Attached files
| file | filename |
|---|---|
| 8-K - 8-K - DELCATH SYSTEMS, INC. | form8k.htm |
Exhibit 99.1

Investor Presentation
(NASDAQ: DCTH)
(NASDAQ: DCTH)
March 2015

2 DELCATH SYSTEMS, INC
Forward-looking Statements
This presentation contains forward-looking statements, within the meaning of the federal securities laws, related to future
events and future financial performance which include statements about our expectations, beliefs, plans, objectives,
intentions, goals, strategies, assumptions and other statements that are not historical facts. Forward-looking statements
are subject to known and unknown risks and uncertainties and are based on potentially inaccurate assumptions, which
could cause actual results to differ materially from expected results, performance or achievements expressed or implied
by statements made herein. Our actual results could differ materially from those anticipated in forward-looking statements
for many reasons, including, but not limited to, uncertainties relating to: the timing and results of future clinical trials
including without limitation the OM, HCC, ICC, and mCRC trials in the Company’s Clinical Development Program, clinical
adoption, use and resulting sales, if any, for the CHEMOSAT system in Europe, our ability to obtain reimbursement for
the CHEMOSAT system in various markets including without limitation Germany and the United Kingdom, our ability to
successfully commercialize the Melphalan/HDS system and the potential of the Melphalan/HDS system as a treatment for
patients with primary and metastatic disease in the liver, the Company's ability to satisfy the requirements of the FDA's
Complete Response Letter relating to the ocular melanoma indication and the timing of the same, approval of the
Melphalan/HDS system by the U.S. FDA, acceptance of the Phase 3 trial publication, the impact of the presentations at
ESSO and SSO and future clinical results consistent with the data presented, approval of the current or future
Melphalan/HDS system for delivery and filtration of melphalan or other chemotherapeutic agents for various indications in
the U.S. and/or in foreign markets, actions by the FDA or other foreign regulatory agencies, our ability to successfully
enter into strategic partnership and distribution arrangements in foreign markets and the timing and revenue, if any, of the
same, uncertainties relating to the timing and results of research and development projects, and uncertainties regarding
our ability to obtain financial and other resources for any clinical trials, research, development, and commercialization
activities. These factors, and others, are discussed from time to time in our filings with the Securities and Exchange
Commission including the section entitled ‘‘Risk Factors’’ in our most recent Annual Report on Form 10-K and our Reports
on Form 10-Q and Form 8-K.
events and future financial performance which include statements about our expectations, beliefs, plans, objectives,
intentions, goals, strategies, assumptions and other statements that are not historical facts. Forward-looking statements
are subject to known and unknown risks and uncertainties and are based on potentially inaccurate assumptions, which
could cause actual results to differ materially from expected results, performance or achievements expressed or implied
by statements made herein. Our actual results could differ materially from those anticipated in forward-looking statements
for many reasons, including, but not limited to, uncertainties relating to: the timing and results of future clinical trials
including without limitation the OM, HCC, ICC, and mCRC trials in the Company’s Clinical Development Program, clinical
adoption, use and resulting sales, if any, for the CHEMOSAT system in Europe, our ability to obtain reimbursement for
the CHEMOSAT system in various markets including without limitation Germany and the United Kingdom, our ability to
successfully commercialize the Melphalan/HDS system and the potential of the Melphalan/HDS system as a treatment for
patients with primary and metastatic disease in the liver, the Company's ability to satisfy the requirements of the FDA's
Complete Response Letter relating to the ocular melanoma indication and the timing of the same, approval of the
Melphalan/HDS system by the U.S. FDA, acceptance of the Phase 3 trial publication, the impact of the presentations at
ESSO and SSO and future clinical results consistent with the data presented, approval of the current or future
Melphalan/HDS system for delivery and filtration of melphalan or other chemotherapeutic agents for various indications in
the U.S. and/or in foreign markets, actions by the FDA or other foreign regulatory agencies, our ability to successfully
enter into strategic partnership and distribution arrangements in foreign markets and the timing and revenue, if any, of the
same, uncertainties relating to the timing and results of research and development projects, and uncertainties regarding
our ability to obtain financial and other resources for any clinical trials, research, development, and commercialization
activities. These factors, and others, are discussed from time to time in our filings with the Securities and Exchange
Commission including the section entitled ‘‘Risk Factors’’ in our most recent Annual Report on Form 10-K and our Reports
on Form 10-Q and Form 8-K.

