Attached files
| file | filename |
|---|---|
| EXCEL - IDEA: XBRL DOCUMENT - iNeedMD Holdings, Inc. | Financial_Report.xls |
| EX-3.1 - iNeedMD Holdings, Inc. | ex3_1.htm |
| EX-31.1 - CERTIFICATION - iNeedMD Holdings, Inc. | f10k2014ex31i_ineedmdhold.htm |
| EX-32.1 - CERTIFICATION - iNeedMD Holdings, Inc. | f10k2014ex32i_ineedmdhold.htm |
| EX-31.2 - CERTIFICATION - iNeedMD Holdings, Inc. | f10k2014ex31ii_ineedmdhold.htm |
| EX-32.2 - CERTIFICATION - iNeedMD Holdings, Inc. | f10k2014ex32ii_ineedmdhold.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
☒ ANNUAL REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended: December 31, 2014
or
☐ TRANSITION REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
Commission file number: 333-187248
INEEDMD HOLDINGS, INC.
(Exact name of registrant as specified in its charter)
| Nevada | 45-4487461 | |
| (State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
650 First Avenue, Third Floor
New York, New York 10016
(Address of principal executive offices)
(212) 256-9669
(Registrant’s telephone number, including area code)
Securities registered under Section 12(b) of the Exchange Act: None
Securities registered under Section 12(g) of the Exchange Act: Common Stock, par value $0.001 per share
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports) and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by checkmark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definition of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.:
| Large accelerated filer | ☐ | Non-accelerated filer | ☐ | |
| Accelerated filer | ☐ | Smaller reporting company | ☒ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
Aggregate market value of the voting and non-voting common equity held by non-affiliates as of June 30, 2014: $0.
As of April 15, 2015, the registrant has one class of common equity, and the number of shares issued and outstanding of such common equity is 48,014,424.
Documents Incorporated By Reference: None.
TABLE OF CONTENTS
| 2 |
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
Included in this Annual Report on Form 10-K are “forward-looking” statements, as well as historical information. Although we believe that the expectations reflected in these forward-looking statements are reasonable, we cannot assure you that the expectations reflected in these forward-looking statements will prove to be correct. Our actual results could differ materially from those anticipated in forward-looking statements as a result of certain factors, including matters described in the section titled “Risk Factors.” Forward-looking statements include those that use forward-looking terminology, such as the words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “project,” “plan,” “will,” “shall,” “should,” and similar expressions, including when used in the negative. Although we believe that the expectations reflected in these forward-looking statements are reasonable and achievable, these statements involve risks and uncertainties and we cannot assure you that actual results will be consistent with these forward-looking statements. Important factors that could cause our actual results, performance or achievements to differ from these forward-looking statements include the following:
| ● | the availability and adequacy of our cash flow to meet our requirements; |
| ● | economic, competitive, demographic, business and other conditions in our local and regional markets; |
| ● | changes in our business and growth strategy; |
| ● | changes or developments in laws, regulations or taxes in the healthcare industry; |
| ● | actions taken or not taken by third-parties, including our contractors and competitors; |
| ● | the availability of additional capital; and |
| ● | other factors discussed under the section entitled “Risk Factors” or elsewhere in this Annual Report. |
All forward-looking statements attributable to us are expressly qualified in their entirety by these and other factors. We undertake no obligation to update or revise these forward-looking statements, whether to reflect events or circumstances after the date initially filed or published, to reflect the occurrence of unanticipated events or otherwise unless required by applicable law.
| 3 |
BUSINESS OF INEEDMD
Corporate History
Clutterbug Move Management, Inc. (“Clutterbug”) was incorporated on February 1, 2012 under the laws of the State of Nevada. Clutterbug provided personalized moving assistance and organization support services to the elderly who were seeking a transition to a new location. As senior citizen move managers, the Clutterbug team serviced all aspects of a life move for the elderly including, but not limited to, space and timetable planning, downsizing belongings, sorting possessions, organizing sales and donations, overseeing the transition of items to storage, packing and unpacking and setting up our client’s new residence.
Due to the lack of results in Clutterbug’s attempt to implement its original business plan, management determined it was in the best interests of the shareholders to look for other potential business opportunities that might be available. Effective December 24, 2014, Clutterbug, Clutterbug Acquisition Corp., a Nevada corporation and wholly-owned subsidiary of Clutterbug (“Merger Sub”), iNeedMD, Inc., a privately-held Delaware corporation headquartered in New York and Victoria Young an individual (the “Majority Shareholder”), entered into an Acquisition Agreement and Plan of Merger (the “Agreement”) pursuant to which the Merger Sub was merged with and into iNeedMD, Inc. with iNeedMD, Inc. surviving as a wholly-owned subsidiary of Clutterbug (the “Merger”). The transaction (the “Closing”) took place on December 24, 2014 (the “Closing Date”). Immediately following the Closing of the Agreement, Clutterbug changed its business plan to that of iNeedMD, Inc.
On February 12, 2015, Clutterbug filed an amendment to its Articles of Incorporation with the State of Nevada and changed its corporate name from Clutterbug Move Management, Inc. to iNeedMD Holdings, Inc. On February 25, 2015, the Company received notice from the Financial Industry Regulatory Authority (“FINRA”) that the Company’s name change application has been approved and effective February 27, 2015, Clutterbug began trading under its new name, iNeedMD Holdings, Inc. (“iNeedMD”). Additionally, on February 25, 2015, Clutterbug received notice from FINRA that effective February 27, 2015, it will be quoted on the OTC Markets under its new trading symbol, “NEMD”.
iNeedMD, Inc. is a medical device development company that was incorporated in the State of Delaware on February 16, 2000. Over the course of the last twelve years, it has built a balanced portfolio of intellectual property to support the mobile health and telehealth industry. Our pioneering product development programs focus on easy-to-use systems for collecting a plurality of diagnostic information and transmitting that data to local and/or remote locations.
iNeedMD Holdings, Inc. Overview
One of the Company’s commercialized products, The EKG GloveTM is an FDA-cleared and CE-marked medical device that we believe will transform the way 12-lead, diagnostic electrocardiograms (EKG/ECG) are administered. The ECG is a key component of every cardiac monitoring program for “at risk” patients. According to the American Heart Association, factors that classify patients as “at risk” are old age, tobacco smoking, high blood triglyceride levels, high low-density lipoprotein (LDL) cholesterol levels, low high-density lipoprotein (HDL) cholesterol levels, high blood pressure, diabetes, obesity, chronic kidney disease, excessive alcohol consumption, anxiety, drug abuse, family history and having experienced a prior cardiac event.
According to the World Health Organization, ischemic heart disease (“IHD”), which includes Myocardial Infarctions (MI/AMI), angina, and heart failure due to MI, was the leading cause of death, for both men and women, in the United States and worldwide in 2011. According to the Agency for Healthcare Research and Quality, five of the top 20 most expensive conditions treated in U.S. hospitals by all payers in 2011 were due to cardiac related events (10.4%) with aggregate hospital costs of more than $45 billion. According to the U.S. Department of Health and Human Services, in 2010, 24% of all deaths within the United States were caused by heart disease and in 2011, 45% of men and 31% of women aged 75 years or older had reported that they were told they had heart disease at some point in their life by a physician or other health care professional.
Given the expansive, global impact of various cardiac diseases, The EKG Glove provides healthcare workers with a standardized ECG solution that will enable them to quickly and accurately assess an individual’s cardiac health. As a result, iNeedMD’s products may assist in the management of healthcare costs and ultimately save lives. The sanitary, disposable EKG Glove is applied to the chest in under 30 seconds as the electrodes and conductive circuitry are fully integrated into a single, flexible shell. A single coaxial cable connects The EKG Glove to most ECG acquisition devices, eliminating the use of the numerous “spaghetti wires” that have been indicated in the spread of Hospital Acquired Infections (HAIs) and are easily positioned incorrectly leading to erroneous test results. In essence, The EKG Glove is an ECG “peel-and-stick” solution that facilitates the faster and more accurate collection of data that remains the mainstay in the assessment of cardiac health. We believe The EKG Glove can immediately be put into service in hospitals and other medical facilities that are looking for time-saving and infection-limiting disposable products.
| 4 |
iNeedMD is also focused on innovation as it relates to the acquisition and transmission of ECGs and other health data so that doctors, regardless of their physical proximity to a patient, can diagnose or rule-out a variety of medical disorders. The Company’s research efforts include the development of portable, wireless platform technologies that are Bluetooth- and WIFI-enabled. When paired with iNeedMD’s easy-to-use EKG Glove and similar products, these devices will enable individuals of various skill levels to obtain critical patient information and transmit results to skilled medical professionals at any location. In addition, the flexibility of The EKG Glove and evolving products, make partnering with other medical device manufacturers feasible where synergistic and volume-driven market opportunities exist in the point-of-care and telehealth markets. The Company is currently in discussions to pair The EKG Glove with 510(k) cleared wireless ECG devices (“EKG Glove System”) to enable immediate penetration into a variety of target markets.
Target Markets
The EKG Glove has six distinct markets:
| 1. | Hospitals - Gaining acceptance of The EKG Glove as an essential component of institutions’ cardiac screen programs, will assist the Company in solidifying the benefits of the product within the aggregate medical community. The emergency department is a targeted user base in hospitals as minimizing time and maximizing accuracy are keys to effective patient triage and treatment. In addition, the disposability of The EKG Glove, along with its all-in-one design and single cable connection to existing EKG machines supports the Center for Disease Control’s (CDC) directive that patient care equipment as it relates to infection prevention and control, preferably be disposable. Since hospital acquired infections are always of concern, and in light of the preparatory steps that many hospitals are taking to prevent the spread of infectious disease, The EKG Glove can be a helpful solution for delivering efficient, infection-limiting cardiac monitoring services throughout the hospital. The Company has already conducted several successful clinical studies and trials in a variety of New York hospitals including NYU Medical, Mount Sinai Hospital and Peconic Bay Hospital and is working to expand its reach to other private, public and military institutions in the United States and internationally. |
| 2. | Nursing Homes and Skilled Nursing Facilities - According to the American Association of Cardiovascular and Pulmonary Rehabilitation, acute cardiac events such as myocardial infarction and heart failure affect over two million individuals in the United States each year, and over half of these individuals are over the age of 65. Also common in older adults are cardiac surgical procedures such as coronary artery bypass and valve surgery. After cardiac events, these older adults often require rehabilitation at a skilled nursing facility (SNF) or Nursing Home; these centers often lack in-house cardiac care programs. The Company’s pairing of its easy–to-use EKG Glove with a wireless, portable ECG acquisition device (the “EKG Glove System”) provides the Company with an opportunity to deliver one of the most important and basic cardiac testing methodologies to these facilities to improve patient care. Healthcare workers, of any skill level, can be trained to adhere The EKG Glove to the patient and obtain an accurate ECG; the data is then captured for immediate interpretation by a physician and/or transmitted to the facility’s electronic health record for remote analysis or interpretation at a later time based on the medical staff’s schedule. As a result, the Company believes that integration of the EKG Glove System into nursing facilities will fill an unmet market need. |
| 3. | Heart Rhythm Monitoring Market - With the rise in the number of patients in the United States suffering from various stages of heart disease, it is becoming increasingly necessary to monitor a patient’s heart function on a regular basis. Most people are familiar with the use of ambulatory Holter Monitors used by cardiologists to record a patient’s heart function over a 24 or 48 hour period. In addition, according to the National Center on Sleep Disorders Research, about 70 million Americans suffer from sleep problems, with nearly 60 percent of those individuals being categorized as having a chronic disorder. Sleep studies aimed at diagnosis of these disorders like sleep apnea, have ECG monitoring at the core of the assessment. In both cardiac and sleep monitoring applications, The EKG Glove when paired with the Company’s wireless, portable ECG acquisition device that is currently in development (the “iNeedMD EKG Glove System”) plus a lightweight vest to hold the devices in place, will have the added advantage of recording and transmitting ECG data via a wireless connection through a software interface to a tablet, personal computer or cell phone back to the doctor’s office in real-time. This feature will provide physicians with the capability to discover serious problems before the end of the test and ultimately help patients understand and monitor their own health status. The remote communications capability of the iNeedMD EKG Glove System is likely to alleviate the burden on the medical services by allowing the ECG test to be self-administered at home. |
| 5 |
Other attractive segments within the Heart Rhythm Monitoring market include the use of The EKG Glove, with existing ECG machines or paired with a wireless portable ECG acquisition device, by Clinical Research Organizations (CROs) involved in pharmaceutical drug development. Throughout the multi-phase process, assessment of a chemical compound’s potential impact on cardiac health must be completed. A research professional can utilize The EKG Glove to quickly and precisely position leads on the patient or volunteer and acquire an accurate ECG tracing while effectively minimizing the risk of lead misplacement or transmission of infection as compared to the utilization of traditional reusable, “spaghetti wire” leads.
| 4. | Medical Transport - When emergency medical technicians (EMTs) wirelessly transmit electrocardiograms (ECG) directly to a cardiologist or attending physician’s Bluetooth-enabled device for immediate interpretation, heart attack patients can potentially receive an artery-opening procedure in half the usual time. The physician, upon observing definitive signs of a heart attack via remote acquisition, can direct hospital staff to have the patient bypass the emergency department upon arrival and go directly to the catheterization laboratory for treatment. Since The EKG Glove can facilitate accurate ECG lead placement in under 30 seconds, the product’s ease-of-use can improve the efficiency of the EMT and helps ensure the precision of the ECG tracing is of diagnostic quality. The American College of Cardiology (ACC) and the American Heart Association recommend that patients have their arteries opened directly within 90 minutes of arriving at the hospital; as a result if the EKG Glove System facilitates a quicker "door-to-reperfusion" rate, the more likely the heart muscle and the patient will be saved. | |
| 5. | Remote Medical Assessment - As previously noted above,wirelessly transmitting electrocardiograms (ECG) via Bluetooth-enabled devices directly to physicians for immediate assessment and interpretation improves the quality of patient care. This is especially true in geographically isolated regions or other settings where cardiac care physicians are absent or inaccessible. In remote locations such as in rural communities, telehealth adoption is significantly higher as compared to hospitals in urban areas. For example, Alaska hospitals reported the highest percent of telehealth adoption at 75 percent; Arkansas at 71 percent; South Dakota at 70 percent; and Maine at 69 percent. In addition, the Department of Veterans Affairs recently reported that 690,000 veterans received care via 2 million telehealth visits in fiscal year 2014 which ended in September 2014 – that’s 12 percent of all veterans enrolled in VA health care. Home telehealth is a growing segment of the category and often only encompasses the acquisition of vital signs such as pulse, weight, blood pressure and temperature. Since The EKG Glove is capable of being self-adhered given its all-in-one design, and the Bluetooth ECG acquisition device can be configured to facilitate ECG transmission with minimal patient interface, this user-friendly system has the potential to enable off-site cardiac assessments into a home telehealth menu of services. |
In addition, according to the Western Journal of Emergency Medicine, medical incidents that occur during commercial air travel, often require assistance from a medical professional facilitated through the services of remote emergency response centers. “MedAire,” “The First Call,” and the University of Pittsburgh Medical Center’s “StatMD,” for example, offer 24-hour consultations via call centers staffed by physicians. MedAire responds to an average of 17,000 cases per year. Emergency medical kits onboard aircraft are not required to include cardiac monitoring equipment such as The EKG Glove System. Adding this device system to an emergency response protocol to enable the acquisition ECGs for interpretation by doctors at a call center, can assist in ruling out of events initially perceived as cardiac, or confirm the need for the crew/pilot to request further medical assistance that may require diversion of the flight to the nearest airport capable of landing the aircraft. The act of diverting a full aircraft is expensive and is estimated to range between $3,000 and $100,000 depending on the size of the plane and the basic costs of refueling, plus the financial consequences of re-routing passengers. We believe the development of the iNeedMd EKG Glove System is timely as on-board Wi-Fi access becomes more prominent on commercial airlines which will facilitate the remote transmission of critical cardiac data. We believe this will allow medical and airline professionals to make well-informed and potentially life-and cost-saving decisions.
| 6 |
| 6. | Urgent Care Health Clinics - According to AMN Healthcare, walk-in facilities afford widespread access to care for minor illnesses and a less-expensive alternative to after-hours care. The professional staff is also trained to recognize an urgent matter and refer the patient to the nearest emergency department. Many of the established retail clinics, such as Walgreens’ Healthcare Clinic, are expanding into diagnosing and treating chronic illnesses. Regardless of the treatment scenario, the addition of the user-friendly EKG Glove System for a rapid and accurate assessment of an individual’s cardiac health may provide clinics with more efficient and cost-effective care programs that ultimately improve patient outcomes. |
Manufacturing
The services of an outside operator, Wilson-Hurd Manufacturing (WH) of Berlin, Wisconsin, are utilized to run, build and manage the manufacturing process of the EKG Glove. WH has manufacturing and quality control systems that comply with ISO 9001:2008, ISO 13485:2013 and FDA regulatory requirements.
A semi-automated process is currently used to manufacture the EKG Glove, where human interaction is necessary to move each individual glove through the assembly line. Under the current assembly line configuration, bulk production of the disposable EKG Glove is approximately 25,000 gloves per month (per shift). WH can expand production capacity by adding up to 2 additional shifts for a maximum of 75,000 gloves per month. Should volume requirements warrant an incremental increase in production capacity, iNeedMD is ready to commence the construction of one fully automated, no-touch assembly line. Such a line can be operated in two eight hour shifts producing 83,000 EKG Gloves monthly (per shift). This would increase the production capacity to approximately 166,000 gloves per month (assuming 2 shifts per day) and it is estimated that the fully automated line will reduce the per-unit cost of The EKG Glove by approximately 25% (assuming two shifts).
Products and Usage
| 1. | The EKG Glove |
The EKG Glove is a single-use, disposable medical device that replaces the traditional EKG lead wires and electrodes used in the diagnosis of and screening for cardiovascular disease. The EKG Glove has been designed to obtain a full, diagnostic, 12 lead, resting electrocardiogram (ECG/EKG) using embedded printed circuitry connected to pre-positioned electrodes affixed to the underside of a glove-like sheath (or shell).
The EKG Glove is FDA-cleared, CE Marked and protected by eight (8) U.S. and three (3) international patents:
The key distinction between The EKG Glove and the traditional method of EKG administration is the removal of the 10 wire leads, commonly referred to as “spaghetti wires” and having the electrodes embedded into the form factor of The EKG Glove.
To obtain a tracing, the operator need only (i) remove the backing; (ii) slip on The EKG Glove; (iii) properly position and apply it to the patient’s chest; (iv) attach the three extendable electrodes, (vv) plug the 10 pin connector of the accessory cable into The EKG Glove, and (v) plug the other connector to any compatible ECG monitoring device. A full 12-Lead, diagnostic ECG will be produced and viewable. This entire procedure can be completed in under 1 minute. The EKG Glove can be utilized with most existing EKG machines in hospitals or doctors’ offices.
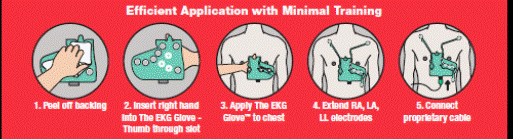
| 7 |
| 2. | Proprietary Acquisition Devices |
iNeedMD is in the process of formalizing relationships with third party device manufacturers to supply wireless, FDA-cleared, Bluetooth-enabled (with USB-connected capability) ECG acquisition devices that will allow the cardiac tracings to be transmitted to a wide variety of devices including laptops, tablets and ultimately cell phones. Device software will permit live streaming of the ECG and the saving and forwarding of various file types that are compatible with data stored within Electronic Health Record (EHR) systems. All partners will provide some customization of their software interface to ensure compatibility with existing EHRs.
The Company is also developing its own wireless, portable health information acquisition device that will be Bluetooth- and Wi-Fi-enabled, and be application driven to offer a seamless, user-friendly interface with laptops, tablets and cell phones. iNeedMD is also exploring an agreement with an India-based interpretation services providers that will allow the Company to provide healthcare clients with interpretations service from certified cardiologists and other specialists if local access is not feasible.
The Company is dependent on third-party service providers to develop, deliver and maintain certain aspects of its product and operations, including the manufacturing of its ECG acquisition devices. Until such time as the Company’s own acquisition device is fully developed, the Company will rely on third parties for the initial pairing, development and clearance of the ECG acquisition device with The EKG Glove. The Company has limited control over these third parties. Any discontinuation of these third-party services, or any reduction in performance or increase in price that would require the Company to replace such services, would disrupt its business. In the event that these service providers are unable to operate to the Company’s satisfaction, it would be forced to seek other firms to provide these services. There can be no assurance that services from substitute providers would be available on reasonable terms, if at all. Such an occurrence would involve significant delay and expense and would have a material adverse effect on the Company’s business, prospects, results of operations and financial condition.
Competition
We believe the Company is in an unprecedented position in having the only FDA-cleared 12-lead diagnostic EKG product that is sanitary, disposable and is easy enough to apply that even a patient has the potential to position accurately on himself/herself which can improve patient comfort and care. The product features may also decrease the personnel demands within the healthcare system as it can allow less technical staff to assist healthcare professionals in preparing the patient for an electrocardiogram. It may also help facilitate the implementation of telehealth initiatives related to heart health in a more streamlined, accessible manner. Its innovations are protected by 8 patents (international patents are held in China, India and Israel), which we believe will make it difficult for any company to compete with a similar product. In addition, iNeedMD’s intellectual property portfolio encompasses a system for collecting and transmitting a plurality of diagnostic information to remote locations, as well as receiving data which helps to safeguard future product development efforts. Aside from an EKG diagnostic device, patent-protections include blood pressure, pulse rate, and temperature monitoring, as well as an auscultation device, all within the easy-to-use glove framework.
The Company believes the primary obstacle for market penetration will come from apathy to change, fear of financial uncertainty due to change, fear of the application of new and innovative technologies and the unwillingness to learn new products and methods even if that change would enhance the workplace. Primary competition should be segmented by two distinct markets. For The EKG Glove used in tandem with standard legacy ECG machinery via a single coaxial cable, other non-disposable or single-use leadwire systems that are less user-friendly pose a competitive threat as they may have long terms contracts (purchase orders and maintenance agreements) and/or be associated with larger companies.
| 8 |
Snapshot of EKG Glove versus Leadwire Competitors:
| Company EKG Devices | iNeedMD,
Inc. EKG Glove |
LifeSYNC, Corp. LeadWear |
Various Manufacturers Traditional ECG Electrodes |
| Universally Compatible | YES | No | Yes |
| Prescription Ready | YES | Yes | Yes |
| Always Sanitary | YES | Yes | No |
| Electromagnetic Interference Protected | YES | No | No |
| Limited/No Patient Prep Required | YES | No | No |
| Disposable | YES | Yes | Available |
| No “Spaghetti Wire” | YES | Yes | No |
| No Additional Electrodes | YES | No | No |
| Wireless Availability | YES | Yes | No |
| Can be applied in under 1 minute | YES | No | No |
| Radiolucent | YES | Yes | No |
| Compact Light Weight | YES | Yes | Yes |
| Mobile | YES | Yes | Yes |
| No External leads from device needed | YES | No | No |
| Hypoallergenic | YES | Yes | Yes |
| No Arms and Legs Leads | YES | Yes | No |
| Made in the USA | YES | No | Yes/No |
| MSRP for 12 Lead | $20.00 | $15.00 | $0.85 to $3.00 |
As noted in the table above, The EKG Glove is currently the only disposable, all-in-one, 12-Lead system that offers a time-efficient, accurate application in less than 1 minute, regardless of the skill level of the individual/technician positioning the device on the patient.
When The EKG Glove is paired with an ECG acquisition device, different competitive profiles emerge. The institutional/hospital market is dominated by existing, mainline EKG machines sold by large well-known manufacturers and their distributors. In this scenario, we define our competition as companies that sell fixed or large, ECG machines that are far from portable, or complex PC-based ECG systems. Many of these competitors have greater access to capital than iNeedMD which can help maintain or expand their market-share. As a result, this market may have substantial barriers to entry when the pairing of The EKG Glove with a more portable ECG acquisition device. However, iNeedMD’s EKG Glove has the benefit of being uniquely compatible with these devices via a single coaxial cable; therefore, The EKG Glove can be integrated into most hospital systems as a valued accessory that can improve workflow and patient comfort and care.
