Attached files
| file | filename |
|---|---|
| EX-32.2 - EX-32.2 - ASENSUS SURGICAL, INC. | trxc-ex322_6.htm |
| EX-32.1 - EX-32.1 - ASENSUS SURGICAL, INC. | trxc-ex321_7.htm |
| EX-31.2 - EX-31.2 - ASENSUS SURGICAL, INC. | trxc-ex312_8.htm |
| EX-31.1 - EX-31.1 - ASENSUS SURGICAL, INC. | trxc-ex311_9.htm |
| EX-23.1 - EX-23.1 - ASENSUS SURGICAL, INC. | trxc-ex231_10.htm |
| EX-21.1 - EX-21.1 - ASENSUS SURGICAL, INC. | trxc-ex211_11.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
|
☒ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2016
OR
|
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number 0-19437
TRANSENTERIX, INC.
(Exact name of registrant as specified in its charter)
|
Delaware |
|
11-2962080 |
|
(State or other jurisdiction of incorporation or organization) |
|
(I.R.S. Employer Identification No.) |
635 Davis Drive, Suite 300, Morrisville, NC 27560
(Address of principal executive offices) (Zip Code)
Registrant’s telephone number, including area code: (919) 765-8400
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class |
|
Name of each exchange on which registered |
|
Common Stock $0.001 par value per share |
|
NYSE MKT |
Securities registered pursuant to Section 12(g) of the Act:
None
(Title of class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒.
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒.
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐.
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒ No ☐.
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K ☒.
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer |
|
☐ |
|
Accelerated filer |
|
☒ |
|
|
|
|
|
|||
|
Non-accelerated filer |
|
☐ (Do not check if a smaller reporting company) |
|
Smaller reporting company |
|
☐ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act) Yes ☐ No ☒.
On June 30, 2016, the last business day of the registrant’s most recently completed second fiscal quarter, the aggregate market value (based on the average bid and asked price of its common stock on that date) of the voting stock held by non-affiliates of the registrant was $88,862,336.
The number of shares outstanding of the registrant’s common stock, as of February 28, 2017 was 122,723,238.
Documents Incorporated By Reference: None
ANNUAL REPORT ON FORM 10-K
DECEMBER 31, 2016
Table of Contents
|
|
|
|
|
Page |
|
|
|
|||
|
ITEM 1. |
|
|
4 |
|
|
ITEM 1.A. |
|
|
12 |
|
|
ITEM 1.B. |
|
|
25 |
|
|
ITEM 2. |
|
|
25 |
|
|
ITEM 3. |
|
|
25 |
|
|
ITEM 4. |
|
|
26 |
|
|
|
|
|
||
|
|
|
|||
|
ITEM 5. |
|
|
27 |
|
|
ITEM 6. |
|
|
30 |
|
|
ITEM 7. |
|
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
|
31 |
|
ITEM 7.A. |
|
|
42 |
|
|
ITEM 8. |
|
|
44 |
|
|
ITEM 9. |
|
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
|
79 |
|
ITEM 9.A. |
|
|
79 |
|
|
ITEM 9.B. |
|
|
79 |
|
|
|
|
|
||
|
|
|
|||
|
ITEM 10. |
|
|
80 |
|
|
ITEM 11. |
|
|
83 |
|
|
ITEM 12. |
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS. |
|
94 |
|
ITEM 13. |
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE. |
|
95 |
|
ITEM 14. |
|
|
96 |
|
|
|
|
|
||
|
|
|
|||
|
ITEM 15. |
|
|
98 |
|
|
ITEM 16. |
|
|
100 |
|
2
This Annual Report on Form 10-K (“Annual Report”) contains certain “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). Such forward-looking statements contain information about our expectations, beliefs or intentions regarding our product development and commercialization efforts, business, financial condition, results of operations, strategies or prospects. You can identify forward-looking statements by the fact that these statements do not relate strictly to historical or current matters. Rather, forward-looking statements relate to anticipated or expected events, activities, trends or results as of the date they are made. Because forward-looking statements relate to matters that have not yet occurred, these statements are inherently subject to risks and uncertainties that could cause our actual results to differ materially from any future results expressed or implied by the forward-looking statements.
Many factors could cause our actual operations or results to differ materially from the operations and results anticipated in forward-looking statements. These factors include, but are not limited to:
|
|
• |
our history of operating losses and uncertainty as to our ability to continue as a going concern; |
|
|
• |
our need to obtain additional funding to continue our operations; |
|
|
• |
our ability to successfully transition from a research and development company to a company focused on marketing, sales and distribution of our products; |
|
|
• |
our ability to successfully develop, clinically test and commercialize our products; |
|
|
• |
the timing and outcome of the regulatory review process for our products; |
|
|
• |
our ability to attract and retain key management, marketing and scientific personnel; |
|
|
• |
competition from existing and new market entrants; |
|
|
• |
our ability to successfully prepare, file, prosecute, maintain, defend and enforce patent claims and other intellectual property rights; |
|
|
• |
changes in the health care and regulatory environments of the United States, Italy and other countries in which the Company operates; |
|
|
• |
our ability to identify and pursue development of additional products; and |
|
|
• |
other factors contained in the section entitled “Risk Factors” contained in this Annual Report. |
We do not undertake any obligation to update our forward-looking statements, except as required by applicable law.
3
Overview
TransEnterix, Inc. (the “Company”) is a medical device company that is pioneering the use of robotics to improve minimally invasive surgery by addressing the clinical challenges associated with current laparoscopic and robotic options. The Company is focused on the commercialization and further development of its Senhance™ Surgical Robotic System (formerly known as the ALF-X ® Surgical Robotic System) (the “Senhance System”), a multi-port robotic system that brings the advantages of robotic surgery to patients while enabling surgeons with innovative technology. The Company also developed the SurgiBot™ System, a single-port, robotically enhanced laparoscopic surgical platform. The Senhance System has been granted a CE Mark in Europe for laparoscopic abdominal and pelvic surgery, as well as limited thoracic operations excluding cardiac and vascular surgery, but is not available for sale in the U.S. The SurgiBot System is not available for sale in any market.
The Senhance System is a multi-port robotic surgery system which allows multiple arms to control robotic instruments and a camera. The system features advanced technology to enable surgeons with haptic feedback and the ability to move the camera via eye movement. The system replicates laparoscopic motion that is familiar to experienced surgeons, and features three-dimensional high definition (“3DHD”) vision technology. The Senhance System also offers responsible economics to hospitals by offering robotic technology with reusable instruments with minimal additional costs per surgery.
The SurgiBot System is designed to utilize flexible instruments through articulating channels controlled directly by the surgeon, with robotic assistance, while the surgeon remains patient-side within the sterile field. In June 2015, the Company submitted a 510(k) application to the FDA for the SurgiBot System. On April 19, 2016, the FDA notified the Company that the SurgiBot System did not meet the criteria for substantial equivalence based on the data and information submitted by TransEnterix in the 510(k) submission. After interactions with the FDA, the Company determined that a new 510(k) application would need to be submitted in order to obtain clearance for the SurgiBot System. Based on this fact, we have evaluated the operational and financial feasibility of pursuing two 510(k) applications in parallel and have elected to primarily focus our near term U.S. regulatory efforts on the 510(k) submission for the Senhance System, and our current strategy is to focus our resources on the commercialization of and U.S. regulatory clearance for the Senhance System.
Consequently, in May 2016, the Company implemented a restructuring plan. The restructuring plan resulted in: 1) reducing the Company’s workforce; 2) abandoning certain equipment; 3) cancelling certain contracts; 4) writing down inventory related to the SurgiBot System; and 5) writing off certain patents.
We believe that future outcomes of minimally invasive surgery will be enhanced through our combination of more advanced tools and robotic functionality which are designed to: (i) empower surgeons with improved precision, dexterity and visualization; (ii) improve patient satisfaction and enable a desirable post-operative recovery; and (iii) provide a cost-effective robotic system, compared to existing alternatives today, for a potentially wide range of clinical applications. Our strategy is to focus on the development and commercialization of the Senhance System.
Recent Corporate Transaction
On September 18, 2015, the Company entered into a Membership Interest Purchase Agreement, (the “Purchase Agreement”) with Sofar S.p.A., (“Sofar”) as seller, Vulcanos S.r.l. (“Vulcanos”), as the acquired company, and TransEnterix International, Inc. (“TransEnterix International”), a direct, wholly owned subsidiary of the Company which was incorporated in September 2015, as buyer. The closing of the transactions occurred on September 21, 2015 (the “Closing Date”) pursuant to which the Company acquired all of the membership interests of Vulcanos from Sofar (now known as the “Senhance Acquisition”), and changed the name of Vulcanos to TransEnterix Italia S.r.l (“TransEnterix Italia”). For a description of the Senhance Acquisition and related transactions, see the disclosure titled “Senhance Acquisition and Related Transactions” under Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in this Annual Report.
As used herein, the term “Company” refers to TransEnterix, Inc., including its subsidiaries, TransEnterix International, TransEnterix Italia, TransEnterix Europe S.Á.R.L and TransEnterix Europe S.Á.R.L, Bertrange, Swiss Branch, Lugano and TransEnterix Asia PTE. LTD. after giving effect to the Senhance Acquisition.
The Company operates in one business segment. Please see the disclosure in Note 2 “Summary of Significant Accounting Policies – Segments” in the Notes to our Consolidated Financial Statements in Item 8 of this Annual Report regarding our business operations in the U.S. and elsewhere.
4
Market Overview
Over the past two decades, laparoscopic surgery has emerged as a minimally invasive alternative to open surgery. In laparoscopic surgery, multiple incisions are necessary to provide surgical access ports. Carbon dioxide gas insufflation is then used to create room in the body cavity, and long rigid instruments are introduced through ports placed in the incisions to perform surgical tasks. Millions of laparoscopic surgical procedures across a broad range of clinical applications are now performed each year worldwide, though many surgeries are still performed in an open fashion.
While laparoscopy has improved the invasive nature of many previously open procedures, it still has many limitations. Traditional, or rigid, laparoscopy still requires multiple incisions to achieve the visualization and instrument triangulation required to perform successful surgery. Rigid laparoscopy also creates physical challenges by forcing the surgeon’s hands and arms into awkward angles, requiring the surgeon to hold instruments in fixed positions for long periods of time, and requiring an assistant to stabilize and move a laparoscopic camera. Another challenge associated with rigid laparoscopic surgery is the creation of a cumbersome and potentially tissue-damaging fulcrum at the patient’s abdominal wall where instruments are manipulated. Nearly all laparoscopic instruments are rigid instruments that lack the internal articulation required to enhance dexterity in complex tasks. Most laparoscopic surgeries are performed with two-dimensional (“2-D”) visualization of the operative field, making depth perception difficult.
Despite such limitations, traditional laparoscopy remains the prevalent technique in minimally invasive surgery. We believe that robotic devices that replicate laparoscopic motion are more comfortable for surgeons to adopt, thereby increasing the opportunity to enhance traditional surgical methods with robotics. Our Senhance System mimics laparoscopic surgery.
Robotic and computer controlled assistance have developed as technologies that offer the potential to improve upon many aspects of the laparoscopic surgical experience. Hundreds of thousands of robotic-assisted surgical procedures are now performed each year worldwide, but they still represent a small fraction of the total laparoscopic procedures performed. While initial widespread adoption of robotic-assisted surgery was focused on urologic and gynecologic procedures that were primarily performed in an open fashion prior to robotics, recently developed robotic approaches have been applied to many other clinical applications, particularly in general surgery. Despite recent advances, we believe there remain many limitations associated with current robotic-assisted surgery systems used in connection with laparoscopic surgeries.
Product Overview
We are addressing the challenges in laparoscopy and robotic-assisted surgery with innovative products and product candidates that leverage the best features of both approaches to minimally invasive surgery.
Current Product Offering
Senhance System
The Senhance System is a multi-port robotic surgery system which allows up to four arms to control robotic instruments and a camera. The system builds on the success of laparoscopy by enhancing the traditional features that surgeons have come to expect from existing products and by addressing some of the limitations associated with robotic surgery systems for laparoscopic procedures. The Senhance System also offers responsible economics to hospitals by offering robotic technology with reusable instruments with minimal additional costs per surgery when compared to laparoscopy. The Senhance System has been granted a CE Mark in Europe for use in abdominal and pelvic surgery, but is not available for sale in the U.S.
Key features of the Senhance System are:
|
|
• |
Haptic Feedback: The Senhance System’s haptic feedback feature provides the surgeon with the ability to feel the tactile response of the body during a procedure. |
|
|
• |
Enhanced Vision: The Senhance System features 3DHD vision technology and gives the surgeon the ability to move the camera via eye movement so that the camera is centered in the surgeon’s field of vision. |
|
|
• |
Laparoscopic Motion: The Senhance System utilizes laparoscopic motion that is similar to the motion used during traditional laparoscopic surgeries. |
|
|
• |
View of the Sterile Field: The Senhance System offers the user an open view of the operating room and sterile field from the console. |
|
|
• |
Enables Use of Standard Trocars: The Senhance System allows for standard laparoscopic trocars to be used and does not require that robotic arms be docked directly to the patient. |
5
The Senhance System is manufactured for us under contract by a third party manufacturer. We or our manufacturer acquire raw materials and components of the Senhance System from vendors, some of which are sole suppliers. We believe our relationships with our vendors and manufacturing contractor are good. We further believe that we have the manufacturing and inventory reserves to meet our anticipated Senhance System sales for the foreseeable future. We are currently taking steps to develop redundant manufacturing and supply alternatives.
Products in Development
SurgiBot System Robotic Platform
The SurgiBot System is designed to utilize flexible instruments through articulating channels controlled directly by the surgeon, with robotic assistance, while the surgeon remains patient-side within the sterile field. In June 2015, the Company submitted a 510(k) application to the FDA for the SurgiBot System. On April 19, 2016, the FDA notified the Company that the SurgiBot System did not meet the criteria for substantial equivalence based on the data and information submitted by TransEnterix in the 510(k) submission. After interactions with the FDA, the Company determined that a new 510(k) application would need to be submitted in order to obtain clearance for the SurgiBot System. Based on this fact, we have evaluated the operational and financial feasibility of pursuing two 510(k) applications in parallel and have elected to primarily focus our near term regulatory efforts on the 510(k) submission for the Senhance System, and our current strategy is to focus our resources on the commercialization of and regulatory clearance for the Senhance System.
Key features of the SurgiBot System are:
|
|
• |
Patient Side: The SurgiBot System is positioned next to the operating table, thereby allowing the surgeon, as operator, to control the system from within the sterile field next to the patient. |
|
|
• |
Precision with Scaling: The SurgiBot System allows the user to adjust the level of mechanized movement using scaled ratios. |
|
|
• |
Strength: The SurgiBot System features powered motion driven by motors controlled by the surgeon. |
|
|
• |
Ergonomics: The SurgiBot System stabilizes multiple instruments and a laparoscope and allows the surgeon to reposition his or her hands in an ergonomic fashion. |
|
|
• |
Internal Triangulation: The SurgiBot System utilizes a deployment mechanism to achieve triangulation of multiple instruments inside the body as contrasted with other single-port robotic systems that rely on crossing instruments at the patient’s abdominal wall. The SurgiBot System allows for triangulation that can be adjusted in the surgical field during a procedure and be maintained at positions throughout a body cavity. |
|
|
• |
Direct Surgeon Connection to the Instruments: The SurgiBot System allows the surgeon-operator to maintain human tactile feedback along several degrees of motion. Existing robotic systems lack any such tactile feedback. |
Other Products
SPIDER Surgical System
Prior to 2015, TransEnterix Surgical developed and commercialized the SPIDER ® Surgical System (the “SPIDER System”), a manual laparoscopic system, in the United States, Europe and the Middle East. The SPIDER System utilized flexible instruments and articulating channels that were controlled directly by the surgeon, allowing for multiple instruments to be introduced via a single site. The SPIDER System was cleared by the FDA in 2009 and CE Marked in August 2011. The Company also manufactured multiple instruments that could be deployed using the disposable SPIDER System. As of December 31, 2014, we ceased all commercialization efforts with respect to the SPIDER System in order to fully focus our efforts on our other products.
Surgical Instruments
The Company has developed and manufactures flexible and rigid laparoscopic surgical instruments that are used in abdominal surgery, such as scissors, graspers, clip appliers, and suction and irrigation instruments. Such instruments were sold in limited volumes in connection with the SPIDER System, and have been adapted for use with the SurgiBot System.
In the year ended December 31, 2016, we had one European customer, Humanitas Hospital in Milan, Italy, who accounted for 100% of our revenue of our Senhance System products. On February 23, 2017, the Company announced that it made its second sale of the Senhance System to a customer in Germany. In the year ended December 31, 2015, the Company had no revenue, as we focused our efforts on the SurgiBot System development and the Senhance Acquisition. In the year ended December 31, 2014, we had one U.S. customer who accounted for 37% of our revenue for products that included the SPIDER System. The Company is not dependent on
6
current customers. Please see the disclosure in Note 2 “Summary of Significant Accounting Policies – Segments” in the Notes to our Consolidated Financial Statements in Item 8 of this Annual Report regarding the geographic locations of our revenues and assets in the U.S. and elsewhere.
Business Strategy
Our current strategy is to focus our resources on the commercialization of and U.S. regulatory clearance for the Senhance System. After interactions with the FDA, we determined that a new 510(k) application would need to be submitted in order to obtain clearance for the SurgiBot System. Based on this fact, we have evaluated the operational and financial feasibility of pursuing two 510(k) applications in parallel and have elected to primarily focus our near term efforts on the 510(k) submission for the Senhance System.
We believe that:
|
|
• |
there are a number of hospitals and an increasing number of ambulatory surgery centers in the U.S. and internationally that could benefit from the addition of robotic-assisted minimally invasive surgery and, with the Senhance System, lower operational costs; |
|
|
• |
with the Senhance System, surgeons can benefit from the haptic feedback, enhanced 3DHD vision and open architecture consistent with current laparoscopic surgery procedures; and |
|
|
• |
patients will continue to seek a minimally invasive option for many common general abdominal and gynecologic surgeries, which are addressed by the Senhance System. |
Sales and Marketing
We have recruited an initial sales and marketing team and have initiated initial commercialization of the CE-marked Senhance System in Europe, the Middle East and Africa and limited countries in Asia. We utilize distributors and sales agents in a number of jurisdictions where we do not sell directly. Our distribution agreements typically provide exclusivity in a specific territory or jurisdiction. To date we have agreements with ten distributors or sales agents for the Senhance System.
We have initiated a Clinical Leadership Program with leading surgical centers in Europe to utilize the Senhance System. We believe the program helps improve our visibility in Europe and provides more widespread opportunity for observation of robotic surgery with the Senhance System. We opened three such Clinical Leadership Program sites in 2016 and anticipate adding a fourth in early 2017. In addition, in December 2016 we opened a new European training and research and development center in Milan, Italy.
Research and Development
During the fiscal years ended December 31, 2016, 2015 and 2014, we incurred research and development expenses of approximately $29.3 million, $29.7 million and $27.9 million, respectively. In 2016, such expenses primarily related to the SurgiBot System development and the Senhance System development, including the preparation and submission of regulatory filings. In 2015 and 2014, such expenses primarily related to the SurgiBot System development, including the preparation and submission of regulatory filings. We fund our research and development expenses primarily from proceeds raised from equity and debt financing transactions. We expect to continue to use equity and debt financing transactions to fund our research and development activities. No customers are obligated to pay any material portion of such research and development expenses.
Intellectual Property
We believe that our intellectual property and expertise is an important competitive resource. Our experienced research and development team has created a substantial portfolio of intellectual property, including patents, patent applications, trade secrets and proprietary know-how. We maintain an active program of intellectual property protection, both to assure that the proprietary technology developed by us is appropriately protected and, where necessary, to assure that there is no infringement of our proprietary technology by competitive technologies.
The following summarizes our current patent and patent application portfolio.
Patents and Patent Applications Owned by the Company: The Company holds nine United States patents, three Italian patents, one Russian patent, a European patent, two Japanese patents, two Australian patents and a Chinese patent, and it has more than forty patent applications filed in the United States and abroad. In each instance, we own all right, title and interest, and no licenses, security interests or other encumbrances have been granted on such patents and patent applications.
7
Several of our issued patents resulted from filings related to the Senhance System. These include one of the United States patents, and the Italian, Russian and Chinese patents. The earliest to expire patents within this part of our portfolio will remain in force until 2030. Two of our United States patents resulted from filings relating to the SurgiBot System developed by the Company and will remain in force until 2033. Three of our United States patents and the European, Japanese and Australian patents resulted from filings relating to the SPIDER System, which the Company stopped selling as of December 31, 2014. The earliest to expire patents within this part of our portfolio will remain in force until 2027. The patent applications relate to the Senhance System, the SurgiBot System and the SPIDER System, and other instruments and systems for minimally invasive surgical procedures. We intend to seek further patent and other intellectual property protection in the United States and internationally, where available and when appropriate, as we continue our product development efforts.
Patents and Patent Applications Licensed to the Company: TransEnterix Italia has exclusively licensed technology, know-how, patents and patent applications relating to the Senhance System from the European Union. This licensed portfolio includes two issued US patents which will remain in force until 2030, and at least fifteen patents issued in other countries, including two European patents, three Korean patents, three Japanese patents and three Chinese patents, which expire in 2027. At least fifteen additional patent applications are pending, including three in the United States.
The license agreement with the European Union has a term which runs until the final licensed patent expires, unless the agreement is terminated earlier by mutual consent of the parties or for breach. The Company is currently in compliance with the terms of this license agreement.
Competition
Our industry is highly competitive, subject to change and significantly affected by new product introductions and other activities of industry participants. Many of our competitors have significantly greater financial and human resources than we do and have established reputations with our target customers, as well as worldwide distribution channels that are more established and developed than ours.
There are many competitive offerings in the field of minimally invasive surgery. Several companies have launched devices that enable reduced incision or single incision laparoscopic surgery with or without robotic assistance. Our surgical competitors include, but are not limited to: Applied Medical, Medtronic plc, Intuitive Surgical, Titan Medical and Verb Surgical.
In addition to surgical device manufacturer competitors, there are many products and therapies that are designed to reduce the need for or attractiveness of surgical intervention. These products and therapies may impact the overall volume of surgical procedures and negatively impact our business.
Our ability to compete may be affected by the failure to fully educate physicians in the use of our products and products in development, or by the level of physician expertise. This may have the effect of making our products less attractive. Among currently available surgical robotic systems, we expect the Senhance System to differentiate on the basis of its use of standard trocars and lower per procedure costs; and we expect the Senhance System to differentiate, in most cases, its ability to provide the surgeon with tactile feedback. Several medical device companies are actively engaged in research and development of robotic systems or other medical devices and tools used in minimally invasive surgery procedures. We cannot predict the basis upon which we will compete with new products marketed by others.
Government Regulation of our Product Development Activities
The U.S. government and foreign governments regulate the medical device industry through various agencies, including but not limited to, the U.S. FDA, which administers the Federal Food, Drug and Cosmetic Act (the “FDCA”). The design, testing, manufacturing, storage, labeling, distribution, advertising, and marketing of medical devices are subject to extensive regulation by federal, state and local governmental authorities in the United States, including the FDA, and by similar agencies in other countries, including the European Union. Any device product that we develop must receive all requisite regulatory approvals or clearances, as the case may be, before it may be marketed in a particular country.
Device Development, Marketing Clearance and Approval
Medical devices are subject to varying levels of pre-market regulatory requirements. The FDA classifies medical devices into one of three classes: (i) Class I devices are relatively simple and can be manufactured and distributed with general controls; (ii) Class II devices are somewhat more complex and receive greater scrutiny from the FDA and have heightened regulatory requirements; and (iii) Class III devices are new, high risk devices, and frequently are permanently implantable or help sustain life and generally require a Pre-Market Approval (“PMA”) by the FDA.
8
In the United States, a company generally can obtain permission to distribute a new medical device in one of two ways. The first applies to any device that is substantially equivalent to a device first marketed prior to May 1976, or to another device marketed after that date, but which was substantially equivalent to a pre-May 1976 device. These devices are either Class I or Class II devices. To obtain FDA clearance to distribute the medical device, a company generally must submit a Section 510(k) notification, and receive an FDA order finding substantial equivalence to a predicate device (pre-May 1976 device or post-May 1976 device that was substantially equivalent to a pre-May 1976 device) and permitting commercial distribution of that medical device for its intended use. A 510(k) notification must provide information supporting a claim of substantial equivalence to a single medical device, the predicate device. If clinical data from human experience are required to support the 510(k) notification, these data must be gathered in compliance with investigational device exemption (“IDE”) regulations for investigations performed in the United States. The 510(k) process is normally used for products of the type that we are developing and propose to market and sell. The FDA review process for premarket notifications submitted pursuant to Section 510(k) takes, pursuant to statutory requirements, 90 days, but it can take substantially longer if the FDA has questions regarding the regulatory submission. It is possible for Section 510(k) clearance procedures to take from six to twenty-four months, depending on the concerns raised by the FDA and the complexity of the device. There is no guarantee that the FDA will “clear” a medical device for marketing, in which case the device cannot be distributed in the United States. There is also no guarantee that the FDA will deem the applicable device subject to the 510(k) process, as opposed to the more time-consuming, resource-intensive and problematic PMA process described below. In 2011, the FDA issued a series of draft guidance documents designed to reform the 510(k) clearance process. Similarly, the Medical Device User Fee Amendments of 2012 authorized the FDA to collect user fees for the review of certain pre-market submissions received on or after October 1, 2012, including 510(k) notifications.
The second, more comprehensive, approval process applies to a new device that is not substantially equivalent to a pre-1976 product or that is to be used in supporting or sustaining life or preventing impairment. These devices are normally Class III devices. For example, most implantable devices are subject to the approval process as a Class III device. Two steps of FDA approval are generally required before a company can market a product in the United States that is subject to approval, as opposed to clearance, as a Class III device. First, a company must comply with IDE regulations in connection with any human clinical investigation of the device. These regulations permit a company to undertake a clinical study of a “non-significant risk” device without formal FDA approval. Prior express FDA approval is required if the device is a significant risk device. Second, the FDA must approve the company’s PMA application, which contains, among other things, clinical information acquired under the IDE. Additionally, devices subject to PMA approval may be subject to a panel review to obtain marketing approval and are required to pass a factory inspection in accordance with the current “good manufacturing practices” standards in order to obtain approval. The FDA will approve the PMA application if it finds there is reasonable assurance that the device is safe and effective for its intended use. The PMA process takes substantially longer than the 510(k) process, approximately one to two years or more. However, in some instances the FDA may find that a device is new and not substantially equivalent to a predicate device but is also not a high risk device as is generally the case with Class III PMA devices. In these instances FDA may allow a device to be down classified from Class III to Class I or II. The de novo classification option is an alternate pathway to classify novel devices of low to moderate risk that had automatically been placed in Class III after receiving a “not substantially equivalent” (“NSE”) determination in response to a 510(k) notification. The regulations have also been amended to allow a sponsor to submit a de novo classification request to the FDA for novel low to moderate risk devices without first being required to submit a 510(k) application. These types of applications are referred to as “Evaluation of Automatic Class III Designation” or “de novo.” In instances where a device is deemed not substantially equivalent to a Class II predicate device, the candidate device may be filed as a de novo application which may lead to delays in regulatory decisions by the FDA. FDA review of a de novo application may lead the FDA to identify the device as either a Class I or II device and worthy of either an exempt or 510(k) regulatory pathway.
We believe that the Senhance System and related products are Class II devices, and we are in the process of pursuing Section 510(k) clearance for the Senhance System. The FDA might find that the 510(k) submission does not provide the evidence required to prove that the Senhance System is substantially equivalent to marketed Class II devices. If that were to occur, we would be required to undertake the more complex and costly PMA process or perhaps be considered for a de novo reclassification. For either the 510(k), de novo, or the PMA process, the FDA could require us to conduct clinical trials, which would take more time, cost more money and pose other risks and uncertainties.
Clinical studies conducted in the U.S. or used in any U.S. application on an unapproved medical device require approval from the FDA prior to initiation. Even when a clinical study has been approved by the FDA or deemed approved, the study is subject to factors beyond a manufacturer’s control, including, but not limited to, the fact that the institutional review board (IRB) at a specified clinical site might not approve the study, might decline to renew approval, or might suspend or terminate the study before its completion. There is no assurance that a clinical study at any given site will progress as anticipated. In addition, there can be no assurance that the clinical study will provide sufficient evidence to assure the FDA that the product is safe and effective, a prerequisite for FDA approval of a PMA, or substantially equivalent in terms of safety and effectiveness to a predicate device, a prerequisite for clearance under Section 510(k). Even if the FDA approves or clears a device, it may limit its intended uses in such a way that manufacturing and distribution of the device may not be commercially feasible.
9
After clearance or approval to market is given, the FDA and foreign regulatory agencies, upon the occurrence of certain serious adverse events, are authorized under various circumstances to withdraw the clearance or approval of the device, or require changes to a device, its manufacturing process or its labeling or require additional proof that regulatory requirements have been met.
A manufacturer of a device approved through the PMA process is not permitted to make changes to the device which affect its safety or effectiveness without first submitting a supplement application to its PMA and obtaining FDA approval for that supplement, prior to marketing the modified device. In some instances, the FDA may require clinical trials to support a supplement application. A manufacturer of a device cleared through the 510(k) process must submit an additional premarket notification if it intends to make a change or modification in the device that could significantly affect the safety or effectiveness of the device, such as a significant change or modification in design, material, chemical composition, energy source, labeling or manufacturing process. Any change in the intended uses of a PMA device or a 510(k) device requires an approval supplement or new cleared premarket notification. Exported devices are subject to the regulatory requirements of each country to which the device is exported, as well as certain FDA export requirements.
Continuing FDA Regulation
After a device is placed on the market, numerous FDA and other regulatory requirements continue to apply. These include:
|
|
• |
establishment registration and device listing with the FDA; |
|
|
• |
quality system regulations, which require manufacturers to follow stringent design, testing; |
|
|
• |
process control, documentation and other quality assurance procedures; |
|
|
• |
labeling regulations, which prohibit the promotion of products for unapproved, i.e. “off label,” uses and impose other restrictions on labeling; |
|
|
• |
Medical Device Reporting regulations, which require that manufacturers report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if it were to recur; |
|
|
• |
corrections and removal reporting regulations, which require that manufacturers report to the FDA field corrections and product recalls or removals if undertaken to reduce a risk to health posed by the device or to remedy a violation of the FDCA that may present a risk to health; and |
|
|
• |
requirements to conduct postmarket surveillance studies to establish continued safety data. |
We are required to, and have, registered with the FDA and the International Organization for Standardization (“ISO”) as medical device manufacturers and must obtain all necessary permits and licenses to operate our business. As manufacturers, we and our suppliers are subject to announced and unannounced inspections by the FDA to determine our compliance with the Quality System Regulation (“QSR”) and other regulations.
In Europe, we need to comply with the requirements of the Medical Devices Directive (“MDD”) and appropriately affix the CE Mark on our products to attest to such compliance. To achieve compliance, our products must meet the “Essential Requirements” of the MDD relating to safety and performance and we must successfully undergo verification of our regulatory compliance, or conformity assessment, by a notified body selected by us. The level of scrutiny of such assessment depends on the regulatory class of the product. We are subject to continued surveillance by our notified body and will be required to report any serious adverse incidents to the appropriate authorities. We also must comply with additional requirements of individual countries in which our products are marketed. In the European Community, we are required to maintain certain ISO certifications in order to sell products. These regulations require us or our manufacturers to manufacture products and maintain documents in a prescribed manner with respect to design, manufacturing, testing, labeling and control activities.
Impact of Regulation
Failure to comply with the applicable regulatory requirements can result in enforcement action by the FDA, which may include, among other things, any of the following sanctions:
|
|
• |
warning letters, fines, injunctions, consent decrees and civil penalties; |
|
|
• |
repair, replacement, refund or seizure of our products; |
|
|
• |
operating restrictions, partial suspension or total shutdown of production; |
|
|
• |
refusing our request for 510(k) clearance or premarket approval of new products or modifications to existing products; |
10
|
|
• |
withdrawing or suspending clearances or approvals that are already granted; |
|
|
• |
criminal prosecution; and |
|
|
• |
disgorgement of profits. |
Further, the levels of revenues and profitability of medical device companies like us may be affected by the continuing efforts of government and third party payors to contain or reduce the costs of health care through various means. For example, in certain foreign markets, pricing or profitability of products is subject to governmental control. In the United States, there have been, and we expect that there will continue to be, a number of federal and state proposals to implement similar governmental controls.
Therefore, we cannot assure you that any of our products will be considered cost effective, or that, following any commercialization of our products, coverage and reimbursement will be available or sufficient to allow us to manufacture and sell them competitively and profitably.
Health Care Regulation
Our business activities are subject to additional healthcare regulation and enforcement by the federal government and by authorities in the states and foreign jurisdictions in which we conduct our business. Such laws include, without limitation, state and federal anti-kickback, fraud and abuse, false claims, privacy and security and physician payment transparency laws. If our operations are found to be in violation of any of such laws that apply to us, we may be subject to penalties, including, without limitation, civil and criminal penalties, damages, fines, the curtailment or restructuring of our operations, exclusion from participation in federal and state healthcare programs and imprisonment, any of which could adversely affect our ability to operate our business and our financial results.
In the United States, there have been, and we expect there to continue to be, a number of legislative and regulatory initiatives, at both the federal and state government levels, to change the healthcare system in ways that, if approved, could affect our ability to sell our products profitably. At the current time, our products are not defined as durable medical equipment (“DME”). Non-DME devices used in surgical procedures are normally paid directly by the hospital or health care provider and not reimbursed separately by third-party payors. Instead, the hospital or health care provider is reimbursed based on the procedure performed and the inpatient or outpatient stay. As a result, these types of devices are subject to intense price competition that can place a small manufacturer at a competitive disadvantage as hospitals, ambulatory surgery centers and health care providers attempt to negotiate lower prices for products such as the ones we develop and sell.
In March 2010, President Obama signed into law both the Patient Protection and Affordable Care Act (the “Affordable Care Act”) and the reconciliation law known as Health Care and Education Reconciliation Act (the “Reconciliation Act,” and, with the Affordable Care Act, the “2010 Health Care Reform Legislation”). The constitutionality of the 2010 Health Care Reform Legislation was confirmed twice by the Supreme Court of the United States. Both Congressional leaders and newly elected President Trump have announced plans to repeal or modify the 2010 Health Care Reform Legislation. At this time the Company is not certain as to the impact of federal health care legislation on its business.
The 2010 Health Care Reform Legislation subjects manufacturers of medical devices to an excise tax of 2.3% on certain U.S. sales of medical devices beginning in January 2013. This excise tax was suspended in December 2015 for two years, and we anticipate that this may be repealed. If eventually implemented, this excise tax will likely increase our expenses in the future.
Further, the 2010 Health Care Reform Legislation includes the Open Payments Act (formerly referred to as the Physician Payments Sunshine Act), which, in conjunction with its implementing regulations, requires certain manufacturers of certain drugs, biologics, and devices that are reimbursed by Medicare, Medicaid and the Children’s Health Insurance Program to report annually certain payments or “transfers of value” provided to physicians and teaching hospitals and to report annually ownership and investment interests held by physicians and their immediate family members during the preceding calendar year. We have provided reports under the Open Payments Act to the Centers for Medicare & Medicaid Services since 2014. The failure to report appropriate data accurately, timely, and completely could subject us to significant financial penalties. Other countries and several states currently have similar laws and more may enact similar legislation.
International Regulation and Potential Impact
Through the Senhance Acquisition, the Company has expanded into international markets and intends to pursue continued expansion. Some of these markets maintain unique regulatory requirements outside of or in addition to those of the U.S. FDA and the European Union. The Senhance System is CE marked, which allows us to offer the product for sale in a number of jurisdictions, including select countries in Europe, the Middle East and Asia. Due to the variations in regulatory requirements within territories, the Company may
11
be required to perform additional safety or clinical testing or fulfill additional agency requirements for specific territories. The Company may also be required to apply for registration using third parties within those territories and may be dependent upon the third parties’ successful regulatory processes to file, register and list the product applications and associated labeling, which could lead to significant investments and resource use. These additional requirements may result in delays in international registrations and commercialization of our products in certain countries.
In addition, we are utilizing distributors and sales agents in various territories throughout Europe, the Middle East and Africa, and need to ensure that our activities, and the activities of our distributors and sales agents, are compliant with local law and U.S. laws governing the sales of medical devices. The laws governing the registration, approval, clearance and sales of medical devices, such as the Senhance System, in multiple jurisdictions are complex, and the failure to comply with such laws in any given jurisdiction could subject us to financial penalties or suspension or termination of our ability to sell our products in the applicable jurisdiction.
Employees
As of December 31, 2016, we had 104 employees, including 103 full time employees. The Company considers its relationships with its employees to be good.
Corporate Information
The Company’s principal executive offices are located at 635 Davis Drive, Suite 300, Morrisville, NC 27560. TransEnterix Surgical was originally incorporated under the laws of the State of Delaware on July 12, 2006. On September 3, 2013, TransEnterix Surgical merged with and into a merger subsidiary of SafeStitch Medical, Inc. and became a wholly owned subsidiary of SafeStitch in a reverse merger transaction. SafeStitch was originally incorporated on August 19, 1988 as NCS Ventures Corp. under the laws of the State of Delaware. Its name was changed to Cellular Technical Services Company, Inc. on May 31, 1991. On September 4, 2007, SafeStitch acquired SafeStitch LLC, and, in January 2008, changed its name to SafeStitch Medical, Inc. On December 6, 2013, SafeStitch’s name was changed to TransEnterix, Inc. On September 21, 2015, TransEnterix International, a wholly owned subsidiary of the Company formed by the Company in conjunction with the Senhance Acquisition, acquired all of the membership interests of Vulcanos and changed the name of Vulcanos to TransEnterix Italia.
As of December 31, 2016, the active subsidiaries of the Company are TransEnterix Surgical, Inc., SafeStitch LLC, TransEnterix International, Inc. and TransEnterix Italia, S.r.l., TransEnterix Europe S.Á.R.L, TransEnterix Europe S.Á.R.L, Bertrange, Swiss Branch, Lugano and TransEnterix Asia PTE. LTD.
Available Information
The Company maintains a website at www.transenterix.com. Our Code of Business Conduct and Ethics, as reviewed and updated on October 26, 2016, is available on our website. Our annual reports on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K, and amendments to those reports, filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act, are available free of charge on our website as soon as practicable after electronic filing of such material with, or furnishing it to, the U.S. Securities and Exchange Commission (the “SEC”). This information may be read and copied at the Public Reference Room of the SEC at 100 F Street, N.E., Washington D.C. 20549. The SEC also maintains an internet website that contains reports, proxy statements, and other information about issuers, like TransEnterix, Inc., who file electronically with the SEC. The address of the site is http://www.sec.gov.
We are currently highly dependent on the success of a single product, the Senhance System. We cannot give any assurance that the Senhance System can be successfully commercialized or that it will receive regulatory clearance in the U.S.
We are currently highly dependent on the commercial success of the Senhance System, which is currently CE marked but not FDA cleared. We began our selling efforts for the Senhance System in the fourth quarter of 2015 and we have had limited commercial success to date. We cannot assure you that we will be able to successfully commercialize the Senhance System, or that the FDA will grant regulatory clearance for the Senhance System, for a number of reasons, including, without limitation, failure in our sales and marketing efforts, the potential introduction by our competitors of more clinically effective or cost-effective alternatives, or our ability to obtain regulatory clearance in a timely manner, or at all. Failure to successfully commercialize the Senhance System would have a material and adverse effect on our business. Regulatory authorities may change requirements for the clearance of a product regardless of previous discussions with us. These regulatory authorities may also clear a product for fewer or more limited uses than we request. In addition, the FDA or other non-U.S. regulatory authorities may not approve or clear the labeling claims necessary or desirable for the successful commercialization of our products.
12
We expect that our sales cycle for the Senhance System will be lengthy and unpredictable, which will make it difficult for us to forecast revenue and increase the magnitude of quarterly fluctuations in our operating results.
Purchase of a surgical robotic system such as the Senhance System represents a capital purchase by hospitals and other potential customers. The capital purchase nature of the transaction, the complexity of our product, the relative newness of surgical robotics and the competitive landscape requires us to spend substantial time and effort to assist potential customers in evaluating our robotic systems. We must communicate with multiple surgeons, administrative staff and executives within each potential customer in order to receive all approvals on behalf of such organizations. We may face difficulty identifying and establishing contact with such decision makers. Even after initial acceptance, the negotiation and documentation processes can be lengthy. Additionally, our customers may have strict limitations on spending given the current economic climate. We expect our sales cycle to typically range between six and twelve months, but it may be longer. Any delay in completing sales in a particular quarter could cause our operating results to fall below expectations. We also expect such a lengthy sales cycle makes it more difficult for us to accurately forecast revenue in future periods and may cause revenues and operating results to vary significantly in future periods.
Although we have recently expanded our commercial organization, we currently have limited marketing, sales and distribution capabilities. We intend to distribute our products through direct sales and independent contractor and distribution agreements with companies possessing established sales and marketing operations in the medical device industry, but there can be no assurance that we will be successful in building our sales capabilities. To the extent that we enter into distribution, co-promotion or other arrangements, our product revenue is likely to be lower than if we directly market or sell our products. In addition, any revenue we receive will depend in whole or in part upon the efforts of such third parties, which may not be successful and are generally not within our control. If we are unable to enter into such arrangements on acceptable terms or at all, we may not be able to successfully commercialize our products. If we are not successful in commercializing our existing and future products, either on our own or through collaborations with one or more third parties, our future product revenue will suffer and we may incur significant additional losses.
We will need to ensure that we have procedures in place to require our distributors and sales agents to comply with applicable laws and regulations governing the sales of medical devices in the jurisdictions where they operate. Failure to meet such requirements could subject us to financial penalties or the suspension or termination of the ability to sell our products in such jurisdiction.
We have a history of operating losses, and we may not be able to achieve or sustain profitability. In addition, we may be unable to continue as a going concern.
We have a limited operating history. We are not profitable and have incurred losses since our inception. Substantial doubt exists about our ability to continue as a going concern as a result of anticipated capital needs as well as past recurring losses and an accumulated deficit. Our net loss for the year ended December 31, 2016 was $120.0 million, and our accumulated deficit as of December 31, 2016 was $302.8 million. We believe that our existing cash and cash equivalents, together with cash received from sales of our products, will not be sufficient to meet our anticipated cash needs for the next 12 months.
We expect to continue to incur losses for the foreseeable future, and these losses will likely increase as we continue to develop and commercialize our products and product candidates. We will continue to incur research and development and general and administrative expenses related to our operations, and expect to increase our sales and marketing expenses as we increase our sales and marketing activities for the Senhance System in Europe and other jurisdictions where CE marking provides authorization for commercial activities, and pursue our regulatory strategy in the U.S. If our products fail in development or do not gain regulatory clearance or approval, or if our products do not achieve market acceptance, we may never become profitable. Even if we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods.
We will require substantial additional funding, which may not be available to us on acceptable terms, or at all.
The net proceeds of recent equity financings, including our at-the-market offering in 2016 and the ongoing equity financing with Lincoln Park Capital Fund, LLC commenced in December 2016, will not be sufficient to support development of our products and product candidates and provide us with the necessary resources to commercialize these products and product candidates. While we are currently focused on seeking U.S. regulatory approval for, and commercialization of, our Senhance System, we intend to advance multiple additional products through clinical and pre-clinical development in the future. We will need to raise substantial additional capital in order to continue our operations and achieve our business’ objectives.
We have filed two shelf registration statements which have been declared effective by the SEC. As of December 31, 2016, we had $37.8 million available for future financings. Such capacity will expire in November 2017. We cannot assure you that we will be successful in obtaining such additional financing on terms acceptable to the Company or at all.
13
Our future funding requirements will depend on many factors, including, but not limited to:
|
|
• |
the costs of our Senhance System commercialization and development activities; |
|
|
• |
the costs and timing of seeking and obtaining FDA and other non-U.S. regulatory clearances and approvals for the Senhance System; |
|
|
• |
the costs associated with establishing a sales force and commercialization capabilities; |
|
|
• |
the costs associated with the expansion of our manufacturing capabilities; |
|
|
• |
our need to expand our research and development activities; |
|
|
• |
the costs of acquiring, licensing or investing in businesses, products and technologies; |
|
|
• |
the economic and other terms and timing of our existing licensing arrangement and any collaboration, licensing or other arrangements into which we may enter in the future; |
|
|
• |
our need and ability to hire additional management, scientific, medical and sales and marketing personnel; |
|
|
• |
the effect of competing technological and market developments; |
|
|
• |
our need to implement additional internal systems and infrastructure, including financial and reporting systems, quality systems and information technology systems; and |
|
|
• |
our ability to maintain, expand and defend the scope of our intellectual property portfolio. |
Until we generate a sufficient amount of product revenue to finance our cash requirements, which may never occur, we expect to finance future cash needs primarily through public or private equity offerings, debt financings or strategic collaborations. We do not know whether additional funding will be available on acceptable terms, or at all. If we are not able to secure additional funding when needed, we may have to delay, reduce the scope of or eliminate one or more of our research and development programs. To the extent that we raise additional funds by issuing equity securities, our stockholders may experience significant dilution; and debt financing, if available, may involve restrictive covenants that limit our operations. To the extent that we raise additional funds through collaboration and licensing arrangements, it may be necessary to relinquish some rights to our products or grant licenses on terms that may not be favorable to us.
The surgical robotics industry is increasingly competitive, which can negatively impact our commercial opportunities.
