Attached files
| file | filename |
|---|---|
| EXCEL - IDEA: XBRL DOCUMENT - GeneSYS ID, Inc. | Financial_Report.xls |
| EX-31.1 - CERTIFICATION - GeneSYS ID, Inc. | f10k2014ex31i_rxsafesinc.htm |
| EX-32.1 - CERTIFICATION - GeneSYS ID, Inc. | f10k2014ex32i_rxsafesinc.htm |
| EX-31.2 - CERTIFICATION - GeneSYS ID, Inc. | f10k2014ex31ii_rxsafesinc.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
☒ ANNUAL REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2014
☐ TRANSITION REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from _________ to ________
Commission file number: 333-193800
| RX Safes, Inc. | ||
| (Exact name of registrant as specified in its charter) |
| Nevada | 27-2928918 | |
(State
or other jurisdiction of |
(I.R.S.
Employer Identification No.) | |
170 Green Valley Parkway, Suite 300 Henderson, NV |
89012 | |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number: 516-983-9144
Securities registered under Section 12(b) of the Exchange Act:
| Title of each class | Name of each exchange on which registered |
| none | not applicable |
Securities registered under Section 12(g) of the Exchange Act:
| Title of each class |
| Common Stock, par value of $0.001 |
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by checkmark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 229.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 232.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. Yes ☐ No ☒
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company.
| ☐ Large accelerated filer | ☐ Accelerated filer |
| ☐ Non-accelerated filer | ☒ Smaller reporting company |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
State the aggregate market value of the voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold, or the average bid and asked price of such common equity, as of the last business day of the registrant’s most recently completed second fiscal quarter. Not Available
Indicate the number of shares outstanding of each of the registrant’s classes of common stock, as of the latest practicable date. 115,591,472 common shares as of February 24, 2015

TABLE OF CONTENTS
Business Overview
Rx Safes, Inc. designs, develops, engineers and markets fingerprint medical security storage solutions for consumers and healthcare professionals. We are led by our CEO and director, Lorraine Yarde, who has been a pioneer in the embedded biometric marketplace. Ms. Yarde successfully launched the first nationwide mass market distribution of consumer biometric products, selling through market leaders such as the Home Depot, Sears and Costco. Ms. Yarde has a proven ability to establish strategic product development and marketing relationships with brand name Original Equipment Manufacturers (OEMs) and has been responsible for selling tens of thousands of fingerprint-enabled products into the US market.
Founded in 2010, Rx Safes, Inc. is an emerging leading provider of standalone, biometric medical security solutions in the new targeted sector of Prescription (Rx) and over-the-counter (OTC) drug security, providing real solutions to address Rx and OTC drug diversion and abuse. The Rx DrugSAFE™ product line of medical safes and drug security products offer consumers and healthcare professionals proprietary, patented fingerprint solutions specifically designed to safely and securely store medications within the home, in healthcare facilities as well as out in the field. These secure storage products help control access to medications, addressing both loss prevention and safety concerns within a variety of healthcare settings. We believe Rx DrugSAFE™ products are easy to set up and operate, convenient and competitively priced.
In an ongoing effort to keep up with ever changing technology, after recently completing a redesign of the core electronics technology and mechanical locking mechanisms of our products to provide further security and convenience for our customers, we are once again looking to redesign our fingerprint interface to introduce enhanced features such as audit tracking capabilities, enterprise system integration, reporting and interoperability with IOS and Android devices. We have taken our experience in safe and security product design and fingerprint technology and our understanding of consumer’s wants and needs and developed a product line that we believe can help save thousands of lives.
Our strategy over the next twenty-four months will be to penetrate the consumer and healthcare medicine storage markets through comprehensive, multi-tiered marketing, distribution and educational efforts that leverage both traditional and online retail pharmacies, medical supply distributors, government funded initiatives and strategic partnerships. We plan to engage the support of credible professional and celebrity endorsements to position and market our products directly to consumers. We further plan to leverage relationships we have established with complementary businesses and organizations to provide education and raise awareness on the issue of Rx and OTC drug abuse and the increase in occurrences of accidental poisonings caused by infants, toddlers, adolescents and others accessing unsecured medications in the home.
The Problem and Challenge
A study by the National Drug Abuse Warning Network (DAWN) found the overall, medical emergencies related to nonmedical use of pharmaceuticals increased 132 percent in the period from 2004 to 2011, with opiate/opioid involvement rising 183 percent. DAWN also estimates that over 1.2 million ER (Emergency Room) visits involved nonmedical use of prescription medicines, over-the-counter drugs, or other types of pharmaceuticals in 2011.
The US population consumes more prescription medication than any other country in the world and the vast majority do not lock up these potentially dangerous drugs. According to the Partnership Attitude Tracking Study (PATS), one in five teens has admitted to abusing prescription medications. Moreover, according to ______________, 70% of these drugs are stolen from the homes of unsuspecting family and friends.
In addition, drug theft and diversion is a major problem facing the healthcare community resulting in businesses losing billions of dollars each year. Every American is affected by this epidemic due to sky-rocketing insurance costs as a result of the extra burden on the healthcare system caused by prescription drug abuse.
| 1 |
With prescription drugs overdoses causing more than 100 deaths each day in the US according to the National Institute of Drug Abuse, parents and providers alike must remain vigilant about keeping medications secured and out of the hands of unauthorized users. Children and toddlers present special concerns, because they often don't yet understand the risks or threats posed by drugs in their surroundings. They are curious about their environment so parents and caregivers must ensure that the environment they explore is always a safe one. Younger toddlers are inclined to put things into their mouths, and preschoolers are curious about items found in drawers and cabinets.
The reality is that all medicines are dangerous and potentially deadly for young children. Even common household medications such as Tylenol and vitamin supplements may appear harmless, but can actually cause harm when accidentally consumed by children.
These problems are also uncovered when physicians and pharmacists either write, or fulfill prescriptions. The traditional protocol to “lock up your medications at home to keep kids away from accessing drugs” has been the advice of these professionals for many years, yet there haven’t been any products patients could turn to that effectively, and conveniently, did the job. We believe our Rx DrugSAFE products provide real security against unauthorized persons, yet convenient access to prescribed users. We also believe families will make use of these products to keep medications locked up and their homes Rx and OTC abuse free.
We plan to offer various sizes of safes for consumer use, professional office use, and professional mobile use.
Principal Products and Potential Markets
According to the National Survey on Drug Use and Health, in 2012, an estimated 23.9 million Americans aged 12 or older were current (past month) illicit drug users, meaning they had used an illicit drug during the month prior to the survey interview. This estimate represents 9.2 percent of the population aged 12 or older. Illicit drugs include marijuana/hashish, cocaine (including crack), heroin, hallucinogens, inhalants, or prescription-type psychotherapeutics used non-medically. Of that number, there were 2.6 percent of persons aged 12 or older who used prescription-type psychotherapeutic drugs non-medically in the past month. These estimates were similar to estimates in 2009 (7.0 million or 2.8 percent) and to estimates in 2002 (6.3 million or 2.7 percent).
An estimated 52 million people (20% aged 12 and older) have used prescription drugs for nonmedical reasons in their lifetimes.
According to a report on Prescription Drug Trends by the Kaiser Family Foundation, the number of prescriptions dispensed in the US in 2009 increased 2.1% (from 3.8 billion to 3.9 billion), a larger growth rate than the 1.0% increase in 2008 over 2007. From 1999 to 2009, the number of prescriptions increased 39% (from 2.8 billion to 3.9 billion), compared to a US population growth of 9 %. Because of the new prevalence of these drugs in US households along with the fact that these medications are typically not locked up and easily accessible, such availability is thought to be one of the main factors contributing to the growing issue of abuse among young adults.
According to the National Institute on Drug Abuse, drug abuse and addiction have extreme negative consequences for individuals and for society as a whole. Estimates of the total overall costs of substance abuse in the United States, including productivity and health and crime-related costs, exceed $600 billion annually. As indicated above, the prevalence of prescription drugs in our society continues to grow and is a leading factor in the overall abuse cycle. The US government has committed $25.4 billion in 2015 to address the country’s drug abuse issue,, the largest percentage of which is being spent on prevention and treatment.
We believe that by preventing access we can prevent abuse. Our current product, the RxDrugSAFE stores up to 8 regular size pill bottles, as well as cough syrups. In addition our future planned launch of the RxDrugSAFE PRO will increase that capacity to over 30 regular size pill bottles. These products offer a real solution to potentially100% of the addressable market where there is a need to keep medications on hand and easily accessible but secured when not being used.
| 2 |
The Solution to the Problem
At the forefront of the educational information disseminated by pharmaceutical companies and drug abuse awareness organizations is the promotion of the responsible, secure storage of all potentially dangerous medications in the home. Current child resistant packaging, while helpful, is not alone sufficient to prevent children’s access to medications and does not prevent teens from accessing drugs. Pin code safes or combination lock boxes are easy for a child to thwart and not a viable solution for some prescribed users such as the elderly. A safe uses a key and keys can easily be misplaced or found by a determined teen. The keys can be stolen and copied and further, many elderly patients suffer from arthritis and find it difficult to turn a key. Because of the many deficiencies and problems inherent with other storage methods, it is not easy for most families to lock up their medications today.
Until now, there has not been a medicine security lock box product available to families and healthcare professionals that offers the level of security required, coupled with convenient, quick and reliable access by the authorized user(s) when needed. Rx Safes, Inc. plans to fill the void in the market with its Rx DrugSAFE line of fingerprint activated drug security solutions that can be used to secure medications and prevent unauthorized access in the home and within healthcare facilities.
Consumer Biometrics Market and Applications
Biometrics (or biometric authentication) refers generally to the identification of humans by their characteristics or traits. Biometric identifiers are the distinctive, measurable characteristics used to label and describe individuals. Biometric identifiers are often categorized as physiological versus behavioral characteristics. A physiological biometric would identify by one's voice, DNA, hand print or finger print. Behavioral biometrics are related to the behavior of a person, including but not limited to, typing rhythm, gait, and voice. Automated fingerprint identification system and live-scan biometric fingerprint identification systems, which automatically match one or many unknown fingerprints against a database of known prints and are primarily used for governmental and criminal justice applications. Historically, the adoption of biometrics technology has been concentrated in the government sector. However, there has been a shift in the dynamics of the market, with an increased number of biometric products being developed for other applications. The main use of biometrics in the consumer sector to date has been in the wireless and PC/network security markets, with a range of select high-end models of biometrically-enabled wireless handsets, PCs and laptops appearing on the market.
At present, consumer biometrics products are in the early adoption phase but with companies such as Apple and Samsung incorporating fingerprint readers on smart phones that people use on a daily basis, consumer familiarity and acceptance of fingerprint technology is increasing. These companies are paving the way for other fingerprint-activated products to make their way into every day usage. Rx Safes, Inc. offers biometric security products for securing prescription drugs and other controlled substances in the home and within a variety of healthcare settings. We believe that there is a unique opportunity in the marketplace today for us to establish a trusted brand of fingerprint-enabled drug/healthcare security solutions.
Rx DrugSAFE Products
Our products were originally launched in 2010 and are marketed under the trademark Rx DrugSAFE (“Rx DrugSAFE”). They provide consumers and healthcare professionals with professional grade, secure, yet convenient medicine safes, all using state of the art fingerprint recognition technology. Rx DrugSAFE is a metal safe, not a flimsy plastic container with a cheap digital PIN or tumbler briefcase style lock. Rx DrugSAFE is not designed as a deterrent. It is designed to securely store prescription medications to prevent access.
All Rx DrugSAFE products share these features in common:
| ● | Use secure fingerprint recognition technology to prevent unauthorized access yet provide convenient access to responsible adults, parents, caregivers and healthcare professionals. The authorized user’s fingerprint is their unique key which can never be lost, stolen or forgotten. | |
| ● | Our products are embedded and battery operated and do not require a computer or external power source. | |
| ● | No pin codes or combinations needed – making Rx DrugSAFE products simple and convenient for everyone to use but impossible for kids to thwart. | |
| ● | A key is not required to access the safe, although available as a back-up – eliminates the risk of the keys being stolen or misplaced and prevents the difficulties associated with turning a key for arthritis sufferers. |
| 3 |
| ● | Each safe can store up to 120 unique users making it possible to enroll other responsible family members in the event medications need to be accessed in an emergency, thereby making the products ideal for use within healthcare settings where multiple users may require access to the safe contents. | |
| ● | Durable 17 gauge steel construction – a real safe, not just a deterrent. | |
| ● | Uses a reinforced steel lock and hinges for extra security. | |
| ● | Permanent mounting capabilities so the safe can be secured horizontally in a drawer or other horizontal surface or vertically on a wall, maybe inside a closet or in the bathroom. Future Rx DrugSAFE solutions will mount within an existing medicine cabinet providing a secure compartment within the cabinet to place medicines and other controlled or dangerous items. |
At the end of 2011, we sent the Rx DrugSAFE to a professional security expert who identified a number of deficiencies with the mechanical/industrial design of the earlier version of the product. Because security is the key attribute of the product, the company chose to undergo a redesign, not changing the basic look and feel of the product but improving the locking mechanism as well as other design features that had been identified as potential vulnerabilities. In addition, we further enhanced the fingerprint technology utilized in the product making it easier to program and operate. We did this by shortening the number of steps required for consumers to enroll in the product and also lessened the matching sensitivity of the device to reduce single use failure. We also added additional features to make the product more consumer friendly including a hinge to hold the cover open when the patient is accessing the contents of the safe, serialized keys so that if the keys are lost or misplaced, we are able to replace them and finally a back-up battery pack, so if the user ignores the warning signs (intermittent red and green flashing led) and does not have the keys to hand, they are still able to gain access to the safe to get their medications and then change the batteries. As a result of this re-design, we now feel strongly that the Rx DrugSAFE product is now ready to re-enter the marketplace. We received a sample order of 100 of the newly designed safes in mid-2013 and have spent the last several months testing them internally as well as providing a number as marketing units to prospective business partners and selling a small number of safes to consumers for further feedback. From the immediate feedback, we believe that the modifications that were made addressed the security and user concerns.
Technology
We plan to offer several products under the trademark Rx DrugSAFE, designed to securely store prescription medications, pharmaceutical samples and other controlled substances. The products are designed around identity based technology allowing only authorized users to access the products.
Our Rx DrugSAFE products will all incorporate an embedded high performance micro-processor, flash memory, which stores the encrypted
fingerprint data, and a radio frequency (“RF’) based silicon fingerprint chip to develop superior performance in a
stand-alone device. Based on our many years of experience with this technology, we believe that we offer the industry’s
most efficient technology to deliver accurate fingerprint imaging, secure matching, low power consumption to conserve battery
life and reduced processor load for fast image capture and matching. In addition, other prescription drug safe products use older
mechanical key locks and/or pincodes that are inherently less secure and easier to bypass.
Our current design incorporates a fingerprint sensor from Authentec, with its patented TruePrint technology, which utilizes low power radio frequency signals to read beneath the skin’s surface to the living skin layer, which means that unlike any other fingerprint technologies, the solution we use is capable of reading virtually every fingerprint every time, regardless of skin conditions. Authentec was recently acquired by Apple and the sensors have been rebranded and are being manufactured and sold under license, through a company called Digital Persona. We have signed an NDA Agreement with Digital Persona, and are able to continue purchasing sensors from them or through a third party source via our manufacturing partner. Each sensor sold comes with its own license as part of the sale. Because the direction and further development of the Digital Persona product line is unknown, we are presently evaluating other options including a touch sensor from a company called Next Biometrics. Because the fingerprint reader from Next Biometrics requires the user to place their finger on the fingerprint sensor instead of swiping, we believe this will improve the overall user experience. We have written a design document and are presently speaking with a number of familiar engineering resources with specific experience working with fingerprint biometrics, with a view to re-engineer a new fingerprint interface incorporating the Next Biometrics product. This new interface will then be integrated into our existing and future products and will also incorporate new features to address the needs of commercial healthcare customers including audit tracking and reporting capabilities as well as the ability to interface with applications on external smart devices so that data can then be integrated with a healthcare facilities enterprise systems.
| 4 |
RxDrugSAFE at Home
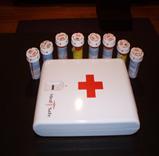
The original RxDrugSAFE product, featured below, offers a real solution to families looking to not just send a message to their kids, but to take serious measures to protect them within their home environment. Rx DrugSAFE works by recognizing an authorized user’s finger before it unlocks. It is specifically designed for use in home environments and can be permanently mounted in a bathroom/bedroom drawer or closet. It also provides families with additional flexibility in that it can be easily transported if medications are needed while traveling. The safe comes with a twisted steel security cable, allowing it to be secured to a fixed item in a room or vehicle, such as a car seat. Our Rx DrugSAFE Home Medicine Security Safe can hold up to 8 regular size prescription bottles. It can also secure other controlled or potentially dangerous substances such as cough syrups containing dextromethorphan, morphine vials, insulin and syringes. For less than the cost of a video game console, parents can now protect their children from the dangers of prescription medicine.
RxDrugSAFE™
 |
 |
We have begun preliminary design on and intend to introduce to market the Rx DrugSAFE IC (In Cabinet), featured below, a version of the safe which mounts directly into an existing medicine cabinet and can hold up to 18 pill bottles. The “IC” version is designed to be securely mounted on the bottom shelf of an existing medicine cabinet, and will be screwed into the back of the cabinet (and into the wall). We expect to have physical prototypes of the IC product by the end of 2015.
 |
R x DrugSAFE IC ™ |
| 5 |
RxDrugSAFE in the Facility
We recognize that the Rx DrugSAFE products and technology can also provide a viable drug security solution for certain areas of the healthcare marketplace, as well as other facilities where medications may be present, such as schools and early learning centers. The Rx DrugSAFE is ideal for use in medical offices as well as by home healthcare providers to store and transport controlled substances. Medical practices can also utilize the product to securely store their prescription pads, an item which is commonly stolen from doctor’s offices. Rx DrugSAFE has also been purchased by hospice facilities and nursing homes, environments where many potentially dangerous drugs are dispensed to individual patients and need to be secured. We have also previously sold units to ambulance services to secure medicines transported in the back of the ambulances. RxDrugSAFE’s sleek, compact design makes its suitable for use by mobile healthcare providers. We also offer an optional carry handle enhancing the product’s portability.
In addition, based on feedback received from the healthcare community, Rx Safes, Inc. has designed and prototyped a larger version of the product called Rx DrugSAFE PRO. This version is designed specifically for use within a variety of healthcare environments. It is much larger than the RxDrugSAFE product, comes with adaptable foam inserts to secure pill bottles of various sizes in the upright position and can store between 16 to 30 pill bottles, and other controlled items and equipment, depending on the configuration used.
| RxDrugSAFE PRO™ | |
 |
 |
 |
 |
| 6 |
We had originally anticipated that the PRO would be available for sale at the end of 2014. However, due to delays in obtaining the necessary funding to move this project forward, we now expect the PRO will be available for order in the fall of 2015.
Rx DrugSAFE in the Field
The Affordable Care Act of 2010, Section 2703, created an optional Medicaid State Plan benefit for states to establish Health Homes to coordinate care for people with Medicaid within their home environment. States receive a 90% enhanced Federal Medical Assistance Percentage (FMAP) for the specific health home services. With a goal to provide the ability for mobile healthcare workers to securely and safely transport medications and provide HIPAA compliant care in the field, Rx Safes, Inc, has prototyped the Mobile Medical Station (MMS). The MMS is a waterproof, shockproof and secure rolling case with telescopic handle that is secured with Rx Safes’ patented fingerprint technology. The case incorporates state of the art vital statistics monitoring equipment, a rugged field tablet containing patient H&P data which can be securely uploaded to the primary healthcare enterprise system and one of our Rx DrugSAFE fingerprint lockboxes for the safe and secure transportation of medications.
 |
 |
We also plan to incorporate mobile printing capabilities, which are often required in a remote healthcare setting. The prototype was developed by Rx Safes as a proof of concept using non-standardized equipment. However, it is our intention to develop strategic relationships with OEM suppliers for each of the required devices and offer a standard MMS along with customizable options, depending upon the specific needs of the healthcare provider group. We anticipate that the MMS will be available for initial order in the fall of 2015.
Rx DrugSAFE in the Future
Our mission is as simple as it is powerful – to become the leading provider of standalone biometric medical security solutions for consumer and healthcare markets. To this end, we intend to consider adding products that complement our current and developing productline including, for instance, biometrically controlled dosing devices, portable HIPAA compliant biometric EMR devices, biometric tele-medicine solutions, products to biometrically secure physical and electronic medical health records and secure, remote healthcare monitoring products.
Initially we are looking to offer a comprehensive approach to drug security and diversion. In 2015/16, we plan to introduce additional products and solutions for home and professional use to safeguard medicines and other types of medical equipment and data. As noted above, we intend to introduce a fingerprint activated lockbox called Rx DrugSAFE-IC, which will provide consumers with the ability to convert a portion of an existing medicine cabinet into a secure lockbox to store their medicines and other controlled substances. This product is expected to be ready for purchase at the end of 2015. Rx DrugSAFE PRO is estimated to reach the market in the fall of 2015.
| 7 |
We envision a three tiered product catalogue and product roadmap. Our first products are based on the need to secure controlled substances in the home and in certain institutional and healthcare environments where a smaller storage solution is needed. This includes the two current RxDrugSAFE products, as well as two additional products for future release. The two new products will use the same technology, but the actual safe sizes will be different. We plan on offering a new intermediate size called Rx DrugSAFE II that is between the sizes of the RxDRUGSAFE and RxDURGSAFE PRO, as well as a fourth size, as mentioned before, that will be designed to fit inside a home medicine cabinet.
The second line of products will utilize the same technology but will focus on larger storage products, such as lateral and vertical file cabinets and vertical storage cabinets. These storage cabinets would be used primarily in professional healthcare offices and facilities to store physical medical files and sample pharmaceutical products.
The third line of storage products will be rolling medicine carts and other storage cabinets that can be used by healthcare facilities to store controlled substances in between patient rooms, as well as fixed medicine storage products for the same facilities. Once again, these products will incorporate the same secure and convenient fingerprint technology embedded in current storage units, but may also be tied into an enterprise where data relating to the patient, the type, dose and time of medication dispensed, and by whom can be wirelessly communicated to the main system.
If we are able to build brand recognition and establish market acceptance of our technology, we will then look to introduce the other healthcare security products we have identified above, to offer a comprehensive suite of healthcare related security solutions.
