Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - STERIS CORP | d804033d8k.htm |
| EX-99.4 - EX-99.4 - STERIS CORP | d804033dex994.htm |
| EX-99.1 - EX-99.1 - STERIS CORP | d804033dex991.htm |
| EX-10.2 - EX-10.2 - STERIS CORP | d804033dex102.htm |
| EX-10.1 - EX-10.1 - STERIS CORP | d804033dex101.htm |
| EX-2.2 - EX-2.2 - STERIS CORP | d804033dex22.htm |
| EX-2.1 - EX-2.1 - STERIS CORP | d804033dex21.htm |
| EX-99.3 - EX-99.3 - STERIS CORP | d804033dex993.htm |
 STERIS Acquisition
of
Synergy Health
Creating a Global Leader in
Infection Prevention
and
Sterilization
October 13, 2014
Exhibit 99.2 |
 1
Forward Looking Statements
NOT FOR RELEASE, PUBLICATION OR DISTRIBUTION, IN WHOLE OR IN PART, DIRECTLY OR
INDIRECTLY, IN, INTO OR FROM ANY JURISDICTION WHERE TO DO SO WOULD CONSTITUTE
A VIOLATION OF THE RELEVANT LAWS OR REGULATIONS OF SUCH JURISDICTION
No Offer or Solicitation
This document is provided for informational purposes only and does not constitute
an offer to sell, or an invitation to subscribe for, purchase or
exchange,
any
securities
or
the
solicitation
of
any
vote
or
approval
in
any
jurisdiction,
nor
shall
there
be
any
sale,
issuance,
exchange
or
transfer
of
the securities referred to in this document in any jurisdiction in contravention of
applicable law. Forward-Looking Statements
This document may contain statements concerning certain trends, expectations,
forecasts, estimates, or other forward-looking information affecting or
relating to Synergy or STERIS or its industry, products or activities that are intended to qualify for the protections afforded "forward-looking
statements" under the Private Securities Litigation Reform Act of 1995 and
other laws and regulations. Forward-looking statements speak only as to
the date of this document and may be identified by the use of
forward-looking terms such as "may," "will,"
"expects," "believes," "anticipates,"
"plans," "estimates," "projects," "targets,"
"forecasts," "outlook," "impact," "potential," "confidence," "improve," "optimistic," "deliver," "comfortable,"
"trend", and "seeks," or the negative of such terms or other
variations on such terms or comparable terminology. Many important factors could
cause actual results to differ materially from those in the forward-looking
statements including, without limitation, disruption of production or
supplies, changes in market conditions, political events, pending or future claims
or litigation, competitive factors, technology advances, actions of
regulatory agencies, and changes in laws, government regulations, labeling or
product approvals or the application or interpretation thereof. Other risk
factors are described herein and in STERIS and Synergy's other securities filings, including Item 1A of STERIS's Annual Report on Form 10-K
for the year ended March 31, 2014 dated May 29, 2014 and in Synergy's annual report
and accounts for the year ended 30 March 2014 (section headed "principal
risks and uncertainties"). Many of these important factors are outside of STERIS’s or Synergy's control. No assurances can be
provided as to any result or the timing of any outcome regarding
matters described herein or otherwise with respect to any regulatory action,
administrative proceedings, government investigations, litigation, warning
letters, consent decree, cost reductions, business strategies, earnings or
revenue trends or future financial results. References to products and the consent
decree are summaries only and should not be considered the specific
terms
of
the
decree
or
product
clearance
or
literature.
