Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Diffusion Pharmaceuticals Inc. | a15-2015_28k.htm |
Exhibit 99.1
|
|
[LOGO] |
|
|
This presentation includes "forward-looking statements" under the Private Securities Litigation Reform Act of 1995. All statements other than statements of historical facts contained in this presentation, including statements regarding our anticipated future clinical and regulatory events, future financial/position, business strategy and plans and objectives of management for future operations, are forward-looking statements. Forward-looking statements can be identified by words such as “potential,” "may," "will," "should," "forecast,“ "project," "could," "expect," "believe," "estimate," "anticipate," "intend," "plan,“ “continue”, other words of similar meaning, derivations of such words and the use of future dates. Forward-looking statements in this presentation include, without limitation, statements regarding our current business strategies, the potential future commercialization of our product candidates, potential estimated market sizes for our product candidates, anticipated start dates, durations and completion dates, as well as potential future results, of our future clinical trials, anticipated designs of our future clinical trials, and anticipated future regulatory submissions and events. Uncertainties and risks may cause actual results to be materially different than those expressed in or implied by our forward-looking statements. Particular uncertainties and risks include, among others, uncertainties regarding our ability to license out our existing and license in additional products and technologies and the terms of such licenses; uncertainties involved in clinical testing, the difficulty of developing pharmaceutical products, obtaining regulatory and other approvals and achieving market acceptance, and other risks and uncertainties described in our filings with the Securities and Exchange Commission, including our most recent annual report on Form 10-K/A, subsequent quarterly reports on Form 10-Q and final prospectus dated July 31, 2014. All forward-looking statements in this presentation speak only as of the date of this presentation and we undertake no obligation to update or revise any forward-looking statement, whether as a result of new information, future events or otherwise. 2 |
