Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Tonix Pharmaceuticals Holding Corp. | v420039_8k.htm |
Exhibit 99.01

NASDAQ: TNXP Investor Presentation September 2015 © 2015 Tonix Pharmaceuticals Holding Corp.

2 Cautionary note on forward - looking statements Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995 . These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others . These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially . There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements . These factors include, but are not limited to, substantial competition ; our possible need for additional financing ; uncertainties of patent protection and litigation ; uncertainties of government or third party payor reimbursement ; limited research and development efforts and dependence upon third parties ; and risks related to failure to obtain U . S Food and Drug Administration clearances or approvals and noncompliance with its regulations . As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products . The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by the Company on its website or otherwise . Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law . Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31 , 2014 and Quarterly Report on Form 10 - Q for the period ended June 30 , 2015 , as filed with the Securities and Exchange Commission (the “SEC”) on February 27 , 2015 and August 7 , 2015 , respectively, and future periodic reports filed with the SEC on or after the date hereof . All of the Company's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements .

3 Developing innovative medicines for large and growing markets Common and chronic disorders of the central nervous system (CNS) Late - stage candidates supported by human experience Capitalized to achieve key readouts in all of our clinical - stage programs 2016 to feature Phase 3 and Phase 2 clinical trial results

4 Candidate Indication Near - term Catalyst Tonmya Fibromyalgia Top line data 2H 2016 TNX - 102 SL Post - Traumatic Stress Disorder Top line data 1H 2016 TNX - 201 Episodic Tension - Type Headache Top line data 1Q 2016 Pipeline led by Tonmya™ for fibromyalgia Preclinical Phase 1 Phase 2 Phase 3 NDA Market NDA = New Drug Application Tonmya / T NX - 102 SL (cyclobenzaprine HCl sublingual tablets, 2.8 mg) and TNX - 201 ( dexisometheptene mucate ) are Investigational New Drugs and are not approved for any indication.

