Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - ZIOPHARM ONCOLOGY INC | d67763d8k.htm |
 Wells
Fargo Healthcare Conference
SEPTEMBER 2015 Exhibit 99.1 |
 2 Forward-looking Statements This presentation contains certain
forward-looking information about ZIOPHARM Oncology,
Inc. that is intended to be
covered by the safe harbor for "forward-looking statements" provided by the
Private Securities Litigation Reform Act of 1995, as amended.
Forward-looking statements are statements that are not historical facts, and in some cases can be identified by terms such as "may," "will," "could," "expects," "plans," "anticipates," and "believes." These
statements include, but are not limited to, statements regarding the
progress, timing and results of preclinical and clinical trials involving the Company's drug candidates, and the progress of the Company's research and development programs. All of such statements are subject to certain risks and
uncertainties, many of which are difficult to predict and generally beyond the control
of the Company, that could cause actual results to differ materially from
those expressed in, or implied by, the forward-looking statements. These risks and uncertainties include, but are not limited to: whether chimeric antigen receptor T cell (CAR T) approaches, Ad-RTS-IL-12, TCR and NK
cell- based therapies, or any of our other therapeutic candidates will
advance further in the pre-clinical or clinical trials process and
whether and when, if at all, they will receive final approval from the U.S. Food and
Drug Administration or equivalent foreign regulatory agencies and for
which indications; whether chimeric antigen receptor T cell (CAR T) approaches, Ad-RTS-IL-12, TCR and NK cell-based therapies, and our other therapeutic products will be successfully marketed if approved; the strength and
enforceability of our intellectual property rights; competition from other
pharmaceutical and biotechnology companies; and the other risk factors
