Attached files
| file | filename |
|---|---|
| 8-K - 8-K - LUMINEX CORP | form8-k011215.htm |

N AC H U M “ H O MI ” S H A MI R P R E S I D E N T A N D C H I E F E X E C U T I V E O F F I C E R 3 3 R D A N N UA L J . P. M O R G A N H E A LT H C A R E C O N F E R E N C E J A N U A R Y 1 5 , 2 0 1 5

SAFE HARBOR STATEMENT 2 | © Copyright 2015 Luminex Corporation | Breakthrough solutions to improve health and advance science Certa in statements made dur ing the course o f th is presentat io n may not be purely h istor ica l and conseque nt l y may be forward lo o k ing statements w ith in the meaning o f the Pr ivate Secur i t i es L i t igat ion Reform Act o f 1995, inc ludi n g but not l imite d to statements made regardin g: our partner model and the abi l i ty o f our partners and insta l le d base to dr ive future growth; the abi l i ty o f o ur techno logy to enhance product iv i t y and ef f ic iency; our f inanc ia l pos i t ion and long-term revenue growth; our molecular d iagnost ic bus ines s model , the markets we are target ing, market segmentat io n, expected growth o f such markets , and the abi l i ty o f o ur products to address those markets ; sa les o f our products , thei r technica l capabi l i t i es , and the ant ic ipate d market s i ze and acceptance, demand and regulatory env ironm e nt and approva ls therefor ; our d i rect sa les ef forts ; our system placement s; our system and assay product p ipel i n e and ant ic ipated t imel i n es for regulatory approva ls and market re leases , inc ludi n g for ARIES instrum e ntat io n and assays; market opportuni ty for ARIES and NxTAG RPP product ; funct iona l i t y and benef i t s o f ARIES and NxTAG RPP and compet i t i v e po s i t io n; re imbu rs e me n t t rends; develop me n t and growth o f our PGx strategy , our abi l i ty to dr ive growth thro ugh investm e nt in R&D and focus on operat ing leverage and managing operat ing costs ; our long term f inanc ia l targets ; our key steps and strategies fo r gro wth; o ur st rategic out look and growth p lan for our bus ines s for 2015 and beyond; operat iona l t rends, inc ludi n g those re lated to sa les o f systems, assays , consumables , and roya l ty revenue s; compet i t iv e threats and products o f fered by o ther companies; 2014 revenue guidance; our bus ines s out look, f inanc ia l targets and pro ject ions about revenu es, cash f low, system shipme nt s , expense s and market condit ions , and their ant ic ipate d impact on Luminex for 2015 and beyond; and, any statement s o f the p lans , s t rategie s and object ives o f managemen t for future operat ions. These forward look ing statements speak only as o f the date hereof and are based on our current bel iefs and expectat ions and are subject to known or unknown r i sks and uncerta int i es so me of which are beyond our contro l that could cause actua l resul ts or p lans to d i f fer mater ia l l y and adverse l y f rom those ant ic ipated in the forward look ing statements . Factors that could cause or contr ibute to such d i f ferenc es are deta i le d in our annual , quarter l y , o r o ther f i l ing s w ith the Secur i t ie s and Exchange Commiss ion. We undertake no obl igat io n to update these forward look ing statements . A lso , certa in non-GAAP f inanc ia l measures as def ined by SEC Regulat io n G, may be covered in th is presentat io n. To the extent that any non-GAAP f inanc ia l measures are covered, a presentat io n o f and reconc i l ia t io n to the most d i rect ly comparable GAAP f inanc ia l measures wi l l be inc lude d in th is presentat ion may be ava i lable on our websi te at www. luminexcor p.com in accordance with Regulat ion G.
