Attached files
| file | filename |
|---|---|
| EX-31.1 - EX-31.1 - Avinger Inc | a15-7478_1ex31d1.htm |
| EX-32.1 - EX-32.1 - Avinger Inc | a15-7478_1ex32d1.htm |
| EX-31.2 - EX-31.2 - Avinger Inc | a15-7478_1ex31d2.htm |
| EX-23.1 - EX-23.1 - Avinger Inc | a15-7478_1ex23d1.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
(Mark One)
x ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the Fiscal Year Ended December 31, 2014
or
o TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
Commission File Number: 001-36817
AVINGER, INC.
(Exact name of registrant as specified in its charter)
|
Delaware |
|
20-8873453 |
|
(State or other jurisdiction of |
|
(I.R.S. Employer |
|
incorporation or organization) |
|
Identification Number) |
400 Chesapeake Drive
Redwood City, California 94063
(Address of principal executive offices and zip code)
(650) 241-7900
(Telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
|
Title of Each Class: |
|
Name of Each Exchange on which Registered |
|
Common Stock, par value $0.001 per share |
|
The NASDAQ Global Market |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes o No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definitions of “large accelerated filer,” “accelerated filer,” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer o |
|
Accelerated filer o |
|
Non-accelerated filer o |
|
Smaller reporting company x |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes o No x
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the registrant, based on the closing price of a share of the registrant’s common stock on January 30, 2015 as reported by the NASDAQ Global Market on such date was approximately $13.50. The registrant has elected to use January 30, 2015, which was the initial trading date on the NASDAQ Global Market, as the calculation date because on June 30, 2014 (the last business day of the registrant’s mostly recently completed second fiscal quarter), the registrant was a privately-held company. Shares of the registrant’s common stock held by each executive officer, director and holder of 5% or more of the outstanding common stock have been excluded in that such persons may be deemed to be affiliates. This calculation does not reflect a determination that certain persons are affiliates of the registrant for any other purpose.
As of March 26, 2015, the number of outstanding shares of the registrant’s common stock, par value $0.001 per share, was 12,228,260.
DOCUMENTS INCORPORATED BY REFERENCE
None.
AVINGER, INC.
ANNUAL REPORT ON FORM 10-K
FOR THE FISCAL YEAR ENDED DECEMBER 31, 2014
“Avinger,” “Ocelot,” “Pantheris,” and “Lumivascular”are trademarks of our company. Our logo and our other trade names, trademarks and service marks appearing in this Annual Report on Form 10-K are our property. Other trade names, trademarks and service marks appearing in this Annual Report on Form 10-K are the property of their respective owners. Solely for convenience, our trademarks and trade names referred to in this Annual Report on Form 10-K appear without the ™ symbol, but those references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights, or the right of the applicable licensor to these trademarks and trade names. Certain market and industry data used in this Annual Report on Form 10-K, where noted, is attributable to Millennium Research Group, Inc. Millennium Research Group asserts copyright protection over the use of such information and reserves all rights with respect to its use. This information has been reprinted with Millennium Research Group’s permission and the reproduction, distribution, transmission or publication of such information is prohibited without its consent.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains forward-looking statements concerning our business, operations and financial performance and condition, as well as our plans, objectives and expectations for our business, operations and financial performance and condition. Any statements contained herein that are not statements of historical facts may be deemed to be forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “anticipate,” “assume,” “believe,” “contemplate,” “continue,” “could,” “due,” “estimate,” “expect,” “goal,” “intend,” “may,” “objective,” “plan,” “predict,” “potential,” “positioned,” “seek,” “should,” “target,” “will,” “would” and other similar expressions that are predictions of or indicate future events and future trends, or the negative of these terms or other comparable terminology. These forward-looking statements include, but are not limited to, statements about:
· the outcome of our clinical studies, including VISION, and plans to conduct further clinical studies;
· our plans to modify our current products, or develop new products, to address additional indications;
· the expected timing of submission of a 510(k) to FDA for Pantheris;
· the expected growth in our business and our organization;
· our expectations regarding government and third-party payor coverage and reimbursement;
· our ability to retain and recruit key personnel, including the continued development of a sales and marketing infrastructure;
· our ability to obtain and maintain intellectual property protection for our products;
· our estimates of our expenses, ongoing losses, future revenue, capital requirements and our needs for, or ability to obtain, additional financing;
· our expectations regarding the time during which we will be an emerging growth company under the Jumpstart Our Business Startups Act or a smaller reporting company under the Securities Act;
· our ability to identify and develop new and planned products and acquire new products;
· our financial performance; and
· developments and projections relating to our competitors or our industry.
We believe that it is important to communicate our future expectations to our investors. However, there may be events in the future that we are not able to accurately predict or control and that may cause our actual results to differ materially from the expectations we describe in our forward-looking statements. These forward-looking statements are based on management’s current expectations, estimates, forecasts and projections about our business and the industry in which we operate and management’s beliefs and assumptions and are not guarantees of future performance or development and involve known and unknown risks, uncertainties and other factors that are in some cases beyond our control. As a result, any or all of our forward-looking statements in this Annual Report on Form 10-K may turn out to be inaccurate. Factors that may cause actual results to differ materially from current expectations include, among other things, those listed under “Risk Factors” and elsewhere in this Annual Report on Form 10-K. Potential investors are urged to consider these factors carefully in evaluating the forward-looking statements. These forward-looking statements speak only as of the date of this Annual Report on Form 10-K. We assume no obligation to update or revise these forward-looking statements for any reason, even if new information becomes available in the future.
You should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward-looking statements will be achieved or occur. We undertake no obligation to update publicly any forward-looking statements for any reason after the date of this Annual Report on Form 10-K to conform these statements to actual results or to changes in our expectations.
You should read this Annual Report on Form 10-K and the documents that we reference in this Annual Report on Form 10-K and have filed with the SEC as exhibits to the Annual Report on Form 10-K with the understanding that our actual future results, levels of activity, performance and events and circumstances may be materially different from what we expect.
Overview
We are a commercial-stage medical device company that designs, manufactures and sells image-guided, catheter-based systems that are used by physicians to treat patients with peripheral arterial disease, or PAD. Patients with PAD have a build-up of plaque in the arteries that supply blood to the arms and legs. Our mission is to dramatically improve the treatment of vascular disease through the introduction of products based on our lumivascular platform, the only intravascular image-guided system available in this market. We manufacture and sell a suite of products in the United States and select European markets. Our current products include our Lightbox imaging console, as well as our Wildcat, Kittycat, and the Ocelot family of catheters, which are designed to allow physicians to penetrate a total blockage in an artery, known as a chronic total occlusion, or CTO. We are also developing Pantheris, our image-guided atherectomy device, designed to allow physicians to precisely remove arterial plaque in PAD patients. Pantheris is currently undergoing a U.S. clinical trial intended to support a 510(k) submission in the second half of 2015 to the U.S. Food and Drug Administration, or FDA. We believe that Pantheris, if cleared by FDA, will significantly enhance our market opportunity within PAD and can expand the overall addressable market for PAD endovascular procedures.
According to an article published in The Lancet, the global prevalence of PAD was estimated at 202 million people in 2010. The prevalence of PAD in the United States alone was estimated at 18 million people in 2010 and is projected to grow to 21 million people by 2020 according to the Sage Group. Despite its prevalence, PAD is underdiagnosed and undertreated relative to many other serious vascular conditions, including coronary artery disease, or CAD, in part because many PAD patients are asymptomatic or dismiss their symptoms as normal signs of aging. Despite the relative undertreatment of PAD, Millennium Research Group estimates that over 570,000 catheter-based PAD procedures in the pelvis and legs were performed in the United States in 2013, which corresponded to a $1.0 billion market. Millennium Research Group also estimates that the number of catheter-based PAD procedures will grow to almost 700,000 in 2017, representing a $1.2 billion market in the United States. Higher diagnosis and intervention rates resulting from greater physician and patient awareness of PAD, as well as higher prevalence, may significantly expand the market opportunity for PAD treatments, according to the Millennium Research Group.
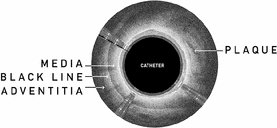
Current treatments for PAD, including bypass surgery, can be costly and may result in complications, high levels of post-surgery pain and lengthy hospital stays and recovery times. Minimally invasive, or endovascular, treatments include stents, angioplasty, and atherectomy devices, which are catheter-based products for the removal of plaque. These treatments also have limitations in their safety or efficacy profiles and frequently result in recurrence of the disease, also known as restenosis. We believe one of the main contributing factors to high restenosis rates for PAD patients treated with endovascular technologies is the amount of vascular injury that occurs during an intervention. Specifically, these treatments often disrupt the membrane between the outermost layers of the artery, which we refer to as the black line.
Our lumivascular platform is the only technology that offers real-time visualization of the inside of the artery during PAD treatment. We believe this approach will significantly improve patient outcomes by providing physicians with a clearer picture of the artery using radiation-free image guidance during treatment, enabling them to better differentiate between plaque and healthy arterial structures. Our lumivascular platform is designed to improve patient safety by enabling physicians to direct treatment towards the plaque, while avoiding healthy portions of the artery.
In March 2015, we completed enrollment of 134 patients in VISION, a 133-patient clinical trial designed to support a filing with FDA for our Pantheris atherectomy device. VISION is designed to evaluate the safety and efficacy of Pantheris to perform atherectomy using intravascular imaging. We believe the data from VISION will also allow us to demonstrate that avoiding the black line reduces the likelihood of restenosis in these patients. If Pantheris is cleared by FDA, we plan to commercialize it as part of our lumivascular platform in the United States and in select European countries after obtaining any required marketing authorizations.
We have assembled a team with extensive medical device development and commercialization capabilities, including our founder, John B. Simpson, Ph.D., M.D., who founded Advanced Cardiovascular Systems, FoxHollow Technologies and Perclose, among other vascular medical device companies. We began commercializing our initial non-lumivascular platform products in 2009 and introduced our lumivascular platform products in the United States in late 2012. We generated revenues of $11.2 million in 2014, $13.0 million in 2013 and $8.6 million in 2012.
Overview of Peripheral Arterial Disease
Atherosclerosis is a progressive, degenerative condition in which plaque, consisting of lipids, cholesterol, calcium and other substances found in the blood stream, accumulates on the arterial wall. The accumulation of plaque can result in the narrowing of an artery, which may lead to serious health problems. Plaque can occur in many areas of the body and may vary in composition, density and size. These blockages
sometimes contain hard areas, characterized as calcified plaque, as well as softer deposits consisting of fibrous or fatty tissue. As plaque continues to accumulate, it can completely block the artery, making it particularly difficult for physicians to treat.
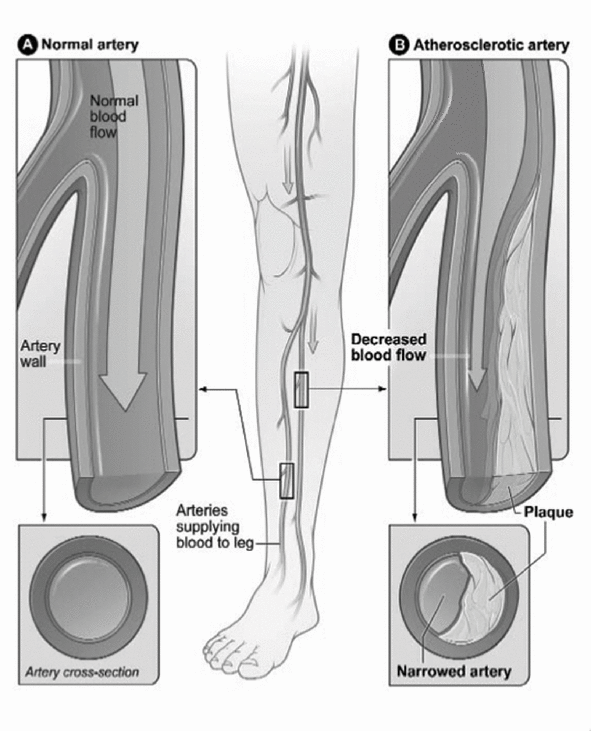
Comparison of a normal artery to an atherosclerotic artery
PAD is atherosclerosis in the arteries that supply blood to the arms and legs, and may lead to serious symptoms such as pain, fatigue or numbness. Genetic predisposition, diabetes, smoking, hypertension, physical inactivity, high cholesterol, obesity and aging all increase the risk of developing PAD. In extreme cases, PAD can lead to critical limb ischemia, or CLI, which, if left untreated, can result in ulceration, infection, or gangrene in the feet and legs and eventually limb amputation or death. The Transatlantic Intersociety Consensus for the Management of Peripheral Arterial Disease, or TASC II, estimates that 55% of CLI patients will undergo amputation or die within one year after the diagnosis.
Current Treatments for PAD and Their Limitations
Physicians have several options available to treat PAD. For mild cases, lifestyle changes or drug therapy may slow or stabilize progression of the disease and alleviate symptoms. For more advanced cases of PAD, a physician may employ minimally-invasive endovascular procedures, or surgical interventions such as bypass or amputation.
Medical Management
The large majority of cases of diagnosed PAD in the United States are medically managed, according to the Society of Interventional Radiology. For this population, lifestyle changes, including improved diet, regular exercise and smoking cessation, as well as drug treatment are often prescribed. Although these measures can be effective, many people are unable to sustain them. In addition, these measures may reduce the symptoms, but do not treat the underlying causes of the disease. Physicians may also prescribe medications that lower cholesterol and reduce blood pressure. These drug therapies are generally prescribed for the life of the patient and do not treat the obstruction, making them an ineffective treatment for many patients. As a result, many of these patients will ultimately require more aggressive treatments.
Surgery
Bypass Surgery. More severe cases of PAD may be treated by surgeons with bypass surgery. This procedure entails using a synthetic graft or harvesting a healthy vessel from another area of the body and grafting it around a blocked portion of an artery. This procedure diverts blood flow around the occluded area to ensure that the tissue supplied by these arteries receives sufficient blood flow. Given its invasive nature, bypass surgery is performed by physicians in an operating room with the patient under general anesthesia. Bypass surgery involves multi-day hospital stays for healing and rehabilitation. General anesthesia and the potential for surgical infections make this approach less suitable for patients with conditions such as high blood pressure, heart failure, chronic obstructive pulmonary disease or poor kidney function. We estimate there were over 150,000 lower extremity bypass surgeries performed in the United States in 2013.
Amputation. CLI is a serious form of PAD caused by severe lack of blood flow to the legs and often results in pain at rest and tissue breakdown. Physicians may recommend full or partial amputation of the leg or foot for patients with CLI. TASC II estimates that 30% of patients with CLI will require an amputation within one year of diagnosis, and 15% of patients who undergo amputation of one leg will undergo amputation of the other leg within two years of the first amputation. According to TASC II, the mortality rate for patients with CLI is 25% at one year from the development of the condition. The Sage Group estimates that approximately 200,000 amputations occur annually as a result of CLI.
Endovascular Interventions
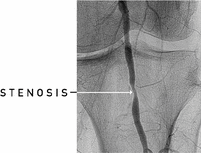
In recent years, technologies and techniques have improved such that many forms of PAD can now be treated by physicians with endovascular approaches. We believe PAD endovascular interventions will continue to increase due to improved safety and effectiveness of endovascular procedures relative to surgical alternatives, together with greater physician and patient awareness of the disease. The most common endovascular treatments include balloon angioplasty, stenting and atherectomy. These procedures involve a physician feeding a catheter over a guidewire through a small incision, typically while using fluoroscopy, or x-ray, as a visual guide. In the event that the patient has a CTO, the physician may require a specialized guidewire, support catheter or other device to cross the CTO prior to treatment consisting of balloon angioplasty, stenting, atherectomy or some combination thereof.
Fluoroscopy is the primary imaging tool currently used during endovascular treatments but delivers limited information to physicians. This technology provides an external view of the artery and does not allow physicians to differentiate between plaque and healthy arterial structures. Additionally, fluoroscopy exposes physicians, hospital staff and patients to radiation, which can lead to cataracts, cancer and abnormal blood cell counts. In addition, physicians frequently perform angiography in combination with fluoroscopy to assess the location and severity of the blockage. Angiography requires the use of contrast dye, which can increase the risk of kidney damage and may lead to acute kidney failure.
Importance of the Black Line. Scientific research has identified the importance of minimizing vascular injury during an endovascular intervention, and specifically the disruption of the membrane between the outer most layers of the artery, which we call the black line. A study by the Sanford Burnham Institute concluded that disruption of the area around the black line creates an inflammatory response significantly greater than when the black line is not injured, ultimately leading to accelerated narrowing of the artery. This narrowing of the artery is known as restenosis, which can lead to the restriction of blood flow. Black line disruption can be caused by wire-based CTO crossing, dissection from balloon angioplasty, stent placement, or an atherectomy device cutting through this area.
|
Lumivascular View |
Cross-Sectional View |
|
|
|
|
|
|
Image of the black line using our visualization compared to a cross sectional view of an artery.
A study from New York’s Mount Sinai Hospital, published in the Journal of the American College of Cardiology, or JACC, demonstrated the correlation between restenosis rates and vascular injury during directional atherectomy procedures. Specifically, the study examined the composition of the tissue removed during treatment of 102 patients and assessed restenosis rates after one year. The study found that in 54% of the patients, the extracted portion contained healthy tissue, indicating disruption of the black line. In this group of patients the restenosis rate, one-year after treatment, was 96%, while in the group of patients without evidence of black line disruption, the restenosis rate was only 15%.
The data from the Mount Sinai Hospital study are summarized in the following chart:
Atherectomy Procedures — Restenosis Rates at 1-Year
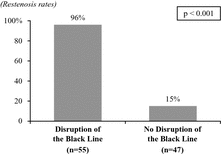
We believe balloon angioplasty, stenting and current atherectomy procedures often result in vascular injury, limiting their safety and efficacy, and increase restenosis rates associated with these treatments.
Balloon Angioplasty. In an angioplasty procedure, a miniature balloon attached to the tip of the treatment catheter opens the blood vessel by expanding the vessel and compressing plaque against the arterial wall. While angioplasty catheters are relatively easy to use, they stretch the arterial wall, often leading to dissections of, and damage to, the black line. Furthermore, angioplasty does not actually remove the plaque, which remains in the artery. Different variations of balloon catheters have been developed for the treatment of PAD, claiming additional benefits compared to standard angioplasty. These include cutting or scoring balloons designed to treat blockages with lower inflation pressures, as well as drug-coated balloons designed to suppress the inflammatory response to minimize restenosis. According to TASC II, 35% of angioplasty treatments result in restenosis at one year and 52% at three years. Millennium Research Group estimates that 500,000 PAD angioplasty procedures in the pelvis and legs were performed in the United States in 2013, 62% of which required the additional use of a stent.
Stenting. A stent is a wire-mesh tube that acts as a scaffold inside the artery to maintain adequate blood flow. Stents are currently available in bare metal and drug-coated varieties, with the latter designed to inhibit restenosis. Since stents rely on a similar expansion mechanism as balloons, we believe they also cause injury to the arterial wall and disrupt the black line during placement. According to TASC II, 27% of PAD stent treatments result in restenosis at one year and 36% at three years. Additionally, according to a study in JACC, stents placed in the legs fracture in approximately 25% of cases and have one-year patency, or absence of restenosis, rates of 41%, compared to 84% in cases with no stent fractures. Stents placed in the legs are often longer than coronary stents due to the diffuse nature of the lesions and the arterial anatomy, and longer stents have significantly higher fracture rates. Once a stent is implanted, it cannot be removed, which may limit future treatment options such as angioplasty, additional stenting, atherectomy and bypass surgery. Millennium Research Group estimates that 370,000 PAD stent procedures in the pelvis and legs were performed in the United States in 2013.
Atherectomy. Atherectomy is a procedure in which plaque is cleared from the arterial walls using a catheter-based technology with a mechanism to remove or displace diseased tissue. There are several types of atherectomy devices, including directional, rotational and laser, each with different mechanisms of action to remove or displace plaque. Currently available atherectomy devices rely on fluoroscopy rather than on- board imaging to provide visual guidance throughout the entire procedure. Atherectomy treatments frequently require the use of a stent or balloon to achieve the desired outcome and cannot selectively target the removal of only diseased tissue. As a result, current atherectomy technologies can damage the black line, which we believe increases the risk of restenosis. According to an article published in the Journal of Invasive Cardiology reviewing published clinical data, one-year restenosis rates for existing atherectomy technologies range from 22% to 46%. According to Millennium Research Group, there were 80,000 atherectomy procedures performed in the pelvis and legs in the United States in 2013, 86% of which required the use of a stent or balloon.
Our Solution
Our pioneering lumivascular platform combines best-in-class interventional devices with optical coherence tomography, or OCT, a high resolution, light-based, radiation-free intravascular imaging technology. Our lumivascular platform currently provides physicians with real-time OCT images from the inside of an artery during CTO crossing, and we believe Pantheris will be the first product to offer intravascular visualization during atherectomy.
|
|
|
Visualization using our lumivascular technology compared to standard fluoroscopy imaging
We believe the combination of enhanced visualization and the ability to precisely target the diseased portion of an artery will allow physicians to access difficult-to-treat areas and significantly improve the safety and efficacy of endovascular procedures for patients. Market acceptance of our lumivascular platform products may be hindered if physicians are not presented with compelling data from long-term studies of the safety and efficacy of our lumivascular platform products as compared to alternative procedures such as angioplasty, stenting, bypass surgery or other atherectomy procedures. Physicians will also need to appreciate the value of real-time imaging in improving patient outcomes in order to change current methods for treating PAD patients. We believe that our lumivascular platform provides the following benefits to physicians, hospitals and patients, as compared to balloons, stents and other atherectomy procedures:
· Improved efficacy through reduced risk of restenosis. Clinical evidence supports the proposition that more desirable outcomes in treating PAD are achieved by minimizing black line disruption, thereby reducing the risk of restenosis. Our lumivascular platform is designed to provide physicians with a clear picture from inside the artery during treatment. This visualization helps physicians to avoid disrupting the black line during an intervention, which we believe reduces the risk of restenosis. In addition, the directional nature of our catheters is designed to enable physicians to accurately target the diseased area, resulting in less damage to arterial structures and allowing for the precise removal of plaque. Our human feasibility trials have demonstrated that Pantheris avoided cutting the black line in 99% of the 86 tissue samples collected. Additionally, a study conducted at Mount Sinai Hospital, New York involving 102 patients found one-year restenosis rates of 96% and 15% in patients with and without black line disruption, respectively. The Mount Sinai Hospital study was not conducted using our products. Although we believe that our products would achieve similar results to those achieved with existing atherectomy devices in which no black line disruption occurred, we can provide no assurance that this would have been the case.
· Safety of endovascular procedures. Serious adverse events such as perforations and dissections may be reduced during endovascular procedures using our lumivascular platform. The results of our CONNECT II trial showed the benefit of our lumivascular platform, as demonstrated by the 97% efficacy and 98% safety rates in CTO cases using Ocelot.
· Expanded patient population eligible for endovascular treatment of PAD. Our lumivascular platform is designed to allow physicians to treat complex PAD cases where a traditional guidewire may not be successful due to the high CTO crossing success rates of Ocelot in such cases. There are 150,000 peripheral bypass procedures and 200,000 amputations performed each year in the United States. We believe these procedures are frequently performed as a result of an inability to cross a CTO with endovascular techniques. In our CONNECT II trial, Ocelot demonstrated a 97% CTO crossing rate in cases where a traditional guidewire was not successful. This crossing effectiveness enables the endovascular treatment of patients who may have previously been required to undergo bypass surgery or amputation. In addition, due to improved safety of our lumivascular platform products, we believe physicians will be more likely to use our products to treat patients who would otherwise be medically managed.
· Decreased radiation exposure for physicians and patients. In current endovascular treatments for PAD, physicians use fluoroscopy as the primary means of imaging and navigating to the target vessel and assessing results of the treatment. This standard practice exposes physicians, hospital staff and patients to harmful x-ray radiation for a significant period of time. Radiation exposure can be especially high for physicians and hospital staff who may perform multiple endovascular PAD procedures per day. Our lumivascular platform, which utilizes radiation-free OCT imaging, provides real-time visualization from the inside of the artery. When using our lumivascular platform, physicians may elect to use less fluoroscopy during a procedure as a result of having an additional means of visualization that does not involve radiation.
· Reduced use of balloons and stents and preservation of future treatment options. Pantheris, if cleared by FDA, is designed to enable physicians to successfully perform atherectomy procedures and remove plaque blockages in PAD patients using fewer balloons and stents. Current atherectomy procedures often require the use of balloons and stents, which may result in restenosis and
limit future treatment options. By avoiding the use of stents in atherectomy procedures, we believe that Pantheris better preserves future treatment options. We believe our lumivascular platform can replace other endovascular technologies, lower restenosis rates and reduce overall healthcare costs.
· Lumivascular platform designed for ease of adoption by physicians and hospitals. Our lumivascular platform products, while providing image-guided assistance to physicians, are used in a similar fashion to traditional catheters. Consequently, we believe the more than 10,000 interventional cardiologists, vascular surgeons and interventional radiologists in the United States that are trained in endovascular techniques can generally adopt our lumivascular platform and products without extensive training. We are designing future products to be compatible with our lumivascular platform, which we expect will enhance the value proposition for hospitals to invest in our technology. We also expect that Pantheris will qualify for existing reimbursement codes currently utilized by other atherectomy products, further facilitating adoption of our products.
Risks of using the lumivascular platform include the risks that are common to endovascular procedures and generally may include perforation, dissection, embolization, bleeding, infection, restenosis and limb loss. We are aware of certain characteristics and features of our lumivascular platform that may prevent widespread market adoption, including that the current model of Pantheris may require two physicians to operate the catheter and that training for technicians and physicians will be required to enable them to effectively operate our lumivascular platform products. Our Pantheris product is not cleared or approved by FDA for commercial sale. Pantheris may not be sold in the United States without clearance from FDA. Our current products are contraindicated, and therefore should not be used, in the iliac, coronary, cerebral, renal and carotid arteries.
Our Strategy
Our goal is to become the leading provider of image-guided medical devices for physicians to treat vascular diseases. The key elements of our strategy are to:
· Successfully complete the Pantheris VISION clinical trial. In March 2015, we completed enrollment of patients in the VISION clinical trial, which is a pivotal, non-randomized, prospective, single-arm trial to evaluate the safety and effectiveness of Pantheris in performing atherectomy procedures. The trial includes 134 patients at 20 sites within the United States and Europe. Data collection from the VISION trial is ongoing, and data monitoring and auditing of the acute procedural data and 30-day and six-month follow-up data is currently underway. We intend to use the data from our VISION trial to support an FDA 510(k) submission in the second half of 2015 for Pantheris. If Pantheris is cleared by FDA and other regulatory authorities, we plan to commercialize it as part of our lumivascular platform in the United States and in select European countries after obtaining any required marketing authorizations.
· Increase the installed base and penetration of our lumivascular platform. We have a direct sales organization that is divided into two distinct roles, sales of capital equipment and sales of disposable products. Our current sales efforts focus on establishing new lumivascular platform sites by marketing our products to physicians and hospital administrators. Additionally, we seek to increase the use of our lumivascular platform products by our current customers through case coverage, clinical training and other programs. We expect to grow our sales force in preparation for the commercial launch of Pantheris in order to increase the base of customers using our lumivascular platform products. We believe that expanding our U.S. commercial infrastructure and establishing distributor relationships in select regions outside the United States will drive further adoption of our lumivascular platform.
· Perform additional post-market studies to demonstrate the clinical and economic benefits of our lumivascular platform. We intend to initiate post-market studies that will examine clinical outcomes of our lumivascular platform products compared to other endovascular treatments for PAD, and demonstrate the benefits of our lumivascular platform. We plan to conduct studies comparing the safety, efficacy and cost of our lumivascular platform products to competitive products and may also conduct studies to gain additional clinical indications.
· Assist hospitals in raising awareness of our lumivascular platform for patients suffering from PAD. We are focused on increasing the awareness of our lumivascular platform and the benefits it offers to patients and physicians. We work with our hospital customers to build a lumivascular platform-based program through clinical training, public relations and physician education. The main focus of our clinical value proposition is to demonstrate how the lumivascular platform allows physicians to avoid injury to the black line during intervention, while addressing the other limitations of competing endovascular approaches. We plan to continue working with our customers to position our lumivascular platform as an offering they can use to demonstrate their commitment to using the most advanced technologies in caring for their patients.
· Leverage our technology platform to develop new products and further enhance our intellectual property portfolio. We intend to continue to invest in initiatives to improve the safety, efficacy and ease of use of our lumivascular platform, as well as to reduce
costs and procedure times. We have also identified a number of future expansion opportunities designed to position our lumivascular platform as the standard of care for vascular disease. We expect our Pantheris atherectomy device to be an important addition to our lumivascular platform if it is cleared for commercialization. We also intend to explore the feasibility of seeking new indications for our lumivascular platform to address unmet clinical needs within the CAD market. We believe we have a strong intellectual property portfolio and will continue to enhance this portfolio as we develop new technologies.
· Optimize our manufacturing operations to achieve cost and production efficiencies while maintaining quality. We design, develop and manufacture all of our products in-house at our headquarters in Redwood City, California using some components and sub-assemblies provided by third-party suppliers. We believe that controlling the manufacturing and assembly of our products allows us to innovate more quickly and produce higher quality products than if we outsourced manufacturing. We have the capacity to significantly increase our manufacturing volume within our current facilities. We intend to use our design, engineering and manufacturing capabilities to further advance and improve the efficiency of our manufacturing processes, which we believe will reduce unit costs and increase our gross margins. In the future, we may seek to manufacture certain of our products outside the United States to further reduce costs.
Our Products
Our current products include our Lightbox console and our various catheters used in PAD treatment. Each of our current products is, and our future products will be, designed to address significant unmet clinical needs in the treatment of vascular disease.
LUMIVASCULAR PRODUCTS
|
Name |
|
Clinical |
|
Size (Length, Diameter) |
|
Regulatory Status |
|
Original |
|
Lightbox(1) |
|
OCT Imaging |
|
N/A |
|
FDA Cleared |
|
November 2012 |
|
|
|
|
|
|
|
CE Mark |
|
September 2011 |
|
Pantheris |
|
Atherectomy |
|
130cm, 8 French (F) |
|
IDE Trial |
|
N/A |
|
Ocelot(2) |
|
CTO Crossing |
|
110cm, 6F |
|
FDA Cleared |
|
November 2012 |
|
|
|
|
|
|
|
CE Mark |
|
September 2011 |
|
Ocelot MVRX(2) |
|
CTO Crossing |
|
110cm, 6F |
|
FDA Cleared |
|
December 2012 |
|
Ocelot PIXL(2) |
|
CTO Crossing |
|
135/150cm, 5F |
|
FDA Cleared |
|
December 2012 |
|
|
|
|
|
|
|
CE Mark |
|
October 2012 |
(1) Lightbox is cleared for use with compatible Avinger products.
(2) The Ocelot system is intended to facilitate the intra-luminal placement of conventional guidewires beyond stenotic lesions including sub and chronic total occlusions in the peripheral vasculature prior to further percutaneous interventions using OCT-assisted orientation and imaging. The system is an adjunct to fluoroscopy and provides images of vessel lumen and wall structures. The Ocelot system is contraindicated for use in the iliac, coronary, cerebral, renal and carotid vasculature.
NON-IMAGING PRODUCTS
|
Name |
|
Indication |
|
Size (Length, Diameter) |
|
Regulatory Status |
|
Original |
|
Wildcat(1) |
|
Guidewire Support |
|
110cm, 6F |
|
FDA Cleared |
|
February 2009(3) |
|
|
|
CTO Crossing |
|
110cm, 6F |
|
FDA Cleared |
|
August 2011 |
|
|
|
|
|
|
|
CE Mark |
|
May 2011 |
|
Kittycat 2(2) |
|
CTO Crossing |
|
150cm, 5F |
|
FDA Cleared |
|
October 2011 |
|
|
|
|
|
|
|
CE Mark |
|
September 2011 |
(1) The Wildcat catheter is intended to facilitate the intraluminal placement of conventional guidewires beyond stenotic lesions (including sub and chronic total occlusions) in the peripheral vasculature prior to further percutaneous intervention. The Wildcat catheter is contraindicated for use in the iliac, coronary, cerebral, renal and carotid vasculature. The Wildcat catheter is intended to be used to support steerable guidewires in accessing discrete regions of the peripheral vasculature. It may be used to facilitate placement and exchange of guidewires and other interventional devices. It may also be used to deliver saline or contrast.
(2) The Kittycat 2 catheter is intended to facilitate the intraluminal placement of conventional guidewires beyond stenotic lesions (including sub and chronic total occlusions) in the peripheral vasculature prior to further percutaneous intervention. The Kittycat 2 catheter is contraindicated for use in the iliac, coronary, cerebral, renal and carotid vasculature.
(3) This original clearance date is for the 7F version of Wildcat. The commercially available version of Wildcat is listed and was cleared in August 2010.
Lumivascular Platform Overview
Our lumivascular platform integrates OCT visualization with interventional catheters and is the industry’s only system that provides real-time intravascular imaging during the treatment portion of PAD procedures. Our lumivascular platform consists of a capital component, Lightbox, and a variety of disposable catheter products, including Ocelot, Ocelot PIXL, Ocelot MVRX and, if cleared by FDA, Pantheris.
Lightbox
Lightbox is our proprietary imaging console, which enables the use of lumivascular catheters during PAD procedures. The console contains an optical transceiver that transmits light into the artery through an optical fiber and displays a cross-sectional image of the vascular tissue to the physician on a high definition monitor during the procedure. Lightbox is configured with two monitors, one for the physicians, and one for the Lightbox technician.
|
|
|
|
|
|
|
Lightbox |
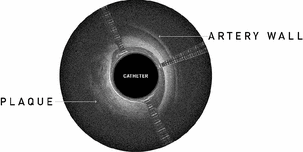
OCT image, showing layered structures (artery wall) on the right and non-layered structures (atherosclerotic plaque) on the left. |
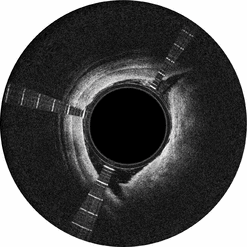
Lightbox displays a cross-sectional view of the vessel, which provides physicians with detailed information about the orientation of the catheter and the surrounding artery and plaque. Layered structures represent relatively healthy portions of the artery and non-layered structures represent the plaque that is blocking blood flow in the artery. Navigational markers allow the physician to orient the catheter toward the treatment area, helping to avoid damage to the black line during a procedure. Lightbox received FDA 510(k) clearance in November 2012 and CE Mark in Europe in September 2011.
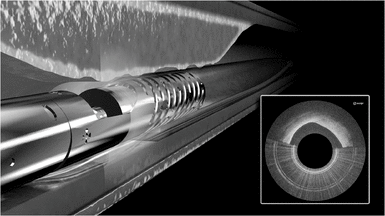
Pantheris
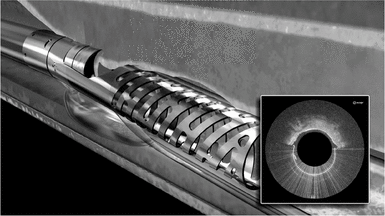
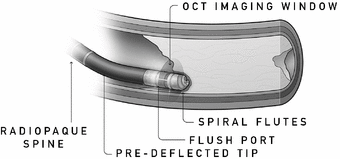
We believe Pantheris will be the first atherectomy catheter to incorporate real-time OCT intravascular imaging. Pantheris may be used alone or following a CTO crossing procedure using Ocelot or other products. Pantheris is a single-use product and will provide physicians with the ability to see a cross-sectional view of the artery throughout the procedure. The device restores blood flow by shaving thin strips of plaque using a high-speed directional cutting mechanism that specifically targets the portion of the artery where the plaque resides while minimizing disruption to the black line. The excised plaque is deposited in the nosecone of the device and removed from the artery. We believe Pantheris, if cleared by FDA, will represent a meaningful advancement in the treatment of PAD and will expand the existing treatable market.
|
|
|
|
|
Pantheris positioned prior to a cut |
|
|
|
|
|
|
|
Pantheris excising plaque |
To perform atherectomy procedures using Pantheris, physicians advance Pantheris to the diseased portion of the vessel using fluoroscopy prior to activating the cutting tip. The OCT image provides the physician with a cross- sectional view of the treatment site and the relative orientation of the cutter. Visual cues are used to orient the cutting mechanism to target diseased sections of the artery and the plaque is removed by activating the cutter and advancing the catheter through the blockage. A balloon beneath the cutter is inflated to move the catheter closer to the plaque, enabling the physician to stabilize the device and adjust the cut depth into the plaque as necessary. Multiple cuts can be made with the same device until sufficient plaque has been removed to restore adequate blood flow in the artery. In July 2014, FDA granted us an investigational device exemption, or IDE, for Pantheris and we commenced enrollment of our 133-patient VISION trial. We completed enrollment of the VISION trial in March 2015 with 134 patients and expect to complete 6-month patient follow-up and submit for 510(k) clearance from FDA during the second half of 2015. We have made minor modifications and may make further modifications to the design of Pantheris prior to widespread commercialization, which could require regulatory clearances or approvals.
Ocelot, Ocelot PIXL and Ocelot MVRX
Ocelot is the first ever CTO crossing catheter to incorporate real-time OCT imaging, which allows physicians to see the inside of an artery during a CTO crossing procedure. Physicians have traditionally relied solely on fluoroscopy and tactile feedback to guide catheters through complicated blockages. Ocelot allows physicians to accurately navigate through CTOs by utilizing the OCT images to precisely guide the device through the arterial blockage, while minimizing disruption to the black line.
|
|
|
|
|
|
|
Ocelot crossing a chronic total occlusion, or CTO |
Lightbox visualization |
Ocelot has a corkscrew-like tip that rotates to facilitate advancement of the catheter through a CTO. Marker bands are displayed on the OCT image and allow the tip of the catheter to be steered towards the blockage and away from the arterial wall as it moves through the blockage. Once through the blockage, a guidewire can be extended and Ocelot is removed, leaving the wire in place for additional therapies such as the use of an atherectomy catheter like Pantheris. We received CE Mark for Ocelot in September 2011 and received FDA 510(k) clearance in November 2012.
We also offer Ocelot PIXL, a lower profile CTO-crossing device for below-the-knee arteries and Ocelot MVRX, which offers a different tip design for above-the-knee arteries. We received CE Mark for Ocelot PIXL in October 2012 and received FDA 510(k) clearance in December 2012. We received FDA 510(k) clearance for Ocelot MVRX in December 2012.
Other Products
Our first-generation CTO-crossing catheters, Wildcat and Kittycat 2, employ a proprietary design that uses a rotational spinning technique, allowing the physician to switch between a passive and active mode when navigating across a CTO. Once across the CTO, Wildcat and Kittycat 2 allow for placement of a guidewire and removal of the catheter while leaving the wire in place for additional therapies. Both products require the use of fluoroscopy rather than our lumivascular platform for imaging. Wildcat was our first commercial product and has received both FDA 510(k) clearance in the United States and CE Mark in Europe for crossing peripheral artery CTOs. Kittycat 2 has FDA 510(k) clearance in the United States and CE Mark clearance in Europe for the treatment of peripheral artery CTOs.
Clinical Development
We have conducted several clinical trials to evaluate the safety and efficacy of our products and we received FDA clearance for Wildcat and Ocelot for CTO crossing in 2011 and 2012, respectively. We are currently evaluating the safety and efficacy of Pantheris in our VISION clinical trial and expect to file a 510(k) submission with FDA in the second half of 2015.
CONNECT (Wildcat)
Our clinical trial for the Wildcat catheter, known as the CONNECT trial, was a prospective, multi-center, non-randomized trial that evaluated the safety and efficacy of Wildcat in crossing CTOs in arteries of the upper leg. The CONNECT trial enrolled 88 patients with CTOs at 15 centers in the United States. Patients were followed for 30 days post-procedure and an independent group of physicians verified the results to determine crossing efficacy and safety endpoints. The CONNECT trial demonstrated that Wildcat was able to cross 89% of CTOs following unsuccessful attempts to cross with standard guidewire techniques. The trial demonstrated a 95% freedom from major adverse events, or MAEs. In the CONNECT trial, MAEs were defined as clinically significant perforations or embolizations and/or Grade C or greater dissections occurring within 30 days of the procedure. These results represent the second-highest reported CTO crossing rate of any published CTO clinical trial, exceeded only by our subsequent CONNECT II clinical trial results.
CONNECT II (Ocelot)
Our clinical trial for Ocelot, known as CONNECT II, was a prospective, multi- center, non-randomized trial that evaluated the safety and efficacy of Ocelot in crossing CTOs in arteries of the upper leg using OCT intravascular imaging. The CONNECT II trial enrolled 100 patients with CTOs at 14 centers in the United States and two centers in Europe. Patients were followed for 30 days post-procedure and an independent group of physicians verified the results to confirm the primary efficacy and safety endpoints. Results from the CONNECT II trial demonstrated
that Ocelot surpassed its primary efficacy endpoint by successfully crossing the CTO in 97% of the cases following unsuccessful attempts to cross with standard guidewire techniques. Ocelot achieved these rates with 98% freedom from MAEs.
VISION (Pantheris)
VISION is our pivotal, non-randomized, prospective, single-arm trial to evaluate the safety and effectiveness of Pantheris across 20 sites within the United States and Europe. The objective of the clinical trial is to demonstrate that Pantheris can be used to effectively remove plaque from diseased lower extremity arteries while using on-board visualization as an adjunct to fluoroscopy. Two groups of patients are being treated in VISION: (1) optional roll-ins, which are typically the first two procedures at a site, and (2) the primary cohort, which will be the analyzable group of patients. The data for these two groups will be reported separately in our 510(k) submission to FDA. Based on final enrollment, the primary cohort includes 134 patients. In March 2015, we completed enrollment of patients in the VISION clinical trial.
VISON’s primary efficacy endpoint requires that at least 87% of lesions treated by physicians using Pantheris have a residual stenosis of less than 50%, as verified by an independent core laboratory. The primary safety endpoint requires that less than 43% of patients experience an MAE through six-month follow-up as adjudicated by an independent Clinical Events Committee, or CEC. MAEs for VISION include cardiovascular-related death, unplanned major index limb amputation, clinically driven target lesion revascularization, or TLR, heart attack, clinically significant perforation, dissection, embolus, and pseudoaneurysm. Both primary safety and effectiveness study endpoints must be met in order for the VISION trial to be successful.
Data collection from the VISION trial is ongoing, and data monitoring and auditing of the acute procedural data and 30-day follow-up data is currently underway. As of January 12, 2015, preliminary acute procedural data were available for 116 patients, comprised of 28 roll-in patients and 88 patients in the primary cohort. 30-day follow-up data were available for 35 of these patients. Within the 116-patient group as of January 12, 2015, there were four potential MAEs, consisting of two emboli and two TLRs, all reported by the investigator to be related or possibly related to the use of Pantheris. Both emboli were resolved with routine therapy without adverse clinical consequences for the patients, both of whom had good outcomes from the Pantheris atherectomy. Both TLRs were treated with standard balloon angioplasty and stenting without adverse clinical consequences for the patients.
The four potential MAEs occurred after the initial meeting, which to date is the only meeting, of the CEC. The CEC will make the final determination as to whether these events were related to use of Pantheris or another part of the treatment procedure. Additional MAEs may be reported during the 30-day and six-month follow-up period for patient outcomes. The final analysis for the safety endpoint will not be conducted until enrollment is complete and all patients are followed through six months.
With respect to our primary efficacy endpoint analysis, as of January 12, 2015, results reviewed by the independent core lab were available for 113 lesions (31 from roll-in patients and 82 from primary cohort patients) from a total of 91 patients. An independent core laboratory has verified residual restenosis of less than 50% in 98% of these lesions. Based on the currently available data, we believe that we are on track to meet or exceed the requirements necessary to meet the primary efficacy endpoint. The final efficacy endpoint analysis will not be completed until all patients are enrolled and all lesions have been analyzed by the core lab.
Although not mandated by FDA to support the market clearance of Pantheris, the protocol for the VISION trial indicates that routine histopathological analysis of the tissue extracted by Pantheris will be conducted. This process allows us to determine the amount of adventitia present in the tissue, which in turn indicates the extent to which the black line has been disrupted during Pantheris procedures. As of January 12, 2015, we had completed histopathological analysis on tissue from 109 patients (28 roll-in and 81 primary cohort), representing 138 lesions (35 roll-in and 103 primary cohort) and have determined that the average percent area of adventitia is only 1.0% of the total excised tissue. We believe the low level of black line disruption will correlate to lower restenosis rates and improved long-term outcomes for patients treated with Pantheris, but we do not intend to make any promotional claims to that effect based on the data from this study. We intend to publish the results of the histopathological analysis in conjunction with the primary safety and efficacy endpoint data from the VISION trial.
Preliminary VISION trial data available for reporting as of January 12, 2015 is summarized in the table below. The preliminary data for our VISION trial may not be predictive of its final results and failure of the trial can occur at any time. A number of companies in the medical device field have suffered significant setbacks during clinical trials due to lack of efficacy or unacceptable safety issues, notwithstanding promising preliminary results. We cannot assure you that the final results of the VISION trial will meet its primary efficacy or safety endpoints, that the trial will be successful overall or that the data will support FDA clearance of Pantheris.
|
|
|
Roll-In |
|
Primary |
|
Total |
|
|
Patients Enrolled (complete enrollment n=161) |
|
28 |
|
88 |
|
116 |
|
|
|
|
|
|
|
|
(72.0 |
)% |
|
Lesions treated |
|
35 |
|
107 |
|
142 |
|
|
Primary Efficacy Endpoint |
|
|
|
|
|
|
|
|
Lesions analyzed by core lab |
|
31 |
|
82 |
|
113 |
|
|
Lesions meeting primary efficacy endpoint criterion of residual restenosis of less than 50% by core lab |
|
100 |
% |
98 |
% |
98 |
% |
|
|
|
(31/31 |
) |
(80/82 |
) |
(111/113 |
) |
|
Primary Safety Endpoint (MAEs through 6 months) |
|
|
|
|
|
|
|
|
Total MAEs Reported (116 subjects with acute data and 35 subjects with 30-day follow-up data) |
|
1 |
|
3 |
|
4 |
|
|
Reported MAEs as a percentage of patients enrolled |
|
3.6 |
% |
3.4 |
% |
3.4 |
% |
|
|
|
(1/28 |
) |
(3/88 |
) |
(4/116 |
) |
|
Histopathology Results (Non-Endpoint Data) |
|
|
|
|
|
|
|
|
Lesions with histopathology results |
|
35 |
|
103 |
|
138 |
|
|
Average percent area of adventitia in all lesions with histopathology results |
|
0.5 |
% |
1.2 |
% |
1.0 |
% |
Sales and Marketing
We focus our sales and marketing efforts primarily on the approximately 10,000 interventional cardiologists, vascular surgeons and interventional radiologists in the United States that are potential users of our lumivascular platform products. Our marketing efforts are focused on developing strong relationships with physicians and hospitals that we have identified as key opinion leaders based on their knowledge of our products, clinical expertise and reputation. We also use continuing medical education programs and other opportunities to train interventional cardiologists, vascular surgeons, and interventional radiologists in the use of our lumivascular platform products and educate them as to the benefits of our products as compared to alternative procedures such as angioplasty, stenting, bypass surgery or other atherectomy procedures. In addition, we work with physicians to help them develop their practices and with hospitals to market themselves as centers of excellence in PAD treatment by making our products available to physicians for treating patients.
Our sales team consists of a vice president, regional directors, sales managers and implementation specialists. Our sales managers are divided into two primary roles, one focused on sale and use of our disposable catheters and the other focused on sale and service of our Lightbox console. We have an extensive hands-on sales training program, focused on our technologies, lumivascular image interpretation, case management, sales processes, sales tools and implementing our sales and marketing programs and compliance with applicable federal and state laws and regulations. Our sales team is supported by a highly specialized marketing team, which is divided into three areas of focus: clinical education, marketing program implementation and technology awareness and product development. We also have a small team of field engineers responsible for installation, service and maintenance of our Lightbox consoles.
As of December 31, 2014, we had 42 employees focused on sales and marketing. Our sales, general and administrative expenses for the years ended December 31, 2014, 2013 and 2012 were $18.5 million, $25.8 million and $22.8 million, respectively.
Competition
The medical device industry is highly competitive, subject to rapid change and significantly affected by new product introductions, results of clinical research, corporate combinations and other factors relating to our industry. Because of the market opportunity and the high growth potential of the PAD treatment market, competitors and potential competitors have historically dedicated, and will continue to dedicate, significant resources to aggressively develop and commercialize their products.
Our products compete with a variety of products or devices for the treatment of PAD, including other CTO crossing devices, stents, balloons and atherectomy catheters, as well as products used in vascular surgery. Large competitors in the CTO crossing, stent and balloon market segments include Abbott Laboratories, BARD, Boston Scientific, Cook Medical, Johnson & Johnson and Medtronic. Competitors in the atherectomy market include Boston Scientific, Cardiovascular Systems, Medtronic, Philips and Spectranetics. Some competitors have attempted to combine intravascular imaging with atherectomy and may have current programs underway to do so. These and other companies may attempt to incorporate on-board visualization into their products in the future. Other competitors include pharmaceutical companies that manufacture drugs for the treatment of symptoms associated with mild to moderate PAD and companies that provide products used by surgeons in peripheral and coronary bypass procedures. These competitors and other companies may introduce new products that compete with our solution.
Many of our competitors have substantially greater financial, manufacturing, marketing and technical resources than we do. Furthermore, many of our competitors have well-established brands, widespread distribution channels and broader product offerings, and have established stronger and deeper relationships with target customers.