|
|
Investment Highlights • Two core technologies – PI3K/Akt/mTOR Pathway Inhibitor – “Soft” Anti-Androgen • Three clinical trials to be initiated in 2016 – Age related macular degeneration – Glioblastoma – Acne • Strong preclinical in-vitro and in-vivo data • Lead product completed two Phase I clinical trials • Experienced board of directors and management |
|
|
Overview Specialty pharmaceutical company initially focused on developing products for ophthalmology, oncology and dermatology Headquartered in Chicago Eight employees One MD Three PhDs Indications in development Age related macular degeneration Glioblastoma Acne 4 |
|
|
Stephen M. Simes - Chief Executive Officer BioSante, Unimed, Bio-Technology General, Gynex, Searle Phillip B. Donenberg – Chief Financial Officer BioSante, Unimed, Gynex Mark Weinberg, MD, MBA – Senior VP, Clinical Development Astellas, Lundbeck, Ovation, Takeda, Abbott David Sherris, Ph.D. - Chief Scientific Officer Paloma/Vasculomedics, OXiGENE, Serono, Unilever, Centocor Ph.D. in Biochemistry and Molecular Genetics Yael Schwartz, Ph.D. – Executive VP, Preclinical Development Hygeia/Canterbury Labs, Sepracor, Parexel International, Dana-Farber Cancer Institute Ph.D. in Endocrine Physiology 5 Management |
|
|
Board of Directors Sol Barer, PhD - Chairman Former CEO & Executive Chairman, and Chairman, Celgene Corporation Isaac Blech - Vice-Chairman Leading biotechnology entrepreneur and investor Genetic Systems, Nova, Celgene, ICOS, Texas BioTechnology, Pathogenesis Stephen M. Simes – CEO BioSante, Unimed, Bio-Technology General, Gynex, Searle Rex Bright SkinMedica, J&J, GlaxoSmithKline, Allergan Nelson Stacks Waveguide Corporation Yael Schwartz, PhD – Executive VP, Preclinical Development Hygeia/Canterbury Labs, Sepracor, Parexel International, Dana-Farber Cancer Institute David Sherris, PhD – Chief Scientific Officer Paloma/Vasculomedics, OXiGENE, Serono, Unilever, Centocor 6 |
|
|
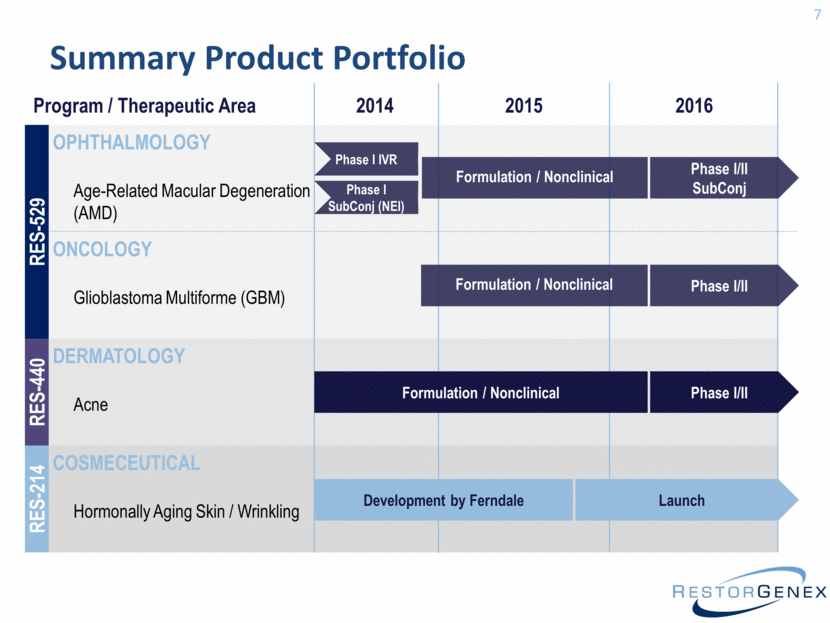
Summary Product Portfolio 7 Program / Therapeutic Area 2014 2015 2016 RES-529 OPHTHALMOLOGY Age-Related Macular Degeneration (AMD) ONCOLOGY Glioblastoma Multiforme (GBM) RES-440 DERMATOLOGY Acne RES-214 COSMECEUTICAL Hormonally Aging Skin / Wrinkling Phase I IVR Formulation / Nonclinical Phase I/II SubConj Formulation / Nonclinical Phase I/II Formulation / Nonclinical Phase I/II Launch Development by Ferndale Phase I SubConj (NEI) |
