Attached files
| file | filename |
|---|---|
| 8-K - 8-K - HERON THERAPEUTICS, INC. /DE/ | d894174d8k.htm |
| EX-99.1 - EX-99.1 - HERON THERAPEUTICS, INC. /DE/ | d894174dex991.htm |
Exhibit 99.2

Heron Post-Operative Pain Program: HTX-011 Phase 1 Single-Ascending-Dose Study HERON THERAPEUTICS Advancing medicine. Improving Health.

Legal 4Disclaimer This presentation contains “forward-looking statements” as defined by the Private Securities Litigation Reform Act of 1995. These forward-looking statements involve risks and uncertainties, including uncertainties associated with the development, regulatory approval, manufacture, launch and acceptance of new products, completion of clinical studies and the results thereof, the ability to establish strategic alliances and/or acquire desirable assets, progress in research and development programs and other risks and uncertainties identified in the Company’s filings with the Securities and Exchange Commission. Actual results may differ materially from the results anticipated in our forward looking statements. We caution investors that forward-looking statements reflect our analysis only on their stated date. We do not intend to update them except as required by law. 2 HERON THERAPEUTICS Advancing medicine. Improving Health.

Heron Post-Operative Pain Program Objective: Develop a best-in-class therapeutic for post-operative pain 3 HERON THERAPEUTICS Advancing medicine. Improving Health.

Heron Post-Operative Pain Program Target Product Profile: Maximal pain relief that lasts for 2-3 days Maximal reduction of opioid use Maximal reduction of length of hospital stay Elimination of dose-limiting peak of bupivacaine 4 HERON THERAPEUTICS Advancing medicine. Improving Health.

