Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - ASENSUS SURGICAL, INC. | d938528d8k.htm |
| EX-99.2 - EX-99.2 - ASENSUS SURGICAL, INC. | d938528dex992.htm |
 Todd M.
Pope, President & CEO Joe Slattery, EVP & CFO
June 8, 2015 Exhibit 99.1 |
 Cautionary Statement TransEnterix, Inc. (“TransEnterix,” “we” or “our”) has filed a registration statement (including a prospectus) and a preliminary prospectus supplement with the Securities and Exchange Commission (“SEC”) for the offering to which this presentation relates. Before you invest you should read the prospectus and the preliminary prospectus supplement in that registration statement and the other documents TransEnterix has filed with the SEC for more complete information about TransEnterix and the offering. Any statements contained in this presentation that do not describe historical facts, including statements about the beliefs and expectations of TransEnterix and other statements regarding our future plans and goals, may constitute forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, and are intended to qualify for the safe harbor from liability established by the Private Securities Litigation Reform Act of 1995. These forward-looking statements can be identified by terminology such as "will," "expect," "anticipate," "future," "intend," "plan," "believe," "estimate," "confident" and similar statements. Any forward-looking statements contained herein are based on current expectations, but are subject to a number of risks and uncertainties that may cause actual results to differ materially from expectations. Such statements are subject to risks and uncertainties that are often difficult to predict, are beyond our control, and which may cause results to differ materially from expectations. Factors that could cause our results to differ materially from those described include, but are not limited to, whether the SurgiBot TM System's 510(k) application submitted on June 1, 2015 will be cleared by the U.S. FDA, the pace of adoption of our products by surgeons, the success and market opportunity of our products, most notably the SurgiBot System, the effect on our business of existing and new regulatory requirements, and other economic and competitive factors. For a discussion of the most significant risks and uncertainties associated with TransEnterix's business, please review our filings with the SEC, including our Annual Report on Form 10-K for the year ended December 31, 2014 filed with the SEC on February 20, 2015, and other filings we make with the SEC. You are cautioned not to place undue reliance on these forward looking statements, which are based on our expectations as of the date of this presentation and speak only as of the date of this presentation. We undertake no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise. 2 |
