Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - ZIOPHARM ONCOLOGY INC | d944220d8k.htm |
 June 2015 www.ziopharm.com The Future of Cancer Therapy Exhibit 99.1 |
 This presentation contains certain forward-looking information about ZIOPHARM Oncology that is intended to be covered by the safe harbor for "forward-looking statements" provided by the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts. Words such as "expect(s)," "feel(s)," "believe(s)," "will," "may," "anticipate(s)" and similar expressions are intended to identify forward-looking statements. These statements include, but are not limited to, statements regarding our ability to successfully develop and commercialize our therapeutic products, our ability to expand our long-term business opportunities; financial projections and estimates and their underlying assumptions; and future performance. All of such statements are subject to certain risks and uncertainties, many of which are difficult to predict and generally beyond the control of the Company, that could cause actual results to differ materially from those expressed in, or implied or projected by, the forward-looking information and statements. These risks and uncertainties include, but are not limited to: whether any of our therapeutic candidates will advance further in the clinical trials process and whether and when, if at all, they will receive final approval from the U.S. Food and Drug Administration or equivalent foreign regulatory agencies and for which indications; whether any of our therapeutic candidates will be successfully marketed if approved; whether our DNA-based biotherapeutics discovery and development efforts will be successful; our ability to achieve the results contemplated by our collaboration agreements; the strength and enforceability of our intellectual property rights; competition from pharmaceutical and biotechnology companies; the development of and our ability to take advantage of the market for DNA-based