Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Intersect ENT, Inc. | d850341d8k.htm |
| EX-99.1 - EX-99.1 - Intersect ENT, Inc. | d850341dex991.htm |
| Exhibit 99.2
|
Lisa Earnhardt, President & CEO
NASDAQ: XENT
JANUARY 2015
|
|
Forward-Looking Statements
Certain statements in this presentation constitute “forward-looking statements” within the meaning of the Securities Act of 1933, as amended (the “Securities Act”), and Securities Exchange Act of 1934, as amended (“Exchange Act”), including, without limitation, statements regarding our outlook for financial performance, sales force growth, clinical studies, approval of new products and indications and the receipt of reimbursement. We intend these forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in the Securities Act and the Exchange Act and are making this statement for purposes of complying with those safe harbor provisions. These forward-looking statements reflect our current views about our plans, intentions, expectations, strategies and prospects, which are based on the information currently available to us and on assumptions we have made. Although we believe that our plans, intentions, expectations, strategies and prospects as reflected in or suggested by those forward-looking statements are reasonable, we can give no assurance that the plans, intentions, expectations or strategies will be attained or achieved. Furthermore, actual results may differ materially from those described in the forward-looking statements and will be affected by a variety of risks and factors that are beyond our control, including those risks and uncertainties discussed under “Risk Factors” in our S-1A filing dated July 18, 2014 and subsequent quarterly filings with the SEC. All information in this presentation is as of the date of this presentation, and we undertake no duty to update this information unless required by law. In addition, the preliminary revenue for the three months and year ended December 31, 2014 represent our estimates based on currently available information and have not been audited or reviewed by our independent registered public accounting firm. These estimates do not present all necessary information necessary for an understanding of our financial condition and may be adjusted when we complete our unaudited financial statements for the quarter and year periods ended December 31, 2014.
| 1 |
|
|
|
ADVANCING CLINICALLY-PROVEN THERAPIES for Ear, Nose and Throat (ENT)
Physicians and Patients
Focus on Chronic Sinusitis (CS)
29M
Patients in US
IMPROVING QUALITY OF LIFE from Surgery to Office Care
| 2 |
|
|
|
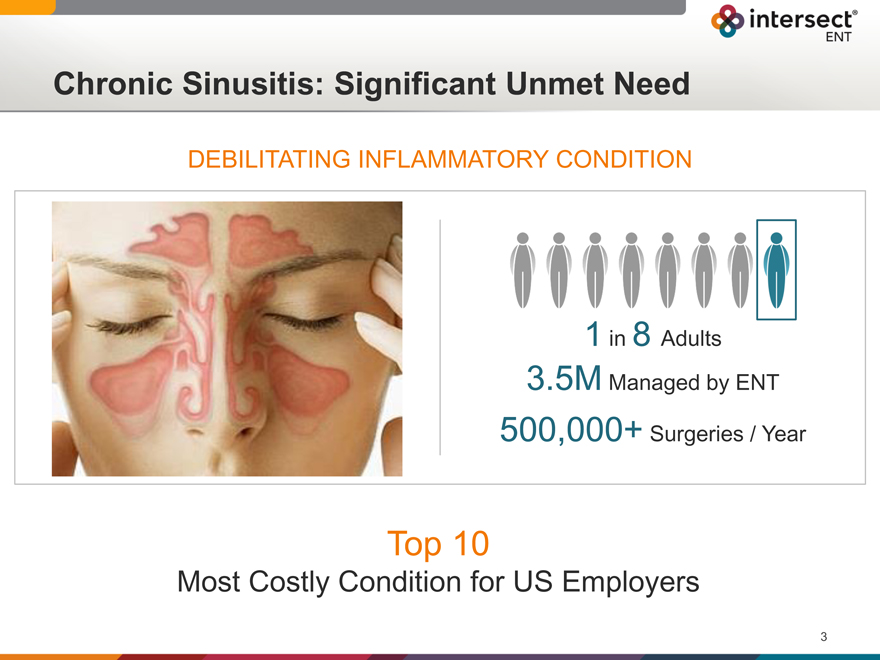
Chronic Sinusitis: Significant Unmet Need
DEBILITATING INFLAMMATORY CONDITION
| 1 |
|
in 8 Adults |
3.5M Managed by ENT
500,000+ Surgeries / Year
Top 10
Most Costly Condition for US Employers
| 3 |
|
|
|
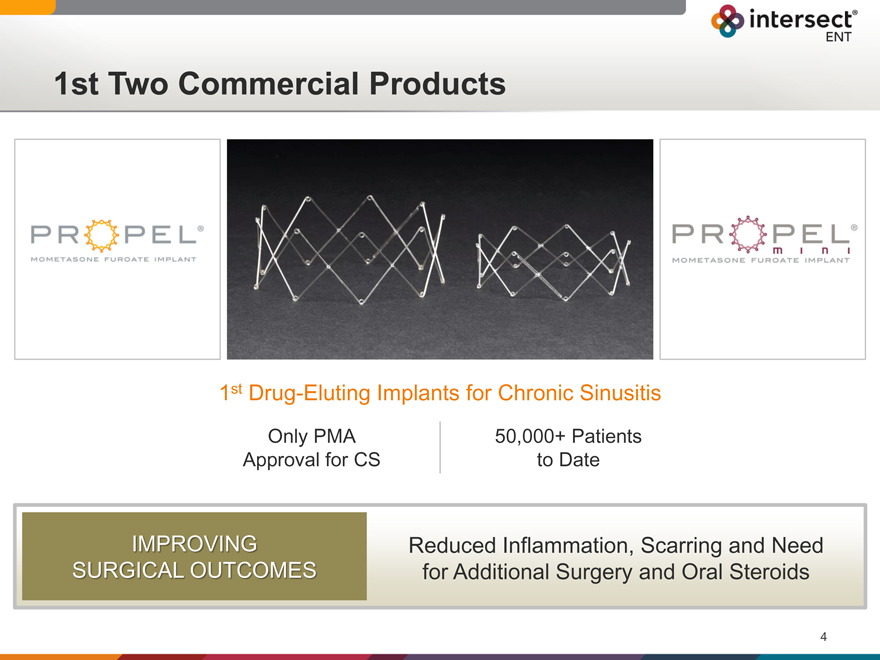
1st Two Commercial Products
1st Drug-Eluting Implants for Chronic Sinusitis
Only PMA 50,000+ Patients
Approval for CS to Date
IMPROVING
SURGICAL OUTCOMES
Reduced Inflammation, Scarring and Need for Additional Surgery and Oral Steroids
| 4 |
|
|
|
