Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Fibrocell Science, Inc. | form8-kfiliing1x12x15.htm |

Fibrocell Corporate Presentation David Pernock, Chairman & CEO Biotech Showcase 2015 January 12, 2015

2 Forward-Looking Statements This presentation contains, and our officers and representatives may from time to time make, statements that are “forward-looking statements” within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995. Examples of forward-looking statements include, among others, statements we make regarding our development strategy, timing and potential advantages of our product candidates. These forward-looking statements rely on a number of assumptions concerning future events and are subject to a number of risks, uncertainties, and other factors, many of which are outside of Fibrocell Science’s control. Important factors that could cause our actual results and financial condition to differ materially from those indicated in the forward-looking statements include, among others, the following: (i) uncertainties relating to the initiation and completion of clinical trials; and (ii) whether clinical trial results will validate and support the safety and efficacy of our product candidates, as well as those set forth under the caption “Item 1A. Risk Factors” in Fibrocell Science’s most recent Form 10-K filing, as amended, as updated in “Item 1A. Risk Factors” in Fibrocell Science’s most recent Form 10-Q filing. Any forward-looking statement made by us in this presentation is based only on information currently available to us and speaks only as of the date on which it is made. In addition, Fibrocell Science operates in a highly competitive and rapidly changing environment, and new risks may arise. Accordingly, you should not place any reliance on forward-looking statements as a prediction of actual results. Fibrocell Science disclaims any intention to, and undertakes no obligation to, update or revise any forward-looking statement. You are also urged to carefully review and consider the various disclosures in Fibrocell Science’s most recent annual report on Form 10-K, as amended, our most recent Form 10-Q as well as other public filings with the SEC since the filing of Fibrocell Science’s most recent annual report.

Investment Highlights • Fibrocell is the scientific leader of autologous fibroblast therapy for skin and connective tissue diseases • Developing gene-therapies for orphan skin diseases using the autologous fibroblast cell as the delivery vehicle for the desired gene • Our lead gene-therapy program is focused on RDEB, a rare, congenital orphan skin disease. Potential to expand into other orphan skin diseases. – Devastating, progressive, painful blistering disease that leads to death – IND expected to be filed by mid 2015, Phase I expected to be initiated in 2H 2015 – Developed in collaboration with Intrexon Corporation (NYSE: XON) • Phase II sBLA program: autologous fibroblast for vocal cord scarring (efficacy results expected 2H 2015) 3

The Science of Autologous Fibroblasts • Fibroblasts repair tissue infrastructure by producing extracellular matrix proteins including collagen and growth factors • Most common cell in skin and connective tissue − Autologous cells reduce risk of patient rejection • Fibroblasts have distinct advantages for gene transduction for use in orphan skin diseases • Our proprietary technology is used to create personalized biologics 4

Gene Therapy Product Engine Integrates Intrexon’s synthetic biology to develop gene therapies for orphan diseases using the autologous fibroblast as the delivery vehicle Autologous Fibroblast Product Engine BLA label extension in Phase II • Vocal Cord Scarring Autologous Fibroblast Platform Technology Autologous Fibroblast Platform Technology 5

Gene Therapy Product Engine Autologous Fibroblast Product Engine Two Product Engines – Multiple Therapeutics in Development Collection Culture Administration Vector Preparation Gene Packaging Gene Integration Fibrocell Proprietary Personalized Biologics Approach 6

