Attached files
| file | filename |
|---|---|
| EX-99.3 - EX-99.3 - Unilife Corp | d866731dex993.htm |
| EX-99.1 - EX-99.1 - Unilife Corp | d866731dex991.htm |
| 8-K - FORM 8-K - Unilife Corp | d866731d8k.htm |
 2
nd
Quarter of Fiscal 2015 Earnings Call
Confidential-Unilife Corporation
February 9, 2015
Exhibit 99.2 |
 Confidential-Unilife Corporation
2
This
presentation contains forward looking statements under the safe harbor provisions of
the US securities laws. These forward-looking
statements
are
based
on
management’s
beliefs
and
assumptions
and
on
information
currently
available
to
our management. Our management believes that these forward-looking
statements are reasonable as and when made. However you should not place
undue reliance on any such forward looking statements as these are subject to risks and
uncertainties.
Please
refer
to
our
press
releases
and
our
SEC
filings
for
more
information
regarding
the
use
of
forward
looking statements.
Cautionary Note Regarding Forward-Looking Statements
|
 Confidential-Unilife Corporation
Investor Highlights
Continuing our Strong Momentum
3
Our large market
opportunity is proven and
expanding
Our ability to win uniquely is
well-demonstrated and
gaining strength
Our execution to support
existing customer programs
is strong and remains firmly
on track
Our attractive model is
validated and supported
by our improving revenue
performance |
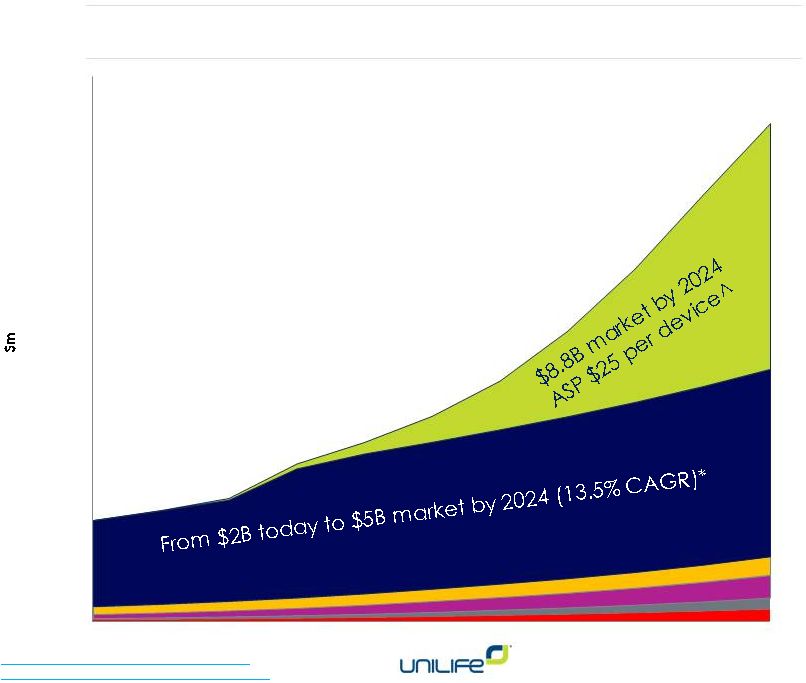 Projected growth
from $4B (2015) -
$15B (2025)**
CAGR 14%
Wearable
injectors
^
(wearable disposal
self-injectors)
Prefilled
syringes*
Auto-injectors
Reconstitution
Ocular delivery
Novel delivery
^
Roots
Resarch.
Wearable
Bolus
Injectors.
2014.
VisionGain
Medical
Device
Leader
Series
2014-2024.