Product Specifications
The RxDrugSAFE™ products are made from hardened 17 gauge steel with steel pin reinforced hinges. They have an advanced steel lock, which is controlled using state of the art fingerprint technology. They are finished in bright white powder coat with the RxDrugSAFE logo and our own personalized medical cross symbol, to distinguish brand identity. The safes come with two sets of back up keys, (most customers seem to require a back-up), and a security cable that is used to temporarily affix the safe to an immovable object. Standard features include:
| ● | Programmable for use by up to 120 individuals |
| ● | Uses unique fingerprint technology to prevent unauthorized access |
| ● | Simplified programming – user is enrolled in less than 10 seconds |
| ● | User recognition takes less than 1 second |
| ● | Convenient and portable - runs on 4 AA Batteries |
| ● | Battery failsafe - enrollment data is not lost due to battery failure or removal |
| ● | Back-up battery pack if the batteries die |
| ● | Can hold up to 8/30 standard size pill bottles |
| ● | Tubular pick resistant back up keylock |
| ● | Serialized keys |
| ● | Stainless steel hinge to hold cover in the open position |
| ● | The portable Rx DrugSAFE weighs less than 3 lbs |
| 8 |
Dimensions of existing product offerings:
Rx DrugSAFE and RX DrugSAFE PRO
 |
 |
Optional accessories include:
| ● | Carry handle – the detachable handle simply screws on to the RxDrugSAFE, and now you have a portable travel drug safe. |
| ● | Mounting bracket – allows a user to mount the Rx DrugSAFE and PRO horizontally in a drawer or on a counter top, or vertically on a wall inside a closet. This is a more permanent solution than using the security cable and provides additional security. |
Trademark, Copyright and Patent Protection
We believe that we have begun to establish a recognizable brand behind the Rx DrugSAFE product name in the market. To protect the Rx DrugSAFE brand name and mark, we filed an application with the US Patent and Trademark Office for the Rx DrugSAFE mark, as a standard character mark. This mark was published on March 1, 2011 and registered on May 17, 2011. The serial number for the trademark is 85141627. We established “first use” of the mark in May 2010 and due to aggressive sales and business development efforts were able to justify a claim to the mark based on first use and customer familiarity with the name as it relates to our product and market.
We have secured an exclusive license to use patent number 6,766,040, which covers the system and method for capturing, enrolling and verifying a fingerprint and covers the technology used in the Rx DrugSAFE products. The patent was acquired by us in the asset purchase agreement we entered with Axius Consulting Group, Inc. The patent was originally licensed to Axius by bioMETRX.
The patent license is exclusive to Rx Safes, Inc. as it relates to any embedded, battery operated product sold into the healthcare industry, or for related consumer or commercial healthcare applications such as narcotic and prescription pill and gun safes. The patent covers various aspects of the technology used in our products including independent claims on the size of the fingerprint template, the peak power consumption used to process and match a fingerprint image, as well as the fact that it is powered using one or more batteries. The relevant claims of the patent as they relate to the design of the fingerprint user interface used in our products are as follows:
| ● | A biometric verification device for providing secure access to a unit connected to the device, the device comprising: a. a biometric sensor capable of sensing a biometric trait of a user that is unique to said user and providing a first signal containing information representing said biometric trait; and b. a processing unit connected to said biometric sensor so as to receive said first signal, said processing unit being adapted to compare said information with biometric data stored in said processing unit representing a biometric trait of an enrolled person, and provide a verification signal indicating whether or not said information corresponds sufficiently with said biometric data to verify said user is said enrolled person, wherein said processing unit completes said comparison and generates said verification signal within 20 seconds of when said biometric sensor senses said biometric trait, said biometric sensor and processing unit, together being configured to use no more than 1W of peak power. |
| 9 |
| ● | wherein said biometric trait is a fingerprint | |
| ● | wherein said processing unit completes said comparison and generates said verification signal using no more than 400 mW of peak power. | |
| ● | wherein said processing unit completes said comparison and generates said verification signal within 7 seconds of when said biometric sensor senses said biometric trait. | |
| ● | wherein said processing unit stores said biometric data representing a biometric trait of an enrolled person using no more than 1 K bytes of data. | |
| ● | further including one or more batteries that comprise the sole source of power for the device. | |
| ● | further including a wired interface for connecting the device with the unit |
We believe that any practical application used for a similar purpose within our market will likely infringe upon this patent, meaning that Rx Safes, Inc. has the ability to protect its market, prevent illegal competition and offer the only practical, effective fingerprint based solutions to store pills, medicine bottles and other controlled substances.
We intend to file design patents for our products and will continue to pursue all available protection for our current and future product offerings. In order to do this, however, we will need additional capital.
Marketing and Distribution Plan
As previously discussed, we originally launched the Rx DrugSAFE product in 2010 but took it off the market at the end of 2011 to address some vulnerability issues. The core technology and mechanical locking mechanism have been redesigned to address these issues. We also added some additional features to include a hinge for the cover, serialized keys and a back-up battery system. As part of the redesign, we also focused on pricing and have been able to reduce the overall cost of the Rx DrugSAFE product while improving its overall functionality and usability and adding the new features mentioned.
During the initial launch in 2010, we took steps to validate the demand and need for the product by consumers, the health care segment and professionals alike. We conducted beta testing and initial market testing to ensure we were positioning and pricing the product correctly for our initial launch. To facilitate this market testing, we produced physical prototypes and tested these in typical usage situations, initially with friends and family and then a more expansive group of testers and initial customers. We also conducted informal focus groups with the test groups, at trade shows and during grass roots community health and wellness events and events targeting child safety. We received overwhelming positive acceptance from all groups we spoke with. The main areas we focused on were: product features, functionality, pricing, operation and construction. An important, but not the only goal of the plan, was to increase awareness of our products and fingerprint technology in the market.
With the completion of the redesign, we intend to ramp up our business development efforts. Our plan is to have independent sales and marketing teams focusing on each market segment to include consumer, as well as the health care and B2B professional segment, such as schools, early learning centers, government facilities and corporations, among other entities.
Consumer Market
The overall goal when attempting to create a footprint in the consumer market is to use the Rx DrugSAFE as a tool to educate parents on the prescription abuse issue because raised awareness translates to increased demand. Sensitivities as to need and price have to be considered but it is clear that to be effective, broad market penetration through a variety of channels is a necessity. We have identified a number of key channels that we must sell through in order to successfully reach the consumer in a meaningful way.
| 10 |
Government Funded Initiatives
Prescription drug abuse has become one of the greatest challenges facing our society and more people overdose from prescription drugs than marijuana and cocaine combined. Many government organizations have begun to gather and study data and have established programs to educate and inform people about the issues of prescription drug abuse. These include the National Institute on Drug Abuse, the CDC and SAMHSA. There are many grants available through SAMSHA, NIDA, Bureau of Justice and other federal programs. In addition, many states have established their own task forces and funds to tackle the prescription drug abuse epidemic. As part of the National Drug Control Budget, President Obama has requested $25.4 billion in 2015 alone, to reduce drug abuse and its consequences and the largest portion of this budget is to be spent on prevention and treatment. The Rx DrugSAFE product can offer a significant positive impact on the prevention element of the government’s efforts. By preventing access to prescription drugs in the home and other healthcare settings, we can reduce the number of teenagers becoming addicted to these drugs, prevent accidental ingestion by toddlers and deter theft by addicts or dealers.
The primary goal is to get our product into the hands of at risk families through the multitude of state and federal programs. To this end, we have begun to make contact with all National Association of Drug Diversion Groups (NADDI) throughout the US, offering the Rx DrugSAFE as a product they can purchase and donate, using one of the many grants available. Recently, we successfully shipped 500 Rx DrugSAFE units to the Oklahoma Bureau of Narcotics and Drug Diversion. The Oklahoma project was supported by a grant awarded by the Bureau of Justice Assistance, which is a component of the Office of Justice Programs. Now that we have proven our product can be recognized as an integral part of one of these such programs and at least one appropriate funding source has been identified, we intend to take this program countrywide as step one in our efforts to benefit from the many government funded prescription drug abuse initiatives.
Retail Pharmacies
In focusing on consumers, we have determined the various potential entry points to consumers in the market, which are primarily “bricks and mortar” retail pharmacies, national chains and community retail pharmacists. These are obvious first points of market penetration, considering that these are the places where the majority of potentially dangerous drugs are dispensed to consumers. Patients often look to their pharmacist for advice and they are often a trusted part of a patient’s care regime.
The national chains operate approximately 39,000 pharmacies and fill nearly 2.6 billion prescriptions annually, which is estimated to be more than 72 percent of annual prescriptions in the United States. Storing medications securely is an initiative that has become more important to these pharmacies due to the increased number of prescriptions being written and the concern that these medications are getting into the wrong hands.
During our initial launch, chain drug stores, Rite Aid and Walgreens and online pharmacy, drugstore.com, piloted the product online. In addition, we established a relationship with OCI, a tier one distributor to Walmart and have also sold a small amount of product at walmart.com. Clearly, we did not want to sell an inferior product through these channels and consequently pulled the product from these sites during the redesign. We are not presently selling product to any of these online retailers. Now that we have completed our redesigned product we plan to reengage with these partners and resume selling on these sites. In addition, we have reached out to the major bricks and mortar retail pharmacy chains proposing a program to use the Rx DrugSAFE as a patient acquisition and retention tool as well as to place Rx DrugSAFE product displays at the pharmacy counter point of sale, making it accessible to patients and available for the pharmacists to point to when dispensing potentially dangerous medication as they advise the patients about the importance to lock up their medications. We plan to work on a coupon program with the stores whereby the customer can obtain a significant discount if they fill a certain amount of prescriptions within a specified period of time. These programs are at the preliminary stages of development and we anticipate closing on one or more such deals within the next 6 months.
In order to successfully penetrate the retail pharmacy market, it is essential to establish Rx DrugSAFE as an item that can be purchased through one of the 3 largest US based health care distribution companies, McKesson, Cardinal Health and Amerisource Bergen. These three companies service approximately 98% of all US based retail pharmacies, national chains and community retail pharmacies.
| 11 |
McKesson is the second largest medical products and health care services distributor in the world next to Cardinal Health. We established a vendor relationship with McKesson and the Rx DrugSAFE product was added as the first product we are able to sell through McKesson Pharmaceuticals Consumer Products Group to their various customers. McKesson serves more than 25,000 pharmacies nationwide and these customers include many of the top retail pharmacy chains, including Walgreens and Rite Aid. McKesson is also the main distributor to Walmart Pharmacies and has its own franchised chain of over 2,600 community pharmacies that are branded under the “Health Mart” umbrella. Health Mart Pharmacy ranked “Highest in Customer Satisfaction with Chain Drug Pharmacies” in JD Power and Associates 2009 National Pharmacy Study. Health Mart Pharmacies are independently owned and fall under the category of community retail pharmacies. They are usually owner operated and are well established within their communities. These pharmacies struggle on a daily basis to compete with larger chains, which frequently have the ability to offer more competitive pricing and more comprehensive services. These pharmacies typically focus their efforts on offering a higher quality of service to customers and are constantly looking for ways to distinguish themselves from the national chains.
We exhibited the Rx DrugSAFE at the 2010 McKesson Pharmacy Strategies Conference, where we were given an opportunity to present our product to hundreds of McKesson community pharmacy owners, many Health Mart franchise owners and independent pharmacy owners and pharmacists. The feedback regarding the product was overwhelmingly positive and we secured a number of orders through the conference. Once again, due to the redesign effort, we subsequently stopped shipping product under the McKesson agreement. We have made initial contact with the new buyer for our category with a goal to re-launch the new improved product through its channels in the coming months.
Prior to our pulling the original Rx DrugSAFE products, we were establishing a vendor relationship with Cardinal Health, the nation’s largest distributor of healthcare products and actually entered into a formal vendor agreement with the company. The plan was that Cardinal Health would offer Rx DrugSAFE products through its various channels, including its retail pharmacy customers, the largest of which is Walgreens, and through its own franchise group of independent pharmacies, The Medicine Shoppe, which has over 850 pharmacy locations across the US and another 400 international pharmacy locations in Canada, China, Japan, Indonesia and the Middle East. To date, we have not sold any product through Cardinal Health but have recently reengaged with them with a view to launch the redesigned product through its various channels.
We were also working on establishing a similar relationship with Amerisource Bergen Drug Corporation, which provides a comprehensive line of consumer products to pharmacy customers, including over the counter (OTC), health and beauty care (HBC), home healthcare (HHC), private label (PL), and general merchandise (GM) customers. They also operate Good Neighbor Pharmacy, a distribution, marketing and purchasing group that provides over 3,000 independently owned pharmacies with branded medical supplies and services. We plan to resume discussions with Amerisource Bergen in the coming months.
We are not a supplier to Amerisource Bergen Drug Corporation, but we hope to work with Amerisource Bergen to reach hospitals, alternate care facilities and other health care businesses throughout the United States, Canada and selected global markets. Amerisource Bergen’s Specialty Group brings specialty pharmaceutical and medical related products from the manufacturer to such entities, as well as primary care physicians, group purchasing organizations, physician practice management companies and large group practices.
Finally, we will seek to establish distribution relationships with pharmacy cooperative buying groups, and have established such a relationship with Epic Pharmacies, under our standard reseller agreement, so that the product may be offered directly to members of such groups. Epic has over 1,200 independent pharmacy members who purchase products for their pharmacies through Epic. We have already established a reseller relationship with Epic, where its members will enjoy the benefit of special discounted pricing for the Rx DrugSAFE product.
Online pharmacies are also a growing segment in the US pharmacy market and the largest of these is DrugStore.com. DrugStore.com sells more consumer-based medical supplies than all of the other online pharmacies combined and has over 3,000,000 worldwide purchasers annually. DrugStore.com is also the online service provider for Riteaid.com. We solicited DrugStore.com by phone, and we sent the company a product sample which they reviewed and determined that it would complement their product offerings and they established an agreement with us. We are an approved vendor to DrugStore.com and have successfully shipped product to their customers in the past through a drop ship relationship, which is facilitated by a company called CommerceHub, a third party fee based service which processes all of the DrugStore.com orders. We have re-established communications with DrugStore.com and plan on re-engaging with CommerceHub so that the Rx DrugSAFE product will once again be available for purchase at Drugstore.com.
| 12 |
Online Resellers
To expand our online presence beyond our own website (www.rxdrugsafe.com), we have created a reseller program to attract a variety of online resellers who have established customers and have already expended resources to position their respective sites at the top of the page on major search engines. Our Reseller Agreement offers qualified resellers the ability to purchase our products at a discount to the MSRP for the Rx DrugSAFE. The greater volume of business a reseller conducts with Rx Safes, Inc. the greater the discount the reseller will enjoy. We presently have 5 Reseller discount levels. To maintain status, a Reseller must agree to abide by our Minimum Advertised Pricing (MAP) policy for the Rx DrugSAFE. To date, we have signed up over 20 online resellers who range from home health and medical supply companies to companies that sell all types of products for the home.
We plan to continue to build upon this reseller network and plan to provide monthly incentives and special offers to encourage the resellers to give the Rx DrugSAFE product high profile placement on each of their respective web sites.
Medical Professionals
We have received endorsements from physicians in various specialties. For instance, Dr. Mary Ellen Renna (pediatrician) appears on Fox and Friends as well as WPIX Channel 11 with our products, covering prescription drug abuse and endorses use of our safe as a way families can safeguard their medicines. Although Dr. Renna’s agreement has expired, she still continues to endorse our products during the course of her business by recommending the safe storage of drugs to her patients and suggesting the Rx DrugSAFE as the best option. They will not receive any additional compensation for this. Doctors are called upon to prescribe medications to their patients for various reasons. Just like pharmacists, doctors typically have a trusted relationship with their patients, so it makes perfect sense that we should look at doctors as a channel through which our product could be marketed to consumers. For example, pediatricians support parents from pregnancy, through birth and often up to 18 years of age. They readily dispense advice to parents on what they can do to prepare their homes for their new babies and children in general and a major part of that is ensuring the home environment is safe. Other physicians also dispense medications that people can potentially abuse and that in the wrong hands can be deadly. In addition, pain management clinics primarily take a pharmacologic approach to treating long term pain and various medical practitioners, clinical psychologists, physiotherapists, occupational therapists and nurse practitioners frequently prescribe various narcotic drugs such as opioids and antidepressants to help manage pain over time. These channels as well as drug rehabilitation centers, recovery centers and sober living facilities also provide a conduit through which the Rx DrugSAFE can be introduced to the general public.
Physicians are typically well respected by patients and their advice is taken seriously. Having such respected professionals recommend our product to patients in effect extends our sales force to qualified medical and related professionals, a team we would not be able to engage in any other manner.
To help further develop these channels, Rx Safes, Inc. intends to set up a program where medical professionals will be able to display literature on the Rx DrugSAFE product in their waiting rooms and exam rooms. Making this literature available to patients at a physician’s office will provide the Rx DrugSAFE product some credibility in the eyes of the patient. The medical practices would then be able to choose to take a passive or active role in selling the Rx DrugSAFE product, but either way will be incentivized to a degree to sell the product as they automatically receive a percentage of the sales generated from their practice. This program has not been established as yet and is a proposed program that we are discussing internally and with medical practices. As such, we have not established any details on percentages that participating medical practices would receive. Each physician’s office would be assigned a unique code which would be indicated on the back of the literature available to patients to take away with them. The patient will be required to enter that code when they go to our site to order an Rx DrugSAFE in order to receive a small discount on their purchase and this code would tie back specifically to the physician’s office where the literature was displayed. At the end of every month, the physician’s office would receive a check from Rx Safes, Inc. to cover their commission on sales the office generated.
| 13 |
Health Care Systems
Our hospital marketing program is designed to provide health care systems with an effective outreach program within their local communities, with the visibility to attract patients to their healthcare services. ER doctors often take an active role in advising patients to secure their medications in their homes upon discharge. To facilitate this effort, we plan to supply the hospital system with pre-printed custom Rx DrugSAFE prescription pads that will allow a patient to acquire an Rx DrugSAFE product through our professional referral program at a significant discount to the Management’s Suggested Retail Price (MSRP). In addition, we also plan to provide an informational flyer sponsored by Rx Safes, Inc. offering valuable information on steps discharged patients can take at home to secure their medications. The hospitals/health systems may distribute the pads and flyers throughout their facilities. Physicians and other discharging medical professionals would provide patients with a prescription for an Rx DrugSAFE whenever they issue pain, stimulant or anti-depressant medication. Each safe sold will provide the health system with a 10% commission to further support outreach efforts dealing with prescription drug abuse in their catchment area. This program offers the health systems a tremendous community service opportunity as well as creating increased, credible awareness for the Rx DrugSAFE product. The program is designed to be compliant with regulations specifically pertaining to healthcare advertising and marketing and will likely launch as a pilot by Q3 2015. We intend to begin a recruiting campaign in the coming months to identify qualified regionally based personnel to target healthcare facilities/systems to sign up for the program.
Fundraising/Community Collaboration
Good corporate citizenship is a major part of our business philosophy. We believe in “Doing Well By Doing Good” and feel strongly about our mission to arm families, educators and other community organizations with the information necessary to keep our kids safe. To support this effort, we have developed various fundraising partnership initiatives which tie the Rx DrugSAFE product in with educational material on drug safety which can be disseminated by schools, church groups, youth and community organizations, PTA groups and other non-profit groups. We will support these groups in their educational programs by providing product materials for them to use or make available to their membership. In exchange, we plan to ask these nonprofit groups to include the Rx DrugSAFE product in their marketing materials for various events. We also plan to offer discounts for nonprofit groups and other organizations who want to advertise the Rx DrugSAFE products directly on their websites or at fundraising events for their groups. In establishing these relationships, we are hoping to reach a broader audience than we would be capable of independently utilizing the resources of already established organizations to reach target demographics.
We have already informal relationships with drug awareness outreach programs embedded in communities around the United States, such as D.A.R.E., Partnership at DrugFree.org and NOT MY KID, as well as various PTA and other community based drug free and child safety advocacy groups. These organizations deliver and lead community education programs and create public awareness campaigns relating to the issues of drug and alcohol abuse.
Strategic Corporate Partnerships
We hope to develop relationships to promote our products and the benefits of the products with one or more of the large pharmaceutical companies that are responsible for manufacturing some of the medications that people can potentially abuse. Some of the pharmaceutical companies we have contacted to date are Purdue, Pfizer and Abbott. Each of these companies manufactures a least one heavily abused prescription drug and each has received a great deal of negative media coverage about the addictive effects of the drugs and the level of abuse that has been seen. To date, pharmaceutical companies have primarily attempted to curb such abuse by providing educational materials when medications are dispensed, in the hope that the users of the medications would pay attention and read the literature. Unfortunately, in most cases this information goes unread and, as a result, efforts to date to curb abuse in this manner have been ineffective, as the levels of prescription drug abuse has more than doubled in the last 5 years.
| 14 |
We have proposed a variety of initiatives to pharmaceutical companies to address this abuse, such as sponsoring a donation of Rx DrugSAFE products to at-risk families and a coupon drive, which would be offered through national print and online publications to pharmacies, doctor’s offices and others where the coupon would be given in the name of the pharmaceutical company for a patient to purchase an Rx DrugSAFE product at a significant discount to the MSRP. We would then redeem the coupon value back from the pharmaceutical company to cover the cost of the discount provided. These programs were not established due to the redesign of the Rx DrugSAFE product but we plan to resume discussions with the various pharmaceutical companies to move these relationships forward in the coming months.
Prior to our taking the product off the market to engage in the redesign, Purdue Pharmaceuticals had already taken various steps to implement an alliance with us, featuring our product in the “Safeguard My Meds” campaign, an initiative used by the National Community Pharmacists Association (NCPA) and sponsored by Purdue. For example, Purdue has used a short vignette which appears on the “Safeguard My Meds” home page, showing the Rx DrugSAFE as a recommended mechanism to secure medications in the home. The product was also on display at a special “Safeguard My Meds” booth at the NCPA annual convention, which took place October 23rd – 27th, 2011. We intend to reengage in discussions regarding future initiatives with Purdue Pharmaceuticals and hope to work together more significantly on joint efforts to educate families about the importance of locking up medications at home.