contained in our periodic and interim SEC reports filed from time to time with the Securities and Exchange Commission, including but not limited to, our Annual Report on Form 10-K for the fiscal year ended December 31, 2014, and our
Quarterly Report on Form 10Q for the quarter ended June 30, 2015. Readers are cautioned
not to place undue reliance on these forward-looking statements that
speak only as of the date hereof, and we do not undertake any obligation to revise and disseminate forward-looking statements to reflect events or circumstances after the date hereof, or to reflect the occurrence of or
non-occurrence of any events.
|
 3 • NASDAQ: ZIOP • Robust synthetic immunology pipeline Virotherapy & adoptive cell therapy Multiple partnerships • Differentiated technology platforms Gene switch, non-viral integration and viral-based delivery • Multiple trial launches in 2015/2016 • Well capitalized Cash and equivalents of $176.1 million (Pro forma) Sufficient to fund our planned operations into Q1 2018 Highlights |
 4 Delivering on the Premise and Promise of Gene and Immune-based Therapies Fully Aligned with research and development (Intrexon) Big pharma partnership (CARs) Bio-processing for next-generation products Aligned with academic centers for manufacturing of cells for investigator-initiated trials Contract manufacturing for ZIOP- sponsored trials in place Established suite of correlative studies Undertaking multi-center gene therapy trials Multi-faceted approach to gene therapy (non-viral and viral) Ability to manipulate the immune response in situ (generate immune response within patient) Ability to provide an immune response (infuse immune cells) Ability to manipulate virus after infusion Ability to manipulate immune cells after infusion Immune cells targeting tumor through CAR, TCR, and NK Infuse patient-derived immune cells Infuse 3 rd party (allogeneic) immune cells |
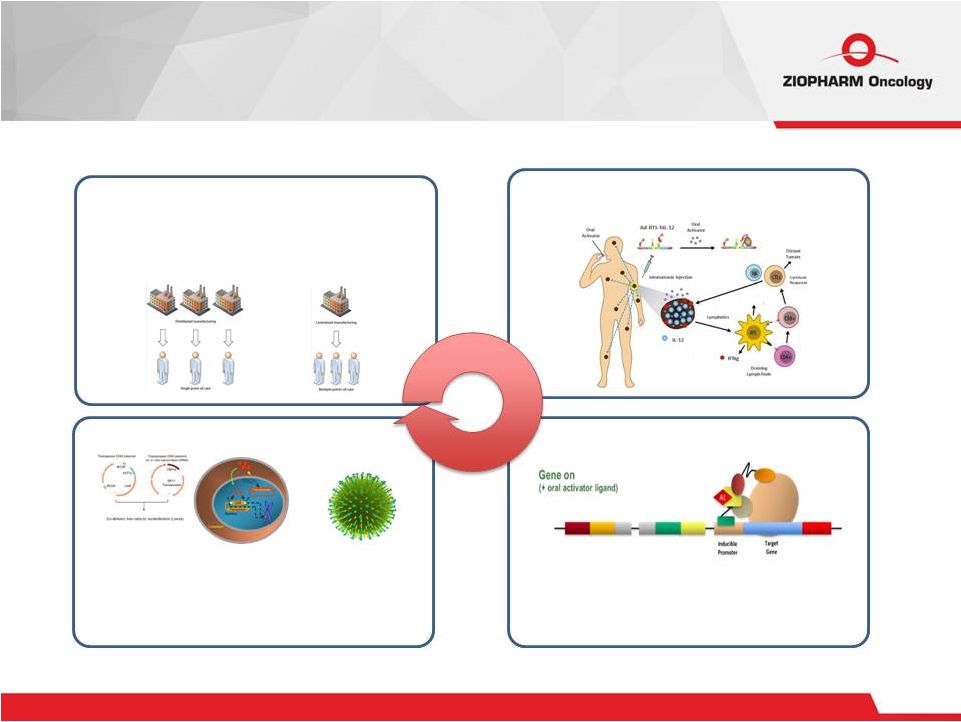 Virotherapy RTS ® Gene Switches Viral & Non-viral Gene Integration Cell Therapy CAR-T, TCR and NK Cells Autologous and Allogeneic Our Approach 5 |
