Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Endo International plc | d831842d8k.htm |
Exhibit 99.1

Exhibit 99.1
Endo International plc
Capital Research Presentation
December 3, 2014
©2014 Endo Pharmaceuticals Inc. All rights reserved.

Additional Information
ADDITIONAL INFORMATION
This communication does not constitute an offer to buy or solicitation of an offer to sell any securities. This communication relates to a proposed business combination transaction between Endo International plc (“Endo”) and Auxilium Pharmaceuticals, Inc. (“Auxilium”). In furtherance of this proposed transaction, Endo and Auxilium intend to file one or more registration statements, prospectuses, proxy statements or other documents with the U.S. Securities and Exchange Commission (“SEC”). This communication is not a substitute for any registration statement, prospectus, proxy statement or other document Endo and/or Auxilium file with the SEC in connection with the proposed transaction. INVESTORS AND SECURITY
HOLDERS OF AUXILIUM ARE URGED TO READ THE REGISTRATION STATEMENT, PROSPECTUS, PROXY STATEMENT AND OTHER DOCUMENTS FILED WITH THE SEC CAREFULLY IN THEIR ENTIRETY WHEN THEY BECOME AVAILABLE AS THEY WILL CONTAIN IMPORTANT INFORMATION ABOUT THE PROPOSED TRANSACTION. The definitive proxy statement (when available) will be mailed to stockholders of Auxilium. Investors and security holders will be able to obtain free copies of these documents (when available) and other documents filed with the SEC by Endo through the web site maintained by the SEC at http://www.sec.gov.
CERTAIN INFORMATION REGARDING PARTICIPANTS
Endo and Auxilium and certain of their respective directors and executive officers may be deemed to be participants in the solicitation of proxies from Auxilium stockholders with respect to the proposed transaction under the rules of the SEC. Security holders may obtain information regarding the names and interests of Endo’s directors and executive officers in Endo Health Solutions Inc.‘s (“EHSI”) Annual Report on Form 10-K for the year ended December 31, 2013, which was filed with the SEC on March 3, 2014, and Endo’s proxy statement for the 2014 Annual General Meeting of Shareholders, which was filed with the SEC on April 29, 2014. Security holders may obtain information regarding the names and interests of Auxilium’s directors and executive officers in Auxilium’s Annual Report on Form 10-K for the year ended December 31, 2013, which was filed with the SEC on February 28, 2014, Auxilium’s proxy statement for the 2014 Annual Meeting of Stockholders, which was filed with the SEC on April 10, 2014, and the materials that will be filed with the SEC in connection with the proposed transaction. These documents can be obtained free of charge from the sources indicated above. Additional information regarding the interests of these participants in the proxy solicitation and a description of their direct and indirect interests, by security holdings or otherwise, will also be included in the proxy statement and other relevant materials to be filed with the SEC when they become available.
All trademarks, service marks, trade names, product names and logos appearing in this presentation are the property of their respective owners. XIAFLEX®, Testim®, TESTOPEL®, STENDRA®, edex®, Osbon® ErecAid®, STRIANT®, Theo24®, Semprex®-D, dilatrate®-SR and robaxin® and the related logos are the property of Auxilium. All other trademarks, service marks, trade names, product names and logos appearing in this presentation are the property of Endo
1
©2014 Endo Pharmaceuticals Inc. All rights reserved.
|
|
Forward Looking Statements; Non-GAAP Financial Measures
This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and Canadian securities legislation. Statements including words such as “believes,” “expects,” “anticipates,” “intends,” “estimates,” “plan,” “will,” “may,” “look forward,” “intend,” “guidance,” “future” or similar expressions are forward-looking statements. Because these statements reflect our current views, expectations and beliefs concerning future events, these forward-looking statements involve risks and uncertainties. Although Endo believes that these forward-looking statements and information are based upon reasonable assumptions and expectations, readers should not place undue reliance on them, or any other forward looking statements or information in this news release. Investors should note that many factors, as more fully described in the documents filed by Endo with securities regulators in the United States and Canada including under the caption “Risk Factors” in Endo’s and EHSI’s Form 10-K, Form 10-Q and Form 8-K filings, as applicable, with the Securities and Exchange Commission and with securities regulators in Canada on System for Electronic Document Analysis and Retrieval (“SEDAR”) and as otherwise enumerated herein or therein, could affect Endo’s future financial results and could cause Endo’s actual results to differ materially from those expressed in forward-looking statements contained in EHSI’s Annual Report on Form 10-K. The forward-looking statements in this presentation are qualified by these risk factors. These are factors that, individually or in the aggregate, could cause our actual results to differ materially from expected and historical results. Endo assumes no obligation to publicly update any forward-looking statements, whether as a result of new information, future developments or otherwise, except as may be required under applicable securities law.