Indication 2014 2015 2016 Recessive Dystrophic Epidermolysis Bullosa (RDEB) GM-HDF-COL7 Vocal Cord Scarring Linear Scleroderma Development Pipeline Lead Rare Disease Program Azficel-T sBLA Programs Phase II/III Phase I Phase II Primary Endpoint Phase III Product Optimization and PoC Studies/Pre-Clinical Rare Disease Program 7 Product Optimization and PoC Studies/ Pre-Clinical
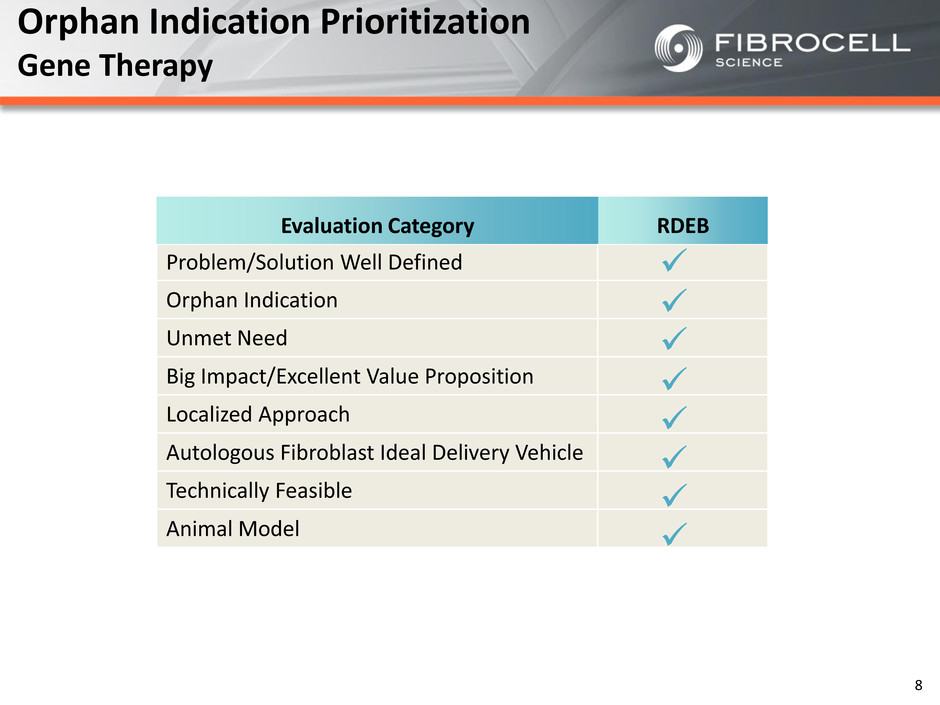
Orphan Indication Prioritization Gene Therapy RDEB Problem/Solution Well Defined Orphan Indication Unmet Need Big Impact/Excellent Value Proposition Localized Approach Autologous Fibroblast Ideal Delivery Vehicle Technically Feasible Animal Model Evaluation Category RDEB 8
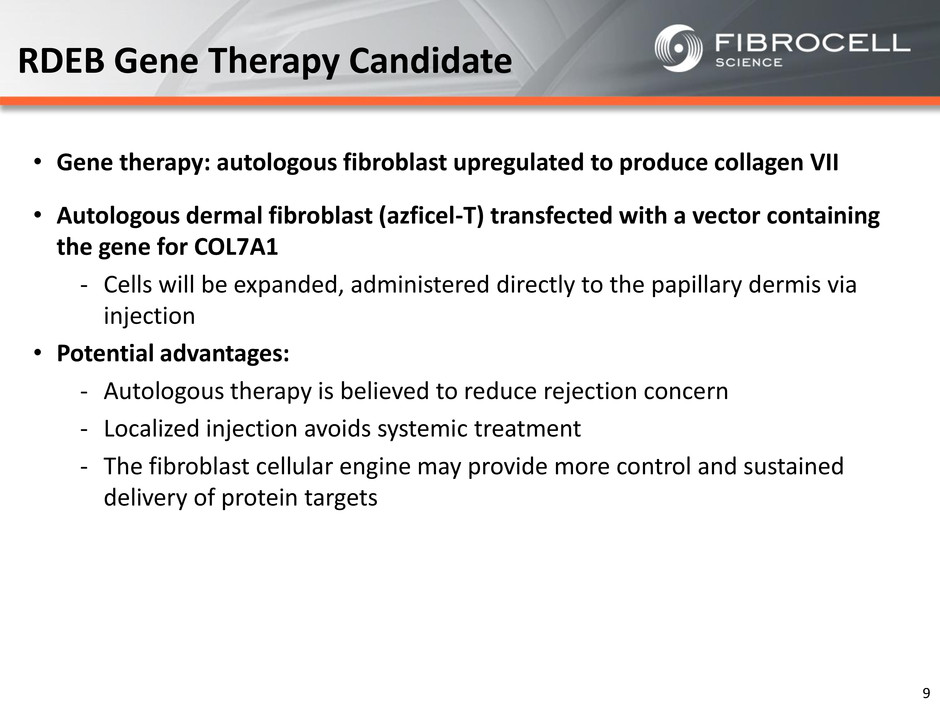
• Gene therapy: autologous fibroblast upregulated to produce collagen VII • Autologous dermal fibroblast (azficel-T) transfected with a vector containing the gene for COL7A1 ‐ Cells will be expanded, administered directly to the papillary dermis via injection • Potential advantages: ‐ Autologous therapy is believed to reduce rejection concern ‐ Localized injection avoids systemic treatment ‐ The fibroblast cellular engine may provide more control and sustained delivery of protein targets RDEB Gene Therapy Candidate 9

