Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Ignyta, Inc. | d850063d8k.htm |
 Catalyzing
Precision Medicine with Integrated Rx/Dx in Oncology January 2015
®
Exhibit 99.1 |
 Safe Harbor Statement
2
This document contains forward-looking statements, as that term is defined in Section 27A of the
Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, about Ignyta,
Inc. (“us” or the “Company”). Statements that are not purely historical are
forward-looking statements. These include statements regarding, among other things: the clinical and/or
non-clinical data or plans underlying entrectinib, RXDX-103 or any of our other development
programs; our ability to design and conduct development activities for entrectinib,
RXDX-103 and our other development programs; our ability to develop or access companion
diagnostics for our product candidates; our ability to obtain and maintain intellectual property protection for
our product candidates; our ability to adequately fund our development programs; our ability to obtain
regulatory approvals in order to market any of our product candidates; and our ability to
successfully commercialize any approved products. Forward-looking statements involve known and unknown risks that relate to future events or the
Company’s future financial performance, some of which may be beyond our control, and the
actual results could differ materially from those discussed in this document.
Accordingly, the Company cautions investors not to place undue reliance on the forward-looking statements
contained in, or made in connection with, this document.
Important factors that could cause actual results to differ materially from those indicated by such
forward-looking statements, include, among others, the potential for results of past or
ongoing clinical or non-clinical studies to differ from expectations or previous results;
the interpretation of data from our clinical and non-clinical studies; our ability to initiate and complete clinical
trials and non-clinical studies; regulatory developments; the potential advantages of our product
candidates; the markets any approved products are intended to serve; and our capital needs; as
well as those set forth under the headings “Special Note Regarding Forward-Looking
Statements,” “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition
and Results of Operations” contained in the Company’s Form 10-K filed with the
Securities and Exchange Commission (“SEC”) on February 28, 2014, and similar
disclosures made in the Company’s Form 10-Q filings and other SEC filings and press
releases. The forward-looking statements contained in this document represent our estimates and assumptions
only as of the date of this document, and we undertake no duty or obligation to update or
revise publicly any forward-looking statements contained in this document as a result of
new information, future events or changes in our expectations.
Third-party information included herein has been obtained from sources believed to be reliable,
but the accuracy or completeness of such information is not guaranteed by, and should not be
construed as a representation by, the Company. |
 Company History and Financial Highlights
San Diego based biotechnology company (NASDAQ: RXDX), incorporated in 2011
~50 employees: more than half with M.D.’s, Ph.D.’s or other graduate degrees
Acquired Actagene Oncology in May 2013 to enter oncology precision medicine
market
Licensed from Nerviano Medical Sciences exclusive worldwide rights to
entrectinib
&
RXDX-102
1
in
Oct.
2013,
and
RXDX-103
&
RXDX-104
2
in
Aug.
2014
Raised over $140 million between Nov. 2013 and Sept. 2014
Cash, cash equivalents & marketable securities of $94.7 million at Q3 2014
3
1
Back-up compound
2
Discontinued
Ignyta’s vision is to become the world’s leading precision medicine
company, with an integrated approach to "Rx/Dx" in oncology.
|
 Jonathan Lim,
M.D., Chairman, CEO, and Co-Founder. Former Chair, CEO of Eclipse;
CEO at Halozyme; McKinsey; NIH Postdoc at Harvard; surgical resident at NYH-Cornell
Jacob Chacko, M.D., Chief Financial Officer.
Former Vice President at TPG Capital;
McKinsey; Marshall Scholar at Oxford University; UCLA Med; Harvard Business School
Zachary Hornby, Chief Operating Officer.
Former Senior Director of Business
Development at Fate Therapeutics; Director of BD at Halozyme; L.E.K. Consulting; HBS
Adrian Senderowicz, M.D., Chief Medical Officer.
Former VP, Global Regulatory
Oncology at Sanofi; CMO at Tokai; Sr. Med. Dir. at Astra Zeneca;
U.S. FDA; NCI/NIH
Robert Wild, Ph.D., Chief Scientific Officer.
Former CSO, Oncology Research/Drug
Discovery at Eli Lilly; Sr. Dir., Oncology Research at OSI Pharma; BMS; SUGEN
Matt Onaitis, General Counsel and Secretary.