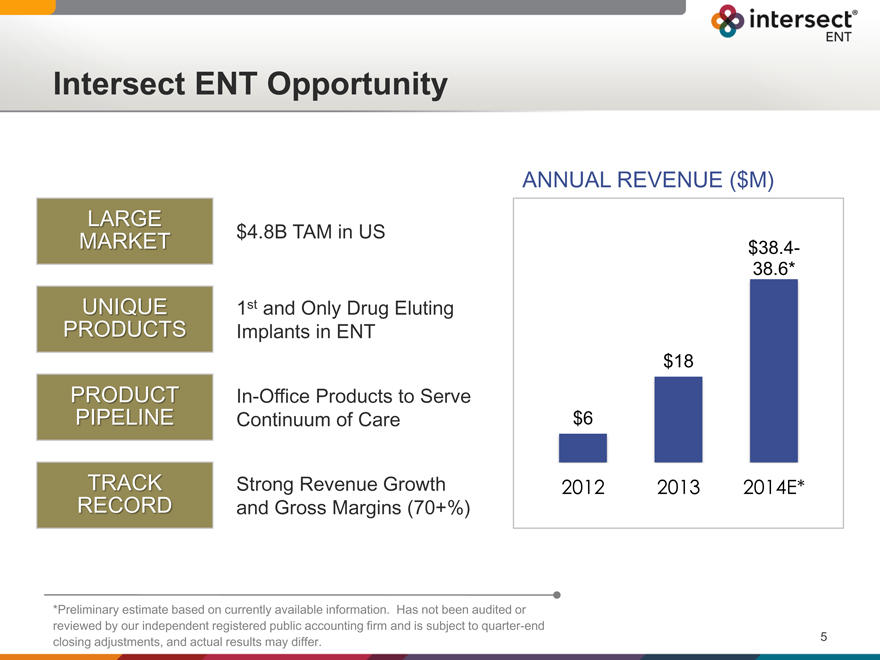
Intersect ENT Opportunity
LARGE
MARKET $4.8B TAM in US
UNIQUE 1st and Only Drug Eluting
PRODUCTS Implants in ENT
PRODUCT In-Office Products to Serve
PIPELINE Continuum of Care
TRACK Strong Revenue Growth
RECORD and Gross Margins (70+%)
ANNUAL REVENUE ($M)
$38.4-
38.6*
$18
$6
2012 2013 2014E*
*Preliminary estimate based on currently available information. Has not been audited or reviewed by our independent registered public accounting firm and is subject to quarter-end closing adjustments, and actual results may differ.
| 5 |
|
|
|
CS Treatment Pathway
MEDICATION TO TREAT
Oral Steroids Reduce Inflammation but Cause Side Effects
SURGERY TO OPEN (FESS)
Surgery Opens Pathways but Can Result in Post-Operative Scarring and Inflammation
MANAGEMENT TO MAINTAIN
Within One Year, 64% Symptom Recurrence and 10% Revision Surgery
Challenges Across the Continuum of Care to Treat Inflammation and Deliver Steroid Safely and Effectively
| 6 |
|
|
|
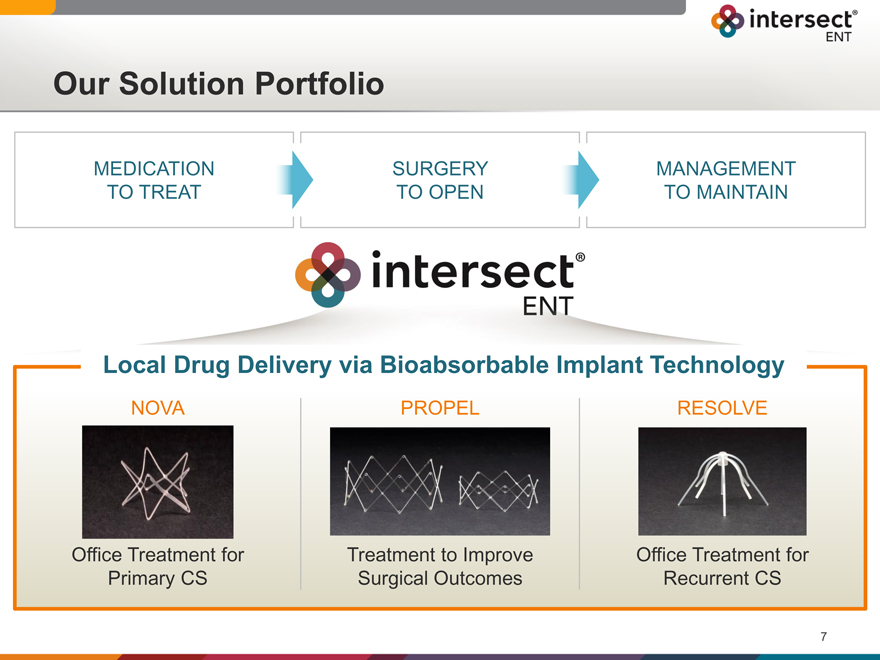
Our Solution Portfolio
MEDICATION SURGERY MANAGEMENT
TO TREAT TO OPEN TO MAINTAIN
Local Drug Delivery via Bioabsorbable Implant Technology
NOVA PROPEL RESOLVE
Office Treatment for Treatment to Improve Office Treatment for
Primary CS Surgical Outcomes Recurrent CS
| 7 |
|
|
|
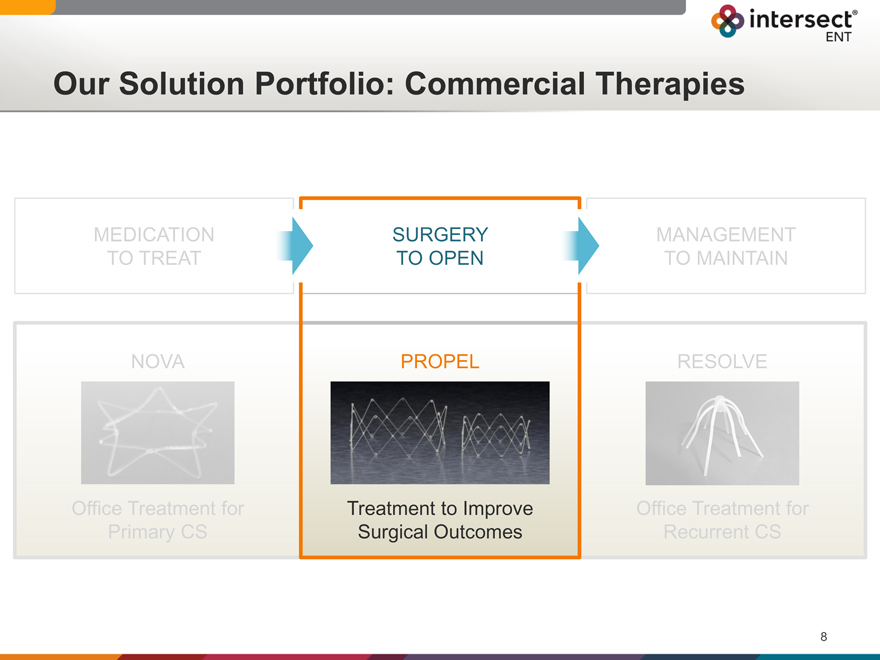
Our Solution Portfolio: Commercial Therapies
SURGERY
TO OPEN
PROPEL
Treatment to Improve
Surgical Outcomes
| 8 |
|
|
|
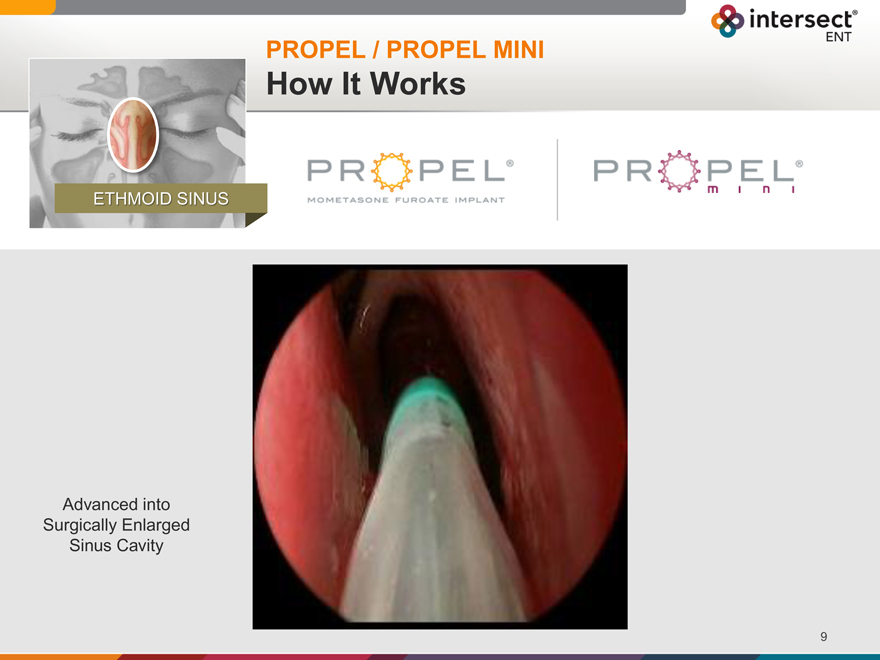
PROPEL / PROPEL MINI
How It Works
ETHMOID SINUS
Advanced into Surgically Enlarged Sinus Cavity
