Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Ignyta, Inc. | d933944d8k.htm |
| EX-99.2 - EXHIBIT 99.2 - Ignyta, Inc. | d933944dex992.htm |
| EX-99.4 - EXHIBIT 99.4 - Ignyta, Inc. | d933944dex994.htm |
| EX-99.1 - EXHIBIT 99.1 - Ignyta, Inc. | d933944dex991.htm |
 Entrectinib
(formerly RXDX-101) is a potent, selective, small molecule inhibitor of the tyrosine kinases TrkA (coded
by the gene NTRK1), TrkB (coded by the gene NTRK2), TrkC (coded by the gene
NTRK3), ROS1 (coded by the gene ROS1), and
ALK
(coded
by
the
gene
ALK).
Molecular
alterations
to
these
targets
are
present
in
several
different
tumor types, including non-small cell lung cancer (NSCLC), colorectal cancer (CRC),
papillary thyroid cancer, pancreatic cancer, and neuroblastoma, among others.
Potent in vitro and in vivo antitumor effects have been observed in
several TRK, ROS1 and ALK-dependent tumor models. Antitumor effects in the
preclinical models were associated with apoptosis and downstream signaling effects,
leading to MAPK and AKT modulation. In the First-In-Human Study
ALKA-372-001, entrectinib demonstrated early clinical activity (1 CR, 5 PRs among 25
patients) when given intermittently under fasting conditions (ASCO 2014; updated data
abstract 2517). Here we report on the ongoing Phase 1 portion of a companion
Phase 1/2a study (STARTRK-1) where entrectinib was administered on a continuous
daily dosing regimen under fed conditions. STARTRK-1: Phase
1/2a Study of Entrectinib, an Oral Pan-Trk, ROS1, and ALK Inhibitor,
in Patients with Advanced Solid Tumors with Relevant Molecular
Alterations Manish
R.
Patel
1
,
Todd
M.
Bauer²,
Stephen
V.
Liu³,
Alexander
Drilon
4
,
Jennifer
Wheler
5
,
Alice
Shaw
6
,
Anna
Farago
6
,
Sai-Hong
I.
Ou
7
,
David
Luo
8
,
Litain
Yeh
8
,
Zachary
Hornby
8
,
Adrian
Senderowicz
8
,
and
Jonathan
E.
Lim
8
1
Sarah
Cannon
Research
Institute/Florida
Cancer
Specialists,
Sarasota,
FL;
2
Sarah
Cannon
Research
Institute/Tennessee
Oncology,
PLLC,
Nashville,
TN;
3
Georgetown
Lombardi
Comprehensive
Cancer
Center,
Washington,
D.C.;
4
Memorial
Sloan
Kettering
Cancer
Center,
New
York,
NY;
5
The
University
of
Texas
MD
Anderson
Cancer
Center,
Houston,
TX;
6
Massachusetts
General
Hospital,
Boston,
MA;
7
Chao
Family
Comprehensive
Cancer
Center,
University
of
California,
Irvine;
8
Ignyta,
Inc.,
San Diego, CA
Abstract
#2596
–
Presented
at
the
American
Society
of
Clinical
Oncology
(ASCO)
Annual
Meeting,
May
29
–
June
2,
2015,
Chicago,
IL.
Thank
you
to
all
the
patients
and
their
families
who
participated
in
this
study.
Background
Exhibit 99.3 |
 STARTRK-1: Phase 1/2a Study of Entrectinib, an Oral Pan-Trk, ROS1,
and ALK Inhibitor, in Patients with Advanced Solid Tumors with
Relevant Molecular Alterations Manish
R.
Patel
1
,
Todd
M.
Bauer²,
Stephen
V.
Liu³,
Alexander
Drilon
4
,
Jennifer
Wheler
5
,
Alice
Shaw
6
,
Anna
Farago
6
,
Sai-Hong
I.
Ou
7
,
David
Luo
8
,
Litain
Yeh
8
,
Zachary
Hornby
8
,
Adrian
Senderowicz
8
,
and
Jonathan
E.