Heron Post-Operative Pain Program Introducing HTX-011: An injectable pain therapeutic that utilizes proprietary Biochronomer® polymer-based drug delivery platform technology Contains both bupivacaine (anesthetic) and meloxicam (anti-inflammatory) Designed to deliver both drugs evenly over 2-3 days without a large initial peak HTX-011 builds on other innovations in the category and has best-in-class potential 5 HERON THERAPEUTICS Advancing medicine. Improving Health.
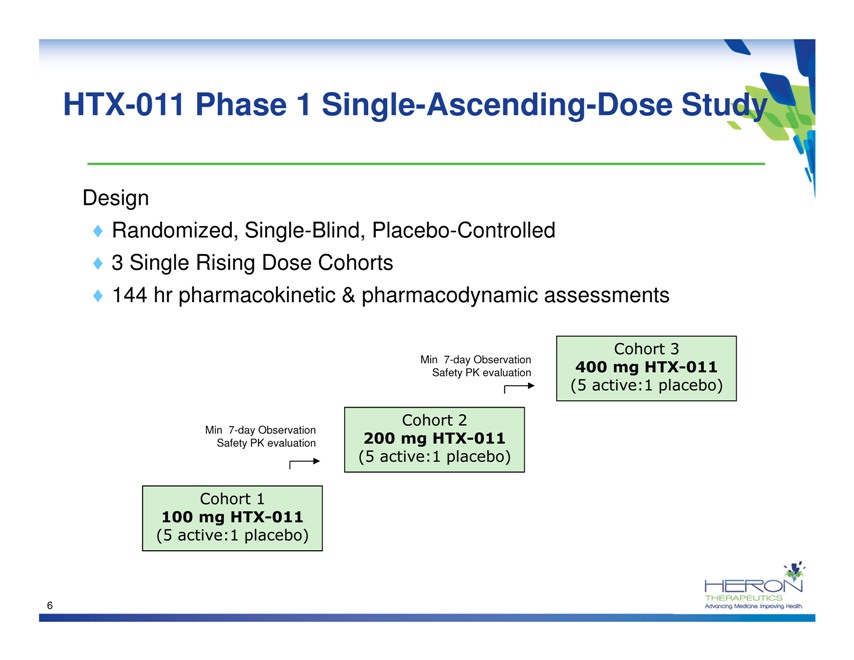
HTX-011 Phase 1 Single-Ascending-Dose Study Design Randomized, Single-Blind, Placebo-Controlled 3 Single Rising Dose Cohorts 144 hr pharmacokinetic & pharmacodynamic assessments Cohort 3 Min 7-day Observation Safety PK evaluation 400 mg HTX-011 (5 active:1 placebo) Min 7-day Observation Cohort 2 Safety PK evaluation 200 mg HTX-011 (5 active:1 placebo) Cohort 1 100 mg HTX-011 (5 active:1 placebo) 6 HERON THERAPEUTICS Advancing medicine. Improving Health.
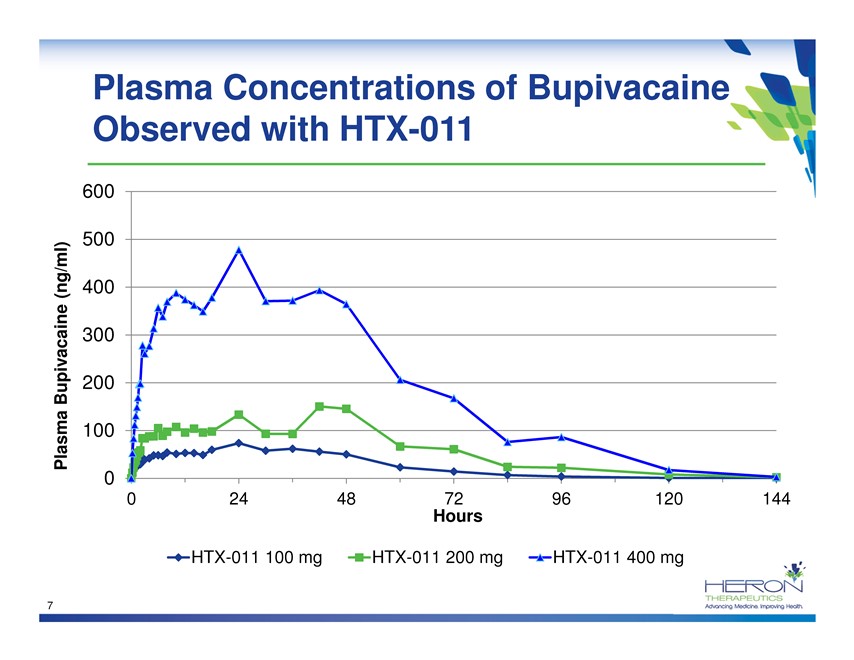
Plasma Concentrations of Bupivacaine Observed with HTX-011 600 500 (ng/ml) 400 300 Bupivacaine 200 Plasma 100 0 0 24 48 72 96 120 144 Hours HTX-011 100 mg HTX-011 200 mg HTX-011 400 mg 7 HERON THERAPEUTICS Advancing medicine. Improving Health.
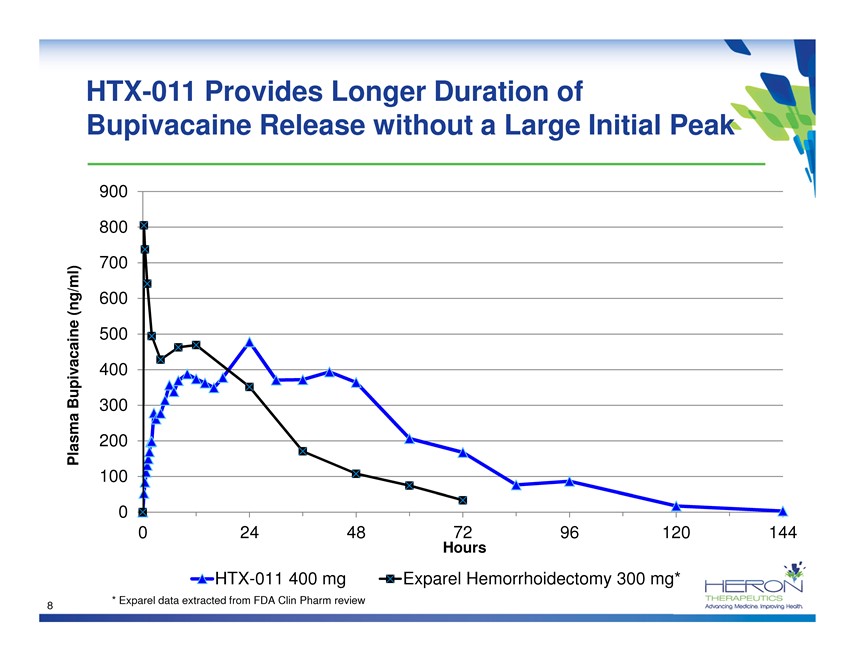
HTX-011 Provides Longer Duration of Bupivacaine Release without a Large Initial Peak 900 800 700 (ng/ml) 600 500 Bupivacaine 400 300 Plasma 200 100 0 0 24 48 72 96 120 144 Hours HTX-011 400 mg Exparel Hemorrhoidectomy 300 mg* * Exparel data extracted from FDA Clin Pharm review 8 HERON THERAPEUTICS Advancing medicine. Improving Health.
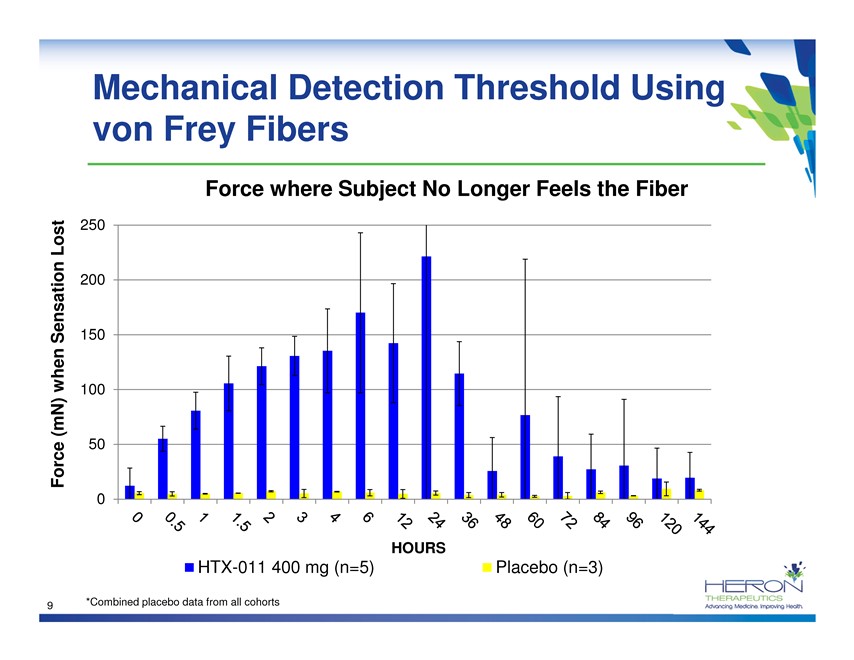
