Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Fortress Biotech, Inc. | v422405_8k.htm |
Exhibit 99.1

Avenue Therapeutics, Inc. Corporate Overview October 2015

Forward Looking Statements Statements in this presentation that are not descriptions of historical facts are forward - looking statements within the meaning of the “ safe harbor ” provisions of the Private Securities Litigation Reform Act of 1995. We have attempted to identify forward - looking statements by terminology including “ anticipates, ” “ believes, ” “ can, ” “ continue, ” “ could, ” “ estimates, ” “ expects, ” “ intends, ” “ may, ” “ plans, ” “ potential, ” “ predicts, ” “ should, ” or “ will ” or the negative of these terms or other comparable terminology. Forward - looking statements are based on management ’ s current expectations and are subject to risks and uncertainties that could negatively affect our business, operating results, financial condition and stock price. Factors that could cause actual results to differ materially from those currently anticipated are risks relating to: our growth strategy; results of research and development activities; uncertainties relating to preclinical and clinical testing; our dependence on third party suppliers; our ability to obtain, perform under and maintain financing and strategic agreements and relationships; our ability to attract, integrate, and retain key personnel; the early stage of products under development; our need for substantial funds; government regulation; patent and intellectual property matters; competition; as well as other risks described in our SEC filings. We expressly disclaim any obligation or undertaking to update or revise any statements contained herein to reflect any change in our expectations or any changes in events, conditions or circumstances after the date of this presentation. 2

Business Overview x We seek to in - license or acquire, develop and commercialize pharmaceutical products for the acute/intensive care hospital setting x Our lead asset is intravenous (IV) of Tramadol, in - licensed from Revogenex Ireland Ltd. x We have an experienced management team and advisors x We are a subsidiary of Fortress Biotech 3

Executive Team x Lindsay A. Rosenwald, MD, Executive Chairman ▪ Chairman and CEO of Fortress Biotech, Inc. ▪ Prolific and successful investor in the life sciences industry for over 20 years previously as Chairman of Paramount BioCapital ▪ Co - founder and Co - Portfolio Manager of Opus Point Partners x Lucy Lu, MD, President & Chief Executive Officer ▪ EVP and CFO of Fortress Biotech, Inc. ▪ Former Senior Analyst at Citi Investment Research ▪ 15 years of experience in life sciences x David Horin, Interim Chief Financial Officer ▪ Managing Partner, Chord Advisors, LLC. ▪ Former Chief Financial Officer of Rodman & Renshaw Capital Group, Inc. ▪ Former Managing Director of Accounting Policy and Financial Reporting at Jefferies Group, Inc. 4