Lim
8
1
Sarah
Cannon
Research
Institute/Florida
Cancer
Specialists,
Sarasota,
FL;
2
Sarah
Cannon
Research
Institute/Tennessee
Oncology,
PLLC,
Nashville,
TN;
3
Georgetown
Lombardi
Comprehensive
Cancer
Center,
Washington,
D.C.;
4
Memorial
Sloan
Kettering
Cancer
Center,
New
York,
NY;
5
The
University
of
Texas
MD
Anderson
Cancer
Center,
Houston,
TX;
6
Massachusetts
General
Hospital,
Boston,
MA;
7
Chao
Family
Comprehensive
Cancer
Center,
University
of
California,
Irvine;
8
Ignyta,
Inc.,
San Diego, CA
Abstract
#2596
–
Presented
at
the
American
Society
of
Clinical
Oncology
(ASCO)
Annual
Meeting,
May
29
–
June
2,
2015,
Chicago,
IL.
Thank
you
to
all
the
patients
and
their
families
who
participated
in
this
study.
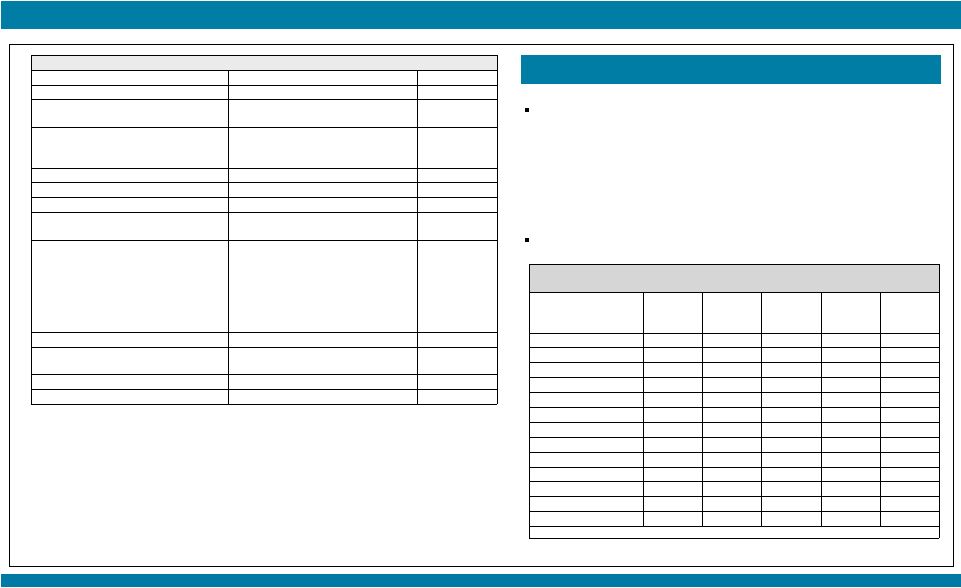
Patient Population:
Locally advanced or metastatic solid tumors with NTRK1/2/3, ROS1 or ALK molecular alterations,
for whom no alternative effective standard therapy is available or for whom standard
therapy is considered unsuitable or intolerable. Patients were assigned to escalating
doses of entrectinib until determination of the maximum tolerated dose (MTD) and/or
recommended Phase 2 dose (RP2D) using standard 3+3 criteria.
As of 01 May 2015, 29 patients were enrolled across 4 dose levels.
Table 1. Patient Disposition and Baseline Characteristics, n (%)
100 mg/m²
200 mg/m²
400 mg/m²
800 mg
TOTAL
Enrolled
5
5
10
9
29
Discontinued
5
5
7 (70)
4 (44)
21 (72)
Primary reason for discontinuation
Disease Progression
Adverse Event
Consent Withdrawn
4
0
1
4
1
0
6
0
1
3
0
1
17 (59)
1 (3)
3 (10)
Age, years, median (range)
68 (28-73)
63 (45-70)
50 (42-66)
54 (38-76)
55 (28-76)
Sex, male/female (%)
100/0
20/80
30/70
11/89
34/66
ECOG performance status
0
1
2
2 (40)
3 (60)
0
2 (40)
3 (60)
0
2 (20)
5 (50)
3 (30)
2 (22)
7 (78)
0
8 (28)
18 (62)
3 (10)
Cohort
Total Dose
(QD)
1
100 mg/m²
2
200 mg/m²
3
400 mg/m²
4
800 mg
The MTD and/or RP2D was defined as the dose with
1 out of 6 patients with DLT (Dose Limiting Toxicity)
DLTs were evaluated during Cycle 1 and graded according to the NCI CTCAE v4.03
Phase 1 Trial Design
Controlled asymptomatic CNS involvement was allowed, with steroids at a stable or decreasing
dose for at least 2 weeks prior to entrectinib treatment
ECOG performance status
2 |
 STARTRK-1: Phase 1/2a Study of Entrectinib, an Oral Pan-Trk, ROS1,
and ALK Inhibitor, in Patients with Advanced Solid Tumors with
Relevant Molecular Alterations Manish
R.