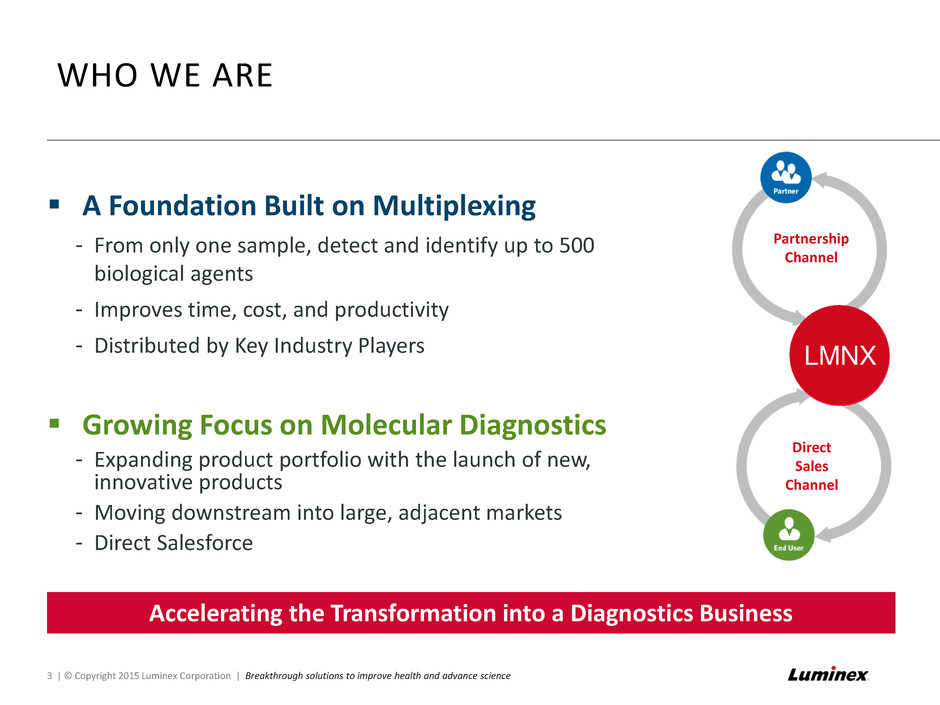
WHO WE ARE 3 | © Copyright 2015 Luminex Corporation | Breakthrough solutions to improve health and advance science A Foundation Built on Multiplexing - From only one sample, detect and identify up to 500 biological agents - Improves time, cost, and productivity - Distributed by Key Industry Players Growing Focus on Molecular Diagnostics - Expanding product portfolio with the launch of new, innovative products - Moving downstream into large, adjacent markets - Direct Salesforce Shifting the Company into a Diagnostics Business Partnership Channel Direct Sales Channel Accelerati g the Transf rmation into a Diagnostics Business LMNX
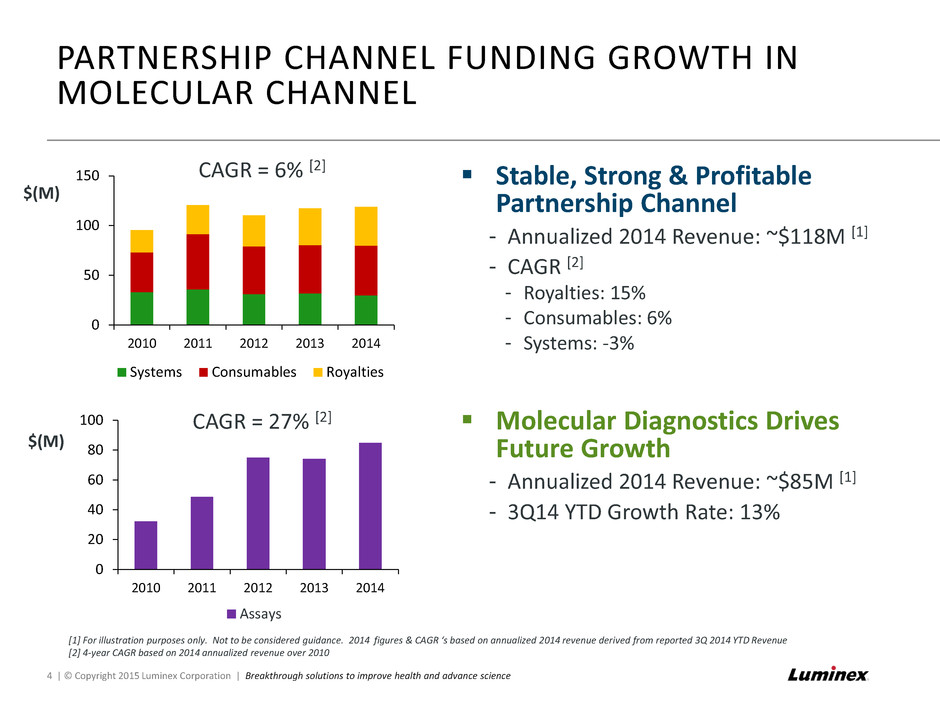
PARTNERSHIP CHANNEL FUNDING GROWTH IN MOLECULAR CHANNEL 4 | © Copyright 2015 Luminex Corporation | Breakthrough solutions to improve health and advance science Stable, Strong & Profitable Partnership Channel - Annualized 2014 Revenue: ~$118M [1] - CAGR [2] - Royalties: 15% - Consumables: 6% - Systems: -3% Molecular Diagnostics Drives Future Growth - Annualized 2014 Revenue: ~$85M [1] - 3Q14 YTD Growth Rate: 13% [1] For illustration purposes only. Not to be considered guidance. 