Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Celldex Therapeutics, Inc. | a15-5217_28k.htm |
| EX-99.1 - EX-99.1 - Celldex Therapeutics, Inc. | a15-5217_2ex99d1.htm |
| EX-99.2 - EX-99.2 - Celldex Therapeutics, Inc. | a15-5217_2ex99d2.htm |
Exhibit 99.3
|
|
2014 Business/Financial Results and 2015 Corporate Strategy Presentation February 24, 2015 |
|
|
Forward Looking Statement This communication contains "forward-looking" statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements other than statements of historical fact are statements that could be forward-looking statements. You can identify these forward-looking statements through our use of words such as “may,” “will,” “can,” “anticipate,” “assume,” “should,” “indicate,” “would,” “believe,” “contemplate,” “expect,” “seek,” “estimate,” “continue,” “plan,” “point to,” “project,” “predict,” “could,” “intend,” “target,” “potential” and other similar words and expressions of the future. These forward-looking statements are subject to risks and uncertainties that may cause actual future experience and results to differ materially from those discussed in these forward-looking statements. Important factors that might cause such a difference include, but are not limited to, the timing, cost and uncertainty of obtaining regulatory approvals for product candidates; our ability to develop and commercialize products before competitors that are superior to the alternatives developed by such competitors; the validity of our patents and our ability to avoid intellectual property litigation, which can be costly and divert management time and attention; and the other factors listed under “Risk Factors” in our filings with the SEC, including Forms 10-K, 10-Q and 8-K. Celldex does not undertake any obligation to release publicly any revisions to such forward-looking statement to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated events. 2 |
|
|
2014 – Another Significant Year for Celldex 3 Enrollment completed – 745 EGFRvIII patients Screened >4,800 patients from more than 200 clinical trial sites across 22 countries (30% positivity) Most comprehensive study conducted by a biotech to date Positive interim data at SNO 2014 All endpoints favored rindo Statistically significant and clinically meaning OS benefit Rindopepimut granted Breakthrough Designation |
|
|
2014 – Another Significant Year for Celldex 4 Commercial Preparation Underway Optimizing product uptake, minimizing access hurdles and supporting customers through innovative high-touch service programs while achieving financial targets Have hired key talent with focused expertise Goal: field a lean commercial organization that is nimble and scalable as key development, regulatory and access milestones are achieved Celldex global commercial footprint ~150 FTEs at launch Intend to work with regional marketing and distribution partners for Rintega in other key markets Rintega® – new trade name for Rindopepimut |
|
|
5 Glembatumumab Vedotin Continued site activation – almost 100 to date Now open in the United States, Australia and Canada; plans to enter the EU Program expanding into other indications 2014 – Another Significant Year for Celldex Varlilumab Proof of concept data at ASCO 2014 & SITC 2014 Phase 1/2 study with Opdivo® (BMS) initiated Phase 1b study with ONT-10 (Oncothyreon) initiated Multiple other studies in queue |
|
|