By aligning with a product such as the Rx DrugSAFE, the pharmaceutical companies can demonstrate to their customers that they are taking an active role in addressing drug abuse and offer real solutions for families who use and store medications safely. In turn, Rx Safes, Inc. will benefit from the association with such companies and their extensive global outreach capabilities. As part of each relationship, the pharmaceutical company would not only assist us in reaching potential customers directly but would also become a customer of ours, purchasing Rx DrugSAFE units to give away as part of positive public relation campaign and purchasing the coupons that we could use to make the product affordable for more people.
Health insurers are another group that could become strategically and financially vested in our efforts to prevent unauthorized access to prescription drugs in the home. It is the private health insurers, HMOs, Medicaid and Medicare that feel the financial burden when their members end up in the ER, in treatment or rehabilitation and have to be prescribed medications to treat addiction. The cycle of addiction is perpetuating and we believe costs insurers millions of dollars annually. If insurers provide Rx DrugSAFE units to their members, this could have a positive impact on the number of people, particularly toddlers and teenagers, ending up in the ER and in subsequent in/outpatient treatment, reducing the overall costs to the insurers and demonstrating a real ROI if used as designed by plan members. We have begun initial outreach to some of the larger health insurers and plan to move these discussions forward over the coming months.
Trade Shows and Community Events
Trade shows and grass roots community events are an excellent way to educate the buying public, whether direct consumers, industry buyers or others, about a company’s products and/or services. We plan to look for every opportunity to exhibit our products and talk to trade show attendees. Some of the industry and community sponsored events we have targeted in the past include The McKesson Pharmacy Strategies Conference, The University of Southern Nevada Drug Abuse Awareness Team’s Annual “We Pledge” event, Nevada Youth Alliance’s Back to School Health Fair, The University of Southern Nevada Annual Health Festival and Boulder City Annual Health Festival and University Medical Center’s Annual Pediatric Trauma Conference, among others. With proper resources at our disposal, there are a number of large industry conferences that we would like to target for 2014, including the National Community Pharmacists Association (NCPA) Convention and Trade Expo, Senior Care Pharmacy Annual Meeting and Exhibition, Community Anti-Drug Coalitions of America’s (CADCA) National Leadership Forum and the National Association of Chain Drug Stores (NACDS) Market Place Conference. We have already registered to exhibit at the Mid-America Healthcare Venture Forum in Chicago on March 9-11th and the International Cannabis Expo in NYC, scheduled for June 17-19, 2015 and hope to have the opportunity to attend/exhibit at some of the other conferences referenced.
| 15 |
Medical Marijuana Market
To date, 23 states and DC have enacted laws to legalize medical marijuana and four states have legalized recreational use. As yet another drug available to patients to treat various ailments, medical marijuana and cannabis infused products need to be securely locked up in the home, primarily to prevent access by children. With cannabis infused candy, chocolate, cookies and brownies available for purchase it is not surprising that there were an increased number of ER visits due to children ingesting these drugs. We plan on positioning the Rx DrugSAFE as the go to product for patients who use medical marijuana and have already developed new branding to target this emerging market. We have exhibited at a handful of local medical marijuana events and, as referenced above, we will be participating at the upcoming ICA event in NYC where our CEO, Lorraine Yarde, will be a guest speaker.
In addition to the channels focused on consumers that were discussed above, there are other avenues available to increase our sales to consumers that we would like to pursue once the resources are available to do so. These include targeting corporate health and wellness programs of companies by offering the Rx DrugSAFE product as an incentive for company employees to participate in such programs or offering the product at a discounted price so the company employees can lock medications.
Health Care Organizations and Providers
As discussed above, the Rx DrugSAFE products also provide a viable drug security solution to certain areas of the healthcare marketplace including medical offices, hospitals, home health care providers, nursing homes, rehab and sober living centers, assisted living facilities, hospice facilities and ambulance services. This list will become broader as we begin to expand our product line into larger storage solutions. There are three main established channels through which we will market our products into the health care arena: health care and medical distributors; independent manufacturer’s representatives; and online medical supply stores.
Health Care Distributors
There are many smaller distributors in the health care field, some of whom are more industry category focused or support certain geographic regions. However, if we are to make any significant impact selling our products into the health care and medical arena, we believe that we will have to sell through one or more of the global distribution leaders discussed above.
As previously noted, we are already a vendor for McKesson and we plan to sell our products through the McKesson home healthcare group to the consumer market. However, McKesson Pharmaceutical distribution also supplies branded, generic and over-the-counter pharmaceuticals and medical products to more than 40,000 customers spanning institutional providers such as hospitals, health systems, integrated delivery networks and long-term care providers. Through our already established relationship with McKesson, we hope to also service other McKesson distribution groups including McKesson Medical-Surgical. This McKesson group delivers a comprehensive offering of medical-surgical supplies, medical equipment and health care technology-related services to the alternate site market, including physician offices, surgery centers, long-term care facilities and home care businesses across the country and distributes more than 150,000 medical-surgical products to more than 300,000 customers. Finally, McKesson also owns Moore Medical, an internet-enabled, multichannel, specialty direct marketer and distributor of medical, surgical and pharmaceutical products to nearly 100,000 healthcare practices and facilities in non-hospital settings nationwide, including: physicians, emergency medical technicians, schools, correctional institutions, municipalities, occupational healthcare professionals and other specialty practice communities.
Independent Manufacturer’s Representatives
Independent Sales Representatives play an important role within the health care industry and form a unique bridge between manufacturers and health care facilities, allowing companies like us to effectively get a new product to market and utilize a national sales force, which is purely performance driven, without the burden of the monthly overhead to hire inside sales personnel. By forming relationships with independent sales representatives, we hope to take immediate advantage of their already established relationships in various areas of health care, including hospitals, ambulatory centers, home health care providers, doctor’s offices, dental practices, assisted living and nursing facilities and hospice care centers.
| 16 |
Through membership with the Healthcare Independent Representative Association (HIRA), we intend to expand our network of independent sales representatives. HIRA is a national organization that focuses on furthering the function and professionalism of independent sales agents in the health care marketplace and works to match manufacturer’s products with the right sales agencies and markets.
The advantages to us of establishing a network of independent sales agents is that they will provide us with immediate entry into various territories, permit regular calls to be made on existing customers of the representatives and prospective customers, provide quality salesmanship and help control expenses.
Online Medical Supply Stores
With the newfound dependence on everything electronic, many small to mid-size health care providers and even purchasing departments of hospitals and other medical facilities are turning to reputable sites on the internet to procure a variety of medical products. There are many sites that are recognized as leaders in the online medical supply space. Allegro Medical was one of the first companies to sell medical supplies online back in 1997. Their B2B program serves schools, government agencies, hospital, doctors, nursing homes, rehab facilities, durable medical equipment stores and more. Upon receipt of the new product shipment, we plan to launch the Rx DrugSAFE product on the Allegro site. In addition, we were already selling the Rx DrugSAFE product through a number of online medical supply companies such as Medical Supplies and Equipment Company, Mednetdirect, Universal Medical and Devon Medical Products and we plan to reengage with these groups as well as work with other online medical supply companies to get them to carry their products.
B2B Professional Sales
We are also attempting to market our products to a variety of other organizations and businesses such as educational facilities, day care centers, rehabilitation facilities, government agencies, the military and corporations in general. We have spent a limited amount of time targeting these particular businesses, but plan to increase our efforts in this area and establish a team of independent representatives who will focus on B2B channels on our behalf.
In 2014, we plan to target the largest health care supplier to school nurses nationwide, School Nurses Supply, Inc., to add our products to their catalog. We will also reestablish discussions with Phoenix House, a non-profit residential and outpatient drug and alcohol rehabilitation organization with 120 programs in 10 states, and Hazelden Treatment Centers, one of the world’s largest and most respected private not for profit alcohol and drug addiction treatment centers, to share the benefits of using the Rx DrugSAFE product with its patients. In addition, we also plan on targeting Kinder Care Learning Centers, a Knowledge Learning Corporation Company and Tutor Time, two of the nation’s leading providers of early childhood learning and school age education and care, which are required to lock up medications by law.
Hospice Leasing Program
In any business, it is generally desirable to have some form of recurring revenue model, where the business can expect to receive payment on products or services on a monthly or other regular basis, such as through a leasing arrangement. Although Rx DrugSAFE products may be difficult to market on a recurring revenue basis, we have identified hospice facilities as a segment of the market that could benefit from leasing as opposed to purchasing Rx DrugSAFE and Rx DrugSAFE PRO units.
Medications used by hospice patients are often stored and dispensed at the point of care in the patient’s hospice room. The family and friends of a hospice patient may be concerned the security of these medications. When family members and friends visit the patient, these visits are often unsupervised and it is easy for an inquisitive teen, other family members or friends with the wrong motivation to steal a patient’s pain medications without the knowledge of the patient, the family or the hospice staff.
| 17 |
To eliminate this concern, we plan to develop a leasing program for hospice facilities. For the family of a hospice patient, it may not make practical sense for them to purchase the Rx DrugSAFE. However, leasing the product from the hospice may make more sense. The hospice facilities we have spoken with believe such a program could be effective and some appear interested in offering the product as part of the services available patients. For each Rx DrugSAFE product leased, the facility would receive a percentage of the monthly lease cost. The benefit for Rx Safes, Inc. is that each unit is easily reprogrammable back to factory default once a patient has passed and the family no longer needs the unit. One single Rx DrugSAFE unit can be used over and over within the same facility and continue generating revenue for us and the hospice.
Shipping
Orders for our products are either placed on line at our website or certain customers will call and place a telephone or fax order which we enter into our online ecommerce store. Presently with minimal inventory, orders are shipped directly by the company from our offices but if a larger order comes in, we plan to ship to our warehousing and distribution partner Exxtra Express and products will be shipped from there. Exxtra Express, is located at 8640 Slauson Avenue, Pico Rivera, CA 90660. Orders are expected to be sent to the warehouse on a daily basis, either by fax, email or my providing access to our online order system.
Government Regulation and Legislative Developments
Food and Drug Administration Registration
Our ultimate goal is to facilitate accessibility to our products for the entire patient population. In order to accomplish this, we will need to obtain Food and Drug Administration (FDA) recognition and approval of the Rx DrugSAFE as a medical device to enable us to offer the product through health plans and have the majority of the acquisition costs reimbursable by Medicaid, Medicare and/or other health insurance providers.
The FDA regulates medical devices through the Center for Devices and Radiological Health (CDRH). The Food, Drug and Cosmetic Act and implementing regulations regulate the certification of medical devices. Medical devices must comply with the standards enforced by the CDRH to pass certification. Once a device has been registered with the FDA, the device will appear on a list of FDA registered devices which is accessible through a searchable database by the general buying public. It is also a requirement of many insurance providers that such devices must be registered with the FDA and comply with applicable regulations and controls in order to be covered under insurance plans.
In 2011, we began the process of establishing our business as a “device establishment” to be entered into the FDA Registration and Device Listing Database. We were assigned an Owner Operator Number 10036866, a Listing Number D124488 and requested a new Product Code for our products so that they could be recognized as medical devices. Due to the lack of capital at the time, we did not complete the registration process, which incidentally has to be renewed on an annual basis. With our newly designed product, it is our intention to once again pursue device registration in order to move to the next step of making our product accessible and reimbursable through Medicaid, Medicare and other insurance providers.
Commonwealth of Massachusetts
On August 9, 2010, Massachusetts Governor Deval Patrick signed a bill into law requiring every pharmacy located in the Commonwealth of Massachusetts to make available lock boxes for sale at each location commencing January 1, 2011. The new law, Chapter 283 of the Acts of 2010, added Safeguards to the Prescription Monitoring Program and to Substance Abuse Education and Prevention.
This new legislation mandates that pharmacies offer for sale, and stock, a secure prescription medication safe in all stores located within the Commonwealth. This is the first such law in the United States and similar legislation may be introduced in the near future. This may be a very significant event for our company and products if other states and local governmental units take the initiative to require pharmacies to carry locking prescription safes and there is compulsory enforcement by the government.
| 18 |
Manufacturing/R&D
We have an established relationship with a contract manufacturer/sourcing company called Good Promotion, LTD., located in Shenzen, China. We have been working with this company for over 4 years from the manufacturing of our original safe product through the redesign and cost reduction effort we completed in 2013. Good Promotion acts as our representative in China and sources materials, components and contracts with a specialist electronics manufacturer for the production of the fingerprint module that is used in our products. Good Promotion then manufactures the body of the safe and assembles the mechanical and electronic components, delivering us a finished, packaged product. Good Promotion also offers custom design and development services and was also responsible for the re-design of the mechanical locking mechanism and other hardware features of our current product.
We have no written agreement with Good Promotions to manufacture our products. Our arrangement with our manufacturer to acquire inventory is strictly through purchase orders.
As referenced above, because the direction and further development of the current technology used in our products is unknown we are presently evaluating other options including a touch sensor from a company called Next Biometrics. Because the fingerprint reader from Next Biometrics requires the user to place their finger on the fingerprint sensor instead of swiping, we believe this will improve the overall user experience. We have written a design document and are presently speaking with a number of familiar engineering resources with specific experience working with fingerprint biometrics, with a view to re-engineer a new fingerprint interface incorporating the Next Biometrics product. This new interface will then be integrated into our existing and future products and will also incorporate new features to address the needs of commercial healthcare customers including audit tracking and reporting capabilities as well as the ability to interface with applications on external smart devices so that data can then be integrated with a healthcare facilities enterprise systems.
While we may look to change the fingerprint module we use in our product as technology advances, purchase a module from a different electronics contract manufacturer or design and develop our own module, it is our intention to continue our relationship with Good Promotion or another Chinese based manufacturer to keep the costs of our electronics down. However, as part of the proposed redesign, we are evaluating the option of bringing the manufacturing of the metal boxes and final assembly of the product to the US. This would allow us to cut down on the cost and the time it takes for us to ship finished product and would provide us with a tighter control over the lead time and quality of our finished product.
The fingerprint sensor that is used in the current fingerprint module in our Rx DrugSAFE product contains intellectual property that is not specifically owned by Rx Safes, Inc, However, each sensor chip comes with an individual license to use the intellectual property from the manufacturer and this license cost is factored into the cost of the sensor. The design of the overall fingerprint module that is produced by our electronics manufacturer, incorporates the fingerprint sensor as a part of the design and it is the overall design of the electronic module and its functionality that is covered by the claims in the patent to which we have a license.
Competitive Position Of Rx Safes, Inc.
The following narrative generally describes our competitors in the market for professional grade security safes that protect medications and other controlled substances/items from those who may abuse such products.
Professional Narcotics/Drug Safes
There are several leading manufacturers of Professional Grade Narcotic Safes/lock boxes, designed for medication storage, offering a large selection of medication lockable boxes, locking drug boxes, divisible lock boxes, refrigerator lock boxes, locking drug boxes, narcotic cabinets, medical bins, organizers, narcotic boxes, digital narcotic boxes, keyed boxes, combination boxes, lockable medicine cabinets, and drug storage cabinets in many different sizes, shapes, and styles.
The safes are priced from $99.00 to over $900 for larger storage cabinets. They are manufactured using a range of materials from 20 gauge steel to polyethylene terephthalate (PETG) plastic. The safes use a variety of locking mechanisms, including single and double key locks, mechanical thumb wheel combination locks and electronic keypads with back-up keys.
| 19 |
Medicine Storage Products
Currently, there are several other products that are available to consumers to deter prescription drug abuse in the home. We believe that these products are much less secure and much less tamper resistant than our products.
Because the products are made from plastic or lightweight aluminum and do not use the advanced security technology found in the Rx DrugSAFE products, they are less expensive to manufacture and have a lower cost than the Rx DrugSAFE product. As such, we believe these products do not provide the same level of safety and security as the Rx DrugSAFE products and in the case of one of the products, are easy to break into without the use of any tools. In addition, these products use pin/combination codes or keys so they are not as convenient as the Rx DrugSAFE and are easy to thwart. Since consumers have so many access codes and numbers to remember, there is a chance they will use a pin that they already use frequently. In our opinion, this is much less secure approach. In addition, it is easy to forget a pin code, which causes people to write such codes down, which opens the device up to potential undetected tampering. Further, the competitor products will not be suitable for use by the elderly, especially Alzheimer patients, stroke victims or those with arthritis who have difficulty remembering numbers and access codes or turning a key. Finally, none of these products have a back-up entry method so that in case of an emergency, life-saving medications may not be easily accessible if a family member has not been told the pin code or combination in advance.
Plastic Storage Containers
Other manufacturers have produced competing products using plastic containers designed to resemble several pill bottles adjoined together. These products have sold at retail for approximately $14.95 - $39.99. The main security feature of these storage containers is an antiquated tri-tumbler locking mechanism which can be easily accessed. In addition to the low level of security afforded by the locking mechanism, many of these product can only store up to 4 standard pill bottles and, most cannot be securely affixed to a surface, and therefore can also easily be stolen. One of the major brands of these types of storage containers was forced to recall its product from the market. Since our product is technologically more advanced and more secure than the plastic containers, we do not believe that the plastic containers will be able to effectively compete with our products.
Employees
We presently employ Lorraine Yarde full-time as our CEO and Director, and Susan Kutzner part-time as our Treasurer and Director.
Risks Related to Our Financial Condition and Our Business
Because we have a limited operating history, you may not be able to accurately evaluate our operations.
We have had limited operations to date and have generated only a small amount of revenue. Therefore, we have a limited operating history upon which to evaluate the merits of investing in our company. Because we are in the early stages of operating our business, we are subject to many of the same risks inherent in the operation of a business with a limited operating history, including the potential inability to continue as a going concern.
Our investors may lose their entire investment because our financial status creates a doubt whether we will continue as a going concern.
Our auditors, in their opinion dated March 14, 2015 have stated that currently we do not have sufficient cash nor do we have a significant source of revenues to cover our operational costs and allow us to continue as a going concern. We seek to raise operating capital to implement our business plan. Our company's plan specifies a minimum amount of $585,000 in additional operating capital to operate for the next twelve months. However, there can be no assurance that we will be successful. You may lose your entire investment.
| 20 |
We are dependent on outside financing for continuation of our operations.
Because we have generated only a small amount of revenue and currently operate at a significant loss, we are completely dependent on the continued availability of financing in order to continue our business. There can be no assurance that financing sufficient to enable us to continue our operations will be available to us in the future. Our failure to obtain future financing or to produce levels of revenue to meet our financial needs could result in our inability to continue as a going concern and, as a result, investors in the Company could lose their entire investment.
Our business will not grow unless the market for prescription and OTC drug safety products expands both domestically and internationally.
Our revenues will be derived from the sale of fingerprint activated drug safety products, predominantly into the consumer market. Drug safety products have not gained widespread commercial acceptance by consumers. We cannot accurately predict the future growth rate, if any, or the ultimate size of the drug safety market. The expansion of the market for our products depends on a number of factors including without limitation:
| ● | national or international events which may affect the need for or interest in drug safety products; | |
| ● | the cost, performance and reliability of our products and those of our competitors; | |
| ● | customers’ perception of the perceived benefit by drug safety products and their satisfaction with the performance and reliablity our products; | |
| ● | public perceptions regarding the confidentiality of private information; | |
| ● | proposed or enacted legislation related to the safe storage of prescription and OTC medication; and | |
| ● | marketing efforts and publicity regarding these products. |
We may face intense competition from other drug safety product providers who offer less expensive solutions to lock up medications
A significant number of established and startup companies are marketing or developing drug safety products that may eventually compete with our current offerings.
The drug safety product market is a rapidly evolving and intensely competitive, and we believe that additional significant long-term competitors will continue to enter the market. We expect competition in the market to increase and intensify in the near term. Companies competing with us may introduce products that are targeted at our target markets and competitively priced, have increased performance or functionality or incorporate technological advances we have not yet developed or implemented. Some present and potential competitors have financial, marketing, research, and manufacturing resources substantially greater than ours. Other players in the drug safety market do have the potential to directly compete with us. Among these companies are Medicus Health, Graham Field, Medsafe and LockMed. The former two companies primarily focus on the professional healthcare market and their products are costly and cumbersome for home consumer use. We believe the latter two companies offer inferior and less effective solutions when compared the Rx DrugSAFE, but at a price less expensive than ours. . This competitive disadvantage we face could mean less market share for our company.
The drug safety product market is a fledgling industry and the growth and success of it is dependent upon the perceived need by families to lock up medications in the home and the subsequent adoption of drug safety products consumers.
The majority of both teens and young adults obtain prescription drugs they abuse from friends and relatives, sometimes without their knowledge. And according to the 2012 Monitoring the Future survey, which is an ongoing study of the behaviors, attitudes and values of American secondary school students, funded by research grants from the National Institute on Drug Abuse, about 50 percent of high school seniors said that opioid drugs other than heroin (e.g., Vicodin) would be fairly or very easy to get. Many teens think these drugs are safe because they have legitimate uses, but taking them without a prescription to get high or self-medicate can be as dangerous – and addictive – as using street narcotics and illicit drugs.
| 21 |
This public health threat requires an all-out effort to raise awareness in the public about proper use, storage, and disposal of these powerful drugs. Retail pharmacies, pharmaceutical companies, as well as the government, have only offered educational programs in the hopes that education will prevent teen prescription drug abuse. It has not. The fact is that more teens take prescription drugs now than they did 5 years ago, deaths from prescription drug overdoses have skyrocketed, yet still, the public and the profession have not provided consumers a real solution. But regardless of these scary statistics and the more widespread publicity on the issue of prescription drug abuse, based on our own independent findings and industry knowledge, it still appears that many US households do not lock up their medications and these drugs are easily accessible. according to a statement by Nora Volkow, Director of the National Institute on Drug Abuse (NIDA), on Scientific Research on Prescription Drug Abuse before the US Senate Judiciary Committee, Subcomittee on Crime and Drugs, Wednesday March 12, 2008, most people don't lock up their prescription medications, nor do they discard them when they are no longer needed for their intended use, making them vulnerable to theft or misuse.
There are many potential customers that do not even know that drug safety products exist. If the public does not grasp or is ignorant of the benefits of our drug safety products, we may not be able to reach out and persuade them to purchase our products.
Because we do not have an exclusive agreement with our third party Chinese manufacturer that will manufacture our products, we may be unable to effectively manufacture and distribute our products or distribute them at all, which would adversely affect our reputation and materially reduce our revenues.
