-Resectable Disease Chemotherapy CLINICAL UTILTY Diagnosis Screening for cancer RESECTABLE DISEASE Success of surgery Re-staging of cancer Chemo or not? Advanced and Metastatic Unresectable Prognosis of disease for determining aggressiveness of therapy Clinical trials targeting KRAS Prognostic for Survival KRAS G12/13 + CA-19-9 KRAS G12/13 KRAS G12/13 Minimal Residual Disease Response Monitoring Pancreatic Cancer Screening KRAS G12/13 CA 19-9 Response Monitoring Pancreatic Cancer Copyright ©2015 Trovagene, Inc. Confidential
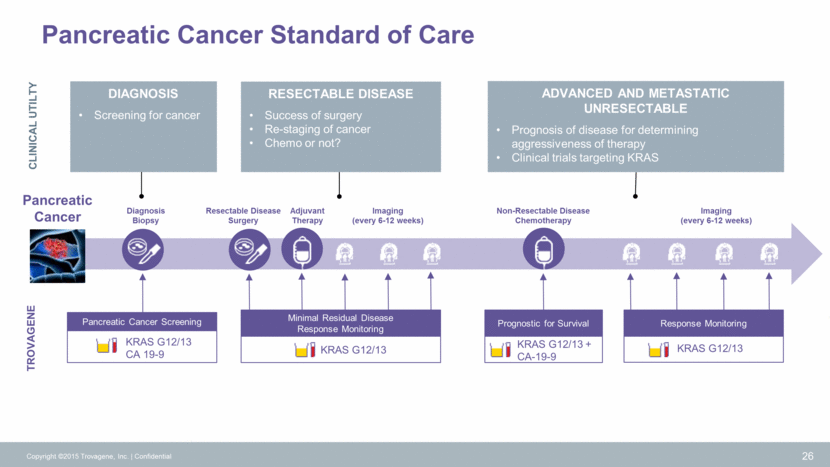
Melanoma Melanoma Standard of Care TROVAGENE First Line Treatment Second Line Treatment Imaging (every 6-8 weeks) Imaging (every 6-8 weeks) Response Monitoring BRAF – Drug Holidays Imaging (every 6-8 weeks) 27 CLINICAL UTILTY Stage IV Metastatic MELANOMA Monitoring response allows selection or early switch to optimal therapy: BRAF inhibitors or Immunotherapy? Optimal intervals on intermittent BRAF therapy Immunotherapy “Pseudoprogression” or not? Copyright ©2015 Trovagene, Inc. Confidential
30+ Clinical Studies to Validate & Integrate Our PCM Platform Copyright ©2015 Trovagene, Inc. Confidential 28 Disease Ongoing Pending Protocol in Dev/Approval Under Evaluation Market Size U.S. (# of pts) Lung Cancer (NSCLC) 7 0 – 399,000 Colorectal Cancer 3 3 – 1,154,000 Pancreatic Cancer 4 – 1 42,000 Melanoma 2 2 – 922.000 ECD/LCH 2 1 – 5,000 Brain Cancer 1 1 – 142,000 Prostate Cancer – 1 – 2,618,000 All-Comers, Metastatic Cancers 2 – – 525,000 HPV 4 – 3 Total # of Studies 25 8 4 This image cannot currently be displayed.

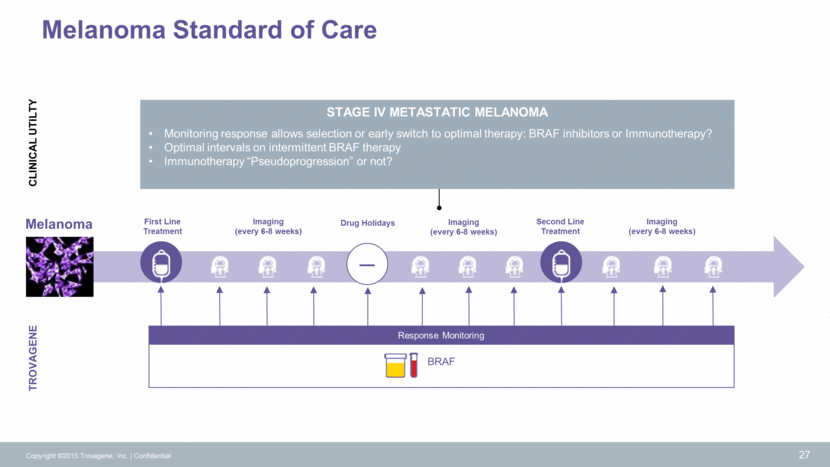
Clinical Studies Continue to Drive Presentations & Publications Copyright ©2015 Trovagene, Inc. Confidential 29 NEXTGEN DX SUMMIT Oral Presentation, Technology IPV Meeting Poster Presentation HR-HPV Publication Cancer Discovery Histiocytic BRAF 3Q’14 Publication Blood ECD Clinical Guidelines 4Q’14 1Q’15 2Q’15 Eurogin Congress Clinical Results Presented HR-HPV Large-scale Trial ELCC Meeting Oral Presentation EGFR T790M 2 ASCO GI Posters Pancreatic KRAS University