|
|
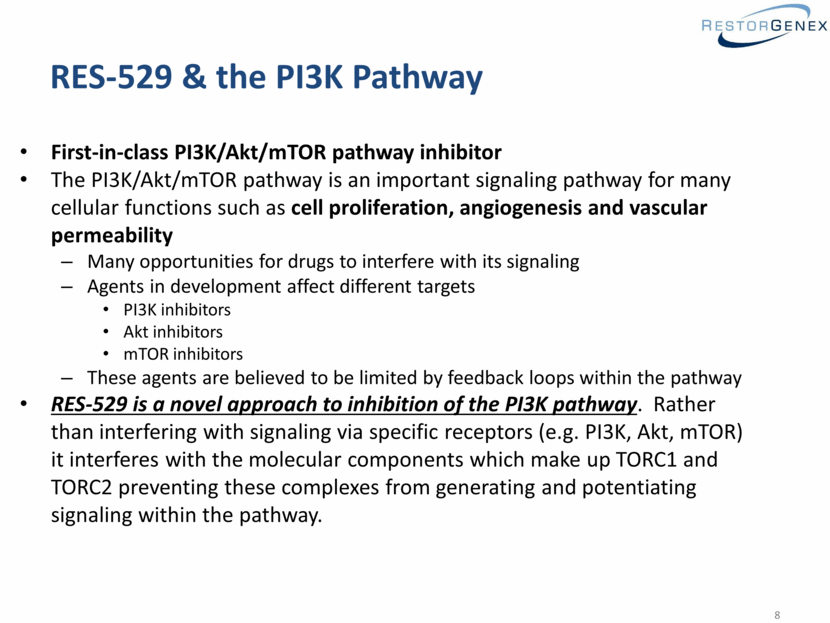
RES-529 & the PI3K Pathway First-in-class PI3K/Akt/mTOR pathway inhibitor The PI3K/Akt/mTOR pathway is an important signaling pathway for many cellular functions such as cell proliferation, angiogenesis and vascular permeability Many opportunities for drugs to interfere with its signaling Agents in development affect different targets PI3K inhibitors Akt inhibitors mTOR inhibitors These agents are believed to be limited by feedback loops within the pathway RES-529 is a novel approach to inhibition of the PI3K pathway. Rather than interfering with signaling via specific receptors (e.g. PI3K, Akt, mTOR) it interferes with the molecular components which make up TORC1 and TORC2 preventing these complexes from generating and potentiating signaling within the pathway. |
|
|
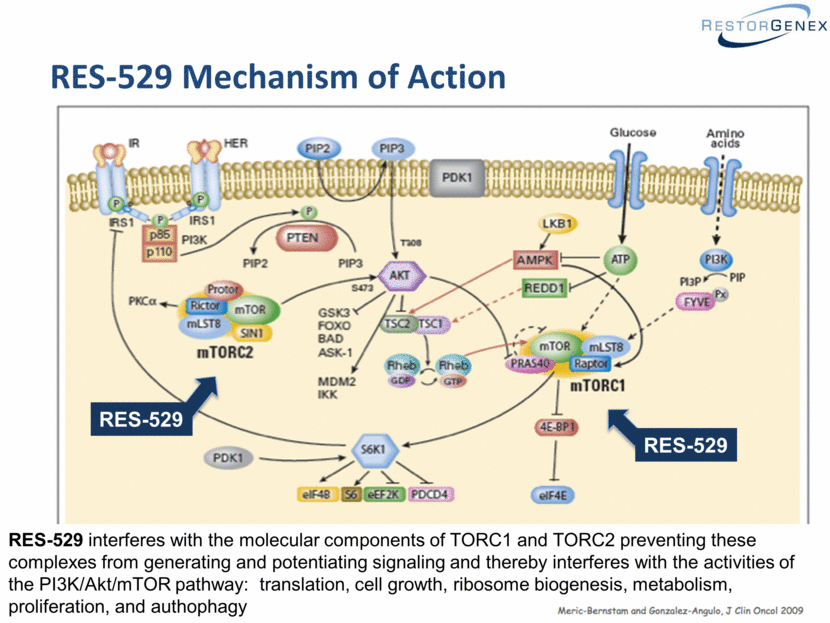
RES-529 Mechanism of Action RES-529 RES-529 RES-529 interferes with the molecular components of TORC1 and TORC2 preventing these complexes from generating and potentiating signaling and thereby interferes with the activities of the PI3K/Akt/mTOR pathway: translation, cell growth, ribosome biogenesis, metabolism, proliferation, and authophagy |
|
|
Ophthalmology |
|
|
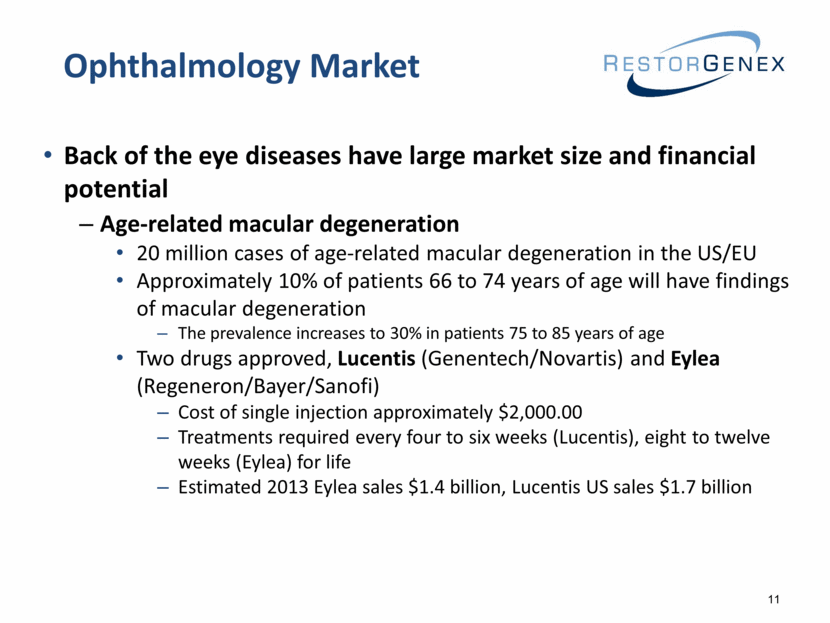
Back of the eye diseases have large market size and financial potential Age-related macular degeneration 20 million cases of age-related macular degeneration in the US/EU Approximately 10% of patients 66 to 74 years of age will have findings of macular degeneration The prevalence increases to 30% in patients 75 to 85 years of age Two drugs approved, Lucentis (Genentech/Novartis) and Eylea (Regeneron/Bayer/Sanofi) Cost of single injection approximately $2,000.00 Treatments required every four to six weeks (Lucentis), eight to twelve weeks (Eylea) for life Estimated 2013 Eylea sales $1.4 billion, Lucentis US sales $1.7 billion Ophthalmology Market 11 |
|
|
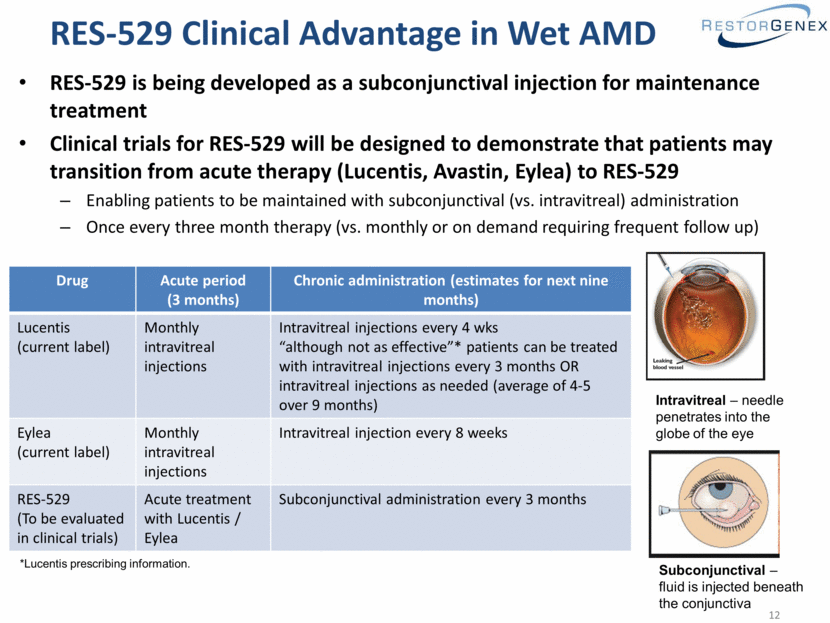