Unless
legally
required,
STERIS
and
Synergy
do
not
undertake
to
update
or
revise
any forward-looking statements even if events make clear that any projected
results, express or implied, will not be realized. Other potential risks and
uncertainties that could cause actual results to differ materially from those in the forward-looking statements include, without limitation, (a) the
receipt of approval of both STERIS’s shareholders and Synergy’s
shareholders, (b) the regulatory approvals required for the transaction not being
obtained
on
the
terms
expected
or
on
the
anticipated
schedule,
(c)
the
parties’
ability
to
meet
expectations
regarding
the
timing,
completion
and
accounting and tax treatments of the transaction, (d) the possibility that the
parties may be unable to achieve expected synergies and operating
efficiencies in connection with the transaction within the expected time-frames
or at all and to successfully integrate Synergy’s operations into those
of STERIS, (e) the integration of Synergy’s operations into those of STERIS
being more difficult, time-consuming or costly than expected, |
 2
Forward Looking Statements
(f) operating costs, customer loss and business disruption (including, without
limitation, difficulties in maintaining relationships with employees,
customers, clients or suppliers) being greater than expected following the
transaction, (g) the retention of certain key employees of Synergy being
difficult,
(h)
changes
in
tax
laws
or
interpretations
that
could
increase
our
consolidated
tax
liabilities,
including,
if
the
transaction
is
consummated,
changes in tax laws that would result in New STERIS being treated as a domestic
corporation for United States federal tax purposes, (i) the potential for
increased pressure on pricing or costs that leads to erosion of profit margins, (j) the possibility that market demand will not develop for
new technologies, products or applications or services, or business initiatives
will take longer, cost more or produce lower benefits than anticipated,
(k)
the
possibility
that
application
of
or
compliance
with
laws,
court
rulings,
certifications,
regulations,
regulatory
actions,
including
without
limitation
those relating to FDA warning notices or letters, government investigations, the
outcome of any pending FDA requests, inspections or submissions, or
other
requirements
or
standards
may
delay,
limit
or
prevent
new
product
introductions,
affect
the
production
and
marketing
of
existing
products
or services or otherwise affect Company performance, results, prospects or value,
(l) the potential of international unrest, economic downturn or effects of
currencies, tax assessments, adjustments or anticipated rates, raw material costs or availability, benefit or retirement plan costs, or other
regulatory compliance costs, (m) the possibility of reduced demand, or reductions
in the rate of growth in demand, for products and services, (n) the
possibility that anticipated growth, cost savings, new product acceptance, performance or approvals, or other results may not be achieved, or
that transition, labor, competition, timing, execution, regulatory, governmental,
or other issues or risks associated with STERIS and Synergy's businesses,
industry or initiatives including, without limitation, the consent decree or those matters described in STERIS's Form 10-K for the year
ended
March
31,
2014
and
other
securities
filings,
may
adversely
impact
Company
performance,
results,
prospects
or
value,
(o)
the
possibility
that
anticipated financial results or benefits of recent acquisitions, or of STERIS's
restructuring efforts will not be realized or will be other than
anticipated, (p) the effects of the contractions in credit availability, as well as
the ability of STERIS and Synergy's customers and suppliers to adequately
access the credit markets when needed, and (q) those risks described in STERIS's Annual Report on Form 10-K for the year ended
March 31, 2014, and other securities filings.
Important Additional Information Regarding the Transaction Will Be Filed With The
SEC It is expected that the shares of New STERIS to be issued by New STERIS
to Synergy Shareholders in the U.K. law scheme of arrangement transaction that forms a part of the transaction will be issued in
reliance upon the exemption from the registration requirements of the Securities
Act of 1933, as amended, provided by Section 3(a)(10) thereof. In connection
with the issuance of New STERIS shares to STERIS shareholders pursuant to the merger that forms a part of the transaction, New
STERIS will file with the SEC a registration statement on Form S-4 that will
contain a prospectus of New STERIS as well as a proxy statement of
STERIS relating to the merger that forms a part of the transaction, which we refer
to together as the Form S-4/Proxy Statement. INVESTORS AND SECURITY
HOLDERS ARE URGED TO READ THE FORM S-4/PROXY STATEMENT, AND OTHER DOCUMENTS FILED
WITH
THE
SEC
IN
CONNECTION
WITH
THE
TRANSACTION
CAREFULLY
AND
IN
THEIR
ENTIRETY,
BECAUSE
THEY
WILL
CONTAIN
IMPORTANT INFORMATION ABOUT THE TRANSACTION, THE PARTIES TO THE TRANSACTION AND THE
RISKS ASSOCIATED WITH THE TRANSACTION.. . |
 3
Forward Looking Statements
Those documents, if and when filed, as well as STERIS’S and New STERIS’s
other public filings with the SEC may be obtained without charge at the
SEC’s website at www.sec.gov, at STERIS’s website at www.steris-ir.com. Security holders and other interested parties will also be able to
obtain, without charge, a copy of the Form S-4/Proxy Statement and other
relevant documents (when available) by directing a request by mail or
telephone Julie_Winter@steris.com or (440) 392-7245. Security holders
may also read and copy any reports, statements and other information
filed
with
the
SEC
at
the
SEC
public
reference
room
at
100
F
Street
N.E.,
Room
1580,
Washington,
D.C.
20549.