To compete effectively, we have to demonstrate that our products are attractive alternatives to other devices and treatments on the basis of:
· procedural safety and efficacy;
· acute and long-term outcomes;
· ease of use and procedure time;
· price;
· size and effectiveness of sales force;
· radiation exposure for physicians, hospital staff and patients; and
· third-party reimbursement.
Intellectual property
In order to remain competitive, we must develop and maintain protection of the proprietary aspects of our technologies. We rely on a combination of patents, copyrights, trademarks, trade secret laws and confidentiality and invention assignment agreements to protect our intellectual property rights.
It is our policy to require our employees, consultants, contractors, outside scientific collaborators and other advisors to execute non-disclosure and assignment of invention agreements on commencement of their employment or engagement. Agreements with our employees also forbid them from using the proprietary rights of third parties in their work for us. We also require confidentiality or material transfer agreements from third parties that receive our confidential data or materials.
As of December 31, 2014, we held five issued U.S. patents and had 14 U.S. utility patent applications and 8 PCT applications pending. As of December 31, 2014, we also had one issued patent from the Japan Patent Office, one issued patent from the Chinese patent office, and one European patent which has been nationalized in Germany, France, Great Britain, Italy and Ireland. As of December 31, 2014, we had 29 pending patent applications outside of the United States, including in Australia, Canada, Europe, India and Japan. As we continue to research and develop our Pantheris technology, we intend to file additional U.S. and foreign patent applications related to the design, manufacture and therapeutic uses of our atherectomy devices. Our issued patents expire between the years 2028 and 2032.
Our patent applications may not result in issued patents and our patents may not be sufficiently broad to protect our technology. Any patents issued to us may be challenged by third parties as being invalid, or third parties may independently develop similar or competing technology that avoids our patents. The laws of certain foreign countries do not protect our intellectual property rights to the same extent as do the laws of the United States.
As of December 31, 2014, we held two allowed U.S. trademarks and two registered marks in Europe. We have one pending trademark application in the United States.
Research and Development
Our ongoing research and development activities are primarily focused on improving and enhancing our lumivascular platform, specifically our core competency of integrating OCT intravascular imaging onto therapeutic catheters. Our research objectives target areas of unmet clinical need, increase the utility of the lumivascular platform and adoption of our products by healthcare providers.
· Product line improvements and extensions. We are developing improvements to our lumivascular platform, including additional catheters for use in different clinical applications. For example, we are developing a next-generation CTO crossing device to target the coronary market and enhanced versions of Pantheris.
· Additional treatment indications. We intend to seek additional regulatory clearances from FDA to expand the indications for which our products can be marketed within PAD, as well as in other areas of the body. This includes both expanding the marketed indications for our current products, as well as development of new products.
· Next-generation console. We are focusing our console development efforts on miniaturization, equipment integration and increased processing power in anticipation of future catheter products. We may also develop a version of our lumivascular platform that integrates OCT imaging into existing catheterization lab and operating room imaging systems.
· Improved software and user interface. We are actively improving our software to provide more information and control to our end users during a procedure. We use physician and staff feedback to improve the features and user functionality of our lumivascular platform.
As of December 31, 2014, we had 19 employees focused on research and development. In addition to our internal team, we retain third-party-contractors from time to time to provide us with assistance on specialized projects. We also work closely with experts in the medical
community to supplement our internal research and development resources. Research and development expenses for the years ended December 31, 2014, 2013 and 2012 were $11.2 million, $16.0 million and $15.4 million, respectively.
Manufacturing
Prior to the introduction of our lumivascular platform, our non-imaging catheter products were manufactured by a third-party. All of our products are now manufactured in-house using components and sub-assemblies manufactured both in-house at our facilities in Redwood City, California and by outside vendors. We expect our current manufacturing facility will be sufficient to meet our anticipated growth through at least 2016. We assemble all of our products at our manufacturing facility but certain critical processes such as coating and sterilization are done by outside vendors.
Our manufacturing operations are subject to regulatory requirements of 21 CFR part 820, the Quality System Regulation for medical devices sold in the United States, which is enforced by FDA, the Medical Devices Directive 93/42/EEC, which is required for doing business in the European Union, and applicable requirements relating to the environment, waste management and health and safety matters, including measures relating to the release, use, storage, treatment, transportation, discharge, disposal and remediation of hazardous substances, and the sale, labeling, collection, recycling, treatment and disposal of products containing hazardous substances. We cannot ensure that we will not incur material costs or liability in connection with our operations, or that our past or future operations will not result in claims by or injury to employees or the public.
Order quantities and lead times for components purchased from outside suppliers are based on our forecasts derived from historical demand and anticipated future demand. Lead times for components may vary significantly depending on the size of the order, time required to fabricate and test the components, specific supplier requirements and current market demand for the components and subassemblies. To date, we have not experienced significant delays in obtaining any of our components or subassemblies.
We rely on single and limited source suppliers for several of our components. For example, we rely on one vendor for, among other components, our torque shaft and drive cable. These components are critical to our products and there are relatively few alternative sources of supply for them. We do not carry a significant inventory of these components. Identifying and qualifying additional or replacement suppliers for any of the components used in our products could involve significant time and cost. Any supply interruption from our vendors or failure to obtain additional vendors for any of the components used to manufacture our products would limit our ability to manufacture our product and could therefore harm our business, financial condition and results of operations.
Our suppliers have no contractual obligations to supply us with, and we are not contractually obligated to purchase from them, any of our supplies. Any supply interruption from our vendors or failure to obtain additional vendors for any of the components would limit our ability to manufacture our product and could have a material adverse effect on our business, financial condition and results of operations.
We have registered with FDA as a medical device manufacturer and have obtained a manufacturing license from CDRH. We and our component suppliers are required to manufacture our products in compliance with FDA’s Quality System Regulation, or QSR in 21 CFR part 820 of the Federal Food, Drug and Cosmetic Act. The QSR regulates extensively the methods and documentation of the design, testing, control, manufacturing, labeling, quality assurance, packaging, storage and shipping of our products. FDA enforces the QSR through periodic unannounced inspections that may include the manufacturing facilities of our subcontractors. Since we began manufacturing onsite, our Quality System has undergone 13 external audits, the last of which occurred on August 6, 2014 and resulted in zero non-conformances.
Our failure or the failure of our component suppliers to maintain compliance with the QSR requirements could result in the shutdown of our manufacturing operations or the recall of our products, which would harm our business. In the event that one of our suppliers fails to maintain compliance with our or governmental quality requirements, we may have to qualify a new supplier and could experience manufacturing delays as a result. We have opted to maintain quality assurance and quality management certifications to enable us to market our products in the member states of the European Union, the European Free Trade Association and countries which have entered into Mutual Recognition Agreements with the European Union. Our Redwood City facilities meet the requirements set forth by ISO 13485:2003 Medical devices—Quality management systems—Requirements for regulatory purposes and MDD 93/42/EEC European Union Council Medical Device Directive.
Government Regulation
In general, medical device companies must navigate a challenging regulatory environment. The Food and Drug Administration, or FDA, regulates the medical device market to ensure the safety and efficacy of these products. FDA allows for two primary pathways for a medical device to gain approval for commercialization: a successful PMA application or 510(k) clearance. A completely novel product must go through the more rigorous PMA process, or premarket approval, if it cannot receive authorization through a 510(k). FDA has established three different classes of medical devices that indicate the level of risk associated with using a device and consequent degree of regulatory controls needed to govern its safety and efficacy. Level I and Level II devices are considered lower risk and often can gain approval for commercial distribution by
submitting a notification request to FDA, generally known as the 510(k) process. The devices regarded as the highest risk by FDA are designated Class III status and generally require the submission of a PMA application for approval to commercialize a product. These generally include life- sustaining, life-supporting, or implantable devices or devices without a known predicate technology already approved by FDA.
The 510(k) clearance path can be significantly less time-consuming and arduous than PMA approval, making this route preferable for a medical device company. Through a 510(k), a company must provide documentation that its device is substantially equivalent to a technology already approved through a 510(k) or in distribution before May 28, 1976 for which FDA has not yet required a PMA submission. FDA has 90 days from the date of the premarket equivalence submission to authorize or decline commercial distribution of the device. However, similar to the PMA process, approval may take longer than this three-month window, as FDA can request additional data. If FDA resolves that the product is not substantially equivalent to a predicate device, then the device acquires a Class III designation. All of our currently marketed products have received commercial clearance and associated indications for use through the 510(k) regulatory pathway with FDA, some with the support of clinical data.
A PMA application must be accompanied by substantial data that supports the safety and efficacy of the device, which includes the provision of preclinical, clinical, technical, manufacturing and labeling information. If FDA deems the application acceptable to pass through the first level of scrutiny, it has 180 days to review the submission, but it can typically take longer (up to several years) as this regulatory body can request additional information or clarifications. FDA may also impose additional regulatory hurdles for a premarket approval, including the institution of an outside advisory panel of experts to assess the application or provide recommendations as to whether to approve the device. Although FDA in the end approves or disapproves the device, in nearly all cases FDA follows the recommendation from the independent panel concerning approvability of the new device. As part of this process, FDA will also inspect the manufacturing operations of the company requesting approval to verify compliance with quality control regulations. Significant changes in the fabrication of a device, or alterations in the labeling or design of a product require new PMA applications or PMA supplements for a product originally approved under a PMA. This creates substantial regulatory risk for devices undergoing the PMA route.
Pervasive and Continuing Regulation
After a device is placed on the market, numerous regulatory requirements continue to apply. These include:
· FDA’s Quality System Regulation, or QSR, which requires manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all aspects of the manufacturing process;
· labeling regulations and FDA prohibitions against the promotion of products for uncleared, unapproved or off-label uses;
· clearance or approval of product modifications that could significantly affect safety or efficacy or that would constitute a major change in intended use;
· medical device reporting, or MDR, regulations, which require that manufacturers report to FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if the malfunction were to recur; and
· post-market surveillance regulations, which apply when necessary to protect the public health or to provide additional safety and effectiveness data for the device.
After a device receives 510(k) clearance or PMA approval, any modification that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, will require a new clearance or approval. FDA requires each manufacturer to make this determination initially, but FDA can review any such decision and can disagree with a manufacturer’s determination. If FDA disagrees with the determination not to seek a new 510(k) clearance or PMA, FDA may retroactively require a new 510(k) clearance or premarket approval. FDA could also require a manufacturer to cease marketing and distribution and/or recall the modified device until 510(k) clearance or premarket approval is obtained. Also, in these circumstances, it may be subject to significant regulatory fines, penalties, and warning letters.
The MDR regulations require that we report to FDA any incident in which our product may have caused or contributed to a death or serious injury or in which our product malfunctioned and, if the malfunction were to recur, would likely cause or contribute to death or serious injury.
We have registered with FDA as a medical device manufacturer and have obtained a manufacturing license from the California Department of Health Services, or CDHS. FDA has broad post-market and regulatory enforcement powers. We are subject to unannounced inspections by FDA and the Food and Drug Branch of CDHS to determine our compliance with the QSR and other regulations, and these inspections may include the manufacturing facilities of our suppliers. BSI, our European Notified Body, inspected our facility in 2013 and found
zero non-conformances. Our current facility has been inspected by FDA in 2009, 2011 and 2013, and two, three and zero observations, respectively, were noted during those inspections. In the latest FDA audit, there were no findings that involved a material violation of regulatory requirements, and no non-conformances were noted. Our responses to these observations noted in 2009 and 2011 have been accepted by FDA, and we believe that we are in substantial compliance with the QSR.
Failure to comply with applicable regulatory requirements can result in enforcement action by FDA, which may include any of the following sanctions:
· warning letters, fines, injunctions, consent decrees and civil penalties;
· repair, replacement, refunds, recall or seizure of our products;
· operating restrictions, partial suspension or total shutdown of production;
· refusing our requests for 510(k) clearance or premarket approval of new products, new intended uses or modifications to existing products;
· withdrawing 510(k) clearance or premarket approvals that have already been granted; and
· criminal prosecution.
Regulatory System for Medical Devices in Europe
The European Union consists of 25 member states and has a coordinated system for the authorization of medical devices. The E.U. Medical Devices Directive, or MDD, sets out the basic regulatory framework for medical devices in the European Union. This directive has been separately enacted in more detail in the national legislation of the individual member states of the European Union.
The system of regulating medical devices operates by way of a certification for each medical device. Each certificated device is marked with CE mark which shows that the device has a Certificat de Conformité. There are national bodies known as Competent Authorities in each member state which oversee the implementation of the MDD within their jurisdiction. The means for achieving the requirements for CE mark varies according to the nature of the device. Devices are classified in accordance with their perceived risks, similarly to the U.S. system. The class of a product determines the requirements to be fulfilled before CE mark can be placed on a product, known as a conformity assessment. Conformity assessments for our products are carried out as required by the MDD. Each member state can appoint Notified Bodies within its jurisdiction. If a Notified Body of one member state has issued a Certificat de Conformité, the device can be sold throughout the European Union without further conformance tests being required in other member states.
Health Insurance Portability and Accountability Act
The Health Insurance Portability and Accountability Act of 1996, or HIPAA, established for the first time comprehensive federal protection for the privacy and security of health information. The HIPAA standards apply to three types of organizations, or Covered Entities: health plans, healthcare clearing houses, and healthcare providers which conduct certain healthcare transactions electronically. Title II of HIPAA, the Administrative Simplification Act, contains provisions that address the privacy of health data, the security of health data, the standardization of identifying numbers used in the healthcare system and the standardization of certain healthcare transactions. The privacy regulations protect medical records and other protected health information by limiting their use and release, giving patients the right to access their medical records and limiting most disclosures of health information to the minimum amount necessary to accomplish an intended purpose. The HIPAA security standards require the adoption of administrative, physical, and technical safeguards and the adoption of written security policies and procedures. HIPAA requires Covered Entities to obtain a written assurance of compliance from individuals or organizations who provide services to Covered Entities involving the use or disclosure of protected health information (“Business Associates”).
On February 17, 2009, Congress enacted Subtitle D of the Health Information Technology for Economic and Clinical Health Act, or HITECH, provisions of the American Recovery and Reinvestment Act of 2009. HITECH amends HIPAA and, among other things, expands and strengthens HIPAA, creates new targets for enforcement, imposes new penalties for noncompliance and establishes new breach notification requirements for Covered Entities and Business Associates. Regulations implementing major provisions of HITECH were finalized on January 25, 2013 through publication of the HIPAA Omnibus Rule, or the Omnibus Rule. The Omnibus Rule contained significant changes for Covered Entities and Business Associates with respect to permitted uses and disclosures of Protected Health Information.
Under HITECH’s new breach notification requirements, Covered Entities must report breaches of protected health information that has not been encrypted or otherwise secured in accordance with guidance from the Secretary of the U.S. Department of Health and Human Services,
or the Secretary. Required breach notices must be made as soon as is reasonably practicable, but no later than 60 days following discovery of the breach. Reports must be made to affected individuals and to the Secretary and in some cases, they must be reported through local and national media, depending on the size of the breach. We are currently subject to the HIPAA regulations. We are subject to audit under HHS’s HITECH-mandated audit program. We may also be audited in connection with a privacy complaint. We are subject to prosecution and/or administrative enforcement and increased civil and criminal penalties for non-compliance, including a new, four-tiered system of monetary penalties adopted under HITECH. We are also subject to enforcement by state attorneys general who were given authority to enforce HIPAA under HITECH. To avoid penalties under the HITECH breach notification provisions, we must ensure that breaches of protected health information are promptly detected and reported within the company, so that we can make all required notifications on a timely basis. However, even if we make required reports on a timely basis, we may still be subject to penalties for the underlying breach.
In addition to the federal privacy regulations, there are a number of state laws regarding the privacy and security of health information and personal data that are applicable to clinical laboratories. The compliance requirements of these laws, including additional breach reporting requirements, and the penalties for violation vary widely and new privacy and security laws in this area are evolving. Requirements of these laws and penalties for violations vary widely. We believe that we have taken the steps required of us to comply with health information privacy and security statutes and regulations in all jurisdictions, both state and federal. However, we may not be able to maintain compliance in all jurisdictions where we do business. Failure to maintain compliance, or changes in state or federal laws regarding privacy or security, could result in civil and/or criminal penalties and could have a material adverse effect on our business.
If we or our operations are found to be in violation of HIPAA, HITECH or their implementing regulations, we may be subject to penalties, including civil and criminal penalties, fines, and exclusion from participation in U.S. federal or state health care programs, and the curtailment or restructuring of our operations. HITECH increased the civil and criminal penalties that may be imposed against Covered Entities, their Business Associates and possibly other persons, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorney’s fees and costs associated with pursuing federal civil actions.
In addition to federal privacy regulations, there are a number of state laws governing confidentiality of health information that are applicable to our operations. New laws governing privacy may be adopted in the future as well. We have taken steps to comply with health information privacy requirements that are applicable to us.
Federal, State and Foreign Fraud and Abuse Laws
Because of the significant federal funding involved in Medicare and Medicaid, Congress and the states have enacted, and actively enforce, a number of laws to eliminate fraud and abuse in federal healthcare programs. Our business is subject to compliance with these laws. In March 2010, the Recipient Protection and Affordable Care Act, as amended by the Healthcare and Education Affordability Reconciliation Act, which we refer to collectively as the Affordable Care Act, was enacted in the United States. The provisions of the Affordable Care Act are effective on various dates. The Affordable Care Act expands the government’s investigative and enforcement authority and increases the penalties for fraud and abuse, including amendments to both the Anti-Kickback Statute and the False Claims Act, to make it easier to bring suit under these statutes. The Affordable Care Act also allocates additional resources and tools for the government to police healthcare fraud, with expanded subpoena power for HHS, additional funding to investigate fraud and abuse across the healthcare system and expanded use of recovery audit contractors for enforcement.
Anti-Kickback Statutes. The federal healthcare programs’ Anti-Kickback Statute prohibits persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual, or the furnishing or arranging for a good or service, for which payment may be made under a federal healthcare program such as Medicare or Medicaid.
The definition of “remuneration” has been broadly interpreted to include anything of value, including, for example, gifts, certain discounts, the furnishing of free supplies, equipment or services, credit arrangements, payment of cash and waivers of payments. Several courts have interpreted the statute’s intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals of federal healthcare covered businesses, the statute has been violated. Penalties for violations include criminal penalties and civil sanctions such as fines, imprisonment and possible exclusion from Medicare, Medicaid and other federal healthcare programs. In addition some kickback allegations have been claimed to violate the Federal False Claims Act, discussed in more detail below.
The Anti-Kickback Statute is broad and prohibits many arrangements and practices that are otherwise lawful in businesses outside of the healthcare industry. Recognizing that the Anti-Kickback Statute is broad and may technically prohibit many innocuous or beneficial arrangements, Congress authorized the Office of Inspector General (OIG) of the U.S. Department of Health and Human Services to issue a series of regulations known as “safe harbors.” These safe harbors set forth provisions that, if all their applicable requirements are met, will assure healthcare providers and other parties that they will not be prosecuted under the Anti-Kickback Statute. The failure of a transaction or arrangement to fit precisely within one or more safe harbors does not necessarily mean that it is illegal or that prosecution will be pursued.
However, conduct and business arrangements that do not fully satisfy an applicable safe harbor may result in increased scrutiny by government enforcement authorities such as OIG.
Many states have adopted laws similar to the Anti-Kickback Statute. Some of these state prohibitions apply to referral of recipients for healthcare items or services reimbursed by any source, not only the Medicare and Medicaid programs.
Government officials have focused their enforcement efforts on the marketing of healthcare services and products, among other activities, and recently have brought cases against companies, and certain individual sales, marketing and executive personnel, for allegedly offering unlawful inducements to potential or existing customers in an attempt to procure their business.
Federal False Claims Act. Another development affecting the healthcare industry is the increased use of the federal False Claims Act, and in particular, action brought pursuant to the False Claims Act’s “whistleblower” or “qui tam” provisions. The False Claims Act imposes liability on any person or entity that, among other things, knowingly presents, or causes to be presented, a false or fraudulent claim for payment by a federal healthcare program. The qui tam provisions of the False Claims Act allow a private individual to bring actions on behalf of the federal government alleging that the defendant has violated the False Claims Act and to share in any monetary recovery. In recent years, the number of suits brought against healthcare providers by private individuals has increased dramatically. In addition, various states have enacted false claims law analogous to the False Claims Act, and many of these state laws apply where a claim is submitted to any third-party payor and not just a federal healthcare program.
When an entity is determined to have violated the False Claims Act, it may be required to pay up to three times the actual damages sustained by the government, plus civil penalties of between $5,500 and $11,000 for each separate instance of false claim. As part of any settlement, the government may ask the entity to enter into a corporate integrity agreement, which imposes certain compliance, certification and reporting obligations. There are many potential bases for liability under the False Claims Act. Liability arises, primarily, when an entity knowingly submits, or causes another to submit, a false claim for reimbursement to the federal government. The federal government has used the False Claims Act to assert liability on the basis of inadequate care, kickbacks and other improper referrals, and improper use of Medicare numbers when detailing the provider of services, in addition to the more predictable allegations as to misrepresentations with respect to the services rendered. In addition, the federal government has prosecuted companies under the False Claims Act in connection with off-label promotion of products. Our future activities relating to the reporting of wholesale or estimated retail prices of our products, the reporting of discount and rebate information and other information affecting federal, state and third-party reimbursement of our products and the sale and marketing of our products may be subject to scrutiny under these laws.
While we are unaware of any current matters, we are unable to predict whether we will be subject to actions under the False Claims Act or a similar state law, or the impact of such actions. However, the costs of defending such claims, as well as any sanctions imposed, could significantly affect our financial performance.
The Sunshine Act. The Physician Payment Sunshine Act, or the Sunshine Act, which was enacted as part of the Affordable Care Act, requires all entities that operate in the United States and manufacturers of a drug, device, biologic or other medical supply that is covered by Medicare, Medicaid or the Children’s Health Insurance Program to report annually to the Secretary of the Department of Health and Human Services: (i) payments or other transfers of value made by that entity, or by a third-party as directed by that entity, to physicians and teaching hospitals or to third parties on behalf of physicians or teaching hospitals; and (ii) physician ownership and investment interests in the entity. The payments required to be reported include the cost of meals provided to a physician, travel reimbursements and other transfers of value, including those provided as part of contracted services such as speaker programs, advisory boards, consultation services and clinical trial services. The final rule implementing the Sunshine Act requires data collection on payments to begin on August 1, 2013. The first annual report, comprised of data collected from August 1, 2013 to December 31, 2013, was due March 31, 2014. The statute requires the federal government to make reported information available to the public starting September 2014, which it has. Failure to comply with the reporting requirements can result in significant civil monetary penalties ranging from $1,000 to $10,000 for each payment or other transfer of value that is not reported (up to a maximum per annual report of $150,000) and from $10,000 to $100,000 for each knowing failure to report (up to a maximum per annual report of $1.0 million). Additionally, there are criminal penalties if an entity intentionally makes false statements in such reports. We are subject to the Sunshine Act and the information we disclose may lead to greater scrutiny, which may result in modifications to established practices and additional costs. Additionally, similar reporting requirements have also been enacted on the state level domestically, and an increasing number of countries worldwide either have adopted or are considering similar laws requiring transparency of interactions with healthcare professionals.
Foreign Corrupt Practices Act. The Foreign Corrupt Practices Act, or FCPA, prohibits any United States individual or business from paying, offering, or authorizing payment or offering of anything of value, directly or indirectly, to any foreign official, political party or candidate for the purpose of influencing any act or decision of the foreign entity in order to assist the individual or business in obtaining or retaining business. The FCPA also obligates companies whose securities are listed in the United States to comply with accounting provisions requiring us
to maintain books and records that accurately and fairly reflect all transactions of the corporation, including international subsidiaries, if any, and to devise and maintain an adequate system of internal accounting controls for international operations.
International Laws. In Europe various countries have adopted anti-bribery laws providing for severe consequences, in the form of criminal penalties and/or significant fines, for individuals and/or companies committing a bribery offence. Violations of these anti-bribery laws, or allegations of such violations, could have a negative impact on our business, results of operations and reputation. For instance, in the United Kingdom, under the Bribery Act 2010, which went into effect in July 2011, a bribery occurs when a person offers, gives or promises to give a financial or other advantage to induce or reward another individual to improperly perform certain functions or activities, including any function of a public nature. Bribery of foreign public officials also falls within the scope of the Bribery Act 2010. Under the new regime, an individual found in violation of the Bribery Act of 2010, faces imprisonment of up to 10 years. In addition, the individual can be subject to an unlimited fine, as can commercial organizations for failure to prevent bribery.
There are also international privacy laws that impose restrictions on the access, use, and disclosure of health information. All of these laws may impact our business. Our failure to comply with these privacy laws or significant changes in the laws restricting our ability to obtain required patient information could significantly impact our business and our future business plans.
U.S. Healthcare Reform
Changes in healthcare policy could increase our costs and subject us to additional regulatory requirements that may interrupt commercialization of our current and future solutions. Changes in healthcare policy could increase our costs, decrease our revenues and impact sales of and reimbursement for our current and future solutions. The Affordable Care Act substantially changes the way healthcare is financed by both governmental and private insurers, and significantly impacts our industry. The Act contains a number of provisions that impact our business and operations, some of which in ways we cannot currently predict, including those governing enrollment in federal healthcare programs and reimbursement changes.
There will continue to be proposals by legislators at both the federal and state levels, regulators and third-party payors to reduce costs while expanding individual healthcare benefits. Certain of these changes could impose additional limitations on the prices we will be able to charge for our current and future solutions or the amounts of reimbursement available for our current and future solutions from governmental agencies or third-party payors. While in general it is too early to predict specifically what effect the Affordable Care Act and its implementation or any future healthcare reform legislation or policies will have on our business, current and future healthcare reform legislation and policies could have a material adverse effect on our business and financial condition.
Third-Party Reimbursement
Payment for patient care in the United States is generally made by third-party payors, including private insurers and government insurance programs, such as Medicare and Medicaid. The Medicare program, the largest single payor in the United States, is a federal governmental health insurance program administered by the Centers for Medicare and Medicaid Services, or CMS, and covers certain medical care expenses for eligible elderly and disabled individuals. Because a large percentage of the population with PAD includes Medicare beneficiaries, and private insurers may follow the coverage and payment policies of Medicare, Medicare’s coverage and payment policies are significant to our operations.
Medicare pays PAD treatment facilities, including hospitals and physician office-based labs, pre-determined amounts for each procedure performed. These payment amounts differ based on a variety of factors, including:
· Type of procedure performed—angioplasty, stent or atherectomy;
· Patient-specific complexities and comorbidities;
· Type of facility—hospital, teaching hospital or office-based lab;
· Inpatient or outpatient status; and
· Geographic region.
We receive payment from the treatment facility for our products, and the Medicare reimbursement to the facility is intended to cover the overall cost of treatment, including the cost of products used during the procedure as well as the overhead cost associated with the facility where the procedure is performed. For procedures performed in hospitals, the physician who performs the procedure is reimbursed separately under the Medicare physician fee schedule. Claims for PAD procedures are typically submitted by the treatment facility and physician to Medicare or other
health insurers using established billing codes. These codes identify the procedures performed and are relied upon to determine third-party payor reimbursement amounts.
Medicare reimbursement levels for fiscal year 2015 went into effect as of October 1, 2014. National average Medicare payment rates for PAD procedures for fiscal year 2015 are $10,150 - $19,148 for inpatient procedures and, $4,537 - $14,841 for outpatient procedures. These amounts include the cost of disposable catheters such as Ocelot and Pantheris, and additional device-specific reimbursement is not available. The amount of reimbursement can vary substantially by geographical region and by facility. Payment rates of other third-party payors may follow Medicare rates, or they may be higher or lower, depending on their particular reimbursement methodology. Because of the wide variability, it is not possible to identify an average rate for third-party payors other than Medicare.
Employees
As of December 31, 2014, we had 116 employees, including 24 in manufacturing and operations, 42 in sales and marketing, 19 in research and development, 16 in clinical affairs, regulatory affairs, and quality assurance and 15 in finance, general administrative and executive administration. All 116 employees are full time employees. None of our employees are represented by a labor union or are parties to a collective bargaining agreement and we believe that our employee relations are good.
Corporate and other Information
We were incorporated in Delaware on March 8, 2007. Our principal executive offices are located at 400 Chesapeake Drive, Redwood City, California 94063, and our telephone number is (650) 241-7900. Our website address is www.avinger.com . References to our website address do not constitute incorporation by reference of the information contained on the website, and the information contained on the website is not part of this document.
We make available, free of charge on our corporate website, copies of our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, Proxy Statements, and all amendments to these reports, as soon as reasonably practicable after such material is electronically filed with or furnished to the Securities and Exchange Commission pursuant to Section 13(a) or 15(d) of the Securities Exchange Act. We also show detail about stock trading by corporate insiders by providing access to SEC Forms 3, 4 and 5. This information may also be obtained from the SEC’s on-line database, which is located at www.sec.gov. Our common stock is traded on the NASDAQ Stock Market under the symbol “AVGR”.
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012. As such, we are eligible for exemptions from various reporting requirements applicable to other public companies that are not emerging growth companies, including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002 and reduced disclosure obligations regarding executive compensation. We will remain an emerging growth company until the earlier of (1) December 31, 2019, (2) the last day of the fiscal year (a) in which we have total annual gross revenue of at least $1.0 billion or (b) in which we are deemed to be a large accelerated filer, which means the market value of our common stock that is held by non-affiliates exceeds $700 million as of the prior June 30th, and (3) the date on which we have issued more than $1.0 billion in non-convertible debt securities during the prior three-year period.
We have identified the following risks and uncertainties that may have a material adverse effect on our business, financial condition, results of operations and future growth prospects. Our business could be harmed by any of these risks. The risks and uncertainties described below are not the only ones we face. The trading price of our common stock could decline due to any of these risks, and you may lose all or part of your investment. In assessing these risks, you should also refer to the other information contained in this Annual Report on Form 10-K, including our financial statements and related notes. Please also see “Cautionary Notes Regarding Forward-Looking Statements.”
Risks Related to Our Business
Our quarterly and annual results may fluctuate significantly, may not fully reflect the underlying performance of our business and may result in decreases in the price of our common stock.
Our quarterly and annual results of operations, including our revenues, profitability and cash flow, may vary significantly in the future and period-to-period comparisons of our operating results may not be meaningful. Accordingly, the results of any one quarter or period should not be relied upon as an indication of future performance. Our quarterly and annual financial results may fluctuate as a result of a variety of factors, many of which are outside our control and, as a result, may not fully reflect the underlying performance of our business. Fluctuation in quarterly and annual results may decrease the value of our common stock. Factors that may cause fluctuations in our quarterly and annual results include, without limitation:
· our ability to obtain and maintain FDA clearance and approval from foreign regulatory authorities for our products, particularly our Pantheris atherectomy device, which has not yet been approved for marketing;
· market acceptance of our lumivascular platform;
· the availability of reimbursement for our lumivascular platform products;
· our ability to attract new customers and grow our business with existing customers;
· results of our clinical trials, particularly our VISION trial for Pantheris;
· the timing and success of new product and feature introductions by us or our competitors or any other change in the competitive dynamics of our industry, including consolidation among competitors, customers or strategic partners;
· the amount and timing of costs and expenses related to the maintenance and expansion of our business and operations;
· changes in our pricing policies or those of our competitors;
· general economic, industry and market conditions;
· the regulatory environment;
· the hiring, training and retention of key employees, including our ability to expand our sales team;
· litigation or other claims against us;
· our ability to obtain additional financing; and
· advances and trends in new technologies and industry standards.
We have a history of net losses and we may not be able to achieve or sustain profitability.
We have incurred significant losses in each period since our inception in 2007. We incurred net losses of $32.0 million in 2014, $39.9 million in 2013 and $33.9 million in 2012. As of December 31, 2014, we had an accumulated deficit of approximately $146.5 million. These losses and our accumulated deficit reflect the substantial investments we have made to develop our lumivascular platform and acquire customers.
We expect our costs and expenses to increase in the future due to anticipated increases in cost of revenues, sales and marketing expenses, research and development expenses and general and administrative expenses and, therefore, we expect our losses to continue for the foreseeable future as we continue to make significant future expenditures to develop and expand our business. In addition, as a public company, we will incur significant legal, accounting and other expenses that we did not incur as a private company. Accordingly, we cannot assure you that we will achieve profitability in the future or that, if we do become profitable, we will sustain profitability. Our failure to achieve and sustain profitability would negatively impact the market price of our common stock.
Our limited commercialization experience and number of approved products makes it difficult to evaluate our current business, predict our future prospects and forecast our financial performance and growth.
We were incorporated in 2007, began commercializing our initial non-lumivascular platform products in 2009 and introduced our first lumivascular platform products in the United States in late 2012. Our limited commercialization experience and number of approved products make it difficult to evaluate our current business and predict our future prospects. We have encountered and will continue to encounter risks and difficulties frequently experienced by companies in rapidly-changing industries. These risks and uncertainties include the risks inherent in clinical trials and increasing and unforeseen expenses as we continue to attempt to grow our business.
Our short commercialization experience and limited number of approved products also make it difficult for us to forecast our future financial performance and growth and such forecasts are limited and subject to a number of uncertainties, including our ability to successfully complete our VISION clinical trial and obtain FDA clearance for, and successfully commercialize, Pantheris in the United States. If our assumptions regarding the risks and uncertainties we face, which we use to plan our business, are incorrect or change due to circumstances in our business or our markets, or if we do not address these risks successfully, our operating and financial results could differ materially from our expectations and our business could suffer.
Our success depends in large part on our ability to obtain FDA clearance for, and successfully commercialize, Pantheris. This device is still in clinical trials, has been used in only a limited number of procedures and there is no long-term data on its safety and efficacy.
The long-term viability of our company is largely dependent on the successful development and commercialization of Pantheris. In March 2015, we completed enrollment of patients in a clinical study called VISION that will be used to support regulatory clearance of Pantheris, and we do not have significant long term data on Pantheris’ safety and efficacy. While we expect to successfully complete the on-going study and file our 510(k) submission for Pantheris in the second half of 2015 with FDA, there can be no guarantee that the study will be completed, that the primary endpoints will be achieved, or that we will receive regulatory clearance for the sale and marketing of Pantheris in the United States. Although we have collected preliminary data for our VISION trial, this preliminary data may not be predictive of its final results and failure of the trial can occur at any time. A number of companies in the medical device field have suffered significant setbacks during clinical trials due to lack of efficacy or unacceptable safety issues, notwithstanding promising preliminary results. Because we are depending heavily on sales of Pantheris to achieve our revenue goals, failure to successfully complete the study and receive FDA clearance, in a timely manner or at all, will harm our financial results and ability to become profitable. Even if we obtain regulatory clearance, our ability to successfully market this product will be limited due to a number of factors including regulatory restrictions in our labeling. In addition, there can be no guarantee that Pantheris will be accepted by the medical community as a valid alternative to currently available devices. If we cannot sell Pantheris as planned, our financial results will be harmed.
Failure to successfully complete our Pantheris clinical study would significantly impair our financial results. Such a failure could (i) delay or prevent Pantheris from obtaining regulatory clearance, (ii) require us to perform another clinical trial, which will be expensive, may not be successful and will significantly delay our ability to commercialize Pantheris and (iii) impair our ability to convince hospitals and physicians of the benefits of our lumivascular platform products.
We may not be able to secure additional financing on favorable terms, or at all, to meet our future capital needs and our failure to obtain additional financing when needed could force us to delay, reduce or eliminate our product development programs and commercialization efforts.
We believe that the net proceeds from our initial public offering in January of $56.8 million, total cash proceeds of $6.2 million from our Series E preferred stock financing in January 2015 together with our cash and cash equivalents at December 31, 2014 and expected revenues from operations will be sufficient to satisfy our capital requirements and fund our operations for at least 18 months following the date of this Annual Report on Form 10-K. We will likely need additional funds to meet our operational needs and capital requirements for product development, clinical trials and commercialization.
Prior to our initial public offering, we have financed our operations primarily through sales of our products, net proceeds from the issuance of our preferred stock and debt financings. We do not know when or if our operations will generate sufficient cash to fund our ongoing operations. In the future, we may require additional capital in order to (i) continue to conduct research and development activities, (ii) conduct
post-market clinical studies, as well as clinical trials to obtain regulatory clearances and approvals necessary to commercialize our lumivascular platform products, (iii) expand our sales and marketing infrastructure and (iv) acquire complementary business technology or products; or (v) respond to business opportunities, challenges, a decline in sales, increased regulatory obligations or unforeseen circumstances. Our future capital requirements will depend on many factors, including:
· the costs, timing and outcomes of clinical trials and regulatory reviews associated with our future products, including Pantheris;
· the costs and expenses of expanding our sales and marketing infrastructure and our manufacturing operations;
· the costs and timing of developing variations of our lumivascular platform products, especially Pantheris, and, if necessary, obtaining FDA clearance of such variations;
· the degree of success we experience in commercializing our lumivascular platform products, particularly Pantheris;
· the extent to which our lumivascular platform is adopted by hospitals for use by interventional cardiologists, vascular surgeons and interventional radiologists in the treatment of PAD;
· the number and types of future products we develop and commercialize;
· the costs of preparing, filing and prosecuting patent applications and maintaining, enforcing and defending intellectual property-related claims; and
· the extent and scope of our general and administrative expenses.
We may raise funds in equity or debt financings following our initial public offering or enter into credit facilities in order to access funds for our capital needs. Any debt financing obtained by us in the future would cause us to incur additional debt service expenses and could include restrictive covenants relating to our capital raising activities and other financial and operational matters, which may make it more difficult for us to obtain additional capital and pursue business opportunities. If we raise additional funds through further issuances of equity or convertible debt securities, our existing stockholders could suffer significant dilution in their percentage ownership of our company, and any new equity securities we issue could have rights, preferences and privileges senior to those of holders of our common stock. If we are unable to obtain adequate financing or financing on terms satisfactory to us when we require it, we may terminate or delay the development of one or more of our products, delay clinical trials necessary to market our products, or delay establishment of sales and marketing capabilities or other activities necessary to commercialize our products. If this were to occur, our ability to continue to grow and support our business and to respond to business challenges could be significantly limited.
We have a significant amount of debt, which may affect our ability to operate our business and secure additional financing in the future.
As of December 31, 2014, we had $20.4 million in principal and interest outstanding under our credit facility, or the credit agreement, with PDL Biopharma, or PDL, and $8.6 million in principal plus interest outstanding under convertible promissory notes, or the notes. We must make significant annual debt payments under the credit agreement, which diverts resources from other activities. Our debt with PDL is collateralized by substantially all of our assets and contains customary financial and operating covenants limiting our ability to, among other things, dispose of assets, undergo a change in control, merge or consolidate, enter into certain transactions with affiliates, make acquisitions, incur debt, incur liens, pay dividends, repurchase stock and make investments, in each case subject to certain exceptions. In addition to the interest and principal payments due under the credit agreement, we are obligated to pay PDL a royalty at the rate of 1.8% of our quarterly revenues through the maturity date of April 18, 2018. To the extent that we prepay the borrowings under the credit agreement, our royalty obligations will continue and will be payable through the maturity date at the higher of a reduced rate of 0.9% of our quarterly revenues or certain minimum amounts. During this period, we must continue to comply with covenants limiting our ability to, among other things, undergo a change in control and dispose of assets, in each case subject to certain exceptions. These covenants may make it difficult to operate our business. We are also subject to standard event of default provisions both under the credit agreement and the notes that, if triggered, would allow the debt to be accelerated, which could significantly deplete our cash resources, cause us to raise additional capital at unfavorable terms, require us to sell portions of our business or result in us becoming insolvent. The existing collateral pledged under the credit agreement, the covenants to which we are bound and the obligation to pay a certain percentage of our future revenues to PDL, even after the PDL debt is repaid, may prevent us from being able to secure additional debt or equity financing on favorable terms, or at all, or to pursue business opportunities, including potential acquisitions. In addition, as of December 31, 2014, we are obligated to pay our former financial advisor a transaction fee of $487,500.
We depend on a limited number of products, which we only recently introduced in the United States. If these products fail to gain, or lose, market acceptance, our business will suffer.
Ocelot, Ocelot PIXL, Ocelot MVRX, Lightbox, Wildcat and Kittycat 2 are our only products currently cleared for sale, and our current revenues are wholly dependent on them. Sales of Wildcat and Kittycat 2 have declined and are continuing to decline as we focus on the promotion of our lumivascular platform products. We expect that sales of our current and future lumivascular platform products in the United States will account for substantially all of our revenues for the foreseeable future. Because of their recent commercial introduction, our lumivascular platform products have limited product and brand recognition. We do not know if our lumivascular platform products will be successful over the long term and market acceptance may be hindered if physicians are not presented with compelling data from long-term studies of the safety and efficacy of our lumivascular platform products compared to alternative procedures, such as angioplasty, stenting, bypass surgery or other atherectomy procedures. For example, if patients undergoing treatment with our lumivascular platform products have retreatment rates higher than or comparable with the retreatment rates of alternative procedures, it will be difficult to demonstrate the value of our lumivascular platform products. Any studies we may conduct comparing our lumivascular platform with alternative procedures will be expensive, time consuming and may not yield positive results. Physicians will also need to appreciate the value of real-time imaging in improving patient outcomes in order to change current methods for treating PAD patients. In addition, demand for our lumivascular platform products may decline or may not increase as quickly as we expect. Failure of our lumivascular platform products to significantly penetrate current or new markets would harm our business, financial condition and results of operations.
We are also aware of certain characteristics and features of our lumivascular platform that may prevent widespread market adoption. For example, the current model of Pantheris may require two physicians to operate the catheter and a technician to operate the Lightbox, making it less financially attractive for physicians. It may take significant time and expense to modify our products to allow a single physician to operate the entire system and we can provide no guarantee that we will be able to make such modifications, or obtain any additional and necessary regulatory clearances for such modifications. Also, although the OCT images created by our Lightbox may make it possible for physicians to reduce the degree to which fluoroscopy and contrast dye are used when using our lumivascular platform products compared to competing endovascular products, physicians are still using both fluoroscopy and contrast dye, particularly with Pantheris. As a result, risks of complications from radiation and contrast dye are still present and may limit the commercial success of our products. Finally, it will require training for technicians and physicians to effectively operate our lumivascular platform products, including interpreting the OCT images created by our Lightbox, which may affect adoption of our products by physicians. These or other characteristics and features of our lumivascular platform may cause our products not to be widely adopted and harm our business, financial condition and results of operation.
Our ability to compete is highly dependent on demonstrating the benefits of our lumivascular platform to physicians, hospitals and patients.
In order to generate sales, we must be able to clearly demonstrate that our lumivascular platform is both a more effective treatment system and less costly than the alternatives offered by our competitors. If we are unable to convince physicians that our lumivascular platform leads to significantly lower restenosis, or narrowing of the artery, rates and fewer adverse events during surgery than those using competing technologies, our business will suffer. In order to use our Ocelot family of catheters or, if cleared, Pantheris, hospitals must make an investment in our Lightbox. Accordingly, we must convince hospitals and physicians that our lumivascular platform results in significantly better patient outcomes at a competitive overall cost. For example, we may need to demonstrate that the investment hospitals must make when purchasing our Lightbox and the incremental costs of having up to two physicians operate Pantheris can be justified based on the benefits to patients, physicians and hospitals. If we are unable to develop robust clinical data to support these claims we will be unable to convince hospitals and third-party payors of these benefits and our business will suffer.
Our value proposition to physicians and hospitals is largely dependent upon our contention that the rate of disruption of the black line when physicians are using our products is lower than with competing products. If minimizing disruption to the black line does not significantly impact patient outcomes, meaning either (i) that restenosis is often triggered without disrupting the black line, or (ii) the black line can often be disrupted without triggering restenosis, then we may be unable to demonstrate our lumivascular platform’s benefits are any different than competing technologies. Furthermore, physicians may find our imaging system difficult to use and we may not provide physicians with adequate training to be able to realize the benefits of our lumivascular platform. If physicians do not value the benefits of on-board imaging and the enhanced visualization enabled by our products during an endovascular intervention as compared to our competitor’s products, or do not believe that such benefits improve clinical outcomes, our lumivascular platform products may not be widely adopted.
The use, misuse or off-label use of the products in our lumivascular platform may result in injuries that lead to product liability suits, which could be costly to our business.
We require limited training in the use of our lumivascular platform products because we market primarily to physicians who are experienced in the interventional techniques required to use our device. If demand for our lumivascular platform continues to grow, less experienced physicians will likely use the device, potentially leading to more injury and an increased risk of product liability claims. The use or
misuse of our lumivascular platform products has in the past resulted, and may in the future result, in complications, including damage to the treated artery, infection, internal bleeding, and limb loss, potentially leading to product liability claims. Our lumivascular platform products are not FDA-cleared or approved for use in the carotid, cerebral, coronary, iliac, or renal arteries. Our sales force does not promote the use of our products for off-label indications, and our U.S. instructions for use specify that our lumivascular platform products are not intended for use in the carotid, cerebral, coronary, iliac or renal arteries. However, we cannot prevent a physician from using our lumivascular platform products for these off-label applications. The application of our lumivascular platform products to coronary arteries, as opposed to peripheral arteries, is more likely to result in complications that have serious consequences. For example, if excised plaque were not captured properly in our device, it could be carried by the bloodstream to a more narrow location, blocking a coronary artery, leading to a heart attack, or blocking an artery to the brain, leading to a stroke. If our lumivascular platform products are defectively designed, manufactured or labeled, contain defective components or are misused, we may become subject to costly litigation initiated by our customers or their patients. Product liability claims are especially prevalent in the medical device industry and could harm our reputation, divert management’s attention from our core business, be expensive to defend and may result in sizable damage awards against us. Although we maintain product liability insurance, the amount or breadth of our coverage may not be adequate for the claims that are made against us.
The expense and potential unavailability of insurance coverage for liabilities resulting from our products could harm us and our ability to sell our lumivascular platform products.
We may not have sufficient insurance coverage for future product liability claims. We may not be able to obtain insurance in amounts or scope sufficient to provide us with adequate coverage against all potential liabilities. Any product liability claims brought against us, with or without merit, could increase our product liability insurance rates or prevent us from securing continuing coverage, harm our reputation in the industry, significantly increase our expenses, and reduce product sales. Product liability claims in excess of our insurance coverage would be paid out of cash reserves, harming our financial condition and operating results.
Some of our customers and prospective customers may have difficulty in procuring or maintaining liability insurance to cover their operations and use of our lumivascular platform products. Medical malpractice carriers are withdrawing coverage in certain states or substantially increasing premiums. If this trend continues or worsens, our customers may discontinue using our lumivascular platform products and potential customers may opt against purchasing our lumivascular platform products due to the cost or inability to procure insurance coverage.
Our ability to compete depends on our ability to innovate successfully.
The market for medical devices in general, and in the PAD market in particular, is highly competitive, dynamic, and marked by rapid and substantial technological development and product innovation. There are few barriers that would prevent new entrants or existing competitors from developing products that compete directly with ours. Demand for our lumivascular platform products could be diminished by equivalent or superior products and technologies offered by competitors. If we are unable to innovate successfully, our lumivascular platform products could become obsolete and our revenues would decline as our customers purchase our competitors’ products.
The medical device market is characterized by extensive research and development and rapid technological change. Technological progress or new developments in our industry could harm sales of our products. Our products could be rendered obsolete because of future innovations in the treatment of PAD. In order to remain competitive, we must continue to develop new product offerings and enhancements to our existing lumivascular platform products. Maintaining adequate research and development personnel and resources to meet the demands of the market is essential. If we are unable to develop products, applications or features due to certain constraints, such as insufficient cash resources, inability to raise sufficient cash in future equity or debt financings, high employee turnover, inability to hire sufficient research and development personnel or a lack of other research and development resources, we may miss market opportunities. Furthermore, many of our competitors expend a considerably greater amount of funds on their research and development programs than we do, and those that do not may be acquired by larger companies that would allocate greater resources to our competitors’ research and development programs. Our failure or inability to devote adequate research and development resources or compete effectively with the research and development programs of our competitors could harm our business.
We compete against companies that have longer operating histories, more established products and greater resources, which may prevent us from achieving significant market penetration, increasing our revenues or becoming profitable.
Our products compete with a variety of products and devices for the treatment of PAD, including other CTO crossing devices, stents, balloons and atherectomy catheters, as well as products used in vascular surgery. Large competitors in the CTO crossing, stent and balloon markets include Abbott Laboratories, BARD, Boston Scientific, Cook Medical, Covidien, Johnson & Johnson and Medtronic. Competitors in the atherectomy market include Boston Scientific, Cardiovascular Systems, Covidien, Spectranetics and Volcano. Some competitors have previously attempted to combine intravascular imaging with atherectomy and may have current programs underway to do so. These and other companies may attempt to incorporate on-board visualization into their products in the future and may remain competitive with us in marketing traditional technologies. Other competitors include pharmaceutical companies that manufacture drugs for the treatment of symptoms associated with mild to moderate PAD and companies that provide products used by surgeons in peripheral and coronary bypass procedures. These competitors and other
companies may introduce new products that compete with our products. Many of our competitors have significantly greater financial and other resources than we do and have well-established reputations, as well as broader product offerings and worldwide distribution channels that are significantly larger and more effective than ours. Competition with these companies could result in price-cutting, reduced profit margins and loss of market share, any of which would harm our business, financial condition and results of operations.
Our ability to compete effectively depends on our ability to distinguish our company and our lumivascular platform from our competitors and their products, and includes such factors as:
· procedural safety and efficacy;
· acute and long-term outcomes;
· ease of use and procedure time;
· price;
· size and effectiveness of sales force;
· radiation exposure for physicians, hospital staff and patients; and
· third-party reimbursement.