This presentation may refer to non-GAAP financial measures, including adjusted diluted EPS, that are not prepared in accordance with accounting principles generally accepted in the United States and that may be different from non-GAAP financial measures used by other companies. Investors are encouraged to review Endo’s current report on Form 8-K filed with the SEC for Endo’s reasons for including those non-GAAP financial measures in this presentation. Reconciliation of non-GAAP financial measures to the nearest comparable GAAP amounts have been provided within the appendix at the end of this presentation.
2
©2014 Endo Pharmaceuticals Inc. All rights reserved.
|
|
Discussion Topics
Endo’s Strategy and Operating Model Recent Accomplishments Auxilium Update 2014 Financial Guidance Q&A
3
©2014 Endo Pharmaceuticals Inc. All rights reserved.
|
|
Who is Endo International plc
Business Overview
Roots dating back to 1920
Originally a family-run pharmaceutical company
Became Endo Products in 1935
Management buyout in 1997 from DuPont Merck
Became publically traded in 2000
Develop, manufacture, market, and distribute quality branded pharmaceutical, generic and device products through five (5) operating companies Global headquarters in Dublin, Ireland and U.S. headquarters in Malvern, PA Focus on higher margin specialty therapeutics such as pain management, urology and endocrinology
Financial Overview
Market cap of ~$11B
Close to 5,000 employees worldwide 2014 revenue guidance of ~$2.8+B ~85% pharmaceuticals based on Q3 2014 revenues
4
|
|
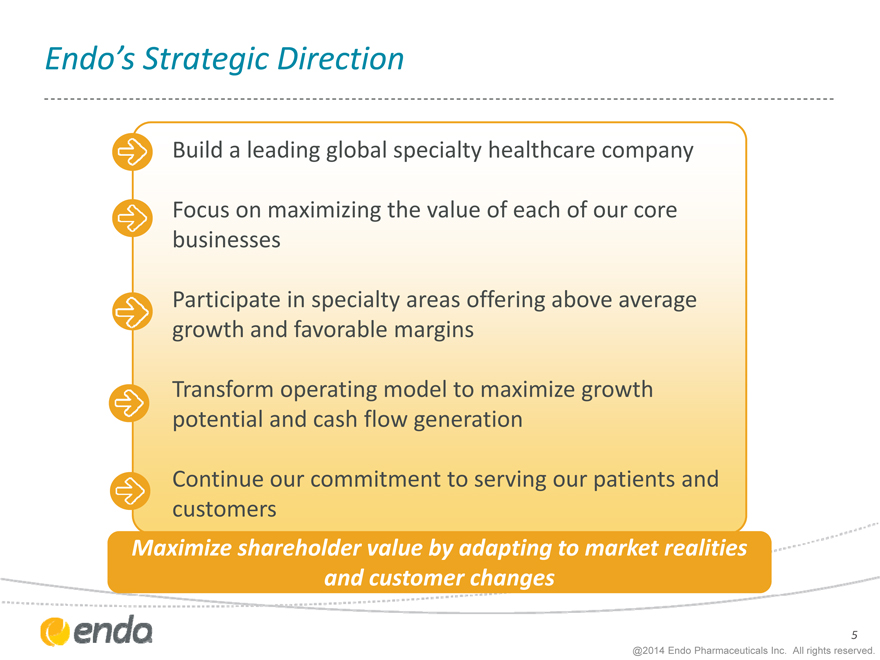
Endo’s Strategic Direction
Build a leading global specialty healthcare company
Focus on maximizing the value of each of our core businesses
Participate in specialty areas offering above average growth and favorable margins
Transform operating model to maximize growth potential and cash flow generation
Continue our commitment to serving our patients and customers
Maximize shareholder value by adapting to market realities and customer changes
5
@2014 Endo Pharmaceuticals Inc. All rights reserved.
