Attached files
| file | filename |
|---|---|
| EX-10.7 - EX-10.7 - MARRONE BIO INNOVATIONS INC | d90912dex107.htm |
| EX-31.1 - EX-31.1 - MARRONE BIO INNOVATIONS INC | d90912dex311.htm |
| EX-10.6 - EX-10.6 - MARRONE BIO INNOVATIONS INC | d90912dex106.htm |
| EX-32.1 - EX-32.1 - MARRONE BIO INNOVATIONS INC | d90912dex321.htm |
| EX-23.1 - EX-23.1 - MARRONE BIO INNOVATIONS INC | d90912dex231.htm |
| EX-31.2 - EX-31.2 - MARRONE BIO INNOVATIONS INC | d90912dex312.htm |
| EX-10.9 - EX-10.9 - MARRONE BIO INNOVATIONS INC | d90912dex109.htm |
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
| x | Annual report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 |
For the fiscal year ended December 31, 2014
or
| ¨ | Transition report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 |
For the transition period from to
Commission File Number: 001-36030
Marrone Bio Innovations, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 20-5137161 | |
| (State or other jurisdiction of Incorporation or organization) |
(I.R.S. Employer Identification No.) |
1540 Drew Avenue, Davis, CA 95618
(Address of principal executive offices and zip code)
(530) 750-2800
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| Class |
Exchange on which registered | |
| Common Stock, $0.00001 par value | NASDAQ Global Market |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ¨ No x
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ¨ No x
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 or Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not be contained, to the best of the registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act:
| Large accelerated filer | ¨ | Accelerated filer | ¨ | |||
| Non-accelerated filer | ¨ (Do not check if a smaller reporting company) | Smaller reporting company | x | |||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
As of June 30, 2015, the last day of the registrant’s most recently completed second quarter, the aggregate market value of the registrant’s voting and non-voting common stock held by non-affiliates was $20,508,703, based upon the closing price of the common stock as reported on the NASDAQ Global Market. This calculation excludes the shares of common stock held by each officer, director and holder of 5% or more of the outstanding common stock as of June 30, 2015. This calculation does not reflect a determination that such persons are affiliates for any other purposes.
Indicate the number of shares outstanding of each of the issuer’s classes of common stock, as of the latest practicable date.
| Class |
Shares Outstanding at October 31, 2015 | |
| Common Stock, $0.00001 par value |
24,464,582 |
Table of Contents
EXPLANATORY NOTE
In this Annual Report on Form 10-K, or this Form 10-K, Marrone Bio Innovations, Inc., or the Company, is restating its consolidated financial statements, selected financial data (as applicable) and certain financial data in management’s discussion and analysis and other information for the following periods: (i) the fiscal year ended December 31, 2013, including related quarterly periods; and (ii) the quarterly periods ended March 31 and June 30, 2014 (the Restatement). This is our first periodic report since our Quarterly Report on Form 10-Q for the period ended June 30, 2014. This report covers the fiscal years ended December 31, 2014, 2013 (as restated) and 2012 and includes unaudited condensed consolidated financial statements and supplemental information as applicable for quarterly periods ended March 31, 2014 (as restated), June 30, 2014 (as restated), September 30, 2014, December 31, 2014, March 31, 2013 (as restated), June 30, 2013 (as restated), September 30, 2013 (as restated) and December 31, 2013 (as restated).
The Company has not amended, and does not intend to amend, its Annual Report on Form 10-K for the year ended December 31, 2013 or any of its Quarterly Reports on Form 10-Q for periods prior to December 31, 2014. The Company also does not intend to file a Quarterly Report on Form 10-Q for the quarter ended September 30, 2014. The financial statements and related financial information for the restated periods contained in any of the Company’s filings prior to this Annual Report on Form 10-K for the year ended December 31, 2014 should no longer be relied upon.
Summary of the Restatement
On September 3, 2014, the Company announced that the Audit Committee of the Company’s board of directors had commenced an independent investigation after learning of documents calling into question the recognition of revenue in the fourth quarter of 2013 for an $870,000 transaction. The Audit Committee concluded, after consultation with management, that the Company’s previously reported financial statements as of and for the fiscal year ended December 31, 2013, the related report of the independent auditors on those 2013 financial statements dated March 25, 2014, and the unaudited interim financial statements as of and for the three months, the three and six months and the three and nine months ended March 31, June 30 and September 30, 2013, respectively, and as of and for the three months and the three and six months ended March 31 and June 30, 2014, respectively, should no longer be relied upon.
In February 2015, the Company announced the conclusion and findings of the Audit Committee’s independent investigation. As discussed further below, the Audit Committee principally determined that as a result of the failure of certain employees to share with the Company’s finance department or the external auditors important transaction terms with distributors, including “inventory protection” arrangements that would permit the distributors to return to the Company certain unsold products, the Company inappropriately recognized revenue for certain historical sales transactions with these distributors prior to satisfying the criteria for revenue recognition required under U.S. Generally Accepted Accounting Principles (GAAP).
In light of the foregoing, the Company’s management evaluated the necessity, nature and scope of any restatements of its previously filed financial statements, as discussed further below. Based on such evaluation, the Company, among other things, determined to change its revenue recognition methodology from “sell-in” to “sell-through” for sales to certain distributors. The Company has now recognized, in the aggregate, approximately $6.7 million less in product revenues than previously reported for 2013 and the first six months of 2014, as discussed further below. Of this amount, an aggregate of approximately $2.0 million in product was returned by certain distributors subsequent to June 30, 2014 pursuant to “inventory protection” rights and will not result in recognition of revenue in future periods.
Audit Committee Investigation
In late August 2014, a written agreement executed on behalf of the Company with one its distributors was identified by management that had not previously been disclosed by certain sales personnel to the Company’s
i
Table of Contents
finance department. This arrangement was promptly communicated to the Audit Committee, which decided to conduct an independent investigation of the issues identified by management. The Audit Committee promptly retained independent legal counsel to assist in the investigation.
The Audit Committee and its advisors investigated certain sales to distributors to determine whether undisclosed commitments were made that could have an impact on the timing and treatment of revenue recognition and whether the Company’s internal controls over financial reporting and disclosure controls and procedures were sufficient. The Audit Committee and its advisors also considered risk areas other than revenue recognition.
The investigation focused on revenue recorded in 2013 and the first two quarters of 2014. During the course of the investigation, the Audit Committee and its advisors collected and reviewed hard copy documents from individual custodians, electronically stored information, and various Company files. The Audit Committee also conducted 44 interviews with both current and former employees.
Audit Committee Findings
In February 2015, the Audit Committee completed its internal investigation. The principal findings of the Audit Committee were as follows:
| • | certain employees did not share with the Company’s finance department or the external auditors certain important transactional terms related to historical sales transactions; |
| • | certain sales personnel executed inaccurate “sales representation” letters, which are intended to inform the Company’s finance department and the external auditors of any commitments not included on a customer purchase order provided to the finance department; and |
| • | certain employees mischaracterized expenses related to agreements to pay for the storage and freight fees associated with certain transactions. |
As a result the Audit Committee concluded that the Company recognized revenue for certain transactions prior to satisfying the criteria for revenue recognition required under GAAP. In addition, the Audit Committee found that supply chain personnel were directed to ship the wrong product to a customer because the Company did not have the ordered product readily available.
The employees primarily responsible for the foregoing conduct are no longer with the Company. The Audit Committee concluded that the Company can rely on current management to accurately prepare the Company’s financial statements.
Restatement Process and Impact
Based on the results of the investigation, the Company’s management carried out a further evaluation to determine whether and by what amounts to restate any of the Company’s previously filed financial statements. This evaluation included collection and review of additional electronically stored information, transactional records from certain customers and correspondence with certain customers. Specifically, the Company’s management evaluated all distributor sales transactions during 2013 and the first two quarters of 2014 on a customer-by-customer and transaction-by-transaction basis. With respect to each individual transaction, the Company’s management evaluated relevant facts and circumstances that came to light in their review in order to apply the Company’s revenue recognition policy to such transactions.
Historically, the Company had determined that with limited exceptions, the criteria for revenue recognition were met at the point at which title was transferred to the distributor. However, based on the review, the following circumstances were identified for certain transactions that would result in the criteria for revenue recognition not being met with respect to such transactions until the Company’s products were sold through by the distributor, including:
| • | promises to certain distributors to accept returns of unsold inventory where the amount of future returns could not be reasonably estimated and/or we had significant obligations for future performance to bring about resale of the product by the distributor; and |
ii
Table of Contents
| • | arrangements with certain distributors that did not require payment of amounts due until product was resold by the distributor. |
Accordingly, in the restated periods, and for the foreseeable future, the Company is now using a “sell-through” method for sales to certain distributors rather than the “sell-in” method previously used by the Company. In general, under the “sell-in” method, sales by the Company to distributors are recognized at the point at which title was transferred to the distributors, in contrast to the “sell-through” method, whereby sales by the Company to distributors are not recognized as product revenues until the distributors sell the product through to end-users. The principal impact of switching from a “sell-in” to a “sell-through” method is that product revenues with respect to the applicable distributors are deferred to later periods.
Primarily as a result of the change in methodology from “sell-in” to “sell-through” for certain sales to certain distributors, the Company recognized approximately $6.1 million and $0.6 million less revenue for the year ended December 31, 2013 and the six months ended June 30, 2014, respectively, than had been previously reported. Of these amounts, an aggregate of approximately $2.0 million in product was returned by certain distributors subsequent to June 30, 2014 pursuant to “inventory protection” rights and will not result in recognition of revenue in future periods. The restatement of previously issued financial statements reduced the Company’s gross profit for the year ended December 31, 2013 and the six months ended June 30, 2014 by $2.6 million and $0.6 million, respectively. Net loss, basic loss per share and diluted loss per share for the year ended December 31, 2013 was increased by approximately $2.8 million, $0.32 per share and $0.31 per share, respectively, and the six months ended June 30, 2014 was increased by approximately $0.4 million, $0.02 per share and $0.02 per share, respectively.
For additional discussion of the accounting errors identified and the restatement adjustments, see Note 2, Restatement of Previously Issued Consolidated Financial Statements, and Note 21, Quarterly Financial Information (Unaudited), to the consolidated financial statements included in Part II–Item 8–“Financial Statements and Supplementary Data” in this Annual Report on Form 10-K.
Controls and Procedures
Management has assessed the adequacy of its internal control over financial reporting, and based on the Audit Committee’s recommendations, Company management has concluded that the following deficiencies related to the Audit Committee’s investigation constituted, individually or in the aggregate, material weaknesses in our internal control over financial reporting as of December 31, 2014:
| • | Control Environment - The control environment, which includes the Company’s Code of Conduct, is the responsibility of senior management, sets the tone of our organization, influences the control consciousness of employees, and is the foundation for the other components of internal control over financial reporting. The Audit Committee determined, based on the results of its independent investigation, that relevant information related to historical sales transactions, to which certain sales personnel were aware of, was consistently not shared with the finance department or the Company’s external auditors, certain sales personnel executed inaccurate representation letters, and certain sales personnel mischaracterized expense reports to pay for storage or freight charges associated with certain sales transactions. As a result of these findings, we determined that certain former sales personnel did not project an attitude of integrity and control consciousness, leading to insufficient attention to their responsibilities and internal controls. Further, effective mitigating controls were not in place to discourage, prevent or detect management override of internal control by certain sales personnel related to the Company’s process for recognizing revenue. |
| • | Revenue Recognition – The Company’s internal controls were not effectively designed to identify instances when sales personnel made unauthorized commitments with certain distributors, including “inventory protection” arrangements that would permit the distributors to return to the Company certain unsold products. In addition, controls were not in place to identify instances of management |
iii
Table of Contents
| override of internal controls by sales personnel related to the recognition of sales to the Company’s distributors. Consequently, revenue for certain transactions was recognized prior to satisfaction of all required revenue recognition criteria. |
While the Company has implemented the plan for remediation of these material weaknesses, the Company is still in the process of testing and evaluating the effectiveness of the remediation measures we have taken to date. In addition, many of these remediation efforts focus on continued training and communication of the Company’s enhanced policies and procedures. More specifically, the Company’s plan includes, among other things, improvement of the Company’s Code of Conduct and whistleblower policies; enhancement of training for all employees on the Company’s Code of Conduct and for sales personnel on the Company’s revenue recognition policy and the need for timely communication with the finance department; and expansion of the Company’s formal internal certification process to additional individuals within the Company. Additionally, as part of the Company’s remediation process the Company has taken steps to ensure that sales personnel primarily responsible for accounting improprieties are no longer employed by the Company.
The Company intends to continue to identify and implement actions to improve the effectiveness of its internal control over financial reporting. For more information on the status of the Company’s remediation efforts, please see Part II-Item 9A-“Controls and Procedures” in this Annual Report on Form 10-K.
iv
Table of Contents
Special Note Regarding Forward-Looking Statements and Trade Names
This Annual Report on Form 10-K includes a number of forward-looking statements that involve many risks and uncertainties. Forward-looking statements may be identified by the use of the words “would,” “could,” “will,” “may,” “expect,” “believe,” “should,” “anticipate,” “outlook,” “if,” “future,” “intend,” “plan,” “estimate,” “predict,” “potential,” “targets,” “seek” or “continue” and similar words and phrases, including the negatives of these terms, or other variations of these terms, that denote future events. These forward-looking statements include: our plans to target our existing products or product variations for new markets and for new uses and applications; our plans and expectations with respect to growth in sales of our product lines; our ability and plans to develop, register and commercialize additional new product candidates and bring new products to market across multiple categories faster and at a lower cost than other developers of pest management products; our expectations regarding registering new products and new formulations and expanded use labels for existing products, including submitting new products to the EPA; our belief that challenges facing the use of conventional chemical pesticides will continue to grow; our beliefs regarding the growth of markets for, and unmet demand for, bio-based products; our beliefs regarding market adoption for our products and our ability to compete in our target markets; our intention to maintain existing, and develop new, supply, sales and distribution channels and extend market access; expectations regarding potential future payments under strategic collaboration and development agreements; our plans to grow our business while improving efficiency, including by focusing on a limited number of product candidates, taking measures to reduce expenses and expanding our sales and marketing team; our plans with respect to manufacturing; our plans to seek third-party collaborations to develop and commercialize more early stage product candidates; our intention to continue to devote significant resources toward our proprietary technology and research and development; our expectations that sales will be seasonal and the impact of continued drought and other weather-related conditions; our ability to protect our intellectual property in the United States and abroad; our beliefs regarding the effects of the outcome of certain legal matters; our expectations regarding incurring additional costs related to the Audit Committee investigation, restatement of financial statements and director and officer liability insurance; our plans regarding remediation activities related to weakness in our internal control over financial reporting; our anticipated impact of certain accounting pronouncements; our ability to use carryforwards; our expectations regarding market risk, including interest rate changes, foreign currency fluctuations and commodity price changes; and our future financial and operating results. These statements reflect our current views with respect to future events and our potential financial performance and are subject to risks and uncertainties that could cause our actual results and financial position to differ materially and adversely from what is projected or implied in any forward-looking statements included in this Annual Report on Form 10-K. These factors include, but are not limited to, the risks described under Part I–Item 1A—“Risk Factors,” Part II–Item 7—“Management’s Discussion and Analysis of Financial Condition and Results of Operations,” elsewhere in this Annual Report on Form 10-K and those discussed in other documents we file with the U.S. Securities and Exchange Commission (“SEC”). We make these forward-looking statements based upon information available on the date of this Annual Report on Form 10-K, and we have no obligation (and expressly disclaim any such obligation) to update or alter any forward-looking statements, whether as a result of new information or otherwise except as otherwise required by securities regulations.
As used herein, “MBI,” the “Company,” “we,” “our” and similar terms refer to Marrone Bio Innovations, Inc., unless the context indicates otherwise.
Except as context otherwise requires, references in this Annual Report on Form 10-K to our product lines, such as Regalia, refer collectively to all formulations of the respective product line, such as Regalia Maxx, Regalia Rx or Regalia SC, and all trade names under which our distributors sell such product lines internationally, such as Sakalia, Sentry R or Milsana. Our logos, Grandevo®, Regalia®, Venerate®, Zequanox®, HavenTM, MajesteneTM and other trade names, trademarks or service marks of Marrone Bio Innovations, Inc. appearing herein are the property of Marrone Bio Innovations, Inc. This Annual Report on Form 10-K contains additional trade names, trademarks and service marks of other companies. We do not intend our use or display of other companies’ trade names, trademarks or service marks to imply relationships with, or endorsement or sponsorship of us by, these other companies.
v
Table of Contents
| Page | ||||||
| Item 1. |
1 | |||||
| Item 1A. |
27 | |||||
| Item 1B. |
48 | |||||
| Item 2. |
48 | |||||
| Item 3. |
48 | |||||
| Item 4. |
49 | |||||
| Item 5. |
50 | |||||
| Item 6. |
51 | |||||
| Item 7. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
54 | ||||
| Item 7A. |
90 | |||||
| Item 8. |
91 | |||||
| Item 9. |
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
163 | ||||
| Item 9A. |
163 | |||||
| Item 9B. |
166 | |||||
| Item 10. |
167 | |||||
| Item 11. |
175 | |||||
| Item 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
185 | ||||
| Item 13. |
Certain Relationships and Related Transactions, and Director Independence |
188 | ||||
| Item 14. |
190 | |||||
| Item 15. |
191 | |||||
| 192 | ||||||
vi
Table of Contents
We make bio-based pest management and plant health products. Bio-based products are comprised of naturally occurring microorganisms, such as bacteria and fungi, and plant extracts. Our current products target the major markets that use conventional chemical pesticides, including certain agricultural and water markets, where our bio-based products are used as alternatives for, or mixed with, conventional chemical products. We also target new markets for which there are no available conventional chemical pesticides, the use of conventional chemical products may not be desirable or permissible because of health and environmental concerns (including for organically certified crops) or because the development of pest resistance has reduced the efficacy of conventional chemical pesticides. All of our current products are EPA-approved and registered as “biopesticides.” We expect our future products will include plant health products qualified as “biostimulants,” which may require state registrations but do not require EPA registration. We believe our current portfolio of products and our pipeline address the growing global demand for effective, efficient and environmentally responsible products to control pests, increase crop yields and reduce crop stress.
We currently primarily sell to the crop protection market. Our three commercially available crop protection product lines, are Regalia, for plant disease control and plant health, and Grandevo and Venerate, for insect and mite control. These products can be used in both conventional and organic crop production, and are sold to growers of specialty crops such as grapes, citrus, tomatoes, vegetables, nuts, leafy greens and ornamental plants. We have also had some sales of Regalia for large-acre row crops such as corn and soybeans. We have also developed a commercially available product line that we sell to the water treatment market. Zequanox selectively controls invasive mussels that cause significant infrastructure and ecological damage across a broad range of in-pipe and open-water applications, including hydroelectric and thermoelectric power generation, industrial applications and recreation. We believe that our existing crop protection products, or variations thereof, can also be specifically targeted for industrial and institutional, turf and ornamental, home and garden and animal health uses such as controlling grubs, ants, flies and mosquitoes in and around schools, parks, golf courses and other public-use areas.
Since the second half of 2014, we have been implementing a prioritization plan to focus our resources on continuing to improve and promote our commercially available products, advancing product candidates that are expected to have the greatest impact on near-term growth potential and expanding international presence and commercialization. Our goal has been to reduce expenses, conserve cash and improve operating efficiencies, to extract greater value from our products and product pipeline and to improve our communication to and connection with the global sustainability movement that is core to our cultural values.
In connection with this new strategy, we have significantly reduced overall headcount, while building a new sales organization with increased training and ability to educate and support customers, as well as providing our product development staff with greater responsibility for technical sales support, field-trials and demonstrations to promote sales growth. In addition, while we believe that we have developed a robust pipeline of novel product candidates, we are currently limiting our internal efforts to five product candidates, Majestene (MBI-305), a biopesticide for parasitic roundworm control, or “bionematicide,” based on the microbe used in Venerate, for which we have initiated a targeted placement to select customers, MBI-010, a bioherbicide that is also based on the microorganism in Venerate and Majestene, and MBI-110, a biofungicide, both of which we plan to submit to the EPA in 2016, Haven (MBI-505), a plant health product that does not require EPA registration, and MBI-601, a biopesticide that produces gaseous natural compounds, or “biofumigant,” which we submitted to the EPA in April 2014. Simultaneously, we are seeking collaborations with third parties to develop and commercialize more early stage candidates on which we have elected not to expend significant resources given our reduced budget.
We believe that collectively, these measures will best position us to respond to the business challenges reflected in our financial results for recent periods, but our long-term, global vision for our business and our commitment to that vision remains fundamentally unchanged.
1
Table of Contents
Industry Overview
Pest management is an important global industry. Phillips McDougall, an independent advisory firm, estimates the 2014 agrichemical market (crop protection) at $56.6 billion, with Brazil ranking first at $11.6 billion in sales, followed by the United States at $9.2 billion. Most of the markets we currently target or plan to target primarily rely on conventional chemical pesticides, supplemented in certain agricultural markets by the use of genetically modified crops. Conventional chemical pesticides are generally synthetic materials that directly kill or inactivate pests. However, demand for effective and environmentally responsible bio-based products continues to increase. The global market for biopesticides, which control pests by non-toxic mechanisms such as attracting pests to traps or interfering with their ability to digest food, was valued at $3.6 billion for 2014 and projected to grow to $6.9 billion in 2019, reflecting a 13.9% compound annual growth rate of over the period, according to BCC Research, an independent market research firm. In comparison, global synthetic pesticides sales were projected at 5.7% compound annual growth for the same period. We believe these trends will continue as the benefits of using bio-based pest management and plant health products become more widely known.
Crop Protection
Conventional Production. Growers are constantly challenged to supply the escalating global demand for food, while reducing the negative impact of crop protection practices on consumers, farm workers and the environment. The dominant technologies for crop protection are conventional chemical pesticides and genetically modified crops. Major agrichemical companies have invested billions of dollars to develop genetically modified crops that resist pests or have high tolerance to conventional chemical pesticides. The market for genetically modified crops was estimated at $21 billion in 2014, according to Phillips McDougall. In addition, according to the International Service for the Acquisition of Agri-biotech Applications, a third-party not-for-profit organization, in 2014, 182 million hectares (484 million acres) were planted with genetically modified crops in 28 countries, with the United States, Brazil, Argentina, India and Canada planting the most (in that order). Soybean, corn, cotton and canola plantings have made the greatest inroads, accounting for 50%, 30%, 14% and 9%, respectively, of genetically modified seeds planted globally.
Conventional chemical pesticides and genetically modified crops have historically been effective in controlling pests. However, there are increasing challenges facing the use of conventional chemical pesticides such as pest resistance and environmental, consumer and worker safety concerns. Governmental agencies are further pressuring growers, distributors and manufacturers by restricting or banning certain forms of conventional chemical pesticide usage, particularly in the European Union, as some conventional chemical pesticide products are being phased out, as well as at local levels, where many city and county governments have prohibited the sale of certain conventional chemical pesticide products, magnifying the complexity of agrichemical companies’ distribution and regulatory compliance. At the same time, a number of supermarket chains and food processors, key purchasers of specialty fruits, nuts and vegetables, are imposing synthetic chemical residue restrictions, limiting options available to growers close to harvest. Consumers, scientists and environmental groups have also voiced concerns about the unintended effects of genetically modified crops, including pest resistance and contamination of non-genetically modified crops. In response to consumer and environmental group concerns and restrictions by importing countries, several large-scale food purchasers have demanded that their contracted growers supply them only non-genetically modified crops.
These factors are significant market drivers for conventional producers, and their impact is continuing to grow. An increasing number of growers are implementing integrated pest management (IPM) programs that, among other things, combine bio-based pest management products and crop cultivating practices and techniques such as crop rotation, with conventional chemical pesticides and genetically modified crops. Bio-based pest management products are becoming a larger component of IPM programs due in part to the challenges associated with conventional chemical pesticides and genetically modified crops.
Organic Production. Certified organic crops such as food, cotton and ornamental plants, are produced without the use of synthetic chemicals, genetic modification or any other bioengineering or adulteration. As such, organic
2
Table of Contents
growers are limited in the number of alternatives for pest management. The U.S. Department of Agriculture, or the USDA, approved national production and labeling standards for organic food marketed in the United States in late 2000. These standards have contributed to the growth of organic food consumption in the United States, and other countries have implemented similar programs. According to the Organic Trade Association, a business association, consumer demand for organic food has outpaced the available acreage in the United States, with $1.4 billion of organic food imported in 2013 and $49 billion of domestic organic food sales in 2014, or 5% of all food sales, up 11% over 2013. In addition, U.S. sales of non-GMO-labeled foods were estimated at $8.5 billion across 2,100 brands and 22,000 verified items in 2014, according to SPINS, a third party consulting firm. We believe this growing demand is primarily driven by concerns about food safety and the adverse environmental effects of conventional chemical pesticides and genetically modified crops.
Water Treatment
Global demand for water treatment products was estimated to be $48 billion in 2012, according to The Freedonia Group, an independent market research firm, and the global market for specialty biocide chemicals for water treatment was projected to be $5.2 billion in 2013, according to BCC Research. Invasive and native pest species are increasingly a concern in diverse applications such as hydroelectric and thermoelectric power generation, industrial applications, drinking water, aquaculture, irrigation and recreation. However, discharge of water treatment chemicals to target these pests is highly regulated, and in many cases, such as with management of open waters and sensitive environmental habitats, use of conventional chemicals is prohibited.
One particular area of concern has been the damage caused by invasive zebra and quagga mussels, which clog pipes, disrupt ecosystems, encrust infrastructure and blanket beaches with razor-sharp shells. These species initially infested the Great Lakes region and have spread across the United States. Industry reports estimate that these mussels cause approximately $1.0 billion in damage and associated control costs annually in parts of the United States alone. There are limited treatment options available, many of which are toxic to aquatic flora and fauna. To date, most treatment options have been focused either on manual removal of the mussels, which is time consuming and costly, or conventional chemical treatments, which potentially jeopardize the environment and are thus controlled tightly by regulatory agencies.
The water treatment market also includes products to control algae, aquatic weeds and unwanted microorganisms. For example, one of the most effective and popular methods for controlling algae and unwanted microorganisms is chlorination. One of the major concerns in using chlorination in surface water supplies is that chlorine combines with various organic compounds to form by-products, some of which are considered possible carcinogens.
Other Target Markets
We are also taking steps through strategic collaborations to commercialize our existing crop protection products, or variations thereof, for other markets. Although conventional chemical pesticides have traditionally serviced the industrial and institutional, professional turf and ornamental, home and garden and animal health markets, governmental regulations are restricting their use, and reports indicate that end users increasingly value environmentally friendly products, with some households willing to forego pest control treatments entirely if alternatives to conventional chemical pesticides are not available.
Benefits of Bio-Based Pest Management and Plant Health Products
While conventional chemical pesticides are often effective in controlling pests, some of these chemicals are acutely toxic, some are suspected carcinogens and some can have other harmful effects on the environment and other animals. Health and environmental concerns have prompted stricter legislation around the use of conventional chemical pesticides, particularly in Europe, where the use of some highly toxic or endocrine-disrupting chemical pesticides is banned or severely limited and the importation of produce is subject to strict regulatory standards on
3
Table of Contents
pesticide residues. In addition, the European Union has passed the Sustainable Use Directive, which requires EU-member countries to reduce the use of conventional chemical pesticides and to use alternative pest management methods, including bio-based pest management products. Over the past two decades, U.S. regulatory agencies have also developed stricter standards and regulations. Furthermore, a growing shift in consumer preference towards organic and sustainable food production has led many large, global food retailers to require their supply chains to implement these practices, including the use of bio-based pest management and fertilizer solutions, water and energy efficiency practices, and localized food product sourcing.
Aside from the health and environmental concerns, conventional chemical pesticide users face additional challenges such as pest resistance and reduced worker productivity, as workers may not return to the fields for a certain period of time after treatment. Similar risks and hazards are also prevalent in the water treatment market, as chlorine and other chemicals used to control invasive water pests contaminate and endanger natural waterways. Costs of using conventional chemical pesticides are also increasing due to a number of factors, including raw materials costs such as rising costs of petroleum, stringent regulatory requirements and pest resistance to conventional chemical pesticides, which requires increasing application rates or the use of more expensive alternative products.
As the cost of conventional chemical pesticides increases and the use of conventional chemical pesticides and genetically modified crops meets increased opposition from government agencies and consumers, and the efficacy of bio-based pest management and plant health products becomes more widely recognized among growers, bio-based pest management products are gaining popularity and represent a strong growth sector within the market for pest management technologies. Growers are increasingly incorporating bio-based pest management products into IPM programs, and bio-based pest management products help create the type of sustainable agriculture programs that growers and food companies increasingly emphasize.
Bio-based pest management products include biopesticides, as well as minerals such as copper and sulfur. The EPA registers biopesticides in two major categories: (i) microbial pesticides, which contain a microorganism such as a bacterium or fungus as the active ingredient and (ii) biochemical pesticides, which are naturally occurring substances such as insect sex pheromones, certain plant extracts and fatty acids. Biostimulasts, which are not registered by the EPA absent additional pest control usages, are microorganisms or natural substances derived from microorganisms or plants that growers use to reduce plant stress, stimulate plant physiology to increase yield, manage pest resistance and reduce chemical residues.
We believe many bio-based pest management products perform as well as or better than conventional chemical pesticides. When used in rotation or in spray tank mixtures with conventional chemical pesticides, bio-based pest management products can increase crop yields and quality over chemical-only programs. Agricultural industry reports, as well as our own research, indicate that bio-based pest management products can affect plant physiology and morphology in ways that may improve crop yield and can increase the efficacy of conventional chemical pesticides. In addition, pests rarely develop resistance to bio-based pest management products due to their complex modes of action. Likewise, bio-based pest management products have been shown to extend the product life of conventional chemical pesticides and limit the development of pest resistance, a key issue facing users of conventional chemical pesticides, by eliminating pests that survive conventional chemical pesticide treatments. Most bio-based pest management products are listed for use in organic farming, providing those growers with compelling pest control options to protect yields and quality. Given their generally lower toxicity compared with many conventional chemical pesticides, bio-based pest management products can add flexibility to harvest timing and worker re-entry times and can improve worker safety. Many bio-based pest management products are also exempt from conventional chemical residue tolerances, which are permissible levels of chemical residue at the time of harvest set by governmental agencies. Bio-based pest management products may not be subject to restrictions by food retailers and governmental agencies limiting chemical residues on produce, which enables growers to export to wider markets.
In addition to performance attributes, bio-based pest management products registered with the EPA as biopesticides can offer other advantages over conventional chemical pesticides. From an environmental
4
Table of Contents
perspective, biopesticides have low toxicity, posing low risk to most non-target organisms, including humans, other mammals, birds, fish and beneficial insects. Biopesticides are biodegradable, resulting in less risk to surface water and groundwater and generally have low air-polluting volatile organic compound content. Because biopesticides tend to pose fewer risks than conventional pesticides, the EPA offers a more streamlined registration process for these products, which generally requires significantly less toxicological and environmental data and a lower registration fee. As a result, both the time and money required to bring a new product to market are reduced.
Our Solution
We produce bio-based pest management and plant health products that are effective and generally designed to be compatible with existing pest control equipment and infrastructure. This allows them to be used as alternatives for, or mixed with, conventional chemical pesticides, as well as in markets for which there are no available conventional chemical pesticides or the use of conventional chemical products may not be desirable or permissible because of health and environmental concerns. We believe that compared with conventional chemical pesticides, our products:
| • | can be competitive in both price and efficacy; |
| • | provide viable alternatives where conventional chemical pesticides and genetically modified crops are subject to regulatory restrictions; |
| • | comply with market-imposed requirements for pest management programs by food processors and retailers; |
| • | are environmentally friendly; |
| • | meet stringent organic farming requirements; |
| • | improve worker productivity by shortening field re-entry times after spraying and allowing spraying up to the time of harvest; |
| • | are exempt from residue restrictions applicable to conventional chemical pesticides in both the agriculture and water markets; and |
| • | are less likely to result in the development of pest resistance. |
In addition, our experience has shown that when our products are mixed with conventional chemical pesticides, they can:
| • | increase the effectiveness of conventional chemical pesticides while reducing their required application levels; |
| • | increase levels of pest control and consistency of control; |
| • | increase crop yields; |
| • | increase crop quality, including producing crops with higher levels of protein, better taste and color and more attractive flowers; and |
| • | delay the development of pest resistance to conventional chemical pesticides. |
We believe that the benefits of our products will encourage sustained adoption by end users. For example, we have seen that growers that have used our products on a trial basis in one year have generally continued to use our products in higher levels in subsequent years.
5
Table of Contents
Our Competitive Strengths
Focus on Bio-Based Products
Our belief in and commitment to our vision is our greatest strength. We believe that the world needs more organic and sustainable products and practices, and our goal is to champion that cause. Our experience has shown that by using bio-based pest management and plant health products, growers can benefit the environment and produce more healthy food while improving yields. However, bio-based products have application methods and modes of action that differ fundamentally from conventional chemical products. While major agrichemical companies sell bio-based products, we do not believe that those companies have sufficiently prioritized bio-based products or invested in the internal and external education that is essential to successfully promote these products, and those companies are often conflicted when marketing both conventional chemical products and bio-based products. In contrast, we believe MBI has long been recognized as a thought leader in the bio-based product industry, and we have consistently sought to educate growers in the use and benefits of these products, both alone and mixed with conventional chemical products. We believe our drive to convert acres to these sustainable practices will make us disruptive.
Commercially Available Products
We have four commercially available product lines: Regalia, Grandevo, Venerate and Zequanox. All four of these product lines are EPA approved, and Regalia is also approved in Canada, nine Latin American countries (including Brazil), South Africa and parts of Europe. Zequanox is approved in Canada, and is the only product EPA-approved for open water application other than copper, which is rarely used due to its negative environmental effects. All four of these commercialized lines are subject to patents and trade secrets related to the work we have done to characterize, formulate, develop and manufacture marketable products. We believe these product lines, along with our other EPA-approved and EPA-submitted products and other pipeline product candidates, provide us the foundation for continuing to build the leading portfolio of bio-based pest management products.
Robust Pipeline of Novel Product Candidates
Our pipeline of early-stage discoveries and new product candidates extends across a variety of product types for different end markets, including herbicides, fungicides, nematicides, insecticides, algaecides (for algae control), molluscicides (for mussel and snail control) and plant growth and plant stress regulators. Our product candidates are developed both internally and sourced from third parties. Our research and development process enables us to discover, source and develop multiple products in parallel, which keeps our pipeline robust. In August 2014, we received EPA approval of MBI-011, a weed-controlling biochemical, sarmentine, discovered and isolated from a pepper plant species, and we are currently pursuing third-party manufacturers to synthesize the natural compound at a cost that allows us to introduce the product to the market. We are currently developing the active ingredient in Venerate, a Burkholderia rinojensis microbe we isolated using our discovery process, for commercial release as Majestene bionematicide (MBI-305). We also have additional product candidates at various other stages of development, including MBI-601, a fungus that produces volatile compounds and works as a soil biofumigant, which was submitted to the EPA in April 2014, and a new Bacillus-based fungicide, MBI-110 that has demonstrated activity against downy mildew, Sclerotinia and other crop diseases, which we expect to submit to the EPA by early 2016.
Rapid and Efficient Development Process
We believe we can develop and commercialize novel and effective products faster and at a lower cost than many other developers of pest management products. For example, we have moved each of Regalia, Grandevo, Venerate and Zequanox through development, EPA approval and first U.S. launch in approximately four years or less at a cost of $3 million to $6 million. Thereafter, we have continued to develop and refine these products producing new formulations, applying for expanded use labels, and seeking new markets, in each case at a cost of less than $10 million per product line. In comparison, a report from Phillips McDougall shows that the average
6
Table of Contents
cost for major agrichemical companies to bring a new crop protection product to market has been over $250 million, and these products have historically taken an average of nearly ten years to move through development, regulatory approval and market launch.
Proprietary Discovery Process
Our discovery process allows us to efficiently discover microorganisms and plant extracts that produce or contain compounds that display a high level of pesticidal activity against various pests. After we identify pesticidal activity, we subject the microorganisms and plant extracts to tests to determine effects on plant growth, nutrient uptake and drought and salt stress. We then use various analytical chemistry techniques to identify and characterize the natural product chemistry of the compounds, which we optimize and patent. Four of our product candidates, one of which is EPA-approved, are what we believe to be newly identified microorganism species. We believe that four of our product candidates produce novel compounds that we identified, and four of our product candidates have been found to have, or produce compounds with, a novel mode of action. Our proprietary discovery process is protected by patents on the microorganisms, their natural product compounds and their uses for pest management, as well as a patent application we have filed on a screening process to identify enzyme-inhibiting herbicides. We also maintain trade secrets related to the discovery, formulation, process development and manufacturing capabilities. By conducting our own discovery as well as working with outside collaborators, we are able to access the broadest range of products for commercialization, giving us an advantage over other natural bio-based pest management companies.
Management Team with Significant Industry Experience
Our management team has deep experience in bio-based pest management products and the broader agriculture industry. Our chief executive officer and other key employees average over 25 years of experience and include individuals who have led agrichemical sales and marketing organizations, top scientists and industry experts, some of whom have served in leadership roles at large multinational corporations and governmental agencies, commercialized multiple products, brought multiple products through EPA, state and foreign regulatory processes, filed patent applications and received patents, led groundbreaking research studies and published numerous scientific articles. In addition, our chief financial officer brings over 30 years of financial management experience spanning a variety of industries, including over 13 years of service as several public companies’ chief financial officer. Our general counsel has over 30 years of experience (25 years with public companies) in senior legal, sales and operating roles, including general counsel, vice president of sales and chief operating officer.
Our Growth Strategy
Accelerate Adoption of New Products, Product Applications and Product Lines
Our goal is to provide growers with complete and effective solutions to a broad range of pest management and plant health needs. Due to the competitive nature of the industry and the seasonality of crop growing, speed is essential to ensure widespread adoption. Accordingly, we have launched targeted placements of our products with early adopters in the United States relatively early in the product commercialization stage and for a limited number of indications. These growers, many of whom have unmet market needs, help us to troubleshoot and refine our products and to maximize their value proposition, enabling us to efficiently develop new formulations and expand uses and market penetration with minimal up-front capital investment per product line. We also believe we will be able to leverage growers’ positive experiences using our Regalia, Grandevo and Venerate product lines to accelerate adoption of new products, product applications and product lines. We believe product diversity allows us to compete with larger companies, to strengthen relationships with growers and distributors and not to be dependent on any one product or product category. Further, by offering and developing multiple products simultaneously, we believe we are perceived as a technology leader and can gain the benefits of increased momentum with distributors and end users. We will continue to target early adopters of new pest management and plant health technologies with controlled product launches and to educate growers and water resource managers about the benefits of bio-based pest management products through demonstrations to accelerate commercial adoption of our products.
7
Table of Contents
Deliberately Expand Applications of Our Product Lines
We want growers to know and trust that our products work. Although our initial EPA-approved master labels cover our products’ anticipated crop-pest use combinations, we launch early formulations of our pest management and plant health products to targeted customers under commercial labels that list a limited number of crops and applications that our initial efficacy data can best support. We then gather new data from experiments, field trials and demonstrations, gain product knowledge and get feedback to our research and development team from customers, researchers and agricultural agencies. Based on this information, we enhance our products, refine our recommendations for their use in optimal integrated pest management programs, expand our commercial labels, and submit new product formulations to the EPA and other regulatory agencies. For example, we began sales of Regalia SC, an earlier formulation of Regalia, in the Florida fresh tomatoes market in 2008, while a more effective formulation of Regalia with an expanded master label, including listing for use in organic farming, was under review by the EPA. When approved, we launched this new formulation into the Southeast United States in 2009 and nationally in 2010. In 2011, we received EPA approval of a newly expanded Regalia master label covering hundreds of crops and various new uses for applications to soil and through irrigation systems, and we recently expanded sales of Regalia in large-acre row crops as a plant stimulus product, in addition to its beneficial uses as a fungicide. Similarly, ongoing field development research on the microbe used in our insecticide product Venerate led to our October 2015 registration of Majestene (MBI-305) as a nematicide. We believe we have opportunities to broaden the commercial applications and expand the use of our existing products lines into several key end markets, including large-acre row crop applications, seed treatment, forestry, and public health to help drive significant growth for our company.
Focus on Proven Technology Families
We discover and develop more than one product line based on the same technology. For example, the Burkholderia microbe on which Venerate is based is also active against a broad range of nematodes, enabling development as our bionematicide product candidate, Majestene (MBI-305), and produces several herbicidal compounds, enabling development as our bioherbicide product candidate, MBI-010. In addition, our product candidates MBI-110 and MBI-507 are based on microbial fermentations of a newly identified Bacillus strain we isolated using our proprietary screening platform, and the Chromobacterium species on which Grandevo is based may also yield a promising bionematicide product, which we have begun development as MBI-304. Developing multiple products based on the same microbe allows for a more efficient use of research, development and manufacturing resources and enables us to leverage capital invested in existing technologies.
Continue to Develop and Commercialize New Products in Both Existing and New Markets
Our goal is to rapidly and efficiently develop, register and commercialize new products each year, with the goal of developing a full suite of pest management and plant health products. For example, while our current crop protection products address plant diseases and insects, we are developing products that can also control nematodes and weeds as well as products for improving fertilizer efficiency and reducing drought and salt stress. Our bioassay screening has identified at least four microbes that display activity against blue-green algae associated with toxic algal blooms, which have resulted in seasonal closures of some drinking water supplies in the Great Lakes region, and we are seeking partners to move these early-stage discoveries forward.
Target International Markets
Expanding international sales is an important component of our growth strategy, but the global markets for pest management products are intensely competitive. Our plan is to focus on key countries and regions with the largest and fastest growing biopesticide and plant health product markets for specialty crops and selected row crops. We intend to work with regional and country distributors who have brand recognition and established customer bases and who can conduct field trials and grower demonstrations and lead or assist in regulatory processes and market development.
8
Table of Contents
Leverage Manufacturing Capabilities
We initially used third-party manufacturers to produce all of our products on a commercial scale. In 2014, we completed the repurpose of a manufacturing facility that we purchased in July 2012 by installing three 20,000 liter fermentation tanks and constructing a dedicated building to house them, which has enabled us to manufacture in-house the majority of our products. We believe that greater control of our own manufacturing capacity allows us to scale-up processes and institute process changes more quickly and efficiently while ultimately lowering manufacturing costs over time to achieve the desired margins and protecting the proprietary position of our products. We continue to use third party manufacturers for Venerate and for spray-dried powder formulations of Grandevo and Zequanox.
Our Products
Commercially Available Products
The table below summarizes our current portfolio of commercially available biopesticide products, which have been able to move through development, EPA approval and first U.S. market launch in four years or less and at a cost of $3.0 million to $6.0 million. We have continued to develop and refine these products after initial launch, producing new formulations, applying for expanded use labels, and seeking new markets.
| NAME |
MARKET |
TARGET |
USE |
STATUS | ||||
| Regalia | Crop Protection, Home and Garden, Turf | Plant Disease/ Plant Health |
Protects against fungal and bacterial diseases and enhances yields | Commercially Available Domestically and Internationally | ||||
| Grandevo | Crop Protection, Home and Garden, Turf | Insects and Mites | Controls a broad range of sucking and chewing insects through feeding | Commercially Available Domestically | ||||
| Zequanox | Water Treatment | Invasive Mussels (In-Pipe and Open Water Habitat Restoration) | Controls invasive mussels that restrict water flow in industrial and power facilities and harm recreational waters | Commercially Available Domestically and in Canada | ||||
| Venerate | Crop Protection, Home and Garden, Turf, Animal Health | Insects and Mites | Controls sucking and chewing insects on contact | Commercially Available Domestically | ||||
Regalia
| • | Biofungicide |
| • | Crop Protection, Home and Garden, Turf: Targets Plant Disease, Improves Plant Health, Increases Yields |
| • | Commercially Available Internationally |
Regalia, a plant extract-based fungicidal biopesticide, or “biofungicide,” is EPA-registered for crop and non-crop uses and approved for use on foliage and roots in all states in the United States, including California and Florida, where the majority of the specialty crops are grown. It is also approved for sale in Brazil (tomatoes, potatoes, dried beans), Ecuador (flowers), Mexico (citrus and tree fruit, berries, tomatoes, peppers, potatoes, cucurbits, flowers, potatoes and grapes), Turkey (covered vegetables), Canada (tomatoes, grapes, strawberries, cucurbits, apples, turf, blueberries, hops (emergency use), ornamental plants and wheat), Peru (grapes and quinoa), South
9
Table of Contents
Africa (grapes), and Panama, Dominican Republic, El Salvador, Guatemala and Honduras (potatoes, tomatoes, peppers, tobacco, cucurbits, beans, avocados, citrus, peanuts, papayas and strawberries). Registration efforts are currently underway in China, with Regalia demonstrating efficacy in government-conducted trials on tomatoes, cucurbits, strawberries and grapes. University researchers have extensively tested the product against several important plant diseases, especially against mildews. We, and our commercial partners, have also conducted hundreds of trials in the United States and abroad, including five years of crop trials in Europe. The data show that Regalia is an effective addition to a disease management program against a broad range of diseases and can increase yields in crops such as strawberries, tomatoes, potatoes, soybeans, rice, wheat, alfalfa, sugarcane and corn.
Regalia is made from an extract of the giant knotweed plant and acts by turning on a plant’s “immune system,” a process called induced systemic resistance. Regalia also enhances the efficacy of major conventional chemical fungicides, and we have received a patent application on this synergism. Regalia also is effective for seed treatment of soybean, corn and cotton, for which we have filed a patent application, and we have received a patent on the effects on root growth and yield when Regalia is applied to the seed or as a root stimulant. For example, in field tests and in actual grower use, Regalia has shown significant yield increases on strawberries, tomatoes, potatoes, soybeans, rice, wheat, alfalfa, sugarcane and corn, with less irrigation required for strawberries treated with Regalia.
We obtained an exclusive license relating to the technology used in our Regalia product line while Regalia was in the process development and formulation stage of product development. In addition to developing the supply chain to commercially market the product, using our natural product chemistry expertise, we developed an analytical method to measure and characterize the major compounds in the plant extract, and we enhanced these compounds several times in new formulations, providing Regalia with a broader spectrum of activity and better efficacy than the original licensed product. In addition, we improved the physical properties of our Regalia formulations and developed four formulations that meet organic farming standards. We have filed several patent applications with respect to these innovations. In addition, we have received a U.S. patent for modulating plant growth by treating roots of plants with Regalia (or other compounds or extracts of knotweed), and transplanting the plants into soil. We have also received a patent on the synergistic combination of Regalia or knotweed extract and some important chemical fungicides.
We launched Regalia SC, an earlier formulation of Regalia, into the Florida fresh tomatoes market in December 2008. This formulation had a limited label with a few crops and uses on the label and it was not compliant for organic listing. In 2009, we began sales of Regalia based plant health products in the United Kingdom (under the name Sentry R by Plant Health Care) and Ecuador (under the name Milsana), and we later received a revised, broader label with hundreds of crops for a new organic formulation, which we subsequently launched into the Florida vegetables and Arizona leafy greens markets. In January 2010, we received state approval in California and immediately launched Regalia into the leafy greens and walnuts markets. Key markets include vegetables in the southeast, citrus in Florida, leafy greens and vegetables in California and Arizona, walnuts and stone fruit in California and pome fruit and grapes in California and the Pacific Northwest. In December 2011 and August 2012, we received EPA approval and California regulatory approval, respectively, for an expanded Regalia label that includes new soil applications, instructions for yield improvement in corn and soybeans and additional crops and target pathogens. Our product for row crops is sold separately as Regalia Rx and for international markets, where the Regalia trademark is allowed, as Regalia Maxx. We submitted Regalia for registration in the European Union, which is one of the largest fungicide markets in the world. We received regulatory approval for Regalia in South Africa in June 2013, in El Salvador, Guatemala and Honduras in December 2013, in Peru in March 2014, in Colombia in June 2014, in Tunisia and Morocco in late 2014 and in Brazil, for tomato, potato and dry beans, in December 2014. We have recently received EPA approval for three new formulations (12%, 16% and a solvent-free 5%), which will be used for market segmentation and replacement of existing formulations.
Regalia, Regalia Maxx and Regalia Rx are USDA National Organic Program compliant and OMRI-USA/OMRI-Canada certified.
10
Table of Contents
Grandevo
| • | Bioinsecticide |
| • | Crop Protection, Home and Garden, Turf: Targets Insects and Mites |
| • | Commercially Available Domestically, International Expansion Efforts Underway |
Grandevo is based on a new species of microorganism, Chromobacterium subtsugae, which was discovered by a scientist at the USDA in Beltsville, Maryland, and which we have licensed and commercialized. Grandevo is a powerful feeding inhibitor: insects and mites become agitated when encountering it and will not feed and starve, or, if they do ingest it, die from disruption to their digestive system. Grandevo also has repellent effects on and reduces egg hatching and reproduction of target insects and mites. Grandevo is particularly effective against chewing insects (such as caterpillars and beetles) and sucking insects (such as stinkbugs and mealybugs, as well as thrips and psyllids, which are respectively known as “corn lice” and “plant lice”). Trials to date and reports from grower use have shown instances of commercial levels of efficacy as good as the leading conventional chemical pesticides on a range of chewing and sucking insect and mite pests, including two invasive species of psyllid affecting citrus and potato crops. Grandevo has also shown significant control of other pests such as plant-feeding fly larvae, mosquitoes, and white grubs in turf grass, “leafmining” caterpillar larvae and other leaf-eating caterpillars. Grandevo has also shown efficacy against corn rootworm, a major pest of corn, which has reportedly been resistant to corn engineered for rootworm control. Grandevo has shown efficacy against other soil pests, including white grubs, wireworms, and root maggots. Field trials are ongoing to further characterize Grandevo’s efficacy.
We obtained a co-exclusive license for the bacterial strain used in our Grandevo product line while Grandevo was undergoing primary screening as a potential product candidate. Since licensing the microorganism, we completed the testing and development necessary to produce and commercialize an EPA-approved product and have filed our own patent applications with respect to the microorganism, including its genome, synergistic combinations with conventional chemical pesticides, product formulations containing the bacterial strain as well as the chemistry produced by the microorganism upon which Grandevo is based. We have an issued U.S. patent on one of these novel compounds produced by the bacteria and novel insecticidal and nematicidal uses.
We placed a prototype liquid formulation of Grandevo on a targeted basis under a limited label into the Florida citrus crop market in 2011. Commencing in the summer of 2012, we launched a dry formulation of Grandevo in markets across the United States where state registrations have been approved, targeting key markets, including citrus, tomatoes, peppers, strawberries, potatoes, leafy greens and other fruits and vegetables. This dry formulation was approved by the EPA in May 2012 and has been registered in 49 of 50 states (Hawaii pending) as well as Puerto Rico. In May 2013, we received EPA approval for a revised label reflecting Grandevo’s safety for bees. In addition, we submitted the registration dossier for Grandevo to Mexico and Canada and for emergency use in Brazil in October 2014. Grandevo has received completeness determination from the European Commission and is now cleared to begin the evaluation for Annex 1 listing and commercialization in the European Union. A June 2015 policy decision by the European Commission, the European Food Safety Authority and a Working Group of EU Member States has allowed Grandevo, which contains only non-viable Chromobacterium subtsugae cells, to be evaluated as a microbial pesticide. Until this recent EU decision, only pesticides containing live microbes could be evaluated under EU regulation. Grandevo is being assessed under the Netherlands Government’s “Green Deal” Initiative, which has been created with an aim to “speed up the sustainability of PPPs (plant protection products) in agriculture and horticulture by facilitating the authorization of green PPPs with a low risk for humans, animals and the environment.” Efficacy trials recently completed in Europe will be used to support uses of Grandevo for the control of whitefly and thrips in Solanaceae (tomato, pepper and aubergine) and Cucurbitaceae (melon, cucumber and squash) crops.
Grandevo is USDA National Organic Program compliant and OMRI-USA/OMRI-Canada certified.
11
Table of Contents
Zequanox
| • | Biomolluscicide |
| • | Water Treatment: Targets Invasive Mussels (In-Pipe and Open Water Habitat Restoration) |
| • | Commercially Available in United States and Canada |
| • | USDA “BioPreferred” Program Certified Product |
Zequanox addresses the problem of invasive zebra and quagga mussels, which clog pipes, disrupt ecosystems, encrust infrastructure and blanket beaches with razor-sharp shells. These mussels cause approximately $1.0 billion in damage and associated control costs annually in parts of the United States alone. There are limited treatment options available, many of which are time-consuming and costly, or harm aquatic flora and fauna. Zequanox is a biomolluscicide derived from a common microbe found in soil and water bodies, Pseudomonas fluorescens. Zequanox is an environmentally friendly, bio-based pest management product that is designed to kill over 75% of invasive mussels in treated pipe systems without causing collateral ecological damage. In July 2012, we conducted an open water trial in Deep Quarry Lake, Illinois, where the Zequanox treatment killed more than 90% of the tested mussels on the lake bed. This level of control in open water treatments was repeated in 2013. We generated revenues for treating an Oklahoma Gas & Electric facility 2012 and 2013 and a First Light & Power facility along the Housatonic River in Connecticut in 2014. In addition, Zequanox was used by the Minnesota Department of Natural Resources and the Minnehaha Creek Watershed District’s Aquatic Invasive Species Program to treat a recent infestation of these invasive mussels in Christmas Lake, resulting in 100% control of the mussels in the tested area. Zequanox is approved in Canada and is the only product EPA-approved for open water application in the United States other than copper, which is rarely used due to its negative environmental effects.
At recommended application rates, Zequanox is not toxic to other aquatic life, including ducks, fish, crustaceans and other bivalve species such as native clams or mussels. Zequanox is safe to workers, less labor intensive and requires shorter treatment times compared with conventional chemical pesticides. Zequanox can be used by power plants and raw water treatment facilities as an alternative to conventional chemical treatments such as chlorine, or as a complement to those products.
We entered into a license agreement with The University of the State of New York pursuant to which we were granted an exclusive license under the University’s rights relating to the bacterial strain used in our Zequanox product line while the product’s natural product chemistry was still under investigation. Since then, we have developed dry powder formulations, significantly improved the fermentation process for higher cell yield, allowing us to increase manufacturing scale, and we have filed patent applications relating to natural product compounds in the Zequanox cells we have identified and product formulations we have developed. In addition, we have received $1.1 million in grants from the National Science Foundation for work needed to commercialize the bacterial strain in Zequanox, which is currently being marketed and sold directly to U.S. power and industrial companies.
Due to prioritization constraints, we have not committed resources to Zequanox sufficient to market it full-scale and substantially improve margins. However, we are currently in discussions with large water treatment companies to further develop Zequanox and expand it commercially. In addition, we continue to work with state, federal and bi-national partners via the Great Lakes Commission’s Invasive Mussel Collaborative and the EPA’s Great Lakes Restoration Initiative to further develop Zequanox in the Great Lakes/Upper Mississippi River Basin as a habitat restoration tool and potential harmful algal bloom management tool (as zebra and quagga mussels selectively feed on beneficial algae while rejecting toxic blue-green algae).
12
Table of Contents
Venerate
| • | Bioinsecticide |
| • | Crop Protection, Home and Garden, Turf and Ornamentals, Animal Health: Targets Insects and Mites |
| • | Commercially Available Domestically |
Venerate is based on a microbial fermentation of a new bacterial species we isolated using our proprietary discovery process. We have identified compounds produced by the microorganism in Venerate that control a broad range of chewing and sucking insects and mites, as well as flies and plant parasitic nematodes, on contact, which is complementary to the anti-feeding effects of Grandevo. In addition, because we currently sell Venerate in a liquid formulation and Grandevo in a powder formulation, we are seeking to exploit opportunities for market segmentation, including for combinations with liquid fertilizer and for low-volume aerial applications. Venerate was approved by the EPA in February 2014 and we began to sell in May 2014. We submitted Venerate for the Canadian Pest Management Regulatory Agency registration in April 2014 and submitted the registration dossier for Venerate to Mexico in April 2014. We have conducted field trials on several crops and insects and mites, many of which show efficacy as good as leading conventional chemical pesticides. Venerate has shown positive results in field trials against soil insects of corn, wheat and soybeans, applied both in-furrow and as seed treatments, and has shown broad spectrum activity across a wide range of pests, including Asian citrus psyllid, corn rootworm, stinkbugs, caterpillars and weevils. Additional trials are in progress in 2015.
We have filed patent applications on the microorganism and the natural product compounds that demonstrate insecticidal and nematicidal activity, as well as product formulations containing the microorganism. Venerate is USDA National Organic Program compliant and OMRI-USA/OMRI-Canada certified.
Product Pipeline
Our pipeline consists of product candidates in various stages of development, including biostimulant and plant health products that do not require EPA registration, products submitted to the EPA for registration, and other promising product candidates under development, as well as other early-stage discoveries. In 2014, we prioritized our pipeline candidates, focusing first on those are expected to have the greatest near-term growth potential. We are seeking collaborations with third parties to develop and commercialize more early stage candidates.
Majestene (MBI-305)
| • | Bionematicide |
| • | Crop Protection, Turf: Targets Nematodes |
| • | EPA Approved; Under Development |
Majestene (MBI-305) is a bionematicide product candidate that we are developing based on the microorganism used in Venerate. This nematicide is active against a broad range of nematodes, and in field trials it has been as effective as or better than the leading conventional chemical nematicide against soybean cyst, root knot, lesion, stunt, reniform, lance and burrowing nematodes. Crops tested include soybean, corn, cotton, turf, tomato, potato and banana. Usage for Majestene as a nematicide was approved by the EPA in connection with its approval of the labels for Venerate in 2014, and a modified label with refined rates, nematode species and crops was approved in October 2015. We have filed a patent application for use of the bacterial strain in Majestene for use as a nematicide. We expect to do a targeted placement of Majestene with key, early adopter growers in 2015, with a broader launch in 2016.
13
Table of Contents
Haven (MBI-505)
| • | Anti-transpirant |
| • | Crop Protection, Turf, Ornamentals: Enhances Crop Yields and Plant Health |
| • | EPA Exempt; Under Development |
Haven (MBI-505), a plant health product candidate that helps prevent plants from drying out, or “anti-transpirant,” is based on a technology of naturally-derived, plant-based compounds that we licensed from Kao Corporation for use in the United States. The licensed patents are directed to methods of promoting plant growth and increasing biomass and crop yield. We have been actively developing new formulations and conducting field trials showing that Haven protects and enhances yields by reflecting heat from leaves, which reduces plant water lass, allowing plants to thrive better in sun-stressed environments. 2014 field trials in the United States and Chile demonstrated a reduction in sun-stressed fruit and an increase in quality characteristics on citrus, apples and grapes, increased yields on walnuts, almonds and wheat, often equal to or better than the commercial standard, and increased turf growth. Unlike competitor products, Haven does not leave an undesirable white deposit on crops.
MBI-010
| • | Bioherbicide |
| • | Crop Protection, Home and Garden, Turf: Targets Weeds |
| • | Under Development |
MBI-010 is based on the same species of bacteria used to produce Venerate, which we isolated using a proprietary discovery process that identifies herbicides that inhibit a certain plant enzyme. MBI-010 produces several herbicidal compounds, some of which are novel, that are rapidly taken up by germinating seeds and by the roots of seedling and mature weeds. MBI-010 has demonstrated effectiveness against a range of weeds, including weeds resistant to leading conventional chemical herbicides, either after or before the weeds’ emergence. MBI-010 has also demonstrated a novel mode of action (inhibiting histone deacetylase enzymes), and some of its active compounds are transmitted systemically through the vascular structure of weeds. We have filed a patent application with respect to the MBI-010 formulation uses, and its associated natural product compounds as an herbicide. We also received a U.S. patent on the process we used to discover MBI-010, and certain other bioherbicides. We are working on formulations to provide the needed commercial shelf life stability and efficacy and fermentation processes to increase yields and lower costs. We expect to submit MBI-010 to the EPA in 2016.
MBI-601
| • | Biofumigant |
| • | Crop Protection, Home, Industrial: Targets Plant Disease, Nematodes and Insects |
| • | Under Development |
MBI-601 is a biofumigant based on a novel and proprietary genus of fungus, Muscodor, which was discovered by a scientist at Montana State University. We obtained a co-exclusive license for several strains and species of this fungus, which produces a suite of gaseous natural product compounds that have been shown to control certain species of harmful fungi and bacteria that cause plant diseases and to control nematodes and some insect species. We believe that MBI-601 may be used for agricultural and industrial applications, including post-harvest control of fruit and flower decay and pre-planting control of plant diseases and nematodes, as a viable alternative to methyl bromide and other chemical fumigants, which are subject to significant regulatory restrictions and for which few effective, non-toxic alternatives are available. We submitted MBI-601 to the EPA in April 2014. In 2014, we obtained a license to an artificial mixture of the gaseous compounds produced by the Muscodor fungus, which extends the potential uses of this technology by enabling development of products at a potentially lower cost and better shelf stability than versions using the living fungus.
14
Table of Contents
MBI-110
| • | Biofungicide and Plant Health |
| • | Crop Protection, Home and Garden: Targets Plant Disease, Improves Plant Health |
| • | Under Development |
MBI-110 is based on microbial fermentations of a newly identified Bacillus strain we isolated using our proprietary screening platform. MBI-110 is being developed as a biofungicide, targeting difficult to control plant diseases such as Sclerotinia white molds, gray mold and downy mildews. We have identified compounds, some of which are novel, produced by the microorganism in MBI-110 that control a broad range of plant diseases. We have filed a U.S. patent application covering fungicidal uses and have received notice of allowance of related claims. We expect to submit MBI-110 product to the EPA by early 2016. Several field trials were conducted in 2014 in Europe and the United States in 2013 and 2014 that showed good efficacy against white molds and downy mildews.
Other Candidates
In addition to the above, we have developed patented technology relating to a number of other product candidates, including MBI-304, a bionematicide product candidate based on the microorganism used in Grandevo, MBI-011 and MBI-005, bioherbicides that have received EPA approval, MBI-302, a bionematicide with an EPA registration package that is nearly complete. We are also developing MBI-507, a plant health product and plant root and growth biostimulant based on the living spores of the microorganism used in Venerate, and we filed a U.S. patent application covering uses for growth promotion applications and have received notice of allowance of these claims. We are seeking collaborations with third parties to develop and commercialize certain of these and other promising early-stage candidates, but as resources permit, we may choose to move some of these product candidates forward internally.
We have also discovered several microorganisms with algaecidal activity and over 25 additional fungicide, herbicide, insecticide and nematicide candidates using our proprietary screening platform. In addition, we have produced a collection of microorganisms from taxonomic groups that research suggests may enhance nutrient uptake in plants, reduce stress and otherwise increase plant growth.
Our Discovery and Product Development Process
Our proprietary technology comprises a sourcing process for microorganisms and plant extracts, an extensive proprietary microorganism collection, microbial fermentation technology, screening technology and a process to identify and characterize natural compounds with pesticidal activity. Our technology enables us to isolate and screen naturally occurring microorganisms and plant extracts in an efficient manner and to identify those that may have novel, effective and safe pest management or plant health promoting characteristics. We then analyze and characterize the structures of compounds either produced by selected microorganisms or found in plant extracts to identify product candidates for further development and commercialization. We have screened more than 18,000 microorganisms and 350 plant extracts, and we have identified multiple product candidates that display significant levels of activity against insects, nematodes, weeds, plant diseases and invasive species such as zebra and quagga mussels, aquatic weeds and algae. We also have produced a collection of microorganisms from taxonomic groups that may enhance nutrient uptake in plants, reduce stress and otherwise increase plant growth. Our product candidates come primarily from our own discovery and development as well as in-licensed technology from universities, corporations and governmental entities.
Our proprietary product development process includes several important components. For all of our product candidates, we develop an analytical method to detect the quantity of the active natural product compounds that are produced by the microorganism or that are extracted from plants. For microbial products, we develop unique proprietary fermentation processes that increase the active natural compounds produced by the microorganisms. We also scale-up fermentation volumes to maximize yields consistently in each batch. Similarly, for our plant extract-based products, we develop a manufacturing process that increases the amount of active natural compounds extracted from plant materials.
15
Table of Contents
Our deep understanding of natural product chemistry allows us to develop fermentation and formulations that optimize the concentrations, efficacy and stability of compounds produced by microorganisms or plants. These methods allow us to produce products that are highly effective and of a consistent quality on a commercial scale. With the successful commissioning of our manufacturing facility, we have added a wealth of know-how and demonstrated an ability to produce products that are effective and of a consistent quality on a commercial scale.
Our commercial products are sold in various formulations are tailored to meet customers’ needs and display performance characteristics such as effectiveness, shelf life, compatibility with other pesticides and ease of use. Our senior management’s numerous years of experience in the development of commercial products and formulations have resulted in a highly efficient product development process.
Our discovery and development process is illustrated in the following diagram:
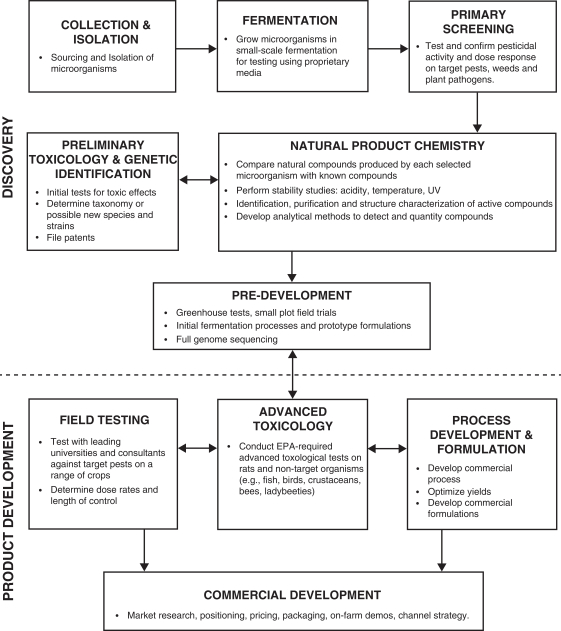
16
Table of Contents
Discovery
We have found over 25 candidates for commercial development from our proprietary discovery process, including Venerate, a new bacterial species and bioinsecticide, MBI-011, a burndown bioherbicide, MBI-010, a systemic bioherbicide, MBI-302 and MBI-303, bionematicides, MBI-110, a biofungicide, and MBI-507, for plant health, as well as several bioalgaecides, additional biofungicides, bioherbicides and bionematicides and plant growth enhancers. Key aspects of our discovery process include:
Collection and isolation. Using our years of experience, we target selected habitats and niches of high biodiversity to collect soil, compost, insects, flowers, or other biological matter from which we isolate our proprietary microorganisms on proprietary media. We capture information in a microorganism database such as taxonomic groups, geographical locations, types of samples, niches and habitats where collected and biological activity. We also isolate microorganisms that improve the efficiency of plants to uptake nitrogen and phosphorous. In addition to isolating our own microorganisms, which make up approximately 90% of our collection, we have had collaborations with three companies plus the Scripps Institution of Oceanography to diversify our sourcing of microorganisms.
Fermentation. For our microbial products, before testing the selected microorganisms for activity against pests, we ferment them to produce sufficient quantities for testing. We grow the selected microorganisms in proprietary media, which maximizes their pesticidal properties. In addition, we use proprietary fermentation processes that are designed to replicate those that would be required for large-scale fermentation and commercial production, avoiding the time and expense of an unsuccessful scale-up.
Primary screening. We use automated, miniaturized biological assays to test the selected microorganism’s or plant extract’s effectiveness against several weed, insect and nematode pests and plant pathogens and algae. We compare those results to conventional chemical pesticide standards. When a microorganism shows a high level of pesticidal activity, we conduct further tests to determine the spectrum of activity, mode of action, stability and activity on plants. We also test for the microorganisms’ ability to reduce plant stress and promote growth.
Novel and proprietary screening methods for weeds and nematodes. We have used proprietary assays based on specific enzymes that find systemic herbicidal compounds from microorganisms, one of which is subject of a pending patent covering identification of compounds that act systemically through plants’ vascular systems. We have developed a rapid, efficient method to find microorganisms that produce compounds with a high level of activity against plant parasitic nematodes.
Natural product chemistry. Using high-performance liquid chromatography (HPLC) with diode array detection technology, liquid chromatography-mass spectroscopy (LCMS), gas chromatography-mass spectroscopy (GC-MS) and nuclear magnetic resonance (NMR), we compare the natural product compounds produced by each of the selected microorganisms with known compounds. This allows us to eliminate those microorganisms that produce known toxins and to select those that we believe are novel and safe. From the selected microorganisms, we identify and characterize the natural product compounds responsible for their pesticidal activity by using HPLC, LCMS, GC-MS and NMR equipment. We then develop analytical methods to measure the quantity of these compounds in individual fermentation batches, determine the quantities needed to maximize efficacy and to insure consistent levels of these compounds from batch to batch.
Genetic identification. After confirming pesticidal activity during our primary screen, we perform the initial genetic identification of the microorganisms. Further characterization of the genome of our early stage candidates is contracted with one of several genome sequencing service companies. This characterization allows us to
determine novelty compared to discoveries from others, the relatedness to human or animal pathogens, genes for compounds that are not expressed in fermentation or detected by our chemists, and information about the
possible mode of action on the target pest. We also file additional patent applications based on the results of these genetic identification processes.
17
Table of Contents
Product Development
We believe that by maintaining a strong reputation in the industry, many opportunities come to us for development in addition to our own discoveries from our in-house efforts. Once we discover or are brought an opportunity, we make a preliminary assessment of the commercial potential of a natural product determined through laboratory, greenhouse and initial field tests. We then select product candidates we have discovered in-house or in-licensed for further development. Key aspects of our product development include:
Development of the manufacturing process that maximizes the active natural product compounds. For our microbial biopesticide products, we develop proprietary processes that increase the yield of both the microorganism and the active natural product compounds produced by the microorganism during fermentation. Similarly, for our plant extract-based products, we develop proprietary processes that increase the amount of active natural compounds extracted from plant materials. This process development allows us to produce products that have superior performance. For our microbial products, we then scale-up these proprietary processes in progressively larger fermentation tanks. We develop quality control methods based on the active natural product compounds rather than just the microorganisms or plant extracts. This approach results in a more consistent and effective product.
Formulation. We are able to develop proprietary water-soluble powder, liquid and granule formulations that allow us to tailor our products to customers’ needs. This allows us to develop product formulations with enhanced performance characteristics such as effectiveness, value, shelf life, suitability for organic agriculture, water solubility, rain resistance, compatibility with other pesticides and ease of use. Formulation is critical to ensuring a bio-based pest management and plant health product’s performance. Our understanding of the natural product chemistry allows us to develop formulations that maximize the effectiveness and stability of the compounds produced by the microorganisms or plants.
Field testing. We conduct numerous field trials for each product candidate that we develop. These field trials are conducted in small plots on commercial farms or research stations by our own field development specialists as well as private and public researchers to determine large-scale effectiveness, use rates, spray timing and crop safety. We conduct crop protection product field trials globally in both hemispheres to accelerate the results of our field trials and provide alternate season learning opportunities. As the crop protection product candidate nears commercialization, we conduct demonstration trials on the farm. These trials are conducted with distributors, influential growers and food processors on larger acreages. For Zequanox, we have been working with large power and industrial customers both in the United States and Canada to obtain field trial data to help with product commercialization efforts and to obtain efficacy data.
Sales, Marketing and Distribution
In the United States, we sell our products through our own internal sales force, which consists of nine employees focused on managing distributor relationships and creating grower demand for our products. In addition, a dedicated team of four employees provide technical service support to both our customers and sales representatives on the use of our products in integrated pest management programs, both for conventional growers as well as for an expanding number of organic growers. Our sales force covers all major regions in the United States, including California and the Pacific Northwest, the Southeast, the Northeast, the Mid-Atlantic and the Midwest regions. We currently sell our crop protection product lines, Regalia, Grandevo and Venerate, through leading agricultural distributors such as Crop Production Services, Chem-Nut and members of the Integrated Agribusiness Professionals group. These are the same distribution partners that most major agrichemical companies use for delivering solutions to growers across the country. For our water treatment product line, Zequanox, we are seeking sales and distribution partners for in-pipe and open water uses. Zequanox is currently being marketed and sold directly to a selected group of U.S. power and industrial companies.
With respect to sales outside of the United States, we have signed exclusive international distribution agreements for Regalia with major international distributors such as FMC (for certain markets in Latin America), Syngenta
18
Table of Contents
(for markets in Africa, Europe and the Middle East) and Engage Agro (for markets in Canada and professional turf and ornamental plant markets in the United States). We also intend to work with regional and country distributors who have brand recognition and established customer bases and who can conduct field trials and grower demonstrations and lead or assist in regulatory processes and market development. For example, we are in discussions and have testing agreements with distributors in Brazil, Australia, New Zealand, and Europe and for certain foreign markets for Grandevo and Venerate.
We derived approximately 91%, 95%, 96% and 94% of our revenues from Regalia and Grandevo for the years ended December 31, 2014, 2013 and 2012 and the six months ended June 30, 2015, respectively. In addition, we currently rely, and expect to continue to rely, on a limited number of distributors for a significant portion of our revenues since we sell through highly concentrated, traditional distribution channels. For the year ended December 31, 2014, our top two distributors accounted for 43% of our total revenues, with Crop Production Services and Helena Chemicals accounting for 30% and 13% of our total revenues, respectively.
While the biopesticide industry has been growing, customers in the crop production sector and the water treatment sector are generally cautious in their adoption of new products and technologies and may perceive bio-based pest management products as less attractive than conventional chemical pesticides. Growers often require on-farm demonstrations of a given pest management or plant health product, and given the relative novelty of our water treatment products, consumers of those products will continue to require education on their use. We are implementing the following strategies to accelerate adoption rates and promote sales of our bio-based pest management and plant health products:
Establish a focused and effective sales and marketing team that shares our values. We were seriously and negatively impacted by the departures of our former chief operating officer, who led our sales and marketing teams, and significant members of our sales staff in the third quarter of 2014. Over the last year, we have been rebuilding our sales and marketing teams, including hiring highly experienced personnel to train our sales force and seeking a new head of marketing to guide an expanded marketing department. In addition, we are more effectively organizing the data and educational material that we have amassed over nine years of operations on our bio-based products as well as organic and sustainable agricultural practices in order to train and equip our sales staff to communicate with and educate distributors and growers. We believe that hiring and training a sales and marketing staff with high level of technical expertise and knowledge regarding the capabilities of our bio-based products is essential to expanding adoption of our products by growers and sales to distributors.
Develop an extensive demonstration program. We believe that for growers to be convinced that a bio-based pesticide or plant health product works, they often must see it for themselves. Growers risk their crop each time they try a new product, and often produce only one crop per year on any given plot of land. Further, bio-based pesticide and plant health products are often applied differently and at different times than conventional chemical pesticides, so may be used incorrectly by an inexperienced grower or advisor, decreasing efficacy. We typically conduct on-farm demonstrations with growers in the first year they try one of our products on smaller plots of land, to ensure successful application, promoting the continued use of our products in future years across more acres. In addition, we work with distributors to determine which crops to emphasize in a given year and area to maximize the effectiveness of our demonstration program.
Target early adopters of new pest management technologies. For crop protection products, we target large commercial growers in the United States, who generally set industry standards through more widespread adoption of new pest management technologies they initially test on portions of their crops. We also target organic growers, who are more willing to take risks on new products as they have had few alternatives and great demand for increased yields. We plan to continue to recruit these growers and their consultants to participate in demonstrations and field trials, enabling them to become familiar with our bio-based pest management and plant health products, to experience their benefits firsthand and to promote the use of our products with other growers in their regions. For Zequanox, we have developed strategic relationships with early adopters in the power generation business to do efficacy demonstrations while perfecting the formulations and application of the product.
