Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Radius Health, Inc. | a15-2164_1ex99d1.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of
the Securities Exchange Act of 1934
Date of report (Date of earliest event reported): January 12, 2015
RADIUS HEALTH, INC.
(Exact name of registrant as specified in its charter)
|
Delaware |
|
001-35726 |
|
80-0145732 |
|
(State or other jurisdiction of |
|
(Commission |
|
(I.R.S. Employer |
950 Winter Street
Waltham, MA 02451
(Address of principal executive offices) (Zip Code)
(617) 551-4000
(Registrant’s telephone number, include area code)
N/A
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
¨ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
¨ Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
¨ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
¨ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Item 7.01. Regulation FD Disclosure.
On January 12, 2015, Radius Health, Inc. announced a series of follow-ups on the positive top-line 18 month facture results that it announced on December 21, 2014 from its Phase 3 clinical trial (ACTIVE) evaluating the investigational drug abaloparatide-SC for potential use in the reduction of fractures in post-menopausal osteoporosis. The full text of the press release issued in connection with the announcement is furnished as Exhibit 99.1 to this Current Report on Form 8-K.
The information in Item 7.01 of this Form 8-K (including Exhibit 99.1) shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly provided by specific reference in such a filing.
Item 8.01. Other Events.
Radius Health, Inc., which is referred to herein as the “Company,” “we,” “us” or “our”, recently updated its business information as follows:
Abaloparatide-SC
On December 21, 2014, the Company announced positive top-line data from the Phase 3 clinical trial (ACTIVE) of the investigational drug abaloparatide-SC, which is referred to herein as the “ACTIVE Trial”, evaluating the investigational drug abaloparatide-SC for potential use in the reduction of fractures in postmenopausal osteoporosis. On the primary endpoint, abaloparatide-SC (n=690, fracture rate 0.72%) achieved a statistically significant 83% reduction of incident vertebral fractures (defined as new and worsening vertebral fractures) as compared to the placebo-treated group (n=711, fracture rate 4.36%) (p<0.0001). The ACTIVE trial included an open-label teriparatide [rDNA origin] injection treatment group (n=717, fracture rate 0.98%) that showed a statistically significant 78% reduction of incident vertebral fractures as compared to the placebo-treated group (p<0.0001). On the secondary endpoints, as compared to placebo, abaloparatide-SC achieved: a statistically significant fracture-rate reduction of 43% in the adjudicated non-vertebral fracture subset of patients; a statistically significant reduction of 45% in the adjudicated clinical fracture group (this value was incorrectly reported as 41% in the press release issued by the Company on December 21, 2014), which includes both vertebral and non-vertebral fractures; and a statistically significant difference in the time to first incident of non-vertebral fracture in both the adjudicated non-vertebral fracture (p=0.0489) and the clinical fracture subset of patients (p=0.0112). The open-label teriparatide injection treatment group, as compared to placebo, achieved a fracture-rate reduction of 28% in the adjudicated non-vertebral fracture subset of patients and a reduction of 29% in the adjudicated clinical fracture group. The fracture-rate reduction observed in the abaloparatide-SC treatment group, as compared to open-label teriparatide, was not statistically significant.
On January 8, 2015, the U.S. Food and Drug Administration, or FDA, provided the Company comments on the draft Statistical Analysis Plan, or SAP, that was used for the analysis of the top-line data from the ACTIVE Trial. In its correspondence, FDA made several recommendations for changes in the data analyses undertaken in the SAP. The Company has performed these analyses and believes that, as noted below, there are no material changes from the top-line results the Company announced on December 21, 2014. The Company believes that the abaloparatide-SC program is on-track for submission of a new drug application for abaloparatide-SC to the FDA, and submission of a marketing authorization application to the European Medicines Agency, or EMA, in the second half of 2015. However, FDA and EMA have not reviewed any of the data from the ACTIVE trial. The results from the ACTIVE trial and from the first six months of the ACTIVExtend trial, together with the entire data set from the abaloparatide development program, are subject to regulatory review, and only FDA and EMA can separately determine whether the data in the new drug application, once submitted, support approval of the investigational drug abaloparatide-SC for its potential use in the reduction of fractures in postmenopausal osteoporosis.