3 DELCATH SYSTEMS, INC
About Delcath Systems
• A specialty pharmaceutical and medical device oncology company with a
principal therapeutic focus on the treatment of primary liver cancer and other
cancers that have metastasized to the liver
principal therapeutic focus on the treatment of primary liver cancer and other
cancers that have metastasized to the liver
• Proprietary system isolates the liver from circulation, delivers a substantially
higher concentration of chemotherapy (melphalan) compared with systemic
doses, and filters most of the chemotherapy out of the blood prior to returning
it to the patient to reduce myleosuppressive side effects
higher concentration of chemotherapy (melphalan) compared with systemic
doses, and filters most of the chemotherapy out of the blood prior to returning
it to the patient to reduce myleosuppressive side effects
• In late-stage clinical development in the U.S. with initial commercial activities
underway in Europe
underway in Europe
• Initially pursuing orphan indications in metastatic ocular melanoma,
hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma
(ICC)
hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma
(ICC)
Seeking to Make a Clinically Meaningful Difference For Cancer Patients With Liver Dominant Disease

DELCATH SYSTEMS, INC
Investment Highlights
Believe We are Positioned to Capitalize on Large, Compelling Market Opportunity
• Late-stage asset – demonstrated clinically meaningful efficacy in more than
600 procedures and multiple tumor types
600 procedures and multiple tumor types
• Active clinical program – initiating global Phase 3 study in ocular
melanoma; HCC/ICC Phase 2 program ongoing
melanoma; HCC/ICC Phase 2 program ongoing
• Unique, highly differentiated solution – orphan designations create
barriers to entry
barriers to entry
• Large market opportunity – cancers of the liver remain a multibillion-dollar
unmet medical need; early commercial activity EU
unmet medical need; early commercial activity EU
• Imminent valuation milestones – 2015 value drivers include publications,
reimbursement and clinical data
reimbursement and clinical data
• Attractive business model – initial orphan focus and anticipated high gross
margins form basis of profitable long-term model
margins form basis of profitable long-term model
• Experienced management team – aligned with requirements of clinically
driven pharmaceutical industry
driven pharmaceutical industry

5 DELCATH SYSTEMS, INC
2014-2015 Milestones
2014 Accomplishments
ü Phase 2 HCC trial open and first patient treated
ü 100th patient treated in Europe (commercial and clinical)
ü Positive efficacy data from three institutions presented at ESSO 2014
ü Product revenue increased 118% Y/Y to $1.1 million
ü Cash burn reduced by 50% Y/Y
1H-2015
ü NUB reimbursement decision in Germany – Value 4 awarded for 2015
ü Submit Phase 3 metastatic melanoma trial results for publication
o EU registry open for enrollment
o ICC cohort open for enrollment
2H-2015
o Initiation of Phase 3 metastatic ocular melanoma program
o Interim evaluation on HCC/ICC program
Executing on Multiple Fronts to Create Value

6 DELCATH SYSTEMS, INC
The Liver: A Life-Limiting Organ
• Cancers of the liver remain a major unmet medical need
o Large global patient population – approximately 1.2 million* patients
diagnosed annually with primary or metastatic liver cancer
diagnosed annually with primary or metastatic liver cancer
o The liver is often the life-limiting organ for cancer patients and one of the
leading causes of cancer death
leading causes of cancer death
o Prognosis after liver involvement is poor, with overall survival generally
less than 12 months
less than 12 months
• CHEMOSAT® Melphalan/HDS is a proprietary product
uniquely positioned to potentially treat the entire liver as a
standalone or complementary therapy
uniquely positioned to potentially treat the entire liver as a
standalone or complementary therapy
* SOURCE - 2008 GLOBOCAN
Effective Liver Cancer Treatment Remains a Major Unmet Medical Need