2014 figures & CAGR ‘s based on annualized 2014 revenue derived from reported 3Q 2014 YTD Revenue [2] 4-year CAGR based on 2014 annualized revenue over 2010 0 50 100 150 2010 2011 2012 2013 2014 $(M) Systems Consumables Royalties 0 20 40 60 80 100 2010 2011 2012 2013 2014 $(M) Assays CAGR = 6% [2] CAGR = 27% [2]
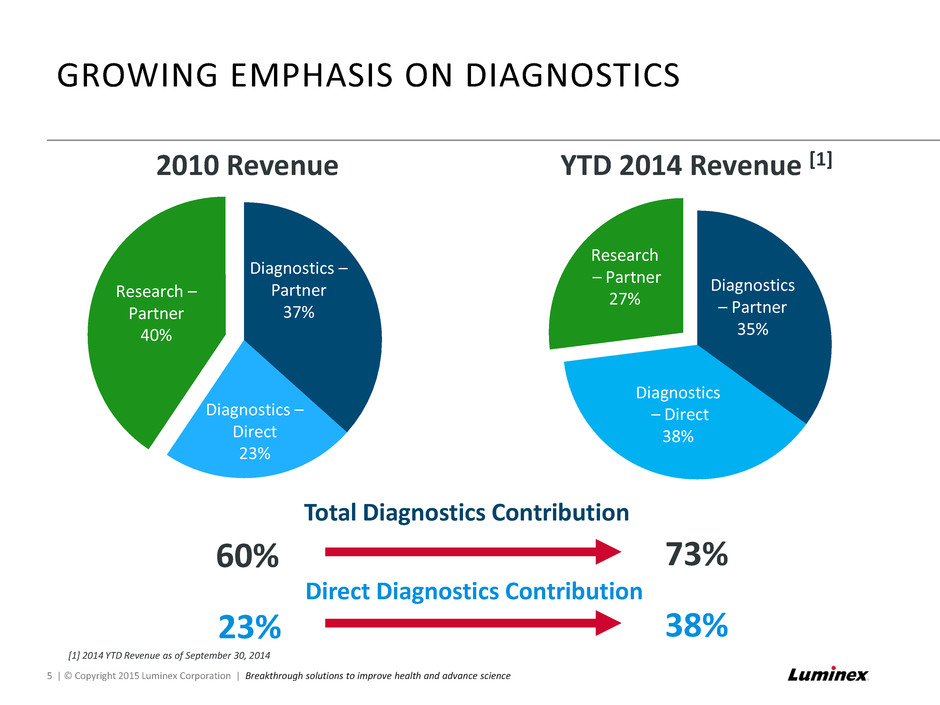
Diagnostics – Direct 38% Diagnostics – Partner 35% Research – Partner 27% GROWING EMPHASIS ON DIAGNOSTICS 5 | © Copyright 2015 Luminex Corporation | Breakthrough solutions to improve health and advance science YTD 2014 Revenue [1] Research – Partner 40% Diagnostics – Direct 23% Diagnostics – Partner 37% 2010 Revenue Total Diagnostics Contribution 60% 73% Direct Diagnostics Contribution 23% 38% [1] 2014 YTD Revenue as of September 30, 2014

Positioning for Accelerating Growth in Molecular Diagnostics With Launches of ARIES and NxTAG 2015 – YEAR OF TRANSITION 6 | © Copyright 2015 Luminex Corporation | Breakthrough solutions to improve health and advance science ATTRACTIVE Markets Leadership position in growing multibillion dollar markets with multiple revenue streams DIFFERENTIATED Technology Solution-based product innovation to Lab customers NEW PRODUCT Launches NxTAG® RPP ARIES® US & EU Launch OPERATIONAL Improvement New leadership streamlining operational functions across business units FINANCIAL Leverage Strong gross margins & Profitability