The traditional ECG machines and PC-based systems are costly to acquire and service. For example, General Electric, sells its smallest PC-based system for approximately $3,500 in the U.S. The overwhelming majority require a certified trained professional to place a complicated system of individual lead wires on an individual in the correct configuration to obtain an ECG tracing. For office-based practitioners, retail health clinics, and other non-institutional healthcare environments, the acquisition cost and personnel requirements for traditional ECG systems may prohibit healthcare professionals from offering their patients one of the basic assessment tools to determine cardiac health. As a result, portable ECQ acquisition devices, some with USB and/or wireless capability, have emerged in the marketplace as viable alternatives. However, none of the current offerings provide the convenience and accuracy of the “peel-and-stick”, disposable EKG Glove design that takes the complexity out of proper lead placement and can improve the accuracy of ECG tracings.
| 9 |
Snapshot of The EKG Glove paired with a Wireless ECG Acquisition Device versus the Competition:
| Company EKG Devices | iNeedMD,
Inc. EKG Glove + Acquisition Device |
LifeSYNC, Corp. LeadWear System | Commwell Medical, Inc. Physio Glove ET | SHL SmartHeartPRO |
| Universally Compatible | YES | No | No | No |
| Prescription Ready | YES | Yes | Yes | Yes |
| Always Sanitary | YES | Yes | No | No |
| Electromagnetic Interference Protected | YES | Yes | No | No |
| Limited/No Patient Prep Required | YES | No | Yes | Yes |
| Disposable | YES | Yes | No | No |
| No “Spaghetti Wire” | YES | Yes | Yes | Yes |
| No Additional Electrodes | YES | No | Yes | Yes |
| Wireless Availability | YES | Yes | Yes | Yes |
| Can be applied in under 1 minute | YES | No | Yes | Yes |
| Radiolucent | YES | Yes | No | No |
| Compact Light Weight | YES | Yes | No | No |
| Mobile | YES | Yes | Yes | Yes |
| No External leads from device needed | YES | No | Yes | Yes |
| Hypoallergenic | YES | Yes | Yes | Yes |
| No Arms and Legs Leads | YES | Yes | No | Yes |
| Made in the USA | YES | No | No | No |
| MSRP for ECG Acquisition Device + 12 Lead System | $2,500.00 | $3,000.00 | $2,400.00 | $1,225.00 |
The overwhelming majority of competitive offerings still require the assistance of a certified trained professional to place a complicated system of ten lead wires on an individual in the correct configuration to obtain an electrocardiogram. Overall, the Company believes that the primary competitive factors in the EKG/ECG markets include:
| ● | The quality and reliability of the overall patient data delivery solution; | |
| ● | Access to distribution channels necessary to achieve broad distribution and use of products; | |
| ● | The availability of diagnostic equipment with the ability to generate digital results that can be delivered over the Internet and the access to necessary intellectual property rights; | |
| ● | The ability to license or develop and support secure formats for digital patient data delivery; | |
| ● | The ability to obtain appropriate regulatory clearances and certifications when required; | |
| ● | The ability to license and support popular and emerging media formats for digital media delivery, particularly video in live and replay formats, in a market where competitors may control the intellectual property rights for these formats; | |
| ● | The size of the patient community for streaming and digital media and its appeal to content providers and the medical community; |
| 10 |
| ● | Features for care givers to act on and their adaption to the diagnostic information gained over the Internet; | |
| ● | Ease of use and interactive user features in products; | |
| ● | Ease of finding and accessing patient information over the Internet; | |
| ● | Ability of streaming media, media delivery, and network capacity technology and cost per user; | |
| ● | Pricing and licensing terms; | |
| ● | Compatibility with new and existing electronic medical/health record systems; | |
| ● | Compatibility with the user’s existing network components and software systems; | |
| ● | The size and financial resources of our competitors; | |
| ● | The customer loyalty and in-elasticity of the consumer markets for medical supplies; and | |
| ● | Challenges caused by bandwidth constraints and other limitations of the Internet infrastructure. |
When The EKG Glove is paired with portable, wireless ECG acquisition devices, the system should have appeal in a variety of healthcare scenarios previously noted in the Target Market analysis, even as compared to other wireless device systems. Currently, our system is the only FDA-cleared, user-friendly EKG monitoring device system that has a sanitary, single-patient disposable lead wire system that helps ensure proper lead placement regardless of the skill level of the individual facilitating the ECG tracing. These factors comprise iNeedMD’s belief that the Company’s products will be valued by various target markets in the United States and abroad.
| 1) | The EKG Glove offers a sanitary, disposable model, with all circuitry and leads built-in to the single, easy- to-use device. |
| 2) | The EKG Glove is the only disposable ECG lead system that does not require the assistance of a skilled technician to accurately position on a patient. |
| 3) | The EKG Glove is compatible with most existing ECG machines via a single connector cable. |
| 4) | When paired with a wireless ECG acquisition device and software, The EKG Glove is the only disposable, sanitary ECG system with the potential for self-administration. |
Sales and Marketing
The Company’s marketing strategy is to stress the ease-of-use, accuracy, speed and flexibility of The EKG Glove. Our research suggests that the Company should initially introduce The EKG Glove paired with a wireless ECG acquisition device to Health Care Professionals with an aggressive campaign targeting Nursing Homes/Skilled Nursing Facilities, Medical Transport Companies, Retail Health Clinics and Hospitals.
iNeedMD is currently forging relationships with established sales agents and healthcare distributors, both domestic and international, with strong relationships in the targeted market segments. The Company is also building a technical sales and service team that will facilitate training of the aforementioned outside sales network and drive product integration and account servicing. iNeedMD is also planning to participate in market-specific trade shows and conferences that will expose the Company and its products to potential users within the nursing home, telehealth, government and urgent care sectors.
Price: The EKG Glove does not require a skilled technician for precise placement on an individual and therefore minimizes the potential for repeat testing that often exists when traditional lead-wires are positioned incorrectly. Pairing with a portable, compact ECG acquisition device can eliminate the need for healthcare professionals to purchase bulky, expensive ECG machines. These benefits have numerous cost-saving implications related to reducing expensive capital equipment purchases and limiting the need for highly compensated technical personnel. Although The EKG Glove will be priced higher than traditional “spaghetti wire” lead systems, the speed, accuracy, cost-reducing and other tangible benefits justify the user cost of approximately $20. In addition, through market research and sales trials conducted by iNeedMD, management, the cost to a hospital, per EKG done in the conventional manner is calculated to be approximately $22 to $26. Based on the same research, management believes that The EKG Glove can reduce the per unit (EKG) cost down to approximately $21. This creates approximately a $5 saving, per EKG. If the integration of The EKG Glove will be paired with the purchase of a wireless ECG acquisition device, the cost of the hardware will be expected to range between $1500 and $2500, depending on a facility’s need to also integrate the purchase of a Bluetooth or Wi-Fi enabled device.
| 11 |
Compensation: Compensation for the sales efforts of the Company is commission-based. The structure rewards external sales agents and an initially small internal staff with a target compensation of 20% of gross sales.
Distribution: The Company is strategically partnering with carefully-selected distributors (national and international) with existing sales relationships with customers within the varied target markets previously noted.
International Scale: Given the scope of some of the technology to be deployed, the Company must think globally. iNeedMD is currently negotiating licensing arrangements with groups in Saudi Arabia (for the Middle East Market), India, South America, and China.
Strategic Partnerships: In order to take advantage of immediate market opportunities with customers looking to adopt The EKG Glove paired with a portable ECG device, iNeedMD has solidified relationships with suppliers of FDA-cleared ECG acquisition devices; therefore at this time, iNeedMD relies on third parties for the manufacturing of such devices. Other business alliances with companies in the related healthcare industries may be considered if opportunities warrant such collaborations.
Intellectual Property Rights
iNeedMD, Inc. has patented technology on mounting biosensors on a glove (mitten) platform that will generate vital signs, including blood pressure, pulse rate, oxygen saturation, temperature and a full twelve-lead EKG, which can be transmitted via Internet, wireless or commercial telephone lines to any remote location. iNeedMD currently has eight patents issued (US patent Nos. 6,224,548; 6,248,064; 6,595,918; 6,540,673; 6757, 556; 7,112,175; 7,435,222 and; 7,753,845), two for the original glove design and two for a two-glove mapping system intended for use in future product offerings beyond the first four products.
The Company also has a number of additional patents for the original glove design and a two-glove system, as well as applications for its electrodes and twelve-lead design. International patents are held in China, India and Israel and will be applied for in Canada, Europe, Brazil and Mexico. All of the applications are for utility patents and some include method claims or technology patents.
U.S. PATENT No. 6,224,548 - Tele-diagnostic device
File Date: May 26, 1998
Issued Date: May 1, 2001
Expire Date: May 26, 2018
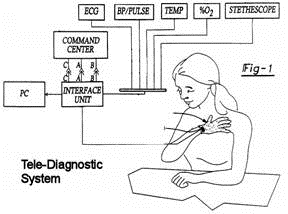 |
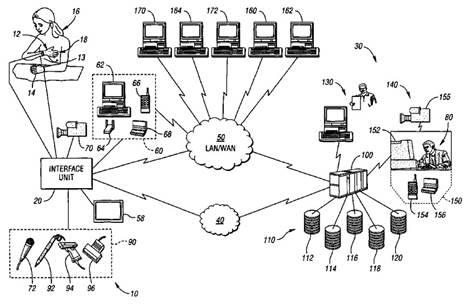
The objective of this patent is to provide a system for collecting a plurality of diagnostic information and transmitting the diagnostic information to a remote location. The system (Fig-1) comprises a glove member (Fig-2) adaptable to be worn on a person's hand and an interface unit (Fig. 3) in electrical communication with the glove member. The interface unit is capable of transmitting information to, and receiving information from, a remote location. The glove member comprises a palm portion, a wrist portion and five phalange portions. The glove member further comprises an EKG diagnostic device, a blood pressure and pulse rate diagnostic device and a temperature device. The glove member may also further comprise a diagnostic device and an auscultation device. |
| 12 |
U.S. PATENT No. 6,248,064 - Continuation of U.S. PATENT No. 6,224,548
File Date: Nov 10, 1998
Issued Date: Jun 19, 2001
Expire Date: May 26, 2018
Added: Additional
Glove apparatus (Two Glove system)
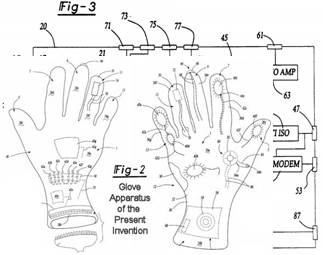
The objective of this patent is to create a system for collecting a plurality of diagnostic information and transmitting the diagnostic information to a remote location for providing emergency treatment. The system comprises a first member adaptable to be worn on a person's first hand and a second member adaptable to be worn on a person's second hand. The members comprise a plurality of diagnostic devices and a defibrillator device. A transmitting unit for transmitting information to, and receiving information from, a remote location is provided.
The patents granted below are all related to the Two Glove system and were issued as a continuation of U.S. PATENT No. 6,224,548.:
U.S. PATENT No. 6,595,918 - Continuation of U.S. PATENT No. 6,224,548
File Date: Jun 19, 2001
Issued Date: Jul 22, 2003
Expire Date: May 26, 2018
U.S. PATENT No. 6,540,673 - Continuation of U.S. PATENT No. 6,224,548
File Date: Dec 19, 2000
Issued Date: Apr 1, 2003
Expire Date: May 26, 2018
U.S. PATENT No. 7,753,845 - Divisional of U.S. PATENT No. 6,540,673 which is a Continuation of U.S. PATENT No. 6,224,548
File Date: Mar 27, 2003
Issued Date: Jul 13, 2010
Expire Date: May 26, 2018
| 13 |
U.S. PATENT No. 7,112,175 - Continuation of U.S. PATENT No. 6,595,918 which is a Continuation of U.S. PATENT No. 6,224,548
File Date: Dec 18, 2001
Issued Date: Sep 26, 2006
Expire Date: May 26, 2018
U.S. PATENT No. 7,435,222 - Continuation of U.S. PATENT No. 7,112,175 which is a Continuation of U.S. PATENT No. 6,595,918 which is a Continuation of U.S. PATENT No. 6,224,548
File Date: Sep 25, 2006
Issued Date: Oct 14, 2008
Expire Date: May 26, 2018
U.S. PATENT No. 6,757,556 - Continuation of U.S. PATENT No. 6,595,918 and U.S. PATENT No. 6,540,673 and both are a Continuation of U.S. PATENT No. 6,224,548
File Date: Oct 4, 2001
Issued Date: Jun 29, 2004
Expire Date: May 26, 2018
Spring loaded Electrode Sensor.
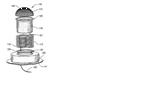 |
The patent related to the spring loaded electric sensor involves an electrode sensor attachable to a substrate for sensing electrical activity of a patient. The electrode sensor comprises an elongated conductive body having first and second ends, wherein the first end is adapted to contact the patient for sensing electrical activity of the patient. The second end is configured to conductively attach to the substrate. The elongated conductive body is greater than about 2 millimeters in length and is configured to extend from the substrate. |
INTERNATIONAL PATENTS
CANADA PATENT No. 2332892 - Same as U.S. PATENT No. 6,224,548
File Date: May 19, 1999
Issued Date: Nov 3, 2009
Expire Date: May 19, 2019
INDIA PATENT No. 246790 - Same as U.S. PATENT No. 6,224,548
File Date: May 19, 1999
Issued Date: Mar 16, 2011
Expire Date: May 19, 2019
CHINA PATENT No. ZL98815542.X - Same as U.S. PATENT No. 6,224,548
File Date: Dec Nov 1, 1999
Issued Date: Mar 27, 2002
Expire Date: Nov 1, 2019
ISRAEL PATENT No. 139781 - Same as U.S. PATENT No. 6,224,548
File Date: May 19, 1999
Issued Date: Oct 6, 2006
Expire Date: May 19, 2019
| 14 |
METHOD FOR REMOTE MEDICAL CONSULT AND CARE (TELE-HEALTH)
U.S. PATENT No. 7,860,725
File Date: Dec 19, 2000
Issued Date: Apr 1, 2003
Expire Date: May 19, 2019
A method for remote medical consulting includes collecting diagnostic data using at least one wearable device contoured to at least a portion of a person's hand, transmitting the diagnostic data to a remote location, transmitting audio data and video images of the patient to the remote location, and communicating diagnosis and/or treatment information to the patient based at least in part on the diagnostic data. The treatment information may include a prescription electronically transmitted to the patient or a pharmacy. The method includes billing of the patient via credit or debit card, bank account, or a third party, such as an insurance company. The diagnostic data as well as the audio and video data may be transmitted wirelessly via cellular or satellite communication networks and/or using a wide area computer network such as the internet.
U.S. PATENT No. 8,285,560 - Continuation of U.S. PATENT No. 7,860,725
File Date: Dec 23, 2010
Issued Date: Oct 9, 2012
Expire Date: May 19, 2019
In October 2013, the Company was also granted a provisional patent for a Glove base ultra sound. This patent is currently pending approval.
DESCRIPTION OF PROPERTY
We currently lease commercial office space located at 650 First Avenue, Third Floor, New York, New York 10016 for $3,855 per month. The Company entered into the lease in July 2014 for a term of 5 years with the option to renew.
RISK FACTORS
You should carefully consider the risks described below together with all of the other information included in this Annual Report on Form 10-K before making an investment decision with regard to our securities. The statements contained in or incorporated herein that are not historic facts are forward-looking statements that are subject to risks and uncertainties that could cause actual results to differ materially from those set forth in or implied by forward-looking statements. If any of the following risks actually occurs, our business, financial condition or results of operations could be harmed. In that case, you may lose all or part of your investment.
| 15 |
RISKS RELATED TO OUR COMPANY AND OUR INDUSTRY
THE COMPANY OPERATES IN A COMPETITIVE ENVIRONMENT.
Many of the Company’s current and potential competitors have longer operating histories, greater name recognition, more employees, and significantly greater financial, technical, marketing, public relations, and distribution resources than the Company. The competitive environment may require the Company to make changes in the Company’s products, pricing, licensing, services, or marketing to maintain and extend the Company’s current brand and technology franchise. Price concessions or the emergence of other pricing or distribution strategies of competitors may diminish the Company’s revenues, impact the Company’s margins, or lead to a reduction in the Company’s market share, any of which will harm the Company’s business.
The Company believes that the primary competitive factors in the medical device market include:
| ● | access to distribution channels necessary to achieve broad distribution and use of products; | |
| ● | the availability for delivery over the Internet and ownership of necessary intellectual property rights; | |
| ● | the ability to license or develop and support its devices; | |
| ● | the ability to license and support its products where competitors may control the market; | |
| ● | the market penetration and size of product use and its appeal to hospitals, EMS, and other medical institutions; | |
| ● | features for the analysis of the data that is obtained; | |
| ● | ease of use and interactive user features in products; | |
| ● | compatibility with new and existing software; | |
| ● | compatibility with the user’s existing network components and software systems; and | |
| ● | challenges caused by the introduction of new medical devices into the established market. |
The Company’s failure to adequately address any of the above factors could harm the Company’s business strategy and operating results.
WE HAVE LIMITED OPERATING HISTORY.
To date, our efforts have been focused primarily on the development and marketing of our business model. We have limited operating history for investors to evaluate the potential of our business development. We may not operate profitably and we may not have adequate working capital to meet our obligations as they become due.
THE COMPANY’S QUARTERLY FINANCIAL RESULTS MAY FLUCTUATE SIGNIFICANTLY.
The Company expects its quarterly revenues, expenses and operating results to fluctuate significantly in the future as a result of a variety of factors, some of which are outside of the Company’s control. These factors include:
| ● | the number of the Company customers; | |
| ● | the Company’s ability to establish and strengthen brand awareness; | |
| ● | the Company’s success, and the success of its strategic partners, in marketing the Company’s products and services; | |
| ● | the amount and timing of the costs relating to marketing efforts or other initiatives; | |
| ● | the timing of contracts with strategic partners and other parties; | |
| ● | fees the Company may pay for distribution, service agreements and promotional arrangements or other costs the Company incurs as it expands operations; | |
| ● | the level of acceptance of the Internet by the healthcare industry; | |
| ● | the Company’s ability to compete in a highly competitive market, and the introduction of new sites and services by the Company or its competitors; | |
| ● | technical difficulties, system downtime, undetected software errors and other problems affecting the Internet generally or the operation of the Company Web site; and | |
| ● | economic conditions specific to the Internet and online media and general economic conditions. |
| 16 |
In addition, in an attempt to enhance the Company’s long-term competitive position, the Company may from time to time, make decisions regarding pricing, marketing, services and technology that could have a near-term material adverse effect on its business, financial condition and operating results. Due to the foregoing factors, the Company believes that quarter-to-quarter comparisons of its operating results are not a good indication of its future performance.
THE MARKET STUDIES DESCRIBED HEREIN WERE COMMISSIONED AND PAID FOR BY THE COMPANY AND ARE NOT INDEPENDENT.
The Company’s market studies were not prepared by or reviewed by an independent source. The results of these studies may not be representative or indicative of the actual consumer response to the Company’s products. The Company has only made minimal sales to date. There can be no assurance that the Company’s projections will or can be realized and actual results may differ materially from those set forth in the Company’s projections. Because of the above limitations, investors are cautioned against placing undue reliance on these market studies.
THE IMPLEMENTATION OF THE COMPANY’S BUSINESS STRATEGY WILL REQUIRE SIGNIFICANT EXPENDITURE OF CAPITAL AND WILL REQUIRE ADDITIONAL FINANCING.
The implementation of the Company’s business strategy will require significant expenditures of capital, and the Company will require additional financing. Additional funds may be sought through equity or debt financings. The Company cannot offer any assurances that commitments for such financings will be obtained on favorable terms, if at all. Equity financings could result in dilution to holders and debt financing could result in the imposition of significant financial and operational restrictions on the Company. The Company’s inability to access adequate capital on acceptable terms could have a material adverse effect on the Company’s business, results of operations and financial condition.
If the Company fails to generate revenue as anticipated or if its development costs continue to exceed available funds, the Company may be required to undertake unplanned additional financing(s). If such additional financing is required, the Company can offer no assurance that additional funds will be available or that they will be obtained on economically acceptable terms.
THE COMPANY MAY NOT SUCCESSFULLY DEVELOP NEW PRODUCTS AND SERVICES.
The Company’s growth depends on its ability to develop leading edge medical device products and services. The Company’s business and operating results would be harmed if the Company fails to develop products and services that achieve widespread market acceptance or that fail to generate significant revenues to offset development costs. The Company may not be able to timely and successfully identify, develop and market new product and service opportunities. If the Company introduces new products and services, they may not attain broad market acceptance or contribute meaningfully to the Company’s revenues or profitability.
Because the markets for the Company’s products and services are rapidly changing, the Company must develop new offerings of its products and services quickly. The Company has experienced development delays and cost overruns in its development efforts in the past and the Company may encounter such problems in the future. Delays and cost overruns could affect its ability to respond to technological changes, evolving industry standards, competitive developments, or customer requirements. The Company’s products also may contain undetected errors that could cause increased development costs, loss of revenues, adverse publicity, reduced market acceptance of the products or lawsuits by customers.
| 17 |
THE COMPANY MAY NOT SUCCESSFULLY MANAGE ITS GROWTH.
The Company cannot successfully implement its business model if the Company fails to manage its growth. The Company must rapidly and significantly expand its operations domestically and internationally and anticipate the need for further expansion to take advantage of market opportunities. Managing this substantial expansion will place a significant strain on the Company’s management, operational and financial resources. If the Company’s growth accelerates, the Company will need to continue to improve its financial and managerial control and reporting systems and procedures.
The Company will have to implement new management information software systems. This will affect many aspects of its business, including its accounting, operations, electronic commerce, customer service, purchasing, and sales and marketing functions. The purchase, implementation and testing of these systems will result in significant capital expenditures and could disrupt the Company’s day-to-day operations. If these systems are not implemented as expected, the Company’s ability to provide products and services to its customers on a timely basis will suffer and delays in the recording and reporting of its operating results could occur.
THE COMPANY MAY NOT SUCCESSFULLY DEVELOP ITS MARKETING CAPABILITY.
The Company has limited experience developing and marketing its products and related services. Developing and assembling a technical development team and sales and marketing strategy and team to adequately support its business will require substantial effort and require significant management and financial resources. The Company may be unable to find the appropriate employees or consultants to assist with the commercialization of its products, build a suitable sales force, or enter into satisfactory marketing arrangements with third parties, and its sales and marketing efforts may be unsuccessful.
THE COMPANY RELIES ON THIRD-PARTY PROVIDERS.
The Company will be dependent upon third-party service providers to develop, deliver and maintain certain aspects of its product and operations, including the manufacturing of its ECG acquisition devices. The Company relies on third parties for the initial pairing, development and clearance of the ECG acquisition device with The EKG Glove. The Company has limited control over these third parties. Any discontinuation of these third-party services, or any reduction in performance or increase in price that would require the Company to replace such services, would disrupt its business. In the event that these service providers are unable to operate to the Company’s satisfaction, it would be forced to seek other firms to provide these services. There can be no assurance that services from substitute providers would be available on reasonable terms, if at all. Such an occurrence would involve significant delay and expense and would have a material adverse effect on the Company’s business, prospects, results of operations and financial condition.
THE SUCCESS OF THE COMPANY DEPENDS ON ITS ABILITY TO ATTRACT A CRITICAL MASS OF CUSTOMERS.
The Company business strategy is to create a new paradigm for connecting doctors to patients for both urgent and non-urgent medical care, including the monitoring of vital statistics of medical patients and the marketing of related medical devices. The volume of business depends in part on the perceived value of the Company’s monitoring and assessments products and services. In order for the Company’s business to become valuable in the marketplace, it needs to attract customers, which may be hospitals, medical professionals, medical education institutions, insurance companies and individuals, as well as a large number of vendors, sponsors and advertisers. The Company cannot assure you that it will be successful in attracting a large number of vendors, sponsors or advertisers, nor can it assure you that consumers will use its online services. If medical professionals do not purchase the Company’s product and/or consumers do not use its Web site, it will have a material adverse effect on the Company’s business.
| 18 |
THE COMPANY COULD LOSE STRATEGIC RELATIONSHIPS THAT ARE ESSENTIAL TO ITS BUSINESS.
The loss of certain current strategic relationships, the inability to find other strategic partners or the failure of the Company’s existing relationships to achieve meaningful positive results could harm the Company’s business. The Company intends to rely in part on strategic relationships to help it:
| ● | maximize adoption of the Company’s products through distribution arrangements; | |
| ● | increase the amount and type of data that can be processed to help boost the demand for the Company’s products and services; | |
| ● | enhance the Company’s brand; | |
| ● | expand the range of commercial activities based on the Company’s technology; | |
| ● | increase the performance and utility of the Company’s products and services. |
Many of these goals are beyond the Company’s expertise. The Company anticipates that the efforts of the Company’s strategic partners will become more important as the medical device business matures. In addition, the efforts of the Company’s strategic partners may be unsuccessful. Furthermore, these strategic relationships may be terminated before the Company realizes any benefit.
THE COMPANY’S BUSINESS WILL SUFFER IF ITS SYSTEMS FAIL OR BECOME UNAVAILABLE.
A reduction in the performance, reliability and availability of the Company’s website and network infrastructure will harm the Company’s ability to distribute its products and services to its users, as well as its reputation and ability to attract and retain users and customers. The Company’s systems and operations could be damaged or interrupted by fire, flood, power loss, telecommunications failure, Internet breakdown, earthquake, acts of terrorism, and similar events. The Company’s systems are also subject to break-ins, sabotage, intentional acts of vandalism and similar misconduct. The Company does not have fully redundant systems or a formal disaster recovery plan, and the Company does not carry adequate business interruption insurance to compensate the Company for losses that may occur from a system outage.
A sudden and significant increase in traffic on the Company’s website could strain the capacity of the software, hardware and telecommunications systems that the Company deploys or uses. This could lead to slower response times or system failures. The Company depends on Web browsers, Internet service providers (“ISPs”) and online service providers to provide Internet users access to the Company’s website. Many of these providers have experienced significant outages in the past, and could experience outages, delays, and other difficulties due to system failures unrelated to the Company’s systems.
THE COMPANY’S NETWORK IS SUBJECT TO SECURITY RISKS THAT COULD HARM THE COMPANY’S REPUTATION AND EXPOSE IT TO LITIGATION OR LIABILITY.
Online communications depend on the ability to transmit confidential information securely over public networks. Any compromise of the Company’s ability to transmit confidential information securely, and costs associated with preventing or eliminating any problems, could harm the Company’s business. Online transmissions are subject to a number of security risks, including:
| ● | the Company’s encryption architecture or licensed encryption and authentication technology may be compromised, breached or otherwise be insufficient to ensure the security of customer information; | |
| ● | the Company could experience unauthorized access, computer viruses and other disruptive problems, whether intentional or accidental; | |
| ● | a third party could circumvent the Company’s security measures and misappropriate proprietary information or interrupt operations; and |
The occurrence of any of these or similar events could damage the Company’s reputation and expose it to litigation or liability. The Company may also be required to expend significant capital or other resources to protect against the threat of security breaches or to alleviate problems caused by such breaches.
| 19 |
THE COMPANY MAY BE UNABLE TO ADEQUATELY PROTECT ITS PROPRIETARY RIGHTS.