The life sciences industry is highly competitive, and we face significant competition from many medical device companies that are researching and marketing products designed to address minimally invasive and robotic-assisted surgery, including new entrants in the competitive market. We are currently commercializing the Senhance System in Europe which accepts a CE Mark, the Middle East and selected countries in Asia and face significant competition in such markets. Many of our competitors, including Intuitive Surgical, have significantly greater financial, manufacturing, marketing and product development resources than we do. Some of the medical device companies we compete with or expect to compete with include Intuitive Surgical, Applied Medical, Titan Medical, Medtronic plc, Verb Surgical and a number of minimally invasive surgical device and robotic surgical device manufacturers and providers of products and therapies that are designed to reduce the need for or attractiveness of surgical intervention. In addition, many other universities and private and public research institutions are or may become active in research involving surgical devices for minimally invasive and robotic-assisted surgery.
We believe that our ability to successfully compete will depend on, among other things:
|
|
• |
the efficacy, safety and reliability of our products; |
|
|
• |
the speed at which we develop our products; |
|
|
• |
our ability to commercialize and market any of our products that may receive regulatory clearance or approval; |
|
|
• |
the cost of our products in relation to alternative devices; |
|
|
• |
the timing and scope of regulatory clearances or approvals; |
|
|
• |
whether our competitors substantially reduce the cost of ownership of an alternative device; |
|
|
• |
our ability to protect and defend intellectual property rights related to our products; |
|
|
• |
our ability to have our partners manufacture and sell commercial quantities of any approved products to the market; |
14
|
|
• |
the availability of adequate coverage and reimbursement by third-party payors for the procedures in which our products are used; |
|
|
• |
the effectiveness of our sales and marketing efforts; and |
|
|
• |
acceptance of future products by physicians and other health care providers. |
If our competitors market products that are more effective, safer, easier to use or less expensive than our products or future products, or that reach the market sooner than our products, we may not achieve commercial success. In addition, the medical device industry is characterized by rapid technological change. It may be difficult for us to stay abreast of the rapid changes in each technology. If we fail to stay at the forefront of technological change, we may be unable to compete effectively. Technological advances or products developed by our competitors may render our technologies or products obsolete or less competitive.
We anticipate that the highly competitive surgical robotics environment can lead our competitors to attempt to slow or derail our commercial progress. We are using our best efforts to enter the commercial markets effectively and efficiently while maintaining compliance with all regulatory and legal requirements. Responding to the actions of our competitors will require the attention of our management and may distract the management team from its focus on our commercial operations and lead to increased costs of commercialization, which could have a negative impact on our financial position.
The regulatory approval and clearance processes are expensive, time-consuming and uncertain and may prevent us from obtaining approvals or clearances, as the case may be, for the commercialization of some or all of our products.
In June 2015 the Company submitted a 510(k) application to the FDA for the SurgiBot System and worked with the FDA to provide additional information as requested. On April 19, 2016, the FDA informed the Company that the SurgiBot System did not meet the substantial equivalence test based on the 510(k) application. After interactions with the FDA, the Company determined that a new 510(k) application would need to be submitted in order to obtain clearance for the SurgiBot System. Based on this fact, we have evaluated the operational and financial feasibility of pursuing two 510(k) applications in parallel and have elected to primarily focus our near term regulatory efforts on the 510(k) submission for the Senhance System. We expect to file a 510(k) submission for the Senhance System in the 2017 second quarter. The product development and design, testing, manufacturing, labeling, approval, clearance, selling, marketing and distribution of medical devices are subject to extensive regulation by the FDA and other non-U.S. regulatory authorities, which regulations differ from country to country. We are not permitted to market our products in the United States until we receive a clearance letter under the 510(k) process or approval of a PMA from the FDA, depending on the nature of the device. Obtaining approval of any PMA can be a lengthy, expensive and uncertain process. We can provide no assurance, even if our products are reviewed under the 510(k) premarket notification process that the FDA will review our application expeditiously or determine that the device is substantially equivalent to a lawfully marketed non-PMA device. If the FDA asks questions during a 510(k) process, the time required to answer the questions can extend the time to market up to an additional six months. If we cannot sufficiently answer the questions, or for a variety of other reasons the FDA does not provide clearance for a product candidate, such as the Senhance System or the SurgiBot System, we cannot market the device.
Regulatory approval of a PMA, PMA supplement or clearance pursuant to a 510(k) premarket notification is not guaranteed, and the approval or clearance process, as the case may be, is expensive, uncertain and may, especially in the case of the PMA application, take several years. The FDA also has substantial discretion in the medical device clearance process or approval process. Despite the time and expense exerted, failure can occur at any stage, and we could encounter problems that cause us to repeat or perform additional development, standardized testing, pre-clinical studies and clinical trials. The number of pre-clinical studies and clinical trials that will be required for FDA clearance or approval varies depending on the medical device candidate, the disease or condition that the medical device candidate is designed to address, and the regulations applicable to any particular medical device candidate. The FDA or other non-U.S. regulatory authorities can delay, limit or deny clearance or approval of a medical device candidate for many reasons, including:
|
|
• |
a medical device candidate may not be deemed safe or effective, in the case of a PMA application; |
|
|
• |
a medical device candidate may not be deemed to be substantially equivalent to a device lawfully marketed either as a grandfathered device or one that was cleared through the 510(k) premarket notification process; |
|
|
• |
a medical device candidate may not be deemed to be in conformance with applicable standards and regulations; |
|
|
• |
FDA or other regulatory officials may not find the data from pre-clinical studies and clinical trials sufficient; |
|
|
• |
the FDA might not approve our processes or facilities or those of any of our third-party manufacturers for a Class III PMA device; |
15
|
|
• |
other non-U.S. regulatory authorities may not approve our processes or facilities or those of any of our third-party manufacturers, thereby restricting export; or |
|
|
• |
the FDA or other non-U.S. regulatory authorities may change clearance or approval policies or adopt new regulations. |
The laws governing the regulatory approval or clearance pathways in jurisdictions outside of the United States are complex. We need to ensure that our activities, and the activities of our distributors and agents, comply with such laws. If we do not comply with such laws, we may not be able to sell our products, including the Senhance System, in all jurisdictions we have targeted, which could have an adverse effect on our business operations and financial condition.
In the second quarter of 2016 we recorded a significant charge to earnings related to our evaluation of the recoverability of identifiable intangibles, other long-lived assets, and product inventory related to our SurgiBot System.
We assess the recoverability of identifiable intangibles with finite lives and other long-lived assets, such as equipment, for impairment whenever events or changes in circumstances indicate that the carrying value may not be recoverable in accordance with the provisions of FASB ASC 360, “Property, Plant and Equipment.” In accordance with FASB ASC 350, “Intangibles-Goodwill and Other,” goodwill and intangible assets with indefinite lives from acquisitions are evaluated annually, or more frequently, if events or circumstances indicate there may be an impairment, to determine whether any portion of the remaining balance of goodwill and indefinite lived intangibles may not be recoverable. Factors that may be considered a change in circumstances indicating that the carrying value of our goodwill or other intangible assets may not be recoverable include a decline in our stock price and market capitalization, future cash flows or external economic or industry changes. We may be required to record a significant charge to earnings in our financial statements during the period in which any impairment of our goodwill or other intangible assets is determined, which could adversely impact our results of operations.
On April 19, 2016, the FDA notified the Company that the SurgiBot System did not meet the criteria for substantial equivalence based on the data submitted in the 510(k) submission. As a result, we reprioritized our efforts on the commercialization of and regulatory clearance for the Senhance System. Consequently, in May 2016, we implemented a restructuring plan. The restructuring changes amounted to $5.7 million, of which $2.6 million was included as inventory write down related to restructuring and $3.1 million was included as restructuring and other charges in the consolidated statements of operations and comprehensive loss, during the second quarter of 2016. The restructuring and other charges of $3.1 million included: (i) $0.5 million to be paid in cash, of which $0.4 million related to employee severance costs and $0.1 million related to cancellation of certain contracts; and (ii) $2.6 million for other non-cash charges, of which $1.0 million related to the write-off of long-lived assets for the abandonment of certain equipment and tooling directly relating to the SurgiBot System and $1.6 million related to the write-off of intellectual property for certain patents also relating to the SurgiBot System.
Our global operations expose us to additional risks and challenges associated with conducting business internationally.
The international expansion of our business may expose us to risks inherent in conducting foreign operations. These risks include:
|
|
• |
challenges associated with managing geographically diverse operations, which require an effective organizational structure and appropriate business processes, procedures and controls; |
|
|
• |
the increased cost of doing business in foreign jurisdictions, including compliance with international and U.S. laws and regulations that apply to our international operations; |
|
|
• |
currency exchange and interest rate fluctuations and the resulting effect on our revenue and expenses, and the cost and risk of entering into hedging transactions, if we chose to do so in the future; |
|
|
• |
potentially adverse tax consequences; |
|
|
• |
complexities and difficulties in obtaining protection and enforcing our intellectual property; |
|
|
• |
compliance with additional regulations and government authorities in a highly regulated business; and |
|
|
• |
general economic and political conditions outside of the U.S. |
The risks that we face in our international operations may continue to intensify as we further develop and expand our international operations.
16
Our business may become subject to economic, political, regulatory and other risks associated with domestic and international operations.
Our business is subject to risks associated with conducting business domestically and internationally, in part due to some of our suppliers being located outside the U.S. Accordingly, our future results could be harmed by a variety of factors, including:
|
|
• |
difficulties in compliance with U.S. and non-U.S. laws and regulations; |
|
|
• |
changes in U.S. and non-U.S. regulations and customs; |
|
|
• |
changes in non-U.S. currency exchange rates and currency controls; |
|
|
• |
changes in a specific country’s or region’s political or economic environment; |
|
|
• |
trade protection measures, import or export licensing requirements or other restrictive actions by U.S. or non-U.S. governments; |
|
|
• |
negative consequences from changes in tax laws; and |
|
|
• |
difficulties associated with staffing and managing foreign operations, including differing labor relations. |
As we ramp up our manufacturing capabilities we face risks arising from sole suppliers of components and our ability to meet delivery schedules for sales of our products.
The Senhance System is manufactured for us under contract by a third party manufacturer. We or our manufacturer acquire raw materials and components of the Senhance System from vendors, some of which are sole suppliers. Although we believe that we have the manufacturing and inventory reserves to meet our anticipated Senhance System sales for the foreseeable future, we are currently taking steps to develop redundant manufacturing and supply alternatives. We cannot assure you that we will be successful in developing these redundant supply and manufacturing capabilities. If we are not successful, our business operations could suffer.
Our stock price has been volatile and may experience additional fluctuation in the future.
The market price of our common stock has been, and may continue to be, highly volatile, and such volatility could cause the market price of our common stock to decrease and could cause you to lose some or all of your investment in our common stock. During the two year period ended December 31, 2016, the market price of our common stock fluctuated from a high of $6.10 per share to a low of $1.03 per share, and our stock price continues to fluctuate. The market price of our common stock may continue to fluctuate significantly in response to numerous factors, some of which are beyond our control, such as:
|
|
• |
the announcement of favorable or unfavorable news regarding us, including our product development efforts and regulatory clearance activities; |
|
|
• |
the achievement of commercial sales of our products; |
|
|
• |
the announcement of new products or product enhancements by us or our competitors; |
|
|
• |
the impact of litigation, including class action litigation, on us; |
|
|
• |
developments concerning intellectual property rights and regulatory approvals; |
|
|
• |
variations in our and our competitors’ results of operations; |
|
|
• |
changes in earnings estimates or recommendations by securities analysts, if our common stock is covered by analysts; |
|
|
• |
developments in surgical robotics; |
|
|
• |
the results of product liability or intellectual property lawsuits; |
|
|
• |
future issuances of common stock or other securities; |
|
|
• |
the addition or departure of key personnel; |
|
|
• |
announcements by us or our competitors of acquisitions, investments or strategic alliances; and |
|
|
• |
general market conditions and other factors, including factors unrelated to our operating performance. |
Further, the stock market in general, and the market for medical device companies in particular, has recently experienced extreme price and volume fluctuations. Continued market fluctuations could result in extreme volatility in the price of our common stock, which could cause a decline in the value of our common stock and the loss of some or all of your investment.
17
Fluctuations in foreign currency exchange rates may adversely affect our financial results.
We conduct operations in several different countries, including the U.S. and throughout Europe, and portions of our revenues, expenses, assets and liabilities are denominated in U.S. dollars or other currencies, including Euros. Since our consolidated financial statements are presented in U.S. dollars, we must translate revenues, income and expenses, as well as assets and liabilities, into U.S. dollars at exchange rates in effect during or at the end of each reporting period. We have not historically hedged our exposure to foreign currency fluctuations. Accordingly, increases or decreases in the value of the U.S. dollar against the other currencies could materially affect our net operating revenues, operating income and the value of balance sheet items denominated in foreign currencies.
We have a substantial amount of indebtedness, which may adversely affect our financial resources and our ability to operate our business.
We are party with Silicon Valley Bank and Oxford Finance LLC (the “Lenders”), to, and jointly and severally liable with certain of our U.S. subsidiaries for, $20.0 million of outstanding debt under term loans issued under our Amended and Restated Loan Agreement, as amended (the “Loan Agreement”). The maturity date of the outstanding term loan is July 1, 2018. Our resulting substantial level of indebtedness and other financial obligations increase the possibility that we may be unable to pay, when due, the principal of, interest on, or other amounts due in respect of, our indebtedness. Further, under the Loan Agreement, we are subject to certain restrictive covenants that, among other things, may limit our ability to obtain additional financing for working capital requirements, product development activities, debt service requirements, and general corporate or other purposes. These restrictive covenants include, without limitation, restrictions on our ability to: (1) change the nature of our business; (2) incur additional indebtedness; (3) incur liens; (4) make certain investments; (5) make certain dispositions of assets; (6) merge, dissolve, consolidate or sell all or substantially all of our assets; (7) enter into transactions with affiliates; and (8) transfer more than designated amounts to our foreign subsidiaries during the term of the Loan Agreement. If we breach any of these restrictive covenants or are unable to pay our indebtedness under the Loan Agreement when due, this could result in a default under the Loan Agreement. In such event, the Lenders may elect (after the expiration of any applicable notice or grace periods) to declare all outstanding borrowings, together with accrued and unpaid interest and other amounts payable under the Loan Agreement, to be immediately due and payable. Any such occurrence would have an immediate and materially adverse impact on our business and results of operations. The Loan Agreement is secured by a security interest in all assets of the Company and its current and future U.S. subsidiaries, including a security interest in intellectual property proceeds, but excluding a current security interest in intellectual property.
We are party with Sofar to the Purchase Agreement pursuant to which we made the Senhance Acquisition. A significant portion of the purchase price is payable in tranches upon the achievement of designated milestone events, which are described in Item 8, Notes to Consolidated Financial Statements, Note 3 “Acquisition of Senhance Surgical Robotic System” of this Annual Report. As of January 4, 2017, the maximum amount of the aggregate milestone payments under the Purchase Agreement could be €21.4 million. If we are unable to make such milestone payments when due, such failure could have a material adverse effect on our financial condition.
Even if we obtain regulatory clearances or approvals for our products, the terms thereof and ongoing regulation of our products may limit how we manufacture and market our products, which could materially impair our ability to generate anticipated revenues.
Once regulatory clearance or approval has been granted, the cleared or approved product and its manufacturer are subject to continual review. Any cleared or approved product may be promoted only for its indicated uses. In addition, if the FDA or other non-U.S. regulatory authorities clear or approve any of our products, the labeling, packaging, adverse event reporting, storage, advertising and promotion for the product will be subject to extensive regulatory requirements. We and any outsourced manufacturers of our products are also required to comply with the FDA’s Quality System Regulation, or similar requirements of non-U.S. regulatory authorities which includes requirements relating to quality control and quality assurance, as well as the corresponding maintenance of records and documentation as well as other quality system requirements and regulations from non-U.S. regulatory authorities. Further, regulatory agencies must approve our manufacturing facilities for Class III devices before they can be used to manufacture our products, and all manufacturing facilities are subject to ongoing regulatory inspection. If we fail to comply with the regulatory requirements of the FDA, either before or after clearance or approval, or other non-U.S. regulatory authorities, or if previously unknown problems with our products, manufacturers or manufacturing processes are discovered, we could be subject to administrative or judicially imposed sanctions, including:
|
|
• |
restrictions on the products, manufacturers or manufacturing process; |
|
|
• |
adverse inspectional observations (“Form 483”), warning letters, non-warning letters incorporating inspectional observations; |
|
|
• |
civil or criminal penalties or fines; |
|
|
• |
injunctions; |
18
|
|
• |
product seizures, detentions or import bans; |
|
|
• |
voluntary or mandatory product recalls and publicity requirements; |
|
|
• |
suspension or withdrawal of regulatory clearances or approvals; |
|
|
• |
total or partial suspension of production; |
|
|
• |
imposition of restrictions on operations, including costly new manufacturing requirements; |
|
|
• |
refusal to clear or approve pending applications or premarket notifications; and |
|
|
• |
import and export restrictions. |
Any of these sanctions could have a material adverse effect on our reputation, business, results of operations and financial condition. Furthermore, our key component suppliers may not currently be or may not continue to be in compliance with all applicable regulatory requirements, which could result in our failure to produce our products on a timely basis and in the required quantities, if at all.
In addition, the FDA and other non-U.S. regulatory authorities may change their policies and additional regulations may be enacted that could prevent or delay regulatory clearance or approval of our products. We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad. If we are not able to maintain regulatory compliance, we would likely not be permitted to market our future products and we may not achieve or sustain profitability.
Once our products are cleared or approved, modifications to our products may require new 510(k) clearances, premarket approvals or new or amended CE Certificates of Conformity, and may require us to cease marketing or recall the modified products until clearances, approvals or the relevant CE Certificates of Conformity are obtained.
Any modification to a 510(k)-cleared device that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, design, or manufacture, requires a new 510(k) clearance or, possibly, a PMA. The FDA requires every manufacturer to make this determination in the first instance, but the FDA may review such determinations. The FDA may not agree with our decisions regarding whether new clearances or approvals are necessary. If the FDA disagrees with our determinations for any future changes, or prior changes to previously marketed products, as the case may be, we may be required to cease marketing or to recall the modified products until we obtain clearance or approval, and we may be subject to significant regulatory fines or penalties.
Furthermore, the FDA’s ongoing review of the 510(k) program may make it more difficult for us to make modifications to our products, either by imposing more strict requirements on when a new 510(k) for a modification to a previously cleared product must be submitted, or applying more onerous review criteria to such submissions. In July and December 2011, respectively, the FDA issued draft guidance documents addressing when to submit a new 510(k) due to modifications to 510(k) cleared products and the criteria for evaluating substantial equivalence. The July 2011 draft guidance document was ultimately withdrawn as the result of the FDASIA, and as a result, the FDA’s original guidance document regarding 510(k) modifications, which dates back to 1997, remains in place. It is uncertain when the FDA will seek to issue new guidance on product modifications. Any efforts to do so could result in a more rigorous review process and make it more difficult to obtain clearance for device modifications.
Even after clearance or approval for our products is obtained, we are subject to extensive post-market regulation by the FDA. Our failure to meet strict regulatory requirements could require us to pay fines, incur other costs or even close our facilities.
Even after we have obtained the proper regulatory clearance or approval to market a product, the FDA has the power to require us to conduct post-market studies. These studies can be very expensive and time-consuming to conduct. Failure to complete such studies in a timely manner could result in the revocation of clearance or approval and the recall or withdrawal of the product, which could prevent us from generating sales from that product in the United States. The FDA has broad enforcement powers, and any regulatory enforcement actions or inquiries, or other increased scrutiny on us, could dissuade some surgeons from using our products and adversely affect our reputation and the perceived safety and efficacy of our products.
We are also required to comply with the FDA’s QSR, which covers the methods used in, and the facilities and controls used for, the design, manufacture, quality assurance, labeling, packaging, sterilization, storage, shipping, installation and servicing of our marketed products. The FDA enforces the QSR through periodic announced and unannounced inspections of manufacturing facilities. In addition, in the future, regulatory authorities and/or customers may require specific packaging of sterile products, which could increase our costs and the price of our products. Later discovery of previously unknown problems with our products, including unanticipated adverse events or adverse events of unanticipated severity or frequency, manufacturing problems, or failure to comply with regulatory requirements such as QSR, may result in changes to labeling, restrictions on such products or manufacturing
19
processes, withdrawal of the products from the market, voluntary or mandatory recalls, a requirement to repair, replace or refund the cost of any medical device we manufacture or distribute, fines, suspension of regulatory approvals, product seizures, injunctions or the imposition of civil or criminal penalties which would adversely affect our business, operating results and prospects.
If one of our products, or a malfunction of one of our products, causes or contributes to a death or a serious injury, we will be subject to medical device reporting regulations, which can result in voluntary corrective actions or agency enforcement actions.
Under the FDA’s medical device reporting (“MDR”) regulations, we are required to report to the FDA any incident in which our product may have caused or contributed to a death or serious injury or in which our product malfunctioned and, if the malfunction were to recur, would likely cause or contribute to death or serious injury. Repeated product malfunctions may result in a voluntary or involuntary product recall, which could divert managerial and financial resources, impair our ability to manufacture our products in a cost-effective and timely manner, and have an adverse effect on our reputation, results of operations and financial condition. We are also required to follow detailed recordkeeping requirements for all firm-initiated medical device corrections and removals, and to report such corrective and removal actions to the FDA if they are carried out in response to a risk to health and have not otherwise been reported under the MDR regulations.
All manufacturers bringing medical devices to market in the European Economic Area (“EEA”) are legally bound to report any incident that led or might have led to the death or serious deterioration in the state of health of a patient, user or other person, and which the manufacturer’s device is suspected to have caused, to the competent authority in whose jurisdiction the incident occurred. In such case, the manufacturer must file an initial report with the relevant competent authority, which would be followed by further evaluation or investigation of the incident and a final report indicating whether further action is required.
Any adverse event involving our products could result in future voluntary corrective actions, such as recalls or customer notifications, or agency action, such as inspection or enforcement action. Adverse events involving our products have been reported to us in the past, and we cannot guarantee that they will not occur in the future. Any corrective action, whether voluntary or involuntary, will require the dedication of our time and capital, distract management from operating our business and may harm our reputation and financial results.
A recall of our products, either voluntarily or at the direction of the FDA or another governmental authority, or the discovery of serious safety issues with our products, could have a significant adverse impact on us.
The FDA and similar foreign governmental authorities such as the competent authorities of the EEA countries have the authority to require the recall of commercialized products in the event of material deficiencies or defects in design or manufacture or in the event that a product poses an unacceptable risk to health. Manufacturers may, under their own initiative, recall a product if any material deficiency in a device is found. A government-mandated or voluntary recall by us or one of our distributors could occur as a result of an unacceptable risk to health, component failures, manufacturing errors, design or labeling defects or other deficiencies and issues.
Any future recalls of any of our products would divert managerial and financial resources and could have an adverse effect on our reputation, results of operations and financial condition, which could impair our ability to produce our products in a cost-effective and timely manner in order to meet our customers’ demands. We may also be required to bear other costs or take other actions that may have a negative impact on our future sales and our ability to generate profits.
U.S. legislative or FDA regulatory reforms may make it more difficult and costly for us to obtain regulatory approval of our product candidates and to manufacture, market and distribute our products after approval is obtained.
From time to time, legislation is drafted and introduced in Congress that could significantly change the statutory provisions governing the regulatory approval, manufacture and marketing of regulated products. In addition, FDA regulations and guidance are often revised or reinterpreted by the FDA in ways that may significantly affect our business and our products. Any new regulations or revisions or reinterpretations of existing regulations may impose additional costs or lengthen review times of future products. In addition, FDA regulations and guidance are often revised or reinterpreted by the agency in ways that may significantly affect our business and our products. It is impossible to predict whether legislative changes will be enacted or FDA regulations, guidance or interpretations changed, and what the impact of such changes, if any, may be.
20
Even if we receive regulatory clearance or approval to market our products, the market may not be receptive to our products, which could undermine our financial viability.
Even if our products obtain regulatory clearance or approval, resulting products may not gain market acceptance among physicians, patients, health care payors and/or the medical community. We have experienced minimal sales of our Senhance System, to date. We believe that the degree of market acceptance will depend on a number of factors, including:
|
|
• |
timing of market introduction of competitive products; |
|
|
• |
safety and efficacy of our products; |
|
|
• |
physician training in the use of our products; |
|
|
• |
prevalence and severity of any side effects; |
|
|
• |
potential advantages or disadvantages over alternative treatments; |
|
|
• |
strength of marketing and distribution support; and |
|
|
• |
price of our future products, both in absolute terms and relative to alternative treatments. |
If applicable, availability of coverage and reimbursement from government and other third-party payors can also impact the acceptance of our product offerings.
If we fail to attract and retain key management and professional personnel, we may be unable to successfully commercialize or develop our products.
We will need to effectively manage our operational, sales and marketing, development and other resources in order to successfully pursue our commercialization and research and development efforts for our existing and future products. Our success depends on our continued ability to attract, retain and motivate highly qualified personnel. If we are not successful in retaining and recruiting highly qualified personnel, our business may be harmed as a result.
We may be subject, directly or indirectly, to federal and state anti-kickback, fraud and abuse, false claims, privacy and security and physician payment transparency laws. If we are unable to comply, or have not fully complied, with such laws, we could face substantial penalties.
Our business activities are subject to additional healthcare regulation and enforcement by the federal government and by authorities in the states and foreign jurisdictions in which we conduct our business. Such laws include, without limitation, state and federal anti-kickback, fraud and abuse, false claims, privacy and security and physician payment transparency laws. If our operations are found to be in violation of any of such laws that apply to us, we may be subject to penalties, including, without limitation, civil and criminal penalties, damages, fines, the curtailment or restructuring of our operations, exclusion from participation in federal and state healthcare programs and imprisonment, any of which could adversely affect our ability to operate our business and our financial results.
Current legislation and future legislative or regulatory reform of the health care system may affect our ability to sell our products profitably.
In the United States, there have been, and we expect there to continue to be, a number of legislative and regulatory initiatives, at both the federal and state government levels, to change the healthcare system in ways that, if approved, could affect our ability to sell our products profitably. While many of the proposed policy changes require congressional approval to implement, we cannot assure you that reimbursement payments under governmental and private third-party payor programs to health care providers will remain at levels comparable to present levels or will be sufficient to cover the costs allocable to patients eligible for reimbursement under these programs. Any changes that lower reimbursement rates under Medicare, Medicaid or private payor programs could negatively affect our business.
To the extent that any of our products are deemed to be durable medical equipment (“DME”), they may be subject to distribution under Medicare’s Competitive Acquisition regulations, which could adversely affect the amount that we can seek from payors. Non‑DME devices used in surgical procedures are normally paid directly by the hospital or health care provider and not reimbursed separately by third-party payors. As a result, these types of devices are subject to intense price competition that can place a small manufacturer at a competitive disadvantage as hospitals and health care providers attempt to negotiate lower prices for products such as the ones we develop and sell.
21
Most significantly, in March 2010, President Obama signed into law both the Patient Protection and Affordable Care Act (the “Affordable Care Act”) and the reconciliation law known as Health Care and Education Reconciliation Act (the “Reconciliation Act”, and, with the Affordable Care Act, the “2010 Health Care Reform Legislation”). Both Congressional leaders and President Trump have announced plans to repeal or modify the 2010 Health Care Reform Litigation. At this time, the Company is not certain as to the impact of federal health care legislation on its business.
The 2010 Health Care Reform Legislation subjects manufacturers of medical devices to an excise tax of 2.3% on certain U.S. sales of medical devices beginning in January 2013. This excise tax was suspended in December 2015 for two years, however, if eventually implemented, this excise tax will likely increase our expenses in the future.
Further, the 2010 Health Care Reform Legislation includes the Open Payments Act (formerly referred to as the Physician Payments Sunshine Act), which, in conjunction with its implementing regulations, requires certain manufacturers of certain drugs, biologics, and devices that are reimbursed by Medicare, Medicaid and the Children’s Health Insurance Program to report annually certain payments or “transfers of value” provided to physicians and teaching hospitals and to report annually ownership and investment interests held by physicians and their immediate family members during the preceding calendar year. We provided reports under the Open Payments Act to the Centers for Medicare & Medicaid Services (“CMS”). The failure to report appropriate data accurately, timely, and completely could subject us to significant financial penalties. Other countries and several states currently have similar laws and more may enact similar legislation.
We are unable to predict what additional legislation or regulation relating to the health care industry or third-party coverage and reimbursement may be enacted in the future or what effect such legislation or regulation would have on our business. Any cost containment measures or other health care system reforms that are adopted could have a material and adverse effect on our ability to commercialize our existing and future products successfully.
Because our design, development and manufacturing capabilities are limited, we may rely on third parties to design, develop, manufacture or supply some of our products. An inability to find additional or alternate sources for these services and products could materially and adversely affect our financial condition and results of operations.
We have used third-party design and development sources to assist in the design and development of our medical device products. In the future, we may choose to use additional third-party sources for the design and development of our products. If these design and development partners are unable to provide their services in the timeframe or to the performance level that we require, we may not be able to establish a contract and obtain a sufficient alternative supply from another supplier on a timely basis and in the manner that we require.
Our products require precise, high quality manufacturing. We and our contract manufacturers will be subject to ongoing periodic unannounced inspection by the FDA and non-U.S. regulatory authorities to ensure strict compliance with the quality systems regulations, current “good manufacturing practices” and other applicable government regulations and corresponding standards. If we or our contract manufacturers fail to achieve and maintain high manufacturing standards in compliance with QSR, we may experience manufacturing errors resulting in patient injury or death, product recalls or withdrawals, delays or interruptions of production or failures in product testing or delivery, delay or prevention of filing or approval of marketing applications for our products, cost overruns or other problems that could seriously harm our business.
Any performance failure by us or on the part of our design and development partners or contract manufacturers could delay product development or regulatory clearance or approval of our products, or commercialization of our products and future products, depriving us of potential product revenue and resulting in additional losses. In addition, our dependence on any third party for design, development or manufacturing could adversely affect our future profit margins. Our ability to replace any then-existing manufacturer may be difficult because the number of potential manufacturers is limited and, in the case of Class III devices, the FDA must approve any replacement manufacturer before manufacturing can begin. It may be difficult or impossible for us to identify and engage a replacement manufacturer on acceptable terms in a timely manner, or at all.
We may become subject to potential product liability claims, and we may be required to pay damages that exceed our insurance coverage.
Our business exposes us to potential product liability claims that are inherent in the design, testing, manufacture, sale and distribution of our products and each of our product candidates that we are seeking to introduce to the market. Surgical medical devices involve significant risks of serious complications, including bleeding, nerve injury, paralysis, infection, and even death. Any product liability claim brought against us, with or without merit, could result in the increase of our product liability insurance rates or in our inability to secure coverage in the future on commercially reasonable terms, if at all. In addition, if our product liability insurance proves to be inadequate to pay a damage award, we may have to pay the excess of this award out of our cash reserves, which could significantly harm our financial condition. If longer-term patient results and experience indicate that our products or any component of a product
22
causes tissue damage, motor impairment or other adverse effects, we could be subject to significant liability. A product liability claim, even one without merit, could harm our reputation in the industry, lead to significant legal fees, and result in the diversion of management’s attention from managing our business.
If we are unable to obtain and enforce patent protection for our products, our business could be materially harmed.
Our success depends, in part, on our ability to protect proprietary methods and technologies that we develop or license under the patent and other intellectual property laws of the United States and other countries, so that we can prevent others from unlawfully using our inventions and proprietary information. However, we may not hold proprietary rights to some patents required for us to commercialize our proposed products. We hold nine United States patents, three Italian patents, one Russian patent, a European patent, two Japanese patents, two Australian patents and a Chinese patent, and have more than forty patent applications filed in the United States and abroad. Because certain U.S. patent applications are confidential until patents issue, such as applications filed prior to November 29, 2000, or applications filed after such date which will not be filed in foreign countries, third parties may have filed patent applications for technology covered by our pending patent applications without our being aware of those applications, and our patent applications may not have priority over those applications. For this and other reasons, we or our third-party collaborators may be unable to secure desired patent rights, thereby losing desired exclusivity. If licenses are not available to us on acceptable terms, we will not be able to market the affected products or conduct the desired activities, unless we challenge the validity, enforceability or infringement of the third-party patent or otherwise circumvent the third-party patent.
Our strategy depends on our ability to promptly identify and seek patent protection for our discoveries. In addition, we will rely on third-party collaborators to file patent applications relating to proprietary technology that we develop jointly during certain collaborations. The process of obtaining patent protection is expensive and time-consuming. If our present or future collaborators fail to file and prosecute all necessary and desirable patent applications at a reasonable cost and in a timely manner, our business will be adversely affected. Despite our efforts and the efforts of our collaborators to protect our proprietary rights, unauthorized parties may be able to develop and use information that we regard as proprietary.
The issuance of a patent provides a presumption, but does not guarantee that it is valid. Any patents we have obtained, or obtain in the future, may be challenged or potentially circumvented. Moreover, the United States Patent and Trademark Office (the “USPTO”) may commence interference proceedings involving our patents or patent applications. Any such challenge to our patents or patent applications would be costly, would require significant time and attention of our management and could have a material adverse effect on our business. In addition, future court decisions may introduce uncertainty in the enforceability or scope of any patent, including those owned by medical device companies.
Our pending patent applications may not result in issued patents. The patent position of medical device companies, including ours, is generally uncertain and involves complex legal and factual considerations. The standards that the USPTO and its foreign counterparts use to grant patents are not always applied predictably or uniformly and can change. There is also no uniform, worldwide policy regarding the subject matter and scope of claims granted or allowable in medical device patents. Accordingly, we do not know the degree of future protection for our proprietary rights or the breadth of claims that will be allowed in any patents issued to us or to others. The legal systems of certain countries do not favor the aggressive enforcement of patents, and the laws of foreign countries may not protect our rights to the same extent as the laws of the United States. Therefore, the enforceability or scope of our owned or licensed patents in the United States or in foreign countries cannot be predicted with certainty, and, as a result, any patents that we own or license may not provide sufficient protection against competitors. We may not be able to obtain or maintain patent protection for our pending patent applications, those we may file in the future, or those we may license from third parties.
We cannot assure you that any patents that will issue, that may issue or that may be licensed to us will be enforceable or valid or will not expire prior to the commercialization of our products, thus allowing others to more effectively compete with us. Therefore, any patents that we own or license may not adequately protect our future products.
For our Senhance System, we rely on our license from the European Union, and any loss of our rights under such license agreement, or failure to properly prosecute, maintain or enforce the patent applications underlying such license agreement, could materially adversely affect our business prospects for the Senhance System.
Some of the patents and patent applications in our patent portfolio related to the Senhance System are licensed to TransEnterix Italia under a license agreement with the European Union. Presently, we rely on such licensed technology for our Senhance System products and may license additional technology from the European Union or other third parties in the future. The EU license agreement gives us rights for the commercial exploitation of the licensed patents, patent applications and know-how, subject to certain provisions of the license agreement. Failure to comply with these provisions could result in the loss of our rights under the EU license agreement. Our inability to rely on these patents and patent applications which are the basis of certain aspects of our Senhance System technology would have an adverse effect on our business.
23
Further, our success will depend in part on the ability of us, the European Union and other third-party licensors to obtain, maintain and enforce patent protection for our licensed intellectual property and, in particular, those patents to which we have secured exclusive rights. We, the European Union or other third-party licensors may not successfully prosecute the patent applications which are licensed to us, may fail to maintain these patents, and may determine not to pursue litigation against other companies that are infringing these patents, or may pursue such litigation less aggressively than necessary to obtain an acceptable outcome from any such litigation. Without protection for the intellectual property we have licensed, other companies might be able to offer substantially identical products for sale, which could materially adversely affect our competitive business position, business prospects and results of operations.
If we or our licensors are unable to protect the confidentiality of our proprietary information and know-how, the value of our technology and products could be adversely affected.
In addition to patent protection, we also rely on other proprietary rights, including protection of trade secrets, know-how and confidential and proprietary information. To maintain the confidentiality of trade secrets and proprietary information, we will seek to enter into confidentiality agreements with our employees, consultants and collaborators upon the commencement of their relationships with us. These agreements generally require that all confidential information developed by the individual or made known to the individual by us during the course of the individual’s relationship with us be kept confidential and not disclosed to third parties. Our agreements with employees also generally provide and will generally provide that any inventions conceived by the individual in the course of rendering services to us shall be our exclusive property. However, we may not obtain these agreements in all circumstances, and individuals with whom we have these agreements may not comply with their terms. In the event of unauthorized use or disclosure of our trade secrets or proprietary information, these agreements, even if obtained, may not provide meaningful protection, particularly for our trade secrets or other confidential information. To the extent that our employees, consultants or contractors use technology or know-how owned by third parties in their work for us, disputes may arise between us and those third parties as to the rights in related inventions. Adequate remedies may not exist in the event of unauthorized use or disclosure of our confidential information. The disclosure of our trade secrets would impair our competitive position and may materially harm our business, financial condition and results of operations.
Our commercial success depends significantly on our ability to operate without infringing the patents and other proprietary rights of third parties.
Other entities may have or obtain patents or proprietary rights that could limit our ability to manufacture, use, sell, offer for sale or import products or impair our competitive position. In addition, to the extent that a third party develops new technology that covers our products, we may be required to obtain licenses to that technology, which licenses may not be available or may not be available on commercially reasonable terms, if at all. If licenses are not available to us on acceptable terms, we will not be able to market the affected products or conduct the desired activities, unless we challenge the validity, enforceability or infringement of the third-party patent or circumvent the third-party patent, which would be costly and would require significant time and attention of our management. Third parties may have or obtain valid and enforceable patents or proprietary rights that could block us from developing products using our technology. Our failure to obtain a license to any technology that we require may materially harm our business, financial condition and results of operations.
If we become involved in patent litigation or other proceedings related to a determination of rights, we could incur substantial costs and expenses, substantial liability for damages or be required to stop our product development and commercialization efforts, any of which could materially adversely affect our liquidity, business prospects and results of operations.
Third parties may sue us for infringing their patent rights. Likewise, we may need to resort to litigation to enforce a patent issued or licensed to us or to determine the scope and validity of proprietary rights of others. In addition, a third party may claim that we have improperly obtained or used its confidential or proprietary information. Furthermore, in connection with our third-party license agreements, we generally have agreed to indemnify the licensor for costs incurred in connection with litigation relating to intellectual property rights. The cost to us of any litigation or other proceeding relating to intellectual property rights, even if resolved in our favor, could be substantial, and the litigation would divert our management’s efforts. Some of our competitors may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources. Uncertainties resulting from the initiation and continuation of any litigation could limit our ability to continue our operations.
If any parties successfully claim that our creation or use of proprietary technologies infringes upon their intellectual property rights, we might be forced to pay damages, potentially including treble damages, if we are found to have willfully infringed on such parties’ patent rights. In addition to any damages we might have to pay, a court could require us to stop the infringing activity or obtain a license. Any license required under any patent may not be made available on commercially acceptable terms, if at all. In addition, such licenses are likely to be non-exclusive and, therefore, our competitors may have access to the same technology licensed to us. If we fail to obtain a required license and are unable to design around a patent, we may be unable to effectively market some of our technology and products, which could limit our ability to generate revenues or achieve profitability and possibly prevent us from generating revenue sufficient to sustain our operations.
24
Our stockholders have experienced dilution of their percentage ownership of our stock and may experience additional dilution in the future.
We have raised significant capital through the issuance of our common stock and will need to raise substantial additional capital in order to continue our operations and achieve our business objectives. The future issuance of the Company’s equity securities will further dilute the ownership of our outstanding common stock. The market price of our common stock has been, and may continue to be, highly volatile, and such volatility could cause the market price of our common stock to decrease and could cause you to lose some or all of your investment in our common stock.
Sales by stockholders of substantial amounts of our shares of common stock, the issuance of new shares of common stock by us or the perception that these sales may occur in the future could materially and adversely affect the market price of our common stock.
As of December 31, 2016, our directors, executive officers, principal stockholders and affiliated entities beneficially owned, in the aggregate, approximately 38% of our outstanding voting securities. As a result, if some or all of them acted together, they would have the ability to exert substantial influence over the election of our board of directors and the outcome of issues requiring approval by our stockholders. This concentration of ownership may also have the effect of delaying or preventing a change in control of the Company that may be favored by other stockholders. This could prevent transactions in which stockholders might otherwise recover a premium for their shares over current market prices.
We have been named as a defendant in a class action lawsuit with claims of violations of the federal securities laws.
We have been named as a defendant in a class action lawsuit alleging claims under the federal securities laws. See the description in Item 3 below. Based on the limited nature of the plaintiff’s allegations, the stage of the proceedings, and because significant legal issues have yet to be raised or decided, we have determined that the amount of any possible loss or range of possible loss in connection with this matter is not reasonably estimable. Our management believes the alleged claims are without merit, but if we are not successful in our defense of this action, we could be liable for significant damages. The class action lawsuit may also divert our attention from our ordinary business operations, and we may incur expenses associated with the defense. Accordingly, the ultimate resolution of the matter could have a material adverse effect on our business, results of operations, financial condition and liquidity and, consequently, could negatively impact the trading price of our common stock.
None.
Our principal corporate office is located at 635 Davis Drive, Suite 300, Morrisville, North Carolina. We lease this facility, which consists of 37,328 square feet, for a five-year term, under a lease that commenced on April 1, 2010. An amendment to this lease was signed on June 13, 2014, extending the lease term until June 30, 2018. Pursuant to a lease entered into on October 24, 2013, we also lease 24,000 square feet of warehouse and office space in Durham, North Carolina. That lease commenced in January 2014 and has a 52-month term, with a six-year renewal option.
Our Italian research and development and demonstration facilities are located at Viale dell'Innovazione 3, 20126 Milan, Italy. We lease these facilities, which consist of 11,273 square feet, for a six-year term ending on July 31, 2022, under a lease that commenced on May 12, 2016.
On June 2, 2016, a stockholder filed a putative class action complaint, Ashok V. Bankley, individually and on behalf of all others similarly situated vs. TransEnterix, Inc., Todd M. Pope and Joseph P. Slattery , in the United States District Court for the Eastern District of North Carolina (Case No. 5:16-cv-00313-D) (the “Initial Complaint”), against the Company and two of its executive officers on behalf of all persons who purchased or otherwise acquired the Company’s common stock between February 10, 2016 and May 10, 2016. On August 4, 2016, the defendants filed a motion to dismiss the Initial Complaint for failure to state a claim under the securities laws. On August 30, 2016, the court appointed Randall Clark, Samir Patel, the Underhill Cemetery Association, and the North Underhill Cemetery Association as the lead plaintiffs in the Initial Complaint, and also provided the plaintiffs an opportunity to amend the Initial Complaint. On September 26, 2016, the lead plaintiffs filed an Amended Complaint. Among other things, the Amended Complaint asserts revised claims against the Company and Messrs. Pope and Slattery, and adds claims against certain current and former members of the Company’s Board of Directors, and Cantor Fitzgerald & Co., the sales agent under the 2016 Sales Agreement, under which the Company offered and sold, through Cantor, shares of common stock in its 2016 ATM Offering. The Amended Complaint alleges that the defendants made false and misleading public statements related to the Company's SurgiBot
25
System and its 510(k) application in violation of certain federal securities laws. The Amended Complaint seeks class certification of a class consisting of all persons who purchased or otherwise acquired the Company’s common stock between February 10, 2016 and May 10, 2016, class certification of a subclass of persons who purchased or otherwise acquired the Company’s common stock in connection with the 2016 ATM Offering between February 9, 2016 and April 19, 2016, unspecified monetary damages, costs, and attorneys’ fees. On November 8, 2016, the defendants moved to dismiss the Amended Complaint, which the plaintiffs later opposed. As of January 23, 2017, the motions to dismiss were fully briefed and deemed submitted to the court for decision.
On June 9, 2016, a different stockholder filed another putative class action complaint, Thomas Ravey, individually and on behalf of all others similarly situated vs. TransEnterix, Inc., Todd M. Pope and Joseph P. Slattery, in the United States District Court for the Middle District of North Carolina (Case No. 1:16-cv-599) (the “Ravey Action”). The Ravey Action asserted substantially similar claims against the same defendants and sought substantially similar relief as the Initial Complaint. On August 4, 2016, the plaintiff in the Ravey Action voluntarily dismissed the Ravey Action.
On July 8, 2016, a stockholder filed a putative derivative complaint, Otto Pikal v. Todd M. Pope, et al., in the General Court of Justice, Superior Court Division, Wake County, North Carolina (case number 16CV008930), on behalf of the Company against certain of our current officers and directors. The complaint alleges, among other things, that the defendants breached their fiduciary duties by disseminating false and misleading information to the Company’s shareholders relating to the Company’s SurgiBot System and its 510(k) application in violation of certain federal securities laws and by failing to ensure that the Company maintained adequate internal controls. The complaint seeks, among other things, unspecified monetary damages and an order directing the Company to take steps to improve its corporate governance and to protect the Company and its stockholders from future wrongdoing such as that alleged in the complaint. On September 29, 2016, the court entered an order staying the litigation pending resolution of the motion to dismiss the Amended Complaint in the Bankley Action.