We do not own or operate any manufacturing facilities. We use only one third party manufacturers in China to manufacture our products, Good Promotions, Ltd. If we lose the services of our third party manufacturer, we may be unable to secure the services of replacement manufacturers. In addition, because we do not have a written agreement with our manufacturer, it could refuse to supply some or all of our products, reduce the number of products that it supplies or change the terms and prices under which it normally supplies our products. The occurrence of any such conditions will have a materially negative effect upon our reputation and our ability to distribute our products, which will cause a material reduction in our revenues.
In addition, certain Chinese manufacturers and the products they export have recently been the source of safety concerns and recalls, which is generally attributed to lax regulatory, quality control and safety standards. Should Chinese manufacturers continue to draw public criticism for exporting unsafe products, whether those products relate to our products or not we may be adversely affected by the stigma associated with Chinese production, which could have a material adverse effect on our business, results of operations and financial condition.
The occurrence of any such conditions will have a materially negative effect upon our reputation and our ability to distribute our products, which will cause a material reduction in our revenues.
We may be forced to re-design the core technology for our product line if Authentec (whose technology is now sold and manufactured under license by Digital Persona) stops supplying us with Sensors.
Our products currently incorporate a fingerprint sensor, that could possible become EOL (end of life) which means that the manufacturer will no longer make or support the product. This would cause us to have to redesign our core technology and would mean that we may not have product to sell. The redesign could be costly and time consuming and may allow potential competitors to take opportunities in the market place. Moreover, if we are unable to redesign our core technology, we could go out of business.
Our financial and operating results often vary significantly from quarter to quarter and may be negatively affected by a number of factors.
Our financial and operating results may fluctuate from quarter to quarter because of the following reasons:
| ● | reduced demand for products and services caused, for example, by product offerings from new competitors; |
| 22 |
| ● | the inability to timely and successfully (i) complete development of designs, components and products, (ii) complete new product introductions that may result in improved gross margins, or (iii) manufacture in volume; | |
| ● | changes in the mix of products and services we or our distributors sell; | |
| ● | the readiness of customers to accept delivery of new products on a timely basis; | |
| ● | unforeseen legal expenses, including litigation costs; | |
| ● | expenses related to acquisitions or mergers; | |
| ● | impairment charges arising out of our assessments of goodwill and intangibles; | |
| ● | other one-time financial charges; | |
| ● | the lack of availability or increase in cost of key components and subassemblies; | |
| ● | competitive pricing pressures; and | |
| ● | unpredictable product production schedules. |
Particularly important is the need to invest in planned technical development programs to maintain and enhance our competitiveness, and to successfully develop and launch new products and services on a timely basis. Managing and improving the likelihood of success of such programs requires the development of budgets, plans and schedules for the execution of these programs and the adherence to such budgets, plans and schedules. The majority of such program costs are payroll and related staff expenses, and secondarily materials, subcontractors and promotional expenses. These costs are very difficult to adjust in response to short-term fluctuations in our revenues, compounding the difficulty of achieving profitability in the event of a revenue downturn.
Our lengthy and variable sales cycle will make it difficult to predict operating results.
We have had limited sales to date. Nevertheless, we anticipate certain of our products will have a lengthy sales cycle. Although our target is consumers, we plan to access consumers through various distribution channels and not direct to the consumer, and it is the distributors that take the time to evaluate the products to determine if they fit into, or complement their existing product offerings. In addition, in our efforts to become approved as a medical device, this will require a great deal of time and effort getting insurance companies to pick up the product and then following that, introducing the product to the doctors, hospitals and other healthcare providers and facilities to encourage them to write prescriptions for the product.
If, after expending significant funds and effort, we fail to receive an order, a negative impact on our financial results and stock price could result. It is difficult to predict accurately the sales cycle of any order for any of our products. If we do not ship one or more orders as forecast for a fiscal quarter, our total revenues and operating results for that quarter could be materially and adversely affected.
The substantial lead-time required for ordering parts and materials may lead to inventory problems.
The lead-time for ordering parts and materials and building many of our products can be many months. As a result, we must order parts and materials and build our products based on forecasted demand. If demand for our products lags significantly behind our forecasts, we may produce more products than we can sell, which can result in cash flow problems and write-offs or write-downs of obsolete inventory.
We will rely in part upon sales reps, retailers and distribution partners to distribute our products, and we may be adversely affected if those parties do not actively promote our products or pursue customers who would have a potential demand for our products.
We estimate that a significant portion of our revenue will come from sales to partners including reps, retailers, distributors and resellers. Some of these relationships have not been formalized in a detailed contract, and may be subject to termination at any time. Even where these relationships are formalized in a detailed contract, the agreements are often terminable with little or no notice and subject to periodic amendment. We cannot control the amount and timing of resources that our partners devote to activities on our behalf.
We intend to continue to seek strategic relationships to distribute, license and sell certain of our products. We, however, may not be able to negotiate acceptable relationships in the future and cannot predict whether current or future relationships will be successful.
| 23 |
The loss of sole or limited source suppliers may result in delays or additional expenses.
We obtain certain hardware components and complete products, as well as software applications, from a single source or a limited group of suppliers. We do not have long-term agreements with any of our suppliers. We will experience significant delays in manufacturing and shipping of products to customers if we lose these sources or if supplies from these sources are delayed.
As a result, we may be required to incur additional development, manufacturing and other costs to establish alternative sources of supply. It may take several months to locate alternative suppliers, if required, or to re-tool our products to accommodate components from different suppliers. We cannot predict if we will be able to obtain replacement components within the time frames we require at an affordable cost, or at all. Any delays resulting from suppliers failing to deliver components or products on a timely basis in sufficient quantities and of sufficient quality or any significant increase in the price of components from existing or alternative suppliers could have a severe negative impact on our financial results and stock price.
We may be subject to loss in market share and market acceptance as a result of performance failures, manufacturing errors, delays or shortages.
There is a risk that for unforeseen reasons we may be required to repair or replace a substantial number of products in use or to reimburse customers for products that fail to work or meet strict performance criteria. To date, we have experienced some product failures related to customer use of the fingerprint technology. It took some customers some time to get used to how the fingerprint technology worked, and customer service was needed to correct this problem. Also, prior to our redesign there was a material deficiency with the mechanical design and construction of the metal case that would allow a possible breach of the safe. We have taken steps to limit remedies for product failure to the repair or replacement of malfunctioning or noncompliant products or services, and also attempt to exclude or minimize exposure to product and related liabilities by including in our standard agreements warranty disclaimers and disclaimers for consequential and related damages as well as limitations on our aggregate liability. From time to time, in certain sales transactions, we may negotiate liability provisions that vary from such standard forms. There is a risk that our contractual provisions may not adequately minimize our product and related liabilities or that such provisions may be unenforceable. We intend to carry product liability insurance, but coverage we secure may not be adequate to cover potential claims. Moreover, to the extent we have to repair, reimburse or expend funds to cover customer service issues, our results of operations will be negatively affected.
We may be subject to repair, replacement, reimbursement and liability claims as a result of products that fail to work or to meet applicable performance criteria.
There is a risk that for unforeseen reasons we may be required to repair or replace a substantial number of products in use or to reimburse customers for products that fail to work or meet strict performance criteria. We attempt to limit remedies for product failure to the repair or replacement of malfunctioning or noncompliant products or services, and also attempt to exclude or minimize exposure to product and related liabilities by including in our standard agreements warranty disclaimers and disclaimers for consequential and related damages as well as limitations on our aggregate liability. From time to time, in certain complex sale or licensing transactions, we may negotiate liability provisions that vary from such standard forms. There is a risk that our contractual provisions may not adequately minimize our product and related liabilities or that such provisions may be unenforceable. We intend to carry product liability insurance, but coverage we secure may not be adequate to cover potential claims.
Failure by us to maintain the proprietary nature of our technology, intellectual property and manufacturing processes could have a material adverse effect on our business, operating results, financial condition, stock price, and on our ability to compete effectively.
We principally rely upon patent, trademark, copyright, trade secret and contract law to establish and protect our proprietary rights. There is a risk that claims allowed on any patent licenses or trademarks we hold may not be broad enough to protect our technology. In addition, our patent licenses or trademarks may be challenged, invalidated or circumvented and we cannot be certain that the rights granted thereunder will provide competitive advantages to us. Moreover, any current or future issued or licensed patents, or trademarks, or currently existing or future developed trade secrets or know-how may not afford sufficient protection against competitors with similar technologies or processes, and the possibility exists that certain of our already issued patents or trademarks may infringe upon third party patents or trademarks or be designed around by others. In addition, there is a risk that others may independently develop proprietary technologies and processes, which are the same as, substantially equivalent or superior to ours, or become available in the market at a lower price.
| 24 |
In addition, foreign laws treat the protection of proprietary rights differently from laws in the United States and may not protect our proprietary rights to the same extent as U.S. laws. The failure of foreign laws or judicial systems to adequately protect our proprietary rights or intellectual property, including intellectual property developed on our behalf by foreign contractors or subcontractors may have a material adverse effect on our business, operations, financial results and stock price.
There is a risk that we have infringed or in the future will infringe patents or trademarks owned by others, that we will need to acquire licenses under patents or trademarks belonging to others for technology potentially useful or necessary to us, and that licenses will not be available to us on acceptable terms, if at all.
We may have to litigate to enforce our patents or trademarks or to determine the scope and validity of other parties’ proprietary rights. Litigation could be very costly and divert management’s attention. An adverse outcome in any litigation may have a severe negative effect on our financial results and stock price. To determine the priority of inventions, we may have to participate in interference proceedings declared by the United States Patent and Trademark Office or oppositions in foreign patent and trademark offices, which could result in substantial cost and limitations on the scope or validity of our patents or trademarks.
We also rely on trade secrets and proprietary know-how, which we seek to protect by confidentiality agreements with our employees, consultants, service providers and third parties. There is a risk that these agreements may be breached, and that the remedies available to us may not be adequate. In addition, our trade secrets and proprietary know-how may otherwise become known to or be independently discovered by others.
Compliance with changing regulation of corporate governance and public disclosure may result in additional expenses.
Changing laws, regulations and standards relating to corporate governance and public disclosure, including the Sarbanes-Oxley Act of 2002 and new SEC regulations, are creating uncertainty for companies such as ours. These new or changed laws, regulations and standards are subject to varying interpretations in many cases due to their lack of specificity, and as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies, which could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices. We are committed to maintaining high standards of corporate governance and public disclosure. As a result, we intend to invest resources to comply with evolving laws, regulations and standards, and this investment may result in increased general and administrative expenses and a diversion of management time and attention from revenue-generating activities to compliance activities. If our efforts to comply with new or changed laws, regulations and standards differ from the activities intended by regulatory or governing bodies due to ambiguities related to practice, our reputation may be harmed.
If we fail to adequately manage the size of our business, it could have a severe negative effect on our financial results or stock price.
Our management believes that in order to be successful we must appropriately manage the size of our business. This may mean reducing costs and overhead in certain economic periods, and selectively growing in periods of economic expansion. In addition, we will be required to implement operational, financial and management information procedures and controls that are efficient and appropriate for the size and scope of our operations. The management skills and systems currently in place may not be adequate and we may not be able to manage any significant cost reductions or effectively provide for our growth.
| 25 |
If we fail to attract and retain qualified senior executive and key technical personnel, our business will not be able to expand.
We are dependent on the continued availability of the services of our employees, many of whom are individually key to our future success, and the availability of new employees to implement our business plans. We rely heavily on the marketing experience of Lorraine Yarde to move our product into distribution for sales. As we move forward we will need to engage professionals with various specific experience. We will need engineers with specific knowledge in designing and developing biometric products and there are limited resources available that have this specific experience. We will also need to hire professionals who understand the process of selling product through CMS (Centers for Medicare and Medicaid Services) as well as people familiar with working with medical devices as it relates to reimbursement by private insurance companies. We will also need to identify sales and marketing professionals with specific experience in selling into certain channels of distribution such as mass merchant retail, hospitals, doctors and healthcare facilities.
The market for skilled employees is highly competitive, especially for employees in technical fields. There can be no assurance that we will be able to retain the services of all our key employees or a sufficient number to execute our plans, nor can there be any assurance we will be able to continue to attract new employees as required.
Our personnel may voluntarily terminate their relationship with us at any time, and competition for qualified personnel, especially engineers, is intense. The process of locating additional personnel with the combination of skills and attributes required to carry out our strategy could be lengthy, costly and disruptive.
If we lose the services of key personnel, or fail to replace the services of key personnel who depart, we could experience a severe negative effect on our financial results and stock price. In addition, there is intense competition for highly qualified engineering and marketing personnel in the locations where we principally operate. The failure to acquire the services of any key engineering, marketing or other personnel or our failure to attract, integrate, motivate and retain additional key employees could have a material adverse effect on our business, operating and financial results and stock price.
If we are unable to compensate Ms. Yarde under her employment agreement, she may not be able or willing to devote a sufficient amount of time to our business operations, causing our business to fail.
We have an employment agreement with Ms. Yarde to compensate her, among other things, a salary of $175,000 per year. If we are unable to pay her, she may be unwilling to devote time to our business, which could cause us to fail.
If we fail to comply with the new rules under the Sarbanes-Oxley Act related to accounting controls and procedures, or if material weaknesses or other deficiencies are discovered in our internal accounting procedures, our stock price could decline significantly.
Section 404 of the Sarbanes-Oxley Act requires annual management assessments of the effectiveness of our internal controls over financial reporting and a report by our independent auditors addressing these assessments. We are in the process of documenting and testing our internal control procedures, and we may identify material weaknesses in our internal control over financial reporting and other deficiencies. If material weaknesses and deficiencies are detected, it could cause investors to lose confidence in our Company and result in a decline in our stock price and consequently affect our financial condition. In addition, if we fail to achieve and maintain the adequacy of our internal controls, we may not be able to ensure that we can conclude on an ongoing basis that we have effective internal controls over financial reporting in accordance with Section 404 of the Sarbanes-Oxley Act. Moreover, effective internal controls, particularly those related to revenue recognition, are necessary for us to produce reliable financial reports and are important to helping prevent financial fraud. If we cannot provide reliable financial reports or prevent fraud, our business and operating results could be harmed, investors could lose confidence in our reported financial information, and the trading price of our Common Stock could drop significantly. In addition, we cannot be certain that additional material weaknesses or significant deficiencies in our internal controls will not be discovered in the future.
| 26 |
Insiders will continue to have substantial control over us and will be able to influence corporate matters.
Our directors and officers, whose interests may differ from other stockholders, have the ability to exercise significant control over us. Presently, these individuals beneficially own approximately 33% of our common stock. These stockholders are able to exercise significant influence over all matters requiring approval by our stockholders, including the election of directors, the approval of significant corporate transactions, and any change of control of our company. These individuals could prevent transactions, which would be in the best interests of the other shareholders. The interests of our officers and directors may not necessarily be in the best interests of the shareholders in general.
Risks Related to Our Securities
If a market for our common stock does not develop, shareholders may be unable to sell their shares.
Our common stock is quoted under the symbol “RXSF” on the OTCPink operated by OTC Markets Group, Inc, an electronic inter-dealer quotation medium for equity securities. We do not currently have an active trading market. There can be no assurance that an active and liquid trading market will develop or, if developed, that it will be sustained.
Our securities are very thinly traded. Accordingly, it may be difficult to sell shares of our common stock without significantly depressing the value of the stock. Unless we are successful in developing continued investor interest in our stock, sales of our stock could continue to result in major fluctuations in the price of the stock.
Our common stock price may be volatile and could fluctuate widely in price, which could result in substantial losses for investors.
The market price of our common stock is likely to be highly volatile and could fluctuate widely in price in response to various factors, many of which are beyond our control, including:
| ● | technological innovations or new products and services by us or our competitors; |
| ● | government regulation of our products and services; |
| ● | the establishment of partnerships with other technology companies; |
| ● | intellectual property disputes; |
| ● | additions or departures of key personnel; |
| ● | sales of our common stock |
| ● | our ability to integrate operations, technology, products and services; |
| ● | our ability to execute our business plan; |
| ● | operating results below expectations; |
| ● | loss of any strategic relationship; |
| ● | industry developments; |
| ● | economic and other external factors; and |
| ● | period-to-period fluctuations in our financial results. |
Because we are a development stage company with nominal revenues to date, you should consider any one of these factors to be material. Our stock price may fluctuate widely as a result of any of the above.
In addition, the securities markets have from time to time experienced significant price and volume fluctuations that are unrelated to the operating performance of particular companies. These market fluctuations may also materially and adversely affect the market price of our common stock.
We have not paid cash dividends in the past and do not expect to pay cash dividends in the future on our common stock. Any return on investment may be limited to the value of our common stock.
We have never paid cash dividends on our common stock and do not anticipate paying cash dividends in the foreseeable future. The payment of cash dividends on our common stock will depend on earnings, financial condition and other business and economic factors at such time as the board of directors may consider relevant. If we do not pay cash dividends, our common stock may be less valuable because a return on your investment will only occur if its stock price appreciates.
| 27 |
In the event that we are unable to sell sufficient shares of common stock we may need additional capital in the future, which may not be available to us on favorable terms, or at all, and the raising of additional capital at a later time may dilute your ownership of our common stock.
We expect that $2,460,000, will be sufficient to meet our expected needs for working capital and capital expenditures to fully implement and carry out our business plan. If we need to raise capital in the future, through private or public offerings, depending on the terms of the offerings, such sales of additional shares may dilute the holdings of investors who purchase our shares. We cannot be certain that additional financing through private placements of our stock or borrowing will be available to us when required, on favorable terms, or at all. Our inability to obtain adequate capital or financing may limit our ability to achieve the level of corporate growth that we believe to be necessary to succeed in our business or may require us to cease business operations entirely.
We must raise at $585,000 to pursue our business plan on a limited basis.
We have no alternative plan of operation if we are unable to raise $585,000. We plan to remain in business through our website sales for as long as possible. Those investing in our common stock are taking substantial risk in that they may lose their entire investment if we do not raise $585,000
As a new investor, you will experience substantial dilution as a result of future equity issuances.
In the event we are required to raise additional capital it may do so by selling additional shares of common stock thereby dilutin the shares and ownership interests of existing shareholders.
Because we are subject to the “Penny Stock” rules, the level of trading activity in our stock may be reduced.
The Securities and Exchange Commission has adopted regulations which generally define "penny stock" to be any listed, trading equity security that has a market price less than $5.00 per share or an exercise price of less than $5.00 per share, subject to certain exemptions. The penny stock rules require a broker-dealer, prior to a transaction in a penny stock not otherwise exempt from the rules, to deliver a standardized risk disclosure document that provides information about penny stocks and the risks in the penny stock market. The broker-dealer must also provide the customer with current bid and offer quotations for the penny stock, the compensation of the broker-dealer and its salesperson in the transaction, and monthly account statements showing the market value of each penny stock held in the customer’s account. In addition, the penny stock rules generally require that prior to a transaction in a penny stock, the broker-dealer make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written agreement to the transaction. These disclosure requirements may have the effect of reducing the level of trading activity in the secondary market for a stock that becomes subject to the penny stock rules which may increase the difficulty Purchasers may experience in attempting to liquidate such securities.
Provisions in the Nevada Revised Statutes and our Bylaws could make it very difficult for an investor to bring any legal actions against our directors or officers for violations of their fiduciary duties or could require us to pay any amounts incurred by our directors or officers in any such actions.
Members of our board of directors and our officers will have no liability for breaches of their fiduciary duty of care as a director or officer, except in limited circumstances, pursuant to provisions in the Nevada Revised Statutes and our Bylaws as authorized by the Nevada Revised Statutes. Specifically, Section 78.138 of the Nevada Revised Statutes provides that a director or officer is not individually liable to the company or its shareholders or creditors for any damages as a result of any act or failure to act in his or her capacity as a director or officer unless it is proven that (1) the director’s or officer’s act or failure to act constituted a breach of his or her fiduciary duties as a director or officer and (2) his or her breach of those duties involved intentional misconduct, fraud or a knowing violation of law. This provision is intended to afford directors and officers protection against and to limit their potential liability for monetary damages resulting from suits alleging a breach of the duty of care by a director or officer. Accordingly, you may be unable to prevail in a legal action against our directors or officers even if they have breached their fiduciary duty of care. In addition, our Bylaws allow us to indemnify our directors and officers from and against any and all costs, charges and expenses resulting from their acting in such capacities with us. This means that if you were able to enforce an action against our directors or officers, in all likelihood, we would be required to pay any expenses they incurred in defending the lawsuit and any judgment or settlement they otherwise would be required to pay. Accordingly, our indemnification obligations could divert needed financial resources and may adversely affect our business, financial condition, results of operations and cash flows, and adversely affect prevailing market prices for our common stock.
| 28 |
Currently, we do not own any real estate. Our principal executive offices are located at 170 Green Valley Parkway, Suite 300 Henderson, NV 89012. We have entered into a one year lease for the facility, with a cost of approximately $450 per month. We believe that our properties are adequate for our current needs, but growth potential may require larger facilities due to anticipated addition of personnel. We do not have any policies regarding investments in real estate, securities or other forms of property.
Aside from that below, we are not a party to any pending legal proceeding. We are not aware of any pending legal proceeding to which any of our officers, directors, or any beneficial holders of 5% or more of our voting securities are adverse to us or have a material interest adverse to us.
On March 10, 2015, we filed a complaint in the District Court for Clark County, Nevada, against Wall Street Buy Sell Hold Inc., a New York corporation, for Fraud in the Inducement, Deceptive Trade Practices and Unjust Enrichment in connection with a Consulting Agreement dated February 10, 2014. As a result of the misrepresentations and actions perpetuated by Wall Street Buy Sell Hold Inc., we are asking court to award us damages, recession of the Consulting Agreement and a return of monies paid thereunder, and for punitive damages.
Item 4. Mine Safety Disclosures
Not applicable.
Item 5. Market for Registrant’s Common Equity and Related Stockholder Matters and Issuer Purchases of Equity Securities
Market Information
We recently acquired our trading symbol, “RXFS,” on December 18, 2014. Only a limited market exists for our securities. There is no assurance that a regular trading market will develop, or if developed, that it will be sustained. Therefore, a shareholder may be unable to resell his securities in our company.
On February 18, 2015, the last sales price per share of our common stock was $0.29.