Disease Current Treatments Epidemiology • Devastating, progressive, painful blistering disease that leads to death • RDEB life expectancy: 30 years • Cause: A mutation in the COL7A1 gene which encodes for type VII collagen • Bandaging & antibiotics – up to $20,000 per month • Feeding tubes • Surgery, including hand and esophageal Dystrophic EB (DEB) 25% of all EB sufferers1 = 5,500 – 12,500 US • RDEB ~5% of all EB sufferers US2 = 1,100 – 2,500 • DDEB ~20% of all EB sufferers US3 = 4,400 – 10,000 Recessive Dystrophic Epidermolysis Bullosa 10
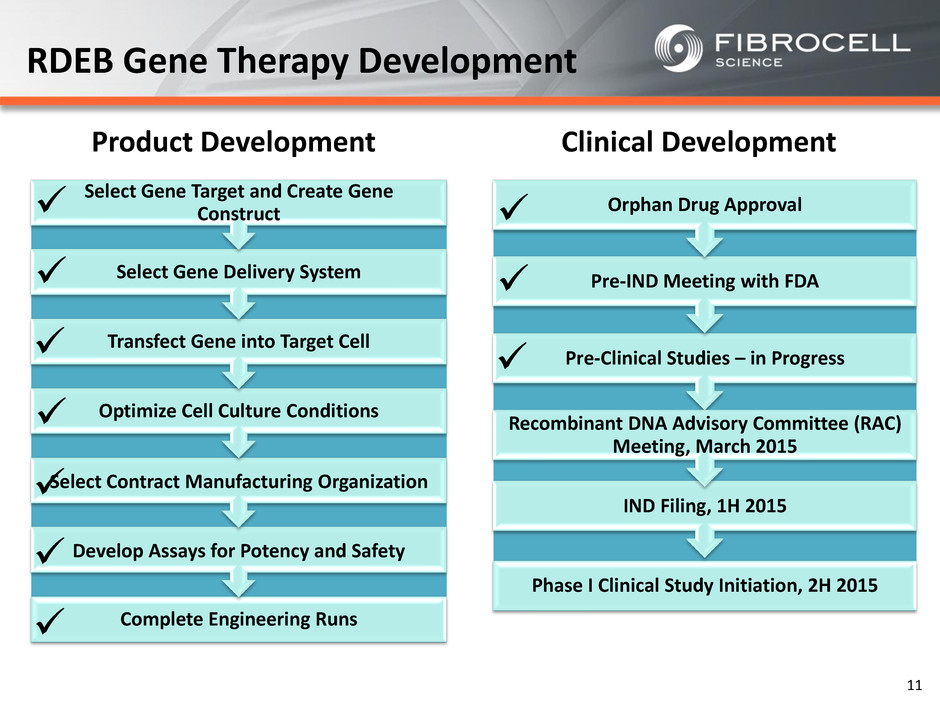
Phase I Clinical Study Initiation, 2H 2015 IND Filing, 1H 2015 Pre-Clinical Studies – in Progress Recombinant DNA Advisory Committee (RAC) Meeting, March 2015 Pre-IND Meeting with FDA Orphan Drug Approval RDEB Gene Therapy Development Complete Engineering Runs Develop Assays for Potency and Safety Select Contract Manufacturing Organization Optimize Cell Culture Conditions Transfect Gene into Target Cell Select Gene Delivery System Select Gene Target and Create Gene Construct 11 Product Development Clinical Development

• Multiple published studies in previous animal models4,5,6 demonstrate that fibroblasts genetically modified with COL7A1: ‐ Deposited collagen VII and formed anchoring fibrils at the dermal-epidermal junction ‐ Exhibited stable expression ‐ Corrected blistering • Autologous cells are preferred over allogeneic cells due to increased proliferation, less inflammation and lack of immunogenic response7,8,9 Rationale Supporting Our Approach in RDEB 12

• Precedent for FDA-allowed IND using GM cells to treat RDEB ‐ An NIH-funded Phase I clinical trial using autologous keratinocyte sheets genetically modified with COL7A1 to treat RDEB is ongoing ‐ Establishes a basis and precedent pathway for our Phase I clinical trial design for GM-HDF-COL7 ‐ The NIH clinical trial at Stanford is being conducted by the same experts working with Fibrocell on our preclinical studies and expected Phase I clinical trial • Fibroblast cells have distinct advantages for development of gene therapy for RDEB: ‐ Express higher levels of collagen VII ‐ Injected on localized basis ‐ Fibroblasts are readily available ‐ Protected from infection when injected into papillary dermis ‐ Administered outpatient Rationale Supporting Our Approach in RDEB 13

Transforming Lives of RDEB Patients 14

Disease Epidemiology • Excess production of extracellular matrix characterized by skin fibrosis and linear scars • The linear areas of skin thickening may extend to underlying tissue and muscle in children which may impair growth in affected legs and arms or forehead • Lesions appearing across joints impair motion and may be permanent • Localized Scleroderma ~200,000 sufferers US10 comprised of many different sub-types - Linear Scleroderma Initial target is a group of ~40,000 patients who have scleroderma over a major joint and exhibit severe joint pain10 Current Treatments • Systemic or topical corticosteroids • UVA light therapy • Physical therapy Linear Scleroderma Photo: © 2015 American College of Rheumatology. Used with permission. 15

