Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - PALISADE BIO, INC. | v404363_8-k.htm |
| EX-99.02 - EXHIBIT 99.02 - PALISADE BIO, INC. | v404363_ex99-02.htm |
Exhibit 99.01

Barclay’s Global Healthcare Conference March 12, 2015

Safe Harbor statements under the Private Securities Litigation Reform Act of 1995 : This presentation contains forward - looking statements as defined in Section 27 A of the Securities Act of 1933 as amended, and section 21 E of the Securities Exchange Act of 1934 , as amended . Such forward - looking statements are based upon Neuralstem, Inc . ’s management’s current expectations, estimates, beliefs, assumptions, and projections about Neuralstem’s business and industry . Words such as “anticipates,” “expects,” “intends,” “plans,” “predicts,” “believes,” “seeks,” “estimates,” “may,” “will,” “should,” “would,” “potential,” “continue,” and variations of these words (or negatives of these words) or similar expressions, are intended to identify forward - looking statements . In addition, any statements that refer to expectations, projections, or other characterizations of future events or circumstances, including any underlying assumptions, are forward - looking statements . These forward - looking statements are not guarantees of future performance and are subject to certain risks, uncertainties, and assumptions that are difficult to predict . Therefore, our actual results could differ materially and adversely from those expressed in any forward - looking statements as a result of various risk factors . These risks and uncertainties include the risks associated with the effect of changing economic conditions, trends in the products markets, variations in Neuralstem’s cash flow, market acceptance risks, technical development risks and other risk factors detailed in Neuralstem’s Securities and Exchange Commission filings . For links to SEC documents please visit the company’s Web site : neuralstem . com . For links to SEC documents please visit the company’s Web site : neuralstem . com . 2 Neuralstem , Inc. Safe Harbor Statement

Neuralstem Technology 3 Neuralstem's technology enables the isolation, expansion and controlled differentiation of neural stem cells into mature, physiologically relevant human neurons and glial cells. Spinal Cord Hippocampus GABAergic Cholingergic Midbrain Dopaminergic • Discovered at NIH • Regionally specific CNS neural stem cells : brain and spinal cord • Fully characterized: • Expanded under defined conditions: no animal - derived reagents, serum or feeder cells • Reproducible differentiation: constitutive behavior of cells • FDA cGMP manufacturing • Worldwide patents

Neuralstem Overview Midbrain Neuralstem’s proprietary dual platform technology uses regionally specific neural stem cells for the development of CNS cell therapies and small molecule drug discovery. Neuralstem’s proprietary platform technology Transplantation Cell Therapies NSI - 566 Cell transplantation: Spinal Cord Delivery Platform Lead Indication: ALS Drug Screening Small Molecules NSI - 189 Novel neurogenic MOA, oral formulation Lead Indication: Major Depressive Disorder 4

Neuralstem Pipeline 5 One Technology, Two Neurogenic Platforms, Multiple Indications Compound / Indication Preclinical Phase 1 Phase 1b Phase 2 Phase 2b Data Small Molecule NSI-189 US Major Depression Disorder P2 start 2Q15 NSI-189 US Cognitive Deficit in Schizoprenia PIb start mid15 Cell Therapy NSI-566 US Amyotrophic Lateral Sclerosis Controlled P2 start 2015 NSI-566 US Chronic Spinal Cord Injury P1b data 4Q15 NSI-566 China Ischemic Stroke P2 start 2015 NSI-566 South Korea Acute Spinal Cord Injury Awaiting IND approval NSI-523.IGF Alzheimer's Disease Efficacy in animal data
Human Proof of Concept Safe and tolerable: • Completed 49 cell therapy transplantations world wide • No toxicity Biological Activity: • Definitive evidence of long - term cell survival via DNA fingerprinting • Synaptic integration – nursing, protecting and “bridging the gaps” Three ongoing clinical trials: • Amyotrophic Lateral Sclerosis (ALS): Phase 2 • Chronic Spinal Cord Injury ( cSCI ): Phase Ib • Ischemic Stroke: Phase I/II NSI - 566: Physiologically relevant human neural stem cells 6 Cell Therapy

NSI - 566/ALS Phase I Long Term ALSFRS - R Data 7 Phase Ib Patient Follow - up: 1200 day efficacy data • Patients 10, 11, 12, received ten lumbar and five cervical injections of 100,000 cells each • Safe and well tolerated; Average hospital stay 3 - 4 days • Proof of “Activity” – cells provide neurotrophic support acting as “nurse cells” in motor neuron pools in the spinal cord • L ong term cell survival in 6 autopsy patients Annals Of Clinical And Translational Neurology, Glass et.al