Former GC at Trius and Somaxon;
Associate GC at Biogen Idec; Director of Legal Affairs at Elan; Stanford Law School
Management Team
4 |
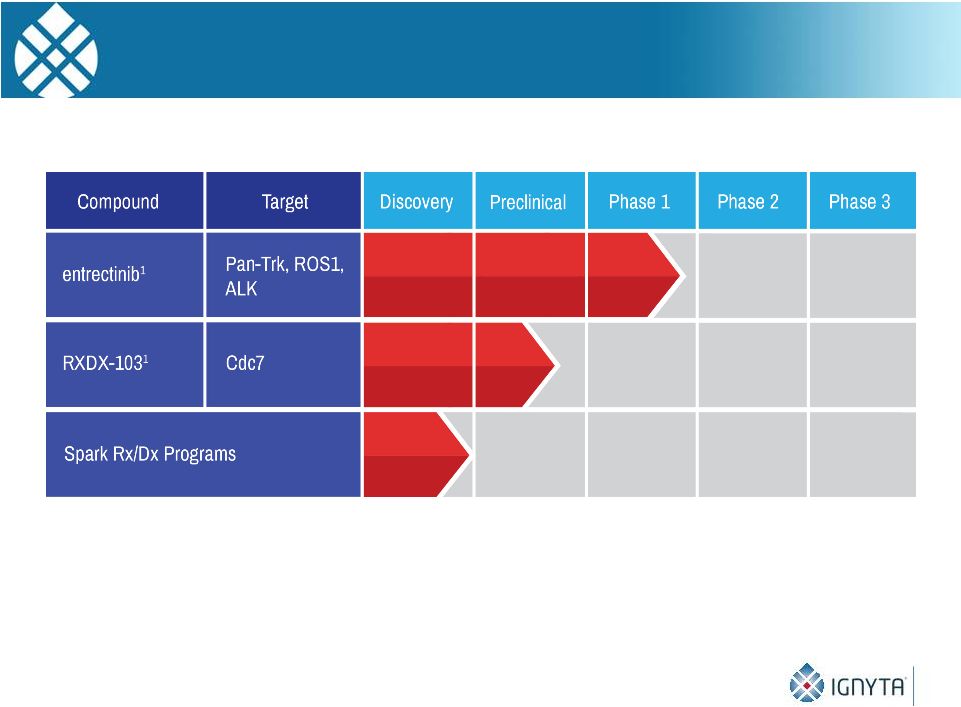 Ignyta’s
Pipeline 5
1
In-licensed from Nerviano Medical Sciences (NMS) |
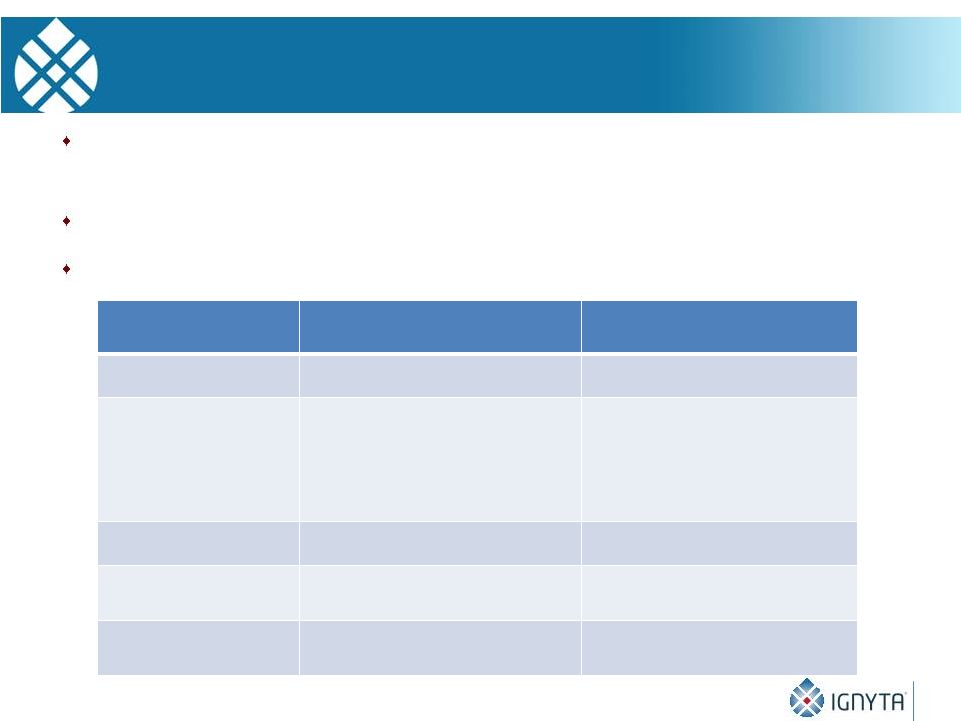 Entrectinib,
RXDX-102* and RXDX-103: Exclusive Worldwide Licenses
6
Compounds in-licensed from Nerviano, a former Pfizer/Pharmacia R&D site
with kinase expertise and 40 years of experience in oncology R&D
Nerviano had invested ~$40MM across the compounds
Deal terms compare favorably with other high profile oncology in-licenses
* Back-up compound
Deal Term
Entrectinib & 102*
(Oct. 2013)
RXDX-103
(Aug. 2014)
Upfront payment
$7M
$3.5M
Aggregate
development and
regulatory milestones
for WW approval
$55M for 1
st
product;
$50M for additional products
and/or indications
$68M
Sales milestones
None
None
Total upfront and
potential milestones
$112M
$71.5M
Royalties
Single digit to low double
digit %
Single digit to low double
digit % |
 Timeline of Oncogenic Driver Discovery in Lung Cancers
7
1999
2004
2009
2014
Drilon Am J Hematol Oncol 2014 |
 Targeted Therapy in Lung Cancers
8 |
 TrkA/B/C
Signaling Pathways 9 |
 Trk
Rearrangements in Human Malignancy TM
Kinase Domain
NTRK1
MPRIP-NTRK1
CD74-NTRK1
NSCLC
TPM3-NTRK1
TFG-NTRK1
TPR-NTRK1
Papillary Thyroid
Cancer
TPM3-NTRK1
Colorectal Cancer
NTRK1, NTRK2
or
NTRK3
fusions have also been identified in
glioblastoma, AML and secretory breast cancer
Greco A, et al. Mol Cell Endocrinol 2009; Alberti L, et al. J Cell Physiol 2003;
Martin-Zanca D et al. Nature 1986; Wiesner T, et al. Nat Commun 2013; Vaishnavi A,
et al, Nat Med 2013. 10 |
 Entrectinib
Next Generation Kinase Inhibitor
Potent inhibitor of 5 oncogenic driver targets:
Crosses the blood brain barrier, enabling targeting of CNS lesions
In Phase 1/2 study (Ph 1 dose escalation ongoing) with multiple future
“pipeline in a product”
opportunities
Composition of matter patent issued in the US and allowed in Europe,
with commercial protection out to 2029 (excluding patent term
extension)
11
Target
TrkA
TrkB
TrkC
ROS1
ALK
IC50 (nM)
1.7
0.1
0.1
0.2
1.6 |