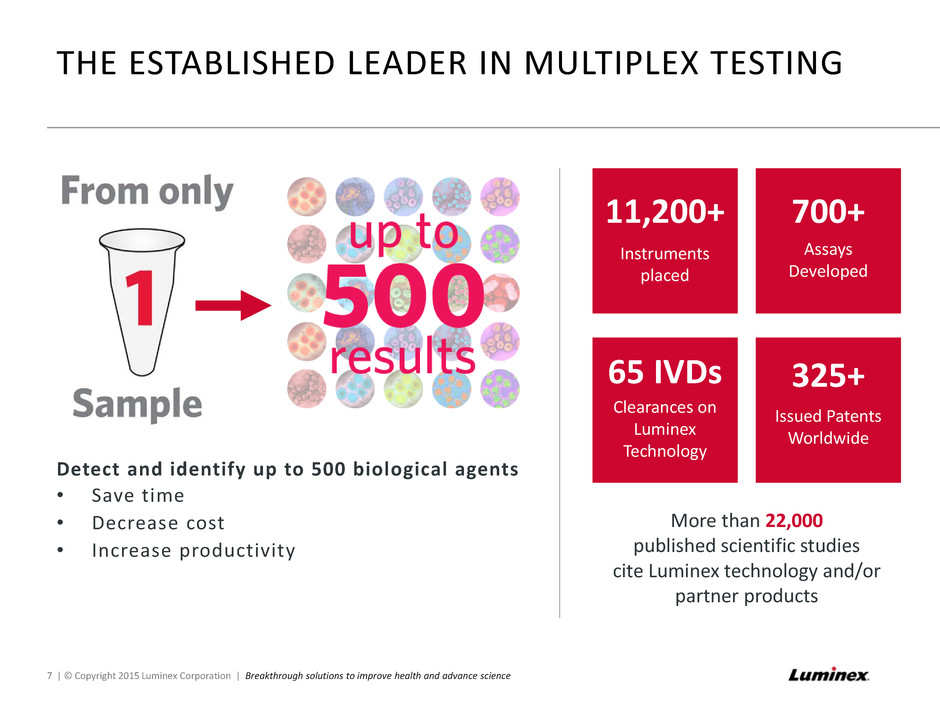
THE ESTABLISHED LEADER IN MULTIPLEX TESTING 7 | © Copyright 2015 Luminex Corporation | Breakthrough solutions to improve health and advance science Detect and identify up to 500 biological agents • Save time • Decrease cost • Increase productivity 11,200+ Instruments placed 700+ Assays Developed 65 IVDs Clearances on Luminex Technology 325+ Issued Patents Worldwide More than 22,000 published scientific studies cite Luminex technology and/or partner products
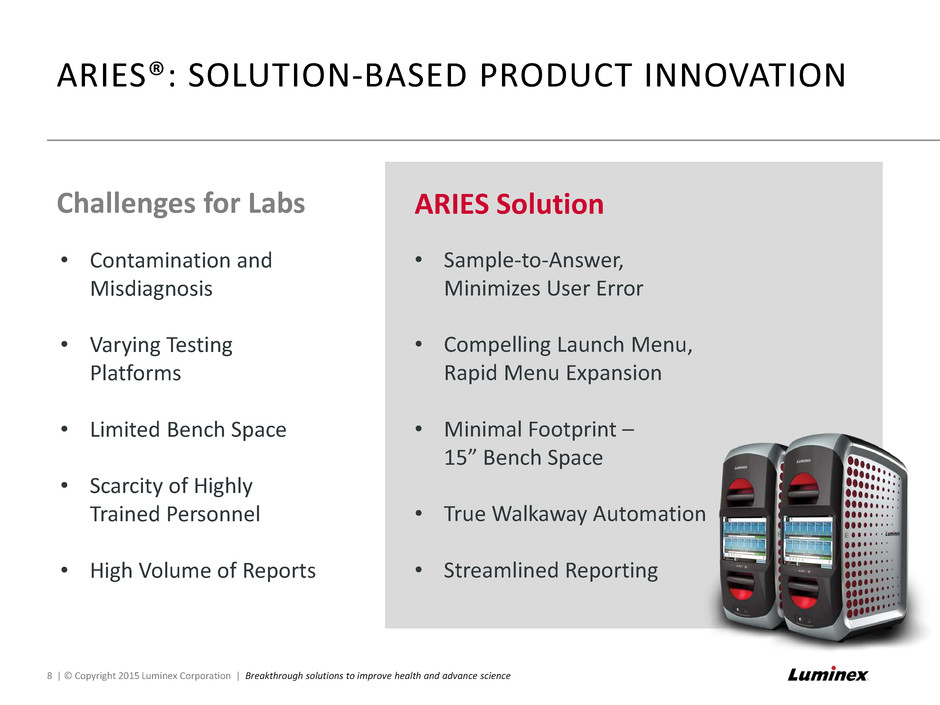
ARIES®: SOLUTION-BASED PRODUCT INNOVATION 8 | © Copyright 2015 Luminex Corporation | Breakthrough solutions to improve health and advance science • Contamination and Misdiagnosis • Varying Testing Platforms • Limited Bench Space • Scarcity of Highly Trained Personnel • High Volume of Reports Challenges for Labs ARIES Solution • Sample-to-Answer, Minimizes User Error • Compelling Launch Menu, Rapid Menu Expansion • Minimal Footprint – 15” Bench Space • True Walkaway Automation • Streamlined Reporting
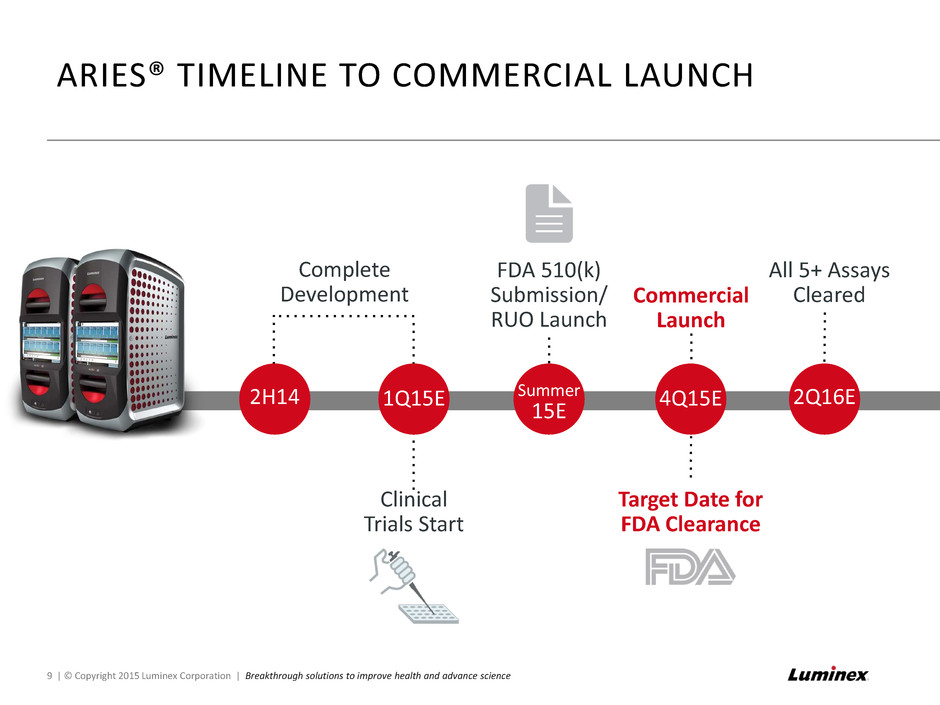
