Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Endo International plc | d928010d8k.htm |
| EX-99.1 - EX-99.1 - Endo International plc | d928010dex991.htm |
Exhibit 99.2

Endo International plc
Par Pharmaceutical
Acquisition
May 18, 2015
©2015 Endo Pharmaceuticals Inc. All rights reserved.

Forward Looking Statements
This presentation contains information relating to the acquisition of Par by Endo that includes or is based on “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act and Canadian securities legislation. These statements include statements regarding the timing and the closing of the transaction, the expected benefits of the transaction, the expected accretion to earnings resulting from the transaction, expected product approvals and Endo’s plans to operate Par. Forward-looking statements include the information concerning our possible or assumed results of operations. We have tried, whenever possible, to identify such statements by words such as “believes,”
“expects,” “anticipates,” “intends,” “estimates,” “plan,” “projected,” “forecast,” “will,” “may” or similar expressions. Wehave based these forward-looking statements on our current expectations of future events. Because these statements reflect our current views concerning future events, these forward-looking statements involve risks and uncertainties. If underlying assumptions prove inaccurate or unknown, or unknown risks or uncertainties materialize, actual results could differ material from those expressed in the forward-looking statements contained in this presentation. Risks and uncertainties include, among other things, uncertainties as to the timing of the acquisition; the possibility that various closing conditions to the transaction may not be satisfied or waived, including that a governmental entity may prohibit, delay, or refuse to grant approval for the consummation of the transaction; that the FDA or other regulatory authorities do not approve any product(s) in the manner desired by Endo on a timely basis, or at all; that there is a material adverse change to Par; that the integration of Par business into Endo is not as successful as expected; the failure of Endo to achieve the expected financial and commercial results from the transaction; other business effects, including effects of industry, economic or political conditions outside Endo’s control; transaction costs; the outcome of litigation, actual or contingent liabilities; as well as other cautionary statements contained elsewhere herein and in Endo’s periodic reports filed with the Securities and Exchange Commission (SEC) and with securities regulators in Canada on the System for Electronic Document Analysis and Retrieval (SEDAR), including current reports on Form 8-K, quarterly reports on Form 10-Q and annual reports on Form 10-K. We do not undertake any obligation to update our forward-looking statements after the date of this presentation for any reason, even if new information becomes available or other events occur in the future, except as may be required under applicable securities law. You should understand that it is not possible to predict or identify all such factors. Consequently, you should not consider this to be a complete discussion of all potential risks or uncertainties.
©2015 Endo Pharmaceuticals Inc. All rights reserved.
1
Non-GAAP Financial Measures
This presentation may refer to non-GAAP financial measures, including EBITDA and adjusted gross margin, which are financial measures that are not prepared in conformity with accounting principles generally accepted in the United States (GAAP). We define adjusted gross margin as total revenues, less cost of revenues, adjusted for amortization of intangible assets; certain upfront and milestone payments to partners; certain cost reduction and integration-related initiatives; inventory step-up recorded as part of our acquisitions; certain excess costs that will be eliminated pursuant to integration plans and certain other items that we believe do not reflect our core operating performance. Our presentation of EBITDA and adjusted gross margin may be different from non-GAAP financial measures presented by other companies. We believe that our presentation of non-GAAP financial measures provides useful supplementary information regarding operational performance because it enhances an investor’s overall understanding of the financial performance and prospects for future core business activities by providing a basis for the comparison of results of core business operations between current, past and future periods. Management uses non-GAAP financial measures to prepare operating budgets and forecasts and to measure performance against those budgets and forecasts on a corporate and segment level. Endo also uses non-GAAP financial measures for evaluating management performance for compensation purposes. Reconciliation of non-GAAP financial measures to the nearest comparable GAAP amounts have been provided within the appendix at the end of this presentation.
Additional Information
This presentation is provided for informational purposes only and is neither an offer to purchase nor a solicitation of an of fer to sell shares of Endo. Endo and Par shareholders should read any filings made by Endo with the SEC in connection with the proposed combination, as they will contain important information. Those documents, if and when filed, as well as Endo’s other public filings with the SEC, may be obtained without charge at the SEC’s website at www.sec.gov and at Endo’s website at endo.com. 2
©2015 Endo Pharmaceuticals Inc. All rights reserved.

Par Pharmaceutical Acquisition: Overview
Endo to acquire privately-held Par for $8.05 billion
$1.55 billion in equity to Par shareholders
$6.5 billion cash consideration
Fully committed financing from Barclays and Deutsche Bank
Expected to be financed by combination of cash, term loans, bonds and an equity offering of ~$1.5 to $2 billion
Creates leading specialty pharmaceutical company with top five generics business as measured by U.S. sales1
2014 pro forma revenues of $4.2 billion
Unanimously approved by both companies’ Boards of Directors
Par CEO Paul Campanelli joins Endo to lead Generics business
Expected to close in 2H 2015, subject to regulatory and other customary closing conditions
No shareholder approvals required
1 Source: IMS Health LTM as of 10/31/14
©2015 Endo Pharmaceuticals Inc. All rights reserved.
3
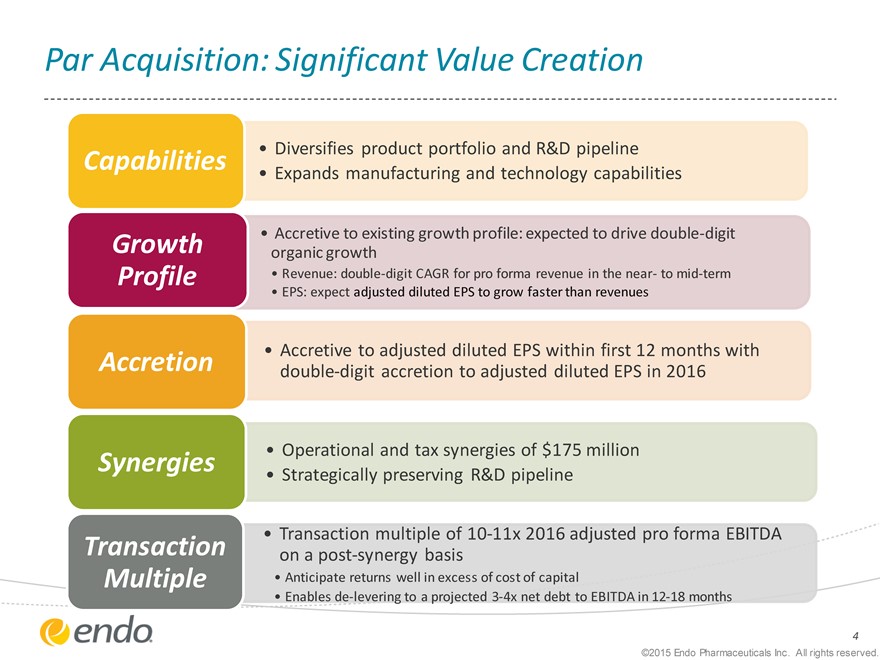
Par Acquisition: Significant Value Creation
• Diversifies product portfolio and R&D pipeline
Capabilities
• Expands manufacturing and technology capabilities
Growth • Accretive to existing growth profile: expected to drive double-digit organic growth
Profile • Revenue: double-digit CAGR for pro forma revenue in the near- to mid-term
• EPS: expect adjusted diluted EPS to grow faster than revenues
Accretion • Accretive to adjusted diluted EPS within first 12 months with double-digit accretion to adjusted diluted EPS in 2016
• Operational and tax synergies of $175 million
Synergies
• Strategically preserving R&D pipeline
• Transaction multiple of 10-11x 2016 adjusted pro forma EBITDA
Transaction on a post-synergy basis
Multiple • Anticipate returns well in excess of cost of capital
• Enables de-levering to a projected 3-4x net debt to EBITDA in 12-18 months
4
©2015 Endo Pharmaceuticals Inc. All rights reserved.