In the January 8, 2015 correspondence, FDA recommended that the primary endpoint of incident vertebral fracture reduction be performed excluding worsening vertebral fracture and including only new vertebral fracture. Using the FDA-recommended analysis, on the primary endpoint of reduction of new vertebral fractures (excluding worsening), abaloparatide-SC (n=690, fracture rate 0.58%) achieved a statistically significant 86% reduction as
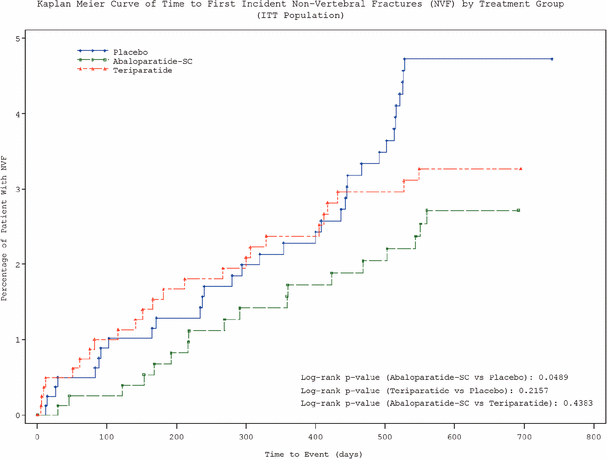
compared to the placebo-treated group (n=711, fracture rate 4.22%) (p<0.0001). The open-label teriparatide injection treatment group (n=717, fracture rate 0.84%) showed a statistically significant 80% reduction of new vertebral fractures (excluding worsening) as compared to the placebo-treated group (p<0.0001). FDA also recommended, for the secondary endpoint of non-vertebral fractures, that the Company’s definition was generally acceptable provided that sternal (breast bone) and patella (knee cap) fractures were excluded. In the previously announced top-line data for the secondary endpoint of non-vertebral fracture reduction noted above, the Company had excluded sternum and patella. For that analysis, abaloparatide-SC (n=824, Kaplan-Meier estimated, or KM, fracture rate 2.7%) achieved a statistically significant reduction compared to the placebo-treated group (n=821, KM fracture rate 4.7%), and the hazard ratio for abaloparatide vs. placebo was 0.57 (p=0.0489); the open label teriparatide injection treatment group (n=818, KM fracture rate 3.3%) had a hazard ratio of 0.72 (p=NS) compared to the placebo-treated group. FDA also recommended, for the secondary endpoint of bone mineral density, or BMD, that the Company use an ANCOVA, approach with the last observation carried forward for missing data. The Mixed-Effect Model For Repeated Measures, or MMRM, method, which was used in the BMD secondary endpoint in the top-line data announced in December 2014, is to be applied for sensitivity analysis.
The top-line results announced in December 2014 included the following results of comparative analyses of abaloparatide-SC versus teriparatide using the MMRM method on these BMD secondary endpoints.
Mean Percent Change In Bone Mineral Density (BMD) From Baseline (MMRM approach)
|
|
|
Lumbar Spine |
|
Total Hip |
|
Femoral Neck |
| ||||||||||||
|
|
|
6 mo |
|
12 mo |
|
18 mo |
|
6 mo |
|
12 mo |
|
18 mo |
|
6 mo |
|
12 mo |
|
18 mo |
|
|
Placebo |
|
0.60 |
% |
0.45 |
% |
0.63 |
% |
0.31 |
% |
0.09 |
% |
-0.10 |
% |
-0.13 |
% |
-0.41 |
% |
-0.43 |
% |
|
abaloparatide-SC |
|
6.58 |
%** |
9.77 |
%** |
11.20 |
%* |
2.32 |
%** |
3.41 |
%** |
4.18 |
%** |
1.72 |
%** |
2.65 |
%** |
3.60 |
%** |
|
teriparatide |
|
5.25 |
%* |
8.28 |
%* |
10.49 |
%* |
1.44 |
%* |
2.29 |
%* |
3.26 |
%* |
0.87 |
%* |
1.54 |
%* |
2.66 |
%* |
** p<0.0001 vs. placebo and teriparatide
* p<0.0001 vs. placebo