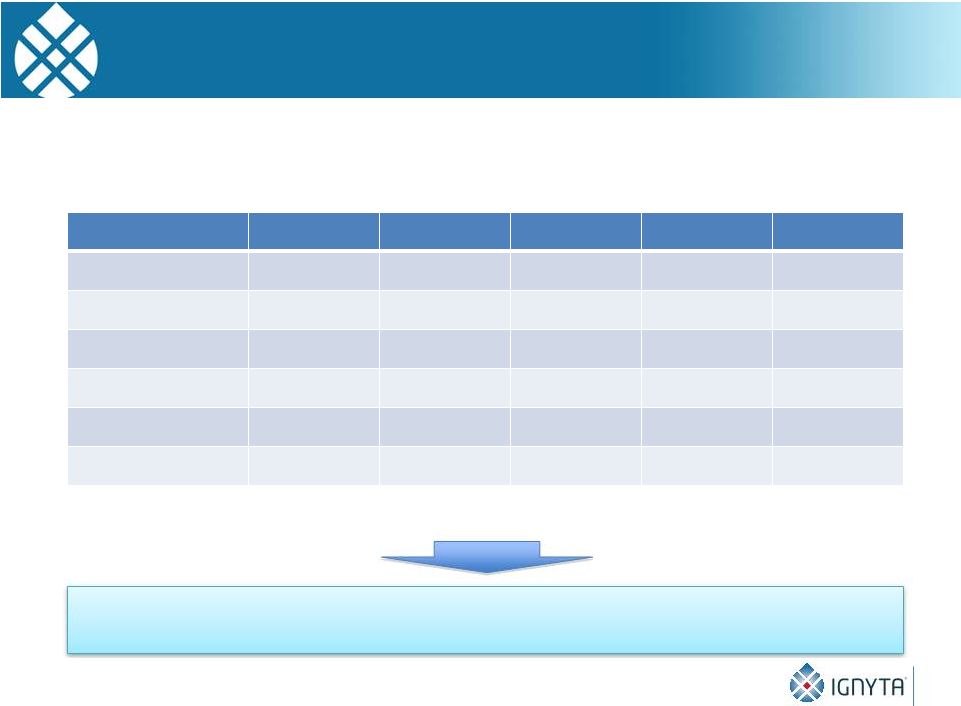 Molecular Alterations Targeted by Entrectinib
Are Present in a Large Number of Tumors
We estimate that 25,000 -
35,000 tumors newly detected in the US
each year have an alteration* to TrkA, TrkB, TrkC, ROS1 or ALK
12
These tumors represent segments of the “Big 4”
(NSCLC, CRC, PC, BC), as well as
cancers with extremely high unmet need (pancreatic, neuroblastoma)
TrkA
TrkB
TrkC
ROS1
ALK
NSCLC
X
X
X
X
Colorectal
X
X
X
Prostate
X
Pancreatic
X
Melanoma
X
Neuroblastoma
X
X
X
* Alterations
include
translocations,
splice
variants,
mutations
and
overexpression
Others include papillary thyroid, breast, AML, glioblastoma, gastric,
ovarian |
 Entrectinib Has Both First-in-Class and
Best-in-Class Opportunities
Trk: No Trk-targeting agents in Ph 2 or later
ROS1: No agents approved for targeting ROS1
ALK: Xalkori (crizotinib) and Zykadia (ceritinib) are approved ALK
inhibitors
Both approved only for NSCLC; but being studied in other histologies
NSCLC patients on Xalkori develop de novo ALK mutations that cause
Xalkori resistance; median duration of effect is ~10 months
13
Xalkori estimated to have sales >$350M in 2014 |
 Entrectinib Induces
Robust Tumor Regression and Stabilization in TrkA and ROS1 Rearranged In Vivo Cancer
Models TrkA KM12 CRC Tumor Xenograft Model
Continuous dosing treatments started at
Day 9, bid; 21 days of treatment completed
Continuous dosing treatments started at
Day 15, bid; 10 days of treatment completed
ROS1 BaF3 Allograft Model
14 |
 STARTRK-2 and Beyond
* “RP2D”
= Recommended Phase 2 Dose; “ORR”
= Objective Response Rate
ALKA-372-001
Phase 1 dose
escalation study of
intermittent dosing
schedule in Italy:
20-30 cancer
patients with ALK,
ROS1 or TrkA
alterations
ALKi
treated
cohort
STARTRK-1
Dose escalation of daily continuous
dosing schedule in 6-24 patients with Trk,
ROS1 or ALK molecular alterations
“Basket trial”
expansion cohorts in 100
patients with TrkA, TrkB, TrkC, ROS1 or
ALK molecular alterations, treated with
RP2D*
Global Phase 1/2 study in U.S., EU and Asia
Pivotal registration
studies in most
promising tumor types
and targets
Accelerated approval
and/or breakthrough
therapy designation
possible
15
Trk A+