On April 25, 2016, Intuitive Surgical, Inc. and its French subsidiary, Intuitive Surgical SAS (collectively, “Intuitive”), brought a request for unilateral measures of enquiry in front of the President of the Commercial Court of Toulon (France) (the “President”) against two employees of TransEnterix International, Inc. alleging that the Company, through these two employees, engaged in acts of unfair competition. On May 3, 2016, the President rendered an order granting Intuitive’s request for unilateral measures of enquiry with respect to its allegations (the “Order”). On June 28, 2016, TransEnterix International filed a writ challenging the Order and requesting that it be withdrawn by the President. On September 7, 2016, the President rendered his decision on TransEnterix International’s challenge (the “Ruling”) and ruled in favor of TransEnterix International. Under the Ruling, the Intuitive unilateral measures of enquiry were declared to be unjustified and the Order was withdrawn. The President also declared that the Ruling was provisionally enforceable. Intuitive has filed an appeal against the Ruling (the “Appeal”) with the Court of Appeal of Aix-en-Provence (France). Because Intuitive did not comply with the Ruling, TransEnterix International filed a dismissal request against the Appeal with the same Court of Appeal (the “Dismissal Request”). Currently, Intuitive’s counsel advised TransEnterix International’s counsel of their intent to waive the Appeal (the “Waiver”). A hearing regarding the Dismissal Request has been scheduled for March 7, 2017 in order to leave time for the parties to formalize the Waiver. The writ of Waiver from Intuitive’s French counsel has been provided to TransEnterix. Once the Waiver is formalized in connection with the March 7, 2017 hearing, the Dismissal Request, as well as the Appeal, will be struck off the agenda of the Court of Appeal and the Ruling in favor of TransEnterix International will become final.
Based on the stage of the proceedings, we have determined that the amount of any possible loss or range of possible loss in connection with the above matters is not reasonably estimable.
Not applicable.
26
|
ITEM 5. |
MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Since April 2, 2014, our common stock has been listed on the NYSE MKT under the symbol “TRXC.” The table below sets forth, for the respective periods indicated, the high and low bid prices for our common stock on the NYSE MKT. The bid prices represent inter-dealer transactions, without adjustments for retail mark-ups, mark-downs or commissions and may not necessarily represent actual transactions.
On March 31, 2014, the Company effectuated a reverse stock split of its issued and outstanding shares of common stock at a ratio of 1 for 5 (the “Reverse Stock Split”). As a result of the Reverse Stock Split, the Company’s issued and outstanding stock decreased from 244,276,923 to 48,855,255 shares of common stock, all with a par value of $0.001. All information related to common stock, stock options, RSUs, warrants and earnings per share for prior periods has been retroactively adjusted in this Annual Report to give effect to the Reverse Stock Split.
|
|
|
Bid Prices |
|
|||||
|
|
|
High |
|
|
Low |
|
||
|
2017 |
|
|
|
|
|
|
|
|
|
First Quarter (through February 28, 2017) |
|
$ |
1.57 |
|
|
$ |
1.30 |
|
|
2016 |
|
|
|
|
|
|
|
|
|
First Quarter |
|
$ |
4.79 |
|
|
$ |
1.54 |
|
|
Second Quarter |
|
|
6.10 |
|
|
|
1.03 |
|
|
Third Quarter |
|
|
1.97 |
|
|
|
1.16 |
|
|
Fourth Quarter |
|
|
2.33 |
|
|
|
1.30 |
|
|
2015 |
|
|
|
|
|
|
|
|
|
First Quarter |
|
$ |
3.50 |
|
|
$ |
2.52 |
|
|
Second Quarter |
|
|
4.87 |
|
|
|
2.70 |
|
|
Third Quarter |
|
|
3.66 |
|
|
|
2.07 |
|
|
Fourth Quarter |
|
|
2.90 |
|
|
|
2.10 |
|
As of February 28, 2017, there were approximately 243 record holders of our common stock (counting all shares held in single nominee registration as one stockholder).
We paid no dividends or made any other distributions in respect of our common stock during our fiscal years ended December 31, 2016, 2015 and 2014, and we have no plans to pay any dividends or make any other distributions in the future. In addition, the terms of the Loan Agreement prohibit the Company from paying any dividends without the consent of the Lenders.
27
Securities Authorized for Issuance Under Equity Compensation Plans.
The Company currently has one equity compensation plan under which it makes awards, the TransEnterix, Inc. Amended and Restated Incentive Compensation Plan, as amended (the “Plan”). The Plan was originally approved by the Board of Directors and adopted by the majority of our stockholders on November 13, 2007, and amended and restated and approved by the Board of Directors and approved by the majority of our stockholders on May 7, 2015 to increase the number of shares of common stock authorized under the Plan to 11,940,000 shares, and to make other changes. The Plan was amended on June 8, 2016 to increase in the number of shares reserved for issuance under the Plan to 18,940,000 shares. The Plan is used for plan-based awards for officers, other employees, consultants, advisors and non-employee directors. In connection with the Merger, we assumed all of the options that were issued and outstanding immediately prior to the Merger as issued by TransEnterix Surgical, and adjusted based on the Merger at the exchange ratio, which are now exercisable for approximately 1,870,892 shares of common stock. Such options were granted under the TransEnterix, Inc. 2006 Stock Plan (the “2006 Plan”) which was assumed by the Company in the Merger. The 2006 Plan is maintained solely for the purpose of the stock options granted under such 2006 Plan that remain outstanding; no future awards are authorized to be made under the 2006 Plan.
The following table gives information about the Company’s common stock that may be issued upon the exercise of options and other equity awards as of December 31, 2016:
|
Plan Category |
|
Number of securities to be issued upon exercise of outstanding options (1) |
|
|
Weighted average exercise price of outstanding options |
|
|
Number of securities remaining available for future issuance (2) |
|
|||
|
Equity compensation plans approved by security holders |
|
|
11,512,987 |
|
|
$ |
2.75 |
|
|
|
7,283,351 |
|
|
Equity compensation plans not approved by security holders (3) |
|
|
1,870,892 |
|
|
$ |
0.80 |
|
|
|
— |
|
|
Total |
|
|
13,383,879 |
|
|
|
|
|
|
|
7,283,351 |
|
|
(1) |
Includes 10,617,659 shares underlying outstanding stock options awarded under the Plan and 895,328 restricted stock units awarded under the Plan. |
|
(2) |
These shares are all available for future awards under the Plan. |
|
(3) |
Represents 1,870,892 shares underlying outstanding stock options awarded prior to the Merger under the 2006 Plan and assumed in the Merger. |
28
The graph below matches TransEnterix, Inc.'s cumulative 5-year total shareholder return on common stock with the cumulative total returns of the NYSE MKT Composite index and the RDG SmallCap Medical Devices index. The graph tracks the performance of a $100 investment in our common stock and in each index (with the reinvestment of all dividends) from December 31, 2011 to December 31, 2016.
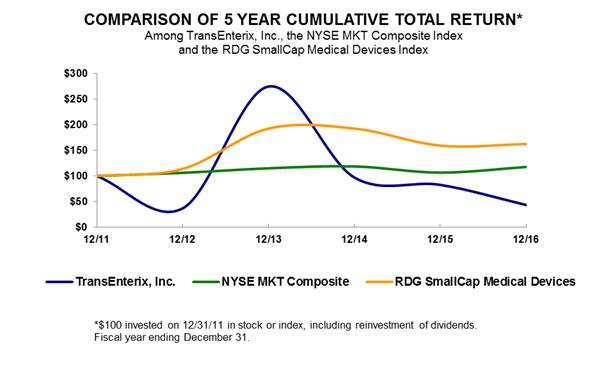
Unregistered Sales of Equity Securities and Use of Proceeds.
On September 14, 2016, the Board of Directors approved the issuance of up to 150,000 shares of common stock to a vendor of the Company in lieu of a cash payment. As previously reported, during the third quarter of 2016, the Company issued 81,032 shares of the Company’s common stock, and during the fourth quarter of 2016, the Company issued 14,646 shares of the Company’s common stock to such vendor. To date, the Company has issued a total of 95,678 shares of the Company’s common stock to such vendor. The issuance of the foregoing securities were exempt from the registration requirements of the Securities Act of 1933, as amended (the "Securities Act") afforded by Section 3(a)(9) or 4(a)(2) thereof and Regulation D promulgated thereunder, which exception we believe is available because the securities were not offered pursuant to a general solicitation and such issuances were otherwise made in compliance with the requirements of Regulation D and Rule 506. The securities issued in this transaction may not be resold except pursuant to an effective registration statement filed under the Securities Act or pursuant to a valid exemption from the registration requirements of the Securities Act.
The following table summarizes the Company’s purchases of its common stock for the quarter ended December, 2016:
|
Period |
|
Total Number of Shares Purchased (1) |
|
|
Average Price Paid per Share |
|
||
|
October 1-31, 2016 |
|
|
22,133 |
|
|
$ |
1.69 |
|
|
November 1-30, 2016 |
|
|
— |
|
|
|
— |
|
|
December 1-31, 2016 |
|
|
— |
|
|
|
— |
|
|
(1) |
Consists of shares we acquired from employees associated with the withholding of shares to pay certain withholding taxes upon the vesting of RSUs by delivering to us shares of our common stock in accordance with the terms of our equity compensation plan that were previously approved by our stockholders. We purchased these shares at their fair market value, as determined by reference to the closing price of our common stock on the day of vesting of the RSUs. |
29
The table below shows selected consolidated financial data. The statements of operations and comprehensive loss data for the years ended December 31, 2016, 2015 and 2014 and the balance sheet data at December 31, 2016 and 2015 are derived from our financial statements included elsewhere in this report. The statements of operations and comprehensive loss data for the year ended December 31, 2013 and 2012 and the balance sheet data at December 31, 2014, 2013 and 2012 are derived from our financial statements not included in this report. The historical results presented below are not necessarily indicative of financial results to be achieved in future periods.
|
Year ended December 31, |
|
2016(1) |
|
|
2015 (1) |
|
|
2014 (2) |
|
|
2013 (3)(4) |
|
|
2012 (4) |
|
|||||
|
|
|
|
|
|
|
(in thousands) |
|
|||||||||||||
|
Statement of Operations and Comprehensive Loss Data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sales |
|
$ |
1,519 |
|
|
$ |
— |
|
|
$ |
401 |
|
|
$ |
1,431 |
|
|
$ |
2,115 |
|
|
Income (loss) from continuing operations |
|
$ |
(119,980 |
) |
|
$ |
(46,948 |
) |
|
$ |
(37,652 |
) |
|
$ |
(28,358 |
) |
|
$ |
(15,425 |
) |
|
Income (loss) from continuing operations per common share |
|
$ |
(1.07 |
) |
|
$ |
(0.59 |
) |
|
$ |
(0.64 |
) |
|
$ |
(2.23 |
) |
|
$ |
(2.86 |
) |
|
Balance Sheet Data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets |
|
$ |
176,249 |
|
|
$ |
248,602 |
|
|
$ |
135,111 |
|
|
$ |
116,714 |
|
|
$ |
17,560 |
|
|
Long-term obligations and redeemable preferred stock (5) |
|
$ |
27,690 |
|
|
$ |
40,253 |
|
|
$ |
9,175 |
|
|
$ |
4,602 |
|
|
$ |
83,595 |
|
|
(1) |
Includes the assets and liabilities of TransEnterix Italia acquired and assumed in the Senhance Acquisition, which occurred on September 21, 2015. See the description titled “Senhance Acquisition and Related Transactions” under Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in this Annual Report. |
|
(2) |
On March 31, 2014, we effectuated a reverse stock split of our issued and outstanding shares of common stock at a ratio of 1 for 5. As a result of the Reverse Stock Split, our issued and outstanding stock decreased from 244,276,923 to 48,855,255 shares of common stock, all with a par value of $0.001. All information related to common stock, preferred stock and earnings per share for prior periods has been retroactively adjusted to give effect to the Reverse Stock Split. |
|
(3) |
On September 3, 2013, TransEnterix Surgical, Inc. a Delaware corporation (“TransEnterix Surgical”), and SafeStitch Medical, Inc., a Delaware corporation (“SafeStitch”) consummated a merger transaction whereby TransEnterix Surgical merged with a merger subsidiary of SafeStitch, with TransEnterix Surgical as the surviving entity in the merger (the “Merger”). As a result of the Merger, TransEnterix Surgical became a wholly owned subsidiary of SafeStitch. On December 6, 2013, SafeStitch changed its name to TransEnterix, Inc. The Merger was a reverse merger for accounting purposes with TransEnterix Surgical as the acquiring company. Therefore, from September 3, 2013 forward the financial statements of the Company are the historical financial statements of TransEnterix Surgical with the addition of SafeStitch as of the date of the Merger. |
|
(4) |
Represent the financial statements of TransEnterix Surgical as of and for the year ended December 31, 2012, and for the period from January 1, 2013 to September 2, 2013. |
|
(5) |
Long-term obligations include: (1) Cash Consideration installments to be paid to Sofar in connection with the Senhance Acquisition; (2) outstanding amounts under our Loan Agreement, first entered into by TransEnterix Surgical in January 2012 and amended from time to time since such time; (3) in 2016 and 2015 net deferred tax liabilities; and (4) in 2013, promissory notes of SafeStitch, which were converted into equity securities of the Company in 2013. In addition, concurrent with the closing of the Merger on September 3, 2013, the Company consummated a private placement (the “Private Placement”) transaction in which it issued and sold shares of its Series B Convertible Preferred Stock, par value $0.01 per share (the “Series B Preferred Stock”) to provide funding to support the Company’s operations following the Merger. The Company issued 7,544,704.4 shares of Series B Preferred Stock, each share of which was convertible, subject to certain conditions. Each share of Series B Preferred Stock was converted into two shares of our common stock on December 6, 2013. |
30
The following discussion of our financial condition and results of operations should be read in conjunction with our “Risk Factors” and our consolidated financial statements and the related notes to our consolidated financial statements included in this Annual Report. The following discussion contains forward-looking statements. See cautionary note regarding “Forward-Looking Statements” at the beginning of this Annual Report.
Overview
TransEnterix, Inc. (the “Company,” “we” or “us”) is a medical device company that is pioneering the use of robotics to improve minimally invasive surgery by addressing the clinical challenges associated with current laparoscopic and robotic options. We are focused on the commercialization and further development of our Senhance™ Surgical Robotic System (formerly known as the ALF-X ® Surgical Robotic System) (the “Senhance System”), a multi-port robotic system that brings the advantages of robotic surgery to patients while enabling surgeons with innovative technology. The Senhance System has been granted a CE Mark in Europe for laparoscopic abdominal and pelvic surgery, as well as limited thoracic operations excluding cardiac and vascular surgery, but is not available for sale in the U.S. We have also developed the SurgiBot™ System (the “SurgiBot System”), a single-port, robotically enhanced laparoscopic surgical platform. The SurgiBot System is not available for sale in any market.
The Senhance System is a multi-port robotic surgery system which allows multiple robotic arms to control instruments and a camera. The system features advanced technology to enable surgeons with haptic feedback and the ability to move the camera via eye movement. The system replicates laparoscopic motion that is familiar to experienced surgeons, and integrates three-dimensional high definition (“3DHD”) vision technology. The Senhance System also offers responsible economics to hospitals by offering robotic technology with reusable instruments thereby reducing additional costs per surgery when compared to other robotic solutions.
The SurgiBot System is designed to utilize flexible instruments through articulating channels controlled directly by the surgeon, with robotic assistance, while the surgeon remains patient-side within the sterile field. In June 2015, the Company submitted a 510(k) application to the FDA for the SurgiBot System. On April 19, 2016, the FDA notified the Company that the SurgiBot System did not meet the criteria for substantial equivalence based on the data and information submitted by TransEnterix in the 510(k) submission. After interactions with the FDA, the Company determined that a new 510(k) application would need to be submitted in order to obtain clearance for the SurgiBot System. Based on this fact, we have evaluated the operational and financial feasibility of pursuing two 510(k) applications in parallel and have elected to primarily focus our near term U.S. regulatory efforts on the 510(k) submission for the Senhance System and our current strategy is to focus our resources on the commercialization of and U.S. regulatory clearance for the Senhance System.
Consequently, in May 2016, the Company implemented a restructuring plan. The restructuring plan resulted in: 1) reducing the Company’s workforce; 2) abandoning certain equipment; 3) cancelling certain contracts; 4) writing down inventory related to the SurgiBot System; and 5) writing off certain patents.
We believe that future outcomes of minimally invasive surgery will be enhanced through our combination of more advanced tools and robotic functionality which are designed to: (i) empower surgeons with improved precision, dexterity and visualization; (ii) improve patient satisfaction and enable a desirable post-operative recovery; and (iii) provide a cost-effective robotic system, compared to existing alternatives today, for a potentially wide range of clinical applications. Our strategy is to focus on the development and commercialization of the Senhance System.
From our inception, we devoted a substantial percentage of our resources to research and development and start-up activities, consisting primarily of product design and development, clinical studies, manufacturing, recruiting qualified personnel and raising capital.
Since inception, we have been unprofitable. As of December 31, 2016 we had an accumulated deficit of $302.8 million.
We expect to continue to invest in research and development and related clinical studies, and increase selling, general and administrative expenses as we grow. As a result, we will need to generate significant revenue in order to achieve profitability.
In 2015 we incurred $4.2 million of Senhance Acquisition-related expenses (described below), which were included in operating expenses for the year ended December 31, 2015.
We operate in one business segment.
31
Recent Events
Lincoln Park Purchase Agreement
On December 16, 2016, we entered into a purchase agreement (the “LPC Purchase Agreement”) with Lincoln Park Capital Fund, LLC, an Illinois limited liability company (“Lincoln Park”), pursuant to which we have the right to sell to Lincoln Park up to an aggregate of $25,000,000 in shares of our common stock, subject to certain limitations and conditions set forth in the LPC Purchase Agreement.
From time to time on any trading day we select, we have the right, in our sole discretion, subject to the conditions and limitations in the LPC Purchase Agreement, to direct Lincoln Park to purchase up to 150,000 shares of common stock (each such purchase, a “Regular Purchase”) over the 36-month term of the LPC Purchase Agreement. The purchase price of shares of common stock pursuant to the LPC Purchase Agreement will be based on the prevailing market price at the time of sale as set forth in the LPC Purchase Agreement. There are no trading volume requirements or restrictions under the LPC Purchase Agreement. Lincoln Park’s obligation under each Regular Purchase shall not exceed $2,000,000. There is no upper limit on the price per share that Lincoln Park must pay for common stock under the LPC Purchase Agreement, but in no event will shares be sold to Lincoln Park on a day our closing price is less than the floor price as set forth in the LPC Purchase Agreement. We may, in our sole discretion, direct Lincoln Park to purchase additional amounts as accelerated purchases if on the date of a Regular Purchase the closing sale price of the common stock is not below the threshold price as set forth in the LPC Purchase Agreement. The Company and Lincoln Park may mutually agree to increase the amount of Common Stock sold to Lincoln Park on any accelerated purchase date.
The offer and sale of shares of common stock under the LPC Purchase Agreement was made under our previously filed and currently effective Registration Statement on Form S-3 (File No. 333-199998). We intend to use the net proceeds of this offering for general corporate purposes, including working capital, product development and capital expenditures.
We issued to Lincoln Park 312,538 shares of common stock as of December 31, 2016 as commitment shares in consideration for entering into the LPC Purchase Agreement. We shall issue pro rata up to an additional 152,434 shares of Common Stock to Lincoln Park as determined by the number of shares of Common Stock purchased by Lincoln Park pursuant to the LPC Purchase Agreement.
Controlled Equity Offering
On February 9, 2016, we entered into a Controlled Equity Offering SM Sales Agreement (the “2016 Sales Agreement”) with Cantor Fitzgerald & Co. (“Cantor”) under which we can offer and sell, through Cantor, up to approximately $43.6 million in shares of common stock in an at-the market offering (the “2016 ATM Offering”). On February 20, 2015, we had entered into a Controlled Equity Offering SM Sales Agreement (the “2015 Sales Agreement”) with Cantor, as sales agent, pursuant to which we offered and sold, through Cantor, $25.0 million in shares of common stock in an at-the-market offering from February 2015 through February 2016 (the “2015 ATM Offering”). All sales of shares were made pursuant to an effective shelf registration statement on Form S-3 filed with the Securities and Exchange Commission (the “SEC”). We pay Cantor a commission of approximately 3% of the aggregate gross proceeds received from all sales of common stock under the 2015 Sales Agreement and the 2016 Sales Agreement.
The following table summarizes the total sales under the 2015 Sales Agreement and 2016 Sales Agreement for the periods indicated (in thousands, except per share amounts):
|
|
|
2016 Sales Agreement |
|
|
2015 Sales Agreement |
|
|
||||||
|
|
|
Year Ended December 31, 2016 |
|
|
Year Ended December 31, 2016 |
|
|
Year Ended December 31, 2015 |
|
|
|||
|
Total shares of common stock sold |
|
|
8,763.4 |
|
|
|
5,710.2 |
|
|
|
2,014.3 |
|
|
|
Average price per share |
|
$ |
4.70 |
|
|
$ |
3.23 |
|
|
$ |
3.25 |
|
|
|
Gross proceeds |
|
$ |
41,156 |
|
|
$ |
18,454 |
|
|
$ |
6,546 |
|
|
|
Commissions earned by Cantor |
|
$ |
1,235 |
|
|
$ |
553 |
|
|
$ |
197 |
|
|
|
Other issuance costs |
|
$ |
185 |
|
|
$ |
— |
|
|
$ |
259 |
|
|
Senhance Acquisition and Related Transactions
Amendment to Membership Interest Purchase Agreement
On September 21, 2015, the Company announced that it had entered into a Membership Interest Purchase Agreement, dated September 18, 2015 (the “Purchase Agreement”) with Sofar S.p.A., (the “Seller”), Vulcanos S.r.l., as the acquired company, and TransEnterix International, Inc., a wholly owned subsidiary of the Company (the “Buyer”). The closing of the transactions contemplated by the Purchase Agreement occurred on September 21, 2015 (the “Closing Date”) pursuant to which the Buyer acquired
32
all of the membership interests of the acquired company from the Seller, and changed the name of the acquired company to TransEnterix Italia S.r.l (“TransEnterix Italia”). On the Closing Date, pursuant to the Purchase Agreement, the Company completed the strategic acquisition from Sofar S.p.A. of all of the assets, employees and contracts related to the advanced robotic system for minimally invasive laparoscopic surgery now known as the Senhance System (the “Senhance Acquisition”).
Under the terms of the Purchase Agreement, the consideration consisted of the issuance of 15,543,413 shares of the Company’s common stock (the “Securities Consideration”) and approximately $25,000,000 U.S. Dollars and 27,500,000 Euro in cash consideration (the “Cash Consideration”). The Securities Consideration was issued in full at closing of the acquisition; the Cash Consideration was or will be paid in four tranches, with US $25,000,000 paid at closing and the remaining Cash Consideration of 27,500,000 Euro to be paid in three additional tranches based on achievement of negotiated milestones. On December 30, 2016, the Company entered into an amendment to restructure the terms of the Second Tranche of the Cash Consideration (the “Amendment”). Under the Amendment, the Second Tranche was restructured to reduce the contingent cash consideration by €5.0 million in exchange for the issuance of 3,722,685 shares of the Company’s common stock with an aggregate fair market value of €5.0 million. On January 4, 2017, the Company issued 3,722,685 shares of the common stock with a fair value of €5.0 million. The price per share was $1.404 and was calculated based on the average of the closing prices of the Company’s common stock on ten consecutive trading days ending one day before the execution of the Amendment.
The issuance of the Securities Consideration was effected as a private placement of securities under Section 4(a)(2) of the Securities Act of 1933, as amended (the “Securities Act”), and Regulation D promulgated thereunder.
The Purchase Agreement contains customary representations and warranties of the parties and the parties have customary indemnification obligations, which are subject to certain limitations described further in the Purchase Agreement.
Registration Rights and Lock-Up Agreements
In connection with the Senhance Acquisition, we also entered into a Registration Rights Agreement, dated as of September 21, 2015, with the Seller, pursuant to which we agreed to register the Securities Consideration shares for resale following the end of the lock-up periods described below. The resale Registration Statement has been filed and is effective, pending lapse of the lock-up restrictions described below.
In connection with the Senhance Acquisition, Sofar entered into a Lock-Up Agreement with us pursuant to which Sofar agreed, subject to certain exceptions, not to sell, transfer or otherwise convey any of the Securities Consideration for one year following the Closing Date. On September 21, 2016, fifty percent of the Securities Consideration was released from the lock-up restrictions and is eligible to be resold under the effective resale registration statement. With respect to the remaining fifty percent of the Securities Consideration, the Lock-up Agreement provides that an additional twenty-five percent of the Securities Consideration remains locked-up until the eighteen-month anniversary of the Closing Date, and the remaining twenty-five percent of the Securities Consideration remains locked-up until the two-year anniversary of the Closing Date. The restrictions on transfer contained in the Lock-up Agreement cease to apply to all of the Securities Consideration following the second anniversary of the Closing Date, or earlier upon certain other conditions.
2015 Events
Public Offering
On June 11, 2015, we sold 16,666,667 shares of common stock at a public offering price of $3.00 per share for aggregate gross proceeds of $50.0 million in an underwritten firm commitment public offering. We granted the underwriters an option, exercisable for 30 days, to purchase up to an additional 2,500,000 shares of common stock to cover over-allotments. The common stock was offered and sold pursuant to our shelf registration statement on Form S-3 (File No. 333-199998) registering an aggregate of $100.0 million of our designated securities. The closing of the public offering occurred on June 17, 2015. On July 10, 2015, the underwriters exercised a portion of their over-allotment option to acquire an additional 2,075,000 shares at the public offering price of $3.00 per share for aggregate additional gross proceeds of $6.2 million. The purchase of the over-allotment shares closed on July 15, 2015. Total proceeds were $52.2 million, net of issuance costs of $4.0 million.
2014 Events
Reverse Stock Split
On March 31, 2014, we effectuated a reverse stock split of our issued and outstanding shares of common stock at a ratio of 1 for 5 (the “Reverse Stock Split”). As a result of the Reverse Stock Split, our issued and outstanding stock decreased from 244,276,923 to 48,855,255 shares of common stock, all with a par value of $0.001. All information related to common stock, stock options, RSUs, warrants and earnings per share for prior periods has been retroactively adjusted to give effect to the Reverse Stock Split.
33
Public Offering
On April 14, 2014, we sold 12,500,000 shares of common stock at a public offering price of $4.00 per share for aggregate gross proceeds of $50.0 million in an underwritten firm commitment public offering. We granted the underwriters an option, exercisable for 30 days, to purchase up to an additional 1,875,000 shares of common stock to cover over-allotments. Certain of our existing stockholders that are affiliated with certain of our directors purchased $10.0 million of common stock in the public offering. The common stock was offered and sold pursuant to our shelf registration statement on Form S-3 (File No. 333-193235) registering an aggregate of $100.0 million of our designated securities. The closing of the public offering occurred on April 21, 2014. On April 30, 2014, the underwriters exercised a portion of their over-allotment option to acquire an additional 1,610,000 shares at the public offering price of $4.00 per share for aggregate additional gross proceeds of $6.4 million. The purchase of the over-allotment shares closed on May 5, 2014. Total proceeds were $52.4 million, net of issuance costs of $4.0 million.
In connection with the public offering, our common stock was eligible to be listed on the NYSE MKT and began trading on such exchange on April 1, 2014.
Results of Operations
Our results of operations include the operations of TransEnterix Italia from the Senhance Acquisition date of September 21, 2015 forward.
Revenue
In 2016, our revenue consisted of product and service revenue resulting from the sale in Europe of a Senhance System, instruments and accessories, and related services. We recognize revenue when persuasive evidence that an arrangement exists, delivery has occurred or service has been rendered, the price is fixed or determinable, and collectability is reasonably assured. Amounts billed in excess of the associated revenue recognized are deferred.
In 2014, we derived sales from our SPIDER System and other distributed products through limited direct sales in the United States and international distributors. We recorded revenue when persuasive evidence of an arrangement existed, delivery had occurred which was typically at shipping point, the fee was fixed or determinable and collectability was reasonably assured. Shipping and handling costs billed to customers are included in revenue.
We expect to experience some unevenness in the number and trend, and average selling price, of units sold on a quarterly basis given the early stage of commercialization of our products.
Product and service revenue for the year ended December 31, 2016 increased to $1.5 million compared to $0 for the year ended December 31, 2015. The $1.5 million increase was the result of the revenue recognized on the sale of one Senhance System, net of deferred revenue, during the third quarter of 2016.
Product revenue for the year ended December 31, 2015 decreased to $0 compared to $0.4 million for the year ended December 31, 2014. The $0.4 million decrease was the result of our decision to focus resources on the SurgiBot System development and therefore away from continued investment in sales and marketing of the SPIDER System. The SPIDER System remained on the market for existing customers through December 31, 2014. We discontinued sales of the SPIDER System on December 31, 2014.
Cost of Revenue
In 2016, cost of revenues consists primarily of costs related to contract manufacturing, materials, and manufacturing overhead. We expense all inventory provisions as cost of revenues. The manufacturing overhead costs include the cost of quality assurance, material procurement, inventory control, facilities, equipment depreciation and operations supervision and management. We expect overhead costs as a percentage of revenues to become less significant as our production volume increases. We expect cost of revenues to increase in absolute dollars to the extent our revenues grow and as we continue to invest in our operational infrastructure to support anticipated growth.
In 2014, cost of revenue consisted of materials, labor and overhead incurred internally to produce our products and the impairment and write down of excess and obsolete inventory. Shipping and handling costs we incurred were included in cost of goods sold.
Cost of revenue for the year ended December 31, 2016 increased to $1.1 million as compared to $0 for the year ended December 31, 2015. This increase over the prior year period was the result of the costs recognized in connection with the sale of one Senhance System during the third quarter of 2016. The $1.1 million cost of revenue primarily represents the fair value of the Senhance System determined using the acquisition method of accounting at the Senhance Acquisition date.
34
Cost of revenue for the year ended December 31, 2015 decreased to $0 as compared to $1.1 million for the year ended December 31, 2014. The $1.1 million decrease was primarily the result of our reduction in sales as we limited sales of our SPIDER System to our existing customers and discontinued the production of our SPIDER System as of December 31, 2014.
Research and Development
Research and development (“R&D”) expenses primarily consist of engineering, product development and regulatory expenses incurred in the design, development, testing and enhancement of our products and legal services associated with our efforts to obtain and maintain broad protection for the intellectual property related to our products. In future periods, we expect R&D expenses to remain consistent or be modestly higher as we continue to invest in basic research, clinical studies, product development and intellectual property supporting the evolution of our Senhance System. R&D expenses are expensed as incurred.
R&D expenses for the year ended December 31, 2016 decreased 1% to $29.3 million as compared to $29.7 million for the year ended December 31, 2015. The $0.4 million decrease resulted primarily from decreased supplies expense of $3.7 million and decreased contract engineering services, consulting and other outside services of $3.6 million, offset by increased preclinical lab expense of $3.8 million, increased facility costs of $0.9 million, increased stock compensation costs of $0.8 million, increased travel related expenses of $0.5 million, increased other costs of $0.6 million and increased personnel related costs of $0.3 million.
R&D expenses for the year ended December 31, 2015 increased 6% to $29.7 million as compared to $27.9 million for the year ended December 31, 2014. The $1.8 million increase resulted primarily from increased preclinical lab expense of $1.4 million, increased personnel related costs of $0.8 million, increased stock compensation costs of $0.3 million, and increased other costs of $0.4 million, offset by decreased supplies expense of $1.5 million and decreased contract engineering services, consulting and other outside services of $0.8 million related to product development of our SurgiBot System. In addition, R&D expenses incurred for development of the Senhance System from September 21, 2015 to December 31, 2015 were $1.2 million.
Sales and Marketing
Sales and marketing expenses include costs for sales and marketing personnel, travel, demonstration product, market development, physician training, tradeshows, marketing clinical studies and consulting expenses. We expect sales and marketing expenses to increase significantly in 2017 in support of our Senhance System product launch. We cannot assure you that the Senhance System will be cleared by the FDA, or that we will meet our anticipated product launch target for the Senhance System in 2017.
Sales and marketing expenses for the year ended December 31, 2016 increased 217% to $9.2 million compared to $2.9 million for the year ended December 31, 2015. The $6.3 million increase was primarily related to increased personnel related costs of $2.6 million, increased travel related expenses of $1.0 million, increased consulting costs of $0.9 million, increased tradeshow costs of $0.5 million, increased stock compensation costs of $0.5 million, increased other costs of $0.6 million and increased depreciation expense $0.2 million.
Sales and marketing expenses for the year ended December 31, 2015 increased 71% to $2.9 million compared to $1.7 million for the year ended December 31, 2014. The $1.2 million increase was primarily related to increased tradeshow costs of $0.3 million, increased personnel related costs of $0.2 million, increased travel related expenses of $0.1 million, and increased stock compensation costs of $0.1 million. In addition, sales and marketing expenses related to the Senhance Acquisition from September 21, 2015 to December 31, 2015 were $0.5 million.
General and Administrative
General and administrative expenses consist of personnel costs related to the executive, finance and human resource functions, as well as professional service fees, legal fees, accounting fees, insurance costs, and general corporate expenses. In future periods, we expect general and administrative expenses to increase to support our sales, marketing, and research and development efforts.
General and administrative expenses for the year ended December 31, 2016 increased 38% to $10.8 million compared to $7.8 million for the year ended December 31, 2015. The $3.0 million increase was primarily due to increased legal, accounting, and investor relation fees and other public company costs of $1.8 million, increased stock compensation costs of $0.5 million, increased personnel costs of $0.4 million, and increased other costs of $0.3 million.
General and administrative expenses for the year ended December 31, 2015 increased 37% to $7.8 million compared to $5.7 million for the year ended December 31, 2014. The $2.1 million increase was primarily due to increased personnel costs of $1.0 million, increased stock compensation costs of $1.0 million, and increased consulting expenses of $0.1 million, offset by decreased legal, accounting, and investor relation fees and other public company costs of $0.2 million. In addition, general and administrative expenses related to the Senhance Acquisition from September 21, 2015 to December 31, 2015 were $0.2 million.
35
Amortization of Intangible Assets
Amortization of intangible assets for the year ended December 31, 2016 increased to $7.0 million compared to $2.2 million for the year ended December 31, 2015. The $4.8 million increase was primarily the result of amortization of developed technology related to the acquisition of the Senhance System on September 21, 2015.
Amortization of intangible assets for the year ended December 31, 2015 increased to $2.2 million compared to $0.5 million for the year ended December 31, 2014. The $1.7 million increase was primarily the result of amortization of developed technology related to the acquisition of the Senhance System on September 21, 2015.
Change in Fair Value of Contingent Consideration
The change in fair value of contingent consideration in connection with the Senhance Acquisition was $0.5 million for the year ended December 31, 2016 primarily related to the Amendment to the Purchase Agreement for the Second Tranche, the change in expected timelines for the achievement of milestones, the effect of the passage of time on the fair value measurement and the impact of foreign currency exchange rates.
The change in fair value of contingent consideration in connection with the Senhance Acquisition was $0.4 million for the year ended December 31, 2015 related primarily to the foreign currency translation impact.
Inventory write-down related to restructuring
On April 19, 2016, the FDA notified the Company that the SurgiBot System did not meet the criteria for substantial equivalence based on the data submitted in the 510(k) submission. As a result, we reprioritized our near-term regulatory efforts to the 510(k) submission for the Senhance System. Consequently, in May 2016, the Company implemented a restructuring plan. Under this plan, we recorded a $2.6 million write-down of inventory related to the SurgiBot System.
Restructuring and other charges
Under the restructuring plan executed in May 2016, we recorded $3.1 million in restructuring and other charges. The restructuring charges included: (i) $0.5 million to be paid in cash, of which $0.4 million related to employee severance costs and $0.1 million related to cancellation of certain contracts; and (ii) $2.6 million for other non-cash charges, of which $1.0 million related to the write-off of long-lived assets for the abandonment of certain equipment and tooling and $1.6 million related to the write-off of intellectual property for certain patents.
Goodwill impairment
The Company performs an annual impairment test of goodwill at December 31, or more frequently if events or changes in circumstances indicate that the carrying value of our one reporting unit may not be recoverable. During the second quarter of 2016, we were notified by the FDA that the SurgiBot System did not meet the criteria for substantial equivalency, negatively impacting our market capitalization, and warranting an interim two-step quantitative impairment test. Based on the impairment test, we recorded goodwill impairment of $61.8 million during the second quarter of 2016.
Acquisition Related Costs
Acquisition related costs consist primarily of legal, accounting and other professional fees related to the Senhance Acquisition. We incurred $4.2 million of acquisition-related expenses for the year ended December 31, 2015.
Other Expense, Net
Other expense is primarily composed of interest expense on notes payable.
Other expense for the year ended December 31, 2016 increased to $1.9 million compared to $1.6 million for the year ended December 31, 2015. The $0.3 million increase was primarily related to the increase in notes payable of approximately $10.0 million in August 2015.
Other expense for the year ended December 31, 2015 increased to $1.6 million compared to $1.0 million for the year ended December 31, 2014. The $0.6 million increase was related to the increase in notes payable of approximately $10.0 million in August 2015.
36
Income Tax Benefit
Income tax benefit consists primarily of taxes related to the amortization of purchase accounting intangibles in connection with the Italian taxing jurisdiction for TransEnterix Italia as a result of the acquisition of the Senhance System. We recognized $5.5 million and $1.0 million of income tax benefit for the year ended December 31, 2016 and 2015, respectively.
Liquidity and Capital Resources
Sources of Liquidity
Since our inception we have incurred significant losses and, as of December 31, 2016, we had an accumulated deficit of $302.8 million. We have not yet achieved profitability and we cannot assure investors that we will achieve profitability with our existing capital resources. Our recurring losses raise substantial doubt about our ability to continue as a going concern. As a result, the Company’s independent registered public accounting firm included an explanatory paragraph in its report on our consolidated financial statements as of and for the years ended December 31, 2016, 2015 and 2014 with respect to this uncertainty. We expect to continue to fund research and development, sales and marketing and general and administrative expenses at similar to current or higher levels and, as a result, we will need to generate significant revenues to achieve profitability. Our principal sources of cash to date have been proceeds from public offerings of common stock, private placements of common and preferred stock, incurrence of debt and the sale of equity securities held as investments.
We currently have two effective shelf registration statements on file with the SEC, each of which were initially filed to register up to $100.0 million of debt securities, common stock, preferred stock, or warrants, or any combination thereof for future financing transactions. In March 2016, the November 2014 shelf registration statement was increased by $50.0 million. From April 2014 through December 2016, we have raised $179.2 million in gross proceeds and approximately $168.8 million in net proceeds under such shelf registration statements through public offerings of our securities. As of December 31, 2016, we had $37.8 million available for future financings under such shelf registration statements. In addition, approximately $2.4 million is reserved for future issuances under the 2016 ATM Offering and $24.6 million is reserved for future issuances under the Lincoln Park Agreement.
At December 31, 2016, we had cash and cash equivalents, excluding restricted cash, of approximately $24.4 million.
Consolidated Cash Flow Data
|
|
|
Years Ended December 31, |
|
|||||||||
|
|
|
2016 |
|
|
2015 |
|
|
2014 |
|
|||
|
(in millions) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash (used in) provided by |
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating activities |
|
$ |
(52.4 |
) |
|
$ |
(38.8 |
) |
|
$ |
(33.4 |
) |
|
Investing activities |
|
|
(1.4 |
) |
|
|
(26.2 |
) |
|
|
4.0 |
|
|
Financing activities |
|
|
49.9 |
|
|
|
68.4 |
|
|
|
54.0 |
|
|
Net (decrease) increase in cash, cash equivalents and restricted cash |
|
$ |
(3.9 |
) |
|
$ |
3.4 |
|
|
$ |
24.6 |
|
Operating Activities
For the year ended December 31, 2016, cash used in operating activities of $52.4 million consisted of net loss of $120.0 million and cash used for working capital of $8.5 million, offset by non-cash items of $76.1 million. The non-cash items primarily consisted of $61.8 million goodwill impairment, $2.6 million inventory write-down related to restructuring, $2.6 million non-cash restructuring and other charges, $5.0 million of stock-based compensation expense, $1.9 million of depreciation, $7.1 million of amortization, and $0.5 million change in fair value of contingent consideration, offset by $5.6 million deferred income tax benefit. The decrease in cash from changes in working capital included $6.6 million increase in inventories, $0.4 million decrease in accounts payable, $1.5 million increase in other current and long term assets and $1.0 million increase in accounts receivable, offset by $1.1 million increase in accrued expenses.
For the year ended December 31, 2015, cash used in operating activities of $38.8 million consisted of net loss of $46.9 million, offset by non-cash items of $5.5 million and cash provided by working capital of $2.6 million. The non-cash items primarily consisted of $3.3 million of stock-based compensation expense, $1.3 million of depreciation, and $2.3 million of amortization, offset by $1.0 million deferred income tax benefit and $0.4 million change in fair value of contingent consideration. The increase in cash from changes in working capital included $1.9 million increase in inventories and $2.0 million increase in other current and long term assets. These amounts were partially offset by $1.1 million increase in accounts payable, $5.4 million increase in accrued expenses and $0.1 million decrease in accounts receivable.
37
For the year ended December 31, 2014, cash used in operating activities of $33.4 million consisted of net loss of $37.7 million, offset by non-cash items of $3.3 million and cash provided by working capital of $1.0 million. The non-cash items primarily consisted of $1.8 million of stock-based compensation expense, $0.8 million of depreciation, $0.6 million of amortization, and $0.1 million loss on disposal of property and equipment. The increase in cash from changes in working capital included $0.7 million decrease in inventories, $0.4 million increase in accrued expenses, and $0.1 decrease in interest receivable, partially offset by $0.2 million increase in other current and long term assets.
Investing Activities
For the year ended December 31, 2016, net cash used in investing activities was $1.4 million. This amount reflected the purchases of property and equipment.
For the year ended December 31, 2015, net cash used in investing activities was $26.2 million. This amount reflected the $25.0 million payment for the Senhance Acquisition and $1.2 million paid for the purchases of property and equipment.
For the year ended December 31, 2014, net cash provided by investing activities was $4.0 million. This amount reflected $6.2 million proceeds from the sale and maturities of investments, offset by $2.2 million paid for the purchases of property and equipment.
Financing Activities
For the year ended December 31, 2016, net cash provided by financing activities was $49.9 million. This amount was primarily related to $58.0 million in proceeds from the issuance of common stock, net of issuance costs, partially offset by $6.9 million in payments of debt and $1.2 million in payments of contingent consideration.
For the year ended December 31, 2015, net cash provided by financing activities was $68.4 million. This amount was primarily related $58.3 million in proceeds from the issuance of common stock, net of issuance costs, and $9.9 million proceeds from the issuance of debt, and $0.2 million proceeds from the issuance of stock options and warrants.
For the year ended December 31, 2014, net cash provided by financing activities was $54.0 million. This amount was primarily related to $52.5 million in proceeds from the issuance of common stock, net of issuance costs, $4.3 million proceeds from the issuance of debt, and $0.1 million proceeds from the issuance of stock options and warrants, offset by $2.9 million payments on debt.
Operating Capital and Capital Expenditure Requirements
We believe that our existing cash and cash equivalents, together with cash received from sales of our products, will not be sufficient to meet our anticipated cash needs through at least the next 12 months. We intend to spend substantial amounts on commercial activities, on research and development activities, including product development, regulatory and compliance, clinical studies in support of our future product offerings, the enhancement and protection of our intellectual property, on notes payable payments as they come due, and on contingent consideration payments in connection with the acquisition of the Senhance System. We will need to obtain additional financing to pursue our business strategy, to respond to new competitive pressures or to take advantage of opportunities that may arise. To meet our capital needs, we are considering multiple alternatives, including, but not limited to, additional equity financings, debt financings and other funding transactions. There can be no assurance that we will be able to complete any such transaction on acceptable terms or otherwise. If we are unable to obtain the necessary capital, we will need to pursue a plan to license or sell our assets, seek to be acquired by another entity, cease operations and/or seek bankruptcy protection.
Cash and cash equivalents held by our foreign subsidiaries totaled $1.1 million at December 31, 2016. We do not intend or currently foresee a need to repatriate cash and cash equivalents held by our foreign subsidiary. If these funds are needed in the U.S., we believe that the potential U.S. tax impact to repatriate these funds would be immaterial.
Loan Agreement
We are the borrower under an outstanding credit facility with Silicon Valley Bank and Oxford Finance LLC (the “Lenders”), initially entered into in January 2012, as subsequently amended or amended and restated (collectively, the “Loan Agreement”). A number of the amendments related to the Senhance Acquisition or the growth of our business in non-U.S. jurisdictions. Under the Loan Agreement, our current borrowing capacity is $20.0 million, all of which is borrowed under term loans. We have had periods of interest-only payments during the Loan Agreement, and have been making principal payments since January 2016. The maturity date of the term loans are July 1, 2018.
38
In connection with the entry into the Loan Agreement, we are obligated to pay final payment and facility fees. The final payment fee obligation payable is 6.5% of the original principal amount of each term loan.
In addition, in connection with the borrowings under the Loan Agreement, we issued warrants to the Lenders to purchase shares of the Company’s common stock as follows:
|
Date of issuance |
|
Common stock underlying warrants |
|
|
Expiration date |
|
|
01/17/2012 |
|
|
279,588 |
|
|
01/17/2019 |
|
09/26/2014 |
|
|
38,324 |
|
|
09/26/2021 |
|
08/14/2015 |
|
|
112,903 |
|
|
08/14/2022 |
|
TOTAL |
|
|
430,815 |
|
|
|
The Loan Agreement is secured by a security interest in all assets of the Company and its current and future U.S. subsidiaries, including a security interest in intellectual property proceeds, but excluding a current security interest in intellectual property. In addition, our subsidiary TransEnterix International is a co-borrower under the Loan Agreement, and pledged 65% of the equity interests of TransEnterix Europe S.á.r.l., a wholly owned subsidiary of TransEnterix International as additional security. The Loan Agreement contains provisions permitting the Company to transfer designated amounts to TransEnterix Italia during the term of the Loan Agreement. The Loan Agreement contains customary representations (tested on a continual basis) and covenants that, subject to exceptions, restrict our ability to do the following things: declare dividends or redeem or repurchase equity interests; incur additional liens; make loans and investments; incur additional indebtedness; engage in mergers, acquisitions, and asset sales; transact with affiliates; undergo a change in control; add or change business locations; and engage in businesses that are not related to its existing business.
