Attached files
| file | filename |
|---|---|
| 8-K - 8-K IR MEETING - KUBOTA PHARMACEUTICAL HOLDINGS CO LTD | acucelairmeeting8-k052015.htm |

Acucela is a clinical-stage biotechnology company that specializes in discovering and developing novel therapeutics to treat and slow the progression of sight-threatening ophthalmic diseases affecting millions of individuals worldwide. Acucela Inc. IR Meeting Tokyo May 20, 2015

DISCLAIMER This presentation contains forward-looking statements concerning our product development, our technology, our competitors, our intellectual property, our financial condition and our plans for research and development programs that involve risks, uncertainties and assumptions. These statements are based on the current estimates and assumptions of the management of Acucela as of the date of this presentation and are subject to uncertainty and changes in circumstances. Given these uncertainties, you should not place undue reliance upon these forward-looking statements. Such forward-looking statements are subject to risks, uncertainties, assumptions and other factors that may cause the actual results of Acucela to be materially different from those reflected in such forward-looking statements. Important factors that could cause actual results to differ materially from those indicated by such forward- looking statements include, among others, those set forth in our reports on file with the Tokyo Securities Exchange and the United States Securities and Exchange Commission. The Company does not undertake any obligation to release publicly any revisions to these forward-looking statements to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated events. All statements contained in this presentation are made only as of the date of this presentation. May IR Meeting [ticker: 4589] 2

Key areas of strength May IR Meeting [ticker: 4589] 3 An ophthalmology-focused, science-driven biotechnology company People and Strategy • Executive leadership with experience in health care management, life science administration and technology • Broad-skilled employee base in research, development and operations • Strategic plan to develop an innovative portfolio of ophthalmology products Partnership • Long-time partnership with Otsuka Pharmaceutical • Potential high-reward alliance • Acucela has rights for lead investigational candidate (emixustat hydrochloride) in Europe, South and Central America and most of Africa Technology • Unique mechanism of action in visual cycle modulation (VCM) • Lead clinical trial program in geographic atrophy (GA) associated with dry age-related macular degeneration (AMD); no treatments currently available • 117 granted patents; 181 pending patents (as of 3/31/15) Financials • Successful IPO; $163M (gross) raised • Cash, short-term and long-term investments for the three months ended 3/31/15 was $187M ready to invest in ongoing programs, business development and internal research and development

People and Strategy

Management Team On May 1, 2015 the new board brought in a seasoned management team with significant experience in health care management, life science administration and technology. Ryo Kubota, MD, PhD Chairman, President and CEO Ted Danse, MBA Chief Business Officer Steve Tarr, PhD Chief Operating Officer John Gebhart, MBA Chief Financial Officer Qliance Medical Management, Inc Remote Medical International Ventripoint, PhysioSonics Carena, Clarity Health Nexcura, and DS-IQ Emeritus Senior Living Santa Clara Valley Health System Scottsdale Lincoln Health Network University of Washington Medicine Neurotech Pharmaceuticals, Inc. ISTA Pharmaceuticals Inc. Allergan Coopervision, Bausch & Lomb Schering-Plough May IR Meeting [ticker: 4589] 5