9
|
|
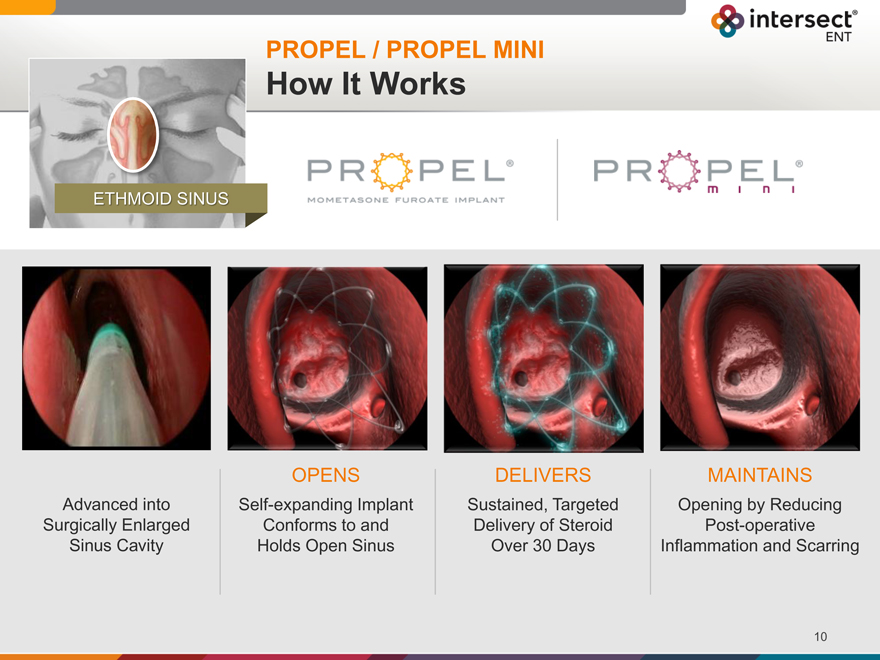
PROPEL / PROPEL MINI
How It Works
ETHMOID SINUS
Advanced into Surgically Enlarged Sinus Cavity
OPENS
Self-expanding Implant Conforms to and Holds Open Sinus
DELIVERS
Sustained, Targeted Delivery of Steroid Over 30 Days
MAINTAINS
Opening by Reducing Post-operative Inflammation and Scarring
10
|
|
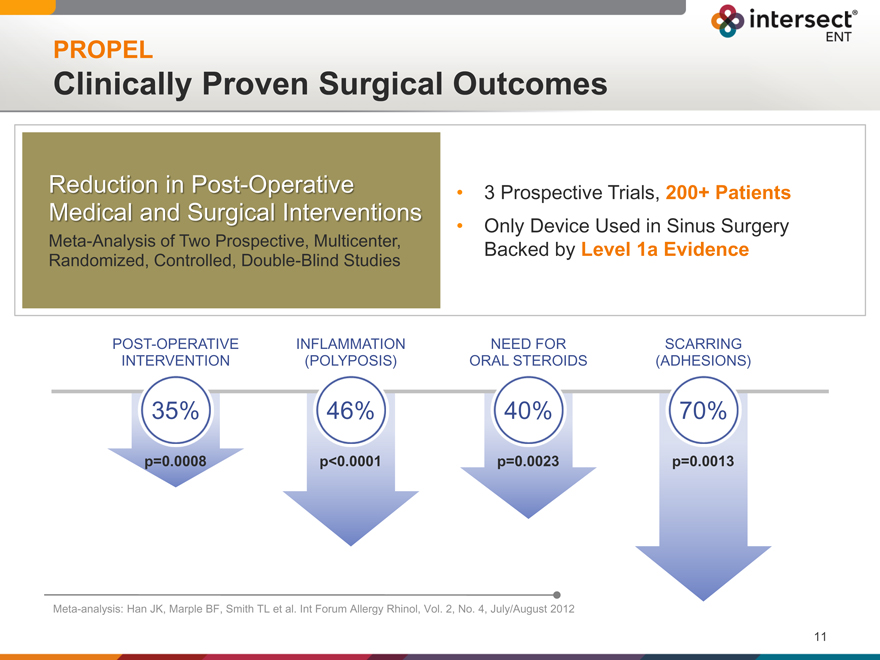
PROPEL
Clinically Proven Surgical Outcomes
Reduction in Post-Operative
Medical and Surgical Interventions
Meta-Analysis of Two Prospective, Multicenter, Randomized, Controlled, Double-Blind Studies
| 3 |
|
Prospective Trials, 200+ Patients Only Device Used in Sinus Surgery |
Backed by Level 1a Evidence
POST-OPERATIVE INFLAMMATION NEED FOR SCARRING
INTERVENTION (POLYPOSIS) ORAL STEROIDS (ADHESIONS)
35% 46% 40% 70%
p=0.0008 p<0.0001 p=0.0023 p=0.0013
Meta-analysis: Han JK, Marple BF, Smith TL et al. Int Forum Allergy Rhinol, Vol. 2, No. 4, July/August 2012
11
|
|
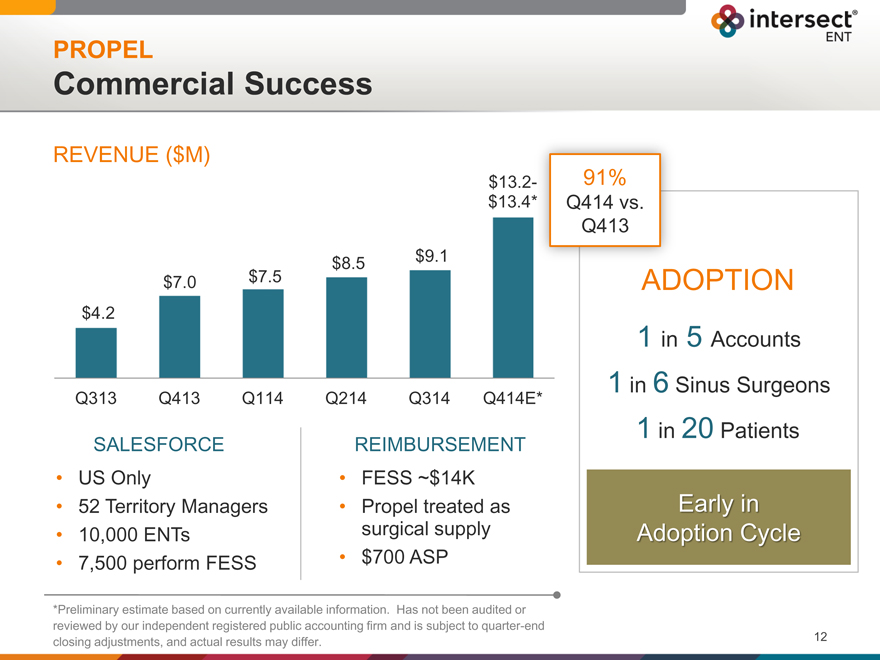
PROPEL
Commercial Success
REVENUE ($M)
$ 13.2
$ 13.4
$8.5 $ 9.1
$7.0 $7.5
$4.2
Q313 Q413 Q114 Q214 Q314 Q414E*
91%
Q414 vs.
Q413
ADOPTION
| 1 |
|
in 5 Accounts |
| 1 |
|
in 6 Sinus Surgeons |
| 1 |
|
in 20 Patients |
Early in
Adoption Cycle
SALESFORCE REIMBURSEMENT
US Only FESS ~$14K
52 Territory Managers Propel treated as
10,000 ENTs surgical supply
7,500 perform FESS $700 ASP
*Preliminary estimate based on currently available information. Has not been audited or reviewed by our independent registered public accounting firm and is subject to quarter-end closing adjustments, and actual results may differ.