On September 7, 2016, we and our U.S. subsidiaries entered into the Fifth Amendment to the Loan Agreement (the “Fifth Amendment”). The Fifth Amendment provides more flexibility to the Company with respect to its intercompany activities with its foreign subsidiaries and adds an affirmative covenant requiring the Company to maintain a $10.0 million cash balance in accounts held with a Lender.
Contractual Obligations and Commercial Commitments
The following table summarizes our contractual obligations as of December 31, 2016 (in millions):
|
|
|
Payments due by period |
|
|
|
|
|
|||||||||||||
|
|
|
Total |
|
|
Less than 1 year |
|
|
1 to 3 years |
|
|
3 to 5 years |
|
|
Thereafter |
|
|||||
|
Long-term debt obligations (1) |
|
$ |
15.2 |
|
|
$ |
8.8 |
|
|
$ |
6.4 |
|
|
$ |
— |
|
|
$ |
— |
|
|
Operating leases |
|
$ |
2.6 |
|
|
$ |
0.9 |
|
|
$ |
1.3 |
|
|
$ |
0.4 |
|
|
$ |
— |
|
|
License and supply agreements |
|
$ |
13.3 |
|
|
$ |
7.4 |
|
|
$ |
2.2 |
|
|
$ |
1.6 |
|
|
$ |
2.1 |
|
|
Total contractual obligations (2) |
|
$ |
31.1 |
|
|
$ |
17.1 |
|
|
$ |
9.9 |
|
|
$ |
2.0 |
|
|
$ |
2.1 |
|
|
(1) |
Long-term debt obligations include principal and interest payments on our notes payable. |
|
(2) |
As of December 31, 2016, the contingent consideration that may be paid under the Purchase Agreement with Sofar upon the achievement of milestones is approximately €21.4 million. Due to uncertainty regarding the timing and amount of future payments related to these liabilities, these amounts are excluded from the contractual obligations table above. |
Long-term debt obligations include future payments under the Loan Agreement.
Operating lease amounts include future minimum lease payments under all our non-cancelable operating leases with an initial term in excess of one year. We rent office space in North Carolina under an operating lease which expires in 2018, with options to extend the lease through 2021. We also rent space for a warehouse facility in North Carolina which expires in 2018, with options to extend the lease through 2024. In Italy, we rent space for research and development and demonstration facilities under an operating lease which expires in 2022. This table does not include obligations for any lease extensions.
License and supply agreements include agreements assumed as part of the Senhance Acquisition.
39
Off-Balance Sheet Arrangements
As of December 31, 2016, we did not have any off-balance sheet arrangements.
Critical Accounting Policies and Estimates
The discussion and analysis of our financial condition and results of operations set forth above under the headings “Results of Operations” and “Liquidity and Capital Resources” have been prepared in accordance with U.S. GAAP and should be read in conjunction with our financial statements and notes thereto appearing in Item 8 of this Annual Report. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. On an on-going basis, we evaluate our critical accounting policies and estimates, including identifiable intangible assets and goodwill, business combinations, in-process research and development, contingent consideration, stock-based compensation, inventory, and revenue recognition. We base our estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. A more detailed discussion on the application of these and other accounting policies can be found in Note 2 in the Notes to the Financial Statements set forth in our financial statements for the years ended December 31, 2016, 2015, and 2014 which are included as Item 8 of this Annual Report. Actual results may differ from these estimates under different assumptions and conditions.
While all accounting policies impact the financial statements, certain policies may be viewed as critical. Critical accounting policies are those that are both most important to the portrayal of financial condition and results of operations and that require management’s most subjective or complex judgments and estimates. Our management believes the policies that fall within this category are the policies on accounting for identifiable intangible assets and goodwill, business acquisitions, in-process research and development, contingent consideration, stock-based compensation, inventory and revenue recognition.
Identifiable Intangible Assets and Goodwill
Identifiable intangible assets consist of purchased patent rights recorded at cost and developed technology acquired as part of a business acquisition recorded at estimated fair value. Intangible assets are amortized over 7 to 10 years. We periodically evaluate identifiable intangible assets for impairment whenever events or changes in circumstances indicate that the carrying amount may not be recoverable.
Indefinite-lived intangible assets, such as goodwill, are not amortized. We test the carrying amounts of goodwill for recoverability on an annual basis or when events or changes in circumstances indicate evidence of potential impairment exists by performing either a qualitative evaluation or a two-step quantitative test. The qualitative evaluation is an assessment of factors, including industry, market and general economic conditions, market value, and future projections to determine whether it is more likely than not that the fair value of a reporting unit is less than it’s carrying amount, including goodwill. We may elect to bypass this qualitative assessment and perform a two-step quantitative test. The quantitative goodwill impairment test is performed using a two-step approach. In the first step, the fair value of the reporting unit is determined and compared to the reporting unit's carrying value, including goodwill. If the fair value of the reporting unit is less than its carrying value, the second step of the goodwill impairment test is performed to measure the amount of impairment, if any. In the second step, the fair value of the reporting unit is allocated to the assets and liabilities of the reporting unit as if it had been acquired in a business combination and the purchase price was equivalent to the fair value of the reporting unit. The excess of the fair value of the reporting unit over the amounts assigned to its assets and liabilities is referred to as the implied fair value of goodwill. The implied fair value of the reporting unit's goodwill is then compared to the actual carrying value of goodwill. If the implied fair value of goodwill is less than the carrying value of goodwill, an impairment loss is recognized for the difference. We performed a qualitative assessment during the annual impairment review for fiscal 2015 as of December 31, 2015 and concluded that it is not more likely than not that the fair value of our single reporting unit is less than its carrying amount. Therefore, the two-step goodwill impairment test for the reporting unit was not necessary in fiscal 2015. During the second quarter of 2016, the FDA notified the Company that the SurgiBot System did not meet the criteria for substantial equivalency, negatively impacting the Company’s market capitalization, and warranting an interim two-step quantitative impairment test. We determined the fair value of our reporting unit using a discounted cash flow analysis derived from our long-term plans. The fair value of the reporting unit was corroborated using market prices for TransEnterix, Inc. The inputs used to determine the fair values were classified as Level 3 in the fair value hierarchy. Based on the impairment test, we recorded goodwill impairment of $61.8 million during the second quarter of 2016. We performed a qualitative assessment during the annual impairment review for fiscal 2016 as of December 31, 2016 and concluded that it is not more likely than not that the fair value of our single reporting unit is less than its carrying amount. Therefore, the two-step goodwill impairment test for the reporting unit was not necessary as of December 31, 2016.
40
Business Acquisitions
Business acquisitions are accounted for using the acquisition method of accounting in accordance with Accounting Standards Codification (“ASC”) 805, “Business Combinations.” ASC 805 requires, among other things, that assets acquired and liabilities assumed be recognized at their fair values, as determined in accordance with ASC 820, “Fair Value Measurements,” as of the acquisition date. For certain assets and liabilities, book value approximates fair value. In addition, ASC 805 establishes that consideration transferred be measured at the closing date of the acquisition at the then-current market price. Under ASC 805, acquisition-related costs (i.e., advisory, legal, valuation and other professional fees) and certain acquisition-related restructuring charges impacting the target company are expensed in the period in which the costs are incurred. The application of the acquisition method of accounting requires the Company to make estimates and assumptions related to the estimated fair values of net assets acquired.
Significant judgments are used during this process, particularly with respect to intangible assets. Generally, intangible assets are amortized over their estimated useful lives. Goodwill and other indefinite-lived intangibles are not amortized, but are annually assessed for impairment. Therefore, the purchase price allocation to intangible assets and goodwill has a significant impact on future operating results.
In-Process Research and Development
In-process research and development, or IPR&D, assets are considered to be indefinite-lived until the completion or abandonment of the associated research and development projects. IPR&D assets represent the fair value assigned to technologies that we acquire, which at the time of acquisition have not reached technological feasibility and have no alternative future use. During the period that the assets are considered indefinite-lived, they are tested for impairment on an annual basis, or more frequently if we become aware of any events occurring or changes in circumstances that indicate that the fair value of the IPR&D assets are less than their carrying amounts. If and when development is complete, which generally occurs when we have regulatory approval and are able to commercialize products associated with the IPR&D assets, these assets are then deemed definite-lived and are amortized based on their estimated useful lives at that point in time. If development is terminated or abandoned, we may have a full or partial impairment charge related to the IPR&D assets, calculated as the excess of carrying value of the IPR&D assets over fair value.
Contingent Consideration
Contingent consideration is recorded as a liability and measured at fair value using a discounted cash flow model utilizing significant unobservable inputs including the probability of achieving each of the potential milestones and an estimated discount rate associated with the risks of the expected cash flows attributable to the various milestones. Significant increases or decreases in any of the probabilities of success or changes in expected timelines for achievement of any of these milestones would result in a significantly higher or lower fair value of these milestones, respectively, and commensurate changes to the associated liability. The fair value of the contingent consideration at each reporting date will be updated by reflecting the changes in fair value in our statement of operations.
Stock-Based Compensation
We recognize as expense, the grant-date fair value of stock options and other stock based compensation issued to employees and non-employee directors over the requisite service periods, which are typically the vesting periods. We use the Black-Scholes-Merton model to estimate the fair value of our stock-based payments. The volatility assumption used in the Black-Scholes-Merton model is based on the calculated historical volatility based on an analysis of reported data for a peer group of companies. The expected term of options granted by us has been determined based upon the simplified method, because we do not have sufficient historical information regarding our options to derive the expected term. Under this approach, the expected term is the mid-point between the weighted average of vesting period and the contractual term. The risk-free interest rate is based on U.S. Treasury rates whose term is consistent with the expected life of the stock options. We have not paid and do not anticipate paying cash dividends on our shares of common stock; therefore, the expected dividend yield is assumed to be zero. We estimate forfeitures based on our historical experience and adjust the estimated forfeiture rate based upon actual experience.
Inventory
Inventory, which includes material, labor and overhead costs, is stated at cost, and determined on a first-in, first-out basis, not in excess of market value. We record reserves, when necessary, to reduce the carrying value of inventory to its net realizable value. At the point of loss recognition, a new, lower-cost basis for that inventory is established, and any subsequent improvements in facts and circumstances do not result in the restoration or increase in that newly established cost basis.
41
Revenue Recognition
Our revenue consists of product revenue resulting from the sales of systems, instruments and accessories, and service revenue. We recognize revenue when persuasive evidence of an arrangement exists, delivery has occurred or service has been rendered, the price is fixed or determinable, and collectability is reasonably assured. Revenue is presented net of taxes collected from customers that are remitted to government authorities. We generally recognize revenue at the following points in time:
|
|
• |
System sales. For systems sold directly to end customers, revenue is recognized when acceptance occurs, which is deemed to have occurred upon customer acknowledgment of delivery or installation, depending on the terms of the arrangement. The Senhance Systems are delivered with a software component. However, because the software and non-software elements function together to deliver the system’s essential functionality, our arrangements are excluded from being accounted for under software revenue recognition guidance. |
|
|
• |
Instruments and accessories. Revenue from sales of instruments and accessories is generally recognized at the time of shipment. |
|
|
• |
Service. Service revenue is recognized ratably over the term of the service period. Revenue related to services performed on a time-and-materials basis is recognized when it is earned and billable. |
Our system sale arrangements contain multiple elements including a system(s), instruments, accessories, and system service. We generally deliver all of the elements, other than service, within days of entering into the system sale arrangement. Each of these elements is a separate unit of accounting. System accessories, instruments, accessories and service are also sold on a stand-alone basis.
For multiple-element arrangements, revenue is allocated to each unit of accounting based on their relative selling prices. Relative selling prices are based first on vendor specific objective evidence of fair value (“VSOE”), then on third-party evidence of selling price (“TPE”) when VSOE does not exist, and then on management's best estimate of the selling price (“BESP”) when VSOE and TPE do not exist.
Our system sale arrangements generally include a one-year period of free service, and the right for the customer to purchase service annually thereafter. The revenue allocated to the free service period is deferred and recognized ratably over the free service period. Deferred revenue was primarily comprised of deferred revenue related to service contracts for the periods presented.
Because we have neither VSOE nor TPE for our systems, the allocation of revenue is based on BESP for the systems sold. The objective of BESP is to determine the price at which we would transact a sale, had the product been sold on a stand-alone basis. We determine BESP for our systems by considering multiple factors, including, but not limited to, features and functionality of the system, geographies, type of customer, and market conditions. We regularly review BESP and maintain internal controls over establishing and updating these estimates.
Recent Accounting Pronouncements
See “Note 2. Summary of Significant Accounting Policies” of the Notes to Consolidated Financial Statements in “Item 8. Financial Statements and Supplementary Data” of this Annual Report for a full description of recent accounting pronouncements including the respective expected dates of adoption and effects on our Consolidated Balance Sheets and Consolidated Statements of Operations and Comprehensive Loss.
General
We have limited exposure to market risks from instruments that may impact the Balance Sheets, Statements of Operations and Comprehensive Loss, and Statements of Cash Flows. Such exposure is due primarily to changing interest rates.
Interest Rates
The primary objective for our investment activities is to preserve principal while maximizing yields without significantly increasing risk. This is accomplished by investing excess cash in money market funds and Treasury securities. As of December 31, 2016, approximately 93% of the investment portfolio was in cash equivalents with very short term maturities and therefore not subject to any significant interest rate fluctuations.
42
Foreign Currency Exchange Rate Risk
We conduct operations in several different countries, including the U.S. and throughout Europe, and portions of our revenues, expenses, assets and liabilities are denominated in U.S. dollars or other currencies, including Euros. Since our consolidated financial statements are presented in U.S. dollars, we must translate revenues, income and expenses, as well as assets and liabilities, into U.S. dollars at exchange rates in effect during or at the end of each reporting period. We have not historically hedged our exposure to foreign currency fluctuations. Accordingly, increases or decreases in the value of the U.S. dollar against the other currencies could materially affect our net operating revenues, operating income and the value of balance sheet items denominated in foreign currencies.
During the year ended December 31, 2016, 100% of our revenue and approximately 27% of our expenses were denominated in currencies other than the U.S. dollar, most notably the Euro. Based on actual results over the past year, a hypothetical 10% increase or decrease in the U.S. dollar against the Euro would have increased or decreased revenue by approximately $0.2 million and operating expenses by approximately $3.7 million.
43
|
|
|
Page |
|
|
45 |
|
|
|
|
|
|
Consolidated Financial Statements : |
|
|
|
Consolidated Balance Sheets as of December 31, 2016 and 2015 |
|
47 |
|
|
48 |
|
|
|
49 |
|
|
|
50 |
|
|
|
51 |
44
Report of Independent Registered Public Accounting Firm
Board of Directors and Stockholders
TransEnterix, Inc.
Morrisville, North Carolina
We have audited the accompanying consolidated balance sheets of TransEnterix, Inc. (the “Company”) as of December 31, 2016 and 2015 and the related consolidated statements of operations and comprehensive loss, stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2016. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of TransEnterix, Inc. at December 31, 2016 and 2015, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2016, in conformity with accounting principles generally accepted in the United States of America.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As described in Note 2 to the consolidated financial statements, the Company has suffered recurring losses from operations and has not generated significant revenue or positive cash flows from operations. These factors raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), TransEnterix, Inc.’s internal control over financial reporting as of December 31, 2016, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) and our report dated March 6, 2017 expressed an unqualified opinion thereon.
/s/ BDO USA, LLP
Raleigh, North Carolina
March 6, 2017
45
Report of Independent Registered Public Accounting Firm
Board of Directors and Stockholders
TransEnterix, Inc.
Morrisville, North Carolina
We have audited TransEnterix Inc.’s internal control over financial reporting as of December 31, 2016, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). TransEnterix, Inc.’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Item 9A, “Management’s Report on Internal Control Over Financial Reporting”. Our responsibility is to express an opinion on the company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, TransEnterix, Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2016, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of TransEnterix, Inc. as of December 31, 2016 and 2015, and the related consolidated statements of operations and comprehensive loss, stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2016 and our report dated March 6, 2017 expressed an unqualified opinion thereon.
/s/ BDO USA, LLP
Raleigh, North Carolina
March 6, 2017
46
TransEnterix, Inc.
(in thousands, except share amounts)
|
|
|
December 31, |
|
|
December 31, |
|
||
|
|
|
2016 |
|
|
2015 |
|
||
|
Assets |
|
|
|
|
|
|
|
|
|
Current Assets |
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
24,165 |
|
|
$ |
38,449 |
|
|
Accounts receivable, net |
|
|
621 |
|
|
|
76 |
|
|
Inventories |
|
|
7,883 |
|
|
|
6,625 |
|
|
Interest receivable |
|
|
12 |
|
|
|
6 |
|
|
Other current assets |
|
|
5,335 |
|
|
|
3,987 |
|
|
Total Current Assets |
|
|
38,016 |
|
|
|
49,143 |
|
|
Restricted cash |
|
|
10,425 |
|
|
|
— |
|
|
Accounts receivable, net of current portion |
|
|
266 |
|
|
|
— |
|
|
Inventories, net of current portion |
|
|
— |
|
|
|
709 |
|
|
Property and equipment, net |
|
|
5,772 |
|
|
|
4,408 |
|
|
Intellectual property, net |
|
|
37,090 |
|
|
|
46,898 |
|
|
In-process research and development |
|
|
15,920 |
|
|
|
16,511 |
|
|
Goodwill |
|
|
68,697 |
|
|
|
130,869 |
|
|
Other long term assets |
|
|
63 |
|
|
|
64 |
|
|
Total Assets |
|
$ |
176,249 |
|
|
$ |
248,602 |
|
|
Liabilities and Stockholders’ Equity |
|
|
|
|
|
|
|
|
|
Current Liabilities |
|
|
|
|
|
|
|
|
|
Accounts payable |
|
$ |
3,984 |
|
|
$ |
4,450 |
|
|
Accrued expenses |
|
|
8,206 |
|
|
|
7,395 |
|
|
Contingent consideration – current portion |
|
|
10,502 |
|
|
|
12,500 |
|
|
Notes payable - current portion, net of debt discount |
|
|
7,997 |
|
|
|
6,727 |
|
|
Total Current Liabilities |
|
|
30,689 |
|
|
|
31,072 |
|
|
Long Term Liabilities |
|
|
|
|
|
|
|
|
|
Contingent consideration – less current portion |
|
|
12,298 |
|
|
|
11,000 |
|
|
Notes payable - less current portion, net of debt discount |
|
|
4,995 |
|
|
|
12,990 |
|
|
Net deferred tax liabilities |
|
|
10,397 |
|
|
|
16,263 |
|
|
Total Liabilities |
|
|
58,379 |
|
|
|
71,325 |
|
|
Commitments and Contingencies |
|
|
|
|
|
|
|
|
|
Stockholders’ Equity |
|
|
|
|
|
|
|
|
|
Common stock $0.001 par value, 750,000,000 shares authorized at December 31, 2016 and 2015, respectively; 115,781,030 and 100,180,872 shares issued at December 31, 2016 and 2015, respectively; and 115,687,351 and 100,149,453 shares outstanding at December 31, 2016 and 2015, respectively |
|
|
115 |
|
|
|
100 |
|
|
Additional paid-in capital |
|
|
426,609 |
|
|
|
363,280 |
|
|
Accumulated deficit |
|
|
(302,844 |
) |
|
|
(182,864 |
) |
|
Treasury stock at cost, 93,679 and 31,419 shares at December 31, 2016 and 2015, respectively |
|
|
(241 |
) |
|
|
(73 |
) |
|
Accumulated other comprehensive loss |
|
|
(5,769 |
) |
|
|
(3,166 |
) |
|
Total Stockholders’ Equity |
|
|
117,870 |
|
|
|
177,277 |
|
|
Total Liabilities and Stockholders’ Equity |
|
$ |
176,249 |
|
|
$ |
248,602 |
|
See accompanying notes to consolidated financial statements.
47
TransEnterix, Inc.
Consolidated Statements of Operations and Comprehensive Loss
(in thousands except per share amounts)
|
|
|
Year Ended December 31, |
|
|||||||||
|
|
|
2016 |
|
|
2015 |
|
|
2014 |
|
|||
|
Revenue |
|
$ |
1,519 |
|
|
$ |
— |
|
|
$ |
401 |
|
|
Cost of revenue |
|
|
1,069 |
|
|
|
— |
|
|
|
1,095 |
|
|
Gross profit (loss) |
|
|
450 |
|
|
|
— |
|
|
|
(694 |
) |
|
Operating Expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
|
29,273 |
|
|
|
29,669 |
|
|
|
27,944 |
|
|
Sales and marketing |
|
|
9,151 |
|
|
|
2,855 |
|
|
|
1,727 |
|
|
General and administrative |
|
|
10,813 |
|
|
|
7,831 |
|
|
|
5,741 |
|
|
Amortization of intangible assets |
|
|
6,967 |
|
|
|
2,185 |
|
|
|
503 |
|
|
Change in fair value of contingent consideration |
|
|
482 |
|
|
|
(400 |
) |
|
|
— |
|
|
Inventory write-down related to restructuring |
|
|
2,565 |
|
|
|
— |
|
|
|
— |
|
|
Restructuring and other charges |
|
|
3,064 |
|
|
|
— |
|
|
|
— |
|
|
Goodwill impairment |
|
|
61,784 |
|
|
|
— |
|
|
|
— |
|
|
Acquisition related costs |
|
|
— |
|
|
|
4,231 |
|
|
|
— |
|
|
Total Operating Expenses |
|
|
124,099 |
|
|
|
46,371 |
|
|
|
35,915 |
|
|
Operating Loss |
|
|
(123,649 |
) |
|
|
(46,371 |
) |
|
|
(36,609 |
) |
|
Other Expense |
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest expense, net |
|
|
(1,889 |
) |
|
|
(1,601 |
) |
|
|
(1,043 |
) |
|
Other income |
|
|
35 |
|
|
|
— |
|
|
|
— |
|
|
Total Other Expense, net |
|
|
(1,854 |
) |
|
|
(1,601 |
) |
|
|
(1,043 |
) |
|
Loss before income taxes |
|
$ |
(125,503 |
) |
|
$ |
(47,972 |
) |
|
$ |
(37,652 |
) |
|
Income tax benefit |
|
|
5,523 |
|
|
|
1,024 |
|
|
|
— |
|
|
Net loss |
|
$ |
(119,980 |
) |
|
$ |
(46,948 |
) |
|
$ |
(37,652 |
) |
|
Other comprehensive loss |
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign currency translation loss |
|
|
(2,603 |
) |
|
|
(3,166 |
) |
|
|
— |
|
|
Comprehensive loss |
|
$ |
(122,583 |
) |
|
$ |
(50,114 |
) |
|
$ |
(37,652 |
) |
|
Net loss per share - basic and diluted |
|
$ |
(1.07 |
) |
|
$ |
(0.59 |
) |
|
$ |
(0.64 |
) |
|
Weighted average common shares outstanding - basic and diluted |
|
|
112,185 |
|
|
|
79,628 |
|
|
|
58,714 |
|
See accompanying notes to consolidated financial statements.
48
TransEnterix, Inc.
Consolidated Statements of Stockholders’ Equity
(in thousands)
|
|
|
Common Stock (1) |
|
|
Treasury Stock |
|
|
Additional Paid-in |
|
|
Accumulated |
|
|
Accumulated Other Comprehensive |
|
|
Total Stockholders’ |
|
||||||||||||||
|
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
Deficit |
|
|
Loss |
|
|
Equity |
|
||||||||
|
Balance, December 31, 2013 |
|
|
48,842 |
|
|
$ |
49 |
|
|
|
— |
|
|
$ |
— |
|
|
$ |
203,238 |
|
|
$ |
(98,264 |
) |
|
|
— |
|
|
$ |
105,023 |
|
|
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1,840 |
|
|
|
— |
|
|
|
— |
|
|
|
1,840 |
|
|
Issuance of common stock, net of issuance costs |
|
|
14,110 |
|
|
|
14 |
|
|
|
— |
|
|
|
— |
|
|
|
52,419 |
|
|
|
— |
|
|
|
— |
|
|
|
52,433 |
|
|
Exercise of stock options and restricted stock units |
|
|
221 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
75 |
|
|
|
— |
|
|
|
— |
|
|
|
75 |
|
|
Exercise of warrants |
|
|
10 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
16 |
|
|
|
— |
|
|
|
— |
|
|
|
16 |
|
|
Issuance of warrants |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
54 |
|
|
|
— |
|
|
|
— |
|
|
|
54 |
|
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(37,652 |
) |
|
|
— |
|
|
|
(37,652 |
) |
|
Balance, December 31, 2014 |
|
|
63,183 |
|
|
$ |
63 |
|
|
|
— |
|
|
$ |
— |
|
|
$ |
257,642 |
|
|
$ |
(135,916 |
) |
|
$ |
— |
|
|
$ |
121,789 |
|
|
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
3,311 |
|
|
|
— |
|
|
|
— |
|
|
|
3,311 |
|
|
Issuance of common stock, net of issuance costs |
|
|
20,756 |
|
|
|
21 |
|
|
|
— |
|
|
|
— |
|
|
|
58,310 |
|
|
|
— |
|
|
|
— |
|
|
|
58,331 |
|
|
Issuance of common stock, acquisition |
|
|
15,543 |
|
|
|
15 |
|
|
|
|
|
|
|
|
|
|
|
43,662 |
|
|
|
|
|
|
|
|
|
|
|
43,677 |
|
|
Exercise of stock options and restricted stock units |
|
|
698 |
|
|
|
1 |
|
|
|
— |
|
|
|
— |
|
|
|
258 |
|
|
|
— |
|
|
|
— |
|
|
|
259 |
|
|
Return of common stock to pay withholding taxes on restricted stock |
|
|
— |
|
|
|
— |
|
|
|
(31 |
) |
|
|
(73 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(73 |
) |
|
Issuance of warrants |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
97 |
|
|
|
— |
|
|
|
— |
|
|
|
97 |
|
|
Other comprehensive loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(3,166 |
) |
|
|
(3,166 |
) |
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(46,948 |
) |
|
|
— |
|
|
|
(46,948 |
) |
|
Balance, December 31, 2015 |
|
|
100,180 |
|
|
$ |
100 |
|
|
|
(31 |
) |
|
$ |
(73 |
) |
|
$ |
363,280 |
|
|
$ |
(182,864 |
) |
|
$ |
(3,166 |
) |
|
$ |
177,277 |
|
|
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
5,033 |
|
|
|
— |
|
|
|
— |
|
|
|
5,033 |
|
|
Issuance of common stock, net of issuance costs |
|
|
15,086 |
|
|
|
15 |
|
|
|
— |
|
|
|
— |
|
|
|
58,014 |
|
|
|
— |
|
|
|
— |
|
|
|
58,029 |
|
|
Exercise of stock options and restricted stock units |
|
|
419 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
166 |
|
|
|
— |
|
|
|
— |
|
|
|
166 |
|
|
Return of common stock to pay withholding taxes on restricted stock |
|
|
— |
|
|
|
— |
|
|
|
(63 |
) |
|
|
(168 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(168 |
) |
|
Issuance of common stock for services |
|
|
96 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
116 |
|
|
|
— |
|
|
|
— |
|
|
|
116 |
|
|
Other comprehensive loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(2,603 |
) |
|
|
(2,603 |
) |
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(119,980 |
) |
|
|
— |
|
|
|
(119,980 |
) |
|
Balance, December 31, 2016 |
|
|
115,781 |
|
|
$ |
115 |
|
|
|
(94 |
) |
|
$ |
(241 |
) |
|
$ |
426,609 |
|
|
$ |
(302,844 |
) |
|
$ |
(5,769 |
) |
|
$ |
117,870 |
|
See accompanying notes to consolidated financial statements.
|
(1) |
Adjusted for 1:5 reverse stock split on March 31, 2014. |
49
TransEnterix, Inc.
Consolidated Statements of Cash Flows
(in thousands)
|
|
|
Twelve Months Ended |
|
|||||||||
|
|
|
December 31, |
|
|||||||||
|
|
|
2016 |
|
|
2015 |
|
|
2014 |
|
|||
|
Operating Activities |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(119,980 |
) |
|
$ |
(46,948 |
) |
|
$ |
(37,652 |
) |
|
Adjustments to reconcile net loss to net cash and cash equivalents used in operating activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation |
|
|
1,942 |
|
|
|
1,248 |
|
|
|
807 |
|
|
Amortization of intangible assets |
|
|
6,967 |
|
|
|
2,185 |
|
|
|
503 |
|
|
Amortization of debt discount and debt issuance costs |
|
|
177 |
|
|
|
142 |
|
|
|
83 |
|
|
Stock-based compensation |
|
|
5,033 |
|
|
|
3,311 |
|
|
|
1,840 |
|
|
Common stock issued for services |
|
|
116 |
|
|
|
— |
|
|
|
— |
|
|
Inventory write-down related to restructuring |
|
|
2,565 |
|
|
|
— |
|
|
|
— |
|
|
Loss on disposal of property |
|
|
— |
|
|
|
34 |
|
|
|
86 |
|
|
Non-cash restructuring and other charges |
|
|
2,556 |
|
|
|
— |
|
|
|
— |
|
|
Goodwill impairment |
|
|
61,784 |
|
|
|
— |
|
|
|
— |
|
|
Deferred tax benefit |
|
|
(5,562 |
) |
|
|
(1,024 |
) |
|
|
— |
|
|
Change in fair value of contingent consideration |
|
|
482 |
|
|
|
(400 |
) |
|
|
— |
|
|
Changes in operating assets and liabilities, net of effect of acquisition: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts receivable |
|
|
(1,041 |
) |
|
|
133 |
|
|
|
55 |
|
|
Interest receivable |
|
|
(6 |
) |
|
|
(5 |
) |
|
|
67 |
|
|
Inventories |
|
|
(6,647 |
) |
|
|
(4,630 |
) |
|
|
701 |
|
|
Other current and long term assets |
|
|
(1,528 |
) |
|
|
728 |
|
|
|
(170 |
) |
|
Accounts payable |
|
|
(356 |
) |
|
|
1,096 |
|
|
|
(36 |
) |
|
Accrued expenses |
|
|
1,112 |
|
|
|
5,371 |
|
|
|
363 |
|
|
Net cash and cash equivalents used in operating activities |
|
|
(52,386 |
) |
|
|
(38,759 |
) |
|
|
(33,353 |
) |
|
Investing Activities |
|
|
|
|
|
|
|
|
|
|
|
|
|
Payment for acquisition of a business |
|
|
— |
|
|
|
(25,000 |
) |
|
|
|
|
|
Proceeds from sale and maturities of investments |
|
|
— |
|
|
|
— |
|
|
|
6,191 |
|
|
Proceeds from sale of property and equipment |
|
|
— |
|
|
|
— |
|
|
|
25 |
|
|
Purchase of property and equipment |
|
|
(1,361 |
) |
|
|
(1,234 |
) |
|
|
(2,174 |
) |
|
Net cash and cash equivalents (used in) provided by investing activities |
|
|
(1,361 |
) |
|
|
(26,234 |
) |
|
|
4,042 |
|
|
Financing Activities |
|
|
|
|
|
|
|
|
|
|
|
|
|
Payment of debt |
|
|
(6,902 |
) |
|
|
— |
|
|
|
(2,877 |
) |
|
Payment of contingent consideration |
|
|
(1,182 |
) |
|
|
— |
|
|
|
— |
|
|
Proceeds from issuance of common stock, net of issuance costs |
|
|
58,029 |
|
|
|
58,331 |
|
|
|
52,433 |
|
|
Proceeds from issuance of debt, net of debt discount |
|
|
— |
|
|
|
9,887 |
|
|
|
4,291 |
|
|
Taxes paid related to net share settlement of vesting of restricted stock units |
|
|
(168 |
) |
|
|
(73 |
) |
|
|
— |
|
|
Proceeds from exercise of stock options and warrants |
|
|
166 |
|
|
|
259 |
|
|
|
91 |
|
|
Net cash and cash equivalents provided by financing activities |
|
|
49,943 |
|
|
|
68,404 |
|
|
|
53,938 |
|
|
Effect of exchange rate changes on cash and cash equivalents |
|
|
(55 |
) |
|
|
22 |
|
|
|
— |
|
|
Net (decrease) increase in cash, cash equivalents and restricted cash |
|
|
(3,859 |
) |
|
|
3,433 |
|
|
|
24,627 |
|
|
Cash, cash equivalents and restricted cash, beginning of period |
|
|
38,449 |
|
|
|
35,016 |
|
|
|
10,389 |
|
|
Cash, cash equivalents and restricted cash, end of period |
|
$ |
34,590 |
|
|
$ |
38,449 |
|
|
$ |
35,016 |
|
|
Supplemental Disclosure for Cash Flow Information |
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest paid |
|
$ |
1,289 |
|
|
$ |
973 |
|
|
$ |
904 |
|
|
Supplemental Schedule of Noncash Investing Activities |
|
|
|
|
|
|
|
|
|
|
|
|
|
Transfer of inventory to property and equipment |
|
$ |
3,198 |
|
|
|
— |
|
|
|
— |
|
|
Issuance of common stock warrants |
|
|
— |
|
|
$ |
97 |
|
|
$ |
54 |
|
|
Contingent consideration related to acquisition |
|
|
— |
|
|
$ |
23,900 |
|
|
|
— |
|
|
Issuance of common stock related to acquisition |
|
|
— |
|
|
$ |
43,677 |
|
|
|
— |
|
See accompanying notes to consolidated financial statements.
50
TransEnterix, Inc.
Notes to Consolidated Financial Statements
|
1. |
Organization and Capitalization |
TransEnterix, Inc. (the “Company”) is a medical device company that is pioneering the use of robotics to improve minimally invasive surgery by addressing the clinical challenges associated with current laparoscopic and robotic options. The Company is focused on the commercialization and further development of its Senhance™ Surgical Robotic System (formerly known as the ALF-X ® Surgical Robotic System) (the “Senhance System”), a multi-port robotic system that brings the advantages of robotic surgery to patients while enabling surgeons with innovative technology. The Company also developed the SurgiBot™ System, a single-port, robotically enhanced laparoscopic surgical platform. The Senhance System has been granted a CE Mark in Europe for laparoscopic abdominal and pelvic surgery, as well as limited thoracic operations excluding cardiac and vascular surgery, but is not available for sale in the U.S. The SurgiBot System is not available for sale in any market.
The Senhance System is a multi-port robotic surgery system which allows multiple arms to control robotic instruments and a camera. The system features advanced technology to enable surgeons with haptic feedback and the ability to move the camera via eye movement. The system replicates laparoscopic motion that is familiar to experienced surgeons, and features three-dimensional high definition (“3DHD”) vision technology. The Senhance System also offers responsible economics to hospitals by offering robotic technology with reusable instruments with minimal additional costs per surgery.
The SurgiBot System is designed to utilize flexible instruments through articulating channels controlled directly by the surgeon, with robotic assistance, while the surgeon remains patient-side within the sterile field. In June 2015, the Company submitted a 510(k) application to the FDA for the SurgiBot System. On April 19, 2016, the FDA notified the Company that the SurgiBot System did not meet the criteria for substantial equivalence based on the data and information submitted by TransEnterix in the 510(k) submission. As a result, the Company has reprioritized its near-term regulatory efforts to focus on the 510(k) submission for the Senhance System. Consequently, in May 2016, the Company implemented a restructuring plan. See Note 17 to the consolidated financial statements for further details regarding the restructuring.
On September 3, 2013, TransEnterix Surgical, Inc. a Delaware corporation (“TransEnterix Surgical”), and SafeStitch Medical, Inc., a Delaware corporation (“SafeStitch”) consummated a merger transaction whereby TransEnterix Surgical merged with a merger subsidiary of SafeStitch, with TransEnterix Surgical as the surviving entity in the merger (the “Merger”). As a result of the Merger, TransEnterix Surgical became a wholly owned subsidiary of SafeStitch. On December 6, 2013, SafeStitch changed its name to TransEnterix, Inc. and increased the authorized shares of common stock from 225,000,000 to 750,000,000, and authorized 25,000,000 shares of preferred stock, par value $0.01 per share.
On September 18, 2015, the Company entered into a Membership Interest Purchase Agreement, (the “Purchase Agreement”) with Sofar S.p.A., (“Sofar”) as seller, Vulcanos S.r.l. (“Vulcanos”), as the acquired company, and TransEnterix International, Inc. (“TransEnterix International”), a direct, wholly owned subsidiary of the Company which was incorporated in September 2015, as buyer. The closing of the transactions occurred on September 21, 2015 (the “Closing Date”) pursuant to which the Company acquired all of the membership interests of Vulcanos from Sofar (now known as the “Senhance Acquisition”), and changed the name of Vulcanos to TransEnterix Italia S.r.l (“TransEnterix Italia”). The Senhance Acquisition included all of the assets, employees and contracts related to the Senhance System. See Note 3 for a description of the related transactions.
As used herein, the term “Company” refers to the combination of SafeStitch and TransEnterix Surgical after giving effect to the Merger, and includes TransEnterix International, TransEnterix Italia, TransEnterix Europe S.Á.R.L, TransEnterix Europe S.Á.R.L, Bertrange, Swiss Branch, Lugano and TransEnterix Asia PTE. LTD. after giving effect to the Senhance Acquisition, the term “SafeStitch” refers to the historic business of SafeStitch Medical, Inc. prior to the Merger, and the term “TransEnterix Surgical” refers to the historic business of TransEnterix Surgical, Inc. prior to the Merger.
The Company operates in one business segment.
51
|
2. |
Summary of Significant Accounting Policies |
Basis of Presentation
The accompanying Consolidated Financial Statements have been prepared in accordance with U.S. generally accepted accounting principles (“U.S. GAAP”) and include the accounts of the Company and its direct and indirect wholly owned subsidiaries, SafeStitch LLC, TransEnterix Surgical, Inc., TransEnterix International, Inc., TransEnterix Italia S.r.l., TransEnterix Europe S.Á.R.L, TransEnterix Europe S.Á.R.L, Bertrange, Swiss Branch, Lugano and TransEnterix Asia PTE. LTD. All inter-company accounts and transactions have been eliminated in consolidation.
Going Concern
The accompanying consolidated financial statements have been prepared on a going concern basis. The Company has accumulated a deficit of approximately $302.8 million as of December 31, 2016, a net loss of approximately $120.0 million for the year ended December 31, 2016, and has not generated significant revenue or positive cash flows from operations. These factors raise substantial doubt about the Company’s ability to continue as a going concern. The accompanying consolidated financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or amounts of liabilities that might result from the outcome of this uncertainty. To meet its capital needs, the Company is considering multiple alternatives, including, but not limited to, additional equity financings, debt financings and other funding transactions. There can be no assurance that the Company will be able to complete any such transaction on acceptable terms or otherwise. If the Company is unable to obtain the necessary capital, it will need to pursue a plan to license or sell its assets, seek to be acquired by another entity and/or cease operations.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. Significant items subject to such estimates and assumptions include identifiable intangible assets and goodwill, contingent consideration, stock compensation expense, restructuring and other charges, excess and obsolete inventory reserves, and deferred tax asset valuation allowances.
Reverse Stock Split
On March 31, 2014, the Company effectuated a reverse stock split of its issued and outstanding shares of common stock at a ratio of 1 for 5 (the “Reverse Stock Split”). As a result of the Reverse Stock Split, the Company’s issued and outstanding stock decreased from 244,276,923 to 48,855,255 shares of common stock, all with a par value of $0.001. All information related to common stock, stock options, restricted stock units, warrants and earnings per share for prior periods has been retroactively adjusted to give effect to the Reverse Stock Split.
Cash and Cash Equivalents, Restricted Cash, and Short-Term Investments
The Company considers all highly liquid investments with original maturities of 90 days or less at the time of purchase to be cash equivalents and investments with original maturities of between 91 days and one year to be short-term investments. In order to manage exposure to credit risk, the Company invests in high-quality investments rated at least A2 by Moody’s Investors Service or A by Standard & Poor.
Restricted cash at December 31, 2016 includes $10.0 million in a money market account, held in connection with the Company’s notes payable and $425,000 in cash accounts held as collateral primarily under the terms of an office operating lease and automobile leases.
The Company held no investments as of December 31, 2016, 2015, and 2014 as it sold all its investment securities during 2014.
Realized gains and losses on sales of investment securities are determined based on the specific-identification method and are recorded in interest expense, net. The amortized cost of debt securities is adjusted for amortization of premiums and accretion of discounts to maturity computed under the effective interest method. Such amortization and accretion is included in interest expense, net.
52
Concentrations and Credit Risk
The Company’s principal financial instruments subject to potential concentration of credit risk are cash and cash equivalents and investments held in money market accounts. The Company places cash deposits with a federally insured financial institution. The Company maintains its cash at banks and financial institutions it considers to be of high credit quality; however, the Company’s cash deposits may at times exceed the FDIC insured limit. Balances in excess of federally insured limitations may not be insured. The Company has not experienced losses on these accounts, and management believes that the Company is not exposed to significant risks on such accounts.
The Company’s accounts receivable are derived from net revenue to customers located throughout the world. The Company evaluates its customers’ financial condition and, generally, requires no collateral from its customers. The Company provides reserves for potential credit losses but has not experienced significant losses to date. The Company had one customer who constituted 100% of the Company’s net accounts receivable at December 31, 2016 and one customer who constituted 100% of the Company’s net accounts receivable at December 31, 2015. The Company had one customer who accounted for 100% of sales in 2016 and two customers who accounted for 37% and 10% of sales in 2014. There were no sales in 2015.
Accounts Receivable
Accounts receivable are recorded at net realizable value, which includes an allowance for estimated uncollectable accounts. The allowance for uncollectible accounts was determined based on historical collection experience.
Inventories
Inventories are stated at the lower of cost (determined on a first-in, first-out basis) or market. Inventory costs include direct materials, direct labor, and normal manufacturing overhead. The Company records reserves, when necessary, to reduce the carrying value of inventory to its net realizable value. Management considers forecast demand in relation to the inventory on hand, competitiveness of product offerings, market conditions and product life cycles when determining excess and obsolescence and net realizable value adjustments. At the point of loss recognition, a new, lower-cost basis for that inventory is established, and any subsequent improvements in facts and circumstances do not result in the restoration or increase in that newly established cost basis. Any inventory on hand at the measurement date in excess of the Company’s current requirements based on anticipated levels of sales is classified as long-term on the Company’s consolidated balance sheets. The Company’s classification of long-term inventory requires it to estimate the portion of on-hand inventory that can be realized over the next 12 months.
Identifiable Intangible Assets and Goodwill
Identifiable intangible assets are recorded at cost, or when acquired as part of a business acquisition, at estimated fair value. Certain intangible assets are amortized over 7 to 10 years. Similar to tangible personal property and equipment, the Company periodically evaluates identifiable intangible assets for impairment whenever events or changes in circumstances indicate that the carrying amount may not be recoverable.
Intellectual property consists of purchased patent rights and developed research and development acquired as part of a business acquisition. Amortization of the patent rights is recorded using the straight-line method over the estimated useful life of the patents of 10 years. Amortization of the developed research and development is recorded using the straight-line method over the estimated useful life of 7 years. This method approximates the period over which the Company expects to receive the benefit from these assets. See Note 17 for additional information related to the write-off of purchased patents in connection with the restructuring plan executed in May 2016. No impairment existed at December 31, 2015 or 2014.
Indefinite-lived intangible assets, such as goodwill, are not amortized. The Company tests the carrying amounts of goodwill for recoverability on an annual basis at December 31 or when events or changes in circumstances indicate evidence a potential impairment exists, using a fair value based test. The Company continues to operate in one segment, which is considered to be the sole reporting unit and therefore, goodwill is tested for impairment at the enterprise level. See Note 10 for additional information related to goodwill impairment recorded during the second quarter of 2016. No impairment existed at December 31, 2015 or 2014.
In-Process Research and Development
In-process research and development (“IPR&D”) assets represent the fair value assigned to technologies that were acquired, which at the time of acquisition have not reached technological feasibility and have no alternative future use. IPR&D assets are considered to be indefinite-lived until the completion or abandonment of the associated research and development projects. During the period that
53
the IPR&D assets are considered indefinite-lived, they are tested for impairment on an annual basis, or more frequently if the Company becomes aware of any events occurring or changes in circumstances that indicate that the fair value of the IPR&D assets are less than their carrying amounts. If and when development is complete, which generally occurs upon regulatory approval, and the Company is able to commercialize products associated with the IPR&D assets, these assets are then deemed definite-lived and are amortized based on their estimated useful lives at that point in time. If development is terminated or abandoned, the Company may have a full or partial impairment charge related to the IPR&D assets, calculated as the excess of carrying value of the IPR&D assets over fair value. The IPR&D was acquired on September 21, 2015. No impairment existed at December 31, 2016 and 2015.
Property and Equipment
Property and equipment consists primarily of machinery, manufacturing equipment, demonstration equipment, computer equipment, furniture, and leasehold improvements, which are recorded at cost.
Depreciation is recorded using the straight-line method over the estimated useful lives of the assets as follows:
|
Machinery, manufacturing and demonstration equipment |
|
3-5 years |
|
Computer equipment |
|
3 years |
|
Furniture |
|
5 years |
|
Leasehold improvements |
|
Lesser of lease term or 3 to 10 years |
Upon retirement or sale, the cost of assets disposed of and the related accumulated depreciation and amortization are removed from the accounts and any resulting gain or loss is credited or charged to operations. Repairs and maintenance costs are expensed as incurred.
Impairment of Long-Lived Assets
The Company reviews its long-lived assets for possible impairment whenever events or changes in circumstances indicate that the carrying amount of the assets may not be fully recoverable. To determine the recoverability of its long-lived assets, the Company evaluates the probability that future estimated undiscounted net cash flows will be less than the carrying amount of the assets. If such estimated cash flows are less than the carrying amount of the long-lived assets, then such assets are written down to their fair value. The Company’s estimates of anticipated cash flows and the remaining estimated useful lives of long-lived assets could be reduced in the future, resulting in a reduction to the carrying amount of long-lived assets.