6 CDX-301 Pilot study of CDX-301 alone and with Mozobil® in HSCT initiated Investigator sponsored Phase 1/2 study of CDX-301/Hiltonol®/radiation in B-cell lymphoma ongoing Hiltonol® is a registered trademark of Oncovir; Mozobil® is a registered trademark of Genzyme Corporation CDX-1401 Phase 1 study demonstrates CDX-1401 induces strong immune responses and may predispose patients to better outcome on checkpoint inhibitors NCI sponsored Phase 2 study of CDX-1401 and CDX-301 in metastatic melanoma initiated Investigator sponsored study of CDX-1401 and IDO inhibitor in NY-ESO-1+ ovarian cancer initiated 2014 – Another Significant Year for Celldex |
|
|
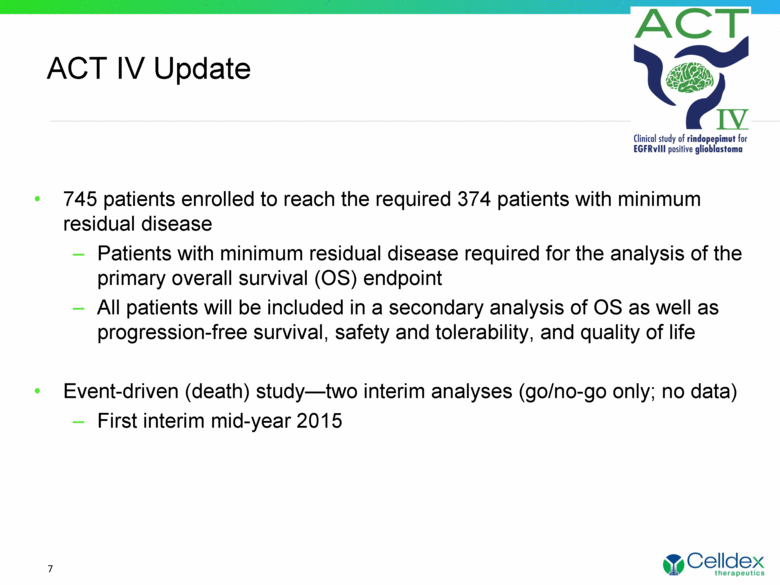
ACT IV Update 7 745 patients enrolled to reach the required 374 patients with minimum residual disease Patients with minimum residual disease required for the analysis of the primary overall survival (OS) endpoint All patients will be included in a secondary analysis of OS as well as progression-free survival, safety and tolerability, and quality of life Event-driven (death) study—two interim analyses (go/no-go only; no data) First interim mid-year 2015 |
|
|
ReACT Interim Data SNO 2014—PFS: Bev-Naïve Relapsed GBM (Group 1) HR = 0.67 (0.39, 1.14) p = 0.134*** Primary Endpoint: PFS-6 (Crude rate) Rintega + bevacizumab (n=35) 9/33 (27%)* p = 0.048** control + bevacizumab (n=37) 4/35 (11%)* * 6-month status pending for 4 patients (2 in each treatment arm) ** Chi-square test (1-sided). Study is designed to detect a PFS-6 difference with 1-sided = 0.2. Data based on Investigator Review *** Logrank test (2-sided) Rintega + bevacizumab (n=35) control + bevacizumab (n=37) 8 Months Progression-Free Survival(%) 0 5 10 15 20 25 0 25 50 75 100 |
|
|
ReACT Interim Data SNO 2014—Improved OS: Bev-Naïve Relapsed GBM (Group 1) Median (95% CI) Rintega + bevacizumab (n=35) 12.0 (9.7, --) control + bevacizumab (n=37) 8.8 (6.8, 11.4) HR = 0.47 (0.25, 0.91) p = 0.0208 Patients have not yet experienced progression of disease on study treatment 9 Overall Survival (%) Patients have not yet experienced progression of disease on study treatment |
|
|
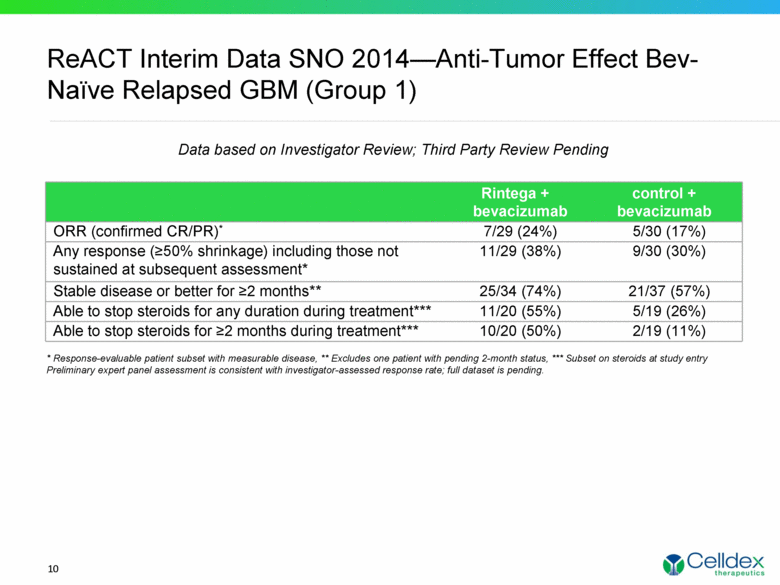
ReACT Interim Data SNO 2014—Anti-Tumor Effect Bev-Naïve Relapsed GBM (Group 1) Data based on Investigator Review; Third Party Review Pending Rintega + bevacizumab control + bevacizumab ORR (confirmed CR/PR)* 7/29 (24%) 5/30 (17%) Any response (>50% shrinkage) including those not sustained at subsequent assessment* 11/29 (38%) 9/30 (30%) Stable disease or better for >2 months** 25/34 (74%) 21/37 (57%) Able to stop steroids for any duration during treatment*** 11/20 (55%) 5/19 (26%) Able to stop steroids for >2 months during treatment*** 10/20 (50%) 2/19 (11%) * Response-evaluable patient subset with measurable disease, ** Excludes one patient with pending 2-month status, *** Subset on steroids at study entry Preliminary expert panel assessment is consistent with investigator-assessed response rate; full dataset is pending. 10 |
|
|
ReACT Interim Data SNO 2014—Early Anti-EGFRvIII Titer Response Correlates with Survival (Group 1) Bevacizumab-Naïve HR (95% CI): 0.47 (0.18, 1.27) p = 0.128 Median OS Months (95% CI) 6-Month OS (%) Good Titers* (n=14) 20.8 (9.9, 20.8) 95% All others (n=21) 10.4 (5.8, --) 69% * Based on the generation of anti-EGFRvIII antibody titer > 1:12,800 by Day 57. Similar trends were observed across a range of cut-off values (1:800 to 1:12,800). 11 Months Overall Survival (%) 0 5 10 15 20 25 30 0 25 50 75 100 |
|
|
Glembatumumab Vedotin Update 12 Breast cancer Revised protocol design reviewed by FDA and central European regulators 95 sites now open to screening in the US, Canada and Australia; plans to open in EU Expanded development Metastatic melanoma study initiated Squamous cell lung cancer CRADA with the NCI – initial studies in uveal melanoma and pediatric sarcoma |