Board of Directors Directors Background Ryo Kubota, MD PhD Chairman. President and Chief Executive Officer and also founder of Acucela Inc. Yoshitaka Kitao (through June 25, 2015) Chief Executive Officer - SBI Holdings, Inc. Previously: Executive Vice President and Chief Financial Officer, Board of Directors - SOFTBANK Corp. , Chief Executive Officer and Representative Director - SOFTBANK Finance Corporation Shiro Mita, PhD President and Chief Executive Officer - M’s Science Corporation Previously: Executive Director of Drug Discovery, Director - Santen Pharmaceuticals Co., Ltd Eisaku Nakamura Directors - Koinobori Associates Inc. Previously: Director and General Manager - Bio Sight Capital Co., Ltd , Chief Executive Officer and President - Berevno Corporation, Board of Directors - CanBas Corporation and Activus Pharma Co. Ltd. Robert Takeuchi President - RT Consulting, Inc. Previously: President - SOFTBANK Finance, America Corporation, Director of International Equity Sales - Credit Suisse First Boston, Board of Directors SBI Investment Co., Ltd. and Quark Pharmaceuticals, Inc. Shintaro Asako (nominated for election on June 25, 2015) Chief Executive Officer - DeNA West Previously, Chief Financial Officer - MediciNova, Inc. May IR Meeting [ticker: 4589] 6
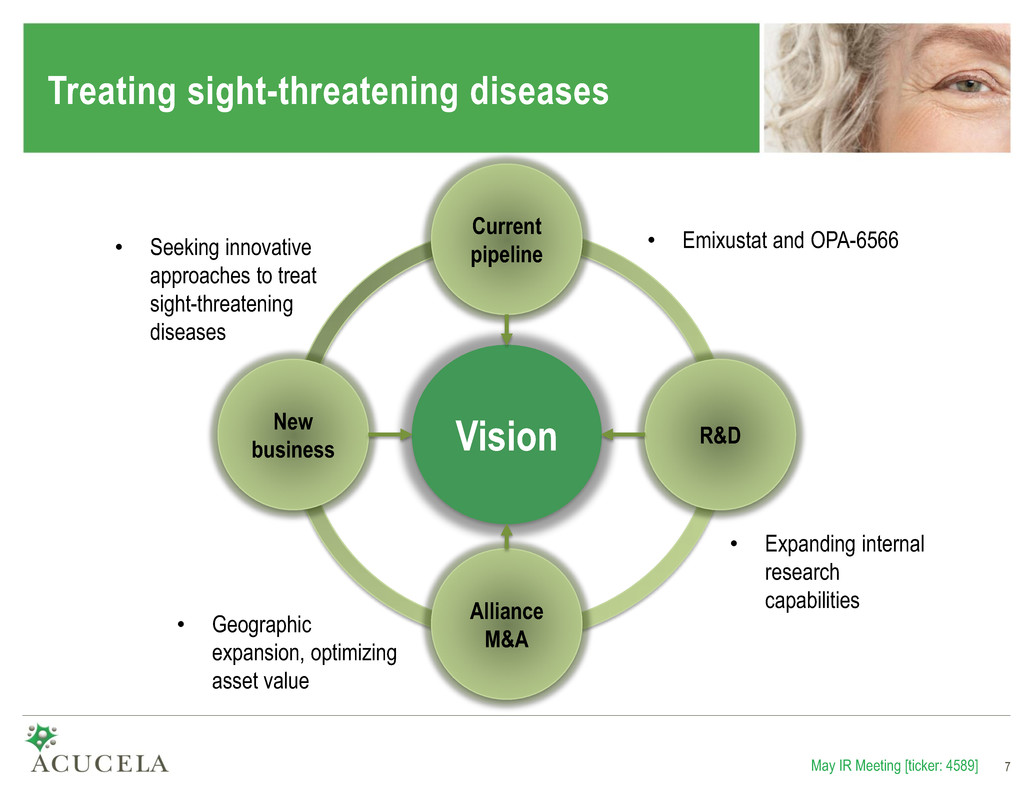
Treating sight-threatening diseases Vision R&D Alliance M&A New business Current pipeline • Expanding internal research capabilities • Emixustat and OPA-6566 • Geographic expansion, optimizing asset value • Seeking innovative approaches to treat sight-threatening diseases May IR Meeting [ticker: 4589] 7
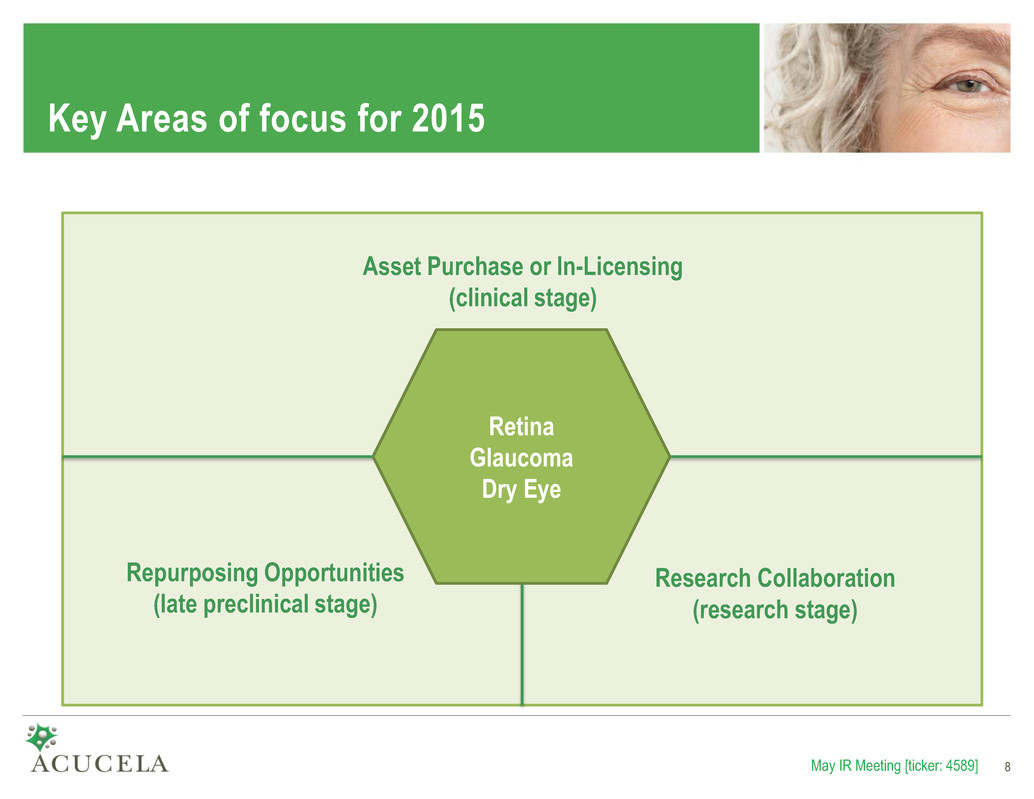
Key Areas of focus for 2015 Acucela is actively seeking new partnering opportunities to expand our product pipeline. May IR Meeting [ticker: 4589] 8 Retina Glaucoma Dry Eye Asset Purchase or In-Licensing (clinical stage) Repurposing Opportunities (late preclinical stage) Research Collaboration (research stage)