 IL-12 Virotherapy Adenoviral vector engineered to express IL-12 utilizing RheoSwitch Therapeutic System ® (RTS ® ) gene switch Injected intra-tumor with IL-12 expression controlled via administration of oral activator ligand veledimex Regulated intra-tumoral expression of IL-12 promotes activation of tumor-infiltrating lymphocytes to drive cytotoxic immune response against distant tumors 6 |
 Improved persistence and
survival in tumor microenvironment Limits on-target, off-tumor toxicities Administer virus locally to achieve systemic anti-tumor effects (Breast) Administer virus locally to achieve local anti-tumor effects (GBM) RTS® Gene Switch Technology 7 |
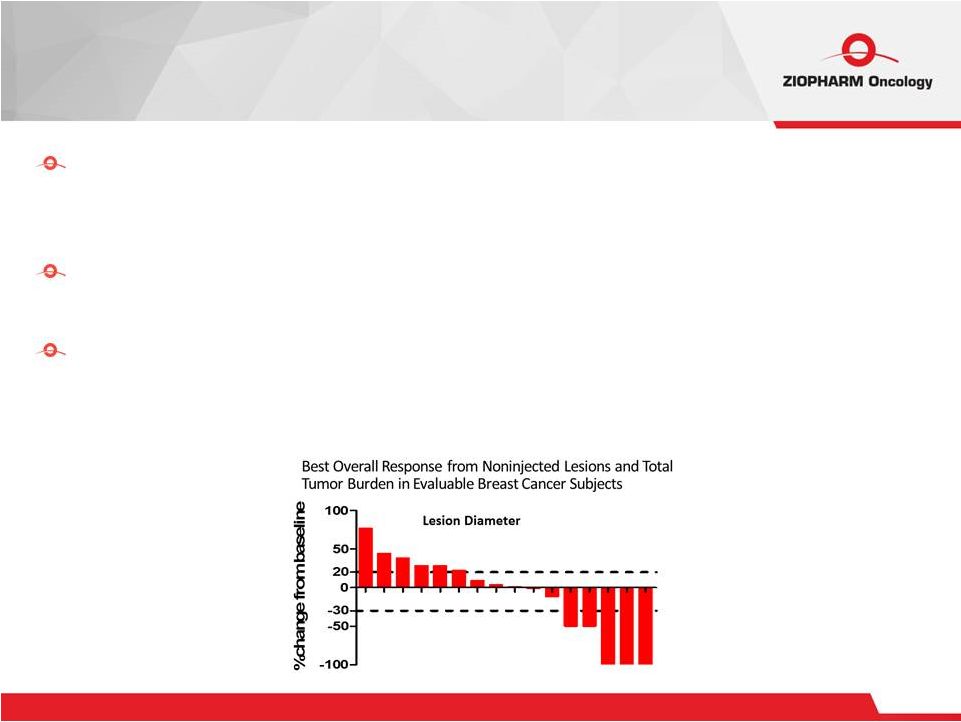 Expression of functional IL-12 in human subjects by direct intra-tumoral
injection of Ad-RTS-hIL-12 + oral veledimex generates
downstream IFNg production and rapid systemic elevation of IL-10 and
IP-10 Documented systemic immune activation and clinical effect in advanced, heavily pretreated melanoma and breast cancer patients Adverse event profile, including cytokine release syndrome, of Ad-RTS-hIL-12
+ veledimex is predictable, controllable, and fully and rapidly reversible on
stopping veledimex. Several subjects then restarted on veledimex Ad-RTS-IL-12 Gene Therapy Clinical Response To Date 8 |
 Early
Response Data Expected in 4Q 2015 Phase 1 Study of IL-12 Gene Therapy
in Recurrent or Progressive Glioblastoma/Grade III Malignant
Glioma A Multi-Center Gene Therapy Study
A single cycle of Ad-RTS-hIL-12 +
escalating veledimex
1O : Safety and tolerability
2O : Determine MTD and immune response, including ORR, PFS and OS N = 72 Recurrent or progressive glioblastoma or Grade III malignant glioma Stratified according to clinical indication for tumor resection: Resection plus injection vs. stereotactic injection alone 9 |
 Immunotherapy phase of treatment
A single cycle of Ad-RTS-hIL-12 +
veledimex goal of maintaining or improving pre- study response 1 O : Safety and tolerability 2 O : ORR, disease control and biomarkers N = 40 locally advanced or metastatic breast cancer of all subtypes up to 20% (8 subjects) with HER2+ breast cancer Response (PR or SD) to first- or second-line standard therapy Suspend chemotherapy phase of
