Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - ASSEMBLY BIOSCIENCES, INC. | v402614_8k.htm |
Exhibit 99.1

Assembly Biosciences February 2015 1

Forward - Looking Statements This presentation contains forward - looking statements regarding future events. Forward - looking statements involve known and unknown risks that could cause actual results to differ materially from expected results. These risks and uncertainties include, among others: risks related to the scientific bases, costs, timing, regulatory review and results of our studies and clinical trials; our ability to obtain FDA and other regulatory approval of our product candidates; our anticipated capital expenditures, our estimates regarding our capital requirements, and our need for future capital; the unpredictability of the size of the markets for, and market acceptance of, any of our product candidates; our ability to sell any approved products and the price we are able to realize; our ability to obtain future funding on acceptable terms; our ability to retain and hire necessary employees and to staff our operations appropriately; our ability to compete in our industry and innovation by our competitors; our ability to stay abreast of and comply with new or modified laws and regulations that currently apply or become applicable to our business; and the risks set out in our filings with the SEC . 2
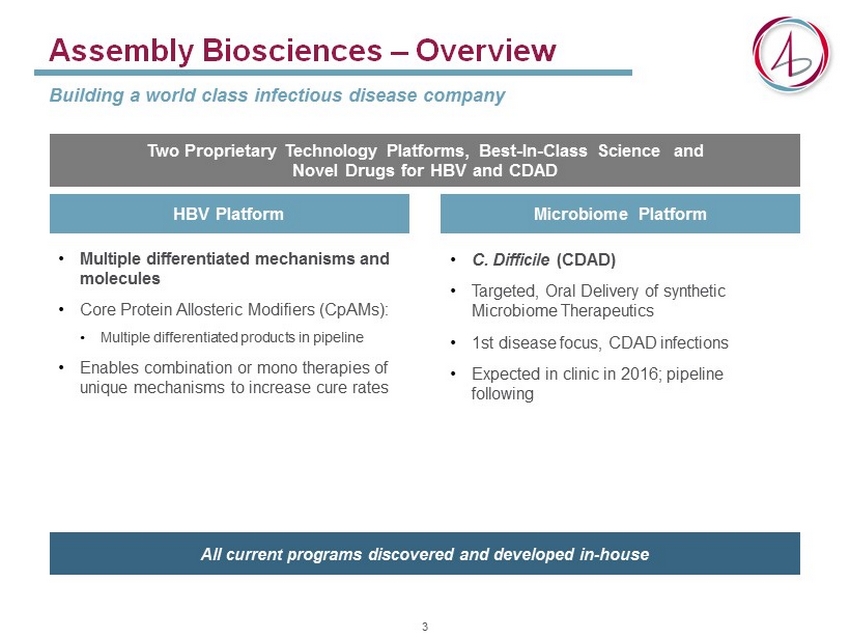