7 DELCATH SYSTEMS, INC
Existing Liver Cancer Treatments Landscape
Existing Liver Cancer Treatments Have Limitations
|
Treatment
|
Advantages
|
Disadvantages
|
|
Systemic
|
– Non-invasive
– Repeatable
|
– Systemic toxicities
– Limited efficacy in liver
|
|
Regional
(e.g., Isolated Hepatic Perfusion)
|
– Therapeutic effect
– Targeted
|
– Invasive/limited repeatability
– Multiple treatments are
required but not possible |
|
Focal
(e.g., surgery, radioembolization,
chemoembolization, radiofrequency ablation) |
– Partial removal or
treatment of tumors |
– Only 10%-20% are resectable
– Invasive and/or limited
repeatability – Treatment is limited by tumor
size, number of lesions and location – Tumor revascularization
– Cannot treat diffuse disease
|

8 DELCATH SYSTEMS, INC
Concentrating the Power of Chemotherapy for Disease Control in the Liver
Our Solution: Whole Organ-Focus Disease Control
• Our proprietary system isolates the liver circulation, delivers a
high concentration of chemotherapy (melphalan) and filters
most of the chemotherapy out of the blood prior to returning it
to the patient
high concentration of chemotherapy (melphalan) and filters
most of the chemotherapy out of the blood prior to returning it
to the patient
• The procedure typically takes approximately 2-3 hours to
complete and involves a team including the interventional
radiologist and perfusionist
complete and involves a team including the interventional
radiologist and perfusionist
• We believe more than 180 treatments with improved device
and procedure in the U.S. and EU provide confidence safety
can be validated in a controlled setting
and procedure in the U.S. and EU provide confidence safety
can be validated in a controlled setting
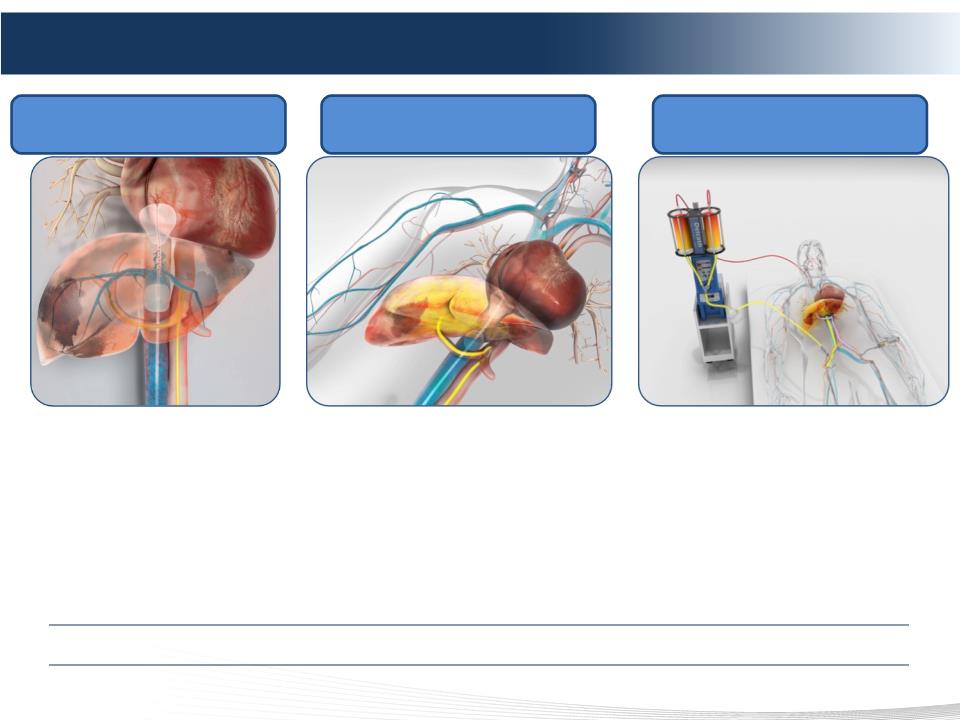
9 DELCATH SYSTEMS, INC
The Melphalan Hepatic Delivery System (HDS)
• Device designed to administer high-dose chemotherapy to the liver while reducing
systemic exposure
systemic exposure
• Marketed as Delcath Hepatic CHEMOSAT® Delivery System (device only) in EU
• Investigational drug/device combination product regulated as a drug in the U.S.
Liver Isolated Via Double Balloon
Catheter In IVC
Catheter In IVC
Melphalan Infused Directly Into Liver
Via Catheter In Hepatic Artery
Via Catheter In Hepatic Artery
Blood Exiting The Liver Filtered By
Proprietary Extra-corporeal Filters
Proprietary Extra-corporeal Filters
More Than 300 Patients Treated To Date