19
Table of Contents
Educate growers and water resource managers about the benefits of our bio-based pest management products. We will continue to perform on-farm and in-facility demonstrations and provide field data packages to support and validate our products claims. We will also continue to participate in trade shows and conferences to educate growers, their licensed pest control advisors and water resource managers about the benefits of our bio-based pest management products. When in the field, our sales and technical service team members have access to a wealth of information regarding our products and on pre-loaded tablet computers to assist in solving growers’ and distributors’ problems real-time. We have provided a free application for mobile phones users to assist in calculating tank mix quantities, as well as a webinar and an online course on bio-based pest management products, which can be taken by growers for continuing education credit to maintain crop protection product applicator licenses. We intend to continue and expand our efforts to work with utilities, which we believe will create increased demand for Zequanox in adjacent market spaces beyond the power and industrial treatment opportunities we are currently targeting.
Develop and leverage relationships with key industry influencers. We will continue to develop relationships early in the product development process with influential members within our target markets, including large innovative growers, technical experts at leading agricultural universities, licensed pest control advisors, wineries, food processors, produce packers, retailers and power facilities. We believe that educating industry influencers about the benefits of Regalia, Grandevo, Venerate, Zequanox and our future products increases the likelihood that they will recommend our products to our distributors and end users.
Focus our own sales and marketing on the United States, while signing strategic agreements for international markets, turf, ornamental plants and consumer retail. Because of the concentration of large growers in the United States, we can access these customers through our own sales force. For international markets for Zequanox, we intend to develop strategic partnerships with large suppliers and distributors of water products. For Regalia, we have signed distribution agreements with leading agrichemical companies and regional distributors. For Grandevo, Venerate and future products, distribution agreements will be developed with regional and national distributors or large multinationals on a case-by-case basis, depending on their expertise in the regions. We have engaged distributors that are selling Regalia in Canada for specialty crops, in the United States for turf and ornamental plants, and in parts of the Midwest United States for row crops. We have also engaged a distributor, Engage Agro, who is selling Grandevo and Regalia in the United States for turf and ornamental plants, as Grandevo PTO and Regalia PTO, respectively. We have an exclusive relationship with Scotts Miracle-Gro for the consumer retail market, and have recently submitted dossiers to Canadian regulators for consumer retail uses of Regalia and Grandevo, as part of our relationship with Scotts Miracle-Gro.
Manufacturing
We have substantially transitioned our manufacturing process in-house to our Bangor, Michigan facility which was formerly used as a biodiesel plant prior to our acquisition in July 2012. Biopesticide formulation, microbial fermentation and product packaging are among the facility’s core competencies. We believe in-house manufacturing enhances control and flexibility in production while lowering manufacturing costs over time to achieve desired margins, in addition to strengthening intellectual property security. The facility has significant room for expansion to install drying capacity and larger fermenters to accommodate production of multiple products at higher volumes.
We produced the first test batch of Grandevo and produced small-scale amounts of Regalia at this facility in the last quarter of 2013. We now ferment our Grandevo and Zequanox products in our manufacturing facility, but use a third-party contractor for formulating them into spray-dried powders. The facility also accommodates full-scale production of Regalia. While we have the ability to produce the majority of our products using our own manufacturing capacity, we currently exclusively use third parties to manufacture Venerate, and we expect to continue to utilize third-party manufactures for supplemental production capacity to meet excess seasonal demand. Once manufactured, we may use our own facility or third parties to package and label products.
20
Table of Contents
The active ingredient in our Regalia product line is derived from the giant knotweed plant, which we obtain from China. We have scaled production of Regalia using a single supplier to acquire raw knotweed from numerous regional sources and perform an extraction process on this plant and create a dried extract that is shipped to our manufacturing plant for production and packaging. We do not maintain a long-term supply contract with this supplier. While there can be no assurance that we will continue to be able to obtain dried giant knotweed plant extract from our supplier in China at a competitive price point, we estimate that our current supply of the ingredient will be sufficient to manufacture product to meet the next 12 months’ demand. Should we elect or be required to do so, we do not believe that we would have substantial difficulty in finding an alternative supplier as we have identified and received quality knotweed from a number of new possible suppliers, although there can be no assurance that we will continue to be able to obtain dried extract from China at a competitive price point.
Research and Development
As of September 30, 2015, we had 45 full-time equivalent employees dedicated to research and development and patent related activities, seven of whom hold Ph.D. degrees, plus four sales and field development personnel who focus on technical support and demonstration and research field trials. Our research and development team has technical expertise in microbiology, natural product and analytical chemistry, biochemistry, fermentation, entomology, nematology, weed science, plant physiology, plant pathology and aquatic sciences. Our research and development activities are principally conducted at our Davis, California facility as well as by our field development specialists on crops and mussel-infested facilities in their respective regions. We have reduced the size of our research and development staff compared to prior periods as part of our measures to streamline business operations, but we have made, and will continue to make, substantial investments in research and development. Our research and development expenses, including patent expenses, were $19.3 million, $17.9 million, $12.7 million and $6.8 million in fiscal years 2014, 2013 and 2012 and the six months ended June 30, 2015, respectively.
Intellectual Property Rights
We rely on patents and other proprietary right protections, including trade secrets and proprietary know-how, to preserve our competitive position. As of September 30, 2015, we had 23 issued U.S. patents and 64 issued foreign patents (of which 5 U.S. patents and 25 foreign patents were in-licensed), 31 pending provisional and non-provisional patent applications (of which one was in-licensed), and 189 pending foreign patent applications (of which 6 were in-licensed) relating to microorganisms and natural product compounds, uses and related technologies. As of September 30, 2015, we had received 16 U.S. trademark registrations and had 7 trademark applications pending in the United States. As of September 30, 2015, we also had received 59 trademark registrations and had 12 trademark applications pending in various other countries.
When we find a microbial product in our screen that kills or inhibits one or more pests or pathogens in at least three replicated tests and identify the microorganism and its associated chemistry, we file a patent application claiming any one or more of the following:
| • | the microorganism, its DNA products, as well as mutations and other derivatives; |
| • | the use of the microorganism for pest management; |
| • | novel natural product compounds, their analogs and unique mixtures of compounds produced by the microorganism; |
| • | the new use of known natural product compounds for pest management; |
| • | formulations of the microorganism or compounds; and |
| • | synergistic mixtures of the microorganism or compounds with conventional chemical or other pesticides. |
21
Table of Contents
One of our commercially available products and certain of our lead product candidates are based on microbes we have identified using our proprietary discovery process, including Venerate, MBI-305 and MBI-010, which are based on a Burkholderia bacterium, and with respect to which we have 7 issued patents and have 44 pending patent applications (both U.S. and foreign), and MBI-110 and MBI-507, which are based on a Bacillus strain with respect to which we have one issued patents and have 12 pending patent applications (both U.S. and foreign).
We have also entered into in-license and research and development agreements with respect to the use and commercialization of Regalia, Grandevo and Zequanox, as well as certain products under development, including Haven (MBI-505) and MBI-601. Under the licensing arrangements for our commercially available products, we are obligated to pay royalty fees between 2% and 5% of net sales of these products, subject in certain cases to aggregate dollar caps. The exclusivity and royalty provisions of these agreements are generally tied to the expiration of underlying patents. For Regalia, the licensed patent is related to a method of extraction of knotweed. These patents acquired for Regalia and in-licensed for Zequanox will expire in 2017, although we have filed separate patent applications with respect to both product lines and have been issued two U.S. patents with respect to Regalia and one for Zequanox. In addition, the in-licensed U.S. patent for Grandevo is expected to expire in 2024, but there is a pending in-licensed patent application relating to Grandevo that could expire later than 2024, if issued, and we have also filed separate patent applications for Grandevo of which three have been issued on a novel compound and uses for nematodes and corn rootworm. While third parties thereafter may develop products using the technology under the expired patents, we do not believe that they can produce competitive products without infringing other aspects of our proprietary technology, and we therefore do not expect the expiration of the patents or the related exclusivity obligations to have a significant adverse financial or operational impact on our business. Certain additional information regarding the intellectual property associated with commercially available products based in part on in-licensed technology follows:
| • | Regalia. We entered into an exclusive license agreement with a company co-founded by Dr. Hans von Amsberg, a former employee of German chemical producer BASF, in May 2007 for U.S. and limited international use of a U.S. patent and technology used in our Regalia product line. We have also filed patent applications with respect to new formulations of Regalia. Two U.S. patents have been issued on the synergistic combinations with biopesticides and conventional chemical pesticides and one patent has been issued on the new uses for soil and roots. |
| • | Grandevo. We entered into a co-exclusive license agreement with the USDA in November 2007 for the use in the United States of a U.S.-issued patent and a U.S. patent application relating to the Chromobacterium subtsugae bacteria used in our Grandevo product line. We have filed patent applications on the compounds produced in the bacterial cells, gene sequences, new uses for the Chromobacterium subtsugae bacteria and for new uses and new formulations of our Grandevo product line. Four U.S. patents have been issued, on a novel compound produced by the bacteria, use for corn rootworm populations and for nematode control. While a second company has licensed the USDA’s patent with respect to the Chromobacterium subtsugae bacteria and could develop products based on the same underlying intellectual property, we have not provided this company access to the proprietary technology we have developed relating to Grandevo. |
| • | Zequanox. We entered into a license agreement with The University of the State of New York in December 2009 pursuant to which we were granted an exclusive license under the University’s rights for the worldwide use of a U.S.-issued patent and a Canadian-issued patent relating to the Pseudomonas fluorescens bacteria used in our Zequanox product line. One U.S. patent has been issued on the natural, mussel-killing compounds in the bacteria, and we have filed patent applications relating to various Zequanox active ingredients. |
Regulatory Considerations
Our activities are subject to extensive federal, state, local and foreign governmental regulations. These regulations may prevent us or our collaborators from developing or commercializing products in a timely manner
22
Table of Contents
or under technically or commercially feasible conditions and may impose expenses, delays and other impediments to our product development and registration efforts. In the United States, the EPA regulates our bio-based pest management products under the Federal Insecticide, Fungicide and Rodenticide Act (FIFRA), the Federal Food, Drug and Cosmetics Act (FFDCA) and the Food Quality Protection Act (FQPA). In addition, some of our plant health products are regulated as fertilizers or biostimulants in each of the fifty states.
In 2004, the United States Congress passed the Pesticide Registration Improvement Renewal Act, which was reauthorized in 2007 and 2012, a result of efforts from an industry coalition of pesticide companies and environmental groups, to codify pesticide approval times in return for user fees. This law facilitates faster approval times for biopesticides, with EPA approvals typically received within 16 to 24 months, compared with 36 months or longer for conventional chemical pesticides. Registration processes for state and foreign governments vary between jurisdictions and can take up to 12 months for state governments such as California and New York and up to 36 months or more for foreign governments. In some instances, California and Canada will conduct joint reviews with the EPA, which allows some pesticides to receive concurrent approvals in California, Canada and the United States. However, in most instances, most foreign government submissions will not occur until after a U.S. registration has been secured. To register a crop protection product with the EPA, companies must demonstrate the product is safe to mammals, non-target organisms, endangered species and the environment. To demonstrate the bio-based pest management product’s safety, required studies must be conducted that evaluate mammalian toxicology, toxicological effects to non-target organisms in the environment (ecotoxicological exposures) and physical and chemical properties of the product. The registration dossier is subject to both scientific and administrative reviews by EPA scientists and management before registration approval. The scientific review involves thorough evaluation of submitted data and completion of risk assessments for human dietary and ecotoxicological exposures. Upon completion of this process, the registration package, including the proposed label, is sent to the Office of General Council for legal review. The final step in the registration process is administrative sign-off by the EPA director of the Biopesticides and Pollution Prevention Division.
In addition to EPA approval, we are required to obtain regulatory approval from the appropriate state regulatory authority in individual states and foreign regulatory authorities before we can market or sell any pest management product in those jurisdictions. Foreign governments typically require up to two seasons of locally generated field efficacy data on crop-pest combinations before a product dossier can be submitted for review. California and foreign jurisdictions also require us to submit product efficacy data, which the EPA historically has not required, but may request.
While these regulations substantially increase the time and cost associated with bringing our products to market, we believe that our management team’s significant experience in bringing our and other companies’ technologies through EPA, state and foreign regulatory approval, efficient development process, and ability to leverage our strategic collaborations to assist with registrations, particularly in Europe and Latin America, will enable us to overcome these challenges.
Since our plant health products (which are classified by the EPA as biostimulants) are not used to control pests, they currently fall outside the legal scope of FIFRA, FFDCA and FQPA and, therefore, we do not need to submit applications for EPA registrations for such products. However, we must still submit state registrations for our plant health products, and those containing microbes of foreign origin may also need to “deregulated” (or determined not to be a plant pest) under the Plant Protection Act by the USDA Animal and Plant Health Inspection Service prior to use in field trials or for large scale release. Nevertheless, the regulatory process is significantly accelerated compared to that for biopesticides.
Regalia. The EPA granted approval for the Regalia SC formulation in August 2008, for the Regalia 5% (Regalia) formulation in May 2009, for the Regalia 20% (Regalia Maxx) formulation in January 2010 and for a “ready to use” consumer formulation in January 2010. Regalia is currently registered in all U.S. states. We have also
23
Table of Contents
registered Regalia Maxx in Brazil, Mexico, Canada, South Africa, Ecuador, Turkey, Panama, El Salvador, Guatemala, Honduras, Peru, the Dominican Republic, Morocco and Tunisia. We submitted an Annex 1 registration dossier to the European Union. Our Regalia registration package has completed initial review by regulatory authorities in the United Kingdom, which is serving as lead for completing the Annex 1 (active substance) listing of Regalia for the European Union. The UK-generated risk assessment has completed its technical review by the European Food Safety Authority, and it is currently being reviewed by the European Commission for Annex 1 listing consideration. In addition to obtaining the Annex 1 listing, we must obtain Annex 3 authorization approval from each country in which we plan to market and sell products. “Regalia Maxx” will be marketed as “Sakalia” by Syngenta in Europe, the Middle East and Africa.
Grandevo. In August 2011 and May 2012, the EPA granted approval for the Grandevo insecticide “technical grade active ingredient” and a wettable powder formulation, respectively. This wettable powder formulation is registered in all 50 states as well as Puerto Rico and the District of Columbia. In May 2013, we received EPA approval for a revised label reflecting Grandevo’s safety for bees. In addition, we submitted the registration dossier for Grandevo to Mexico and we also received permission to field test Grandevo in Brazil, Australia, New Zealand and South Africa allowing us to prepare the dossiers for submission in those countries. We submitted dossiers for Grandevo registration in Europe and Canada in 2015, with the Netherlands recently finding the Grandevo dossier meeting “completeness check” requirements in July 2015 and officially starting the dossier review for the EU. Grandevo is also currently being field evaluated in a Brazilian government sponsored emergency use program to control an outbreak of Heliocoverpa armigera infestations in cotton and soya. Grandevo was submitted to the emergency use program in October 2014 and is under active review and field evaluation by Brazilian regulatory agencies. Concurrently, we have other field trials either underway or planned in Brazil for 2015 on a variety of insect pests, in order to have additional crop-pest uses added to the regulatory dossier and label.
Zequanox. In July 2011, the EPA granted a conditional approval of the “technical grade active ingredient” in an early formulation of Zequanox. A spray-dried powder formulation, which is an improvement over the “end product” approved in July 2011, was approved in March 2012, and this formulation is now commercially available. We have also received approval for Zequanox use in hydroelectric plants in Canada in November of 2012. We received EPA approval for open water uses in June 2014. Currently, Zequanox is being evaluated by several U.S. and Canadian federal, state and provincial entities as an invasive mussel eradication, native mussel habitat restoration and harmful algal bloom prevention tool in the Great Lakes regional under the auspices of government programs. In pipe and open water labels have been approved in all targeted states, with the exception of California where in pipe uses are currently registered and the open water use label is under evaluation.
Venerate. In February 2014, the EPA granted approval for Venerate. Venerate is currently registered in 49 states and Puerto Rico, with registration pending in Hawaii. In 2014, we submitted Venerate registration dossiers in Canada and Mexico. Several key regulatory efficacy trials to support Venerate Annex 1 listing in Europe have been completed and ongoing 2015 work will enable us to submit a dossier for Venerate in 2016. As with Grandevo, Venerate is also currently being field evaluated in a Brazilian government sponsored emergency use program to control an outbreak of Heliocoverpa armigera infestations in cotton and soya. Venerate also was submitted to the emergency use program in October 2014 and is under active review by Brazilian regulatory agencies. Concurrently, we have other field trials either underway or planned in Brazil for 2015 on a variety of insect pests, in order have broader uses available.
As with any pesticide, our pest management products will continue to be subject to review by the EPA and state regulatory agencies. The EPA has the authority to revoke the registration or impose limitations on the use of any of our pest management products if we do not comply with the regulatory requirements, if unexpected problems occur with a product or the EPA receives other newly discovered adverse information. See Part I-Item 1A-“Risk Factors—Risks Relating to Our Business and Strategy—Our inability to obtain regulatory approvals, or to comply with ongoing and changing regulatory requirements, could delay or prevent sales of the products we are developing and commercializing.” Our research and development activities are also subject to federal, state and
24
Table of Contents
local worker safety, air pollution, water pollution and solid and hazardous waste regulatory programs and periodic inspection. We believe that our facilities are in substantial compliance with all applicable environmental regulatory requirements.
Competition
For pest management products, performance and value are critical competitive factors. To compete against manufacturers of conventional chemical pesticides and genetically modified crops, we need to demonstrate the advantages of our products over these more established pest management products. Many large agrichemical companies are developing and have introduced new conventional chemical pesticides and genetically modified products that they believe are safer and more environmentally friendly than older conventional chemical products.
The pest management market is very competitive and is dominated by multinational chemical and life sciences companies such as Arysta, BASF, Bayer, Dow Agrosciences, DuPont, FMC, Monsanto, Sumitomo Chemical and Syngenta. Universities, research institutes and government agencies may also conduct research, seek patent protection and, through collaborations, develop competitive pest management products. Other companies, including bio-specialized biopesticide businesses such as AgraQuest (now a part of Bayer), Certis USA (now a part of Mitsui), Novozymes (in a joint venture with Monsanto) and Valent Biosciences (now a part of Sumitomo) may prove to be significant competitors in the bio-based pest management and plant health market.
In many instances, agrichemical companies have substantially greater financial, technical, development, distribution and sales and marketing resources than we do. Moreover, these companies may have greater name recognition than we do and may offer discounts as a competitive tactic. There can be no assurance that our competitors will not succeed in developing pest management products that are more effective or less expensive than ours or that would render our products obsolete or less competitive. Our success will depend in large part on our ability to maintain a competitive position with our technologies and products.
Employees
In connection with our recent changes in business strategy, we have significantly reduced overall headcount, while building a new sales and marketing organization which provides for increased training and a better ability to educate and support customers as well as transitioning our product development staff to undertake greater responsibility for technical sales support, field trials and demonstrations to promote sales growth. As of September 30, 2015, we had 86 full-time equivalent employees, of whom nine hold Ph.D. degrees. Approximately 45 employees are engaged in research and development and patent related activities, 16 in sales and marketing (including four sales and field development personnel who focus on technical support and demonstration and research field trials) and 25 in management, operations, accounting/finance and administration. None of our employees are represented by a labor union.
Restatement
Matters relating to or arising from the Audit Committee investigation, the Restatement and weaknesses in our internal controls, including adverse publicity, regulatory inquiries and litigation matters, have caused us to incur significant legal, accounting and other professional fees and other costs, have exposed us to greater risks associated with other civil litigation, regulatory proceedings and government enforcement actions, have diverted resources and attention that would otherwise be directed toward our operations and implementation of our business strategy and may have impacted our ability to attract and retain customers, employees and vendors. The impacts on our business related to these matters are discussed further elsewhere in this Annual Report on Form 10-K, including under the Explanatory Note to this report, in Part I–Item 1A–“Risk Factors–Risks Relating to our Financial Reporting Process,” Note 21, Quarterly Financial Information (Unaudited), to the consolidated financial statements included in this report and elsewhere in this report.
25
Table of Contents
Corporate Information
We were originally incorporated in the State of Delaware in June 2006 as Marrone Organic Innovations, Inc. Our principal executive offices are located at 1540 Drew Avenue, Davis, CA 95618. Our telephone number is (530) 750-2800. Our website address is www.marronebioinnovations.com.
26
Table of Contents
Our operations and financial results are subject to various risks and uncertainties, including those described below, which could adversely affect our business, financial condition, results of operations, cash flows, growth prospects and the trading price of our common stock.
Risks Relating to Our Business and Strategy
We have a limited number of commercialized products, have incurred significant losses to date and anticipate continuing to incur losses in the future, and we may not achieve or maintain profitability.
We are an early stage company with a limited number of commercialized products. We have incurred operating losses since our inception in June 2006, and we expect to continue to incur operating losses for the foreseeable future. At December 31, 2014 and 2013 and June 30, 2015, we had an accumulated deficit of $159.8 million, $108.2 million and $182.7 million, respectively. For the years ended December 31, 2014, 2013 and 2012 and the six months ended June 30, 2015, we had a net loss attributable to common stockholders of $51.7 million, $32.6 million, $40.8 million and $22.9 million, respectively. As a result, we will need to generate significant revenues to achieve and maintain profitability. If our revenues grow more slowly than anticipated, or if operating expenses exceed expectations, then we may not be able to achieve profitability in the near future or at all, which may depress our stock price.
Through June 30, 2015, we have derived substantially all of our revenues from sales of Regalia and Grandevo. In addition, we have derived revenues from strategic collaboration and development agreements for the achievement of testing validation, regulatory progress and commercialization events, and from sales of other products. Accordingly, there is only a limited basis upon which to evaluate our business and prospects. Our future success depends, in part, on our ability to market and sell other products, such as Zequanox and Venerate, as well as our ability to increase sales of Regalia and Grandevo and to introduce new products. An investor in our stock should consider the challenges, expenses, and difficulties we will face as a company seeking to develop and manufacture new types of products in a relatively established market. We expect to derive future revenues primarily from sales of Regalia, Grandevo, Zequanox, Venerate and other products, but we cannot guarantee the magnitude of such sales, if any. We expect to continue to devote substantial resources to expand our research and development activities, further increase manufacturing capabilities and expand our sales and marketing activities for the further commercialization of Regalia, Grandevo, Zequanox, and Venerate, and other product candidates. We expect to incur additional losses for the next several years and may never become profitable.
We may require additional financing in the future to meet our business requirements and to service our debt. Such capital raising may be costly or difficult to obtain and could dilute current stockholders’ equity interests, and we may be unable to repay our secured indebtedness.
In our August 2015 private placement transaction, we issued senior secured promissory notes in the initial aggregate principal amount of $40.0 million, which accrues interest at a rate of 8% per annum, with $10 million payable 3 years from the closing, $10 million payable 4 years from the closing and $20 million due 5 years from the closing. In addition, in June 2014, we borrowed $10.0 million pursuant to a promissory note with a bank, which accrues interest at a variable interest rate, currently at 5.25% per annum, and which is repayable in monthly payments through June 2036, and we completed private placements in October 2012 and April 2013 of promissory notes in the aggregate principal amount of $12.45 million, which accrues interest at 18% through maturity in October 2017. The debt agreements with respect to these transactions contain various financial and other covenants, as discussed below, and our obligations under the loan agreements are secured by all of our personal property assets and general intangibles.
As we expect to continue to incur losses until we are able to significantly grow our revenue, we may need additional financing to meet the financial covenants or pay the principal and interest under our debt agreements,
27
Table of Contents
as well as to maintain and expand our business. We may seek additional funds from public and private stock offerings, corporate collaborations and licenses, borrowings under lease lines of credit or other sources. Additional capital may not be available on terms acceptable to us, or at all. Any additional equity financing may be dilutive to stockholders, and debt financing, if available, may include restrictive covenants. In addition, our existing loan agreements contain certain restrictive covenants that either limit our ability to, or require a mandatory prepayment if we incur additional indebtedness and liens and enter into various specified transactions. We therefore may not be able to engage in any of the foregoing transactions unless we obtain the consent of our lenders or prepay the outstanding amounts under the term loan agreements, which could require us to pay additional prepayment penalties. In addition, we may incur substantial costs in pursuing future capital financing, including investment banking fees, legal fees, accounting fees, securities law compliance fees, printing and distribution expenses and other costs. We also may be required to recognize non-cash expenses in connection with certain securities we issue, such as warrants, which may adversely impact our financial results.
Certain of our debt agreements also contain financial covenants, including maintaining minimum current, debt-to-worth and loan-to-value ratios and certain cash balance requirements, in addition to provisions providing for an event of default if there is a material adverse change in our financial condition and if we are in default under certain of our other agreements. While we are not currently in default under any of these agreements, and none of our lenders have previously declared an event of default on our indebtedness, prior to our recent receipt of waivers from our lenders, we had not been in compliance with certain of these covenants. In addition, if we fail to pay any principal or interest under our indebtedness when due, or are otherwise in violation of the covenants under our debt agreements, this may result in the acceleration of our indebtedness, and we may not have sufficient funds to repay that indebtedness.
We expect that our current resources and future operating revenue will be sufficient to fund operations for at least the next 12 months. If we cannot raise more money when needed, or are unable to use our future working capital, borrowings or equity financing to repay or refinance the amounts outstanding under our debt agreements or to renegotiate our debt arrangements with lenders, we may have to reduce our capital expenditures, scale back our development of new products, reduce our workforce or license to others products that we otherwise would seek to commercialize ourselves. Further, we may not be able to continue operating if we do not generate sufficient revenue from operations needed to stay in business, and we may be required to seek protection from creditors through bankruptcy proceedings. See Part II-Item 7-“Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources” below.
Our business may fail if we are not able to increase sales of our four commercialized products.
Our future success will depend on our ability to significantly increase sales from the bio-based pest management products we have commercialized, both domestically and abroad. Our initial sales of our primary formulation of Regalia and our initial formulation of Grandevo occurred in the fourth quarter of 2009 and the fourth quarter of 2011, respectively, we began selling Zequanox in the second half of 2012, and we began to sell Venerate, a bioinsecticide, in May 2014. However, while we have invested considerable resources in the launch of these products, to date, various factors have impeded anticipated growth in sales of these products.
For example, we believe adverse conditions in the U.S. agricultural industry, including low commodity prices, may have reduced demand for our products. Further delays in regulatory approvals of certain of our products in Europe and other jurisdictions may slow international growth, and any delay in a product launch that causes us to miss a growing season may require us to wait a year to enter that market. The extended drought in California and other markets has reduced demand for our products, as fewer acres are planted and certain of our strategic collaborations have not resulted in anticipated increases in sales of Regalia outside of the Unites States. Due to prioritization constraints, we have not committed resources to Zequanox sufficient to market it full-scale, and our collaboration efforts with regard to this product may not result in increased sales. In addition, the departures of our former chief operating officer and significant members of our sales staff in the third quarter of 2014 and subsequent turnover in our sales and marketing department disrupted the 2014 launch of Venerate as well as growth in sales of our other commercialized products, including Regalia and Grandevo.
28
Table of Contents
Lower than expected sales growth may increase write-offs and inventory obsolescence if we are not be able to use raw materials or sell finished goods before they expire, and may result in higher proportional operating expense levels, increases in our costs of goods sold and decreases in product margins, as we are unable to manufacture products as efficiently at low volumes and underutilization of our Bangor, Michigan manufacturing facility results in increased relative overhead and operating costs, in addition to decreased allocation of depreciation and other costs to production and inventory. If we are unable to establish a successful sales and marketing infrastructure internally and increase sales of our commercialized products, our financial results will be adversely affected, our available cash and ability to raise additional capital will decrease, and our business may fail.
We have limited experience in marketing and selling our products and will need to expand our sales and marketing infrastructure.
We currently have limited sales and marketing experience and capabilities. As of September 30, 2015, we employed 16 full-time equivalent sales and marketing personnel, four of which focus on technical support and demonstration and conducting field trials. Many of these sales personnel are recent hires for us following the departures of our former chief operating officer, who led our sales and marketing teams, and significant members of our sales staff in the third quarter of 2014. These personnel have required significant training to attain a high level of technical expertise and knowledge regarding the capabilities of our bio-based products compared with conventional chemical pest management products and techniques in order to educate growers and independent distributors on the uses and benefits of our products. We will need to further develop our sales and marketing capabilities and find partners in order to successfully increase sales of our commercially available products and to commercialize other products we are developing, which may involve substantial costs. There can be no assurance that our specialists and other members of our sales and marketing team will successfully compete against the sales and marketing teams of our current and future competitors, many of which may have more established relationships with distributors and growers. Our inability to recruit, train and retain sales and marketing personnel or their inability to effectively market and sell the products we are developing could impair our ability to gain market acceptance of our products and cause our sales to suffer.
If we are unable to maintain and further establish successful relations with the third-party distributors that are our principal customers, or they do not focus adequate resources on selling our products or are unsuccessful in selling them to end users, sales of our products will be adversely affected.
In the United States, we rely on independent distributors of agrichemicals such as Crop Production Services and Wilbur Ellis to distribute and assist us with the marketing and sale of Regalia, Grandevo, Venerate and other products we are developing. These distributors are our principal customers, and our future revenue growth will depend in large part on our success in establishing and maintaining this sales and distribution channel. However, there can be no assurance that our distributors will be successful in selling our products to end users, or will focus adequate resources on selling them, and they may not continue to purchase or market our products for a number of reasons.
For example, many distributors lack experience in marketing bio-based pest management and plant health products, which generally must be used differently than conventional chemical pesticides. Distributors may not continue to market our products if they receive negative feedback from end users, even if we believe our products are being blamed for damage to treated plants caused by other pesticides with which our products have been combined (whether properly or improperly). In addition, many of our distributors are in the business of distributing and manufacturing other, possibly competing, pest management and plant health products, including internally developed and commercialized bio-based products as well as bio-based products developed by larger agrichemical companies that negotiate to “bundle” such specialty products with other high demand products. As a result, our distributors may earn higher margins by selling competing products or combinations of competing products. Further, in 2014 and 2015, our Audit Committee investigation, Restatement work and external investigations imposed additional work on our distributors, which has been perceived negatively in some cases, and distributors may also react negatively to additional “sell-through” reporting requirements we may require of
29
Table of Contents
them to apply our own revenue recognition policies. If we are unable to establish or maintain successful relationships with independent distributors, we will need to further develop our own sales and distribution capabilities, which would be expensive and time-consuming and the success of which would be uncertain.
The product candidates we select for development and commercialization may fail to generate significant revenues, and we may not be able to successfully enter into strategic collaborations with respect to our other product candidates.
In 2014, we began to implement a prioritization plan to focus our research and development on products that are expected to have the greatest near-term growth potential. Accordingly, we are currently limiting our internal efforts on five product candidates, Majestene (MBI-305), a bionematicide for which we have initiated a target placement, MBI-010, a bioherbicide, and MBI-110, a biofungicide, both of which we plan to submit to the EPA in 2016, Haven, a plant health product that does not require EPA registration, and MBI-601, a biofumigant that we submitted to the EPA in April 2014. We are also seeking collaborations with third parties to develop and commercialize certain promising early-stage candidates.
Successful development of product candidates will require significant additional investment, including costs associated with research and development, completing field trials and obtaining regulatory approval, as well as the ability to manufacture our products in large quantities at acceptable costs while also preserving high product quality. Difficulties often encountered in scaling up production include problems involving production yields, quality control and assurance, shortage of qualified personnel, production costs and process controls. In addition, we are subject to inherent risks associated with new products and technologies. These risks include the possibility that any product candidate may:
| • | be found unsafe; |
| • | be ineffective or less effective than anticipated; |
| • | fail to receive or take longer to receive necessary regulatory approvals; |
| • | be difficult to competitively price relative to alternative pest management solutions; |
| • | be harmful to consumers, growers, farm workers or the environment; |
| • | be harmful to crops when used in connection with conventional chemical pesticides; |
| • | be difficult or impossible to manufacture on an economically viable scale; |
| • | be subject to supply chain constraints for raw materials; |
| • | fail to be developed and accepted by the market prior to the successful marketing of similar products by competitors; |
| • | be impossible to market because it infringes on the proprietary rights of third parties; or |
| • | be too expensive for commercial use. |
Our decisions regarding which product candidates to pursue may cause us to fail to capitalize on product candidates that could have given rise to viable commercial products and profitable market opportunities. In addition, we may not be successful in entering into new arrangements with third parties, on favorable terms or at all, with respect to product candidates we do not pursue internally.
Adverse weather conditions and other natural conditions can reduce acreage planted or incidence of crop disease or pest infestations, which can adversely affect our results of operations.
Production of the crops on which our products are typically applied is vulnerable to extreme weather conditions such as heavy rains, hurricanes, hail, tornadoes, freezing conditions, drought, fires and floods. Weather conditions can be impacted by climate change resulting from global warming, including changes in precipitation patterns and the increased frequency of extreme weather events, or other factors. Unfavorable weather conditions
30
Table of Contents
can reduce both acreage planted and incidence (or timing) of certain crop diseases or pest infestations, each of which may reduce demand for our products. For example, in 2013 and 2012, the United States experienced nationwide abnormally low rainfall or drought, reducing the incidence of fungal diseases such as mildews and the demand for fungicides, such as Regalia. These conditions have been present in some of our key markets through both 2014 and 2015 as well, and have also resulted in further reductions in acreage planted throughout California and the Pacific Northwest. Shortened bloom cycles relating to changes in weather patterns also could reduce the amount of pesticides and plant health products used during a growing season. For example, in 2014, the Florida citrus market experienced a shortened bloom cycle as a result of changes in weather patterns, which negatively affected our sales of Grandevo in the Florida market. In addition, ideal weather conditions can reduce the incidence of diseases and pest infestations and increase yields without the use of additional pesticide and plant health applications. Increased yields can also reduce commodity prices causing growers to make a decision not to increase costs by reducing the amount of pesticides and plant health products used during a growing season. Since all of our products have different margins, changes in product mix due to these conditions could affect our overall margins.
If our ongoing or future field trials are unsuccessful, we may be unable to obtain regulatory approval of, or commercialize, our products on a timely basis.
The successful completion of multiple field trials in domestic and foreign locations on various crops and water infrastructures is critical to the success of our product development and marketing efforts. If our ongoing or future field trials are unsuccessful or produce inconsistent results or unanticipated adverse side effects on crops or on non-target organisms, or if we are unable to collect reliable data, regulatory approval of our products could be delayed or we may be unable to commercialize our products. In addition, more than one growing or treatment season may be required to collect sufficient data and we may need to collect data from different geographies to prove performance for customer adoption. Although we have conducted successful field trials on a broad range of crops, we cannot be certain that additional field trials conducted on a greater number of acres, or on crops for which we have not yet conducted field trials, will be successful. Moreover, the results of our ongoing and future field trials are subject to a number of conditions beyond our control, including weather-related events such as drought or floods, severe heat or frost, hail, tornadoes and hurricanes, or low or no natural occurrence of the pests intended for testing. Generally, we pay third parties such as growers, consultants and universities, to conduct field tests on our behalf. Incompatible crop treatment practices or misapplication of our products by these third parties or lack of sufficient occurrence of the identified pests in nature for a particular trial could impair the success of our field trials.
Our inability to obtain regulatory approvals, or to comply with ongoing and changing regulatory requirements, could delay or prevent sales of the products we are developing and commercializing.
The field testing, manufacture, sale and use of pest management products, including Regalia, Grandevo, Zequanox, Venerate, Majestene and other products we are developing, are extensively regulated by the EPA and state, local and foreign governmental authorities. These regulations substantially increase the time and cost associated with bringing our products to market. If we do not receive the necessary governmental approvals to test, manufacture and market our products, or if regulatory authorities revoke our approvals, do not grant approvals in a timely manner or grant approvals subject to restrictions on their use, we may be unable to sell our products in the United States or other jurisdictions, which would result in our future revenues being less than anticipated.
We have received approval from the EPA for the active ingredients and certain end product formulations for Regalia, Grandevo, Zequanox, Venerate, Majestene, and MBI-011. As we introduce new formulations of and applications for our products, we will need to seek EPA approval prior to commercial sale. For any such approval, the EPA may require us to fulfill certain conditions within a specified period of time following initial approval. We are also required to obtain regulatory approval from other state and foreign regulatory authorities before we market our products in their jurisdictions, some of which have taken, and may take, longer than anticipated.
31
Table of Contents
Some of these states and foreign countries may apply different criteria than the EPA in their approval processes. Although federal pesticide law preempts separate state and local pesticide registration requirements to some extent, state and local governments retain authority to control pesticide use within their borders.
There can be no assurance that we will be able to obtain regulatory approval for marketing our additional products or new product formulations and applications we are developing. Although the EPA has in place a registration procedure for biopesticides like Regalia and Grandevo that is streamlined in comparison to the registration procedure for conventional chemical pesticides, there can be no assurance that all of our products or product extensions will be eligible for this streamlined procedure or that additional requirements will not be mandated by the EPA that could make the procedure more time consuming and costly for our future products.
Additionally, for California state registration and registration in jurisdictions outside of the United States, all products need to be proven efficacious for each proposed crop-pest combination, which can require costly field trial testing and a favorable result is not assured. Because many of the products that may be sold by us must be registered with one or more government agencies, the registration process can be time consuming and expensive, and there is no guarantee that the product will obtain all needed registrations. We have intentionally obtained registration in some jurisdictions and not in others. California is one of the largest and most important producers of agricultural products in the world. Because of its stringent regulation of pesticides and environmental focus, we also view California as one of the most natural and attractive markets for our products. However, California is also very stringent, is generally more time consuming and lacks legally mandated deadlines for its reviews of reduced-risk biopesticides. Therefore, gaining concurrent approvals with the EPA, other states and sometimes even other countries may not always be achievable. Even if we obtain all necessary regulatory approvals to market and sell our products, they will be subject to continuing review and extensive regulatory requirements, including periodic re-registrations. The EPA, as well as state and foreign regulatory authorities, could withdraw a previously approved product from the market upon receipt of newly discovered information, including an inability to comply with their regulatory requirements or the occurrence of unanticipated problems with our products, or for other reasons.
Bio-based pest management and plant health products are not well understood, which necessitates investment in customer education and makes effectively marketing and selling our products difficult.
The market for bio-based pest management and plant health products is underdeveloped when compared to conventional pesticides. Customers in the crop production sector and the water treatment sector are generally cautious in their adoption of new products and technologies. Growers often require on-farm demonstrations of a given pest management or plant health product. Initial purchases of the product tend to be conservative, with the grower testing on a small portion of their overall crop. As the product is proven, growers incorporate the product into their rotational programs and deploy it on a greater percentage of their operations. As a result, large scale adoption can take several growing seasons. Water treatment products must also pass efficacy and ecological toxicity tests. In addition, given the relative novelty of our water treatment products, consumers of those products will continue to require education on their use, which may delay their adoption.
In addition, customers have historically perceived bio-based pest management products as more expensive and less effective than conventional chemical pesticides. To succeed, we will need to continue to change that perception. To the extent that the market for bio-based pest management products does not further develop or customers elect to continue to purchase and rely on conventional chemical pesticides, our market opportunity will be limited.
The high level of competition in the market for pest management and plant health products may result in pricing pressure, reduced margins or the inability of our products to achieve market acceptance.
The markets for pest management and plant health products are intensely competitive, rapidly changing and undergoing consolidation. We may be unable to compete successfully against our current and future competitors, which may result in price reductions, reduced margins and the inability to achieve market acceptance for our products.
32
Table of Contents
Many entities are engaged in developing pest management and plant health products. Our competitors include major multinational agrichemical companies such as Arysta, BASF, Bayer, Dow Agrosciences, DuPont, FMC, Monsanto, Sumitomo Chemical and Syngenta, some of which have developed bio-based products for our target markets, as well as specialized bio-based pesticide and plant health businesses such as AgraQuest (now a part of Bayer), Certis USA (now a part of Mitsui), Novozymes (in a joint venture with Monsanto) and Valent Biosciences (now a part of Sumitomo). Many of these organizations have longer operating histories, significantly greater resources, greater brand recognition and a larger base of customers than we do. As a result, they may be able to devote greater resources to the manufacture, promotion or sale of their products, receive greater resources and support from independent distributors, initiate or withstand substantial price competition or more readily take advantage of acquisition or other opportunities. Further, many of the large agrichemical companies have a more diversified product offering than we do, which may give these companies an advantage in meeting customers’ needs by enabling them to offer a broader range of pest management and plant health solutions.
Our product sales are expected to be seasonal and subject to weather conditions and other factors beyond our control, which may cause our operating results to fluctuate significantly quarterly and annually.
Sales of our crop protection products have been, and are generally expected to be, seasonal. Regalia and Grandevo, which accounted for the majority of revenues in recent periods, have historically been sold and applied to crops in greater quantity in the second and fourth quarters, with lowest sales in third quarter when there is the lowest pest and disease pressure in the United States. We expect this trend to continue in the future, but this seasonality could be reduced, or we could experience seasonality in different periods than anticipated, as a result of various factors, including if we expand into new geographical territories or introduce new fermentations or products with different applicable growing seasons, or if a more significant component of our revenue becomes comprised of sales of Zequanox, which has a separate seasonal sales cycle compared to our crop protection products.
Notwithstanding anticipated seasonality, we expect substantial fluctuation in sales year over year and quarter over quarter, and seasonality could be reduced, as a result of a number of variables on which sales of our products are dependent. Weather conditions, natural disasters and other factors affect planting and growing seasons and incidence of pests and plant disease, and accordingly affect decisions by our distributors, direct customers and end users about the types and amounts of pest management and plant health products to purchase and the timing of use of such products. In addition, disruptions that cause delays by growers in harvesting or planting can result in the movement of orders to a future quarter, which would negatively affect the quarter and cause fluctuations in our operating results. For example, late snows and cold temperatures in the Midwestern and Eastern United States in the first and second quarters of 2014 delayed planting and pesticide and other plant health applications. Customers also may purchase large quantities of our products in a particular quarter to store and use over long periods of time or time their purchases to manage their inventories, which may cause significant fluctuations in our operating results for a particular quarter or year. For example, we believe that we experienced higher sales of Regalia in the first quarters of 2012 and 2011 than in the second quarters of those years as a result of distributors ordering in advance of the application season, and low commodity prices may discourage growers from purchasing our products in an effort to reduce their costs and increase their margins for a growing season.
Our expense levels are based in part on our expectations regarding future sales. As a result, any shortfall in sales relative to our expectations could cause significant fluctuations in our operating results from quarter to quarter, which could result in uncertainty surrounding our level of earnings and possibly a decrease in our stock price.
We rely on the experience and expertise of our senior management team and other key personnel, and if we are unable to recruit or retain qualified personnel, our development and commercialization efforts may be significantly delayed.
We depend heavily on the principal members of our management, particularly Pamela G. Marrone, Ph.D., our founder, President and Chief Executive Officer, the loss of whose services might significantly delay or prevent
33
Table of Contents
the achievement of our scientific or business objectives. Although we maintain and are the beneficiary of $10.0 million in key person life insurance policies for the life of Dr. Marrone, we do not believe the proceeds would be adequate to compensate us for her loss.
We have a lean staff, and rely on qualified sales and marketing, research and development and management personnel to succeed. For example, the departures of our former chief operating officer and significant members of our sales staff in the third quarter of 2014 and subsequent turnover in our sales and marketing department adversely impacted our business by disrupting the 2014 launch of Venerate as well as the growth in sales of our other commercialized products, including Regalia and Grandevo. The process of hiring, training and successfully integrating qualified personnel into our operation is a lengthy and expensive one. The market for qualified personnel such as experienced fermentation engineers and formulation chemists is very competitive because of the limited number of people available with the necessary technical skills and understanding of our technology and anticipated products, and few sales and marketing personnel have prior experience with bio-based products. Perceived instability and risk in our business has made it difficult to retain qualified personnel could impair our ability to meet our business objectives and adversely affect our results of operations and financial condition.
If we or our third-party manufactures are unable to produce our products at a satisfactory quality, in a timely manner, in sufficient quantities or at an acceptable cost, our business could be negatively impacted.
We have transitioned the majority of our manufacturing processes in-house to our facility in Bangor, Michigan. If severe weather, a fire or natural disaster occurs, a contaminant grows in our fermentations, or a mechanical or labor problem leads to a reduced capacity or shutdown of our fermenters or other equipment, we may not be successful in producing the amount and quality of product we anticipate in the facility and our results of operations may suffer as a result.
We also continue to rely on third parties to formulate Grandevo and Zequanox into spray-dried powders and for all of our production of Venerate, and from time to time, we expect to use third-party manufacturers for supplemental production capacity to meet excess seasonal demand and for packaging. Our reliance on third parties to manufacture our products presents significant risks to us, including the following:
| • | reduced control over delivery schedules, yields and product reliability; |
| • | price increases; |
| • | manufacturing deviations from internal and regulatory specifications; |
| • | the failure of a key manufacturer to perform its obligations to us for technical, market or other reasons; |
| • | challenges presented by introducing our fermentation processes to new manufacturers or deploying them in new facilities; |
| • | difficulties in establishing additional manufacturers if we are presented with the need to transfer our manufacturing process technologies to them; |
| • | misappropriation of our intellectual property; and |
| • | other risks in potentially meeting our product commercialization schedule or satisfying the requirements of our distributors, direct customers and end users. |
We have not entered into any long-term manufacturing or supply agreements for any of our products, and we may need to enter into additional agreements for the commercial development, manufacturing and sale of our products. There can be no assurance that we can do so on favorable terms, if at all.
Our products have been produced in quantities sufficient to meet commercial demand. However, our dependence upon others for the production of a portion of our products, or for a portion of the manufacturing process,
34
Table of Contents
particularly for drying, may adversely affect our ability to develop and commercialize any products on a timely and competitive basis. If manufacturing capacity is reduced or eliminated at one or more of our third-party manufacturers’ facilities, we could have difficulties fulfilling our customer orders, and our net revenues and results of operations could decline.
We must accurately forecast demand for our products to obtain adequate and cost-effective capacity from our third-party manufacturers and to purchase certain of the raw materials used in our products at cost-effective rates. Our third-party manufacturers are not required to supply us products until we place and they accept our purchase orders, which generally occurs approximately one month prior to the anticipated product delivery date based on our own rolling forecasts. Our purchase orders may not be accepted and our third-party manufacturers may not be willing to provide us with additional products on a timely basis if they prioritize orders placed by other companies, many of whom are more established than us and order larger volumes of products. In addition, while raw material orders are generally placed one month in advance, because certain of the raw materials used in our products are in short supply or are subject to capacity demands, we place some raw material orders approximately six months in advance to avoid paying higher prices. Accordingly, if we inaccurately forecast demand for our products, we may be unable to meet our customers’ delivery requirements, or we may accumulate excess inventories of products and raw materials.
Failure to achieve expected manufacturing yields for our products could negatively impact our operating results.
Low yields may result from product design, development stage or process technology failures. We do not know whether a yield problem exists until our products are manufactured based on our design. When a yield issue is identified, the product is analyzed and tested to determine the cause. As a result, yield deficiencies may not be identified until well into the production process. We may experience inability to ramp up yields in our own manufacturing facility. In the event we continue to rely on third-party manufacturers, resolution of yield problems requires cooperation among, and communication between, us and our manufacturers. We have limited experience producing a number of our products at commercial scale, and we will not succeed if we cannot maintain or decrease our production costs and effectively scale our technology and manufacturing processes.
We rely on a single supplier based in China for a key ingredient of Regalia.
The active ingredient in our Regalia product line is derived from the giant knotweed plant, which we obtain from China. Our single supplier acquires raw knotweed from numerous regional sources and performs an extraction process on this plant, creating a dried extract that is shipped to our third-party manufacturer in the United States. Although we have identified additional sources of knotweed that appear to be reliable and of appropriate quality, there can be no assurance that we will continue to be able to obtain dried extract from China at a competitive price point.
Other ingredients used in the manufacturing of our products are also sourced from a limited number of suppliers. There can be no assurance that we will continue to be able to obtain such ingredients reliably and of appropriate quality at a competitive price point.
Our Zequanox product line requires additional development to become profitable, and our collaborative efforts on this product may not result in increased sales or gross margins.
Our Zequanox product line is principally designed to control invasive mussels that restrict critical water flow in industrial and power facilities and impinge on access to recreational waters. Although Zequanox requires additional development to become profitable, due to prioritization constraints, we have not committed resources to this product sufficient to market it full-scale and substantially improve gross margins. Our ability to generate significant revenues from Zequanox has been dependent on our ability to persuade customers to evaluate the costs of our Zequanox products compared to the overall cost of the chlorine treatment process, the primary
35
Table of Contents
current alternative to using Zequanox, rather than the cost of purchasing chemicals alone. Sales of Zequanox have also remained lower than our other products due to the length of the treatment cycle, the longer sales cycle (the bidding process with utility companies and government agencies occurs on a yearly or multi-year basis) and the unique nature of the treatment approach for each customer based on the extent of the infestation and the design of the facility. We expect our near-term sales of Zequanox will continue to be to governmental agencies and regulated industries, which typically take longer to negotiate and approve contracts than the private sector. Further, we currently expect that our governmental sales may be subject to bidding procedures as well as uncertainties surrounding these agencies’ budget approval processes. We are pursuing partnerships to assist us in further developing Zequanox and expanding it commercially, but we may be unsuccessful in securing and maintaining collaboration efforts with regard to this product, and any such efforts may not result in increased sales or gross margins.
We depend on a limited number of distributors.
Our current revenues are derived from a limited number of key customers, each of which serves as a third-party distributor to our products’ end users. For the six months ended June 30, 2015, our top distributor, Crop Production Services, accounted for 27% of our total revenues. For the year ended December 31, 2014, our top two distributors accounted for 43% of our total revenues, with Crop Production Services and Helena Chemical accounting for 30% and 13% of our total revenues, respectively. For the year ended December 31, 2013, our top distributor, Crop Production Services, accounted for 20% of our total revenues. We expect a limited number of distributors to continue to account for a significant portion of our revenues for the foreseeable future. This customer concentration increases the risk of quarterly fluctuations in our revenues and operating results. The loss or reduction of business from one or a combination of our significant distributors could materially adversely affect our revenues, financial condition and results of operations.
Any decline in U.S. agricultural production could have a material adverse effect on the market for pesticides and on our results of operations and financial position.
Conditions in the U.S. agricultural industry significantly impact our operating results. The U.S. agricultural industry has contracted in recent periods, and can be affected by a number of factors, including weather patterns and field conditions, current and projected grain inventories and prices, domestic and international demand for U.S. agricultural products and U.S. and foreign policies regarding trade in agricultural products. State and federal governmental policies, including farm subsidies and commodity support programs, as well as the prices of fertilizer products and the prices at which produce may be sold, may also directly or indirectly influence the number of acres planted, the mix of crops planted and the use of pesticides for particular agricultural applications. There are various proposals pending before the U.S. Congress to cut or eliminate various agricultural subsidies. If such proposals are implemented, they may adversely impact the U.S. agricultural industry and suppliers to that industry such as us.
Our intellectual property is integral to our business. If we are unable to protect our patents and proprietary rights in the United States and foreign countries, our business could be adversely affected.
Our success depends in part on our ability to obtain and maintain patent and other proprietary rights protection for our technologies and products in the United States and other countries. If we are unable to obtain or maintain these protections, we may not be able to prevent third parties from using our proprietary rights. It is also possible that we will fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection. As of September 30, 2015, we had 23 issued U.S. patents and 64 issued foreign patents (of which five U.S. patents and 25 foreign patents were in-licensed), 31 pending provisional and non-provisional U.S. patent applications (of which one was in-licensed), and 189 pending foreign patent applications (of which six were in-licensed).
The patent position of biotechnology and biochemical companies generally is highly uncertain, involves complex legal and factual questions and has in recent years been the subject of much litigation. As a result, the issuance,
36
Table of Contents
scope, validity, enforceability and commercial value of our patent rights are highly uncertain. Our pending and future patent applications may not result in patents being issued which protect our technology or products, in whole or in part, or which effectively prevent others from commercializing competitive technologies and products. Changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of our patents or narrow the scope of our patent protection. For example, the U.S. Supreme Court has ruled on several patent cases in recent years, either narrowing the scope of patent protection available in certain circumstances or weakening the rights of patent owners in certain situations. In addition, recent changes to the patent laws of the United States provide additional procedures for third parties to challenge the validity of issued patents, some of which allow a lower evidentiary standard to hold a patent claim invalid. The laws of some foreign countries do not protect proprietary rights to the same extent as the laws of the United States, and we may encounter significant problems and costs in protecting our proprietary rights in these foreign countries.
Our patents and those patents for which we have license rights may be challenged, narrowed, invalidated or circumvented. In addition, our issued patents may not contain claims sufficiently broad to protect us against third parties with similar technologies or products or provide us with any competitive advantage. We are not certain that our pending patent applications will be issued. Moreover, our competitors could challenge or circumvent our patents or pending patent applications. It is also not possible to patent and protect all knowledge and know-how associated with our products so there may be areas that are not protected such as certain formulations and manufacturing processes. Costly and time-consuming litigation could be necessary to enforce and determine the scope of our proprietary rights, and failure to obtain or maintain trade secret protection could adversely affect our competitive business position.
For certain of our products, we hold co-exclusive licenses to certain of the intellectual property related to these products. Although our products that are derived from intellectual property licensed to us on a co-exclusive basis also include our own proprietary technology, the third parties with whom we share co-exclusive rights may develop products based on the same underlying intellectual property. This could adversely affect the sale of our products.
Intellectual property litigation could cause us to spend substantial resources and could distract our personnel from their normal responsibilities.
Even if resolved in our favor, litigation or other legal proceedings relating to intellectual property claims may cause us to incur significant expenses, and could distract our technical and management personnel from their normal responsibilities. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock. Such litigation or proceedings could substantially increase our operating losses and reduce the resources available for development, sales, marketing or distribution activities. We may not have sufficient financial or other resources to adequately conduct such litigation or proceedings. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater financial resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position could be harmed.
We have taken measures to protect our trade secrets and know-how, including the use of confidentiality agreements with our employees, consultants, advisors and third-party manufacturers. It is possible that these agreements may be breached and that any remedies for a breach will not make us whole. In addition, some courts inside and outside of the United States are less willing or unwilling to protect trade secrets. We generally control and limit access to, and the distribution of, our product documentation and other proprietary information. Despite
37
Table of Contents
our efforts to protect these proprietary rights, our trade secret-protected know-how could fall into the public domain, unauthorized parties may copy aspects of our products and obtain and use information that we regard as proprietary. We also cannot guarantee that other parties will not independently develop our knowhow or otherwise obtain access to our technologies.
Third parties may misappropriate our microbial strains.
Third parties, including contract manufacturers, often have custody or control of our microbial strains. If our microbial strains were stolen, misappropriated or reverse engineered, they could be used by other parties who may be able to reproduce the microbial strains for their own commercial gain. If this were to occur, it would be difficult for us to challenge and prevent this type of use, especially in countries with limited intellectual property protection.
Other companies may claim that we infringe their intellectual property or proprietary rights, which could cause us to incur significant expenses or prevent us from selling our products.
Our success depends in part on our ability to operate without infringing the patents and proprietary rights of third parties. Product development is inherently uncertain in a rapidly evolving technological environment such as ours in which there may be numerous patent applications pending, many of which are confidential when filed, with regard to similar technologies. Patents issued to third parties may contain claims that conflict with our patents and that may place restrictions on the commercial viability of our products and technologies. Third parties could assert infringement claims against us in the future. We may become party to, or threatened with, future adversarial proceedings or litigation regarding intellectual property rights with respect to our products, product candidates and technology. We may not be aware of all such third-party intellectual property rights potentially relevant to our products and product candidates.
Any litigation, adversarial proceeding or proceeding before governmental authorities regarding intellectual property rights, regardless of its outcome, would probably be costly and require significant time and attention of our key management and technical personnel. Litigation, adversarial proceedings or proceedings before governmental authorities could also force us to:
| • | stop or delay using our proprietary screening technology; |
| • | stop or delay selling, manufacturing or using products that incorporate the challenged intellectual property; |
| • | pay damages; and/or |
| • | enter into licensing or royalty agreements which, if available at all, may only be available on unfavorable terms. |
Claims that we have misappropriated the confidential information or trade secrets of third parties could have a similar negative impact on our business.
We use hazardous materials in our business and are subject to potential liability under environmental laws. Any claims relating to improper handling, storage or disposal of hazardous materials could be time consuming and costly to resolve.
We are subject to federal, state and local laws and regulations governing the use, manufacture, storage, handling, disposal and release of hazardous materials and certain waste products. Our research and development and manufacturing activities involve the controlled use of hazardous materials and biological waste. Some of these materials may be novel, including bacteria with novel properties and bacteria that produce biologically active compounds. We cannot eliminate the risk of accidental contamination or discharge and any injury resulting from these materials. In addition, although we have not currently identified any environmental liabilities, the
38
Table of Contents
manufacturing facility we purchased in July 2012 may have existing environmental liabilities associated with it that may also result in successor liabilities for us, and we will be subject to increased exposure to potential environmental liabilities as we manufacture our products on a larger scale. We may also be held liable for hazardous materials brought onto the premises of our manufacturing facility before we acquired title, without regard for fault for, or knowledge of, the presence of such substances, as well as for hazardous materials that may be discovered after we no longer own the property if we sell it in the future. In the event of an accident, or if any hazardous materials are found within our operations or on the premises of our manufacturing facility in violation of the law at any time, we may be liable for all cleanup costs, fines, penalties and other costs. This liability could exceed our resources, and, if significant losses arise from hazardous substance contamination, our financial viability may be substantially and adversely affected.
In addition, we may have to incur significant costs to comply with future environmental laws and regulations. In addition, we cannot predict the impact of new governmental regulations that might have an adverse effect on the research, development, production and marketing of our products. We may be required to incur significant costs to comply with current or future laws or regulations. Our business may be harmed by the cost of compliance.
Our collaborators may use hazardous materials in connection with our collaborative efforts. To our knowledge, their work is performed in accordance with applicable biosafety regulations. In the event of a lawsuit or investigation, however, we could be held responsible for any injury caused to persons or property by exposure to, or release of, hazardous materials used by these parties. Further, we may be required to indemnify our collaborators against all damages and other liabilities arising out of our development activities or products produced in connection with these collaborations.
Our headquarters and facility and certain manufacturers and suppliers are located in regions that are subject to natural disasters, as well as in some cases geopolitical risks and social upheaval.
Our Davis, California headquarters is located near a known earthquake fault. The impact of a major earthquake, fire or other natural disaster, including floods, on our Davis facilities, Bangor, Michigan manufacturing plant, infrastructure and overall operations is difficult to predict and any natural disaster could seriously disrupt our entire business process. In addition, Regalia is produced by a third-party manufacturer in Florida in a location that could be impacted by hurricane activity, and certain of our raw materials are sourced in China, which is subject to risks associated with uncertain political, economic and other conditions such as the outbreak of contagious diseases, such as avian flu, swine flu and SARS, and natural disasters. The insurance we maintain may not be adequate to cover our losses resulting from natural disasters or other business interruptions. Although these risks have not materially adversely affected our business, financial condition or results of operations to date, there can be no assurance that such risks will not do so in the future.
Inability to comply with regulations applicable to our facilities and procedures could delay, limit or prevent our research and development or manufacturing activities.
Our research and development and manufacturing facilities and procedures are subject to continual review and periodic inspection. We must spend funds, time and effort in the areas of production, safety and quality control and assurance to ensure full technical compliance with the regulations applicable to these facilities and procedures. If the EPA or another regulatory body determines that we are not in compliance with these regulations, regulatory approval of our products could be delayed or we may be required to limit or cease our research and development or manufacturing activities or pay a monetary fine. If we are required to limit or cease our research and development activities, our ability to develop new products would be impaired. In addition, if we are required to limit or cease our manufacturing activities, our ability to produce our products in commercial quantities would be impaired or prohibited, which would harm our business.
We may be exposed to product liability and remediation claims, which could harm our business.
The use of certain bio-based pest management and plant health products is regulated by various local, state, federal and foreign environmental and public health agencies. These regulations may include requirements that
39
Table of Contents
only certified or professional users apply the product or that certain products be used only on certain types of locations, may require users to post notices on properties to which products have been or will be applied, may require notification to individuals in the vicinity that products will be applied in the future or may ban the use of certain ingredients. Even if we are able to comply with all such regulations and obtain all necessary registrations, we cannot provide assurance that our products will not cause injury to crops, the environment or people under all circumstances. For example, our products may be improperly combined with other pesticides or, even when properly combined, our products may be blamed for damage caused by these other pesticides. The costs of remediation or products liability could materially adversely affect our future quarterly or annual operating results.
We may be held liable for, or incur costs to settle, liability and remediation claims if any products we develop, or any products that use or incorporate any of our technologies, cause injury or are found unsuitable during product testing, manufacturing, marketing, sale or use. These risks exist even with respect to products that have received, or may in the future receive, regulatory approval, registration or clearance for commercial use. We cannot guarantee that we will be able to avoid product liability exposure.
We currently maintain product liability insurance at levels we believe are sufficient and consistent with industry standards for companies at our stage of development. We cannot guarantee that our product liability insurance is adequate and, at any time, it is possible that this insurance coverage may not be available on commercially reasonable terms or at all. A product liability claim could result in liability to us greater than our assets or insurance coverage. Moreover, even if we have adequate insurance coverage, product liability claims or recalls could result in negative publicity or force us to devote significant time and attention to those matters, which could harm our business.
Our ability to use our net operating loss carry-forwards to offset future taxable income may be subject to certain limitations.
As of December 31, 2014, we had approximately $128.2 million of federal and $117.9 million of state operating loss carry-forwards available to offset future taxable income, which expire in varying amounts beginning in 2026 for federal and 2016 for state purposes if unused. It is possible that we will not generate taxable income in time to use these loss carry-forwards before their expiration.
Section 382 of the Internal Revenue Code imposes restrictions on the use of a corporation’s net operating losses, as well as certain recognized built-in losses and other carryforwards, after an “ownership change” occurs. A Section 382 “ownership change” occurs if one or more stockholders or groups of stockholders who own at least 5% of our stock increase their ownership by more than 50 percentage points over their lowest ownership percentage within a rolling three-year period. Future issuances or sales of our stock (including certain transactions involving our stock that are outside of our control) could also result in an ownership change under Section 382. If an “ownership change” occurs, Section 382 would impose an annual limit on the amount of pre-change net operating losses and other losses we can use to reduce our taxable income generally equal to the product of the total value of our outstanding equity immediately prior to the “ownership change” (subject to certain adjustments) and the applicable federal long-term tax-exempt interest rate for the month of the “ownership change.” The applicable rate for ownership changes occurring in the month of December 2014 was 2.80%.
Because U.S. federal net operating losses generally may be carried forward for up to 20 years, the annual limitation may effectively provide a cap on the cumulative amount of pre-ownership change losses, including certain recognized built-in losses that may be utilized. Such pre-ownership change losses in excess of the cap may be lost. In addition, if an ownership change were to occur, it is possible that the limitations imposed on our ability to use pre-ownership change losses and certain recognized built-in losses could cause a net increase in our U.S. federal income tax liability and U.S. federal income taxes to be paid earlier than otherwise would be paid if such limitations were not in effect. Further, if the amount or value of these deferred tax assets is reduced, such reduction would have a negative impact on the book value of our common stock.
40
Table of Contents
We completed a Section 382 analysis as of December 31, 2013 and concluded that approximately $0.5 million in federal net operating losses and approximately $0.2 million in federal research and development credits are expected to expire prior to utilization as a result of our previous ownership changes and corresponding annual limitations. We have not conducted an analysis to determine the amount of state net operating losses that are also expected to expire prior to utilization. Our existing net operating loss carry-forwards or credits may be subject to significant limitations due to events occurring since December 31, 2013, and we have not updated our Section 382 analysis to consider events since December 31, 2013, including the effect of issuing common stock pursuant to a public offering in June 2014. Our inability to use these net operating loss carry-forwards as a result of the Section 382 limitations could harm our financial condition.
Our business is subject to various governmental regulations, and compliance with these regulations may cause us to incur significant expenses. If we fail to maintain compliance with applicable regulations, we may be forced to recall products and cease their manufacture and distribution, which could subject us to civil or criminal penalties.
The complex legal and regulatory environment exposes us to compliance and litigation costs and risks that could materially affect our operations and financial results. These laws and regulations may change, sometimes significantly, as a result of political or economic events. They include environmental laws and regulations, tax laws and regulations, import and export laws and regulations, government contracting laws and regulations, labor and employment laws and regulations, securities and exchange laws and regulations, and other laws such as the Foreign Corrupt Practices Act. In addition, proposed laws and regulations in these and other areas could affect the cost of our business operations. We face the risk of changes in both domestic and foreign laws regarding trade, potential loss of proprietary information due to piracy, misappropriation or foreign laws that may be less protective of our intellectual property rights. Violations of any of these laws and regulations could subject us to criminal or civil enforcement actions, any of which could have a material adverse effect on our business, financial condition or results of operations.
Risks Related to Ownership of our Common Stock
Our principal stockholders will have significant voting power and may take actions that may not be in the best interest of other stockholders.
As of September 30, 2015, our executive officers and directors and their affiliates beneficially owned or controlled, directly or indirectly, an aggregate of approximately 2.8 million shares, or 11.2% of our common stock. In addition, affiliates of Waddell & Reed Financial Inc., which beneficially own 19.99% of our common stock, hold $40 million of principal in senior secured promissory notes issued in August 2015. If all of these security holders act together, they will be able to exert significant control over our management and affairs, which could result in some corporate actions that our other stockholders do not view as beneficial such as failure to approve change of control transactions that could offer holders of our common stock a premium over the market value of our company. As a result, the market price of our common stock could be adversely affected.
Our common stock may experience extreme price and volume fluctuations, and you may not be able to resell shares of our common stock at or above the price you paid.
We are an early stage company with a limited trading history and a history of losses. Since shares of our common stock were sold in our initial public offering in August 2013 at a price of $12.00 per share, our stock price has ranged between $1.74 and $20.00 through September 30, 2015. The trading price of our common stock will likely continue to be highly volatile and could be subject to wide fluctuations in price in response to various factors, some of which are beyond our control. These factors include:
| • | the announcement of the Audit Committee investigation, the Restatement and the SEC investigation and lawsuits arising out of related matters; |
41
Table of Contents
| • | our small public float relative to the total number of shares of common stock that are issued and outstanding; |
| • | quarterly variations in our results of operations, those of our competitors or those of our customers; |
| • | announcements of technological innovations, new products or services or new commercial relationships by us or our competitors; |
| • | our ability to develop and market new products on a timely basis; |
| • | disruption to our operations; |
| • | media reports and publications about pest management products; |
| • | announcements concerning our competitors or the pest management industry in general; |
| • | our entry into, modification of or termination of key license, research and development or collaborative agreements; |
| • | new regulatory pronouncements and changes in regulatory guidelines or the status of our regulatory approvals; |
| • | general and industry-specific economic conditions; |
| • | any major change in our board of directors or management; |
| • | commencement of, or our involvement in, litigation; |
| • | changes in financial estimates, including our ability to meet our future net revenues and operating profit or loss projections; and |
| • | changes in earnings estimates or recommendations by securities analysts. |
Substantial future sales of our common stock, or the perception in the public markets that these sales may occur, may depress our stock price.
Sales of substantial amounts of our common stock in the public market, or the perception that such sales could occur, could adversely affect the market price of our common stock. As of September 30, 2015, we had approximately 24.5 million shares of common stock outstanding, 2.2 million which were are held by our directors and officers and their affiliates and an additional 11.9 million shares which were held by other beneficial holders of 5% or more of our common stock. Although these shares are subject in some cases to volume and manner of sale restriction of Rule 144 of the Securities Act, any determination by holders of a substantial number of such shares to sell our stock, or the perception that such sales may occur, could cause our share price to fall. In addition, as of September 30, 2015 we had 4.8 million shares of our common stock either issued or available for issuance under our equity incentive plans. These shares may be sold in the public market upon issuance and once vested.
Because we have no plans to pay dividends on our common stock, investors must look solely to stock appreciation for a return on their investment in us.
We have never declared or paid any cash dividends on our capital stock, and we do not anticipate paying any cash dividends on our common stock in the foreseeable future. We currently intend to retain all future earnings to fund the development and growth of our business. Any payment of future dividends will be at the discretion of our board of directors and will depend on, among other things, our earnings, financial condition, capital requirements, level of indebtedness, statutory and contractual restrictions applying to the payment of dividends and other considerations that the board of directors deems relevant. Investors must rely on sales of their common stock after price appreciation, which may never occur, as the only way to realize a return on their investment. Investors seeking cash dividends should not purchase our common stock.
42
Table of Contents
We are an “emerging growth company” and we cannot be certain if the reduced disclosure requirements applicable to emerging growth companies will make our common stock less attractive to investors.
We are an “emerging growth company” as defined in the JOBS Act. For as long as we continue to be an emerging growth company we may choose to take advantage of certain exemptions from various reporting requirements applicable to other public companies but not to emerging public companies, which includes, among other things:
| • | exemption from the auditor attestation requirements under Section 404 of the Sarbanes-Oxley Act of 2002; |
| • | reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements; |
| • | exemption from the requirements of holding non-binding stockholder votes on executive compensation arrangements; and |
| • | exemption from any rules requiring mandatory audit firm rotation and auditor discussion and analysis and, unless the SEC otherwise determines, any future audit rules that may be adopted by the Public Company Accounting Oversight Board. |
We could be an emerging growth company until the last day of the fiscal year following the fifth anniversary after our initial public offering, or until the earliest of (i) the last day of the fiscal year in which we have annual gross revenues of $1 billion or more; (ii) the date on which we have, during the previous three year period, issued more than $1 billion in non-convertible debt or (iii) the date on which we are deemed to be a large accelerated filer under the federal securities laws. We will qualify as a large accelerated filer as of the first day of the first fiscal year after we have (i) more than $700 million in outstanding common equity held by our non-affiliates and (ii) been public for at least 12 months. The value of our outstanding common equity will be measured each year on the last day of our second fiscal quarter.
Under the JOBS Act, emerging growth companies are also permitted to elect to delay adoption of new or revised accounting standards until companies that are not subject to periodic reporting obligations are required to comply, if such accounting standards apply with non-reporting companies. We have made an irrevocable decision to opt out of this extended transition period for complying with new or revised accounting standards.
We cannot predict if investors will find our common stock less attractive if we rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile.
We incur significant costs as a result of operating as a public company, and our management is required to devote substantial time to comply with the laws and regulations affecting public companies, which costs will increase after we are no longer an “emerging growth company.”
As a public company, we will incur significant legal, accounting and other expenses, including costs associated with public company reporting and corporate governance requirements, in order to comply with the rules and regulations imposed by the Sarbanes-Oxley Act, as well as rules implemented by the SEC and NASDAQ. Such costs will increase after we cease to qualify as an emerging growth company. Our management and other personnel have needed to devote a substantial amount of time to these compliance initiatives and our legal and accounting compliance costs have increased. We also may need to hire additional staff or consultants in the areas of investor relations, legal and accounting to continue to operate as a public company. The expenses incurred by public companies for reporting and corporate governance purposes have increased dramatically over the past several years. We expect these rules and regulations to continue to increase our legal and financial compliance costs substantially and to make some activities more time consuming and costly. We are currently unable to
43
Table of Contents
estimate these costs with any degree of certainty. Greater expenditures may be necessary in the future with the advent of new laws and regulations pertaining to public companies. We also expect that, as a public company, it will continue to be expensive for us to obtain director and officer liability insurance.
The Sarbanes-Oxley Act requires, among other things, that we maintain effective internal control over financial reporting and disclosure controls and procedures. In particular, as a public company, we are required to perform system and process evaluations and testing of our internal control over financial reporting to allow management to report on the effectiveness of our internal control over financial reporting, as required by Section 404 of the Sarbanes-Oxley Act. As described above, as an emerging growth company, we are not yet required to comply with the auditor attestation provisions of Section 404. However, we are required to comply with management attestations of Section 404, and our testing, or the subsequent testing by our independent registered public accounting firm, may reveal deficiencies in our internal control over financial reporting that are deemed to be material weaknesses.We expect to incur substantial accounting expense and management time on compliance-related issues with respect to Section 404. Moreover, if we are not able to comply with the requirements of Section 404 in a timely manner, or if we or our independent registered public accounting firm identify deficiencies in our internal control over financial reporting that are deemed to be material weaknesses, we could lose investor confidence in the accuracy and completeness of our financial reports, which could cause our stock price to decline.
For example, as more fully described in the Explanatory Note to this Annual Report on Form 10-K, in September 2014, our Audit Committee initiated an independent investigation regarding certain accounting matters concerning recognition of revenue in a sales transaction. As a result of the matters relating to the Audit Committee’s investigation, we announced that certain of our previously filed financial statements could no longer be relied upon and subsequently completed the Restatement. While we believe we have appropriately determined the errors made in our previously reported consolidated financial statements, recorded the correct adjustments in preparing our restated consolidated financial statements and remediated the material weaknesses identified in our internal control over financial reporting related to the subject matter of the Audit Committee’s investigation, we can provide no assurances that other material weaknesses in our internal control over financial reporting, such as the material weakness we have identified with respect to stock option grants, will not be identified in the future. See also “—Risks Related to our Financial Reporting Process.”
Provisions in our charter documents and under Delaware law could discourage a takeover that stockholders may consider favorable.
Provisions in our amended and restated certificate of incorporation and bylaws may have the effect of delaying or preventing a change of control or changes in our management. These provisions include the following:
| • | the right of our board of directors to elect directors to fill a vacancy created by the expansion of our board of directors or the resignation, death or removal of a director, which prevents stockholders from being able to fill vacancies on our board of directors; |
| • | the establishment of a classified board of directors requiring that only a subset of the members of our board of directors be elected at each annual meeting of stockholders; |
| • | the prohibition of cumulative voting in our election of directors, which would otherwise allow less than a majority of stockholders to elect director candidates; |
| • | the requirement that stockholders provide advance notice to nominate individuals for election to our board of directors or to propose matters that can be acted upon at a stockholders’ meeting. These provisions may discourage or deter a potential acquirer from conducting a solicitation of proxies to elect the acquirer’s own slate of directors or otherwise attempting to obtain control of our company; |
| • | the ability of our board of directors to issue, without stockholder approval, shares of undesignated preferred stock with terms set by the board of directors, which rights could be senior to those of |
44
Table of Contents
| our common stock. The ability to authorize undesignated preferred stock makes it possible for our board of directors to issue preferred stock with voting or other rights or preferences that could impede the success of any attempt to acquire us; |
| • | the ability of our board of directors to alter our bylaws without obtaining stockholder approval; |
| • | the inability of our stockholders to call a special meeting of stockholders and to take action by written consent in lieu of a meeting; |
| • | the required approval of the holders of at least two-thirds of the shares entitled to vote at an election of directors to adopt, amend, or repeal our bylaws; |
| • | the required approval of the holders of at least two-thirds of the shares entitled to vote at an election of directors to repeal or adopt any provision of our certificate of incorporation regarding the election of directors; |
| • | the required approval of the holders of at least 80% of such shares to amend or repeal the provisions of our bylaws regarding the election and classification of directors; and |
| • | the required approval of the holders of at least two-thirds of the shares entitled to vote at an election of directors to remove directors without cause. |
As a Delaware corporation, we are also subject to certain Delaware anti-takeover provisions. Under Delaware law, a corporation may not engage in a business combination with any holder of 15% or more of its capital stock unless the holder has held the stock for three years or, among other things, the board of directors has approved the transaction. Our board of directors could rely on Delaware law to prevent or delay an acquisition of us.
Risks Related to Our Financial Reporting Processes
The restatement of our previously issued financial statements has been time consuming and expensive, and matters relating to or arising from the restatement and material weaknesses in our internal control over financial reporting, including adverse publicity and potential concerns from our customers and prospective customers, regulatory inquiries, and litigation matters, could have a material adverse effect on our business, financial condition or stock price.