Assembly Biosciences – Overview Building a world class infectious disease company Two Proprietary Technology Platforms, Best - In - Class Science and Novel Drugs for HBV and CDAD All current programs discovered and developed in - house • Multiple differentiated mechanisms and molecules • Core Protein Allosteric Modifiers ( CpAMs ): • Multiple differentiated products in pipeline • Enables combination or mono therapies of unique mechanisms to increase cure rates HBV Platform Microbiome Platform • C. D ifficile (CDAD) • Targeted, Oral Delivery of synthetic Microbiome Therapeutics • 1st disease focus, CDAD infections • Expected in clinic in 2016; pipeline following 3
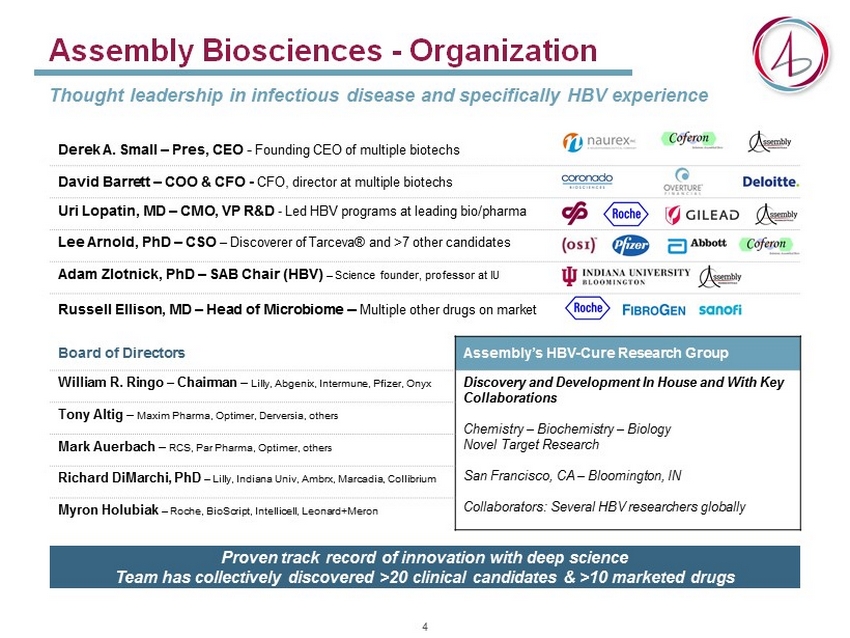
Assembly Biosciences - Organization Thought leadership in infectious disease and specifically HBV experience Derek A. Small – Pres , CEO - Founding CEO of multiple biotechs David Barrett – COO & CFO - CFO, director at multiple biotechs Uri Lopatin , MD – CMO, VP R&D - Led HBV programs at leading bio/ pharma Lee Arnold, PhD – CSO – Discoverer of Tarceva ® and >7 other candidates Adam Zlotnick , PhD – SAB Chair (HBV) – Science founder, professor at IU Russell Ellison, MD – Head of Microbiome – M ultiple other drugs on market Board of Directors Assembly’s HBV - Cure Research Group William R. Ringo – Chairman – Lilly, Abgenix , Intermune , Pfizer, Onyx Discovery and Development In House and With Key Collaborations Chemistry – Biochemistry – Biology Novel Target Research San Francisco, CA – Bloomington, IN Collaborators: Several HBV researchers globally Tony Altig – Maxim Pharma , Optimer , Derversia , others Mark Auerbach – RCS, Par Pharma , Optimer , others Richard DiMarchi , PhD – Lilly, Indiana Univ , Ambrx , Marcadia , Collibrium Myron Holubiak – Roche, BioScript , Intellicell , Leonard+Meron Proven track record of innovation with deep science Team has collectively discovered >20 clinical candidates & >10 marketed drugs 4