Please
call
the
SEC
at
(800)
732-
0330 or visit the SEC’s website for further information on its public
reference room. STERIS, its directors and certain of its executive officers
may be considered participants in the solicitation of proxies in connection with the
transactions contemplated by the Proxy Statement. Information about the directors
and executive officers of STERIS is set forth in its Annual Report on Form
10-K for the year ended 31 March, 2014, which was filed with the SEC on 29 May, 2014, and its proxy statement for its 2014
annual
meeting
of
shareholders,
which
was
filed
with
the
SEC
on
9
June,
2014.
Other
information
regarding
potential
participants
in
the
proxy
solicitations and a description of their direct and indirect interests, by security
holdings or otherwise, will be contained in the Form S-4/Proxy Statement
when it is filed. Synergy and New STERIS are each organised under the laws
of England. Some of the officers and directors of Synergy and New STERIS may be
residents of countries other than the United States. As a result, it may not be
possible to sue Synergy, New STERIS or such persons in a non-US
court
for
violations
of
US
securities
laws.
It
may
be
difficult
to
compel
Synergy,
New
STERIS
and
their
respective
affiliates
to
subject
themselves
to
the jurisdiction and judgment of a US court or for investors to enforce against
them the judgments of US courts. Participants in the Solicitation
STERIS, its directors and certain of its executive officers may be considered
participants in the solicitation of proxies in connection with the
transactions contemplated by the Proxy Statement. Information about the directors
and executive officers of STERIS is set forth in its Annual Report on Form
10-K for the year ended 31 March, 2014, which was filed with the SEC on 29 May, 2014, and its proxy statement for its 2014
annual
meeting
of
shareholders,
which
was
filed
with
the
SEC
on
9
June,
2014.
Other
information
regarding
potential
participants
in
the
proxy
solicitations and a description of their direct and indirect interests, by security
holdings or otherwise, will be contained in the Proxy Statement/Prospectus
when it is filed. Responsibility
The
directors
of
STERIS
accept
responsibility
for
the
information
contained
in
this
document
and,
to
the
best
of
their
knowledge
and
belief
(having
taken
all
reasonable
care
to
ensure
that
such
is
the
case),
the
information
contained
in
this
document
is
in
accordance
with
the
facts
and
it
does
not
omit anything likely to affect the import of such information.
|
 4
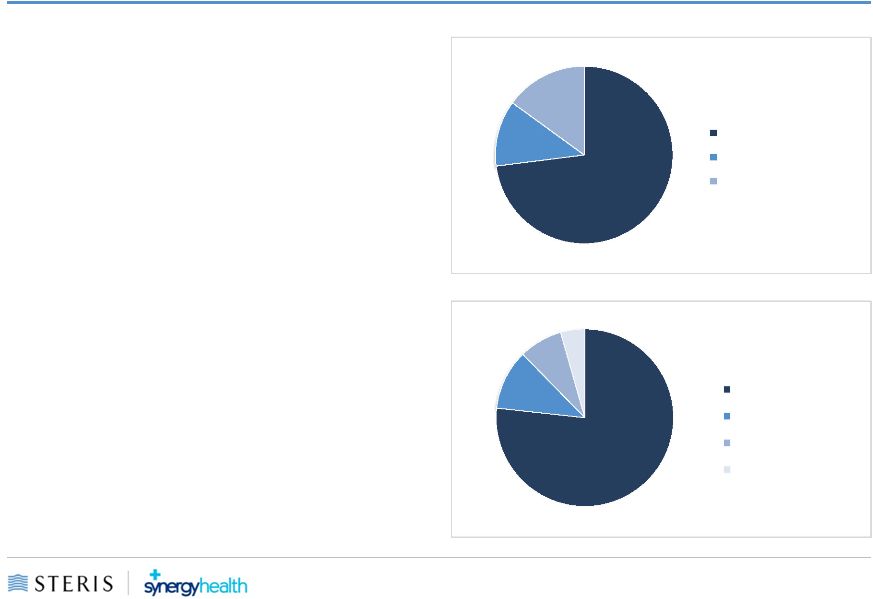
STERIS Overview
•
A global leader in infection prevention,
decontamination, GI and surgical products
and services
•
$1.9
billion
in
revenue
in
FY15
•
Approximately
8,000
people worldwide
•
Direct sales and service force of over 2,500
team members
•
77%
of
revenue
in
the
United
States
•
Nine
acquisitions in the past three years
Business Mix
by Segment
Note:
Based
on
FY2014
actual
revenues
Geographic Mix
73%
12%
15%
77%
11%
8%
4%
Healthcare
Isomedix
Life Sciences
United States
EMEA
APAC / LATAM
Canada |
 5
Synergy Overview
•
Global leader in outsourced sterilization
services for medical device manufacturers,
hospitals and other industries.