The Company’s inability to protect its proprietary rights, and the costs of doing so, could harm its business. The Company’s success and ability to compete partly depend on the superiority, uniqueness or value of its technology, including both internally developed technology and technology licensed from third parties. To protect its proprietary rights, the Company plans to rely on a combination of patent, trademark, copyright and trade secret laws, confidentiality agreements with its employees and third parties, protective contractual provisions and its ability to encrypt its programming. The Company currently has eight patents issued (US patent Nos. 6,224,548; 6,248,064; 6,595,918; 6,540,673; 6757,556; 7,112,175; 7,435,222; and 7,753,845), two for the original glove design and two for a two-glove mapping system intended for use in future product offerings. The Company also has a number of additional patents for the original glove design and a two-glove system, as well as applications for its electrodes and twelve-lead design. International patents are held in China, India, Israel, Canada, and will be applied for in Europe, Brazil and Mexico. Despite the Company’s efforts to protect its proprietary rights, unauthorized parties may copy or infringe aspects of its technology, products, services, or trademarks, or obtain and use information the Company regards as proprietary. The Company’s proprietary rights may be especially difficult to protect in foreign countries, where unrelated third parties may have registered the Company’s domain names and trademarks under their own names in an attempt to prevent the Company from using the domain names and trademarks in those countries without paying them a significant sum of money. This could prevent the Company from using the Company’s valuable brands in those countries, and reduce the value of the Company’s intellectual property. In addition, others may independently develop technologies that are similar or superior to ours, which could reduce the value of the Company’s intellectual property.
Companies in the medical device industry have frequently resorted to litigation regarding intellectual property rights. The Company may have to litigate to enforce the Company’s intellectual property rights, to protect the Company’s trade secrets or to determine the validity and scope of other parties’ proprietary rights. The Company may lack the financial and legal resources to effectively pursue infringers of its intellectual property rights. Moreover, the Company’s products and services may possibly infringe upon proprietary rights held by others, and in the event there are patent infringement suits by other companies, any litigation could result in its incurring substantial costs, could prevent the sale of a specific product or service, or could require the Company to direct its efforts away from its business plan.
THE EXPIRATION OR LOSS OF PATENT PROTECTION MAY ADVERSELY AFFECT OUR FUTURE REVENUES AND OPERATING INCOME.
iNeedMD will rely on patent, trademark and other intellectual property protection in the discovery, development, manufacturing, and sale of its products. In particular, patent protection is, in the aggregate, important in the Company’s marketing of The EKG Glove in the United States and most major markets outside of the United States. The expiration or loss of patent protection for a product typically is followed promptly by substitutes that may significantly reduce sales for that product in a short amount of time. If our competitive position is compromised it could have a material adverse effect on our business and results of operations.
THE COMPANY MAY NEED TO INCUR LITIGATION EXPENSES IN ORDER TO DEFEND INTELLECTUAL PROPERTY RIGHTS AND MIGHT NEVERTHELESS BE UNABLE TO ADEQUATELY PROTECT THESE RIGHTS.
The Company believes that its future success will depend in part on its ability to protect its internally developed technologies, which it seeks to protect through a combination of patent, trademark, copyright and trade secret laws. Protection of the Company’s trademarks is crucial as it attempts to build its brand name and reputation. Despite actions the Company takes to protect its intellectual property rights, it may be possible for third parties to copy or otherwise obtain and use its intellectual property without authorization or to develop similar technology independently. The Company may need to engage in costly litigation to enforce its intellectual property rights, to protect its trade secrets or to determine the validity and scope of the intellectual property rights of others. The Company cannot assure you that its efforts to prevent misappropriation or infringement of its intellectual property will be successful. An adverse determination in any litigation of this type could require the Company to make significant changes to the structure and operation of its products or online services and features or to license alternative technology from another party. Implementation of any of these alternatives could be costly and time- consuming and may not be successful. Any intellectual property litigation would be likely to result in substantial costs and diversion of resources and management attention. In addition, legal standards relating to the validity, enforceability and scope of protection of intellectual property rights in Internet-related businesses are uncertain and still evolving.
| 20 |
THE COMPANY MAY NOT BE ABLE TO DEVELOP NEW PRODUCT OR SERVICE OFFERINGS IF IT IS UNABLE TO OBTAIN NEEDED TECHNOLOGY.
The Company relies upon third parties to develop technologies that will enhance its planned product and service offerings. If the Company’s relationships with these third parties are impaired or terminated, then the Company would have to find other developers on a timely basis or develop technology completely on its own. The Company cannot predict whether it will be able to obtain the third-party technology necessary for continued development and introduction of new and enhanced products and services.
THE COMPANY MAY INCUR SUBSTANTIAL COSTS AND DIVERSION OF THE COMPANY’S MANAGEMENT’S RESOURCES IF THE COMPANY INFRINGES UPON THE PROPRIETARY RIGHTS OF OTHERS.
The Company intends to have the permission of, and, in some cases, licenses from, any developer of a software program that the Company uses in the Company’s software. Although the Company has not, at this time, obtained an opinion of counsel, the Company does not believe that the software or the trademarks the Company uses or will use or any of the other elements of the Company’s business infringe on the proprietary rights of any third parties. Third parties may assert claims against the Company for infringement of their proprietary rights and these claims may be successful.
The Company could incur substantial costs and diversion of management resources in the defense of any claims relating to proprietary rights. Parties making these claims could secure a judgment awarding substantial damages as well as injunctive or other equitable relief that could effectively block the Company’s ability to license the Company’s products in the United States or abroad. If a third party asserts a claim relating to proprietary technology or information against the Company, the Company may seek licenses to the intellectual property from the third party. The Company cannot be certain, however, that third parties will extend licenses to it on commercially reasonable terms, or at all. If the Company fails to obtain the necessary licenses or other rights, it could materially and adversely affect the Company’s ability to operate the Company’s business.
The Company could be exposed to significant legal liability if new case law is decided, or new government regulation is enacted, regarding the Internet and Internet service providers. The law relating to the Company’s business and operations is evolving and no clear legal precedents have been established. The adoption of new laws or the application of existing laws may decrease the growth in the use of the Internet, affect telecommunications costs or increase the likelihood or scope of competition from regional telephone companies. These results could decrease the demand for the Company’s services or increase the Company’s cost of doing business, each of which would hurt the Company’s projected gross margins and revenues.
THE COMPANY MAY BE UNABLE TO SUCCESSFULLY COMPETE WITH ESTABLISHED ECG MANUFACTURERS AND TELEMEDICINE COMPANIES IN THE MEDICAL DEVICE AND TELEHEALTH MARKETS.
The market for telemedicine software and services, for the delivery, diagnosis, and care of patient over the Internet is relatively new, constantly changing, and intensely competitive. As medical care evolves into a central component of the Internet experience, more companies are entering the market for, and expending increasing resources to develop, patient diagnostic delivery software and services. The Company expects that competition will continue to intensify.
Many of the Company’s current and potential competitors have longer operating histories, greater name recognition, more employees, and significantly greater financial, technical, marketing, public relations, and distribution resources than the Company does. The competitive environment may require us to make changes in the Company’s products, pricing, licensing, services, or marketing to maintain and extend the Company’s current brand and technology franchise. Price concessions or the emergence of other pricing or distribution strategies of competitors may diminish the Company’s revenues, impact the Company’s margins, or lead to a reduction in the Company’s market share, any of which will harm the Company’s business. The Company’s failure to adequately address any of the above factors could harm the Company’s business strategy and operating results.
| 21 |
General Electric and Cisco are the principal competitors in the development and distribution of ECG machines and real-time telemedicine network technology. General Electric currently competes in the market for ECG equipment and controls over 50% of the U.S. market. Cisco is currently the market leader in assisting large medical institutions in developing and deploying their telehealth networks. The Company believes that General Electric’s and Cisco’s commitments to, and presences in, the telehealth and telemedicine markets has increased and that both companies will continue to increase competitive pressure in the overall market for medical product distribution.
In addition to General Electric and Cisco, multiple other Fortune 500 companies are entering this marketplace which will lead to the Company face increasing competition from other well-established companies that are developing and marketing telemedical products. The Company expects that other large communication and software companies will devote more resources to developing and marketing medical applications, and will seek to compete more vigorously with us in the marketplace. As more companies enter the market with products that compete with the Company’s solutions and tools the competitive landscape could change rapidly to the Company’s disadvantage.
THE COMPANY IS REGULATED BY THE FDA.
The Company’s products are subject to regulation by the federal Food and Drug Administration (“FDA”) and, in some jurisdictions, by state and foreign governmental authorities. In particular, the Company must obtain specific clearance from the FDA before it can market products in the United States. The process of obtaining such clearances can be time-consuming and expensive, and there can be no assurance that all clearances sought by the Company will be granted or that FDA review will not involve delays adversely affecting the marketing and sale of Company products. Current FDA enforcement policy prohibits the promotion or labeling of approved medical devices for unapproved uses. The Company is also required to adhere to the manufacturing, testing, control, labeling, documentation and product surveillance requirements of the FDA. These regulations may have a material impact on the Company’s business. Medical device laws exist in many of the foreign countries where the Company will do business. Federal state and foreign regulations regarding the manufacture and sale of medical devices are subject to future changes. Such revisions may have a material impact on the Company’s business. If the FDA believes that a company is not in compliance with applicable regulations, it can institute proceedings to detain or seize products, issue a recall, impose operating restrictions, enjoin future violations and assess civil and criminal penalties against the Company, its officers or its employees and can recommend criminal prosecution to the Department of Justice. Other regulatory agencies may have similar powers. In addition, product clearances could be withdrawn due to the failure to comply with regulatory standards or the occurrence of unforeseen problems following initial marketing. An adverse regulatory action, depending on its magnitude, may have a material adverse effect on the Company.
THE COMPANY MAY BE SUBJECT TO INCREASED GOVERNMENT REGULATION IN THE FUTURE.
There are currently few laws or regulations that specifically regulate communications or commerce on the Internet. However, laws and regulations may be adopted in the future that address issues such as user privacy, pricing and characteristics and quality of products and services. If more stringent regulatory requirements and/or safety and quality standards are issued in the future, they may have an adverse effect on the Company’s business. In addition, use of the Company’s website could be adversely affected if its Internet system provider or the Internet system providers of its customers adopts stricter safety standards or become more heavily regulated by the Federal Communications Commission.
Internet user privacy has become a significant issue both in the United States and abroad. Current United States privacy law consists of a few disparate statutes directed at specific industries that collect personal data, none of which specifically covers the collection of personal information online. The Company cannot guarantee that the United States or foreign nations will not adopt legislation purporting to protect such privacy. Any such legislation could affect the way in which the Company is allowed to conduct business, especially those aspects that involve the collection or use of personal information, and could have a material adverse effect on the Company’s business, financial condition and operating results. Moreover, it may take years to determine the extent to which existing laws governing issues such as property ownership, libel, negligence and personal privacy are applicable to the Internet. Currently, the Company’s operations are not regulated by any healthcare agency. However, with regard to healthcare issues on the Internet, the Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), mandates the use of standard transactions, standard identifiers, security and other provisions. It will be necessary for the Company platform and for the applications that the Company provides to be in compliance with the regulations. The Company may need to comply with Federal privacy and security regulations promulgated under HIPAA and expanded significantly under the Health Information Technology for Economic and Clinical Health Act, or HITECH Act, as well as certain state privacy and security laws. Compliance with these laws may require substantial expenditure or changes to our operations, and failure to comply with these laws may have a significant effect on the Company.
| 22 |
THE COMPANY COULD BE SUBJECT TO SALE OR OTHER TAXES.
The tax treatment of the Internet and e-commerce is currently unsettled. A number of proposals have been made at the federal, state and local level and by certain foreign governments that could impose taxes on the sale of goods and services and certain other Internet activities. The Company cannot predict the effect of current attempts at taxing or regulating commerce over the Internet. Any legislation that substantially impairs the growth of e-commerce could have a material adverse effect on the Company’s business, financial condition and operating results.
THE COMPANY MAY EXPERIENCE THE ADVERSE EFFECT OF ECONOMIC DOWNTURN.
In the event of an economic downturn or change in consumer patterns, the Company’s business could be adversely affected. For example, if the economy declines, the Company’s customers could experience decreased consumer activity, which could lessen their need for a third-party information management system.
THE COMPANY MAY BE UNABLE TO RESPOND TO THE RAPID TECHNOLOGICAL CHANGE IN ITS INDUSTRY AND SUCH CHANGE MAY INCREASE COSTS AND COMPETITION THAT MAY ADVERSELY AFFECT ITS BUSINESS.
Rapidly changing technologies, frequent new product and service introductions and evolving industry standards characterize the Company’s market. The continued growth of the Internet and intense competition in the Company’s industry exacerbate these market characteristics. The Company’s future success will depend on its ability to adapt to rapidly changing technologies by continually improving the performance features and reliability of its products and services. The Company may experience difficulties that could delay or prevent the successful development, introduction or marketing of its products and services. In addition, any new enhancements must meet the requirements of its current and prospective users and must achieve significant market acceptance. The Company could also incur substantial costs if it needs to modify its products and services or infrastructures to adapt to these changes.
The Company also expects that new competitors may introduce products, systems or services that are directly or indirectly competitive with the Company. These competitors may succeed in developing, products, systems and services that have greater functionality or are less costly than the Company’s products, systems and services, and may be more successful in marketing such products, systems and services. Technological changes have lowered the cost of operating communications and computer systems and purchasing software. These changes reduce the Company’s cost of providing services but also facilitate increased competition by reducing competitors’ costs in providing similar services. This competition could increase price competition and reduce anticipated profit margins.
| 23 |
THE COMPANY’S SERVICES ARE NEW AND ITS INDUSTRY IS EVOLVING.
You should consider the Company’s prospects in light of the risks, uncertainties and difficulties frequently encountered by companies in their early stage of development, particularly companies in the rapidly evolving Internet market. To be successful in this market, the Company must, among other things:
| ● | develop and introduce functional and attractive service offerings; | |
| ● | attract and maintain a large base of subscribers and consumers; | |
| ● | increase awareness of the Company brand and develop subscriber and consumer loyalty; | |
| ● | establish and maintain strategic relationships with distribution partners and service and content providers; | |
| ● | respond to competitive and technological developments; | |
| ● | build an operations structure to support the Company business; and | |
| ● | attract, retain and motivate qualified personnel. |
The Company cannot guarantee that it will succeed in achieving these goals, and its failure to do so would have a material adverse effect on its business, prospects, financial condition and operating results.
The Company’s products and services are new and are only in early stages of commercialization. The Company is not certain that these products and services will function as anticipated or be desirable to its intended market. Also, some of the Company’s products and services may have limited functionalities, which may limit their appeal to subscribers and consumers and put the Company at a competitive disadvantage. If the Company’s current or future products and services fail to function properly or if the Company does not achieve or sustain market acceptance, it could lose subscribers or could be subject to claims which could have a material adverse effect on the Company business, financial condition and operating results.
As is typical in a new and rapidly evolving industry, demand and market acceptance for recently introduced products and services are subject to a high level of uncertainty and risk. Because the market for the Company is new and evolving, it is difficult to predict with any certainty the size of this market and its growth rate, if any. The Company cannot guarantee that a market for the Company will develop or that demand for Company services will emerge or be sustainable. If the market fails to develop, develops more slowly than expected or becomes saturated with competitors, the Company’s business, financial condition and operating results would be materially adversely affected.
MARKET ACCEPTANCE IS UNCERTAIN.
There is no guarantee that products developed by the Company will become a commercial success. There can be no assurance that the Company will obtain any significant degree of market acceptance among customers. The Company may be required to devote substantial resources to educate the market, but it cannot sure you that a sufficient number of customers will use the Company’s products for a commercial success to be achieved. The failure of the Company’s products to gain market acceptance would have a material adverse effect on the Company’s business, financial condition and results of operations.
THE COMPANY’S BUSINESS COULD BE SHUT DOWN OR SEVERELY IMPACTED IF A DISASTER OCCURS.
The Company’s future operations and services depend on the extent to which the Company’s computer equipment and the telecommunications infrastructure of the Company’s third-party network providers is protected against damage from fire, earthquakes, power loss, telecommunications failures, acts of terrorism, and similar events. If an earthquake or act of terrorism damages equipment at the Company’s network operations center, the Company may have no means of replacing this equipment on a timely basis or at all and the Company’s service would be shut down. The Company will not maintain fully redundant or back-up Internet services, backbone facilities or other fully redundant computing and telecommunications facilities. Furthermore, the Company does not currently have any business interruption insurance. Any prolonged disruption of the Company’s services due to system failure could result in user turnover, decreased revenues, or business failure.
| 24 |
WE MAY INCUR SIGNIFICANT COSTS TO ENSURE COMPLIANCE WITH UNITED STATES CORPORATE GOVERNANCE AND ACCOUNTING REQUIREMENTS.
We may incur significant costs associated with our public company reporting requirements, costs associated with newly applicable corporate governance requirements, including requirements under the Sarbanes-Oxley Act of 2002 and other rules implemented by the U.S. Securities and Exchange Commission (the “SEC”). We expect all of these applicable rules and regulations to significantly increase our legal and financial compliance costs and to make some activities more time consuming and costly. We also expect that these applicable rules and regulations may make it more difficult and more expensive for us to obtain director and officer liability insurance and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. As a result, it may be more difficult for us to attract and retain qualified individuals to serve on our board of directors or as executive officers. We are currently evaluating and monitoring developments with respect to these newly applicable rules, and we cannot predict or estimate the amount of additional costs we may incur or the timing of such costs.
WHILE NO CURRENT LAWSUITS ARE FILED AGAINST THE COMPANY, THE POSSIBILITY EXISTS THAT A CLAIM OF SOME KIND MAY BE MADE IN THE FUTURE.
While no current lawsuits are filed against us, the possibility exists that a claim of some kind may be made in the future. While we will work to ensure high product and service quality and accuracy, no assurance can be given that some claims for damages will not arise. We currently carry product liability insurance. We cannot make any assurances that such insurance will completely cover any potential claims against the Company.
WE DEPEND ON OUR KEY MANAGEMENT PERSONNEL AND THE LOSS OF THEIR SERVICES COULD ADVERSELY AFFECT OUR BUSINESS.
We place substantial reliance upon the efforts and abilities of our Chief Executive Officer and President, Thomas A. Nicolette and our other executive officer. Though no individual is indispensable, the loss of the services of any of these executive officers could have a material adverse effect on our business, operations, revenues or prospects. We do not maintain key man life insurance on the lives of these individuals.
THE COMPANY’S FAILURE TO CONTINUE TO ATTRACT, TRAIN, OR RETAIN HIGHLY QUALIFIED PERSONNEL COULD HARM THE COMPANY’S BUSINESS.
The Company’s success also depends on the Company’s ability to attract, train, and retain qualified personnel, specifically those with management and product development skills. In particular, the Company must hire additional skilled software engineers to further the Company’s research and development efforts. Competition for such personnel is intense, particularly in high-technology centers. If the Company does not succeed in attracting new personnel or retaining and motivating the Company’s current personnel, the Company’s business could be harmed.
| 25 |
IF WE FAIL TO ESTABLISH AND MAINTAIN AN EFFECTIVE SYSTEM OF INTERNAL CONTROL, WE MAY NOT BE ABLE TO REPORT OUR FINANCIAL RESULTS ACCURATELY OR TO PREVENT FRAUD. ANY INABILITY TO REPORT AND FILE OUR FINANCIAL RESULTS ACCURATELY AND TIMELY COULD HARM OUR REPUTATION AND ADVERSELY IMPACT THE TRADING PRICE OF OUR COMMON STOCK.
Effective internal control is necessary for us to provide reliable financial reports and prevent fraud. If we cannot provide reliable financial reports or prevent fraud, we may not be able to manage our business as effectively as we would if an effective control environment existed, and our business and reputation with investors may be harmed. As a result, our small size and any current internal control deficiencies may adversely affect our financial condition, results of operation and access to capital. We currently have insufficient written policies and procedures for accounting and financial reporting with respect to the requirements and application of US GAAP and SEC disclosure requirements. Additionally, there is a lack of formal process and timeline for closing the books and records at the end of each reporting period and such weaknesses restrict the Company’s ability to timely gather, analyze and report information relative to the financial statements.
Because of the Company’s limited resources, there are limited controls over information processing. There is inadequate segregation of duties consistent with control objectives. Our Company’s management is composed of a small number of individuals resulting in a situation where limitations on segregation of duties exist. In order to remedy this situation we would need to hire additional staff. Currently, the Company is unable to hire additional staff to facilitate greater segregation of duties but will reassess its capabilities after completion of the Offering.
RISKS RELATED TO OUR COMMON STOCK
OUR SHARES OF COMMON STOCK HAVE NO TRADING AND THERE CAN BE NO ASSURANCE THAT THERE WILL BE AN ACTIVE MARKET FOR OUR SHARES OF COMMON STOCK EITHER NOW OR IN THE FUTURE.
Our shares of common stock have not been publicly traded, and the price if traded may not reflect our value. There can be no assurance that there will be an active market for our shares of common stock either now or in the future. The market liquidity will be dependent on the perception of our operating business and any steps that our management might take to bring us to the awareness of investors. There can be no assurance given that there will be any awareness generated. Consequently, investors may not be able to liquidate their investment or liquidate it at a price that reflects the value of the business. If a more active market should develop, the price may be highly volatile. Because there may be a low price for our shares of common stock, many brokerage firms may not be willing to effect transactions in the securities. Even if an investor finds a broker willing to effect a transaction in the shares of our common stock, the combination of brokerage commissions, transfer fees, taxes, if any, and any other selling costs may exceed the selling price. Further, many lending institutions will not permit the use of such shares of common stock as collateral for any loans.
THE SECURITIES LAWS MAY RESTRICT THE TRANSFERABILITY OF THE SECURITIES BEING ISSUED.
The shares of common stock have not been registered under the Securities Act or registered or qualified under any state or foreign securities laws. Such securities are being issued based upon the Company’s reliance upon an exemption from registration under the Securities Act for an offer and sale of securities that does not involve a public offering. Unless such securities are so registered, they may not be offered or sold except pursuant to an exemption from, or in a transaction not subject to, the registration requirements of the Securities Act and applicable state or foreign securities laws. Investors subscribing for shares will first be required to make representations and covenants concerning these transfer restrictions which are necessary to satisfy the requirements of the exemption from registration being relied upon by the Company for the issuance of the common stock. The certificates representing shares of the common stock will bear a legend indicating that they are so restricted.
| 26 |
Holders of our common stock will only be able to resell their securities pursuant to an effective registration statement or through an exemption from federal and state registration or qualification requirements. There can be no assurance that an exemption will be available when an investor desires to liquidate. Accordingly, purchasers of the securities in this Offering must be prepared to bear the economic risks of investment for an indefinite period of time since the securities cannot be resold unless they are subsequently registered or an exemption from resale is available.
WE MAY BE SUBJECT TO PENNY STOCK RULES WHICH WILL MAKE THE SHARES OF OUR COMMON STOCK MORE DIFFICULT TO SELL.
We may be subject now and in the future to the SEC’s “penny stock” rules if our shares common stock sell below $5.00 per share. Penny stocks generally are equity securities with a price of less than $5.00. The penny stock rules require broker-dealers to deliver a standardized risk disclosure document prepared by the SEC which provides information about penny stocks and the nature and level of risks in the penny stock market. The broker-dealer must also provide the customer with current bid and offer quotations for the penny stock, the compensation of the broker- dealer and its salesperson, and monthly account statements showing the market value of each penny stock held in the customer’s account. The bid and offer quotations, and the broker-dealer and salesperson compensation information must be given to the customer orally or in writing prior to completing the transaction and must be given to the customer in writing before or with the customer’s confirmation.
In addition, the penny stock rules require that prior to a transaction the broker dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written agreement to the transaction. The penny stock rules are burdensome and may reduce purchases of any offerings and reduce the trading activity for shares of our common stock. As long as our shares of common stock are subject to the penny stock rules, the holders of such shares of common stock may find it more difficult to sell their securities.
SHARES OF OUR CURRENTLY ISSUED AND OUTSTANDING STOCK MAY BECOME FREELY TRADABLE PURSUANT TO RULE 144 AND MAY DILUTE THE MARKET FOR YOUR SHARES AND HAVE A DEPRESSIVE EFFECT ON THE PRICE OF THE SHARES OF OUR COMMON STOCK.
A substantial majority of our outstanding shares of common stock are “restricted securities” within the meaning of Rule 144 under the Securities Act. As restricted shares, these shares may be resold only pursuant to an effective registration statement or under the requirements of Rule 144 or other applicable exemptions from registration under the Act and as required under applicable state securities laws. Rule 144 provides in essence that an Affiliate (as such term is defined in Rule 144(a)(1)) of an issuer who has held restricted securities for a period of at least six months (one year after filing Form 10 information with the SEC for shell companies and former shell companies, such as Pubco) may, under certain conditions, sell every three months, in brokerage transactions, a number of shares that does not exceed the greater of 1% of a company’s outstanding shares of common stock or the average weekly trading volume during the four calendar weeks prior to the sale (the four calendar week rule does not apply to companies quoted on the OTC Bulletin Board). Rule 144 also permits, under certain circumstances, the sale of securities, without any limitation, by a person who is not an Affiliate of the Company and who has satisfied a one- year holding period. A sale under Rule 144 or under any other exemption from the Act, if available, or pursuant to subsequent registrations of our shares of common stock, may have a depressive effect upon the price of our shares of common stock in any active market that may develop.
YOU WILL EXPERIENCE DILUTION OF YOUR OWNERSHIP INTEREST BECAUSE OF THE FUTURE ISSUANCE OF ADDITIONAL SHARES OF OUR COMMON STOCK AND OUR PREFERRED STOCK.