Our Approach: Leverage HBc to Target cccDNA Our Core Competencies and Competitive Advantage Our Approach to an HBV Cure Prevent NEW cccDNA Inhibit Existing cccDNA Eliminate / Silence cccDNA HBV CURE Intellectual Property HBV Biology Proprietary Enabling Assays World Class Chemistry Clinical Expertise 5
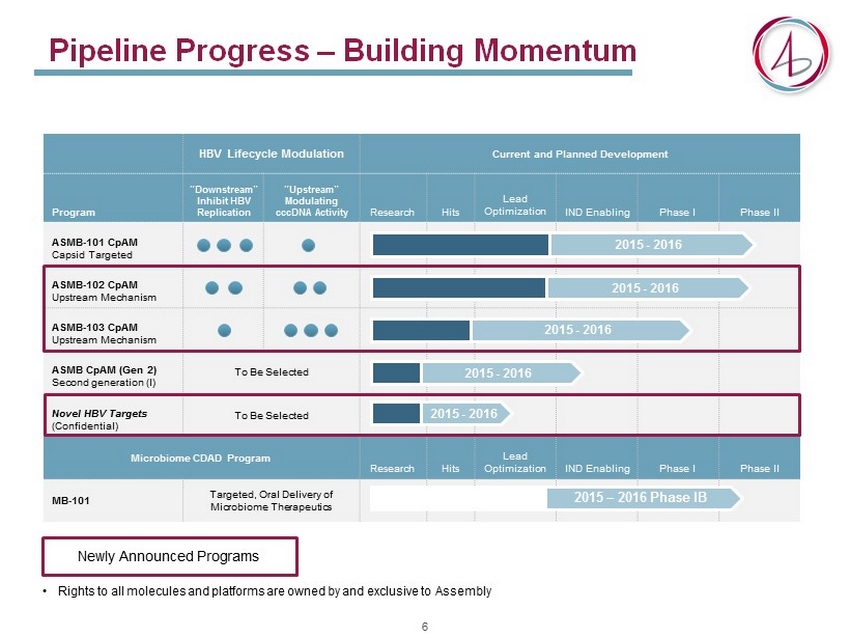
Pipeline Progress – Building Momentum HBV Lifecycle Modulation Current and Planned Development Program “Downstream” Inhibit HBV Replication “Upstream” Modulating cccDNA Activity Research Hits Lead Optimization IND Enabling Phase I Phase II ASMB - 101 CpAM Capsid Targeted ASMB - 102 CpAM Upstream Mechanism ASMB - 103 CpAM Upstream Mechanism ASMB CpAM (Gen 2) Second generation (I) To Be Selected Novel HBV Targets (Confidential) To Be Selected Microbiome CDAD Program Research Hits Lead Optimization IND Enabling Phase I Phase II MB - 101 Targeted, Oral Delivery of Microbiome Therapeutics 2015 - 2016 2015 - 2016 2015 - 2016 2015 - 2016 2015 – 2016 Phase IB 2015 - 2016 Newly Announced Programs • Rights to all molecules and platforms are owned by and exclusive to Assembly 6

HBV Background & Assembly Approach 7
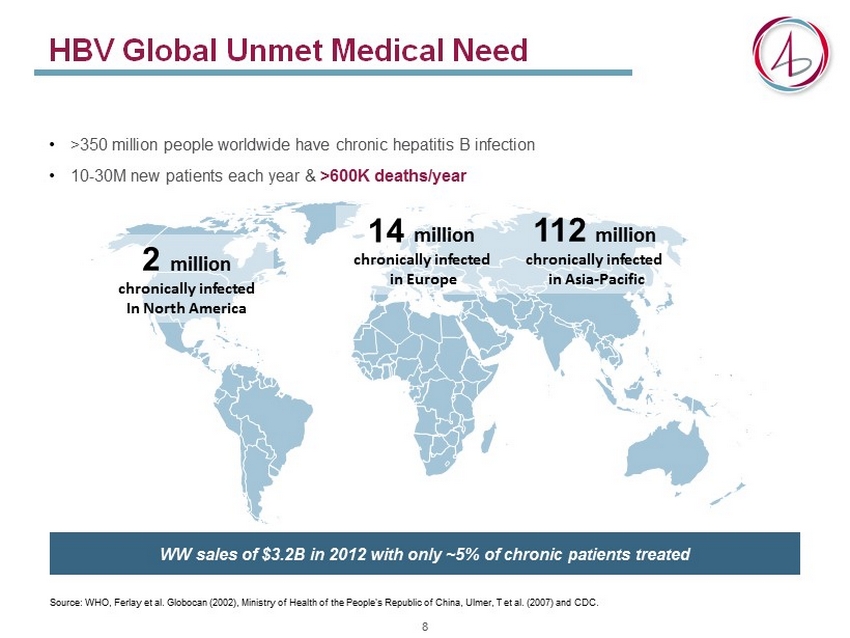
HBV Global Unmet Medical Need • >350 million people worldwide have chronic hepatitis B infection • 10 - 30M new patients each year & >600K deaths/year WW sales of $3.2B in 2012 with only ~5% of chronic patients treated Source: WHO, Ferlay et al. Globocan (2002), Ministry of Health of the People's Republic of China, Ulmer , T et al. ( 2007) and CDC. 2 million chronically infected In North America 14 million chronically infected in Europe 112 million chronically infected in Asia - Pacific 8