Disease Current Treatments Epidemiology Damage to the fibroblast layer causes scarring and edema which limits air flow and results in severe and significant limitations in voice quality, often loss of voice • Voice therapy • Surgery - Injection (collagen, fat, calcium, hyaluronic acid) - Implant (PTFE, silastic) Estimated 147,000 people currently suffering from chronic or severe dysphonia from vocal cord scarring Vocal Cord Scarring 16

Positive Phase I clinical trial results published in peer-reviewed journal11 • All patients completed the clinical trial, successive injections of autologous fibroblasts were well-tolerated, and no serious adverse events reported; n=5 • Statistically significant and sustained improvement: - Efficacy analysis for wave grade improvements was significant starting in month 3 of the 12 month clinical trial (p=0.04) - Voice Handicap Index and Voice Quality assessments showed an improvement in voice quality overall for the duration of the clinical trial 17 Rationale Supporting Our Approach in Vocal Cord Scarring

• Trial initiated; Enrollment active • Multi-center trial: 3 major centers • Double-blind, randomized, placebo-controlled, 20 subjects • 4-month efficacy endpoint • FDA Validated Study Endpoints Vocal Cord Scarring Phase II 18

• Cash position $44.2 million cash position at Q3-2014 • HQ facility: Exton, PA • ~50 employees • 40.9 million shares outstanding - 6.0 million warrants; 2.3 million options • Clean capital structure • Analyst research: - Wedbush Securities, Inc. — David Nierengarten, PhD - Griffin Securities, Inc. — Keith Markey, PhD Financial and Corporate Information 19

Investment Highlights • Fibrocell is the scientific leader of autologous fibroblast therapy for skin and connective tissue diseases • Developing gene-therapies for orphan skin diseases using the autologous fibroblast cell as the delivery vehicle for the desired gene • Our lead gene-therapy program is focused on RDEB, a rare, congenital orphan skin disease. Potential to expand into other orphan skin diseases. – Devastating, progressive, painful blistering disease that leads to death – IND expected to be filed by mid 2015, Phase I expected to be initiated in 2H 2015 – Developed in collaboration with Intrexon Corporation (NYSE: XON) • Phase II sBLA program: autologous fibroblast for vocal cord scarring (efficacy results expected 2H 2015) • NASDAQ listed: FCSC 20

References 1 Petrof G., et. al. Fibroblast cell therapy enhances initial healing in recessive dystrophic epidermolysis bullosa wounds: results of a randomised, vehicle-controlled trial. Brit J Dermatol. 2013 Nov;169(5):1025-33. 2 DEBRA International. What is EB Infographic. http://www.debra-international.org/epidermolysis-bullosa.html. Accessed 10/06/2014. 3 Calculated as follows: 25% of DEB - 5% RDEB = 20% DDEB 4Ortiz-Urda S. et al. Injection of genetically engineered fibroblasts corrects regenerated human epidermolysis bullosa skin tissue. J Clin Invest. 2003. 111:251–255. 5Woodley DT. et. al. Normal and Gene-Corrected Dystrophic Epidermolysis Bullosa Fibroblasts Alone Can Produce Type VII Collagen at the Basement Membrane Zone. Journal of Investigative Dermatology. 2003. 1021-1028. 6Chen M et al. NC1 domain of type VII collagen binds to the beta 3 chain of laminin 5 via a unique subdomain within the fibronectin-like repeats. J Invest Dermatol. 1999 Feb;112(2):177-83 7 Petrof, G. et al., Fibroblast cell therapy enhances initial healing in recessive dystrophic epidermolysis bullosa wounds: results of a randomized, vehicle-controlled trial. Br J Dermatol. 2013 Nov;169(5):1025-33. 8 Morimoto N. et. al.; Viability and function of autologous and allogeneic fibroblasts Seeded in dermal substitutes after implantation. J Surg Res. 2005 May 1;125(1):56-67. 9 Lamme, E. N., Van Leeuwen, R. T. J., Mekkes, J. R. and Middelkoop, E. (2002), Allogeneic fibroblasts in dermal substitutes induce inflammation and scar formation. Wound Repair and Regeneration, 10: 152-160. 10 The Scleroderma Foundation. What is Scleroderma? www.scleroderma.orgl; accessed 10/09/2014. States that systemic scleroderma is one third of all scleroderma cases. 11 Chhetri, Dinesh, Injection of Cultured Autologous Fibroblasts for Human Vocal Fold Scars. The Laryngoscope 121(4):785-792, 2011 21