Patel
1
,
Todd
M.
Bauer²,
Stephen
V.
Liu³,
Alexander
Drilon
4
,
Jennifer
Wheler
5
,
Alice
Shaw
6
,
Anna
Farago
6
,
Sai-Hong
I.
Ou
7
,
David
Luo
8
,
Litain
Yeh
8
,
Zachary
Hornby
8
,
Adrian
Senderowicz
8
,
and
Jonathan
E.
Lim
8
1
Sarah
Cannon
Research
Institute/Florida
Cancer
Specialists,
Sarasota,
FL;
2
Sarah
Cannon
Research
Institute/Tennessee
Oncology,
PLLC,
Nashville,
TN;
3
Georgetown
Lombardi
Comprehensive
Cancer
Center,
Washington,
D.C.;
4
Memorial
Sloan
Kettering
Cancer
Center,
New
York,
NY;
5
The
University
of
Texas
MD
Anderson
Cancer
Center,
Houston,
TX;
6
Massachusetts
General
Hospital,
Boston,
MA;
7
Chao
Family
Comprehensive
Cancer
Center,
University
of
California,
Irvine;
8
Ignyta,
Inc.,
San Diego, CA
Abstract
#2596
–
Presented
at
the
American
Society
of
Clinical
Oncology
(ASCO)
Annual
Meeting,
May
29
–
June
2,
2015,
Chicago,
IL.
Thank
you
to
all
the
patients
and
their
families
who
participated
in
this
study.
Three treatment-related Grade 3 AEs were observed: neutropenia
(400 mg/m
2
, reported at Cycle 2 and resolved with dose reduction
to 200 mg/m
2
) and 2 DLTs at a fixed daily dose of 800 mg:
cognitive impairment and worsened fatigue; both resolved upon
study drug interruption. As such, the MTD was exceeded at 800
mg and 400 mg/m
2
per day was selected as the BSA-based RP2D
for both studies. Further exploration of a fixed daily dose regimen
is ongoing.
No treatment-related SAEs have been reported.
Table 3. Most Common (>10%), Treatment-Related, All Grades, Adverse
Events, n (%)
100
mg/m²
(n=5)
200
mg/m²
(n=5)
400
mg/m²
(n=9)
800 mg
(n=8)
TOTAL
(n=27
a
)
Fatigue
1 (20)
2 (40)
5 (56)
1 (13)
9 (33)
Dysgeusia
0
2 (40)
4 (44)
1 (13)
7 (26)
Constipation
1 (20)
2 (40)
2 (22)
1 (13)
6 (22)
Nausea
1 (20)
3 (60)
2 (22)
0
6 (22)
Paresthesia
1 (20)
0
4 (44)
1 (13)
6 (22)
Dizziness
0
1 (20)
3 (33)
1 (13)
5 (19)
Cognitive disorder
0
0
1 (11)
3 (38)
4 (15)
Vomiting
0
2 (40)
1 (11)
1 (13)
4 (15)
Arthralgia
0
0
2 (22)
1 (13)
3 (11)
Asthenia
0
0
2 (22)
1 (13)
3 (11)
Diarrhea
0
1 (20)
1 (11)
1 (13)
3 (11)
Attention disturbance
0
0
2 (22)
1 (13)
3 (11)
Peripheral neuropathy
0
1 (20)
1 (11)
1 (13)
3 (11)
a
total excluded 2 patients currently in Cycle 1 with no AE reporting
Table 2. Tumor Type and Molecular Alterations (n=29)
Primary Diagnosis
Molecular Alteration
N
Acinic cell carcinoma
NTRK3
fusion
1
Breast
NTRK3
ALK
SNP
SNP
1
1
CRC
NTRK1
NTRK3
ALK
SNP
SNP
SNP
2
2
1
Cholangiocarcinoma
NTRK3
SNP
1
Endometrial
ALK
fusion
1
Esthesioneuroblastoma
NTRK1
amplification
1
Large cell neuroendocrine
No NTRK1/2/3, ROS1, or ALK
alterations
1
NSCLC (70% prior ALK/ROS1 inhibitor)
NTRK1
NTRK1
NTRK3
ROS1
ROS1
ALK
ALK
amplification
fusion
SNP
amplification
fusion
fusion
SNP
1
1
1
1
2
6
1
Ovarian
ALK/NTRK
amplification
1
Pancreatic
No NTRK1/2/3, ROS1, or ALK
alterations
1
Renal cell carcinoma
NTRK2
SNP
1
Squamous cell carcinoma
ALK
amplification
1
RESULTS
Safety |
 STARTRK-1: Phase 1/2a Study of Entrectinib, an Oral Pan-Trk, ROS1,
and ALK Inhibitor, in Patients with Advanced Solid Tumors with
Relevant Molecular Alterations Manish
R.