Contingent Consideration
Contingent consideration is recorded as a liability and is the estimate of the fair value of potential milestone payments related to business acquisitions. Contingent consideration is measured at fair value using a discounted cash flow model utilizing significant unobservable inputs including the probability of achieving each of the potential milestones and an estimated discount rate associated with the risks of the expected cash flows attributable to the various milestones. Significant increases or decreases in any of the probabilities of success or changes in expected timelines for achievement of any of these milestones would result in a significantly higher or lower fair value of these milestones, respectively, and commensurate changes to the associated liability. The fair value of the contingent consideration at each reporting date is updated by reflecting the changes in fair value in our statement of operations and comprehensive loss.
Translation of Foreign Currencies
The functional currency of the Company’s operational foreign subsidiaries is Euros. The assets and liabilities of the Company’s foreign subsidiaries are translated into U.S. dollars at exchange rates in effect at the balance sheet date. Income and expense items are translated at the average exchange rates prevailing during the period. The cumulative translation effect for a subsidiary using a functional currency other than the U.S. dollar is included in accumulated other comprehensive income or loss as a separate component of stockholders’ equity.
54
The Company’s intercompany accounts are denominated in the functional currency of the foreign subsidiary. Gains and losses resulting from the remeasurement of intercompany receivables that the Company considers to be of a long-term investment nature are recorded as a cumulative translation adjustment in accumulated other comprehensive income or loss as a separate component of stockholders’ equity, while gains and losses resulting from the remeasurement of intercompany receivables from a foreign subsidiary for which the Company anticipates settlement in the foreseeable future are recorded in the consolidated statement of operations and comprehensive loss. The net gains and losses included in net loss in the consolidated statements of operations and comprehensive loss for the years December 31, 2016, 2015, and 2014 were not significant.
Business Acquisitions
Business acquisitions are accounted for using the acquisition method of accounting in accordance with ASC 805, “Business Combinations.” ASC 805 requires, among other things, that assets acquired and liabilities assumed be recognized at their fair values, as determined in accordance with ASC 820, “Fair Value Measurements,” as of the acquisition date. For certain assets and liabilities, book value approximates fair value. In addition, ASC 805 establishes that consideration transferred be measured at the closing date of the acquisition at the then-current market price. Under ASC 805, acquisition related costs (i.e., advisory, legal, valuation and other professional fees) and certain acquisition-related restructuring charges impacting the target company are expensed in the period in which the costs are incurred. The application of the acquisition method of accounting requires the Company to make estimates and assumptions related to the estimated fair values of net assets acquired.
Significant judgments are used during this process, particularly with respect to intangible assets. Generally, intangible assets are amortized over their estimated useful lives. Goodwill and other indefinite-lived intangibles are not amortized, but are annually assessed for impairment. Therefore, the purchase price allocation to intangible assets and goodwill has a significant impact on future operating results.
Risk and Uncertainties
The Company is subject to a number of risks similar to other similarly-sized companies in the medical device industry. These risks include, without limitation, the historical lack of profitability; the Company’s ability to raise additional capital; its ability to successfully integrate the Senhance System into its business; its ability to successfully develop, clinically test and commercialize its products; the timing and outcome of the regulatory review process for its products; changes in the health care and regulatory environments of the United States, Italy, other countries in the European Union, and other countries in which the Company intends to operate; its ability to attract and retain key management, marketing and scientific personnel; competition from new entrants; its ability to successfully prepare, file, prosecute, maintain, defend and enforce patent claims and other intellectual property rights; its ability to successfully transition from a research and development company to a marketing, sales and distribution concern; competition in the market for robotic surgical devices; and its ability to identify and pursue development of additional products.
Revenue Recognition
The Company’s revenue consists of product revenue resulting from the sales of systems, instruments and accessories, and service revenue. The Company recognizes revenue when persuasive evidence of an arrangement exists, delivery has occurred or service has been rendered, the price is fixed or determinable, and collectability is reasonably assured. Revenue is presented net of taxes collected from customers that are remitted to government authorities. The Company generally recognizes revenue at the following points in time:
|
|
• |
System sales. For systems sold directly to end customers, revenue is recognized when acceptance occurs, which is deemed to have occurred upon customer acknowledgment of delivery or installation, depending on the terms of the arrangement. The Senhance Systems are delivered with a software component. However, because the software and non-software elements function together to deliver the system’s essential functionality, the Company's arrangements are excluded from being accounted for under software revenue recognition guidance. |
|
|
• |
Instruments and accessories. Revenue from sales of instruments and accessories is generally recognized at the time of shipment. |
|
|
• |
Service. Service revenue is recognized ratably over the term of the service period. Revenue related to services performed on a time-and-materials basis is recognized when it is earned and billable. |
The Company's system sale arrangements contain multiple elements including a system(s), instruments, accessories, and system service. The Company generally delivers all of the elements, other than service, within days of entering into the system sale arrangement. Each of these elements is a separate unit of accounting. System accessories, instruments, and service are also sold on a stand-alone basis.
55
For multiple-element arrangements, revenue is allocated to each unit of accounting based on their relative selling prices. Relative selling prices are based first on vendor specific objective evidence of fair value (“VSOE”), then on third-party evidence of selling price (“TPE”) when VSOE does not exist, and then on management's best estimate of the selling price (“BESP”) when VSOE and TPE do not exist.
The Company’s system sale arrangements generally include a one-year period of free service, and the right for the customer to purchase service annually thereafter. The revenue allocated to the free service period is deferred and recognized ratably over the free service period.
Because the Company has neither VSOE nor TPE for its systems, the allocation of revenue is based on BESP for the systems sold. The objective of BESP is to determine the price at which the Company would transact a sale, had the product been sold on a stand-alone basis. The Company determines BESP for its systems by considering multiple factors, including, but not limited to, features and functionality of the system, geographies, type of customer, and market conditions. The Company regularly reviews BESP and maintains internal controls over establishing and updating these estimates.
Cost of Goods Sold
Cost of goods sold consists of contract manufacturing, materials, labor and manufacturing overhead incurred internally to produce the products. Shipping and handling costs incurred by the Company are included in cost of goods sold.
Research and Development Costs
Research and development expenses primarily consist of engineering, product development and regulatory expenses, incurred in the design, development, testing and enhancement of our products and legal services associated with our efforts to obtain and maintain broad protection for the intellectual property related to our products. Research and development costs are expensed as incurred.
Stock-Based Compensation
The Company follows ASC 718 (“Stock Compensation”) and ASC 505-50 (“Equity-Based Payments to Non-employees”), which provide guidance in accounting for share-based awards exchanged for services rendered and requires companies to expense the estimated fair value of these awards over the requisite service period. For awards granted to non-employees, the Company determines the fair value of the stock-based compensation awards granted as either the fair value of the consideration received or the fair value of the equity instruments issued, whichever is more reliably measurable. If the fair value of the equity instruments issued is used, it is measured using the stock price and other measurement assumptions as of the earlier of either (1) the date at which a commitment for performance by the counterparty to earn the equity instruments is reached, or (2) the date at which the counterparty’s performance is complete.
The Company recognizes compensation expense for stock-based awards based on estimated fair values on the date of grant for awards granted to employees. The Company uses the Black-Scholes-Merton option pricing model to determine the fair value of stock options. The fair value of restricted stock units is determined by the market price of the Company’s common stock on the date of grant. The expense associated with stock-based compensation is recognized on a straight-line basis over the requisite service period of each award.
The Company records as expense the fair value of stock-based compensation awards, including stock options and restricted stock units. Compensation expense for stock-based compensation was approximately $5,033,000, $3,311,000 and $1,840,000 for the years ended December 31, 2016, 2015 and 2014, respectively.
Income Taxes
The Company accounts for income taxes using the asset and liability method, which requires the recognition of deferred tax assets or liabilities for the temporary differences between financial reporting and tax basis of the Company’s assets and liabilities, and for tax carryforwards at enacted statutory rates in effect for the years in which the asset or liability is expected to be realized. The effect on deferred taxes of a change in tax rates is recognized in income during the period that includes the enactment date. In addition, valuation allowances are established when necessary to reduce deferred tax assets and liabilities to the amounts expected to be realized.
56
Comprehensive Loss
Comprehensive loss is defined as the change in equity of a business enterprise during a period from transactions and other events and circumstances from non-owner sources.
Segments
The Company operates in one business segment—the research, development and sale of medical device robotics to improve minimally invasive surgery. The Company’s chief operating decision maker (determined to be the Chief Executive Officer) does not manage any part of the Company separately, and the allocation of resources and assessment of performance are based on the Company’s consolidated operating results. Approximately 49% of the Company’s total consolidated assets are located within the U.S. as of December 31, 2016. The remaining assets are mostly located in Europe and are primarily related to the Company’s facility in Italy, and include goodwill, intellectual property, in-process research and development, other current assets, property and equipment, cash, accounts receivable and inventory of $90.4 million at December 31, 2016, associated with the Senhance Acquisition in September 2015. Total assets outside of the U.S. excluding goodwill amounted to 40% and 28% of total consolidated assets at December 31, 2016 and 2015, respectively. The Company recognizes sales by geographic area based on the country in which the customer is based. For the years ended December 31, 2016, 2015, and 2014, 0%, 0%, and 90%, respectively, of net revenue were generated in the United States and 100%, 0%, and 0% were generated in Europe.
Impact of Recently Issued Accounting Standards
In January 2017, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2017-04, Simplifying the Test for Goodwill Impairment. Under the new standard, goodwill impairment would be measured as the amount by which a reporting unit’s carrying value exceeds its fair value, not to exceed the carrying value of goodwill. This ASU eliminates existing guidance that requires an entity to determine goodwill impairment by calculating the implied fair value of goodwill by hypothetically assigning the fair value of a reporting unit to all of its assets and liabilities as if that reporting unit had been acquired in a business combination. This ASU is effective prospectively to annual and interim impairment tests beginning after December 15, 2019, with early adoption permitted. The Company is currently evaluating the new guidelines.
In August 2016, the FASB issued ASU 2016-18, Statement of Cash Flows (Topic 230)—Restricted Cash. ASU 2016-18 requires the statement of cash flows to be a reconciliation between beginning and ending cash balances inclusive of restricted cash balances. ASU 2016-18 is effective for fiscal years beginning after December 15, 2017 and is to be applied using a retrospective transition method to each period presented. Early adoption is permitted. The Company adopted this ASU for the year ended December 31, 2016. The adoption of this standard resulted in the removal of changes in Restricted Cash from the Consolidated Statements of Cash Flows of $250,000 and $125,000 for the years ended December 31, 2015 and 2014, respectively and inclusion of these amounts as part of the starting and ending cash balances.
In August 2016, the FASB issued ASU 2016-15, Statement of Cash Flows (Topic 230) which addresses changes to reduce the presentation diversity of certain cash receipts and cash payments in the statement of cash flows, including debt prepayment or extinguishment costs, settlement of certain debt instruments, contingent consideration payments made after a business combination, proceeds from the settlement of insurance claims, and distributions received from equity method investees. The guidance becomes effective for fiscal years beginning after December 15, 2017, including interim periods within those fiscal years, with early adoption permitted. An entity that elects early adoption must adopt all of the amendments in the same period. The new standard will be applied retrospectively, but may be applied prospectively if retrospective application would be impracticable. The Company is currently evaluating the new guidance and has not determined the impact this standard may have on its consolidated statement of cash flows.
In March 2016, the FASB issued ASU 2016-09, Compensation – Stock Compensation (Topic 718) – Improvements to Employee Share-Based Payment Accounting. Under ASU 2016-09, the tax effects of stock compensation will be recognized as income tax expense or benefit in the income statement and the tax effects of exercised or vested awards will be treated as discrete items in the reporting period in which they occur. Along with other income tax cash flows, excess tax benefits will be classified as operating activities, and cash paid by an employer when directly withholding shares for tax withholding purposes will be classified as financing activities. Entities may make an entity-wide accounting policy election to either estimate the number of awards that are expected to vest (current GAAP) or account for forfeitures when they occur. The threshold to qualify for equity classification permits withholding up to the maximum statutory tax rates in the applicable jurisdictions. For public companies, ASU 2016-09 is effective for annual periods beginning after December 15, 2016, and interim periods within those annual periods. Early adoption is permitted, however, an entity that elects early adoption must adopt all amendments under the new standard in the same period. The Company does not expect this ASU will have a material impact on its consolidated financial statements.
57
In February 2016, the FASB issued ASU 2016-02, Leases. The new standard establishes a right-of-use (ROU) model that requires a lessee to record a ROU asset and a lease liability on the balance sheet for all leases with terms longer than 12 months. Leases will be classified as either finance or operating, with classification affecting the pattern of expense recognition in the income statement. The new standard is effective for fiscal years beginning after December 15, 2018, including interim periods within those fiscal years. A modified retrospective transition approach is required for lessees for capital and operating leases existing at, or entered into after, the beginning of the earliest comparative period presented in the financial statements, with certain practical expedients available. The Company currently expects that upon adoption, ROU assets and lease liabilities will be recognized in the balance sheet in amounts that the Company does not expect will have a material impact on the consolidated financial statements based on the Company’s current leases.
In May 2014, the FASB issued ASU 2014-09, Revenue from Contracts with Customers (ASU 2014-09), which supersedes nearly all existing revenue recognition guidance under U.S. GAAP. The core principle of ASU 2014-09 is to recognize revenue when promised goods or services are transferred to customers in an amount that reflects the consideration to which an entity expects to be entitled for those goods or services. ASU 2014-09 defines a five step process to achieve this core principle and, in doing so, more judgment and estimates may be required within the revenue recognition process than are required under existing U.S. GAAP.
The updated guidance is effective for annual periods beginning after December 15, 2017, and interim periods therein, using either of the following transition methods: (i) a full retrospective approach reflecting the application of the standard in each prior reporting period with the option to elect certain practical expedients, or (ii) a retrospective approach with the cumulative effect of initially adopting ASU 2014-09 recognized at the date of adoption (which includes additional footnote disclosures). Due to limited sales, the Company has evaluated its contracts and has concluded that the impact of adopting the standard will have no material impact on its consolidated financial statements and related disclosures.
In August 2014, the FASB issued ASU 2014-15, Presentation of Financial Statements—Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern (“ASU 2014-15”). The amendments in ASU 2014-15 are intended to define management’s responsibility to evaluate whether there is substantial doubt about an organization’s ability to continue as a going concern and to provide related footnote disclosures. Under U.S. GAAP, financial statements are prepared under the presumption that the reporting organization will continue to operate as a going concern, except in limited circumstances. The going concern basis of accounting is critical to financial reporting because it establishes the fundamental basis for measuring and classifying assets and liabilities. Currently, U.S. GAAP lacks guidance about management’s responsibility to evaluate whether there is substantial doubt about the organization’s ability to continue as a going concern or to provide related footnote disclosures. This ASU provides guidance to an organization’s management, with principles and definitions that are intended to reduce diversity in the timing and content of disclosures that are commonly provided by organizations today in the financial statement footnotes. This update is effective for annual periods ending after December 15, 2016, and interim periods within annual periods beginning after December 15, 2016. Early application is permitted for annual or interim reporting periods for which the financial statements have not previously been issued. The Company adopted this ASU in the current year, noting no material impact on the Company’s consolidated financial statements.
In July 2015, the FASB issued ASU 2015-11, Simplifying the Measurement of Inventory (Topic 330). This update requires inventory within the scope of the standard to be measured at the lower of cost and net realizable value. Previous guidance required inventory to be measured at the lower of cost or market (where market was defined as replacement cost, with a ceiling of net realizable value and floor of net realizable value less a normal profit margin). This update is effective for annual and interim periods beginning after December 15, 2016, which will require us to adopt these provisions in the first quarter of fiscal year 2017. Early adoption is permitted. The Company does not expect this ASU will have a material impact on its consolidated financial statements.
In January 2016, the FASB issued ASU 2016-01, Recognition and Measurement of Financial Assets and Financial Liabilities . ASU 2016-01 requires equity investments to be measured at fair value with changes in fair value recognized in net income; simplifies the impairment assessment of equity investments without readily determinable fair values by requiring a qualitative assessment to identify impairment; eliminates the requirement for public business entities to disclose the method(s) and significant assumptions used to estimate the fair value that is required to be disclosed for financial instruments measured at amortized cost on the balance sheet; requires public business entities to use the exit price notion when measuring the fair value of financial instruments for disclosure purposes; requires an entity to present separately in other comprehensive income the portion of the total change in the fair value of a liability resulting from a change in the instrument-specific credit risk when the entity has elected to measure the liability at fair value in accordance with the fair value option for financial instruments; requires separate presentation of financial assets and financial liabilities by measurement category and form of financial assets on the balance sheet or the accompanying notes to the financial statements and clarifies that an entity should evaluate the need for a valuation allowance on a deferred tax asset related to available-for-sale securities in combination with the entity’s other deferred tax assets. ASU 2016-01 is effective for financial statements issued for fiscal years beginning after December 15, 2017, and interim periods within those fiscal years. The Company does not believe the adoption of this guidance will have a material impact on its consolidated financial statements or related footnote disclosures.
58
Reclassifications
As a result of a recent acquisition, certain financial statement captions have been added and we have reclassified certain prior-period amounts on our consolidated balance sheet and statement of operations and comprehensive loss to conform to the presentation for the current period. Such reclassifications have no effect on previously reported total assets, liabilities, stockholders’ equity or net loss.
|
3. |
Acquisition of Senhance Surgical Robotic System |
On September 21, 2015, the Company completed the strategic acquisition, through its wholly owned subsidiary TransEnterix International, from Sofar, of all of the assets, employees and contracts related to the advanced robotic system for minimally invasive laparoscopic surgery now known as the Senhance System and changed the name of the acquired company from Vulcanos S.r.l. to TransEnterix Italia S.r.l.
Under the terms of the Purchase Agreement, the consideration consisted of the issuance of 15,543,413 shares of the Company’s common stock (the “Securities Consideration”) and approximately $25.0 million U.S. Dollars and €27.5 million Euro in cash consideration (the “Cash Consideration”). The Securities Consideration was issued in full at the closing of the Senhance Acquisition; the Cash Consideration was or will be paid in four tranches, as follows:
(1) $25.0 million of the Cash Consideration was paid at closing.
(2) On December 30, 2016, the Company entered into an Amendment to the Purchase Agreement (the “Amendment”) to restructure the terms of the second tranche of the Cash Consideration (the “Second Tranche”). Under the Amendment, the Second Tranche was restructured to be paid through the (A) the issuance of 3,722,685 shares of the Company’s common stock with an aggregate fair market value of €5.0 million and (B) the payment of €5.0 million in cash upon the occurrence of either (i) receipt of clearance from the FDA for the Senhance System; or (ii) the Company having cash on hand of at least $50.0 million, or (iii) successfully completing a financing, raising at least $50.0 million in gross proceeds after September 2015, exclusive of any financing proceeds related to the December 2016 purchase agreement between the Company and Lincoln Park Capital Fund, LLC.; with payment of simple interest at a rate of 9.0% per annum beginning on December 31, 2016. Prior to December 30, 2016, the second tranche of the Cash Consideration of €10.0 million was payable after the achievement of both of the following milestones (i) the earlier of approval from the FDA for the Senhance System or December 31, 2016, and (ii) the Company having cash on hand of at least $50.0 million, or successfully completing a financing, raising at least $50.0 million in gross proceeds; with payment of simple interest at a rate of 9.0% per annum between the achievement of the first milestone event and the payment date.
(3) The third tranche of the Cash Consideration (the “Third Tranche”) of €15.0 million shall be payable upon achievement of trailing revenues from sales or services contracts of the Senhance System of at least €25.0 million over a calendar quarter.
(4) The fourth tranche of the Cash Consideration of €2.5 million shall be payable by December 31 of each year as reimbursement for certain debt payments made by Sofar under an existing Sofar loan agreement in such year, with payments beginning as of December 31, 2016. As of December 31, 2016, the Company had paid €1.1 million of the fourth tranche.
The Third Tranche will be payable even if the Second Tranche is not then payable. In addition, the Second Tranche and Third Tranche payments will be accelerated in the event that (i) the Company or TransEnterix International is acquired, (ii) the Company significantly reduces or suspends selling efforts of the Senhance System, or (iii) the Company acquires a business that offers alternative products that are directly competitive with the Senhance System.
Under the Purchase Agreement, 10% of the Securities Consideration was being held in escrow to support Sofar’s representations and warranties under the Purchase Agreement. In accordance with a related escrow agreement, the escrowed shares were released in September 2016. The Company, a subsidiary and Sofar also entered into a Security Agreement, which provides that 10% of the membership interests of TransEnterix Italia have a lien placed thereon by and in favor of Sofar to support the Company’s representations and warranties under the Purchase Agreement. The security interest period is twenty-four months after the closing of the Senhance Acquisition.
The Purchase Agreement contains customary representations and warranties of the parties and the parties have customary indemnification obligations, which are subject to certain limitations described further in the Purchase Agreement.
In connection with the Senhance Acquisition, the Company also entered into a Registration Rights Agreement, dated as of September 21, 2015, with Sofar, pursuant to which the Company agreed to register the Securities Consideration shares for resale following the end of the lock-up periods described below. The resale registration statement has been filed and is effective, pending lapse of the lock-up restrictions as described below.
59
In connection with the Senhance Acquisition, Sofar entered into a Lock-Up Agreement with the Company pursuant to which Sofar agreed, subject to certain exceptions, not to sell, transfer or otherwise convey any of the Securities Consideration for one year following the Closing Date. On September 21, 2016, fifty percent of the Securities Consideration was released from the lock-up restrictions and is eligible to be resold under the effective resale registration statement. With respect to the remaining fifty percent of the Securities Consideration, the Lock-up Agreement provides that an additional twenty-five percent of the Securities Consideration remains locked-up until the eighteen-month anniversary of the Closing Date, and the remaining twenty-five percent of the Securities Consideration remains locked-up until the two-year anniversary of the Closing Date. The restrictions on transfer contained in the Lock-up Agreement cease to apply to all of the Securities Consideration following the second anniversary of the Closing Date, or earlier upon certain other conditions.
The Senhance Acquisition was accounted for as a business combination utilizing the methodology prescribed in ASC 805. The purchase price for the Senhance Acquisition has been allocated to the assets acquired and liabilities assumed based on their estimated fair values.
The Senhance Acquisition-date fair value of the consideration is as follows (in thousands, except for per share amounts):
|
Common shares issued |
|
|
15,543 |
|
|
Closing price per share |
|
$ |
2.81 |
|
|
|
|
$ |
43,677 |
|
|
Cash consideration |
|
|
25,000 |
|
|
Contingent consideration |
|
|
23,900 |
|
|
Total consideration |
|
$ |
92,577 |
|
The following table summarizes the estimated fair values of the assets acquired and liabilities assumed on September 21, 2015, the date of acquisition (in thousands):
|
Accounts receivable |
|
$ |
78 |
|
|
Inventories |
|
|
2,800 |
|
|
Current deferred tax asset |
|
|
526 |
|
|
Other current assets |
|
|
4,180 |
|
|
Property and equipment |
|
|
1,384 |
|
|
Intellectual property |
|
|
48,500 |
|
|
In-process research and development |
|
|
17,100 |
|
|
Goodwill |
|
|
38,348 |
|
|
Total assets acquired |
|
$ |
112,916 |
|
|
Accounts payable and other liabilities |
|
|
1,915 |
|
|
Long-term deferred tax liabilities |
|
|
18,424 |
|
|
Net assets acquired |
|
$ |
92,577 |
|
The Company allocated $48.5 million of the purchase price to identifiable intangible assets of intellectual property that met the separability and contractual legal criterion of ASC 805. The intellectual property is being amortized using the straight-line method over 7 years.
IPR&D is principally the estimated fair value of the Senhance System technology which had not reached commercial technological feasibility nor had alternative future use at the time of the acquisition and therefore the Company considered IPR&D, with assigned values to be allocated among the various IPR&D assets acquired.
Goodwill is calculated as the difference between the acquisition-date fair value of the consideration transferred and the fair values of the assets acquired and liabilities assumed. The goodwill resulting from this acquisition arises largely from synergies expected from combining the operations of TransEnterix Italia with the Company’s existing operations. The goodwill is not deductible for income tax purposes.
All legal, consulting and other costs related to the acquisition, aggregating approximately $4.2 million, have been expensed as incurred and are included in operating expenses in the Company’s consolidated statement of operations and comprehensive loss for the year ended December 31, 2015. The results of operations for TransEnterix Italia are included in the Company’s consolidated statements of operations and comprehensive loss for the period from the September 21, 2015 acquisition date.
60
The following unaudited pro forma information presents the combined results of operations for the years ended December 31, 2015 and 2014, as if the Company had completed the ALF-X Acquisitions at the beginning of fiscal 2014. The pro forma financial information is provided for comparative purposes only and is not necessarily indicative of what actual results would have been had the acquisition occurred on the date indicated, nor does it give effect to synergies, cost savings, fair market value adjustments, immaterial amortization expense and other changes expected to result from the acquisition. Accordingly, the pro forma financial results do not purport to be indicative of consolidated results of operations as of the date hereof, for any period ended on the date hereof, or for any other future date or period. The pro forma consolidated financial information has been calculated after applying the Company’s accounting policies and includes adjustments for transaction-related costs and amortization of intellectual property.
|
|
|
Year Ended December 31, |
|
|||||
|
|
|
2015 |
|
|
2014 |
|
||
|
|
|
(In thousands except per share amounts) |
|
|||||
|
Revenue |
|
$ |
77 |
|
|
$ |
401 |
|
|
Net loss |
|
|
53,994 |
|
|
|
46,874 |
|
|
Net loss per share |
|
$ |
0.57 |
|
|
$ |
0.63 |
|
|
4. |
Cash, Cash Equivalents, and Restricted Cash |
Cash, cash equivalents and restricted cash consist of the following:
|
|
|
December 31, |
|
|
December 31, |
|
||
|
|
|
2016 |
|
|
2015 |
|
||
|
|
|
(In thousands) |
|
|||||
|
Cash |
|
$ |
1,975 |
|
|
$ |
1,666 |
|
|
Money market |
|
|
22,190 |
|
|
|
36,783 |
|
|
Total cash and cash equivalents |
|
$ |
24,165 |
|
|
$ |
38,449 |
|
|
Restricted cash |
|
$ |
10,425 |
|
|
$ |
— |
|
|
Total |
|
$ |
34,590 |
|
|
$ |
38,449 |
|
Restricted cash at December 31, 2016 includes $10.0 million in a money market account, held in connection with the Company’s notes payable and $425,000 in cash accounts held as collateral primarily under the terms of an office operating lease and automobile leases.
The Company held no investments at December 31, 2016 and 2015 as it sold all its investment securities during 2014. There were no realized gains or losses for the years ended December 31, 2016, 2015 or 2014. There have been no unrealized gains or losses reclassified to accumulated other comprehensive loss.
|
5. |
Fair Value |
The Company held certain assets and liabilities that are required to be measured at fair value on a recurring basis. These assets and liabilities include available for sale securities classified as cash and cash equivalents, restricted cash and contingent consideration. ASC 820-10 (“Fair Value Measurement Disclosure”) requires the valuation using a three-tiered approach, which requires that fair value measurements be classified and disclosed in one of three tiers. These tiers are: Level 1, defined as quoted prices in active markets for identical assets or liabilities; Level 2, defined as valuations based on observable inputs other than those included in Level 1, such as quoted prices for similar assets and liabilities in active markets, or other inputs that are observable or can be corroborated by observable input data; and Level 3, defined as valuations based on unobservable inputs reflecting the Company’s own assumptions, consistent with reasonably available assumptions made by other market participants.
For assets and liabilities recorded at fair value, it is the Company’s policy to maximize the use of observable inputs and minimize the use of unobservable inputs when developing fair value measurements, in accordance with the fair value hierarchy. Fair value measurements for assets and liabilities where there exists limited or no observable market data and therefore, are based primarily upon estimates, are often calculated based on the economic and competitive environment, the characteristics of the asset or liability and other factors. Therefore, the results cannot be determined with precision and may not be realized in an actual sale or immediate settlement of the asset or liability. Additionally, there may be inherent weaknesses in any calculation technique, and changes in the underlying assumptions used, including discount rates and estimates of future cash flows, could significantly affect the results of current or future values. The Company utilizes fair value measurements to record fair value adjustments to certain assets and liabilities and to determine fair value disclosures.
61
As prescribed by U.S. GAAP, the Company groups assets and liabilities at fair value in three levels, based on the markets in which the assets and liabilities are traded and the reliability of the assumptions used to determine fair value. An adjustment to the pricing method used within either Level 1 or Level 2 inputs could generate a fair value measurement that effectively falls in a lower level in the hierarchy.
The determination of where an asset or liability falls in the hierarchy requires significant judgment. The Company evaluates its hierarchy disclosures and based on various factors, it is possible that an asset or liability may be classified differently from period to period. However, the Company expects changes in classifications between levels will be rare.
The carrying values of accounts receivable, inventories, interest receivable, accounts payable, and certain accrued expenses at December 31, 2016 and 2015, approximate their fair values due to the short-term nature of these items. The Company’s notes payable balance also approximates fair value as of December 31, 2016 and 2015, as the interest rates on the notes payable approximate the rates available to the Company as of these dates.
The following are the major categories of assets measured at fair value on a recurring basis as of December 31, 2016 and 2015, using quoted prices in active markets for identical assets (Level 1); significant other observable inputs (Level 2); and significant unobservable inputs (Level 3):
|
|
|
December 31, 2016 |
|
|||||||||||||
|
|
|
(In thousands) |
|
|||||||||||||
|
|
|
(unaudited) |
|
|||||||||||||
|
Description |
|
Quoted Prices in Active Markets for Identical Assets (Level 1) |
|
|
Significant Other Observable Inputs (Level 2) |
|
|
Significant Unobservable Inputs (Level 3) |
|
|
Total |
|
||||
|
Assets measured at fair value |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
24,165 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
24,165 |
|
|
Restricted cash |
|
|
10,425 |
|
|
|
— |
|
|
|
— |
|
|
|
10,425 |
|
|
Total Assets measured at fair value |
|
$ |
34,590 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
34,590 |
|
|
Liabilities measured at fair value |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Contingent consideration |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
22,800 |
|
|
$ |
22,800 |
|
|
Total liabilities measured at fair value |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
22,800 |
|
|
$ |
22,800 |
|
|
|
|
December 31, 2015 |
|
|||||||||||||
|
|
|
(In thousands) |
|
|||||||||||||
|
Description |
|
Quoted Prices in Active Markets for Identical Assets (Level 1) |
|
|
Significant Other Observable Inputs (Level 2) |
|
|
Significant Unobservable Inputs (Level 3) |
|
|
Total |
|
||||
|
Assets measured at fair value |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
38,449 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
38,449 |
|
|
Restricted cash |
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
Total Assets measured at fair value |
|
$ |
38,449 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
38,449 |
|
|
Liabilities measured at fair value |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Contingent consideration |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
23,500 |
|
|
$ |
23,500 |
|
|
Total liabilities measured at fair value |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
23,500 |
|
|
$ |
23,500 |
|
The Company’s financial liabilities consisted of contingent consideration potentially payable to Sofar related to the Senhance Acquisition in September 2015 (Note 3). This liability is reported as Level 3 as estimated fair value of the contingent consideration related to the acquisition requires significant management judgment or estimation and is calculated using the income approach, using various revenue and cost assumptions and applying a probability to each outcome. The change in fair value of the contingent consideration of $0.5 million for the year ended December 31, 2016 was primarily due to the Amendment to the Purchase Agreement for the Second Tranche (Note 3), the change in expected timelines for the achievement of milestones, the effect of the passage of time on the fair value measurement and the impact of foreign currency exchange rates. The change in fair value of the contingent consideration of $0.4 million for the year ended December 31, 2015 was primarily due to the impact of foreign currency exchange rates. Adjustments associated with the change in fair value of contingent consideration are included in the Company’s consolidated statements of operations and comprehensive loss.
62
The following table presents quantitative information about the inputs and valuation methodologies used for the Company’s fair value measurements classified in Level 3 as of September 21, 2015 and December 31, 2015 and 2016:
|
|
|
Valuation Methodology |
|
Significant Unobservable Input |
|
Weighted Average (range, if applicable) |
|
Contingent consideration |
|
Probability weighted income approach |
|
Milestone dates |
|
2016 to 2017 |
|
|
|
|
|
Discount rate Probability of occurrence |
|
7.5% to 9.0% 100% |
The following table summarizes the change in fair value, as determined by Level 3 inputs, for all assets and liabilities using unobservable Level 3 inputs for the years ended December 31, 2016 and 2015:
|
|
|
Fair Value Measurement at Reporting Date (Level 3) |
|
|
|
|
|
(In thousands) |
|
|
|
Balance at December 31, 2014 |
|
$ |
— |
|
|
Additions for contingent consideration |
|
|
23,900 |
|
|
Change in fair value |
|
|
(400 |
) |
|
Balance at December 31, 2015 |
|
|
23,500 |
|
|
Payment for contingent consideration |
|
|
(1,182 |
) |
|
Change in fair value |
|
|
482 |
|
|
Balance at December 31, 2016 |
|
$ |
22,800 |
|
|
Current portion |
|
|
10,502 |
|
|
Long-term portion |
|
|
12,298 |
|
|
Balance at December 31, 2016 |
|
$ |
22,800 |
|
|
6. |
Accounts Receivable, Net |
The following table presents the components of accounts receivable:
|
|
|
December 31, |
|
|
December 31, |
|
||
|
|
|
2016 |
|
|
2015 |
|
||
|
|
|
(In thousands) |
|
|||||
|
Gross accounts receivable |
|
$ |
960 |
|
|
$ |
76 |
|
|
Allowance for uncollectible accounts |
|
|
(73 |
) |
|
|
— |
|
|
Total accounts receivable, net |
|
$ |
887 |
|
|
$ |
76 |
|
|
Short-term portion |
|
$ |
621 |
|
|
$ |
76 |
|
|
Long-term portion |
|
|
266 |
|
|
|
— |
|
|
Total accounts receivable |
|
$ |
887 |
|
|
$ |
76 |
|
|
7. |
Inventories |
The components of inventories are as follows:
|
|
|
December 31, |
|
|
December 31, |
|
||
|
|
|
2016 |
|
|
2015 |
|
||
|
|
|
(In thousands) |
|
|||||
|
Finished goods |
|
$ |
4,698 |
|
|
$ |
2,704 |
|
|
Raw materials |
|
|
3,185 |
|
|
|
4,630 |
|
|
Total inventories |
|
$ |
7,883 |
|
|
$ |
7,334 |
|
|
Short-term portion |
|
$ |
7,883 |
|
|
$ |
6,625 |
|
|
Long-term portion |
|
|
— |
|
|
|
709 |
|
|
Total inventories |
|
$ |
7,883 |
|
|
$ |
7,334 |
|
63
As disclosed in Note 17, the Company executed a restructuring plan in May 2016 and wrote down inventory related to the SurgiBot System. The write down of inventory of $2.6 million is included in the accompanying consolidated statements of operations and comprehensive loss for the year ended December 31, 2016.
|
8. |
Other Current Assets |
The following table presents the components of other current assets:
|
|
|
December 31, |
|
|
December 31, |
|
||
|
|
|
2016 |
|
|
2015 |
|
||
|
|
|
(In thousands) |
|
|||||
|
Prepaid expenses |
|
$ |
2,186 |
|
|
$ |
750 |
|
|
Advances to vendors |
|
|
1,806 |
|
|
|
2,701 |
|
|
Other receivables |
|
|
1,343 |
|
|
|
536 |
|
|
Total |
|
$ |
5,335 |
|
|
$ |
3,987 |
|
|
9. |
Property and Equipment |
Property and equipment consisted of the following:
|
|
|
December 31, |
|
|
December 31, |
|
||
|
|
|
2016 |
|
|
2015 |
|
||
|
|
|
(In thousands) |
|
|||||
|
Machinery, manufacturing and demonstration equipment |
|
$ |
7,579 |
|
|
$ |
5,846 |
|
|
Computer equipment |
|
|
2,124 |
|
|
|
1,875 |
|
|
Furniture |
|
|
614 |
|
|
|
374 |
|
|
Leasehold improvements |
|
|
2,028 |
|
|
|
1,700 |
|
|
Total property and equipment |
|
|
12,345 |
|
|
|
9,795 |
|
|
Accumulated depreciation and amortization |
|
|
(6,573 |
) |
|
|
(5,387 |
) |
|
Property and equipment, net |
|
$ |
5,772 |
|
|
$ |
4,408 |
|
As disclosed in Note 17, the Company executed a restructuring plan in May 2016 and disposed of certain long-lived assets, primarily equipment and fixtures related to the SurgiBot System. The disposal of long-lived assets of $1.0 million is included as a component of restructuring and other charges in the accompanying consolidated statements of operations and comprehensive loss for the year ended December 31, 2016. There were no such disposals for the year ended December 31, 2015.
Depreciation expense was $1,942,000, $1,248,000 and $807,000, for the years ended December 31, 2016, 2015 and 2014, respectively.
|
10. |
Goodwill, In-Process Research and Development and Intellectual Property |
Goodwill
Goodwill of $93.8 million was recorded in connection with the Merger, as described in Note 1, and goodwill of $38.3 million was recorded in connection with the Senhance Acquisition, as described in Note 3. The carrying value of goodwill and the change in the balance for the years ended December 31, 2016 and 2015 is as follows:
|
|
|
Goodwill |
|
|
|
|
|
(In thousands) |
|
|
|
Balance at December 31, 2014 |
|
$ |
93,842 |
|
|
Additions |
|
|
38,348 |
|
|
Foreign currency translation impact |
|
|
(1,321 |
) |
|
Balance at December 31, 2015 |
|
|
130,869 |
|
|
Foreign currency translation impact |
|
|
(388 |
) |
|
Impairment loss |
|
|
(61,784 |
) |
|
Balance at December 31, 2016 |
|
$ |
68,697 |
|
64
The Company performs an annual impairment test of goodwill at December 31, or more frequently if events or changes in circumstances indicates that the carrying value of the Company’s one reporting unit may not be recoverable. During the second quarter of 2016, the FDA notified the Company that the SurgiBot System did not meet the criteria for substantial equivalency, negatively impacting the Company’s market capitalization, and warranting an interim two-step quantitative impairment test. Goodwill is tested for impairment using a two-step approach. In the first step, the fair value of the reporting unit is determined and compared to the reporting unit's carrying value, including goodwill. If the fair value of the reporting unit is less than its carrying value, the second step of the goodwill impairment test is performed to measure the amount of impairment, if any. In the second step, the fair value of the reporting unit is allocated to the assets and liabilities of the reporting unit as if it had been acquired in a business combination and the purchase price was equivalent to the fair value of the reporting unit. The excess of the fair value of the reporting unit over the amounts assigned to its assets and liabilities is referred to as the implied fair value of goodwill. The implied fair value of the reporting unit's goodwill is then compared to the actual carrying value of goodwill. If the implied fair value of goodwill is less than the carrying value of goodwill, an impairment loss is recognized for the difference.
The Company determined the fair value of the reporting unit using a discounted cash flow analysis derived from the Company’s long-term plans. The fair value of the reporting unit was corroborated using market prices for TransEnterix, Inc. The inputs used to determine the fair values were classified as Level 3 in the fair value hierarchy. Based on the impairment test, the Company recorded goodwill impairment of $61.8 million during the second quarter of 2016. No impairment was recorded as of December 31, 2015.
The Company performed a qualitative assessment during the annual impairment review for fiscal 2016 as of December 31, 2016 and concluded that it is not more likely than not that the fair value of the Company’s single reporting unit is less than its carrying amount. Therefore, the two-step goodwill impairment test for the reporting unit was not necessary at December 31, 2016.
In-Process Research and Development
As described in Note 3, on September 21, 2015, the Company acquired all of the assets related to the Senhance System and recorded $17.1 million of IPR&D. The estimated fair value of the IPR&D was determined using a probability-weighted income approach, which discounts expected future cash flows to present value. The projected cash flows were based on certain key assumptions, including estimates of future revenue and expenses, taking into account the stage of development of the technology at the acquisition date and the time and resources needed to complete development. The Company used a discount rate of 45% and cash flows that have been probability adjusted to reflect the risks of product commercialization, which the Company believes are appropriate and representative of market participant assumptions.
The carrying value of the Company’s IPR&D assets and the change in the balance for the years ended December 31, 2015 and 2016 is as follows:
|
|
|
In-Process Research and Development |
|
|
|
|
|
(In thousands) |
|
|
|
Balance at December 31, 2014 |
|
|
— |
|
|
Additions |
|
|
17,100 |
|
|
Foreign currency translation impact |
|
|
(589 |
) |
|
Balance at December 31, 2015 |
|
|
16,511 |
|
|
Foreign currency translation impact |
|
|
(591 |
) |
|
Balance at December 31, 2016 |
|
$ |
15,920 |
|
Intellectual Property
In 2009, the Company purchased certain patents from an affiliated company for $5.0 million in cash and concurrently terminated a license agreement related to the patents. The patent expiration dates begin in 2027. In addition, as described in Note 3, on September 21, 2015, the Company acquired all of the developed technology related to the Senhance System and recorded $48.5 million of intellectual property. The estimated fair value of the intellectual property was determined using a probability-weighted income approach, which discounts expected future cash flows to present value. The projected cash flows were based on certain key assumptions, including estimates of future revenue and expenses, taking into account the stage of development of the technology at the acquisition date and the time and resources needed to complete development. The Company used a discount rate of 45% and cash flows that have been probability adjusted to reflect the risks of product commercialization, which the Company believes are appropriate and representative of market participant assumptions.
65
As disclosed in Note 17, the Company executed a restructuring plan in May 2016 and wrote-off certain intellectual property consisting of patents related to the SurgiBot System. The write-off of intellectual property of $1.6 million is included as a component of restructuring and other charges in the accompanying consolidated statements of operations and comprehensive losses for the year ended December 31, 2016. There were no such write offs for the year ended December 31, 2015.
The components of gross intellectual property, accumulated amortization, and net intellectual property as of December 31, 2016 and 2015 are as follows:
|
|
|
December 31, 2016 |
|
|
|
December 31, 2015 |
|
||||||||||||||||||||||||||||||
|
|
|
(In thousands) |
|
|
|
(In thousands) |
|
||||||||||||||||||||||||||||||
|
|
|
Gross Carrying Amount |
|
|
Accumulated Amortization |
|
|
Foreign currency translation impact |
|
|
Write-off |
|
|
Net Carrying Amount |
|
|
|
Gross Carrying Amount |
|
|
Accumulated Amortization |
|
|
Foreign currency translation impact |
|
|
Net Carrying Amount |
|
|||||||||
|
Patents |
|
$ |
5,000 |
|
|
$ |
(3,438 |
) |
|
$ |
— |
|
|
$ |
(1,562 |
) |
|
$ |
— |
|
|
|
$ |
5,000 |
|
|
$ |
(3,259 |
) |
|
$ |
— |
|
|
$ |
1,741 |
|
|
Developed technology |
|
|
48,500 |
|
|
|
(8,458 |
) |
|
|
(2,952 |
) |
|
|
— |
|
|
|
37,090 |
|
|
|
|
48,500 |
|
|
|
(1,672 |
) |
|
|
(1,671 |
) |
|
|
45,157 |
|
|
Total intellectual property |
|
$ |
53,500 |
|
|
$ |
(11,896 |
) |
|
$ |
(2,952 |
) |
|
$ |
(1,562 |
) |
|
$ |
37,090 |
|
|
|
$ |
53,500 |
|
|
$ |
(4,931 |
) |
|
$ |
(1,671 |
) |
|
$ |
46,898 |
|
The estimated future amortization expense of intangible assets as of December 31, 2016 is as follows:
|
|
|
Years ending December 31, |
|
|
|
|
|
(In thousands) |
|
|
|
2017 |
|
$ |
6,929 |
|
|
2018 |
|
|
6,929 |
|
|
2019 |
|
|
6,929 |
|
|
2020 |
|
|
6,929 |
|
|
2021 |
|
|
6,929 |
|
|
Thereafter |
|
|
2,445 |
|
|
Total |
|
$ |
37,090 |
|
|
11. |
Income Taxes |
The components for the income tax expense (benefit) are as follows for the years ended December 31 (in thousands):
|
|
|
2016 |
|
|
2015 |
|
|
2014 |
|
|||
|
Current income taxes |
|
|
|
|
|
|
|
|
|
|
|
|
|
Federal |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
State |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Foreign |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Deferred income taxes |
|
|
|
|
|
|
|
|
|
|
|
|
|
Federal |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
State |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Foreign |
|
|
(5,523 |
) |
|
|
(1,024 |
) |
|
|
— |
|
|
Total income tax expense (benefit) |
|
$ |
(5,523 |
) |
|
$ |
(1,024 |
) |
|
$ |
— |
|
The United States and foreign components of loss from operations before taxes are as follows for the years ended December 31 (in thousands):
|
|
|
2016 |
|
|
2015 |
|
|
2014 |
|
|||
|
United States |
|
$ |
(88,624 |
) |
|
$ |
(44,438 |
) |
|
$ |
(37,652 |
) |
|
Foreign |
|
|
(36,879 |
) |
|
|
(3,534 |
) |
|
|
— |
|
|
Total loss from operations before taxes |
|
$ |
(125,503 |
) |
|
$ |
(47,972 |
) |
|
$ |
(37,652 |
) |
66
Significant components of the Company’s deferred tax assets consist of the following at December 31 (in thousands):
|
|
|
2016 |
|
|
2015 |
|
||
|
Noncurrent deferred tax assets: |
|
|
|
|
|
|
|
|
|
Stock-based compensation |
|
|
2,300 |
|
|
|
1,543 |
|
|
Inventory |
|
|
204 |
|
|
|
1,819 |
|
|
Accrued expenses and other |
|
|
564 |
|
|
|
936 |
|
|
Contribution carryforward |
|
|
— |
|
|
|
2 |
|
|
Research credit carryforward |
|
|
4,970 |
|
|
|
4,224 |
|
|
Fixed assets |
|
|
557 |
|
|
|
275 |
|
|
Capitalized start-up costs and other intangibles |
|
|
3,586 |
|
|
|
3,864 |
|
|
Net operating loss carryforwards |
|
|
82,298 |
|
|
|
64,867 |
|
|
|
|
|
94,479 |
|
|
|
77,530 |
|
|
Valuation allowance |
|
|
(91,885 |
) |
|
|
(75,897 |
) |
|
Net noncurrent deferred tax asset |
|
|
2,594 |
|
|
|
1,633 |
|
|
Noncurrent deferred tax liabilities |
|
|
|
|
|
|
|
|
|
Fixed assets |
|
|
(292 |
) |
|
|
(973 |
) |
|
Purchase accounting intangibles |
|
|
(12,699 |
) |
|
|
(16,923 |
) |
|
Net noncurrent deferred tax liability |
|
|
(12,991 |
) |
|
|
(17,896 |
) |
|
Net deferred tax asset (liability) |
|
$ |
(10,397 |
) |
|
$ |
(16,263 |
) |
The transaction described in Note 3 was a nontaxable transaction according to ASC 740, and the goodwill recorded under U.S. GAAP purchase accounting is not deductible for tax purposes.