|
|
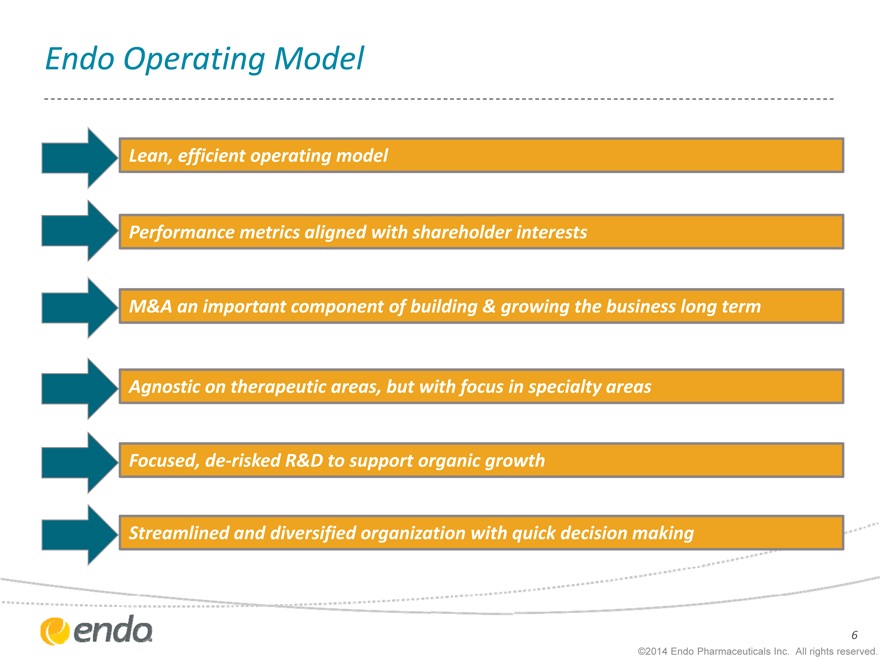
Endo Operating Model
Lean, efficient operating model
Performance metrics aligned with shareholder interests
M&A an important component of building & growing the business long term Agnostic on therapeutic areas, but with focus in specialty areas Focused, de-risked R&D to support organic growth Streamlined and diversified organization with quick decision making
6
©2014 Endo Pharmaceuticals Inc. All rights reserved.
|
|
Executing Our Strategy
Strengthened talent and organization
Implemented a lean operating model to achieve $325 million in savings Completed/initiated multiple accretive, value-creating transactions
Acquisitions and in-licensing deals: Paladin Labs, Boca Pharmacal, DAVA Pharmaceuticals, Grupo Farmaceutico Somar, Sumavel® DosePro® and NatestoTM
Announced agreement to acquire Auxilium Pharmaceuticals
Increased strategic focus
Completed the divestiture of HealthTronics and discovery assets Agreements in principle to settle substantially all U.S. mesh claims
Sharpened focus on near-term organic growth priorities Enhanced capital structure flexibility Delivering on our financial targets
7
©2014 Endo Pharmaceuticals Inc. All rights reserved.