Clinical Advisors x Scott Reines , MD - PhD ▪ Former Vice President/Senior Vice President at Merck Research Laboratories and Johnson & Johnson Pharmaceutical R&D x Robert Dworkin, PhD ▪ Director of the Analgesic, Anesthetic, and Addiction Clinical Trial Translations, Innovations, Opportunities, and Networks (ACTTION) x Harold Minkowitz , MD ▪ Assistant Professor of Anesthesiology at the University of Texas Health Science Center at Houston x Neil Singla , MD ▪ Founder and Chief Scientific Officer, Lotus Clinical Research x Mark Wallace, MD ▪ Director, Center for Pain Medicine, UC San Diego 5
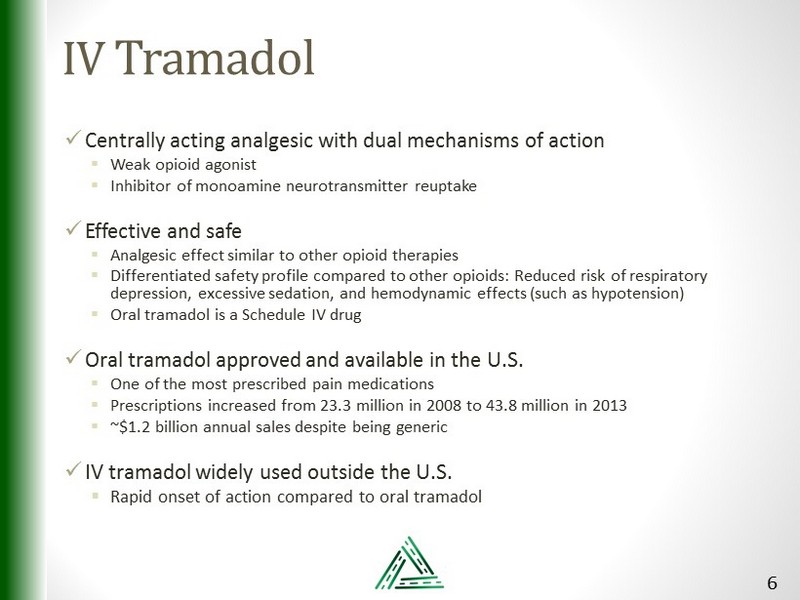
IV Tramadol x Centrally acting analgesic with dual mechanisms of action ▪ Weak opioid agonist ▪ Inhibitor of monoamine neurotransmitter reuptake x Effective and safe ▪ Analgesic effect similar to other opioid therapies ▪ Differentiated safety profile compared to other opioids: Reduced risk of respiratory depression, excessive sedation, and hemodynamic effects (such as hypotension ) ▪ Oral tramadol is a Schedule IV drug x Oral tramadol approved and available in the U.S. ▪ O ne of the most prescribed pain medications ▪ Prescriptions increased from 23.3 million in 2008 to 43.8 million in 2013 ▪ ~$1.2 billion annual sales despite being generic x IV tramadol widely used outside the U.S. ▪ Rapid onset of action compared to oral tramadol 6
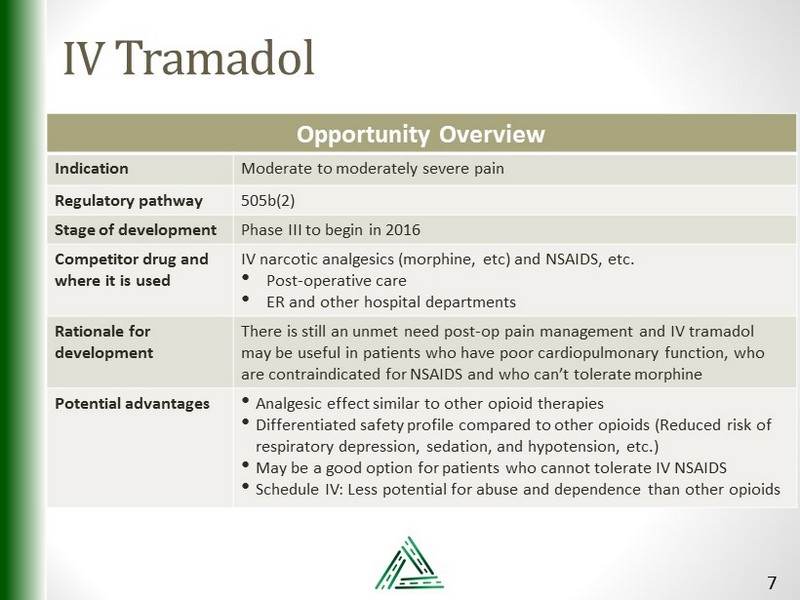