Penny Stock
The SEC has adopted rules that regulate broker-dealer practices in connection with transactions in penny stocks. Penny stocks are generally equity securities with a market price of less than $5.00, other than securities registered on certain national securities exchanges or quoted on the NASDAQ system, provided that current price and volume information with respect to transactions in such securities is provided by the exchange or system. The penny stock rules require a broker-dealer, prior to a transaction in a penny stock, to deliver a standardized risk disclosure document prepared by the SEC, that: (a) contains a description of the nature and level of risk in the market for penny stocks in both public offerings and secondary trading; (b) contains a description of the broker's or dealer's duties to the customer and of the rights and remedies available to the customer with respect to a violation of such duties or other requirements of the securities laws; (c) contains a brief, clear, narrative description of a dealer market, including bid and ask prices for penny stocks and the significance of the spread between the bid and ask price; (d) contains a toll-free telephone number for inquiries on disciplinary actions; (e) defines significant terms in the disclosure document or in the conduct of trading in penny stocks; and (f) contains such other information and is in such form, including language, type size and format, as the SEC shall require by rule or regulation.
| 29 |
The broker-dealer also must provide, prior to effecting any transaction in a penny stock, the customer with (a) bid and offer quotations for the penny stock; (b) the compensation of the broker-dealer and its salesperson in the transaction; (c) the number of shares to which such bid and ask prices apply, or other comparable information relating to the depth and liquidity of the market for such stock; and (d) a monthly account statement showing the market value of each penny stock held in the customer's account.
In addition, the penny stock rules require that prior to a transaction in a penny stock not otherwise exempt from those rules, the broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser's written acknowledgment of the receipt of a risk disclosure statement, a written agreement as to transactions involving penny stocks, and a signed and dated copy of a written suitability statement.
These disclosure requirements may have the effect of reducing the trading activity for our common stock. Therefore, stockholders may have difficulty selling our securities.
Holders of Our Common Stock
As of February 24, 2015, we had 115,591,472 shares of our common stock issued and outstanding, held by 79 shareholders of record.
Dividends
We currently intend to retain future earnings for the operation of our business. We have never declared or paid cash dividends on our common stock, and we do not anticipate paying any cash dividends in the foreseeable future.
In the event that a dividend is declared, common stockholders on the record date are entitled to share ratably in any dividends that may be declared from time to time on the common stock by our board of directors from funds legally available.
There are no restrictions in our articles of incorporation or bylaws that restrict us from declaring dividends. The Nevada Revised Statutes, however, do prohibit us from declaring dividends where, after giving effect to the distribution of the dividend:
| 1. | We would not be able to pay our debts as they become due in the usual course of business; or |
| 2. | Our total assets would be less than the sum of our total liabilities, plus the amount that would be needed to satisfy the rights of shareholders who have preferential rights superior to those receiving the distribution. |
Securities Authorized for Issuance under Equity Compensation Plans
On January 5, 2015, our board of directors and majority shareholders approved the adoption of the RX Safes, Inc. 2015 Equity Incentive Plan (the “2015 Equity Incentive Plan”).
The purpose of the 2015 Equity Incentive Plan is to foster and promote our long-term financial success and increase stockholder value by motivating performance through incentive compensation. The 2015 Equity Incentive Plan is intended to encourage participants to acquire and maintain ownership interests in our company and to attract and retain the services of talented individuals upon whose judgment and special efforts the successful conduct of our business is largely dependent.
The 2015 Equity Incentive Plan became effective upon its approval by the majority of stockholders on January 1, 2015 and, unless earlier terminated, will continue until January 1, 2025. A total of 25,000,000 shares of common stock may be issued under the 2015 Equity Incentive Plan.
| 30 |
The 2015 Equity Incentive Plan provides for the granting of incentive stock options, non-qualified stock options, stock appreciation rights, restricted stock, stock units, performance shares and performance units to our employees, officers, directors and consultants, including incentive stock options, non-qualified stock options, restricted stock, and other benefits.
Equity Compensation Plans as of December 31, 2014
| A | B | C | ||||||||||
| Plan Category | Number of securities to be issued upon exercise of outstanding options, warrants and rights | Weighted-average exercise price of outstanding options, warrants and right | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (A)) | |||||||||
| Equity compensation plans approved by security holders | 0 | 0 | 0 | |||||||||
| Equity compensation plans not approved by security holders | 8,250,000 | 0.005 | 8,250,000 | |||||||||
| Total | 8,250,000 | 0.005 | 8,250,000 | |||||||||
Recent Sales of Unregistered Securities
On January 1, 2015, we entered into an employment agreement with Lorraine Yarde and granted her an option under our 2015 Equity Incentive Plan to purchase 10,000,000 shares of our common stock, as follows:
| i. | 5,000,000 shares vested immediately with a strike price of $0.05 per share; |
| ii. | 2,500,000 shares vested on the six month anniversary of January 1, 2015 if Ms. Yarde is still working for us with a strike price of $0.05 per share. |
| iii. | 1,5000,000 shares vested on the first anniversary of January 1, 2015 if Ms. Yarde is still working for us with a strike price of $0.10 per share. |
| iv. | 1,000,000 shares vested on the second anniversary of January 1, 2015 if Ms. Yarde is still working for us with a strike price of $0.25 per share. |
On February 12, 2015, we executed a consulting agreement with Sean McManus, and agreed to issue him 100,000 shares of common stock.
For the year ending 2014, we issued 296,400 shares of common stock to 27 investors. The proceeds from the issuance totaled $74,100 and was used for working capital.
Since inception, we have issued 10,899,732 share of common stock to 44 investors, proceeds of stock sale total $672,600.
Additionally, we have issued 24,091,740 shares of common stock for services and 80,500,000 shares of common stock to our founders.
| 31 |
These securities were issued pursuant to Section 4(2) of the Securities Act and/or Rule 506 promulgated thereunder. The holders represented their intention to acquire the securities for investment only and not with a view towards distribution. The investors were given adequate information about us to make an informed investment decision. We did not engage in any general solicitation or advertising. We directed our transfer agent to issue the stock certificates with the appropriate restrictive legend affixed to the restricted stock.
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
Forward-Looking Statements
Certain statements, other than purely historical information, including estimates, projections, statements relating to our business plans, objectives, and expected operating results, and the assumptions upon which those statements are based, are “forward-looking statements.” These forward-looking statements generally are identified by the words “believes,” “project,” “expects,” “anticipates,” “estimates,” “intends,” “strategy,” “plan,” “may,” “will,” “would,” “will be,” “will continue,” “will likely result,” and similar expressions. Forward-looking statements are based on current expectations and assumptions that are subject to risks and uncertainties which may cause actual results to differ materially from the forward-looking statements. Our ability to predict results or the actual effect of future plans or strategies is inherently uncertain. Factors which could have a material adverse affect on our operations and future prospects on a consolidated basis include, but are not limited to: changes in economic conditions, legislative/regulatory changes, availability of capital, interest rates, competition, and generally accepted accounting principles. These risks and uncertainties should also be considered in evaluating forward-looking statements and undue reliance should not be placed on such statements.
Overview
We design, develop, engineer and market fingerprint medical security storage solutions for consumers and healthcare professionals. Our CEO and director, Ms. Yarde, has years of experience designing, developing and marketing consumer based security fingerprint products including garage door openers, front door locks, thermostats and mail boxes through multiple distribution channels, in prior companies. Founded in 2010, we are a pioneer in a new targeted sector in Prescription (Rx) and over-the-counter (OTC) drug security, providing real solutions to address Rx and OTC drug abuse. The Rx DrugSAFE™ product line of medical safes and prescription drug products offers consumers security products specifically designed to safely and securely store medications in the home. These secure storage products also offer healthcare professionals a secure and convenient means by which to control access to medications, addressing both loss prevention and safety concerns within a variety of healthcare settings. Our Rx DrugSAFE™ products are easy to set up and operate, convenient and competitively priced.
We have spent over a year redesigning the core electronics technology and mechanical locking mechanisms of our products in order to provide further security and convenience for our customers. We have taken our experience in safe and security product design and fingerprint technology and our understanding of consumer’s wants and needs and developed a product line that we believe can help save thousands of lives.
In the next 12 months, we intend to continue with our research and product development, implement on several areas of our marketing and distribution plan, purchase inventory of our existing product and new product designs and use working capital to hire sales and support personnel, secure necessary business insurances and continue to establish business relationships to generate awareness, increase sales and comply with all necessary reporting requirements.
We will need to raise capital to meet our objectives. We had a registered offering to sell up to $2,460,000 in net proceeds. The offering is now closed. We were only able to raise $72,200 in that offering, but we are continuing our fundraising efforts, which have been somewhat hampered in 2014 because we did not have a symbol and were not yet trading. On December 18, 2014, we were finally able to complete the process with FINRA and we are now trading under the symbol, RXSF. We hope that our trading status will bring more financing opportunities to the table for our company.
| 32 |
On February 25, 2015 we entered into a securities purchase agreement with Coventry Enterprises, LLC (“Coventry”) whereby Coventry agreed to invest up to $100,000 in exchange for our issuance of two convertible promissory, each in the original principal amount of $50,000.00. The Notes bear interest at a rate of 8% per annum. All outstanding principal and accrued interest under the Notes is due and payable on February 25, 2016. In addition, on the same date we entered into an Equity Purchase Agreement with Coventry, whereby Coventry agreed to purchase up to $10,000,000 of our common stock, to be registered in a Form S-1 registration statement. The agreement will have a three-year term unless sooner terminated because $10,000,000 of our common stock has already been sold to Coventry. The registration of the shares is not mandatory and we may choose to look to other forms of financing to support the ongoing efforts of the business.
Despite these recent transactions, we can provide no assurance that we will be able to continue to raise money under terms that are beneficial to us and our shareholders.
On August 26, 2014, we received a Purchase Order from the Oklahoma Division of Narcotics and Dangerous Drug Control, following ourbeing named as the winning bidder of an Invitation to Bid we submitted on August 11, 2014. The Purchase Order was for 500 units of the current Rx DrugSAFE personal Fingerprint Prescription Drug Lock Box and is being funded by a Grant 2012-PMBX-0012 awarded by the Bureau of Justice Assistance, which is a Component of the United States Department of Justice. This order was successfully shipped to the customer on January 29, 2015 and we generated a receivable in the amount of $49,500. As a follow on from the shipment of this order we have begun reaching out to other State’s corresponding Departments, to discuss a similar program, where they would offer the Rx DrugSAFE product to at risk families within each State. We are directing them to the Bureau of Justice Assistance Grant as a possible means of funding such programs.
If we are able to raise enough capital, we plan to use $246,000 for Research and Development and will use this capital to develop additional products to add to our product line. To date, we have funded our Research and Development through the sale of unregistered securities. The majority of the product development work was conducted under our agreement with Axius Consulting Group, Inc. whose principals have extensive experience in designing and developing consumer biometrics products. Based on our needs which were identified from feedback we received from customers as well as from a security specialist who evaluated our product, Axius worked with our sourcing company in China, providing them with the specifications for the product changes that were needed and then assisting them in conjunction with Rx Safes, Inc. to facilitate the prototyping and testing of these various changes to the product. Although we can continue to use this newly designed core technology, if we are able to raise sufficient capital we will further re-design our core electronic fingerprint and mechanical locking systems to incorporate the latest improvements in technology and cost reduction. As part of our product roadmap, we presently have three additional products at various stages of design. Firstly, we have a hand manufactured model of a larger version of the Rx DrugSAFE, the Rx DrugSAFE Pro, which can still be used in the home but is primarily designed to accommodate larger and a greater number of prescription pill bottles to support the needs of a variety of healthcare environments including assisted living facilities, nursing homes and emergency service vehicles. In order to commercialize these products, there are various steps we must take. The second product is the Rx DrugSAFE IC, which is designed to be securely mounted inside of an existing medicine cabinet. Finally, our third product is a proof of concept of the proposed Mobile Medical Station (MMS). The MMS is a waterproof, shockproof and secure rolling case with telescopic handle that is secured with our patented fingerprint technology. The case incorporates state of the art vital statistics monitoring equipment, a rugged field tablet containing patient H&P data which can be securely uploaded to the primary healthcare enterprise system and one of our Rx DrugSAFE fingerprint lockboxes for the safe and secure transportation of medications.
| 33 |
The fingerprint technology we plan on using in the Rx Drug SAFE Pro is identical to that of our current product. However, we will need to create CAD drawings for the assembly and then manufacture tooling for all the parts that make up the metal box. Once commenced, we anticipate that this will take approximately 2 months. We will then need to manufacture several samples of these parts, known as “first shots” from the tooling to test the integrity of the tools. This sampling is expected to take one month. Once we have verified that the tools are sufficient, we will then need to hand-assemble and test approximately 20 samples of the Rx DrugSAFE PRO with the new electronics, mechanical lock and other new design features that were incorporated into the new Rx DrugSAFE. At this point we will need to design in stronger mechanical locking components to support the larger size and greater weight of the Rx DrugSAFE Pro. This step is anticipated to take an additional 2 months. We have the option of working with our existing manufacturing and development partner in China, Good Promotions, to source the necessary components, make the mold and prototype the Rx DrugSAFE Pro so that we can test the product prior to mass manufacturing. Alternatively, if we determine that it makes the most sense and is financially viable, we may decide to identify a metal fabricator in the US to make the molds and prototypes and subsequent production units. This is an important business decision as once the molds have been built we will be somewhat committed to work with the manufacturer who has the molds as they are almost impossible to ship. Once we have paid for the production of the molds, if we wanted to change direction and use a different manufacturer to make the product, we would likely have to pay for new molds to be made.
Regardless of which manufacturing route we decide to take, once these samples are properly tested we will then be able to place an order for production. If we are successful in raising capital by the end of March 2015, we anticipate that this will occur at the end of May 2015 so we will have units of the Rx DrugSAFE PRO available for resale in September 2015. Many of the healthcare facilities we have spoken with to date have requested additional features be available with our products to make them more applicable for their use. As part of the development of the Rx DrugSAFE PRO, we will also evaluate the cost of adding audit tracking capabilities to the safe, which will require additional flash memory, newly developed software and a time clock be added to the Printed Circuit Board. We may consider engaging the services of a US based engineering partner to perform this evaluation and deliver a report on the feasibility and cost differential for adding these additional features. This evaluation is anticipated to take 2 months and will be designed into the electronics over time.
All of the steps needed for the development of the Rx DrugSAFE PRO will be taken with the Rx DrugSAFE IC. This product is an insert designed to be installed inside of most standard medicine cabinets to allow seamless introduction of our secure technology in a place most households are familiar with keeping their medications. Because we do not intend to change the fingerprint module we use in the IC, we will simply focus on the industrial and mechanical design for this product and will work with our manufacturing and development partner, Good Promotions, to complete these designs and create the mold. If we are successful in raising capital by the end of March 2015, we anticipate we will begin the process of developing this product in May 2015 and that the CAD drawing and tooling for this will be completed at the end of June. Sampling of the parts will take place in July 2015 and then testing of the finished sample units will be conducted in August and September 2015. The IC will need to be tested in various standard medicine cabinets offered for sale in the US prior to launching in the market. We should be able to place or first order for this product for availability at the end of 2015. As with the Pro, we have already received a number of enquiries for the IC version of our product.
If we are able to raise $1,835,000 by the end of March 2015 we will be able to commence production design of the Mobile Medical Station. It is our intention to develop strategic relationships with OEM suppliers for each of the required devices including the case and offer a standard MMS along with customizable options, depending upon the specific needs of the healthcare provider group. We will work with the case manufacturer to incorporate our fingerprint interface into the case. This process may require some additional tooling of parts to incorporate the technology but this will not be determined until we begin the project. We anticipate that the MMS will be available for initial order in the fall of 2015.
If we are only able to raise $585,000 necessary to continue our business operations we will have to scale back the number of samples of the Rx DrugSAFE PRO that we produce for testing although the schedule will stay the same. We will not be able to afford evaluation for adding new technology but anticipate the timeline to bring the basic RxDrugSAFE PRO to market will remain the same. However, we anticipate that the development of the Rx DrugSAFE IC will be delayed until additional funds are available that can be further dedicated to Research and Development. We will also have to delay the production design of the MMS.
In order to create a market for our current and future products we recognize the importance of having a strong sales and marketing plan. We have budgeted $369,000 in any capital we are able to raise to be spent on sales and marketing over the next 12 months of operations.
| 34 |
We have already taken steps to establish sales channels for our products and have begun to re-engage with the large distributors McKesson, Cardinal Health and Amerisource Bergen. We plan to invest in the participation of their various marketing programs to provide exposure for our products within their channels of distribution, which are the national pharmacy chains as well as the many independently owned or franchised neighborhood pharmacies as well as commercial healthcare facilities. With McKesson and Cardinal Health, we already have established distribution agreements in place and anticipate it will take a month or two to re-establish parameters for these two accounts. We have yet to establish a distribution agreement with Amerisource Bergen and anticipate that this will take approximately 3 months to develop the necessary relationships and establish an agreement. We will be able to accomplish these two prior goals even if we are only able to raise the minimum necessary to continue operations. However, a key to making these relationships successful is participating in their various “pay to play” programs where each company’s sales force is incentivized to sell particular products based on our participation in such programs. These programs include but are not limited to providing targeted sales material to the reps to help market our product, attending and exhibiting at various trade shows hosted by each respective distributor, offering special discounted pricing and providing special financial incentives to the sales reps to encourage them to sell our products. The participation in such programs is an ongoing effort and if we are only able to raise the minimum necessary to continue our business operations, we will not have the adequate financial resources in place to participate and will therefore have to rely on traditional grass roots efforts, i.e., establishing our own demand at the end user level, in order to make these relationships a success.
Effective March 1, 2015 we have retained an investor/public relations firm called Renmark Financial Communications Financiers, to work with the company to gain exposure and build awareness and visibility of our company in the marketplace.
A critical part of any business success is a strong web and social media presence. To facilitate this, we intend to hire a company to manage our social media presence as well as revamp and optimize our own website with SEO and SEM with an ultimate goal to be in the top 3 of browser searches for our products and related products. If we are able to raise the necessary funds, the goal would be to increase our ranking over a period of 6 months. This is a top priority for us to be able to increase consumer awareness of our business and we will execute this even if we are only able to raise the minimum amount required to continue operations as this is considered a critical component of our ongoing sales and marketing efforts. To support this we will also need to create strong visual branding and have already identified a graphics design consultant we will engage to strengthen our visual presence across the web, promotional material and in any media campaigns. Even if we are only able to raise the minimum amount needed to continue operations, we will still set aside the necessary resource to engage a graphics person although we will scale down the amount of time spent on visual branding to only address the web based needs of the company.
An effective way to reach our targeted consumer audience is through the use of an infomercial. We will set aside approximately $100,000 of our sales and marketing budget to produce both a short form and long form infomercial and buy media time in select target markets to test the success of this for our current product. If successful we will dedicate additional resources from an intended subsequent capital raise to launch a full blown infomercial campaign and will engage with a third party fulfillment company to handle the incoming calls and product fulfillment. We will be able to fund an infomercial at this level if we are able to raise $1,835,000. If we are not able to raise that, we will not have sufficient funds to develop an infomercial or purchase media time and therefore will not be able to execute on this part of our plan.
Our ultimate goal for the consumer version of our product is to make it widely attainable to the public through medical reimbursement. To this end, we will once again pursue FDA registration, set aside approximately $10,000 to pay the necessary product and facility registration fees and will then hire a consultant to assist us in the process of navigating CMS and private health insurance companies to recognize the product as a medical device and offer reimbursement of the majority of the purchase price through their plans so that the customer will only have to pay a small co-payment to purchase the product. If we are successful in raising capital by the end of March 2015, our goal is to have the Rx DrugSAFE product be reimbursable by June 2015. However, if we are only able to raise the minimum amount required to continue operations we will still look to immediately obtain the necessary registrations and pay the corresponding fees but will not be in a position to hire a consultant to assist us in establishing the product as a reimbursable medical device and will instead need to navigate this effort without the assistance of an industry expert. Without the guidance of an industry specialist, we anticipate that the June 2015 goal would be too aggressive and we would likely push back our targeted date to November 2015.
We have also found trade shows to be an effective way to reach a large number of potential customers for a small investment of capital and plan on attending 5 in the next 12 month period. To support our intended growth in facilities within the healthcare industry and once we are able to raise the necessary funds, we will invest approximately $50,000 of our sales and marketing budget to attend and exhibit at a number of industry trade shows, securing booth space, and creating a small trade show display for the company and products. If we are only able to raise the minimum amount required to continue operations we will significantly reduce our trade show budget to $10,000 and will likely select only one event to attend in the initial 12 month period in addition to the already scheduled International Cannabis Association Conference in June, 2015.
| 35 |
Because we will need to regularly inform our customers and the market on our business progress as well as provide updated information on trends in our industry our goal would be to issue a Press Release a minimum of once every two weeks as the business continues to achieve its milestones and the industry landscape changes. However, with limited resources should we only raise the minimum amount required to continue operations, we will have to significantly decrease our commitment to this effort limiting the issuing of a Press Release to once every month. This could have a negative impact on the marketplace as we will not be able to effectively communicate our progress to the market.
Finally, we have identified a number of not for profit organizations with whom we would like to partner. These organizations have access to targeted audiences of potential consumers of our product and would advertise our product on their website, at various events and as part of targeted campaigns. All of these organizations require financial sponsorship in order to take advantage of the wide reach they enjoy and this outreach could be key in creating awareness of our product by qualified potential customers who are already taking the time to investigate the issue of prescription drug abuse by visiting the organizations site or attending an event. We will need to raise at least $1,210,000 of our targeted amount to in order to participate with at least one of these organizations. If we only raise the minimum amount, we will not be able to afford the sponsorship fees and will therefore not be able to pursue this avenue to potential customers.
With our multifaceted effort to create a market for our product it is essential to ensure we have adequate product to meet the potential demand and will therefore budget $615,000 raised through the offering to place an immediate inventory order from our existing manufacturing partner Good Promotions and have it shipped and housed at Exxtra Express with whom we have already established a warehousing and distribution relationship. These units will have a lead time of approximately 90 days to arrive at our warehousing facility from the time of order. We find it effective to invest in a third party warehouse to fulfill our orders as we benefit from their fast turn around and discounted shipping rates and overall believe the company will save a substantial amount of money using them as a partner. We plan to order approximately 7,500 units of our current product and set aside any remaining balance to place a small order for each of the Pro and IC products once we have completed their product design so that we can do a pilot test of those products in select markets. Through our manufacturing sourcing partner we have access to multiple factories should our capacity outgrow the capabilities of our present facility. Thus, we believe 7,500 units will not be a problem. If we are only able to raise $585,000, we will have to significantly reduce the amount of units of the Rx DrugSAFE to 2,000 pieces and will likely only be able to afford to order approximately 300 units of the Rx DrugSAFE PRO product. This will have a negative impact on our pricing as the less units we order, the higher the cost. This will also clearly impact our revenue potential and will mean that the release of the Rx DrugSAFE IC will need to be postponed until we have adequate capital to order the necessary inventory.