|
|
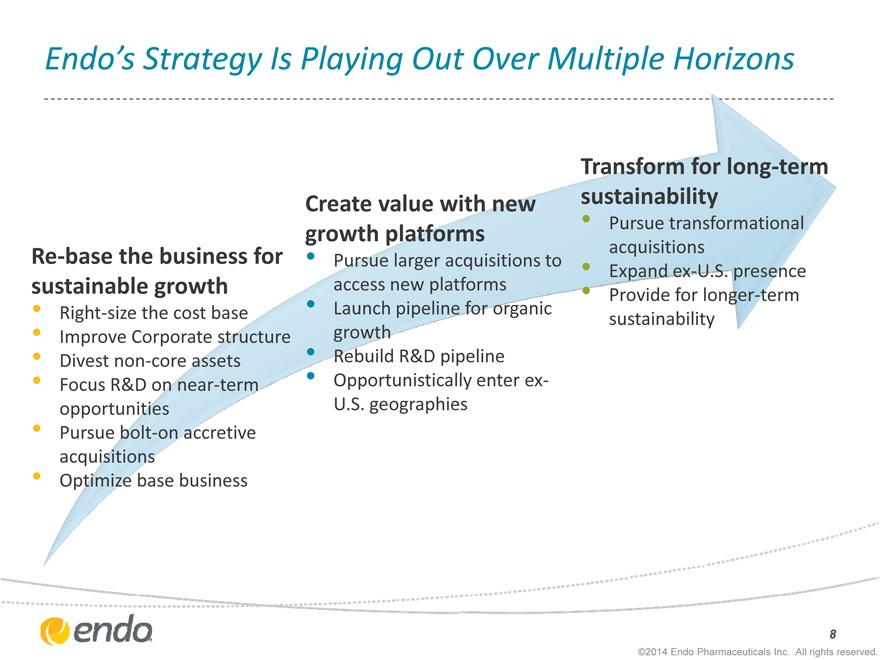
Endo’s Strategy Is Playing Out Over Multiple Horizons
Re-base the business for sustainable growth
Right-size the cost base
Improve Corporate structure
Divest non-core assets
Focus R&D on near-term opportunities
Pursue bolt-on accretive acquisitions
Optimize base business
Create value with new growth platforms
Pursue larger acquisitions to access new platforms
Launch pipeline for organic growth
Rebuild R&D pipeline
Opportunistically enter ex-U.S. geographies
Transform for long-term sustainability
Pursue transformational acquisitions
Expand ex-U.S. presence
Provide for longer-term sustainability
8
©2014 Endo Pharmaceuticals Inc. All rights reserved.
|
|
Progress on Near-Term Strategic Priorities – Q3 2014
Deploying capital to accretive, value-creating opportunities
Announced agreement to acquire Auxilium Pharmaceuticals
Closed acquisitions of Grupo Farmaceutico Somar and DAVA Pharmaceuticals
Enhancing operational focus on organic growth drivers
Delivering double-digit organic growth in U.S. Generics Strong core revenue growth in U.S. Branded Pharmaceuticals Launched Travelan® and Veregen® in Canada
Maximizing base business through LIDODERM® and Fortesta® Gel AG sales
Sharpening R&D focus on near-term opportunities
Focused on filing NDA for BEMA buprenorphine by late-2014 or early-2015 Completed full-year objective of 8 ANDA filings by U.S. Generics business AMS announced positive top-line results for the investigational TOPAS™ system
Meeting our financial targets
Raising 2014 Revenue and Adjusted EPS Financial Guidance based on solid operating results and progress on near-term priorities
9
©2014 Endo Pharmaceuticals Inc. All rights reserved.
|
|
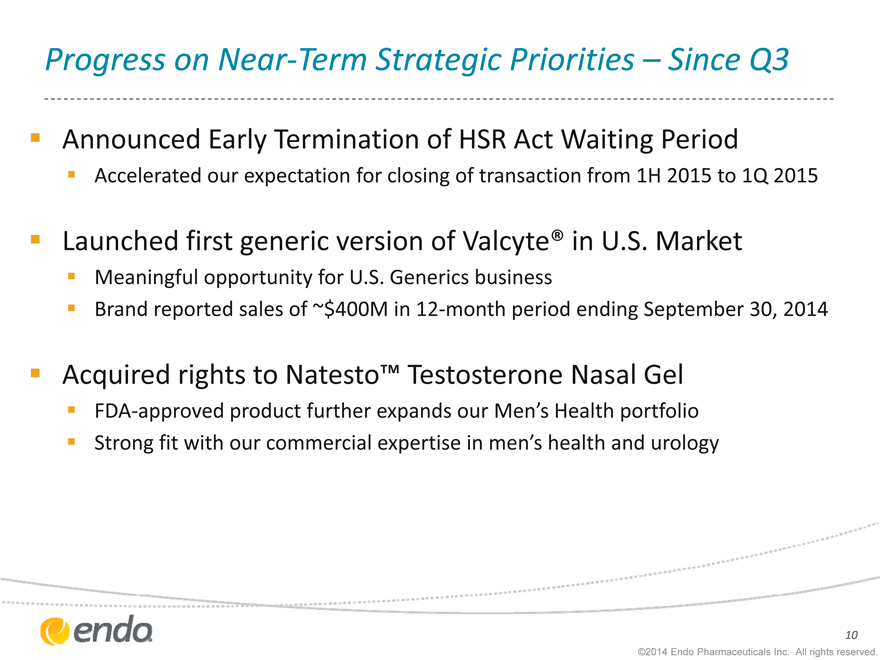
Progress on Near-Term Strategic Priorities – Since Q3
Announced Early Termination of HSR Act Waiting Period
Accelerated our expectation for closing of transaction from 1H 2015 to 1Q 2015
Launched first generic version of Valcyte® in U.S. Market
Meaningful opportunity for U.S. Generics business
Brand reported sales of ~$400M in 12-month period ending September 30, 2014
Acquired rights to Natesto™ Testosterone Nasal Gel
FDA-approved product further expands our Men’s Health portfolio Strong fit with our commercial expertise in men’s health and urology
10
©2014 Endo Pharmaceuticals Inc. All rights reserved.