As more fully described in the Explanatory Note to this Annual Report on Form 10-K, in September 2014, our Audit Committee initiated an independent investigation regarding certain accounting matters concerning recognition of revenue in a sales transaction. Our Audit Committee conducted its investigation and review with the assistance of independent counsel and an independent forensic accounting advisor.
In February 2015, we announced the completion of the investigation. In light of the Audit Committee’s findings, we have evaluated our historical distributor sales transactions to determine whether previously undisclosed commitments were made that have an impact on the timing and treatment of revenue recognition, and whether our internal controls relating to revenue recognition are sufficient. On November 10, 2015 we filed this Annual Report on Form 10-K for the year ended December 31, 2014, which we refer to as the Form 10-K and which includes restated consolidated financial statements, selected financial data (as applicable), certain financial data in management’s discussion and analysis and other information for the fiscal year ended December 31, 2013, including related quarterly periods, and the quarterly periods ended March 31 and June 30, 2014. The circumstances and findings of our Audit Committee’s investigation are more fully described in the Explanatory Note on page i of this report.
We also initiated contact with the staff of the Division of Enforcement of the SEC in September 2014 to advise them of the initiation of the Audit Committee’s investigation. The Division of Enforcement has since initiated a formal investigation of these matters, with which we have fully cooperated. The investigation by the Division of Enforcement is ongoing.
45
Table of Contents
As a result of the matters relating to the Audit Committee’s investigation, we announced that certain of our previously filed financial statements could no longer be relied upon, and we did not file our Quarterly Report on Form 10-Q for the quarters ended September 30, 2014, March 31, 2015, or June 30, 2015 or our Annual Report on Form 10-K for the year ended December 31, 2014 on a timely basis. The non-compliance with public reporting obligations subjected us to delisting proceedings from the NASDAQ Global Market. Subsequently we presented our plan to regain compliance with NASDAQ’s filing requirement at a hearing, and the reviewing panel granted a stay of delisting through November 9, 2015 to regain compliance.
As also further described in Part I-Item 3-“Legal Proceedings”, the Company, our current and former executive officers and members of our board of directors have been named as defendants in various lawsuits asserting claims arising out of the subject matter of the Audit Committee’s investigation.
We have incurred over $11 million in, and where applicable expect to incur additional, significant legal, accounting and other professional fees and other costs in connection with the Audit Committee’s investigation, the preparation of restated consolidated financial statements, our remediation efforts, responding to the SEC investigation, petitioning NASDAQ to stay delisting of our common stock and related matters. While we believe we have made appropriate judgments in determining the errors made in our previously reported consolidated financial statements and recording the correct adjustments in preparing our restated consolidated financial statements, there is a risk that we may have to further restate our historical consolidated financial statements, amend prior filings with the SEC or take other actions not currently contemplated, including as a result of SEC review of our filings. Further, if the SEC were to conclude that enforcement action is appropriate, we could be required to pay large civil penalties and fines. The SEC also could impose other sanctions against us or certain of our current and former directors and officers. Any of these events could have a material adverse effect on our business, results of operations, cash flows or financial condition and cause the price of our securities to decline.
In addition to the pending legal proceedings and SEC investigation, the matters described above have also exposed us to greater risks associated with other civil litigation, regulatory proceedings and government enforcement actions to which we and current and former members of our senior management may in the future be subject. If we do not prevail in the pending legal proceedings or any other litigation, we may be required to pay a significant amount of monetary damages that may be in excess of our insurance coverage, or may have additional penalties or other remedies imposed against us, or our current or former directors or officers, which could harm our reputation, business, results of operations, cash flows or financial condition. In addition, under Delaware law, our bylaws and certain indemnification agreements, we may have an obligation to indemnify certain current and former officers and directors in relation to these matters. Such indemnification may have a material adverse effect on our business, results of operations, or financial condition to the extent insurance does not cover our costs. In addition, we may not have sufficient coverage under directors’ and officers’ insurance policies, in which case our business, results of operations or financial condition may be materially and adversely affected.
Our board of directors, management and other key employees have expended, and may continue to expend, a substantial amount of time on matters relating to the Audit Committee investigation, the Restatement, the SEC investigation, the NASDAQ hearing process and the pending private litigation, diverting resources and attention that would otherwise be directed toward our operations and implementation of our business strategy, all of which could materially adversely affect our business, results of operations, cash flows or financial condition.
Further, we have been and may continue to be the subject of negative publicity focusing on the Restatement and related matters, and may be adversely impacted by negative reactions from our stockholders, creditors or others with which we do business. We believe this negative publicity has impacted our ability to attract and retain customers, employees and vendors, and may continue to do so in the future. Concerns include the perception of the work effort required to address our accounting and control environment, the ability for us to be a long term provider to customers and our ability to timely pay outstanding balances to vendors. Moreover, we believe that our competitors have sought and will continue to seek to leverage the Restatement and related matters to try and raise concerns about us in the minds of our customers and customer prospects. The continued occurrence of any of the foregoing could harm our business and reputation and cause the price of our securities to decline.
46
Table of Contents
If we fail to establish and maintain proper and effective internal controls, our ability to produce accurate financial statements on a timely basis could be impaired, which would adversely affect our consolidated operating results, our ability to operate our business and investors’ views of us.
Our management is responsible for establishing and maintaining adequate internal control over financial reporting to ensure that information regarding the reliability of our financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles in the United States. As discussed under Part II-Item 9A-“Controls and Procedures,” in this Annual Report on Form 10-K, based on the Audit Committee’s investigation management identified material weaknesses in internal control over financial reporting as of December 31, 2014 resulting in revenue transactions that were recognized prior to satisfaction of all required revenue recognition criteria. These include material weaknesses related to our process for recognizing revenue relating to certain former sales personnel who did not project an attitude of integrity and control consciousness, leading to insufficient attention to their responsibilities and internal controls, without management having implemented mitigating controls to discourage, prevent or detect override of internal control by those personnel. In addition, it was determined that our internal controls were not effectively designed to identify instances when sales personnel made unauthorized commitments with certain distributors. While we have implemented a plan to remediate these material weaknesses, which focuses on continued training for and communication with employees regarding our enhanced policies and procedures, we are still in the process of testing and evaluating the effectiveness of the remediation measures we have taken to date.
Also in connection with management’s assessment of our internal control over financial reporting, management has identified an additional deficiency that constituted a material weakness in our internal control over financial reporting as of December 31, 2014 in our governance practices related to ineffective controls over the timeliness and accuracy of documentation related to actions of our board of directors and compensation committee specific to approving stock option grants. While no financial statement accounts or disclosures were misstated, the potential impact could have led to a material misstatement. We are developing a plan to conduct training with our legal department and those charged with governance to ensure that board and compensation committee minutes are prepared more timely and accurately and reviewed with sufficient rigor to ensure the minutes fully and accurately reflect the actions/approvals related to stock option grants.
In connection with the foregoing, our Chief Executive Officer and Chief Financial Officer determined that our internal control over financial reporting was not effective as of December 31, 2014.
Remedying our material weaknesses has required substantial management time and attention, and ensuring that we have adequate internal control over financial reporting and procedures in place to produce accurate financial statements on a timely basis will continue to be a costly and time consuming effort. Any failure to implement effective internal control over financial reporting or to complete and maintain the remediation of our identified control deficiencies may result in additional errors, material misstatements or delays in our financial reporting, failure to meet our financial reporting obligations or failure to avoid or detect fraud in our financial reporting. This in turn would have a material adverse effect on our business and results of operations and could have a substantial adverse impact on the trading price of our common stock and our relationships with customers and suppliers.
Our management does not expect that our internal control over financial reporting will prevent or detect all errors and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud, if any, within the Company will have been detected. As discussed in this Annual Report on Form 10-K, our Audit Committee and management have identified control deficiencies in the past and may identify additional deficiencies in the future.
47
Table of Contents
The processes underlying the preparation of the financial statements contained in this report may not have been adequate and our financial statements remain subject to the risk of future restatement.
The completion of our audit for the year ended December 31, 2014, the Restatement and the revenue recognition review undertaken in connection therewith involved many months of review and analysis, including highly technical analyses of our contracts and business practices, the proper application of paragraph ASC 605-10-S99-1 of the FASB Accounting Standards Codification for revenue recognition, and other accounting rules and pronouncements. The completion of our financial statement audit also followed the completion of an extremely detailed forensic audit as part of the Audit Committee’s investigation. Given the complexity and scope of these exercises, and notwithstanding the very extensive time, effort, and expense that went into them, we cannot assure you that these processes were adequate or that additional accounting errors will not come to light in the future in these or other areas. If additional accounting errors come to light in areas reviewed as part of our processes or otherwise, including as a result of SEC review of our filings, or if ongoing interpretations of applicable accounting rules and pronouncements result in unanticipated changes in our accounting practices or financial reporting, future restatements of our financial statements may be required.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
Our headquarters are located at 1540 Drew Avenue in Davis, California, in a facility consisting of approximately 27,300 square feet of office, laboratory space and greenhouse space under a lease entered into in September 2013. This facility accommodates our research, development, sales, marketing, operations, finance and administrative activities. The facilities include a new, state-of-the-art fermentation lab and pilot plant, expanded formulation lab and pilot with spray drying and granulation capabilities, an insectary, a plant pathology and nematology lab and a plant and weed sciences lab, among others. The initial term of the lease is for a period of 60 months. The lease term commenced on August 20, 2014.
We also purchased an 11,400 square-foot manufacturing facility in Bangor, Michigan, in July 2012, which we have repurposed to accommodate large-scale manufacturing of our products. We believe that our leased facilities and our manufacturing facility are adequate to meet our needs.
On September 5, 2014, September 8, 2014, September 11, 2014, September 15, 2014 and November 3, 2014, we, along with certain of our current and former officers and directors, and others were named as defendants in putative securities class action lawsuits filed in the U.S. District Court for the Eastern District of California. On February 13, 2015, these actions were consolidated as Special Situations Fund III QP, L.P. et al v. Marrone Bio Innovations, Inc. et al, Case No 2:14-cv-02571-MCE-KJN. On September 2, 2015, an initial consolidated complaint was filed on behalf of (i) all persons who purchased or otherwise acquired our publicly traded common stock directly in or traceable to our August 1, 2013 initial public offering; (ii) all persons who purchased or otherwise acquired our common stock directly in our June 6, 2014 secondary offering; and (iii) all persons who purchased or otherwise acquired our common stock on the open market between March 7, 2014 and September 2, 2014 (the “Class Action”). In addition to the Company, the initial consolidated complaint names certain of our current and former officers and directors and our independent registered public accounting firm as defendants. The initial consolidated complaint alleges violations of the Securities Act of 1933, the Securities Exchange Act of 1934, and SEC Rule 10b-5 arising out of the issuance of allegedly false and misleading statements about our business and prospects, including our financial statements, product revenues and system of internal controls. Plaintiffs contend that such statements caused our stock price to be artificially inflated. The action includes claims for damages, fees, and expenses, including an award of attorneys’ and experts’ fees to the putative class.
48
Table of Contents
Pursuant to a stipulation between the parties, and by order of the Court, Defendants need not respond to the initial consolidated complaint. An amended consolidated complaint is to be filed no later than 60 days after the Company announces the restatement(s) after which defendants will have 60 days to respond. The outcome of this matter is not presently determinable.
On September 9, 2014 and November 25, 2014, shareholder derivative actions were filed in the Superior Court of California, County of Yolo (Case No. CV14-1481) and the U.S. District Court for the Eastern District of California (Case No. 1:14-cv-02779-JAM-CKD), purportedly on our behalf, against certain current and former officers and members of our board of directors (the “2014 Derivative Actions”). The plaintiffs in the 2014 Derivative Actions allege that the defendants breached their fiduciary duties, committed waste, were unjustly enriched, and aided and abetted breaches of fiduciary duty by causing us to issue allegedly false and misleading statements. The issues in the 2014 Derivative Actions overlap substantially with those at issue in the Class Action described above. The plaintiffs in the 2014 Derivative Actions seek, purportedly on behalf of the Company, an unspecified award of damages including, but not limited to, various corporate governance reforms, an award of restitution, an award of reasonable costs and expenses, including attorneys’ fees, and other further relief as the Court may deem just and proper. The Courts have granted the parties’ stipulations to defer litigation activity, subject to certain conditions and pending certain developments in the Class Action.
On October 14, 2015, a shareholder derivative action was filed in the Superior Court of California, County of Yolo (Case No. CV15-1423), purportedly on our behalf, against certain current and former officers and members of our board of directors and our independent registered public accounting firm (the “2015 Derivative Action,” and with the 2014 Derivative Actions, the “Derivative Actions”). The plaintiff in the 2015 Derivative Action alleges that the director and officer defendants breached their fiduciary duties, committed waste and were unjustly enriched, by causing the Company to issue allegedly false and misleading statements. The plaintiff in the 2015 Derivative Action also alleges that our independent registered public accounting firm committed professional negligence and malpractice. The issues in the 2015 Derivative Action overlap substantially with those at issue in the 2014 Derivative Actions and the Class Action described above. The parties are negotiating a date by which the defendants’ response to the newly filed complaint will be due. Given the preliminary nature of the Derivative Actions, we are not in a position to express any opinion regarding the outcome in these matters.
We also advised the staff of the SEC’s Division of Enforcement of the Audit Committee’s independent investigation. The SEC commenced a formal investigation of these matters, with which we are cooperating. Though the investigation continues, we have engaged in discussions with the Division of Enforcement staff concerning the resolution of any enforcement action that it may recommend. In accordance with ASC 450, Contingencies, the Company recorded an accrual of $1.8 million in its financial statements for the year ended December 31, 2014 for its estimate of the penalties arising from such enforcement action and has estimated the range of the reasonably possible loss to be between $1.8 million and $4.0 million.
From time to time we may be involved in litigation that we believe is of the type common to companies engaged in our line of business, including intellectual property and employment issues. While the outcome of these other claims cannot be predicted with certainty, we do not believe that the outcome of any of these other legal matters will have a material adverse effect on our results of operations, financial condition or cash flows.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
49
Table of Contents
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information for Common Stock
Our common stock has been listed on the NASDAQ Global Market under the symbol “MBII” since August 2, 2013. Prior to that time, there was no public market for our stock. The following table sets forth for the indicated periods the high and low intra-day sales prices per share for our common stock on the NASDAQ Global Market.
| HIGH | LOW | |||||||
| 2013 |
||||||||
| Third Quarter 2013 (from August 2, 2013) |
$ | 18.58 | $ | 12.27 | ||||
| Fourth Quarter 2013 |
$ | 20.00 | $ | 13.01 | ||||
| 2014 |
||||||||
| First Quarter 2014 |
$ | 19.64 | $ | 13.05 | ||||
| Second Quarter 2014 |
$ | 14.03 | $ | 8.21 | ||||
| Third Quarter 2014 |
$ | 11.84 | $ | 2.41 | ||||
| Fourth Quarter 2014 |
$ | 3.75 | $ | 1.85 | ||||
Holders of Record
As of September 30, 2015, there were 74 stockholders of record of our common stock, and the closing price of our common stock was $2.11 per share as reported on the NASDAQ Global Market. Because some of our shares of common stock are held by brokers and other institutions on behalf of stockholders, we are unable to estimate the total number of stockholders represented by these record holders.
Dividend Policy
We have never declared or paid any cash dividend on our common stock. We intend to retain any future earnings and do not expect to pay dividends in the foreseeable future.
Equity Compensation Plan Information
Information, as of December 31, 2014, regarding equity compensation plans approved and not approved by stockholders is summarized in the following table:
| PLAN CATEGORY |
NUMBER OF SECURITIES TO BE ISSUED UPON EXERCISE OF OUTSTANDING OPTIONS, WARRANTS AND RIGHTS |
WEIGHTED-AVERAGE EXERCISE PRICE OF OUTSTANDING OPTIONS, WARRANTS AND RIGHTS |
NUMBER OF SECURITIES REMAINING AVAILABLE FOR FUTURE ISSUANCE UNDER EQUITY COMPENSATION PLANS (EXCLUDING SECURITIES REFLECTED IN COLUMN (a))(1) |
|||||||||
| (a) | (b) | |||||||||||
| Equity compensation plans approved by security holders |
2,830,653 | $ | 9.39 | 1,086,444 | ||||||||
| Equity compensation plans not approved by security holders |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
2,830,653 | $ | 9.39 | 1,086,444 | ||||||||
|
|
|
|
|
|
|
|||||||
| (1) | Consists of shares available for issuance under our 2013 Stock Incentive Plan. |
50
Table of Contents
Stock Performance Graph
This performance graph shall not be deemed “soliciting material” or to be “filed” with the SEC for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (Exchange Act), or otherwise subject to the liabilities under that Section, and shall not be deemed to be incorporated by reference into any filing of Marrone Bio Innovations, Inc. under the Securities Act of 1933, as amended, or the Exchange Act.
The following graph shows a comparison from August 2, 2013 (the date our common stock commenced trading on the NASDAQ Global Market) through December 31, 2014 of the cumulative total return for our common stock, the Standard & Poor’s 500 Stock Index (S&P 500 Index) and the Nasdaq Composite Index (NASDAQ Composite). The graph assumes that $100 was invested at the market close on August 2, 2013 in the common stock of Marrone Bio Innovations, Inc., the S&P 500 Index and the NASDAQ Composite and data for the S&P 500 Index and the NASDAQ Composite assumes reinvestments of dividends. The stock price performance of the following graph is not necessarily indicative of future stock price performance.
ITEM 6. SELECTED FINANCIAL DATA
You should read the following selected consolidated financial data in connection with Part II-Item 7-“Management’s Discussion and Analysis of Financial Condition and Results of Operation,” and our consolidated financial statements and the related notes included in Part II-Item 8-“Financial Statements and Supplementary Data” in this Annual Report on Form 10-K.
The consolidated statements of operations data for each of the years ended December 31, 2014, 2013 (as restated) and 2012 and the consolidated balance sheet data as of December 31, 2014 and 2013 (as restated) are derived from our audited consolidated financial statements included in Part II-Item 8-“Financial Statements and Supplementary Data” in this Annual Report on Form 10-K. The consolidated statements of operations data for the years ended December 31, 2012 and 2011 and the consolidated balance sheet data as of December 31, 2012 and 2011 are derived from our audited consolidated financial statements that are not included in this Annual Report on Form 10-K. Our historical results are not necessarily indicative of our results in any future period.
51
Table of Contents
Consolidated Statements of Operations Data:
| YEAR ENDED DECEMBER 31 | ||||||||||||||||||||
| 2014 | 2013 | 2012 | 2011 | 2010 | ||||||||||||||||
| As restated(1) | ||||||||||||||||||||
| (in thousands, except per share data) | ||||||||||||||||||||
| Revenues: |
||||||||||||||||||||
| Product |
$ | 7,750 | $ | 7,588 | $ | 6,777 | $ | 5,044 | $ | 3,666 | ||||||||||
| License(2) |
232 | 193 | 179 | 57 | — | |||||||||||||||
| Related party |
1,154 | 665 | 184 | 150 | 31 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total revenues |
9,136 | 8,446 | 7,140 | 5,251 | 3,697 | |||||||||||||||
| Cost of product revenues, including cost of product revenues to related parties of $561, $374, $126, $50 and $10 for the years ended December 31, 2014, 2013, 2012, 2011 and 2010, respectively |
9,438 | 7,243 | 4,333 | 2,172 | 1,738 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Gross profit (loss) |
(302 | ) | 1,203 | 2,807 | 3,079 | 1,959 | ||||||||||||||
| Operating expenses: |
||||||||||||||||||||
| Research, development and patent |
19,281 | 17,905 | 12,741 | 9,410 | 5,563 | |||||||||||||||
| Non-cash charge associated with a convertible note |
— | — | 3,610 | — | — | |||||||||||||||
| Selling, general and administrative |
28,950 | 15,017 | 10,294 | 6,793 | 4,353 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total operating expenses |
48,231 | 32,922 | 26,645 | 16,203 | 9,916 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Loss from operations |
(48,533 | ) | (31,719 | ) | (23,838 | ) | (13,124 | ) | (7,957 | ) | ||||||||||
| Other income (expense): |
||||||||||||||||||||
| Interest income |
59 | 49 | 16 | 22 | 22 | |||||||||||||||
| Interest expense |
(2,907 | ) | (6,056 | ) | (2,466 | ) | (88 | ) | (102 | ) | ||||||||||
| Change in estimated fair value of financial instruments(3) |
— | 6,717 | (12,461 | ) | 1 | — | ||||||||||||||
| Gain on extinguishment of debt |
— | 49 | — | — | — | |||||||||||||||
| Other (expense) income, net |
(278 | ) | (282 | ) | (45 | ) | 9 | 1 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total other income (expense), net |
(3,126 | ) | 477 | (14,956 | ) | (56 | ) | (79 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Loss before income taxes |
(51,659 | ) | (31,242 | ) | (38,794 | ) | (13,180 | ) | (8,036 | ) | ||||||||||
| Income taxes |
— | — | — | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net loss |
(51,659 | ) | (31,242 | ) | (38,794 | ) | (13,180 | ) | (8,036 | ) | ||||||||||
| Deemed dividend on convertible notes |
— | (1,378 | ) | (2,039 | ) | — | — | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net loss attributable to common stockholders |
$ | (51,659 | ) | $ | (32,620 | ) | $ | (40,833 | ) | $ | (13,180 | ) | $ | (8,036 | ) | |||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net loss per common share(4): |
||||||||||||||||||||
| Basic |
$ | (2.32 | ) | $ | (3.74 | ) | $ | (32.48 | ) | $ | (10.64 | ) | $ | (6.58 | ) | |||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Diluted |
$ | (2.32 | ) | $ | (4.25 | ) | $ | (32.48 | ) | $ | (10.64 | ) | $ | (6.58 | ) | |||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Weighted-average shares outstanding used in computing net loss per common share(4): |
||||||||||||||||||||
| Basic |
22,314 | 8,731 | 1,257 | 1,239 | 1,221 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Diluted |
22,314 | 8,911 | 1,257 | 1,239 | 1,221 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (1) | For adjustments related to 2013, see Note 2, Restatement of Previously Issued Consolidated Financial Statements, to the consolidated financial statements included in this Annual Report on Form 10-K. |
52
Table of Contents
| (2) | We receive payments under strategic collaboration and distribution agreements under which we provide third parties with exclusive development, marketing and distribution rights. These payments are initially classified as deferred revenues and are recognized as revenues over the exclusivity period. See Note 3 of our accompanying Notes to Consolidated Financial Statements included in Part II-Item 8-“Financial Statements and Supplementary Data” in this Annual Report on Form 10-K for an explanation of the method used to calculate license revenues. |
| (3) | Prior to the completion of the initial public offering, we accounted for the outstanding warrants exercisable into shares of our Series A, Series B and Series C convertible preferred stock and the outstanding warrants exercisable into a variable number of shares of common stock as liability instruments, as the Series A, Series B and Series C convertible preferred stock and the common stock into which these warrants were convertible were contingently redeemable upon the occurrence of certain events or transactions. In addition, convertible notes were accounted for at estimated fair value. The warrant instruments and convertible notes were adjusted to fair value at each reporting period with the change in fair value recorded in the consolidated statements of operations. These charges did not continue after the completion of the initial public offering because the preferred stock warrants were exercised and the convertible notes automatically converted into common stock in accordance with their terms upon the completion of the initial public offering. The common stock warrants were, in accordance with their terms upon the completion of the initial public offering, either automatically exercised for shares of common stock or represent the right to purchase a fixed number of shares. See Part II-Item 7-“Management’s Discussion and Analysis of Financial Conditions and Results of Operations—Key Components of Our Results of Operations—Change in Estimated Fair Value of Financial Instruments and Deemed Dividend on Convertible Notes.” |
| (4) | Includes the effect of a 1-for-3.138458 reverse stock split, effective August 1, 2013. |
Balance Sheet Data:
| DECEMBER 31 | ||||||||||||||||||||
| 2014 | 2013 | 2012 | 2011 | 2010 | ||||||||||||||||
| As restated (1) | ||||||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Cash and cash equivalents |
$ | 35,324 | $ | 24,455 | $ | 10,006 | $ | 2,215 | $ | 4,287 | ||||||||||
| Short-term investments |
— | 13,677 | — | 2,000 | — | |||||||||||||||
| Working capital (deficit) (2) |
23,521 | 44,221 | (11,468 | ) | 5,030 | 4,935 | ||||||||||||||
| Total assets |
77,182 | 69,918 | 33,778 | 9,818 | 7,937 | |||||||||||||||
| Debt and capital leases (net of unamortized discount) |
24,327 | 14,972 | 16,740 | 806 | 1,106 | |||||||||||||||
| Convertible notes |
— | — | 41,860 | — | — | |||||||||||||||
| Preferred stock warrant liability |
— | — | 1,884 | 27 | 28 | |||||||||||||||
| Common stock warrant liability |
— | — | 301 | — | — | |||||||||||||||
| Total liabilities |
43,951 | 30,887 | 68,413 | 4,306 | 2,689 | |||||||||||||||
| Convertible preferred stock |
— | — | 39,612 | 39,612 | 26,542 | |||||||||||||||
| Total stockholders’ equity (deficit) |
33,231 | 39,031 | (74,247 | ) | (34,100 | ) | (21,204 | ) | ||||||||||||
| (1) | For adjustments related to 2013, see Note 2, Restatement of Previously Issued Consolidated Financial Statements, to the consolidated financial statements included in this Annual Report on Form 10-K. |
| (2) | Working capital (deficit) is defined as total current assets minus total current liabilities. |
53
Table of Contents
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion of our financial condition and results of operations in connection with our consolidated financial statements and the related notes included in Part II-Item 8-“Financial Statements and Supplementary Data” in this Annual Report on Form 10-K. In addition to our historical consolidated financial information, the following discussion contains forward-looking statements that reflect our plans, estimates, and beliefs. Our actual results could differ materially from those discussed in the forward-looking statements. Factors that could cause or contribute to these differences include those discussed below and elsewhere in this Annual Report on Form 10-K, particularly in Part I-Item 1A-“Risk Factors.”
Overview
We make bio-based pest management and plant health products. Bio-based products are comprised of naturally occurring microorganisms, such as bacteria and fungi, and plant extracts. Our current products target the major markets that use conventional chemical pesticides, including certain agricultural and water markets, where our bio-based products are used as alternatives for, or mixed with, conventional chemical products. We also target new markets for which there are no available conventional chemical pesticides, the use of conventional chemical products may not be desirable or permissible because of health and environmental concerns (including for organically certified crops) or because the development of pest resistance has reduced the efficacy of conventional chemical pesticides. We believe our current portfolio of EPA-approved and registered “biopesticide” products and our pipeline address the growing global demand for effective, efficient and environmentally responsible products to control pests, increase crop yields and reduce crop stress.
The agricultural industry is increasingly dependent on effective and sustainable pest management practices to maximize yields and quality in a world of increased demand for agricultural products, rising consumer awareness of food production processes and finite land and water resources. In addition, our research has shown that the global market for biopesticides is growing substantially faster than the overall market for pesticides. This demand is in part a result of conventional growers acknowledging that there are tangible benefits to adopting bio-based pest management products into integrated pest management (IPM) programs, as well as increasing consumer demand for organic food. We seek to capitalize on these global trends by providing both conventional and organic growers with solutions to a broad range of pest management needs through strategies such as adding new products to our product portfolio, continuing to broaden the commercial applications of our existing product lines, leveraging growers’ positive experiences with existing product lines, and educating growers with on-farm product demonstrations and controlled product launches with key target customers and other early adopters. We believe this approach enables us to stay ahead of our competition in providing innovative pest management solutions, enhances our sales process at the distributor level and helps us to capture additional value from our products.
Although our long-term, global vision for our business and our commitment to that vision remain fundamentally unchanged, to date, we have not achieved anticipated growth in sales of our products, which has resulted in an increase in inventory write-offs, higher proportional operating expenses levels, increases in costs of goods sold, and decreases in product margins. In response to the business challenges reflected in our financial results for recent periods, since the second half of 2014, we have been implementing a prioritization plan to focus our resources on continuing to improve and promote our commercially available products, advancing product candidates that are expected to have the greatest impact on near-term growth potential and expanding international presence and commercialization. Our goal has been to reduce expenses, conserve cash and improve operating efficiencies, to extract greater value from our products and product pipeline and to improve our communication to and connection with the global sustainability movement that is core to our cultural values.
In connection with this new strategy, we have significantly reduced overall headcount, while building a new sales and marketing organization with increased training and ability to educate and support customers as well as providing our product development staff with greater responsibility for technical sales support, field trials and
54
Table of Contents
demonstrations to promote sales growth. In addition, while we believe that we have developed a robust pipeline of novel product candidates, we are currently limiting our internal efforts on five promising product candidates. Simultaneously, we are seeking collaborations with third parties to develop and commercialize more early stage candidates on which we have elected not to expend significant resources given our reduced budget. We believe collectively, these measures, together with our competitive strengths, including our leadership in the biologicals industry, commercially available products, robust pipeline of novel product candidates, proprietary discovery and development processes and industry experience, position us for growth.
We sell our crop protection products to leading agrichemical distributors while also working directly with growers to increase existing and generate new product demand. To date, we have marketed our bio-based pest management and plant health products for agricultural applications to U.S. growers, through distributors and our own sales force, and we have focused primarily on high value specialty crops such as grapes, citrus, tomatoes, and leafy greens. A large portion of our sales are currently attributable to conventional growers who use our bio-based pest management products either to replace conventional chemical pesticides or enhance the efficacy of their IPM programs. In addition, a portion of our sales are attributable to organic farmers, who cannot use conventional pesticides and have few alternatives for pest management. As we continue to demonstrate the efficacy of our bio-based pest management and plant health products on new crops or for new applications, we may either continue to sell our product through our in-house sales force or collaborate with third parties for distribution to select markets.
Although we have historically sold a significant majority of our products in the United States, expanding our international presence and commercialization is an important component of our growth strategy. Regalia is currently available in select international markets under distribution agreements with major agrichemical companies. Going forward, our plan is to focus on key countries and regions with the largest and fastest growing biopesticide and plant health product markets for specialty crops and selected row crops. We intend to work with regional and country distributors who have brand recognition and established customer bases and who can conduct field trials and grower demonstrations and lead or assist in regulatory processes and market development.
We currently market our water treatment product, Zequanox, through our sales and technical workforce to a selected group of U.S. power and industrial companies. Due to prioritization constraints, we have not committed resources to Zequanox sufficient to market it full-scale. However, we are seeking sales and distribution partners for in-pipe and open water uses, and are currently in discussions with large water treatment companies to further develop Zequanox and expand it commercially. In addition, we continue to work with state, federal and bi-national partners to further develop Zequanox in the Great Lakes/Upper Mississippi River Basin as a habitat restoration tool and potential harmful algal bloom management tool. We believe that Zequanox presents a unique opportunity for generating long-term revenue, as there are limited water treatment options available to date, most of which are time-consuming, costly or subject to high levels of regulation. Our ability to generate significant revenues from Zequanox is dependent on our ability to persuade customers to evaluate the costs of our Zequanox products compared to the overall cost of the chlorine treatment process, the primary current alternative to using Zequanox, rather than the cost of purchasing chemicals alone. Sales of Zequanox have also remained lower than our other products due to the length of the treatment cycle, the longer sales cycle (the bidding process with utility companies and governmental agencies occurs on a yearly or multi-year basis) and the unique nature of the treatment approach for each customer based on the extent of the infestation and the design of the facility.
Although our initial EPA-approved master labels cover our products’ anticipated crop-pest use combinations, we launch early formulations of our pest management and plant health products to targeted customers under commercial labels that list a limited number of crops and applications that our initial efficacy data can best support. We then gather new data from experiments, field trials and demonstrations, gain product knowledge and get feedback to our research and development team from customers, researchers and agricultural agencies. Based on this information, we enhance our products, refine our recommendations for their use in optimal integrated pest management programs, expand our commercial labels, and submit new product formulations to the EPA and
55
Table of Contents
other regulatory agencies. For example, we began sales of Regalia SC, an earlier formulation of Regalia, in the Florida fresh tomatoes market in 2008, while a more effective formulation of Regalia with an expanded master label, including listing for use in organic farming, was under review by the EPA. In 2011, we received EPA approval of a further expanded Regalia master label covering hundreds of crops and various new uses for applications to soil and through irrigation systems, and we recently expanded sales of Regalia in large-acre row crops as a plant stimulus product, in addition to its beneficial uses as a fungicide. Similarly, ongoing field development research on the microbe used in our insecticide product Venerate led to our October 2015 registration of Majestene (MBI-305) as a nematicide. We believe we have opportunities to broaden the commercial applications and expand the use of our existing products lines to help drive significant growth for our company.
Our total revenues were $9.1 million, $8.4 million and $7.1 million for the years ended December 31, 2014, 2013 and 2012, respectively, and have risen as growers have adopted our products and have used our products on an expanded number of crops. We generate our revenues primarily from product sales, which historically were principally attributable to sales of Regalia and are now increasingly attributable to Grandevo. However, various factors have impeded anticipated growth in sales of these products in recent years, and may continue to impede growth. For example, we believe adverse conditions in the U.S. agricultural industry, including low commodity prices, may have reduced demand for our products. Further delays in regulatory approvals of certain of our products in Europe and other jurisdictions may slow international growth, and any delay in a product launch that causes us to miss a growing season may require us to wait a year to enter that market. The extended drought in California and other markets has reduced demand for our products as fewer acres are planted, and certain of our strategic collaborations have not resulted in anticipated increases in sales of Regalia outside of the Unites States. Due to prioritization constraints, we have not committed resources to Zequanox sufficient to market it full-scale, and our collaboration efforts with regard to this product may not result in increased sales. In addition, the departure of our former chief operating officer and significant members of our sales staff in the third quarter of 2014 and subsequent turnover in our sales and marketing department disrupted the 2014 launch of Venerate as well as growth in sales of our other commercialized products, including Regalia and Grandevo.
Since 2011, we have also recognized revenues from our strategic collaboration and distribution agreements, which amounted to $0.2 million for each of the years ended December 31, 2014, 2013 and 2012, excluding related party revenues. For the years ended December 31, 2014 and 2013, we recognized $0.3 million and $0.1 million, respectively, of related party revenues under these agreements based on the terms of our commercial agreement with Syngenta, an affiliate of one of our 5% stockholders. There were no related party revenues recognized under these agreements for the year ended December 31, 2012.
We currently sell our crop protection products through the same leading agricultural distributors used by the major agrichemical companies. Distributors with 10% or more of our total revenues in any one of the periods presented consist of the following:
| CROP PRODUCTION SERVICES |
ENGAGE AGRO |
HELENA CHEMICALS |
||||||||||
| For the years ended December 31, |
||||||||||||
| 2014 |
30 | % | 2 | % | 13 | % | ||||||
| 2013 |
20 | % | 2 | % | 8 | % | ||||||
| 2012 |
33 | % | 13 | % | 12 | % | ||||||
While we expect product sales to a limited number of distributors to continue to be our primary source of revenues, as we continue to develop our pipeline and introduce new products to the marketplace, we anticipate that our revenues stream will be diversified over a broader product portfolio and customer base.
56
Table of Contents
Our cost of product revenues was $9.4 million, $7.2 million and $4.3 million for the years ended December 31, 2014, 2013 and 2012, respectively. Cost of product revenues included $0.6 million, $0.4 million and $0.1 million of cost of product revenues to related parties for the years ended December 31, 2014, 2013 and 2012, respectively. Gross margins were a negative 3% for the year ended December 31, 2014, compared to 14% and 39% for the years ended December 31, 2013 and 2012, respectively. Cost of product revenues consists principally of the cost of inventory which includes the cost of raw materials, and third-party services and allocation of operating expenses of our manufacturing plant related to procuring, processing, formulating, packaging and shipping our products. Cost of product revenues also include charges recorded for write-downs of inventory which has increased in recent years and, beginning in 2014, idle capacity at our manufacturing plant when the manufacturing plant was placed into service. We expect our cost of product revenues related to the cost of inventory to increase and cost of product revenues relating to write-downs of inventory and idle capacity of our manufacturing plant to decrease as we expand sales and increase production of our existing commercial products Regalia, Grandevo, Venerate and Zequanox and introduce new products to the market. Our cost of product revenues related to the cost of inventory has increased as a percentage of total revenues primarily due to a change in product mix, with Grandevo representing an increased percentage of total revenues as Grandevo is early in its life cycle. We expect to see a gradual increase in gross margin over the life cycle of each of our products, including Grandevo, as we improve production processes, gain efficiencies and increase product yields. These increases may be offset by additional charges for inventory write-downs and idle capacity at our manufacturing plant until overall volume in the plant increases significantly.
Our research, development and patent expenses have historically comprised a significant portion of our operating expenses, amounting to $19.3 million, $17.9 million and $12.7 million for the years ended December 31, 2014, 2013 and 2012, respectively. We have reduced the size of our research and development staff compared to prior periods and are reducing costs spent on various research and development and patent efforts as part of our efforts to streamline business operations and focus on our pipeline product priorities. However, we have made, and will continue to make, substantial investments in research and development and we intend to continue to devote significant resources toward the advancement of product candidates that are expected to have the greatest impact on near-term growth potential. Simultaneously, we are seeking collaborations with third parties to develop and commercialize more early stage candidates, which we have elected not to expend significant resources on given our reduced budget.
Selling, general and administrative expenses incurred to establish and build our market presence and business infrastructure have generally comprised the remainder of our operating expenses, amounting to $29.0 million, $15.0 million and $10.3 million for the years ended December 31, 2014, 2013 and 2012, respectively. While we have reduced headcount in comparison to prior periods overall, in connection with our new strategy, we have been building a new sales and marketing organization which provides for increased training and a better ability to educate and support customers as well as transitioning our product development staff to undertake greater responsibility for technical sales support, field trials and demonstrations to promote sales growth. We expect that in the future, our selling, general and administrative expenses will increase due to our expanded product portfolio and due to additional costs incurred relating to being a public company. In addition, for the year ended December 31, 2014, we incurred $5.8 million in costs related to the Audit Committee’s independent investigation, which commenced in September 2014, and expect to incur significant costs through 2015 related to the Audit Committee investigation and the Restatement. In addition, we have engaged in discussions with the SEC’s Division of Enforcement staff concerning the resolution of any enforcement action that it may recommend. In accordance with ASC 450, Contingencies, we recorded an accrual of $1.8 million in our financial statements for the year ended December 31, 2014 for our estimate of the penalties arising from such enforcement action.
In addition, for the year ended December 31, 2012, in connection with a convertible note, we incurred a non-recurring, non-cash charge of $3.6 million as operating expenses. We also recognized a net gain of $6.7 million for the year ended December 31, 2013 and a net loss of $12.5 million for the year ended December 31, 2012, in non-cash charges attributable to the change in estimated fair value of financial instruments, which were reported in other income (expense).
57
Table of Contents
Historically, we have funded our operations from the issuance of shares of common stock, preferred stock, warrants and convertible notes, the issuance of debt and entry into financing arrangements, product sales, payments under strategic collaboration and distribution agreements and government grants, but we have experienced significant losses as we invested heavily in research and development. We expect to incur additional losses related to our investment in the continued development, expansion and marketing of our product portfolio.
In August 2013, we closed an initial public offering of 5.5 million shares of our common stock (the IPO). The public offering price of the shares sold in the offering was $12.00 per share. Our total gross proceeds from the offering were $65.6 million, and after deducting underwriting discounts and commissions and offering expenses payable by us, the aggregate net proceeds that we received totaled approximately $56.1 million. Upon the closing of the IPO, all shares of our outstanding convertible preferred stock and all of our outstanding convertible notes automatically converted into shares of common stock, and all outstanding warrants to purchase convertible preferred stock and certain warrants to purchase common stock were exercised for shares of common stock. In June 2014 we completed a public offering of 4.6 million shares of its common stock. The public offering price of the shares sold in the offering was $9.50 per share. Our total gross proceeds from the offering to us were $43.5 million, and after deducting underwriting discounts and commissions and offering expenses payable by us, the aggregate net proceeds we received totaled $39.9 million. In addition, in June 2014, we borrowed $10.0 million pursuant to a promissory note with Five Star Bank and in August 2015, we issued and sold to affiliates of Waddell & Reed Financial, Inc. in a private placement senior secured promissory notes in the aggregate principal amount of $40.0 million and warrants to purchase up to 4.0 million shares of our common stock at an exercise price of $1.91 per share for aggregate consideration of $40.0 million.
Critical Accounting Policies and Estimates
Our consolidated financial statements and the related notes included elsewhere in this Annual Report on Form 10-K are prepared in accordance with accounting principles generally accepted in the United States. The preparation of these consolidated financial statements requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities, net revenue, costs, and expenses, and any related disclosures. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances. Changes in accounting estimates are reasonably likely to occur from period to period. Accordingly, actual results could differ significantly from the estimates made by our management. We evaluate our estimates and assumptions on an ongoing basis. To the extent that there are material differences between these estimates and our actual results, our future financial statement presentation, financial condition, results of operations and cash flows will be affected.
We believe that the assumptions and estimates associated with revenue recognition, including assumptions and estimates used in determining the timing and amount of revenue to recognize for those transactions accounted for on a “sell-through” method, and inventory valuation and share-based compensation have the greatest potential impact on our consolidated financial statements. Therefore, we consider these to be our critical accounting policies and estimates. For further information on all of our significant accounting policies, see Note 3 of our accompanying Notes to Consolidated Financial Statements included in Part II-Item 8-“Financial Statements and Supplementary Data” in this Annual Report on Form 10-K.
Key Components of Our Results of Operations
Product Revenues
Product revenues consist of revenues generated primarily from sales to distributors, net of rebates and cash discounts. Our product revenues through 2012 were primarily derived from sales of Regalia, but now are increasingly impacted by new products such as Grandevo. Product revenues, not including related party revenues, constituted 85%, 90% and 95% of our total revenues for the years ended December, 2014, 2013 and 2012, respectively. Product revenues in the United States, not including related party revenues, constituted 78%, 77% and 78% of our total revenues for the years ended December 31, 2014, 2013 and 2012, respectively.
58
Table of Contents
In some cases, we recognize distributor revenue as title and risk of loss passes, provided all other revenue recognition criteria has been satisfied (the “sell-in” method). For certain sales to certain distributors, the revenue recognition criteria for distributor sales are not satisfied at the time title and risk of loss passes to the distributor; specifically, in instances where “inventory protection” arrangements were offered to distributors that would permit these distributors to return to the Company certain unsold products, we consider the arrangement not to be fixed or determinable, and accordingly, revenue is deferred until products are resold to customers of the distributor (the “sell-through” method). The cost of goods sold associated with such deferral are also deferred and classified as deferred cost of product revenues in the consolidated balance sheets. Cash received from customers related to delivered product that may not represent a true sale are classified as customer refund liabilities in the consolidated balance sheets and the related cost of inventory remains in inventory in the consolidated balance sheets until the product is returned or is resold to customers of the distributor and revenue is recognized. For the years ended December 31, 2014 and 2013, 63% and 26%, respectively, of product revenues, not including related party revenues, were recognized on a sell-through basis. During the year ended December 31, 2012, there were no product revenues recognized on a sell-through basis. As of December 31, 2014 and 2013, the Company recorded current deferred product revenues of $3.2 million and $4.7 million, respectively.
License Revenues
License revenues generally consist of revenues recognized under our strategic collaboration and distribution agreements for exclusive distribution rights, either for Regalia or for our broader pipeline of products, for certain geographic markets or for market segments that we are not addressing directly through our internal sales force. Our strategic collaboration and distribution agreements generally outline overall business plans and include payments we receive at signing and for the achievement of testing validation, regulatory progress and commercialization events. As these activities and payments are associated with exclusive rights that we provide over the term of the strategic collaboration and distribution agreements, revenues related to the payments received are deferred and recognized as revenues over the term of the exclusive period of the respective agreements, which we estimate to be between 5 and 17 years based on the terms of the contract and the covered products and regions. For the years ended December 31, 2014, 2013 and 2012, license revenues constituted 2% of total revenues for each year. As of December 31, 2014, including agreements with related parties discussed below, we had received an aggregate of $2.9 million in payments and had $0.8 million recorded in accounts receivable under our strategic collaboration and distribution agreements. In addition, there will be an additional $0.5 million in payments due on certain anniversaries of regulatory approval and an additional $1.6 million in payments under these agreements that we could potentially receive if the testing validation, regulatory progress and commercialization events occur.
Related Party Revenues
Related party revenues consist of both product revenues and license revenues. Les Lyman, a member of our board of directors, is the chairman and significant indirect shareholder of The Tremont Group, Inc., which purchases our products for further distribution and resale. In addition, in December 2012, we issued a convertible note to Syngenta Ventures Pte. LTD. (Syngenta Ventures), an affiliate of one of our international distributors, Syngenta, with whom we entered into a commercial agreement with and sell our products to for further distribution and resale. Syngenta Ventures reduced its ownership stake in the Company below 5% in June 2014. Accordingly, sales subsequent to June 2014 to Syngenta have not been included as related party revenues. For the years ended December 31, 2014, 2013 and 2012, related party revenues constituted 13%, 8% and 3% of total revenues, respectively. For the years ending December 31, 2014 and 2013, 71% and 63%, respectively, of related party revenues, were recognized on a sell-through basis. During the year ending December 31, 2012, there were no related party revenues recognized on a sell through basis.
Cost of Product Revenues and Gross Profit (Loss)
Cost of product revenues consists principally of the cost of raw materials, including inventory costs and third- party services related to procuring, processing, formulating, packaging and shipping our products. As we have
59
Table of Contents
used our Bangor, Michigan manufacturing plant to produce certain of our products, cost of product revenues includes allocation of operating costs including direct and indirect labor, productions supplies, repairs and maintenance depreciation, utilities and property taxes. The amount of indirect labor and overhead allocated to finished goods are determined on a basis presuming normal capacity utilization. Operating costs incurred in excess of production allocations, considered idle capacity, are expensed to cost of goods sold in the period incurred rather than added to the cost of the finished goods produced. Cost of product revenues also may include charges due to inventory adjustments and reserves. Gross profit (loss) is the difference between total revenues and the cost of product revenues. Gross margin is gross profit (loss) expressed as a percentage of total revenues.
We have entered into in-license technology agreements with respect to the use and commercialization of our three commercially available product lines, including Regalia, Grandevo and Zequanox, and certain products under development. Under these licensing arrangements, we typically make royalty payments based on net product revenues, with royalty rates varying by product and ranging between 2% and 5% of net sales, subject in certain cases to aggregate dollar caps. These royalty payments are included in cost of product revenues, but they have historically not been significant. In addition, costs associated with license revenues have been included in cost of product revenues, as they have not been significant. The exclusivity and royalty provisions of these agreements are generally tied to the expiration of underlying patents. The patents for Regalia and Zequanox will expire in 2017 and the in-licensed U.S. patent for Grandevo is expected to expire in 2024. There is, however, a pending in-licensed patent application relating to Grandevo, which could expire later than 2024 if issued. After the termination of these provisions, we may continue to produce and sell these products. While third parties thereafter may develop products using the technology under expired patents, we do not believe that they can produce competitive products without infringing other aspects of our proprietary technology, including pending patent applications related to Regalia, Zequanox and Grandevo, and we therefore do not expect the expiration of the patents or the related exclusivity obligations to have a significant adverse financial or operational impact on our business.
We expect to see increases in gross profit over the life cycle of each of our products because gross margins are expected be increased over time as production processes improve and as we gain efficiencies and increase product yields. While we expect margins to improve on a product-by-product basis, our overall gross margins may vary as we introduce new products. In particular, we are experiencing and expect further near-term downward pressure on overall gross margins as we expand sales of Grandevo, Venerate and Zequanox and when we introduce new products. Gross margin has been and will continue to be affected by a variety of factors, including plant utilization, product manufacturing yields, changes in product production processes, new product introductions, product mix and average selling prices.
To date, we have relied on third parties for the production of our products. In July 2012, we acquired a manufacturing facility, which we repurposed for manufacturing operations and began full-scale manufacturing using this facility in 2014. We continue to use third party manufacturers for Venerate and for spray-dried powder formulations of Grandevo and Zequanox. We expect gross margins to improve using this facility when sales volumes recover enough to reduce overhead and unused capacity charges from our facility.
Research, Development and Patent Expenses
Research, development and patent expenses includes personnel costs, including salaries, wages, benefits and share-based compensation, related to our research, development and patent staff in support of product discovery and development activities. Research, development and patent expenses also include costs incurred for laboratory supplies, field trials and toxicology tests, quality control assessment, consultants and facility and related overhead costs
Beginning in the fourth quarter of 2014, we reduced our research and development staff and prioritized our pipeline candidates, focusing first on those that can be in the market in the next two years. We expect research, development and patent expenses to decrease in the near term as we have reduced headcount and focus our efforts on select pipeline products. We are working to find partners to assist development of other pipeline candidates.
60
Table of Contents
Non-Cash Charge Associated with a Convertible Note
In December 2012, we issued a $12.5 million convertible note to Syngenta Ventures, an affiliate of one of our distributors, and incurred charges of $3.9 million representing the excess of the estimated fair value of the convertible note on the date of issuance compared to the cash received. Because the holder of this convertible note is an affiliate of one of our distributors, we recorded $0.3 million of the charges as a reduction of revenues recognized under our agreements with the affiliated distributor through the date of issuance of the convertible note in December 2012. We recorded the remaining $3.6 million of the charges in operating expenses as a non-recurring non-cash charge associated with a convertible note in December 2012.
Selling, General and Administrative Expenses
Selling, general and administrative expenses consist primarily of personnel costs, including salaries, wages, benefits and share-based compensation, related to our executive, sales, marketing, finance and human resources personnel, as well as professional fees, including legal and accounting fees, and other selling costs incurred related to business development and to building product and brand awareness. We create brand awareness through programs such as speaking at industry events, trade show displays and hosting local-level grower and distributor meetings. In addition, we dedicate significant resources to technical marketing literature, targeted advertising in print and online media, webinars and radio advertising. Costs related to these activities, including travel, are included in selling expenses. Our administrative expenses have increased in recent periods primarily as a result of becoming a public company and incurring significant costs in connection with the Audit Committee’s independent investigation and subsequent restatement of our financial statements.
We expect our selling expenses to increase in the near term, both in absolute dollars and as a percent of total revenues. For the year ended December 31, 2014, we incurred $5.8 million in costs related to the Audit Committee investigation and subsequent restatement of our financial statements. We have continued to incur such costs during the year ending December 31, 2015. Further, as a result of pending litigation, we also expect to incur additional costs relating to directors and officers liability insurance in future periods. In addition, we have engaged in discussions with the SEC’s Division of Enforcement staff concerning the resolution of any enforcement action that it may recommend. In accordance with ASC 450, Contingencies, we recorded an accrual of $1.8 million in our financial statements for the year ended December 31, 2014 for our estimate of the penalties arising from such enforcement action. In the long term, we expect our selling, general and administrative expenses to decline as a percent of total revenues. We expect our overall selling, general and administrative expenses to increase in absolute dollars in order to drive product sales, and we will incur additional expenses associated with operating as a public company. Such increases may include increased insurance premiums, investor relations expenses, legal and accounting fees associated with the expansion of our business and corporate governance, financial reporting expenses, expenses related to Sarbanes-Oxley and other regulatory compliance obligations.
Interest Expense
We recognize interest expense on notes payable, convertible notes and other debt obligations. During 2012, we entered into a $0.5 million term loan, issued $24.1 million in convertible notes and $17.5 million in promissory notes, including a $10.0 million promissory note paid off prior to its maturity date. In October 2012, we issued a $2.5 million convertible note, and we incurred $0.2 million of interest expense for the year ended December 31, 2012 as a result of the excess in the $2.7 million estimated fair value of the convertible note on the date of issuance compared to the cash received. During 2013, we issued $6.5 million in convertible notes and $4.95 million in promissory notes, including the partial conversion of $1.25 million of a convertible note into a promissory note. Accordingly, our interest expense increased both in absolute terms and as a percentage of total revenues. In May 2013, we issued a $3.0 million convertible note, and we incurred $1.2 million of interest expense for the year ended December 31, 2013 as a result of the excess in the $4.2 million estimated fair value of the convertible note on the date of issuance compared to the cash received. Immediately following the
61
Table of Contents
completion of the IPO in August 2013, the convertible notes converted into shares of our common stock. Accordingly, we ceased to incur the interest expense associated with these convertible notes. In addition, in connection with the early repayment of a $10.0 million senior secured promissory note issued in April 2012, we wrote-off the unamortized debt discount totaling $0.8 million and incurred an early termination fee of $0.3 million, which were recorded to interest expense during the year ended December 31, 2013. In June 2014, we entered into a $10.0 million promissory note with a variable interest rate that varies with the prime rate. Accordingly, our interest expense will increase as the prime rate increases. In August 2015, pursuant to a purchase agreement, we issued and sold to affiliates of Waddell & Reed Financial, Inc. senior secured promissory notes in the aggregate principal amount of $40.0 million with a fixed interest rate and warrants to purchase up to 4.0 million shares of common stock of the Company at an exercise price of $1.91 per share for aggregate consideration of $40.0 million. Accordingly, interest expense will increase as a result of the additional debt outstanding.
We have also acquired equipment under capital leases which results in interest expense over the lease term. We increased our capital lease obligations to $2.5 million as of December 31, 2013 from $0.4 million as of December 31, 2012, and our capital lease obligations were $2.0 million as of December 31, 2014.
Interest Income
Interest income consists primarily of interest earned on investments and cash balances. Our interest income will vary each reporting period depending on our average investment and cash balances during the period and market interest rates.
Change in Estimated Fair Value of Financial Instruments and Deemed Dividend on Convertible Notes
Until the effective date of the IPO, we accounted for the outstanding warrants exercisable into shares of our Series A, Series B and Series C convertible preferred stock as liability instruments, as the Series A, Series B and Series C convertible preferred stock into which these warrants were contingently convertible upon the occurrence of certain events or transactions. We also accounted for the outstanding warrants exercisable into a variable number of common shares at a fixed monetary amount as liability instruments. Our convertible notes were recorded at estimated fair value on a recurring basis as the predominant settlement feature of the convertible notes was to settle a fixed monetary amount in a variable number of shares. We adjusted the warrants and the convertible notes to fair value at each reporting period and on the effective date of the IPO with the change in estimated fair value recorded in the consolidated statements of operations.
Based on our operating performance (including the closing of several debt financings and the IPO) and changes in the probability and timing of, and estimated proceeds from, the completion of a an initial public offering in which the Company receives gross cash proceeds, before underwriting discounts, commissions and fees, of at least $30,000,000 (a Qualified IPO) or a sale of substantially all of the Company’s assets or a series of transactions that result in the transfer of more than 50% of the Company’s outstanding voting power (an Acquisition) between reporting dates or the issuance dates of the warrants, we recognized a net gain due to the change in the estimated fair value of financial instruments related to the warrants of $0.4 million for the year ended December 31, 2013 and a net loss of $1.6 million for the year ended December 31, 2012.
We issued $24.1 million in convertible notes during the year ended December 31, 2012. During the year ended December 31, 2013, we issued $6.5 million in convertible notes and converted $1.25 million of a convertible note into a promissory note. Based on our operating performance and changes in the probability and timing of, and estimated proceeds from, the completion of a Qualified IPO or an Acquisition between the reporting dates, or the issuance dates of these notes, we recognized a net gain due to the change in estimated fair value of financial instruments of $6.3 million for the year ended December 31, 2013 and a net loss of $10.9 million for the year ended December 31, 2012, relating to convertible notes. In addition to the ongoing adjustments to the estimated fair value of our convertible notes, we also recognized a one-time deemed dividend in connection with the issuance of certain convertible notes to preferred stockholders because we estimated the fair value of the
62
Table of Contents
convertible notes as of the issuance dates to be greater than the cash proceeds received. Accordingly, we determined that the excess of the estimated fair value of the convertible notes on the dates of issuance over cash proceeds to us represents a deemed dividend to preferred stockholders, and $1.4 million and $2.0 million was reflected in the net loss attributable to common stockholders for the years ended December 31, 2013 and 2012, respectively.
As a result of the automatic exercise of all Series A and Series B convertible preferred stock warrants and certain common stock warrants for shares of common stock, the automatic conversion of all convertible notes into common stock in accordance with their terms, and the exercise of all Series C convertible preferred stock warrants for shares of common stock in connection with our IPO in August 2013, there will not be any further adjustments to these warrants and convertible notes. In addition, upon completion of the IPO, the exercise price and number of shares to be issued upon exercise of the remaining outstanding common stock warrants became known. Accordingly, after the IPO, the fair value of the outstanding common stock warrant liability on the date of the IPO was reclassified to equity and will no longer be adjusted to its estimated fair value on each reporting date.
Income Tax Provision
Since our inception, we have been subject to income taxes principally in the United States. We anticipate that as we further expand our sales into foreign countries, we will become subject to taxation based on the foreign statutory rates and our effective tax rate could fluctuate accordingly.
Income taxes are computed using the asset and liability method, under which deferred tax assets and liabilities are determined based on the difference between the financial statement and tax bases of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized. As of December 31, 2014, based on the available information, it is more likely than not that our deferred tax assets will not be realized, and accordingly we have taken a full valuation allowance against all of our United States deferred tax assets.
As of December 31, 2014, we had net operating loss carry-forwards for federal income tax reporting purposes of $128.2 million, which begin to expire in 2026, and California and other state net operating loss carry-forwards of $87.2 million and $30.7 million, respectively, which begin to expire in 2016. Additionally, as of December 31, 2014, we had federal research and development tax credit carry-forwards of $1.6 million, which begin to expire in 2026, and state research and development tax credit carry-forwards of $1.8 million, which have no expiration date.
Our ability to use its federal and state net operating loss carry-forwards and federal and state tax credit carryforwards to reduce future taxable income and future taxes, respectively, may be subject to restrictions attributable to equity transactions that may have resulted in a change of ownership as defined by Internal Revenue Code Section 382. In the event we have had such a change in ownership, utilization of these carryforwards could be severely restricted and could result in significant amounts of these carryforwards expiring prior to benefitting us.
Restatement
This Management’s Discussion and Analysis of Financial Condition and Results of Operations gives effect to the restatement adjustments made to the previously reported consolidated financial statements for the year ended December 31, 2013. For additional information and a detailed discussion of the Restatement, see “Explanatory Note” on page i of this Annual Report on Form 10-K and Note 2, “Restatement of Previously Issued Consolidated Financial Statements” to the notes to our consolidated financial statements included in Part II-Item 8-“Financial Statements and Supplementary Data” in this Annual Report on Form 10-K.
63
Table of Contents
It also gives effect to the restatement adjustments made to the previously reported condensed consolidated financial statements for the quarterly and year to date periods ended March 31, 2014 (restated), June 30, 2014 (restated), March 31, 2013 (restated), June 30 2013 (restated), and September 30, 2013 (restated) and to the previously reported supplemental information for the quarterly period ended December 31, 2013 (restated). For additional information and a detailed discussion of the quarterly restatement, see Note 21, “Quarterly Financial Information (Unaudited)” to the Notes to our consolidated financial statements included in , “Financial Statements and Supplementary Data” in this Annual Report on Form 10-K.
Results of Operations
The following table sets forth certain statements of operations data as a percentage of total revenues:
| YEAR ENDED DECEMBER 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| As restated | ||||||||||||
| Revenues: |
||||||||||||
| Product |
85 | % | 90 | % | 95 | % | ||||||
| License |
2 | 2 | 2 | |||||||||
| Related party |
13 | 8 | 3 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total revenues |
100 | 100 | 100 | |||||||||
| Cost of product revenues (1) |
103 | 86 | 61 | |||||||||
|
|
|
|
|
|
|
|||||||
| Gross profit (loss) |
(3 | ) | 14 | 39 | ||||||||
| Operating expenses: |
||||||||||||
| Research, development and patent |
211 | 212 | 178 | |||||||||
| Non-cash charge associated with a convertible note |
— | — | 51 | |||||||||
| Selling, general and administrative |
317 | 178 | 144 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
528 | 390 | 373 | |||||||||
|
|
|
|
|
|
|
|||||||
| Loss from operations |
(531 | ) | (376 | ) | (334 | ) | ||||||
| Other income (expense): |
||||||||||||
| Interest income |
1 | 1 | — | |||||||||
| Interest expense |
(32 | ) | (72 | ) | (34 | ) | ||||||
| Change in estimated fair value of financial instruments |
— | 80 | (175 | ) | ||||||||
| Gain on extinguishment of debt |
— | — | — | |||||||||
| Other expense, net |
(3 | ) | (3 | ) | — | |||||||
|
|
|
|
|
|
|
|||||||
| Total other income (expense), net |
(34 | ) | 6 | (209 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Income taxes |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
(565 | )% | (370 | )% | (543 | )% | ||||||
|
|
|
|
|
|
|
|||||||
| (1) | Includes 6%, 4% and 2% in cost of product revenues to related parties for the years ended December 31, 2014 2013, and 2012, respectively. See Note 18 of our accompanying Notes to Consolidated Financial Statements included in Part II-Item 8-“Financial Statements and Supplementary Data” in this Annual Report on Form 10-K for further discussion. |
Comparison of the Years Ended December 31, 2014, 2013 and 2012
Product Revenues
| YEAR ENDED DECEMBER 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| As restated | ||||||||||||
| (Dollars in thousands) | ||||||||||||
| Product revenues |
$ | 7,750 | $ | 7,588 | $ | 6,777 | ||||||
| % of total revenues |
85 | % | 90 | % | 95 | % | ||||||
64
Table of Contents
Our product revenues increased by approximately $0.2 million, or 2%, in 2014 compared to 2013 and $0.8 million, or 12%, in 2013 compared to 2012. Beginning in 2013 and continuing into 2014, as a result of additional terms being offered to customers, revenue was deferred for certain sales transactions and is being recognized on a sell-through basis compared to 2012 where all sales transactions were recognized on a sell-in basis. Product revenues increased in 2014 compared to 2013 primarily due to the recognition of revenue that was deferred in 2013. The extended drought in California and other markets has reduced demand for our products as fewer acres are planted, In addition, the departures of our former chief operating officer and significant members of our sales staff in the third quarter of 2014 and subsequent turnover in our sales and marketing department disrupted the 2014 launch of Venerate as well as growth in sales of our other commercialized products. Increases were partially offset by a decrease in sales of Grandevo and Regalia as a result of the extended drought in California and other markets which has reduced demand for our products as fewer acres are planted, the Florida citrus market experiencing a shortened bloom cycle resulting from changes in weather patterns and fewer pesticide and plant health products being used.
Product revenues increased in 2013 compared to 2012 due to increased acceptance of our products, with Grandevo representing an increased percentage of total sales as we launched the most popular formulation of Grandevo in the summer of 2012. In addition, increases in 2013 were partially offset by certain transactions being recognized on a sell-through basis rather than on a sell-in basis resulting in the deferral of revenue in 2013. There were no transactions recognized on a sell-through basis in 2012.
License Revenues
| YEAR ENDED DECEMBER 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| (Dollars in thousands) | ||||||||||||
| License revenues |
$ | 232 | $ | 193 | $ | 179 | ||||||
| % of total revenues |
2 | % | 2 | % | 2 | % | ||||||
License revenues related to certain strategic collaboration and distribution agreements increased by 20% in 2014 compared to 2013 and increased by 8% in 2013 compared to 2012 but do not comprise a significant portion of our total revenues.
Related Party Revenues
| YEAR ENDED DECEMBER 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| As restated | ||||||||||||
| (Dollars in thousands) | ||||||||||||
| Related party revenues |
$ | 1,154 | $ | 665 | $ | 184 | ||||||
| % of total revenues |
13 | % | 8 | % | 3 | % | ||||||
Related party revenues increased by approximately $0.5 million, or 74%, in 2014 compared to 2013 and $0.5 million, or 261%, in 2013 compared to 2012. Related party revenues increased in 2014 compared to 2013 and in 2013 compared to 2012 due to increased product sales to The Tremont Group, Inc. as they increased sales of our product to a larger number of end users as a result of increased acceptance of our products.
Cost of Product Revenues and Gross Profit (Loss)
| YEAR ENDED DECEMBER 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| As restated | ||||||||||||
| (Dollars in thousands) | ||||||||||||
| Costs of product revenues |
$ | 9,438 | $ | 7,243 | $ | 4,333 | ||||||
| % of total revenues |
103 | % | 86 | % | 61 | % | ||||||
| Gross profit (loss) |
$ | (302 | ) | $ | 1,203 | $ | 2,807 | |||||
| % of total revenues (gross margin) |
(3 | )% | 14 | % | 39 | % | ||||||
65
Table of Contents
Our cost of product revenues increased by $2.2 million, or 30%, in 2014 compared to 2013 and $2.9 million, or 67%, in 2013 as compared to 2012. Our gross margins decreased from 14% to a negative 3% in 2014 compared to 2013 and from 39% to 14% in 2013 compared to 2012. Cost of product revenue increased and gross margin decreased in 2014 compared to 2013, primarily due to adjustments to the Company’s inventory reserve of $0.7 million for inventory that may not be used prior to expiration as a result of lower production and sales forecasts, a $0.9 million write-off of inventory primarily due identification of inventory that would not suitable for sale in future periods either due to the inventory not passing quality inspection or the efficacy had declined, and a $0.3 million write-down of the carrying value of inventory to net realizable value. In addition, our manufacturing plant began full-scale production in 2014, and as a result of actual utilization of the plant being less than what is considered normal capacity, $0.9 million in operating costs of the manufacturing plant were recorded to costs of product revenues in 2014 and not allocated to the cost of inventory. Cost of product revenues and gross margin were also negatively impacted in 2013 by a $0.2 million write-down of the carrying value of inventory to net realizable value, a $0.2 million write-off of inventory primarily due to the identification of inventory that was not suitable for sale and a $0.2 million write-down of the carrying value of deferred cost of product revenues to net realizable value.
Cost of product revenues increased and gross margin decreased in 2013 compared to 2012, in each case primarily due to a change in product mix, with Grandevo representing an increased percentage of total sales as we launched the most popular formulation of Grandevo in the summer of 2012 along with increased product acceptance leading to an overall increase in sales and cost of product revenues. Since Grandevo is early in its life cycle, our gross margins have been negatively affected. However, we expect to see a gradual increase in gross margin over the life cycle of each of our products, including Grandevo, as we improve production processes, gain efficiencies and increase product yields. Cost of product revenues and gross margin were also negatively impacted in 2013 by inventory write-offs as noted above. Cost of product revenues and gross margin were also negatively impacted in 2012 by a $0.9 million write-off of an early formulation of our Zequanox line of products that was not suitable for sale.
Research, Development and Patent Expenses
| YEAR ENDED DECEMBER 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| As restated | ||||||||||||
| (Dollars in thousands) | ||||||||||||
| Research, development and patent expenses |
$ | 19,281 | $ | 17,905 | $ | 12,741 | ||||||
| % of total revenues |
211 | % | 212 | % | 178 | % | ||||||
Research, development and patent expense increased by $1.4 million, or 8%, in 2014 compared to 2013 and $5.2 million, or 41%, in 2013 compared to 2012. Research, development and patent expense was greater in 2014 compared to 2013 primarily due to an increase of $1.1 million in employee-related expenses, which consisted primarily of salaries, wages, severance and share-based compensation, $0.5 million in direct testing costs, $0.1 million in depreciation and $0.2 million in increased rent. This was offset by a decrease of $0.1 million in outside services, $0.2 million in supplies and materials and $0.2 million in travel related expenses.
Research, development and patent expense increased in 2013 compared to 2012 primarily due to an increase of $2.4 million in employee-related expenses, which consisted primarily of salaries, wages and share-based compensation, $1.5 million in direct testing costs, $0.4 million in outside services, $0.3 million in depreciation, a reduction of $0.2 million in grants received, and $0.3 million in travel and general costs.
Non-Cash Charge Associated with a Convertible Note
| YEAR ENDED DECEMBER 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| (Dollars in thousands) | ||||||||||||
| Non-cash charge associated with a convertible note |
$ | — | $ | — | $ | 3,610 | ||||||
| % of total revenues |
— | % | — | % | 51 | % | ||||||
66
Table of Contents
This one time charge was associated with the issuance of a convertible note during 2012 for which the estimated fair value at the date of issuance was greater than the proceeds received from the convertible note. Because the holder of this convertible note was one of our preferred stockholders and was an affiliate of one of our distributors as of the date of issuance, we recorded $0.3 million of the expense as a reduction to the revenues associated with the affiliated distributor from inception through the date of issuance, and the remaining $3.6 million was recorded in operating expenses as a non-recurring non-cash charge associated with a convertible note.
Selling, General and Administrative Expenses
| YEAR ENDED DECEMBER 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| As restated | ||||||||||||
| (Dollars in thousands) | ||||||||||||
| Selling, general and administrative expenses |
$ | 28,950 | $ | 15,017 | $ | 10,294 | ||||||
| % of total revenues |
317 | % | 178 | % | 144 | % | ||||||
Selling, general and administrative expense increased by $13.9 million, or 93%, in 2014 compared to 2013 and $4.7 million, or 46%, in 2013 compared to 2012. Selling, general and administrative expense increased in 2014 compared to 2013 primarily due to an accrual of $1.8 million in 2014 for estimated penalties in connection with the SEC investigation and a $6.7 million increase in outside services, primarily related to approximately $5.8 million in accounting, consulting, and legal fees incurred as a result of the Audit Committee investigation. Employee related expenses, which consisted primarily of salaries, wages, and share based compensation also contributed to $3.2 million of the increase. An additional $1.4 million in startup and operating costs were incurred associated with the manufacturing facility not included in inventory or cost of product revenues, which commenced full-scale inventory production in 2014. General costs increased $0.3 million, primarily resulting from increasing costs associated with being a public company after the IPO in August 2013. Fixed expenses increased $0.4 million, primarily associated with increased rent and depreciation associated with the new corporate headquarters and lab space. In addition, travel and supplies and materials increased $0.2 million.
Selling, general and administrative expense increased in 2013 compared to 2012 primarily due to an increase of $2.3 million in employee-related expenses, driven by increased headcount, which primarily related to salaries, wages and share-based compensation and $0.4 million relating to a transition agreement with our former Chief Financial Officer. In addition, $1.4 million of the increase was attributable to outside services such as consulting and audit fees, as well as other professional services, $0.2 million was attributable to travel expenses and $0.4 million to other costs including rent, depreciation, supplies and materials.
Other Income (Expense), Net
| YEAR ENDED DECEMBER 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| As restated | ||||||||||||
| (Dollars in thousands) | ||||||||||||
| Interest income |
$ | 59 | $ | 49 | $ | 16 | ||||||
| Interest expense |
(2,907 | ) | (6,056 | ) | (2,466 | ) | ||||||
| Change in estimated fair value of financial instruments |
— | 6,717 | (12,461 | ) | ||||||||
| Gain on extinguishment of debt |
— | 49 | — | |||||||||
| Other expense , net |
(278 | ) | (282 | ) | (45 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total other income (expense), net |
$ | (3,126 | ) | $ | 477 | $ | (14,956 | ) | ||||
|
|
|
|
|
|
|
|||||||
Interest income, consisting primarily of interest on cash and short-term investments, was largely unchanged. Interest expense decreased in 2014 compared to 2013 primarily as a result of the conversion of convertible notes to common
67
Table of Contents
stock upon completion of the initial public offering in August 2013. This was partially offset by an increase in interest as a result of the issuance of a $10.0 million variable rate promissory note in June 2014. Interest expense increased in 2013 compared to 2012 as a result of the issuance a $3.0 million convertible note in May 2013, for which we incurred $1.2 million of interest expense for the year ended December 31, 2013 as a result of the excess in the $4.2 million estimated fair value of the convertible note on the date of issuance compared to the cash received. In addition, in connection with the repayment of a promissory note in January 2013 which had been issued in April 2012, we wrote-off the unamortized debt discount totaling $0.8 million and incurred an early termination fee of $0.3 million, which were recorded to interest expense during the year ended December 31, 2013. The remainder of the change in interest expense was due to increased borrowings under notes payable, convertible notes and capital lease agreements.
The change in the estimated fair value of financial instruments was associated with outstanding warrants and convertible notes issued in 2012 and 2013. We issued $30.6 million in convertible notes, warrants to purchase 0.2 million shares of Series C convertible preferred stock and warrants for the issuance of a variable number of shares of common stock based on a fixed monetary amount during that time. This was offset by the decrease in convertible notes of $1.25 million in May 2013 in connection with the conversion of a portion of a convertible note in exchange for a promissory note. Upon the closing of the IPO, all shares of our outstanding convertible preferred stock and convertible notes automatically converted into shares of common stock and outstanding warrants to purchase convertible preferred stock and certain warrants to purchase common stock were exercised for shares of common stock. Accordingly, we ceased incurring the interest expense and change in estimated fair value of financial instruments associated with the convertible preferred stock and convertible notes. See the Notes to Consolidated Financial Statements included in Part II-Item 8-“Financial Statements and Supplementary Data” in this Annual Report on Form 10-K for further discussion.
Other expense for the year ended December 31, 2014 and 2013 primarily reflects a losses on disposal of fixed assets in the amount of $0.2 million in each year. The remainder of other expense related to foreign currency transaction expenses incurred during the year.
68
Table of Contents
The following table sets forth certain statements of operations data as a percentage of total revenues:
| THREE MONTHS ENDED SEPTEMBER 30, |
NINE MONTHS ENDED SEPTEMBER 30, |
|||||||||||||||
| 2014 | 2013 | 2014 | 2013 | |||||||||||||
| As restated | As restated | |||||||||||||||
| (Dollars in thousands) | (Dollars in thousands) | |||||||||||||||
| Revenues: |
||||||||||||||||
| Product |
85 | % | 82 | % | 85 | % | 89 | % | ||||||||
| License |
3 | 3 | 2 | 2 | ||||||||||||
| Related Party |
12 | 15 | 13 | 9 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total revenues |
100 | 100 | 100 | 100 | ||||||||||||
| Cost of product revenues (1) |
159 | 82 | 100 | 74 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Gross profit (loss) |
(59 | ) | 18 | — | 26 | |||||||||||
| Operating expenses: |
||||||||||||||||
| Research, development and patent |
219 | 231 | 166 | 189 | ||||||||||||
| Selling, general and administrative |
336 | 234 | 244 | 170 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
555 | 465 | 410 | 359 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss from operations |
(614 | ) | (447 | ) | (410 | ) | (333 | ) | ||||||||
| Other income (expense) |
||||||||||||||||
| Interest income |
1 | 1 | 1 | — | ||||||||||||
| Interest expense |
(35 | ) | (58 | ) | (28 | ) | (87 | ) | ||||||||
| Change in estimated fair value of financial instruments |
— | 193 | — | 109 | ||||||||||||
| Gain on extinguishment of debt |
— | — | — | 1 | ||||||||||||
| Other income (expense) |
(6 | ) | (3 | ) | (3 | ) | (1 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total other income (expense), net |
(40 | ) | 133 | (30 | ) | 22 | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Income taxes |
— | — | — | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
(654 | )% | (314 | )% | (440 | )% | (311 | )% | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| (1) | Includes cost of product revenues to related parties of 10% and 9% for the three months ended September 30, 2014 and 2013, respectively, and 6% and 5% for the nine months ended September 30, 2014 and 2013, respectively. See Note 18 of our accompanying Notes to Consolidated Financial Statements included in Part II-Item 8-“Financial Statements and Supplementary Data” in this Annual Report on Form 10-K for further discussion. |
Comparison of Three Months Ended September 30, 2014 and 2013
Product Revenues
| THREE MONTHS ENDED SEPTEMBER 30, |
||||||||
| 2014 | 2013 | |||||||
| As restated | ||||||||
| (Dollars in thousands) | ||||||||
| Product revenues |
$ | 1,881 | $ | 1,589 | ||||
| % of total revenues |
85 | % | 82 | % | ||||
Product revenues increased by approximately $0.3 million, or 18%. Beginning in 2013 and continuing into 2014, as a result of additional terms being offered to customers, revenue was deferred for certain sales transactions and is being recognized on a sell-through basis compared to 2012 where all sales transactions were recognized on a sell-in basis. Product revenues increased in 2014 compared to 2013 primarily due to an increase the amount of revenue recognized during the three months ended September 30, 2014 that was deferred in prior periods. This was offset by a decrease in the amount of shipments to customers in the three months ended September 30, 2014
69
Table of Contents
compared to the same period in 2013 primarily as a result of the departures of our former chief operating officer and significant members of our sales and marketing staff in the third quarter of 2014 which disrupted the 2014 launch of Venerate as well as growth in sales of our other commercialized products.