biotherapeutics; our ability to raise additional capital to fund our operations on terms acceptable to us; general economic conditions; and the other risk factors contained in our periodic and interim reports filed with the SEC including, but not limited to, our annual report on Form 10-K for the fiscal year ended December 31, 2014 and other filings with the SEC, which are available at www.sec.gov. Our audience is cautioned not to place undue reliance on these forward-looking statements that speak only as of the date hereof, and we do not undertake any obligation to revise and disseminate forward-looking statements to reflect events or circumstances after the date hereof, or to reflect the occurrence of or non-occurrence of any events. 1 Forward-Looking Statements |
 Financial Highlights
NASDAQ symbol “ZIOP”
Approximately 128.2 million shares outstanding
Cash and cash equivalents of approximately $129.7 million
Cash resources sufficient to fund our operations into 2Q 2017
Excludes 2Q payment of $57.5 million from Intrexon related to the
biopharmaceutical business of Merck KGaA
agreement No debt * As of March 31, 2015 2 |
 Intrexon and ZIOPHARM Collaboration with the Biopharmaceutical Business of Merck KGaA Highlights Significant Value of CAR-T Platform 3 Exclusive strategic collaboration and license agreement to develop and commercialize
CAR-T cancer therapies
Exclusive access to CAR T suite of technologies, including MD Anderson
technologies Intrexon
and ZIOPHARM responsible for all platform and product developments until IND
filing Biopharmaceutical business of Merck KGaA
to nominate targets of interest, lead IND filing and pre-IND
interactions, clinical development and commercialization
Intrexon and ZIOPHARM retain ability to explore targets independently, granting Merck KGaA opt-in rights during clinical development Up to $941M for two targets recognizes value of CAR T programs and technology, de- risks portfolio and adds significant global development expertise Upfront, milestone and royalty payments divided evenly between ZIOPHARM and