Compelling Strategic & Financial Rationale
Strategically expands product portfolio, R&D pipeline, capabilities and long-term growth drivers
Adds extensive range of dosage forms and delivery systems Focus on specialized, market leading products
Designed to accelerate Endo growth:
Double-digit revenue growth in mid-term, accretive to adjusted diluted EPS, meaningful synergies, increased generics adjusted gross margins Strong R&D pipeline capable of fueling long-term organic growth
Drives strategic expansion of overall corporate profile, scope, and size, establishing a powerful platform for future M&A
Strong cash flow expected to lead to rapid de-levering back to 3-4x net debt to EBITDA in 12-18 months
Aligned with Endo’s strategy of pursuing accretive, value-creating growth opportunities
Creates shareholder value and drives benefits for patients & customers
5
©2015 Endo Pharmaceuticals Inc. All rights reserved.

Transaction Aligns with Endo’s Strategic Direction
Build a leading global specialty pharmaceutical company
Focus on maximizing the value of each of our core businesses
Participate in specialty areas offering above average growth and favorable margins
Transform operating model to maximize growth potential and cash flow generation
Continue our commitment to serving our patients & customers
Improving lives while creating value
6
@2015 Endo Pharmaceuticals Inc. All rights reserved.
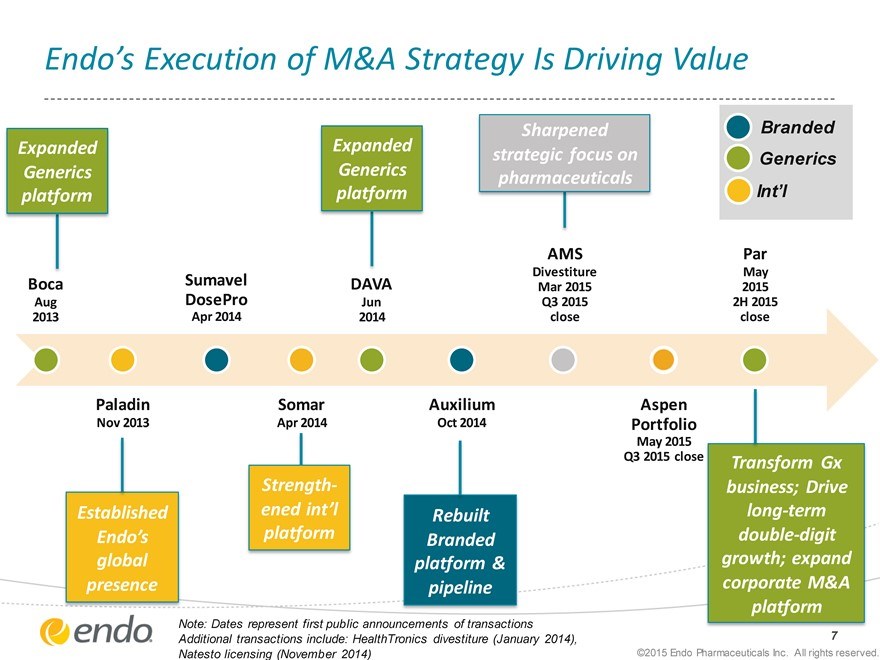
Endo’s Execution of M&A Strategy Is Driving Value
Expanded Generics platform
Expanded Generics platform
Sharpened strategic focus on pharmaceuticals
Branded Generics
Int’l
Boca
Aug 2013
Sumavel DosePro
Apr 2014
DAVA
Jun 2014
AMS
Divestiture Mar 2015 Q3 2015 close
Par
May 2015
2H 2015 close
Paladin
Nov 2013
Established
Endo’s global presence
Somar
Apr 2014
Strengthened int’l platform
Auxilium
Oct 2014
Rebuilt Branded platform & pipeline
Aspen Portfolio
May 2015 Q3 2015 close
Transform Gx business; Drive long-term double-digit growth; expand corporate M&A platform
Note: Dates represent first public announcements of transactions
Additional transactions include: HealthTronics divestiture (January 2014), Natesto licensing (November 2014)
©2015 Endo Pharmaceuticals Inc. All rights reserved.
7
Endo’s Vision: Leading Specialty Pharmaceutical Co.
U.S. Branded
U.S. Generics International Pharmaceuticals
Transaction closed January 2015
8
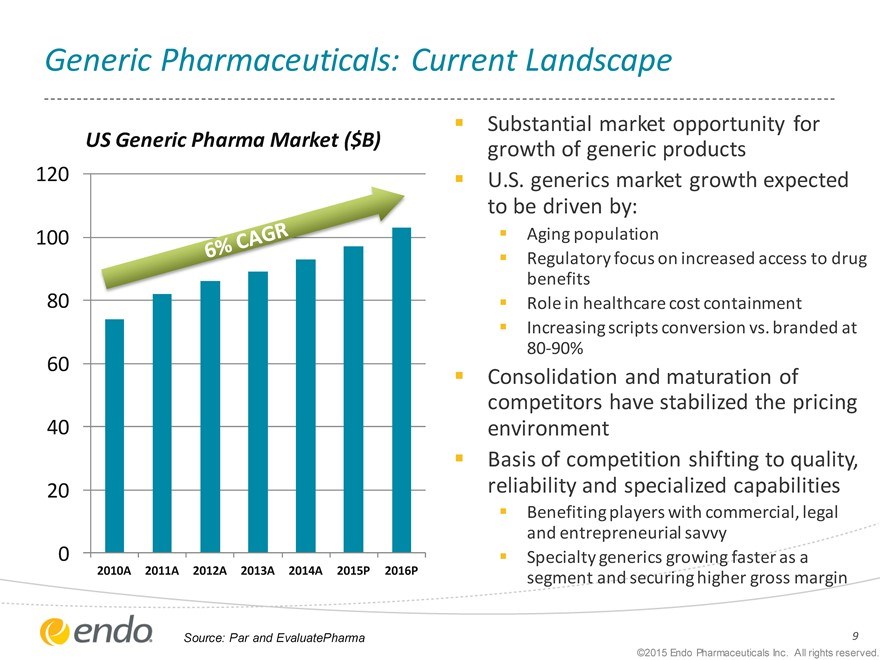
Generic Pharmaceuticals: Current Landscape
US Generic Pharma Market ($B)
120 100 80 60 40 20 0
2010A 2011A 2012A 2013A 2014A 2015P 2016P
Substantial market opportunity for growth of generic products U.S. generics market growth expected to be driven by:
Aging population
Regulatory focus on increased access to drug benefits Role in healthcare cost containment Increasing scripts conversion vs. branded at 80-90%
Consolidation and maturation of competitors have stabilized the pricing environment Basis of competition shifting to quality, reliability and specialized capabilities
Benefiting players with commercial, legal and entrepreneurial savvy Specialty generics growing faster as a segment and securing higher gross margin
Source: Par and EvaluatePharma
9
©2015 Endo Pharmaceuticals Inc. All rights reserved.
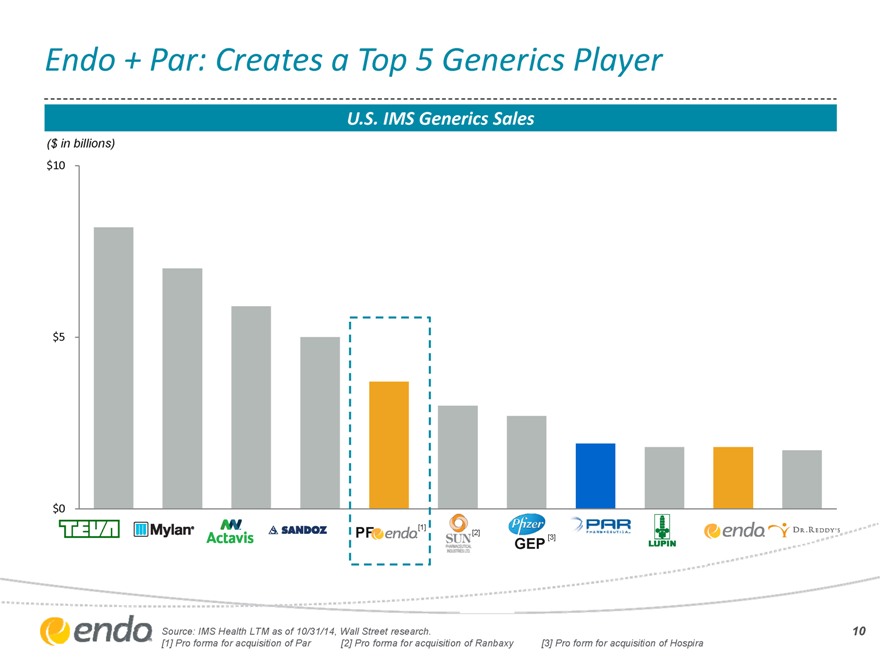
Endo + Par: Creates a Top 5 Generics Player
U.S. IMS Generics Sales
($ in billions) $10
$5
$0
[1]
PF [2]
GEP[3]
Source: IMS Health LTM as of 10/31/14, Wall Street research.