Key Elements of the 2015 Strategic Plan • Work collaboratively with Otsuka on the development of emixustat and OPA-6566 − Emixustat Phase 2b/3 clinical trial is continuing as scheduled − Otsuka is evaluating next steps for OPA-6566, an adenosine A2a receptor agonist for the potential treatment of glaucoma • Initiate partnering efforts for emixustat in Acucela’s territories − Plan to initiate out-licensing efforts under the Emixustat Agreement, primarily for Europe • Leverage expertise in VCM − Evaluating the potential to develop emixustat for additional indications such as diabetic retinopathy (DR) or diabetic macular edema (DME) − Planning to initiate and complete preclinical animal model studies in 2015 to evaluate the potential for development of emixustat in DR • Build a world-class ophthalmic product pipeline through internal research, M&A, and additional partnering or in-licensing opportunities Our goal is to develop an innovative portfolio of ophthalmology products. May IR Meeting [ticker: 4589] 9

Partnership

Partnership with Otsuka Pharmaceutical May IR Meeting [ticker: 4589] 11 Investigational Product Candidate Potential Indication License Territory Financial Terms Emixustat hydrochloride (developed by Acucela) Dry AMD and other ophthalmic indications Joint (50/50) - North America Acucela - Europe, South and Central America and most of Africa Otsuka – Asia-Pacific, some countries in Africa/Middle East • Otsuka paid $5M cash upfront payment to Acucela • Potential milestone payments: $258M total • Currently Otsuka and Acucela are equally sharing all development expenses; Otsuka loans funds to Acucela for the payment of Acucela’s share of the development expenses through to product launch OPA-6566 (developed by Otsuka) Glaucoma and other ophthalmic indications United States • Currently evaluating next steps for the program Unchanged stable relationship with Otsuka Pharmaceutical