5 Fibromyalgia: a chronic, multi - symptom disorder that generates frustration for patients and physicians Fibromyalgia is characterized by : Believed to result from amplified sensory and pain signaling in CNS 1 Causes significant impairment in all areas of life - Lower levels of health - related quality - of - life – reduced daily functioning - Interference with work (loss of productivity, disability ) Inflicts substantial strain on the healthcare system - Average patient has 20 physician office visits per year 2 - Annual direct medical costs are twice those for non - fibromyalgia individuals 3 1 Phillips K & Clauw DJ, Best Pract Res Clin Rheumatol 2011;25:141. 2 Robinson et al, Pain Medicine 2013;14:1400. 3 White et al, J Occupational Environ Med 2008;50:13. – C hronic widespread pain – Unrefreshing sleep – Fatigue – D iminished cognition
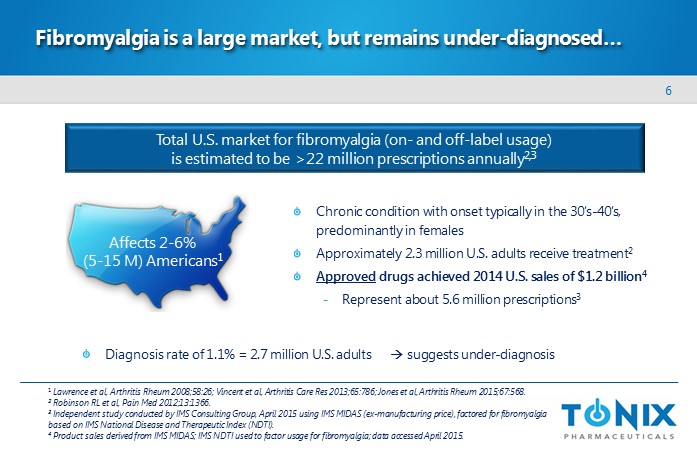
6 Fibromyalgia is a large market, but remains under - diagnosed… 1 Lawrence et al, Arthritis Rheum 2008;58:26; Vincent et al, Arthritis Care Res 2013;65:786; Jones et al, Arthritis Rheum 2015; 67 :568. 2 Robinson RL et al, Pain Med 2012;13:1366. 3 Independent study conducted by IMS Consulting Group, April 2015 using IMS MIDAS ( ex - manufacturing price), factored for fibromyalgia based on IMS National Disease and Therapeutic Index (NDTI). 4 Product sales derived from IMS MIDAS; IMS NDTI used to factor usage for fibromyalgia; data accessed April 2015. Affects 2 - 6% ( 5 - 15 M) Americans 1 Chronic condition with onset typically in the 30’s - 40’s, predominantly in females Approximately 2.3 million U.S. adults receive treatment 2 Approved drugs achieved 2014 U.S. sales of $1.2 billion 4 - Represent about 5.6 million prescriptions 3 Total U.S. market for fibromyalgia (on - and off - label usage ) is estimated to be >22 million prescriptions annually 2,3 Diagnosis rate of 1.1% = 2.7 million U.S. adults suggests under - diagnosis
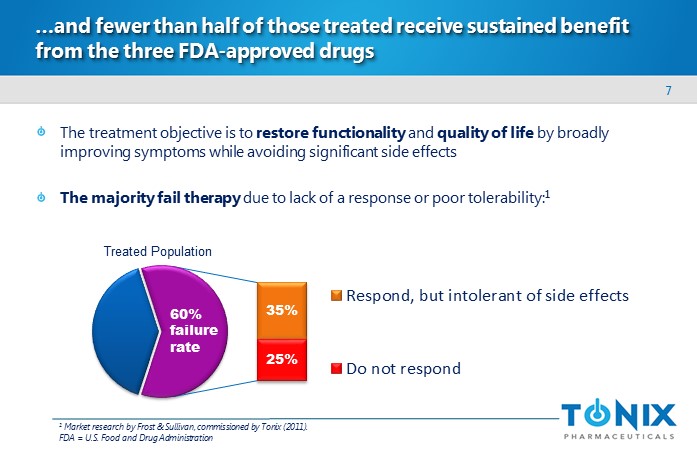
7 …and fewer than half of those treated receive sustained benefit from the three FDA - approved drugs The treatment objective is to restore functionality and quality of life by broadly improving symptoms while avoiding significant side effects The majority fail therapy due to lack of a response or poor tolerability: 1 Respond, but intolerant of side effects Do not respond 25% 35% 60% failure rate 1 Market research by Frost & Sullivan, commissioned by Tonix (2011). FDA = U.S. Food and Drug Administration T reated P opulation

8 Pervasive treatment dissatisfaction creates an opportunity for a differentiated therapeutic option High rates of discontinuation, switching and augmentation - Patients cycle through different medications - A ttempt to treat multiple symptoms and/or avoid intolerable side effects - Two or more medications are used simultaneously, on average 1 - The typical patient has tried six different medications 2 Significant off - label use of prescription painkillers and sleep aids Large need for new therapies that provide broad symptom relief without a significant side effect burden 1 Robinson RL et al, Pain Medicine 2012;13:1366. 2 “Patient Trends: Fibromyalgia”, Decision Resources, 2011.