cohort
Trk B+
cohort
Trk C+
cohort
ROS1+
cohort
ALKi
naive
cohort
Entrectinib Clinical Development Overview:
STARTRK
(“Studies
Targeting
ALK,
ROS1
and/or
TRKA/B/C”)
Program |
 First-in-human study initiated by Nerviano in Oct 2012; Ignyta assumed
control in Nov 2013
Patient population: advanced metastatic solid tumors, with alterations to
TrkA, ROS1 or ALK (detected by IHC or FISH)
Primary objectives: DLT, MTD, RP2D
Secondary objectives: safety, PK, antitumor activity by RECIST
N= 26 enrolled, 25 dosed across 3 schedules (A, B and C)
16
Note: Data as of ESMO September 2014 poster discussion; “DLT” = dose-limiting
toxicity; “MTD” = maximum tolerated dose; “RP2D” = recommended
phase 2 dose ALKA-372-001: Phase I Dose Escalation Study of
Entrectinib in Adult Patients with Advanced Solid Tumors |
 17
Dose
1
2
3
4
Week
Schedule B: PO, QD, fed
Dose Cohorts:
Dose
Break
1
2
3
4
Week
Schedule C: PO, QD (4 on, 3 off) x 4 weeks, fed
Dose
Break
1
2
3
4
Week
Schedule A: PO, QD (4 on, 3 off) x 3 weeks, fasting
Note: Data as of ESMO September 2014 poster discussion; “DLT” = dose-limiting
toxicity; “MTD” = maximum tolerated dose; “RP2D” = recommended
phase 2 dose ALKA-372-001: Dosing Schedules
•
3 patients in
each of 100,
200, 400, 800,
1200, and
1600
mg/m
2
•
3 patients in
200
mg/m
2
•
3 patients in
400
mg/m
2 |
 18
Note: Data as of ESMO September 2014 poster discussion
n = 25
Age, mean (range) years
51
(22-75)
Female, n (%)
15 (60)
Race, %
White
100
Histology, n (%)
NSCLC
Neuroblastoma
CRC
Pancreatic
Leiomyosarcoma
GBM
16 (64)
4 (16)
2 (8)
1 (4)
1 (4)
1 (4)
Performance status (ECOG)
0
1
2
12
12
1
Prior systemic therapy
1
2
3 or more
3
5
17
Demographics and Baseline Characteristics |
 *
7 had prior treatment w/ crizotinib ** Patient had false positive TrkA IHC
result Histology and Molecular Alteration
19
Note: Data as of ESMO September 2014 poster discussion
Primary Diagnosis
Molecular Alteration
N
NSCLC
ALK rearrangement
ROS1 6 rearrangements
1 deletion
1 copy number gain
8*
8
CRC
TrkA rearrangement
ROS1 deletion
1
1
Neuroblastoma
ALK mutation
ROS1 rearrangement
TrkA overexpression
2
1
1
Glioblastoma
None**
1
Pancreatic
ROS1 deletion
1
Leiomyosarcoma
ALK deletion