In addition, competitors with greater financial resources than ours could acquire other companies to gain enhanced name recognition and market share, as well as new technologies or products that could effectively compete with our existing products, which may cause our revenues to decline and would harm our business.
If our clinical trials are unsuccessful or significantly delayed, or if we do not complete our clinical trials, our business may be harmed.
Clinical development is a long, expensive, and uncertain process and is subject to delays and the risk that products may ultimately prove unsafe or ineffective in treating the indications for which they are designed. Completion of clinical trials may take several years or more. We cannot provide any assurance that our clinical trials will meet their primary endpoints or that such trials or their results will be accepted by the FDA or foreign regulatory authorities. Even if we achieve positive early or preliminary results in clinical trials, these results do not necessarily predict final results, and positive results in early trials may not indicate success in later trials. Many companies in the medical device industry have suffered significant setbacks in late-stage clinical trials, even after receiving promising results in earlier trials or in the preliminary results from these late-stage clinical trials.
We may experience numerous unforeseen events during, or because of, the clinical trial process that could delay or prevent us from receiving regulatory clearance or approval for new products or modifications of existing products, including new indications for existing products, including:
· our clinical trials may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional clinical and/or preclinical testing which may be expensive and time consuming;
· trial results may not meet the level of statistical significance required by FDA or other regulatory authorities;
· FDA or similar foreign regulatory authorities may find the product is not sufficiently safe for investigational use in humans;
· FDA or similar foreign regulatory authorities may interpret data from preclinical testing and clinical trials in different ways than we do;
· there may be delays or failure in obtaining approval of our clinical trial protocols from FDA or other regulatory authorities;
· there may be delays in obtaining institutional review board approvals or government approvals to conduct clinical trials at prospective sites;
· FDA or similar foreign regulatory authorities may find our or our suppliers’ manufacturing processes or facilities unsatisfactory;
· FDA or similar foreign regulatory authorities may change their review policies or adopt new regulations that may negatively affect or delay our ability to bring a product to market or receive approvals or clearances to treat new indications;
· we may have trouble in managing multiple clinical sites;
· we may experience delays in agreeing on acceptable terms with third-party research organizations and trial sites that may help us conduct the clinical trials; and
· we, or regulators, may suspend or terminate our clinical trials because the participating patients are being exposed to unacceptable health risks.
Failures or perceived failures in our clinical trials will delay and may prevent our product development and regulatory approval process, damage our business prospects and negatively affect our reputation and competitive position.
From time to time, we engage outside parties to perform services related to certain of our clinical studies and trials, and any failure of those parties to fulfill their obligations could increase costs and cause delays.
From time to time, we engage consultants to help design, monitor, and analyze the results of certain of our clinical studies and trials. The consultants we engage interact with clinical investigators to enroll patients in our clinical trials. We depend on these consultants and clinical investigators to help facilitate the clinical studies and trials and monitor and analyze data from these studies and trials under the investigational plan and protocol for the study or trial and in compliance with applicable regulations and standards, commonly referred to as good clinical practices. We may face delays in our regulatory approval process if these parties do not perform their obligations in a timely, compliant or competent manner. If these third parties do not successfully carry out their duties or meet expected deadlines, or if the quality, completeness or accuracy of the data they obtain is compromised due to the failure to adhere to our clinical trial protocols or for other reasons, our clinical studies or trials may be extended, delayed or terminated or may otherwise prove to be unsuccessful, and we may have to conduct additional studies, which would significantly increase our costs, in order to obtain the regulatory clearances that we need to commercialize our products.
We have no long-term data regarding the safety and efficacy of our lumivascular platform products. Any long-term data that is generated by clinical trials involving our lumivascular platform may not be positive or consistent with our short-term data, which would harm our ability to obtain clearance to market and sell our products.
Our lumivascular platform is a novel system, and our success depends on its acceptance by the medical community as being safe and effective, and improving clinical outcomes. Important factors upon which the efficacy of our lumivascular platform products will be measured are long-term data on the rate of restenosis following our procedure, and the corresponding duration of patency, or openness of the artery, and publication of that data in peer-reviewed journals. Another important factor that physicians will consider is the rate of reintervention, or retreatment, following the use of our lumivascular platform products. The long-term clinical benefits of procedures that use our lumivascular platform products are not known.
The results of short-term clinical experience of our lumivascular platform products do not necessarily predict long-term clinical benefit. Restenosis rates typically increase over time. We believe that physicians will compare the rates of long-term restenosis and reintervention for procedures using our lumivascular platform products against alternative procedures, such as angioplasty, stenting, bypass surgery and other atherectomy procedures. If the long-term rates of restenosis and reintervention do not meet physicians’ expectations, our lumivascular platform products may not become widely adopted and physicians may recommend alternative treatments for their patients. Another significant factor that physicians will consider is acute safety data on complications that occur during the use of our lumivascular platform products. If the results obtained from our VISION trial or any post-market studies that we conduct or post-clearance surveillance indicate that the use of our lumivascular platform products are not as safe or effective as other treatment options or as current short-term data would suggest, adoption of our product may suffer and our business would be harmed. Even if we believe the data collected from clinical studies or clinical experience indicate positive results, each physician’s actual experience with our products will vary. Physicians who are technically proficient participate in our clinical trials and are high-volume users of our lumivascular platform products. Consequently, the results of our clinical trials and their experiences using our products may lead to better patient outcomes than those of physicians that are less proficient, perform fewer procedures or who use our products infrequently.
Our ability to market our current products in the United States is limited to use in peripheral vessels, and if we want to market our products for other uses, we will need to file for FDA clearances or approvals and may need to conduct trials in addition to VISION to support expanded use, which would be expensive, time-consuming and may not be successful.
Our current products are cleared in the United States solely for crossing sub-total and chronic total occlusions in the peripheral vasculature. This clearance prohibits our ability to market or advertise our products for any other indication within the peripheral vasculature, which restricts our ability to sell these products and could affect our growth. Additionally, our products are contraindicated for use in the cerebral, carotid, coronary, iliac, and renal arteries. While off-label uses of medical devices are common and FDA does not regulate physicians’ choice of treatments, FDA does restrict a manufacturer’s communications regarding such off-label use. We are not allowed to actively promote or advertise our products for off-label uses. In addition, we cannot make comparative claims regarding the use of our products against any alternative treatments without conducting head-to-head comparative clinical studies, which would be expensive and time consuming. If our promotional activities fail to comply with FDA’s regulations or guidelines, we may be subject to FDA warnings or enforcement action by FDA and other
government agencies. In the future, if we want to market a variation of Ocelot or Pantheris in the United States for use in coronary arteries, we will need to make modifications to these products, conduct further clinical trials and obtain new clearances or approvals from FDA. There can be no assurance that we will successfully develop these modifications, that future clinical studies will be successful or that the expense of these activities will be offset by additional revenues.
The continuing development of many of our products, including Pantheris, depends upon maintaining strong working relationships with physicians.
The development, marketing, and sale of our products, including Pantheris, depends upon our ability to maintain strong working relationships with physicians. We rely on these professionals to provide us with considerable knowledge and experience regarding the development, marketing and sale of our products. Physicians assist us in clinical trials and as researchers, marketing and product consultants and public speakers. If we cannot maintain our strong working relationships with these professionals and continue to receive their advice and input, the development and marketing of our products could suffer, which could harm our business, financial condition and results of operations. The medical device industry’s relationship with physicians is under increasing scrutiny by the Health and Human Services Office of Inspector General, or OIG, the Department of Justice, or DOJ, state attorneys general, and other foreign and domestic government agencies. Our failure to comply with laws, rules and regulations governing our relationships with physicians, or an investigation into our compliance by the OIG, DOJ, state attorneys general and other government agencies, could significantly harm our business.
If we fail to grow our sales and marketing capabilities and develop widespread brand awareness cost effectively, our growth will be impeded and our business may suffer.
We plan to continue to expand and optimize our sales infrastructure in order to grow our customer base and our business. Identifying and recruiting qualified personnel and training them in the use of our lumivascular platform, and on applicable federal and state laws and regulations and our internal policies and procedures, requires significant time, expense and attention. It can take several months before our sales representatives are fully trained and productive. Our business may be harmed if our efforts to expand and train our sales force do not generate a corresponding increase in revenues. In particular, if we are unable to hire, develop and retain talented sales personnel or if new sales personnel are unable to achieve desired productivity levels in a reasonable period of time, we may not be able to realize the expected benefits of this investment or increase our revenues.
Our ability to increase our customer base and achieve broader market acceptance of our lumivascular platform will depend to a significant extent on our ability to expand our marketing operations. We plan to dedicate significant financial and other resources to our marketing programs. Our business will be harmed if our marketing efforts and expenditures do not generate an increase in revenue.
In addition, we believe that developing and maintaining widespread awareness of our brand in a cost-effective manner is critical to achieving widespread acceptance of our lumivascular platform and attracting new customers. Brand promotion activities may not generate customer awareness or increase revenues, and even if they do, any increase in revenues may not offset the costs and expenses we incur in building our brand. If we fail to successfully promote, maintain and protect our brand, we may fail to attract or retain the customers necessary to realize a sufficient return on our brand-building efforts, or to achieve the widespread brand awareness that is critical for broad customer adoption of our lumivascular platform.
If we are unable to manage the anticipated growth of our business, our future revenues and operating results may be harmed.
Any growth that we experience in the future could provide challenges to our organization, requiring us to expand our sales personnel and manufacturing operations and general and administrative infrastructure. We expect to grow our sales force in anticipation of obtaining marketing clearance for Pantheris. Rapid expansion in personnel could mean that less experienced people produce and sell our products, which could result in inefficiencies and unanticipated costs and disruptions to our operations.
We have limited experience manufacturing our lumivascular platform products in commercial quantities, which could harm our business.
Because we have only limited experience in manufacturing our lumivascular platform products in commercial quantities, we may encounter production delays or shortfalls. Such production delays or shortfalls may be caused by many factors, including the following:
· we intend to significantly expand our manufacturing capacity, and our production processes may have to change to accommodate this growth;
· key components and sub-assemblies of our lumivascular platform products are currently provided by a single supplier or limited number of suppliers, and we do not maintain large inventory levels of these components and sub-assemblies; if we experience a shortage in any of these components or sub-assemblies, we would need to identify and qualify new supply sources, which could increase our expenses and result in manufacturing delays;
· we may experience a delay in completing validation and verification testing for new controlled-environment rooms at our manufacturing facilities;
· we have limited experience in complying with FDA’s Quality System Regulation, which applies to the manufacture of our lumivascular platform products; and
· to increase our manufacturing output significantly, we will have to attract and retain qualified employees, who are in short supply, for our manufacturing operations.
If we are unable to keep up with demand for our lumivascular platform products, our revenues could be impaired, market acceptance for our lumivascular platform products could be harmed and our customers might instead purchase our competitors’ products. Our inability to successfully manufacture our lumivascular platform products would materially harm our business.
Our manufacturing facilities and processes and those of our third-party suppliers are subject to unannounced FDA and state regulatory inspections for compliance with Quality System regulations. Developing and maintaining a compliant quality system is time consuming and expensive. Failure to maintain, or not fully comply with the requirements of, a quality system could result in regulatory authorities initiating enforcement actions against us and our third-party suppliers, which could include the issuance of warning letters, seizures, prohibitions on product sales, recalls and civil and criminal penalties, any one of which could significantly impact our manufacturing supply and impair our financial results.
If our manufacturing facility becomes damaged or inoperable, or we are required to vacate the facility, our ability to manufacture and sell our lumivascular platform products and to pursue our research and development efforts may be jeopardized.
We currently manufacture and assemble our lumivascular platform products in-house. Our products are comprised of components sourced from a variety of contract manufacturers, with final assembly completed at our facility in Redwood City, California. Our facility and equipment, or those of our suppliers, could be harmed or rendered inoperable by natural or man-made disasters, including fire, earthquake, terrorism, flooding and power outages. Any of these may render it difficult or impossible for us to manufacture products for some period of time. If our facility is inoperable for even a short period of time, the inability to manufacture our current products, and the interruption in research and development of any future products, may result in harm to our reputation, increased costs, lower revenues and the loss of customers. Furthermore, it could be costly and time-consuming to repair or replace our facilities and the equipment we use to perform our research and development work and manufacture our products.
We depend on third-party vendors to manufacture some of our components and sub-assemblies, which could make us vulnerable to supply shortages and price fluctuations that could harm our business.
We currently manufacture some of our components and sub-assemblies at our Redwood City facility and rely on third-party vendors for other components and sub-assemblies used in our lumivascular platform. Our reliance on third-party vendors subjects us to a number of risks that could impact our ability to manufacture our products and harm our business, including:
· interruption of supply resulting from modifications to, or discontinuation of, a supplier’s operations;
· delays in product shipments resulting from uncorrected defects, reliability issues or a supplier’s failure to consistently produce quality components;
· price fluctuations due to a lack of long-term supply arrangements with our suppliers for key components;
· inability to obtain adequate supply in a timely manner or on commercially reasonable terms;
· difficulty identifying and qualifying alternative suppliers for components in a timely manner;
· inability of the manufacturer or supplier to comply with Quality System regulations enforced by the FDA and state regulatory authorities;
· inability to control the quality of products manufactured by third parties;
· production delays related to the evaluation and testing of products from alternative suppliers and corresponding regulatory qualifications; and
· delays in delivery by our suppliers due to changes in demand from us or their other customers.
Any significant delay or interruption in the supply of components or sub-assemblies, or our inability to obtain substitute components, sub-assemblies or materials from alternate sources at acceptable prices in a timely manner, could impair our ability to meet the demand of our customers and harm our business.
We depend on single and limited source suppliers for some of our product components and sub-assemblies, and if any of those suppliers are unable or unwilling to produce these components and sub-assemblies or supply them in the quantities that we need, we would experience manufacturing delays.
We rely on single and limited source suppliers for several of our components and sub-assemblies. For example, we rely on single vendors for our optical fiber and drive cables, that are key components of our catheters, and we rely on a single vendor for our data acquisition card in Lightbox. These components are critical to our products and there are relatively few alternative sources of supply. We do not carry a significant inventory of these components. Identifying and qualifying additional or replacement suppliers for any of the components or sub-assemblies used in our products could involve significant time and cost. Any supply interruption from our vendors or failure to obtain additional vendors for any of the components or sub-assemblies incorporated into our products would limit our ability to manufacture our products and could therefore harm our business, financial condition and results of operations.
Our future growth depends on physician adoption of our lumivascular platform products, which may require physicians to change their current practices.
We intend to educate physicians on the capabilities of our lumivascular platform products and advances in treatment for PAD patients. We target our sales efforts to interventional cardiologists, vascular surgeons and interventional radiologists because they are often the physicians diagnosing and treating both coronary artery disease and PAD. However, the initial point of contact for many patients may be general practitioners, podiatrists, nephrologists and endocrinologists, each of whom commonly treat patients experiencing complications or symptoms resulting from PAD. If these physicians are not made aware of our lumivascular platform products, they may not refer patients to interventional cardiologists, vascular surgeons and interventional radiologists for treatment using our lumivascular platform procedure, and those patients may instead be surgically treated or treated with an alternative interventional procedure. In addition, there is a significant correlation between PAD and coronary artery disease, and many physicians do not routinely screen for PAD while screening for coronary artery disease. If we are not successful in educating physicians about screening for PAD and about the capabilities of our lumivascular platform products, our ability to increase our revenues may be impaired.
We depend on our senior management team and the loss of one or more key employees or an inability to attract and retain highly skilled employees could harm our business.
Our success largely depends upon the continued services of our executive management team and key employees and the loss of one or more of our executive officers or key employees could harm us and directly impact our financial results. Our employees may terminate their employment with us at any time. Changes in our executive management team resulting from the hiring or departure of executives, including the recent addition of our chief executive officer, could disrupt our business. In particular, our founder, Dr. John Simpson, is the visionary behind many of our product development activities and he actively supports our clinical trials and physician education and training efforts. If Dr. Simpson was no longer working at our company, our industry credibility, product development efforts and physician relationships would be harmed. We do not currently maintain key person life insurance policies on any of our employees, including Dr. Simpson.
To execute our growth plan, we must attract and retain highly qualified personnel. Competition for skilled personnel is intense, especially for engineers with high levels of experience in designing and developing medical devices and for sales executives. We have, from time to time, experienced, and we expect to continue to experience, difficulty in hiring and retaining employees with appropriate qualifications. Many of the companies with which we compete for experienced personnel have greater resources than we have. If we hire employees from competitors or other companies, their former employers may attempt to assert that these employees or we have breached legal obligations, resulting in a diversion of our time and resources and, potentially, damages. In addition, job candidates and existing employees, particularly in the San Francisco Bay Area, often consider the value of the stock awards they receive in connection with their employment. If the perceived value of our stock awards declines, it may harm our ability to recruit and retain highly skilled employees. In addition, we invest significant time and expense in training our employees, which increases their value to competitors who may seek to recruit them. If we fail to attract new personnel or fail to retain and motivate our current personnel, our business and future growth prospects would be harmed.
We do not currently intend to devote significant additional resources in the near-term to market our lumivascular platform internationally, which will limit our potential revenues from our lumivascular platform products.
Marketing our lumivascular platform outside of the United States would require substantial additional sales and marketing, regulatory and personnel expenses. As part of our product development and regulatory strategy, we plan to expand into select European markets, but we do not currently intend to devote significant additional resources to market our lumivascular platform internationally in order to focus our resources
and efforts on the U.S. market. Our decision to market our products primarily in the United States in the near-term will limit our ability to reach all of our potential markets and will limit our potential sources of revenue. In addition, our competitors will have an opportunity to further penetrate and achieve market share outside of the United States until such time, if ever, that we devote significant additional resources to market our lumivascular platform products or other products internationally.
Our ability to utilize our net operating loss carryovers may be limited.
As of December 31, 2014, we had federal and state net operating loss carryforwards, or NOLs, due to prior period losses of $133.9 million and $134.2 million, respectively, which if not utilized will begin to expire in 2027 for federal purposes and 2015 for state purposes. We may use these NOLs to offset against taxable income for U.S. federal income tax purposes. However, Section 382 of the Internal Revenue Code of 1986, as amended, may limit the NOLs we may use in any year for U.S. federal income tax purposes in the event of certain changes in ownership of our company. A Section 382 “ownership change” generally occurs if one or more stockholders or groups of stockholders who own at least 5% of our stock increase their ownership by more than 50 percentage points over their lowest ownership percentage within a rolling three-year period. Similar rules may apply under state tax laws. This offering or future issuances or sales of our stock (including certain transactions involving our stock that are outside of our control) could cause an “ownership change.” If an “ownership change” occurs, Section 382 would impose an annual limit on the amount of pre-ownership change NOLs and other tax attributes we can use to reduce our taxable income, potentially increasing and accelerating our liability for income taxes, and also potentially causing those tax attributes to expire unused. Any limitation on using NOLs could (depending on the extent of such limitation and the NOLs previously used) result in our retaining less cash after payment of U.S. federal income taxes during any year in which we have taxable income (rather than losses) than we would be entitled to retain if such NOLs were available as an offset against such income for U.S. federal income tax reporting purposes, which could harm our profitability.
The forecasts of market growth included in this Annual Report on Form-10K may prove to be inaccurate, and even if the markets in which we compete achieve the forecasted growth, our business may not grow at similar rates, if at all.
Growth forecasts are subject to significant uncertainty and are based on assumptions and estimates that may not prove to be accurate. The forecasts in this Annual Report on Form 10-K relating to, among other things, the expected growth in PAD prevalence, diagnosis and endovascular PAD procedures and the markets therefor and increased awareness, higher diagnosis, and intervention rates, may prove to be inaccurate.
Even if these markets experience the forecasted growth described in this Annual Report on Form 10-K, we may not grow our business at similar rates, or at all. Our growth is subject to many factors, including whether the market for PAD treatments continues to grow, our ability to successfully complete the VISION trial, obtain 510(k) clearance for Pantheris and commercialize Pantheris, the rate of market acceptance of our lumivascular platform products versus the products of our competitors and our success in implementing our business strategies, each of which is subject to many risks and uncertainties. Accordingly, the forecasts of market growth included in this Annual Report on Form 10-K should not be taken as indicative of our future growth.
We may acquire other companies or technologies, which could divert our management’s attention, result in additional dilution to our stockholders and otherwise disrupt our operations and harm our operating results.
We may in the future seek to acquire or invest in businesses, applications or technologies that we believe could complement or expand our lumivascular platform, enhance our technical capabilities or otherwise offer growth opportunities. The pursuit of potential acquisitions may divert the attention of management and cause us to incur various costs and expenses in identifying, investigating and pursuing suitable acquisitions, whether or not they are consummated. We may not be able to identify desirable acquisition targets or be successful in entering into an agreement with any particular target or obtain the expected benefits of any acquisition or investment.
To date, the growth in our business has been organic, and we have no experience in acquiring other businesses. In any acquisition, we may not be able to successfully integrate acquired personnel, operations and technologies, or effectively manage the combined business following the acquisition. Acquisitions could also result in dilutive issuances of equity securities, the use of our available cash, or the incurrence of debt, which could harm our operating results. In addition, if an acquired business fails to meet our expectations, our operating results, business and financial condition may suffer.
Risks Related to Our Intellectual Property
We may in the future be a party to intellectual property litigation or administrative proceedings that could be costly and could interfere with our ability to sell our lumivascular platform products.
The medical device industry has been characterized by extensive litigation regarding patents, trademarks, trade secrets, and other intellectual property rights, and companies in the industry have used intellectual property litigation to gain a competitive advantage. It is possible
that U.S. and foreign patents and pending patent applications or trademarks controlled by third parties may be alleged to cover our products, or that we may be accused of misappropriating third parties’ trade secrets. Additionally, our products include hardware and software components that we purchase from vendors, and may include design components that are outside of our direct control. Our competitors, many of which have substantially greater resources and have made substantial investments in patent portfolios, trade secrets, trademarks, and competing technologies, may have applied for or obtained or may in the future apply for or obtain, patents or trademarks that will prevent, limit or otherwise interfere with our ability to make, use, sell and/or export our products or to use product names. We may become a party to patent or trademark infringement or trade secret claims and litigation as a result of these and other third-party intellectual property rights being asserted against us. The defense and prosecution of these matters are both costly and time consuming. Vendors from whom we purchase hardware or software may not indemnify us in the event that such hardware or software is accused of infringing a third-party’s patent or trademark or of misappropriating a third-party’s trade secret.
Further, if such patents, trademarks, or trade secrets are successfully asserted against us, this may harm our business and result in injunctions preventing us from selling our products, license fees, damages and the payment of attorney fees and court costs. In addition, if we are found to willfully infringe third-party patents or trademarks or to have misappropriated trade secrets, we could be required to pay treble damages in addition to other penalties. Although patent, trademark, trade secret, and other intellectual property disputes in the medical device area have often been settled through licensing or similar arrangements, costs associated with such arrangements may be substantial and could include ongoing royalties. We may be unable to obtain necessary licenses on satisfactory terms, if at all. If we do not obtain necessary licenses, we may not be able to redesign our lumivascular platform products to avoid infringement.
Similarly, interference or derivation proceedings provoked by third parties or brought by the U.S. Patent and Trademark Office, or USPTO, may be necessary to determine the priority of inventions or other matters of inventorship with respect to our patents or patent applications. We may also become involved in other proceedings, such as re-examination, inter partes review, or opposition proceedings, before the USPTO or other jurisdictional body relating to our intellectual property rights or the intellectual property rights of others. Adverse determinations in a judicial or administrative proceeding or failure to obtain necessary licenses could prevent us from manufacturing and selling our lumivascular platform products or using product names, which would have a significant adverse impact on our business.
Additionally, we may need to commence proceedings against others to enforce our patents or trademarks, to protect our trade secrets or know-how, or to determine the enforceability, scope and validity of the proprietary rights of others. These proceedings would result in substantial expense to us and significant diversion of effort by our technical and management personnel. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. We may not be able to stop a competitor from marketing and selling products that are the same or similar to our products or from using product names that are the same or similar to our product names, and our business may be harmed as a result.
We are aware of patents held by third parties that may be asserted against us in litigation that could be costly and could limit our ability to sell our lumivascular platform products.
We are aware of patent families related to catheter positioning, optical coherence tomography, occlusion cutting and atherectomy owned by third parties. With regard to atherectomy patents, one of our founders, Dr. John Simpson, founded FoxHollow Technologies prior to founding our company. FoxHollow Technologies developed an atherectomy device that is currently sold by Covidien, and Dr. Simpson and our Chief Technology Officer, Himanshu Patel, are listed as inventors on patents covering that device that are now held by Covidien. We are not currently aware of any claims Covidien has made or intends to make against us with respect to Pantheris or any other product or product under development. Because of a doctrine known as “assignor estoppel,” if any of Dr. Simpson’s earlier patents are asserted against us by Covidien, we may be prevented from asserting an invalidity defense regarding those patents, and our defense may be compromised. Covidien has significantly greater financial resources than we do to pursue patent litigation and could assert these patent families against us at any time. Adverse determinations in any such litigation could prevent us from manufacturing or selling Pantheris or other products or products under development, which would significantly harm our business.
Intellectual property rights may not provide adequate protection, which may permit third parties to compete against us more effectively.
In order to remain competitive, we must develop and maintain protection of the proprietary aspects of our technologies. We rely on a combination of patents, copyrights, trademarks, trade secret laws and confidentiality and invention assignment agreements to protect our intellectual property rights. As of December 31, 2014, we held five issued U.S. patents and had 14 U.S. utility patent applications and 8 PCT applications pending. As of December 31, 2014, we also had one issued patent from the Japan Patent Office, one issued patent from the Chinese patent office, and one European patent which has been nationalized in Germany, France, Great Britain, Italy and Ireland. As of December 31, 2014, we had 29 pending patent applications outside of the United States, including in Australia, Canada, Europe, India and Japan. Our patents and patent applications include claims covering key aspects of the design, manufacture and therapeutic use of OCT imaging catheters, occlusion-crossing catheters, atherectomy devices and our imaging console. Our patent applications may not result in issued patents and our
patents may not be sufficiently broad to protect our technology. Any patents issued to us may be challenged by third parties as being invalid, or third parties may independently develop similar or competing technology that avoids our patents. Should such challenges be successful, competitors might be able to market products and use manufacturing processes that are substantially similar to ours. We may not be able to prevent the unauthorized disclosure or use of our technical knowledge or other trade secrets by consultants, vendors or former or current employees, despite the existence generally of confidentiality agreements and other contractual restrictions. Monitoring unauthorized use and disclosure of our intellectual property is difficult, and we do not know whether the steps we have taken to protect our intellectual property will be adequate. In addition, the laws of many foreign countries will not protect our intellectual property rights to the same extent as the laws of the United States. Consequently, we may be unable to prevent our proprietary technology from being exploited abroad, which could affect our ability to expand to international markets or require costly efforts to protect our technology. To the extent our intellectual property protection is incomplete, we are exposed to a greater risk of direct competition. In addition, competitors could purchase our products and attempt to replicate some or all of the competitive advantages we derive from our development efforts or design around our protected technology. Our failure to secure, protect and enforce our intellectual property rights could substantially harm the value of our lumivascular platform, brand and business.
We use certain open source software in Lightbox. We may face claims from companies that incorporate open source software into their products or from open source licensors, claiming ownership of, or demanding release of, the source code, the open source software or derivative works that were developed using such software, or otherwise seeking to enforce the terms of the applicable open source license. These claims could result in litigation and could require us to cease offering Lightbox unless and until we can re-engineer it to avoid infringement. This re-engineering process could require significant additional research and development resources, and we may not be able to complete it successfully. These risks could be difficult to eliminate or manage, and, if not addressed, could harm our business, financial condition and operating results.
Risks Related to Government Regulation
Failure to comply with laws and regulations could harm our business.
Our business is subject to regulation by various federal, state, local and foreign governmental agencies, including agencies responsible for monitoring and enforcing employment and labor laws, workplace safety, environmental laws, consumer protection laws, anti-bribery laws, import/export controls, federal securities laws and tax laws and regulations. In certain jurisdictions, these regulatory requirements may be more stringent than those in the United States and in other circumstances these requirements may be more stringent in the United States. Noncompliance with applicable regulations or requirements could subject us to investigations, sanctions, mandatory recalls, enforcement actions, adverse publicity, disgorgement of profits, fines, damages, civil and criminal penalties or injunctions and administrative actions. If any governmental sanctions, fines or penalties are imposed, or if we do not prevail in any possible civil or criminal litigation, our business, operating results and financial condition could be harmed. In addition, responding to any action will likely result in a significant diversion of management’s attention and resources and substantial costs. Enforcement actions and sanctions could further harm our business, operating results and financial condition.
If we fail to obtain and maintain necessary regulatory clearances or approvals for our lumivascular platform products, or if clearances or approvals for future products and indications are delayed or not issued, our commercial operations would be harmed.
Our lumivascular platform products are medical devices that are subject to extensive regulation by FDA in the United States and by regulatory agencies in other countries where we do business. Government regulations specific to medical devices are wide-ranging and govern, among other things:
· product design, development and manufacture;
· laboratory, preclinical and clinical testing, labeling, packaging storage and distribution;
· premarketing clearance or approval;
· record keeping;
· product marketing, promotion and advertising, sales and distribution; and
· post-marketing surveillance, including reporting of deaths or serious injuries and recalls and correction and removals.
Before a new medical device, or a new intended use for, an existing product can be marketed in the United States, a company must first submit and receive either 510(k) clearance or premarketing approval from FDA, unless an exemption applies. Either process can be expensive, lengthy and unpredictable. We may not be able to obtain the necessary clearances or approvals or may be unduly delayed in doing so,
which could harm our business. Furthermore, even if we are granted regulatory clearances or approvals, they may include significant limitations on the indicated uses for the product, which may limit the market for the product. Although we have obtained 510(k) clearance to market our Ocelot family of catheters for crossing sub and total occlusions in the peripheral vasculature, our clearance can be revoked if safety or efficacy problems develop. To market Pantheris in the United States, we must successfully complete a clinical trial, submit an application to FDA for 510(k) clearance and obtain such clearance. Therefore, even if we believe we have successfully developed Pantheris, we may not be permitted to market Pantheris in the United States if we do not obtain FDA regulatory clearance to market the product. Delays in obtaining clearance or approval could increase our costs and harm our revenues and growth.
In addition, we are required to timely file various reports with the FDA, including reports required by the medical device reporting regulations, or MDRs, that require that we report to the regulatory authorities if our devices may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if the malfunction were to recur. If these reports are not filed timely, regulators may impose sanctions and sales of our products may suffer, and we may be subject to product liability or regulatory enforcement actions, all of which could harm our business. For example, we have submitted to the FDA five MDRs regarding our Ocelot family of catheters, which included four for perforations and one related to removal of the guidewire coating.
If we initiate a correction or removal for one of our devices to reduce a risk to health posed by the device, we would be required to submit a publically available Correction and Removal report to the FDA and in many cases, similar reports to other regulatory agencies. This report could be classified by the FDA as a device recall which could lead to increased scrutiny by the FDA, other international regulatory agencies and our customers regarding the quality and safety of our devices. Furthermore, the submission of these reports has been and could be used by competitors against us in competitive situations and cause customers to delay purchase decisions or cancel orders and would harm our reputation.
The FDA and the Federal Trade Commission, or FTC, also regulate the advertising and promotion of our products to ensure that the claims we make are consistent with our regulatory clearances, that there are adequate and reasonable data to substantiate the claims and that our promotional labeling and advertising is neither false nor misleading in any respect. If the FDA or FTC determines that any of our advertising or promotional claims are misleading, not substantiated or not permissible, we may be subject to enforcement actions, including Warning Letters, and we may be required to revise our promotional claims and make other corrections or restitutions.
FDA and state authorities have broad enforcement powers. Our failure to comply with applicable regulatory requirements could result in enforcement action by FDA or state agencies, which may include any of the following sanctions:
· adverse publicity, warning letters, fines, injunctions, consent decrees and civil penalties;
· repair, replacement, refunds, recall or seizure of our products;
· operating restrictions, partial suspension or total shutdown of production;
· refusing our requests for 510(k) clearance or premarket approval of new products, new intended uses or modifications to existing products;
· withdrawing 510(k) clearance or premarket approvals that have already been granted; and
· criminal prosecution.
If any of these events were to occur, our business and financial condition would be harmed.
Material modifications to our lumivascular platform products may require new 510(k) clearances or premarket approvals or may require us to recall or cease marketing our lumivascular platform products until clearances are obtained.
Material modifications to the intended use or technological characteristics of our lumivascular platform products will require new 510(k) clearances or premarket approvals or require us to recall or cease marketing the modified devices until these clearances or approvals are obtained. Based on FDA published guidelines, FDA requires device manufacturers to initially make and document a determination of whether or not a modification requires a new approval, supplement or clearance; however, FDA can review a manufacturer’s decision. Any modification to an FDA-cleared device that would significantly affect its safety or efficacy or that would constitute a major change in its intended use would require a new 510(k) clearance or possibly a premarket approval. We may not be able to obtain additional 510(k) clearances or premarket approvals for new products or for modifications to, or additional indications for, our lumivascular platform products in a timely fashion, or at all. Delays in obtaining required future clearances would harm our ability to introduce new or enhanced products in a timely manner, which in turn would harm our future growth. We have made modifications to our lumivascular platform products in the past and will make additional modifications in the future that we believe do not or will not require additional clearances or approvals. If FDA disagrees and requires new clearances or approvals for the modifications, we may be required to recall and to stop selling or marketing our lumivascular platform products as modified, which could harm our operating results and require us to redesign our lumivascular platform products. In these circumstances, we may be subject to significant enforcement actions. We have made minor modifications and may make further modifications to the design of Pantheris prior to widespread commercialization, which could require regulatory clearances or approvals.
If we or our suppliers fail to comply with FDA’s Quality System Regulation, our manufacturing operations could be delayed or shut down and lumivascular platform sales could suffer.
Our manufacturing processes and those of our third-party suppliers are required to comply with FDA’s Quality System Regulation, which covers the procedures and documentation of the design, testing, production, control, quality assurance, labeling, packaging, storage and shipping of our lumivascular platform products. We are also subject to similar state requirements and licenses. In addition, we must engage in extensive recordkeeping and reporting and must make available our manufacturing facilities and records for periodic unannounced inspections by governmental agencies, including FDA, state authorities and comparable agencies in other countries. If we fail a Quality System inspection, our operations could be disrupted and our manufacturing interrupted. Failure to take adequate corrective action in response to an adverse Quality System inspection could result in, among other things, a shut-down of our manufacturing operations, significant fines, suspension of marketing clearances and approvals, seizures or recalls of our device, operating restrictions and criminal prosecutions, any of which would cause our business to suffer. Furthermore, our key component suppliers may not currently be or may not continue to be in compliance with applicable regulatory requirements, which may result in manufacturing delays for our product and cause our revenues to decline.
We have registered with FDA as a medical device manufacturer and have obtained a manufacturing license from the California Department of Health Services, or CDHS. FDA has broad post-market and regulatory enforcement powers. We are subject to unannounced inspections by FDA and the Food and Drug Branch of CDHS to determine our compliance with the QSR and other regulations, and these inspections may include the manufacturing facilities of our suppliers. Our current facility has been inspected by FDA in 2009, 2011 and 2013, and two, three and zero observations, respectively, were noted during those inspections. BSI, our European Notified Body, inspected our facility in 2013 and found zero non-conformances. We can provide no assurance that we will continue to remain in compliance with the QSR. If FDA, CDHS or BSI inspect our facility and discover compliance problems, we may have to shut down our facility and cease manufacturing until we can take the appropriate remedial steps to correct the audit findings. Taking corrective action may be expensive, time consuming and a distraction for management and if we experience a shutdown or delay at our manufacturing facility we may be unable to produce our lumivascular platform products, which would harm our business.
Our lumivascular platform products may in the future be subject to product recalls that could harm our reputation.
FDA and similar governmental authorities in other countries have the authority to require the recall of commercialized products in the event of material regulatory deficiencies or defects in design or manufacture. A government mandated or voluntary recall by us could occur as a result of component failures, manufacturing errors or design or labeling defects. Recalls of our lumivascular platform products would divert managerial attention, be expensive, harm our reputation with customers and harm our financial condition and results of operations. A recall announcement would negatively affect our stock price.
Changes in coverage and reimbursement for procedures using our lumivascular platform products could affect the adoption of our lumivascular platform and our future revenues.
Currently, our lumivascular platform procedure is typically reimbursed by third-party payors, including Medicare and private healthcare insurance companies, under existing reimbursement codes. These payors may change their coverage and reimbursement policies, as well as payment amounts, in a way that would prevent or limit reimbursement for our products, which would significantly harm our business. Also, healthcare reform legislation or regulation may be proposed or enacted in the future, which may adversely affect such policies and amounts. We cannot predict whether and to what extent existing coverage and reimbursement will continue to be available. If physicians, hospitals and other providers are unable to obtain adequate coverage and reimbursement for procedures performed using our lumivascular platform products, they are significantly less likely to use our lumivascular platform products and our business would be harmed.
Healthcare reform measures could hinder or prevent our planned products’ commercial success.
In the United States, there have been, and we expect there will continue to be, a number of legislative and regulatory changes to the healthcare system in ways that could harm our future revenues and profitability and the future revenues and profitability of our potential customers. Federal and state lawmakers regularly propose and, at times, enact legislation that would result in significant changes to the healthcare system, some of which are intended to contain or reduce the costs of medical products and services. For example, one of the most significant healthcare reform measures in decades, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act, or Affordable Care Act, was enacted in 2010. The Affordable Care Act contains a number of provisions, including those governing enrollment in federal healthcare programs, reimbursement changes and fraud and abuse measures, all of which will impact existing government healthcare programs and will result in the development of new programs. The Affordable Care Act, among other things, imposes an excise tax of 2.3% on the sale of most medical devices, including ours, and any failure to pay this amount could result in the imposition of an injunction on the sale of our products, fines and penalties.
It remains unclear whether changes will be made to the Affordable Care Act. We cannot assure you that the Affordable Care Act, as currently enacted or as amended in the future, will not harm our business and financial results and we cannot predict how future federal or state legislative or administrative changes relating to healthcare reform will affect our business.
There likely will continue to be legislative and regulatory proposals at the federal and state levels directed at containing or lowering the cost of health care. We cannot predict the initiatives that may be adopted in the future or their full impact. The continuing efforts of the government, insurance companies, managed care organizations and other payors of healthcare services to contain or reduce costs of health care may harm:
· our ability to set a price that we believe is fair for our products;
· our ability to generate revenues and achieve or maintain profitability; and
· the availability of capital.
If we fail to comply with healthcare regulations, we could face substantial penalties and our business, operations and financial condition could be adversely affected.
Even though we do not and will not control referrals of healthcare services or bill directly to Medicare, Medicaid or other third-party payors, certain federal and state healthcare laws and regulations pertaining to fraud and abuse and patients’ rights are and will be applicable to our business. We could be subject to healthcare fraud and abuse and patient privacy regulation by both the federal government and the states in which we conduct our business. The regulations that will affect how we operate include:
· the federal healthcare program Anti-Kickback Statute, which prohibits, among other things, any person from knowingly and willfully offering, soliciting, receiving or providing remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual for, or the purchase, order or recommendation of, any good or service for which payment may be made under federal healthcare programs, such as the Medicare and Medicaid programs;
· the federal False Claims Act, which prohibits, among other things, individuals or entities from knowingly presenting, or causing to be presented, false claims, or knowingly using false statements, to obtain payment from the federal government;
· federal criminal laws that prohibit executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters;
· the federal Physician Payment Sunshine Act, created under the Affordable Care Act, and its implementing regulations, which require manufacturers of drugs, medical devices, biologicals and medical supplies for which payment is available under Medicare, Medicaid, or the Children’s Health Insurance Program to report annually to the U.S. Department of Health and Human Services, or HHS, information related to payments or other transfers of value made to physicians and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members;
· HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, which governs the conduct of certain electronic healthcare transactions and protects the security and privacy of protected health information; and
· state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws which may apply to items or services reimbursed by any third-party payor, including commercial insurers.
The Affordable Care Act, among other things, amends the intent requirement of the Federal Anti-Kickback Statute and criminal healthcare fraud statutes. A person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it. In addition, the Affordable Care Act provides that the government may assert that a claim including items or services resulting from a violation of the Federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the False Claims Act.
Efforts to ensure that our business arrangements will comply with applicable healthcare laws may involve substantial costs. It is possible that governmental and enforcement authorities will conclude that our business practices do not comply with current or future statutes, regulations or case law interpreting applicable fraud and abuse or other healthcare laws and regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of civil, criminal and administrative penalties, damages, disgorgement, monetary fines, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, contractual damages, reputational harm, diminished profits and future earnings, and curtailment of our operations, any of which could harm our ability to operate our business and our results of operations. In addition, the clearance or approval and commercialization of any of our products outside the United States will also likely subject us to foreign equivalents of the healthcare laws mentioned above, among other foreign laws.
Compliance with environmental laws and regulations could be expensive. Failure to comply with environmental laws and regulations could subject us to significant liability.
Our research and development and manufacturing operations involve the use of hazardous substances and are subject to a variety of federal, state, local and foreign environmental laws and regulations relating to the storage, use, discharge, disposal, remediation of, and human exposure to, hazardous substances and the sale, labeling, collection, recycling, treatment and disposal of products containing hazardous substances. In addition, our research and development and manufacturing operations produce biological waste materials, such as human and animal tissue, and waste solvents, such as isopropyl alcohol. These operations are permitted by regulatory authorities, and the resultant waste materials are disposed of in material compliance with environmental laws and regulations. Liability under environmental laws and regulations can be joint and several and without regard to fault or negligence. Compliance with environmental laws and regulations may be expensive and non-compliance could result in substantial liabilities, fines and penalties, personal injury and third part property damage claims and substantial investigation and remediation costs. Environmental laws and regulations could become more stringent over time, imposing greater compliance costs and increasing risks and penalties associated with violations. We cannot assure you that violations of these laws and regulations will not occur in the future or have not occurred in the past as a result of human error, accidents, equipment failure or other causes. The expense associated with environmental regulation and remediation could harm our financial condition and operating results.
Risks related to ownership of our common stock
Our stock price may be volatile, and purchasers of our common stock could incur substantial losses.
Our stock price has fluctuated since our initial public offering and is likely to continue to fluctuate substantially. As a result of this price fluctuation, investors may experience losses on their investments in our stock. In addition, the development stage of our operations may make it difficult for investors to evaluate the success of our business to date and to assess our future viability. The market price for our common stock may be influenced by many factors, including:
· the results of our clinical trials, including our VISION trial;
· changes in analysts’ estimates, investors’ perceptions, recommendations by securities analysts or our failure to achieve analysts’ estimates;
· quarterly variations in our or our competitors’ results of operations;
· the financial projections we may provide to the public, any changes in these projections or our failure to meet these projections;
· general market conditions and other factors unrelated to our operating performance or the operating performance of our competitors;
· changes in operating performance and stock market valuations of other technology companies generally, or those in the medical device industry in particular;
· the loss of key personnel, including changes in our board of directors and management;
· legislation or regulation of our business;
· lawsuits threatened or filed against us;
· the announcement of new products or product enhancements by us or our competitors;
· announcements related to patents issued to us or our competitors and to litigation; and
· developments in our industry.
In addition, the stock prices of many companies in the medical device industry have experienced wide fluctuations that have often been unrelated to the operating performance of those companies. In the past, stockholders have instituted securities class action litigation following periods of market volatility. If we were to become involved in securities litigation, it could subject us to substantial costs, divert resources and the attention of management from our business and harm our business, results of operations, financial condition, reputation and cash flows. These factors may materially and adversely affect the market price of our common stock.
If securities or industry analysts do not publish research or reports about our business, or publish negative reports about our business, our share price and trading volume could decline.
The trading market for our common stock will depend in part on the research and reports that securities or industry analysts publish about us or our business, our market and our competitors. We do not have any control over these analysts. If one or more of the analysts who cover
us downgrade our shares or change their opinion of our shares, our share price would likely decline. If one or more of these analysts cease coverage of our company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which could cause our share price or trading volume to decline. If our operating results fail to meet the forecast of analysts, our stock price will likely decline.
Sales of a substantial number of shares of our common stock in the public market by our existing stockholders could cause our stock price to fall.
Sales of a substantial number of shares of our common stock in the public market, or the perception that these sales might occur, could depress the market price of our common stock and could impair our ability to raise capital through the sale of additional equity securities. We recently completed our initial public offering and a substantial number of shares will become available for sale six months thereafter. We are unable to predict the effect that these sales and others may have on the prevailing market price of our common stock.
Our directors, officers and principal stockholders have significant voting power and may take actions that may not be in the best interests of our other stockholders.
As of February 28, 2015, our directors, officers and each stockholder holding more than 5% of our common stock collectively control approximately 35.7% of our outstanding common stock, assuming the exercise of all options and warrants held by such persons. As a result, these stockholders, if they act together, would be able to exert significant influence over the management and affairs of our company and most matters requiring stockholder approval, including the election of directors and approval of significant corporate transactions. This concentration of ownership may have the effect of delaying or preventing a change in control, might adversely affect the market price of our common stock and may not be in the best interests of our other stockholders.
We previously identified and remediated a material weakness in our internal control over financial reporting. We may identify additional material weaknesses in the future that may cause us to fail to meet our reporting obligations or result in material misstatements of our financial statements. If we fail to remediate any material weaknesses or if we fail to establish and maintain effective control over financial reporting, our ability to accurately and timely report our financial results could be adversely affected.
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements in accordance with US generally accepted accounting principles. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of annual or interim financial statements will not be prevented or detected on a timely basis.
Prior to the completion of our initial public offering, we were a private company with limited accounting personnel and other resources to address our internal control over financial reporting. During the course of preparing for our initial public offering, we determined that we had a material weakness in our internal control over financial reporting as of December 31, 2013 and 2012. The material weakness we identified related to not maintaining sufficient compliment of resources with an appropriate level of accounting knowledge, experience and training commensurate with our structure and financial reporting requirements.
For a discussion of our remediation and the actions that we have executed during 2014 to remediate the material weakness see “Item 9A. Controls and Procedures — Changes in Internal Control over Financial Reporting” The actions we have taken are subject to continued review, supported by confirmation and testing by management as well as audit committee oversight. While we have remediated this weakness, we cannot assure you that additional material weaknesses or significant deficiencies in our internal control over financial reporting will not be identified in the future. Any failure to maintain or implement required new or improved controls, or any difficulties we encounter in their implementation, could result in additional material weaknesses or significant deficiencies, cause us to fail to meet our periodic reporting obligations or result in material misstatements in our financial statements. Any such failure could also adversely affect the results of periodic management evaluations regarding the effectiveness of our internal control over financial reporting. The existence of a material weakness or significant deficiency could result in errors in our financial statements that could result in a restatement of financial statements, cause us to fail to meet our reporting obligations and cause investors to lose confidence in our reported financial information, leading to a decline in our stock price.
The requirements of being a public company may strain our resources, divert management’s attention and affect our ability to attract and retain executive management and qualified board members.
As a public company, we are subject to the reporting requirements of the Securities Exchange Act of 1934, as amended, or the Exchange Act, the Sarbanes-Oxley Act, the Dodd-Frank Act, the listing requirements of The NASDAQ Stock Market and other applicable securities laws, rules and regulations. Compliance with these laws, rules and regulations have increased our legal and financial compliance costs and will make some activities more difficult, time-consuming or costly and increase demand on our systems and resources, particularly after we are no longer an “emerging growth company.” The Exchange Act requires, among other things, that we file annual, quarterly and current reports with respect to our business and operating results. The Sarbanes-Oxley Act requires, among other things, that we maintain effective disclosure controls and procedures and internal control over financial reporting. In order to maintain and, if required, improve our disclosure controls and procedures and
internal control over financial reporting to meet this standard, significant resources and management oversight may be required. Our management and other personnel now need to devote a substantial amount of time to these compliance initiatives. As a result, management’s attention may be diverted from other business concerns and our costs and expenses will increase, which could harm our business and operating results. We may need to hire more employees in the future or engage outside consultants to comply with these requirements, which will increase our costs and expenses.
In addition, changing laws, regulations and standards relating to corporate governance and public disclosure are creating uncertainty for public companies, increasing legal and financial compliance costs and making some activities more time consuming. These laws, regulations and standards are subject to varying interpretations, in many cases due to their lack of specificity and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices. We intend to invest resources to comply with evolving laws, regulations and standards, and this investment may result in increased general and administrative expenses and a diversion of management’s time and attention from revenue-generating activities to compliance activities. If our efforts to comply with new laws, regulations and standards differ from the activities intended by regulatory or governing bodies due to ambiguities related to their application and practice, regulatory authorities may initiate legal proceedings against us and our business may be harmed.
We will incur additional compensation costs in the event that we decide to pay our executive officers cash compensation closer to that of executive officers of other public medical device companies, which would increase our general and administrative expense and could harm our profitability. Any future equity awards will also increase our compensation expense. We also expect that being a public company and compliance with applicable rules and regulations will make it more expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced coverage or incur substantially higher costs to obtain coverage. These factors could also make it more difficult for us to attract and retain qualified executive officers and members of our board of directors, particularly to serve on our audit committee and compensation committee.
As a result of disclosure of information in this Annual Report on Form 10-K and in filings required of a public company, our business and financial condition will become more visible, which could be advantageous to our competitors and clients and could result in threatened or actual litigation, including by competitors and other third parties. If such claims are successful, our business and operating results could be harmed, and even if the claims are resolved in our favor, these claims, and the time and resources necessary to resolve them, could divert the resources of our management and harm our business and operating results.