At December 31, 2016 and 2015, the Company has provided a full valuation allowance against its net deferred assets in the U.S. Luxembourg, and Swiss tax jurisdiction, since realization of these benefits is not more likely than not. The valuation allowance increased approximately $16.0 million from the prior year. At December 31, 2016, the Company had federal and state net operating loss tax carryforwards of approximately $221.5 million and $176.5 million, respectively. These net operating loss carryforwards expire in various amounts starting in 2027 and 2018, respectively. The Company’s federal and state net operating loss carryforwards include approximately $0.4 million of excess tax benefits related to deductions from the exercise of stock options. The tax benefit of these deductions has not been recognized in deferred tax assets. If utilized, the benefits from these deductions will be recorded as adjustments to additional paid-in capital. At December 31, 2016, the Company had federal research credit carryforwards in the amount of $5.0 million. These carryforwards begin to expire in 2027. The utilization of the federal net operating loss carryforwards and credit carryforwards will depend on the Company’s ability to generate sufficient taxable income prior to the expiration of the carryforwards. In addition, the maximum annual use of net operating loss and research credit carryforwards is limited in certain situations where changes occur in stock ownership. At December 31, 2016, the Company had foreign operating loss carryforwards in Italy of approximately $10.0 million, which can be carried forward indefinitely; foreign operating loss carryforwards in Luxembourg of approximately $0.1 million, which can be carried forward indefinitely; and foreign operating loss carryforwards in Switzerland of approximately $4.3 million, which begin to expire in 2023. The Company has no unremitted foreign earnings as of December 31, 2016.
The Company has evaluated its tax positions to consider whether it has any unrecognized tax benefits. As of December 31, 2016 the Company had gross unrecognized tax benefits of approximately $1.0 million. Of the total, none would reduce the Company’s effective tax rate if recognized. The Company does not anticipate a significant change in total unrecognized tax benefits or the Company’s effective tax rate due to the settlement of audits or the expiration of statutes of limitations within the next twelve months. Furthermore, the Company does not expect any cash settlement with the taxing authorities as a result of these unrecognized tax benefits as the Company has sufficient unutilized carryforward attributes to offset the tax impact of these adjustments.
The following is a tabular reconciliation of the Company’s change in gross unrecognized tax positions at December 31 (in thousands):
|
|
|
2016 |
|
|
2015 |
|
|
2014 |
|
|||
|
Beginning balance |
|
$ |
862 |
|
|
$ |
606 |
|
|
$ |
— |
|
|
Gross increases for tax positions related to current periods |
|
|
186 |
|
|
|
256 |
|
|
|
606 |
|
|
Gross increases for tax positions related to prior periods |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Ending balance |
|
$ |
1,048 |
|
|
$ |
862 |
|
|
$ |
606 |
|
The Company recognizes interest and penalties related to uncertain tax positions in the provision for income taxes. As of December 31, 2016 and 2015, the Company had no accrued interest or penalties related to uncertain tax positions.
67
The Company has analyzed its filing positions in all significant federal, state, and foreign jurisdictions where it is required to file income tax returns, as well as open tax years in these jurisdictions. With few exceptions, the Company is no longer subject to United States Federal, state, and local tax examinations by tax authorities for years before 2013, although carryforward attributes that were generated prior to 2013 may still be adjusted upon examination by the taxing authorities if they either have been or will be used in a future period. No income tax returns are currently under examination by taxing authorities.
Taxes computed at the statutory federal income tax rate of 34% are reconciled to the provision for income taxes as follows for the years ended December 31:
|
|
|
2016 |
|
|
2015 |
|
|
2014 |
|
|||||||||||||||
|
|
|
|
|
|
|
% of Pretax |
|
|
|
|
|
|
% of Pretax |
|
|
|
|
|
|
% of Pretax |
|
|||
|
|
|
Amount |
|
|
Earnings |
|
|
Amount |
|
|
Earnings |
|
|
Amount |
|
|
Earnings |
|
||||||
|
United States federal tax at statutory rate |
|
$ |
(42,671 |
) |
|
|
34.0 |
% |
|
$ |
(16,311 |
) |
|
|
34.0 |
% |
|
$ |
(12,801 |
) |
|
|
34.0 |
% |
|
State taxes (net of deferred benefit) |
|
|
(2,487 |
) |
|
|
2.0 |
% |
|
|
(1,121 |
) |
|
|
2.3 |
% |
|
|
(786 |
) |
|
|
2.0 |
% |
|
Nondeductible expenses |
|
|
667 |
|
|
|
(0.5 |
%) |
|
|
1,797 |
|
|
|
(3.7 |
%) |
|
|
253 |
|
|
|
(0.7 |
%) |
|
Research & Development credits |
|
|
(922 |
) |
|
|
0.7 |
% |
|
|
(1,281 |
) |
|
|
2.7 |
% |
|
|
(1,532 |
) |
|
|
4.1 |
% |
|
Change in unrecognized tax benefits |
|
|
186 |
|
|
|
(0.1 |
%) |
|
|
256 |
|
|
|
(0.5 |
%) |
|
|
606 |
|
|
|
(1.6 |
%) |
|
Foreign tax rate differential |
|
|
3,969 |
|
|
|
(3.2 |
%) |
|
|
175 |
|
|
|
(0.4 |
%) |
|
|
— |
|
|
|
0.0 |
% |
|
Goodwill impairment |
|
|
20,816 |
|
|
|
(16.6 |
%) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Change in enacted tax rates and other, net |
|
|
(1,069 |
) |
|
|
0.8 |
% |
|
|
532 |
|
|
|
(1.2 |
%) |
|
|
392 |
|
|
|
(1.0 |
%) |
|
Change in valuation allowance |
|
|
15,988 |
|
|
|
(12.7 |
%) |
|
|
14,929 |
|
|
|
(31.1 |
%) |
|
|
13,868 |
|
|
|
(36.8 |
%) |
|
Income tax benefit |
|
$ |
(5,523 |
) |
|
|
4.4 |
% |
|
$ |
(1,024 |
) |
|
|
2.1 |
% |
|
$ |
— |
|
|
|
0.0 |
% |
|
12. |
Accrued Expenses |
The following table presents the components of accrued expenses:
|
|
|
December 31, |
|
|
December 31, |
|
||
|
|
|
2016 |
|
|
2015 |
|
||
|
|
|
(In thousands) |
|
|||||
|
Taxes and other assessments |
|
$ |
2,676 |
|
|
$ |
3,112 |
|
|
Compensation and benefits |
|
|
2,328 |
|
|
|
2,492 |
|
|
Interest and final payment fee |
|
|
1,000 |
|
|
|
411 |
|
|
Deferred rent |
|
|
323 |
|
|
|
278 |
|
|
Consulting and other vendors |
|
|
1,428 |
|
|
|
553 |
|
|
Legal and professional fees |
|
|
243 |
|
|
|
268 |
|
|
Royalties |
|
|
159 |
|
|
|
— |
|
|
Other |
|
|
49 |
|
|
|
281 |
|
|
Total |
|
$ |
8,206 |
|
|
$ |
7,395 |
|
|
13. |
Notes Payable |
On January 17, 2012, TransEnterix Surgical entered into a loan and security agreement with Silicon Valley Bank and Oxford Finance LLC (the “Lenders”), which loan and security agreement, as subsequently amended and restated is referred to as the “Loan Agreement.” In connection with the Merger, the Company assumed and became the borrower under the Loan Agreement.
On August 14, 2015, the Company entered into the First Amendment to the Loan Agreement (the “First Amendment”) with the Lenders. The first tranche of the First Amendment increased the Company’s borrowings at August 14, 2015 from $10,000,000 to $20,000,000. The First Amendment allowed for interest-only payments at 7.5% per annum through April 30, 2016 and had a maturity date of October 1, 2018.
On September 18, 2015, in connection with entry into the Purchase Agreement with Sofar S.p.A. (see Note 3 for a description of the related transactions), the Company and the Lenders entered into the Consent and Second Amendment (the “Second Amendment”) to the Loan Agreement. The Second Amendment modified the period in which the Company could make interest-only payments at 7.5% per annum on the term loans until January 31, 2016. The Second Amendment has a maturity date of July 1, 2018.
In connection with the entry into the Loan Agreement and its amendments, the Company became obligated to pay final payment and facility fees. To date, the Company has paid $498,920 in final payment obligations and $255,000 in facility fees under the Loan Agreement. The final payment fee obligation payable under the Second Amendment is 6.5% of the original principal amount of each term loan without the interest only extension and 8.0% with both interest-only extensions.
68
In addition, in connection with the borrowings, the Company issued warrants to the Lenders to purchase shares of the Company’s common stock amounting to an aggregate of 430,815 warrants under the Loan Agreement. Additional warrants will be issued if additional tranche term loans are made. The warrants expire seven years from their respective issue date.
The Loan Agreement is secured by a security interest in all assets of the Company and its current and future U.S. subsidiaries, including a security interest in intellectual property proceeds, but excluding a current security interest in intellectual property. The Loan Agreement contains customary representations (tested on a continual basis) and covenants that, subject to exceptions, restrict the Company’s ability to do the following things: declare dividends or redeem or repurchase equity interests; incur additional liens; make loans and investments; incur additional indebtedness; engage in mergers, acquisitions, and asset sales; transact with affiliates; undergo a change in control; add or change business locations; and engage in businesses that are not related to its existing business.
Further, under the Second Amendment, the Lenders consented to the formation of TransEnterix International, the entry of the Company into the Purchase Agreement and other transaction documents, and the name change of TransEnterix Italia. The Company agreed to pledge 100% of the common stock of TransEnterix International as additional security for the borrowings under the Loan Agreement. The Second Amendment added a provision permitting the Company to transfer designated amounts to TransEnterix Italia during the term of the Loan Agreement. This provision for the transfer of designated amount was amended on November 13, 2015 with the Third Amendment to the Loan Agreement. On April 19, 2016, the Company and its U.S. subsidiaries entered into the Consent and Fourth Amendment to the Loan Agreement (the “Fourth Amendment”) pursuant to which TransEnterix International, joined the Loan Agreement and the existing promissory notes as a co-borrower thereunder and pledged, as collateral for the obligations under the Loan Agreement, substantially all of its non-intellectual property assets, including up to 65% of the equity interests owned by TransEnterix International in TransEnterix Europe S.Á.R.L, a wholly owned subsidiary of TransEnterix International. On September 7, 2016, the Company and its U.S. subsidiaries entered into the Fifth Amendment to the Loan Agreement (the “Fifth Amendment”). The Fifth Amendment provides more flexibility to the Company with respect to its intercompany activities with its foreign subsidiaries and adds an affirmative covenant requiring the Company to maintain a $10.0 million cash balance in accounts held with the Lender. Cash amounts related to this requirement are included in restricted cash on the consolidated balance sheet. The Company is in compliance with all covenants as of December 31, 2016.
In accordance with ASC 470-50 Debt – Modifications and Extinguishments, it was determined that the debt refinancing on September 26, 2014, was considered to be a debt modification. Accordingly, the Company recorded approximately $129,000 of debt discount, consisting of the $75,000 facility fee and the relative fair value of warrants on the issue date of $54,000. Additionally, approximately $30,000 of legal fees were recorded as a result of the transaction. The debt discount and deferred financing costs will be amortized over the life of the new debt agreement using the effective interest method into interest expense, net.
In accordance with ASC 470-50 Debt – Modifications and Extinguishments, it was determined that the debt refinancings on August 14, 2015, September 18, 2015, April 19, 2016 and September 7, 2016 were considered to be debt modifications. Additionally, during the third quarter of 2015, the Company adopted ASU No. 2015-03, “Interest—Imputation of Interest (Subtopic 835-30): Simplifying the Presentation of Debt Issuance Costs”. ASU 2015-03 requires debt issuance costs to be presented in the balance sheet as a direct deduction from the carrying value of the associated debt liability, consistent with the presentation of a debt discount. The Company recorded a debt discount of approximately $210,000 for these amendments. Accordingly, the unamortized debt discount is presented as a reduction of the related debt liability in the Company’s balance sheet. In accordance with ASU 2015-03, this adopted guidance was applied retrospectively. The debt discount will be amortized over the life of the new debt agreement using the effective interest method into interest expense, net.
In connection with the issuance of the notes payable and its amendments, TransEnterix Surgical incurred approximately $371,000 in debt issuance costs paid to lenders and third parties and $280,000 in debt issuance costs related to issuance of warrants to the lenders. The unamortized balance of $107,000 as of December 31, 2016 will be amortized using the effective interest method.
As of December 31, 2016 future principal payments under the Company’s notes payable agreements are as follows:
|
Years ending December 31, |
|
|
|
|
|
(In thousands) |
|
|
|
|
|
2017 |
|
$ |
8,090 |
|
|
2018 |
|
|
5,007 |
|
|
Total |
|
$ |
13,097 |
|
69
|
14. |
Stock-Based Compensation |
The Company’s stock-based compensation plans include the TransEnterix, Inc. Amended and Restated Incentive Compensation Plan, as amended, previously named the TransEnterix, Inc. 2007 Incentive Compensation Plan (the “Plan”), as well as options outstanding under the TransEnterix, Inc. Stock Option Plan (the “2006 Plan”). As part of the Merger, options outstanding, whether vested or unvested, under the 2006 Plan were adjusted by the Exchange Ratio of 1.1533, and assumed by the Company concurrent with the closing of the Merger.
The Plan was initially approved by the majority of the stockholders on November 13, 2007. The Plan was amended on June 19, 2012 to increase the number of shares of common stock available for issuance to 1,000,000 and was amended on October 29, 2013 to (a) increase the number of shares of common stock authorized for issuance under the Plan from 1,000,000 shares of common stock to 4,940,000 shares of common stock, (b) increase the per-person award limitations for options or stock appreciation rights from 200,000 to 1,000,000 shares and for restricted stock, deferred stock, performance shares and/or other stock-based awards from 100,000 to 500,000 shares, and (c) change the name of the Plan to reflect the Merger-related change. The Plan was again amended on May 7, 2015 to (i) increase the number of shares reserved for issuance under the Plan to 11,940,000 shares; (ii) extend the term of the Plan until May 7, 2025; and (iii) make other changes and updates to the Plan and was further amended in October 2015 to add French Sub-Plan amendments applicable to awards made to France-based employees. The Plan was further amended on June 8, 2016 to (a) approve an increase in the number of shares reserved for issuance under the Plan to 18,940,000 shares and (b) establish maximum equity award limits for initial awards and annual awards to non-employee directors. The October 2013, May 2015 and June 2016 amendments were approved by the Board of Directors and stockholders; the French Sub-Plan was approved by the Board of Directors. Under the Plan, which is administered by the Compensation Committee, the Company may grant stock options, stock appreciation rights, restricted stock and/or deferred stock to employees, officers, directors, consultants and vendors. The exercise price of stock options or stock appreciation rights may not be less than the fair market value of the Company’s shares at the date of grant. Additionally, no stock options or stock appreciation rights granted under the Plan may have a term exceeding ten years.
The 2006 Plan was adopted and approved by stockholders in September 2006 and provided for the granting of up to 80,000 stock options to employees, directors, and consultants. Under the 2006 Plan, both employees and non-employees were eligible for such stock options. In 2009, the 2006 Plan was amended to increase the total options pool to 1,110,053. In 2011, the 2006 Plan was amended to increase the total options pool to 3,378,189. The amendments were approved by the Board of Directors and stockholders. The Board of Directors had the authority to administer the plan and determine, among other things, the exercise price, term and dates of the exercise of all options at their grant date. Under the 2006 Plan, options become vested generally over four years, and expire not more than 10 years after the date of grant. As part of the Merger, options outstanding under the 2006 Plan were adjusted by the Conversion Ratio, and remain in existence as options of TransEnterix.
During the years ended December 31, 2016, 2015 and 2014, the Company recognized $5,033,000, $3,311,000 and $1,840,000, respectively, of stock-based compensation expense, including stock options and restricted stock units. During 2014, the Company granted options with performance-based features. As of December 31, 2014, the Company determined that achievement of the pre-defined corporate performance goals was not probable and no expense was recognized. The performance options were cancelled in 2015.
The Company recognizes as expense, the grant-date fair value of stock options and other stock based compensation issued to employees and non-employee directors over the requisite service periods, which are typically the vesting periods. The Company uses the Black-Scholes-Merton model to estimate the fair value of its stock-based payments. The volatility assumption used in the Black-Scholes-Merton model is based on the calculated historical volatility based on an analysis of reported data for a peer group of companies. The expected term of options granted by the Company has been determined based upon the simplified method, because the Company does not have sufficient historical information regarding its options to derive the expected term. Under this approach, the expected term is the mid-point between the weighted average of vesting period and the contractual term. The risk-free interest rate is based on U.S. Treasury rates whose term is consistent with the expected life of the stock options. The Company has not paid and does not anticipate paying cash dividends on its shares of common stock; therefore, the expected dividend yield is assumed to be zero. The Company estimates forfeitures based on the historical experience of the Company and adjusts the estimated forfeiture rate based upon actual experience.
The fair value of options granted were estimated using the Black-Scholes-Merton option pricing model based on the assumptions in the table below:
|
|
|
Years ended December 31, |
|
|||||||||
|
|
|
2016 |
|
|
2015 |
|
|
2014 |
|
|||
|
Expected dividend yield |
|
|
0% |
|
|
|
0% |
|
|
|
0% |
|
|
Expected volatility |
|
|
47% |
|
|
45% - 56% |
|
|
46% - 63% |
|
||
|
Risk-free interest rate |
|
1.13% - 2.09% |
|
|
1.44% - 1.95% |
|
|
1.60% - 2.30% |
|
|||
|
Expected life (in years) |
|
5.5 - 6.3 |
|
|
5.5 - 6.3 |
|
|
5.2 - 7.0 |
|
|||
70
The following table summarizes the Company’s stock option activity, including grants to non-employees, for the year ended December 31, 2016:
|
|
|
|
|
|
|
|
|
|
|
Weighted- |
|
|
|
|
|
|
|
|
|
|
|
|
|
Average |
|
|
|
|
|
|
|
|
|
Weighted- |
|
|
Remaining |
|
||
|
|
|
Number of |
|
|
Average |
|
|
Contractual |
|
|||
|
|
|
Shares |
|
|
Exercise Price |
|
|
Term (Years) |
|
|||
|
Options outstanding at December 31, 2015 |
|
|
8,300,819 |
|
|
$ |
2.63 |
|
|
|
8.23 |
|
|
Granted |
|
|
5,368,755 |
|
|
|
2.81 |
|
|
|
|
|
|
Forfeited |
|
|
(797,301 |
) |
|
|
3.53 |
|
|
|
|
|
|
Cancelled |
|
|
(152,946 |
) |
|
|
4.61 |
|
|
|
|
|
|
Exercised |
|
|
(230,776 |
) |
|
|
0.71 |
|
|
|
|
|
|
Options outstanding at December 31, 2016 |
|
|
12,488,551 |
|
|
$ |
2.66 |
|
|
|
8.08 |
|
The following table summarizes information about stock options outstanding at December 31, 2016:
|
|
|
|
|
|
|
|
|
|
|
Weighted |
|
|
|
|
|
|
|
|
|
|
|
|
|
Average |
|
|
|
|
|
|
|
|
|
Weighted |
|
|
Remaining |
|
||
|
|
|
Number of |
|
|
Average |
|
|
Contractual |
|
|||
|
|
|
Shares |
|
|
Exercise Price |
|
|
Term (Years) |
|
|||
|
Exercisable at December 31, 2016 |
|
|
4,822,183 |
|
|
$ |
2.43 |
|
|
|
6.65 |
|
|
Vested or expected to vest at December 31, 2016 |
|
|
11,987,933 |
|
|
$ |
2.66 |
|
|
|
8.03 |
|
The aggregate intrinsic value of stock options outstanding, exercisable, and vested or expected to vest at December 31, 2016 was approximately $1.3 million, $1.3 million, and $1.3 million, respectively. This amount is before applicable income taxes and represents the closing market price of the Company’s common stock at December 31, 2016 less the grant price, multiplied by the number of stock options that had a grant price that is less than the closing market price. This amount represents the amount that would have been received by the optionees had these stock options been exercised on that date.
The total intrinsic value of options exercised during 2016, 2015 and 2014 was approximately $519,000, $1,603,000 and $996,000, respectively.
The Company granted 5,368,755, 4,407,758 and 2,422,309 options to employees and non-employees during the years ended December 31, 2016, 2015 and 2014, respectively, with a weighted-average grant date fair value of $1.30, $1.37 and $2.87, respectively.
As of December 31, 2016, the Company had future employee stock-based compensation expense of approximately $7,953,000 related to unvested share awards, which is expected to be recognized over an estimated weighted-average period of 2.9 years.
|
15. |
Restricted Stock Units |
In 2015 and 2016, the Company issued Restricted Stock Units (“RSUs”) to certain employees which vest over three years. By their terms, the RSUs become immediately vested upon the earlier of (i) a change of control and (ii) defined vesting dates, subject to the continuous service with the Company at the applicable vesting event. When vested, the RSUs represent the right to be issued the number of shares of the Company’s common stock that is equal to the number of RSUs granted. The fair value of each RSU is estimated based upon the closing price of the Company’s common stock on the grant date. Share-based compensation expense related to RSUs is recognized over the requisite service period as adjusted for estimated forfeitures.
71
The following is a summary of the RSU activity for the years ended December 31, 2016, 2015 and 2014:
|
|
|
|
|
|
|
Weighted |
|
|
|
|
|
Number of |
|
|
Average |
|
||
|
|
|
Restricted |
|
|
Grant |
|
||
|
|
|
Stock Units |
|
|
Date Fair |
|
||
|
|
|
Outstanding |
|
|
Value |
|
||
|
Unvested, December 31, 2013 |
|
|
210,000 |
|
|
$ |
7.19 |
|
|
Granted |
|
|
— |
|
|
|
— |
|
|
Vested |
|
|
(70,000 |
) |
|
|
7.19 |
|
|
Unvested, December 31, 2014 |
|
|
140,000 |
|
|
$ |
7.19 |
|
|
Granted |
|
|
380,000 |
|
|
|
2.94 |
|
|
Vested |
|
|
(70,000 |
) |
|
|
7.19 |
|
|
Forfeited |
|
|
(27,500 |
) |
|
|
2.94 |
|
|
Unvested, December 31, 2015 |
|
|
422,500 |
|
|
$ |
3.64 |
|
|
Granted |
|
|
660,331 |
|
|
|
3.74 |
|
|
Vested |
|
|
(187,503 |
) |
|
|
4.53 |
|
|
Unvested, December 31, 2016 |
|
|
895,328 |
|
|
$ |
3.53 |
|
As of December 31, 2016, 2015 and 2014, the Company recorded approximately $1,463,000, $816,000 and $503,000, respectively, in compensation expense for the RSUs. As of December 31, 2016, the unrecognized stock-based compensation expense related to unvested RSUs was approximately $2.1 million, which is expected to be recognized over a weighted average period of approximately 1.9 years. No restricted stock units were granted in 2014. The weighted average grant date fair value of the RSUs granted in 2015 was $2.94. The weighted average grant date fair value of the RSUs granted in 2016 was $3.74.
|
16. |
Warrants |
On March 22, 2013, SafeStitch entered into a stock purchase agreement with approximately 17 investors (the “2013 PIPE Investors”) pursuant to which the 2013 PIPE Investors purchased an aggregate of approximately 2,420,000 shares of common stock at a price of $1.25 per share for aggregate consideration of approximately $3.0 million. Included in this private placement was the issuance of warrants to purchase approximately 1,209,600 common shares, representing one warrant for every two common shares purchased, with an exercise price of $1.65 per share and five year expiration. Among the 2013 PIPE Investors purchasing shares were related parties who purchased 1.28 million shares and received 640,000 warrants. There were approximately 1.2 million warrants outstanding that were assumed as of the Merger. During the years ended December 31, 2016, 2015 and 2014, 0, 0, and 10,000, respectively of these warrants were exercised.
On January 17, 2012, TransEnterix Surgical entered into the original Loan Agreement with Silicon Valley Bank and Oxford Finance LLC. (collectively, the “Lenders”). Pursuant to such agreement, TransEnterix Surgical issued preferred stock warrants to the Lenders on January 17, 2012 and December 21, 2012, respectively, to purchase shares of TransEnterix Surgical preferred stock. The preferred stock warrants expire 10 years from the issue date. The preferred stock warrants were remeasured immediately prior to the Merger. As of the Merger, the preferred stock warrants converted to common stock warrants, adjusted based on a Merger exchange ratio of 1.1533, and the preferred stock warrant liability was reclassified to additional paid-in capital. These warrants are exercisable for an aggregate of approximately 279,588 shares of common stock, with an exercise price of $1.45. During the year ended December 31, 2013, 139,794 of these warrants were exercised in a cashless transaction for 112,766 shares of common stock. None of these warrants were exercised during the years ended December 31, 2016, 2015 or 2014.
On September 26, 2014, the Company entered into an amendment to the Loan Agreement with the Lenders. In connection with the first tranche borrowings under such amendment, the Company issued 38,325 common stock warrants to the Lenders to purchase shares of the Company’s common stock, with an exercise price of $4.015 per share. Additional common stock warrants will be issued if additional tranche term loans are made under the Loan Agreement. The warrants expire seven years from their respective issue date. The Company concluded that the warrants are considered equity instruments. The warrants were recognized at the relative fair value on the issuance date as a debt discount and will be amortized using the effective interest method from issuance to the maturity of the term loans. None of these warrants were exercised during the year ended December 31, 2016, 2015 and 2014.
On August 14, 2015, in connection with an amendment to the Loan Agreement and first tranche borrowings thereunder, the Company issued 112,903 common stock warrants to the Lenders to purchase shares of the Company’s common stock, with an exercise price of $3.10 per share. Additional common stock warrants will be issued if additional tranche term loans are made under the Loan Agreement. The warrants expire seven years from their respective issue date. The Company concluded that the warrants are considered equity instruments. The warrants were recognized at the relative fair value on the issuance date as a debt discount and will
72
be amortized using the effective interest method from issuance to the maturity of the note. None of these warrants were exercised during the year ended December 31, 2016 and 2015.
|
|
|
|
|
|
|
|
|
|
|
Weighted |
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted |
|
|
Average |
|
|
|
|
|
||
|
|
|
|
|
|
|
Average |
|
|
Remaining |
|
|
Weighted |
|
|||
|
|
|
Number of |
|
|
Exercise |
|
|
Contractual |
|
|
Average |
|
||||
|
|
|
Warrants |
|
|
Price |
|
|
Life (in years) |
|
|
Fair Value |
|
||||
|
Outstanding at December 31, 2013 |
|
|
1,285,394 |
|
|
$ |
1.45 |
|
|
|
4.7 |
|
|
$ |
1.75 |
|
|
Granted |
|
|
38,325 |
|
|
|
4.02 |
|
|
|
6.7 |
|
|
|
1.41 |
|
|
Exercised |
|
|
(10,000 |
) |
|
|
1.65 |
|
|
|
— |
|
|
|
— |
|
|
Outstanding at December 31, 2014 |
|
|
1,313,719 |
|
|
$ |
1.70 |
|
|
|
3.9 |
|
|
$ |
1.75 |
|
|
Granted |
|
|
112,903 |
|
|
|
3.10 |
|
|
|
6.6 |
|
|
|
0.86 |
|
|
Exercised |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Outstanding at December 31, 2015 |
|
|
1,426,622 |
|
|
$ |
1.81 |
|
|
|
3.2 |
|
|
$ |
1.54 |
|
|
Granted |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Exercised |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Outstanding at December 31, 2016 |
|
|
1,426,622 |
|
|
$ |
1.81 |
|
|
|
2.2 |
|
|
$ |
1.54 |
|
The aggregate intrinsic value of the common stock warrants in the above table was $0, $1.0 million and $1.6 million at December 31, 2016, 2015 and 2014, respectively. The aggregate intrinsic value is before applicable income taxes and is calculated based on the difference between the exercise price of the warrants and the estimated fair market value of the applicable stock as of the respective dates.
|
17. |
Restructuring |
On April 19, 2016, the FDA notified the Company that the SurgiBot System did not meet the criteria for substantial equivalence based on the data submitted in the 510(k) submission. As a result, the Company has reprioritized its efforts to focus on the commercialization of and regulatory clearance for the Senhance System. Consequently, in May 2016, the Company implemented a restructuring plan. Under the restructuring plan, the Company reduced headcount, discontinued efforts on the SurgiBot System, and cancelled certain contracts. The restructuring charges amounted to $5.7 million, of which $2.6 million was included as inventory write down related to restructuring and $3.1 million was included as restructuring and other charges in the consolidated statements of operations and comprehensive loss, during the second quarter of 2016.
The restructuring and other charges of $3.1 million included: (i) $0.5 million to be paid in cash, of which $0.4 million related to employee severance costs and $0.1 million related to cancellation of certain contracts; and (ii) $2.6 million for other non-cash charges, of which $1.0 million related to the disposal of long-lived assets for the abandonment of certain equipment and tooling directly relating to the SurgiBot System and $1.6 million related to the write-off of intellectual property for certain patents also relating to the SurgiBot System. There are no future payments under the restructuring plan as of December 31, 2016.
|
18. |
Purchase Agreement, Controlled Equity Offering and Public Offering of Common Stock |
On December 16, 2016, the Company entered into a purchase agreement (the “Purchase Agreement”) with Lincoln Park Capital Fund, LLC, (“Lincoln Park”), pursuant to which the Company has the right to sell to Lincoln Park up to an aggregate of $25.0 million in shares of the Company’s common stock, (the “Common Stock”), subject to certain limitations and conditions set forth in the Purchase Agreement. From time to time on any trading day the Company selects, the Company has the right, in its sole discretion, subject to the conditions and limitations in the Purchase Agreement, to direct Lincoln Park to purchase up to 150,000 shares of Common Stock (each such purchase, a “Regular Purchase”) over the 36-month term of the Purchase Agreement. The purchase price of shares of Common Stock pursuant to the Purchase Agreement will be based on the prevailing market price at the time of sale as set forth in the Purchase Agreement. There are no trading volume requirements or restrictions under the Purchase Agreement. Lincoln Park’s obligation under each Regular Purchase shall not exceed $2.0 million. There is no upper limit on the price per share that Lincoln Park must pay for Common Stock under the Purchase Agreement, but in no event will shares be sold to Lincoln Park on a day the Company’s closing price is less than the floor price as set forth in the Purchase Agreement. Both the amount and frequency of the Regular Purchases can be increased upon the mutual agreement of the Company and Lincoln Park. The Company will control the timing and amount of any sales of shares of Common Stock to Lincoln Park. The Company may, in its sole discretion, direct Lincoln Park to purchase additional amounts as accelerated purchases if on the date of a Regular Purchase the closing sale price of the Common Stock is not below the threshold price as set forth in the Purchase Agreement. The Company and Lincoln Park may mutually agree to increase the amount of Common Stock sold to Lincoln Park on any accelerated purchase date. The Company issued to Lincoln Park 312,538 shares of Common Stock as commitment shares in consideration for the Purchase Agreement through December
73
31, 2016. The Company shall issue pro rata up to an additional 152,434 shares of Common Stock to Lincoln Park as determined by the number of shares Common Stock purchased by Lincoln Park pursuant to the Purchase Agreement. All sales of shares have been and will continue to be made pursuant to an effective shelf registration statement on Form S-3 filed with the SEC. Sales under the Purchase Agreement for the year ended December 31, 2016 were 300,000 shares, with gross proceeds of $412,500 and net proceeds of $392,500.
On February 20, 2015, the Company entered into a Controlled Equity Offering SM Sales Agreement (the “2015 Sales Agreement”) with Cantor Fitzgerald & Co. (“Cantor”), as sales agent, pursuant to which the Company sold through Cantor, from time to time, up to $25.0 million in shares of common stock in an at-the-market offering. All sales of shares were made pursuant to an effective shelf registration statement on Form S-3 filed with the SEC. The Company pays Cantor a commission of approximately 3% of the aggregate gross proceeds received from all sales of common stock under the Sales Agreement. Sales under the 2015 Sales Agreement have been fully sold as of February 9, 2016, with cumulative shares of 7,724,488, gross proceeds of $25.0 million and net proceeds of $24.0 million.
On June 11, 2015, the Company sold 16,666,667 shares of common stock at a public offering price of $3.00 per share for aggregate gross proceeds of $50.0 million in an underwritten firm commitment public offering. Net proceeds after issuance costs were $46.4 million. The closing of the public offering occurred on June 17, 2015. The Company granted the underwriters an option, exercisable for 30 days, to purchase up to an additional 2,500,000 shares of Common Stock.
On July 10, 2015, the underwriters exercised a portion of their option to acquire an additional 2,075,000 shares at the public offering price of $3.00 per share for aggregate additional gross proceeds of $6.2 million. Net proceeds after issuance costs were $5.8 million. The purchase of the option shares closed on July 15, 2015. Total proceeds (including the option) were $52.2 million, net of issuance costs of $4.0 million. The common stock was offered and sold pursuant to the Shelf Registration Statement filed in November 2014 (the “November 2014 Shelf Registration Statement”), which was declared effective on December 19, 2014. The November 2014 Shelf Registration Statement allowed the Company to raise up to $100.0 million through the sale of debt securities, common stock, preferred stock, warrants, or any combination thereof. On March 3, 2016, the Company filed an amendment to the November 2014 Shelf Registration Statement increasing the amount available from $100.0 million to $150.0 million.
On February 9, 2016, the Company entered into a Controlled Equity Offering SM Sales Agreement (the “2016 Sales Agreement”) with Cantor, as sales agent, pursuant to which the Company can sell through Cantor, from time to time, up to $43.6 million in shares of common stock in an at-the-market offering. All sales of shares have been and will continue to be made pursuant to an effective shelf registration statement on Form S-3 filed with the SEC. The Company pays Cantor a commission of approximately 3% of the aggregate gross proceeds received from all sales of common stock under the 2016 Sales Agreement. Unless otherwise terminated earlier, the 2016 Sales Agreement continues until all shares available under the Sales Agreement have been sold.
The following table summarizes the total sales under the 2015 Sales Agreement and 2016 Sales Agreement for the periods indicated (in thousands, except per share amounts):
|
|
|
2016 Sales Agreement |
|
|
2015 Sales Agreement |
|
||||||
|
|
|
Year Ended December 31, 2016 |
|
|
Year Ended December 31, 2016 |
|
|
Year Ended December 31, 2015 |
|
|||
|
Total shares of common stock sold |
|
|
8,763.4 |
|
|
|
5,710.2 |
|
|
|
2,014.3 |
|
|
Average price per share |
|
$ |
4.70 |
|
|
$ |
3.23 |
|
|
$ |
3.25 |
|
|
Gross proceeds |
|
$ |
41,156 |
|
|
$ |
18,454 |
|
|
$ |
6,546 |
|
|
Commissions earned by Cantor |
|
$ |
1,235 |
|
|
$ |
553 |
|
|
$ |
197 |
|
|
Other issuance costs |
|
$ |
185 |
|
|
$ |
— |
|
|
$ |
259 |
|
On April 14, 2014, the Company sold 12,500,000 shares of common stock at a public offering price of $4.00 per share for aggregate gross proceeds of $50.0 million in an underwritten firm commitment public offering. Certain of the Company’s existing stockholders that are affiliated with certain of the Company’s directors purchased $10.0 million of common stock in the public offering. The closing of the public offering occurred on April 21, 2014. The Company granted the underwriters an option, exercisable for 30 days, to purchase up to an additional 1,875,000 shares of Common Stock to cover over-allotments. On April 30, 2014, the underwriters exercised a portion of their over-allotment option to acquire an additional 1,610,000 shares at the public offering price of $4.00 per share for aggregate additional gross proceeds of $6.4 million. The purchase of the over-allotment shares closed on May 5, 2014. Total proceeds were $52.4 million, net of issuance costs of $4.0 million. The common stock was offered and sold pursuant to the Shelf Registration Statement filed in January 2014 (the “January 2014 Shelf Registration Statement”), which was declared effective on April 2, 2014. The January 2014 Shelf Registration Statement allowed the Company to raise up to $100.0 million through the sale of debt securities, common stock, preferred stock, warrants, or any combination thereof. As of December 31, 2016, the Company had
74
$37.8 million available for future financings under such shelf registration statements. In addition, approximately $2.4 million is reserved for future issuances under the 2016 ATM Offering and $24.6 million is reserved for future issuances under the Lincoln Park Agreement.
|
19. |
Basic and Diluted Net Loss per Share |
Basic net loss per common share is computed by dividing net loss attributable to common stockholders by the weighted average number of common shares outstanding during the period. Diluted net loss per common share is computed giving effect to all dilutive potential common shares that were outstanding during the period. Diluted potential common shares consist of incremental shares issuable upon exercise of stock options, warrants and restricted stock units. In computing diluted net loss per share for the years ended December 31, 2016, 2015, and 2014, no adjustment has been made to the weighted average outstanding common shares as the assumed exercise of outstanding options, warrants and restricted stock units would be anti-dilutive.
Potential common shares not included in calculating diluted net loss per share are as follows:
|
|
|
December 31, |
|
|||||||||
|
|
|
2016 |
|
|
2015 |
|
|
2014 |
|
|||
|
Stock options |
|
|
12,488,551 |
|
|
|
8,300,819 |
|
|
|
5,423,741 |
|
|
Stock warrants |
|
|
1,426,622 |
|
|
|
1,426,622 |
|
|
|
1,313,719 |
|
|
Nonvested restricted stock units |
|
|
895,328 |
|
|
|
422,500 |
|
|
|
140,000 |
|
|
Total |
|
|
14,810,501 |
|
|
|
10,149,941 |
|
|
|
6,877,460 |
|
|
20. |
Related Person Transactions |
Synergy Life Science Partners, L.P. and Synecor, LLC collectively owned approximately 5% and 6% of the Company’s common stock at December 31, 2016 and 2015, respectively. A member of the Company’s Board of Directors is managing partner of Synergy Life Science Partners, L.P. and an executive officer of Synecor, LLC. Various research and development services were purchased by the Company from Synecor LLC and its wholly owned subsidiary Synchrony Labs LLC pursuant to arms’ length terms approved by the Audit Committee and totaled approximately $5,000, $435,000 and $66,000 for the years ended December 31, 2016, 2015 and 2014, respectively.
On September 18, 2015, TransEnterix Italia entered into a services agreement for receipt of administrative services from Sofar and payment of rent to Sofar, a stockholder that owned approximately 13% and 16% of the Company’s common stock at December 31, 2016 and 2015, respectively. Expenses under this agreement were approximately $232,000 and $89,000 for the years ended December 31, 2016 and 2015, respectively. The services agreement terminated in August 2016.
In November 2016, the Company agreed to enter into a technology and patents purchase agreement with Sofar to acquire from Sofar certain technology and intellectual property rights related to the Senhance Acquisition, and formerly licensed by the Company. The acquisition price was €375,000.
As discussed in Note 3, in September 2015, the Company completed the Senhance Acquisition using a combination of cash, stock and potential post-acquisition milestone payments. On December 30, 2016, the Company entered into an Amendment to the Senhance Acquisition purchase agreement with Sofar to restructure the terms of the Second Tranche of the Cash Consideration. Under the Amendment, the Second Tranche was restructured to reduce the contingent cash consideration by €5.0 million in exchange for the issuance of 3,722,685 shares of the Company’s common stock with an aggregate fair market value of €5.0 million. On January 4, 2017, the Company issued to Sofar 3,722,685 shares of the common stock with a fair value of €5.0 million. The price per share was $1.404 and was calculated based on the average of the closing prices of the Company’s common stock on ten consecutive trading days ending one day before the execution of the Amendment.
|
21. |
Commitments and Contingencies |
Contingent Consideration
As discussed in Note 3, in September 2015, the Company completed the Senhance Acquisition using a combination of cash, stock and potential post-acquisition milestone payments. These milestone payments may be payable in the future, depending on the achievement of certain regulatory and commercial milestones. On December 30, 2016, the Company entered into an Amendment to restructure the terms of the Second Tranche of the Cash Consideration. Under the Amendment, the Second Tranche was restructured to reduce the contingent cash consideration by €5.0 million in exchange for the issuance of 3,722,685 shares of the Company’s common stock with an aggregate fair market value of €5.0 million. As of December 31, 2016, the fair value of the contingent consideration was $22.8
75
million. On January 4, 2017, after the issuance of 3,722,685 shares of the Company’s common stock, the fair value of the contingent consideration was $17.6 million. The maximum amount of the aggregate milestone payments, after the issuance of the shares on January 4, 2017, could be €21.4 million.
Legal Proceedings
When determining the estimated probable loss or range of losses, significant judgment is required to be exercised in order to estimate the amount and timing of the loss to be recorded. Estimating an amount or range of possible losses resulting from litigation proceedings is inherently difficult and requires an extensive degree of judgment, particularly where the matters involve indeterminate claims for monetary damages, are in the early stages of the proceedings, and are subject to appeal. In addition, because most legal proceedings are resolved over extended periods of time, potential losses are subject to change due to, among other things, new developments, changes in legal strategy, the outcome of intermediate procedural and substantive rulings and other parties’ settlement posture and their evaluation of the strength or weakness of their case against the Company. For these reasons, the Company is currently unable to predict the ultimate timing or outcome of, or reasonably estimate the possible losses or a range of possible losses resulting from, the matters described above. Based on information currently available, the Company does not believe that any reasonably possible losses arising from currently pending legal matters will be material to the Company’s results of operations or financial condition. However, in light of the inherent uncertainties involved in such matters, an adverse outcome in one or more of these matters could materially and adversely affect the Company’s financial condition, results of operations or cash flows in any particular reporting period.
No liability or related charge was recorded to earnings in the Company’s consolidated financial statements for legal contingencies for the year ended December 31, 2016.
On June 2, 2016 a stockholder filed a putative class action complaint, Ashok V. Bankley, individually and on behalf of all others similarly situated vs. TransEnterix, Inc., Todd M. Pope and Joseph P. Slattery , in the United States District Court for the Eastern District of North Carolina (Case No. 5:16-cv-00313-D) (the “Initial Complaint”), against the Company and two of its executive officers on behalf of all persons who purchased or otherwise acquired the Company’s common stock between February 10, 2016 and May 10, 2016. On August 4, 2016, the defendants filed a motion to dismiss the Initial Complaint for failure to state a claim under the securities laws. On August 30, 2016, the court appointed Randall Clark, Samir Patel, the Underhill Cemetery Association, and the North Underhill Cemetery Association as the lead plaintiffs in the Initial Complaint, and also provided the plaintiffs an opportunity to amend the Initial Complaint. On September 26, 2016, the lead plaintiffs filed an Amended Complaint. Among other things, the Amended Complaint asserts revised claims against the Company and Messrs. Pope and Slattery, and adds claims against certain current and former members of the Company’s Board of Directors, and Cantor Fitzgerald & Co., the sales agent under the 2016 Sales Agreement, under which the Company offered and sold, through Cantor, shares of common stock in its 2016 ATM Offering. The Amended Complaint alleges that the defendants made false and misleading public statements related to the Company's SurgiBot System and its 510(k) application in violation of certain federal securities laws. The Amended Complaint seeks class certification of a class consisting of all persons who purchased or otherwise acquired the Company’s common stock between February 10, 2016 and May 10, 2016, class certification of a subclass of persons who purchased or otherwise acquired the Company’s common stock in connection with the 2016 ATM Offering between February 9, 2016 and April 19, 2016, unspecified monetary damages, costs, and attorneys’ fees. On November 8, 2016, the defendants moved to dismiss the Amended Complaint, which the plaintiffs later opposed. As of January 23, 2017, the motions to dismiss were fully briefed and deemed submitted to the court for decision.
On June 9, 2016, a different stockholder filed another putative class action complaint, Thomas Ravey, individually and on behalf of all others similarly situated vs. TransEnterix, Inc., Todd M. Pope and Joseph P. Slattery, in the United States District Court for the Middle District of North Carolina (Case No. 1:16-cv-599) (the “Ravey Action”). The Ravey Action asserted substantially similar claims against the same defendants and sought substantially similar relief as the Initial Complaint. On August 4, 2016, the plaintiff in the Ravey Action voluntarily dismissed the Ravey Action.