Complete Development ARIES® TIMELINE TO COMMERCIAL LAUNCH Clinical Trials Start 2H14 1Q15E Summer 15E FDA 510(k) Submission/ RUO Launch 4Q15E Target Date for FDA Clearance All 5+ Assays Cleared 2Q16E 9 | © Copyright 2015 Luminex Corporation | Breakthrough solutions to improve health and advance science Commercial Launch
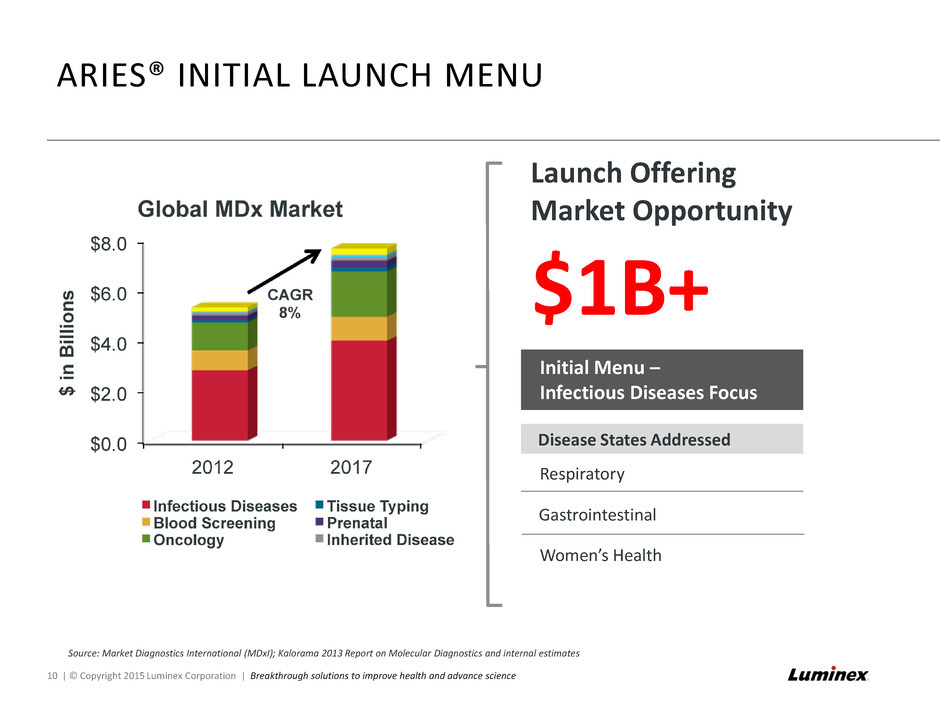
Launch Offering Market Opportunity ARIES® INITIAL LAUNCH MENU 10 | © Copyright 2015 Luminex Corporation | Breakthrough solutions to improve health and advance science Source: Market Diagnostics International (MDxI); Kalorama 2013 Report on Molecular Diagnostics and internal estimates $1B+ Initial Menu – Infectious Diseases Focus Disease States Addressed Respiratory Gastrointestinal Women’s Health

NXTAG® RPP: STREAMLINES WORKFLOW WITHOUT SACRIFICING THROUGHPUT 11 | © Copyright 2015 Luminex Corporation | Breakthrough solutions to improve health and advance science Hands-on Time: 20-25 min Time to Results: < 4 hrs • Comprehensive Multiplex Panel • Minimal Hands-on Time • Rapid Turnaround Time • Outstanding Performance • Closed-tube Workflow • Highly Scalable Throughput
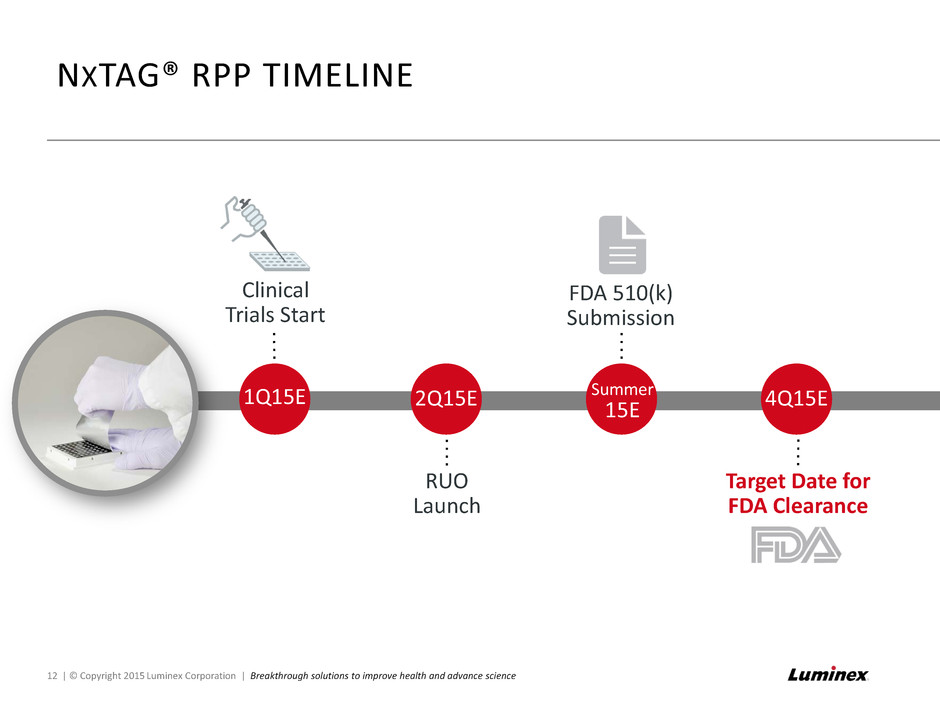
