Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Corium International, Inc. | a15-16423_18k.htm |
| EX-99.1 - EX-99.1 - Corium International, Inc. | a15-16423_1ex99d1.htm |
|
|
© 2015 Corium All Rights Reserved. Corium International, Inc. July 28, 2015 MicroCor hPTH(1-34) Phase 2a Interim Topline Results |
|
|
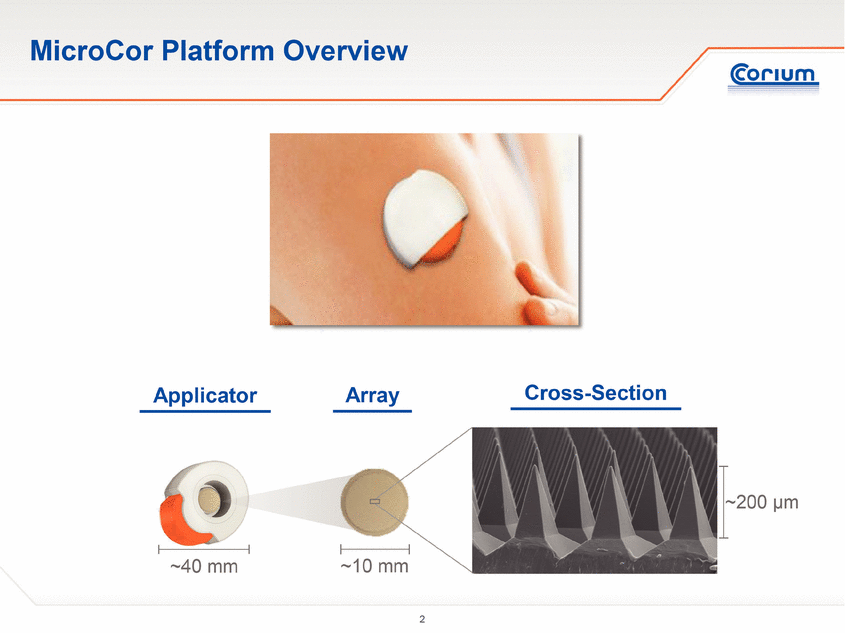
MicroCor Platform Overview ~10 mm ~200 µm ~40 mm Applicator Array Cross-Section |
|
|
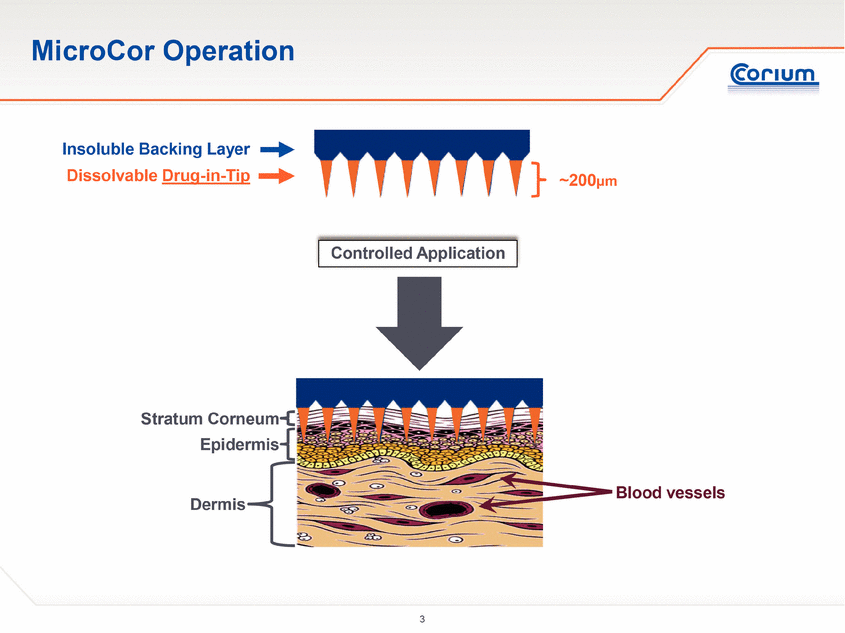
MicroCor Operation Dissolvable Drug-in-Tip Insoluble Backing Layer Controlled Application Stratum Corneum Dermis Blood vessels Epidermis ~200µm |
|
|
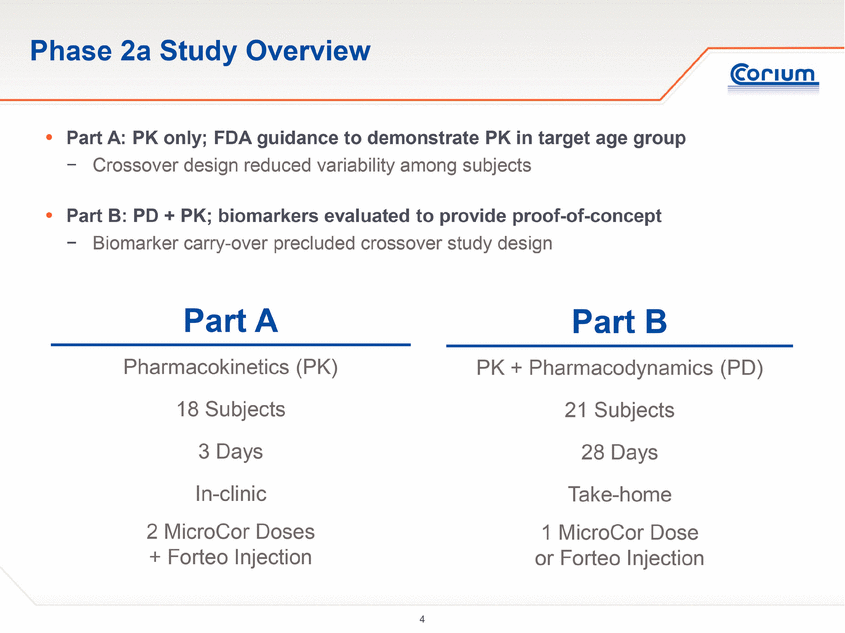
Phase 2a Study Overview Part A Pharmacokinetics (PK) 18 Subjects 3 Days In-clinic 2 MicroCor Doses + Forteo Injection Part B PK + Pharmacodynamics (PD) 21 Subjects 28 Days Take-home 1 MicroCor Dose or Forteo Injection . Part A: PK only; FDA guidance to demonstrate PK in target age group - Crossover design reduced variability among subjects . Part B: PD + PK; biomarkers evaluated to provide proof-of-concept - Biomarker carry-over precluded crossover study design |
|
|