Applied
Sterilisation
Technologies
(AST)
provider of outsourced sterilization to medical device
manufacturers
Hospital Sterilization Services (HSS)
provider of outsourced decontamination services for
reusable medical and surgical equipment
Healthcare Solutions (HCS)
provider of a range of products and services in
managing the environment in a healthcare setting
•
$604
million in revenue in Fiscal Year 2014
•
UK-domiciled
FTSE
250
company
•
6,000
employees worldwide
•
Three
acquisitions
in
the
past
two
years
Note:
Business mix by segment figures pertain to Q1 FY2015
Geographic Mix relates to FY14 results
Business Mix
by Segment
Geographic Mix
25%
Applied Sterilization
Technologies (AST)
Healthcare Solutions
(HCS)
Hospital Sterilisation
Services (HSS)
34%
41%
43%
31%
5%
21%
UK & Ireland
Europe & Middle East
Asia & Africa
Americas |
 6
Transaction Overview
Transaction Terms
•
Acquisition of Synergy for $1.9
billion in cash and New STERIS
stock
•
STERIS shareholders to receive
one share of New STERIS for
each STERIS share
•
Synergy shareholders to receive
per-share consideration valued
at £19.50, consisting of £4.39
cash and 0.4308 share of New
STERIS
•
STERIS has secured committed
funding for the transaction
•
New STERIS ownership: 70%
STERIS shareholders / 30%
Synergy shareholders
Substantial Premium to
Synergy Shareholders
•
% premium to last closing
share price
•
% premium to 3-month VWAP
•
% premium to 52-week high
Transaction Structure
•
STERIS and Synergy each to
merge into subsidiaries of a UK
holding company New STERIS
•
New STERIS incorporated in the
UK; New STERIS expected to
retain NYSE listing under ticker
STE
•
New STERIS to maintain
operational headquarters in
Mentor, Ohio
39
32
27 |
 7
Strategic Rationale
Create a global
leader in infection
prevention and
sterilization
•
Allows STERIS to better
provide comprehensive
solutions to device
companies and
hospitals around the
world
•
Build on STERIS’s
recent acquisitions to
provide an expanded
suite of integrated,
value-added products
and services to
hospitals globally
Increase portfolio
diversity
•
Create a more balanced
portfolio from which
New STERIS could
deliver products and
services tailored to best
serve the evolving
needs of global
Customers
Increase
geographic
diversity
•
Combines STERIS’s
strength in North
America with Synergy’s
strong positions in
Europe
•
Increases exposure to
emerging markets such
as Asia Pacific and
Latin America
•
Brings together the
geographically
complementary STERIS
Isomedix and Synergy
AST device sterilization
businesses to create a
leading global supplier
to best serve medical
device Customers
Accelerate
growth profile
•
Estimated annual pre-
tax cost synergies of
$30
million
or
more,
which will be phased
50% in fiscal 2016 and
100% thereafter
•
Expected to be
significantly accretive to
adjusted earnings per
diluted
share
in
fiscal
year 2016
and beyond |
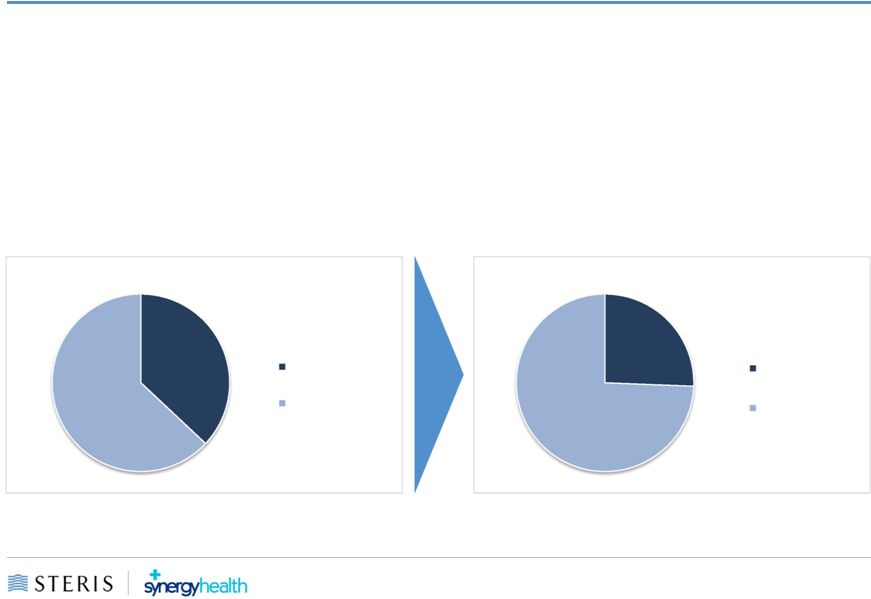 Greater
Portfolio Diversity STERIS
New STERIS
Source:
Company filings and investor presentation.