License Revenues
| THREE MONTHS ENDED SEPTEMBER 30, |
||||||||
| 2014 | 2013 | |||||||
| (Dollars in thousands) | ||||||||
| License revenues |
$ | 65 | $ | 48 | ||||
| % of total revenues |
3 | % | 3 | % | ||||
License revenues related to certain strategic collaboration and distribution agreements increased by 35% in the three months ended September 30, 2014 compared to the same period in 2013, but do not comprise a significant portion of our total revenues.
Related Party Revenues
| THREE MONTHS ENDED SEPTEMBER 30, |
||||||||
| 2014 | 2013 | |||||||
| As restated | ||||||||
| (Dollars in thousands) | ||||||||
| Related party revenues |
$ | 254 | $ | 296 | ||||
| % of total revenues |
12 | % | 15 | % | ||||
Related party revenues decreased by less than $0.1 million in the three months ended September 30, 2014 compared to the three months ended September 30, 2013 as we continue to recognize revenue upon resale of products by the Tremont Group, Inc. to its customers.
Cost of Product Revenues and Gross Profit (Loss)
| THREE MONTHS ENDED SEPTEMBER 30, |
||||||||
| 2014 | 2013 | |||||||
| As restated | ||||||||
| (Dollars in thousands) | ||||||||
| Cost of product revenues |
$ | 3,502 | $ | 1,594 | ||||
| % of total revenues |
159 | % | 82 | % | ||||
| Gross profit (loss) |
$ | (1,302 | ) | $ | 339 | |||
| % of total revenues |
(59 | )% | 18 | % | ||||
Cost of product revenues increased by $1.9 million, or 120% in the three months ended September 30, 2014 compared to the three months ended September 30, 2013. Gross margin decreased from 18% in the three months ended September 30, 2013 to a negative 59% in the three months ended September 30, 2014. Cost of product revenue increased and gross margin decreased in 2014 primarily due to adjustments to the Company’s inventory reserve of $0.5 million for inventory that may not be used prior to expiration as a result of lower production and sales forecasts, a $0.6 million write-off of inventory primarily due to the identification of inventory that was not suitable for sale and a $0.2 million write-down of the carrying value of inventory to net realizable value. In addition, our manufacturing plant began full-scale production in 2014, and as a result of actual utilization of the plant being less than what is considered normal capacity, $0.3 million in operating costs of the manufacturing plant were recorded to costs of product revenues
70
Table of Contents
in 2014 and not allocated to the cost of inventory. Cost of product revenues and gross margin were also negatively impacted in 2013 by a $0.2 million write down of the carrying value of deferred cost of product revenues to net realizable value.
Research, Development and Patent Expenses
| THREE MONTHS ENDED SEPTEMBER 30, |
||||||||
| 2014 | 2013 | |||||||
| (Dollars in thousands) | ||||||||
| Research, development and patent expenses |
$ | 4,817 | $ | 4,454 | ||||
| % of total revenues |
219 | % | 231 | % | ||||
Research, development and patent expenses increased by approximately $0.4 million, or 8%, primarily due to an increase of $0.2 million in direct research and development testing, an increase of $0.1 million in fixed expenses primarily associated with depreciation, an increase of $0.1 million associated with increased general and outside services expenses primarily associated with consulting services.
Selling, General and Administrative Expenses
| THREE MONTHS ENDED SEPTEMBER 30, |
||||||||
| 2014 | 2013 | |||||||
| As restated | ||||||||
| (Dollars in thousands) | ||||||||
| Selling, general and administrative expenses |
$ | 7,394 | $ | 4,527 | ||||
| % of total revenues |
336 | % | 234 | % | ||||
Selling, general and administrative expenses increased by approximately $2.9 million, or 63%, due primarily to an increase of $1.1 million in outside services, primarily related to accounting, consulting, and legal fees incurred as a result of the Audit Committee’s independent investigation, and an accrual in 2014 of $1.8 million for estimated penalties in connection with the SEC investigation.
Other Income (Expense), Net
| THREE MONTHS ENDED SEPTEMBER 30, |
||||||||
| 2014 | 2013 | |||||||
| As restated | ||||||||
| (Dollars in thousands) | ||||||||
| Interest income |
$ | 21 | $ | 24 | ||||
| Interest expense |
(769 | ) | (1,122 | ) | ||||
| Change in estimated fair value of financial instruments |
— | 3,730 | ||||||
| Other expense, net |
(139 | ) | (67 | ) | ||||
|
|
|
|
|
|||||
| Total other income (expense), net |
$ | (887 | ) | $ | 2,565 | |||
|
|
|
|
|
|||||
Interest expense decreased primarily due to the conversion of convertible notes into shares of our common stock immediately following the completion of the IPO in August 2013. Accordingly, we ceased to incur the interest expense associated with these convertible notes. This was partially offset by an increase in interest expense as we issued promissory notes in the amount of $10.0 million in June 2014.
The change in the estimated fair value of financial instruments was associated with outstanding warrants and convertible notes issued in 2012 and 2013. Upon the closing of the IPO, all shares of our outstanding convertible
71
Table of Contents
preferred stock and convertible notes automatically converted into shares of common stock and outstanding warrants to purchase convertible preferred stock and certain warrants to purchase common stock were exercised for shares of common stock. Accordingly, we ceased to incur the interest expense and change in estimated fair value of financial instruments associated with the convertible preferred stock and convertible notes.
Comparison of Nine Months Ended September 30, 2014 and 2013
Product Revenues
| NINE MONTHS ENDED SEPTEMBER 30, |
||||||||
| 2014 | 2013 | |||||||
| As restated | ||||||||
| (Dollars in thousands) | ||||||||
| Product revenues |
$ | 6,812 | $ | 5,473 | ||||
| % of total revenues |
85 | % | 89 | % | ||||
Product revenues increased by approximately $1.3 million, or 24%. Beginning in 2013 and continuing into 2014, as a result of additional terms being offered to customers, revenue was deferred for certain sales transactions and is being recognized on a sell-through basis compared to 2012 where all sales transactions were recognized on a sell-in basis. Product revenues increased in 2014 compared to 2013 primarily due to an increase the amount of revenue recognized during the nine months ended September 30, 2014 that was deferred in prior periods. This was offset by a decrease in the amount of shipments to customers in the nine months ended September 30, 2014 compared to the same period in 2013 primarily as a result of the departures of our former chief operating officer and significant members of our sales and marketing staff in the third quarter of 2014 which disrupted the 2014 launch of Venerate as well as growth in sales of our other commercialized products.
License Revenues
| NINE MONTHS ENDED SEPTEMBER 30, |
||||||||
| 2014 | 2013 | |||||||
| (Dollars in thousands) | ||||||||
| License revenues |
$ | 161 | $ | 144 | ||||
| % of total revenues |
2 | % | 2 | % | ||||
License revenues related to certain strategic collaboration and distribution agreements increased by 12% in the nine months ended September 30, 2014 and 2013, but do not comprise a significant portion of our total revenues.
Related Party Revenues
| NINE MONTHS ENDED SEPTEMBER 30, |
||||||||
| 2014 | 2013 | |||||||
| As restated | ||||||||
| (Dollars in thousands) | ||||||||
| Related party revenues |
$ | 1,065 | $ | 555 | ||||
| % of total revenues |
13 | % | 9 | % | ||||
Related party revenues increased by $0.5 million, or 92%, in the nine months ended September 30, 2014 compared to the nine months ended September 30, 2013 as we continue to recognize revenue upon resale of products by the Tremont Group, Inc. to its customers.
72
Table of Contents
Cost of Product Revenues and Gross Profit (Loss)
| NINE MONTHS ENDED SEPTEMBER 30, |
||||||||
| 2014 | 2013 | |||||||
| As restated | ||||||||
| (Dollars in thousands) | ||||||||
| Cost of product revenues |
$ | 8,006 | $ | 4,593 | ||||
| % of total revenues |
100 | % | 74 | % | ||||
| Gross profit (loss) |
$ | 32 | $ | 1,579 | ||||
| % of total revenues |
0 | % | 26 | % | ||||
Our cost of product revenues increased by $3.4 million, or 74% in the nine months ended September 30, 2014 compared to the nine months ended September 30, 2013. Our gross margin decreased from 26% in the nine months ended September 30, 2013 to 0% in the nine months ended September 30, 2014. Cost of product revenue increased and gross margin decreased, primarily due to adjustments to our inventory reserve of $0.7 million for inventory that may not be used prior to expiration as a result of lower production and sales forecasts, a $0.8 million write-off of inventory primarily due to the identification of inventory that was not suitable for sale and a $0.3 million write-down of the carrying value of inventory to net realizable value. In addition, our manufacturing plant began full-scale production in 2014, and as a result of actual utilization of the plant being less than what is considered normal capacity, $0.3 million in operating costs of the manufacturing plant were recorded to costs of product revenues in 2014 and not allocated to the cost of inventory. Cost of product revenues and gross margin were also negatively impacted in 2013 by a $0.2 million write down of the carrying value of deferred cost of product revenues to net realizable value.
Research, Development and Patent Expenses
| NINE MONTHS ENDED SEPTEMBER 30, |
||||||||
| 2014 | 2013 | |||||||
| (Dollars in thousands) | ||||||||
| Research, development and patent expenses |
$ | 13,378 | $ | 11,678 | ||||
| % of total revenues |
166 | % | 189 | % | ||||
Research, development and patent expense increased by $1.7 million, or 14%, in the nine months ended September 30, 2014 compared to the nine months ended September 30, 2013. Research, development and patent expense was greater in 2014 compared to 2013 primarily due to an increase of $1.2 million in employee-related expenses, which consisted primarily of salaries, wages, severance and share-based compensation, $0.3 million in fixed expenses consisting primarily of rent and depreciation, $0.2 million in direct testing costs.
Selling, General and Administrative Expenses
| NINE MONTHS ENDED SEPTEMBER 30, |
||||||||
| 2014 | 2013 | |||||||
| As restated | ||||||||
| (Dollars in thousands) | ||||||||
| Selling, general and administrative expenses |
$ | 19,636 | $ | 10,491 | ||||
| % of total revenues |
244 | % | 170 | % | ||||
Selling, general and administrative expense increased by $8.9 million, or 85%, in the nine months ended September 30, 2014 compared to the nine months ended September 30, 2013 primarily due to an increase of $3.2 million employee-related expenses, primarily related to salaries, wages and severance-related costs, an increase in of $1.8 million in outside services, primarily related to accounting, consulting, and legal fees incurred as a result of the Audit Committee’s independent investigation, an accrual of $1.8 million in 2014 for estimated penalties in
73
Table of Contents
connection with the SEC investigation, and increase of $1.4 million associated with the manufacturing plant prior to beginning full-scale manufacturing in the second quarter of 2014 which were not recorded to inventory or cost of product revenues, an increase of $0.3 million in fixed expenses primarily related to insurance as a result of becoming a public company in August 2013, an increase of $0.3 million in general expenses primarily resulting from reporting costs associated with being a public company, an increase of $0.3 million in travel expenses primarily related to airfare and lodging, and an increase of $0.1 million in supplies and materials.
Other Income (Expense), Net
| NINE MONTHS ENDED SEPTEMBER 30, |
||||||||
| 2014 | 2013 | |||||||
| As restated | ||||||||
| (Dollars in thousands) | ||||||||
| Interest income |
$ | 42 | $ | 25 | ||||
| Interest expense |
(2,238 | ) | (5,341 | ) | ||||
| Change in estimated fair value of financial instruments |
— | 6,717 | ||||||
| Gain on extinguishment of debt |
— | 49 | ||||||
| Other expense, net |
(246 | ) | (81 | ) | ||||
|
|
|
|
|
|||||
| Total other expense, net |
$ | (2,442 | ) | $ | 1,369 | |||
|
|
|
|
|
|||||
Interest expense decreased primarily due to the conversion of convertible notes into shares of our common stock immediately following the completion of the IPO in August 2013. Accordingly, we ceased to incur the interest expense associated with these convertible notes. In addition, interest expense decreased as a result of issuing a $3.0 million convertible note in May 2013, and recording $1.2 million in interest expense, during the nine months ended September 30, 2013 as a result of the excess in the $4.2 million estimated fair value of the convertible note on the date of issuance compared with the cash received. This was partially offset by an increase in interest expense as we issued promissory notes in the amount of $10.0 million in June 2014.
The change in the estimated fair value of financial instruments was associated with outstanding warrants and convertible notes issued in 2012 and 2013. We issued $30.6 million in convertible notes, warrants to purchase 0.2 million shares of Series C convertible preferred stock and warrants for the issuance of a variable number of shares of common stock based on a fixed monetary amount during that time. This was offset by the decrease in convertible notes of $1.25 million in May 2013 in connection with the conversion of a portion of a convertible note in exchange for a promissory note. Upon the closing of the IPO, all shares of our outstanding convertible preferred stock and convertible notes automatically converted into shares of common stock and outstanding warrants to purchase convertible preferred stock and certain warrants to purchase common stock were exercised for shares of common stock. Accordingly, we ceased incurring the interest expense and change in estimated fair value of financial instruments associated with the convertible preferred stock and convertible notes.
Seasonality and Quarterly Results
Sales of our crop protection products have been, and are generally expected to be, seasonal. Regalia and Grandevo, which accounted for the majority of revenues in recent periods, have historically been sold and applied to crops in greater quantity in the second and fourth quarters, with lowest sales in third quarter when there is the lowest pest and disease pressure in the United States. We expect this trend to continue in the future, but this seasonality could be reduced, or we could experience seasonality in different periods than anticipated, as a result of various factors, including if we expand into new geographical territories or introduce new products with different applicable growing seasons, or if a more significant component of our revenue becomes comprised of sales of Zequanox, which has a separate seasonal sales cycle compared to our crop protection products.
74
Table of Contents
Notwithstanding anticipated seasonality, we expect substantial fluctuation in sales year over year and quarter over quarter, and seasonality could be reduced or enhanced, as a result of a number of variables on which sales of our products are dependent. Weather conditions, natural disasters and other factors affect planting and growing seasons and incidence of pests and plant disease, and accordingly affect decisions by our distributors, direct customers and end users about the types and amounts of pest management and plant health products to purchase and the timing of use of such products. In addition, disruptions that cause delays by growers in harvesting or planting can result in the movement of orders to a future quarter, which would negatively affect the quarter and cause fluctuations in our operating results. For example, late snows and cold temperatures in the Midwestern and Eastern United States in the first and second quarters of 2014 delayed planting and pesticide and plant health applications. Customers also may purchase large quantities of our products in a particular quarter to store and use over long periods of time or time their purchases to manage their inventories, which may cause significant fluctuations in our operating results for a particular quarter or year, and low commodity prices may discourage growers from purchasing our products in an effort to reduce their costs and increase their margins for a growing season.
The level of seasonality in our business overall is difficult to evaluate as a result of our relatively early stage of development, our relatively limited number of commercialized products, our expansion into new geographical territories, the introduction of new products and the timing of introductions of new formulations and products. It is possible that our business may be more seasonal, or experience seasonality in different periods, than anticipated. For example, if sales of Zequanox become a more significant component of our revenue, the separate seasonal sales cycles could cause further shifts in our quarterly revenue. Other factors may also contribute to the unpredictability of our operating results, including the size and timing of significant distributor transactions, the delay or deferral of use of our products and the fiscal or quarterly budget cycles of our distributors, direct customers and end users. Customers may purchase large quantities of our products in a particular quarter to store and use over long periods of time or time their purchases to manage their inventories, which may cause significant fluctuations in our operating results for a particular quarter or year.
75
Table of Contents
The following tables set forth our unaudited quarterly consolidated statements of operations data in dollars and as a percentage of total revenues for each of the four quarters covering fiscal years 2013 and 2014. We have prepared the quarterly consolidated statements of operations data on a basis consistent with the audited consolidated financial statements included in Part II-Item 8-“Financial Statements and Supplementary Data” in this Annual Report on Form 10-K. In the opinion of management, the financial information reflects all adjustments, consisting only of normal recurring adjustments, which we consider necessary for a fair presentation of this data. This information should be read in connection with the audited consolidated financial statements and related notes included in Part II-Item 8-“Financial Statements and Supplementary Data” in this Annual Report on Form 10-K. The results of historical periods are not necessarily indicative of the results of operations for any future period.
Fiscal Year 2013:
| MARCH 31, 2013 |
JUNE 30, 2013 |
SEPTEMBER 30, 2013 |
DECEMBER 31, 2013 |
|||||||||||||
| As restated(1) | As restated(1) | As restated(1) | As restated(1) | |||||||||||||
| (In thousands) | ||||||||||||||||
| (Unaudited) | ||||||||||||||||
| Revenues: |
||||||||||||||||
| Product |
$ | 2,089 | $ | 1,795 | $ | 1,589 | $ | 2,115 | ||||||||
| License |
48 | 48 | 48 | 49 | ||||||||||||
| Related party |
54 | 205 | 296 | 110 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total revenues |
2,191 | 2,048 | 1,933 | 2,274 | ||||||||||||
| Cost of product revenues (2) |
1,414 | 1,585 | 1,594 | 2,650 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Gross profit (loss) |
777 | 463 | 339 | (376 | ) | |||||||||||
| Operating expenses: |
||||||||||||||||
| Research, development and patent |
3,283 | 3,941 | 4,454 | 6,227 | ||||||||||||
| Selling, general and administrative |
2,847 | 3,117 | 4,527 | 4,526 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
6,130 | 7,058 | 8,981 | 10,753 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss from operations |
(5,353 | ) | (6,595 | ) | (8,642 | ) | (11,129 | ) | ||||||||
| Other income (expense): |
||||||||||||||||
| Interest income |
1 | — | 24 | 24 | ||||||||||||
| Interest expense |
(1,968 | ) | (2,251 | ) | (1,122 | ) | (715 | ) | ||||||||
| Change in estimated fair value of financial instruments |
(3,563 | ) | 6,550 | 3,730 | — | |||||||||||
| Gain on extinguishment of debt |
— | 49 | — | — | ||||||||||||
| Other expense, net |
(7 | ) | (7 | ) | (67 | ) | (201 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total other income (expense), net |
(5,537 | ) | 4,341 | 2,565 | (892 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Income taxes |
— | — | — | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
$ | (10,890 | ) | $ | (2,254 | ) | $ | (6,077 | ) | $ | (12,021 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
76
Table of Contents
| MARCH 31, 2013 |
JUNE 30, 2013 |
SEPTEMBER 30, 2013 |
DECEMBER 31, 2013 |
|||||||||||||
| As restated(1) | As restated(1) | As restated(1) | As restated(1) | |||||||||||||
| (Unaudited) | ||||||||||||||||
| Revenues: |
||||||||||||||||
| Product |
95 | % | 88 | % | 82 | % | 93 | % | ||||||||
| License |
2 | 2 | 3 | 2 | ||||||||||||
| Related party |
3 | 10 | 15 | 5 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total revenues |
100 | 100 | 100 | 100 | ||||||||||||
| Cost of product revenues (3) |
64 | 78 | 83 | 117 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Gross profit (loss) |
36 | 22 | 17 | (17 | ) | |||||||||||
| Operating expenses: |
||||||||||||||||
| Research, development and patent |
150 | 192 | 230 | 274 | ||||||||||||
| Selling, general and administrative |
130 | 152 | 234 | 199 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
280 | 344 | 464 | 473 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss from operations |
(244 | ) | (322 | ) | (447 | ) | (490 | ) | ||||||||
| Other income (expense): |
||||||||||||||||
| Interest income |
— | — | 1 | 1 | ||||||||||||
| Interest expense |
(90 | ) | (110 | ) | (58 | ) | (31 | ) | ||||||||
| Change in estimated fair value of financial instruments |
(163 | ) | 320 | 193 | — | |||||||||||
| Gain on extinguishment of debt |
— | 2 | — | — | ||||||||||||
| Other expense, net |
— | — | (3 | ) | (9 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total other income (expense), net |
(253 | ) | 212 | 133 | (39 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Income taxes |
— | — | — | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
(497 | )% | (110 | )% | (314 | )% | (529 | )% | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| (1) | For adjustments related to 2013, see Note 2, Restatement of Previously Issued Consolidated Financial Statements, to the consolidated financial statements included in this Annual Report on Form 10-K. |
| (2) | Includes $0.1 million, $0.2 million and $0.2 million in cost of product revenues to related parties for the quarters ended June 30, 2013, September 30, 2013 and December 31, 2013, respectively. There were no cost of product revenues to related parties for the quarter ended March 31, 2013. See Note 18 of our accompanying Notes to Consolidated Financial Statements included in Part II-Item 8-“Financial Statements and Supplementary Data” of this Annual Report on Form 10-K for further discussion. |
| (3) | Includes 0%, 6%, 9% and 3% in cost of product revenues to related parties for the quarters ended March 31, 2013, June 30, 2013, September 30, 2013 and December 31, 2013, respectively. See Note 18 of our accompanying Notes to Consolidated Financial Statements included in Part II-Item 8-“Financial Statements and Supplementary Data” of this Annual Report on Form 10-K for further discussion. |
77
Table of Contents
Fiscal Year 2014:
| MARCH 31, 2014 |
JUNE 30, 2014 |
SEPTEMBER 30, 2014 |
DECEMBER 31, 2014 |
|||||||||||||
| As restated(1) | As restated(1) | |||||||||||||||
| (In thousands) | ||||||||||||||||
| (Unaudited) | ||||||||||||||||
| Revenues: |
||||||||||||||||
| Product |
$ | 2,054 | $ | 2,877 | $ | 1,881 | $ | 938 | ||||||||
| License |
45 | 51 | 65 | 71 | ||||||||||||
| Related party |
605 | 206 | 254 | 89 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total revenues |
2,704 | 3,134 | 2,200 | 1,098 | ||||||||||||
| Cost of product revenues (2) |
1,793 | 2,711 | 3,502 | 1,432 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Gross profit (loss) |
911 | 423 | (1,302 | ) | (334 | ) | ||||||||||
| Operating expenses: |
||||||||||||||||
| Research, development and patent |
4,297 | 4,264 | 4,817 | 5,903 | ||||||||||||
| Selling, general and administrative |
6,324 | 5,920 | 7,394 | 9,312 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
10,621 | 10,184 | 12,211 | 15,215 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss from operations |
(9,710 | ) | (9,761 | ) | (13,513 | ) | (15,549 | ) | ||||||||
| Other income (expense): |
||||||||||||||||
| Interest income |
10 | 11 | 21 | 17 | ||||||||||||
| Interest expense |
(606 | ) | (863 | ) | (769 | ) | (669 | ) | ||||||||
| Other expense, net |
(9 | ) | (98 | ) | (139 | ) | (32 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total other income (expense), net |
(605 | ) | (950 | ) | (887 | ) | (684 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Income taxes |
— | — | — | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
$ | (10,315 | ) | $ | (10,711 | ) | $ | (14,400 | ) | $ | (16,233 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| MARCH 31, 2014 |
JUNE 30, 2014 |
SEPTEMBER 30, 2014 |
DECEMBER 31, 2014 |
|||||||||||||
| As restated(1) | As restated(1) | |||||||||||||||
| (Unaudited) | ||||||||||||||||
| Revenues: |
||||||||||||||||
| Product |
76 | % | 92 | % | 85 | % | 85 | % | ||||||||
| License |
2 | 2 | 3 | 7 | ||||||||||||
| Related party |
22 | 6 | 12 | 8 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total revenues |
100 | 100 | 100 | 100 | ||||||||||||
| Cost of product revenues (3) |
66 | 87 | 159 | 130 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Gross profit (loss) |
34 | 13 | (59 | ) | (30 | ) | ||||||||||
| Operating expenses: |
||||||||||||||||
| Research, development and patent |
159 | 136 | 219 | 538 | ||||||||||||
| Selling, general and administrative |
234 | 188 | 336 | 848 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
393 | 324 | 555 | 1,386 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss from operations |
(359 | ) | (311 | ) | (614 | ) | (1,416 | ) | ||||||||
| Other income (expense): |
||||||||||||||||
| Interest income |
— | — | 1 | 2 | ||||||||||||
| Interest expense |
(22 | ) | (28 | ) | (35 | ) | (61 | ) | ||||||||
| Other expense, net |
— | (3 | ) | (6 | ) | (3 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total other income (expense), net |
(22 | ) | (31 | ) | (40 | ) | (62 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Income taxes |
— | — | — | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
(381 | )% | (342 | )% | (654 | )% | (1,478 | )% | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
78
Table of Contents
| (1) | For adjustments related to 2014, see Note 2, Restatement of Previously Issued Consolidated Financial Statements, to the consolidated financial statements included in this Annual Report on Form 10-K. |
| (2) | Includes $0.2 million, $0.1 million, $0.2 million and less than $0.1 million in cost of product revenues to related parties for the quarters ended March 31, 2014, June 30, 2014, September 30, 2014 and December 31, 2014, respectively. See Note 18 of our accompanying Notes to Consolidated Financial Statements included in Part II, Item 8, “Financial Statements and Supplementary Data” of this Annual Report on Form 10-K for further discussion. |
| (3) | Includes 6%, 4%, 10% and 4% in cost of product revenues to related parties for the quarters ended March 31, 2014, June 30, 2014, September 30, 2014 and December 31, 2014, respectively. See Note 18 of our accompanying Notes to Consolidated Financial Statements included in Part II-Item 8-“Financial Statements and Supplementary Data” in this Annual Report on Form 10-K for further discussion. |
Liquidity and Capital Resources
From our inception until the IPO in August 2013, our operations have been financed primarily by net proceeds from the private placements of convertible preferred stock, convertible notes, promissory notes, term loans, as well as proceeds from the sale of our products and payments under strategic collaboration and distribution agreements and government grants.
In the IPO, we issued 5.5 million shares of our common stock (inclusive of 0.7 million shares of common stock sold upon the exercise of the underwriters’ option to purchase additional shares). The public offering price of the shares sold in the offering was $12.00 per share. The total gross proceeds from the offering to us were $65.6 million, and after deducting underwriting discounts and commissions and offering expenses payable by us, the aggregate net proceeds received totaled approximately $56.1 million.
In June 2014, we completed a public offering of 4.6 million shares of our common stock (inclusive of 0.7 million shares of common stock sold upon the exercise of the underwriters’ option to purchase additional shares). The public offering price of the shares sold in the offering was $9.50 per share. The total gross proceeds from the offering to us were $43.5 million, and after deducting underwriting discounts and commissions and offering expenses payable by us, the aggregate net proceeds received totaled $39.9 million. In addition, in June 2014, we borrowed $10.0 million pursuant to a promissory note with a bank. This note requires us to maintain a deposit balance with the lender of $1.6 million. In addition, until we provide documentation that proceeds were used for construction of the manufacturing plant, proceeds from the loan will be maintained in a restricted deposit account. As of September 30, 2015, we had $1.9 million remaining in the restricted deposit account. In August 2015, we also borrowed $40.0 million pursuant to senior secured promissory notes with lenders. These notes require us to maintain a cash and cash equivalents balance of $15.0 million.
As of December 31, 2014, our cash and cash equivalents totaled $35.3 million. In August 2015, we issued and sold senior secured promissory notes to three affiliated lenders in the aggregate principal amount of $40.0 million. The notes bear interest at a rate of 8% per annum payable semi-annually on June 30 or December 31 of each year, commencing on December 31, 2015, with $10 million payable three years from the closing, $10 million payable four years from the closing, and $20 million payable five years from the closing. After this financing, as of September 30, 2015, our cash and cash equivalents totaled $27.8 million and we had restricted cash of $18.4 million in total relating to cash that we are contractually obligated to maintain in accordance with our debt agreements. We believe our current cash and cash equivalents, available proceeds from the August 2015 issuance and sale of $40.0 million in senior secured promissory notes and cash from revenues, will be sufficient to satisfy our liquidity requirements for the next 12 months. However, we may seek additional funding through debt or equity financings that may be used, among other things, to expand our product development and sales and marketing efforts, to increase capacity of our manufacturing facility, to complete strategic transactions and/or for working capital. Adequate funds for this and the other purposes may not be available to us when needed or on acceptable terms, and we may need to raise capital that may not be available on favorable or acceptable terms, if at all. If we cannot raise money when needed, we may have to reduce or slow product development activities, further reduce operating expenses and/or reduce capital investment.
79
Table of Contents
Since our inception, we have incurred significant net losses, and, as of December 31, 2014, we had an accumulated deficit of $157.8 million, and we expect to incur additional losses related to the continued development and expansion of our business. Our liquidity may be negatively impacted as a result of slower than expected adoption of our products. We have certain strategic collaboration and distribution agreements under which we receive payments for the achievement of testing validation, regulatory progress and commercialization events. As of December 31, 2014, we had received an aggregate of $2.9 million in payments and had $0.8 million currently due and payable under these agreements. In addition, there will be an additional $0.5 million in payments due on certain anniversaries of regulatory approval and an additional $1.6 million in payments under these agreements that we could potentially receive if the testing validation, regulatory progress and commercialization events occur.
For the years ended December 31, 2014, 2013 and 2012, we used $13.0 million, $4.0 million and $2.8 million, respectively, in cash to fund capital expenditures. In July 2012, we acquired a manufacturing facility, formerly used as a biodiesel plant. Repurposing of the facility was completed in 2014 and included installation of fermentation tanks and the construction of a dedicated building to house them. In December 2013, we produced the first test batch of Grandevo at this facility and began full-scale production of our products using our own manufacturing capacity in 2014. The facility now accommodates full-scale production of Regalia, which we successfully produced in small-scale in 2013, and full-scale fermentation of Grandevo and Zequanox.
We had the following debt arrangements in place as of December 31, 2014, in each case as discussed below (dollars in thousands):
| DESCRIPTION |
STATED ANNUAL INTEREST RATE |
PRINCIPAL AMOUNT BALANCE (INCLUDING ACCRUED INTEREST) |
PAYMENT/MATURITY | |||||||
| Term Loan (1) |
7.00 | % | $ | 183 | Monthly/April 2016 | |||||
| Promissory Notes (2) |
12.00 | % | $ | 12,575 | Monthly(4)/October 2015 | |||||
| Promissory Note (3) |
5.25 | % | $ | 9,899 | Monthly/June 2036 | |||||
| (1) | See “—Term Loan.” |
| (2) | See “—October 2012 and April 2013 Secured Promissory Notes.” |
| (3) | See “—June 2014 Secured Promissory Note.” |
| (4) | Monthly payments are interest only until maturity. |
Term Loan
In March 2012, we entered into a term loan agreement with Five Star Bank, which replaced our existing revolving line of credit with the bank. Under the term loan agreement, we are obligated to repay the loan at a rate of approximately $12,000 per month through maturity.
Under the terms of the term loan agreement, all of our outstanding debt to Five Star Bank is secured by all of our inventory, chattel paper, accounts receivable, equipment and general intangibles (excluding certain financed equipment and any intellectual property). Among other things, a payment default with respect to each of the promissory notes and the term loan, as well as other events such as a default under other loans or agreements that would materially affect us, constitute events of default. Upon an event of default, Five Star Bank may declare the entire unpaid principal and interest immediately due and payable. This loan was paid off in August 2015.
October 2012 and April 2013 Secured Promissory Notes
In October 2012, we completed the sale of promissory notes in the aggregate principal amount of $7.5 million to 12 lenders in a private placement. In addition, in April 2013, we completed the sale of an additional $4.95 million of promissory notes to 10 lenders in a private placement under an amendment to the note purchase agreement in exchange for $3.7 million in cash and $1.25 million in cancellation of indebtedness under a
80
Table of Contents
previously outstanding convertible note. Maturity, currently October 2015, may be extended in one year increments for a period of no more than two years. In the event the maturity date is extended, the interest rate increases to 13% in the first year of the extension and the note matures in October 2016, and if extended for an additional year thereafter, the interest rate increases to 14% in the second year of extension and the note matures in October 2017. These promissory notes are secured by a security interest in all of our present and future accounts receivable, chattel paper, commercial tort claims, goods, inventory, equipment, personal property, instruments, investment properties, documents, letter of credit rights, deposit accounts, general intangibles, records, real property, appurtenances and fixtures, tenant improvements and intellectual property, which consists of our patents, copyrights and other intangibles.
As of December 31, 2014, we were in breach of our covenants under the agreement as a result of our failure to provide annual financial statements in a timely manner and our being in breach of covenants on the June 2014 Secured Promissory Note as described below. However, in November 2015, the Company received an extension from the lending agent with respect to compliance with the requirements to deliver annual financial statements to the earlier of (i) November 15, 2015 or (ii) such time such financial statements are filed with the SEC. This covenant breach was cured in November 2015, as a result of obtaining this extension and the waiver of certain of its covenants with respect to the June 2014 Secured Promissory Note, as described below.
In August 2015, the terms of the notes were amended, resulting in an increase in the interest rate to 18% effective September 1, 2015 for the remaining term of the notes. The Company also provided written notice in September 2015 to extend the maturity date to October 2, 2017.
June 2014 Secured Promissory Note
In June 2014, we borrowed $10.0 million pursuant to a business loan agreement and promissory note with Five Star Bank which bears interest at prime rate plus 2.00% per annum. The interest rate is subject to change from time to time to reflect changes in the prime rate; however, the interest rate shall not be less than 5.25% or more than the maximum rate allowed by applicable law. If the interest rate increases, the lender, may, at its option, increase the amount of each monthly payment to ensure that the note would be paid in full by the maturity date, increase the amount of each monthly payment to reflect the change in interest rate, increase the number of monthly payments, or keep the monthly payments the same and increase the final payment amount. As of December 31, 2014, the interest rate was 5.25%.
The June 2014 Secured Promissory Note is repayable in monthly payments of $64,390 commencing July 13, 2014, with the final payment due on June 13, 2036. Certain of our deposit accounts and our subsidiary’s inventories, chattel paper, accounts, equipment and general intangibles have been pledged as collateral for the promissory note. We are required to maintain a deposit balance with the lender of $1.6 million, which was recorded as restricted cash included in noncurrent assets. In addition, until we provide documentation that the proceeds were used for construction of the manufacturing plant, proceeds from the loan will be maintained in a restricted deposit account. As of December 31, 2014, we had $1.9 million remaining in this restricted deposit account, which was recorded as restricted cash included in current assets.
We may prepay 20% of the outstanding principal loan balance each year without penalty. A prepayment fee of 10% will be charged if prepayments exceed 20% in the first year, and the prepayment fee will decrease by 1% each year for the first ten years of the loan.
We are required to maintain a current ratio of not less than 1.25-to-1.0, a debt-to-worth ratio of no greater than 4.0-to-1.0 and maintain a loan-to-value ratio of no greater than 70% as determined by the lender. We are also required to comply with certain affirmative and negative covenants under the loan agreement discussed above. In the event of default on the debt, the lender may declare the entire unpaid principal and interest immediately due and payable. As of December 31, 2014, we were in breach of the covenants under the loan agreement as a result of our annual and quarterly reports not being filed within the prescribed time period and our being in breach of
81
Table of Contents
covenants on the October 2012 and April 2013 Secured Promissory Notes, described above. Effective, September 30, 2015, our debt-to-worth ratio was greater than 4.0-to-1.0 as a result of the issuance of $40.0 million in promissory notes in August 2015, described below, increasing our debt and the continuing net loss increasing our accumulated deficit. However, in November 2015, we received a waiver from the lender with respect to compliance with the requirements to as of September 30, 2015, the Company was in breach of its covenants under the notes as the Company was in breach of its covenants under its October 2012 and April 2013 Secured Promissory Notes and June 2014 Secured Promissory Note as discussed above. However, these breaches were cured in November 2015, as a result of the Company obtaining an extension to deliver its annual financial statements with respect to the October 2013 and April 2013 Secured Promissory Notes and the waiver of certain of the Company’s covenants with respect to the June 2014 Secured Promissory Note as described above.
August 2015 Senior Secured Promissory Notes
On August 20, 2015, we issued and sold senior secured promissory notes to three affiliated lenders in the aggregate principal amount of $40.0 million. The notes bear interest at a rate of 8% per annum payable semi-annually on June 30 or December 31 of each year, commencing on December 31, 2015, with $10.0 million payable three years from the closing, $10.0 million payable four years from the closing, and $20.0 million payable five years from the closing. The notes contain customary covenants, in addition to the obligation to maintain cash and cash equivalents of at least $15.0 million.
The notes are secured by substantially our personal property assets. The lenders shall be entitled to have a first priority lien on our intellectual property assets, pursuant to intercreditor arrangements with certain of our existing lenders. The notes provide for various events of default, including, among others, default in payment of principal or interest, breach of any representation or warranty by us or any subsidiary under any agreement or document delivered in connection with the notes, a continued breach of any other condition or obligation under any loan documents, certain bankruptcy, liquidation, reorganization or change of control events, the acquisition by any person or persons acting as group, other than the lenders, of beneficial ownership of 40% or more of our outstanding voting stock and certain events in which Pamela G. Marrone, Ph.D. ceases to serve as our Chief Executive Officer. Upon an event of default, the entire unpaid principal and interest may be declared immediately due and payable. As of September 30, 2015, we were in breach of certain of the covenants under the August 2015 note because we were in breach of certain of the covenants under our October 2012 and April 2013 notes and June 2014 note, each as discussed above. However, these breaches were cured in November 2015, as a result of our obtaining an extension to deliver our annual financial statements with respect to the October 2013 and April 2013 notes and the waiver of certain of our covenants with respect to the June 2014 note, each as described above.
The following table sets forth a summary of our cash flows for the periods indicated:
| YEAR ENDED DECEMBER 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| (In thousands) | ||||||||||||
| Net cash used in operating activities |
$ | (35,935 | ) | $ | (34,064 | ) | $ | (22,425 | ) | |||
| Net cash provided by (used in) investing activities |
671 | (17,620 | ) | (757 | ) | |||||||
| Net cash provided by financing activities |
46,133 | 66,133 | 30,973 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net increase in cash and cash equivalents |
$ | 10,869 | $ | 14,449 | $ | 7,791 | ||||||
|
|
|
|
|
|
|
|||||||
Cash Flows from Operating Activities
Net cash used in operating activities of $35.9 million during the twelve months ended December 31, 2014 primarily resulted from our net loss of $51.7 million, which included $4.6 million of share-based compensation expense, $2.6 million of depreciation and amortization expense, loss on disposal assets of $0.2 million and $0.8 million of non-cash interest expense. The net loss also included approximately $5.8 million in accounting, consulting, and legal fees incurred as a result of the Audit Committee’s independent investigation. In addition, net cash used in operating activities resulted from decreases in deferred revenue of $0.1 million and deferred
82
Table of Contents
revenue from related parties of $0.7 million and increases in prepaid expenses and other assets of $0.3 million. This was offset by decreases in accounts receivable of $2.0 million, deferred cost of product revenues of $1.1 million, inventory of $0.1, million and accounts receivable from related parties of $0.9 million, and increases in customer refund liabilities of $1.0 million, accounts payable of $1.7 million and accrued and other liabilities of $1.9 million.
Net cash used in operating activities of $34.1 million during the twelve months ended December 31, 2013 primarily resulted from our net loss of $31.2 million, which included a gain of $6.7 million in connection with a change in the fair value of financial instruments and $4.3 million in non-cash interest expense, $2.3 million in share-based compensation expense, $1.0 million in depreciation and amortization expense and $0.2 million in loss on disposal of equipment. In addition, net cash used in operating activities resulted from increases in accounts receivable of $1.0 million, accounts receivable due from related parties of $0.8 million, inventory of $7.8 million and deferred cost of product revenues of $2.9 million. This was offset by increases in deferred revenue of $3.5 million, deferred revenue from related parties of $0.9 million, accounts payable of $1.7 million, and accrued and other liabilities of $1.1 million as well as decreases in prepaid expenses and other assets of $1.4 million.
Net cash used in operating activities of $22.4 million during the twelve months ended December 31, 2012 primarily resulted from our net loss of $38.8 million, which included non-cash charges of $12.5 million in connection with a change in fair value of financial instruments, $3.9 million in connection with the issuance of a convertible note, $1.2 million of non-cash interest expense, $0.7 million in share-based compensation and $0.6 million in depreciation and amortization. In addition, net cash used in operating activities resulted from net changes in operating assets and liabilities of $2.5 million, primarily due to increases in inventory of $1.6 million, $2.5 million in accounts receivable, $0.1 million in accounts receivable from related parties and $2.1 million in prepaid expenses and other assets, offset by an increase of $0.3 million in deferred revenue, $0.9 million in deferred revenue from related parties and $2.6 million in accounts payable, accrued liabilities and other liabilities.
Cash Flows from Investing Activities
Net cash provided by investing activities of $0.7 million during the twelve months ended December 31, 2014 primarily resulted from $13.0 million used for the purchase of property, plant and equipment, primarily associated improvements related to the manufacturing plant, which was offset by $13.7 million in cash provided from maturities of short-term investments.
Net cash used in investing activities of $17.6 million during the twelve months ended December 31, 2013 primarily resulted from $4.0 million used for the purchase of property, plant and equipment, primarily associated with improvements related to the manufacturing plant and $17.5 million in cash for the purchase of short-term investments, offset by $3.8 million in cash provided by maturities of short-term investments.
Net cash used in investing activities was $0.8 million during the twelve months ended December 31, 2012, consisting of approximately $2.8 million used for purchase of property, plant and equipment, primarily associated with the purchase of the manufacturing plant and its subsequent improvement, offset by $2.0 million provided from the maturity of a short-term investment.
Cash Flows from Financing Activities
Net cash provided by financing activities of $46.1 million during the twelve months ended December 31, 2014 consisted primarily of $39.9 million in proceeds from the secondary offering, net of offering costs and underwriter commissions, $9.7 million from the issuance of debt and warrants, net of financing costs, $4.7 million in proceeds from the line of credit and $1.4 million in proceeds from the exercise of stock options and warrants. This was offset by $4.7 million in payments on the line of credit, $1.5 million in payments on our debt and capital leases and $3.4 million transferred to restricted cash.
83
Table of Contents
Net cash provided by financing activities of $66.1 million during the twelve months ended December 31, 2013 consisted primarily of $56.1 million in proceeds from the initial public offering, net of offering costs and underwriter commissions, $6.5 million from the issuance of convertible notes, $3.7 million from the issuance of debt, net of financing costs, $9.1 million from the release of restricted cash, $2.9 million in proceeds from secured borrowing and $0.3 million in proceeds from the exercise of stock options. This was offset by $9.6 million in payments on our debt and capital leases and $2.9 million in reductions of secured borrowing.
Net cash provided by financing activities of $31.0 million during the twelve months ended December 31, 2012 consisted primarily of $24.1 million from the issuance of convertible notes, $17.4 million from the issuance of debt, net of financing costs and $0.5 million in draws on our line of credit, partially offset by $9.1 million transferred from cash to restricted cash as part of our obligations under a debt agreement to repay a then-outstanding note payable and $1.9 million in payments on our line of credit, debt and capital lease obligations.
Contractual Obligations
The following is a summary of our contractual obligations as of December 31, 2014:
| TOTAL | 2015 | 2016-2017 | 2018-2019 | 2020 AND BEYOND |
||||||||||||||||
| (In thousands) | ||||||||||||||||||||
| Operating lease obligations |
$ | 4,645 | $ | 1,111 | $ | 1,970 | $ | 1,564 | $ | — | ||||||||||
| Debt and capital leases |
24,529 | 14,677 | 779 | 610 | 8,463 | |||||||||||||||
| Interest payments relating to debt and capital leases |
7,957 | 1,748 | 1,006 | 935 | 4,268 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total |
$ | 37,131 | $ | 17,536 | $ | 3,755 | $ | 3,109 | $ | 12,731 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
Operating leases consist of contractual obligations from agreements for non-cancelable office space and leases used to finance the acquisition of equipment. Debt and capital equipment leases payments and the interest payments relating thereto include promissory notes and capital lease obligations in accordance with payment terms under the agreements.
In September 2013 and then amended in April 2014, we entered into a lease agreement for a new 27,300 square foot office and laboratory facility located in Davis, California. The initial term of the lease is for a period of 60 months and commenced in August 2014. The monthly base rent is $44,000 for the first 12 months with a 3% increase each year thereafter. We have an option to extend the lease term twice for a period of five years each. In addition, concurrent with the amendment in April 2014, we entered into a lease agreement with an affiliate of the landlord to lease approximately 17,400 square feet of office and laboratory space in the same building complex in Davis, California. The initial term of the lease is for a period of 60 months and commenced in August 2014. The monthly base rent is $28,000 with a 3% increase each year thereafter.
In addition, we continue to lease a portion of our old headquarters’ location on Second Street in Davis until that lease expires in October 2016. We expect to enter into agreements to sublease the portions of the current and old office facilities that we are not currently utilizing.
Since December 31, 2014, we have not added any additional leases that would qualify as operating leases. In August 2015, we issued and sold senior secured promissory notes to three affiliated lenders in the aggregate principal amount of $40.0 million. The notes bear interest at a rate of 8% per annum payable semi-annually on June 30 or December 31 of each year, commencing on December 31, 2015, with $10 million payable three years from the closing, $10 million payable four years from the closing, and $20 million payable five years from the closing.
Inflation
We believe that inflation has not had a material impact on our results of operations for the years ended December 31, 2014, 2013, and 2012.
84
Table of Contents
Off-Balance Sheet Arrangements
We have not been involved in any material off-balance sheet arrangements.
Recently Issued Accounting Pronouncements
In May 2014, the Financial Accounting Standards Board (the FASB) issued ASU No. 2014-09, Revenue from Contracts with Customers (Topic 606) (ASU 2014-09). ASU 2014-09 requires entities to recognize revenue through the application of a five-step model, which includes identification of the contract, identification of the performance obligations, determination of the transaction price, allocation of the transaction price to the performance obligations and recognition of revenue as the entity satisfies the performance obligations. ASU 2014-09 will become effective for us beginning January 1, 2017. We are currently evaluating the guidance to determine the potential impact on our financial condition, results of operations, cash flows and financial statement disclosures.
In August 2014, the FASB issued ASU No. 2014-15, Presentation of Financial Statements – Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern (ASU 2014-15). ASU 2014-15 requires that management assess an entity’s ability to continue as a going concern by incorporating and expanding upon certain principles that are currently in U.S. auditing standards. ASU 2014-15 is effective for annual periods ending after December 15, 2016, and for annual periods and interim periods thereafter. We are currently evaluating the guidance to determine the potential impact on our financial statement disclosures.
In April 2015, the FASB issued ASU No. 2015-03, Interest – Imputation of Interest (Topic 835-30): Simplifying the Presentation of Debt Issuance Costs (ASU 2015-03). ASU 2015-03 requires that debt issuance costs related to a recognized debt liability be presented in the balance sheet as a direct deduction from the carrying amount of that debt liability, consistent with debt discounts. The recognition and measurement guidance for debt issuance costs is not affected by ASU 2015-03. ASU 2015-03 is effective for financial statements issued for fiscal years beginning after December 15, 2015, and interim periods within those fiscal years. Early adoption is permitted. Upon adoption, we will reclassify debt issuance costs from prepaid expenses and other current assets and other assets to debt, current portion on the consolidated balance sheets; we do not otherwise anticipate adoption will materially impact our statements of financial position or results of operations.
In July 2015, the FASB issued ASU No. 2015-11, Inventory (Topic 330): Simplifying the Measurement of Inventory (ASU 2015-11), which applies guidance on subsequent measurement of inventory. An entity should measure inventory at the lower of cost and net realizable value. Net realizable value is the estimated selling prices in the ordinary course of business, less reasonable predictable costs of completion, disposal and transportation. The guidance excludes inventory measured using LIFO or the retail inventory method. ASU 2015-11 will be effective for interim and annual reporting periods beginning after December 15, 2016. Early application is permitted. The Company does not anticipate that the adoption of this ASU will materially change the presentation of its consolidated financial statements.
Critical Accounting Policies and Estimates
Inventories
Inventories are stated at the lower of cost or market (net of realizable value or replacement cost) and include the cost of material and external and internal labor and manufacturing costs. Cost is determined on the first-in, first-out basis. We provide for inventory reserves when conditions indicate that the selling price may be less than cost due to physical deterioration, obsolescence, changes in price levels, or other factors. Additionally, we provide reserves for excess and slow-moving inventory to its estimated net realizable value. The reserves are based upon estimates about future demand from our customers and distributors and market conditions.
85
Table of Contents
Fair Value of Financial Instruments
Fair value is defined as an exit price that would be received from the sale of an asset or paid to transfer a liability in an orderly transaction between market participants on the measurement date. A three tier fair value hierarchy has been established, which prioritizes the inputs used in measuring fair value as follows: Level 1, observable inputs such as quoted prices in active markets; Level 2, inputs other than the quoted prices in active markets that are observable either directly or indirectly; and Level 3, unobservable inputs in which there is little or no market data, which requires that we develop our own assumptions. This hierarchy requires the use of observable data, when available, and minimizes the use of unobservable inputs when determining fair value.
Until the effective date of the IPO, we accounted for the outstanding warrants exercisable into shares of our Series A, Series B and Series C convertible preferred stock as liability instruments, as these warrants were convertible into Series A, Series B and Series C convertible preferred stock upon the occurrence of certain events or transactions. We also accounted for the outstanding warrants exercisable into a variable number of shares of common stock at a fixed monetary amount as liability instruments. Our convertible notes were recorded at estimated fair value on a recurring basis as the predominant settlement feature of the convertible notes was to settle a fixed monetary amount in a variable number of shares. We adjusted the warrants and the convertible notes to estimated fair value at each reporting period and on the effective date of the IPO with the change in estimated fair value recorded in the consolidated statements of operations.
Starting with fiscal year 2012, due to our closing several debt financings and an initial public offering becoming more probable as we began investing significant time and resources into the initial public offering process, we changed our valuation methodology to estimate the fair value of our financial instruments, including our outstanding warrants and convertible notes, from the option method to the probability weighted expected return method, which we refer to as the “expected return method.” The expected return method analyzes the returns afforded to common equity holders under multiple possible future scenarios. Under the expected return method, share value is based upon the probability-weighted present value of expected future net cash flows (distributions to shareholders) under each of the possible scenarios, giving consideration to the rights and preferences of each share class. This method is most appropriate when the long-term outlook for an enterprise is largely known and multiple possible future scenarios can be reasonably estimated. As the expected return method estimated the fair value of our warrants and convertible notes using unobservable inputs, they were both considered to be Level 3 fair value measurements. Changes in the probability weights and discount rates used in the expected return method valuation model and the estimated time to a liquidity event may have a significant impact on the estimated fair value of the preferred and common stock warrant liabilities and the convertible notes.
As a result of the automatic exercise of all Series A and Series B convertible preferred stock warrants and certain common stock warrants for shares of common stock, the automatic conversion of all convertible notes into common stock in accordance with their terms, and the exercise of all Series C convertible preferred stock warrants for shares of common stock in connection with our IPO in August 2013, there will not be any further adjustments to these warrants and convertible notes. In addition, upon completion of the IPO, the exercise price and number of shares to be issued upon exercise of the remaining outstanding common stock warrants became known. Accordingly, after the IPO, the fair value of the common stock warrant liability on the date of the IPO was reclassified to equity and will no longer be adjusted to its estimated fair value on each reporting date.
Revenue Recognition
We recognize revenues when persuasive evidence of an arrangement exists, delivery and transfer of title has occurred or services have been rendered, the price is fixed or determinable and collectability is reasonably assured, unless contractual obligations, acceptance provisions or other contingencies exist. If such obligations or provisions exist, revenue is recognized after such obligations or provisions are fulfilled or expire.
Product revenues consist of revenues generated from sales to distributors and from sales of our products to direct customers, net of rebates and cash discounts. For sales of products made to distributors, we recognize revenue either
86
Table of Contents
on a sell-in or sell-through basis depending on the specific facts and circumstances of the distributor. Factors considered include, but are not limited to, whether the payment terms offered to the distributor are structured to correspond to when product is re-sold, the distributor history of adhering to the terms of its contractual arrangements with us, whether we have a pattern of granting concessions for the benefit of the distributor, and whether there are other conditions that may indicate that the sale to the distributor is not substantive.
In some cases, we recognize distributor revenue as title and risk of loss passes, provided all other revenue recognition criteria has been satisfied (the “sell-in” method). For certain sales to distributors, the revenue recognition criteria for distributor sales are not satisfied at the time of shipment or receipt; specifically, in instances where additional contractual terms or other arrangements offered distributors include a general right to return, we consider the arrangement not to be fixed or determinable, and accordingly, revenue is deferred until products are resold to customers of the distributor (the “sell-through” method). The costs of goods sold associated with such deferral are also deferred and classified in deferred cost of product revenues in the consolidated balance sheets. Cash received from customers related to delivered product that may not represent a true sale are classified as customer refund liabilities in the consolidated balance sheets and the related cost of inventory remains in inventory in the consolidated balance sheets until the product is returned or is resold to customers of the distributor and revenue is recognized. For the years ended December 31, 2014 and 2013, 53% and 23%, respectively, of revenue was recognized on a sell-through basis. During the year ended December 31, 2012, there was no revenue recognized on a sell through basis. As of December 31, 2014 and 2013, the Company recorded current deferred product revenues of $3.2 million and $4.7 million, respectively.
From time to time, the Company offers certain product rebates to its distributors and to growers, which are estimated and recorded as reductions to product revenues and an accrued liability is recorded at the later of when the revenues are recorded or the rebate is being offered.
The Company recognizes license revenues pursuant to strategic collaboration and distribution agreements under which the Company receives payments for the achievement of testing validation, regulatory progress and commercialization events. As these activities and payments are associated with exclusive rights that the Company provides in connection with strategic collaboration and distribution agreements over the term of the agreements, revenues related to the payments received are deferred and recognized over the term of the exclusive distribution period of the respective agreement. For the year ended December 31, 2014, the Company received payments totaling $0.5 million and as of December 31, 2014 had $0.8 million recorded in accounts receivable. No payments were received under these agreements during the year ended December 31, 2013. For the year ended December 31, 2012, the Company received payments totaling $1.5 million, of which $1.0 million was received from a related party. For each of the years ended December 31, 2014, 2013 and 2012, the Company recognized approximately $0.2 million as license revenues, excluding related party revenues, in the accompanying consolidated statements of operations.
The Company has a strategic collaboration and distribution agreement with Syngenta, an affiliate of one of its 5% stockholders Syngenta Ventures, until June 2014 when, in connection with the secondary offering, Syngenta Ventures sold 600,000 common shares, reducing its ownership percentage below 5%. Beginning in June 2014, revenue recognized under this arrangement has been included in license revenues. For the year ended December 31, 2014, the Company recognized $0.3 million of related party revenues relating to the period that Syngenta Ventures was one of our 5% stockholders. For the years ended December 31, 2013, the Company recognized $0.1 million of related party revenues under these agreements.
At December 31, 2014, the Company recorded current and non-current deferred revenues of $0.3 million and $2.1 million, respectively, related to payments received under these agreements. At December 31, 2013, the Company recorded current and non-current deferred revenues of $0.3 million and $1.4 million, respectively, related to payments received under these agreements, of which $0.1 million and $0.6 million, respectively, related to deferred revenues from related parties based on the terms of the Company’s commercial agreement with Syngenta.
87
Table of Contents
Share-Based Compensation
We recognize share-based compensation expense for all stock options made to employees and directors based on estimated fair values.
We estimate the fair value of stock options on the date of grant using an option-pricing model. The value of the portion of the stock options that is ultimately expected to vest is recognized as expense over the requisite service periods using the straight-line method. Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates.
The estimated fair value of options vested during the years ended December 31, 2014, 2013 and 2012 was $3.9 million, $1.3 million, and $0.5 million, respectively. The weighted-average estimated fair value of options granted during the years ended December 31, 2014, 2013 and 2012 was $6.10 per share, $10.35 per share and $4.24 per share, respectively. During the years ended December 31, 2014, 2013 and 2012, we recorded share-based compensation expense of $4.6 million, $2.3 million and $0.7 million, respectively. As of December 31, 2014, the total share-based compensation expense related to unvested stock options granted to employees under our share-based compensation plans but not yet recognized was $8.3 million. These costs will be amortized to expense on a straight-line basis over a weighted-average remaining term of 2.7 years.
In connection with the decision of our former Chief Financial Officer, Mr. Glidewell, to retire, we entered into a transition agreement with Mr. Glidewell which provided, among other things, for the vesting of his outstanding equity awards through the transition date. For the years ended December 31, 2014 and 2013, we recorded share-based compensation expense of $0.4 million and $0.3 million, respectively, relating to the acceleration of vesting of Mr. Glidewell’s option awards. As of December 31, 2014 there was no share-based compensation expense related to unvested options granted to Mr. Glidewell under our share-based compensation plans but not yet recognized. Refer to Note 7 in Part II, Item 8, “Financial Statements and Supplementary Data” of this Annual Report on Form 10-K for further discussion regarding Mr. Glidewell’s transition agreement.
For purposes of determining our historical share-based compensation expense, we used the Black-Scholes-Merton (BSM) option-pricing model to calculate the estimated fair value of stock options on the measurement date (generally, the grant date). This model requires inputs for the expected life of the stock option, estimated volatility factor, risk-free interest rate and expected dividend yield. Our estimates of forfeiture rates also affect the amount of aggregate compensation expense. Prior to our initial public offering, our board of directors considered numerous objective and subjective factors to determine the fair value of our common stock at each meeting at which stock options were granted and approved. These inputs are subjective and generally require significant judgment. For the years ended December 31, 2014, 2013 and 2012, we calculated the fair value of stock options granted using the following assumptions:
| YEAR ENDED DECEMBER 31 | ||||||
| 2014 | 2013 | 2012 | ||||
| Expected life (years) |
5.46-6.08 | 5.29-7.71 | 5.00-6.08 | |||
| Estimated volatility factor |
49%-71% | 70%-75% | 72%-0.76% | |||
| Risk-free interest rate |
1.63%-2.05% | 1.27%-2.11% | 0.74%-1.16% | |||
| Expected dividend yield |
— | — | — | |||
Expected Life—Our expected life represents the period that our share-based payment awards are expected to be outstanding. We use the “simplified method” in accordance with Staff Accounting Bulletin (SAB) No. 107, Share-Based Payment, and SAB No. 110, Simplified Method for Plain Vanilla Share Options, to develop the expected term of options determined to be “plain vanilla.” Under this approach, the expected term is presumed to be the midpoint between the vesting date and the contractual end of the option grant. For stock options granted with an exercise price not equal to the determined fair market value, we estimate the expected life based on historical data and management’s expectations about exercises and post-vesting termination behavior. We will use the simplified method until we have sufficient historical data necessary to provide a reasonable estimate of expected life in accordance with SAB No. 107 and SAB No. 110.
88
Table of Contents
Estimated Volatility Factor— Since we have a limited trading history in our common stock, we calculate volatility based upon the trading history and calculated volatility of the common stock of comparable agricultural biotechnology companies in determining an estimated volatility factor.
Risk-Free Interest Rate—We base the risk-free interest rate on the implied yield currently available on U.S. Treasury constant-maturity securities with the same or substantially equivalent remaining term.
Expected Dividend Yield—We have not declared dividends nor do we expect to in the foreseeable future. Therefore, a zero value was assumed for the expected dividend yield.
Estimated Forfeitures—When estimating forfeitures, we consider voluntary and involuntary termination behavior and actual option forfeitures.
If in the future we determine that other methods are more reasonable, or other methods for calculating these assumptions are prescribed by authoritative guidance, the fair value calculated for our stock options could change significantly. Higher volatility and longer expected lives result in an increase to share-based compensation expense determined at the grant date. Share-based compensation expense affects our research, development and patent expense and selling, general and administrative expense.
The BSM option-pricing model was developed for use in estimating the fair value of traded options that have no vesting restrictions and are fully transferable, characteristics not present in our stock options. Existing valuation models, including the BSM option-pricing model, may not provide reliable measures of the fair values of our stock options. Consequently, there is a risk that our estimates of the fair values of the stock options on the grant dates may bear little resemblance to the actual values realized upon exercise. Stock options may expire or otherwise result in zero intrinsic value as compared to the fair values originally estimated on the grant date and reported in the consolidated financial statements. Alternatively, value may be realized from these instruments that is significantly higher than the fair values originally estimated on the grant date and reported in the consolidated financial statements.
Income Taxes
We use the asset and liability method of accounting for income taxes. Deferred tax assets and liabilities are recognized for the estimated future tax consequences attributable to the differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. To the extent deferred tax assets cannot be recognized under the preceding criteria, we establish valuation allowances as necessary to reduce deferred tax assets to the amounts expected to be realized. As of December 31, 2014 and 2013, all deferred tax assets were fully offset by a valuation allowance. Realization of deferred tax assets is dependent upon future federal, state and foreign taxable income. Our judgments regarding deferred tax assets may change as we expand into international jurisdictions, due to future market conditions, changes in U.S. or international tax laws and other factors. These changes, if any, may require possible material adjustments to these deferred tax assets, resulting in a reduction in net income or an increase in net loss in the period when such determinations are made.
We recognize liabilities for uncertain tax positions based upon a two-step process. To the extent a tax position does not meet a more-likely-than-not level of certainty; no benefit is recognized in the consolidated financial statements. If a position meets the more-likely-than-not level of certainty, it is recognized in the consolidated financial statements at the largest amount that has a greater than 50% likelihood of being realized upon ultimate settlement. Our policy is to analyze our tax positions taken with respect to all applicable income tax issues for all open tax years (in each respective jurisdiction). As of December 31, 2014 and 2013, we have concluded that no uncertain tax positions were required to be recognized in our consolidated financial statements. It is our practice to recognize interest and penalties related to income tax matters in income tax expense. No amounts were recognized for interest and penalties during the years ended December 31, 2014, 2013 and 2012.
89
Table of Contents
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We currently have minimal exposure to the effect of interest rate changes, foreign currency fluctuations and changes in commodity prices. We are exposed to changes in the general economic conditions in the countries where we conduct business, which currently is substantially all in the United States. Our current investment strategy is to invest in financial instruments that are highly liquid, readily convertible into cash and which mature within six months from the date of purchase. To date, we have not used derivative financial instruments to manage any of our market risks or entered into transactions using derivative financial instruments for trading purposes.
We do not believe our cash equivalents and short-term investments have significant risk of default or illiquidity. While we believe our cash equivalents and short-term investments do not contain excessive risk, we cannot provide absolute assurance that in the future our investments will not be subject to adverse changes in market value.
Interest Rate Risk
We had cash and cash equivalents of $35.3 million at December 31, 2014, which was held for working capital purposes. We do not enter into investments for trading or speculative purposes. We entered into a promissory note in June 2014, which bears interest at the prime rate plus 2.00%. A change in market interest rates of 1% would have an impact of approximately $0.1 million on our future annual interest expense. All of our other debt, including the promissory notes we issued and sold in August 2015, is at fixed interest rates and thus a change in market interest rates would not have an impact on interest expense.
Foreign Currency Risk
Revenue and expenses have been primarily denominated in U.S. dollars and foreign currency fluctuations have not had a significant impact on our historical results of operations. In addition, our strategic collaboration and distribution agreements for current products provide for payments in U.S. dollars. As we market new products internationally, our product revenues and expenses may be in currencies other than U.S. dollars, and accordingly, foreign currency fluctuations may have a greater impact on our financial position and operating results.
Commodity Risk
Our exposure to market risk for changes in commodity prices currently is minimal. As our commercial operations grow, our exposure will relate mostly to the demand side as our end users are exposed to fluctuations in prices of agricultural commodities.
90
Table of Contents
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
91
Table of Contents
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders of
Marrone Bio Innovations, Inc.
We have audited the accompanying consolidated balance sheets of Marrone Bio Innovations, Inc. (“the Company”) as of December 31, 2014 and 2013, and the related consolidated statements of operations, comprehensive loss, convertible preferred stock and stockholders’ equity (deficit), and cash flows for each of the three years in the period ended December 31, 2014. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company’s internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Marrone Bio Innovations, Inc. at December 31, 2014 and 2013, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2014, in conformity with U.S. generally accepted accounting principles.
As discussed in Note 2 to the consolidated financial statements, the 2013 financial statements have been restated to correct accounting errors primarily related to revenue recognition.
| /s/ Ernst & Young LLP |
| Sacramento, California |
| November 10, 2015 |
92
Table of Contents
Consolidated Balance Sheets
(In Thousands, Except Par Value)
| DECEMBER 31 | ||||||||
| 2014 | 2013 | |||||||
| As restated | ||||||||
| Assets |
||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 35,324 | $ | 24,455 | ||||
| Restricted cash, current portion |
1,856 | — | ||||||
| Short-term investments |
— | 13,677 | ||||||
| Accounts receivable |
1,787 | 3,784 | ||||||
| Accounts receivable from related parties |
— | 903 | ||||||
| Inventories, net |
12,644 | 12,717 | ||||||
| Deferred cost of product revenue, including deferred cost of product revenues to related parties of $333 and $610 as of December 31, 2014 and 2013, respectively |
1,797 | 2,861 | ||||||
| Prepaid expenses and other current assets |
1,315 | 1,354 | ||||||
|
|
|
|
|
|||||
| Total current assets |
54,723 | 59,751 | ||||||
| Property, plant and equipment, net |
20,166 | 9,361 | ||||||
| Restricted cash, less current portion |
1,560 | — | ||||||
| Other assets |
733 | 806 | ||||||
|
|
|
|
|
|||||
| Total assets |
77,182 | 69,918 | ||||||
|
|
|
|
|
|||||
| Liabilities and stockholders’ equity |
||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 5,841 | $ | 4,460 | ||||
| Accrued liabilities |
6,321 | 4,534 | ||||||
| Deferred revenue, current portion |
2,861 | 3,850 | ||||||
| Deferred revenue from related parties, current portion |
660 | 1,128 | ||||||
| Customer refund liabilities |
1,044 | — | ||||||
| Capital lease obligations, current portion |
1,839 | 1,401 | ||||||
| Debt, current portion |
12,636 | 157 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
31,202 | 15,530 | ||||||
| Deferred revenue, less current portion |
2,050 | 744 | ||||||
| Deferred revenue from related parties, less current portion |
— | 628 | ||||||
| Capital lease obligations, less current portion |
185 | 1,134 | ||||||
| Debt, less current portion |
9,667 | 12,280 | ||||||
| Other liabilities |
847 | 571 | ||||||
|
|
|
|
|
|||||
| Total liabilities |
43,951 | 30,887 | ||||||
| Commitments and contingencies (Note 15) |
||||||||
| Stockholders’ equity: |
||||||||
| Preferred stock: $0.00001 par value; 20,000 shares authorized, no shares issued or outstanding at December 31, 2014 and December 31, 2013 |
— | — | ||||||
| Common stock: $0.00001 par value; 250,000 shares authorized and 24,465 shares issued and outstanding at December 31, 2014; 250,000 shares authorized and 19,323 shares issued and outstanding at December 31, 2013 |
— | — | ||||||
| Additional paid in capital |
193,079 | 147,220 | ||||||
| Accumulated deficit |
(159,848 | ) | (108,189 | ) | ||||
|
|
|
|
|
|||||
| Total stockholders’ equity |
33,231 | 39,031 | ||||||
|
|
|
|
|
|||||
| Total liabilities and stockholders’ equity |
$ | 77,182 | $ | 69,918 | ||||
|
|
|
|
|
|||||
See accompanying notes.
93
Table of Contents
Consolidated Statements of Operations
(In Thousands, Except Per Share Data)
| YEAR ENDED DECEMBER 31 | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| As restated | ||||||||||||
| Revenues: |
||||||||||||
| Product |
$ | 7,750 | $ | 7,588 | $ | 6,777 | ||||||
| License |
232 | 193 | 179 | |||||||||
| Related party |
1,154 | 665 | 184 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total revenues |
9,136 | 8,446 | 7,140 | |||||||||
| Cost of product revenues, including cost of product revenues to related parties of $561, $374 and $126 for the years ended December 31, 2014, 2013 and 2012, respectively |
9,438 | 7,243 | 4,333 | |||||||||
|
|
|
|
|
|
|
|||||||
| Gross profit (loss) |
(302 | ) | 1,203 | 2,807 | ||||||||
| Operating expenses: |
||||||||||||
| Research, development and patent |
19,281 | 17,905 | 12,741 | |||||||||
| Non-cash charge associated with a convertible note |
— | — | 3,610 | |||||||||
| Selling, general and administrative |
28,950 | 15,017 | 10,294 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
48,231 | 32,922 | 26,645 | |||||||||
|
|
|
|
|
|
|
|||||||
| Loss from operations |
(48,533 | ) | (31,719 | ) | (23,838 | ) | ||||||
| Other income (expense): |
||||||||||||
| Interest income |
59 | 49 | 16 | |||||||||
| Interest expense |
(2,907 | ) | (6,056 | ) | (2,466 | ) | ||||||
| Change in estimated fair value of financial instruments |
— | 6,717 | (12,461 | ) | ||||||||
| Gain on extinguishment of debt |
— | 49 | — | |||||||||
| Other expense, net |
(278 | ) | (282 | ) | (45 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total other income (expense), net |
(3,126 | ) | 477 | (14,956 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Loss before income taxes |
(51,659 | ) | (31,242 | ) | (38,794 | ) | ||||||
| Income taxes |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
(51,659 | ) | (31,242 | ) | (38,794 | ) | ||||||
| Deemed dividend on convertible notes |
— | (1,378 | ) | (2,039 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Net loss attributable to common stockholders |
$ | (51,659 | ) | $ | (32,620 | ) | $ | (40,833 | ) | |||
|
|
|
|
|
|
|
|||||||
| Net loss per common share: |
||||||||||||
| Basic |
$ | (2.32 | ) | $ | (3.74 | ) | $ | (32.48 | ) | |||
|
|
|
|
|
|
|
|||||||
| Diluted |
$ | (2.32 | ) | $ | (4.25 | ) | $ | (32.48 | ) | |||
|
|
|
|
|
|
|
|||||||
| Weighted-average shares outstanding used in computing net loss per common share: |
||||||||||||
| Basic |
22,314 | 8,731 | 1,257 | |||||||||
|
|
|
|
|
|
|
|||||||
| Diluted |
22,314 | 8,911 | 1,257 | |||||||||
|
|
|
|
|
|
|
|||||||
See accompanying notes.
94
Table of Contents
Consolidated Statements of Comprehensive Loss
(In Thousands)
| YEAR ENDED DECEMBER 31 | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| As restated | ||||||||||||
| Net loss |
$ | (51,659 | ) | $ | (31,242 | ) | $ | (38,794 | ) | |||
| Other comprehensive loss |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Comprehensive loss |
$ | (51,659 | ) | $ | (31,242 | ) | $ | (38,794 | ) | |||
|
|
|
|
|
|
|
|||||||
See accompanying notes.
95
Table of Contents
Consolidated Statements of Convertible Preferred Stock and Stockholders’ Equity (Deficit)
(In Thousands)
| CONVERTIBLE PREFERRED STOCK | ||||||||||||||||||||||||||||||||
| SERIES A | SERIES B | SERIES C | TOTAL | |||||||||||||||||||||||||||||
| SHARES | AMOUNT | SHARES | AMOUNT | SHARES | AMOUNT | SHARES | AMOUNT | |||||||||||||||||||||||||
| Balance at December 31, 2011 |
1,484 | $ | 3,747 | 2,242 | $ | 10,758 | 4,778 | $ | 25,107 | 8,504 | $ | 39,612 | ||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Exercise of stock options |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Share-based compensation |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Deemed dividend, convertible notes |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Balance at December 31, 2012 |
1,484 | 3,747 | 2,242 | 10,758 | 4,778 | 25,107 | 8,504 | 39,612 | ||||||||||||||||||||||||
| Net loss, as restated |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Exercise of stock options |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Share-based compensation |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Deemed dividend, convertible notes |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Cash exercise of preferred stock warrants |
— | — | 10 | 47 | — | — | 10 | 47 | ||||||||||||||||||||||||
| Net exercise of preferred stock warrants |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Conversion of preferred stock into common stock |
(1,484 | ) | (3,747 | ) | (2,252 | ) | (10,805 | ) | (4,778 | ) | (25,107 | ) | (8,514 | ) | (39,659 | ) | ||||||||||||||||
| Convertible notes converted into common stock |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Cash exercise of common stock warrants |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Net exercise of common stock warrants |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Reclassification of warrants from liability to equity |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Issuance of common stock upon initial public offering, net of offering costs and underwriter commission |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Balance at December 31, 2013, as restated |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Exercise of stock options |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Share-based compensation |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Cash exercise of common stock warrants |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Issuance of common stock in follow-on offering, net of offering costs and underwriter commission |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Balance at December 31, 2014 |
— | $ | — | — | $ | — | — | $ | — | — | $ | — | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
96
Table of Contents
MARRONE BIO INNOVATIONS, INC.
Consolidated Statements of Convertible Preferred Stock and Stockholders’ Equity (Deficit) - Continued
(In Thousands)
|
COMMON STOCK |
ADDITIONAL PAID IN CAPITAL |
ACCUMULATED DEFICIT |
TOTAL STOCKHOLDERS’ EQUITY (DEFICIT) |
|||||||||||||||||
| SHARES | AMOUNT | |||||||||||||||||||
| Balance at December 31, 2011 |
1,247 | $ | — | $ | 636 | $ | (34,736 | ) | $ | (34,100 | ) | |||||||||
| Net loss |
— | — | — | (38,794 | ) | (38,794 | ) | |||||||||||||
| Exercise of stock options |
20 | — | 24 | — | 24 | |||||||||||||||
| Share-based compensation |
— | — | 662 | — | 662 | |||||||||||||||
| Deemed dividend, convertible notes |
— | — | — | (2,039 | ) | (2,039 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balance at December 31, 2012 |
1,267 | — | 1,322 | (75,569 | ) | (74,247 | ) | |||||||||||||
| Net loss, as restated |
— | — | — | (31,242 | ) | (31,242 | ) | |||||||||||||
| Exercise of stock options |
217 | — | 250 | — | 250 | |||||||||||||||
| Share-based compensation |
— | — | 2,300 | — | 2,300 | |||||||||||||||
| Deemed dividend, convertible notes |
— | — | — | (1,378 | ) | (1,378 | ) | |||||||||||||
| Cash exercise of preferred stock warrants |
— | — | — | — | — | |||||||||||||||
| Net exercise of preferred stock warrants |
71 | — | — | — | — | |||||||||||||||
| Conversion of preferred stock into common stock |
8,514 | — | 39,659 | — | 39,659 | |||||||||||||||
| Convertible notes converted into common stock |
3,741 | — | 44,890 | — | 44,890 | |||||||||||||||
| Cash exercise of common stock warrants |
3 | — | 25 | — | 25 | |||||||||||||||
| Net exercise of common stock warrants |
47 | — | — | — | — | |||||||||||||||
| Reclassification of warrants from liability to equity |
— | — | 2,669 | — | 2,669 | |||||||||||||||
| Issuance of common stock upon initial public offering, net of offering costs and underwriter commission |
5,463 | — | 56,105 | — | 56,105 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balance at December 31, 2013, as restated |
19,323 | — | 147,220 | (108,189 | ) | 39,031 | ||||||||||||||
| Net loss |
— | — | — | (51,659 | ) | (51,659 | ) | |||||||||||||
| Exercise of stock options |
561 | — | 1,305 | — | 1,305 | |||||||||||||||
| Share-based compensation |
— | — | 4,555 | — | 4,555 | |||||||||||||||
| Cash exercise of common stock warrants |
6 | — | 50 | — | 50 | |||||||||||||||
| Issuance of common stock in follow-on offering, net of offering costs and underwriter commission |
4,575 | — | 39,949 | — | 39,949 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balance at December 31, 2014 |
24,465 | $ | — | $ | 193,079 | $ | (159,848 | ) | $ | 33,231 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
See accompanying notes.