[1] Pro forma for acquisition of Par
[2] Pro forma for acquisition of Ranbaxy
[3] Pro form for acquisition of Hospira
10

About Qualitest
Founded in 1983 Acquired by Endo in 2010 Headquarters: Huntsville, AL
Manufacturing Facilities: Huntsville, AL & Charlotte, NC 1,750 employees
Double digit organic growth performance and outlook
Strong volume growth across balanced portfolio of over 700 products
Controlled substances, specialty generics, Liquids / semi-solids, commodity generics
Pipeline of approximately 90 programs Strong expertise in controlled substances
11
©2015 Endo Pharmaceuticals Inc. All rights reserved.

About Par Pharmaceutical
Founded in 1978
Headquarters: Woodcliff Lake, NJ
Manufacturing Facilities: NY/CT, MI, CA and India 2,000 employees
Privately-held pharmaceutical company operating in the U.S. as two business segments:
Generics : approx. 95 products with 215 pipeline programs
Multiple dosage forms and delivery systems with a focus on high barrier-to-entry products, Paragraph IV, first-to-file and first-to-market opportunities
Branded : 2 approved, marketed products
12
©2015 Endo Pharmaceuticals Inc. All rights reserved.
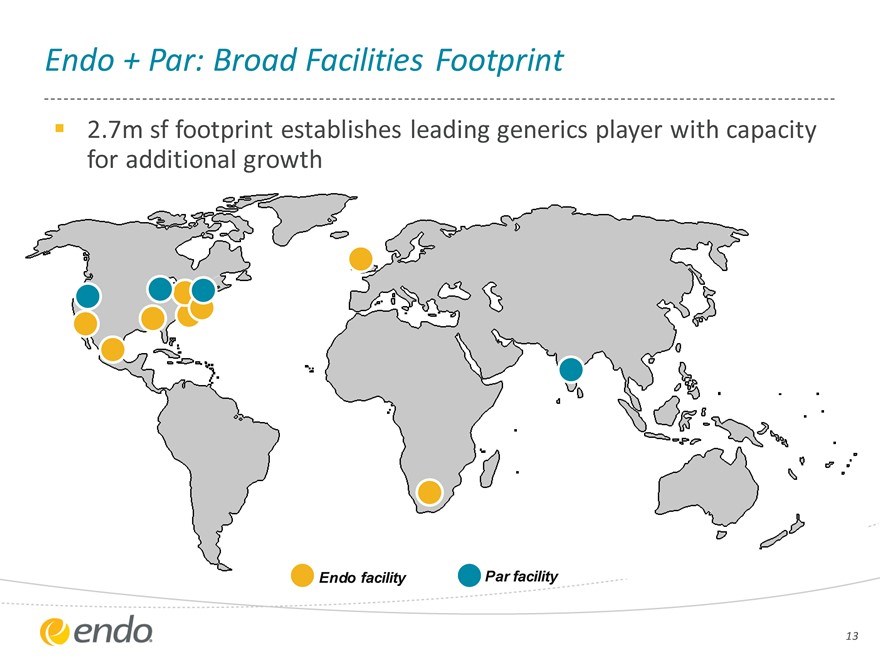
Endo + Par: Broad Facilities Footprint
2.7m sf footprint establishes leading generics player with capacity for additional growth
Endo facility Par facility
13
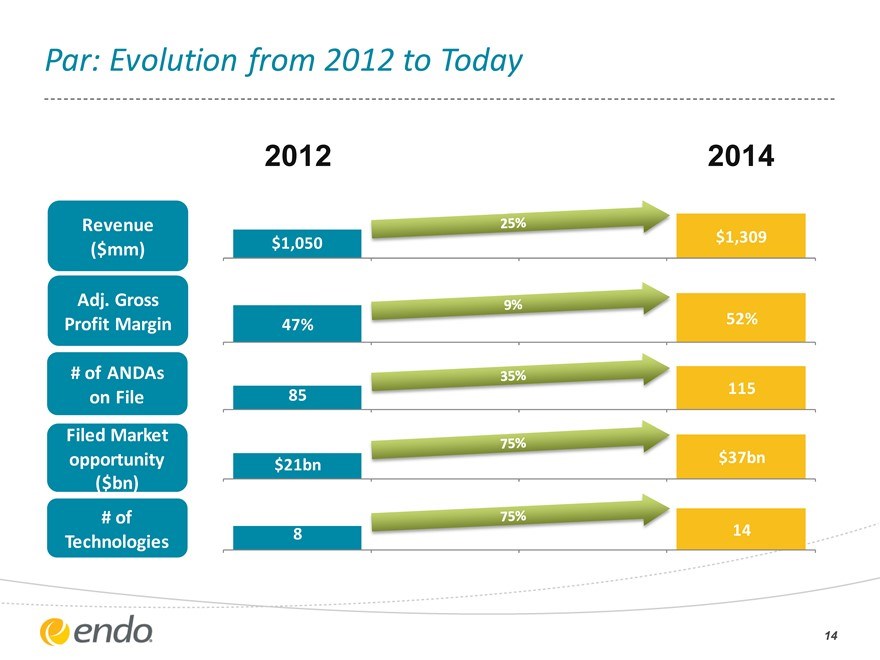
Par: Evolution from 2012 to Today
2012
2014
Revenue ($mm)
Adj. Gross Profit Margin
# of ANDAs on File
Filed Market opportunity ($bn)
# of Technologies
$1,050 47% 85 $21bn
8
$1,309 52% 115 $37bn
14
14

Par Today: High-Value, Long-Term Growth Products
Focus of core business is attractive high-value generics
Paragraph IV, first-to-file and first-to-market opportunities
Specialized products that are difficult to manufacture and/or present complex legal and regulatory challenges, including the generics of:
Lovaza® (complex and difficult-to-source API) Precedex™ (unique dosage form) Luvox CR® (controlled-release product) Focalin XR (controlled substance)
Emphasis on market leading products
Majority of Par’s top 10 generic drugs by revenue are market leaders
Significant number of products are either exclusive or have two or fewer competitors