8
Capital
Recurring
Capital
Recurring
63%
37%
74%
26%
•
More balanced portfolio
from which New STERIS could deliver products and services
tailored
to
best
serve
the
evolving
needs
of
global
Customers
•
Differentiated product
and service offering
•
Potential
to
cross-sell
STERIS’s
products
and
services
to
Synergy’s
Customers
and
vice versa
•
Increases
recurring
revenue
mix
–
combined
58
Isomedix/AST
facilities
in
18
countries |
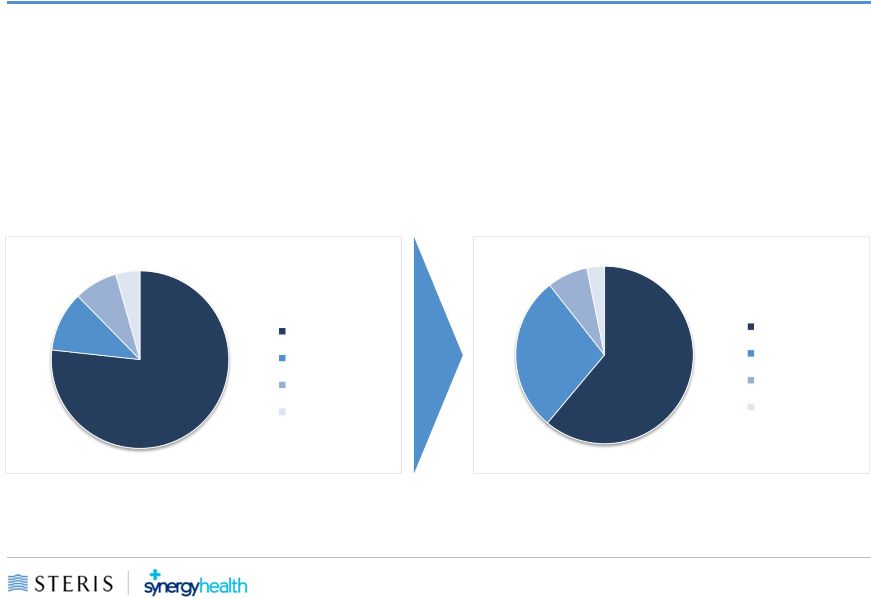 9
Greater Geographic Diversity
•
Combines
STERIS’s strength in North America with
Synergy’s strong positions in
Europe
•
Brings together the geographically complementary
STERIS’s Isomedix and
Synergy’s AST
device sterilization businesses to create a leading global supplier to best serve medical device Customers
Source:
Company filings and investor presentation.