Assembly’s Approach to HBV Cure 1. HBV is a DNA virus – unlike HCV, HBV viral reservoir ( cccDNA ) is in the nucleus 2. Curative therapy for HBV will require modulation and destabilization or silencing of cccDNA • Limited cure is seen on current therapies (3 - 10% of cases) (1) • Preliminary research in the HBV field suggests that selective degradation of cccDNA is achievable, and that it may be HBc dependent (2) Our goal is to develop small molecule oral therapies to sustainably suppress or eliminate cccDNA by leveraging our expertise in HBV Core Protein (1) New therapeutic targets and drugs for the treatment of chronic hepatitis B. Seimars in Liv Dis 33 , 130 – 137 (2013). (2) Lucifora , J. et al. Science 343, 1221 – 1228 (2014 ). Prevent NEW cccDNA Inhibit Existing cccDNA Eliminate / Silence cccDNA HBV CURE 9
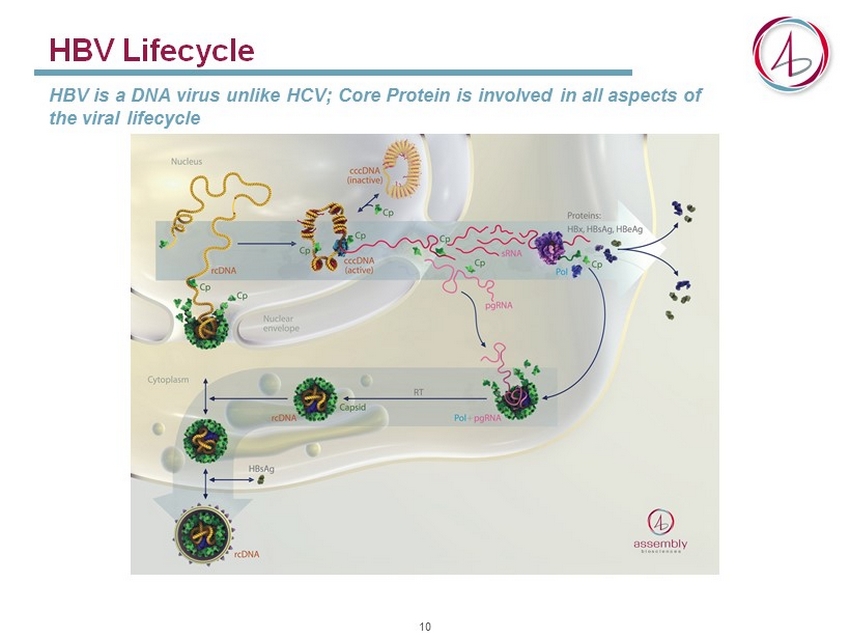
HBV Lifecycle HBV is a DNA virus unlike HCV; Core Protein is involved in all aspects of the viral lifecycle 10
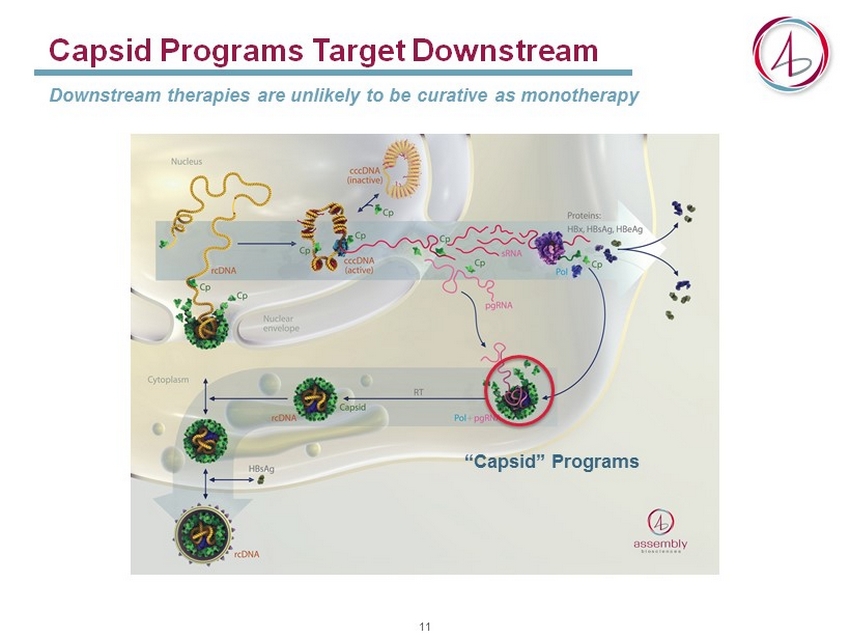
Capsid Programs Target Downstream Downstream therapies are unlikely to be curative as monotherapy “Capsid” Programs 11
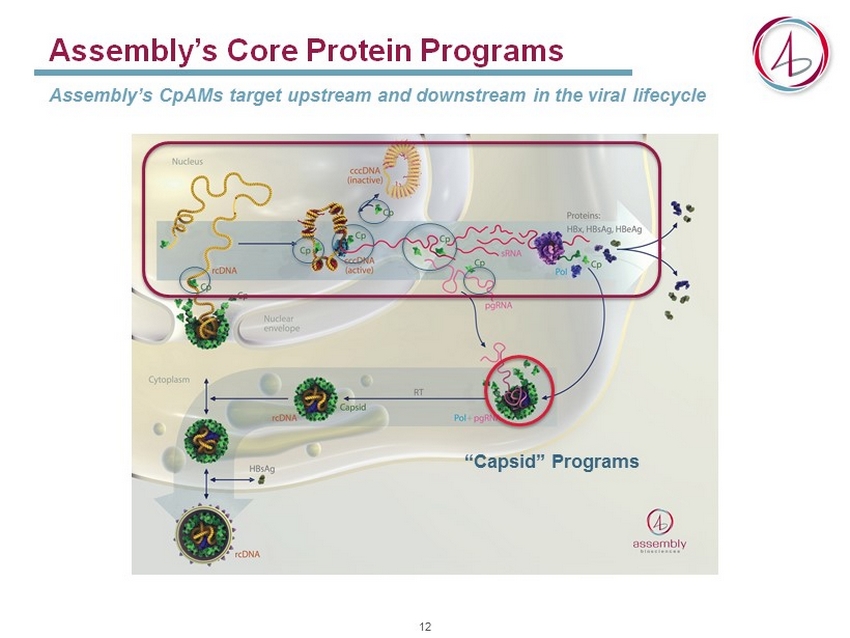
Assembly’s Core Protein Programs Assembly’s CpAMs target upstream and downstream in the viral lifecycle “Capsid” Programs 12

Program and Pipeline Overview Core Protein Rationale 13
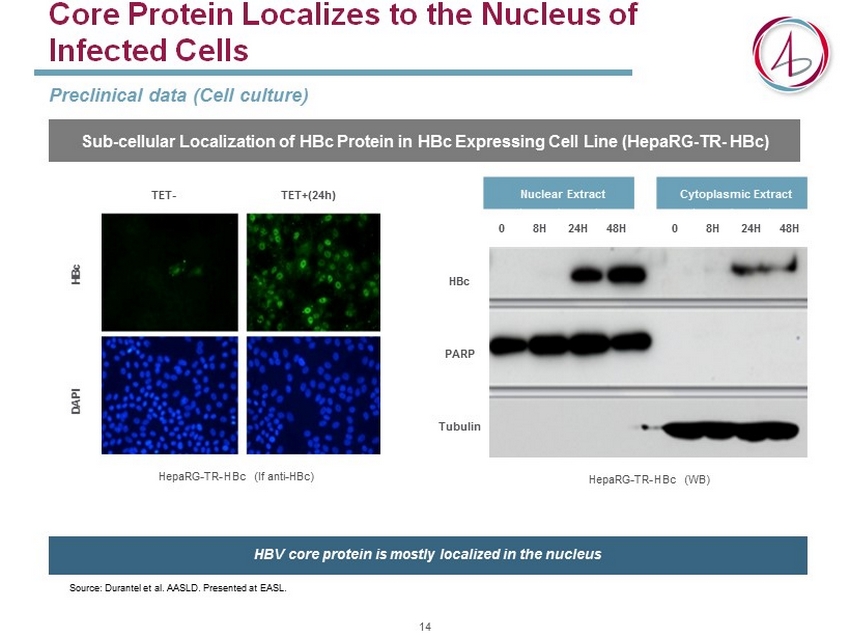