9 Tonmya in Phase 3 clinical development for fibromyalgia Advanced sublingual tablet containing cyclobenzaprine (CBP) 2.8 mg - Eutectic formulation rapidly delivers a low dose of CBP, avoids first - pass metabolism - Designed for chronic bedtime administration, no titration Tonmya demonstrated broad activity and was very well - tolerated in Phase 2b study - Statistically - significant improvements across core fibromyalgia symptoms - Positive trend in primary endpoint of change in mean pain intensity - Systemic tolerability similar to placebo - Transient administration site reactions were more common with Tonmya, no impact on completion rate Tonix approaches the treatment of fibromyalgia by targeting sleep quality - Non - restorative sleep is a common clinical and diagnostic feature 1 - Evolving understanding of the role of sleep in pain control and fibromyalgia development 2 - Tonmya targets CNS receptors believed to play key roles in sleep physiology 1 Swick TJ, Ther Adv Musculoskel Dis 2011;3:167 - 178 . 2 Choy EHS, Nat Rev Rheumatol adv online pub 28 April 2015. Tonmya is an Investigational New Drug and is not approved for any indication.
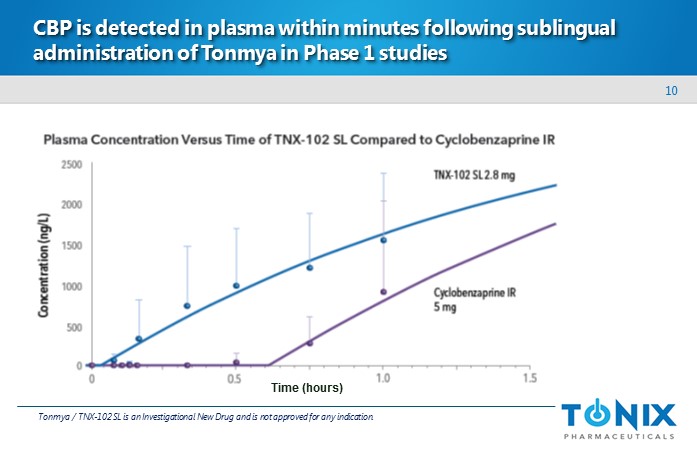
10 CBP is detected in plasma within minutes following sublingual administration of Tonmya in Phase 1 studies Tonmya / TNX - 102 SL is an Investigational New Drug and is not approved for any indication. Time (hours)
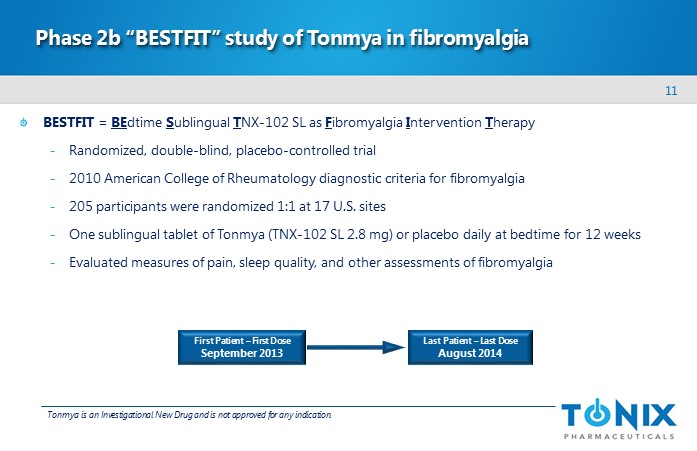
11 Phase 2b “BESTFIT” study of Tonmya in fibromyalgia BESTFIT = BE dtime S ublingual T NX - 102 SL as F ibromyalgia I ntervention T herapy - Randomized , double - blind, placebo - controlled trial - 2010 American College of Rheumatology diagnostic criteria for fibromyalgia - 205 participants were randomized 1:1 at 17 U.S. sites - One sublingual tablet of Tonmya (TNX - 102 SL 2.8 mg) or placebo daily at bedtime for 12 weeks - Evaluated measures of pain, sleep quality, and other assessments of fibromyalgia First Patient – First Dose September 2013 Last Patient – Last Dose August 2014 Tonmya is an Investigational New Drug and is not approved for any indication.