Applying the ANCOVA approach with the last observation carried forward that FDA recommended in its January 8, 2015 correspondence results in the following comparative analysis of the BMD secondary endpoints:
Mean Percent Change In Bone Mineral Density (BMD) From Baseline (ANCOVA approach)
|
|
|
Lumbar Spine |
|
Total Hip |
|
Femoral Neck |
| ||||||||||||
|
|
|
6 mo |
|
12 mo |
|
18 mo |
|
6 mo |
|
12 mo |
|
18 mo |
|
6 mo |
|
12 mo |
|
18 mo |
|
|
Placebo |
|
0.55 |
% |
0.39 |
% |
0.48 |
% |
0.29 |
% |
0.10 |
% |
-0.08 |
% |
-0.12 |
% |
-0.37 |
% |
-0.44 |
% |
|
abaloparatide-SC |
|
5.90 |
%** |
8.19 |
%*** |
9.20 |
%* |
2.07 |
%** |
2.87 |
%** |
3.44 |
%**** |
1.54 |
%** |
2.21 |
%** |
2.90 |
%***** |
|
teriparatide |
|
4.84 |
%* |
7.40 |
%* |
9.12 |
%* |
1.33 |
%* |
2.03 |
%* |
2.81 |
%* |
0.80 |
%* |
1.41 |
%* |
2.26 |
%* |
* vs. placebo p<0.0001
** vs. teriparatide p<0.0001
*** vs. placebo p< 0.0001 AND vs. teriparatide p=0.0087
**** vs. placebo p< 0.0001 AND vs. teriparatide p=0.0003
***** vs. placebo p< 0.0001 AND vs. teriparatide p=0.0016
The ACTIVE Trial also evaluated several potential safety measures, including blood calcium levels, orthostatic hypotension, nausea, dizziness and injection-site reactions. Among the most frequently reported adverse events, the following incidence rates were reported in the trial:
· back pain: placebo (n=820) (10.0%), abaloparatide (n=822) (8.6%), teriparatide (n=818) (7.2%)
· arthralgia: placebo (9.8%), abaloparatide (8.5%), teriparatide (8.6%)
· upper respiratory tract infection: placebo (8.9%), abaloparatide (9.0%), teriparatide (9.8%)
· hypercalciuria: placebo (8.9%), abaloparatide (10.9%), teriparatide (12.5%)
· dizziness: placebo (6.1%), abaloparatide (10.0%), teriparatide (7.3%)
In December 2014, the Company reported hypercalcemia event rates using uncorrected serum calcium values of 1.2% for the placebo group (n=820), 6.0% for the abaloparatide-SC group (n=822) and 10.8% for the teriparatide group (n=818). The results for the primary analysis of the hypercalcemia event rate based on albumin corrected serum calcium are now available and are as follows: 0.37% for the placebo group (n=820), 3.41% for the abaloparatide-SC group (n=822) and 6.36% for the teriparatide group (n=818). Each of the abaloparatide group and teriparatide group had statistically significantly higher hypercalcemia event rates as compared to the placebo group, and the abaloparatide group had a statistically significant lower hypercalcemia event rate as compared to the teriparatide group (p=0.0055).
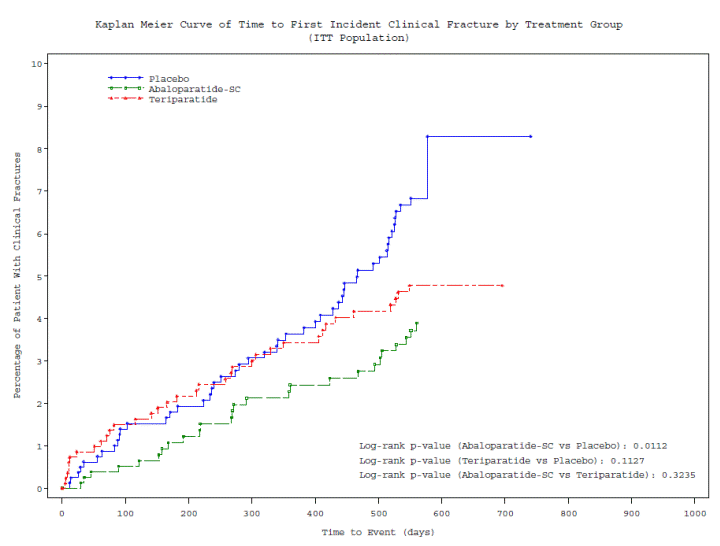
As part of the top-line results, the Company reported the results for several exploratory endpoints which are being updated in this Current Report. For clinical fractures, abaloparatide-SC (n=824, KM fracture rate 3.9%) statistically significantly reduced clinical fractures compared to placebo (n=821, KM fracture rate 8.3%) with a hazard ratio=0.55 (p=0.0112); teriparatide (n=818, KM fracture rate 4.8%) had a hazard ratio = 0.71 (p=NS) compared to the placebo treated group.