Technology Emixustat Hydrochloride (Emixustat) for GA Associated with Dry AMD
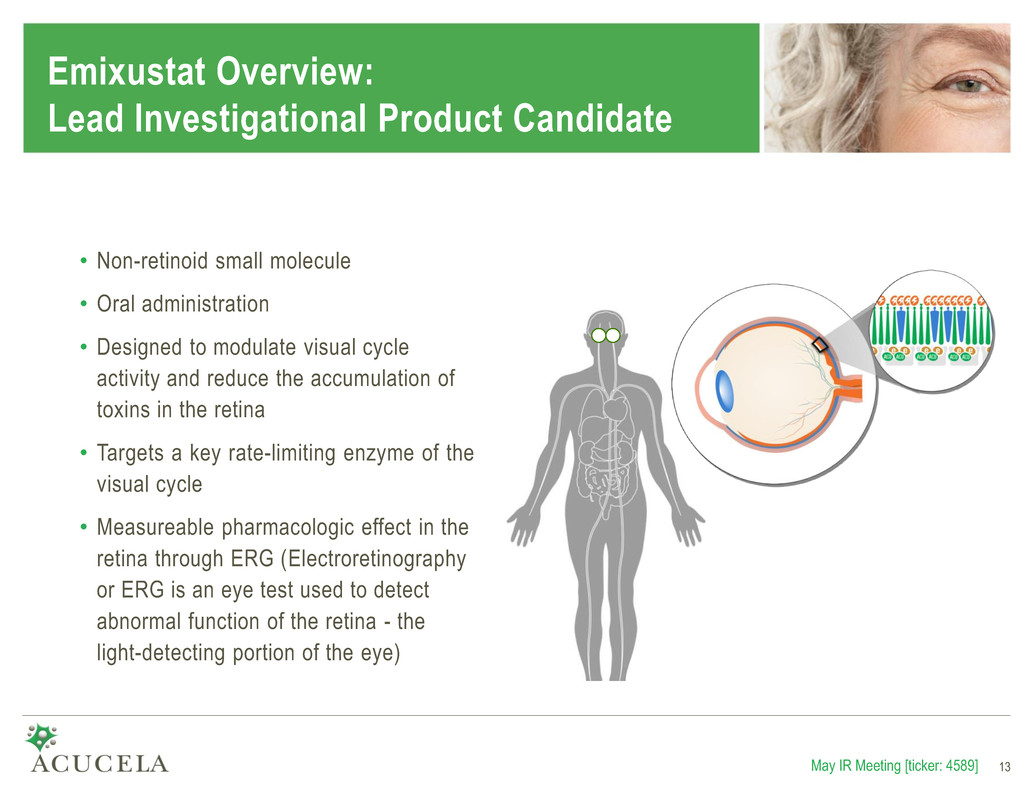
Emixustat Overview: Lead Investigational Product Candidate • Non-retinoid small molecule • Oral administration • Designed to modulate visual cycle activity and reduce the accumulation of toxins in the retina • Targets a key rate-limiting enzyme of the visual cycle • Measureable pharmacologic effect in the retina through ERG (Electroretinography or ERG is an eye test used to detect abnormal function of the retina - the light-detecting portion of the eye) May IR Meeting [ticker: 4589] 13
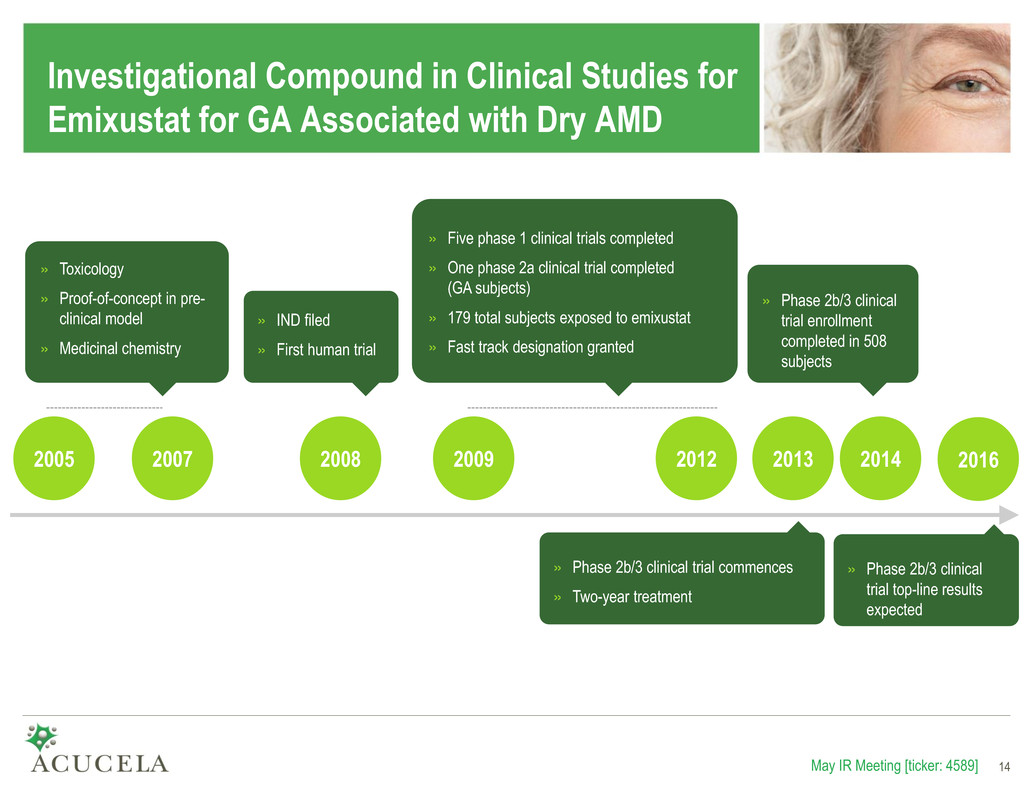
Investigational Compound in Clinical Studies for Emixustat for GA Associated with Dry AMD May IR Meeting [ticker: 4589] 14 2005 2007 2009 2012 2013 » Phase 2b/3 clinical trial commences » Two-year treatment » Toxicology » Proof-of-concept in pre- clinical model » Medicinal chemistry » Five phase 1 clinical trials completed » One phase 2a clinical trial completed (GA subjects) » 179 total subjects exposed to emixustat » Fast track designation granted » IND filed » First human trial 2008 14 2014 » Phase 2b/3 clinical trial enrollment completed in 508 subjects 2016 » Phase 2b/3 clinical trial top-line results expected