10 DELCATH SYSTEMS, INC
Melphalan Dosing & Background
• Well-understood, dose-dependent, tumor-preferential, alkylating cytotoxic agent
that demonstrates little to no hepatic toxicity
that demonstrates little to no hepatic toxicity
• Dose administered directly to liver is substantially higher than that of systemic IV
chemotherapy
chemotherapy
• Melphalan, an established chemotherapy agent, is proven active at high doses
with broad antitumor activity
with broad antitumor activity
|
Type
|
Dosing (mg/kg)
|
|
Multiple Myeloma (label)
|
0.25
|
|
Chemoembolization
|
0.62
|
|
Surgical Isolated Hepatic Perfusion (IHP)
|
1.50
|
|
Myeloablation
|
2.50-3.50
|
|
Chemosaturation (PHP)
|
3.00
|
An Established Drug for Liver Cancer Therapy

11 DELCATH SYSTEMS, INC
Total Available EU & U.S. Market Opportunity
Effective Liver Cancer Treatment Remains a Major Unmet Medical Need
Notes:
1) Source: Globocan, American Cancer Society
2) Source: LEK, Strategy&, Company estimates
3) Assumes an average of two treatments per patient
|
Cancer Type
|
Annual Incidence1
|
Eligible Pts2
|
Revenue per Patient3
|
Annual Potential
Market Opportunity (millions) |
|
Ocular Melanoma (OM)
|
5,700-8,600
|
2,600-4,300
|
$40,000-$50,000
|
$104-$215
|
|
Cholangio Carcinoma (ICC)
|
11,500
|
6,500
|
$40,000-$50,000
|
$260-$330
|
|
Hepatocellular Carcinoma (HCC)
|
64,500
|
7,600-14,700
|
$40,000-$50,000
|
$304-$735
|
|
Colorectal (CRC)
|
411,000
|
40,000-55,000
|
$40,000-$50,000
|
$1,600-$2,750
|
|
Total EU and US
|
492,700-495,600
|
56,700-80,500
|
|
$2,268-$4,030
|

12 DELCATH SYSTEMS, INC
Clinically Differentiated Results
• Phase 1, 2 and 3 trials produced positive results in multiple histologies
• Melanoma Liver Mets
o Positive Phase 3 results in hepatic metastatic melanoma
o n=93 (90% ocular melanoma, 10% cutaneous melanoma)
• Neuroendocrine Tumor (NET) Liver Mets
o mNET cohort in Phase 2 trial showed encouraging 42% objective response rate
(ORR) vs. ~10% for approved targeted therapy
(ORR) vs. ~10% for approved targeted therapy
o Median overall survival of ~32 months on ITT basis
• Hepatocellular Carcinoma (HCC)
o Positive signal with high-dose melphalan in HCC cohort of Phase 2 trial (5/8 patients)
is encouraging when approved systemic therapies have modest efficacy and
challenges with tolerability
is encouraging when approved systemic therapies have modest efficacy and
challenges with tolerability
• Colorectal Cancer (CRC) Liver Mets
o Data from surgical Isolated Hepatic Perfusion (IHP) with melphalan indicates strong
potential in well-defined patient population with earlier stage CRC yielding ~50-60%
median response rate and median OS of 17.4-24.8 mos
potential in well-defined patient population with earlier stage CRC yielding ~50-60%
median response rate and median OS of 17.4-24.8 mos
Encouraging Initial Results on a Broad Range of Histologies