12
|
|
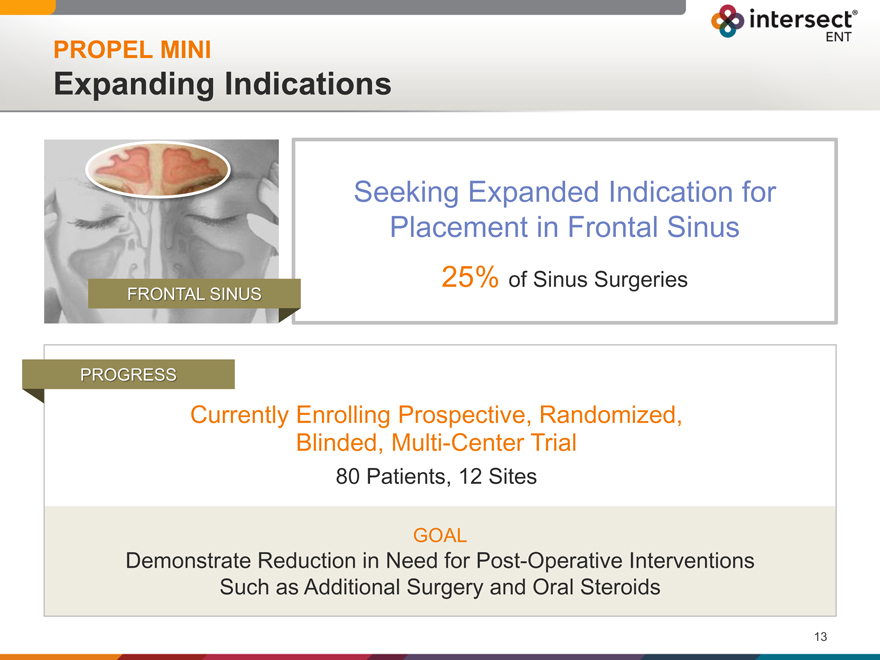
PROPEL MINI
Expanding Indications
Seeking Expanded Indication for Placement in Frontal Sinus
25% of Sinus Surgeries
FRONTAL SINUS
PROGRESS
Currently Enrolling Prospective, Randomized,
Blinded, Multi-Center Trial
80 Patients, 12 Sites
GOAL
Demonstrate Reduction in Need for Post-Operative Interventions Such as Additional Surgery and Oral Steroids
13
|
|
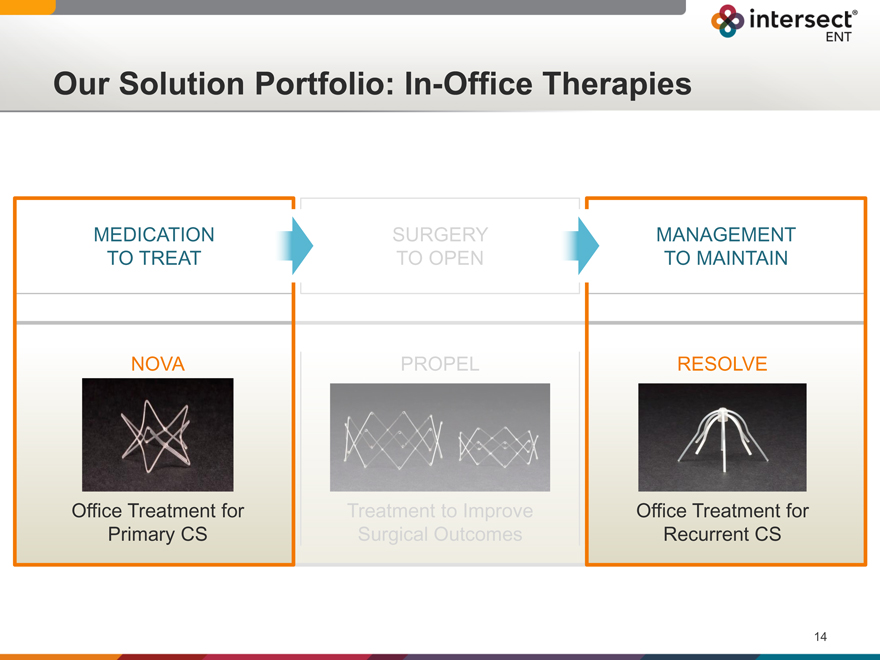
Our Solution Portfolio: In-Office Therapies
MEDICATION TO TREAT
NOVA
Office Treatment for Primary CS
MANAGEMENT
TO MAINTAIN
RESOLVE
Office Treatment for
Recurrent CS
14
|
|
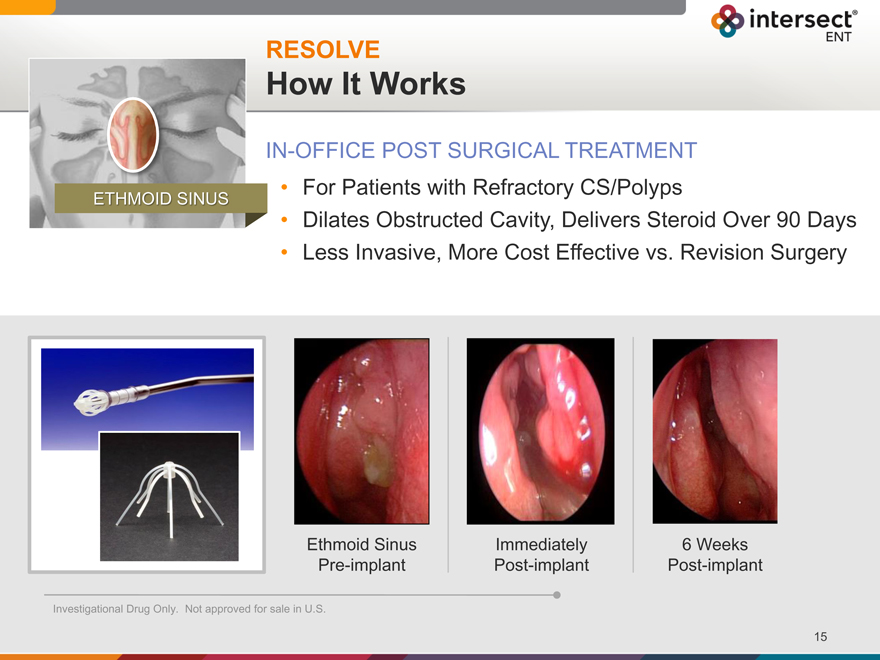
RESOLVE
How It Works
IN-OFFICE POST SURGICAL TREATMENT
For Patients with Refractory CS/Polyps
Dilates Obstructed Cavity, Delivers Steroid Over 90 Days
Less Invasive, More Cost Effective vs. Revision Surgery
ETHMOID SINUS
Ethmoid Sinus Immediately 6 Weeks
Pre-implant Post-implant Post-implant
Investigational Drug Only. Not approved for sale in U.S.
15
|
|
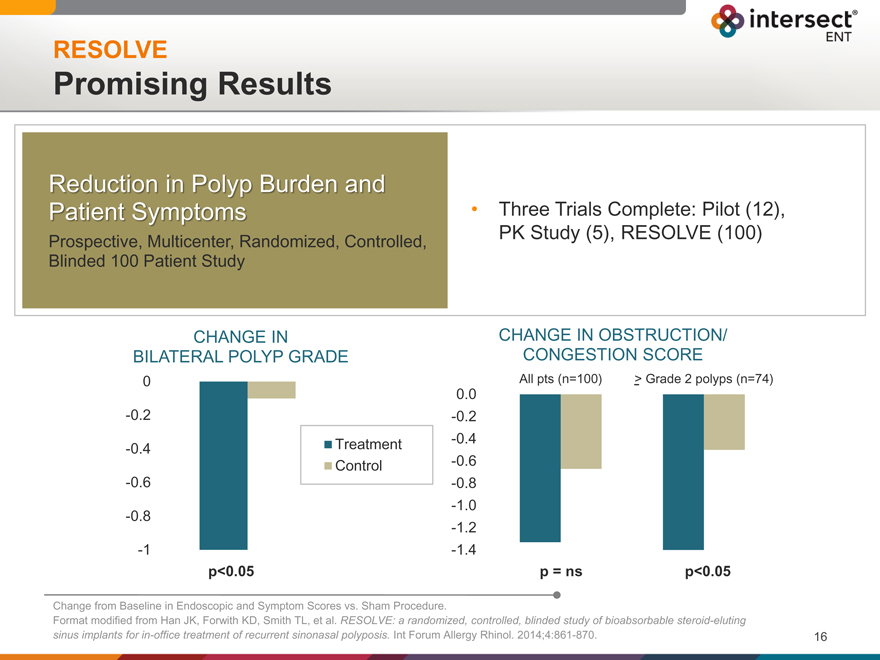
RESOLVE
Promising Results
Reduction in Polyp Burden and
Patient Symptoms
Prospective, Multicenter, Randomized, Controlled, Blinded 100 Patient Study
Three Trials Complete: Pilot (12), PK Study (5), RESOLVE (100)
CHANGE IN
BILATERAL POLYP GRADE
0
-0.2
-0.4 Treatment
Control
-0.6
-0.8
-1
p<0.05
CHANGE IN OBSTRUCTION/
CONGESTION SCORE
All pts (n=100) > Grade 2 polyps (n=74)
0.0
-0.2
-0.4
-0.6
-0.8
-1.0
-1.2
-1.4
p = ns p<0.05
Change from Baseline in Endoscopic and Symptom Scores vs. Sham Procedure.