Phase 2a—Part A Design (PK Only) . Single-dose, randomized, crossover study .18 healthy post-menopausal women (50-85 years of age) . Per FDA guidance, two MicroCor doses to establish dose proportionality: – 16 mcg targeted to achieve Cmaxcomparable to Forteo – 38 mcg targeted to achieve AUC* comparable to Forteo . Forteo20 mcg subcutaneous injection as comparator . Three consecutive days of treatment, each subject receiving all three treatments . Primary endpoint: compare MicroCor and Forteo PK . Secondary endpoints: safety, tolerability, and dose proportionality *AUC = Area Under the Curve, a measure of the total drug delivered to the bloodstream |
|
|
Phase 2a—Part B Design (PK/PD) . Multiple-dose, randomized, parallel groups .21 healthy post-menopausal women (50-85 years of age) . Two treatment groups: – 38 mcg MicroCor(n=15) – 20 mcg Forteosubcutaneous injection (n=6) . Each subject dosed once per day for 28 consecutive days . First 4 doses applied by clinic staff, remainder self-administered by subjects . Primary endpoint: compare MicroCor and Forteo PK at Days 1 and 28 . Secondary endpoints: safety, tolerability, and changes in bone biomarkers |
|
|
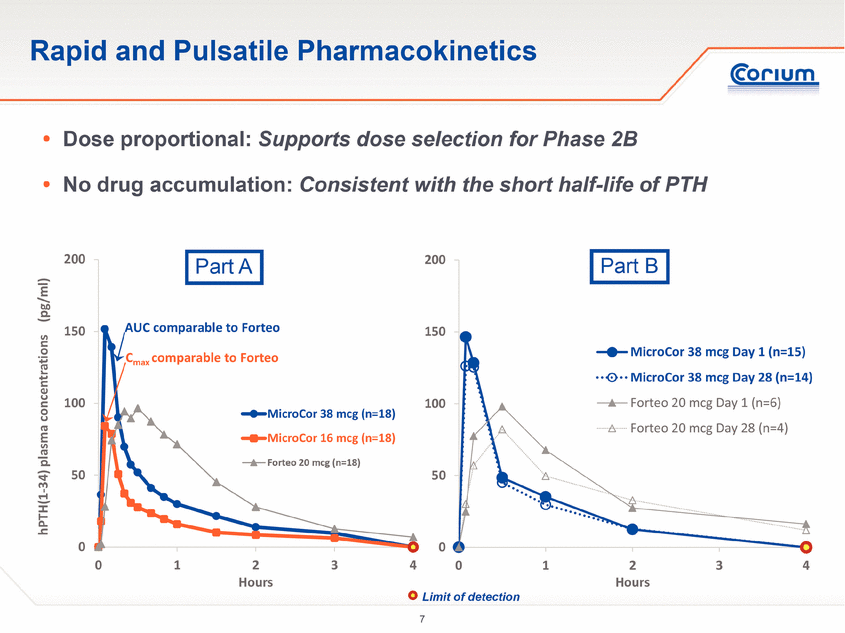