Patel
1
,
Todd
M.
Bauer²,
Stephen
V.
Liu³,
Alexander
Drilon
4
,
Jennifer
Wheler
5
,
Alice
Shaw
6
,
Anna
Farago
6
,
Sai-Hong
I.
Ou
7
,
David
Luo
8
,
Litain
Yeh
8
,
Zachary
Hornby
8
,
Adrian
Senderowicz
8
,
and
Jonathan
E.
Lim
8
1
Sarah
Cannon
Research
Institute/Florida
Cancer
Specialists,
Sarasota,
FL;
2
Sarah
Cannon
Research
Institute/Tennessee
Oncology,
PLLC,
Nashville,
TN;
3
Georgetown
Lombardi
Comprehensive
Cancer
Center,
Washington,
D.C.;
4
Memorial
Sloan
Kettering
Cancer
Center,
New
York,
NY;
5
The
University
of
Texas
MD
Anderson
Cancer
Center,
Houston,
TX;
6
Massachusetts
General
Hospital,
Boston,
MA;
7
Chao
Family
Comprehensive
Cancer
Center,
University
of
California,
Irvine;
8
Ignyta,
Inc.,
San Diego, CA
Abstract
#2596
–
Presented
at
the
American
Society
of
Clinical
Oncology
(ASCO)
Annual
Meeting,
May
29
–
June
2,
2015,
Chicago,
IL.
Thank
you
to
all
the
patients
and
their
families
who
participated
in
this
study.
Exposures of entrectinib administered on a
continuous daily dosing regimen increased in a
dose proportional manner and reached steady-
state within a week of dosing
Plasma half-life is estimated to be ~ 20-24 hours,
compatible with QD dosing
At the RP2D (400 mg/m
2
QD), the plasma protein
binding corrected mean C
trough
is ~ 2.5X to 3X
that of concentrations observed in animal tumor
models with complete tumor growth inhibition
RESULTS
Pharmacokinetics
100
1000
10000
0
6
12
18
24
Time (hr)
100 mg/m
2
200 mg/m
2
400 mg/m
2
Target Conc. |
 STARTRK-1: Phase 1/2a Study of Entrectinib, an Oral Pan-Trk, ROS1,
and ALK Inhibitor, in Patients with Advanced Solid Tumors with
Relevant Molecular Alterations Manish
R.
Patel
1
,
Todd
M.
Bauer²,
Stephen
V.
Liu³,
Alexander
Drilon
4
,
Jennifer
Wheler
5
,
Alice
Shaw
6
,
Anna
Farago
6
,
Sai-Hong
I.
Ou
7
,
David
Luo
8
,
Litain
Yeh
8
,
Zachary
Hornby
8
,
Adrian
Senderowicz
8
,
and
Jonathan
E.
Lim
8
1
Sarah
Cannon
Research
Institute/Florida
Cancer
Specialists,
Sarasota,
FL;
2
Sarah
Cannon
Research
Institute/Tennessee
Oncology,
PLLC,
Nashville,
TN;
3
Georgetown
Lombardi
Comprehensive
Cancer
Center,
Washington,
D.C.;
4
Memorial
Sloan
Kettering
Cancer
Center,
New
York,
NY;
5
The
University
of
Texas
MD
Anderson
Cancer
Center,
Houston,
TX;
6
Massachusetts
General
Hospital,
Boston,
MA;
7
Chao
Family
Comprehensive
Cancer
Center,
University
of
California,
Irvine;
8
Ignyta,
Inc.,
San Diego, CA
Abstract
#2596
–
Presented
at
the
American
Society
of
Clinical
Oncology
(ASCO)
Annual
Meeting,
May
29
–
June
2,
2015,
Chicago,
IL.