Core Protein Localizes to the Nucleus of Infected Cells Preclinical data (Cell culture) TET - TET+(24h) HBc DAPI HepaRG - TR - HBc (If anti - HBc ) Nuclear Extract Cytoplasmic Extract 0 8H 24H 48H 0 8H 24H 48H HBc PARP Tubulin HepaRG - TR - HBc (WB) Source: Durantel et al. AASLD. Presented at EASL. Sub - cellular Localization of HBc Protein in HBc Expressing Cell Line ( HepaRG - TR - HBc ) HBV core protein is mostly localized in the nucleus 14
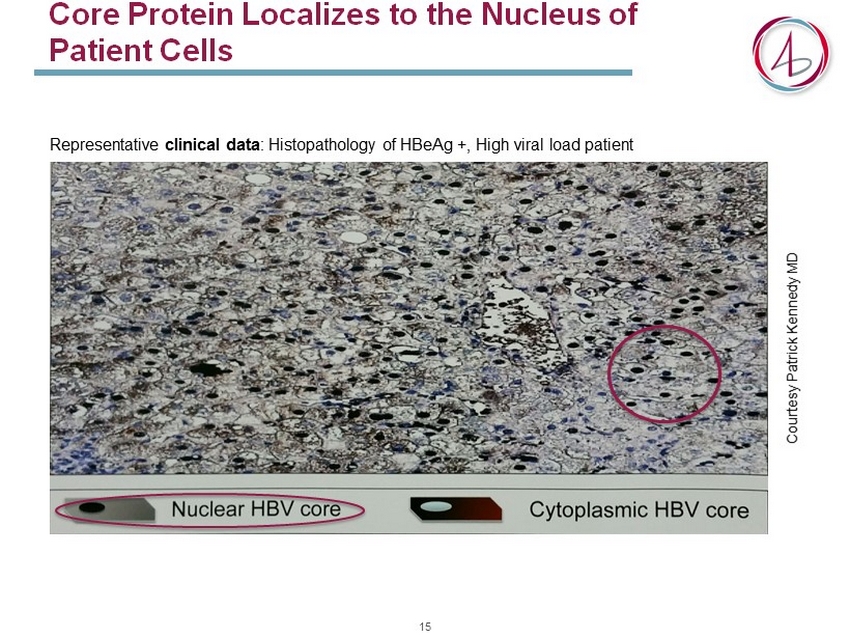
Core Protein Localizes to the Nucleus of Patient Cells Representative clinical data : Histopathology of HBeAg +, High viral load patient Courtesy Patrick Kennedy MD 15
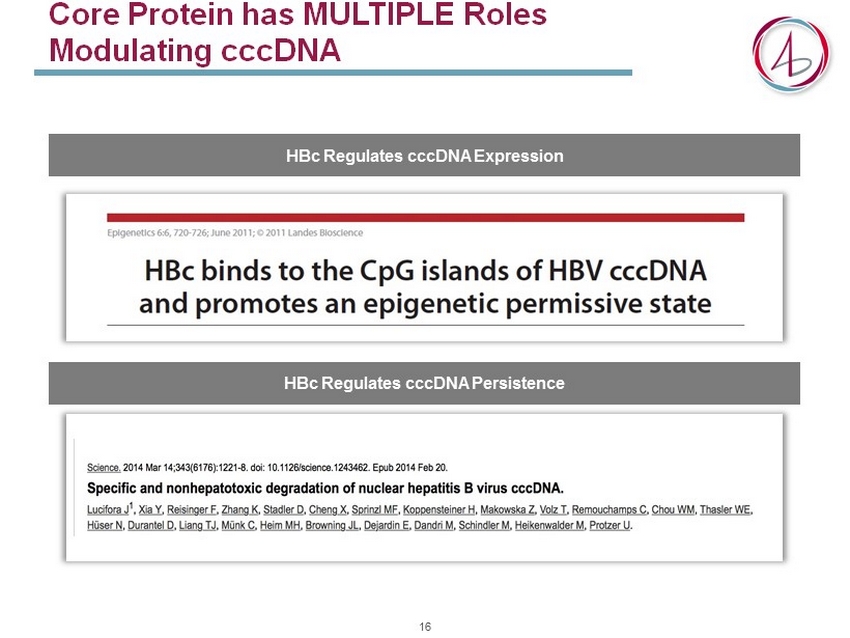
Core Protein has MULTIPLE Roles Modulating cccDNA HBc Regulates cccDNA Expression HBc Regulates cccDNA Persistence 16
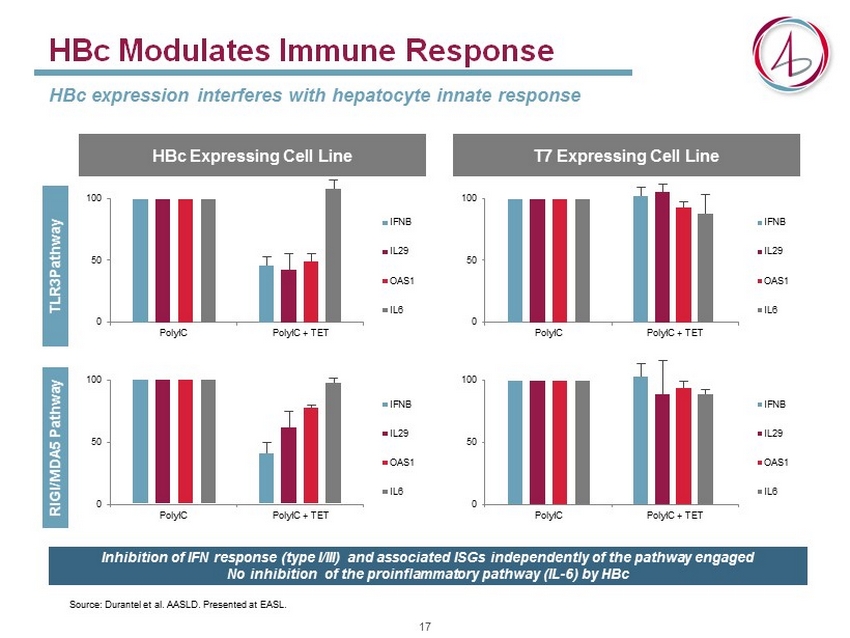
HBc Expressing Cell Line HBc Modulates Immune Response HBc expression interferes with hepatocyte innate response 0 50 100 PolyIC PolyIC + TET IFNB IL29 OAS1 IL6 T7 Expressing Cell Line TLR3Pathway RIGI/MDA5 Pathway 0 50 100 PolyIC PolyIC + TET IFNB IL29 OAS1 IL6 0 50 100 PolyIC PolyIC + TET IFNB IL29 OAS1 IL6 0 50 100 PolyIC PolyIC + TET IFNB IL29 OAS1 IL6 Source: Durantel et al. AASLD. Presented at EASL. Inhibition of IFN response (type I/III) and associated ISGs independently of the pathway engaged No inhibition of the proinflammatory pathway (IL - 6) by HBc 17
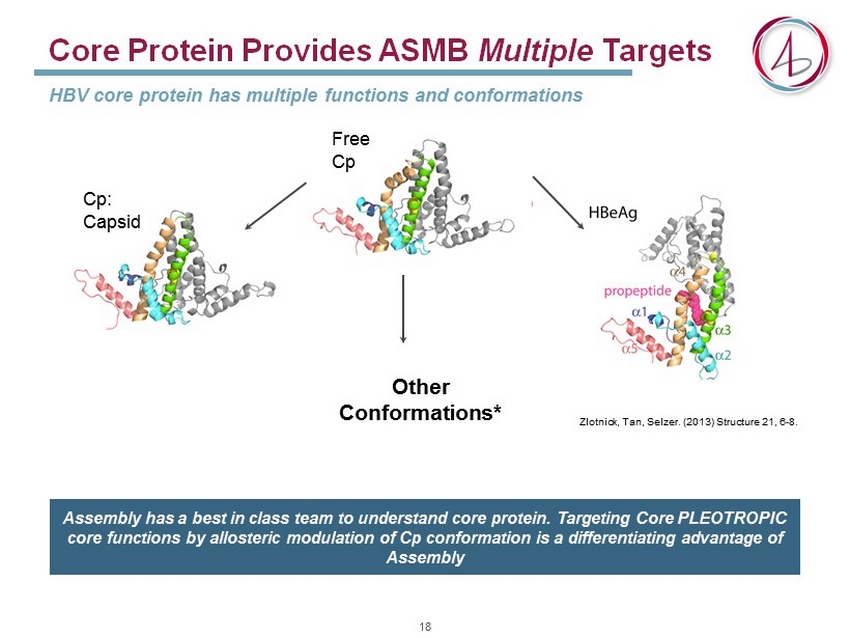