 Advancing Surgery Through Innovation |
 Transaction Overview Issuer: TransEnterix, Inc. Ticker (Exchange): TRXC (NYSE MKT) Amount Offered: $50 million common stock Option to Purchase Additional Shares: 15% Lock-up Provision: 90 days Expected Pricing: XXXXX Bookrunners: Stifel / RBC Capital Markets Use of Proceeds: For research and development, sales and marketing, and commercialization related to our SurgiBot TM System, working capital and other general corporate purposes. |
 Investment Highlights The SurgiBot System is currently in development • Surgical robotics Focus • Developing patient-side robotic platform, SurgiBot System Technology • Addresses unmet needs in today’s robotic offerings • Cost effective • Broad procedure applicability Solution • Large addressable market – ~2M US procedures Market • Strong management team Experience |
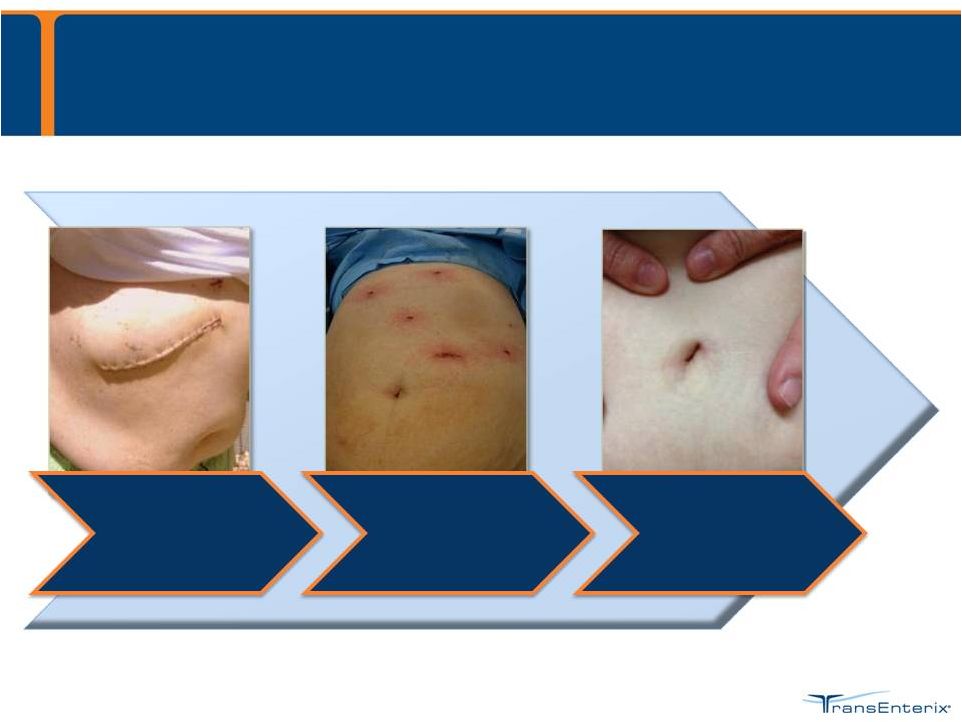 Surgical
Progress = Less Invasive 6
Open Surgery
Rigid Laparoscopy (1989) Flexible Laparoscopy (2010) |
 Spider
Surgical System Enabling Flexible Laparoscopy
• Internal (intra-abdominal) triangulation • Flexible, articulating instruments for dissection and retraction • True left/right instrumentation • Lower profile port of access • Over 3,500 procedures performed • Broad procedural mix 7 |
 Robotically-enabled
Laparoscopic Platform
The SurgiBot System is currently in development |
 Benefits
of Surgical Robotics 9
Benefits of
Surgical Robotics Improved dissection and retraction strength Enhanced precision and control Improved surgeon ergonomics Advanced visualization |
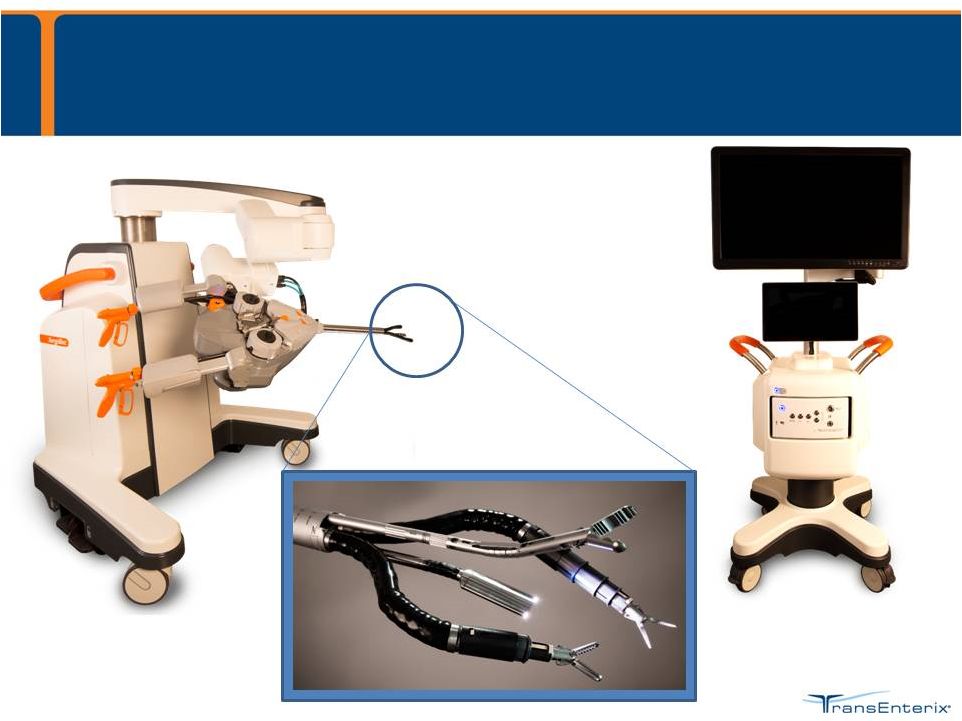 SurgiBot Patient-side Robotic Platform 3DHD Vision Cart SurgiBot The SurgiBot System is currently in development |
 SurgiBot Patient-side Robotic Platform The SurgiBot System is currently in development [Video of SurgiBot™ System] |
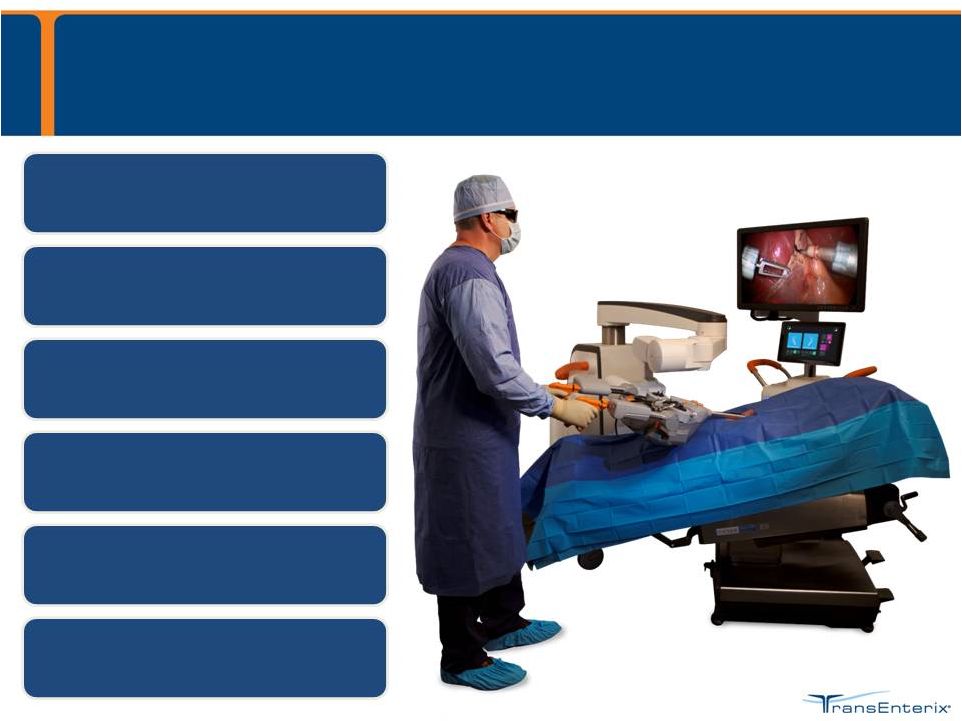 SurgiBot Patient-side Robotic Platform The SurgiBot System is currently in development Surgeon scrubbed-in Easily repositioned for multi- quadrant surgery Flexible, articulating channels & instruments Steerable 3DHD Vision Small, mobile platform Cost effective platform |
 Procedure Market Opportunity
13 Bariatric Surgery Cholecystectomy Colorectal Gynecology/Urology Targeted US Procedures 160K 1,140K 363K 230K Source: Millennium Research Group US Markets Laparoscopic Devices 2014 ~2M US Laparoscopic Procedures |