For wrist fractures, abaloparatide-SC (n=824, KM fracture rate 0.5%) and teriparatide (n=818, KM fracture rate 2.0%) were not statistically significantly reduced compared to placebo (n=821, KM fracture rate 1.5%); wrist fractures were statistically significantly less for abaloparatide-SC than for the teriparatide treated group (p=0.0149).
The following table sets forth the Kaplan-Meier curve of time to first incident non-vertebral fractures by treatment group in the intent-to-treat population.
The following table sets forth the Kaplan-Meier curve of time to first incident clinical fractures by treatment group in the intent-to-treat population.
The Company anticipates the first results from the ongoing six-month extension study (the “ACTIVExtend trial”) in the second quarter of 2015, and plans to submit a new drug application to the FDA, and a marketing authorization application to the EMA, in the second half of 2015. The results from the ACTIVE trial and from the first six months of the ACTIVExtend trial, together with the entire data set from the abaloparatide development program, are subject to regulatory review. The Company holds worldwide commercialization rights to Abaloparatide-SC, other than in Japan, and with a favorable regulatory outcome, the Company anticipates the first commercial sales of Abaloparatide-SC will take place in 2016.
Abaloparatide-TD
On December 21, 2014, the Company also reported that it made progress towards the development of an optimized, short-wear-time transdermal patch, for its investigational drug abaloparatide-TD based on 3M Drug Delivery Systems’ sMTS platform. Together with 3M Drug Delivery Systems, the Company evaluated a number of new TD patch configurations with the goal of selecting a configuration that may be capable of demonstrating therapeutic comparability to abaloparatide-SC injection. In preliminary, non-human primate pharmacokinetic studies, prototype A7 achieved a desirable pharmacokinetic profile, with comparable AUC, Cmax, Tmax and T1/2 relative to abaloparatide-SC. The Company believes that these results support continued clinical development toward future global regulatory submissions as a potential post-approval line extension of the investigational drug abaloparatide-SC. The Company expects to initiate the clinical evaluation of the optimized abaloparatide-TD patch in the second half of 2015, with the goal of achieving pharmacokinetic equivalence to abaloparatide-SC.
RAD1901
On December 21, 2014, the Company also reported that it continues to advance its novel oral agent, RAD1901, a selective estrogen receptor down-regulator/degrader, and expects to report progress on its Phase 1 clinical study in the United States for the treatment of metastatic breast cancer in the first half of 2015 and to initiate two Phase 1 clinical studies in the European Union in 2015. Following FDA acceptance of the IND for the U.S. Phase 1 clinical study in December 2014, the Company submitted the U.S. Phase 1 clinical study protocol to the NIH ClinicalTrials.gov registry and the clinical trial record is expected to be available online in January 2015. The Phase 1 study that is the subject of the IND is a multicenter, open-label, two-part, dose-escalation study of RAD1901 in postmenopausal women with advanced estrogen receptor positive and HER2-negative breast cancer that is designed to determine the recommended dose for a Phase 2 study and includes a preliminary evaluation of the potential anti-tumor effect of RAD1901. This U.S. Phase 1 clinical study of RAD1901 for the treatment of metastatic breast cancer is now open for patient screening and enrollment. The Company also expects to initiate a Phase 2b clinical trial of RAD1901 for vasomotor systems in the second half of 2015.