Current product base is relatively more profitable and longer-lived than competitors
Lovaza® is a registered trademark of GlaxoSmithKline; Precedex™ is a registered trademark of Hospira; Luvox CR® is a
15 registered trademark of Jazz Pharmaceuticals; Focalin XR is a trademark of Novartis AG
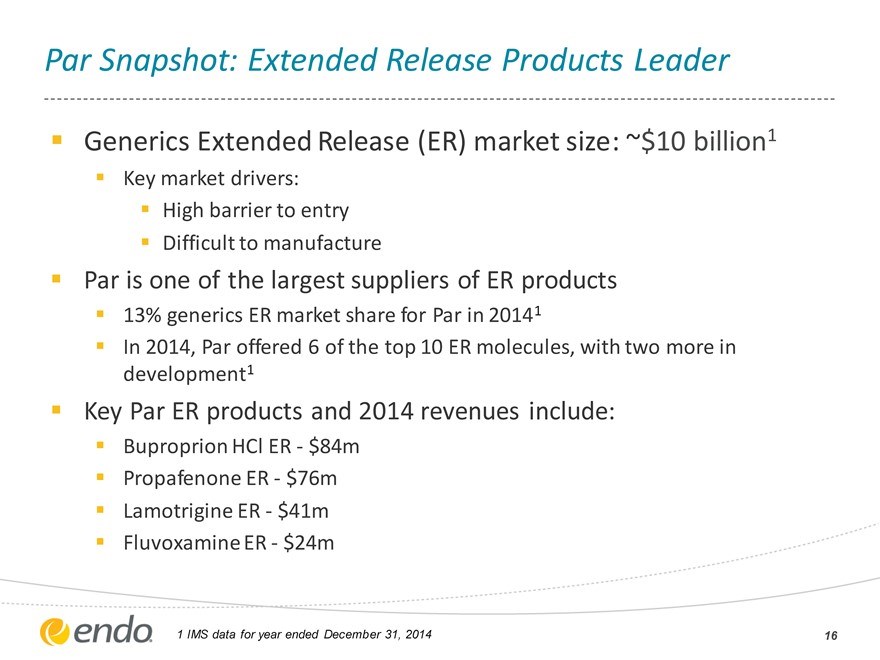
Par Snapshot: Extended Release Products Leader
Generics Extended Release (ER) market size: ~$10 billion1
Key market drivers: High barrier to entry Difficult to manufacture
Par is one of the largest suppliers of ER products
13% generics ER market share for Par in 20141
In 2014, Par offered 6 of the top 10 ER molecules, with two more in development1
Key Par ER products and 2014 revenues include:
Buproprion HCl ER - $84m Propafenone ER - $76m Lamotrigine ER - $41m Fluvoxamine ER - $24m
1 IMS data for year ended December 31, 2014
16
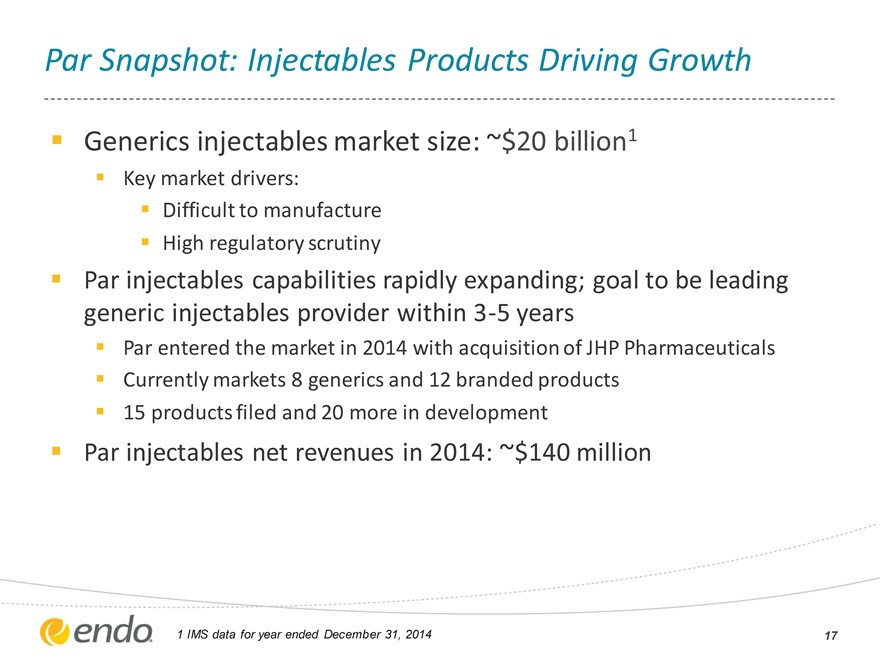
Par Snapshot: Injectables Products Driving Growth
Generics injectables market size: ~$20 billion1
Key market drivers: Difficult to manufacture High regulatory scrutiny
Par injectables capabilities rapidly expanding; goal to be leading generic injectables provider within 3-5 years
Par entered the market in 2014 with acquisition of JHP Pharmaceuticals Currently markets 8 generics and 12 branded products 15 products filed and 20 more in development
Par injectables net revenues in 2014: ~$140 million
1 IMS data for year ended December 31, 2014
17
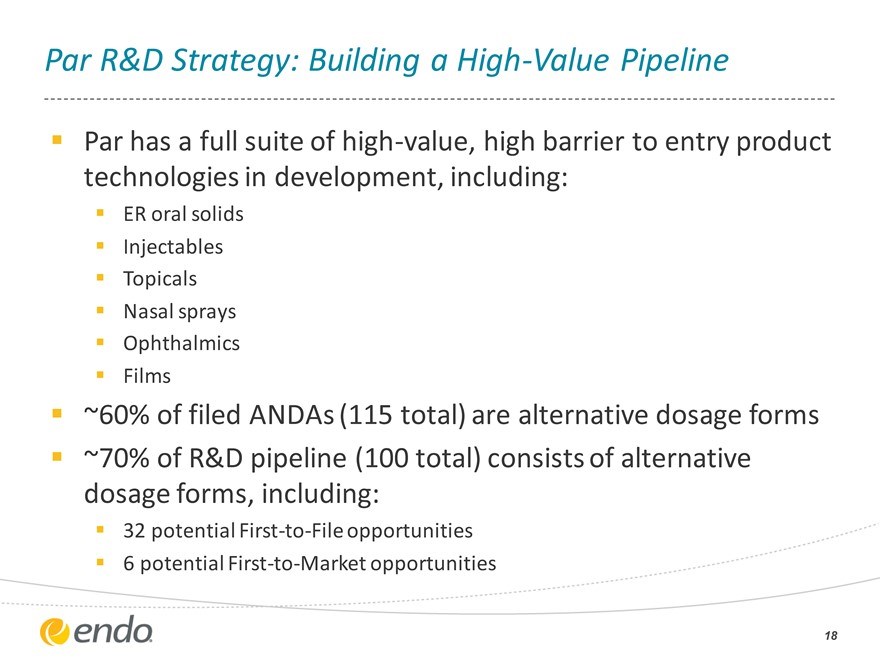
Par R&D Strategy: Building a High-Value Pipeline
Par has a full suite of high-value, high barrier to entry product technologies in development, including:
ER oral solids Injectables Topicals Nasal sprays Ophthalmics Films
~60% of filed ANDAs (115 total) are alternative dosage forms ~70% of R&D pipeline (100 total) consists of alternative dosage forms, including:
32 potential First-to-File opportunities 6 potential First-to-Market opportunities
18
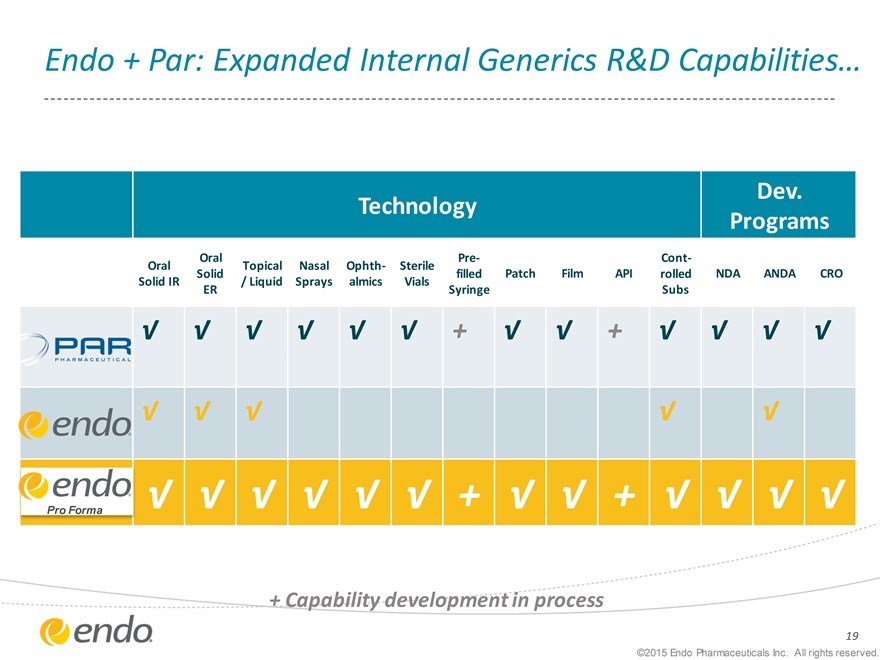
Endo + Par: Expanded Internal Generics R&D Capabilities…
Technology
Dev. Programs
Oral Pre- Cont-Oral Topical Nasal Ophth- Sterile
Solid filled Patch Film API rolled NDA ANDA CRO Solid IR / Liquid Sprays almics Vials ER Syringe Subs
+ Capability development in process
19
©2015 Endo Pharmaceuticals Inc. All rights reserved.