Clinical Development Progress to Date • Completed Clinical Trials to Date − Five phase 1 clinical trials (normal healthy adults) − One phase 2a clinical trial (GA subjects) − Total of 179 human subjects exposed to emixustat • On-going Clinical Trial – phase 2b/3 “SEATTLE” Study − Design – Two year, randomized, double-masked, dose-ranging study comparing the safety and efficacy of emixustat with placebo in patients with GA associated with dry AMD – A total of 508 patients with GA associated with dry AMD were enrolled − Objectives – Primary: Determine if emixustat reduces lesion growth rate compared to placebo – Secondary: – Evaluate safety and tolerability – Assess changes in best-corrected visual acuity – Evaluate the effect on development of wet AMD − Next Milestone – Top-line 2-year trial results anticipated in mid-2016 May IR Meeting [ticker: 4589] 15
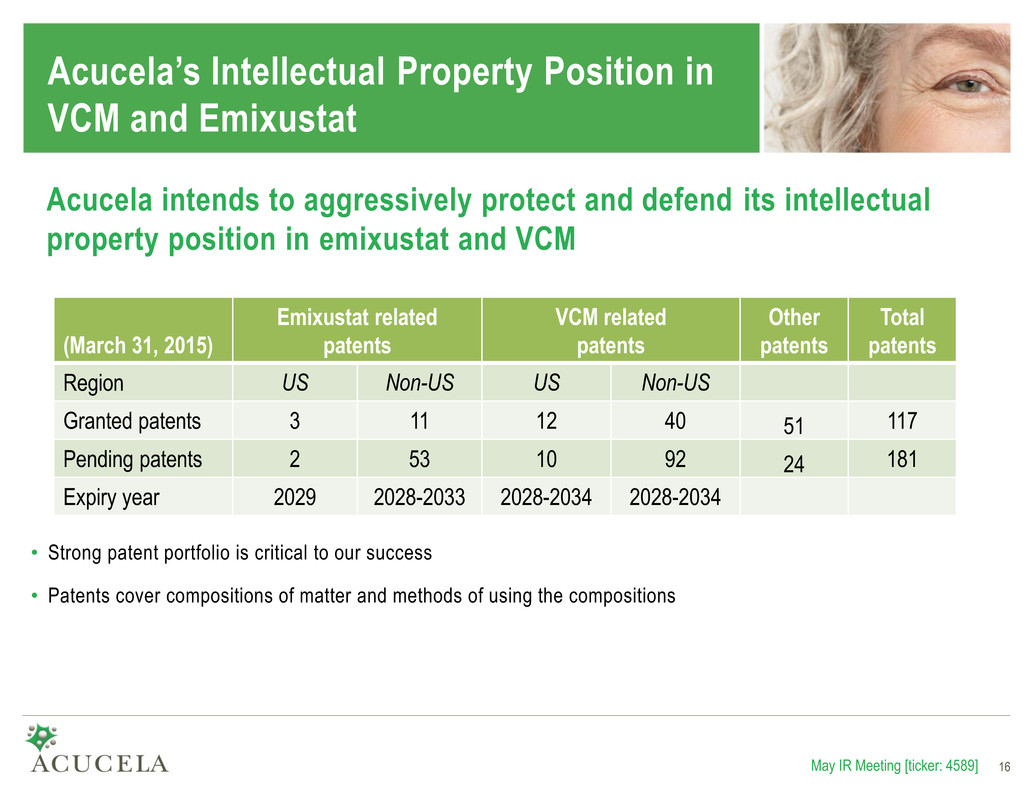
Acucela’s Intellectual Property Position in VCM and Emixustat (March 31, 2015) Emixustat related patents VCM related patents Other patents Total patents Region US Non-US US Non-US Granted patents 3 11 12 40 51 117 Pending patents 2 53 10 92 24 181 Expiry year 2029 2028-2033 2028-2034 2028-2034 May IR Meeting [ticker: 4589] 16 Acucela intends to aggressively protect and defend its intellectual property position in emixustat and VCM • Strong patent portfolio is critical to our success • Patents cover compositions of matter and methods of using the compositions