In the future, we may issue our authorized but previously unissued equity securities, resulting in the dilution of the ownership interests of our present stockholders. We are currently authorized to issue an aggregate of 75,000,000 shares of capital stock consisting of 65,000,000 shares of common stock, par value $0.001 and 10,000,000 shares of blank check preferred stock, par value $0.001.
| 27 |
We may also issue additional shares of our common stock or other securities that are convertible into or exercisable for common stock in connection with hiring or retaining employees or consultants, future acquisitions, future sales of our securities for capital raising purposes, or for other business purposes. The future issuance of any such additional shares of our common stock or other securities may create downward pressure on the trading price of our common stock. There can be no assurance that we will not be required to issue additional shares, warrants or other convertible securities in the future in conjunction with hiring or retaining employees or consultants, future acquisitions, future sales of our securities for capital raising purposes or for other business purposes, including at a price (or exercise prices) below the price at which shares of our common stock are trading.
WE DO NOT EXPECT TO PAY DIVIDENDS AND INVESTORS SHOULD NOT BUY OUR COMMON STOCK EXPECTING TO RECEIVE DIVIDENDS.
We have not paid any dividends on our common stock in the past, and do not anticipate that we will declare or pay any dividends in the foreseeable future. Consequently, investors will only realize an economic gain on their investment in our common stock if the price appreciates. Investors should not purchase our common stock expecting to receive cash dividends. Because we do not pay dividends, and there may be limited trading, investors may not have any manner to liquidate or receive any payment on their investment. Therefore, our failure to pay dividends may cause investors to not see any return on investment even if we are successful in our business operations. In addition, because we do not pay dividends we may have trouble raising additional funds, which could affect our ability to expand our business operations.
WE MAY HAVE SOLD SECURITIES IN VIOLATION OF APPLICABLE SECURITIES LAWS, WHICH COULD GIVE PURCHASERS OF OUR SECURITIES THE RIGHT TO SEEK REFUNDS OR DAMAGES, WHICH COULD REDUCE THE PROCEEDS AVAILABLE FROM OUR OFFERING.
In issuing securities in our offering, we relied upon certain provisions of federal and state securities laws which provide “private placement exemptions” from the registration requirements of the securities laws. Because we may not have complied with all of the applicable exemption requirements, certain investors who acquired our securities may have a right to obtain a recovery of the consideration paid in connection with their purchase of our securities. In addition, we could be subject to additional liabilities if state or federal authorities were to assert violations of the securities laws against us.
Item 1B. Unresolved Staff Comments.
Not applicable.
| 28 |
We currently lease commercial office space located at 650 First Avenue, Third Floor, New York, New York 10016 for $3,855 per month. The Company entered into the lease in July 2014 for a term of 5 years with the option to renew.
We are not currently involved in any litigation that we believe could have a materially adverse effect on our financial condition or results of operations. There is no action, suit, proceeding, inquiry or investigation before or by any court, public board, government agency, self-regulatory organization or body pending or, to the knowledge of the executive officers of our Company or any of our subsidiaries, threatened against or affecting our Company, our common stock, any of our subsidiaries or of our Company’s or our Company’s subsidiaries’ officers or directors in their capacities as such, in which an adverse decision could have a material adverse effect.
Item 4. Mine Safety Disclosures.
Not applicable.
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
(a) Market Information
Our shares of common stock are currently quoted on the OTC Markets under the symbol “NEMD”. At this time our shares of common stock do not trade. We expect trading to commence in the second quarter of 2015.
(b) Holders
As of April 15, 2015, a total of 48,014,424 shares of the Company’s common stock are currently outstanding held by 210 shareholders of record.
(c) Dividends
We have not declared or paid any dividends on our common stock and intend to retain any future earnings to fund the development and growth of our business. Therefore, we do not anticipate paying dividends on our common stock for the foreseeable future. There are no restrictions on our present ability to pay dividends to stockholders of our common stock, other than those prescribed by Nevada law.
(d) Securities Authorized for Issuance under Equity Compensation Plans
At this time, the Company does not have an equity compensation plan.
Rule 10B-18 Transactions
During the year ended December 31, 2014, there were no repurchases of the Company’s common stock by the Company.
Recent Sales of Unregistered Securities
During the year ended December 31, 2014, we have not issued any securities which were not registered under the Securities Act and not previously disclosed in the Company’s Quarterly Reports on Form 10-Q or Current Reports on Form 8-K.
| 29 |
Item 6. Selected Financial Data.
Not applicable.
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
This annual report on Form 10-K and other reports filed by iNeedMD Holdings, Inc. (the “Company”) from time to time with the SEC (collectively, the “Filings”) contain or may contain forward-looking statements and information that are based upon beliefs of, and information currently available to, the Company’s management as well as estimates and assumptions made by Company’s management. Readers are cautioned not to place undue reliance on these forward-looking statements, which are only predictions and speak only as of the date hereof. When used in the Filings, the words “anticipate,” “believe,” “estimate,” “expect,” “future,” “intend,” “plan,” or the negative of these terms and similar expressions as they relate to the Company or the Company’s management identify forward-looking statements. Such statements reflect the current view of the Company with respect to future events and are subject to risks, uncertainties, assumptions, and other factors, including the risks relating to the Company’s business, industry, and the Company’s operations and results of operations. Should one or more of these risks or uncertainties materialize, or should the underlying assumptions prove incorrect, actual results may differ significantly from those anticipated, believed, estimated, expected, intended, or planned.
Although the Company believes that the expectations reflected in the forward-looking statements are reasonable, the Company cannot guarantee future results, levels of activity, performance, or achievements. Except as required by applicable law, including the securities laws of the United States, the Company does not intend to update any of the forward-looking statements to conform these statements to actual results.
Our financial statements are prepared in accordance with accounting principles generally accepted in the United States (“GAAP”). These accounting principles require us to make certain estimates, judgments and assumptions. We believe that the estimates, judgments and assumptions upon which we rely are reasonable based upon information available to us at the time that these estimates, judgments and assumptions are made. These estimates, judgments and assumptions can affect the reported amounts of assets and liabilities as of the date of the financial statements as well as the reported amounts of revenues and expenses during the periods presented. Our financial statements would be affected to the extent there are material differences between these estimates and actual results. In many cases, the accounting treatment of a particular transaction is specifically dictated by GAAP and does not require management’s judgment in its application. There are also areas in which management’s judgment in selecting any available alternative would not produce a materially different result. The following discussion should be read in conjunction with our financial statements and notes thereto appearing elsewhere in this report.
Overview
We intend for this discussion to provide information that will assist in understanding our financial statements, the changes in certain key items in those financial statements, and the primary factors that accounted for those changes, as well as how certain accounting principles affect our financial statements.
Business Overview
iNeedMD Holdings, Inc. (formerly Clutterbug Move Management, Inc.) was incorporated on February 1, 2012, under the laws of the State of Nevada. The Company is a medical device company that has developed a disposable device used in the diagnosis, prevention, and monitoring of cardiovascular disease.
| 30 |
Results of Operations
Summary of Statements of Operations for the Years Ended December 31, 2014 and 2013:
| For the Years Ended | ||||||||
| December 31, 2014 | December 31, 2013 | |||||||
| Revenue | $ | 910 | $ | 7,539 | ||||
| Gross profit | 604 | 1,659 | ||||||
| Compensation* | 35,038,488 | 64,761,772 | ||||||
| Consulting fees | 1,015,522 | 297,258 | ||||||
| Consulting fees – related party | 278,000 | 107,600 | ||||||
| Sales and marketing | 336,944 | 149,976 | ||||||
| Sales and marketing – related party | 20,000 | 18,500 | ||||||
| Research and development | 697,142 | 37,069 | ||||||
| General and administrative | 1,207,946 | 281,921 | ||||||
| Interest Income | 237 | -- | ||||||
| Proceeds from insurance settlement | 36,365 | -- | ||||||
| Interest Expense | 96,444 | 99,393 | ||||||
| Amortization of debt discount | 1,233,334 | 250,000 | ||||||
| Amortization of debt issuance costs | 129,459 | 28,626 | ||||||
| Debt Conversion Expense | 100,000 | -- | ||||||
| Derivative Expense | 1,141,407 | 133,087 | ||||||
| Change in fair value of derivative liabilities | 944,994 | (139,280 | ) | |||||
| Net Loss | 40,312,486 | 66,302,823 | ||||||
| Loss per common A share- basic | $ | 1.45 | $ | 1,318.23 | ||||
* For the year ended December 31, 2013, the compensation of $64,761,772 is due to the issuance of approximately 32 million shares (Pre-Reverse Stock Split), to certain individuals (the "2013 Compensation Shares"). The issuance of the 2013 Compensation Shares was due to a management restructuring in January 2013 and the compensation charge affiliated with such issuance is solely based on the way the value of the 2013 Compensation Shares is accounted for under U.S. Generally Accepted Accounting Principles (“GAAP”). The 2013 Compensation Shares were valued at $2.00 per share based on the Company's most recent capital raise pursuant to which the Company raised approximately $475,000 at $2.00 per share (the "2013 Capital Raise"). Accordingly, such compensation charge is a one-time non-cash charge that has no effect on cash flows from operating activities. Net loss from operations was affected by approximately $64 million due to these issuances.
For the year ended December 31, 2014, substantially all of the compensation of $35,038,488 is due to the issuance of approximately 34 million shares (Post-Reverse Stock Split), to certain individuals (the "2014 Compensation Shares"). The issuance of the 2014 Compensation Shares was due to a management restructuring in April 2014 and the compensation charge affiliated with such issuance is solely based on the way the value of the 2014 Compensation Shares is accounted for under U.S. Generally Accepted Accounting Principles (“GAAP”). The 2014 Compensation Shares were valued at $1.00 per share based on the Company's most recent capital raise pursuant to which the Company raised approximately $450,000 at $1.00 per share. The Company also raised $8,400,000 at $1.00 per share in 2014 (the “2014 Capital Raise”). Accordingly, such compensation charge is a one-time non-cash charge that has no effect on cash flows from operating activities. Net loss from operations was affected by approximately $34 million due to these issuances.
Revenue
Revenue was $910 for the year ended December 31, 2014 as compared to $7,539 for the year ended December 31, 2013, a decrease of $6,629. The decrease in revenue is primarily attributable to the Company focusing their efforts on research and development and marketing their products.
Gross Profit
Gross profit percentage increased from a gross profit of 22% during the year ended December 31, 2013 to a gross profit of 66% during the year ended December 31, 2014. The increase in gross profit is primarily attributable to the decrease in the cost to manufacture The EKG Glove due to larger orders placed with the supplier during the latter half of 2014.
| 31 |
Compensation
Compensation decreased from $64,761,772 for the year ended December 31, 2013 to $35,038,488 for the year ended December 31, 2014.
For the year ended December 31, 2013, the compensation of $64,761,772 is due to the issuance of approximately 32 million shares (Pre-Reverse Stock Split), to certain individuals (the "2013 Compensation Shares"). The issuance of the 2013 Compensation Shares was due to a management restructuring in January 2013 and the compensation charge affiliated with such issuance is solely based on the way the value of the 2013 Compensation Shares is accounted for under U.S. Generally Accepted Accounting Principles (“GAAP”). The 2013 Compensation Shares were valued at $2.00 per share based on the Company's most recent capital raise pursuant to which the Company raised approximately $475,000 at $2.00 per share (the "2013 Capital Raise"). Accordingly, such compensation charge is a one-time non-cash charge that has no effect on cash flows from operating activities. Net loss from operations was affected by approximately $64 million due to these issuances.
For the year ended December 31, 2014, substantially all of the compensation of $35,038,488 is due to the issuance of approximately 34 million shares (Post-Reverse Stock Split), to certain individuals (the "2014 Compensation Shares"). The issuance of the 2014 Compensation Shares was due to a management restructuring in April 2014 and the compensation charge affiliated with such issuance is solely based on the way the value of the 2014 Compensation Shares is accounted for under U.S. Generally Accepted Accounting Principles (“GAAP”). The 2014 Compensation Shares were valued at $1.00 per share based on the Company's most recent capital raise pursuant to which the Company raised approximately $450,000 at $1.00 per share. The Company also raised $8,400,000 at $1.00 per share in 2014 (the “2014 Capital Raise”). Accordingly, such compensation charge is a one-time non-cash charge that has no effect on cash flows from operating activities. Net loss from operations was affected by approximately $34 million due to these issuances.
Consulting Fees
Consulting fees, including related party consulting fees, were $1,293,522 for the year ended December 31, 2014 as compared to $408,858 for the year ended December 31, 2013, an increase of $888,664. The increase is attributable to the increase in capital resources as compared to the prior year and the work associated with transitioning from a private company to a publicly traded company. Although the Company entered into employment agreements in the third quarter 2014, the Company mainly utilized consultants to carry out its business functions during the year ended December 31, 2014.
Sales and Marketing
Sales and marketing expense, including related party sales and marketing fees, were $356,944 for the year ended December 31, 2014 as compared to $168,476 for the year ended December 31, 2013, an increase of $188,468. The increase is attributable to the increase in capital resources as compared to the prior year and the Company’s efforts to increase awareness of its products to the medical community.
Research and Development
Research and development expenses were $697,142 for the year ended December 31, 2014 versus $37,069 for the year ended December 31, 2013, an increase of $660,073. The Company had increased capital resources as compared to the prior year and focused its attention on refining the design of its EKG glove and interface.
General and Administrative Expenses
General and administrative expenses were $1,207,946 for the year ended December 31, 2014 versus $281,921 for the year ended December 31, 2013, an increase of $926,025. The increase is attributable to professional fees incurred in relation to the transition from a private company to a publicly traded company and travel.
Interest Expense
Interest expense was $96,444 for the year ended December 31, 2014 as compared to $99,393 for the year ended December 31, 2013, a decrease of $2,949.
| 32 |
Amortization of Debt Discount
Amortization of debt discount was $1,233,334 for the year ended December 31, 2014 as compared to $250,000 for the year ended December 31, 2013, an increase of $983,334. The increase was attributable to the warrants issued in conjunction with Ayer Notes I & II, along with the common stock issued with the Manchanda Note.
Amortization of Debt Issuance Costs
Amortization of debt issuance costs was $129,459 for the year ended December 31, 2014 as compared to $28,626 for the year ended December 31, 2013, an increase of $100,833. Warrants issued to the investment advisor, underwriter fees and broker fees paid in conjunction with Ayer Notes I & II accounted for the increase.
Debt Conversion Expense
Debt conversion expense was $100,000 for the year ended December 31, 2014 as compared to $- for the year ended December 31, 2013. In 2014, the Company induced Richard Pinto to convert the remaining $100,000 of his note principal by offering a conversion price of $0.50, a $0.50 discount from the market price of $1.00.
Derivative Expense
Derivative expense was $1,141,407 for the year ended December 31, 2014 versus $133,087 for the year ended December 31, 2013, an increase of $1,008,320. The increase in the derivative expense was associated with the doubling of the warrant coverage on Ayer Notes I & II, which was triggered by the respective maturity date extensions.
Change in Fair Value of Derivative Liabilities
Change in fair value of derivative liabilities was a gain of $944,994 for the year ended December 31, 2014 versus a loss of $139,280 for the year ended December 31, 2013, an increase of $1,084,274. The increase was due to the increase of the exercise price of the warrants associated with Ayer Notes I & II and the passage of time as the derivatives neared closer to their expiration dates.
Net Loss
For the reasons mentioned above, net loss for the year ended December 31, 2014 was $40,312,486, or a loss per share of $1.45 to Common A shareholders, in comparison to the net loss for the year ended December 31, 2013 of $66,302,823, or loss per share of $1,318.23 to Common A shareholders.
Liquidity and Capital Resources
The following table summarizes total current assets, liabilities and working capital at December 31, 2014, compared to December 31, 2013:
| December 31, 2014 | December 31, 2013 | Increase (Decrease) | ||||||||||
| Current Assets | $ | 2,616,029 | $ | 953,449 | $ | 1,662,580 | ||||||
| Current Liabilities | $ | 2,324,338 | $ | 1,820,702 | $ | 503,636 | ||||||
| Working Capital (Deficit) | $ | 291,691 | $ | (867,253 | ) | $ | 1,158,944 | |||||
At December 31, 2014, we had working capital of $291,691 as compared to a working capital deficit of $867,253 at December 31, 2013, an increase in working capital of $1,158,944. The increase is primarily attributable to an increase in cash of approximately $7,500,000 due to the equity capital raised and $600,000 proceeds received from the issuance of a convertible note payable. This increase in current assets was offset by the repayment of convertible notes totaling $1,350,000 and the net cash used from operating activities of approximately $4,600,000.
| 33 |
Net Cash
Net cash used in operating activities for the years ended December 31, 2014 and 2013 was $4,566,234 and $952,403, respectively. The net loss for the years ended December 31, 2014 and 2013 was $40,312,486 and $66,302,823, respectively. During 2014, the net loss was offset by adjustments due to amortization of debt issuance costs and debt discount of the notes payable of $129,459 and $1,233,334, respectively, the derivative expense of $1,141,407, the change in fair value of the derivative of $(944,994) and the share based payments for Class A common stock and warrants issued to consultants totaling $34,408,043. During 2013, the net loss was offset by adjustments due to amortization of debt discount of the notes payable of $250,000, the derivative expense of $133,087, the change in fair value of the derivative of $139,280 and the share based payments for Class A common stock of $64,779,372. The Company incurred significant expenses executing its business development plan in order to generate future revenue.
Net cash used in investing activities during the year ended December 31, 2014 was $417,017, of which $67,017 was used for the purchase of equipment and $350,000 was used for reverse merger fees, as compared to $47,000 provided by investing activities for the repayment of loans to consultants for the 2013 comparable period.
Financings
During the year ended December 31 2014, we received net proceeds of approximately $7,500,000 from the issuance of Class A common shares compared to $1,135,000 received during the year ended December 31, 2013. During the year ended December 31, 2014 and 2013, we also received proceeds of $600,000 and $750,000, respectively, for the issuance of convertible notes. The issuance of additional debt during the year ended December 31, 2014 resulted in the payment of $27,000 in debt issuance costs. We paid $1,350,000 for the repayment of convertible notes during the year ended December 31, 2014. During the year ended December 31, 2013, we repaid related party loans totaling $87,300 to our largest shareholder.
Going Concern
As reflected in the accompanying financial statements, the Company had a net loss and net cash used in operations of $40,312,486 and $4,566,234, respectively, for the year ended December 31, 2014 and an accumulated deficit totaling $115,340,329. The Company does not generate significant revenue. These factors raise substantial doubt about the Company’s ability to continue as a going concern.
The ability of the Company to continue its operations as a going concern is dependent on Management's plans, which include the raising of capital through debt and/or equity markets with some additional funding from other traditional financing sources, including term notes, until such time that funds provided by operations are sufficient to fund working capital requirements. The Company may need to incur additional liabilities with certain related parties to sustain the Company’s existence.
The Company will require additional funding to finance the growth of its current and expected future operations as well as to achieve its strategic objectives. The Company believes its current available cash along with anticipated revenues may be insufficient to meet its cash needs for the near future. There can be no assurance that financing will be available in amounts or terms acceptable to the Company, if at all. The accompanying financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. These financial statements do not include any adjustments relating to the recovery of the recorded assets or the classification of the liabilities that might be necessary should the Company be unable to continue as a going concern.
Off-Balance Sheet Arrangements
As of December 31, 2014 and December 31, 2013, the Company had no off-balance sheet arrangements.
| 34 |
Critical Accounting Policies
We believe that the following accounting policies are the most critical to aid you in fully understanding and evaluating this “Management’s Discussion and Analysis of Financial Condition and Results of Operation.”
Revenue Recognition
The Company follows paragraph 605-10-S99-1 of the FASB Accounting Standards Codification for revenue recognition. The Company recognizes revenue when it is realized or realizable and earned. The Company considers revenue realized or realizable and earned when all of the following criteria are met: (i) persuasive evidence of an arrangement exists, (ii) the product has been shipped to the customer, (iii) the sales price is fixed or determinable, and (iv) collectability is reasonably assured.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period.
The Company’s significant accounting estimates and assumptions affecting the consolidated financial statements were the allowance for doubtful accounts, valuation allowance for deferred tax assets and estimates and assumptions used in valuation of equity and derivative instruments. Those significant accounting estimates or assumptions bear the risk of change due to the fact that there are uncertainties attached to those estimates or assumptions, and certain estimates or assumptions are difficult to measure or value.
Management bases its estimates on historical experience and on various assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources.
Management regularly reviews its estimates utilizing currently available information, changes in facts and circumstances, historical experience and reasonable assumptions. After such reviews, and if deemed appropriate, those estimates are adjusted accordingly. Actual results could differ from those estimates.
Stock Based Compensation
All stock-based payments to employees, non-employee consultants, and to nonemployee directors for their services as directors, including any grants of restricted stock and stock options, are measured at fair value on the grant date and recognized in the statements of operations as compensation or other expense over the relevant service period. Stock-based payments to nonemployees are recognized as an expense over the period of performance. Such payments are measured at fair value at the earlier of the date a performance commitment is reached or the date performance is completed. In addition, for awards that vest immediately and are non-forfeitable the measurement date is the date the award is issued.
Fair Value of Financial Instruments
We follow paragraph 825-10-50-10 of the FASB Accounting Standards Codification for disclosures about fair value of our financial instruments and paragraph 820-10-35-37 of the FASB Accounting Standards Codification (“Paragraph 820-10-35-37”) to measure the fair value of our financial instruments. Paragraph 820-10-35-37 establishes a framework for measuring fair value in accounting principles generally accepted in the United States of America (“U.S. GAAP”), and expands disclosures about fair value measurements. To increase consistency and comparability in fair value measurements and related disclosures, Paragraph 820-10-35-37 establishes a fair value hierarchy which prioritizes the inputs to valuation techniques used to measure fair value into three (3) broad levels. The fair value hierarchy gives the highest priority to quoted prices (unadjusted) in active markets for identical assets or liabilities and the lowest priority to unobservable inputs.
| 35 |
Derivative Instruments
We evaluate our convertible debt and warrants to determine if those contracts or embedded components of those contracts qualify as derivatives to be separately accounted for in accordance with Paragraph 810-10-05-4 of the Codification and Paragraph 815-40-25 of the Codification. The result of this accounting treatment is that the fair value of the embedded derivative is marked-to-market each balance sheet date and recorded as a liability. In the event that the fair value is recorded as a liability, the change in fair value is recorded in the statements of operations as other income or expense. Upon conversion or exercise of a derivative instrument, the instrument is marked to fair value at the conversion date and then that fair value is reclassified to equity.
Recent Accounting Pronouncements
In May 2014, the Financial Accounting Standards Board issued Accounting Standards Update 2014-09, Revenue from Contracts with Customers. Amendments in this Update create Topic 606, Revenue from Contracts with Customers, and supersede the revenue recognition requirements in Topic 605, Revenue Recognition, including most industry-specific revenue recognition guidance throughout the Industry Topics of the Codification. In addition, the amendments supersede the cost guidance in Subtopic 605-35, Revenue Recognition—Construction-Type and Production-Type Contracts, and create new Subtopic 340-40, Other Assets and Deferred Costs—Contracts with Customers. In summary, the core principle of Topic 606 is that an entity recognizes revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. This Accounting Standards Update is the final version of Proposed Accounting Standards Update 2011-230—Revenue Recognition (Topic 605) and Proposed Accounting Standards Update 2011–250—Revenue Recognition (Topic 605): Codification Amendments, both of which have been deleted. The amendments in this Update are effectively for the Company for annual reporting periods beginning after December 15, 2016, including interim periods within that reporting period. The Company is currently evaluating the effects of ASU 2014-09 on the consolidated financial statements.
In June 2014, the Financial Accounting Standards Board issued Accounting Standards Update 2014-12, Compensation- Stock Compensation. The amendments in this update apply to reporting entities that grant their employees share-based payments in which the terms of the award provide that a performance target can be achieved after the requisite service period. This Accounting Standards Update is the final version of Proposed Accounting Standards Update EITF–13D–Compensation–Stock Compensation (Topic 718): Accounting for Share-Based Payments When the Terms of an Award Provide That a Performance Target Could Be Achieved after the Requisite Service Period, which has been deleted. The amendments require that a performance target that affects vesting and that could be achieved after the requisite service period be treated as a performance condition. A reporting entity should apply existing guidance in Topic 718 as it relates to awards with performance conditions that affect vesting to account for such awards. As such, the performance target should not be reflected in estimating the grant-date fair value of the award. Compensation cost should be recognized in the period in which it becomes probable that the performance target will be achieved and should represent the compensation cost attributable to the period(s) for which the requisite service has already been rendered. If the performance target becomes probable of being achieved before the end of the requisite service period, the remaining unrecognized compensation cost should amount of compensation cost recognized during and after the requisite service period should reflect the number of awards that are expected to vest and should be adjusted to reflect those awards that ultimately vest. The requisite service period ends when the employee can cease rendering service and still be eligible to vest in the award if the performance target is achieved. As indicated in the definition of vest, the stated vesting period (which includes the period in which the performance target could be achieved) may differ from the requisite service period. The amendments in this Update are effective for annual periods and interim periods within those annual periods beginning after December 15, 2015, and early adoption is permitted. The Company is currently evaluating the effects of ASU 2014-12 on the consolidated financial statements.
In August 2014, the Financial Accounting Standards Board issued Accounting Standards Update 2014-15, Presentation of Financial Statements- Going Concern. The Update provides U.S. GAAP guidance on management’s responsibility in evaluating whether there is substantial doubt about a company’s ability to continue as a going concern and about related footnote disclosures. For each reporting period, management will be required to evaluate whether there are conditions or events that raise substantial doubt about a company’s ability to continue as a going concern within one year from the date the financial statements are issued. This Accounting Standards Update is the final version of Proposed Accounting Standards Update 2013-300–Presentation of Financial Statements (Topic 205): Disclosure of Uncertainties about an Entity’s Going Concern Presumption, which has been deleted. The amendments in this Update are effective for the annual period ending after December 15, 2016, and for annual periods and interim periods thereafter. The Company is currently evaluating the effects of ASU 2014-15 on the consolidated financial statements.