treatment (HER2 therapy permitted) Phase 1b/2 Study of IL-12 Gene Therapy in Locally Advanced/Metastatic Breast Cancer Early Response Data Expected in 4Q 2015 10 |
 11 Pipeline Demonstrated the ability of Veledimex to cross blood-brain barrier in animal models (AACR 2014) Proof-of-concept in humans being explored in ongoing multi-center gene therapy trial Combination therapies Veledimex for activation of genetically modified T cells in brain |
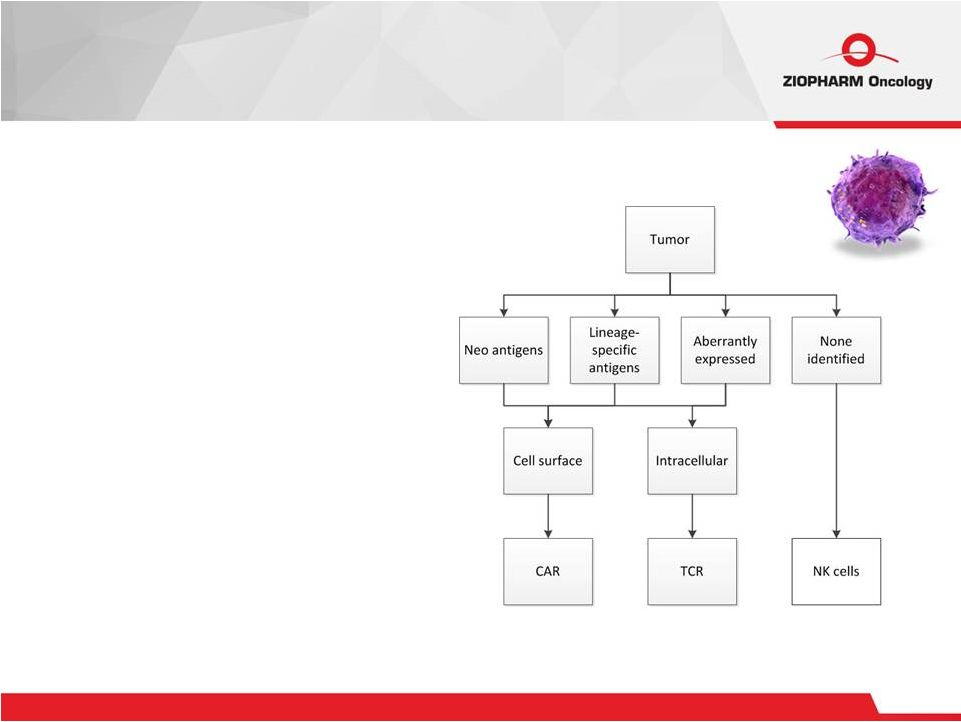 12 CAR + T cells • Target cell surface tumor- associated antigens (TAAs) independent of HLA • “Public” cell surface antigens TCR + T cells • Target intracellular TAAs dependent on HLA • “Private” intra-cellular antigens NK cells • Target tumor with loss of HLA • No “antigens” Cell Therapy |
 13 Sleeping Beauty (SB)
Non-viral Gene Integration Step 1 (safety) Step 2 (efficacy) Step 3 (scale) |
 14 Pre-emptive donor lymphocyte Infusion with CD19- directed CAR + T cells infused after autologous hematopoietic stem-cell transplantation (HSCT) Population 7 patients with advanced non-Hodgkin lymphoma (NHL) Safety Patients have not demonstrated any acute or late toxicity to CAR + T-cell infusions Efficacy 100% of patients remain alive following a single CAR + T-cell infusion with a median follow-up of 24 months 86% of patients (n=6) remain alive and in complete remission (CR) Trial at MDACC; Will be submitted for publication |
 15 Pre-emptive donor lymphocyte infusion with CD19- directed, CAR + T cells infused after allogeneic HSCT *Wilhelm et al. J Oncol Pharm Practice, 2014, 20:257 Population 19 patients with advanced CD19 + ALL (n=17) or NHL (n=2) Safety 3 patients developed GVHD which is lower than the expected range after allogeneic HSCT alone Efficacy 58% of patients (n=11/19) remain in complete remission (CR) and 10/19 remain alive and in CR (1 death in CR) following CAR + T-cell infusion with a median follow-up of 6.5 months post transplant