We are an emerging growth company and a smaller reporting company and we cannot be certain if the reduced disclosure requirements applicable to emerging growth companies and smaller reporting companies will make our common stock less attractive to investors.
We are an emerging growth company and a smaller reporting company. For as long as we continue to be an emerging growth company, we may take advantage of certain exemptions from reporting requirements that are applicable to other public companies including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. We will remain eligible for these exemptions, other than with respect to stockholder approval of golden parachute payments, after we are no longer an emerging growth company for as long as we remain a smaller reporting company. We cannot predict if investors will find our common stock less attractive because we will rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile or decline.
We will remain an emerging growth company until the earlier of (1) the last day of the fiscal year (a) following the fifth anniversary of our initial public offering, (b) in which we have total annual gross revenue of at least $1.0 billion, or (c) in which we are deemed to be a large accelerated filer, which means the market value of our common stock that is held by non- affiliates exceeds $700 million as of the prior June 30th, and (2) the date on which we have issued more than $1.0 billion in non-convertible debt securities during the prior three-year period. We cannot predict if investors will find our common stock less attractive because we may rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may suffer or be more volatile.
Anti-takeover provisions in our amended and restated certificate of incorporation and bylaws and Delaware law could discourage a takeover.
Our amended and restated certificate of incorporation and bylaws, as amended and restated, contain provisions that might enable our management to resist a takeover. These provisions include:
· a classified board of directors;
· advance notice requirements applicable to stockholders for matters to be brought before a meeting of stockholders and requirements as to the form and content of a stockholder’s notice;
· a supermajority stockholder vote requirement for amending certain provisions of our amended and restated certificate of incorporation and bylaws;
· the right to issue preferred stock without stockholder approval, which could be used to dilute the stock ownership of a potential hostile acquirer;
· allowing stockholders to remove directors only for cause;
· a requirement that the authorized number of directors may be changed only by resolution of the board of directors;
· allowing all vacancies, including newly created directorships, to be filled by the affirmative vote of a majority of directors then in office, even if less than a quorum, except as otherwise required by law;
· a requirement that our stockholders may only take action at annual or special meetings of our stockholders and not by written consent;
· limiting the forum for certain litigation against us to Delaware; and
· limiting the persons that can call special meetings of our stockholders to our board of directors, the chairperson of our board of directors, the chief executive officer or the president (in the absence of a chief executive officer).
These provisions might discourage, delay or prevent a change in control of our company or a change in our management. The existence of these provisions could adversely affect the voting power of holders of common stock and limit the price that investors might be willing to pay in the future for shares of our common stock. In addition, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which generally prohibits a Delaware corporation from engaging in any of a broad range of business combinations with any “interested” stockholder for a period of three years following the date on which the stockholder became an “interested” stockholder.
Our amended and restated certificate of incorporation will provide that the Court of Chancery of the State of Delaware will be the sole and exclusive forum for substantially all disputes between us and our stockholders, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers or employees.
Our amended and restated certificate of incorporation provides that, unless we consent to the selection of an alternative forum, the Court of Chancery of the State of Delaware is the sole and exclusive forum for (i) any derivative action or proceeding brought on our behalf, (ii) any action asserting a claim of breach of fiduciary duty owed by any of our directors, officers or other employees to us or to our stockholders, (iii) any action asserting a claim arising pursuant to the Delaware General Corporation Law or our certificate of incorporation or bylaws (iv) any action to interpret apply, enforce or determine the validity of our certificate of incorporation or bylaws or (v) any action asserting a claim governed by the internal affairs doctrine. The choice of forum provision may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers or other employees, which may discourage such lawsuits against us and our directors, officers and other employees. Alternatively, if a court were to find the choice of forum provision contained in our amended and restated certificate of incorporation to be inapplicable or unenforceable in an action, we may incur additional costs associated with resolving such action in other jurisdictions, which could harm our business, operating results and financial condition.
We have not paid dividends in the past and do not expect to pay dividends in the future, and any return on investment may be limited to the value of our stock.
We have never paid cash dividends and do not anticipate paying cash dividends in the foreseeable future. The payment of dividends will depend on our earnings, capital requirements, financial condition, prospects and other factors our board of directors may deem relevant. In addition, our credit agreement prohibits us from, among other things, paying any dividends or making any other distribution or payment on account of our common stock. If we do not pay dividends, our stock may be less valuable because a return on your investment will only occur if you sell our common stock after our stock price appreciates.
An active trading market for our common stock may not be maintained.
Our stock is currently approved for quotation on the NASDAQ Stock Market, but we can provide no assurance that we will be able to maintain an active trading market on NASDAQ or any other exchange in the future. If an active market for our common stock is not maintained, it may be difficult for our stockholders to sell shares without depressing the market price for the shares or at all.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
We maintain our principal executive offices, comprising 44,200 square feet in two buildings in Redwood City, California, under a lease agreement that expires in November 2016. We have the option to extend the lease through November 2022. Our facility houses our research and development, sales, marketing, manufacturing, finance and administrative activities. We believe that our current facilities are adequate for our current and anticipated future needs through 2016.
We are not currently a party to any material legal proceedings. From time to time we may be involved in legal proceedings or investigations, which could have an adverse impact on our reputation, business and financial condition and divert the attention of our management from the operation of our business.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
MARKET INFORMATION FOR COMMON STOCK
Our common stock began trading on The NASDAQ Global Market on January 30, 2015 and trades under the symbol “AVGR”. Prior to January 30, 2015, there was no public market for our common stock. As a result we have not set forth quarterly information with respect to the high and low prices for our common stock for the two most recent fiscal years or provided a performance graph. On March 26, 2015, the last reported sale price of our common stock as reported on The NASDAQ Global Market was $11.02 per share.
HOLDERS OF RECORD
As of March 26, 2015, there were 12,228,260 shares of our common stock held by 345 holders of record of our common stock. The actual number of stockholders is greater than this number of record holders, and includes stockholders who are beneficial owners, but whose shares are held in street name by brokers and other nominees. This number of holders of record also does not include stockholders whose shares may be held in trust by other entities.
DIVIDEND POLICY
We have never declared or paid, and do not anticipate declaring or paying, any cash dividends on any of our capital stock. We do not anticipate paying any dividends in the foreseeable future, and we currently intend to retain all available funds and any future earnings for use in the operation of our business and to finance the growth and development of our business. Future determination as to the declaration and payment of dividends, if any, will be at the discretion of our board of directors and will depend on then existing conditions, including our operating results, financial condition, contractual restrictions, capital requirements, business prospects and other factors our board of directors may deem relevant. Our credit agreement prohibits us from paying any dividends or making any other distribution or payment on account of our common stock.
SECURITIES AUTHORIZED FOR ISSUANCE UNDER EQUITY COMPENSATION PLANS
See Note 12 of the financial statements herein regarding information about securities authorized for issuance under our equity compensation plans.
RECENT SALES OF UNREGISTERED SECURITIES
We have issued and sold the following securities from January 1, 2014 to December 31, 2014:
1. From January 2014 through December 2014 we granted options to purchase 2,720,174 shares of our common stock with exercise prices ranging from $4.50 to $20.25 per share. From January 2014 through November 2014, 2,568 options were exercised with exercise prices ranging from $4.95 to $20.25 per share.
2. From May 2014 through July 2014, we issued convertible promissory notes to 15 accredited investors in an aggregate principal amount of $4,719,950 with an interest rate of the 30-day LIBOR plus 6%. In connection therewith, we issued warrants to purchase 56,181 shares of our common stock at an exercise price of $12.60.
3. From September 2014 through December 2014 we issued and sold to 76 accredited investors an aggregate of 2,617,883 shares of Series E preferred stock (convertible into an aggregate of 2,617,883 shares of common stock) at a purchase price per share of $12.60 or, for purchasers who were investing by converting their promissory notes, at a price per share of $10.71. In connection therewith, we issued warrants to purchase an aggregate of 1,308,918 shares of our common stock at an exercise price of $12.60 per share.
4. From August 2014 through December 2014, we granted options to purchase 59,230 shares of our Series E preferred stock at $12.60 per share, 53,744 of which were exercised at $12.60 per share. In connection therewith, we issued warrants to purchase an aggregate of 26,861 shares of our common stock at an exercise price of $12.60 per share.
The sales of the above securities were deemed to be exempt from registration under the Securities Act with respect to items 2 and 3 above in reliance on Section 4(2) of the Securities Act, or Regulation D promulgated thereunder, with respect to item 4 above in reliance on Rule 701 promulgated under Section 3(b) of the Securities Act as transactions by an issuer not involving a public offering or transactions pursuant to compensatory benefit plans and contracts relating to compensation as provided under such Rule 701 and with respect to item 1 above in reliance on both section 4(2) of the Securities Act and Rule 701 promulgated under section 3(b) of the Securities Act. The recipients of securities in each such transaction represented their intention to acquire the securities for investment only and not with a view to or for sale in connection with any
distribution thereof and appropriate legends were affixed to the share certificates and warrants issued in such transactions. All recipients had adequate access, through their relationships with us, to information about us.
USE OF PROCEEDS FROM PUBLIC OFFERING OF COMMON STOCK
Our initial public offering of 5,000,000 shares of common stock was effected through a registration statement on Form S-1 (File No. 333-201322), which was declared effective on January 29, 2015. Our initial public offering closed on February 4, 2015 and resulted in net proceeds of approximately $56.8 million, after deducting underwriting discounts and commissions of approximately $4.5 million and other expenses of approximately $3.7 million. No payments for such expenses were made directly or indirectly to any of our officers or directors.
Canaccord Genuity Inc., Cowen and Company, LLC, Oppenheimer & Co. Inc., BTIG, LLC and Stephens Inc. acted as the underwriters. There has been no material change in the planned use of proceeds from our initial public offering as described in our final prospectus filed with the SEC on January 30, 2015 pursuant to Rule 424(b) of the Securities Act.
PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS
None.
ITEM 6. SELECTED FINANCIAL DATA
You should read the following selected financial data together with the section of this Annual Report on Form 10-K entitled “Management’s discussion and analysis of financial condition and results of operations” and our financial statements and the related notes included in this Annual Report on Form 10-K. The statement of operations data for the years ended December 31, 2014, 2013 and 2012 and the balance sheet data as of December 31, 2014 and 2013 are derived from our audited financial statements included elsewhere in this Annual Report on Form 10-K. We have included, in our opinion, all adjustments, consisting only of normal recurring adjustments that we consider necessary for a fair presentation of the financial information set forth in those statements. Our historical results are not necessarily indicative of the results to be expected in the future or any other period.
Statements of Operations Data:
|
|
|
Year Ended |
| |||||||
|
|
|
December 31, |
| |||||||
|
|
|
2014 |
|
2013 |
|
2012 |
| |||
|
|
|
(in thousands, except per share data) |
| |||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenues |
|
$ |
11,213 |
|
$ |
12,964 |
|
$ |
8,560 |
|
|
Cost of revenues |
|
6,513 |
|
8,205 |
|
4,151 |
| |||
|
Gross profit |
|
4,700 |
|
4,759 |
|
4,409 |
| |||
|
|
|
|
|
|
|
|
| |||
|
Operating expenses: |
|
|
|
|
|
|
| |||
|
Research and development |
|
11,224 |
|
15,973 |
|
15,416 |
| |||
|
Selling, general and administrative |
|
18,503 |
|
25,758 |
|
22,848 |
| |||
|
Total operating expenses |
|
29,727 |
|
41,731 |
|
38,264 |
| |||
|
Loss from operations |
|
(25,027 |
) |
(36,972 |
) |
(33,855 |
) | |||
|
|
|
|
|
|
|
|
| |||
|
Interest income (expense), net |
|
(6,014 |
) |
(2,923 |
) |
19 |
| |||
|
Other income (expense), net |
|
(909 |
) |
5 |
|
(19 |
) | |||
|
Loss before provision for income taxes |
|
(31,950 |
) |
(39,890 |
) |
(33,855 |
) | |||
|
Provision for income taxes |
|
14 |
|
11 |
|
9 |
| |||
|
Net loss and comprehensive loss |
|
$ |
(31,964 |
) |
$ |
(39,901 |
) |
$ |
(33,864 |
) |
|
|
|
|
|
|
|
|
| |||
|
Net loss per share, basic and diluted |
|
$ |
(132.63 |
) |
$ |
(170.52 |
) |
$ |
(162.03 |
) |
|
|
|
|
|
|
|
|
| |||
|
Weighted average common shares used to compute net loss per share, basic and diluted |
|
241 |
|
234 |
|
209 |
| |||
Balance Sheets Data:
|
|
|
As of December 31, |
| ||||
|
|
|
2014 |
|
2013 |
| ||
|
|
|
(in thousands) |
| ||||
|
Cash and cash equivalents |
|
$ |
12,316 |
|
$ |
12,221 |
|
|
Working capital |
|
10,054 |
|
15,874 |
| ||
|
Total assets |
|
24,780 |
|
25,008 |
| ||
|
Long-term borrowings |
|
18,537 |
|
20,052 |
| ||
|
Convertible notes and accrued interest |
|
8,643 |
|
13,731 |
| ||
|
Convertible preferred stock |
|
132,260 |
|
99,654 |
| ||
|
Accumulated deficit |
|
(146,533 |
) |
(114,569 |
) | ||
|
Total stockholders’ deficit |
|
(143,868 |
) |
(112,782 |
) | ||
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations together with the section of this Annual Report on Form 10-K entitled “Selected financial data” and our financial statements and related notes included elsewhere in this Annual Report on Form 10-K. This discussion and other parts of this Annual Report on Form 10-K contain forward-looking statements that involve risks and uncertainties, such as statements of our plans, objectives, expectations and intentions. Our actual results could differ materially from those discussed in these forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those discussed in the section of this Annual Report on Form 10-K entitled “Risk factors.”
Overview
We are a commercial-stage medical device company that designs, manufactures and sells image-guided, catheter-based systems that are used by physicians to treat patients with peripheral arterial disease, or PAD. Patients with PAD have a build-up of plaque in the arteries that supply blood to the arms and legs. Our mission is to dramatically improve the treatment of vascular disease through the introduction of products based on our lumivascular platform, the only intravascular image-guided system available in this market. We manufacture and sell a suite of products in the United States and select European markets. Our current products include our Lightbox imaging console, as well as our Wildcat, Kittycat, and the Ocelot family of catheters, which are designed to allow physicians to penetrate a total blockage in an artery, known as a chronic total occlusion, or CTO. We are also developing Pantheris, our image-guided atherectomy device, designed to allow physicians to precisely remove arterial plaque in PAD patients. Pantheris is currently undergoing a U.S. clinical trial intended to support a 510(k) submission in the second half of 2015 to the U.S. Food and Drug Administration, or FDA. We believe that Pantheris, if cleared by FDA, will significantly enhance our market opportunity within PAD and can expand the overall addressable market for PAD endovascular procedures.
During the first quarter of 2015, we completed enrollment of patients in VISION, a clinical trial designed to support a filing with FDA for our Pantheris atherectomy device. VISION is designed to evaluate the safety and efficacy of Pantheris to perform atherectomy using intravascular imaging. We believe the data from VISION will also allow us to demonstrate that avoiding the black line reduces the likelihood of restenosis in these patients. If Pantheris is cleared by FDA, we plan to commercialize it as part of our lumivascular platform in the United States and in select European countries after obtaining any required marketing authorizations.
We focus our direct sales force, marketing efforts and promotional activities on interventional cardiologists, vascular surgeons and interventional radiologists in the United States and select European countries. We also work on developing strong relationships with physicians and hospitals that we have identified as key opinion leaders. Although our sales and marketing efforts are directed at these physicians because they are the primary users of our technology, we consider the hospitals and medical centers where the procedure is performed to be our customers, as they typically are responsible for purchasing our products. We are designing future products to be compatible with our lumivascular platform, which we expect to enhance the value proposition for hospitals to invest in our technology. We also expect that Pantheris will qualify for existing reimbursement codes currently utilized by other atherectomy products, further facilitating adoption of our products.
Prior to the introduction of our lumivascular platform our non-imaging catheter products were manufactured by third parties. All of our products are now manufactured in-house using components and sub-assemblies manufactured both in-house at our facilities in Redwood City, California and by outside vendors. We expect our current manufacturing facility will be sufficient to meet our anticipated growth through at least 2016. We assemble all of our products at our manufacturing facility, but certain critical processes such as coating and sterilization are done by outside vendors.
As of December 31, 2014, we had approximately $8.6 million in principal and accrued interest underlying outstanding promissory notes, or the notes, that are due and payable upon the earlier of October 29, 2018 or upon certain specified events. We have also borrowed $20.0 million under our credit facility, or the credit agreement, with PDL Biopharma, or PDL. All outstanding amounts under this credit agreement must be repaid on April 18, 2018. We are required to make certain royalty payments on our net sales until April 18, 2018, regardless of whether we prepay the term loan, and we are required to pay an exit fee at maturity, or earlier prepayment in full, based on a percentage of the original principal amount borrowed.
We began commercializing our initial non-lumivascular platform products in 2009 and introduced our lumivascular platform products in the United States in late 2012. We generated revenues of $11.2 million in 2014, $13.0 million in 2013 and $8.6 million in 2012. During the years ended December 31, 2014, 2013 and 2012, our net loss was $32.0 million, $39.9 million and $33.9 million, respectively. We have not been profitable since inception and as of December 31, 2014, our accumulated deficit was $146.5 million. Since inception, we have financed our operations primarily through private placements of our preferred securities and, to a lesser extent, debt financing arrangements. In January 2015, we completed an initial public offering of 5.0 million shares. As a result of our initial public offering, which closed in February 2015, we received net proceeds of approximately $56.8 million, after underwriting discounts and commissions of approximately $4.5 million and other expenses associated with our initial public offering of approximately $3.7 million.
During the third and fourth quarters of 2013, we effected a reduction in force, lowering our total headcount from 168 employees at June 30, 2013 to 115 employees at December 31, 2013. We implemented this reduction to better align resource utilization with our corporate strategy as we transitioned our focus from non-imaging products to lumivascular platform products, including Pantheris.
Components of Our Results of Operations
Revenues
All of our revenues are currently derived from sales of our Lightbox console and our various PAD catheters and related services in the United States and select European markets. We expect our revenues to increase as we continue to expand our sales and marketing infrastructure and introduce new lumivascular platform products including, if cleared by FDA, Pantheris. No single customer accounted for more than 10% of our revenues during 2012, 2013 or 2014.
We expect our revenues to fluctuate from quarter-to-quarter due to a variety of factors. In the first quarter, our results can be harmed by adverse weather and by resetting of annual patient healthcare insurance plan deductibles, both of which may cause patients to delay elective procedures. In the third quarter, the number of elective procedures nationwide is historically lower than other quarters throughout the year, which we believe is primarily attributable to the summer vacations of physicians and their patients.
Cost of Revenues and Gross Profit
Cost of revenues consists primarily of costs related to manufacturing overhead, materials and direct labor. A significant portion of our cost of revenues currently consists of manufacturing overhead costs. These overhead costs include the cost of quality assurance, material procurement, inventory control, facilities, equipment and operations supervision and management. We expect overhead costs as a percentage of revenues to become less significant as our production volume increases. Cost of revenues also includes depreciation expense for production equipment, depreciation and related maintenance expense for leased equipment held by customers and certain direct costs such as those incurred for shipping our products. We expect cost of revenues to increase in absolute dollars to the extent our revenues grow.
We calculate gross margin as gross profit divided by revenues. Our gross margin has been and will continue to be affected by a variety of factors, primarily production volumes, manufacturing costs, product yields, headcount and cost-reduction strategies. We expect our gross margin to increase over the long term as our production volume increases and as we spread the fixed portion of our manufacturing overhead costs over a larger number of units produced, thereby reducing our per unit manufacturing costs. We intend to use our design, engineering and manufacturing capabilities to further advance and improve the efficiency of our manufacturing processes, which we believe will reduce costs and increase our gross margin. In the future, we may seek to manufacture certain of our products outside the United States to further reduce costs. Our gross margin will likely fluctuate from quarter-to-quarter as we continue to introduce new products and adopt new manufacturing processes and technologies.
Research and Development Expenses
Research and development, or R&D, expenses consist primarily of engineering, product development, clinical and regulatory affairs, consulting services, materials, depreciation and other costs associated with products and technologies in development. These expenses include employee compensation, including stock-based compensation, supplies, materials, quality assurance expenses allocated to R&D programs, consulting, related travel expenses and facilities expenses. Clinical expenses include clinical trial design, clinical site reimbursement, data management, travel expenses and the cost of manufacturing products for clinical trials. In the future, we expect R&D expenses to increase in absolute dollars as we continue to develop new products and enhance existing products and technologies. However, we expect R&D expenses as a percentage of revenues to vary over time depending on the level and timing of our new product development efforts, as well as our clinical development, clinical trial and other related activities.
Selling, General and Administrative Expenses
We have a direct sales organization that is divided into two distinct roles - sales of capital equipment, such as our Lightbox, and sales of disposable products, such as our catheters. Our current sales efforts focus on establishing new lumivascular platform sites by marketing our products to physicians and hospital administrators. Additionally, we seek to increase the use of our lumivascular platform products by our current customers through case coverage, clinical training and other programs.
Selling, general and administrative, or SG&A, expenses consist primarily of compensation for personnel, including stock-based compensation, related to selling and marketing functions, physician education programs, business development, finance, information technology and human resource functions. Other SG&A expenses include commissions, training, travel expenses, educational and promotional activities, marketing initiatives, market research and analysis, conferences and trade shows, professional services fees, including legal, audit and tax fees, insurance costs, a 2.3% tax on U.S. sales of medical devices, general corporate expenses and allocated facilities-related expenses. We expect to grow our sales force in preparation for the commercial launch of Pantheris in order to increase the base of customers using our lumivascular
platform products. We believe that expanding our U.S. sales infrastructure and establishing distributor relationships in select regions outside the United States will drive further adoption of our lumivascular platform. We expect SG&A expenses to continue to increase in absolute dollars and as a percentage of revenues through at least 2015 as we expand our infrastructure to both drive and support anticipated growth in revenues and due to additional legal, accounting, insurance and other expenses associated with being a public company.
Interest Income (Expense), net
Interest income (expense), net consists primarily of interest incurred on our outstanding indebtedness, our royalty obligation to PDL and non-cash interest related to the amortization of debt discount and issuance costs associated with our various debt agreements. Due to the conversion of $7.8 million and $3.8 million in principal amount of the notes and related accrued interest into shares of our Series E preferred stock in September and November 2014, respectively, we expect that our interest expense will decrease.
Other Income (Expense), net
Other income (expense), net primarily consists of gains and losses resulting from the remeasurement of the fair value of our common stock warrant liability and the compound embedded derivative instrument associated with the notes. We continued to record adjustments to the estimated fair value of the common stock warrants until the Series E preferred stock issuance in September 2014, upon which the common stock warrant exercise price was fixed at $12.60 per share. At this time we re-evaluated the terms of the common stock warrants and determined that the common stock warrants issued with the convertible notes met the requirements for equity classification and the fair value of the warrant liability was reclassified to additional paid-in capital. We will continue to record adjustments to the estimated fair value of the compound embedded derivative instrument associated with the notes until the notes are converted into shares of our capital stock or are repaid. Additionally, for the year ended December 31, 2014, other income (expense), net includes the loss on the extinguishment of our notes.
Results of Operations:
|
|
|
Year Ended |
| |||||||
|
|
|
December 31, |
| |||||||
|
|
|
2014 |
|
2013 |
|
2012 |
| |||
|
|
|
(in thousands, except percentages) |
| |||||||
|
|
|
|
|
|
|
|
| |||
|
Revenues |
|
$ |
11,213 |
|
$ |
12,964 |
|
$ |
8,560 |
|
|
Cost of revenues |
|
6,513 |
|
8,205 |
|
4,151 |
| |||
|
Gross profit |
|
4,700 |
|
4,759 |
|
4,409 |
| |||
|
Gross margin |
|
42 |
% |
37 |
% |
52 |
% | |||
|
|
|
|
|
|
|
|
| |||
|
Operating expenses: |
|
|
|
|
|
|
| |||
|
Research and development |
|
11,224 |
|
15,973 |
|
15,416 |
| |||
|
Selling, general and administrative |
|
18,503 |
|
25,758 |
|
22,848 |
| |||
|
Total operating expenses |
|
29,727 |
|
41,731 |
|
38,264 |
| |||
|
Loss from operations |
|
(25,027 |
) |
(36,972 |
) |
(33,855 |
) | |||
|
|
|
|
|
|
|
|
| |||
|
Interest income (expense), net |
|
(6,014 |
) |
(2,923 |
) |
19 |
| |||
|
Other income (expense), net |
|
(909 |
) |
5 |
|
(19 |
) | |||
|
Loss before provision for income taxes |
|
(31,950 |
) |
(39,890 |
) |
(33,855 |
) | |||
|
Provision for income taxes |
|
14 |
|
11 |
|
9 |
| |||
|
Net loss and comprehensive loss |
|
$ |
(31,964 |
) |
$ |
(39,901 |
) |
$ |
(33,864 |
) |
Comparison of Years Ended December 31, 2014 and 2013
Revenues. Revenues decreased $1.8 million, or 14%, to $11.2 million during the year ended December 31, 2014, compared to $13.0 million during the year ended December 31, 2013. For the year ended December 31, 2014, sales of our Lightbox imaging console increased by $1.1 million, or 37%, to $3.9 million while sales of our disposable catheters decreased by $2.8 million, or 28%, to $7.3 million. The decrease in total revenues in 2014 and changes in revenue mix related to strategic decisions made by the Company in the fourth quarter of 2013 to focus commercial efforts on its lumivascular programs to broaden physician exposure to optical coherence tomography (OCT) image interpretation and build the installed base of the Lightbox imaging console.
Cost of Revenues and Gross Margin. Cost of revenues decreased $1.7 million, or 21%, to $6.5 million during the year ended December 31, 2014, compared to $8.2 million during the year ended December 31, 2013. This decrease was attributable to the decrease in revenues from sales of our Wildcat and Kittycat non-imaging catheters, as well as a decrease in personnel-related expenses associated with our headcount reduction during the third and fourth quarters of 2013.
Research and Development Expenses. R&D expenses decreased $4.8 million, or 30%, to $11.2 million during the year ended December 31, 2014, compared to $16.0 million during the year ended December 31, 2013. This decrease was primarily due to a $2.2 million decrease in personnel-related expenses associated with our headcount reduction during the third and fourth quarters of 2013, a decrease of $1.5 million in product development materials and related costs and a reduction of $1.1 million in outside services and, as we focused our research and development efforts on our lumivascular platform products, particularly Pantheris.
Selling, General and Administrative Expenses. SG&A expenses decreased $7.3 million, or 28%, to $18.5 million during the year ended December 31, 2014, compared to $25.8 million during the year ended December 31, 2013. This decrease was primarily due to a $6.1 million decrease in personnel-related expenses associated with our headcount reduction during the third and fourth quarters of 2013 and a reduction of $1.4 million in consulting, legal and professional fees, associated with our reduction in headcount and cost reduction actions taken in the second half of 2013 partially offset by an increase of $0.3 million in tradeshow and travel-related expenses.
Interest Income (Expense), Net. Interest income (expense), net increased $3.1 million, or 106%, to an expense of $6.0 million during the year ended December 31, 2014, compared to an expense of $2.9 million during the year ended December 31, 2013. This increased expense was attributable to interest expense incurred on our credit agreement with PDL, entered into during the second quarter of 2013, and the notes issued during the fourth quarter of 2013, and non-cash interest related to the amortization of debt discount and issuance costs associated with the notes and the credit agreement.
Other Income (Expense), Net. Other income (expense), net decreased to an expense of $0.9 million during the year ended December 31, 2014, compared to income of $5,000 during the year ended December 31, 2013. The increase in other expense was primarily attributable to the $1.2 million loss on the extinguishment of our notes that were converted into Series E preferred stock in September 2014, partially offset by the remeasurement of the fair value of our common stock warrant liability through the issuance of the Series E preferred stock in September 2014, and the derivative instruments associated with our notes which are accounted for as a compound embedded derivative instrument and marked-to-market at each reporting date.
Comparison of Years Ended December 31, 2013 and 2012
Revenues. Revenues increased $4.4 million, or 51%, to $13.0 million during the year ended December 31, 2013, compared to $8.6 million during the year ended December 31, 2012. $8.3 million of this increase was attributable to the U.S. launch of our lumivascular platform in December 2012, partially offset by a decrease in sales of our non-imaging catheters of $3.9 million. Our average selling prices per unit were substantially consistent for both periods.
Cost of Revenues and Gross Margin. Cost of revenues increased $4.0 million, or 98%, to $8.2 million during the year ended December 31, 2013, compared to $4.2 million during the year ended December 31, 2012. This increase was primarily attributable to the growth in sales of our lumivascular platform products, which commenced in the United States in December 2012.
Gross margin for the year ended December 31, 2013, decreased to 37%, compared to 52% for the year ended December 31, 2012. This decrease was primarily due to the costs associated with the introduction of a new manufacturing process related to the launch of our lumivascular platform and the transition of the manufacturing process of our non-imaging catheters in-house, as well as continued investments in our manufacturing infrastructure, primarily in personnel, which resulted in an increased allocation of facilities expense to cost of revenues.
Research and Development Expenses. R&D expenses increased $0.6 million, or 4%, to $16.0 million during the year ended December 31, 2013, compared to $15.4 million during the year ended December 31, 2012. This increase was primarily due to an increase in personnel-related expenses.
Selling, General and Administrative Expenses. SG&A expenses increased $3.0 million, or 13%, to $25.8 million during the year ended December 31, 2013, compared to $22.8 million during the year ended December 31, 2012. This increase was primarily due to an increase of $3.4 million in employee-related expenses from an increase in headcount. SG&A expenses also increased $0.3 million due to the medical device tax, which became effective on January 1, 2013. These increases were partially offset by a decreased allocation of facilities expense to SG&A due to our decision to manufacture our lumivascular platform products in-house.
Interest Income (Expense), Net. Interest income (expense), net increased to an expense of $2.9 million during the year ended December 31, 2013, compared to income of $19,000 during the year ended December 31, 2012. The increase in interest expense was attributable to interest expense incurred on our outstanding indebtedness, including the credit agreement, entered into during the second quarter of 2013, and the notes issued during the fourth quarter of 2013, and non-cash interest related to the amortization of debt discount and issuance costs associated with the notes and the credit agreement.
Other Income (Expense), Net. Other income (expense), net increased $24,000 to an income of $5,000 during the year ended December 31, 2013, compared to an expense of $19,000 during the year ended December 31, 2012. The increase in other income was attributable to the remeasurement of the fair value of our common stock warrant liability and the derivative instruments associated with the notes, which are accounted for as a compound embedded derivative instrument, and marked-to-market at each reporting date.
Liquidity and Capital Resources
As of December 31, 2014, we had cash and cash equivalents of $12.3 million and an accumulated deficit of $146.5 million, compared to cash and cash equivalents of $12.2 million and an accumulated deficit of $114.6 million as of December 31, 2013. We currently believe that the net proceeds of approximately $56.8 million from our initial public offering in January 2015, total cash proceeds of $6.2 million from our Series E preferred stock financing in January 2015, together with our existing cash and cash equivalents and expected revenues, will be sufficient to meet our capital requirements and fund our operations for at least 18 months. If these sources are insufficient to satisfy our liquidity requirements, we may seek to sell additional equity or debt securities or obtain an additional credit facility. Prior to our initial public offering in January 2015, our primary sources of capital have been private placements of preferred stock and debt financing agreements. In April 2013, we entered into a credit agreement with PDL, under which we could borrow up to $40.0 million, of which $20.0 million was immediately available and drawn by us. The remaining $20.0 million would have been available based upon the achievement of certain net revenue milestones prior to June 30, 2014. We did not achieve the net revenue milestones and, accordingly, cannot borrow additional funds under the credit agreement. As of December 31, 2014, we had $20.4 million outstanding under the credit agreement. See section titled “Contractual Obligations — PDL Credit and Security Agreements.”
If we raise additional funds by issuing equity securities, our stockholders would experience dilution. Debt financing, if available, may involve covenants restricting our operations or our ability to incur additional debt. Any debt financing or additional equity that we raise may contain terms that are not favorable to us or our stockholders and require significant debt service payments, which diverts resources from other activities. Additional financing may not be available at all, or in amounts or on terms acceptable to us. If we are unable to obtain additional financing, we may be required to delay the development, commercialization and marketing of our products and scale back our business and operations.
Cash Flows
|
|
|
Year Ended |
| |||||||
|
|
|
December 31, |
| |||||||
|
|
|
2014 |
|
2013 |
|
2012 |
| |||
|
|
|
(in thousands) |
| |||||||
|
Net cash (used in) provided by: |
|
|
|
|
|
|
| |||
|
Operating activities |
|
$ |
(21,801 |
) |
$ |
(40,655 |
) |
$ |
(35,234 |
) |
|
Investing activities |
|
(117 |
) |
(496 |
) |
(288 |
) | |||
|
Financing activities |
|
22,013 |
|
32,755 |
|
37,303 |
| |||
|
|
|
|
|
|
|
|
| |||
|
Net (decrease) increase in cash and cash equivalents |
|
$ |
95 |
|
$ |
(8,396 |
) |
$ |
1,781 |
|
Net Cash Used in Operating Activities
Net cash used in operating activities for 2014 was $21.8 million, consisting primarily of a net loss of $32.0 million, partially offset by a decrease in net operating assets of $3.6 million and by non-cash charges of $6.6 million. The decrease in net operating assets was primarily due to decreases in inventory, and an increase in accrued expenses and other current liabilities related to interest payable to PDL and transaction fees related to our Series E financing. The non-cash charges primarily consisted of depreciation, stock-based compensation, non-cash interest expense related to our credit agreement with PDL, and losses on the extinguishment of our notes.
Net cash used in operating activities for 2013 was $40.7 million, consisting primarily of a net loss of $39.9 million and an increase in net operating assets of $4.3 million, partially offset by non-cash charges of $3.6 million. The increase in net operating assets was primarily due to the expansion of our sales and marketing organizations to support the ongoing commercialization of our lumivascular platform resulting in increases in accounts receivable and inventory as well as a decrease in accounts payable and accrued expenses and other current liabilities due to timing of payments. Non-cash charges consisted primarily of depreciation, stock-based compensation, and non-cash interest expense related to our credit agreement with PDL.
Net cash used in operating activities for 2012 was $35.2 million, consisting primarily of a net loss of $33.9 million and an increase in net operating assets of $2.6 million, partially offset by non-cash charges of $1.3 million. The increase in net operating assets was primarily due to increases in inventory as we launched our lumivascular platform in late 2012, partially offset by an increase in accounts payables, accrued
compensation and accrued expenses and other current liabilities related to expansion of our sales, and marketing and manufacturing supply chain to support the launch of our lumivascular platform. Non-cash charges consisted primarily of depreciation and stock-based compensation.
Net Cash Used in Investing Activities
Net cash used in investing activities in 2014, 2013 and 2012 was $0.1 million, $0.5 million and $0.3 million, respectively, consisting of purchases of property and equipment.
Net Cash Provided by Financing Activities
Net cash provided by financing activities in 2014 was $22.0 million, consisting of net proceeds of $4.7 million from the issuance of convertible notes and net proceeds of $19.2 million from the issuance of our Series E preferred stock. Cash paid for deferred IPO Costs was $1.8 million.
Net cash provided by financing activities in 2013 was $32.8 million, consisting primarily of net proceeds of $19.3 million under our credit agreement with PDL and net proceeds of $13.4 million from the issuance of convertible notes.
Net cash provided by financing activities in 2012 was $37.3 million, consisting primarily of net proceeds of $37.1 million from the issuance of our Series D preferred stock.
Off-Balance Sheet Arrangements
We currently have no off-balance sheet arrangements, such as structured finance, special purpose entities, or variable interest entities.
Contractual Obligations
Our principal obligations consist of the operating lease for our facilities, capital leases related to office equipment, the credit agreement with PDL, the notes and non-cancellable purchase commitments. The following table sets out, as of December 31, 2014, our contractual obligations due by period (in thousands):
|
|
|
Payments Due by Period |
| |||||||||||||
|
|
|
Less Than 1 |
|
1 - 3 Years |
|
3-5 Years |
|
More Than 5 |
|
Total |
| |||||
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Operating lease obligations |
|
$ |
1,125 |
|
$ |
1,060 |
|
$ |
— |
|
$ |
— |
|
$ |
2,185 |
|
|
Capital lease obligations |
|
12 |
|
1 |
|
— |
|
— |
|
13 |
| |||||
|
Credit agreement with PDL |
|
4,272 |
|
21,772 |
|
— |
|
— |
|
26,044 |
| |||||
|
Convertible promissory notes |
|
— |
|
17,479 |
|
— |
|
— |
|
17,479 |
| |||||
|
Noncancellable purchase commitments |
|
1,334 |
|
— |
|
— |
|
— |
|
1,334 |
| |||||
|
|
|
$ |
6,743 |
|
$ |
40,312 |
|
$ |
— |
|
$ |
— |
|
$ |
47,055 |
|
In addition to the interest and principal payments due under our credit agreement with PDL, we are obligated to pay PDL a royalty at the rate of 1.8% of our quarterly revenues through the maturity date of April 18, 2018. To the extent that we prepay the borrowings under our credit agreement, our royalty obligations will continue and will be payable through the maturity date at a reduced rate of the greater of 0.9% of our quarterly revenues or specified minimum payments. Because we are unable to estimate the actual royalty amounts payable under the PDL credit agreement, the table above excludes the minimum annual royalty payments and percentage-based royalties due thereunder.
Convertible Promissory Notes
On October 29, 2013, we entered into a Note and Warrant Purchase Agreement, or the Convertible Note Agreement, with certain existing preferred stockholders, third-parties and employees for the issuance of convertible notes up to an aggregate principal amount of $25.0 million. Under the terms of the Convertible Note Agreement, we issued convertible notes, or the notes, in October and November 2013 for total proceeds of $13.5 million, in May 2014 for $4.2 million in total proceeds and in July 2014 for $0.5 million in total proceeds. The notes bear interest equal to 30-day LIBOR, plus 6% per annum subject to a minimum internal rate of return of 20% per annum. The principal and accrued interest thereon will mature on the earlier of: (i) October 29, 2018, (ii) an event of default or (iii) a change of control event.
The principal and the accrued interest on the notes was convertible, at the option of the holder, upon a future issuance of our preferred stock or common stock into that same stock at a conversion price equal to 85% of the price paid by other investors in the financing event. If the holder does not elect to convert the notes upon the closing of such a financing and such financing raises net proceeds of at least $20.0 million, we may repay the notes at 125% of the outstanding principal and accrued and unpaid interest. Upon a change of control, at the election of the holder, we are obligated to make a payment to such holder equal to the greater of (i) 125% of the outstanding principal and accrued and unpaid interest,
(ii) an amount equal to the return the holders of Series D preferred stock would be entitled to receive in such change of control, or (iii) the amount providing the investor with an annual 20% minimum internal rate of return, provided that in the event that the change of control includes any contingent payments based on future performance, the amount due and payable under clause (ii) will be recalculated at the time each installment or contingent payment is made. In September 2014, in connection with the issuance of the Series E preferred stock, $7.8 million of principal and accrued interest outstanding under the notes was converted into shares of Series E preferred stock. In November 2014, an additional $3.8 million of principal and accrued interest outstanding under the notes was converted into shares of Series E preferred stock. Upon the conversion of the notes, we recorded a loss from the extinguishment of the debt in the amount of $1.2 million, which is reflected in other income (expense), net in the statement of operations and comprehensive loss. As of December 31, 2014, $8.6 million in principal and accrued interest remained outstanding under the notes.
We may, at our sole election, prepay such outstanding principal and accrued and unpaid interest under the notes by paying each holder an amount equal to 125% of the principal and accrued and unpaid interest under the notes at any time prior to their maturity date.
Lease Agreement
We lease our headquarters in Redwood City, California pursuant to a lease agreement with HCP LS Redwood City dated July 30, 2010, or the 2010 Lease, as amended by the First Amendment to Lease dated September 30, 2011 and together with the 2010 Lease, the Amended Lease. The Amended Lease has a rental commencement date of December 1, 2011 and a term of five years and expires in November 2016. We have two options to extend the lease term for a period of three years each. Each option must be exercised no more than 12 months and no less than nine months prior to the expiration of the applicable term. The Amended Lease is for an aggregate of approximately 44,200 rentable square feet.
PDL Credit and Security Agreements
On April 18, 2013, we, as borrower, entered into a credit agreement with PDL, as lender and agent. The credit agreement provided for an aggregate term loan facility of up to $40.0 million, available in two tranches of up to $20.0 million each. We borrowed $20.0 million as a term loan under tranche one of the credit agreement on April 18, 2013. We also paid closing fees to PDL of approximately $200,000, which were deducted from the tranche one funds we received, plus legal and brokerage fees. Tranche two of the credit agreement, the availability of which was conditioned on our satisfaction of certain milestones, never became available to us as we did not reach those milestones. The proceeds from tranche one were used for working capital, capital expenditures and general corporate purposes.
The tranche one term loan bears interest at a rate equal to 12.0% per annum. Interest on the tranche one term loan is due and payable quarterly in arrears, provided that we may elect to add up to 1.5% percent of interest per annum to increase the outstanding principal balance of such loan for the first eight interest payment dates after the closing date with respect to the tranche one loan. Pursuant to this provision, we converted $529,000 of the interest on the tranche one loan amount into principal and, as of December 31, 2014, there was $20.4 million outstanding under the credit agreement. Principal is payable in equal quarterly installments beginning December 2015. All outstanding amounts under the tranche one term loan must be repaid on April 18, 2018.
At maturity of the tranche one term loan, or upon its full prepayment, we are obligated to pay an exit fee equal to 1.0% of the original principal amount borrowed. Additionally, until April 18, 2018, even if the term loan is prepaid, we are obligated to pay to PDL a certain percentage of our net revenue each quarter. Until the end of the quarter in which prepayment occurs, we are required to pay to PDL a quarterly amount equal to 1.8% of our net revenues for such quarter. If we prepay the loan, we are still required to pay to PDL a quarterly amount equal to the greater of 0.9% of our net revenues for each calendar month during such quarter and certain minimum amounts, starting at $65,000 per quarter in 2013 and increasing annually to $310,000 per quarter in 2018. On April 18, 2013, we entered into a security agreement with PDL, as agent, pursuant to which we secured our obligations under the tranche one term loan by granting to PDL a security interest on substantially all of our assets.
The credit agreement and the security agreement contain customary affirmative covenants and customary negative covenants limiting our ability to, among other things, dispose of assets, undergo a change in control, merge or consolidate with affiliates, make acquisitions, incur debt, incur liens, pay dividends, enter into restrictive agreements, repurchase stock and make investments, in each case subject to certain exceptions. Additionally, even if the term loan is prepaid, until there are no further obligations to periodically pay to lender a percentage of our net revenue, we must comply with certain affirmative covenants and negative covenants limiting our ability to, among other things, undergo a change in control or dispose of assets, in each case subject to certain exceptions. The credit agreement and the security agreement also contain customary events of default including, among others, payment defaults, breaches of covenants, bankruptcy and insolvency events, cross defaults with certain material indebtedness, judgment defaults, and breaches of representations and warranties. Upon an event of default, PDL may declare all or a portion of our outstanding obligations payable to be immediately due and payable and exercise other rights and remedies provided for under the credit agreement, the security agreement and any guaranty. Additionally, upon an event of default, the interest rate would likely be increased to a default rate of 14.0% per annum. We were in compliance with the covenants under the credit agreement and the security agreement as of December 31, 2014.
Indemnification
In the normal course of business, we enter into contracts and agreements that contain a variety of representations and warranties and provide for indemnification of the counterparty. Our exposure under these agreements is unknown because it involves claims that may be made against us in the future, but have not yet been made. To date, we have not been subject to any claims or been required to defend any action related to our indemnification obligations. However, we may incur significant expense in the future as a result of these indemnification obligations.
In accordance with our amended and restated certificate of incorporation and our amended and restated bylaws, we have indemnification obligations to our officers and directors, subject to some limits, with respect to their service in such capacities. We have also entered into indemnification agreements with our directors and certain of our officers. To date, we have not been subject to any claims, and we have director and officer insurance that may enable us to recover a portion of any amounts paid for future potential claims. However, we may incur significant expense in the future as a result of these indemnification obligations.
Related Parties
For a description of our related party transactions, see “Certain Relationships and Related Transactions, and Director Independence.”
Critical Accounting Policies and Estimates
Management’s discussion and analysis of our financial condition and results of operations is based on our financial statements, which have been prepared in accordance with U.S. generally accepted accounting principles. The preparation of these financial statements requires us to make estimates and assumptions for the reported amounts of assets, liabilities, revenues, expenses and related disclosures. Our estimates are based on our historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions and any such differences may be material.
While our significant accounting policies are more fully described in Note 2 of our financial statements included in this Annual Report on Form 10-K, we believe the following discussion addresses our most critical accounting policies, which are those that are most important to our financial condition and results of operations and require our most difficult, subjective and complex judgments.
Revenue Recognition
All of our revenues are currently derived from sales of our lumivascular platform products, various non-imaging PAD catheters and related services in the United States and select European markets. We recognize revenues when the following revenue recognition criteria are met:
· Persuasive evidence of an arrangement exists. We consider this criterion satisfied when we have an agreement or contract in place with the customer.
· Delivery has occurred or services have been rendered. We principally determine this criterion to be satisfied as follows:
· Lightbox console: upon our receipt of a form executed by the customer acknowledging that the training and installation process is complete.
· PAD catheters: when the product has been shipped and risk of loss and title has passed to the customer.
· Service: recognized ratably over the term of the service period. To date service revenues have been insignificant.
· The fee is fixed or determinable and collectability is reasonably assured. We determine the satisfaction of these criteria based on our judgment regarding the nature of the fee charged for products, contractual agreements entered into, and the collectability of those fees under any contract or agreement.
We offer our customers the ability to purchase or lease our Lightbox. When a customer leases the Lightbox, we recover the cost of providing the system by charging that customer a premium on sales of the Ocelot family of catheters. When a Lightbox is leased, we retain title to the equipment and it remains capitalized on our balance sheet under property and equipment. The costs to maintain these leased Lightboxes held by customers are charged to cost of revenues as incurred.
We evaluate our lease agreements and account for these contracts under the guidance pertaining to accounting for leases and for revenue arrangements with multiple deliverables. The guidance requires arrangement consideration to be allocated between a lease deliverable and a non-lease deliverable based upon the relative selling prices of the deliverables, using a specific hierarchy. The hierarchy is as follows: vendor-specific objective evidence of fair value of the respective elements, third-party evidence of selling price, or best estimate of selling price, or BESP. We allocate arrangement consideration using BESP.
We assessed whether the embedded lease is an operating lease or sales-type lease and determined that collectability of the minimum lease payments is not reasonably predictable given that any payments under the lease agreements are dependent upon contingent future Ocelot catheter sales. We concluded, therefore, that the embedded lease did not meet the criteria of a sales-type lease and we account for it as an operating lease. We recognize revenue allocated to the lease as the Ocelot catheters are delivered.
We must make significant assumptions regarding the future collectability of accounts receivable from customers to determine whether revenue recognition criteria have been met. If collectability is not assured at the time of shipment, we defer revenues until such criterion has been met. We estimate reductions in revenue for potential returns of products by customers. In making such estimates, we analyze historical returns, current economic trends and changes in customer demand and acceptance of our products.
Common Stock Valuation
Our intent has been to grant all options with an exercise price not less than the fair value of our common stock underlying those options on the date of grant. Prior to our initial public offering in January 2015, we have determined the estimated fair value of our common stock at each valuation date in accordance with the guidelines outlined in the American Institute of Certified Public Accountants Practice Aid, Valuation of Privately-Held-Company Equity Securities Issued as Compensation. Our board of directors, with the assistance of independent third-party valuation firms and management, developed these valuations using significant judgment and taking into account numerous factors, including valuation reports; developments at our company; the rights, preferences and privileges of our preferred stock relative to those of our common stock; market conditions; the lack of marketability of our common stock; and contemporaneous debt and equity financing events.
For all option grant prior to our initial public offering in January 2015, our board of directors determined the enterprise value based on the application of the market approach and the income approach. Under the market approach we estimate the value based upon analysis of similar companies. We then apply these derived multiples or values to our financial metrics to estimate our market value. The income approach, or discounted cash flow method, estimates value based on the expectation of future net cash flows, which are then discounted back to the present using a rate of return derived from companies of similar type and risk profile. The allocation of these enterprise values to each part of our capital structure, including our common stock, was done based on the option pricing method, or OPM. OPM treats the rights of the holders of preferred and common stock as equivalent to call options on any value of the enterprise above certain break points of value based upon the liquidation preferences of the holders of the preferred stock, as well as their rights to participation and conversion. Thus, the estimated value of the common stock can be determined by estimating the value of its portion of each of these call option rights. OPM derives the implied equity value of a company from a recent transaction involving the company’s own securities issued on an arms-length basis. Following the closing of our initial public offering in 2015, the fair value of our common stock is determined based on the closing price of our common stock on The NASDAQ Stock Market.
We recorded total non-cash stock-based compensation expense of $0.6 million, $0.7 million and $0.4 million for the years ended December 31, 2014, 2013 and 2012, respectively. At December 31, 2014, we had $15.8 million of total unrecognized employee stock-based compensation expense, net of estimated forfeitures, related to stock option grants. This amount will be recognized as expense over a weighted-average period of 3.9 years. We expect to continue to grant stock options in the future, and, to the extent that we do, our actual stock-based compensation expense recognized in future periods will likely increase. The stock-based compensation expense that we recognize in the first quarter of 2015 and each quarter thereafter through 2018 will be increased, as a result of our determination to calculate that expense based on deemed fair values of our common stock that are higher than the exercise prices of certain stock options granted prior to our initial public offering.