In April 2015, the Financial Accounting Standards Board issued Accounting Standards Update 2015-03, Interest—Imputation of Interest. To simplify presentation of debt issuance costs, the amendments in this Update would require that debt issuance costs be presented in the balance sheet as a direct deduction from the carrying amount of debt liability, consistent with debt discounts or premiums. The recognition and measurement guidance for debt issuance costs would not be affected by the amendments in this Update. This Accounting Standards Update is the final version of Proposed Accounting Standards Update 2014-250—Interest—Imputation of Interest (Subtopic 835-30), which has been deleted. The amendments in this Update are effective for financial statements issued for fiscal years beginning after December 15, 2015, and interim periods within those fiscal years. The Company is currently evaluating the effects of ASU 2015-03 on the consolidated financial statements.
| 36 |
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
We do not hold any derivative instruments and do not engage in any hedging activities.
Our consolidated financial statements are contained in pages F-1 through F-24 which appear at the end of this Annual Report.
'Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
There are no reportable events under this item for the year ended December 31, 2014.
Item 9A. Controls and Procedures.
(a) EVALUATION OF DISCLOSURE CONTROLS AND PROCEDURES
Based on their evaluation as of the end of the period covered by this Annual Report on Form 10-K, our principal executive officer and principal financial officer have concluded that our disclosure controls and procedures (as defined in Rules 13a-15(c) and 15d-15(e) under the Exchange Act) are not effective to ensure that information required to be disclosed by us in report that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the U.S. Securities and Exchange Commission’s rules and forms and to ensure that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is accumulated and communicated to our management, including our chief executive officer and chief financial officer, as appropriate to allow timely decisions regarding required disclosure.
(b) MANAGEMENT’S REPORT ON INTERNAL CONTROL OVER FINANCIAL REPORTING
This Company’s management is responsible for establishing and maintaining internal controls over financial reporting and disclosure controls. Internal Control Over Financial Reporting is a process designed by, or under the supervision of, the Company’s principal executive and principal financial officers, or persons performing similar functions, and effected by the issuer’s board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
| (1) | Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of the assets of the issuer; |
| (2) | Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the issuer are being made only in accordance with authorizations of management and directors of the registrant; and | |
| (3) | Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the issuer’s assets that could have a material effect on the financial statements. |
Disclosure controls and procedures are designed to ensure that information required to be disclosed in reports filed under the Securities Exchange Act of 1934, as amended, is appropriately recorded, processed, summarized and reported within the specified time periods.
| 37 |
Management has conducted an evaluation of the effectiveness of our internal control over financial reporting as of December 31, 2014, based on the framework established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”).
Based on this assessment, management concluded that as of December 31, 2014, it had material weaknesses in its internal control procedures.
A material weakness is a control deficiency, or combination of control deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements would not be prevented or detected on a timely basis. As of December 31, 2014, we have concluded that our internal control over financial reporting was ineffective. The Company’s assessment identified certain material weaknesses which are set forth below:
Functional Controls and Segregation of Duties
Because of the company’s limited resources, there are limited controls over information processing, and no internal controls over the accuracy, completeness and authorization of transactions.
There is an inadequate segregation of duties consistent with control objectives. Our Company’s management is composed of a small number of individuals resulting in a situation where limitations on segregation of duties exist. In order to remedy this situation we would need to hire additional staff to provide greater segregation of duties. Currently, it is not feasible to hire additional staff to obtain optimal segregation of duties. Management will reassess this matter in the following year to determine whether improvement in segregation of duty is feasible.
There is a lack of top level reviews in place to review targets, product development, joint ventures or financing. All major business decisions are carried out by the officers with board of director approval when needed.
Accordingly, as the result of identifying the above material weaknesses we have concluded that these control deficiencies resulted in a reasonable possibility that a material misstatement of the annual or interim financial statements will not be prevented or detected on a timely basis by the Company’s internal controls.
Management believes that the material weaknesses set forth above were the result of the scale of our operations and are intrinsic to our small size. Management believes these weaknesses did not have a material effect on our financial results and intends to take remedial actions upon receiving funding for the Company’s business operations.
This annual report does not include an attestation report of our registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by our registered public accounting firm pursuant to temporary rules of the SEC that permit the Company to provide only management’s report herein.
(c) CHANGES IN INTERNAL CONTROLS OVER FINANCIAL REPORTING
We are committed to improving our financial organization. As part of this commitment, we will create a position to segregate duties consistent with control objectives and will increase our personnel resources and technical accounting expertise within the accounting function when funds are available to us by preparing and implementing sufficient written policies and checklists which will set forth procedures for accounting and financial reporting with respect to the requirements and application of US GAAP and SEC disclosure requirements.
Management believes that preparing and implementing sufficient written policies and checklists will remedy the material weaknesses pertaining to insufficient written policies and procedures for accounting and financial reporting with respect to the requirements and application of US GAAP and SEC disclosure requirements. We intend to take appropriate and reasonable steps to make the necessary improvements to remediate these deficiencies, including:
| (1) | We will document a formal code of ethics. | |
| (2) | We will revise processes to provide for a greater role of independent board members in the oversight and review until such time that we are adequately capitalized to permit hiring additional personnel to address segregation of duties issues, ineffective controls over the revenue cycle and insufficient supervision and review by our corporate management. | |
| (3) | We will continue to update the documentation of our internal control processes, including formal risk assessment of our financial reporting processes. |
| 38 |
We intend to consider the results of our remediation efforts and related testing as part of our year-end 2014 assessment of the effectiveness of our internal control over financial reporting.
Subsequent to December 31, 2014, we have undertaken the following steps to address the deficiencies stated above:
| ● | Continued the development and documentation of internal controls and procedures surrounding the financial reporting process, primarily through the use of account reconciliations, and supervision. |
Not applicable.
Item 10. Directors, Executive Officers and Corporate Governance.
Directors and Executive Officers
The following table discloses our directors and executive officers as of April 15, 2015. There are no familial relationships between or among the directors or executive officers of the Company.
| Name | Age | Position | ||
| Govindan Gopinathan, MD | 76 | Chairman of the Board of Directors | ||
| Thomas Nicolette | 64 | President, Chief Executive Officer and Director | ||
| Patrice McMorrow | 48 | Executive Vice President of Business Development |
Dr. Govindan Gopinathan, 76, Chairman of the Board of Directors
Dr. Gopinathan, 76, is a former Clinical Professor of Neurology at New York University Langone Medical Center and the primary inventor of The EKG Glove, and co-founder of iNeedMD, Inc., where he had served as Chairman and CEO for the last 14 years. Previously he had served as Chief of the Department of Neurology at the Manhattan VA hospital from 1975 to 1978 where he was involved in administrative and management responsibilities.
Doctor Gopinathan is board certified in Internal Medicine by the Royal College of Physicians of Canada, and the University of Kerala, India. Doctor Gopinathan is also board certified in neurology by the American Academy of Neurology and the Royal College of Physicians of Canada. He is a Fellow of the American Academy of Neurology, the Royal Society of Medicine, London, and National Institute of Health, Clinical Fellowships.
Thomas Nicolette, 64, President, Chief Executive Officer and Director
Mr. Nicolette has served as the principal of Nicolette Consulting Group Limited, a business management consulting firm, since founding it in 1984. He served as President, Chief Executive Officer and director of Akers Biosciences, Inc. from 2006 until early 2014 and was instrumental in a $15 million IPO that facilitated Akers Biosciences’ listing on the NASDAQ capital market.
| 39 |
From 1997 through 2012, Mr. Nicolette was the Corporate Secretary, Treasurer and director of Sentech EAS Corp., a designer and manufacturer of electronic security systems for retail, commercial and industrial firms. From 2003 through 2006, Mr. Nicolette was the director of international business development for November AG a developer of methods of authentication for anti-counterfeiting based in Germany. From 2001 to 2004, Mr. Nicolette served as Chairman of Exaqt Sa de CV a manufacturer and installer of electronic security systems. From 2001 through 2003, Mr. Nicolette served as Executive Director of Tri-Mex Group Limited, a developer of monitoring and response solutions to protect high value or hazardous cargo. Mr. Nicolette served as President, Chief Executive Officer and Director of DNA Technologies, Inc., a holder of patented technology providing solutions for counterfeiting, forgery and product diversion, from 2000 through 2003. From 1995 through 2001, Mr. Nicolette was the President, Chief Executive Officer and director of Sentry Technology Corporation which owned Knogo North America, Inc. and Video Sentry Corporation, designers and manufacturers of electronic articles surveillance systems and closed circuit television systems worldwide. Also, Mr. Nicolette served as President, Chief Executive Officer and director of Knogo Corporation, a New York Stock Exchange listed multi-national company and purveyor of electronic article surveillance, from 1986 through 1994.
Mr. Nicolette is a graduate of Michigan State University School of Criminal Justice.
Patrice McMorrow, 48, Executive Vice President of Business Development
Ms. McMorrow, age 48, combines over 20 years of experience in marketing and business development senior management with proficiencies in strategic and tactical planning and implementation, sales training and operations, and IPO prospectus development.
Ms. McMorrow joined iNeedMD, Inc. in August of 2014 as Executive Vice President of Business Development. Previously, she had been involved in a number of companies most notably within the medical device and pharmaceutical industries, holding positions ranging from product manager to Vice President of Marketing. After gaining experience in management consulting and pharmaceutical sales and earning a graduate degree, from 1992 through 1995, Ms. McMorrow served as the Director of REC SPECS Sports Eyewear marketing for the now Liberty Sport, Inc. From 1995 through 1996, she served as a product manager at pharmaceutical manufacturer, Organon, Inc. and launched the company’s first antidepressant into the US market. From 1996 through 2003, Ms. McMorrow held a variety of marketing consulting positions, with a focus in the fashion and pharmaceutical industries, and secured a project management role with a contract pharmaceutical sales company, Innovex to assist in the re-launch of a Novartis mature migraine headache line. She also helped establish the national distribution network for then pharmaceutical manufacturer, Genta Incorporated. From 2003 until 2004, Ms. McMorrow transitioned to a product management role at Genta. Immediately prior to joining iNeedMD, Ms. McMorrow spent 10 years at Akers Biosciences, Inc. an in vitro diagnostic medical device company. She held positions of Director of Marketing where she helped launch the company’s entrance into the commercial medical device market and established and managed a network of national distributors and sales representatives. Later as Vice President of Marketing, Ms. McMorrow continued to assist in the launch of new product entrants in the laboratory and health and wellness sectors. She was also a member of the management team that facilitated Akers Biosciences’ listing on the NASDAQ capital market in early 2014.
Ms. McMorrow holds an undergraduate degree in Business with a concentration in Marketing from Albright College and an MBA in Marketing from Seton Hall University.
Key Personnel
Nitin Patel, MD, Age 40, Vice President of Research and Development
Dr. Nick Patel, age 40, is an internist from Columbia, S.C. He received his undergraduate degree from the University of South Carolina Honors College and attended medical school at the Medical University of South Carolina in Charleston, S.C. Following medical school, Dr. Patel completed his residency in internal medicine at Orlando Regional Medical Center in Florida and Palmetto Health in Columbia, S.C. He has been at Palmetto Health for over 10 years and serves as the Medical Director of Midlands Internal Medicine. He is also the head of Ambulatory Medical Informatics at Palmetto Health. Dr. Patel has given multiple presentations in topics ranging from Healthcare IT transformation, workflow enhancements, and population health. He is managing the largest, first of its kind.
| 40 |
Microsoft Surface pilot in the nation, to improve workflow at Palmetto Health. Dr. Patel has also been instrumental in the development of the Bluetooth wireless transmitter and mobile application.
Robert Riola, Age 50, Vice President of Operations
Mr. Robert Riola, age 50, combines over 25 years of experience in Information Technology management with experience in data center design, application development, risk management, digital rights management, and online marketing and sales. He joined the Company as Chief Technology Officer in 2010. Previously, he had been involved with a number of companies holding positions including Vice President of Operations Disaster Recovery Specialist and CTO.
Family Relationships
There are no family relationships among our directors, executive officers, or persons nominated or chosen by the Company to become directors or executive officers.
Committees of the Board of Directors
Our Board currently does not have an Audit Committee, a Compensation Committee or a Nominating and Corporate Governance Committee (the “Committees”). We plan to form the Committees in the second quarter of 2015.
Compliance with Section 16(a) of the Exchange Act
Section 16(a) of the Exchange Act requires the Company’s directors, executive officers and persons who beneficially own 10% or more of a class of securities registered under Section 12 of the Exchange Act to file reports of beneficial ownership and changes in beneficial ownership with the SEC. Directors, executive officers and greater than 10% stockholders are required by the rules and regulations of the SEC to furnish the Company with copies of all reports filed by them in compliance with Section 16(a).
Based solely on our review of certain reports filed with the Securities and Exchange Commission pursuant to Section 16(a) of the Securities Exchange Act of 1934, as amended, the reports required to be filed with respect to transactions in our common stock during the fiscal year ended December 31, 2014, were timely.
Code of Ethics
We have not yet adopted a code of ethics. We plan to adopt a code of ethics in the second quarter of 2015.
Legal Proceedings
There are no material proceedings to which any director or officer, or any associate of any such director or officer, is a party that is adverse to our Company or any of our subsidiaries or has a material interest adverse to our Company or any of our subsidiaries. No director or executive officer has been a director or executive officer of any business which has filed a bankruptcy petition or had a bankruptcy petition filed against it during the past ten years. No director or executive officer has been convicted of a criminal offense or is the subject of a pending criminal proceeding during the past ten years. No director or executive officer has been the subject of any order, judgment or decree of any court permanently or temporarily enjoining, barring, suspending or otherwise limiting his involvement in any type of business, securities or banking activities during the past ten years. No director or officer has been found by a court to have violated a federal or state securities or commodities law during the past ten years.
| 41 |
Item 11. Executive Compensation.
2014 SUMMARY COMPENSATION TABLE
EXECUTIVE COMPENSATION
Summary Compensation
The following summary compensation table sets forth all compensation awarded to, earned by, or paid to the named executive officers paid by us during the periods ended December 31, 2014, 2013 and 2012.
| Name and Principal Position | Year | Salary ($) | Bonus ($) | Stock Awards ($)(1) | Option Awards ($) | Non-Equity Incentive Plan Compensation ($) | Non- Qualified Deferred Compensation Earnings ($) | All Other Compensation ($)(3) | Totals ($) | |||||||||||||||||||||||||
| Govindan Gopinathan, MD (2) | 2014 | 139,902 | 0 | 0 | 0 | 0 | 0 | 211,385 | 351,287 | |||||||||||||||||||||||||
| Chairman of the Board | 2013 | 0 | 0 | 0 | 0 | 0 | 0 | 94,600 | 94,600 | |||||||||||||||||||||||||
| 2012 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||||||||||
| Thomas Nicolette (4) | 2014 | 0 | 0 | 0 | 0 | 0 | 0 | 778,454 | 778,454 | |||||||||||||||||||||||||
| Chief Executive Officer | 2013 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||||||||||||||||||||
| 2012 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||||||||||
| Patrice McMorrow(6) | 2014 | 40,625 | 0 | 0 | 0 | 0 | 0 | 0 | 40,625 | |||||||||||||||||||||||||
| Executive Vice President | 2013 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||||||||||||||||||||
| of Business Development | 2012 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||||||||||||||||||||
| Robert Riola (5) | 2014 | 102,500 | 0 | 0 | 0 | 0 | 0 | 32,500 | 135,000 | |||||||||||||||||||||||||
| Former Chief Technology | 2013 | 0 | 0 | 0 | 0 | 0 | 0 | 58,262 | 58,262 | |||||||||||||||||||||||||
| Officer | 2012 | 0 | 0 | 0 | 0 | 0 | 0 | 60,566 | 60,566 | |||||||||||||||||||||||||
| Victoria Young(7) | 2014 | 25,000 | 0 | 0 | 0 | 0 | 0 | 0 | 25,000 | |||||||||||||||||||||||||
| Former Chief Executive | 2013 | 25,000 | 0 | 0 | 0 | 0 | 0 | 0 | 25,000 | |||||||||||||||||||||||||
| Officer and Director | 2012 | 21,552 | 0 | 8,000 | 0 | 0 | 0 | 0 | 29,552 | |||||||||||||||||||||||||
| 1. | During 2013, the Company completed a capital raise of approximately $475,000 at $2.00 per share. Dr. Govindan Gopinathan was issued approximately 21.3 million shares post capital raise (Pre-Reverse Stock Split) which were subsequently valued at $2.00 per share for the 2013 fiscal year end. The compensation charge affiliated with such issuance is solely based on the way the value of these shares is accounted for under GAAP. The shares issued to Dr. Govindan Gopinathan were valued at $2.00 per share. |
| 2. | On December 24, 2014, Dr. Govindan Gopinathan was appointed Chairman of the Board of the Company. |
| 42 |
| 3. | All Other Compensation reflects consulting fees paid. |
| 4. | On December 24, 2014, upon the resignation of Ms. Young, Mr. Nicolette was appointed President, Chief Executive Officer and Director of the Company. In accordance with SEC rules, the amounts shown reflect the aggregate grant date fair value of the warrants granted to Mr. Nicolette during 2014, computed in accordance with Financial Accounting Standards Board Accounting Standards Codification Topic 718. The 1,000,000 warrants issued were valued at the grant date value of $0.6885 based upon a Black-Scholes-Merton model resulting in a grant date fair value of $688,454. The warrants outstanding are being amortized over their respective vesting periods. The Company expensed an aggregate $516,340 during 2014 in relation to the warrants granted to Mr. Nicolette. For a description of the assumptions used to calculate the amounts in the table, see Note 2 – Summary of Significant Accounting Policies and Note 13—Commitments and Contingencies found in the F-pages. |
| 5. | On December 24, 2014, Mr. Riola was appointed Vice President of Operations of the Company. |
| 6. | On December 24, 2014, Ms. McMorrow was appointed Executive Vice President of Business Development of the Company. |
| 7. | On December 24, 2014, Ms. Victoria Young resigned as the Company’s Chief Executive Officer and Director. |
Consulting Agreements
Mr. Thomas Nicolette
On July 1, 2014, Mr. Thomas Nicolette entered into a two year consulting agreement to serve as the Company’s Chief Operating Officer (“COO”). The COO will receive monthly fees of $15,000. In addition, the COO received warrants to purchase 1,000,000 shares of the Class A common stock, with 500,000 warrants vesting on July 1, 2014 and 500,000 warrants vesting on June 30, 2015. The warrants are exercisable for five years at an exercise price of $0.50. During the year ended December 31, 2014, the Company recorded $516,340 in compensation expense pertaining to these warrants. On December 24, 2014, Mr. Nicolette was appointed President, Chief Executive Officer and Director of the Company. Mr. Nicolette’s consulting agreement remains in effect until amended to reflect his new expanded responsibilities.
Outstanding Equity Awards
As of December 31, 2014, the Company has no outstanding equity awards.
DIRECTOR COMPENSATION
The Company did not award any compensation to directors for their service as such during the years ended December 31, 2014, 2013 and 2012.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
Our authorized capital stock consists of 75,000,000 shares, of which 65,000,000 are for shares of common stock, par value $0.001 per share, and 10,000,000 are for shares of blank check preferred stock, par value $0.001 per share. As of April 15, 2015, there were 48,014,424 shares of our common stock issued and outstanding, all of which were fully paid, non-assessable and entitled to vote. Each share of our common stock entitles its holder to one vote on each matter submitted to the stockholders.
| 43 |
The following table provides the names and addresses of each person known to us to own more than 5% of our outstanding shares of common stock as of April 15, 2015, and by the officers and directors, individually and as a group. Except as otherwise indicated, all shares are owned directly and the shareholders listed possesses sole voting and investment power with respect to the shares shown.
| Name(1) | Number of Common Shares Beneficially Owned(2) | Percent of Class(3) | ||||||
| Dr. Govindan Gopinathan (4) Chairman of the Board of Directors | 3,206,581 | 6.70 | % | |||||
| Thomas Nicolette(4)(5) President, Chief Executive Officer and Director | 2,500,000 | 5.20 | % | |||||
| Patrice McMorrow (7) Executive Vice President of Business Development | 250,000 | * | % | |||||
| Three Officers and Directors as a Group | 5,956,581 | 12.40 | % | |||||
| 5% or Greater Stockholders | ||||||||
| Gopinathan Family Retained Annuity Trust(6) | 4,000,000 | 8.33 | % | |||||
| Govindan Gopinathan Irrevocable Trust(6) | 4,400,000 | 9.16 | % | |||||
*less than 1%
| 1. | Except as otherwise indicated, the persons named in this table have sole voting and investment power with respect to all shares of Common Stock shown as beneficially owned by them, subject to community property laws where applicable and to the information contained in the footnotes to this table. Unless otherwise indicated, the address of the beneficial owner is 650 First Avenue, Third Floor, New York, New York 10016. |
| 2. | Pursuant to Rules 13d-3 and 13d-5 of the Exchange Act, beneficial ownership includes any shares as to which a shareholder has sole or shared voting power or investment power, and also any shares which the shareholder has the right to acquire within 60 days, including upon exercise of common shares purchase options or warrants. There are 48,014,424 shares of Common Stock issued and outstanding as of April 15, 2015. |
| 3. | Based on 48,014,424 issued and outstanding shares of Common Stock as of April 15, 2015. |
| 4. | On December 24, 2014, Dr. Govindan Gopinathan was appointed Chairman of the Board of Directors of the Company. On the same date, Mr. Nicolette was appointed President, Chief Executive Officer and Director of the Company. |
| 5. | Mr. Nicolette holds warrants to purchase 1,000,000 shares of Common Stock of the Company with 500,000 shares of Common Stock that vested on July 1, 2014 and 500,000 shares which will vest on June 30, 2015 exercisable for five years at an exercise price of $0.50 per share. |
| 6. | Dr. Govindan Gopinathan has no beneficial ownership of the shares held in the Gopinathan Family Retained Annuity Trust and the Govindan Gopinathan Irrevocable Trust. |
| 7. | Ms. McMorrow holds warrants to purchase 500,000 shares of Common Stock of the Company with 250,000 shares of Common Stock that vested on January 1, 2015 and 250,000 shares of Common Stock which will vest on September 30, 2015 exercisable for five years at an exercise price of $0.50 per share. On December 24, 2014, Ms. McMorrow was appointed Executive Vice President of Business Development of the Company. |
DESCRIPTION OF SECURITIES
General
Our authorized capital stock consists of 75,000,000 shares, of which 65,000,000 shares are common stock, par value $0.001 per share and 10,000,000 are for shares of blank check preferred stock, par value $0.001 per share.
| 44 |
Common Stock
As of April 15, 2015, there were 48,014,424 shares of our common stock issued and outstanding held by 210 beneficial owners of record.
Voting Rights
Each share of stock entitles the holder to one vote for each share on all matters submitted to a stockholder vote. Directors are elected by a plurality of the votes of the shares present in person or represented by proxy at an annual shareholders’ meeting and entitled to vote on the election of directors. Any other action shall be authorized by a majority of the votes cast, unless otherwise provided under the Nevada Revised Statutes. Holders of our stock representing a majority of the voting power of our capital stock issued, outstanding and entitled to vote, represented in person or by proxy, are necessary to constitute a quorum at any meeting of our stockholders.
Dividend Rights
Holders of our common stock are entitled to share in all dividends that our board of directors, in its discretion, declares from legally available funds.
Anti-Takeover Provisions
Our charter and bylaws contain provisions that may make it more difficult for a third party to acquire or may discourage acquisition bids for us. Our Board may, without action of our stockholders, issue authorized but unissued shares of preferred stock. The existence of unissued preferred stock may enable the Board, without further action by the stockholders, to issue such stock to persons friendly to current management or to issue such stock with terms that could render more difficult or discourage an attempt to obtain control of us, thereby protecting the continuity of our management. Our shares of preferred stock could therefore be issued quickly with terms that could delay, defer, or prevent a change in control of us, or make removal of management more difficult.
Item 13. Certain Relationships and Related Transactions.
Except as disclosed below, none of our officers, directors, proposed director nominees, beneficial owners of more than 10% of our shares of common stock, or any relative or spouse of any of the foregoing persons, or any relative of such spouse who has the same house as such person or who is a director or officer of any parent or subsidiary of our Company, has any direct or indirect material interest in any transaction to which we are a party since our incorporation or in any proposed transaction to which we are proposed to be a party. In the event a related party transaction is proposed, such transaction will be presented to our board of directors for consideration and approval. Any such transaction will require approval by a majority of the disinterested directors and such transactions will be on terms no less favorable than those available to disinterested third parties.
Dr. Govindan Gopinathan provided consulting services for the company. Consulting expenses pertaining to his services were $148,000 and $94,600 for the years ended December 31, 2014 and 2013, respectively.
Director Independence
The common stock of the Company is currently quoted on the OTC Markets quotation systems which currently does not have director independence requirements. On an annual basis, each director and executive officer will be obligated to disclose any transactions with the Company in which a director or executive officer, or any member of his or her immediate family, have a direct or indirect material interest in accordance with Item 407(a) of Regulation S-K. Following completion of these disclosures, the Board will make an annual determination as to the independence of each director using the current standards for “independence” that satisfy the criteria for the NASDAQ.
As of April 15, 2015, the Board has determined that none of its directors are independent under these standards:
| 45 |
Item 14. Principal Accountant Fees and Services.
Audit Fees
(a) The aggregate fees billed by RRBB Accountants and Advisors for the audit of the Company’s financial statements for the fiscal years ended December 31, 2014 and 2013, were $38,000 and $30,000, respectively.
Audit Related Fees
(b) RRBB Accountants and Advisors did not bill the Company any amounts for assurance and related services that were related to its audit or review of the Company’s financial statements during the fiscal years ended December 31, 2014 and 2013, respectively.