Core Protein Provides ASMB Multiple Targets HBV core protein has multiple functions and conformations Free Cp Cp : C apsid Zlotnick, Tan, Selzer. (2013) Structure 21 , 6 - 8. Other Conformations* Assembly has a best in class team to understand core protein. Targeting Core PLEOTROPIC core functions by allosteric modulation of Cp conformation is a differentiating advantage of Assembly 18
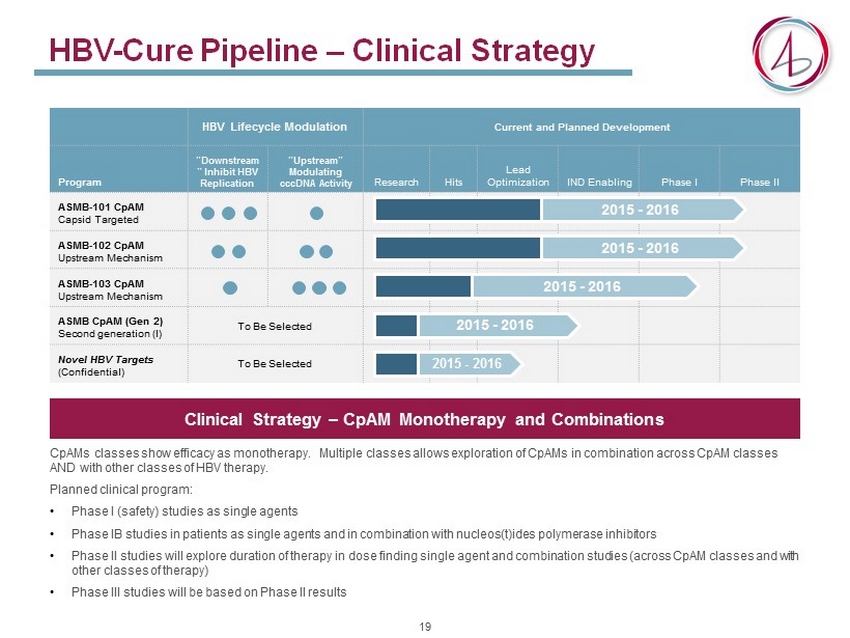
HBV - Cure Pipeline – Clinical Strategy CpAMs classes show efficacy as monotherapy. Multiple classes allows exploration of CpAMs in combination across CpAM classes AND with other classes of HBV therapy. Planned clinical program: • Phase I (safety) studies as single agents • Phase IB studies in patients as single agents and in combination with nucleos (t)ides polymerase inhibitors • Phase II studies will explore duration of therapy in dose finding single agent and combination studies (across CpAM classes and with other classes of therapy) • Phase III studies will be based on Phase II results HBV Lifecycle Modulation Current and Planned Development Program “Downstream ” Inhibit HBV Replication “Upstream” Modulating cccDNA Activity Research Hits Lead Optimization IND Enabling Phase I Phase II ASMB - 101 CpAM Capsid Targeted ASMB - 102 CpAM Upstream Mechanism ASMB - 103 CpAM Upstream Mechanism ASMB CpAM (Gen 2) Second generation (I) To Be Selected Novel HBV Targets (Confidential) To Be Selected 2015 - 2016 2015 - 2016 2015 - 2016 2015 - 2016 2015 - 2016 Clinical Strategy – CpAM Monotherapy and Combinations 19
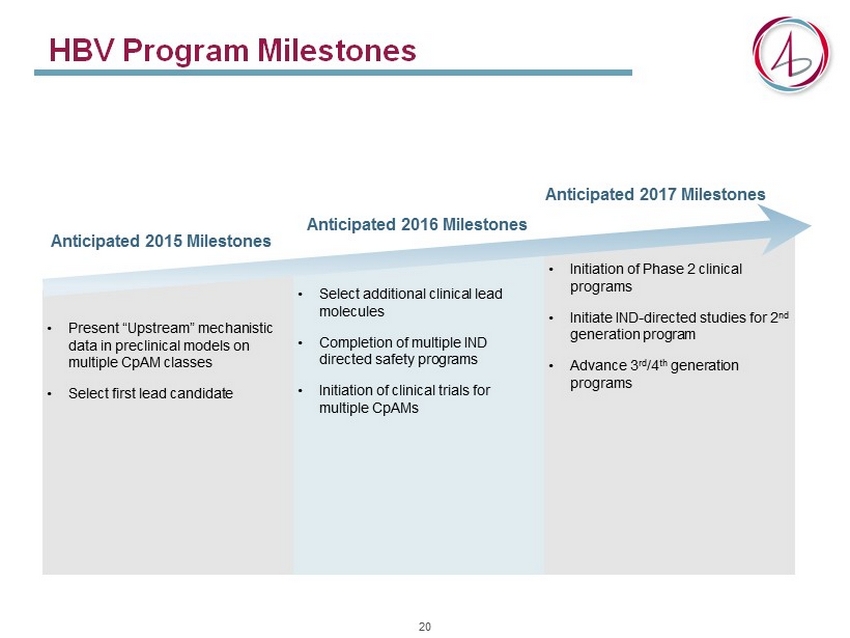