97
Table of Contents
Consolidated Statements of Cash Flows
(In Thousands)
| YEAR ENDED DECEMBER 31 | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| As restated | ||||||||||||
| Cash flows from operating activities |
||||||||||||
| Net loss |
$ | (51,659 | ) | $ | (31,242 | ) | $ | (38,794 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||||
| Depreciation and amortization |
2,581 | 976 | 613 | |||||||||
| Loss on disposal of equipment |
243 | 231 | — | |||||||||
| Share-based compensation |
4,555 | 2,300 | 662 | |||||||||
| Non-cash interest expense |
780 | 4,315 | 1,224 | |||||||||
| Reduction of revenue associated with a convertible note |
— | — | 245 | |||||||||
| Non-cash charge associated with a convertible note |
— | — | 3,610 | |||||||||
| Change in estimated fair value of financial instruments |
— | (6,717 | ) | 12,461 | ||||||||
| Gain on extinguishment of debt |
— | (49 | ) | — | ||||||||
| Amortization of investment securities premiums/discounts, net |
10 | 18 | — | |||||||||
| Net changes in operating assets and liabilities: |
||||||||||||
| Accounts receivable |
1,997 | (950 | ) | (2,464 | ) | |||||||
| Accounts receivable from related parties |
903 | (767 | ) | (59 | ) | |||||||
| Inventories |
73 | (7,845 | ) | (1,625 | ) | |||||||
| Prepaid expenses and other assets |
(309 | ) | 1,374 | (2,097 | ) | |||||||
| Deferred cost of product revenues |
1,064 | (2,861 | ) | — | ||||||||
| Accounts payable |
1,667 | 1,682 | 1,174 | |||||||||
| Accrued and other liabilities |
1,895 | 1,141 | 1,381 | |||||||||
| Deferred revenue |
(108 | ) | 3,464 | 354 | ||||||||
| Deferred revenue from related parties |
(671 | ) | 866 | 890 | ||||||||
| Customer refund liabilities |
1,044 | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in operating activities |
(35,935 | ) | (34,064 | ) | (22,425 | ) | ||||||
| Cash flows from investing activities |
||||||||||||
| Purchases of property, plant and equipment |
(13,002 | ) | (3,966 | ) | (2,757 | ) | ||||||
| Proceeds from sale of equipment |
6 | 41 | — | |||||||||
| Purchase of short-term investments |
(49 | ) | (17,477 | ) | — | |||||||
| Maturities of short-term investments |
13,716 | 3,782 | 2,000 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by (used in) investing activities |
671 | (17,620 | ) | (757 | ) | |||||||
| Cash flows from financing activities |
||||||||||||
| Proceeds from public offerings, net of offering costs and underwriter commissions |
39,949 | 56,105 | — | |||||||||
| Proceeds from issuance of convertible notes payable |
— | 6,529 | 24,076 | |||||||||
| Proceeds from issuance of debt, net of financing costs |
9,696 | 3,700 | 17,375 | |||||||||
| Proceeds from line of credit |
4,687 | — | 500 | |||||||||
| Repayment of line of credit |
(4,687 | ) | — | (500 | ) | |||||||
| Repayment of debt |
(378 | ) | (9,433 | ) | (1,154 | ) | ||||||
| Repayment of capital leases |
(1,073 | ) | (229 | ) | (209 | ) | ||||||
| Proceeds from secured borrowing |
— | 2,880 | — | |||||||||
| Reductions in secured borrowing |
— | (2,880 | ) | — | ||||||||
| Change in restricted cash |
(3,416 | ) | 9,139 | (9,139 | ) | |||||||
| Proceeds from exercise of stock options |
1,305 | 250 | 24 | |||||||||
| Proceeds from exercise of preferred stock warrants |
— | 47 | — | |||||||||
| Proceeds from exercise of common stock warrants |
50 | 25 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by financing activities |
46,133 | 66,133 | 30,973 | |||||||||
| Net increase in cash and cash equivalents |
10,869 | 14,449 | 7,791 | |||||||||
| Cash and cash equivalents, beginning of year |
24,455 | 10,006 | 2,215 | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash and cash equivalents, end of year |
$ | 35,324 | $ | 24,455 | $ | 10,006 | ||||||
|
|
|
|
|
|
|
|||||||
| Supplemental disclosure of cash flow information |
||||||||||||
| Cash paid for interest, net of capitalized interest of $668, $636 and $106 for the years ended December 31, 2014, 2013 and 2012, respectively |
$ | 2,102 | $ | 1,692 | $ | 1,136 | ||||||
|
|
|
|
|
|
|
|||||||
| Supplemental disclosure of non-cash investing and financing activities |
||||||||||||
| Property, plant and equipment included in accounts payable and accrued liabilities |
$ | 204 | $ | 1,009 | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
| Equipment acquired under capital leases |
$ | 834 | $ | 2,106 | $ | 317 | ||||||
|
|
|
|
|
|
|
|||||||
| Equipment acquired in association with operating lease |
$ | 285 | $ | — | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
| Interest added to the principal of convertible notes |
$ | — | $ | 1,623 | $ | 837 | ||||||
|
|
|
|
|
|
|
|||||||
| Reclassification of warrants from liabilities to equity |
$ | — | $ | 2,669 | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
| Conversion of convertible notes to common stock |
$ | — | $ | 44,890 | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
| Conversion of preferred stock to common stock |
$ | — | $ | 39,659 | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
See accompanying notes.
98
Table of Contents
Notes to Consolidated Financial Statements
December 31, 2014
1. Summary of Business, Basis of Presentation and Liquidity
Marrone Bio Innovations, Inc. (Company), formerly Marrone Organic Innovations, Inc., was incorporated under the laws of the State of Delaware on June 15, 2006, and is located in Davis, California. In July 2012, the Company formed a wholly-owned subsidiary, Marrone Michigan Manufacturing LLC (MMM LLC), which holds the assets of a manufacturing plant the Company purchased in July 2012. The Company makes bio-based pest management and plant health products. The Company targets the major markets that use conventional chemical pesticides, including certain agricultural and water markets where its bio-based products are used as alternatives for, or mixed with, conventional chemical pesticides. The Company also targets new markets for which there are no available conventional chemical pesticides, the use of conventional chemical pesticides may not be desirable or permissible because of health and environmental concerns (including organically certified crops), or because the development of pest resistance has reduced the efficacy of conventional chemical pesticides. The Company delivers EPA-approved and registered biopesticide products and other bio-based products that address the global demand for effective, safe and environmentally responsible products.
In August 2013, the Company closed its initial public offering of 5,462,500 shares of its common stock (inclusive of 712,500 shares of common stock sold upon the exercise of the underwriters’ option to purchase additional shares) (IPO). The public offering price of the shares sold in the offering was $12.00 per share. The total gross proceeds from the offering to the Company were $65,550,000, and after deducting underwriting discounts and commissions and offering expenses payable by the Company, the aggregate net proceeds received by the Company totaled approximately $56,105,000. Upon the closing of the IPO, all shares of the Company’s outstanding convertible preferred stock and convertible notes automatically converted into shares of common stock and outstanding warrants to purchase convertible preferred stock and certain warrants to purchase common stock were exercised for shares of common stock (See Note 20). In connection with the IPO, on August 1, 2013, the Company amended and restated its certificate of incorporation to effect a 1-for-3.138458 reverse stock split (See Note 19).
In June 2014, the Company completed a public offering of 4,575,000 shares of its common stock (inclusive of 675,000 shares of common stock sold upon the exercise of the underwriters’ option to purchase additional shares). The public offering price of the shares sold in the offering was $9.50 per share. The total gross proceeds from the offering to the Company were $43,463,000, and after deducting underwriting discounts and commissions and offering expenses payable by the Company, the aggregate net proceeds received by the Company totaled $39,949,000.
The Company is an early stage company with a limited operating history and has a limited number of commercialized products. As of December 31, 2014, the Company had an accumulated deficit of $159,848,000, has incurred significant losses since inception and expects to continue to incur losses for the foreseeable future. Until the completion of the IPO in August 2013, the Company had funded operations primarily with the net proceeds from the private placements of convertible preferred stock, convertible notes, promissory notes, term loans, as well as proceeds from the sale of its products and payments under strategic collaboration agreements and government grants. The Company will need to generate significant revenue growth to achieve and maintain profitability. As of December 31, 2014, the Company had working capital of $23,521,000, including cash and cash equivalents of $35,324,000. In addition, as of December 31, 2014, the Company had debt totaling $22,303,000 under these agreements which contain various financial and non-financial covenants, as well as certain material adverse change clauses. If the Company breaches any of the covenants contained within the debt agreements or the material adverse change clauses are triggered, the entire unpaid principal and interest balance would be due and payable upon demand. Management believes that currently available resources combined with the additional $40,000,000 in proceeds raised from the issuance of secured promissory notes in August 2015 (see Note 22) will be sufficient to fund the Company’s cash requirements through at least December 31, 2015.
99
Table of Contents
The Company participates in a heavily regulated and highly competitive crop protection industry and believes that adverse changes in any of the following areas could have a material effect on the Company’s future financial position, results of operations, or cash flows: inability to obtain regulatory approvals, increased competition in the pesticide market, market acceptance of the Company’s products, weather and other seasonal factors beyond the Company’s control, litigation or claims against the Company related to intellectual property, patents, products, or governmental regulation, and the Company’s ability to support increased growth.
Although management recognizes that it will likely need to raise additional funds in the future, there can be no assurance that such efforts will be successful or that, in the event that they are successful, the terms and conditions of such financing will not be unfavorable. Any future equity financing may result in dilution to existing shareholders and any debt financing may include additional restrictive covenants. Any failure to obtain additional financing or not achieve the revenue growth necessary to fund the Company with cash flows from operations will have a material adverse effect upon the Company and will likely result in a substantial reduction in the scope of the Company’s operations and impact the Company’s ability to achieve its planned business objectives. In addition, any future breach of covenants included in the Company’s debt agreements which could result in the lender demanding payment of the unpaid principal and interest balances will have a material adverse effect upon the Company and would likely require the Company to seek to renegotiate these debt arrangements with the lenders. If such negotiations are unsuccessful the Company may be required to seek protection from creditors through bankruptcy proceedings.
2. Restatement of Previously Issued Consolidated Financial Statements
On September 3, 2014, the Company announced that the Audit Committee of the Company’s board of directors had commenced an independent investigation after learning of documents calling into question the recognition of revenue in the fourth quarter of 2013 for an $870,000 transaction.
In February 2015, the Company announced the conclusion and findings of the Audit Committee’s independent investigation. The Audit Committee principally determined that as a result of the failure of certain employees to share with the Company’s finance department or the external auditors important transaction terms with certain distributors, including “inventory protection” arrangements that would permit these distributors to return to the Company certain unsold products, the Company inappropriately recognized revenue for certain historical sales transactions with these distributors prior to satisfying the criteria for revenue recognition required under U.S. Generally Accepted Accounting Principles (GAAP).
Historically, the Company had determined that with limited exceptions, the criteria for revenue recognition were met at the point at which title was transferred to the distributor. However, based on the Audit Committee’s independent investigation the following circumstances were identified for certain transactions that would result in the criteria for revenue recognition not being met with respect to such transactions until the Company’s products were sold through by the distributor, including:
| • | promises to certain distributors to accept returns of unsold inventory where the amount of future returns could not be reasonably estimated and/or we had significant obligations for future performance to bring about resale of the product; and |
| • | arrangements with certain distributors that did not require payment of amounts due until product was resold. |
In light of the foregoing, the Company’s management evaluated the necessity, nature and scope of any restatements of its previously filed financial statements. Based on such evaluation, the Company, among other things, determined to change its revenue recognition methodology from “sell-in” to “sell-through” for sales to certain distributors. In addition to the resulting deferral of revenue recognition to later periods, an aggregate of approximately $2.0 million in product was returned by certain distributors subsequent to June 30, 2014 pursuant to “inventory protection” rights and will not result in recognition of revenue in future periods.
100
Table of Contents
Within these financial statements, the Company has included the restated consolidated financial statements for the year ended December 31, 2013, the restated unaudited condensed consolidated financial statements for the interim periods in 2013 (see Note 21), and the interim periods ended March 31, 2014 and June 30, 2014 (see Note 21), which is referred to as the restatement. The restatement corrects accounting errors related to the following:
Revenue related adjustments:
| • | Sales to certain distributors on a sell-through basis - Adjustments were recorded to reflect recognition of revenue on a sell-through basis, resulting in reductions to product revenue, related party revenue, cost of product revenues and increases to deferred revenue, deferred revenue from related parties, and deferred cost of product revenues. |
| • | Balance sheet reclassifications related to shipments of incorrect product – Adjustments were recorded to reduce accounts receivable balances and increase inventory balances previously recorded for instances where the Company shipped the incorrect product to a customer and would not have had a right to payment. |
| • | Balance sheet reclassifications related to delivered product that may not represent a true sale- Adjustments were recorded to reduce accounts receivable balances or increase customer refund liabilities when cash was received and increase inventory balances previously recorded relating to product that was returned subsequent to June 30, 2014 by certain distributors pursuant to “inventory protection” rights. |
| • | Other individually immaterial errors including certain corrections that had been previously identified but not recorded because they were not material, individually or in the aggregate, to the Company’s consolidated financial statements related to adjustments to sales and cost of sales to correct cutoff on immaterial revenue transactions. While none of these other adjustments were individually material, they are being recorded as part of the restatement process |
Other miscellaneous adjustments:
| • | Adjustments also include items related to other individually immaterial errors including certain corrections that had been previously identified but not recorded because they were not material, individually or in the aggregate, to the Company’s consolidated financial statements. These corrections include adjustments to forfeiture rates applied in determining stock based compensation expense, interest expense on a capital lease, interest expense capitalized into property, plant and equipment, certain accrued liabilities, miscellaneous reclassification entries and disclosures relating to unrecognized tax benefits. While none of these other adjustments were individually material, they are being recorded as part of the restatement process |
101
Table of Contents
The table below summarizes the effects of the restatement adjustments on the consolidated balance sheet as of December 31, 2013 (in thousands):
| DECEMBER 31, 2013 | ||||||||||||
| As reported | Adjustments | As restated | ||||||||||
| Assets |
||||||||||||
| Current assets: |
||||||||||||
| Cash and cash equivalents |
$ | 24,455 | $ | — | $ | 24,455 | ||||||
| Restricted cash, current portion |
— | — | — | |||||||||
| Short-term investments |
13,677 | — | 13,677 | |||||||||
| Accounts receivable |
6,215 | (2,431 | ) | 3,784 | ||||||||
| Accounts receivable from related parties |
903 | — | 903 | |||||||||
| Inventories, net |
11,666 | 1,051 | 12,717 | |||||||||
| Deferred cost of product revenue, including deferred cost of product revenue to related parties of $610 as of December 31, 2013 |
— | 2,861 | 2,861 | |||||||||
| Prepaid expenses and other current assets |
1,737 | (383 | ) | 1,354 | ||||||||
|
|
|
|
|
|
|
|||||||
| Total current assets |
58,653 | 1,098 | 59,751 | |||||||||
| Property, plant and equipment, net |
9,420 | (59 | ) | 9,361 | ||||||||
| Other assets |
806 | — | 806 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total assets |
68,879 | 1,039 | 69,918 | |||||||||
|
|
|
|
|
|
|
|||||||
| Liabilities and stockholders’ equity |
||||||||||||
| Current liabilities: |
||||||||||||
| Accounts payable |
$ | 4,460 | $ | — | $ | 4,460 | ||||||
| Accrued liabilities |
4,380 | 154 | 4,534 | |||||||||
| Deferred revenue, current portion |
1,209 | 2,641 | 3,850 | |||||||||
| Deferred revenue from related parties, current portion |
131 | 997 | 1,128 | |||||||||
| Capital lease obligations, current portion |
1,401 | — | 1,401 | |||||||||
| Debt, current portion |
157 | — | 157 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total current liabilities |
11,738 | 3,792 | 15,530 | |||||||||
| Deferred revenue, less current portion |
744 | — | 744 | |||||||||
| Deferred revenue from related parties, less current portion |
628 | — | 628 | |||||||||
| Capital lease obligations, less current portion |
1,134 | — | 1,134 | |||||||||
| Debt, less current portion |
12,280 | — | 12,280 | |||||||||
| Other liabilities |
571 | — | 571 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total liabilities |
27,095 | 3,792 | 30,887 | |||||||||
| Commitments and contingencies |
||||||||||||
| Stockholders’ equity : |
||||||||||||
| Preferred stock: $0.00001 par value; 20,000 shares authorized, no shares issued or outstanding at December 31, 2013(1) |
— | — | — | |||||||||
| Common stock: $0.00001 par value; 250,000 shares authorized and 19,323 shares issued and outstanding at December 31, 2013(1) |
— | — | — | |||||||||
| Additional paid in capital |
147,220 | — | 147,220 | |||||||||
| Accumulated deficit |
(105,436 | ) | (2,753 | ) | (108,189 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total stockholders’ equity |
41,784 | (2,753 | ) | 39,031 | ||||||||
|
|
|
|
|
|
|
|||||||
| Total liabilities and stockholders’ equity |
$ | 68,879 | $ | 1,039 | $ | 69,918 | ||||||
|
|
|
|
|
|
|
|||||||
| (1) | Par value, shares authorized and shares issued and outstanding represents the as reported and as restated amounts as there were no adjustments to these totals. |
102
Table of Contents
The table below summarizes the effects of the restatement adjustments on the consolidated statement of operations for the year ended December 31, 2013 (in thousands except per share amounts):
| YEAR ENDED DECEMBER 31, 2013 | ||||||||||||||||
| As reported | Revenue Related Adjustments |
Other Miscellaneous Adjustments |
As restated |
|||||||||||||
| Revenues: |
||||||||||||||||
| Product |
$ | 12,657 | $ | (5,069 | ) | $ | — | $ | 7,588 | |||||||
| License |
193 | — | — | 193 | ||||||||||||
| Related party |
1,693 | (1,028 | ) | — | 665 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total revenues |
14,543 | (6,097 | ) | — | 8,446 | |||||||||||
| Cost of product revenues, including cost of product revenues to related parties of $374 (1) for the year ended December 31, 2013 |
10,736 | (3,421 | ) | (72 | ) | 7,243 | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Gross profit |
3,807 | (2,676 | ) | 72 | 1,203 | |||||||||||
| Operating expenses: |
||||||||||||||||
| Research, development and patent |
17,814 | — | 91 | 17,905 | ||||||||||||
| Selling, general and administrative |
15,018 | (10 | ) | 9 | 15,017 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
32,832 | (10 | ) | 100 | 32,922 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss from operations |
(29,025 | ) | (2,666 | ) | (28 | ) | (31,719 | ) | ||||||||
| Other income (expense): |
||||||||||||||||
| Interest income |
49 | — | — | 49 | ||||||||||||
| Interest expense |
(5,997 | ) | — | (59 | ) | (6,056 | ) | |||||||||
| Change in estimated fair value of financial instruments |
6,717 | — | — | 6,717 | ||||||||||||
| Gain on extinguishment of debt |
49 | — | — | 49 | ||||||||||||
| Other expense, net |
(282 | ) | — | — | (282 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total other income, net |
536 | — | (59 | ) | 477 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss before income taxes |
(28,489 | ) | (2,666 | ) | (87 | ) | (31,242 | ) | ||||||||
| Income taxes |
— | — | — | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
(28,489 | ) | (2,666 | ) | (87 | ) | (31,242 | ) | ||||||||
| Deemed dividend on convertible notes |
(1,378 | ) | — | — | (1,378 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss attributable to common stockholders |
$ | (29,867 | ) | $ | (2,666 | ) | $ | (87 | ) | $ | (32,620 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss per common share: |
||||||||||||||||
| Basic |
$ | (3.42 | ) | $ | (0.31 | ) | $ | (0.01 | ) | $ | (3.74 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Diluted |
$ | (3.94 | ) | $ | (0.30 | ) | $ | (0.01 | ) | $ | (4.25 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Weighted-average shares outstanding used in computing net loss per common share: |
||||||||||||||||
| Basic |
8,731 | — | — | 8,731 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Diluted |
8,911 | — | — | 8,911 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| (1) | Cost of product revenues to related parties for the year ended December 31, 2013 was reported as $984. Revenue related adjustments for the year ended December 31, 2013 totaled $(610). There were no other miscellaneous adjustments affecting cost of product revenues to related parties. |
103
Table of Contents
The table below summarizes the effects of the restatement adjustments on the consolidated statement of comprehensive loss for the year ended December 31, 2013 (in thousands):
| YEAR ENDED DECEMBER 31, 2013 | ||||||||||||
| As reported | Adjustments | As restated | ||||||||||
| Net loss |
$ | (28,489 | ) | $ | (2,753 | ) | $ | (31,242 | ) | |||
| Other comprehensive loss |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Comprehensive loss |
$ | (28,489 | ) | $ | (2,753 | ) | $ | (31,242 | ) | |||
|
|
|
|
|
|
|
|||||||
The table below summarizes the effects of the restatement adjustments on the consolidated statement of convertible preferred stock and stockholders’ equity (deficit) for the year ended December 31, 2013 (in thousands):
As reported
| CONVERTIBLE PREFERRED STOCK | ||||||||||||||||||||||||||||||||
| SERIES A | SERIES B | SERIES C | TOTAL | |||||||||||||||||||||||||||||
| SHARES | AMOUNT | SHARES | AMOUNT | SHARES | AMOUNT | SHARES | AMOUNT | |||||||||||||||||||||||||
| Balance at December 31, 2012 |
1,484 | $ | 3,747 | 2,242 | $ | 10,758 | 4,778 | $ | 25,107 | 8,504 | $ | 39,612 | ||||||||||||||||||||
| Net loss, as reported |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Exercise of stock options |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Share-based compensation |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Deemed dividend, convertible notes |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Cash exercise of preferred stock warrants |
— | — | 10 | 47 | — | — | 10 | 47 | ||||||||||||||||||||||||
| Net exercise of preferred stock warrants |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Conversion of preferred stock into common stock |
(1,484 | ) | (3,747 | ) | (2,252 | ) | (10,805 | ) | (4,778 | ) | (25,107 | ) | (8,514 | ) | (39,659 | ) | ||||||||||||||||
| Convertible notes converted into common stock |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Cash exercise of common stock warrants |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Net exercise of common stock warrants |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Reclassification of warrants from liability to equity |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Issuance of common stock upon initial public offering, net of offering costs and underwriter commission |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Balance at December 31, 2013, as reported |
— | $ | — | — | $ | — | — | $ | — | — | $ | — | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
104
Table of Contents
|
COMMON STOCK |
ADDITIONAL PAID IN CAPITAL |
ACCUMULATED DEFICIT |
TOTAL STOCKHOLDERS’ EQUITY (DEFICIT) |
|||||||||||||||||
| SHARES | AMOUNT | |||||||||||||||||||
| Balance at December 31, 2012 |
1,267 | $ | — | $ | 1,322 | $ | (75,569 | ) | $ | (74,247 | ) | |||||||||
| Net loss, as reported |
— | — | — | (28,489 | ) | (28,489 | ) | |||||||||||||
| Exercise of stock options |
217 | — | 250 | — | 250 | |||||||||||||||
| Share-based compensation |
— | — | 2,300 | — | 2,300 | |||||||||||||||
| Deemed dividend, convertible notes |
— | — | — | (1,378 | ) | (1,378 | ) | |||||||||||||
| Cash exercise of preferred stock warrants |
— | — | — | — | — | |||||||||||||||
| Net exercise of preferred stock warrants |
71 | — | — | — | — | |||||||||||||||
| Conversion of preferred stock into common stock |
8,514 | — | 39,659 | — | 39,659 | |||||||||||||||
| Convertible notes converted into common stock |
3,741 | — | 44,890 | — | 44,890 | |||||||||||||||
| Cash exercise of common stock warrants |
3 | — | 25 | — | 25 | |||||||||||||||
| Net exercise of common stock warrants |
47 | — | — | — | — | |||||||||||||||
| Reclassification of warrants from liability to equity |
— | — | 2,669 | — | 2,669 | |||||||||||||||
| Issuance of common stock upon initial public offering, net of offering costs and underwriter commission |
5,463 | — | 56,105 | — | 56,105 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balance at December 31, 2013, as reported |
19,323 | $ | — | $ | 147,220 | $ | (105,436 | ) | $ | 41,784 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
105
Table of Contents
Adjustments
| CONVERTIBLE PREFERRED STOCK | ||||||||||||||||||||||||||||||||
| SERIES A | SERIES B | SERIES C | TOTAL | |||||||||||||||||||||||||||||
| SHARES | AMOUNT | SHARES | AMOUNT | SHARES | AMOUNT | SHARES | AMOUNT | |||||||||||||||||||||||||
| Balance at December 31, 2012 |
— | $ | — | — | $ | — | — | $ | — | — | $ | — | ||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Exercise of stock options |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Share-based compensation |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Deemed dividend, convertible notes |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Cash exercise of preferred stock warrants |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Net exercise of preferred stock warrants |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Conversion of preferred stock into common stock |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Convertible notes converted into common stock |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Cash exercise of common stock warrants |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Net exercise of common stock warrants |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Reclassification of warrants from liability to equity |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Issuance of common stock upon initial public offering, net of offering costs and underwriter commission |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Balance at December 31, 2013 |
— | $ | — | — | $ | — | — | $ | — | — | $ | — | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
|
COMMON STOCK |
ADDITIONAL PAID IN CAPITAL |
ACCUMULATED DEFICIT |
TOTAL STOCKHOLDERS’ EQUITY (DEFICIT) |
|||||||||||||||||
| SHARES | AMOUNT | |||||||||||||||||||
| Balance at December 31, 2012 |
— | $ | — | $ | — | $ | — | $ | — | |||||||||||
| Net loss |
— | — | — | (2,753 | ) | (2,753 | ) | |||||||||||||
| Exercise of stock options |
— | — | — | — | — | |||||||||||||||
| Share-based compensation |
— | — | — | — | — | |||||||||||||||
| Deemed dividend, convertible notes |
— | — | — | — | — | |||||||||||||||
| Cash exercise of preferred stock warrants |
— | — | — | — | — | |||||||||||||||
| Net exercise of preferred stock warrants |
— | — | — | — | — | |||||||||||||||
| Conversion of preferred stock into common stock |
— | — | — | — | — | |||||||||||||||
| Convertible notes converted into common stock |
— | — | — | — | — | |||||||||||||||
| Cash exercise of common stock warrants |
— | — | — | — | — | |||||||||||||||
| Net exercise of common stock warrants |
— | — | — | — | — | |||||||||||||||
| Reclassification of warrants from liability to equity |
— | — | — | — | — | |||||||||||||||
| Issuance of common stock upon initial public offering, net of offering costs and underwriter commission |
— | — | — | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balance at December 31, 2013 |
— | $ | — | $ | — | $ | (2,753 | ) | $ | (2,753 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
106
Table of Contents
As restated
| CONVERTIBLE PREFERRED STOCK | ||||||||||||||||||||||||||||||||
| SERIES A | SERIES B | SERIES C | TOTAL | |||||||||||||||||||||||||||||
| SHARES | AMOUNT | SHARES | AMOUNT | SHARES | AMOUNT | SHARES | AMOUNT | |||||||||||||||||||||||||
| Balance at December 31, 2012 |
1,484 | $ | 3,747 | 2,242 | $ | 10,758 | 4,778 | $ | 25,107 | 8,504 | $ | 39,612 | ||||||||||||||||||||
| Net loss, as restated |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Exercise of stock options |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Share-based compensation |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Deemed dividend, convertible notes |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Cash exercise of preferred stock warrants |
— | — | 10 | 47 | — | — | 10 | 47 | ||||||||||||||||||||||||
| Net exercise of preferred stock warrants |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Conversion of preferred stock into common stock |
(1,484 | ) | (3,747 | ) | (2,252 | ) | (10,805 | ) | (4,778 | ) | (25,107 | ) | (8,514 | ) | (39,659 | ) | ||||||||||||||||
| Convertible notes converted into common stock |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Cash exercise of common stock warrants |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Net exercise of common stock warrants |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Reclassification of warrants from liability to equity |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Issuance of common stock upon initial public offering, net of offering costs and underwriter commission |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Balance at December 31, 2013, as restated |
— | $ | — | — | $ | — | — | $ | — | — | $ | — | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
|
COMMON STOCK |
ADDITIONAL PAID IN CAPITAL |
ACCUMULATED DEFICIT |
TOTAL STOCKHOLDERS’ EQUITY (DEFICIT) |
|||||||||||||||||
| SHARES | AMOUNT | |||||||||||||||||||
| Balance at December 31, 2012 |
1,267 | $ | — | $ | 1,322 | $ | (75,569 | ) | $ | (74,247 | ) | |||||||||
| Net loss, as restated |
— | — | — | (31,242 | ) | (31,242 | ) | |||||||||||||
| Exercise of stock options |
217 | — | 250 | — | 250 | |||||||||||||||
| Share-based compensation |
— | — | 2,300 | — | 2,300 | |||||||||||||||
| Deemed dividend, convertible notes |
— | — | — | (1,378 | ) | (1,378 | ) | |||||||||||||
| Cash exercise of preferred stock warrants |
— | — | — | — | — | |||||||||||||||
| Net exercise of preferred stock warrants |
71 | — | — | — | — | |||||||||||||||
| Conversion of preferred stock into common stock |
8,514 | — | 39,659 | — | 39,659 | |||||||||||||||
| Convertible notes converted into common stock |
3,741 | — | 44,890 | — | 44,890 | |||||||||||||||
| Cash exercise of common stock warrants |
3 | — | 25 | — | 25 | |||||||||||||||
| Net exercise of common stock warrants |
47 | — | — | — | — | |||||||||||||||
| Reclassification of warrants from liability to equity |
— | — | 2,669 | — | 2,669 | |||||||||||||||
| Issuance of common stock upon initial public offering, net of offering costs and underwriter commission |
5,463 | — | 56,105 | — | 56,105 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balance at December 31, 2013, as restated |
19,323 | $ | — | $ | 147,220 | $ | (108,189 | ) | $ | 39,031 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
107
Table of Contents
The table below summarizes the effects of the restatement adjustments on the consolidated statement of cash flows for the year ended December 31, 2013 (in thousands):
| YEAR ENDED DECEMBER 31, 2013 | ||||||||||||
| As reported | Adjustments | As restated | ||||||||||
| Cash flows from operating activities |
||||||||||||
| Net loss |
$ | (28,489 | ) | $ | (2,753 | ) | $ | (31,242 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||||
| Depreciation and amortization |
976 | — | 976 | |||||||||
| Loss on disposal of equipment |
231 | — | 231 | |||||||||
| Share-based compensation |
2,300 | — | 2,300 | |||||||||
| Non-cash interest expense |
4,315 | — | 4,315 | |||||||||
| Change in estimated fair value of financial instruments |
(6,717 | ) | — | (6,717 | ) | |||||||
| Gain on extinguishment of debt |
(49 | ) | — | (49 | ) | |||||||
| Amortization of investment securities premiums/discounts, net |
18 | — | 18 | |||||||||
| Net changes in operating assets and liabilities: |
||||||||||||
| Accounts receivable |
(3,381 | ) | 2,431 | (950 | ) | |||||||
| Accounts receivable from related parties |
(767 | ) | — | (767 | ) | |||||||
| Inventories |
(6,794 | ) | (1,051 | ) | (7,845 | ) | ||||||
| Prepaid expenses and other assets |
991 | 383 | 1,374 | |||||||||
| Deferred cost of product revenues |
— | (2,861 | ) | (2,861 | ) | |||||||
| Accounts payable |
1,682 | — | 1,682 | |||||||||
| Accrued and other liabilities |
987 | 154 | 1,141 | |||||||||
| Deferred revenue |
823 | 2,641 | 3,464 | |||||||||
| Deferred revenue from related parties |
(131 | ) | 997 | 866 | ||||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in operating activities |
(34,005 | ) | (59 | ) | (34,064 | ) | ||||||
| Cash flows from investing activities |
||||||||||||
| Purchases of property, plant and equipment |
(4,025 | ) | 59 | (3,966 | ) | |||||||
| Proceeds from sale of equipment |
41 | — | 41 | |||||||||
| Purchase of short-term investments |
(17,477 | ) | — | (17,477 | ) | |||||||
| Maturities of short-term investments |
3,782 | — | 3,782 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in investing activities |
(17,679 | ) | 59 | (17,620 | ) | |||||||
| Cash flows from financing activities |
||||||||||||
| Proceeds from public offerings, net of offering costs and underwriter commissions |
56,105 | — | 56,105 | |||||||||
| Proceeds from issuance of convertible notes payable |
6,529 | — | 6,529 | |||||||||
| Proceeds from issuance of debt, net of financing costs |
3,700 | — | 3,700 | |||||||||
| Repayment of debt |
(9,433 | ) | — | (9,433 | ) | |||||||
| Repayment of capital leases |
(229 | ) | — | (229 | ) | |||||||
| Proceeds from secured borrowing |
2,880 | — | 2,880 | |||||||||
| Reductions in secured borrowing |
(2,880 | ) | — | (2,880 | ) | |||||||
| Change in restricted cash |
9,139 | — | 9,139 | |||||||||
| Proceeds from exercise of stock options |
250 | — | 250 | |||||||||
| Proceeds from exercise of preferred stock warrants |
47 | — | 47 | |||||||||
| Proceeds from exercise of common stock warrants |
25 | — | 25 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by financing activities |
66,133 | — | 66,133 | |||||||||
| Net increase in cash and cash equivalents |
14,449 | — | 14,449 | |||||||||
| Cash and cash equivalents, beginning of year |
10,006 | — | 10,006 | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash and cash equivalents, end of year |
$ | 24,455 | $ | — | $ | 24,455 | ||||||
|
|
|
|
|
|
|
|||||||
| Supplemental disclosure of cash flow information |
||||||||||||
| Cash paid for interest, net of capitalized interest of $636 for the year ended December 31, 2013(1) |
$ | 1,682 | $ | 10 | $ | 1,692 | ||||||
|
|
|
|
|
|
|
|||||||
| Supplemental disclosure of non-cash investing and financing activities |
||||||||||||
| Property, plant and equipment included in accounts payable and accrued liabilities |
$ | 1,009 | $ | — | $ | 1,009 | ||||||
|
|
|
|
|
|
|
|||||||
| Equipment acquired under capital leases |
$ | 2,106 | $ | — | $ | 2,106 | ||||||
|
|
|
|
|
|
|
|||||||
| Interest added to the principal of convertible notes |
$ | 1,623 | $ | — | $ | 1,623 | ||||||
|
|
|
|
|
|
|
|||||||
| Reclassification of warrants from liabilities to equity |
$ | 2,669 | $ | — | $ | 2,669 | ||||||
|
|
|
|
|
|
|
|||||||
| Conversion of convertible notes to common stock |
$ | 44,890 | $ | — | $ | 44,890 | ||||||
|
|
|
|
|
|
|
|||||||
| Conversion of preferred stock to common stock |
$ | 39,659 | $ | — | $ | 39,659 | ||||||
|
|
|
|
|
|
|
|||||||
| (1) | Capitalized interest was previously reported as $695. An adjustment of ($59) was recorded as a result of the restatement. |
108
Table of Contents
3. Significant Accounting Policies
Basis of Presentation
The consolidated financial statements include the accounts of the Company and its wholly-owned subsidiary. All significant intercompany balances and transactions have been eliminated in consolidation.
Use of Estimates
The preparation of financial statements in conformity with U.S. generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Cash and Cash Equivalents
The Company considers all highly liquid financial instruments purchased with a maturity of three months or less to be cash equivalents. Cash and cash equivalents consist of cash on deposit, money market funds and certificates of deposit accounts (CDs) with U.S. financial institutions. The Company is exposed to credit risk in the event of default by financial institutions to the extent that cash and cash equivalents balances with financial institutions are in excess of amounts that are insured by the Federal Deposit Insurance Corporation. The Company has not experienced any losses on these deposits.
Restricted Cash
The Company’s restricted cash consists of cash that the Company is contractually obligated to maintain on deposit at a bank in accordance with the promissory note entered into in June 2014. See Note 9 for further discussion.
Short-Term Investments
The Company’s short-term investments consist of certificates of deposit with original maturities less than one year but greater than three months which are classified as held-to-maturity. Certificates of deposit are stated at their amortized cost with realized gains or losses, if any, reported as other income or expenses in the consolidated statements of operations. The Company routinely evaluates the realizability of its short-term investments and recognizes an impairment charge when a decline in the estimated fair value of a short-term investment is below the amortized cost and determined to be other-than-temporary. The Company considers various factors in determining whether to recognize an impairment charge, including the duration of time and the severity to which the fair value has been less than amortized cost, any adverse changes in the investee’s financial condition, and the Company’s intent and ability to hold the short-term investment for a period of time sufficient to allow for any anticipated recovery in market value. To date, the Company has not recognized any losses on its short-term investments.
The amortized cost and estimated fair values of short-term investments are summarized in the following table (in thousands):
| DECEMBER 31, 2013 | ||||||||||||||||
| AMORTIZED COST |
GROSS UNREALIZED GAINS |
GROSS UNREALIZED LOSSES |
ESTIMATED FAIR VALUE |
|||||||||||||
| Securities Held-to-Maturity |
||||||||||||||||
| Certificates of deposit, with maturities less than 1 year |
$ | 13,677 | $ | — | $ | (4 | ) | $ | 13,673 | |||||||
|
|
|
|
|
|
|
|
|
|||||||||
109
Table of Contents
The short-term investments at December 31, 2013 were in inactive markets and, therefore, the estimated fair value is measured based on the Level 2 valuation hierarchy. The Company did not have any investments in securities as of December 31, 2014.
Fair Value of Financial Instruments
ASC 820, Fair Value Measurements (ASC 820), clarifies that fair value is an exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or liability.
ASC 820 requires that the valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. ASC 820 establishes a three tier value hierarchy, which prioritizes inputs that may be used to measure fair value as follows:
| • | Level 1—Quoted prices in active markets for identical assets or liabilities. |
| • | Level 2—Observable inputs other than quoted prices in active markets for identical assets and liabilities, quoted prices for identical or similar assets or liabilities in inactive markets, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. |
| • | Level 3—Inputs that are generally unobservable and typically reflect management’s estimate of assumptions that market participants would use in pricing the asset or liability. |
The following table presents the Company’s financial assets measured at fair value on a recurring basis as of December 31, 2014 and 2013 (in thousands):
| DECEMBER 31, 2014 | ||||||||||||||||
| TOTAL | LEVEL 1 | LEVEL 2 | LEVEL 3 | |||||||||||||
| Assets |
||||||||||||||||
| Money market funds |
$ | 14,746 | $ | 14,746 | $ | — | $ | — | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| DECEMBER 31, 2013 | ||||||||||||||||
| TOTAL | LEVEL 1 | LEVEL 2 | LEVEL 3 | |||||||||||||
| Assets |
||||||||||||||||
| Money market funds |
$ | 6,717 | $ | 6,717 | $ | — | $ | — | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
The Company’s money market funds are held at registered investment companies and as of December 31, 2014 and 2013 were in active markets and, therefore, are measured based on the Level 1 valuation hierarchy.
The Company estimated the fair value of the common and preferred stock warrant liabilities as of December 31, 2012 using the Probability Weighted Expected Return Method (PWERM), which analyzes the returns afforded to common equity holders under multiple future scenarios. Under the PWERM, share value is based upon the probability-weighted present value of expected future net cash flows (distributions to stockholders), considering each of the possible future events and giving consideration to the rights and preferences of each share class. This method is most appropriate when the long-term outlook for an enterprise is largely known and multiple future scenarios can be reasonably estimated.
The common and preferred stock warrant liabilities were valued by a PWERM valuation using six scenarios, which included three initial public offering scenarios, two merger scenarios and a sale of the Company’s intellectual property. An annual discount rate of 35% was applied to the PWERM valuations as of December 31, 2012. The common stock warrant liability valuation also included an 18% discount for lack of marketability as of December 31, 2012. As the PWERM estimates the fair value of the common and preferred stock warrant liabilities using unobservable inputs, it is considered to be a Level 3 fair value measurement.
110
Table of Contents
Effective on the date of the IPO, under ASC 815-40-15, Contracts in Entity’s Own Equity (ASC 815-40-15), the common and preferred stock warrant liabilities were considered to be indexed to the Company’s stock, and accordingly, the total warrants liability of $2,669,000 was reclassified and included in stockholders’ equity (deficit) as of December 31, 2013. The Company revalued the warrants immediately prior to the IPO. The fair value of the warrants which would have expired on the date of the IPO unless exercised was determined using the intrinsic method based on the IPO price of $12.00 per share, which is deemed a Level 2 fair value measurement. The fair value of the warrants that would not have expired on the date of the IPO regardless of whether or not they were exercised was determined using the Black-Scholes-Merton option-pricing model, which is deemed a Level 3 fair value measurement.
As a result of the change in estimated fair value between December 31, 2012 or the issuance dates of the warrants issued during the year ended December 31, 2013 and the closing of the IPO, the Company recognized a net gain from the total change in estimated fair value of the common and preferred stock warrant liabilities as shown in the tables below.
The following table provides a reconciliation of the beginning and ending balances for the common and preferred stock warrant liabilities measured at fair value using significant unobservable inputs (Level 3). The amounts included in the “Transfers out of Level 3” represent the beginning balance in the interim quarter during which it was transferred (in thousands):
| COMMON STOCK WARRANT LIABILITY |
||||
| Fair value at December 31, 2012 |
$ | 301 | ||
| Warrants issued |
900 | |||
| Change in fair value recorded in change in fair value of financial instruments |
377 | |||
| Transfers out of Level 3 |
(434 | ) | ||
| Reclassified to stockholders’ equity (deficit) |
(1,144 | ) | ||
|
|
|
|||
| Fair value at December 31, 2013 |
$ | — | ||
|
|
|
|||
| PREFERRED STOCK WARRANT LIABILITY |
||||
| Fair value at December 31, 2012 |
$ | 1,884 | ||
| Change in fair value recorded in change in fair value of financial instruments |
(823 | ) | ||
| Transfers out of Level 3 |
(140 | ) | ||
| Reclassified to stockholders’ equity (deficit) |
(921 | ) | ||
|
|
|
|||
| Fair value at December 31, 2013 |
$ | — | ||
|
|
|
|||
Effective on the date of the IPO, all of the Company’s convertible notes were converted into shares of common stock. Prior to the IPO, convertible notes were valued by a PWERM valuation utilizing inputs similar to those used for estimating fair values of the common and preferred stock warrant liabilities described above. A discount rate of 25% was used for valuing the March and October 2012 Convertible Notes, defined in Note 9, as of December 31, 2012. A discount rate of 18% was used for valuing the October 2012 Subordinated Convertible Notes and the December 2012 Convertible Note, both defined in Note 9, as of December 31, 2012. These annual discount rates were applied in the PWERM valuation as of December 31, 2012. The Company revalued the convertible notes immediately prior to the IPO. As a result of the IPO, the number of shares to be issued became known and the Company estimated the fair value of the convertible notes using the intrinsic method based on the IPO price of $12.00 per share, which is deemed a Level 2 fair value measurement. Due to the change in estimated
111
Table of Contents
fair values between December 31, 2012 or the issuance dates of the convertible notes issued during the year ended December 31, 2013 and the closing of the IPO, the Company recognized a gain from the change in estimated fair value of the convertible notes as shown in the table below.
The following table provides a reconciliation of the beginning and ending balances for the convertible notes measured at fair value using significant unobservable inputs (Level 3). The amounts included in the “Transfers out of Level 3” represent the beginning balance in the interim quarter during which it was transferred (in thousands):
| Fair value at December 31, 2012 |
$ | 41,860 | ||
| Convertible notes issued |
9,069 | |||
| Convertible notes cancelled |
(1,360 | ) | ||
| Accrued interest |
1,299 | |||
| Change in fair value recorded in change in fair value of financial instruments |
(2,634 | ) | ||
| Transfers out of Level 3 |
(48,234 | ) | ||
|
|
|
|||
| Fair value at December 31, 2013 |
$ | — | ||
|
|
|
During the year ended December 31, 2013, as noted above, there were $574,000 of preferred and common stock warrants and $48,234,000 of convertible notes transferred from the Level 3 to Level 2 category. There were no such transfers from the Level 3 to Level 2 category during the year ended December 31, 2012. Further, there were no transfers from the Level 2 to Level 1 category during the years ended December 31, 2013 or 2012.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk consist principally of cash, cash equivalents, short-term investments, accounts receivable and debt. The Company deposits its cash, cash equivalents and short-term investments with high credit quality domestic financial institutions with locations in the U.S. Such deposits may exceed federal deposit insurance limits. The Company believes the financial risks associated with these financial instruments are minimal.
The Company’s customer base is dispersed across many different geographic areas, and currently most customers are pest management distributors in the U.S. Generally, receivables are due up to 120 days from the invoice date and are considered past due after this date, although the Company may offer extended terms from time to time.
During the years ended December 31, 2014, 2013 and 2012, 12%, 16% and 20%, respectively, of the Company’s revenues were generated from international customers.
From inception through December 31, 2012, the Company’s principal source of revenues was its Regalia product line. During the years ended December 31, 2014 and 2013, Grandevo and Regalia were the principal sources of the Company’s total revenues. During the years ended December 31, 2014, 2013 and 2012, these two product lines accounted for 91%, 95% and 96%, respectively, of the Company’s total revenues.
Customers with 10% or more of the Company’s total revenues for any one of the years presented consist of the following:
| CUSTOMER A |
CUSTOMER B |
CUSTOMER C |
||||||||||
| For the years ended December 31, |
||||||||||||
| 2014 |
30 | % | 2 | % | 13 | % | ||||||
| 2013 (as restated) |
20 | % | 2 | % | 8 | % | ||||||
| 2012 |
33 | % | 13 | % | 12 | % | ||||||
112
Table of Contents
Customers with 10% or more of the Company’s outstanding accounts receivable as of either December 31, 2014 or 2013 consist of the following:
| CUSTOMER A |
CUSTOMER B |
CUSTOMER C (1) |
CUSTOMER D |
|||||||||||||
| December 31, 2014 |
37 | % | 23 | % | 0 | % | 10 | % | ||||||||
| December 31, 2013 (as restated) |
11 | % | 0 | % | 19 | % | 17 | % | ||||||||
| (1) | Represents accounts receivable from related parties. See Note 18 for further discussion. |
Concentrations of Supplier Dependence
The active ingredient in the Company’s Regalia product line is derived from the giant knotweed plant, which the Company obtains from China. The Company’s single supplier acquires raw knotweed from numerous regional sources and performs an extraction process on this plant, creating a dried extract that is shipped to the Company’s manufacturing plant. A disruption at this supplier’s manufacturing site or a disruption in trade between the U.S. and China could negatively impact sales of Regalia. The Company currently uses one supplier and does not have a long-term supply contract with this supplier. Although the Company has identified additional sources of knotweed, there can be no assurance that the Company will continue to be able to obtain dried extract from China at a competitive price.
Accounts Receivable
The carrying value of the Company’s receivables represents their estimated net realizable values. The Company generally does not require collateral and estimates any required allowance for doubtful accounts based on historical collection trends, the age of outstanding receivables, and existing economic conditions. If events or changes in circumstances indicate that specific receivable balances may be impaired, further consideration is given to the collectibility of those balances and the allowance is recorded accordingly. Past-due receivable balances are written-off when the Company’s internal collection efforts have been unsuccessful in collecting the amount due. During the years ended December 31, 2014, 2013 and 2012, no receivable balances were written-off. As of December 31, 2014 and 2013, the Company had no allowance for doubtful accounts.
Inventories
Inventories are stated at the lower of cost or market value (net realizable value or replacement cost) and include the cost of material and external and internal labor and manufacturing costs. Cost is determined on the first-in, first-out basis. The Company provides for inventory reserves when conditions indicate that the selling price may be less than cost due to physical deterioration, obsolescence, changes in price levels, or other factors. Additionally, the Company provides reserves for excess and slow-moving inventory on hand that is not expected to be sold to reduce the carrying amount of excess and slow-moving inventory to its estimated net realizable value. The reserves are based upon estimates about future demand from the Company’s customers and distributors and market conditions. As of December 31, 2014, the Company had $668,000 in reserves against its inventories. As of December 31, 2013, the Company had $45,000 reserves against its inventories. During the year ended December 31, 2014, the Company recorded, as a component of cost of product revenues, adjustments to the Company’s inventory reserve of $695,000 due to quantities on hand that may not be used prior to expiration as a result of lower production and sales forecasts.
During the year ended December 31, 2014, the Company recorded, as a component of cost of product revenues, an $894,000 write-off of inventory primarily due the identification of inventory that would not be suitable for sale in future periods either due to the inventory not passing quality inspection or the efficacy had declined, an adjustment of $890,000 as a result of actual utilization of the plant being less than what is considered normal capacity of the Company’s manufacturing facilities and a $270,000 write-down of the carrying value of inventory to net realizable value. During the year ended December 31, 2013, the Company recorded, as a component of
113
Table of Contents
cost of product revenues, an inventory write-off of $205,000 primarily due the identification of inventory that would not be suitable for sale and an adjustment of $194,000 to write-down its Zequanox inventory to net realizable value.
Inventories, net consist of the following (in thousands):
| DECEMBER 31 | ||||||||
| 2014 | 2013 | |||||||
| As restated | ||||||||
| Raw materials |
$ | 5,692 | $ | 5,355 | ||||
| Work in progress |
1,150 | 2,989 | ||||||
| Finished goods |
5,378 | 3,733 | ||||||
| Finished goods held at customers |
424 | 640 | ||||||
|
|
|
|
|
|||||
| $ | 12,644 | $ | 12,717 | |||||
|
|
|
|
|
|||||
Deferred Cost of Product Revenues
Deferred cost of product revenues are stated at the lower of cost or net realizable value and include product sold where title has transferred but the criteria for revenue recognition have not been met. As of December 31, 2014 and 2013, the Company recorded current deferred cost of product revenues of $1,797,000 and $2,861,000, respectively. During the year ended December 31, 2013, the Company recorded an adjustment of $174,000 to write down the carrying value of deferred cost of product revenues to net realizable value.
Property, Plant and Equipment
Property, plant and equipment are recorded at cost and are depreciated using the straight-line method over their estimated useful lives. The Company generally uses the following estimated useful lives for each asset category:
| ASSET CATEGORY |
ESTIMATED USEFUL LIFE | |||
| Building |
30 years | |||
| Computer equipment |
2-3 years | |||
| Machinery and equipment |
3-20 years | |||
| Office equipment |
3-5 years | |||
| Furniture |
3-5 years | |||
| Leasehold improvements |
Shorter of lease term or useful life | |||
| Software |
3 years | |||
Amortization of assets under capital leases is included in depreciation expense. Maintenance, repairs and minor renewals are expensed as incurred. Expenditures that substantially increase an asset’s useful life are capitalized.
Deferred Financing Costs
Deferred financing costs, net include fees and costs incurred to obtain long-term financing. The costs are being amortized over the terms of the respective loans on a basis that approximates level yield. Unamortized deferred financing fees are written-off when debt is retired before the maturity date. Upon the amendment or termination of debt, unamortized deferred financing fees are accounted for in accordance with ASC 470-50-40, Debt Modifications and Extinguishments (ASC 470-50-40). As of December 31, 2014, $172,000 and $269,000 of the deferred financing costs were recorded as a component of current and non-current other assets, respectively, and are being amortized to interest expense. As of December 31, 2013, $458,000 and $148,000 of the deferred financing costs were recorded as a component of current and non-current other assets, respectively, and are being amortized to interest expense.
114
Table of Contents
Impairment of Long-Lived Assets
Impairment losses related to long-lived assets are recognized in the event the net carrying value of such assets is not recoverable and exceeds fair value. The Company evaluates the recoverability of its long-lived assets whenever events or changes in circumstances indicate that the carrying value of an asset may not be recoverable. The carrying amount of a long-lived asset (asset group) is not recoverable if it exceeds the sum of the undiscounted cash flows expected to result from the use and eventual disposition of the asset (asset group). If an asset is considered is not recoverable, the impairment loss is measured as the amount by which the carrying value of the asset group exceeds its estimated fair value. To date, the Company has not recognized any such impairment loss associated with its long-lived assets.
Preferred Stock Warrant Liability
The Company accounted for outstanding warrants exercisable into shares of its preferred stock as liability instruments as the preferred stock into which these warrants were convertible were contingently redeemable upon the occurrence of certain events or transactions. The Company adjusted the warrant instruments to fair value at each reporting period with the change in fair value recorded as a component of change in estimated fair value of financial instruments in the consolidated statements of operations. Effective on the date of the IPO, under ASC 815-40-15, the preferred stock warrant liabilities were considered to be indexed to the Company’s stock, and accordingly, the total warrants liability was reclassified and included in stockholders’ equity (deficit) as of December 31, 2013.
Common Stock Warrant Liability
The Company issued detachable common stock warrants in connection with the October 2012 and April 2013 Secured Promissory Notes as defined and discussed in Note 9 to purchase a variable number of the Company’s shares of common stock based on a fixed monetary amount. As the predominant settlement feature of these common stock warrants was to settle a fixed monetary amount in a variable number of shares, these common stock warrants fell within the scope of ASC 480, Distinguishing Liabilities from Equity (ASC 480). Accordingly, these common stock warrants were recorded at estimated fair value on their issuance date and were adjusted to their estimated fair value as of each reporting date with the change in estimated fair value recorded as a component of change in estimated fair value of financial instruments in the accompanying consolidated statements of operations. Effective on the date of the IPO, under ASC 815-40-15, the common stock warrant liabilities were considered to be indexed to the Company’s stock, and accordingly, the total warrants liability was reclassified and included in stockholders’ equity (deficit) as of December 31, 2013.
Revenue Recognition
The Company recognizes revenues when persuasive evidence of an arrangement exists, transfer of title has occurred or services have been rendered, the price is fixed or determinable and collectability is reasonably assured. If contractual obligations, acceptance provisions or other contingencies exist which indicate that the price is not fixed and determinable, revenue is recognized after such obligations or provisions are fulfilled or expire.
Product revenues consist of revenues generated from sales to distributors and from sales of the Company’s products to distributors and direct customers, net of rebates and cash discounts. For sales of products made to distributors, the Company recognizes revenue either on a sell-in or sell-through basis depending on the specific facts and circumstances of the transaction(s) with the distributor. Factors considered include, but are not limited to, whether the payment terms offered to the distributor are structured to correspond to when product is resold, the distributor history of adhering to the terms of its contractual arrangements with the Company, whether the Company has a pattern of granting concessions for the benefit of the distributor and whether there are other conditions that may indicate that the sale to the distributor is not substantive.
115
Table of Contents
In some cases, the Company recognizes distributor revenue as title and risk of loss passes, assuming all other revenue recognition criteria has been satisfied (the “sell-in” method). For certain sales to certain distributors, the revenue recognition criteria for distributor sales are not satisfied at the time title and risk of loss passes; specifically, in instances where “inventory protection” arrangements were offered to distributors that would permit these distributors to return to the Company certain unsold products, the Company considers the arrangement not to be fixed or determinable, and accordingly, revenue is deferred until products are resold to customers of the distributor (the “sell-through” method). As of December 31, 2014 and 2013, the Company recorded current deferred product revenues of $3,190,000 and $4,654,000, respectively. The cost of goods sold associated with such deferral are also deferred and classified as deferred cost of product revenues in the consolidated balance sheets. Cash received from customers related to delivered product that may not represent a true sale are classified as customer refund liabilities in the consolidated balance sheets and the related cost of inventory remains in inventory in the consolidated balance sheets until the product is returned or is resold to customers of the distributor and revenue is recognized. For the years ending December 31, 2014 and 2013, 53% and 23%, respectively, of total revenue was recognized on a sell-through basis. During the year ending December 31, 2012, there was no revenue recognized on a sell-through basis.
From time to time, the Company offers certain product rebates to its distributors and to growers, which are estimated and recorded as reductions to product revenues, and an accrued liability is recorded at the later of when the revenues are recorded or the rebate is being offered.
The Company recognizes license revenues pursuant to strategic collaboration and distribution agreements under which the Company receives payments for the achievement of testing validation, regulatory progress and commercialization events. As these activities and payments are associated with exclusive rights that the Company provides in connection with strategic collaboration and distribution agreements over the term of the agreements, revenues related to the payments received are deferred and recognized over the term of the exclusive distribution period of the respective agreement. For the year ended December 31, 2014, the Company received payments totaling $500,000 and as of December 31, 2014 had $750,000 included in accounts receivable. No payments were received under these agreements during the year ended December 31, 2013. For the year ended December 31, 2012, the Company received payments totaling $1,533,000, of which $1,000,000 was received from a related party. For the years ended December 31, 2014, 2013 and 2012, the Company recognized $232,000, $193,000 and $179,000, respectively, as license revenues, excluding related party revenues, in the accompanying consolidated statements of operations.
The Company has a strategic collaboration and distribution agreement with Syngenta, an affiliate of, Syngenta Ventures Pte. LTD (Syngenta Ventures), which until June 2014, was a 5% stockholder. In connection with the secondary offering, Syngenta Ventures sold 600,000 common shares, reducing its ownership percentage below 5%. Beginning in June 2014, revenue recognized under this arrangement has been included in license revenues. For the year ended December 31, 2014, the Company recognized $333,000 of related party revenues relating to the period that Syngenta Ventures was one of its 5% stockholders. For the years ended December 31, 2013, the Company recognized $131,000 of related party revenues under these agreements. There were no related party license revenues recognized for the year ended December 31, 2012. At December 31, 2014, the Company recorded current and non-current deferred revenues of $331,000 and $2,050,000, respectively, related to payments received under these agreements. At December 31, 2013, the Company recorded current and non-current deferred revenues of $324,000 and $1,372,000, respectively, related to payments received under these agreements, of which $131,000 and $628,000, respectively, related to deferred revenues from related parties based on the terms of the Company’s commercial agreement with Syngenta.
Research, Development and Patent Expenses
Research and development expenses, includes payroll-related expenses, toxicology costs, regulatory costs, consulting costs and lab costs. Patent expenses includes legal costs relating to the patents and patent filing costs. These expenses are expensed to operations as incurred. For the years ended December 31, 2014, 2013 and 2012,
116
Table of Contents
research and development expenses totaled $18,110,000, $16,918,000 and $12,140,000, respectively, and patent expenses totaled $1,171,000, $987,000 and $601,000, respectively.
Shipping and Handling Costs
Amounts billed for shipping and handling are included as a component of product revenues. Related costs for shipping and handling have been included as a component of cost of product revenues.
Advertising
The Company expenses advertising costs as incurred. Advertising costs for the years ended December 31, 2014, 2013 and 2012, were $557,000, $760,000 and $609,000, respectively.
Share-Based Compensation
The Company recognizes share-based compensation expense for all stock options made to employees and directors based on estimated fair values.
The Company estimates the fair value of stock options on the date of grant using an option-pricing model. The value of the portion of the stock options that is ultimately expected to vest is recognized as expense over the requisite service periods using the straight-line method. Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates.
For purposes of determining the Company’s historical share-based compensation expense, it used the Black-Scholes-Merton (BSM) option-pricing model to calculate the estimated fair value of stock options on the measurement date (generally, the grant date). This model requires inputs for the expected life of the stock options, estimated volatility factor, risk-free interest rate, and expected dividend yield. The Company’s estimates of forfeiture rates also affect the amount of aggregate compensation expense. These inputs are subjective and generally require significant judgment. For the years ended December 31, 2014, 2013 and 2012, the Company calculated the fair value of stock options granted using the following assumptions:
| YEAR ENDED DECEMBER 31 | ||||||
| 2014 | 2013 | 2012 | ||||
| Expected life (years) |
5.46-6.08 | 5.29-7.71 | 5.00-6.08 | |||
| Estimated volatility factor |
49%-71% | 70%-75% | 72%-76% | |||
| Risk-free interest rate |
1.63%-2.05% | 1.27%-2.11% | 0.74%-1.16% | |||
| Expected dividend yield |
— | — | — | |||
Expected Life—The Company’s expected life represents the period that its share-based payment awards are expected to be outstanding. The Company uses the “simplified method” in accordance with Staff Accounting Bulletin (SAB) No. 107, Share-Based Payment, and SAB No. 110, Simplified Method for Plain Vanilla Share Options, to develop the expected term of options determined to be “plain vanilla.” Under this approach, the expected term is presumed to be the midpoint between the vesting date and the contractual end of the option grant. For stock options granted with an exercise price not equal to the determined fair market value, the Company estimates the expected life based on historical data and management’s expectations about exercises and post-vesting termination behavior. The Company will use the simplified method until it has sufficient historical data necessary to provide a reasonable estimate of expected life in accordance with SAB No. 107 and SAB No. 110.
Estimated Volatility Factor—Since the Company has a limited trading history in its common stock, the Company uses the calculated volatility based upon the trading history and calculated volatility of the common stock of comparable agricultural biotechnology companies in determining an estimated volatility factor.
117
Table of Contents
Risk-Free Interest Rate—The Company bases the risk-free interest rate on the implied yield currently available on U.S. Treasury constant-maturity securities with the same or substantially equivalent remaining term.
Expected Dividend Yield—The Company has not declared dividends nor does it expect to in the foreseeable future. Therefore, a zero value was assumed for the expected dividend yield.
Estimated Forfeitures—When estimating forfeitures, the Company considers voluntary and involuntary termination behavior and actual option forfeitures.
If in the future the Company determines that other methods are more reasonable, or other methods for calculating these assumptions are prescribed by authoritative guidance, the fair value calculated for the Company’s stock options could change significantly. Higher volatility and longer expected lives result in an increase to share-based compensation expense determined at the grant date. Share-based compensation expense is recorded in the Company’s research, development and patent expense and selling, general and administrative expense.
Other Income (Expense), Net
Other income (expense), net included net losses resulting from foreign currency transactions in the amount of $47,000, $53,000, and $54,000 for the years ended December 31, 2014, 2013 and 2012, respectively. In addition, other income (expense), net included losses on disposal of fixed assets totaling $243,000 and $231,000 for the years ended December 31, 2014 and 2013, respectively. There were no losses on disposals of fixed assets during the year ended December 31, 2012.
Income Taxes
The Company uses the asset and liability method of accounting for income taxes. Deferred tax assets and liabilities are recognized for the estimated future tax consequences attributable to the differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. To the extent deferred tax assets cannot be recognized under the preceding criteria, the Company establishes valuation allowances as necessary to reduce deferred tax assets to the amounts expected to be realized. As of December 31, 2014 and 2013, all deferred tax assets were fully offset by a valuation allowance. Realization of deferred tax assets is dependent upon future federal, state, and foreign taxable income. The Company’s judgments regarding deferred tax assets may change as the Company expands into international jurisdictions, due to future market conditions, changes in U.S. or international tax laws, and other factors. These changes, if any, may require possible material adjustments to these deferred tax assets, resulting in a reduction in net income or an increase in net loss in the period when such determinations are made.
The Company recognizes liabilities for uncertain tax positions based upon a two-step process. To the extent a tax position does not meet a more-likely-than-not level of certainty; no benefit is recognized in the consolidated financial statements. If a position meets the more-likely-than-not level of certainty, it is recognized in the consolidated financial statements at the largest amount that has a greater than 50% likelihood of being realized upon ultimate settlement. The Company’s policy is to analyze the Company’s tax positions taken with respect to all applicable income tax issues for all open tax years (in each respective jurisdiction). As of December 31, 2014 and 2013, the Company has concluded that no uncertain tax positions were required to be recognized in its consolidated financial statements. It is the Company’s practice to recognize interest and penalties related to income tax matters in income tax expense. No amounts were recognized for interest and penalties during the years ended December 31, 2014, 2013 and 2012.
118
Table of Contents
Comprehensive Loss
Comprehensive loss represents the net loss for the period plus the results of certain changes to stockholders’ equity (deficit) that are not reflected in the consolidated statements of operations, if applicable. The only component of the Company’s comprehensive loss for the periods presented is net loss.
Net Loss Per Share
Basic net loss per share, which excludes dilution, is computed by dividing the net loss attributable to common stockholders by the weighted-average number of shares of common stock outstanding during the period. Diluted net loss per share reflects the potential dilution that could occur if securities or other contracts to issue common stock, such as stock options, convertible notes, convertible preferred stock and warrants, result in the issuance of common stock which share in the losses of the Company. Certain potential shares of common stock have been excluded from the computation of diluted net loss per share for certain periods as their effect would be anti-dilutive. Such potentially dilutive shares are excluded when the effect would be to reduce the loss per share. The treasury stock method has been applied to determine the dilutive effect of warrants. See Note 5 for further discussion.
Segment Information
The Company is organized as a single operating segment, whereby its chief operating decision maker assesses the performance of and allocates resources to the business as a whole.
Recently Issued Accounting Pronouncements
In May 2014, the Financial Accounting Standards Board (the FASB) issued ASU No. 2014-09, Revenue from Contracts with Customers (Topic 606) (ASU 2014-09). ASU 2014-09 requires entities to recognize revenue through the application of a five-step model, which includes identification of the contract, identification of the performance obligations, determination of the transaction price, allocation of the transaction price to the performance obligations and recognition of revenue as the entity satisfies the performance obligations. ASU 2014-09 will become effective for us beginning January 1, 2018. The Company is currently evaluating the guidance to determine the potential impact on its statements of financial position, results of operations, cash flows and financial statement disclosures.
In August 2014, the FASB issued ASU No. 2014-15, Presentation of Financial Statements – Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern (ASU 2014-15). ASU 2014-15 requires that management assess an entity’s ability to continue as a going concern by incorporating and expanding upon certain principles that are currently in U.S. auditing standards. ASU 2014-15 is effective for annual periods ending after December 15, 2016, and for annual periods and interim periods thereafter. The Company is currently evaluating the guidance to determine the potential impact to its statements of financial position, results of operations and financial statement disclosures.
In April 2015, the FASB issued ASU No. 2015-03, Interest - Imputation of Interest (Topic 835-30): Simplifying the Presentation of Debt Issuance Costs (ASU 2015-03). ASU 2015-03 requires that debt issuance costs related to a recognized debt liability be presented in the balance sheet as a direct deduction from the carrying amount of that debt liability, consistent with debt discounts. The recognition and measurement guidance for debt issuance costs is not affected by ASU 2015-03. ASU 2015-03 is effective for financial statements issued for fiscal years beginning after December 15, 2015, and interim periods within those fiscal years. Early adoption is permitted. Upon adoption, the Company will reclassify debt issuance costs from prepaid expenses and other current assets and other assets as a reduction to debt on the consolidated balance sheets; the Company does not otherwise anticipate adoption will materially impact its statements of financial position or results of operations. The Company is not planning to early adopt ASU 2015-03.
119
Table of Contents
In July 2015, the FASB issued ASU No. 2015-11, Inventory (Topic 330): Simplifying the Measurement of Inventory, which applies guidance on subsequent measurement of inventory. An entity should measure inventory at the lower of cost and net realizable value. Net realizable value is the estimated selling prices in the ordinary course of business, less reasonable predictable costs of completion, disposal and transportation. The guidance excludes inventory measured using LIFO or the retail inventory method. ASU 2015-11 will be effective for interim and annual reporting periods beginning after December 15, 2016. Early application is permitted. The Company is currently evaluating the guidance to determine the potential impact to its statements of financial position, results of operations, cash flows and financial statement disclosures. The Company is not planning to early adopt ASU 2015-11.
4. Property, Plant and Equipment
Property, plant and equipment consist of the following (in thousands):
| DECEMBER 31 | ||||||||
| 2014 | 2013 | |||||||
| As restated | ||||||||
| Land |
$ | 1 | $ | 1 | ||||
| Buildings |
6,583 | — | ||||||
| Computer equipment and software |
522 | 459 | ||||||
| Furniture, fixtures and office equipment |
347 | 293 | ||||||
| Machinery and equipment |
15,519 | 4,624 | ||||||
| Leasehold improvements |
1,259 | 497 | ||||||
| Construction in progress |
1,023 | 6,444 | ||||||
|
|
|
|
|
|||||
| 25,254 | 12,318 | |||||||
| Less accumulated depreciation |
(5,088 | ) | (2,957 | ) | ||||
|
|
|
|
|
|||||
| $ | 20,166 | $ | 9,361 | |||||
|
|
|
|
|
|||||
The Company has granted to third parties interests in specific property and equipment as part of certain financing arrangements (see Note 9).
Depreciation and amortization expense for the years ended December 31, 2014, 2013 and 2012, was $2,581,000, $976,000 and $613,000, respectively, which included amortization expense related to capital leases for those periods (see Note 14).
5. Net Loss Per Share
Basic net loss per share, which excludes dilution, is computed by dividing the net loss attributable to common stockholders by the weighted-average number of shares of common stock outstanding during the period. Diluted net loss per share reflects the potential dilution that could occur if securities or other contracts to issue common stock, such as stock options, convertible notes, convertible preferred stock and warrants, result in the issuance of common stock which share in the losses of the Company.
The following table sets forth the potential shares of common stock that are not included in the calculation of diluted net loss per share because to do so would be anti-dilutive as of the end of each period presented (in thousands). Such potentially dilutive shares are excluded when the effect would be to reduce the loss per share.
| DECEMBER 31 | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| Convertible preferred stock |
— | — | 8,504 | |||||||||
| Convertible notes (1) |
— | — | — | |||||||||
| Stock options outstanding |
2,831 | 2,608 | 2,067 | |||||||||
| Warrants to purchase convertible preferred stock |
— | — | 207 | |||||||||
| Warrants to purchase common stock (2) |
145 | 151 | — | |||||||||
120
Table of Contents
| (1) | As of December 31, 2012, the Company had approximately $41,860,000, in contingently convertible notes payable and related accrued interest for which the contingencies related to conversion had not been met as of December 31, 2012. Therefore, it would have no dilutive or anti-dilutive impact until the contingency had been met effective upon the IPO in August 2013. All convertible notes converted to common stock in connection with the IPO. See Note 9 for further discussion. |
| (2) | In October 2012 and April 2013, the Company issued warrants to purchase a number of shares of common stock equal to 15% of the funded principal amount of the October 2012 and April 2013 Secured Promissory Notes as defined in Note 8, divided by 70% of the value of common stock in a sale of the Company or if the Company closes an initial public offering in which the Company receives gross cash proceeds, before underwriting discounts, commissions and fees, of at least $30,000,000 (a Qualified IPO), with an exercise price of 70% of the value of common stock in a sale of the Company or a Qualified IPO. In June 2013, the Company issued warrants to purchase a number of shares of common stock equal to 10% of the total committed amount of the June 2013 Credit Facility as defined in Note 9, divided by 70% of the value of common stock in a sale of the Company or a Qualified IPO, with an exercise price of 70% of the value of common stock in a sale of the Company or a Qualified IPO. These warrants were contingently exercisable for which the contingencies related to exercise had not been met until the IPO in August 2013. Therefore, they would have no dilutive or anti-dilutive impact until the contingency had been met in August 2013. See Note 9 for further discussion. |
The numbers of shares of common stock issuable upon the exercise of warrants to purchase convertible preferred stock and upon the conversion of convertible preferred stock were at a ratio of one-to-one.
| YEAR ENDED DECEMBER 31 | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| As restated | ||||||||||||
| (In thousands, except per share data) | ||||||||||||
| Numerator: |
||||||||||||
| Net loss |
$ | (51,659 | ) | $ | (31,242 | ) | $ | (38,794 | ) | |||
| Deemed dividend on convertible notes |
— | (1,378 | ) | (2,039 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Net loss attributable to common stockholders |
$ | (51,659 | ) | $ | (32,620 | ) | $ | (40,833 | ) | |||
|
|
|
|
|
|
|
|||||||
| Effect of potentially dilutive securities: |
||||||||||||
| Convertible notes |
— | (4,392 | ) | — | ||||||||
| Warrants to purchase preferred stock |
— | (840 | ) | — | ||||||||
|
|
|
|
|
|
|
|||||||
| Net loss for diluted net loss per share |
$ | (51,659 | ) | $ | (37,852 | ) | $ | (40,833 | ) | |||
|
|
|
|
|
|
|
|||||||
| Denominator |
||||||||||||
|
|
|
|
|
|
|
|||||||
| Weighted average shares used for basic net loss per share |
22,314 | 8,731 | 1,257 | |||||||||
|
|
|
|
|
|
|
|||||||
| Effect of potentially dilutive securities: |
||||||||||||
| Convertible notes |
— | 127 | — | |||||||||
| Warrants to purchase preferred stock |
— | 53 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Weighted average shares outstanding for diluted net loss per share |
22,314 | 8,911 | 1,257 | |||||||||
|
|
|
|
|
|
|
|||||||
| Basic net loss per share: |
$ | (2.32 | ) | $ | (3.74 | ) | $ | (32.48 | ) | |||
|
|
|
|
|
|
|
|||||||
| Diluted net loss per share: |
$ | (2.32 | ) | $ | (4.25 | ) | $ | (32.48 | ) | |||
|
|
|
|
|
|
|
|||||||
121
Table of Contents
6. Other Assets
Other assets consist of the following (in thousands):
| DECEMBER 31 | ||||||||
| 2014 | 2013 | |||||||
| Prepaid distribution fees |
$ | 115 | $ | 125 | ||||
| Deferred financing costs, less current portion |
269 | 148 | ||||||
| Deposits for equipment |
— | 256 | ||||||
| Deposits on building and equipment leases |
205 | 177 | ||||||
| Other assets |
144 | 100 | ||||||
|
|
|
|
|
|||||
| $ | 733 | $ | 806 | |||||
|
|
|
|
|
|||||
7. Accrued Liabilities
Accrued liabilities consist of the following (in thousands):
| DECEMBER 31 | ||||||||
| 2014 | 2013 | |||||||
| As restated | ||||||||
| Accrued compensation |
$ | 1,348 | $ | 2,040 | ||||
| Accrued severance |
— | 100 | ||||||
| Accrued expenses |
4,760 | 1,724 | ||||||
| Accrued warranty costs |
202 | 60 | ||||||
| Accrued inventory costs |
11 | 610 | ||||||
|
|
|
|
|
|||||
| $ | 6,321 | $ | 4,534 | |||||
|
|
|
|
|
|||||
On November 7, 2013, the Company announced that its Chief Financial Officer, Donald Glidewell, had decided to retire from the Company. To facilitate the transition, Mr. Glidewell agreed to remain as the Company’s Chief Financial Officer for up to five months while the Company searched for a successor Chief Financial Officer, and the Company entered into a transition agreement with Mr. Glidewell that provides, among other things, for continued vesting of his outstanding equity awards through his retirement date and that upon his separation from the Company, Mr. Glidewell received:
| • | an amount equal to six months of his then-current annual base salary payable monthly for a period of six months from his retirement date in the form of salary continuation; |
| • | medical and dental coverage, plus disability and life insurance premiums, for a period of six months following his retirement; and |
| • | full acceleration of vesting of his outstanding equity awards that are unvested as of his retirement date. |
As of December 31, 2013, the Company recorded accrued severance expenses in the amount of $100,000 based on the terms of the transition agreement for salary, COBRA, and transition service related costs, which was subsequently paid. See Note 13 for further discussion regarding the acceleration of vesting of Mr. Glidewell’s outstanding equity awards.
In addition, during the year ended December 31, 2014, the Company reduced the size of its staff compared to prior periods as part of its measures to streamline business operations and to reduce operating expenses and conserve cash and recorded severance charges of $258,000 to research, development and patent expenses and of $147,000 to selling, general and administrative expenses.