Tax Fees
(c) The aggregate fees billed by RRBB Accountants and Advisors for tax compliance, advice and planning were $0 for the fiscal year ended December 31, 2014 and $0 for the fiscal year ended December 31, 2013.
All Other Fees
(d) RRBB Accountants and Advisors did not bill the Company for any products and services other than the foregoing during the fiscal years ended December 31, 2014 and 2013, respectively.
| 46 |
Item 15. Exhibits, Financial Statement Schedules.
(d) Exhibits. Exhibit No. Description
| Exhibit No. |
Description | |
| 2.1 | Acquisition and Plan of Merger Agreement dated December 17, 2014 by and among Clutterbug Move Management, Inc., Clutterbug Acquisition Corp., and iNeedMD Holdings, Inc. (incorporated herein by reference to Exhibit 2.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on December 30, 2014). | |
| 3.1 | Certificate of Incorporation of Clutterbug Move Management, Inc. (incorporated herein by reference to Exhibit 3.1 to the Company’s Form S-1 filed with the Securities and Exchange Commission on March 14, 2013). | |
| 3.2 | By-Laws of Clutterbug Move Management, Inc. (incorporated herein by reference to Exhibit 3.2 to the Company’s Form S-1 filed with the Securities and Exchange Commission on March 14, 2013). | |
| 3.3 | Certificate of Incorporation of iNeedMD, Inc. (incorporated herein by reference to Exhibit 3.3 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on December 30, 2014). | |
| 3.4 | By-Laws of iNeedMD, Inc. (incorporated herein by reference to Exhibit 3.4 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on December 30, 2014). | |
| 3.5 | Amended Certificate of Incorporation of iNeedMD Holdings, Inc.* | |
| 16.1 | Letter by KLJ & Associates LLP dated December 24, 2014 (incorporated herein by reference to Exhibit 16.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on December 30, 2014). | |
| 31.1 | Certification by the Principal Executive Officer of Registrant pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 (Rule 13a-14(a) or Rule 15d-14(a)).* | |
| 31.2 | Certification by the Principal Executive Officer of Registrant pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 (Rule 13a-14(a) or Rule 15d-14(a)).* | |
| 32.1 | Certification by the Principal Executive Officer of Registrant pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 (Rule 13a-14(a) or Rule 15d-14(a)).* | |
| 32.2 | Certification by the Principal Executive Officer of Registrant pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 (Rule 13a-14(a) or Rule 15d-14(a)).* | |
| 99.1 | Letter of Resignation from Victoria Young, dated December 24, 2014 (incorporated herein by reference to Exhibit 99.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on December 30, 2014). | |
| * | Filed Herewith. |
| 47 |
Pursuant to the requirements of Section 13 or 15(d) of the Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| INEEDMD HOLDINGS, INC. | ||
| Date: April 15, 2015 | By: | /s/ Thomas A. Nicolette |
| Name: | Thomas A. Nicolette | |
| Title: | Chief Executive Officer | |
| (Principal Executive Officer) | ||
| (Principal Financial Officer) | ||
| (Principal Accounting Officer) | ||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ Dr. Govindan Gopinathan | Chairman | April 15, 2015 | ||
| Dr. Govindan Gopinathan | ||||
| /s/ Thomas A. Nicolette | Chief Executive Officer, Principal Executive | April 15, 2015 | ||
| Thomas A. Nicolette | Officer, Principal Financial Officer, Principal | |||
| Accounting Officer | ||||
| /s/ Patrice McMorrow | Executive Vice President of Business Development | April 15, 2015 | ||
| Patrice McMorrow |
| 48 |
iNeedMD Holdings, Inc.
Financial Statements
December 31, 2014 and 2013
iNeedMD Holdings, Inc.
Index to the Financial Statements
December 31, 2014 and 2013
| Contents | Page(s) |
| Report of Independent Registered Public Accounting Firm | F-2 |
| Balance Sheets at December 31, 2014 and 2013 | F-3 |
| Consolidated Statements of Operations for the Year Ended December 31, 2014 and 2013 | F-4 |
| Consolidated Statements of Stockholders’ Equity (Deficit) at December 31, 2014 and 2013 | F-5 |
| Consolidated Statements of Cash Flows for the Year Ended December 31, 2014 and 2013 | F-6 |
| Notes to the Financial Statements | F-7 |
| F-1 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
iNeedMD Holdings, Inc.
We have audited the accompanying balance sheets of iNeedMD Holdings, Inc. as of December 31, 2014 and 2013, and the related statements of operations, stockholders equity (deficit), and cash flows for the two years then ended. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of iNeedMD Holdings, Inc. as of December 31, 2014 and 2013, and the results of its operations and its cash flows for the two years then ended, in conformity with U.S. generally accepted accounting principles.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 3 to the financial statements, the Company had a net loss and net cash used in operations of $40,312,486 and $4,566,234, respectively and has an accumulated deficit totaling $115,340,329 and does not generate any significant revenue. These conditions raise substantial doubt about its ability to continue as a going concern. Management’s plans regarding those matters also are described in Note 3. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Somerset, New Jersey
April 15, 2015
| F-2 |
Consolidated Balance Sheets
| December 31, | ||||||||
| 2014 | 2013 | |||||||
| Assets | ||||||||
| Current Assets | ||||||||
| Cash | $ | 2,457,622 | $ | 853,908 | ||||
| Accounts receivable, net | — | — | ||||||
| Inventory | 148,476 | — | ||||||
| Prepaid expenses | 9,931 | 588 | ||||||
| Prepaid Interest | — | 10,473 | ||||||
| Debt issuance costs, net | — | 88,480 | ||||||
| Total Current Assets | 2,616,029 | 953,449 | ||||||
| Property and equipment, net | 48,549 | — | ||||||
| Other Assets | ||||||||
| Security Deposits | 7,941 | — | ||||||
| Total Other Assets | 7,941 | — | ||||||
| Total Assets | $ | 2,672,519 | $ | 953,449 | ||||
| Liabilities and Stockholders' Equity (Deficit) | ||||||||
| Current Liabilities: | ||||||||
| Accounts payable and accrued liabilities | $ | 247,904 | $ | 489,057 | ||||
| Deferred revenue | 36,975 | — | ||||||
| Convertible notes payable, net of debt discount of $59,177 and $566,667, respectively | 140,823 | 183,333 | ||||||
| Note payable | — | 250,000 | ||||||
| Derivative Liability | 1,898,636 | 898,312 | ||||||
| Total Current Liabilities | 2,324,338 | 1,820,702 | ||||||
| Non-current Liabilities: | ||||||||
| Convertible notes payable, net of debt discount of $0 and $125,844 respectively | — | 74,156 | ||||||
| Derivative liability | — | 389,932 | ||||||
| Total Non-current Liabilities | — | 464,088 | ||||||
| Total Liabilities | 2,324,338 | 2,284,790 | ||||||
| Commitments and contingencies | ||||||||
| Stockholders' Equity (Deficit) | ||||||||
| Preferred Stock par value $0.001: 10,000,000 shares authorized; 400 and 400 shares issued and outstanding, respectively | — | — | ||||||
| Class A Common Stock par value $0.001: 65,000,000 shares authorized; 48,014,424 and 52,271 shares issued and outstanding, respectively | 48,014 | 52 | ||||||
| Additional paid-in capital | 115,640,496 | 73,696,450 | ||||||
| Accumulated deficit | (115,340,329 | ) | (75,027,843 | ) | ||||
| Total Stockholders' Equity (Deficit) | 348,181 | (1,331,341 | ) | |||||
| Total Liabilities and Stockholders' Equity (Deficit) | $ | 2,672,519 | $ | 953,449 | ||||
See accompanying notes to the financial statements.
| F-3 |
Consolidated Statements of Operations
| For the Year Ended December 31, | ||||||||
| 2014 | 2013 | |||||||
| Revenue | $ | 910 | $ | 7,539 | ||||
| Cost of revenue | 306 | 5,880 | ||||||
| Gross margin | 604 | 1,659 | ||||||
| Operating expenses | ||||||||
| Compensation | 35,038,488 | 64,761,772 | ||||||
| Consulting fees | 1,015,522 | 297,258 | ||||||
| Consulting fees - related party | 278,000 | 107,600 | ||||||
| Sales and marketing | 336,944 | 149,976 | ||||||
| Sales and marketing - related party | 20,000 | 18,500 | ||||||
| Research and development | 697,142 | 37,069 | ||||||
| General and administrative | 1,207,946 | 281,921 | ||||||
| Total operating expenses | 38,594,042 | 65,654,096 | ||||||
| Income from operations | (38,593,438 | ) | (65,652,437 | ) | ||||
| Other income (expenses) | ||||||||
| Interest income | 237 | - | ||||||
| Proceeds from insurance settlement | 36,365 | - | ||||||
| Interest expense | (96,444 | ) | (99,393 | ) | ||||
| Amortization of debt discount | (1,233,334 | ) | (250,000 | ) | ||||
| Amortization of deferred financing costs | (129,459 | ) | (28,626 | ) | ||||
| Debt conversion expense | (100,000 | ) | - | |||||
| Derivative expense | (1,141,407 | ) | (133,087 | ) | ||||
| Change in fair value of derivative liabilities | 944,994 | (139,280 | ) | |||||
| Total other income (expenses) | (1,719,048 | ) | (650,386 | ) | ||||
| Net loss | $ | (40,312,486 | ) | $ | (66,302,823 | ) | ||
| Net loss per common share | ||||||||
| Basic and diluted- Common A shares | $ | (1.45 | ) | $ | (1,318.23 | ) | ||
| Weighted average common A shares outstanding - basic and diluted | 27,865,356 | 50,297 | ||||||
See accompanying notes to the financial statements.
| F-4 |
Consolidated Statements of Stockholders’ Equity (Deficit)
For the Years Ended December 31, 2014 and 2013
| Preferred Stock | Common A Stock | Additional | Total Stockholders' | |||||||||||||||||||||||||
| Par Value $0.0001 | Par Value $0.0001 | Paid-in | Accumulated | Equity | ||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Capital | Deficit | (Deficit) | ||||||||||||||||||||||
| Balance - December 31, 2012 | 400 | $ | — | 17,836 | $ | 18 | $ | 7,782,1112 | $ | (8,725,020 | ) | $ | (942,890 | ) | ||||||||||||||
| Share-based compensation - Class A common stock | — | — | 32,765 | 33 | 64,779,339 | — | 64,779,372 | |||||||||||||||||||||
| Issuance of Class A common stock for cash | — | — | 1,670 | 1 | 1,134,999 | — | 1,135,000 | |||||||||||||||||||||
| Net loss | — | — | — | — | — | (66,302,823 | ) | (66,302,823 | ) | |||||||||||||||||||
| Balance - December 31, 2013 | 400 | $ | — | 52,271 | $ | 52 | $ | 73,696,450 | $ | (75,027,843 | ) | $ | (1,331,341 | ) | ||||||||||||||
| Share-based compensation - Class A common stock | — | — | 33,891,703 | 3,3892 | 33,857,811 | — | 33,891,703 | |||||||||||||||||||||
| Issuance of Class A common stock in conjunction with convertible note | — | — | 100,000 | 100 | 66,567 | — | 66,667 | |||||||||||||||||||||
| Conversion of Notes payable into Class A common stock | — | — | 200,000 | 200 | 199,800 | — | 200,000 | |||||||||||||||||||||
| Issuance of Class A common stock in conjunction with legal settlement | — | — | 20,000 | 20 | 19,980 | — | 20,000 | |||||||||||||||||||||
| Beneficial Conversion Feature on convertible note | — | — | — | — | 133,333 | — | 133,333 | |||||||||||||||||||||
| Issuance of Class A common stock for cash | — | — | 450 | — | 450,000 | — | 450,000 | |||||||||||||||||||||
| Issuance of Class A common stock in an offering- net proceeds | — | — | 8,400,000 | 8,400 | 7,055,565 | — | 7,063,965 | |||||||||||||||||||||
| Issuance of warrants to consultants | — | — | — | — | 516,340 | — | 516,340 | |||||||||||||||||||||
| Reverse merger fees | — | — | — | — | (350,000 | ) | — | (350,000 | ) | |||||||||||||||||||
| Additional Shares resulting from the reverse merger | — | — | 5,350,000 | 5,350 | (5,350 | ) | — | — | ||||||||||||||||||||
| Net loss | — | — | — | — | — | (40,312,486 | ) | (40,312,486 | ) | |||||||||||||||||||
| Balance - December 31, 2014 | 400 | $ | — | 48,014,424 | $ | 48,014 | $ | 115,640,496 | $ | (115,340,329 | ) | $ | 348,181 | |||||||||||||||
See accompanying notes to the financial statements.
| F-5 |
Consolidated Statements of Cash Flows
| For the Year Ended December 31, | ||||||||
| 2014 | 2013 | |||||||
| Cash Flows From Operating Activities: | ||||||||
| Net loss | $ | (40,312,486 | ) | $ | (66,302,823 | ) | ||
| Adjustments to reconcile net loss to net cash used by operating activities: | ||||||||
| Depreciation expense | 8,680 | — | ||||||
| Loss on disposal of property and equipment | 9,788 | — | ||||||
| Amortization of debt issuance costs on notes payable | 129,459 | 28,626 | ||||||
| Amortization of debt discount on notes payable | 1,233,334 | 250,000 | ||||||
| Derivative expense | 1,141,407 | 133,087 | ||||||
| Change in fair value of derivative liabilities | (944,994 | ) | 139,280 | |||||
| Share based payments- Class A common stock | 33,891,703 | 64,779,372 | ||||||
| Share based payments- warrants issued to consultants | 516,340 | — | ||||||
| Debt Conversion Expense | 100,000 | — | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Inventory | (148,476 | ) | — | |||||
| Prepaid expenses | (9,343 | ) | 7,178 | |||||
| Prepaid interest | 10,473 | (4,715 | ) | |||||
| Security deposits | (7,941 | ) | — | |||||
| Accounts payable and accrued liabilities | (221,153 | ) | 17,592 | |||||
| Deferred revenue | 36,975 | — | ||||||
| Net Cash Used by Operating Activities | (4,566,234 | ) | (952,403 | ) | ||||
| Cash Flows from Investing Activities: | ||||||||
| Purchase of property and equipment | (67,017 | ) | — | |||||
| Repayment of loans to consultants | — | 47,000 | ||||||
| Reverse merger fees | (350,000 | ) | — | |||||
Net Cash (Used In) Provided by Investing Activities | (417,017 | ) | 47,000 | |||||
| Cash Flows from Financing Activities: | ||||||||
| Net repayment to related party | — | (87,300 | ) | |||||
| Payment of debt issuance costs | (27,000 | ) | (90,000 | ) | ||||
| Proceeds from the issuance of convertible notes | 600,000 | 750,000 | ||||||
| Repayment of notes payable | (150,000 | ) | — | |||||
| Repayment of convertible notes | (1,350,000 | ) | — | |||||
| Net proceeds from the issuance of Class A common stock | 7,513,965 | 1,135,000 | ||||||
| Net Cash Provided By Financing Activities | 6,586,965 | 1,707,700 | ||||||
| Net change in cash | 1,603,714 | 802,297 | ||||||
| Cash at beginning of year | 853,908 | 51,611 | ||||||
| Cash at end of year | $ | 2,457,622 | $ | 853,908 | ||||
| Supplemental disclosures of cash flow information: | ||||||||
| Interest paid | $ | 67,444 | $ | 83,320 | ||||
| Income taxes | — | — | ||||||
| Supplemental disclosure of non-cash investing and financing activities: | ||||||||
| Debt issuance costs paid in the form of stock warrants on convertible notes payable | $ | 13,979 | $ | 27,106 | ||||
| Debt discount in conjunction with the recording of the original value of the derivative liability | $ | 400,000 | $ | 750,000 | ||||
| Debt discount in conjunction with Class A common stock issued with convertible notes payable | $ | 66,667 | $ | — | ||||
| Debt discount in conjunction with beneficial conversion feature | $ | 133,333 | $ | — | ||||
| Issuance of Class A common stock in conjunction with a legal settlement | $ | 20,000 | $ | — | ||||
| Conversion of notes payable into Class A common stock | $ | 200,000 | $ | — | ||||
See accompanying notes to the financial statements.
| F-6 |
Notes to the Consolidated Financial Statements
December 31, 2014 and 2013
Note 1 - Organization and Operations
INeedMD Holdings, Inc. (“iNeedMD” or the “Company”) was incorporated on February 16, 2000, under the laws of the State of Delaware. The Company is a medical device company that has developed a disposable device used in the diagnosis, prevention, and monitoring of cardiovascular disease. On January 27, 2014, a new subsidiary was formed in India called iNeedMD Medical Device Private Ltd. The purpose of the formation of the subsidiary was to provide sales and distribution in India.
Effective December 24, 2014, Clutterbug Move Management, Inc. a Nevada corporation (“Clutterbug), Clutterbug Acquisition Corp, a Nevada corporation and wholly-owned subsidiary of Clutterbug (“Merger Sub”), iNeedMD and Victoria Young an individual (the “Majority Shareholder”), entered into an Acquisition Agreement and Plan of Merger (the “Agreement”) pursuant to which the Merger Sub was merged with and into iNeedMD, with iNeedMD surviving as a wholly-owned subsidiary of Clutterbug (the “Merger”). The transaction (the “Closing”) took place on December 24, 2014 (the “Closing Date”). Clutterbug acquired, through a reverse triangular merger, all of the outstanding capital stock of iNeedMD in exchange for issuing iNeedMD’s shareholders (the “iNeedMD Shareholders”), pro-rata, a total of 42,464,424 shares of Clutterbug’s common stock. Immediately after the Merger was consummated, the Majority Shareholder of Clutterbug cancelled 5,350,000 shares of her restricted common stock of Clutterbug (the “Cancellation”). In consideration of the Cancellation of such common stock, iNeedMD paid the Majority Shareholder an aggregate of $350,000 and released the other affiliates from certain liabilities. In addition, iNeedMD agreed to spinout to the Majority Shareholder any and all assets related to the Company’s senior move management and assistance services business within 30 days after the Closing. As a result of the Merger and the Cancellation, the iNeedMD Shareholders became the majority shareholders of the Company.
Our consolidated financial statements include the accounts of the Company and its subsidiary. All significant intercompany accounts and transactions have been eliminated in consolidation.
Note 2 - Significant and Critical Accounting Policies and Practices
The Management of the Company is responsible for the selection and use of appropriate accounting policies and the appropriateness of accounting policies and their application. Critical accounting policies and practices are those that are both most important to the portrayal of the Company’s financial condition and results and require management’s most difficult, subjective, or complex judgments, often as a result of the need to make estimates about the effects of matters that are inherently uncertain. The Company’s significant and critical accounting policies and practices are disclosed below as required by generally accepted accounting principles.
Basis of Presentation
The Company’s financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“US GAAP”) and the rules and regulations of the Securities and Exchange Commission.
Use of Estimates and Assumptions and Critical Accounting Estimates and Assumptions
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period.
| F-7 |
Management bases its estimates on historical experience and on various assumptions that are believed to be reasonable in relation to the financial statements taken as a whole under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources.
Management regularly evaluates the key factors and assumptions used to develop the estimates utilizing currently available information, changes in facts and circumstances, historical experience and reasonable assumptions. After such evaluations, if deemed appropriate, those estimates are adjusted accordingly.
Fair Value of Financial Instruments
The Company follows paragraph 825-10-50-10 of the FASB Accounting Standards Codification for disclosures about fair value of its financial instruments and paragraph 820-10-35-37 of the FASB Accounting Standards Codification (“Paragraph 820-10-35-37”) to measure the fair value of its financial instruments. Paragraph 820-10-35-37 establishes a framework for measuring fair value in accounting principles generally accepted in the United States of America (U.S. GAAP), and expands disclosures about fair value measurements. To increase consistency and comparability in fair value measurements and related disclosures, Paragraph 820-10-35-37 establishes a fair value hierarchy which prioritizes the inputs to valuation techniques used to measure fair value into three (3) broad levels. The fair value hierarchy gives the highest priority to quoted prices (unadjusted) in active markets for identical assets or liabilities and the lowest priority to unobservable inputs. The three (3) levels of fair value hierarchy defined by Paragraph 820-10-35-37 are described below:
| Level 1 | Quoted market prices available in active markets for identical assets or liabilities as of the reporting date. | |
| Level 2 | Pricing inputs other than quoted prices in active markets included in Level 1, which are either directly or indirectly observable as of the reporting date. | |
| Level 3 | Pricing inputs that are generally unobservable inputs and not corroborated by market data. |
Financial assets are considered Level 3 when their fair values are determined using pricing models, discounted cash flow methodologies or similar techniques and at least one significant model assumption or input is unobservable.
| F-8 |
The fair value hierarchy gives the highest priority to quoted prices (unadjusted) in active markets for identical assets or liabilities and the lowest priority to unobservable inputs. If the inputs used to measure the financial assets and liabilities fall within more than one level described above, the categorization is based on the lowest level input that is significant to the fair value measurement of the instrument.
The carrying amount of the Company’s financial assets and liabilities, such as cash, accounts receivable, accounts payable and accrued liabilities approximate their fair value because of the short maturity of those instruments.
The Company uses Level 3 of the fair value hierarchy to measure the fair value of the derivative liabilities and revalues its derivative liabilities at every reporting period and recognizes gains or losses in the statements of operations that are attributable to the change in the fair value of the derivative liabilities.
Fair Value of Financial Assets and Liabilities Measured on a Recurring Basis
Level 3 Financial Liabilities – Derivative conversion features and warrant liabilities
Financial assets and liabilities measured at fair value on a recurring basis are summarized below and disclosed on the consolidated balance sheets as of December 31, 2014:
| Carrying | Fair Value Measurement Using | |||||||||||||||||||
| Value | Level 1 | Level 2 | Level 3 | Total | ||||||||||||||||
| Derivative conversion features and warrant liabilities | $ | 1,898,636 | $ | - | $ | - | $ | 1,898,636 | $ | 1,898,636 | ||||||||||
Financial assets and liabilities measured at fair value on a recurring basis are summarized below and disclosed on the consolidated balance sheets as of December 31, 2013:
| Carrying | Fair Value Measurement Using | |||||||||||||||||||
| Value | Level 1 | Level 2 | Level 3 | Total | ||||||||||||||||
| Derivative conversion features and warrant liabilities | $ | 1,288,244 | $ | - | $ | - | $ | 1,288,244 | $ | 1,288,244 | ||||||||||
The table below provides a summary of the changes in fair value, including net transfers in and/or out, of all financial assets and liabilities measured at fair value on a recurring basis using significant unobservable inputs (Level 3) during the years ended December 31, 2014 and 2013:
| Fair Value Measurement Using Level 3 Inputs Total Derivative Liabilities | ||||
| Balance, December 31, 2012 | $ | 238,771 | ||
| Total (gains) or losses (realized/unrealized) included in statement of operations | 139,280 | |||
| Purchases, issuances and settlements | 910,193 | |||
| Balance, December 31, 2013 | 1,288,244 | |||
| Total (gains) or losses (realized/unrealized) included in statement of operations | (944,994 | ) | ||
| Purchases, issuances and settlements | 1,555,386 | |||
| Balance, December 31, 2014 | $ | 1,898,636 | ||
| F-9 |
Changes in the unobservable input values could potentially cause material changes in the fair value of the Company’s Level 3 financial instruments. The significant unobservable inputs used in the fair value measurements are the expected volatility assumption and fair value of the Company’s common stock price. A significant increase (decrease) in the expected volatility and/or fair value of the Company’s common stock price assumptions could potentially result in a higher (lower) fair value measurement.
Cash Equivalents
Cash is maintained in an operating account and a savings account. The Company considers all highly liquid investments with a maturity of three months or less when purchased to be cash equivalents.
Concentration of Credit Risk
The Company maintains its cash in bank deposit accounts that at times exceed federally insured limits. These cash balances are maintained at creditworthy financial institutions. The Company has not experienced any losses in such accounts and believes it is not exposed to any significant credit risk.
Accounts Receivable and Allowance for Doubtful Accounts
Accounts receivable are recorded at the invoiced amount, net of an allowance for doubtful accounts. The Company performs on-going credit evaluations of its customers and adjusts credit limits based upon payment history and the customer’s current credit worthiness, as determined by the review of their current credit information; and determines the allowance for doubtful accounts based on historical write-off experience, customer specific facts and economic conditions.
Account balances are charged off against the allowance after all means of collection have been exhausted and the potential for recovery is considered remote. The Company determines when receivables are past due or delinquent based on how recently payments have been received.
Outstanding account balances are reviewed individually for collectability. The allowance for doubtful accounts is the Company’s best estimate of the amount of probable credit losses in the Company’s existing accounts receivable. Bad debt expense is included in general and administrative expenses, if any.
The Company does not have any off-balance-sheet credit exposure to its customers.
The allowance for doubtful accounts was $0 and $4,119 at December 31, 2014 and 2013, respectively.
Inventory
Inventory are stated using the first-in, first-out (FIFO) valuation method. Inventory was comprised solely of finished goods of $148,476 and $0 at December 31, 2014 and December 31, 2013, respectively.
Property and Equipment
Property and equipment are recorded at cost. Depreciation expense is computed using the straight-line method over the estimated useful lives. Asset lives for financial statement reporting of depreciation are:
| Computer equipment | 3-5 years |
| Leasehold improvements | 5 years |
| F-10 |
Debt Issuance Costs
Debt issuance costs are amortized using the straight-line method over the term of the notes.
Derivative Instruments
The Company evaluates its convertible debt and warrants to determine if those contracts or embedded components of those contracts qualify as derivatives to be separately accounted for in accordance with Paragraph 810-10-05-4 of the Codification and Paragraph 815-40-25 of the Codification. The result of this accounting treatment is that the fair value of the embedded derivative is marked-to-market each balance sheet date and recorded as a liability. In the event that the fair value is recorded as a liability, the change in fair value is recorded in the statements of operations as other income or expense. Upon conversion or exercise of a derivative instrument, the instrument is marked to fair value at the conversion date and then that fair value is reclassified to equity.