HBV Program Milestones • Present “Upstream” mechanistic data in preclinical models on multiple CpAM classes • Select first lead candidate • Select additional clinical lead molecules • Completion of multiple IND directed safety programs • Initiation of clinical trials for multiple CpAMs • Initiation of Phase 2 clinical programs • Initiate IND - directed studies for 2 nd generation program • Advance 3 rd /4 th generation programs Anticipated 2015 Milestones Anticipated 2016 Milestones Anticipated 2017 Milestones 20
Intellectual Property Portfolio: HBV & CpAMs The patent applications fall broadly into two categories: 1. Platform patent applications – Core Protein • Multiple platform patent applications filed, with others in process • Patent applications cover novel mechanism of action, methods of treatment, novel assays, and other aspects of novel HBV Core Protein mechanisms 2. Composition of matter patent applications – Novel CpAMs • Several compound, structure, and composition of matter applications pending We have a portfolio of filings to date, and expect to be filing more Our patent portfolio is intended to cover significant geography in HBV 21

Microbiome Program: C. D ifficile 22

Microbiome Therapeutics: Overview • We have in - licensed technology for targeted delivery of complex agents to select regions of the GI tract • Our delivery technology utilizes state of the art encapsulation technology and new coating technologies in conjunction to exploit specific pH gradients across the gut. • This is designed to deliver complex agents to the proximal colon and / or terminal ileum • C. Difficile infection provides an excellent path for proof of concept of microbiome therapy approach • C. Difficile is a recognized major health problem and increasing in incidence • FMT has provided