RES-529 Clinical Advantage in Wet AMD RES-529 is being developed as a subconjunctival injection for maintenance treatment Clinical trials for RES-529 will be designed to demonstrate that patients may transition from acute therapy (Lucentis, Avastin, Eylea) to RES-529 Enabling patients to be maintained with subconjunctival (vs. intravitreal) administration Once every three month therapy (vs. monthly or on demand requiring frequent follow up) Drug Acute period (3 months) Chronic administration (estimates for next nine months) Lucentis (current label) Monthly intravitreal injections Intravitreal injections every 4 wks “although not as effective”* patients can be treated with intravitreal injections every 3 months OR intravitreal injections as needed (average of 4-5 over 9 months) Eylea (current label) Monthly intravitreal injections Intravitreal injection every 8 weeks RES-529 (To be evaluated in clinical trials) Acute treatment with Lucentis / Eylea Subconjunctival administration every 3 months Intravitreal – needle penetrates into the globe of the eye Subconjunctival – fluid is injected beneath the conjunctiva *Lucentis prescribing information. |
|
|
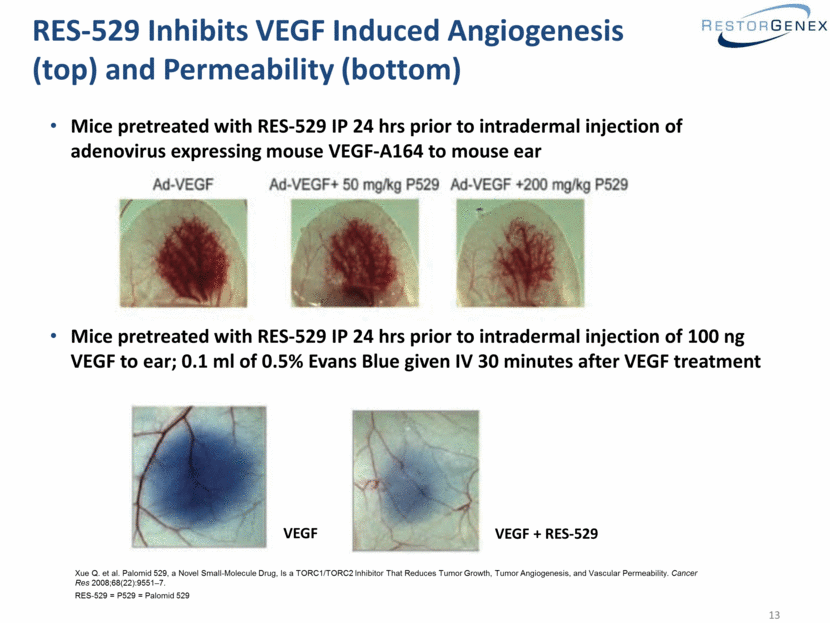
RES-529 Inhibits VEGF Induced Angiogenesis (top) and Permeability (bottom) Mice pretreated with RES-529 IP 24 hrs prior to intradermal injection of adenovirus expressing mouse VEGF-A164 to mouse ear Mice pretreated with RES-529 IP 24 hrs prior to intradermal injection of 100 ng VEGF to ear; 0.1 ml of 0.5% Evans Blue given IV 30 minutes after VEGF treatment VEGF VEGF + RES-529 Xue Q. et al. Palomid 529, a Novel Small-Molecule Drug, Is a TORC1/TORC2 Inhibitor That Reduces Tumor Growth, Tumor Angiogenesis, and Vascular Permeability. Cancer Res 2008;68(22):9551–7. RES-529 = P529 = Palomid 529 |
|
|
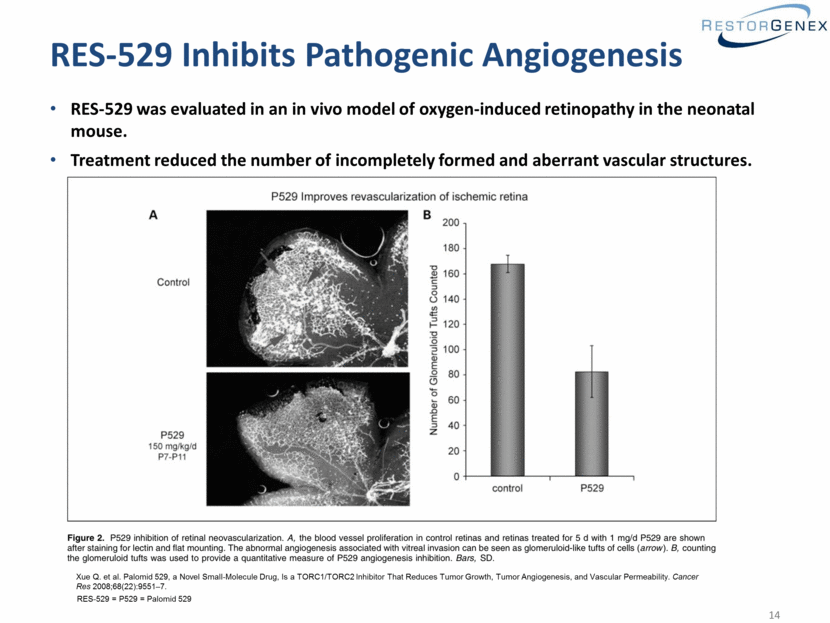
RES-529 Inhibits Pathogenic Angiogenesis RES-529 was evaluated in an in vivo model of oxygen-induced retinopathy in the neonatal mouse. Treatment reduced the number of incompletely formed and aberrant vascular structures. Xue Q. et al. Palomid 529, a Novel Small-Molecule Drug, Is a TORC1/TORC2 Inhibitor That Reduces Tumor Growth, Tumor Angiogenesis, and Vascular Permeability. Cancer Res 2008;68(22):9551–7. RES-529 = P529 = Palomid 529 |
|
|
RES-529: Completed Phase I Trials in AMD Protocol P52901 sponsored Company and conducted by Dr. Jeffrey Heier (Eye Boston) and Dr. David Brown (Retinal Consultants Houston) A Phase I Open-Label Study to Investigate the Safety, Tolerability and Pharmacokinetic Profile of Single Intravitreal and Subconjunctival Doses of RES-529 in Patients with Advanced Neovascular Age-Related Macular Degeneration (AMD) 