RUO Launch NXTAG® RPP TIMELINE Clinical Trials Start 1Q15E 2Q15E Summer 15E FDA 510(k) Submission 4Q15E Target Date for FDA Clearance 12 | © Copyright 2015 Luminex Corporation | Breakthrough solutions to improve health and advance science

A R I E S Full MDx (500) Moderately Complex MDx (1000) Limited MDx (5,000) No MDx Capability (7,000) EXPANDING DOWNSTREAM WITH ARIES Source: Mdxi Market Diagnostics Int’l, Emmes Database (2013): 1,000 U.S. laboratory survey, Scientia Analysis, AHA Registered U.S. Hospitals, CMS CLIA 13 | © Copyright 2015 Luminex Corporation | Breakthrough solutions to improve health and advance science Luminex 2015 and Beyond Key Positioning • Access to Attractive, Fast Growing Markets Segments • Simple, Easy to Use Solutions Drive Adoption • Offering Complete Testing Solution: - xTAG® /NxTAG® RPP (High Multiplex) - ARIES® (Real-time PCR) xTAG NxTAG
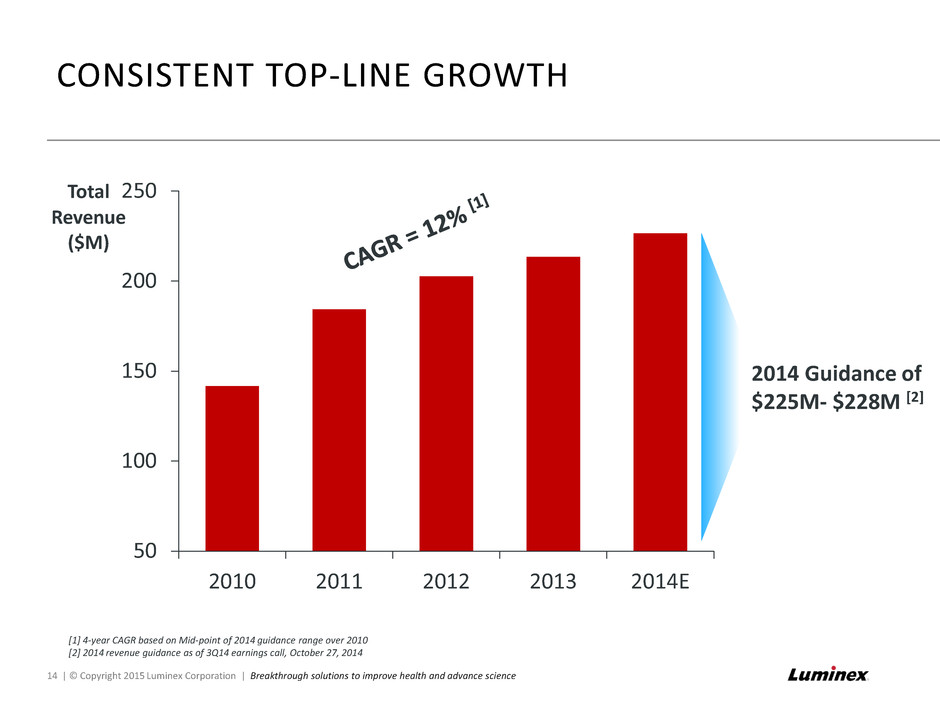
50 100 150 200 250 2010 2011 2012 2013 2014E Total Revenue ($M) CONSISTENT TOP-LINE GROWTH [1] 4-year CAGR based on Mid-point of 2014 guidance range over 2010 [2] 2014 revenue guidance as of 3Q14 earnings call, October 27, 2014 14 | © Copyright 2015 Luminex Corporation | Breakthrough solutions to improve health and advance science 2014 Guidance of $225M- $228M [2]
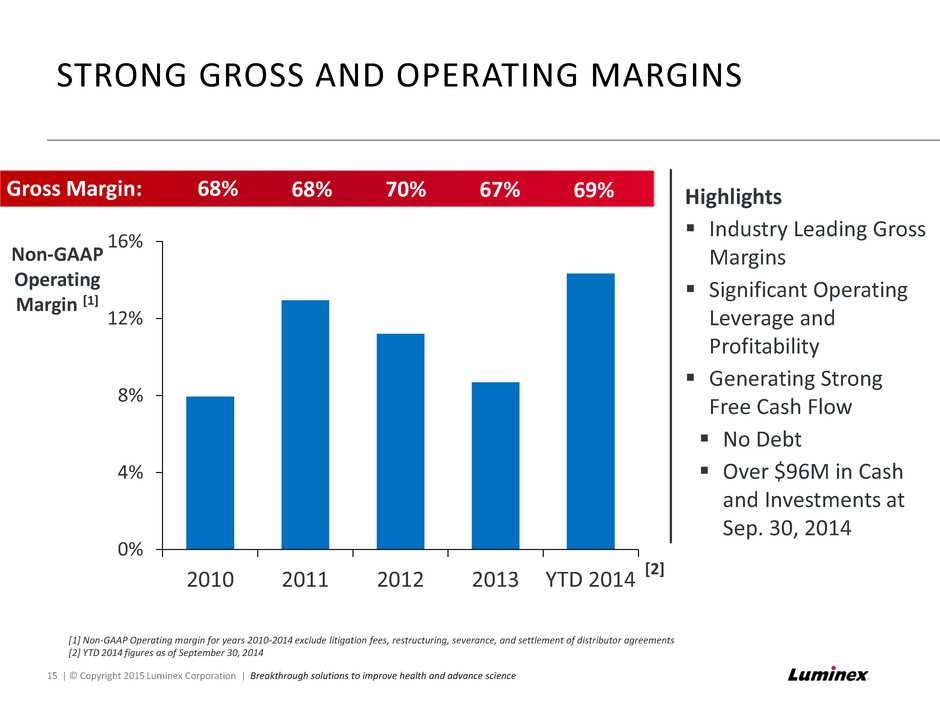