the most clear success in therapy to date: Durable cure within 24hrs • Success reported with minimal mixtures of bacterial strains Assembly’s approach : Selected strains (GMP product) delivered in an oral - capsule based therapy to recapitulate cure rates seen in FMT 23
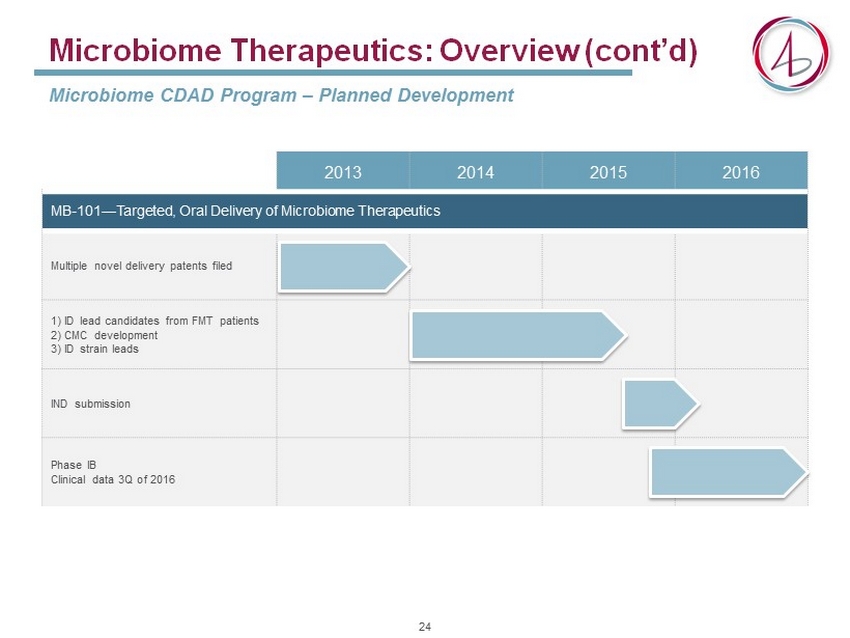
2013 2014 2015 2016 MB - 101 — Targeted, Oral Delivery of Microbiome Therapeutics Multiple novel delivery patents filed 1) ID lead candidates from FMT patients 2) CMC development 3) ID strain leads IND submission Phase IB Clinical data 3Q of 2016 Microbiome Therapeutics: Overview (cont’d) Microbiome CDAD Program – Planned Development 24

Financials & Key Investment Highlights 25
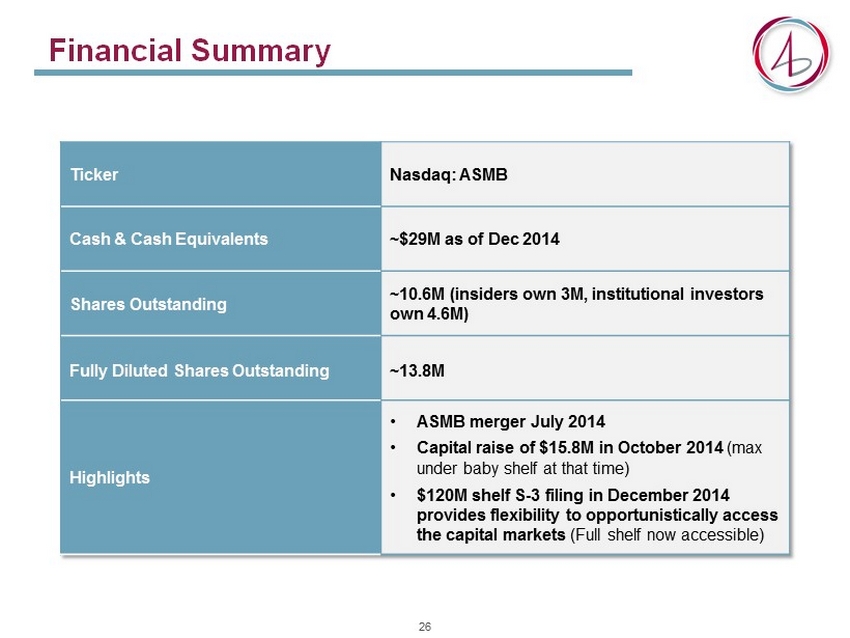
Financial Summary Ticker Nasdaq: ASMB Cash & Cash Equivalents ~$29M as of Dec 2014 Shares Outstanding ~10.6M (insiders own 3M, institutional investors own 4.6M) Fully Diluted Shares Outstanding ~13.8M Highlights • ASMB merger July 2014 • Capital raise of $15.8M in October 2014 (max under baby shelf at that time) • $120M shelf S - 3 filing in December 2014 provides flexibility to opportunistically access the capital markets (Full shelf now accessible) 26
Experienced Management Team and Scientific Advisory Board with Leadership in HBV Core Protein Research Proven, In - House Discovery and Development Capabilities with Multiple Shots on Goal Novel Microbiome Program for C.Difficile Potential First - and Best - in - Class HBV Platform With Potential to Cure HBV World Class Infectious Disease Company with Two Novel Platforms Investment Highlights 27



