Format modified from Han JK, Forwith KD, Smith TL, et al. RESOLVE: a randomized, controlled, blinded study of bioabsorbable steroid-eluting sinus implants for in-office treatment of recurrent sinonasal polyposis. Int Forum Allergy Rhinol. 2014;4:861-870.
16
|
|
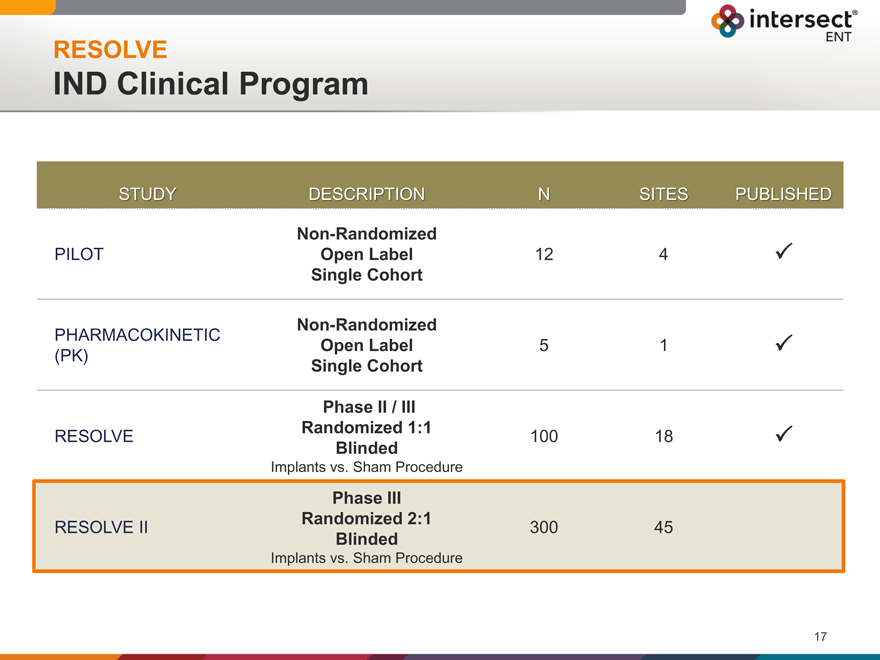
RESOLVE
IND Clinical Program
STUDY DESCRIPTION N SITES PUBLISHED
Non-Randomized
PILOT Open Label 12 4
Single Cohort
Non-Randomized
PHARMACOKINETIC Open Label 5 1
(PK) Single Cohort
Phase II / III
RESOLVE Randomized 1:1 100 18
Blinded
Implants vs. Sham Procedure
Phase III
Randomized 2:1
RESOLVE II 300 45
Blinded
Implants vs. Sham Procedure
17
|
|
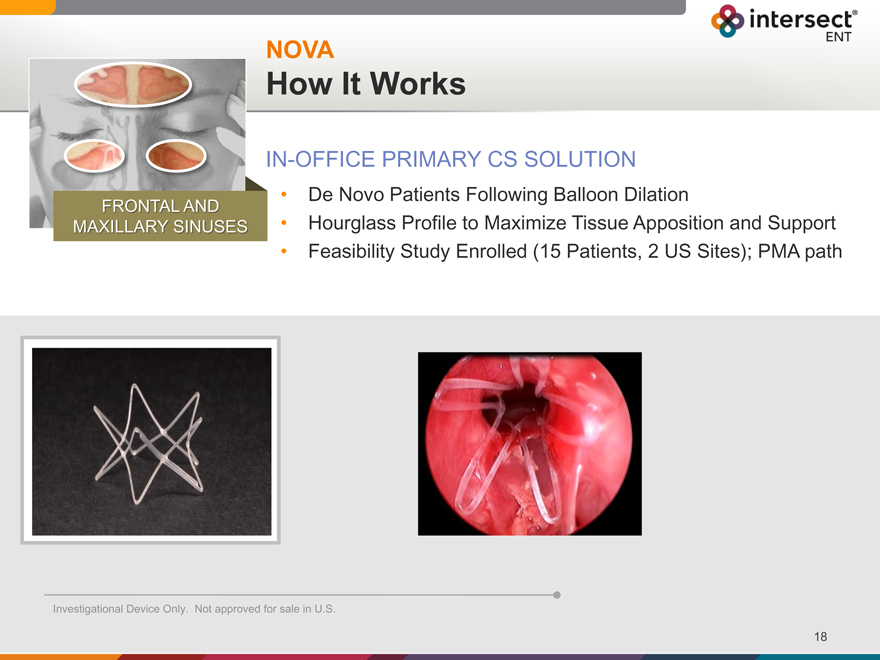
NOVA
How It Works
IN-OFFICE PRIMARY CS SOLUTION
De Novo Patients Following Balloon Dilation
Hourglass Profile to Maximize Tissue Apposition and Support
Feasibility Study Enrolled (15 Patients, 2 US Sites); PMA path
FRONTAL AND
MAXILLARY SINUSES
Investigational Device Only. Not approved for sale in U.S.