15 patients treated via intravitreal injection Doses between 0.004mg and 0.5mg Protocol 11-EI-0066 sponsored by National Eye Institute (NEI) and conducted by Dr. Catherine Meyerle A Phase I Unmasked Study to Investigate the Safety and Tolerability of Subconjunctival Injections of RES-529 in Patients with Neovascular Age-Related Macular Degeneration* 5 patients treated with subconjunctival injections of 2mg qmo x 3 *Dalal M. et al. Subconjunctival Palomid 529 in the treatment of neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol (2013) 251:2705–2709. RES-529 = P529 = Palomid 529 |
|
|
RES-529: Clinical Data Patients enrolled: End stage patients, refractory to VEGF therapy Most had significant visual deficits and retinal fluid on OCT Safety/Tolerability: Intravitreal administration (Company sponsored study) 0.5 mg (highest dose studied) associated with temporary visual disturbance from haze in vitreous attributed to drug particles in the vitreous. This effect resolved. Subconjunctival administration (NEI study) 2mg monthly x 3 administered Depot formed at injection site Efficacy: Preliminary evidence of biologic activity based on: fluid pocket reduction retinal thinning cyst reduction |
|
|
RES-529: Status in AMD 2015: Synthesis of additional API Formulation of subconjunctival drug product Toxicology studies to support future trials with subconjunctival formulation 2016: Phase I/II clinical trial Additional assessment of safety and tolerability Determine MTD for subconjunctival administration Clinical efficacy |
|
|
Oncology |
|
|
RES-529 in Glioblastoma Glioblastoma is lead oncology indication Why glioblastoma: Tremendous unmet medical need Proving efficacy is challenging but upside is significant PI3K/Akt/mTOR pathway significantly up-regulated in glioblastoma Plan to conduct phase 2a studies in other tumors once MTD is determined Orally available Potential orphan indication Market size: WW market about $1 billion in 2013 to $4.5 billion by 2020 at a Compound Annual Growth Rate of approximately 28%.* Growth will be driven primarily by new agents. * EvaluatePharma, Adis R&D Insight ** Treatment options and outcomes for glioblastoma in the elderly patient, N.D. Arvold and D. A. Reardon, lin Interv Aging. 2014; 9: 357–367 |
|
|