|
|
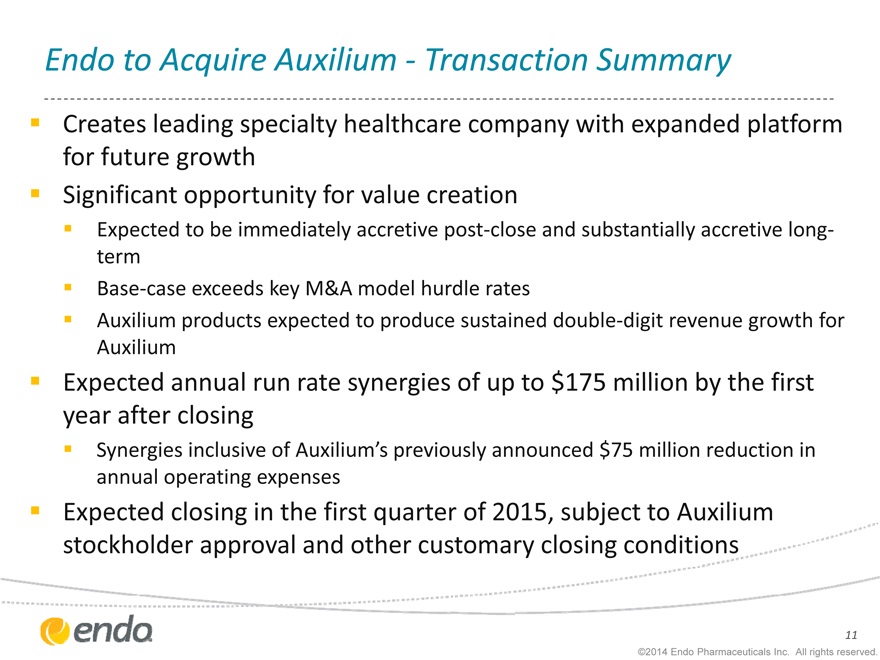
Endo to Acquire Auxilium - Transaction Summary
Creates leading specialty healthcare company with expanded platform for future growth Significant opportunity for value creation
Expected to be immediately accretive post-close and substantially accretive long-term Base-case exceeds key M&A model hurdle rates Auxilium products expected to produce sustained double-digit revenue growth for Auxilium
Expected annual run rate synergies of up to $175 million by the first year after closing
Synergies inclusive of Auxilium’s previously announced $75 million reduction in annual operating expenses
Expected closing in the first quarter of 2015, subject to Auxilium stockholder approval and other customary closing conditions
11
©2014 Endo Pharmaceuticals Inc. All rights reserved.
|
|
Focus on Accessing and Fostering Organic Growth Drivers
U.S. Branded Pharmaceuticals
Expect to submit NDA for BEMA® buprenorphine by end of 2014 or early 2015 Leading indicators for AVEEDTM on-track Auxilium transaction
U.S. Generic Pharmaceuticals
Building ANDA portfolio
Maximizing high value launch opportunities (e.g., valganciclovir launch) Investing to increase manufacturing capacity to support business
International Pharmaceuticals
New product launches in current markets by Somar and Paladin Potential Latin American extensions for Somar portfolio
Devices
On-track to return to organic revenue growth
Potential new product launches including the investigational TOPAS™ system
12
©2014 Endo Pharmaceuticals Inc. All rights reserved.