Stock-Based Compensation
We maintain an equity incentive plan to provide long-term incentive for employees, consultants and members of our board of directors. The plan allows for the issuance of non-statutory and incentive stock options to employees and non-statutory stock options to consultants and non-employee directors.
We are required to determine the fair value of equity incentive awards and recognize compensation expense for all equity incentive awards, including employee stock options. We recognize this expense over the requisite service period. In addition, we recognize stock-based compensation expense in the statements of operations and comprehensive loss based on awards expected to vest and, therefore, the amount of expense has been reduced for estimated forfeitures. We use the straight-line method for expense attribution.
The valuation model we used for calculating the fair value of awards for stock-based compensation expense is the Black-Scholes option-pricing model, or the Black-Scholes model. The Black-Scholes model requires us to make assumptions and judgments about the variables used in the calculation, including the weighted average period of time that the options granted are expected to be outstanding, the volatility of common stock, an assumed risk-free interest rate and an estimated forfeiture rate.
The following table summarizes the assumptions we used to determine the fair value of stock options:
|
|
|
Year Ended December 31, |
| ||||
|
|
|
2014 |
|
2013 |
|
2012 |
|
|
|
|
|
|
|
|
|
|
|
Expected term (years) |
|
6.3 |
|
6.9 |
|
6.9 |
|
|
Expected volatility |
|
59.1 |
% |
52.1 |
% |
48.7 |
% |
|
Risk-free interest rate |
|
1.8 |
% |
1.4 |
% |
1.2 |
% |
|
Dividend rate |
|
— |
|
— |
|
— |
|
Fair Value of Common Stock. As discussed above, the fair value of the shares of our common stock underlying the stock options has historically been determined by our board of directors after considering independent third-party valuation reports. Because there had previously been no public market for our common stock, our board of directors determined the fair value of our common stock at the time of grant of the option by considering a number of objective and subjective factors, including valuations of comparable companies, sales of our preferred stock, our operating and financial performance and the general and industry-specific economic outlook.
Expected Term. We do not believe we are able to rely on our historical exercise and post-vesting termination activity to provide accurate data for estimating the expected term for use in determining the fair value-based measurement of our options. Therefore, we have opted to use the “simplified method” for estimating the expected term of options, which is the average of the weighted average vesting period and contractual term of the option.
Expected Volatility. Since there had previously been no public market for our common stock and lack of company specific historical volatility, we have determined the share price volatility for options granted based on an analysis of the volatility of a peer group of publicly traded companies. In evaluating similarity, we consider factors such as stage of development, risk profile, enterprise value and position within the industry.
Risk-free Interest Rate. The risk-free interest rate is based on the U.S. Treasury yield in effect at the time of the grant for zero-coupon U.S. Treasury notes with remaining terms similar to the expected term of the options.
Dividend Rate. We assumed the expected dividend to be zero as we have never paid dividends and have no current plans to do so.
Expected Forfeiture Rate. We are required to estimate forfeitures at the time of grant, and revise those estimates in subsequent periods if actual forfeitures differ from those estimates. We use historical data to estimate pre-vesting option forfeitures and record share-based compensation expense only for those awards that are expected to vest. To the extent actual forfeitures differ from the estimates, we record the difference as a cumulative adjustment in the period that the estimates are revised.
Service period. We amortize all stock-based compensation over the requisite service period of the awards, which is generally the same as the vesting period of the awards. We amortize the stock-based compensation cost on a straight-line basis over the expected service periods.
If factors change and we employ different assumptions, stock-based compensation expense may differ significantly from what we have recorded in the past. If there are any modifications or cancellations of the underlying unvested securities, we may be required to accelerate, increase or cancel any remaining unearned stock-based compensation expense. To the extent that our assumptions are incorrect, the amount of stock-based compensation recorded will change.
Common Stock Warrant Liability
For a warrant classified as a derivative liability, we record the fair value of that warrant on the balance sheet at the inception of such classification and adjust to fair value at each financial reporting date. We record the changes in the fair value of the warrants in the statement of operations and comprehensive loss as a component of other income (expense), net. We continued to adjust the carrying value of the common stock warrant liability for changes in the fair value of the warrants until the Series E preferred stock issuance in September 2014, upon which the common stock warrant exercise price was fixed at $12.60 per share. At this time we re-evaluated the terms of the common stock warrants and determined that the common stock warrants issued with the convertible notes now met the requirements for equity classification at which time we reclassified the fair value of the warrant liability to stockholders’ deficit. Our assumptions with regard to the warrant valuation were based on
estimates of the valuation of the underlying common stock, the volatility of the common stock and interest rates and such estimates could vary significantly.
Compound Embedded Derivative
We have derivative instruments related to redemption features embedded within the outstanding convertible notes. The compound embedded derivatives were accounted for as a liability at the inception of the obligation and are remeasured to fair value as of each balance sheet date, with the related remeasurement adjustment recognized as other income (expense), net in the statement of operations and comprehensive loss. The fair value of the compound embedded derivative is determined based on an income approach that identified the cash flows using a “with-and-without” valuation methodology. The inputs used to determine estimated fair value of the derivative instruments include the probabilities of the underlying events triggering the embedded derivative and their timing. We will record adjustments to the estimated fair value of the compound embedded derivative associated with convertible notes until the notes are converted into shares of our capital stock or are repaid.
JOBS Act Accounting Election
As an emerging growth company under the Jumpstart Our Business Startups Act of 2012, we are eligible to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, are subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Interest Rate Risk
The risk associated with fluctuating interest rates is primarily limited to our cash equivalents, which are carried at quoted market prices. Due to the short-term maturities and low risk profile of our cash equivalents, an immediate 100 basis point change in interest rates would not have a material effect on the fair value of our cash equivalents. We do not currently use or plan to use financial derivatives in our investment portfolio.
We are also exposed to market risk related to fluctuations in interest rates indexed to LIBOR, which determines the variable interest payments we make on the notes, as they bear interest equal to 30-day LIBOR, plus 6% per annum. However, we do not believe we are subject to any material market risk exposure as the notes are subject to, and interest is accrued at, a minimum internal rate of return of 20%.
Credit Risk
As of December 31, 2014, our cash and cash equivalents were maintained with one financial institution in the United States, and our current deposits are likely in excess of insured limits. We have reviewed the financial statements of this institution and believe it has sufficient assets and liquidity to conduct its operations in the ordinary course of business with little or no credit risk to us.
Our accounts receivable primarily relate to revenues from the sale of our lumivascular platform products to hospitals and medical centers in the United States. None and three customers represented more than 10% of our accounts receivable as of December 31, 2014 and 2013, respectively.
Foreign Currency Risk
Our business is primarily conducted in U.S. dollars. Any transactions that may be conducted in foreign currencies are not expected to have a material effect on our results of operations, financial position or cash flows.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The information required by this item appears in a separate section of this Annual Report on Form 10-K beginning on page 78 and is incorporated herein by reference.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in our reports under the Securities Exchange Act of 1934, as amended, or the Exchange Act, and the rules and regulations thereunder, is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms and that such information is accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate, to allow for timely decisions regarding required disclosure. In designing and evaluating the disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and management is required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
As required by Rule 13a-15(b) under the Exchange Act, our management, under the supervision and with the participation of our principal executive officer and principal financial officer, has evaluated the effectiveness of the design and operation of our disclosure controls and procedures (as such term is defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) as of December 31, 2014. Based on such evaluation, our principal executive officer and principal financial officer have concluded that, as of December 31, 2014, our disclosure controls and procedures were effective, having implemented the remediation measures relating to the material weakness identified as of December 31, 2012 and 2013 as described below.
A “material weakness” is defined under SEC rules as a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of a company’s annual or interim financial statements will not be prevented or detected on a timely basis by the company’s internal controls. As of December 31, 2012 and 2013, as a result of management’s evaluation of our disclosure controls and procedures, and other internal reviews and evaluations that were completed after the year ended December 31, 2013, management concluded that we had a material weakness in our internal control over financial reporting. The material weakness we identified related to not maintaining sufficient compliment of resources with an appropriate level of accounting knowledge, experience and training commensurate with our structure and financial reporting requirements.
Management’s Remediation Activities
With the oversight of senior management and our audit committee, we executed the implementation of remediation steps in 2014. These efforts focused on (i) the hiring of personnel with technical accounting and financial reporting experience; (ii) the implementation of improved accounting and financial reporting procedures, to improve the completeness, timeliness and accuracy of our financial reporting and disclosures including the assessment of more judgmental areas of accounting, and (iii) utilizing outside technical accounting consultants when necessary.
We believe the measures described above have remediated the material weakness we identified and strengthened our internal control over financial reporting. These improvements to our internal control infrastructure were implemented in the second half of 2014, and were in place in connection with the preparation of our financial statements for the year ended December 31, 2014. As such, we believe that the remediation initiative outlined above was sufficient to remediate the material weakness in internal control over financial reporting as discussed above. We are committed to continuing to improve our internal control processes and will continue to diligently and vigorously review our financial reporting controls and procedures.
Management’s Annual Report on Internal Control Over Financial Reporting
This Annual Report on Form 10-K does not include a report of management’s assessment regarding internal control over financial reporting or an attestation report of our registered public accounting firm due to a transition period established by rules of the SEC for newly public companies.
Changes in Internal Control Over Financial Reporting
Other than the changes described above under “Management’s Remediation Activities,” there were no changes in our internal controls over financial reporting identified in management’s evaluation pursuant to Rules 13a-15(d) and 15d-15(d) of the Exchange Act that occurred during the fourth quarter of 2014 that materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
None.
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Executive Officers, Directors and Key Employees
The following table sets forth information, as of February 28, 2015, regarding our executive officers, directors and key employees.
|
|
Age |
|
Title | |
|
Jeffrey M. Soinski |
|
53 |
|
President, Chief Executive Officer and Director |
|
John B. Simpson, Ph.D., M.D.(1) |
|
71 |
|
Director and Executive Chairman of the Board of Directors |
|
Matthew B. Ferguson |
|
47 |
|
Chief Business Officer and Chief Financial Officer |
|
Sougata Banerjee |
|
48 |
|
Senior Vice President, Operations and Quality |
|
Bart C. Beasley |
|
46 |
|
Vice President, Marketing |
|
Arjun M. Desai, M.D. |
|
33 |
|
Chief Medical Officer |
|
Daniel V. George |
|
45 |
|
Vice President, Finance |
|
Patricia A. Hevey |
|
49 |
|
Vice President, Clinical, Quality & Regulatory Affairs |
|
Himanshu N. Patel |
|
55 |
|
Chief Technology Officer |
|
Philip R. Preuss |
|
37 |
|
Vice President, Corporate Development |
|
John D. Simpson |
|
36 |
|
Vice President, Sales |
|
James G. Cullen(2)(3) |
|
72 |
|
Director |
|
Thomas J. Fogarty(3) |
|
81 |
|
Director |
|
Donald A. Lucas(1)(2)(3) |
|
52 |
|
Director |
|
James B. McElwee(1)(2)(3) |
|
63 |
|
Director |
(1) Member of the audit committee.
(2) Member of the compensation committee.
(3) Member of the nominating and governance committee.
Jeffrey M. Soinski has served as our President, Chief Executive Officer and a member of our Board of Directors since December 2014. From its formation in September 2009 until the acquisition of its Unisyn business by GE Healthcare in May 2013, Mr. Soinski served as Chief Executive Officer of Medical Imaging Holdings and its primary operating company Unisyn Medical Technologies, a national provider of technology-enabled products and services to the medical imaging industry. Mr. Soinski remains a Director of Medical Imaging Holdings and its remaining operating company Consensys Imaging Service. From July 2008 to June 2013, Mr. Soinski served periodically as a Special Venture Partner for Galen Partners, a leading healthcare-focused private equity firm, which has Medical Imaging Holdings as one of its portfolio companies. From 2001 until its acquisition by C.R. Bard in 2008, Mr. Soinski was President and CEO of Specialized Health Products International, a publicly-traded manufacturer and marketer of proprietary safety medical products. Mr. Soinski served as a consultant to BLOXR Corporation, a venture-backed medical device company, from October 2013 until September 2014. He has served on the board of directors of Merriman Holdings, parent of Merriman Capital, a San Francisco-based investment banking and brokerage firm, since 2008. Mr. Soinski holds a B.A. degree from Dartmouth College.
We believe Mr. Soinski is qualified to serve as a member of our board of directors because of his extensive corporate finance and business strategy experience as well as his experience with public companies.
John B. Simpson, Ph.D., M.D. founded our company in March 2007 and has served as a member of our board of directors since March 2007. From March 2007 to December 2014, Dr. Simpson served as our Chief Executive Officer. Since March 2000 Dr. Simpson has served in various positions at De Novo Ventures, a venture capital fund, including managing director and clinical director. Since 1983, Dr. Simpson has been a partner at Cardiovascular Medicine and Coronary Interventions, a cardiology physician group. Prior to founding our company, Dr. Simpson founded several other interventional cardiology companies, including Perclose, a manufacturer of femoral artery access site closure devices, Devices for Vascular Intervention, a manufacturer of atherectomy devices, Advanced Cardiovascular Systems, a manufacturer of balloon angioplasty devices and FoxHollow Technologies, a manufacturer of atherectomy devices. Dr. Simpson holds a B.S. in Agriculture from Ohio State University, an M.D. from the Duke University School of Medicine and an M.S. and a Ph.D. in Biomedical Science from the University of Texas.
We believe Dr. Simpson is qualified to serve as a member of our board of directors because of his medical background, extensive knowledge of medical device company operations, and his experience working with companies, regulators and other stakeholders in the medical device industry.
Matthew B. Ferguson has served as our Chief Business Officer and Chief Financial Officer since January 2011, and also as our Co-President from August 2012 to October 2013. From December 2009 to December 2010, Mr. Ferguson served as the Chief Financial Officer at Tethys Bioscience, a provider of molecular diagnostic tests for cardiometabolic conditions. From January 2008 to April 2009 he served as the Chief Financial Officer at Proteolix, a developer of novel drugs for the treatment of cancer and autoimmune diseases. Mr. Ferguson also served as the Chief Financial Officer and Vice President of Finance at FoxHollow Technologies. Mr. Ferguson holds a B.S. in Civil Engineering from Stanford University, an M.S. in Mechanical Engineering from the University of Pennsylvania and an M.B.A. from the University of California at Berkeley.
Sougata (Bunty) Banerjee has served as our Senior Vice President of Operations since January 2012. From November 2009 to January 2012, Mr. Banerjee was Vice President of Operations and Quality at Evalve where he oversaw the acquisition of Evalve by Abbott Laboratories in 2009 and led the post-acquisition integration and business expansion as Head of Operations at Abbott Vascular, Structural Heart. Prior to Evalve, Mr. Banerjee served as Plant Manager at Epicor, holding general management responsibilities including operations, quality, product development, finance, human resources, and providing leadership in product commercialization and new product introductions. Prior to Epicor, Mr. Banerjee held several operations leadership positions at several business units of Boston Scientific. Earlier in his career, Mr. Banerjee held various engineering positions at Crompton-Greaves, Caterpillar, and Larsen-Toubro. Mr. Banerjee received a B.S. in Electrical Engineering from Jadavpur University, India and an M.S. in Industrial Management from Clemson University.
Bart C. Beasley has been our Vice President of Marketing since January 2013. From January 2009 to January 2013, he served as the Senior Director of Marketing at Transcend Medical. From January 2007 to January 2009, Mr. Beasley worked as an independent consultant providing consulting on sales and marketing strategy matters within the medical device industry. Mr. Beasley holds a B.S. in Economics from Santa Clara University and an M.B.A. from IESE, University of Navarra in Spain.
Arjun M. Desai, M.D. joined our company in January 2012 and has served as our Chief Medical Officer since November 2013. From July 2010 to December 2011, Dr. Desai served as a consultant and advisor for Incline Therapeutics, developing the IONSYS transdermal fentanyl delivery system and other companies. From 2008 to December 2011, Dr. Desai was a Staff Physician at Stanford University in the Department of Anesthesia where he completed his advanced anesthesia residency training. Dr. Desai continues to be affiliated with Stanford University. Dr. Desai has also served as a fellow in the United States House Policy Committee, advising members of Congress on healthcare legislation. Additionally, Dr. Desai represented the United States State Department and Rotary International as an Ambassador of Goodwill to Singapore where he led vaccine prophylaxis campaigns and lectured in the department of health economics at the National University of Singapore. Dr. Desai holds an M.D. from the University of Miami Miller School of Medicine and a B.A. in Economics from the University of Oklahoma.
Daniel V. George has served as our Vice President, Finance since August 2014. From June 2012 to August 2014, Mr. George served as a consultant and Vice President of Finance for ApniCure, a medical device company specializing in the treatment of sleep apnea. From March 2009 to June 2012, Mr. George worked for Avantis Medical Systems, a manufacturer of colonoscopy visualization technology, where he was both a consultant and Chief Financial Officer. Mr. George was also the Sr. Director of Finance at FoxHollow Technologies and worked for PricewaterhouseCoopers in the assurance and business advisory practice. Mr. George holds B.S. degrees in both Accounting and Finance from California State University, Long Beach.
Patricia A. Hevey has served as our Vice President of Clinical, Regulatory and Quality Affairs since September 2014. From April 2014 to September 2014, Ms. Hevey was our Vice President of Clinical and Regulatory Affairs and from February 2011 to February 2014, she served as our Director of Clinical and Regulatory Affairs. From July 2010 until February 2011, Ms. Hevey was the President of Hevey Clinical Consulting and from October 2008 to July 2010, she was the Director of Clinical and Regulatory Affairs at Baxano. Ms. Hevey holds a B.S. in Clinical Research Administration from George Washington University Medical School and an associate of science in Radiology Science from Canada College.
Himanshu N. Patel served as our Chief Technology Officer from January 2011 to November 2011 and since October 2013. From September 1999 to February 2007, Mr. Patel led research and development activities as the Director of Advanced Technologies at FoxHollow Technologies. Mr. Patel holds a B.S. in Mechanical Engineering from M.S. University of Baroda, India, and an M.S. in Mechanical Engineering from the University of Florida.
Philip R. Preuss has served as our Vice President, Corporate Development since September 2014. From September 2012 to August 2014, Mr. Preuss served as our Vice President, Finance and Corporate Development. Mr. Preuss joined our company in August 2009 and has held the positions of Vice President, Corporate Development and Vice President, Finance. Prior to joining our company, Mr. Preuss was a Manager of Business Development at another medical device company founded by Dr. Simpson. Mr. Preuss was also a Senior Associate of Corporate Development at FoxHollow Technologies, where he worked on internal strategic priorities and the exploration of external business opportunities. Before entering the medical device industry, Mr. Preuss held various roles in the financial services sector, and specifically within the field of equity research. Mr. Preuss holds an M.B.A. from the Kellogg School of Management and a B.A. in both Economics and History from Stanford University.
John D. Simpson has served as our Vice President of Sales since August 2011 and served as a member of our board of directors from December 2009 to January 2015. Mr. Simpson joined our company in 2008 and has held the positions of Chief Marketing Officer and Co-President. From 2001 to 2005, Mr. Simpson worked at FoxHollow Technologies in a Clinical Affairs, Sales and Marketing role. From 2005 to 2006, Mr. Simpson worked at Palo Alto Investors, an independent, privately held investment advisor. Mr. Simpson rejoined FoxHollow Technologies 2006 where he worked in Corporate Development. Mr. Simpson is a Founder and the former Chief Executive Officer of Recreation, which is a full service creative, digital and media agency focused on brand strategy and implementation for life changing innovations. Mr. Simpson holds a B.A. in Sociology from Duke University.
James G. Cullen has served as a member of our board of directors since December 2014. During the last five years, Mr. Cullen has held board and committee positions with various companies. Mr. Cullen is currently the non-executive Chairman of the board of Neustar, Inc., a neutral provider of real-time information services and analytics, a director and member of the investment and finance committees of Prudential Financial, non-executive Chairman of the Board of Agilent Technologies, a director of Keysight Technologies, and a director and chairman of the audit committee of Johnson & Johnson. From 1993 to 2000, Mr. Cullen was President, Vice Chairman and Chief Operating Officer of Bell Atlantic Corporation (now Verizon). From 1989 to 1993, he was President and Chief Executive Officer of Bell Atlantic-New Jersey. Mr. Cullen holds a B.A. in Economics from Rutgers University and an M.S. in Management Science from the Massachusetts Institute of Technology.
We believe Mr. Cullen is qualified to serve as a member of our board of directors because of his extensive experience serving on the boards of public companies as well as his financial and business expertise.
Thomas J. Fogarty, M.D. has served as a member of our board of directors since December 2014. Dr. Fogarty is a managing director of Emergent Medical Partners, an investment firm focused on private medical device companies, which he founded in 2007. Prior to Emergent Medical Partners, Dr. Fogarty held various positions at Stanford University where he performed both cardiac and peripheral vascular surgery. His positions at Stanford University included Professor of Cardiovascular Surgery and President of the Medical Staff. Dr. Fogarty holds a B.S. degree in Biology from Xavier University and an M.D. from Cincinnati College of Medicine.
We believe Dr. Fogarty is qualified to serve as a member of our board of directors because of his medical background and extensive knowledge of medical device company operations.
Donald A. Lucas has served as a member of our board of directors since 2013 and has been an investor in our company since 2011. Mr. Lucas has been a venture capitalist since 1985, having invested in companies such as Oracle, Macromedia and Cadence Design alongside his father Donald L. Lucas. Mr. Lucas has sourced or led investments in companies such as Intuitive Surgical, Coulter Pharmaceutical, Dexcom, Infinera, Reputation.com, Chegg, Palantir and Theranos. Mr. Lucas has served on the boards of Dexcom and the Silicon Valley Chapter of the JDRF and is a member of the UCSF Diabetes Center Leadership Council. Mr. Lucas holds a B.A. from Santa Clara University.
We believe Mr. Lucas is qualified to serve as a member of our board of directors because of his substantial corporate finance, business strategy and corporate development expertise gained from his significant experience in the venture capital industry, analyzing, investing in, serving on the boards of, and providing guidance to various technology companies.
James B. McElwee has served as a member of our board of directors since March 2011. Mr. McElwee served as general partner of Weston Presidio, a private equity and venture capital firm, from 1992 to 2010. During his tenure as a general partner and member of the investment committee, Weston Presidio led the start up financing of JetBlue Airways and made investments in Fender Musical Instruments, The Coffee Connection, Guitar Center, Mapquest, Party City, Petzazz, RE/MAX, and others. Prior to Weston Presidio, Mr. McElwee was Senior Vice President of the Security Pacific Venture Capital Group and the founding Managing Director of its Menlo Park office where he was responsible for early private investments in Costco, Universal Health Services, Cypress Semiconductor, Aspect Telecommunications, Xilinx, MIPS Computer Systems, Harmonic, Microchip, Vitesse and others. Prior to entering the venture capital industry in 1979, Mr. McElwee was a Senior Consultant with Accenture working on a variety of clients in the retailing, healthcare and technology industries. Mr. McElwee holds a B.A. in Economics from Claremont McKenna College and an M.B.A. from the Wharton Graduate School of Business.
We believe Mr. McElwee is qualified to serve as a member of our board of directors because of his substantial corporate development and business strategy expertise gained in the venture capital industry.
Executive Officers
Each of our executive officers serves at the discretion of our board of directors and holds office until his or her successor is duly elected and qualified or until his or her earlier resignation or removal. John B. Simpson, the Executive Chairman of our board of directors, is the father of John D. Simpson, our Vice President, Sales.
Board of Directors
Our business is managed under the direction of our board of directors, which consists of five directors. Our directors hold office until the earlier of their death, resignation, removal or disqualification, or until their successors have been elected and qualified. We are actively searching for qualified candidates to add to our board of directors or to replace current members. Our board of directors does not have a formal policy on whether the roles of Chief Executive Officer and Chairman of our board of directors should be separate. Prior to our initial public offering, the members of our board of directors were elected in compliance with the provisions of our amended and restated certificate of incorporation and a voting agreement among certain of our stockholders. The voting agreement terminated upon the closing of our initial public offering, on February 5, 2015, and none of our stockholders have any special rights regarding the election or designation of members of our board of directors.
Our amended and restated certificate of incorporation provides that the authorized number of directors may be changed only by resolution of the board of directors. Our board of directors is divided into three classes with staggered three-year terms. We do not expect to have an annual meeting of stockholders in 2015 and our first annual meeting of stockholders will be in 2016. At each annual meeting of stockholders, the successors to directors whose terms then expire will be elected to serve from the time of election and qualification until the third annual meeting following election or until their earlier death, resignation or removal. Our directors have been divided among the three classes as follows:
· The Class I directors are Jeffrey M. Soinski and John B. Simpson, and their terms will expire at our annual meeting of stockholders to be held in 2016
· The Class II directors are Donald A. Lucas and James B. McElwee, and their terms will expire at our annual meeting of stockholders to be held in 2017; and
· The Class III directors are James G. Cullen and Thomas J. Fogarty and their terms will expire at our annual meeting of stockholders to be held in 2018.
This classification of the board of directors, together with the ability of the stockholders to remove our directors only for cause and the inability of stockholders to call special meetings, may have the effect of delaying or preventing a change in control or management.
Director Independence
Under the rules of The NASDAQ Stock Market, independent directors must comprise a majority of a listed company’s board of directors within a specified period of time after listing on The NASDAQ Stock Market. In addition, the rules of The NASDAQ Stock Market require that, subject to specified exceptions, each member of a listed company’s audit, compensation, and nominating and governance committees be independent. Our board of directors has reviewed the independence of each director and determined that Messrs. Cullen, Fogarty, Lucas and McElwee are independent under the rules of The NASDAQ Stock Market. Our board of directors will review the independence of each director at least annually. During these reviews, the board of directors will consider transactions and relationships between each director (and his or her immediate family and affiliates) and our company and its management to determine whether any such transactions or relationships are inconsistent with a determination that the director is independent. This review will be based primarily on responses of the directors to questions in a directors’ and officers’ questionnaire regarding employment, business, familial, compensation and other relationships with our company including its management.
We believe that the composition of our board of directors meets the requirements for independence under the current requirements of The NASDAQ Stock Market. As required by The NASDAQ Stock Market, we anticipate that our independent directors will meet in regularly scheduled executive sessions at which only independent directors are present. We intend to comply with future governance requirements to the extent they become applicable to us.
Corporate Governance
We believe that good corporate governance is important to ensure that, as a public company, we will be managed for the long-term benefit of our stockholders. We and our board of directors have been reviewing the corporate governance policies and practices of other public companies, as well as those suggested by various authorities in corporate governance. We have also considered the provisions of the Sarbanes-Oxley Act and the rules of the SEC and The NASDAQ Stock Market.
Based on this review, our board of directors has taken steps to implement many of these provisions and rules. In particular, we have established and expect to enhance charters for the audit committee, compensation committee and nominating and governance committee, as well as a code of business conduct and ethics applicable to all of our directors, officers and employees.
Board Committees
Our board of directors has established a standing audit committee, a compensation committee, and a nominating and governance committee. Our board of directors has assessed the independence of the members of each of these standing committees as defined under the rules of The NASDAQ Stock Market and, in the case of the audit committee, the independence requirements of Rule 10A-3 under the Securities Exchange Act of 1934, as amended.
Audit Committee. Messrs. Lucas, McElwee and Dr. Simpson serve on our audit committee. Mr. Lucas serves as the chair of the audit committee and we are actively searching for a financial expert within the meaning of the regulations of the SEC. Our board of directors has assessed whether all members of the audit committee meet the composition requirements of The NASDAQ Stock Market, including the requirements regarding financial literacy and financial sophistication. Our board of directors found that Messrs. Lucas, McElwee and Dr. Simpson have met the financial literacy and financial sophistication requirements and that Messrs. Lucas and McElwee are independent under SEC and The NASDAQ Stock Market rules. Before the expiration of the phase-in period applicable to initial public offerings under SEC and The NASDAQ Stock Market rules, all members of our audit committee will be independent for audit committee purposes. The audit committee’s primary responsibilities include:
· appointing, approving the compensation of, and assessing the qualifications and independence of our independent registered public accounting firm, which currently is Ernst & Young LLP;
· reviewing and discussing with management and our independent registered public accounting firm our annual and quarterly financial statements and related disclosures;
· preparing the audit committee report required by SEC rules to be included in our annual proxy statements;
· monitoring our internal control over financial reporting, disclosure controls and procedures;
· reviewing our risk management status;
· establishing policies regarding hiring employees from our independent registered public accounting firm and procedures for the receipt and retention of accounting related complaints and concerns;
· meeting independently with our independent registered public accounting firm and management; and
· monitoring compliance with the code of business conduct and ethics for financial management.
All audit and non-audit services must be approved in advance by the audit committee. Our board of directors has adopted a written charter for the audit committee which will be available on our website at www.avinger.com.
Compensation Committee. Messrs. Lucas, Cullen and McElwee serve on our compensation committee. Mr. McElwee serves as the chair of the compensation committee. The compensation committee’s responsibilities include:
· annually reviewing and approving corporate goals and objectives relevant to compensation of our chief executive officer and our other executive officers;
· determining the compensation of our chief executive officer and our other executive officers;
· reviewing and making recommendations to our board of directors with respect to director compensation; and
· overseeing and administering our equity incentive plans.
Our chief executive officer and chief financial officer make compensation recommendations for our other executive officers and initially proposes the corporate and departmental performance objectives under our Executive Bonus Plan to the compensation committee. From time to time, the compensation committee may use outside compensation consultants to assist it in analyzing our compensation programs and in determining appropriate levels of compensation and benefits. For example, in 2014, we engaged Radford Consulting to advise us on compensation philosophy as we transitioned towards becoming a publicly-traded company, selection of a group of peer companies to use for compensation benchmarking purposes and cash and equity compensation levels for our directors, executives and other employees based on current market practices. Our board of directors has adopted a written charter for the compensation committee which is available on our website at www.avinger.com.
Nominating and Governance Committee. Messrs. Lucas, Cullen, McElwee and Dr. Fogarty serve on our nominating and governance committee. Mr. Cullen serves as the chair of the nominating and governance committee. The nominating and governance committee’s responsibilities include:
· identifying individuals qualified to become members of our board of directors;
· recommending to our board of directors the persons to be nominated for election as directors and to each of our board’s committees;
· reviewing and making recommendations to our board of directors with respect to management succession planning;
· developing, updating and recommending to our board of directors corporate governance principles and policies; and
· overseeing the evaluation of our board of directors and committees.
Our board of directors has adopted a written charter for the nominating and governance committee which is available on our website at www.avinger.com.
Lead Independent Director
Our board of directors has appointed James G. Cullen to serve as our lead independent director. As lead independent director, Mr. Cullen is expected to preside over periodic meetings of our independent directors, to serve as a liaison between our Executive Chairman and the independent directors, and to perform such additional duties as our Board may otherwise determine and delegate. At the end of each board meeting, the independent directors are expected to meet without Mr. Soinski and Dr. Simpson present. Following each meeting, Mr. Cullen is expected to provide feedback to Mr. Soinski and Dr. Simpson on their performance and the performance of our employees during the meeting and to recommend new agenda items for the next meeting.
Code of Business Conduct and Ethics
We have adopted a code of business conduct and ethics that applies to all of our employees, officers and directors, including those officers responsible for financial reporting. The code of business conduct and ethics is available on our website at www.avinger.com. Changes to or waivers of the code will be disclosed on the same website. We intend to satisfy the disclosure requirement under Item 5.05 of Form 8-K regarding any amendment to, or waiver of, any provision of the code in the future by disclosing such information on our website.
Limitation on Liability and Indemnification Matters
Our amended and restated certificate of incorporation contains provisions that limit the liability of our directors for monetary damages to the fullest extent permitted by Delaware law. Consequently, our directors will not be personally liable to us or our stockholders for monetary damages for any breach of fiduciary duties as directors, except liability for:
· any breach of the director’s duty of loyalty to us or our stockholders;
· any act or omission not in good faith or that involves intentional misconduct or a knowing violation of law;
· unlawful payments of dividends or unlawful stock repurchases or redemptions as provided in Section 174 of the Delaware General Corporation Law; and
· any transaction from which the director derived an improper personal benefit.
Our amended and restated certificate of incorporation and amended and restated bylaws provide that we are required to indemnify our directors and officers, in each case to the fullest extent permitted by Delaware law. Our amended and restated bylaws also provide that we are obligated to advance expenses incurred by a director or officer in advance of the final disposition of any action or proceeding, and permit us to secure insurance on behalf of any officer, director, employee or other agent for any liability arising out of his or her actions in that capacity regardless of whether we would otherwise be permitted to indemnify him or her under Delaware law. We have entered, and expect to continue to enter, into agreements to indemnify our directors, executive officers and other employees as determined by our board of directors. With specified exceptions, these agreements provide for indemnification for related expenses including, among other things, attorneys’ fees, judgments, fines and settlement amounts incurred by any of these individuals in any action or proceeding. We believe that these bylaw provisions and indemnification agreements are necessary to attract and retain qualified persons as directors and officers. We also maintain directors’ and officers’ liability insurance.
The limitation of liability and indemnification provisions in our amended and restated certificate of incorporation and amended and restated bylaws may discourage stockholders from bringing a lawsuit against our directors and officers for breach of their fiduciary duty. They may also reduce the likelihood of derivative litigation against our directors and officers, even though an action, if successful, might benefit us and our stockholders. Further, a stockholder’s investment may be adversely affected to the extent that we pay the costs of settlement and damages.
Section 16(a) Beneficial Ownership Reporting Compliance
We did not have any class of equity securities registered pursuant to Section 12 of the Exchange Act during our most recent fiscal year. As a result, none of our directors, officers or other affiliated persons were subject to Section 16 of the Exchange Act during such year.
ITEM 11. EXECUTIVE COMPENSATION
Summary Compensation Table
This discussion contains forward looking statements that are based on our current plans, considerations, expectations and determinations regarding future compensation programs. Actual compensation programs that we adopt may differ materially from currently planned programs as
summarized in this discussion. As an “emerging growth company” as defined in the JOBS Act and a smaller reporting company we are not required to include a Compensation Discussion and Analysis section and have elected to comply with the scaled disclosure requirements applicable to emerging growth companies and smaller reporting companies.
The following table provides information regarding the total compensation for services rendered in all capacities that was earned by each individual who served as our principal executive officer at any time in 2014, and our two other most highly compensated executive officers who were serving as executive officers as of December 31, 2014. These individuals were our named executive officers for 2014.
|
Name and Principal |
|
Year |
|
Salary |
|
Bonus |
|
Stock |
|
Option |
|
Non-Equity |
|
Non-Qualified |
|
All Other |
|
Total |
|
|
John B. Simpson, Ph.D., M.D.(4) |
|
2014 |
|
362,917 |
|
— |
|
— |
|
1,994,872 |
|
— |
|
— |
|
— |
|
2,357,789 |
|
|
Executive Chairman |
|
2013 |
|
340,584 |
|
63,410 |
|
— |
|
301,061 |
|
— |
|
— |
|
— |
|
705,055 |
|
|
Jeffrey M. Soinski(4) |
|
2014 |
|
4,327 |
|
— |
|
— |
|
1,474,016 |
|
— |
|
— |
|
— |
|
1,478,343 |
|
|
President and Chief Executive Officer |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Matthew B. Ferguson |
|
2014 |
|
300,917 |
|
— |
|
— |
|
227,229 |
|
— |
|
— |
|
— |
|
528,146 |
|
|
Chief Financial Officer and Chief Business Officer |
|
2013 |
|
282,584 |
|
44,618 |
|
— |
|
72,543 |
|
— |
|
— |
|
— |
|
399,745 |
|
|
Sougata Banerjee |
|
2014 |
|
241,333 |
|
60,000 |
|
— |
|
168,771 |
|
— |
|
— |
|
— |
|
470,104 |
|
|
Senior Vice President of Operations |
|
2013 |
|
226,666 |
|
71,976 |
|
— |
|
11,826 |
|
— |
|
— |
|
— |
|
310,468 |
|
(1) The amounts reported include salary paid and 200% of salary deferred in each of the fiscal years. No more than 10% of a named executive officer’s salary was deferred in each fiscal year.
(2) The amount reported for 2014 was paid at the discretion of our board of directors and not pursuant to any plan. The 2013 bonus amounts were paid pursuant to an executive bonus plan based on quarterly performance in five areas: Pantheris development, sales, cash burn, Lightbox placements and Ocelot development.
(3) The amounts reported represent the aggregate grant-date fair value of the stock options awarded to the named executive officer in 2014, calculated in accordance with ASC Topic 718. Such grant-date fair value does not take into account any estimated forfeitures related to service-vesting conditions. The assumptions used in calculating the grant-date fair value of the options reported in this column are set forth in the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Critical Accounting Policies and Estimates—Stock-Based Compensation.”
(4) Mr. Soinski was appointed our President and Chief Executive Officer on December 29, 2014, succeeding our founder and then-Chief Executive Officer, Dr. John B. Simpson. Dr. Simpson became our Executive Chairman upon Mr. Soinski’s appointment.
Executive Officer Employment Letters
John B. Simpson
We entered into an employment offer letter in November 2014 with John B. Simpson. The letter has no specific term and provides for at-will employment. The letter does not provide for any bonus. Effective November 1, 2014, Dr. Simpson’s annual base salary is $335,000.
Jeffrey M. Soinski
We entered into an employment offer letter in December 2014 with Jeffrey M. Soinski, our President and Chief Executive Officer. The letter has no specific term and provides for at-will employment. The letter also provides that, in 2015, Mr. Soinski is eligible to receive an annual performance bonus of up to 40% of his annual salary based on the achievement of certain goals mutually agreed upon by him and our board of directors. Effective December 29, 2014, Mr. Soinski’s annual base salary is $375,000.
Pursuant to Mr. Soinski’s employment offer letter, if, within the 12 month period following a “change in control,” we terminate Mr. Soinski’s employment without “cause,” or Mr. Soinski resigns for “good reason” (as such terms are defined in Mr. Soinski’s employment offer letter), Mr. Soinski will receive accelerated vesting as to 100% of his outstanding unvested stock options. If we experience a change in control, and Mr. Soinski remains our employee through such date, Mr. Soinski will receive accelerated vesting as to 50% of his outstanding unvested stock options and/or restricted stock.
If we terminate Mr. Soinski without cause at any time, he will be entitled to receive 12 months of base salary and COBRA medical and dental insurance coverage, in each case payable in substantially equal installments in accordance with our payroll practices, as severance, in exchange for signing and not revoking a severance agreement and general release against us and our affiliates within 60 days following his termination of employment.
The letter provides that Mr. Soinski may receive payments or reimbursements from us for up to $30,000 of reasonable and documented expenses related to temporary lodging, travel, and commuting costs incurred by Mr. Soinski prior to August 2015 in connection with his transition from Utah to Redwood City, California, and reimbursements of up to $100,000 related to the sale of Mr. Soinski’s home in Utah and relocation to California.
Matthew B. Ferguson
We entered into an employment offer letter in December 2010 with Matt Ferguson, our Chief Financial Officer and Chief Business Officer. The letter has no specific term and provides for at-will employment. The letter did not provide for any bonus. Effective November 1, 2014, Mr. Ferguson’s annual base salary is $275,000.
Sougata Banerjee
We entered into an employment offer letter in November 2011 with Sougata (Bunty) Banerjee, our Senior Vice President of Operations. The letter has no specific term and provides for at-will employment. The letter also provides that Mr. Banerjee is eligible to earn quarterly bonuses targeted at $60,000 annually based on the satisfaction of milestones mutually agreed upon by us and Mr. Banerjee. Effective January 1, 2015, Mr. Banerjee’s annual base salary is $235,000.
Pension Benefits and Nonqualified Deferred Compensation
We do not provide a pension plan for our employees, and none of our named executive officers participated in a nonqualified deferred compensation plan in 2014.
Outstanding Equity Awards at 2014 Year-End
The following table sets forth information regarding outstanding stock options and stock awards held by our named executive officers as of December 31, 2014:
|
|
|
Option Awards |
|
Stock Awards |
| ||||||||||
|
Name |
|
Grant Date(1) |
|
Number of |
|
Number of |
|
Option |
|
Option |
|
Number of |
|
Market |
|
|
John B. Simpson |
|
5/1/2013 |
(5) |
28,888 |
|
— |
|
22.50 |
|
5/1/2018 |
|
— |
|
— |
|
|
|
|
12/31/2014 |
(6) |
838,250 |
|
— |
|
4.95 |
|
12/31/2024 |
|
— |
|
— |
|
|
Jeffrey M. Soinski |
|
12/31/2014 |
(6) |
619,385 |
|
— |
|
4.50 |
|
12/31/2024 |
|
— |
|
— |
|
|
Matthew B. Ferguson |
|
7/29/2011 |
(7) |
33,965 |
|
— |
|
12.60 |
|
7/29/2021 |
|
— |
|
— |
|
|
|
|
5/1/2013 |
(5) |
6,815 |
|
— |
|
20.25 |
|
5/1/2023 |
|
— |
|
— |
|
|
|
|
12/31/2014 |
(6) |
95,482 |
|
— |
|
4.50 |
|
12/31/2024 |
|
— |
|
— |
|
|
Sougata Banerjee |
|
1/20/2012 |
(8) |
20,878 |
|
— |
|
14.85 |
|
1/20/2022 |
|
— |
|
— |
|
|
|
|
5/1/2013 |
(5) |
1,111 |
|
— |
|
20.25 |
|
5/1/2023 |
|
— |
|
— |
|
|
|
|
12/31/2014 |
(6) |
70,918 |
|
— |
|
4.50 |
|
12/31/2024 |
|
— |
|
— |
|
(1) Each of the outstanding equity awards was granted pursuant to our 2009 Stock Plan. Effective as of January 29, 2015, no additional awards will be granted under the 2009 Stock Plan, and all awards granted under the 2009 Stock Plan that are repurchased, forfeited, expire, are cancelled or otherwise not issued will become available for grant under the 2015 Plan in accordance with its terms.
(2) All of our options are early exercisable subject to the Company’s right to repurchase any unvested shares.
(3) This column represents the fair value of a share of our common stock on the date of grant, as determined by our board of directors.
(4) Because Avinger’s common stock was not traded on a public market on December 31, 2014, the market value has been determined based on a per-share common stock value of $9.45, which was the per share value of our common stock as determined by an independent valuation firm as of December 31, 2014.
(5) 25% of the shares of our common stock subject to this option vested on January 1, 2014, and the balance vests in 36 successive equal monthly installments, subject to continued service through each such vesting date.
(6) 25% of the shares of our common stock subject to this option vests on December 31, 2015, and the balance vests in 36 successive equal monthly installments, subject to continued service through each such vesting date.
(7) 25% of the shares of our common stock subject to this option vested on December 31, 2011, and the balance vested in 36 successive equal monthly installments, subject to continued service through each such vesting date.
(8) 25% of the shares of our common stock subject to this option vested on January 3, 2013, and the balance vests in 36 successive equal monthly installments, subject to continued service through each such vesting date.
Executive Officer Change in Control Severance Agreements
In March 2012, we entered into change of control and severance agreements with each of John B. Simpson, Matt Ferguson, and Sougata Banerjee that superseded all previous severance and change of control arrangements we had entered into with these employees. Under each of these agreements, if, within the 18 month period following a “change of control,” we terminate the employment of the applicable employee other than for “cause,” death or disability, or the employee resigns for “good reason” (as such terms are defined in the employee’s employment agreement) and, within 60 days following the employee’s termination, the employee executes an irrevocable separation agreement and release of claims, the employee is entitled to receive (i) continuing payments of severance pay at a rate equal to the employee’s base salary and target bonus, as then in effect, for 12 months for Dr. Simpson and Mr. Ferguson and 6 months for Mr. Banerjee, (ii) reimbursement of premiums to maintain group health insurance continuation benefits pursuant to “COBRA” for employee and employee’s dependents for up to 12 months for Dr. Simpson and Mr. Ferguson and 6 months for Mr. Banerjee, (iii) accelerated vesting as to 100% of the employee’s outstanding unvested stock options and/or restricted stock, and (iv) the extension of the post-termination exercise period of any options held by the employee for a period of 1 year. Additionally, if we experience a change in control, 50% of the employee’s outstanding unvested stock options and/or restricted stock will vest.
Employee Benefit and Stock Plans
2015 Equity Incentive Plan
Our board of directors adopted, and our stockholders approved, our 2015 Equity Incentive Plan, or the 2015 Plan, in January 2015. Our 2015 Plan permits the grant of incentive stock options, within the meaning of Section 422 of the Code, to our employees and any parent and subsidiary corporations’ employees, and for the grant of nonstatutory stock options, restricted stock, restricted stock units, stock appreciation rights, performance units and performance shares to our employees, directors and consultants and our parent and subsidiary corporations’ employees and consultants.
Authorized shares. A total of 1,320,000 shares of our common stock are reserved for issuance pursuant to the 2015 Plan. In addition, the shares reserved for issuance under our 2015 Plan will also include shares reserved but not issued under the 2009 Stock Plan, as amended, or the 2009 Plan, and shares subject to stock options or similar awards granted under the 2009 Plan that expire or terminate without having been exercised in full and shares issued pursuant to awards granted under the 2009 Plan that are forfeited to or repurchased by us (provided that the maximum number of shares that may be added to the 2015 Plan pursuant to this sentence is 3,000,000 shares). In addition, shares may become available under the 2015 Plan as described below.
The number of shares available for issuance under the 2015 Plan includes an annual increase on the first day of each fiscal year beginning in fiscal 2016, equal to the lesser of:
· 1,690,000 shares;
· 5.0% of the outstanding shares of common stock as of the last day of our immediately preceding fiscal year; or
· such other amount as our board of directors may determine.
If an award expires or becomes unexercisable without having been exercised in full, is surrendered pursuant to an exchange program, or, with respect to restricted stock, restricted stock units, performance units or performance shares, is forfeited or repurchased due to failure to vest, the unpurchased shares (or for awards other than stock options or stock appreciation rights, the forfeited or repurchased shares) will become available for future grant or sale under our 2015 Plan. With respect to stock appreciation rights, the net shares issued will cease to be available under the 2015 Plan and all remaining shares will remain available for future grant or sale under the 2015 Plan. Shares used to pay the exercise price of an award or satisfy the tax withholding obligations related to an award will become available for future grant or sale under our 2015 Plan. To the extent an award is paid out in cash rather than shares, such cash payment will not result in reducing the number of shares available for issuance under our 2015 Plan.
Plan administration. Our board of directors or one or more committees appointed by our board of directors will administer our 2015 Plan. In the case of awards intended to qualify as “performance-based compensation” within the meaning of Section 162(m) of the Code, the committee will consist of two or more “outside directors” within the meaning of Section 162(m). In addition, if we determine it is desirable to qualify transactions under the 2015 Plan as exempt under Rule 16b-3 of the Securities Exchange Act of 1934, as amended, or Rule 16b-3, such transactions will be structured to satisfy the requirements for exemption under Rule 16b-3. Subject to the provisions of our 2015 Plan, the administrator has the power to administer the plan, including but not limited to, the power to interpret the terms of our 2015 Plan and awards granted under it, to create, amend and revoke rules relating to our 2015 Plan, including creating sub-plans, and to determine the terms of the awards, including the exercise price, the number of shares subject to each such award, the exercisability of the awards and the form of consideration, if any, payable upon exercise. The administrator also has the authority to amend existing awards to reduce or increase their exercise price, to allow participants the opportunity to transfer outstanding awards to a financial institution or other person or entity selected by the administrator and to institute an exchange program by which outstanding awards may be surrendered in exchange for awards of the same type which may have a higher or lower exercise price or different terms, awards of a different type and/or cash.
Stock options. Stock options may be granted under our 2015 Plan. The exercise price of options granted under our 2015 Plan must at least be equal to the fair market value of our common stock on the date of grant. The term of an incentive stock option may not exceed 10 years, except that with respect to any participant who owns more than 10% of the voting power of all classes of our outstanding stock, the term must not exceed five years and the exercise price must equal at least 110% of the fair market value on the grant date. The administrator will determine the methods of payment of the exercise price of an option, which may include cash, shares or other property acceptable to the administrator, as well as other types of consideration permitted by applicable law. After the termination of service of an employee, director or consultant, he or she may exercise his or her option for the period of time stated in his or her option agreement. Generally, if termination is due to death or disability, the option will remain exercisable for 12 months. In all other cases, the option will generally remain exercisable for three months following the termination of service. However, in no event may an option be exercised later than the expiration of its term. Subject to the provisions of our 2015 Plan, the administrator determines the other terms of options.
Stock appreciation rights. Stock appreciation rights may be granted under our 2015 Plan. Stock appreciation rights allow the recipient to receive the appreciation in the fair market value of our common stock between the exercise date and the date of grant. Stock appreciation rights may not have a term exceeding 10 years. After the termination of service of an employee, director or consultant, he or she may exercise his or her stock appreciation right for the period of time stated in his or her option agreement. However, in no event may a stock appreciation right be exercised later than the expiration of its term. Subject to the provisions of our 2015 Plan, the administrator determines the other terms of stock appreciation rights, including when such rights become exercisable and whether to pay any increased appreciation in cash or with shares of our common stock, or a combination thereof, except that the per share exercise price for the shares to be issued pursuant to the exercise of a stock appreciation right will be no less than 100% of the fair market value per share on the date of grant.