RES-529 in Glioblastoma Current products: Current standard of care products Temodar, Avastin, Gliadel Wafer Median survival with only supportive care, less than 6 months** Median survival with aggressive chemotherapy in combination with radiotherapy, 12 to 15 months** RES-529 benefits: Activity shown in multiple in vitro and in vivo animal models for GBM with evidence that it can pass the blood brain barrier First-in-class mechanism of action exerting PI3K/Akt/mTOR pathway control above other drugs targeting this pathway (PI3K pathway significantly up-regulated in GBM) * EvaluatePharma, Adis R&D Insight ** Treatment options and outcomes for glioblastoma in the elderly patient, N.D. Arvold and D. A. Reardon, lin Interv Aging. 2014; 9: 357–367 |
|
|
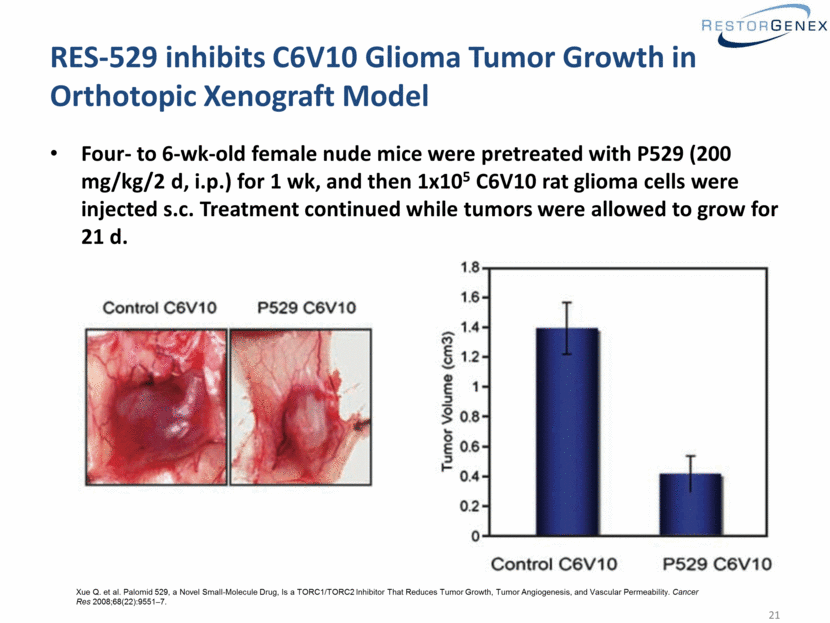
RES-529 inhibits C6V10 Glioma Tumor Growth in Orthotopic Xenograft Model Four- to 6-wk-old female nude mice were pretreated with P529 (200 mg/kg/2 d, i.p.) for 1 wk, and then 1x105 C6V10 rat glioma cells were injected s.c. Treatment continued while tumors were allowed to grow for 21 d. Xue Q. et al. Palomid 529, a Novel Small-Molecule Drug, Is a TORC1/TORC2 Inhibitor That Reduces Tumor Growth, Tumor Angiogenesis, and Vascular Permeability. Cancer Res 2008;68(22):9551–7. |
|
|
RES-529 has been extensively studied by academia in preclinical oncology models Similar efficacy in glioma orthotopic xenograft model regardless of route of administration (iv, po, or ip)1 Potent effects in NCI60 cell line panel2 Penetration of the blood brain barrier with pharmacologically active levels reached in murine brain3 Efficacy in breast cancer xengorafts4 Synergy with XRT and chemotherapy in prostate cancer xenografts5,6 1Xue Q. et al. Palomid 529, a Novel Small-Molecule Drug, Is a TORC1/TORC2 Inhibitor That Reduces Tumor Growth, Tumor Angiogenesis, and Vascular Permeability. Cancer Res 2008;68(22):9551–7 and Xue Q. unpublished data. 2Diaz R. et al. The novel Akt inhibitor Palomid 529 (P529) enhances the effect of radiotherapy in prostate cancer. Br J Cancer 2009Mar24;100(6):932-40. 3Lin F. et al. Dual mTORC1 and mTORC2 inhibitor Palomid 529 penetrates the Blood–Brain Barrier without restriction by ABCB1 and ABCG2. Int J Cancer 2013Sep1;133(5):1222-33. 4Xiang T. et al. Targeting the Akt/mTOR pathway in Brca1-deficient cancers. Oncogene 20011May26;30(21):2443-50. 