18
|
|
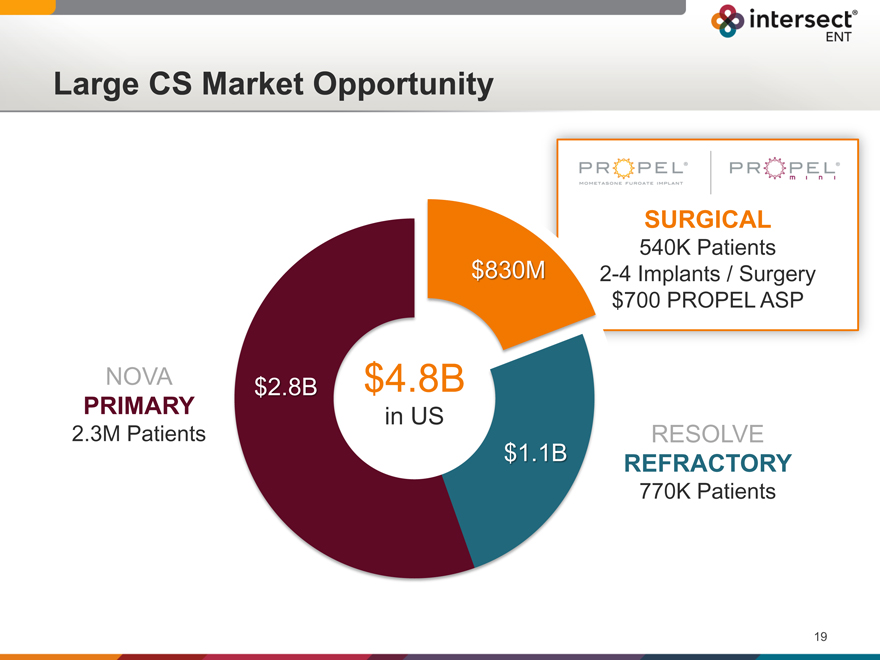
Large CS Market Opportunity
SURGICAL
540K Patients 2-4 Implants / Surgery $700 PROPEL ASP
$830M
NOVA $2.8B $4.8B
PRIMARY in US
2.3M Patients
$1.1B
RESOLVE
REFRACTORY
770K Patients
19
|
|
Industry Landscape
MEDICATION SURGERY MANAGEMENT
TO TREAT TO OPEN TO MAINTAIN
STEROIDS
SURGICAL TOOLS / SUPPLIES
We Are a Unique Solution – Complementary to Other Technologies
20
|
|
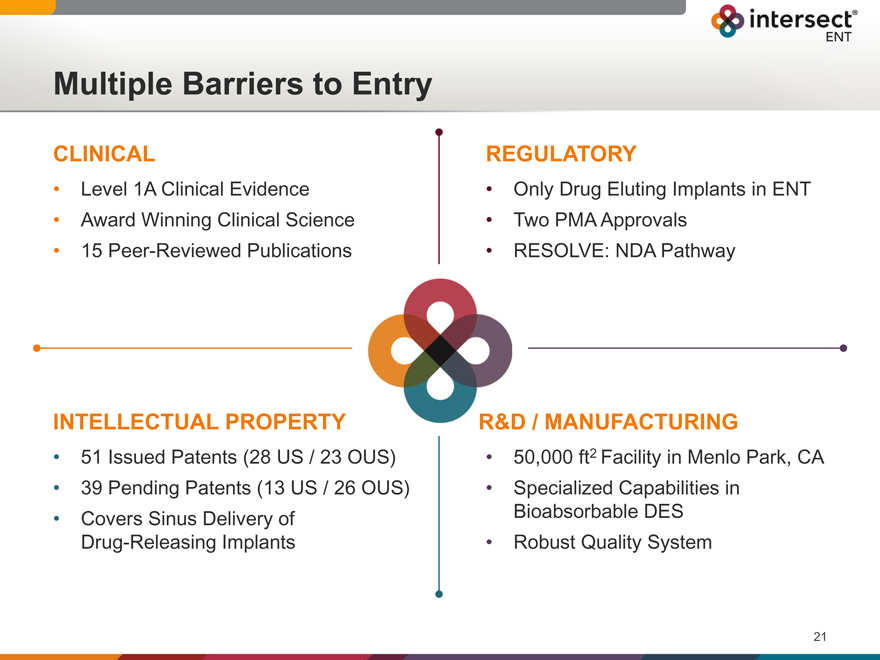
Multiple Barriers to Entry
CLINICAL
Level 1A Clinical Evidence
Award Winning Clinical Science
15 Peer-Reviewed Publications
REGULATORY
Only Drug Eluting Implants in ENT
Two PMA Approvals
RESOLVE: NDA Pathway
INTELLECTUAL PROPERTY
51 Issued Patents (28 US / 23 OUS)
39 Pending Patents (13 US / 26 OUS)
Covers Sinus Delivery of Drug-Releasing Implants
R&D / MANUFACTURING
50,000 ft2 Facility in Menlo Park, CA
Specialized Capabilities in Bioabsorbable DES
Robust Quality System
21
|
|
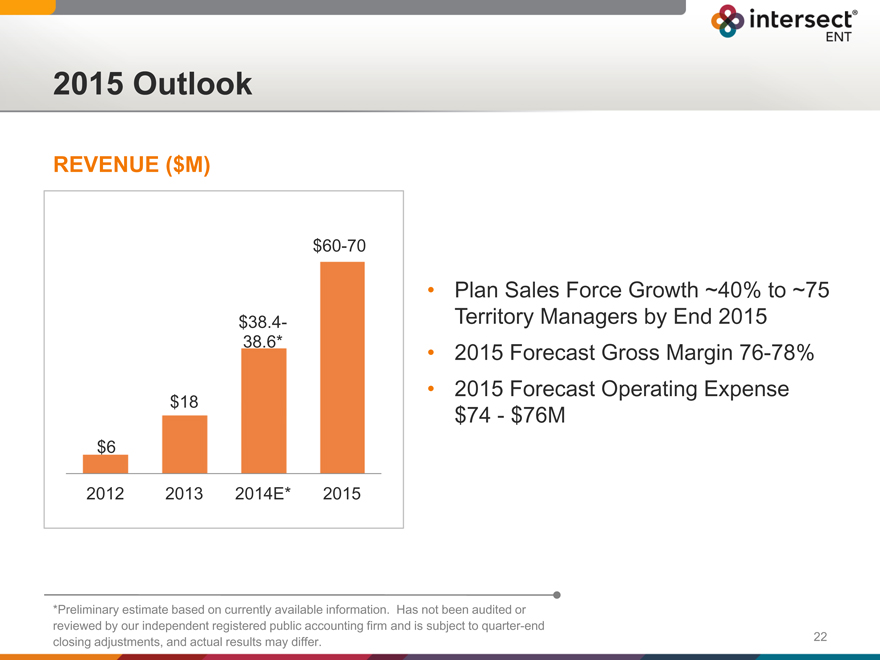
2015 Outlook
REVENUE ($M)
$60-70
$38.4-3
8.6*
$18
$6
2012 2013 2014E* 2015
Plan Sales Force Growth ~40% to ~75 Territory Managers by End 2015 2015 Forecast Gross Margin 76-78% 2015 Forecast Operating Expense
$74—$76M
*Preliminary estimate based on currently available information. Has not been audited or reviewed by our independent registered public accounting firm and is subject to quarter-end closing adjustments, and actual results may differ.