Intrexon Upfront payment of $115 million For the first two targets, up to $826 million development, regulatory and commercial milestones ($413 million
per product)
Tiered royalties up to lower-double digits on net sales
Merck KGaA may elect additional targets at additional cost |
 Key Investment Highlights
Robust synthetic immunology pipeline targeting hematologic
malignancies and solid tumors
Non-viral and viral CAR-T, TCR, and NK-cell therapies
IL-12 gene therapy: adenoviral vector for IL-12
expression controlled via oral activator Differentiated technology
platforms In vivo control of gene expression by oral pill Non-viral: faster and less complex lower cost Dual approach: Point-of-Care (autologous) & Off-the-Shelf (allogeneic)
Agreement with biopharmaceutical business of Merck KGaA: to
elect initial 2 CAR-T targets
Multiple trial launches in 2015/2016 with potential data in Q4 2015
Well capitalized
4 |
 Milestones 2015 / 2016
Intra-tumoral IL-12 RheoSwitch
® Phase 1/2 Breast Cancer with SOC initiated Q2 2015 Phase 1 GBM initiated Q2 2015 – potential early data Q4 2015 CAR-T Initiate Phase 1 next-generation CD19 CAR trials Potential early data on Phase 1 CARs studies in Q4 2015 Initiate novel CAR for myeloid malignancies in late 2015/early 2016 Other Leukemia and Solid Tumor CAR-T trials in 2016 Allogenic, Off-the-Shelf T-Cell Studies in 2016 5 |
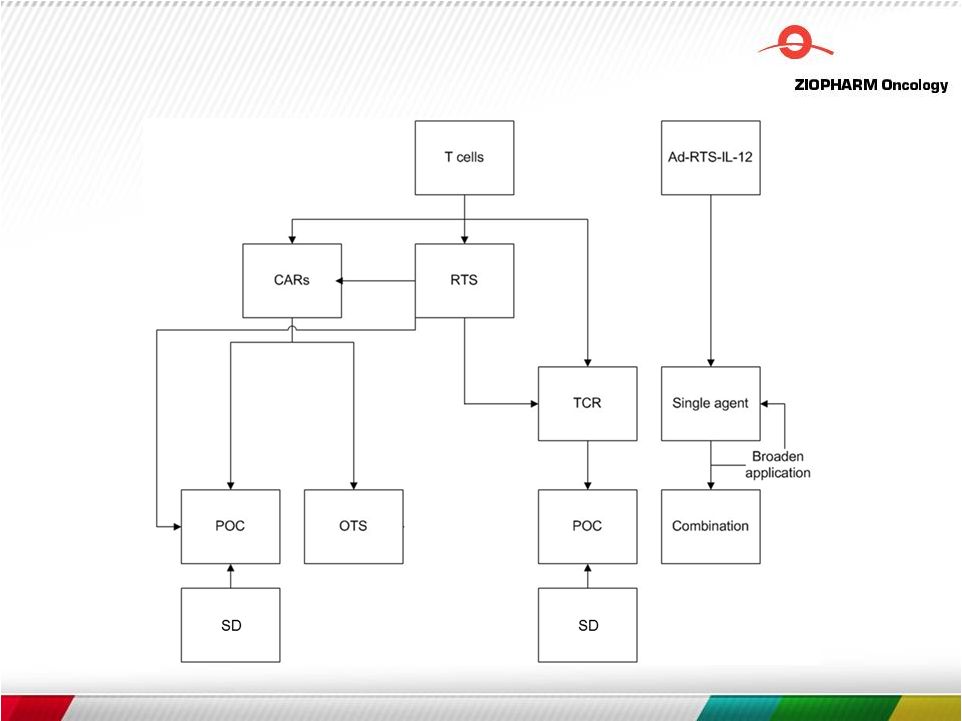 Overall strategy and differentiation
6 |
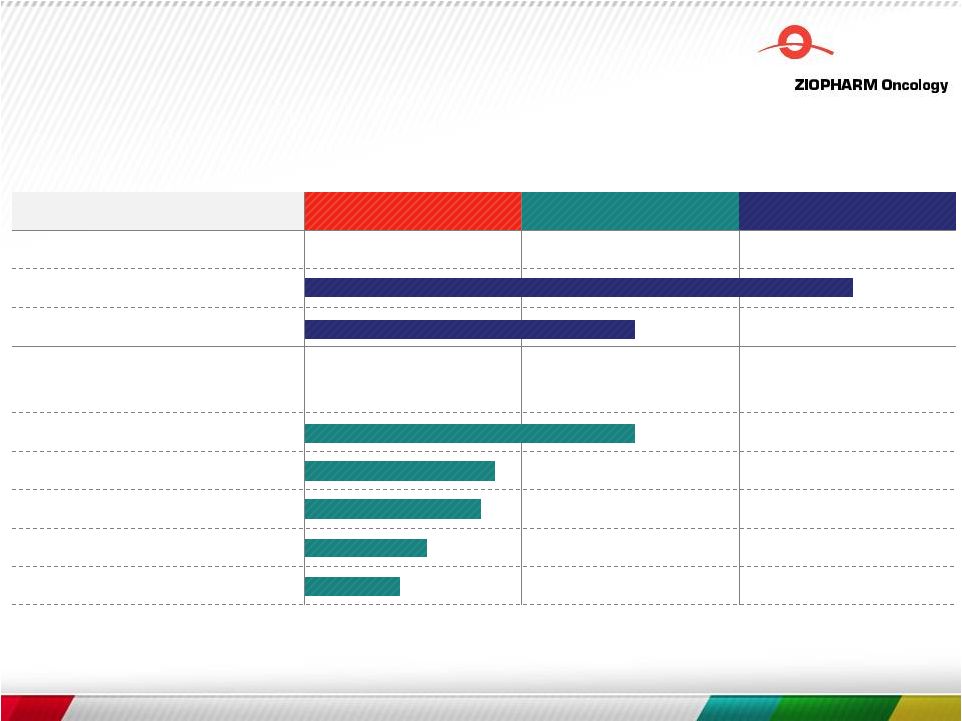 Robust Pipeline In
Synthetic Immunology
Compound Pre Clinical Phase 1 Phase 2 Ad-RTS-IL-12 gene Rx IND Breast GBM Adoptive Cell Therapies B-cell Malignancies Myeloid Malignancies RTS-controlled T cell Solid Tumors Universal Donor 7 |
 IL-12 Gene Therapy
Adenoviral vector engineered
to express IL-12 utilizing
RheoSwitch Therapeutic
System® gene switch
Injected intra-tumor with IL-12
expression controlled via