Thank
you
to
all
the
patients
and
their
families
who
participated
in
this
study.
Patients who would have qualified for future
Phase 2 studies based on fusion, dose, and
treatment history; response as per RECIST
v1.1 and based upon local assessment.
*
10 responses among 11 patients
treated at or above the RP2D, leading
to a combined 91% response rate; 9
patients remain on study treatment
with durable responses for up to 16
cycles
Phase 2-eligible patients* (n=11)
Among the other 50 non-Phase 2 eligible patients (e.g., non-fusion alterations,
ALKi-
or ROS1i-resistant), 13 patients (26%) remain on study:
RESULTS
STARTRK-1 and ALKA-372-001 Studies (n=67) Show Preliminary Antitumor Activity
of Entrectinib in ALKi and ROS1i-Naïve Patients (n=17) with NTRK1/2/3,
ROS1, or ALK Fusions NTRK1/2/3 SNPs, IHC+, amplifications: n=15 (6 ongoing)
ROS1 fusions, ROS1i-resistant: n=3 (1 ongoing)
ROS1 amplifications, deletions: n=4
ALK fusions, ALKi-resistant: n=17 (4 ongoing)
ALK SNPs, amplifications, deletions: n=7 (2 ongoing; 1 patient with
neuroblastoma had a PR for 9 cycles and remains on study)
False positives: n=2
No alterations: n=2 |
 STARTRK-1: Phase 1/2a Study of Entrectinib, an Oral Pan-Trk, ROS1,
and ALK Inhibitor, in Patients with Advanced Solid Tumors with
Relevant Molecular Alterations Manish
R.
Patel
1
,
Todd
M.
Bauer²,
Stephen
V.
Liu³,
Alexander
Drilon
4
,
Jennifer
Wheler
5
,
Alice
Shaw
6
,
Anna
Farago
6
,
Sai-Hong
I.
Ou
7
,
David
Luo
8
,
Litain
Yeh
8
,
Zachary
Hornby
8
,
Adrian
Senderowicz
8
,
and
Jonathan
E.
Lim
8
1
Sarah
Cannon
Research
Institute/Florida
Cancer
Specialists,
Sarasota,
FL;
2
Sarah
Cannon
Research
Institute/Tennessee
Oncology,
PLLC,
Nashville,
TN;
3
Georgetown
Lombardi
Comprehensive
Cancer
Center,
Washington,
D.C.;
4
Memorial
Sloan
Kettering
Cancer
Center,
New
York,
NY;
5
The
University
of
Texas
MD
Anderson
Cancer
Center,
Houston,
TX;
6
Massachusetts
General
Hospital,
Boston,
MA;
7
Chao
Family
Comprehensive
Cancer
Center,
University
of
California,
Irvine;
8
Ignyta,
Inc.,
San Diego, CA
Abstract
#2596
–
Presented
at
the
American
Society
of
Clinical
Oncology
(ASCO)
Annual
Meeting,
May
29
–
June
2,
2015,
Chicago,
IL.
Thank
you
to
all
the
patients
and
their
families
who
participated
in
this
study.
Entrectinib was well tolerated in patients with relapsed or refractory
metastatic cancers harboring NTRK1/2/3, ROS1, or ALK molecular
alterations.
A
BSA-based
RP2D
has
been
defined
as
400
mg/m
2
QD,
which
provides exposure consistent with complete tumor inhibition in
animal tumor models.
Pharmacokinetic profile showed linear dose increases from 100 to
400 mg/m
2
.
Among
patients
with
ALK
inhibitor-
or
ROS1
inhibitor-naïve
NTRK1/2/3,
ROS1,
or
ALK
fusions,
10/11
(91%)
patients
treated
at or above the RP2D exhibited objective responses as early as
Cycle 1 with durable responses for up to 16 cycles.
These preliminary data support further development of entrectinib.
42 year-old female (MSKCC)
4 prior therapies, ECOG PS 1
Acinic cell carcinoma with NTRK3 fusion
entrectinib 400 mg/m²
QD
46 year-old male (MGH)
4 prior therapies, ECOG PS 2
NSCLC with NTRK1 fusion
entrectinib 400 mg/m²
QD
Jan 2015
Mar 2015
Mar 2015
Apr 2015
Copies of this poster obtained through QRC are for personal
use and may not be reproduced without permission from ASCO
and the author of this poster.
RESULTS
Conclusions
Exploration of a fixed daily dose regimen is ongoing. |