12 BESTFIT: Tonmya broadly improved fibromyalgia Category Endpoint – week 12 1 p value Pain 30% responder analysis 2 0.033 Sleep Daily Sleep Quality PROMIS Sleep Disturbance <0.001 0.005 Overal l response to therapy PGIC 0.025 Assessment of disease impact FIQ - R Total score 0.014 1 Intent - to - treat analysis, N=205 (Tonmya N=103, placebo N=102) 2 FDA - accepted primary endpoint in current Phase 3 AFFIRM study Source : Phase 2b BESTFIT study data. Tonmya is an Investigational New Drug and is not approved for any indication. BESTFIT pre - specified primary endpoint: change in week 12 mean pain score (p=0.172) PROMIS = Patient - Reported Outcomes Measurement Information System PGIC = Patient Global Impression of Change FIQ - R = Fibromyalgia Impact Questionnaire - Revised p < 0.05 s tatistically significant
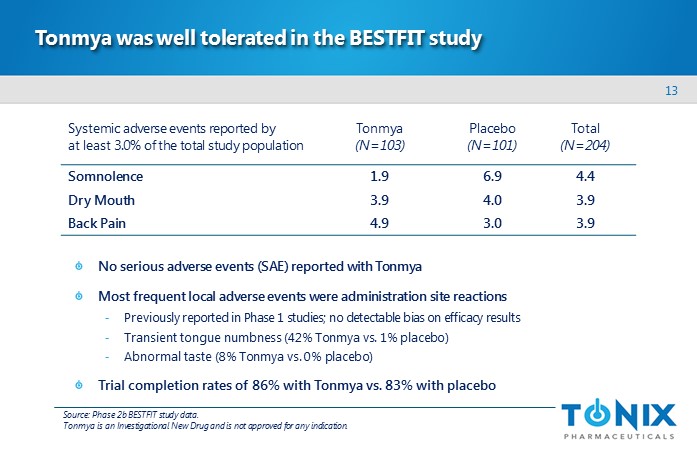
13 Tonmya was well tolerated in the BESTFIT study No serious adverse events (SAE) reported with Tonmya Most frequent local adverse events were administration site reactions - Previously reported in Phase 1 studies; no detectable bias on efficacy results - Transient tongue numbness (42% Tonmya vs . 1% placebo) - Abnormal taste (8% Tonmya vs . 0% placebo) Trial completion rates of 86% with Tonmya vs . 83% with placebo Systemic adverse events reported by at least 3.0% of the total study population Tonmya (N=103) Placebo (N=101) Total (N=204) Somnolence 1.9 6.9 4.4 Dry Mouth 3.9 4.0 3.9 Back Pain 4.9 3.0 3.9 Source: Phase 2b BESTFIT study data. Tonmya is an Investigational New Drug and is not approved for any indication.
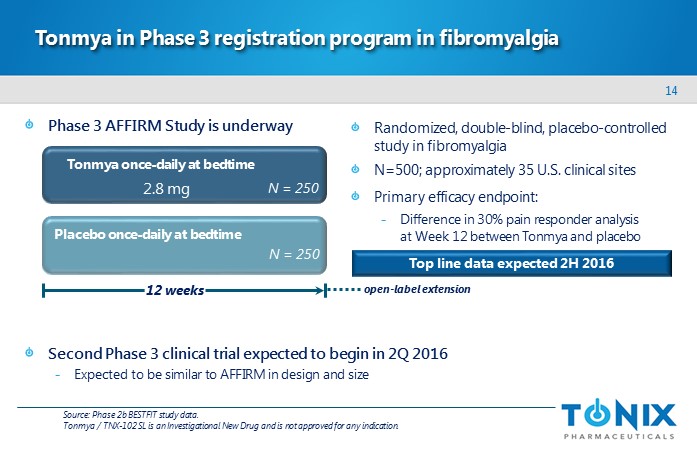
14 Phase 3 AFFIRM Study is underway Second Phase 3 clinical trial expected to begin in 2Q 2016 - E xpected to be similar to AFFIRM in design and size Tonmya in Phase 3 registration program in fibromyalgia Randomized, double - blind, placebo - controlled study in fibromyalgia N=500; approximately 35 U.S. clinical sites Primary efficacy endpoint: - Difference in 30% pain responder analysis at Week 12 between Tonmya and placebo Placebo once - daily at bedtime 12 weeks Tonmya once - daily at bedtime N = 250 N = 250 2.8 mg open - label extension Source: Phase 2b BESTFIT study data. Tonmya / TNX - 102 SL is an Investigational New Drug and is not approved for any indication. Top line data expected 2H 2016