Restricted stock. Restricted stock may be granted under our 2015 Plan. Restricted stock awards are grants of shares of our common stock that vest in accordance with terms and conditions established by the administrator. The administrator will determine the number of shares of restricted stock granted to any employee, director or consultant and, subject to the provisions of our 2015 Plan, will determine the terms and conditions of such awards. The administrator may impose whatever conditions for lapse of the restriction on the shares it determines to be appropriate (for example, the administrator may set restrictions based on the achievement of specific performance goals or continued service to us); provided, however, that the administrator, in its sole discretion, may accelerate the time at which any restrictions will lapse or be removed. Recipients of restricted stock awards generally will have voting and dividend rights with respect to such shares upon grant without regard to the restriction, unless the administrator provides otherwise. Shares of restricted stock as to which the restrictions have not lapsed are subject to our right of repurchase or forfeiture.
Restricted stock units. Restricted stock units may be granted under our 2015 Plan. Restricted stock units are bookkeeping entries representing an amount equal to the fair market value of one share of our common stock. Subject to the provisions of our 2015 Plan, the administrator will determine the terms and conditions of restricted stock units, including the vesting criteria (which may include accomplishing specified performance criteria or continued service to us) and the form and timing of payment. Notwithstanding the foregoing, the administrator, in its sole discretion, may accelerate the time at which any restricted stock units will vest.
Performance units and performance shares. Performance units and performance shares may be granted under our 2015 Plan. Performance units and performance shares are awards that will result in a payment to a participant only if performance goals established by the administrator are achieved or the awards otherwise vest. The administrator will establish organizational or individual performance goals or other vesting criteria in its discretion, which, depending on the extent to which they are met, will determine the number and/or the value of performance units and performance shares to be paid out to participants. After the grant of a performance unit or performance share, the administrator, in its sole discretion, may reduce or waive any performance criteria or other vesting provisions for such performance units or performance shares. Performance units shall have an initial dollar value established by the administrator prior to the grant date. Performance shares shall have an initial value equal to the fair market value of our common stock on the grant date. The administrator, in its sole discretion, may pay earned performance units or performance shares in the form of cash, in shares or in some combination
Outside directors. Our 2015 Plan provides that all non-employee directors are eligible to receive all types of awards (except for incentive stock options) under the 2015 Plan. Our 2015 Plan provides that in any given fiscal year, a non-employee director may not receive under the 2015 Plan awards having a grant date fair value greater than $500,000 increased to $1,500,000 in connection with his or her initial service, as grant fair value is determined under generally accepted accounting principles. Our 2015 Plan further provides that, in the event of a merger or change in control, as defined in our 2015 Plan, each outstanding equity award granted under our 2015 Plan that is held by a non-employee director will fully vest, all restrictions on the shares subject to such award will lapse, and with respect to awards with performance-based vesting, all performance goals or other vesting criteria will be deemed achieved at 100% of target levels, and all of the shares subject to such award will become fully exercisable, if applicable.
Non-transferability of awards. Unless the administrator provides otherwise, our 2015 Plan generally does not allow for the transfer of awards and only the recipient of an award may exercise an award during his or her lifetime.
Certain adjustments. In the event of certain changes in our capitalization, to prevent diminution or enlargement of the benefits or potential benefits available under our 2015 Plan, the administrator will adjust the number and class of shares that may be delivered under our 2015 Plan and/or the number, class and price of shares covered by each outstanding award and the numerical share limits set forth in our 2015 Plan. In the event of our proposed liquidation or dissolution, the administrator will notify participants as soon as practicable and all awards will terminate immediately prior to the consummation of such proposed transaction.
Merger or change in control. Our 2015 Plan provides that in the event of a merger or change in control, as defined under the 2015 Plan, each outstanding award will be treated as the administrator determines, except that if a successor corporation or its parent or subsidiary does not assume or substitute an equivalent award for any outstanding award, then such award will fully vest, all restrictions on the shares subject to such award will lapse, all performance goals or other vesting criteria applicable to the shares subject to such award will be deemed achieved at 100% of target levels and all of the shares subject to such award will become fully exercisable, if applicable, for a specified period prior to the transaction. The award will then terminate upon the expiration of the specified period of time.
Amendment, termination. The administrator will have the authority to amend, suspend or terminate the 2015 Plan provided such action will not impair the existing rights of any participant. Our 2015 Plan will automatically terminate in 2025, unless we terminate it sooner.
2015 Employee Stock Purchase Plan
Our board of directors adopted, and our stockholders approved, our 2015 Employee Stock Purchase Plan, or ESPP, in January 2015. The ESPP became effective upon our initial public offering.
The ESPP includes a component that is intended to qualify as an “employee stock purchase plan” under Section 423 of the Internal Revenue Code of 1986, as amended, or the 423 Component, and a component that does not comply with Section 423, or the Non-423 Component. For purposes of this disclosure, a reference to the “ESPP” will mean the 423 Component. Unless determined otherwise by the administrator, each of our future non-U.S. subsidiaries, if any, will participate in a separate offering under the Non-423 Component.
Authorized shares. A total of 500,000 shares of our common stock are available for sale. In addition, our ESPP provides for annual increases in the number of shares available for issuance under the ESPP on the first day of each fiscal year beginning in fiscal year 2016, equal to the lesser of:
· 1.5% of the outstanding shares of our common stock on the last day of the previous fiscal year;
· 493,000 shares; or
· such other amount as may be determined by our board of directors.
As of the date hereof, no shares of our common stock have been purchased under the ESPP.
Plan administration. Our board of directors or a committee appointed by our board of directors will administer the ESPP. The administrator has authority to administer the plan, including but not limited to, full and exclusive authority to interpret the terms of the ESPP, determine eligibility to participate subject to the conditions of our ESPP as described below, and to establish procedures for plan administration necessary for the administration of the Plan, including creating sub-plans.
Eligibility. Generally, all of our employees are eligible to participate if they are employed by us, or any participating subsidiary, for at least 20 hours per week and more than five months in any calendar year. However, an employee may not be granted an option to purchase stock under the ESPP if such employee:
· immediately after the grant would own stock constituting 5% or more of the total combined voting power or value of all classes of our capital stock; or
· holds rights to purchase stock under all of our employee stock purchase plans that accrue at a rate that exceeds $25,000 worth of stock for each calendar year in which the option is outstanding.
Offering periods. Our ESPP is intended to qualify under Section 423 of the Code, and provides for 6 month offering periods. The offering periods generally start on the first trading day on or after March 1 and September 1 of each year, except for our first offering period which commenced on January 30, 2015, our first trading day following the effective date of our initial public offering. The administrator may, in its discretion, modify the terms of future offering periods.
Payroll deductions. Our ESPP permits participants to purchase common stock through payroll deductions of up to 20% of their eligible compensation, which includes a participant’s base straight time gross earnings, but exclusive of payments for incentive compensation, bonuses, payments for overtime and shift premium, equity compensation income and other similar compensation. A participant may purchase a maximum of 2,500 shares during an offering period.
Exercise of purchase right. Amounts deducted and accumulated by the participant are used to purchase shares of our common stock at the end of each six-month offering period. The purchase price of the shares will be 85% of the lower of the fair market value of our common stock on the first trading day of each offering period or on the exercise date. Participants may end their participation at any time during an offering period, and will be paid their accrued payroll deductions that have not yet been used to purchase shares of common stock. Participation ends automatically upon termination of employment with us.
Non-transferability. A participant may not transfer rights granted under our ESPP other than by will, the laws of descent and distribution, or as otherwise provided under our ESPP.
Merger or change in control. In the event of our merger or change in control, as defined under the ESPP, a successor corporation may assume or substitute for each outstanding option. If the successor corporation refuses to assume or substitute for the option, the offering period then in progress will be shortened, and a new exercise date will be set. The administrator will notify each participant that the exercise date has been changed and that the participant’s option will be exercised automatically on the new exercise date unless prior to such date the participant has withdrawn from the offering period.
Amendment, termination. Our ESPP will automatically terminate in 2035, unless we terminate it sooner. The administrator has the authority to amend, suspend or terminate our ESPP at any time.
2009 Stock Plan, as Amended
Our board of directors adopted, and our stockholders approved, our 2009 Stock Plan, or the 2009 Plan, in March 2009. Our 2009 Plan was most recently amended in December 2014. Effective as of January 29, 2015, the 2009 Plan was terminated, and accordingly, no additional awards will be granted under the 2009 Stock Plan. All awards granted under the 2009 Stock Plan that are repurchased, forfeited, expire, are cancelled or otherwise not issued will become available for grant under the 2015 Plan in accordance with its terms. Our 2009 Plan allowed for the grant of incentive stock options, within the meaning of Section 422 of the Internal Revenue Code of 1986, as amended, to our employees and our parent and subsidiary corporations’ employees, and for the grant of nonstatutory stock options and shares of common stock to our employees, directors and consultants and our parent and subsidiary corporations’ employees, directors and consultants.
Authorized Shares. The 2009 Plan was terminated, and accordingly, no additional awards will be granted under the 2009 Stock Plan. Our 2009 Plan will continue to govern outstanding awards granted thereunder. As of December 31, 2014, options to purchase 3,010,373 shares of our common stock remained outstanding under our 2009 Plan. In the event that an outstanding option or other right for any reason expires or is canceled, the shares allocable to the unexercised portion of such option or other right shall be added to the number of shares then available for issuance under the 2015 Plan.
Plan Administration. Our board of directors or a committee of our board (the administrator) administers our 2009 Plan. Subject to the provisions of the 2009 Plan, the administrator has the full authority and discretion to take any actions it deems necessary or advisable for the administration of the 2009 Plan. All decisions, interpretations and other actions of the administrator are final and binding on all participants in the 2009 Plan.
Options. The 2009 Plan was terminated, and accordingly, no additional awards will be granted under the 2009 Stock Plan. For stock options previously granted under our 2009 Plan, the exercise price per share of all options must equal at least 100% of the fair market value per share of our common stock on the date of grant, as determined by the administrator. The term of a stock option may not exceed 10 years. With respect to any participant who owns 10% of the voting power of all classes of our outstanding stock as of the grant date, the term of an incentive stock option granted to such participant must not exceed five years and the exercise price per share of such incentive stock option must equal at least 110% of the fair market value per share of our common stock on the date of grant, as determined by the administrator. The 2009 Plan administrator determines the terms and conditions of options.
After termination of an employee, director or consultant, he or she may exercise his or her option for the period of time as specified in the 2009 Plan. If termination is due to death, it is expected that the option will remain exercisable for 12 months, and if termination is due to disability, it is expected that the option will remain exercisable for 6 months. In all other cases, it is expected that the option will remain exercisable for three months. However, an option generally may not be exercised later than the expiration of its term.
Shares of Common Stock. Prior to the termination of the 2009 Plan shares of our common stock may be granted under our 2009 Plan, either as a purchasable award or as a direct grant. The administrator determined the purchase price and the number of shares granted to the award recipient. Stock purchase rights must be exercised within 30 days of grant.
Transferability of Awards. Our 2009 Plan generally does not allow for the transfer or assignment of options, except by will or by the laws of descent and distribution. Shares issued upon exercise of an option will be subject to such special forfeiture conditions, rights of repurchase, rights of first refusal, and other transfer restrictions as the administrator may determine.
Certain Adjustments. In the event of a subdivision of our outstanding stock, a declaration of a dividend payable in shares, a combination or consolidation of our outstanding stock into a lesser number of shares, a reclassification, or any other increase or decrease in the number of issued shares of stock effected without receipt of consideration by us, the 2009 Plan will be appropriately adjusted by the administrator as to the class and maximum number of securities subject to the 2009 Plan and the class, number of securities and price per share of common stock subject to outstanding awards under the 2009 Plan, provided that our administrator will make any adjustments as may be required by Section 25102(o) of the California Corporations Code.
Merger or Change in Control. Our 2009 Plan provides that, in the event of a merger or consolidation, all shares acquired under the 2009 Plan and all options shall be subject to the agreement of merger or consolidation. Such agreement need not treat all options in an identical manner, and it shall provide for one or more of the following with respect to each option:
· the continuation of the option by us (if we are the surviving corporation);
· the assumption of the option by the surviving corporation or its parent in a manner that complies with Section 424(a) of the Internal Revenue Code of 1986, as amended;
· the substitution by the surviving corporation or its parent of a new option in a manner that complies with Section 424(a) of the Internal Revenue Code of 1986, as amended.
· full acceleration of vesting of the option, followed by cancellation of the option if it is not exercised prior to the merger or consolidation, provided that option holders shall be able to exercise the option during a period of at least 5 days, subject to the terms of the 2009 Plan; or
· the cancellation of the option and the payment to the option holder of an amount equal to the excess of (A) the fair market value of the shares subject to the option (whether or not the option is then exercisable or such shares are then vested) as of the closing date of the merger or consolidation over (B) the exercise price of the option.
Amendment; Termination. Our board of directors terminated the 2009 Plan in January 2015. All outstanding awards will continue to be governed by their existing terms.
Executive Incentive Compensation Plan
Our board of directors has adopted an Executive Incentive Compensation Plan, or the Bonus Plan. The Bonus Plan will be administered by our compensation committee following the completion of this offering. The Bonus Plan allows our compensation committee to provide cash incentive awards to selected employees, including our named executive officers, based upon performance goals established by our compensation committee.
Under the Bonus Plan, our compensation committee determines the performance goals applicable to any award, which goals may include, without limitation: attainment of research and development milestones, sales bookings, business divestitures and acquisitions, cash flow, cash position, earnings (which may include any calculation of earnings, including but not limited to earnings before interest and taxes, earnings before taxes, earnings before interest, taxes, depreciation and amortization and net earnings), earnings per share, net income, net profit, net sales, operating cash flow, operating expenses, operating income, operating margin, overhead or other expense reduction, product defect measures, product release timelines, productivity, profit, return on assets, return on capital, return on equity, return on investment, return on sales, revenue, revenue growth, sales results, sales growth, stock price, time to market, total stockholder return, working capital, and individual objectives such as peer reviews or other subjective or objective criteria. Performance goals that include our financial results may be determined in accordance with GAAP or such financial results may consist of non-GAAP financial measures and any actual results may be adjusted by the compensation committee for one-time items or unbudgeted or unexpected items when performance goals that include our financial results may be determined in accordance with GAAP, or such financial results may consist of non-GAAP financial measures, and any actual results may be adjusted by the compensation committee for one-time items or unbudgeted or unexpected items when determining whether the performance goals have been met. The goals may be on the basis of any factors the compensation committee determines relevant, and may be adjusted on an individual, divisional, business unit or company-wide basis. The performance goals may differ from participant to participant and from award to award.
Our compensation committee may, in its sole discretion and at any time, increase, reduce or eliminate a participant’s actual award, and/or increase, reduce or eliminate the amount allocated to the bonus pool for a particular performance period. The actual award may be below, at or above a participant’s target award, in the compensation committee’s discretion. Our compensation committee may determine the amount of any reduction on the basis of such factors as it deems relevant, and it is not required to establish any allocation or weighting with respect to the factors it considers.
Actual awards are paid in cash only after they are earned, which usually requires continued employment through the date a bonus is paid. Our compensation committee has the authority to amend, alter, suspend or terminate the Bonus Plan provided such action does not impair the existing rights of any participant with respect to any earned bonus.
401(k) Plan
We maintain a tax-qualified retirement plan that provides eligible employees with an opportunity to save for retirement on a tax advantaged basis. We may make a discretionary matching contribution to the 401(k) plan, and may make a discretionary employer contribution to each eligible employee each year. To date, we have not made any matching or profits sharing contributions into the 401(k) plan. All participants’
interests in our matching and profit sharing contributions, if any, vest pursuant to a six-year graded vesting schedule from the time of contribution. Pre-tax contributions are allocated to each participant’s individual account and are then invested in selected investment alternatives according to the participants’ directions. The 401(k) plan is intended to qualify under Sections 401(a) and 501(a) of the Code. As a tax-qualified retirement plan, contributions to the 401(k) plan and earnings on those contributions are not taxable to the employees until distributed from the 401(k) plan, and all contributions are deductible by us when made.
Director Compensation
Historically non-employee members of our board of directors did not receive any cash compensation for service on our board of directors or committees, including attending board and committee meetings. However, we did reimburse our non-employee directors for travel, lodging and other reasonable expenses incurred in attending board, committee and other company-related meetings. In addition, from time to time we have granted stock options to some of our directors. In fiscal year 2014, we granted Donald Lucas, one of our non-employee directors, a non-statutory stock option to purchase 4,444 shares of our common stock at an exercise price of $20.25 per share. One quarter of the shares underlying this option vested on November 26, 2014, and the remaining shares underlying this option vest in equal monthly installments thereafter. Additionally, in fiscal year 2014, each of James Cullen, Thomas Fogarty, Donald Lucas and James McElwee, constituting all of our non-employee directors, was granted a non-statutory option to purchase 24,444 shares of our common stock at an exercise price of $4.50 per share. One quarter of the shares underlying these options vest on December 31, 2015 and the remaining shares underlying these options vest in equal monthly installments thereafter.
The following table sets forth a summary of the compensation received by our directors that are not named executive officers who received compensation during our fiscal year ended December 31, 2014:
|
Name(1) |
|
Option Awards |
|
Total ($) |
| |
|
James G. Cullen |
|
$ |
58,172 |
|
58,172 |
|
|
Thomas J. Fogarty |
|
58,172 |
|
58,172 |
| |
|
Donald A. Lucas |
|
105,763 |
|
105,763 |
| |
|
James B. McElwee |
|
58,172 |
|
58,172 |
| |
|
James Muzzy(4) |
|
— |
|
— |
| |
|
John D. Simpson(4) |
|
— |
|
— |
| |
(1) Mr. Cullen was appointed to our board of directors on December 30, 2014 and Dr. Fogarty was appointed to our board of directors on December 31, 2014.
(2) Amounts shown represent the grant date fair value of options granted during 2014, as calculated in accordance with ASC Topic 718. The assumptions used in calculating the grant-date fair value of the options reported in this column are set forth in the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Critical Accounting Policies and Estimates—Stock-Based Compensation.”
(3) As of December 31, 2014, Messrs. Cullen, Lucas, McElwee, Muzzy, Simpson and Dr. Fogarty had outstanding options to purchase a total of 24,444, 28,888, 31,110, 4,444, 92,905 and 24,444 shares of our common stock, respectively.
(4) Mr. Muzzy resigned from our board of directors on October 31, 2014. Mr. Simpson resigned from our board of directors on January 13, 2015.
Directors who are also our employees receive no additional compensation for their service as directors. During 2014, John B. Simpson, John D. Simpson and Jeffrey M. Soinski, three of our directors, were also our employees. See “Executive Compensation—Summary Compensation Table” for additional information about the compensation for Dr. Simpson and Mr. Soinski.
Outside Director Compensation Policy
We compensate each non-employee director for his or her service consisting of annual cash retainers and equity awards. Our board of directors will have the discretion to revise non-employee director compensation as it deems necessary or appropriate.
Cash Compensation. All non-employee directors will be entitled to receive the following cash compensation for their services following the completion of this offering:
· $35,000 per year for service as a board member;
· $20,000 per year additionally for service as chairman of the audit committee;
· $10,000 per year additionally for service as an audit committee member;
· $15,000 per year additionally for service as chairman of the compensation committee;
· $7,500 per year additionally for service as a compensation committee member;
· $10,000 per year additionally for service as chairman of the nominating and corporate governance committee; and
· $5,000 per year additionally for service as a nominating and corporate governance committee member.
All cash payments to non-employee directors, or the Retainer Cash Payments, will be paid biannually with the first biannual installment payable on the date of the Company’s annual meeting of stockholders or, if no annual meeting occurs in a given year, May 1, and the second biannual installment payable on November 1 of each year.
Election to Receive Stock Options in Lieu of Cash Payments. All non-employee directors may elect to convert a Retainer Cash Payment into a nonstatutory stock option, or a Retainer Option, with a grant date fair value equal to the applicable Retainer Cash Payment. Each Retainer Option will be granted on the date that the applicable Retainer Cash Payment was scheduled to be paid, and all of the shares underlying the Retention Option will vest and become exercisable one year from the date of grant, subject to continued service as a director through the applicable vesting date. The Retainer Option will be subject to certain terms and conditions as described below under the section titled “Equity Compensation.”
Elections to convert a Retainer Cash Payment into a Retainer Option must generally be made on or prior to December 31 of the year prior to the year in which the Retainer Cash Payment is scheduled to be paid, or such earlier deadline as established by our board of directors or compensation committee. A newly appointed non-employee director will be permitted to elect to convert Retainer Cash Payments payable in the same calendar year into Retainer Options, provided that such election is made prior to the date the individual becomes a non-employee director.
Equity Compensation. Nondiscretionary, automatic grants of nonstatutory stock options will be made to our non-employee directors.
· Initial option. Each person who first becomes a non-employee director will be granted an option to purchase shares having a grant date fair value equal to $115,000, or the Initial Option. The Initial Option will be granted on the date of the first meeting of our board of directors or compensation committee occurring on or after the date on which the individual first became a non-employee director. The shares underlying the Initial Option will vest and become exercisable as to one thirty-sixth (1/36th) of the shares subject to such Initial Option on each monthly anniversary of the commencement of the non-employee director’s service as a director, subject to the continued service as a director through the applicable vesting date.
· Annual Option. On the date occurring once each calendar year on the same date that our board of directors grants annual equity awards to our senior executives, each non-employee director will be granted an option to purchase shares having a grant date fair value equal to $75,000, or the Annual Option. All of the shares underlying the Annual Option will vest and become exercisable one year from the date of grant, subject to continued service as a director through the applicable vesting date.
The exercise price per share of each stock option granted under our outside director compensation policy, including Retainer Options, Initial Options and Annual Options, will be the fair market value of a share of our common stock, as determined in accordance with our 2015 Equity Incentive Plan, or the 2015 Plan, on the date of the option grant. The grant date fair value is computed in accordance with the Black-Scholes option valuation methodology or such other methodology our board of directors or compensation committee may determine.
Any stock option granted under our outside director compensation policy will fully vest and become exercisable in the event of a change in control, as defined in our 2015 Plan, provided that the optionee remains a director through such change in control. Further, our 2015 Plan, provides that in the event of a merger or change in control, as defined in our 2015 Plan, each outstanding equity award granted under our 2015 Plan that is held by a non-employee director will fully vest, all restrictions on the shares subject to such award will lapse, and with respect to awards with performance-based vesting, all performance goals or other vesting criteria will be deemed achieved at 100% of target levels, and all of the shares subject to such award will become fully exercisable, if applicable, provided such optionee remains a director through such merger or change in control.
Compensation Committee Interlocks and Insider Participation
None of our executive officers serves as a member of the board of directors or compensation committee, or other committee serving an equivalent function, of any other entity that has one or more of its executive officers serving as a member of our board of directors or its compensation committee. None of the current members of the compensation committee of our board of directors has ever been one of our employees.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The following table provides information concerning beneficial ownership of our common stock as of February 28, 2015, by:
· each stockholder, or group of affiliated stockholders, that we know owns more than 5% of our outstanding common stock;
· each of our named executive officers;
· each of our directors and director nominees; and
· all of our executive officers, directors and director nominees as a group.
The percentage of shares beneficially owned is computed on the basis of 12,228,260 shares of our common stock outstanding as of February 28, 2015.
Beneficial ownership is determined in accordance with the rules of the SEC, and generally includes voting power and/or investment power with respect to the securities held. In addition, the rules include shares of common stock issuable pursuant to the exercise of stock options or warrants that are either immediately exercisable or exercisable on or before April 29, 2015, which is 60 days after February 28, 2015. These shares are deemed to be outstanding and beneficially owned by the person holding those options or warrants for the purpose of computing the percentage ownership of that person, but they are not treated as outstanding for the purpose of computing the percentage ownership of any other person.
Except as indicated in the footnotes to this table, the persons or entities named have sole voting and investment power with respect to all shares of our common stock shown as beneficially owned by them. Except as indicated in the footnotes to this table, the address for each beneficial owner is c/o Avinger, Inc., 400 Chesapeake Drive, Redwood City, CA 94063.
|
|
|
Shares Beneficially Owned |
| ||
|
Name of Beneficial |
|
Number of |
|
Percentage |
|
|
5% and Greater Stockholders: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Entities affiliated with John B. Simpson(1) |
|
1,675,752 |
|
13.48 |
% |
|
Funds affiliated with Lucas Venture Group(2) |
|
845,659 |
|
6.78 |
% |
|
PV 1114, LLC(3) |
|
566,664 |
|
4.55 |
% |
|
Black Diamond Ventures, XVIII, LLC(4) |
|
514,774 |
|
4.14 |
% |
|
Emergent Medical Partners II, LP(5) |
|
472,221 |
|
3.81 |
% |
|
|
|
|
|
|
|
|
Named Executive Officers and Directors: |
|
|
|
|
|
|
Jeffrey M. Soinski(6) |
|
619,385 |
|
4.83 |
% |
|
John B. Simpson, Ph.D., M.D.(7) |
|
2,542,890 |
|
19.12 |
% |
|
Matthew Ferguson(8) |
|
159,743 |
|
1.30 |
% |
|
Sougata Banerjee(9) |
|
92,907 |
|
* |
|
|
James G. Cullen(10) |
|
136,242 |
|
1.11 |
% |
|
Thomas J. Fogarty, M.D.(11) |
|
496,665 |
|
4.00 |
% |
|
Donald A. Lucas(12) |
|
874,547 |
|
7.00 |
% |
|
James B. McElwee(13) |
|
51,721 |
|
* |
|
|
All executive officers and directors as a group (10 individuals)(14) |
|
5,376,559 |
|
35.70 |
% |
* Represents ownership of less than 1%
(1) Includes warrants to purchase 222,220 shares of common stock. John B. Simpson has sole voting and dispositive power with respect to shares held by the Simpson Family Trust, GIGL Investments II, L.P., GIGL Investments L.P., and FoxHollow ACLP. John B. Simpson disclaims beneficial ownership in GIGL Investments II, L.P., GIGL Investments L.P., and FoxHollow ACLP, except to the extent of his pecuniary interest therein.
(2) Includes 562,038 shares and warrants to purchase 260,392 shares of common stock held by Lucas Venture Group IX, LLC and 23,230 shares held by Lucas Venture Group III, LP. Mr. Lucas has sole voting and dispositive power with respect to shares held by Lucas Venture Group IX, LLC and Lucas Venture Group III, LP. Mr. Lucas disclaims beneficial ownership of these shares, except to the extent of his pecuniary interest in the named funds.
(3) Includes warrants to purchase 233,332 shares of common stock.
(4) Includes warrants to purchase 211,966 shares of common stock.
(5) Includes warrants to purchase 194,444 shares of common stock.
(6) Includes 619,385 shares issuable upon exercise of options exercisable within 60 days of February 28, 2015.
(7) Includes 1,264,289 shares and warrants to purchase 222,220 shares of common stock held by John B. Simpson & Rita Lynn Simpson, Trustees of the Simpson Family Trust Dated 1/12/90, 39,003 shares held by GIGL II Investments, L.P., 25,484 shares held by GIGL Investments, L.P., 124,757 shares held by FoxHollow ACLP, and 867,138 shares issuable upon exercise of options exercisable within 60 days of February 28, 2015.
(8) Includes warrants to purchase 9,653 shares of common stock and 136,263 shares issuable upon exercise of options exercisable within 60 days of February 28, 2015.
(9) Includes 92,907 shares of common stock issuable upon exercise of options exercisable within 60 days of February 28, 2015.
(10) Includes 73,834 shares and warrants to purchase 24,862 shares of common stock held by Gilbert Investments, LLC, 13,102 shares held by 2000 James Cullen Generation Skipping Family Trust and 24,444 shares of common stock issuable upon exercise of options exercisable within 60 days of February 28, 2015. Mr. Cullen has sole voting and dispositive power with respect to shares held by Gilbert Investments, LLC and James Cullen Generation Skipping Family Trust. Mr. Cullen does not have a pecuniary interest in the James Cullen Generation Skipping Family Trust and disclaims beneficial ownership in Gilbert Investments, LLC except to the extent of his pecuniary interest therein.
(11) Includes 277,777 shares and warrants to purchase 194,444 shares of common stock held by Emergent Medical Partners II, L.P. and 24,444 shares issuable upon exercise of options exercisable within 60 days of February 28, 2015. Dr. Fogarty shares voting and dispositive power with respect to shares held by Emergent Medical Partners II, L.P. with John Kirtland and Robert Brownell. Dr. Fogarty disclaims beneficial ownership in Emergent Medical Partners II, L.P. except to the extent of his pecuniary interest therein.
(12) Includes 562,038 shares and warrants to purchase 260,392 shares of common stock held by Lucas Ventures Group IX, LLC and 23,230 shares held by Lucas Venture Group III, LP and 28,888 shares issuable upon exercise of options exercisable within 60 days of February 28, 2015.
(13) Includes warrants to purchase 5,521 shares of common stock and 31,110 shares issuable upon exercise of options and warrants exercisable within 60 days of February 28, 2015.
(14) Includes warrants to purchase 735,414 shares of common stock and 2,113,098 shares issuable upon exercise of options exercisable within 60 days of February 28, 2015.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
As a smaller reporting company, we are required to disclose certain transactions to which we are or will be a party and in which any of our directors, executive officers, or holders of more than 5% of our common stock, or any member of the immediate family of the foregoing persons, had or will have a direct or indirect material interest in the event the amount of such transaction exceeds the lesser of $120,000 or one percent of the average of our total assets at year end for the last two completed fiscal years. The average of our 2013 and 2014 year-end assets multiplied by one percent is greater than $120,000.
Other than compensation arrangements, we describe below transactions and series of similar transactions, since January 1, 2012, to which we were a party or will be a party, in which:
· the amounts involved exceeded or will exceed $120,000; and
· any of our directors, executive officers, or holders of more than 5% of our common stock, or any member of the immediate family of the foregoing persons, had or will have a direct or indirect material interest.
Compensation arrangements for our directors and named executive officers are described elsewhere in this Annual Report on Form 10-K.
We entered into a Master Consulting Agreement in November 2013 with Recreation, Inc., a brand strategy and design agency, for marketing services. John D. Simpson is the founder and was the Chief Executive Officer of Recreation at the time we entered into the Master Consulting Agreement and is also our Vice President, Sales. Pursuant to this Consulting Agreement and the current Statement of Work in effect from March 2015 through February 2016, Recreation provides marketing services to us at a $20,000 per month retainer with an aggregate cap of $240,000 for the contract period. The Master Consulting Agreement has no specific term. Periodically Recreation may continue to provide marketing services to us at reasonable and customary rates. The amounts we paid to Recreation in 2014 and 2013 were $984,000 and $107,000, respectively.
During the years ended December 31, 2013 and 2012, we paid $146,000 and $140,000, respectively, to Baysinger Search & Associates, or Baysinger, for recruiting services. Baysinger’s management included the wife of our former Vice President, Sales.
We entered into a Time Sharing Agreement with JBS Consulting, or JBS, in June 2011. JBS is owned and controlled by our Executive Chairman of our board of directors, John B. Simpson. Pursuant to this Time Sharing Agreement, we leased the right to use an airplane owned by JBS for business-related travel by our employees. We agreed to pay to JBS expenses related to the operation of the airplane and the aggregate incremental cost of each specific flight leased by us.
Concurrently with the Time Sharing Agreement, we entered into a Reimbursement Agreement with JBS and John B. Simpson, pursuant to which JBS agreed to reimburse us for certain costs and expenses incurred by us under the Time Sharing Agreement, except for (i) the cost of a first class fare equivalent commercial airline ticket for all flights when Dr. Simpson or one of our directors is aboard the airplane in connection with Company business, and (ii) the cost of a coach fare equivalent commercial airline ticket for all flights when any of our employees or consultants are aboard the airplane in connection with company business. Neither the Time Sharing Agreement nor the Reimbursement Agreement have any specific term. The net amounts paid to JBS under the Time Sharing Agreement and Reimbursement Agreement in 2014, 2013 and 2012 were $0, $568,000 and $611,000, respectively, which represented only a fraction of the total cost of the airplane. Travel by our employees on this airplane ended in August 2013.
Series E Preferred Stock Financing
From September 2014 to January 2015, the Company issued a total of 3,162,098 shares of Series E convertible preferred stock at $12.60 per share for cash proceeds of $26,217,933, and pursuant to the conversion of outstanding convertible promissory notes in the amount of $11,582,000, at 85% of the issuance price, or $10.71 per share. Investors received warrants to purchase up to the number of shares of common stock equal to seventy percent (70%) of the number of shares of Series E preferred stock purchased by such investor. The table below sets forth the number of shares of Series E preferred stock sold to our directors, executive officers and holders of more than 5% of our capital stock:
|
Name |
|
Number of |
|
Number of |
|
Aggregate |
| |
|
Persons and entities associated with John B. Simpson, Ph.D., M.D. |
|
255,552 |
|
365,076 |
|
$ |
4,599,970 |
|
|
Lucas Venture Group IX, LLC |
|
290,417 |
|
371,990 |
|
$ |
4,222,870 |
|
|
Matthew Ferguson |
|
10,843 |
|
13,791 |
|
$ |
155,205 |
|
2013 Bridge Loan
In October 2013, November 2013, May 2014 and July 2014, we issued subordinated convertible promissory notes with an aggregate principal amount of $18,192,134. The subordinated convertible promissory notes accrued interest at the rate of the 30-day LIBOR rate plus 6% per annum subject to 20% internal rate of return. The table below sets forth the amount of subordinated convertible promissory notes sold to our directors, executive officers and holders of more than 5% of our capital stock:
|
Name |
|
Principal Amount |
| |
|
Lucas Venture Group IX, LLC |
|
$ |
2,522,193.57 |
|
|
James McElwee |
|
$ |
50,000 |
|
|
Matthew Ferguson |
|
$ |
100,000 |
|
|
Himanshu Patel |
|
$ |
150,000 |
|
|
Entities associated with Jim and Carolyn Milgard |
|
$ |
3,000,000 |
|
From September 2014 to November 2014, the outstanding principal and interest accrued under certain of the subordinated convertible promissory notes converted into shares of Series E preferred stock at a conversion price of $10.71 per share. The table below sets forth the number of shares of Series E preferred stock issued to our directors, executive officers and holders of more than 5% of our capital stock in connection with this conversion of subordinated convertible promissory notes:
|
Name |
|
Number of |
|
|
Lucas Venture Group IX, LLC |
|
245,619 |
|
|
James McElwee |
|
4,911 |
|
|
Himanshu Patel |
|
14,270 |
|
|
Matthew Ferguson |
|
9,823 |
|
Series D Preferred Stock Financing
In June 2012, August 2012 and September 2012, we issued an aggregate of 722,367 shares of our Series D preferred stock at a price per share of $52.20. In connection with our initial public offering the Series D preferred stock automatically converted into shares of common stock at a conversion rate of 1.61688. The table below sets forth the number of shares of Series D preferred stock sold to our directors, executive officers and holders of more than 5% of our capital stock:
|
Name |
|
Number of |
|
Aggregate |
| |
|
Entities associated with John B. Simpson, Ph.D., M.D. |
|
37,509 |
|
$ |
1,958,003 |
|
|
Entities associated with Donald A. Lucas |
|
84,029 |
|
$ |
4,386,447 |
|
|
James McElwee |
|
4,444 |
|
$ |
232,000 |
|
|
Entities associated with Jim and Carolyn Milgard |
|
95,785 |
|
$ |
5,000,000 |
|
Policies and Procedures for Related Party Transactions
Our board of directors has adopted a policy, effective upon the closing of our initial public offering, that our executive officers, directors, nominees for election as a director, beneficial owners of more than 5% of any class of our common stock and any members of the immediate family of any of the foregoing persons are not permitted to enter into a related person transaction with us without the prior consent of our audit committee. Any request for us to enter into a transaction with an executive officer, director, nominee for election as a director, beneficial owner of more than 5% of any class of our common stock or any member of the immediate family of any of the foregoing persons in which the amount involved exceeds $120,000 and such person would have a direct or indirect interest must first be presented to our audit committee for review, consideration and approval. In approving or rejecting any such proposal, our audit committee is to consider the material facts of the transaction,
including, but not limited to, whether the transaction is on terms no less favorable than terms generally available to an unaffiliated third-party under the same or similar circumstances and the extent of the related person’s interest in the transaction. We did not have a formal review and approval policy for related party transactions at the time of any of the transactions described above. However, all of the transactions described above were entered into after presentation, consideration and approval by our board of directors and/or our audit committee.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The following table represents aggregate fees billed to us for the years ended December 31, 2014 and 2013 by Ernst & Young LLP. All fees below were approved by our Audit Committee.
|
Year ending December 31, |
|
2014 |
|
2013 |
| ||
|
|
|
|
|
|
| ||
|
Audit fees |
|
$ |
2,090,000 |
(1) |
$ |
50,000 |
|
|
Audit related fees |
|
— |
|
— |
| ||
|
Tax fees |
|
— |
|
— |
| ||
|
All other fees |
|
— |
|
— |
| ||
|
Total |
|
$ |
2,090,000 |
|
$ |
50,000 |
|
(1) Audit fees consist of fees incurred for professional services rendered for the audit of our annual financial statements and review of the quarterly financial statements that are normally provided by Ernst & Young LLP in connection with regulatory filings or engagements. For the year ended December 31, 2014, audit fees also includes fees related to our initial public offering and review of documents filed with the SEC of $1,610,000.
Pre-approval Policies and Procedures
Our Audit Committee has responsibility for establishing policies and procedures for the pre-approval of audit and non-audit services rendered by our independent registered public accounting firm, Ernst & Young LLP. The policy generally pre-approves specified services in the defined categories of audit services, audit-related services, and tax services up to specified amounts. Pre-approval may also be given as part of the Audit Committee’s approval of the scope of the engagement of the independent registered public accounting firm or on an individual explicit case-by-case basis before the independent registered public accounting firm is engaged to provide each service. The pre-approval of services may be delegated to one or more of the Audit Committee’s members, but the decision must be reported to the full Audit Committee at its next scheduled meeting.
The Audit Committee has determined that the rendering of the above services by Ernst & Young LLP is compatible with the SEC’s policies on auditor independence.
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(a)(1) Financial Statements
The following Financial Statements are filed as part of this Annual Report on Form 10-K:
|
|
|
79 | |
|
|
Financial Statements |
|
|
|
|
|
80 | |
|
|
|
81 | |
|
|
Statements of Convertible Preferred Stock and Stockholders’ Deficit |
|
82 |
|
|
|
83 | |
|
|
|
84 |
(a)(2) Financial Statement Schedules
All other schedules have been omitted because the information required to be set forth therein is not applicable or is shown in the financial statements or notes thereto.
|
Description |
|
Balance at |
|
Charged to costs |
|
Write offs |
|
Balance |
| ||||
|
|
|
|
|
|
|
|
|
|
| ||||
|
Allowance for doubtful accounts receivable: |
|
|
|
|
|
|
|
|
| ||||
|
Fiscal year ended 2012 |
|
$ |
30 |
|
$ |
36 |
|
$ |
12 |
|
$ |
54 |
|
|
Fiscal year ended 2013 |
|
$ |
54 |
|
$ |
45 |
|
$ |
79 |
|
$ |
20 |
|
|
Fiscal year ended 2014 |
|
$ |
20 |
|
$ |
— |
|
$ |
— |
|
$ |
20 |
|
|
|
|
|
|
|
|
|
|
|
| ||||
|
|
|
Balance at |
|
Charged to costs |
|
Write offs |
|
Balance |
| ||||
|
|
|
|
|
|
|
|
|
|
| ||||
|
Allowance for sales returns: |
|
|
|
|
|
|
|
|
| ||||
|
Fiscal year ended 2012 |
|
$ |
— |
|
$ |
235 |
|
$ |
— |
|
$ |
235 |
|
|
Fiscal year ended 2013 |
|
$ |
235 |
|
$ |
310 |
|
$ |
457 |
|
$ |
88 |
|
|
Fiscal year ended 2014 |
|
$ |
88 |
|
$ |
135 |
|
$ |
146 |
|
$ |
77 |
|
(a)(3) Exhibits
The exhibits listed in the accompanying index to exhibits are filed as part of, or incorporated by reference into, this Annual Report on Form 10-K.
AVINGER, INC.
INDEX TO FINANCIAL STATEMENTS
As of December 31, 2014 and 2013, and the
Years Ended December 31, 2014, 2013 and 2012
|
79 | |
|
Financial Statements: |
|
|
80 | |
|
81 | |
|
Statements of Convertible Preferred Stock and Stockholders’ Deficit |
82 |
|
83 | |
|
84 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders of Avinger, Inc.
We have audited the accompanying balance sheets of Avinger, Inc. as of December 31, 2014 and 2013, and the related statements of operations and comprehensive loss, convertible preferred stock and stockholders’ deficit, and cash flows for each of the three years in the period ended December 31, 2014. Our audits also included the financial statement schedule included in the Index at Item 15(a). These financial statements and schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements and schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company’s internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Avinger, Inc. at December 31, 2014 and 2013, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2014, in conformity with U.S. generally accepted accounting principles. Also, in our opinion, the related financial statement schedule, when considered in relation to the basic financial statements taken as a whole, presents fairly in all material respects the information set forth therein.
/s/ Ernst & Young LLP
Redwood City, California
March 27, 2015
AVINGER, INC.
(In thousands, except share and per share data)
|
|
|
As of December 31, |
| ||||
|
|
|
2014 |
|
2013 |
| ||
|
Assets |
|
|
|
|
| ||
|
Current assets: |
|
|
|
|
| ||
|
Cash and cash equivalents |
|
$ |
12,316 |
|
$ |
12,221 |
|
|
Accounts receivable, net of allowance for doubtful accounts of $20 at December 31, 2014 and 2013 |
|
2,068 |
|
1,627 |
| ||
|
Inventories |
|
3,991 |
|
4,741 |
| ||
|
Prepaid expenses and other current assets |
|
562 |
|
1,011 |
| ||
|
Total current assets |
|
18,937 |
|
19,600 |
| ||
|
|
|
|
|
|
| ||
|
Property and equipment, net |
|
2,608 |
|
4,858 |
| ||
|
Other assets |
|
3,235 |
|
550 |
| ||
|
Total assets |
|
$ |
24,780 |
|
$ |
25,008 |
|
|
|
|
|
|
|
| ||
|
Liabilities, convertible preferred stock and stockholders’ deficit |
|
|
|
|
| ||
|
Current liabilities: |
|
|
|
|
| ||
|
Accounts payable |
|
$ |
1,013 |
|
$ |
996 |
|
|
Accrued compensation |
|
1,147 |
|
1,274 |
| ||
|
Accrued expenses and other current liabilities |
|
4,850 |
|
1,456 |
| ||
|
Borrowings |
|
1,873 |
|
— |
| ||
|
Total current liabilities |
|
8,883 |
|
3,726 |
| ||
|
|
|
|
|
|
| ||
|
Borrowings, net of current portion |
|
18,537 |
|
20,052 |
| ||
|
Convertible notes and accrued interest |
|
8,643 |
|
13,731 |
| ||
|
Other long-term liablities |
|
325 |
|
627 |
| ||
|
Total liabilities |
|
36,388 |
|
38,136 |
| ||
|
|
|
|
|
|
| ||
|
Commitments and contingencies (Note 10) |
|
|
|
|
| ||
|
|
|
|
|
|
| ||
|
Convertible preferred stock issuable in series, par value of $0.001 |
|
|
|
|
| ||
|
Shares authorized: 6,819,197 at December 31, 2014 and 2,668,794 at December 31, 2013 |
|
|
|
|
| ||
|
Shares issued and outstanding: 5,262,728 at December 31, 2014 and 2,591,102 at December 31, 2013 |
|
|
|
|
| ||
|
Liquidation preference: $231,836 at December 31, 2014 and $97,186 at December 31, 2013 |
|
132,260 |
|
99,654 |
| ||
|
Stockholders’ deficit: |
|
|
|
|
| ||
|
Common stock, par value of $0.001 |
|
|
|
|
| ||
|
Shares authorized: 15,555,555 at December 31, 2014 and 3,762,452 at December 31, 2013 |
|
|
|
|
| ||
|
Shares issued and outstanding: 243,260 at December 31, 2014 and 240,692 at December 31, 2013 |
|
— |
|
— |
| ||
|
Additional paid-in capital |
|
2,665 |
|
1,787 |
| ||
|
Accumulated deficit |
|
(146,533 |
) |
(114,569 |
) | ||
|
Total stockholders’ deficit |
|
(143,868 |
) |
(112,782 |
) | ||
|
Total liabilities, convertible preferred stock, and stockholders’ deficit |
|
$ |
24,780 |
|
$ |
25,008 |
|
See accompanying notes.
AVINGER, INC.
STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(In thousands, except per share data)
|
|
|
Year Ended |
| |||||||
|
|
|
December 31, |
| |||||||
|
|
|
2014 |
|
2013 |
|
2012 |
| |||
|
|
|
|
|
|
|
|
| |||
|
Revenues |
|
$ |
11,213 |
|
$ |
12,964 |
|
$ |
8,560 |
|
|
Cost of revenues |
|
6,513 |
|
8,205 |
|
4,151 |
| |||
|
Gross profit |
|
4,700 |
|
4,759 |
|
4,409 |
| |||
|
|
|
|
|
|
|
|
| |||
|
Operating expenses: |
|
|
|
|
|
|
| |||
|
Research and development |
|
11,224 |
|
15,973 |
|
15,416 |
| |||
|
Selling, general and administrative |
|
18,503 |
|
25,758 |
|
22,848 |
| |||
|
Total operating expenses |
|
29,727 |
|
41,731 |
|
38,264 |
| |||
|
Loss from operations |
|
(25,027 |
) |
(36,972 |
) |
(33,855 |
) | |||
|
|
|
|
|
|
|
|
| |||
|
Interest income |
|
2 |
|
11 |
|
25 |
| |||
|
Interest expense |
|
(6,016 |
) |
(2,934 |
) |
(6 |
) | |||
|
Other income (expense), net |
|
(909 |
) |
5 |
|
(19 |
) | |||
|
Loss before provision for income taxes |
|
(31,950 |
) |
(39,890 |
) |
(33,855 |
) | |||
|
Provision for income taxes |
|
14 |
|
11 |
|
9 |
| |||
|
Net loss and comprehensive loss |
|
$ |
(31,964 |
) |
$ |
(39,901 |
) |
$ |
(33,864 |
) |
|
|
|
|
|
|
|
|
| |||
|
Net loss per share, basic and diluted |
|
$ |
(132.63 |
) |
$ |
(170.52 |
) |
$ |
(162.03 |
) |
|
|
|
|
|
|
|
|
| |||
|
Weighted average common shares used to compute net loss per share, basic and diluted |
|
241 |
|
234 |
|
209 |
| |||
See accompanying notes.
AVINGER, INC.
STATEMENTS OF CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS’ DEFICIT
(In thousands, except share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Series A Convertible |
|
Series A-1 Convertible |
|
Series B Convertible |
|
Series C Convertible |
|
Series D Convertible |
|
Series E Convertible |
|
Common Stock |
|
Additional |
|
Accumulated |
|
Total Stockholders’ |
| ||||||||||||||||||||||||
|
|
|
Shares |
|
Amount |
|
Shares |
|
Amount |
|
Shares |
|
Amount |
|
Shares |
|
Amount |
|
Shares |
|
Amount |
|
Shares |
|
Amount |
|
Shares |
|
Amount |
|
Capital |
|
Deficit |
|
Deficit |
| ||||||||||
|
Balance at December 31, 2011 |
|
326,591 |
|
$ |
6,183 |
|
225,235 |
|
$ |
6,649 |
|
755,486 |
|
$ |
27,272 |
|
562,671 |
|
$ |
22,450 |
|
— |
|
$ |
— |
|
— |
|
$ |
— |
|
201,088 |
|
$ |
— |
|
$ |
391 |
|
$ |
(40,804 |
) |
$ |
(40,413 |
) |
|
Issuance of common stock, net of repurchases |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
30,014 |
|
|
|
184 |
|
— |
|
184 |
| ||||||||||
|
Employee stock-based compensation |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
449 |
|
— |
|
449 |
| ||||||||||
|
Repurchase of Series C Convertible Preferred Stock and issuance costs |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(1,248 |
) |
(53 |
) |
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
| ||||||||||
|
Issuance of Series D Convertible Preferred stock, net of issuance costs |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
722,367 |
|
37,158 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
| ||||||||||
|
Net and comprehensive loss |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(33,864 |
) |
(33,864 |
) | ||||||||||
|
Balance at December 31, 2012 |
|
326,591 |
|
6,183 |
|
225,235 |
|
6,649 |
|
755,486 |
|
27,272 |
|
561,423 |
|
22,397 |
|
722,367 |
|
37,158 |
|
— |
|
— |
|
231,102 |
|
— |
|
1,024 |
|
(74,668 |
) |
(73,644 |
) | ||||||||||
|
Issuance of common stock, net of repurchases |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
9,590 |
|
— |
|
109 |
|
— |
|
109 |
| ||||||||||
|
Employee stock-based compensation |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
654 |
|
— |
|
654 |
| ||||||||||
|
Series D Convertible Preferred Stock issuance costs |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(5 |
) |
— |
|
— |
|
— |
|
— |
|
|
|
— |
|
— |
| ||||||||||
|
Net and comprehensive loss |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(39,901 |
) |
(39,901 |
) | ||||||||||
|
Balance at December 31, 2013 |
|
326,591 |
|
6,183 |
|
225,235 |
|
6,649 |
|
755,486 |
|
27,272 |
|
561,423 |
|
22,397 |
|
722,367 |
|
37,153 |
|
— |
|
— |
|
240,692 |
|
— |
|
1,787 |
|
(114,569 |
) |
(112,782 |
) | ||||||||||
|
Issuance of common stock |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
2,568 |
|
— |
|
28 |
|
— |
|
28 |
| ||||||||||
|
Employee stock-based compensation |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
641 |
|
— |
|
641 |
| ||||||||||
|
Issuance of Series E Convertible Preferred stock, net of issuance costs |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
2,671,626 |
|
32,606 |
|
— |
|
— |
|
— |
|
— |
|
— |
| ||||||||||
|
Issuance of common stock warrants |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
175 |
|
— |
|
175 |
| ||||||||||
|
Reclass of warrant liability to additional paid-in capital |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
34 |
|
— |
|
34 |
| ||||||||||
|
Net and comprehensive loss |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(31,964 |
) |
(31,964 |
) | ||||||||||
|
Balance at December 31, 2014 |
|
326,591 |
|
$ |
6,183 |
|
225,235 |
|
$ |
6,649 |
|
755,486 |
|
$ |
27,272 |
|
561,423 |
|
$ |
22,397 |
|
722,367 |
|
$ |
37,153 |
|
2,671,626 |
|
$ |
32,606 |
|
243,260 |
|
$ |
— |
|
$ |
2,665 |
|
$ |
(146,533 |
) |
$ |
(143,868 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
See accompanying notes.