administration of oral activator
ligand veledimex
Regulated intra-tumoral
expression of IL-12 promotes
activation of tumor-infiltrating
lymphocytes to drive cytotoxic
immune response against
distant tumors
8 |
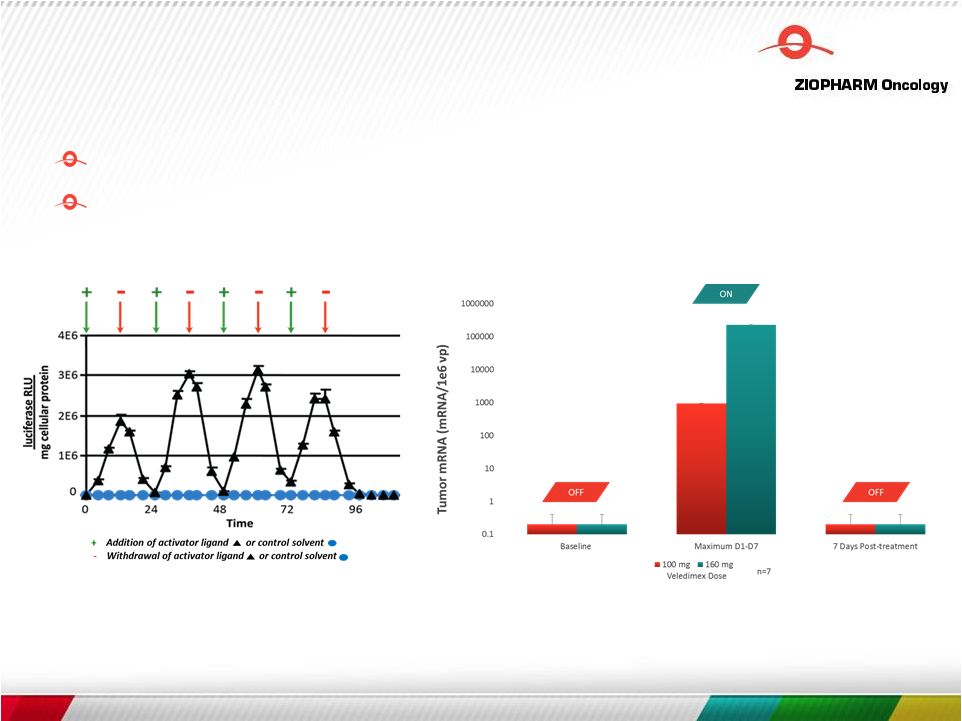 Ad-RTS-IL-12 —
Oral Veledimex Precisely Controls Expression of IL-12 (in vivo control)
9 Improved persistence and survival in tumor microenvironment Limits on-target, off-tumor toxicities |
 Phase 1b/2 Study of IL-12 Gene Therapy in
Locally Advanced/Metastatic Breast Cancer
Immunotherapy phase of treatment
A single cycle of Ad-RTS-hIL-12 +
veledimex goal of maintaining or improving pre- study response 1 O : Safety and tolerability 2 O : ORR, disease control and biomarkers Immunotherapy phase of treatment A single cycle of Ad-RTS-hIL-12 + veledimex goal of maintaining or improving pre- study response 1 O : Safety and tolerability 2 O : ORR, disease control and biomarkers N = 40 locally advanced or metastatic breast cancer of all subtypes up to 20% (8 subjects) with HER2+ breast cancer Response (PR or SD) to first- or second-line standard therapy N = 40 locally advanced or metastatic breast cancer of all subtypes up to 20% (8 subjects) with HER2+ breast cancer Response (PR or SD) to first- or second-line standard therapy Suspend chemotherapy phase of
treatment (HER2 therapy
permitted) Suspend chemotherapy phase of
treatment (HER2 therapy
permitted) 10 |
 Phase 1 Study of IL-12 Gene Therapy in Recurrent
or Progressive Glioblastoma/Gr.III
Malignant Glioma
A single cycle of Ad-RTS-hIL-12 +
escalating veledimex
1 O : Safety and tolerability 2 O : Determine MTD and immune response, including ORR, PFS and OS A single cycle of Ad-RTS-hIL-12 + escalating veledimex 1 O : Safety and tolerability 2 O : Determine MTD and immune response, including ORR, PFS and OS N = 72 Recurrent or progressive glioblastoma or Grade III malignant glioma Stratified according to clinical indication for tumor resection: Resection plus injection vs. stereotactic injection alone N = 72 Recurrent or progressive glioblastoma or Grade III malignant glioma Stratified according to clinical indication for tumor resection: Resection plus injection vs. stereotactic injection alone 11 |