We have budgeted $1,205,000 of any monies we are able to raise for general working capital. Up to February 2014 we had been operating the business from a home office but in March 2014 we identified suitable office space to relocate the company within a Regus Executive Suite facility. The space we have secured is in an executive office and although we presently have only one small office, we have the ability to easily expand the space needed as we bring on additional personnel. We expect to incur additional office expense for telephone/internet, office furniture and equipment postage and utilities and have budgeted another $50,000 to cover these expenses. We will then look to hire personnel to help support the company’s intended growth and execute on our business plan. These resources will include three executive personnel, a CEO, currently held by Ms. Yarde, CFO and COO, two senior sales executives, one to focus on developing our market in the consumer space and the other focusing on the healthcare industry in general, each with their respective sales support administrator, an in-house bookkeeper, and a receptionist/office administrator. This hiring effort will begin immediately once we have raised the necessary capital and we are budgeting approximately $750,000 within the next 12 months of operations for payroll costs and benefits. Effective January 1, 2015, we entered into an employment agreement with our CEO, Lorraine Yarde, which secures her ongoing commitment to the company. However, until we are able to raise enough money, we are not able to compensate Ms. Yarde under her agreement and her salary and benefits are presently accruing.
The remaining $380,000 of working capital will be used as needed to continue to support our business. The money will be spent in the areas of T&E, consulting and professional fees (we will place our securities counsel, The Doney Law Firm, our audit firm ZBS Group, LLP and our accountants, Tichy and Ioanides, CPA on monthly retainers), inventory management and distribution (Exxtra Express), and to build on our intellectual property portfolio through the filing of design patents for each of our products and other day to day operating expenses. If we are only able to raise $585,000 it will force us to significantly scale back our proposed operations. We will still need to maintain suitable office space but will simply keep 1- 2 small offices in the Executive Suite and reduce the monthly budget to approx. $1,000 per month, including phones and internet. We will also then eliminate the need to purchase costly office furniture and equipment.
| 36 |
Results of Operations for the Years Ended December 31, 2014 and 2013
Revenues
Our total revenue reported for the year ended December 31, 2014 was approximately $5,761, as compared with $3,024 for the year ended December 31, 2013. The revenues we had for the year ended December 31, 2014 were from online sales.
Gross Profit
Gross profit for the year ended December 31, 2014 was $3,253, or approximately 56% of sales.
Operating Expenses
Operating expenses increased to approximately $176,279 for the year ended December 31, 2014 from approximately $172,665 for the year ended December 31, 2013. The detail by major category is reflected in the table below.
| Years Ended December 31 | ||||||||
| 2014 | 2013 | |||||||
| Merchant Account Fees | $ | 1,682 | $ | 898 | ||||
| Advertising and Promotion | 3,030 | 440 | ||||||
| Automobile Expense | 2,089 | 654 | ||||||
| Bank Service Charges | 350 | 821 | ||||||
| Consulting | 20,000 | 120,000 | ||||||
| Meals and Entertainment | 3,413 | 107 | ||||||
| Marketing Expenses | 27,008 | 1,758 | ||||||
| Computer and Internet Expenses | 1,810 | 1,894 | ||||||
| Freight and Shipping | 1,070 | 54 | ||||||
| Business Licenses | 1,155 | 500 | ||||||
| Printing and Stationary | 189 | - | ||||||
| Insurance Expenses | 285 | - | ||||||
| Trade Shows | 1,301 | - | ||||||
| Office Supplies | 1,696 | 176 | ||||||
| Office Expense | 493 | - | ||||||
| Postage | 1,029 | 280 | ||||||
| Professional Fees | 93,169 | 43,605 | ||||||
| Rent Expense | 7,337 | - | ||||||
| Product Development | 773 | - | ||||||
| Samples | 834 | - | ||||||
| Telephone Expenses | 264 | - | ||||||
| Travel Expense | 6,985 | 1,478 | ||||||
| Utilities | 317 | - | ||||||
| Total Operating Expense | $ | 176,279 | $ | 172,665 | ||||
Net Loss
We finished the year ended December 31, 2014 with a loss of approximately $177,067, as compared to a loss of approximately $166,007 during the year ended December 31, 2013. The reasons for specific components are discussed above.
| 37 |
Liquidity and Capital Resources
As of December 31, 2014, we had total current assets of $38,785 and total current liabilities of $341,293. We had a working capital deficit of $302,508 as of December 31, 2014.
Operating activities used $138,234 in cash for the year ended December 31, 2014. Our net loss of $177,067 was the main component of our negative operating cash flow.
Cash flows provided by financing activities during the year ended December 31, 2014 amounted to $149,600 from related party loan advances of $75,500 and proceeds from the sale of our common stock of 74,100.
We have $13,087 in cash available as of December 31, 2014. Management believes that we will need $2,500,000 to implement and fully carry out our business plan and no further funding will be required. However, we must raise at least $585,000 in net proceeds in the next two to twelve months otherwise we may temporarily or permanently have to cease operations.
On February 25, 2015 we entered into a securities purchase agreement with Coventry whereby Coventry agreed to invest up to $100,000 in exchange for our issuance of two convertible promissory, each in the original principal amount of $50,000.00. The Notes bear interest at a rate of 8% per annum. All outstanding principal and accrued interest under the Notes is due and payable on February 25, 2016. In addition, on the same date we entered into an Equity Purchase Agreement with Coventry, whereby Coventry agreed to purchase up to $10,000,000 of our common stock, to be registered in a Form S-1 registration statement. The agreement will have a three-year term unless sooner terminated because $10,000,000 of our common stock has already been sold to Coventry. The registration of the shares is not mandatory and we may choose to look to other forms of financing to support the ongoing efforts of the business.
Based upon our current financial condition, we do not have sufficient cash to operate our business at the current level for the next twelve months. We intend to fund operations through increased sales and debt and/or equity financing arrangements, which may be insufficient to fund expenditures or other cash requirements. We plan to seek additional financing in a private equity offering to secure funding for operations. There can be no assurance that we will be successful in raising additional funding. If we are not able to secure additional funding, the implementation of our business plan will be impaired. There can be no assurance that such additional financing will be available to us on acceptable terms or at all.
Going Concern
We have minimal revenues for the years ending December 31, 2014 and December 31, 2013, respectively. We currently have negative working capital, and have not completed our efforts to establish a stabilized source of revenues sufficient to cover operating costs over an extended period of time.
Management anticipates that we will be dependent, for the near future, on additional investment capital to fund operating expenses. We intend to position the company so that it may be able to raise additional funds through the capital markets. In light of management’s efforts, there are no assurances that we will be successful in this or any of our endeavors or become financially viable and continue as a going concern.
Off Balance Sheet Arrangements
As of December 31, 2014, there were no off balance sheet arrangements.
| 38 |
Critical Accounting Policies
In December 2001, the SEC requested that all registrants list their most “critical accounting polices” in the Management Discussion and Analysis. The SEC indicated that a “critical accounting policy” is one which is both important to the portrayal of a company’s financial condition and results, and requires management’s most difficult, subjective or complex judgments, often as a result of the need to make estimates about the effect of matters that are inherently uncertain. We do not believe that any accounting policies currently fit this definition.
Recently Issued Accounting Pronouncements
On May 1, 2014, the FASB issued ASU 2014-09, “Revenue from Contracts with Customers (Topic 606),” which is the new comprehensive revenue recognition standard that will supersede all existing revenue recognition guidance under GAAP. The standard’s core principle is that a company will recognize revenue when it transfers promised goods or services to a customer in an amount that reflects the consideration to which the company expects to be entitled in exchange for those goods or services. This ASU is effective for annual and interim periods beginning on or after December 15, 2016, and early adoption is not permitted. Entities will have the option of using either a full retrospective approach or a modified approach to adopt the guidance in the ASU. The Company will continue to assess the impact on its financial statements.
In June 2014, the FASB issued ASU 2014-12, “Compensation - Stock Compensation (Topic 718): Accounting for Share-Based Payments When the Terms of an Award Provide That a Performance Target Could be Achieved after the Requisite Service Period.” This ASU provides more explicit guidance for treating share-based payment awards that require a specific performance target that affects vesting and that could be achieved after the requisite service period as a performance condition. The new guidance is effective for annual and interim reporting periods beginning after December 15, 2015. The Company does not expect the adoption of this guidance to have a material impact on the financial statements.
On June 10, 2014, the Financial Accounting Standards Board (“FASB”) issued update ASU 2014-10, Development Stage Entities (Topic 915). Amongst other things, the amendments in this update removed the definition of development stage entity from Topic 915, thereby removing the distinction between development stage entities and other reporting entities from US GAAP. In addition, the amendments eliminate the requirements for development stage entities to (1) present inception-to-date information on the statements of income, cash flows and shareholder’s equity, (2) label the financial statements as those of a development stage entity; (3) disclose a description of the development stage activities in which the entity is engaged and (4) disclose in the first year in which the entity is no longer a development stage entity that in prior years it had been in the development stage. The amendments are effective for annual reporting periods beginning after December 31, 2014 and interim reporting periods beginning after December 15, 2015, however entities are permitted to early adopt for any annual or interim reporting period for which the financial statements have yet to be issued. The Company has elected to early adopt these amendments and accordingly have not labeled the financial statements as those of a development stage entity and have not presented inception-to-date information on the respective financial statements.
In August 2014, the FASB issued ASU 2014-15, “Presentation of Financial Statements – Going Concern (Topic 205-40)”, which requires management to evaluate whether there is substantial doubt about an entity’s ability to continue as a going concern for each annual and interim reporting period. If substantial doubt exists, additional disclosure is required. This new standard will be effective for the Company for annual and interim periods beginning after December 15, 2016. Early adoption is permitted. The Company expects to adopt this new standard for the fiscal year ending December 31, 2015 and the Company will continue to assess the impact on its financial statements.
| 39 |
Item 8. Financial Statements and Supplementary Data
Index to Financial Statements Required by Article 8 of Regulation S-X:
| 40 |

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders
RX Safes Inc.
Henderson, NV
We have audited the accompanying balance sheets of RX Safes Inc. as of December 31, 2014 and 2013, and the related statements of operations, stockholders’ deficit and cash flows for each of the years in the two year period ended December 31, 2014. RX Safes, Inc. management is responsible for these financial statements. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement. The company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Rx Safes, Inc. as of December 31, 2014 and 2013, and the results of its operations and its cash flows for each of the years in the two year period ended December 31, 2014 are in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 10 to the financial statements, the Company is an early stage company with limited operations and resources, which raises substantial doubt about its ability to continue as a going concern. Management’s plans regarding those matters are also described in Note 10. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ ZBS Group LLP
Plainview, New York
March 14, 2015
255 Executive Drive, Suite 400 Plainview, New York 11803
Tel: (516) 394-3344 Fax: (516) 908-7867
www.zbscpas.com
| F-1 |
BALANCE SHEETS
| December 31, | December 31, | |||||||
| ASSETS | 2014 | 2013 | ||||||
| Current Assets | ||||||||
| Cash and cash equivalents | $ | 13,087 | $ | 1,721 | ||||
| Inventory | 25,698 | 5,187 | ||||||
| Total Assets | $ | 38,785 | $ | 6,908 | ||||
| LIABILITIES AND | ||||||||
| STOCKHOLDERS' DEFICIT | ||||||||
| Current Liabilities | ||||||||
| Accounts Payable | $ | 226,274 | $ | 196,071 | ||||
| Loans Payable | 110,274 | - | ||||||
| Interest Payable | 4,745 | - | ||||||
| Total Current Liabilities | 341,293 | 196,071 | ||||||
| Long Term Liabilities | ||||||||
| Loans Payable | - | 34,774 | ||||||
| Interest Payable | - | 604 | ||||||
| Total Long Term Liabilities | - | 35,378 | ||||||
| Total Liabilities | 341,293 | 231,449 | ||||||
| Stockholders' Deficit | ||||||||
| Common stock, par value $.001, 250,000,000 authorized | ||||||||
| 115,491,472 and 115,095,072 shares issued and outstanding | ||||||||
| as of December 31, 2014 and December 31, 2013 | 115,490 | 115,094 | ||||||
| Additional paid in capital | 1,742,336 | 1,643,632 | ||||||
| Additional paid in capital - Warrants | 389,235 | 389,235 | ||||||
| Additional paid in capital - expired options | 21,302 | 21,302 | ||||||
| Accumulated deficit | (2,570,871 | ) | (2,393,804 | ) | ||||
| Total Stockholders' Deficit | (302,508 | ) | (224,541 | ) | ||||
| Total Liabilities and Stockholders' Deficit | $ | 38,785 | $ | 6,908 | ||||
The accompanying notes are an integral part of these financial statements.
| F-2 |
The accompanying notes are an integral part of these financial statements.
| F-3 |
| RX SAFES INC. | |||||||
| STATEMENTS OF STOCKHOLDERS' DEFICIT | |||||||
| FOR THE YEARS ENDED DECEMBER 31, 2014 AND 2013 |
| Additional | Total | |||||||||||||||||||
| Common Stock | Paid-in | Accumulated | Stockholders' | |||||||||||||||||
| Shares | Amount | Capital | Deficit | Equity | ||||||||||||||||
| Balance, January 1, 2013 | 114,061,739 | $ | 114,061 | $ | 2,035,202 | $ | (2,227,797 | ) | $ | (78,534 | ) | |||||||||
| On 6/26/13, stock options exercised | 1,000,000 | 1,000 | 9,000 | 10,000 | ||||||||||||||||
| On 6/26/13, shares issued for cash | 33,333 | 33 | 9,967 | 10,000 | ||||||||||||||||
| Net loss for year ended December 31, 2013 | - | - | - | (166,007 | ) | (166,007 | ) | |||||||||||||
| Balance, December 31, 2013 | 115,095,072 | $ | 115,094 | $ | 2,054,169 | $ | (2,393,804 | ) | $ | (224,541 | ) | |||||||||
| On 8/14/14, shares issued for service | 100,000 | 100 | 24,900 | - | 25,000 | |||||||||||||||
| Total shares sold from 9/15/14 to 11/18/14 | 296,400 | 296 | 73,804 | 74,100 | ||||||||||||||||
| Net loss for year ended December 31, 2014 | - | - | - | (177,067 | ) | (177,067 | ) | |||||||||||||
| Balance, December 31, 2014 | 115,491,472 | $ | 115,490 | $ | 2,152,873 | $ | (2,570,871 | ) | $ | (302,508 | ) | |||||||||
The accompanying notes are an integral part of these financial statements.
| F-4 |
STATEMENTS OF CASH FLOWS
| Year Ended | Year Ended | |||||||
| December 31, 2013 | December 31, 2014 | |||||||
| OPERATING ACTIVITIES | ||||||||
| Net loss | $ | (177,067 | ) | $ | (166,007 | ) | ||
| Adjustments to reconcile net loss to net cash used in operations: | ||||||||
| Common Stock issued for services | 25,000 | - | ||||||
| Decrease (increase) in assets | ||||||||
| Inventory | (20,511 | ) | (4,655 | ) | ||||
| Advance to Vendor | - | 3,050 | ||||||
| Increase (decrease) in liabilities | ||||||||
| Accounts payable | 30,203 | 82,274 | ||||||
| Interest Payable | 4,141 | 604 | ||||||
| Net cash used in operating activities | (138,234 | ) | (84,734 | ) | ||||
| INVESTING ACTIVITIES | - | - | ||||||
| FINANCING ACTIVITIES | ||||||||
| Proceeds from issuance of Common stock | 74,100 | 20,000 | ||||||
| Proceeds from related party loan advances | 75,500 | 34,774 | ||||||
| Loans from shareholders | - | (325 | ) | |||||
| Net cash from financing activities | 149,600 | 54,449 | ||||||
| NET INCREASE IN CASH | 11,366 | (30,285 | ) | |||||
| Cash and cash equivalents beginning of year | 1,721 | 32,006 | ||||||
| Cash and cash equivalents end of year | $ | 13,087 | $ | 1,721 | ||||
| Supplemental Disclosure of Cash Flow Information | ||||||||
| Cash Paid for Interest | $ | - | $ | - | ||||
| Cash Paid for Taxes | $ | - | $ | - | ||||
| Non-cash investing and financing activities: | ||||||||
| Common stock issued for conversion of debt | $ | - | $ | - | ||||
| Cancellation of common stock | $ | - | $ | - | ||||
The accompanying notes are an integral part of these financial statements.
| F-5 |
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2014
NOTE 1.
Nature of Operations:
Rx Safes, Inc. (“the Company”) was incorporated under the laws of the State of Nevada on June 1, 2010. The Company develops, designs, manufactures, and sells finger print activated medication safes and other health care storage equipment.
NOTE 2.
Summary of Significant Accounting Policies:
The financial statements of the Company have been prepared using generally accepted accounting principles (“GAAP”) in the United States (“U.S.”) of America that are applicable to a going concern which contemplates that the Company will continue in operation for the foreseeable future and will be able to realize its assets and discharge its liabilities in the normal course of business.
Management’s use of estimates
The preparation of financial statements in conformity with U.S. generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Cash and cash equivalents
Cash and cash equivalents include funds on hand and short-term investments with original maturities of three months or less. Cash deposits are insured in limited amounts by the Federal Deposit Insurance Corporation (FDIC).
Inventories
Inventories consist of safes and brackets. All inventories are valued at lower of average cost or market. The company periodically reviews inventories and items considered obsolete or outdated are reduced to their estimated net realizable value.
The components of inventory as of December 31, 2014 and December 31, 2013 are valued as follows:
| December 31, 2014 | December 31, 2013 | |||||||
| Safes | $ | 25,652 | $ | 5,072 | ||||
| Brackets | 46 | 115 | ||||||
| Total Inventory | $ | 25,698 | $ | 5,187 | ||||
Shipping and Handling Freight Fees and Costs
All amounts billed to a customer in a sales transaction related to shipping and handling represent revenues earned and are reported as revenue. The costs incurred by the Company for shipping and handling are reported as part of cost of goods sold.
| F-6 |
RX SAFES INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2014
Revenue recognition
Revenue is recognized when the four criteria for revenue recognition are met: (1) persuasive evidence of an arrangement exists; (2) shipment or delivery has occurred; (3) the price is fixed or determinable and (4) collectability is reasonably assured. The Company has recognized revenue associated with its mission as stated above in the nature of operations footnote. Sales to customers are recorded when the goods are shipped to the customer. Sales are reported net of allowances for estimated returns and allowances in the accompanying statements of income. Allowances for returns are estimated based on historical customer return rates. The Company has not had any product returns since inception. Customers pre-pay for orders though a website with their credit cards prior to the shipment of the goods, which takes place within a few days after the order is placed.
Advertising expense
The Company expenses advertising costs as incurred. Advertising expense charged to operating expenses was $3,030 and $440 for the years ended December 31, 2014 and 2013, respectively.
Rent expense
The Company pays rent on a month to month basis. Rent expense charged to operating expenses was $7,337 and $0 for the years ended December 31, 2014 and 2013, respectively.
Research and development costs
Research and development costs, consisting primarily of expenditures paid to our manufacturing and development partner in China, are expensed as incurred. Research and development expense charged to operating expenses was $0 for the years ended December 31, 2014 and 2013, respectively.
Fair value measurements
Generally accepted accounting principles require disclosing the fair value of financial instruments to the extent practicable for financial instruments which are recognized or unrecognized in the balance sheet. The fair value of the financial instruments disclosed herein is not necessarily representative of the amount that could be realized or settled, nor does the fair value amount consider the tax consequences of realization or settlement.
In assessing the fair value of financial instruments, the Company uses a variety of methods and assumptions, which are based on estimates of market conditions and risks existing at the time. For certain instruments, including cash and cash equivalents, accounts receivable, accounts payable, and accrued expenses, it was estimated that the carrying amount approximated fair value because of the short maturities of these instruments. All debt is based on current rates at which the Company could borrow funds with similar remaining maturities and approximates fair value.
GAAP establishes a hierarchy for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use on unobservable inputs by requiring that the most observable inputs be used when available. Observable inputs are inputs that market participants would use in pricing the asset or liability developed based on market data obtained from sources independent of the Company. Unobservable inputs are inputs that reflect the Company’s assumptions about the assumptions market participants would use in pricing the asset or liability developed based on the best information available in the circumstances. The hierarchy is described below:
Level 1: Quoted prices (unadjusted) in active markets that are accessible at the measurement date for assets or liabilities. The fair value hierarchy gives the highest priority to Level 1 inputs.
| F-7 |
RX SAFES INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2014
Level 2: Observable prices that are based on inputs not quoted on active markets, but corroborated by market data.
Level 3: Unobservable inputs are used when little or no market data is available. The fair value hierarchy gives the lowest priority to Level 3 inputs.
Determining which category an asset or liability falls within the hierarchy requires significant judgment. We evaluate our hierarchy disclosures each quarter
Recent Accounting Pronouncements
On May 1, 2014, the FASB issued ASU 2014-09, “Revenue from Contracts with Customers (Topic 606),” which is the new comprehensive revenue recognition standard that will supersede all existing revenue recognition guidance under GAAP. The standard’s core principle is that a company will recognize revenue when it transfers promised goods or services to a customer in an amount that reflects the consideration to which the company expects to be entitled in exchange for those goods or services. This ASU is effective for annual and interim periods beginning on or after December 15, 2016, and early adoption is not permitted. Entities will have the option of using either a full retrospective approach or a modified approach to adopt the guidance in the ASU. The Company will continue to assess the impact on its financial statements.
In June 2014, the FASB issued ASU 2014-12, “Compensation - Stock Compensation (Topic 718): Accounting for Share-Based Payments When the Terms of an Award Provide That a Performance Target Could be Achieved after the Requisite Service Period.” This ASU provides more explicit guidance for treating share-based payment awards that require a specific performance target that affects vesting and that could be achieved after the requisite service period as a performance condition. The new guidance is effective for annual and interim reporting periods beginning after December 15, 2015. The Company does not expect the adoption of this guidance to have a material impact on the financial statements.