13 DELCATH SYSTEMS, INC
Clinical Development Program Overview
• Initiating global Phase 3 trial 2H 2015 in Ocular Melanoma
(OM) Liver Mets
(OM) Liver Mets
• Establish proof-of-concept in Hepatocellular Carcinoma
(HCC) and Intrahepatic Cholangiocarcinoma (ICC)
(HCC) and Intrahepatic Cholangiocarcinoma (ICC)
o Commenced global Phase 2 program in HCC in 2014
o Expanding program to include ICC cohort in EU trial
• Initiating EU registry to collect efficacy and safety data in
the commercial setting
the commercial setting
• Supporting Investigator Initiated Trials in HCC and in
mCRC
mCRC
Focused on Liver Dominant, Orphan Diseases With High Unmet Need

14 DELCATH SYSTEMS, INC
Clinical Development Program Detail
|
Trials
|
Tumor
|
Objectives
|
|
Phase 3 Pivotal Trial
|
OM liver mets
|
§ Global Phase 3 trial to start 2H-2015
§ Primary endpoint: Overall Survival (OS)
§ Believed to be fastest pathway to NDA approval in the U.S.
|
|
Phase 2 Trial
|
HCC
(unresectable
confined to the liver) |
§ Protocol 201 (U.S. only)
§ Safety, efficacy of melphalan/HDS treatment followed by sorafenib
§ Evaluate ORR (mRECIST)
§ Assess safety, PFS
§ Characterize systemic exposure of melphalan
§ Assess patient QoL
|
|
§ Protocol 202 (EU only)
§ Safety, efficacy of melphalan/HDS treatment w/o sorafenib in patients with
unresectable liver cancer § Evaluate ORR (mRECIST)
§ Assess safety, PFS
§ Characterize systemic exposure of melphalan
§ Assess patient QoL
|
||
|
Phase 2 Cohort
|
ICC
(unresectable
confined to the liver) |
§ To be added to 202 HCC trial protocol
§ ORR of melphalan/HDS treatment in patients with intra‑hepatic
cholangiocarcinoma (ICC) § Other measures as specified in the 202 EU protocol
§ Signal-seeking go/no-go decision 2H - 2015
|
|
Investigator Initiated
Trials |
mCRC
|
§ University of Leiden study; ~6 patients treated to date
|
|
HCC
|
§ Johannes Wolfgang Goethe University Hospital (Frankfurt) study; different patient
selection from 202 study; open for enrollment |
|
|
EU Commercial
Registry |
EU Commercial
Cases |
§ Data collection on safety, QoL assessments
§ Potential efficacy signals in additional tumor types
§ Support reimbursement in key markets
|

15 DELCATH SYSTEMS, INC
OM Metastases Rationale
• OM has high incidence of liver metastases
o Up to 90% of patients with metastases will have liver involvement
o Life expectancy of approximately 6 months
o 5,700 - 8,600 cases of OM liver metastases diagnosed in U.S. and EU
annually
annually
5,700-8,600
Cases of Ocular
Melanoma
Melanoma
~50-55%
Metastasize
~90%
Show Liver Mets
~2,600 - 4,300
Eligible Patients
Limited TX Options
U.S./EU Market Size
*Sources: ACS, SEER, NIH, OMF, KOL Interviews, 2014 3rd Party
Analysis
Analysis
Proven Efficacy in Attractive Orphan Opportunity
• Clear efficacy signal seen in prior Phase 3 trial of melphalan/HDS
• Currently no standard of care
• Believed to be fastest pathway to NDA approval in the U.S.
• FDA granted melphalan/HDS orphan drug status for treatment of OM

15 DELCATH SYSTEMS, INC
Intent-to-Treat Analysis (June 2012)
§ 5.3 mos improvement in hPFS
§ Hazard ratio = 0.50
§ (95% CI 0.31-0.80)
§ p=0.0029
Months
7.0
1.7
1.0
1.0
0.8
0.8
0.6
0.6
0.4
0.4
0.2
0.2
0.0
0.0
Proportion of patients surviving
5.3 mo
Chemosaturation (CS-PHP)
Previous Ocular Melanoma Mets Phase 3 Results
0
0
5
5
10
10
15
15
20
20
25
25
30
30
Best alternative care (BAC)
Hepatic Progression Free Survival (hPFS)
Chemosaturation (CS-PHP)
§ 3.8 mos improvement in PFS
§ Hazard ratio = 0.42
§ (95% CI 0.27-0.64)
§ p<0.0001
Overall Progression Free Survival (Investigator)
Proportion of patients surviving
Months
5.4
1.6
1.0
1.0
0.8
0.8
0.6
0.6
0.4
0.4
0.2
0.2
0.0
0.0
3.8 mo
Intent-to-Treat Analysis (June 2012)
Best alternative care (BAC)
0
0
5
5
10
10
15
15
20
20
25
25
30
30
35
35
40
40
45
45
50
50
55
55
Clinically Meaningful Benefit Previously Demonstrated for Metastatic OM Patients
4 subjects remain
alive at 5-8 years
alive at 5-8 years