On July 8, 2016, a stockholder filed a putative derivative complaint, Otto Pikal v. Todd M. Pope, et al., in the General Court of Justice, Superior Court Division, Wake County, North Carolina (case number 16CV008930), on behalf of the Company against certain of our current officers and directors. The complaint alleges, among other things, that the defendants breached their fiduciary duties by disseminating false and misleading information to the Company’s shareholders relating to the Company’s SurgiBot System and its 510(k) application in violation of certain federal securities laws and by failing to ensure that the Company maintained adequate internal controls. The complaint seeks, among other things, unspecified monetary damages and an order directing the Company to take steps to improve its corporate governance and to protect the Company and its stockholders from future wrongdoing such as that alleged in the complaint. On September 29, 2016, the court entered an order staying the litigation pending resolution of the motion to dismiss the Amended Complaint in the Bankley Action.
76
On April 25, 2016, Intuitive Surgical, Inc. and its French subsidiary, Intuitive Surgical SAS (collectively, “Intuitive”), brought a request for unilateral measures of enquiry in front of the President of the Commercial Court of Toulon (France) (the “President”) against two employees of TransEnterix International, Inc. alleging that the Company, through these two employees, engaged in acts of unfair competition. On May 3, 2016, the President rendered an order granting Intuitive’s request for unilateral measures of enquiry with respect to its allegations (the “Order”). On June 28, 2016, TransEnterix International filed a writ challenging the Order and requesting that it be withdrawn by the President. On September 7, 2016, the President rendered his decision on TransEnterix International’s challenge (the “Ruling”) and ruled in favor of TransEnterix International. Under the Ruling, the Intuitive unilateral measures of enquiry were declared to be unjustified and the Order was withdrawn. The President also declared that the Ruling was provisionally enforceable. Intuitive has filed an appeal against the Ruling (the “Appeal”) with the Court of Appeal of Aix-en-Provence (France). Because Intuitive did not comply with the Ruling, TransEnterix International filed a dismissal request against the Appeal with the same Court of Appeal (the “Dismissal Request”). Currently, Intuitive’s counsel advised TransEnterix International’s counsel of their intent to waive the Appeal (the “Waiver”). A hearing regarding the Dismissal Request has been scheduled for March 7, 2017 in order to leave time for the parties to formalize the Waiver. The writ of Waiver from Intuitive’s French counsel has been provided to TransEnterix. Once the Waiver is formalized in connection with the March 7, 2017 hearing, the Dismissal Request, as well as the Appeal, will be struck off the agenda of the Court of Appeal and the Ruling in favor of TransEnterix International will become final.
Operating Leases
On November 2, 2009, TransEnterix Surgical entered into an operating lease for its corporate offices for a period of five years commencing in April 2010. On June 12, 2014, the Company entered into a lease amendment extending the term of the lease for a period of 3 years and 2 months commencing on May 1, 2015 and expiring on June 30, 2018, with an option to renew for an additional three years. On October 25, 2013, the Company entered into an operating lease for its warehouse for a period of four years and four months commencing in January 2014, with an option to renew for an additional six years. On May 12, 2016 TransEnterix Italia entered into an operating lease for research and development and demonstration facilities for a period of 6 years commencing in July 2016. Rent expense was approximately $907,000, $513,000 and $424,000 for the years ended December 31, 2016, 2015 and 2014, respectively. The Company’s approximate future minimum payments for its operating lease obligations that have initial or remaining noncancelable terms in excess of one year are as follow:
|
|
|
Years ending December 31, |
|
|
|
|
|
(In thousands) |
|
|
|
2017 |
|
$ |
861 |
|
|
2018 |
|
|
733 |
|
|
2019 |
|
|
273 |
|
|
2020 |
|
|
274 |
|
|
2021 |
|
|
274 |
|
|
Thereafter |
|
|
160 |
|
|
Total |
|
$ |
2,575 |
|
SafeStitch leased various office space on a month to month basis from a company controlled by a shareholder. Rent expense under these leases was $0, $0 and $89,000 for the years ended December 31, 2016, 2015 and 2014, respectively.
License and Supply Agreements
As discussed in Note 3, in September 2015, the Company completed the Senhance Acquisition. As part of this transaction, the Company assumed certain license and supply agreements. Commitments under these agreements amount to approximately $7,403,000 in 2017, $841,000 in 2018, $841,000 in 2019, $526,000 in 2020, $526,000 in 2021 and $3.2 million thereafter until termination in 2027.
On February 13, 2014, TransEnterix Surgical, Inc., a wholly owned subsidiary of the Company, entered into a Robotic Development and Supply Agreement (the “Robotic Agreement”) with Microline Surgical, Inc. (“Microline”). Under the Robotic Agreement, Microline was developing a flexible sealer product for exclusive use by the Company with the SurgiBot System in open, minimally invasive and laparoscopic surgery. Payments under the Robotic Agreement were $0 and $400,000 for the year ended December 31, 2016 and 2015, respectively. As part of the restructuring related to the SurgiBot System, the Robotic Agreement was terminated in 2016.
The Company has placed orders with various suppliers for the purchase of certain tooling, supplies and contract engineering and research services. Each of these orders has a duration or expected completion within the next twelve months.
77
|
22. |
Quarterly Results of Operation (Unaudited) |
The following is a summary of the Company’s unaudited quarterly results of operations for the fiscal years ended December 31, 2016 and 2015 (in thousands, except per share amounts):
|
|
|
Fiscal Year Ended December 31, 2016 |
|
|||||||||||||||||
|
|
|
1st |
|
|
2nd |
|
|
3rd |
|
|
4th |
|
|
Total |
|
|||||
|
|
|
Quarter |
|
|
Quarter |
|
|
Quarter |
|
|
Quarter |
|
|
Year |
|
|||||
|
Total revenues |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
1,466 |
|
|
$ |
53 |
|
|
$ |
1,519 |
|
|
Cost of goods sold |
|
|
— |
|
|
|
— |
|
|
|
1,031 |
|
|
|
38 |
|
|
|
1,069 |
|
|
Amortization of intangible assets |
|
|
1,817 |
|
|
|
1,786 |
|
|
|
1,709 |
|
|
|
1,655 |
|
|
|
6,967 |
|
|
Goodwill impairment |
|
|
— |
|
|
|
61,784 |
|
|
|
— |
|
|
|
— |
|
|
|
61,784 |
|
|
Restructuring and other charges |
|
|
— |
|
|
|
3,085 |
|
|
|
— |
|
|
|
(21 |
) |
|
|
3,064 |
|
|
Inventory write-down related to restructuring |
|
|
— |
|
|
|
2,565 |
|
|
|
— |
|
|
|
— |
|
|
|
2,565 |
|
|
Other operating expenses |
|
|
13,163 |
|
|
|
11,509 |
|
|
|
12,278 |
|
|
|
12,769 |
|
|
|
49,719 |
|
|
Interest expense, net |
|
|
578 |
|
|
|
394 |
|
|
|
462 |
|
|
|
420 |
|
|
|
1,854 |
|
|
Loss before income taxes |
|
|
(15,558 |
) |
|
|
(81,123 |
) |
|
|
(14,014 |
) |
|
|
(14,808 |
) |
|
|
(125,503 |
) |
|
Income tax benefit |
|
|
2,645 |
|
|
|
992 |
|
|
|
1,070 |
|
|
|
816 |
|
|
|
5,523 |
|
|
Net loss |
|
$ |
(12,913 |
) |
|
$ |
(80,131 |
) |
|
$ |
(12,944 |
) |
|
$ |
(13,992 |
) |
|
$ |
(119,980 |
) |
|
Net loss per share - basic and diluted |
|
$ |
(0.12 |
) |
|
$ |
(0.70 |
) |
|
$ |
(0.11 |
) |
|
$ |
(0.12 |
) |
|
$ |
(1.07 |
) |
|
|
|
Fiscal Year Ended December 31, 2015 |
|
|||||||||||||||||
|
|
|
1st |
|
|
2nd |
|
|
3rd |
|
|
4th |
|
|
Total |
|
|||||
|
|
|
Quarter |
|
|
Quarter |
|
|
Quarter |
|
|
Quarter |
|
|
Year |
|
|||||
|
Total revenues |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
Cost of goods sold |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Amortization of intangible assets |
|
|
125 |
|
|
|
126 |
|
|
|
338 |
|
|
|
1,596 |
|
|
|
2,185 |
|
|
Acquisition related costs |
|
|
— |
|
|
|
— |
|
|
|
4,003 |
|
|
|
228 |
|
|
|
4,231 |
|
|
Other operating expenses |
|
|
9,714 |
|
|
|
8,942 |
|
|
|
9,223 |
|
|
|
12,076 |
|
|
|
39,955 |
|
|
Interest expense, net |
|
|
281 |
|
|
|
280 |
|
|
|
436 |
|
|
|
604 |
|
|
|
1,601 |
|
|
Loss before income taxes |
|
|
(10,120 |
) |
|
|
(9,348 |
) |
|
|
(14,000 |
) |
|
|
(14,504 |
) |
|
|
(47,972 |
) |
|
Income tax benefit |
|
|
— |
|
|
|
— |
|
|
|
99 |
|
|
|
925 |
|
|
|
1,024 |
|
|
Net loss |
|
$ |
(10,120 |
) |
|
$ |
(9,348 |
) |
|
$ |
(13,901 |
) |
|
$ |
(13,579 |
) |
|
$ |
(46,948 |
) |
|
Net loss per share - basic and diluted |
|
$ |
(0.16 |
) |
|
$ |
(0.14 |
) |
|
$ |
(0.16 |
) |
|
$ |
(0.13 |
) |
|
$ |
(0.59 |
) |
|
23. |
Subsequent Events |
As discussed in Note 3, in September 2015, the Company completed the Senhance Acquisition using a combination of cash, stock and potential post-acquisition milestone payments. On December 30, 2016, the Company entered into an Amendment to restructure the terms of the Second Tranche of the Cash Consideration. Under the Amendment, the Second Tranche was restructured to reduce the contingent cash consideration by €5.0 million in exchange for the issuance of 3,722,685 shares of the Company’s common stock with an aggregate fair market value of €5.0 million. On January 4, 2017, The Company issued 3,722,685 shares of the common stock with a fair value of €5.0 million. The price per share was $1.404 and was calculated based on the average of the closing prices of the Company’s common stock on ten consecutive trading days ending one day before the execution of the Amendment.
78
None.
Disclosure Controls and Procedures
Our management, with the participation of our principal executive officer and principal financial officer, evaluated the effectiveness of our disclosure controls and procedures (as such term is defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) as of December 31, 2016. We maintain disclosure controls and procedures that are designed to provide reasonable assurance that information required to be disclosed in our reports filed or submitted under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms and that such information is accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate, to allow for timely decisions regarding required disclosure. Our management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Based on such evaluation, our Chief Executive Officer and Chief Financial Officer concluded that, as of December 31, 2016, our disclosure controls and procedures were effective at the reasonable assurance level.
Management’s Report on Internal Control over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. Internal control over financial reporting includes those policies and procedures that: (i) pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of the assets of the Company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
For the year ended December 31, 2016, pursuant to Section 404 of the Sarbanes-Oxley Act of 2002, management (with the participation of our principal executive officer and principal financial officer) conducted an evaluation of the effectiveness of our internal control over financial reporting based on the original framework established in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Based on this evaluation, management concluded that, as of December 31, 2016, our internal control over financial reporting was effective.
The Company’s independent registered public accounting firm, BDO USA, LLP, audited the effectiveness of the Company’s internal control over financial reporting as of December 31, 2016. BDO USA, LLP’s report on the effectiveness of the Company’s internal control over financial reporting as of December 31, 2016 is set forth herein.
Changes in Internal Controls Over Financial Reporting
There were no changes in the Company’s internal control over financial reporting during the last quarter that have materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting.
None.
79
Directors and Executive Officers
Our executive officers are elected by the Board of Directors, and serve for a term of one year and until their successors have been elected and qualified or until their earlier resignation or removal by the Board of Directors. There are no family relationships among any of the directors and executive officers of the Company. In accordance with our amended and restated certificate of incorporation, as amended, incumbent directors are elected to serve until our next annual meeting and until each director’s successor is duly elected and qualified. No director or executive officer has been involved in any legal proceeding during the past ten years that is material to an evaluation of his or her ability or integrity to serve as a member of our Board.
The following table sets forth names, ages and positions with the Company for all directors and executive officers of the Company as of March 6, 2017:
|
Name |
|
Age |
|
Position |
|
Director |
|
Directors |
|
|
|
|
|
|
|
Andrea Biffi |
|
35 |
|
Director |
|
2015 |
|
Jane H. Hsiao, Ph.D., MBA |
|
69 |
|
Director |
|
2005 |
|
William N. Kelley, M.D. |
|
77 |
|
Director |
|
2015 |
|
Aftab R. Kherani, M.D. |
|
43 |
|
Director |
|
2013 |
|
Paul A. LaViolette |
|
59 |
|
Director and Chairman of the Board |
|
2013 |
|
David B. Milne |
|
54 |
|
Director |
|
2013 |
|
Richard C. Pfenniger, Jr. |
|
61 |
|
Director |
|
2005 |
|
Todd M. Pope |
|
51 |
|
Chief Executive Officer, President, and Director |
|
2013 |
|
William N. Starling |
|
63 |
|
Director |
|
2013 |
|
|
|
|
|
|||
|
Other Executive Officers |
|
|
|
|
|
|
|
Anthony Fernando |
|
45 |
|
Chief Technology Officer |
|
|
|
Joseph P. Slattery |
|
52 |
|
Executive Vice President and Chief Financial Officer |
|
|
The following information summarizes, for each of our directors, his or her principal occupations and other public company directorships for at least the last five years and information regarding the specific experiences, qualifications, attributes and skills of such director:
Andrea Biffi. Mr. Biffi is currently the Chief Executive Officer of Sofar S.p.A., a position he has held since June 2015 and he has served as a member of the Board of Directors of Sofar S.p.A. since November 2012. Mr. Biffi has worked for Sofar, or companies owned by Sofar, since January 2008. Prior to becoming Chief Executive Officer, Mr. Biffi was General Manager of Sofar from November 2012 until June 2015. From January 2008 until November 2013, Mr. Biffi served as General Manager of SOVETA BALTICA UAB, a Lithuanian subsidiary of Sofar. Since the date of its incorporation in February 2013, Mr. Biffi has served as CEO and President of Sofar SWISS S.A. and since March 2016, Chairman of the Board of Directors of Sofar Americas Inc.
Jane H. Hsiao, Ph.D., MBA. Dr. Hsiao has served since May 2007 as Vice-Chairman and Chief Technical Officer of OPKO. Since October 2008, Dr. Hsiao has served as Chairman of the Board and, since February 2012, Interim CEO of medical device developer, Non-Invasive Monitoring Systems, Inc. (NIMS). Additionally, Dr. Hsiao serves as a director to Neovasc, Inc., a company developing and marketing medical specialty vascular devices, and Cocrystal Pharma, formerly Biozone Pharmaceuticals, Inc., a publicly traded biotechnology company developing new treatments for viral diseases. Dr. Hsiao previously served as the Vice Chairman-Technical Affairs and Chief Technical Officer of IVAX, from 1995 until IVAX was acquired in January 2006 by Teva. Dr. Hsiao also served as Chairman, CEO and President of IVX Animal Health, IVAX’s veterinary products subsidiary, from 1998 until 2006, and as IVAX’s Chief Regulatory Officer from 1992 to 1995. Dr. Hsiao previously served on the board of directors of Prolor and Sorrento Therapeutics, Inc., a development stage biopharmaceutical company. Dr. Hsiao received her B.S. from National Taiwan University and her Ph.D. from the University of Illinois, Chicago. Dr. Hsiao’s background in building and growing companies in the pharmaceutical and medical device industry, her strong technical expertise, as well as her senior management experience and extensive board service allow her to play an integral role as a member of our Board. Her broad experience in many biotechnology and life science companies gives her a keen understanding and appreciation of the many regulatory and developmental issues confronting medical device, pharmaceutical and biotechnology companies.
80
William N. Kelley, M.D. Dr. Kelley is currently Professor of Medicine at the School of Medicine of the University of Pennsylvania. He is also a director of GenVec, Inc., since June 2002. From 1989 to 2000, Dr. Kelley served as Executive Vice President of the University of Pennsylvania with responsibilities as Chief Executive Officer for the Medical Center, founding CEO of the University of Pennsylvania Health System, Dean of the School of Medicine, and the Robert G. Dunlop Professor of Medicine and Biochemistry and Biophysics. In the national leadership arena, Dr. Kelley has served as President of the American Society for Clinical Investigation, President of the American College of Rheumatology, Chair of the American Board of Internal Medicine, and Chair of the Residency Review Committee for Internal Medicine. Within the past five years, Dr. Kelley served on the board of directors of Merck & Co. Inc. and Beckman Coulter, Inc. Dr. Kelley’s experience as a practicing physician and a chief executive of a large healthcare system, as well as his experience as a director on other publicly traded healthcare company boards are the primary skills, qualifications and experience that bring value to our Board.
Aftab R. Kherani, M.D. Since September 2008, Dr. Kherani has served as an investment professional of Aisling Capital, where he is currently a Partner. Previously, Dr. Kherani was an Engagement Manager at McKinsey & Company, where he was a member of the Pharmaceutical, Medical Product and Private Equity practices. Prior to McKinsey, Dr. Kherani was a Chief Resident in Surgery at Duke University Medical Center, where he completed his residency in general surgery. He completed a two-year post-doctoral research fellowship at Columbia University, College of Physicians & Surgeons from 2001 to 2003. Dr. Kherani currently serves as a Director of Spirox, Inc. and as a board observer at EarLens, Inc., a privately-held company. Dr. Kherani is also a board observer at Loxo Oncology, Inc. Dr. Kherani received his M.D. from Duke, and his B.S. in Biology and A.B. in Economics from Duke. The Board of Directors believes that Dr. Kherani’s qualifications, skills and attributes, including his experience as a general surgeon, coupled with his strong investment background and healthcare consulting experience, position him to provide unique insights and be a valuable contributor to our Board.
Paul A. LaViolette. Mr. LaViolette has served as Chairman of our Board since September 2013. Mr. LaViolette is Managing Partner and Chief Operating Officer at SV Life Sciences (SVLS), a medical device value fund. He joined SVLS in 2009 and has over 33 years of global medical technology management experience. Prior to joining SVLS, Mr. LaViolette was most recently Chief Operating Officer at Boston Scientific Corporation (BSC), an $8 billion medical device leader. During his 15 years at BSC, he served as COO, Group President, President-Cardiology and President-International. Mr. LaViolette integrated two dozen acquisitions and led extensive product development, operations and worldwide commercial organizations. Mr. LaViolette previously held marketing and general management positions at CR Bard, and various marketing roles at Kendall (Covidien). Mr. LaViolette serves on the boards of Axon Therapies, Bardy Diagnostics, Inc., Cardiofocus, Inc., CardioKinetix, Inc., Cibiem, Inc., CSA Medical Inc., Corvia Medical, Inc., Endotronix, Inc., Soffio Medical, Inc., ValenTx, Inc., Ximedica, each of which are privately-held, as well as the Medical Device Manufacturers Association. Mr. LaViolette received his B.A. in Psychology from Fairfield University and his MBA from Boston College. Mr. LaViolette’s broad experience and keen business judgment qualify him to serve on our Board, and as the Chairman of our Board. Mr. LaViolette’s vast medical device operating experience makes him knowledgeable in the areas of product launches, new product development, clinical and regulatory affairs, plant management, quality systems, international sales and marketing, acquisitions and integrations and the analysis of investment opportunities.
David B. Milne. Mr. Milne is a Managing Partner at SVLS. He joined SVLS in 2005 and has 30 years of experience in the healthcare industry having worked at several leading public and private medical technology companies. From 1999 until joining SVLS in 2005, he held the position of Vice President of Corporate Business Development at BSC and was responsible for over 50 transactions totaling nearly $2 billion in acquisitions, equity investments and development partnerships. Mr. Milne currently sits on the board of the following privately-held companies: EBR Systems, Inc., Entellus Medical, Inc., Lombard Medical, Inc., ReShape Medical, Inc., and Spinal Kinetics, Inc. Previously Mr. Milne worked at Scimed Life Systems, Becton Dickinson and Parker Laboratories. He holds an MBA in Marketing/Finance from New York University and a BS in Biology from Rutgers University. The Board of Directors believes Mr. Milne brings his managerial, leadership and operational experience, particularly his acquisition, equity investment, licensing and collaboration experience to provide insights and substantial contributions to our Board.
Richard C. Pfenniger, Jr. Mr. Pfenniger served as the Interim CEO of Vein Clinics of America, Inc., a privately held company, from May 2014 through February 2015, and as the Interim CEO of IntegraMed America, Inc., a privately held company (IntegraMed), from January 2013 through June 2013. Previously, Mr. Pfenniger served as Chief Executive Officer and President of Continucare Corporation, a provider of physician services, from October 2003 until December 2011, and the Chairman of Continucare’s board of directors from September 2002 until December 2011. Additionally, Mr. Pfenniger served as CEO and Vice Chairman of Whitman Education Group, Inc., a post-secondary education provider, from 1997 until 2003. From 1994 to 1997, Mr. Pfenniger served as Chief Operating Officer of IVAX Corporation, and from 1989 to 1994 he served as Senior Vice President-Legal Affairs and General Counsel of IVAX Corporation. Prior to that, Mr. Pfenniger was engaged in the private practice of law, and earlier in his career, Mr. Pfenniger worked as a C.P.A. with Price Waterhouse & Co. Mr. Pfenniger is a director of GP Strategies, Inc., a corporate education and training company; Wright Investors’ Services Holdings, Inc., an investment management and financial advisory firm; BioCardia, Inc., a regenerative medicine company; OPKO Health, Inc.; Vein Clinics of America, Inc. and IntegraMed. Mr. Pfenniger received his B.B.A. from Florida Atlantic University and his J.D. from the University of Florida. As a result of Mr. Pfenniger’s multi-faceted experience as a chief executive officer, chief operating officer and general counsel, he is able to provide valuable business,
81
leadership and management advice to the Board of Directors in many critical areas. In addition, Mr. Pfenniger’s knowledge of the healthcare business has given him insight into many aspects of our business. Mr. Pfenniger also brings financial expertise to the Board of Directors, including through his service as Chairman of our Audit Committee.
Todd M. Pope. Mr. Pope became our President and Chief Executive Officer on September 3, 2013 in connection with the consummation of the Merger. Prior to the Merger, he was the president and chief executive officer of TransEnterix Surgical, Inc., one of the parties to the Merger, from September 2008. Mr. Pope has spent more than 25 years working in key leadership positions within the medical device industry. Prior to joining TransEnterix Surgical, Mr. Pope served as worldwide president of Cordis, a multi-billion-dollar division within Johnson & Johnson’s medical device business. Mr. Pope previously held a number of leadership positions within Johnson & Johnson and Boston Scientific Corporation. Mr. Pope received his bachelor’s degree from University of North Carolina at Chapel Hill, and currently serves on the University’s Kenan-Flagler Board of Visitors, and Educational Foundation Executive Board. The Board of Directors believes that Mr. Pope’s more than 25 years’ leadership experience in the medical device industry, at both privately held and multi-national companies, and his knowledge of the industry, coupled with his deep understanding of our technologies, product candidates, market and history make him an essential contributor to our Board of Directors.
William N. Starling. William N. Starling is Managing Director of Synergy Life Science Partners, LP, a life science venture capital firm founded in 2006, and Chief Executive Officer and co-founder, in 2000, of Synecor, LLC, an incubator/accelerator for new medical device companies. As CEO of Synecor, Mr. Starling is a cofounder of BaroSense Inc., Bioerodible Vascular Solutions, Inc., InnerPulse, Inc., TransEnterix, Interventional Autonomics Corporation, NeuroTronik Limited, Aegis Surgical, Limited, and Atrius Limited, all of which are privately-held companies. Mr. Starling currently serves as CEO of Aegis Surgical and Atrius Limited, and as a board member of EBR Systems, Inc., which are privately-held. He began his career in the medical technology device industry at American Edwards Laboratories and subsequently was part of the founding management team and Director of Marketing for Advanced Cardiovascular Systems, Inc.; a cofounder, Vice President and board member of Ventritex, Inc.; and a cofounder and Chairman of the Board of Directors and President/CEO of Cardiac Pathways Corporation. Mr. Starling received his BSBA degree from the University of North Carolina at Chapel Hill and his MBA degree from the University of Southern California. The Board of Directors believes that Mr. Starling’s experience in working with companies throughout their life cycle from start-up, through IPO to publicly traded, his extensive contributions to the medical device industry and his public company board experience make him a valuable contributor to our Board.
Executive Officers (Non-Board Members)
Anthony Fernando. Mr. Fernando has served as our Chief Technology Officer since January 19, 2016 and served as Vice President, International Development, from August 2015 until his appointment as Chief Technology Officer on January 19, 2016. Previously, Mr. Fernando served as Vice President, Innovation and Technology, International, of Stryker Singapore Pvt. Ltd, a global medical technology company, from October 2013 until July 2015. From August 2010 until October 2013, Mr. Fernando served as Director of Research and Development, greater Asia, for Becton Dickinson & Company, a global medical technology company engaged in the development, manufacture and sale of medical devices. From July 2007 until July 2010, Mr. Fernando served as the Director of Research and Development, Asia – Environmental Health, at Perkinelmer Singapore Pvt. Ltd. Until July 2015, Mr. Fernando also served as a director of Stryker India Private Limited and Stryker Global Technology Center (India).
Joseph P. Slattery. Mr. Slattery has served as our Executive Vice President and Chief Financial Officer since October 2013. Previously, Mr. Slattery served as Executive Vice President and Chief Financial Officer of Baxano Surgical, Inc., a minimally invasive spine company, from April 2010 until September 2013. Mr. Slattery served as a member of the Baxano Surgical board of directors from November 2007 until April 2010 and resigned in connection with his appointment as an officer. From February 1996 through August 2007, Mr. Slattery served in various roles of increasing responsibility at Digene Corporation, a molecular diagnostics company that was acquired by Qiagen, N.V. in August 2007, including from October 2006 through August 2007 as Chief Financial Officer and Senior Vice President of Finance and Information Systems. Mr. Slattery served on the board of directors of Micromet, Inc., a publicly-held biopharmaceutical company, which was acquired by Amgen in March 2012, and currently serves on the board of directors of CVRx, Inc., a privately-held medical device company, and Exosome Diagnostics, a privately-held molecular diagnostics company. Mr. Slattery received a B.S. degree in Accountancy from Bentley University and is a Certified Public Accountant.
Section 16(a) Beneficial Ownership Reporting Compliance
Under Section 16(a) of the Exchange Act, the Company’s directors, executive officers and persons who own more than ten percent (10%) of our Common Stock are required to file with the SEC initial reports of ownership and reports of changes in ownership of the Common Stock and other equity securities of the Company. To the Company’s knowledge, based solely on a review of copies of such reports furnished to the Company during and/or with respect to the year ended December 31, 2016, the Company is not aware of any late or delinquent filings required under Section 16(a) of the Exchange Act in respect of the Company’s equity securities.
82
We have adopted a Code of Business Conduct and Ethics that applies to our principal executive officer, principal financial officer and other persons performing similar functions. A copy of our Code of Business Conduct and Ethics is available on our website at www.transenterix.com. We intend to post amendments to, or waivers from a provision of, our Code of Business Conduct and Ethics that apply to our principal executive officer, principal financial officer or persons performing similar functions on our website.
Interested parties who want to communicate with the independent or non-management directors as a group, with the Board as a whole, any Board committee or any individual Board members should address their communications to the Board, the Board members or the Board committee, as the case may be, and send them to c/o Corporate Secretary, TransEnterix, Inc., 635 Davis Drive, Suite 300, Morrisville, North Carolina 27560, or call the Corporate Secretary at (305) 575-4602. The Corporate Secretary will forward all such communications directly to such Board members. Any such communications may be made on an anonymous and confidential basis.
There have been no changes to the procedures by which interested parties may communicate with the Board.
Board Nominations by Security Holders
The Board will consider candidates recommended by our stockholders pursuant to written applications submitted to our Corporate Secretary, TransEnterix, Inc., 635 Davis Drive, Suite 300, Morrisville, North Carolina 27560.
There have been no changes to the procedures by which security holders may recommend nominees to our Board.
Compensation Discussion and Analysis
This Compensation Discussion and Analysis (“CD&A”) describes our compensation program for our named executive officers (“Named Executive Officers”) during the year ended December 31, 2016. The following discussion focuses on our compensation program and compensation‑related decisions for 2016 and also addresses why we believe our compensation program is appropriate for the Company.
Business Overview for 2016
The Company is a medical device company that is pioneering the use of robotics to improve minimally invasive surgery by addressing the clinical challenges associated with current laparoscopic and robotic options. The Company is focused on the commercialization and further development of its Senhance System, a multi-port robotic system that brings the advantages of robotic surgery to patients while enabling surgeons with innovative technology. The Company also developed the SurgiBot System, a single-port, robotically enhanced laparoscopic surgical platform. The Senhance System has been granted a CE Mark in Europe for laparoscopic abdominal and pelvic surgery, as well as limited thoracic operations excluding cardiac and vascular surgery, but is not available for sale in the U.S. The SurgiBot System is not available for sale in any market.
The Senhance System is a multi-port robotic surgery system which allows multiple arms to control robotic instruments and a camera. The system features advanced technology to enable surgeons with haptic feedback and the ability to move the camera via eye movement. The system replicates laparoscopic motion that is familiar to experienced surgeons, and features 3DHD vision technology. The Senhance System also offers responsible economics to hospitals by offering robotic technology with reusable instruments with minimal additional costs per surgery.
The SurgiBot System is designed to utilize flexible instruments through articulating channels controlled directly by the surgeon, with robotic assistance, while the surgeon remains patient-side within the sterile field. In June 2015, the Company submitted a 510(k) application to the FDA for the SurgiBot System. On April 19, 2016, the FDA notified the Company that the SurgiBot System did not meet the criteria for substantial equivalence based on the data and information submitted by TransEnterix in the 510(k) submission. After interactions with the FDA, the Company determined that a new 510(k) application would need to be submitted in order to obtain clearance for the SurgiBot System. Based on this fact, the Company evaluated the operational and financial feasibility of pursuing two 510(k) applications in parallel and elected to primarily focus the Company’s near term regulatory efforts on the 510(k) submission for the Senhance System, and its current strategy is to focus the Company’s resources on the commercialization of and regulatory clearance for the Senhance System.
Consequently, in May 2016, the Company implemented a restructuring plan. The restructuring plan resulted in: 1) reducing the Company’s workforce; 2) abandoning certain equipment; 3) cancelling certain contracts; 4) writing off inventory related to the SurgiBot System; and 5) writing off certain patents.
83
Compensation philosophy
The Company believes it is vital to link executive compensation to corporate performance and to create incentives for management to enhance Company value. In accordance with its compensation philosophy, the Company seeks to attract and retain employees through salary levels that are competitive with the local market and similarly situated companies but generally to follow the market rather than lead the market, particularly with respect to cash compensation, and offer attractive equity and cash-based incentive components to align compensation with Company performance objectives. The Company desires, over time, to move total direct compensation toward the median of comparable companies, while remaining more aggressive in the use of equity-based compensation, but not in a market leader position. The Company believes this approach allows it to attract and retain candidates that support the Company culture of being motivated by aggressive goals and optimism about the future, while permitting the Company to preserve the use of cash for incentive compensation.
The Company is the result of a reverse merger of a privately held, venture‑backed company and a publicly traded company, consummated on September 3, 2013, and the subsequent Senhance Acquisition described in the Business section of this Annual Report. The Compensation Committee’s focus for 2016 was to establish a program to provide compensation to the executives aligned with the Company’s need to integrate the Senhance System development and early commercialization activities and the SurgiBot System development activities, with a focus on performance‑based incentive compensation designed to incentivize the Named Executive Officers to pursue regulatory approval for the SurgiBot System in the United States, finance the Company through such development stage and support the early commercialization activities related to the Senhance System in identified countries in Europe, the Middle East and Asia.
Procedures for determining compensation
Our Compensation Committee has the overall responsibility for designing and evaluating the compensation policies and programs for our Named Executive Officers. In 2016, the Compensation Committee reviewed updated information procured, aggregated and summarized from public sources regarding similarly situated companies, and compensation of the Named Executive Officers in prior years. The Compensation Committee also relied on input from our Chief Executive Officer regarding the Named Executive Officers (other than himself), and on its analysis of our corporate performance.
With respect to the compensation for the Chief Executive Officer, each year the Compensation Committee evaluates the Chief Executive Officer’s performance, sets his compensation and approves his compensation. In 2016, the Compensation Committee approved the salary, annual incentive bonus and the long-term equity awards for the Chief Executive Officer.
Our Chief Executive Officer plays a significant role in the compensation-setting process of the other Named Executive Officers and makes recommendations to the Compensation Committee concerning performance objectives and salary and bonus levels for the other Named Executive Officers and executive team. The Compensation Committee, at least annually, discusses such recommendations with the Chief Executive Officer. The Compensation Committee may, in its sole discretion, approve, in whole or in part, the recommendations of the Chief Executive Officer. In 2016, the Compensation Committee approved the Chief Executive Officer’s recommendations for salary, bonus and long‑term equity awards for each of the other Named Executive Officers.
At each of the annual meetings of stockholders held in 2014, 2015 and 2016, stockholders holding approximately 98% of the votes cast approved, on an advisory basis, the compensation paid to our Named Executive Officers for the prior calendar year. The Compensation Committee monitors and considers these advisory vote results in making compensation decisions. The Compensation Committee will continue to monitor the annual say-on-pay results and include such results in its annual executive compensation analysis.
Elements of compensation
The compensation of our Named Executive Officers consists primarily of four major components:
|
|
• |
base salary; |
|
|
• |
annual cash-based incentive awards; |
|
|
• |
long-term equity awards; and |
|
|
• |
other benefits. |
Base salary
The base salary of each of our Named Executive Officers is determined based on an evaluation of the responsibilities of that particular position, each Named Executive Officer’s historical salary earned in similar management positions with the Company or other companies, and a review of the information procured by the Committee as described above. A significant portion of each Named
84
Executive Officer’s total compensation is in the form of base salary. The base salary component is designed to provide the Named Executive Officers with consistent income and to attract and retain talented and experienced executives capable of leading our product development, operations and strategic growth.
In February 2016, Messrs. Pope and Slattery received merit increases in their base salary, retroactive to January 1, 2016, and Mr. Fernando was determined to be an executive officer of the Company. At such time, Mr. Fernando’s received an increase to his base salary in connection with his promotion to Chief Technology Officer. The 2016 base salaries for the Named Executive Officers are set forth in the Summary Compensation Table following this CD&A.
Annual incentive plan
The Compensation Committee established the 2016 annual cash incentive plan for management (the “Incentive Plan”) at its February 2016 meeting, and finalized 2016 Incentive Plan goals in June 2016. The Incentive Plan is designed to recognize and reward our executives, including the Named Executive Officers, for contributing towards the achievement of our annual corporate business plan. The annual Incentive Plan awards are designed to reward near-term operating performance and the achievement of milestones critical to the Company’s success. The Compensation Committee believes the Incentive Plan serves as a valuable short-term incentive program for providing cash bonus opportunities for executives upon achievement of targeted product development and operating results. The maximum annual cash incentive plan award opportunity was 75% for Mr. Pope and 50% of base salary for each of Messrs. Slattery and Fernando. For the Named Executive Officers, the 2016 goals were 100% weighted on the approved corporate goals. The 2016 Incentive Plan corporate goals, which were each weighted by the Committee, were:
|
|
1. |
Receipt of FDA clearance for the SurgiBot System; |
|
|
2. |
Achievement of revenue goals for early commercialization of the Senhance System in Europe and, if cleared in the U.S., of the SurgiBot System; |
|
|
3. |
Regulatory milestones for the Company’s products; and |
|
|
4. |
Development and execution of financing strategies to fund the Company’s operations during development and early commercialization periods. |
Additional stretch goals related to regulatory clearances and commercialization were also established.
At a meeting held in February 2017, the Compensation Committee reviewed the achievement of the corporate goals under the 2016 Incentive Plan. The Compensation Committee noted the FDA’s decision not to provide clearance for the SurgiBot System, which occurred in April 2016, and the impact of such event on the Company’s business and operations. The Compensation Committee reviewed the impact of the FDA decision on the Company’s strategic focus, the 2016 activities of the management team following the FDA decision, including the decisions made to re-focus the Company’s U.S. regulatory strategy on the Senhance System, the financial status of the Company at the end of the year, the results of the early commercialization activities for the Senhance System, and the integration of the Senhance Acquisition during 2016. The Compensation Committee also considered the bonuses that had been paid under the 2015 incentive plan. After considering the foregoing factors, the Compensation Committee approved annual incentive plan payouts at 30% of the target bonus levels under the 2016 Incentive Plan. The 2016 Incentive Plan bonuses for the Named Executive Officers are set forth in the Summary Compensation Table following this CD&A. The incentive compensation awarded was paid in the first quarter of 2017.
Long-term equity awards
The Compensation Committee believes that it is essential to align the interests of the Named Executive Officers with the interests of our stockholders, and believes the best way to accomplish this alignment is through awards of long-term, equity-based compensation. Such awards are made under the Company’s Amended and Restated Incentive Compensation Plan, as amended (the “Plan”).
For 2016, the Compensation Committee discussed the form such equity-based grants should take and determined that providing a mixture of time-based stock options and restricted stock units (“RSU”) best met the needs of the Company and its executives as a retention incentive. Such stock option and RSU awards were granted under the Plan in February 2016. The time-based stock option awards typically vest over a four year period with the first 25% cliff vesting on the first anniversary of the date of grant and then vesting monthly thereafter. For the RSU awards, the awards vest one-third on each of the first three anniversaries of the date of grant. In October 2016, the Compensation Committee made additional stock option grants to the Named Executive Officers as a retention incentive.
The grant date value of the awards made to the Named Executive Officers is set forth in the Summary Compensation Table following this CD&A.
85
The Compensation Committee periodically reviews long-term incentives to assure that our executive officers and other key employees are appropriately motivated and rewarded in a way that is aligned with our long-term financial results.
Other benefits
Perquisites and other benefits - We offer our Named Executive Officers modest perquisites and other personal benefits that we believe are reasonable and in our best interest and generally in line with benefits we offer to all of our employees. See the disclosure in the Summary Compensation Table for more information.
Employment agreements and severance benefits - We have entered into employment agreements with each Named Executive Officer. These agreements provide our Named Executive Officers with certain severance benefits in the event of involuntary termination. See “Executive Compensation — Agreements with Named Executive Officers.”
Pension benefits - The Company has no defined benefit plans, supplemental executive retirement plans or actuarial plans.
Nonqualified defined contribution and other deferred compensation plans - The Company does not have a defined contribution plan and has not contributed to a deferred compensation plan.
86
Summary Compensation Tables
SUMMARY COMPENSATION TABLE
The following table lists the compensation of our named executive officers for 2016 and, where applicable, for the prior two years:
|
Name and Principal Position |
|
Year |
|
|
Salary |
|
|
Bonus |
|
|
Stock |
|
|
Option |
|
|
Non-Equity |
|
|
Nonqualified |
|
|
All Other |
|
|
Total |
|
|||||||||
|
Todd M. Pope |
|
|
2016 2015 2014 |
|
|
$ $ $ |
453,200 440,002 400,000 |
|
|
$ $ $ |
— — — |
|
|
$ $ $ |
464,768 294,000 — |
|
|
$ $ $ |
1,213,610 1,205,100 367,802
|
|
|
$ $ $ |
101,970 429,000 90,000 |
(4) (4) (4) |
|
$ $ $ |
— — — |
|
|
$ $ $ |
— — — |
|
|
$ $ $ |
2,233,548 2,368,102 947,802 |
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||
|
Joseph P. Slattery, |
|
|
2016 2015 2014 |
|
|
$ $ $ |
324,450 315,041 282,808
|
|
|
$ $ $ |
— — — |
|
|
$ $ $ |
280,132 176,400 —
|
|
|
$ $ $ |
692,160 541,060 1,118,718 |
|
|
$ $ $ |
48,668 204,750 51,300
|
(4) (4) (4) |
|
$ $ $ |
— — — |
|
|
$ $ $
|
— — — |
|
|
$ $ $ |
1,345,410 1,237,251 1,452,826
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Anthony Fernando, |
|
|
2016
|
|
|
$
|
300,000
|
|
|
$
|
—
|
|
|
$
|
280,132
|
|
|
$
|
692,160
|
|
|
$
|
45,000
|
(4)
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
1,317,992
|
|
|
(1) |
Represents time-based restricted stock units (“RSU”) awarded to the named executive officers as part of the long-term incentive awards. The RSU awards vests in three equal installments on the first three anniversaries of the date of grant. |
|
(2) |
For all RSUs and stock options, the values reflect the aggregate grant date fair value computed in accordance with FASB ASC Topic 718. Assumptions made in the calculation of these amounts are described in Notes 14 and 15 to the Company’s audited financial statements, included in this Annual Report. |
|
(3) |
In the event of termination of his employment in connection with a Change in Control, all of Mr. Pope’s unvested outstanding equity awards shall accelerate and vest upon the date of termination. |
|
(4) |
Represents bonuses paid under a TransEnterix incentive bonus plan for 2016, 2015 and 2014. Corporate performance goals were established by the Compensation Committee for each year. The incentive bonus was based on the achievement of corporate performance goals only. |
|
(5) |
Mr. Slattery became our Executive Vice President and Chief Financial Officer on October 2, 2013. |
|
(6) |
Mr. Fernando became our Chief Technology Officer on January 19, 2016. |
87
GRANTS OF PLAN-BASED AWARDS
The following table sets forth information regarding all plan-based awards granted to our Named Executive Officers during the fiscal year ended December 31, 2016. All equity awards to our Named Executive Officers in 2016 were granted under our Amended and Restated Incentive Compensation Plan, as amended.
|
|
|
|
|
Estimated Future Payouts Under Non-Equity Incentive Plan Awards |
|
Estimated Future Payouts Under Equity Incentive Plan Awards |
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
Name |
|
Grant Date |
|
Threshold ($) |
|
Target ($) |
|
Maximum ($)(1) |
|
Threshold (#) |
|
Target (#) |
|
Maximum (#) |
|
All Other Stock Awards: Number of Shares of Stock or Units (#)(2) |
|
All Other Option Awards: Number of Securities Underlying Options (#)(3) |
|
|
Exercise or Base Price of Option Awards ($/Sh) |
|
|
Grant Date Fair Value of Stock and Option Awards ($)(2)(3) |
||||||
|
Todd M. Pope |
|
02/12/2016 10/25/2016 |
|
|
—
|
|
339,900
|
|
509,850
|
|
|
|
|
|
|
|
|
|
121,667
|
|
547,500 340,000 |
|
3.82 1.53 |
|
|
1,434,938 243,440 |
||||
|
Joseph P. Slattery |
|
02/12/2016 10/25/2016 |
|
|
—
|
|
162,225 |
|
243,338 |
|
|
|
|
|
|
|
|
|
73,333 |
|
330,000 150,000 |
|
3.82 1.53 |
|
|
864,892 107,400 |
||||
|
Anthony Fernando |
|
02/12/2016 10/25/2016 |
|
|
—
|
|
150,000
|
|
225,000
|
|
|
|
|
|
|
|
|
|
73,333
|
|
330,000 150,000 |
|
3.82 1.53 |
|
|
864,892 107,400 |
||||
|
(1) |
Represents the potential payout at 150% of target for the 2016 annual incentive bonuses. Please see the description of the 2016 annual incentive bonus in the CD&A section of this Annual Report, and the Summary Compensation Table for the 2016 annual incentive bonuses earned. |
|
(2) |
The value of stock awards in the table is based on the closing price of the Company’s Common Stock on the date of grant. |
|
(3) |
For all stock options in the table, the option values reflect the aggregate grant date fair value computed in accordance with FASB ASC Topic 718. Assumptions made in the calculation of these amounts are described in Note 14 to the Company’s audited financial statements, included in this Annual Report. |
88
OUTSTANDING EQUITY AWARDS AT FISCAL YEAR-END
The following table lists the outstanding equity awards held by the Named Executive Officers as of December 31, 2016:
|
|
|
OPTION AWARDS |
|
STOCK AWARDS |
|
||||||||||||||
|
Name |
|
(1) |
|
(1) |
|
Equity |
|
Option |
|
Option |
|
Number of |
|
Market |
|
Equity |
|
Equity |
|
|
Todd M. Pope |
|
104,686 |
|
__ |
|
__ |
|
0.35 |
|
12/14/2019 |
|
__ |
|
__ |
|
__ |
|
__ |
|
|
|
|
929,263 |
|
__ |
|
__ |
|
0.35 |
|
04/12/2022 |
|
__ |
|
__ |
|
__ |
|
__ |
|
|
|
|
281,118 |
|
64,872 |
|
__ |
|
2.00 |
|
08/12/2023 |
|
__ |
|
__ |
|
__ |
|
__ |
|
|
|
|
34,000 |
|
14,000 |
|
__ |
|
8.00 |
|
02/13/2024 |
|
__ |
|
__ |
|
__ |
|
__ |
|
|
|
|
53,345 |
|
29,255 |
|
__ |
|
3.94 |
|
05/27/2024 |
|
__ |
|
__ |
|
__ |
|
__ |
|
|
|
|
206,250 |
|
243,750 |
|
__ |
|
2.95 |
|
02/04/2025 |
|
66,667 |
|
86,667 |
|
__ |
|
__ |
|
|
|
|
131,250 |
|
318,750 |
|
__ |
|
2.36 |
|
10/05/2025 |
|
__ |
|
__ |
|
__ |
|
__ |
|
|
|
|
__ |
|
547,500 |
|
__ |
|
3.82 |
|
02/12/2026 |
|
121,667 |
|
158,167 |
|
__ |
|
__ |
|
|
|
|
__ |
|
340,000 |
|
__ |
|
1.53 |
|
10/25/2026 |
|
__ |
|
__ |
|
__ |
|
__ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Joseph P. Slattery |
|
395,833 |
|
104,167 |
|
__ |
|
4.02 |
|
04/21/2024 |
|
__ |
|
__ |
|
__ |
|
__ |
|
|
|
|
16,727 |
|
9,173 |
|
__ |
|
3.94 |
|
05/27/2024 |
|
__ |
|
__ |
|
__ |
|
__ |
|
|
|
|
123,750 |
|
146,250 |
|
__ |
|
2.94 |
|
02/04/2025 |
|
40,000 |
|
52,000 |
|
__ |
|
__ |
|
|
|
|
29,166 |
|
70,834 |
|
__ |
|
2.45 |
|
10/28/2025 |
|
__ |
|
__ |
|
__ |
|
__ |
|
|
|
|
__ |
|
330,000 |
|
__ |
|
3.82 |
|
02/12/2026 |
|
73,333 |
|
95,333 |
|
__ |
|
__ |
|
|
|
|
__ |
|
150,000 |
|
__ |
|
1.53 |
|
10/25/2026 |
|
__ |
|
__ |
|
__ |
|
__ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Anthony Fernando |
|
66,666 |
|
133,334 |
|
__ |
|
2.97 |
|
08/17/2025 |
|
__ |
|
__ |
|
__ |
|
__ |
|
|
|
|
29,166 |
|
70,834 |
|
__ |
|
2.45 |
|
10/28/2025 |
|
__ |
|
__ |
|
__ |
|
__ |
|
|
|
|
__ |
|
330,000 |
|
__ |
|
3.82 |
|
02/12/2026 |
|
73,333 |
|
95,333 |
|
__ |
|
__ |
|
|
|
|
__ |
|
150,000 |
|
__ |
|
1.53 |
|
10/25/2026 |
|
__ |
|
__ |
|
__ |
|
__ |
|
|
(1) |
One-fourth of the shares underlying each option award vests on the first anniversary of the grant date of such option award, and 1/48th of the shares underlying the full award vest each month thereafter for 36 months. |
|
(2) |
Each of the stock options granted have a ten-year term beginning on the date of grant. |
|
(3) |
Based on the closing price of the Company’s Common Stock on December 30, 2016 of $1.30 per share. |
89
OPTION EXERCISES AND STOCK VESTED
The following table provides information with respect to stock options exercised by our Named Executive Officers and stock in which our Named Executive Officers vested during the year ended December 31, 2016 upon the lapse of forfeiture restrictions on previously granted RSUs.
|
|
|
OPTION AWARDS |
|
STOCK AWARDS |
||||
|
Name |
|
Number of Shares Acquired on Exercise (#) |
|
Value Realized |
|
Number of Shares |
|
Value Realized |
|
Todd M. Pope |
|
150,000 |
|
235,500 |
|
33,334 |
|
108,669 |
|
Joseph P. Slattery |
|
— |
|
— |
|
86,666 |
|
177,866 |
|
Anthony Fernando |
|
— |
|
— |
|
— |
|
— |
Agreements with Named Executive Officers
Todd M. Pope
On February 3, 2015, the Company entered into an employment agreement with Todd M. Pope regarding Mr. Pope’s continued employment with the Company as its President and Chief Executive Officer. The initial employment period under the employment agreement commenced on September 3, 2013 and continued until December 31, 2015. The term of the employment agreement automatically renews for successive one-year terms, unless terminated in accordance with the terms of the employment agreement. Mr. Pope’s annual base salary under the employment agreement for the year ended December 31, 2016 was $453,200. Mr. Pope’s salary is subject to increase in accordance with the employment agreement. He is eligible to receive annually, or otherwise, an incentive compensation award opportunity, payable in cash, as determined by the Compensation Committee of the Board, and he is eligible for long term incentive equity compensation. Mr. Pope’s target annual cash incentive compensation opportunity will not be less than 50% of his base salary for the portion of the employment period falling within a given fiscal year, and performance goals are based on both Company performance metrics and personal performance metrics, as established and approved by the Compensation Committee or the Board annually. The equity-based compensation will be awarded under the Company’s Amended and Restated Incentive Compensation Plan, or any successor thereto, in the discretion of the Compensation Committee or the Board. Mr. Pope is entitled to severance benefits, paid by the Company or any successor, as follows. If the employment agreement is terminated without cause or for good reason, or if the employment agreement is not extended at the end of the then-current term, Mr. Pope will receive severance and continued health and welfare benefits for twelve months following termination. If Mr. Pope’s employment is terminated in connection with a Change in Control of the Company (as defined in the employment agreement), his severance benefits would be expanded to twenty-four (24) months. The severance payable is the sum of (a) his annual rate of base salary immediately preceding his termination of employment, and (b) his target annual bonus for the fiscal year in which the termination occurs. In addition, Mr. Pope would continue to receive payment for health care benefits for such period. Such severance benefit can be paid in a lump sum in the Change in Control context, subject to a payment delay required by applicable law. In addition, in the event of termination of his employment in connection with a Change in Control, to the extent not previously accelerated, all of Mr. Pope’s unvested outstanding equity awards shall accelerate and vest upon the date of termination. Mr. Pope is subject to non-solicitation and non-competition covenants during the terms of the employment agreement and for one (1) year immediately following the termination of his employment.