0 50 100 150 200 0 1 2 3 4 hPTH(1-34) plasma concentration (pg/ml) Hours MicroCor 38 mcg Day 1 (n=15) MicroCor 38 mcg Day 28 (n=14) Forteo 20 mcg Day 1 (n=6) Forteo 20 mcg Day 28 (n=4) Rapid and Pulsatile Pharmacokinetics . Dose proportional: Supports dose selection for Phase 2B . No drug accumulation: Consistent with the short half-life of PTH 0 50 100 150 200 0 1 2 3 4 hPTH(1-34) plasma concentrations (pg/ml) Hours MicroCor 38 mcg (n=18) MicroCor 16 mcg (n=18) Forteo 20 mcg (n=18) AUC comparable to Forteo Cmaxcomparable to Forteo Part A Part B Limit of detection |
|
|
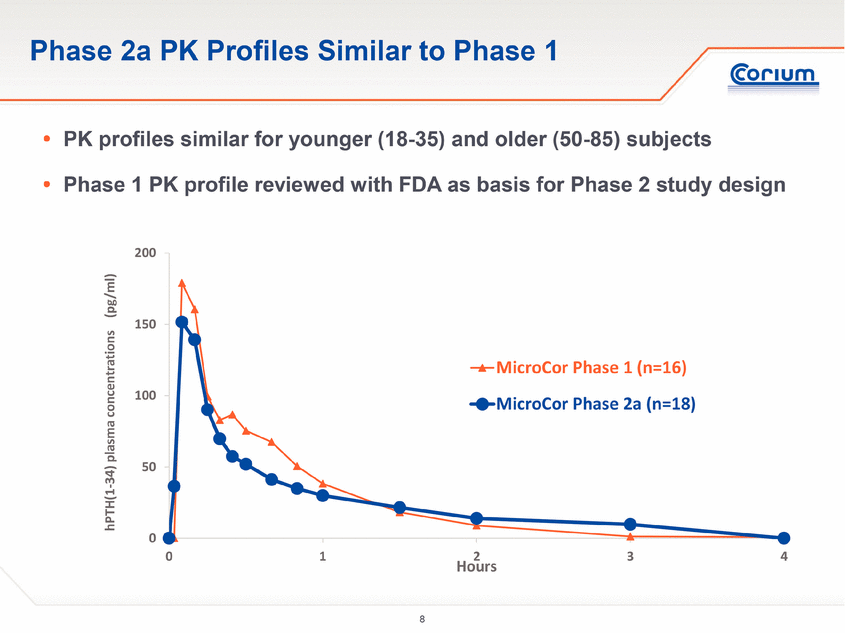
. PK profiles similar for younger (18-35) and older (50-85) subjects . Phase 1 PK profile reviewed with FDA as basis for Phase 2 study design Phase 2a PK Profiles Similar to Phase 1 0 50 100 150 200 0 1 2 3 4 hPTH(1-34) plasma concentrations (pg/ml) Hours MicroCor Phase 1 (n=16) MicroCor Phase 2a (n=18) |
|
|
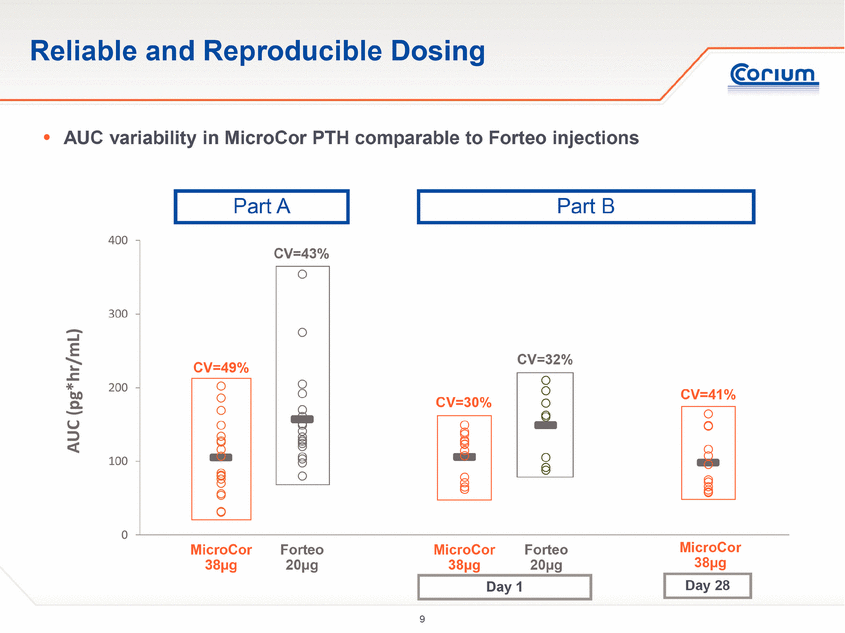
0 100 200 300 400 AUC (pg*hr/mL) Reliable and Reproducible Dosing CV=49% CV=30% . AUC variability in MicroCor PTH comparable to Forteoinjections MicroCor 38µg Forteo 20µg CV=43% MicroCor 38µg Forteo 20µg CV=32% Part A Part B CV=41% Day 1 Day 28 MicroCor 38µg |
|
|