|
|
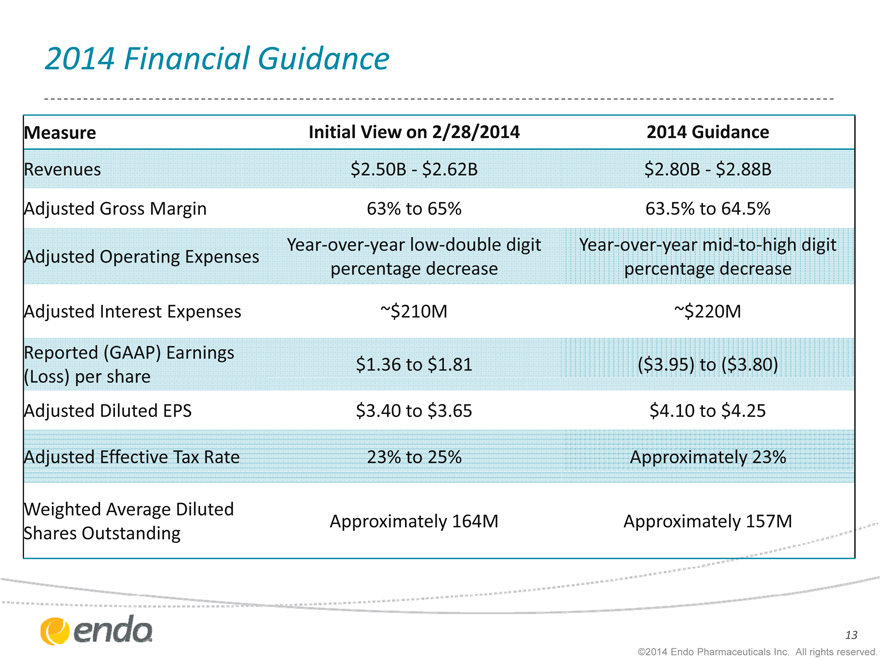
2014 Financial Guidance
Measure
Revenues
Adjusted Gross Margin
Adjusted Operating Expenses
Adjusted Interest Expenses
Reported (GAAP) Earnings
Adjusted Diluted EPS
Adjusted Effective Tax Rate
Weighted Average Diluted Shares Outstanding
Initial View on 2/28/2014
$2.50B - $2.62B
63% to 65%
Year-over-year low-double digit percentage decrease
~$210M $1.36 to $1.81 $3.40 to $3.65 23% to 25% Approximately 164M
2014 Guidance $2.80B - $2.88B 63.5% to 64.5%
Year-over-year mid-to-high digit percentage decrease
~$220M
($3.95) to ($3.80) $4.10 to $4.25 Approximately 23% Approximately 157M
13
©2014 Endo Pharmaceuticals Inc. All rights reserved.
|
|
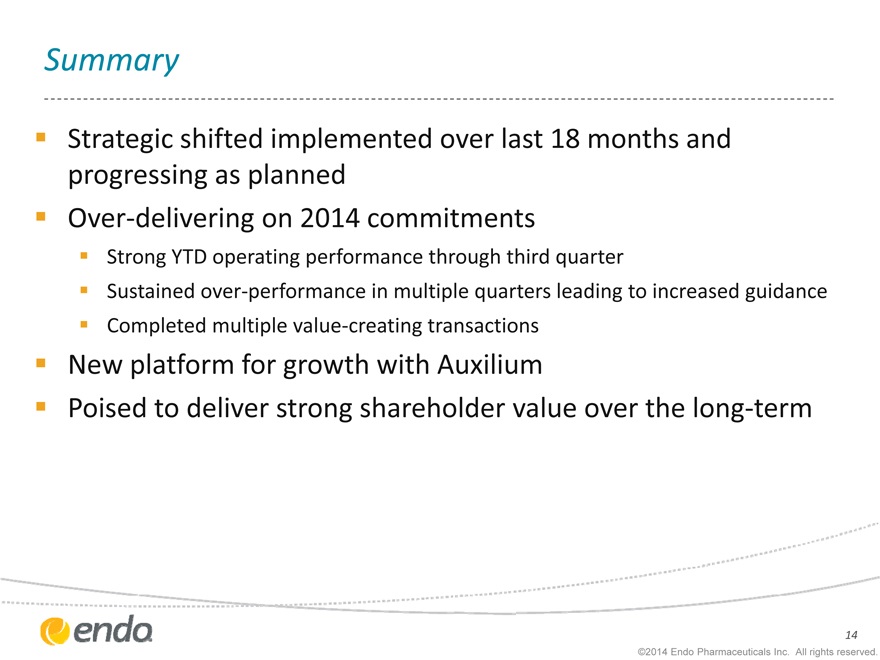
Summary
Strategic shifted implemented over last 18 months and progressing as planned Over-delivering on 2014 commitments
Strong YTD operating performance through third quarter
Sustained over-performance in multiple quarters leading to increased guidance Completed multiple value-creating transactions
New platform for growth with Auxilium
Poised to deliver strong shareholder value over the long-term
14
©2014 Endo Pharmaceuticals Inc. All rights reserved.