IV Tramadol Opportunity Overview Indication Moderate to moderately severe pain Regulatory pathway 505b(2) Stage of development Phase III to begin in 2016 Competitor drug and where it is used IV narcotic analgesics (morphine, etc ) and NSAIDS, etc. • Post - operative care • ER and other hospital departments Rationale for development There is still an unmet need post - op pain management and IV tramadol may be useful in patients who have poor cardiopulmonary function, who are contraindicated for NSAIDS and who can’t tolerate morphine Potential advantages • Analgesic effect similar to other opioid therapies • Differentiated safety profile compared to other opioids (Reduced risk of respiratory depression, sedation, and hypotension, etc.) • May be a good option for patients who cannot tolerate IV NSAIDS • Schedule IV: Less potential for abuse and dependence than other opioids 7
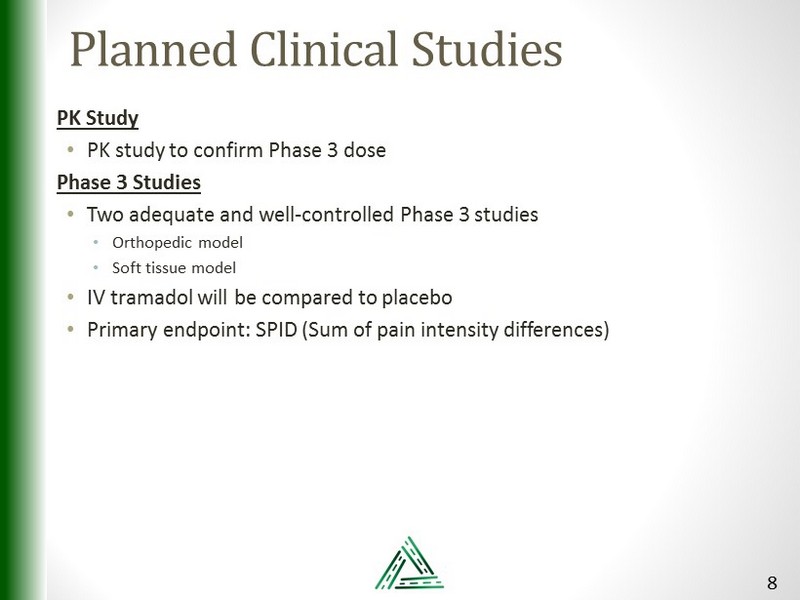
Planned Clinical Studies PK Study • PK study to confirm Phase 3 dose Phase 3 Studies • Two adequate and well - controlled Phase 3 studies • Orthopedic model • Soft tissue model • IV tramadol will be compared to placebo • Primary endpoint: SPID (Sum of pain intensity differences) 8
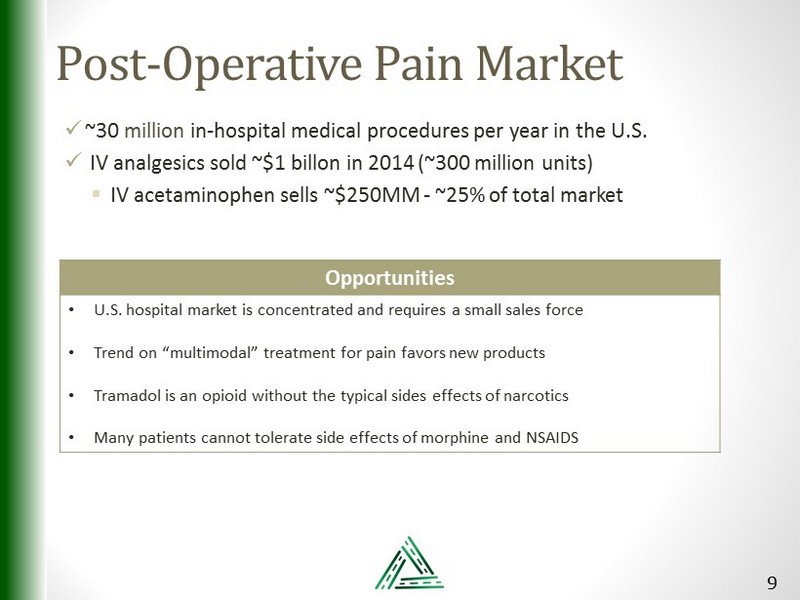
Post - Operative Pain Market x ~30 million in - hospital medical procedures per year in the U.S. x IV analgesics sold ~$1 billon in 2014 (~300 million units) ▪ IV acetaminophen sells ~$250MM - ~25% of total market Opportunities • U.S. hospital market is concentrated and requires a small sales force • Trend on “multimodal” treatment for pain favors new products • Tramadol is an opioid without the typical sides effects of narcotics • Many patients cannot tolerate side effects of morphine and NSAIDS 9