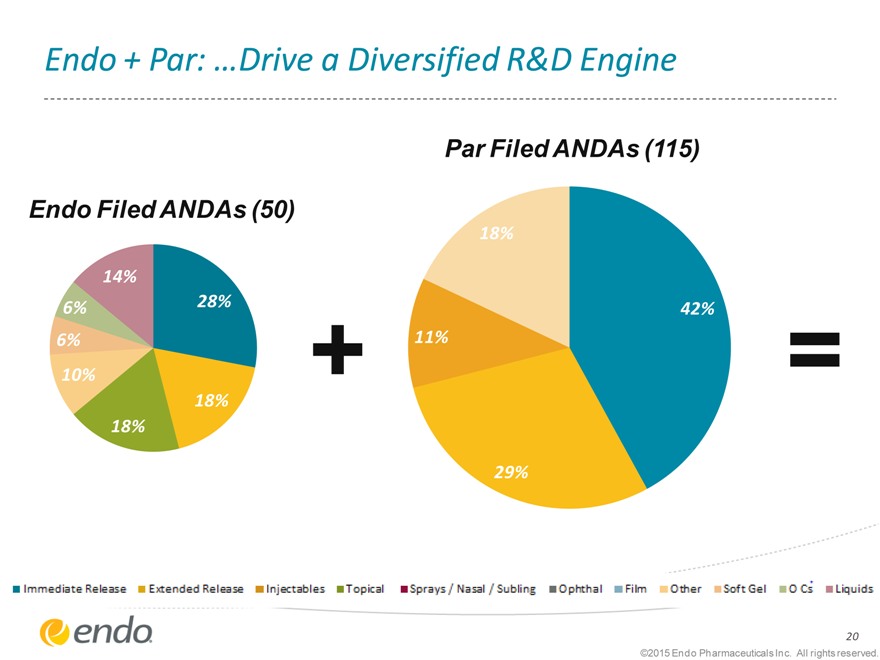
Endo + Par: …Drive a Diversified R&D Engine
Endo Filed ANDAs (50)
Par Filed ANDAs (115)
14%
6% 28%
6%
10%
0 18%
0 0 18%
18%
42%
11%
29%
Immediate Release Extended Release Injectables Topical Sprays/Nasal/Subling Ophthal Film Other SoftGel O C’s Liquids
20
©2015 Endo Pharmaceuticals Inc. All rights reserved.
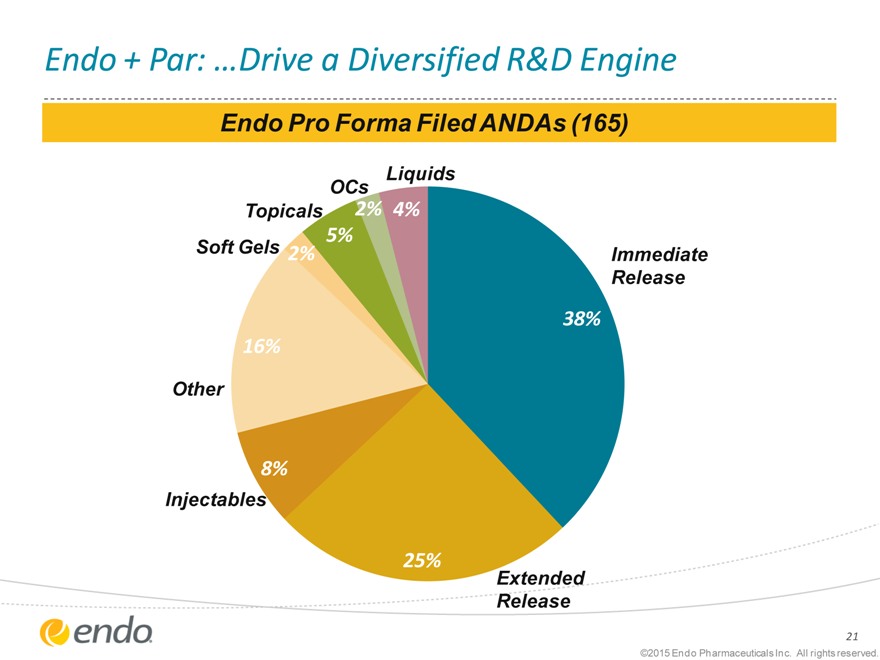
Endo + Par: …Drive a Diversified R&D Engine
Endo Pro Forma Filed ANDAs (165)
Liquids
OCs
Topicals 2% 4%
Soft Gels 5%
0 0 2% Immediate
0% Release
38%
16%
Other
8%
Injectables
25%
Extended
Release
21
©2015 Endo Pharmaceuticals Inc. All rights reserved.
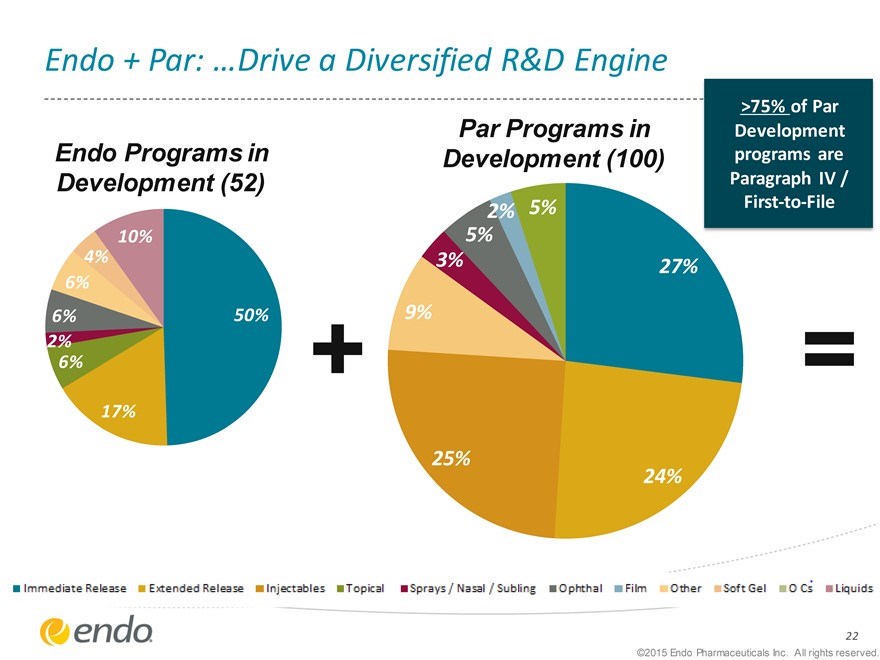
Endo + Par: …Drive a Diversified R&D Engine
Endo Programs in Development (52)
Par Programs in
Development (100)
>75% of Par Development programs are Paragraph IV / First-to-File
10% 4%
0 6%
6% 50% 2% 6%
0
17%
2% 5% 5%
3% 27% 9% 25% 24%
22
©2015 Endo Pharmaceuticals Inc. All rights reserved.