15 Candidate Indication Near - term Catalyst TNX - 102 SL Post - Traumatic Stress Disorder Top line data 1H 2016 TNX - 102 SL in Phase 2 development for post - traumatic stress disorder (PTSD) Preclinical Phase 1 Phase 2 Phase 3 NDA Market Tonmya / T NX - 102 SL (cyclobenzaprine HCl sublingual tablet, 2.8 mg) and TNX - 201 ( dexisometheptene mucate ) are Investigational New Drugs and are not approved for any indication.

16 PTSD: An important and growing public health problem PTSD is a chronic disorder following a traumatic event and is characterized by: Considered a stress response, but prolonged and does not resolve with time - 20% of women and 8 % of men who experience significant trauma develop PTSD 1 Associated with significant life disruption - Social isolation, inability to maintain employment, loss of independent living - Unpredictable acts of violence, suicidal thoughts – re - experiencing the triggering event – negative alterations in mood/cognition – situation/stimulus avoidance – hypervigilance (anxiety, difficulty sleeping) 1 Kessler et al, Arch Gen Psychiatry 1995;52:1048.
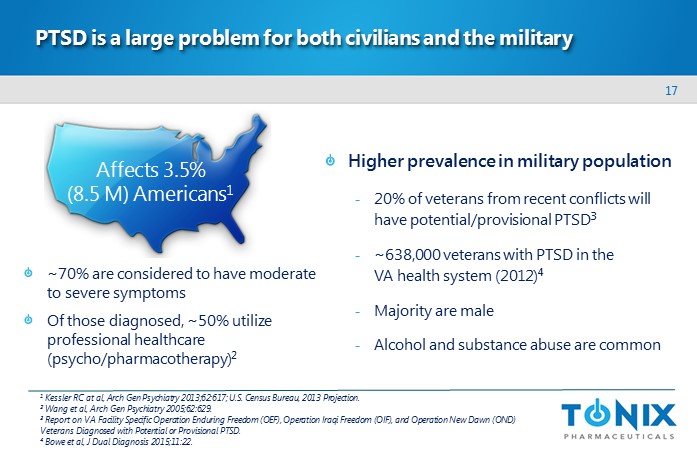
17 PTSD is a large problem for both civilians and the military ~70% are considered to have moderate to severe symptoms Of those diagnosed, ~50% utilize professional healthcare (psycho/pharmacotherapy) 2 1 Kessler RC at al, Arch Gen Psychiatry 2013;62:617; U.S. Census Bureau, 2013 Projection. 2 Wang et al, Arch Gen Psychiatry 2005;62:629. 3 Report on VA Facility Specific Operation Enduring Freedom (OEF), Operation Iraqi Freedom (OIF), and Operation New Dawn (OND) Veterans Diagnosed with Potential or Provisional PTSD. 4 Bowe et al, J Dual Diagnosis 2015;11:22. Affects 3.5% (8.5 M) Americans 1 Higher prevalence in military population - 20% of veterans from recent conflicts will have potential/provisional PTSD 3 - ~638,000 veterans with PTSD in the VA health system ( 2012) 4 - Majority are male - Alcohol and substance abuse are common