** Compilation of Unilife estimates and market research reports
4
Large, Growing Markets
$-
$2,000
$4,000
$6,000
$8,000
$10,000
$12,000
$14,000
$16,000
$18,000
2015
2016
2017
2018
2019
2020
2021
2022
2023
2024
2025 |
 Our
Demonstrated Ability to Win Uniquely 5
5
Compelling Technologies
Platform Specific Solutions
Comprehensive Portfolio
Robust Supply Chain with Continuity of Supply
Addressing unmet needs
Adding value for patients,
prescribers and payers
Mitigating customer risk
Customizable to needs of specific
drugs in broad portfolio
Preferred one-stop partner for
economies of scope
Addressing immediate and long-
term needs
Initial due diligence streamlines
path to incremental agreements
Flexible supply chain for
components and materials
Alternative sources of production
and supply
Maximize efficiencies and minimize
risk
Create distinctive brand identity for
each drug
Integration with standard
equipment and processes |
 An
Attractive Business Model 6
•
Unifill Finesse for
Lovenox
•
10-Years
•
$50MM
in upfront payments
•
Min. 150MM units^
•
Unifill platform for 20
generic injectables
•
15-Years
•
$40MM
in upfront / milestone payments
•
Min. 175MM units^
•
Unilife sole provider
for all applicable
wearable drugs
•
5-10 biologics*
•
15-Years minimum
•
With biologics R&D
division MedImmune
•
Long-term agreement
(duration not disclosed)
•
Several target biologics
6
+ for innovative, differentiated delivery systems. Supply agreements in negotiation.
Avg. 5MM units p.a. wearable drug* with device ASP $25 Supply Agreements for
Prefilled Syringes Supply Agreements for Wearable Injectors
^ Minimum annual volumes per year following initial ramp program
* Unilife estimates based on industry averages and internal estimates
Cornerstone Supply Agreements Signed To-Date
Model validated and supported by growing financial performance
o
Can generate a flow of incremental programs and supply agreements
Capacity
to
generate
recurring,
predictable
revenue
for
10
–
15
years
Low sales and marketing costs supports attractive blended
operating margins of 40% or more over time |