|
|
Questions & Answers
©2014 Endo Pharmaceuticals Inc. All rights reserved.
|
|
Appendix
©2014 Endo Pharmaceuticals Inc. All rights reserved.
|
|
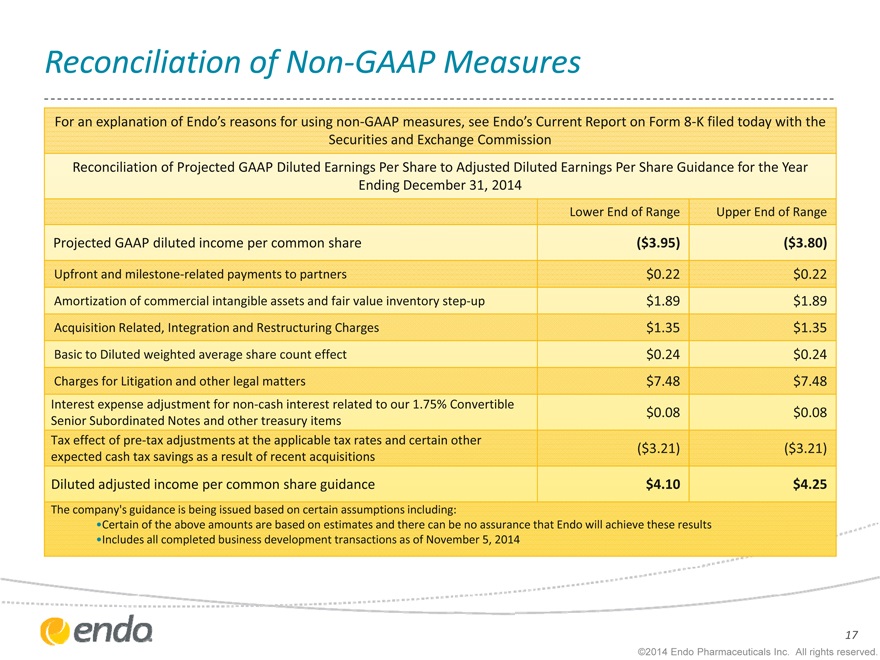
Reconciliation of Non-GAAP Measures
For an explanation of Endo’s reasons for using non-GAAP measures, see Endo’s Current Report on Form 8-K filed today with the Securities and Exchange Commission
Reconciliation of Projected GAAP Diluted Earnings Per Share to Adjusted Diluted Earnings Per Share Guidance for the Year Ending December 31, 2014
Projected GAAP diluted income per common share
Upfront and milestone-related payments to partners
Amortization of commercial intangible assets and fair value inventory step-up Acquisition Related, Integration and Restructuring Charges Basic to Diluted weighted average share count effect
Charges for Litigation and other legal matters
Interest expense adjustment for non-cash interest related to our 1.75% Convertible Senior Subordinated Notes and other treasury items Tax effect of pre-tax adjustments at the applicable tax rates and certain other expected cash tax savings as a result of recent acquisitions
Diluted adjusted income per common share guidance
The company’s guidance is being issued based on certain assumptions including:
Certain of the above amounts are based on estimates and there can be no assurance that Endo will achieve these results
Includes all completed business development transactions as of November 5, 2014
Lower End of Range
($3.95) $0.22 $1.89 $1.35 $0.24 $7.48 $0.08
($3.21) $4.10
Upper End of Range
($3.80)
$0.22
$1.89
$1.35
$0.24
$7.48
$0.08
($3.21)
$4.25
17
©2014 Endo Pharmaceuticals Inc. All rights reserved.
|
|
Endo International plc
Capital Research Presentation
December 3, 2014
©2014 Endo Pharmaceuticals Inc. All rights reserved.