NSI - 566 Lead Program: Amyotrophic Lateral Sclerosis 8 Phase II ALS 9 M onth Data Primary Endpoints x NSI - 566 cells and surgery are well tolerated x Maximum tolerate dose: 400,000 cells per injection, 20 injections for each lumbar and cervical surgery Secondary Endpoints Investigating ALSFRS - R and subset muscle groups correlated to injection sites • ALSFRS - R: rating scale of 12 functional activities • H and grip (bilateral): lower arm muscular strength • Seated vital capacity: breathing Goals • Identify likely responders and refine patient population • Increase confidence of providing a meaningful therapeutic benefit
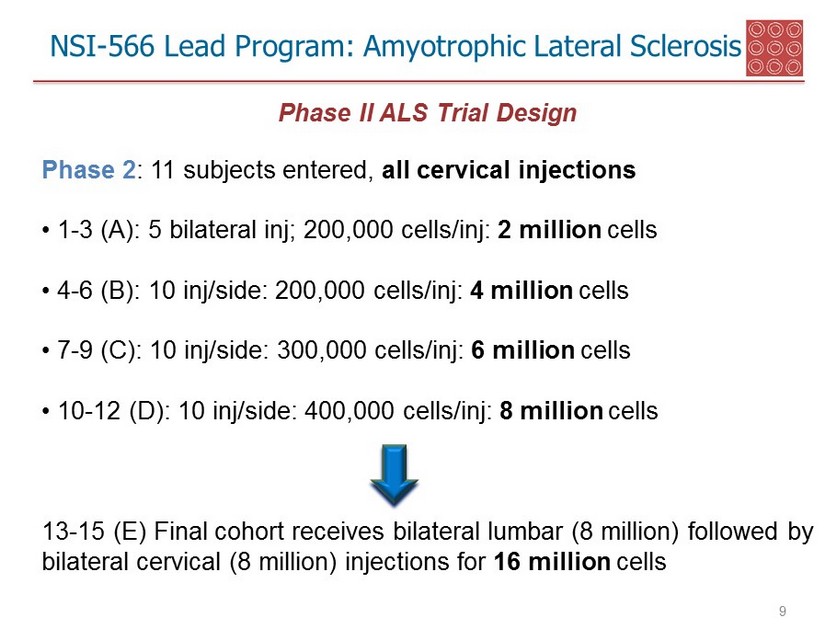
NSI - 566 Lead Program: Amyotrophic Lateral Sclerosis 9 Phase II ALS Trial Design Phase 2 : 11 subjects entered, all cervical injections • 1 - 3 (A): 5 bilateral inj ; 200,000 cells/ inj : 2 million cells • 4 - 6 (B): 10 inj /side: 200,000 cells/ inj : 4 million cells • 7 - 9 (C): 10 inj /side: 300,000 cells/ inj : 6 million cells • 10 - 12 (D): 10 inj /side: 400,000 cells/ inj : 8 million cells 13 - 15 (E) Final cohort receives bilateral lumbar (8 million) followed by bilateral cervical (8 million) injections for 16 million cells

ALS Phase II 9 month data 10 Statistically significant between responders and non - responders - 47 % response rate to the stem cell treatment Responder measurements • ALSFRS: 7 of 15 patients had a near - zero slope of decline or positive slope • Grip Strength : 7 of 15 patients had a near - zero decline, or positive strengthening
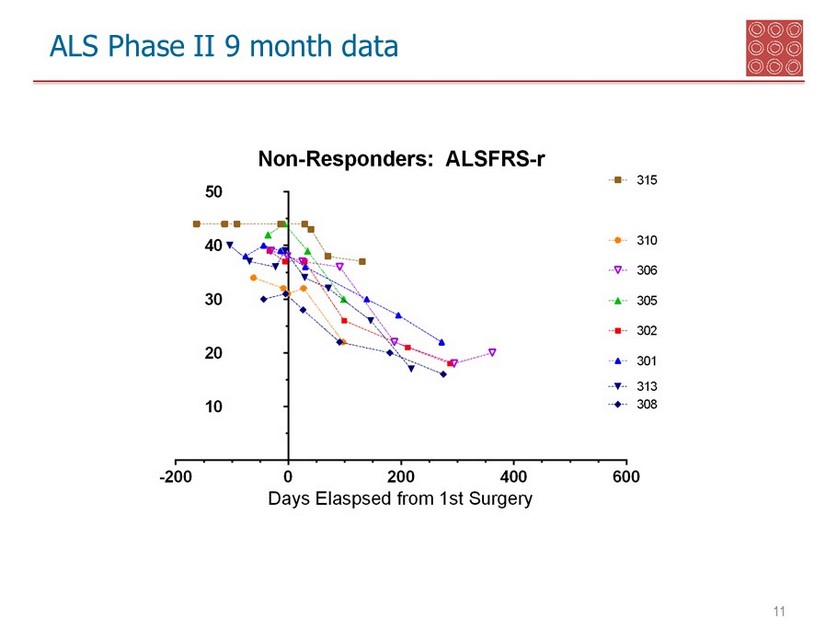
ALS Phase II 9 month data 11