Increase In Bone Formation Biomarker *P1NP – Procollagen Type 1 N-terminal propeptide 0 50 100 150 0 7 14 21 28 35 42 49 56 P1NP (% change from baseline) Days MicroCor 38 mcg (n=14) Forteo 20 mcg (n=4) Treatment stopped . Serum P1NP* levels indicative of bone formation activity . Increase in P1NP with MicroCor similar to Forteo during 28-day treatment . Marker is predictive of BMD response (primary endpointin Phase 2b/3) P1NP |
|
|
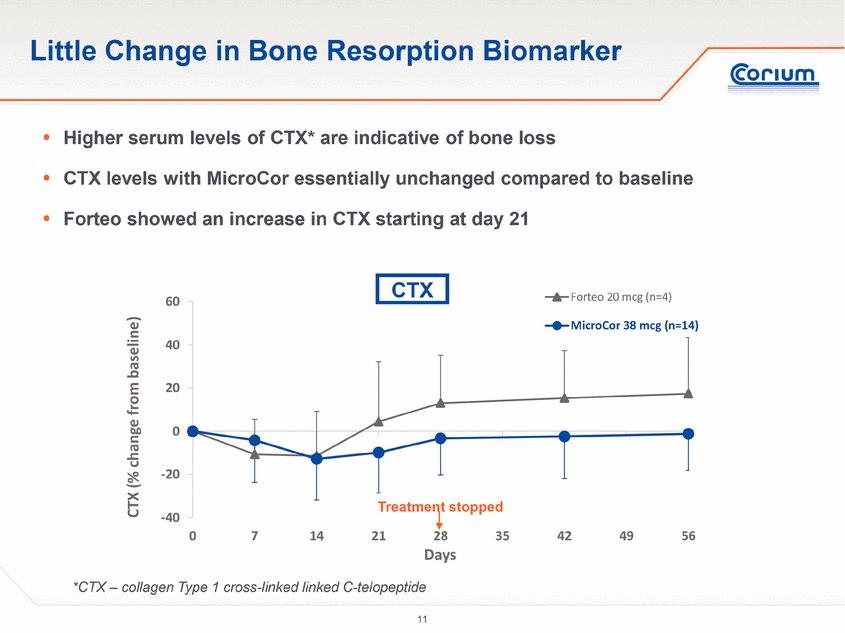
-40 -20 0 20 40 60 0 7 14 21 28 35 42 49 56 CTX (% change from baseline) Days Forteo 20 mcg (n=4) MicroCor 38 mcg (n=14) Little Change in Bone Resorption Biomarker *CTX – collagen Type 1 cross-linked linked C-telopeptide . Higher serum levels of CTX* are indicative of bone loss . CTX levels with MicroCor essentially unchanged compared to baseline . Forteo showed an increase in CTX starting at day 21 Treatment stopped CTX |
|
|
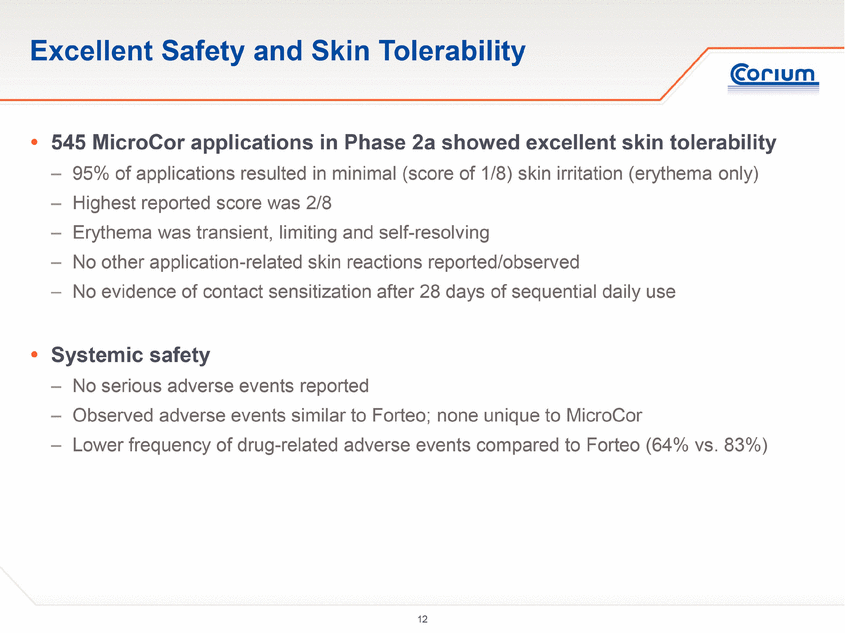
Excellent Safety and Skin Tolerability .545 MicroCor applications in Phase 2a showed excellent skin tolerability –95% of applications resulted in minimal (score of 1/8) skin irritation (erythema only) – Highest reported score was 2/8 – E rythema was transient, limiting and self-resolving – No other application-related skin reactions reported/observed – No evidence of contact sensitization