Competitive Landscape x Acetaminophen & NSAIDS ▪ Lack potency and may not be effective as monotherapy for pain management after major surgery ▪ E nhance quality of analgesia in conjunction with opioids ▪ Side Effects (NSAIDS) : bleeding , GI and renal impairment, etc. x Opioids ▪ Inexpensive and standard of care in many post - op settings ▪ Can be used in conjunction with other agents ▪ Side Effects : sedation , dizziness, nausea, vomiting, constipation (high incidence), physical dependence, and respiratory depression, etc. IV Tramadol Opportunity: Patients with poor cardiopulmonary function (including the elderly), patients contraindicated for NSAIDS and patients who cannot tolerate narcotics such as morphine 10
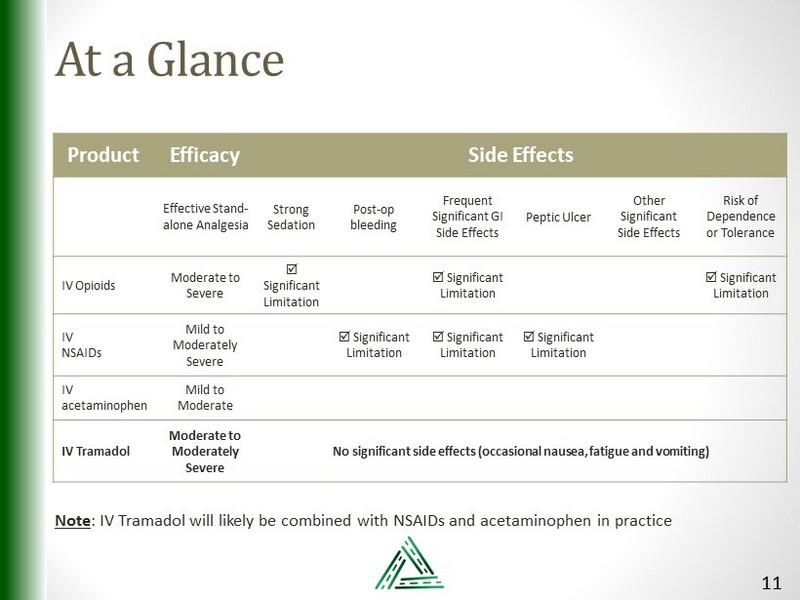
At a Glance Product Efficacy Side Effects Effective Stand - alone Analgesia Strong Sedation Post - op bleeding Frequent Significant GI Side Effects Peptic Ulcer Other Significant Side Effects Risk of Dependence or Tolerance IV Opioids Moderate to Severe Significant Limitation Significant Limitation Significant Limitation IV NSAIDs Mild to Moderately Severe Significant Limitation Significant Limitation Significant Limitation IV acetaminophen Mild to Moderate IV Tramadol Moderate to Moderately Severe No significant side effects (occasional nausea, fatigue and vomiting) Note : IV Tramadol will likely be combined with NSAIDs and acetaminophen in practice 11
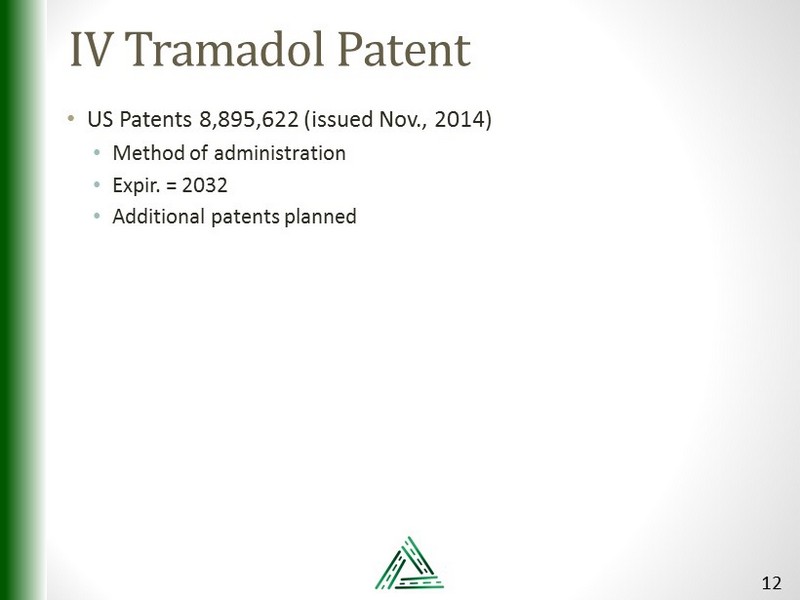
IV Tramadol Patent • US Patents 8,895,622 (issued Nov., 2014) • Method of administration • Expir. = 2032 • Additional patents planned 12

Upcoming Milestones x Commence PK study in 1Q2016 x Commence Phase 3 program in 2016 x Phase 3 Data 2017 x ~$20 million to complete Phase 3 Program 13

Avenue Therapeutics, Inc. Contact: Lucy Lu, M.D. 3 Columbus Circle, 15 th Floor New York, NY 10019 Tel: 781 - 652 - 4511 Email: llu@fortressbiotech.com 14