17 DELCATH SYSTEMS, INC
ICC Rationale
• Significant market opportunity in U.S. and EU
o ~15% of new HCC cases diagnosed annually
o ~90% of patients are not suitable for surgical resection
o ~20-30% are candidates for focal interventions
*Sources: ACS, SEER, NIH, KOL Interviews, 2014 3rd Party Analysis
Encouraging Early Commerical Activity in Disease With Limited Treatment Options
76,000
HCC Diagnosis
~90%
Unresectable
~6,500
Eligible Patients
U.S./EU Market Size
~15%
Of HCC
Cases
Cases
~20-30%
Focal
Interventions
Interventions
• Unmet medical need – Delcath will pursue a melphalan orphan drug
designation from the FDA for patients with ICC
designation from the FDA for patients with ICC
o Efficacy signals from early commercial uses in EU
18 DELCATH SYSTEMS, INC
HCC Rationale
• Significant opportunity in the U.S. and EU
o HCC most common primary cancer of the liver
o ~76,000* cases diagnosed annually
• Large unmet medical need in first-line therapy
76,000
Cases of Primary
Liver Cancer
Liver Cancer
~90%
HCC
~80-90%
Unresectable
~20-30%
Child Pugh Class A
~7,600 - 14,700
Eligible Patients
~20-30%
Interventional
TX
TX
U.S./EU Market Size
*Sources: WHO, KOL Interviews, 2014 3rd Party Analysis
Large, Deadly Disease in Need of Better Treatments
o Only one approved systemic therapy in the U.S., EU and certain Asian
markets
markets
o ~90% of patients not candidates for surgical resection
o 20-30% of patients are candidates for focal interventions
• FDA granted melphalan/HDS orphan drug status for treatment of
unresectable HCC
unresectable HCC

19 DELCATH SYSTEMS, INC
Prior FDA Experience
• New Drug Application (NDA) submitted August 2012 seeking
indication in OM liver metastases with first-generation filters
and procedure
indication in OM liver metastases with first-generation filters
and procedure
• ODAC meeting in May 2013
o Efficacy shown with statistical significance
o Negative vote due to benefit/risk analysis
o Complete FDA & Delcath ODAC briefing materials available at
www.delcath.com/clinical-research/clinical-bibliography/
www.delcath.com/clinical-research/clinical-bibliography/
• Complete Response Letter (CRL) issued September 2013
• FDA requests include, but not limited to:
o Well-controlled randomized trial(s) to establish the safety and
efficacy using the to-be-marketed device configuration
efficacy using the to-be-marketed device configuration
o Overall survival as the primary efficacy outcome measure
o Demonstrate clinical benefits outweigh risks
FDA Learnings Provide Beneficial Clinical Study Roadmap

20 DELCATH SYSTEMS, INC
Risks Observed in Previous Clinical Program
• Risks observed with prior product and procedure protocol
• Integrated safety population of patients showed risks associated with
melphalan/HDS included:
melphalan/HDS included:
o 4.1% incidence of death due to adverse reactions
o 4% incidence of stroke
o 2% reported incidence of myocardial infarction in the setting of an incomplete cardiac
risk assessment
risk assessment
o ≥70% incidence of grade 4 bone marrow suppression with a median time of recovery
of greater than 1 week
of greater than 1 week
o 18% incidence of febrile neutropenia, along with the additive risk of hepatic injury,
severe hemorrhage and gastrointestinal perforation
severe hemorrhage and gastrointestinal perforation
• Deaths due to certain adverse reactions did not occur again during the
clinical trials following the adoption of related protocol amendments
clinical trials following the adoption of related protocol amendments
Treating Physicians in U.S. and EU Report Improved Safety Profile