 Cell Therapy Portfolio of Effector Cells 1. Chimeric Antigen Receptor (CAR+) T cells: Target cell surface tumor-associated antigens (TAAs) independent of HLA
“Public” antigens
2. T Cell Receptor (TCR
+ ) cells: Target intracellular TAAs dependent on HLA “Private” antigens 3. Natural Killer (NK) cells: Target tumor with loss of HLA No “antigens” 12 |
 Improving the Therapeutic Potential of
CAR-T Understanding of the effector cell biology Co-stimulation with cytokine Signals 1 & 2 Signal 3 13 |
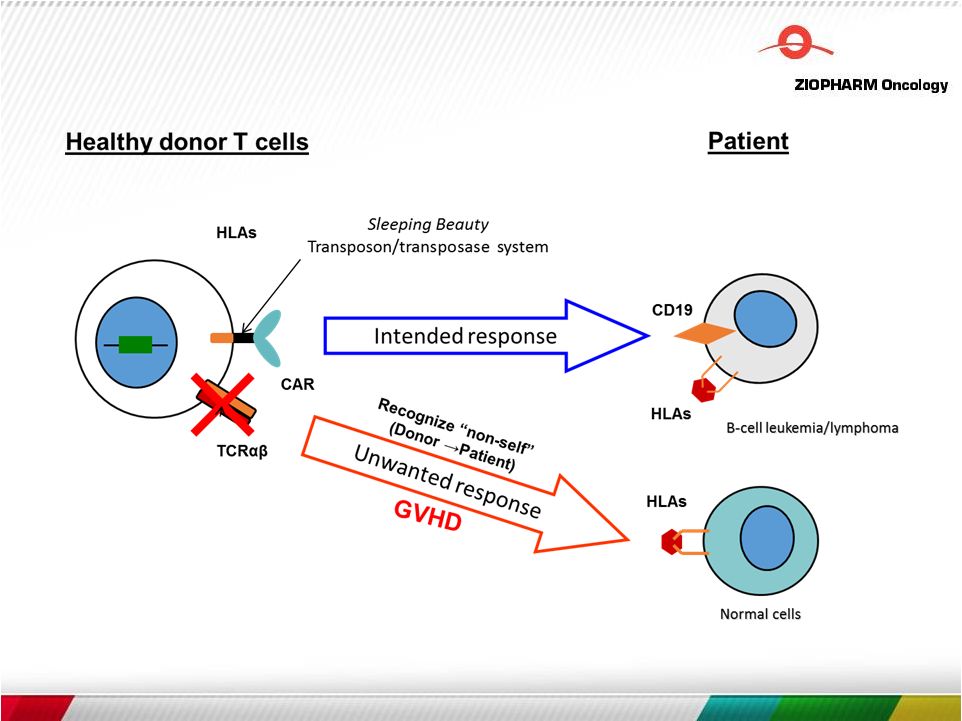
 Advantages of Non-Viral Gene Integration Sleeping Beauty transposon/transposase system
Fast, efficient and nimble gene transfer method
Improves cost, time, and complexity compared to viral-based integration 14 |
 Management of Potential Toxicity
through Inducible Immunoreceptor
Expression (in vivo control)
Many attractive target antigens are also expressed on normal cells
Inducible CAR + T Cell Inducible TCR + T Cell CAR + T Cell TCR + T Cell 15 Veledimex Next Generation |
 Building the Next-Generation of
Inducible Cytokines (in vivo
control) Therapeutic potential of effector cells depends on
recognition of tumor cells and recycling effector function in tumor
microenvironment. 16
Veledimex Next Generation |
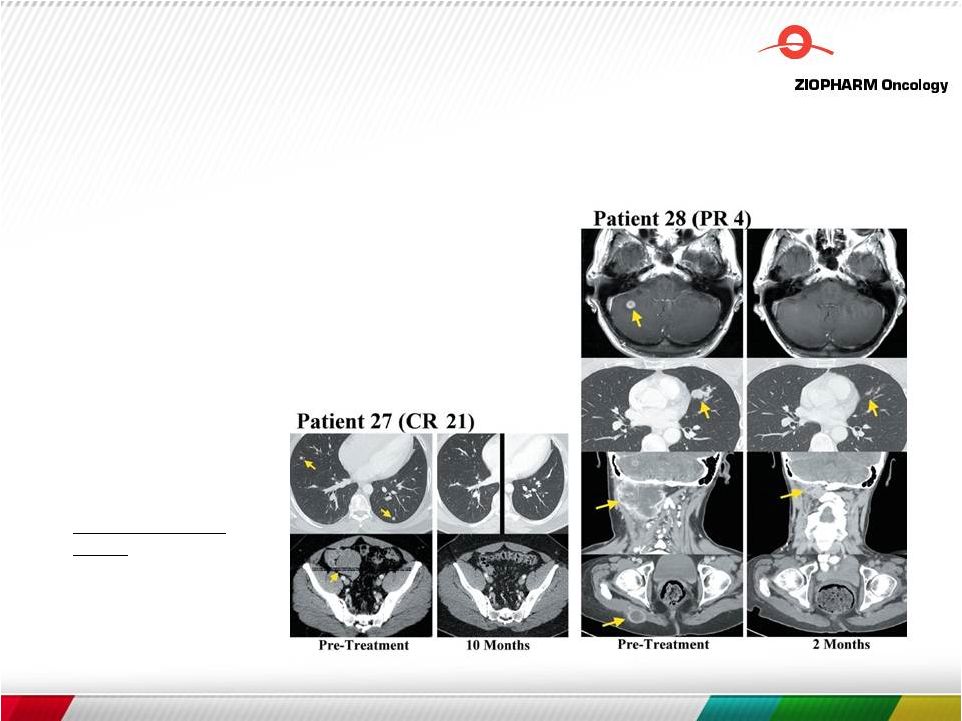 IL-12 Cell Therapy
Zhang, Rosenberg et al., Feb 2015, Clinical
Cancer Research Tumor Infiltrating Lymphocytes Genetically
Engineered with an Inducible Gene Encoding Interleukin-12 for
the Immunotherapy of Metastatic Melanoma •
TIL plus IL-12 can mediate objective responses in up to 70% of
patients with metastatic melanoma