Note:
Based on STERIS FY2014 actual revenues and Synergy management
STERIS
New STERIS
77%
11%
8%
4%
United States
EMEA
APAC / LATAM
Canada
61%
28%
8%
3%
US
EMEA
APAC/LATAM
Canada |
 10
Accelerated Growth Profile
Accelerated growth
profile
•
Leverage STERIS's
capabilities and infrastructure
to make Synergy’s products
and services more successful
•
Access Synergy’s Customer
base to cross-sell current and
new STERIS products and
services
$30 million or more
estimated annual pre-
tax cost synergies
•
Optimizing global back-office
•
Leveraging best-
demonstrated practices
across plants
•
In-sourcing consumables
•
Eliminating redundant public
company costs
•
Phased in 50% in fiscal 2016
and 100% thereafter
Compelling financial
benefits and improved
financial flexibility
•
Expected to be significantly
accretive to adjusted earnings
per diluted share in fiscal year
2016 and beyond
•
New STERIS is expected to
have an effective tax rate of
approximately 25% by fiscal
year 2016
•
Provide more flexible access
to New STERIS’s global cash
flows |
 11
Logical Extension of STERIS’s Growth Strategy
STERIS track record of executing
value-creating M&A transactions
•
Strengthen core business (VTS Medical and
Eschmann, Biotest)
•
Expand GI business (US Endoscopy)
•
National consolidator of instrument repair
business (Spectrum, TRE, Florida Surgical, Life
Systems, IMS)
Acquisition of Synergy is
consistent with long-term goals
•
Mid-
to high-single digit revenue growth
Market growth
Success with new products
Acquisitions
•
Double-digit earnings per share growth
•
Generate robust annual free cash flows |
 12
Efficient Capital Allocation
Reasonable debt-to-capital levels
with access to additional funds
•
Anticipated debt to EBITDA of ~2.9x (an
increase from 2.2x as of June 30, 2014)
•
More flexible access to global cash
•
Revolving credit facility in place to support
operational needs
•
In conjunction with the transaction, STERIS obtained
a 364-Day Bridge Credit Agreement. Bank of
America Merrill Lynch, J.P. Morgan and Key Bank
provided committed financing in conjunction with the
transaction in the amount of approximately $1.6
billion.
Disciplined capital allocation
policies
•
Maintain and grow our dividend responsibly
relative to our growth
•
Invest for growth in our organic businesses
•
Targeted acquisitions in adjacent product and
market areas
•
Reduce total company leverage
•
Share buybacks |
 13
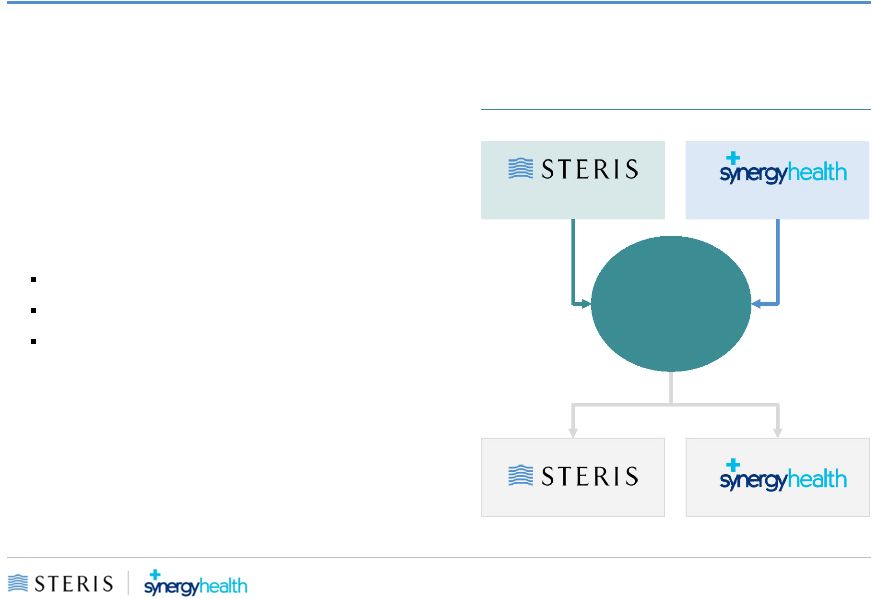
New STERIS Corporate Structure
•
STERIS and Synergy to each merge into
subsidiaries of a new holding company New
STERIS
•
New STERIS to be incorporated in the UK
•
STERIS’s operational headquarters will
remain in Mentor, Ohio
•
New STERIS expected to have a 13 member
Board of Directors
10 current STERIS Directors
Synergy CEO
Two additional members from Synergy Board
•
New STERIS shares expected to be listed on
the New York Stock Exchange under the
ticker STE
~70%
~30%
Note:
Excludes,
for
clarity
only,
US
Acquisition
Co,
into
which
STERIS
will
initially merge, and UK merger sub, into which Synergy will merge.
Post-Closing
Organizational Structure
shareholders
shareholders
New STERIS
(UK-
Incorporated) |
 14
Next Steps
Combination to be
implemented by UK
Scheme of Arrangement
•
New STERIS S-4/Proxy
expected to be filed in
November
•
Scheme to become effective
and transaction expected to
be completed by the end of
fiscal 2015
Regulatory approvals
•
Subject to STERIS and
Synergy shareholder
approvals
•
Subject to regulatory review in
the U.S. and U.K.
Next Communications
•
STERIS and Synergy’s 2Q15
earnings calls are scheduled
for November 5, 2014 |