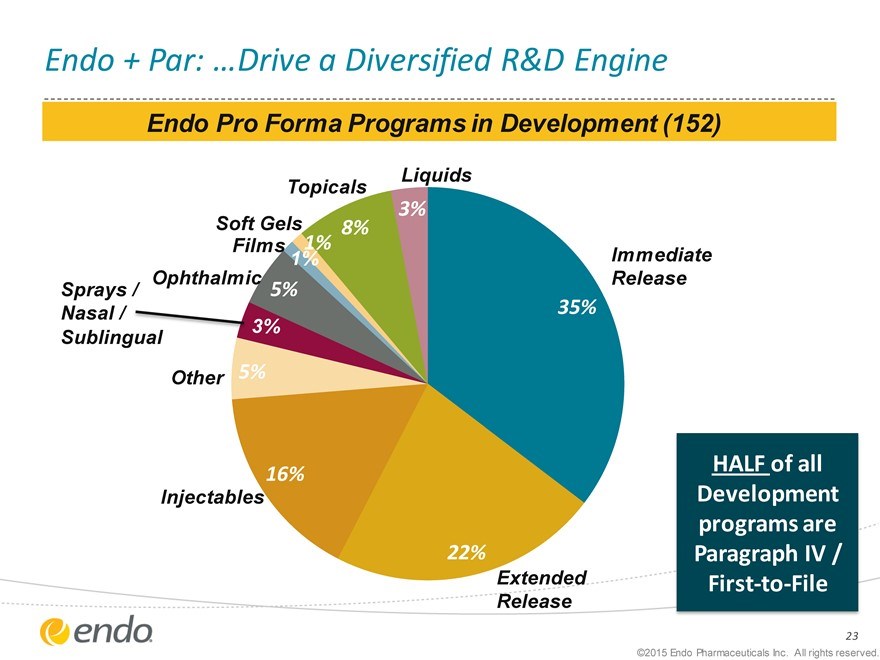
Endo + Par: …Drive a Diversified R&D Engine
Endo Pro Forma Programs in Development (152)
Liquids Topicals
3%
Soft Gels 8%
1%Films 1% 1%
Ophthalmic Release Sprays / 5% Nasal / 35% 3% Sublingual Other 5%
16%
Injectables
22%
Extended Release
HALF of all Development programs are Paragraph IV / First-to-File
23
©2015 Endo Pharmaceuticals Inc. All rights reserved.
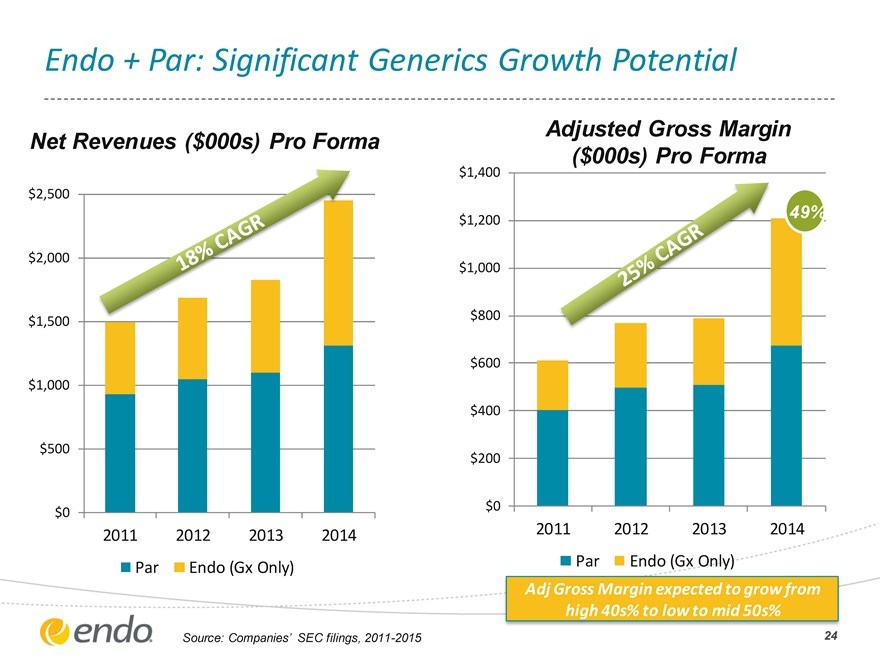
Endo + Par: Significant Generics Growth Potential
Net Revenues ($000s) Pro Forma
$2,500 $2,000 $1,500 $1,000 $500
$0
2011 2012 2013 2014
Par Endo (Gx Only)
Adjusted Gross Margin
($000s) Pro Forma
$1,400
49%
$1,200
$1,000
$800
47%
$600 $400 $200
$0
2011 2012 2013 2014
Par Endo (Gx Only)
Adj Gross Margin expected to grow from high 40s% to low to mid 50s%
Source: Companies’ SEC filings, 2011-2015
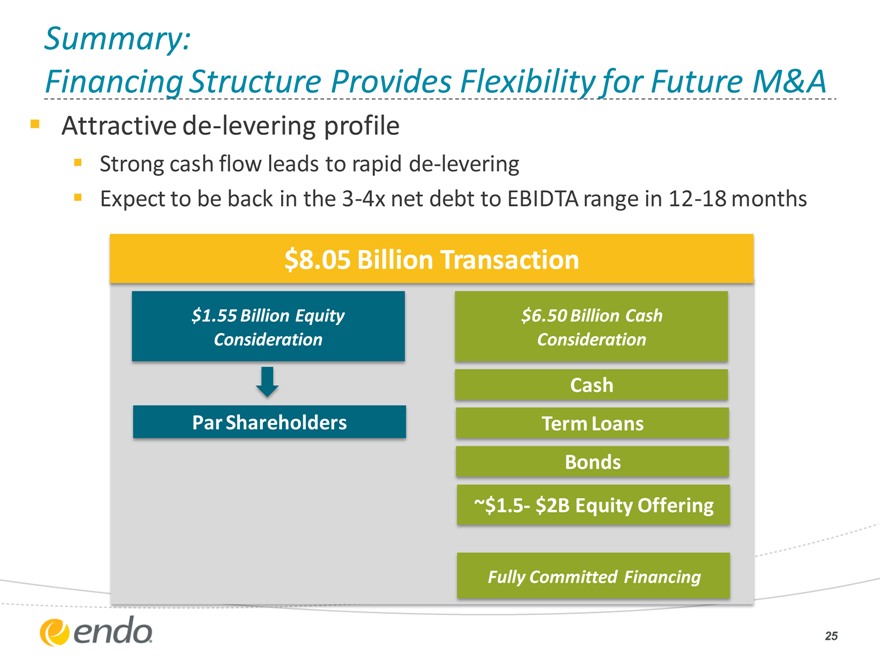
Summary:
Financing Structure Provides Flexibility for Future M&A
Attractive de-levering profile
Strong cash flow leads to rapid de-levering
Expect to be back in the 3-4x net debt to EBIDTA range in 12-18 months
$8.05 Billion Transaction
$1.55 Billion Equity $6.50 Billion Cash
Consideration Consideration
Cash
Par Shareholders Term Loans
Bonds
~$1.5- $2B Equity Offering
Fully Committed Financing
25
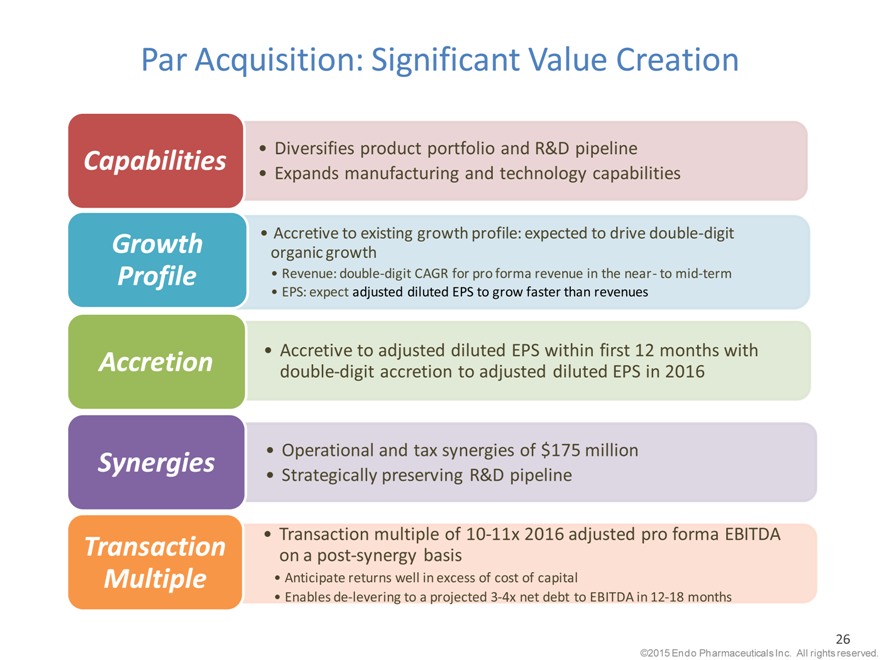
Par Acquisition: Significant Value Creation
Capabilities Diversifies product portfolio and R&D pipeline
Expands manufacturing and technology capabilities
Accretive to existing growth profile: expected to drive double-digit
Growth organic growth
Profile Revenue: double-digit CAGR for pro forma revenue in the near - to mid-term
EPS: expect adjusted diluted EPS to grow faster than revenues
Accretive to adjusted diluted EPS within first 12 months with
Accretion double-digit accretion to adjusted diluted EPS in 2016
Synergies Operational and tax synergies of $175 million
Strategically preserving R&D pipeline
Transaction multiple of 10-11x 2016 adjusted pro forma EBITDA
Transaction on a post-synergy basis
Multiple Anticipate returns well in excess of cost of capital
Enables de-levering to a projected 3-4x net debt to EBITDA in 12-18 months
26
©2015 Endo Pharmaceuticals Inc. All rights reserved.