122
Table of Contents
The Company warrants the specifications and/or performance of its products through implied product warranties and has extended product warranties to qualifying customers on a contractual basis. The Company estimates the costs that may be incurred during the warranty period and records a liability in the amount of such costs at the time product is shipped. The Company’s estimate is based on historical experience and estimates of future warranty costs as a result of increasing usage of the Company’s products. The Company periodically assesses the adequacy of its recorded warranty liabilities and adjusts the amounts as necessary. Changes in the Company’s accrued warranty costs during the period are as follows (in thousands):
| Balance at December 31, 2013 |
$ | 60 | ||
| Warranties issued during the period |
220 | |||
| Settlements made during the period |
(78 | ) | ||
|
|
|
|||
| Balance at December 31, 2014 |
$ | 202 | ||
|
|
|
8. Factoring and Security Agreement
On June 13, 2013, the Company entered into a factoring and security agreement (Factoring and Security Agreement) with a third-party that would enable the Company to sell the entire interest in certain accounts receivable up to $5,000,000. Under the Factoring and Security Agreement, 15% of the sales proceeds will be held back by the purchaser until collection of such receivables. Such holdbacks are not considered legal securities, nor are they certificated. Upon the sale of the receivable, the Company will not maintain servicing. The purchaser may require the Company to repurchase accounts receivable if (i) the payment is disputed by the account debtor, with the purchaser being under no obligation to determine the bona fides of such dispute; (ii) the account debtor has become insolvent or (iii) upon the effective date of the termination of the Factoring and Security Agreement. The purchaser will retain its security interest in any accounts repurchased by the Company. The Factoring and Security Agreement is secured by all of the Company’s personal property and fixtures, and proceeds thereof, including accounts receivable, inventory, equipment and general intangibles other than intellectual property. Upon sale of the receivable, the Company may elect to set up a reserve where upon the cash for the sale remains with the third-party and the Company can draw on the available amount on the reserve account at any time. The Company elected to utilize the reserve account. On November 11, 2013, the Company terminated the Factoring and Security Agreement effective January 10, 2014.
The Company accounted for sales of accounts receivable under the Factoring and Security Agreement as a secured borrowing in accordance with ASC 860, Transfers and Servicing (ASC 860). As of December 31, 2014 and 2013, the Company did not have excess funds available on the reserve account and did not have secured borrowings outstanding under the arrangement. As of December 31, 2013, the Company had $479,000 included in accounts receivable that were transferred under this arrangement.
123
Table of Contents
9. Debt
Debt consists of the following (in thousands):
| DECEMBER 31 | ||||||||
| 2014 | 2013 | |||||||
| Term Loan (Term Loan) bearing interest at 7.00% per annum which is payable monthly through April 2016. The Term Loan is collateralized by all of the Company’s inventories, chattel paper, accounts receivable, equipment and general intangibles (excluding certain financed equipment and intellectual property) pledged as collateral under the Term Loan, subordinated |
$ | 183 | $ | 309 | ||||
| Promissory note (Promissory Note) bearing interest at 7.00% per annum which is payable monthly through November 2014, collateralized by all of the Company’s inventories, chattel paper, accounts receivable, equipment and general intangibles (excluding certain financed equipment and intellectual property), net of unamortized debt discount at December 31, 2013 of $2, subordinated. The Promissory Note was repaid in November 2014. |
— | 123 | ||||||
| Secured promissory notes (October 2012 and April 2013 Secured Promissory Notes) bearing interest at 12.00% per annum which are payable monthly through October 2015, collateralized by substantially all of the Company’s assets, net of unamortized debt discount at December 31, 2014 and 2013 of $203 and $445, respectively |
12,247 | 12,005 | ||||||
| Secured promissory note (June 2014 Secured Promissory Note) bearing interest at prime plus 2% (5.25% as of December 31, 2014) per annum, which is payable monthly through June 2036, collateralized by all of the Company’s deposit accounts and MMM LLC’s inventories, chattel paper, accounts, equipment and general intangibles |
9,873 | — | ||||||
|
|
|
|
|
|||||
| Debt |
22,303 | 12,437 | ||||||
| Less current portion |
(12,636 | ) | (157 | ) | ||||
|
|
|
|
|
|||||
| $ | 9,667 | $ | 12,280 | |||||
|
|
|
|
|
|||||
As of December 31, 2014, aggregate contractual future principal payments on the Company’s debt, by year, are due as follows (in thousands):
| Years ending December 31: |
||||
| 2015 |
$ | 12,839 | ||
| 2016 |
313 | |||
| 2017 |
282 | |||
| 2018 |
297 | |||
| 2019 |
313 | |||
| Thereafter |
8,462 | |||
|
|
|
|||
| Total future principal payments |
22,506 | |||
|
|
|
The fair value of the Company’s outstanding debt obligations was $22,587,000 and $13,950,000 as of December 31, 2014 and 2013, respectively, which was estimated based on a discounted cash flow model using an estimated market rate of interest of 11.25% for the fixed rate debt and 5.25% for the variable rate debt as of December 31, 2014 and 7.0% for the fixed rate debt and 5.25% for the variable rate debt as of December 31, 2013, and is classified as Level 3 within the fair value hierarchy.
124
Table of Contents
Promissory Notes, Term Loan, Revolving Line of Credit and Credit Facility
Term Loan
In March 2009, October 2010 and October 2011, the Company and Five Star Bank agreed to modify the terms of its existing revolving line of credit (Revolver). Under the modified terms of the Revolver, the Company’s borrowings under the Revolver were limited to 75% of qualifying accounts receivable with a maximum borrowing limit of $500,000. In March 2012, the Company entered into a change in terms agreement with the bank under which the existing Revolver was replaced by the Term Loan in the amount of $500,000 with a rate of 7.00% per annum, maturing April 1, 2016. The Company’s inventories, chattel paper, accounts receivable, equipment and general intangibles (excluding certain financed equipment and intellectual property) have been pledged as collateral under the Term Loan. The Term Loan provides for various events of default including breach of any term, obligation, covenant or condition under any other agreement between the Company and Five Star Bank. As of December 31, 2014, the Company was in breach of its covenants under the Term Loan as the Company was in breach of its covenants under its June 2014 Secured Promissory Note. However, this breach was cured in November 2015 as a result of the Company obtaining a waiver of certain of the Company’s covenants with respect to the June 2014 Secured Promissory Note as discussed below. The Term Loan was fully paid off in August 2015.
Promissory note
In March 2009, the Company borrowed $650,000 pursuant to a promissory note Five Star Bank which bears interest at the rate of 7.00% per annum and is repayable in six monthly interest only payments starting May 1, 2009, followed by 60 equal monthly installments of $13,000 commencing November 1, 2009, with the final payment due on November 1, 2014. All of the Company’s inventories, chattel paper, accounts receivable, equipment and general intangibles (excluding certain financed equipment and any intellectual property) were pledged as collateral for the promissory note. This note was paid in full on November 1, 2014.
Secured promissory notes
On October 2, 2012, the Company borrowed $7,500,000 pursuant to senior notes (October 2012 Secured Promissory Notes) with a group of lenders. The October 2012 Secured Promissory Notes have an initial term of three years and can be extended for an additional two years in one year increments at the option of the Company. During the initial three-year term, the October 2012 Secured Promissory Notes bear interest at 12% per annum. If the term of the October 2012 Secured Promissory Notes is extended an additional year, the interest rate increases to 13% during the fourth year. If the term of the October 2012 Secured Promissory Notes is extended for an additional two years, the interest rate is 14% during the fifth year. Interest on the October 2012 Secured Promissory Notes is payable monthly through the initial maturity date of the loan which is October 2, 2015 or through any extension period. The principal and all unpaid interest are due on the maturity date, as may be extended.
As part of the terms of the October 2012 Secured Promissory Notes, the Company is required to pay a fee of 5% of the funded principal amount to the agent that facilitated the borrowing and provides management of the relationship with the group of lenders (Agent Fee). This Agent Fee is payable within 30 days after all interest and principal have been paid. For each year the Company extends the maturity date of the October 2012 Secured Promissory Notes beyond the initial term, the agent will receive an additional 1% fee based on the funded principal amount. The present value of the unpaid Agent Fee, based on 5% of the funded principal amount, or $261,000, as of the closing date of the October 2012 Secured Promissory Notes was recorded as both deferred financing costs as a component of current and non-current other assets and non-current other liabilities. The amortization of the deferred financing costs and the accretion of the Agent Fee are recorded to interest expense over the term of the arrangement. As of December 31, 2014, $569,000 of the Agent Fee, including the amounts relating to the additional funds received from the issuance of the April 2013 Secured Promissory Notes discussed below, was recorded under accrued liabilities. As of December 31, 2013, $502,000 was recorded under non-current other liabilities. In addition, the Company incurred an additional $66,000 in financing-related costs,
125
Table of Contents
primarily legal fees. These costs were recorded as deferred financing costs as a component of current and non-current other assets and are being amortized to interest expense over the term of the arrangement.
The October 2012 Secured Promissory Notes are secured by the Company’s ownership interest in MMM LLC, a security interest in the assets of the Manufacturing Plant, and all of the Company’s other assets, subject to certain permitted liens. This security interest was subordinate to the security interest held by the holder of a previously outstanding promissory note issued in April 2012 and repaid in January 2013 (April 2012 Note), which also had a security interest in MMM LLC.
The Company also issued warrants (Common Stock Warrants) to the group of lenders to purchase a number of shares of common stock equal to 15% of the funded principal amount of the October 2012 Secured Promissory Notes divided by 70% of the value of common stock in a sale of the Company or a Qualified IPO, with such Common Stock Warrants having an exercise price of 70% of the value of common stock in a sale of the Company or a Qualified IPO. The Common Stock Warrants would be automatically exercised immediately prior to expiration on the earlier to occur of a Qualified IPO or a sale of the Company or the maturity of the October 2012 Secured Promissory Notes. The October 2012 Secured Promissory Notes could be prepaid six months after the initial funding date or earlier if a Qualified IPO or a sale of the Company occurs. As the predominant settlement feature of the Common Stock Warrants is to settle a fixed monetary amount in a variable number of shares, the Common Stock Warrants were accounted for under ASC 480. Accordingly, the Common Stock Warrants were recorded at estimated fair value on their issuance date and were adjusted to their estimated fair value as of each reporting date with the change in estimated fair value recorded as a component of change in estimated fair value of financial instruments in the Company’s consolidated statements of operations. The fair value of the Common Stock Warrants at the date of issuance of $282,000 was recorded as a discount to the October 2012 Secured Promissory Notes and is being amortized to interest expense over the term of the arrangement. Until the effective date of the IPO, the Company estimated the fair value of the Common Stock Warrants using a PWERM valuation based on unobservable inputs, and, therefore, the Common Stock Warrants were considered to be Level 3 liabilities. Upon closing of the IPO, the exercise price of the Common Stock Warrants was determined to be $8.40 per share and the number of shares to be issued upon exercise of the warrants was no longer variable. As a result of the IPO, the Common Stock Warrants were considered to be indexed to the Company’s stock, and accordingly, the common stock warrants liability was reclassified and included in stockholders’ equity (deficit) during the year ended December 31, 2013.
The October 2012 Secured Promissory Notes contain certain covenant requirements which include a requirement to maintain a minimum cash balance of the lesser of the April 2012 Note indebtedness described above or $5,000,000. As the April 2012 Note was fully paid off in January 2013, the Company no longer has a minimum cash balance requirement under the October 2012 Secured Promissory Notes. The Company is also precluded from adding additional debt without lender approval but allowance is made if such debt is subordinated to the October 2012 Secured Promissory Notes or if such additional debt is not more than $2,000,000 in the aggregate. In the event of default on the October 2012 Secured Promissory Notes, the lenders may declare the entire unpaid principal and interest immediately due and payable.
On April 10, 2013 (Conversion Date), the Company entered an amendment to increase, by up to $5,000,000, the amount available under the terms of the loan agreement with respect to the October 2012 Secured Promissory Notes. Under this amendment, an additional $4,950,000 was issued in partial consideration for $3,700,000 in cash received and in partial conversion for the cancellation of $1,250,000 of the total principal balance of the October 2012 Subordinated Convertible Note described below (collectively, April 2013 Secured Promissory Notes). The total amount borrowed under the amended loan agreement for the October 2012 Secured Promissory Notes and the April 2013 Secured Promissory Notes increased from $7,500,000 to $12,450,000 as of the Conversion Date. The accrued interest of $74,000 for the partially converted October 2012 Subordinated Convertible Note as of the Conversion Date shall be repaid or converted on the applicable maturity date of the October 2012 Subordinated Convertible Note.
126
Table of Contents
In connection with the issuance of the April 2013 Secured Promissory Notes, the Company issued additional warrants (Additional Common Stock Warrants) to purchase a number of shares of common stock equal to 20% of the funded principal amount of the April 2013 Secured Promissory Notes divided by 70% of the value of common stock in a sale of the Company or a Qualified IPO, with such Additional Common Stock Warrants to have an exercise price of 70% of the value of common stock in a sale of the Company or a Qualified IPO. As the predominant settlement feature of the Additional Common Stock Warrants was to settle a fixed monetary amount in a variable number of shares, the Common Stock Warrants were accounted for under ASC 480. Accordingly, the Additional Common Stock Warrants were recorded at estimated fair value on their issuance date and were adjusted to their estimated fair value as of each reporting date with the change in estimated fair value recorded as a component of change in estimated fair value of financial instruments in the Company’s consolidated statements of operations. The fair value of the Additional Common Stock Warrants at the date of issuance was estimated to be $465,000. The Company estimated the fair value of the Additional Common Stock Warrants using a PWERM valuation based on unobservable inputs and, therefore, the Additional Common Stock Warrants were considered to be Level 3 liabilities. Upon closing of the IPO, the exercise price of the Common Stock Warrants was determined to be $8.40 per share and the number of shares to be issued upon exercise of the warrants was no longer variable. As a result of the IPO, the Common Stock Warrants were considered to be indexed to the Company’s stock, and accordingly, the common stock warrants liability was reclassified and included in stockholders’ equity (deficit) during the year ended December 31, 2013.
The debt holder who converted $1,250,000 principal balance of the October 2012 Subordinated Convertible Note (with a fair value of $1,360,000 on the date of conversion) also loaned an additional $2,500,000 in cash as part of the April 2013 Secured Promissory Notes (collectively, the $3,750,000 Notes). The Company accounted for the conversion as an extinguishment of debt in accordance with ASC 470-50-40. The $1,360,000 fair value of the partially converted October 2012 Subordinated Convertible Note on the Conversion Date was derecognized and the fair value of the $3,750,000 Notes with the portion of the fair value of the Additional Common Stock Warrants issued to this debt holder on the date of issuance was recorded. The Company recorded the $49,000 excess of the total fair value of the $3,750,000 Notes and the related Additional Common Stock Warrants on the issuance date over total consideration received as a gain on extinguishment of debt in the accompanying consolidated statements of operations for the year ended December 31, 2013.
The following table shows the consideration received, fair values of the notes and common stock warrants issued and calculation of the gain on extinguishment of debt for the $3,750,000 Notes (in thousands):
| Consideration received |
||||
| Fair Value of October 2012 Subordinated Convertible Note |
$ | 1,360 | ||
| Cash |
2,500 | |||
|
|
|
|||
| Total Consideration Received (a) |
$ | 3,860 | ||
| Notes and Warrants Issued |
||||
| Principal Balance of Notes Issued |
$ | 3,750 | ||
| Debt Discount (1) |
(291 | ) | ||
|
|
|
|||
| Fair Value of Notes Issued |
3,459 | |||
| Fair Value of Additional Common Stock Warrants Issued |
352 | |||
|
|
|
|||
| Total Fair Value of Notes and Warrants Issued (b) |
$ | 3,811 | ||
|
|
|
|||
| Gain on Extinguishment of Debt (a—b) |
$ | 49 | ||
|
|
|
| (1) | The amortization of this account is being recorded in interest expense in the consolidated statements of operations over the term of the arrangement. |
127
Table of Contents
The remaining fair value to the Additional Common Stock Warrants of $113,000, net of the fair value of the Additional Common Stock Warrants issued of $352,000 related to the $3,750,000 Notes discussed above, was recorded as a debt discount to the April 2013 Secured Promissory Notes and is being amortized to interest expense over the term of the arrangement.
As a result of the amendment described above, the Company is also required to pay the Agent Fee, 5% of the $3,700,000 in cash received from the April 2013 Secured Promissory Notes, under the same terms as the October 2012 Secured Promissory Notes. In addition, the portion of the Agent Fee relating to the converted October 2012 Subordinated Convertible Note that would be due under the terms of the October 2012 Subordinated Convertible Note will be paid under the terms of the October 2012 and April 2013 Secured Promissory Notes. The present value of the unpaid Agent Fee of $172,000, based on 5% of the funded principal amount of $4,950,000, as of the closing date of the April 2013 Secured Promissory Notes was recorded as both deferred financing costs as a component of current and non-current other assets and non-current other liabilities. The amortization of the deferred financing costs and the accretion of the Agent Fee are being amortized to interest expense over the term of the arrangement.
In addition, the Company incurred an additional $24,000 in financing-related costs, primarily legal fees. These costs were recorded as deferred financing costs as a component of current and non-current other assets and are being amortized to interest expense over the term of the arrangement.
The amendment to the loan agreement also amended the interest provision applicable to the October 2012 and April 2013 Secured Promissory Notes to allow any holder of the October 2012 and April 2013 Secured Promissory Notes to request the Company to defer all interest due monthly to the applicable maturity date, and the optional prepayment provision applicable to the October 2012 and April 2013 Secured Promissory Notes to allow the Company to repay the outstanding amount of the October 2012 and April 2013 Secured Promissory Notes, either (i) with the written consent of the lender or the agent on such lenders’ behalf or (ii) without such consent provided that the Company pays the interest that would have been due from the prepayment date to the initial maturity date.
Activity related to the October 2012 and April 2013 Secured Promissory Notes from December 31, 2013 through December 31, 2014 consisted of the following (in thousands):
| DECEMBER 31, 2013 |
ADDITIONS | AMORTIZATION OF DEBT DISCOUNT |
PRINCIPAL PAYMENTS |
DECEMBER 31, 2014 |
||||||||||||||||
| Principal |
$ | 12,450 | $ | — | $ | — | $ | — | $ | 12,450 | ||||||||||
| Debt discount related to issuance of common stock warrants (1) |
(241 | ) | — | 135 | — | (106 | ) | |||||||||||||
| Discount related to the $3,750,000 Notes (1) |
(204 | ) | — | 107 | — | (97 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| $ | 12,005 | $ | — | $ | 242 | $ | — | $ | 12,247 | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (1) | The amortization of this account is included in interest expense in the consolidated statements of operations and as non-cash interest expense in the consolidated statements of cash flows. |
As of December 31, 2014, the Company was in breach of its covenants under the October 2012 and April 2013 Secured Promissory Notes as a result of its failure to provide annual financial statements in a timely manner and as the Company was in breach of certain of its covenants under the June 2014 Secured Promissory Note as described below. However, in November 2015, the Company received an extension from the lending agent with respect to compliance with the requirements to deliver annual financial statements to the earlier of
128
Table of Contents
(i) November 15, 2015 or (ii) such time such financial statements are filed with the SEC. This covenant breach was cured in November 2015, as a result of obtaining this extension and the waiver of certain of its covenants with respect to the June 2014 Secured Promissory Note, as described below.
In August 2015, the terms of the notes were amended, resulting in an increase in the interest rate to 18% effective September 1, 2015 for the remaining term of the notes. The Company also provided a written notice in September 2015 to extend the maturity date to October 2, 2017.
Credit Facility
On June 14, 2013, the Company entered into a credit facility agreement (June 2013 Credit Facility) with a group of lenders that are, or that are affiliated with, existing investors in the Company. Under the June 2013 Credit Facility, the lenders have committed to permit the Company to draw an aggregate of up to $5,000,000, and, subject to the Company’s obtaining additional commitments from lenders, such amount may be increased to up to $7,000,000. The June 2013 Credit Facility expired on June 30, 2014 without having been drawn upon.
During the term of the June 2013 Credit Facility, the Company could request from the lenders up to four advances, with each advance equal to one-quarter of each lender’s aggregate commitment amount. The Company would issue a promissory note in the principal amount of each such advance that would accrue interest at a rate of 10% per annum. The principal and all unpaid interest under the promissory notes would be due on the maturity date, and the Company could not prepay the promissory notes prior to the maturity date without consent of at least a majority in interest of the aggregate principal amount of the promissory notes then outstanding under the June 2013 Credit Facility.
In connection with the June 2013 Credit Facility, the Company agreed to pay a fee of 2% of the total commitment amount to the lenders. In addition, the Company incurred an additional $10,000 in financing-related costs, primarily legal fees. These costs were recorded as deferred financing costs as a component of current other assets and were fully amortized to interest expense as of December 31, 2014.
In connection with the June 2013 Credit Facility, the Company issued warrants (June 2013 Warrants) to purchase a number of shares of common stock equal to 10% of the total committed amount of the June 2013 Credit Facility divided by 70% of the value of common stock in a sale of the Company or a Qualified IPO, with such June 2013 Warrants to have an exercise price of 70% of the value of common stock in a sale of the Company or a Qualified IPO. The June 2013 Warrants expire upon the earlier of June 14, 2023 or the sale of the Company. As the predominant settlement feature of the June 2013 Warrants was to settle a fixed monetary amount in a variable number of shares, the June 2013 Warrants were accounted for under ASC 480. Accordingly, the June 2013 Warrants were recorded at estimated fair value on their issuance date and were adjusted to their estimated fair value as of each reporting date with the change in estimated fair value recorded as a component of change in estimated fair value of financial instruments in the Company’s consolidated statements of operations. The fair value of the June 2013 Warrants at the date of issuance of $435,000 was recorded as a deferred financing cost as a current other asset and was amortized to interest expense over the term of the arrangement. Until the effective date of the IPO, the Company estimated the fair value of the June 2013 Warrants using a PWERM valuation based on unobservable inputs and, therefore, the June 2013 Warrants were considered to be Level 3 liabilities. Upon closing of the IPO, the exercise price of the June 2013 Warrants was determined to be $8.40 per share and the number of shares to be issued upon exercise of the warrants was no longer variable. As a result of the IPO, the June 2013 Warrants were considered to be indexed to the Company’s stock, and accordingly, the common stock warrants liability was reclassified and included in stockholders’ equity (deficit) during the year ended December 31, 2013.
Secured promissory note
On April 11, 2014, the Company entered into a $3,000,000 promissory note with Five Star Bank. On April 14, 2014, the Company entered into an agreement with the bank to modify the terms of the promissory note from a
129
Table of Contents
single payment loan to a revolving line of credit, which allowed the Company to borrow up to $3,000,000. On April 28, 2014, the Company entered into an agreement to modify the terms of the revolving line of credit to increase the borrowing limit up to $5,000,000. In June 2014, the $4,687,000 balance on the revolving line of credit was paid off and the line was closed when the Company borrowed $10,000,000 pursuant to a business loan agreement and promissory note (June 2014 Secured Promissory Note) with the bank (Lender) which bears interest at prime rate (3.25% as of December 31, 2014) plus 2.00% per annum. The interest rate is subject to change from time to time to reflect changes in the prime rate; however, the interest rate shall not be less than 5.25% or more than the maximum rate allowed by applicable law. If the interest rate increases, the Lender, may, at its option, increase the amount of each monthly payment to ensure that the note would be paid in full by the maturity date, increase the amount of each monthly payment to reflect the change in interest rate, increase the number of monthly payments, or keep the monthly payments the same and increase the final payment amount. As of December 31, 2014, the interest rate was 5.25%.
The June 2014 Secured Promissory Note is repayable in monthly payments of $64,390 commencing July 13, 2014, with the final payment due on June 13, 2036. Certain of the Company’s deposit accounts and MMM, LLC’s inventories, chattel paper, accounts, equipment and general intangibles have been pledged as collateral for the promissory note. The Company is required to maintain a deposit balance with the Lender of $1,560,000, which is recorded as restricted cash included in noncurrent assets as a result of the debt being classified as current. In addition, until the Company provides documentation that the proceeds were used for construction of the Manufacturing Plant, proceeds from the loan will be maintained in a restricted deposit account. As of December 31, 2014, the Company had $1,856,000 remaining in this restricted deposit account, which was recorded as restricted cash included in current assets.
In addition, the Company incurred an additional $304,000 in financing-related costs, including USDA guarantee fees. These costs were recorded as deferred financing costs as a component of current and non-current other assets on the date of issuance and are being amortized to interest expense over the term of the arrangement.
The Company may prepay 20% of the outstanding principal loan balance each year without penalty. A prepayment fee of 10% will be charged if prepayments exceed 20% in the first year, and the prepayment fee will decrease by 1% each year for the first ten years of the loan.
The Company is required to maintain a current ratio of not less than 1.25-to-1.0, a debt-to-worth ratio of no greater than 4.0-to-1.0 and maintain a loan-to-value ratio of no greater than 70% as determined by the Lender. The Company is also required to comply with certain affirmative and negative covenants under the loan agreement discussed above. In the event of default on the debt, the lender may declare the entire unpaid principal and interest immediately due and payable. As of December 31, 2014, the Company was in breach of the covenants under the loan agreement as a result of its annual and quarterly reports not being filed within the prescribed time periods the Company being in breach of certain of its covenants under the October 2012 and April 2013 Secured Promissory Notes as described above and its being in breach of certain of its covenants on the October 2012 and April 2013 Secured Promissory Notes described above. Effective September 30, 2015, the Company’s debt-to-worth ratio was greater than 4.0-to-1.0 as a result of the issuance of $40,000,000 in promissory notes in August 2015 as described in Note 22, which increased the Company’s debt while the Company continued to incur net losses, which decreased stockholders’ equity. However, in November 2015, the Company received a waiver from the Lender with respect to compliance with the requirements to (i) deliver annual financial statements (extended to November 15, 2015), (ii) maintain a current ratio greater than 1.25-to-1.0 (extended to December 31, 2015) and (iii) maintain a debt-to-worth ratio less than 4.0-to-1.0 (extended to December 31, 2016). The receipt of this waiver and the extension to provide financial statements under the October 2012 and April 2013 Secured Promissory Notes cured the Company of being in breach of the covenants under the loan agreements, and the Company obtained an extension of to deliver its annual financial statements with respect to the October 2012 and April 2013 Secured Promissory Notes, as described above.
130
Table of Contents
10. Preferred Stock
The Company sold 1,484,000 shares of its Series A convertible preferred stock in private placements in April 2007 for $2.608 per share, including conversion of certain convertible notes payable, 2,242,000 shares of its Series B convertible preferred stock in August 2008 for $4.849 per share, including conversion of convertible notes payable, and 4,778,000 shares of its Series C convertible preferred stock from March 2010 to June 2011 for $5.317 per share, including conversion of the $514,000 of convertible notes payable plus accrued interest of $5,000. The Company recorded the issuance of its Series A, B, and C convertible preferred stock, net of issuance costs.
In May 2012, in connection with the issuance of the Series C Warrant, the Company amended certificate of incorporation to increase the number of shares of common stock the Company is authorized to issue from 12,745,000 shares to 12,936,000 shares and to increase the number of shares of convertible preferred stock the Company is authorized to issue from 8,632,000 shares to 8,823,000, of which 1,489,000 shares were designated as Series A convertible preferred stock, 2,252,000 shares were designated as Series B convertible preferred stock, and 5,082,000 shares were designated as Series C convertible preferred stock.
Upon the closing of the IPO, all shares of the Company’s outstanding convertible preferred stock automatically converted into shares of common stock. Further, in August 2013, the Company amended and restated its certificate of incorporation to effect the conversion of its outstanding convertible preferred stock into common stock on a 1-for-1 basis. The amendment also increased the number of shares of preferred stock authorized for issuance to 20,000,000.
Investors in the Company’s Series C convertible preferred stock were entitled to receive noncumulative dividends, before and in preference to any amounts paid to Series A and Series B convertible preferred stockholders and common stockholders, and investors in the Company’s Series A and B convertible preferred stock were entitled to receive noncumulative dividends, on a pari passu basis, before and in preference to any amounts paid to common stockholders. Dividends would be paid only when and if declared by the board of directors. In addition, these investors were entitled to voting rights equal to the number of shares of the Company’s common stock into which the Series A, B and C convertible preferred stock were convertible as of the close of business on the record date fixed for each stockholder’s meeting. No dividends were declared during the years ended December 31, 2014, 2013 and 2012.
As the Company’s Series A, B and C convertible preferred stock contained redemption features that were outside of the Company’s control, all shares of Series A, B and C convertible preferred stock were presented outside of permanent equity as of December 31, 2012.
11. Warrants
The following table summarizes information about the Company’s common stock warrants outstanding as of December 31, 2014 (in thousands, except exercise price data):
| DESCRIPTION |
ISSUE DATE | EXPIRATION DATE (1) |
NUMBER OF SHARES SUBJECT TO WARRANTS ISSUED |
EXERCISE PRICE |
||||||||
| In connection with April 2013 Secured Promissory Note (Additional Common Stock Warrants) |
April 2013 | October 2015 | 118 | $ | 8.40 | |||||||
| In connection with June 2013 Credit Facility (June 2013 Warrants) |
June 2013 | June 2023 | 27 | $ | 8.40 | |||||||
|
|
|
|||||||||||
| 145 | ||||||||||||
|
|
|
|||||||||||
131
Table of Contents
| (1) | Both common stock warrants expire upon the earlier to occur of (i) the date listed above; (ii) the acquisition of the Company by another entity by means of any transaction or series of related transactions (including, without limitation, any transfer of more than 50% of the voting power of the Company, reorganization, merger or consolidation, but excluding any merger effected exclusively for the purpose of changing the domicile of the Company) or (iii) a sale of all or substantially all of the assets of the Company; unless the Company’s stockholders of record as constituted immediately prior to such acquisition or sale will, immediately after such acquisition or sale (by virtue of securities issued as consideration for the Company’s acquisition or sale or otherwise) hold at least fifty percent (50%) of the voting power of the surviving or acquiring entity. |
The Additional Common Stock Warrants are exercisable 18 months after the consummation of the IPO and the June 2013 Warrants became exercisable on the date of the IPO.
12. Common Stock
In August 2013, the Company amended and restated its certificate of incorporation to increase the number of shares of common stock authorized for issuance to 250,000,000 shares with $0.00001 par value. As of December 31, 2014, the Company had reserved shares of common stock for future issuances as follows (in thousands):
| SHARES | ||||
| Stock options available for future grant |
1,086 | |||
| Stock options outstanding |
2,831 | |||
| Warrants to purchase common stock |
145 | |||
|
|
|
|||
| 4,062 | ||||
|
|
|
|||
13. Stock Option Plans
In July 2006, the Company authorized the 2006 Equity Incentive Plan, as amended, (2006 Plan). The 2006 Plan provided for the issuance of up to 1,434,000 shares of common stock underlying awards. The 2006 Plan was terminated in December 2011. As of December 31, 2014 and 2013, there were no shares available to be granted under the 2006 Equity Incentive Plan.
The 2006 Plan allowed holders to exercise stock options prior to their vesting. The common stock received by the employee is restricted and follows the same vesting schedule as the originally granted option. In the event the employee terminates employment from the Company (whether voluntary or involuntary), the Company retains a right to repurchase the unvested common stock at the original option exercise price. As of December 31, 2014 and 2013, no options had been exercised that would be subject to repurchase.
As of December 31, 2014, options to purchase 363,000 shares of the Company’s common stock at a weighted-average exercise price of $1.00 per share were outstanding under the 2006 Plan, of which 360,000 were vested at December 31, 2014. During the year ended December 31, 2014, 333,000 and 9,000 options were exercised and cancelled, respectively, under the 2006 Plan.
In July 2011 and as amended in September 2012, the Company authorized the 2011 Stock Plan (2011 Plan). The 2011 Plan provided for the issuance of up to 1,167,000 shares of common stock underlying awards, plus any shares of common stock underlying awards previously issued under the 2006 Plan that terminate or expire after the date of authorization of the 2011 Plan, subject to certain adjustments. In addition, the 2011 Plan provided that the Company not deliver more than 2,446,000 shares upon the exercise of incentive stock options issued under both the 2006 Plan and 2011 Plan. The 2011 Plan was terminated in August 2013 and no new stock awards may be granted under the 2011 Plan. As of December 31, 2014, there were no shares available to be granted under the 2011 Plan.
132
Table of Contents
As of December 31, 2014, options to purchase 633,000 shares of the Company’s common stock at a weighted-average exercise price of $8.28 per share were outstanding under the 2011 Plan, of which 391,000 were vested at December 31, 2014. During the year ended December 31, 2014, 228,000 and 271,000 options were exercised and canceled, respectively, under the 2011 Plan.
In August 2013, the Company’s board of directors adopted the 2013 Stock Incentive Plan (2013 Plan) covering officers, employees, directors of, and consultants to, the Company. The 2013 Plan allows for the granting of the following types of “stock awards”: incentive stock options, non-qualified stock options, stock appreciation rights, restricted stock, restricted stock units and dividend equivalent rights. At the time the 2013 Plan was established, the maximum aggregate number of shares of the Company’s common stock that could be issued pursuant to the 2013 Plan was 1,600,000 plus the number of shares of common stock reserved for issuance pursuant to future grants under the 2011 Plan. The number of shares authorized for issuance pursuant to the 2013 Plan is automatically increased by any additional shares that would have otherwise returned to the 2011 Plan as a result of the forfeiture, termination or expiration of awards previously granted under the 2011 Plan. In addition, the number of shares authorized for issuance pursuant to the 2013 Plan will increase by a number equal to the least of (i) 3.5% of the number of shares of the Company’s common stock outstanding on the last day of the immediately preceding fiscal year or (ii) a lesser number of shares determined by the administrator.
As of December 31, 2014, options to purchase 1,835,000 shares of the Company’s common stock at a weighted-average exercise price of $11.44 per share were outstanding under the 2013 Plan, of which 206,000 were vested at December 31, 2014. During the year ended December 31, 2014, no options were exercised and 494,000 options were canceled under the 2013 Plan.
Generally, options vest 25% on the first anniversary from the date of grant and 1/48 per month thereafter; however, options may be granted with different vesting terms as determined by the Company’s board of directors.
The following table summarizes the activity under the Company’s stock option plans for the year ended December 31, 2014 (in thousands, except exercise price and remaining contractual life data):
| SHARES AVAILABLE FOR GRANT |
SHARES OUTSTANDING |
WEIGHTED- AVERAGE EXERCISE PRICE |
WEIGHTED- AVERAGE REMAINING CONTRACTUAL LIFE (IN YEARS) |
AGGREGATE INTRINSIC VALUE |
||||||||||||||||
| Balances at December 31, 2013 |
1,194 | 2,608 | $ | 8.56 | 8.1 | $ | 24,158 | |||||||||||||
| Options authorized |
676 | — | ||||||||||||||||||
| Options granted |
(1,557 | ) | 1,557 | $ | 10.06 | |||||||||||||||
| Options exercised |
— | (561 | ) | $ | 2.33 | |||||||||||||||
| Options canceled |
773 | (773 | ) | $ | 13.05 | |||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Balances at December 31, 2014 |
1,086 | 2,831 | $ | 9.39 | 7.4 | $ | 1,595 | |||||||||||||
|
|
|
|
|
|||||||||||||||||
| Vested and expected to vest at December 31, 2014 |
2,420 | $ | 9.19 | 7.3 | $ | 1,109 | ||||||||||||||
|
|
|
|||||||||||||||||||
| Exercisable at December 31, 2014 |
957 | $ | 7.09 | 5.7 | $ | 380 | ||||||||||||||
|
|
|
|||||||||||||||||||
The total intrinsic value of options exercised for the years ended December 31, 2014, 2013 and 2012 were $6,302,000, $2,801,000 and $93,000, respectively.
133
Table of Contents
The estimated fair value of options vested during the years ended December 31, 2014, 2013 and 2012, was $3,863,000, $1,314,000 and $489,000 respectively. The weighted-average estimated fair value of options granted during the years ended December 31, 2014, 2013 and 2012 was $6.10 per share, $10.35 per share and $4.24 per share, respectively.
During the years ended December 31, 2014, 2013 and 2012, the Company recorded share-based compensation expense of $4,555,000, $2,300,000 and $662,000, respectively. For the years ended December 31, 2014, 2013 and 2012, the Company did not realize any tax benefit associated with its share-based compensation expense. No tax benefit was recognized because a portion of the option grants were ISOs for which stock-based compensation expense is not deductible and also due to the full valuation allowance on the Company’s deferred tax asset that is further discussed in Note 16.
As of December 31, 2014, the total share-based compensation expense related to unvested options granted to employees under the Company’s stock option plans but not yet recognized was $8,265,000. These costs will be amortized to expense on a straight-line basis over a weighted-average remaining term of 2.7 years.
In connection with Mr. Glidewell’s retirement, the Company entered into a transition agreement with Mr. Glidewell (See Note 7) which provided, among other things, for the vesting of his outstanding equity awards through the retirement date. For the years ended December 31, 2014 and 2013, the Company recorded share-based compensation expense of $444,000 and $266,000, respectively, relating to the acceleration of vesting of Mr. Glidewell’s option awards. As of December 31, 2014, there was no unamortized share-based compensation associated with the transition agreement.
14. Capital Leases
The Company accounts for certain equipment acquired under financing arrangements as capital leases. This equipment is included in property, plant and equipment and related amortization is included in depreciation expense.
As of December 31, 2014 and 2013, the cost of this equipment was $3,063,000 and $3,046,000, respectively, and the related accumulated amortization was $1,627,000 and $935,000, respectively.
Amortization of capital leases for the years ended December 31, 2014, 2013 and 2012 was $1,496,000, $470,000 and $175,000, respectively.
As of December 31, 2014, aggregate contractual future minimum lease payments on the capital leases are due as follows (in thousands):
| CAPITAL LEASES |
||||
| Years ending December 31: |
||||
| 2015 |
$ | 1,922 | ||
| 2016 |
173 | |||
| 2017 |
19 | |||
|
|
|
|||
| Total minimum payments required |
2,114 | |||
| Less: amount representing interest |
(90 | ) | ||
|
|
|
|||
| Present value of future payments |
2,024 | |||
| Less: current portion |
(1,839 | ) | ||
|
|
|
|||
| $ | 185 | |||
|
|
|
|||
134
Table of Contents
15. Commitments and Contingencies
Operating Leases
The Company has a non-cancelable lease for an aggregate of approximately 24,500 square feet of non-contiguous office space in an office complex in Davis, California under which a portion of the covered space terminated beginning in February 2014. The remaining portion of the space will terminate by October 2016. The lease includes negotiated annual increases in the monthly rental payments.
On September 9, 2013, the Company entered into a lease agreement for a new 28,700 square foot office and laboratory facility located in Davis, California. The initial term of the lease is for a period of 60 months and commenced on August 20, 2014. The original monthly base rent is $46,000 for the first 12 months with a 3% increase each year thereafter. In April 2014, the Company entered into an agreement to amend this lease agreement. The square footage leased was reduced to approximately 27,300 square feet and the monthly base rent was reduced to $44,000 per month for the first 12 months, with a 3% increase each year thereafter.
Concurrent with this amendment, in April 2014, the Company entered into a lease agreement with an affiliate of the landlord to lease approximately 17,400 square feet of office and laboratory space in the same building complex in Davis, California. The initial term of the lease is for a period of 60 months and commenced on August 20, 2014. The monthly base rent is $28,000 with a 3% increase each year thereafter.
The Company recognizes expense under its leases on a straight-line basis over the lease terms. At December 31, 2014, the Company’s aggregate commitment under non-cancelable lease agreements is as follows (in thousands):
| OPERATING LEASES |
||||
| Years ending December 31: |
||||
| 2015 |
$ | 1,111 | ||
| 2016 |
1,029 | |||
| 2017 |
941 | |||
| 2018 |
949 | |||
| 2019 and beyond |
615 | |||
|
|
|
|||
| Total minimum payments required |
$ | 4,645 | ||
|
|
|
|||
Rental expense charged to operations for all operating leases was $1,134,000, $691,000 and $484,000 for the years ended December 31, 2014, 2013 and 2012, respectively.
Litigation
On September 5, 2014, September 8, 2014, September 11, 2014, September 15, 2014 and November 3, 2014, the Company, along with certain of its current and former officers and directors, and others were named as defendants in putative securities class action lawsuits filed in the U.S. District Court for the Eastern District of California. On February 13, 2015, these actions were consolidated as Special Situations Fund III QP, L.P. et al v. Marrone Bio Innovations, Inc. et al, Case No 2:14-cv-02571-MCE-KJN. On September 2, 2015, an initial consolidated complaint was filed on behalf of (i) all persons who purchased or otherwise acquired our publicly traded common stock directly in or traceable to the Company’s August 1, 2013 initial public offering; (ii) all persons who purchased or otherwise acquired the Company’s common stock directly in our June 6, 2014 secondary offering; and (iii) all persons who purchased or otherwise acquired the Company’s common stock on the open market between March 7, 2014 and September 2, 2014 (the “Class Action”). In addition to the Company, the initial consolidated complaint names certain of the Company’s current and former officers and directors and the Company’s independent registered accounting firm as defendants. The initial consolidated complaint alleges violations of the Securities Act of 1933, the Securities Exchange Act of 1934, and SEC Rule 10b-5 arising out of the issuance of allegedly false and misleading statements about the Company’s business and prospects, including the Company’s financial statements, product revenues and system of internal controls.
135
Table of Contents
Plaintiffs contend that such statements caused the Company’s stock price to be artificially inflated. The action includes claims for damages, fees, and expenses, including an award of attorneys’ and experts’ fees to the putative class. Pursuant to a stipulation between the parties, and by order of the Court, Defendants need not respond to the initial consolidated complaint. An amended consolidated complaint is to be filed no later than 60 days after the Company announces the restatement(s) after which defendants will have 60 days to respond. The outcome of this matter is not presently determinable.
On September 9, 2014 and November 25, 2014, shareholder derivative actions were filed in the Superior Court of California, County of Yolo (Case No. CV14-1481) and the U.S. District Court for the Eastern District of California (Case No. 1:14-cv-02779-JAM-CKD), purportedly on the Company’s behalf, against certain current and former officers and members of our board of directors (the 2014 Derivative Actions). The plaintiffs in the 2014 Derivative Actions allege that the defendants breached their fiduciary duties, committed waste, were unjustly enriched, and aided and abetted breaches of fiduciary duty by causing the Company to issue allegedly false and misleading statements. The issues in the 2014 Derivative Actions overlap substantially with those at issue in the Class Action described above. The plaintiffs in the 2014 Derivative Actions seek, purportedly on behalf of the Company, an unspecified award of damages including, but not limited to, various corporate governance reforms, an award of restitution, an award of reasonable costs and expenses, including attorneys’ fees, and other further relief as the Court may deem just and proper. The Courts have granted the parties’ stipulations to defer litigation activity, subject to certain conditions and pending certain developments in the Class Action.
On October 14, 2015, a shareholder derivative action was filed in the Superior Court of California, County of Yolo (Case No. CV15-1423), purportedly on the Company’s behalf, against certain current and former officers and members of the Company’s board of directors and the Company’s independent registered public accounting firm (the “2015 Derivative Action,” and with the 2014 Derivative Actions, the “Derivative Actions”). The plaintiff in the 2015 Derivative Action alleges that the director and officer defendants breached their fiduciary duties, committed waste and were unjustly enriched, by causing the Company to issue allegedly false and misleading statements. The plaintiff in the 2015 Derivative Action also alleges that the Company’s independent registered public accounting firm committed professional negligence and malpractice. The issues in the 2015 Derivative Action overlap substantially with those at issue in the 2014 Derivative Actions, and the Class Action described above. The parties are negotiating a date by which the defendants’ response to the newly filed complaint will be due. Given the preliminary nature of the Derivative Actions, we are not in a position to express any opinion regarding the outcome in these matters.
The Company is currently unable to estimate a range of reasonably possible loss for the litigation because these matters involve significant uncertainties. Those uncertainties include the legal theory or the nature of the claims, the complexity of the facts, the results of any investigation or litigation, and the timing of resolution of the investigations or litigation. Although the Company cannot estimate a reasonable range of loss based on currently available information, the resolution of these matters could have a material adverse effect on the Company’s financial position, results of operations or cash flows.
SEC Investigation
As previously discussed, the Company advised the staff of the Division of Enforcement of the SEC in September 2014 that the Audit Committee of the Company’s board of directors had commenced an internal investigation. The SEC commenced a formal investigation of these matters, with which the Company is cooperating. Though the investigation continues, the Company has engaged in discussions with the Division of Enforcement staff concerning the resolution of any enforcement action that it may recommend. In accordance with ASC 450, Contingencies, the Company has recorded an accrual of $1,750,000 in its financial statements for the year ended December 31, 2014 for its estimate of the penalties arising from such enforcement action and has estimated the range of the reasonably possible loss to be between $1,750,000 and $4,000,000. Publicity surrounding the foregoing or any enforcement action as a result of the SEC’s investigation, even if ultimately resolved favorably, could have an adverse impact on the Company’s reputation, business, financial position, results of operations or cash flows.
136
Table of Contents
16. Income Taxes
As of December 31, 2014, the Company had net operating loss carry-forwards for federal income tax reporting purposes of $128,160,000, which begin to expire in 2026, and California and various other state net operating loss carry-forwards of $87,200,000 and $30,700,000, respectively, which begin to expire in 2016. Additionally, as of December 31, 2014, the Company had federal research and development tax credit carry-forwards of $1,638,000, which begin to expire in 2026, and state research and development tax credit carry-forwards of $1,775,000, which have no expiration date.
The Company’s ability to use its federal and state net operating loss carry-forwards and federal and state tax credit carryforwards to reduce future taxable income and future taxes, respectively, may be subject to restrictions attributable to equity transactions that may have resulted in a change of ownership as defined by Internal Revenue Code (IRC) Section 382. In the event the Company has had such a change in ownership, utilization of these carryforwards could be severely restricted and could result in significant amounts of these carryforwards expiring prior to benefitting the Company. The Company completed a Section 382 analysis as of December 31, 2013 and concluded that $493,000 in federal net operating losses and $151,000 in federal research and development credits are expected to expire prior to utilization as a result of the Company’s previous ownership changes and corresponding annual limitations. The Company has not, however, conducted a Section 382 study for any periods after December 31, 2013 and, accordingly the Company cannot provide any assurance that an ownership change within the meaning of the IRC has not occurred since that date.
As of December 31, 2014, deferred tax assets of $53,728,000, arising principally as a result of the Company’s net operating loss carry-forwards, tax credits, and certain costs capitalized for tax purposes during the Company’s development stage, were fully offset by a valuation allowance. The valuation allowance increased by $18,459,000, $12,869,000 and $8,134,000 for the years ended December 31, 2014, 2013 and 2012, respectively. The deferred tax asset balances as of December 31, 2014 and 2013 did not include excess tax benefits from stock option exercises. The amount excluded at December 31, 2014 and 2013 was $2,092,000 and $978,000, respectively.
The temporary timing differences that give rise to the deferred tax assets are as follows (in thousands):
| DECEMBER 31 | ||||||||
| 2014 | 2013 | |||||||
| As restated | ||||||||
| Components of deferred taxes: |
||||||||
| Net operating loss carryforwards |
$ | 47,723 | $ | 29,828 | ||||
| Research and development tax credit |
2,106 | 1,630 | ||||||
| Other, net |
3,899 | 3,812 | ||||||
|
|
|
|
|
|||||
| Net deferred tax assets |
53,728 | 35,270 | ||||||
| Less: valuation allowance |
(53,728 | ) | (35,270 | ) | ||||
|
|
|
|
|
|||||
| Net deferred tax assets |
$ | — | $ | — | ||||
|
|
|
|
|
|||||
137
Table of Contents
The Company had no deferred tax liabilities at December 31, 2014 and 2013.
The Company recognized no income tax expense, and did not receive a benefit from income taxes for the years ended December 31, 2014, 2013 and 2012. The provision for income taxes is different than the amount computed using the applicable statutory federal income tax rate with the difference for each year summarized below:
| DECEMBER 31 | ||||||||
| 2014 | 2013 | |||||||
| As restated | ||||||||
| Federal tax benefit at statutory rate |
34 | % | 34 | % | ||||
| State tax benefit, net of federal benefit |
5 | 5 | ||||||
| Interest expense |
(1 | ) | (5 | ) | ||||
| Mark-to-market accounting |
— | 8 | ||||||
| Deemed dividend |
— | (2 | ) | |||||
| Share-based compensation expense |
(2 | ) | (2 | ) | ||||
| Other |
— | 2 | ||||||
| Adjustment due to change in valuation allowance |
(36 | ) | (40 | ) | ||||
|
|
|
|
|
|||||
| Provision for income taxes |
— | % | — | % | ||||
|
|
|
|
|
|||||
As of December 31, 2014, the Company had unrecognized tax benefits of $853,000. The unrecognized tax benefits, if recognized, would not impact the Company’s effective tax rate as the recognition of these tax benefits would be offset by changes in the Company’s valuation allowance. The Company does not believe there will be any material changes in its unrecognized tax position over the next twelve months.
A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows (in thousands):
| 2014 | 2013 | |||||||
| As restated | ||||||||
| Balance at January 1 |
$ | 660 | $ | 418 | ||||
| Increase related to prior year tax positions |
— | 85 | ||||||
| Increase related to current year tax positions |
193 | 157 | ||||||
|
|
|
|
|
|||||
| Balance at December 31 |
$ | 853 | $ | 660 | ||||
|
|
|
|
|
|||||
The Company files income tax returns in the U.S. federal jurisdiction and various state jurisdictions. The Company is subject to U.S. federal and state income tax examination for 2006 through 2013 due to unutilized net operating losses and research credits.
17. Employee Benefit Plan
The Company has a defined contribution plan offered to all eligible employees, which is qualified under Section 401(k) of the Internal Revenue Code. The Company currently provides a matching contribution. Matching contributions are based on a formula which provides for a dollar-for-dollar matching contribution of the employee’s 401(k) contribution up to 3% of eligible pay plus a 50% matching contribution on the employee’s 401(k) contribution between 3% and 5% of eligible pay. Each participant is 100% vested in elective contributions and the Company’s matching contribution. The Company provided 401(k) matching contributions for the years ended December 31, 2014, 2013 and 2012 were $370,000, $294,000 and $229,000, respectively.
18. Related Party Transactions
Les Lyman, a member of the Company’s board of directors, is the chairman and significant indirect shareholder of The Tremont Group, Inc. During the years ended December 31, 2014 and 2013, revenue of $821,000 and $418,000, respectively, was recognized on a sell-through basis relating to product purchased by The Tremont
138
Table of Contents
Group that was resold by the Tremont Group during the period. As of December 31, 2014 the Company had no outstanding accounts receivable due from The Tremont Group Inc. As of December 31, 2013 the Company had outstanding accounts receivable due from The Tremont Group, Inc. of $903,000. As of December 31, 2014 and 2013, the Company recorded deferred cost of product revenues of $333,000 and $610,000, respectively, and deferred product revenue of $660,000 and $997,000, respectively, relating to product sold to The Tremont Group, Inc. where title has transferred but the criteria for revenue recognition had not been met. Although the Company anticipates sales of its products to The Tremont Group, Inc. to continue through 2015, the Company cannot estimate the amount of those sales.
In December 2012, the Company issued a $12,500,000 convertible note to Syngenta Ventures, an affiliate of one of the Company’s distributors, for which there was no outstanding balance as of December 31, 2014 and 2013 as the convertible note converted into shares of the Company’s common stock immediately following the completion of the IPO in August 2013. During the year ended December 31, 2013, the Company recorded revenue of $116,000 relating to sales of product to Syngenta and $131,000 relating to license revenue recognized based on the terms of the Company’s commercial agreement with Syngenta. In connection with the public offering completed in June 2014, Syngenta Ventures sold 600,000 common shares and is no longer a 5% stockholder. As such, beginning in June 2014, the Company included license revenues recognized under these agreements in license revenues. For the year ended December 31, 2014, the Company recognized $333,000 of related party revenues under these agreements prior to Syngenta Ventures reducing its ownership stake.
19. Reverse Stock Split
On August 1, 2013, the Company amended and restated its certificate of incorporation to effect the conversion of its outstanding convertible preferred stock into common stock on a 1-for-1 basis followed immediately by a reverse split of shares of its common stock (including the common stock issued upon conversion of the convertible preferred stock) at a 1-for-3.138458 ratio (the Reverse Stock Split). The amendment also increased the number of shares of common stock authorized for issuance to 250,000,000 shares and the number of shares of preferred stock authorized for issuance to 20,000,000. The par value of the common stock and preferred stock was not adjusted as a result of the Reverse Stock Split.
All issued and outstanding common stock, preferred stock, and warrants for common stock or preferred stock, and the related per share amounts contained in the consolidated financial statements, have been retroactively adjusted to give effect to this Reverse Stock Split for all periods presented.
20. Public Offerings
In August 2013, the Company closed its initial public offering of 5,462,500 shares of its common stock (inclusive of 712,500 shares of common stock sold upon the exercise of the underwriters’ option to purchase additional shares). The public offering price of the shares sold in the offering was $12.00 per share. The total gross proceeds from the offering to the Company were $65,550,000, and after deducting underwriting discounts and commissions and offering expenses payable by the Company, the aggregate net proceeds received by the Company totaled approximately $56,105,000. In connection with the IPO:
| • | all outstanding shares of convertible preferred stock were converted into 8,514,000 shares of common stock, including 10,000 shares issued upon the cash exercise of Series B convertible preferred stock warrants; |
| • | all outstanding principal and accrued interest of the convertible notes were converted into 3,741,000 shares of common stock; |
| • | 47,000 shares of common stock were issued upon the net exercise of common stock warrants; |
| • | 3,000 shares of common stock were issued upon the cash exercise of common stock warrants; and |
| • | the Series A and Series C convertible preferred stock warrants were net exercised for 71,000 shares of common stock. |
139
Table of Contents
After the closing of the IPO, the Company had 19,133,000 shares of common stock and 151,000 warrants to purchase common stock outstanding and there were no shares of convertible preferred stock, preferred stock warrants or balances related to convertible notes outstanding.
In June 2014, the Company completed a secondary offering of 4,575,000 shares of its common stock (inclusive of 675,000 shares of common stock sold upon the exercise of the underwriters’ option to purchase additional shares). The public offering price of the shares sold in the offering was $9.50 per share. The total gross proceeds from the offering to the Company were $43,463,000, and after deducting underwriting discounts and commissions and offering expenses payable by the Company, the aggregate net proceeds received by the Company totaled $39,949,000.