of Copenhagen Colorectal KRAS Genomac AACR Posters Pancreatic & colon cancers KRAS Urine sample has >10x ctDNA vs plasma sample Molecular Medicine Tri Conference Oral Presentation Trovagene Technology and Clinical Evidence Annual Conference of the Association for Value-Based Cancer Care Cost Savings: Urine vs Lung Cancer Biopsies Biomarkers & Diagnostics World Congress Oral Presentation ASCO Posters EGFR Lung KRAS Pancreatic KRAS Colon KRAS in Patients with BRAF Mutation 3Q’15 IPV Conference Barretos Validation Study NEXTGEN DX SUMMIT Oral Presentation Monitoring Tumor Dynamics by EGFR 16th World Conference on Lung Cancer Oral Presentation EGFR Lung Cancer ESMO Abstracts EGFR Lung Cancer KRAS Pancreatic Cancer Personalized Medicine World Congress Oral Presentation Trovagene Technology and Clinical Evidence 7/2014 8/2014 8/2014 10/2014 1/2015 1/2015 2/2015 2/2015 4/2015 4/2015 5/2015 5/2015 6/2015 8/2015 9/2015 9/2015 9/2015
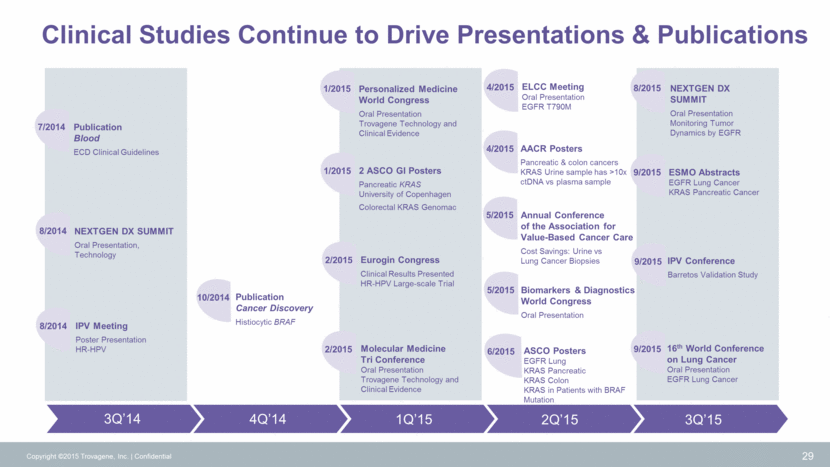
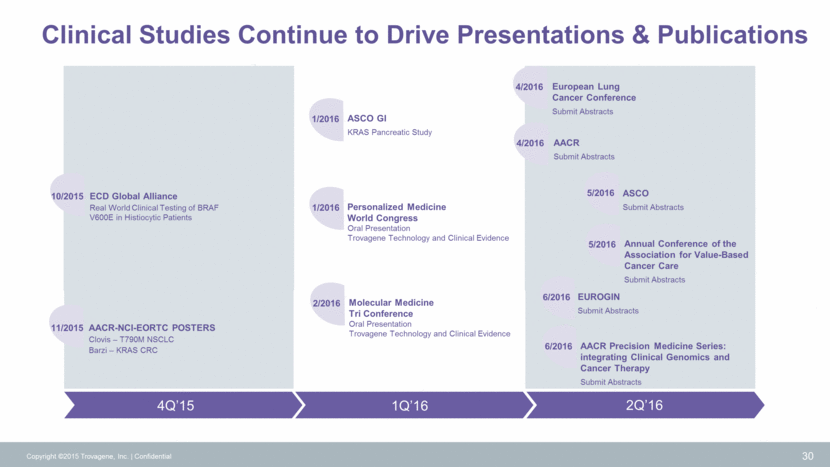
Clinical Studies Continue to Drive Presentations & Publications 30 EUROGIN Submit Abstracts ASCO GI KRAS Pancreatic Study Personalized Medicine World Congress Oral Presentation Trovagene Technology and Clinical Evidence Molecular Medicine Tri Conference Oral Presentation Trovagene Technology and Clinical Evidence AACR Submit Abstracts European Lung Cancer Conference Submit Abstracts ASCO Submit Abstracts Copyright ©2015 Trovagene, Inc. Confidential 2Q’16 1Q’16 4Q’15 ECD Global Alliance Real World Clinical Testing of BRAF V600E in Histiocytic Patients 10/2015 AACR-NCI-EORTC POSTERS Clovis – T790M NSCLC Barzi – KRAS CRC 11/2015 1/2016 1/2016 2/2016 4/2016 4/2016 5/2016 5/2016 Annual Conference of the Association for Value-Based Cancer Care Submit Abstracts 6/2016 6/2016 AACR Precision Medicine Series: integrating Clinical Genomics and Cancer Therapy Submit Abstracts
Creating Physician Awareness & Demand Copyright ©2015 Trovagene, Inc. Confidential 31 The Trovagene Clinical Experience Program (CEP) offers physicians hands-on experience with their patients Physician Access Benefits Choice of 3 Applications Urine as a sample Diagnostic in lieu of a tissue biopsy result Quantitation Baseline & monitor Ultra sensitivity Early therapeutic response