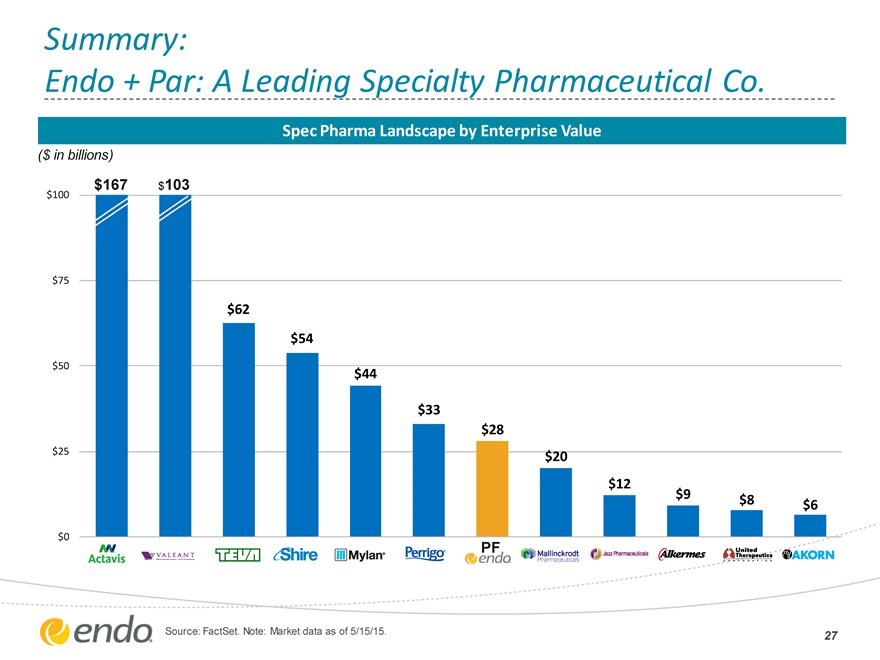
Summary:
Endo + Par: A Leading Specialty Pharmaceutical Co.
Spec Pharma Landscape by Enterprise Value
($ in billions)
$100 $167 $103
$75
$62
$54
$50
$44
$33 $28
$25 $20
$12 $9
$8 $6
$0 PF
Source: FactSet. Note: Market data as of 5/15/15.
27
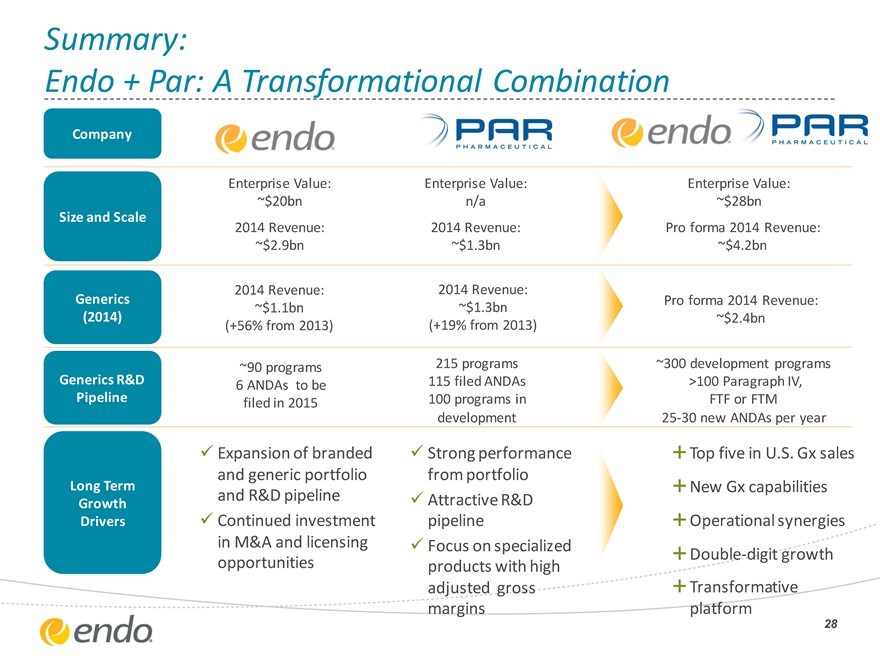
Summary:
Endo + Par: A Transformational Combination
Company
Size and Scale
Generics
(2014)
Generics R&D
Pipeline
Long Term Growth
Drivers
Enterprise Value:
~$20bn 2014 Revenue:
~$2.9bn
2014 Revenue:
~$1.1bn (+56% from 2013)
~90 programs
6 ANDAs to be filed in 2015
Expansion of branded and generic portfolio and R&D pipeline Continued investment in M&A and licensing opportunities
Enterprise Value: n/a 2014 Revenue:
~$1.3bn
2014 Revenue:
~$1.3bn
(+19% from 2013)
215 programs 115 filed ANDAs
100 programs in development
Strong performance from portfolio Attractive R&D pipeline Focus on specialized products with high adjusted gross margins
Enterprise Value:
~$28bn
Pro forma 2014 Revenue:
~$4.2bn
Pro forma 2014 Revenue:
~$2.4bn
~300 development programs >100 Paragraph IV,
FTF or FTM 25-30 new ANDAs per year
Top five in U.S. Gx sales New Gx capabilities
Operational synergies
Double-digit growth
Transformative platform
28

Appendix
©2015 Endo Pharmaceuticals Inc. All rights reserved.