In circumstances where the embedded conversion option in a convertible instrument is required to be bifurcated and there are also other embedded derivative instruments in the convertible instrument that are required to be bifurcated, the bifurcated derivative instruments are accounted for as a single, compound derivative instrument.
The classification of derivative instruments, including whether such instruments should be recorded as liabilities or as equity, is re-assessed at the end of each reporting period. Equity instruments that are initially classified as equity that become subject to reclassification are reclassified to liability at the fair value of the instrument on the reclassification date. Derivative instrument liabilities are classified in the balance sheet as current or non-current to correspong with its host instrument.
The Company marks to market the fair value of the remaining embedded derivative warrants at each balance sheet date and records the change in the fair value of the remaining embedded derivative warrants as other income or expense in the statements of operations.
The Company utilizes the Monte Carlo model that values the liability of the debt conversion feature derivative financial instruments and derivative warrants based on a probability weighted discounted cash flow model with the assistance of the third party valuation firm. The reason the Company selected the Monte Carlo model is that in many cases there may be multiple embedded features or the features of the bifurcated derivatives may be so complex that a Black-Scholes valuation does not consider all of the terms of the instrument. Therefore, the fair value may not be appropriately captured by simple models. In other words, simple models such as Black-Scholes may not be appropriate in many situations given the complex features and terms of conversion option (e.g., combined embedded derivatives). The Monte Carlo model is based on future projections of the various potential outcomes. The features that are analyzed and incorporated into the model include the exercise and full reset features. Based on these features, there are two primary events that can occur; the holder exercises the derivative instrument or the derivative instrument is held until it expires. The Monte Carlo model analyzes the underlying economic factors that influence which of these events would occur, when they are likely to occur, and the specific terms that would be in effect at the time (i.e. stock price, exercise price, volatility, etc.). Projections are then made on the underlying factors which lead to potential scenarios. Probabilities are assigned to each scenario based on management projections. This leads to a cash flow projection and a probability associated with that cash flow. A discounted weighted average cash flow over the various scenarios is completed to determine the value of the derivative instrument.
Related Parties
The Company follows subtopic 850-10 of the FASB Accounting Standards Codification for the identification of related parties and disclosure of related party transactions.
| F-11 |
The financial statements shall include disclosures of material related party transactions, other than compensation arrangements, expense allowances, and other similar items in the ordinary course of business. The disclosures shall include: (a.) the nature of the relationship(s) involved; (b.) a description of the transactions, including transactions to which no amounts or nominal amounts were ascribed, for each of the periods for which income statements are presented, and such other information deemed necessary to an understanding of the effects of the transactions on the financial statements; (c.) the dollar amounts of transactions for each of the periods for which income statements are presented and the effects of any change in the method of establishing the terms from that used in the preceding period; and (d.) amounts due from or to related parties as of the date of each balance sheet presented and, if not otherwise apparent, the terms and manner of settlement.
Commitments and Contingencies
The Company follows subtopic 450-20 of the FASB Accounting Standards Codification to report accounting for contingencies. Certain conditions may exist as of the date the financial statements are issued, which may result in a loss to the Company but which is only be resolved when one or more future events occur or fail to occur. The Company assesses such contingent liabilities, and such assessment inherently involves an exercise of judgment. In assessing loss contingencies related to legal proceedings that are pending against the Company or un-asserted claims that may result in such proceedings, the Company evaluates the perceived merits of any legal proceedings or un-asserted claims as well as the perceived merits of the amount of relief sought or expected to be sought therein.
If the assessment of a contingency indicates that it is probable that a material loss has been incurred and the amount of the liability can be estimated, then the estimated liability would be accrued in the Company’s financial statements. If the assessment indicates that a potential material loss contingency is not probable but is reasonably possible, or is probable but cannot be estimated, then the nature of the contingent liability, and an estimate of the range of possible losses, if determinable and material, would be disclosed.
Loss contingencies considered remote are generally not disclosed unless they involve guarantees, in which case the guarantees would be disclosed. Management does not believe, based upon information available at this time, that these matters will have a material adverse effect on the Company’s financial position, results of operations or cash flows. However, there is no assurance that such matters will not materially and adversely affect the Company’s business, financial position, and results of operations or cash flows.
Revenue Recognition
The Company follows paragraph 605-10-S99-1 of the FASB Accounting Standards Codification for revenue recognition. The Company recognizes revenue when it is realized or realizable and earned. The Company considers revenue realized or realizable and earned when all of the following criteria are met: (i) persuasive evidence of an arrangement exists, (ii) the product has been shipped (iii) the sales price is fixed or determinable, and (iv) collectability is reasonably assured.
Deferred Revenue (Liability)
The Company receives up-front payments for goods. These payments are initially deferred and subsequently recognized when the goods are shipped. Deferred revenue at December 31, 2014 and 2013 was $36,975 and $0, respectively.
Equity Instruments Issued to Parties Other Than Employees for Acquiring Goods or Services
The Company accounts for equity instruments issued to parties other than employees for acquiring goods or services under guidance of Sub-topic 505-50 of the FASB Accounting Standards Codification (“Sub-topic 505-50”).
Pursuant to ASC Section 505-50-30, all transactions in which goods or services are the consideration received for the issuance of equity instruments are accounted for based on the fair value of the consideration received or the fair value of the equity instrument issued, whichever is more reliably measurable. The measurement date used to determine the fair value of the equity instrument issued is the earlier of the date on which the performance is complete or the date on which it is probable that performance will occur. If shares of the Company are thinly traded the use of share prices established in the Company’s most recent PPM, or weekly or monthly price observations would generally be more appropriate than the use of daily price observations as such shares could be artificially inflated due to a larger spread between the bid and asked quotes and lack of consistent trading in the market.
| F-12 |
The fair value of share options and similar instruments is estimated on the date of grant using a Black-Scholes-Merton option-pricing valuation model. The ranges of assumptions for inputs are as follows:
| ● | Expected term of share options and similar instruments: Pursuant to Paragraph 718-10-50-2(f)(2)(i) of the FASB Accounting Standards Codification, the expected term of share options and similar instruments represents the period of time the options and similar instruments are expected to be outstanding taking into consideration of the contractual term of the instruments and holder’s expected exercise behavior into the fair value (or calculated value) of the instruments. Pursuant to paragraph 718-10-S99-1, it may be appropriate to use the simplified method, i.e., expected term = ((vesting term + original contractual term) / 2), if (i) A company does not have sufficient historical exercise data to provide a reasonable basis upon which to estimate expected term due to the limited period of time its equity shares have been publicly traded; (ii) A company significantly changes the terms of its share option grants or the types of employees that receive share option grants such that its historical exercise data may no longer provide a reasonable basis upon which to estimate expected term; or (iii) A company has or expects to have significant structural changes in its business such that its historical exercise data may no longer provide a reasonable basis upon which to estimate expected term. The Company uses the simplified method to calculate expected term of share options and similar instruments as the company does not have sufficient historical exercise data to provide a reasonable basis upon which to estimate expected term. | |
| ● | Expected volatility of the entity’s shares and the method used to estimate it. Pursuant to ASC Paragraph 718-10-50-2(f)(2)(ii), a thinly-traded or nonpublic entity that uses the calculated value method shall disclose the reasons why it is not practicable for the Company to estimate the expected volatility of its share price, the appropriate industry sector index that it has selected, the reasons for selecting that particular index, and how it has calculated historical volatility using that index. The Company uses the average historical volatility of the comparable companies over the expected contractual life of the share options or similar instruments as its expected volatility. If shares of a company are thinly traded the use of weekly or monthly price observations would generally be more appropriate than the use of daily price observations as the volatility calculation using daily observations for such shares could be artificially inflated due to a larger spread between the bid and asked quotes and lack of consistent trading in the market. | |
| ● | Expected annual rate of quarterly dividends. An entity that uses a method that employs different dividend rates during the contractual term shall disclose the range of expected dividends used and the weighted-average expected dividends. The expected dividend yield is based on the Company’s current dividend yield as the best estimate of projected dividend yield for periods within the expected term of the share options and similar instruments. | |
| ● | Risk-free rate(s). An entity that uses a method that employs different risk-free rates shall disclose the range of risk-free rates used. The risk-free interest rate is based on the U.S. Treasury yield curve in effect at the time of grant for periods within the expected term of the share options and similar instruments. |
Pursuant to ASC paragraph 505-50-25-7, if fully vested, non-forfeitable equity instruments are issued at the date the grantor and grantee enter into an agreement for goods or services (no specific performance is required by the grantee to retain those equity instruments), then, because of the elimination of any obligation on the part of the counterparty to earn the equity instruments, a measurement date has been reached. A grantor shall recognize the equity instruments when they are issued (in most cases, when the agreement is entered into). Whether the corresponding cost is an immediate expense or a prepaid asset (or whether the debit should be characterized as contra-equity under the requirements of paragraph 505-50-45-1) depends on the specific facts and circumstances. Pursuant to ASC paragraph 505-50-45-1, a grantor may conclude that an asset (other than a note or a receivable) has been received in return for fully vested, non-forfeitable equity instruments that are issued at the date the grantor and grantee enter into an agreement for goods or services (and no specific performance is required by the grantee in order to retain those equity instruments). Such an asset shall not be displayed as contra-equity by the grantor of the equity instruments. The transferability (or lack thereof) of the equity instruments shall not affect the balance sheet display of the asset. This guidance is limited to transactions in which equity instruments are transferred to other than employees in exchange for goods or services. Section 505-50-30 provides guidance on the determination of the measurement date for transactions that are within the scope of this Subtopic.
| F-13 |
Pursuant to Paragraphs 505-50-25-8 and 505-50-25-9, an entity may grant fully vested, non-forfeitable equity instruments that are exercisable by the grantee only after a specified period of time if the terms of the agreement provide for earlier exercisability if the grantee achieves specified performance conditions. Any measured cost of the transaction shall be recognized in the same period(s) and in the same manner as if the entity had paid cash for the goods or services or used cash rebates as a sales discount instead of paying with, or using, the equity instruments. A recognized asset, expense, or sales discount shall not be reversed if a stock option that the counterparty has the right to exercise expires unexercised.
Pursuant to ASC paragraph 505-50-30-S99-1, if the Company receives a right to receive future services in exchange for unvested, forfeitable equity instruments, those equity instruments are treated as unissued for accounting purposes until the future services are received (that is, the instruments are not considered issued until they vest). Consequently, there would be no recognition at the measurement date and no entry should be recorded.
Advertising Costs
Advertising Costs are expensed as incurred. Advertising Expenses were $35,252 and $2,113 for the years ended December 31, 2014 and 2013, respectively.
Income Tax Provision
The Company accounts for income taxes under Section 740-10-30 of the FASB Accounting Standards Codification. Deferred income tax assets and liabilities are determined based upon differences between the financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that are in effect when the differences are expected to reverse. Deferred tax assets are reduced by a valuation allowance to the extent management concludes it is more likely than not that the assets will not be realized. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the statements of operations in the period that includes the enactment date.
The Company adopted section 740-10-25 of the FASB Accounting Standards Codification (“Section 740-10-25”). Section 740-10-25 addresses the determination of whether tax benefits claimed or expected to be claimed on a tax return should be recorded in the financial statements. Under Section 740-10-25, the Company may recognize the tax benefit from an uncertain tax position only if it is more likely than not that the tax position will be sustained on examination by the taxing authorities, based on the technical merits of the position. The tax benefits recognized in the financial statements from such a position should be measured based on the largest benefit that has a greater than fifty percent (50%) likelihood of being realized upon ultimate settlement. Section 740-10-25 also provides guidance on de-recognition, classification, interest and penalties on income taxes, accounting in interim periods and requires increased disclosures.
The estimated future tax effects of temporary differences between the tax basis of assets and liabilities are reported in the accompanying consolidated balance sheets, as well as tax credit carry-backs and carry-forwards. The Company periodically reviews the recoverability of deferred tax assets recorded on its consolidated balance sheets and provides valuation allowances as management deems necessary.
Management makes judgments as to the interpretation of the tax laws that might be challenged upon an audit and cause changes to previous estimates of tax liability. In addition, the Company operates within multiple taxing jurisdictions and is subject to audit in these jurisdictions. In management’s opinion, adequate provisions for income taxes have been made for all years. If actual taxable income by tax jurisdiction varies from estimates, additional allowances or reversals of reserves may be necessary.
The 2011 through 2013 tax years remain subject to examination by taxing authorities.
| F-14 |
Uncertain Tax Positions
The Company did not take any uncertain tax positions and had no adjustments to its income tax liabilities or benefits pursuant to the provisions of Section 740-10-25 for the reporting periods ended December 31, 2014 or 2013.
Net Income (Loss) per Common Share
Net income (loss) per common share is computed pursuant to Section 260-10-45 of the Codification. Basic net income (loss) per common share is computed by dividing net income (loss) by the weighted average number of shares of common stock outstanding during the period. Diluted net income (loss) per common share is computed by dividing net income (loss) by the weighted average number of shares of common stock and potentially outstanding shares of common stock during the period to reflect the potential dilution that could occur from common shares issuable through convertible debt, stock options or warrants.
The following table shows the outstanding dilutive Class A common shares excluded from the diluted net income (loss) per share calculation as they were anti-dilutive:
| Outstanding Dilutive Class A Common Shares | ||||||||
| For the Year Ended December 31, 2014 | For the Year Ended December 31, 2013 | |||||||
| Conversion features on convertible notes | 686,246 | 1,065,982 | ||||||
| Stock purchase warrants | 2,996,740 | 1,877,369 | ||||||
| Total outstanding dilutive common shares | 3,682,986 | 2,943,351 | ||||||
Subsequent Events
The Company follows the guidance in Section 855-10-50 of the FASB Accounting Standards Codification for the disclosure of subsequent events. The Company evaluated subsequent events through the date when the financial statements were issued.
Recently Issued Accounting Pronouncements
In May 2014, the Financial Accounting Standards Board issued Accounting Standards Update 2014-09, Revenue from Contracts with Customers. Amendments in this Update create Topic 606, Revenue from Contracts with Customers, and supersede the revenue recognition requirements in Topic 605, Revenue Recognition, including most industry-specific revenue recognition guidance throughout the Industry Topics of the Codification. In addition, the amendments supersede the cost guidance in Subtopic 605-35, Revenue Recognition—Construction-Type and Production-Type Contracts, and create new Subtopic 340-40, Other Assets and Deferred Costs—Contracts with Customers. In summary, the core principle of Topic 606 is that an entity recognizes revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. This Accounting Standards Update is the final version of Proposed Accounting Standards Update 2011-230—Revenue Recognition (Topic 605) and Proposed Accounting Standards Update 2011–250—Revenue Recognition (Topic 605): Codification Amendments, both of which have been deleted. The amendments in this Update are effectively for the Company for annual reporting periods beginning after December 15, 2016, including interim periods within that reporting period. The Company is currently evaluating the effects of ASU 2014-09 on the consolidated financial statements.
| F-15 |
In June 2014, the Financial Accounting Standards Board issued Accounting Standards Update 2014-12, Compensation- Stock Compensation. The amendments in this update apply to reporting entities that grant their employees share-based payments in which the terms of the award provide that a performance target can be achieved after the requisite service period. This Accounting Standards Update is the final version of Proposed Accounting Standards Update EITF–13D–Compensation–Stock Compensation (Topic 718): Accounting for Share-Based Payments When the Terms of an Award Provide That a Performance Target Could Be Achieved after the Requisite Service Period, which has been deleted. The amendments require that a performance target that affects vesting and that could be achieved after the requisite service period be treated as a performance condition. A reporting entity should apply existing guidance in Topic 718 as it relates to awards with performance conditions that affect vesting to account for such awards. As such, the performance target should not be reflected in estimating the grant-date fair value of the award. Compensation cost should be recognized in the period in which it becomes probable that the performance target will be achieved and should represent the compensation cost attributable to the period(s) for which the requisite service has already been rendered. If the performance target becomes probable of being achieved before the end of the requisite service period, the remaining unrecognized compensation cost should amount of compensation cost recognized during and after the requisite service period should reflect the number of awards that are expected to vest and should be adjusted to reflect those awards that ultimately vest. The requisite service period ends when the employee can cease rendering service and still be eligible to vest in the award if the performance target is achieved. As indicated in the definition of vest, the stated vesting period (which includes the period in which the performance target could be achieved) may differ from the requisite service period. The amendments in this Update are effective for annual periods and interim periods within those annual periods beginning after December 15, 2015, and early adoption is permitted. The Company is currently evaluating the effects of ASU 2014-12 on the consolidated financial statements.
In April 2015, the Financial Accounting Standards Board issued Accounting Standards Update 2015-03, Interest—Imputation of Interest. To simplify presentation of debt issuance costs, the amendments in this Update would require that debt issuance costs be presented in the balance sheet as a direct deduction from the carrying amount of debt liability, consistent with debt discounts or premiums. The recognition and measurement guidance for debt issuance costs would not be affected by the amendments in this Update. This Accounting Standards Update is the final version of Proposed Accounting Standards Update 2014-250—Interest—Imputation of Interest (Subtopic 835-30), which has been deleted. The amendments in this Update are effective for financial statements issued for fiscal years beginning after December 15, 2015, and interim periods within those fiscal years. The Company is currently evaluating the effects of ASU 2015-03 on the consolidated financial statements.
Note 3- Going Concern
As reflected in the accompanying financial statements, the Company has a net loss and net cash used in operations of $40,312,486 and $4,566,234, respectively, for the year ended December 31, 2014 and an accumulated deficit totaling $115,340,329. The Company does not generate significant revenue. These factors raise substantial doubt about the Company’s ability to continue as a going concern.
The ability of the Company to continue its operations as a going concern is dependent on Management's plans, which include the raising of capital through debt and/or equity markets with some additional funding from other traditional financing sources, including term notes, until such time that funds provided by operations are sufficient to fund working capital requirements. The Company may need to incur additional liabilities with certain related parties to sustain the Company’s existence.
The Company will require additional funding to finance the growth of its current and expected future operations as well as to achieve its strategic objectives. The Company believes its current available cash along with anticipated revenues may be insufficient to meet its cash needs for the near future. There can be no assurance that financing will be available in amounts or terms acceptable to the Company, if at all. The accompanying financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. These financial statements do not include any adjustments relating to the recovery of the recorded assets or the classification of the liabilities that might be necessary should the Company be unable to continue as a going concern.
| F-16 |
Note 4 – Property & Equipment
Property and equipment consists of the following:
| December 31, | December 31, | |||||||
| 2014 | 2013 | |||||||
| Computer Equipment | $ | 46,938 | $ | - | ||||
| Leasehold Improvements | 9,450 | - | ||||||
| 56,388 | - | |||||||
| Accumulated Depreciation | (7,839 | ) | - | |||||
| Net Property and Equipment | $ | 48,549 | $ | - | ||||
Depreciation expense for the years ended December 31, 2014 and 2013 was $8,680 and $0, respectively.
Note 5 – Convertible Notes Payable
Convertible notes payable consist of the following:
| December 31, | December 31, | |||||||
| 2014 | 2013 | |||||||
| Ayer Notes (I and II) | $ | - | $ | 750,000 | ||||
| Chertoff Note | 100,000 | 100,000 | ||||||
| Van Damm Note | 100,000 | 100,000 | ||||||
| Total Convertible Notes | 200,000 | 950,000 | ||||||
| Unamortized Debt Discount | (59,177 | ) | (692,511 | ) | ||||
| Total Convertible Notes, Net of Debt Discount | 140,823 | 257,489 | ||||||
| Current Portion of Convertible Notes | 140,823 | 183,333 | ||||||
| Long-Term Convertible Notes less Current Portion | $ | - | $ | 74,156 | ||||
Chertoff and Van Damm Notes
On November 20, 2012, we entered into two Note Purchase Agreements whereby the investors acquired $200,000 of our convertible promissory notes for an aggregate purchase price of $200,000 in a private placement. The Notes are secured by all of the assets of the Company. The Notes bear interest at 9.5% per annum and mature three years from the date of issuance. Accrued interest on the Notes as of December 31, 2014 and 2013 was $40,186 and $21,186, respectively.
| F-17 |
The Notes are convertible at any time by the investors into Class A common shares equal to the outstanding principal and interest to be converted, at a 30% discount to the last equity round. The notes had an initial conversion price of $1.40 subject to adjustments. If at any time following the issuance date, the company completes an equity or debt offering at a value below the conversion price, then the conversion price adjusts with a 30% discount of the purchase price per share of common stock payable by the investors in such subsequent equity financing. The note adjustment provisions, referred to as a full ratchet reset dilutive feature, require treatment as a derivative liability. The fair market value of the derivative at the commitment date was $242,630. The Company utilized a Monte Carlo model to value the anti-dilutive conversion feature of the Notes. The probability and timing of potential future financing and fundamental transactions as applicable were estimated. The following assumptions were used to value the note conversion feature at December 31, 2013: closing stock price: $1.00; exercise price $0.35; expected volatility 100%; remaining term 1.88 years; risk-free rate 1.75%, expected dividend yield 0%. The following assumptions were used to value the note conversion feature at December 31, 2014: closing stock price: $1.00; exercise price $0.35; expected volatility 89%; remaining term 0.89 years; risk-free rate 0.25%, expected dividend yield 0%.
On the balance sheet, the Notes were originally recorded at $0 ($200,000 net of debt discount of $200,000). The debt discount is amortized over the life of the Notes using the straight-line method as it approximates the effective interest method. As of December 31, 2014 and 2013, the unamortized debt discount on the Note was $59,177 and $125,844, respectively.
Ayer Note I
On December 9, 2013, we entered into a Note Purchase Agreement (the Note) with the Ayer Capital Partners Master Fund, LP whereby the investor acquired a $750,000 convertible promissory note for a purchase price of $750,000 in a private placement. The Note was secured by all of the assets of the Company. The Note bore interest at 1% per annum and matures on March 9, 2014. The maturity date on the Note was extended to June 7, 2014. On June 13, 2014, the noteholder signed a default and covenant waiver. The Note was repaid on July 15, 2014. The investor had the option to convert all or a portion of the Note into equity prior to the maturity date contingent upon an equity financing by the Company at a conversion price equal to the price per share of financing securities paid by the other investors in the financing.
In connection with the Note, the investor received a warrant to purchase shares of Class A common stock at a price equal to 35% of the principal amount of the Note. This warrant was issued to the investor at an exercise price of $0.26878. The warrant expires on December 9, 2018. If the maturity date of the Note is extended, the warrant increases to an amount to purchase shares of Class A common stock at a price equal to 70% of the principal amount of the Note. If at any time prior to the two year anniversary of the issuance date of the Note, the Company completes an equity or debt offering at a value below the exercise price, then the exercise price adjusts to the purchase price per share of common stock payable by the investors in such subsequent equity financing. The Company analyzed the exercise feature for derivative consideration under ASC 815 and determined that the exercise feature qualified for accounting treatment as a derivative liability. The fair market value of the derivative at the commitment date was $883,087. The Company utilized a Monte Carlo model to value the anti-dilutive feature of the warrants. The probability and timing of potential future financing and fundamental transactions as applicable were estimated. The following assumptions were utilized to value the derivative liability at December 9, 2013: closing stock price: $1.00; exercise price $0.26878; expected volatility 101%; remaining term five years; risk-free rate 1.50%, expected dividend yield 0%. The following assumptions were used to value the warrant liability at December 31, 2013: closing stock price: $1,000; exercise price $268.78; expected volatility 100%; remaining term 4.94 years; risk free rate 1.75%, expected dividend yield 0%.The following assumptions were used to value the warrant liability at December 31, 2014: closing stock price: $1.00; exercise price $0.50; expected volatility 94%; remaining term 3.94 years; risk-free rate 1.38%, expected dividend yield 0%.
On March 9, 2014, due to the maturity extension of the Note, the warrant increased to an amount to purchase shares of Class A common stock at a price equal to 70% of the principal amount of the Note. As a result, an additional derivative liability was recorded at a fair market value of $881,433. The following assumptions were used to value the warrant liability at March 9, 2014: closing stock price: $1,000; exercise price $268.78; expected volatility 100%; remaining term 4.75 years; risk-free rate 1.65%, expected dividend yield 0%. The following assumptions were used to value the warrant liability at December 31, 2014: closing stock price: $1.00; exercise price $0.50; expected volatility 94%; remaining term 3.94 years; risk-free rate 1.38%, expected dividend yield 0%.
| F-18 |
Debt issuance costs of $117,406 pertaining to placement agent fees and commissions on the Note were recorded. The debt issuance costs are amortized over the life of the Note. Debt issuance costs were paid in the form of $60,000 in cash and stock warrants to purchase 30,000 Class A common shares. The warrants issued to the placement agent were of the same form as the warrants attached to the Note, and the market value of the derivative liability at the commitment date was $27,106.
On the balance sheet, the Note was originally recorded at $0 ($750,000 net of debt discount of $750,000). The debt discount was amortized over the life of the Note. As of December 31, 2014 the debt discount was fully amortized and at December 31, 2013, the unamortized debt discount on the Note was $566,667.
Ayer Note II
On February 24, 2014, we entered into a Note Purchase Agreement (the Note) with the Ayer Capital Special Situations Fund, LP whereby the investor acquired a $400,000 convertible promissory note for a purchase price of $400,000 in a private placement. The Note was secured by all of the assets of the Company. The Note bore interest at 1% per annum and the original maturity date was May 24, 2014. The maturity was subsequently extended to August 22, 2014. On June 13, 2014, the noteholder signed a default and covenant waiver. The Note was repaid on August 26, 2014. The investor had the option to convert all or a portion of the Note into equity prior to the maturity date contingent upon an equity financing by the Company at a conversion price equal to the price per share of financing securities paid by the other investors in the financing.