On June 10, 2014, the Financial Accounting Standards Board (“FASB”) issued update ASU 2014-10, Development Stage Entities (Topic 915). Amongst other things, the amendments in this update removed the definition of development stage entity from Topic 915, thereby removing the distinction between development stage entities and other reporting entities from US GAAP. In addition, the amendments eliminate the requirements for development stage entities to (1) present inception-to-date information on the statements of income, cash flows and shareholder’s equity, (2) label the financial statements as those of a development stage entity; (3) disclose a description of the development stage activities in which the entity is engaged and (4) disclose in the first year in which the entity is no longer a development stage entity that in prior years it had been in the development stage. The amendments are effective for annual reporting periods beginning after December 31, 2014 and interim reporting periods beginning after December 15, 2015, however entities are permitted to early adopt for any annual or interim reporting period for which the financial statements have yet to be issued. The Company has elected to early adopt these amendments and accordingly have not labeled the financial statements as those of a development stage entity and have not presented inception-to-date information on the respective financial statements.
In August 2014, the FASB issued ASU 2014-15, “Presentation of Financial Statements – Going Concern (Topic 205-40)”, which requires management to evaluate whether there is substantial doubt about an entity’s ability to continue as a going concern for each annual and interim reporting period. If substantial doubt exists, additional disclosure is required. This new standard will be effective for the Company for annual and interim periods beginning after December 15, 2016. Early adoption is permitted. The Company expects to adopt this new standard for the fiscal year ending December 31, 2015 and the Company will continue to assess the impact on its financial statements.
| F-8 |
RX SAFES INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2014
Income Taxes
The Company provides for income taxes under the provisions of Accounting Standards Codification (“ASC”) Topic No. 740, “Income Taxes”, which requires that an asset and liability based approach be used in accounting for income taxes. Deferred income tax assets and liabilities are recorded to reflect the tax consequences on future years of the temporary differences of revenue and expense items for financial statement and income tax purposes. Valuation allowances are provided against assets, which are not likely to be realized.
Stock-based Compensation
The Company adopted ASC 718, "Compensation - Stock Compensation" for stock-based compensation. ASC 718 requires that the fair value of the equity instruments (such as stock options) exchanged for services be recognized as an expense in the financial statements as the related services are performed.
Earnings Per Share
Earnings per share ("EPS") has been calculated in accordance with ASC 260, “Earnings Per Share" which requires the presentation of both basic net income per share and net income per common share assuming dilution. Basic earnings per share are computed by dividing income available to common stockholders by the weighted average number of shares outstanding for the year. Diluted earnings per share reflects the potential dilution that could occur upon the exercise of common stock options resulting in the issuance of common stock to stockholders who would then share in the earnings of the Company. ASC 260 precludes the inclusion of any potential common shares in the computation of any diluted per-share amounts when such inclusion is anti-dilutive.
NOTE 3.
RELATED PARTY TRANSACTIONS
The Company has entered into certain transactions in the normal course of business with entities under common ownership. While being on the Board of Directors for the Company, Mark Basile was also CEO of Axius Consulting Group, Inc. On August 2, 2012 Mr Basile resigned from his position on the Board. Loraine Yarde, CEO of the Company, also has a position with Axius Consulting Group. The Company paid consulting fees that amounted to $0 and $120,000 for the years ended December 31, 2014 and 2013 respectively.
Consulting Agreement
Effective July 1, 2010 the company entered into a consulting agreement with Axius Consulting Group, Inc (“Axius”). The Company engaged Axius to provide sevices in connection with the Company's efforts to seek out business relationships and financing to grow the Company's business. The consulting agreement was not renewed on July 1, 2013. Axius Consulting has at its sole discretion, to have the option to take payment of the accrued fee in cash or as common stock of the business, at a 50% discount to the current market value of the common stock. Certain payments made directly on behalf of the principals of Axius, in the form of health insurance payments of $29,395 in 2013 were applied against payables. Balances included in accounts payable to Axius totaled $171,701 and $176,701 for the years ended December 31 2014, and 2013, respectively.
| F-9 |
RX SAFES INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2014
Loans Payable
Effective May 1, 2013 entered into an agreement with Axius Consulting Group, Inc (“Axius”). It is the intent of both parties to create a line of credit agreement. The company may borrow up to $50,000 from Axius. The unpaid principal of this line of credit shall bear an interest rate of Four percent per annum. Interest on the unpaid balance of the note shall accrue monthly but shall not be due and payable until the principal balance becomes due and payable. The principal balance of the note is due and payable on December 31, 2014. The outstanding balance of the note payable was $25,274 and $34,774 at December 31, 2014 and 2013, respectively. Total interest accrued on the note was $1,895 and $604 for the years ended December 31, 2014 and 2013, respectively. On December 30, 2014 an extension agreement was signed extending the due date of the Note to June 30, 2015 without penalty.
Effective January 1, 2014 entered into an agreement Lorraine Yarde It is the intent of both parties to create a line of credit agreement. The company may borrow up to $100,000 from Lorraine Yarde. The unpaid principal of this line of credit shall bear an interest rate of Four percent per annum. Interest on the unpaid balance of the note shall accrue monthly but shall not be due and payable until the principal balance becomes due and payable. The principal balance of the note is due and payable on December 31, 2014. The outstanding balance of the note payable as of December 31, 2014 was $55,000. Total interest accrued on the note as of December 31, 2014 was $2,050. On December 30, 2014 an extension agreement was signed extending the due date of the Note to June 30, 2015 without penalty.
Effective January 1, 2014 the Company entered into a Master Promissory Note agreement with Naples Family Trust. It is the intent of both parties to create a line of credit agreement. Under the terms of the note the Company may borrow up to $100,000. The unpaid principal of this line of credit shall bear an interest rate of four percent per annum. Interest on the unpaid balance of the note shall accrue monthly but shall not be due and payable until the principal balance becomes due and payable. The principal balance of the note is due and payable on December 31, 2014. The outstanding balance of the note payable at December 31, 2014 was $30,000. Total interest accrued on the note as of December 31, 2014 was $800. On December 30, 2014 an extension agreement was signed extending the due date of the Note to June 30, 2015 without penalty.
NOTE 5.
License Agreements
Included in the assets purchase of Axius Consulting Group, Inc. was a Patent & Licensing Rights Agreement with bioMETRX. The agreement grants the licensee a royalty based, ($.50 per unit) exclusive license under their Patent License to use, manufacture, have manufactured, license and/or sell licensed intellectual property for any legal purpose with North America within the health care and consumer health markets. The term of the agreement is from the effective date (March 1, 2009) to the full end of the term or terms for which Patent Rights have not expired or, if only Technology Rights are licensed and no Patent Rights are applicable, for a term of 8 years.
NOTE 6.
Income Taxes
As of December 31, 2014, the Company has net operating loss carry forwards of approximately $2,571,000 that may be available to reduce future years’ taxable income in varying amounts through 2031. Future tax benefits which may arise as a result of these losses have not been recognized in these financial statements, as their realization is determined not likely to occur and accordingly , the Company has recorded a valuation allowance for the deferred tax asset relating to these tax loss carry-forwards.
| F-10 |
RX SAFES INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2014
The provision for Federal income tax consists of the following:
| Year Ended | Year Ended | |||||||
| December 31, | December 31, | |||||||
| 2014 | 2013 | |||||||
| Federal income tax benefit attributable to: | ||||||||
| Current Operations | $ | 61,973 | $ | 58,102 | ||||
| Less: valuation allowance | (61,973 | ) | (58,102 | ) | ||||
| Net Provision for Federal income taxes | $ | - | $ | - | ||||
The cumulative tax effect at the expected rate of 35% of significant items comprising our net deferred tax amount is as follows:
| Year Ended | Year Ended | |||||||
| December 31, | December 31, | |||||||
| 2014 | 2013 | |||||||
| Statutory tax rate | 35 | % | 35 | % | ||||
| Loss for the year before income taxes | $ | (177,067 | ) | $ | (166,007 | ) | ||
| Deferred tax asset | (61,973 | ) | (58,102 | ) | ||||
| Net operating loss carryover | 61,973 | 58,102 | ||||||
| Less: valuation allowance | (61,973 | ) | (58,102 | ) | ||||
| Net deferred tax asset | $ | - | $ | - | ||||
The Company reviews tax positions taken to determine if it is more likely than not that the position would be sustained upon examination resulting in an uncertain tax position. The Company did not have any material unrecognized tax benefit at December 31 2014 and December 31, 2013, respectively. The Company recognizes interest accrued and penalties related to unrecognized tax benefits in tax expense. The Company recognized no interest and penalties during the years ended December 31, 2014 and December 31, 2013, respectively.
The Company files U.S. federal tax returns and a tax return in states where obligated. All tax periods since inception remain open to examination by the taxing jurisdictions to which the Company is subject.
| F-11 |
RX SAFES INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2014
NOTE 7
Stock options and warrants
A summary of stock option and warrant activity for the period from inception January 1, 2013 to December 31, 2014 follows:
| Stock | ||||||||
| Description | Options | Warrants | ||||||
| Outstanding at January 1, 2013 | 1,000,000 | 10,800,000 | ||||||
| Options exercised June 20, 2013 | (1,000,000 | ) | - | |||||
| Expired September 28, 2013 | - | 2,000,000 | ||||||
| Outstanding at December 31, 2013 | - | 8,800,000 | ||||||
| Expired January 28, 2014 | (50,000 | ) | ||||||
| Expired February 10, 2014 | (500,000 | ) | ||||||
| Outstanding at December 31, 2014 | - | 8,250,000 | ||||||
Pursuant to a Letter Agreement effective June 26, 2010 with Dr. Mary Ellen Renna for marketing services for a term of two years ending on June 25, 2012, the Company issued a total of 1,500,000 post-split (300,000 pre-split) stock options to Dr. Renna. Each option is exercisable into one share of Company common stock at an exercise price of $0.01 post-split ($0.05 pre-split) per share. 500,000 of the options vested June 26, 2010 and expired June 20, 2012, 500,000 of the options vested June 26, 2011 of which 500,000 of the options were exercised on June 20, 2013, and 500,000 of the options vested June 26, 2012 of which 500,000 of the options were exercised on June 13, 2013. The $64,550 estimated fair value of the 1,500,000 stock options (calculated using the Black-Scholes option pricing model and the following assumptions: (i) $0.05 share price, (ii) $0.01 exercise price, (iii) terms of 2 years, 3 years, and 4 years, (iv) 100% expected volatility, and (v) risk free interest rates of 0.65%, 1.07%, and 1.49%) has been expensed evenly over the two year period from June 26, 2010 to June 25, 2012.
On June 20, 2013, Ms. Renna exercised the balance of her stock options amounting to 1,000,000 shares and paid to the Company $10,000 in cash.
Pursuant to a Letter Agreement dated August 20, 2010 with Dr. Reed Karim for marketing services for a term of two years ending on August 31, 2012, the Company issued a total of 1,260,000 post-split (252,000 pre-split) warrants to Dr. Karim. Each warrant is exercisable into one share of Company common stock at an exercise price of $0.005 post-split ($0.025 pre-split) per share. 630,000 of the warrants vested August 20, 2010 and expire August 20, 2015, 315,000 of the warrants vested September 1, 2011 and expire September 1, 2016, and 315,000 of the warrants vested September 1, 2012 and expire September 1, 2017. The $59,693 estimated fair value of the 1,260,000 warrants (calculated using the Black-Scholes option pricing model and the following assumptions: (i) $0.05 share price, (ii) $0.005 exercise price, (iii) terms of 5 years, 6 years, and 7 years, (iv) 100% expected volatility, and (v) risk free interest rates of 1.47%, 1.78%, and 2.08%) has been expensed evenly over the period from August 20, 2010 to August 31, 2012.
On May 23, 2011, pursuant to an amendment to the Letter Agreement dated August 20, 2010 referred to in the preceding paragraph, the Company increased the number of issued warrants to Dr. Karim from a total of 1,260,000 post-split (252,000 pre-split) warrants to a total of 8,800,000 post-split (1,650,000 pre-split) warrants. 2,750,000 of the warrants vested August 20, 2010 and expire August 20, 2015, 2,750,000 warrants vested September 1, 2011 and expire September 1, 2016, and 2,750,000 warrants vested September 1, 2012 and expire September 1, 2017. The $329,542 excess of the $389,235 estimated fair value of the 8,800,000 warrants (calculated using the Black-Scholes option pricing model and the following assumptions: (i) $0.05 share price, (ii) $0.005 exercise price, (iii) terms of 4.25 years, 5.25 years, and 6.25 years, (iv) 100% expected volatility, and (v) risk free interest rates of 1.46%, 1.89%, and 2.23%) over the $59,693 estimated fair value of the 1,260,000 warrants at August 20, 2010 referred to in the preceding paragraph has been expensed evenly over the period from May 23, 2011 to August 31, 2012.
| F-12 |
RX SAFES INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2014
NOTE 8
Equity
Common Stock.
Stock Split
On August 22, 2012 the Company amended its Certificate of Incorporation to split its outstanding shares of the companies’ common stock at a ratio of 5-for-1. The par value of the common stock was decreased to $.001 and increased the number of authorized shares to issue to 250,000,000. The accompanying financial statements have been adjusted to retroactively reflect the stock split.
For the year ended December 31, 2014, the Company sold in its registered offering 296,400 shares of its common stock to 27 investors for an aggregate proceeds of $74,100. The purchase price was $0.25 per common share. Since inception from June 1, 2010 through December 31, 2014, the Company has issued 10,899,732 shares of common stock to 44 investors for proceeds totaling $672,600. Additionally 24,091,740 shares of common stock were issued as compensation for services, with common shares totaling 80,500,000 issued to its founders.
Preferred Stock.
The company is authorized to issue a second class of 50,000,000 preferred shares.
NOTE 9
Consulting Agreement
On February 20, 2014, the Company executed a Consulting Agreement with Wall Street Buy Sell Hold Inc. (the “Consultant) to become effective once the Company’s Form S-1 registration statement becomes effective. The Agreement provides for the Consultant to perform certain specified business and investor relations services for the Company for a term of six months commencing on the effective date of the Company’s Form S-1 registration statement. If an opt-out clause is not exercised at the end of the six months, the term will extend for an additional six month period. The Agreement provides for the payment of monthly cash compensation to the Consultant of $20,000 per month until such time as the Company has raised $1,500,000 in its public offering and $30,000 per month thereafter.
On February 10, 2014, the consultant was issued 5,000,000 post-split shares of common stock for investor relations consulting services. These shares were returned by the consultant to treasury and the consultant was issued a warrant to purchase 5,000,000 shares of common stock. The issued warrant will vest immediately. Additionally, a warrant to purchase 2,500,000 shares will be issued at 6 months of service. A final warrant to purchase 2,500,000 shares will be issued at the end of 12 months if the company continues the agreement to 12 months.
On December 1, 2014 the Company voided and terminated its agreement with the Consultant. The voiding and termination of the agreement will cancel all warrants previously issued under the agreement.
Effective August 19, 2014, the Company executed a Marketing Agreement with Josh Tolley who has become a spokesperson for the Company. In consideration for his services the Company shall issue 100,000 shares of common stock as stock based compensation for this Agreement. The term of the agreement is six months. At the end of the initial six month period, it is the intent of the Company to extend this relationship for an additional period, provided the Parties are in mutual agreement that they have established a good working relationship, at which time a new Agreement will be executed between the Parties. The shares were issued to Mr. Tolley on August 19, 2014.
| F-13 |
RX SAFES INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2014
NOTE 10
Going Concern
The accompanying financial statements have been prepared in conformity with generally accepted accounting principle, which contemplate continuation of the Company as a going concern. However, the Company had minimal revenues for the years ending December 31, 2014 and December 31, 2013, respectively. The Company currently has limited working capital, and has not completed its efforts to establish a stabilized source of revenues sufficient to cover operating costs over an extended period of time.
Management anticipates that the Company will be dependent, for the near future, on additional investment capital to fund operating expenses. The Company intends to position itself so that it may be able to raise additional funds through the capital markets. In light of management’s efforts, there are no assurances that the Company will be successful in this or any of its endeavors or become financially viable and continue as a going concern.
NOTE 11
Subsequent Events
On January 1, 2015, the Company entered into an employment agreement with Lorraine Yarde to be its Chief Executive Officer. Yarde’s initial annual Base Salary is $175,000, with annual salary increases of no less than 10% of the prior year’s annual salary. Yarde will be eligible to earn bonus a Performance Bonus for each complete fiscal year. The Company will immediately grant to Yarde an option under the Company’s 2015 Equity Incentive Plan to purchase 10,000,000 shares of the Company's Common Stock with vesting and strike prices set forth in the Yarde Agreement.
Effective January 6, 2015, the Company executed a business consulting agreement with Paramount Advisors, LLC. The consultant will act as advisor in co connection with the Company’s business matters, investor relations, and advertising services.
The Company shall pay the consultant the following:
(a) A total of 900,000 shares of validly issued unregistered shares of the Company’s common stock par value $0.001 per share, which trades on the OTC under the symbol “RXSF” in two equal installments as follows:
i. 450,000 shares due immediately; and
ii. 450,000 shares due on or before March 1, 2015
(b) A total of $18,000 in cash, payable in equal monthly installments of $3,000 per month. Said cash payments will not be due to consultant until the Company has successfully completed a capital raise of at least one hundred thousand dollars and upon that occurrence, the Company shall pay the consultant all monthly payments that it had accrued from the beginning of this Agreement and remain current on all cash payments from that time. The Agreement will commence on the date first set forth above and shall continue for a term of six months, thereafter.
Effective February 23, 2015 the company terminated its agreement with Paramount advisors due to non-performance.
Effective February 12, 2015, the company executed a consulting agreement with Sean McManus. The Company has engaged Consultant to provide services in connection with the Company’s sale and distribution of its flagship Rx DrugSAFE product. Consultant will introduce the Company to sales opportunities, strategic partners and such other services as the Company and Consultant may deem appropriate. Upon execution of this Agreement, the Company shall pay to Consultant an initial retainer of 100,000, restricted shares of Rx Safes, Inc. common stock. For any sales of the Company’s products directly attributable to the efforts of the Consultant, Consultant will be paid a commission of 10% of the net sales to the Company for as long as any agreement is in effect between the Company and any Client directly introduced to Company by Consultant, and for a tail period of 24 months after the last date of any agreement, whether terminated or otherwise expired between Company and Client or 24 months after delivery of the product to the Client, whichever is later in time. This Agreement shall continue in full force and effect for 6 consecutive months. The Company and Consultant may negotiate to extend the term of this Agreement and the terms and conditions under which the relationship shall continue.
| F-14 |
RX SAFES INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2014
Effective February 25, 2015, the company entered into an equity purchase agreement with Coventry Enterprises, LLC to purchase up to $10,000,000 of the Company’s common stock. The agreement will have a three year term. Coventry shall at no time own more than 4.99% of the Company’s common stock, and is prohibited from short sales of the stock during the term of the agreement.
In addition, Coventry agreed to invest up to $100,000 into the Company in exchange for the issuance of convertible promissory notes each in the amount of $50,000. The notes bear interest at a rate of 8%. All outstanding interest and principal is due and payable February 25, 2016. Note I is convertible by Coventry into shares of the Company’s common stock at any time on or after the Issuance Date. The conversion price for each share is equal to 65% multiplied by the lowest trading price of the Common Stock on the OTC Market for the 20 prior trading days. The Company may prepay Note I within 120 days of the Issuance Date by paying 125% of the outstanding principal plus any accrued but unpaid interest. Note II is only convertible into shares of Common Stock at the Notes Conversion Price after full cash payment by Coventry to the Company of all principal and interest due under Note III. Note II may not be prepaid by the Company. However, if Note I is prepaid by the Company within 6 months of the Issuance Date, then all of Coventry’s obligations and our obligations under Note II and Note III will be deemed satisfied, and Note II and Note III will be cancelled in full. Coventry issued Note III to the Company, which is a back end promissory in the original principal amount of $50,000, at the rate of 8%, and has been initially secured by the Company’s issuance of Note II. All outstanding principal and accrued interest on Note III is due and payable when the Company files its registration statement in connection with the Equity Purchase Agreement, described above, but no later than August 19, 2015.
Effective March 1, 2015, the Company executed an investor relations agreement with Renmark Financial Communications Inc. The investor relations program is a continuous effort to create awareness and build an audience that will follow the growth of the Company. Renmark will establish a liaison between the company and its retail investors and make the company more visible in the market place. The agreement is on a monthly basis. The Company will have the right to terminate the agreement at any time. Compensation for the services rendered is a monthly fee of $4,000.
Effective March 3, 2015, the Company has terminated the agreements dated August 31, 2010 and May 23, 2011 with Dr. Reef Karim. As a result of the termination the Dr. Karim has no further right to purchase or receive stock or warrants from the company and all outstanding, unexercised warrants will be cancelled.
On March 10, 2015, the Company filed a complaint in the District Court for Clark County, Nevada, against Wall Street Buy Sell Hold Inc., a New York corporation, for Fraud in the Inducement, Deceptive Trade Practices and Unjust Enrichment in connection with a Consulting Agreement dated February 10, 2014. As a result of the misrepresentations and actions perpetuated by Wall Street Buy Sell Hold Inc., the Company is asking court to award damages, recession of the Consulting Agreement and a return of monies paid thereunder, and for punitive damages.
| F-15 |
Item 9. Changes In and Disagreements with Accountants on Accounting and Financial Disclosure
During the last two fiscal years, we have had no disagreements with our accountants on accounting and financial disclosure.
Item 9A. Controls and Procedures
a) Disclosure Controls and Procedures
We conducted an evaluation, with the participation of our Chief Executive Officer and Chief Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures, as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended, or the Exchange Act, as of December 31, 2014, to ensure that information required to be disclosed by us in the reports filed or submitted by us under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the Securities Exchange Commission’s rules and forms, including to ensure that information required to be disclosed by us in the reports filed or submitted by us under the Exchange Act is accumulated and communicated to our management, including our principal executive and principal financial officer, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure. Based on that evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that as of December 31, 2014, our disclosure controls and procedures were not effective at the reasonable assurance level due to the material weaknesses identified and described in Item 9A(b).
Our principal executive officers do not expect that our disclosure controls or internal controls will prevent all error and all fraud. Although our disclosure controls and procedures were designed to provide reasonable assurance of achieving their objectives and our principal executive officers have determined that our disclosure controls and procedures are effective at doing so, a control system, no matter how well conceived and operated, can provide only reasonable, not absolute assurance that the objectives of the system are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within the Company have been detected. These inherent limitations include the realities that judgments in decision-making can be faulty, and that breakdowns can occur because of simple error or mistake. Additionally, controls can be circumvented if there exists in an individual a desire to do so. There can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions.