• Enhances the activity of effector CD4+ and CD8+ T cells as well as natural killer NK and NK T cells • Collapse of tumor stroma is triggered by IL-12 induction of Fas* • 50 to 100 fold fewer cells compared to standard TIL protocols and in the absence of IL-2 co-administration • Transferred cells did not persist long-term and were associated with severe dose-limiting toxicity * Kerkar, Rosenberg et al., Mol Ther. 2013 Jul;21(7):1369-77 17 |
 Next-generation CAR
+ T cells Bioprocessing Close system Ex vivo
cytokines Cryopreservation CAR design Humanization Stalk Endodomains Cytokine IL-12 Others Time in culture 0-30 days 18 |
 New CD-19 CAR Design Using Sleeping
Beauty
Prior Sleeping Beauty CAR Design
Modified-1 Sleeping Beauty CAR Design
T cell infusion (day 1)
Next-generation design demonstrates
superior in vivo activity in
pre-clinical models Established Disease Model
Minimal Residual Disease Model
T cell infusion (day 6)
Prior Sleeping Beauty CAR Design
Modified-1 Sleeping Beauty CAR Design
19 |
 Cytokine Signaling Enhances CARs
Persisting T-cell Subsets (in
vitro): Established Disease Model
20 CD95 Expression: + + +
+
Memory Subset:
SCM CM EM EMRA |
 CD19-specific CAR
+ T cells Template for CAR + T cells Safety • Nearing completion Efficacy • New CAR Efficacy POC OTS • Generate in advance of need 21 |
 Dual CMC Distribution Approaches for
Cell Therapies Registration Trials & Global Distribution with Multiple Pharma Partners Centralized Point-of-care (POC) Genetically modified T cells One patient Multiple Patients Autologous T cells Allogeneic T cells Real time In advance of need 22 |
 Self Months Months Weeks Weeks Days Days Hours Hours T-cell manufacturing time T-cell acquisition Genetic modification Infusion Steps Toward Point-of-Care Distribution 23 |
 Off-the-Shelf (OTS) Solution
24 |
 Safely infuse patients
No immediate or late toxicity
No excess GVHD
Outpatient infusions
50% of patients with aggressive
disease remain in remission after
transplant & CAR
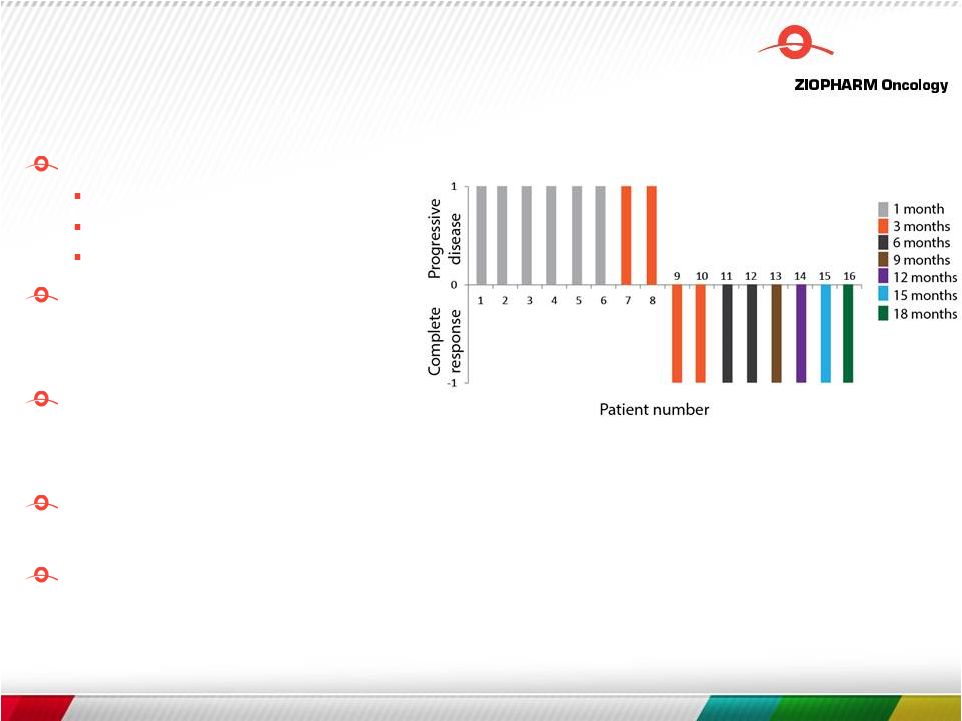