18 Significant gap in current therapeutic landscape for PTSD Medicines for PTSD often provide inadequate and/or inconsistent benefit - FDA - approved medications are limited to two SSRIs, approved >10 years ago - Weak evidence of treatment effect in men 1 - Lack of efficacy evidence in those with a history of combat - related trauma 2 - Carry suicidality warnings, require dose titration Sleep dysfunction in PTSD is resistant to currently - approved options - 95%+ report insomnia, 83% report recurrent dreams of the trauma 3 - Correlated with disease severity, depression, substance abuse and suicide 4 - Poor sleep quality after trauma may increase the risk of developing PTSD - Off - label use of anxiolytics, sedative - hypnotics, opiates, and antipsychotics SSRI = selective serotonin reuptake inhibitor 1 Marshall et al, Am J Psychiatry 2001;158:1982. 2 Jonathan Davidson, personal communications, 2014. 3 Green B. Post - traumatic stress disorder: Symptom profiles in men and women. Curr Med Res Opin 2003;19:200 – 4 . 4 Germain et al, J Anxiety Disord 2005;19:233; Krakow et al, J Nerv Ment Dis 2002;190:442.
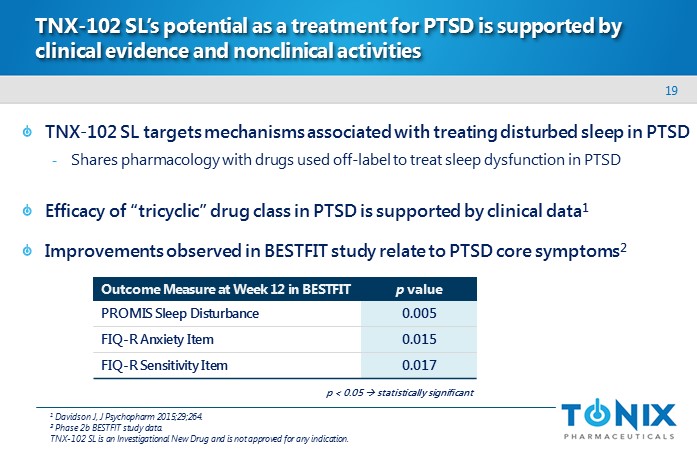
19 TNX - 102 SL’s potential as a treatment for PTSD is supported by clinical evidence and nonclinical activities TNX - 102 SL targets mechanisms associated with treating disturbed sleep in PTSD - Shares pharmacology with drugs used off - label to treat sleep dysfunction in PTSD Efficacy of “tricyclic” drug class in PTSD is supported by clinical data 1 Improvements observed in BESTFIT study relate to PTSD core symptoms 2 Outcome Measure at Week 12 in BESTFIT p value PROMIS Sleep Disturbance 0.005 FIQ - R Anxiety Item 0.015 FIQ - R Sensitivity Item 0.017 1 Davidson J, J Psychopharm 2015;29;264. 2 Phase 2b BESTFIT study data. TNX - 102 SL is an Investigational New Drug and is not approved for any indication . p < 0.05 s tatistically significant

20 Phase 2 “ AtEase ” trial of TNX - 102 SL in PTSD is ongoing Randomized, double - blind, placebo - controlled trial in military - related PTSD N=220; approximately 25 U.S. clinical sites Primary efficacy endpoint: - Difference in Clinician - Administered PTSD Scale (CAPS) score between TNX - 102 SL 2.8 mg and placebo TNX - 102 SL is an Investigational New Drug and is not approved for any indication . Top line data expected 1H 2016 TNX - 102 SL at bedtime once - daily Placebo at bedtime once - daily 12 weeks N = 88 TNX - 102 SL at bedtime once - daily N = 88 N = 44 2.8 mg 5.6 mg open - label extension www.ateasestudy.com

21 Candidate Indication Near - term Catalyst TNX - 201 Episodic Tension - Type Headache Top line data 1Q 2016 TNX - 201 in Phase 2 development for episodic tension - type headache Preclinical Phase 1 Phase 2 Phase 3 NDA Market Tonmya / T NX - 102 SL (cyclobenzaprine HCl sublingual tablet, 2.8 mg) and TNX - 201 ( dexisometheptene mucate ) are Investigational New Drugs and are not approved for any indication.