1 |
 Adverse
Event G1 (%)
G2 (%)
Total (%)**
Nausea
14 (56)
2 (8)
16 (64)
Paresthesia
14 (56)
0 (0)
14 (56)
Asthenia
9 (36)
3 (12)
13 (52)
Vomiting
7 (28)
2 (8)
9 (36)
Diarrhea
6 (24)
2 (8)
8 (32)
Myalgia
6 (24)
1 (4)
7 (28)
Abdominal pain
4 (16)
2 (8)
6 (24)
Back Pain
5 (20)
1 (4)
6 (24)
Arthralgia
3 (12)
3 (12)
6 (24)
Headache
6 (24)
0 (0)
6 (24)
Dyspnea
2 (8)
1 (4)
6 (24)
Pyrexia
6 (24)
0 (0)
6 (24)
Dysgeusia
6 (24)
0 (0)
6 (24)
Cough
4 (16)
1 (4)
5 (20)
* Frequency >
20%, all dosing schedules
Most Common* Adverse Events (n=25)
** Total includes one possibly drug-related G3 asthenia AE (4%) and three unrelated G3
dyspnea AEs (12%) -
Overall, one G4 (increased lipase) and one G5 (respiratory failure) AE have been reported
(both unrelated) Note: Data as of ESMO September 2014 poster discussion
•
Most AEs
Grade 2
•
No DLT observed
at any dose level
•
No drug-related
SAEs
20 |
 •
Exposure to entrectinib
increased in an
approximate dose
proportional manner
up to doses of 800
mg/m
2
•
Mean plasma half-life
was 17-
44 hours
3
3
4
3
3
3
n
Entrectinib Steady State PK Profile Suitable for
Once Daily Dosing
21
Note: Data as of ESMO September 2014 poster discussion
|
 Tumor type
(Alteration)
Dose
(mg/m²)
Treatment Cycles / Months
Best
Response
2
4
6
8
10
12
14
16
18
20
22
24
Neuroblastoma
(ALK)
PR
NSCLC
(ALK)
SD
Pancreatic
(ROS1)
SD
NSCLC
(ALK)
PR
NSCLC
(ROS1)
1200
PR
CRC
(TrkA)
1600
PR
NSCLC
(ROS1)
400**
PR
NSCLC
(ROS1)
400***
CR*
PD at C11
PD at C4
22
* Unconfirmed / ** All PRs were Sch. A except 1 patient with ROS1+ NSCLC in Sch. C /
*** CR was Sch. C Note:
17
patients
had
progressive
disease,
3
of
whom
were
dosed
at
100
mg/m
2
(sub-therapeutic),
3
of
whom
had gene deletions (not thought to be tumor-activating), and 1 of whom did not have a relevant
molecular alteration
Timing of PR
Timing of CR
Note: Data as of ESMO September 2014 poster discussion
200
400
800
1200
200
400
800
400
800
1200
800
Observed Clinical Responses in Patients with Each of TrkA, ROS1
and ALK, in Each of CRC, NSCLC and Neuroblastoma |
 Partial Response in
75 Year-Old Patient with TrkA Rearranged Metastatic Colorectal Cancer
Patient screened and treated at Ospedale Niguarda Ca’