140
Table of Contents
21. Quarterly Financial Information (Unaudited)
The following tables present the condensed consolidated balance sheets for each quarter in 2014 (in thousands, except par value), including adjustments described in Note 2:
| March 31, 2014 (Unaudited) |
||||||||||||
| As reported | Adjustments | As restated | ||||||||||
| Assets |
||||||||||||
| Current assets: |
||||||||||||
| Cash and cash equivalents |
$ | 21,298 | $ | — | $ | 21,298 | ||||||
| Short-term investments |
2,664 | — | 2,664 | |||||||||
| Accounts receivable |
7,231 | (1,987 | ) | 5,244 | ||||||||
| Accounts receivable from related parties |
1,230 | — | 1,230 | |||||||||
| Inventories, net |
12,837 | 754 | 13,591 | |||||||||
| Deferred cost of product revenues, including deferred cost of product revenues to related parties of $641 as of March 31, 2014 |
— | 2,907 | 2,907 | |||||||||
| Prepaid expenses and other current assets |
1,765 | (219 | ) | 1,546 | ||||||||
|
|
|
|
|
|
|
|||||||
| Total current assets |
47,025 | 1,455 | 48,480 | |||||||||
| Property, plant and equipment, net |
15,795 | (59 | ) | 15,736 | ||||||||
| Other assets |
639 | — | 639 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total assets |
63,459 | 1,396 | 64,855 | |||||||||
|
|
|
|
|
|
|
|||||||
| Liabilities and stockholders’ equity |
||||||||||||
| Current liabilities: |
||||||||||||
| Accounts payable |
$ | 8,563 | $ | — | $ | 8,563 | ||||||
| Accrued liabilities |
3,040 | (40 | ) | 3,000 | ||||||||
| Deferred revenue, current portion |
1,017 | 3,128 | 4,145 | |||||||||
| Deferred revenue from related parties, current portion |
31 | 1,040 | 1,071 | |||||||||
| Capital lease obligations, current portion |
1,680 | — | 1,680 | |||||||||
| Debt, current portion |
123 | — | 123 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total current liabilities |
14,454 | 4,128 | 18,582 | |||||||||
| Deferred revenue, less current portion |
695 | — | 695 | |||||||||
| Deferred revenue from related parties, less current portion |
404 | — | 404 | |||||||||
| Capital lease obligations, less current portion |
1,059 | — | 1,059 | |||||||||
| Debt, less current portion |
12,312 | — | 12,312 | |||||||||
| Other liabilities |
574 | — | 574 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total liabilities |
29,498 | 4,128 | 33,626 | |||||||||
| Commitments and contingencies |
||||||||||||
| Stockholders’ equity: |
||||||||||||
| Preferred stock: $0.00001 par value; 20,000 shares authorized, no shares issued or outstanding at March 31, 2014 (1) |
— | — | — | |||||||||
| Common stock: $0.00001 par value; 250,000 shares authorized and 19,707 shares issued and outstanding at March 31, 2014 (1) |
— | — | — | |||||||||
| Additional paid in capital |
149,643 | 90 | 149,733 | |||||||||
| Accumulated deficit |
(115,682 | ) | (2,822 | ) | (118,504 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total stockholders’ equity |
33,961 | (2,732 | ) | 31,229 | ||||||||
|
|
|
|
|
|
|
|||||||
| Total liabilities and stockholders’ equity |
$ | 63,459 | $ | 1,396 | $ | 64,855 | ||||||
|
|
|
|
|
|
|
|||||||
| (1) | Par value, shares authorized and shares issued and outstanding represents the as reported and as restated amounts as there were no adjustments to these totals. |
141
Table of Contents
| June 30, 2014 (Unaudited) |
||||||||||||
| As reported | Adjustments | As restated | ||||||||||
| Assets |
||||||||||||
| Current assets: |
||||||||||||
| Cash and cash equivalents |
$ | 57,630 | $ | — | $ | 57,630 | ||||||
| Restricted cash, current portion |
3,325 | — | 3,325 | |||||||||
| Short-term investments |
249 | — | 249 | |||||||||
| Accounts receivable |
4,110 | (110 | ) | 4,000 | ||||||||
| Accounts receivable from related parties |
490 | — | 490 | |||||||||
| Inventories, net |
12,501 | 756 | 13,257 | |||||||||
| Deferred cost of product revenues, including deferred cost of product revenues to related parties of $584 as of June 30, 2014 |
— | 3,024 | 3,024 | |||||||||
| Prepaid expenses and other current assets |
1,768 | (290 | ) | 1,478 | ||||||||
|
|
|
|
|
|
|
|||||||
| Total current assets |
80,073 | 3,380 | 83,453 | |||||||||
| Property, plant and equipment, net |
18,485 | (59 | ) | 18,426 | ||||||||
| Restricted cash, less current portion |
1,560 | — | 1,560 | |||||||||
| Other assets |
899 | — | 899 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total assets |
101,017 | 3,321 | 104,338 | |||||||||
|
|
|
|
|
|
|
|||||||
| Liabilities and stockholders’ equity |
||||||||||||
| Current liabilities: |
||||||||||||
| Accounts payable |
$ | 5,185 | $ | — | $ | 5,185 | ||||||
| Accrued liabilities |
3,511 | (28 | ) | 3,483 | ||||||||
| Deferred revenue, current portion |
537 | 3,616 | 4,153 | |||||||||
| Deferred revenue from related parties, current portion |
— | 998 | 998 | |||||||||
| Customer refund liabilities |
— | 1,883 | 1,883 | |||||||||
| Capital lease obligations, current portion |
1,836 | — | 1,836 | |||||||||
| Debt, current portion |
340 | — | 340 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total current liabilities |
11,409 | 6,469 | 17,878 | |||||||||
| Deferred revenue, less current portion |
1,043 | — | 1,043 | |||||||||
| Capital lease obligations, less current portion |
886 | — | 886 | |||||||||
| Debt, less current portion |
22,090 | — | 22,090 | |||||||||
| Other liabilities |
577 | — | 577 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total liabilities |
36,005 | 6,469 | 42,474 | |||||||||
| Commitments and contingencies |
||||||||||||
| Stockholders’ equity: |
||||||||||||
| Preferred stock: $0.00001 par value; 20,000 shares authorized, no shares issued or outstanding at June 30, 2014 (1) |
— | — | — | |||||||||
| Common stock: $0.00001 par value; 250,000 shares authorized and 24,380 shares issued and outstanding at June 30, 2014 (1) |
— | — | — | |||||||||
| Additional paid in capital |
191,079 | — | 191,079 | |||||||||
| Accumulated deficit |
(126,067 | ) | (3,148 | ) | (129,215 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total stockholders’ equity |
65,012 | (3,148 | ) | 61,864 | ||||||||
|
|
|
|
|
|
|
|||||||
| Total liabilities and stockholders’ equity |
$ | 101,017 | $ | 3,321 | $ | 104,338 | ||||||
|
|
|
|
|
|
|
|||||||
| (1) | Par value, shares authorized and shares issued and outstanding represents the as reported and as restated amounts as there were no adjustments to these totals. |
142
Table of Contents
| September 30, 2014 |
||||
| (Unaudited) | ||||
| Assets |
||||
| Current assets: |
||||
| Cash and cash equivalents |
$ | 46,281 | ||
| Restricted cash, current portion |
1,856 | |||
| Accounts receivable |
2,635 | |||
| Accounts receivable from related parties |
162 | |||
| Inventories, net |
13,164 | |||
| Deferred cost of product revenues, including deferred cost of product revenues to related parties of $367 as of September 30, 2014 |
1,783 | |||
| Prepaid expenses and other current assets |
1,846 | |||
|
|
|
|||
| Total current assets |
67,727 | |||
| Property, plant and equipment, net |
19,039 | |||
| Other assets |
657 | |||
| Restricted cash, less current portion |
1,560 | |||
|
|
|
|||
| Total assets |
88,983 | |||
|
|
|
|||
| Liabilities and stockholders’ equity |
||||
| Current liabilities: |
||||
| Accounts payable |
$ | 3,116 | ||
| Accrued liabilities |
5,601 | |||
| Deferred revenue, current portion |
2,826 | |||
| Deferred revenue from related parties, current portion |
744 | |||
| Customer refund liabilities |
1,025 | |||
| Capital lease obligations, current portion |
1,931 | |||
| Debt, current portion |
397 | |||
|
|
|
|||
| Total current liabilities |
15,640 | |||
| Deferred revenue, less current portion |
1,433 | |||
| Capital lease obligations, less current portion |
421 | |||
| Debt, less current portion |
21,948 | |||
| Other liabilities |
1,006 | |||
|
|
|
|||
| Total liabilities |
40,448 | |||
| Commitments and contingencies |
||||
| Stockholders’ equity: |
||||
| Preferred stock: $0.00001 par value; 20,000 shares authorized, no shares issued or outstanding at September 30, 2014 |
— | |||
| Common stock: $0.00001 par value; 250,000 shares authorized and 24,460 shares issued and outstanding at September 30, 2014 |
— | |||
| Additional paid in capital |
192,150 | |||
| Accumulated deficit |
(143,615 | ) | ||
|
|
|
|||
| Total stockholders’ equity |
48,535 | |||
|
|
|
|||
| Total liabilities and stockholders’ equity |
$ | 88,983 | ||
|
|
|
|||
143
Table of Contents
The following tables present the condensed consolidated balance sheets for each quarter in 2013 (in thousands, except par value), including adjustments described in Note 2:
| March 31, 2013 (Unaudited) |
||||||||||||
| As reported | Adjustments | As restated | ||||||||||
| Assets |
||||||||||||
| Current assets: |
||||||||||||
| Cash and cash equivalents |
$ | 1,791 | $ | — | $ | 1,791 | ||||||
| Accounts receivable |
3,043 | (565 | ) | 2,478 | ||||||||
| Accounts receivable from related parties |
— | 268 | 268 | |||||||||
| Inventories, net |
5,367 | 197 | 5,564 | |||||||||
| Deferred cost of product revenues, including deferred cost of product revenues to related parties of $194 as of March 31, 2013 |
— | 194 | 194 | |||||||||
| Prepaid expenses and other current assets |
927 | — | 927 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total current assets |
11,128 | 94 | 11,222 | |||||||||
| Property, plant and equipment, net |
3,853 | — | 3,853 | |||||||||
| Other assets |
2,858 | — | 2,858 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total assets |
17,839 | 94 | 17,933 | |||||||||
|
|
|
|
|
|
|
|||||||
| Liabilities, convertible preferred stock and stockholders’ equity (deficit) |
||||||||||||
| Current liabilities: |
||||||||||||
| Accounts payable |
$ | 2,242 | $ | — | $ | 2,242 | ||||||
| Accrued liabilities |
1,716 | (7 | ) | 1,709 | ||||||||
| Deferred revenue, current portion |
324 | (137 | ) | 187 | ||||||||
| Deferred revenue from related parties, current portion |
— | 379 | 379 | |||||||||
| Capital lease obligations, current portion |
245 | — | 245 | |||||||||
| Debt, current portion |
181 | — | 181 | |||||||||
| Preferred stock warrant liability |
1,883 | — | 1,883 | |||||||||
| Common stock warrant liability |
316 | — | 316 | |||||||||
| Convertible notes payable, current portion |
25,803 | — | 25,803 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total current liabilities |
32,710 | 235 | 32,945 | |||||||||
| Deferred revenue, less current portion |
1,615 | (727 | ) | 888 | ||||||||
| Deferred revenue from related parties, less current portion |
— | 727 | 727 | |||||||||
| Capital lease obligations, less current portion |
209 | — | 209 | |||||||||
| Debt, less current portion |
7,723 | — | 7,723 | |||||||||
| Convertible notes payable, less current portion |
20,234 | — | 20,234 | |||||||||
| Other liabilities |
481 | — | 481 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total liabilities |
62,972 | 235 | 63,207 | |||||||||
| Commitments and contingencies |
||||||||||||
| Convertible preferred stock—Series A: $0.00001 par value; 1,489 shares authorized; 1,484 shares issued and outstanding at March 31, 2013(1) |
3,747 | — | 3,747 | |||||||||
| Convertible preferred stock—Series B: $0.00001 par value; 2.252 shares authorized; 2,242 shares issued and outstanding at March 31, 2013(1) |
10,758 | — | 10,758 | |||||||||
| Convertible preferred stock—Series C: $0.00001 par value; 5,082 shares authorized; 4,778 shares issued and outstanding at March 31, 2013(1) |
25,107 | — | 25,107 | |||||||||
| Stockholders’ deficit: |
||||||||||||
| Common stock: $0.00001 par value; 12,936 shares authorized; 1,269 shares issued and outstanding at March 31, 2013(1) |
— | — | — | |||||||||
| Additional paid in capital |
1,573 | — | 1,573 | |||||||||
| Accumulated deficit |
(86,318 | ) | (141 | ) | (86,459 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total stockholders’ deficit |
(84,745 | ) | (141 | ) | (84,886 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total liabilities, convertible preferred stock and stockholders’ deficit |
$ | 17,839 | $ | 94 | $ | 17,933 | ||||||
|
|
|
|
|
|
|
|||||||
| (1) | Par value, shares authorized and shares issued and outstanding represents the as reported and as restated amounts as there were no adjustments to these totals. |
144
Table of Contents
| June 30, 2013 (Unaudited) |
||||||||||||
| As reported | Adjustments | As restated | ||||||||||
| Assets |
||||||||||||
| Current assets: |
||||||||||||
| Cash and cash equivalents |
$ | 4,237 | $ | — | $ | 4,237 | ||||||
| Accounts receivable |
3,905 | (510 | ) | 3,395 | ||||||||
| Accounts receivable from related parties |
— | 267 | 267 | |||||||||
| Inventories, net |
6,928 | 96 | 7,024 | |||||||||
| Deferred cost of product revenues, including deferred cost of product revenues to related parties of $247 as of June 30, 2013 |
— | 2,102 | 2,102 | |||||||||
| Prepaid expenses and other current assets |
1,522 | — | 1,522 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total current assets |
16,592 | 1,955 | 18,547 | |||||||||
| Property, plant and equipment, net |
4,766 | — | 4,766 | |||||||||
| Other assets |
4,217 | — | 4,217 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total assets |
25,575 | 1,955 | 27,530 | |||||||||
|
|
|
|
|
|
|
|||||||
| Liabilities, convertible preferred stock and stockholders’ deficit |
||||||||||||
| Current liabilities: |
||||||||||||
| Accounts payable |
$ | 4,300 | $ | — | $ | 4,300 | ||||||
| Accrued liabilities |
2,272 | (47 | ) | 2,225 | ||||||||
| Deferred revenue, current portion |
324 | 2,279 | 2,603 | |||||||||
| Deferred revenue from related parties, current portion |
— | 469 | 469 | |||||||||
| Capital lease obligations, current portion |
573 | — | 573 | |||||||||
| Debt, current portion |
172 | — | 172 | |||||||||
| Preferred stock warrant liability |
1,308 | — | 1,308 | |||||||||
| Common stock warrant liability |
1,424 | — | 1,424 | |||||||||
| Convertible notes payable, current portion |
18,991 | — | 18,991 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total current liabilities |
29,364 | 2,701 | 32,065 | |||||||||
| Deferred revenue, less current portion |
1,534 | (694 | ) | 840 | ||||||||
| Deferred revenue from related parties, less current portion |
— | 694 | 694 | |||||||||
| Capital lease obligations, less current portion |
357 | — | 357 | |||||||||
| Debt, less current portion |
12,265 | — | 12,265 | |||||||||
| Convertible notes payable, less current portion |
29,243 | — | 29,243 | |||||||||
| Other liabilities |
614 | — | 614 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total liabilities |
73,377 | 2,701 | 76,078 | |||||||||
| Commitments and contingencies |
||||||||||||
| Convertible preferred stock—Series A: $0.00001 par value; 1,489 shares authorized; 1,484 shares issued and outstanding as of June 30,2013(1) |
3,747 | — | 3,747 | |||||||||
| Convertible preferred stock—Series B: $0.00001 par value; 2,252 shares authorized; 2,242 shares issued and outstanding as of June 30,2013(1) |
10,758 | — | 10,758 | |||||||||
| Convertible preferred stock—Series C: $0.00001 par value; 5,082 shares authorized; 4,778 shares issued and outstanding as of June 30,2013(1) |
25,107 | — | 25,107 | |||||||||
| Stockholders’ deficit: |
||||||||||||
| Common stock: $0.00001 par value; 12,936 shares authorized and 1,281 shares issued and outstanding at June 30, 2013(1) |
— | — | — | |||||||||
| Additional paid in capital |
1,921 | 10 | 1,931 | |||||||||
| Accumulated deficit |
(89,335 | ) | (756 | ) | (90,091 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total stockholders’ deficit |
(87,414 | ) | (746 | ) | (88,160 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total liabilities, convertible preferred stock and stockholders’ deficit |
$ | 25,575 | $ | 1,955 | $ | 27,530 | ||||||
|
|
|
|
|
|
|
|||||||
| (1) | Par value, shares authorized and shares issued and outstanding represents the as reported and as restated amounts as there were no adjustments to these totals. |
145
Table of Contents
| September 30, 2013 (Unaudited) |
||||||||||||
| As reported | Adjustments | As restated | ||||||||||
| Assets |
||||||||||||
| Current assets: |
||||||||||||
| Cash and cash equivalents |
$ | 40,775 | $ | — | $ | 40,775 | ||||||
| Short-term investments |
12,684 | — | 12,684 | |||||||||
| Accounts receivable |
2,050 | (636 | ) | 1,414 | ||||||||
| Accounts receivable from related parties |
— | — | — | |||||||||
| Inventories, net |
10,754 | 258 | 11,012 | |||||||||
| Deferred cost of product revenues, including deferred cost of product revenues to related parties of $120 as of September 30, 2013 |
— | 1,777 | 1,777 | |||||||||
| Prepaid expenses and other current assets |
2,357 | (358 | ) | 1,999 | ||||||||
|
|
|
|
|
|
|
|||||||
| Total current assets |
68,620 | 1,041 | 69,661 | |||||||||
| Property, plant and equipment, net |
5,726 | (37 | ) | 5,689 | ||||||||
| Other assets |
1,057 | — | 1,057 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total assets |
75,403 | 1,004 | 76,407 | |||||||||
|
|
|
|
|
|
|
|||||||
| Liabilities and stockholders’ equity |
||||||||||||
| Current liabilities: |
||||||||||||
| Accounts payable |
$ | 5,125 | $ | — | $ | 5,125 | ||||||
| Accrued liabilities |
2,973 | (85 | ) | 2,888 | ||||||||
| Deferred revenue, current portion |
1,094 | 1,446 | 2,540 | |||||||||
| Deferred revenue from related parties, current portion |
— | 322 | 322 | |||||||||
| Capital lease obligations, current portion |
782 | — | 782 | |||||||||
| Debt, current portion |
177 | — | 177 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total current liabilities |
10,151 | 1,683 | 11,834 | |||||||||
| Deferred revenue, less current portion |
1,453 | (661 | ) | 792 | ||||||||
| Deferred revenue from related parties, less current portion |
— | 661 | 661 | |||||||||
| Capital lease obligations, less current portion |
538 | — | 538 | |||||||||
| Debt, less current portion |
12,261 | — | 12,261 | |||||||||
| Other liabilities |
569 | — | 569 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total liabilities |
24,972 | 1,683 | 26,655 | |||||||||
| Commitments and contingencies |
||||||||||||
| Stockholders’ equity: |
||||||||||||
| Preferred stock: $0.00001 par value; 20,000 shares authorized, no shares issued or outstanding at September 30, 2013 (1) |
— | — | — | |||||||||
| Common stock: $0.00001 par value; 250,000 shares authorized and 19,199 shares issued and outstanding at September 30, 2013 (1) |
— | — | — | |||||||||
| Additional paid in capital |
145,876 | 44 | 145,920 | |||||||||
| Accumulated deficit |
(95,445 | ) | (723 | ) | (96,168 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total stockholders’ equity |
50,431 | (679 | ) | 49,752 | ||||||||
|
|
|
|
|
|
|
|||||||
| Total liabilities and stockholders’ equity |
$ | 75,403 | $ | 1,004 | $ | 76,407 | ||||||
|
|
|
|
|
|
|
|||||||
| (1) | Par value, shares authorized and shares issued and outstanding represents the as reported and as restated amounts as there were no adjustments to these totals. |
146
Table of Contents
The following tables present the condensed consolidated statements of operations for each quarter in 2014 (in thousands, except per share data), including adjustments described in Note 2:
| Three months ended March 31, 2014 (Unaudited) |
||||||||||||||||
| As reported | Revenue Related Adjustments |
Other Miscellaneous Adjustments |
As restated | |||||||||||||
| Revenues: |
||||||||||||||||
| Product |
$ | 2,097 | $ | (43 | ) | $ | — | $ | 2,054 | |||||||
| License |
45 | — | — | 45 | ||||||||||||
| Related party |
648 | (43 | ) | — | 605 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total revenues |
2,790 | (86 | ) | — | 2,704 | |||||||||||
| Cost of product revenues, including cost of product revenues to related parties of $161 for the three months ended March 31, 2014(1) |
1,652 | 69 | 72 | 1,793 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Gross profit |
1,138 | (155 | ) | (72 | ) | 911 | ||||||||||
| Operating expenses: |
||||||||||||||||
| Research, development and patent |
4,282 | — | 15 | 4,297 | ||||||||||||
| Selling, general and administrative |
6,330 | (18 | ) | 12 | 6,324 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
10,612 | (18 | ) | 27 | 10,621 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss from operations |
(9,474 | ) | (137 | ) | (99 | ) | (9,710 | ) | ||||||||
| Other income (expense): |
||||||||||||||||
| Interest income |
10 | — | — | 10 | ||||||||||||
| Interest expense |
(773 | ) | — | 167 | (606 | ) | ||||||||||
| Other expense, net |
(9 | ) | — | — | (9 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total other income (expense), net |
(772 | ) | — | 167 | (605 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss before income taxes |
(10,246 | ) | (137 | ) | 68 | (10,315 | ) | |||||||||
| Income taxes |
— | — | — | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
$ | (10,246 | ) | $ | (137 | ) | $ | 68 | $ | (10,315 | ) | |||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss per common share: |
||||||||||||||||
| Basic |
$ | (0.52 | ) | $ | (0.01 | ) | $ | — | $ | (0.53 | ) | |||||
|
|
|
|
|
|
|
|
|
|||||||||
| Diluted |
$ | (0.52 | ) | $ | (0.01 | ) | $ | — | $ | (0.53 | ) | |||||
|
|
|
|
|
|
|
|
|
|||||||||
| Weighted-average shares outstanding used in computing net loss per common share: |
||||||||||||||||
| Basic |
19,518 | — | — | 19,518 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Diluted |
19,518 | — | — | 19,518 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| (1) | Cost of product revenues to related parties for the three months ended March 31, 2014 was reported as $192. Revenue related adjustments for the three months ended March 31, 2014 totaled $(31). There were no other miscellaneous adjustments for the three months ended March 31, 2014. |
147
Table of Contents
| Three months ended June 30, 2014 (Unaudited) |
Six months ended June 30, 2014 (Unaudited) |
|||||||||||||||||||||||||||||||
| As reported |
Revenue Related Adjustments |
Other Miscellaneous Adjustments |
As restated |
As reported |
Revenue Related Adjustments |
Other Miscellaneous Adjustments |
As restated |
|||||||||||||||||||||||||
| Revenues: |
||||||||||||||||||||||||||||||||
| Product |
$ | 3,414 | $ | (537 | ) | $ | — | $ | 2,877 | $ | 5,511 | $ | (580 | ) | $ | — | $ | 4,931 | ||||||||||||||
| License |
51 | — | — | 51 | 96 | — | — | 96 | ||||||||||||||||||||||||
| Related party |
164 | 42 | — | 206 | 812 | (1 | ) | — | 811 | |||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Total revenues |
3,629 | (495 | ) | — | 3,134 | 6,419 | (581 | ) | — | 5,838 | ||||||||||||||||||||||
| Cost of product revenues, including cost of product revenues to related parties of $130 and $291 for the three and six months ended June 30, 2014, respectively(1) |
2,849 | (138 | ) | — | 2,711 | 4,501 | (69 | ) | 72 | 4,504 | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Gross profit |
780 | (357 | ) | — | 423 | 1,918 | (512 | ) | (72 | ) | 1,334 | |||||||||||||||||||||
| Operating expenses: |
||||||||||||||||||||||||||||||||
| Research, development and patent |
4,264 | — | — | 4,264 | 8,546 | — | 15 | 8,561 | ||||||||||||||||||||||||
| Selling, general and administrative |
5,989 | — | (69 | ) | 5,920 | 12,319 | (18 | ) | (57 | ) | 12,244 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Total operating expenses |
10,253 | — | (69 | ) | 10,184 | 20,865 | (18 | ) | (42 | ) | 20,805 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Loss from operations |
(9,473 | ) | (357 | ) | 69 | (9,761 | ) | (18,947 | ) | (494 | ) | (30 | ) | (19,471 | ) | |||||||||||||||||
| Other income (expense): |
||||||||||||||||||||||||||||||||
| Interest income |
11 | — | — | 11 | 21 | — | — | 21 | ||||||||||||||||||||||||
| Interest expense |
(825 | ) | — | (38 | ) | (863 | ) | (1,598 | ) | — | 129 | (1,469 | ) | |||||||||||||||||||
| Other expense, net |
(98 | ) | — | — | (98 | ) | (107 | ) | — | — | (107 | ) | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Total other income (expense), net |
(912 | ) | — | (38 | ) | (950 | ) | (1,684 | ) | — | 129 | (1,555 | ) | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Loss before income taxes |
(10,385 | ) | (357 | ) | 31 | (10,711 | ) | (20,631 | ) | (494 | ) | 99 | (21,026 | ) | ||||||||||||||||||
| Income taxes |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Net loss |
$ | (10,385 | ) | $ | (357 | ) | $ | 31 | $ | (10,711 | ) | $ | (20,631 | ) | $ | (494 | ) | $ | 99 | $ | (21,026 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Net loss per common share: |
||||||||||||||||||||||||||||||||
| Basic |
$ | (0.50 | ) | $ | (0.02 | ) | $ | — | $ | (0.52 | ) | $ | (1.02 | ) | $ | (0.02 | ) | $ | — | $ | (1.04 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Diluted |
$ | (0.50 | ) | $ | (0.02 | ) | $ | — | $ | (0.52 | ) | $ | (1.02 | ) | $ | (0.02 | ) | $ | — | $ | (1.04 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Weighted-average shares outstanding used in computing net loss per common share: |
||||||||||||||||||||||||||||||||
| Basic |
20,775 | — | — | 20,775 | 20,150 | — | — | 20,150 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Diluted |
20,775 | — | — | 20,775 | 20,150 | — | — | 20,150 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| (1) | Cost of product revenues to related parties for the three and six months ended June 30, 2014 was reported as $73 and $265. Revenue related adjustments for the three and six months ended June 30, 2014 totaled $57 and $26, respectively. There were no other miscellaneous adjustments for the three and six months ended June 30, 2014. |
148
Table of Contents
| Three months ended September 30, 2014 |
Nine months ended September 30, 2014 |
Three months ended December 31, 2014 |
||||||||||
| (Unaudited) | (Unaudited) | (Unaudited) | ||||||||||
| Revenues: |
||||||||||||
| Product |
$ | 1,881 | $ | 6,812 | $ | 938 | ||||||
| License |
65 | 161 | 71 | |||||||||
| Related party |
254 | 1,065 | 89 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total revenues |
2,200 | 8,038 | 1,098 | |||||||||
| Cost of product revenues, including cost of product revenues to related parties of $230, $521 and $40 for the three and nine months ended September 30, 2014 and the three months ended December 31, 2014, respectively |
3,502 | 8,006 | 1,432 | |||||||||
|
|
|
|
|
|
|
|||||||
| Gross profit (loss) |
(1,302 | ) | 32 | (334 | ) | |||||||
| Operating expenses: |
||||||||||||
| Research, development and patent |
4,817 | 13,378 | 5,903 | |||||||||
| Selling, general and administrative |
7,394 | 19,638 | 9,312 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
12,211 | 33,016 | 15,215 | |||||||||
|
|
|
|
|
|
|
|||||||
| Loss from operations |
(13,513 | ) | (32,984 | ) | (15,549 | ) | ||||||
| Other income (expense): |
||||||||||||
| Interest income |
21 | 42 | 17 | |||||||||
| Interest expense |
(769 | ) | (2,238 | ) | (669 | ) | ||||||
| Other expense, net |
(139 | ) | (246 | ) | (32 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total other income (expense), net |
(887 | ) | (2,442 | ) | (684 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Loss before income taxes |
(14,400 | ) | (35,426 | ) | (16,233 | ) | ||||||
| Income taxes |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
$ | (14,400 | ) | $ | (35,426 | ) | $ | (16,233 | ) | |||
|
|
|
|
|
|
|
|||||||
| Net loss per common share: |
||||||||||||
| Basic |
$ | (0.59 | ) | $ | (1.64 | ) | $ | (0.66 | ) | |||
|
|
|
|
|
|
|
|||||||
| Diluted |
$ | (0.59 | ) | $ | (1.64 | ) | $ | (0.66 | ) | |||
|
|
|
|
|
|
|
|||||||
| Weighted-average shares outstanding used in computing net loss per common share(1): |
||||||||||||
| Basic |
24,421 | 21,589 | 24,464 | |||||||||
|
|
|
|
|
|
|
|||||||
| Diluted |
24,421 | 21,589 | 24,464 | |||||||||
|
|
|
|
|
|
|
|||||||
| (1) | Stock options totaling 2,903 and common stock warrants totaling 145 were not included in the computation of diluted income per share for the three and nine months ended September 30, 2014 as they were anti-dilutive. |
149
Table of Contents
The following tables present the condensed consolidated statements of operations for each quarter in 2013 (in thousands, except per share data), including adjustments described in Note 2:
| Three months ended March 31, 2013 (Unaudited) |
||||||||||||||||
| As reported | Revenue Related Adjustments |
Other Miscellaneous Adjustments |
As restated | |||||||||||||
| Revenues: |
||||||||||||||||
| Product |
$ | 2,373 | $ | (284 | ) | $ | — | $ | 2,089 | |||||||
| License |
48 | — | — | 48 | ||||||||||||
| Related party |
309 | (255 | ) | — | 54 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total revenues |
2,730 | (539 | ) | — | 2,191 | |||||||||||
| Cost of product revenues (1) |
1,795 | (381 | ) | — | 1,414 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Gross profit |
935 | (158 | ) | — | 777 | |||||||||||
| Operating expenses: |
||||||||||||||||
| Research, development and patent |
3,283 | — | — | 3,283 | ||||||||||||
| Selling, general and administrative |
2,847 | — | — | 2,847 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
6,130 | — | — | 6,130 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss from operations |
(5,195 | ) | (158 | ) | — | (5,353 | ) | |||||||||
| Other income (expense): |
||||||||||||||||
| Interest income |
1 | — | — | 1 | ||||||||||||
| Interest expense |
(1,985 | ) | — | 17 | (1,968 | ) | ||||||||||
| Change in estimated fair value of financial instruments |
(3,563 | ) | — | — | (3,563 | ) | ||||||||||
| Other expense, net |
(7 | ) | — | — | (7 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total other income (expense), net |
(5,554 | ) | — | 17 | (5,537 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss before income taxes |
(10,749 | ) | (158 | ) | 17 | (10,890 | ) | |||||||||
| Income taxes |
— | — | — | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
$ | (10,749 | ) | $ | (158 | ) | $ | 17 | $ | (10,890 | ) | |||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss per common share: |
||||||||||||||||
| Basic |
$ | (8.48 | ) | $ | (0.12 | ) | $ | 0.01 | $ | (8.59 | ) | |||||
|
|
|
|
|
|
|
|
|
|||||||||
| Diluted |
$ | (8.48 | ) | $ | (0.12 | ) | $ | 0.01 | $ | (8.59 | ) | |||||
|
|
|
|
|
|
|
|
|
|||||||||
| Weighted-average shares outstanding used in computing net loss per common share: |
||||||||||||||||
| Basic |
1,268 | — | — | 1,268 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Diluted |
1,268 | — | — | 1,268 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| (1) | Cost of product revenues to related parties for the three months ended March 31, 2013 was reported as $194. Revenue related adjustments for the three months ended March 31, 2013 totaled $(194). There were no other miscellaneous adjustments for the three months ended March 31, 2013. |
150
Table of Contents
| Three months ended June 30, 2013 (Unaudited) |
Six months ended June 30, 2013 (Unaudited) |
|||||||||||||||||||||||||||||||
| As reported |
Revenue Related Adjustments |
Other Miscellaneous Adjustments |
As restated |
As reported |
Revenue Related Adjustments |
Other Miscellaneous Adjustments |
As restated |
|||||||||||||||||||||||||
| Revenues: |
||||||||||||||||||||||||||||||||
| Product |
$ | 4,152 | $ | (2,357 | ) | $ | — | $ | 1,795 | $ | 6,525 | $ | (2,641 | ) | $ | — | $ | 3,884 | ||||||||||||||
| License |
48 | — | — | 48 | 96 | — | — | 96 | ||||||||||||||||||||||||
| Related party |
300 | (95 | ) | — | 205 | 609 | (350 | ) | — | 259 | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Total revenues |
4,500 | (2,452 | ) | — | 2,048 | 7,230 | (2,991 | ) | — | 4,239 | ||||||||||||||||||||||
| Cost of product revenues, including cost of product revenues to related parties of $117 for both the three and six months ended June 30, 2013(1) |
3,398 | (1,813 | ) | — | 1,585 | 5,193 | (2,194 | ) | — | 2,999 | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Gross profit |
1,102 | (639 | ) | — | 463 | 2,037 | (797 | ) | — | 1,240 | ||||||||||||||||||||||
| Operating expenses: |
||||||||||||||||||||||||||||||||
| Research, development and patent |
3,941 | — | — | 3,941 | 7,224 | — | — | 7,224 | ||||||||||||||||||||||||
| Selling, general and administrative |
3,107 | — | 10 | 3,117 | 5,954 | — | 10 | 5,964 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Total operating expenses |
7,048 | — | 10 | 7,058 | 13,178 | — | 10 | 13,188 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Loss from operations |
(5,946 | ) | (639 | ) | (10 | ) | (6,595 | ) | (11,141 | ) | (797 | ) | (10 | ) | (11,948 | ) | ||||||||||||||||
| Other income (expense): |
||||||||||||||||||||||||||||||||
| Interest income |
— | — | — | — | 1 | — | — | 1 | ||||||||||||||||||||||||
| Interest expense |
(2,285 | ) | — | 34 | (2,251 | ) | (4,270 | ) | — | 51 | (4,219 | ) | ||||||||||||||||||||
| Change in estimated fair value of financial instruments |
6,550 | — | — | 6,550 | 2,987 | — | — | 2,987 | ||||||||||||||||||||||||
| Gain on extinguishment of debt |
49 | — | — | 49 | 49 | — | — | 49 | ||||||||||||||||||||||||
| Other expense, net |
(7 | ) | — | — | (7 | ) | (14 | ) | — | — | (14 | ) | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Total other income (expense), net |
4,307 | — | 34 | 4,341 | (1,247 | ) | — | 51 | (1,196 | ) | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Loss before income taxes |
(1,639 | ) | (639 | ) | 24 | (2,254 | ) | (12,388 | ) | (797 | ) | 41 | (13,144 | ) | ||||||||||||||||||
| Income taxes |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Net loss |
(1,639 | ) | (639 | ) | 24 | (2,254 | ) | (12,388 | ) | (797 | ) | 41 | (13,144 | ) | ||||||||||||||||||
| Deemed dividend on convertible notes |
(1,378 | ) | — | — | (1,378 | ) | (1,378 | ) | — | — | (1,378 | ) | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Net loss attributable to common stockholders |
$ | (3,017 | ) | $ | (639 | ) | 24 | $ | (3,632 | ) | $ | (13,766 | ) | $ | (797 | ) | 41 | $ | (14,522 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Net loss per common share: |
||||||||||||||||||||||||||||||||
| Basic |
$ | (2.36 | ) | $ | (0.50 | ) | $ | 0.02 | $ | (2.84 | ) | $ | (10.81 | ) | $ | (0.63 | ) | $ | 0.03 | $ | (11.41 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Diluted |
$ | (2.67 | ) | $ | (0.47 | ) | $ | 0.02 | $ | (3.12 | ) | $ | (10.81 | ) | $ | (0.63 | ) | $ | 0.03 | $ | (11.41 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Weighted-average shares outstanding used in computing net loss per common share: |
||||||||||||||||||||||||||||||||
| Basic |
1,277 | — | — | 1,277 | 1,273 | — | — | 1,273 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Diluted |
1,347 | — | — | 1,347 | 1,273 | — | — | 1,273 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| (1) | Cost of product revenues to related parties for the three and six months ended June 30, 2013 was reported as $170 and $364, respectively. Revenue related adjustments for the three and six months ended June 30, 2013 totaled $(53) and $(247), respectively. There were no other miscellaneous adjustments for the three and six months ended June 30, 2013. |
151
Table of Contents
| Three months ended September 30, 2013 (Unaudited) |
Nine months ended September 30, 2013 (Unaudited) |
|||||||||||||||||||||||||||||||
| As reported |
Revenue Related Adjustments |
Other Miscellaneous Adjustments |
As restated |
As reported |
Revenue Related Adjustments |
Other Miscellaneous Adjustments |
As restated |
|||||||||||||||||||||||||
| Revenues: |
||||||||||||||||||||||||||||||||
| Product |
$ | 1,149 | $ | 440 | $ | — | $ | 1,589 | $ | 7,674 | $ | (2,201 | ) | $ | — | $ | 5,473 | |||||||||||||||
| License |
48 | — | — | 48 | 144 | — | — | 144 | ||||||||||||||||||||||||
| Related party |
149 | 147 | — | 296 | 758 | (203 | ) | — | 555 | |||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Total revenues |
1,346 | 587 | — | 1,933 | 8,576 | (2,404 | ) | — | 6,172 | |||||||||||||||||||||||
| Cost of product revenues, including cost of product revenues to related parties of $178 and $295 for the three and the nine months ended September 30, 2013, respectively(1) |
1,077 | 517 | — | 1,594 | 6,270 | (1,677 | ) | — | 4,593 | |||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Gross profit |
269 | 70 | — | 339 | 2,306 | (727 | ) | — | 1,579 | |||||||||||||||||||||||
| Operating expenses: |
||||||||||||||||||||||||||||||||
| Research, development and patent |
4,454 | — | — | 4,454 | 11,678 | — | — | 11,678 | ||||||||||||||||||||||||
| Selling, general and administrative |
4,493 | — | 34 | 4,527 | 10,447 | — | 44 | 10,491 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Total operating expenses |
8,947 | — | 34 | 8,981 | 22,125 | — | 44 | 22,169 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Loss from operations |
(8,678 | ) | 70 | (34 | ) | (8,642 | ) | (19,819 | ) | (727 | ) | (44 | ) | (20,590 | ) | |||||||||||||||||
| Other income (expense): |
||||||||||||||||||||||||||||||||
| Interest income |
24 | — | — | 24 | 25 | — | — | 25 | ||||||||||||||||||||||||
| Interest expense |
(1,119 | ) | — | (3 | ) | (1,122 | ) | (5,389 | ) | — | 48 | (5,341 | ) | |||||||||||||||||||
| Change in estimated fair value of financial instruments |
3,730 | — | — | 3,730 | 6,717 | — | — | 6,717 | ||||||||||||||||||||||||
| Gain on extinguishment of debt |
— | — | — | — | 49 | — | — | 49 | ||||||||||||||||||||||||
| Other expense, net |
(67 | ) | — | — | (67 | ) | (81 | ) | — | — | (81 | ) | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Total other income (expense), net |
2,568 | — | (3 | ) | 2,565 | 1,321 | — | 48 | 1,369 | |||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Loss before income taxes |
(6,110 | ) | 70 | (37 | ) | (6,077 | ) | (18,498 | ) | (727 | ) | 4 | (19,221 | ) | ||||||||||||||||||
| Income taxes |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Net loss |
(6,110 | ) | 70 | (37 | ) | (6,077 | ) | (18,498 | ) | (727 | ) | 4 | (19,221 | ) | ||||||||||||||||||
| Deemed dividend on convertible notes |
— | — | — | — | (1,378 | ) | — | — | (1,378 | ) | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Net loss attributable to common stockholders |
$ | (6,110 | ) | $ | 70 | $ | (37 | ) | $ | (6,077 | ) | $ | (19,876 | ) | $ | (727 | ) | $ | 4 | $ | (20,599 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Net loss per common share: |
||||||||||||||||||||||||||||||||
| Basic |
$ | (0.47 | ) | $ | — | $ | — | $ | (0.47 | ) | $ | (3.83 | ) | $ | (0.14 | ) | $ | — | $ | (3.97 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Diluted |
$ | (0.80 | ) | $ | — | $ | — | $ | (0.80 | ) | $ | (4.63 | ) | $ | (0.14 | ) | $ | — | $ | (4.77 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Weighted-average shares outstanding used in computing net loss per common share: |
||||||||||||||||||||||||||||||||
| Basic |
12,888 | — | — | 12,888 | 5,187 | — | — | 5,187 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Diluted |
13,422 | — | — | 13,422 | 5,417 | — | — | 5,417 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| (1) | Cost of product revenues to related parties for the three and nine months ended September 30, 2013 was reported as $51 and $415, respectively. Revenue related adjustments for the three and nine months ended September 30, 2013 totaled $127 and $(120), respectively. There were no other miscellaneous adjustments for the three and nine months ended September 30, 2013. |
152
Table of Contents
| Three months ended December 31, 2013 (Unaudited) |
||||||||||||||||
| As reported | Revenue Related Adjustments |
Other Miscellaneous Adjustments |
As restated | |||||||||||||
| Revenues: |
||||||||||||||||
| Product |
$ | 4,983 | $ | (2,868 | ) | $ | — | $ | 2,115 | |||||||
| License |
49 | — | — | 49 | ||||||||||||
| Related party |
935 | (825 | ) | — | 110 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total revenues |
5,967 | (3,693 | ) | — | 2,274 | |||||||||||
| Cost of product revenues, including cost of product revenues to related parties of $79 for the three months ended December 31, 2013(1) |
4,466 | (1,744 | ) | (72 | ) | 2,650 | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Gross profit (loss) |
1,501 | (1,949 | ) | 72 | (376 | ) | ||||||||||
| Operating expenses: |
||||||||||||||||
| Research, development and patent |
6,136 | — | 91 | 6,227 | ||||||||||||
| Selling, general and administrative |
4,571 | (10 | ) | (35 | ) | 4,526 | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
10,707 | (10 | ) | 56 | 10,753 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss from operations |
(9,206 | ) | (1,939 | ) | 16 | (11,129 | ) | |||||||||
| Other income (expense): |
||||||||||||||||
| Interest income |
24 | — | — | 24 | ||||||||||||
| Interest expense |
(608 | ) | — | (107 | ) | (715 | ) | |||||||||
| Other expense, net |
(201 | ) | — | — | (201 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total other income (expense), net |
(785 | ) | — | (107 | ) | (892 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss before income taxes |
(9,991 | ) | (1,939 | ) | (91 | ) | (12,021 | ) | ||||||||
| Income taxes |
— | — | — | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
$ | (9,991 | ) | $ | (1,939 | ) | $ | (91 | ) | $ | (12,021 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss per common share: |
||||||||||||||||
| Basic |
$ | (0.52 | ) | $ | (0.10 | ) | $ | (0.01 | ) | $ | (0.63 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Diluted |
$ | (0.52 | ) | $ | (0.10 | ) | $ | (0.01 | ) | $ | (0.63 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Weighted-average shares outstanding used in computing net loss per common share: |
||||||||||||||||
| Basic |
19,246 | — | — | 19,246 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Diluted |
19,246 | — | — | 19,246 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| (1) | Cost of product revenues to related parties for the three months ended December 31, 2013 was reported as $569. Revenue related adjustments for the three months ended December 31, 2013 totaled $(490). There were no other miscellaneous adjustments for the three months ended December 31, 2013. |
153
Table of Contents
The following tables present the condensed consolidated statements of comprehensive loss for each quarter in 2014 (in thousands), including adjustments described in Note 2:
| Three months ended March 31, 2014 (Unaudited) |
||||||||||||
| As reported | Adjustments | As restated | ||||||||||
| Net loss |
$ | (10,246 | ) | $ | (69 | ) | $ | (10,315 | ) | |||
| Other comprehensive loss |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Comprehensive loss |
$ | (10,246 | ) | $ | (69 | ) | $ | (10,315 | ) | |||
|
|
|
|
|
|
|
|||||||
| Three months ended June 30, 2014 (Unaudited) |
||||||||||||
| As reported | Adjustments | As restated | ||||||||||
| Net loss |
$ | (10,385 | ) | $ | (326 | ) | $ | (10,711 | ) | |||
| Other comprehensive loss |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Comprehensive loss |
$ | (10,385 | ) | $ | (326 | ) | $ | (10,711 | ) | |||
|
|
|
|
|
|
|
|||||||
| Six months ended June 30, 2014 (Unaudited) |
||||||||||||
| As reported | Adjustments | As restated | ||||||||||
| Net loss |
$ | (20,631 | ) | $ | (395 | ) | $ | (21,026 | ) | |||
| Other comprehensive loss |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Comprehensive loss |
$ | (20,631 | ) | $ | (395 | ) | $ | (21,026 | ) | |||
|
|
|
|
|
|
|
|||||||
| Three months ended September 30, 2014 (Unaudited) |
||||
| Net loss |
$ | (14,400 | ) | |
| Other comprehensive loss |
— | |||
|
|
|
|||
| Comprehensive loss |
$ | (14,400 | ) | |
|
|
|
|||
| Nine months ended September 30, 2014 (Unaudited) |
||||
| Net loss |
$ | (35,426 | ) | |
| Other comprehensive loss |
— | |||
|
|
|
|||
| Comprehensive loss |
$ | (35,426 | ) | |
|
|
|
|||
| Three months ended December 31, 2014 (Unaudited) |
||||
| Net loss |
$ | (16,233 | ) | |
| Other comprehensive loss |
— | |||
|
|
|
|||
| Comprehensive loss |
$ | (16,233 | ) | |
|
|
|
|||
154
Table of Contents
The following tables present the condensed consolidated statements of comprehensive loss for each quarter in 2013 (in thousands), including adjustments described in Note 2:
| Three months ended March 31, 2013 (Unaudited) |
||||||||||||
| As reported | Adjustments | As restated | ||||||||||
| Net loss |
$ | (10,749 | ) | $ | (141 | ) | $ | (10,890 | ) | |||
| Other comprehensive loss |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Comprehensive loss |
$ | (10,749 | ) | $ | (141 | ) | $ | (10,890 | ) | |||
|
|
|
|
|
|
|
|||||||
| Three months ended June 30, 2013 (Unaudited) |
||||||||||||
| As reported | Adjustments | As restated | ||||||||||
| Net loss |
$ | (1,639 | ) | $ | (615 | ) | $ | (2,254 | ) | |||
| Other comprehensive loss |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Comprehensive loss |
$ | (1,639 | ) | $ | (615 | ) | $ | (2,254 | ) | |||
|
|
|
|
|
|
|
|||||||
| Six months ended June 30, 2013 (Unaudited) |
||||||||||||
| As reported | Adjustments | As restated | ||||||||||
| Net loss |
$ | (12,388 | ) | $ | (756 | ) | $ | (13,144 | ) | |||
| Other comprehensive loss |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Comprehensive loss |
$ | (12,388 | ) | $ | (756 | ) | $ | (13,144 | ) | |||
|
|
|
|
|
|
|
|||||||
| Three months ended September 30, 2013 (Unaudited) |
||||||||||||
| As reported | Adjustments | As restated | ||||||||||
| Net loss |
$ | (6,110 | ) | $ | 33 | $ | (6,077 | ) | ||||
| Other comprehensive loss |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Comprehensive loss |
$ | (6,110 | ) | $ | 33 | $ | (6,077 | ) | ||||
|
|
|
|
|
|
|
|||||||
| Nine months ended September 30, 2013 (Unaudited) |
||||||||||||
| As reported | Adjustments | As restated | ||||||||||
| Net loss |
$ | (18,498 | ) | $ | (723 | ) | $ | (19,221 | ) | |||
| Other comprehensive loss |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Comprehensive loss |
$ | (18,498 | ) | $ | (723 | ) | $ | (19,221 | ) | |||
|
|
|
|
|
|
|
|||||||
| Three months ended December 31, 2013 (Unaudited) |
||||||||||||
| As reported | Adjustments | As restated | ||||||||||
| Net loss |
$ | (9,991 | ) | $ | (2,030 | ) | $ | (12,021 | ) | |||
| Other comprehensive loss |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Comprehensive loss |
$ | (9,991 | ) | $ | (2,030 | ) | $ | (12,021 | ) | |||
|
|
|
|
|
|
|
|||||||
155
Table of Contents
The following tables present the condensed consolidated statements of cash flows for the three months ended March 2014, the six months ended June 30, 2014 and the nine months ended September, 30 2014 (in thousands), including adjustments described in Note 2:
| THREE MONTHS ENDED MARCH 31, 2014 (Unaudited) |
||||||||||||
| As reported | Adjustments | As restated | ||||||||||
| Cash flows from operating activities |
||||||||||||
| Net loss |
$ | (10,246 | ) | $ | (69 | ) | $ | (10,315 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||||
| Depreciation and amortization |
488 | — | 488 | |||||||||
| Share-based compensation |
1,522 | — | 1,522 | |||||||||
| Non-cash interest expense |
248 | 17 | 265 | |||||||||
| Amortization of investment securities premiums/discounts, net |
9 | — | 9 | |||||||||
| Net changes in operating assets and liabilities: |
||||||||||||
| Accounts receivable |
(1,016 | ) | (444 | ) | (1,460 | ) | ||||||
| Accounts receivable from related parties |
(327 | ) | — | (327 | ) | |||||||
| Inventories |
(1,171 | ) | 297 | (874 | ) | |||||||
| Prepaid expenses and other assets |
(224 | ) | (74 | ) | (298 | ) | ||||||
| Deferred cost of product revenues |
— | (46 | ) | (46 | ) | |||||||
| Accounts payable |
2,737 | — | 2,737 | |||||||||
| Accrued and other liabilities |
(1,337 | ) | (211 | ) | (1,548 | ) | ||||||
| Deferred revenue |
(241 | ) | 487 | 246 | ||||||||
| Deferred revenue from related parties |
(324 | ) | 43 | (281 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in operating activities |
(9,882 | ) | — | (9,882 | ) | |||||||
| Cash flows from investing activities |
||||||||||||
| Purchases of property, plant and equipment |
(5,044 | ) | — | (5,044 | ) | |||||||
| Purchase of short-term investments |
(49 | ) | — | (49 | ) | |||||||
| Maturities of short-term investments |
11,053 | — | 11,053 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by investing activities |
5,960 | — | 5,960 | |||||||||
| Cash flows from financing activities |
||||||||||||
| Repayment of debt |
(67 | ) | — | (67 | ) | |||||||
| Repayment of capital leases |
(69 | ) | — | (69 | ) | |||||||
| Proceeds from exercise of stock options |
851 | — | 851 | |||||||||
| Proceeds from exercise of common stock warrants |
50 | — | 50 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by financing activities |
765 | — | 765 | |||||||||
| Net increase in cash and cash equivalents |
(3,157 | ) | — | (3,157 | ) | |||||||
| Cash and cash equivalents, beginning of year |
24,455 | — | 24,455 | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash and cash equivalents, end of period |
$ | 21,298 | $ | — | $ | 21,298 | ||||||
|
|
|
|
|
|
|
|||||||
| Supplemental disclosure of cash flow information |
||||||||||||
| Cash paid for interest, net of capitalized interest of $469 for the three months ended March 31, 2014(1) |
$ | 525 | $ | (184 | ) | $ | 341 | |||||
|
|
|
|
|
|
|
|||||||
| Supplemental disclosure of non-cash investing and financing activities |
||||||||||||
| Property, plant and equipment included in accounts payable and accrued liabilities |
$ | 2,040 | $ | — | $ | 2,040 | ||||||
|
|
|
|
|
|
|
|||||||
| Equipment acquired under capital leases |
$ | 453 | $ | — | $ | 453 | ||||||
|
|
|
|
|
|
|
|||||||
| (1) | Capitalized interest amount represents the as reported and as restated amount as there were no adjustments to this balance. |
156
Table of Contents
| SIX MONTHS ENDED JUNE 30, 2014 (Unaudited) |
||||||||||||
| As reported | Adjustments | As restated | ||||||||||
| Cash flows from operating activities |
||||||||||||
| Net loss |
$ | (20,631 | ) | $ | (395 | ) | $ | (21,026 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||||
| Depreciation and amortization |
1,081 | — | 1,081 | |||||||||
| Loss on disposal of equipment |
85 | — | 85 | |||||||||
| Share-based compensation |
2,722 | — | 2,722 | |||||||||
| Non-cash interest expense |
495 | 33 | 528 | |||||||||
| Amortization of investment securities premiums/discounts, net |
10 | — | 10 | |||||||||
| Net changes in operating assets and liabilities: |
||||||||||||
| Accounts receivable |
2,105 | (2,321 | ) | (216 | ) | |||||||
| Accounts receivable from related parties |
413 | — | 413 | |||||||||
| Inventories |
(835 | ) | 295 | (540 | ) | |||||||
| Prepaid expenses and other assets |
(350 | ) | (93 | ) | (443 | ) | ||||||
| Deferred cost of product revenues |
— | (163 | ) | (163 | ) | |||||||
| Accounts payable |
330 | — | 330 | |||||||||
| Accrued and other liabilities |
(628 | ) | (215 | ) | (843 | ) | ||||||
| Deferred revenue |
(799 | ) | 976 | 177 | ||||||||
| Deferred revenue from related parties |
(333 | ) | — | (333 | ) | |||||||
| Customer refund liabilities |
— | 1,883 | 1,883 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in operating activities |
(16,335 | ) | — | (16,335 | ) | |||||||
| Cash flows from investing activities |
||||||||||||
| Purchases of property, plant and equipment |
(9,425 | ) | — | (9,425 | ) | |||||||
| Purchase of short-term investments |
(49 | ) | — | (49 | ) | |||||||
| Maturities of short-term investments |
13,467 | — | 13,467 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in investing activities |
3,993 | — | 3,993 | |||||||||
| Cash flows from financing activities |
||||||||||||
| Proceeds from public offerings, net of offering costs and underwriter commissions |
39,959 | — | 39,959 | |||||||||
| Proceeds from issuance of debt, net of financing costs |
9,621 | — | 9,621 | |||||||||
| Proceeds from line of credit |
4,687 | — | 4,687 | |||||||||
| Repayment of line of credit |
(4,687 | ) | — | (4,687 | ) | |||||||
| Repayment of debt |
(137 | ) | — | (137 | ) | |||||||
| Repayment of capital leases |
(219 | ) | — | (219 | ) | |||||||
| Change in restricted cash |
(4,885 | ) | — | (4,885 | ) | |||||||
| Proceeds from exercise of stock options |
1,128 | — | 1,128 | |||||||||
| Proceeds from exercise of common stock warrants |
50 | — | 50 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by financing activities |
45,517 | — | 45,517 | |||||||||
| Net increase (decrease) in cash and cash equivalents |
33,175 | — | 33,175 | |||||||||
| Cash and cash equivalents, beginning of year |
24,455 | — | 24,455 | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash and cash equivalents, end of period |
$ | 57,630 | $ | — | $ | 57,630 | ||||||
|
|
|
|
|
|
|
|||||||
| Supplemental disclosure of cash flow information |
||||||||||||
| Cash paid for interest, net of capitalized interest of $648 for the six months ended June 30, 2014(1) |
$ | 1,103 | $ | (181 | ) | $ | 922 | |||||
|
|
|
|
|
|
|
|||||||
| Supplemental disclosure of non-cash investing and financing activities |
||||||||||||
| Property, plant and equipment included in accounts payable and accrued liabilities |
$ | 834 | $ | — | $ | 834 | ||||||
|
|
|
|
|
|
|
|||||||
| Equipment acquired under capital leases |
$ | 646 | $ | — | $ | 646 | ||||||
|
|
|
|
|
|
|
|||||||
| (1) | Capitalized interest amount represents the as reported and as restated amount as there were no adjustments to this balance. |
157
Table of Contents
| NINE MONTHS ENDED SEPTEMBER 30, 2014 (Unaudited) |
||||
| Cash flows from operating activities |
||||
| Net loss |
$ | (35,426 | ) | |
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||
| Depreciation and amortization |
1,829 | |||
| Loss on disposal of equipment |
209 | |||
| Share-based compensation |
3,631 | |||
| Non-cash interest expense |
646 | |||
| Amortization of investment securities premiums/discounts, net |
10 | |||
| Net changes in operating assets and liabilities: |
||||
| Accounts receivable |
1,149 | |||
| Accounts receivable from related parties |
741 | |||
| Inventories |
(447 | ) | ||
| Prepaid expenses and other assets |
(712 | ) | ||
| Deferred cost of product revenues |
1,078 | |||
| Accounts payable |
(1,172 | ) | ||
| Accrued and other liabilities |
1,705 | |||
| Deferred revenue |
(760 | ) | ||
| Deferred revenue from related parties |
(587 | ) | ||
| Customer refund liabilities |
1,025 | |||
|
|
|
|||
| Net cash used in operating activities |
(27,081 | ) | ||
| Cash flows from investing activities |
||||
| Purchases of property, plant and equipment |
(11,329 | ) | ||
| Proceeds from sale of equipment |
6 | |||
| Purchase of short-term investments |
(49 | ) | ||
| Maturities of short-term investments |
13,716 | |||
|
|
|
|||
| Net cash used in investing activities |
2,344 | |||
| Cash flows from financing activities |
||||
| Proceeds from public offerings, net of offering costs and underwriter commissions |
39,949 | |||
| Proceeds from issuance of debt, net of financing costs |
9,696 | |||
| Proceeds from line of credit |
4,687 | |||
| Repayment of line of credit |
(4,687 | ) | ||
| Repayment of debt |
(271 | ) | ||
| Repayment of capital leases |
(745 | ) | ||
| Change in restricted cash |
(3,416 | ) | ||
| Proceeds from exercise of stock options |
1,300 | |||
| Proceeds from exercise of common stock warrants |
50 | |||
|
|
|
|||
| Net cash provided by financing activities |
46,563 | |||
| Net increase in cash and cash equivalents |
21,826 | |||
| Cash and cash equivalents, beginning of year |
24,455 | |||
|
|
|
|||
| Cash and cash equivalents, end of period |
$ | 46,281 | ||
|
|
|
|||
| Supplemental disclosure of cash flow information |
||||
| Cash paid for interest, net of capitalized interest of $651 for the nine months ended September 30, 2014 |
$ | 1,573 | ||
|
|
|
|||
| Supplemental disclosure of non-cash investing and financing activities |
||||
| Property, plant and equipment included in accounts payable and accrued liabilities |
$ | 249 | ||
|
|
|
|||
| Equipment acquired under capital leases |
$ | 834 | ||
|
|
|
|||
158
Table of Contents
The following tables present the condensed consolidated statements of cash flows for the three months ended March 31, 2013, the six months ended June 30, 2013 and the nine months ended September 30, 2013 (in thousands), including adjustments described in Note 2:
| THREE MONTHS ENDED MARCH 31, 2013 (Unaudited) |
||||||||||||
| As reported | Adjustments | As restated | ||||||||||
| Cash flows from operating activities |
||||||||||||
| Net loss |
$ | (10,749 | ) | $ | (141 | ) | $ | (10,890 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||||
| Depreciation and amortization |
184 | — | 184 | |||||||||
| Share-based compensation |
249 | — | 249 | |||||||||
| Non-cash interest expense |
1,467 | — | 1,467 | |||||||||
| Change in estimated fair value of financial instruments |
3,563 | — | 3,563 | |||||||||
| Net changes in operating assets and liabilities: |
||||||||||||
| Accounts receivable |
59 | 297 | 356 | |||||||||
| Accounts receivable from related parties |
(132 | ) | — | (132 | ) | |||||||
| Inventories |
(495 | ) | (197 | ) | (692 | ) | ||||||
| Prepaid expenses and other assets |
(558 | ) | — | (558 | ) | |||||||
| Deferred cost of product revenues |
— | (194 | ) | (194 | ) | |||||||
| Accounts payable |
138 | — | 138 | |||||||||
| Accrued and other liabilities |
(1,320 | ) | (7 | ) | (1,327 | ) | ||||||
| Deferred revenue |
(48 | ) | (7 | ) | (55 | ) | ||||||
| Deferred revenue from related parties |
(33 | ) | 249 | 216 | ||||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in operating activities |
(7,675 | ) | — | (7,675 | ) | |||||||
| Cash flows from investing activities |
||||||||||||
| Purchases of property, plant and equipment |
(432 | ) | — | (432 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in investing activities |
(432 | ) | — | (432 | ) | |||||||
| Cash flows from financing activities |
||||||||||||
| Repayment of debt |
(9,224 | ) | — | (9,224 | ) | |||||||
| Repayment of capital leases |
(25 | ) | — | (25 | ) | |||||||
| Change in restricted cash |
9,139 | — | 9,139 | |||||||||
| Proceeds from exercise of common stock warrants |
2 | — | 2 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in financing activities |
(108 | ) | — | (108 | ) | |||||||
| Net decrease in cash and cash equivalents |
(8,215 | ) | — | (8,215 | ) | |||||||
| Cash and cash equivalents, beginning of year |
10,006 | — | 10,006 | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash and cash equivalents, end of period |
$ | 1,791 | $ | — | $ | 1,791 | ||||||
|
|
|
|
|
|
|
|||||||
| Supplemental disclosure of cash flow information |
||||||||||||
| Cash paid for interest, net of capitalized interest of $113 for the three months ended March 31, 2013(1) |
$ | 518 | $ | (17 | ) | $ | 501 | |||||
|
|
|
|
|
|
|
|||||||
| Supplemental disclosure of non-cash investing and financing activities |
||||||||||||
| Equipment acquired under capital leases |
$ | 77 | $ | — | $ | 77 | ||||||
|
|
|
|
|
|
|
|||||||
| Interest added to the principal of convertible notes |
$ | 628 | $ | — | $ | 628 | ||||||
|
|
|
|
|
|
|
|||||||
| (1) | Capitalized interest amount represents the as reported and as restated amount as there were no adjustments to this balance. |
159
Table of Contents
| SIX MONTHS ENDED JUNE 30, 2013 (Unaudited) |
||||||||||||
| As reported | Adjustments | As restated | ||||||||||
| Cash flows from operating activities |
||||||||||||
| Net loss |
$ | (12,388 | ) | $ | (756 | ) | $ | (13,144 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||||
| Depreciation and amortization |
356 | — | 356 | |||||||||
| Share-based compensation |
588 | 10 | 598 | |||||||||
| Non-cash interest expense |
3,404 | — | 3,404 | |||||||||
| Change in estimated fair value of financial instruments |
(2,987 | ) | — | (2,987 | ) | |||||||
| Gain on extinguishment of debt |
(49 | ) | — | (49 | ) | |||||||
| Net changes in operating assets and liabilities: |
— | |||||||||||
| Accounts receivable |
(804 | ) | 243 | (561 | ) | |||||||
| Accounts receivable from related parties |
(131 | ) | — | (131 | ) | |||||||
| Inventories |
(2,056 | ) | (96 | ) | (2,152 | ) | ||||||
| Prepaid expenses and other assets |
(1,633 | ) | — | (1,633 | ) | |||||||
| Deferred cost of product revenues |
— | (2,102 | ) | (2,102 | ) | |||||||
| Accounts payable |
2,196 | — | 2,196 | |||||||||
| Accrued and other liabilities |
(743 | ) | (47 | ) | (790 | ) | ||||||
| Deferred revenue |
(96 | ) | 2,409 | 2,313 | ||||||||
| Deferred revenue from related parties |
(66 | ) | 339 | 273 | ||||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in operating activities |
(14,409 | ) | — | (14,409 | ) | |||||||
| Cash flows from investing activities |
||||||||||||
| Purchases of property, plant and equipment |
(1,338 | ) | — | (1,338 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in investing activities |
(1,338 | ) | — | (1,338 | ) | |||||||
| Cash flows from financing activities |
||||||||||||
| Proceeds from issuance of convertible notes payable |
6,529 | — | 6,529 | |||||||||
| Proceeds from issuance of debt, net of financing costs |
3,700 | — | 3,700 | |||||||||
| Repayment of debt |
(9,303 | ) | — | (9,303 | ) | |||||||
| Repayment of capital leases |
(98 | ) | — | (98 | ) | |||||||
| Change in restricted cash |
9,139 | — | 9,139 | |||||||||
| Proceeds from exercise of stock options |
11 | — | 11 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by financing activities |
9,978 | — | 9,978 | |||||||||
| Net decrease in cash and cash equivalents |
(5,769 | ) | — | (5,769 | ) | |||||||
| Cash and cash equivalents, beginning of year |
10,006 | — | 10,006 | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash and cash equivalents, end of period |
$ | 4,237 | $ | — | $ | 4,237 | ||||||
|
|
|
|
|
|
|
|||||||
| Supplemental disclosure of cash flow information |
||||||||||||
| Cash paid for interest, net of capitalized interest of $279 for the six months ended June 30, 2013(1) |
$ | 866 | $ | (100 | ) | $ | 766 | |||||
|
|
|
|
|
|
|
|||||||
| Supplemental disclosure of non-cash investing and financing activities |
||||||||||||
| Equipment acquired under capital leases |
$ | 256 | $ | — | $ | 256 | ||||||
|
|
|
|
|
|
|
|||||||
| Interest added to the principal of convertible notes |
$ | 1,299 | $ | — | $ | 1,299 | ||||||
|
|
|
|
|
|
|
|||||||
| (1) | Capitalized interest amount represents the as reported and as restated amount as there were no adjustments to this balance. |
160
Table of Contents
| NINE MONTHS ENDED SEPTEMBER 30, 2013 (Unaudited) |
||||||||||||
| As reported | Adjustments | As restated | ||||||||||
| Cash flows from operating activities |
||||||||||||
| Net loss |
$ | (18,498 | ) | $ | (723 | ) | $ | (19,221 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||||
| Depreciation and amortization |
594 | — | 594 | |||||||||
| Loss on disposal of equipment |
47 | — | 47 | |||||||||
| Share-based compensation |
1,125 | 44 | 1,169 | |||||||||
| Non-cash interest expense |
4,068 | — | 4,068 | |||||||||
| Change in estimated fair value of financial instruments |
(6,717 | ) | — | (6,717 | ) | |||||||
| Gain on extinguishment of debt |
(49 | ) | — | (49 | ) | |||||||
| Amortization of investment securities premiums/discounts, net |
4 | — | 4 | |||||||||
| Net changes in operating assets and liabilities: |
||||||||||||
| Accounts receivable |
920 | 500 | 1,420 | |||||||||
| Accounts receivable from related parties |
— | 136 | 136 | |||||||||
| Inventories |
(5,882 | ) | (258 | ) | (6,140 | ) | ||||||
| Prepaid expenses and other assets |
509 | 358 | 867 | |||||||||
| Deferred cost of product revenues |
— | (1,777 | ) | (1,777 | ) | |||||||
| Accounts payable |
3,021 | — | 3,021 | |||||||||
| Accrued and other liabilities |
(87 | ) | (84 | ) | (171 | ) | ||||||
| Deferred revenue |
527 | 1,674 | 2,201 | |||||||||
| Deferred revenue from related parties |
— | 93 | 93 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in operating activities |
(20,418 | ) | (37 | ) | (20,455 | ) | ||||||
| Cash flows from investing activities |
||||||||||||
| Purchases of property, plant and equipment |
(2,238 | ) | 37 | (2,201 | ) | |||||||
| Proceeds from sale of equipment |
16 | — | 16 | |||||||||
| Purchase of short-term investments |
(12,688 | ) | — | (12,688 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in investing activities |
(14,910 | ) | 37 | (14,873 | ) | |||||||
| Cash flows from financing activities |
||||||||||||
| Proceeds from public offerings, net of offering costs and underwriter commissions |
56,105 | — | 56,105 | |||||||||
| Proceeds from issuance of convertible notes payable |
6,529 | — | 6,529 | |||||||||
| Proceeds from issuance of debt, net of financing costs |
3,700 | — | 3,700 | |||||||||
| Repayment of debt |
(9,367 | ) | — | (9,367 | ) | |||||||
| Repayment of capital leases |
(162 | ) | — | (162 | ) | |||||||
| Proceeds from secured borrowing |
2,880 | — | 2,880 | |||||||||
| Reductions in secured borrowing |
(2,880 | ) | — | (2,880 | ) | |||||||
| Change in restricted cash |
9,139 | — | 9,139 | |||||||||
| Proceeds from exercise of stock options |
81 | — | 81 | |||||||||
| Proceeds from exercise of preferred stock warrants |
47 | — | 47 | |||||||||
| Proceeds from exercise of common stock warrants |
25 | — | 25 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by financing activities |
66,097 | — | 66,097 | |||||||||
| Net increase in cash and cash equivalents |
30,769 | — | 30,769 | |||||||||
| Cash and cash equivalents, beginning of year |
10,006 | — | 10,006 | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash and cash equivalents, end of period |
$ | 40,775 | $ | — | $ | 40,775 | ||||||
|
|
|
|
|
|
|
|||||||
161
Table of Contents
| NINE MONTHS ENDED SEPTEMBER 30, 2013 (Unaudited) |
||||||||||||
| As reported | Adjustments | As restated | ||||||||||
| Supplemental disclosure of cash flow information |
||||||||||||
| Cash paid for interest, net of capitalized interest of $412 for the nine months ended September 30, 2013(1) |
$ | 1,321 | $ | (97 | ) | $ | 1,224 | |||||
|
|
|
|
|
|
|
|||||||
| Supplemental disclosure of non-cash investing and financing activities |
||||||||||||
| Equipment acquired under capital leases |
$ | 617 | $ | — | $ | 617 | ||||||
|
|
|
|
|
|
|
|||||||
| Interest added to the principal of convertible notes |
$ | 1,623 | $ | — | $ | 1,623 | ||||||
|
|
|
|
|
|
|
|||||||
| Reclassification of warrants from liabilities to equity |
$ | 2,669 | $ | — | $ | 2,669 | ||||||
|
|
|
|
|
|
|
|||||||
| Conversion of convertible notes to common stock |
$ | 44,890 | $ | — | $ | 44,890 | ||||||
|
|
|
|
|
|
|
|||||||
| Conversion of preferred stock to common stock |
$ | 39,659 | $ | — | $ | 39,659 | ||||||
|
|
|
|
|
|
|
|||||||
| (1) | Capitalized interest amount was previously reported as $450. An adjustment of ($38) was recorded as a result of the restatement. |
22. Subsequent Events
Sale of Notes and Warrants
On August 20, 2015, the Company issued and sold to entities affiliated with Waddell & Reed Financial, Inc., one of its 5% stockholders, senior secured promissory notes in the aggregate principal amount of $40,000,000 and warrants to purchase up to 4,000,000 shares of common stock of the Company for aggregate consideration of $40,000,000, pursuant to a purchase agreement, dated August 20, 2015.
The notes will bear interest at a rate of 8% per annum payable semi-annually on June 30 or December 31 of each year, commencing on December 31, 2015, with $10,000,000 payable three years from the closing, $10,000,000 payable four years from the closing, and $20,000,000 payable five years from the closing. The notes contain customary covenants, in addition to the obligation to maintain cash and cash equivalents of at least $15,000,000. The notes provide for various events of default, including, among others, default in payment of principal or interest, breach of any representation or warranty by the Company or any subsidiary under any agreement or document delivered in connection with the notes, a continued breach of any other condition or obligation under any loan document, certain bankruptcy, liquidation, reorganization or change of control events, the acquisition by any person or persons acting as group, other than the lenders, of beneficial ownership of 40% or more of the outstanding voting stock of the Company and certain events in which Pamela Marrone, Ph.D. ceases to serve as the Company’s Chief Executive Officer. As of September 30, 2015, the Company was in breach of its covenants under the notes as the Company was in breach of its covenants under its October 2012 and April 2013 Secured Promissory Notes and June 2014 Secured Promissory Note. However, these breaches were cured in November 2015, as a result of the Company obtaining an extension to deliver its annual financial statements with respect to the October 2013 and April 2013 Secured Promissory Notes and the waiver of certain of the Company’s covenants with respect to the June 2014 Secured Promissory Note. See Note 9 for further discussion.
The notes are secured by substantially all the Company’s personal property assets. The agent, acting behalf of the lenders, shall be entitled to have a first priority lien on the Company’s intellectual property assets, pursuant to intercreditor arrangements with certain of the Company’s existing lenders.
The warrants are immediately exercisable at an exercise price of $1.91 per share (subject to adjustments) and may be exercised at a holder’s option at any time on or before August 20, 2023 (subject to certain exceptions). Proceeds from the sale of the notes and warrants will be allocated to the notes and warrants based on the relative fair value.
162
Table of Contents
In connection with the above transaction, on August 19, 2015, the Company amended the October 2012 and April 2013 Secured Promissory Notes. As a result of the amendment, interest on loans was accrued at a rate of 12% per annum until September 1, 2015, and thereafter is accrued at a rate of 18% per annum, and the Company is permitted to prepay at any time the outstanding indebtedness under the agreement without penalty. In addition, in September 2015, the Company provided written notice exercising its right to extend the maturity through October 2, 2017. The amendments will be accounted for as a modification of the loan agreement with the effective interest rate adjusted prospectively from the amendment date.
Separation Agreement
On January 14, 2015, James Iademarco was appointed as the Company’s President and Chief Operating Officer, effective January 14, 2015. On August 20, 2015, the Company entered into a separation agreement with James Iademarco, the Company’s President and Chief Operating Officer, whereby he resigned effective August 31, 2015, but agreed to remain available to advise the Company in a consulting capacity for an additional period of up to 90 days to assist with the transition of various pending matters. Pursuant to the separation agreement, Mr. Iademarco is entitled to receive, among other things, an amount equal to one-twelfth of his prior base salary of $290,000 on or before the 15th day of each of the twelve months following August 31, 2015 and certain premium payments for health and vision insurance coverage, in partial consideration for Mr. Iademarco granting the Company a general release of liability and claims.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
Not applicable.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
Disclosure controls and procedures are controls and other procedures that are designed to ensure that information required to be disclosed in our reports filed or submitted under the Securities Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the Securities and Exchange Commission’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed in our reports filed under the Exchange Act is accumulated and communicated to management, including our principal executive officer and our principal financial officer, as appropriate, to allow timely decisions regarding required disclosure. As of the end of the period covered by this report, the Company carried out an evaluation under the supervision and with the participation of its management, including the Company’s Chief Executive Officer (CEO) and its Chief Financial Officer (CFO), of the effectiveness of the design and operation of the Company’s disclosure controls and procedures in ensuring that material information required to be disclosed in the Company’s reports filed or submitted under the Exchange Act, has been made known to them in a timely fashion. Based on this evaluation, the CEO and CFO concluded that the Company’s disclosure controls and procedures were not effective as of December 31, 2014 due to material weaknesses in our internal control over financial reporting, which are disclosed below.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as such term is defined in Rule 13a-15(f) of the Exchange Act. Our management assessed, with the oversight of the board of directors, the effectiveness of our internal control over financial reporting as of December 31, 2014. In making this assessment, management used the criteria established in Internal Control-Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway
163
Table of Contents
Commission (“COSO”). Based on this assessment, management has concluded that our internal control over financial reporting was not effective as of December 31, 2014 due to material weaknesses in our internal control over financial reporting, which are disclosed below.
Material Weaknesses and Remediation Activities
A “material weakness” is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the Company’s annual or interim financial statements will not be prevented or detected on a timely basis. In connection with management’s assessment of our internal control over financial reporting described above, management has identified the following deficiencies that constituted individually, or in the aggregate, material weaknesses in our internal control over financial reporting as of December 31, 2014:
Control Environment - The control environment, which includes the Company’s Code of Conduct, is the responsibility of senior management, sets the tone of our organization, influences the control consciousness of employees, and is the foundation for the other components of internal control over financial reporting. The Audit Committee determined, based on the results of its independent investigation, that relevant information related to historical sales transactions, to which certain sales personnel were aware of, was consistently not shared with the finance department or the Company’s external auditors, certain sales personnel executed inaccurate representation letters, and certain sales personnel mischaracterized expense reports to pay for storage or freight charges associated with certain sales transactions. As a result of these findings, we determined that certain former sales personnel did not project an attitude of integrity and control consciousness, leading to insufficient attention to their responsibilities and internal controls. Further, effective mitigating controls were not in place to discourage, prevent or detect management override of internal control by certain sales personnel related to the Company’s process for recognizing revenue.
Revenue Recognition – The Company’s internal controls were not effectively designed to identify instances when sales personnel made unauthorized commitments with certain distributors, including “inventory protection” arrangements that would permit the distributors to return to the Company certain unsold products. In addition, controls were not in place to identify instances of management override of internal controls by sales personnel related to the recognition of sales to the Company’s distributors. Consequently, revenue for certain transactions was recognized prior to satisfaction of all required revenue recognition criteria.
As a result of the material weaknesses related to the control environment and revenue recognition as described above, the Company did not identify that information provided to the finance department by certain sales personnel was not complete and accurate, which would be necessary to enable the Company to correctly assess the timing of revenue recognition. These material weaknesses also prevented the identification of instances of management override of internal control over financial reporting by certain sales personnel. As a result, the Company’s controls were not effective to prevent or detect a material misstatement of recognized revenue.
We have developed and implemented a plan to remediate these material weaknesses, which includes, among other things:
| • | We have enhanced our Code of Conduct and associated training by supplementing materials with examples of improper conduct and resulting implications to individuals as well as the Company. This training will be ongoing. |
| • | We have enhanced our whistleblower policy and associated training to ensure employees have the appropriate awareness of its purpose, how to access the whistleblower hotline, and the Company’s anti-retaliation policy. This training will be ongoing. |
| • | We have enhanced our training provided to individuals in the sales organization, including those involved with executing sales transactions, on the Company’s revenue recognition policy, including |
164
Table of Contents
| illustrative examples of terms and conditions that that can have an impact on the timing of revenue recognition and the need for timely communication with the finance department. This training will be ongoing. |
| • | We have expanded our formal internal certification process to additional individuals in the Company, including the supply chain organization, and incorporated a more comprehensive questionnaire of circumstances, including illustrative examples, which require timely communication with the finance department. |
| • | We have identified customers where facts and circumstances exist that revenue recognition criteria are not satisfied at the time of title transfer and have implemented processes to determine customers’ channel inventory and have implemented internal controls related to our accounting for revenue transactions accounted for under a sell-through basis where applicable, including the performance of physical inventory observations at distributor locations and review and reconciliation of sales and inventory information provided by these customers. |
| • | The personnel identified as responsible for accounting improprieties are no longer employed by the Company. |
With respect to the control environment and revenue recognition weaknesses, while we have implemented a plan for remediation, we are still in the process of testing and evaluating the effectiveness of the remediation measures we have taken to date. In addition, many of the remediation efforts involve continued training and communication of the enhanced policies and procedures.
Also in connection with management’s assessment of our internal control over financial reporting, management has identified the following additional deficiency that constituted a material weakness in our internal control over financial reporting as of December 31, 2014:
Stock Option Grants – We determined that a deficiency exists in our governance practices related to ineffective controls over the timeliness and accuracy of documentation related to actions of our board of directors (board) and compensation committee specific to approving stock option grants. While no financial statement accounts or disclosures were misstated in connection with our stock option grants, the potential impact of not accurately documenting board and committee approvals could have led to a material misstatement. We are developing a plan to conduct training with our legal department and those charged with governance to ensure that board and compensation committee minutes are prepared more timely and accurately and reviewed with sufficient rigor to ensure that the minutes of actions/approvals by the board and compensation committee fully and accurately reflect the actions related to stock option grants.
As we continue to evaluate our internal control over financial reporting, we may determine that additional measures should be taken to address these or other control deficiencies, and/or that we should modify the remediation plan described above. Notwithstanding the identified material weaknessses in our internal control over financial reporting, we have concluded that the financial statements and other financial information included in this Annual Report on Form 10-K fairly present in all material respects our financial condition, results of operations and cash flows as of, and for, the periods presented.
Changes in Internal Control
Other than the remediation actions taken and the controls implemented for sell-through accounting described in this Item 9A, there were no changes in our internal control over financial reporting identified in management’s evaluation pursuant to Rules 13a-15(d) or 15d-15(d) of the Exchange Act during the period covered by this Annual Report on Form 10-K that materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
165
Table of Contents
Limitations on Effectiveness of Controls and Procedures
In designing and evaluating the disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable, not absolute, assurance of achieving the desired control objectives. Because of the inherent limitations in internal control over financial reporting, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within the Company have been detected. These inherent limitations include the realities that judgments in decision making can be faulty and that breakdowns can occur because of a simple error or mistake. Controls can also be circumvented by the individual acts of some persons, by collusion of two or more people, or by management override of the controls. The design of any system of controls is based in part on certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Over time, controls may become inadequate because of changes in conditions or deterioration in the degree of compliance with policies or procedures. In addition, the design of disclosure controls and procedures must reflect the fact that there are resource constraints and that management is required to apply judgment in evaluating the benefits of possible controls and procedures relative to their costs. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected.
None.
166
Table of Contents
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Executive Officers and Directors
The following table sets forth certain information about our executive officers, directors and key employees as of September 30, 2015:
| NAME |
AGE |
POSITION | ||
| Board of Directors: |
||||
| Pamela G. Marrone, Ph.D. |
58 | President, Chief Executive Officer and Director | ||
| Elin Miller (3) |
55 | Chair of the Board | ||
| George Kerckhove ,(2) |
78 | Director | ||
| Pamela Contag, Ph.D. (1) |
58 | Director | ||
| Timothy Fogarty (1) (2) |
54 | Director | ||
| Les Lyman (3) (4) |
68 | Director | ||
| Richard Rominger (1),(3) |
88 | Director | ||
| Shaugn Stanley (2),(3) |
56 | Director | ||
| Other Executive Officers: |
||||
| James B. Boyd |
62 | Vice President, Chief Financial Officer and Assistant Secretary | ||
| Linda V. Moore |
69 | Vice President, General Counsel, Secretary, and Chief Compliance Officer | ||
| Brian R. Ahrens |
44 | Vice President of Sales | ||
| Keith J. Pitts |
52 | Vice President of Regulatory and Government Affairs |
| (1) | Member of the Compensation Committee. |
| (2) | Member of the Audit Committee. |
| (3) | Member of the Nominating and Corporate Governance Committee. |
| (4) | In accordance with NASDAQ listing standards, Mr. Lyman will resign from his position on the nominating and corporate governance committee effective as of the date of the 2015 annual meeting. |
Board of Directors
Pamela G. Marrone, Ph.D. is our founder and has served as our Chief Executive Officer and has been a member of our board of directors since our inception in 2006 and as our President during that period except from January 2015 through August 2015. Prior to founding the Company, in 1995 Dr. Marrone founded AgraQuest, Inc. (acquired by Bayer), where she served as chief executive officer until May 2004 and as president or chairman from such time until March 2006, and where she led teams that discovered and commercialized several bio-based pest management products. She served as founding president and business unit head for Entotech, Inc., a biopesticide subsidiary of Denmark-based Novo Nordisk A/S (acquired by Abbott Laboratories), from 1990 to 1995, and held various positions at the Monsanto Company from 1983 until 1990, where she led the Insect Biology Group, which was involved in pioneering projects in transgenic crops, natural products and microbial pesticides. Dr. Marrone is an author of over a dozen invited publications, is in demand as a speaker and has served on the boards and advisory councils of numerous professional and academic organizations. In 2013, Dr. Marrone was named the Sacramento region’s “Executive of the Year” by the Sacramento Business Journal and “Cleantech Innovator of the Year” by the Sacramento Area Regional Technology Alliance and Best Manager with Strategic Vision by Agrow in 2014. Dr. Marrone earned a B.S. in Entomology from Cornell University and a Ph.D. in Entomology from North Carolina State University. We believe Dr. Marrone’s qualifications to sit on our board of directors include the fact that, as our founder, Dr. Marrone is uniquely familiar with the business, structure, culture and history of our company and that she also brings to the board of directors considerable expertise based on her management and technical and commercialization experience in the biopesticide industry.
167
Table of Contents
Elin D. Miller has served on our board of directors since 2011 and was appointed the Chair of our board in 2013. Ms. Miller has been the Principal of Elin Miller Consulting, LLC since 2009 and also currently serves on the board of directors of Vestaron Corporation, a venture-backed agricultural biotechnology firm, is chair of National FFA Foundation, NeighborWorks Umpqua and Umpqua Community College Board of Trustees and is a board member of the regional board of Umpqua Bank. Appointed by the President of the United States, Ms. Miller assumed regional management of the U.S. Environmental Protection Agency (EPA) in the Pacific Northwest from 2006 to 2009. Prior to serving at the EPA, Ms. Miller led Arysta Lifescience Corporation as president and chief executive officer of North America and Australasia from 2004 to 2006. Ms. Miller also served in various positions at Dow Agroscienses/Dow Chemical from 1996 to 2004, including Vice President of Pest Management, Vice President of Asia Pacific, and Global Vice President of Public Affairs. Ms. Miller’s career also includes serving as director of the California Department of Conservation and serving as chief deputy director of the Department of Pesticide Regulation at the California Environmental Protection Agency. Ms. Miller earned a B.S. in Agronomy and Plant Protection from the University of Arizona and is a graduate of INSEAD’s Advanced Management Program. We believe Ms. Miller’s qualifications to sit on our board of directors include her years of regulatory experience and her perspective gained in management of companies in the life sciences, pesticide and agricultural industries.
Pamela Contag, Ph.D. has served on our board of directors since October 2013. Dr. Contag co-founded ConcentRx, a cell-based immune therapy company, where she has served as chief executive officer since 2012. Dr. Contag has served as the chief executive officer of Cygnet Bio Inc., a private company active in the discovery and adaptation of natural products to applications in healthcare, energy, and food, since its founding in 2009. Dr. Contag founded Cobalt Technologies in 2005, where she served as chief executive officer until 2008, and co-founded Xenogen in 1995, where she served until 2006, serially, as chief executive officer, co-chief executive officer and president, during which time the company completed an initial public offering, listing on NASDAQ. Dr. Contag has been featured as one of the top 25 women in small business by Fortune magazine, and in 2011, she was honored with Astia’s “Cleantech Innovator of the Year” award for her contributions at Cygnet. Dr. Contag has held board positions in the public, private, and not-for-profit sectors, including chairman of the board of trustees of the Molecular Sciences Institute since 2013, where she has served as a trustee since 2011, and board positions at Delcath Systems, Inc. and StartUp America, to which she was appointed by the White House. Dr. Contag also consults in biotechnology for academics and industry and has served as a consulting professor at Stanford University School of Medicine. She is widely published in the field of microbiology and optical imaging, and has over 35 patents in biotechnology. Dr. Contag received her Ph.D. in Microbiology and Immunology at the University of Minnesota Medical School and completed postdoctoral training at Stanford University School of Medicine, specializing in Host-Pathogen Interactions. We believe Dr. Contag’s qualifications to sit on our board of directors include her experience as a biotechnology entrepreneur, her experience as a president in taking a company public and her understanding of new technology.
Timothy Fogarty has served on our board of directors since 2010. As the chief financial officer and a partner of The Contrarian Group, Inc., a private equity fund where he has worked since May 2006, Mr. Fogarty previously served on the boards of TeachTown, Amanzi and Bellwether Marine Acquisition Corporation. From December 2003 to March 2006, Mr. Fogarty worked for Cypress Reinsurance, a startup Bermuda reinsurer, as president and chief operating officer. Mr. Fogarty is a Certified Public Accountant in good standing in California and earned a B.S. in Accounting from California State Polytechnic University, Pomona. We believe Mr. Fogarty’s qualifications to sit on our board of directors include his extensive experience in investment management and accounting and his perspective gained as a board member of various early-stage companies.
George H. Kerckhove joined our board of directors in July 2014. He has served on the board of directors for Gundersen Medical Foundation since 2010 and previously served on the board of directors for Merix Corporation, where he chaired the audit committee, Wellspring International, American Standard Companies and the Mississippi Valley Conservancy Land Trust. He worked with the American Standard Companies from 1988 through 2000, where he served as VP and chief financial officer, executive VP and global sector manager of
168
Table of Contents
various countries and president and general manager of the European Division. Prior to that, he served in a variety of positions from 1962 through 1987 with The Trane Company, from product manager in several product departments, VP and general manager, Process Equipment Division, and executive VP and general manager of both the US and International Commercial Equipment Divisions. Mr. Kerckhove received Bachelor of Science degrees in Agricultural Engineering and Mechanical Engineering, a Master of Science Degree in Mechanical Engineering, and an MBA, all from the University of Wisconsin in Madison. We believe Mr. Kerckhove’s qualifications to sit on our board include his education in agricultural engineering and his extensive experience in finance, accounting and management in global publically-traded companies.
Les Lyman has served on our board of directors since October 2013. Mr. Lyman has served as the Chairman of each of The Lyman Group, Inc. and The Tremont Group, Inc., independent agricultural retail companies with 15 locations in northern California, since 2008. Under his leadership, the organizations have grown to become among the largest independent agricultural retailers in the nation. The organizations were honored with the Agricultural Retailers Associations’ 2011 Retailer of the Year Award and were awarded the Environmental Respect Award in 1996 and 2010 for excellence in environmental stewardship. Mr. Lyman has also directed the founding of Blue Creek Sustainable LLC, MVP Consolidated, FS3, Inland Terminal, and Mar Vista Resources. He previously served as chairman of the Board of Integrated Agribusiness Professionals, and as a member of the board of Western Agricultural Chemicals Association, California Fertilizer Association, and the Agricultural Retailers Association. Mr. Lyman holds a degree in Agricultural Business Management from California Polytechnic State University, San Luis Obispo. We believe Mr. Lyman’s qualifications to sit on our board of directors include his experience with acquisitions, his extensive experience in building and leading agricultural retail businesses and his overall understanding of the agricultural market, competitors in the market and growers’ needs.
Richard Rominger has served on our board since our inception in 2006 and was Chair of our board from 2008 to 2013. Mr. Rominger is a fourth generation Yolo County, California farmer and is active in farm organizations and cooperatives. Mr. Rominger served as Director (Secretary) of the California Department of Food and Agriculture from 1977 to 1982 and was the Deputy Secretary at the U. S. Department of Agriculture in Washington, DC from 1993 to 2001. Mr. Rominger has served as a production agriculture advisor at University of California, Davis, University of California, Riverside, California State University, Fresno and California Polytechnic State University, San Luis Obispo and has served on the advisory committee of the Agricultural Sustainability Institute at University of California, Davis and as a special advisor to the Chancellor at University of California, Davis. He is a member of the University of California President’s Advisory Commission on Agriculture and Natural Resources and the California Roundtable on Agriculture and the Environment and serves on the board of directors of Oryzatech, Inc., a plant based building material company. Mr. Rominger earned a B.S. in Plant Science from University of California, Davis and graduated summa cum laude. We believe Mr. Rominger’s qualifications to sit on our board of directors include his years of government experience and his perspective gained as a leader in keeping American agriculture healthy and sustainable.
Shaugn Stanley has served on our board of directors since 2012. Mr. Stanley currently serves as senior managing director at Stifel Financial Corp., which in 2010 purchased Thomas Weisel Partners, an investment firm that Mr. Stanley co-founded in 1998 and at which Mr. Stanley served in a number of senior positions, including chief financial officer, chief administrative officer and director of private client services. Prior to that, from 1997 to 1998, Mr. Stanley served as chief financial officer for Montgomery Securities and in various executive financial roles at Fidelity Investments Brokerage Group from 1991 to 1997. Mr. Stanley earned a B.B.A. in Accounting from Stephen F. Austin State University and is a Certified Public Accountant. We believe Mr. Stanley’s qualifications to sit on our board of directors include his extensive experience in financial services and his expertise and experience in corporate accounting and financial reporting processes.
Executive Officers
James B. Boyd was appointed as our Vice President and Chief Financial Officer effective February 2014, and Assistant Secretary effective March 2014. Mr. Boyd previously served as chief financial officer of Quantenna
169
Table of Contents
Communications and Link-A-Media Devices, both venture capital backed companies, from 2012 to 2013 and from 2010 to 2012, respectively. From 2007 to 2010, he served as chief financial officer and senior vice president of Silicon Storage Technology and from 2000 to 2007, Mr. Boyd served as chief financial officer and senior vice president of ESS Technology, both NASDAQ listed companies. Mr. Boyd earned an M.B.A. in Finance from the University of Wisconsin and a J.D. from Golden Gate University.
Linda V. Moore was appointed as Vice President, General Counsel, Secretary and Chief Compliance Officer in March 2014. Ms. Moore co-founded The Moore Group, where she served as principal from 2005 to 2007, during which time she also served as chief operating officer and general counsel of Mobius Photonics, and 2009 to 2014. From 2007 to 2009, Ms. Moore served as executive vice president, general counsel, chief compliance officer and secretary of Merix Corporation. Ms. Moore has served as an Executive Mentor to Astia (formerly Women’s Technology Cluster) and as a member of the Advisory Board for Remedy Interactive and Opportunity Works. She has also taught at the University of Detroit Mercy and Santa Clara University as an adjunct professor. Ms. Moore earned a J.D. at Michigan State University School of Law.
Brian Ahrens was appointed as Vice President of Sales in 2014. Previously, from 2005 to 2014 Mr. Ahrens served in various positions, including strategic business leader and innovation leader, at ADAMA (previously MANA), a crop protection solutions company. From 2003 to 2005, he served in various positions, including global strategic marketing manager, at BASF, a leading chemical producer. Mr. Ahrens earned a B.A. in Agriculture Business from Iowa State University.
Keith J. Pitts has served as our Vice President of Regulatory and Government Affairs since July 2008. Previously, from January 2001 to June 2007, Mr. Pitts served as Director of Public Policy at the Pew Initiative on Food and Biotechnology, a non-partisan research and policy organization based in Washington, D.C. From 1986 to 2001, Mr. Pitts worked in senior legislative, administrative, regulatory and public policy roles in both the U.S. Department of Agriculture and the House Committee on Agriculture. Mr. Pitts earned a B.A. in Chemistry from the University of North Carolina.
Board of Directors
Our board of directors currently consists of eight members.
In accordance with our amended and restated certificate of incorporation and amended and restated bylaws, our board of directors has been divided into three classes with staggered three-year terms. At each annual general meeting of stockholders, the successors to directors whose terms then expire will be elected to serve from the time of election and qualification until the third annual meeting following election. Our directors have been divided among the three classes as follows:
| • | The Class I directors are Pamela G. Marrone, Ph.D. and Les Lyman and their terms will expire at the annual general meeting of stockholders to be held in 2017; |
| • | The Class II directors are Timothy Fogarty, Richard Rominger and Shaugn Stanley and their terms will expire at the next annual general meeting of stockholders to be held in 2015; and |
| • | The Class III directors are Pamela Contag, Ph.D., Elin Miller and George H. Kerckhove and their terms will expire at the annual general meeting of stockholders to be held in 2016. |
The board currently separates the role of Chairman and Chief Executive Officer, with Dr. Marrone serving as Chief Executive Officer and Ms. Miller serving as Chairman. The board believes that separating these two roles promotes balance between the board’s independent authority to oversee our business and the Chief Executive Officer and our management team, which manages the business on a day-to-day basis. The current separation of the Chairman and Chief Executive Officer roles allows the Chief Executive Officer to focus her time and energies on operating and managing the Company and leverages the experience and perspectives of the Chairman.
170
Table of Contents
We believe the board maintains effective independent oversight through a number of governance practices, including our strong committee system, open and direct communication with management, input on meeting agendas, and regular executive sessions.
In addition, the board has established the following procedures for selecting the presiding director during the executive sessions of the board. The presiding director will be (i) the Chairman of the board or (ii) another director appointed by the independent directors. In fiscal year 2013, Ms. Miller, our Chairman, presided at each of the executive sessions of our board.
Director Independence
The rules of NASDAQ generally require that a majority of the members of a listed company’s board of directors be independent. In addition, the listing rules generally require that, subject to specified exceptions, each member of a listed company’s audit, compensation, and governance committees be independent. Audit committee members must also satisfy the independence criteria set forth in Rule 10A-3 under the Securities Exchange Act of 1934, as amended (Exchange Act) and compensation committee members must also satisfy the independence criteria set forth in Rule 10-C-1 under the Exchange Act. In order to be considered independent for purposes of Rule 10A-3 and Rule 10C-1, a committee member may not, other than in his or her capacity as a member of the board of directors, or any board committee: accept, directly or indirectly, any consulting, advisory, or other compensatory fee from the listed company or any of its subsidiaries; or be an affiliated person of the listed company or any of its subsidiaries.