22 Episodic tension - type headache ( ETTH) – the most common form of headache 75 million adults in the U.S. experience ETTH each year 1 - Constant band of pressure on the back/sides of head; “squeezed in a vice” feeling - Most are infrequent (<1 per month) ; rarely require medical attention, mainly self - treated 21 million experience frequent ETTH each year 1 - Frequent = one to 14 headaches per month over a three - month period - More likely to seek physician care and receive a prescription product 1,2 ETTH is the major contributor to all ‘non - migraine headaches’ 1 - Non - migraine headaches lead to 9.2 million emergency room or office visits each year 3,4 1 Schwartz et al, JAMA 1998;279:381 - 383; Chowdhury, Ann Ind Acad Neurol 2012;15:83 - 88; Tonix analysis of public literature. 2 Scher et al, Cephalalgia 2010;30:321 - 328; Tonix analysis of public literature . 3 Health Care Utilization Project data, 2011. 4 IMS National Disease and Therapeutic Index™ 2013.

23 Current treatment market for non - migraine headaches (ETTH) “First - line” treatment - NSAIDs are most common - Many patients self - medicate with a course of over - the - counter NSAIDs before seeing a physician 1 - Less than 50% of treated patients are pain - free at two hours 2 - Some are refractory to NSAIDs when they first seek medical attention 1 “Next - step” treatment options for acute/abortive therapy are limited - Butalbital combinations are the only FDA - approved prescription products for ETTH - 3.5 million prescriptions are written per year for non - migraine headaches 1 - Not recommended for extended use because of addiction, tolerance and abuse potential 3 - Opioid narcotic combination products are used off - label to treat non - migraine headaches - Include products that contain hydrocodone, codeine, and tramadol 1 - ~5.3 million prescriptions issued annually for the treatment of non - migraine headaches 1 NSAID = non - steroidal anti - inflammatory drug 1 Based on independent study commissioned by Tonix , based on physician interviews using IMS National Prescription Audit (8/2013 – 7/142014) and IMS National Disease and Therapeutic Index™ Q3 2008 – Q3 2014. 2 Moore et al, Pain 2014;155:2220 - 2228. 3 Fioricet package insert .

24 Isometheptene was used for ETTH, but no longer approved Isometheptene – a brief history - Isometheptene mucate was commonly used in the past - Indicated for tension - type headache, but also used for migraine 1 - Single - agent medicine (pre - 1962) - Component of prescription combination drug products - Annual unit volume of 2.5 million prescriptions at peak (1997) - Midrin ® – NDA withdrawn (2011) - Prodrin ® – marketed under “unapproved drug category ” No product containing any form of isometheptene is FDA - approved for any indication 1 IMS Health, National Prescription Audit, 01/1995 – 12/2000 (extracted 8/2014); IMS Health, IMS National Disease and Therapeutic Index™, 01/1995 – 12/2000 (extracted 8/2014).

25 Scientific rationale for development of TNX - 201 TNX - 201 ( dexisometheptene mucate ) contains the (R) isomer of isometheptene - Pharmacologic profile is distinct from (S) isomer per preclinical studies - Represents a new class of analgesics (selective imidazoline - 1 receptor agonists) (R) isomer (S) isomer • Analgesic in two rat models of headache 1 • Binds to imidazoline - 1 receptor • Inactive on adrenergic receptors • Sympathomimetic x TNX - 201 1 Fried NT et al, Headache 2015;55:184 . TNX - 201 is an Investigational New Drug and is not approved for any indication.

26 Current value proposition for TNX - 201 Key attributes - Unique mechanism of action (imidazoline - 1 receptor agonist) - Differentiated pharmacology as a single isomer of isometheptene - May offer effective, safe, and non - addictive treatment option for ETTH May serve patients who: - Do not adequately respond to an NSAID - Would be candidates for treatment with butalbital and/or opioid narcotics - Suffer from medication overuse headaches - May be caused by NSAIDs, butalbital , or opioid narcotics TNX - 201 is an Investigational New Drug and is not approved for any indication .