Granda, Milan, Italy
Week 4**
April 2014
Prior to Treatment*
March 2014
*
Patient
had
prior
1
st
line
FOLFOX,
2
nd
line
FOLFIRI/Cetuximab
and
3
rd
line Irinotecan
**
Treated
with
Schedule
A,
1600
mg/m
2
23 |
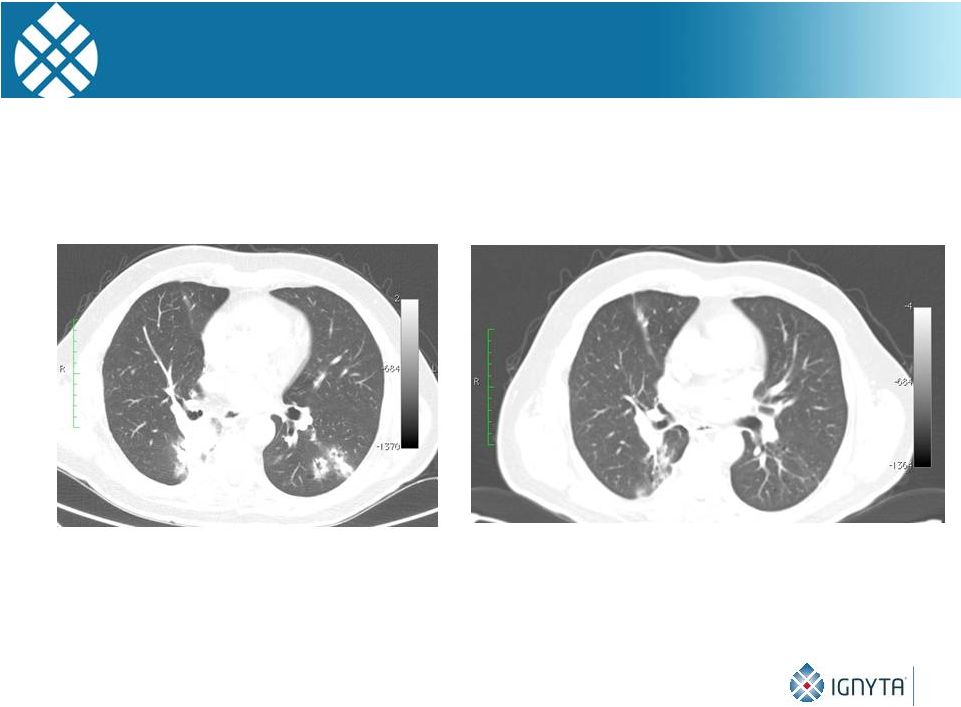 Partial Response in
63 Year-Old Patient with ROS1 Rearranged Non-small Cell Lung Cancer
Week 4**
August 2014
Prior to Treatment*
July 2014
* Patient had 3 prior cycles of chemotherapy
** Treated
with
Schedule
C,
400
mg/m
2
24 |
 Cycle
15** April 2014
Prior to Treatment*
February 2013
25
Partial Response in 22 Year-Old Patient with
Metastatic ALK+ Neuroblastoma
* Patient had 4 prior cycles of chemotherapy
**
Treated
with
Schedule
A,
dose
escalated
to
1200
mg/m
2 |
 Entrectinib
is a potent, selective inhibitor of key oncogenic kinases: Trk family, ROS1 and
ALK -
1 complete response
-
5 partial responses (3 patients > 9 cycles) across multiple tumor types (NSCLC, CRC, and
NB) in patients with TrkA, ROS1, and ALK molecular alterations
-
2 prolonged stable disease (11 and 18 cycles)
Well tolerated at doses up to 1600 mg/m
2
on an intermittent schedule
PK profile suitable for appropriate target coverage with once daily dosing
Responders tend to have higher exposure than non-responders throughout the
entire dosing cycle in Schedule A
Currently testing 3 dosing schedules, including continuous daily
dosing
26
Note: Data as of ESMO September 2014 poster discussion
Entrectinib: Novel Kinase Inhibitor with Promising Antitumor
Response Observed with Intermittent Dosing Schedule |
 RXDX-103
(formerly NMS-P862): Nerviano’s 2 nd
Generation Compound*
27
*
In 2009, NMS advanced NMS-P354, a first generation Cdc7 inhibitor, into 3
phase 1 studies in solid and hematologic tumors; dosed 48 patients in the 3
studies, but was unable to achieve sufficient drug exposure due to rapid
metabolism and generation of a toxic metabolite Nerviano Medical Sciences (NMS) is
world’s leading expert in Cdc7 biology, having worked on the mechanism since
2003 Preclinical development candidate: NMS’
2nd generation compound engineered
with an improved metabolic profile
Good drug-like properties (oral bioavailability, permeability, PK, etc.)