Forward-Looking Statements
This Current Report contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this Current Report that do not relate to matters of historical fact should be considered forward-looking statements, including without limitation, expectations regarding the clinical significance and regulatory review of top-line data from our Phase 3 ACTIVE study and from our extension study of abaloparatide-SC, the submission of an NDA or MAA for abaloparatide-SC, the timing of the completion of the ACTIVExtend study and the availability of results, the timing of regulatory submissions applying for approval of abaloparatide-SC, the clinical development of abaloparatide-TD and the clinical development of RAD1901.
These forward-looking statements are based on management’s current expectations. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the following: we have no product revenues; our need for additional funding, which may not be available; we are not currently profitable and may never become profitable; restrictions imposed on our business by our credit facility, and risks related to default on our obligations under our credit facility; risks related to raising additional capital; our limited operating history; quarterly fluctuation in our financial results; our dependence on the success of
abaloparatide-SC, and our inability to ensure that abaloparatide-SC will obtain regulatory approval or be successfully commercialized; risks related to clinical trials, including having most of our products in early stage clinical trials and uncertainty that results will support our product candidate claims; the risk that adverse side effects will be identified during the development of our product candidates; product candidates for which we obtain marketing approval, if any, could be subject to restrictions or withdrawal from the market and we may be subject to penalties; failure to achieve market acceptance of our product candidates; risks related to the use of our limited resources on particular product candidates and not others; delays in enrollment of patients in our clinical trials, which could delay or prevent regulatory approvals; the dependence of our drug development program upon third-parties who are outside our control; the risk that a regulatory or government official will determine that third-parties with a financial interest in the outcome of the Phase 3 study of abaloparatide-SC affected the reliability of the data from the study; our reliance on third parties to formulate and manufacture our product candidates; failure to establish additional collaborations; our lack of experience selling, marketing and distributing products and our lack of internal capability to do so; failure to compete successfully against other drug companies; developments by competitors may render our products or technologies obsolete or non-competitive; risks related to the fact that our drugs may sell for inadequate prices or patients may be unable to obtain adequate reimbursement; effects of product liability lawsuits on commercialization of our products; failure to comply with obligations of our intellectual property licenses; failure to protect our intellectual property or failure to secure necessary intellectual property related to abaloparatide-SC, abaloparatide-TD, RAD-1901 and/or RAD-140; our or our licensors’ inability to obtain and maintain patent protection for technology and products; risks related to our compliance with patent application requirements; failure to protect the confidentiality of our trade secrets; risks related to our infringement of third parties’ rights; risks related to employees’ disclosure of former employers’ trade secrets; risks associated with intellectual property litigation, including expending substantial resources and distracting personnel from their normal responsibilities; risks associated with healthcare reform; our failure to comply with healthcare laws and regulations; our exposure to claims associated with the use of hazardous materials and chemicals; inability to successfully manage our growth; risks relating to business combinations and acquisitions; our reliance on key executive officers and advisors; our inability to hire additional qualified personnel; volatility in the price of our common stock; capital appreciation is the only source of gain for our common stock; risks related to increased costs and compliance initiatives associated with operating as a public company; our directors, executive officers and principal stockholders have substantial control over us and could delay or prevent a change in control; future sales of our common stock could depress the price of our common stock; inaccurate or unfavorable information about us could cause the price of our common stock to decline; provisions in our charter documents and Delaware law could discourage takeover attempts; and our ability to use our net operating loss carryforwards and certain other tax attributes may be limited. These and other important factors discussed under the caption “Risk Factors” in our Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission, or SEC, on November 10, 2014, and our other reports filed with the SEC could cause actual results to differ materially from those indicated by the forward-looking statements made in this Current Report. Any such forward-looking statements represent management’s estimates as of the date of this Current Report. While we may elect to update such forward-looking statements at some point in the future, we disclaim any obligation to do so, even if subsequent events cause our views to change. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this Current Report.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits
The following exhibit relating to Item 7.01 shall be deemed to be furnished, and not filed:
|
Exhibit |
|
Description |
|
|
|
|
|
99.1 |
|
Press Release issued on January 12, 2015 |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
RADIUS HEALTH, INC. | |
|
|
|
|
|
|
|
|
|
Date: January 12, 2015 |
By: |
/s/ B. Nicholas Harvey |
|
|
|
Name: B. Nicholas Harvey |
|
|
|
Title: Chief Financial Officer |