[1] Non-GAAP Operating margin for years 2010-2014 exclude litigation fees, restructuring, severance, and settlement of distributor agreements [2] YTD 2014 figures as of September 30, 2014 STRONG GROSS AND OPERATING MARGINS 15 | © Copyright 2015 Luminex Corporation | Breakthrough solutions to improve health and advance science 0% 4% 8% 12% 16% 2010 2011 2012 2013 YTD 2014 Non-GAAP Operating Margin [1] [2] Highlights Industry Leading Gross Margins Significant Operating Leverage and Profitability Generating Strong Free Cash Flow No Debt Over $96M in Cash and Investments at Sep. 30, 2014 Gross Margin: 68% 68% 70% 67% 69%

MY 90 DAY INITIAL OBSERVATIONS 16 | © Copyright 2015 Luminex Corporation | Breakthrough solutions to improve health and advance science Focus On Execution Partnership Challenge Next Generation Sequencing Operational Improvements Announced on 3Q14 earnings call: “We expect consumable revenue to be under pressure in the near term, as result of temporary inventory management challenges by our largest partner” Expect this pressure to continue during 2015 Gaining traction within certain Diagnostics applications Could affect trajectory of our Genetics Assay portfolio, primarily our CF business in high volume accounts Not expected to impact our Infectious Disease portfolio Deliver 2014 Guidance Build 2015 Operating Plan Launch ARIES® & NxTAG® by End of 4Q15 Shift from a technology based company to a market driven company Create organizational synergy while leveraging existing resources Streamline global manufacturing to provide incremental GM leverage

KEY TAKEAWAYS: HOW WE WIN 17 | © Copyright 2015 Luminex Corporation | Breakthrough solutions to improve health and advance science • Utilize Brand Recognition and Reputation While Expanding Addressable Market • Offer a Complete Testing Solution • Focus on Execution, Organizational Synergy and Becoming Market Driven • Leverage a Strong Balance Sheet • Growing Emphasis on Diagnostics

THANK YOU
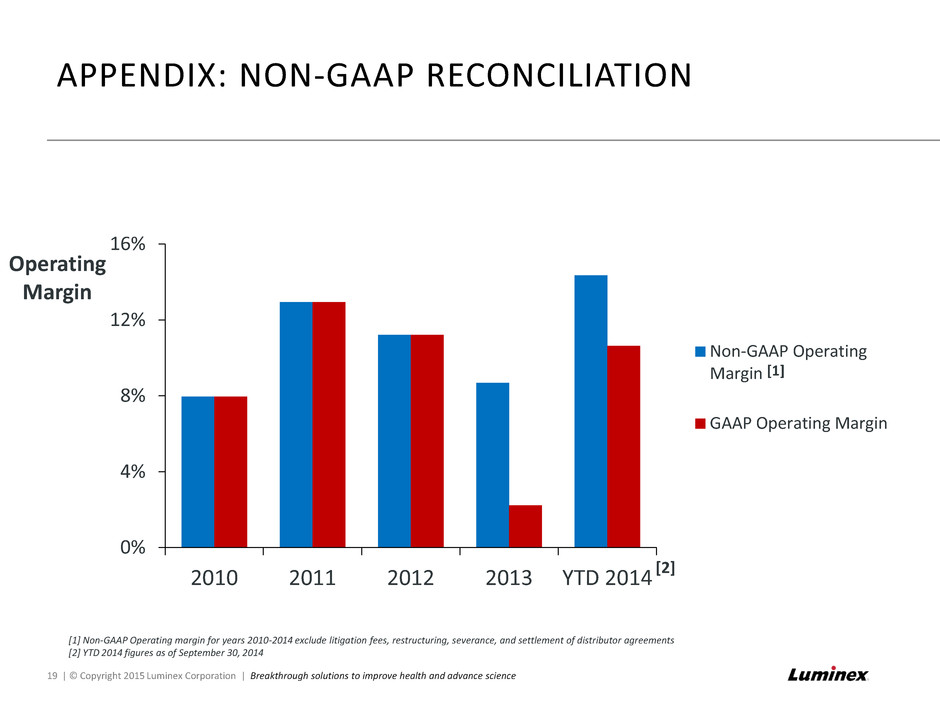
[1] Non-GAAP Operating margin for years 2010-2014 exclude litigation fees, restructuring, severance, and settlement of distributor agreements [2] YTD 2014 figures as of September 30, 2014 APPENDIX: NON-GAAP RECONCILIATION 19 | © Copyright 2015 Luminex Corporation | Breakthrough solutions to improve health and advance science 0% 4% 8% 12% 16% 2010 2011 2012 2013 YTD 2014 Non-GAAP Operating Margin GAAP Operating Margin Operating Margin [2] [1]