AVINGER, INC.
(In thousands)
|
|
|
Year Ended |
| |||||||
|
|
|
December 31, |
| |||||||
|
|
|
2014 |
|
2013 |
|
2012 |
| |||
|
|
|
|
|
|
|
|
| |||
|
Cash flows from operating activities |
|
|
|
|
|
|
| |||
|
Net loss |
|
$ |
(31,964 |
) |
$ |
(39,901 |
) |
$ |
(33,864 |
) |
|
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
| |||
|
Depreciation and amortization |
|
1,451 |
|
1,501 |
|
772 |
| |||
|
Amortization of debt issuance costs and debt discount |
|
212 |
|
133 |
|
— |
| |||
|
Stock-based compensation |
|
641 |
|
654 |
|
449 |
| |||
|
Remeasurement of warrant and embedded derivatives |
|
(378 |
) |
1 |
|
— |
| |||
|
Noncash interest expense |
|
3,485 |
|
1,221 |
|
— |
| |||
|
Loss on extinguishment of convertible notes |
|
1,234 |
|
— |
|
— |
| |||
|
Provision for doubtful accounts receivable |
|
— |
|
45 |
|
36 |
| |||
|
Provision for excess and obsolete inventories |
|
(48 |
) |
15 |
|
1 |
| |||
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
| |||
|
Accounts receivable |
|
(441 |
) |
(443 |
) |
(182 |
) | |||
|
Inventories |
|
1,714 |
|
(3,069 |
) |
(3,851 |
) | |||
|
Prepaid expenses and other current assets |
|
444 |
|
(230 |
) |
(123 |
) | |||
|
Other assets |
|
2 |
|
139 |
|
— |
| |||
|
Accounts payable |
|
17 |
|
(275 |
) |
162 |
| |||
|
Accrued compensation |
|
(128 |
) |
281 |
|
271 |
| |||
|
Accrued expenses and other current liabilities |
|
2,080 |
|
(523 |
) |
952 |
| |||
|
Other liabilities |
|
(122 |
) |
(204 |
) |
143 |
| |||
|
|
|
|
|
|
|
|
| |||
|
Net cash used in operating activities |
|
(21,801 |
) |
(40,655 |
) |
(35,234 |
) | |||
|
|
|
|
|
|
|
|
| |||
|
Cash flows from investing activities |
|
|
|
|
|
|
| |||
|
Purchase of property and equipment |
|
(117 |
) |
(496 |
) |
(288 |
) | |||
|
|
|
|
|
|
|
|
| |||
|
Net cash used in investing activities |
|
(117 |
) |
(496 |
) |
(288 |
) | |||
|
|
|
|
|
|
|
|
| |||
|
Cash flows from financing activities |
|
|
|
|
|
|
| |||
|
Principal paydown of capital lease obligations |
|
(17 |
) |
(18 |
) |
(9 |
) | |||
|
Proceeds from borrowings, net of issuance costs |
|
— |
|
19,281 |
|
— |
| |||
|
Proceeds from convertible notes, net of issuance costs |
|
4,700 |
|
13,399 |
|
— |
| |||
|
Proceeds from the issuance of convertible preferred stock, net of issuance costs |
|
19,155 |
|
(5 |
) |
37,106 |
| |||
|
Payments for deferred initial public offering costs |
|
(1,848 |
) |
— |
|
— |
| |||
|
Proceeds from the issuance of common stock |
|
23 |
|
98 |
|
206 |
| |||
|
|
|
|
|
|
|
|
| |||
|
Net cash provided by financing activities |
|
22,013 |
|
32,755 |
|
37,303 |
| |||
|
|
|
|
|
|
|
|
| |||
|
Net change in cash and cash equivalents |
|
95 |
|
(8,396 |
) |
1,781 |
| |||
|
|
|
|
|
|
|
|
| |||
|
Cash and cash equivalents, beginning of year |
|
12,221 |
|
20,617 |
|
18,836 |
| |||
|
|
|
|
|
|
|
|
| |||
|
Cash and cash equivalents, end of year |
|
$ |
12,316 |
|
$ |
12,221 |
|
$ |
20,617 |
|
|
|
|
|
|
|
|
|
| |||
|
Supplemental disclosure of cash flow information |
|
|
|
|
|
|
| |||
|
Cash paid for interest |
|
$ |
2,281 |
|
$ |
1,587 |
|
$ |
6 |
|
|
|
|
|
|
|
|
|
| |||
|
Noncash investing and financing activities: |
|
|
|
|
|
|
| |||
|
Conversion of convertible notes and accrued interest into Series E convertible preferrred stock |
|
$ |
11,582 |
|
$ |
— |
|
$ |
— |
|
|
Landlord paid tenant improvements |
|
— |
|
— |
|
369 |
| |||
|
Accounts payable for purchases of property and equipment |
|
— |
|
20 |
|
200 |
| |||
|
Capital lease obligations for property and equipment |
|
— |
|
23 |
|
25 |
| |||
|
Reclassification of stock options early exercised to liability |
|
— |
|
— |
|
39 |
| |||
|
Vesting of common stock subject to repurchase |
|
5 |
|
10 |
|
21 |
| |||
|
Embedded derivatives associated with convertible notes |
|
— |
|
179 |
|
— |
| |||
|
Issuance of common stock warrants |
|
175 |
|
1 |
|
— |
| |||
|
Reclass of warrant liability to additional paid-in capital |
|
34 |
|
— |
|
— |
| |||
|
Transfer between inventory and property and equipment |
|
(916 |
) |
1,829 |
|
1,341 |
| |||
See accompanying notes.
AVINGER, INC.
1. Organization
Organization, Nature of Business
Avinger, Inc. (the “Company”), a Delaware corporation, was founded in March 2007 by cardiologist and medical device entrepreneur Dr. John B. Simpson. The Company designs, manufactures and sells image-guided, catheter-based systems that are used by physicians to treat patients with peripheral arterial disease (“PAD”). Patients with PAD have a build-up of plaque in the arteries that supply blood to the arms and legs. The Company manufactures and sells a suite of products in the United States and in select European markets. The Company has developed its lumivascular platform, which integrates optical coherence tomography (“OCT”) visualization with interventional catheters and is the industry’s only system that provides real-time intravascular imaging during the treatment portion of PAD procedures. The Company’s lumivascular platform consists of a capital component, Lightbox, as well as a variety of disposable catheter products. The Company’s current products include its non-imaging catheters, Wildcat and Kittycat, as well as its lumivascular platform products, Ocelot, Ocelot PIXL and Ocelot MVRX, all of which are designed to allow physicians to penetrate a total blockage in an artery, known as a chronic total occlusion (“CTO”). The Company is also developing Pantheris, its image-guided atherectomy device, designed to allow physicians to precisely remove arterial plaque in PAD patients. Pantheris is currently undergoing a U.S. clinical trial intended to support a 510(k) submission to the U.S. Food and Drug Administration (“FDA”) in the second half of 2015. The Company is located in Redwood City, California.
Reverse Stock Split
On January 14, 2015, the Company’s Board of Directors approved an amendment to the Company’s amended and restated certificate of incorporation to effect a 1-for-45 reverse stock split of the Company’s common stock and convertible preferred stock. The par value of the common stock and convertible preferred stock was not adjusted as a result of the reverse stock split. All common stock, convertible preferred stock, stock options and warrants, and per share amounts in the financial statements have been retroactively adjusted for all periods presented to give effect to the reverse stock split. The reverse stock split was effected on January 28, 2015.
Initial Public Offering
In January 2015, the Company issued and sold 5,000,000 shares of its common stock in its initial public offering (“IPO”) at a public offering price of $13.00 per share, for net proceeds of approximately $56,783,000 after deducting underwriting discounts and commissions of approximately $4,550,000 and expenses of approximately $3,667,000. Upon the closing of the IPO, all shares of convertible preferred stock then outstanding converted into an aggregate of 6,967,925 shares of common stock.
2. Summary of Significant Accounting Policies
Basis of Presentation
The financial statements have been prepared in accordance with United States generally accepted accounting principles (“U.S. GAAP”) and pursuant to the rules and regulations of the United States Securities and Exchange Commission (“SEC”).
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the amounts and disclosures reported in the financial statements. Management uses significant judgment when making estimates related to its common stock valuation and related stock-based compensation, the valuation of the common stock warrants, the valuation of compound embedded derivatives,
provisions for doubtful accounts receivable and excess and obsolete inventories, clinical trial accruals, and its reserves for sales returns and warranty costs. Management bases its estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results could differ from those estimates.
Fair Value of Financial Instruments
The Company has evaluated the estimated fair value of its financial instruments as of December 31, 2014 and 2013. Financial instruments consist of cash and cash equivalents, accounts receivable and payable, and other current liabilities, borrowings, convertible notes, warrant liabilities and embedded derivatives. The carrying amounts of cash and cash equivalents, accounts receivable and payable, and other current liabilities approximate their respective fair values because of the short-term nature of those instruments. Based upon the borrowing terms and conditions currently available to the Company, the carrying values of the borrowings and convertible notes approximate fair value. Fair value accounting is applied to the warrant liabilities and embedded derivatives that are recorded at fair value in the financial statements.
Cash and Cash Equivalents
The Company considers all highly liquid investments with an original maturity of three months or less at the time of purchase to be cash equivalents. Cash equivalents are considered available-for-sale marketable securities and are recorded at fair value, based on quoted market prices. As of December 31, 2014 and 2013, the Company’s cash equivalents are entirely comprised of investments in money market funds. Any related unrealized gains and losses are recorded in other comprehensive income (loss) and included as a separate component of stockholders’ deficit. There were no unrealized gains and losses as of December 31, 2014 and 2013. Any realized gains and losses and interest and dividends on available-for-sale securities are included in interest income or expense and computed using the specific identification cost method.
Restricted Cash
At December 31, 2014 and 2013, a deposit of $255,000 was restricted from withdrawal. The restricted cash secures obligations of the Company associated with its corporate credit card. The restricted deposit account is included in prepaid expenses and other current assets.
Concentration of Credit Risk, and Other Risks and Uncertainties
Financial instruments that potentially subject the Company to credit risk consist of cash and cash equivalents and accounts receivable to the extent of the amounts recorded on the balance sheets.
The Company’s policy is to invest in cash and cash equivalents, consisting of money market funds. These financial instruments are held in Company accounts at one financial institution. The counterparties to the agreements relating to the Company’s investments consist of financial institutions of high credit standing.
The Company provides for uncollectible amounts when specific credit problems arise. Management’s estimates for uncollectible amounts have been adequate, and management believes that all significant credit risks have been identified at December 31, 2014 and 2013.
The Company’s accounts receivable are due from a variety of health care organizations in the United States and select European markets. At December 31, 2014 and 2013, there were none and three, respectively, customers that represented 10% or more of the Company’s accounts receivable. For the years ended December 31, 2014, 2013 and 2012, there were no customers that represented 10% or more of revenues. Disruption of sales orders or a deterioration of financial condition of its customers would have a negative impact on the Company’s financial position and results of operations.
The Company commenced in-house manufacture of certain commercial products in December 2012, including the production of the Ocelot family of catheters. Certain of the Company’s product components and sub-assemblies continue to be manufactured by sole suppliers. Disruption in component or sub-assembly supply from these manufacturers or from in-house production would have a negative impact on the Company’s financial position and results of operations.
The Company is subject to certain risks, including that its devices may not be approved or cleared for marketing by governmental authorities or be successfully marketed. There can be no assurance that the Company’s products will continue to be accepted in the marketplace, nor can there be any assurance that any future devices can be developed or manufactured at an acceptable cost and with appropriate performance characteristics. The Company is also subject to risks common to companies in the medical device industry, including, but not limited to, new technological innovations, dependence upon third-party payors to provide adequate coverage and reimbursement, dependence on key personnel and suppliers, protection of proprietary technology, product liability claims, and compliance with government regulations.
Existing or future devices developed by the Company may require approvals or clearances from the FDA or international regulatory agencies. In addition, in order to continue the Company’s operations, compliance with various federal and state laws is required. If the Company were denied or delayed in receiving such approvals or clearances, it may be necessary to adjust operations to align with the Company’s currently approved portfolio. If clearance for the products in the current portfolio were withdrawn by the FDA, this may have a material adverse impact on the Company.
Accounts Receivable
Trade accounts receivable are recorded at the invoiced amount and do not bear interest. The allowance for doubtful accounts is the Company’s best estimate of the amount of probable credit losses in the Company’s existing accounts receivable. The Company determines the allowance for doubtful accounts based upon an aging of accounts receivable, historical experience, and management judgment. Accounts receivable balances are reviewed individually for collectability. To date, the Company has not experienced significant credit- related losses.
Inventories
Inventories are valued at the lower of cost or market. Cost is determined using the first-in, first-out method for all inventories. The Company’s policy is to write down inventory that has become obsolete, inventory that has a cost basis in excess of its expected net realizable value, and inventory in excess of expected requirements. The estimate of excess quantities is subjective and primarily dependent on the estimates of future demand for a particular product. If the estimate of future demand is too high, the Company may have to increase the reserve for excess inventory for that product and record a charge to the cost of revenues. Inventory used in clinical trials is expensed at the time of production and recorded as research and development expense. Prior to receiving FDA approval, costs related to purchases of materials and the manufacturing of the product are recorded as research and development expense. All direct manufacturing costs incurred after FDA approval are capitalized into inventory.
Property and equipment
Property and equipment are recorded at cost. Repairs and maintenance costs are expensed as incurred. Depreciation and amortization are calculated using the straight-line method over the estimated useful lives of the assets of three to five years. Depreciation expense includes the amortization of assets acquired under capital leases and equipment located at customer sites. Equipment held by customers is comprised of the Lightboxes located at customer sites under a lease agreement and are recorded at cost. Upon execution of a lease agreement, the related equipment is reclassified from inventory to the property and equipment account. Depreciation expense for equipment held by customers is recorded as a component of cost of revenues. Leasehold improvements and assets recorded under capital leases are amortized using the straight-line method over the shorter of the lease term or estimated useful economic life of the asset.
Deferred Initial Public Offering Costs
Deferred offering costs, which primarily consist of direct incremental legal and accounting fees relating to the IPO, are capitalized. The deferred offering costs will be offset against IPO proceeds upon the consummation of the offering. In the event the offering is terminated, deferred offering costs will be expensed. As of December 31, 2014, $2,608,000 of deferred offering costs were capitalized in other assets on the balance sheet. No deferred offering costs were capitalized as of December 31, 2013.
Impairment of Long-Lived Assets
The Company reviews long-lived assets, including property and equipment, for impairment whenever events or changes in business circumstances indicate that the carrying amount of the assets may not be fully recoverable. If indicators of impairment exist, an impairment loss would be recognized when estimated undiscounted future cash flows expected to result from the use of the asset and its eventual disposition are less than its carrying amount. Impairment, if any, is measured as the amount by which the carrying amount of the long-lived asset exceeds its fair value. The Company has not recorded any impairment of long-lived assets since inception.
Convertible Preferred Stock
The Company records its convertible preferred stock at fair value on the dates of issuance, net of issuance costs. A redemption event will only occur upon the liquidation or winding up of the Company, a greater than 50% change in control, or sale of substantially all of the assets of the Company. In the event of a change of control of the Company, proceeds received from the sale of such shares will be distributed in accordance with the liquidation preferences set forth in the Company’s amended and restated certificate of incorporation unless the holders of convertible preferred stock otherwise agree or have converted their shares into shares of common stock. Therefore, convertible preferred stock is classified outside of stockholders’ deficit on the balance sheets as events triggering the liquidation preferences are not solely within the Company’s control. The Company has elected not to adjust the carrying values of the convertible preferred stock to the redemption value of such shares since it is uncertain whether or when a redemption event will occur. Subsequent adjustments to increase the carrying values to the redemption values will be made when it becomes probable that such redemption will occur.
Warrant Liability and Embedded Derivative Instruments
The Company accounts for its warrants for shares of common stock in accordance with the accounting guidance for derivatives. The accounting guidance provides a two-step model to be applied in determining whether a financial instrument is indexed to an entity’s own stock and, therefore, qualifies for a scope exception. The two-step model requires a contract for a financial instrument to be both (1) indexed to the entity’s own stock and (2) classified in the stockholders’ deficit section of the balance sheet. If a financial instrument qualifies for a scope exception, it would not be considered a derivative financial instrument.
As the price per share of the common stock warrants issued with the convertible notes was not fixed until the issuance of the Series E Convertible Preferred Stock in September 2014, these warrants were initially classified as a derivative liability. As a derivative liability, the warrants were initially recorded at fair value and were subject to remeasurement at each balance sheet date until September 2014. Any change in fair value as a result of a remeasurement was recognized as a component of other income (expense), net in the statements of operations and comprehensive loss. The Company re-evaluated the terms of the common stock warrants issued with the convertible notes after the issuance of the Series E Convertible Preferred Stock in September 2014 and determined that they then met the first criterion of the two-step model. Accordingly, the associated current fair value of the warrant liability was reclassified to additional paid-in capital in the stockholders’ deficit section of the balance sheet at that time, thus satisfying the second criterion of the two-step model.
The Company records a compound derivative asset or liability related to redemption features embedded within the outstanding convertible notes. The convertible notes issued in 2013 and 2014 included features which were determined to be embedded derivatives requiring bifurcation and separate accounting. The embedded derivatives were initially recorded at fair value and are subject to remeasurement as of each balance sheet date. Any
change in fair value is recognized as a component of other income (expense), net in the statements of operations and comprehensive loss.
Revenue Recognition
The Company’s revenues are derived from (1) sale of its Lightbox (2) sale of disposables, which consist of catheters and accessories, and (3) sale of customer service contracts. The Company recognizes revenue in accordance with Accounting Standards Codification (“ASC”) 605-10, Revenue Recognition, when persuasive evidence of an arrangement exists, the fee is fixed or determinable, collection of the fee is probable and delivery has occurred. For all sales, the Company uses either a signed agreement or a binding purchase order as evidence of an arrangement.
The Company’s revenue recognition policies generally result in revenue recognition at the following points:
1. Lighbox sales: The Company sells its products directly to hospitals and medical centers. Provided all other criteria for revenue recognition have been met, the Company recognizes revenue for Lightbox sales directly to end customers when delivery and acceptance occurs, which is defined as receipt by the Company of an executed form by the customer acknowledging that the training and installation process is complete.
2. Sales of disposables: Disposable revenues consist of sales of the Company’s catheters and accessories and are recognized when the product has shipped, risk of loss and title has passed to the customer and collectability is reasonably assured.
3. Service revenue: Service revenue is recognized ratably over the term of the service period. To date service revenue has been insignificant.
The Company offers its customers the ability to purchase or lease its Lightbox. The Company recovers the cost of providing the leased Lightbox through a premium in the amount charged for its disposable products in comparison to a standalone purchase. When a Lightbox is placed under a lease agreement, the Company retains title to the equipment and it remains capitalized on its balance sheet under property and equipment. Depreciation expense on these leased Lightboxes is recorded to cost of revenues on a straight-line basis. The costs to maintain these leased Lightboxes are charged to cost of revenues as incurred.
The Company evaluates its lease agreements and accounts for these contracts under the guidance in ASC 840, Leases and ASC 605-25, Revenue Recognition—Multiple Element Arrangements. The guidance requires arrangement consideration to be allocated between a lease deliverable and a non-lease deliverable based upon the relative selling-price of the deliverables, using a specific hierarchy. The hierarchy is as follows: vendor- specific objective evidence of fair value of the respective elements, third-party evidence of selling price, or best estimate of selling price (“BESP”). The Company allocates arrangement consideration using BESP.
The Company assessed whether the embedded lease is an operating lease or sales-type lease. Based on the Company’s assessment of the guidance and given that any payments under the lease agreements are dependent upon contingent future sales, it was determined that collectability of the minimum lease payments is not reasonably predictable. Accordingly, the Company concluded the embedded lease did not meet the criteria of a sales-type lease and accounts for it as an operating lease. The Company recognizes revenue allocated to the lease as the contingent disposable product purchases are delivered and are included in revenues within the statement of operations and comprehensive loss.
The Company estimates reductions in revenue for potential returns of products by customers. In making such estimates, management analyzes historical returns, current economic trends and changes in customer demand and acceptance of its products. The Company expenses shipping and handling costs as incurred and includes them in the cost of revenues. In those cases where the Company bills shipping and handling costs to customers, it will classify the amounts billed as a component of revenue.
Cost of Revenues
Cost of revenues consists primarily of manufacturing overhead costs, material costs and direct labor. A significant portion of the Company’s cost of revenues currently consists of manufacturing overhead costs. These overhead costs include the cost of quality assurance, material procurement, inventory control, facilities, equipment and operations supervision and management. Cost of revenues also includes depreciation expense for the Lightboxes under lease agreements and certain direct costs such as shipping costs.
Product Warranty Costs
The Company typically offers a one-year warranty for parts and labor on its products commencing upon the transfer of title and risk of loss to the customer. The Company accrues for the estimated cost of product warranties upon invoicing its customers, based on historical results. The warranty obligation is affected by product failure rates, material usage and service delivery costs incurred in correcting a product failure. Should actual product failure rates, material usage or service delivery costs differ from these estimates, revisions to the estimated warranty liability would be required. Periodically the Company assesses the adequacy of its recorded warranty liabilities and adjusts the amounts as necessary. Warranty provisions and claims are summarized as follows (in thousands):
|
|
|
Year Ended |
| |||||||
|
|
|
2014 |
|
2013 |
|
2012 |
| |||
|
|
|
|
|
|
|
|
| |||
|
Balance beginning of year |
|
$ |
105 |
|
$ |
11 |
|
$ |
— |
|
|
Warranty provision |
|
140 |
|
230 |
|
11 |
| |||
|
Usage |
|
(78 |
) |
(136 |
) |
— |
| |||
|
|
|
|
|
|
|
|
| |||
|
Balance end of year |
|
$ |
167 |
|
$ |
105 |
|
$ |
11 |
|
Research and Development
The Company expenses research and development costs as incurred. Research and development expenses include personnel and personnel-related costs, costs associated with pre-clinical and clinical development activities, and costs for prototype products that are manufactured prior to market approval for that prototype product; internal and external costs associated with the Company’s regulatory compliance and quality assurance functions, including the costs of outside consultants and contractors that assist in the process of submitting and maintaining regulatory filings; and overhead costs, including allocated facility and related expenses.
Clinical Trials
The Company accrues and expenses costs for its clinical trial activities performed by third parties, including clinical research organizations and other service providers, based upon estimates of the work completed over the life of the individual study in accordance with associated agreements. The Company determines these estimates through discussion with internal personnel and outside service providers as to progress or stage of completion of trials or services pursuant to contracts with clinical research organizations and other service providers and the agreed-upon fee to be paid for such services.
Advertising Costs
The Company expenses advertising costs as incurred. Advertising costs include design and production costs, including website development, physician and patient testimonial videos, written media campaigns, and other items. Advertising costs of approximately $720,000, $321,000 and $732,000 were expensed during the years ended December 31, 2014, 2013 and 2012, respectively.
Common Stock Valuation and Stock-Based Compensation
Stock-based awards issued to employees are recorded at fair value as of the grant date using the Black-Scholes option-pricing model and recognized as expense on a straight-line basis over the vesting period of the award. Because noncash stock-based compensation expense is based on awards ultimately expected to vest, it is reduced by an estimate for future forfeitures. Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from estimates.
The fair value of the Company’s common stock is determined by its Board of Directors with assistance from management and third-party valuation specialists. Management’s approach to estimate the fair value of the Company’s common stock is consistent with the methods outlined in the American Institute of Certified Public Accountants Practice Aid, Valuation of Privately-Held Company Equity Securities Issued as Compensation. Management considers several factors to estimate enterprise value, including significant milestones that would generally contribute to increases in the value of the Company’s common stock.
Foreign Currency
The Company records net gains and losses resulting from foreign exchange transactions as a component of foreign currency exchange losses in other income (expense), net. During the years ended December 31, 2014 and 2012, the Company recorded $21,000 and $8,000 of foreign currency exchange net losses, respectively, and $11,000 of net gains during the year ended December 31, 2013.
Income Taxes
The Company utilizes the liability method of accounting for income taxes. Under this method, deferred tax assets and liabilities are determined based on differences between financial reporting and tax reporting bases of assets and liabilities and are measured using enacted tax rates and laws that are expected to be in effect when the differences are expected to reverse. Valuation allowances are established when necessary to reduce deferred tax assets to the amounts expected to be realized. The Company’s policy is to record interest and penalties on uncertain tax positions as income tax expense when they occur. During the years ended December 31, 2014, 2013 and 2012, the Company did not recognize accrued interest or penalties related to unrecognized tax benefits.
Net Loss per Share
Basic net loss per share is computed by dividing the net loss by the weighted average number of shares of common stock outstanding during the period, without consideration for potential dilutive common shares. Diluted net loss per share is computed by dividing the net loss by the weighted average number of shares of common stock and dilutive potential shares of common stock outstanding during the period. Common stock shares subject to repurchase are excluded from the calculations as the continued vesting of such shares is contingent upon the holders’ continued service to the Company. For the computation of net loss per share, common stock shares subject to repurchase of 583, 1,249 and 4,509 were excluded from the calculations as of December 31, 2014, 2013 and 2012, respectively. Since the Company was in a loss position for all periods presented, basic net loss per share is the same as diluted net loss per share as the inclusion of all potential dilutive common shares would have been anti-dilutive.
The Company allocates no loss to participating securities because they have no contractual obligation to share in the losses of the Company. The shares of the Company’s convertible preferred stock participate in any dividends declared by the Company and are therefore considered to be participating securities.
Net loss per share was determined as follows (in thousands, except per share data):
|
|
|
Year Ended |
| |||||||
|
|
|
| ||||||||
|
|
|
2014 |
|
2013 |
|
2012 |
| |||
|
|
|
|
|
|
|
|
| |||
|
Net loss |
|
$ |
(31,964 |
) |
$ |
(39,901 |
) |
$ |
(33,864 |
) |
|
Weighted average common stock outstanding |
|
241 |
|
234 |
|
209 |
| |||
|
Net loss per share, basic and diluted |
|
$ |
(132.63 |
) |
$ |
(170.52 |
) |
$ |
(162.03 |
) |
In addition to the outstanding convertible notes (Note 8), the following potentially dilutive securities outstanding have been excluded from the computations of diluted weighted average shares outstanding because such securities have an antidilutive impact due to losses reported, in common stock equivalent shares:
|
|
|
December 31, |
| ||||
|
|
|
2014 |
|
2013 |
|
2012 |
|
|
|
|
|
|
|
|
|
|
|
Convertible preferred stock outstanding |
|
5,262,728 |
|
2,591,102 |
|
2,591,102 |
|
|
Common stock options |
|
3,010,373 |
|
398,740 |
|
313,153 |
|
|
Common stock warrants |
|
1,552,327 |
|
51,601 |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
9,825,428 |
|
3,041,443 |
|
2,904,255 |
|
Comprehensive Loss
For the years ended December 31, 2014, 2013 and 2012, there was no difference between comprehensive loss and the Company’s net loss.
Segment and Geographical Information
The Company operates in one segment. Primarily all of the Company’s long-lived assets are based in the United States. Long-lived assets are comprised of property and equipment. For the years ended December 31, 2014, 2013 and 2012, 99%, 98%, 98%, respectively, of the Company’s revenues, were in the United States, based on the shipping location of the external customer.
Recent Accounting Pronouncements
In May 2014, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2014-09, Revenue from Contracts with Customers: Topic 606 (ASU 2014-09), to supersede nearly all existing revenue recognition guidance under U.S. GAAP. The core principle of ASU 2014-09 is to recognize revenues when promised goods or services are transferred to customers in an amount that reflects the consideration that is expected to be received for those goods or services. ASU 2014-09 defines a five step process to achieve this core principle and, in doing so, it is possible more judgment and estimates may be required within the revenue recognition process than required under existing U.S. GAAP, including identifying performance obligations in the contract, estimating the amount of variable consideration to include in the transaction price and allocating the transaction price to each separate performance obligation. ASU 2014-09 is effective for the Company in the fiscal year ended December 31, 2017 using either of two methods: (i) retrospective to each prior reporting period presented with the option to elect certain practical expedients as defined within ASU 2014-09; or (ii) retrospective with the cumulative effect of initially applying ASU 2014-09 recognized at the date of initial application and providing certain additional disclosures as defined per ASU 2014-09. The Company is currently evaluating the impact of the pending adoption of ASU 2014-09 on its financial statements.
In August 2014, the FASB issued ASU No. 2014-15—Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern under ASC Subtopic 205-40, Presentation of Financial Statements—Going Concern. ASU No. 2014-15 provides guidance about management’s responsibility to evaluate whether there is substantial doubt about an entity’s ability to continue as a going concern and to provide related footnote disclosures. Management’s evaluation should be based on relevant conditions and events that are known or reasonably knowable at the date that the financial statements are issued (or at the date that the financial statements are available to be
issued when applicable). Substantial doubt about an entity’s ability to continue as a going concern exists when relevant conditions and events, considered in the aggregate, indicate that it is probable that the entity will be unable to meet its obligations as they become due within one year after the date that the financial statements are issued (or available to be issued). ASU No. 2014-15 is effective for the Company in the fiscal year ended December 31, 2016 and early adoption is permitted. The Company is currently evaluating the impact of the pending adoption of ASU 2014-15 on its financial statements.
3. Fair Value Measurements
The Company measures certain financial assets and liabilities at fair value on a recurring basis, including cash equivalents, common stock warrants and the derivative instruments related to redemption features embedded within its outstanding convertible notes. Fair value is an exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or a liability. A three- tier fair value hierarchy is established as a basis for considering such assumptions and for inputs used in the valuation methodologies in measuring fair value:
Level 1—Quoted prices in active markets for identical assets or liabilities.
Level 2—Inputs other than quoted prices included within Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3—Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
As of December 31, 2014 and 2013, cash equivalents and restricted cash were all categorized as Level 1 and consisted of money market funds. The Company issued convertible notes in 2013 and 2014 (Note 8). In connection with the convertible notes, the Company agreed to issue warrants to purchase shares of its common stock. As the price per share of the common stock warrants was not fixed until the issuance of the Series E Convertible Preferred Stock in September 2014, they were classified as a derivative liability and were subject to remeasurement at each balance sheet date until September 2014. The convertible notes also contained redemption features which were determined to be a compound embedded derivative requiring fair value accounting. The common stock warrant liability and embedded derivatives in the convertible notes were categorized as Level 3. When a determination is made to classify a financial instrument within Level 3, the determination is based upon the significance of the unobservable inputs to the overall fair value measurement. However, Level 3 financial instruments typically include, in addition to the unobservable inputs, observable inputs (that is, components that are actively quoted and can be validated to external sources). Any change in fair value is recognized as a component of other income (expense), net, on the statements of operations and comprehensive loss.
There were no transfers in or out of Level 1 and Level 2 fair value measurements during the years ended December 31, 2014, 2013 and 2012.
Common Stock Warrant Liability
The following table sets forth a summary of the changes in the estimated fair value of the Company’s common stock warrant liability, which represents a financial instrument classified as Level 3. Accordingly, the expense in the table below includes changes in fair value due in part to observable factors that are part of the Level 3 methodology (in thousands):
|
|
|
Year Ended |
| ||||
|
|
|
| |||||
|
|
|
2014 |
|
2013 |
| ||
|
|
|
|
|
|
| ||
|
Fair value - beginning of year |
|
$ |
(6 |
) |
$ |
— |
|
|
Issuance of warrants |
|
— |
|
(1 |
) | ||
|
Change in fair value recorded in other income (expense), net |
|
(28 |
) |
(5 |
) | ||
|
Reclass of warrant liability to additional paid-in capital |
|
34 |
|
— |
| ||
|
|
|
|
|
|
| ||
|
Fair value - end of year |
|
$ |
— |
|
$ |
(6 |
) |
The fair value of the common stock warrants was determined by using an option pricing model to allocate the total enterprise value to the various securities within the Company’s capital structure. The model’s inputs reflect assumptions that market participants would use in pricing the instrument in a current period transaction and included:
|
|
|
Year Ended |
|
|
|
|
December 31, |
|
|
|
|
2013 |
|
|
|
|
|
|
|
Time to liquidity (years) |
|
2.0 |
|
|
Expected volatility |
|
55 |
% |
|
Discounted cash flow rate |
|
25 |
% |
|
Risk-free interest rate |
|
0.38 |
% |
|
Marketability discount rate |
|
23 |
% |
The time to liquidity input was based on the Company’s estimate of when potential liquidity could be provided to stockholders. The volatility factor was based on the average historic price volatility for publicly-traded industry peers. The discounted cash flow rate takes into consideration a company specific risk premium, market risk premium and an assumed risk free rate of return. The risk-free interest rate was based on the yields of U.S. Treasury securities with maturities similar to the time to liquidity. The marketability discount is used to reflect that private company securities are generally less liquid than the securities of a public company. These assumptions are inherently subjective and involve significant management judgment. Generally, increases (decreases) in the fair value of the underlying common stock would result in a directionally similar impact to the fair value measurement. As of December 31, 2013, the common stock warrant liability is included in other long-term liabilities on the balance sheet. As the price per share of the common stock warrants was not fixed until the issuance of the Series E Convertible Preferred Stock in September 2014, they were classified as a derivative liability and were subject to remeasurement at each balance sheet date. Contemporaneous with the Series E Convertible Preferred Stock issuance, the Company determined that these common stock warrants met the requirements for equity classification and the fair value of the common stock warrant liability was reclassified to additional paid-in capital.
Embedded Derivatives in Convertible Notes
The following table sets forth a summary of the changes in the estimated fair value of the Company’s compound embedded derivative associated with its convertible notes, which represent a financial instrument classified as Level 3. Accordingly, the income (expense) in the table below includes changes in fair value due in part to observable factors that are part of the Level 3 methodology (in thousands):
|
|
|
Year Ended |
| ||||
|
|
|
| |||||
|
|
|
2014 |
|
2013 |
| ||
|
|
|
|
|
|
| ||
|
Fair value - beginning of year |
|
$ |
(175 |
) |
$ |
— |
|
|
Issuance of convertible notes |
|
— |
|
(179 |
) | ||
|
Change in fair value recorded in other income (expense), net |
|
406 |
|
4 |
| ||
|
|
|
|
|
|
| ||
|
Fair value - end of year |
|
$ |
231 |
|
$ |
(175 |
) |
The Company determined the value of the compound derivative utilizing a Monte Carlo Simulation model. The inputs used to determine the estimated fair value of the derivative instrument include the probability of an
underlying event triggering the embedded derivative occurring and its timing. The fair value measurement is based upon significant inputs not observable in the market. The inputs included the probability that the Company would need to raise additional equity in 2014, as well as various financing and exit events in 2015. These assumptions are inherently subjective and involve significant management judgment. The following table summarizes these various assumptions as of December 31, 2014 and 2013:
|
|
|
Year Ended |
| ||
|
|
|
December 31, |
| ||
|
|
|
2014 |
|
2013 |
|
|
|
|
|
|
|
|
|
Equity financing in 2014 |
|
100.0 |
% |
100.0 |
% |
|
Equity financing in 2015 |
|
14.3 |
% |
58.2 |
% |
|
Liquidation |
|
0.1 |
% |
1.5 |
% |
|
Initial public offering |
|
79.5 |
% |
16.7 |
% |
|
Change of control |
|
6.2 |
% |
25.1 |
% |
The compound embedded derivative liability is included in other long-term liabilities as of December 31, 2013 and the compound embedded derivative asset is included in other long-term assets as of December 31, 2014, on the balance sheets.
4. Inventories
Inventories consisted of the following (in thousands):
|
|
|
December 31, |
| ||||
|
|
|
2014 |
|
2013 |
| ||
|
|
|
|
|
|
| ||
|
Raw materials |
|
$ |
2,265 |
|
$ |
2,563 |
|
|
Work-in-process |
|
61 |
|
181 |
| ||
|
Finished products |
|
1,665 |
|
1,997 |
| ||
|
|
|
|
|
|
| ||
|
Total inventories |
|
$ |
3,991 |
|
$ |
4,741 |
|
5. Property and Equipment, Net
Property and equipment, net, consisted of the following (in thousands):
|
|
|
December 31, |
| ||||
|
|
|
2014 |
|
2013 |
| ||
|
|
|
|
|
|
| ||
|
Computer software |
|
$ |
320 |
|
$ |
314 |
|
|
Computer equipment |
|
867 |
|
775 |
| ||
|
Machinery and equipment |
|
2,993 |
|
2,840 |
| ||
|
Furniture and fixtures |
|
542 |
|
535 |
| ||
|
Leasehold improvements |
|
655 |
|
653 |
| ||
|
Equipment held by customers |
|
1,045 |
|
2,534 |
| ||
|
|
|
6,422 |
|
7,651 |
| ||
|
|
|
|
|
|
| ||
|
Less: Accumulated depreciation and amortization |
|
(3,827 |
) |
(2,813 |
) | ||
|
Add: Construction-in-progress |
|
13 |
|
20 |
| ||
|
|
|
|
|
|
| ||
|
|
|
$ |
2,608 |
|
$ |
4,858 |
|
Depreciation expense for the years ended December 31, 2014, 2013 and 2012, was $1,451,000, $1,501,000 and $772,000, respectively. Amortization of capital leased assets included in depreciation for the years ended
December 31, 2014, 2013 and 2012, was $17,000, $16,000 and $13,000, respectively. Property and equipment includes certain equipment that is leased to customers and located at customer premises. The Company retains the ownership of the leased equipment and has the right to remove the equipment if it is not being utilized according to expectations. Depreciation expense relating to the leased equipment held by customers of $378,000, $425,000, and $14,000, was recorded in cost of revenues during the years ended December 31, 2014, 2013 and 2012. The net book value of this equipment was $760,000 and $2,216,000 at December 31, 2014 and 2013, respectively.
6. Accrued Expenses and Other Current Liabilities
Accrued expenses and other current liabilities consisted of the following (in thousands):
|
|
|
December 31, |
| ||||
|
|
|
2014 |
|
2013 |
| ||
|
|
|
|
|
|
| ||
|
Accrued professional services |
|
$ |
1,917 |
|
$ |
152 |
|
|
Accrued travel expenses |
|
464 |
|
150 |
| ||
|
Accrued sales, use and other taxes |
|
112 |
|
91 |
| ||
|
Accrued clinical trial costs |
|
359 |
|
23 |
| ||
|
Accrued interest payable |
|
1,150 |
|
571 |
| ||
|
Sales return allowance |
|
77 |
|
88 |
| ||
|
Accrued warranty |
|
167 |
|
105 |
| ||
|
Other accrued liabilities |
|
604 |
|
276 |
| ||
|
|
|
|
|
|
| ||
|
|
|
$ |
4,850 |
|
$ |
1,456 |
|
7. Borrowings
On April 18, 2013, the Company entered into a Credit Agreement (“Agreement”) with PDL BioPharma, Inc. (“PDL”) whereby PDL agreed to loan up to $40,000,000. Contemporaneous with the execution of the Agreement the Company borrowed an initial $20,000,000 (“Term Note”). Under the terms of the Agreement, if the Company achieved certain net revenue milestones prior to June 30, 2014, the Company would be eligible to borrow an additional amount between $10,000,000 and $20,000,000 (net of fees) at the Company’s election. The Company did not achieve the net revenue milestones and accordingly, there were no additional available funds to borrow under the Agreement.
The Term Note matures on April 18, 2018, has a stated interest rate of 12.0% per annum and can be prepaid by the Company at any time. A fee of 1.0% ($200,000) of the original principal amount is payable upon maturity or prepayment in full of the Term Note, and is being amortized into the Term Note. The Company pays interest-only through the first ten quarters and, thereafter, will commence repayment of principal in equal installments including accrued and unpaid interest, payable each quarter. Under the terms of the Agreement, for the first eight quarterly interest payments, or through 2015, on the Term Note the Company may elect to convert an amount of interest, up to 1.5% per annum, into additional loans, referred to as paid-in-kind, or PIK, loans. The PIK loans will accrue, be capitalized and compounded, and added to the aggregate principal balance of the Term Note. In addition to the interest and principal payments, the Company also pays a royalty, referred to as Assigned Interests, equal to 1.8% of the Company’s quarterly net revenues. Upon prepayment of the Term Note, the Company’s obligations relating to Assigned Interests continue, and will be payable through the maturity date at a reduced rate of 0.9% of the quarterly net revenues, subject to certain quarterly minimum mandatory amounts as follows (in thousands), which are payable quarterly:
|
Year Ending December 31, |
|
Mandatory |
| |
|
|
|
|
| |
|
2015 |
|
$ |
305 |
|
|
2016 |
|
305 |
| |
|
2017 |
|
305 |
| |
|
2018 |
|
310 |
| |
|
|
|
$ |
1,225 |
|
The Term Note grants PDL a security interest in substantially all current and future assets of the Company and contains customary affirmative covenants and customary negative covenants limiting the Company’s ability to, among other things and for so long as any amounts are due and owing under the Agreement, dispose of assets, undergo a change in control, merge or consolidate, enter into certain transactions with affiliates, make acquisitions, incur debt, incur liens, pay dividends, repurchase stock and make investments, in each case subject to certain exceptions. Additionally, even if the Term Note is prepaid, until there are no further obligations relating to Assigned Interests, it must comply with certain affirmative covenants and negative covenants limiting its ability to, among other things, undergo a change in control and dispose of assets, in each case subject to certain exceptions. The Agreement and the security interest agreement also contain customary events of default including, among others, payment defaults, breaches of covenants, bankruptcy and insolvency events, cross defaults with certain material indebtedness, defaults upon the entry of certain judgments against the Company, and breaches of representations and warranties. Upon an event of default, all obligations may become immediately due and payable and the stated interest rate would likely be increased to a default rate of 14.0% per annum.
The Company incurred fees and legal expenses of $519,000 in connection with the Agreement, which have been recorded as deferred financing costs on the accompanying balance sheets and are amortized using the effective interest method. The Company also paid $200,000 in fees to PDL upon origination of the Term Note, which is reflected as a discount on the debt and is being accreted over the life of the Term Note. The Company calculated an effective interest rate of 27.2% upon origination of the Term Note based on its best estimate of future cash outflows. The Company reviews its estimate of forecasted Assigned Interests payable each quarter and revisions to estimated cash flows are reflected using the retrospective method. Under the retrospective method, the Company computes a new effective interest rate based on the original carrying amount, actual cash flows to date, and remaining estimated cash flows over the maturity of the Term Note. The new effective interest rate, 18.3% as of December 31, 2014, is then used to adjust the carrying amount to the present value of the revised estimated cash flows, discounted at the new effective interest rate. For the years ended December 31, 2014 and 2013, the Company incurred interest expense of $3,380,000 and $2,492,000, respectively.
Principal and PIK loan repayments of the Term Note as of December 31, 2014 are as follows (in thousands):
|
|
|
Principal and PIK |
| |
|
Year Ending December 31, |
|
Loan Repayments |
| |
|
|
|
|
| |
|
2015 |
|
$ |
1,818 |
|
|
2016 |
|
7,273 |
| |
|
2017 |
|
7,273 |
| |
|
2018 |
|
3,636 |
| |
|
|
|
|
| |
|
|
|
20,000 |
| |
|
Add: Payment in kind interest |
|
529 |
| |
|
|
|
|
| |
|
|
|
20,529 |
| |
|
Less: Amount representing debt discount |
|
(119 |
) | |
|
|
|
|
| |
|
|
|
20,410 |
| |
|
Less: Current portion of long-term borrowings |
|
(1,873 |
) | |
|
|
|
|
| |
|
Borrowings, net of current portion |
|
$ |
18,537 |
|
8. Convertible Notes
On October 29, 2013, the Company entered into a Note and Warrant Purchase Agreement (the “Convertible Note Agreement”), as amended in May 2014, with certain existing convertible preferred stockholders, third-parties and employees for the issuance of convertible notes for up to an aggregate principal amount of $25,000,000. Under the terms of the Convertible Note Agreement, the Company issued convertible notes in October and November 2013 for total proceeds of $13,472,000, and in May and July 2014 for additional total proceeds of $4,720,000. The convertible notes bear interest at a rate of 30-day LIBOR, plus 6% per annum subject to a minimum internal rate of return of 20%. The notes will mature and the accrued interest thereon will become payable on the earlier of: (i) October 29, 2018, (ii) an event of default, or (iii) a change of control event.
The principal and accrued interest on the notes are convertible, at the option of the holder, upon a future issuance of the Company’s convertible preferred stock or common stock (the “Equity Financing”) into that same stock at a conversion price equal to 85% of the price paid by other investors in the financing event. If the holder does not elect to convert the notes upon the closing of an Equity Financing, and such financing raises net proceeds of at least $20,000,000, the Company may repay the notes at 125% of the outstanding principal and accrued and unpaid interest. Upon a change of control, the Company will repay the holder, at the election of such holder, a payment equal to the greater of (i) 125% of the outstanding principal and accrued and unpaid interest, (ii) an amount equal to the return the holders of Series D preferred stock would be entitled to receive in such change of control, or (iii) the amount providing the investor with a 20% minimum internal rate of return, provided that in the event that the change of control includes any contingent payments based on future performance, the amount due and payable under clause (ii) will be recalculated at the time each installment or contingent payment is made.
In conjunction with the issuance of the convertible notes, the Company issued warrants to purchase up to the number of shares of common stock equal to 15% of the principal amount of the convertible notes divided by an exercise price per share equal to the lesser of $39.15 per share, or the price per share paid by the investors in the first bona fide preferred stock financing subsequent to the date of the convertible notes. Upon the Series E Convertible Preferred Stock issuance in September 2014, the exercise price per share was fixed at $12.60 per share and the Company issued warrants to purchase a total of 216,547 shares of common stock. The warrants are immediately exercisable and expire upon the earlier of September 2019, the closing of the Company’s IPO or upon the consummation of a change of control of the Company. The estimated fair value of the warrants upon issuance, of $1,000, was based on an option pricing model. The Company recorded the fair value of the warrants at issuance as a debt discount and as a warrant liability. The debt discount is being accreted using the effective interest method as additional interest expense over the term of the convertible notes.
The convertible notes have redemption features that were determined to be compound embedded derivatives requiring bifurcation and separate accounting. The fair value of the compound embedded derivative upon issuance was determined to be a liability of $179,000. The fair value of these derivative instruments was recognized as an additional discount and as a derivative liability on the balance sheets upon issuance of the convertible notes.
The compound embedded derivative associated with the convertible notes requires periodic re-measurements to fair value while the instruments are still outstanding.
Through December 31, 2014, the Company incurred total debt issuance costs of $93,000 in connection with the issuance of the convertible notes. The deferred issuance costs will be amortized over the term of the convertible notes.
In September and November 2014, in connection with the issuance of the Series E Convertible Preferred Stock, $11,582,000 of the outstanding convertible notes and accrued interest thereon was converted into shares of Series E Convertible Preferred Stock (Note 11). Upon the conversion of the convertible notes, the Company recorded a net loss from the extinguishment of the debt in the amount of $1,234,000 which is reflected in other income (expense), net in the statement of operations and comprehensive loss.
The Company’s interest expense associated with the convertible notes amounted to $2,633,000 and $433,000 during the years ended December 31, 2014 and 2013, respectively, based on the minimum internal rate of return of 20%.
9. Capital Leases
Capital lease obligations consist of leased office equipment. As of December 31, 2014 and 2013, the aggregate amount of capital leases recorded within property and equipment, net, on the accompanying balance sheet is $12,000 and $29,000, respectively. The current portion of the capital lease obligations is included in accrued liabilities and the balance included within other long-term liabilities represents the long-term portion.
The future minimum lease payments as of December 31, 2014, are as follows (in thousands):
|
|
|
Future Minimum |
| |
|
Year ending December 31, |
|
Lease Payments |
| |
|
|
|
|
| |
|
2015 |
|
$ |
12 |
|
|
2016 |
|
2 |
| |
|
Total minimum payments |
|
14 |
| |
|
|
|
|
| |
|
Less: Amount representing future interest |
|
1 |
| |
|
|
|
|
| |
|
Present value of minimum lease payments |
|
$ |
13 |
|
10. Commitments and Contingencies
Lease Commitments
The Company’s operating lease obligations primarily consist of leased office, laboratory, and manufacturing space under a non-cancelable operating lease that expires in November 2016. The lease agreement includes two renewal provisions allowing the Company to extend this lease for additional periods of three years each. In addition to the minimum future lease commitments presented below, the lease requires the Company to pay property taxes, insurance, maintenance, and repair costs. The lease includes a rent holiday concession and escalation clauses for increased rent over the lease term. Rent expense is recognized using the straight-line method over the term of the lease. The Company records deferred rent calculated as the difference between rent expense and the cash rental payments. In connection with the facility lease, the landlord also provided incentives of $369,000 to the Company in the form of leasehold improvements. These amounts have been reflected as deferred rent and are being amortized as a reduction to rent expense over the term of the Company’s operating lease. Rent expense was $922,000, $922,000 and $1,003,000 for the years ended December 31, 2014, 2013 and 2012, respectively.