5Gravinia GL. et al. Torc1/Torc2 Inhibitor, Palomid 529, Enhances Radiation Response Modulating CRM1-Mediated Survivin Function and Delaying DNA Repair in Prostate Cancer Models. Prostate 2014JanJun74(8);852-68. 6Gravinia GL. et al. The TORC1/TORC2 inhibitor, Palomid 529, reduces tumor growth and sensitizes to docetaxel and cisplatin in aggressive and hormone-refractory prostate cancer cells. Endocrine Related Cancer (2011);18:385-400 RES-529 = P529 = Palomid 529 |
|
|
RES-529: Status in Glioblastoma 2015: Synthesis of additional API Development of oral formulation Toxicology studies to support systemic administration in oncology 2016: Phase I/II clinical trial Determine MTD in glioblastoma Efficacy assessment in recurrent glioblastoma Launch Phase 2a studies in other oncology indications utilizing MTD dose Including prostate cancer and breast cancer |
|
|
Dermatology |
|
|
RES-440: Our Lead Anti-androgen An Easily Deactivated, Topically Active “Soft” Anti-Androgen for Local Disorders Resulting from Excess Androgen Acne Vulgaris is the Primary Initial Market Opportunity 25 |
|
|
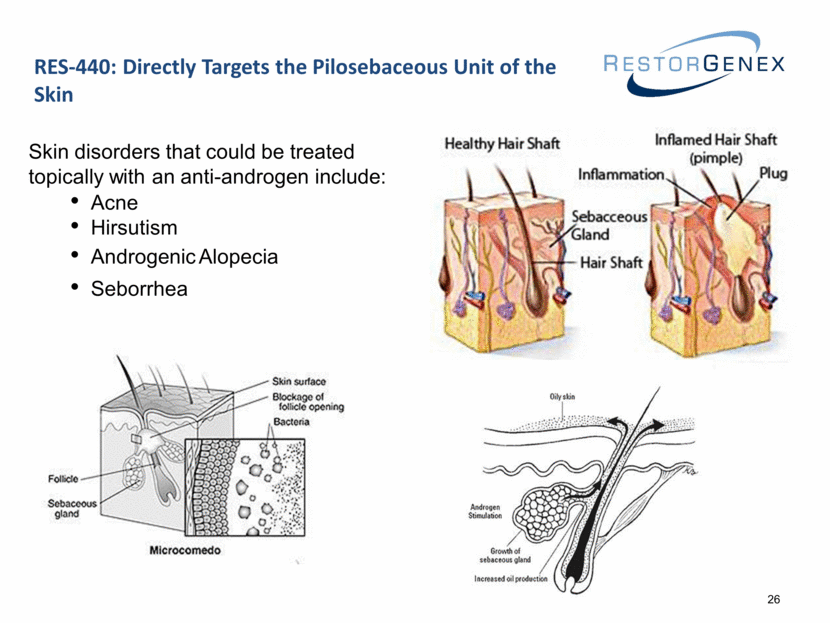
RES-440: Directly Targets the Pilosebaceous Unit of the Skin Skin disorders that could be treated topically with an anti-androgen include: Acne Hirsutism Androgenic Alopecia Seborrhea 26 |
|
|
RES-440 (“soft” anti-androgen) Proposed indication: Acne Market size: $1B-$2B** (Acne affects 40 million to 50 million Americans) Current products: Retinoids: skin irritation, sunlight sensitivity; Accutane: systemic effects, birth defects; Antibiotics: tooth discoloration and resistance RES-440 benefits: A first-in-class topical that directly targets the androgen receptor; no systemic exposure; non-irritating, no sunlight sensitivity Status: Phase I/II Q1 2016 (12 week trial) **IBIS World, 2012; GlobalData, 2010: Acne Drug Pipeline Analysis and Market Forecasts to 2016 27 |
|
|
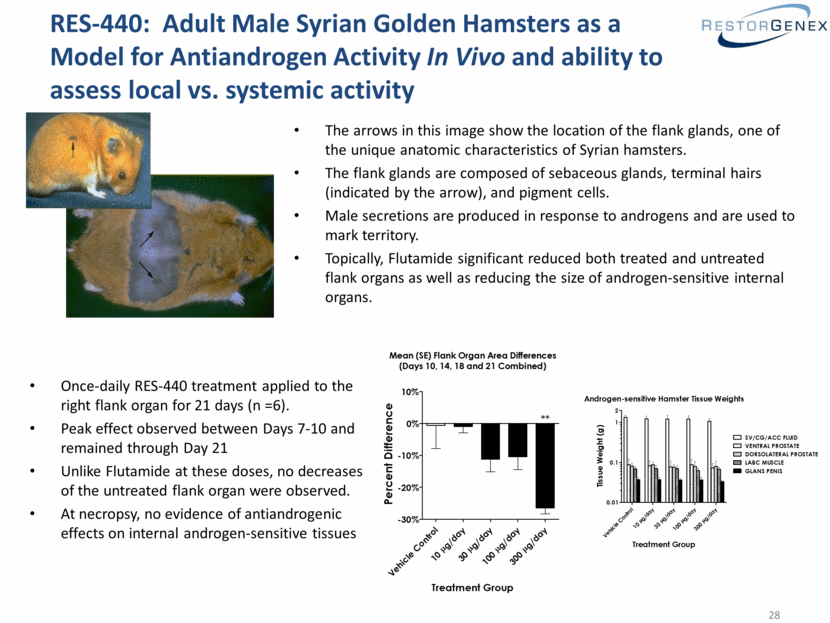
RES-440: Adult Male Syrian Golden Hamsters as a Model for Antiandrogen Activity In Vivo and ability to assess local vs. systemic activity The arrows in this image show the location of the flank glands, one of the unique anatomic characteristics of Syrian hamsters. The flank glands are composed of sebaceous glands, terminal hairs (indicated by the arrow), and pigment cells. Male secretions are produced in response to androgens and are used to mark territory. Topically, Flutamide significant reduced both