among survivors. Among 8 patients who received haplo-HCST and CAR, 100% of remain alive and 75% remain in remission Rate of CMV reactivation after CAR + T-cell administration was 24% vs. 41% previously reported for our patients without infusion Trial at MDACC, Updated from EHA 2015; Will be submitted for publication |
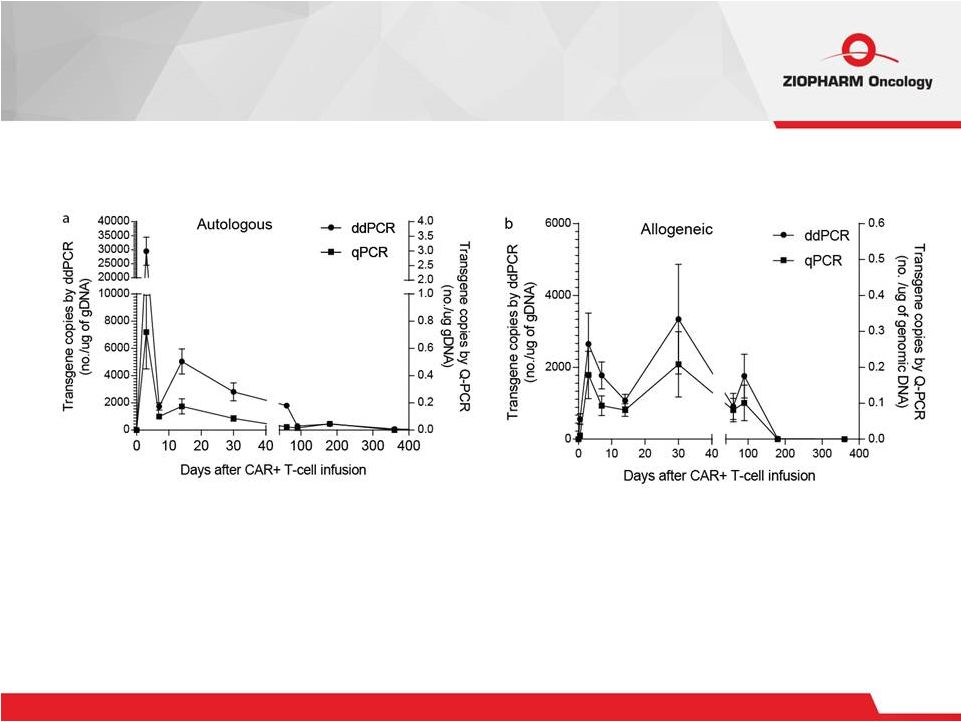 16 Persistence of Infused CAR + T Cells Trial at MDACC, Updated from EHA 2015; Will be submitted for
publication |
 17 Improving Therapeutic Potential of CAR + T Cells Signals 1 & 2 Signal 3 |
 18 1. New CAR designs for patients with hematologic malignancies and solid tumors 2. Combining CAR with cytokines – IL-12 – IL-15 • TCR-based T-cell therapy • NK-cell immunotherapy Next-generation Adoptive Cell-based Clinical Trials |
 19 How do we achieve on-target anti-cancer activity? The Promise and Challenges of DNA-based Immuno-Oncology Avoid |
 20 Strategies to widen therapeutic index to Target Solid Tumors |
 21 Off-the-shelf T Cells |
 22 Elimination of Endogenous TCR |
 23 Fitting Together the Pieces of the Puzzle NK cells T cells TCRs CARs Virotherapy Cytokines Remote Controlled |
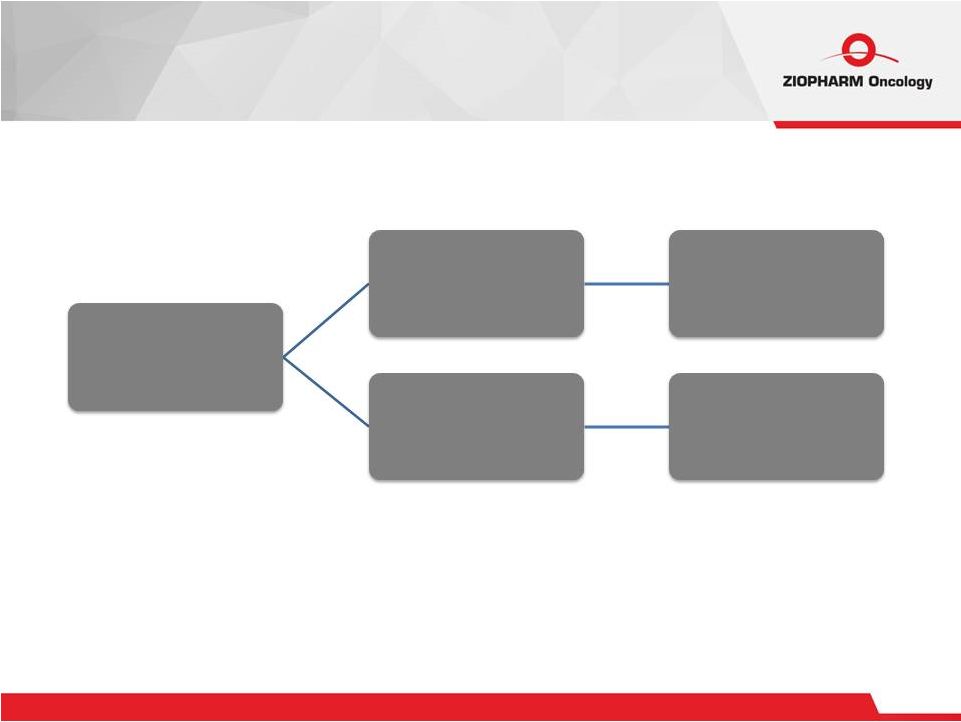 24 Clinical Programs Ziopharm 2015 Ad-RTS-IL12 Multicenter CARs MDACC |
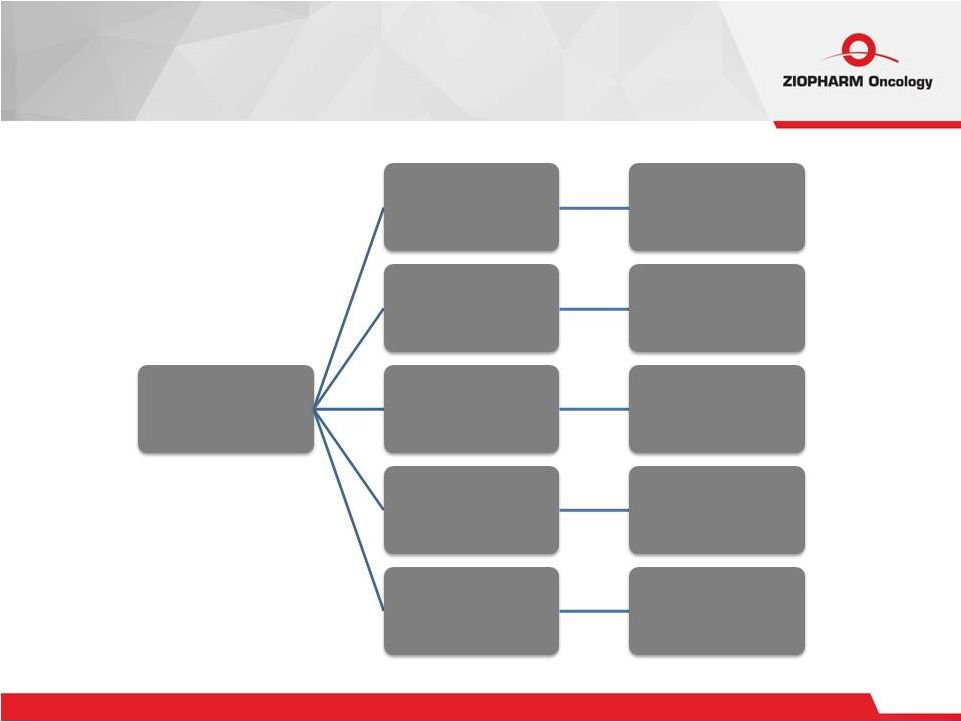 25 Clinical Programs Ziopharm 2016+ Ad-RTS-IL12 CARs and interleukin Multicenter NK cells TCRs CARs Multicenter MDACC MDACC Multicenter |
 26 Abstracts • European Hematology Association (June
2015) –
Donor-derived, CD19-directed, CAR-modified T cells infused after
allogeneic hematopoietic stem-cell transplantation as pre-
emptive donor lymphocyte infusion in patients with CD19+ malignances
• CRI-CIMT-EATI-AACR
(September 2015) – Demonstration of systemic antitumor immunity via intratumoral regulated expression of IL-12 in advanced breast cancer and
melanoma patients – Demonstration of systemic antitumor immunity via intratumoral regulated expression of IL-12 as a gene therapy approach to
treatment of cancer – Bio-engineered Dectin-1 CAR+ T cells to control invasive fungal infection