 Commercial Pipeline to Generate Incremental Growth
7
•
In-depth product-specific
discussions with customer
•
Multiple current and
prospective customers
•
Multiple potential drug
candidates per customer
•
May take 1 -
3 years to
begin an active program
•
Device customization and
other activities to prepare
for commercial launch
•
Signed customer agreements
•
Many programs confidential
•
Upfront or milestone based
revenue from programs
•
Programs may span 1 –
3 years
•
May be more than one
program per customer
•
Supply agreements signed
prior to commercial launch
•
10-15 yr. duration common
•
Potential for multiple supply
agreements per customer
Many active
target
opportunities
across multitude
of pharma
companies and
therapy areas
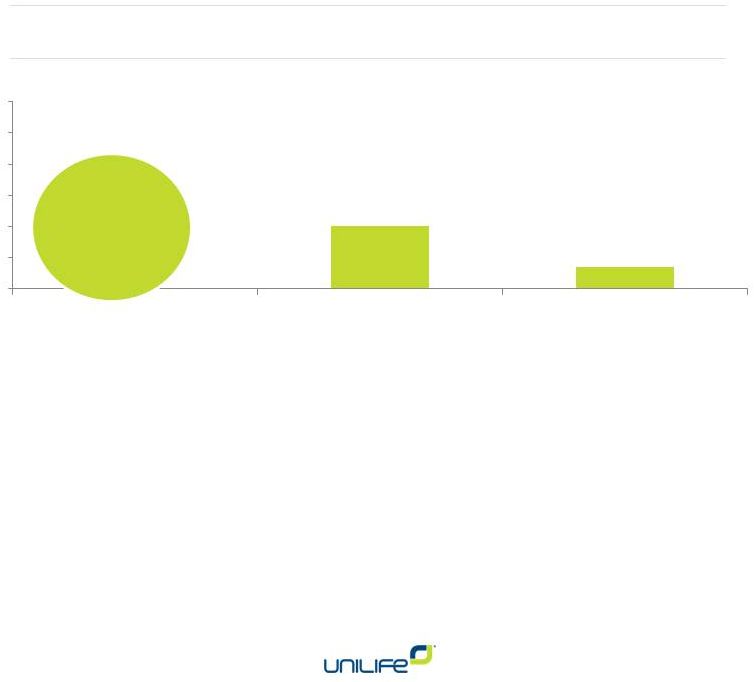
Snapshot as of Jan 1, 2015
Up 33% from last quarter
20
7
0
10
20
30
40
50
60
Commercial Pipeline
Active Programs
Supply Agreements |
 Business Execution
8
•
Commercial shipments commenced
°
1
st
1 million units prior to middle FY16
°
2
nd
line operational
°
3
rd
line nearing completion
•
Shipments to accelerate FY15 -
FY16
Case Study One: Unifill platform of prefilled syringes |
 9
Several customer
programs underway
Shipped over 80,000 devices
or drug containers in H1 FY15
Scaling up capacity
High-volume line with 5MM
unit annual capacity
Flextronics validation
Begin shipping devices
for human drug trials
during first half FY16
Business Execution
Case Study Two: Platform of Wearable Injectors |
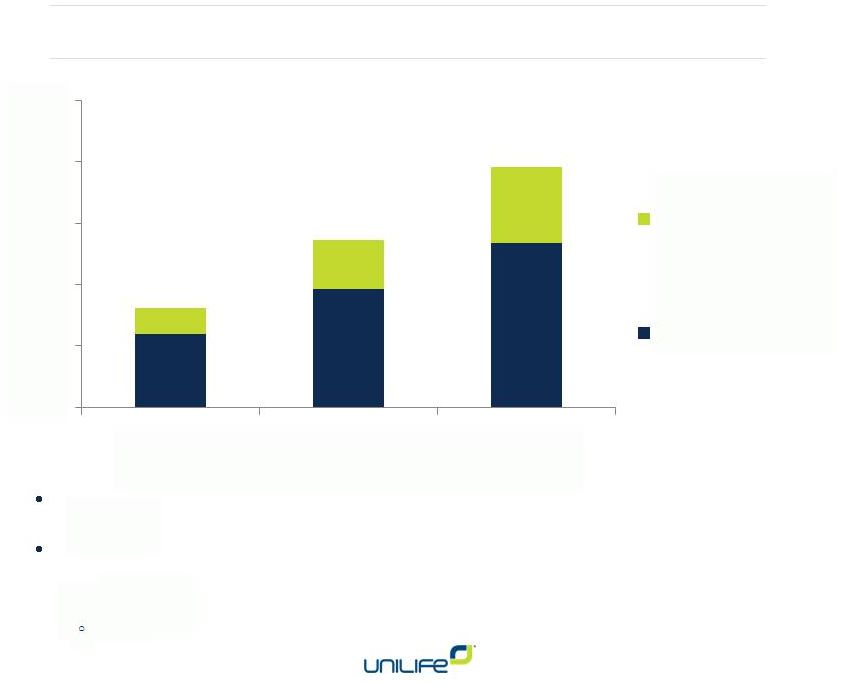 Business Execution
Now 180-plus patents issued worldwide. Over 200 more pending.
R&D investment to improve existing technologies and create new
device categories
Case Study Three: R&D Programs
10
New Patent
Applications
Granted Patents
FY 2012
FY 2013
FY 2014
0
50
100
150
200
250
Patent
return
on
investment
is
increasing
for
each
dollar
of
R&D
spent |
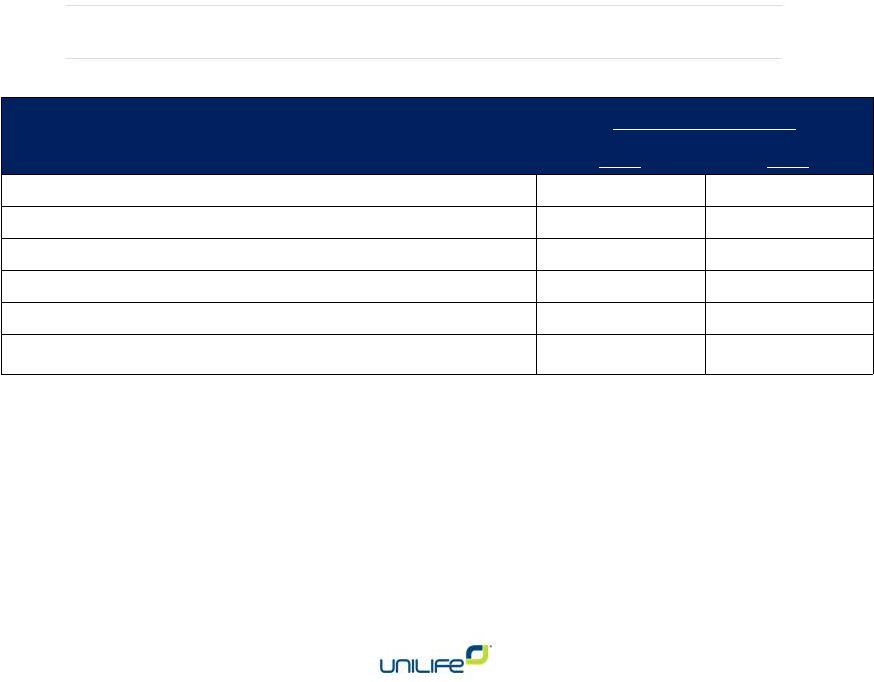 Financial Results
Three Months Ended
2014
2013
Revenues
$5.4MM
$3.6MM
Research & development
$11.3
MM
$7.8MM
Selling, general & administrative
$9.5MM
$6.7MM
Net loss per share