ALS Phase II 9 month data 12 • Responders ’ disease progression was - 0.007 point per day • N on - responders ’ disease progression was - 0.1 point per day • Measurement : an average slope of decline of ALSFRS Statistical Significant Reduction in ALSFRS Slope
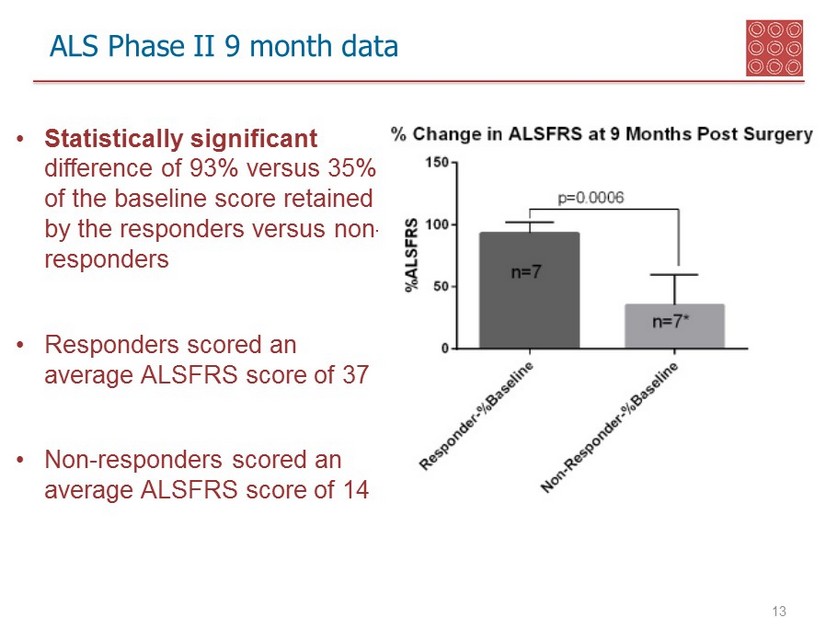
ALS Phase II 9 month data 13 • Statistically significant difference of 93% versus 35% of the baseline score retained by the responders versus non - responders • Responders scored an average ALSFRS score of 37 • Non - responders scored an average ALSFRS score of 14

ALS Phase II 9 month data 14 Seated Vital Capacity : responders remained within 94% of scores vs. 71% for non responders

ALS Phase II Control study 15 ALS Phase II Results • NSI - 566 cells and surgery are well tolerated • Identified max tolerated dose • 47% responder rates • Predictive responder profile ALS Phase II Control Study • ~ 50 patients • 9 month follow - up • L umbar and cervical injections, total 16 million cells • Enhanced screening protocol • Refined responder patient population • Multiple endpoints including ALSFRS, grip strength, vital capacity

Neurogenic Small Molecule Platform 16 NSI - 189: First - in - class Neurogenic Small Molecule Drug, Reversing Hippocampal Atrophy Screening human hippocampal s tem c ells Biological Activity » increase in synaptic connection » neurogenesis in the human hippocampus Major Depressive Disorder (MDD) • Phase II commencement 2Q15: ~150 patients, 20 centers, 90 day dosing, 6 month follow - up Cognitive Deficit in Schizophrenia • NSI - 189 Pipeline Expansion • Phase Ib commencement: mid 2015

Stages of Neurogenesis 17

Screening Path 18 10,269 small molecule compounds primary screen 16 neurogenic compounds in vitro 16 tested in acute toxicity in mice 15 tested for neurogenesis in healthy, adult mice 7 orally active neurogenic leads 1 development candidate selection NSI - 189