Financials
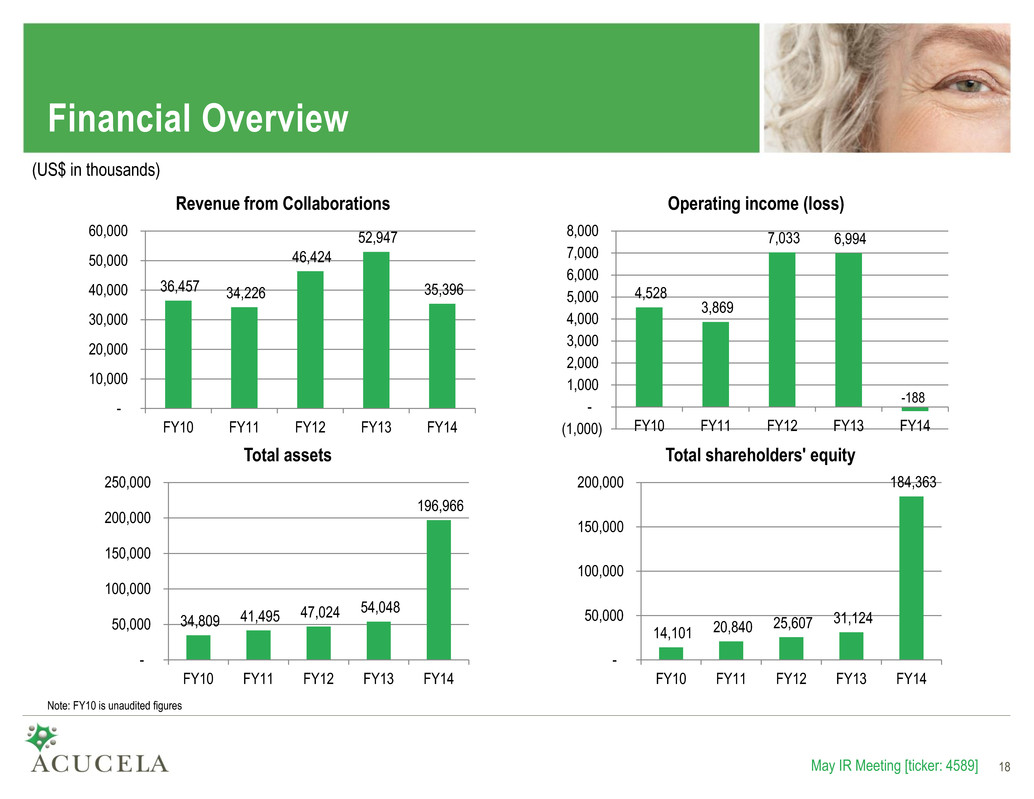
Financial Overview 36,457 34,226 46,424 52,947 35,396 - 10,000 20,000 30,000 40,000 50,000 60,000 FY10 FY11 FY12 FY13 FY14 Revenue from Collaborations 4,528 3,869 7,033 6,994 (1,000) - 1,000 2,000 3,000 4,000 5,000 6,000 7,000 8,000 FY10 FY11 FY12 FY13 FY14 Operating income (loss) 34,809 41,495 47,024 54,048 196,966 - 50,000 100,000 150,000 200,000 250,000 FY10 FY11 FY12 FY13 FY14 Total assets 14,101 20,840 25,607 31,124 184,363 - 50,000 100,000 150,000 200,000 FY10 FY11 FY12 FY13 FY14 Total shareholders' equity May IR Meeting [ticker: 4589] 18 Note: FY10 is unaudited figures (US$ in thousands) -188