|
|
Varlilumab Update 13 Phase 1 Study Very well tolerated; clear biologic activity and promising signs of clinical activity in advanced, treatment-refractory patient populations CR in Hodgkin lymphoma – 18.9+ months PR in renal cell carcinoma – 11+ plus months; continuing to decrease in tumor volume Stable disease from 3 - 30.7+ months Expanded development Clinical trial collaboration with BMS (Opdivo) initiated Clinical trial collaboration with Oncothyreon (ONT-10) initiated Phase 1/2 study of varli and Yervoy® (plus CDX-1401 in NY-ESO-1 positive patients) Multiple other studies in queue Yervoy® is a registered trademark of Bristol-Myers Squibb |
|
|
CDX-1401 Update 14 Phase 1 Study Phase 1 data suggest CDX-1401 may predispose patients to better outcome on subsequent therapy with checkpoint inhibitors 6 of 8 patients received checkpoint blockade following CDX-1401 treatment had significant clinical responses Expanded development Phase 1/2 study of varli and Yervoy (plus CDX-1401 in NY-ESO-1 positive patients) NCI sponsored Phase 2 study of CDX-1401 and CDX-301 in metastatic melanoma initiated Investigator sponsored study of CDX-1401 and IDO inhibitor in NY-ESO-1 positive ovarian cancer initiated |
|
|
CDX-301 Update 15 Pilot study of CDX-301 in hematopoietic stem cell transplant in combination with Mozobil initiated Investigator sponsored Phase 1/2 study of intratumoral injection of CDX-301 and Hiltonol in combo with low-dose radiotherapy for patients with low-grade B-cell lymphoma ongoing |
|
|
New Addition to the Pipeline in 2016: CDX-014 ADC directed to a novel renal cell carcinoma and ovarian target TIM-1 (T-cell Immunoglobulin Mucin-1) expression upregulated in several human cancers, most notably renal cell and ovarian carcinomas; has very restricted expression in healthy tissues Manufacturing and IND-enabling studies to complete in 2015 Planned Phase 1 study in refractory advanced stage RCC and other TIM-1+ cancers 16 |
|
|
2014 Q4 and YE Financials as of 12/31/2014 17 Net loss of $31.8M Q4 2014 vs $22.1M Q4 2013 Net loss of $118.1M for 2014 vs $81.6M for 2013 $201.0M in cash, cash equivalents and marketable securities 89.6M shares outstanding |
|
|
2015 Milestones Overview Rintega Continue execution on the ACT IV Phase 3 registration study in frontline GBM. First interim: mid-year 2015 Report final data from ReACT Phase 2 study in recurrent GBM mid-year 2015 Continued preparation for potential commercial launch of Rintega Glembatumumab vedotin (CDX-011) Continue accrual of METRIC study in triple negative breast cancer and melanoma study Initiate/support multiple studies, CLDX- sponsored Phase 2 study in squamous cell lung cancer / NCI-sponsored studies in uveal melanoma and pediatric sarcoma Varlilumab (CDX-1127) Execution of Phase 1/2 study of varli and Opdivo Initiate Phase 1/2 study of varli and Yervoy plus CDX-1401 in NY-ESO-1 positive patients Initiate multiple combination studies with a broad array of agents CDX-1401 Support Phase 2 study of CDX-1401/CDX-301 in melanoma (NCI sponsored) CDX-301 Continued execution of CDX-301 pilot study alone and with Mozobil in HSCT Support Phase 1/2 study of CDX-301/Hiltonol/radiation in B-cell lymphoma (inv sponsored) CDX-014 Manufacturing and IND-enabling work completing; file IND by YE 2015 18 |
|
|
2014 Business/Financial Results and 2015 Corporate Strategy Presentation February 24, 2015 |