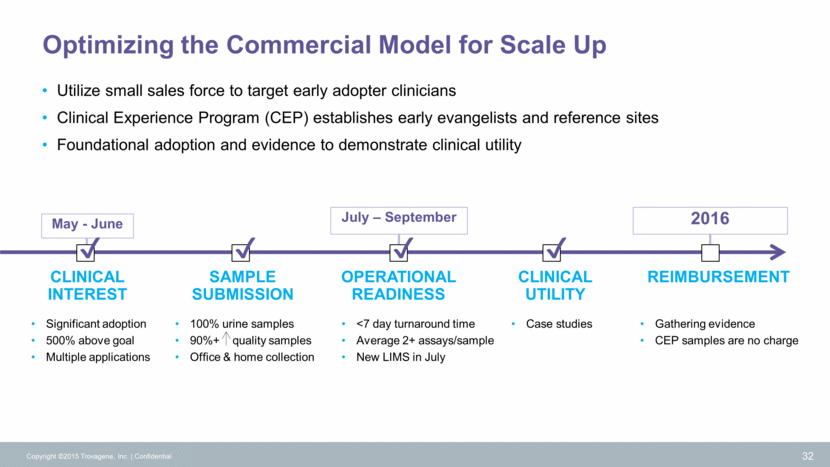
May - June July – September 2016 Optimizing the Commercial Model for Scale Up CLINICAL INTEREST Copyright ©2015 Trovagene, Inc. Confidential 32 SAMPLE SUBMISSION OPERATIONAL READINESS CLINICAL UTILITY REIMBURSEMENT Significant adoption 500% above goal Multiple applications 100% urine samples 90%+ quality samples Office & home collection <7 day turnaround time Average 2+ assays/sample New LIMS in July Utilize small sales force to target early adopter clinicians Clinical Experience Program (CEP) establishes early evangelists and reference sites Foundational adoption and evidence to demonstrate clinical utility Case studies Gathering evidence CEP samples are no charge
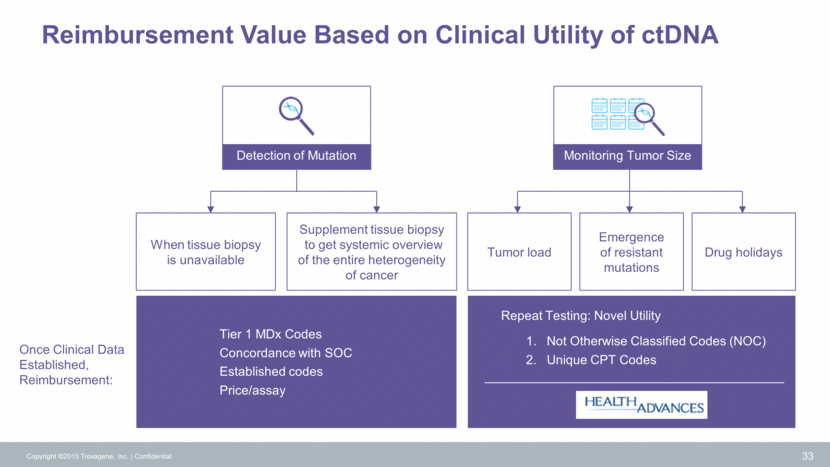
Reimbursement Value Based on Clinical Utility of ctDNA Copyright ©2015 Trovagene, Inc. Confidential 33 Detection of Mutation Monitoring Tumor Size When tissue biopsy is unavailable Tumor load Emergence of resistant mutations Drug holidays Tier 1 MDx Codes Concordance with SOC Established codes Price/assay Repeat Testing: Novel Utility Not Otherwise Classified Codes (NOC) Unique CPT Codes Once Clinical Data Established, Reimbursement: Supplement tissue biopsy to get systemic overview of the entire heterogeneity of cancer
2015 – 2016 Milestones Copyright ©2015 Trovagene, Inc. Confidential 34 Clinical Evidence 20+ abstracts in 2015 5 manuscripts Products 6 new menu assays Multiplex panels in 2016 Clinical Studies 30+ clinical studies Top academic centers Strategic Partnerships Geographic expansion Kit manufacturing Pharma collaboration Marketing & Adoption Real world case studies High clinical interest and strong service levels Reimbursement and sales & marketing scale up in 2016
For further information Please contact: Stephen Zaniboni, CFO szaniboni@trovagene.com Antonius Schuh, CEO aschuh@trovagene.com Matt Posard, CCO mposard@trovagene.com