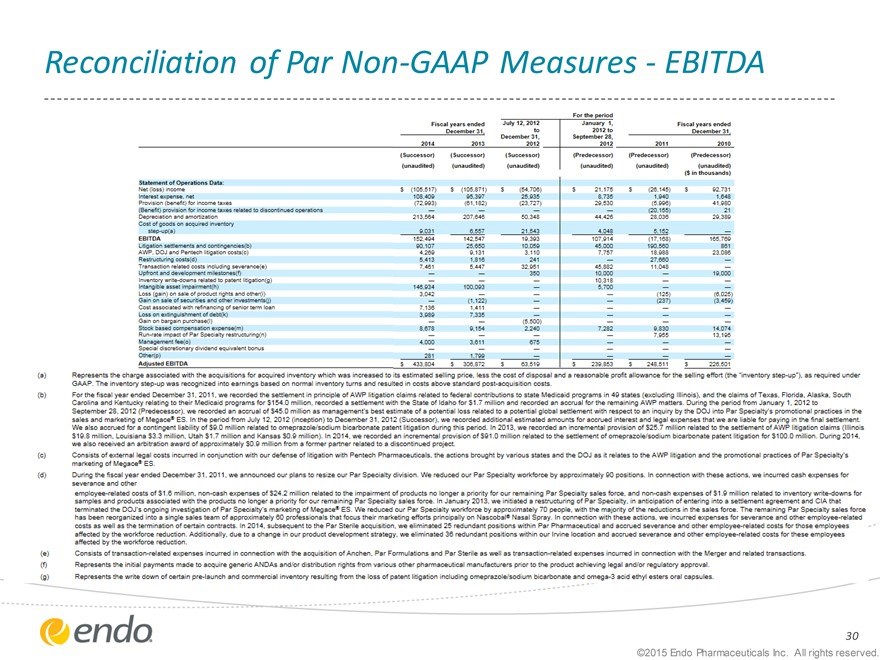
Reconciliation of Par Non-GAAP Measures - EBITDA
30
©2015 Endo Pharmaceuticals Inc. All rights reserved.
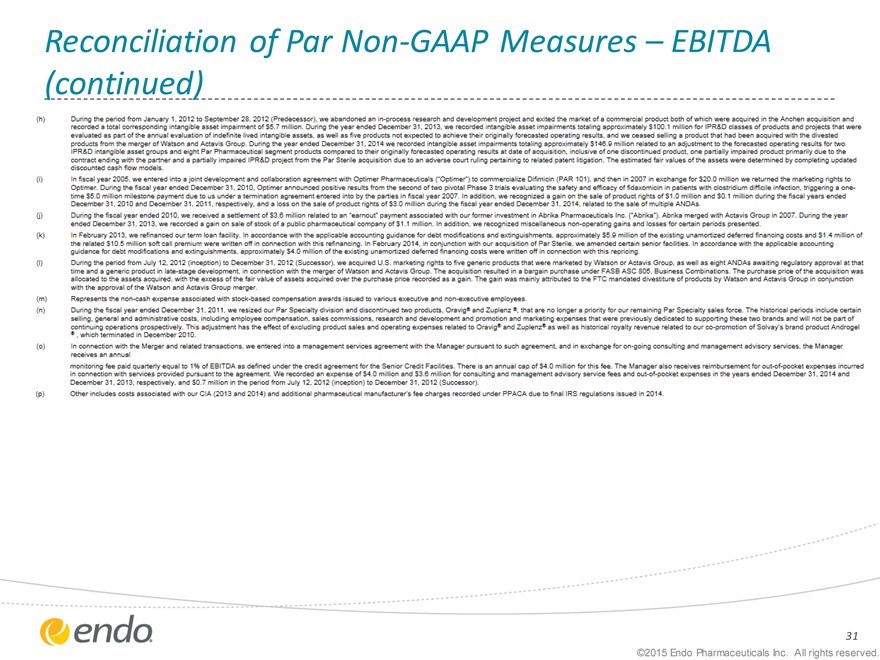
Reconciliation of Par Non-GAAP Measures – EBITDA (continued)
31
©2015 Endo Pharmaceuticals Inc. All rights reserved.
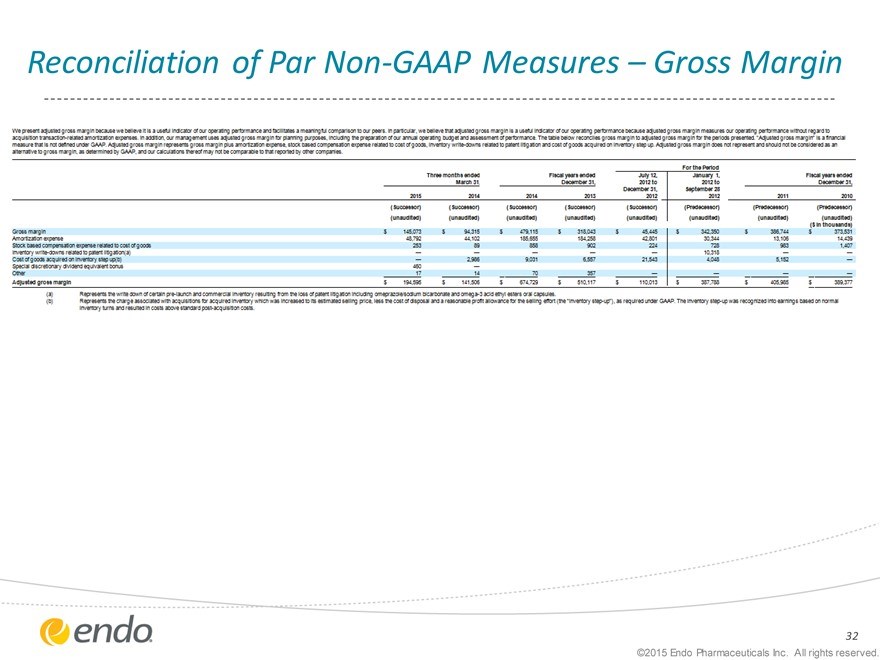
Reconciliation of Par Non-GAAP Measures – Gross Margin
32
©2015 Endo Pharmaceuticals Inc. All rights reserved.
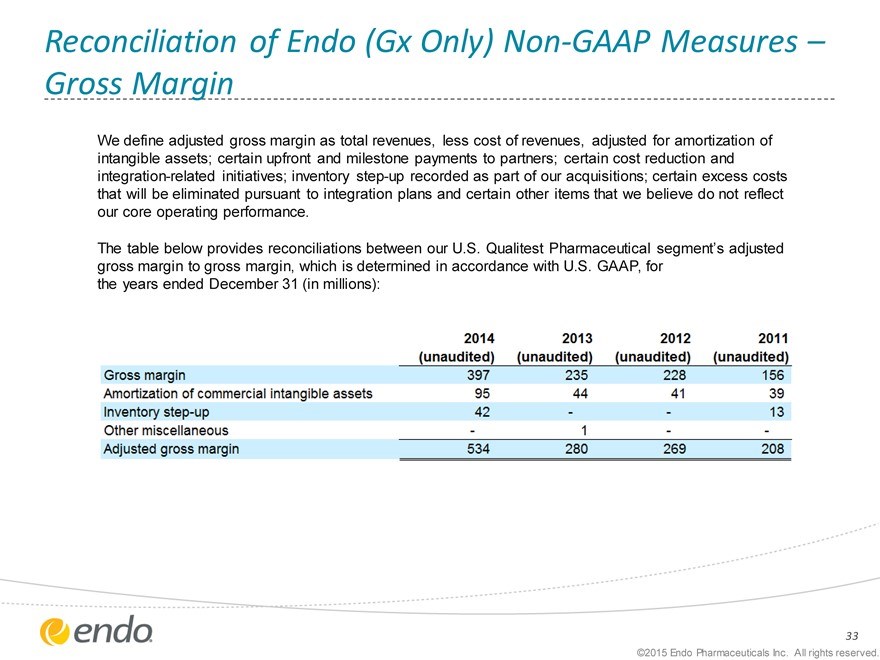
Reconciliation of Endo (Gx Only) Non-GAAP Measures – Gross Margin
We define adjusted gross margin as total revenues, less cost of revenues, adjusted for amortization of intangible assets; certain upfront and milestone payments to partners; certain cost reduction and integration-related initiatives; inventory step-up recorded as part of our acquisitions; certain excess costs that will be eliminated pursuant to integration plans and certain other items that we believe do not reflect our core operating performance.
The table below provides reconciliations between our U.S. Qualitest Pharmaceutical segment’s adjusted gross margin to gross margin, which is determined in accordance with U.S. GAAP, for the years ended December 31 (in millions):
33
©2015 Endo Pharmaceuticals Inc. All rights reserved.

Endo International plc
Par Acquisition
May 18, 2015
©2015 Endo Pharmaceuticals Inc. All rights reserved.