In connection with the Note, the investor received a warrant to purchase shares of Class A common stock at a price equal to 35% of the principal amount of the Note. This warrant was issued to the investor at an exercise price of $268.78. The warrant expires on February 24, 2019. If the maturity date of the Note is extended, the warrant increases to an amount to purchase shares of Class A common stock at a price equal to 70% of the principal amount of the Note. If at any time prior the two year anniversary of the issuance date of the Note, the Company completes an equity or debt offering at a value below the exercise price, then the exercise price adjusts to the purchase price per share of common stock payable by the investors in such subsequent equity financing. The Company analyzed the exercise feature for derivative consideration under ASC 815 and determined that the exercise feature qualified for accounting treatment as a derivative liability. The fair market value of the derivative at the commitment date was $455,430. The Company utilized a Monte Carlo model to value the anti-dilutive feature of the warrants. The probability and timing of potential future financing and fundamental transactions as applicable were estimated. The following assumptions were used to value the warrant liability at February 24, 2014: closing stock price: $1,000; exercise price $268.78; expected volatility 100%; remaining term five years; risk-free rate 1.57%, expected dividend yield 0%. The following assumptions were used to value the warrant liability at December, 2014: closing stock price: $1,000; exercise price $0.50; expected volatility 99%; remaining term 4.15 years; risk-free rate 1.38%, expected dividend yield 0%.
On May 24, 2014, due to the maturity extension of the Note, the warrant increased to an amount to purchase shares of Class A common stock at a price equal to 70% of the principal amount of the Note. As a result, an additional derivative liability was recorded at a fair market value of $204,544. The following assumptions were used to value the warrant liability on May 24, 2014: closing stock price: $1.00; exercise price $1.00; expected volatility 96%; remaining term 4.75 years; risk-free rate 1.55%, expected dividend yield 0%. The following assumptions were used to value the warrant liability at December, 2014: closing stock price: $1,000; exercise price $0.50; expected volatility 99%; remaining term 4.15 years; risk-free rate 1.38%, expected dividend yield 0%.
Debt issuance costs of $40,979 pertaining to placement agent fees and commissions on the Note were recorded. The debt issuance costs are amortized over the life of the Note using the straight-line method as it approximates the effective interest method. Debt issuance costs were paid in the form of $27,000 in cash and stock warrants to purchase 16,000 Class A common shares. The warrants issued to the placement agent were of the same form as the warrants attached to the Note, and the market value of the derivative liability at the commitment date was $13,979. As of September 30, 2014, the debt issuance costs were fully amortized.
On the balance sheet, the Note was originally recorded at $0 ($400,000 net of debt discount of $400,000). The debt discount was amortized over the life of the Note and as of December 31, 2014 the debt discount was fully amortized.
| F-19 |
Manchanda Note
On June 20, 2014, we entered into a Note Purchase Agreement with Kabir Manchanda whereby the investor acquired a $200,000 convertible promissory note for a purchase price of $200,000 in a private placement. The Note bore interest at 12% per annum and matures on March 20, 2015. The Note and accrued interest can convert into Class A common stock at $0.50 per share. The Note was repaid on October 27, 2014. In addition, the noteholder received 100,000 shares of Class A common stock. The beneficial conversion feature and the common stock was recorded as a debt discount of $200,000.
On the balance sheet, the Note was originally recorded at $0 ($200,000 net of debt discount of $200,000). The debt discount was amortized over the life of the Note, and as of December 31, 2014 the debt discount on the Note was fully amortized.
Note 6 – Note Payable
On November 15, 2011, we entered into a Note Purchase Agreement with Richard Pinto whereby the investor acquired a $250,000 promissory note for a purchase price of $250,000 in a private placement. The Note bore interest at 21% per annum and matured on February 15, 2012. The maturity date on the Note was extended to August 1, 2014. On October 27, 2014, $150,000 of the note principal was repaid less $2,987 in previously overpaid interest. Furthermore, on November 24, 2014, the Company induced the investor to convert the remaining $100,000 of note principal by offering a conversion price of $0.50, a $0.50 discount from the market price of $1.00. As a result, the remaining $100,000 of note principal converted into 200,000 shares of Class A common stock, and the investor agreed to cancel outstanding warrants issued in connection with the note. The Company recorded a debt conversion expense of $100,000 in the consolidated Statement of Operations. Since the Company had previously overpaid on the investor’s interest payments, the Company had recorded prepaid interest of $10,473 as of December 31, 2013. This note was personally guaranteed by Govindan Gopinathan, the Executive Board Chairman and largest shareholder of the Company at the time the note was issued, and Fernando Green, the Senior Vice President of the Company at the time the note was issued.
In connection with the Note, warrants to purchase 25 Class A common shares were issued to the investor. The warrants have an exercise price of $2,000 per share and expire on November 1, 2016. The Company valued the warrants at $11,854 on the commitment date using a Black-Scholes-Merton option pricing model utilizing the following assumptions: closing stock price: $1,000; exercise price $2,000; expected volatility 113%; expected term five years; risk-free rate 0.90%, expected dividend yield 0%. The warrants were canceled on November 24, 2014.
On the balance sheet, the Note was originally recorded at $238,146 ($250,000 net of debt discount of $11,854). The debt discount was amortized over the life of the Note and is fully amortized.
Note 7 – Derivative Liabilities
The company identified derivative liabilities associated with the convertible debt and warrants issued from 2012 to 2014.
As a result of the application of ASC No. 815, the fair values of the Company’s derivative liabilities are summarized as follows:
| Note Conversion Features | Warrants | Total | ||||||||||
| Balance December 31, 2012 | 238,771 | - | 238,771 | |||||||||
| Fair value at the commitment date | - | 910,193 | 910,193 | |||||||||
| Change in fair value | 151,161 | (11,881 | ) | 139,280 | ||||||||
| Balance, December 31, 2013 | 389,932 | 898,312 | 1,288,244 | |||||||||
| Fair value at the commitment date | - | 1,555,686 | 1,555,686 | |||||||||
| Change in fair value | 47,453 | (992,447 | ) | (944,994 | ) | |||||||
| Balance, December 31, 2014 | $ | 437,385 | $ | 1,461,251 | $ | 1,898,636 | ||||||
| F-20 |
The Company recorded debt discount to the extent of the gross proceeds of each note, and immediately expensed the remaining derivative value if it exceeded the gross proceeds. The Company recorded a derivative expense of $1,141,407 and $133,087 for the years ended December 31, 2014 and 2013, respectively.
Note 8 – Related Party Transactions
Transactions involving related parties cannot be presumed to be carried out on an arm's-length basis, as the requisite conditions of competitive, free-market dealings may not exist. Representations about transactions with related parties, if made, shall not imply that the related party transactions were consummated on terms equivalent to those that prevail in arm's-length transactions unless such representations can be substantiated.
Periodically, Govindan Gopinathan, the Executive Board Chairman and largest shareholder of the Company, advanced the Company monies and periodically, the Company repaid portions of the advances. The advances are non-interest bearing and have no specified maturity date. On December 31, 2013, the Company settled the remaining $17,600 owed to him in Class A common shares. As of December 31, 2014 and December 31, 2013, the Company owed no monies to him. In addition, Govindan Gopinathan provided consulting services for the company. Consulting expenses pertaining to his services were $148,000 and $94,600 for the years ended December 31, 2014 and 2013, respectively, and are a component of Consulting fees - related party in the Statement of Operations.
Arthur Tilford, a former Board member, provided consulting services for the company. Consulting expenses pertaining to his services were $20,000 and $13,000 for the years ended December 31, 2014 and 2013, respectively, and are a component of Consulting fees - related party in the Statement of Operations.
Andrew Mininger, a former Board member, provided consulting services for the company. Consulting expenses pertaining to his services were $20,000 and $0 for the years ended December 31, 2014 and 2013, respectively, and are a component of Consulting fees - related party in the Statement of Operations.
Dalen Harrison, a former Board member, provided sales and marketing services for the company. Sales and marketing expenses pertaining to his services were $20,000 and $18,500 for the years ended December 31, 2014 and 2013, respectively, and are a component of Sales and marketing- related party in the Statement of Operations.
Thomas Nicolette, the Chief Executive Officer, provided consulting services for the Company. Consulting expenses pertaining to his services were $90,000 and $0 for the years ended December 31, 2014 and 2013, respectively, and are a component of Consulting fees - related party in the Consolidated Statement of Operations.
Note 9 – Proceeds from Insurance Settlement
During the year ended December 31, 2014, the Company experienced two thefts at its Hicksville, New York office. The Company received an insurance settlement for $36,365, which was recorded as Proceeds from insurance settlement in the Consolidated Statement of Operations.
Note 10 – Stockholders’ Equity (Deficit)
Class A Common Stock
In 2013, the Company issued 1,660,000 Class A common shares to investors in private placements at prices ranging from $.50 per share to $1.00 per share for total proceeds of $1,135,000.
| F-21 |
At December 31, 2013, the Board of Directors issued 500,000 Class A common shares to the Chief Executive Officer for services provided to the Company and to settle a $17,600 related party payable. The shares were valued at $1.00 per share and $482,400 was recorded as Compensation Expense in the Statement of Operations.
At December 31, 2013, the Board of Directors issued 250,000 Class A common shares to Blue Ocean Advisors, Inc. for services provided to the Company. The shares were valued at $1.00 per share and $250,000 was recorded as Compensation Expense in the Statement of Operations.
On January 27, 2014, the Company issued 50 Class A common shares to an investor in a private placements at $1,000 per share for total proceeds of $50,000.
On March 6, 2014, the Company issued 400 Class A common shares to an investor in a private placements at $1,000 per share for total proceeds of $400,000.
On April 20, 2014, the Company, with the ratification of the Board of Directors, amended the Company’s Certificate of Incorporation to effect a 1 for 1,000 reverse split of the Company's issued and outstanding shares of common stock. All references to numbers or values of the Company's common shares have been adjusted to reflect this 1 for 1,000 reverse split. All warrant amounts and exercise prices have been adjusted to reflect this 1 for 1,000 reverse split.
On April 20, 2014, the Company, with the ratification of the Board of Directors, issued 33,891,703 shares of Class A common stock to investors and consultants. The shares were valued at $1.00 per share and $33,891,703 was recorded as Compensation Expense in the Statement of Operations.
As discussed in Note 5, on June 20, 2014, a noteholder received 100,000 shares of Class A common stock as part of the consideration in his Note Purchase Agreement.
Between June and August 2014, the Company sold 8,400,000 shares of common stock to investors in exchange for $8,400,000 in gross proceeds in connection with the private placement of the Company’s stock.
In connection with the private placement, the Company incurred fees of $1,336,035. In addition, 840,000 five year warrants with an exercise price of $1.00 were issued to the placement agent. The Company valued the warrants at $664,869 on the commitment date using a Black-Scholes-Merton option pricing model. The value of the warrants was a direct cost of the private placement and has been recorded as a reduction in additional paid in capital.
In valuing the warrants issued in conjunction with the above private placements, the Company used the following assumptions:
| ● | The stock price was based upon the issuance price the in private placement, or $1.00 per share. |
| ● | The risk-free interest rate assumption is based on the U.S. Treasury yield for a period consistent with the expected term of the option in effect at the time of the grant. The risk free rate had a range of 1.55% – 1.74%. |
| ● | The Company has not paid any dividends on common stock since its inception and does not anticipate paying dividends on its common stock in the foreseeable future. Therefore, the expected dividend rate was $0. |
| ● | The warrant term is the life of the warrant, which was five years. |
| ● | The expected volatility was benchmarked against similar companies in a similar industry. The expected volatility used had a range of 101% – 117%. |
| ● | The forfeiture rate is based on the historical forfeiture rate for the Company’s unvested warrants, which was 0%. |
| F-22 |
As discussed in Note 13, on October 10, 2014, the Company reached a settlement with a former sales consultant for and issued 20,000 shares of Class A common stock valued at $1.00 per share.
As discussed in Note 6, on November 24, 2014, a noteholder received 200,000 shares of Class A common stock valued at $1.00 as consideration for converting $100,000 of note principal.
On December 24, 2014, as a result of the reverse merger with Clutterbug, 3,800,000 of Clutterbug’s shares were cancelled.
Class B Common Stock
On January 2, 2013, the Compensation Committee, with the ratification of the Board of Directors, awarded 32,014,686 Class B common shares to reward certain individuals of the Company for past and current performance that they have contributed to the success of the Company. The shares were valued at $2.00 per share and $64,029,372 was recorded as Compensation Expense in the Statement of Operations.
Class B common shares carry the same voting rights as Class A common shares, but there are a few key differences:
| 1. | Class B common shares can only be issued by the Company, without cost, to senior management for their contributions to the Company. |
| 2. | A Class B common shareholder forfeits his Class B common shares upon termination of employment for any reason other than the shareholder’s death or disability. |
| 3. | Class B common shareholders have no dividend rights. |
| 4. | Transferability of Class B common shares carries significant restrictions. |
| 5. | Upon a liquidity event, each Class B common share automatically converts in to one Class A common share, but the Class A common shares get liquidation preference. |
On April 20, 2014, the Company, with the ratification of the Board of Directors, amended the Company’s Certificate of Incorporation to eliminate the common B class of stock. All of the existing Class B common shares as of April 20, 2014 were converted into the same number of Class A common shares. All references to numbers or values of the Company's common shares have been adjusted to reflect this share conversion.
Warrants
The following is a summary of the Company’s warrant activity during the years ended December 31, 2013 and 2014:
| Warrants | Weighted Average Exercise Price |
Weighted Average Remaining Contractual Life |
||||||||||
| Outstanding – December 31, 2012 | 1,040 | $ | 1,050.00 | 2.57 | ||||||||
| Granted | 1,008 | 268.78 |
4.94 | |||||||||
| Exercised | - | - | ||||||||||
| Forfeited/Cancelled | (170 | ) | 1,010.00 | |||||||||
| Outstanding – December 31, 2013 | 1,878 | $ | 647.55 | 3.52 | ||||||||
| Granted | 3,494,992 | 0.62 | 4.30 | |||||||||
| Exercised | - | - | ||||||||||
| Forfeited/Cancelled | (130 | ) | 1,384.62 | |||||||||
| Outstanding – December 31, 2014 | 3,496,740 | $ | 0.83 | 4.30 | ||||||||
| Exercisable – December 31, 2014 | 2,996,740 | $ | 0.89 | 4.26 | ||||||||
| F-23 |
At December 31, 2014 and 2013, the total intrinsic value of warrants outstanding and exercisable was $1,328,000 and $714,672, respectively.
As of December 31, 2014 and 2013, unrecognized compensation costs related to non-vested warrants was $172,114 and $-, respectively. The cost is expected to be amortized over a weighted average period of 0.50 years.
Note 11 – Income Taxes
Deferred Tax Assets
At December 31, 2014, the Company has available for federal income tax purposes a net operating loss (“NOL”) carry-forward of approximately $25,554,000 net of (i) $100,801,000 in accumulated share-based compensation (ii) $2,681,000 of accumulated interest and derivative expense associated with the derivative liabilities (iii) $100,000 in debt conversion expense and (iv) $(860,000) of accumulated change in the fair value of derivative liabilities included in the Statements of Operations, that may be used to offset future taxable income through the fiscal year ending December 31, 2034. No tax benefit has been reported with respect to these net operating loss carry-forwards in the accompanying financial statements since the Company believes that the realization of its net deferred tax asset of approximately $5,112,000 was not considered more likely than not based upon the Company’s losses since inception. Accordingly, the potential tax benefits of the net loss carry-forwards are fully offset by a valuation allowance of $5,112,000. The valuation allowance increased approximately $1,805,000 and $408,000 for the years ended December 31, 2014 and 2013, respectively.
Components of deferred tax assets are as follows:
| December 31, | ||||||||
| 2014 | 2013 | |||||||
| Net deferred tax assets – Non-current: | ||||||||
| Expected income tax benefit from NOL carry-forwards | $ | 5,112,000 | $ | 3,307,000 | ||||
| Less valuation allowance | (5,112,000 | ) | (3,307,000 | ) | ||||
| Deferred tax assets, net of valuation allowance | $ | - | $ | - | ||||
Income Tax Provision in the Consolidated Statements of Operations
A reconciliation of the federal statutory income tax rate and the effective income tax rate as a percentage of income before income taxes is as follows:
| For the Year Ended | ||||||||
| December 31, | ||||||||
| 2014 | 2013 | |||||||
| U.S. statutory federal tax rate | (34.0 | )% | (34.0 | )% | ||||
| State income taxes, net of federal tax benefit | (0.5 | )% | (0.1 | )% | ||||
| Share based compensation | 29.2 | % | 33.3 | % | ||||
| Other permanent differences | 0.8 | % | 0.2 | % | ||||
| Change in valuation allowance | 4.5 | % | 0.6 | % | ||||
| Effective income tax rate | 0.0 | % | 0.0 | % | ||||
| F-24 |
Note 12 – Significant Risks and Uncertainties
Accounts Payable Concentrations
Accounts payable from a single vendor in any one year can exceed 10.0% of our total purchases. As of December 31, 2014, three vendors represented 66% of our accounts payable. As of December 31, 2013, one vendor represented 73% of our accounts payable. The loss of any key vendor would have to be replaced by others or our inability to do so may have a material adverse effect on our business and financial condition.
Note 13 – Commitments and Contingencies
Operating Leases
Prior to December 1, 2013, the Company had a month-to-month commercial lease with ProSuites, LLC, at Glen Cove, New York for office space. The Company paid $926 a month in rent. The Company canceled the lease effective November 30, 2014.
Effective December 1, 2013, the Company entered into a commercial real estate lease with ProSuites, LLC, at 480 Forest Ave. for new office space located in Locust Valley. The initial term of the Lease is for one year ending on November 30, 2014. Pursuant to the Lease, the Company paid base rent of $1,750 per month. The lease expired on November 30, 2014 and was not renewed.
On April 25, 2014, the Company signed a 36 month lease agreement for its office facilities in Hicksville, New York commencing on June 1, 2014 and expiring on June 1, 2017. The lease requires base annual rent of approximately $51,000 for the first year, with 4% increments each year thereafter. Rent expense is recognized on a straight line basis over the term of the lease. Effective September 1, 2014, the lessor released the Company from its remaining obligations under the lease in exchange for the lessor retaining the Company’s three month security deposit totaling $12,750. The release of the security deposit to the lessor resulted in $12,750 of additional rent expense during the year ended December 31, 2014.
On June 18, 2014, the Company signed a 60 month lease agreement for its office facilities in New York, New York commencing on July 1, 2014 and expiring on June 30, 2019. The lease requires base annual rent of approximately $46,260 for the first year, with 3% increments each year thereafter. Rent expense is recognized on a straight line basis over the term of the lease. As of December 31, 2014, the Company has a deferred rent balance of $1,442, which is a component of Accounts Payable and Accrued Liabilities in the Consolidated Balance Sheet.
Rent expense was $71,478 and $11,114 for the years ended December 31, 2014 and 2013, respectively.
Future minimum lease payments under these non-cancelable operating leases are approximately as follows:
| Year Ending December 31 | ||||
| 2015 | $ | 47,070 | ||
| 2016 | 48,363 | |||
| 2017 | 49,813 | |||
| 2018 | 51,308 | |||
| 2019 | 26,033 | |||
| Total | $ | 222,587 | ||
| F-25 |
Financial Advisory and Investment Banking Agreement
The Company entered into an exclusive Financial Advisory and Investment Banking Agreement with Spartan Capital Securities, LLC (“Spartan”) effective January 17, 2014 and amended on June 9, 2014 (the “Spartan Advisory Agreement”). Pursuant to the Spartan Advisory Agreement, Spartan is acting as the Company’s exclusive financial advisor and placement agent to assist the Company in connection with a best efforts private placement (the “Financing”) of up to $7.4 million of the Company’s equity securities (the “Securities”) and a reverse merger. The Company upon closing of the Financing shall pay consideration to Spartan, in cash, a fee in an amount equal to 10% of the aggregate gross proceeds raised in the Financing. The Company shall grant and deliver to Spartan at the closing of the Financing, for nominal consideration, five year warrants (the “Warrants”) to purchase a number of shares of the Company’s Common Stock equal to 10% of the number of shares of Common Stock (and/or shares of Common Stock issuable upon exercise of securities or upon conversion or exchange of convertible or exchangeable securities) sold at such closing. The Warrants shall be exercisable at any time during the five year period commencing on the closing to which they relate at an exercise price equal to the purchase price per share of Common Stock paid by investors in the Financing or, in the case of exercisable, convertible, or exchangeable securities, the exercise, conversion or exchange price thereof. If the Financing is consummated by means of more than one closing, Spartan shall be entitled to the fees provided herein with respect to each such closing.
Along with the above fees, the Company shall pay (i) a $10,000 engagement fee upon execution of the agreement, (ii) up to $60,000 for expenses incurred by Spartan in connection with this Financing, together with cost of background checks on the officers and directors of the Company and (iii) a monthly fee of $10,000 for 36 months contingent upon Spartan successfully completing the $6 million Financing.
Consulting Agreement
On July 1, 2014, Thomas Nicolette entered into a two year consulting agreement to serve as the Company’s Chief Operating Officer (“COO”) conditioned upon and effective upon the Company receiving investment funding of at least $5,000,000. On July 18, 2014, the Company had received the minimum investment funding and the agreement became effective. The COO received monthly fees of $15,000. In addition, the COO received warrants to purchase 1,000,000 shares of the Class A common stock, with 500,000 warrants vesting on July 1, 2014 and 500,000 warrants vesting on June 30, 2015. The warrants are exercisable for five years at an exercise price of $0.50. During the year ended December 31, 2014, the Company recorded $516,340 in compensation expense pertaining to these warrants.
In valuing the warrants issued to Mr. Nicolette, the Company used the following assumptions:
| ● | The stock price was based upon the issuance price in the private placement, or $1.00 per share. |
| ● | The risk-free interest rate assumption is based on the U.S. Treasury yield for a period consistent with the expected term of the option in effect at the time of the grant. The risk free rate was 1.66%. |
| ● | The Company has not paid any dividends on common stock since its inception and does not anticipate paying dividends on its common stock in the foreseeable future. Therefore, the expected dividend rate was $0. |
| ● | The warrant term is the expected life of the warrant, which was 2.5 years. |
| ● | The expected volatility was benchmarked against similar companies in a similar industry. The expected volatility used was 90%. |
| ● | The forfeiture rate is based on the historical forfeiture rate for the Company’s unvested warrants, which was 0%. |
On November 12, 2014, Thomas Nicolette resigned from his position as Company’s Chief Operating Officer and was promoted to Chief Executive Officer. He also was added as a member of the Board of Directors. Mr. Nicolette’s consulting agreement remains in effect until amended to reflect his new expanded responsibilities.
Employment Agreements
On May 5, 2014, Robert Riola entered into a three year employment agreement to serve as the Company’s Chief Technology Officer (“CTO”) conditioned upon and effective upon the Company receiving investment funding of at least $5,000,000. On July 18, 2014, the Company had received the minimum investment funding and the agreement became effective. The CTO shall receive a base salary of $185,000. Until the Company reaches a quarterly adjusted EBITDA as defined in the agreement, 50% of the CTO’s salary is deferred. The CTO shall be entitled to consideration for a discretionary annual bonus payable in cash and/or share-based compensation.
| F-26 |
On November 12, 2014, Robert Riola resigned from his position as the Company’s Chief Technology Officer. He remained an employee of the Company. His deferred salary was forfeited as the Company did not reach the quarterly adjusted EBITDA target.
On May 27, 2014, Govindan Gopinathan entered into a three year employment agreement to serve as the Company’s Chief Executive Officer (“CEO”) conditioned upon and effective upon the Company receiving investment funding of at least $5,000,000. On July 18, 2014, the Company had received the minimum investment funding and the agreement became effective. The CEO shall receive a base salary of $380,000. Until the Company reaches a quarterly adjusted EBITDA as defined in the agreement, 50% of the CEO’s salary is deferred. The CEO shall be entitled to consideration for a discretionary annual bonus payable in cash and/or share-based compensation.
On November 12, 2014, Govindan Gopinathan resigned from his position as Company’s Chief Executive Officer. His deferred salary was forfeited as the Company did not reach the quarterly adjusted EBITDA target. Effective December 24, 2014, Govindan Gopinathan was appointed as the Executive Chairman of the Board of Directors.
Management Transition Agreement
On September 12, 2014, the Company signed a management transition agreement with a former management consultant of the Company. The management consultant assisted with the orderly transition of management and the transfer of information, documentation and knowledge that the consultant may possess about the business and affairs of the Company. In exchange, the Company paid the management consultant a nonrefundable fee of $125,000 upfront, and commencing on October 15, 2014, the Company paid the management consultant $12,500 per month for the next twelve months contingent upon the management consultant’s continued cooperation. For the year ended December 31, 2014, the Company paid the management consultant $162,500 in connection with the management transition agreement, which is recorded as a component of Consulting expense in the Consolidated Statement of Operations.
Litigations, Claims and Assessments
In 2013, a former sales consultant who was terminated by the Company commenced an action against the Company. The former sales consultant claimed that he had a signed employment agreement with the Company and that the Company breached the terms of his employment agreement by improperly terminating him. He sought unpaid salary for the duration of the term of the employment agreement, together with stock warrants he claimed was allegedly due him. INeedMD claimed that the alleged employment agreement that the former consultant presented in court is a fraudulent document, the document was not executed by Dr. Gopinathan, the Company’s CEO, and such employment and the effective date of the employment agreement was specifically conditioned upon the Company first being properly funded. On October 10, 2014, the matter was settled for $35,000 in cash and 20,000 shares of Class A common stock valued at $1.00 per share.
Note 14 – Subsequent Events
On January 1, 2015, the Company issued warrants to purchase 500,000 shares of the Company’s common stock to Patrice McMorrow, Executive Vice President of Business Development. The warrants are exercisable for five years at an exercise price of $0.50 per share. 250,000 of the warrants vest on January 1, 2015 and the other 250,000 warrants vest on September 30, 2015.
F-27