Single agent and combination therapy activity in multiple in vivo models; potential
efficacy in drug resistance setting or in combination with cell cycle inhibitors
Patient selection hypothesis and pharmacodynamic biomarkers
Potential clinical benefit in selected cancer patient populations (e.g., breast, colon)
Targeting FIH study in early 2016
RXDX-103 Cdc7 Kinase Inhibitor: First-in-Class Opportunity
|
 Role of Cdc7 in
Cancer Cdc7 is upregulated in cancer
-
Breast cancer: Cdc7 overexpression linked to genomic instability, poor
prognosis and reduced survival
-
Epithelial ovarian cancer: Cdc7 overexpression correlated with poor
prognosis (specifically disease free survival) and advanced clinical stage
-
DLBCL and melanoma: increased Cdc7 activity associated with poor
clinical outcome and lower relapse free survival
Cdc7 overexpression associated with acquired chemotherapy resistance
28
Sources: Rodriguez, 2010; Choshzick, 2010; Kulkarni et. al., 2009; Nambiar et al., 2007;
Krawczyk et al., 2009 |
 Cell Division
Cycle Background Cell cycle kinases promote progression through different phases of cell
division cycle These proteins are deregulated in cancer, and considered druggable
targets Cdc7 is a serine threonine kinase essential for initiating DNA replication
during S phase 29
Source: Malumbres, 2011 |
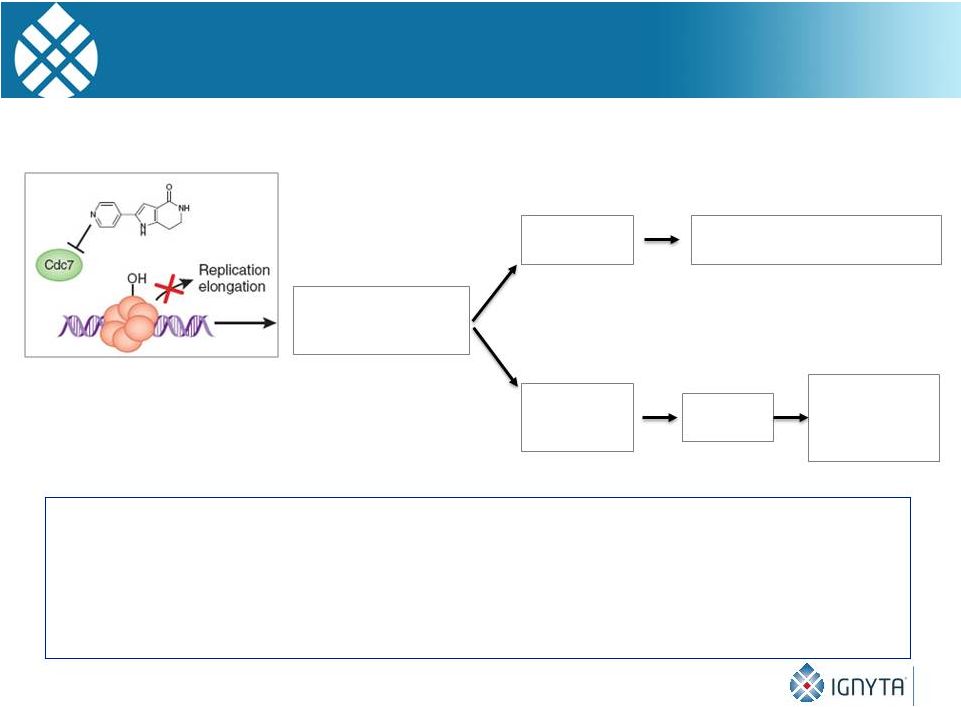 30
Inhibition of Cdc7
arrests DNA
replication initiation
Checkpoint
failure
Tumor cells
DNA breaks, mitotic catastrophe,
apoptotic cell death
Normal cells
Intact
replication
checkpoint
Cell cycle
arrest
Resume
replication after
removal of
Cdc7 inhibition
Figure modified from Nature Chemical Biology 4, 331-32 (2008)
Highly Targeted, Selective Cdc7 Inhibition
Kills Cancer Cells while Sparing Healthy Cells
•
Cdc7
inhibition
leads
to
apoptotic
cell
death
in
tumor
cells
due
to
their
lack
of
functional
checkpoint
response (checkpoint proteins are often mutated in human cancer)
•
In contrast, normal cells arrest reversibly in a p53 dependent fashion
•
A Cdc7 inhibitor could potentially act in synergy with chemotherapies that target the DNA
replication elongation process (e.g., targeted cell cycle inhibitors,
anti-metabolites, topoisomerase inhibitors, and intercalating agents)
|
 31
RXDX-103 (IC
50
nM)
Cdc7/Dbf4
9
Cdk9/CycT
150
Gsk3 beta
170
Cdk2/CycA
450
RXDX-103 Kinase Screen
Source: Nerviano Medical Sciences
RXDX-103 Is a Highly Potent, Selective
ATP-Competitive Cdc7 Inhibitor |
 32
Source: Nerviano Medical Sciences
RXDX-103 15 mg/kg QD (1-18)
Vehicle
RXDX-103 Demonstrates Potent Single Agent
Anti-Tumor Activity in a Rat Breast Cancer Model |
 RXDX-103
Demonstrates Synergistic Anti-Tumor Activity in Combination with Docetaxel in a
Mouse Breast Cancer Model 33
MX-1 Triple Negative mouse xenograft model
Source: Nerviano Medical Sciences |
 34
2014
2015
2016
2017
2018
Preclinical/GLP
Ph1/2a –
single agent
Ph1/2a –
combo (breast)
Ph1/2a –
combo (ovarian)
Ph1/2a –