Summary

Summary of Investor Rationale • People and Strategy − Executive management team with a combined eight decades of experience in executive leadership, health care management, life science administration and technology − Strategic plan put into place to grow the company’s pipeline • Partnership − Stable relationship with Otsuka Pharmaceutical − Financially beneficial collaboration agreement • Technology − Visual Cycle Modulation, a unique mechanism of action that differentiates the product candidate (emixustat) and that allows for oral administration – Ongoing phase 2b/3 clinical trial (ClinicalTrials.gov identifier: NCT01802866) − Significant unmet medical need (GA associated with dry AMD) − Competitive and defensible IP position (as of Marcy 31, 2015): 117 granted patents; 181 pending patent applications • Finance − Healthy balance sheet to invest in on-going programs, business development and internal research and development May IR Meeting [ticker: 4589] 20

Message to our investors “With our lead investigational drug in clinical trials and our expertise in the science of visual cycle modulation (VCM), Acucela has the potential to greatly improve the lives of millions of people who suffer from sight-threatening diseases like age-related macular degeneration (AMD) and glaucoma. We continue on as an innovator in the treatment of ophthalmic diseases.” Ryo Kubota, Chairman, President and CEO May IR Meeting [ticker: 4589] 21

Acucela is a clinical-stage biotechnology company that specializes in discovering and developing novel therapeutics to treat and slow the progression of sight-threatening ophthalmic diseases affecting millions of individuals worldwide.