after 28 days of sequential daily use . Systemic safety – No serious adverse events reported – Observed adverse events similar to Forteo; none unique to MicroCor – Lower frequency of drug-related adverse events compared to Forteo(64% vs. 83%) |
|
|
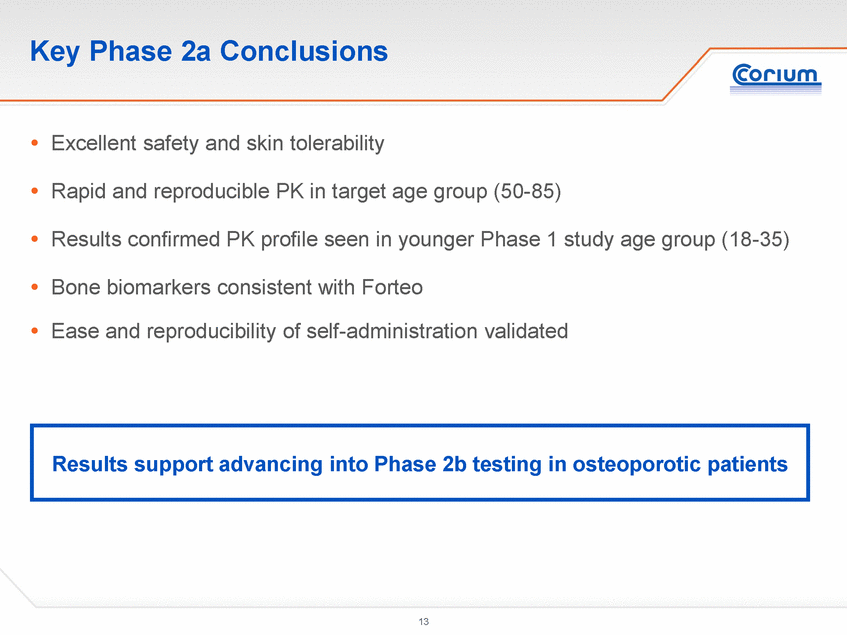
Key Phase 2a Conclusions .Excellent safety and skin tolerability .Rapid and reproducible PK in target age group (50-85) .Results confirmed PK profile seen in younger Phase 1 study age group (18-35) .Bone biomarkersconsistent with Forteo .Ease and reproducibility of self-administration validated Results support advancing into Phase 2b testing in osteoporotic patients |
|
|
Planned Phase 2b Trial Design .Efficacy andsafety study in 150-200 post-menopausal osteoporotic women .Three MicroCor doses with Forteo 20 mcg as active comparator .Six-month study duration with daily self-administration of treatments .Trial could commence in late CY 2016 .Partnering or co-development could affect timing Primary Endpoint: Changes in BMD at lumbar spine at 6 months Secondary Endpoints:Changes in BMD at hip, bone biomarkers, safety, and skin tolerability |
|
|
© 2015 Corium All Rights Reserved. Corium International, Inc. July 28, 2015 Nasdaq: CORI |