$0.18
$0.17
Adjusted net loss*
$12.5MM
$8.3MM
Adjusted
net
loss
per
share
-
diluted
$0.12
$0.08
* Adjusted net loss excludes non-cash share-based compensation
expense, depreciation and amortization, interest expense and change
in fair value of financial statements. ^ Excluding non-cash items
•
Compared to prior quarter (Q1, FY 2015):
o
Increased revenue by $4MM
o
Decreased adjusted net loss by $3.4MM (21%)
•
Deferred revenue increased by $3.9MM since 6/30/2014
•
Current cash of $10.8MM
o
Does not include net proceeds of $44.7MM from Feb 2015
offering 11 |
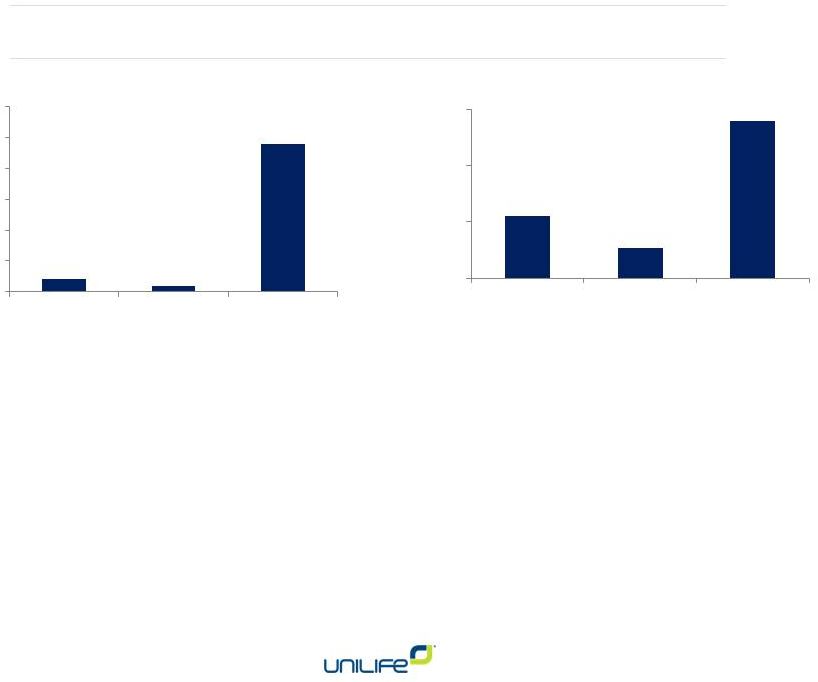 Increasing Cash Receipts and Revenue
12
Cash Receipts
Revenue
$MM
$MM
12
•
$8MM in cash receipts from
customers during the first half FY 2015
o
On schedule to generate an additional $20MM during second half FY 2015
•
Additional $45
million from U.S. offering of common stock added in February 2015 to $10 million on balance sheet as of December 31
•
Sufficient cash on hand and to be generated from customer
programs and device sales to support operating activities through to
at least the end of fiscal 2016
o
Additional agreements may further extend cash runway.
$-
$5.00
$10.00
$15.00
$20.00
$25.00
$30.00
FY 2012
FY 2013
FY 2014
$-
$5.00
$10.00
$15.00
FY 2012
FY 2013
FY 2014 |
 Summary
13
Existing Agreements
•
Continued scale-up to commercial launch
•
Commencement of new programs with additional molecules
•
Expected human drug studies with some target molecules
Incremental Agreements
•
Multiple new agreements: commercial supply agreements with AbbVie and others
Other Commercial and Operational Highlights
•
Increase in the total number of active programs
•
Development of additional Unilife products / platforms
Financial Highlights
•
Substantial increase in year on year revenue for the current quarter
•
Increasing revenue from product sales, customization programs and milestone-based
fees •
Potential for significant fees from customers seeking exclusivity access
|
 Confidential-Unilife Corporation
Questions
14 |
 Confidential-Unilife Corporation
Final Comments |