21 DELCATH SYSTEMS, INC
Safety Improvements Implemented
• New generation filter
o Improve filter efficiency and consistency
• Vasopressors and methylprednisolone
o Reduce cardiovascular risk
• Prophylactic transfusions and growth factors
o Reduce risk of myelosuppression
• Intra-arterial nitroglycerin
o Prevent hepatic arterial spasm
• Liver tumor burden not to exceed >50%
o Address risk of liver failure
Decisive Measures to Assure Improved Safety

22 DELCATH SYSTEMS, INC
Positive Developments
• Improved device and procedure since prior trials
o >180 treatments with improved device and procedure in U.S. and
EU
EU
o Many issues raised at ODAC have not been reported
• Current device/procedure permitting multiple treatment cycles
• Recent scientific presentations at ESSO for OM from 3 centers in
U.S. and EU
U.S. and EU
o University Southampton reported 63% positive response (47% had a
partial response and 16% had a complete response)
partial response and 16% had a complete response)
o Moffitt reported 67% positive response (partial response and one
complete response)
complete response)
o Leiden reported 80% positive response (partial response)
Patients Report Improved Quality of Life

23 DELCATH SYSTEMS, INC
European Commercial Activity
CHEMOSAT®
Hepatic Delivery System
•Approved as Class IIb Medical Device; kit supplied
without melphalan
without melphalan
•Broad indication for intra-hepatic administration of
melphalan hydrochloride and subsequent filtration of
the venous blood return
melphalan hydrochloride and subsequent filtration of
the venous blood return
•>160 commercial procedures performed in 15 leading
cancer centers across the EU
cancer centers across the EU
•Reimbursement via Individual Funding Requests;
NUB Value 4 Status in Germany
NUB Value 4 Status in Germany
•UK Block Grants pending and private pay insurance
•Published data in peer-reviewed journal needed to
support reimbursement efforts in certain EU countries
support reimbursement efforts in certain EU countries

24 DELCATH SYSTEMS, INC
CHEMOSAT® Commercial Treatments in Europe
Multiple Tumor Types Treated Since EU Launch
Treatments/Re-treatments Increasing

25 DELCATH SYSTEMS, INC
Cash & Capital Resources
|
Cash & Cash Equivalents
|
$20.5 million at December 31, 2014
|
|
Debt
|
None
|
|
ATM Program1
|
$40 million available at December 31, 2014
|
|
Shares Outstanding
|
12.2 million (14.5 million fully diluted3) at
February 17, 2015 |
1) Subject to market conditions and certain limitations
2) Fully diluted includes approximate 0.2 million options and 0.9 million warrants
|
|
2014 Operating Cash Spend (Unaudited)
|
||||
|
|
Q1 A
|
Q2 A
|
Q3 A
|
Q4 Est.
|
FY Est.
|
|
Quarterly Guidance
|
$5-6M
|
$5-6M
|
$4-5M
|
$4-5M
|
$16.5-17.5M
|
|
Quarterly Actual
|
$4.5M
|
$4.0M
|
$4.0M
|
$3.7M
|
$16.2M
|
Focused Spending and Resources to Support Execution of Near-term Plan

26 DELCATH SYSTEMS, INC
In Summary
Melphalan Hydrochloride for Injection for use with the
Delcath Hepatic Delivery System (Melphalan/HDS)
Delcath Hepatic Delivery System (Melphalan/HDS)
• Cancers of the liver remain a
multibillion-dollar unmet medical
need
multibillion-dollar unmet medical
need
• Unique, highly differentiated solution
• Late-stage asset in U.S., early
commercial activity EU
commercial activity EU
• Compelling emerging data
• Imminent valuation milestones
• Attractive orphan drug business
model
model
• Experienced pharmaceutical
management team executing a data
-driven plan
management team executing a data
-driven plan

* DELCATH SYSTEMS, INC
© 2015 DELCATH SYSTEMS, INC. ALL RIGHTS RESERVED
Delcath and CHEMOSAT are registered trademarks of Delcath Systems, Inc.