(b) Management Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rules 13a-15(f) and 15d-15(f) promulgated under the Exchange Act, as amended, as a process designed by, or under the supervision of, our principal executive and principal financial officer and effected by our board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles in the United States and includes those policies and procedures that:
| ● | Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect our transactions and any disposition of our assets; |
| ● | Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and |
| ● | Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements. |
| 41 |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect all misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis. Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2014. In making this assessment, our management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control-Integrated Framework. Based on this assessment, Management identified the following two material weaknesses that have caused management to conclude that, as of December 31, 2014, our disclosure controls and procedures, and our internal control over financial reporting, were not effective at the reasonable assurance level:
| 1. | We do not have written documentation of our internal control policies and procedures. Written documentation of key internal controls over financial reporting is a requirement of Section 404 of the Sarbanes-Oxley Act as of the year ending December 31, 2014. Management evaluated the impact of our failure to have written documentation of our internal controls and procedures on our assessment of our disclosure controls and procedures and has concluded that the control deficiency that resulted represented a material weakness. |
| 2. | We do not have sufficient segregation of duties within accounting functions, which is a basic internal control. Due to our size and nature, segregation of all conflicting duties may not always be possible and may not be economically feasible. However, to the extent possible, the initiation of transactions, the custody of assets and the recording of transactions should be performed by separate individuals. Management evaluated the impact of our failure to have segregation of duties on our assessment of our disclosure controls and procedures and has concluded that the control deficiency that resulted represented a material weakness. |
| 3. | Effective controls over the control environment were not maintained. Specifically, a formally adopted written code of business conduct and ethics that governs our employees, officers, and directors was not in place. Additionally, management has not developed and effectively communicated to employees its accounting policies and procedures. This has resulted in inconsistent practices. Further, our Board of Directors does not currently have any independent members and no director qualifies as an audit committee financial expert as defined in Item 407(d)(5)(ii) of Regulation S-K. Since these entity level programs have a pervasive effect across the organization, management has determined that these circumstances constitute a material weakness. |
To address these material weaknesses, management performed additional analyses and other procedures to ensure that the financial statements included herein fairly present, in all material respects, our financial position, results of operations and cash flows for the periods presented. Accordingly, we believe that the financial statements included in this report fairly present, in all material respects, our financial condition, results of operations and cash flows for the periods presented.
(c) Remediation of Material Weaknesses
To remediate the material weakness in our documentation, evaluation and testing of internal controls we plan to engage a third-party firm to assist us in remedying this material weakness once resources become available.
We intend to remedy our material weakness with regard to insufficient segregation of duties by hiring additional employees in order to segregate duties in a manner that establishes effective internal controls once resources become available.
| 42 |
(d) Changes in Internal Control over Financial Reporting
No change in our system of internal control over financial reporting occurred during the period covered by this report, the fiscal year ended December 31, 2014, that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
None
Item 10. Directors, Executive Officers and Corporate Governance
The current executive officers and directors of RX SAFES, INC. are as follows:
| Name | Age | Position | Director Since | |||
| Lorraine Yarde | 44 | President, Chief Executive Officer, Principal Executive Officer Chief Financial Officer, Principal Financial Officer, Principal Accounting Officer, Secretary & Director | June 2010 | |||
| Susan Von Kutzner | 62 | Treasurer & Director | August 2012 |
Officer/Director Experience 2008 to present:
Lorraine Yarde:
2004-2009: COO, bioMETRX, Inc. a public company that simplified state of the art fingerprint technology and incorporated it into every day consumer products. Ms. Yarde was responsible for providing strategic direction, driving revenue and securing and maintaining business relationships with major corporations. Ms. Yarde served as Chief Operating Officer and a member of the Board of Directors of bioMETRX
2008-2010: Co-Founder, Partner - Axius Healthcare Security, Inc., a company focusing on delivering products to support patient safety and loss prevention in the Healthcare arena
2009-Present: Co-Founder, Axius Consulting Group, Inc., a company that offers entrepreneurs assistance in financing and commercializing their new products and ideas.
2010-Present: Co-Founder, CEO Rx Safes, Inc... Ms. Yarde is currently responsible for all aspects of managing, guiding and operating this fledgling business including the overall direction of the business, it’s marketing, distribution and pricing strategies, product design, development and market introduction, general day to day operations, sales, marketing, and business development. Ms. Yarde has already developed strong relationships with industry partners and has been responsible for establishing all of the distribution channels and publicity to date..
2013-Present: Co-Founder, Principal Axius Entertainment Group, Inc., a company that focuses on the production of unscripted video content for television and alternative media.
Ms. Yarde received a B.A. in Business Administration from Croydon College.
Aside from that provided above, Ms. Yarde does not hold and has not held over the past five years any other directorships in any company with a class of securities registered pursuant to Section 12 of the Exchange Act or subject to the requirements of Section 15(d) of the Exchange Act or any company registered as an investment company under the Investment Company Act of 1940. Ms. Yarde’s career spans over 20 years of sales, marketing, business development and senior operational management and she has specific experience in commercializing new, innovative products through variety of channels. Specfically, Ms. Yarde has over 9 years of experience in the biometric industry focusing on integrating fingerprint technology into every day consumer products. The Board believes that Ms. Yarde has the experience, qualifications, attributes and skills necessary to serve on the Board because of her many years of experience in the biometric’s industry and her strong relationships with industry partners.
| 43 |
Susan Von Kutzner:
2009-Present: For the past five years Dr. Kutzner has been an advisor for Axius Consulting Group, Inc. and Rx Safes, Inc. which involved review of documents and recommendations for contacts to further business transactions. For instance, she profiled corporations and governmental agencies that had a specific reference to health care supplies, health care provision involving drug prescriptions which required security in the home (personal drug safe) and professional health setting (i.e. hospital, ambulatory and mobile clinics), retail drug stores (sites with pharmacies that would recommend a personal safe for prescription drug security in the home). Since August of 2012, she has served as Treasurer and Director of RX Safes, Inc.
Dr. Kutzner received her Ph.D. from the University of Michigan (Research Methodology and Clinical Research); an M.S. from the University of Pennsylvania (Clinical Care of Women); and a B.S. from University of Connecticut.
Dr. Kutzner does not hold and has not held over the past five years any other directorships in any company with a class of securities registered pursuant to Section 12 of the Exchange Act or subject to the requirements of Section 15(d) of the Exchange Act or any company registered as an investment company under the Investment Company Act of 1940. Susan Von. Kutzner, Ph.D., has 39 years of experience as an OB/ Gyn clinician & Research Scientist. The Board believes that Dr. Kutzner has the experience, qualifications, attributes and skills necessary to serve on the Board because of her valued education, familiarity with hospital practices, work ethic and strong organizational and analytical capabilities.
Term of Office
Our Directors are appointed for a one-year term to hold office until the next annual general meeting of our shareholders or until removed from office in accordance with our bylaws. Our officers are appointed by our board of directors and hold office until removed by the board, subject to their respective employment agreements.
Significant Employees
We have no significant employees other than our officers and directors.
Family Relationships
There are no family relationships between or among the directors, executive officers or persons nominated or chosen by us to become directors or executive officers.
Involvement in Certain Legal Proceedings
During the past ten years, none of the following occurred with respect to a present or former director, executive officer, or employee: (1) any bankruptcy petition filed by or against any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time; (2) any conviction in a criminal proceeding or being subject to a pending criminal proceeding (excluding traffic violations and other minor offenses); (3) being subject to any order, judgment or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining, barring, suspending or otherwise limiting his or her involvement in any type of business, securities or banking activities; and (4) being found by a court of competent jurisdiction (in a civil action), the SEC or the Commodities Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended or vacated.
| 44 |
Committees
We do not currently have an audit, compensation or nominating committee.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act requires our directors and executive officers and persons who beneficially own more than ten percent of a registered class of the Company’s equity securities to file with the SEC initial reports of ownership and reports of changes in ownership of common stock and other equity securities of the Company. Officers, directors and greater than ten percent beneficial shareholders are required by SEC regulations to furnish us with copies of all Section 16(a) forms they file. To the best of our knowledge based solely on a review of Forms 3, 4, and 5 (and any amendments thereof) received by us, the following persons have failed to file, on a timely basis, the identified reports required by Section 16(a) of the Exchange Act during fiscal year ended December 31, 2014:
| Name and principal position | Number of late reports | Transactions not timely reported | Known failures to file a required form | |||||||||
| Lorraine Yarde President, CEO and Director | 0 | 0 | 0 | |||||||||
| Susan Von Kutzner Treasurer and Director | 0 | 0 | 0 | |||||||||
Naples Trust 10% Holder | 0 | 0 | 0 | |||||||||
Code of Ethics
As of December 31, 2014, we had not adopted a Code of Ethics for Financial Executives, which would include our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions.
Item 11. Executive Compensation
The table below summarizes all compensation awarded to, earned by, or paid to our current executive officers for the fiscal years ended December 31, 2014 and 2013.
| SUMMARY COMPENSATION TABLE | ||||||||||||||||||||||||||||||||||||
| Name and principal position | Year | Salary ($) | Bonus ($) | Stock Awards ($) | Option Awards ($) | Non-Equity Incentive Plan Compensation ($) | Nonqualified Deferred Compensation Earnings ($) | All Other Compensation ($) | Total ($) | |||||||||||||||||||||||||||
| Lorraine Yarde | 2013 | - | - | - | - | - | - | 11,542 | 11,542 | |||||||||||||||||||||||||||
| President, CEO and Director | 2014 | - | - | - | - | - | - | - | - | |||||||||||||||||||||||||||
| Susan Von Kutzner | 2014 | - | - | - | - | - | - | - | - | |||||||||||||||||||||||||||
| Treasurer and Director | 2013 | - | - | - | - | - | - | - | - | |||||||||||||||||||||||||||
Narrative Disclosure to the Summary Compensation Table
Ms. Yarde received compensation through our agreement with Axius Consulting Group, Inc. Certain payments made directly on behalf of the principals of Axius, in the form of health insurance payments of $32,977 in 2013. Ms. Yarde received $11,542 of those health insurance payments, which are listed as “Other Compensation” above in the Summery Compensation Table for 2013.
| 45 |
On January 1, 2015, we entered into an employment agreement with Ms. Yarde to be our Chief Executive Officer. The following is a summary of the material terms of the agreement.
| ● | The term commences on January 1, 2015 and ends on the earlier of (i) Yarde’s death or mental or physical disability or incapacity, (ii) Yarde’s resignation or (iii) termination by us at any time. |
| ● | Ms. Yarde’s initial annual Base Salary is $175,000, with annual salary increases of no less than 10% of the prior year’s annual salary to commence on the first day after each year anniversary. |
| ● | Ms. Yarde will be eligible to earn a Performance Bonus for each complete fiscal year, which will be equal to fifty percent (50%) of her Base Salary for such fiscal year (the “Target Bonus”). The actual amount of the Performance Bonus payable to Ms. Yarde for any fiscal year may be greater than or less than the Target Bonus for such fiscal year and will be determined by the decision of the disinterested members of the board of directors based on the achievement of certain financial and individual performance goals to be established annually by the board of directors. |
| ● | We will immediately grant to Ms. Yarde an option under the Company’s 2015 Equity Incentive Plan to purchase 10,000,000 shares of our Common Stock with vesting and strike prices set forth in the agreement. |
| ● | Ms. Yarde will have the right to convert any then unpaid compensation to our stock at a 50% discount to the then market rate of our Common Stock based on the closing price of the prior days trading, in the form of registered securities (S-8 shares). |
| ● | Ms. Yarde will be entitled to participate in our health and welfare benefit programs and vacation and other benefit programs for which other employees of our company are generally eligible, subject to any eligibility requirements of such plans and programs. If we do not offer a health and welfare benefit program, Ms. Yarde will be entitled to reimbursement of expenses paid by employee for a private health and benefit program up to $1,000 each calendar month. |
| ● | Upon termination of Ms. Yarde’s employment, she may be entitled to receive certain post-termination severance benefits depending upon whether such termination is by us without Cause, in relation to a Change of Control, a resignation by Ms. Yarde for Good Reason, or by reason of Ms. Yarde’s death or disability (as such terms are defined in the agreement). In the event we terminate Ms. Yarde’s employment without Cause or Ms. Yarde elects a resignation for Good Reason, Ms. Yarde shall be entitled to receive as severance her Base Salary for a period equal to the number of complete months she has worked for us, up to a maximum of twelve (12) months. |
Outstanding Equity Awards at Fiscal Year-End
The table below summarizes all unexercised options, stock that has not vested, and equity incentive plan awards for each named executive officers as of December 31, 2014.
| OUTSTANDING EQUITY AWARDS AT FISCAL YEAR-END | ||||||||||||||||||||||||||||||||||||
| OPTION AWARDS | STOCK AWARDS | |||||||||||||||||||||||||||||||||||
| Name | Number of Securities Underlying Unexercised Options (#) Exercisable | Number of Securities Underlying Unexercised Options (#) Unexercisable | Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) | Option Exercise Price ($) | Option Expiration Date | Number of Shares or Units of Stock That Have Not Vested (#) | Market Value of Shares or Units of Stock That Have Not Vested ($) | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested (#) | |||||||||||||||||||||||||||
| Lorraine Yarde | - | - | - | - | - | - | - | - | - | |||||||||||||||||||||||||||
| Susan Von Kutzner | - | - | - | - | - | - | - | - | - | |||||||||||||||||||||||||||
| 46 |
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related
The following table presents certain information regarding beneficial Ownership of our Common Stock as of February 24, 2015, by (i) each person known by us to be the beneficial owner of more than 5% of the outstanding shares of Common Stock, (ii) each director of our company, (iii) each Named Executive Officer and (iv) all directors and executive officers as a group. Unless otherwise indicated, each person in the table has sole voting and investment power as to the shares shown. Unless otherwise indicated, the address of each beneficial owner is 170 Green Valley Parkway, Suite 300 Henderson, NV 89012.
Title of Class | Name and Address of Beneficial Owner | Shares Beneficially Owned (1) | Percent of Common Stock (2) | |||||||||
| Common | Lorraine Yarde(3) | 36,990,000 | 30.6 | % | ||||||||
| Common | Susan Von Kutzner | 3,008,000 | 2.6 | % | ||||||||
| Officers and Directors as a Group | 39,998,000 | 33.1 | % | |||||||||
| 5% Shareholders | ||||||||||||
| Common | The Naples Trust(4) 736 Carlisle Road Jericho, NY 11753 | 33,270,000 | 28.7 | % | ||||||||
| (1) | As used in this table, "beneficial ownership" means the sole or shared power to vote, or to direct the voting of, a security, or the sole or shared investment power with respect to a security (i.e., the power to dispose of, or to direct the disposition of, a security).In addition, for purposes of this table, a person is deemed, as of any date, to have "beneficial ownership" of any security that such person has the right to acquire within 60 days after such date. |
| (2) | The above table is based on current outstanding shares of 115,591,472 as of February 24, 2015. |
| (3) | Based on 31,990,000 shares of common stock held in her name and a vested option to purchase 5,000,000 shares of common stock. | |
| (4) | Maria Curcio has voting and disposition authority over the trust. |
Item 13. Certain Relationships and Related Transactions, and Director Independence
Other than described below or the transactions described under the heading “Executive Compensation” (or with respect to which such information is omitted in accordance with SEC regulations), there have not been, and there is not currently proposed, any transaction or series of similar transactions to which we were or will be a participant in which the amount involved exceeded or will exceed the lesser of $120,000 or one percent of the average of our total assets at year-end for the last two completed fiscal years, and in which any director, executive officer, holder of 5% or more of any class of our capital stock or any member of the immediate family of any of the foregoing persons had or will have a direct or indirect material interest.
Lorraine Yarde, our CEO, is the co-founder and minority shareholder with Axius Consulting Group, Inc. (“Axius”). Mr. Mark Basile, our former director, is also a co-founder and majority shareholder of Axius. Effective July 1, 2010, we entered into a consulting agreement with Axius. We engaged Axius to provide services in connection with our efforts to seek out business relationships and financing to grow our business. We agreed to pay Axius a retainer of $20,000 per month for a 12 month consecutive period. We renewed the yearlong agreement on July 1, 2011 and again on July 1, 2012. The consulting agreement was not renewed on July 1, 2013. Axius has at its sole discretion, to have the option to take payment of the accrued fee in cash or as common stock of the business, at a 50% discount to the current market value of the common stock. Certain payments made directly on behalf of the principals of Axius, in the form of health insurance payments of $32,977 in 2013. Balances included in accounts payable to Axius totaled $171,701 and $176,701 for the years ended December 31 2014, and 2013, respectively. The value of Mr. Basile’s interest in the Axius Consulting Agreement was $128,775 and Ms. Yarde’s interest was $2,926 for the year ended December 31, 2014.
| 47 |
On January 1, 2014, Lorraine Yarde executed a Master Promissory Note that allows us to borrow up to $100,000, at Ms. Yarde’s sole discretion, bearing simple interest in the amount of 4% per annum with a maturity date of December 31, 2014. The outstanding balance of the note payable as of December 31, 2014 was $55,000. Total interest accrued on the note as of December 31, 2014 was $2,050. On December 30, 2014 an extension agreement was signed extending the due date of the Note to June 30, 2015 without penalty.
On July 1, 2010, we agreed to purchase all assets of Axius for the amount of $16,262. Included in the asset purchase was inventory valued at $26,000. In connection with the sale, we agreed to purchase Aaron Kapner’s interest in the assets of Axius for the original amount invested. Mr Kapner was issued 260,000 shares of our common stock for full settlement for the purchase of his interest in Axius.
Included in the assets purchase of Axius was a Patent & Licensing Rights Agreement with bioMETRX. The agreement grants the licensee a royalty based ($.50 per unit on the sale) exclusive license under the Patent License to use, manufacture, have manufactured, license and/or sell licensed intellectual property for any legal purpose with North America within the health care and consumer health markets. The term of the agreement is from the effective date (March 1, 2009) to the full end of the term or terms for which Patent Rights have not expired or, if only Technology Rights are licensed and no Patent Rights are applicable, for a term of 8 years. No royalties have been paid to bioMETRX to date.
Effective May 1, 2013, we entered into a Master Promissory Note with Axius that allows us to borrow up to $50,000 at 4% interest that matures on December 31, 2014. The outstanding balance of the note payable was $25,274 and $34,774 at December 31, 2014 and 2013, respectively. Total interest accrued on the note was $1,895 and $604 for the years ended December 31, 2014 and 2013, respectively. On December 30, 2014 an extension agreement was signed extending the due date of the Note to June 30, 2015 without penalty.
Effective January 1, 2014 we entered into a Master Promissory Note with Naples Trust, a majority shareholder of the company, which allows us to borrow up to $100,000 at 4% interest that matures on December 31, 2014. The outstanding balance of the note payable at December 31, 2014 was $30,000. Total interest accrued on the note as of December 31, 2014 was $800. On December 30, 2014 an extension agreement was signed extending the due date of the Note to June 30, 2015 without penalty.
Item 14. Principal Accounting Fees and Services
Below is the table of Audit Fees (amounts in US$) billed by our auditor in connection with the audit of the Company’s annual financial statements for the years ended:
| Financial Statements for the Year Ended December 31 | Audit Services | Audit Related Fees | Tax Fees | Other Fees | ||||||||||||
| 2014 | $ | 21,000 | $ | 0 | $ | $ | 0 | |||||||||
| 2013 | $ | 8,000 | $ | 0 | $ | $ | 0 | |||||||||
| 48 |
Item 15. Exhibits, Financial Statements Schedules
(a) Financial Statements and Schedules
The following financial statements and schedules listed below are included in this Form 10-K.
Financial Statements (See Item 8)
(b) Exhibits
| Exhibit Number | Description | |
| 3.1 | Amended and Restated Articles of Incorporation of RX Safes, Inc. (the “Company”)1 | |
| 3.2 | Bylaws of the Company1 | |
| 10.1 | Sale of Assets, dated July 1, 20101 | |
| 10.2 | Patent and Licensing Rights Agreement2 | |
| 10.3 | Master Promissory Note3 | |
| 10.4 | Master Promissory Note3 | |
| 10.5 | Consulting Agreement4 | |
| 10.6 | Amendment No. 1 to Consulting Agreement4 | |
| 10.7 | Master Promissory Note4 | |
| 10.8 | Employment Agreement, dated January 5, 20155 | |
| 31.1** | Certification of Chief Executive Officer pursuant to Securities Exchange Act Rule 13a-14(a)/15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | |
| 31.2** | Certification of Chief Financial Officer pursuant to Securities Exchange Act Rule 13a-14(a)/15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | |
| 32.1** | Certification of Chief Executive Officer and Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | |
| 101** | The following materials from the Company’s Annual Report on Form 10-K for the year ended December 31, 2014 formatted in Extensible Business Reporting Language (XBRL). |
1 Incorporated by reference to the Form S-1, filed by the Company with the Securities and Exchange Commission on February 7, 2014.
2 Incorporated by reference to the Form S-1/A filed by the Company with the Securities and Exchange Commission on April 29, 2014.
3 Incorporated by reference to the Form S-1/A filed by the Company with the Securities and Exchange Commission on June 19, 2014.
4 Incorporated by reference to the Form S-1/A filed by the Company with the Securities and Exchange Commission on July 2, 2014.
5 Incorporated by reference to the Form 8-K, filed by the Company with the Securities and Exchange Commission on January 5, 2015.
| 49 |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| RX Safes, Inc. | ||
| By: | /s/ Lorraine Yarde | |
Lorraine Yarde Chief Executive Officer, Principal Executive Officer, Chief Financial Officer, Principal Financial Officer, Principal Accounting Officer, President, Secretary and Director |
||
| March 20, 2015 |
| By: | /s/ Susan Von Kutzner | |
| Susan Von Kutzner | ||
| Title: | Treasurer and Director | |
| Date: | March 20, 2015 |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| By: | /s/ Lorraine Yarde | |
Lorraine Yarde Chief Executive Officer, Principal Executive Officer, Chief Financial Officer, Principal Financial Officer, Principal Accounting Officer, President, Secretary and Director |
||
| March 20, 2015 |
| By: | /s/ Susan Von Kutzner | |
| Susan Von Kutzner | ||
| Title: | Treasurer and Director | |
| Date: | March 20, 2015 |
50