27 Phase 2 proof - of - concept trial of TNX - 201 in ETTH is ongoing Randomized, double - blind, placebo - controlled trial in episodic tension - type headache N=200 enrollment goal at approximately 10 U.S . clinical sites Top line data expected 1Q 2016 Placebo TNX - 201 N = 100 N = 100 140 mg A proof - of - concept study to evaluate: - Proportion of subjects who report “pain free” at several intervals post - dose - Proportion of subjects who use rescue medication during the 24 hours post - dose - Change from baseline in pain severity score at several intervals post - dose Results will be used to support discussion with FDA on Phase 3 study design TNX - 201 is an Investigational New Drug and is not approved for any indication.

28 Intellectual property Composition - of - matter (eutectic) - Patents filed - Protection expected to 2034 Pharmacokinetics (PK) - Patents filed - Protection expected to 2033 Method - of - use - Fibromyalgia: patents issued, 2020 expiry - PTSD: patents filed Tonmya / TNX - 102 SL Fibromyalgia, PTSD Composition - of - matter (isomer) - Patents filed - Protection expected to 2033 TNX - 201 Headache Tonmya, TNX - 102 SL and TNX - 201 are Investigational New Drugs and are not approved for any indication. Wholly - owned by Tonix with no obligations to others

29 Financial overview NASDAQ: TNXP Cash reported at June 30, 2015 $ 48.7 million Net proceeds from July 2015 financing 18.7 million Cash used in operations in 1H 2015 $ 18.3 million Shares outstanding (September 9, 2015) 18.8 million

30 Management team Seth Lederman, MD President & CEO Leland Gershell, MD, PhD Chief Financial Officer Bruce Daugherty, PhD Chief Scientific Officer Ronald Notvest, PhD SVP, Commercial Planning & Development Gregory Sullivan, MD Chief Medical Officer

31 Board of directors Seth Lederman, MD Chairman Ernest Mario, PhD ALZA, Glaxo, Reliant Pharma John Rhodes NYSERDA, NRDC, Booz Allen Hamilton Samuel Saks, MD Jazz Pharma, ALZA, Johnson & Johnson Charles Mather BTIG, Janney, Jefferies, Cowen, Smith Barney Stuart Davidson Labrador Ventures, Alkermes , Combion Patrick Grace Apollo Philanthropy, WR Grace, Chemed Donald Landry, MD, PhD Chair of Medicine, Columbia University

32 Milestones – recent and upcoming Tonmya – Fibromyalgia □ May 2015 Began Phase 3 AFFIRM study □ June 2015 Presented additional data from Phase 2b BESTFIT study at EULAR □ 2H 2016 Report top - line results from AFFIRM study TNX - 102 SL – Post - Traumatic Stress Disorder □ January 2015 Began Phase 2 AtEase study in military - related PTSD □ 1H 2016 Report top - line results from AtEase study TNX - 201 – Episodic Tension - Type Headache □ June 2015 Began randomization in proof - of - concept Phase 2 study □ June 2015 Presented non - clinical data at AHS (receptor, animal models) □ 1Q 2016 Report top - line results from proof - of - concept Phase 2 study x x x Tonmya / TNX - 102 SL and TNX - 201 are Investigational New Drugs and are not approved for any indication. x x

33 Developing innovative medicines for large and growing markets Common and chronic disorders of the central nervous system (CNS) Late - stage candidates supported by human experience Capitalized to achieve key readouts in all of our clinical - stage programs 2016 to feature Phase 3 and Phase 2 clinical trial results

NASDAQ: TNXP 509 Madison Avenue New York, NY 10022 (212) 980 - 9155 www.tonixpharma.com