• SDQ combined treatment group showed statistically significant improvement (p=0.02) • Clinically meaningful reduction in depressive & cognitive symptoms (40mg, 80mg) • P ersistent efficacy over the non - dosing 8 - week follow - up period p=0.02 d=0.90 Study Day -20 0 20 40 60 80 100 Symptoms of Depression Questionnaire 2.0 2.2 2.4 2.6 2.8 3.0 3.2 3.4 3.6 3.8 Placebo NS-189 NS-189 1x per day NS-189 2x per day NS-189 3x per day p=0.03 d=1.10 Day 84 Day 28 Symptoms of Depression Questionnaire (SDQ) p=0.09 d=0.95 Study Day 0 20 40 60 80 100 Montgomery and Asberg Depression Rating Scale 5 10 15 20 25 30 Placebo NS-189 NS-189 1x per day NS-189 2x per day NS-189 3x per day p=0.19 d=0.84 Montgomery - Asberg Depression Rating Scale (MADRS) Day 28 Day 84 NSI - 189 MDD Phase Ib Clinically Meaningful Results 19

p=0.01 d=0.94 Study Day -20 0 20 40 60 80 100 Cognitive and Physical Functioning Questionnaire 2.0 2.5 3.0 3.5 4.0 4.5 5.0 Placebo NS-189 NS-189 1x per day NS-189 2x per day NS-189 3x per day p<0.01 d=1.20 • Significant effect size in cognitive function improvement; improvement persisted over 8 - week follow up period • NSI - 189 CPFQ was significantly better than the placebo group (p=0.01) at Day 28; Large effect size of 0.94 Cognitive and Physical Functioning Questionnaire (CPFQ) Day 28 Day 84 NSI - 189 MDD Phase Ib Clinically Meaningful Results All: A Phase 1B, Randomized, Double - Blind, Placebo - Controlled, Multiple - Dose Escalation Study Evaluating the Effects of NSI - 189 Phosp hate, a Neurogenic Compound, in Patients with Major Depressive Disorder (MDD) , presented June 2014, by Maurizio Fava, M.D., Karl Johe , Ph.D., Lev G. Gertsik , MD, Larry Ereshefsky , PharmD , Bettina Hoeppner , Ph.D., Martina Flynn, David Mischoulon , M.D., Ph.D., Gustavo Kinrys , M.D., and Marlene Freeman, M.D 20

NSI - 189 MDD Phase Ib Biomarkers Quantitative EEG ( qEEG ) measurements, electrophysical biomarker : • Day 28 statistical significance • Significantly increased brain wave patterns in the hippocampal region 10 Plasma biomarkers : • Identified a rapid response • C onsistent with therapeutic effects shown by the traditional clinical measures • (A1AT , Apo C3, BDNF, Cortisol, EGF, MPO, Prolactin , Resistin , sTNFR2 and TSH) High - frequency alpha at Day 28: 10 - 12 Hz 21 A Phase 1B, Randomized, Double - Blind, Placebo - Controlled, Multiple - Dose Escalation Study Evaluating the Effects of NSI - 189 Phosp hate, a Neurogenic Compound, in Patients with Major Depressive Disorder (MDD) , presented June 2014, by Maurizio Fava, M.D., Karl Johe , Ph.D., Lev G. Gertsik , MD, Larry Ereshefsky , PharmD , Bettina Hoeppner , Ph.D., Martina Flynn, David Mischoulon , M.D., Ph.D., Gustavo Kinrys , M.D., and Marlene Freeman

NSI - 189 Program Expansion • Phase Ib to commence mid 2015 • Hippocampal atrophy well documented in this patient population • Psychotic and hallucinations are distinct from cognitive deficit • High unmet patient population; limited therapies available • 3.5 million (1%) of Americans diagnosed 22 NSI - 189 for the treatment of cognitive deficit in schizophrenia The National of Mental Health (NIMH)

Neuralstem Timeline 23 NSI - 189 neurogenic small molecule platform Phase II MDD commence in 2Q15 Phase Ib full data; Dr. Maurizio Fava publication Phase Ib cognitive deficit in schizophrenia commence mid2015 NSI - 566 cell therapy platform Phase II ALS full data 1H15 Phase II ALS expected commence mid 2015 Phase Ib cSCI currently enrolling, data 4Q15 Phase I/II ischemic stroke expect to roll over into Phase II 2015