Mechanical Detection Threshold Using von Frey Fibers Force where Subject No Longer Feels the Fiber Lost 250 200 Sensation 150 when 100 (mN) Force 50 0 HOURS HTX-011 400 mg (n=5) Placebo (n=3) 9 *Combined placebo data from all cohorts HERON THERAPEUTICS Advancing medicine. Improving Health.
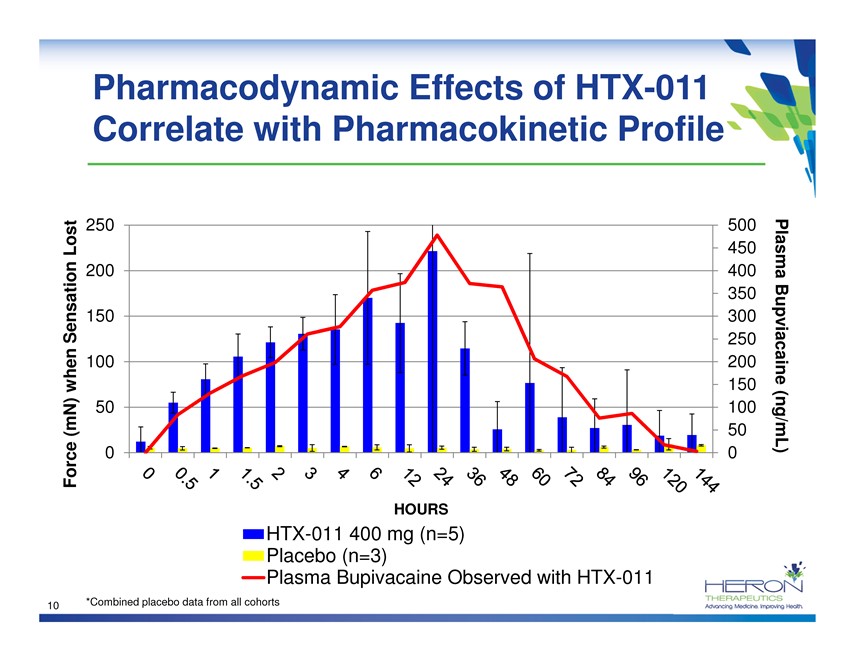
Pharmacodynamic Effects of HTX-011 Correlate with Pharmacokinetic Profile 250 500 Lost 450 200 400 Plasma 350 Sensation 150 300 250 100 200 Bupviacaine 150 when(mN) 50 100 50 (ng/mL) Force 0 0 HOURS HTX-011 400 mg (n=5) Placebo (n=3) Plasma Bupivacaine Observed with HTX-011 10 *Combined placebo data from all cohorts HERON THERAPEUTICS Advancing medicine. Improving Health.

Safety • No serious adverse events or premature discontinuations • No clinically relevant ECG changes • No clinically relevant laboratory changes • Only adverse events considered possibly related to drug were associated with the subcutaneous administration of the product: mild redness and bruising at some injection sites 11 HERON THERAPEUTICS Advancing medicine. Improving Health.
Summary • Initial Phase 1 experience validates target product profile for HTX-011 • Desired pharmacokinetic profile for both bupivacaine and meloxicam achieved • Strong pharmacodynamic activity that correlated with pharmacokinetic profile observed – Rapid on-set of action without a large initial peak – 2-3 days of stable bupivacaine plasma levels correlated to 2-3 days of anesthetic effects • All three doses were well-tolerated • Phase 1 results support immediate advancement into Phase 2 12 HERON THERAPEUTICS Advancing medicine. Improving Health.