Our board of directors has undertaken a review of its composition, the composition of its committees and the independence of each director and considered whether any director has a material relationship with us that could compromise his or her ability to exercise independent judgment in carrying out his or her responsibilities. Our board of directors has also reviewed whether the directors that comprise our audit committee and compensation committee satisfy the independence standards for those committees established by the applicable SEC rules and NASDAQ rules. In making this determination, our board of directors has considered the relationships that each of these non-employee directors has with our company and all other facts and circumstances our board of directors deem relevant in determining their independence, including the beneficial ownership of our capital stock held by each non-employee director. Based on this determination, the board of directors determined that each of its non-employee members was independent except for Les Lyman.
Board Committees
In fiscal year 2014, our board of directors had three standing committees: an audit committee, a compensation committee and a nominating and corporate governance committee. The composition and responsibilities of each of our committees are below.
Audit Committee
Our audit committee is comprised of Mr. Kerckhove, Mr. Fogarty, and Mr. Stanley, each of whom is a non-employee member of our board of directors. Mr. Stanley is our audit committee chair and is our audit committee financial expert, as currently defined under the SEC rules. Our board of directors has determined that each of Mr. Kerckhove, Mr. Fogarty and Mr. Stanley is independent within the meaning of the applicable SEC rules and the listing standards of NASDAQ.
Our audit committee oversees our corporate accounting and financial reporting process. Among other matters, the audit committee evaluates the independent registered public accounting firm’s qualifications, independence and performance; determines the engagement of the independent registered public accounting firm; reviews and approves the scope of the annual audit and the audit fee; discusses with management and the independent registered public accounting firm the results of the annual audit and the review of our quarterly consolidated financial statements; approves the retention of the independent registered public accounting firm to perform any proposed permissible non-audit services; monitors the rotation of partners of the independent registered public accounting
171
Table of Contents
firm on our engagement team as required by law; reviews our critical accounting policies and estimates; and annually reviews the audit committee charter and the committee’s performance. The audit committee operates under a written charter adopted by the board that satisfies the applicable standards of NASDAQ.
Compensation Committee
Our compensation committee is comprised of Dr. Contag, Mr. Fogarty and Mr. Rominger, each of whom is a non-employee member of our board of directors. Dr. Contag is our compensation committee chair. Our board of directors has determined that each of Dr. Contag, Mr. Fogarty and Mr. Rominger is independent within the meaning of the applicable SEC rules and the listing standards of NASDAQ.
Our compensation committee reviews and recommends programs, arrangements and policies relating to the compensation and benefits of our officers and employees. The compensation committee reviews and approves corporate goals and objectives relevant to the compensation of our chief executive officer and other executive officers, evaluates the performance of these officers in light of those goals and objectives and sets the compensation of these officers based on such evaluations. The compensation committee administers the issuance of stock options and other awards under our stock plans. The compensation committee reviews and evaluates, at least annually, the performance of the compensation committee and its members. The compensation committee operates under a written charter adopted by the board that satisfies the applicable standards of NASDAQ. The compensation committee may form and delegate authority under its charter to subcommittees or other persons when appropriate.
Nominating and Corporate Governance Committee
Our nominating and corporate governance committee is comprised of Mr. Lyman, Ms. Miller, Mr. Rominger and Mr. Stanley, each of whom is a non-employee member of our board of directors. Ms. Miller is our nominating and corporate governance committee chair. Our board of directors has determined that each of Ms. Miller, Mr. Rominger and Mr. Stanley is independent within the meaning of the applicable SEC rules and the listing standards of NASDAQ. The board determined that Mr. Lyman should serve on the nominating and corporate governance committee for up to two years as permitted under NASDAQ listing standards due to exceptional circumstances. In accordance with such two year limitation, Mr. Lyman will resign from his position on the nominating and corporate governance committee effective as of the date of the 2015 annual meeting.
Our nominating and corporate governance committee is responsible for making recommendations regarding candidates for directorships and the size and the composition of our board of directors. Candidates for directorships are identified and considered on the basis of experience, areas of expertise and other factors relative to the overall composition of our board of directors. The nominating and corporate governance committees will consider candidates for directorship recommended by stockholders that are submitted in compliance with its charter. In addition to making recommendations for director candidates, the nominating and corporate governance committee is responsible for overseeing our corporate governance principles and making recommendations concerning governance matters. The nominating and corporate governance committee operates under a written charter adopted by the board that satisfies the applicable standards of NASDAQ.
Compensation Committee Interlocks and Insider Participation
None of our executive officers currently serve, or in the past year have served, as a member of the board of directors or compensation committee of any other entity that has one or more executive officers serving on our board of directors.
Director Compensation
Directors who are employees of ours do not receive any compensation for their service on our board of directors. The following compensation policy has historically been applicable to all of our non-employee directors:
| • | Initial Equity Grants. Each non-employee director who joins the board will receive an option to purchase 16,000 shares of our common stock. |
172
Table of Contents
| • | Annual Retainers. Each non-employee director will receive an annual retainer for service on the board valued at $50,000, consisting, at each director’s option, of up to $25,000 in cash and the remainder in options, in addition to annual retainers for service as chair of our board of directors, or committees of our board of directors, valued as follows, consisting in each case, at each director’s option, of up to 50% in cash and the remainder in options. Each director who is an affiliate of an investor holding more than 5% of our outstanding shares of common stock will receive the entire value of their eligible retainers in options. |
| Annual retainer fee for services on the board of directors |
$ | 50,000 | ||
| Additional annual retainer fees for service as chair of: |
||||
| Board of Directors |
$ | 15,000 | ||
| Audit Committee |
$ | 10,000 | ||
| Compensation Committee |
$ | 7,500 | ||
| Nominating and Corporate Governance Committee |
$ | 7,500 |
We seek to maintain our ability to attract well qualified directors. In October 2014, our board of directors first engaged Pearl Meyer & Partners, LLC (“Pearl Meyer”) to prepare a competitive assessment of non-employee director compensation. Pearl Meyer’s report noted that while we compared approximately to the 35th percentile of peers in our industry, director compensation was below competitive levels and suggested that we consider phasing in changes to the mix and levels of cash and stock-based director compensation, including increases to committee compensation. In light of the matters related to the Audit Committee investigation, we postponed any changes to director compensation, and in August 2015, Pearl Meyer delivered an updated report to the board of directors regarding market practices, recommending changes, based on our lower financial and size metrics, that would bring our director compensation more comparable to the 25th percentile of peers in our industry. In November 2015, in consideration of the Pearl Meyer report and our financial situation, our board of directors adopted the following update to our compensation policy applicable to all of our non-employee directors:
| • | Initial Equity Grants. Each non-employee director who joins the board will receive restricted stock units valued at $40,000, with one-third of the restricted stock units vesting on the first anniversary of the director’s service and the remainder vesting monthly thereafter. |
| • | Annual Retainers. Each non-employee director will receive an annual retainer for service on the board valued at $50,000, consisting of $25,000 in cash and the remainder in restricted stock units, in addition to annual cash retainers for service as chair of our board of directors, or as a member or chair of committees of our board of directors, as set forth in the table below. Cash retainers will be paid on a quarterly basis, with restricted stock units awarded at our annual stockholders meeting and vesting after one year. |
| Additional annual retainer fees for service as a Chair of the Board |
$ | 20,000 | ||||||
| Additional annual retainer fees for service as a member or chair of (with chair fees inclusive of fees for service as a member): |
Member | Chair | ||||||
| Audit Committee |
$ | 7,500 | $ | 15,000 | ||||
| Compensation Committee |
$ | 5,000 | $ | 10,000 | ||||
| Nominating and Corporate Governance Committee |
$ | 3,750 | $ | 7,500 | ||||
This updated policy will be effective as of our 2015 annual meeting of stockholders, with modified retroactive application in consideration of the seven months of additional service by our directors beyond their expected terms as a result of the delay in the anticipated timing of our 2015 annual meeting of stockholders. Accordingly, on the date of our 2015 annual meeting, each of our non-employee directors will receive restricted stock units valued at $25,000, 7/12ths of which will be vested immediately and the remainder vesting monthly thereafter, and we will make a payment to each of our non-employee directors equal to 7/12ths of the cash annual retainer fees to which he or she would be entitled under the updated policy for such director’s current positions on our board of directors and the committees thereof.
173
Table of Contents
In addition, our board of directors from time to time may consider additional payments to our directors in respect of extraordinary service by such director. For example, in 2015, our board of directors approved of payments of $25,000 to George Kerckhove and $10,000 to each of Elin Miller and Pamela Contag, Ph.D., each of whom provided, on behalf of the board of directors, input and guidance to our leadership team and executive officers regarding business operations, and $15,000 to Shaugn Stanley and $12,500 to Timothy Fogarty in respect of their time and efforts relating to the Audit Committee investigation.
Director Compensation Table
Our non-employee directors who served during the fiscal year ended December 31, 2014 received the following compensation for their service on our board of directors:
| NAME |
FEES EARNED OR PAID IN CASH ($) |
OPTION AWARDS ($) (1),(2) |
TOTAL ($) |
|||||||||
| Elin Miller |
18,125 | 54,371 | (3) | 72,496 | ||||||||
| Pamela Contag, Ph.D. |
28,750 | 28,749 | (4) | 57,499 | ||||||||
| Timothy Fogarty |
— | 49,996 | (5) | 49,996 | ||||||||
| Les Lyman |
25,000 | 25,000 | (6) | 50,000 | ||||||||
| Richard Rominger |
— | 49,996 | (7) | 49,996 | ||||||||
| Shaugn Stanley |
30,000 | 29,997 | (8) | 59,997 | ||||||||
| George Kerckhove |
25,000 | 114,548 | (9) | 139,548 | ||||||||
| (1) | This column reflects the aggregate grant date fair value of option awards granted to our directors estimated pursuant to FASB ASC 718, Compensation – Share based compensation (ASC 718). Valuation assumptions are described under Note 2 of our accompanying Notes to Consolidated Financial Statements included in Part II-Item 8-“Financial Statements and Supplementary Data” in this Annual Report on Form 10-K. |
| (2) | The following table sets forth the aggregate number of option awards held by each non-employee director as of December 31, 2014: |
| NAME |
AGGREGATE NUMBER OF OPTION AWARDS |
|||
| Elin Miller |
32,071 | |||
| Pamela Contag, Ph.D. |
24,124 | |||
| Timothy Fogarty |
17,335 | |||
| Les Lyman |
23,388 | |||
| Richard Rominger |
33,357 | |||
| Shaugn Stanley |
26,346 | |||
| George Kerckhove |
20,866 | |||
| (3) | On May 29, 2014, we granted Ms. Miller an option to purchase 10,674 shares of our common stock with a per share exercise price of $10.90. All of the shares subject to the option will fully vest upon the date of the 2015 annual meeting of the stockholders. |
| (4) | On May 29, 2014, we granted Dr. Contag an option to purchase 5,644 shares of our common stock with a per share exercise price of $10.90. All of the shares subject to the option will fully vest upon the date of the 2015 annual meeting of the stockholders. |
| (5) | On May 29, 2014, we granted Mr. Fogarty an option to purchase 9,815 shares of our common stock with a per share exercise price of $10.90. All of the shares subject to the option will fully vest upon the date of the 2015 annual meeting of the stockholders. |
| (6) | On May 29, 2014, we granted Mr. Lyman an option to purchase 4,908 shares of our common stock with a per share exercise price of $10.90. All of the shares subject to the option will fully vest upon the date of the 2015 annual meeting of the stockholders. |
174
Table of Contents
| (7) | On May 29, 2014, we granted Mr. Rominger an option to purchase 9,815 shares of our common stock with a per share exercise price of $10.90. All of the shares subject to the option will fully vest upon the date of the 2015 annual meeting of the stockholders. |
| (8) | On May 29, 2014, we granted Mr. Stanley an option to purchase 5,889 shares of our common stock with a per share exercise price of $10.90. All of the shares subject to the option will fully vest upon the date of the 2015 annual meeting of the stockholders. |
| (9) | On July 1, 2014, we granted Mr. Kerckhove an option to purchase 16,000 shares of our common stock with a per share exercise prices of $11.25 upon joining the board. One-third of the total shares subject to his option vest on the date of each of the 2015, 2016 and 2017 annual meetings of the stockholders, such that all of the shares subject to the option will be fully vested upon the date of the 2017 annual meeting of the stockholders. On July 1, 2014, we also granted Mr. Kerckhove an option to purchase 4,866 shares of our common stock with a per share exercise price of $11.25. All of the shares subject to the option will fully vest upon the date of the 2015 annual meeting of the stockholders. |
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act requires our directors and executive officers, and persons who own more than ten percent of a registered class of our equity securities, to file with the SEC initial reports of ownership and reports of changes in ownership of common stock and other equity securities of MBI. Officers, directors and greater than ten percent stockholders are required by SEC regulation to furnish us with copies of all Section 16(a) forms they file.
To our knowledge, based solely on a review of the copies of such reports furnished to us and written representations that no other reports were required, during the fiscal year ended December 31, 2014 all Section 16(a) filing requirements applicable to our officers, directors and greater than ten percent beneficial owners were filed in a timely manner.
Code of Business Conduct and Ethics
We have adopted the Marrone Bio Innovations Code of Business Conduct and Ethics that applies to all officers, directors and employees. Our Code of Business Conduct and Ethics is available on the investor relations section of our website (at investors.marronebio.com) under the heading “Corporate Governance.” If we make any substantive amendments to our Code of Business Conduct and Ethics or grant any waiver from a provision of the Code of Business Conduct and Ethics to any executive officer or director, we will promptly disclose the nature of the amendment or waiver on the investor relations section of our website at investors.marronebio.com under the heading “Corporate Governance.” We intend to satisfy the disclosure requirement under Item 5.05 of Form 8-K regarding amendment to, or waiver from, a provision of our Code of Business Conduct and Ethics by posting such information on our website at the address and location specified above.
ITEM 11. EXECUTIVE COMPENSATION
We refer to our chief executive officer and our two other most highly compensated executive officers discussed below as our “named executive officers.” Our named executive officers for fiscal year 2014 were as follows:
| • | Pamela G. Marrone, Ph. D., President and Chief Executive Officer |
| • | James B. Boyd, Chief Financial Officer |
| • | Linda V. Moore, Vice President, General Counsel, Secretary and Chief Compliance Officer |
175
Table of Contents
Summary Compensation Table
The following table presents information regarding compensation earned by or awards to our named executive officers during fiscal years 2014, 2013 and 2012.
| NAME AND PRINCIPAL POSITION |
YEAR | SALARY ($) |
BONUS ($) |
OPTION AWARDS ($)(1) |
NON-EQUITY INCENTIVE PLAN COMPENSATION ($) |
ALL OTHER COMPENSATION ($)(2) |
TOTAL ($) | |||||||||||||||||||||
| Pamela G. Marrone, Ph.D |
||||||||||||||||||||||||||||
| President and Chief Executive Officer |
2014 | 300,000 | — | — | — | 11,973 | 311,973 | |||||||||||||||||||||
| 2013 | 250,000 | 25,835 | (3) | 1,014,461 | 60,023 | 11,206 | 1,361,525 | |||||||||||||||||||||
| 2012 | 250,000 | — | 452,144 | 75,375 | 11,804 | 789,323 | ||||||||||||||||||||||
| James B. Boyd |
||||||||||||||||||||||||||||
| Chief Financial Officer |
2014 | 202,769 | 10,000 | (4) | 1,704,699 | — | 1,855 | 1,919,323 | ||||||||||||||||||||
| Linda V. Moore |
||||||||||||||||||||||||||||
| Vice President, General Counsel, Secretary and Chief Compliance Officer |
2014 | 178,125 | — | 935,460 | 19,372 | 15,781 | 1,148,738 | |||||||||||||||||||||
| (1) | This column reflects the aggregate grant date fair value of option awards granted to our named executive officers estimated pursuant to FASB ASC 718, Compensation – Share based compensation (ASC 718). Valuation assumptions are described in Note 2 of our accompanying Notes to Consolidated Financial Statements included in Part II-Item 8-“Financial Statements and Supplementary Data” in this Annual Report on Form 10-K. |
| (2) | This column includes our 401(k) retirement savings plan matching, payment of life insurance premiums, long-term disability and other insurance-related reimbursements. In addition, Ms. Moore’s other compensation includes certain reimbursements for relocation expenses. |
| (3) | Represents a discretionary bonus. |
| (4) | Represents a signing bonus. |
Non-Equity Incentive Awards
We structure our annual non-equity incentive awards to reward named executive officers for the successful performance of our company as a whole and of each participating named executive officer as an individual. For the 2014 fiscal year, our compensation committee established a bonus plan available to all of our executive officers and other key employees. The bonus plan provides for a target cash award of up to 30% of the named executive officer’s salary, with 75% of the target award based upon the achievement of company-wide goals, and 25% of the target award based upon the achievement of individual goals. The progress of the goals is tracked by the compensation committee on a quarterly basis. Each company-wide goal and individual goal received a weighting, such that a named executive officer would receive a portion of the target non-equity incentive award for each goal achieved. The company-wide goals were based on our forecasts and plans for fiscal year 2014 and took into account factors, including net revenues objectives, based on anticipated timing and volume of new customer activity, and product development events such as completion of development work and EPA submissions for new products, processing international registrations and introduction of products into new markets. Based upon these factors, the compensation committee determined that 20% of the company-wide goals were achieved in 2014. Therefore, the participants in the bonus plan were entitled to 15% of their target bonuses based upon the company-wide goals component.
In addition to the company-wide goals, 25% of each named executive officer’s 2014 bonus target was comprised of achievement of individual goals.
176
Table of Contents
For 2014, each named executive officer was generally evaluated with respect to individual goals on the basis of the overall performance of our company, including the success of public offerings and private placements and the extent to which we were successful in achieving net revenues goals, developing strategic collaborations, product development, commercialization targets, geographical expansion, organizational development and growth. Based upon the compensation committee’s consideration of the matters related to our recent audit committee investigation and financial restatement, as described above in the Explanatory Note to this Annual Report, its discussions with Dr. Marrone, our Chief Executive Officer, and Mr. Boyd, our Chief Financial Officer, and its subsequent recommendation to the board related to the foregoing, the board determined not to make an award to Dr. Marrone or Mr. Boyd under the bonus plan for 2014. Although the board adopted a policy for 2014 of capping the bonus pool for employees generally at 80%, based on Ms. Moore’s achievement level of 100% of her bonus targets for her individual goals, the board exercised its discretion to increase Ms. Moore’s cap to 85% (representing 21% of her aggregate bonus target) for an aggregate non-equity incentive award equal to 36% of her bonus target (with 41% of this award based on the achievement of 20% of the company-wide goals).
The non-equity incentive award can either be paid out or deferred to a future payout time at the discretion of the board of directors. Payments are not guaranteed and are subject to approval by the board of directors. In addition, the determination of goal achievement (full or partial) is made by the compensation committee and approved by the board of directors.
Outstanding Equity Awards at the End of Fiscal Year 2014
The following table provides information regarding unexercised stock options held by each of the named executive officers as of the end of fiscal year 2014.
| NAME |
GRANT DATE |
SECURITIES UNDERLYING UNEXERCISED OPTIONS EXERCISABLE (#)(1) |
SECURITIES UNDERLYING UNEXERCISED OPTIONS UNEXERCISABLE (#) |
OPTION EXERCISE PRICE ($) |
OPTION EXPIRATION DATE | |||||||||||
| Pamela G. Marrone, Ph.D. |
5/1/2007 | 53,378 | (2) | — | 0.47 | 5/1/2017 | ||||||||||
| 10/22/2008 | 47,794 | (3) | — | 1.19 | 10/22/2018 | |||||||||||
| 1/28/2009 | 9,559 | (4) | — | 1.19 | 1/28/2019 | |||||||||||
| 1/11/2010 | 4,779 | (5) | — | 1.19 | 1/11/2020 | |||||||||||
| 1/24/2011 | 19,092 | (6) | — | 1.19 | 1/24/2021 | |||||||||||
| 1/24/2011 | 31,206 | (7) | 657 | 1.19 | 1/24/2021 | |||||||||||
| 12/15/2011 | 1,593 | (8) | 12,214 | 1.41 | 12/15/2021 | |||||||||||
| 2/20/2012 | 13,390 | (9) | — | 3.11 | 2/20/2022 | |||||||||||
| 10/29/2012 | 34,524 | (10) | 29,201 | 12.08 | 10/29/2022 | |||||||||||
| 8/1/2013 | 637 | (11) | 1,274 | 12.00 | 8/1/2023 | |||||||||||
| 9/27/2013 | 26,251 | (12) | 57,749 | 18.01 | 9/27/2023 | |||||||||||
| 11/6/2013 | 140 | (13) | 342 | 16.77 | 11/6/2023 | |||||||||||
| James B. Boyd |
2/26/2014 | — | 190,000 | (14) | 14.03 | 2/26/2024 | ||||||||||
| Linda V. Moore |
3/17/2014 | — | 100,000 | (15) | 14.61 | 3/17/2024 | ||||||||||
| (1) | Options granted under the Marrone Bio Innovations, Inc. Stock Option Plan, which we refer to as the 2006 Plan, are immediately exercisable in full, regardless of vesting. Any unvested shares issued upon the exercise of these options are subject to a right of repurchase. |
| (2) | The option vested with respect to one-quarter of the total shares subject to the option on the first anniversary of the vesting commencement date of May 1, 2007, and with respect to 1/48th of the total shares subject to the option monthly thereafter for 36 months, such that all the shares were fully vested upon the fourth anniversary of the option’s vesting commencement date. |
| (3) | The option vested with respect to one-quarter of the total shares subject to the option on the first anniversary of the vesting commencement date of November 1, 2008, and with respect to 1/48th of the total shares subject to the option monthly thereafter for 36 months, such that all the shares were fully vested upon the fourth anniversary of the option’s vesting commencement date. |
177
Table of Contents
| (4) | The option vested with respect to one-quarter of the total shares subject to the option on the first anniversary of the vesting commencement date of January 1, 2009, and with respect to 1/48th of the total shares subject to the option monthly thereafter for 36 months, such that all the shares were fully vested upon the fourth anniversary of the option’s vesting commencement date. |
| (5) | The option vested with respect to 100% of the total shares subject to the option on the vesting commencement date of January 1, 2010. |
| (6) | The options vested with respect to 100% of the total shares subject to the option on the vesting commencement date of January 1, 2011. |
| (7) | Includes 8,625 shares underlying exercisable options that are unvested. The options vest with respect to one-quarter of the total shares subject to the option on the first anniversary of the vesting commencement date of January 1, 2011, and with respect to 1/48th of the total shares subject to the options monthly thereafter for 36 months, such that all the shares will be fully vested upon the fourth anniversary of the options’ vesting commencement date. |
| (8) | The options vest with respect to 1/60th of the total shares subject to the options one month after the vesting commencement date of November 1, 2011, and with respect to 1/60th of the total shares subject to the options monthly thereafter for 59 months, such that all the shares will be fully vested upon the fifth anniversary of the options’ vesting commencement date. |
| (9) | The options vested with respect to 100% of the total shares subject to the options on the vesting commencement date of February 20, 2012. |
| (10) | The options vest with respect to one-quarter of the total shares subject to the options on October 18, 2013, and with respect to 1/48th of the total shares subject to the options monthly thereafter for 36 months, such that all the shares will be fully vested upon the fourth anniversary of the options’ vesting commencement date. |
| (11) | The options vest with respect to one-quarter of the total shares subject to the options on August 1, 2014, and with respect to 1/48th of the total shares subject to the options monthly thereafter for 36 months, such that all the shares will be fully vested upon the fourth anniversary of the options’ vesting commencement date. |
| (12) | The options vest with respect to one-quarter of the total shares subject to the options on September 27, 2014, and with respect to 1/48th of the total shares subject to the options monthly thereafter for 36 months, such that all the shares will be fully vested upon the fourth anniversary of the options’ vesting commencement date. |
| (13) | The option vests with respect to one-quarter of the total shares subject to the option on October 1, 2014, and with respect to 1/48th of the total shares subject to the option monthly thereafter for 36 months, such that all the shares will be fully vested upon the fourth anniversary of the option’s vesting commencement date. |
| (14) | The option vests with respect to one-quarter of the total shares subject to the option on February 26, 2015, and with respect to 1/48th of the total shares subject to the option monthly thereafter for 36 months, such that all the shares will be fully vested upon the fourth anniversary of the option’s vesting commencement date. |
| (15) | The option vests with respect to one-quarter of the total shares subject to the option on March 17, 2015, and with respect to 1/48th of the total shares subject to the option monthly thereafter for 36 months, such that all the shares will be fully vested upon the fourth anniversary of the option’s vesting commencement date. |
Option Exercises and Stock Vested
The following table summarizes for each named executive officer the stock option exercises and stock award shares vested during fiscal 2014:
| OPTION AWARDS | STOCK AWARDS | |||||||||||||
| NUMBER OF SHARES ACQUIRED ON EXERCISE (#) |
VALUE REALIZED ON EXERCISE ($) |
NUMBER OF SHARES ACQUIRED ON VESTING (#) |
VALUE REALIZED ON VESTING ($) |
|||||||||||
| Name | ||||||||||||||
| Pamela G. Marrone, Ph.D. |
20,056 | 133,486 | — | — | ||||||||||
| James B. Boyd |
— | — | — | — | ||||||||||
| Linda V. Moore |
— | — | — | — | ||||||||||
178
Table of Contents
Employment Agreements
We have entered into employment offer letters with each of our named executive officers described below, and employee proprietary information and inventions assignment agreements, under which each of our named executive officers has agreed not to disclose our confidential information or induce us to use proprietary information or trade secrets of others at any time.
Pamela G. Marrone, Ph.D.
Effective as of June 29, 2006, we entered into an offer letter with Pamela G. Marrone, Ph.D., our President and Chief Executive Officer. Under the offer letter, Dr. Marrone is entitled to an annual base salary which was $250,000 for 2013, and was increased to $300,000 commencing in 2014 in connection with our initial public offering. Dr. Marrone is eligible for our benefit programs on the same terms as our other executives. In addition, in accordance with the terms of the offer letter, our board of directors granted Dr. Marrone a restricted stock award of 97,424 shares, which completely vested on June 29, 2010, and an option to purchase 53,378 shares of our common stock on May 1, 2007, which completely vested on May 1, 2011.
The letter agreement provides that either party may terminate the employment arrangement for any reason or no reason, but four weeks’ notice is requested if the agreement is terminated by Dr. Marrone. In addition, the agreement provides that if we actively or constructively terminate Dr. Marrone’s employment without cause (whether or not in connection with a change of control), Dr. Marrone will be eligible to receive:
| • | an amount equal to twelve months of her then-current annual base salary payable in the form of salary continuation; and |
| • | medical and dental coverage, plus disability and life insurance premiums, for a period of twelve months following her termination. |
James B. Boyd
Effective as of February 26, 2014, we entered into an offer letter with James B. Boyd, our successor Vice President and Chief Financial Officer. Under the offer letter, Mr. Boyd is entitled to an annual base salary of $240,000, and is eligible for our benefit programs, vacation benefits, medical benefits and 401(k) plan participation. In addition, in satisfaction of obligations to Mr. Boyd in the offer letter with respect to option awards, our board of directors granted Mr. Boyd an option to purchase 190,000 shares of our common stock on February 13, 2014, which vests, subject to continued employment on each vesting date, with respect to one-quarter of the total shares subject to the option on the first anniversary of the option’s vesting commencement date of February 26, 2014 and with respect to 1/48th of the total shares subject to the option monthly thereafter for 36 months, such that all shares subject to the option will be fully vested on the fourth anniversary of such option’s vesting commencement date.
The offer letter also provided for a $10,000 signing bonus upon Mr. Boyd’s acceptance, relocation expenses of $20,000 and three months temporary housing. The letter agreement provides that either party may terminate the employment arrangement for any reason or no reason, but four weeks’ notice is requested if Mr. Boyd terminates his employment. In addition, the agreement provides that if we actively or constructively terminate Mr. Boyd’s employment without cause (whether or not in connection with a change of control), Mr. Boyd will be eligible to receive:
| • | an amount equal to six months of his then-current annual base salary payable in the form of salary continuation; and |
| • | medical and dental coverage, plus disability and life insurance premiums, for a period of six months following his termination. |
179
Table of Contents
Effective March 3, 2015, Mr. Boyd’s terms of employment were revised pursuant to a letter agreement to increase his base salary to $250,000 and to provide that if a change in control occurs that is not pursuant to an agreement signed within nine months of March 3, 2015, and termination other than “for cause” occurs within twelve months of such change in control, then in addition to the severance amount set forth in his offer letter, he will be entitled to receive a lump-sum payment equal to six months’ base salary. If we enter into an agreement with respect to a change in control transaction within nine months of the date of the letter agreement, then upon the closing of such transaction, a lump sum payment equal to 18 months’ base salary would be made.
Linda V. Moore
Effective as of March 17, 2014, we entered into an offer letter with Linda Moore, our General Counsel. Under the offer letter, Ms. Moore is entitled to an annual base salary of $225,000, and is eligible for our benefit programs, vacation benefits, medical benefits and 401(k) plan participation. In addition, in satisfaction of obligations to Ms. Moore in the offer letter with respect to option awards, our board of directors granted Ms. Moore an option to purchase 100,000 shares of our common stock on March 17, 2014, which vests, subject to continued employment on each vesting date, with respect to one-quarter of the total shares subject to the option on the first anniversary of the option’s vesting commencement date of March 17, 2014 and with respect to 1/48th of the total shares subject to the option monthly thereafter for 36 months, such that all shares subject to the option will be fully vested on the fourth anniversary of such option’s vesting commencement date.
The offer letter also provided for relocation expenses of $10,000 and one month temporary housing. The letter agreement provides that either party may terminate the employment arrangement for any reason or no reason, but four weeks’ notice is requested if Ms. Moore terminates her employment. In addition, the agreement provides that if we actively or constructively terminate Ms. Moore’s employment without cause (whether or not in connection with a change of control), Ms. Moore will be eligible to receive:
| • | an amount equal to six months of his then-current annual base salary payable in the form of salary continuation; and |
| • | medical and dental coverage, plus disability and life insurance premiums, for a period of six months following his termination. |
Effective February 9, 2015, Ms. Moore’s terms of employment were revised pursuant to a letter agreement to increase her base salary to $240,000 and to provide that if a change in control occurs that is not pursuant to an agreement signed within nine months of March 3, 2015, and termination other than “for cause” occurs within twelve months of such change in control, then in addition to the severance amount set forth in her offer letter, a lump-sum payment equal to six months’ base salary. If the Company enters into an agreement with respect to a change in control transaction within nine months of the date of the letter agreement, then upon the closing of such transaction, a lump sum payment equal to 12 months’ base salary would be made.
Compensation Risk Management
We have considered the risks associated with our compensation policies and practices for all employees, and we believe we have designed our compensation policies and practices in a manner that does not create incentives that could lead to excessive risk taking that would have a material adverse effect on our Company.
Employee Benefit and Stock Plans
As a result of our announcement that certain of our previously filed financial statements should no longer be relied upon, we ceased using our registration statement on Form S-8 to make equity grants to employees. Accordingly, on September 3, 2014, we suspended option exercises under our equity incentive plans for all employees, including executive officers, provided that any options that would have terminated unexercised as a result of such suspension will be exercisable for thirty days following the date that the registration statement becomes available for use. In addition, we did not make any equity awards to employees, including executive officers, after September 3, 2014.
180
Table of Contents
Marrone Bio Innovations, Inc. Stock Option Plan
We established the Marrone Bio Innovations, Inc. Stock Option Plan, which we refer to as the 2006 Plan, effective as of July 26, 2006. We ceased granting options under our 2006 Plan after, and the 2006 Plan terminated upon, the adoption of our 2011 Plan on July 19, 2011. Our 2006 Plan provided for the grant of incentive stock options, within the meaning of Section 422 of the Internal Revenue Code of 1986, as amended (the Code), to our employees and any parent and subsidiary corporations’ employees, and for the grant of non-qualified stock options to our employees, outside directors and consultants and our parent and subsidiary corporations’ employees and consultants.
Administration: Our board of directors administered our 2006 Plan. The administrator’s powers include the power to: determine the fair market value of our common stock; select the individuals to whom options may be granted; determine the number of shares of stock covered by each option; approve forms of award agreement; determine the terms and conditions of options granted to employees and consultants (e.g., the exercise price, the times when options may be exercised (which may be based on performance criteria), any vesting acceleration or waiver of forfeiture restrictions, and any restriction or limitation regarding any option or the underlying shares of stock); reduce the exercise price of any option granted to employees and consultants to the then current fair market value of our common stock if such fair market value has declined since the date of grant; prescribe, amend and rescind rules and regulations relating to our 2006 Plan; modify or amend each option; institute an option exchange program; and make all other determinations deemed necessary or advisable for administering our 2006 Plan.
Transferability of Options: Our 2006 Plan allows for the transfer of options only (i) by will; and (ii) by the laws of descent and distribution. Only the recipient of an option may exercise such option during his or her lifetime.
Certain Adjustments: In the event of certain changes in our capitalization our board of directors will make adjustments to one or more of (i) the number of shares that are covered by outstanding options; (ii) the exercise price of outstanding options, and (iii) the numerical share limits contained in our 2006 Plan. In the event of our complete liquidation or dissolution, recipients must be notified at least ten (10) days prior to the proposed transaction and may exercise all vested and unvested options until ten (10) days prior to such transaction; all outstanding options will terminate immediately prior to the consummation of such transaction.
Corporate Transactions: Our 2006 Plan provides that in the event of a corporate transaction, as defined in our 2006 Plan, each outstanding option will become immediately vested. In the event of a corporate transaction involving a merger or sale of assets, options will be exercisable for a period of fifteen (15) days from the date that notice of the transaction is provided; the option will then terminate upon the expiration of that period.
2011 Stock Plan
We established our 2011 Stock Plan, which we refer to as the 2011 Plan, effective as of July 19, 2011. Our 2011 Plan provided for the grant of incentive stock options, within the meaning of Section 422 of the Code, to our employees and any parent and subsidiary corporations’ employees, and for the grant of non-qualified stock options and stock purchase rights to our employees, directors and consultants and any parent and subsidiary corporations’ employees, directors and consultants. We ceased granting options under our 2011 Plan after, and the 2011 Plan terminated upon, the adoption of our 2013 Plan on August 1, 2013.
Administration: Our board of directors administered our 2011 Plan. The administrator’s powers include the power to: determine the persons to whom, and the times at which, awards shall be granted and the number of shares of our common stock subject to each award; determine the fair market value of our common stock; determine the terms, conditions and restrictions applicable to each award (e.g. the exercise price, the method of payment, the method for satisfaction of any tax withholding obligation, the timing, terms and conditions of the exercisability and vesting of the award, the time of the expiration of the award, and the effect of the recipient’s
181
Table of Contents
termination of service); approve forms of award agreement; amend, modify, extend, cancel or renew any award or waive any restrictions or conditions applicable to any award; accelerate, continue, extend or defer the exercisability of any award; prescribe, amend or rescind rules guidelines and policies relating to the 2011 Plan; and make all other determinations and take such other actions with respect to the 2011 Plan or any award as it deems advisable and that is consistent with applicable law, regulations and rules.
Stock Options: Our 2011 Plan allowed for the grant of incentive stock options that qualify under Section 422 of the Code only to our employees and employees of any parent or subsidiary of ours. Non-qualified stock options could be granted to our employees, directors, and consultants and those of any parent or subsidiary of ours. The exercise price of all options granted under our 2011 Plan was required to be at least equal to the fair market value of our common stock on the date of grant. The term of an option may not exceed ten (10) years, except that with respect to any employee who owns more than ten percent (10%) of the voting power of all classes of our outstanding stock or the outstanding stock of any parent or subsidiary corporation as of the grant date (i) the term of an incentive stock option must not exceed five (5) years; and (ii) the exercise price of an incentive stock option must equal at least one hundred ten percent (110%) of the fair market value of our common stock on the grant date.
After the continuous service of an employee, director or consultant terminates, he or she may exercise his or her option, to the extent vested, for the period of time specified in the award agreement. If his or her continuous service terminates for cause, however, the option shall immediately terminate. An option may not be exercised later than the expiration of its term.
Stock Purchase Rights: Our 2011 Plan allowed for the grant of stock purchase rights. Stock purchase rights are rights to purchase our common stock for at least one hundred percent (100%) of the fair market value of our common stock and which are exercisable for thirty (30) days from the date of grant. The purchase price of a stock purchase right may be paid in cash or in the form of services rendered. The board of directors may subject a stock purchase right to vesting conditions.
Transferability of Awards: Our 2011 Plan allowed for the transfer of awards only (i) by will; (ii) by the laws of descent and distribution and (iii) for non-qualified stock options, to the extent authorized by the board of directors. Only the recipient of an award may exercise such award during his or her lifetime except that non-qualified stock options may be transferred to certain trusts and certain family members.
Certain Adjustments: In the event of certain changes in our capitalization, to prevent diminution or enlargement of the benefits or potential benefits available under the 2011 Plan, the board of directors will make adjustments to one or more of (i) the number and class of shares subject to the 2011 Plan and that are covered by outstanding awards; (ii) the exercise price of outstanding awards and (iii) the incentive stock option share limit contained in the 2011 Plan.
Changes in Control: Our 2011 Plan provides that in the event of a change in control, as defined in the 2011 Plan, the board of directors, in its discretion may provide that (i) the vesting and exercisability of any outstanding awards shall accelerate; or (ii) that each outstanding award (including, at the board of directors’ discretion, unvested awards) shall be cashed out; payment due with respect to unvested awards would then be payable in accordance with the existing vesting schedule. Further, the successor corporation may assume or substitute an equivalent award for each outstanding award; if the successor corporation does not do so, awards held by recipients who have not terminated employment with us will vest in full as of the change in control.
2013 Stock Incentive Plan
In August 2013, our board of directors adopted the 2013 Stock Incentive Plan (2013 Plan). The 2013 Plan serves as the successor to our 2011 Plan. Our 2013 Plan provides for the grant of incentive stock options, within the meaning of Section 422 of the Code, to our employees and any parent and subsidiary corporations’ employees, and for the grant of non-qualified stock options, stock appreciation rights, restricted stock, restricted stock units and dividend equivalent rights to our employees, directors and consultants and our parent and subsidiary corporations’ employees, directors and consultants.
182
Table of Contents
Shares: We initially authorized a total of 1,600,000 shares of our common stock for issuance pursuant to the 2013 Plan, plus the number of shares of common stock reserved for issuance pursuant to future grants under the 2011 Plan upon the adoption of the 2013 Plan. In addition, the number of shares authorized for issuance pursuant to the 2013 Plan will be increased by any additional shares that would otherwise return to the 2011 Plan after the date of adoption of the 2013 Plan as a result of the forfeiture, termination or expiration of awards previously granted under the 2011 Plan. Further, our 2013 Plan provides for annual increases in the number of shares available for issuance thereunder equal to the least of (i) 3.5% of the number of shares of the Company’s common stock outstanding on the last day of the immediately preceding fiscal year or (ii) a lesser number of shares determined by the administrator. Based on and subject to the foregoing, as of January 1, 2015, including such annual increase, 3,770,733 shares of our common stock, plus any additional shares which are subject to options granted under our 2006 Plan or 2011 Plan but are forfeited or otherwise terminate or expire subsequent to January 1, 2015, were authorized for issuance pursuant to the 2013 Plan. In addition, as of January 1, 2015, under the 2013 Plan, 1,835,098 shares of common stock were issuable upon the exercise of outstanding options granted and 1,935,635 additional shares of common stock were reserved for issuance pursuant to future grants.
Administration: Our board of directors or a committee of our board of directors administers our 2013 Plan. In the case of awards intended to qualify as “performance based compensation” within the meaning of Section 162(m) of the Code, the committee consists of two (2) or more “outside directors” within the meaning of Section 162(m) of the Code. The administrator has the power to determine and interpret the terms and conditions of the awards, including the employees, directors and consultants who will receive awards, the exercise price, the number of shares subject to each such award, the vesting schedule and exercisability of the awards, the restrictions on transferability of awards and the form of consideration payable upon exercise. The administrator also has the authority to institute an exchange program whereby the exercise prices of outstanding awards may be reduced or outstanding awards may be surrendered or cancelled in exchange for other awards of the same type (which may have higher or lower exercise prices) or awards of a different type.
Stock Options: Our 2013 Plan allows for the grant of incentive stock options that qualify under Section 422 of the Code only to our employees and employees of any parent or subsidiary of ours. Non-qualified stock options may be granted to our employees, directors and consultants and those of any parent or subsidiary of ours. The exercise price of all options granted under our 2013 Plan must at least be equal to the fair market value of our common stock on the date of grant. The term of an incentive stock option may not exceed ten (10) years, except that with respect to any employee who owns more than ten percent (10%) of the voting power of all classes of our outstanding stock or any parent or subsidiary corporation as of the grant date, the term must not exceed five (5) years and the exercise price must equal at least one hundred ten percent (110%) of the fair market value on the grant date.
After the continuous service of an employee, director or consultant terminates, he or she may exercise his or her option, to the extent vested, for the period of time specified in the option agreement. However, an option may not be exercised later than the expiration of its term.
Stock Appreciation Rights: Our 2013 Plan allows for the grant of stock appreciation rights. Stock appreciation rights allow the recipient to receive the appreciation in the fair market value of our common stock between the date of grant and the exercise date. The administrator will determine the terms of stock appreciation rights, including when such rights become exercisable and whether to pay the increased appreciation in cash or with shares of our common stock, or a combination thereof, except that the base appreciation amount for the cash or shares to be issued pursuant to the exercise of a stock appreciation right will be no less than one hundred percent (100%) of the fair market value per share on the date of grant. After the continuous service of an employee, director or consultant terminates, he or she may exercise his or her stock appreciation right, to the extent vested, only to the extent provided in the stock appreciation right agreement.
Restricted Stock Awards: Our 2013 Plan allows for the grant of restricted stock. Restricted stock awards are shares of our common stock that vest in accordance with terms and conditions established by the administrator. The administrator will determine the number of shares of restricted stock granted to any employee, director or
183
Table of Contents
consultant. The administrator may impose whatever conditions on vesting it determines to be appropriate. For example, the administrator may set restrictions based on the achievement of specific performance goals. Shares of restricted stock that do not vest are subject to our right of repurchase or forfeiture.
Restricted Stock Units: Our 2013 Plan allows for the grant of restricted stock units. Restricted stock units are awards that will result in payment to a recipient at the end of a specified period only if the vesting criteria established by the administrator are achieved or the award otherwise vests. The administrator may impose whatever conditions to vesting, restrictions and conditions to payment it determines to be appropriate. The administrator may set restrictions based on the achievement of specific performance goals or on the continuation of service or employment. Payments of earned restricted stock units may be made, in the administrator’s discretion, in cash, with shares of our common stock or other securities, or a combination thereof.
Dividend Equivalent Rights: Our 2013 Plan allows for the grant of dividend equivalent rights. Dividend equivalent rights are awards that entitle the recipients to compensation measured by the dividends we pay with respect to our common stock.
Transferability of Awards: Our 2013 Plan allows for the transfer of awards under the 2013 Plan only (i) by will; (ii) by the laws of descent and distribution and (iii) for awards other than incentive stock options, to the extent authorized by the administrator. Only the recipient of an incentive stock option may exercise such award during his or her lifetime.
Certain Adjustments: In the event of certain changes in our capitalization, to prevent diminution or enlargement of the benefits or potential benefits available under the 2013 Plan, the administrator will make adjustments to one or more of the number or class of shares that are covered by outstanding awards, the exercise or purchase price of outstanding awards, the numerical share limits contained in the 2013 Plan and any other terms that the administrator determines require adjustment. In the event of our complete liquidation or dissolution, all outstanding awards will terminate immediately upon the consummation of such transaction.
Corporate Transactions and Changes in Control: Our 2013 Plan provides that in the event of a corporate transaction, as defined in the 2013 Plan, each outstanding award will terminate upon the consummation of the corporate transaction to the extent that such awards are not assumed by the acquiring or succeeding corporation. Prior to or upon the consummation of a corporate transaction or a change in control, as defined in the 2013 Plan, an outstanding award may vest, in whole or in part, to the extent provided in the award agreement or as determined by the administrator in its discretion. The administrator may condition the vesting of an award upon the subsequent termination of the recipient’s service or employment within a specified period of time following the consummation of a corporate transaction or change in control. The administrator will not be required to treat all awards similarly in the event of a corporate transaction or change in control.
Plan Amendments and Termination: Our 2013 Plan will automatically terminate ten (10) years following the date it becomes effective, unless we terminate it sooner. In addition, our board of directors has the authority to amend, suspend or terminate the 2013 Plan provided such action does not impair the rights under any outstanding award unless mutually agreed to in writing by the recipient and us.
401(k) Plan
We maintain a 401(k) retirement savings plan. Each participant who is a U.S. employee may contribute to the 401(k) plan, through payroll deductions, up to a statutorily prescribed annual limit imposed by the Internal Revenue Service (which limit was $18,000 in 2014). All amounts contributed by employee participants and earnings on these contributions are fully vested at all times and are not taxable to participants until withdrawn. Employee participants may elect to invest their contributions in various established funds. We may make contributions to the accounts of plan participants.
184
Table of Contents
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The following table sets forth certain information with respect to the beneficial ownership of our common stock as of September 30, 2015, for:
| • | each person or group of affiliated persons known by us to be the beneficial owner of more than 5% of our common stock; |
| • | each of our named executive officers; |
| • | each of our directors; and |
| • | all current executive officers and directors as a group. |
We have determined beneficial ownership in accordance with SEC rules. The information does not necessarily indicate beneficial ownership for any other purpose. Under these rules, the number of shares of common stock deemed outstanding includes shares issuable upon exercise of options held by the respective person or group that may be exercised within 60 days after September 30, 2015. For purposes of calculating each person’s or group’s percentage ownership, stock options and warrants exercisable within 60 days after December 31, 2014 are included for that person or group but not the stock options of any other person or group.
Applicable percentage ownership is based on 24,464,582 shares of common stock outstanding at December 31, 2014. In computing the number of shares of common stock beneficially owned by a person and the percentage ownership of that person, we deemed to be outstanding all shares of common stock subject to options and warrants exercisable within 60 days of September 30, 2015. We did not deem these shares outstanding, however, for the purpose of computing the percentage ownership of any other person.
Unless otherwise indicated and subject to applicable community property laws, to our knowledge, each stockholder named in the following table possesses sole voting and investment power over the shares listed. Unless otherwise noted below, the address of each person listed in the table is c/o Marrone Bio Innovations, Inc., 1540 Drew Avenue, Davis, CA 95618.
| SHARES BENEFICIALLY OWNED | ||||||||
| NAME AND ADDRESS OF BENEFICIAL OWNER |
SHARES (#) |
SHARES (%) |
||||||
| 5% Stockholders: |
||||||||
| Entities affiliated with Waddell & Reed Financial, Inc. (1) |
4,921,045 | 19.9 | ||||||
| 6300 Lamar Avenue |
||||||||
| Overland Park, KS 66202 |
||||||||
| PRIMECAP Management Company(2) |
3,242,100 | 13.3 | ||||||
| 225 South Lake Avenue #400 |
||||||||
| Pasadena, CA 91101 |
||||||||
| Stuart Mill Venture Partners, L.P. (3) |
1,347,317 | 5.5 | ||||||
| 252 North Washington Street |
||||||||
| Falls Church, VA 22046 |
||||||||
| Entities affiliated with Saffron Hill Ventures (4) |
1,287,983 | 5.3 | ||||||
| 130 Wood Street |
||||||||
| London EC2V 6DL |
||||||||
| United Kingdom |
||||||||
| Richard Mashall (5) |
1,303,070 | 5.3 | ||||||
| c/o Senvest Management, LLC |
||||||||
| 540 Madison Avenue, 32nd Floor |
||||||||
| New York, New York 10022 |
||||||||
| Entities affiliated with CGI Opportunity Fund II, L.P. (6) |
1,272,465 | 5.2 | ||||||
185
Table of Contents
| SHARES BENEFICIALLY OWNED | ||||||||
| NAME AND ADDRESS OF BENEFICIAL OWNER |
SHARES (#) |
SHARES (%) |
||||||
| Directors and Executive Officers: |
||||||||
| Pamela G. Marrone, Ph.D. (7) |
1,074,761 | 4.3 | ||||||
| Elin Miller (8) |
20,830 | * | ||||||
| Pamela Contag, Ph.D. (9) |
13,147 | * | ||||||
| Timothy Fogarty (10) |
8,710 | * | ||||||
| George Kerckhove (11) |
500 | * | ||||||
| Les Lyman (12) |
14,147 | * | ||||||
| Richard Rominger (13) |
120,389 | * | ||||||
| Shaugn Stanley (14) |
17,466 | * | ||||||
| James B. Boyd (15) |
83,136 | * | ||||||
| Linda V. Moore (16) |
41,672 | * | ||||||
| All current directors and executive officers as a group (12 persons) (17) |
1,539,742 | 6.1 | ||||||
| * | Represents beneficial ownership of less than 1% of our outstanding common stock. |
| (1) | As reported in the Schedule 13G filed February 13, 2015, the securities reported on herein are beneficially owned by one or more open-end investment companies or other managed accounts which are advised or sub-advised by Ivy Investment Management Company (IICO), the direct holder of 2,660,992 shares and an investment advisory subsidiary of Waddell & Reed Financial, Inc. (WDR) or Waddell & Reed Investment Management Company (WRIMCO), the direct holder of 2,107,100 shares and an investment advisory subsidiary of WDR. The investment sub-advisory contracts grant IICO and WRIMCO investment power over securities owned by such sub-advisory clients and, in most cases, voting power. Any investment restriction of a sub-advisory contract does not restrict investment discretion or power in a material manner. Therefore, IICO and/or WRIMCO may be deemed the beneficial owner of the securities covered by this statement under Rule 13d-3 of the Securities Exchange Act of 1934. Also includes warrants to purchase in the aggregate 152,953 shares of common stock held by Ivy Science & Technology Fund, Waddell & Reed Advisors Science & Technology Fund and Ivy Funds VIP Science & Technology, each an open-end fund of a series trust managed by either IICO or WRIMCO, which are exercisable within 60 days. |
| (2) | PRIMECAP Management Company is an independent investment management company. |
| (3) | Includes warrants to purchase 8,929 shares of common stock held by Stuart Mill Venture Partners, L.P. Walter Lubsen Jr., Jeffrey Salinger and Jana Hernandes are the Managing Partners and Lawrence Hough is the Managing Director of Stuart Mill Partners, LLC, the general partner of Stuart Mill Venture Partners, L.P., and therefore may be deemed to share voting control and investment power over the securities held by Stuart Mill Venture Partners, L.P. |
| (4) | Includes 191,782 shares of common stock held by Saffron Hill Ventures L.P., 1,096,201 shares of common stock held by Saffron Hill Ventures 2, L.P. Shawn Luetchens and Ranjeet Bhatia are Directors of Saffron Hill MGP Ltd and Saffron Hill MGP2 Ltd, the General Partners of Saffron Hill Ventures L.P. and Saffron Hill Ventures 2, L.P., respectively, and therefore may be deemed to share voting control and investment power over the securities held by Saffron Hill Ventures L.P. and Saffron Hill Ventures 2, L.P. |
| (5) | As reported in the Schedule 13G filed June 5, 2015, the reported securities are held in the accounts of Senvest Master Fund, L.P. and Senvest International L.L.C. (the Investment Vehicles). Senvest Management, LLC serves as investment manager of Senvest Master Fund, L.P. Richard Mashaal is the managing member of Senvest Management, LLC and is president of, exercising investment and voting powers over, Senvest International L.L.C. Mr. Mashaal may be deemed to have voting and dispositive powers over the securities held by the Investment Vehicles. Senvest Management, LLC may be deemed to beneficially own the securities held by Senvest Master Fund, L.P. by virtue of Senvest Management, LLC’s position as investment manager of Senvest Master Fund, L.P. Mr. Mashaal may be deemed to beneficially own the securities held by the Investment Vehicles by virtue of Mr. Mashaal’s status as the managing member of Senvest Management, LLC and his investment and voting powers over Senvest International L.L.C. |
186
Table of Contents
| (6) | Includes 1,270,085 shares held by CGI Opportunity Fund II, L.P. and 2,380 shares held by Ueberroth Family Trust dated June 27, 1986. Peter V. Ueberroth and Joseph Ueberroth are Partners of CGI Opportunity Gen Par II, LLC, the sole General Partner of CGI Opportunity Fund II, L.P. and therefore may be deemed to share voting control and investment power over the securities held by CGI Opportunity Fund II, L.P., and Peter V. Ueberroth is the trustee of, and holds voting control and investment power over the securities held by, Ueberroth Family Trust dated June 27, 1986. See also Note 10 to this section. |
| (7) | Includes 731,931 shares of common stock held by Dr. Marrone, 283,254 shares of common stock issuable to Dr. Marrone upon the exercise of outstanding options exercisable within 60 days, 6,442 shares of common stock held by Florence H. Marrone TOD Pamela G. Marrone, Ph.D. and 53,134 shares of common stock held by Dr. Marrone and Michael Rogers. Does not include 60,526 shares of common stock issuable to Dr. Marrone upon the exercise of outstanding options not exercisable within 60 days. |
| (8) | Includes 833 shares of common stock and 19,997 shares of common stock issuable upon the exercise of outstanding options exercisable within 60 days. Does not include 12,074 shares of common stock issuable upon the exercise of outstanding options not exercisable within 60 days. |
| (9) | Includes 13,147 shares of common stock issuable upon the exercise of outstanding options exercisable within 60 days. Does not include 10,977 shares of common stock issuable upon the exercise of outstanding options not exercisable within 60 days. |
| (10) | Includes 1,190 shares of common stock held by Timothy and Patricia Fogarty 2011 Trust, Dated August 1, 2011 and 7,520 shares of common stock issuable upon the exercise of outstanding options exercisable within 60 days. Timothy Fogarty is a Partner of the Contrarian Group, an affiliate of CGI Opportunity Fund II, L.P. but does not hold voting control or investment power over the securities held by CGI Opportunity Fund II, L.P. Does not include 9,815 shares of common stock issuable upon the exercise of outstanding options not exercisable within 60 days. See also Note 6 to this section. |
| (11) | Includes 500 shares of common stock. Does not include 20,866 shares of common stock issuable upon the exercise of outstanding options not exercisable within 60 days. |
| (12) | Includes 1,000 shares of common stock held by Leslie F. Lyman as custodian for Jackson WH Lyman UCAUTMA and 13,147 shares of common stock issuable upon the exercise of outstanding options exercisable within 60 days. Does not include 10,241 shares of common stock issuable upon the exercise of outstanding options not exercisable within 60 days. |
| (13) | Includes 99,522 shares of common stock held by The Richard and Mary Rominger Community Trust and 20,867 shares of common stock usable to Mr. Rominger upon the exercise of outstanding options exercisable within 60 days. Does not include 12,490 shares of common stock issuable upon the exercise of outstanding options not exercisable within 60 days. |
| (14) | Includes 17,466 shares of common stock issuable upon the exercise of outstanding options exercisable within 60 days. Does not include 8,880 shares of common stock issuable upon the exercise of outstanding options not exercisable within 60 days. |
| (15) | Includes 83,136 shares of common stock issuable upon the exercise of outstanding options exercisable within 60 days. Does not include 106,864 shares of common stock issuable upon the exercise of outstanding options not exercisable within 60 days. |
| (16) | Includes 41,672 shares of common stock issuable upon the exercise of outstanding options exercisable within 60 days. Does not include 58,328 shares of common stock issuable upon the exercise of outstanding options not exercisable within 60 days. |
| (17) | Includes 894,522 shares of common stock, 645,190 shares of common stock issuable upon the exercise of outstanding options held by current directors and executive officers exercisable within 60 days of common stock. Does not include 436,873 shares of common stock issuable upon the exercise of outstanding options held by current directors and executive officers not exercisable within 60 days. See also Notes 3, 4, and 6 to this section. |
187
Table of Contents
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
We describe below the transactions and series of similar transactions, since December 31, 2013, to which we were a participant or will be a participant, in which:
| • | the amounts involved exceeded or will exceed $120,000; and |
| • | any of our directors, executive officers, holders of more than 5% of our capital stock (which we refer to as 5% stockholders) or any member of their immediate family had or will have a direct or indirect material interest, other than compensation arrangements with directors and executive officers, which are described where required under Part III, Item 11, “Executive Compensation.” |
Executive Compensation and Employment Arrangements
Please see “Executive Compensation” for information on compensation arrangements with our executive officers and agreements with, and offer letters to, our executive officers containing compensation and termination provisions, among others.
Syngenta Commercial Agreement
In February 2011, we entered into an agreement with Syngenta Crop Protection AG, an affiliate of Syngenta Ventures Pte. LTD, which was a 5% stockholder from December 2012 to June 2014, whereby we have designated Syngenta as our exclusive distributor for Regalia in specialty crop markets in Europe, Africa and the Middle East. During the six months ended June 30, 2014 prior the reduction in ownership percentage, we recorded revenue of $333,000 in license revenue based on the terms of our commercial agreement with Syngenta. For a description of the agreement, see Part I-Item 1-“Business—Strategic Collaborations and Relationships” in this Annual Report on Form 10-K.
The Lyman/Tremont Groups
Les Lyman, a member of our board of directors, is the chairman and significant indirect shareholder of The Tremont Group, Inc. During the year ended December 31, 2014, we recognized revenue of $821,000 related to the sale of our products for further distribution and resale. As of December 31, 2014, we had no outstanding accounts receivable due from The Tremont Group, Inc. Although we anticipate sales of our products to The Tremont Group, Inc. to continue through 2015, we cannot estimate the amount of those sales.
Waddell & Reed
In August 2015, we entered into a purchase agreement with Ivy Science & Technology Fund, Waddell & Reed Advisors Science & Technology Fund and Ivy Funds VIP Science & Technology, each an affiliate of Waddell & Reed which is a 5% stockholder. Pursuant to such purchase agreement, we sold, to such affiliates, senior secured promissory notes in the aggregate principal amount of $40,000,000, which remains outstanding, and warrants to purchase up to 4,000,000 shares of our common stock at an exercise price of $1.91 per share for an aggregate consideration of $40,000,000. The notes bear interest at a rate of 8% per annum payable semi-annually on June 30 or December 31 of each year, commencing on December 31, 2015, with $10 million payable 3 years from the closing, $10 million payable 4 years from the closing, and $20 million due 5 years from the closing. For a description of the agreement, see Note 22 of our accompanying Notes to Consolidated Financial Statements included in Part II-Item 8-“Financial Statements and Supplementary Data” in this Annual Report on Form 10-K .
Director and Officer Indemnification and Insurance
We have adopted provisions in our current certificate of incorporation that limit or eliminate the liability of our directors for monetary damages for breach of their fiduciary duties, except for liability that cannot be eliminated
188
Table of Contents
under the Delaware General Corporation Law. Accordingly, our directors will not be personally liable for monetary damages for breach of their fiduciary duties as directors, except with respect to of the following:
| • | any breach of their duty of loyalty to us or our stockholders; |
| • | acts or omissions not in good faith or that involve intentional misconduct or a knowing violation of law; |
| • | unlawful payments of dividends or unlawful stock repurchases or redemptions as provided in Section 174 of the Delaware General Corporation Law; or |
| • | any transaction from which the director derived an improper personal benefit. |
This limitation of liability does not apply to liabilities arising under the federal securities laws and does not affect the availability of equitable remedies such as injunctive relief or rescission. If Delaware law is amended to authorize the further elimination or limiting of director liability, then the liability of our directors will be eliminated or limited to the fullest extent permitted by Delaware law as so amended.
Our certificate of incorporation and our bylaws also provide that we shall indemnify our directors and executive officers and shall indemnify our other officers and employees and other agents to the fullest extent permitted by law. We believe that indemnification under our bylaws covers at least negligence and gross negligence on the part of indemnified parties. Our bylaws, as currently in effect, also permit us to secure insurance on behalf of any officer, director, employee or other agent for any liability arising out of his or her actions in this capacity, regardless of whether our bylaws would permit indemnification.
We have entered and intend to continue to enter into separate indemnification agreements with certain of our directors and executive officers that are, in some cases, broader than the specific indemnification provisions provided by Delaware law and our charter documents, and may provide additional procedural protection. These agreements will require us, among other things, to:
| • | indemnify officers and directors against certain liabilities that may arise because of their status as officers and directors; |
| • | advance expenses, as incurred, to officers and directors in connection with a legal proceeding subject to limited exceptions; and |
| • | cover officers and directors under any general or directors’ and officers’ liability insurance policy maintained by us. |
We believe that these provisions and agreements are necessary to attract and retain qualified persons as directors and executive officers. Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling our company pursuant to the foregoing provisions, the opinion of the Securities and Exchange Commission is that such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
In addition, we maintain standard policies of insurance under which coverage is provided to our directors and officers against loss arising from claims made by reason of breach of duty or other wrongful act, and to us with respect to payments which may be made by us to such directors and officers pursuant to the above indemnification provisions or otherwise as a matter of law. We also make available standard life insurance and accidental death and disability insurance policies to our employees.
Policies and Procedures Regarding Related Party Transactions
Our board of directors reviews related party transactions for potential conflict of interest issues. Our board of directors has adopted a written related person transaction policy to set forth the policies and procedures for the review and approval or ratification of related person transactions. This policy covers any transaction,
189
Table of Contents
arrangement or relationship, or any series of similar transactions, arrangements or relationships in which we were or are to be a participant, the amount involved exceeds $120,000 and a related person had or will have a direct or indirect material interest, including, without limitation, purchases of goods or services by or from the related person or entities in which the related person has a material interest, indebtedness, guarantees of indebtedness or employment by us or a related person.
Director Independence
For a discussion of the independence of our directors, please see Part III-Item 10-“Directors, Executive Officers and Corporate Governance—Director Independence” above.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
The following table summarizes the fees of Ernst & Young LLP, our independent registered public accounting firm, for each of the last two fiscal years.
| FEE CATEGORY |
FISCAL 2014 | FISCAL 2013 | ||||||
| Audit fees (1) |
$ | 3,723,000 | $ | 1,133,000 | ||||
| Audit-related fees (2) |
12,000 | 50,000 | ||||||
| Tax fees (3) |
— | 32,000 | ||||||
| All other fees |
— | — | ||||||
|
|
|
|
|
|||||
| Total fees |
$ | 3,735,000 | $ | 1,215,000 | ||||
|
|
|
|
|
|||||
| (1) | Audit fees consist of professional services rendered in connection with the audit of our consolidated financial statements and review of our quarterly consolidated financial statements. Audit fees for fiscal 2014 also include fees associated with the Audit Committee’s independent investigation and related Restatement and our public offering of common stock completed in June 2014, as well as the delivery of comfort letters, consents and reviews of documents filed with the SEC. Audit fees for fiscal 2013 also include fees associated with our initial public offering of common stock completed in August 2013, which included a review of our quarterly consolidated financial statements included in our registration statement on Form S-1 filed with the SEC, as well as the delivery of comfort letters, consents and reviews of documents filed with the SEC. |
| (2) | Audit-related fees consist of professional services for assurance and related services that are reasonably related to the performance of the audit or review of our consolidated financial statements and are not reported under “Audit Fees.” These services include accounting consultations concerning financial accounting and reporting standards. |
| (3) | Tax fees consist of fees for professional services rendered for tax compliance, tax planning and tax advice. |
The Audit Committee pre-approves all audit and non-audit services to be, and has approved all of the foregoing audit and non-audit services, performed by the independent registered public accounting firm in accordance with the Audit Committee Charter.
190
Table of Contents
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
We have filed the following documents as part of this Form 10-K:
| 1. | Consolidated financial statements: |
| 2. | Financial Statement Schedules |
All schedules have been omitted because they are not required, not applicable, not present in amounts sufficient to require submission of the schedule, or the required information is otherwise included.
| 3. | Exhibits |
See the Exhibit Index immediately following the signature page of this Annual Report on Form 10-K, which is incorporated by reference here.
191
Table of Contents
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this Annual Report on Form 10-K to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Davis, State of California, on November 10, 2015.
| MARRONE BIO INNOVATIONS, INC. |
| /S/ PAMELA G. MARRONE |
| Pamela G. Marrone President and Chief Executive Officer |
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Pamela G. Marrone her or his true and lawful attorney-in-fact and agent, with full power of substitution and, for her or him and in her or his name, place and stead, in any and all capacities to sign any and all amendments to this Annual Report on Form 10-K, and to file the same, with all exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorney-in-fact and agent full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as she or he might or could do in person, hereby ratifying and confirming all that said attorney-in-fact and agent, or her substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this Annual Report on Form 10-K has been signed by the following persons on behalf of the Registrant and in the capacities and on the dates indicated:
| SIGNATURE |
TITLE |
DATE | ||
| /s/ Pamela G. Marrone Pamela G. Marrone |
President and Chief Executive Officer (Principal Executive Officer) |
November 10, 2015 | ||
| /s/ James B. Boyd James B. Boyd |
Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) |
November 10, 2015 | ||
| /s/ Elin Miller Elin Miller |
Chair of the Board | November 10, 2015 | ||
| /s/ Pamela Contag Pamela Contag |
Director | November 10, 2015 | ||
| /s/ Tim Fogarty Tim Fogarty |
Director | November 10, 2015 | ||
| /s/ George Kerckhove George Kerckhove |
Director | November 10, 2015 | ||
| /s/ Les Lyman Les Lyman |
Director | November 10, 2015 | ||
192
Table of Contents
| SIGNATURE |
TITLE |
DATE | ||
| /s/ Richard Rominger Richard Rominger |
Director | November 10, 2015 | ||
| /s/ Shaugn Stanley Shaugn Stanley |
Director | November 10, 2015 | ||
193
Table of Contents
INDEX TO EXHIBITS
| INCORPORATED BY REFERENCE | FILED HEREWITH | |||||||||||
| EXHIBIT |
EXHIBIT DESCRIPTION |
FORM |
FILE NO. |
EXHIBIT |
FILING DATE |
|||||||
| 3.1 | Fourth Amended and Restated Certificate of Incorporation of Marrone Bio Innovations, Inc. | 10-K | 001-36030 | 3.1 | March 25, 2014 | |||||||
| 3.2 | Amended and Restated Bylaws of Marrone Bio Innovations, Inc. | 10-K | 001-36030 | 3.2 | March 25, 2014 | |||||||
| 4.1 | Form of Marrone Bio Innovations, Inc.’s common stock certificate. | S-1/A | 333-189753 | 10.4 | July 22, 2013 | |||||||
| 4.2 | Form of promissory note warrants. | 001-36030 | 4.2 | |||||||||
| 4.3 | Form of credit facility warrants. | 001-36030 | 4.3 | |||||||||
| 10.1† | Marrone Bio Innovations, Inc. Stock Option Plan and related documents. | S-1 | 333-189753 | 10.1 | July 1, 2013 | |||||||
| 10.2† | Marrone Bio Innovations, Inc. 2011 Stock Plan and related documents. | S-1 | 333-189753 | 10.2 | July 1, 2013 | |||||||
| 10.3† | Marrone Bio Innovations, Inc. 2013 Stock Incentive Plan and related documents. | S-1/A | 333-189753 | 10.3 | July 22, 2013 | |||||||
| 10.4† | Indemnification Agreement by and between Marrone Bio Innovations, Inc. and each of its directors and executive officers. | S-1/A | 333-189753 | 10.4 | July 22, 2013 | |||||||
| 10.5† | Offer letter, dated June 29, 2006, between Marrone Organic Innovations, Inc. and Dr. Pamela G. Marrone. | S-1 | 333-189753 | 10.5 | July 1, 2013 | |||||||
| 10.6† | Offer letter, dated February 26, 2014, between Marrone Bio Innovations, Inc. and Linda V. Moore. | X | ||||||||||
| 10.7† | Letter Agreement, dated March 3, 2015, between Marrone Bio Innovations, Inc. and Linda V. Moore. | X | ||||||||||
| 10.8† | Offer letter, dated February 10, 2014, between Marrone Bio Innovations, Inc. and James B. Boyd. | 10-K | 001-36030 | 10.8 | March 25, 2014 | |||||||
| 10.9† | Letter Agreement, dated March 3, 2015, between Marrone Bio Innovations, Inc. and James B. Boyd. | X | ||||||||||
194
Table of Contents
| INCORPORATED BY REFERENCE | FILED HEREWITH | |||||||||||
| EXHIBIT |
EXHIBIT DESCRIPTION |
FORM |
FILE NO. |
EXHIBIT |
FILING DATE |
|||||||
| 10.10 | Office Lease, dated September 9, 2013, by and between Bio Innovations, Inc. and Six Davis, LLC. | 10-Q | 001-36030 | 10.1 | September 13, 2013 | |||||||
| 10.11 | First Amendment to Lease, dated April 30, 2014, by and between Marrone Bio Innovations, Inc. and Six Davis, LLC. | 10-Q | 001-36030 | 10.3 | May 15, 2014 | |||||||
| 10.12 | Office Lease, dated April 30, 2014, by and between Marrone Bio Innovations, Inc. and Seven Davis, LLC. | 10-Q | 001-36030 | 10.4 | May 15, 2014 | |||||||
| 10.13 | Business Loan Agreement, dated June 13, 2014, by and between Five Star Bank and jointly and severally Marrone Michigan Manufacturing LLC and Marrone Bio Innovations, Inc. | 10-Q | 001-36030 | 10.4 | August 13, 2014 | |||||||
| 10.14 | Convertible Note Purchase Agreement, dated March 15, 2012, by and among Marrone Bio Innovations, Inc. and the Investors party thereto, including form of convertible promissory note. | S-1 | 333-189753 | 10.15 | July 1, 2013 | |||||||
| 10.15 | Amendment and Consent, dated August 30, 2012, by and among Marrone Bio Innovations, Inc. and the Investors party thereto, including form of convertible promissory note. | S-1 | 333-189753 | 10.16 | July 1, 2013 | |||||||
| 10.16 | Loan Agreement, dated October 2, 2012, by and among Marrone Bio Innovations, Inc., the Investors party thereto and the administrative and collateral agent, including form of promissory note and warrant. | S-1 | 333-189753 | 10.17 | July 1, 2013 | |||||||
| 10.17 | Security Agreement, dated October 2, 2012, by and among Marrone Bio Innovations, Inc. and the administrative and collateral agent. | S-1 | 333-189753 | 10.18 | July 1, 2013 | |||||||
195
Table of Contents
| INCORPORATED BY REFERENCE | FILED HEREWITH | |||||||||||
| EXHIBIT |
EXHIBIT DESCRIPTION |
FORM |
FILE NO. |
EXHIBIT |
FILING DATE |
|||||||
| 10.18 | Loan Agreement, dated October 16, 2012, by and among Marrone Bio Innovations, Inc., the Investor party thereto and the administrative and collateral agent, including form of convertible promissory note. | S-1 | 333-189753 | 10.19 | July 1, 2013 | |||||||
| 10.19 | Security Agreement, dated October 16, 2012, by and among Marrone Bio Innovations, Inc. and the administrative and collateral agent. | S-1 | 333-189753 | 10.20 | July 1, 2013 | |||||||
| 10.20 | Note Purchase Agreement, dated December 6, 2012, by and between Marrone Bio Innovations, Inc. and Syngenta Ventures Pte. Ltd., including convertible promissory note. | S-1 | 333-189753 | 10.21 | July 1, 2013 | |||||||
| 10.21 | Intercreditor Agreement, dated December 6, 2012, by and among Marrone Bio Innovations, Inc., Syngenta Ventures Pte. Ltd. and the administrative agent and collateral agent. | S-1 | 333-189753 | 10.22 | July 1, 2013 | |||||||
| 10.22 | Amendment and Consent, dated April 10, 2013, by and among Marrone Bio Innovations, Inc. and the administrative agent party thereto. | S-1 | 333-189753 | 10.23 | July 1, 2013 | |||||||
| 10.23 | Amendment and Consent, dated April 10, 2013, by and among Marrone Bio Innovations, Inc. and the administrative agent party thereto. | S-1 | 333-189753 | 10.23 | July 1, 2013 | |||||||
| 10.24 | Credit Facility Agreement, dated June 14, 2013, by and among Marrone Bio Innovations, Inc. and the Investors party thereto, including form of promissory note and warrant. | S-1 | 333-189753 | 10.33 | July 1, 2013 | |||||||
| 10.25†† | License Agreement, dated May 22, 2007, between the KHH Biosci, Inc. and Marrone Organic Innovations, Inc. | S-1/A | 333-189753 | 10.24 | July 31, 2013 | |||||||
196
Table of Contents
| INCORPORATED BY REFERENCE | FILED HEREWITH | |||||||||||
| EXHIBIT |
EXHIBIT DESCRIPTION |
FORM |
FILE NO. |
EXHIBIT |
FILING DATE |
|||||||
| 10.26†† | License Agreement, dated November 13, 2007, between the U.S. Government, as represented by the U.S. Department of Agriculture, Agricultural Research Service, and Marrone Organic Innovations, Inc. | S-1 | 333-189753 | 10.25 | July 1, 2013 | |||||||
| 10.27†† | License Agreement, dated December 28, 2009, between the University of the State of New York and Marrone Bio Innovations, Inc. | S-1/A | 333-189753 | 10.26 | July 31, 2013 | |||||||
| 10.28†† | Commercial Agreement, dated February 1, 2011, between Syngenta Crop Protection AG and Marrone Bio Innovations, Inc. | S-1/A | 333-189753 | 10.27 | July 31, 2013 | |||||||
| 10.29†† | Commercial Agreement, dated August 26, 2011, between FMC Corporation and Marrone Bio Innovations, Inc. | S-1/A | 333-189753 | 10.28 | July 31, 2013 | |||||||
| 10.30†† | Technology Evaluation and Master Development Agreement, dated September 13, 2011, between The Scotts Company LLC and Marrone Bio Innovations, Inc. | S-1/A | 333-189753 | 10.29 | July 31, 2013 | |||||||
| 10.31 | Asset Purchase Agreement, dated May 25, 2012, between Bankruptcy Trustee for Michigan BioDiesel, LLC and Marrone Bio Innovations, Inc. | S-1 | 333-189753 | 10.30 | July 1, 2013 | |||||||
| 21.1 | Subsidiary List of Marrone Bio Innovations, Inc. | S-1/A | 333-189753 | 21.1 | July 22, 2013 | |||||||
| 23.1 | Consent of Ernst & Young LLP, Independent Registered Public Accounting Firm. | X | ||||||||||
| 24.1 | Power of Attorney (included on signature page). | X | ||||||||||
| 31.1 | Certification of Principal Executive Officer Required Under Rule 13a-14(a) and 15d-14(a) of the Securities Exchange Act of 1934, as amended. | X | ||||||||||
197
Table of Contents
| INCORPORATED BY REFERENCE | FILED HEREWITH | |||||||||||
| EXHIBIT |
EXHIBIT DESCRIPTION |
FORM |
FILE NO. |
EXHIBIT |
FILING DATE |
|||||||
| 31.2 | Certification of Principal Financial Officer Required Under Rule 13a-14(a) and 15d-14(a) of the Securities Exchange Act of 1934, as amended. | X | ||||||||||
| 32.1 | Certification of Principal Executive Officer and Principal Financial Officer Required Under Rule 13a-14(b) of the Securities Exchange Act of 1934, as amended, and 18 U.S.C. §1350 | X | ||||||||||
| 101 | Interactive Data Files Pursuant to Rule 405 of Regulation S-T: (i) Consolidated Balance Sheets as of December 31, 2013 and 2012; (ii) Consolidated Statements of Operations for the Years ended December 31, 2013, 2012 and 2011; (iii) Consolidated Statements of Comprehensive Loss for the Years ended December 31, 2013, 2012 and 2011; (iv) Consolidated Statements of Convertible Preferred Stock and Stockholders’ Equity (Deficit) as of December 31, 2013, 2012 and 2011; (v) Consolidated Statements of Cash Flows for the Years ended December 31, 2013, 2012 and 2011 and (vi) Notes to Consolidated Financial Statements | X | ||||||||||
| † | Indicates a management contract or compensatory plan or arrangement. |
| †† | Confidential portions of this document have been redacted and filed separately with the Securities and Exchange Commission. |
198