– Ex-vivo generation of clinical grade T cells by using activating and propagating feeder cells to cross-link T cell receptor
– Designing CARs for personalized immunotherapy: rapid assembly of CARs from principal components using “EZ-CAR”
platform –
Transcriptional and epigenetic signatures of ex vivo propagated three distinct TCR V 1, TCR V 2 and TCR V 3 cell subtypes with broad specificity for malignancies Papers – Sleeping Beauty Transposition of Chimeric Antigen Receptors Targeting Receptor Tyrosine Kinase-Like Orphan Receptor-1
(ROR1) into Diverse Memory T-Cell Populations. PLoS
One. 2015 Jun 1;10(6):e0128151 PMID: 26030772
– Genetic Engineering of T Cells to Target HERV-K, an Ancient Retrovirus on Melanoma. Clin
Cancer Res. 2015 Mar 31. [Epub
ahead of print] PMID: 25829402
– Moving from tinkering in the garage to assembly line production: the manufacture of genetically modified T cells expressing
chimeric antigen receptors (CARs) comes on line. Cancer Gene Ther. 2015
Mar;22(2):64-6. PMID: 25675874 –
Manufacture of T cells using the Sleeping Beauty system to enforce expression of a
CD19-specific chimeric antigen receptor. Cancer Gene Ther. 2015
Mar;22(2):95-100. PMID: 25591810 –
Tuning Sensitivity of CAR to EGFR Density Limits Recognition of Normal Tissue While
Maintaining Potent Antitumor Activity. Cancer Res. 2015 Sep
1;75(17):3505-18. PMID: 26330164 Extensive Data
Outreach… |
 27 CRI-CIMT-EATI-AACR Immunotherapy Conference September
16-19, NY, NY SITC November 4-8, National
Harbor, MD SNO November 19-23, San Antonio, TX CAR-T Summit November
12-13, Boston, MA ASH December 5-8, Orlando FL SABCS December 9-12, San Antonio TX Additional papers …With More to Come for 2015 |
 28 ZIOPHARM Oncology Pipeline Solid Tumors 2016+ Heme 2015+ Virotherapy Multi- center trials 2015+ Multi Modal 2016+ Expanded Indications 2016+ Single Agent 2015+ CAR Cytokine 2016+ Adoptive Cell Therapy TCR 2016+ CAR 2015+ NK cells 2016+ T cells 2015+ Allogeneic 2016+ Autologous 2015+ |
 Wells
Fargo Healthcare Conference
SEPTEMBER 2015 |