+ T cells Safely re-infuse CAR + T cells from patient-specific cryopreserved banks (n=3 re-treated) CAR + T cells apparently effective in the adjuvant disease setting Approximately 28-day CAR + T-cell persistence Ongoing Phase 1 Safety Study: First Generation Proof of Concept N = 16 pts treated, 8 remain in CR with median 7.5 mo. follow-up Kebriaei et. al, EHA 2015 25 |
 Future of T-cell therapy
26 |
 Drive Pipeline Forward To Create Value
How Are We Differentiated
Translation of immunology to
immunotherapies
T cells NK cells Non-viral gene transfer (personalized therapies) CAR TCR Cytokine Cellular engineering Expression Control 27 Personalized and orally controlled cytokine transcription CAR + T cells Ad-RTS- IL-12 |
 Differentiated Technology Platforms
Lower cost, controllable toxicity, autologous and allogeneic
28 Technology Intrexon / ZIOPHARM / MD Anderson RheoSwitch Therapeutic System® Most advanced family of ligands and switches available for dynamic range, safety, and temporal control (on-off-on etc.) Non-Viral Integration Platform First in-human testing of Sleeping Beauty system in hematopoietic
cells UltraVector® Industrialized assembly and screening of multigenic DNA modules for synthetic biology Adoptive Cell Therapy Expertise in development and implementation of novel immunotherapy trials Laser-enabled Analysis and Processing (LEAP®) Computerized image-based selection and laser processing for cell identification and purification AttSite® Recombinases Stable, targeted gene integration and expression with proprietary serine recombinases |
 Personalized Therapy for Disease
and Patient Infuse T cells with more than one specificity Personalized for the disease Intra-tumor Inter-tumor Heterogeneity of tumor-associated antigen (TAA) Infuse T cells with one or more specificity Personalized for the patient “N=1” trial paradigm 29 |
 Power-Law Curve
The New Industrialization of T cells
30 |
 The N=1 approach has been shown for
non-genetically modified T cells
31 |
 www.ziopharm.com
The Future of Cancer Therapy |