 Hospital Market Opportunity
14 Hospitals without Robotic Capability • Current robotic offerings are not cost effective • System price • Limited procedure volume • Surgery centers – untapped market for robotics • Losing market share to robotic facilities • Large underpenetrated OUS opportunity Hospitals with
Robotic Capability • Invested in the strategic and competitive value of robotic surgery • ROI expectations changing/under pressure • Potential for diversification of robotic solutions • Procedures • Price • Facilities |
 Significant Base of Underserved Hospitals
15 Mid/Small Hospitals • >3,000 w/ <300 beds • >80% lack robotic offering • Losing market share to robotic facilities • Represent >800K target annual procedures Surgery Centers • ~5,000 in US • Economics driving rapid procedure growth • SurgiBot will offer versatility and cost benefits |
 Combining Advanced Technology AND Maximizing Value
16 Price Surgical Robotics >$2B Revenue High Growth Traditional Laparoscopy >$10B Revenue Low Growth |
 Recurring Revenue Model
• US Pricing: • Capital sale ~$500K • Instruments ~$1,000-1,500/procedure • Service ~$50K/year • International: • Market-specific pricing • Distributors provide service/support 17 Instruments and Accessories Service Capital Equipment The SurgiBot System is currently in development |
 Highlights – Instrumentation 18 Instrumentation Needs By Procedure Status Chole Bariatrics GYN SPIDER SurgiBot Grasper Launched Developed Dissector Launched Developed Hook Launched Developed Clip Applier Launched Developed Shears Launched Developed Suction Irrigator Launched Developed Needle Driver Launched Developed Advanced Energy Launched Development Bipolar Energy 3 rd Party Development Stapler 3 rd Party 3 rd Party The SurgiBot System is currently in development |
 Advanced Energy Superior Visualization – 360 Degree Articulation [Video of Flex Ligating Shears as Compared to Competition] |
 Milestones • 510(k) submitted June 2015 • Anticipate 6-9 month review cycle • Anticipate commercial launch Q2 2016 20 The SurgiBot System is currently in development |
 Operating Capabilities & Talent
21 Rapid Prototyping R&D Team Broad Experience cGMP Manufacturing |
 Prior Experience Todd M. Pope President and Chief Executive Officer Joe Slattery EVP and Chief Financial Officer Mohan Nathan Vice President of Global Marketing Vernon Brown Vice President, Quality and Regulatory Affairs Nicole Bell Vice President, Research and Development Larry Pope Vice President, Operations Management Team 22 |
 |