combo (CRC)
Preclinical data suggests efficacy in specific patient populations:
Current Program Status:
Completed preliminary rodent PK
and in vitro ADME studies
Preparing for manufacturing GMP
scale-up and GLP tox studies
Breast cancer with biomarker 1 overexpression
Biomarker 2 positive ovarian cancer
Biomarker 2 positive colorectal cancer
RXDX-103 Clinical Development Strategy
Preliminary
Plan; Subject to Change |
 35
Central
Lab
Clinical Sites
Specimens
•
GXP
•
CLIA
Platforms
Output
FFPE
Fresh Tissue
FISH
IHC
PCR
NGS
Oncolome®
Trial Enrollment
CDx
Ignyta’s Dx Capabilities Enable Leadership
in Precision Medicine
•
CNVs
•
Splice variants
•
Mutations
•
Overexpression
•
Rearrangements
|
 Ignyta’s
Two-Pronged Approach to Generating Value from Novel Targets Identified by
Oncolome Oncolome Mining & Pathway Mapping
36
Target Prioritization by Ignyta Oncology Team
Compound Database Screen
Drug Developer Outreach
Program /Company Selection
External Targets
Engage in discussions with
biopharma companies with
existing programs for
potential in-licensing (e.g.,
Nerviano)
Internal Targets
If no attractive clinical or
preclinical stage programs
exist for target of interest,
then initiate Spark discovery
program (e.g., Spark-1)
Drug Candidate Diligence
Deprioritized Clinical Assets
Oncology assets developed
without a biomarker strategy
and that failed due to lack of
sufficient clinical efficacy
Repositioned asset, with
biomarker strategy revealed
by Oncolome |
 2015
Corporate Milestones 37
2030 Vision
2015 Milestones
Obtain US orphan drug designation for at least one indication
Report clinical data (preliminary) from ALKA-372-001 and STARTRK-1 at ASCO, 2Q15;
and at ESMO and/or ENA, 2H15
Implement internally developed CDx to support Phase 2a of STARTRK-1, 2Q15
Identify RP2D and preferred dosing schedule, mid-2015
Enroll first patient in Phase 2a of STARTRK-1, 3Q15
Potentially initiate STARTRK-2+ pivotal registration study(ies), 2H15
Initiate lead optimization for one Spark program, YE15
Obtain CAP licensure of Dx lab, YE15
Complete IND enabling activities for RXDX-103 to support 1Q16 IND filing
2011 –
2015
Advance clinical pipeline
2016 –
2020
Commercialize RXDX
lead
2021 –
2025
Scale pipeline revenue
2026 –
2030
Drive sustainable
profitability
Leading precision
medicine
company |
 38
Precision oncology company with integrated approach to Rx/Dx development
Experienced management team, excellent track record in oncology
Pipeline targeting first-in-class opportunities in
cancer Lead oncology asset: Phase 1/2 highly potent, selective kinase inhibitor with a
complete response and observed partial responses in each of TrkA, ROS1 and
ALK, in 3 tumor types
Multiple potential clinical readouts in 2015
Comprehensive diagnostic capabilities and biomarker strategies for patient
screening and confirmation
Composition of matter IP for clinical and preclinical drug candidates
Strong financial position
Company Highlights |