The future minimum lease payments as of December 31, 2014, are as follows (in thousands):
|
|
|
Future Minimum |
| |
|
Year ending December 31, |
|
Lease Payments |
| |
|
|
|
|
| |
|
2015 |
|
$ |
1,125 |
|
|
2016 |
|
1,060 |
| |
|
|
|
|
| |
|
Total minimum lease payments |
|
$ |
2,185 |
|
Purchase Obligations
Purchase obligations consist of agreements to purchase goods and services entered into in the ordinary course of business. The Company had noncancellable commitments to suppliers for purchases totaling $1,334,000 and $1,316,000 as of December 31, 2014 and 2013, respectively.
Indemnification
In the normal course of business, the Company enters into contracts and agreements that contain a variety of representations and warranties and may provide for indemnification of the counterparty. The Company’s exposure under these agreements is unknown because it involves claims that may be made against it in the future, but have not yet been made. To date, the Company has not been subject to any claims or been required to defend any action related to its indemnification obligations.
In accordance with the Company’s amended and restated certificate of incorporation and its amended and restated bylaws, the Company has indemnification obligations to its officers and directors, subject to some limits, with respect to their service in such capacities. The Company has also entered into indemnification agreements with its directors and certain of its officers. To date, the Company has not been subject to any claims, and it maintains director and officer insurance that may enable it to recover a portion of any amounts paid for future potential claims. The Company’s exposure under these agreements is unknown because it involves claims that may be made against it in the future, but have not yet been made. The Company believes that the fair value of these indemnification obligations is minimal, and accordingly, it has not recognized any liabilities relating to these obligations for any period presented.
Legal Proceedings
The Company was not party to any legal proceedings at December 31, 2014 and 2013. The Company assesses, in conjunction with its legal counsel, the need to record a liability for litigation and contingencies. Reserve estimates are recorded when and if it is determined that a loss-related matter is both probable and reasonably estimable.
On February 15, 2014, the Company entered into an engagement letter with a financial advisor which provided for such firm to serve as its placement agent and for the Company to make certain payments to them in connection with its Series E Convertible Preferred Stock financing. After the entry into such engagement letter, the financial advisor did not provide the level of service the Company was expecting and was not responsible for introducing the Company to any of the Series E Convertible Preferred Stock investors. In December 2014, the Company and its former financial advisor agreed to amend and to terminate their engagement letter, effective immediately. Pursuant to the terms of the amended engagement letter, the Company agreed to pay the former financial advisor a transaction fee of $650,000, to be paid in four equal quarterly installments starting on December 31, 2014, and ending on September 30, 2015 and $35,000 for reimbursement of the former financial advisor’s out-of-pocket expenses, which were due upon execution of the amendment. The transaction fee and out-of-pocket expenses were reflected as additional Series E Convertible Preferred Stock issuance costs during the year ended December 31, 2014.
11. Convertible Preferred Stock
At December 31, 2013, convertible preferred stock authorized and outstanding consisted of the following (in thousands except share amounts):
|
Series |
|
Shares |
|
Shares |
|
Carrying |
|
Preferential |
| ||
|
Series A |
|
326,595 |
|
326,591 |
|
$ |
6,183 |
|
$ |
6,212 |
|
|
Series A-1 |
|
225,235 |
|
225,235 |
|
6,649 |
|
3,243 |
| ||
|
Series B |
|
755,516 |
|
755,486 |
|
27,272 |
|
27,538 |
| ||
|
Series C |
|
561,448 |
|
561,423 |
|
22,397 |
|
22,485 |
| ||
|
Series D |
|
800,000 |
|
722,367 |
|
37,153 |
|
37,708 |
| ||
|
|
|
2,668,794 |
|
2,591,102 |
|
$ |
99,654 |
|
$ |
97,186 |
|
At December 31, 2014, convertible preferred stock authorized and outstanding consisted of the following (in thousands except share amounts):
|
|
|
|
|
Shares |
|
|
|
Preferential |
| ||
|
|
|
Shares |
|
Issued and |
|
Carrying |
|
Liquidation |
| ||
|
Series |
|
Authorized |
|
Outstanding |
|
Value |
|
Value |
| ||
|
|
|
|
|
|
|
|
|
|
| ||
|
Series A |
|
326,595 |
|
326,591 |
|
$ |
6,183 |
|
$ |
6,212 |
|
|
Series A-1 |
|
225,235 |
|
225,235 |
|
6,649 |
|
3,243 |
| ||
|
Series B |
|
755,516 |
|
755,486 |
|
27,272 |
|
27,538 |
| ||
|
Series C |
|
561,448 |
|
561,423 |
|
22,397 |
|
22,485 |
| ||
|
Series D |
|
800,000 |
|
722,367 |
|
37,153 |
|
37,708 |
| ||
|
Series E |
|
4,150,403 |
|
2,671,626 |
|
32,606 |
|
134,650 |
| ||
|
|
|
6,819,197 |
|
5,262,728 |
|
$ |
132,260 |
|
$ |
231,836 |
|
As of December 31, 2014, the rights, privileges, and preferences of the Company’s Series A, Series A-1, Series B, Series C, Series D and Series E Convertible Preferred Stock were as follows:
Conversion
Shares of Series A, Series A-1, Series B, Series C, Series D and Series E Convertible Preferred Stock are convertible into shares of common stock at the holders’ option at any time or automatically (i) immediately prior to the closing of a firmly underwritten public offering in which the offering price per share is not less than $25.20 and the aggregate gross proceeds received by the Company are not less than $50,000,000 or (ii) upon receipt by the Company of a written request for such conversion from the holders of the majority of the Series A, Series A-1, Series B, Series C, Series D and Series E Convertible Preferred Stock then outstanding, voting as a single class and on an as-converted basis. Each share of Series A, Series A-1, Series B, Series C, Series D, and Series E Convertible
Preferred Stock shall be convertible into the number of fully paid, non-assessable shares of common stock that results from dividing the original issue price per share of $19.0215, $14.40, $36.45, $40.05, $52.20 and $12.60, respectively, by the conversion price in effect for Series A, Series A-1, Series B, Series C, Series D and Series E Convertible Preferred Stock at the time of the conversion. Upon any increase or decrease of the conversion price for the Series A, Series A-1, Series B, Series C, Series D and Series E Convertible Preferred Stock, the conversion rate for such series shall be appropriately increased or decreased.
The issuance price of the Series E Convertible Preferred Stock was lower than the conversion price of previously issued Convertible Preferred Stock. Accordingly, the conversion prices of the affected series of Convertible Preferred Stock was reduced pursuant to a defined adjustment formula which will result in the issuance of an increased number of shares of common stock upon conversion of such series of Convertible Preferred Stock. At December 31, 2014, the adjusted conversion prices of the Series A, Series A-1 Series B, Series C and Series D Convertible Preferred Stock were $15.76725, $13.23859, $25.30326, $27.273 and $33.92087 per share, respectively. At December 31, 2014, the conversion price of the Series E Convertible Preferred Stock was $12.60 per share. As of December 31, 2014, the increased number of shares of common stock upon conversion of each series of Convertible Preferred Stock and the Series E Convertible Preferred Stock is as follows:
|
|
|
Actual Shares |
|
As Converted |
|
Additional Shares |
|
|
|
|
Issued and |
|
Shares Issued |
|
Due to Anti-Dilution |
|
|
Series |
|
Outstanding |
|
And Outstanding |
|
Adjustment |
|
|
|
|
|
|
|
|
|
|
|
Series A |
|
326,591 |
|
393,996 |
|
67,405 |
|
|
Series A-1 |
|
225,235 |
|
244,995 |
|
19,760 |
|
|
Series B |
|
755,486 |
|
1,088,300 |
|
332,814 |
|
|
Series C |
|
561,423 |
|
824,444 |
|
263,021 |
|
|
Series D |
|
722,367 |
|
1,111,636 |
|
389,269 |
|
|
Series E |
|
2,671,626 |
|
2,671,626 |
|
— |
|
|
|
|
5,262,728 |
|
6,334,997 |
|
1,072,269 |
|
The shares are subject to adjustment upon a recapitalization, which shall mean any stock dividend, stock split, combination of shares, reorganization, recapitalization, or other similar events.
Dividends
The holders of Series E Convertible Preferred Stock are entitled to receive dividends at the rate of $1.008 per share per annum when and if declared by the Board of Directors. After payment of the aforementioned preferential amount to the holders of the Series E Convertible Preferred stock, the holders of Series A, Series A-1, Series B, Series C, and Series D Convertible Preferred Stock are entitled to receive dividends, when, and if declared by the Board of Directors, out of any assets at the time legally available therefor, at the dividend rate of $1.5219, $1.17, $2.916, $3.204, and $4.176 per share, per annum, respectively, on a pari passu basis (subject to adjustment from time to time for recapitalization), payable in preference and priority to any declaration or payment of any distribution on common stock of the Company in such calendar year. No distributions shall be made with respect to the common stock unless dividends on the Series A, Series A-1, Series B, Series C, Series D and Series E Convertible Preferred Stock have been declared in accordance with the preferences stated herein and all declared dividends on the Series A, Series A-1, Series B, Series C, Series D and Series E Convertible Preferred Stock have been paid or set aside for payment to these stockholders. The right to receive dividends on shares of Series A, Series A-1, Series B, Series C, Series D and Series E Convertible Preferred Stock is not cumulative, and no right to dividends accrues to holders of Series A, Series A-1, Series B, Series C, Series D and Series E Convertible Preferred Stock by reason of the fact that dividends on said shares are not declared or paid. As long as any of the Series A, Series A-1, Series B, Series C, Series D and Series E Convertible Preferred Stock is issued and outstanding, the Company may not declare or pay dividends without first obtaining the approval of the holders of more than sixty percent (60%) of the outstanding shares of Series A, Series A-1, Series B, Series C, Series D and Series E Convertible Preferred Stock. Since inception, no dividends have been declared or paid.
Voting
Each holder of shares of Series A, Series A-1, Series B, Series C, Series D and Series E Convertible Preferred Stock is entitled to voting rights equivalent to the number of shares of common stock into which the respective shares are convertible and votes together as one class with the common stock, except as provided by law or under the Company’s certificate of incorporation. Certain financing, acquisition, disposition, and recapitalization transactions require the vote of a majority of the shares of the outstanding Series A, Series A-1, Series B, Series C, Series D and Series E Convertible Preferred Stock.
Liquidation Preference
In the event of a liquidation, dissolution or winding up of the Company, whether voluntary or involuntary, the holders of Series E Convertible Preferred Stock are entitled to receive a per share liquidation preference in the amount of $50.40 plus all declared and unpaid dividends on such shares prior and in preference to any distribution or payment is made to the holders of the Series A, Series A-1, Series B, Series C, and Series D Convertible Preferred Stock. If upon the liquidation, dissolution or winding up of the Company, the assets are insufficient to make payments in full to the holders of Series E Convertible Preferred Stock, then the entire assets of the Company legally available for distribution will be distributed with equal priority and pro rata among the holders of the Series E Convertible Preferred Stock.
After payment of the full Series E liquidation preference, the holders of the Series A, Series A-1, Series B, Series C and Series D Convertible Preferred Stock shall be entitled to receive a per share liquidation preference on a pari passu basis in the amount of $19.0215, $14.40, $36.45, $40.05, and $52.20, respectively, plus all declared and unpaid dividends on such shares, prior and in preference to any distribution or payout of any of the assets of the Company to the holders of common stock. If assets are insufficient to make payments in full to all holders of Series A, Series A 1, Series B, Series C, and Series D Convertible Preferred Stock, then the entire assets of the Company legally available for distribution will be distributed with equal priority and pro rata among the holders of the Series A, Series A-1, Series B, Series C, and Series D Convertible Preferred Stock.
The remaining assets shall be distributed among the holders of the common stock on a pro rata basis based on the number of shares of common stock held by them.
Shares of Series A, Series A-1, Series B, Series C, Series D and Series E Convertible Preferred Stock shall not be entitled to be converted into shares of common stock in order to participate in any distribution as shares of common stock without first foregoing participation in such distribution as Series A, Series A-1, Series B, Series C, Series D and Series E Convertible Preferred Stock.
Redemption
The convertible preferred shares do not have redemption rights in favor of the Company or the holder thereof.
2012 Preferred Stock Plan
The 2012 Preferred Stock Plan (the “2012 Plan”) was adopted on July 19, 2012. The 2012 Plan was established to allow employees the opportunity to participate in the Series D Convertible Preferred Stock issuance. Under the 2012 Plan, 126,435 shares were authorized for issuance. In September 2012, the Company granted 19,952 fully vested options to purchase shares of Series D Convertible Preferred Stock at $52.20 per share. In September 2012, 10,267 of the options were exercised, the remaining options to purchase 9,685 shares expired unexercised at that time and were returned to the 2012 Plan. As of December 31, 2013, there were 116,168 shares available for grant and no options were outstanding under the 2012 Plan.
On November 3, 2014, the Company’s Board of Directors approved the termination of the 2012 Plan effective immediately.
2014 Preferred Stock Plan
In August 2014, the Company’s Board of Directors adopted the 2014 Preferred Stock Plan (the “2014 Plan”). The 2014 Plan was established to allow employees the opportunity to participate in the Series E Convertible Preferred Stock issuance. Under the 2014 Plan, 88,888 shares were authorized for issuance. In August through December 2014, the Company granted 59,230 fully vested options to purchase shares of Series E Convertible Preferred Stock at $12.60 per share. In September through December 2014, 53,744 of the options were exercised and the remaining options expired. As of December 31, 2014, there were 35,144 shares available for grant and no options were outstanding under the 2014 Plan.
On January 14, 2015, the Company’s Board of Directors approved the termination of the 2014 Preferred Stock Plan effective immediately prior to consummation of the Company’s IPO.
12. Stockholders’ Deficit
Common Stock
At December 31, 2014 the Company’s certificate of incorporation, as amended and restated, authorizes the Company to issue 15,555,555 shares of common stock with $0.001 par value. Holders of common stock are entitled to one vote per share on all matters to be voted upon by the stockholders. Common stockholders are entitled to dividends when and if declared by the Board of Directors, subject to the preferences that may be applicable to any outstanding shares of Series A, Series A-1, Series B, Series C, Series D and Series E Convertible Preferred Stock. No dividends have been declared to date.
Restricted Stock
In May 2012, the Company entered into two Restricted Stock Purchase Agreements with two individuals in return for certain intellectual property (“IP”) and ongoing consulting services. 1,666 shares of common stock were issued under each Restricted Stock Purchase Agreement for a total of 3,332 shares at a fair market value of $14.85 per share for a total purchase price of $49,500. The shares are subject to repurchase at cost, or $14.85 per share, with 20% being released from the repurchase option at the date of assignment of the IP and 1/48th of the remaining 80% being released monthly thereafter. Stock compensation expense of $49,500, representing the intrinsic value of the shares was recorded to consulting expense in 2012. Since it was not possible to value the IP, this noncash compensation expense was calculated at the fair market value of the shares of $14.85 per share.
As of December 31, 2014 and 2013, a total of 583 and 1,249 shares, respectively, were subject to repurchase, at cost, under the Restricted Stock Purchase Agreements.
Common Stock Warrants
In connection with the issuance of the Company’s Series E Convertible Preferred Stock in September through December 2014, the Company issued warrants, to each investor who purchased shares of Series E Convertible Preferred Stock, to purchase up to the number of shares of common stock equal to 50% of the number of shares of the Company’s Series E Convertible Preferred Stock purchased.
In connection with the issuance of the Company’s Series E Convertible Preferred Stock in September and November 2014, the Company issued warrants, to the holders of the outstanding convertible notes, to purchase up to the number of shares of common stock equal to 50% of the number of shares of the Company’s Series E Preferred Stock purchased through the conversion of an outstanding convertible note.
As of December 31, 2014, in connection with the issuance of its Series E Convertible Preferred Stock, the Company issued warrants to purchase an aggregate of 1,335,779 shares of common stock. The warrants are immediately exercisable, at an exercise price per share of $12.60, and expire upon the earlier of September 2, 2019 or upon the consummation of a change of control of the Company. The Company determined that these common
stock warrants meet the requirements for equity classification. Accordingly, the common stock warrants were recorded at their allocated fair value of $175,000 within stockholders’ deficit.
2009 Stock Plan
The Avinger, Inc. 2009 Stock Plan, as adopted March 25, 2009 (the “2009 Plan”), provides for the grant of incentive stock options (“ISOs”) and nonstatutory stock options (“NSOs”) to purchase common shares. ISOs may be granted only to Company employees (including officers and directors who are also employees). NSOs may be granted to Company employees, consultants and directors. ISOs and NSOs may be granted with exercise prices at not less than 100% of the fair value of the common stock on the date of grant. Pursuant to the 2009 Plan, the exercise price of ISOs granted to a stockholder, who, at the time of grant, owns stock representing more than 10% of the voting power of all classes of the stock of the Company, shall be not less than 110% of the fair market value per share of common stock on the date of grant. The Company’s Board of Directors determines the vesting schedule of the options. Options granted generally vest over four years and expire ten years from the date of grant.
Activity under the 2009 Plan is set forth below:
|
|
|
|
|
Options Outstanding |
| ||||||
|
|
|
Shares |
|
|
|
Weighted |
|
Aggregate |
| ||
|
|
|
Available for |
|
Number of |
|
Average |
|
Intrinsic Value |
| ||
|
|
|
Grant |
|
Shares |
|
Exercise Price |
|
(in thousands) |
| ||
|
|
|
|
|
|
|
|
|
|
| ||
|
Balance at December 31, 2011 |
|
85,097 |
|
299,915 |
|
$ |
10.03 |
|
$ |
1,447 |
|
|
Additional shares reserved |
|
111,111 |
|
— |
|
|
|
|
| ||
|
Granted |
|
(128,086 |
) |
128,086 |
|
$ |
17.94 |
|
|
| |
|
Exercised |
|
— |
|
(26,682 |
) |
$ |
7.35 |
|
|
| |
|
Cancelled |
|
88,166 |
|
(88,166 |
) |
$ |
10.48 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
| ||
|
Balance at December 31, 2012 |
|
156,288 |
|
313,153 |
|
$ |
13.45 |
|
$ |
2,226 |
|
|
Granted |
|
(272,308 |
) |
272,308 |
|
$ |
20.49 |
|
|
| |
|
Exercised |
|
— |
|
(12,183 |
) |
$ |
7.89 |
|
|
| |
|
Cancelled |
|
174,538 |
|
(174,538 |
) |
$ |
14.13 |
|
|
| |
|
Shares repurchased |
|
2,593 |
|
— |
|
$ |
14.85 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
| ||
|
Balance at December 31, 2013 |
|
61,111 |
|
398,740 |
|
$ |
16.15 |
|
$ |
— |
|
|
Additional shares reserved |
|
2,574,795 |
|
— |
|
|
|
|
| ||
|
Granted |
|
(2,720,174 |
) |
2,720,174 |
|
$ |
4.68 |
|
|
| |
|
Exercised |
|
— |
|
(2,568 |
) |
$ |
8.85 |
|
|
| |
|
Cancelled |
|
105,973 |
|
(105,973 |
) |
$ |
16.62 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
| ||
|
Balance at December 31, 2014 |
|
21,705 |
|
3,010,373 |
|
$ |
5.78 |
|
$ |
13,188 |
|
Additional information related to the status of options as of December 31, 2014 is summarized as follows:
|
Options Outstanding and Vested as of December 31, 2014 |
| |||||||||||||
|
Options Outstanding |
|
Options Vested |
| |||||||||||
|
|
|
|
|
Weighted |
|
Weighted |
|
|
|
Weighted |
| |||
|
|
|
|
|
Average |
|
Average |
|
|
|
Average |
| |||
|
Exercise |
|
Options |
|
Remaining |
|
Exercise |
|
Number |
|
Exercise |
| |||
|
Price |
|
Outstanding |
|
Contractual Life |
|
Price |
|
Exercisable |
|
Price |
| |||
|
|
|
|
|
|
|
|
|
|
|
|
| |||
|
$ |
4.05 |
|
4,928 |
|
4.44 |
|
$ |
4.05 |
|
3,961 |
|
$ |
4.05 |
|
|
$ |
4.50 |
|
1,874,150 |
|
10.00 |
|
$ |
4.50 |
|
— |
|
$ |
4.50 |
|
|
$ |
4.95 |
|
863,221 |
|
9.87 |
|
$ |
4.95 |
|
24,971 |
|
$ |
4.95 |
|
|
$ |
12.60 |
|
72,593 |
|
6.53 |
|
$ |
12.60 |
|
70,026 |
|
$ |
12.60 |
|
|
$ |
14.85 |
|
51,549 |
|
7.06 |
|
$ |
14.85 |
|
38,609 |
|
$ |
14.85 |
|
|
$ |
20.25 |
|
105,879 |
|
8.53 |
|
$ |
20.25 |
|
44,254 |
|
$ |
20.25 |
|
|
$ |
22.05 |
|
9,165 |
|
7.77 |
|
$ |
22.05 |
|
5,355 |
|
$ |
22.05 |
|
|
$ |
22.50 |
|
28,888 |
|
8.34 |
|
$ |
22.50 |
|
13,842 |
|
$ |
22.50 |
|
|
|
|
3,010,373 |
|
9.75 |
|
$ |
5.78 |
|
201,018 |
|
$ |
5.38 |
| |
Additional information related to the status of options as of December 31, 2013 is summarized as follows:
|
Options Outstanding and Vested as of December 31, 2013 |
| ||||||||||||||
|
Options Outstanding |
|
Options Vested |
| ||||||||||||
|
|
|
|
|
Weighted |
|
Weighted |
|
|
|
Weighted |
| ||||
|
|
|
|
|
Average |
|
Average |
|
|
|
Average |
| ||||
|
Exercise |
|
Options |
|
Remaining |
|
Exercise |
|
Number |
|
Exercise |
| ||||
|
Price |
|
Outstanding |
|
Contractual Life |
|
Price |
|
Exercisable |
|
Price |
| ||||
|
|
|
|
|
|
|
|
|
|
|
|
| ||||
|
$ |
4.05 |
|
7,174 |
|
5.65 |
|
$ |
4.05 |
|
7,174 |
|
$ |
4.05 |
| |
|
$ |
4.95 |
|
40,069 |
|
6.59 |
|
$ |
4.95 |
|
37,075 |
|
$ |
4.95 |
| |
|
$ |
12.60 |
|
81,764 |
|
7.55 |
|
$ |
12.60 |
|
61,173 |
|
$ |
12.60 |
| |
|
$ |
14.85 |
|
68,783 |
|
8.34 |
|
$ |
14.85 |
|
35,316 |
|
$ |
14.85 |
| |
|
$ |
20.25 |
|
149,801 |
|
9.51 |
|
$ |
20.25 |
|
3,608 |
|
$ |
20.25 |
| |
|
$ |
22.05 |
|
22,261 |
|
9.08 |
|
$ |
22.05 |
|
12,306 |
|
$ |
22.05 |
| |
|
$ |
22.50 |
|
28,888 |
|
8.57 |
|
$ |
22.50 |
|
— |
|
$ |
22.50 |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| ||||
|
|
|
398,740 |
|
8.45 |
|
$ |
16.15 |
|
156,652 |
|
$ |
11.82 |
| ||
The weighted-average grant date fair value of stock options granted during the years ended December 31, 2014, 2013 and 2012 was $6.60, $7.20, and $8.55 per share, respectively. As of December 31, 2014, the aggregate intrinsic value of options outstanding and vested was $134,000. The aggregate intrinsic value of options exercised was none, $153,000 and $332,000 during the years ended December 31, 2014, 2013 and 2012, respectively. The aggregate intrinsic value was calculated as the difference between the exercise prices of the underlying options and the estimated fair value of the common stock on the date of exercise. Because of the Company’s net operating losses, the Company did not realize any tax benefits from share-based payment arrangements for the years ended December 31, 2014, 2013 and 2012.
2015 Equity Incentive Plan
In January 2015, the Company’s Board of Directors adopted and the Company’s stockholders approved the 2015 Equity Incentive Plan (“2015 Plan”). The 2015 Plan provides for the grant of ISOs to employees and for the grant of NSOs, restricted stock, restricted stock units, stock appreciation rights, performance units and performance shares to employees, directors and consultants. A total of 1,320,000 shares of common stock were reserved for issuance pursuant to the 2015 Plan. In addition, the shares reserved for issuance under the 2015 Plan will also include shares reserved but not issued under the 2009 Plan, plus any share awards granted under the 2009 Plan that expire or terminate without having been exercised in full or that are forfeited or repurchased. In addition, the number
of shares available for issuance under the 2015 Plan will also include an annual increase on the first day of each fiscal year beginning in fiscal 2016, equal to the lesser of:
· 1,690,000 shares;
· 5.0% of the outstanding shares of common stock as of the last day of the immediately preceding fiscal year; or
· An amount as determined by the Board of Directors.
2015 Employee Stock Purchase Plan
In January 2015, the Company’s Board of Directors adopted and the Company’s stockholders approved the 2015 Employee Stock Purchase Plan (“ESPP”) under which eligible employees are permitted to purchase common stock at a discount through payroll deductions. 500,000 shares of common stock are reserved for issuance and will be increased on the first day of each fiscal year, commencing in 2016, by an amount equal to the lesser of (i) 493,000 shares (ii) 1.5% of the outstanding shares of common stock as of the last day of the immediately preceding fiscal year; or (iii) an amount as determined by the Board of Directors. The price of the common stock purchased will be the lower of 85% of the fair market value of the common stock at the beginning of an offering period or at the end of a purchase period. The ESPP was effective upon adoption by the Company’s Board of Directors but will not be in use until the completion of the IPO. The ESPP is intended to qualify as an “employee stock purchase plan” within the meaning of Section 423 of the Internal Revenue Code of 1986, as amended.
13. Stock-Based Compensation
The Company estimates the fair value of stock-based compensation on the date of grant using the Black-Scholes option-pricing model. The Black-Scholes model determines the fair value of stock-based payment awards based on the fair market value of the Company’s common stock on the date of grant and is affected by assumptions regarding a number of complex and subjective variables. These variables include, but are not limited to, the fair value of the Company’s common stock, and the volatility over the expected term of the awards. The Company has opted to use the “simplified method” for estimating the expected term of options, whereby the expected term equals the arithmetic average of the vesting term and the original contractual term of the option. Due to the Company’s limited operating history and a lack of company specific historical and implied volatility data, the Company has based its estimate of expected volatility on the historical volatility of a group of similar companies that are publicly traded. When selecting these public companies on which it has based its expected stock price volatility, the Company selected companies with comparable characteristics to it, including enterprise value, stage of development, risk profile, and position within the industry as well as selecting companies with historical share price information sufficient to meet the expected life of the stock-based awards. The historical volatility data was computed using the daily closing prices for the selected companies’ shares during the equivalent period of the calculated expected term of the share-based payments. The Company will continue to analyze the historical stock price volatility and expected term assumptions as more historical data for the Company’s common stock becomes available. The risk-free rate assumption is based on the U.S. Treasury instruments with maturities similar to the expected term of the Company’s stock options. The expected dividend assumption is based on the Company’s history of not paying dividends and its expectation that it will not declare dividends for the foreseeable future.
As stock-based compensation expense recognized in the financial statements is based on awards ultimately expected to vest, it has been reduced for estimated forfeitures. Forfeitures are estimated at the time of grant and revised, if necessary, over the service period to the extent that actual forfeitures differ, or are expected to differ, from prior estimates. Forfeitures are estimated based on estimated future employee turnover and historical experience. The fair value for the Company’s employee stock options was estimated at the date of grant using the Black-Scholes valuation model with the following average assumptions:
|
|
|
Year Ended December 31, |
| ||||
|
|
|
2014 |
|
2013 |
|
2012 |
|
|
|
|
|
|
|
|
|
|
|
Expected term (years) |
|
6.3 |
|
6.9 |
|
6.9 |
|
|
Expected volatility |
|
59.1 |
% |
52.1 |
% |
48.7 |
% |
|
Risk-free interest rate |
|
1.8 |
% |
1.4 |
% |
1.2 |
% |
|
Dividend rate |
|
— |
|
— |
|
— |
|
As of December 31, 2014 and 2013, the total unamortized compensation expense related to stock-based awards granted to employees and directors was $18,938,000 and $1,528,000, which is expected to be amortized over the next 3.92 and 2.70 years, respectively.
Total stock-based compensation expense recognized, before taxes, during the years ended December 31, 2014, 2013 and 2012, is as follows (in thousands):
|
|
|
Year Ended December 31, |
| |||||||
|
|
|
2014 |
|
2013 |
|
2012 |
| |||
|
|
|
|
|
|
|
|
| |||
|
Cost of revenues |
|
$ |
55 |
|
$ |
62 |
|
$ |
51 |
|
|
Research and development expenses |
|
155 |
|
165 |
|
123 |
| |||
|
Selling, general and administrative expenses |
|
431 |
|
427 |
|
275 |
| |||
|
|
|
$ |
641 |
|
$ |
654 |
|
$ |
449 |
|
14. Income Taxes
For the years ended December 31, 2014, 2013 and 2012, the Company’s provision for income taxes consisted of state income tax expense of $14,000, $11,000 and $9,000, respectively.
A reconciliation of the statutory U.S. federal rate to the Company’s effective tax rate is as follows (in thousands):
|
|
|
Year Ended |
| |||||||
|
|
|
December 31, |
| |||||||
|
|
|
2014 |
|
2013 |
|
2012 |
| |||
|
|
|
|
|
|
|
|
| |||
|
Tax at federal statutory rate |
|
$ |
(10,863 |
) |
$ |
(13,563 |
) |
$ |
(11,507 |
) |
|
State taxes, net of federal benefit |
|
14 |
|
11 |
|
10 |
| |||
|
Permanent differences |
|
730 |
|
298 |
|
235 |
| |||
|
Change in valuation allowance |
|
10,316 |
|
13,450 |
|
11,648 |
| |||
|
Research credits |
|
(219 |
) |
(179 |
) |
(377 |
) | |||
|
Other |
|
36 |
|
(6 |
) |
— |
| |||
|
|
|
|
|
|
|
|
| |||
|
Provision for taxes |
|
$ |
14 |
|
$ |
11 |
|
$ |
9 |
|
Significant components of the Company’s net deferred tax assets as of December 31, 2014 and 2013 consist of the following (in thousands):
|
|
|
As of December 31, |
| ||||
|
|
|
2014 |
|
2013 |
| ||
|
|
|
|
|
|
| ||
|
Deferred tax assets: |
|
|
|
|
| ||
|
Federal, state, and foreign net operating losses |
|
$ |
52,433 |
|
$ |
40,807 |
|
|
Research and other credits |
|
2,157 |
|
1,771 |
| ||
|
Fixed assets |
|
58 |
|
2 |
| ||
|
Other |
|
1,652 |
|
1,391 |
| ||
|
Total deferred tax assets |
|
56,300 |
|
43,971 |
| ||
|
|
|
|
|
|
| ||
|
Less: Valuation allowance |
|
(56,108 |
) |
(43,915 |
) | ||
|
|
|
|
|
|
| ||
|
Deferred tax liabilities: |
|
|
|
|
| ||
|
Interest |
|
(192 |
) |
(56 |
) | ||
|
|
|
|
|
|
| ||
|
Net deferred tax assets |
|
$ |
— |
|
$ |
— |
|
The valuation allowance increased by $12,193,000, $15,927,000 and $13,675,000 during the years ended December 31, 2014, 2013 and 2012, respectively.
As of December 31, 2014, the Company had federal net operating loss carryforwards of approximately $133,851,000, which begin to expire in 2027, and state net operating loss carryforwards of approximately $134,174,000, which begin to expire in 2015.
As of December 31, 2014, the Company had federal research and development credit carryforwards of approximately $1,807,000, which expire in the years 2027 through 2033, and state research and development credit carryforwards of approximately $1,930,000. The state research and development credit can be carried forward indefinitely.
Federal and state tax laws impose substantial restrictions on the utilization of the net operating loss, and credit carryforwards in the event of an ownership change as defined in Section 382 of the Internal Revenue Code. Accordingly, the Company’s ability to utilize these carryforwards may be limited as a result of such ownership change. Such a limitation could result in the expiration of carryforwards before they are utilized.
The Company had unrecognized tax benefits of approximately $1,121,000 and $919,000, as of December 31, 2014 and 2013, of which $924,000 and $759,000, respectively, would affect the effective tax rate if recognized, before consideration of the valuation allowance.
A reconciliation of the unrecognized tax benefits from January 1, 2012 through December 31, 2014 is as follows (in thousands):
|
|
|
As of December 31, |
| |||||||
|
|
|
2014 |
|
2013 |
|
2012 |
| |||
|
|
|
|
|
|
|
|
| |||
|
Balance at beginning of year |
|
$ |
919 |
|
$ |
592 |
|
$ |
430 |
|
|
Additions based on tax positions related to current year |
|
202 |
|
165 |
|
162 |
| |||
|
Additions for tax positions of prior years |
|
— |
|
162 |
|
— |
| |||
|
Balance at end of year |
|
$ |
1,121 |
|
$ |
919 |
|
$ |
592 |
|
The Company does not expect a significant change to its unrecognized tax benefits over the next twelve months. The unrecognized tax benefits may increase or change during the next twelve months for items that arise in the ordinary course of business.
The Company files income tax returns in the U.S. federal jurisdiction and various state jurisdictions. In the normal course of business, the Company is subject to examination by taxing authorities throughout the nation. The Company is not currently under audit by the Internal Revenue Service or other similar state and local authorities. All tax years remain open to examination by major taxing jurisdictions to which the Company is subject.
15. Related-Party Transactions
The Company entered into an agreement with JBS Consulting, LLC (“JBS Consulting”) regarding the use of a private aircraft owned by JBS Consulting for company business-related travel by the Company’s directors, officers and employees. Dr. John B. Simpson, the Company’s founder and the Executive Chairman of the Board of Directors, and its former CEO, is the president and managing officer of JBS Consulting. Pursuant to the agreement, JBS Consulting will be reimbursed for the cost of first class airfare for all flights in connection with company business-related travel by Dr. John B. Simpson and the cost of coach airfare for all flights in connection with company business-related travel by other directors, officers, and employees. For the years ended December 31, 2014, 2013 and 2012, JBS Consulting provided private plane service to the Company totaling approximately none, $568,000 and $611,000, respectively.
During the years ended December 31, 2014, 2013 and 2012, the Company purchased marketing services from Recreation, Inc., a brand strategy and design agency headquartered in San Francisco, California for $984,000, $107,000 and none, respectively. John D. Simpson, the Company’s Vice President of Sales, was the Chief Executive Officer of Recreation, Inc. until March 2015 and is the son of Dr. John B. Simpson, the Company’s founder and the Executive Chairman of the Board of Directors, and its former Chief Executive Officer. As of December 31, 2014 and 2013, amounts due to Recreation, Inc., included in accounts payable and accrued liabilities, were $298,000 and $56,000, respectively.
During the years ended December 31, 2014, 2013 and 2012, Baysinger Search & Associates, Inc. (Baysinger), a company whose management includes the wife of the Company’s then-Vice President of Sales, provided recruiting services to the Company totaling approximately none, $146,000 and $140,000.
From October 2013 through July 2014, the Company entered into convertible notes with certain investors, including existing stockholders, some members of the Board of Directors and their affiliated companies and some members of management for a total aggregate principal amount of $18,192,000 (Note 8) and issued warrants to purchase shares of the Company’s common stock at an exercise price of $12.60 per share. The issuance of $5,122,000 of the total aggregate principal amount of the convertible notes was considered a related-party transaction. As of December 31, 2014 and 2013, the carrying value of the related-party convertible notes was $2,793,000 and $4,719,000, respectively. For the years ended December 31, 2014 and 2013, the Company recognized $1,021,000 and $140,000, respectively, of interest expense related to the related-party convertible notes within interest expense in the Company’s statements of operations and comprehensive loss.
16. 401(k) Plan
The Company has a qualified retirement plan under section 401(k) of the Internal Revenue Code (“IRC”) under which participants may contribute up to 100% of their eligible compensation, subject to maximum deferral limits specified by the IRC. The Company may make a discretionary matching contribution to the 401(k) plan, and may make a discretionary employer contribution to each eligible employee each year. Eligible employees vest in the Company’s contributions over a graded six year schedule. To date, the Company has made no contributions to the 401(k) plan.
17. Subsequent Events
Amended and Restated Certificate of Incorporation
On January 14, 2015, the Company’s Board of Directors approved an amended and restated certificate of incorporation that became effective upon its filing with the Secretary of State of the State of Delaware on February 4, 2015, immediately prior to the closing of the IPO. The amended and restated certificate of incorporation
increased the authorized share capital to 100,000,000 shares of common stock, $0.001 par value per share, and 5,000,000 shares of preferred stock, $0.001 par value per share.
2009 Stock Plan
On January 14, 2015, the Company’s Board of Directors approved the termination of the 2009 Stock Plan effective immediately prior to consummation of the Company’s IPO.
Series E Convertible Preferred Stock Issuance
On January 9, 2015, the Company issued a total of 490,472 shares of Series E Convertible Preferred Stock at $12.60 per share for total cash proceeds of $6,180,000.
In connection with the issuance of the Company’s Series E Convertible Preferred Stock in January 2015, the Company issued common stock warrants to each investor who acquired shares of Series E Convertible Preferred Stock equal to 50% of the number of shares of the Company’s Series E Convertible Preferred Stock acquired by such investor. The warrants to purchase 245,235 shares of common stock are immediately exercisable, at an exercise price per share of $12.60, and expire upon the earlier of September 2, 2019 or upon the consummation of a change in control of the Company.
Upon conversion of the Convertible Preferred Stock to common stock, an additional 142,456 shares of common stock will be issued due to anti-dilution adjustments triggered by the issuances of Series E Convertible Preferred Stock in January 2015.
On January 14, 2015, the Company amended its Series E Convertible Preferred Stock Purchase agreement to provide for the issuance of common stock warrants to each investor who purchased shares of Series E Convertible Preferred Stock equal to 70% of the number of shares of the Company’s Series E Convertible Preferred Stock purchased by such investor. As with the common stock warrants previously issued, any new common stock warrants are immediately exercisable, at an exercise price of $12.60 per share, and expire upon the earlier of September 2, 2019 or upon consummation of a change in control of the Company. As a result of this amendment, the Company issued additional warrants to purchase 632,381 shares of common stock to investors who previously acquired shares of Series E Convertible Preferred Stock from September 2014 through January 2015.
18. Pro Forma Balance Sheet Data (Unaudited)
The table below shows, on a pro forma basis, the impact of the IPO and the issuance of additional Series E Convertible Preferred stock in January 2015 on certain condensed balance sheet items. The as-adjusted condensed balance sheet data below gives effect to the sale of 5,000,000 shares of common stock from the IPO at the initial public offering price of $13.00 per share, after deducting the underwriting discounts and commissions and estimated offering related transaction expenses, to the sale of 490,472 shares of Series E Convertible Preferred Stock at $12.60 per share for total cash proceeds of $6,180,000 and to the automatic conversion of all outstanding shares of our preferred stock into 6,967,925 shares of our common stock (in thousands):
Condensed Balance Sheets Data:
|
|
|
December 31, 2014 |
| ||||
|
|
|
Actual |
|
Pro Forma |
| ||
|
|
|
|
|
(unaudited) |
| ||
|
Cash and cash equivalents |
|
$ |
12,316 |
|
$ |
77,128 |
|
|
Working capital |
|
10,054 |
|
75,625 |
| ||
|
Total assets |
|
24,780 |
|
86,984 |
| ||
|
Long-term borrowings |
|
18,537 |
|
18,537 |
| ||
|
Convertible notes and accrued interest |
|
8,643 |
|
8,643 |
| ||
|
Convertible preferred stock |
|
132,260 |
|
— |
| ||
|
Common stock |
|
— |
|
12 |
| ||
|
Additional paid-in capital |
|
2,665 |
|
197,877 |
| ||
|
Accumulated deficit |
|
(146,533 |
) |
(146,533 |
) | ||
|
Total stockholders’ equity (deficit) |
|
(143,868 |
) |
51,356 |
| ||
19. Selected Quarterly Financial Information (Unaudited)
The following table represents certain unaudited quarterly information for the eight quarters ended December 31, 2014. This data has been derived from unaudited financial statements that, in the opinion of the Company’s management, include all adjustments, consisting only of normal recurring adjustments, necessary for a fair presentation of such information when read in conjunction with the Company’s annual audited financial statements and notes thereto appearing elsewhere in this report. These operating results are not necessarily indicative of results for any future period. Net loss per share for all periods presented has been retroactively adjusted to reflect the 1-for-45 reverse stock split effected on January 28, 2015 (in thousands, except per share data):
|
|
|
Three Months Ended |
|
Three Months Ended |
| ||||||||||||||||||||
|
|
|
Mar 31, |
|
Jun 30, |
|
Sep 30, |
|
Dec 31, |
|
Mar 31, |
|
Jun 30, |
|
Sep 30, |
|
Dec 31, |
| ||||||||
|
|
|
(unaudited) |
|
(unaudited) |
| ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||
|
Revenues |
|
$ |
2,119 |
|
$ |
3,389 |
|
$ |
2,632 |
|
$ |
3,073 |
|
$ |
2,926 |
|
$ |
3,253 |
|
$ |
3,390 |
|
$ |
3,395 |
|
|
Gross profit |
|
616 |
|
1,421 |
|
1,161 |
|
1,502 |
|
1,268 |
|
877 |
|
1,563 |
|
1,051 |
| ||||||||
|
Operating expenses |
|
6,995 |
|
6,949 |
|
7,306 |
|
8,477 |
|
11,477 |
|
11,760 |
|
10,005 |
|
8,489 |
| ||||||||
|
Net loss |
|
(7,971 |
) |
(7,078 |
) |
(8,780 |
) |
(8,135 |
) |
(10,215 |
) |
(11,804 |
) |
(9,281 |
) |
(8,601 |
) | ||||||||
|
Net loss per share, basic and diluted |
|
$ |
(33.21 |
) |
$ |
(29.37 |
) |
$ |
(36.43 |
) |
$ |
(33.62 |
) |
$ |
(44.41 |
) |
$ |
(50.88 |
) |
$ |
(39.66 |
) |
$ |
(36.14 |
) |
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this Annual Report on Form 10-K to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
Avinger, Inc. |
|
|
(Registrant) |
|
|
|
|
|
|
|
Date: March 27, 2015 |
/s/ JEFFERY M. SOINSKI |
|
|
Jeffrey M. Soinski |
|
|
Chief Executive Officer |
|
|
(Principal Executive Officer) |
|
|
|
|
Date: March 27, 2015 |
/s/ MATTHEW B. FERGUSON |
|
|
Matthew B. Ferguson |
|
|
Chief Financial Officer |
|
|
(Principal Financial and Accounting Officer) |
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Jeffrey Soinski and Matthew Ferguson, jointly and severally, as his or her true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution, for him or her, and in his or her name, place and stead, in any and all capacities, to sign any and all amendments to this Annual Report on Form 10-K, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite or necessary to be done in and about the premises hereby ratifying and confirming all that said attorneys-in-fact and agents, or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, this Annual Report on Form 10-K has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
|
Signature |
|
Title |
|
Date |
|
|
|
|
|
|
|
/s/ JEFFREY M. SOINSKI |
|
Chief Executive Officer (Principal Executive Officer); Director |
|
March 27, 2015 |
|
Jeffrey M. Soinski |
|
|
| |
|
|
|
|
|
|
|
/s/ MATTHEW B. FERGUSON |
|
Chief Financial Officer and Chief Business Officer (Principal Financial and Accounting Officer) |
|
March 27, 2015 |
|
Matthew B. Ferguson |
|
|
| |
|
|
|
|
|
|
|
/s/ DONALD A. LUCAS |
|
Director |
|
March 27, 2015 |
|
Donald A. Lucas |
|
|
|
|
|
Signature |
|
Title |
|
Date |
|
|
|
|
|
|
|
/s/ JOHN B. SIMPSON |
|
Executive Chairman of the Board of Directors; Director |
|
March 27, 2015 |
|
John B. Simpson, Ph.D., M.D. |
| |||
|
|
|
|
|
|
|
/s/ JAMES B. MCELWEE |
|
Director |
|
March 27, 2015 |
|
James B. McElwee |
|
|
|
|
|
|
|
|
|
|
|
/s/ JAMES G. CULLEN |
|
Director |
|
March 27, 2015 |
|
James G. Cullen |
|
|
|
|
|
|
|
|
|
|
|
/s/ THOMAS J. FOGARTY |
|
Director |
|
March 27, 2015 |
|
Thomas J. Fogarty |
|
|
|
|
|
Exhibit |
|
Exhibit Title |
|
|
|
|
|
3.1 (1) |
|
Amended and Restated Certificate of Incorporation of the registrant. |
|
|
|
|
|
3.2 (1) |
|
Bylaws of the registrant. |
|
|
|
|
|
4.1 (2) |
|
Specimen Common Stock certificate of the registrant. |
|
|
|
|
|
10.1 (1) |
|
Form of Indemnification Agreement for directors and executive officers. |
|
|
|
|
|
10.2 (3) |
|
2009 Stock Plan and Form of Option Agreement thereunder. |
|
|
|
|
|
10.3 (3) |
|
2014 Preferred Stock Plan. |
|
|
|
|
|
10.4 (1) |
|
2015 Equity Incentive Plan. |
|
|
|
|
|
10.5 (1) |
|
Form of Restricted Stock Unit Award Agreement. |
|
|
|
|
|
10.6 (1) |
|
Form of Stock Option Agreement. |
|
|
|
|
|
10.7 (1) |
|
2015 Employee Stock Purchase Plan. |
|
|
|
|
|
10.8 (1) |
|
Executive Incentive Compensation Plan. |
|
|
|
|
|
10.9 (3) |
|
Amended and Restated Investors’ Rights Agreement dated September 2, 2014 by and among the registrant and certain stockholders. |
|
|
|
|
|
10.10 (3) |
|
Lease Agreement, dated July 30, 2010, by and between the registrant and HCP LS Redwood City, LLC for office space located at 400 and 600 Chesapeake Drive, Redwood City, California. |
|
|
|
|
|
10.11 (3) |
|
First Amendment to Lease Agreement dated September 30, 2011 by and between registrant and HCP LS Redwood City, LLC. |
|
|
|
|
|
10.12 (3) |
|
Credit Agreement dated April 18, 2013 by and between registrant and PDL Biopharma. |
|
|
|
|
|
10.13 (3) |
|
Security Agreement dated April 18, 2013 by and between registrant and PDL BioPharma. |
|
|
|
|
|
10.14 (3) |
|
Employment Letter dated November 5, 2014 by and between registrant and John B. Simpson. |
|
|
|
|
|
10.15 (3) |
|
Employment Letter dated April 2, 2014 by and between registrant and John D. Simpson. |
|
|
|
|
|
10.16 (3) |
|
Employment Letter dated December 29, 2010 by and between registrant and Matthew B. Ferguson. |
|
|
|
|
|
10.17 (3) |
|
Employment Letter dated November 28, 2011 by and between registrant and Sougata Banerjee. |
|
|
|
|
|
10.18 (3) |
|
Change of Control and Severance Agreement dated March 1, 2012 by and between registrant and John B. Simpson. |
|
|
|
|
|
10.19 (3) |
|
Change of Control and Severance Agreement dated March 1, 2012 by and between registrant and Matthew B. Ferguson. |
|
|
|
|
|
10.20 (3) |
|
Change of Control and Severance Agreement dated March 1, 2012 by and between registrant and Sougata Banerjee. |
|
|
|
|
|
10.21 (3) |
|
Note and Warrant Purchase Agreement dated October 29, 2013 by and between registrant and holders of convertible promissory notes. |
|
|
|
|
|
10.22 (3) |
|
Amendment No. 1 to the Note and Warrant Purchase Agreement dated May 6, 2014 by and between registrant and holders of convertible promissory notes. |
|
|
|
|
|
10.23 (3) |
|
Employment Letter dated December 17, 2014 by and between registrant and Jeffrey M. Soinski. |
|
|
|
|
|
23.1 |
|
Consent of Independent Registered Public Accounting Firm. |
|
|
|
|
|
24.1 |
|
Power of Attorney (included on signature page). |
|
31.1 |
|
Certification of the Chief Executive Officer pursuant to Securities Exchange Act Rules 13a-14(a) and 15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
|
|
|
|
|
31.2 |
|
Certification of the Chief Financial Officer pursuant to Securities Exchange Act Rules 13a-14(a) and 15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
|
|
|
|
|
32.1 |
|
Certifications of the Chief Executive Officer and Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
|
|
(1) |
Incorporated by reference to Amendment No. 1 to the Registrant’s Registration Statement on Form S-1 (File No. 333-201322) filed with the SEC on January 20, 2015. |
|
|
|
|
|
|
(2) |
Incorporated by reference to Amendment No. 2 to the Registrant’s Registration Statement on Form S-1 (File No. 333-201322) filed with the SEC on January 28, 2015. |
|
|
|
|
|
|
(3) |
Incorporated by reference to the Registrant’s Registration Statement on Form S-1 (File No. 333-201322), filed with the SEC on December 30, 2014. |