22
|
|
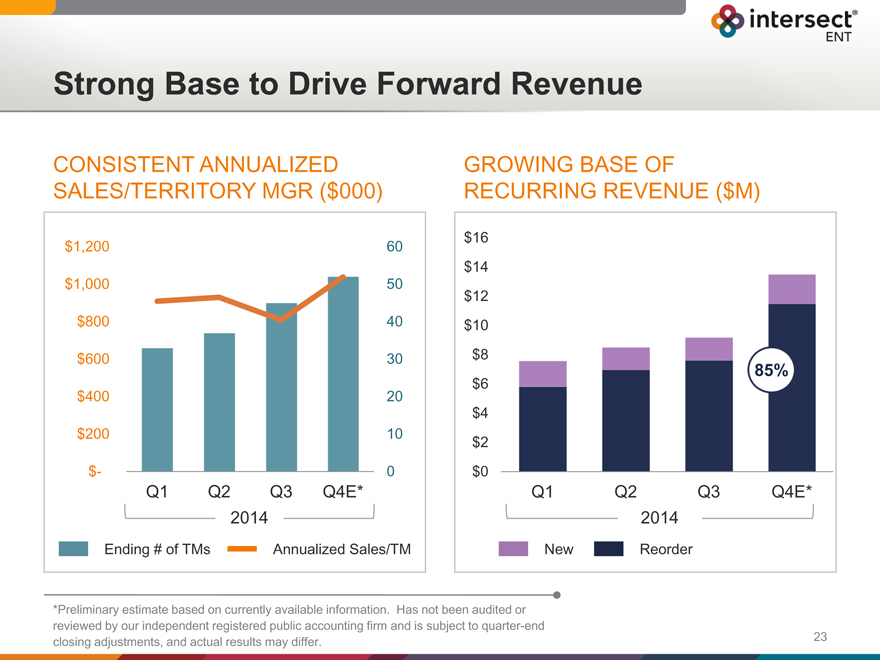
Strong Base to Drive Forward Revenue
CONSISTENT ANNUALIZED
SALES/TERRITORY MGR ($000)
$1,200 60
$1,000 50
$800 40
$600 30
$400 20
$200 10
$- 0
Q1 Q2 Q3 Q4E*
2014
Ending # of TMs Annualized Sales/TM
GROWING BASE OF
RECURRING REVENUE ($M)
$16
$14
$12
$10
$8
85%
$6
$4
$2
$0
Q1 Q2 Q3 Q4E*
2014
New Reorder
*Preliminary estimate based on currently available information. Has not been audited or reviewed by our independent registered public accounting firm and is subject to quarter-end closing adjustments, and actual results may differ.
23
|
|
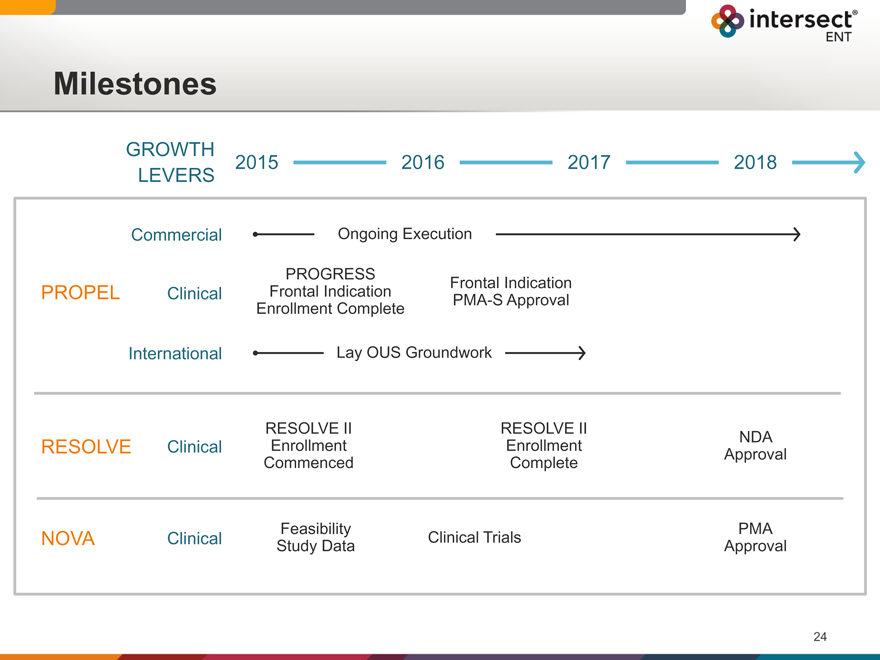
Milestones
GROWTH
2015 2016 2017 2018
LEVERS
Commercial Ongoing Execution
PROGRESS
PROPEL Clinical Frontal Indication Frontal PMA-S Indication Approval
Enrollment Complete
International Lay OUS Groundwork
RESOLVE II RESOLVE II
RESOLVE Clinical Enrollment Enrollment Approval NDA
Commenced Complete
Feasibility PMA
NOVA Clinical Study Data Clinical Trials Approval
24
|
|
Experienced Leadership
MANAGEMENT TEAM
LISA EARNHARDT
President & CEO
JERI HILLEMAN RICH KAUFMAN
CFO COO, SVP R&D & Operations
ROB BINNEY JAMES STAMBAUGH
VP Sales VP Clinical & Reimbursement
SUSAN STIMSON AMY WOLBECK
VP Marketing VP Regulatory Affairs & Quality
EXPERIENCE INCLUDES
25
|
|
$4.8B US Market
1st and ONLY Drug Eluting Implants in ENT
EMERGING In-Office Portfolio
TRACK RECORD Strong Revenue Growth & GMs
26
|
|
NASDAQ: XENT
28