Joseph P. Slattery
In connection with his hiring, we entered into an offer letter, which constituted an employment agreement, with Mr. Slattery in September 2013. Under the employment agreement, Mr. Slattery is entitled to receive a base salary and an annual bonus, as determined by the Compensation Committee. Mr. Slattery also received a grant of 200,000 RSUs, which vested one-third (1/3) per year on the first three anniversaries of Mr. Slattery’s start date with the Company (the “Initial RSU Grant”). As of October 2016, the Initial RSU Grant vested fully.
Under the employment agreement, Mr. Slattery was entitled to a stock option grant exercisable for 500,000 shares of the Company’s common stock (the “Fundraising Option Grant”) following the successful closing of a Company fundraising in which at least $20.0 million in proceeds was raised for the Company and where at least 50% of the funds raised come from non-insiders (the “Fundraising”). The Fundraising Option Grant was awarded on April 21, 2014, with an exercise price of $4.02 per share, with vesting of 25% on the one (1) year anniversary of Mr. Slattery’s start date and thereafter vesting in thirty-six (36) equal monthly installments. Mr. Slattery was prohibited from exercising any the Fundraising Option Grant for a period of six (6) months following the date of grant.
Any remaining unvested portion of the Fundraising Stock Option Grant will accelerate and vest in the event of Mr. Slattery’s involuntary termination from employment with the Company at the time of or within twelve (12) months following a change of control.
90
In the event that there is a change of control of the Company affecting his employment, Mr. Slattery shall be entitled to receive a lump sum payment, paid by the Company or any successor, equal to 12 months of his base salary and reimbursement for COBRA premiums for a period of up to 12 months, subject to signing a release of claims in favor of TransEnterix.
Anthony Fernando
On August 14, 2015, Mr. Fernando entered into an employment agreement with the Company as its Vice President, International Development. The initial employment period under the employment agreement commenced on August 16, 2015 and continued until December 31, 2016. The term of the employment agreement automatically renews for successive one-year terms, unless terminated in accordance with the terms of the employment agreement. Under the agreement, Mr. Fernando is entitled to receive a base salary and annual bonus, as determined by the Compensation Committee, and a one-time signing bonus.
Mr. Fernando will be eligible to receive annually or otherwise any incentive compensation awards, payable in cash, which the Company, the Compensation Committee or such other authorized committee of the Board determines to award. For each fiscal year of the Company falling in whole or in part during the Employment Period, Mr. Fernando's target annual cash incentive compensation opportunity will be no less than 25% of his base salary for the portion of the Employment Period falling within that fiscal year. With respect to the annual cash incentive compensation award, the performance goals shall be based on performance metrics approved by the Compensation Committee or the Board annually.
Mr. Fernando also received an initial stock option grant of 200,000 shares of TransEnterix common stock. These options will vest 25% one year following the grant date and 1/48 per month thereafter for the next three years. After four years, the full amount of this option grant will be vested. The grant date for this initial option grant was August 17, 2015.
In the event of termination of Mr. Fernando's employment by the Company without Cause or by Mr. Fernando for Good Reason, each as defined in the employment agreement, Mr. Fernando shall be entitled to his base salary and any other compensation and benefits to the extent actually earned by him under the agreement or under any benefit plan or program of the Company as of the date of such termination at the normal time for payment of such salary, compensation or benefits; business reimbursements; and severance and continued health and welfare benefits for a six-month period. If Mr. Fernando’s employment is terminated in connection with a Change in Control of the Company (as defined in the employment agreement), his severance benefits will be expanded to twelve (12) months. In addition, in the event of termination of his employment in connection with a Change in Control, to the extent not previously accelerated, all of Mr. Fernando’s unvested outstanding equity awards shall accelerate and vest upon the date of termination.
Mr. Fernando is subject to non-solicitation and non-competition covenants during the terms of the employment agreement and for one (1) year immediately following the termination of his employment.
The named executive officers get no compensation, other than accrued obligations, in other termination events, including voluntary termination by the executive or termination on death or disability of the executive.
91
The following table calculates what the severance compensation would have been for the named executive officers if a qualifying termination had occurred at December 31, 2016:
|
Named Executive Officer |
|
Benefit |
|
Termination without Cause ($) |
|
Termination for Good Reason ($) |
|
Change In Control (Single Trigger) ($) (1) |
|
Change In Control (Double Trigger) ($) |
|
|
Todd M. Pope |
|
Severance (2) |
|
793,100 |
|
793,100 |
|
— |
|
1,586,200 |
|
|
|
|
Equity Awards (3) |
|
— |
|
— |
|
— |
|
1,227,084 |
|
|
|
|
Health Care Benefits |
|
17,112 |
|
17,112 |
|
|
|
34,224 |
|
|
|
|
Total |
|
810,212 |
|
810,212 |
|
|
|
2,847,508 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Joseph P. Slattery |
|
Severance (2) |
|
— |
|
— |
|
— |
|
324,450 |
|
|
|
|
Equity Awards (3) |
|
— |
|
— |
|
— |
|
147,333 |
|
|
|
|
Health Care Benefits |
|
— |
|
— |
|
|
|
17,258 |
|
|
|
|
Total |
|
— |
|
— |
|
— |
|
489,041 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Anthony Fernando |
|
Severance (2) |
|
150,000 |
|
150,000 |
|
— |
|
300,000 |
|
|
|
|
Equity Awards |
|
— |
|
— |
|
— |
|
95,333 |
|
|
|
|
Heath Care Benefits |
|
8,809 |
|
8,809 |
|
— |
|
17,618 |
|
|
|
|
Total |
|
158,809 |
|
158,809 |
|
— |
|
412,951 |
|
|
(1) |
No severance benefits or equity award acceleration occurs automatically on the event of a Change of Control. |
|
(2) |
Receipt of severance is contingent upon executing a release of claims. Severance is paid over a one-year period for Mr. Pope and Mr. Slattery and a six-month period for Mr. Fernando, if there is a qualifying termination without cause or termination with good reason outside of the Change in Control context. Mr. Pope and Mr. Slattery may receive their severance payment on termination in a lump sum. Mr. Fernando shall receive his severance payment on termination on a monthly basis or in a lump sum in the event of a Change of Control. Severance payments are subject to applicable law and will be paid by the Company or any successor. |
|
(3) |
Consists of the difference between the fair market value of our Common Stock and the exercise price of the stock option for each in-the-money stock option grant and the fair market value of any RSUs for which vesting is accelerated. The closing price of the Company’s common stock on December 30, 2016 was $1.30 per share; therefore no value was added for stock options that were out-of-the-money as of such date. |
Equity Compensation Plan
The Company currently has one equity compensation plan under which it makes awards, the TransEnterix, Inc. Amended and Restated Incentive Compensation Plan, as amended (the “Plan”). The Plan was originally approved by the Board of Directors and adopted by the majority of our stockholders on November 13, 2007, and amended and restated and approved by the Board of Directors and approved by the majority of our stockholders on May 7, 2015 to increase the number of shares of common stock authorized under the Plan to 11,940,000 shares, and to make other changes. The Plan was amended on June 8, 2016 to increase in the number of shares reserved for issuance under the Plan to 18,940,000 shares. The Plan is used for plan-based awards for officers, other employees, consultants, advisors and non-employee directors. The Company can issue stock options, stock appreciation rights, restricted stock units and other stock-based awards under the Plan. In connection with the Merger, we assumed all of the options that were issued and outstanding immediately prior to the Merger as issued by TransEnterix Surgical, and adjusted based on the Merger at the exchange ratio, which are now exercisable for approximately 1,870,892 shares of common stock. Such options were granted under the TransEnterix, Inc. 2006 Stock Plan (the “2006 Plan”) which was assumed by the Company in the Merger. The 2006 Plan is maintained solely for the purpose of the stock options granted under such 2006 Plan that remain outstanding; no future awards are authorized to be made under the 2006 Plan.
92
Director Compensation
The following table lists the compensation paid during 2016 to the non-employee directors of the Company:
DIRECTOR COMPENSATION
|
Name |
|
Fees Earned or Paid in Cash ($) |
|
Stock Awards ($) |
|
Option Awards ($) (1) |
|
Non-Equity Incentive Plan Compensation ($) |
|
Nonqualified Deferred Compensation Earnings |
|
All Other Compensation ($) |
|
Total ($) |
|
Andrea Biffi |
|
|
|
|
|
13,360 |
|
|
|
|
|
|
|
13,360 |
|
Jane H. Hsiao, Ph.D., MBA |
|
|
|
|
|
13,360 |
|
|
|
|
|
|
|
13,360 |
|
William N. Kelley |
|
|
|
|
|
16,700 |
|
|
|
|
|
|
|
16,700 |
|
Aftab R. Kherani, M.D. |
|
|
|
|
|
13,360 |
|
|
|
|
|
|
|
13,360 |
|
Paul A. LaViolette |
|
|
|
|
|
20,040 |
|
|
|
|
|
|
|
20,040 |
|
David B. Milne |
|
|
|
|
|
13,360 |
|
|
|
|
|
|
|
13,360 |
|
Richard C. Pfenniger, Jr. |
|
|
|
|
|
16,700 |
|
|
|
|
|
|
|
16,700 |
|
William N. Starling |
|
|
|
|
|
16,700 |
|
|
|
|
|
|
|
16,700 |
|
(1) |
For all stock options in the table, the option values reflect the aggregate grant date fair value computed in accordance with FASB ASC Topic 718. Assumptions made in the calculation of these amounts are described in Note 14 to the Company’s audited financial statements, included in this Annual Report. |
Director Compensation Arrangements
On May 28, 2014, the Board approved a plan of compensation for its non-employee directors. Under the compensation plan, each new non-employee director receives a stock option grant to purchase 30,000 shares of Common Stock, vesting in equal installments on the first three anniversaries of the date of grant. In addition, each non-employee member of the Board receives an annual stock option grant to purchase 20,000 shares of Common Stock; the Chair of the Board receives an annual stock option grant to purchase an additional 10,000 shares of Common Stock; and the Chair of each of the Audit, Compensation and Corporate Governance and Nominating Committee receives an annual additional stock option grant to purchase 5,000 shares of Common Stock. The annual stock option grants vest quarterly over one year. The term of each stock option is ten years and all such stock options are awarded under, and subject to the provisions of, the Plan.
Compensation Committee Interlocks and Insider Participation
The current members of the Company’s Compensation Committee are Mr. Starling, Chair, Mr. LaViolette and Dr. Kherani. No member of the Compensation Committee is or has ever been an officer or employee of the Company. In addition, during the year ended December 31, 2016, none of our executive officers served as a member of the board of directors or the compensation committee of any other entity that has one or more executive officers serving on our Board of Directors or our Compensation Committee.
Report of the Compensation Committee
The Compensation Committee has reviewed and discussed the foregoing “Compensation Discussion and Analysis” with the Company’s management. Based on this review and discussion, the Compensation Committee has recommended to the Board of Directors that the “Compensation Discussion and Analysis” be included in the Company’s Annual Report on Form 10-K and in its proxy statement for the 2017 Annual Meeting of Stockholders.
William N. Starling, Chair
Paul A. LaViolette
Aftab R. Kherani, M.D.
93
|
ITEM 12. |
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS. |
The following table sets forth certain information concerning the beneficial ownership of Common Stock by: (i) each person known by us to be the beneficial owner of more than 5% of our outstanding Common Stock currently; (ii) each of our current directors (iii) each of our named executive officers; and (iv) all of our executive officers and directors as a group. Ownership information is set forth as of February 28, 2017. Unless otherwise noted, each of the following disclaims any beneficial ownership of the shares, except to the extent of his, her or its pecuniary interest, if any, in such shares. Unless otherwise indicated, the mailing address of each individual is c/o TransEnterix, Inc., 635 Davis Drive, Suite 300, Morrisville, NC 27560.
|
|
|
As of February 28, 2017 |
|
|||||
|
Name and Address of Beneficial Owner |
|
Number of Shares of |
|
|
Percentage of |
|
||
|
Directors and Executive Officers |
|
|
|
|
|
|
|
|
|
Paul LaViolette (3) |
|
7,634,958 |
|
|
|
|
6.2 |
% |
|
Andrea Biffi (4) |
|
115,000 |
|
|
|
|
* |
|
|
Jane H. Hsiao, Ph.D., MBA (5) |
|
4,965,230 |
|
|
|
|
4.0 |
% |
|
William N. Kelley, M.D. (6) |
|
78,750 |
|
|
|
|
* |
|
|
Aftab R. Kherani, M.D. (7) |
|
55,000 |
|
|
|
|
* |
|
|
David Milne (8) |
|
7,601,692 |
|
|
|
|
6.2 |
% |
|
Richard C. Pfenniger, Jr. (9) |
|
131,750 |
|
|
|
|
* |
|
|
Todd M. Pope (10) |
|
2,231,624 |
|
|
|
|
1.8 |
% |
|
William N. Starling (11) |
|
5,936,689 |
|
|
|
|
4.8 |
% |
|
Anthony Fernando (12) |
|
259,178 |
|
|
|
|
* |
|
|
Joseph P. Slattery (13) |
|
1,005,750 |
|
|
|
|
* |
|
|
All Executive Officers and Directors as a group (11 persons) (14) |
|
22,468,930 |
|
|
|
|
17.7 |
% |
|
5% or More Stockholders |
|
|
|
|
|
|
|
|
|
Sofar S.p.A. |
|
19,266,098 |
|
|
|
|
15.7 |
% |
|
Aisling Capital III, L.P. (15) |
|
8,335,819 |
|
|
|
|
6.8 |
% |
|
SV Life Sciences Fund (16) |
|
7,546,692 |
|
|
|
|
6.1 |
% |
* Holds less than 1%
|
(1) |
A person is deemed to be the beneficial owner of shares of Common Stock underlying options and warrants held by that person that are exercisable as of February 28, 2017 or that will become exercisable within 60 days thereafter. |
|
(2) |
Based on 122,723,238 shares of Common Stock outstanding as of February 28, 2017. Each beneficial owner’s percentage ownership is determined assuming that options and warrants that are held by such person (but not those held by any other person) and that are exercisable as of February 28, 2017, or that will become exercisable within 60 days thereafter, have been exercised into Common Stock. The additional shares resulting from such exercise are included in both the numerator and denominator for such beneficial owner for purposes of their calculation. |
|
(3) |
Includes 7,338,352 shares held by SV Life Sciences Fund IV, L.P. and 208,340 shares held by SV Life Sciences Fund IV Strategic Partners, L.P. Paul LaViolette is a partner of SVLSF IV, LLC and a control person of both SV Life Sciences Fund IV, L.P. and SV Life Sciences Fund IV Strategic Partners, L.P. Also includes options to purchase 88,266 shares of Common Stock. |
|
(4) |
Includes 90,000 shares of Common Stock directly held by Mr. Biffi and options to purchase 25,000 shares of Common Stock. |
|
(5) |
Includes options to purchase 145,000 shares of Common Stock, and warrants to acquire 400,000 shares of Common Stock. Dr. Hsiao’s Common Stock holdings also include beneficial ownership of shares held by Hsu Gamma Investments, L.P. (“Hsu Gamma”), which holds 1,257,694 shares of Common Stock. Dr. Hsiao is the general partner of Hsu Gamma. Dr. Hsiao’s address is 4400 Biscayne Blvd, Miami, FL 33137. |
|
(6) |
Includes 20,000 shares of Common Stock held by Dr. Kelley and options to purchase 58,750 shares of Common Stock. |
|
(7) |
Consists of options to purchase 55,000 shares of Common Stock. |
|
(8) |
Includes 7,338,352 shares held by SV Life Sciences Fund IV, L.P. and 208,340 shares held by SV Life Sciences Fund IV Strategic Partners, L.P. David Milne is a managing partner of SVLSF IV, LLC and a control person of both SV Life Sciences Fund IV, L.P. and SV Life Sciences Fund IV Strategic Partners, L.P. Also includes options to purchase 55,000 shares of Common Stock. |
|
(9) |
Includes 48,000 shares of Common Stock held by Mr. Pfenniger and options to purchase 83,750 shares of Common Stock. |
|
(10) |
Consists of 217,309 shares of Common Stock held by Mr. Pope and options to purchase 2,014,315 shares of Common Stock. |
|
(11) |
Includes 5,318,969 shares of Common Stock held by Synergy Life Science Partners, L.P., and 392,122 shares of Common Stock held by Synecor, L.L.C. William N. Starling is a managing director of Synergy Life Science Partners, L.P. and the chief executive officer of Synecor, L.L.C. Based on information made available to the Company, William N. Starling, Richard S. Stack and Mudit K. Jain share voting and investment control over the shares of Common Stock held by such entities. Also includes 135,223 shares held by W. Starling and D. Starling, Trustees of the Starling Family Trust, UDT August 15, 1990. Further includes options to purchase 90,375 shares of Common Stock. |
|
(12) |
Includes 42,095 shares of Common Stock held by Mr. Fernando and options to purchase 217,083 shares of Common Stock. |
94
|
(13) |
Includes 244,365 shares of common stock directly held and jointly owned by Mr. Slattery and his spouse, 25,000 shares of common stock held in the Joseph Slattery IRA, and options to purchase 736,385 shares of Common Stock. |
|
(14) |
Includes options to purchase 3,550,174 shares of Common Stock and warrants to purchase 400,000 shares of Common Stock. |
|
(15) |
The address of Aisling Capital III, LP is 888 Seventh Avenue, 30th Floor, New York, NY 10106. Based on information made available to the Company and on the Schedule 13D filings made by Aisling Capital III, LP, Steve Elms, Dennis Purcell and Andrew Schiff share voting and investment control over the shares of Common Stock held by Aisling Capital III, LP. |
|
(16) |
Consists of 7,338,352 shares held by SV Life Sciences Fund IV, L.P. and 208,340 shares held by SV Life Sciences Fund IV Strategic Partners, L.P. The address of each of SV Life Sciences Fund IV, L.P., SV Life Sciences Fund IV Strategic Partners, L.P. and SVLSF IV, LLC, their control person, is One Boston Place Suite 3900, 201 Washington Street, Boston, MA 02108. Based on information made available to the Company and on the Schedule 13G filings made by SV Life Sciences Fund IV, L.P. and SV Life Sciences Fund IV Strategic Partners, L.P., David Milne shares voting and investment control over the shares of Common Stock owned by such entities. |
The Company is not aware of any arrangements with any of the foregoing stockholders or any other stockholder of the Company that may result in a change in control of the Company.
Securities Authorized for Issuance Under Equity Compensation Plans
Please see the disclosure in Item 5 of this Annual Report regarding the securities authorized for issuance under the Company’s equity compensation plan.
Certain Relationships and Related Transactions
TransEnterix Italia, S.r.l., our wholly owned Italian subsidiary, is party to a services agreement for receipt of administrative services from Sofar and payment of rent to Sofar, a stockholder that owned approximately 13% and 16% of the Company’s common stock at December 31, 2016 and 2015, respectively. Expenses under this agreement were approximately $232,000 and $89,000 for the years ended December 31, 2016 and 2015, respectively. The services agreement was terminated in August 2016.
In November 2016, the Company agreed to enter into a technology and patents purchase agreement with Sofar to acquire from Sofar certain technology and intellectual property rights related to the Senhance Acquisition, and formerly licensed by the Company. The acquisition price was €375,000.
Review and Approval of Transactions with Related Persons
In accordance with our Code of Business Conduct and Ethics, and Audit Committee procedures, the Audit Committee of our Board reviews and approves all transactions that are required to be reported under Item 404(a) of Regulation S-K, including each transaction described above. In order to approve a related person transaction, the Audit Committee requires that (i) such transactions be fair and reasonable to us at the time it is authorized by the Audit Committee and (ii) such transaction must be authorized, approved or ratified by the affirmative vote of a majority of the members of the Audit Committee who have no interest, either directly or indirectly, in any such related person transaction.
Board of Directors
The Board, in the exercise of its reasonable business judgment, has determined that each of our current directors qualify as independent directors pursuant to the applicable NYSE MKT and SEC rules and regulations, except Mr. Pope, who is currently employed as our President and Chief Executive Officer, and Mr. Biffi, who is currently employed as the Chief Executive Officer and member of the Board of Directors, of Sofar S.p.A., an affiliate of TransEnterix owning approximately 16% of the Company’s outstanding Common Stock.
Audit Committee
The current members of the Company’s Audit Committee are Mr. Pfenniger, Chair, Mr. Milne and Dr. Kherani. Due to each member’s extensive experience in serving operating companies in both managerial and director capacities, the Board determined that each member has the requisite knowledge of financial statements and general understanding of financial and reporting matters to allow each such member to serve on the Audit Committee. The Audit Committee Charter is available on our website at www.transenterix.com.
The Board, in the exercise of its reasonable business judgment and utilizing the general standards it applies for determining the independence of directors, has determined that each of the current and incoming Audit Committee members qualifies as independent pursuant to NYSE MKT Rule 803.
95
The Board has determined that Mr. Pfenniger is an audit committee financial expert as defined in Item 407(d)(5)(ii) of Regulation S-K. The Board made this determination based on Mr. Pfenniger’s extensive career and background serving as an accountant and auditor as well as his serving various operating companies in both managerial and director capacities.
Corporate Governance and Nominating Committee
The current members of the Company’s Nominating Committee are Dr. Kelley, Chair, Mr. LaViolette and Mr. Milne. Due to each member’s extensive experience in serving operating companies in both managerial and director capacities, the Board determined that each member has the requisite knowledge and skills to allow each such member to serve on the Nominating Committee, and qualifies as independent pursuant to NYSE MKT Rule 803. The Corporate Governance and Nominating Committee charter is available on our web site at www.transenterix.com.
Compensation Committee
The current members of the Company’s Compensation Committee are Mr. Starling, Chair, Mr. LaViolette and Dr. Kherani. Due to each member’s extensive experience in serving operating companies in both managerial and director capacities, the Board determined that each member has the requisite knowledge and skills to allow each such member to serve on the Compensation Committee. The Compensation Committee Charter is available on our website at www.transenterix.com.
The Board, in the exercise of its reasonable business judgment and utilizing the general standards it applies for determining the independence of directors, has determined that each of the Compensation Committee members qualifies as independent pursuant to NYSE MKT Rule 803.
Audit Fees
BDO has served as the independent registered public accounting firm of the Company since 2012. The following table sets forth the fees billed to the Company by BDO and BDO S.p.A. for its audits of the Company’s consolidated annual financial statements and other services for the years ended December 31, 2016 and 2015.
|
|
|
2016 |
|
|
2015 |
|
||
|
Audit Fees |
|
$ |
429,536 |
|
|
$ |
341,000 |
|
|
Audit Related Fees |
|
$ |
— |
|
|
$ |
99,000 |
|
|
Tax Fees |
|
$ |
— |
|
|
|
— |
|
|
All Other Fees |
|
$ |
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
Total Fees |
|
$ |
429,536 |
|
|
$ |
440,000 |
|
Audit Fees. This category includes fees billed by BDO USA, LLP and BDO S.p.A. in 2015 and 2016 for professional services for the audit of our annual financial statements, review of financial statements included in our quarterly reports on Form 10-Q, and services that are normally provided by the independent auditor in connection with statutory and regulatory filings or engagements for the relevant fiscal years.
Audit-Related Fees. This category includes fees billed in the fiscal years shown for assurance and related services that are reasonably related to the performance of the audit or review of our financial statements and are not reported under the category “Audit Fees.” The “Audit-Related Fees” during 2015 pertained to services provided by BDO S.p.A. in relation to the carve-out audits of the Senhance System (a carve-out of Sofar S.p.A.).
Tax Fees. This category includes fees billed in the fiscal years shown for professional services for tax compliance, tax advice, and tax planning.
All Other Fees. This category includes fees billed in the fiscal years shown for products and services provided by the principal accountant that are not reported in any other category.
Pre-Approval Policies and Procedures
Our Audit Committee has a policy in place that requires its review and pre-approval of all audit and permissible non-audit services provided by our independent auditors. The services requiring pre-approval by the audit committee may include audit services, audit-related services, tax services and other services. The pre-approval requirement is waived with respect to the provision of non-audit services if (i) the aggregate amount of all such non-audit services provided to us constitutes not more than 5% of the total amount of revenues paid by us to our independent auditors during the fiscal year in which such non-audit services were provided, (ii) such
96
services were not recognized at the time of the engagement to be non-audit services, and (iii) such services are promptly brought to the attention of the Audit Committee or by one or more of its members to whom authority to grant such approvals has been delegated by the Audit Committee. All audit-related services, tax services and all other services provided by BDO are pre-approved by the Audit Committee. The Audit Committee has considered and determined that the provision of all non-audit services set forth in the table above is compatible with maintaining BDO’s independence.
97
|
|
(a) |
(1) The following consolidated financial statements are filed as a part of this Annual Report: |
|
|
|
Page |
|
Consolidated Financial Statements : |
|
|
|
|
|
|
|
|
45 |
|
|
Consolidated Balance Sheets as of December 31, 2016 and 2015 |
|
47 |
|
|
48 |
|
|
|
49 |
|
|
|
50 |
(2) Consolidated Financial Statement Schedules: The information required by this item is included in the consolidated financial statements contained in Item 8 of this Annual Report.
(3) Exhibits: The following exhibits are filed as part of, or incorporated by reference into, this Annual Report.
|
Exhibit No. |
|
Description |
|
|
|
|
|
1.1 |
|
Controlled Equity Offering SM Sales Agreement by and between TransEnterix, Inc. and Cantor Fitzgerald & Co. dated February 20, 2015 (filed as Exhibit 10.1 to our Current Report on Form 8-K, filed with the SEC on February 20, 2015 and incorporated by reference herein). |
|
|
|
|
|
1.2 |
|
Underwriting Agreement by and among TransEnterix, Inc. and Stifel Nicolaus & Company, Incorporated and RBC Capital Markets, LLC dated June 11, 2015 (filed as Exhibit 1.1 to our Current Report on Form 8-K, filed with the SEC on June 12, 2015 and incorporated by reference herein). |
|
|
|
|
|
1.3 |
|
Controlled Equity OfferingSM Sales Agreement by and between TransEnterix, Inc. and Cantor Fitzgerald & Co. dated February 9, 2016 (filed as Exhibit 1.1 to the Company’s Current Report on Form 8-K, filed with the SEC on February 9, 2016, and incorporated by reference herein). |
|
|
|
|
|
2.1 |
|
Membership Interest Purchase Agreement, dated September 18, 2015, by and among Sofar S.p.A., Vulcanos S.r.l., the Company and TransEnterix International, Inc. filed as Exhibit 2.1 to our Current Report on Form 8-K, filed with the SEC on September 21, 2015 and incorporated by reference herein). |
|
|
|
|
|
2.1(a) |
|
Amendment to Membership Interest Purchase Agreement by and among TransEnterix, Inc., TransEnterix International, Inc., and Sofar, S.p.A., dated December 30, 2016 (filed as Exhibit 10.1 to the Company's Current Report on Form 8-K, filed with the SEC on January 5, 2017 and incorporated by reference herein). |
|
|
|
|
|
3.1 |
|
Amended and Restated Certificate of Incorporation of TransEnterix, Inc. (filed as Exhibit 3.1 to our Current Report on Form 8-K, filed with the SEC on December 9, 2013 and incorporated by reference herein). |
|
|
|
|
|
3.1.1 |
|
Certificate of Amendment to the Amended and Restated Certificate of Incorporation of TransEnterix, Inc. (filed as Exhibit 3.1 to our Current Report on Form 8-K filed with the SEC on April 1, 2014 and incorporated herein by reference). |
|
|
|
|
|
3.2 |
|
Amended and Restated Bylaws of TransEnterix, Inc. (filed as Exhibit 3.2 to our Current Report on Form 8-K, filed with the SEC on December 9, 2013 and incorporated by reference herein). |
|
|
|
|
|
4.1 |
|
Specimen Certificate for Common Stock of TransEnterix, Inc. (filed as Exhibit 4.1 to the Registrant’s Registration Statement on Form S-3, File No. 333-193235, filed with the SEC on January 8, 2014 and incorporated by reference herein). |
|
|
|
|
|
4.2 |
|
Form of Warrant to Purchase Common Stock for warrants issued to Oxford Finance LLC and Silicon Valley Bank (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K, filed September 30, 2014). |
|
|
|
|
|
4.3 |
|
Form of Common Stock Warrant (filed as Exhibit 4.1 to our Current Report on Form 8-K filed on September 10, 2007 and incorporated by reference herein). |
|
|
|
|
|
4.4 |
|
Form of Common Stock Warrant dated March 22, 2013 (filed as part of Exhibit 10.1 to our Current Report on Form 8-K filed on March 26, 2013 and incorporated by reference herein). |
98
|
Exhibit No. |
|
Description |
|
|
|
|
|
10.1 |
|
Purchase Agreement by and between TransEnterix, Inc. and Lincoln Park Capital, LLC dated December 16, 2016 (filed as exhibit 10.1 to the Company's Current Report on Form 8-K, filed with the SEC on December 20, 2016 and incorporated by reference herein). |
|
|
|
|
|
10. 2++ |
|
License Contract between the European Union and Vulcanos s.r.l. (now known as TransEnterix Italia S.r.l.), dated September 18, 2015 (filed as Exhibit 10.5 to our Quarterly Report on Form 10-Q, filed with the SEC on November 9, 2015 and incorporated by reference herein). |
|
|
|
|
|
10.3 |
|
Registration Rights Agreement, dated September 21, 2015, by and between the Company and Sofar S.p.A. (filed as Exhibit 10.3 to our Current Report on Form 8-K, filed with the SEC on September 21, 2015 and incorporated by reference herein). |
|
|
|
|
|
10.4 |
|
Lock-Up Agreement, dated September 21, 2015, by and between the Company and Sofar S.p.A. (filed as Exhibit 10.4 to our Current Report on Form 8-K, filed with the SEC on September 21, 2015 and incorporated by reference herein). |
|
|
|
|
|
10.5 + |
|
Employment Agreement, dated as of February 3, 2015, by and between the Registrant and Todd M. Pope (filed as Exhibit 10.1 to our Current Report on Form 8-K, filed with the SEC on February 6, 2015, and incorporated by reference herein). |
|
|
|
|
|
10.6 + |
|
Offer letter, dated September 12, 2013, by and between the Registrant and Joseph P. Slattery (filed as Exhibit 10.1 to our Current Report on Form 8-K, filed with the SEC on September 23, 2013 and incorporated by reference herein). |
|
|
|
|
|
10.7 + |
|
Employment Agreement, dated as of August 14, 2015, by and between the Registrant and Anthony Fernando (filed as Exhibit 10.12 to our Annual Report on Form 10-K for the year ended December 31, 2015, filed March 3, 2016 and incorporated herein by reference). |
|
|
|
|
|
10.8 + |
|
TransEnterix, Inc. Amended and Restated Incentive Compensation Plan, effective as of May 7, 2015 (filed as Exhibit 10.1 to the Registrant’s Registration Statement on Form S-8, File No. 333-203950, filed with the SEC on May 7, 2015 and incorporated by reference herein). |
|
|
|
|
|
10.8.1 + |
|
Exhibit A to the TransEnterix, Inc. Amended and Restated Incentive Compensation Plan (French Sub-Plan) (filed as Exhibit 10.13.1 to our Annual Report on Form 10-K for the year ended December 31, 2015, filed March 3, 2016 and incorporated herein by reference). |
|
|
|
|
|
10.8.2+ |
|
Amendment to the TransEnterix, Inc. Amended and Restated Incentive Compensation Plan, dated June 8, 2016 (filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K, filed with the SEC on June 10, 2016 and incorporated herein by reference). |
|
|
|
|
|
10.9 + |
|
Form of Employee Stock Option Agreement pursuant to the Plan (filed as Exhibit 10.15 to our Annual Report on Form 10-K for the year ended December 31, 2013, filed March 5, 2014 and incorporated by reference herein). |
|
|
|
|
|
10.10 + |
|
Form of Employee Stock Option Agreement (performance stock options) pursuant to the Plan (filed as Exhibit 10.16 to our Annual Report on Form 10-K for the year ended December 31, 2013, filed March 5, 2014 and incorporated herein by reference). |
|
|
|
|
|
10.11 + |
|
Form of Non-Employee Stock Option Agreement pursuant to the Plan (filed as Exhibit 10.17 to our Annual Report on Form 10-K for the year ended December 31, 2013, filed March 5, 2014 and incorporated herein by reference). |
|
|
|
|
|
10.12 + |
|
Form of Restricted Stock Unit Agreement pursuant to the Plan (filed as Exhibit 10.18 to our Annual Report on Form 10-K for the year ended December 31, 2013, filed March 5, 2014 and incorporated herein by reference). |
|
|
|
|
|
10.13 + |
|
Restricted Stock Unit Agreement, dated as of October 2, 2013, by and between the Company and Joseph P. Slattery (filed as Exhibit 10.19 to our Annual Report on Form 10-K for the year ended December 31, 2013, filed March 5, 2014 and incorporated herein by reference). |
|
|
|
|
|
10.14 |
|
Amended and Restated Loan and Security Agreement, dated September 26, 2014, among the Borrowers and the Lenders and Collateral Agent (filed as Exhibit 10.1 to our Current Report on Form 8-K filed with the SEC on September 30, 2014 and incorporated by reference herein) |
|
|
|
|
|
10.14.1 |
|
First Amendment to Amended and Restated Loan and Security Agreement, dated August 14, 2015, by and among TransEnterix, Inc., TransEnterix Surgical, Inc. and SafeStitch LLC, as Borrower, and Oxford Finance LLC, as Lender and Collateral Agent, and Silicon Valley Bank, as Lender (filed as Exhibit 10.1 to our Current Report on Form 8-K filed with the SEC on August 17, 2015 and incorporated by reference herein). |
99
|
Exhibit No. |
|
Description |
|
|
|
|
|
10.14.2 |
|
Consent and Second Amendment to Amended and Restated Loan Agreement, dated September 18, 2015, by and among the Company, its subsidiaries TransEnterix Surgical, Inc. and SafeStitch LLC (collectively, the “Borrowers”), and SVB, as Lender, and Oxford, as Lender and Collateral Agent (filed as Exhibit 10.1 to our Current Report on Form 8-K filed with the SEC on September 21, 2015 and incorporated by reference herein). |
|
|
|
|
|
10.14.3 |
|
Third Amendment to Amended and Restated Loan and Security Agreement, dated November 13, 2015, by and among TransEnterix, Inc., TransEnterix Surgical, Inc. and SafeStitch LLC, as Borrower, and Oxford Finance LLC, as Lender and Collateral Agent, and Silicon Valley Bank, as Lender (filed as Exhibit 10.1 to our Current Report on Form 8-K filed with the SEC on November 16, 2015 and incorporated by reference herein). |
|
|
|
|
|
10.14.4 |
|
Consent and Fourth Amendment to Amended and Restated Loan and Security Agreement, dated April 19, 2016, by and among TransEnterix, Inc., TransEnterix Surgical, Inc., SafeStitch LLC and TransEnterix International, Inc., Oxford Finance LLC, and Silicon Valley Bank (filed as Exhibit 10.2 to our Quarterly Report on Form 10-Q for the quarter ended March 31, 2016, filed with the SEC on May 10, 2016 and incorporated by reference herein). |
|
|
|
|
|
10.14.5 |
|
Fifth Amendment to Amended and Restated Loan and Security Agreement, dated September 7, 2016, by and among TransEnterix, Inc., TransEnterix Surgical, Inc., SafeStitch LLC and TransEnterix International, Inc., Oxford Finance LLC, and Silicon Valley Bank (filed as Exhibit 10.1 to the Company's Current Report on Form 10-K, filed with the SEC on September 8, 2016 and incorporated by reference herein). |
|
|
|
|
|
10.15 |
|
Lease Agreement, dated as of December 11, 2009, by and between TransEnterix, Inc. and GRE Keystone Technology Park Three LLC (filed as Exhibit 10.25 to Amendment No. 2 to our Annual Report on Form 10-K for the year ended December 31, 2013, filed with the SEC on March 31, 2014 and incorporated herein by reference). |
|
|
|
|
|
10.15.1 |
|
Lease Modification Agreement No. 1, dated as of May 4, 2010, by and between TransEnterix, Inc. and GRE Keystone Technology Park Three LLC (filed as Exhibit 10.25.1 to Amendment No. 2 to our Annual Report on Form 10-K for the year ended December 31, 2013, filed with the SEC on March 31, 2014 and incorporated herein by reference). |
|
|
|
|
|
14.1 |
|
Code of Ethics Pursuant to Section 406 of the Sarbanes-Oxley Act of 2002 (incorporated by reference to the Registrant’s website – see Item 1. “BUSINESS – Available Information.”) |
|
|
|
|
|
21.1 * |
|
Subsidiaries of the Registrant. |
|
|
|
|
|
23.1 * |
|
Consent of BDO USA, LLP. |
|
|
|
|
|
31.1 * |
|
Certification of Chief Executive Officer pursuant to Rule 13a-14(a)/15d-14(a). |
|
|
|
|
|
31.2 * |
|
Certification of Chief Financial Officer pursuant to Rule 13a-14(a)/15d-14(a). |
|
|
|
|
|
32.1 * |
|
Certification of Chief Executive Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
|
|
|
|
|
32.2 * |
|
Certification of Chief Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
|
|
|
|
|
101.INS * |
|
XBRL Instance Document. |
|
|
|
|
|
101.SCH * |
|
XBRL Taxonomy Extension Schema Document. |
|
|
|
|
|
101.CAL * |
|
XBRL Taxonomy Extension Calculation Linkbase Document. |
|
|
|
|
|
101.DEF * |
|
XBRL Taxonomy Extension Definition Linkbase Document. |
|
|
|
|
|
101.LAB * |
|
XBRL Taxonomy Extension Label Linkbase Document. |
|
|
|
|
|
101.PRE * |
|
XBRL Taxonomy Extension Presentation Linkbase Document. |
|
|
|
|
|
+ |
A management contract, compensatory plan or arrangement required to be separately identified. |
|
++ |
Confidential treatment has been granted for certain portions of the agreement pursuant to a confidential treatment request filed with the Commission on November 9, 2015. Such provisions have been filed separately with the Commission. |
|
* |
Filed herewith. |
Registrants may voluntarily include a summary of information required by Form 10-K under this Item 16. The Company has elected not to include such summary information.
100
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
Date: March 6, 2017 |
|
TransEnterix, Inc. |
||
|
|
|
|
|
|
|
|
|
By: |
|
/s/ Todd M. Pope |
|
|
|
|
|
Todd M. Pope |
|
|
|
|
|
President, Chief Executive Officer |
|
|
|
|
|
and a Director |
|
|
|
|
|
(principal executive officer) |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed by the following persons on behalf of the Registrant in the capacities and on the dates indicated.
|
Signature |
|
Title(s) |
|
Date |
|
|
|
|
||
|
/s/ Todd M. Pope |
|
President, Chief Executive Officer and a Director (principal executive officer) |
|
March 6, 2017 |
|
Todd M. Pope |
|
|
|
|
|
|
|
|
||
|
/s/ Joseph P. Slattery |
|
Executive Vice President and Chief Financial Officer (principal financial officer and principal accounting officer) |
|
March 6, 2017 |
|
Joseph P. Slattery |
|
|
|
|
|
|
|
|
|
|
|
/s/ Paul A. LaViolette |
|
Chairman of the Board and a Director |
|
March 6, 2017 |
|
Paul A. LaViolette |
|
|
|
|
|
|
|
|
||
|
/s/ Andrea Biffi |
|
Director |
|
March 6, 2017 |
|
Andrea Biffi |
|
|
|
|
|
|
|
|
||
|
/s/ Jane H. Hsaio |
|
Director |
|
March 6, 2017 |
|
Jane H. Hsaio, Ph.D. |
|
|
|
|
|
|
|
|
||
|
/s/ William N. Kelley |
|
Director |
|
March 6, 2017 |
|
William N. Kelley, M.D. |
|
|
|
|
|
|
|
|
||
|
/s/ Aftab R. Kherani |
|
Director |
|
March 6, 2017 |
|
Aftab R. Kherani |
|
|
|
|
|
|
|
|
||
|
/s/ David B. Milne |
|
Director |
|
March 6, 2017 |
|
David B. Milne |
|
|
|
|
|
|
|
|
||
|
/s/ Richard C. Pfenniger, Jr. |
|
Director |
|
March 6, 2017 |
|
Richard C. Pfenniger, Jr. |
|
|
|
|
|
|
|
|
||
|
/s/ William N. Starling, Jr. |
|
Director |
|
March 6, 2017 |
|
William N. Starling, Jr. |
|
|
|
|