treated and untreated flank organs as well as reducing the size of androgen-sensitive internal organs. Once-daily RES-440 treatment applied to the right flank organ for 21 days (n =6). Peak effect observed between Days 7-10 and remained through Day 21 Unlike Flutamide at these doses, no decreases of the untreated flank organ were observed. At necropsy, no evidence of antiandrogenic effects on internal androgen-sensitive tissues Mean (SE) Flank Organ Area Differences (Days 10, 14, 18 and 21 Combined) Vehicle Control g/day m 10 g/day m 30 g/day m 100 g/day m 300 -30% -20% -10% 0% 10% Treatment Group Percent Difference ** Androgen-sensitive Hamster Tissue Weights Vehicle Control g/day m 10 g/day m 30 g/day m 100 g/day m 300 0.01 0.1 1 SV/CG/ACC FLUID VENTRAL PROSTATE DORSOLATERAL PROSTATE LABC MUSCLE GLANS PENIS 2 Treatment Group Tissue Weight (g) |
|
|
RES-440: Status in Acne 2015: Synthesis of API Development of topical formulation for clinical trials in acne Toxicology work to support IND 2016: Phase I/II clinical trial Assess safety and tolerability Efficacy in treatment of acne |
|
|
RestorGenex Summary Financial Information as of December 31, 2014 Cash: $22,000,000 Accounts payable: $94,000 Debt: $0 Burn rate into 2015: Approximately $600,000/month Shares outstanding: 18,621,153 Options outstanding: 3,658,701 Warrants outstanding: 4,571,799 Fully diluted: 26,851,653 30 |
|
|
Overview Specialty pharmaceutical company initially focused on developing products for ophthalmology, oncology and dermatology Indications in development Ophthalmology Age-related macular degeneration (AMD); Two Phase I trials completed Current market: over $3 billion Oncology Initially targeting glioblastoma, follow-on to other oncology indications Current market: over $1 billion in glioblastoma; multi-billion in several oncology indications Dermatology: Acne is lead indication Current market: over $1 billion |
|
|
Investment Opportunity Board and management have built major value in public and private biotechnology companies Multiple products in development Relatively short/inexpensive trials leading to rapid and numerous valuation inflection points Multi-billion dollar indications Proposed products based on proprietary platforms Financial security Near-term goal: increased stockholder value through development programs and licensing 32 |
|
|
[LOGO] |